Aspirina o heparina o ambas para mejorar los resultados del embarazo en mujeres con anticuerpos antifosfolipídicos persistentes y aborto de repetición
Resumen
Antecedentes
La aspirina y la heparina se utilizan ampliamente como estrategia preventiva para reducir el riesgo alto de aborto de repetición en pacientes con anticuerpos antifosfolipídicos (aPL).
Esta revisión reemplaza una revisión anterior, desactualizada, que evaluó todas las posibles terapias para prevenir el aborto de repetición en pacientes con aPL. Esta revisión se centra en un ámbito más restringido porque la práctica clínica actual se limita al uso de aspirina o heparinas, o ambas, para las pacientes con aPL en un intento de reducir las complicaciones del embarazo.
Objetivos
Evaluar los efectos de la aspirina o la heparina, o de ambas, para mejorar los resultados del embarazo en pacientes con aPL de repetición (en dos ocasiones distintas), ya sea anticoagulante lúpico (LAC), anticardiolipina (aCL) o anticuerpos de aβ2‐glicoproteína‐I (aβ2GPI) o una combinación, y aborto de repetición (dos o más, que no son necesariamente consecutivos).
Métodos de búsqueda
Se hicieron búsquedas en el Registro de Ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group), ClinicalTrials.gov en la Plataforma de Registros Internacionales de Ensayos Clínicos de la OMS (ICTRP, por sus siglas en inglés) (3 de junio 2019) y en las listas de referencias de los estudios recuperados. Cuando fue necesario, se intentó establecer contacto con los autores del ensayo.
Criterios de selección
Se consideraron elegibles los ensayos controlados aleatorizados, cuasialeatorizados y por grupos que evaluaron los efectos de la aspirina, la heparina (ya sea heparina de bajo peso molecular [HBPM] o heparina no fraccionada [HNF]), o una combinación de aspirina y heparina en comparación con ningún tratamiento, placebo u otro tratamiento, sobre los resultados del embarazo en pacientes con aPL persistente y aborto de repetición. Se tuvieron en cuenta todos los regímenes de tratamiento.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los ensayos con relación a los criterios de inclusión y al riesgo de sesgo. Dos autores de la revisión extrajeron los datos de forma independiente y comprobaron su exactitud, y la certeza de la evidencia se evaluó con los criterios GRADE.
Resultados principales
Once estudios (1672 mujeres) cumplieron los criterios de inclusión; nueve ensayos controlados aleatorizados y dos cuasialeatorizados. Los estudios se realizaron en los Estados Unidos, Canadá, Reino Unido, China, Nueva Zelanda, Irak y Egipto. Uno de los ensayos incluidos tenía 1015 participantes, el resto de los ensayos incluidos tuvieron un número sensiblemente inferior de participantes (es decir, 141 participantes o menos).
Algunos estudios tuvieron riesgos de sesgo de selección y de desgaste altos, y muchos no incluyeron suficiente información para determinar el riesgo de sesgo de notificación. En general, la certeza de la evidencia es baja o muy baja debido al escaso número de participantes en los estudios y al riesgo de sesgo.
La dosis y el tipo de heparina y de aspirina variaron entre los estudios. Un estudio comparó la aspirina sola con el placebo; ningún estudio comparó la heparina sola con el placebo; además, no hubo ensayos que tuvieran un brazo de comparación de ningún tratamiento durante el embarazo; cinco estudios exploraron la eficacia de la heparina (ya sea HNF o HBPM) combinada con aspirina en comparación con la aspirina sola; un ensayo comparó HBPM con aspirina; dos ensayos compararon la combinación de HBPM más aspirina con la combinación de HNF más aspirina; dos estudios evaluaron la combinación de diferentes dosis de heparina combinada con aspirina. Todos los ensayos utilizaron aspirina en dosis baja.
Aspirina versus placebo
Existe muy poca certeza de que la aspirina tenga algún efecto sobre los nacidos vivos en comparación con el placebo (riesgo relativo [RR] 0,94; intervalo de confianza [IC] del 95%: 0,71 a 1,25; 1 ensayo, 40 participantes, evidencia de certeza muy baja).
Existe muy poca certeza de que la aspirina tenga algún efecto en el riesgo de preeclampsia, de aborto espontáneo, de parto prematuro de un recién nacido vivo, de restricción del crecimiento intrauterino o de eventos adversos neonatales, en comparación con el placebo. Existe muy poca certeza de que la aspirina tenga algún efecto sobre los eventos adversos (hemorragia) en la madre en comparación con el placebo (RR 1,29; IC del 95%: 0,60 a 2,77; 1 estudio, 40 participantes). La certeza de la evidencia de estos resultados es muy baja, debido a la imprecisión, al escaso número de participantes incluidas y a los IC del 95% amplios, además del riesgo de sesgo.
Los estudios incluidos no informaron de la tromboembolia venosa ni de la tromboembolia arterial.
Heparina más aspirina versus aspirina sola
La heparina más aspirina podría aumentar el número de nacidos vivos (RR 1,27; IC del 95%: 1,09 a 1,49; 5 estudios, 1295 participantes, evidencia de certeza baja).
No hay certeza de que la heparina más la aspirina tenga algún efecto en el riesgo de preeclampsia, de parto prematuro de un recién nacido vivo o de restricción del crecimiento intrauterino, en comparación con la aspirina sola, debido al riesgo de sesgo y la imprecisión por el bajo número de mujeres incluidas y los IC del 95% amplios. Existe muy poca certeza de que la heparina más la aspirina tenga algún efecto sobre los eventos adversos (hemorragia) en la madre en comparación con la aspirina sola (RR 1,65; IC del 95%: 0,19 a 14,03; 1 estudio, 31 participantes).
Ninguna paciente, ya sea del grupo de heparina más aspirina o del grupo de aspirina sola presentó trombocitopenia inducida por la heparina, reacciones alérgicas o tromboembolia venosa ni arterial. Del mismo modo, ningún niño presentó malformaciones congénitas.
La heparina más aspirina podría reducir el riesgo de aborto espontáneo (RR 0,48; IC del 95%: 0,32 a 0,71; 5 estudios, 1295 participantes, evidencia de certeza baja).
Cuando se comparó la HBPM más aspirina con la aspirina sola, el RR agrupado para los recién nacidos vivos fue de 1,20 (IC del 95%: 1,04 a 1,38; 3 ensayos, 1155 participantes). En la comparación de HNF más aspirina versus aspirina sola, el RR de nacidos vivos fue de 1,74 (IC del 95%: 1,28 a 2,35, 2 ensayos, 140 participantes).
Conclusiones de los autores
La combinación de heparina (HNF o HBPM) más aspirina durante el embarazo podría aumentar la tasa de nacidos vivos en pacientes con aPL persistente en comparación con el tratamiento con aspirina sola. El efecto beneficioso observado de la heparina fue impulsado por un estudio amplio en el que se comparó la HBPM más la aspirina con la aspirina sola. En los estudios incluidos, los eventos adversos no se informaron con frecuencia, o bien no se informaron de manera uniforme. Se necesitan más investigaciones sobre este tema para evaluar más a fondo los posibles riesgos y beneficios de dicha estrategia de tratamiento, especialmente entre las pacientes con aPL y aborto de repetición, a fin de llegar a un acuerdo sobre la prevención ideal del aborto de repetición, en función de un perfil de riesgos.
PICO
Resumen en términos sencillos
Fármacos anticoagulantes para la prevención del aborto de repetición en pacientes con anticuerpos antifosfolipídicos
Se propuso determinar si los fármacos antitrombóticos mejoran los resultados del embarazo en pacientes con niveles persistentes de anticuerpos antifosfolipídicos que han tenido varios abortos.
¿Cuál es el problema?
Las moléculas de fosfolípidos ayudan a conformar las membranas celulares y son fundamentales para que la célula pueda funcionar. El sistema inmunológico puede desarrollar anticuerpos que se dirigen contra las proteínas unidas a los fosfolípidos. Existen diferentes tipos de anticuerpos antifosfolipídicos. La presencia de estos anticuerpos puede producir el desarrollo de coágulos de sangre en las venas o en las arterias, pero también puede provocar abortos de repetición.
¿Por qué es esto importante?
Los anticuerpos antifosfolipídicos se asocian con un mayor riesgo de complicaciones del embarazo, incluido el riesgo de aborto. El consumo de fármacos antitrombóticos durante el embarazo podría ayudar a prevenir el aborto de repetición en mujeres que han tenido abortos habituales. La aspirina es un fármaco antiinflamatorio que reduce la agregación plaquetaria y la coagulación de la sangre. La heparina es un anticoagulante potente que evita la formación de coágulos sanguíneos. La aspirina y la heparina podrían reducir el riesgo de aborto espontáneo asociado con los anticuerpos antifosfolipídicos. La heparina de bajo peso molecular es más fácil de usar y causa menos efectos secundarios para la madre que la heparina no dividida o no fraccionada.
¿Qué evidencia se encontró?
Se realizaron búsquedas de ensayos controlados aleatorizados en la literatura médica hasta junio 2019. Se identificaron 11 estudios en los que participaron 1672 mujeres que ya habían tenido al menos dos abortos y tenían anticuerpos antifosfolipídicos persistentes en la sangre. La mayoría de los estudios comenzaron a administrar aspirina a las mujeres elegibles antes de la concepción. Las participantes se aleatorizaron para recibir heparina adicional, o no, una vez que se confirmó el embarazo. La dosis y el tipo de heparina variaron entre los estudios, al igual que el momento en que se inició el tratamiento y la duración del mismo.
La evidencia se consideró de certeza baja, debido al escaso número de participantes en los estudios y al riesgo de sesgo.
En comparación con el placebo, existe muy poca certeza de que la aspirina tenga algún efecto sobre los nacidos vivos, la preeclampsia, el aborto espontáneo, el parto prematuro de un recién nacido vivo, la restricción del crecimiento intrauterino o los eventos adversos maternoinfantiles. En los estudios que investigaron la aspirina en comparación con el placebo, no se informó de tromboembolia venosa ni de tromboembolia arterial.
La heparina más la aspirina podría aumentar el número de recién nacidos vivos y podría reducir el riesgo de aborto espontáneo.
En comparación con la aspirina sola, no hay certeza de que la heparina más la aspirina tenga algún efecto en el riesgo de preeclampsia, de parto prematuro de un recién nacido vivo, de restricción del crecimiento intrauterino ni de hemorragia materna.
Ninguna paciente, ya sea del grupo de heparina más aspirina o del grupo de aspirina sola presentó trombocitopenia inducida por la heparina, reacciones alérgicas o tromboembolia venosa ni arterial. Del mismo modo, ningún niño presentó malformaciones congénitas.
¿Qué significa esto?
La combinación de heparina con aspirina durante el transcurso del embarazo en pacientes con anticuerpos antifosfolipídicos persistentes podría provocar un mayor número de nacidos vivos que el tratamiento con aspirina sola. No existe certeza sobre la seguridad de la heparina y la aspirina para las madres y los recién nacidos, debido a la falta de informes de eventos adversos. En los ensayos futuros se debería reclutar un número adecuado de mujeres y evaluar exhaustivamente los riesgos y beneficios de esta estrategia de tratamiento.
Authors' conclusions
Summary of findings
| Aspirin compared to placebo for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Patient or population: improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with Aspirin | |||||
| Live birth | Study population | RR 0.94 | 40 | ⊕⊝⊝⊝ | ||
| 850 per 1,000 | 799 per 1,000 | |||||
| Pre‐eclampsia | Study population | RR 1.06 | 33 | ⊕⊝⊝⊝ | ||
| 176 per 1,000 | 187 per 1,000 | |||||
| Adverse events in the mother ‐ Bleeding | Study population | RR 1.29 | 40 | ⊕⊝⊝⊝ | ||
| 350 per 1,000 | 451 per 1,000 | |||||
| Venous thromboembolism | Not reported | |||||
| Arterial thromboembolism | Not reported | |||||
| Pregnancy loss | Study population | RR 1.33 | 40 | ⊕⊝⊝⊝ | ||
| 150 per 1,000 | 200 per 1,000 | |||||
| Preterm delivery of a live infant | 2/16 in the aspirin group and 0/17 in the placebo group had a preterm delivery of a live infant | RR 5.29 | 33 | ⊕⊝⊝⊝ | ||
| Intrauterine growth restriction | Study population | RR 0.27 | 33 | ⊕⊝⊝⊝ | ||
| 235 per 1,000 | 64 per 1,000 | |||||
| Adverse events in the child ‐ Congenital malformations | Study population | RR 1.06 | 33 | ⊕⊝⊝⊝ | ||
| 59 per 1,000 | 62 per 1,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of selection and attrition bias 2 Downgraded two levels due to very serious imprecision: few participants and wide confidence intervals crossing the line of no effect | ||||||
| Heparin plus aspirin compared to aspirin for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Patient or population: women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with Aspirin | Risk with Heparin (UFH or LMWH) and aspirin | |||||
| Live birth | Study population | RR 1.27 | 1295 | ⊕⊕⊝⊝ | Subgroup analysis: UFH + aspirin v aspirin: RR 1.74 LMWH + aspirin v aspirin: RR 1.20 | |
| 675 per 1.000 | 857 per 1.000 | |||||
| Pre‐eclampsia | Study population | RR 0.57 (0.10 to 3.14) | 82 | ⊕⊕⊝⊝ | ||
| 67 per 1.000 | 48 per 1.000 | |||||
| Adverse events in the mother ‐ Bleeding | Study population | RR 1.65 | 31 | ⊕⊕⊝⊝ | ||
| 91 per 1.000 | 150 per 1.000 | |||||
| Adverse events in the mother ‐ Heparin‐induced thrombocytopenia | 0/70 women in the heparin plus aspirin group had heparin‐induced thrombocytopenia, compared with 0/70 in the aspirin only group. | 140 (2 RCTs) | ‐ | |||
| Adverse events in the mother ‐ Allergic reactions | 0/45 women in the heparin plus aspirin group had allergic reactions, compared with 0/45 in the aspirin only group. | 90 | ‐ | |||
| Venous thromboembolism | 0/92 women in the heparin plus aspirin group had venous thromboembolism, compared with 0/90 in the aspirin only group. | 182 (3 RCTs) | ‐ | |||
| Arterial thromboembolism | 0/92 women in the heparin plus aspirin group had venous thromboembolism, compared with 0/90 in the aspirin only group. | 182 (3 RCTs) | ‐ | |||
| Pregnancy loss | Study population | RR 0.48 | 1295 | ⊕⊕⊝⊝ | ||
| 325 per 1.000 | 156 per 1.000 | |||||
| Preterm delivery of a live infant | Study population | RR 0.93 (0.42 to 2.07 | 156 | ⊕⊝⊝⊝ | ||
| 141 per 1.000 | 131 per 1.000 | |||||
| Intrauterine growth restriction | Study population | RR 0.85 (0.33 to 2.19) | 151 | ⊕⊝⊝⊝ | ||
| 125 per 1.000 | 106 per 1.000 | |||||
| Adverse events in the child ‐ Congenital malformations | 0/32 infants the heparin plus aspirin group had congenital malformations, compared with 0/19 in the aspirin only group. | 51 | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias for limitations (selection and attrition bias) 2 Downgraded one level due to serious inconsistency: heterogeneity in interventions (I² > 45%) 3 Downgraded one level due to serious risk of bias for limitations (selection and reporting bias) 4 Downgraded one level due to serious imprecision: few participants and wide confidence interval crossing the line of no effect 5 Downgraded one level due to serious risk of bias for limitations (selection, attrition and reporting bias) 6 Downgraded two levels due to very serious imprecision: few participants and wide confidence interval crossing the line of not effect | ||||||
Background
Description of the condition
