Ab interno trabecular bypass surgery with iStent for open‐angle glaucoma
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012743.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 marzo 2019see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Salud ocular y de la visión
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
JL, AB, and TL designed and wrote the review.
JL, LW, and TL screened studies for inclusion.
JL and LW extracted data from studies.
JL, AB, LW, and TL drafted the review and will be responsible for updates.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Eye Institute (NEI), National Institutes of Health (NIH), USA.
Cochrane Eyes and Vision US Project (and JL during his time with the Project) is supported by cooperative agreement UG1EY020522, NEI, NIH
-
National Institute for Health Research (NIHR), UK.
-
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
-
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
-
National Institute of Aging (NIA), National Institutes of Health (NIH), USA.
JL is supported by the Epidemiology and Biostatistics of Aging Training Program, Grant Number T32 AG000247 from the NIA, NIH.
Declarations of interest
JL: This Cochrane Review was prepared while Dr. Le was a doctoral candidate at the Johns Hopkins Bloomberg School of Public Health. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
AB: none.
LW: none.
TL: none.
Acknowledgements
We thank Kay Dickersin, Henry Jampel, Gus Gazzard, Barbara Hawkins, Kuang Hu, Riaz Qureshi, Anupa Shah, and Richard Wormald for their comments and suggestions during title registration and preparation of this review. This protocol was adapted from a Cochrane Review assessing the evidence for another minimally invasive glaucoma surgical (MIGS) procedure (Hu 2016). Cochrane Eyes and Vision (CEV) created and executed the electronic search strategies. We thank Anupa Shah for assisting with the review process. We thank Nitin Anand for peer reviewing the protocol and Jennifer Evans for her comments on the protocol. We are grateful to the following peer reviewers for their time and comments on the review: Henry Jampel, Ian Pitha, and Mark Lesselroth.
We thank the members of the MIGS Consortium for their input in this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2019 Mar 28 | Ab interno trabecular bypass surgery with iStent for open‐angle glaucoma | Review | Jimmy T Le, Amanda K Bicket, Lin Wang, Tianjing Li | |
| 2017 Aug 14 | Ab interno trabecular bypass surgery with iStent for open angle glaucoma | Protocol | Jimmy T Le, Amanda K Bicket, Tianjing Li | |
Differences between protocol and review
The protocol for this review did not specify time windows for the outcomes analyzed. Working with other author teams of the MIGS Consortium, we clarified time windows of interest as short‐term (six months or less), medium‐term (six to 18 months or less), long‐term (greater than 18 months but less than or equal to 36 months), and over 36 months. When conducting meta‐analyses, we used fixed‐effect models in lieu random‐effects models due to small numbers of randomized controlled trials included. Given that the iStent devices were approved in people undergoing cataract surgery (phacoemulsification), we regarded combined phacoemulsification and MIGS procedure versus phacoemulsification alone as a separate comparison instead of a subgroup analysis.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antihypertensive Agents [administration & dosage];
- Combined Modality Therapy [methods];
- *Glaucoma Drainage Implants;
- Glaucoma, Open-Angle [*surgery];
- Ocular Hypertension [therapy];
- Ophthalmic Solutions [administration & dosage];
- Phacoemulsification;
- Randomized Controlled Trials as Topic;
- *Stents;
- *Trabecular Meshwork;
Medical Subject Headings Check Words
Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.1 Proportion of participants who were drop‐free.
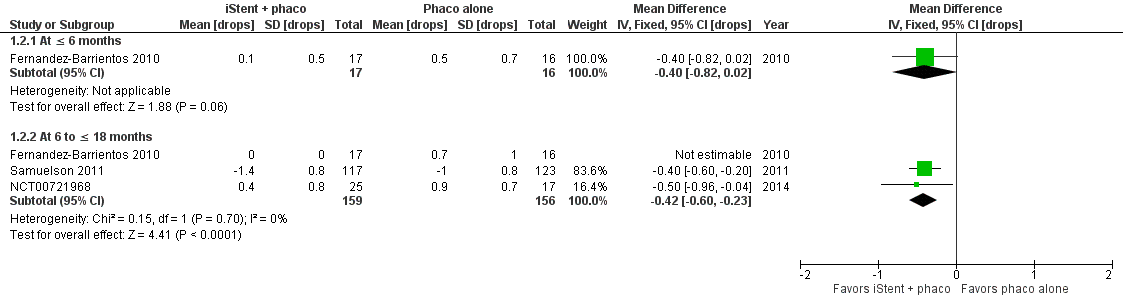
Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline.

