Intervenciones para la promoción de la actividad física en pacientes con enfermedad pulmonar obstructiva crónica (EPOC)
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | DESIGN 2 groups DATES January 2013 to January 2017 SETTING PR, 7 metropolitan hospitals (Australia) SAMPLE SIZE ESWT, CRQ "For the physical activity outcome, 82 participants will be sufficient to provide 80% power to detect as significant, at the (two‐sided) 5% level, a minimum of 1845 step difference in the mean steps per day between the groups, assuming SD 2968" | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS (TOTAL n = 111)
AGE mean 69 (SD 7) years; SEX 30 (52%) male; FEV1 mean 47 (SD 17)% predicted
AGE mean 69 (SD 8) years; SEX 31 (59%) male; FEV1 mean 45 (SD 16)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP 6 months SUPERVISION yes COMMON INTERVENTION exercise training DURATION 8 weeks SETTING supervised outpatient group CONTACT 3 sessions a week (minimum 20 sessions) AEROBIC TRAINING treadmill walking, stationary cycling
STRENGTH TRAINING, OTHER COMPONENTS, EDUCATION nil INTERVENTION supplemental oxygen, 5 litres a minute from oxygen concentrator SHAM intranasal air, 5 litres a minute from modified oxygen concentrator | |
| Outcomes | DEVICE SenseWear
ASSESSMENT TIME POINTS
PRIMARY OUTCOMES
SECONDARY OUTCOMES
| |
| Notes | FUNDING "The study was funded by a National Health and Medical Research Council, Australia, project grant APP1019989. Funding information for this article has been deposited with the Crossref Funder Registry." CONFLICT OF INTEREST statement provided CONTACT Jenny Alison [email protected] University of Sydney (Australia) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sequence generation will be determined using a computerised random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment will be achieved by the use of a central telephone randomisation system coordinated through the NHMRC Clinical Trials Centre at The University of Sydney" |
| Blinding of participants (performance bias) | Low risk | Quote: "blinding of participants, therapists and assessors" |
| Blinding of personnel (performance bias) | Low risk | Quote: "blinding of participants, therapists and assessors" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Quote: "blinding of participants, therapists and assessors" |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Quote: "blinding of participants, therapists and assessors" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT figure provided |
| Selective reporting (reporting bias) | Low risk | Registry, published protocol and paper in agreement Paper additionally reports all CRQ domains, adherence, adverse events and intervention fidelity |
| Other bias | Low risk | Prospective registration |
| Methods | DESIGN 2 groups (3 subgroups according to location of recruitment)
DATES November 2006 to November 2010 SETTING general practice, outpatient hospital clinics, PR centre (The Netherlands) SAMPLE SIZE calculation based on step count | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS of subgroups (not provided by intervention groups)
AGE median 65 (IQR 58 to 72) years; SEX 32 (66%) male; FEV1 median 78 (IQR 66 to 95)% predicted
AGE median 68 (IQR 61 to 72) years; SEX 34 (74%) male; FEV1 median 58 (IQR 40 to 69)% predicted
AGE median 54 (IQR 50 to 63) years; SEX 36 (59%) male; FEV1 median 43 (IQR 28 to 58)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP 12 months post‐intervention SUPERVISION yes INTERVENTION PAC (in‐person, as in De Blok 2006, Hospes 2009) INTERFACE 5 individual sessions ACTIVITY lifestyle physical activity (e.g. walking, cycling, stair‐climbing, gardening) STEP‐TRACKING pedometer (direct feedback) RECORD diary: daily step count, other activities (e.g. cycle, swim) GOALS “Maximal” step count goal, end intervention: set personal “physical activity norm” (between mean and maximal step count) goal EDUCATION/RESOURCES NO INTERVENTION "care appropriate to their health status" SUBGROUP PR DURATION 9 weeks SETTING centre‐based outpatient group CONTACT 3 sessions per week, one to two hours AEROBIC TRAINING "cycling, walking, swimming & sports" STRENGTH TRAINING nil OTHER COMPONENTS "psychological and/or nutritional support as needed" EDUCATION "educational courses" | |
| Outcomes | DEVICE Digiwalker SW‐2000 (pedometer)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study was supported by an unrestricted grant from Boehringer Ingelheim B.V. and by the University Medical Centre Groningen" CONFLICT OF INTEREST statement provided CONTACT Wytske Altenburg [email protected] University of Groningen (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computerized" |
| Allocation concealment (selection bias) | High risk | Quote: "Allocation was open to the researcher, counsellor and patient" |
| Blinding of participants (performance bias) | High risk | Quote: "Allocation was open to the researcher, counsellor and patient" |
| Blinding of personnel (performance bias) | High risk | Quote: "Allocation was open to the researcher, counsellor and patient" |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity: step count was reported by participants |
| Blinding of outcome assessment [other] (detection bias) | High risk | Quote: "Allocation was open to the researcher, counsellor and patient" |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow chart provided but not specified by group according to site of recruitment (as data are presented) N.B. high number of dropouts (45% from the counselling arm of the study) in the pulmonary rehabilitation group |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOMES Paper: additional outcomes reported
Registry: 3 months for intervention group, 6 months for both groups (additional follow‐up time points not reported) SECONDARY OUTCOMES Registry: upper limb, lower limb and respiratory muscle strength; COPD‐related costs (not reported) Paper: SF36 (described in Methods, not reported) |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES October 2013 to January 2016 SETTING 33 primary care centres and hospitals from 5 seaside municipalities (Spain) SAMPLE SIZE calculation based on step count | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA registry
BASELINE CHARACTERISTICS
AGE mean 69 (SD 9) years SEX 170 (84%) male; FEV1 mean 56 (SD 17)% predicted
AGE mean 69 (SD 8) years; SEX 176 (86%) male; FEV1 mean 57 (SD 18)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 months FOLLOW‐UP no SUPERVISION no INTERVENTION PAC (Urban Training) INTERFACE
ACTIVITY walking (trail of intensity as in baseline dyspnoea and 6MWD) STEP‐TRACKING pedometer (direct feedback) RECORD personalised calendar GOALS
EDUCATION/RESOURCES
NO INTERVENTION brochure with recommendation to complete MPA ≥ 30 minutes, ≥ 5 days per week | |
| Outcomes | DEVICE Dynaport MoveMonitor (centre of lower back with an elastic strap)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Judith Garcia‐Aymerich [email protected] ISGlobal, Barcelona (Spain) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A statistician blinded to study objectives and not involved in any study procedure or analysis created the randomisation sequence using Stata 12.0 (StataCorp, College Station, TX, USA) software" |
| Allocation concealment (selection bias) | Low risk | Quote: "At the second study visit, a physiotherapist allocated patients to the corresponding group using a secured computer file, where allocations were ordered according to the randomisation sequence and only available one at a time" |
| Blinding of participants (performance bias) | Unclear risk | Unable to blind participants to intervention BUT Quote: "Patients were not aware of the existence of the alternative group…" Quote: "We implemented diverse measures to avoid contamination (i.e., that participants did not receive the intervention to which they were randomised)" |
| Blinding of personnel (performance bias) | High risk | Quote: "The physiotherapists who administered the intervention and knew the allocated groups" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "outcome examiners and data analysts remained blinded to the allocation" |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow chart provided |
| Selective reporting (reporting bias) | Low risk | As per registry |
| Other bias | Unclear risk | Original estimated enrolment (July 2013) n = 600; Actual enrolment (April 2016) n = 412 |
| Methods | DESIGN cross‐over trial (only pre‐cross‐over data used), 2 groups DATES November 2011 to June 2012 SETTING 14 sites (Germany, Spain, UK) SAMPLE SIZE calculation based on endurance time (cycle ergometry) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
MEDICATIONS
BASELINE CHARACTERISTICS
AGE mean 61 (SD 8) years; SEX 35 (61%) male; FEV1 mean 57 (SD 12)% predicted
AGE mean 59 (SD 8) years SEX 42 (76%) male; FEV1 mean 56 (SD 12)% predicted | |
| Interventions | DURATION OF INTERVENTION 3 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION LAMA (aclidinium bromide, 400 μg) twice daily (09:00, 21:00 ± 1 hour) with a dry powder inhaler (Genuair®/Pressair®) PLACEBO twice daily (09:00, 21:00 ± 1 hour) with a dry powder inhaler (Genuair®/Pressair®) | |
| Outcomes | DEVICE SenseWear Pro3 (software version not reported)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study was supported by Almirall S.A., Barcelona, Spain, and Forest Laboratories LLC, a subsidiary of Actavis, New York, NY, USA. The study sponsors (Almirall S.A., Barcelona, Spain, and Forest Laboratories LLC, a subsidiary of Actavis, New York, NY, USA) were responsible for the conception and design of the study, collection of the data, and data analysis. The sponsors placed no restrictions on statements made in the final version of the manuscript or on the decision to submit the manuscript for publication." CONFLICT OF INTEREST statement provided CONTACT Henrik Watz [email protected] German Center for Lung Research, Grosshansdorf (Germany) Additional data provided: as in Almirall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed according to unique patient identification numbers and a computer‐generated random allocation sequence" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | Low risk | Quote: "Patients and investigators were blinded to treatment allocation throughout the study" |
| Blinding of personnel (performance bias) | Low risk | Quote: "Patients and investigators were blinded to treatment allocation throughout the study" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity, exercise capacity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Low risk | Paper: physical activity (additional outcome) |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES May 2013 to September 2014 SETTING pulmonary outpatient clinics (USA) SAMPLE SIZE "As this was a pilot study, the study was not powered" | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 65 (SD 8) years; SEX 25 (44%) male; FEV1 post‐bronchodilator mean 56 (SD 12)% predicted
AGE mean 66 (SD 8) years; SEX 23 (40%) male; FEV1 post‐bronchodilator mean 52 (SD 12)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION PAC (wellness coaching) INTERFACE
ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD diary: daily step count GOALS personally meaningful activity goal; increase 15% steps a month EDUCATION/RESOURCES nil INTERVENTION pedometer INTERFACE 5 phone calls (fortnightly, report step count) ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD Diary: daily step count GOALS, EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE Omron pedometer
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study was funded by a grant from GlaxoSmithKline." CONFLICT OF INTEREST "DS is an employee of GlaxoSmithKline and holds stock/shares in GlaxoSmithKline. AE, now an employee of PAREXEL International, was an employee of GlaxoSmithKline at the time the study was conducted and holds stock/shares in GlaxoSmithKline. SS was an employee of GlaxoSmithKline at the time the study was conducted and holds stock/shares in GlaxoSmithKline. All other authors have confirmed that they have no conflict of interest related to this manuscript." CONTACT Bruce Bender [email protected] National Jewish Health, Denver (US) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized" insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity: step count was reported by participants |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | High risk | No participant flow diagram provided Total number randomised provided; no details re exclusion or attrition Quote: "Forty‐nine of 57 patients in the Goal group and 50 of the 58 in the Control group completed the study and provided a Week 12 mean steps/day assessment forming the Completer population" |
| Selective reporting (reporting bias) | High risk | No trial registry Paper:
|
| Other bias | High risk |
|
| Methods | DESIGN 2 groups DATES September 2010 to July 2016 SETTING 2 hospitals (USA) SAMPLE SIZE calculation based on readmission rates | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 68 (SD 9) years; SEX 52 (48%) male; FEV1 mean 41 (SD 17)% predicted
AGE mean 68 (SD 9) years; SEX 51 (48%) male; FEV1 mean 40 (SD 17)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 months FOLLOW‐UP no SUPERVISION no INTERVENTION self‐management (health coaching) INTERFACE
ACTIVITY Stamina InMotion Elliptical Trainer (provided) STEP‐TRACKING, RECORD nil GOALS 20 minutes a day (intensity not prescribed) EDUCATION/RESOURCES
OPTIONAL PR "Referred for conventional PR" care according to GOLD guidelines | |
| Outcomes | DEVICE Sensewear armband (model and software version not reported)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Roberto Benzo [email protected] Mayo Clinic, Minnesota (US) Additional information requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly assigned subjects using an online, computer‐generated, simple binomial randomization program to one of the two groups, stratified by center" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL: not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | CONSORT diagram provided 3 people died but full count in the analysis? Quote: "Patients with missing or unknown outcomes were excluded from this analysis… Intent‐to‐treat analyses were also run to account for the missing values (almost none for the primary outcome). Patients with missing values were considered to have died or to have had COPD hospitalisation in these analyses. Because there were very few missing values and results from intent‐to‐treat analyses were similar to the original analyses, no imputations were done." |
| Selective reporting (reporting bias) | High risk | Discrepancy with study dates
PRIMARY OUTCOME
SECONDARY OUTCOMES
N.B. selective reporting of CRQ domains |
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES January 2009 to May 2013 SETTING Duke University Medical Center and Ohio State University (USA) SAMPLE SIZE "based on two primary outcomes: (1) combined death and hospitalizations/COPD‐related physician visits and (2) QoL (mental health and physical functioning) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 8) years; SEX 101 (62%) male; FEV1 mean 45 (SD 17)% predicted
AGE mean 67 (SD 9) years; SEX 98 (60%) male; FEV1 mean 46 (SD 17)% predicted | |
| Interventions | DURATION OF INTERVENTION 16 weeks FOLLOW‐UP annual follow‐up intervals for up to 4 years SUPERVISION no INTERVENTION telephone‐based enhanced coping‐skills training (CST) SETTING home‐based CONTACT telephone calls Weeks 1 to 12: 1 session a week, 30 minutes Weeks 13 and 14: 2 bi‐weekly “booster sessions“ CONTENT coping skills for symptom management
INTERVENTION usual care plus education and symptom monitoring SETTING home‐based CONTACT telephone calls
CONTENT assess health status, providing support, COPD education | |
| Outcomes | DEVICE accelerometer (Kenz Lifecorder Plus NL‐216) (hip)
ASSESSMENT TIME POINTS
PRIMARY OUTCOMES
SECONDARY OUTCOME
| |
| Notes | FUNDING “This research was supported by Grant No. HL 065503 from the National Institutes of Health, Bethesda, Maryland” CONFLICT OF INTEREST not stated CONTACT James Blumenthal [email protected] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed centrally by computer" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Blinded outcomes assessor |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk |
|
| Other bias | Unclear risk |
PROTOCOL “600 COPD patients and their respective caregivers” REPORTED ON REGISTRY “746 participants (patients and caregivers) were consented… of these, 326 patients were randomized and participated in the study intervention along with 252 consented participants who acted as a caregiver; in total 578 participants (patients and caregivers) were involved with the study intervention” |
| Methods | DESIGN 2 groups DATES April 2009 to October 2010 SETTING hospital (Brazil) SAMPLE SIZE calculation based on quadriceps strength | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 64 (SD 13) years SEX 8 (53%) male FEV1 mean 42 (SD 14)% predicted
AGE mean 68 (SD 9) years; SEX 10 (71%) male; FEV1 mean 39 (SD 16)% predicted | |
| Interventions | DURATION OF INTERVENTION duration of hospital admission FOLLOW‐UP 4 weeks SUPERVISED yes INTERVENTION exercise training (whole‐body resistance training) DURATION during admission SETTING individual sessions CONTACT began Day 3 until discharge ≥ 3 sessions AEROBIC TRAINING nil STRENGTH TRAINING upper and lower limbs (2 sets, 8 repetitions)
OTHER COMPONENTS, EDUCATION nil N.B. MONITORING
NO INTERVENTION Normal daily care
No exercise programme or recommendation to exercise after hospital discharge | |
| Outcomes | DEVICE Dynaport MoveMonitor
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Rodrigo Borges [email protected] University of Sao Paulo, Sao Paulo (Brazil) Additional data provided: 6MWD; time lying, sitting, standing and walking | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was computer generated by 1 investigator who was not involved in the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "allocation was concealed in sequentially numbered, sealed, opaque envelopes" |
| Blinding of participants (performance bias) | High risk | Unable to be blinded to the intervention |
| Blinding of personnel (performance bias) | High risk | Quote: "Masking: None (Open Label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "evaluations were performed by a blinded evaluator" |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow diagram provided Randomised n = 46, "completed" n = 29: Quote: "Despite anticipating a patient attrition rate of 40%, it was necessary to randomise more 4 patients to obtain the calculated sample size" "we had a 37% loss to follow‐up that can be considered large and probably reflects the patients’ severity because 35% of patients were referred to the ICU. Another reason for the loss during follow up was the high rate of early discharge... In addition, there was a greater loss to follow‐up in the CG because 2 patients died for reasons unrelated to COPD (rupture of aortic aneurysm, sepsis of abdominal origin) and 2 patients refused to attend the hospital 1 month after discharge" |
| Selective reporting (reporting bias) | High risk | SECONDARY OUTCOMES Paper: exercise capacity, HRQOL, systemic inflammatory mediators, arterial blood gases (baseline), lung function, length of stay, number of sessions, adherence to sessions (additional outcomes reported) |
| Other bias | Unclear risk | Retrospectively registered
Participants performed exercises with NIV in 35% of the sessions because of dyspnoea (Borg ≥ 6). |
| Methods | DESIGN 2 groups DATES March 2006 to March 2007 SETTING (Austria) SAMPLE SIZE "The present study was the first to investigate the effect of Nordic Walking on the physical activity of COPD patients. Therefore, we were unable to reliably estimate the effect size and variances prior to the study." | |
| Participants | "All patients were retired at time of inclusion or on sick leave" INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 62 (SD 9) years; SEX 14 (47%) male; FEV1 mean 48 (SD 19)% predicted
AGE mean 59 (SD 8) years; SEX 13 (43%) male; FEV1 mean 47 (SD 16)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP 6 months SUPERVISION yes INTERVENTION exercise training (Nordic walking) SETTING "mostly performed outdoors", group CONTACT 3 sessions a week AEROBIC TRAINING Nordic walking “bearable dyspnoea, optimal oxygen saturation”
STRENGTH TRAINING, OTHER COMPONENTS nil INTERVENTION education Weekly session: pulmonary pathophysiology, management of breathlessness and exacerbations, clearance of pulmonary secretions, smoking cessation, medication, nutrition | |
| Outcomes | DEVICE DynaPort Activity Monitor (box on a waist belt, leg sensor: left upper leg)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Marie Breyer [email protected] Otto Wagner Hospital, Vienna (Austria) Additional information requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation to either the Nordic Walking or the control group was done by a computer‐generated algorithm maintained by SPSS version 15.01" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT flow diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOME Registry: 6MWD (secondary outcome in paper) Paper
SECONDARY OUTCOMES Paper: SF36 (additional outcome reported) |
| Other bias | Unclear risk | Retrospectively registered No information as to whether participants had completed PR or whether they undertook PR during the follow‐up period which may have influenced results. |
| Methods | DESIGN 2 groups DATES April 2009 to December 2010 SETTING hospital outpatient PR (Belgium) SAMPLE SIZE calculation based on daily walking time | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 7) years; SEX 34 (86%) male; FEV1 mean 45 (SD 14)% predicted
AGE mean 67 (SD 8) years; SEX 32 (79%) male; FEV1 mean 46 (SD 18)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 months FOLLOW‐UP no SUPERVISION yes COMMON INTERVENTION PR SETTING centre‐based outpatient group CONTACT 60 sessions (1 week break for evaluation at Month 3)
AEROBIC TRAINING cycling exercise, treadmill walking, stair climbing, arm ergometry
STRENGTH TRAINING upper and lower limbs (3 sets, 8 repetitions)
OTHER COMPONENTS individual appointments with other healthcare providers as needed (pulmonologist, psychologist, occupational therapist, dietician, social worker, respiratory nurse) EDUCATION programme topics: understanding their disease, role of exercise training, dealing with breathlessness, adequate inhaler use, advice on how to adapt daily life activities, psychological aspects, nutritional aspects, social and financial aspects INTERVENTION PAC (in‐person) INTERFACE 8 individual sessions ACTIVITY not specified STEP‐TRACKING Sensewear Pro armband (no direct feedback) GOALS feedback provided during sessions RECORD, EDUCATION/RESOURCES nil SHAM INTERFACE 8 individual sessions STEP‐TRACKING Sensewear Pro armband (no direct feedback) ACTIVITY, RECORD, GOALS, EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE Dynaport Minimod1 (McRoberts) (lower back at the height of the second lumbar vertebra): time walking, step count Sensewear Pro (software version not reported) (upper arm): time in mild (2 to 3.6 METs) physical activity, MVPA (≥ 3.6 METs)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Thierry Troosters [email protected] KU Leuven (Belgium) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study is a two‐armed randomized controlled trial" |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation will be performed using sealed opaque envelopes in random block sizes (unknown by the investigators) after stratification for daily number of steps at baseline" |
| Blinding of participants (performance bias) | Low risk | Comment: Not possible to blind participants to the intervention BUT ‘sham attention’ provided to control group Quote: "Patients in the control group received a sham attention program. Duration and timing of the individualized sessions were similar to the intervention group, but the general health status of the patient and the progression during training was discussed during the conversations. Intermediate evaluation of physical activity was performed, but no structured feedback was provided" |
| Blinding of personnel (performance bias) | Low risk | Quote: "The multidisciplinary team providing pulmonary rehabilitation was also blinded to group allocation" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "all tests were performed by experienced health professionals that were blinded to group allocation" |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk | SECONDARY OUTCOMES
|
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES not reported SETTING 5 sites of the COPD Clinical Research Network (USA) SAMPLE SIZE calculation based on oxygen use | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 67 (SD 8) years; SEX 6 (55%) male; FEV1 mean 37 (SD 13)% predicted
AGE mean 67 (SD 10) years SEX 8 (73%) male; FEV1 mean 30 (SD 8)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 months FOLLOW‐UP no SUPERVISION no COMMON INTERVENTION clinical co‐ordinator: education session focused on increasing LTOT understanding and encouraging ambulation instructed to use supplemental oxygen 24 hours a day INTERVENTION supplemental oxygen (lightweight ambulatory) INTERVENTION supplemental oxygen (E‐cylinder) | |
| Outcomes | DEVICE RT3 (tri‐axial accelerometer) (waist belt)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Richard Casaburi [email protected] Harbor‐UCLA Medical Center, California (USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were then randomized (stratified by enrollment site) to either continued E‐cylinder use or lightweight device use" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Quote: "Masking: None (Open Label)" |
| Blinding of personnel (performance bias) | High risk | Quote: "Masking: None (Open Label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow chart provided |
| Selective reporting (reporting bias) | Unclear risk | PRIMARY OUTCOME Paper: average oxygen use per hour, variability in oxygen use pattern over the course of the day (additional outcomes reported) SECONDARY OUTCOMES Registry: spirometry (Month 3, Month 6), haemoglobin level, functional exercise capacity, body weight, health status, ambulatory status, survival, number of exacerbations, physician office visits, hospitalisations, exercise endurance (not reported) |
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES May 2013 to July 2015 SETTING PR at university hospitals, primary care and rehabilitation services (UK) SAMPLE SIZE "The study will aim to recruit as many suitable patients as are referred to the PR service within the operational phase. One of the main objectives of this feasibility study is to provide data on recruitment and to enable an accurate estimation of sample size for a planned RCT. Based on calculations from previous studies carried out in the PR service, we anticipate a recruitment rate of around 100 patients during our operational phase. This feasibility study will enable us to estimate the required sample size for the subsequent RCT based on a realistic recruitment strategy." | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 10) years; SEX 38 (75%) male; FEV1 mean 59 (SD 29)% predicted
AGE mean 66 (SD 8) years SEX 33 (64%) male FEV1 mean 55 (SD 21)% predicted | |
| Interventions | DURATION OF INTERVENTION web‐based: predicted to be 6 to 7 weeks, mean 11 weeks observed; centre‐based: 7 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION web‐based PR SETTING home CONTACT
AEROBIC TRAINING “encouraged to exercise on a daily basis” walking
STRENGTH TRAINING upper and lower limbs, hand‐held weights
OTHER COMPONENTS
EDUCATION individualised web page featuring a personalised action plan and educational content based on SPACE for COPD manual, work through content at their own pace INTERVENTION centre‐based PR SETTING outpatient group
CONTACT twice a week, exercise 60 min, education 60 min AEROBIC TRAINING
STRENGTH TRAINING upper and lower limbs, dumbbells, Borg scale rating of perceived exertion (rating 13 – 15)
OTHER COMPONENTS
EDUCATION variety of relevant self‐management topics: medication, relaxation skills, chest clearance, breathlessness management and energy conservation | |
| Outcomes | DEVICE Sensewear armband (model and software version not reported)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Emma Chaplin emma.chaplin@uhl‐tr.nhs.uk University Hospitals Of Leicester NHS Trust, Leicester (UK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation to treatment group allocation will be a 1:1 ratio to either group and will use internet based ‘Sealed Envelope’ randomisation codes where treatment group allocation is sent by automated email to the research physiotherapist" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation to treatment group allocation will be a 1:1 ratio to either group and will use internet based ‘Sealed Envelope’ randomisation codes where treatment group allocation is sent by automated email to the research physiotherapist" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "clinical measures... were conducted by a research physiotherapist who was blinded to treatment group allocation" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOME Registry: ISWD; Paper: only baseline ISWD data reported N.B. paper: "physical activity data are to be presented in future publications" |
| Other bias | Unclear risk |
REGISTRY 06/05/2015: The following changes were made to the trial record... The target number of participants was changed from 100 to 120 however 100 participants specified in the protocol and 103 people were randomised
REGISTRY "We anticipate that it will take approximately 6 to 7 weeks to work through the online programme". PROTOCOL "We anticipate from our work… that it will take approximately 6 weeks to work through the online programme". PAPER METHODS "It was anticipated from previous work that it would take ∼6– 8 weeks to work through the online programme" PAPER RESULTS "The average number of weeks to complete the website was 11±4"
Could this have been a reason for higher drop‐out rate? Not mentioned in Discussion ABSTRACT "Dropout rates were higher in the web‐based programme (57% vs 23%)." PAPER METHODS "7 weeks (4 weeks supervised; 3 weeks unsupervised)… Patients were classed as a completer if they had reached stage 3 or above of the web programme, achieving 75% of the programme which is standard in clinical practice for those attending classes".
REGISTRY Follow Up Length: 3 months PAPER METHODS "Clinical measures were performed at baseline and repeated again at the discharge assessment following completion of either rehabilitation programme (usually ∼6–7 weeks after starting the programme)"
PROTOCOL "patients’ ability to exercise safely will be monitored." PAPER METHODS "Non‐clinical outcomes included a web‐usage audit for the internet‐based programme, recruitment rates, eligibility and patient preference as well as dropout and completion rates in both treatment groups. Any serious adverse events were reported to the sponsor. A serious adverse event was defined as an acute exacerbation of their COPD that resulted in a hospital admission. In order to assess the patients’ ability to exercise safely, an exercise safety quiz was completed online before being able to progress onto stage 2 of the programme which involved exercising. Patients were then monitored online and through the weekly contacts." |
| Methods | DESIGN two groups DATES February 2012 to October 2016 SETTING hospitals (Belgium, Germany, The Netherlands, Canada) SAMPLE SIZE calculation based on 6MWD | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 8) years; SEX 52 (47%) male; FEV1 mean 40 (SD 15)% predicted
AGE mean 65 (SD 7) years; SEX 43 (39%) male; FEV1 mean 43 (SD 17)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION no COMMON INTERVENTION PR SETTING centre‐based outpatient group CONTACT 3 to 5 sessions a week, 1 hour (20 sessions Germany, 36 sessions other centres) AEROBIC TRAINING cycling exercise, treadmill walking, stair climbing, arm ergometry
STRENGTH TRAINING upper and lower limbs, Borg Scale dyspnoea and rating of perceived exertion
OTHER COMPONENTS inspiratory muscle training as by group allocation EDUCATION nil INTERVENTION inspiratory muscle training Powerbreathe KH1 device ‘resistance training’ at high intensity (≥ 50% Pimax) 21 min: 6 cycles of 30 breaths; cycle: approx 3.5 min of resistive breathing, 1 min rest; 2 cycles, 3 sessions a day SHAM Powerbreathe KH1 device ‘endurance training’ at a low training intensity (≤ 10% Pimax) 21 min: 6 cycles of 30 breaths; cycle: approx 3.5 min of resistive breathing, 1 min rest; 3 cycles, 2 sessions a day | |
| Outcomes | DEVICE Dynaport Minimod
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "HaB International (Southam, UK) and McRoberts (The Hague, The Netherlands) provided equipment for testing and training in this study on loan... Disclaimer: None of the sponsors had any role in the preparation of the trial design, patient recruitment, data collection, data analysis, interpretation of the data, approval of the report or the decision to submit this manuscript for publication." CONFLICT OF INTEREST "AM acknowledges a previous (now expired) beneficial interest in the POWERbreathe inspiratory muscle trainers in the form of a share of royalty income to the University of Birmingham, and a potential share of royalty income to Brunel University. In the past, she has also provided consultancy services to POWERbreathe International, but no longer does so. She is named on two patents relating to POWERbreathe products, including the device used in the present study, as well as being the author of two books on inspiratory muscle training. FM reports research support from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Grifols and Novartis, advisory board participation for Boehringer Ingelheim and GlaxoSmithKline, and speaking engagements for Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Grifols and Novartis." CONTACT Daniel Langer [email protected] KU Leuven (Belgium) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group allocation will be performed by simple randomisation using sealed opaque envelopes in random block sizes of four and six (order unknown to investigators)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Group allocation will be performed by simple randomisation using sealed opaque envelopes in random block sizes of four and six (order unknown to investigators)" |
| Blinding of participants (performance bias) | Low risk | Quote: "(sham) intervention described to patients as ‘endurance training’ at a low training intensity" |
| Blinding of personnel (performance bias) | Low risk | Quote: "Physiotherapists providing this intervention will be blinded to group allocation of patients" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity and exercise capacity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL: Quote: "all tests will be performed by experienced investigators who are blinded to group allocation" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Low risk | Protocol: anxiety and depression (not reported) |
| Other bias | Unclear risk | Quote: "data were analysed using a modified intention‐to‐treat approach... we did not consider patients who had missing outcome data due to loss to follow‐up in the analysis. Consequently, no imputation for missing data was performed and a so‐called ‘complete/available case analysis’ was performed" Quote: "Interactions between centres and between‐group post‐treatment differences in PImax, progression of exercise training intensity, endurance cycling time and 6MWD were observed." Quote: "One of the centres offering a 36 session programme (32% of total inclusions) consistently exceeded between group differences in the centre offering 20 sessions (36% of total inclusions). In other centres offering 36 sessions (32% of total inclusions), between‐group differences in these outcomes were consistently smaller than in the centre offering a lower training volume" Quote: "The sham intervention... might even have constituted an endurance‐type training stimulus for these patients in addition to the endurance‐type training stimulus provided by the exercise training sessions" |
| Methods | DESIGN 2 groups DATES April to July 2014 SETTING 3 primary care centres, district hospital (Portugal) SAMPLE SIZE calculation based on MVPA time | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 69 (SD 8) years; SEX 13 (81%) male; FEV1 mean 66 (SD 21)% predicted
AGE mean 64 (SD 8) years; SEX 14 (88%) male; FEV1 mean 68 (SD 20)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 months FOLLOW‐UP no SUPERVISION yes COMMON INTERVENTION PR SETTING outpatient group CONTACT 3 sessions a week, 1 hour AEROBIC TRAINING walking, 20 minutes, modified Borg Scale dyspnoea and fatigue (rating 4 to 6)
STRENGTH TRAINING upper and lower limbs, 15 minutes (2 sets, 10 repetitions)
OTHER COMPONENTS
EDUCATION one session per week, 90 minutes
INTERVENTION PAC INTERFACE
ACTIVITY walking STEP‐TRACKING pedometer Yamax Power Walker EX‐510 (direct feedback) RECORD diary: daily step count, short‐term step count goals GOALS ≥ 800 steps if previous goal met EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE AGT3X+ (Actilife v6.10.4)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Alda Marques [email protected] University of Aveiro, Aveiro (Portugal) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was a randomised controlled trial. Patients were randomly assigned…using a computer‐generated schedule in random blocks of two" |
| Allocation concealment (selection bias) | Low risk | Quote: "One researcher kept the allocation sequence in sealed opaque envelopes, drew the envelopes and scheduled patients" |
| Blinding of participants (performance bias) | Unclear risk | Quote: "Patients knew about the existence of two groups but not the differences between interventions" |
| Blinding of personnel (performance bias) | High risk | Quote: "All measures were administrated in a face‐to‐face interview conducted by the same researchers who implemented the intervention. Thus, assessor blinding was not possible" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL and exercise capacity: Quote: "all measures were administrated in a face‐to‐face interview conducted by the same researchers who implemented the intervention. Thus, assessor blinding was not possible" |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOMES Registry: time spent in different postures (not reported) SECONDARY OUTCOMES Registry: patients' perspectives post intervention, change in number and duration of respiratory exacerbations and hospitalisations (not reported) Paper: upper‐limb isometric muscle strength, behavioural regulations in exercise (additional outcomes) |
| Other bias | Unclear risk | Both groups underwent 12 weeks of PR between April and July 2014. The intervention group also received an additional 12 weeks of physical activity‐focused behavioural intervention
|
| Methods | DESIGN 2 groups DATES December 2012 to March 2015 SETTING Royal Brompton Hospital, London (UK) SAMPLE SIZE calculation based on peak workload (incremental cycle ergometry) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA (PAPER)
EXCLUSION CRITERIA (REGISTRY)
BASELINE CHARACTERISTICS
AGE mean 66 (SD 10) years; SEX 14 (45%) male; FEV1 mean 48 (SD 23)% predicted
AGE mean 68 (SD 7) years; SEX 20 (59%) male; FEV1 mean 52 (SD 20)% predicted | |
| Interventions | DURATION OF INTERVENTION AND FOLLOW‐UP 10 weeks (PR 8 weeks) SUPERVISION no COMMON INTERVENTION PR DURATION 8 weeks SETTING multidisciplinary outpatient group: goal‐setting and progressive approach, continuous reassessment to allow progression as tolerated CONTACT 3 exercise sessions a week (2 supervised) 1 hour exercise, 1 hour education AEROBIC TRAINING treadmill and cycle exercise; intensity 60% to 80% predicted VO2 peak STRENGTH TRAINING upper and lower limb, weights, progression as tolerated OTHER COMPONENTS 1 unsupervised home‐based exercise session EDUCATION self‐management topics including exercise, medication use, diet, coping strategies, increasing physical activity, recognising and managing infections INTERVENTION ACE inhibitor (10 mg enalapril) once daily Started treatment 1 week before PR PLACEBO (microcrystalline cellulose) once daily Started treatment 1 week before PR | |
| Outcomes | DEVICE SenseWear Pro Armband (professional version 7.0) (body of the triceps muscle of right arm)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "Supported by the Medical Research Council (grant reference MR/J000620/1), which provided the salary for K.J.C. The salary of M.I.P. is partly funded by the National Institute for Health Research (NIHR) Biomedical Research Unit. H.M. is partly funded by the NIHR University College London Hospitals Biomedical Research Centre. W.D.‐C.M. is funded by a NIHR Clinical Scientist Award (CS/7/007), a NIHR Clinical Trials Fellowship (NIHR‐CTF‐01‐12‐04), a Medical Research Council New Investigator Grant (G1002113), and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for NW London." CONFLICT OF INTEREST provided CONTACT Nicholas S Hopkinson [email protected] Imperial College, London (UK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated… using block randomization and a block size of four. Randomization was performed by Imperial College Trials Unit" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | Low risk | Quote: "Both subjects and the assessor were blind to treatment allocation" |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Quote: "Both subjects and the assessor were blind to treatment allocation" |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Quote: "Both subjects and the assessor were blind to treatment allocation"Quote: |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | 6MWD listed as secondary outcome in the registry; not reported in the paper |
| Other bias | Low risk | Quote: “Analysis was performed on a per protocol basis” Comment: Prospectively registered |
| Methods | DESIGN 2 groups DATES not reported SETTING outpatients..."regularly attending our units" (Italy) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 75 (SD 5) years; SEX 32 (73%) male; FEV1 mean 0.8 (SD 0.4) litres per second
AGE mean 73 (SD 8) years; SEX 29 (66%) male; FEV1 mean 0.8 (SD 0.2) litres per second | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION essential amino acids supplement, oral mixture (4 grams, Aminotrofic) dose at 10:00 and 17:00 PLACEBO isocaloric undistinguishable dose oral mixture dose at 10:00 and 17:00 | |
| Outcomes | DEVICE SenseWear Armband Pro3 (software version not reported) (upper right arm)
ASSESSMENT TIME POINTS
PRIMARY/SECONDARY OUTCOMES
| |
| Notes | FUNDING not reported CONFLICT OF INTEREST statement not provided CONTACT R Dal Negro [email protected] Federica Boschi federica [email protected] Universita degli Studi di Pavia, Pavia (Italy) Additional data requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eight‐eight outpatients…were selected from those regularly attending our units and enrolled… randomisation table" |
| Allocation concealment (selection bias) | Low risk | Quote: "investigators were blinded to the randomisation table, the code assignments…as subjects were enrolled, they were assigned a progressive number" |
| Blinding of participants (performance bias) | Low risk | Quote: "indistinguishable dose of placebo" |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "double‐blind… investigators were blinded to…the procedure" Comment: not clear if this refers to personnel or outcome assessors |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL: Quote: "double‐blind… investigators were blinded to…the procedure" Comment: not clear if this refers to personnel or outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk | No trial registry; results presented as in Methods Unclear whether some results represent change from baseline or post‐intervention values |
| Other bias | Unclear risk | Unclear relationship with other publication (Dal Negro 2010) |
| Methods | DESIGN 2 groups DATES not reported SETTING PR (The Netherlands) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 10) years; SEX 5 (50%) male; FEV1 mean 52 (SD 22)% predicted
AGE mean 63 (SD 12) years; SEX 4 (36%) male; FEV1 mean 43 (SD 13)% predicted | |
| Interventions | DURATION OF INTERVENTION 9 weeks FOLLOW‐UP no SUPERVISION yes COMMON INTERVENTION PR DURATION 9 weeks SETTING centre‐based outpatient group CONTACT not stated EXERCISE TRAINING “according to evidence‐based guidelines” OTHER COMPONENTS dietary intervention EDUCATION psycho‐educational modules INTERVENTION PAC (in‐person, Altenburg 2015, Hospes 2009) INTERFACE 4 individual sessions ACTIVITY lifestyle physical activity (e.g. walking, cycling, stair‐climbing, gardening) STEP‐TRACKING pedometer (direct feedback) RECORD diary: daily step count, other activities (e.g. cycle, swim) GOALS “maximal” step‐count goal, end intervention: set personal “physical activity norm” (between mean and maximal step count) goal EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE Yamax Digi‐Walker SW‐200 (pedometer) (belt or waistband)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING not reported CONFLICT OF INTEREST statement not provided CONTACT Mathieu HG de Greef [email protected] University of Groningen (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who were referred for pulmonary rehabilitation were randomly assigned" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention. |
| Blinding of personnel (performance bias) | Low risk | Quote: "clinical staff…blinded for group assignment" |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity: unclear if step count was reported by participants |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as in Methods |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES not reported SETTING primary physiotherapy care centres (The Netherlands) SAMPLE SIZE calculation based on walking time | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 69 (SD 10) years; SEX 8 (31%) male; FEV1 mean 68 (SD 8)% predicted
AGE mean 71 (SD 9) years; SEX 10 (38%) male; FEV1 mean 65 (SD 10)% predicted | |
| Interventions | DURATION OF INTERVENTION 10 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION exercise training SETTING group‐based circuit training in primary physiotherapy care centres CONTACT 2 sessions a week, 1 hour AEROBIC TRAINING 10 minutes treadmill walking, 10 minutes cycling STRENGTH TRAINING extremity resistance exercises OTHER COMPONENTS unsupervised home exercise programme once a week (≥ 30 minutes) “Emphasis on continuity rather than increasing training intensity and goal setting” EDUCATION 5 minutes a week: exercise compliance, importance of staying active in daily life "further details on onset intensity, frequency, duration and progression of the workload are described in Appendix A, see online supplementary material" (not available in online supp) NO INTERVENTION standard medical care from their general physician, self‐referral consultation in case of worsening symptoms | |
| Outcomes | DEVICE Personal activity monitor (uniaxial accelerometer) (waist) Quantifies amount of motion in vertical plane with EE ≥ 0.8 METs
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "Eight activity monitors were provided without charge by PAM. PAM had no involvement in the study." CONFLICT OF INTEREST statement provided CONTACT Pieter de Roos [email protected], [email protected] Physiotherapy Centre De Oppers (The Netherlands) Additional information provided: confirmed threshold for MPA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocation was randomised… All possible sequences in permuted blocks of four with two intervention and two control tickets were created and placed at random in sequentially numbered order by an individual not affiliated to the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "concealed using opaque sealed envelopes… At intake and under the supervision of the physiotherapist in the primary care centre, participants were instructed to open the first envelope" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to intervention |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "there was an inevitable lack of blinding of the physiotherapists" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL and exercise capacity: Quote: "due to the setting of the research, the outcome assessor was not blinded" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT flow diagram provided |
| Selective reporting (reporting bias) | Low risk | Results presented as in Methods |
| Other bias | Unclear risk | Clinical trial registration number NL24766.018.08. but unable to locate Email confirmation from author that trial was registered as per Dutch regulations, unable to access in English |
| Methods | DESIGN 2 groups DATES May 2014 to March 2015 SETTING centres across Europe (Belgium, Greece, UK, Switzerland, The Netherlands) SAMPLE SIZE calculation based on step count | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 8) years; SEX 111 (65%) male; FEV1 mean 55 (SD 20)% predicted
AGE mean 67 (SD 8) years; SEX 108 (63%) male; FEV1 mean 57 (SD 21)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION physical activity intervention INTERFACE semi‐automated telecoaching
ACTIVITY "favourite activities" STEP‐TRACKING Step counter (Fitbug Air©; direct feedback) RECORD Fitbug sends step count to app GOALS
EDUCATION/RESOURCES Home exercise booklet NO INTERVENTION Individual in‐person discussion (5 to 10 minutes at Visit 2) Standard leaflet explaining the importance of physical activity in COPD and information about physical activity recommendations | |
| Outcomes | DEVICES (waist) Dynaport MoveMonitor: time walking, walking intensity Actigraph GT3x (software version not reported): step count, MVPA time
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "Swisscom AG who provided 30 sim cards and data usage of up to 1 GB per month" CONFLICT OF INTEREST statement provided CONTACT Heleen Demeyer [email protected] KU Leuven (Belgium) Additional information provided: physical activity, HRQOL, exercise capacity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence was generated with varying block sizes of 4, 6 or 8 and stratified by centre using a statistical software (STATA V.12.0, StataCorp, College Station, Texas, USA)" |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators obtained group allocation using an online system that ensured concealment of random allocation" |
| Blinding of participants (performance bias) | High risk | Quote: "Neither patients nor investigators were blinded to treatment allocation" |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "Neither patients nor investigators were blinded to treatment allocation" Comment: Not clear if this also refers to personnel responsible for implementation |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL and exercise capacity: Quote: "neither patients nor investigators were blinded to treatment allocation... nevertheless, we do acknowledge the lack of blinding could have minimally influenced the 6MWD results" Comment: infers 'investigators' refers to assessors |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | High risk | SECONDARY OUTCOMES Registry: Proportion of patients showing an increase in physical activity > 20%; Paper: "for responder analysis a clinically significant increase in step count was defined as ≥ 1000 steps" Registry: anxiety and depression, PROactive instrument, satisfaction, compliance (not reported) Paper: time walking, walking intensity, sedentarism (defined as step count < 5000), AECOPD in the last 12 months (additional outcomes reported) |
| Other bias | Unclear risk | Sample size calculation
|
| Methods | DESIGN 2 groups DATES November 2004 SETTING 9 rehabilitation centres (The Netherlands) SAMPLE SIZE calculation based on CRQ | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 63 (SD 10) years; SEX 18 (58%) male; FEV1 not reported
AGE mean 61 (SD 7) years; SEX 17 (49%) male; FEV1 not reported | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION yes COMMON INTERVENTION PR SETTING in‐hospital or outpatient (depending on travel distance to centre) CONTACT 3 sessions a week, 1 hour AEROBIC TRAINING Weeks 3 to 12:
STRENGTH TRAINING upper and lower limbs OTHER COMPONENTS
EDUCATION group sessions: disease, treatment strategies, medication, coping, role of rehabilitation, how to recognise an exacerbation, breathing exercises INTERVENTION NIPPV (nocturnal, spontaneous/timed mode, nasal or full face mask) In the hospital within a week after the baseline measurements (before starting PR) Maximal tolerated inspiratory airway pressure, titrated for optimal correction of nocturnal ABG In‐hospital practice period until ≥ 6 hours sleep | |
| Outcomes | DEVICE Digiwalker SW‐200 (pedometer)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOME
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT ML Duiverman [email protected] University Medical Center Groningen (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was computerised and performed by an independent statistician" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Quote: "Masking: None (Open Label)" |
| Blinding of personnel (performance bias) | High risk | Quote: "Masking: None (Open Label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity and exercise capacity (cycle ergometry) |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL and exercise capacity (6MWD, ESWT): Quote: "Masking: None (Open Label)" |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow diagram provided: differential attrition noted Quote: "In the NIPPV + PR group, seven patients did not complete the study. Five patients could not adapt to the NIPPV (16%), one patient withdrew because of rheumatic complaints and one patient died of progressive respiratory failure due to a COPD exacerbation after 69 days on NIPPV, despite initial blood gas improvements... In the PR group, three patients (9%) did not complete the study because of non‐compliance" |
| Selective reporting (reporting bias) | High risk | Registry: polysomnography, baseline dyspnoea index, exercise capacity, electromyography, respiratory muscle strength (not reported) Paper: additional outcomes, step count, HRQOL (Maugeri Respiratory Failure questionnaire, Severe Respiratory Insufficiency questionnaire), anxiety and depression, dyspnoea, arterial blood gases, treatment compliance (additional outcomes) |
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES November 2004 to July 2008 SETTING hospital outpatient pulmonary clinic (The Netherlands) SAMPLE SIZE calculation based on ISWD | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 63 (SD 8) years; SEX 42 (54%) male; FEV1 mean 50 (SD 14)% predicted
AGE mean 64 (SD 8) years; SEX 44 (58%) male; FEV1 mean 51 (SD 17)% predicted | |
| Interventions | DURATION OF INTERVENTION 11 months FOLLOW‐UP yes SUPERVISION yes COMMON INTERVENTION self‐management 4 sessions (2 hours) follow‐up phone calls allowed to attend regular, non‐COPEactive physiotherapy sessions if this was prescribed as part of regular care INTERVENTION exercise training (COPE‐active) Programme development: problematic activities incorporated (bicycling, walking, climbing stairs, and lifting weights) SETTING exercise training in private physiotherapy practices, small groups (2 to 3 participants) CONTACT
AEROBIC TRAINING bicycling, walking, climbing stairs
STRENGTH TRAINING upper and lower extremities
OTHER COMPONENTS
EDUCATION nil N.B. n = 25 (33%) received non‐standardised physiotherapy | |
| Outcomes | DEVICE Yamax Digi‐Walker SW200 (pedometer)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING not reported CONFLICT OF INTEREST statement provided CONTACT Tanja Effing [email protected] University of South Australia, Adelaide (Australia) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised into two study groups, using a minimisation programme" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention. |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOMES Registry: duration and severity of exacerbations measured by daily diaries (not reported) SECONDARY OUTCOMES Registry: Social Support List, self‐efficacy list, EQ5D (not reported) |
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES June 2007 to July 2010 SETTING PR (UK) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS not reported
| |
| Interventions | DURATION OF INTERVENTION 7 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION PR SETTING centre‐based outpatient group CONTACT 2 sessions a week, 1 hour exercise, 1 hour education AEROBIC TRAINING exercise bike
STRENGTH TRAINING light dumbbells and body resistance
OTHER COMPONENTS
EDUCATION
| |
| Outcomes | DEVICE SenseWear Pro (software version 6.0) (right triceps)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING not reported CONFLICT OF INTEREST statement provided CONTACT Richard Costello [email protected] Beaumont Hospital, Dublin (Ireland) Additional information provided: confirmed methodology as per 2012 publication, results as in abstract (no group comparison in paper) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective single‐blinded randomised controlled study" (abstract) Quote: "random allocation to parallel assignment"(registry) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: Quote: "assessor blinded" |
| Incomplete outcome data (attrition bias) | Unclear risk | Total number randomised and group numbers provided; no details re attrition or exclusion |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (abstract only) |
| Other bias | Unclear risk | Only PR group data subsequently published |
| Methods | DESIGN 2 groups DATES July 2010 to October 2014 SETTING university‐based outpatient clinic (Brazil) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA (REGISTRY)
INCLUSION CRITERIA (PAPER)
EXCLUSION CRITERIA (REGISTRY)
EXCLUSION CRITERIA (PAPER)
BASELINE CHARACTERISTICS
AGE mean 69 (SD 9) years; SEX 14 (41%) male; FEV1 mean 48 (SD 17)% predicted
AGE mean 68 (SD 8) years; SEX 9 (25%) male; FEV1 mean 46 (SD 14)% predicted | |
| Interventions | DURATION OF INTERVENTION AND FOLLOW‐UP 6 months FOLLOW‐UP no SUPERVISION yes INTERVENTION water‐based exercise training SETTING outpatient group; heated pool (33 oC), 1 metre depth; up to 4 participants CONTACT 60 sessions, 1 hour
AEROBIC TRAINING high‐intensity, individualised training load, revised weekly
STRENGTH TRAINING (3 sets, 8 repetitions)
OTHER COMPONENTS nil EDUCATION 8 group sessions held every 2 weeks, 15 minutes: disease features and treatment, such as physical training, energy conservation techniques, symptoms, nutrition INTERVENTION land‐based exercise training SETTING centre‐based outpatient group CONTACT 60 sessions, 1 hour
AEROBIC TRAINING high‐intensity, individualised training load
STRENGTH TRAINING upper and lower limbs
OTHER COMPONENTS nil EDUCATION 8 group sessions held every 2 weeks, 15 minutes: disease features and treatment, such as physical training, energy conservation techniques, symptoms, nutrition | |
| Outcomes | DEVICE Power Walker‐PW610: monitor set individually taking into consideration body weight and step length (measured in a 10‐metre walk at usual speed)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Fabio Pitta [email protected] Universidade Estadual de Londrina, Parana (Brazil) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed in two stages: generation of numbers (table of random numbers)" |
| Allocation concealment (selection bias) | Low risk | Quote: "blind allocation (opaque and sealed envelopes)" |
| Blinding of participants (performance bias) | High risk | Quote: "Due to the characteristics of the intervention, it was not possible to blind participants and therapists who applied the training" |
| Blinding of personnel (performance bias) | High risk | Quote: "Due to the characteristics of the intervention, it was not possible to blind participants and therapists who applied the training" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "outcome assessors were not informed about the allocation of patients in the respective groups" |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | Registry (Time Frame: 4 years) SECONDARY OUTCOMES Registry: static balance, timed up and go test (not reported) Paper: spirometry, peripheral and respiratory muscle strength, anthropometry, dyspnoea, anxiety and depression (additional outcomes) |
| Other bias | Unclear risk | Registry
|
| Methods | DESIGN 2 groups DATES screened for inclusion July to December 2016 SETTING Rehabilitation Center, Walenstadtberg (Switzerland) SAMPLE SIZE "aim to... estimate the required sample size for a RCT" | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 7) years; SEX 5 (63%) male; FEV1 not reported
AGE mean 63 (SD 9) years; SEX 5 (63%) male; FEV1 not reported | |
| Interventions | DURATION OF INTERVENTION 3 weeks FOLLOW‐UP 3 months SUPERVISION yes COMMON INTERVENTION exercise training DURATION 3 weeks SETTING inpatient (commence Day 3 of admission), supervised CONTACT 6 sessions a week, 30 minutes AEROBIC TRAINING voluntary exhaustion, 8 to 12 minutes
INTERVENTION outdoor walking
INTERVENTION cycle ergometer
| |
| Outcomes | DEVICE Fitbit One (Fitbit Inc., San Francisco, CA, USA)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study was financially supported by Valens Clinics". CONFLICT OF INTEREST "The authors state that they have no financial, consulting or personal relationships to people or organizations that could influence the authors’ work. There are no conflicts of interests". CONTACT Esther Gamper [email protected] Zurich University of Applied Science, Winterthur (Switzerland) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "concealed block randomization procedure" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "concealed block randomization procedure" Comment: insufficient information |
| Blinding of participants (performance bias) | High risk | REGISTRY Blinding: Open (masking not used) |
| Blinding of personnel (performance bias) | High risk | REGISTRY Blinding: Open (masking not used) |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Insufficient information (?participant diary to self‐report) |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | Blinded assessor for 6MWT, otherwise assessor unblinded |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Low risk | Registry and paper in agreement |
| Other bias | Unclear risk | Retrospective registration |
| Methods | DESIGN 2 groups DATES not reported SETTING hospital and pulmonary rehabilitation centre (The Netherlands) SAMPLE SIZE calculation based on BMI | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
"None of the patients participated in an outpatient or home‐based PR programme" BASELINE CHARACTERISTICS
AGE mean 61 (SD 12) years; SEX 6 (55%) male; FEV1 mean 40 (SD 13)% predicted
AGE mean 62 (SD 10) years; SEX 6 (67%) male; FEV1 mean 40 (SD 16)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION nutritional supplement(energy dense) 3 125 ml Respifor (sip‐feed, Nutricia, The Netherlands) morning, afternoon and evening (daily) provided 2.38 MJ a day (20% protein, 20% fat, 60% carbohydrate) NO INTERVENTION nutritional advice from a dietitian on how to increase their energy intake | |
| Outcomes | DEVICE Tracmor (triaxial accelerometer) (belt at the back of the waist)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
| |
| Notes | FUNDING not reported CONFLICT OF INTEREST statement not provided CONTACT Marja Vermeeren [email protected] University Hospital Maastricht (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were then at random assigned (with a randomisation table) to the intervention group or to the control group" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | Unclear risk | Not specified |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity (energy expenditure) |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Unclear risk | No participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as per methods |
| Other bias | Unclear risk | Quote: "Depleted COPD patients (n=20) were studied 1 and 3 months after discharge from the university hospital… or from the pulmonary rehabilitation centre" Comment: participants post‐admission might respond differently to people who have just completed PR |
| Methods | DESIGN cross‐over trial (only pre‐cross‐over data used), 2 groups DATES May 2011 to May 2013 SETTING hospital (The Netherlands) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 59 (SD 10) years; SEX 6 (32%) male; FEV1 mean 32 (SD 8)% predicted
AGE mean 59 (SD 8) years; SEX 4 (17%) male; FEV1 mean 30 (SD 7)% predicted | |
| Interventions | DURATION OF INTERVENTION surgery FOLLOW‐UP 6 months SUPERVISION yes INTERVENTION endobronchial valve surgery | |
| Outcomes | DEVICE Dynaport MoveMonitor (around the waist at the lower back)
ASSESSMENT TIME POINTS
PRIMARY/SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST "NHT and JH have no conflicts of interest, KK received travel grants and a speakers fee from PulmonX, DJS is a physician advisor to PulmonX, received travel grants and lecture fees for educational and scientific meetings from PulmonX and participates in other clinical trials funded by PulmonX. All authors had complete access to the data, reviewed and approved the manuscript. PulmonX was not involved at any stage during the trial." CONTACT Jorine Hartman [email protected] University Medical Center Groningen (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomized controlled crossover trial" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "Masking/Blinding: none" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: Quote: "Masking/Blinding: none" |
| Incomplete outcome data (attrition bias) | High risk | Participant flow diagram provided Attrition in intervention group attributable to group allocation (8 participants had valves removed and not included at 6‐month assessment) |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOMES Manuscript: daily physical activity (not in registry) SECONDARY OUTCOMES Paper: SGRQ, dyspneoa (additional outcomes) |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES October 2011 to September 2014 SETTING 2 hospital‐based outpatient PR programmes (Australia) SAMPLE SIZE calculation based on CRQ and 6MWD | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 69 (SD 13) years; SEX 48 (60%) male; FEV1 mean 52 (SD 19)% predicted
AGE mean 69 (SD 10) years; SEX 51 (60%) male; FEV1 mean 49 (SD 19)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP 12 months SUPERVISION No INTERVENTION home‐based PR SETTING home CONTACT
AEROBIC TRAINING
STRENGTH TRAINING functional activities and equipment accessible in home, including sit‐to‐stand from a dining chair, step ups on an internal or external step, and water bottles for upper limb weights OTHER COMPONENTS
EDUCATION
INTERVENTION centre‐based PR SETTING outpatient group CONTACT 2 sessions a week AEROBIC TRAINING ≥ 30 minutes
STRENGTH TRAINING functional activities such as stair climbing and sit‐to‐stand practice, as well as free weights for the upper limbs OTHER COMPONENTS Unsupervised home exercise programme 3 sessions a week EDUCATION Self‐management training: structured (lecture‐based) and unstructured disease management education and goal setting (management of exacerbations, understanding medications, ongoing participation in exercise) | |
| Outcomes | DEVICE SenseWear Armband (software version 7.0) (left upper arm)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Anne Holland [email protected] Alfred Health, Melbourne (Australia) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomised, controlled equivalence trial… using a computer generated sequence… generated by an individual unrelated to the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised to treatment groups… using a computer generated sequence that was concealed using opaque envelopes" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention. |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | SECONDARY OUTCOMES Protocol: Spirometry will be repeated at 12 months (only baseline reported) |
| Other bias | Low risk | Quote: "We made every attempt to assess all participants at all time points, even if they were unwell or had not completed the programme. This may have affected the overall magnitude of benefit, but is reflective of the real world challenges of pulmonary rehabilitation" "Importantly, the programme completion rate in the centre‐based group was low, which may not reflect completion rates in all real world centre‐based services and may limit the generalisability of results" |
| Methods | DESIGN 2 groups DATES March 2013 to April 2014 SETTING hospital (Belgium) SAMPLE SIZE calculation based on walking time | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 66 (SD 7) years; SEX 8 (53%) male; FEV1 mean 38 (17%)% predicted
AGE mean 68 (SD 6) years; SEX 9 (60%) male; FEV1 mean 48 (18%)% predicted | |
| Interventions | DURATION OF INTERVENTION 4 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION PAC INTERFACE 3 phone calls a week ACTIVITY not specified STEP‐TRACKING pedometer (direct feedback) GOALS progress report with tips to increase physical activity posted Week 2 and Week 4 RECORD, EDUCATION/RESOURCES nil NO INTERVENTION provided with advice about increasing physical activity during the hospital stay from a physiotherapist | |
| Outcomes | DEVICE Dynaport MoveMonitor (lower back at the height of the second lumbar vertebra)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Thierry Troosters [email protected] KU Leuven (Belgium) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomized" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOMES Registry: step count Paper: step count, time walking (used for sample size calculation), walking intensity SECONDARY OUTCOMES Paper: spirometry, peripheral muscle strength, dyspnoea, CAT (additional outcomes reported) Paper: hospital readmission, medications, PR enrolment (results presented, not in methods) |
| Other bias | Unclear risk | Quote: "By executing this study, we experienced that including patients after an exacerbation of COPD is very difficult. After 1 year, only 30 patients agreed to participate in the study. Intermediate analyses did not reveal better improvements in physical activity in the group that received maximal counselling and real‐time feedback compared to usual care. Based on these conclusions and the fact that the study was very time consuming for the researcher, we decided not to continue with the study" |
| Methods | DESIGN 2 groups DATES not reported SETTING hospital pulmonary outpatient clinic (The Netherlands) SAMPLE SIZE "a power analysis was calculated" (outcome not stated) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 63 (SD 9) years; SEX 10 (50%) male; FEV1 mean 68 (SD 18)% predicted
AGE mean 61 (SD 9) years; SEX 11 (58%) male; FEV1 mean 62 (SD 14)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION pac (in‐person, Altenburg 2015, De Blok 2006) INTERFACE 5 individual sessions ACTIVITY lifestyle physical activity (e.g. walking, cycling, stair‐climbing, gardening) STEP‐TRACKING pedometer (direct feedback) RECORD Diary: daily step count, other activities (e.g. cycle, swim) GOALS “maximal” step count goal, end intervention: set personal “physical activity norm” (between mean and maximal step count) goal EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE Digiwalker SW‐2000 (pedometer)
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING "This study was supported by a research grant from Boehringer Ingelheim B.V. The funding source(s) had no involvement in the study." CONFLICT OF INTEREST "Herewith we state that none of the authors had any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within 3 years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work." CONTACT Linda Bossenbroek [email protected] University Medical Center Groningen (The Netherlands) Additional information requested. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned…" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity: unclear if step count was reported by participants |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as in Methods apart from subgroups for step count in Results (justification presented in Discussion) |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES March 2014 to December 2016 SETTING 71 general practices (UK) SAMPLE SIZE calculation based on SGRQ | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
N.B. use of population descriptor as 'mild' refers to mMRC grade FEV1 ≥ 80% predicted: 89 of 289 (31%) participants in intervention group and 104 of 288 (36%) participants in no‐intervention group so does not meet review definition for subgroup analyses BASELINE CHARACTERISTICS
AGE mean 71 (SD 9) years, SEX 183 (63%) male; FEV1 mean 71 (SD 19)% predicted
AGE mean 70 (SD 8) years; SEX 183 (64%) male; FEV1 mean 72 (SD 19)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 months FOLLOW‐UP no SUPERVISION no INTERVENTION self‐management (telephone health coaching) INTERFACE telephone by a nurse with
STEP‐TRACKING pedometer RECORD self‐monitoring diary EDUCATION/RESOURCES
| |
| Outcomes | DEVICE GENEActiv [non‐dominant wrist]
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Kate Jolly [email protected] University of Birmingham, Birmingham (UK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "individually randomised in a 1:1 ratio to the telephone health coaching or usual care group stratified by centre. The allocation was made using a web‐based programme hosted by the Primary Care Clinical Research and Trials Unit, University of Birmingham. Centre specific randomisation lists were produced by a statistician at the trials unit" |
| Allocation concealment (selection bias) | Low risk | Quote: "Only the Primary Care Clinical Research and Trials Unit had access to the allocation sequence. Patients were informed of their allocation at the end of the recruitment appointment" |
| Blinding of participants (performance bias) | High risk | Quote: "Patients… were not masked to treatment allocation" |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL: |
| Incomplete outcome data (attrition bias) | Unclear risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | PRIMARY OUTCOME (SGRQ) Registry: 6 and 12 months; Paper: 12 months only SECONDARY OUTCOMES Registry: none specified |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES not reported (abstract only) SETTING "following discharge from a respiratory‐related admission" (UK) SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION/EXCLUSION CRITERIA not reported BASELINE CHARACTERISTICS
AGE mean 68 (SD 9) years; SEX 7 (47%) male; FEV1 mean 36 (SD 12)% predicted
AGE mean 67 (SD 4) years; SEX 4 (40%) male; FEV1 mean 48 (SD 13)% predicted | |
| Interventions | DURATION OF INTERVENTION 7 days FOLLOW‐UP no SUPERVISION no INTERVENTION self‐management (SPACE) (as in Mitchell 2013) brief physical activity advice in the form of a self‐management manual | |
| Outcomes | DEVICE SenseWear armband Pro3 (software version not reported)
ASSESSMENT TIME POINTS daily for 7 days OUTCOMES
| |
| Notes | FUNDING, CONFLICT OF INTEREST not reported (abstract only) CONTACT Sally Singh sally.singh@uhl‐tr.nhs.uk University Hospitals Of Leicester NHS Trust, Leicester (UK) Additional information provided: group demographics, physical activity and exercise outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomised controlled trial" Comment: Insufficient information (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Not specified (abstract only) |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified (abstract only) |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | Exercise capacity: not specified (abstract only) |
| Incomplete outcome data (attrition bias) | Unclear risk | Total number randomised and group numbers provided; no details re attrition or exclusion (abstract only) |
| Selective reporting (reporting bias) | High risk | Paper:
|
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES not reported SETTING (Japan) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 64 (SD 8) years; SEX 16 (83%) male; FEV1 mean 58 (SD 23)% predicted
AGE mean 75 (SD 9) years; SEX 19 (93%) male; FEV1 mean 61 (SD 21)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 months FOLLOW‐UP no SUPERVISION no COMMON INTERVENTION PR SETTING home CONTACT
AEROBIC TRAINING daily, ≥ 15 minutes, level walking
STRENGTH TRAINING upper and lower limbs, daily OTHER COMPONENTS
EDUCATION monthly, 45 minutes: equipment use, nutrition, stress management, relaxation techniques, home exercises, the benefits of pulmonary rehabilitation INTERVENTION PAC INTERFACE monthly (or fortnightly) individual session ACTIVITY not specified STEP‐TRACKING Pedometer Kens Lifecorder EX (no direct feedback) GOALS 8000 steps a day
RECORD, EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE Activity Monitoring and Evaluation System (A‐MES tri‐axial accelerometer) (attached on thigh and chest in clothing pockets)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING not reported CONFLICT OF INTEREST statement provided CONTACT Atsuyoshi Kawagoshi [email protected] Akita City Hospital, Akita (Japan) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to one of two groups" Comment: Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Quote: "Patients were not blinded to the randomization" |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as in Methods |
| Other bias | Low risk | N/A |
| Methods | DESIGN 3 groups (2 comparisons)
DATES not reported SETTING (USA) SAMPLE SIZE calculation based on LIPA time | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 71 (SD 8) years; *SEX not reported; FEV1 mean 61 (SD 20)% predicted
AGE mean 72 (SD 9) years; *SEX not reported; FEV1 mean 54 (SD 17)% predicted
AGE mean 71 (SD 8) years; *SEX not reported; FEV1 mean 56 (SD 17)% predicted *"small proportion of women" | |
| Interventions | DURATION OF INTERVENTION 4 months FOLLOW‐UP 12 months SUPERVISION yes INTERVENTION upper‐body resistance training SETTING outpatient group CONTACT 2 sessions a week, 90 minutes supervised upper‐limb training
AEROBIC TRAINING nil STRENGTH TRAINING cable cross‐over system: shoulder shrug, modified latissimus dorsi pulldown, overhead pulldown, front pulldown, front raise, upright row, biceps curl, triceps extension
INTERVENTION exercise‐specific self‐efficacy intervention general principles of exercise, coping with respiratory infections by modifying PA, overcoming barriers to exercise, establishing an active lifestyle INTERVENTION health education basic lung physiology, pathophysiology of COPD, commonly used medications, breathing techniques, healthy eating, relaxation, travel considerations, energy conservation, osteoarthritis, cardiovascular risk factors, cholesterol INTERVENTION gentle chair exercises stretching and toning exercises that were not aerobic | |
| Outcomes | DEVICE Actigraph (model 7164) (software version not reported) (waist)
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Janet Larson [email protected] University of Michigan (USA) Additional information requested. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was stratified by sex and disease severity (GOLD stages) using a customized computer program that blinded investigators to group assignment [software program (biased coin algorithm to ensure equivalent groups)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "concealed allocation process" Comment: Not specified |
| Blinding of participants (performance bias) | Low risk | "All subjects were assigned to an active intervention without being told which intervention was the experimental intervention of interest. We explained that all three interventions were potentially beneficial and that we were comparing the effects of each" |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | PRIMARY OUTCOMES Trial registry: not reported
Paper: physical activity (additional outcome) |
| Other bias | Unclear risk | Retrospectively registered in January 2010; study started September 2003, primary completion January 2009 |
| Methods | DESIGN 2 groups DATES, SETTING, SAMPLE SIZE not provided (abstract only) | |
| Participants | INCLUSION CRITERIA "Patients with COPD enrolled in a conventional 6 month OP PRP were screened for eligibility. After 3 months of PR and one week of PA measurements" EXCLUSION CRITERIA not provided (abstract only)
AGE not reported; SEX 25 (50%) male; FEV1 mean 49 (SD 20)% predicted
| |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP 6 months SUPERVISION yes INTERVENTION PAC "Coaching through a semi‐automated telecoaching program consisting of a step counter and a project‐tailored smartphone application" (Demeyer 2017) INTERVENTION PR | |
| Outcomes | DEVICE not reported (abstract only) ASSESSMENT TIME POINTS
PRIMARY/SECONDARY OUTCOMES
| |
| Notes | FUNDING, CONFLICT OF INTEREST not provided (abstract only) CONTACT Matthias Loeckx [email protected] KU Leuven (Belgium) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information (abstract only) |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information (abstract only) |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information (abstract only) |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Insufficient information (abstract only) |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | Insufficient information (abstract only) |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information (abstract only) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (abstract only) |
| Other bias | Unclear risk | Insufficient information (abstract only) |
| Methods | DESIGN 2 groups DATES January 2009 to August 2009 SETTING respiratory clinics (UK) SAMPLE SIZE calculation based on SF36 | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA not reported BASELINE CHARACTERISTICS
AGE mean 69 (SD 11) years; SEX not reported; FEV1 mean 44 (SD 14)% predicted
AGE mean 68 (SD 9) years; SEX not reported; FEV1 mean 64 (SD 26)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION singing
SHAM film workshops
| |
| Outcomes | DEVICE SenseWear Pro (software version not reported)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Nicholas Hopkinson [email protected] Imperial College, London (UK) Additional information requested | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the end of the baseline visit, patients were randomized to either the singing classes or the film workshops, using randomization in blocks of 4…The sequence was developed by NSH who was not involved with the day to day conduct of the trial" |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutive sequentially numbered sealed envelopes" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention. |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "The co‐ordinators of each session were unaware of the tests measured at baseline" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: Quote: "Following attendance to either group for eight weeks, baseline measurements were again assessed by the same respiratory physiotherapists, who were blinded to treatment allocation" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOME Registry: daily physical activity Paper: SF36 (not in registry) SECONDARY OUTCOMES Paper: control of breathing, qualitative assessments (additional outcomes reported) |
| Other bias | Unclear risk | Retrospectively registered |
| Methods | DESIGN 2 groups DATES October 2013 to July 2016 SETTING outpatient PR programme (Greece) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA (as in Nasis 2015)
EXCLUSION CRITERIA (as per Nasis 2015)
BASELINE CHARACTERISTICS
AGE mean 65 (SD 8) years; SEX 68 (80%) male; FEV1 mean 49 (SD 19)% predicted
AGE mean 67 (SD 8) years; SEX 36 (84%) male; FEV1 mean 45 (SD 19)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION exercise training (HIIT) SETTING centre‐based outpatient group CONTACT 3 sessions a week AEROBIC TRAINING cycling 45 minutes, alternating 30‐second exercise intervals with 30‐second rest periods
STRENGTH TRAINING upper and lower limbs
OTHER COMPONENTS nil EDUCATION education programme: breathing retraining, dietary advice | |
| Outcomes | DEVICE Actigraph GT3X (ActiLife software (version 5.10.0)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Ioannis Vogiatzis [email protected] Zafeiris Louvaris [email protected] National and Kapodistrian University of Athens (Greece) Additional information provided:
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised…in random block sizes of three and six." |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention. |
| Blinding of personnel (performance bias) | Unclear risk | Not specified (research letter only) |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity and exercise capacity (cycle ergometry) |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity (6MWD): Quote: "experienced, health professionals who were blinded to group allocation performed all categories of assessment" |
| Incomplete outcome data (attrition bias) | Unclear risk | No participant flow diagram (research letter only) Quote: "150 consecutive COPD patients were screened… Patients were randomised in a 2:1 ratio into the interval training group (n=85) or the usual care group (n=43)" Attrition and exclusions not reported. |
| Selective reporting (reporting bias) | High risk | Referral to trial registry is not study‐specific.
PRIMARY OUTCOMES Registry: PROactive tool (not reported) Paper: physical activity variables (daily activity levels amount and intensity, sedentarism, wear time, step count, VMU, time in sedentary, light, lifestyle and moderate activities) SECONDARY OUTCOME Registry: daily physical activity (number of steps, VMU per activity minute, walking intensity) Paper: spirometry, anthropometry, exercise capacity, muscle strength, dyspnoea, HRQOL (CAT, CCQ, CRQ), anxiety and depression (additional outomces reported) |
| Other bias | Unclear risk | Registry: no reference to HIIT protocol |
| Methods | DESIGN 2 groups DATES Screened between June 2012 and July 2014 SETTING multidisciplinary respiratory and palliative care meetings and across PR services, 3 National Health Service trusts, London, UK SAMPLE SIZE calculation based on 6MWD | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 70 (SD 11) years; SEX 11 (44%) male; FEV1 mean 31 (SD 11)% predicted
AGE mean 69 (SD 9) years; SEX 10 (37% male); FEV1 mean 31 (SD 13)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 weeks FOLLOW‐UP 6 weeks SUPERVISION no COMMMON INTERVENTION neuromuscular electrical stimulation
ADMINISTRATION self‐administered, 30 minutes daily frequency 50Hz, pulse width 350µs, duty cycle 11% to 50% (equivalent to 15% to 25% of maximum voluntary contraction) INSTRUCTION standardised 30 minutes face‐to‐face training, supervise first set‐up in hospital or home (participant preference)
INTERVENTION amplitude 0 to 120mA over 1000O proportion of active treatment duration: increase weekly from 11% to 25% to 50%, then constant PLACEBO amplitude 0 to 20mA "levels of stimulation detectable by the patient but not able to elicit a tetanic muscle contraction" | |
| Outcomes | DEVICE activPAL (PAL Technologies,Glasgow, UK)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. This study was funded by National Institute for Health Research (NIHR; PDF‐2011‐04‐048). This study represents independent research supported by the UK Clinical Research Collaboration‐registered King’s Clinical Trials Unit at King’s Health Partners, and the NIHR/Wellcome Trust King’s Clinical Research Facility, which are part funded by the NIHR Biomedical Research Centre for Mental Health and the Dementia Unit at South London and Maudsley NHS Foundation Trust, King’s College London and the NIHR Evaluation, Trials and Studies Coordinating Centre. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health … MM is supported by an NIHR Post‐Doctoral Fellowship (PDF‐2011‐04‐048) and Clinical Trials Fellowship (CTF‐2013‐02‐009). CMN is supported by an NIHR Doctoral Fellowship (DRF‐2014‐07‐089). WD‐CM is supported by an NIHR Clinician Scientist Award, a Medical Research Council (UK) New Investigator Research Grant, an NIHR Clinical Trials Fellowship and by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for Northwest London. MIP is supported by the NIHR Respiratory Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London. IJH is an NIHR Senior Investigator and is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for South London and Cicely Saunders International." CONFLICT OF INTEREST "MIP reports receiving personal or institutional payment for consultancy or for research from GlaxoSmithKline, Novartis, Lilly, Pfizer, AstraZeneca, Regeneron, and Biomarin. MM, CMN, WD‐CM, NH, and IJH report holding grants from the National Institute for Health Research during the conduct of the trial. All other authors declare no competing interests." CONTACT Matthew Maddocks [email protected] Kings College, London (UK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned (1:1) at the individual level, using an independent web‐based randomisation system within the independent UK Clinical Research Collaboration‐registered King’s Clinical Trials Unit (London, UK). Using a hybrid minimisation method, 20% of participants were entered using simple randomisation and 80% entered using computer‐generated probabilistic minimisation to balance three potential confounders" |
| Allocation concealment (selection bias) | Low risk | Quote: "Following randomisation to active or placebo NMES, the Clinical Trials Unit informed trial staff via secure email. The trial coordinator, who arranged subsequent masked assessment visits, was informed of trial entry but not group allocation" |
| Blinding of participants (performance bias) | Low risk | Quote: "Participants were not informed of group allocation" |
| Blinding of personnel (performance bias) | High risk | Quote: "Trial physiotherapists and nurses were informed of group allocation and selected an active or placebo NMES device accordingly" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Quote: "Two linked Good Clinical Practice compliant online data entry systems (InferMed, London, UK; MACRO version 4) were created to maintain blinding; the first was used by physiotherapists and nurses for data regarding compliance and safety, and the second was used by trial assessors for outcome data" "trial coordinator (masked to group allocation) undertook physical assessments; questionnaires were self‐completed independently by participants" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Low risk | Paper additionally reports 4‐metre gait speed and adverse events |
| Other bias | Unclear risk | Retrospectively registered Quote: "One participant from each group commenced pulmonary rehabilitation classes during the follow‐up period, with three cumulative attendances between the 6‐week and 12‐week assessments in the active group and two in the placebo group." |
| Methods | DESIGN cross‐over trial (only pre‐cross‐over data used), 2 groups DATES April 2014 to June 2015 SETTING 2 centres (Germany) SAMPLE SIZE "As this was a pilot study, no formal sample size calculation was performed" | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
·MEDICATIONS
BASELINE CHARACTERISTICS
AGE mean 64 (SD 7) years; SEX 8 (53%) male; FEV1 median 1.4 (IQR 1.3 to 2.0) litres
AGE mean 65 (SD 8) years; SEX 7 (47%) male; FEV1 median 1.4 (IQR 0.9 to 1.8) litres | |
| Interventions | DURATION OF INTERVENTION 3 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION LAMA (aclidinium bromide, 400 μg) twice daily (09:00, 21:00 ± 1 hour) with a dry‐powder inhaler (Genuair®/Pressair®) PLACEBO twice daily (09:00, 21:00 ± 1 hour) via a dry‐powder inhaler (Genuair®/Pressair®) | |
| Outcomes | DEVICE SenseWear Pro3 (software version not reported)
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING "Support statement: This study was sponsored by Almirall S.A., Barcelona, Spain. Almirall S.A. were involved in the study design, collection, analysis, interpretation of data and development of this manuscript. Almirall S.A. and AstraZeneca Plc were involved in the review of the manuscript; the decision to submit the manuscript for publication was made jointly by the funders and authors. Funding information for this article has been deposited with the Crossref Funder Registry." CONFLICT OF INTEREST "Principal Investigators are NOT employed by the organization sponsoring the study. There IS an agreement between the Principal Investigator and the Sponsor (or its agents) that restricts the PI's rights to discuss or publish trial results after the trial is completed. Publication of the results by the Investigator will be subject to mutual agreement between the investigator and sponsor." (registry) CONTACT Henrik Watz [email protected] German Center for Lung Research, Grosshansdorf (Germany) Additional data provided: as in Almirall | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized" Comment: insufficient information (research letter only) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | Low risk | Quote: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of personnel (performance bias) | Low risk | Quote: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL: Quote: "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" |
| Incomplete outcome data (attrition bias) | Low risk | No participant flow diagram (research letter only) |
| Selective reporting (reporting bias) | Low risk | Results presented as in registry |
| Other bias | Low risk | Quote: "per‐protocol population (all patients in the safety population who had no major protocol deviations)" |
| Methods | DESIGN 2 groups DATES February 2015 to March 2017 SETTING Community Treatment Centre and hospitals (UK) SAMPLE SIZE calculation based on "physical activity levels" | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS (total n = 44) AGE mean 69 (SD 11) years; SEX 22 (50%) male; FEV1 mean 54 (SD 19)% predicted
| |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION no COMMON INTERVENTION PR DURATION 6 to 10 weeks SETTING outpatient group CONTACT 1 to 2 sessions a week, one hour AEROBIC TRAINING treadmill, cycle ergometer
STRENGTH TRAINING upper and lower limbs OTHER COMPONENTS, EDUCATION nil INTERVENTION PAC (web‐based human coaching) DURATION 12 weeks INTERFACE website ACTIVITY not specified STEP‐TRACKING Activity monitor (Tracmor D): (direct feedback, daily target) RECORD nil GOALS
EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE
ASSESSMENT TIME POINTS
PRIMARY
SECONDARY
| |
| Notes | FUNDING "Sponsor: University of Edinburgh. Collaborator: Philips Healthcare" CONFLICT OF INTEREST not reported (abstract only) CONTACT not reported (abstract only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized controlled trial" Comment: Insufficient information (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Not specified (abstract only) |
| Blinding of participants (performance bias) | High risk | Quote: "None (Open Label)" |
| Blinding of personnel (performance bias) | High risk | Quote: "None (Open Label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL and exercise capacity: Quote: "None (Open Label)" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not specified (abstract only) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (abstract only) |
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES January 2011 to April 2013 SETTING outpatient clinics at private and public hospitals, private clinics, public primary health centres (Chile) SAMPLE SIZE calculation based on step count | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 69 (SD 10) years; SEX 29 (56%) male; FEV1 mean 66 (SD 18)% predicted
AGE mean 68 (SD 8) years; SEX 33 (66%) male; FEV1 mean 66 (SD 21)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION no COMMON INTERVENTION PAC (in‐person) INTERFACE monthly individual session ACTIVITY walking RECORD diary: daily symptoms GOALS walk ≥ 30 minutes a day STEP‐TRACKING, EDUCATION/RESOURCES nil INTERVENTION pedometer INTERFACE monthly individual session ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD diary: daily step count, symptoms GOALS protocol‐driven (revised weekly)
EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE Tanita PD724 (pedometer)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Laura Mendoza [email protected] Hospital Clínico Universidad de Chile, Santiago (Chile) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study was a parallel group, assessor‐blind, randomised controlled trial… Consecutive patients who consented to participate were randomly assigned to one of two groups…by the investigators based on a random number sequence generated in Excel (Microsoft, Redmond, WA, USA) before enrolment commenced" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention. |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "assessor‐blind…Assessments were performed by investigators blinded to treatment allocation…All assessments were made by technicians of the pulmonary function laboratory at Hospital Clínico Universidad de Chile, who were blinded to the allocation of the patients" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | Paper: anthropometrics, spirometry, dyspnoea, using data from the participant diaries, the incidence of (AECOPD)…was determined (additional outcomes reported) |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES December 2009 to April 2012 SETTING 30 primary care COPD registers (UK) SAMPLE SIZE calculation based on CRQ dyspnoea domain | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 69 (SD 8) years; SEX 54 (61%) male; FEV1 mean 56 (SD 17)% predicted
AGE mean 69 (SD 10) years; SEX 47 (50%) male; FEV1 mean 60 (SD 17)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 weeks FOLLOW‐UP 6 months SUPERVISION no INTERVENTION self‐management (SPACE) (as in Kanabar 2015)
NO INTERVENTION Managed within primary care, no PR during the study period | |
| Outcomes | DEVICE SenseWear armband (software version not reported)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Sally Singh sally.singh@uhl‐tr.nhs.uk University Hospitals Of Leicester NHS Trust, Leicester (UK) Additional data provided: group numbers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were assigned to either usual care or SPACE FOR COPD via a web‐based, concealed allocation programme, using simple randomisation codes prepared by the trial statistician" |
| Allocation concealment (selection bias) | Low risk | Quote: "concealed allocation programme" |
| Blinding of participants (performance bias) | High risk | Quote: "Lack of participant blinding may have increased motivation when receiving the treatment and attempts to satisfy the researchers might have increased the observed treatment effect in the intervention arm. We cannot, therefore, rule out the possible impact of attention" |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "Randomisation was conducted by the trial investigator responsible for administering the intervention" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "assessments at 6 weeks and 6 months were conducted by a member of the research team who was blind to randomisation allocation" |
| Incomplete outcome data (attrition bias) | Unclear risk | CONSORT diagram provided for all study participants but subgroup with physical activity data unclear |
| Selective reporting (reporting bias) | Unclear risk | SECONDARY OUTCOMES Registry: task completion, adherence, exacerbation rates (not reported) |
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES December 2011 to January 2013 SETTING Veterans identified from a national database (USA, Puerto Rico) SAMPLE SIZE calculation based on SGRQ | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 67 (SD 9) years; SEX 146 (95%) male; FEV1 not assessed
AGE mean 66 (SD 9) years; SEX 77 (92%) male; FEV1 not assessed | |
| Interventions | DURATION OF INTERVENTION 12 months FOLLOW‐UP no SUPERVISION no COMMON INTERVENTION pedometer No instructions about exercise INTERFACE website ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD website: step count at least monthly GOALS, EDUCATION/RESOURCES nil INTERVENTION PAC (web‐based, as in Wan 2017) DURATION initial intensive 4‐month phase followed by an 8‐month maintenance phase INTERFACE website ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD website: step count at least weekly GOALS Website: display progress, protocol‐driven goal (revised weekly) EDUCATION/RESOURCES website: education and motivational content, community forum | |
| Outcomes | DEVICE Omron HJ‐720 ITC (pedometer)
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Caroline Richardson [email protected] University of Michigan (USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random sample of 28,957 veterans (one‐half urban, one‐half rural) were sent a recruitment letter… Group assignment was computer generated" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to intervention. |
| Blinding of personnel (performance bias) | High risk | Quote: "Masking: None (Open Label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL: Quote: "Masking: None (Open Label)" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | Paper: adherence, engagement, safety, dyspnoea (Methods: baseline, month 4, month 12; Results: baseline) (additional outcomes reported) |
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES not reported (abstract only) SETTING multicentre (Japan) SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA not reported BASELINE CHARACTERISTICS not reported
| |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION LAMA (aclidinium bromide, 400 μg) twice daily INTERVENTION LAMA (tiotropium, 18 μg) once daily | |
| Outcomes | DEVICE GT3X‐BT (software version not reported)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study was funded by Kyorin Pharmaceutical Co., Ltd. (UMIN000020020)" CONFLICT OF INTEREST not reported (abstract only) CONTACT not provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized" Comment: Insufficient information (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Not specified (abstract only) |
| Blinding of participants (performance bias) | High risk | Quote: "open‐label" |
| Blinding of personnel (performance bias) | High risk | Quote: "open‐label" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL: Quote: "open‐label" |
| Incomplete outcome data (attrition bias) | Low risk | Appears that all randomised participants completed the study |
| Selective reporting (reporting bias) | High risk | No registry available; abstract only No data presented that can be used for analysis; only between‐group P‐values provided |
| Other bias | Unclear risk | Abstract only |
| Methods | DESIGN 2 groups, phase 3 DATES September 2003 to October 2005 SETTING Canada, Germany, Spain SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS not reported
| |
| Interventions | DURATION OF INTERVENTION AND FOLLOW‐UP 12 weeks SUPERVISION no INTERVENTION LAMA (tiotropium bromide, 18 mcg) oral inhalation capsules, once daily in the morning with HandiHaler PLACEBO oral inhalation capsules, once daily in the morning with HandiHaler | |
| Outcomes | DEVICE Stayhealthy RT3 accelerometer
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING Boehringer Ingelheim CONFLICT OF INTEREST not stated CONTACT not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Quote: "Double blind" Comment: unclear to whom this refers |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "Double blind" Comment: unclear to whom this refers |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Objectively assessed physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | Quote: "Double blind" Comment: unclear to whom this refers |
| Incomplete outcome data (attrition bias) | Unclear risk | No participant flow chart |
| Selective reporting (reporting bias) | Unclear risk | No data presented suitable for meta‐analysis |
| Other bias | Unclear risk | No participant features reported Not published in peer‐reviewed journal |
| Methods | DESIGN 2 groups DATES September 2011 to November 2012 SETTING 7 centres (The Netherlands) SAMPLE SIZE not reported | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS not reported
| |
| Interventions | DURATION OF INTERVENTION 4‐week run‐in phase, 12 week intervention FOLLOW‐UP no SUPERVISION no COMMON RUN‐IN PHASE 4 weeks, Symbicort (200/6 µg/unit dose) 1 inhalation twice a day INTERVENTION Foster (beclomethasone dipropionate 100 µg plus formoterol 6 µg/unit dose), 2 inhalations twice a day | |
| Outcomes | DEVICE pedometer ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOME
| |
| Notes | FUNDING Chiesi Farmaceutici Parma (Italy) CONFLICT OF INTEREST CONTACT Chiesi Farmaceutici [email protected] Eddi Bindi [email protected] Gabriele Nicolini [email protected] Marteen van den Berge [email protected] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomised” Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | Low risk | Quadruple (participant, care provider, investigator, outcomes assessor) (NCT) |
| Blinding of personnel (performance bias) | Low risk | Quadruple (participant, care provider, investigator, outcomes assessor) (NCT) |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Quadruple (participant, care provider, investigator, outcomes assessor) (NCT) |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Quadruple (participant, care provider, investigator, outcomes assessor) (NCT) |
| Incomplete outcome data (attrition bias) | Unclear risk | EUCTR: target size n = 170 NCT: target size n = 130, actual recruitment n = 113 Study report: randomised Foster n = 31, symbicort n = 29 |
| Selective reporting (reporting bias) | High risk | Results not published in peer‐reviewed journal; only data available are from study report |
| Other bias | Unclear risk | Prospectively registered Quote: “prematurely ended; low number of evaluable patients (which was less than half of the planned number)” |
| Methods | DESIGN cross‐over trial (only pre‐cross‐over data used), 2 groups DATES not reported (abstract only) SETTING PR (country?) SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA not reported BASELINE CHARACTERISTICS (total n = 19) AGE mean 72 (SD 8) years; SEX 11 (58%) males; FEV1 mean 38 (SD 19)% predicted
| |
| Interventions | DURATION OF INTERVENTION AND FOLLOW‐UP 4 weeks (only pre‐cross‐over data) FOLLOW‐UP no SUPERVISION no INTERVENTION four‐wheeled walker | |
| Outcomes | DEVICE StepWatch
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Kylie Hill [email protected] Cindy Ng [email protected] Curtin University, Perth (Australia) Additional information provided: CRQ, physical activity pre‐cross‐over data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL: not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | Not specified
|
| Selective reporting (reporting bias) | Unclear risk | ActivPAL results provided in author correspondence but not presented otherwise |
| Other bias | Unclear risk | No baseline assessment |
| Methods | DESIGN 2 groups DATES October 2006 to April 2008 SETTING PR programmes SAMPLE SIZE feasibility study; no power calculation | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 72 (SD 9) years; SEX 3 (33%) male; FEV1 mean 47 (SD 19)% predicted
AGE mean 64 (SD 12) years; SEX 3 (38%) male; FEV1 mean 34 (SD 15)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 months FOLLOW‐UP no SUPERVISION no INTERVENTION PAC (coached, app) INTERFACE
ACTIVITY Individual home exercise programme (as in exercise test performance, dyspnoea, community‐based facilities, preferred mode) STEP‐TRACKING pedometer (direct feedback) RECORD daily symptoms and exercise log via phone, transmitted real‐time to central server GOALS
EDUCATION/RESOURCES
INTERVENTION PAC (self‐managed, app) INTERFACE
ACTIVITY individual home exercise programme (as in exercise test performance, dyspnoea, community‐based facilities, preferred mode) STEP‐TRACKING pedometer (direct feedback) RECORD daily symptoms and exercise log by phone GOALS
EDUCATION/RESOURCES
| |
| Outcomes | DEVICE Stepwatch 3 (right ankle)
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING reported "Omron Healthcare donated the pedometers" CONFLICT OF INTEREST statement provided CONTACT Huong Nguyen [email protected] University of Washington, Seattle (USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A biostatistician who was not involved in the day‐to‐day study operations generated the randomization sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "A biostatistician who was not involved in the day‐to‐day study operations…placed the randomization in separate sealed opaque envelopes" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention. |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "the interventionist was not blind to group assignment" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity and exercise capacity (cycle ergometry) |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity (6MWD): Quote: "the outcome assessments were performed by a research assistant who was blinded" |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOME Registry: exercise behaviour (not defined) Paper: autonomous self‐regulation for exercise (additional outcome) SECONDARY OUTCOMES Registry: AECOPD (not reported) Paper: technical issues, beliefs and attitudes towards exercise and self care, validation of self‐reported exercise, intervention time and costs (additional outcomes) |
| Other bias | High risk |
|
| Methods | DESIGN 2 groups DATES July 2012 to June 2014 SETTING hospital PR unit (UK) SAMPLE SIZE calculation based on time in moderate‐intensity physical activity | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 68 (SD 9) years; SEX 56 (74%) male; FEV1 mean 51 (SD 21)% predicted
AGE mean 68 (SD 8) years; SEX 54 (71%) male; FEV1 mean 51 (SD 22)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 months FOLLOW‐UP no SUPERVISION no INTERVENTION PR DURATION 8 weeks SETTING outpatient group CONTACT 2 sessions a week (15 minutes warm‐up, 60 minutes exercise, 45 minutes education) AEROBIC TRAINING walking, aim 15 minutes continuously, Borg scale dyspnoea (rating 3 to 4)
STRENGTH TRAINING upper and lower limbs (2 sets, 10 repetitions)
OTHER COMPONENTS at least once a week, home‐based exercise session EDUCATION
INTERVENTION PAC (in‐person) INTERFACE 8 individual sessions (weekly during PR) ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD diary: daily step count GOALS
EDUCATION/RESOURCES nil | |
| Outcomes | DEVICE SenseWear (Software version not reported) and Yamax DigiWalker CW700 (pedometer)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTERESTstatement provided CONTACT Claire Nolan [email protected] Imperial College, London (UK) Additional information provided: confirmed distribution of variables precluding inclusion in meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "parallel, two‐group, assessor‐blinded randomized controlled trial… following baseline assessment, participants were randomly allocated 1:1 to… the allocation sequence was computer generated and accessed by a researcher independent of (study processes)" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Quote: "It was not possible to conceal group allocation from participants" |
| Blinding of personnel (performance bias) | Low risk | Quote: "The clinical team delivering the PR program were blinded to the participants' group allocation" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "assessors blinded to group allocation" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | SECONDARY OUTCOMES Paper: accelerometer and pedometer step counts, adverse events, hospitalisations and deaths (additional outcomes) |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES February 2014 to January 2016 SETTING PR within 2 Health and Social Care Trusts (Northern Ireland) SAMPLE SIZE "this study will inform a future large scale RCT" | |
| Participants | INCLUSION CRITERIA (registry)
EXCLUSION CRITERIA (registry)
BASELINE CHARACTERISTICS
AGE mean 61 (SD 9); SEX 13 (57%) male; FEV1 mean 54 (SD 23)% predicted
AGE mean 67 (SD 8); SEX 11 (42%) male; FEV1 mean 57 (SD 24)% predicted | |
| Interventions | DURATION OF INTERVENTION PAC 12 weeks, PR 6 weeks FOLLOW‐UP no SUPERVISION yes COMMON INTERVENTION participants given the Living Well With COPD for PR booklet INTERVENTION physical activity intervention DURATION 12 weeks INTERFACE weekly contact with the physiotherapist or nurse
ACTIVITY walking STEP‐TRACKING unsealed Yamax Digiwalker CW700 pedometers each day for motivation and feedback RECORD manual with weekly step diary GOALS COM‐B model of behaviour change (capability, opportunity, motivation, behaviour)
EDUCATION/RESOURCES during consultations clinicians focused on helping participants to build self‐efficacy, encouraging social support, providing disease‐specific education INTERVENTION pulmonary rehabilitation DURATION 6 weeks SETTING hospital or health‐centre outpatient departments CONTACT 2 x week, also given a booklet with exercises and encouraged to perform these independently on a third occasion AEROBIC TRAINING exercise component usually lasted for 1 hour, generally consisted of cardiovascular exercises STRENGTH TRAINING lower‐ and upper‐body strengthening exercises OTHER COMPONENTS A diary was used to record the exercises undertaken and the level of breathlessness measured with the BORG scale EDUCATION centre‐based disease‐specific education, at which time participants could engage in discussion and ask questions at least once weekly (30 – 60 minutes) | |
| Outcomes | DEVICE ActigraphVR GT3X accelerometer, sealed Yamax Digiwalker CW700 pedometer worn around the waist for 7 days during all waking hours Only Actigraph data that contained a minimum of 5 days of 10 hours wear‐time were used for analysis Only sealed pedometer data that had a minimum of 5 days of 100 – 50,000 steps were used for analysis ASSESSMENT TIME POINTS 4 study visits for outcome assessment
PRIMARY OUTCOME / SECONDARY OUTCOMES
| |
| Notes | FUNDING "The study was funded by the Northern Ireland Chest Heart and Stroke (NICHS). PhD student (O’Shea O) was funded by the Department of Employment and Learning. The study was sponsored by the Ulster University and the Western and Belfast Health and Social Care Trusts. The study was supported by the Northern Ireland Clinical Research Network (NICRN) Respiratory Health interest group." CONFLICT OF INTEREST "The authors have no conflicts of interest to declare." CONTACT Brenda O'Neill [email protected] Ulster University, Newtownabbey (UK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned to two groups using computer‐generated block random numbers by a member of team not involved in any other aspect of the study in order to ensure allocation concealment" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was retained in sealed envelopes which were opened to reveal group allocation only after consent and after the completion of baseline assessment" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to intervention |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Quote: "All data was collected by a trained independent assessor not involved in the delivery of either intervention; a physiotherapist and/or a research assistant" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Participants’ flow through the study is shown in Figure 1. Quote: "The patients were assessed and randomised to the PAI (n = 24) or PR (n = 26). One participant who was randomised to the PAI made a mistake and attended PR. Therefore, n = 27 attended PR and n = 23 attended the PAI. A further n = 1 participant randomised to PR was excluded from the analysis as subsequent information about their diagnosis revealed that the person did not meet the GOLD criteria for COPD; therefore, n = 49 have been included in the analysis: n = 23 PAI; n = 26 PR." |
| Selective reporting (reporting bias) | Unclear risk | Results reported as in Methods, apart from time points for assessment where follow‐up data not presented |
| Other bias | Unclear risk | Retrospectively registered |
| Methods | DESIGN 2 groups DATES November 2014 to October 2017 SETTING single centre (Japan) SAMPLE SIZE calculation based on lean body mass index | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 77 (SD 10) years; SEX 21 (88%) male; FEV1 mean 64 (SD 25)% predicted
AGE mean 79 (7) years; SEX 20 (95%) male; FEV1 mean 68 (SD 35)% predicted | |
| Interventions | DURATION OF INTERVENTION hospital length of stay FOLLOW‐UP no SUPERVISION yes COMMON INTERVENTION
INTERVENTION nutritional supplement with eicosapentaenoic acid (ProSure) 1 pack or can a day INTERVENTION nutritional supplement without eicosapentaenoic acid (ENSURE®) 1 pack or can a day | |
| Outcomes | DEVICE pedometer (HJA‐401F, Omron) step length measured as 0.37 * height (cm) (input for device)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors" CONFLICT OF INTEREST "None of the authors declared a conflict of interest" CONTACT Takashi Ogasawara [email protected] Hamamatsu Medical Centre, Shizuoka (Japan) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computer‐generated randomization scheme with blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | High risk | Quote: "open‐label" |
| Blinding of personnel (performance bias) | High risk | Quote: "open‐label" |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Quote: "open‐label" |
| Blinding of outcome assessment [other] (detection bias) | High risk | Quote: "open‐label" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT figure provided Quote: "In total, 50 eligible patients were enrolled in the study and evaluated prospectively (Fig. 1). One patient in the EPA group was excluded, because his body weight at the time of admission was erroneously input to the BIA, resulting in the BMI and LBMI being characterized as overweight. Three patients in the control group were also excluded because they could not continue taking ONS orally due to dysphagia or intubation, and one patient withdrew informed consent" |
| Selective reporting (reporting bias) | Low risk | Registry and paper in agreement |
| Other bias | Unclear risk | Prospectively registered Unable to access supplementary data: Quote: "Data not available / The authors do not have permission to share data" |
| Methods | DESIGN 3 groups (3 comparisons)
DATES February to June 2016 SETTING hospital (UK) SAMPLE SIZE "The study will aim to recruit as many patients as are admitted to hospital for an acute COPD exacerbation within the operational period. One of the main objectives of this feasibility study is to provide data on eligibility and recruitment and to enable an accurate estimation of the required sample size for a future trial based on a realistic recruitment plan." | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS AGE mean 71 (SD 20) years; SEX 10 (30%) male; FEV1 not reported
| |
| Interventions | DURATION OF INTERVENTION 2 weeks following discharge from hospital FOLLOW‐UP no SUPERVISION no INTERVENTION feedback INTERFACE
ACTIVITY break up sedentary bouts STEP‐TRACKING inclinometer (direct haptic feedback: sedentary time > threshold) RECORD inclinometer sends data to app GOALS app: step count, sitting, standing, lying down, sit‐to‐stand transitions INTERVENTION education EDUCATION/RESOURCES verbal and written information: booklet Sit Less, Move More, Live Healthier adapted for COPD | |
| Outcomes | DEVICE ActiGraph wGT3X‐BT (version 6.13.2; firmware 1.6.1) (waistband on the right anterior axillary line)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Mark Orme mark.orme@uhl‐tr.nhs.uk University Hospitals Of Leicester NHS Trust, Leicester (UK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomization was conducted... by an individual independent of the research team" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "sequentially numbered sealed envelopes ... balanced combinations of group allocations within blocks will be conducted by a researcher at Loughborough University who is independent of the research team. This will ensure study team researchers will be blinded to group allocation prior to patients deciding whether to take part in the study. Owing to limited study team members and logistical barriers, study team researchers will be made aware of group allocation before consent. Patients will only be informed of their group allocation after providing informed consent" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL: Quote: "researchers will not be blinded to treatment allocation for qualitative interviews and study measurements" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | High risk | Registry: 20 metre gait analysis using foot‐worn inertial sensors (not reported) Registry and protocol: EQ5D, grip strength (not reported) Protocol:
Paper: PR attendance, information on the ownership and usage of computers and smartphones, index of multiple deprivation (additional outcomes reported) |
| Other bias | High risk | No physical activity data available per group Registry: changes made on 31 May 2016 (following completion of recruitment)
|
| Methods | DESIGN 2 groups DATES December 2015 to August 2016 SETTING hospital (China) SAMPLE SIZE "In the absence of pilot data, a formal power calculation was not possible" | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE, SEX not reported; FEV1 post‐bronchodilator mean 49 (SD 13)% predicted
AGE, SEX not reported; FEV1 post‐bronchodilator mean 47 (SD 15)% predicted | |
| Interventions | DURATION OF INTERVENTION 2 weeks indacaterol, 12 weeks indacaterol and intervention FOLLOW‐UP 12 weeks indacaterol SUPERVISION yes INTERVENTION Tai Chi DURATION 12 weeks SETTING "big exercise hall" CONTACT 5 sessions a week, 1 hour TRAINING Yang style
OTHER COMPONENTS LABA (indacaterol, 150 ug per day) EDUCATION "educational input" INTERVENTION PR DURATION 12 weeks SETTING supervised, outpatient, group CONTACT 3 sessions a week, 1 hour AEROBIC TRAINING 50%
STRENGTH TRAINING 50% shoulder abduction, bicep curls, sit to stand, lunge walks with dumbbells
OTHER COMPONENTS LABA (indacaterol, 150 ug per day) EDUCATION "educational sessions" | |
| Outcomes | DEVICE ActiGraph
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES (visits 2, 3, 6, 9)
| |
| Notes | FUNDING "The study was in part funded by Novartis China as an investigator‐initiated trial; the State Key Laboratory of Respiratory Disease (Guangzhou Medical University) was the sponsor. The study drug (indacaterol) was provided by Novartis China. Both the Key State Laboratory (Guangzhou) and the NIHR Respiratory Biomedical Unit provided in‐kind support, the latter through partial salary support of M. I. P. Space for the study was made available by the governors of Xing‐Ning People’s Hospital. Y.M. L. was also partially supported by the National Key Research and Development Program of China (Project No. 2016YFC1304200)." CONFLICT OF INTEREST "Financial/nonfinancial disclosures: None declared. Role of sponsors: Novartis China had no role in writing the manuscript or the decision to publish." CONTACT Yuan‐Ming Luo [email protected] State Key Laboratory of Respiratory Disease, Guangzhou (China) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation: Randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to exercise intervention |
| Blinding of personnel (performance bias) | High risk | Quote: "None (Open Label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL and exercise capacity: Quote: "None (Open Label)" |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOME Registry: only specified primary outcomes
Paper: SGRQ at Visits 2, 3, 6, 9 SECONDARY OUTCOMES not specified in registry Paper:
|
| Other bias | Unclear risk |
|
| Methods | DESIGN 2 groups DATES, SETTING, SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION/EXCLUSION CRITERIA not reported BASELINE CHARACTERISTICS (n = 21)
AGE mean 70 (SD 8) years; SEX not reported; FEV1 mean 44 (SD 14)% predicted
AGE mean 68 (SD 5) years; SEX not reported; FEV1 mean 43 (SD 12)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION PAC (automated coaching) INTERFACE automated coaching messages GOALS "weekly goals, daily feedback" ACTIVITY, STEP‐TRACKING, RECORD, EDUCATION/RESOURCES nil NO INTERVENTION"blinded to their physical activity" | |
| Outcomes | DEVICE DirectLife (activity monitor)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING, CONFLICT OF INTEREST not reported (abstract only) 7 co‐authors on this abstract are employed by Philips (as in Saini abstract) also no disclaimer re Philips (funded as in Saini abstract?) CONTACT Rita Priori [email protected] Phillips (The Netherlands) Additional information provided: confirmed this is a stand‐alone study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized" Comment: Insufficient information (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Not specified (abstract only) |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified (abstract only) |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity: unclear if participant reported |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "21 patients were recruited… Ten IG… and 8 CG…patients completed the study" Comment: Insufficient information (abstract only) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (abstract only) |
| Other bias | Unclear risk | Abstract only |
| Methods | DESIGN 2 groups DATES July 2006 to July 2009 SETTING University Hospital, Universidade Estadual de Londrina, Londrina, Parana (Brazil) SAMPLE SIZE post hoc power calculation… time spent in activities of intensity > 3 METs | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
MEDICATIONS "Pharmacologic treatment was not changed during the course of the study" BASELINE CHARACTERISTICS (n = 63)
AGE mean 65 (SD 10) years; SEX 11 (58%) male; FEV1 mean 39 (SD 14)% predicted
AGE mean 67 (SD 7) years; SEX 10 (50%) male; FEV1 mean 40 (SD 13)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION exercise training (calisthenics and breathing exercises) DURATION 12 weeks SETTING outpatient group CONTACT 3 sessions a week, 1 hour STRENGTH TRAINING
AEROBIC TRAINING, OTHER COMPONENTS, EDUCATION nil INTERVENTION exercise training (endurance and strength training) DURATION 12 weeks SETTING outpatient group CONTACT 3 sessions a week, 1 hour AEROBIC TRAINING
