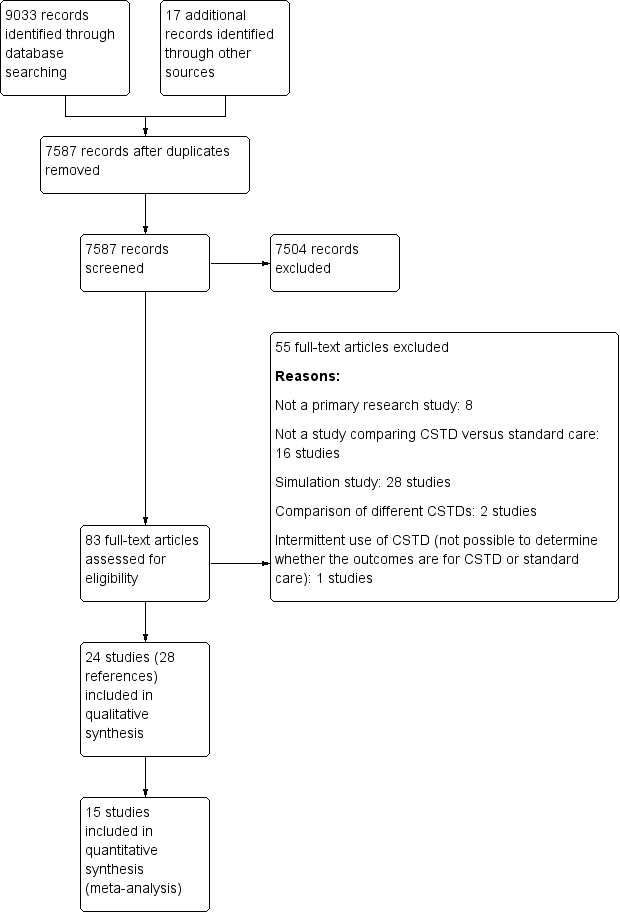
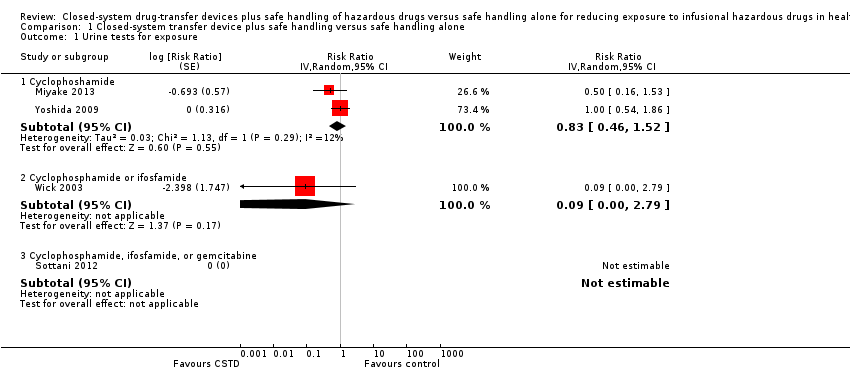
| 1 Urine tests for exposure (ICC = 0.05) Show forest plot | 4 | | Risk Ratio (Random, 95% CI) | Subtotals only |
|
| 1.1 Cyclophoshamide | 2 | | Risk Ratio (Random, 95% CI) | 0.82 [0.44, 1.52] |
| 1.2 Cyclophosphamide or ifosfamide | 1 | | Risk Ratio (Random, 95% CI) | 0.09 [0.00, 1.99] |
| 1.3 Cyclophosphamide, ifosfamide, or gemcitabine | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Urine tests for exposure (ICC = 0.01) Show forest plot | 4 | | Risk Ratio (Random, 95% CI) | Subtotals only |
|
| 2.1 Cyclophoshamide | 2 | | Risk Ratio (Random, 95% CI) | 0.81 [0.43, 1.51] |
| 2.2 Cyclophosphamide or ifosfamide | 1 | | Risk Ratio (Random, 95% CI) | 0.09 [0.01, 1.48] |
| 2.3 Cyclophosphamide, ifosfamide, or gemcitabine | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
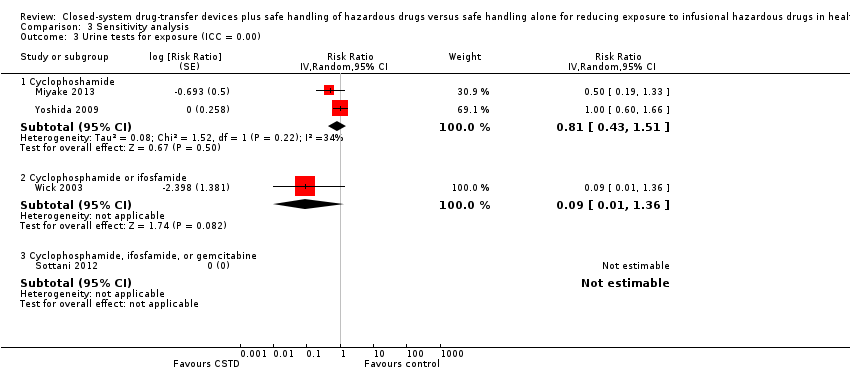
| 3 Urine tests for exposure (ICC = 0.00) Show forest plot | 4 | | Risk Ratio (Random, 95% CI) | Subtotals only |
|
| 3.1 Cyclophoshamide | 2 | | Risk Ratio (Random, 95% CI) | 0.81 [0.43, 1.51] |
| 3.2 Cyclophosphamide or ifosfamide | 1 | | Risk Ratio (Random, 95% CI) | 0.09 [0.01, 1.36] |
| 3.3 Cyclophosphamide, ifosfamide, or gemcitabine | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
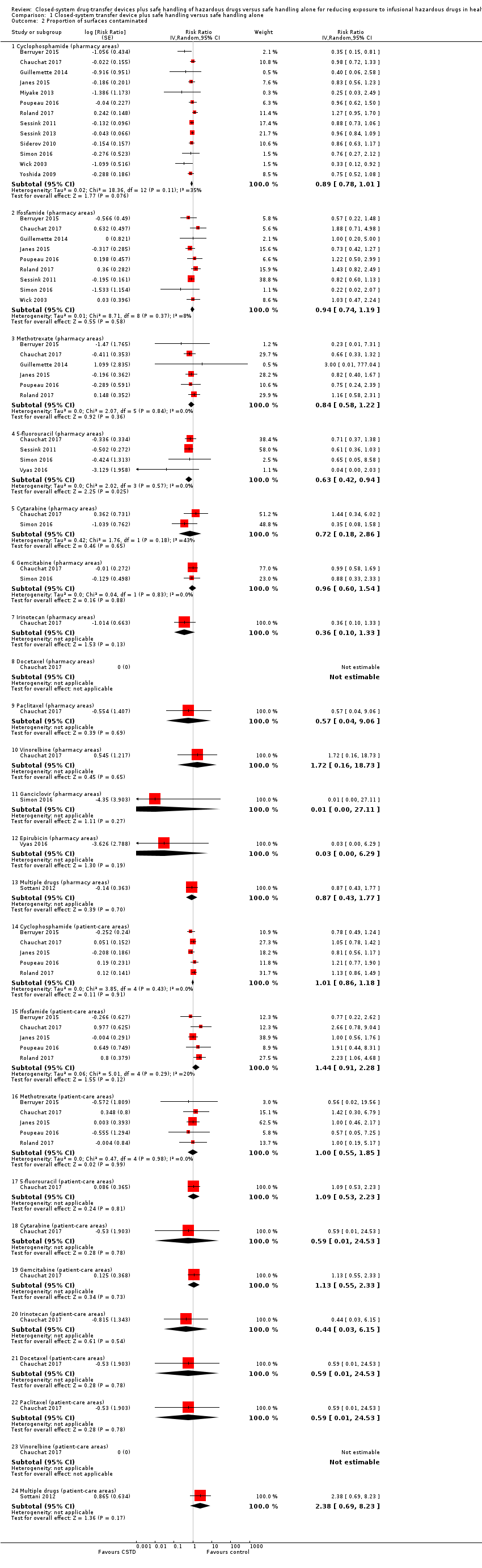
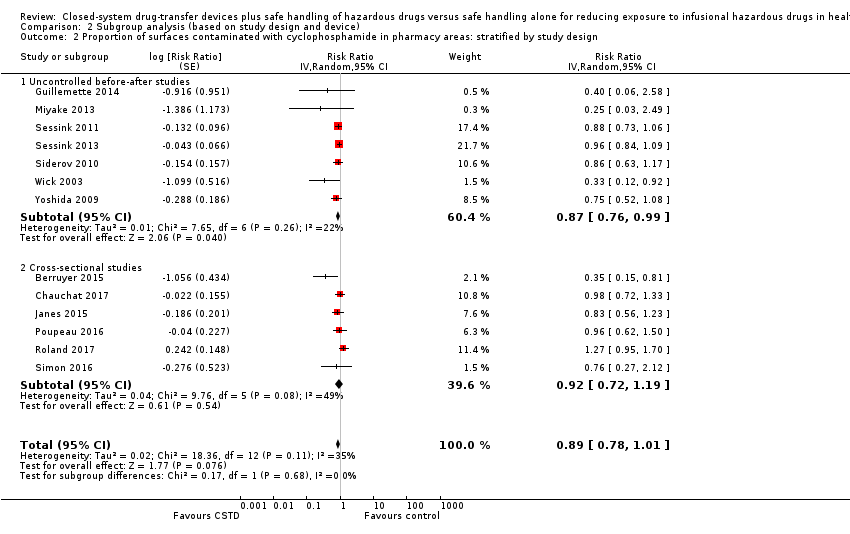
| 4 Proportion of surfaces contaminated (ICC = 0.05) Show forest plot | 15 | | Risk Ratio (Random, 95% CI) | Subtotals only |
|
| 4.1 Cyclophosphamide (pharmacy areas) | 13 | | Risk Ratio (Random, 95% CI) | 0.87 [0.76, 0.99] |
| 4.2 Ifosfamide (pharmacy areas) | 9 | | Risk Ratio (Random, 95% CI) | 0.94 [0.73, 1.23] |