Antiphospholipid antibodies (aPL) are directed against phospholipids and include lupus anticoagulant (LAC), immunoglobulin G (IgG) or immunoglobulin M (IgM) anticardiolipin (aCL) and IgG or IgM anti‐β2‐glycoprotein‐I (aβ2GPI) antibodies. The presence of aPL is associated with a hypercoagulable state (Harris 1983), which is an abnormally increased tendency toward clotting of the blood. aPL are predominantly known for their role in antiphospholipid syndrome (APS), also known as antiphospholipid antibody syndrome or Hughes syndrome. APS is an autoimmune disorder characterised by the occurrence of a clinical event (recurrent pregnancy loss and/or thrombosis) in the persistent presence of aPL. Currently, the diagnosis of APS is made according to the Sydney criteria established in 2006 (also known as the revised Sapporo criteria), and is based on both clinical and biochemical findings (Miyakis 2006). The clinical criteria include venous and/or arterial thrombosis and well‐defined pregnancy complications such as (recurrent) pregnancy loss (miscarriage or fetal loss) and pre‐eclampsia, whereas the biochemical criteria include persistent (after a 12‐week window) presence of aPL. The diagnosis of APS is made if a woman meets at least one of the clinical criteria and at least one of the biochemical criteria.
Antiphospholipid antibodies are reported to be present in 1% to 5.6% of healthy individuals, with prevalence increasing with age (Durcan 2016). In women with recurrent first trimester pregnancy losses, the presence of these antibodies has been detected in 15% (Rai 1995). Presence of antibodies without clinical events does not indicate treatment, as only a minority of individuals with aPL will develop APS (Ruiz‐Irastorza 2010). The prevalence of APS is estimated to range from 40 to 50 per 100,000 individuals, and is especially increased in women with autoimmune and rheumatic diseases, such as systemic lupus erythematosus (SLE) (Gómez‐Puerta 2014; Love 1990).
Knowledge on the mechanisms and triggers inducing the development and persistence of aPL and the different clinical manifestations are poorly understood. It is thought that beside the presence of the antibodies, a trigger such as pregnancy, hormonal therapy, malignancy, smoking or infection, plays a key role in disease initiation (Meroni 2018). As for APS, knowledge and understanding of the disorder evolve constantly, but uncertainty regarding pathogenesis, diagnosis, as well as optimal treatment remains (Schreiber 2018).
Recently it has been suggested that women with different disease manifestations may represent different subgroups with subsequently, a different course of disease in terms of recurrence risk and type of events. For example, women presenting with thrombotic events may represent a different subgroup from women presenting with pregnancy complications, or women presenting with venous events might be a different subgroup again from women presenting with arterial events (Meroni 2012; Lockshin 2013). Moreover, it has been suggested that the risk of (recurrent) pregnancy complications may differ between groups of women. For example, the risk of pregnancy complications (and type of complication) may differ in women with previous complications compared with women with no previous complication, women with high and low aPL titres, and women with positive versus negative LAC antibodies (Erkan 2002; Ioannou 2010; Lockshin 2012).
Description of the intervention
Aspirin and heparins, either unfractionated heparin (UFH) or low‐molecular‐weight heparin (LMWH), are antithrombotic drugs, often prescribed with the intention to prevent excessive clotting of the blood. Aspirin, also known as acetylsalicylic acid, prevents the formation of thromboxane A2, and inhibits platelet aggregation (Vane 1971; Vane 2003). Heparins inhibit thrombus formation by binding to the natural anticoagulant antithrombin, which results in a potent activation of this enzyme (Chaung 2001). The preferred route of UFH administration is either by a continuous intravenous administration or by subcutaneous injection, whereas LMWH is administered by subcutaneous injection. Important side effects of heparin therapy include haemorrhage, heparin‐induced thrombocytopenia and osteoporosis. Heparins do not cross the placenta and are considered safe for the fetus. Treatment with therapeutic doses of UFH requires frequent monitoring, which LMWH treatment does not. For several indications, when studied in the non‐pregnant population, LMWH was found to have similar efficacy and a superior safety profile (Green 1994; Nurmohamed 1992). The antithrombotic effects in pregnant women may not be alike, due to differences in protein binding. Inconsistent findings have been reported and a direct comparison in a large clinical trial has not yet been made (Ensom 2004; Pariente 2016).
How the intervention might work
Antithrombotic therapy has been found to reduce the risk of recurrent (either venous or arterial) thrombosis in APS (ACOG 2012). Traditionally it is hypothesised that pregnancy complications in APS are also the result of a hypercoagulable state, partially by thrombosis of the placental vasculature. Recent hypotheses describe a more intertwined pathophysiological mechanism in which both the coagulation system, as well as inflammation are involved (Meroni 2018; Redecha 2008; Samarkos 2012). Aspirin and heparin may both have a beneficial effect on coagulation and inflammation (Kozlowski 2011; Vane 2003; Vignoli 2006), and are thought to reduce the risk of pregnancy loss in APS. Antiphospholipid antibodies directly inhibit trophoblast proliferation and differentiation, which can lead to defective placentation (Meroni 2018). This inhibitory effect of aPL on proliferation of trophoblasts has been proposed as the pathogenic mechanism in early pregnancy loss, whereas late obstetrical complications have been attributed to a dysfunctional placenta (Burton 2009; Di Simone 2000; Derksen 2008). The effects of UFH on trophoblast proliferation have not been evaluated, but LMWH has the capacity to stimulate proliferation and protect against apoptosis (cell death) of trophoblasts (Shomer 2016). For this reason, if LMWH administration had a beneficial effect in prevention of early miscarriage, the effects would be observed early during pregnancy. Moreover, it has been suggested that aPLs affect the production of several chemokines and angiogenic factors by human endometrial endothelial cells, which may contribute to impaired placentation and vascular transformation. Noticeably, one recent study demonstrated that LMWH and aspirin, alone or in combination, exacerbated the changes in human endometrial endothelial function mediated by aPL, rather than protecting against them (Quao 2018).
Why it is important to do this review
This is a new review which will supersede the previous, out‐of‐date review by Empson and colleagues (Empson 2005), which included all potential therapies for preventing recurrent pregnancy loss in women with aPL. This new review has a narrower scope than Empson 2005, as currently in clinical practice only aspirin or heparins, or both are used in women with aPL in an attempt to reduce pregnancy complications. However, it is uncertain whether these antithrombotic therapies improve pregnancy outcome and reduce the risk of pregnancy complications in women with persistent (on two occasions) aPL.
Objectives
To assess the effects of aspirin or heparin, or both for improving pregnancy outcomes in women with persistent (on two separate occasions) antiphospholipid antibodies (aPL), either lupus anticoagulant (LAC), anticardiolipin (aCL) or aβ2‐glycoprotein‐I antibodies (aβ2GPI), or a combination, and recurrent pregnancy loss (two or more, which do not have to be consecutive).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs), cluster‐randomised trials and quasi‐randomised controlled trials evaluating aspirin or heparin, or both for improving pregnancy outcome in women with recurrent pregnancy loss and persistent antiphospholipid antibodies (aPL). Cross‐over trials were excluded due to the nature of outcomes considered. Studies published in abstract form only were included if sufficient data were available to determine eligibility.
Types of participants
This review includes women with recurrent (two or more, which do not have to be consecutive) pregnancy loss in the presence of persistent (on two separate occasions) aPL. Pregnancy loss entailed any miscarriage or fetal loss, however defined by the trial authors. aPL presence was determined by either positive LAC, aCL or aβ2 antibodies, or a combination.
Types of interventions
Any comparison of aspirin, heparin (either low‐molecular‐weight heparin (LMWH) or unfractionated heparin (UFH)) or a combination of aspirin and heparin with no treatment, placebo or another was included. Any treatment regimen was considered.
Types of outcome measures
Primary outcomes
-
Live birth
Secondary outcomes
For the mother
-
Pre‐eclampsia (definition according to original study)
-
Adverse events in the mother (definitions according to original study: (A) bleeding, (B) heparin‐induced thrombocytopenia, (C) allergic reactions)
-
Venous thromboembolism
-
Arterial thromboembolism
-
Pregnancy loss
For the child
-
Preterm delivery of a live infant (before 37 weeks, 24 to 28 weeks, 28 to 32 weeks and 32 to 37 weeks)
-
Intrauterine growth restriction (definition according to original study)
-
Adverse events in the child (definitions according to original study: (A) congenital malformations, (B) neonatal bleeding)
Search methods for identification of studies
The following search methods section was based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (3 June 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (3 June 2019) for unpublished, planned and ongoing trial reports using the search methods detailed in Appendix 1.
Searching other resources
We handsearched the reference lists of retrieved studies and relevant review articles. We did not apply any language or date restrictions.
Data collection and analysis
The following methods section was based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all potential studies identified as a result of the search strategy. All disagreements were resolved through discussion and if necessary a third author was involved to have the final vote.
We created a study flow diagram to map out the number of records identified, included and excluded (Moher 2009).
Data extraction and management
We designed a form to extract data. Two review authors independently extracted data for every eligible study using the agreed form. A consensus meeting was held to deal with differences in the extracted data, and if necessary a third review author was involved to have the final vote.
All extracted data were entered into the Review Manager 5 (RevMan 5) software (RevMan 2014) and checked for accuracy. In case of uncertainties regarding the study data, we contacted authors of the specific study for additional information.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random‐number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered, sealed, opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed risk of bias by blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed the methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as‐treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest have been reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We did not include any continuous outcome data in the current review. In future updates, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
There were no cluster‐randomised trials identified to date for inclusion; we will however include them in future updates in the analyses along with individually‐randomised trials. We will adjust for sample sizes, guided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). If possible, we will use the estimate of the intra‐cluster correlation coefficient (ICC) derived from the study, from a similar study or from a study with a similar population. When we use ICCs from external sources, we will mention it explicitly in the review, and we will conduct appropriate sensitivity analyses. When both cluster‐randomised trials and individually‐randomised trials are encountered, we will use relevant data for the review. We will combine results from both cluster‐randomised trials and individually‐randomised trials if little heterogeneity is observed between study designs, provided that the interaction between the effect of intervention and choice of randomisation unit is considered to be unlikely. Heterogeneity will be acknowledged in the randomisation unit and we will conduct a sensitivity analysis to explore the effects of this randomisation unit.
Multiple‐arm studies
There were no multi‐arm trials identified to date for inclusion. For future updates, all intervention arms will be reported and described in the Characteristics of included studies table, including the number of women randomised to each arm. We will combine groups, to create a single pair‐wise comparison if possible. Appropriate pair‐wise comparisons will then be selected for the meta‐analysis, in order to avoid double‐counting of one of the arms. We will declare in the Characteristics of included studies table if a trial has an intervention arm that is not applicable or relevant to our review question. We will only include the intervention and control groups that meet the eligibility criteria in the analyses.
Cross‐over trials
We considered cross‐over trials an inappropriate design for this intervention.
Dealing with missing data
For every individual included study, we determined the level of attrition. We evaluated the impact on the overall assessment of the intervention of including studies with high proportions of missing data by conducting a sensitivity analysis without these studies.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis; we attempted to include all participants randomised in the analyses, and analysed these participants according to their allocated treatment assignment, regardless of whether the allocated intervention was received. For each outcome in every trial, the denominator was the number of randomised participants minus the participants whose outcomes are missing. In studies with more than 5% loss to follow‐up, we planned to perform a best‐case scenario analysis (losses to follow‐up assumed to have a positive outcome, e.g. primary outcome) and a worst‐case scenario analysis (losses to follow‐up assumed to have a negative outcome, e.g. no primary outcome) ‐ we did not need to perform best case/worst case analyses because the primary outcome was available for all women.
Assessment of heterogeneity
In all meta‐analyses we assessed statistical heterogeneity using the Tau², I² (Higgins 2003) and Chi² statistics (Deeks 2011). We regarded heterogeneity as substantial if Tau² was greater than zero and either the I² statistic was greater than 30%, or there was a P value equal to or less than 0.10 in the Chi² test for heterogeneity.
Assessment of reporting biases
None of the meta‐analyses in the current review concerned 10 or more studies. If in future updates, if the meta‐analysis includes 10 or more studies, we will explore potential reporting bias (mainly publication bias) using funnel plots and visually assess them. We will prepare funnel plots and visually assess them for asymmetry. If visual assessment leads us to suspect asymmetry, we will conduct additional analyses to explore these potential biases.
Data synthesis
We carried out statistical analysis using RevMan 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I² statistic.
Subgroup analysis and investigation of heterogeneity
For the comparison 'heparin with or without aspirin versus aspirin alone', we did subgroup analysis per type of heparin, as follows:
-
UFH plus aspirin versus aspirin alone
-
LMWH plus aspirin versus aspirin alone
Over the last two decades or so, clinical practice with regard to heparin treatment has changed from using UFH subcutaneously to the current standard of care of LMWH. For this reason, we consider reporting the subgroup results for both UFH and LMWH to be a more detailed description of the evidence and highly relevant for current clinical practice.
Where we identified substantial heterogeneity, we planned to investigate it using subgroup analyses and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, used random‐effects analysis to produce it.