STRENGTH TRAINING quadriceps, biceps, triceps
OTHER COMPONENTS, EDUCATION nil | |
| Outcomes | DEVICE DynaPort Activity Monitor (waist): time walking, standing, sitting, and lying SenseWear (upper‐posterior region of the right arm): step count, MVPA time, energy expenditure
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Fabio Pitta [email protected] Universidade Estadual de Londrina, Parana (Brazil) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized trial" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | Unclear risk | Not specified |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity and exercise capacity (cycle ergometry) |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity (6MWD): not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | No participant flow diagram Group numbers prior to dropout not stated |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as in Methods (other than results for respiratory muscle force after intervention) |
| Other bias | Unclear risk | Post hoc power calculation identified in study limitations in Discussion |
| Methods | DESIGN 2 groups DATES January 2013 to January 2014, followed up until November 2014 SETTING Fitness Centre of the Exercise and Sport Science School of Verona University (Italy) SAMPLE SIZE calculation based on 6MWD | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA Unstable cardiac disease, pneumonia, pulmonary embolism, pulmonary vascular disease, respiratory infections, lung cancer, thoracic malignancy, and bone fractures BASELINE CHARACTERISTICS
AGE mean 66 (SD 5) years; SEX 100% male; FEV1 mean 72 (SD 19)% predicted
AGE mean 66 (SD 4) years; SEX 100% male; FEV1 mean 60 (SD 24)% predicted | |
| Interventions | DURATION OF INTERVENTION 28 weeks FOLLOW‐UP 14 weeks SUPERVISION no INTERVENTION exercise training with tapered supervision physical activity education programme approach, based on a periodically supervised protocol of different exercise modalities DURATION 28 weeks SETTING outpatient group CONTACT 60 minutes
AEROBIC/STRENGTH TRAINING
OTHER COMPONENTS nil EDUCATION "Participants also received brochures with information about the local physical activity facilities" INTERVENTION supervised exercise training training protocol was in line with published recommendations "The participants self‐monitored the intensity and duration of endurance and resistance exercises to avoid exertional dyspnoea" DURATION 28 weeks SETTING outpatient group CONTACT 3 times a week, 1 hour AEROBIC TRAINING cycling, treadmill walking or upper limb ergometer
STRENGTH TRAINING upper limb, lower limb, trunk; 4 sets
OTHER COMPONENTS flexibility and balance exercises EDUCATION "All participants were instructed to continue with their prescribed exercise program during the 14‐week follow‐up period." | |
| Outcomes | DEVICE SenseWear (software version not reported)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "The authors received no financial support." CONFLICT OF INTEREST "The authors have no conflict to declare." CONTACT Giuseppe Coratella [email protected] University of Verona (Italy) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a restricted block (size = 4) randomization, generated by free online software (www.randomization.com) was used to allocate the participants within the 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "allocation and randomization were completed by one of the researchers without any contact or knowledge of the participants…. Thus, no allocation concealment mechanisms were necessary" |
| Blinding of participants (performance bias) | Unclear risk | Not specified |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Low risk | No trial registry; results presented as in Methods |
| Other bias | Unclear risk | only male participants |
| Methods | DATES, SETTING, SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION CRITERIA COPD patients BASELINE CHARACTERISTICS (TOTAL n = 28) AGE mean 64 (SD 7) years; SEX 15 (54%) males; FEV1 not reported | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION online physical activity coaching system designed to support maintenance of physical activity at home after PR NO INTERVENTION | |
| Outcomes | DEVICE Philips DirectLife activity monitor
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOME
| |
| Notes | FUNDING This abstract is funded by Philips CONFLICT OF INTEREST None stated CONTACT Privender Saini [email protected] Phillips (The Netherlands) NB. Abstract states that this study is ongoing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | Unclear risk | Insufficient information |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Insufficient information |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | This abstract is funded by Philips |
| Methods | DESIGN 2 groups DATES not reported SETTING hospital PR department (UK) SAMPLE SIZE calculation based on arbitrary activity counts | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA not reported BASELINE CHARACTERISTICS
AGE mean 71 (SD 4) years; SEX 6 (60%) male; FEV1 mean 43 (SD 16)% predicted
AGE mean 76 (SD 8) years; SEX 8 (80%) male; FEV1 mean 44 (SD 29)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP no SUPERVISION no COMMON INTERVENTION 2 litres a minute through nasal cannula backpack no limit on cylinder usage standard advice
INTERVENTION supplemental oxygen PLACEBO air | |
| Outcomes | DEVICE Z80 32K VI (waist)
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING ACKNOWLEDGMENT: "The authors acknowledge Air Products for the supply of cylinders." CONFLICT OF INTEREST statement provided CONTACT Carolyn Sandland carolyn.sandland@uhl‐tr.nhs.uk University Hospitals Of Leicester NHS Trust, Leicester (UK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized to receive cylinder oxygen or cylinder air for 8 weeks. Prior to the study, a randomization list was prepared" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "prior to the study, a randomization list was prepared and transferred to sealed envelopes" |
| Blinding of participants (performance bias) | Low risk | Quote: "double‐blinded… the cylinders were prefllIed with oxygen or air and were disguised and only identifiable by a colour code" |
| Blinding of personnel (performance bias) | Low risk | Quote: "double‐blinded… the cylinders were prefllIed with oxygen or air and were disguised and only identifiable by a colour code" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | no participant flow diagram by group provided |
| Selective reporting (reporting bias) | Unclear risk | Results as in Methods except SF36 results not presented other than "there were no significant changes in the SF36" |
| Other bias | Unclear risk | Trial registration: National Research Register N0123109178. Unable to access |
| Methods | DESIGN 2 groups DATES May 2008 to December 2010 SETTING 31 general practices in rural, remote and metropolitan areas (Australia) SAMPLE SIZE calculation based on SF36 | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS AGE mean 68 (SD 8) years; SEX 97 (53%) male; FEV1 mean 55 (SD 13)% predicted
| |
| Interventions | DURATION OF INTERVENTION 12 months FOLLOW‐UP no SUPERVISION no INTERVENTION self‐management (health mentoring) Up to 16 phone calls, trained community nurses
SHAM Usual care as provided by GP Monthly “social” phone calls without specific advice or skills training | |
| Outcomes | DEVICE ActiGraph GT1M
ASSESSMENT TIME POINTS
OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Julia Walters [email protected] University of Tasmania, Hobart (Australia) Additional data provided: step count, HRQOL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "cluster randomized controlled trial… Participating practices were randomised using a code generated from a random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "concealment of allocation was provided by sequentially numbered opaque, sealed envelopes" |
| Blinding of participants (performance bias) | Unclear risk | Quote: "randomisation occurred at general practice level to avoid contamination between the intervention and control groups" |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "randomisation occurred at general practice level to avoid contamination between the intervention and control groups" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL: Quote: "blinded assessor" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | High risk | Registry (not in paper)
|
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups DATES, SETTING, SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS not reported
| |
| Interventions | DURATION OF INTERVENTION 10 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION exercise training (eccentric cycle training) SETTING outpatient CONTACT 3 sessions a week AEROBIC TRAINING high‐intensity: target 4 times 80% baseline concentric maximal work capacity STRENGTH TRAINING, OTHER COMPONENTS, EDUCATION nil INTERVENTION exercise training (concentric cycle training) SETTING outpatient CONTACT 3 sessions a week AEROBIC TRAINING high‐intensity: target 80% baseline concentric maximal work capacity STRENGTH TRAINING, OTHER COMPONENTS, EDUCATION nil | |
| Outcomes | DEVICE SenseWear Pro3 (software version not reported)
TIME POINTS
OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement not provided (abstract only) CONTACT Riany Sena [email protected] McGill University Health Center, Montreal (Canada) Additional information provided: type of device, risk of bias assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by an investigator not involved in the study with variable block design (maximum size of 4) with equal allocation ratio (1:1), using a computer‐generated sequence (http://www.randomizer.org)" (correspondence) |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence was placed in double‐sealed opaque envelopes. The trainer opened the appropriate envelope only once the baseline evaluation had been completed" (correspondence) |
| Blinding of participants (performance bias) | Unclear risk | Quote: "Given the kind of intervention, a blinded design was not possible for patients... (but) patients were unaware of our primary outcome... patients allotted to the (eccentric training) group exercised at a different time than did the patients in the (concentric training) group and were asked not to discuss their exercises with assessors and their peers" (correspondence) |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "Given the kind of intervention, a blinded design was not possible for patients and trainers... however, trainers were not involved in the data collection" (correspondence) |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity, exercise capacity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "We had a total of 4 dropouts. There were 11 participants in the eccentric group and 13 participants in the concentric group, but 10 participants in each group completed all the training sessions. For the final study, we did intention to treat analyses and included all 24 participants" (correspondence) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (abstract only) |
| Other bias | Unclear risk | Abstract only |
| Methods | DESIGN 2 groups DATES not reported (abstract only) SETTING PR programme (UK) SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA not reported BASELINE CHARACTERISTICS
AGE mean 67 (SD 10) years; SEX 7 (78%) males; FEV1 mean 1.2 (SD 0.3) litres
AGE mean 71 (SD 9) years; SEX 8 (80%) males; FEV1 mean 1.1 (SD 0.4) litres | |
| Interventions | DURATION OF INTERVENTION 1 week FOLLOW‐UP no SUPERVISION no INTERVENTION PR SETTING outpatient group INTERVENTION unaware of purpose of pedometer, no knowledge of the true purpose of the activity monitor INTERVENTION aware of purpose of pedometer, full knowledge of the purpose of the activity monitor | |
| Outcomes | DEVICE Gaewiler Electronic (waist)
ASSESSMENT TIME POINTS daily for 7 days OUTCOMES
| |
| Notes | FUNDING, CONFLECT OF INTEREST not reported (abstract only) CONTACT Sally Singh sally.singh@uhl‐tr.nhs.uk University Hospitals Of Leicester NHS Trust, Leicester (UK) Additional information requested. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised into two groups" Comment: Insufficient information (abstract only) |
| Allocation concealment (selection bias) | Unclear risk | Not specified (abstract only) |
| Blinding of participants (performance bias) | Unclear risk | Quote: "Patients were randomised into two groups; Group 1, no knowledge of the true purpose of the activity monitor… group 2, full knowledge of the purpose of the activity monitor" |
| Blinding of personnel (performance bias) | Unclear risk | Not specified (abstract only) |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) | Unclear risk | Not specified (abstract only) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information (abstract only) |
| Other bias | Unclear risk | Abstract only |
| Methods | DESIGN 2 groups DATES 2009 to 2012 SETTING VA Puget Sound Health Care System, Seattle (USA) SAMPLE SIZE calculation based on step count | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
AGE mean 67 (SD 10) years; SEX 100% male; FEV1 mean 32 (SD 14) % predicted
AGE mean 67 (SD 9) years; SEX 100% male; FEV1 mean 32 (SD 10)% predicted N.B. group numbers at 6 months not provided | |
| Interventions | DURATION OF INTERVENTION 8 weeks PR, 6 months adherence intervention FOLLOW‐UP yes SUPERVISION no COMMON INTERVENTION exercise sessions SETTING outpatient AEROBIC AND STRENGTH TRAINING goal: treadmill walking 20 minutes
5 minutes warm‐up: upper‐ and lower‐extremity stretching 20 minutes NuStep Borg scale (rating 4 to 6) 5 minutes free arm weights 5 minutes arm ergometry 5 minutes cool‐down: upper‐ and lower‐extremity stretching INTERVENTION adherence intervention
DURATION 4 weeks SETTING outpatient CONTACT 4 hours CONTENT exercise and integrated self‐management education, home safety evaluation
DURATION 3 months SETTING home‐based CONTACT 1 phone call a week CONTENT
DURATION 2 months SETTING home‐based CONTACT 1 phone call a fortnight CONTENT as per stage 2
SETTING outpatient CONTACT 1 session per month CONTENT supervised exercise session INTERVENTION PR DURATION 8 weeks CONTACT 2 hours CONTENT exercise and self‐management instruction Following the 8‐week programme
| |
| Outcomes | DEVICE Stepwatch Activity Monitor (Orthocare Innovations, LLC, Mountlake Terrace, WA) (above dominant ankle)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING VA HSR&D, NRI 04–242 CONFLICT OF INTEREST "The authors declare no conflicts of interest" CONTACT Cynthia Dougherty [email protected] University of Washington, Seattle (USA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized … using a computerized adaptive treatment assignment algorithm" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants (performance bias) | High risk | Quote: "Masking: none (open label)" |
| Blinding of personnel (performance bias) | High risk | Quote: "Masking: none (open label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Quote: "Post‐program measures were completed... by a team member blinded to group assignment" |
| Incomplete outcome data (attrition bias) | Unclear risk | CONSORT diagram provided No details about attrition according to diagnosis group |
| Selective reporting (reporting bias) | Unclear risk | Primary outcomes Registry: 6MWD Paper: 6MWD and physical activity Secondary outcomes Registry: SF36 PCS Paper: SF36 PCS and MCS, clinical COPD questionnaire, Geriatric Depression Scale, cardiopulmonary function outcomes 12‐month outcomes not presented according to group allocation |
| Other bias | Unclear risk | Prospectively registered First Posted: April 30, 2007 Study Start Date: December 2007 Actual Primary Completion Date: December 2011 May 2007; Quote: "Improvement in functional capability, quality of life, self‐efficacy, cardiopulmonary function, gait & balance, mediating effects of self‐regulation model on functional capability, reduction in hospital admissions, outpatient visits & expenditures" August 30, 2010; Enrolment: 200; Revised to 82 February 7, 2012; Enrolment: 100 August 30, 2017; Enrolment: 90 |
| Methods | DESIGN 2 groups DATES October 2010 to April 2011 SETTING "recruited by a chest physician or nurse practitioner" (The Netherlands) SAMPLE SIZE feasibility (limited availability of activity coach) | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 65 (SD 9) years; SEX 8 (44%) male; FEV1 mean 49 (SD 17)% predicted
AGE mean 68 (SD 6) years; SEX 11 (67%) male; FEV1 mean 56 (SD 11)% predicted | |
| Interventions | DURATION OF INTERVENTION 3 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION PAC (telerehabilitation) INTERFACE
ACTIVITY "Try to be active in such a way during the day that the displayed reference line is closely approached" STEP‐TRACKING Accelerometer (MTx‐W sensor) RECORD
GOALS
EDUCATION/RESOURCES
INTERVENTION optional supervised exercise SETTING optional, weekly, group sessions, local physiotherapy practices | |
| Outcomes | DEVICE Yamax Digiwalker 200 (pedometer)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Monique Tabak [email protected] Roessingh Research and Development, Roessinghsbleekweg (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned…according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Quote: "participants were allocated in order of inclusion following the randomization list. Recruitment, randomization, and allocation were performed by different persons" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity: unclear if step count was reported by participants |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL: not specified |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | High risk | SECONDARY OUTCOMES Registry: distribution of activities throughout the day, correlation between pedometer and MTX‐W motion sensor (not reported) Paper: CCQ, dyspnoea, fatigue, number of visits to the web portal, time the activity sensor was worn, adherence (additional outcomes) |
| Other bias | Unclear risk | Physiotherapy‐supervised exercise training in both groups: participation only described at baseline |
| Methods | DESIGN 2 groups DATES December 2011 to July 2013 SETTING Medisch Spectrum Twente Hospital and primary care physiotherapy practices, Enschede (The Netherlands) SAMPLE SIZE Registry: n = 30 Paper: “sample size was based upon the estimated number of patients that could be included within the recruitment period and the availability of technology” | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA (registry)
BASELINE CHARACTERISTICS
AGE mean 64 (SD 9) years; SEX 6 (50%) male; FEV1 median 50 (IQR 33 to 62)% predicted
AGE mean 63 (SD 7) years; SEX 6 (50%) male; FEV1 median 36 (IQR 26 to 54)% predicted | |
| Interventions | DURATION OF INTERVENTION 9 months FOLLOW‐UP no SUPERVISION no INTERVENTION “Condition Coach” technology‐supported care programme Pre‐program: 2 90‐minute self‐management teaching sessions given by a nurse practitioner
4 modules 1. Web‐based exercise programme: breathing exercises, relaxation, mobilisation, resistance and endurance training, mucus clearance created by physiotherapist; every exercise has description and movie care professionals could check progress on the web portal 2. Accelerometer‐based activity sensor and smartphone ambulant activity registration and display, real‐time feedback; goal: reference activity line motivational cues 3. Web‐based self‐management module: participants to treat exacerbations themselves; decision‐support system that automatically formed advice to start medication if worsening clinical condition 4. Teleconsultation module for communication with primary care physiotherapist NO INTERVENTION in case of impending AECOPD, contact medical doctor allowed to attend regular physiotherapy sessions if prescribed as part of usual care | |
| Outcomes | DEVICE accelerometer‐based activity sensor (Inertia Technology, Enschede, The Netherlands)
ASSESSMENT TIME POINTS
PRIMARY OUTCOMES
SECONDARY OUTCOMES
| |
| Notes | FUNDING “Financial support was provided by the NL Agency, a division of the Dutch Ministry of Economic Affairs (grant CALLOP9089).” CONFLICT OF INTEREST “The authors report no conflicts of interest in this work.” CONTACT Monique Tabak [email protected] University of Twente, Drienerlolaan (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomized using a computer‐generated randomization list (Blocked Stratified Randomization version 5; Steven Piantadosi), where randomization was applied in random blocks of two and four.” |
| Allocation concealment (selection bias) | Low risk | Quote:“Participants were allocated by a data manager in order of inclusion following the randomization list, placed in a sealed envelope.” |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to intervention |
| Blinding of personnel (performance bias) | High risk | Masking: None (registry) |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity (unclear if assessed during intervention) |
| Blinding of outcome assessment [other] (detection bias) | High risk | Masking: None (registry) |
| Incomplete outcome data (attrition bias) | High risk | Flow chart provided and attrition documented Quote:“Although 101 patients fulfilled the COPE II study criteria, only 29 patients (29%) were able and willing to participate. The reason for not participating was that patients did not fulfill the additional criterion of having a computer with Internet access at home…. A large number of patients were not able or willing to continue study participation: 33% in the intervention group and 86% in the control group… Although some patients in the intervention group quit the physiotherapy modules (exercising and activity coach) due to weak (n=1, ,T1) or unstable condition (n=1, ,T2) and personal circumstances (n=2, ,T4), they persisted in using the web portal and triage diary till T4.” |
| Selective reporting (reporting bias) | High risk | Results only presented for T0, T1, T2 |
| Other bias | Unclear risk | SAMPLE SIZE Registry: n = 30 Paper: “sample size was based upon the estimated number of patients that could be included within the recruitment period and the availability of technology” |
| Methods | DESIGN 2 groups DATES not reported (abstract only) SETTING hospital (? Malaysia) SAMPLE SIZE not reported (abstract only) | |
| Participants | INCLUSION CRITERIA (as in registry)
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 62 (SD 7) years; SEX 20 (100%) male; FEV1 mean 33 (SD 14)% predicted
AGE mean 66 (SD 8) years; SEX 17 (94%) male; FEV1 mean 34 (14)% predicted | |
| Interventions | DURATION OF INTERVENTION length of stay FOLLOW‐UP no SUPERVISION yes INTERVENTION exercise training (progressive walking and functional resistance exercises) DURATION during admission for AECOPD SETTING inpatient CONTACT commenced within 48 hours of admission, until discharge
AEROBIC TRAINING walking
STRENGTH TRAINING functional resistance exercises
OTHER COMPONENTS afternoon session unsupervised EDUCATION nil NO INTERVENTION as per Malaysian COPD clinical practice guidelines usual physiotherapy care may comprise airway clearance (if required), strategies to minimise dyspnoea (e.g. pursed lip breathing and positioning), and encouragement to mobilise in the ward | |
| Outcomes | DEVICE Stepwatch
ASSESSMENT TIME POINTS
PRIMARY OUTCOMES baseline and prior to discharge
SECONDARY OUTCOMES:
| |
| Notes | FUNDING, CONFLICT OF INTEREST not reported (abstract only) CONTACT Fatim Tahirah [email protected] Curtin University, Perth (Australia) Additional information provided: baseline characteristics, physical activity, exercise capacity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation table created by computerised sequence generation" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants (performance bias) | Unclear risk | Quote: "Participants unable to be blinded to intervention but unaware of other treatment group’s intervention" |
| Blinding of personnel (performance bias) | High risk | Quote: "Not blinded: personnel" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | Exercise capacity: Quote: "assessor blinded" |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information (abstract only) |
| Selective reporting (reporting bias) | Unclear risk | Registry: sit to stand test (not reported) |
| Other bias | Unclear risk | Abstract only |
| Methods | DESIGN 2 groups DATES April 2007 to July 2010 SETTING 70 centres in 10 countries SAMPLE SIZE calculation based on FEV1 area under the curve | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
MEDICATIONS
BASELINE CHARACTERISTICS
AGE mean 61 (SD 8) years; SEX 167 (70%) male; FEV1 post‐bronchodilator mean 66 (SD 8)% predicted
AGE mean 62 (SD 9) years; SEX 147 (67%) male; FEV1 post‐bronchodilator mean 66 (SD 8)% predicted | |
| Interventions | DURATION OF INTERVENTION 24 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION behavioral modification INTERFACE monthly individual session ACTIVITY walking/cycling STEP‐TRACKING nil RECORD activity diary GOALS
EDUCATION/RESOURCES educational DVDs INTERVENTION LAMA (tiotropium, 18 μg) once daily self‐administered morning via HandiHaler PLACEBO once daily self‐administered morning via HandiHaler | |
| Outcomes | DEVICE SenseWear Armband (software version 6.1) (right upper arm)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "The study was funded by Pfizer Inc and Boehringer Ingelheim Pharma GmbH & Co KG." CONFLICT OF INTEREST statement provided CONTACT Thierry Troosters [email protected] KU Leuven (Belgium) Additional information requested: unable to source from Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized, double‐blind, placebo‐controlled, multinational study" (protocol) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | Low risk | Quote: "placebo" |
| Blinding of personnel (performance bias) | Unclear risk | Not specified Quote: "double‐blind treatment phase" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | Protocol: LIPA time, patient diary (not reported) Paper: safety evaluation, adverse events, serous adverse events, AECOPD (additional outcomes reported) |
| Other bias | Unclear risk | Quote: "When this study was designed (in 2006/7), physical activity monitoring was in its infancy. We therefore did not include physical activity as a primary endpoint. Today there is more clarity on factors affecting the outcome of such monitoring, number of days of assessment needed, hours/day and validity of activity monitors in COPD. We measured physical activity using a validated activity monitor as an exploratory endpoint, which allowed for some flexibility in the analysis" |
| Methods | DESIGN 4 groups N.B. all groups received 12 weeks behaviour modification (5 comparisons)
DATES March 2014 to October 2015 SETTING "17 academic institutes, 15 secondary care and 5 primary care centres" (Australia, New Zealand, the USA, Canada and Europe) SAMPLE SIZE calculation based on "EET for each arm compared to self‐management with placebo" "Comparisons between the three non‐placebo arms were also performed; however, the trial was not designed or powered for these comparisons" | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
FROM ONLINE SUPPLEMENT "Patients on continuous home oxygen were excluded from the study... Willingness to increase physical activity is not an inclusion criterion but patients have to be willing to take part in the 8‐week exercise training program (1 in 4 chance of being randomised to the treatment arm that includes exercise training) and take part in the behaviour‐change self‐management program." MEDICATIONS "Patients are permitted to continue with inhaled corticosteroids but not LAMAs, LABAs or short‐acting muscarinic antagonists during the trial, and all are provided with open‐label salbutamol for rescue medication use throughout the trial. Acute exacerbations can be treated as medically necessary." BASELINE CHARACTERISTICS
AGE mean 64 (SD 7) years; SEX 46 (71%) male; FEV1 post‐bronchodilator mean 56 (SD 14)% predicted
AGE mean 66 (SD 6) years; SEX 51 (76%) male; FEV1 post‐bronchodilator mean 57 (SD 13)% predicted
AGE mean 65 (SD 7) years; SEX 45 (63%) male; FEV1 post‐bronchodilator mean 59 (SD 11)% predicted
AGE mean 65 (SD 7) years; SEX 42 (60%) male; FEV1 post‐bronchodilator mean 57 (SD 13)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks (exercise training first 8 weeks) FOLLOW‐UP no SUPERVISION no INTERVENTION placebo once daily INTERVENTION LAMA (tiotropium 5 µg) once daily INTERVENTION LAMA/LABA (tiotropium+olodaterol 5/5 µg) once daily INTERVENTION LAMA/LABA (tiotropium+olodaterol 5/5 µg) once daily with exercise training INTERVENTION behaviour modification DURATION 12 weeks INTERFACE
ACTIVITY Not specified STEP‐TRACKING Pedometer OMRON HJ‐321 (direct feedback) RECORD "patient worksheet" GOALS
EDUCATION/RESOURCES
INTERVENTION high‐intensity whole‐body exercise training SETTING outpatient CONTACT 3 sessions per week AEROBIC TRAINING 30 minutes stationary leg cycling or treadmill walking
STRENGTH TRAINING upper and lower limbs, 45 minutes
OTHER COMPONENTS, EDUCATION nil | |
| Outcomes | DEVICE MoveMonitor
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "Troosters: Funding This work was supported by Boehringer Ingelheim Pharma GmbH & Co. KG. Medical writing assistance was provided by Claire Scofield of Complete HealthVizion, which was contracted and compensated by Boehringer Pharma GmbH & Co. KG. The PROactive project is funded by the IMI‐JU (#115011). The PHYSACTO trial is an in‐kind contribution of the sponsor Boehringer Ingelheim to the PROactive project. In this project, the innovative patient‐reported outcome tool and the activity monitor were developed and validated. Bourbeau: The PROactive project is funded by the IMI‐JU grant number #115011. The PHYSACTO trial is an in‐kind contribution of the sponsor Boehringer Ingelheim to the PROactive project Acknowledgements Medical writing assistance was provided by Laura George, PhD, of Complete HealthVizion, which was contracted and compensated by Boehringer Ingelheim Pharma Inc." CONFLICT OF INTEREST "Competing interests JB has received grants from the Canadian Institute of Health Research R&D collaborative programme (AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Nycomed, Novartis), the Canadian Respiratory Research Network, the Respiratory Health Network of the FRQS and the Research Institute of the MUHC. KLL reports personal fees from the study sponsor for personnel training, as well as a grant from AbbVie and personal fees from Bayer, Janssen, Novartis, AbbVie, Mundipharma and Almirall. MS is employed by Respiplus, a non‐profit organisation that was contracted by the study sponsor to develop the educational component of the training programme. DDS, DE and AH are employees of Boehringer Ingelheim. FM has received grants from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Nycomed and Pfizer, personal fees from Boehringer Ingelheim, GlaxoSmithKline and Novartis, and other financial support from GlaxoSmithKline. TT has received grants from the Innovative Medicines Initiative Joint Undertaking and speaker fees from Boehringer Ingelheim and GlaxoSmithKline. NL is employed by Evidera, a healthcare research firm that provides consulting and other research services to pharmaceutical and other organisations including the study sponsor. The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors. They take full responsibility for the scope, direction, content of, and editorial decisions relating to the manuscript, were involved in reviewing and revising the manuscript at all stages of development, and have approved the submitted manuscript. Troosters: Competing interests TT has received grants from the Innovative Medicines Initiative Joint Undertaking and speaker fees from Boehringer Ingelheim and GlaxoSmithKline. JB has received grants from the Canadian Institute of Health Research R&D collaborative programme (AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck, Nycomed, Novartis), the Canadian Respiratory Research Network, the Respiratory Health Network of the FRQS and the Research Institute of the MUHC. FM has received grants from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, Nycomed and Pfizer, personal fees from Boehringer Ingelheim, GlaxoSmithKline and Novartis, and other financial support from GlaxoSmithKline. NL is employed by Evidera, a healthcare research firm that provides consulting and other research services to pharmaceutical and other organisations including the study sponsor. DE, DDS, LK and AH are employees of Boehringer Ingelheim. DE, DDS and AH are employees of the sponsor and were involved in the design, implementation and interpretation of the study results, together with the investigators Registry: Principal Investigators are NOT employed by the organization sponsoring the study. There IS an agreement between Principal Investigators and the Sponsor (or its agents) that restricts the PI's rights to discuss or publish trial results after the trial is completed. Restriction Description: Boehringer Ingelheim (BI) acknowledges that investigators have the right to publish the study results. Investigators shall provide BI with a copy of any publication or presentation for review prior to any submission. Such review will be done with regard to proprietary information, information related to patentable inventions, medical, scientific, and statistical accuracy within 60 days. BI may request a delay of the publication in order to protect BI’s intellectual property rights." CONTACT Thierry Troosters [email protected] KU Leuven (Belgium) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation is performed using a pseudo‐random number generator and block randomisation is used to achieve balanced allocation" |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients are assigned to treatment arms using a web‐based and telephone‐based response system, and provided with numbered medication boxes" |
| Blinding of participants (performance bias) | Unclear risk | Quote: "A double‐blind design is used for the groups receiving placebo & behaviour modification, (LAMA) & behaviour modification, and (LAMA/LABA) & behaviour modification" Quote: "It is not possible to blind the participants or staff against the group receiving (exercise training)" |
| Blinding of personnel (performance bias) | Unclear risk | Quote: "A double‐blind design is used for the groups receiving placebo+behaviour modification, tiotropium monotherapy+behaviour modification, and tiotropium +olodaterol+behaviour modification" Quote: "It is not possible to blind the participants or staff against the group receiving (exercise training)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: Quote: "A double‐blind design is used for the groups receiving placebo+behaviour modification, tiotropium monotherapy+behaviour modification, and tiotropium +olodaterol+behaviour modification" Quote: "It is not possible to blind the participants or staff against the group receiving (exercise training)" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Low risk | Paper: CRQ, dyspnoea, medication adherence (additional outcomes reported) |
| Other bias | Unclear risk | Registry: Study start March 2014 Study completion October 2015 Original entry: 3 arms Modifications made November 2016: four arms |
| Methods | DESIGN 2 groups DATES not reported SETTING tertiary hospital PR programme (Australia) SAMPLE SIZE calculation based on ESWT | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 73 (8) years; SEX 12 (63%) male; FEV1 mean 60 (SD 23)% predicted
AGE mean 75 (SD 9) years; SEX 6 (35%) male; FEV1 mean 68 (SD 19)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP no SUPERVISION yes INTERVENTION PR (telerehabilitation) SETTING home (laptop computer with an in‐built camera), outpatient group (maximum 4 participants) CONTACT
AEROBIC TRAINING
STRENGTH TRAINING sit to stand, squats (3 sets, 10 repetitions) OTHER COMPONENTS, EDUCATION nil | |
| Outcomes | DEVICE SenseWear (software version 8.0) (left upper arm)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement not provided CONTACT Ling Ling Tsai [email protected] University of Sydney (Australia) Adiitional information provided: clarification for total energy expenditure units (kcal), thresholds for classification of physical activity according to intensity (light, moderate) and results for all time in physical activity greater than three METs (to enable inclusion in meta‐analysis). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This study was a prospective, blinded (assessor and statistician) RCT. Participants were randomized by one of the investigators (L.L.Y.T.) using a computer‐generated sequence" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "concealed allocation" |
| Blinding of participants (performance bias) | High risk | Unable to blind participant to intervention. |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "this study was a prospective, blinded (assessor and statistician) RCT… measurements which were performed by a research assistant who was blind to group allocation" |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | SECONDARY OUTCOMES Registry: satisfaction, semi‐structured interview post telerehabilitation (not reported in paper) |
| Other bias | Low risk | N/A |
| Methods | DESIGN Stage 1 2 groups (intervention (nutritional supplement) with PR vs. PR) Stage 2 2 groups (intervention (nutritional supplement) with PAC vs. PAC) DATES September 2011 to April 2014 SETTING outpatient PR programme in 7 hospitals (The Netherlands) SAMPLE SIZE calculation based on peak torque | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 63 (SEM 1) years; SEX 18 (43%) male; FEV1 mean 57 (SEM 3)% predicted
AGE mean 62 (SEM 1) years; SEX 23 (59%) male; FEV1 mean 53 (SEM 3)% predicted | |
| Interventions | DURATION OF INTERVENTION Phase 1: 4‐month nutritional intervention Phase 2: 8‐month maintenance phase FOLLOW‐UP Phase 3: up for 3 months, without any intervention in either group SUPERVISION no PHASE 1 COMMON INTERVENTION 4‐month outpatient centre‐based pulmonary rehabilitation
INTERVENTION protein, carbohydrates, fat and micronutrients, enriched with leucine, omega‐3 PUFA, and vitamin D (Nutricia NV, Zoetermeer, The Netherlands) PLACEBO flavoured non‐caloric cloudified aqueous solution, no active components PHASE 2 COMMON INTERVENTION Both groups received feedback on their physical activity behaviour twice, based on accelerometry INTERVENTION 1 oral nutritional supplement a day, four nutritional counselling sessions | |
| Outcomes | DEVICE GT3X Actigraph accelerometer
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study was financially supported by a Public–Private Consortium of Maastricht University/NUTRIM, CIRO+ BV Horn, Lung Foundation Netherlands (grant 3.4.09.003), and Nutricia Research." CONFLICT OF INTEREST "ER, FF and EW declare that they have no conflict of interest regarding the present manuscript. AvH is employed by Nutricia Research. CvdB and AS report grants from Netherlands Lung Foundation and Nutricia." Research, during the conduct of the study. AS is a member of International Scientific Advisory Board of Nutricia Advanced Medical Nutrition." CONTACT Annemie Schols [email protected] Maastricht University Medical Center, (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization sequence was generated using the PLAN procedure of SAS statistical software (Enterprise Guide version 4.3) by a statistician from Nutricia Research who had no further involvement in the conduct of the study. The permuted block randomization was stratified for site, with a 1:1 allocation ratio" |
| Allocation concealment (selection bias) | Low risk | Quote: "All details of the randomization, including block size, were unknown to the investigator, site staff, and study staff, except for the statistician who was responsible for generating the randomization sequence, and the supplies manager who needed to be unblinded in order to label the study products and to create unblinding envelopes, etc" |
| Blinding of participants (performance bias) | Low risk | Quote: "The packaging of the test product and control product were identical in appearance. The study product was labelled using product numbers. Which product numbers corresponded to which treatment was only known to the statistician who was responsible for generating the randomization sequence and the clinical studies supplies manager" |
| Blinding of personnel (performance bias) | Low risk | Quote: "The packaging of the test product and control product were identical in appearance. The study product was labelled using product numbers. Which product numbers corresponded to which treatment was only known to the statistician who was responsible for generating the randomization sequence and the clinical studies supplies manager" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity and exercise capacity (cycle ergometry) |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | Exercise capacity (6MWD):"double blind" (paper), "triple blind"(registry) |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided Quote: "Two of the 81 randomized patients did not start the treatment. During the PR, the drop‐out rate was 9.5% (four patients) in NUTRITION and 5.4% (two patients) in PLACEBO" |
| Selective reporting (reporting bias) | Unclear risk | PRIMARY OUTCOME Registry: skeletal muscle strength (baseline, 12 months) Paper: quadriceps muscle strength (baseline, 4 months) SECONDARY OUTCOMES Registry:
Paper:
|
| Other bias | Unclear risk | Quote "Patient inclusion was prematurely discontinued because the test product could not be produced within the appropriate quality specifications due to discontinuation of the supply of one of the ingredients, but justifiable based on other RCTs published in the meantime as argued in the discussion" |
| Methods | DESIGN 2 groups DATES not reported SETTING University (Spain) SAMPLE SIZE not reported | |
| Participants | "Recruitment was conducted by the pulmonology consultants of the aforementioned hospitals, using an intentional system of consecutive series" INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 70 (SD 7) years; SEX 18 (86%) male; FEV1 mean 46 (SD 17)% predicted
AGE 65 (SD 9) years; SEX 13 (68%) male; FEV1 mean 52 (SD 16)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks FOLLOW‐UP 12 months SUPERVISION no INTERVENTION PAC and exercise training INTERFACE weekly phone call ACTIVITY walking 5 sessions a week, 30 to 60 minutes (15‐ to 20‐minute cycles) (as for ISWT) STEP‐TRACKING pedometer (direct feedback, control walking speed) RECORD diary: daily step count GOALS revised weekly, 10% to 20% increase EDUCATION/RESOURCES nil INTERVENTION pedometer ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) GOALS general recommendations to walk more every day EDUCATION/RESOURCES 5 group sessions of respiratory physiotherapy (ventilation techniques, bronchial clearance, thoracic mobilisation, education). "This initial intervention, considered as “standard care” in a PR programme, has shown no influence in the variables we analysed" (Spruit 2013) INTERFACE, RECORD nil | |
| Outcomes | DEVICE OMRON Walking Style X Pocket HJ‐320e (pedometer) (waist, next to right hip)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Jordi Vilaró [email protected] Universidad Autónoma de Madrid (Spain) Additional information provided: 'Risk of bias' assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to one of two groups (experimental or control) through simple randomization, using a system of random numbers generated by Microsoft® Excel 2010" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The allocation sequence was concealed" (correspondence) |
| Blinding of participants (performance bias) | Low risk | Quote: "Participants were not aware of the interventions applied to the other group, and contact between the two groups was avoided at all times" |
| Blinding of personnel (performance bias) | High risk | Quote: "Correspondence between numerical codes and study group was only available to researchers in charge of the treatment, not to evaluators" |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity: Quote: "obtained the data recorded in the memory of the device and the data collected by the patients every day in a diary. We confirmed that either data coincident or, we accepted when there was a variation lower than 10%" (correspondence) |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "correspondence between numerical codes and study group was only available to researchers in charge of the treatment, not to evaluators" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as in Methods |
| Other bias | Unclear risk |
|
| Methods | DESIGN 3 groups, 2 phases
DATES not reported SETTING outpatient clinic (Greece) SAMPLE SIZE calculation based on number of hospitalisations for AECOPD | |
| Participants | "The majority of patients were referred to the PR programme because of persistent respiratory symptoms, but also following hospitalisation for acute exacerbation of COPD (four patients in group A and six patients in group B). These patients were included in the study at least 8 weeks after the hospitalisation. None of these patients had previously participated in a PR programme." INCLUSION CRITERIA
Additional as per registry
EXCLUSION CRITERIA
Additional as in registry
BASELINE CHARACTERISTICS
AGE mean 67 (SD 10) years; SEX 44 (94%) male; FEV1 mean 50 (SD 22)% predicted
AGE mean 67 (SD 7) years; SEX 38 (76%) male; FEV1 mean 52 (SD 17)% predicted
AGE mean 64 (SD 8) years; SEX 37 (74%) male; FEV1 mean 52 (SD 21)% predicted | |
| Interventions | DURATION OF INTERVENTION 14 months FOLLOW‐UP no SUPERVISION yes INTERVENTION exercise training (HIIT) DURATION 8 weeks SETTING centre‐based outpatient group CONTACT 3 sessions a week AEROBIC TRAINING cycling 45 minutes, alternating 30‐second exercise intervals with 30‐second rest periods
STRENGTH TRAINING upper and lower limbs, 15 minutes (3 sets, 10 repetitions) OTHER COMPONENTS advice from dietician EDUCATION instructions on breathing control, self‐management techniques INTERVENTION maintenance (telerehabilitation) DURATION 12 month SETTING home CONTACT 1 session a week, 1 hour (144 sessions) telephone or video conference (tablet) AEROBIC TRAINING individually‐tailored home‐based exercise programme: walking drills STRENGTH TRAINING upper and lower limbs, 3 sets, 10 to 12 repetitions OTHER COMPONENTS
EDUCATION individualised action plan, brochure of prescribed exercises, video demonstrations: home exercises, breathing retraining techniques, educational leaflets for COPD, instruction on management of anxiety and depression symptoms, dietary and self‐management advice INTERVENTION maintenance (centre‐based) DURATION 12 months SETTING outpatient group CONTACT 2 sessions a week (96 sessions) AEROBIC and STRENGTH TRAINING “exercise training” OTHER COMPONENTS nil EDUCATION physiotherapy, dietary and psychological advice | |
| Outcomes | DEVICE Actigraph GT3X (ActiLife software version 5.10.0) (waist, above right hip)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME (12 months following completion of HIIT)
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Ioannis Vogiatzis [email protected], Maroula Vasilopoulou [email protected] National and Kapodistrian University of Athens (Greece) Additional results provided: scale for graph of time in moderate‐intensity physical activity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised into 3 groups using a set of computer‐generated random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | High risk | Quote: "Masking: None (Open Label)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Unclear risk | HRQOL and exercise capacity: Quote: "Masking: None (Open Label)" |
| Incomplete outcome data (attrition bias) | Unclear risk | CONSORT diagram provided Quote: "During the 2‐month primary PR programme, three patients from group A were discontinued from the study because of transport barriers" |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOMES discrepancy between registry and paper Registry:
Methods and Results:
SECONDARY OUTCOMES Paper: cycle ergometry, adherence (additional outcomes) |
| Other bias | Unclear risk | Quote: "During the period spanning from December 2013 to July 2015, patients in groups A and B initially completed a multidisciplinary intense hospital‐based, outpatient, pulmonary rehabilitation programme lasting for 2 months, which was followed by a 12‐month maintenance rehabilitation programme... patients in group C followed the usual care treatment throughout the 14‐month period" Registry: First submitted: November 2015 Primary completion date: July 2015 (definition: final data collection date for primary outcome measure) Study completion date: July 2016 (definition: final data for the primary outcome measures, secondary outcome measures and adverse events (the last participant's last visit). |
| Methods | DESIGN 2 groups DATES not reported SETTING 32 physiotherapy practices (The Netherlands) SAMPLE SIZE calculation based on weekday step count | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 62 (SD 9) years; SEX 42 (50%) male; FEV1 mean 59 (SD 20)% predicted
AGE mean 63 (SD 8) years; SEX 36 (49%) male; FEV1 mean 63 (SD 15)% predicted | |
| Interventions | DURATION OF INTERVENTION 6 months FOLLOW‐UP no SUPERVISION no INTERVENTION PAC (app) INTERFACE
ACTIVITY not specified STEP‐TRACKING accelerometer (in smartphone, direct feedback) GOALS 30 intensive minutes a day
RECORD, EDUCATION/RESOURCES nil INTERVENTION optional supervised exercise SETTING optional, 1 to 2 sessions a week, group training sessions, local physiotherapy practices | |
| Outcomes | DEVICE SenseWear Pro or MF‐SW mini armband (software version 7.1)
ASSESSMENT TIME POINTS
PRIMARY/SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Sigrid Vorrink [email protected] Utrecht University of Applied Sciences (The Netherlands) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was a multicentre, investigator‐blinded, randomised controlled trial…The patients included in the study were randomly assigned to the intervention or usual care group, independent of physiotherapy practice, based on a random number sequence generated in Excel (Microsoft, Redmond, WA, USA) before enrolment. These numbers ranged between 0 and 1. The values were categorised into 0 (usual care) and 1 (intervention), based on a 0.5 threshold. Subsequently, each newly recruited participant was given the first available number and enrolled in the corresponding group. Patients with and without long‐term physiotherapy after pulmonary rehabilitation…were separately randomised via stratification, because this was seen as a confounder" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "assessments were performed by two researchers that were blinded to the group allocation" |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Drop‐out in the intervention group was higher (39% in the intervention group versus 27% in the usual care group), and it was also higher among females. Initial worries about the telephone contract (linked to a personal bank account) and fear of losing the device were reasons for patients to drop out of the study. After the consent form was adjusted to state explicitly that there would not be any financial ramifications, the drop‐out rate decreased" |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as in Methods |
| Other bias | Unclear risk | Quote: "Patients with GOLD stage 4 disease were not included in the study, resulting in a sample not fully similar to other pulmonary rehabilitation studies. This was because their low physical activity level renders an intervention effect improbable" Quote: "The present study was set up in primary care and did not measure outcomes at the start of the pulmonary rehabilitation programme. This makes it difficult to compare the decline in physical activity with the potentially beneficial effects of the preceding pulmonary rehabilitation programme" Quote: "The intervention might have yielded different results in patients that did not complete a pulmonary rehabilitation programme. As there could be more room for improvement, physical activity levels could have increased. This has been shown in previous pedometer studies. Nevertheless, as the intervention was not successful in maintaining physical activity in patients after pulmonary rehabilitation, the question remains whether it is capable of improving physical activity in patients without pulmonary rehabilitation" |
| Methods | DESIGN 2 groups DATES April 2012 to August 2015 SETTING general pulmonary clinics (USA) SAMPLE SIZE calculation based on step count | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS
AGE mean 68 (SD 9) years; SEX 56 (98%) male; FEV1 mean 60 (SD 21)% predicted
AGE mean 69 (SD 8) years; SEX 51 (98%) male; FEV1 mean 66 (SD 22)% predicted | |
| Interventions | DURATION OF INTERVENTION 12 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION PAC (web‐based, as per Moy 2015a) INTERFACE website ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD website: step count weekly GOALS website: display progress, protocol‐driven goal (revised weekly) minimum value of 3 possibilities: 1) 400 step increase from previous goal; 2) 400 step increase from 7‐day average; or 3) 10,000 steps EDUCATION/RESOURCES website: education and motivational content, community forum INTERVENTION pedometer No instructions about exercise INTERFACE website ACTIVITY walking STEP‐TRACKING pedometer (direct feedback) RECORD website: step count monthly GOALS nil EDUCATION/RESOURCES written materials about exercise at study entry | |
| Outcomes | DEVICE Omron HJ‐720 ITC (pedometer)
ASSESSMENT TIME POINTS PRIMARY OUTCOME
SECONDARY OUTCOMES
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Marilyn Moy [email protected], Emily Wan [email protected] VA Boston Healthcare System (USA) Additional information provided: confirmed that data in text and Table 3 are SD (not standard errors) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were then randomized (1:1) by a computer algorithm" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization assignments were generated with random block sizes which were not disclosed to study staff. Assignments were communicated to study staff through the ESC website, and subjects were notified by telephone of their assignment groups" |
| Blinding of participants (performance bias) | High risk | Quote: "Due to the nature of the intervention, participant blinding was not possible" |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "the research assistant conducting assessments at the study conclusion (3 months) was blinded to group assignment" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | High risk | PRIMARY OUTCOME Registry: Originally 6MWD (January 2013) then changed to step count (March 2017) as in paper Between April 2012 and August 2015, 114 participants were enrolled and randomised SECONDARY OUTCOMES Paper: 6MWD, exercise adherence, HRQOL, dyspnoea, depression, COPD knowledge, exercise self‐efficacy, social support, motivation, confidence to exercise daily (additional outcomes reported) |
| Other bias | Low risk | N/A |
| Methods | DESIGN cross‐over trial (only pre‐cross‐over data used), 2 groups DATES April 2014 to February 2015 SETTING 30 secondary care/pulmonology practices (Germany) SAMPLE SIZE calculation based on physical activity | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
MEDICATIONS
BASELINE CHARACTERISTICS
AGE mean 64 (SD 8) years; SEX 64 (67%) male; FEV1 mean 63 (SD 10)% predicted
AGE mean 61 (SD 8) years; SEX 63 (64%) male; FEV1 mean 60 (SD 11)% predicted | |
| Interventions | DURATION OF INTERVENTION 3 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION LAMA/LABA (indacaterol/glycopyrronium (QVA149) 110/50 μg) once daily Breezhaler® PLACEBO once daily Breezhaler® | |
| Outcomes | DEVICE Sensewear (software version not reported)
TIME POINTS
PRIMARY OUTCOMES
SECONDARY OUTCOMES
| |
| Notes | FUNDING "This study was funded by Novartis Pharma GmbH. Employees of Novartis Pharma GmbH were involved in the design of the study and analysis, and interpretation of data and oversaw the collection of data. Two of the authors (CM and MB) are authors of the manuscript." CONFLICT OF INTEREST statement provided CONTACT Henrik Watz [email protected] German Center for Lung Research, Grosshansdorf (Germany) Additional information provided: pre‐cross‐over results as per Novartis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised to one of two treatment sequences in a ratio of 1:1 using a randomisation number from a list generated by the study sponsor. This list was produced using a validated automated system." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | Low risk | Quote: "Masking: Double (Participant, Care Provider)" |
| Blinding of personnel (performance bias) | Low risk | Quote: "Masking: Double (Participant, Care Provider)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Low risk | All data reported as in registry |
| Other bias | Low risk | N/A |
| Methods | DESIGN 2 groups (data from first 4 weeks only) DATES April 2015 to July 2016 SETTING 26 sites (Canada, Germany, Hungary, Spain) SAMPLE SIZE calculation based on spirometry | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
MEDICATIONS
BASELINE CHARACTERISTICS
AGE mean 63 (SD 8) years; SEX 82 (61%) male; FEV1 post‐bronchodilator mean 60 (SD 11)% predicted
AGE mean 62 (SD 8) years; SEX 80 (60%) male; FEV1 post‐bronchodilator mean 61 (SD 11)% predicted | |
| Interventions | DURATION OF INTERVENTION 4 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION LAMA/LABA (aclidinium bromide/formoterol fumarate, 400/12 μg) dry‐powder inhaler twice daily (Genuair™/Pressair®) PLACEBO dry‐powder inhaler twice daily (Genuair™/Pressair®) | |
| Outcomes | DEVICE DynaPort MoveMonitor
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING "Complete Medical Communications, Macclesfield, UK, for providing medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines. This study was sponsored by Menarini Group through its affiliate Berlin‐Chemie and AstraZeneca. Menarini Group and AstraZeneca were involved in the study design, collection, analysis, interpretation of data, and development of this manuscript. Menarini Group and AstraZeneca were also involved in the review of the manuscript." CONFLICT OF INTEREST statement provided CONTACT Henrik Watz [email protected] German Center for Lung Research, Grosshansdorf (Germany) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation: Randomized" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | Low risk | Quote: "Masking: Double (Participant, Investigator)" |
| Blinding of personnel (performance bias) | Low risk | Quote: "Masking: Double (Participant, Investigator)" |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity, exercise capacity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow diagram provided |
| Selective reporting (reporting bias) | Unclear risk | SECONDARY OUTCOMES Paper: additional outcomes
|
| Other bias | Unclear risk | Quote: "When considering the design of the ACTIVATE study and any impact this may have had on the results, it should be noted that statistical significance for the primary end point (trough FRC) was reached only after the use of a nonparametric test or after excluding four potential outliers (the sample size was too small to overcome random error in the presence of these extreme outliers). It was unexpected that the improvements in trough FRC did not reach statistical significance, given that previous evidence showed that aclidinium 400 μg improved trough FRC in patients with moderate‐to‐severe COPD, in addition to improvements in other lung hyperinflation end points both in patients with moderate‐to‐severe COPD and in patients with severe‐to‐very severe COPD. However, when considered collectively with the post‐dose data, this post‐hoc analysis supports the conclusion that AB/FF significantly reduced lung hyperinflation. In addition to this, the 2‐month study period of ACTIVATE is too short to show long‐term treatment effects on physical activity, considering that it can be challenging to encourage long‐term changes in physical activity in patients with COPD and that data for long‐term improvements of physical activity by bronchodilators are lacking." |
| Methods | DESIGN 2 groups DATES October 2016 to January 2017 SETTING hospital outpatient clinic (Indonesia) SAMPLE SIZE calculation based on 6MWT | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
MEDICATION "Patients maintained their usual drug medication during the entire study" BASELINE CHARACTERISTICS (total n = 40)
AGE mean 68 (7) years; SEX 16 (89%) male; FEV1 mean 1.1 (SD 0.5) litres
AGE mean 61 (7) years; SEX 15 (83%) male; FEV1 mean 0.9 (SD 0.4) litres | |
| Interventions | DURATION OF INTERVENTION 6 weeks FOLLOW‐UP no SUPERVISION no INTERVENTION PAC (in‐person) DURATION 6 weeks INTERFACE
ACTIVITY walking ≥ 30 minutes a day, fastest pace possible STEP‐TRACKING pedometer RECORD daily log book: step count, change in clinical condition GOALS aim > 6500 steps a day
EDUCATION/RESOURCES nil INTERVENTION exercise training DURATION 6 weeks SETTING centre‐based supervised outpatient CONTACT 3 sessions a week, 30 minutes AEROBIC TRAINING treadmill
OTHER COMPONENTS Encouraged to be more active at home (i.e. no goals)
STRENGTH TRAINING, EDUCATION nil | |
| Outcomes | DEVICE Omron HJ 321 (pedometer)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING not reported CONFLICT OF INTEREST statement provided CONTACT Kiki Widyastuti [email protected] Dr Moewardi Hospital, Central Jawa (Indonesia) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants (performance bias) | High risk | Quote: "Patients were not blind to treatment" |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Unclear risk | Physical activity: unclear if step count data was reported by participants |
| Blinding of outcome assessment [other] (detection bias) | High risk | HRQOL and exercise capacity: Quote: "researchers evaluating results and patients were not blind to treatment" |
| Incomplete outcome data (attrition bias) | Low risk | CONSORT diagram provided |
| Selective reporting (reporting bias) | Unclear risk | No trial registry; results presented as in Methods |
| Other bias | Unclear risk | Quote: "During the first week, all patients were taught how to use pedometers in three face to face sessions with a researcher checking patients’ ability to use the device properly. After the training week, subjects were randomly assigned to two groups." Comment: unclear if this was the same week as baseline data collection |
| Methods | DESIGN Phase 1 2 groups (intervention (supervised walking training) vs. no intervention) Phase 2 supervised walking training group from Phase 1 randomised into 2 groups (intervention (PAC and pedometer with walking) vs. intervention (walking)) DATES May 2009 to June 2012 SETTING 5 sites, outpatient PR, 2 metropolitan cities (Australia) SAMPLE SIZE Phase 1: calculation based on CRQ Phase 2: calculation based on total SGRQ score at the 14‐month assessment | |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS Phase 1
AGE mean 69 (SD 8) years; SEX 56 (59%) male; FEV1 mean 43 (SD 15)% predicted
AGE mean 68 (SD 9) years; SEX 28 (58%) male; FEV1 mean 43 (SD 15)% predicted Phase 2 (participants from the intervention group in phase 1)
AGE mean 70 (SD 7) years; SEX 25 (51%) male; FEV1 mean 43 (SD 15)% predicted
AGE mean 69 (SD 9) years; SEX 30 (65%) male; FEV1 mean 43 (SD 15)% predicted | |
| Interventions | DURATION OF INTERVENTION 8 weeks exercise training, 12‐month maintenance period FOLLOW‐UP no SUPERVISION Phase 1 supervised, Phase 2 unsupervised Phase 1 INTERVENTION exercise training (ground‐based walking) with follow‐up advice SETTING centre‐based outpatient group, supervised CONTACT 3 sessions a week (20 sessions within 8 to 10 weeks) AEROBIC TRAINING walking, flat indoor track
STRENGTH TRAINING, OTHER COMPONENTS, EDUCATION nil
SETTING home‐based CONTACT telephone
AEROBIC TRAINING adjusted according to the number of steps achieved in the past 2 training sessions NO INTERVENTION medical management optimised at baseline, including individualised COPD action plan; "did not participate in any exercise training and were not given any instructions regarding exercise" Phase 2 Participants in the intervention group in phase 1 were asked to complete a further 12 months of home‐based walking SETTING home‐based ACTIVITY walk ≥ 3 days a week
RECORD weekly diary card (number of days, number of minutes walked) EDUCATION/RESOURCES written instructions, scoring system for self‐assessment of breathlessness and effort INTERVENTION PAC and pedometer INTERFACE
STEP‐TRACKING pedometer (G‐Sensor accelerometer, direct feedback) GOALS based on step count (immediate past 2 training sessions) | |
| Outcomes | DEVICE SenseWear® Pro3 (software version 6.1)
ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
| |
| Notes | FUNDING reported CONFLICT OF INTEREST statement provided CONTACT Sally Wootton [email protected] University of Sydney (Australia) Additional results provided: physical activity and exercise capacity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This study was a prospective, blinded (assessor and statistician), multicentre, randomised controlled trial with concealed allocation. Following baseline assessment participants were randomised via an independent telephone randomisation service using computerised random number generator sequencing to either the walking group or control group. Randomisation was stratified according to baseline lung function, SGRQ, 6MWD and study centre. Randomisation was biased towards the walking group with a 2:1 ratio for the purposes of a long‐term follow‐on study to 12 months." |
| Allocation concealment (selection bias) | Low risk | Quote: "concealed allocation… following baseline assessment participants were randomised via an independent telephone randomisation service" |
| Blinding of participants (performance bias) | High risk | Unable to blind participants to the intervention |
| Blinding of personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment [objective] (detection bias) | Low risk | Physical activity |
| Blinding of outcome assessment [other] (detection bias) | Low risk | HRQOL and exercise capacity: Quote: "assessor blinded" |
| Incomplete outcome data (attrition bias) | Unclear risk | Lack of clarity on participant numbers |
| Selective reporting (reporting bias) | Unclear risk | Physical activity results not reported at 8 months (as in registry) |
| Other bias | Unclear risk | Quote: "Retrospectively registered" (as per www.anzctr.org.au) |
ACE: angiotensin‐converting enzyme; AECOPD: acute exacerbation of COPD; BMI: body mass index; CAT: COPD assessment test; CCQ: clinical COPD questionnaire; COPD: chronic obstructive pulmonary disease; COPEactive: community‐based physiotherapeutic exercise programme; cpm: counts per minute; CRQ: chronic respiratory disease questionnaire; CT: computerised tomography; ECG: electrocardiogram; EE: energy expenditure; ESWT: time walked on endurance shuttle walk test; EQ5D: EuroQol 5 dimensions questionnaire; FER: forced expiratory ratio; FEV1: forced expiratory volume in 1 second; FRC: functional residual capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HIIT: high‐intensity interval training; HRmax: maximum heart rate; HRQOL: health‐related quality of life; ICD‐10: International Statistical Classification of Diseases and Related Health Problems 10th Revision; ICS: inhaled corticosteroid; ISWD: distance walked on incremental shuttle walk test; ITT: intention‐to‐treat; IQR: interquartile range; LABA: long‐acting beta2 agonist; LAMA: long‐acting muscarinic antagonist; LIPA: light‐intensity physical activity; LTOT: long‐term oxygen therapy; MRC: Medical Research Council; METs: metabolic equivalents; min: minutes; MVPA: moderate‐to‐vigorous physical activity; n: number; NIPPV: non‐invasive positive pressure ventilation; 1RM: one repetition maximum; PAC: physical activity counselling; PaCO2: partial pressure of carbon dioxide; PAL: physical activity level; PaO2: partial pressure of oxygen; Pimax: maximal inspiratory pressure; PR: pulmonary rehabilitation; RAND‐36: Dutch version of SF36; RV: residual volume; sec: seconds; SF36: Medical Outcomes Survey 36‐item short‐form health survey questionnaire; SGRQ: St George's respiratory questionnaire; 6MWD: distance walked on six‐minute walk test; 6MWT: six‐minute walk test; SD: standard deviation; SEM: standard error of measurement; SPACE: self‐management programme of activity, coping and education; SpO2: peripheral oxygen saturation; TLC: total lung capacity; VMU: vector magnitude unit; Wmax: maximum work rate.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| study not completed/withdrawn from registry | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| not randomised | |
| not randomised | |
| not randomised | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| observational | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| tried to contact author: no physical activity data presented | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| not randomised | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| Quote: "a complete control group was not possible; therefore, the control group in the present study was monitored on 2 separate occasions 8 weeks apart, and then randomized to either the rehabilitation group or acupuncture and rehabilitation" | |
| no group comparison | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| attempted to contact author: no objective physical activity outcomes published | |
| attempted to contact author: unable to obtain pre‐cross‐over data | |
| physical activity not objectively assessed outcome | |
| secondary analyses | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| observational | |
| not randomised | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| contacted author: unable to obtain pre‐cross‐over data | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| attempted to contact author: no physical activity data for control group | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity listed as objectively assessed outcome in registry, no results reported in paper | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| not randomised | |
| physical activity not objectively assessed outcome | |
| observational | |
| attempted to contact author: unable to obtain pre‐cross‐over data | |
| no group comparison | |
| observational | |
| not randomised | |
| observational | |
| observational | |
| physical activity not objectively assessed outcome | |
| attempted to contact author: unable to obtain pre‐cross‐over data | |
| contacted author; unable to access pre‐cross‐over data from Novartis | |
| author correspondence: pre‐cross‐over data not available | |
| author correspondence: physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| author correspondence: pre‐cross‐over data not available | |
| physical activity not objectively assessed outcome | |
| author correspondence: unable to obtain results just for participants with COPD | |
| physical activity not objectively assessed outcome | |
| study not completed/withdrawn from registry | |
| physical activity not objectively assessed outcome | |
| not randomised | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| author correspondence: study ceased soon after trial registration, no data | |
| physical activity not objectively assessed outcome | |
| not randomised | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| observational | |
| no group comparison | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| observational | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| no group comparison | |
| no group comparison | |
| physical activity not objectively assessed outcome | |
| no group comparison | |
| author correspondence: unable to obtain results just for participants with COPD | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| observational | |
| no group comparison | |
| physical activity not objectively assessed outcome | |
| attempted to contact author: unable to obtain results just for participants with COPD | |
| physical activity not objectively assessed outcome | |
| not randomised | |
| attempted to contact author: unable to determine if randomised | |
| not randomised | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome | |
| physical activity not objectively assessed outcome |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Telerehabilitation versus traditional centre‐based PR for people with chronic respiratory disease: protocol for a randomised controlled trial (REACH) |
| Methods | DESIGN 2 groups, equivalence trial; COMPARISON intervention vs. intervention; SETTING Alfred Health and Austin Health (metropolitan, Melbourne), Wimmera Health Care Group (regional, Horsham) (Australia); SAMPLE SIZE n = 142 (based on CRQ dyspnea domain) Selection bias: "Participants will be randomly allocated (1:1) …. A computer‐generated, block randomisation scheme will be used with stratification …. Sequence generation will be performed by an individual who is independent of the research team and randomisation will occur using an online database. The randomisation sequence will be concealed from investigators"; Performance bias: "Given the nature of the intervention (exercise training) participants will not be blinded to the intervention", also personnel; Detection bias: "assessor‐blinded" |
| Participants | Primary diagnosis of a chronic lung disease (COPD, bronchiectasis, asthma, ILD); age ≥ 40 years |
| Interventions | DURATION OF INTERVENTION 8 weeks COMMON COMPONENTS Protocol for exercise training prescription and progression Disease‐specific education and collaborative self‐management training
OTHER COMPONENTS encouraged to perform an additional 3 unsupervised sessions each week, home diary reviewed weekly; encouraged to continue with a regular exercise regimen following programme completion INTERVENTION Telerehabilitation SETTING home‐based, remotely supervised, groups of 4 to 6 participants
CONTACT
AEROBIC TRAINING cycle training, 30 minutes, ≥ 2 bouts
STRENGTH TRAINING use equipment readily available in the home environment
INTERVENTION Centre‐based PR SETTING outpatient, groups of 8 to 12 participants CONTACT 2 sessions a week AEROBIC TRAINING cycling and walking, 30 minutes, ≥ 2 bouts
STRENGTH TRAINING use equipment readily available in the home environment
|
| Outcomes | DEVICE GeneActiv (wrist), Wear instructions: 7 days ASSESSMENT TIME POINTS
PRIMARY OUTCOME HRQOL: CRQ dyspnoea domain SECONDARY OUTCOMES
SAFETY "The trial will be monitored by an independent Data Safety Monitoring Board (DSMB) comprising a respiratory physician and two clinical research physiotherapists, with consultation with a statistician as required. The DSMB will review data relating to the primary outcome (CRQ‐D) as well as 6MWD, endurance cycle time and Hospital Anxiety and Depression Scale scores. Data will be presented to the DSMB in a blinded fashion. The DSMB will initially review data at a time 6 months from the commencement of recruitment. Any serious adverse events will be notified immediately to the overseeing ethics committee (Alfred Health) and the relevant site governance committee, as well as to the DSMB. If there are concerns about the safety of participants, the DSMB will make a recommendation to the trial steering committee about continuing, stopping, or modifying the trial." |
| Starting date | Prospectively registered; recruitment commenced August 2016; last participant enrolment January 2019 |
| Contact information | Narelle Cox [email protected] La Trobe University, Melbourne (Australia) |
| Notes | FUNDING "This study is funded by a National Health and Medical Research Council (NHMRC) project grant (GNT1101616). NSC is supported by an NHMRC Early Career Fellowship (GNT 1119970). The NHMRC will not interfere with the independence of the authors in regard to the conduct of the trial and will not delay or prevent publication of the study" N.B. will need data only for COPD participants for inclusion |
| Trial name or title | Effect of opioids on outcomes of PR: a randomised, double‐blind, multi‐site, parallel arm, fixed dose, placebo‐controlled trial of the effects of morphine on exercise capacity and other outcomes of PR in COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 260 (based on 6MWD) Selection bias: "schedules have been developed by an organisation not involved in this study... block randomisation schedule held by the Central Registry Clinical Trials Pharmacist"; Blinding: participants, personnel and outcome assessors |
| Participants | Diagnosis of COPD (post‐bronchodilator FER < 0.7); Age ≥ 18 years; modified MRC dyspnoea scale (score 3 or 4); Stable medications for breathlessness for 1 week; Eligible for PR as judged by the treating physician and the investigator |
| Interventions | DURATION OF INTERVENTION 8 weeks INTERVENTION morphine and PR oral sustained‐release morphine 20 mg in the morning and 2 capsules of blinded laxative INTERVENTION placebo and PR oral placebo capsule and 2 capsules of placebo laxative |
| Outcomes | DEVICE (accelerometer) PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; last participant enrolment September 2017; final recruitment n = 10; "stopped early: participant recruitment difficulties; data collected is being analysed" |
| Contact information | David Currow [email protected] Flinders University, Adelaide (Australia) |
| Notes | FUNDING Commonwealth Department of Health |
| Trial name or title | A behaviour‐change intervention to reduce sedentary time in people with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. sham; SETTING PR waiting lists, Royal Prince Alfred Hospital and Prince of Wales Hospital, Sydney (Australia); SAMPLE SIZE n = 70 (based on sedentary time) |
| Participants | Diagnosis of COPD (FER < 0.7); clinically stable (no change in medication within 4 weeks); can mobilise independently with or without a walking aid; expected to wait ≥ 8 weeks for PR |
| Interventions | DURATION OF INTERVENTION 6 weeks INTERVENTION PAC (behaviour change) INTERFACE weekly
GOALS 2 target behaviours
ACTIVITY TRACKING Jawbone UP3 (wrist), all waking hours, real‐time feedback on prolonged bouts of sedentary time, vibrate after 30 minutes of sustained inactivity CONTENT
SHAM No instructions about physical activity or exercise CONTACT weekly phone calls, 15 minutes CONTENT Enquire about change in health status, directed to contact their local doctor if they have any health issues |
| Outcomes | DEVICE activPAL3, Wear instructions: 7 days, 24 hours a day (remove if skin irritation) ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: sedentary time, number of bouts of sedentary time (> 30 minutes), number of sit‐to‐stand transitions SECONDARY OUTCOMES
|
| Starting date | Anticipated to begin February 2017, complete March 2019 |
| Contact information | Zoe McKeough [email protected] University of Sydney (Australia) |
| Notes | FUNDING "This research study is supported by a Physiotherapy Research Foundation Seeding Grant (2016). The principal researcher Sonia Cheng is supported by a Better Breathing Foundation scholarship." CONFLICT OF INTEREST "The authors have no conflicts of interest." |
| Trial name or title | Effect of a PR programme of 8 weeks duration compared to 12 weeks on exercise capacity in people with COPD (PuRe Duration): a randomised controlled trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 68 (based on ESWT) Selection bias: "will be determined using a computer‐generated randomisation program with a minimisation algorithm... completed for each consenting participant by a person independent of the study"; Blinding: outcome assessor, data analysts |
| Participants | Diagnosis of COPD (FEV1 20% to 80% predicted, FER < 0.7); age 40 to 85 years; referred to PR |
| Interventions | COMMON INTERVENTION PR SETTING outpatient, hospital CONTACT 2 sessions a week, 60 to 90 minutes AEROBIC TRAINING
STRENGTH TRAINING upper and lower limbs (3 sets, 8 to 10 repetitions) INTERVENTION PR (12 weeks) INTERVENTION PR (8 weeks) |
| Outcomes | DEVICE Actigraph and ActivPAL, Wear instructions: 1 week ASSESSMENT TIME POINTS
PRIMARY OUTCOME Exercise capacity: ESWT SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; first participant enrolment February 2017 |
| Contact information | Joshua Bishop [email protected] Balmain Hospital, Sydney (Australia) |
| Notes |
| Trial name or title | Does Tai Chi increase cerebral and peripheral oxygenation and do these changes relate to improved cognitive and physical performance in people with COPD? A randomised controlled trial |
| Methods | DESIGN 3 groups; COMPARISONS intervention vs. intervention, intervention vs. no intervention; SAMPLE SIZE n = 66 Selection bias: "sealed opaque envelope"; Blinding: outcome assessors, data analysts |
| Participants | Diagnosis of COPD (GOLD stage ≥ II: FEV1 < 80% predicted; FER < 0.7); age ≥ 55 years |
| Interventions | DURATION OF INTERVENTION 12 weeks COMMON INTERVENTION Exercise and Tai Chi groups SETTING outpatient, group of 4 to 6 participants CONTACT 2 sessions a week, 1 hour TRAINING warm up, exercise, cool down accelerometer (wrist) to record physical activity, exercise log book INTERVENTION Exercise (ergometer) modified Borg Scale (moderate intensity: rating 3 to 4) INTERVENTION Tai Chi (sun style) advised to practice daily at home NO INTERVENTION |
| Outcomes | DEVICE (accelerometer) PRIMARY OUTCOME Exercise capacity: CPET SECONDARY OUTCOMES
|
| Starting date | Prospectively registered February 2017; last data collection September 2018 |
| Contact information | Shirley Ngai [email protected] Hong Kong Polytechnic University (Hong Kong) |
| Notes | FUNDING Research Grants Council, University Grants Committee Secretariat |
| Trial name or title | Randomised controlled trial of a non‐pharmacological integrated care intervention to reduce breathlessness in patients with severe or very severe COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 94 (based on CRQ) Selection bias: "randomisation table created by computer software (i.e. computerised sequence generation)", "sealed opaque envelopes"; Blinding: personnel, outcome assessors |
| Participants | Diagnosis of COPD (GOLD moderate to very severe: FEV1 < 60% predicted; FER < 0.7); age ≥ 18 years; modified MRC dyspnoea scale (score ≥ 2); PR within 1 year or unwilling to attempt PR due to breathlessness |
| Interventions | DURATION OF INTERVENTION eight weeks INTERVENTION range of interventions to address breathlessness
NO INTERVENTION waitlist; basic information about COPD, assessment and correction of inhaler technique, action plan |
| Outcomes | DEVICE (pedometer), Wear instructions: 7 days PRIMARY OUTCOME HRQOL: CRQ mastery domain SECONDARY OUTCOMES
|
| Starting date | Retrospectively registered; first participant enrolment October 2016; registered April 2017; last data collection anticipated June 2021 |
| Contact information | John Wheatley [email protected]; Tracy Smith [email protected] Westmead Hospital, Sydney (Australia) |
| Notes | FUNDING HCF Research Foundation |
| Trial name or title | Effect of cognitive behaviour therapy on anxiety, depression and breathlessness in patients with COPD: a randomised controlled trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 60 Selection bias: "computer‐generated randomisation table", "sealed opaque envelopes"; Blinding: personnel |
| Participants | Diagnosis of COPD (GOLD severe or very severe : FEV1 < 60% predicted; FER < 0.7); age ≥ 18 years; modified MRC dyspnoea scale (score ≥ 2); Completion of the Westmead Hospital Breathlessness Clinic within 9 months; HADS anxiety or depression score > 8 |
| Interventions | DURATION OF INTERVENTION 10 weeks INTERVENTION Cognitive behaviour therapy CONTACT individual weekly sessions, 1 hour, experienced clinical psychologist SETTING
COMPONENTS
NO INTERVENTION waitlist |
| Outcomes | DEVICE (pedometer) Wear instructions: 7 days ASSESSMENT TIME POINTS
PRIMARY OUTCOME HRQOL: CRQ SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; last participant enrolment anticipated September 2021 |
| Contact information | John Wheatley [email protected] Westmead Hospital, Sydney (Australia) |
| Notes |
| Trial name or title | In people with COPD does PR combined with a cognitive behavioural therapy programme for the sensation of breathlessness (BREVE) compared to PR alone improve anxiety and functional exercise capacity at 1, 6 and 12 months? |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SETTING PR at Repatriation General Hospital, Adelaide (Australia); SAMPLE SIZE n = 120 |
| Participants | Diagnosis of COPD (FEV1 < 80% predicted, FER < 0.7); referred and intend to undertake PR |
| Interventions | DURATION OF INTERVENTION 8 weeks COMMON INTERVENTION PR 3 exercise sessions, 1 education session each week INTERVENTION cognitive behavioural therapy with PR INTERVENTION social interaction with PR |
| Outcomes | DEVICE Actigraph GT1X3e ASSESSMENT TIME POINTS
PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; first participant enrolment May 2011; last participant enrolment September 2013 |
| Contact information | Marie Williams [email protected] University of South Australia (Australia) |
| Notes | Physical activity assessed but no results in abstract that can be used for meta‐analysis. Publication pending. |
| Trial name or title | Exacerbations in patients with COPD receiving physical therapy: a cohort‐nested randomised controlled trial (protocol]) Reducing exacerbations in patients with COPD with physiotherapy (DO‐IT COPD) (registry) |
| Methods | DESIGN 2 groups, cohort‐nested; SETTING physical therapy practices, GPs and pulmonologists (southern districts, The Netherlands); SAMPLE SIZE n = 300 (based on exacerbation frequency) |
| Participants | Diagnosis of COPD (GOLD stage II to IV; post‐bronchodilator FEV1 < 80% predicted; FER < 0.7); age > 18 years; AECOPD within 56 days (defined as: unscheduled visit to general practitioner / pulmonologist, hospitalisation, course of antibiotics and/or prednisone); adequate and optimal medication (inhalation) regimen; eligible for reimbursement by health insurance companies for physical therapy (post‐bronchodilator Tiffeneau‐index < 0.6) |
| Interventions | "The project in total will take 5 years. During the first 2 years of the project, COPD patients will be recruited at participating physiotherapy practices. The included patients will be assigned to one of two treatment arms, and followed for 2 years by means of the COPD electronic documentation system, clinical tests and questionnaires. Duration of the treatment in both the intervention and control group will be one year." INTERVENTION "early physical therapy" starting within 56 days of AECOPD DURATION 1 year SETTING outpatient, physiotherapy practices CONTACT 2 sessions a week, one hour AEROBIC TRAINING "high‐intensity"
STRENGTH TRAINING upper and lower limbs
OTHER COMPONENTS
INTERVENTION optional sham No or very low‐intensity exercise training (if the participant insists on training; ≤ 30 minutes once a week, ≤ 15% maximum; modified Borg scale dyspnoea and fatigue (rating ≤ 2) > 30 minutes of moderately‐intense physical activity ≥ 5 days a week |
| Outcomes | DEVICE Dynaport ASSESSMENT TIME POINTS
SECONDARY OUTCOMES
|
| Starting date | Trial registered August 2009; planned start January 2008; planned completion January 2013; protocol published 2014; registry amended March 2016 |
| Contact information | Emmylou Beekman [email protected] Maastricht University (The Netherlands) |
| Notes |
| Trial name or title | Effects of PR prescription on COPD patients |
| Methods | DESIGN 5 groups; COMPARISONS intervention vs. intervention, intervention vs. no intervention; SAMPLE SIZE n = 100 Sequence generation: "a random sequence was generated by a simple random grouping method by a staff member who was not directly involved in the inclusion of the objects"; Allocation concealment: " opaque sealed envelope" |
| Participants | Diagnosis of COPD (GOLD); Age 40 to 80 years; clinically stable ≥ 4 weeks; Have not exercised regularly for ≥ 6 months before the study (≥ twice a week) |
| Interventions | NO INTERVENTION Conventional treatment INTERVENTION Conventional treatment and walking INTERVENTION Conventional treatment and PR INTERVENTION Conventional treatment and upper‐ and lower‐extremities resistance movement INTERVENTION Conventional treatment and upper‐ and lower‐extremities resistance movement + PR |
| Outcomes | PRIMARY OUTCOME Exercise capacity: (not defined) SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; anticipated study completion November 2019 |
| Contact information | Peijun Li [email protected] Shanghai University of Sport; Xiaodan Liu [email protected] Shanghai University of Traditional Chinese Medicine (China) |
| Notes |
| Trial name or title | "Medical Vulnerability": Impact of hospital room cooling on vulnerable patients with lung disease during periods of extreme weather (UCaHS) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 300 Blinding: none |
| Participants | Diagnosis of COPD, asthma, pneumonia, lung fibrosis, or pulmonary hypertension; age ≥ 18 years |
| Interventions | INTERVENTION room with radiant cooling NO INTERVENTION room without radiant cooling |
| Outcomes | SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; first enrolment June 2014; recruitment ongoing (registry) |
| Contact information | Christian Witt [email protected] Arbeitsbereich Ambulante Pneumologie der Charité Universitätsmedizin, Berlin (Germany) |
| Notes | FUNDING Deutsche Forschungsgemeinschaft N.B. will need data only for COPD participants for inclusion |
| Trial name or title | The influence of the maintenance of physical activity on mental health of patients with occupational lung diseases after an inpatient rehabilitation in the BG clinic of Falkenstein |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 194 Blinding: none |
| Participants | Recognised occupational diseases; age 18 to 80 years |
| Interventions | INTERVENTION behaviour‐oriented exercise intervention with inpatient rehabilitation 9 sessions, 45 minutes Influence the participant's attitude towards an active lifestyle with the help of motivational and volitional strategies e.g. action‐planning INTERVENTION inpatient rehabilitation |
| Outcomes | DEVICE ActiGraphGT3x+ TIME POINTS
PRIMARY OUTCOMES Physical activity: (not defined) SECONDARY OUTCOMES
|
| Starting date | Retrospective registration; first enrolment 2016; registered December 2017; recruiting complete, follow‐up continuing |
| Contact information | Petra Wagner petra.wagner@uni‐leipzig.de Universität Leipzig, Sportwissenschaftliche Fakultät, Institut für Gesundheitssport und Public Health (Germany) |
| Notes | FUNDING Deutsche Gesetzliche Unfallversicherung (Germany) N.B. will need data only for COPD participants for inclusion, also to confirm diagnosis |
| Trial name or title | Effect of Erdosteine on inflammatory and oxidative biomarkers in sputum and exhaled breath in patients with COPD |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. placebo; SAMPLE SIZE n = 32 Blinding: double |
| Participants | Diagnosis of COPD (GOLD moderate and severe; FER < 0.7, FEV1 < 15% reversibility after 200 µg salbutamol); smoking history (current or former) > 10 pack‐years; age 38 to 80 years; stable COPD (no chest infection requiring antibiotics or oral steroids or both within 2 months); BMI 19 to 32 kg/m2 |
| Interventions | INTERVENTION Erdosteine (Erdotin) oral capsule PLACEBO |
| Outcomes | DEVICE (pedometer) SECONDARY OUTCOMES
|
| Starting date | Prospective registration; registered November 2007; first enrolment January 2008 |
| Contact information | Imperial College London (UK); no details provided |
| Notes | Unable to identify any publications N.B. will need pre‐cross‐over data for inclusion |
| Trial name or title | Benefits of liquid oxygen in COPD patients without evidence of domiciliary oxygen therapy, presenting desaturation on exertion |
| Methods | DESIGN 2 groups, cross‐over COMPARISON intervention vs, placebo; SAMPLE SIZE n = 112 |
| Participants | Diagnosis of COPD (GesEPOC Stadium II to IV/GOLD III to IV: FEV1 < 80%, FER < 0.7, TLC > 80% predicted); clinical stability for 1 month; optimised medical treatment; do not meet conventional criteria for LTOT (SEPAR Guide, GOLD); SpO2 < 88% on 6MWT; active life |
| Interventions | DURATION OF INTERVENTION 12 weeks INTERVENTION Liquefied medicinal oxygen (Visionox ® device) Concentration number: 1 to 6 PLACEBO |
| Outcomes | DEVICE (accelerometer) ASSESSMENT TIME POINT End intervention: 12 weeks PRIMARY OUTCOME HRQOL: CAT SECONDARY OUTCOMEs
|
| Starting date | Prospective registration; global end of the trial February 2019 "prematurely ended" |
| Contact information | Maria Rosa Güell‐Rous [email protected] Grup ROV de la Societat Catalana de Pneumologia (Spain) |
| Notes | FUNDING Sociedad Española de Neumología y Cirugía Torácica (Spain) N.B. will need pre‐cross‐over data for inclusion |
| Trial name or title | Iron deficiency in patients with COPD: impact of topping with iron carboxymaltose. FACE study; Assessment in patients with ferinject and iron deficiency COPD to improve exercise tolerance |
| Methods | DESIGN 2 groups COMPARISON intervention vs. placebo Blinding: single |
| Participants | Diagnosis of COPD; age 45 to 80 years; clinically stable ≥ 8 weeks (no change in pharmacotherapy treatment since last antibiotics intake or systemic steroids or both for AECOPD) |
| Interventions | INTERVENTION ferric carboxymaltose (Ferinject 50 mg/ml) solution for injection PLACEBO |
| Outcomes | DEVICE SenseWear Pro2, Wear instructions: 7 days ASSSESSMENT TIME POINT 4 weeks PRIMARY OUTCOME Exercise capacity: endurance time (cycle ergometer, 75%) SECONDARY OUTCOMES
|
| Starting date | Prospective registration; first enrolment June 2016; "data collection is anticipated to be completed by the end of 2019" |
| Contact information | Diego Rodriguez Chiaradía [email protected] Consorci Mar Parc de Salut de Barcelona (Spain) |
| Notes |
| Trial name or title | A randomised, placebo‐controlled, blinded, cross‐over, pilot study to explore safety and efficacy of NBMI treatment of patients with mild, moderate and severe COPD |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. placebo; SAMPLE SIZE: n = 12 Blinding: "double blind" |
| Participants | Diagnosis of COPD (GOLD stages I to III; post‐bronchodilator FEV1 > 30% predicted, FER < 0.7); smoking history ≥ 10 pack‐years (ex‐smokers > 6 months); age 45 to 75 years; CAT score > 10; bronchitis with cough and sputum production during many days of the last month, and ≥ 3 months during the last year |
| Interventions | STUDY DURATION 14 days INTERVENTION Emeramide (NBMI) Capsule, oral PLACEBO |
| Outcomes | PRIMARY OUTCOME Adverse events: frequency and severity SECONDARY OUTCOMES
EXPLORATORY OUTCOME Physical activity: (not defined) |
| Starting date | Prospective registration; global end of the trial May 2018 |
| Contact information | Ragnar Klingberg [email protected] NBMI Science AB, Stockholm (Sweden) |
| Notes | FUNDING NBMI Science AB N.B. will need pre‐cross‐over data |
| Trial name or title | Effectiveness of a physical exercise training programme COPD in primary care: a randomised controlled trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. sham; SETTING primary care, Limburg (The Netherlands); SAMPLE SIZE based on 6MWD |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA Physical exercise training programme or rehabilitation therapy in the past year, respiratory tract infections ≤ 8 weeks, serious comorbid conditions which may interfere with exercise BASELINE CHARACTERISTICS
AGE mean 62 (SD 9) years; SEX 27 (59%) male; FEV1 mean 74 (SD 15) % predicted
AGE mean 63 (SD 11) years; SEX 17 (39%) male; FEV1 mean 74 (SD 12) % predicted |
| Interventions | DURATION OF INTERVENTION four months INTERVENTION exercise training SETTING physiotherapy practice CONTACT 2 supervised and 1 unsupervised training sessions a week
AEROBIC TRAINING endurance/interval treadmill training
STRENGTH TRAINING upper and lower limbs (2 to 3 sets, 8 to 12 repetitions)
OTHER COMPONENTS
SHAM "exercise training" SETTING physiotherapy practice CONTACT 1 session a week, 30 minutes TRAINING modified Borg scale dyspnoea and fatigue (rating ≤ 2) OTHER COMPONENTS
|
| Outcomes | DEVICE Dynaport, Wear instructions: 3 days and nights ASSESSMENT TIME POINTS
PRIMARY OUTCOME Exercise capacity: 6MWD (30 metre corridor was not always feasible, minimal corridor length 10 metres) SECONDARY OUTCOMES Physical activity: step count, MVPA time, time walking, time active, movement intensity during walking, PAL
|
| Starting date | Registered 2008, planned closing 2010, protocol published 2014, abstract published 2015 |
| Contact information | Annemieke Fastenau [email protected] Maastricht University (The Netherlands) |
| Notes | FUNDING "The funding of this study is provided by the MUMC MOVE programme of Maastricht University, and an unconditional grant of Boehringer‐Ingelheim, the Netherlands" CONFLICT OF INTEREST "The authors declare that they have no competing interests." email correspondence with author: results are under preparation for publication and advised to use abstract and protocol only pending publication of results |
| Trial name or title | Histological, cellular and molecular investigation of steroid responsiveness in COPD: the HISTORIC study |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 188 Blinding: double |
| Participants | Diagnosis of COPD (GOLD groups B to D); smoking history (current or former) ≥ 10 pack‐years; age ≥ 40 years; no AECOPD or any respiratory infection requiring medical attention or leading to a change in medication within 4 weeks; unchanged respiratory medication regimen within 8 weeks; ≥ 1 AECOPD in previous year |
| Interventions | DURATION OF INTERVENTION 12 months RUN‐IN PERIOD 6 weeks LAMA (aclidinium 400 mcg), LABA (formoterol 12 mcg) and ICS (budesonide 400 mcg), twice daily INTERVENTION ICS, LAMA, LABA (groups A1 and B1) INTERVENTION placbo, LAMA, LABA (groups A2 and B2) |
| Outcomes | ASSESSMENT TIME POINTS
SECONDARY OUTCOME MEASURES
|
| Starting date | Prospective registration; overall trial end April 2021 |
| Contact information | Daiana Stolz [email protected] University Hospital Basel (Switzerland) |
| Notes | FUNDING AstraZeneca |
| Trial name or title | The effectiveness of a physical activity intervention versus PR on cardiovascular risk markers for individuals with COPD: a feasibility study (PARC) |
| Methods | DESIGN: 3 groups; COMPARISON intervention vs. intervention, intervention vs. no intervention; SAMPLE SIZE n = 60 Selection bias: www.sealedenvelope.com |
| Participants | Diagnosis of COPD; age 40 to 85 years |
| Interventions | DURATION OF INTERVENTION 6 weeks INTERVENTION PAC FitBit Charge 2 (heart rate and fitness wristband); tracks activity and exercise; send data to investigators (physical activity level, intensity, time) CONTACT send messages to participant through device FEEDBACK/GOAL SETTING target calculation: mean of the 4 most active days plus 500 steps
INTERVENTION PR SETTING outpatient, group CONTACT 2 sessions a week, 2 hours CONTENT exercise training and education NO INTERVENTION |
| Outcomes | DEVICE Actigraph; Wear instructions: 1 week SECONDARY OUTCOMES
|
| Starting date | Retrospectively registered; registered April 2019; study dates January 2018 to October 2020 |
| Contact information | Sally Singh sally.singh@uhl‐tr.nhs.uk Glenfield Hospital, Leicester (UK) |
| Notes | FUNDING National Centre for Sport and Exercise Medicine, Loughborough University; University Hospital Leicester NHS Trust (UK) |
| Trial name or title | The role of nasal high‐flow to reduce 30‐day hospital readmissions following severe exacerbations of COPD: a mixed‐methods feasibility study: NHF Post‐AECOPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 80 |
| Participants | Emergency hospital admission with a primary diagnosis of AECOPD to St Thomas’ Hospital, London; smoking history ≥ 10 pack‐years; age 40 to 80 years; BMI ≤ 35 kg/m2; home environment safe for visits; live in catchment area |
| Interventions | "followed up for 30 days after hospital discharge" INTERVENTION nasal high‐flow device, daily NO INTERVENTION |
| Outcomes | DEVICE wrist‐worn physical activity monitor; Wear instructions: measured continuously for 30‐day follow‐up period SECONDARY OUTCOMES
|
| Starting date | Retrospectively registered; registered February 2019; study dates September 2018 to August 2021 |
| Contact information | Nicholas Hart [email protected] Rebecca D’Cruz [email protected] Guy’s and St Thomas’ NHS Foundation Trust (UK) |
| Notes | FUNDING National Institute for Health Research (UK) |
| Trial name or title | A self‐management programme of activity coping and education ‐ SPACE for COPD(C) ‐ in primary care: the protocol for a pragmatic trial |
| Methods | DESIGN 2 groups COMPARISON intervention vs. no intervention; SETTING primary care, COPD registers, Respiratory Biomedical Research Unit at University Hospitals of Leicester (UK); SAMPLE SIZE n = 193 (based on CAT) |
| Participants | Diagnosis of COPD (GOLD) |
| Interventions | DURATION OF INTERVENTION 5 months INTERVENTION SPACE for COPD(C) SETTING group‐based, up to 10 participants, community venues, times and locations to suit participants CONTACT 6 sessions, 2 hours CONTENT
NO INTERVENTION "If referred to PR duing study, they will not be denied access to the programme; however, they will not be included in the final analysis due to use of 'intention‐to‐treat' analysis" |
| Outcomes | DEVICE SenseWear Armband [back of right arm], Wear instructions: seven days, 24 hours ASSESSMENT TIME POINTS
PRIMARY OUTCOME HRQOL: CAT SECONDARY OUTCOMES
|
| Starting date | Retrospective registration; start February 2015, end June 2017; preliminary results available October 2018 from trial registry; HRQOL and exercise capacity, not physical activity data |
| Contact information | Sally Singh Sally.Singh@uhl‐tr.nhs.uk University Hospital Leicester NHS Trust (UK) |
| Notes | FUNDING National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care East Midlands (UK) |
| Trial name or title | CELEB trial: Comparative effectiveness of lung volume reduction surgery for emphysema and bronchoscopic lung volume reduction with valve placement: a protocol for a randomised controlled trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SETTING outpatient clinics at hospital sites which have an established multidisciplinary team meeting dedicated to identifying suitable candidates for lung volume reduction (UK); SAMPLE SIZE n = 76 (based on BODE score) Random sequence (low risk): "If the person has a CV negative lobar target for treatment they will be immediately allocated randomly to one of the treatment arms, using an online system. Randomisation will be on a 1:1 basis based on a computer‐generated random sequence with random block sizes generated by Sealed Envelope (London, UK); Allocation concealment (high risk): Patients, the study coordinator and those providing clinical care will not be blinded to treatment allocation; Blinding of participants (high risk): unable to blind participants to intervention; Blinding of personnel (low risk): Investigators and all individuals involved in trial conduct or analysis remained blinded to the randomized treatment until after data lock; Detection bias (low risk): The primary endpoint measures will be performed by staff blinded to treatment allocation and patients will be asked not to reveal this in order to reduce bias." |
| Participants | Diagnosis of COPD (FEV1 < 60% predicted); significant hyperinflation (TLC > 100% predicted, RV > 170% predicted); ex‐smoker > 3 months; Age ≥ 18 years; modified MRC dyspnoea scale (score ≥ 3); CT scan: intact interlobar fissures (> 90%) and heterogeneous emphysema |
| Interventions | INTERVENTION lung volume reduction surgery unilateral video‐assisted thoracoscopic surgery approach intended to remove the most emphysematous area of lung high dependency unit postoperatively, management will include attention to wound discomfort, management of chest drains and prompt mobilisation. INTERVENTION Bronchoscopic lung volume reduction with valve placement bronchoscopy; procedures including valve adjustment and replacement will be permitted to ensure treatment is optimised. |
| Outcomes | DEVICE DynaPort MoveMonitor, Wear instructions: 7 days ASSESSMENT TIME POINTS
PRIMARY OUTCOME Exercise capacity: ISWD SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; opened for recruitment in October 2016; overall trial end March 2020 |
| Contact information | Nicholas Hopkinson [email protected] Imperial College, London (UK) |
| Notes | FUNDING "The study is funded by a grant to Royal Brompton and Harefield NHS Foundation Trust, from the NIHR, through the Research for Patient Benefit scheme (PB‐PG‐1014‐35051). Imperial College, London will support the reporting of this manuscript. Trial sponsor representative: Patrik Pettersson, Royal Brompton and Harefield NHS Foundation Trust (RB&HFT), Royal Brompton Hospital." CONFLICT OF INTEREST "Royal Brompton has received reimbursement of clinical trial expenses from PneumRx, PulmonX, Olympus, Uptake Medical, Holaira and Creo Medical" |
| Trial name or title | ON‐EPIC Oral nitrate supplementation to enhance PR in COPD |
| Methods | DESIGN 2 groups, cross‐over (registry); COMPARISON intervention vs. intervention; SETTING Royal Brompton and Harefield NHS Foundation Trust (UK); SAMPLE SIZE total final enrolment n = 165 Blinding: double |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS (n = 166, 122 completed) AGE mean 68 (SD 10) years; SEX not reported; FEV1 mean 49 (SD 17)% predicted |
| Interventions | COMMON INTERVENTIONS
DURATION 8 weeks CONTACT 2 sessions a week CONTENT supervised, strength and endurance training, home exercise programme
INTERVENTION Nitrate‐rich beetroot juice (containing 12.9 mmol nitrate) with PR INTERVENTION Nitrate‐deplete beetroot juice with PR |
| Outcomes | ASSESSMENT TIME POINTS
PRIMARY OUTCOME Exercise capacity: ISWD SECONDARY OUTCOMES
|
| Starting date | Prospectively registered September 2014; study dates January 2015 to April 2018; intention to publish September 2019 |
| Contact information | Nicholas Hopkinson [email protected] Imperial College, London (UK) |
| Notes | FUNDING The JP Moulton Medical Foundation (UK) N.B. need to confirm: objective assessment of physical activity for inclusion; if cross‐over study, will need pre‐cross‐over data |
| Trial name or title | Training to Improve Dyspnoea (TIDe): a randomised controlled trial to investigate the use of high frequency airway oscillations as training to relieve dyspnoea in COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. sham; SAMPLE SIZE n = 106 (based on CRQ) Selection bias: "web‐based programme (www.sealedenvelope.com)", "randomisation will be allocated by an unblinded assessor and concealed from the patient and the outcome assessor"; Performance bias: "randomisation will be... concealed from the patient" ; Detection bias: "randomisation will be... concealed from the outcome assessor" |
| Participants | Diagnosis of COPD (FER < 0.7); modified MRC dyspnoea scale (score ≥ 2) |
| Interventions | DURATION OF INTERVENTION 8 weeks INTERVENTION Aerosure Medic (HFAO device) battery‐operated, dual‐action device providing oscillations at 25 Hz flow resistance from 0 to 50 cmH2O dependent on the participant’s flow rate inhale and exhale through a mouthpiece deeply for ≥ 5 minutes, 3 times a day SHAM device appears identical, valve is removed (no flow resistance or oscillations) inhale and exhale through a mouthpiece deeply for ≥ 5 minutes, 3 times a day |
| Outcomes | DEVICE ActiGraph GT3X (around the waist, above right hip), Wear instructions: 7 days ASSESSMENT TIME POINTS
PRIMARY OUTCOME HRQOL: CRQ dyspnoea domain SECONDARY OUTCOMES
EXPLORATORY Physical activity: (not defined) |
| Starting date | Retrospectively registered; study dates June 2017 to December 2020 |
| Contact information | Enya Daynes enya.daynes@uhl‐tr.nhs.uk Glenfield Hospital (UK) |
| Notes | FUNDING Actegy Ltd, Bracknell (UK) |
| Trial name or title | Domiciliary application of non‐invasive positive pressure ventilation with average volume assured pressure support to subjects with COPD who remain hypercapnic following the application of non‐invasive positive pressure ventilation (NPPV) for an acute exacerbation: AVAPS‐COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 60 |
| Participants | Diagnosis of COPD (European Respiratory Society/American Thoracic Society); age 50 to 80 years; minimum of 48 hours without NPPV after using NPPV or invasive ventilation in hospital during AECOPD; persistent hypercapnia (PaCO2 ≥ 50 mmHg, < 65 mmHg; pH > 7.32; room air) |
| Interventions | DURATION OF INTERVENTION 1 year INTERVENTION Long‐term domiciliary non‐invasive positive pressure ventilation with average volume assured pressure support ventilatory support function that dynamically determines the pressure support level, which generates the target or control level of exhaled tidal volume by producing a gradual pressure change based on the preceding several breaths NO INTERVENTION |
| Outcomes | DEVICE Actiwatch ASSESSMENT TIME POINTS
SECONDARY OUTCOMES
|
| Starting date | Retrospectively registered; registered December 2008; first enrolment November 2008; trial end November 2010 |
| Contact information | JL Pepin JPepin@chu‐grenoble.fr Laboratoire Exploration Fonctionnelle Cardio‐Respiratoire, Grenoble (France) |
| Notes | FUNDING Respironics International, Inc. (France) email sent 23 August 2019 |
| Trial name or title | Effect of the noninvasive mechanical ventilation on the daily physical activity and the inflammatory biomarkers in stable patients with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 50 Blinding: none |
| Participants | Diagnosis of COPD (post‐bronchodilator FEV1 < 45% predicted, FER < 0.7 for ≥ 6 months); smoking history > 15 pack‐years; age 45 to 75 years; clinically stable ≥ 3 months; pharmacological treatment optimised within 2 years; baseline pH 7.35 to 7.45, PaCO2 > 45 mmHg (room air) |
| Interventions | INTERVENTION nocturnal BiPAP (IPAP 10 to 20 cmH2O, EPAP 4 to 6 cmH2O) NO INTERVENTION |
| Outcomes | PRIMARY OUTCOME Physical activity: (not defined) SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; study start December 2009; estimated study completion June 2021 |
| Contact information | Francisco Garcia‐Rio [email protected] Hospital Universitario La Paz, Madrid (Spain) |
| Notes |
| Trial name or title | Effects of a long‐term physical training programme on pulmonary and systemic aspects in patients with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 82 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD (GOLD); age ≥ 40 years; clinical stability (absence of AECOPD for 3 months); absence of any unstable/severe cardiac, osteoarticular or neuromuscular disorders which could limit physical activities in daily life; no participation in PR within 1 year |
| Interventions | DURATION OF INTERVENTION 6 months INTERVENTION Low‐intensity training 3 sessions a week, 1 hour Calisthenic and breathing exercises; 7 different sets; progressive degrees of difficulty INTERVENTION High‐intensity training 3 sessions a week Aerobic exercises (treadmill and cyclo‐ergometer) ≥ 20 minutes
Resistive exercises upper and lower limbs (3 sets, 8 repetitions)
|
| Outcomes | PRIMARY (up to 4 years) Physical activity: time in activities ≥ moderate intensity SECONDARY (up to 4 years)
|
| Starting date | Retrospectively registered; first posted February 2012; study start November 2009; estimated study completion July 2018 |
| Contact information | Fabio Pitta [email protected] Hospital Universitário Norte do Paraná, Londrina (Brazil) |
| Notes | Sponsor: Universidade Estadual de Londrina |
| Trial name or title | Behavioural medicine intervention to maintain physical capacity and level of physical activity in patients with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 100 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD; age 40 to 80 years; participated in 12 weeks of physical training at Uppsala and Umeå university hospitals |
| Interventions | INTERVENTION PAC personal meeting with the physiotherapist: advice about the value of physical activity, recommendations on how to be physically active. motivational interviewing telephone calls
NO INTERVENTION personal meeting with the physiotherapist: advice about the value of physical activity, recommendations on how to be physically active. |
| Outcomes | PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Actual study start September 2010; estimated study completion December 2020 |
| Contact information | Margareta Emtner [email protected] Uppsala University, Sweden |
| Notes |
| Trial name or title | Effects on exercise capacity, physical activity and quality of life using ambulatory oxygen in patients with COPD who desaturate only during exercise |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 144 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD (post‐bronchodilator FEV1 < 80% predicted, FER < 0.7); non‐smoker (smoke‐free ≥ 6 months); age ≥ 18 years; no AECOPD within 4 weeks; PaO2 > 8 kilopascal at rest; SpO2 ≤ 88%, fall ≥ 4% during 6MWT (room air); SpO2 ≥ 92% during a walk test in self‐selected pace with supplemental oxygen |
| Interventions | DURATION OF INTERVENTION 6 months COMMON INTERVENTION PAC stimulated by a physiotherapist to be more physically active; “a behavioural medicine intervention” INTERVENTION supplemental oxygen during physical activity with PAC INTERVENTION PAC |
| Outcomes | DEVEICE (accelerometer) PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOME Physical activity: “physical activity level” OTHER OUTCOMES HRQOL: EQ5D, SGRQ, CAT |
| Starting date | Retrospective registration; actual study start November 2012; estimated study completion December 2020 |
| Contact information | Margareta Emtner [email protected] Uppsala University (Sweden) |
| Notes | Collaborators: Umeå University, Karolinska Institutet, University of California, Los Angeles |
| Trial name or title | The effect of reflective breathing therapy compared with conventional breathing therapy on dyspnoea, activity and parasympathetic activities in patients with COPD III to IV |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 44 Blinding: none |
| Participants | Diagnosis of COPD (GOLD stage III and IV); age 50 to 75 years; inpatient rehabilitation |
| Interventions | INTERVENTION Conventional breathing therapy, 4 sessions, 30 minutes INTERVENTION Reflectory breathing therapy, 2 sessions, 60 minutes |
| Outcomes | DEVICE Sensewear, Wear instruction: 48 hours post‐intervention SECONDARY OUTCOMES
|
| Starting date | Prospective registration; estimated study completion March 2020 |
| Contact information | Klaus Kennkklenn@schoen‐klinik.de Klinikum Berchtesgadener Land der Schön‐Kliniken (Germany) |
| Notes | N.B. will need pre‐cross‐over data for inclusion |
| Trial name or title | Long‐term Exercise After PR (LEAP): design and rationale of a randomised controlled trial of Tai Chi |
| Methods | DESIGN 3 groups, participants will be enrolled in cohorts; COMPARISON intervention vs. intervention, intervention vs. no intervention; SETTING PR at Beth Israel Deaconess Medical Center, VA Boston Healthcare System, Brigham and Women's Hospital, and Boston Medical Center, as well as referrals from other sites in Massachusetts (USA); SAMPLE SIZE n = 90 (based on 6MWD) |
| Participants | Diagnosis of COPD (FER < 0.7 or CT evidence of emphysema); age > 40 years; Completion of standard PR ≥ 8 weeks duration within 24 weeks |
| Interventions | DURATION OF INTERVENTION 24‐week intervention, 1 year follow‐up INTERVENTION Tai Chi CONTACT total 36 classes
CONTENT 5 formal movements
INTERVENTION Group walking CONTACT total 36 classes
CONTENT Same approximate amount of physical activity (low to moderate aerobic exercise with gentle stretching)
NO INTERVENTION "general recommendations for exercise at home" e.g. walking in the community, local gym, equipment at facility‐based PR programme reommend strength training 3 to 4 sessions a week, 20 minutes Participants are allowed to participate in the maintenance programmes of their usual PR programme |
| Outcomes | DEVICE Omron HJ‐720ITC (pedometer) (waist), Wear instructions: waking hours for 14 days, excluding periods of bathing or other water activities |
| Starting date | Retrospective registration; study start August 2012; first posted December 2013; final data collection for primary outcome measure June 2018 |
| Contact information | Marilyn Moy [email protected] VA Boston Healthcare System (USA) |
| Notes | FUNDING "This study was supported by an award from the National Center for Complementary and Integrative Health, National Institutes of Health (Moy and Yeh, R01AT006358)." CONFLICT OF INTEREST "Peter Wayne is the founder and sole owner of the Tree of Life Tai Chi Center. Peter Wayne's interests were reviewed and are managed by the Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict of interest policy." |
| Trial name or title | The effect of physical activity promotion on short‐ and long‐term outcomes in COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 185 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD (FER < 0.70 or chest CT evidence of emphysema); Age ≥ 40 years; Medical clearance to participate in an exercise programme; Active email account and can check email at least weekly; Access to a computer with Internet connection or willing to come to use study computers; Pedometer with > 90% accuracy compared to manual counts on short clinic walk |
| Interventions | INTERVENTION Pedometer and PAC Website: feedback, goal setting, educational and motivational content and community forum NO INTERVENTION Verbal instructions and written materials about exercise |
| Outcomes | DEVICE (pedometer) PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Prospective registration; estimated study completion February 2020 |
| Contact information | Marilyn Moy [email protected] VA Boston Healthcare System (USA) |
| Notes |
| Trial name or title | BACE Trial ‐ physical activity as a crucial patient‐reported outcome in COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. placebo Blinding: participant, care provider, investigator |
| Participants | Diagnosis of COPD (clinical history OR spirometry); smoking history ≥ 10 pack‐years; age ≥ 18 years; current hospitalisation for potential infectious AECOPD treated with standard therapy; history of at least 1 AECOPD during the last year (prior to the current hospital admission) with systemic steroids and or antibiotics, or both; ECG at admission |
| Interventions | INTERVENTION Azithromycin (oral) n = 250 Day 1 to Day 3: 500 mg azithromycin once a day; Day 4 to Day 90: 250 mg azithromycin once every 2 days PLACEBO (oral) n = 250 Day 1 to Day 3: 500 mg placebo once a day; Day 4 to Day 90: 250 mg placebo once every 2 days |
| Outcomes | DEVICE Dynaport, Wear instructions: 7 days BOTH GROUPS physical activity assessed in subgroups (n = 30 each) Baseline (post‐discharge from hospital), 3 months, 9 months PRIMARY OUTCOME Physical activity: step count SECONDARY OUTCOME Physical activity: step count, time MVPA, time sedentary, time active |
| Starting date | Prospective registration; estimated study completion April 2018 |
| Contact information | Wim Janssens [email protected] KU Leuven (Belgium) |
| Notes | Collaborator: Pro‐Active Medical Pty Ltd |
| Trial name or title | Examine the impact of early chronic disease management education following hospital discharge in acute exacerbation of COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 150 Blinding: none |
| Participants | Diagnosis of COPD; age 50 to 85 years; admitted to the pulmonary ward for AECOPD |
| Interventions | INTERVENTION Early pulmonary education focused education sessions by Certified Respiratory Educator within 2 weeks of discharge NO INTERVENTION general education sessions by Certified Respiratory Educator within 1 month of discharge |
| Outcomes | DEVICE (accelerometer) (wrist or upper arm) ASSESSMENT TIME POINTS
SECONDARY
|
| Starting date | Prospectively registered; estimated study completion December 2019 |
| Contact information | Brandie Walker [email protected] Lisette Machado [email protected] University of Calgary (Canada) |
| Notes |
| Trial name or title | Effect of counselling during PR on self‐determined motivation towards physical activity in people with COPD – protocol of a mixed‐methods study |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SETTING outpatient PR, Canton Hospital Winterthur (Switzerland); SAMPLE SIZE n = 62 Blinding: single |
| Participants | Diagnosis of COPD (GOLD stages B to D); age 40 to 90 years |
| Interventions | COMMON INTERVENTION Pneumofit (PR) DURATION 12 weeks SETTING outpatient CONTACT 3 sessions a week, 90 minutes (36 sessions) AEROBIC TRAINING cycle ergometer or treadmill
STRENGTH TRAINING seated
INTERVENTION PAC with PR CONTACT Week 12 of PR
CONTENT motivational interviewing techniques INTERVENTION PR |
| Outcomes | DEVICE SenseWear Pro (upper right arm wear); Wear instructions: 7 consecutive days (remove for bathing or showering) ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: step count SECONDARY OUTCOMES
|
| Starting date | Prospectively registered May 2015; study start October 2015, final data collection for primary outcome measure January 2020 |
| Contact information | Anne‐Kathrin Rausch‐Osthoff [email protected] Zurich University of Applied Sciences (Switzerland) |
| Notes | FUNDING "This study is funded by the Swiss Lung Association. Funding includes devices (accelerometer) and some of the working hours." CONFLICT OF INTEREST "All authors declare that they have no competing interests." |
| Trial name or title | Short‐course PR and exacerbations and activity of COPD patients over 1 year |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention Blinding: none |
| Participants | INCLUSION CRITERIA
EXCLUSION CRITERIA
BASELINE CHARACTERISTICS (TOTAL n = 136; intervention n = 68, usual care n = 68) AGE mean 75 (SD 7) years; SEX 132 (97%) males; FEV1 mean 47 (SD 16)% predicted |
| Interventions | DURATION OF INTERVENTION 12 months INTERVENTION exercise training SETTING outpatient physiotherapy training CONTACT
NO INTERVENTION |
| Outcomes | DEVICE Actigraph GT3XP ASSESSMENT TIME POINT End intervention: 12 months SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; study start July 2015; study completion November 2018 |
| Contact information | Fanny Ko [email protected] The Chinese University of Hong Kong (Hong Kong) |
| Notes | Need physical activity data "At 12 months, there was no change in activity measured by steps per day between the IG and UG groups" [abstract] |
| Trial name or title | Patient‐centered physical activity coaching in COPD (Walk On!): a study protocol for a pragmatic randomised controlled trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; ; SETTING 14 hospitals, 16 medical services, Kaiser Permanente Southern California (USA); SAMPLE SIZE actual recruitment n = 2702 (composite primary outcome of all‐cause hospitalisations, emergency department visits, observational stays and death) |
| Participants | Any COPD‐related hospitalisation, emergency department visit or observational stay within 12 months; on at least a bronchodilator or steroid inhaler prior to the encounter or if not on an inhaler, previous COPD diagnosis; age > 40 years |
| Interventions | DURATION OF INTERVENTION 12 months INTERVENTION PAC 'Walk On!' Week 0 In‐person individual/group orientation visit
Month 1 ‘intensive’ weekly coaching phone calls
Months 2 to 12
NO INTERVENTION access to all health services e.g. primary, specialty care, PR, health education and lifestyle programmes No instructions to exercise only informed that their medical centre is participating in a study to improve outcomes for members with COPD |
| Outcomes | DEVICES
SECONDARY OUTCOMES
|
| Starting date | Study start June 2015; estimated study completion December 2018 |
| Contact information | Huong Nguyen [email protected] Kaiser Permanente, Pasadena, California (USA) |
| Notes | FUNDING "Funding in part by the Patient‐Centered Outcomes Research Institute, PCORI 1403‐14117, 2015–2018 to Dr. Nguyen" |
| Trial name or title | Home‐based health management of COPD patients |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. no intervention ; SAMPLE SIZE n = 166 Blinding: none |
| Participants | Diagnosis of COPD (GOLD stage II, III or IV); smoking history (current of former) ≥ 10 pack‐years; age ≥ 40 years, hospitalised for AECOPD |
| Interventions | INTERVENTION PAC: activity monitor plus health coaching Actigraph daily during weeks 1, 9, and 17; daily steps and activity will be measured Health coaching NO INTERVENTION |
| Outcomes | DEVICE SenseWear Pro PRIMARY OUTCOMES
|
| Starting date | Prospective registration; actual study completion March 2019 |
| Contact information | Sara Seifert, Minnesota Health Solutions (USA) |
| Notes | Collaborators: National Heart, Lung, and Blood Institute (NHLBI), Mayo Clinic, “Minnesota HealthSolutions Corporation (MHS) proposes to develop and evaluate a program to motivate and monitor people with COPD to complete home exercise as part of PR” N.B. will need pre‐cross‐over data for inclusion |
| Trial name or title | COPD online‐rehabilitation versus conventional COPD rehabilitation – rationale and design for a multicenter randomised controlled trial study protocol (CORe trial) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SETTING Respiratory and Physiotherapy Departments of 8 hospitals in the capital region of Denmark: Amager, Hvidovre, Bispebjerg, Frederiksberg, Herlev, Gentofte, Frederikssund and Hillerød University Hospitals, University of Copenhagen (Denmark); SAMPLE SIZE n = 134 (based on 6MWD) Blinding: assessor and data analyst blinded |
| Participants | Diagnosis of COPD (severe or very severe; FEV1 < 50% predicted ; FER < 0.7); modified MRC dyspnoea scale (score ≥ ) |
| Interventions | COMMON COMPNENT life with COPD, participants topics, medication, daily activity, nutrition, smoking cessation, respiratory and relaxation exercises INTERVENTION online rehabilitation DURATION 10 weeks SETTING home‐based, web‐cam, group of 4 to 8 participants CONTACT 3 sessions a week, 1 hour EXERCISE TRAINING