| 4.3 Methotrexate (pharmacy areas) | 6 | | Risk Ratio (Random, 95% CI) | 0.84 [0.60, 1.19] |
| 4.4 5‐fluorouracil (pharmacy areas) | 4 | | Risk Ratio (Random, 95% CI) | 0.62 [0.42, 0.92] |
| 4.5 Cytarabine (pharmacy areas) | 2 | | Risk Ratio (Random, 95% CI) | 0.68 [0.17, 2.67] |
| 4.6 Gemcitabine (pharmacy areas) | 2 | | Risk Ratio (Random, 95% CI) | 0.95 [0.64, 1.43] |
| 4.7 Irinotecan (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.36 [0.11, 1.19] |
| 4.8 Docetaxel (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Paclitaxel (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.57 [0.05, 7.13] |
| 4.10 Vinorelbine (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.72 [0.20, 15.25] |
| 4.11 Ganciclovir (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.01 [0.00, 3.43] |
| 4.12 Epirubicin (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.03 [0.00, 2.02] |
| 4.13 Multiple drugs (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.87 [0.49, 1.53] |
| 4.14 Cyclophosphamide (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.00 [0.86, 1.17] |
| 4.15 Ifosfamide (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.46 [0.91, 2.34] |
| 4.16 Methotrexate (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.01 [0.57, 1.76] |
| 4.17 5‐fluorouracil (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.09 [0.57, 2.10] |
| 4.18 Cytarabine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 18.10] |
| 4.19 Gemcitabine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.13 [0.58, 2.20] |
| 4.20 Irinotecan (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.44 [0.04, 4.97] |