Forest plot of comparison: 1 iStent in combination with phacoemulsification versus phacoemulsification alone, outcome: 1.3 Mean change in IOP.

Forest plot of comparison: 3 One iStent (without phacoemulsification) versus two iStents (without phacoemulsification), outcome: 3.1 Proportion of participants who were drop‐free.

Forest plot of comparison: 4 One iStent (without phacoemulsification) versus three iStents (without phacoemulsification), outcome: 4.1 Proportion of participants who were drop‐free.
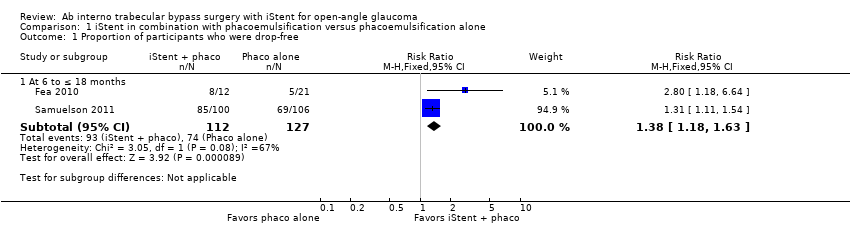
Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 1 Proportion of participants who were drop‐free.
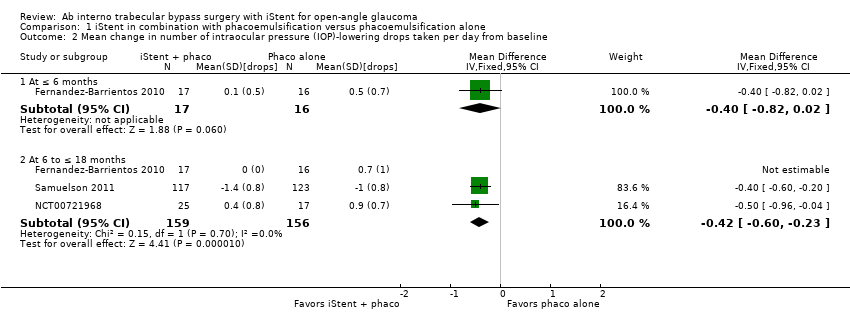
Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline.
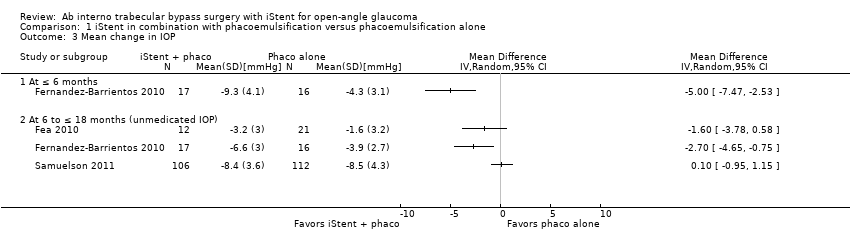
Comparison 1 iStent in combination with phacoemulsification versus phacoemulsification alone, Outcome 3 Mean change in IOP.
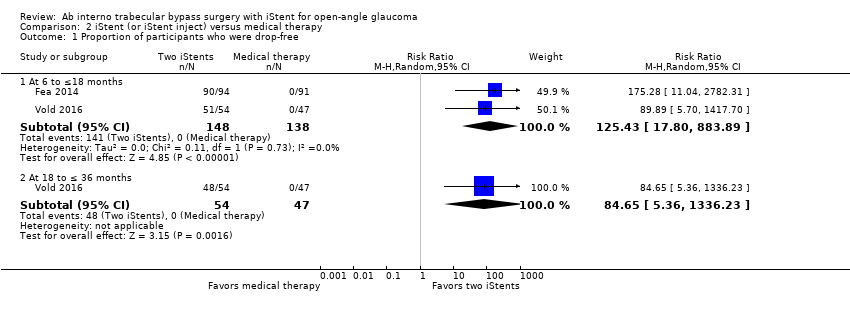
Comparison 2 iStent (or iStent inject) versus medical therapy, Outcome 1 Proportion of participants who were drop‐free.