EDUCATION 25 minutes INTERVENTION centre‐based rehabilitation DURATION 8 to 12 weeks SETTING outpatient, group CONTACT 2 sessions a week, 1 to 2 hours EXERCISE TRAINING 1 hour
EDUCATION 1 session a week, 90 minutes |
| Outcomes | DEVICE ActivPAL
ASSESSMENT TIME POINTS
PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Study start March 2016, scheduled to continue until December 2017 |
| Contact information | Henrik Hansen [email protected]; [email protected] University of Copenhagen, Copenhagen (Denmark) |
| Notes | FUNDING "This research project received specific grants from the Danish Lung Foundation (Charitable funding), Telemedical Center Regional Capital Copenhagen (governmental funding), TrygFonden foundation (Charitable funding). The Grants covers expenses conducting the trial, salary for project employed, and University fee for the PhD education for Henrik Hansen." CONFLICT OF INTEREST "The authors declare that they have no competing interests." |
| Trial name or title | Use of the SMART COPD physical activity app in PR: a randomised feasibility study |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 30 Blinding: none |
| Participants | Diagnosis of COPD; age ≥ 18 years; attending PR in Sheffield, Rotherham or Doncaster |
| Interventions | INTERVENTION PAC (app) with PR and pedometer During PR: app and pedometer Post‐PR: app and pedometer
App and pedometer: set physical activity goals, monitor progress, provide feedback INTERVENTION PR and pedometer During PR: blinded pedometer Post‐PR (8 weeks): blinded pedometer |
| Outcomes | DEVICE Fitbit® Charge pedometer SECONDARY OUTCOMES
|
| Starting date | First posted February 2016; study start January 2016; actual study completion September 2018 |
| Contact information | Mark Hawley [email protected] University of Sheffield (UK) |
| Notes | Sponsor: Sheffield Teaching Hospitals NHS Foundation Trust Collaborators: University of Sheffield, National Institute for Health Research: CLAHRC YH, The Rotherham NHS Foundation Trust, Doncaster And Bassetlaw Hospitals NHS Foundation Trust |
| Trial name or title | Sustaining Training Effects through Physical activity (STEP): enhancing physical activity to achieve sustainable benefits in extrapulmonary consequences of COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 84 Blinding: none |
| Participants | Diagnosis of COPD (internationally‐accepted guidelines); smoking history (current or former) ≥ 10 pack‐years; age 40 to 80 years; completed 3 months of outpatient PR (Gasthuisberg University Hospital, Leuven, Belgium); ability to manage electronic devices (smartphone, step counter) |
| Interventions | DURATION OF INTERVENTION 6 months INTERVENTION PAC (tele‐coaching) with PR feasible goal‐setting and feedback to enhance participant's motivation and commitment Pedometer‐based goals and tele‐coaching support INTERVENTION PR general advices regarding physical activity |
| Outcomes | ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: step count SECONDARY OUTCOMES
|
| Starting date | Retrospective registration; estimated study completion June 2018 |
| Contact information | Thierry Troosters [email protected] KU Leuven (Belgium) |
| Notes | Collaborator: Conselho Nacional de Desenvolvimento Científico e Tecnológico |
| Trial name or title | The role of ambulatory oxygen in improving the effectiveness of PR for COPD patients |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 20 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD; fulfil clinical criteria for PR; exercise‐induced desaturation (fall in SaO2 ≥ 4% to ≤ 90%, or any fall < 90%) and demonstrate improvement with ambulatory oxygen |
| Interventions | INTERVENTION ambulatory oxygen with PR INTERVENTION placebo with PR |
| Outcomes | DEVICE (pedometer )ASSESSMENT TIME POINTS Baseline, 6 weeks, 14 weeks, 18 weeks PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Prospective registration; actual study completion December 2017 |
| Contact information | Vijayaragavan Padmanaban, Imperial College Healthcare NHS Trust (UK) |
| Notes | Collaborator: National Institute for Health Research (UK) |
| Trial name or title | Breathlessness, Exertion And Morphine Sulfate (BEAMS) study |
| Methods | DESIGN "A five stage, national, multi‐site, double‐blind, parallel arm, block randomised, placebo controlled, factorial, dose increment phase III study"; COMPARISON intervention vs. placebo; SETTING 19 centres (Australia); SAMPLE SIZE n = 171 PROTOCOL "The BEAMS study will be promoted to patients with COPD that interferes with ADLs through LungNet (Lung Foundation Australia) and the Primary Health Networks in each recruitment catchment area. Potentially eligible participants will be identified and approached by both primary and secondary care clinicians at participating sites across Australia who will then refer them to the research team. Research team attendance at relevant clinics and study advertisements will help to remind clinical staff of study recruitment and encourage patients to self‐refer. Permission will be sought from consultants in charge of the care of potential participants for research staff to approach them directly. Case identification in both inpatient units and outpatient clinics will also occur following case‐note review." Selection bias: "Randomisation will occur through the development of randomisation tables using random number tables generated by an independent provider", "Randomisation requests will take the form of receipt of a prescription for study medicines by site pharmacists. Site pharmacists will receive the next randomisation number available through telephone contact with the central registry"; Performance bias: "All research staff, treating clinicians and patients will remain blinded to the treatment allocation" |
| Participants | Diagnosis of COPD (post‐bronchodilator FER < 0.7); age ≥ 18 years; modified MRC dyspnoea scale (score 3 or 4); atable medications over prior week; respiratory physician‐confirmed optimisation of treatment; worst breathlessness intensity in the previous 24 hours was at least 3/10 |
| Interventions | COMMON COMPONENTS Written advice detailing standard therapeutic strategies for managing breathlessness Battery‐operated, hand‐held fan and instructions for use throughout the study period as standard breathlessness management strategies INTERVENTION sustained‐release morphine sulfate 1 double‐blind capsule in the morning (8 mg, 16 mg, 24 mg or 32 mg) laxative (Docusate with senna) PLACEBO 1 double‐blind capsule in the morning, placebo laxative optional |
| Outcomes | DEVICE FitBit Charge HR ASSESSMENT TIME POINTS "weeks one and three of the study" OUTCOME MEASURES Physical activity: steps per day, "activity" SAFETY "Participant safety will remain of paramount importance throughout the study period. Rescue medication will therefore be available for participants for treatment of common opioid side effects including nausea and constipation. Opioid toxicity is defined by physician assessment of respiratory depression (≤10 breaths per minute), drowsiness, myoclonus, myosis or National Cancer Institute Common Terminology Criteria for Adverse Events version 4 (NCI CTCAEv4) grade ≥3 for cognitive impairment, confusion or somnolence. Signs suggestive of opioid toxicity will result in urgent physician assessment, investigation of contributing factors and treated with either opioid dose reduction or naloxone according to the severity of the toxicity and degree of respiratory compromise. Reasons for cessation of study drug or withdrawal from the study include treatment failure as defined by unacceptable side effects of NCI CTCAEv4 grade 3 that do not settle with symptomatic intervention or grade 4 or 5 harms. Participants may also be withdrawn if treatment is deemed ineffective by treating clinician, increasing breathlessness scores despite study treatment or withdrawal of participant consent." |
| Starting date | Study start August 2016, estimated primary completion June 2019 |
| Contact information | David Currow [email protected] Flinders University, Adelaide (Australia) |
| Notes | FUNDING "This study was funded by the National Health and Medical Research Council, Australia (Grant Number APP1065571) and sponsored by Flinders University, Adelaide, Australia. The funders and study sponsors had no role in the study design and will have no role in the data collection, analysis or dissemination of study results." CONFLICT OF INTEREST "DCC has received an unrestricted research grant from Mundipharma, is an unpaid member of an advisory board for Helsinn Pharmaceuticals and has consulted Mayne Pharma and received intellectual property payments from them. MJJ has received consulting payments from Mayne Pharma." |
| Trial name or title | Hospital‐level care at home for acutely ill adults: a pilot randomised controlled trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SETTING emergency departments, academic medical centre and community hospital (USA); SAMPLE SIZE "We had limited funding and could only continue our pilot for at most 2.25 months. Thus, irrespective of enrollment, we a priori planned to stop the pilot when funds were depleted." Selection bias: "Participants were randomized to usual care admission or home hospital admission by research study staff. Randomization was stratified by condition with randomly selected block sizes between 4 and 6", "allocation concealment via sealed envelopes"; Performance/detection bias: "Given the nature of the study, blinding of patients, study staff, and physicians was not possible" |
| Participants | Primary or possible diagnosis of any infection, heart failure exacerbation; AECOPD or asthma exacerbation; age ≥ 18 years; resides within 5‐mile radius; caregiver to stay for first 24 hours of admission; primary diagnosis of cellulitis, heart failure, complicated urinary tract infection, or pneumonia that requires inpatient admission |
| Interventions | COMMON COMPONENTS minimum 1 daily visit from an attending general internist 2 daily visits from a home health registered nurse additional visits as needed Also tailored to participant need: medical meals, home health aide, social worker, physical therapist, occupational therapist All participants had continuous monitoring of heart rate, respiratory rate, telemetry, movement, falls, and sleep by a small skin patch (physIQ, Chicago, IL; VitalConnect, San Jose, CA). Monitoring was performed through machine‐based algorithms, and clinical staff reviewed alarms INTERVENTION "Home hospital" could provide oxygen therapy, respiratory therapies, intravenous medications via infusion pump, in‐home radiology, and point‐of‐care blood diagnostics Participants communicated with their home hospital team by telephone, encrypted video, and encrypted short message service physician available 24 hours a day NO INTERVENTION |
| Outcomes | DEVICE VitalConnect VitalPatch ASSESSMENT TIME POINTS
SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; study start September 2016 |
| Contact information | David Levine [email protected] Brigham and Women's Hospital, Boston (USA) |
| Notes | FUNDING "Partners HealthCare Population Health Management provided funding support for the home hospital clinical program. Dr. Levine received funding support from an Institutional National Research Service Award from (T32HP10251) and the Ryoichi Sasakawa Fellowship Fund. The NIH had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript." CONFLICT OF INTEREST "The authors declare that they do not have a conflict of interest." Author correspondence: "Please note that this was the pilot study of ˜20 patients. There are too few patients with COPD to make cuts in the data, sadly. Please note our larger study will be published soon." |
| Trial name or title | The role of activity monitors in improving physical activity in COPD patients participating in PR |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 20 Blinding: none |
| Participants | Diagnosis of COPD (FER < 0.7); optimised pharmacology intervention; referred for PR |
| Interventions | DURATION OF INTERVENTION 7 weeks INTERVENTION PAC with PR wear activity monitor: weekly feedback: amount of activity, steps taken, active minutes each day, wear time tailored advice on how to improve activity levels INTERVENTION PR wear activity monitor, no feedback |
| Outcomes | DEVICE "data recorded on the device, throughout study, up to seven weeks" ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
|
| Starting date | First posted September 2016; estimated study completion August 2017 |
| Contact information | Patricia Kelly [email protected] Glenn Cardwell [email protected] Natalie Garratt [email protected] Salford Royal NHS Foundation Trust, Greater Manchester (UK) |
| Notes |
| Trial name or title | The CaNadian Standardized Pulmonary Rehabilitation Efficacy Trial (CoNSPiRE) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 200 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD (post‐bronchodilator FER < 0.7); enrolled in PR within affiliated sites or satellite tele‐health programme; as part of standard rehabilitation referral procedures; ambulatory, no unstable cardiovascular disease |
| Interventions | COMMON INTERVENTION PR DURATION 6 or 8 weeks SETTING centre‐based or tele‐rehabilitation CONTACT 2 days a week CONTENT 2 hours of exercise training (aerobic and resistance), 1 hour of education designed to promote self‐management INTERVENTION traditional educational approach with PR CONTACT
CONTENT exercise, anatomy, pulmonary diseases, healthier breathing, pulmonary medications, pulmonary devices, exercise action plan, allergies and pulmonary function tests, health and air quality, healthier eating, travel, stress management INTERVENTION new Canadian standardised educational approach with PR CONTACT
CONTENT exercise, living well with chronic lung disease, breathing management, conserving energy, pulmonary medications, inhaler devices, integrating exercise in your life, management of respiratory infections, management of aggravating environmental factors, management of stress and anxiety, nutrition, leisure and travel, getting a good night's sleep, enjoying intimacy, living in a smoke‐free environment, integrating long‐term oxygen into your life, keeping a healthy lifestyle |
| Outcomes | DEVICE Fitbit ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: step count SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; first posted September 2016; actual study start January 2017; estimated study completion August 2019 |
| Contact information | Anne‐Marie Selzler [email protected]; Michael K Stickland [email protected] University of Alberta (Canada) |
| Notes | Collaborators: McGill University, University of Toronto, Université de Sherbrooke |
| Trial name or title | Long‐term effect of a health education programme on daily physical activity in patients with moderate‐to‐very severe COPD (EA‐EPOC) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 128 Blinding: none |
| Participants | Diagnosis of moderate‐to‐very severe COPD (GesEPOC criteria: FEV1 < 80% predicted, ≥ 3 months); smoking history (current or former) > 10 pack‐years; age > 35 years; hospital admission for AECOPD |
| Interventions | INTERVENTION health education 2 nursing health education sessions, 15 and 30 days after discharge
NO INTERVENTION Treatment and follow‐up according to conventional clinical practice including recommendations on healthy habits and lifestyle |
| Outcomes | PRIMARY OUTCOME Physical activity: “physical activity level” SECONDARY OUTCOMES
|
| Starting date | Prospective registration; estimated study completion March 2020 |
| Contact information | Francisco Garcia‐Rio [email protected] Hospital Universitario La Paz, Madrid (Spain) |
| Notes |
| Trial name or title | Assessing the effect of different efficiency indoor air filters on respiratory symptoms in former smokers |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. sham; SAMPLE SIZE n = 52 |
| Participants | Smoking history; does not currently smoke and no‐one currently smokes inside the home; history of AECOPD or development of respiratory symptoms with periods of high outdoor air pollution; age ≥ 40 years; access to Wi‐Fi, cell phone, tablet or personal computer |
| Interventions | INTERVENTION portable HEPA air filtering device with MERV17 air filter SHAM portable air filtering device with basic carbon filter |
| Outcomes | ASSESSMENT TIME POINTS Baseline, 12 weeks, 3 months, 6 months PRIMARY OUTCOME HRQOL: SGRQ SECONDARY/OTHER OUTCOMES
|
| Starting date | Prospective registration; actual study completion June 2019 |
| Contact information | Denitza Blagev, Intermountain Medical Center, Salt Lake City, Utah (USA) |
| Notes | N.B. will need to confirm specific diagnosis of COPD for inclusion, also will need pre‐cross‐over data |
| Trial name or title | Pedometer‐based behavioural intervention for individuals with COPD to Stay Active After Rehabilitation (STAR) |
| Methods | DESIGN 2 groups COMPARISON intervention vs. placebo; SETTING PR clinic Bad Reichenhall (Germany); SAMPLE SIZE n = 502 |
| Participants | Primary reason for referral to PR: diagnosis of COPD (classifications A – D; post‐bronchodilator FER < 0.7); age ≥ 18 years |
| Interventions | COMMON INTERVENTION DURATION 3 weeks, extension 1 to 2 weeks if required (centre average 25 days) SETTING inpatient PR AEROBIC TRAINING 4 to 5 sessions, 45 minutes STRENGTH TRAINING 3 sessions, 45 minutes OTHER COMPONENTS 7 sessions whole‐body vibration muscle training EDUCATION Structured COPD patient education
Intervention components addressing the promotion of physical activity INTERVENTION PAC: pedometer‐based behaviour change intervention SETTING open groups, 6 to 12 participants CONTACT 2 sessions, 45 minutes
CONTENT
PLACEBO “behavior placebo” "Playful" physical activity and revisions of information on physical activity (knowledge of exercise recommendations, knowledge of possibilities of self‐regulation of endurance‐training exercise intensity) as in standard education Booklet: looks identical to intervention group diary, contains a repetition of physical activity‐related information as for standard education |
| Outcomes | DEVICE Actigraph wGT3X‐BT
Sedentary < 100 counts, MVPA > 1952 counts a minute ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: time sedentary (< 100 cpm), MVPA time (> 1952 cpm) |
| Starting date | "recruitment starts in June 2016 and is likely to be finished in September 2017" |
| Contact information | Wolfgang Geidl [email protected] Friedrich‐Alexander University, Erlangen (Germany) |
| Notes | FUNDING "This study is funded by the German Pension Insurance Association, Section Bavaria South (Deutsche Rentenversicherung Bayern Süd; Abteilung Rehabilitation und Sozialmedizin, Am Alten Viehmarkt 2, 84028 Landshut, Germany) (Reference number: 5.011‐6.031.115) (www.deutscherentenversicherung‐ bayernsued.de) which was not involved in the design of the study and collection, management, analysis, interpretation of data, in writing the manuscript, or the decision to submit the report for publication." CONFLICT OF INTEREST "The authors declare that they have no competing interests." |
| Trial name or title | Development and feasibility of a home PR program with health coaching |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 120 |
| Participants | Diagnosis of COPD (GOLD stage II to IV; "as documented by PFT"); smoking history (current or former) ≥10 pack‐years; age ≥ 40 years; eligible for PR |
| Interventions | DURATION OF INTERVENTION 8 weeks INTERVENTION home‐based PR with health coaching SETTING home INTERFACE
ACTIVITY
MONITORING
NO INTERVENTION |
| Outcomes | DEVICE Actigraph ASSESSMENT TIME POINTS
OUTCOME MEASURES
|
| Starting date | Estimated study completion June 2019 |
| Contact information | Roberto Benzo [email protected] Mayo Clinic, Minnesota (USA) |
| Notes | FUNDING "Dr Kramer and Ms Seifert are affiliated with the Minnesota Health Solutions Corporation, Saint Paul, Minnesota. Dr Benzo, Ms Hoult, Ms Seifert, and Dr Kramer are supported by National Institutes of Health Grant SBIRHL 114162‐2 (to Ms Seifert [PI] and Dr Benzo [clinical trial PI]). Ms Seifert has disclosed a relationship with NovuHealth." CONFLICT OF INTEREST "The other authors have disclosed no conflicts of interest" |
| Trial name or title | The feasibility of working memory training in COPD patients and the efficacy on cognitive performance, self‐control and stress response |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 75 Blinding: participant, care provider, investigator, outcomes assessor |
| Participants | Diagnosis of COPD (GOLD); age ≥ 18 years; motivated as evaluated by the self‐determination questionnaire |
| Interventions | COMMON COMPONENTS
INTERVENTION working memory training and healthy lifestyle coaching domain‐general cognitive working memory training programme training increases with difficulty if participants answer 2 subsequent questions correctly INTERVENTION sham working memory training and healthy lifestyle coaching training that does not increase in difficulty |
| Outcomes | DEVICE (accelerometer )PRIMARY OUTCOMES Adherence: training SECONDARY OUTCOMES
|
| Starting date | Prospective registration; estimated study completion July 2019 |
| Contact information | Sarah Mount [email protected]; Martijn van Beers [email protected] Maastricht University Medical Center, Limburg (The Netherlands) |
| Notes | Collaborator: Eatwell |
| Trial name or title | Impact of inspiratory muscle training on daily physical activity (INAF) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. sham; SAMPLE SIZE n = 20 Blinding: participant |
| Participants | Diagnosis of COPD; age 45 to 80 years; clinically stable ≥ 4 weeks; inspiratory muscle weakness (PImax < 70%); pulmonary hyperinflation (TLC > 120%) |
| Interventions | DURATION OF INTERVENTION 5 weeks INTERVENTION inspiratory muscle training OXYGEN DUAL Inspiratory valve with increase resistance INTERVENTION sham inspiratory muscle training Inspiratory valve without resistance |
| Outcomes | DEVICE (accelerometer) ASSESSMENT TIME POINTS
PRIMARY OUTCOME
|
| Starting date | Retrospective registration; estimated study completion March 2018 |
| Contact information | Diego Agustin Rodriguez [email protected] Parc de Salut Mar, Hospital Del Mar (Spain) |
| Notes |
| Trial name or title | Efficacy of a coaching programme to promote physical activity and reduce sedentary behaviour after a COPD exacerbation that require hospitalisation |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 66 Blinding: participant, investigator, outcomes assessor |
| Participants | Diagnosis of COPD (post‐bronchodilator FER < 0.7); smoking history > 10 pack‐years; age > 40 years; hospitalisation due to AECOPD |
| Interventions | DURATION OF INTERVENTION 12 weeks INTERVENTION PAC individualised physical activity and sedentary behaviour coaching motivational interview; usual exercise habits, possible barriers and facilitators, self‐efficacy and motivation progressive program with specific goals‐setting and self‐monitoring NO INTERVENTION |
| Outcomes | DEVICE (accelerometer) ASSESSMENT TIME POINTS
PRIMARY OUTCOME
SECONDARY OUTCOMES
|
| Starting date | First posted March 2017; estimated study start March 2017; estimated study completion December 2018 |
| Contact information | Maria A Ramon [email protected]; Esther Rodriguez [email protected] Hospital Universitari Vall d'Hebron Research Institute, Barcelona (Spain) |
| Notes | Collaborator: Spanish Society of Pneumology and Thoracic Surgery |
| Trial name or title | Long‐term effects of a 3‐month pedometer‐based programme to enhance physical activity in patients with severe COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 74 Blinding: none |
| Participants | Diagnosis of COPD (GOLD severe; FEV1 < 50%); age ≥ 40 years |
| Interventions | DURATION OF INTERVENTION 12 months INTERVENTION Pedometer and PAC 3 months coaching followed by 9 months (keep pedometer, encouraged to sustain an increased level of daily physical activity) “encouraged to be more active” Daily step count goal: 15% increase compared to baseline Monthly telephone calls: encourage compliance, motivate NO INTERVENTION |
| Outcomes | DEVICE SenseWear Pro (upper left arm); Wear instructions: 7 consecutive days ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: step count SECONDARY OUTCOME HRQOL: CAT, SF36 |
| Starting date | Prospectively registered; actual study start May 2017; estimated study completion August 2019 |
| Contact information | Christian Clarenbach [email protected]; Noriane Sievi [email protected] University of Zurich (Switzerland) |
| Notes |
| Trial name or title | Effects of upper‐limb endurance exercise training addition to a conventional high‐intensity exercise training programme on physical activity level and activities of daily living performance in patients with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 64 Blinding: none |
| Participants | Diagnosis of COPD (GOLD); age 40 to 95 years; absence of AECOPD in the previous month (clinical stability); absence of severe or non‐stable cardiac disease; no exercise training programme within 1 year |
| Interventions | DURATION OF INTERVENTION 3 months INTERVENTION High‐intensity endurance exercise of lower limb strengthening exercise of upper and lower limb INTERVENTION High‐intensity endurance exercise of upper and lower limb strengthening exercise of upper and lower limb |
| Outcomes | PRIMARY OUTCOME Physical activity: sedentary time (< 1.5 METs), active time (> 3 METs) SECONDARY OUTCOMES
|
| Starting date | Retrospective registration; first posted April 2017; actual study start January 2017; estimated study completion January 2020 |
| Contact information | Fabio Pitta [email protected] Universidade Estadual de Londrina (Brazil) |
| Notes | Collaborator: National Council for Scientific and Technological Development (Brazil) |
| Trial name or title | Active for life: COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. sham; SAMPLE SIZE n = 183 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD; Age ≥ 50 years; no AECOPD or major illnesses requiring hospitalisation within 8 weeks; no history of other major lung diseases as primary pulmonary problem, history of a recent heart attack or recent onset of chest pains with activity or increasing episodes of chest pain; no other health or mobility problems that limit physical activity; sedentary (< 30 minutes of moderate activity, 3 days a week) |
| Interventions | DURATION OF INTERVENTION
INTERVENTION "Active Life" focus on increasing light physical activity; encouraged to increase total PA by ≥ 60 minutes a day
SHAM chair exercises, behavioural relaxation and health education
|
| Outcomes | DEVICE ActivPal and Actigraph; Wear instructions: 1 week TIME POINTS
PRIMARY OUTCOME Physical activity: time in physical activity, time sedentary (ActivPal), time in light‐intensity physical activity, MVPA time (Actigraph) SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; actual study start July 2017; estimated study completion February 2022 |
| Contact information | She'Lon Tucker [email protected]; Ron Dechert [email protected], Janet Larson, School of Nursing, University of Michigan (USA) |
| Notes |
| Trial name or title | The effect of twice‐daily aclidinium bromide/formoterol fumarate 340/12 mcg vs. once‐daily tiotropium 'respimat' 5 mcg on static and dynamic hyperinflation in patients with COPD during 24 hours |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 49 Blinding: none |
| Participants | Diagnosis of COPD (moderate‐to‐very severe > 12 months before screening; post‐bronchodilator FEV1 < 80% predicted, FER < 0.7); smoking history (current or former) ≥ 10 pack‐years; age ≥ 40 years; modified MRC dyspnoea scale (score at least 2); entering PR at CIRO; severe static hyperinflation (RV > 150% predicted); co‐operative attitude and ability to use correctly the inhalers |
| Interventions | DURATION OF INTERVENTION 4 days INTERVENTION LAMA/LABA (Aclidinium Bromide/Formoterol Fumarate 340/12 mcg) twice daily INTERVENTION LAMA (Tiotropium 'Respimat' 5 mcg) once daily |
| Outcomes | DEVICE (accelerometer); Wear instructions: 4 days SECONDARY OUTCOME Physical activity: “Nighttime physical activity” (inverse surrogate for sleep quality) |
| Starting date | Restrospective registration; first posted September 2017; actual study start July 2017, estimated study completion November 2018 |
| Contact information | Lowie Vanfleteren lowievanfleteren@ciro‐horn.nl; Maud Koopman maudkoopman@ciro‐horn.nl CIRO, Horn (The Netherlands) |
| Notes | Sponsor: Maastricht University Medical Center Collaborators: AstraZeneca N.B. will need pre‐cross‐over data for inclusion, also clarification of physical activity |
| Trial name or title | The effects of singing training for patients with COPD |
| Methods | DESIGN 2 groups, cluster‐randomised; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 220 Blinding: investigator, outcomes assessor |
| Participants | Diagnosis of COPD; age ≥ 18 years; modified MRC scale (score at least 2); recommended for PR; sufficient mobility to attend |
| Interventions | DURATION OF INTERVENTION 10 weeks COMMON INTERVENTION PR SETTING outpatient, group CONTACT 2 sessions a week, 90 minutes (total 20 sessions) CONTENT supervised warm‐ups, aerobic and strength training, breathing exercises INTERVENTION singing training with PR technical instruction in order to achieve better respiratory control and primary muscular strength focus on techniques for efficient expiration focus on musical content and interpretation as well as interaction, the social aspects and joy of singing together. INTERVENTION PR |
| Outcomes | DEVICE (pedometer); Wear instructions: 1 week PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Retrospective registration; actual study start August 2017; first posted September 2017; estimated study completion November 2018 |
| Contact information | Mette Kaasgaard [email protected]; Uffe Bodtger [email protected] University of Aarhus (Denmark) |
| Notes |
| Trial name or title | Social incentives to increase mobility among hospitalised patients: the MOVE IT randomised trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 227 Blinding: investigator, outcomes assessor |
| Participants | Pneumonia, diabetes, congestive heart failure, COPD; age ≥ 18 years; admitted to medicine or oncology floor in the hospital |
| Interventions | DURATION OF INTERVENTION 12 weeks post‐discharge INTERVENTION pedometer with "social incentives" daily step counts Week 2 to Week 13 after hospital discharge weekly step goal: increases from baseline by 10% each week social incentive‐based gamification based on points and levels daily feedback for the step counts, weekly feedback for levels support partner will receive weekly reports INTERVENTION pedometer daily step counts Week 2 to Week 13 after hospital discharge |
| Outcomes | DEVICE pedometer (Nokia Steel) PRIMARY OUTCOME Physical activity: step count |
| Starting date | Prospectively registered; study start January 2018; estimated study completion October 2019 |
| Contact information | Ryan Greysen, Mitesh Patel, University of Pennsylvania (USA) |
| Notes | N.B. will need data only for participants with COPD |
| Trial name or title | A randomised, double‐blind (sponsor unblind), placebo‐controlled, multi‐centre phase IIa study to evaluate the safety and efficacy of 13 weeks of once daily oral dosing of the selective androgen receptor modulator (SARM) GSK2881078 in older men and post‐menopausal women with COPD and muscle weakness, participating in home exercise |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. placebo; SAMPLE SIZE n = 100 Blinding: participant, investigator |
| Participants | Diagnosis of COPD (American Thoracic Society/European Respiratory Society criteria: post‐bronchodilator FEV1 30% to 65% predicted, FER < 0.7); smoking history (current or former) ≥ 10 pack‐years; age 50 to 75 years; BMI 18 to 32 kg/m2; able to read and write in the language used for the diary and able to operate electronic device; if participating in a structured exercise programme must be willing to convert their current exercise programme to the study programme |
| Interventions | INTERVENTION GSK2881078 Cohort 1 Males 2 mg capsules, once daily, oral administration PLACEBO Cohort 1 men 2 capsules of placebo once daily, oral administration INTERVENTION GSK2881078 Cohort 2 women 1 mg capsule, once daily, oral administration PLACEBO Cohort 2 women 2 capsules of placebo once daily, oral administration |
| Outcomes | DEVICE (accelerometer); Wear instructions: 1 week PRIMARY OUTCOME Adverse events and serious adverse events SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; study start February 2018; estimated study completion November 2019 |
| Contact information | |
| Notes | Sponsor: GlaxoSmithKline Collaborator: Parexel (USA, Germany, UK) |
| Trial name or title | Portable oxygen concentrator improvements to physical activity, oxygen usage, and quality of life in COPD patients using LTOT (POC‐STEP) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 190 Blinding: none |
| Participants | Diagnosis of COPD; age ≥ 40 years; qualifies for continuous LTOT, prescribed oxygen at ≤ 5 litres a minute; has not used a portable oxygen concentrator prior to enrolling in this study; able to tolerate pulsed oxygen therapy |
| Interventions | INTERVENTION portable oxygen concentrator with LTOT INTERVENTION LTOT |
| Outcomes | DEVICE "Actigraphy" PRIMARY OUTCOME Physical activity: (not defined) SECONDARY OUTCOME HRQOL: SGRQ |
| Starting date | Prospectively registered; study start July 2018; estimated study completion February 2021 |
| Contact information | Cindy Wen [email protected] ResMed (USA) |
| Notes | Collaborator: Inogen, Inc (USA) |
| Trial name or title | Non‐invasive ventilation and nocturnal alveolar hypoventilation in patients with COPD treated by long‐term oxygen therapy at home |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 38 Blinding: none |
| Participants | Diagnosis of COPD; age 18 to 85 years; LTOT |
| Interventions | INTERVENTION non‐invasive ventilation (respiratory assistance by a facial mask without intubation or tracheotomy) with LTOT INTERVENTION LTOT |
| Outcomes | SECONDARY OUTCOMES
|
| Starting date | Actual study start June 2018; first posted July 2018; estimated study completion April 2020 |
| Contact information | Jean‐Louis Pépin JPepin@chu‐grenoble.fr Marjorie Dole mdole2@chu‐grenoble.fr University Hospital, Grenoble (France) |
| Notes | Collaborators: AGIR à Dom, ResMed |
| Trial name or title | A multicentre, randomised‐controlled trial of extracorporeal CO2 removal (ECCO2R) to facilitate early extubation compared to invasive mechanical ventilation in patients with severe AECOPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 202 Blinding: none |
| Participants | Known history of COPD; age 18 to 75 years; AECOPD requiring invasive mechanical ventilation; acute and potentially reversible cause of acute respiratory failure |
| Interventions | INTERVENTION Extracorporeal carbon dioxide removal Invasive mechanical ventilation treated with vv‐ECCO2R (Extracorporeal carbon dioxide removal) to facilitate early extubation Standard configuration with either double lumen cannula (20 to 22 Fr) or 2 small single vessel cannulas (15 to 19 Fr), allowing a blood flow rate between 1 and 2 litres a minute INTERVENTION conventional care Invasive mechanical ventilation; attempt to extubate and switch to non‐invasive ventilation If extubation fails, tracheostomy can be performed according to treating physician |
| Outcomes | DEVICE Actigraph; Wear instructions: up to 60 days SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; estimated study start January 2019; estimated study completion July 2023 |
| Contact information | Christian Karagiannidis christian.karagiannidis@uni‐wh.de Anne Hage‐Hülsmann Anne.hage‐huelsmann@uni‐wh.de University of Witten/Herdecke (Germany) |
| Notes |
| Trial name or title | Evidence generation for the clinical efficacy and cost effectiveness of myCOPD in patients with mild and moderate newly‐diagnosed COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 60 Blinding: outcomes assessor |
| Participants | Diagnosis of COPD (mild or moderate; FEV1 > 50% predicted) or diagnosed within 12 months (FER < 0.7); smoking history (current or former); age 40 to 80 years; currently taking inhaled medications; access to the internet at home, use of mobile technology and the ability to operate a web platform in English |
| Interventions | INTERVENTION "myCOPD" web‐based application designed to support people in long‐term management of COPD NO INTERVENTION |
| Outcomes | DEVICE FITBIT (pedometer)
PRIMARY OUTCOME HRQOL: CAT SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; actual study start November 2018; estimated study completion February 2019 |
| Contact information | Mal North [email protected] Chloe Barker [email protected] my mhealth Limited, Bournemouth (UK) |
| Notes | Collaborators: Innovate UK, Imperial College London, Hull and East Yorkshire Hospitals NHS Trust Hampshire Hospitals NHS Foundation Trust, Central London Community Healthcare NHS Trust |
| Trial name or title | Effects of a long‐term home‐based exercise training programme using minimal equipment vs. usual care in COPD patients: a study protocol for 2 multicentre randomised controlled trials (HOMEX‐1 and HOMEX‐2 trials) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention SETTING Canton of Zurich (Switzerland); SAMPLE SIZE n = 120 (based on CRQ dyspnoea domain) Blinding: none |
| Participants | Diagnosis of COPD (GOLD stages II to IV; post‐bronchodilation FEV1 < 80% predicted; FER < 0.7); age ≥ 40 years; no PR within 2 years |
| Interventions | DURATION OF INTERVENTION 12 months INTERVENTION home‐based exercise SETTING home CONTACT
TRAINING 6 days a week, 15 to 20 minutes, individualised and progressive strength‐training, 3 different levels CONTENT
NO INTERVENTION |
| Outcomes | DEVICE ActiGraph, Wear instructions: 1 week ASSESSMENT TIME POINTS
PRIMARY OUTCOME HRQOL: CRQ dyspnoea domain SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; study start October 2018; estimated primary completion October 2020 |
| Contact information | Anja Frei [email protected] University of Zurich (Switzerland) |
| Notes | FUNDING "HOMEX‐2 is supported by LUNGE ZÜRICH, Switzerland.The funding bodies had/have no role in the design of the study, data collection, analysis and interpretation of data and in writing the manuscript." CONFLICT OF INTEREST "The authors declare that they have no competing or financial interests." N.B. physical activity not assessed in HOMEX‐1 |
| Trial name or title | Increasing physical activity in COPD through rhythmically‐enhanced music |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 170 Blinding: investigator, outcomes assessor |
| Participants | Diagnosis of COPD (FEV1 < 70% predicted, FER < 0.7); age ≥ 40 years; mean SpO2 88% at peak exercise (with or without oxygen supplementation); ability to hear music |
| Interventions | DURATION OF INTERVENTION 12 weeks INTERVENTION home‐based exercise programme with rhythmically auditory stimulation‐enhanced music INTERVENTION home‐based exercise programme without music |
| Outcomes | DEVICE Actigraph (hip); Wear instructions: 1 week PRIMARY OUTCOME Exercise capacity: 6MWD SECONDARY OUTCOMES
|
| Starting date | Prospective recruitment; study start October 2018; estimated study completion September 2022 |
| Contact information | Susan A O'Connell Schnell [email protected] Franco Laghi [email protected] Edward Hines Jr. VA Hospital, Illinois (USA) |
| Notes | Sponsor: VA Office of Research and Development |
| Trial name or title | Physical activity following PR in patients with COPD |
| Methods | DESIGN 2 groups, cluster; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 60 Blinding: outcomes assessor, "Physiotherapists will be aware of the allocation of the programmes (not possible to blind) as they will be involved with delivering information/training of intervention components. A researcher blinded to group allocation will perform quantitative analysis." |
| Participants | Diagnosis of COPD; age 30 to 100 years; enrolled in PR within Lincolnshire Community Health Services; for telephone interviews: access to a telephone |
| Interventions | DURATION OF INTERVENTION 12 months following PR INTERVENTION PAC
NO INTERVENTION |
| Outcomes | ASSESSMENT TIME POINTS
PRIMARY OUTCOME MEASURE Adherence : “acceptability” number of participants who comply with intervention SECONDARY OUTCOMES
|
| Starting date | Retrospectively registered; actual study start June 2018; first posted September 2018; estimated study completion October 2019 |
| Contact information | Hayley Robinson [email protected] Arwel Jones [email protected] University of Lincoln (UK) |
| Notes | Collaborators: NHS PR clinics across Lincolnshire, United Kingdom, British Lung Foundation, University College, University of Oxford |
| Trial name or title | A web‐based self‐management intervention to increase level of physical activity and decrease use of health care for people with COPD in primary care: a protocol for a randomised controlled clinical trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 144 Blinding: none |
| Participants | Diagnosis of COPD (ICD‐10:J44:9); age ≥ 40 years; visit included primary care units due to COPD; have a smartphone, tablet or computer with access to internet |
| Interventions | INTERVENTION PAC (COPD Web) with pedometer written information about the importance of physical activity website, self‐managed
INTERVENTION pedometer written information about the importance of physical activity |
| Outcomes | DEVICE DynaPort MiniMod; Wear instructions: 1 week ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: (not defined) SECONDARY OUTCOME HRQOL: CAT, CRQ |
| Starting date | Study start November 2018; estimated study completion March 2020 |
| Contact information | Tobias Stenlund [email protected]; Karin Wadell [email protected] Umeå University (Sweden) |
| Notes |
| Trial name or title | A feasibility study assessing the inclusion of physical activity promotion to standard care PR and cognitive behavioural therapy in patients with COPD who are anxious and depressed |
| Methods | DESIGN 2 groups; COMPARISON; intervention vs. intervention; SAMPLE SIZE n = 40 Blinding: single, principal investigator will be blinded from the randomisation as this member will conduct cognitive behavioural therapy |
| Participants | Diagnosis of COPD (obstructive spirometry); age ≥ 40 years; clinically stable; optimised medical therapy; HADS score ≥ 8 |
| Interventions | DURATION OF INTERVENTION 8 weeks INTERVENTION PAC with PR and cognitive behavioural therapy SETTING consultation sessions CONTACT 2 sessions a week (total 16 sessions) CONTENT
INTERVENTION PR and cognitive behavioural therapy |
| Outcomes | DEVICE (triaxial accelerometer); Wear instructions: 1 week ASSESSMENT TIME POINTS
PRIMARY OUTCOME Adherence: PAC
SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; study start November 2018; estimated study completion May 2020 |
| Contact information | Matthew Armstrong [email protected] Northumbria University; Karen Heslop‐Marshell [email protected] Newcastle Upon Tyne, Tyne And Wear (UK) |
| Notes |
| Trial name or title | Residential cleaning of indoor air to reduce acute exacerbations of COPD (CARE): a pilot randomised cross‐over trial |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. placebo; SAMPLE SIZE n = 20 Blinding: participant, investigator, outcomes assessor |
| Participants | Diagnosis of COPD (severe; FEV1 < 50% predicted); age ≥ 18 years; live in Monroe County; referred for PR; AECOPD within 1 year; standard‐sized windows in their bedroom and living room amenable to device installation; expect to sleep each night of the study (2 months of period 1, 2 months of period 2) in either their bedroom or living room ≥ 6 hours a night, no other air filtering devices |
| Interventions | INTERVENTION HEPAirX air filter medical re‐circulating air cleaner 99.97% efficient filter for particles 0.3 µm in size PLACEBO provides only recirculation (without filtration or ventilation using outdoor air) and temperature control of room air |
| Outcomes | PRIMARY OUTCOMES
|
| Starting date | Retrospective registration; actual study start March 2019; first posted November 2018; estimated study completion October 2020 |
| Contact information | Daniel Croft [email protected] University of Rochester Medical Center, New York (USA) |
| Notes | N.B. will need pre‐cross‐over data for inclusion |
| Trial name or title | Promoting activity after COPD exacerbations: Aim 2 (PACE2) |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 64 Selection bias: "Allocation sequence will be concealed"; Blinding: outcomes assessor |
| Participants | Diagnosis of COPD (physician‐diagnosed); age ≥ 18 years; hospitalised as an inpatient, 23‐hour observation, or clinical decision unit; admitting respiratory conditions sensitive to the Centers for Medicare and Medicaid Services Hospital Readmission Reduction Program as listed (AECOPD, asthma/COPD overlap, decompensated heart failure, pneumonia, chronic airway disease) |
| Interventions | DURATION OF INTERVENTION 12 weeks INTERVENTION PAC (PACE2) with pedometer
INTERVENTION pedometer |
| Outcomes | PRIMARY OUTCOME Physical activity: step count (averaged over 1 week, over time using repeated measures over 12 weeks) |
| Starting date | First posted January 2019; estimated study completion May 2022 |
| Contact information | Valentin Prieto‐Centurion, University of Illinois, Chicago (USA) |
| Notes |
| Trial name or title | Leveraging technology to address access and adherence to conventional hospital‐based PR in veterans with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 120 Blinding: outcomes assessor, "study staff communicating randomization assignments to subjects will be different from study staff conducting follow‐up outcome assessments" |
| Participants | Diagnosis of COPD (FER < 0.70 or chest CT evidence of emphysema or prior documentation of FER < 0.70 and clinical evidence of COPD defined as 10 pack‐year cigarette smoking history, dyspnoea or on bronchodilators); age ≥ 40 years; declined participation in conventional PR; medical clearance to participate in exercise; access to computer with Internet connection, a USB port or Bluetooth capability, and Windows XP/Vista/7/8/10 or higher, or Mac OSX 10.5 or higher operating system, or willing to come to VA Medical Center to use study computers; pedometer and accelerometer with > 90% accuracy compared to manual counts on short clinic walk |
| Interventions | DURATION OF INTERVENTION 12 weeks INTERVENTION PAC (Every Step Counts)
NO INTERVENTION
|
| Outcomes | DEVICE (pedometer) PRIMARY OUTCOME Physical activity: step count SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; estimated start September 2019; estimated completion April 2024 |
| Contact information | Marilyn Moy [email protected]; Eric Garshick [email protected] VA Boston Healthcare System (USA) |
| Notes | SPONSOR VA Office of Research and Development |
| Trial name or title | The effect of targeted nutrient supplementation on physical activity and HRQOL in advanced COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 166 Blinding: participant, investigator, outcomes assessor |
| Participants | Diagnosis of COPD (GOLD); age ≥ 18 years; medically stable; history of ≥ 2 moderate AECOPDs, ≥ 1 severe AECOPD (GOLD stage C and D) or FEV1 < 50% predicted and ≥ 1 moderate or severe AECOPD within 12 months |
| Interventions | DURATION OF INTERVENTION 12 months COMMON COMPONENT Counselling CONTACT once a month CONTENT healthy lifestyle (physical activity, smoking cessation, weight management) by motivational interviewing INTERVENTION Targeted nutrient supplement (once daily) with counselling INTERVENTION Placebo supplement (once daily) with counselling |
| Outcomes | DEVICE activPAL; Wear instructions: 1 week ASSESSMENT TIME POINTS
PRIMARY OUTCOMES
SECONDARY/OTHER OUTCOMES
|
| Starting date | First posted January 2019; estimated study completion May 2023 |
| Contact information | Rosanne Beijers [email protected]; Harry Gosker [email protected] Maastricht University Medical Center (The Netherlands) |
| Notes | Collaborator: Nutricia Research |
| Trial name or title | EfiKroniK research programme: effectiveness of physical exercise for people with chronic pathologies. Hybrid, clinical and implementation randomised trial |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 370 Blinding: care provider, outcomes assessor |
| Participants | Diagnosis of COPD; Age 18 to 75 years; BODE index (score 3 to 7); clinical stability (absence of exacerbation, antibiotic treatment, systemic corticosteroids or hospitalisation within 30 days); life expectancy > 2 years; colon, breast or lung solid cancers stage IV non‐small cell with standard first‐line chemotherapy treatment; malignant haemopathy with autologous transplant or lymphomas not localised, in treatment with immunotherapy; schizophrenia; adequate renal, hepatic and haematological function |
| Interventions | DURATION OF INTERVENTION 12 weeks INTERVENTION "personalised exercise program" SETTING supervised nursing in primary and autonomous care afterwards, with support from community resources NO INTERVENTION |
| Outcomes | DEVICE Actigraph xGT3X‐BT (right iliac crest); Wear instructions: 1 week ASSESSMENT TIME POINTS
PRIMARY OUTCOMES
|
| Starting date | Retrospective registration; actual start January 2018; first posted January 2019; estimated study completion December 2020 |
| Contact information | Nere Mendizabal [email protected] Primary Care Research Unit of Bizkaia, Bilbao (Spain) |
| Notes | SPONSOR Basque Health Service (Spain) N.B. will need COPD‐only data for inclusion in this review |
| Trial name or title | Personalised exercise training in COPD ‐ exploring the interaction between exercise physiology, exercise perception and training progression |
| Methods | DESIGN "pilot/feasibility study aiming to characterise exercise limitation at baseline and then conducting an abridged RCT with four training arms"; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 60 |
| Participants | Diagnosis of COPD (FEV1 < 80% predicted, FER < 0.7); age ≥ 40 years; MRC dyspnoea scale (score ≥ 3); stable dose of current regular medication within 4 weeks; clinically‐acceptable ECG at enrolment |
| Interventions | INTERVENTION Eccentric cycling INTERVENTION Concentric cycling INTERVENTION Single‐leg cycling INTERVENTION Lower‐limb resistance training |
| Outcomes | DEVICE “seven‐day activity monitor” PRIMARY OUTCOME Adherence: training attendance SECONDARY OUTCOMES
|
| Starting date | Retrospectively registered; first posted January 2019; actual start October 2018; estimated study completion August 2019 |
| Contact information | Tom Ward [email protected] Loughborough University (UK) |
| Notes |
| Trial name or title | The effectiveness of a physical activity intervention versus PR on cardiovascular risk markers for individuals with COPD: a feasibility study |
| Methods | DESIGN 3 groups; COMPARISONS intervention vs. intervention, intervention vs. no intervention; SAMPLE SIZE n = 60 |
| Participants | Diagnosis of COPD; age 40 to 85 years |
| Interventions | DURATION OF INTERVENTION 6 weeks INTERVENTION PAC SETTING home‐based, unsupervised CONTENT FitBit device: step targets (increase by 500 steps a week) INTERVENTION PR SETTING outpatient, supervised, group CONTENT exercise and education NO INTERVENTION |
| Outcomes | DEVICE Actigraph ASSESSMENT TIME POINTS
PRIMARY OUTCOMES
|
| Starting date | First posted March 2019; estimated study completion February 2020 |
| Contact information | Tareq Alotaibi [email protected]; David Stensel [email protected]; Sally Singh sally.singh@uhl‐tr.nhs.uk University Hospitals of Leicester NHS Trust (UK) |
| Notes |
| Trial name or title | The role of humidified nasal high‐flow to reduce 30‐day hospital re‐admissions following severe exacerbations of COPD: a mixed‐methods feasibility study |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 80 Blinding: none |
| Participants | Emergency hospital admission with a primary diagnosis of AECOPD; smoking history ≥ 10 pack‐years; age 40 to 80 years; BMI ≤ 35kg/m2; to be discharged to home environment deemed safe to perform home assessments; live in the catchment area |
| Interventions | DURATION OF INTERVENTION hospital length of stay INTERVENTION Humidified nasal high‐flow device warmed, humidified air at flow rates of up to 60 litres a minute through a nasal cannula interface (intended delivery 30 litres a minute at 37 ºC if tolerated) NO INTERVENTION |
| Outcomes | DEVICE (triaxial accelerometer) (wrist) ASSESSMENT TIME POINTS Follow‐up: 30 days following discharge SECONDARY OUTCOMES
|
| Starting date | First posted April 2019; estimated study completion August 2021 |
| Contact information | Nicholas Hart [email protected]; Rebecca D'Cruz [email protected] Guy's and St Thomas' NHS Foundation Trust (UK) |
| Notes |
| Trial name or title | PRACTISS COPD; PR of COPD: a trial of sustained internet based self‐management support |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 100 Blinding: single |
| Participants | Pulmonologist‐diagnosed COPD (FER < 0.7); COPD most important limiting factor; PR completion |
| Interventions | DURATION OF INTERVENTION 1 year following PR INTERVENTION self‐management by internet "PatientCoach‐platform" Tools are available to guide discussion; participant determines goal, identifies barriers and plans for overcoming the barriers NO INTERVENTION |
| Outcomes | ASSESSMENT TIME POINTS
PRIMARY OUTCOME HRQOL: CRQ SECONDARY OUTCOMES
|
| Starting date | Study dates February 2013 to February 2015 |
| Contact information | Jacob Sont [email protected] Leids Universitair Medisch Centrum (The Netherlands) |
| Notes | FUNDING Netherlands Asthma Foundation Email sent to enquire regarding objective assessment of physical activity and availability of results |
| Trial name or title | Efficacy of a physical activity coaching system for patients with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 90 Blinding: none |
| Participants | Diagnosis of COPD (GOLD); age > 45 years; referred for PR; clinical stability within 4 weeks (pulmonary infections or AECOPD); absence of recent myocardial Infarction (within 3 months), unstable angina, other significant cardiac problems, resting systolic blood pressure > 180 mmHg, resting diastolic blood pressure > 100 mmHg or tachycardia; absence of significant orthopaedic, neurological, cognitive and/or psychiatric impairment restricting mobility; internet access at home |
| Interventions | DURATION OF INTERVENTION 8 weeks following inpatient PR INTERVENTION PAC Philips physical activity coaching system NO INTERVENTION wears activity monitor no coaching or insight into physical activity |
| Outcomes | ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: (not defined) |
| Starting date | Study dates April 2015 to March 2018 |
| Contact information | Marian Dekker [email protected] Philips Research |
| Notes | FUNDING Philips Research and CIRO+ expertise Center (The Netherlands) Email sent 23 August 2019 to ask about possible relationship with Priori 2017 or Saini 2017 |
| Trial name or title | Effects of the inclusion of a functional circuit to aerobic and resistance training on functionality, physical activity in daily life and immuno‐metabolic response of patients with COPD: a randomised clinical trial with follow‐up |
| Methods | DESIGN 3 groups; COMPARISONS intervention vs. intervention, intervention vs. no intervention; SAMPLE SIZE n = 75 Blinding: none |
| Participants | Diagnosis of COPD (GOLD); age 18 to 100 years; clinically stable (no AECOPD or changes in medications within 30 days); non‐smokers; no home oxygen therapy; no pathological conditions that prevent physical activity, severe or unstable heart disease, other pathological condition that may influence the systemic inflammatory process; not participating in another systematic exercise programme |
| Interventions | COMMON COMPONENT Aerobic training (treadmill)
INTERVENTION "GTF"
INTERVENTION "GTC" Aerobic training only NO INTERVENTION including respiratory physiotherapy techniques |
| Outcomes | OUTCOME MEASURES
|
| Starting date | Prospectively registered; planned last enrolment July 2019 |
| Contact information | Ercy Mara Cipulo Ramos [email protected]; Fabiano Lima [email protected] Universidade Estadual Julio de Mesquita Filho, Presidente Prudente, São Paulo (Brazil) |
| Notes | FUNDING "Fundação de Amparo a Pesquisa do Estado de São Paulo (Brazil)" N.B. need to confirm objective assessment of physical activity for inclusion |
| Trial name or title | The effect of the combination treatment with tiotropium and olodaterol compared to tiotropium on symptoms, respiratory functions and physical activity in maintenance‐naïve Japanese patients with COPD |
| Methods | DESIGN 2 groups; COMPARISON intervention vs. intervention; SAMPLE SIZE n = 80 Blinding: none |
| Participants | Diagnosis of COPD (FEV1 < 80% predicted, FER < 0.7); smoking history (current or former); age 40 to 85 years; maintenance treatment‐naïve |
| Interventions | DURATION OF INTERVENTION 12 weeks INTERVENTION LAMA/LABA (tiotropium and olodaterol) INTERVENTION LAMA (tiotropium) |
| Outcomes | DEVICE (triaxial accelerometer) ASSESSMENT TIME POINTS
SECONDARY OUTCOMES
|
| Starting date | Disclosure of study information April 2017; last follow‐up May 2019; recruitment status: no longer recruiting |
| Contact information | Koichiro Takahashi [email protected]‐u.ac.jp Division of Respiratory Medicine, Saga University (Japan) |
| Notes | FUNDING Nippon Boehringer Ingelheim Co, Ltd |
| Trial name or title | Combined effect of progressive resistance training and physical activity counselling in patients with COPD: a randomised controlled cross‐over study |
| Methods | DESIGN 2 groups, cross‐over; COMPARISON intervention vs. no intervention; SAMPLE SIZE n = 15 Blinding: none |
| Participants | Diagnosis of COPD (GOLD); age 18 to 90 years |
| Interventions | DURATION OF INTERVENTION 8 weeks INTERVENTION exercise training and PAC CONTACT 1 session a week CONTENT
NO INTERVENTION |
| Outcomes | DEVICE (pedometer) ASSESSMENT TIME POINTS
PRIMARY OUTCOME Physical activity: step count SECONDARY OUTCOMES
|
| Starting date | Disclosure of study information February 2018; Recruitment status: completed |
| Contact information | Chiharu Fujisawa [email protected] Shinko Hospital Rehabilitation Center (Japan) |
| Notes | Funding: Japan Science and Technoligy Agency N.B. will need pre‐cross‐over data for inclusion |
| Trial name or title | The effect of comprehensive respiratory rehabilitation using cloud system on exacerbation of patients with COPD at home |
| Methods | DESIGN 2 groups; COMPARISON intervention vs..no intervention; SAMPLE SIZE n = 50 Blinding: none |
| Participants | Diagnosis of COPD (Guidelines for Diagnosis and Treatment of COPD 4th Edition, Japan Respiratory Society); age ≥ 65 years; stable; attending Shinshu University Medical School Hospital for the purpose of diagnosis and treatment of COPD |
| Interventions | INTERVENTION "comprehensive respiratory rehabilitation" SETTING home CONTACT multi‐occupational co‐operation tool CONTENT Exercise; physical activity instruction in daily life; education NO INTERVENTION |
| Outcomes | SECONDARY OUTCOME Physical activity |
| Starting date | Disclosure of the study information July 2018 |
| Contact information | Shohei Kawachi drodman@shinshu‐u.ac.jp Shinshu University Hospital Rehabilitation Department (Japan) |
| Notes | N.B. need to confirm objective assessment of physical activity for inclusion |
| Trial name or title | Long‐term integrated telerehabilitation of COPD patients: a multicentre randomised controlled trial (iTrain) |
| Methods | DESIGN 3 groups; COMPARISONS intervention vs. intervention, intervention vs. no intervention; SETTING Australia, Denmark, Norway; SAMPLE SIZE n = 120 |
| Participants | Diagnosis of COPD (FEV1 < 80% predicted, FER < 0.7); age 40 to 80 years; ≥ 1 COPD‐related emergency department presentation or hospitalisation within 12 months |
| Interventions | STUDY DURATION two years "Any participant in the trial can undertake a traditional PR program at any time during the study period if it is considered clinically indicated by their usual treating team" INTERVENTION Telerehabilitation SETTING home CONTACT
AEROBIC TRAINING treadmill, ≥ 30 minutes
STRENGTH TRAINING 2 to 3 sessions a week INTERVENTION Unsupervised exercise training SETTING unsupervised, home TRAINING treadmill Individualised, prescribed as for telerehabilitation arm, no review or progression Record sessions in paper‐based diary NO INTERVENTION |
| Outcomes | DEVICE SenseWear Armband (software version 7.0) (left upper arm)
ASSESSMENT TIME POINTS
SECONDARY OUTCOMES
|
| Starting date | Prospectively registered; study start October 2014; final data collection for primary outcome measure December 2018 |
| Contact information | Paolo Zanaboni [email protected] University Hospital of North Norway, Tromsø (Norway) |
| Notes | FUNDING "This study was funded by the Research Council of Norway (Project Grant 228919/H10) and the Northern Norway Regional Health Authority (Project Grants HST1117‐13 and HST1118‐13)." CONFLICT OF INTEREST "The authors declare that they have no competing interests." |
AECOPD: acute exacerbation of COPD; BMI: body mass index; CAT: COPD assessement test; CCQ: clinical COPD questionnaire; COPD: chronic obstructive pulmonary disease; CPET: cardiopulmonary exercise test; CRQ: chronic respiratory disease questionnaire; CT: computerised tomography; ECG: electrocardiogram; EE: energy expenditure; EPAP: expiratory positive airway pressure; ESWT: time walked on endurance shuttle walk test; EQ5D: EuroQol 5 dimensions questionnaire; FER: forced expiratory ratio; FEV1: forced expiratory volume in one second; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HRQOL: health‐related quality of life; ICD‐10: International Statistical Classification of Diseases and Related Health Problems 10th Revision; ICS: inhaled corticosteriod; ILD: intersitial lung disease; IPAP: inspiratory positive airway pressure; ISWD: distance walked on incremental shuttle walk test; LABA: long‐acting beta2 agonist; LAMA: long‐acting muscarinic antagonist; LIPA: light intensity physical activity; LTOT: long term oxygen therapy; MRC: Medical Research Council; METs: metabolic equivalents; MVPA: moderate to vigorous physical activity; 1RM: one repetition maximum; PAL: physical activity level; Pimax: maximal inspiratory pressure; PR: pulmonary rehabilitation; RV: residual volume; SAE: serious adverse event; SF36: Medical Outcomes Survey 36‐item short‐form health survey questionnaire; SGRQ: St George's respiratory questionnaire; SpO2: oxygen saturation; 6MWD: distance walked on six‐minute walk test; 6MWT: six‐minute walk test; TLC: total lung capacity; VO2: peak oxygen uptake
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 1 change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 1.1 End intervention | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 208.24 [‐164.91, 581.39] |
| 2 time/change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 2 time/change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 2.1 End intervention | 3 | 190 | Mean Difference (IV, Random, 95% CI) | 3.62 [‐1.90, 9.14] |
| 3 change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 3 change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 3.1 End intervention | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐28.35, 24.61] |
| 4 change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 4 change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 4.1 End intervention | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐41.54 [‐89.97, 6.90] |
| 5 change in time in physical activity (total; minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 5 change in time in physical activity (total; minutes per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 5.1 End intervention | 2 | 88 | Mean Difference (IV, Random, 95% CI) | 23.01 [6.12, 39.90] |
| 6 change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 6 change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 6.1 End intervention | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 16.56 [‐27.06, 60.18] |
| 7 time in "lifestyle" physical activity (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 7 time in "lifestyle" physical activity (minutes per day); Intervention: high‐intensity interval training. | ||||
| 7.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 9.40 [3.87, 14.93] |
| 8 time in light‐intensity physical activity (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 8 time in light‐intensity physical activity (minutes per day); Intervention: high‐intensity interval training. | ||||
| 8.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 28.12 [15.64, 40.60] |
| 9 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 9 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: high‐intensity interval training. | ||||
| 9.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 6.24 [4.00, 8.48] |
| 10 sedentary time (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 10 sedentary time (minutes per day); Intervention: high‐intensity interval training. | ||||
| 10.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | ‐34.25 [‐55.90, ‐12.60] |
| 11 step count (steps per day); Intervention: physical activity counselling Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.11  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 11 step count (steps per day); Intervention: physical activity counselling. | ||||
| 11.1 Step count: in‐person (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Change in step count: telecoaching (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Step count: 'Urban Training' (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 "IMA" (counts per minute); Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 12 "IMA" (counts per minute); Intervention: self‐management. | ||||
| 12.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 physical activity level; Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 13 physical activity level; Intervention: nutritional supplement. | ||||
| 13.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 total energy expenditure (MJ); Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 14 total energy expenditure (MJ); Intervention: nutritional supplement. | ||||
| 14.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 subgroup analysis (supervised vs. unsupervised); change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 15 subgroup analysis (supervised vs. unsupervised); change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 15.1 Supervised pulmonary rehabilitation/exercise training | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 68.86 [‐386.29, 524.01] |
| 15.2 Unsupervised pulmonary rehabilitation/exercise training | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 494.0 [‐157.70, 1145.70] |
| 16 supgroup analysis (supervised vs. unsupervised); change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 16 supgroup analysis (supervised vs. unsupervised); change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 16.1 Supervised pulmonary rehabilitation/exercise training | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 9.87 [‐9.22, 28.96] |
| 16.2 Unupervised pulmonary rehabilitation/exercise training | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐44.0 [‐87.04, ‐0.96] |
| 17 subgroup analysis (supervised vs. unsupervised); change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.17  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 17 subgroup analysis (supervised vs. unsupervised); change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 17.1 Supervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Unsupervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 subgroup analysis (supervised vs. unsupervised); change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.18  Comparison 1 Physical activity: intervention vs. no intervention, Outcome 18 subgroup analysis (supervised vs. unsupervised); change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 18.1 Supervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Unsupervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 step count (steps per day); Intervention: self‐management (health mentoring) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 1 step count (steps per day); Intervention: self‐management (health mentoring). | ||||
| 1.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in step count (steps per day); Intervention: LAMA/LABA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 2 change in step count (steps per day); Intervention: LAMA/LABA. | ||||
| 2.1 End intervention | 2 | 426 | Mean Difference (IV, Random, 95% CI) | 531.30 [167.10, 895.49] |
| 3 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day): Intervention: LAMA/LABA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 3 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day): Intervention: LAMA/LABA. | ||||
| 3.1 End intervention | 2 | 423 | Mean Difference (IV, Random, 95% CI) | 9.74 [4.23, 15.24] |
| 4 change in active energy expenditure (kcal); Intervention: LAMA/LABA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 4 change in active energy expenditure (kcal); Intervention: LAMA/LABA. | ||||
| 4.1 End intervention | 2 | 423 | Mean Difference (IV, Random, 95% CI) | 43.89 [17.92, 69.86] |
| 5 step count (steps per day); Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 5 step count (steps per day); Intervention: nutritional supplement. | ||||
| 5.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 energy expenditure for ambulation (kcal/step/FFM kg); Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 6 energy expenditure for ambulation (kcal/step/FFM kg); Intervention: nutritional supplement. | ||||
| 6.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in step count (steps per day); Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 7 change in step count (steps per day); Intervention: neuromuscular electrical stimulation. | ||||
| 7.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in up/down transitions (number); Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 8 change in up/down transitions (number); Intervention: neuromuscular electrical stimulation. | ||||
| 8.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in time upright (hours); Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.9  Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 9 change in time upright (hours); Intervention: neuromuscular electrical stimulation. | ||||
| 9.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 movement intensity (m/s2); Interventions: nordic walking with education vs. education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 1 movement intensity (m/s2); Interventions: nordic walking with education vs. education. | ||||
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow‐up (9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in step count (steps per day); Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 2 change in step count (steps per day); Interventions: exercise training (COPE‐active) with self‐management vs. self management. | ||||
| 2.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Follow‐up (18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Follow‐up (24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in time in light‐intensity physical activity (minutes per day); Interventions: upper body resistance training with health education vs. health education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 3 change in time in light‐intensity physical activity (minutes per day); Interventions: upper body resistance training with health education vs. health education. | ||||
| 3.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 step count (steps per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 4 step count (steps per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification. | ||||
| 4.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 time walking (minutes per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.5  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 5 time walking (minutes per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification. | ||||
| 5.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 walking intensity (m/s2); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 6 walking intensity (m/s2); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification. | ||||
| 6.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 step count (steps per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 7 step count (steps per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 7.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 time walking (minutes per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.8  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 8 time walking (minutes per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 8.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 walking intensity (m/s2); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.9  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 9 walking intensity (m/s2); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 9.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 step count (steps per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.10  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 10 step count (steps per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification. | ||||
| 10.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 time walking (minutes per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.11  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 11 time walking (minutes per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification. | ||||
| 11.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 walking intensity (m/s2); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.12  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 12 walking intensity (m/s2); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification. | ||||
| 12.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 change in step count (steps per day); Interventions: exercise training and physical activity counselling with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.13  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 13 change in step count (steps per day); Interventions: exercise training and physical activity counselling with pedometer vs. pedometer. | ||||
| 13.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Follow‐up (3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Follow‐up (12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 change in step count (weekday, steps per day); Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.14  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 14 change in step count (weekday, steps per day); Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise. | ||||
| 14.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 METs; Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.15  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 15 METs; Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise. | ||||
| 15.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 step count (steps per day); Interventions: physical activity counselling with pedometer vs. pedometer Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.16  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 16 step count (steps per day); Interventions: physical activity counselling with pedometer vs. pedometer. | ||||
| 16.1 Step count: web‐based (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Step count: app (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Step count: app (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Change in step count: web‐based (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Change in step count: web‐based (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 time active (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.17  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 17 time active (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer. | ||||
| 17.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 time inactive (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.18  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 18 time inactive (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer. | ||||
| 18.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 peak performance (steps per minute); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.19  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 19 peak performance (steps per minute); Interventions: physical activity counselling (app) with pedometer vs. pedometer. | ||||
| 19.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.20  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 20 step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 20.1 End intervention (7 days including PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 End intervention (6 days excluding PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 End intervention (4 days excluding PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.21  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 21 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 21.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.22  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 22 time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 22.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 time sedentary (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.23  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 23 time sedentary (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 23.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 change in step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.24  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 24 change in step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 24.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.25  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 25 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 25.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 change in time walking (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.26  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 26 change in time walking (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 26.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 change in time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.27  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 27 change in time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 27.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28 change in time in light‐intensity physical activity (minutes per day); Interventions: exercise‐specific self‐efficacy training with upper body resistance training vs. upper body resistance training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.28  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 28 change in time in light‐intensity physical activity (minutes per day); Interventions: exercise‐specific self‐efficacy training with upper body resistance training vs. upper body resistance training. | ||||
| 28.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 29 step count (steps per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.29  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 29 step count (steps per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification. | ||||
| 29.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 29.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 30 time walking (minutes per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.30  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 30 time walking (minutes per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification. | ||||
| 30.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 30.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 31 walking intensity (m/s2); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.31  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 31 walking intensity (m/s2); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification. | ||||
| 31.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 31.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 32 step count (steps per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.32  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 32 step count (steps per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 32.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 32.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 time walking (minutes per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.33  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 33 time walking (minutes per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 33.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 33.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 34 walking intensity (m/s2); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.34  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 34 walking intensity (m/s2); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 34.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 34.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 35 step count (steps per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.35  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 35 step count (steps per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 35.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 35.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 36 time walking (minutes per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.36  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 36 time walking (minutes per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 36.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 walking intensity (m/s2); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.37  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 37 walking intensity (m/s2); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 37.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 37.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 38 change in step count (steps per day); Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.38  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 38 change in step count (steps per day); Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation. | ||||
| 38.1 End intervention (stage 1) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.2 End intervention (stage 2) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.3 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 change in step count (steps per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.39  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 39 change in step count (steps per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 39.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 39.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 40 change in time in moderate‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.40  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 40 change in time in moderate‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 40.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 40.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 41 change in time in vigorous‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.41  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 41 change in time in vigorous‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 41.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 41.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 42 change in time in light‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.42  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 42 change in time in light‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 42.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 42.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 43 change in total energy expenditure (kcal); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.43  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 43 change in total energy expenditure (kcal); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 43.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 43.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 44 change in sedentary time (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.44  Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 44 change in sedentary time (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 44.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 44.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in step count (steps per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Physical activity: intervention vs. intervention, Outcome 1 change in step count (steps per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Physical activity: intervention vs. intervention, Outcome 2 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 2.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in number of bouts of moderate‐to‐vigorous intensity physical activity; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Physical activity: intervention vs. intervention, Outcome 3 change in number of bouts of moderate‐to‐vigorous intensity physical activity; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 3.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 change in time in bouts of moderate‐to‐vigorous intensity physical activity(minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Physical activity: intervention vs. intervention, Outcome 4 change in time in bouts of moderate‐to‐vigorous intensity physical activity(minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 4.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 change in sedentary time (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.5  Comparison 4 Physical activity: intervention vs. intervention, Outcome 5 change in sedentary time (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 5.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 change in sedentary time (awake; minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Physical activity: intervention vs. intervention, Outcome 6 change in sedentary time (awake; minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 6.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in number of sedentary bouts; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.7  Comparison 4 Physical activity: intervention vs. intervention, Outcome 7 change in number of sedentary bouts; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 7.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in time in sedentary bouts (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.8  Comparison 4 Physical activity: intervention vs. intervention, Outcome 8 change in time in sedentary bouts (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 8.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in METs; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.9  Comparison 4 Physical activity: intervention vs. intervention, Outcome 9 change in METs; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 9.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 change in total energy expenditure (kcal); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.10  Comparison 4 Physical activity: intervention vs. intervention, Outcome 10 change in total energy expenditure (kcal); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 10.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 change in step count (steps per day); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.11  Comparison 4 Physical activity: intervention vs. intervention, Outcome 11 change in step count (steps per day); Interventions: water‐based exercise training vs. land‐based exercise training. | ||||
| 11.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 change in total energy expenditure (kcal); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.12  Comparison 4 Physical activity: intervention vs. intervention, Outcome 12 change in total energy expenditure (kcal); Interventions: water‐based exercise training vs. land‐based exercise training. | ||||
| 12.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 step count (steps per day); Interventions: Tai Chi vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.13  Comparison 4 Physical activity: intervention vs. intervention, Outcome 13 step count (steps per day); Interventions: Tai Chi vs. pulmonary rehabilitation. | ||||
| 13.1 Mid‐intervention (6 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Mid‐intervention (22 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 step count (steps per day); Interventions: outdoor walking vs. cycle ergometry Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.14  Comparison 4 Physical activity: intervention vs. intervention, Outcome 14 step count (steps per day); Interventions: outdoor walking vs. cycle ergometry. | ||||
| 14.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 step count (steps per day); Interventions: physical activity counselling vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.15  Comparison 4 Physical activity: intervention vs. intervention, Outcome 15 step count (steps per day); Interventions: physical activity counselling vs. pulmonary rehabilitation. | ||||
| 15.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 total energy expenditure (kcal); Interventions: exercise training with tapered supervision vs. supervised exercise training Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.16  Comparison 4 Physical activity: intervention vs. intervention, Outcome 16 total energy expenditure (kcal); Interventions: exercise training with tapered supervision vs. supervised exercise training. | ||||
| 16.1 End intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 mid day activity (vector magnitude units per minute); Interventions: supplemental oxygen (lightweight ambulatory) vs. supplemental oxygen (E‐cylinder) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 4.17  Comparison 4 Physical activity: intervention vs. intervention, Outcome 17 mid day activity (vector magnitude units per minute); Interventions: supplemental oxygen (lightweight ambulatory) vs. supplemental oxygen (E‐cylinder). | ||||
| 17.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in SGRQ total score; Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 1 change in SGRQ total score; Intervention: pulmonary rehabilitation/exercise training. | ||||
| 1.1 End intervention | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐8.79 [‐14.08, ‐3.51] |
| 2 change in SGRQ domain scores. Intervention: pulmonary rehabilitation/exercise training (ground‐based walking) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 2 change in SGRQ domain scores. Intervention: pulmonary rehabilitation/exercise training (ground‐based walking). | ||||
| 2.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in CRQ domain scores; Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 3 change in CRQ domain scores; Intervention: pulmonary rehabilitation/exercise training. | ||||
| 3.1 Dyspnoea (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 1.69 [0.02, 3.36] |
| 3.2 Emotional function (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 2.41 [0.48, 4.35] |
| 3.3 Fatigue (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐0.16, 2.83] |
| 3.4 Mastery (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.47, 1.69] |
| 3.5 Total score (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 6.70 [2.55, 10.86] |
| 4 CAT score; Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 4 CAT score; Intervention: high‐intensity interval training. | ||||
| 4.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | ‐3.15 [‐4.82, ‐1.47] |
| 5 SGRQ domain scores (%change); Intervention: exercise training [inpatient] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.5 ![Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 5 SGRQ domain scores (%change); Intervention: exercise training [inpatient].](/cdsr/doi/10.1002/14651858.CD012626.pub2/media/CDSR/CD012626/image_n/nCD012626-CMP-005-05.png) Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 5 SGRQ domain scores (%change); Intervention: exercise training [inpatient]. | ||||
| 5.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Symptoms (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Activity (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Impacts (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Total score (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 SGRQ domain scores; Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.6  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 6 SGRQ domain scores; Intervention: physical activity counselling. | ||||
| 6.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 CCQ domain scores: Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.7  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 7 CCQ domain scores: Intervention: physical activity counselling. | ||||
| 7.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Functional state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Mental state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in CCQ domain scores; Intervention: physical activity counselling (telecoaching) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.8  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 8 change in CCQ domain scores; Intervention: physical activity counselling (telecoaching). | ||||
| 8.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Functional state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Mental state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in CRQ domain and total scores; Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.9  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 9 change in CRQ domain and total scores; Intervention: physical activity counselling. | ||||
| 9.1 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 change in SGRQ domain and total scores; Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.10  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 10 change in SGRQ domain and total scores; Intervention: physical activity counselling. | ||||
| 10.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 change in CRQ domain scores; Intervention: self‐management (SPACE) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.11  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 11 change in CRQ domain scores; Intervention: self‐management (SPACE). | ||||
| 11.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Dyspnoea (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Emotional domain (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.7 Fatigue (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.8 Mastery (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 CCQ total score; Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.12  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 12 CCQ total score; Intervention: self‐management. | ||||
| 12.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 EQ5D index score; Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.13  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 13 EQ5D index score; Intervention: self‐management. | ||||
| 13.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 EQ5D visual analogue scale score; Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.14  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 14 EQ5D visual analogue scale score; Intervention: self‐management. | ||||
| 14.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 SGRQ domain scores; Intervention: self‐management (telephone health coaching) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.15  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 15 SGRQ domain scores; Intervention: self‐management (telephone health coaching). | ||||
| 15.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.5 Symptoms (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.6 Activity (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.7 Impacts (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.8 Total score (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 EQ5D score; Intervention: self‐management (telephone health coaching) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.16  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 16 EQ5D score; Intervention: self‐management (telephone health coaching). | ||||
| 16.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 CRQ domain scores; Intervention: four‐wheeled walker Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.17  Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 17 CRQ domain scores; Intervention: four‐wheeled walker. | ||||
| 17.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in SF36 component scores; Intervention: singing Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.1  Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 1 change in SF36 component scores; Intervention: singing. | ||||
| 1.1 Physical component (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Mental component (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in CRQ total score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 2 change in CRQ total score; Intervention: neuromuscular electrical stimulation. | ||||
| 2.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in SGRQ total score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 3 change in SGRQ total score; Intervention: neuromuscular electrical stimulation. | ||||
| 3.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 change in EQ5D index score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 4 change in EQ5D index score; Intervention: neuromuscular electrical stimulation. | ||||
| 4.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 change in EQ5D visual analogue scale score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 6.5  Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 5 change in EQ5D visual analogue scale score; Intervention: neuromuscular electrical stimulation. | ||||
| 5.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SF36 component scores (score < 50); Interventions: Nordic walking with education vs. education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 1 SF36 component scores (score < 50); Interventions: Nordic walking with education vs. education. | ||||
| 1.1 Physical component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Physical component score (follow‐up, 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Physical component score (follow‐up, 9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Mental component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Mental component score (follow‐up, 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Mental component score (follow‐up, 9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in CRQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 2 change in CRQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management. | ||||
| 2.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Dyspnoea (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Emotional function (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Fatigue (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Mastery (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Dyspnoea (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Emotional function (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Fatigue (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Mastery (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in CCQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 3 change in CCQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management. | ||||
| 3.1 Symptoms (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Functional state (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Mental state (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Total (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Functional state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Mental state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Symptoms (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Functional state (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Mental state (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Total (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Symptoms (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 Functional state (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Mental state (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Total (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CRQ domain scores; Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.4  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 4 CRQ domain scores; Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification. | ||||
| 4.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 CRQ domain scores; Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.5  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 5 CRQ domain scores; Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 5.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 CRQ domain scores; Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.6  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 6 CRQ domain scores; Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification. | ||||
| 6.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in SGRQ domain and total scores; Intervention: exercise training and physical activity counselling with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.7  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 7 change in SGRQ domain and total scores; Intervention: exercise training and physical activity counselling with pedometer vs. pedometer. | ||||
| 7.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Symptoms (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Activity (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 Impacts (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 Total (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.9 Symptoms (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.10 Activity (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.11 Impacts (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.12 Total (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in CRQ domain scores; Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.8  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 8 change in CRQ domain scores; Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise. | ||||
| 8.1 Dyspnoea (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Emotional function (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Fatigue (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Mastery (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Dyspnoea (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Emotional function (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Fatigue (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 Mastery (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.9 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.10 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.11 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.12 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in SGRQ total score; Interventions: physical activity counselling with pedometer vs. pedometer Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.9  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 9 change in SGRQ total score; Interventions: physical activity counselling with pedometer vs. pedometer. | ||||
| 9.1 web‐based (12 weeks, end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 web‐based (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 web‐based (12 months, end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 SGRQ total: Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.10  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 10 SGRQ total: Interventions: physical activity counselling (app) with pedometer vs. pedometer. | ||||
| 10.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 SF36: Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.11  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 11 SF36: Interventions: physical activity counselling (app) with pedometer vs. pedometer. | ||||
| 11.1 Physical component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Mental component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 change in SGRQ domain scores; Interventions: physical activity counselling (web‐based) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.12  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 12 change in SGRQ domain scores; Interventions: physical activity counselling (web‐based) with pedometer vs. pedometer. | ||||
| 12.1 Symptoms (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Impacts (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Activities (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.5 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.6 Activities (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 SGRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.13  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 13 SGRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 13.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 RAND36 domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.14  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 14 RAND36 domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 14.1 Physical function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Vitality (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Bodily pain (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 General health perception (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.5 Health status (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 change in CRQ dyspnoea domain score; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.15  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 15 change in CRQ dyspnoea domain score; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 15.1 Mid‐intervention (end PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 SGRQ scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.16  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 16 SGRQ scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 16.1 Symptoms (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Activity (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Impacts (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Total score (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.6 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.7 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.8 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 CRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.17  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 17 CRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 17.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.5 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 change in CRQ total score; Interventions: physical activity counselling with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.18  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 18 change in CRQ total score; Interventions: physical activity counselling with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation. | ||||
| 18.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 change in CRQ scores; Interventions: self‐management (health coaching) with pulmonary rehabilitation referral vs. pulmonary rehabilitation referral Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.19  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 19 change in CRQ scores; Interventions: self‐management (health coaching) with pulmonary rehabilitation referral vs. pulmonary rehabilitation referral. | ||||
| 19.1 Physical function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Physical function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.4 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 CRQ domain scores; Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.20  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 20 CRQ domain scores; Interventions: LAMA with behaviour modification vs. placebo with behaviour modification. | ||||
| 20.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 CRQ domain scores; Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.21  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 21 CRQ domain scores; Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 21.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 CRQ domain scores; Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.22  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 22 CRQ domain scores; Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 22.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 change in SGRQ domain and total scores; Interventions: ACE inhibitor with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.23  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 23 change in SGRQ domain and total scores; Interventions: ACE inhibitor with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation. | ||||
| 23.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Activity (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.3 Impacts (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.4 Total (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 change in SGRQ total score; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.24  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 24 change in SGRQ total score; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation. | ||||
| 24.1 End intervention (stage 2) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 change in EQ5D; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.25  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 25 change in EQ5D; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation. | ||||
| 25.1 End intervention (stage 2) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 change in CRQ domain scores; Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.26  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 26 change in CRQ domain scores; Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation. | ||||
| 26.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.5 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.6 Dyspnoea (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.7 Emotional function (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.8 Fatigue (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.9 Mastery (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.10 Total score (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 CRQ domain scores; Interventions: non‐invasive ventilation with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 7.27  Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 27 CRQ domain scores; Interventions: non‐invasive ventilation with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 27.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.5 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 6MWD (metres); Intervention; neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 8.1  Comparison 8 Exercise capacity: intervention vs. placebo/sham, Outcome 1 change in 6MWD (metres); Intervention; neuromuscular electrical stimulation. | ||||
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 6MWD (metres); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 1 change in 6MWD (metres); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 1.1 End intervention | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 29.06 [14.38, 43.75] |
| 2 change in ISWD (metres); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.2  Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 2 change in ISWD (metres); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 2.1 End intervention | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 19.02 [2.20, 35.85] |
| 3 change in ESWT (seconds); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.3  Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 3 change in ESWT (seconds); Intervention: pulmonary rehabilitation/exercise training. | ||||
| 3.1 End intervention | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 237.87 [147.59, 328.16] |
| 4 6MWD (metres); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.4  Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 4 6MWD (metres); Intervention: high‐intensity interval training. | ||||
| 4.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 46.55 [24.66, 68.45] |
| 5 work rate (watts); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 9.5  Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 5 work rate (watts); Intervention: high‐intensity interval training. | ||||
| 5.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 15.47 [8.76, 22.17] |
| 6 change in ISWD (metres); Intervention: self‐management (SPACE) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 9.6  Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 6 change in ISWD (metres); Intervention: self‐management (SPACE). | ||||
| 6.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in ESWT (seconds); Intervention: self‐management (SPACE) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 9.7  Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 7 change in ESWT (seconds); Intervention: self‐management (SPACE). | ||||
| 7.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 6MWD (metres); Intervention: exercise training [inpatient] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 9.8 ![Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 8 6MWD (metres); Intervention: exercise training [inpatient].](/cdsr/doi/10.1002/14651858.CD012626.pub2/media/CDSR/CD012626/image_n/nCD012626-CMP-009-08.png) Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 8 6MWD (metres); Intervention: exercise training [inpatient]. | ||||
| 8.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in CRQ domains; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.1  Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 1 change in CRQ domains; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 1.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Dyspnoea (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Emotional function (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Fatigue (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Mastery (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in CRQ domains; Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.2  Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 2 change in CRQ domains; Interventions: water‐based exercise training vs. land‐based exercise training. | ||||
| 2.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Total score (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 SGRQ domain and total scores; Interventions: Tai Chi vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.3  Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 3 SGRQ domain and total scores; Interventions: Tai Chi vs. pulmonary rehabilitation. | ||||
| 3.1 Symptoms (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Activity (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Impacts (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Total (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Symptoms (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Activity (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Impacts (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Total (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CRQ total score; Interventions: outdoor walking vs. cycle ergometry Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.4  Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 4 CRQ total score; Interventions: outdoor walking vs. cycle ergometry. | ||||
| 4.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Maugeri Respiratory Failure questionnaire; Interventions: exercise training with tapered supervision vs. supervised exercise training Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 10.5  Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 5 Maugeri Respiratory Failure questionnaire; Interventions: exercise training with tapered supervision vs. supervised exercise training. | ||||
| 5.1 End intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 SF36 domain scores; Interventions: self‐management vs. education and symptom monitoring Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 10.6  Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 6 SF36 domain scores; Interventions: self‐management vs. education and symptom monitoring. | ||||
| 6.1 Mental health (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Role emotional (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Vitality (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Social functioning (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Pain (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Role physical (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 General health (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Physical functioning (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 6MWD (metres); Interventions: Nordic walking with education vs. education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.1  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 1 6MWD (metres); Interventions: Nordic walking with education vs. education. | ||||
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow‐up (9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in ISWD (metres); Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.2  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 2 change in ISWD (metres); Interventions: exercise training (COPE‐active) with self‐management vs. self management. | ||||
| 2.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Follow‐up (18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Follow‐up (24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in ESWT (seconds); Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.3  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 3 change in ESWT (seconds); Interventions: exercise training (COPE‐active) with self‐management vs. self management. | ||||
| 3.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Follow‐up (18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Follow‐up (24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 6MWD (metres); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.4  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 4 6MWD (metres); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 4.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 ESWT (seconds); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.5  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 5 ESWT (seconds); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 5.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 6MWD (metres); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.6  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 6 6MWD (metres); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 6.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 ESWT (seconds); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.7  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 7 ESWT (seconds); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification. | ||||
| 7.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 6MWD (metres); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.8  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 8 6MWD (metres); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification. | ||||
| 8.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 ESWT (seconds); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.9  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 9 ESWT (seconds); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification. | ||||
| 9.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 change in ESWT (seconds); Intervention: physical activity counselling and exercise training with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.10  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 10 change in ESWT (seconds); Intervention: physical activity counselling and exercise training with pedometer vs. pedometer. | ||||
| 10.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Follow‐up (3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Follow‐up (12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 6MWD (metres); Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.11  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 11 6MWD (metres); Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise. | ||||
| 11.1 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Mid‐intervention (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 6MWD (metres); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.12  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 12 6MWD (metres); Interventions: physical activity counselling (app) with pedometer vs. pedometer. | ||||
| 12.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.13  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 13 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation. | ||||
| 13.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 change in 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.14  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 14 change in 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation. | ||||
| 14.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 6MWD (metres); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.15  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 15 6MWD (metres); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification. | ||||
| 15.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 ESWT (seconds); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.16  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 16 ESWT (seconds); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification. | ||||
| 16.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.17  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 17 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 17.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 ESWT (s); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.18  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 18 ESWT (s); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification. | ||||
| 18.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.19  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 19 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification. | ||||
| 19.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 ESWT (seconds); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.20  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 20 ESWT (seconds); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification. | ||||
| 20.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 change in ISWD (metres); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.21  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 21 change in ISWD (metres); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation. | ||||
| 21.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 change in ESWT (seconds); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 11.22  Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 22 change in ESWT (seconds); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation. | ||||
| 22.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 6MWD (metres); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 1 change in 6MWD (metres); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation. | ||||
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in 6MWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 12.2  Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 2 change in 6MWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training. | ||||
| 2.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in ISWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 12.3  Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 3 change in ISWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training. | ||||
| 3.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 change in VO2max (ml/min); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 12.4  Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 4 change in VO2max (ml/min); Interventions: water‐based exercise training vs. land‐based exercise training. | ||||
| 4.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 6MWD (metres); Interventions: Tai Chi vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 12.5  Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 5 6MWD (metres); Interventions: Tai Chi vs. pulmonary rehabilitation. | ||||
| 5.1 Mid‐intervention (2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mid‐intervention (14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 6MWD (metres); Interventions: exercise training with tapered supervision vs. supervised exercise training Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.6  Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 6 6MWD (metres); Interventions: exercise training with tapered supervision vs. supervised exercise training. | ||||
| 6.1 End intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison 1: Intervention vs. no intervention
Outcome 1.1: Physical activity: change in step count (steps per day)