The risk of (recurrent) pregnancy complications may differ between different subgroups of women, such as previous placenta‐mediated complications, number of pregnancy losses, high‐ or low‐titre antibodies and positive or negative lupus anticoagulant (LAC) antibodies. For this reason, the following subgroup analyses were pre‐specified.
-
Previous placenta‐mediated complication (pre‐eclampsia; intrauterine growth restriction or placental abruption, or both) versus no previous placenta‐mediated complication
-
Two versus three or more pregnancy losses (which do not have to be consecutive)
-
High‐titre antibodies versus low‐titre antibodies
-
Positive lupus anticoagulant (LAC) antibodies versus negative LAC antibodies
We planned to use the primary outcome (live birth) in subgroup analyses.
We planned to assess subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We planned to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² statistic value.
Sensitivity analysis
Where possible, we carried out sensitivity analyses to explore the effect of use of the full Sapporo criteria for APS, with studies not using the full criteria excluded from the analyses; and trial quality (including quasi‐randomised trials), assessed by random sequence generation and concealment of allocation, with studies assessed as high risk of bias on these domains being excluded from the analyses. Sensitivity analyses were limited to the primary outcome.
In future updates, where cluster‐randomised trials are included, we plan to carry out sensitivity analyses to explore the effects of variation in intra‐cluster correlation coefficient (ICC) values and in the randomisation unit (i.e. individual versus cluster trials).
Summary of findings and assessment of the certainty of the evidence
The certainty of evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes for the two main comparisons.
-
Aspirin versus placebo
-
Heparin plus aspirin versus aspirin alone
Primary outcomes
-
Live birth
Secondary outcomes
For the mother
-
Pre‐eclampsia (definition according to original study)
-
Adverse events in the mother (definitions according to original study: (A) bleeding, (B) heparin‐induced thrombocytopenia, (C) allergic reactions)
-
Venous thromboembolism
-
Arterial thromboembolism
-
Pregnancy loss
For the child
-
Preterm delivery of a live infant (before 37 weeks, 24 to 28 weeks, 28 to 32 weeks and 32 to 37 weeks)
-
Intrauterine growth restriction (definition according to original study)
-
Adverse events in the child (definitions according to original study: (A) congenital malformations, (B) neonatal bleeding)
We used the GRADEpro Guideline Development Tool to import data from RevMan 5 (RevMan 2014) in order to create 'Summary of findings' tables for comparison 1 (aspirin versus placebo) and comparison 2 (heparin plus aspirin versus aspirin alone). A summary of the intervention effect and a measure of certainty for each of the above outcomes in these comparisons was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See: Figure 1
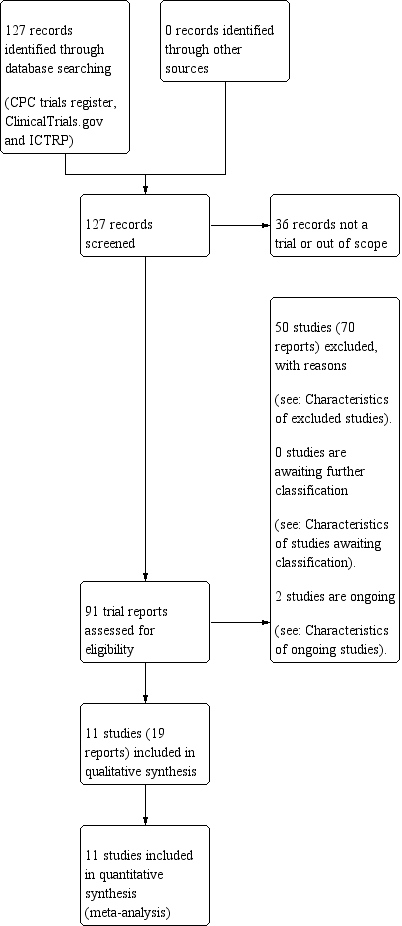
Study flow diagram.
As of June 2019, the search strategy identified 127 records through database screening. The title and abstract screening identified 91 potentially eligible citations. The full‐text screening of these 91 citations identified 11 eligible randomised controlled trials published as full reports (Alalaf 2012; Bao 2017; Farquharson 2002; Fouda 2010; Fouda 2011; Kutteh 1996a; Kutteh 1996b; Laskin 2009; Pattison 2000; Rai 1997; Stephenson 2004. We identified two registered, but unpublished trials (Abdelhafez 2014; Rodger 2017). We did not identify any cluster‐randomised trials that met our inclusion criteria. None of the studies only published as abstracts were included, as insufficient data were available to determine eligibility.
Included studies
A total of 1672 women were enrolled in the 11 included trials; nine were randomised controlled trials and two were quasi‐randomised controlled trials (Kutteh 1996a; Kutteh 1996b). The study designs, inclusion and exclusion criteria and interventions are shown in the Characteristics of included studies tables. The studies were conducted in the USA, Canada, the UK, China, New Zealand, Iraq and Egypt. One included trial involved 1015 women (Bao 2017), all other included trials had considerably lower numbers of participants (i.e. 141 women or fewer).
One study compared aspirin with placebo (n = 40) (Pattison 2000). No study compared heparin alone with placebo and we did not identify trials with a no treatment comparator arm during pregnancy. Five studies explored the efficacy of heparin plus aspirin with aspirin alone; two studies evaluated the combination of unfractionated heparin (UFH) plus aspirin in comparison with aspirin alone (n = 140) (Kutteh 1996a; Rai 1997), three studies used low‐molecular weight heparin (LWMH) plus aspirin and compared this to aspirin alone (n = 1155) (Bao 2017; Farquharson 2002; Laskin 2009). One trial compared LMWH with aspirin (n = 141) (Alalaf 2012). Two trials compared LMWH with UFH, both combined with aspirin (n = 86) (Fouda 2011; Stephenson 2004). Two studies investigated the combination of different doses of heparin plus aspirin; one compared high‐dose UFH with low‐dose UFH, both combined with aspirin (n = 50) (Kutteh 1996b), whereas the other study compared high‐dose LMWH with low‐dose LWMH, both combined with aspirin (n = 60) (Fouda 2010).
Characteristics of participants
The characteristics of the trial participants are summarised in Table 1, though these were not completely reported in all studies. One trial also included participants who did not have antiphospholipid antibodies (aPL) (Laskin 2009) and we included only data from the subgroup of participants with aPL from this study (n = 42/88) (Laskin 2009); we contacted the authors to provide data on the secondary outcomes for the subgroup of aPL‐positive participants, but we did not receive a reply.
| Total No. | Participants per group | Mean age (years) | Mean total prior miscarriages/woman | aCL IgM | aCL IgG | LAC | aCL and LAC | aβ2GPI | |||||||||
| Studies | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | |
| 141 | 80 | 61 | 31.4 ± 5.8 | 30.6 ± 6.3 | 3.3 ± 1.7 | 3.4 ± 1.8 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 1015 | 497 | 518 | median 35 (25‐47) | median 34 (24‐43) | median 4 (2‐11) | median 3 (2‐8) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 98 | 51 | 47 | 33 ± 4.8 | 33 ± 4.9 | 3 ± 0.8 | 3 ± 0.9 | 3/51 | 5/47 | 6/51 | 2/47 | 23/51 | 18/47 | 18/51 | 22/47 | NA | NA | |
| 60 | 30 | 30 | 27.1 ± 3.7 | 28.9 ± 4.2 | 4.0 ± 1.2 | 4.1 ± 1.1 | 4/30 | 5/30 | 8/30 | 6/30 | 10/30 | 9/30 | 8/30 | 10/30 | NA | NA | |
| 60 | 30 | 30 | 27.5 ± 3.2 | 28.6 ± 3.5 | 4.4 ± 1.2 | 4.2 ± 1.2 | 5/30 | 8/30 | 7/30 | 5/30 | 12/30 | 10/30 | 6/30 | 7/30 | NA | NA | |
| 50 | 25 | 25 | 33.2 ± 4.2 | 33.5 ± 5.8 | 3.9 ± 1.4 | 3.7 ± 1.0 | 6/25 | 5/25 | NR | NR | NA | NA | NA | NA | NA | NA | |
| 50 | 25 | 25 | 33.3 ± 4.2 | 33.2 ± 3.9 | 3.9 ± 1.4 | 3.6 ± 1.0 | NR | NR | NR | NR | NA | NA | NA | NA | NA | NA | |
| 42 | 22 | 20 | 34.6 ± 3.9 | 33.8 ± 4.1* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NA | NA | |
| 40 | 20 | 20 | 31 ± 4.5 | 30.9 ± 3.9 | NR | NR | 6/20 | 3/20 | 6/20 | 9/20 | 4/20 | 5/20 | 3/20 | 3/20 | NA | NA | |
| 90 | 45 | 45 | median 32 (23‐40) | median 34 (22‐44) | median 4 (3‐15) | median 4 (3‐8) | 0/45 | 1/45 | 3/45 | 4/45 | 40/45 | 34/45 | 6/45 | 2/45 | NA | NA | |
| 26 | 13 | 13 | 34 (27‐40) | 34 (28‐43) | 3.8 (3‐7) | 3.9 (3‐7) | 4/14 | 11/14 | 11/14 | 7/14 | 6/14 | 3/14 | 4/14 | 2/14 | NA | NA | |
aβ2GPI: anti‐β2‐glycoprotein‐I antibodies; aCL: anticardiolipin antibodies,aPL: antiphospholipid antibodies; LAC: lupus anticoagulant,.LMWH: low‐molecular weight heparin; NA: outcome not assessed;NR: outcome not reported,UFH: unfractionated heparin
* mean age in years for the entire study population (N = 88), not separately reported for the subgroup with positive aPL specifically.
** aPL profiles given for entire study population (N = 28), not separately reported the subgroup of patients who conceived and were subsequently randomised.
-
Alalaf 2012: group A = LMWH, group B = aspirin
-
Bao 2017: group A = LMWH + aspirin, group B = aspirin
-
Farquharson 2002: group A = LMWH + aspirin, group B = aspirin
-
Fouda 2010: group A = high‐dose LMWH plus aspirin, group B = low‐dose LMWH plus aspirin;
-
Fouda 2011: group A = LMWH + aspirin, group B = UFH + aspirin
-
Kutteh 1996a: group A = UFH + aspirin, group B = aspirin
-
Kutteh 1996b: group A = high‐dose UFH plus aspirin, group B = low‐dose UFH + aspirin
-
Laskin 2009: group A = LMWH + aspirin, group B = aspirin
-
Pattison 2000: group A = aspirin, group B = placebo
-
Rai 1997: group A = UFH + aspirin, group B = aspirin
-
Stephenson 2004: group A = LMWH + aspirin, group B = UFH + aspirin
Prior pregnancy losses
The mean number of previous pregnancy losses in the studies ranged from 3 to 4.3 (Alalaf 2012; Farquharson 2002; Fouda 2010;Fouda 2011; Kutteh 1996a; Kutteh 1996b; Rai 1997; Stephenson 2004). In eight trials, participants met the clinical criteria for antiphospholipid syndrome (APS) with three or more early miscarriages. Three trials included women with two or more consecutive pregnancy losses (Alalaf 2012; Bao 2017; Laskin 2009). Previous pregnancy losses concerned mostly early pregnancy losses, but less than half of the included studies specified this (Kutteh 1996a; Kutteh 1996b; Laskin 2009; Pattison 2000; Rai 1997). Full details on pregnancy losses are provided in the characteristics of included studies (Included studies).
Presence of antiphospholipid antibodies (aPL)
All trials included participants with persistent presence of aPL, but the time‐frame between tests differed per study. Two trials included patients with aPL tested at least six weeks apart (Farquharson 2002; Stephenson 2004); three trials with tests at least eight weeks apart (Alalaf 2012; Laskin 2009; Rai 1997); three trials with tests at least 12 weeks apart (Bao 2017; Fouda 2010; Fouda 2011); and three trials did not mention the time‐frame (Kutteh 1996a; Kutteh 1996b; Pattison 2000). None of the included trials reported women with aβ2‐GPI antibodies. Table 1 lists the aPL‐profiles for trial participants.
Dose and type of aspirin and heparin
Low‐dose aspirin was used in all trials. In six trials a dose of aspirin of 75 mg/day was used (Bao 2017; Farquharson 2002; Fouda 2010; Fouda 2011; Pattison 2000; Rai 1997), in four trials the dose was 81 mg/day (Kutteh 1996a; Kutteh 1996b; Laskin 2009; Stephenson 2004), and in one trial the dose used was 100 mg/day (Alalaf 2012). The types of LMWH included bemiparin in a dose of 2500 IU/day (Alalaf 2012), enoxaparin in a dose of 20 mg or 40 mg per day (Fouda 2010, Fouda 2011), dalteparin in a dose of 5000 IU/day (Laskin 2009) or 2500 IU/day (Stephenson 2004), nadroparin in a dose of 4100 IU/day (Bao 2017), and in one trial the type of LMWH was not mentioned, but administered in a dose of 5000 IU/day (Farquharson 2002). The dose of UFH was 5000 IU twice daily in both the trial of Kutteh 1996a and the trial of Stephenson 2004, with the latter with increasing the dose of administered heparin (LMWH or UFH) during the trial. One trial compared a lower and a higher dose of UFH (Kutteh 1996b); in the high‐dose UFH group, the doses of heparin were adjusted to maintain 1.2 to 1.5 times the baseline partial thromboplastin time (PTT) and were increased by 1000 U/dose weekly until the desired range was achieved, whereas in the low‐dose UFH group the dose of heparin was adjusted to maintain the PTT at the upper limits of the normal range in the reference laboratory.
Initiation and duration of treatment
There was a wide variation in treatment initiation and duration between trials. One trial randomised women to aspirin, which was started preconceptionally and continued upon pregnancy confirmation or to LMWH commencing at the confirmation of pregnancy, continuing either treatment until 36 weeks of gestation (Alalaf 2012). In one trial, participants were randomised before 12 weeks of gestation, with a mean gestation age of 6.7 weeks at randomisation, and received treatment until delivery (Farquharson 2002). In two trials, aspirin was started preconceptionally up to 36 gestational weeks, with a heparin (LMWH or UFH) started when the serum pregnancy test became positive until delivery, when it was switched to twice‐daily UFH (Fouda 2010; Fouda 2011). Two trials initiated aspirin preconceptionally in all participants and at the first confirmed pregnancy test, patients were instructed to continue aspirin alone or to add subcutaneous injections of heparin twice‐daily (Kutteh 1996a), or all participants were started on heparin injections (Kutteh 1996b) until full term. Four trials initiated treatment at the first confirmation of pregnancy and treatment was continued until 34 weeks of gestation (Rai 1997), 35 weeks of gestation (Bao 2017; Laskin 2009) or study duration (Pattison 2000). One trial started aspirin before conception, with heparin (LMWH or UFH) started in the luteal phase for a maximum of three cycles until delivery and continued postpartum in a prophylactic dose (Stephenson 2004).
Placebo
One trial randomised between aspirin and placebo (Pattison 2000). No studies were identified that compared heparin with placebo or aspirin and/or heparin with no treatment.
Outcomes reported
The reported outcomes per included trial are summarised in Table 2. Our primary outcome live birth was reported in all studies, in contrast to the secondary outcomes that were only reported in a subset of included trials. All included trials contributed data to at least one comparison in the meta‐analysis.
| Live birth | Pre‐eclampsia | Maternal bleeding | Thrombo cytopenia | Allergic Reactions | VTE ATE | Preterm delivery | IUGR | Congenital Malformations | Neonatal Bleeding | |||||||||||
| Studies | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| 69/80 | 44/61 | 1/69 | 0/44 | 5/81 | NA | NA | NA | NA | NA | 0/80 | 0/61 | 3/69 | 2/44 | NA | NA | NA | NA | NA | NA | |
| 449/497 | 363/518 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 40/51 | 34/47 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 2/40 | 4/34 | NA | NA | NA | NA | NA | NA | |
| 23/30 | 21/30 | 3/30 | 2/30 | 0/30 | 0/30 | 0/30 | 0/30 | NA | NA | 0/30 | 0/30 | 3/23 | 2/21 | 2/30 | 1/30 | 0/23 | 0/21 | 0/23 | 0/21 | |
| 24/30 | 20/30 | 2/24 | 1/20 | 0/30 3/30 | 0/30 3/30 | 0/30 | 0/30 | 0/30 | 1/30 | 0/30 | 0/30 | 3/24 | 2/20 | 1/24 | 2/20 | 0/24 | 0/20 | 0/24 | 0/20 | |
| 20/25 | 11/25 | 2/20 | 1/11 | 0/20 3/20 | 0/11 1/11 | 0/25 | 0/25 | NA | NA | 0/25 | 0/25 | 3/20 | 1/11 | 3/20 | 1/11 | NA | NA | NA | NA | |
| 20/25 | 19/25 | 2/20 | 1/19 | 0/20 3/20 | 0/19 4/19 | 0/25 | 0/25 | NA | NA | NA | NA | 3/20 | 1/19 | 3/20 | 0/19 | NA | NA | NA | NA | |
| 17/22 | 15/20 | NA | NA | NA | NA | NA | NA | NA | NA | 0/22 | 0/20 | NA | NA | NA | NA | NA | NA | NA | NA | |
| 16/20 | 17/20 | 3/16 | 3/17 | 9/20 | 7/20 | NA | NA | NA | NA | NA | NA | 2/16 | 0/17 | 1/16 | 4/17 | 1/16 | 1/17 | NA | NA | |
| 32/45 | 19/45 | 0/32 | 1/19 | 0/45 | NA | 0/45 | NA | 0/45 | NA | 0/45 | 0/45 | 8/32 | 4/19 | 3/32 | 1/19 | 0/32 | 0/19 | NA | NA | |
| 9/13 | 4/13 | 1/13 | 0/13 | 0/13 | 0/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
ATE: arterial thromboembolism;LMWH: low‐molecular weight heparin; NA: outcome not assessed; UFH: unfractionated heparin; VTE: venous thromboembolism.
-
Alalaf 2012: group A = LMWH, group B = aspirin
-
Bao 2017: group A = LMWH + aspirin, group B = aspirin
-
Farquharson 2002: group A = LMWH + aspirin, group B = aspirin
-
Fouda 2010: group A = high‐dose LMWH plus aspirin, group B = low‐dose LMWH plus aspirin
-
Fouda 2011: group A = LMWH + aspirin, group B = UFH + aspirin, *no bleeding in either group, subcutaneous bruising 3/30 in both group
