Pars plana vitrectomy combined with scleral buckle versus pars plana vitrectomy for giant retinal tear
Abstract
Background
A giant retinal tear (GRT) is a full‐thickness neurosensory retinal break extending for 90° or more in the presence of a posterior vitreous detachment.
Objectives
To evaluate the effectiveness and safety of pars plana vitrectomy combined with scleral buckle versus pars plana vitrectomy alone for eyes with giant retinal tear.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 8), which contains the Cochrane Eyes and Vision Trials Register; Ovid MEDLINE; Embase.com; PubMed; Latin American and Caribbean Literature on Health Sciences (LILACS); ClinicalTrials.gov; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP). We did not use any date or language restrictions in our electronic search. We last searched the electronic databases on 16 August 2018.
Selection criteria
We included only randomized controlled trials (RCTs) comparing pars plana vitrectomy combined with scleral buckle versus pars plana vitrectomy alone for giant retinal tear regardless of age, gender, lens status (e.g. phakic or pseudophakic eyes) of the affected eye(s), or etiology of GRT among participants enrolled in these trials.
Data collection and analysis
Two review authors independently assessed titles and abstracts, then full‐text articles, using Covidence. Any differences in classification between the two review authors were resolved through discussion. Two review authors independently abstracted data and assessed risk of bias of included trials.
Main results
We found two RCTs in abstract format (105 participants randomized). Neither RCT was published in full. Based on the data presented in the abstracts, scleral buckling might be beneficial (relative risk of re‐attachement ranged from 3.0 to 4.4), but the findings are inconclusive due to a lack of peer reviewed publication and insufficient information for assessing risk of bias.
Authors' conclusions
We found no conclusive evidence from RCTs on which to base clinical recommendations for scleral buckle combined with pars plana vitrectomy for giant retinal tear. RCTs are clearly needed to address this evidence gap. Such trials should be randomized, and patients should be classified by giant retinal tear characteristics (extension (90º, 90º to 180º, > 180º), location (oral, anterior, posterior to equator)), proliferative vitreoretinopathy stage, and endotamponade. Analysis should include both short‐term (three months and six months) and long‐term (one year to two years) outcomes for primary retinal reattachment, mean change in best corrected visual acuity, study eyes that required second surgery for retinal reattachment, and adverse events such as elevation of intraocular pressure above 21 mmHg, choroidal detachment, cystoid macular edema, macular pucker, proliferative vitreoretinopathy, and progression of cataract in initially phakic eyes.
PICO
Plain language summary
Scleral buckling and vitrectomy in retinal detachment
What is the aim of this review?
To find out the effectiveness and safety of vitrectomy combined with scleral buckle versus vitrectomy alone for eyes with giant retinal tear.
Key messages
Currently no conclusive evidence is available from randomized controlled trials comparing the effectiveness and safety of using a scleral buckle in a combined procedure with vitrectomy for giant retinal tear against using vitrectomy alone.
What was studied in this review?
The retina is a thin inner layer that lines the back of the eye and helps to send visual stimuli to the brain. The retina partially lines the vitreous body, a jelly‐like fluid inside the eye. A giant retinal tear occurs when the vitreous body detaches from the retina (known as a retinal detachment) and creates a hole (retinal tear) of a certain size. For people who suffer retinal detachment associated with giant retinal tear, vitrectomy is usually indicated right away to prevent permanent vision loss. Vitrectomy is a type of eye surgery in which a doctor removes the vitreous and replace it with another solution.
For people with retinal detachment who are subjected to vitrectomy, this procedure can be combined with scleral buckling. Scleral buckling is a procedure to repair a retinal detachment in which a doctor attaches a piece of silicone or a sponge onto the white of the eye (scleral). Using scleral buckling may assist attachment of the retina.
What are the main results of the review?
Cochrane researchers searched for all relevant studies to answer this question and found only two conference abstracts, neither of which was published in full. Cochrane researchers also reached out to the investigators of these two studies but did not receive additional information.
People affected by giant retinal tears and retinal surgeons treating them need evidence to show whether it is useful to combine scleral buckle with vitrectomy. They need more information about the surgical procedure associated with a higher rate of retinal reattachment and reduced surgical risk. Future studies need to obtain information on outcomes for people affected by this condition, such as eye and systemic medical history, eye and retinal tear findings, vision gained from surgery, and adverse events associated with surgery.