| 4.21 Docetaxel (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 18.10] |
| 4.22 Paclitaxel (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 18.10] |
| 4.23 Vinorelbine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.24 Multiple drugs (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 2.38 [0.79, 7.17] |
| 5 Proportion of surfaces contaminated (ICC = 0.01) Show forest plot | 15 | | Risk Ratio (Random, 95% CI) | Subtotals only |
|
| 5.1 Cyclophosphamide (pharmacy areas) | 13 | | Risk Ratio (Random, 95% CI) | 0.83 [0.73, 0.96] |
| 5.2 Ifosfamide (pharmacy areas) | 9 | | Risk Ratio (Random, 95% CI) | 0.89 [0.66, 1.21] |
| 5.3 Methotrexate (pharmacy areas) | 6 | | Risk Ratio (Random, 95% CI) | 0.85 [0.62, 1.16] |
| 5.4 5‐fluorouracil (pharmacy areas) | 4 | | Risk Ratio (Random, 95% CI) | 0.57 [0.33, 0.97] |
| 5.5 Cytarabine (pharmacy areas) | 2 | | Risk Ratio (Random, 95% CI) | 0.64 [0.17, 2.51] |
| 5.6 Gemcitabine (pharmacy areas) | 2 | | Risk Ratio (Random, 95% CI) | 0.93 [0.69, 1.24] |
| 5.7 Irinotecan (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.36 [0.12, 1.08] |
| 5.8 Docetaxel (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.9 Paclitaxel (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.57 [0.06, 5.79] |
| 5.10 Vinorelbine (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.72 [0.23, 12.73] |
| 5.11 Ganciclovir (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.01 [0.00, 0.27] |
| 5.12 Epirubicin (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.03 [0.00, 0.62] |
| 5.13 Multiple drugs (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.87 [0.57, 1.32] |