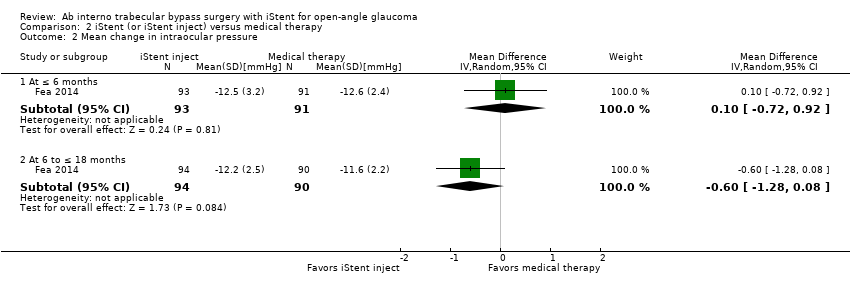
Comparison 2 iStent (or iStent inject) versus medical therapy, Outcome 2 Mean change in intraocular pressure.

Comparison 3 One iStent versus two iStents, Outcome 1 Proportion of participants who were drop‐free.
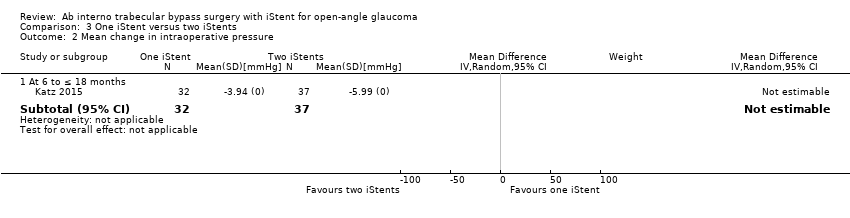
Comparison 3 One iStent versus two iStents, Outcome 2 Mean change in intraoperative pressure.
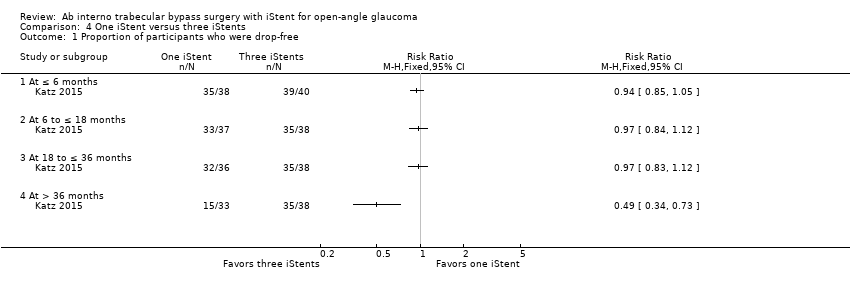
Comparison 4 One iStent versus three iStents, Outcome 1 Proportion of participants who were drop‐free.
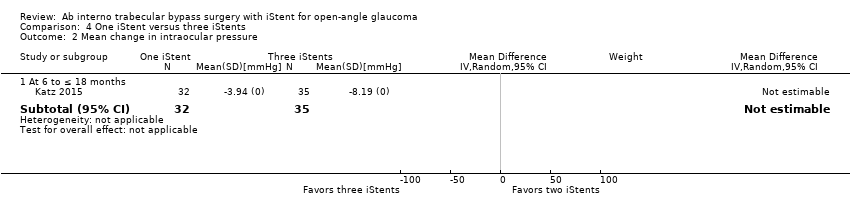
Comparison 4 One iStent versus three iStents, Outcome 2 Mean change in intraocular pressure.