Forest plot of comparison 1: Intervention vs. no intervention
Outcome 1.2: Physical activity: change in time in moderate‐to‐vigorous intensity physical activity (minutes per day)

Forest plot of comparison 1: Intervention vs. no intervention
Outcome 1.4: Physical activity: change in time in light‐intensity physical activity (minutes per day)

Forest plot of comparison 1: Intervention vs. no intervention
Outcome 1.3: Physical activity: change in total energy expenditure (kcal)
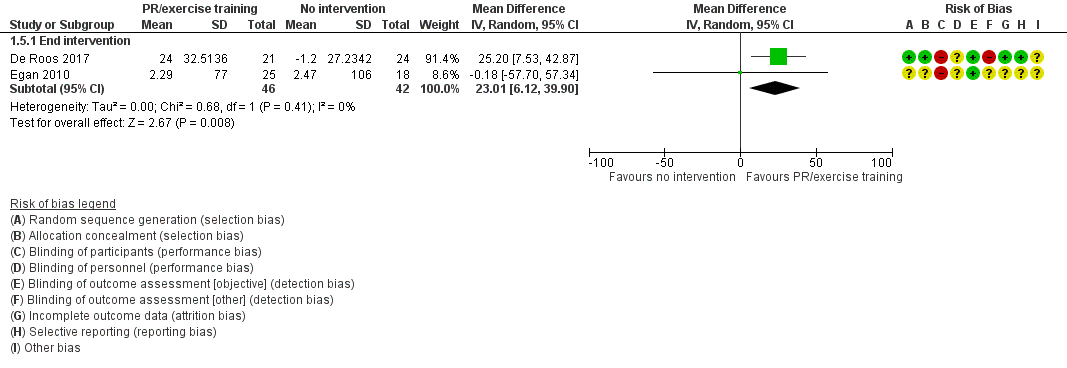
Forest plot of comparison 1: Intervention vs. no intervention
Outcome 1.6: Physical activity: change in time in physical activity (total, minutes per day)