-
Kutteh 1996a: group A = UFH + aspirin, group B = aspirin; **no major bleeding, reports are minor bleeding events
-
Kutteh 1996b: group A = high‐dose UFH plus aspirin, group B = low‐dose UFH + aspirin; **no major bleeding events, reports are minor bleeding events
-
Laskin 2009: group A = LMWH + aspirin, group B = aspirin
-
Pattison 2000: group A = aspirin, group B = placebo
-
Rai 1997: group A = UFH + aspirin, group B = aspirin
-
Stephenson 2004: group A = LMWH + aspirin, group B = UFH + aspirin
-
Live birth rate: all 11 included trials reported our primary outcome live birth.
-
Pre‐eclampsia: eight trials reported on pre‐eclampsia (Alalaf 2012; Fouda 2010; Fouda 2011; Kutteh 1996a; Kutteh 1996b; Pattison 2000; Rai 1997; Stephenson 2004).
-
Maternal bleeding: eight trials mentioned maternal bleeding rates; three trials reported on postpartum haemorrhage or vaginal bleeding during pregnancy (Fouda 2010; Fouda 2011; Stephenson 2004), two trials reported on both major and minor maternal bleeding (Kutteh 1996a; Kutteh 1996b), one trial reported any maternal bleeding without specification (Pattison 2000) and two trials reported bruising at injection site in case of heparin use (Alalaf 2012, Fouda 2010).
-
Heparin‐induced thrombocytopenia (HIT): 10 of 11 trials had heparin as an intervention arm and five trials reported on thrombocytopenia (Fouda 2010; Fouda 2011; Kutteh 1996a; Kutteh 1996b; Rai 1997).
-
Allergic reactions: one trial reported allergic reactions to study medication (Fouda 2011).
-
Venous thromboembolism (VTE): six trials reported on thromboembolic events, without discerning arterial or venous origin (Alalaf 2012; Fouda 2010; Fouda 2011; Kutteh 1996a; Laskin 2009; Rai 1997).
-
Arterial thromboembolism (ATE): six trials reported on thromboembolic events, without discerning arterial or venous origin (Alalaf 2012; Fouda 2010; Fouda 2011; Kutteh 1996a; Laskin 2009; Rai 1997).
-
Preterm delivery of a live infant: eight of 11 trials reported on preterm delivery of a live infant, defined as delivery between 32 to 37 weeks of gestation (Alalaf 2012), between 30 to 36 weeks or before 30 weeks (Farquharson 2002), between 24 to 37 weeks (Pattison 2000) or preterm was not specifically defined (Fouda 2010; Fouda 2011; Kutteh 1996a; Kutteh 1996b; Rai 1997).
-
Intrauterine growth restriction (IUGR): IUGR was reported in six of 11 studies (Fouda 2010; Fouda 2011; Kutteh 1996a; Kutteh 1996b; Pattison 2000; Rai 1997).
-
Congenital malformations: four trials reported on congenital malformations (Fouda 2010; Fouda 2011; Pattison 2000; Rai 1997).
-
Neonatal bleeding: two trials reported on neonatal bleeding (Fouda 2010; Fouda 2011).
Trial registries and dates
Only two of 11 trials had registered their study in a clinical trials registry (Laskin 2009; Fouda 2011), as at the time of publication for most of the other studies clinical trials registries were not operational. Three relatively more recent trials were published, but had not registered their study in a trials registry (Alalaf 2012; Bao 2017; Fouda 2010). More than half of the included studies were published before 2005. Three studies did not report on the recruitment period (Kutteh 1996a; Kutteh 1996b; Rai 1997), with one study only mentioning the overall recruitment time being 39 months (Pattison 2000).
Funding sources
Two trials were funded by governmental and non‐governmental research grants from the UK (Farquharson 2002; Rai 1997), and two by governmental and non‐governmental grants from Canada (Laskin 2009; Stephenson 2004). One study reported supply of study medication and sponsorship from a pharmaceutical company (Laskin 2009). One trial reported financial support by governmental research grants from China (Bao 2017). One study specifically thanked a pharmaceutical company for donation of study medication, but did not clarify the relationship (Stephenson 2004). Four trials did not mention any support (Fouda 2010; Kutteh 1996a; Kutteh 1996b; Pattison 2000).
Declarations of Interest
Five trials explicitly declared no conflict of interest (Alalaf 2012; Fouda 2010; Fouda 2011; Rai 1997; Stephenson 2004) and the other six trials did not state if any interests existed (Bao 2017; Farquharson 2002; Kutteh 1996a; Kutteh 1996b; Pattison 2000; Laskin 2009).
Excluded studies
Reasons for exclusion are stated in the Characteristics of excluded studies table. Eight studies were excluded based on abstract only, as full text could not be retrieved and information in the abstract was insufficient to critically evaluate if inclusion criteria were met (Bu 2009; Dendrinos 2007; Guo 2013; Malathi 2011; Malinowski 2003; Mankuta 1999; Quenby 1992; Zhou 2012). On obtaining the full papers, three trials were found to be non‐randomised (Mohamed 2014; Noble 2005; Shefras 1995) and three trials had a different study design (Gibbins 2018; De Veciana 2001; Kahwa 2006). Eighteen trials considered a different study population, such as women without recurrent miscarriage or no persistent presence of aPL (Agarwal 2018; Cowchock 1997; De Vries 2012; Goel 2006; Golding 1998; Gris 1995; Ismail 2016; Kaaja 1993; Kaandorp 2010; Kahwa 2006; Mahmoud 2004; Radin 2017; Saad 2014; Tulppala 1997; Schisterman 2014; Vahid 1999; van Hoorn 2016; Visser 2011). Sixteen trials evaluated a different intervention or aspirin and/or heparin in combination with for instance intravenous immunoglobulin (IVIG) or prednisone (Branch 2000; Carta 2005; Christiansen 1995; Cowchock 1992; Dendrinos 2009; Eid 2019; Fu 2004; Geva 1998; Laskin 1997; Rai 2005; Shu 2002; Silver 1993; Tang 2012; Triolo 2003; Vaquero 2001; Xiao 2013). Two studies investigated a different outcome; thrombotic sequelae after 20 years (Clark 2009) and drug exposure throughout pregnancy (Ensom 2004), respectively.
Risk of bias in included studies
See Figure 2; Figure 3 for a summary of risk of bias in the included trials.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eight of 11 included trials, had low risk of bias for random sequence generation; seven trials used a computer‐generated list of study numbers or other adequate methods of randomisation (Bao 2017; Farquharson 2002; Fouda 2010; Fouda 2011; Laskin 2009; Pattison 2000; Rai 1997) and one trial used a random numbers table with block of 12 (Stephenson 2004). One trial used some form of alternation, but the method of randomisation, or what treatment allocation was based on (e.g. date of birth or medical record number) was not described and therefore risk of bias is regarded as high (Alalaf 2012). Two quasi‐randomised controlled trials used non‐random alternative assignment to treatment groups (Kutteh 1996a) or a sequential block of 25 allocated to one treatment group and a second sequential block of 25 allocated to the other treatment group (Kutteh 1996b) ‐ both trials were assessed as high risk of bias.
Allocation concealment was considered adequate and thus at low risk of bias in eight of 11 studies (Bao 2017; Farquharson 2002; Fouda 2010; Fouda 2011; Laskin 2009; Pattison 2000; Rai 1997; Stephenson 2004) that used central or telephone randomisation or sealed, opaque envelopes. Three trials were assessed as high risk of bias ‐ two quasi‐randomised controlled trials did not conceal allocation of treatment (Kutteh 1996a; Kutteh 1996b), and one trial did not report methods to conceal allocation (Alalaf 2012).
Blinding
In only one trial both participant and treating physician were unaware of the treatment allocation (Pattison 2000). All other trials did not blind or did not report on blinding participants and treatment providers. Few trials stated explicitly who performed the outcome assessment and whether outcome assessors were blinded to treatment allocation. However, the primary outcome live birth was considered unlikely to be influenced by knowledge of treatment allocation, therefore these trials were assessed as low risk of bias, as suggested in the Cochrane Handbook for Systematic Reviews for Interventions (Higgins 2017).
Incomplete outcome data
The majority of trials had low rates of attrition and reasons for exclusion or the numbers of participants included in each stage of the analysis were clearly reported. Three trials were considered to be at high risk of attrition bias (Alalaf 2012; Bao 2017; Pattison 2000). Alalaf 2012 did not report on exclusions, reasons for exclusions, numbers included in the analysis at each stage or loss to‐follow‐up. Bao 2017, did a per‐protocol analysis; 37 of the 1052 women receiving treatment failed to follow up or could not continue the trial due to change of intervention or specific allergies and outcomes of these censored participants were not reported; we assessed this trial as high risk of bias. In the trial by Pattison 2000, in each arm 5/25 (20%) of participants were excluded because of inappropriate inclusion. Analyses were performed with and without these participants but results from included participants only were published, not an analysis by intent‐to‐treat.
We assessed Kutteh 1996a and Kutteh 1996b as having an unclear risk of attrition bias; in those trials, exclusions, reasons for exclusion and numbers included in the analysis at each stage were not reported and it was unclear whether all evaluated participants started low‐dose aspirin before conception, prior to randomisation. Also, analysis by intent‐to‐treat and losses to follow‐up were unclear.
Selective reporting
Only a minority of trials were registered in a clinical trials registry and had a published study protocol (Fouda 2011; Laskin 2009), and we judged these trials to have a low risk of selective reporting. A clinical trial registry did not exist at the time of publication of some of the included studies (and for those more recently published studies, trial protocols were not available), and we therefore assessed reporting bias as unclear in eight unregistered studies, (Alalaf 2012; Bao 2017; Farquharson 2002; Fouda 2010; Kutteh 1996a; Kutteh 1996b; Rai 1997;Stephenson 2004). One trial excluded 20% of included participants due to inappropriate inclusion and performed analyses with and without these participants, but only provided results from included participants; we did not have sufficient information to assess whether all outcomes were reported and thus judged this trial to be at an unclear risk of reporting bias (Pattison 2000).
Other potential sources of bias
All of the included trials were assessed as low risk of other potential sources of bias.
Effects of interventions
See: Summary of findings 1 Aspirin compared to placebo for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss; Summary of findings 2 Heparin plus aspirin compared to aspirin for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss
See: summary of findings Table 1; summary of findings Table 2
Eleven trials (1672 women) met the inclusion criteria and all trials contributed data to our analyses. We present five different comparisons, with meta‐analysis only possible in three of our comparisons; comparison 2 (heparin [UFH or LMWH] plus aspirin versus aspirin alone), comparison 4 (LMWH plus aspirin versus UFH plus aspirin) and comparison 5 (higher dose heparin [LMWH or UFH] plus aspirin versus lower dose heparin [LMWH or UFH] plus aspirin).
Comparison 1: Aspirin versus placebo
Primary outcome ‐ Live birth
We are uncertain if there is any difference in live birth rates when comparing aspirin with placebo (risk ratio (RR) of 0.94, 95% confidence interval (CI) 0.71 to 1.25, 1 trial, 40 women; very low‐certainty evidence; Analysis 1.1; summary of findings Table 1). In one small trial (Pattison 2000) there were similar numbers of live births in the aspirin group (16/20) and the placebo group (17/20).
Secondary outcomes (maternal)
Pre‐eclampsia
We are uncertain if there is any difference in the risk of pre‐eclampsia between aspirin and placebo (Pattison 2000; RR 1.06, 95% CI 0.25 to 4.52; 1 trial, 33 women; very low‐certainty evidence; Analysis 1.2; summary of findings Table 1).
Adverse events in the mother (definitions according to original study: (A) bleeding, (B) heparin‐induced thrombocytopenia, (C) allergic reactions)
It is uncertain if there is any difference in the risk of bleeding events during pregnancy when aspirin was compared with placebo (Pattison 2000; RR 1.29, 95% CI 0.60 to 2.77; 1 trial, 40 women; very low‐certainty evidence; Analysis 1.3; summary of findings Table 1). Heparin‐induced thrombocytopenia was not reported, as heparin was not evaluated in this trial; allergic reactions to aspirin or placebo were not reported either.
Venous thromboembolism
Not reported.
Arterial thromboembolism
Not reported
Pregnancy loss
We are very uncertain if there is any difference in the risk of pregnancy loss when comparing aspirin with placebo (RR 1.33, 95% CI 0.34 to 5.21; 40 women; 1 study; very low‐certainty evidence; summary of findings Table 1; Analysis 1.7).
Secondary outcomes (for the child)
Preterm delivery of a live infant
It is uncertain if there is any difference between aspirin and placebo in the risk of preterm delivery did not occur in the 17 placebo‐treated women and in two of 16 women receiving aspirin (Pattison 2000; RR 5.29, 95% CI 0.27 to 102.49; 1 trial, 33 children; very low‐certainty evidence; Analysis 1.4; summary of findings Table 1).
Intrauterine growth restriction (IUGR)
It is uncertain if there is any difference in the risk of IUGR between women receiving placebo or aspirin during pregnancy, the RR for IUGR was 0.27 (95% CI 0.03 to 2.13; 1 trial, 33 children; very low‐certainty evidence; Analysis 1.5; summary of findings Table 1),
Adverse events in the child (definitions according to original study: (A) congenital malformations, (B) neonatal bleeding)
It is uncertain if there is any difference between aspirin and placebo in the risk of adverse events. One child in both treatment groups was diagnosed with a congenital malformation in the trial of Pattison 2000, but malformations were not specified (RR 1.06; 95% CI 0.07 to 15.60; 1 trial, 33 children; very low‐certainty evidence; Analysis 1.6; summary of findings Table 1). Neonatal bleeding was not reported.
Comparison 2: Heparin plus aspirin versus aspirin alone
Primary outcome ‐ Live birth
Five studies (1295 women) which compared heparin (either UFH or LMWH) combined with aspirin to aspirin alone, were included in a random‐effects meta‐analysis for the primary outcome live birth. Heparin plus aspirin may increase the number of live births compared with aspirin alone (RR 1.27; 95% CI 1.09 to 1.49; Tau² = 0.01; Chi² = 7.71, df = 4 (P = 0.10); I² = 48%; low‐certainty evidence; Analysis 2.1; summary of findings Table 2).
Subgroup analysis
We carried out a non‐pre‐specified subgroup analysis comparing trials that used LMWH and those that used UFH. There was evidence of a subgroup difference, as indicated by the subgroup interaction test (test for subgroup differences: Chi² = 4.74, df = 1 (P = 0.03), I² = 78.9%), possibly suggesting a larger treatment effect (benefit) with the use of UFH compared with LMWH. Both subgroups demonstrated higher rates of live birth when heparin was combined with aspirin as compared to aspirin alone (trials with LMWH: RR 1.20, 95% CI 1.04 to 1.38, 3 trials, 1155 women; trials with UFH: RR 1.74, 95% CI 1.28 to 2.35, 2 trials, 140 women; Analysis 2.1).
Farquharson 2002 reported 92.3% (12/13) pregnancy losses < 24 weeks of gestation and 7.7% (1/13) ≥ 24 weeks of gestation in the aspirin group, compared with all pregnancy losses in the LMWH plus aspirin group occurring before 24 weeks of gestation. All other included trials only reported total numbers of live birth.