How up‐to‐date is this review?
Cochrane researchers searched for studies published up to 16 August 2018.
Authors' conclusions
Background
Description of the condition
A giant retinal tear (GRT) is a full‐thickness neurosensory retinal break extending for 90° or more in the presence of a posterior vitreous detachment (Freeman 1978; Glasspool 1973; Kanski 1975; Schepens 1967; Scott 1975).
The annual incidence of GRT in the general population is estimated to be between 0.094 and 0.15 per 100,000 individuals (Ang 2010; Mitry 2011). The mean age of people with GRT ranges from 30 to 53 years (Ambresin 2003; Freeman 1981; Ghosh 2004; Goezinne 2008; Lee 2009; Norton 1969; Sirimaharaj 2005; Wolfensberger 2003). By gender, men represent more cases of GRT ‐ up to 91% of all cases (Ang 2010). GRTs are estimated to be the cause of rhegmatogenous retinal detachment in 0.5% to 8.3% of cases (Chou 2007; Freeman 1978; Malbran 1990; Yorston 2002).
GRTs can be classified as primary or secondary. Primary GRTs are idiopathic, and secondary GRTs can be caused by trauma or by peripheral retinal degeneration (lattice‐related and white‐without‐pressure); hereditary vitreoretinopathies, such as Stickler's (Donoso 2003; Stickler 2001), Ehlers‐Danlos, or Marfan syndrome (Dotrelova 1997); or high myopia (> 6 diopters) (Ang 2010); or as a complication of other surgical procedures such as pars plana vitrectomy (PPV) (Shinoda 2008), refractive surgery (Navarro 2005; Ozdamar 1998; Schipper 2000; Vilaplana 1999), excessive diathermy, or photocoagulation (Schepens 1962). Most GRTs are judged to be idiopathic (55% to 66%); next in frequency are those caused by trauma (31%) (Ghosh 2004; Goezinne 2008; Holland 1977; Kanski 1975; Leaver 1981; Leaver 1984; Norton 1969; Sirimaharaj 2005; Vidaurri‐Leal 1984), high myopia (9%) (Holland 1977; Kanski 1975; Lee 2009), and hereditary vitreoretinopathies (1%) (Donoso 2003; Stickler 2001). Other rare associations have included aniridia, lens coloboma, buphthalmos, microspherophakia, retinitis pigmentosa, endogenous endophthalmitis, and acute retinal necrosis (Cahill 1998; Cooling 1980; Dowler 1995; Hovland 1968; Pal 2005).
Up to 13% of GRTs present as bilateral, non‐traumatic tears (Ambresin 2003; Ang 2012; Freeman 1978; Ghosh 2004; Goezinne 2008; Holland 1977; Kanski 1975; Leaver 1984; Lee 2009; Schepens 1962; Schepens 1967; Sirimaharaj 2005; Vidaurri‐Leal 1984). Nearly 10% of cases of GRT have been associated with an earlier GRT in the fellow eye (Ang 2012). GRTs frequently are found just posterior to the ora serrata. In cases of blunt trauma, the GRT usually appears at the superonasal quadrant associated with vitreous base avulsion, secondary to anterior‐to‐posterior compression followed by transverse distention of the globe (Schepens 1967). When GRTs are presented at the equatorial zone with posterior extensions, the slits at either end of the GRT may extend farther posteriorly in a radial fashion (Scott 1976), giving the tear's edge increased independent mobility that tends to make it invert or fold on itself.
At diagnosis, visual acuity varies from counting fingers to light perception; in some cases, visual acuity can be as good as 20/40 (Ang 2010). More than 50% of all cases of GRT are associated with retinal detachment in which the fovea is compromised (fovea‐off retinal detachment). People with GRT usually present with acute painless loss of vision that may be preceded by the perception of lights, floaters, a shadow over the field of vision, or a combination of these symptoms (Ambresin 2003; Ang 2012; Aylward 1993; Ghosh 2004; Goezinne 2008; Leaver 1984).