Forest plot of comparison 1: Intervention vs. no intervention
Outcome 1.5: Physical activity: change in sedentary time (minutes per day)

Forest plot of comparison 1: Intervention vs. no intervention
Outcome: 1.8 Physical activity: time in "lifestyle" physical activity (minutes per day)
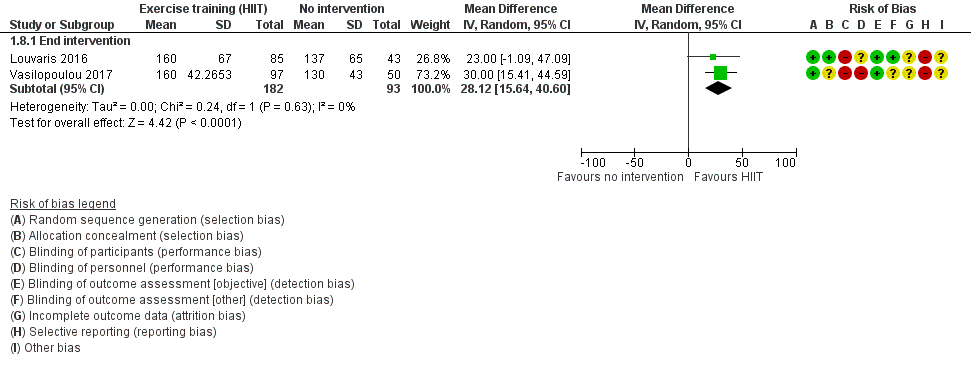
Forest plot of comparison: 1 Intervention vs. no intervention
Outcome: 1.7 Physical activity: time in light‐intensity physical activity (minutes per day)

Forest plot of comparison 1: Intervention vs. vs. no intervention
Outcome 1.9: Physical activity: time in MVPA (minutes per day)

Forest plot of comparison 1: Intervention vs. no intervention
Outcome 1.10: Physical activity: sedentary time (minutes per day)

Forest plot of comparison 2: Intervention vs. placebo
Outcome 2.2: Physical activity: change in step count (steps per day)

Forest plot of comparison 2: Intervention vs. placebo
Outcome 2.3: Physical activity: change in time in moderate‐to‐vigorous intensity physical activity (minutes per day)
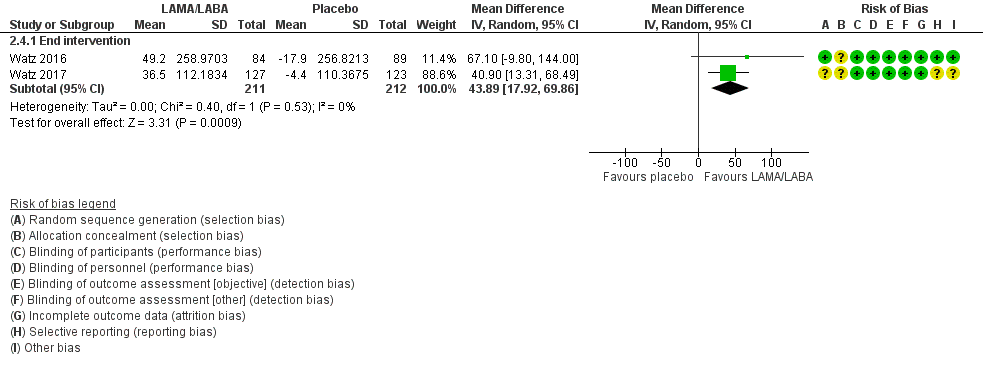
Forest plot of comparison 2: Intervention vs. placebo
Outcome 2.4: Physical activity: change in active energy expenditure (kcal)

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 1 change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training.
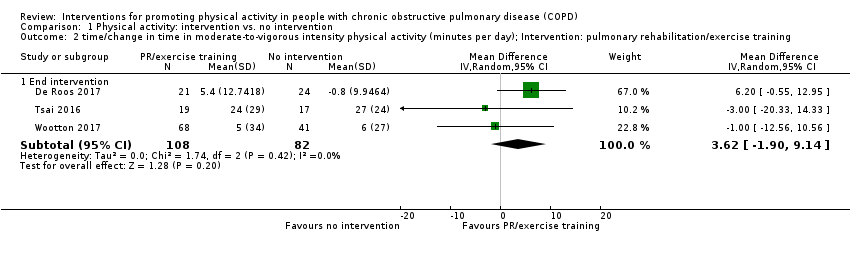
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 2 time/change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training.
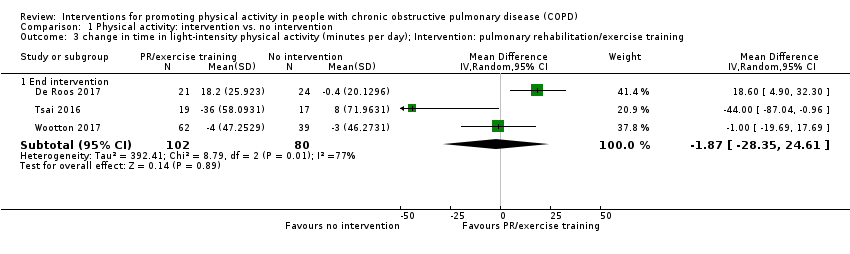
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 3 change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training.
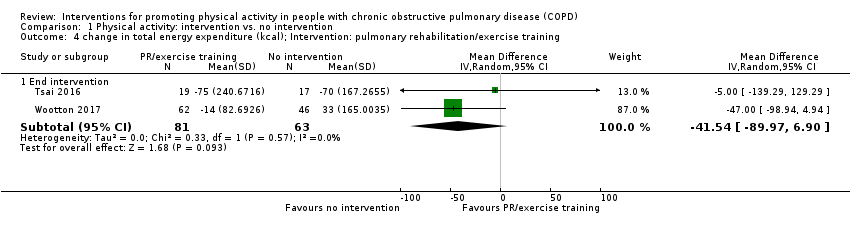
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 4 change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training.