We were not able to perform a subgroup analysis based on history of previous miscarriages, as three trials only included women with three or more recurrent pregnancy losses (Farquharson 2002; Kutteh 1996a; Rai 1997) and one trial explicitly stated that live birth did not differ between those with a history of two versus three pregnancy losses, without reporting numbers of participants (Laskin 2009). The largest trial did not report the numbers of previous miscarriages for participants in either group, but described that this number (two versus three versus more than four) had no significant association with live birth (Bao 2017). Subgroup analyses based on previous placenta‐mediated complications and positivity of lupus anticoagulant (LAC) antibodies were also not possible, since these were not specified for the primary outcome live birth, if reported at all. This also applied to a subgroup analysis based on aPL titers, with only one trial (Kutteh 1996a) reporting and no specification for live birth.
Sensitivity analysis
A sensitivity analysis excluding one quasi‐randomised trial with a higher risk of bias (Kutteh 1996a) did not materially change the treatment effect. We did not carry out a sensitivity analysis to explore the effect of the full Sydney criteria for antiphospholipid syndrome (APS). Technically none of the participants in four trials met the current laboratory criteria for APS (positivity on two separate occasions, tested at least 12 weeks apart; Miyakis 2006), as the time between testing varied between six weeks (Farquharson 2002) and eight weeks (Laskin 2009; Rai 1997), or was undefined (Kutteh 1996a). The participants in the largest trial met the laboratory criteria, but it was unclear if the clinical criteria were met, as the trial did not differentiate between previous early and late pregnancy loss (Bao 2017).
Secondary outcomes (maternal)
Pre‐eclampsia
It is uncertain if there is any difference in the risk of pre‐eclampsia comparing UFH plus aspirin with aspirin alone (RR 0.57 95% CI 0.10 to 3.14; 2 trials, 82 women; low‐certainty evidence; Analysis 2.2; summary of findings Table 2).
Adverse events in the mother (definitions according to original study: (A) bleeding, (B) heparin‐induced thrombocytopenia, (C) allergic reactions)
Major maternal bleeding was reported in one quasi‐randomised controlled trial, but did not occur in any participant (Kutteh 1996a). It is uncertain if there is any difference in the risk of minor bleeding in the mother (RR 1.65; 95% CI, 0.19 to 14.03; 1 trial, 31 women; low‐certainty evidence; Analysis 2.3), but this point estimate may not be reproduced in a larger sample size as implied by the wide confidence interval. Minor bleeding events, not further specified, did occur in 3/20 in the UFH and aspirin treated group, versus 1/11 in the aspirin only group. Data on heparin‐induced thrombocytopenia and allergic reactions were collected, but none of the participating women reported either.
Venous thromboembolism
Three trials reported venous thromboembolism but none of the study participants was diagnosed with a new event during study participation (Kutteh 1996a; Rai 1997; Laskin 2009) (Analysis 2.4)..
Arterial thromboembolism
Three trials reported venous thromboembolism but none of the study participants was diagnosed with a new event during study participation (Kutteh 1996a; Rai 1997; Laskin 2009) (Analysis 2.5)
Pregnancy loss
Heparin plus aspirin may reduce the risk of pregnancy loss compared with aspirin alone (RR 0.48, 95% CI 0.32, 0.71; 1295 women; 5 studies; low‐certainty evidence; summary of findings Table 2) (Analysis 2.9).
Secondary outcomes (for the child)
Preterm delivery of a live infant
It is uncertain if there is any difference in the risk of preterm delivery comparing heparin plus aspirin to aspirin alone (RR 0.93, 95% CI 0.42 to 2.07; 3 trials, 156 women; very low‐certainty of evidence; summary of findings Table 2; Farquharson 2002; Kutteh 1996a, Rai 1997; Analysis 2.6). Farquharson 2002 specified preterm delivery at gestational age between 30 to 36 weeks (1/40 and 3/34 in the heparin plus aspirin and aspirin alone groups, respectively) and delivery before 30 weeks (1/40 and 1/34 in the heparin plus aspirin and aspirin alone groups respectively).
Intrauterine growth restriction
It is uncertain if there is any difference in the risk comparing heparin plus aspirin to aspirin alone (RR 0.85; 95% CI 0.33 to 2.19; 3 trials, 151 women; very low‐certainty of evidence; summary of findings Table 2; Analysis 2.7).
Adverse events in the child (definitions according to original study: (A) congenital malformations, (B) neonatal bleeding)
The study of Rai 1997 comparing UFH plus aspirin with aspirin alone found no congenital malformations in either treatment group (Analysis 2.8). Neonatal bleeding was not reported in any of the trials comparing a combination of heparin and aspirin with aspirin treatment alone.
Comparison 3: LMWH versus aspirin
Primary outcome ‐ Live birth
We identified one study in which LMWH was compared with aspirin (Alalaf 2012). Women in the group treated with LMWH had a higher live birth rate of 86.3%, compared with a 72.1% live birth rate in the women treated with aspirin (RR 1.20, 95% CI 1.00 to 1.43, 1 trial, 141 women; Analysis 3.1).
Secondary outcomes (maternal)
Pre‐eclampsia
One case of pre‐eclampsia occurred in the LMWH‐treated group, compared with none in the aspirin group (RR 1.93, 95% CI 0.08 to 46.31; Analysis 3.2).
Adverse events in the mother (definitions according to original study: (A) bleeding, (B) heparin‐induced thrombocytopenia, (C) allergic reactions)
Alalaf 2012 reported ecchymosis at injection site in 5 of 80 participants in the LMWH‐treated group (RR 8.42, 95% CI 0.47 to 149.41; Analysis 3.3, 1 trial, 141 women). Heparin‐induced thrombocytopenia and allergic reactions to either LMWH or aspirin were not reported.
Venous thromboembolism
One trial reported zero events in both arms (Alalaf 2012) (Analysis 3.4).
Arterial thromboembolism
One trial reported zero events in both arms (Alalaf 2012) (Analysis 3.5).
Pregnancy loss
In one trial there were 11/80 pregnancy losses in the LMHW arm compared to 17/61 in the aspirin arm (RR 0.49, 95% CI 0.25, 0.98; Analysis 3.7).
Secondary outcomes (for the child)
Preterm delivery of a live infant
The rate of preterm delivery was low and no clear difference was observed between treatment groups in the trial that compared heparin alone to aspirin alone (Alalaf 2012; RR 0.96, 95% CI 0.17 to 5.50, 1 trial, 113 women; Analysis 3.6).
Intrauterine growth restriction (IUGR)
IUGR was not reported as an outcome in the trial that compared heparin alone to aspirin alone (Alalaf 2012).
Adverse events in the child (definitions according to original study: (A) congenital malformations, (B) neonatal bleeding)
Neither congenital malformations nor neonatal bleeding were assessed in the trial by Alalaf 2012.
Comparison 4: LMWH plus aspirin versus UFH plus aspirin
Primary outcome ‐ Live birth
There was no clear difference between LMWH and aspirin versus UFH and aspirin for the outcome live birth (RR 1.44, 95% CI 0.80 to 2.62, 2 trials, 86 women; Tau² = 0.11; Chi² = 1.91, df = 1 (P = 0.17); I² = 48%; Analysis 4.1) (Fouda 2011; Stephenson 2004).
Secondary outcomes (maternal)
Pre‐eclampsia
Pre‐eclampsia occurred in three of 37 women in the LMWH plus aspirin treated group versus one woman of 33 in the UFH plus aspirin treated group (RR 2.09, 95% CI 0.33 to 13.22; Analysis 4.2; Fouda 2011; Stephenson 2004).
Adverse events in the mother (definitions according to original study: (A) bleeding, (B) heparin‐induced thrombocytopenia, (C) allergic reactions)
Both the trials by Fouda 2011 and Stephenson 2004 did not establish any major bleeding events in either treatment group, but three of 30 women in each treatment arm in the trial by Fouda 2011 reported subcutaneous bruises (RR 1.00, 95% CI 0.22 to 4.56, 2 trials, 206 women; Analysis 4.3; Fouda 2011; Stephenson 2004). Data on heparin‐induced thrombocytopenia and allergic reactions were collected, but none of the participating women reported in the studies by Fouda 2010;Fouda 2011 reported either.
Venous thromboembolism
One trial reported zero venous thromboembolism events in both arms (Fouda 2011), whereas the trial by Stephenson 2004 did not assess these (Analysis 4.4).
Arterial thromboembolism
One trial reported zero arterial thromboembolism events in both arms (Fouda 2011), whereas the trial by Stephenson 2004 did not assess these (Analysis 4.5).
Pregnancy loss
Based on two studies, there may be a lower risk of pregnancy loss with LMWH plus aspirin compared to UFH plus aspirin (RR 0.53, 95% CI 0.28, 0.99; 83 women; Analysis 4.9).
Secondary outcomes (for the child)
Preterm delivery of a live infant
No clear difference in the risk of preterm delivery was observed with a higher compared with a lower dose of LMWH (Analysis 4.6; Fouda 2011). This outcome could not be evaluated for different doses of UFH, as data were lacking.
Intrauterine growth restriction
The rates of IUGR in the trial by Fouda and colleagues were low, 1 of 24 in the higher dose of LMWH treatment arm versus 2 of 20 in the lower dose of LMWH treated group respectively (RR 0.42, 95% CI 0.04 to 4.27; Analysis 4.7; Fouda 2011).
Adverse events in the child (definitions according to original study: (A) congenital malformations, (B) neonatal bleeding)
Both congenital malformations and neonatal bleeding were reported as an outcome, but the trial by Fouda 2011 had no cases in either treatment arm (Analysis 4.8).
Comparison 5: Higher dose heparin plus aspirin versus lower dose heparin plus aspirin
Primary outcome ‐ Live birth
A higher dose of LMWH did not improve the live birth rate (RR 1.10, 95% CI 0.81 to 1.49, 1 trial, 60 women; Analysis 5.1), similar to the effects of a higher dose of UFH (RR 1.05, 95% CI 0.78 to 1.41, 1 trial, 50 women; Analysis 5.1).
Secondary outcomes (maternal)
Pre‐eclampsia
The incidence of pre‐eclampsia did not clearly differ in the groups treated with a higher or a lower dose of either heparin (RR 1.64, 95% CI 0.41 to 6.48; Analysis 5.2. 2 trials, 90 women; Fouda 2010; Kutteh 1996b).
Adverse events in the mother (definitions according to original study: (A) bleeding, (B) heparin‐induced thrombocytopenia, (C) allergic reactions)
Major maternal bleeding events did not occur in any participant, whereas the rate of minor bleeding events was similar in the high‐dose UFH group (3/20) compared with the low‐dose UFH group (4/19) (RR 0.71, 95% CI 0.18 to 2.77. 2 trials, 99 women; Analysis 5.3; Kutteh 1996b). The incidence of postpartum haemorrhage was evaluated in one trial, but was not diagnosed (Fouda 2010). Heparin‐induced thrombocytopenia was not reported in either trial, while allergic reactions were not assessed (Fouda 2010; Kutteh 1996b).
Venous thromboembolism
Fouda 2010 reported no events in any of the participants during study participation (Fouda 2010; Analysis 5.4).
Arterial thromboembolism
Fouda 2010 reported no events in any of the participants during study participation (Fouda 2010; Analysis 5.5).
Pregnancy loss
Based on two studies, there may be a lower risk of pregnancy loss with LMWH plus aspirin compared to UFH plus aspirin (RR 0.80, 95% CI 0.41, 1.55; 110 women; Analysis 5.9).
Secondary outcomes (for the child)
Preterm delivery of a live infant
The rate of preterm delivery was low and the difference between treatment groups was not clear in the trials that compared a higher‐dose heparin (LMWH or UFH) plus aspirin versus lower‐dose heparin (LMWH or UFH) plus aspirin (RR 1.96, 95% CI 0.52 to 7.32; Analysis 5.6; Fouda 2010; Kutteh 1996b).
Intrauterine growth restriction
IUGR was reported in a small minority of cases and rates with no clear difference between interventions; 2/30 and 3/20 in the respective groups treated with a higher dose heparin (LMWH or UFH) versus 1/30 and 0/19 in the groups treated with a lower dose of heparin (Fouda 2010; Kutteh 1996b; Analysis 5.7).
Adverse events in the child (definitions according to original study: (A) congenital malformations, (B) neonatal bleeding)
Congenital malformations and neonatal bleeding were assessed in the trial by Fouda 2010, but there were no cases in either treatment arm (Fouda 2010; Analysis 5.8).
Discussion
Summary of main results
The aim of this review was to assess the effects of aspirin or heparin or both for improving pregnancy outcome in women with persistent antiphospholipid antibodies (aPL) and recurrent pregnancy loss. There were no trials that had a no treatment comparator arm during pregnancy.
It is uncertain if aspirin alone has any effect on live birth when compared to placebo. There were no studies that investigated heparin alone.
The results of the meta‐analyses suggest that the combination of heparin, started after a positive pregnancy test, plus aspirin may slightly improve live birth rates compared with aspirin alone. This result was mostly driven by one large single‐centre trial (n = 1015) using low‐molecular‐weight heparin (LMWH), that found a 90.3% live birth rate in the LMWH plus aspirin group, compared to 70.1% in the group treated with aspirin alone. Two small trials evaluating unfractionated heparin (UFH) also demonstrated the combination of UFH plus aspirin to be superior to aspirin alone.
The pooled risk ratio for live birth in a head‐to‐head comparison of LMWH with UFH, both in combination with aspirin, did not demonstrate a clear benefit of one heparin over the other. Two small trials compared a higher and a lower dose of heparin (LMWH or UFH) both combined with aspirin, but there were insufficient data for meaningful analyses.
It is very uncertain if aspirin compared with placebo has any effect on the risk of pre‐eclampsia, pregnancy loss, preterm delivery of a live infant, intrauterine growth restriction or adverse events in the mother or child.
Similarly, it is very uncertain if heparin plus aspirin compared with aspirin alone has any effect on the risk of pre‐eclampsia, preterm delivery of a live infant, intrauterine growth restriction or adverse events in the mother or child.
Overall completeness and applicability of evidence
Eleven trials met our predefined inclusion criteria, with differences in treatment regimens and types of intervention. The substantial heterogeneity in study populations and numbers of enrolled participants reflect the clinical heterogeneity of antiphospholipid syndrome (APS) and at the same time point out the potential difficulties that can be encountered in conducting research in this population. The current review focused on recurrent pregnancy loss, whereas obstetric APS also has other manifestations. We strictly adhered to the inclusion criteria to maintain uniformity in this review and consequently excluded a large number of trials. Results from these trials may be of equal clinical importance and should not be neglected because they did not exactly meet the set criteria for the current review.
The evidence from this review stems from an overall small number of trials, though largely driven by the results from one trial with a large number of enrolled participants. The predefined criteria for inclusion and exclusion were followed consistently, though we broadened the criteria for persistent antibodies, as only three trials adhered to the time‐frame of at least 12 weeks between aPL‐testing. The heterogeneity in study populations, the variety of inclusion criteria and the interventions in the included trials form limitations in this review, but for the main comparisons findings were consistent.