Surgical management of GRT can be extremely challenging because of the high incidence of scarring on retinal surfaces and in the vitreous cavity following retinal detachment, which may lead to surgical failure even following initially successful surgical repair. Proliferative vitreoretinopathy (PVR) may be due to retinal pigment epithelium (RPE) cells from the large area of exposed RPE and blood‐borne cells from any concurrent clinical or subclinical associated vitreous hemorrhage. RPE cells migrate toward the vitreous cavity and proliferate into the epiretinal and subretinal space with an increase in cytokine production followed by formation of cellular membranes, which may grow and contract (Duquesne 1996; Girard 1994; Kon 1999; Leaver 1984; Malbran 1990; Miller 1986; Ryan 1985; Tseng 2004; Weller 1990; Wiedemann 1988; Yeung 2008; Yoshino 1989). Proliferative vitreoretinopathy has been estimated to cause up to 49% of retinal redetachment of a GRT (Ghosh 2004; Kertes 1997; Malbran 1990; Rofail 2005; Scott 2002).
A long‐standing debate has surrounded the role of the scleral buckle procedure combined with PPV in the surgical management of GRT. Management includes performing complete vitrectomy, unfolding the retinal flap, sealing the tear with chorioretinal adhesion, and providing intraocular tamponade (Adelman 2013; Chang 1989; Kreiger 1992).
The role of scleral buckling is controversial. Some surgeons consider the procedure beneficial for facilitating attachment of the retina because it reduces early and late vitreoretinal traction, supports areas where unrecognized retinal breaks developed after surgery, and counteracts late traction on the peripheral retina from contracture of residual vitreous gel. Some surgeons believe that scleral buckling complicates closure of a GRT by causing gaping of retinal tissue, producing redundant retinal folds, and being a real cause of posterior retinal slippage due to its role in changing ocular contour and scleral shortening relative to retina. Alternatively, some surgeons consider performing a scleral buckle only for recurrent cases of GRT. This remains the topic of an ongoing discussion among experienced surgeons (Al‐Khairi 2008; Hoffman 1986; May 1992; Scott 2002).
Description of the intervention
One of two surgical interventions is used to repair GRTs: PPV combined with scleral buckle or PPV alone.
PPV is a surgical procedure that involves removal of the vitreous gel. Perfluorocarbon liquids are then used to unfold the retina and provide countertraction and retinal stabilization during removal of fibrous membranes adherent to the retina (epiretinal and subretinal membranes) (Machemer 1972). Once retinal reattachment is complete, the surgeon performs two or three rows of endophotocoagulation under air or a perfluorocarbon liquid bubble at the border of the tear. At the end of the PPV, silicone oil or a gas bubble is usually injected into the eye to provide retinal tamponade, while the retina heals and reattaches (Chang 1989; Machemer 1972; Mathis 1992).
Scleral buckle procedures involve the use of an explant made of silicon sponge or a solid silicone band sutured to the sclera circumferentially around the equator of the eye (Wilkinson 1999; Williams 2006).
Scleral buckle and PPV may be combined for treatment of GRTs (Goezinne 2008; Holland 1977; Weichel 2006).
How the intervention might work
In PPV, the vitreous body and the vitreous base are removed; intraoperatively, perfluorocarbon liquids (perfluoro n‐octano) are used to unfold the retina. Fibrous membranes are removed to relieve the vitreoretinal traction, while the retina flattens. Once the retina is flattened, the tear is sealed by chorioretinal adhesion induced by endophotocoagulation. At completion of the procedure, the vitreous cavity is filed with silicone oil or a gas bubble to promote adhesion between the retina and the RPE (Ambresin 2003; Freeman 1981; Leaver 1981; Leaver 1984; Sirimaharaj 2005).
The scleral buckle creates an indentation in the wall of the eye, which brings the detached retina closer to the eye wall and relieves the vitreoretinal traction by supporting the vitreous base. Thus, the combined procedure may hasten healing and result in better postsurgery anatomical and visual outcomes (Goezinne 2008; Holland 1977; Weichel 2006).
Why it is important to do this review
GRTs are an uncommon cause of retinal detachment. Even though primary and final retinal reattachment rates are achieved in up to 90% of cases (Chang 1989; Goezinne 2008), visual recovery may be limited. Because of the extensive area of RPE exposed, GRTs usually progress rapidly to proliferative vitreoretinopathy, leading to surgical failure or reduced vision. Surgical management of a GRT may be challenging; recurrent retinal detachment secondary to proliferative vitreoretinopathy occurs in up to 40% of cases (Ghosh 2004; Kertes 1997; Malbran 1990; Rofail 2005; Scott 2002).