| iStent in combination with phacoemulsification compared to phacoemulsification alone for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with phacoemulsification alone | Risk with iStent in combination with phacoemulsification | |||||
| Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months | 583 per 1000 | 804 per 1000 | RR 1.38 | 239 | ⊕⊝⊝⊝ | Estimate based on data from 2 trials. |
| Mean change in number of IOP‐lowering drops from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in number of IOP‐lowering drops from baseline ranged from –1.0 to 0.9 drops | MD 0.42 drops fewer | — | 282 | ⊕⊝⊝⊝ | In addition, Fernandez‐Barrientos 2010 reported the change in number of IOP‐lowering drops was 0 (SD 0) in the iStent in combination of phacoemulsification treatment group and 0.7 (SD 1) in the phacoemulsification alone group. |
| Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in IOP from baseline ranged from –8.5 to –1.6 mmHg | MD 1.24 mmHg lower | — | 284 | ⊕⊝⊝⊝ | — |
| Health‐related quality of life | — | — | — | — | Not reported in any of the 4 studies. | |
| Intraoperative complications | — | — | — | — | Samuelson 2011 reported that "[i]n an eye with intraoperative stent malposition, a second stent was implanted during the same surgery." | |
| Postoperative complications | Based on available data, participants who were randomized to treatment with phacoemulsification in combination with iStent were less likely to experience elevated IOP (or IOP spikes) and loss of vision than those randomized to phacoemulsification alone. | — | 334 (4 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| Secondary glaucoma surgery Follow‐up: range 6 to ≤ 18 months | 1 participant randomized to treatment with phacoemulsification in combination with iStent and 1 participant randomized to phacoemulsification alone underwent selective laser trabeculoplasty at 12 months. | — | 290 (3 RCTs) | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for high or unclear risk of bias for blinding of outcome assessor. | ||||||
| IStent (or iStent inject) compared to medical therapy for open‐angle glaucoma | ||||||
| Patient or population: open‐angle glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with medical therapy | Risk with iStent (or iStent inject) | |||||
| Proportion of participants who were drop‐free Follow‐up: range 6 to ≤ 18 months | At 12 months, 0/138 participants randomized to medical therapy were drop‐free at 12 months, while 141/148 (95%) participants randomized to treatment with iStent were drop‐free at 12 months. We did not derive an RR because no events occurred in the control groups of either trial. | — | 286 | ⊕⊝⊝⊝ | In addition, Vold 2016 noted that 48/54 (88%) participants in the iStent treatment group were drop‐free at 36 months. | |
| Mean change in number of IOP‐lowering drops from baseline | — | — | — | — | — | Not reported in either study. |
| Mean change in IOP from baseline Follow‐up: range 6 to ≤ 18 months | The mean change in IOP from baseline was –11.6 mmHg | MD 0.6 mmHg lower | — | 184 | ⊕⊝⊝⊝ | Vold 2016 did not report mean change in IOP but did provide mean IOP (without SD) at 6 months (14.2 mmHg), 18 months (13.5 mmHg), and 36 months (14.6 mmHg) in the iStent treatment groups; and at 6 months (13.8 mmHg), 18 months (14.6 mmHg), and 36 months (15.3 mmHg) in the medical therapy group. |
| Health‐related quality of life | — | — | — | — | Not reported in either study. | |
| Intraoperative complications | 1 participant in the iStent treatment group experienced hyphema which resolved by day 1 | — | 101 (1 RCT) | — | We did not conduct a meta‐analysis of complications. | |
| Postoperative complications | Vold 2016 noted that best‐corrected visual acuity was stable between both groups and did not report on any other postoperative complications. Fea 2014 reported that 1 participant in the iStent inject group experienced IOP decompensation with an elevated IOP of 48 mmHg. | — | 286 | — | We did not conduct a meta‐analysis of complications. | |
| Secondary glaucoma surgery | Fea 2014 reported that 1 participant needed laser treatment to remove an apparent obstruction | — | 286 | — | We did not conduct a meta‐analysis of complications. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOP: intraocular pressure; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision due to small sample size/wide confidence interval. | ||||||