Currently, suggested and widely employed management strategies to improve pregnancy outcomes in women with recurrent pregnancy loss and positive aPL include antepartum administration of prophylactic‐ or intermediate‐dose UFH or prophylactic‐dosed LMWH combined with aspirin over no treatment, but a risk and benefit evaluation per patient is advised (Bates 2012; Skeith 2018). The pharmacokinetic profiles of UFH and LMWH differ and perhaps their biological effects as well. Possible effects on complement activation may be of more importance and it has been hypothesised that the non‐anticoagulant effects of heparins on inflammatory processes, vascular function or placental pathology may play a role in prevention of pre‐eclampsia, a disorder highly associated with APS (Wat 2018). In a mouse model of the APS, the prevention of fetal loss by both LMWH and UFH was mediated through complement activation inhibition (Girardi 2004). A prophylactic dose of LMWH was sufficient to reduce classical complement activity in pregnant women with a history of venous thromboembolism, but this has not been evaluated for different dosages of heparin, or in presence of aPL (Oberkersch 2010). The role of non‐thrombotic processes involved in the pathogenesis of recurrent miscarriage in presence of aPL has become more clear over the years (Schreiber 2018). As defined in the protocol, we planned to carry out a subgroup analysis based on a history of previous placenta‐mediated complications, such as pre‐eclampsia, intrauterine growth restriction and/or placental abruption. The rationale for this was the different pathogenesis of recurrent early (i.e. first‐trimester) miscarriage associated with presence of aPL from the pathogenesis of aPL‐associated late pregnancy morbidity. A minority of trials reported our predefined secondary outcomes and a subgroup analysis was not possible for this reason.
Clinical criteria for pregnancy morbidity associated with APS include ≥ 1 unexplained fetal death after ≥ 10 weeks of gestation, one or more premature delivery < 34 weeks of gestation due to severe (pre)eclampsia or placental insufficiency or ≥ 3 unexplained consecutive miscarriages < 10 weeks of gestation. Though all participants in the included studies had at least two, and most at least three pregnancy losses and persistent presence of aPL, baseline characteristics differed largely between study populations. A substantial part of the studied population in the current review are not considered to be patients with obstetric APS and it is known that differences exist in risk for obstetric complications in subgroups of patients with APS (Meroni 2012; Lockshin 2013; Schreiber 2018). Most of our studied population differs from women described as being a classic APS case (late fetal death, lupus anticoagulant, history of thrombosis). In obstetric APS, presence of lupus anticoagulant has been identified as the main predictor of adverse pregnancy outcomes and thrombotic events (Buyon 2015, Lockshin 2012). As demonstrated in Table 1, in patients whose aPL antibody profiles were reported, the majority of the studied population was positive for lupus anticoagulant alone or both lupus anticoagulant and anticardiolipin antibodies. Bao 2017 reported results on a large number of women with aPL and recurrent miscarriage, but did not report details on aPL‐profiles in either treatment group; therefore it remains unknown if clinically relevant subgroup differences exist. Attempts to contact the authors of this trial in order to retrieve additional data relevant for the current review, were unfruitful. This trial also did not exclude patients with a previous thrombosis, whereas most other trials explicitly did. We ourselves did not further define miscarriage, as both recurrent early or late pregnancy losses could be included (as long as a history of two miscarriages had been established) and only a minority of the studies reported additional data on gestational age at the time of pregnancy loss. Hence, patients with classic APS may have been included in the studied population. We cannot state that the risk for obstetric complications or thrombotic complications for that matter, is much lower in our studied population, as we simply lack the data to conclude this.
As the main predictor for the next pregnancy outcome is the number of previous pregnancy losses (Carp 2007), possibly the trial by Laskin and colleagues (Laskin 2009) would have demonstrated different outcomes for subgroups with ≥ 2 or ≥ 3 previous miscarriages. The largest trial (Bao 2017), which was a large contributor to the pooled effect, included women with a history of at least two consecutive miscarriages, but did not report the number of previous miscarriages per treatment group. It should be noted that the currently employed Sydney classification criteria for APS (Miyakis 2006) form an aid in the diagnosis of APS, but were originally developed for research purposes. In the current review, we were not able to carry out subgroup analyses based on aPL‐profiles, due to the small numbers of included trials and/or limited reporting of aPL‐profiles in correlation to the primary outcome live birth.
Antibody cut‐off levels differed greatly between trials and only one trial explicitly reported aPL‐titres in participants. The association between aPL and recurrent pregnancy loss varies per type of aPL and also differs for early and late pregnancy loss (Opatrny 2006). For this reason, current guidelines recommend testing for aPL ( lupus anticoagulant (LAC) and anticardiolipin (ACA) IgG and IgM) after two pregnancy losses, consecutive or non‐consecutive, whereas testing for aβ2GPI can be considered (Vermeulen 2018). None of the included trials in the current review reported women with aβ2‐GPI antibodies. Clinical studies ideally should report results on women with homogenous aPL‐profiles and women with strongly positive tests or high‐titre antibodies should be analysed separately. As APS is a heterogeneous disease with a wide variation in both clinical presentation and laboratory parameters, an accurate evaluation of two interventions in a homogeneous subset of APS‐patients is challenging.
Uncertainty also remains regarding the ideal timing of initiation and duration of treatment. Most trials included in this review started eligible participants on aspirin preconceptionally, with heparin added once pregnancy was confirmed, in order to compare heparin and/or aspirin with aspirin alone during pregnancy. A recent study by Eid 2019 evaluated early initiation of LMWH, i.e. once positive pregnancy test was established in the fifth week of gestation, and later initiation of LMWH, i.e. after sonographic confirmation of fetal cardiac pulsation in week seven, both regimens combined with aspirin started preconceptionally. Early initiation led to an ongoing pregnancy rate of 81% at 12 weeks' gestation compared to 61% in the later initiation group. However, live birth rates and the incidence of late obstetrical complications were similar in both groups (Eid 2019). In another trial, comparing LMWH and aspirin with placebo given preconceptionally, women in the treatment group had a higher ongoing pregnancy rate within six months after randomisation when compared with women in the placebo group, but live birth overall was not affected. Additionally, the incidence of pre‐eclampsia was higher in the placebo‐treated women; 24% versus 11% in the intervention group (Ismail 2016). Initiation of heparin preconceptionally in all women with APS and recurrent early pregnancy loss would be undesirable from a patients' perspective, but whether heparin can be safely discontinued after the first trimester of pregnancy with regard to pregnancy outcome, requires further investigation.
Women with persistent presence of aPL and a history of thrombosis as well as recurrent miscarriage require thromboprophylaxis during pregnancy, as aspirin only would not be considered sufficient to prevent recurrent thrombosis. However, there are no randomised clinical trials that have evaluated anticoagulant treatment strategies, i.e. between different doses of anticoagulants, in this high‐risk population. In women with obstetric APS without a personal history of venous or arterial thrombosis receiving antepartum anticoagulant prophylaxis continuation postpartum can be considered (Bates 2018). However, the incidence of postpartum thrombosis in this population is unclear, hence the aim of postpartum thromboprophylaxis and duration thereof in this population, cannot be substantiated with the currently available evidence. Further trials should investigate the role of LMWH for prevention of recurrent pregnancy loss and of placenta‐mediated complications in women with APS.
In the trial by Farquharson 2002, a substantial part of the study population was non‐adherent (24/98), which challenges interpretation of the reported results. The authors reported no significant differences in live birth in the non‐adherent group compared to either the adherent group or the whole group. Adherence to therapy may have been higher in women receiving UFH‐ compared to LMWH‐treated women, as the dose of UFH is adjusted per patient through activated partial thromboplastin time (aPTT) monitoring. The optimal dose of LMWH or heparin, with maximal benefit and minimal risks, is unknown. Various doses of aspirin and or heparin were used in the included studies, but we did not account for these differences in the analyses, due to small sizes of the studies and limited data. Studies comparing a high or low dose of either UFH or LMWH in combination with aspirin did not show significant differences between treatment groups, though it should be noted that the quasi‐randomised controlled trial comparing different dosages of UFH lacked the power to detect any significant differences and had methodological limitations (no allocation concealment). Variation in initiation of treatment, in duration of treatment, as well as different doses and agents used, limits the possibilities of a cross‐study comparison. Two included trials continued heparin postpartum.
Noticeably, adverse events associated with heparin therapy, easy bruising at injection site or allergies, did not occur frequently or were not reported. The improvement in pregnancy outcome observed in the UFH‐treated women seemed to be associated with a non‐significant increase in risk of preterm delivery, as assessed in the subgroup of live births. For intrauterine growth restriction a comparison in the subgroup of live births was not possible, as the trial by Laskin at al (Laskin 2009) only reported adverse outcomes for all participants. As we do not know the baseline risk for preterm delivery and intrauterine growth restriction in the subgroups of live birth and this baseline risk likely differs, this comparison is prone to bias.
Important side effects of UFH therapy, such as haemorrhage and heparin‐induced thrombocytopenia did not occur or were not reported and only minor bleeding events and bruising at injection site were mentioned as possible adverse effects of heparin therapy. In the general population (i.e. without anticoagulant use during pregnancy), estimated incidences of bleeding after delivery > 500 mL typically range from 4% to 6% (Scheres 2019). Hence, the possibility of underreporting should be considered in interpreting these figures. Thrombocytopenia occurs in 5% to 10% of all pregnant women, with a slow decrease in platelet counts starting in the second trimester, most likely a consequence of haemodilution (Cines 2017). Osteoporosis is associated with heparin treatment and should be taken into consideration when treatment is long term, but was not evaluated as a secondary outcome in this review. UFH therapy when given in a low dose does not require monitoring and likely is effective in preventing recurrent pregnancy loss, though the unexpectedly low live birth rates in the comparator arms in the UFH studies may have led to an overestimation of the effect. LMWH, which has a similar efficacy and a superior safety profile compared with UFH, is a reasonable alternative treatment and currently most often used in clinical practice.
Quality of the evidence
Most of the trials were judged to be at low risk of bias for most categories. Two quasi‐randomised trials were at high risk of selection bias due to lack of allocation concealment (Kutteh 1996a; Kutteh 1996b),and in one trial, allocation concealment was unclear, therefore considered as at high risk of selection bias as well (Alalaf 2012). Three trials reported incomplete outcome data (attrition bias), including one trial at high risk for reporting bias as well (Alalaf 2012; Bao 2017; Pattison 2000).
Additonally, a majority of trials were not registered in a clinical trial registry nor had published a study protocol. For this reason, it remains unclear whether selective reporting occurred in these trials and we assessed reporting bias as unclear in unregistered studies published in the last 10 years.
Though in only two trials participants and personnel both were made unaware of treatment allocation, we assessed all trials at low risk for performance bias, since live birth is an unequivocal outcome and knowledge of treatment allocation is unlikely to influence this.
Certainty of the evidence
The certainty of evidence is low to very low. We downgraded the evidence for imprecision (due to low numbers of women participating in the studies and wide 95% confidence intervals, which are consistent with appreciable harms and benefits) and for risk of bias limitations.
Potential biases in the review process
We minimised the risk of bias in the selection of studies, with an extensive search strategy and no language or publication date restriction, to identify all relevant studies. Two review authors independently assessed study eligibility, performed data extraction and GRADE assessments. There was no funding provided for this review. Lastly, none of the review authors were involved in any of the trials evaluated for this review. We were unaware of any potential bias by focusing specifically on aspirin and/or heparin versus placebo or another for prevention of recurrent pregnancy loss in women with persistent aPL in this revision of the previous review. Formal assessment of reporting bias by means of a funnel plot, was not possible due to the small number of trials in the meta‐analyses for the main comparisons.
Agreements and disagreements with other studies or reviews
For aspirin, we only identified one study which investigated the effect of aspirin alone by comparing it with placebo (Pattison 2000). However, due to its small sample size and considerable limitations, no conclusions could be drawn based on this single study. In studies with women with recurrent pregnancy loss without persistent aPL, aspirin does not seem to improve live birth rates (De Jong 2014). Aspirin has been studied extensively in the context of reduction of the risk of pre‐eclampsia. Here, also outside of the population with persistent aPL, aspirin reduces the risk of pre‐eclampsia (Askie 2007; Rolnik 2017). Based on the lack of direct evidence and available indirect evidence, it is reasonable to suggest aspirin for prevention of pre‐eclampsia in women with recurrent pregnancy loss and persistent aPL.
A cohort study of 693 women with recurrent miscarriage evaluated live birth rates in aPL‐positive women and in women with unexplained recurrent miscarriage; overall live birth rates were 69% in aPL‐positive women and 63% in women with unexplained recurrent miscarriage. Stratification by treatment demonstrated 79% live birth in the group with aPL‐positive women treated with aspirin and heparin compared to 62% in the group treated with aspirin alone. Stratification by treatment did not show differences in outcome in the group of women with recurrent miscarriage (Cohn 2010). In non‐aPL populations evidence from an individual‐patient level meta‐analysis suggests no role for LMWH in a prophylactic dose during pregnancy to prevent recurrent placenta‐mediated pregnancy complications (Rodger 2014). Another recent meta‐analysis including eight trials involving 483 women with inherited thrombophilia and recurrent miscarriage found no significant difference in live birth rates with LMWH use compared to no LMWH (RR 0.81, 95% CI 0.55 to 1.19) (Skeith 2016).