| 5.14 Cyclophosphamide (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.00 [0.85, 1.17] |
| 5.15 Ifosfamide (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.47 [0.91, 2.38] |
| 5.16 Methotrexate (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.01 [0.60, 1.69] |
| 5.17 5‐fluorouracil (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.09 [0.59, 2.00] |
| 5.18 Cytarabine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 13.92] |
| 5.19 Gemcitabine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.13 [0.61, 2.09] |
| 5.20 Irinotecan (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.44 [0.05, 4.13] |
| 5.21 Docetaxel (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 13.92] |
| 5.22 Paclitaxel (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 13.92] |
| 5.23 Vinorelbine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.24 Multiple drugs (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 2.38 [0.89, 6.33] |
| 6 Proportion of surfaces contaminated (ICC = 0.00) Show forest plot | 15 | | Risk Ratio (Random, 95% CI) | Subtotals only |
|
| 6.1 Cyclophosphamide (pharmacy areas) | 13 | | Risk Ratio (Random, 95% CI) | 0.82 [0.72, 0.94] |
| 6.2 Ifosfamide (pharmacy areas) | 9 | | Risk Ratio (Random, 95% CI) | 0.85 [0.59, 1.21] |
| 6.3 Methotrexate (pharmacy areas) | 6 | | Risk Ratio (Random, 95% CI) | 0.86 [0.63, 1.16] |
| 6.4 5‐fluorouracil (pharmacy areas) | 4 | | Risk Ratio (Random, 95% CI) | 0.55 [0.32, 0.94] |
| 6.5 Cytarabine (pharmacy areas) | 2 | | Risk Ratio (Random, 95% CI) | 0.64 [0.16, 2.46] |
| 6.6 Gemcitabine (pharmacy areas) | 2 | | Risk Ratio (Random, 95% CI) | 0.91 [0.73, 1.13] |