| Study | Diagnosis | Intraocular pressure | Number of glaucoma medication currently taking | Visual acuity (Snellen; BCVA) | Prior incisional glaucoma surgery | Prior laser surgery | Washout period |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | |||||||
| OAG in need of cataract surgery | > 18 mmHg (medicated) | ≥ 1 | 20/80 or worse | Excluded | NR | NR | |
| OHT or OAG in need of cataract surgery | > 17 mmHg and < 31 mmHg (medicated); > 21 mmHg and < 36 mmHg (unmedicated) | ≤ 2 | 20/40 or worse | Excluded | Excluded | Yes | |
| OAG in need of cataract surgery | NR | NR | NR | NR | NR | NR | |
| OAG, PEXG, or PG, in need of cataract surgery | ≤ 24 mmHg (medicated); ≥ 22 mmHg and ≤ 36 mmHg (unmedicated) | ≥ 1 and ≤ 3 | 20/40 or worse | Excluded (except for iridectomy) | Excluded | Yes | |
| Comparison 2: iStent or iStent inject vs medical therapy | |||||||
| OAG, PEXG, or PG | ≥ 22 mmHg and < 38 mmHg (unmedicated) | 1 | 20/200 or better | Excluded | Includeda | Yes | |
| OHT, OAG, or PEXG | ≥ 21 mmHg and ≤ 40 mmHg (unmedicated) | 0 | NR | Excluded | Excluded | NA | |
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | |||||||
| OAG, PEXG, or PG; and phakic | ≥ 18 mmHg and ≤ 30 mmHg (medicated); > 22 mmHg and < 38 mmHg (unmedicated) | 2 | 20/200 or better | Excluded | Includeda | Yes | |
| aAs long as the procedure was not performed within 30 days prior to screening. | |||||||
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| — | — | |
| Adverse events | "No postoperative stent‐related adverse events were observed in these eyes [N = 24] through 48 months. IOP was well controlled in both groups throughout the entire follow‐up period; no secondary surgical intervention was required to control IOP." | |
| 2 iStents in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year | |
| Stent malposition | Authors noted that "six of the 34 (18%) implanted stents appeared to be malpositioned" | NA |
| Need for selective trabeculoplasty | 0 | 1/16 |
| iStent in combination with phacoemulsification at 1 year | Phacoemulsification alone at 1 year | |
| Posterior capsule opacification | 4/27 | 1/17 |
| IOP increase ≥ 10 mmHg vs baseline IOP at any visit | 3/27 | 9/17 |
| Conjunctivitis | 3/27 | 2/17 |
| Corneal abrasion | 2/27 | 1/17 |
| Iritis | 2/27 | 0 |
| Punctate corneal staining | 1/27 | 1/17 |
| Superficial punctate keratitis | 1/27 | 1/17 |
| Blurry vision | 1/27 | 1/17 |
| BCVA loss ≥ 1 line after 3 months postoperative | 0 | 2/17 |
| Eye pain | 0 | 2/17 |
| Retinal detachment | 0 | 1/17 |
| iStent in combination with phacoemulsification at 2 years | Phacoemulsification alone at 2 years | |
| Anticipated early postoperative event (as defined by investigators) | 20/116 | 22/117 |
| Posterior capsule opacification | 7/116 | 12/117 |
| Elevated IOP | 5/116 | 8/117 |
| Stent obstruction | 5/116 | NA |
| Blurry vision or visual disturbance | 4/116 | 8/117 |
| Stent malposition | 3/116 | NA |
| Iritis | 1/116 | 6/117 |
| Conjunctival irritation due to hypotensive medication | 1/116 | 3/117 |
| Disk hemorrhage | 1/116 | 3/117 |
| Comparison 2: iStent (or iStent inject) vs medical therapy | ||
| iStent inject at 1 year (94 eyes of 94 participants) | Medical therapy at 1 year (98 eyes of 98 participants) | |
| IOP decompensation | 1/94 | 0 |
| Soreness/discomfort | 1/94 | 0 |
| Eye burning | 0 | 1/98 |
| Medical allergy | 0 | 1/98 |
| Secondary glaucoma surgery | 1/94 | NA |
| "Safety was favorable in both groups [Two iStents, N = 54; Medical therapy, N = 47; at 36 months). Two complications were reported during stent insertion in the surgery group, both of which were attributed to subject movement during surgery: one of these subjects had hyphema which resolved by day 1 and one subject had a small iridodialysis which resulted in no postoperative ocular sequelae...In the remaining non‐operated subjects, three‐year BCVA was 20/40 or better in 6 eyes (2 in stent group and 4 in med group), 20/100 in 1 eye (stent group), and 20/200 in 6 eyes (3 per group). No other post‐treatment adverse events were reported in either group." | ||
| Additional comparison: 1 iStent vs 2 iStents vs 3 iStents | ||