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Aspirin versus placebo, Outcome 1: Live birth

Comparison 1: Aspirin versus placebo, Outcome 2: Pre‐eclampsia

Comparison 1: Aspirin versus placebo, Outcome 3: Adverse events in the mother

Comparison 1: Aspirin versus placebo, Outcome 4: Preterm delivery of a live infant

Comparison 1: Aspirin versus placebo, Outcome 5: Intrauterine growth restriction

Comparison 1: Aspirin versus placebo, Outcome 6: Adverse events in the child

Comparison 1: Aspirin versus placebo, Outcome 7: Pregnancy loss

Comparison 2: Heparin + aspirin versus aspirin, Outcome 1: Live birth

Comparison 2: Heparin + aspirin versus aspirin, Outcome 2: Pre‐eclampsia

Comparison 2: Heparin + aspirin versus aspirin, Outcome 3: Adverse events in the mother

Comparison 2: Heparin + aspirin versus aspirin, Outcome 4: Venous thromboembolism

Comparison 2: Heparin + aspirin versus aspirin, Outcome 5: Arterial thromboembolism

Comparison 2: Heparin + aspirin versus aspirin, Outcome 6: Preterm delivery of a live infant

Comparison 2: Heparin + aspirin versus aspirin, Outcome 7: Intrauterine growth restriction

Comparison 2: Heparin + aspirin versus aspirin, Outcome 8: Adverse events in the child

Comparison 2: Heparin + aspirin versus aspirin, Outcome 9: Pregnancy loss

Comparison 3: LMWH versus aspirin, Outcome 1: Live birth

Comparison 3: LMWH versus aspirin, Outcome 2: Pre‐eclampsia

Comparison 3: LMWH versus aspirin, Outcome 3: Adverse events in the mother

Comparison 3: LMWH versus aspirin, Outcome 4: Venous thromboembolism

Comparison 3: LMWH versus aspirin, Outcome 5: Arterial thromboembolism

Comparison 3: LMWH versus aspirin, Outcome 6: Preterm delivery of a live infant

Comparison 3: LMWH versus aspirin, Outcome 7: Pregnancy loss

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 1: Live birth

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 2: Pre‐eclampsia

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 3: Adverse events in the mother

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 4: Venous thromboembolism

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 5: Arterial thromboembolism

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 6: Preterm delivery of a live infant

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 7: Intrauterine growth restriction

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 8: Adverse events in the child

Comparison 4: LMWH+ aspirin versus UFH + aspirin, Outcome 9: Pregnancy loss

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 1: Live birth

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 2: Pre‐eclampsia

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 3: Adverse events in the mother

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 4: Venous thromboembolism

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 5: Arterial thromboembolism

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 6: Preterm delivery of a live infant

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 7: Intrauterine growth restriction

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 8: Adverse events in the child