| 6.7 Irinotecan (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.36 [0.13, 1.05] |
| 6.8 Docetaxel (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.9 Paclitaxel (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.57 [0.06, 5.48] |
| 6.10 Vinorelbine (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.72 [0.24, 12.15] |
| 6.11 Ganciclovir (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.01 [0.00, 0.09] |
| 6.12 Epirubicin (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.03 [0.00, 0.42] |
| 6.13 Multiple drugs (pharmacy areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.87 [0.60, 1.26] |
| 6.14 Cyclophosphamide (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.00 [0.85, 1.17] |
| 6.15 Ifosfamide (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.48 [0.91, 2.38] |
| 6.16 Methotrexate (patient‐care areas) | 5 | | Risk Ratio (Random, 95% CI) | 1.01 [0.61, 1.67] |
| 6.17 5‐fluorouracil (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.09 [0.60, 1.97] |
| 6.18 Cytarabine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 14.36] |
| 6.19 Gemcitabine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 1.13 [0.62, 2.06] |
| 6.20 Irinotecan (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.44 [0.05, 3.93] |
| 6.21 Docetaxel (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 14.36] |
| 6.22 Paclitaxel (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.59 [0.02, 14.36] |
| 6.23 Vinorelbine (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.24 Multiple drugs (patient‐care areas) | 1 | | Risk Ratio (Random, 95% CI) | 2.38 [0.92, 6.12] |
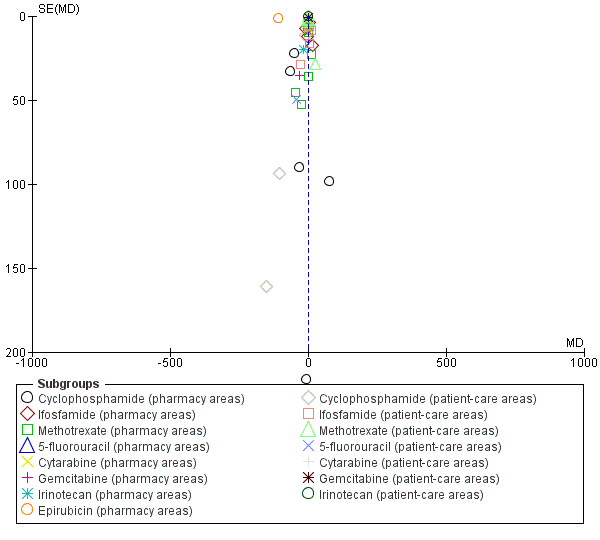
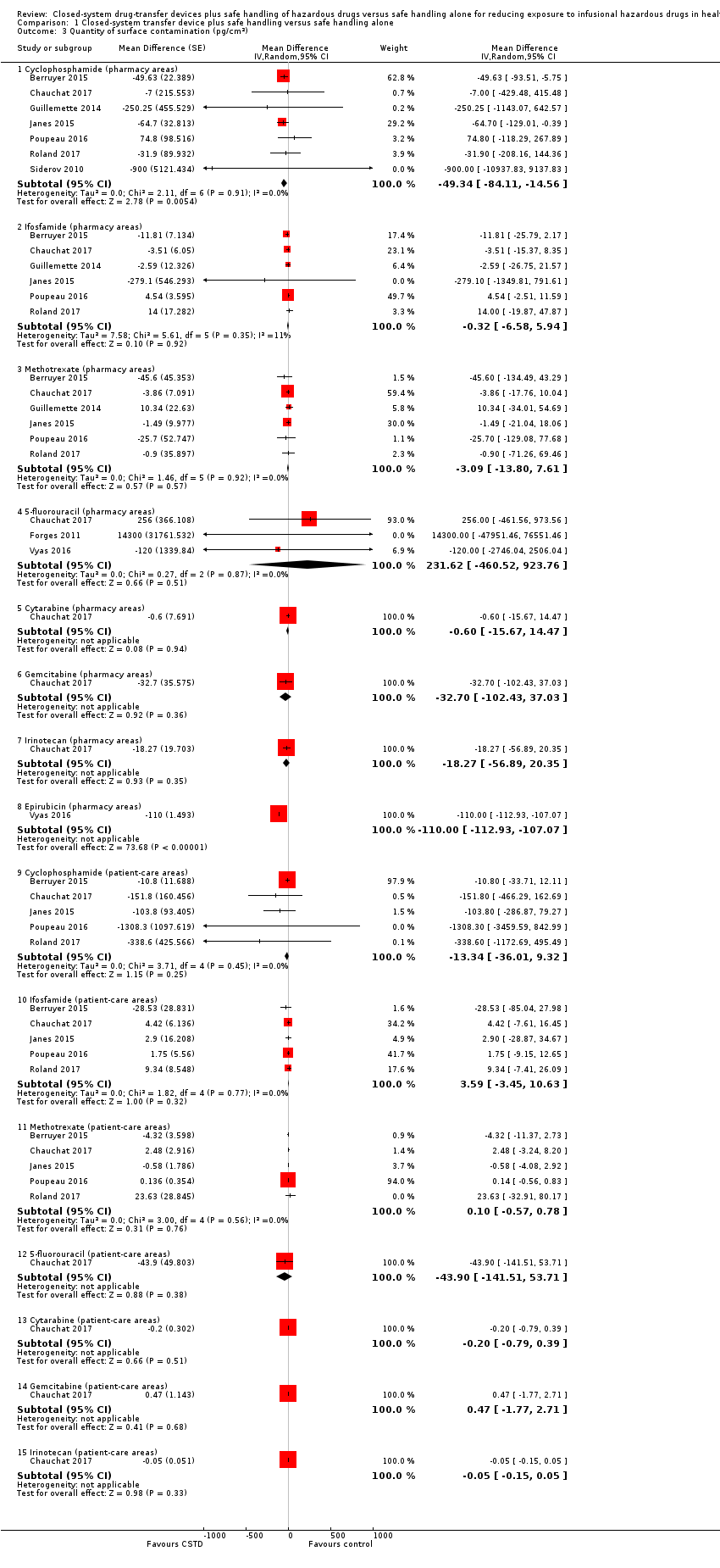