GRT currently is managed with PPV; the use of adjunctive scleral buckling is debated because it is unclear whether applying a scleral buckle provides an anatomical or visual advantage. Although general consensus favors the combined procedure in cases of GRT with proliferative vitreoretinopathy, some surgeons choose not to use scleral buckling because it may distort the shape of the eye and enhance risks of slippage of the retina posteriorly, secondary axial lengthening, gaping of retinal tissue, redundant retinal folds, and fish mouthing (Chang 1989; Machemer 1972; Mathis 1992). Other surgeons favor the combined procedure because it is thought to reduce early and late traction within the vitreous base, thus decreasing the risk of recurrent retinal detachment (Goezinne 2008; Holland 1977; Weichel 2006).
It is therefore important to review the current evidence to compare PPV alone versus combined with scleral buckling to determine the better option that results in higher rates of surgery success, while reducing the number of secondary surgeries and the occurrence of adverse events. A separate Cochrane Review has been published about interventions for prevention of GRT in the fellow eye because people with unilateral GRT are at high risk of developing retinal tears and retinal detachment in the other eye (Ang 2012).
Objectives
To evaluate the effectiveness and safety of pars plana vitrectomy combined with scleral buckle versus pars plana vitrectomy alone for eyes with giant retinal tear.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs), irrespective of their publication status or language.
Types of participants
We included trials that enrolled participants with either unilateral or bilateral GRT. We define GRT as a full‐thickness retinal break extending circumferentially for 90° or greater in the presence of a posterior vitreous detachment, or as defined by eligible trials. We included trials with more than three months of follow‐up, regardless of age, gender, ethnicity, lens status (e.g. phakic or pseudophakic eyes) of the affected eye(s), or etiology of GRT among participants enrolled in the trials.
Types of interventions
We included trials that compared PPV combined with scleral buckle versus PPV alone for GRT.
Types of outcome measures
Primary outcomes
-
Primary surgical success defined as primary retinal reattachment, or as defined by trial investigators, after initial surgery. We considered outcomes reported at different time points (less than six months, six to 12 months, and more than 12 months' follow‐up)
Secondary outcomes
-
Mean change in best corrected visual acuity (BCVA) in logMAR units from baseline to last follow‐up visit, reported at different time points (less than six months, six to 12 months, and more than 12 months' follow‐up)
-
Proportion of study eyes that required a second surgery for retinal reattachment after the initial surgery. We planned to analyze second surgeries reported from day one up to the last reported follow‐up visit after surgery
Adverse events
We planned to investigate the proportion of study eyes with adverse events after surgeries, such as retinal detachment recurrence, elevation of intraocular pressure above 21 mmHg, choroidal detachment, cystoid macular edema, macular pucker, proliferative vitreoretinopathy, or progression of cataract in initially phakic eyes, and any other adverse events reported by included trials at any time from day one up to the last reported follow‐up visit after surgery.
Economic outcome
It was not possible to determine differences in costs between treatment groups because of missing data.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials. There were no restrictions on language or year of publication. Electronic databases were last searched on 16 August 2018.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 8) (which contains the Cochrane Eyes and Vision Trials Register), in the Cochrane Library (searched 16 August 2018) (Appendix 1).
-
MEDLINE Ovid (1946 to 16 August 2018) (Appendix 2).
-
Embase.com (1947 to 16 August 2018) (Appendix 3).
-
PubMed (1948 to 16 August 2018) (Appendix 4).
-
Latin American and Caribbean Health Science Information Database (LILACS) (1982 to 16 August 2018) (Appendix 5).
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 16 August 2018) (Appendix 6).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 16 August 2018) (Appendix 7).
Searching other resources
We searched the reference lists of reports from trials we identified, to look for additional trials. We did not conduct manual searches of conference proceedings or abstracts specifically for this review. We used the Science Citation Index to find studies that cited the identified trials (last searched 21 July 2019).