Comparison 5: Higher dose heparin + aspirin versus lower dose heparin + aspirin, Outcome 9: Pregnancy loss
| Aspirin compared to placebo for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Patient or population: improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with Aspirin | |||||
| Live birth | Study population | RR 0.94 | 40 | ⊕⊝⊝⊝ | ||
| 850 per 1,000 | 799 per 1,000 | |||||
| Pre‐eclampsia | Study population | RR 1.06 | 33 | ⊕⊝⊝⊝ | ||
| 176 per 1,000 | 187 per 1,000 | |||||
| Adverse events in the mother ‐ Bleeding | Study population | RR 1.29 | 40 | ⊕⊝⊝⊝ | ||
| 350 per 1,000 | 451 per 1,000 | |||||
| Venous thromboembolism | Not reported | |||||
| Arterial thromboembolism | Not reported | |||||
| Pregnancy loss | Study population | RR 1.33 | 40 | ⊕⊝⊝⊝ | ||
| 150 per 1,000 | 200 per 1,000 | |||||
| Preterm delivery of a live infant | 2/16 in the aspirin group and 0/17 in the placebo group had a preterm delivery of a live infant | RR 5.29 | 33 | ⊕⊝⊝⊝ | ||
| Intrauterine growth restriction | Study population | RR 0.27 | 33 | ⊕⊝⊝⊝ | ||
| 235 per 1,000 | 64 per 1,000 | |||||
| Adverse events in the child ‐ Congenital malformations | Study population | RR 1.06 | 33 | ⊕⊝⊝⊝ | ||
| 59 per 1,000 | 62 per 1,000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of selection and attrition bias 2 Downgraded two levels due to very serious imprecision: few participants and wide confidence intervals crossing the line of no effect | ||||||
| Heparin plus aspirin compared to aspirin for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Patient or population: women with persistent antiphospholipid antibodies and recurrent pregnancy loss | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with Aspirin | Risk with Heparin (UFH or LMWH) and aspirin | |||||
| Live birth | Study population | RR 1.27 | 1295 | ⊕⊕⊝⊝ | Subgroup analysis: UFH + aspirin v aspirin: RR 1.74 LMWH + aspirin v aspirin: RR 1.20 | |
| 675 per 1.000 | 857 per 1.000 | |||||
| Pre‐eclampsia | Study population | RR 0.57 (0.10 to 3.14) | 82 | ⊕⊕⊝⊝ | ||
| 67 per 1.000 | 48 per 1.000 | |||||
| Adverse events in the mother ‐ Bleeding | Study population | RR 1.65 | 31 | ⊕⊕⊝⊝ | ||
| 91 per 1.000 | 150 per 1.000 | |||||
| Adverse events in the mother ‐ Heparin‐induced thrombocytopenia | 0/70 women in the heparin plus aspirin group had heparin‐induced thrombocytopenia, compared with 0/70 in the aspirin only group. | 140 (2 RCTs) | ‐ | |||
| Adverse events in the mother ‐ Allergic reactions | 0/45 women in the heparin plus aspirin group had allergic reactions, compared with 0/45 in the aspirin only group. | 90 | ‐ | |||
| Venous thromboembolism | 0/92 women in the heparin plus aspirin group had venous thromboembolism, compared with 0/90 in the aspirin only group. | 182 (3 RCTs) | ‐ | |||
| Arterial thromboembolism | 0/92 women in the heparin plus aspirin group had venous thromboembolism, compared with 0/90 in the aspirin only group. | 182 (3 RCTs) | ‐ | |||
| Pregnancy loss | Study population | RR 0.48 | 1295 | ⊕⊕⊝⊝ | ||
| 325 per 1.000 | 156 per 1.000 | |||||
| Preterm delivery of a live infant | Study population | RR 0.93 (0.42 to 2.07 | 156 | ⊕⊝⊝⊝ | ||
| 141 per 1.000 | 131 per 1.000 | |||||
| Intrauterine growth restriction | Study population | RR 0.85 (0.33 to 2.19) | 151 | ⊕⊝⊝⊝ | ||
| 125 per 1.000 | 106 per 1.000 | |||||
| Adverse events in the child ‐ Congenital malformations | 0/32 infants the heparin plus aspirin group had congenital malformations, compared with 0/19 in the aspirin only group. | 51 | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias for limitations (selection and attrition bias) 2 Downgraded one level due to serious inconsistency: heterogeneity in interventions (I² > 45%) 3 Downgraded one level due to serious risk of bias for limitations (selection and reporting bias) 4 Downgraded one level due to serious imprecision: few participants and wide confidence interval crossing the line of no effect 5 Downgraded one level due to serious risk of bias for limitations (selection, attrition and reporting bias) 6 Downgraded two levels due to very serious imprecision: few participants and wide confidence interval crossing the line of not effect | ||||||
| Total No. | Participants per group | Mean age (years) | Mean total prior miscarriages/woman | aCL IgM | aCL IgG | LAC | aCL and LAC | aβ2GPI | |||||||||
| Studies | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | |
| 141 | 80 | 61 | 31.4 ± 5.8 | 30.6 ± 6.3 | 3.3 ± 1.7 | 3.4 ± 1.8 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 1015 | 497 | 518 | median 35 (25‐47) | median 34 (24‐43) | median 4 (2‐11) | median 3 (2‐8) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| 98 | 51 | 47 | 33 ± 4.8 | 33 ± 4.9 | 3 ± 0.8 | 3 ± 0.9 | 3/51 | 5/47 | 6/51 | 2/47 | 23/51 | 18/47 | 18/51 | 22/47 | NA | NA | |
| 60 | 30 | 30 | 27.1 ± 3.7 | 28.9 ± 4.2 | 4.0 ± 1.2 | 4.1 ± 1.1 | 4/30 | 5/30 | 8/30 | 6/30 | 10/30 | 9/30 | 8/30 | 10/30 | NA | NA | |
| 60 | 30 | 30 | 27.5 ± 3.2 | 28.6 ± 3.5 | 4.4 ± 1.2 | 4.2 ± 1.2 | 5/30 | 8/30 | 7/30 | 5/30 | 12/30 | 10/30 | 6/30 | 7/30 | NA | NA | |
| 50 | 25 | 25 | 33.2 ± 4.2 | 33.5 ± 5.8 | 3.9 ± 1.4 | 3.7 ± 1.0 | 6/25 | 5/25 | NR | NR | NA | NA | NA | NA | NA | NA | |
| 50 | 25 | 25 | 33.3 ± 4.2 | 33.2 ± 3.9 | 3.9 ± 1.4 | 3.6 ± 1.0 | NR | NR | NR | NR | NA | NA | NA | NA | NA | NA | |
| 42 | 22 | 20 | 34.6 ± 3.9 | 33.8 ± 4.1* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NA | NA | |
| 40 | 20 | 20 | 31 ± 4.5 | 30.9 ± 3.9 | NR | NR | 6/20 | 3/20 | 6/20 | 9/20 | 4/20 | 5/20 | 3/20 | 3/20 | NA | NA | |
| 90 | 45 | 45 | median 32 (23‐40) | median 34 (22‐44) | median 4 (3‐15) | median 4 (3‐8) | 0/45 | 1/45 | 3/45 | 4/45 | 40/45 | 34/45 | 6/45 | 2/45 | NA | NA | |
| 26 | 13 | 13 | 34 (27‐40) | 34 (28‐43) | 3.8 (3‐7) | 3.9 (3‐7) | 4/14 | 11/14 | 11/14 | 7/14 | 6/14 | 3/14 | 4/14 | 2/14 | NA | NA | |
| aβ2GPI: anti‐β2‐glycoprotein‐I antibodies; aCL: anticardiolipin antibodies,aPL: antiphospholipid antibodies; LAC: lupus anticoagulant,.LMWH: low‐molecular weight heparin; NA: outcome not assessed;NR: outcome not reported,UFH: unfractionated heparin * mean age in years for the entire study population (N = 88), not separately reported for the subgroup with positive aPL specifically. ** aPL profiles given for entire study population (N = 28), not separately reported the subgroup of patients who conceived and were subsequently randomised.
| |||||||||||||||||
| Live birth | Pre‐eclampsia | Maternal bleeding | Thrombo cytopenia | Allergic Reactions | VTE ATE | Preterm delivery | IUGR | Congenital Malformations | Neonatal Bleeding | |||||||||||
| Studies | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B | A | B |
| 69/80 | 44/61 | 1/69 | 0/44 | 5/81 | NA | NA | NA | NA | NA | 0/80 | 0/61 | 3/69 | 2/44 | NA | NA | NA | NA | NA | NA | |
| 449/497 | 363/518 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 40/51 | 34/47 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 2/40 | 4/34 | NA | NA | NA | NA | NA | NA | |
| 23/30 | 21/30 | 3/30 | 2/30 | 0/30 | 0/30 | 0/30 | 0/30 | NA | NA | 0/30 | 0/30 | 3/23 | 2/21 | 2/30 | 1/30 | 0/23 | 0/21 | 0/23 | 0/21 | |
| 24/30 | 20/30 | 2/24 | 1/20 | 0/30 3/30 | 0/30 3/30 | 0/30 | 0/30 | 0/30 | 1/30 | 0/30 | 0/30 | 3/24 | 2/20 | 1/24 | 2/20 | 0/24 | 0/20 | 0/24 | 0/20 | |
| 20/25 | 11/25 | 2/20 | 1/11 | 0/20 3/20 | 0/11 1/11 | 0/25 | 0/25 | NA | NA | 0/25 | 0/25 | 3/20 | 1/11 | 3/20 | 1/11 | NA | NA | NA | NA | |
| 20/25 | 19/25 | 2/20 | 1/19 | 0/20 3/20 | 0/19 4/19 | 0/25 | 0/25 | NA | NA | NA | NA | 3/20 | 1/19 | 3/20 | 0/19 | NA | NA | NA | NA | |
| 17/22 | 15/20 | NA | NA | NA | NA | NA | NA | NA | NA | 0/22 | 0/20 | NA | NA | NA | NA | NA | NA | NA | NA | |
| 16/20 | 17/20 | 3/16 | 3/17 | 9/20 | 7/20 | NA | NA | NA | NA | NA | NA | 2/16 | 0/17 | 1/16 | 4/17 | 1/16 | 1/17 | NA | NA | |
| 32/45 | 19/45 | 0/32 | 1/19 | 0/45 | NA | 0/45 | NA | 0/45 | NA | 0/45 | 0/45 | 8/32 | 4/19 | 3/32 | 1/19 | 0/32 | 0/19 | NA | NA | |
| 9/13 | 4/13 | 1/13 | 0/13 | 0/13 | 0/13 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| ATE: arterial thromboembolism;LMWH: low‐molecular weight heparin; NA: outcome not assessed; UFH: unfractionated heparin; VTE: venous thromboembolism.
| ||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Live birth Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.25] |
| 1.2 Pre‐eclampsia Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.25, 4.52] |
| 1.3 Adverse events in the mother Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Bleeding | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.60, 2.77] |
| 1.4 Preterm delivery of a live infant Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.29 [0.27, 102.49] |
| 1.5 Intrauterine growth restriction Show forest plot | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.13] |
| 1.6 Adverse events in the child Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.6.1 Congenital malformations | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 15.60] |
| 1.7 Pregnancy loss Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.34, 5.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Live birth Show forest plot | 5 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.09, 1.49] |
| 2.1.1 UFH + aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.28, 2.35] |
| 2.1.2 LMWH + aspirin versus aspirin | 3 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.04, 1.38] |
| 2.2 Pre‐eclampsia Show forest plot | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.10, 3.14] |
| 2.3 Adverse events in the mother Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Bleeding | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.19, 14.03] |
| 2.3.2 Heparin‐induced thrombocytopenia | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.3.3 Allergic reactions | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Venous thromboembolism Show forest plot | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.5 Arterial thromboembolism Show forest plot | 3 | 182 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.6 Preterm delivery of a live infant Show forest plot | 3 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.42, 2.07] |
| 2.7 Intrauterine growth restriction Show forest plot | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.33, 2.19] |
| 2.8 Adverse events in the child Show forest plot | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.8.1 Congenital malformations | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.9 Pregnancy loss Show forest plot | 5 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.32, 0.71] |
| 2.9.1 UFH + aspirin versus aspirin | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.29, 0.71] |
| 2.9.2 LMWH + aspirin versus aspirin | 3 | 1155 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.26, 1.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Live birth Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.00, 1.43] |
| 3.2 Pre‐eclampsia Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.08, 46.31] |
| 3.3 Adverse events in the mother Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [0.47, 149.41] |
| 3.3.1 Bleeding | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [0.47, 149.41] |
| 3.4 Venous thromboembolism Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5 Arterial thromboembolism Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.6 Preterm delivery of a live infant Show forest plot | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.17, 5.50] |
| 3.7 Pregnancy loss Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.25, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Live birth Show forest plot | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.80, 2.62] |
| 4.2 Pre‐eclampsia Show forest plot | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.33, 13.22] |
| 4.3 Adverse events in the mother Show forest plot | 2 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.22, 4.56] |
| 4.3.1 Bleeding | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.22, 4.56] |
| 4.3.2 Heparin‐induced thrombocytopenia | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3.3 Allergic reactions | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.4 Venous thromboembolism Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.5 Arterial thromboembolism Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.6 Preterm delivery of a live infant Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.23, 6.76] |
| 4.7 Intrauterine growth restriction Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.04, 4.27] |
| 4.8 Adverse events in the child Show forest plot | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.8.1 Congenital malformations | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.8.2 Neonatal bleeding | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.9 Pregnancy loss Show forest plot | 2 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Live birth Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.33] |
| 5.1.1 Higher dose UFH versus lower dose UFH | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.41] |
| 5.1.2 Higher dose LMWH versus lower dose LMWH | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.81, 1.49] |
| 5.2 Pre‐eclampsia Show forest plot | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.41, 6.48] |
| 5.3 Adverse events in the mother Show forest plot | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.18, 2.77] |
| 5.3.1 Bleeding | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.18, 2.77] |
| 5.3.2 Heparin‐induced thrombocytopenia | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.3.3 Allergic reactions | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.4 Venous thromboembolism Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.5 Arterial thromboembolism Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.6 Preterm delivery of a live infant Show forest plot | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.52, 7.32] |
| 5.7 Intrauterine growth restriction Show forest plot | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [0.61, 21.07] |
| 5.8 Adverse events in the child Show forest plot | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.8.1 Congenital malformations | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.8.2 Neonatal bleeding | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.9 Pregnancy loss Show forest plot | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.55] |
| 5.9.1 Higher dose UFH versus lower dose UFH | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.29, 2.38] |
| 5.9.2 Higher dose LMWH versus lower dose LMWH | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.82] |