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 5 change in time in physical activity (total; minutes per day); Intervention: pulmonary rehabilitation/exercise training.
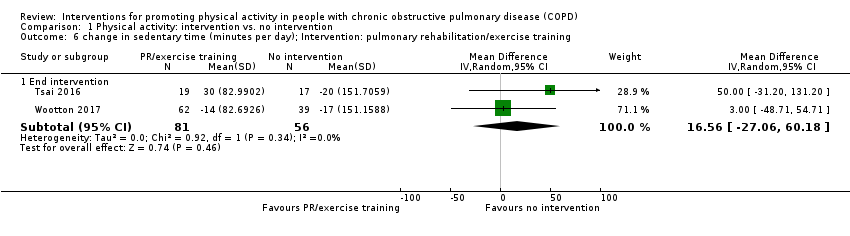
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 6 change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training.

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 7 time in "lifestyle" physical activity (minutes per day); Intervention: high‐intensity interval training.
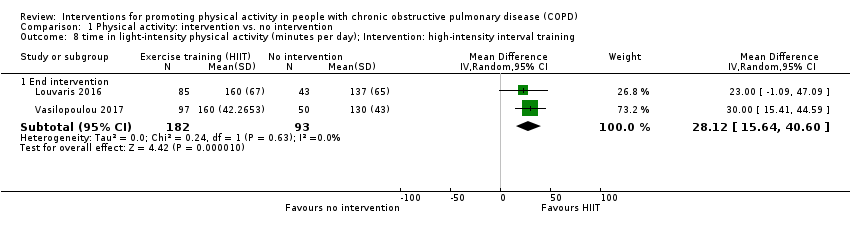
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 8 time in light‐intensity physical activity (minutes per day); Intervention: high‐intensity interval training.

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 9 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: high‐intensity interval training.
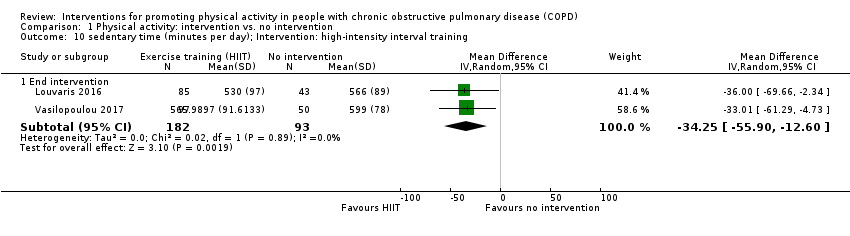
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 10 sedentary time (minutes per day); Intervention: high‐intensity interval training.

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 11 step count (steps per day); Intervention: physical activity counselling.
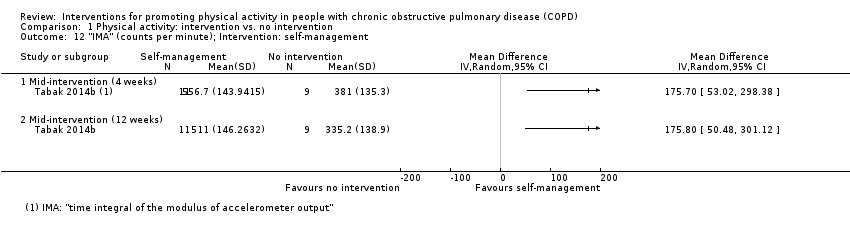
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 12 "IMA" (counts per minute); Intervention: self‐management.

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 13 physical activity level; Intervention: nutritional supplement.
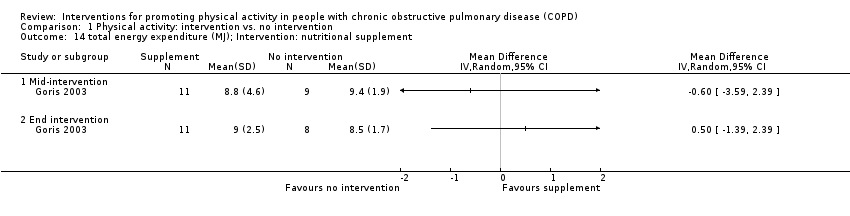
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 14 total energy expenditure (MJ); Intervention: nutritional supplement.

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 15 subgroup analysis (supervised vs. unsupervised); change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training.

Comparison 1 Physical activity: intervention vs. no intervention, Outcome 16 supgroup analysis (supervised vs. unsupervised); change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training.
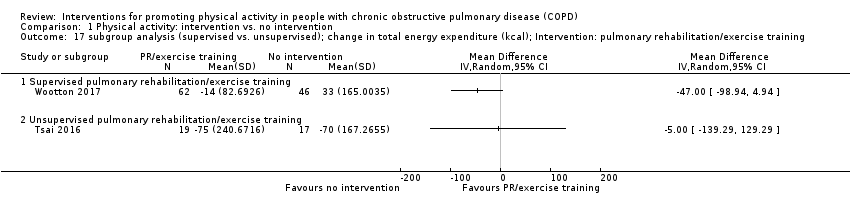
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 17 subgroup analysis (supervised vs. unsupervised); change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training.
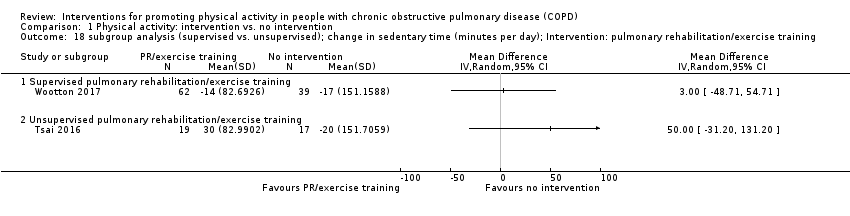
Comparison 1 Physical activity: intervention vs. no intervention, Outcome 18 subgroup analysis (supervised vs. unsupervised); change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training.

Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 1 step count (steps per day); Intervention: self‐management (health mentoring).

Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 2 change in step count (steps per day); Intervention: LAMA/LABA.
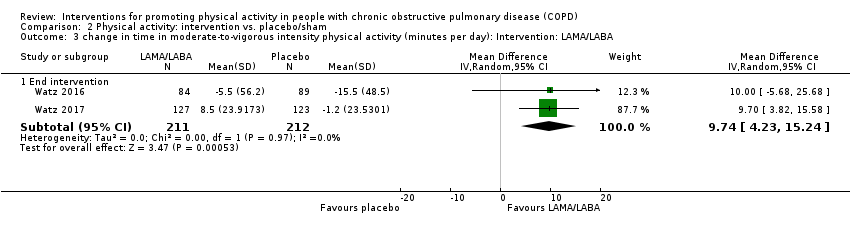
Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 3 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day): Intervention: LAMA/LABA.

Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 4 change in active energy expenditure (kcal); Intervention: LAMA/LABA.

Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 5 step count (steps per day); Intervention: nutritional supplement.
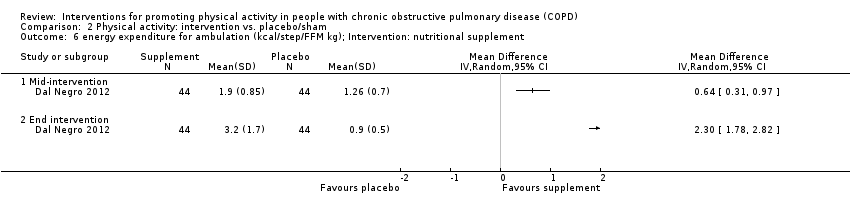
Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 6 energy expenditure for ambulation (kcal/step/FFM kg); Intervention: nutritional supplement.
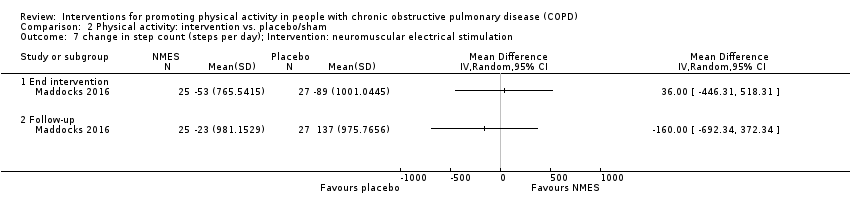
Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 7 change in step count (steps per day); Intervention: neuromuscular electrical stimulation.
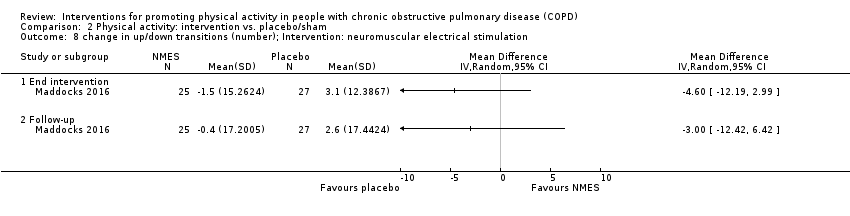
Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 8 change in up/down transitions (number); Intervention: neuromuscular electrical stimulation.

Comparison 2 Physical activity: intervention vs. placebo/sham, Outcome 9 change in time upright (hours); Intervention: neuromuscular electrical stimulation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 1 movement intensity (m/s2); Interventions: nordic walking with education vs. education.
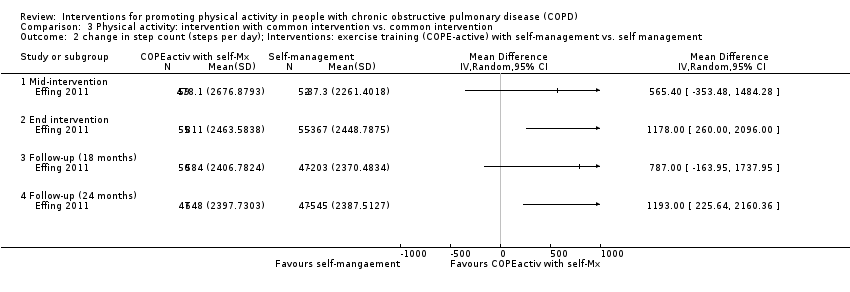
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 2 change in step count (steps per day); Interventions: exercise training (COPE‐active) with self‐management vs. self management.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 3 change in time in light‐intensity physical activity (minutes per day); Interventions: upper body resistance training with health education vs. health education.
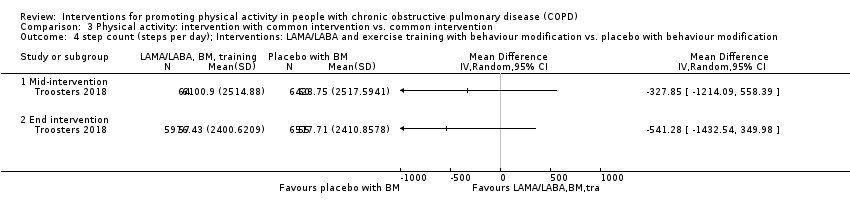
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 4 step count (steps per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 5 time walking (minutes per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 6 walking intensity (m/s2); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 7 step count (steps per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.
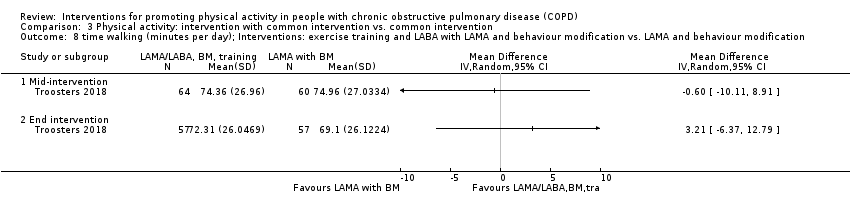
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 8 time walking (minutes per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 9 walking intensity (m/s2); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 10 step count (steps per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification.
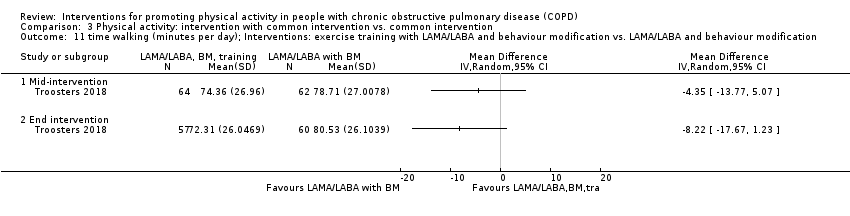
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 11 time walking (minutes per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification.
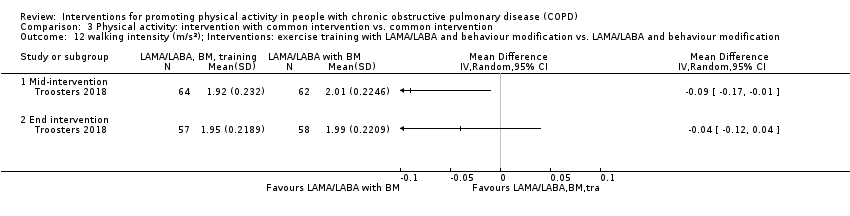
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 12 walking intensity (m/s2); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification.
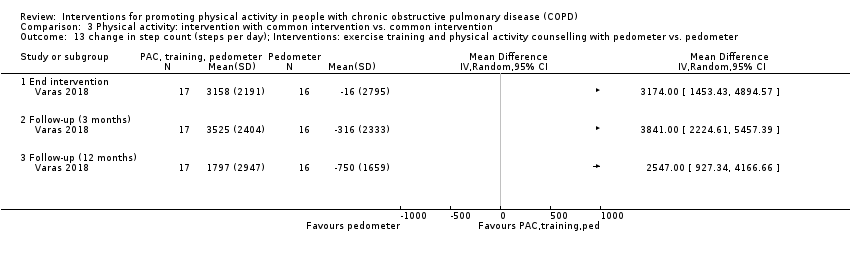
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 13 change in step count (steps per day); Interventions: exercise training and physical activity counselling with pedometer vs. pedometer.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 14 change in step count (weekday, steps per day); Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 15 METs; Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise.
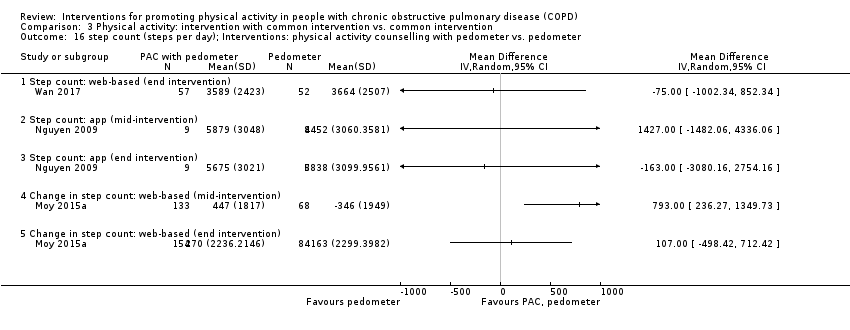
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 16 step count (steps per day); Interventions: physical activity counselling with pedometer vs. pedometer.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 17 time active (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 18 time inactive (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 19 peak performance (steps per minute); Interventions: physical activity counselling (app) with pedometer vs. pedometer.
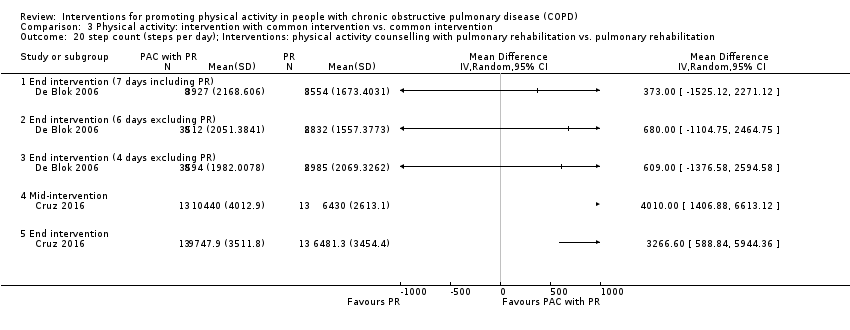
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 20 step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.
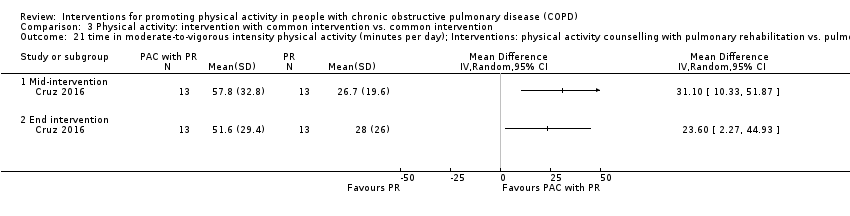
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 21 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.
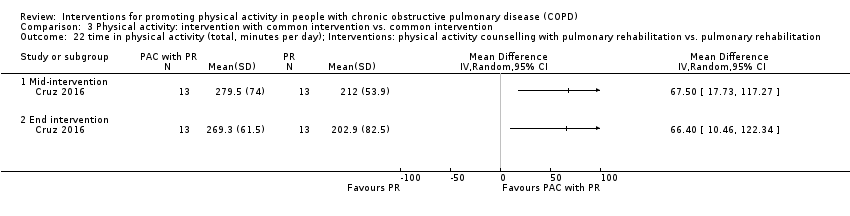
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 22 time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 23 time sedentary (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 24 change in step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 25 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 26 change in time walking (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.
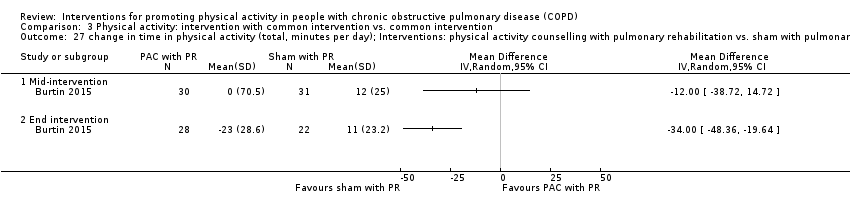
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 27 change in time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.
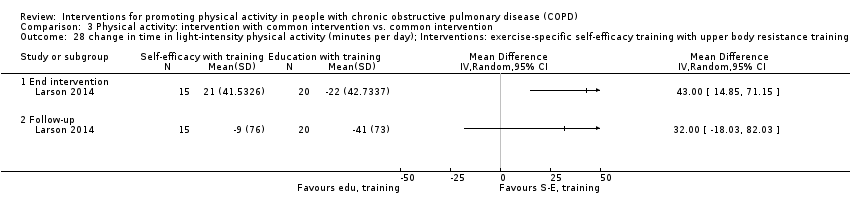
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 28 change in time in light‐intensity physical activity (minutes per day); Interventions: exercise‐specific self‐efficacy training with upper body resistance training vs. upper body resistance training.
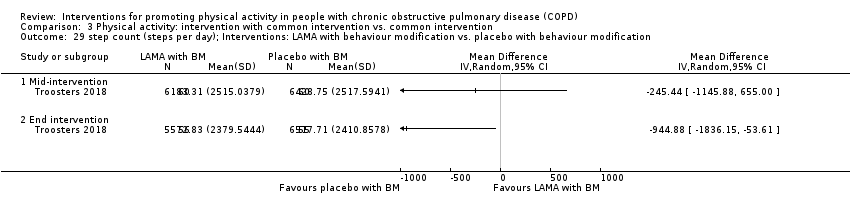
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 29 step count (steps per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification.
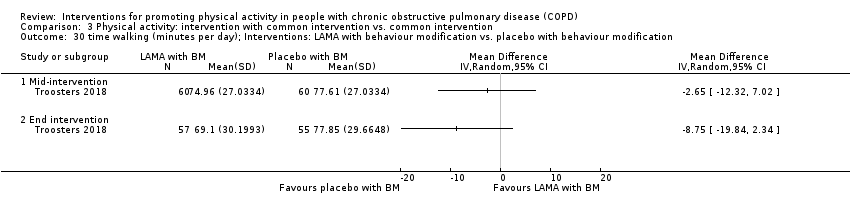
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 30 time walking (minutes per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 31 walking intensity (m/s2); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification.
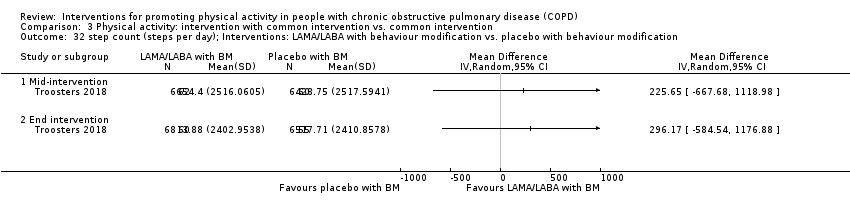
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 32 step count (steps per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 33 time walking (minutes per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification.
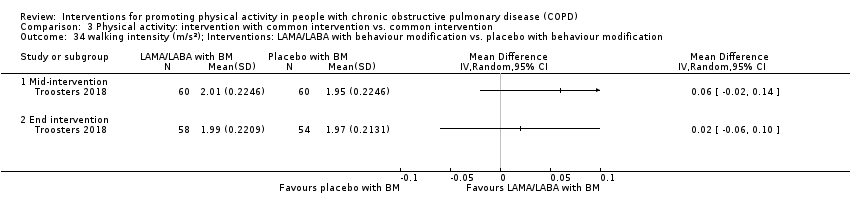
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 34 walking intensity (m/s2); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification.
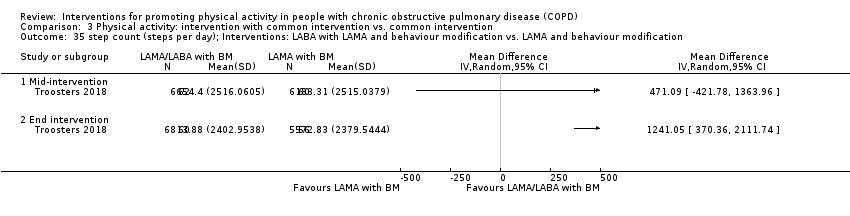
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 35 step count (steps per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 36 time walking (minutes per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 37 walking intensity (m/s2); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 38 change in step count (steps per day); Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 39 change in step count (steps per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 40 change in time in moderate‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.
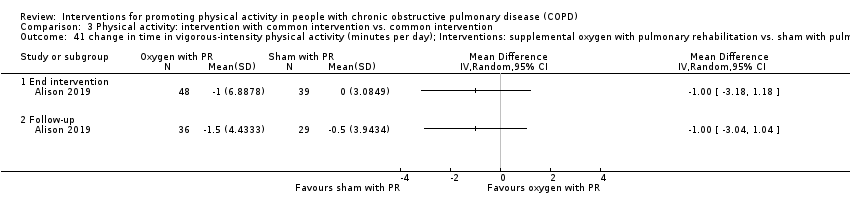
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 41 change in time in vigorous‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 42 change in time in light‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.
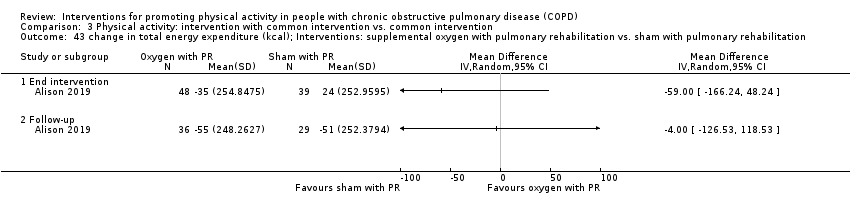
Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 43 change in total energy expenditure (kcal); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.

Comparison 3 Physical activity: intervention with common intervention vs. common intervention, Outcome 44 change in sedentary time (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 1 change in step count (steps per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 2 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 3 change in number of bouts of moderate‐to‐vigorous intensity physical activity; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.
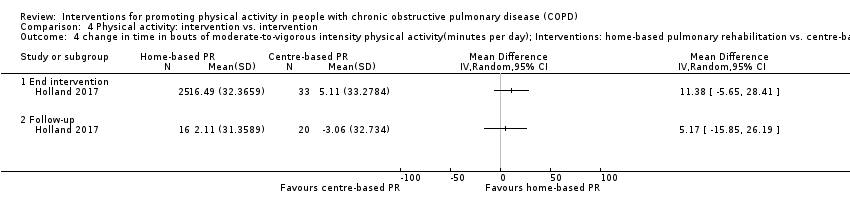
Comparison 4 Physical activity: intervention vs. intervention, Outcome 4 change in time in bouts of moderate‐to‐vigorous intensity physical activity(minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.
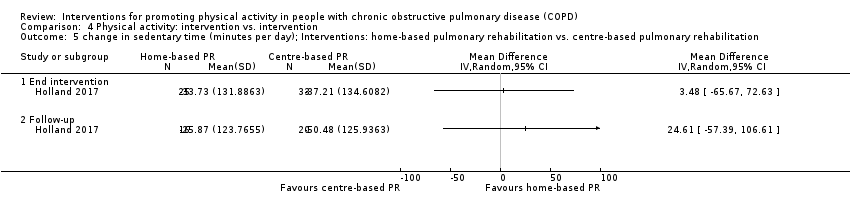
Comparison 4 Physical activity: intervention vs. intervention, Outcome 5 change in sedentary time (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.
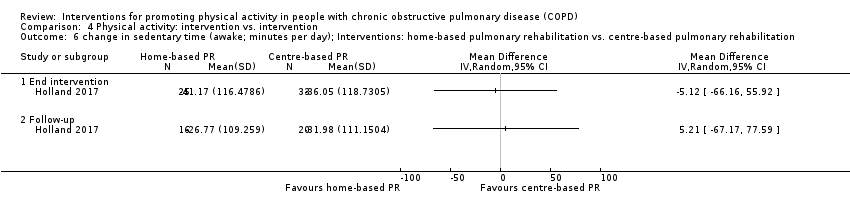
Comparison 4 Physical activity: intervention vs. intervention, Outcome 6 change in sedentary time (awake; minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 7 change in number of sedentary bouts; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.
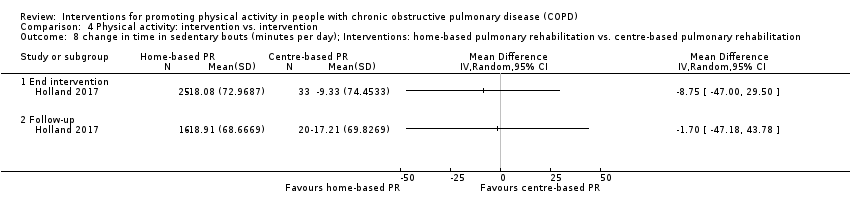
Comparison 4 Physical activity: intervention vs. intervention, Outcome 8 change in time in sedentary bouts (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 9 change in METs; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 10 change in total energy expenditure (kcal); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 11 change in step count (steps per day); Interventions: water‐based exercise training vs. land‐based exercise training.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 12 change in total energy expenditure (kcal); Interventions: water‐based exercise training vs. land‐based exercise training.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 13 step count (steps per day); Interventions: Tai Chi vs. pulmonary rehabilitation.
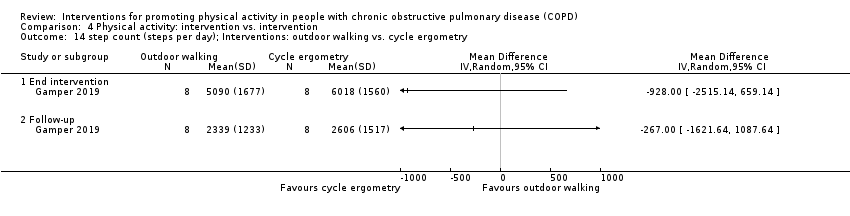
Comparison 4 Physical activity: intervention vs. intervention, Outcome 14 step count (steps per day); Interventions: outdoor walking vs. cycle ergometry.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 15 step count (steps per day); Interventions: physical activity counselling vs. pulmonary rehabilitation.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 16 total energy expenditure (kcal); Interventions: exercise training with tapered supervision vs. supervised exercise training.

Comparison 4 Physical activity: intervention vs. intervention, Outcome 17 mid day activity (vector magnitude units per minute); Interventions: supplemental oxygen (lightweight ambulatory) vs. supplemental oxygen (E‐cylinder).

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 1 change in SGRQ total score; Intervention: pulmonary rehabilitation/exercise training.

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 2 change in SGRQ domain scores. Intervention: pulmonary rehabilitation/exercise training (ground‐based walking).

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 3 change in CRQ domain scores; Intervention: pulmonary rehabilitation/exercise training.
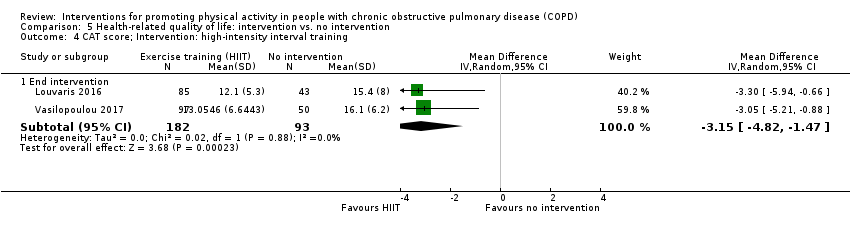
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 4 CAT score; Intervention: high‐intensity interval training.
![Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 5 SGRQ domain scores (%change); Intervention: exercise training [inpatient].](/es/cdsr/doi/10.1002/14651858.CD012626.pub2/media/CDSR/CD012626/image_n/nCD012626-CMP-005-05.png)
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 5 SGRQ domain scores (%change); Intervention: exercise training [inpatient].

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 6 SGRQ domain scores; Intervention: physical activity counselling.
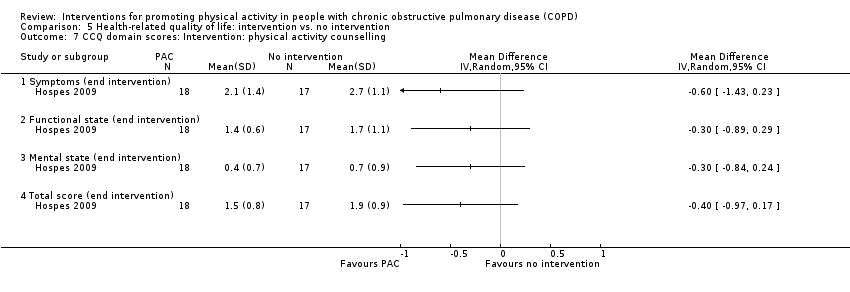
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 7 CCQ domain scores: Intervention: physical activity counselling.
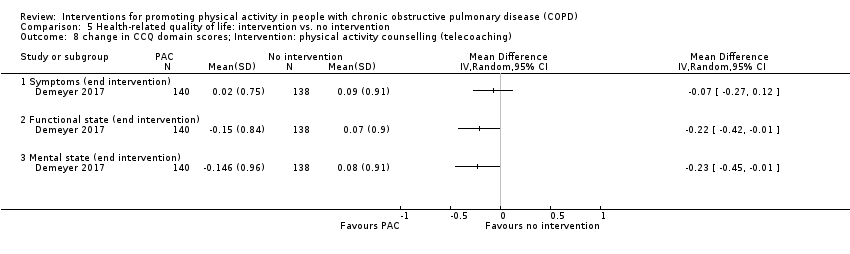
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 8 change in CCQ domain scores; Intervention: physical activity counselling (telecoaching).

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 9 change in CRQ domain and total scores; Intervention: physical activity counselling.

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 10 change in SGRQ domain and total scores; Intervention: physical activity counselling.

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 11 change in CRQ domain scores; Intervention: self‐management (SPACE).
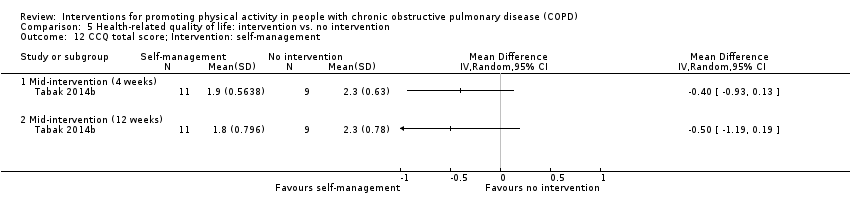
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 12 CCQ total score; Intervention: self‐management.
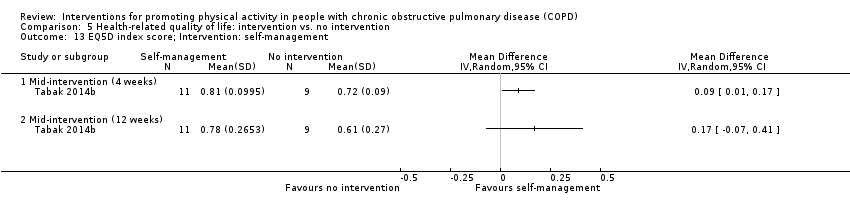
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 13 EQ5D index score; Intervention: self‐management.

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 14 EQ5D visual analogue scale score; Intervention: self‐management.
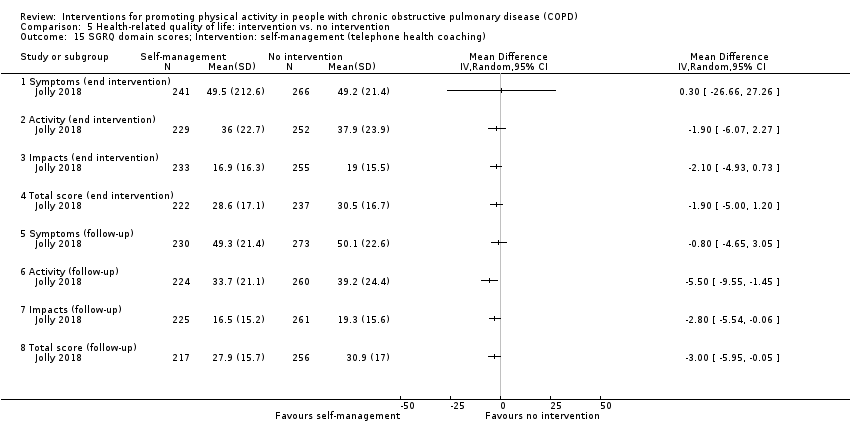
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 15 SGRQ domain scores; Intervention: self‐management (telephone health coaching).
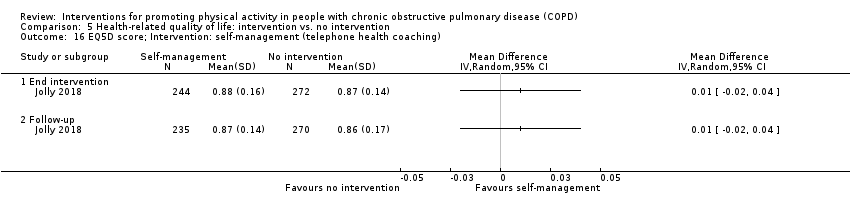
Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 16 EQ5D score; Intervention: self‐management (telephone health coaching).

Comparison 5 Health‐related quality of life: intervention vs. no intervention, Outcome 17 CRQ domain scores; Intervention: four‐wheeled walker.

Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 1 change in SF36 component scores; Intervention: singing.

Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 2 change in CRQ total score; Intervention: neuromuscular electrical stimulation.
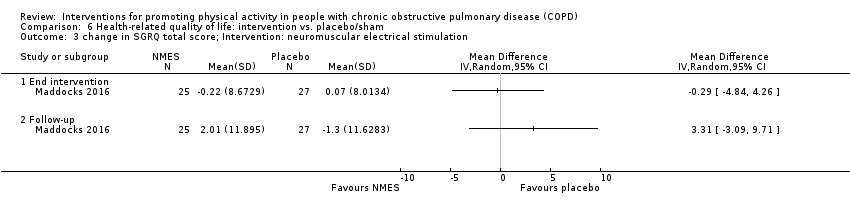
Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 3 change in SGRQ total score; Intervention: neuromuscular electrical stimulation.
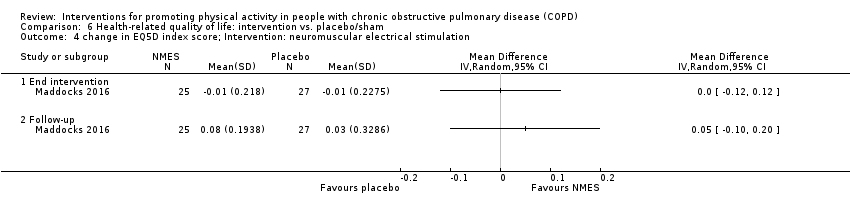
Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 4 change in EQ5D index score; Intervention: neuromuscular electrical stimulation.
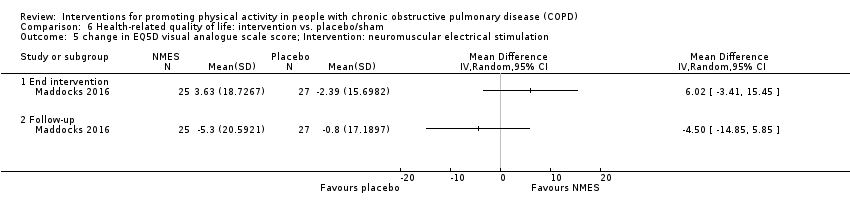
Comparison 6 Health‐related quality of life: intervention vs. placebo/sham, Outcome 5 change in EQ5D visual analogue scale score; Intervention: neuromuscular electrical stimulation.
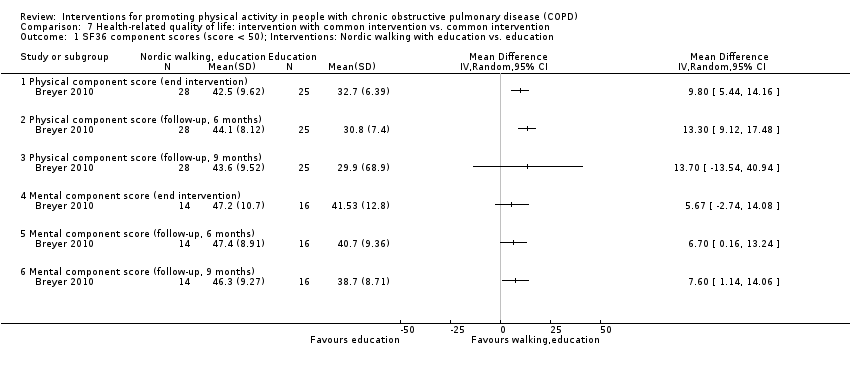
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 1 SF36 component scores (score < 50); Interventions: Nordic walking with education vs. education.
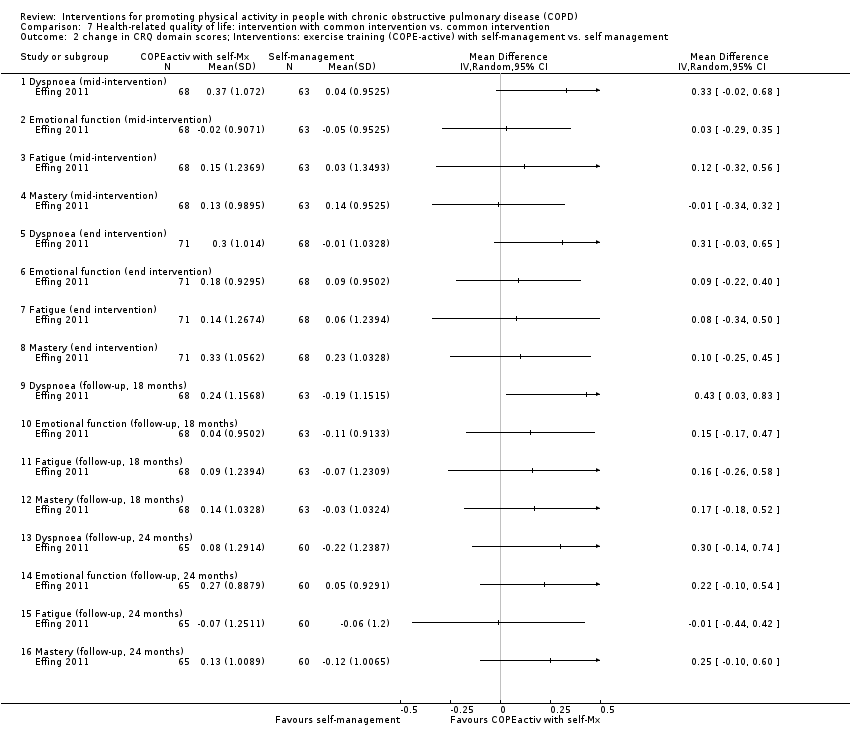
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 2 change in CRQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 3 change in CCQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 4 CRQ domain scores; Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 5 CRQ domain scores; Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 6 CRQ domain scores; Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 7 change in SGRQ domain and total scores; Intervention: exercise training and physical activity counselling with pedometer vs. pedometer.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 8 change in CRQ domain scores; Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 9 change in SGRQ total score; Interventions: physical activity counselling with pedometer vs. pedometer.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 10 SGRQ total: Interventions: physical activity counselling (app) with pedometer vs. pedometer.
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 11 SF36: Interventions: physical activity counselling (app) with pedometer vs. pedometer.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 12 change in SGRQ domain scores; Interventions: physical activity counselling (web‐based) with pedometer vs. pedometer.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 13 SGRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 14 RAND36 domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 15 change in CRQ dyspnoea domain score; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 16 SGRQ scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.
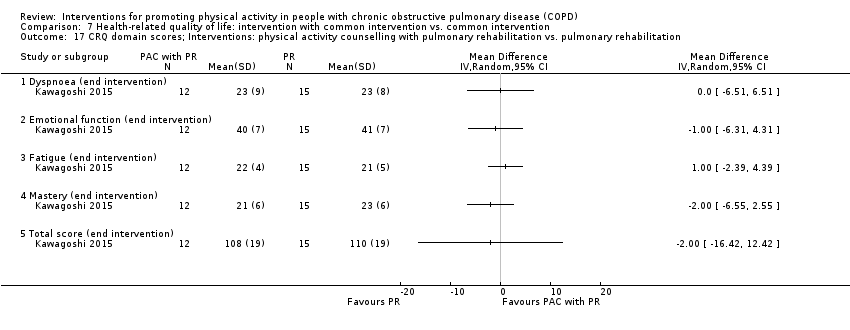
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 17 CRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 18 change in CRQ total score; Interventions: physical activity counselling with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation.
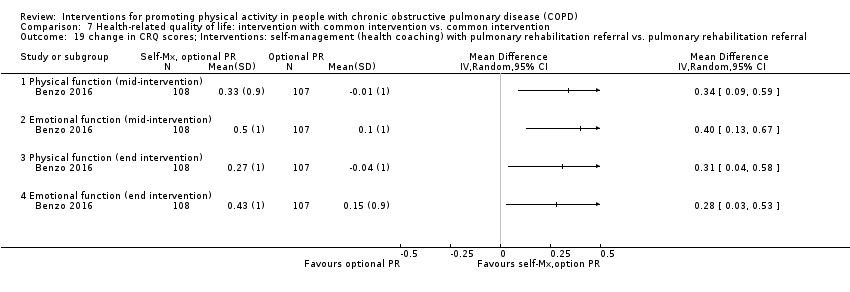
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 19 change in CRQ scores; Interventions: self‐management (health coaching) with pulmonary rehabilitation referral vs. pulmonary rehabilitation referral.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 20 CRQ domain scores; Interventions: LAMA with behaviour modification vs. placebo with behaviour modification.
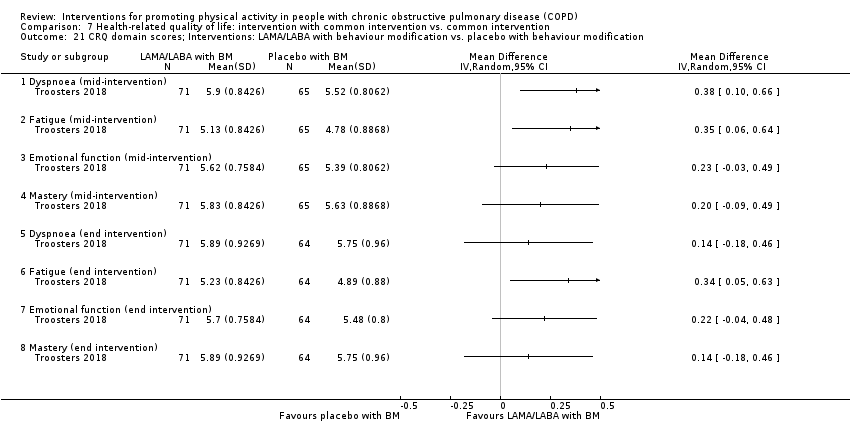
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 21 CRQ domain scores; Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 22 CRQ domain scores; Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.
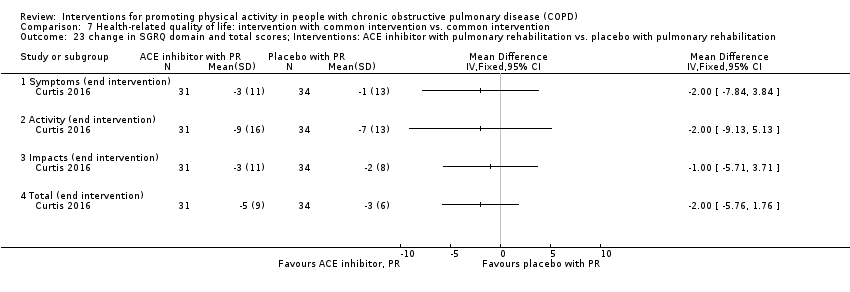
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 23 change in SGRQ domain and total scores; Interventions: ACE inhibitor with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 24 change in SGRQ total score; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 25 change in EQ5D; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation.

Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 26 change in CRQ domain scores; Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation.
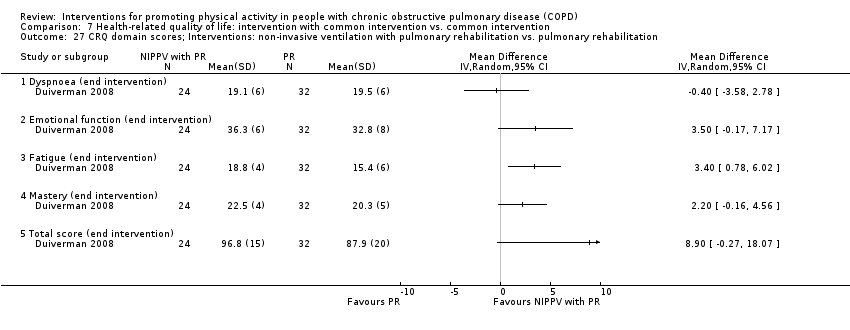
Comparison 7 Health‐related quality of life: intervention with common intervention vs. common intervention, Outcome 27 CRQ domain scores; Interventions: non‐invasive ventilation with pulmonary rehabilitation vs. pulmonary rehabilitation.
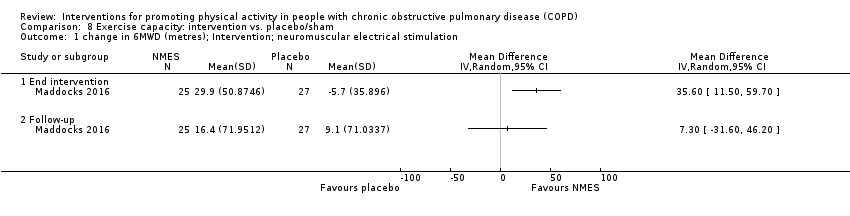
Comparison 8 Exercise capacity: intervention vs. placebo/sham, Outcome 1 change in 6MWD (metres); Intervention; neuromuscular electrical stimulation.
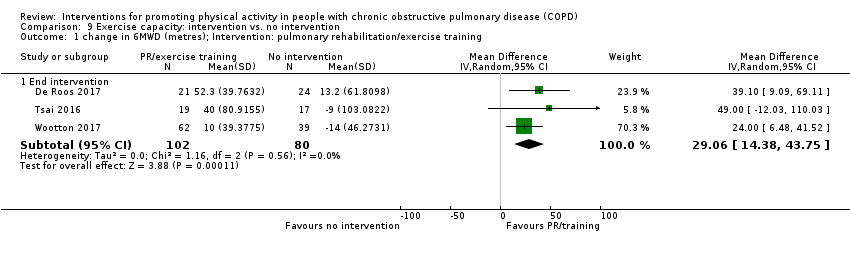
Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 1 change in 6MWD (metres); Intervention: pulmonary rehabilitation/exercise training.
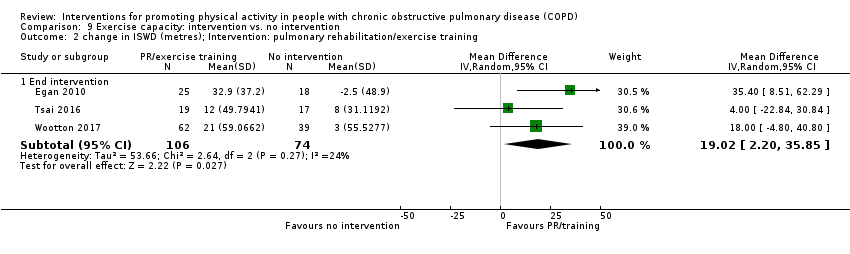
Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 2 change in ISWD (metres); Intervention: pulmonary rehabilitation/exercise training.

Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 3 change in ESWT (seconds); Intervention: pulmonary rehabilitation/exercise training.

Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 4 6MWD (metres); Intervention: high‐intensity interval training.

Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 5 work rate (watts); Intervention: high‐intensity interval training.
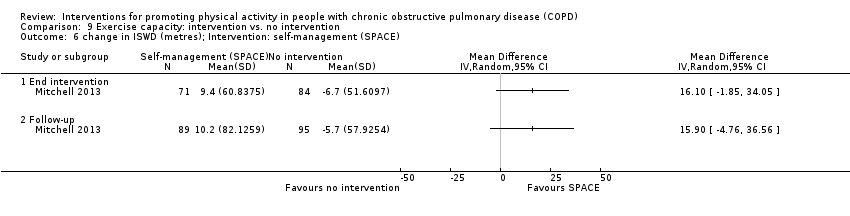
Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 6 change in ISWD (metres); Intervention: self‐management (SPACE).
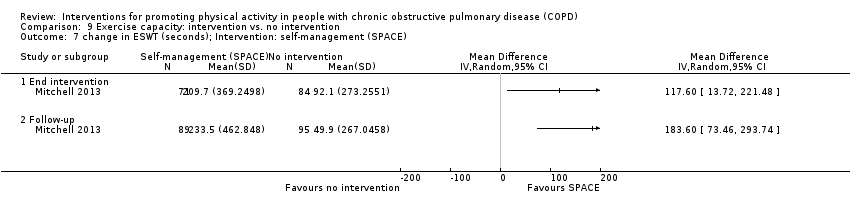
Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 7 change in ESWT (seconds); Intervention: self‐management (SPACE).
![Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 8 6MWD (metres); Intervention: exercise training [inpatient].](/es/cdsr/doi/10.1002/14651858.CD012626.pub2/media/CDSR/CD012626/image_n/nCD012626-CMP-009-08.png)
Comparison 9 Exercise capacity: intervention vs. no intervention, Outcome 8 6MWD (metres); Intervention: exercise training [inpatient].
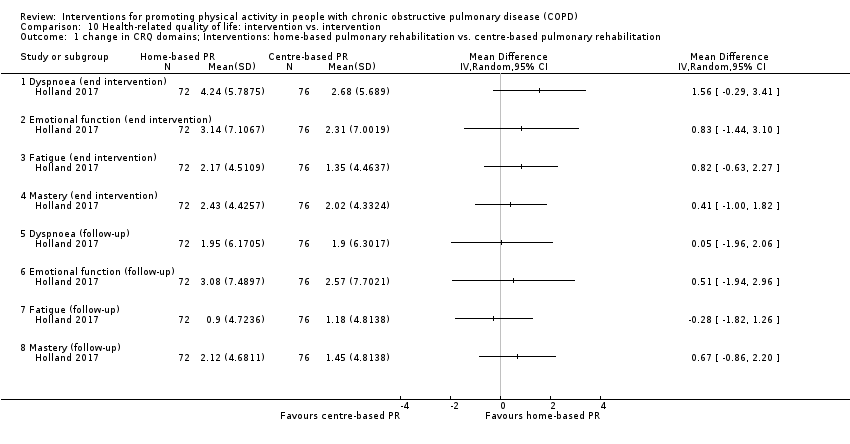
Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 1 change in CRQ domains; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 2 change in CRQ domains; Interventions: water‐based exercise training vs. land‐based exercise training.

Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 3 SGRQ domain and total scores; Interventions: Tai Chi vs. pulmonary rehabilitation.
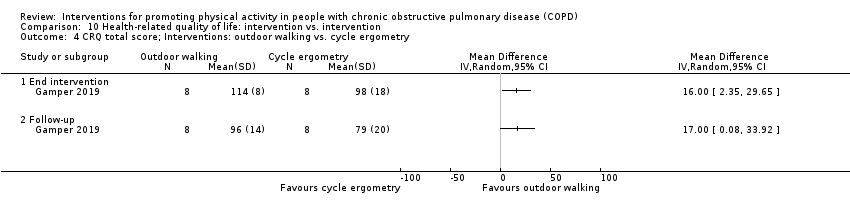
Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 4 CRQ total score; Interventions: outdoor walking vs. cycle ergometry.

Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 5 Maugeri Respiratory Failure questionnaire; Interventions: exercise training with tapered supervision vs. supervised exercise training.

Comparison 10 Health‐related quality of life: intervention vs. intervention, Outcome 6 SF36 domain scores; Interventions: self‐management vs. education and symptom monitoring.
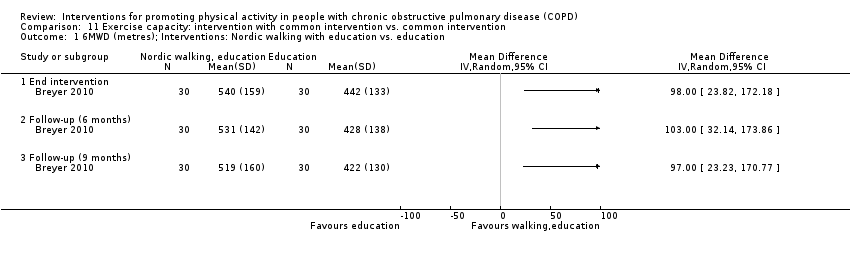
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 1 6MWD (metres); Interventions: Nordic walking with education vs. education.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 2 change in ISWD (metres); Interventions: exercise training (COPE‐active) with self‐management vs. self management.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 3 change in ESWT (seconds); Interventions: exercise training (COPE‐active) with self‐management vs. self management.
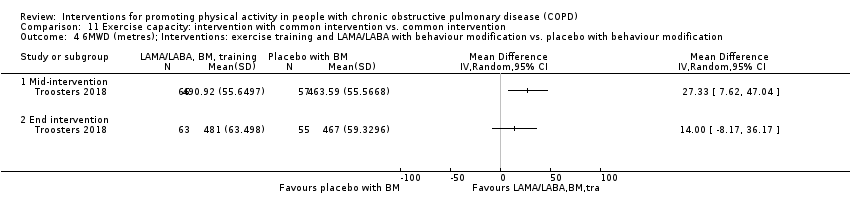
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 4 6MWD (metres); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 5 ESWT (seconds); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 6 6MWD (metres); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 7 ESWT (seconds); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 8 6MWD (metres); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification.
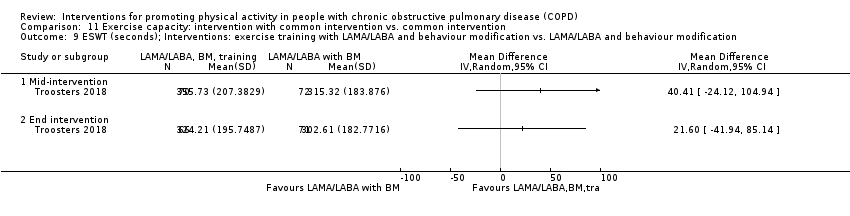
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 9 ESWT (seconds); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 10 change in ESWT (seconds); Intervention: physical activity counselling and exercise training with pedometer vs. pedometer.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 11 6MWD (metres); Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 12 6MWD (metres); Interventions: physical activity counselling (app) with pedometer vs. pedometer.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 13 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 14 change in 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 15 6MWD (metres); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification.
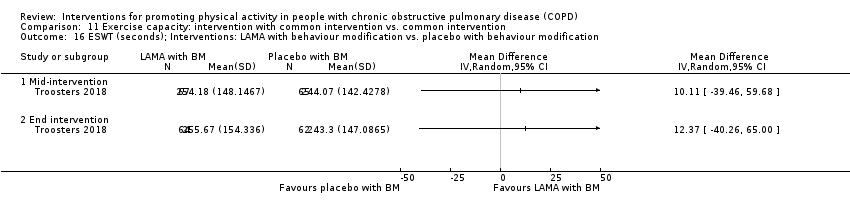
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 16 ESWT (seconds); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 17 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification.
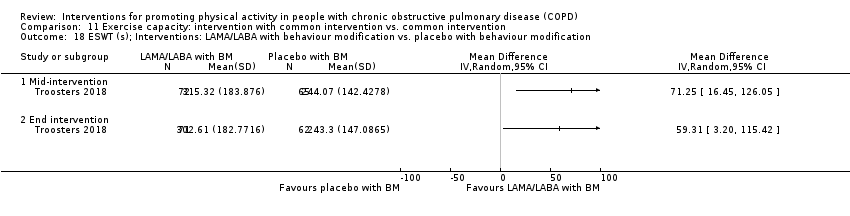
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 18 ESWT (s); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification.
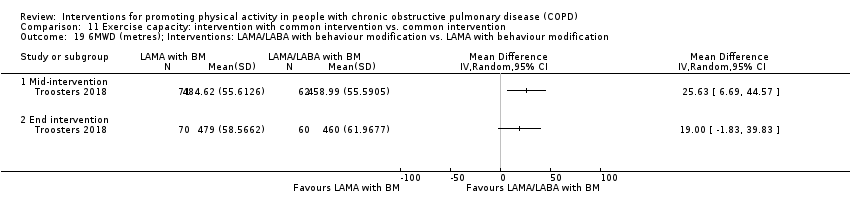
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 19 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification.
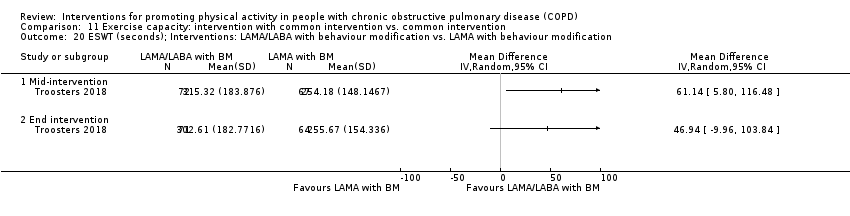
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 20 ESWT (seconds); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification.
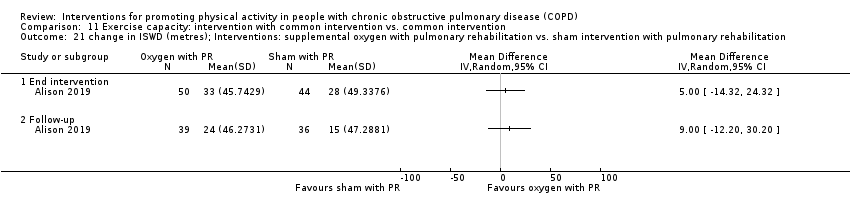
Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 21 change in ISWD (metres); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation.

Comparison 11 Exercise capacity: intervention with common intervention vs. common intervention, Outcome 22 change in ESWT (seconds); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation.
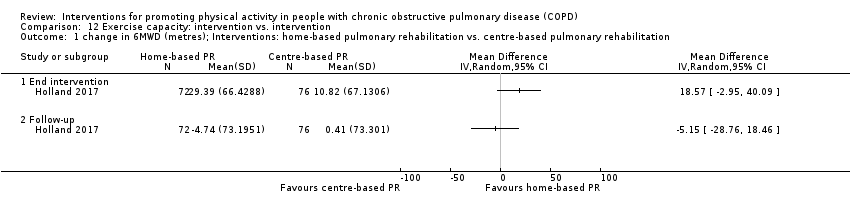
Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 1 change in 6MWD (metres); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation.

Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 2 change in 6MWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training.

Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 3 change in ISWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training.
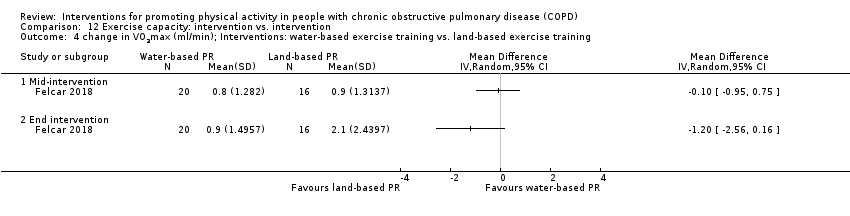
Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 4 change in VO2max (ml/min); Interventions: water‐based exercise training vs. land‐based exercise training.
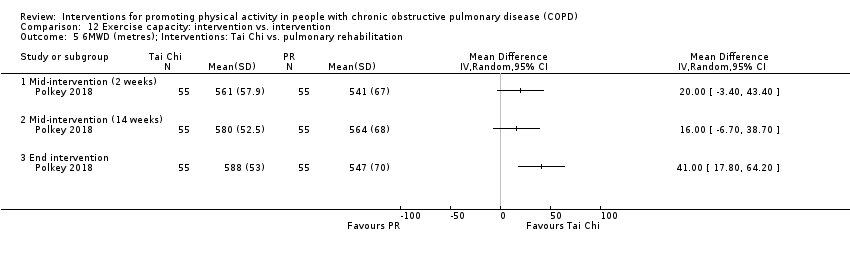
Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 5 6MWD (metres); Interventions: Tai Chi vs. pulmonary rehabilitation.