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of all records identified by electronic and manual searches. Each review author labeled the study referenced in each citation as "definitely relevant," "unsure," or "definitely not relevant." We excluded from the review trials classified as "definitely not relevant." Two review authors independently reassessed the full text of trials labeled as "unsure" and "definitely relevant" according to the inclusion criteria for this review and classified them as "definitely include" or "definitely exclude." We assessed trials labeled as "definitely include" for methodological quality. We resolved any differences in classification between the two review authors through discussion at both stages of the screening process. We documented excluded trials after review of the full‐text reports and provided reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors extracted data independently using Covidence. Two review authors reached consensus before entering data into Review Manager 5 (RevMan 5) (Review Manager 2014). We collected the following information from the included trials: study methods, participants, interventions, and outcomes. We contacted the authors of two conference abstracts that were not published, to ask for more information about the studies; we did not receive any data after two weeks.
Assessment of risk of bias in included studies
We assessed each included trial for risks of bias according to the guidelines provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We considered the following criteria when assessing bias.
-
Random sequence generation: we planned to assess the method used to generate the allocation sequence generation and the allocation concealment method used before randomization.
-
Masking (blinding) of participants and outcome assessors: we planned to assess the methods used to mask participants and outcome assessors.
-
Incomplete outcome data: we planned to assess exclusions after randomization, rates of follow‐up, reasons for losses to follow‐up, and deviations from intention‐to‐treat analysis of outcomes.
-
Selective outcome reporting: we planned to assess selective outcome reporting by comparing protocols and other design documents with trial reports and by noting outcomes that were measured but not reported.
-
Other bias: we planned to assess other sources of bias, for example, industry funding.
We graded each trial on the basis of the above bias criteria as:
-
high risk of bias: when plausible bias seriously weakens the study results;
-
low risk of bias: when plausible bias is unlikely to alter the study results; or
-
unclear risk of bias: when plausible bias raises some doubt about the study results.
For all trials graded as "unclear risk of bias" because of doubt about the study on methods, results, or missing data, we contacted the investigators of the RCTs to request additional information. We assessed risks of bias of using available information if we did not hear back from trial investigators in two weeks, and we noted lack of response from trial investigators. We resolved any disagreements between review authors regarding bias assessment through discussion until we reached consensus.
Measures of treatment effect
We estimated the risk ratio (RR) and its 95% confidence interval (CI) after surgery (PPV combined with scleral buckle vs PPV alone) for the following dichotomous outcomes with information obtained from the included studies.
-
Primary surgical success.
-
Second surgery for retinal reattachment.
-
Development of adverse events such as retinal detachment recurrence, elevation of intraocular pressure above 21 mmHg, choroidal detachment, cystoid macular edema, macular pucker, proliferative vitreoretinopathy, progression of cataract in initially phakic eyes, and any other adverse events reported by included trials at any time from day one up to the last reported follow‐up visit after surgery.
We planned to estimate means and standard deviations of absolute and relative change after surgery (PPV combined with scleral buckle vs PPV alone) for the following outcomes.
-
Absolute and relative change in visual acuity in logMAR from baseline to follow‐up visits in both surgery groups.
-
Absolute and relative change in retinal detachment in GRT from baseline to follow‐up visits in both surgery groups.
To avoid the phenomenon of regression to the mean for the absolute change, we planned to perform an analysis of covariance where the change was defined relative to the value of "y" at baseline.
Unit of analysis issues
For this review, the unit of analysis was the eye; all statistical estimations included within‐individual correlation among analyses. We planned to use covariance and linear regression for repeated measures.
For trials that enrolled both eyes of participants with bilateral GRT and used a paired‐eye design in which one eye was randomized to receive PPV alone and the fellow eye received the combined procedure, the unit of analysis was the eye. If enough data were provided for paired‐eye design trials such as covariance or paired tabulation, we planned to use a paired‐sample t‐test or a McNemar test to assess differences between paired‐eye outcomes.
Dealing with missing data
If trials were not analyzed via an intention‐to‐treat approach, or when data were unclear or missing, we planned to contact trial authors for clarification and further information. We allowed two weeks for trial authors to respond, and if we did not receive a response, we used available information.
Assessment of heterogeneity
We planned to evaluate the heterogeneity of trials by using Cochrane Q and I² tests. The Q value has a Chi² distribution with the null hypothesis that the likelihood ratio is the same for all studies. The I² test evaluates variability of effect. We planned to consider values higher than 50% as evidence of substantial heterogeneity (Deeks 2017). If substantial heterogeneity or inconsistency was present, we did not report the pooled analysis; we planned to report instead a narrative summary of the results.