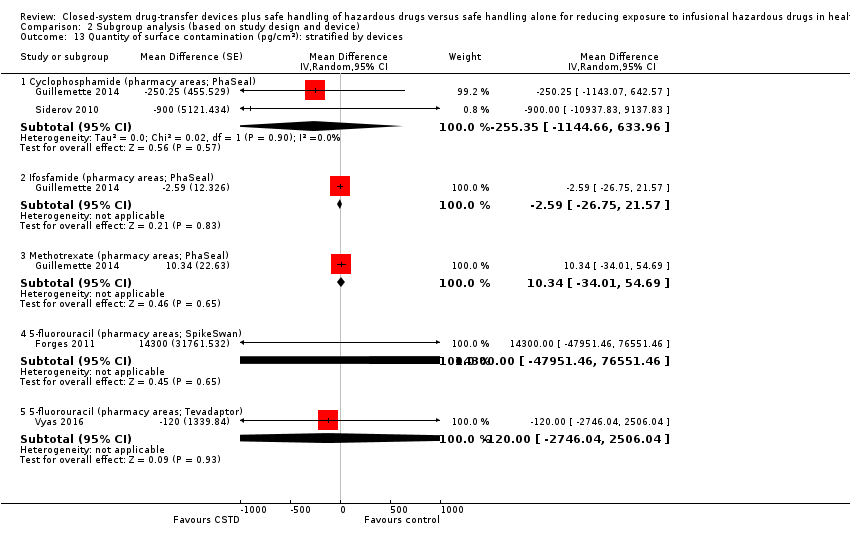
| 7 Quantity of surface contamination (pg/cm²) (ICC = 0.05) Show forest plot | 9 | | Mean Difference (Random, 95% CI) | Subtotals only |
|
| 7.1 Cyclophosphamide (pharmacy areas) | 7 | | Mean Difference (Random, 95% CI) | ‐49.47 [‐81.21, ‐17.73] |
| 7.2 Ifosfamide (pharmacy areas) | 6 | | Mean Difference (Random, 95% CI) | ‐0.95 [‐7.63, 5.72] |
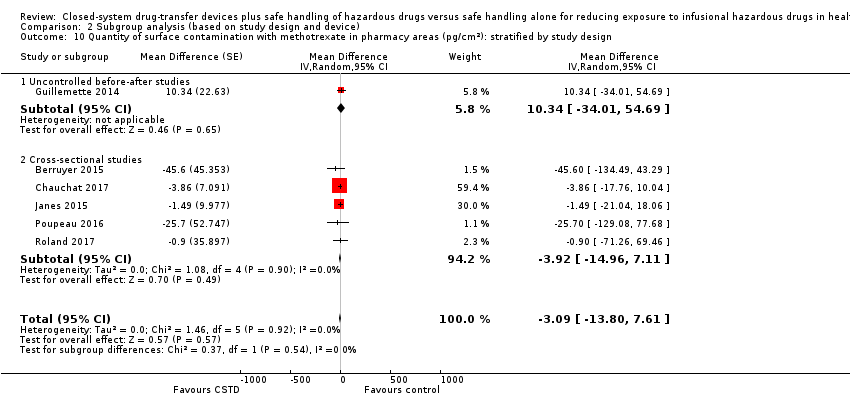
| 7.3 Methotrexate (pharmacy areas) | 6 | | Mean Difference (Random, 95% CI) | ‐2.78 [‐12.45, 6.89] |
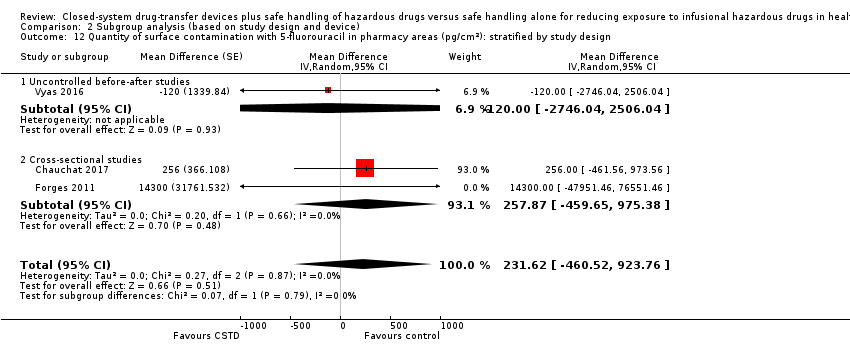
| 7.4 5‐fluorouracil (pharmacy areas) | 3 | | Mean Difference (Random, 95% CI) | 224.25 [‐400.98, 849.49] |
| 7.5 Cytarabine (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.6 [‐14.37, 13.17] |
| 7.6 Gemcitabine (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐32.7 [‐96.40, 31.00] |
| 7.7 Irinotecan (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐18.27 [‐53.55, 17.01] |
| 7.8 Epirubicin (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐110.0 [‐112.32, ‐107.68] |
| 7.9 Cyclophosphamide (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | ‐30.44 [‐90.78, 29.90] |
| 7.10 Ifosfamide (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | 3.59 [‐2.88, 10.07] |
| 7.11 Methotrexate (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | 0.10 [‐0.51, 0.72] |
| 7.12 5‐fluorouracil (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐43.9 [‐133.57, 45.77] |
| 7.13 Cytarabine (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.20 [‐0.74, 0.34] |
| 7.14 Gemcitabine (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | 0.47 [‐1.59, 2.53] |