Comparison 12 Exercise capacity: intervention vs. intervention, Outcome 6 6MWD (metres); Interventions: exercise training with tapered supervision vs. supervised exercise training.
| Population: people with COPD, clinical stability Intervention: pulmonary rehabilitation/exercise training Comparisons: intervention versus no intervention Outcome: time in physical activity (of at least moderate intensity) at end intervention | ||||||
| Interventions | Outcome | Illustrative comparative risks* [mean difference (95% CI) unless indicated] | Number of participants (studies) | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Pulmonary rehabilitation/exercise training | |||||
| Pulmonary rehabilitation vs. no intervention (8 to 10 weeks) | Time/change in time in MVPA | The mean change in time ranged from −1 to 6 minutes per day, mean time 27 minutes per day | The mean difference was 4 (−2 to 9) minutes per day | 190 participants (3 studies; Analysis 1.2) | ⊕⊕⊝⊝ | Baseline values: De Roos 2017 no intervention mean 11 (SD 10), pulmonary rehabilitation 12 (11); Wootton 2017 no intervention 46 (39), pulmonary rehabilitation 54 (43) |
| High‐intensity interval training vs. no intervention (8 to 12 weeks) | Time in MVPA | The mean time ranged from 12 to 14 minutes per day | The mean difference was 6 (4 to 8) minutes per day | 275 participants (2 studies; Analysis 1.9) | ⊕⊕⊕⊝ moderateb | ‐ |
| Maintenance (telerehabilitation) following high‐intensity interval training vs. no intervention (12 months) | Time in moderate intensity physical activity | The mean time was 11 minutes per day | The mean difference was 7 (4 to 10) minutes per day | 97 participants (1 study; Vasilopoulou 2017; Table 1) | ⊕⊕⊕⊝ moderateb | ‐ |
| Maintenance (centre‐based) following high intensity interval training vs. no intervention (12 months) | The mean difference was 11 (8 to 14) minutes per day | 100 participants (1 study; Vasilopoulou 2017; Table 1) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for high risk of performance bias. Downgraded one level for imprecision as CI does not exclude possibility of no effect. | ||||||
| Study | Comparison (setting, if known) Clinical stability unless indicated | Timepoint (end intervention unless indicated) | Outcome (minutes unless indicated) | Intervention group | Comparison group | Between‐group MD (95% CI) where available unless indicated | ||
| n | mean (95% CI) unless indicated | n | mean (95% CI) unless indicated | |||||
| Physical activity counselling vs. no intervention (primary care) | 12 weeks | ∆ step count (n) | 22 | median 537 (IQR −611 to 1740) | 18 | median 431 (IQR −899 to 749) | P = 0.48* | |
| ∆ "daily physical activity" (n) | median 1408 (IQR −2165 to 3304) | median 528 (IQR −966 to 2179) | P = 0.35* | |||||
| follow‐up (12 months post‐intervention) | ∆ step count (n) | 20 | median 157 (IQR −1679 to 994) | 18 | median 48 (IQR −1004 to 885) | P = 0.90* | ||
| ∆ "daily physical activity" (n) | median 353 (IQR −1518 to 3038) | median −576 (IQR −2517 to 1008) | P = 0.26* | |||||
| Physical activity counselling vs. no intervention (secondary care) | 12 weeks | ∆ step count (n) | 21 | median 1002 (IQR −612 to 3077) | 22 | median −814 (IQR −2827 to 1063) | P = 0.007* | |
| ∆ "daily physical activity" (n) | median 1575 (IQR −752 to 3864) | median −1041 (IQR −1971 to 1031) | P = 0.007* | |||||
| follow‐up (12 months post‐intervention) | ∆ step count (n) | 20 | median 1128 (IQR −1322 to 2707) | 19 | median −217 (IQR −1951 to 1147) | P = 0.15* | ||
| ∆ "daily physical activity" (n) | median 1798 (IQR −1994 to 3128) | median −718 (IQR −1812 to 512) | P = 0.11* | |||||
| Physical activity counselling with PR vs. PR | 12 weeks | ∆ step count (n) | 22 | median 547 (IQR 187 to 1323) | 15 | median −211 (IQR −1337 to 1038) | P = 0.03* | |
| ∆ "daily physical activity" (n) | median 1302 (IQR −173 to 1922) | median −849 (IQR −2223 to 961) | P = 0.03* | |||||
| follow‐up (12 months post‐intervention) | ∆ step count (n) | 10 | median −569 (IQR −2512 to 1551) | 13 | median −1137 (IQR −2376 to 1427) | P = 0.58* | ||
| ∆ "daily physical activity" (n) | median −213 (IQR −4525 to 2274) | median −1827 (IQR −3540 to 629) | P = 0.97* | |||||
| Physical activity counselling vs. no intervention (primary care) SUBGROUP: ≥ 10,000 steps per day (baseline) | 12 weeks | ∆ step count (n) | median 675 (IQR 4 to 1853) | median 342 (IQR −955 to 658) | P = 0.20* | |||
| ∆ "daily physical activity" (n) | median 1807 (IQR 164 to 3720) | median 519 (IQR −1089 to 1709] | P = 0.11* | |||||
| follow‐up (12 months post‐intervention) | ∆ step count (n) | median 201 (IQR −693 to 1170) | median 38 (IQR −1071 to 821) | P = 0.55* | ||||
| ∆ "daily physical activity" (n) | median 525 (IQR −545 to 3078) | median −726 (IQR −2954 to 711) | P = 0.06* | |||||
| Physical activity counselling vs. no intervention (secondary care) SUBGROUP: ≤ 10,000 steps per day (baseline) | 12 weeks | ∆ step count (n) | median 1289 (IQR −183 to 3107) | median 34 (IQR −1707 to 1095) | P = 0.02* | |||
| ∆ "daily physical activity" (n) | median 1763 (IQR −763 to 3913) | median −925 (IQR −1452 to 1052) | P = 0.03* | |||||
| follow‐up (12 months post‐intervention) | ∆ step count (n) | median 1436 (IQR −1492 to 2722) | median 0 (IQR −1825 to 1103) | P = 0.12* | ||||
| ∆ "daily physical activity" (n) | median 1928 (IQR −1140 to 3320) | median −526 (IQR −1657 to 435) | P = 0.078* | |||||
| Physical activity counselling with PR vs. PR SUBGROUP: ≤ 10,000 steps per day (baseline) | 12 weeks | ∆ step count (n) | median 547 (IQR 187 to 1323) | median −198 (IQR −1403 to 1051) | P = 0.04* | |||
| ∆ "daily physical activity" (n) | median 1302 (IQR −173 to 1922) | median −843 (IQR −1737 to 1329) | P = 0.052* | |||||
| follow‐up (12 months post intervention) | ∆ step count (n) | median −569 (IQR −1770 to 2170) | median −759 (IQR −2027 to 1641) | P = 0.78* | ||||
| ∆ "daily physical activity" (n) | median −213 (IQR −4525 to 2274) | median −644 (IQR −3706 to 844) | P = 0.91* | |||||
| LAMA vs. placebo | 3 weeks | ∆ step count (n) | 51 | median 69 (IQR −834 to 1262) | 53 | median 125 (IQR −1180 to 1249) | P = 0.73 | |
| ∆ MVPA time | median 1 (IQR −22 to 25) | median −6 (IQR −21 to 15) | P = 0.11 | |||||
| ∆ PAL | median 0.00 (IQR −0.08 to 0.09) | median −0.01 (IQR −0.09 to 0.09) | P = 0.95 | |||||
| ∆ active EE (kcal) | median 10 (−131 to 116) | median −44 (IQR −122 to 40) | P = 0.11 | |||||
| Physical activity counselling with pedometer vs. pedometer | 12 weeks | step count (n) | 49 | x | 50 | x | x | |
| Self‐management (health coaching) with PR referral vs. PR referral | 12 months | ∆ PAL | 108 | mean −0.10 | 106 | mean 0.01 | P = not significant* | |
| step count (n) | only baseline values reported | only baseline values reported | "We did not find a difference in any physical activity outcome between the intervention and control arms at any time point"* | |||||
| time sedentary | ||||||||
| LIPA time | ||||||||
| MPA time | ||||||||
| VPA time | ||||||||
| resting metabolic rate (calories per 24 hours) | ||||||||
| total EE (calories per 24 hours) | ||||||||
| Self‐management vs. education and symptom management | 16 weeks | activity time | 162 | mean 13 (SE 1) | 164 | mean 11 (SE 1) | P = 0.045* | |
| MPA time | mean 6 (SE 0.4) | mean 5.5 (SE 0.4) | 0.5 (−0.6 to 1.6) | |||||
| total EE ("caloric expenditure") | mean 3605 (SE 211) | mean 3113 (SE 212) | P = 0.022* | |||||
| Exercise training (whole‐body resistance training) vs. no intervention (inpatient) | 4‐week follow‐up | time lying | 15 | mean 224 (SD 131) | 14 | mean 203 (SD 140) | 21 (−78 to 120) | |
| time sitting | mean 287 (122) | mean 298 (SD 107) | −11 (−94 to 72) | |||||
| time standing | mean 168 (104) | mean 153 (SD 94) | 15 (−57 to 87) | |||||
| time walking | mean 31 (21) | mean 50 (SD 35) | −19 (−40 to 2) | |||||
| Nordic walking with education vs. education | 12 weeks | ∆ time sitting | 30 | mean −128 (SD 15) | 30 | "Controls did not show any significant change in | P = 0.014* | |
| ∆ time standing | mean 129 (SD 26) | x | ||||||
| ∆ time walking | mean 15 (SD 20) | P = 0.034* | ||||||
| follow‐up (3 months post‐intervention) | ∆ time sitting | mean −120 (SD 32) | P < 0.05* | |||||
| ∆ time standing | mean 133 (SD 14) | P < 0.05* | ||||||
| ∆ time walking | mean 13 (SD 2) | x | ||||||
| follow‐up (6 months post‐intervention) | time sitting | mean 233 (SD 172) | mean 342 (SD 126) | P < 0.01* | ||||
| time standing | mean 320 (SD 178) | mean 220 (SD 130) | P = 0.16 | |||||
| time walking | mean 56 (SD 38) | mean 32 (SD 25) | P < 0.01 | |||||
| Web‐based PR vs. centre‐based PR | 7 weeks | step count (n) | 20 | x | 34 | x | P = 0.37* | |
| "20 min bouts of purposeful activity" (n) | mean change 10% | x | P = 0.26* | |||||
| Inspiratory muscle training with PR vs. sham with PR | 12 weeks (total n = 150) | step count (n) | x | mean 3958 (SD 2253) | x | mean 4506 (SD 1899) | −206 (−923 to 512)* | |
| ACE inhibitor with PR vs. placebo with PR | 10 weeks | ∆ step count (n) | 18 | mean −382 (SD 2082) | 22 | mean 561 (SD 2528) | −943 (−2372 to 486) | |
| ∆ PAL | mean −0.06 (SD 0.16) | mean 0.04 (SD 0.15) | −0.10 (−0.20 to −0.00) | |||||
| Physical activity counselling vs. no intervention | 12 weeks | ∆ time walking | 140 | 7 (1 to 13) | 140 | −10 (−14 to −6) | 17 (10 to 24) | |
| ∆ intensity movement (m/s2) | 0.06 (0.02 to 0.10) | −0.03 (−0.06 to −0.00) | 0.09 (0.04 to 0.14) | |||||
| ∆ MVPA time | 8 (5 to 12) | −3 (−6 to 0.2) | 11 (7 to 15) | |||||
| Non‐invasive ventilation with PR vs. PR | 12 weeks | step count (n) | 24 | median 2799 (IQR 891 to 6135) | 32 | median 2093 (IQR 914 to 3155) | median 1269 (IQR 242 to 2296)* | |
| Water‐based exercise training vs. land‐based exercise training | 6 months | active (n) (> 7500 steps per day) | 20 | baseline 4 end intervention 10 | 16 | baseline 4 end intervention 5 | x | |
| Endobronchial valve surgery vs. no intervention | 6 months post‐surgery | ∆ step count (n) | 19 | 1252 (545 to 1960) | 24 | −148 (−512 to 216) | 1400 (655 to 2145) | |
| ∆ time walking (%) | 1 (0 to 2) | 0 (−1 to 0) | 1 (0 to 2) | |||||
| ∆ time sitting (%) | 0 (−3 to 3) | 2 (1 to 3) | −2 (−5 to 1) | |||||
| ∆ time inactive | −1 (−3 to 1) | 0 (−1 to 1) | −1 (−3 to 1) | |||||
| ∆ intensity movement (g) | 0.01 (0.00 to 0.01) | 0.00 (−0.01 to 0.00) | 0.01 (0.00 to 0.02) | |||||
| Physical activity counselling vs. no intervention | 4 weeks | ∆ step count (n) | 12 | 984 (217 to 1752) | 14 | 1013 (307 to 1719) | −29 (−969 to 911) | |
| ∆ time walking | 13 (3 to 23) | 13 (5 to 21) | 0 (−12 to 12) | |||||
| ∆ intensity movement (m/s2) | 0.06 (0.03 to 0.09) | 0.08 (−0.05 to 0.11) | −0.02 (−0.06 to 0.02) | |||||
| Self‐management vs. no intervention | 12 months | MVPA time a week | 179 | mean 347 (SD 277) | 232 | mean 316 (SD 256) | 12 (−21 to 45)* | |
| Self‐management (SPACE) vs. no intervention (post‐admission) | 7 days | ∆ step count (n) | 15 | −208 (−1146 to 730) | 10 | −518 (−2572 to 1536) | 310 (−1665 to 2285) | |
| ∆ time sedentary | −14 (−71 to 43) | −18 (−87 to 51) | 4 (−75 to 83) | |||||
| ∆time in "physical activity" | −1 (−14 to 12) | −16 (−66 to 34) | 15 (−30 to 60) | |||||
| ∆ MPA time | −1 (−14 to 12) | −14 (−61 to 33) | 13 (−30 to 56) | |||||
| ∆ VPA time | 0 (−1 to 1) | −2 (−61 to 2) | 2 (−1 to 5) | |||||
| ∆ total EE (kcal) | −128 (−236 to −20) | −98 (−292 to 96) | −30 (−225 to 165) | |||||
| ∆ active EE (kcal) | −12 (−77 to 53) | −97 (−310 to 116) | 85 (−108 to 278) | |||||
| Physical activity counselling with PR vs. PR | 12 months | ∆ time sitting | 12 | 59 (−6 to 124) | 15 | 6 (−44 to 56) | 53 (−21 to 127) | |
| ∆ time standing | 43 (24 to 60) | 31 (5 to 57) | 11 (−18 to 40) | |||||
| ∆ time walking | 51 (10 to 92) | 12 (−2 to 27) | 39 (1 to 78) | |||||
| ∆ time lying | −53 (−96 to −10) | −29 (−60 to 2) | −24 (−72 to 24) | |||||
| ∆ frequency postural changes: total (n) | 40 (−2 to 82) | 19 (−5 to 43) | 21 (−23 to 65) | |||||
| ∆ frequency postural changes: getting up (n) | 0 (−16 to 16) | 6 (−11 to 23) | −6 (−27 to 15) | |||||
| ∆ frequency postural changes: standing up (n) | 43 (5 to 81) | 14 (−5 to 33) | 29 (−9 to 67) | |||||
| Exercise‐specific self‐efficacy training with upper‐body resistance training vs. upper‐body resistance training | 4 months | time sedentary | 15 | mean 602 (SD 112) | 20 | mean 577 (SD 107) | 25 (−49 to 99) | |
| time sedentary (% monitored time) | mean 70 (SD 10) | mean 70 (SD 9) | 0 (−6 to 6) | |||||
| MVPA time | mean 6 (SD 6) | mean 4 (SD 3) | 2 (−1 to 5) | |||||
| Upper‐body resistance training with health education vs. health education | time sedentary | 20 | mean 577 (SD 107) | 14 | mean 634 (SD 114) | −57 (−133 to 19) | ||
| time sedentary (% monitored time) | mean 70 (SD 9) | mean 70 (SD 9) | −2 (−8 to 4) | |||||
| MVPA time | mean 4 (SD 3) | mean 3 (SD 2) | 1 (−1 to 3) | |||||
| Physical activity counselling with PR vs. PR | 12 weeks | ∆ step count (n) | 25 | x | 25 | x | MD 1319 (SE 571), P = 0.02* | |
| ∆ MVPA time | MD 8 (SE 4), P = 0.11* | |||||||
| 9 months (follow‐up) | ∆ step count (n) | MD 1348 (SE 628), P = 0.03* | ||||||
| ∆ MVPA time | MD 13 (SE 5), P = 0.02* | |||||||
| Singing vs. sham | 8 weeks | ∆ step count (n) | 13 | −763 (−1758 to 232) | 11 | 1011 (337 to 1685) | −1774 (−2848 to −700) | |
| ∆ time sedentary | −36 (−113 to 41) | −27 (−72 to 18) | −9 (−88 to 71) | |||||
| ∆ “physical activity duration” | −93 (−224 to 38) | 50 (22 to 77) | −142 (−263 to −22) | |||||
| ∆ "activity‐related" EE (kJ) | −144 (−408 to 119) | 229 (131 to 327) | −373 (−625 to −121) | |||||
| High‐intensity interval training vs. no intervention | 12 weeks | step count (n) | 85 | mean 5136 (SD 2866) | 43 | mean 3453 (SD 2493) | 1683 (721 to 2646) | |
| vector magnitude units (n) | mean 495 (SD 213) | mean 406 (SD 205) | 89 (13 to 165) | |||||
| "sedentarism" (%) (< 5000 steps per day) | baseline 69 end intervention 48 | baseline 68 end intervention 69 | x | |||||
| LAMA vs. placebo | 3 weeks | ∆ step count (n) | 14 | median 177 (IQR −222 to 1038) | 15 | median 86 (IQR −366 to 1000) | P = 0.63 | |
| ∆ MVPA time | median −2 (IQR −12 to 26] | median −4 (IQR −16 to 19) | P = 0.51 | |||||
| ∆ PAL | median 0.01 (IQR −0.03 to 0.07] | median 0.01 (IQR −0.06 to 0.04) | P = 0.71 | |||||
| ∆ active EE (kcal) | median 43 (IQR −25 to 153) | median 17 (IQR −69 to 50) | P = 0.51 | |||||
| Physical activity counselling with PR vs. PR | 12 weeks | ∆ step count (n) | 22 | mean 1251 (SD 2408) | 22 | mean −410 (SD 1118) | 1661 (552 to 2770) | |
| Pedometer with physical activity counselling vs. physical activity counselling | 12 weeks | ∆ step count (n) | 50 | mean 3080 (SD 3255) | 47 | mean 138 (SD 1950) | 2942 (1881 to 4003) | |
| Self‐management (SPACE) vs. no intervention | 6 weeks | ∆ step count (n) | 52 | 333 (−85 to 751) | 65 | −214 (−566 to 138) | 547 (12 to 1082) | |
| ∆ time sedentary | −10 (−53 to 33) | 13 (−21 to 47) | −23 (−77 to 31) | |||||
| ∆ total EE (kcal) | −4 (−105 to 97) | −20 (−83 to 43) | 16 (−100 to 132) | |||||
| time in bouts (data from graph) | median 142 (95% CI 91 to 190) | median 96 (95% CI 56 to 135) | P = 0.215* | |||||
| LAMA (aclidinium bromide) vs. LAMA (tiotropium) | 8 weeks | "physical activity with sedentary time" | 22 | x | 22 | x | P = 0.385* | |
| LAMA vs. placebo | 12 weeks | not defined | 123 | x | 125 | x | "increase of activity in tiotropium relative to placebo from 7.9% to 12.23%" "the majority of the mean values over time in the tiotropium group are larger than those in the placebo group" | |
| ICS (beclomethasone) with LABA (formoterol) vs. ICS (budesonide) with LABA (formoterol) | 16 weeks (4‐week run‐in, 12 week intervention) | step count (n) | 30 | mean 3826 (SD 2097) | 29 | mean 3510 (SD 2409) | 316 (−838 to 1470) | |
| Four‐wheeled walker vs. no intervention | 4 weeks | step count (n) | 8 | mean 6465 (SD 4541) minimum 3039, maximum 16,558 | 9 | mean 2384 (SD 1319) minimum 574, maximum 4453 | 4081 (818 to 7344) N.B. no baseline assessment; likely imbalance, data skewed | |
| Physical activity counselling with PR vs. PR | 8 weeks (mid‐intervention, post‐PR) | ∆ step count (n) (SenseWear) | 63 | median 272 (IQR −342 to 782) | 59 | median 155 (IQR −438 to 867) | P = 0.99* | |
| ∆ step count (n) (pedometer) | median 727 (IQR −1493 to 3119) | median 892 (IQR −1187 to 2534) | P = 0.55* | |||||
| ∆ MVPA time | median 11 (IQR −1 to 33) | median 11 (IQR −2 to 28) | P = 0.62* | |||||
| 6 months | ∆ step count (n) (SenseWear) | 56 | median −263 (IQR −778 to 197) | 57 | median −461 (IQR −1168 to −62) | P = 0.09* | ||
| ∆ step count (n) (pedometer) | median 116 (IQR −1698 to 3200) | median 481 (IQR −1931 to 1781) | P = 0.85* | |||||
| ∆ MVPA time | median 2 (IQR −12 to 25) | median 12 (IQR −7 to 31) | P = 0.16* | |||||
| ∆ LIPA time | 44 | median 13 (IQR −38 to 33) | 49 | median 0 (IQR −62 to 36) | P = 0.60* | |||
| ∆ time sedentary | median 2 (IQR −38 to 62) | median 22 (IQR −36 to 81) | P = 0.31 | |||||
| Physical activity counselling vs. PR | 12 weeks physical activity counselling, 6 weeks PR | ∆ MVPA time | 14 | 7 (−10 to 24) | 12 | 1 (−3 to 5) | 6 (−10 to 22) | |
| ∆ MVPA bouts (n) | 0.5 (0.2 to 1.1) | −0.03 (−0.1 to 0.05) | 0.5 (0.3 to 0.8) | |||||
| ∆ MVPA time in bouts | 9 (−4 to 22) | −0.4 (−1 to 1) | 10 (−2 to 21) | |||||
| Feedback and education vs. no intervention; Education vs. no intervention; Feedback with education vs. education (post‐admission) | 14 days | ∆ step count (n) | no group data presented | |||||
| "stationary" time | ||||||||
| "light activity" time | ||||||||
| MVPA time | ||||||||
| Enriched nutritional supplement with inpatient PR vs. nutritional supplement with inpatient PR | hospital discharge | ∆ step count | 24 | mean 1900 (SD 2110) | 21 | mean 1700 (SD 1694) | 200 (−913 to 1313) | |
| ∆ EE (kcal) | mean 1521 (SD 285) | mean 1441 (SD 235) | 80 (−72 to 232) | |||||
| Physical activity counselling vs. no intervention | 4 weeks (mid intervention) | % ∆ MPA time | 10 | mean 19 (SD 30) | 8 | mean −5 (SD 13) | 2 (4 to 45) | |
| 8 weeks | mean 20 (SD 29) | mean −12 (SD 22) | 32 (8 to 55) | |||||
| Exercise training (callisthenics) vs. exercise training (endurance and strength training) | 12 weeks (data from graph) | step count (n) | 20 | mean 4235 (SD 822) | 20 | mean 4198 (SD 680) | "There were no significant inter‐group differences in any variable"* | |
| time walking | mean 43 (SD 26) | mean 53 (SD 39) | ||||||
| time standing | mean 231 (SD 123) | mean 243 (SD 106) | ||||||
| time sitting | mean 334 (SD 126) | mean 318 (SD 108) | ||||||
| time lying | mean 115 (SD 89) | mean 100 (SD 78) | ||||||
| MVPA time | mean 55 (SD 15) | mean 75 (SD 19) | ||||||
| active EE (kcal) | mean 335 (SD 104) | mean 389 (SD 119) | ||||||
| total EE (kcal) | mean 1362 (SD 186) | mean 1318 (SD 113) | ||||||
| Physical activity counselling vs. no intervention | 8 weeks (total n = 28) | % ∆ MPA time | x | mean −9 (SD 24) | x | mean −21 (SD 21) | P = 0.116* | |
| Supplemental oxygen vs. placebo (air) | 8 weeks | % ∆ "domestic activity counts" | 10 | mean 7 (SD 54) | 10 | mean −8 (SD 19) | 15 (−21 to 51) | |
| Exercise training (eccentric cycle training) vs. exercise training (concentric cycle training) | 10 weeks | "physical activity levels" | 8 | x | 8 | x | "unchanged"* | |
| Aware of purpose of pedometer with PR vs. unaware of purpose of pedometer with PR | 7 days | step count (n) | 10 | mean 4098 | 9 | mean 3679 | MD 419 "no significant difference between groups"* | |
| Adherence intervention vs. PR | 6 months (follow‐up) | step count (n) | 32 | mean 5045 (SD 3147) | 31 | mean 5204 (SD 3261) | −159 (−1742 to 1424) | |
| peak performance | mean 56 (SD 19) | mean 56 (SD 19) | 0 (−9 to 9) | |||||
| time inactive (%) | mean 68 (SD 15) | mean 70 (SD 13) | −2 (−9 to 5) | |||||
| Physical activity counselling with optional supervised exercise vs. optional supervised exercise | 4 weeks | step count (n) | 13 | mean 5603 (SD 3475) | 16 | mean 4617 (SD 3460) | 986 (−1553 to 3525) | |
| Exercise training (progressive walking and functional‐resistance exercises) vs. no intervention (inpatient) | hospital discharge | step count (n) | 17 | median 4215 (IQR 2133 to 6693) | 17 | median 2198 (IQR 1242 to 4857) | P = 0.07** | |
| LAMA with behavioural management vs. placebo with behavioural management | 6 months | step count (n) | 221 | mean 6485 | 205 | mean 6122 | x | |
| MVPA time | mean 72 | mean 64 | MD 8 | |||||
| LIPA time | mean 111 (SD 82) | mean 101 (SD 80) | 10 (−6 to 26) | |||||
| inactive (%) (< 6000 steps per day) | mean 40 | mean 43 | OR 0.86 (95% CI 0.57 to 1.30) P = 0.477* | |||||
| PR (telerehabilitation) vs. no intervention | 8 weeks | ∆ PAL | 19 | −0.03 (−0.1 to 0.02) | 17 | −0.02 (−0.1 to 0.1) | 0.08 (−0.1 to 0.1) | |
| Maintenance (telerehabilitation) following HIIT vs. no intervention | 12 months (data from graph) | time sedentary | 47 | mean 584 (SD 98) | 50 | mean 615 (SD 76) | −31 (−66 to 4) | |
| LIPA time | mean 157 (SD 44) | mean 113 (SD 44) | 44 (27 to 62) | |||||
| MPA time | mean 18 (SD 6) | mean 11 (SD 7) | 7 (4 to 10) | |||||
| "lifestyle" physical activity time | mean 41 (SD 16) | mean 34 (SD 16) | 7 (1 to 13) | |||||
| Maintenance (centre‐based) following HIIT vs. no intervention | time sedentary | 50 | mean 551 (SD 83) | 50 | 615 (SD 76) | −64 (−95 to −33) | ||
| LIPA time | mean 159 (SD 43) | 113 (SD 44) | 46 (29 to 63) | |||||
| MPA time | mean 22 (SD 7) | 11 (SD 7) | 11 (8 to 14) | |||||
| "lifestyle" physical activity time | mean 52 (SD 17) | 34 (SD 16) | 18 (12 to 25) | |||||
| Maintenance (telerehabilitation) vs. maintenance (centre‐based) following HIIT | time sedentary | 47 | mean 584 (SD 98) | 50 | mean 551 (SD 83) | 33 (−3 to 69) | ||
| LIPA time | mean 157 (SD 44) | mean 159 (SD 43) | −2 (−19 to 15) | |||||
| MPA time | mean 18 (SD 6) | mean 22 (SD 7) | −4 (−7 to −1) | |||||
| "lifestyle" physical activity time | mean 41 (SD 16) | mean 52 (SD 17) | −11 (−18 to −4) | |||||
| LAMA/LABA vs. placebo | 4 weeks | inactive (%) (< 6000 steps per day) | 127 | mean 41 | 123 | mean 55 | OR 0.27 (95% CI 0.1 to 0.5)* | |
| Physical activity counselling vs. PR | 6 weeks | step count (n) | 18 | mean 6021 (SD 2549) | 18 | mean 6113 (SD 2403) | −92 (−1710 to 1526) | |
| Ground‐based walking vs. no intervention | 8 weeks | time sedentary (% awake time) | 62 | mean 69 (SD 10) | 39 | mean 68 (SD 10) | −2 (−6 to 2)* | |
| LIPA time (% awake time) | mean 25 (SD 7) | mean 25 (SD 7) | 1 (−2 to 4)* | |||||
| MPA time (% awake time) | mean 7 (SD 5) | mean 7 (SD 5) | 1 (−2 to 4)* | |||||
| VPA time (% awake time) | mean 0 (SD 0) | mean 0 (SD 0) | 0 (0 to 0)* | |||||
| Physical activity counselling with pedometer vs. no intervention (following ground‐based walking training) | 12 months | ∆ total EE (kcal) | 23 | −75 (−156 to 6) | 20 | −80 (−166 to 5) | 17 (−131 to 164)* | |
| ∆ step count (n) | −157 (−753 to 439) | −1051 (−1687 to −424) | −617 (−1669 to 453)* | |||||
| ∆ time sedentary | −9 (−34 to 17) | −13 (−40 to 14) | −8 (−50 to 33)* | |||||
| ∆ LIPA time | 8 (−19 to 35) | −16 (−44 to 13) | −27 (−70 to 14)* | |||||
| ∆ MPA time | −11 (−23 to 1) | −1 (−13 to 12) | 20 (−1 to 41)* | |||||
| ∆VPA time | 0 (−1 to 1) | 0 (−1 to 1) | 0 (0 to 1)* | |||||
| * from paper ACE: angiotensin‐converting enzyme; ∆ change from baseline; cpm: counts per minute; "daily physical activity": step count + metabolic equivalents; EE: energy expenditure; ICS: inhaled corticosteroid; LABA: long‐acting beta2 agonist; LAMA: long‐acting muscarinic antagonist; LIPA: light‐intensity physical activity; MD: mean difference; METs: metabolic equivalents; MPA: moderate‐intensity physical activity; MVPA: moderate‐to‐vigorous intensity physical activity; n: number of participants; OR: odds ratio; PAL: physical activity level; PR: pulmonary rehabilitation; SPACE: self‐management programme of activity, coping and education; SD: standard deviation; VPA: vigorous‐intensity physical activity | ||||||||
| Population: people with COPD, clinical stability Intervention: pulmonary rehabilitation/exercise training Comparisons: intervention vs. another intervention Outcome: time in physical activity (of at least moderate intensity) at end intervention | ||||||
| Interventions | Outcome | Illustrative comparative risks* [mean difference (95% CI) unless indicated] | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparator | Intervention of interest | |||||
| Home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation (8 weeks) | Change in time in MVPA | The mean change in time in the centre‐based group was 5 minutes per day | The mean difference was 6 (−19 to 31) minutes per day | 58 participants (1 study; Analysis 4.2) | ⊕⊕⊝⊝ | Baseline values: centre‐based median 79 (IQR 24 to 136), home‐based median 68 (IQR 29 to 121) |
| Calisthenics vs. exercise training (12 weeks) | Time in MVPA | The mean time in the exercise training group was 75 minutes per day | "no significant inter‐group differences in any variable" | 40 participants (1 study; Probst 2011; Table 1) | ⊕⊕⊝⊝ | ‐ |
| Physical activity counselling vs. pulmonary rehabilitation (6 to 12 weeks) | Change in time in MVPA | The mean change in time in the pulmonary rehabilitation group was 1 (−3 to 5) minutes per day | The mean difference was 6 (−10 to 22) minutes per day | 26 participants (1 study; O'Neill 2018; Table 1) | ⊕⊕⊝⊝ | Baseline values: pulmonary rehabilitation mean 15 (SD 5), physical activity counselling mean 14 (SD 15) |
| Telerehabilitation maintenance programme vs. centre‐based maintenance programme (following high‐intensity interval training, 12 months) | Time in moderate‐intensity physical activity | The mean time in the centre‐based group was 22 minutes per day | The mean difference was −4 (−7 to −1) minutes per day | 97 participants (1 study; Vasilopoulou 2017; Table 1) | ⊕⊕⊕⊝ moderatec | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for high risk of performance bias. Downgraded one level for imprecision as CI does not exclude possibility of no effect. bDowngraded one level for unclear risk of selection, performance, detection, attrition and other potential bias. Downgraded one level for imprecision as CI does not exclude possibility of no effect. | ||||||
| Population: people with COPD, clinical stability Intervention: physical activity counselling Comparisons: intervention vs. no intervention, intervention in addition to a standard intervention common to both groups Outcome: time in physical activity (of at least moderate intensity) at end intervention | ||||||
| Interventions | Outcome | Illustrative comparative risks* [mean difference (95% CI) unless indicated] | Number of participants (studies) | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparator | Intervention of interest | |||||
| Comparison: intervention vs. no intervention | ||||||
| Physical activity counselling vs. no intervention (12 weeks) | Change in time in MVPA | The mean change in time was −3 (−0.6 to 0.2) minutes per day | The mean difference was 11 (7 to 15) minutes per day | 280 participants (1 study; Demeyer 2017; Table 1) | ⊕⊕⊕⊝ moderatea | Baseline values: no intervention median 15 (IQR 5 to 35), intervention median 14 (IQR 5 to 26) |
| Physical activity counselling vs. no intervention (following pulmonary rehabilitation, 12 months) | Change in time in moderate intensity physical activity | The mean change was −1 (−13 to 12) minutes per day | The mean difference was 20 (−1 to 41) minutes per day | 43 participants (1 study; Wootton 2017; Table 1) | ⊕⊕⊝⊝ | Baseline values: no intervention mean 51 (SD 49), intervention mean 59 (SD 52) |
| Comparison: intervention in addition to a standard intervention common to both groups | ||||||
| Physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation (6 months) | Time in MVPA | The mean time in the pulmonary rehabilitation group was 28 minutes per day | The mean difference was 24 (2 to 45) minutes per day | 26 participants (1 study; Analysis 3.21) | ⊕⊕⊕⊝ moderatec | P = 0.03 |
| Change in time in MVPA | The median change in time in the pulmonary rehabilitation group was 12 minutes per day | The median change in time was 2 (−12 to 25) minutes per day | 113 participants (1 study; Nolan 2017; Table 1) | ⊕⊕⊝⊝ | P = 0.16 Baseline values: no intervention median 47 (IQR 18 to 103), intervention median 45 (IQR 20 to 81) | |
| Physical activity counselling with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation (6 months) | Change in time in MVPA | The mean change in time in the pulmonary rehabilitation group was 0 minutes per day | The mean difference was −6 (−16 to 3) minutes per day | 50 participants (1 study; Analysis 3.25) | ⊕⊝⊝⊝ | Baseline values: no intervention median 29 (IQR 17 to 44), intervention median 33 (IQR 16 to 47) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for high risk of performance bias and detection bias. | ||||||
| Population: people with COPD, clinical stability Intervention: self‐management Comparisons: intervention vs. no intervention, intervention in addition to a standard intervention common to both groups, intervention vs. another intervention Outcome: time in physical activity (of at least moderate intensity) at end intervention | ||||||
| Interventions | Outcome | Illustrative comparative risks* [mean difference (95% CI) unless indicated] | Number of participants (studies) | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparator | Intervention of interest | |||||
| Comparison: intervention vs. no intervention | ||||||
| Self‐management vs. no intervention (12 months) | Time in MVPA | The mean time was 316 minutes a week | The mean difference was 12 (−21 to 45) minutes a week | 411 participants (1 study; Jolly 2018; Table 1) | ⊕⊕⊝⊝ | P = 0.48 |
| Comparison: intervention in addition to a standard intervention common to both groups | ||||||
| Self‐efficacy training with upper limb exercise vs. education with upper limb exercise (16 weeks) | Time in moderate‐intensity physical activity | The mean time in the education and upper‐limb exercise group was 4 minutes per day | The mean difference was 2 (−1 to 5) minutes per day | 35 participants (1 study; Larson 2014; Table 1) | ⊕⊕⊝⊝ | ‐ |
| Comparison: intervention vs. another intervention | ||||||
| Self‐management vs. education and symptom monitoring (16 weeks) | Time in moderate‐intensity physical activity | The mean time in the self‐management group was 6 minutes per day | The mean difference was 1 (−1 to 2) minutes per day | 326 participants (1 study; Blumenthal 2014; Table 1) | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for high risk of performance bias. Downgraded one level for imprecision as CI does not exclude possibility of no effect. | ||||||
| Population: people with COPD, clinical stability Intervention: pharmacological interventions Comparisons: intervention vs. placebo, intervention in addition to a standard intervention common to both groups Outcome: time in physical activity (of at least moderate intensity) at end intervention | ||||||
| Interventions | Outcome | Illustrative comparative risks* [mean difference (95% CI) unless indicated] | Number of participants (studies) | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Comparator | Intervention of interest | |||||
| Comparison: intervention vs. sham/placebo intervention | ||||||
| LAMA vs. placebo (3 weeks) | Change in time in MVPA | The median change in time was −6 minutes per day | The median change in time was −1 (IQR −17 to 24) minutes per day | 131 participants (2 studies; Beeh 2014; Magnussen 2017; Table 1) | ⊕⊕⊕⊝ moderatea | P = 0.07 Baseline values: Beeh 2014 placebo median 73 (IQR 38 to 135), LAMA median 74 (IQR 32 to 132) Magnussen 2017 placebo mean 88 (SD 65), intervention mean 57 (SD 32) |
| LAMA/LABA vs. placebo (3 to 4 weeks) | Change in time in MVPA | The mean change in time ranged from −16 to −1 minutes per day | The mean difference was 10 (4 to 15) minutes per day | 423 participants (2 studies; Analysis 2.3) | ⊕⊕⊕⊕ high | Baseline values: Watz 2016 placebo mean 130, LAMA/LABA mean 125 |
| Comparison: intervention in addition to a standard intervention common to both groups | ||||||
| LAMA with behaviour modification vs. placebo with behaviour modification (12 weeks to 6 months) | Time in MVPA | The mean time was 64 minutes per day | The mean difference was 8 minutes per day | 426 participants (1 study; Troosters 2014; Table 1) | ⊕⊕⊕⊝ moderateb | P = "not statistically significantly different" |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for imprecision as results do not exclude possibility of no effect. | ||||||
| Comparison | Description of intervention(s) | OUTCOMES | ||||
| Physical activity | Health‐related quality of life | Exercise capacity | Adherence | Adverse events | ||
| TYPE OF INTERVENTION: Pulmonary rehabilitation/exercise training | ||||||
| Clinically stable | ||||||
| Intervention vs. no intervention | Pulmonary rehabilitation vs. no intervention | Y | Y | Y | N | |
| Y | Y | N | N | |||
| Y | Y | Y | Y | |||
| Y | Y | Y | Y | |||
| High‐intensity interval training vs. no intervention | Y | Y | N | N | ||
| Y | Y | Y | Y | |||
| Maintenance (telerehabilitation) following high‐intensity interval training vs. no intervention | ||||||
| Maintenance (centre‐based) following high‐intensity interval training vs. no intervention | ||||||
| Intervention in addition to an intervention common to both groups | Nordic walking with education vs. education | Y | Y | Y | Y | |
| Structure exercise training (COPE‐active) with self‐management vs. self management | Y | Y | Y | Y | ||
| Upper limb exercises with education vs. education | N | N | N | N | ||
| Exercise training and LABA with LAMA and behavioural modification vs. LAMA and behavioural modification | Y | Y | Y | Y | ||
| Exercise training with LAMA/LABA and behavioural modification vs. LAMA/LABA and behavioural modification | ||||||
| Exercise training and LAMA/LABA with behavioural modification vs. placebo with behavioural modification | ||||||
| Exercise training and physical activity counselling with pedometer vs. pedometer | Y | Y | Y | N | ||
| Intervention vs. intervention | Web‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation | Y | Y | N | Y | |
| Exercise training (eccentric cycle training) vs. exercise training (concentric cycle training) | N | Y | N | N | ||
| Home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation | Y | Y | Y | Y | ||
| Water‐based exercise training vs. land‐based exercise training | Y | Y | Y | Y | ||
| Tai Chi vs. pulmonary rehabilitation | Y | Y | Y | Y | ||
| Exercise training (callisthenics) vs. exercise training (endurance and strength training) | Y | Y | Y | N | ||
| Exercise training (outdoor walking) vs. exercise training (cycle ergometry) | Y | Y | N | Y | ||
| physical activity counselling vs. pulmonary rehabilitation | Y | Y | N | N | ||
| Y | Y | Y | Y | |||
| Exercise training with tapering supervision vs. supervised exercise training | Y | Y | Y | N | ||
| Adherence intervention vs. pulmonary rehabilitation | Y | Y | Y | N | ||
| Maintenance following high‐intensity interval training: telerehabilitation vs. centre‐based | Y | Y | Y | N | ||
| Acute | ||||||
| Intervention vs. no intervention | Exercise training vs. no intervention | N | Y | Y | N | |
| Y | Y | Y | Y | |||
| TYPE OF INTERVENTION: Physical activity counselling | ||||||
| Clinically stable | ||||||
| Intervention vs. no intervention | Physical activity counselling vs. no intervention | N | N | Y | N | |
| Y | Y | N | Y | |||
| Y | Y | N | Y | |||
| Y | Y | Y | Y | |||
| Y | Y | Y | Y | |||
| N | N | N | N | |||
| Y | Y | Y | Y | |||
| Intervention in addition to an intervention common to both groups | Physical activity counselling with optional supervised exercise vs. optional supervised exercise | Y | N | Y | N | |
| Y | Y | Y | N | |||
| Pedometer with physical activity counselling vs. physical activity counselling | Y | Y | Y | N | ||
| Physical activity counselling with pedometer vs. pedometer | Y | N | N | Y | ||
| N | Y | Y | Y | |||
| Y | Y | Y | Y | |||
| Y | N | Y | Y | |||
| Pedometer with pulmonary rehabilitation: aware vs. unaware of purpose of pedometer | N | N | N | N | ||
| Physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation | Y | Y | N | N | ||
| Y | Y | Y | N | |||
| Y | Y | N | N | |||
| Y | Y | Y | N | |||
| Y | Y | Y | Y | |||
| Y | Y | Y | Y | |||
| Y | Y | N | N | |||
| Physical activity counselling with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation | Y | Y | Y | N | ||
| Acute | ||||||
| Intervention vs. no intervention | Physical activity counselling vs. no intervention | Y | Y | N | Y | |
| Feedback and education vs. no intervention | Y | N | Y | Y | ||
| Education vs. no intervention | ||||||
| Intervention in addition to an intervention common to both groups | Feedback with education vs. education | |||||
| TYPE OF INTERVENTION: Self‐management | ||||||
| Clinically stable | ||||||
| Intervention vs. no intervention | Self‐management vs. no intervention | Y | Y | N | Y | |
| Y | Y | Y | N | |||
| Y | N | Y | Y | |||
| Intervention vs. sham/placebo intervention | Self‐management (health mentoring) vs. sham | Y | N | Y | Y | |
| Intervention in addition to an intervention common to both groups | Self‐efficacy training with upper limb exercises vs. education with upper limb exercises | N | N | N | N | |
| Intervention vs. intervention | Self‐management (coping skills training) vs. education and symptom monitoring | Y | Y | Y | Y | |
| Acute | ||||||
| Intervention vs. no intervention | Self‐management (SPACE) vs. no intervention | N | Y | N | N | |
| Intervention in addition to an intervention common to both groups | Self‐management (health coaching) with pulmonary rehabilitation referral vs. pulmonary rehabilitation referral | Y | N | Y | Y | |
| TYPE OF INTERVENTION: Pharmacological treatments | ||||||
| Clinically stable | ||||||
| Intervention vs. sham/placebo intervention | LAMA vs. placebo | N | Y | N | Y | |
| Y | N | N | Y | |||
| Y | Y | N | Y | |||
| LAMA/LABA vs. placebo | N | N | Y | Y | ||
| N | Y | N | Y | |||
| Intervention in addition to an intervention common to both groups | LAMA with behavioural modification vs. placebo with behavioural modification | N | N | Y | Y | |
| Y | Y | Y | Y | |||
| LAMA/LABA with behavioural modification vs. placebo with behavioural modification | Y | Y | Y | Y | ||
| LABA with LAMA and behavioural modification vs. LAMA and behavioural modification | ||||||
| ACE inhibitor with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation | Y | Y | Y | Y | ||
| Intervention vs. intervention | ICS and LABA vs. ICS and LABA | Y | N | N | Y | |
| LAMA vs. LAMA | Y | N | Y | Y | ||
| TYPE OF INTERVENTION: Nutritional supplementation | ||||||
| Clinically stable | ||||||
| Intervention vs. no intervention | Nutritional supplement vs. no intervention | N | N | Y | N | |
| Intervention vs. sham/placebo intervention | Nutritional supplement vs. placebo | Y | N | N | N | |
| Intervention in addition to an intervention common to both groups | Nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation | Y | Y | Y | Y | |
| Nutritional supplement with physical activity counselling vs. physical activity counselling | ||||||
| Acute | ||||||
| Intervention in addition to an intervention common to both groups | Enriched nutritional supplement with inpatient pulmonary rehabilitation vs. nutritional supplement with inpatient pulmonary rehabilitation | Y | N | N | N | |
| TYPE OF INTERVENTION: Supplemental oxygen | ||||||
| Clinically stable | ||||||
| Intervention vs. sham/placebo intervention | Supplemental oxygen vs. placebo (air) | Y | Y | Y | Y | |
| Intervention in addition to an intervention common to both groups | Supplemental oxygen with exercise training vs. sham with exercise training | Y | Y | Y | Y | |
| Intervention vs. intervention | Supplemental oxygen (lightweight ambulatory) vs. supplemental oxygen (E‐cylinder) | N | N | Y | Y | |
| TYPE OF INTERVENTION: Other interventions | ||||||
| Clinically stable | ||||||
| Intervention vs. no intervention | Four‐wheeled walker vs. no intervention | Y | N | N | N | |
| Endobronchial valve surgery vs. no intervention | Y | Y | N | Y | ||
| Intervention vs. sham/placebo intervention | Singing vs. sham | Y | Y | Y | N | |
| Neuromuscular electrical stimulation vs. placebo | Y | Y | Y | Y | ||
| Intervention in addition to an intervention common to both groups | Non‐invasive ventilation with pulmonary rehabilitation vs. pulmonary rehabilitation | Y | Y | Y | Y | |
| Inspiratory muscle training with pulmonary rehabilitation vs. sham with pulmonary rehabilitation | Y | Y | Y | N | ||
| ACE: angiotensin‐converting enzyme; ICS: inhaled corticosteroid; LABA: long‐acting beta2 agonist; LAMA: long‐acting muscarinic antagonist; SPACE: self‐management programme of activity, coping and education | ||||||
| Study | Comparison (setting, if known) Clinical stability unless indicated | Timepoint (end intervention unless indicated) | Outcome (score unless indicated) | Intervention group | Comparison group | Between‐group MD (95% CI) where available unless indicated | ||
| n | mean (95% CI) unless indicated | n | mean (95% CI) unless indicated | |||||
| Physical activity counselling vs. no intervention (primary care) | 12 weeks | ∆ CRQ total | 22 | median 2 (IQR −3 to 7) | 18 | median 5 (IQR −3 to 15) | P = 0.398* | |
| ∆ CCQ total | median −0.1 (IQR −0.4 to 0.1) | median −0.1 (IQR −0.4 to 0.3) | P = 0.606* | |||||
| follow‐up (12 months post‐intervention) | ∆ CRQ total | 20 | median 2 (IQR −3 to 14) | 18 | median 13 (IQR −1 to 15) | P = 0.278* | ||
| ∆ CCQ total | median −0.1 (IQR −0.5 to 0.3) | median −0.2 (IQR −0.5 to 0.1) | P = 0.536* | |||||
| Physical activity counselling vs. no intervention (secondary care) | 12 weeks | ∆ CRQ total | 21 | median 2 (IQR −6 to 11) | 22 | median −9 (IQR −14 to 1) | P = 0.006* | |
| ∆ CCQ total | median 0.0 (IQR −0.6 to 0.4) | median 0.1 (IQR −0.2 to 0.5) | P = 0.529* | |||||
| follow‐up (12 months post‐intervention) | ∆ CRQ total | 20 | median 6 (IQR −4 to 10) | 19 | median 1 (IQR −9 to 1) | P = 0.311* | ||
| ∆ CCQ total | median −0.1 (IQR −0.5 to 0.2) | median 0.2 (IQR −0.3 to 0.5) | P = 0.220* | |||||
| Physical activity counselling with PR vs. PR | 12 weeks | ∆ CRQ total | 22 | median 13 (IQR 3 to 20) | 15 | median 8 (IQR 2 to 21) | P = 0.910* | |
| ∆ CCQ total | median −1 (IQR −1 to 0.2) | median −0.2 (IQR −1 to 0.0) | P = 0.345* | |||||
| follow‐up (12 months post‐intervention) | ∆ CRQ total | 10 | median −7 (IQR −16 to 1) | 13 | median −5 (IQR −14 to 2) | P = 0.344* | ||
| ∆ CCQ total | median 0.4 (IQR 0.1 to 1) | median 0.3 (IQR −0.2 to 0.7) | P = 0.368* | |||||
| Urban Training™ vs. no intervention | 12 months | CCQ total | 132 | mean 1 (SD 1) | 148 | mean 1 (SD 1) | 0.1 (−0.3 to 0.1)* | |
| CAT | mean 11 (7) | mean 11 (7) | 0.1 (−1.1 to 1.2)* | |||||
| Physical activity counselling with pedometer vs. pedometer | 12 weeks | ∆ CAT | 49 | x | 50 | x | x | |
| ∆ SGRQ | ||||||||
| Self‐management vs. education and symptom monitoring | 16 weeks | PQLS | 162 | mean 83 (SE 1) | 164 | mean 81 (SE 1) | P = 0.04* | |
| SGRQ | mean 44 (SE 1) | mean 42 (SE 1) | P = 0.068* | |||||
| Web‐based PR vs. centre‐based PR | 7 weeks | ∆ CRQ dyspnoea domain | 22 | 0.7 (0.2 to 1.2) | 40 | 0.8 (0.8 to 0.8) | −0.1 (−0.7 to 0.5) | |
| Inspiratory muscle training with PR vs. sham with PR | 12 weeks (total n = 150) | CRQ dyspnoea domain | x | mean 20 (SD 6) | x | mean 19 (SD 7) | 0.4 (−1.1 to 2.0)* | |
| CRQ emotional function domain | mean 35 (SD 9) | mean 35 (SD 8) | −0.4 (−2.4 to 1.6)* | |||||
| CRQ mastery domain | mean 21 (SD 5) | mean 19 (SD 5) | 0.01 (−1.2 to 1.2)* | |||||
| CRQ fatigue domain | mean 19 (SD 5) | mean 18 (SD 5) | 0.4 (−0.8 to 1.6)* | |||||
| CRQ total | mean 94 (SD 23) | mean 92 (SD 22) | −1.0 (−5.2 to 3.9)* | |||||
| ACE inhibitor with PR vs. placebo with PR | 10 weeks | ∆ CAT | 31 | mean 1 (SD 4) | 34 | mean −1 (SD 3) | 2 (0 to 4) | |
| Nutritional supplementation vs. placebo | 12 weeks | SGRQ total | 44 | mean 69 (SD 10) | 44 | mean 73 (SD 7) | P < 0.001* | |
| Physical activity counselling vs. no intervention | 12 weeks | ∆ CAT** | 140 | 0 (−1 to 1) | 139 | 1 (0 to 2) | −1 (−2 to 1)** | |
| Non‐invasive ventilation with PR vs. PR | 12 weeks | MRF total (%) | 24 | mean 45 (SD 22) | 32 | mean 52 (SD 24) | −10 (−18 to 1)* | |
| MRF daily activities domain (%) | mean 53 (SD 29) | mean 57 (SD 27) | −5 (−17 to 6)* | |||||
| MRF cognition domain (%) | mean 28 (SD 25) | mean 41 (SD 38) | −22 (−35 to −9)* | |||||
| MRF invalidity domain (%) | mean 57 (SD 33) | mean 62 (SD 36) | −6 (−19 to 7)* | |||||
| SRI respiratory complaints domain (%) | mean 58 (SD 13) | mean 52 (SD 17) | 6 (−1 to 12)* | |||||
| SRI physical functioning domain (%) | mean 41 (SD 21) | mean 42 (SD 18) | −2 (−10 to 5)* | |||||
| SRI attendant symptoms and sleep domain (%) | mean 71 (SD 16) | mean 60 (SD 20) | 7 (−1 to 15)* | |||||
| SRI social relationships domain (%) | mean 65 (SD 13) | mean 66 (SD 14) | 1 (−6 to 8)* | |||||
| SRI anxiety domain (%) | mean 63 (SD 17) | mean 57 (SD 22) | 3 (−5 to 11)* | |||||
| SRI well‐being domain (%) | mean 68 (SD 14) | mean 59 (SD 19) | 4 (−3 to 11)* | |||||
| SRI social functioning domain (%) | mean 54 (SD 16) | mean 54 (SD 18) | 1 (−6 to 9)* | |||||
| SRI summary (%) | mean 60 (SD 11) | mean 56 (SD 15) | 3 (−2 to 8)* | |||||
| Endobronchial valve surgery vs. no intervention | 6 months post‐surgery | ∆ SGRQ total | 19 | mean −16 (SD 16) | 24 | mean −3 (SD 9) | MD −13 (SD 4), P = 0.0005* | |
| Physical activity counselling vs. no intervention | 4 weeks | ∆ CAT | 12 | median −3 (IQR −10 to 1) | 15 | median −5 (IQR −7 to 1) | P = 0.78 | |
| Physical activity counselling with PR vs. PR | 12 weeks | ∆ CRQ dyspnoea domain | 25 | x | 25 | x | MD 3 (SE 1), P = 0.04* | |
| 9 months (follow‐up) | MD 3 (SE 2), P = 0.08* | |||||||
| Singing vs. sham | 8 weeks | ∆ CAT | 13 | −1 (−6 to 4) | 11 | 1 (−3 to 5) | −2 (−7 to 4) | |
| High‐intensity interval training vs. no intervention | 12 weeks | CRQ total | 85 | mean 98.4 (SD 21.6) | 43 | mean 89.1 (SD 26.1) | 9.3 (0.3 to 18.4) | |
| CCQ total | mean 2 (SD 1) | mean 2 (SD 1) | −1 (−1 to −0) | |||||
| LAMA vs. placebo | 3 weeks | ∆ CAT | 15 | −2 (−4 to 0) | 15 | −1 (−2 to 1) | 1 (−1 to 4)** | |
| Physical activity counselling with PR vs. PR | 12 weeks | CAT | 22 | median 16 (IQR 8 to 20) | 22 | x | x | |
| Pedometer with physical activity counselling vs. physical activity counselling | 12 weeks | ∆ SGRQ total | 50 | −9 (−12 to −5) | 47 | −4 (−7 to −1) | −5 (−10 to −0.4) | |
| ∆ CAT | −4 (−5 to −2) | −1 (−3 to 1) | −3 (−5 to −1) | |||||
| LAMA (aclidinium bromide) vs. LAMA (tiotropium) | 8 weeks | SGRQ activity domain | 22 | x | 22 | x | P < 0.05* | |
| LAMA vs. placebo | 12 weeks | CRQ | 123 | x | 125 | x | "no significant differences between treatments and the improvement was less than 0.5" | |
| ICS (beclomethasone) with LABA (formoterol) vs. ICS (budesonide) with LABA (formoterol) | 16 weeks (4‐week run‐in, 12‐week intervention) | ∆ CCQ | 30 | mean 0.1 (SD 0.5) | 29 | mean 0.1 (SD 0.5) | 0.01 (−0.24 to 0.26) | |
| Physical activity counselling with PR vs. PR | 8 weeks (mid‐intervention, post‐PR) | ∆ CRQ fatigue domain | 63 | median 2.0 (IQR 0 to 5.0) | 59 | median 4.0 (IQR 2.0 to 6.0) | P = 0.008* | |
| ∆ CRQ emotional function domain | 3.1 (1.9 to 4.4) | 5.3 (3.3 to 7.3) | −2.2 (−4.5 to 0.1) | |||||
| ∆ CRQ mastery domain | 1.8 (1.0 to 2.7) | 3.4 (2.1 to 4.7) | −1.6 (−3.1 to −0.1) | |||||
| ∆ CRQ total | median 11 (IQR 3 to 20) | median 20 (IQR 8 to 27) | P = 0.008* | |||||
| 6 months | ∆ CRQ fatigue domain | 56 | 1 (−0.3 to 2) | 57 | 2 (0.7 to 3.4) | −1.0 (−2.8 to 0.8) | ||
| ∆ CRQ emotion domain | median 0.5 (IQR −3 to 4) | 2 (−1 to 6) | P = 0.12* | |||||
| ∆ CRQ mastery | median 0.5 (IQR −1 to 3) | 2 (−2 to 5) | P = 0.29* | |||||
| ∆ CRQ total | median 3 (IQR −8 to 16) | 10 (−2 to 19) | P = 0.07* | |||||
| Physical activity counselling vs. PR | 12 weeks physical activity counselling, 6 weeks PR | ∆ CAT | 17 | 0.6 (95% CI −3.3 to 4.6) | 19 | −0.4 (−3.5 to 2.7) | 1.1 (−3.6 to 5.7) | |
| ∆ EQ5D index | 18 | −0.0 (95% CI −0.1 to 0.1) | 0.1 (0.0 to 0.2) | −0.10 (−0.22 to 0.01) | ||||
| ∆ EQ5D visual analogue scale | 2.6 (95% CI −14.9 to 20.1) | 13.3 (−0.9 to 27.4) | −10.7 (−31.7 to 10.3) | |||||
| Feedback and education vs. no intervention | 14 days | CAT | 8 | 22 (5) | 6 | 24 (11) | −2 (−12 to 7) | |
| Education vs. no intervention | 3 | 23 (14) | 6 | 24 (11) | −1 (−19 to 17) | |||
| Feedback with education vs. education | 8 | 22 (5) | 3 | 23 (14) | −1 (−17 to 15) | |||
| Enriched nutritional supplement with inpatient PR vs. nutritional supplement with inpatient PR | hospital discharge | CAT | 24 | median 10 (range 2 to 27) | 21 | 10 (3 to 28) | P = 0.75* | |
| Exercise training (callisthenics) vs. exercise training (endurance and strength training) | 12 weeks | SGRQ | 20 | mean 47 (SD 12) | 20 | mean 39 (SD 21) | P = 0.37* | |
| Supplemental oxygen vs. placebo (air) | 8 weeks | CRQ dyspnoea domain | 10 | median 2.2 (IQR 1.8 to 3) | 10 | median 2.5 (IQR 1.5 to 3.2) | P > 0.05* | |
| CRQ emotional function domain | median 4.8 (IQR 3.2 to 5.7) | median 3.8 (IQR 2.6 to 5.6) | ||||||
| CRQ mastery domain | median 5.1 (IQR 3.2 to 5.9) | median 3.6 (IQR 2.3 to 5.0) | ||||||
| CRQ fatigue domain | median 3.4 (IQR 2.8 to 4.8) | median 3.3 (IQR 1.8 to 4.3) | ||||||
| SF36 | x | x | "no significant changes in the SF36"* | |||||
| Self‐management (health mentoring) vs. sham | 6 months (mid‐intervention)** | SGRQ total | 59 | median 39 (IQR 23 to 54) | 81 | median 37 (IQR 29 to 58) | P = 0.439* | |
| SGRQ symptoms domain | median 54 (IQR 32 to 79) | median 51 (IQR 37 to 72) | P = 0.226* | |||||
| SGRQ activities domain | median 54 (IQR 31 to 79) | median 55 (IQR 42 to 73) | P = 0.901* | |||||
| SGRQ impacts domain | median 26 (IQR 14 to 43) | median 27 (IQR 12 to 44) | P = 0.188* | |||||
| SF36 physical function domain | median 39 (IQR 30 to 47) | 82 | median 36 (IQR 26 to 47) | P = 0.212* | ||||
| SF36 role physical domain | median 46 (IQR 40 to 53) | median 46 (IQR 39 to 53) | P = 0.166* | |||||
| SF36 bodily pain domain | median 52 (IQR 38 to 63) | median 51 (IQR 38 to 63) | P = 0.205* | |||||
| SF36 general health domain | median 37 (IQR 30 to 44) | median 37 (IQR 29 to 46) | P = 0.696* | |||||
| SF36 vitality domain | median 47 (IQR 41 to 53) | median 47 (IQR 41 to 53) | P = 0.586* | |||||
| SF36 social functioning domain | median 52 (IQR 41 to 57) | median 52 (IQR 41 to 57) | P = 0.137* | |||||
| SF36 role emotional domain | median 47 (IQR 40to 55) | median 47 (IQR 40 to 55) | P = 0.088* | |||||
| SF36 mental health domain | median 50 (IQR 41 to 58) | median 50 (IQR 41 to 58) | P = 0.808* | |||||
| SF36 physical component | median 41 (IQR 33 to 48) | median 38 (IQR 32 to 47) | P = 0.917* | |||||
| SF36 mental component | median 52 (IQR 43 to 58) | median 50 (IQR 40 to 58) | P = 0.222* | |||||
| 12 months** | SGRQ total | 65 | median 42 (IQR 27 to 56) | 79 | median 41 (IQR 26 to 52) | P = 0.484* | ||
| SGRQ symptoms domain | median 57 (IQR 33 to 71) | median 47 (IQR 35 to 69) | P = 0.253* | |||||
| SGRQ activity domain | median 60 (IQR 42 to 73) | median 55 (IQR 42 to 70) | P = 0.468* | |||||
| SGRQ impacts domain | median 30 (IQR 13 to 40) | median 26 (IQR 14 to 41) | P = 0.669* | |||||
| SF36 physical function domain | 63 | median 36 (IQR 28 to 45) | median 36 (IQR 30 to 45) | P = 0.599* | ||||
| SF36 role physical domain | median 44 (IQR 39 to 51) | median 46 (IQR 40 to 53) | P = 0.354* | |||||
| SF36 bodily pain domain | median 52 (IQR 42 to 63) | median 47 (IQR 38 to 63) | P = 0.392* | |||||
| SF36 general health domain | median 36 (IQR 31 to 46) | median 38 (IQR 31 to 46) | P = 0.476* | |||||
| SF36 vitality domain | median 47 (IQR 38 to 56) | median 50 (IQR 44 to 56) | P = 0.560* | |||||
| SF36 social functioning domain | median 52 (IQR 41 to 57) | median 52 (IQR 41 to 57) | P = 0.812* | |||||
| SF36 role emotional domain | median 53 (IQR 37 to 55) | median 50 (IQR 40 to 55) | P = 0.993* | |||||
| SF36 mental health domain | median 50 (IQR 44 to 58) | median 50 (IQR 41 to 58) | P = 0.690* | |||||
| SF36 physical component | median 40 (IQR 29 to 47) | median 39 (IQR 32 to 46) | P = 0.998* | |||||
| SF36 mental component | median 52 (IQR 44 to 60) | median 52 (IQR 42 to 59) | P = 0.941* | |||||
| Adherence intervention vs. PR | 6 months (follow‐up) | SF36 physical component | 32 | mean 31 (SD 8) | 31 | mean 31 (SD 10) | 0 (−4 to 5) | |
| SF36 mental component | mean 52 (SD 12) | mean 52 (SD 10) | 0 (−6 to 6) | |||||
| CCQ | mean 3 (SD 1) | mean 3 (SD 1) | 0.1 (−0.5 to 0.7) | |||||
| Physical activity counselling with optional supervised exercise vs. optional supervised exercise | 4 weeks | ∆ CCQ total | 14 | −0.3 (−0.6 to −0.01) | 15 | 0 (−0.3 to 0.3) | −0.3 (−0.7 to 0.1)* | |
| PR (telerehabilitation) vs. no intervention | 8 weeks | ∆ CAT | 19 | −1 (−4 to 2) | 17 | 3 (1 to 5) | −3 (−7 to 0)* | |
| Maintenance (telerehabilitation) vs. no intervention | 12 months | SGRQ total | 47 | mean 38 (SD 21) | 50 | mean 50 (SD 18) | −12 (−19 to −4) | |
| CAT | mean 13 (SD 7) | mean 21 (SD 7) | −8 (−11 to −5) | |||||
| Maintenance (centre‐based) vs. no intervention | SGRQ total | 50 | mean 34 (SD 17) | 50 | mean 50 (SD 18) | −16 (−23 to −10) | ||
| CAT | mean 12 (SD 6) | mean 21 (SD 7) | −9 (−12 to −7) | |||||
| Maintenance (telerehabilitation) vs. maintenance (centre‐based) | SGRQ total | 47 | mean 38 (SD 21) | 50 | mean 34 (SD 17) | 5 (−3 to 12) | ||
| CAT | mean 13 (SD 7) | mean 12 (SD 6) | 1 (−2 to 4) | |||||
| Physical activity counselling vs. PR | 6 weeks | CAT | 18 | mean 11 (SD 5) | 18 | mean 9 (SD 3) | 2 (−1 to 4) | |
| * from paper ACE: angiotensin‐converting enzyme; CAT: COPD assessment test; ∆: change from baseline; CCQ: clinical COPD questionnaire; CRQ: chronic respiratory disease questionnaire; EQ5D: EuroQol 5 dimensions questionnaire; ICS: inhaled corticosteroid; IQR: interquartile range; LAMA: long‐acting muscarinic antagonist; MRF: Maugeri respiratory failure questionnaire; MD: mean differences; n: number of participants; PQLS: pulmonary‐specific quality of life scale; RAND36: Dutch translation of SF36 questionnaire; SF36: Medical Outcomes Survey 36‐item short‐form health survey questionnaire; SGRQ: St George's respiratory questionnaire; SRI: severe respiratory insufficiency questionnaire | ||||||||
| Study | Comparison (setting, if known) Clinical stability unless indicated | Time point (end intervention unless indicated) | Outcome | Intervention group | Comparison group | Between‐group MD (95% CI) where available unless indicated | ||
| n | mean (95% CI) unless indicated | n | mean (95% CI) unless indicated | |||||
| Physical activity counselling vs. no intervention (primary care) | 12 weeks | ∆ 6MWD (m) | 22 | median 10 (IQR −7 to 38) | 18 | median 3 (IQR −18 to 22) | P = 0.291* | |
| follow‐up (12 months post‐intervention) | 20 | median 20 (IQR 8 to 54) | 18 | median 13 (IQR −2 to 38) | P = 0.313* | |||
| Physical activity counselling vs. no intervention (secondary care) | 12 weeks | 21 | median 23 (IQR 0 to 51) | 22 | median 4 (IQR −32 to 27) | P = 0.049* | ||
| follow‐up (12 months post intervention) | 20 | median 25 (IQR 4 to 52) | 19 | median 17 (IQR −9 to 57) | P = 0.555* | |||
| Physical activity counselling with PR vs. PR | 12 weeks | 22 | median 17 (IQR −27 to 42) | 15 | median 25 (IQR −15 to 60) | P = 0.605* | ||
| follow‐up (12 months post intervention) | 10 | median 7 (IQR −32 to 51) | 13 | median 10 (IQR −15 to 87) | P = 0.503* | |||
| Urban Training™ vs. no intervention | 12 months | 6MWD (m) | 132 | 488 (106) | 148 | 493 (90) | −2 (−11 to 8)* | |
| LAMA vs. placebo | 3 weeks | ∆ endurance time (sec)** | 54 | median 26 (IQR −106 to 117) | 54 | median −16 (IQR −109 to 18) | P = 0.093* | |
| Self‐management vs. education and symptom monitoring | 16 weeks | 6MWD (m) | 162 | mean 361 (SE 3) | 164 | mean 351 (SE 3) | P = 0.03* | |
| Web‐based PR vs. centre‐based PR | 7 weeks | ∆ ESWT (sec) | 22 | 189 (95 to 283) | 40 | 185 (105 to 264) | 5 (−112 to 121) | |
| Inspiratory muscle training with PR vs. sham with PR | 12 weeks | 6MWD (m) n = 169 | x | mean 388 (SD 113) | x | mean 407 (SD 105) | 1 (−13 to 15)* | |
| endurance time (sec) n = 139 | mean 496 (SD 309) | mean 466 (SD 292) | 98 (17 to 179)* | |||||
| peak work rate (W) n = 92 | mean 64 (SD 26) | mean 59 (SD 22) | 5.2 (−0.4 to 10.8)* | |||||
| VO2peak (mL/min) n = 92 | mean 1048 (SD 313) | mean 966 (SD 323) | 0.01 (−0.1 to 0.1)* | |||||
| VEpeak (L/min) n = 92 | mean 37 (SD 11) | mean 39 (SD 15) | −1 (−3 to 2)* | |||||
| ACE inhibitor with PR vs. placebo with PR | 10 weeks | ∆ peak work rate (W) | 31 | 1 (−2 to 4) | 34 | 9 (5 to 13) | −8 (−13 to −3) | |
| ∆ VO2peak (mL/min/kg) | 0.3 (−0.4 to 1.1) | 1.4 (0.8 to 2.0) | −1.0 (−2.0 to −0.1) | |||||
| ∆ VE/VCO2 slope | −0.9 (−2.1 to 0.4) | −1.3 (−3.2 to 0.7) | 0.4 (−2.0 to 2.8) | |||||
| ∆ oxygen uptake efficiency slope (ml/min) | 29 (−109 to 167) | 151 (40 to 261) | −122 (−292 to 49) | |||||
| Physical activity counselling with PR vs. PR | 9 weeks | 2‐min step test (n steps) | 8 | 57 (32 to 82) | 8 | 55 (37 to 73) | 2 (−24 to 28) | |
| Physical activity counselling vs. no intervention | 12 weeks | ∆ 6MWD (m) | 131 | mean 12 (SD 44) | 136 | mean −1 (SD 44) | 13 (2 to 23) | |
| Non‐invasive ventilation with PR vs. PR | 12 weeks | ESWT (sec) | 24 | median 475 (IQR 295 to 1010) | 32 | 449 (213 to 1042) | 103 (−69 to 276)* | |
| 6MWD (m) | mean 340 (SD 119) | mean 325 (SD 108) | 2 (−19 to 23)* | |||||
| VO2peak (mL/min/kg) | mean 9.8 (SD 2.9) | mean 9.5 (SD 3.0) | 0.3 (−0.9 to 1.4)* | |||||
| Exercise training (outdoor walking) vs. exercise training (cycle ergometry) | 3 weeks | 6MWD (m) | 8 | mean 285 (SD 72) | 8 | mean 440 (SD 49) | −155 (−215 to −95) | |
| Endobronchial valve surgery vs. no intervention | 6 months post‐surgery | ∆ 6MWD (m) | 19 | mean 84 (SD 62) | 24 | mean −20 (SD 35) | MD 104 (SD 165)* | |
| Physical activity counselling vs. no intervention | 4 weeks | ∆ 6MWD (m) | 12 | mean 67 (SD 84) | 15 | mean 64 (SD 59) | 3 (−53 to 59) | |
| Physical activity counselling vs. no intervention | 12 weeks | 6MWD (m) | 18 | mean 387 (SD 47) | 17 | mean 361 (SD SD 67) | 26 (−12 to 64) | |
| Self‐management vs. no intervention (post‐admission) | 7 days | ∆ ISWD (m) | 15 | mean 46 (SD 32) | 10 | mean 44 (SD SD 99) | 2 (−62 to 66) | |
| ∆ ESWT (sec) | mean 369 (SD 355) | mean 290 (SD SD 379) | 80 (−216 to 375) | |||||
| Physical activity counselling with PR vs. PR | 12 months | 6MWD (m) | 12 | mean 445 (SD 138) | 15 | mean 467 (SD SD 151) | −22 (−131 to 87) | |
| Physical activity counselling with PR vs. PR | 12 weeks | ∆ 6MWD (m) | 25 | x | 25 | x | MD 8 (SE 14), P = 0.59* | |
| ∆ endurance time (sec) | MD −80 (SE 71), P = 0.26* | |||||||
| 9 months (follow‐up) | ∆ 6MWD (m) | MD 2 (SE 16), P = 0.90* | ||||||
| ∆ endurance time (sec) | MD 153 (SE 79), P = 0.06* | |||||||
| Singing vs. sham | 8 weeks | ∆ ISWD (m) | 13 | −7 (−35 to 21) | 11 | 15 (−11 to 40) | −22 (−56 to 12) | |
| High‐intensity interval training vs. no intervention | 12 weeks | VO2peak (mL/min/kg) | 85 | mean 17.8 (SD 4) | 43 | mean 15.5 (SD 4.1) | 2.3 (0.8 to 3.8) | |
| Physical activity counselling with PR vs. PR | 12 weeks | ∆ESWT (sec) | 22 | 99 (37 to 161) | 22 | 3 (−34 to 40) | 96 (28 to 164) | |
| Pedometer with PAI vs. PAI | 12 weeks | ∆6MWD (m) | 50 | mean 12 (SD 35) | 47 | mean −1 (SD 24) | 13 (1 to 25) | |
| LAMA vs. placebo | 12 weeks | 6MWD (m) | 123 | x | 125 | x | "trend favouring tiotropium" | |
| Physical activity counselling with pedometer vs. pedometer | 6 months | peak work rate (W) | 9 | mean 49 (SD 24) | 8 | mean 49 (SD 28) | 0 (−25 to 25) | |
| Pedometer with PR vs. PR | 8 weeks (mid‐intervention, post‐PR) | ∆ ISWD (m) | 63 | median 60 (IQR 20 to 90) | 59 | median 50 (IQR 10 to 90) | P = 0.83* | |
| 6 months | 56 | median 30 (IQR 0 to 70) | 57 | median 10 (IQR −30 to 70) | P = 0.25* | |||
| Physical activity counselling vs. PR | 12 weeks physical activity counselling, 6 weeks PR | ∆ ISWD (m) | 16 | −12 (−60 to 36) | 17 | −8 (−44 to 28) | −4 (−60 to 51) | |
| Exercise training (callisthenics) vs. exercise training (endurance and strength training) | 12 weeks | peak work load (W) | 20 | mean 30 (SD 30) | 20 | mean 48 (SD 30) | P = 0.04* | |
| endurance time (min) | mean 8 (SD 7) | mean 17 (SD 24) | P = 0.08* | |||||
| 6MWD (m) | mean 424 (SD 114) | mean 483 (SD 89) | P = 0.30* | |||||
| Supplemental oxygen vs. placebo (air) | 8 weeks | ISWD (m) | 10 | mean 251 (SD 136) | 10 | mean 211 (SD 99) | 40 (−64 to 144) | |
| ESWT (sec) | mean 340 (SD 336) | mean 170 (SD 98) | 170 (−47 to 387) | |||||
| Exercise training (eccentric cycle training) vs. exercise training (concentric cycle training) | 10 weeks | ∆ peak work rate (W) | 8 | 9 (3 to 15) | 8 | 12 (5 to 19) | −3 (−11 to 5) | |
| Adherence intervention vs. PR | 6 months (follow‐up) | 6MWD (m) | 32 | mean 320 (SD 134) | 31 | mean 315 (SD 116) | 5 (−57 to 67) | |
| Self‐management vs. no intervention | 12 weeks (mid‐intervention) | 6MWD (m) | 11 | mean 412 (SE 39) | 9 | mean 312 (SE 44) | 100 (−15 to 215) | |
| Exercise training (progressive walking and functional‐resistance exercises) vs. no intervention (inpatient) | hospital discharge | 2‐min walk distance (m) | 16 | mean 162 (SD 38) | 16 | mean 146 (SD 4) | 13 (3 to 23)* | |
| Nutritional supplementation with PR vs. placebo with PR | 4 months (end intervention) | 6MWD (m) | 38 | mean 500 (SD 111) | 35 | mean 492 (SD 101) | MD −4 (SD 12)* | |
| endurance time (sec) | mean 467 (SD 339) | mean 482 (SD 372) | MD −110 (SD 70)* | |||||
| 12 months (end maintenance) | ∆ endurance time (sec) | 32 | mean 107 (SD 63) | 29 | mean 200 (SD 65) | MD −93 (SD 90)* | ||
| Maintenance (telerehabilitation) vs. no intervention | 12 months | 6MWD (m) | 47 | mean 420 (SD 75) | 50 | mean 340 (SD 110) | 80 (43 to 118) | |
| peak work rate (W) | mean 76 (SD 35) | mean 58 (SD 24) | 18 (6 to 30) | |||||
| Maintenance (centre‐based) vs. no intervention | 6MWD (m) | 50 | mean 428 (SD 63) | 50 | mean 340 (SD 110) | 88 (52 to 123) | ||
| peak work rate (W) | mean 79 (SD 31) | mean 58 (SD 24) | 21 (10 to 32) | |||||
| Maintenance (telerehabilitation) vs. maintenance (centre‐based) | 6MWD (m) | 47 | mean 420 (SD 75) | 50 | mean 428 (SD 63) | −7 (−35 to 20) | ||
| peak work rate (W) | mean 76 (SD 35) | mean 79 (SD 31) | −3 (−16 to 10) | |||||
| Physical activity counselling with pedometer vs. pedometer | 12 weeks | ∆ 6MWD (m) | 57 | mean −1 (SD 56) | 52 | mean 4 (SD 47) | P = 0.72* | |
| LAMA/LABA vs. placebo | 8 weeks (4 weeks medication only, 4 weeks medication and behavioural intervention) | endurance time (sec) | 127 | 51 (15 to 86) | 123 | −5 (−40 to 31) | LSMD 55 (95% CI 6 to 105)* | |
| Physical activity counselling vs. PR | 6 weeks | 6MWD (m) | 18 | mean 242 (SD 79) | 18 | mean 267 (SD 47) | −25 (−68 to 18) | |
| Physical activity counselling with pedometer vs. no intervention (following ground‐based walking training) | 12 months | ∆ 6MWD (m) | 35 | −23 (−41 to −5) | 36 | −39 (−59 to −18) | −16 (−46 to 15)* | |
| ∆ ISWD (m) | 28 | −37 (−60 to −14) | −8 (−34 to 17) | 23 (−13 to 60)* | ||||
| ∆ ESWT (sec) | 29 | −110 (−232 to 12) | −168 (−303 to −33) | −54 (−245 to 137)* | ||||
| * from paper ACE: angiotensin‐converting enzyme; ∆: change from baseline; ESWT: time walked during an endurance shuttle walk test; ISWD: distance walked during an incremental shuttle walk test; IQR: interquartile range; LSMD: least squares mean difference; m: metres; D: mean difference; n: number of participants; sec: seconds; SPACE: self‐management programme of activity, coping and education; 6MWD distance walked on six‐minute walk test; SD: standard deviation; VO2peak: peak oxygen uptake | ||||||||
| Study | Comparison (setting); Clinical stability unless indicated | Data regarding adherence |
| Supplemental oxygen with exercise training vs. sham with exercise training | Attended ≥ 16 sessions; oxygen 48 participants; sham 41 participants | |
| Urban Training™ vs. no intervention | Urban training (132 participants)
| |
| Self‐management (health coaching) with PR referral vs. PR referral | Attendance at PR:
85% of participants completed self‐management intervention (≥ 15 of 21 calls) | |
| Self‐management (coping skills training) vs. education and symptom monitoring | Both groups: median number of completed sessions 14 (100%) | |
| Exercise training (whole‐body resistance training) vs. no intervention (inpatient) | Attendance: 5.6 sessions (95%); Intensity (weight load): increase 9.7% | |
| Exercise training (Nordic walking) with education vs. education | "All patients achieved the preset goal for maximum heart rate to ensure training efficiency (> 75% of the initial maximum heart rate)… none of the patients had any difficulties in performing Nordic Walking adequately" | |
| Physical activity counselling with PR vs. sham with PR | PR: "did not systematically record adherence"; Physical activity counselling/sham (8 scheduled sessions): 82% attendance | |
| Supplemental oxygen (lightweight ambulatory) vs. supplemental oxygen (E‐cylinder) | Oxygen use: lightweight ambulatory 8.9 (4.8) hours per day; E‐cylinder 16.9 (3.9) hours per day | |
| Inspiratory muscle training with PR vs. sham with PR | Completed prescribed sessions of inspiratory muscle training: inspiratory muscle training 79 (4)%; PR 81 (4)% Intensity (inspiratory muscle training load relative to baseline Pimax): 47 (2)% Week 1; 84 (4)% Week 12 | |
| Physical activity counselling with PR vs. PR | "All participants received allocated intervention" | |
| ACE inhibitor with PR vs. placebo with PR |
| |
| Physical activity counselling with PR vs. PR | Physical activity counselling: individual exercise counselling 4 sessions attended, diary‐recorded daily step counts 96% | |
| Exercise training vs. no intervention | Exercise training attendance 18.2 (1.1) of 20 scheduled sessions, home‐based walking 8.6 (0.4) sessions | |
| Physical activity counselling vs. no intervention | PAC median 6 (IQR 4 to 9) contacts (range 0 to 25) | |
| Non‐invasive ventilation with PR vs. PR | PR
NIPPV
| |
| Exercise training (COPE‐active) with self‐management vs. self management | COPE‐active 56 (73%) participants completed (not defined) | |
| Water‐based exercise training vs. land‐based exercise training | "All studied patients completed 60 sessions" | |
| Nutritional supplement vs. no intervention | Adherence: nutritional supplement 95 (6)% Month 1; 89 (14)% Month 3 | |
| Home‐based PR vs. centre‐based PR |
| |
| Self‐management vs. no intervention | Self‐management 999 (86%) of 1156 calls, participated in all 4 scheduled calls 218 (75%) of 289 participants | |
| Physical activity counselling with PR vs. PR | Days of home‐based PR: 239 (25) days a year, 65 (7)%, 4 days a week Days of pedometer wear: 293 (49) days a year, 80 (13)% | |
| Singing vs. sham | Attendance: singing 14.5 of 16 scheduled sessions; sham 7 of 8 scheduled sessions | |
| NMES vs. placebo | Intervention: 42 sessions (1260 minutes)
| |
| Pedometer with physical activity counselling vs. physical activity counselling | Physical activity counselling and pedometer strong correlations between diary and device memory step count (r2 Month 1 0.996, Month 2 0.999, Month 3 0.975) "suggesting a high degree of compliance with the program." | |
| Physical activity counselling with pedometer vs. pedometer | Valid step‐count data
Physical activity counselling with pedometer:
| |
| LAMA (aclidinium bromide) vs. LAMA (tiotropium) | Adherence (both groups) "estimated to be 100% during study period" | |
| Physical activity counselling with pedometer vs. pedometer | Exercise and symptom data submitted: PAC with pedometer 87%; pedometer 66% | |
| Physical activity counselling with PR vs. PR | Physical activity counselling with PR (revised step‐count goal, 5% increments, 8 possible occasions) did not occur 5 (10) occasions | |
| Physical activity counselling vs.PR |
Attended 75% sessions: 100%; mean 11.8 (SD 0.6) of 12 planned consultations
Attended 75% sessions: 70% (9 of 13 participants); mean 10.5 (SD 1.2) of 12 planned classes | |
| Feedback and education vs. no intervention; Education vs. no intervention; Feedback with education vs. education (post‐admission) | Intervention delivery: feedback and education 21 (95%) sessions ; education 1 session not delivered | |
| Tai Chi vs. PR | "Both groups had high compliance in terms of attendance rate (present days/total expected days)" Tai Chi 91 (1)%; PR 87 (2)% | |
| Physical activity counselling vs. no intervention | Days accessed website: Physical activity counselling 87 (15)% | |
| Exercise training (callisthenics) vs. exercise training (endurance and strength training) | Attendance (32 planned sessions): callisthenics 19 (61%) sessions; endurance and strength training 20 (63%) sessions | |
| Exercise training with tapered supervision vs. supervised exercise training | Attendance: exercise training with tapered supervision 100%; supervised exercise training 87% | |
| Supplemental oxygen vs. placebo (air) | Number of cylinders used: oxygen 20.2 (13.7); placebo 10.7 (4.9); P < 0.002 | |
| Self‐management (health mentoring) vs. sham | Attendance: phone contacts median 9.5 of 16 planned sessions (range 1 to 21) | |
| Adherence intervention vs. PR | Attended ≥ 80% sessions: Adherence intervention: 80% (37 of 46 allocated participants); PR: 57% (25 of 44 allocated participants) | |
| Physical activity counselling with optional supervised exercise vs. optional supervised exercise | Physical activity counselling activity coach: 86% "complied" [time activity coach was worn], wear time 17.5 (2.2) days (109% of prescribed), 588 (101) min per day Physical activity counselling diary: 1 participant "complied" (completed diary every day), 1 participant did not use the web portal, 1 participant used web portal for 4 days; completed 17.3 (7.8) times a patient (58% of prescribed) | |
| Self‐management vs. no intervention | Web portal: 86% of days Triage diary: most used module (median 83%) Exercise: 127 exercise schemes performed of 569 schemes prescribed (median 21%) Activity coach‐module: used for 299 days (132 days in monitoring mode, 167 days in feedback mode), "rarely used outside of the measurement weeks" | |
| Physical activity counselling and exercise training with pedometer vs. pedometer | "2 (participants) were excluded for non‐compliance during the intervention phase" Author correspondence states "non‐compliance of the program was established previously, before starting the study, by considering none achieving the individual target of steps/week for three consecutive weeks" | |
| Maintenance (telerehabilitation) vs. no intervention; Maintenance (centre‐based) vs. no intervention; Maintenance (telerehabilitation) vs. maintenance (centre‐based) following high‐intensity interval training | "Adherence to the home‐based maintenance tele‐rehabilitation and hospital‐based, outpatient, maintenance programmes were assessed by the adherence rate (actual number of sessions/total expected number of session×100). Adherence to measurements of vital signs, home exercises, responses to questionnaires and daily steps were recorded for each participant by the number of registrations entered divided by the number of those recommended." "Overall compliance rate" (twice‐weekly supervised exercise training sessions): telerehabilitation 93.5%; centre‐based 91% | |
| Physical activity counselling with optional supervised exercise vs. optional supervised exercise | App accessed: PAC with optional supervised exercise 89 (19)% days, accessed app and personal physical activity goal obtained 34 (16)% days | |
| Physical activity counselling with pedometer vs. pedometer | Pedometer
Website use: Physical activity counselling and pedometer > 4 logins a month "suggests good adherence to the requested weekly logins" (data not shown) | |
| LAMA/LABA vs. placebo | Combined treatment periods 1 and 2: LAMA/LABA 99.5%; placebo 99.6% | |
| Exercise training (ground‐based walking) vs. no intervention | Attendance: 18 (6) of 24 scheduled sessions; Progression (duration): 76 (84%) participants | |
| Physical activity counselling with pedometer vs. no intervention (following ground‐based walking) | % Adherence (number of walks reported divided by the number of prescribed walks) Physical activity counselling: 8 months mean 52 (SD 17); 14 months 52 (55) No intervention: 8 months mean 92 (SD 38); 14 months 77 (54) | |
| Data are mean or mean (SD) unless indicated | ||
| Study | Comparison (setting), if known] Clinical stability unless indicated | Data regarding adverse events |
| Supplemental oxygen with exercise training vs. sham with exercise training | AE: incidence and severity similar in both groups
| |
| Physical activity counselling vs. no intervention (primary care, secondary care); Physical activity counselling with PR vs. PR | Monitoring for AEs described, no results presented: "WA had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any AEs" | |
| Urban Training™ vs. no intervention | Participants experiencing any AE: Urban Training 99 (77%) of 128 participants; no intervention 103 (73%) of 142 participants; P = 0.363
| |
| LAMA vs. placebo | SAEs: none; AEs leading to discontinuation: LAMA 4 events in 57 participants; placebo 1 event in 53 participants | |
| Physical activity counselling with pedometer vs. pedometer | "No withdrawals due to AEs" | |
| Self‐management (health coaching) with PR referral vs. PR referral | "Reasons for not completing the intervention were death during the study period (n = 3)" (108 participants) | |
| Self‐management (coping‐skills training) vs. education and symptom monitoring | Education and symptom monitoring: 3 participants died during the intervention period | |
| Exercise training (whole‐body resistance training) vs. no intervention (inpatient) | "No patients exhibited clinical deterioration except oxygen desaturation (oxygen saturation by pulse oximeter <88%) and increased dyspnoea, which were reversed with oxygen or increasing supplemental oxygen" | |
| Nordic walking with education vs. education | "No (serious) AEs were reported" | |
| Supplemental oxygen (lightweight ambulatory) vs. supplemental oxygen (E‐cylinder) | AEs: lightweight ambulatory 1 death (sudden) in 11 participants; E‐cylinder 1 death (congestive heart failure) in 11 participants | |
| Web‐based PR vs. centre‐based PR | Monitoring for AEs described, no results presented: "Any SAEs were reported to the sponsor. A SAE was defined as an acute exacerbation of their COPD that resulted in a hospital admission" | |
| ACE inhibitor with PR vs. placebo with PR | AECOPD or AE: no difference between groups
| |
| Physical activity counselling vs. no intervention | Respiratory AEs (≥ 1 AECOPD): physical activity counselling 43 (27%) of 159 participants; no intervention 48 (30%) of 159 participants; P = 0.54; 5 participants required hospitalisation Musculoskeletal AEs (back pain, knee pain, rib fracture): physical activity counselling 11 events; no intervention 2 events; P = 0.01; none caused study discontinuation 8 other AEs (not study‐related: cardiovascular problems, melanoma, urinary problems, gastrointestinal problems) | |
| Non‐invasive ventilation with PR vs. PR | Respiratory AEs: Non‐invasive ventilation 1 death ("progressive respiratory failure due to COPD exacerbation") in 24 participants Musculoskeletal AEs: Non‐invasive ventilation 1 withdrawal ("rheumatic complaints") | |
| Exercise training (COPE‐active) with self‐management vs. self management | AEs: none reported | |
| Water‐based exercise training vs. land‐based exercise training | Participant discontinuations due to "health problems"
| |
| Exercise training (outdoor walking) vs. exercise training (cycle ergometry) | During rehabilitation: no AE During follow‐up: ergometry 3 participants AECOPD Walking: unable to use HR monitor as planned, required chest strap; difficult for 70% of participants; resolved using modified Borg scale to guide training intensity | |
| Endobronchial valve surgery vs. no intervention | AEs: endobronchial valve surgery 1 death, 8 valves removed in 19 participants | |
| Home‐based PR vs. centre‐based PR | AEs: none | |
| Physical activity counselling vs. no intervention | Respiratory AEs
| |
| Physical activity counselling vs. no intervention | AEs: no intervention 1 death in 17 participants | |
| Self‐management vs. no intervention | SAEs: self‐management 24 of 179 participants; no intervention 20 of 232 participants
| |
| Physical activity counselling with PR vs. PR | AEs: Physical activity counselling with PR 1 death in 12 participants; PR 1 death in 15 participants | |
| NMES vs. placebo | Similar between groups; AE: NMES 5 (20%) participants; placebo 9 (33%) participants
| |
| LAMA vs. placebo | SAEs: none; AEs: were all "mild or moderate", not study‐related, no withdrawals | |
| Self‐management (SPACE) vs. no intervention | AEs: SPACE five participants with comorbidities, one participant with "worsening of COPD" in 89 participants; no intervention five participants with comorbidities, one death in 95 participants | |
| Physical activity counselling with pedometer vs. pedometer | Musculoskeletal AEs ("mild / minor"):
Deaths:
Four‐month data
12‐month data "no differences between groups with respect to pulmonary, cardiac or other adverse events"
| |
| LAMA (aclidinium bromide) vs. LAMA (tiotropium) | AEs: aclidinium bromide 9 events in 22 participants; tiotropium 7 events in 22 participants | |
| LAMA vs. placebo | “No relevant issues concerning safety were found” Any AE: LAMA 44 (36%) participants, placebo 56 (44%) participants
Most were mild (72 participants, 29%) or moderate (47 participants, 19%) Concerning drug‐related AEs: LAMA 1 (1%) participant; placebo 4 participants SAE: 5 participants (2%) in screening period, 1 died
| |
| ICS (beclomethasone) with LABA (formoterol) vs. ICS (budesonide) with LABA (formoterol) | TEAEs: beclomethasone with formoterol 18 events in 10 participants (33%); budesonide with formoterol 32 events in 15 participants (52%), 1 AECOPD (only severe event) Adverse drug reactions: beclomethasone with formoterol 5 events in 3 participants (10%); budesonide with formoterol 3 events in 3 participants (10%) AEs leading to study discontinuation: beclomethasone with formoterol 3 events in 3 participants (10%); budesonide with formoterol 5 events in 3 participants (10%) "No relevant differences between groups were observed in the proportion of patients with TEAEs, SAEs, adverse drug reactions, severe AEs and AEs leading to discontinuation" | |
| Physical activity counselling with pedometer vs. pedometer | AEs: Physical activity counselling with pedometer "greater number (of AEs)... two falls and three AECOPD" (9 participants) | |
| Physical activity counselling with PR vs. PR |
| |
| Physical activity counselling vs. PR | Study‐related and unexpected AEs; no withdrawals
| |
| Feedback and education vs. no intervention; Education vs. no intervention; Feedback with education vs. education (post‐admission) | Hospital admissions (at least 1 overnight stay, not study‐related): education 2 admissions in 6 participants (1 respiratory, 1 non‐respiratory) Hospital re‐admissions for AECOPD (not considered AE, withdrawn from study): education and feedback 3 admissions in 8 participants; no intervention 1 admission in 6 participants No deaths | |
| Tai Chi vs. PR | AEs: "No difference in AEs was observed between the groups" (undefined); Tai Chi 2 events in 60 participants; PR 2 events in 60 participants Reasons for hospitalisation (SAE) P = 0.769
| |
| Supplemental oxygen vs. placebo (air) | AEs: 1 death during walking test at baseline assessment | |
| Self‐management (health mentoring) vs. sham | AEs: self‐management 2 deaths in 47 participants; sham 2 deaths in 73 participants Respiratory AEs (at least 1 admission for COPD): self‐management 11 (12.2%) participants; sham 5 (5.4%) participants; P = 0.11 | |
| LAMA (tiotropium) with behavioural management vs. placebo with behavioural management | "No SAEs were considered related to study drug and patients recovered from all events"; no deaths TEAEs
| |
| LAMA vs. placebo; LAMA/LABA vs. placebo; LAMA/LABA and exercise training vs. placebo; LAMA/LABA vs. LAMA; LAMA/LABA and exercise training vs. LAMA; LAMA/LABA and exercise training vs. LAMA/LABA N.B. all groups received behavioural modification | Any AE: placebo 46 events in 60 participants; LAMA 51 events in 76 participants; LAMA/LABA 44 events in 76 participants; LAMA/LABA and exercise training 49 events in 76 participants TEAE: placebo 4 events; LAMA 6 events; LAMA/LABA 3 events; LAMA/LABA and exercise training 2 events Severe AE: placebo 5 events; LAMA 10 events; LAMA/LABA 3 events LAMA/LABA and exercise training 8 events "Specific AEs with incidence > 2%"
| |
| Maintenance (telerehabilitation) vs. no intervention; Maintenance (centre‐based) vs. no intervention; Maintenance (telerehabilitation) vs. maintenance (centre‐based) | AEs: none reported | |
| Physical activity counselling with pedometer vs. pedometer | SAEs (not study‐related: abdominal pain, anxiety, mental health crisis, headache, congestion, ear pain, rash, skin abscess, kidney problems, toe fracture, car accident): Physical activity counselling with pedometer 14 events in 9 of 57 participants; pedometer 10 events in 8 of 52 participants; P = 0.54 Respiratory AEs: Physical activity counselling with pedometer 15 events in 13 participants; pedometer 9 events in 8 participants; P = 0.29 | |
| LAMA/LABA vs. placebo | All TEAEs: LAMA/LABA 73 events in 44 of 193 participants (4 participants discontinued); placebo 48 events in 43 of 188 participants (2 participants discontinued) Suspected‐related TEAEs: LAMA/LABA 12 events in 11 participants; placebo 4 events in 4 participants
Serious TEAEs: LAMA/LABA 4 participants, 1 death (not study‐related: suspected myocardial infarction); placebo 2 participants | |
| LAMA/LABA vs. placebo | TEAEs: LAMA/LABA 42.5%, placebo 45.1% SAEs leading to discontinuation: LAMA/LABA 1.5%, placebo 2.3% AEs leading to discontinuation: LAMA/LABA 3%, placebo 4.5% Events reported by > 5% of participants: nasopharyngitis (LAMA/LABA 10.4%; placebo 9.8%), headache (LAMA/LABA 3.0%; placebo 9.0%) | |
| Exercise training (ground‐based walking) vs. no intervention | AEs: none reported | |
| Physical activity counselling with pedometer vs. no intervention (following ground‐based walking) | "No AEs were reported during the study" | |
| ACE: angiotensin‐converting enzyme; AE: adverse event; AECOPD: acute exacerbation of COPD; COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long‐acting beta2‐agonist; LAMA: long‐acting muscarinic antagonist; n: number; NMES: neuromuscular electrical stimulation; OR: odds ratio; PR: pulmonary rehabilitation; SAE: serious adverse event; SPACE: self‐management programme of activity, coping and education; TEAE: treatment‐emergent adverse event | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 End intervention | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 208.24 [‐164.91, 581.39] |
| 2 time/change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End intervention | 3 | 190 | Mean Difference (IV, Random, 95% CI) | 3.62 [‐1.90, 9.14] |
| 3 change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 End intervention | 3 | 182 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐28.35, 24.61] |
| 4 change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 End intervention | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐41.54 [‐89.97, 6.90] |
| 5 change in time in physical activity (total; minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 End intervention | 2 | 88 | Mean Difference (IV, Random, 95% CI) | 23.01 [6.12, 39.90] |
| 6 change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 End intervention | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 16.56 [‐27.06, 60.18] |
| 7 time in "lifestyle" physical activity (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 9.40 [3.87, 14.93] |
| 8 time in light‐intensity physical activity (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 28.12 [15.64, 40.60] |
| 9 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 6.24 [4.00, 8.48] |
| 10 sedentary time (minutes per day); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | ‐34.25 [‐55.90, ‐12.60] |
| 11 step count (steps per day); Intervention: physical activity counselling Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Step count: in‐person (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Change in step count: telecoaching (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Step count: 'Urban Training' (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 "IMA" (counts per minute); Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 physical activity level; Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 total energy expenditure (MJ); Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 subgroup analysis (supervised vs. unsupervised); change in step count (steps per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Supervised pulmonary rehabilitation/exercise training | 2 | 144 | Mean Difference (IV, Random, 95% CI) | 68.86 [‐386.29, 524.01] |
| 15.2 Unsupervised pulmonary rehabilitation/exercise training | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 494.0 [‐157.70, 1145.70] |
| 16 supgroup analysis (supervised vs. unsupervised); change in time in light‐intensity physical activity (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Supervised pulmonary rehabilitation/exercise training | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 9.87 [‐9.22, 28.96] |
| 16.2 Unupervised pulmonary rehabilitation/exercise training | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐44.0 [‐87.04, ‐0.96] |
| 17 subgroup analysis (supervised vs. unsupervised); change in total energy expenditure (kcal); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 Supervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Unsupervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 subgroup analysis (supervised vs. unsupervised); change in sedentary time (minutes per day); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.1 Supervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Unsupervised pulmonary rehabilitation/exercise training | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 step count (steps per day); Intervention: self‐management (health mentoring) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in step count (steps per day); Intervention: LAMA/LABA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End intervention | 2 | 426 | Mean Difference (IV, Random, 95% CI) | 531.30 [167.10, 895.49] |
| 3 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day): Intervention: LAMA/LABA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 End intervention | 2 | 423 | Mean Difference (IV, Random, 95% CI) | 9.74 [4.23, 15.24] |
| 4 change in active energy expenditure (kcal); Intervention: LAMA/LABA Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 End intervention | 2 | 423 | Mean Difference (IV, Random, 95% CI) | 43.89 [17.92, 69.86] |
| 5 step count (steps per day); Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 energy expenditure for ambulation (kcal/step/FFM kg); Intervention: nutritional supplement Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in step count (steps per day); Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in up/down transitions (number); Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in time upright (hours); Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 movement intensity (m/s2); Interventions: nordic walking with education vs. education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow‐up (9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in step count (steps per day); Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Follow‐up (18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Follow‐up (24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in time in light‐intensity physical activity (minutes per day); Interventions: upper body resistance training with health education vs. health education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 step count (steps per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 time walking (minutes per day); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 walking intensity (m/s2); Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 step count (steps per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 time walking (minutes per day); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 walking intensity (m/s2); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 step count (steps per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 time walking (minutes per day); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 walking intensity (m/s2); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 change in step count (steps per day); Interventions: exercise training and physical activity counselling with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Follow‐up (3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Follow‐up (12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 change in step count (weekday, steps per day); Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 METs; Interventions: physical activity counselling with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 step count (steps per day); Interventions: physical activity counselling with pedometer vs. pedometer Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1 Step count: web‐based (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Step count: app (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Step count: app (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Change in step count: web‐based (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Change in step count: web‐based (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 time active (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 time inactive (minutes per day); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 peak performance (steps per minute); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.1 End intervention (7 days including PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 End intervention (6 days excluding PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 End intervention (4 days excluding PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 time sedentary (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 change in step count (steps per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 25.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 change in time walking (minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 26.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 change in time in physical activity (total, minutes per day); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 27.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28 change in time in light‐intensity physical activity (minutes per day); Interventions: exercise‐specific self‐efficacy training with upper body resistance training vs. upper body resistance training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 28.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 29 step count (steps per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 29.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 29.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 30 time walking (minutes per day); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 30.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 30.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 31 walking intensity (m/s2); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 31.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 31.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 32 step count (steps per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 32.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 32.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 33 time walking (minutes per day); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 33.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 33.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 34 walking intensity (m/s2); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 34.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 34.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 35 step count (steps per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 35.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 35.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 36 time walking (minutes per day); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 36.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 36.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 37 walking intensity (m/s2); Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 37.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 37.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 38 change in step count (steps per day); Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 38.1 End intervention (stage 1) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.2 End intervention (stage 2) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 38.3 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 39 change in step count (steps per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 39.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 39.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 40 change in time in moderate‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 40.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 40.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 41 change in time in vigorous‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 41.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 41.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 42 change in time in light‐intensity physical activity (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 42.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 42.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 43 change in total energy expenditure (kcal); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 43.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 43.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 44 change in sedentary time (minutes per day); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 44.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 44.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in step count (steps per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in time in moderate‐to‐vigorous intensity physical activity (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in number of bouts of moderate‐to‐vigorous intensity physical activity; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 change in time in bouts of moderate‐to‐vigorous intensity physical activity(minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 change in sedentary time (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 change in sedentary time (awake; minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in number of sedentary bouts; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in time in sedentary bouts (minutes per day); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in METs; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 change in total energy expenditure (kcal); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 change in step count (steps per day); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 change in total energy expenditure (kcal); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 step count (steps per day); Interventions: Tai Chi vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Mid‐intervention (6 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Mid‐intervention (22 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 step count (steps per day); Interventions: outdoor walking vs. cycle ergometry Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 step count (steps per day); Interventions: physical activity counselling vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 total energy expenditure (kcal); Interventions: exercise training with tapered supervision vs. supervised exercise training Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 End intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 mid day activity (vector magnitude units per minute); Interventions: supplemental oxygen (lightweight ambulatory) vs. supplemental oxygen (E‐cylinder) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in SGRQ total score; Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 End intervention | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐8.79 [‐14.08, ‐3.51] |
| 2 change in SGRQ domain scores. Intervention: pulmonary rehabilitation/exercise training (ground‐based walking) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in CRQ domain scores; Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Dyspnoea (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 1.69 [0.02, 3.36] |
| 3.2 Emotional function (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 2.41 [0.48, 4.35] |
| 3.3 Fatigue (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 1.34 [‐0.16, 2.83] |
| 3.4 Mastery (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.47, 1.69] |
| 3.5 Total score (end intervention) | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 6.70 [2.55, 10.86] |
| 4 CAT score; Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | ‐3.15 [‐4.82, ‐1.47] |
| 5 SGRQ domain scores (%change); Intervention: exercise training [inpatient] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Symptoms (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Activity (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Impacts (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Total score (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 SGRQ domain scores; Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 CCQ domain scores: Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Functional state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Mental state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in CCQ domain scores; Intervention: physical activity counselling (telecoaching) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Functional state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Mental state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in CRQ domain and total scores; Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 change in SGRQ domain and total scores; Intervention: physical activity counselling Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 change in CRQ domain scores; Intervention: self‐management (SPACE) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.5 Dyspnoea (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.6 Emotional domain (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.7 Fatigue (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.8 Mastery (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 CCQ total score; Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 EQ5D index score; Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 EQ5D visual analogue scale score; Intervention: self‐management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Mid‐intervention (4 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 SGRQ domain scores; Intervention: self‐management (telephone health coaching) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.4 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.5 Symptoms (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.6 Activity (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.7 Impacts (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.8 Total score (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 EQ5D score; Intervention: self‐management (telephone health coaching) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 CRQ domain scores; Intervention: four‐wheeled walker Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in SF36 component scores; Intervention: singing Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Physical component (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Mental component (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in CRQ total score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in SGRQ total score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 change in EQ5D index score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 change in EQ5D visual analogue scale score; Intervention: neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SF36 component scores (score < 50); Interventions: Nordic walking with education vs. education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Physical component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Physical component score (follow‐up, 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Physical component score (follow‐up, 9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Mental component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Mental component score (follow‐up, 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Mental component score (follow‐up, 9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in CRQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Dyspnoea (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Emotional function (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Fatigue (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Mastery (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Dyspnoea (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.14 Emotional function (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.15 Fatigue (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.16 Mastery (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in CCQ domain scores; Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Symptoms (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Functional state (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Mental state (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Total (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Functional state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Mental state (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Symptoms (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Functional state (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Mental state (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Total (follow‐up, 18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Symptoms (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 Functional state (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Mental state (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Total (follow‐up, 24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CRQ domain scores; Interventions: LAMA/LABA and exercise training with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 CRQ domain scores; Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 CRQ domain scores; Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in SGRQ domain and total scores; Intervention: exercise training and physical activity counselling with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 Symptoms (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 Activity (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 Impacts (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 Total (follow‐up, 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.9 Symptoms (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.10 Activity (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.11 Impacts (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.12 Total (follow‐up, 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 change in CRQ domain scores; Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Dyspnoea (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Emotional function (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Fatigue (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Mastery (12 weeks, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Dyspnoea (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 Emotional function (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 Fatigue (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 Mastery (6 months, mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.9 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.10 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.11 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.12 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 change in SGRQ total score; Interventions: physical activity counselling with pedometer vs. pedometer Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 web‐based (12 weeks, end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 web‐based (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 web‐based (12 months, end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 SGRQ total: Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 SF36: Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Physical component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Mental component score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 change in SGRQ domain scores; Interventions: physical activity counselling (web‐based) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Symptoms (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Impacts (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Activities (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.5 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.6 Activities (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 SGRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 RAND36 domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Physical function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Vitality (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 Bodily pain (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 General health perception (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.5 Health status (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 change in CRQ dyspnoea domain score; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Mid‐intervention (end PR) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 SGRQ scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1 Symptoms (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Activity (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.3 Impacts (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.4 Total score (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.5 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.6 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.7 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.8 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 CRQ domain scores; Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.5 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 change in CRQ total score; Interventions: physical activity counselling with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 change in CRQ scores; Interventions: self‐management (health coaching) with pulmonary rehabilitation referral vs. pulmonary rehabilitation referral Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Physical function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.3 Physical function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.4 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 CRQ domain scores; Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 CRQ domain scores; Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 CRQ domain scores; Interventions: LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.3 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.5 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.6 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.8 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 change in SGRQ domain and total scores; Interventions: ACE inhibitor with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 23.1 Symptoms (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Activity (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.3 Impacts (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.4 Total (end intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 change in SGRQ total score; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24.1 End intervention (stage 2) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 change in EQ5D; Interventions: nutritional supplement with pulmonary rehabilitation vs. placebo with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 25.1 End intervention (stage 2) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 change in CRQ domain scores; Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 26.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.5 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.6 Dyspnoea (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.7 Emotional function (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.8 Fatigue (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.9 Mastery (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 26.10 Total score (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27 CRQ domain scores; Interventions: non‐invasive ventilation with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 27.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 27.5 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 6MWD (metres); Intervention; neuromuscular electrical stimulation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 6MWD (metres); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 End intervention | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 29.06 [14.38, 43.75] |
| 2 change in ISWD (metres); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 End intervention | 3 | 180 | Mean Difference (IV, Random, 95% CI) | 19.02 [2.20, 35.85] |
| 3 change in ESWT (seconds); Intervention: pulmonary rehabilitation/exercise training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 End intervention | 2 | 137 | Mean Difference (IV, Random, 95% CI) | 237.87 [147.59, 328.16] |
| 4 6MWD (metres); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 46.55 [24.66, 68.45] |
| 5 work rate (watts); Intervention: high‐intensity interval training Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 End intervention | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 15.47 [8.76, 22.17] |
| 6 change in ISWD (metres); Intervention: self‐management (SPACE) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 change in ESWT (seconds); Intervention: self‐management (SPACE) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 6MWD (metres); Intervention: exercise training [inpatient] Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in CRQ domains; Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Dyspnoea (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Emotional function (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Fatigue (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Mastery (follow‐up) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in CRQ domains; Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Dyspnoea (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Emotional function (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Fatigue (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mastery (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Total score (mid‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Dyspnoea (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Emotional function (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Fatigue (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Mastery (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Total score (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 SGRQ domain and total scores; Interventions: Tai Chi vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Symptoms (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Activity (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Impacts (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Total (mid‐intervention, 2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Symptoms (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Activity (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Impacts (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Total (mid‐intervention, 14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Symptoms (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Activity (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Impacts (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Total (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 CRQ total score; Interventions: outdoor walking vs. cycle ergometry Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Maugeri Respiratory Failure questionnaire; Interventions: exercise training with tapered supervision vs. supervised exercise training Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 End intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 SF36 domain scores; Interventions: self‐management vs. education and symptom monitoring Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Mental health (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Role emotional (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Vitality (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Social functioning (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Pain (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Role physical (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 General health (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 Physical functioning (end intervention) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 6MWD (metres); Interventions: Nordic walking with education vs. education Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Follow‐up (9 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in ISWD (metres); Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Follow‐up (18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Follow‐up (24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in ESWT (seconds); Interventions: exercise training (COPE‐active) with self‐management vs. self management Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Follow‐up (18 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Follow‐up (24 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 6MWD (metres); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 ESWT (seconds); Interventions: exercise training and LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 6MWD (metres); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 ESWT (seconds); Interventions: exercise training and LABA with LAMA and behaviour modification vs. LAMA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 6MWD (metres); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 ESWT (seconds); Interventions: exercise training with LAMA/LABA and behaviour modification vs. LAMA/LABA and behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 change in ESWT (seconds); Intervention: physical activity counselling and exercise training with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Follow‐up (3 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Follow‐up (12 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 6MWD (metres); Interventions: physical activity counselling (app) with optional supervised exercise vs. optional supervised exercise Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Mid‐intervention (12 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Mid‐intervention (6 months) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.3 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 6MWD (metres); Interventions: physical activity counselling (app) with pedometer vs. pedometer Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 change in 6MWD (metres); Interventions: physical activity counselling with pulmonary rehabilitation vs. sham with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 6MWD (metres); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 ESWT (seconds); Interventions: LAMA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 16.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 ESWT (s); Interventions: LAMA/LABA with behaviour modification vs. placebo with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 6MWD (metres); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 ESWT (seconds); Interventions: LAMA/LABA with behaviour modification vs. LAMA with behaviour modification Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 change in ISWD (metres); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 change in ESWT (seconds); Interventions: supplemental oxygen with pulmonary rehabilitation vs. sham intervention with pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 change in 6MWD (metres); Interventions: home‐based pulmonary rehabilitation vs. centre‐based pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Follow‐up | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 change in 6MWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 change in ISWD (metres); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 change in VO2max (ml/min); Interventions: water‐based exercise training vs. land‐based exercise training Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Mid‐intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 6MWD (metres); Interventions: Tai Chi vs. pulmonary rehabilitation Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Mid‐intervention (2 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mid‐intervention (14 weeks) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 End intervention | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 6MWD (metres); Interventions: exercise training with tapered supervision vs. supervised exercise training Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 End intervention | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