We expected methodological and clinical sources of heterogeneity from differences in participant characteristics, types or timing of outcome measurements, and intervention characteristics such as surgical methods used, including number of surgeons, PPV gauge number, scleral buckle type (silicon band/silicon sponge), preoperative lens status (phakic, pseudophakic, aphakic), and vitreous substitute endotamponade (gas/silicon oil).
Assessment of reporting biases
When possible, we planned to obtain published protocols or methods papers to compare intended outcomes versus reported outcomes. When we identified 10 or more RCTs, we planned to use funnel plots to judge asymmetry that may indicate publication bias in one or more reported studies in a meta‐analysis.
Data synthesis
We planned to perform data analysis according to the guidelines set forth in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventios (Deeks 2017). We planned to compare PPV combined with scleral buckle versus PPV alone for GRT. When the number of trials was less than three, we planned to use a fixed‐effect model; otherwise we planned to to use a random‐effects model. If we found evidence of substantial heterogeneity, or if we noted large diversity in participant characteristics and trial methods, we planned to present only a narrative summary of the results. We planned to perform a meta‐analysis for all outcomes under Types of outcome measures.
Subgroup analysis and investigation of heterogeneity
We planned to investigate potential explanations for clinical or statistical heterogeneity by comparing outcomes within subgroups to compare the relative effects of PPV combined with scleral buckle and PPV alone by type of tamponade used in surgery (silicon oil or gas), participant age, retinal tear etiology, duration of symptoms, grades or quadrants of retinal tear extension (90° to 180° or one or two quadrants, 180° to 270° or two or three quadrants, and 270° to 360° or three or four quadrants), lens status (phakic, pseudophakic, or aphakic), and presence of proliferative vitreoretinopathy (grade C) according to the Retinal Society classification (Machemer 1991), whenever sufficient data were reported.
Sensitivity analysis
We planned to examine the impact of excluding trials with high risk of bias in all of the following domains ‐ incomplete data, unpublished data, and industry funding ‐ to assess the robustness of estimates with respect to these factors.
"Summary of findings" table
We planned to produce a "Summary of findings" table for the following outcomes: primary surgical success, mean change in BCVA from baseline measured in logMAR units, proportion of study eyes that required a second surgery for retinal reattachment, adverse events, and economic outcomes. We planned to use the GRADE approach to assess the quality of the evidence (Guyatt 2011). Given the certainty, we planned to consider limitations of any included studies, consistency of effect, imprecision of results, indirectness of results, and publication bias.
Results
Description of studies
Results of the search
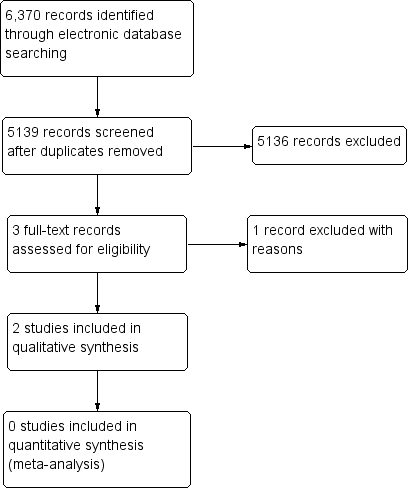
Through electronic searches, we retrieved a total of 5139 unique records, of which 5136 were excluded after review of titles and abstracts. We further excluded one full‐text report because the the population did not meet the eligibility criteria. We included the remaining two conference abstracts in the review (Figure 1). We contacted study authors for more information about the two included studies described in the conference abstracts; we did not obtain any data from investigators after waiting for two weeks.
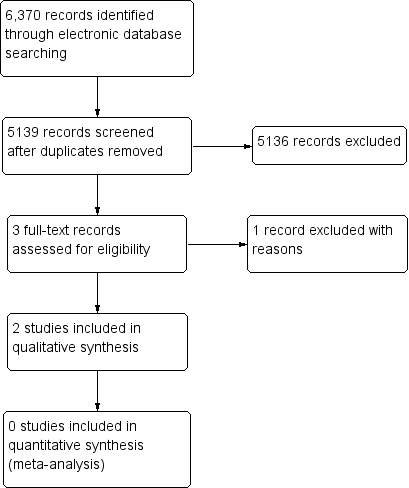
Study flow diagram.
Included studies
The Characteristics of included studies table lists the two included studies in abstract form. In Lewis 1998, 84 eyes were randomized, with a mean follow‐up of 18 months. In Sharma 2000, 21 eyes were randomized, with a follow‐up of 6 months.
Excluded studies
The Characteristics of excluded studies table lists one excluded study along with reasons for exclusion (Falkner Radler 2015).
Risk of bias in included studies
We found only two trials published in abstract form that were eligible for inclusion in the review and for risk of bias assessment.
Allocation
We assessed the two trials as having unclear risk of selection bias because insufficient information was provided in the abstracts.
Blinding
We assessed the two trials as having unclear risk of performance bias and detection bias because insufficient information was provided in the abstracts.
Incomplete outcome data
We assessed the two trials as having unclear risk of attrition bias because insufficient information was provided in the abstracts.
Selective reporting
We assessed the two trials as having unclear risk of reporting bias because we were not able to identify protocols or full reports for these studies.
Other potential sources of bias
We assessed the two trials as having unclear risk of other sources of bias because insufficient information was provided in the abstracts.
Effects of interventions
We foun two RCTs in abstract form (105 participants randomized). Neither RCT was published in full, and insufficient information was available to assess the effectiveness of the interventions.
In Lewis 1998, retinal re‐detachment occurred in 21% of eyes with scleral buckle and in 7% of eyes without scleral buckle (RR 3.0, 95% confidence interval (CI) 0.9 to 10.3). Reoperations were performed in 50% of eyes with and in 14% of eyes without scleral buckle. In Sharma 2000, eight of ten eyes had an attached retina (80%) at six months’ follow‐up in the buckle group, as compared to two of 11 eyes (22%) in the no buckle group (RR 4.4, 95% CI 1.2 to 16.0).
Discussion
Summary of main results
We conducted a search of several electronic literature databases to identify randomized controlled trials (RCTs) that evaluated the role of pars plana vitrectomy combined with scleral buckle versus pars plana vitrectomy in the management of giant retinal tear. Despite using a sensitive search strategy, we identified only two conference abstracts without full‐text reports. Based on these two abstracts, scleral buckling might be beneficial, but the findings are inconclusive due to a lack of peer reviewed publication and insufficient information for assessing risk of bias.
Overall completeness and applicability of evidence
Most of the published literature on this topic is limited to retrospective reports or case series (Ambresin 2003; Dabour 2014; Ghosh 2004; Goezinne 2008; Kumar 2018; Lee 2009; Scott 2002; Rofail 2005). Any attempt to draw conclusions through non‐randomized studies may be misleading. This lack of information demonstrates the importance of publishing the two RCTs reported in the abstract form and conducting properly powered multicenter RCTs on this topic.
Quality of the evidence
Giant retinal tear (GRT) remains an uncommon vitreoretinal pathology; therefore the surgical approach may be challenging for many retinal surgeons. Most published research data have been derived from non‐randomized studies, with primary retinal reattachment ranging from 71.7% up to 94.4% (Ambresin 2003; Batman 1999; Ghosh 2004; Goezinne 2008; Kumar 2018; Lee 2009; Scott 2002; Verstraeten 1995). We found two conference abstracts describing two RCTs without full‐text reports (Lewis 1998; Sharma 2000). Unfortunately, the quality of data and the results cannot be analyzed because of missing information; therefore data should be interpreted cautiously. In summary, it is unclear to date if combining pars plana vitrectomy with scleral buckle offers any advantage in treating giant retinal tears.
Potential biases in the review process
We seek to minimize potential risk of bias by conducting an electronic search of clinical trials with two independent review authors and according to the methods recommended by Cochrane.
Agreements and disagreements with other studies or reviews
Our findings are consistent with those described in a published review (Shunmugam 2014). Both our review and Shunmugam 2014 described the difficulty of determining if using a scleral buckle in a combined procedure with pars plana vitrectomy for giant retinal tear can modify outcomes in GRT surgery. Since evidence from RCTs is lacking, it is difficult to reach conclusions from non‐randomized studies for which patient characteristics and the comparability of surgical techniques cannot be established.
Study flow diagram.