| 7.15 Irinotecan (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.05 [‐0.14, 0.04] |
| 8 Quantity of surface contamination (pg/cm²) (ICC = 0.01) Show forest plot | 9 | | Mean Difference (Random, 95% CI) | Subtotals only |
|
| 8.1 Cyclophosphamide (pharmacy areas) | 7 | | Mean Difference (Random, 95% CI) | ‐49.85 [‐78.93, ‐20.77] |
| 8.2 Ifosfamide (pharmacy areas) | 6 | | Mean Difference (Random, 95% CI) | ‐1.29 [‐7.89, 5.32] |
| 8.3 Methotrexate (pharmacy areas) | 6 | | Mean Difference (Random, 95% CI) | ‐1.93 [‐10.50, 6.64] |
| 8.4 5‐fluorouracil (pharmacy areas) | 3 | | Mean Difference (Random, 95% CI) | 207.62 [‐351.04, 766.28] |
| 8.5 Cytarabine (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.6 [‐13.23, 12.03] |
| 8.6 Gemcitabine (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐32.7 [‐91.14, 25.74] |
| 8.7 Irinotecan (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐18.27 [‐50.63, 14.09] |
| 8.8 Epirubicin (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐110.0 [‐111.68, ‐108.32] |
| 8.9 Cyclophosphamide (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | ‐49.62 [‐133.49, 34.25] |
| 8.10 Ifosfamide (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | 3.59 [‐2.39, 9.58] |
| 8.11 Methotrexate (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | 0.06 [‐0.81, 0.94] |
| 8.12 5‐fluorouracil (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐43.9 [‐126.66, 38.86] |
| 8.13 Cytarabine (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.2 [‐0.70, 0.30] |
| 8.14 Gemcitabine (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | 0.47 [‐1.43, 2.37] |
| 8.15 Irinotecan (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.05 [‐0.13, 0.03] |
| 9 Quantity of surface contamination (pg/cm²) (ICC = 0.00) Show forest plot | 9 | | Mean Difference (Random, 95% CI) | Subtotals only |
|
| 9.1 Cyclophosphamide (pharmacy areas) | 7 | | Mean Difference (Random, 95% CI) | ‐50.15 [‐78.51, ‐21.79] |
| 9.2 Ifosfamide (pharmacy areas) | 6 | | Mean Difference (Random, 95% CI) | ‐1.36 [‐7.81, 5.09] |
| 9.3 Methotrexate (pharmacy areas) | 6 | | Mean Difference (Random, 95% CI) | ‐1.32 [‐9.47, 6.84] |
| 9.4 5‐fluorouracil (pharmacy areas) | 3 | | Mean Difference (Random, 95% CI) | 198.18 [‐338.80, 735.17] |
| 9.5 Cytarabine (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.6 [‐12.93, 11.73] |
| 9.6 Gemcitabine (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐32.7 [‐89.74, 24.34] |
| 9.7 Irinotecan (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐18.27 [‐49.86, 13.32] |
| 9.8 Epirubicin (pharmacy areas) | 1 | | Mean Difference (Random, 95% CI) | ‐110.0 [‐111.48, ‐108.52] |
| 9.9 Cyclophosphamide (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | ‐53.61 [‐141.33, 34.10] |
| 9.10 Ifosfamide (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | 3.60 [‐2.27, 9.46] |
| 9.11 Methotrexate (patient‐care areas) | 5 | | Mean Difference (Random, 95% CI) | 0.03 [‐1.02, 1.08] |
| 9.12 5‐fluorouracil (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐43.9 [‐124.84, 37.04] |
| 9.13 Cytarabine (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.2 [‐0.69, 0.29] |
| 9.14 Gemcitabine (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | 0.47 [‐1.39, 2.33] |
| 9.15 Irinotecan (patient‐care areas) | 1 | | Mean Difference (Random, 95% CI) | ‐0.05 [‐0.13, 0.03] |