| "No complications occurred intraoperatively or perioperatively, including no hypotony, choroidal effusion, hyphema, nor iridodialysis [One iStent, N=38; two iStents, N= 41; three iStents, N=40]. During 42 months of postoperative follow‐up, no device‐related or sight‐threatening adverse events occurred; furthermore, no eyes required additional glaucoma surgery. In this cohort of almost entirely phakic subjects (117 of 119) with mean baseline age between 62 and 69 years, the most common (and expected) adverse event over 3.5 years of follow‐up was progression of preexisting cataract. By Month 42 postoperatively, a total of eight one‐stent eyes, five two‐stent eyes, and seven three‐stent eyes had BCVA loss 1 line due to cataract progression. Of these cases, five one‐stent eyes, two two‐stent eyes, and three three‐stent eyes underwent cataract surgery by Month 42, and their IOP and medication data thereafter were excluded from efficacy analyses; two additional eyes (three‐stent group) had cataract surgery shortly after the Month 42 visit." | ||
| BCVA: best‐corrected visual acuity; IOP: intraocular pressure; N: number; NA: not available. | ||
| Study | Pharmaceutical industry involvement | Other financial support |
| Comparison 1: iStent in combination with phacoemulsification vs phacoemulsification alone | ||
| Glaukos Corporation provided funding/support (including study devices) | Ricerca Finalizaata della Regione Pimonte 2007 | |
| Glaukos Corporation provided funding/support | — | |
| Glaukos Corporation provided funding/support | — | |
| Glaukos Corporation provided funding/support (Investigators were consultants to Glaukos for the conduct of this study) | — | |
| Comparison 2: iStents vs medical therapy | ||
| Glaukos Corporation provided funding/support (including study devices, editorial assistance, payment of article processing charges, financial support, and non‐study financial support) | — | |
| Glaukos Corporation provided funding/support (including non‐financial, financial, and non‐study financial support to some/all authors) | — | |
| Additional comparison: iStent vs 2 iStents vs 3 iStents | ||
| Glaukos Corporation provided funding/support (including study devices and non‐financial, financial, and non‐study financial support to some/all authors) | — | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 6 to ≤ 18 months | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.18, 1.63] |
| 2 Mean change in number of intraocular pressure (IOP)‐lowering drops taken per day from baseline Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At ≤ 6 months | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐0.82, 0.02] |
| 2.2 At 6 to ≤ 18 months | 3 | 315 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.60, ‐0.23] |
| 3 Mean change in IOP Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 At ≤ 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 6 to ≤ 18 months (unmedicated IOP) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 At 6 to ≤18 months | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 125.43 [17.80, 883.89] |
| 1.2 At 18 to ≤ 36 months | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 84.65 [5.36, 1336.23] |
| 2 Mean change in intraocular pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At ≤ 6 months | 1 | 184 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.72, 0.92] |
| 2.2 At 6 to ≤ 18 months | 1 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.28, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At ≤ 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 to ≤ 18 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 18 to ≤ 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At > 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean change in intraoperative pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 6 to ≤ 18 months | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of participants who were drop‐free Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At ≤ 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 to ≤ 18 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 18 to ≤ 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At > 36 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean change in intraocular pressure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 6 to ≤ 18 months | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |

