Continuación versus interrupción del tratamiento antiplaquetario para los episodios isquémicos y hemorrágicos en pacientes adultos sometidos a una cirugía no cardíaca
Resumen
Antecedentes
Los agentes antiplaquetarios se recomiendan en pacientes con infarto de miocardio y síndromes coronarios agudos, ataque isquémico transitorio o accidente cerebrovascular, y en pacientes a los que se les han insertado stents coronarios. Los pacientes que toman agentes antiplaquetarios tienen mayor riesgo de eventos adversos cuando se someten a cirugía no cardíaca debido a estas indicaciones. Sin embargo, la administración del tratamiento antiplaquetario también da lugar a riesgo para el paciente que se somete a cirugía porque aumenta la probabilidad de hemorragia. La interrupción del tratamiento antiplaquetario antes de la cirugía podría reducir este riesgo, pero es más probable que posteriormente provoque problemas trombóticos, como el infarto de miocardio.
Objetivos
Comparar los efectos de continuar versus interrumpir durante al menos cinco días el tratamiento antiplaquetario en la aparición de hemorragia y episodios isquémicos en adultos sometidos a cirugía no cardíaca con anestesia general, espinal o regional.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2018, número 1), MEDLINE (1946 hasta enero 2018) y en Embase (1974 hasta enero 2018). Se buscaron estudios en curso en los registros de ensayos clínicos, y se realizó una búsqueda hacia adelante y hacia atrás de las citas de artículos relevantes.
Criterios de selección
Se incluyeron ensayos controlados aleatorios en adultos que estuvieran tomando tratamiento antiplaquetario único o dual, durante al menos dos semanas, y tuvieran programada una cirugía no cardíaca electiva. Los participantes incluidos tenían al menos un factor de riesgo cardíaco. Se planificó incluir los estudios cuasialeatorios.
Se excluyeron los pacientes programados para cirugías menores con anestesia o sedación local, en los que era poco probable la aparición de hemorragias que necesiten transfusión o cirugía adicional. Se incluyeron los estudios que compararon la continuación preoperatoria del tratamiento antiplaquetario versus la interrupción del tratamiento antiplaquetario o versus la sustitución del tratamiento antiplaquetario con placebo durante al menos cinco días antes de la cirugía.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los estudios para la inclusión, extrajeron los datos, evaluaron el riesgo de sesgo y resumieron los hallazgos. Los resultados primarios fueron: mortalidad por todas las causas en el seguimiento más largo (hasta seis meses); mortalidad por todas las causas (hasta 30 días). Los resultados secundarios incluyeron: pérdida sanguínea que requirió la transfusión de productos sanguíneos; pérdida sanguínea que requirió cirugía adicional; riesgo de episodios isquémicos. Se utilizaron los criterios GRADE para evaluar la calidad de la evidencia para cada resultado
Resultados principales
Se incluyeron cinco ECA con 666 adultos asignados al azar. Se identificaron tres ensayos en curso.
Todos los participantes de los estudios se programaron para cirugía general electiva (incluida cirugía abdominal, urológica, ortopédica y ginecológica) con anestesia general, espinal o regional. Los estudios compararon la continuación del tratamiento antiplaquetario único o dual (aspirina o clopidogrel) con la interrupción del tratamiento durante al menos cinco días antes de la cirugía.
Tres estudios informaron el uso de métodos adecuados de asignación al azar, y dos informaron sobre el uso de métodos para ocultación de la asignación. Tres estudios fueron ensayos controlados con placebo y tuvieron bajo riesgo de sesgo de realización, y tres estudios informaron sobre el uso de métodos adecuados para el cegamiento de los evaluadores de resultado a la asignación a los grupos. El desgaste fue limitado en cuatro estudios, y dos estudios informaron el registro prospectivo en registros de ensayos clínicos y tuvieron bajo riesgo de sesgo de informe selectivo de los resultados.
La mortalidad se informó en dos puntos temporales: el seguimiento más largo informado por los autores de los estudios hasta los seis meses, y el punto temporal informado por los autores de los estudios hasta los 30 días. Cinco estudios informaron la mortalidad hasta los seis meses (en cuatro el seguimiento más largo fue a los 30 días y en uno a los 90 días) y encontraron que continuar o interrumpir el tratamiento antiplaquetario puede lograr poco o ningún cambio en la mortalidad hasta los seis meses (cociente de riesgos [CR] 1,21; intervalo de confianza [IC] del 95%: 0,34 a 4,27; 659 participantes; evidencia de certeza baja); el efecto absoluto es tres muertes más por cada 1000 pacientes en los que se continúan los antiplaquetarios (con una variación de ocho menos a 40 más). El agrupamiento de los cuatro estudios con un seguimiento más largo a los 30 días mostró la misma estimación del efecto, y se encontró que la continuación o la interrupción del tratamiento antiplaquetario puede lograr poco o ningún cambio en la mortalidad a los 30 días después de la cirugía (CR 1,21; IC del 95%: 0,34 a 4,27; 616 participantes; evidencia de certeza baja); el efecto absoluto es tres muertes más por cada 1000 pacientes en los que se continúan los antiplaquetarios (con una variación de nueve menos a 42 más).
Se encontró que la continuación o la interrupción del tratamiento antiplaquetario probablemente produce poco o ningún cambio en la incidencia de hemorragias que requieran transfusión (CR 1,37; IC del 95%: 0,83 a 2,26; 368 participantes; el efecto absoluto es 42 participantes más por cada 1000 que requieren transfusión en el grupo de continuación, con una variación de 19 menos a 119 más; cuatro estudios; evidencia de certeza moderada); y puede lograr poco o ningún cambio en la incidencia de pérdida sanguínea que requiere cirugía adicional (CR 1,54; IC del 95%: 0,31 a 7,58; 368 participantes; el efecto absoluto es seis participantes más por cada 1000 que requieren cirugía adicional en el grupo de continuación, con una variación de siete menos a 71 más; cuatro estudios; evidencia de certeza baja). Se encontró que la continuación o la interrupción del tratamiento antiplaquetario puede producir poco o ningún cambio en la incidencia de episodios isquémicos (que incluyen isquemia periférica, infarto cerebral e infarto de miocardio) en un plazo de 30 días desde la cirugía (CR 0,67; IC del 95%: 0,25 a 1,77; 616 participantes; el efecto absoluto es 17 participantes menos por cada 1000 con un episodio isquémico en el grupo de continuación, con una variación de 39 menos a 40 más; cuatro estudios; evidencia de certeza baja).
Se utilizó el enfoque GRADE para degradar la evidencia para todos los resultados debido a que la evidencia esta limitada a unos pocos estudios. Se observó una amplio intervalo de confianza en las estimaciones del efecto para la mortalidad al final del seguimiento y a los 30 días, y para la hemorragia que requiere transfusión, lo que señala imprecisión. Se observaron diferencias visuales en los resultados de los estudios para los episodios isquémicos, lo que indicó inconsistencia.
Conclusiones de los autores
Se encontró evidencia de baja certeza de que la continuación o la interrupción del tratamiento antiplaquetario antes de la cirugía no cardíaca puede producir poco o ningún cambio en la mortalidad, en la hemorragia que requiere cirugía o en los episodios isquémicos. Se encontró evidencia de moderada certeza de que la continuación o la interrupción del tratamiento antiplaquetario antes de la cirugía no cardíaca probablemente produce poco o ningún cambio en la hemorragia que requiere transfusión. La evidencia se limitó a unos pocos estudios con pocos participantes y pocos episodios. Los tres estudios en curso pueden alterar las conclusiones de la revisión una vez publicados y evaluados.
PICO
Resumen en términos sencillos
Continuar o interrumpir la administración de fármacos antiplaquetarios pocos días antes de la cirugía no cardíaca en adultos
Pregunta de la revisión
Determinar si continuar con la administración de fármacos antiplaquetarios antes de la cirugía no cardíaca que requiera anestesia general, espinal o regional aumenta el riesgo de presentar hemorragia grave, episodios isquémicos o muerte en adultos, en comparación con interrumpir la toma de fármacos antiplaquetarios durante al menos cinco días antes de la cirugía no cardíaca.
Antecedentes
Los fármacos antiplaquetarios como la aspirina o el clopidogrel reducen el riesgo de presentar coágulos sanguíneos, y habitualmente se les prescriben a los pacientes a los que se les han insertado stents coronarios. También se recomiendan en pacientes con angina inestable o cardiopatía, o en pacientes que han tenido un ataque al corazón, cirugía cardíaca o accidente cerebrovascular. La administración del tratamiento antiplaquetario da lugar a un aumento del riesgo de hemorragia, lo que podría provocar problemas si un paciente necesita una cirugía no cardíaca. La interrupción del tratamiento habitual antiplaquetario pocos días antes de la cirugía podría reducir el riesgo de hemorragia grave durante la cirugía. Sin embargo, no tomar estos fármacos antiplaquetarios podría aumentar el riesgo de un ataque al corazón, un accidente cerebrovascular o la muerte.
Características de los estudios
La evidencia de los ensayos controlados aleatorios está actualizada hasta enero de 2018. En la revisión se incluyeron cinco ensayos con 666 adultos. Tres estudios están en curso. Todos los participantes recibían tratamiento antiplaquetario (aspirina o clopidogrel) al comienzo del estudio. Dos estudios interrumpieron la toma de fármacos antiplaquetarios durante al menos cinco días antes de la cirugía, y en tres estudios les administraron a los participantes un placebo en lugar del tratamiento antiplaquetario durante ese tiempo.
Resultados clave
Se encontró evidencia de baja certeza de que continuar o interrumpir el tratamiento antiplaquetario puede producir poco o ningún cambio en el número de pacientes que murieron a los 30 días o a los seis meses después de la cirugía (cinco estudios, 659 participantes). Se encontró evidencia de moderada certeza de que continuar o interrumpir el tratamiento antiplaquetario puede producir poco o ningún cambio en la incidencia de hemorragias lo suficientemente graves como para necesitar una transfusión de sangre durante o inmediatamente después de la cirugía (cuatro estudios, 368 participantes). Se encontró evidencia de baja certeza de que continuar o interrumpir el tratamiento antiplaquetario puede producir poco o ningún cambio en la aparición de hemorragias lo suficientemente graves como para necesitar cirugía adicional (cuatro estudios, 368 participantes), y puede producir poco o ningún cambio en el número de episodios isquémicos como el accidente cerebrovascular o el ataque al corazón. (cuatro estudios, 616 participantes).
Calidad de la evidencia
Algunos estudios tuvieron bajo riesgo de sesgo ya habían informado claramente los métodos para asignar al azar a los pacientes a cada grupo, y tres estudios utilizaron un agente placebo, por lo que los pacientes no sabían si continuaban o no su tratamiento antiplaquetario habitual. Sin embargo, se encontraron pocos estudios con pocos episodios, y hubo una amplia variación en los resultados. Continuar o interrumpir la toma de fármacos antiplaquetarios unos pocos días antes de la cirugía no cardíaca puede producir poco o ningún cambio en el número de pacientes que murieron, que presentaron hemorragia que necesite cirugía adicional o que presentaron episodios isquémicos, y probablemente produce poco o ningún cambio en la hemorragia que necesite una transfusión de sangre. Se encontraron tres estudios que siguen en curso, que aumentarán la certeza en el efecto en futuras actualizaciones de la revisión.
Authors' conclusions
Summary of findings
| Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery | ||||||
| Population: adults undergoing non‐cardiac surgery (including abdominal, urological, orthopaedic, and gynaecological surgery) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of randomized participants | Certainty of the evidence | Comments | |
| Risk with discontinuation | Risk with continuation | |||||
| All‐cause mortality (at longest follow‐up reported by study authors up to 6 months) | Study population | RR 1.21 | 659 | ⊕⊕⊝⊝ | 4 studies reported longest follow‐up at 30 days; 1 study reported longest follow‐up at 90 days | |
| 12 per 1,000 | 15 per 1,000 | |||||
| All‐cause mortality (up to 30 days) | Study population | RR 1.21 | 616 | ⊕⊕⊝⊝ | 4 studies reported mortality at 30 days | |
| 13 per 1,000 | 16 per 1,000 | |||||
| Blood loss requiring transfusion of blood products (intraoperatively and postoperatively) | Study population | RR 1.37 | 368 | ⊕⊕⊕⊝ Moderate2 | ||
| 114 per 1,000 | 156 per 1,000 | |||||
| Blood loss requiring further surgery (intraoperatively and postoperatively) | Study population | RR 1.54 | 368 | ⊕⊕⊝⊝ | ||
| 11 per 1,000 | 17 per 1,000 | |||||
| Ischaemic events: peripheral thrombosis, cerebral myocardial infarction (within 30 days of surgery) | Study population | RR 0.67 | 616 | ⊕⊕⊝⊝ | 2 studies reported data for major thrombotic events (defined as acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, stroke, transient ischaemic attack, acute coronary syndrome, peripheral arterial ischaemia, mesenteric arterial ischaemia, deep proximal or distal venous thrombosis, and pulmonary embolism); 1 study reported data for myocardial infarction; and 1 study reported data for cardio‐cerebrovascular events (which included acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, transient ischaemic attack or stroke) | |
| 52 per 1,000 | 35 per 1,000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded by 2 levels for imprecision because we noted a wide CI for this effect, and because event data were limited to few studies | ||||||
Background
Description of the condition
Platelets play a major role in the pathogenesis of atherosclerotic and thrombotic diseases. Drugs intended to prevent or treat these diseases are widely used. Antiplatelet agents are recommended for people with myocardial infarction and acute coronary syndromes, transient ischaemic attack or stroke, and for those in whom coronary stents have been inserted (NICE 2010). Many people are prescribed two antiplatelet drugs (typically aspirin plus another drug), especially after acute coronary syndromes and coronary artery stenting. In the latter context, these drugs are needed to prevent clots from forming and blocking the stent until normal vascular endothelium grows over the metal of the stent. Stents of different types (‘bare metal’ or ‘drug‐eluting’) require different durations of treatment; for instance, dual antiplatelet therapy with aspirin and clopidogrel is recommended for at least one month after insertion of a bare metal stent, and for at least 12 months after insertion of a drug‐eluting stent (Levine 2016). Interfering with platelet function naturally increases risk of bleeding, but in general this risk is low enough to be acceptable (Sorensen 2009). Anticoagulants such as warfarin are often used to prevent and treat thrombosis, and can also cause bleeding significant enough to warrant pharmacological reversal (Johanson 2015); these are outside the scope of this review.
In the year after stent insertion, about 4% of people will have to undergo non‐cardiac surgery (Berger 2010). Such procedures carry increased risk of an adverse outcome, as both myocardial infarction (Hawn 2013; Wijeysundera 2012), and significant bleeding (Singla 2012), are more likely. However, premature discontinuation of dual antiplatelet therapy can be fatal (Korte 2011), in people with coronary stents. On the other hand, dual antiplatelet therapy used for primary prevention of cardiac or cerebrovascular events can be stopped before surgery without major consequences (Oprea 2013).
Description of the intervention
Commonly used antiplatelet agents fall into three pharmacological classes: thromboxane A2 inhibitors (aspirin), thienopyridines (clopidogrel, prasugrel and ticlopidine) and cyclopentyltriazolopyrimidines (ticagrelor).
Aspirin irreversibly inhibits the enzyme cyclo‐oxygenase 1, leading to loss of platelet aggregation. This effect persists for the lifespan of the platelet, which is seven to 10 days (Oprea 2013). The remaining drugs act on the P2Y12 receptor on the platelet surface, preventing it from binding with adenosine diphosphate (ADP) and thus inhibiting aggregation (Oprea 2013). Again, for clopidogrel, this effect is irreversible and lasts as long as the platelet itself. Thus it is necessary to discontinue these agents for at least one week to allow their effects to wear off. Ticagrelor, on the other hand, is a reversible antagonist at the ADP receptor, although reversal of its clinical effect might not be straightforward.
How the intervention might work
The person on antiplatelet agents undergoing surgery is therefore at risk for two types of complications: bleeding and thrombosis. Which carries greater risk will depend on the indications for the antiplatelet agent and on the type of surgery proposed; these and other factors can be incorporated into a ‘matrix’ to help balance the risks for an individual patient (Korte 2011; Rossini 2014). Discontinuing the antiplatelet agent might make thrombotic problems such as myocardial infarction more likely; on the other hand, performing surgery when antiplatelet drugs are still active in the body is likely to increase bleeding. To complicate matters, the haemodynamic instability caused by severe bleeding might in itself lead to myocardial ischaemia and infarction (Devereaux 2016). Some recent studies (Mantz 2011; Oscarsson 2010), have investigated the effects of continuing or discontinuing aspirin during the perioperative period; results demonstrate no difference in the number of bleeding events (Mantz 2011; Oscarsson 2010), but a reduction in the number of major adverse cardiac events when aspirin is continued during the perioperative period (Oscarsson 2010).
Why it is important to do this review
Current recommendations are to usually continue antiplatelet therapy for people with a coronary artery stent (Fleisher 2014; Oprea 2013), and that very low bleeding risk procedures might be undertaken without stopping dual antiplatelet therapy (Keeling 2016). However, very recent evidence suggests that the relationship between continuation of antiplatelet agents and reduced thrombotic complications might not be as simple as one might suppose (Wasowicz 2016). Given this uncertainty and the large numbers of people affected worldwide, a systematic review of available high‐quality evidence is necessary. The results of this review might inform clinical guidelines and might have implications for the costs of health care.
Objectives
To compare the effects of continuation versus discontinuation for at least five days of antiplatelet therapy on the occurrence of bleeding and ischaemic events in adults undergoing non‐cardiac surgery under general, spinal or regional anaesthesia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We planned to include quasi‐randomized studies (e.g. studies in which participants are assigned by alternation, date of birth or medical record number).
Cluster‐RCTs were not eligible for inclusion.
Types of participants
We included studies involving adults who were taking antiplatelet therapy for at least two weeks, and were scheduled for elective surgery. Antiplatelet therapy was prescribed as single or dual therapy to include all antiplatelet agents, such as aspirin, clopidogrel, prasugrel, ticlopidine or ticagrelor. Included participants had at least one cardiac risk factor.
We included studies involving people scheduled for surgery under general, spinal or regional anaesthesia. We excluded people scheduled for cardiac surgery, which requires different clinical management (Wong 2016). We excluded people scheduled for minor surgeries (such as dental extraction) under local anaesthetic or sedation.
Types of interventions
We included studies that compared perioperative continuation of antiplatelet agents (i.e. continuation of dual or single agent therapy during the preoperative, intraoperative and postoperative periods) with discontinuation of antiplatelet therapy for five days or longer before surgery.
We included studies that administered a placebo or no treatment during the discontinuation phase. We planned to exclude studies that continued only one agent in a dual therapy, and also those which assessed the effects of other drugs initiated before surgery (Sanders 2013; Zhao 2016; Zou 2016).
Types of outcome measures
We aimed to establish whether risk of bleeding is affected by continuation of antiplatelet therapy. Therefore, we collected data on two measures of bleeding: the number of patients requiring transfusion of any blood product owing to blood loss during or after surgery; and the number of patients requiring additional surgical intervention for blood loss during or after surgery. Both continuation and discontinuation of antiplatelets might increase the risk of ischaemic events, and we collected composite data on ischaemic events. We recorded ischaemic events for a follow‐up period of 30 days and recorded the number of deaths at two time points: the longest follow‐up time point reported by study authors up to six months, and time points reported by study authors up to 30 days.
Primary outcomes
-
All‐cause mortality at longest follow‐up (up to six months)
-
All‐cause mortality (up to 30 days)
Secondary outcomes
-
Blood loss requiring transfusion of blood products (intraoperatively and postoperatively)
-
Blood loss requiring further surgical intervention (intraoperatively and postoperatively)
-
Risk of ischaemic events: peripheral thrombosis, cerebral infarction, myocardial infarction within 30 days
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status.
We searched the following databases for relevant trials.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library (searched 2 January 2018)
-
MEDLINE (OvidSP, 1946 to 2 January 2018)
-
Embase (OvidSP, 1974 to 2 January 2018)
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other listed databases. The search strategy was developed in consultation with the Information Specialist. Search strategies can be found in Appendix 1, Appendix 2, and Appendix 3.
We scanned the following trial registries for ongoing and unpublished trials (24 July 2017).
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/AdvSearch.aspx).
-
ClinicalTrials.gov (ClinicalTrials.gov).
Searching other resources
We carried out citation searching of identified included studies in Web of Science (apps.webofknowledge.com), on 3 January 2018 and conducted a search of grey literature through 'Opengrey' (www.opengrey.eu), on 1 August 2017. We carried out backward citation searching of key reviews identified from the searches. We did not need to contact study authors or organizations.
Data collection and analysis
We (Sharon Lewis (SL) and Oliver Schofield‐Robinson (OSR)) independently completed all data collection and analysis before comparing results and reaching consensus. We consulted a third review author (Phil Alderson (PA) or Andrew Smith (AS)) to resolve conflicts when necessary.
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates (Endnote). We used Covidence software to screen the results of the search from titles and abstracts, identify potentially relevant studies (Covidence) and consider whether they met the inclusion criteria (see Criteria for considering studies for this review). We included abstracts at this stage. However, we planned to include abstracts in the review only if they contained sufficient information and relevant results that included denominator figures for each intervention/comparison group. We recorded the number of papers retrieved at each stage, and reported this information using a PRISMA flow chart (Liberati 2009). We reported in the review brief details of closely related, but excluded, papers.
Data extraction and management
We used Covidence software to extract data from individual studies (Covidence). A basic template of the data extraction forms is available at www.covidence.org. We adapted the template to include the following information.
-
Methods: the type of study design; setting; dates of study; funding sources.
-
Participants: the number of participants randomized to each group; baseline characteristics; type of surgery; type of antiplatelet therapy and duration of administration.
-
Interventions/comparison: type of control (placebo or no treatment); time of discontinuation of antiplatelet therapy before surgery.
-
Outcomes: all relevant review outcomes measured and reported by study authors.
-
Outcome data: the results of outcome measures.
We considered the applicability of information from individual studies and the generalizability of data to our intended study population (i.e. the potential for indirectness in our review). Had we identified associated publications from the same study, we planned to create a composite dataset from all eligible publications.
Assessment of risk of bias in included studies
Two review authors (SL and OSR) independently assessed study quality, study limitations and extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We considered the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants, personnel and outcome assessors (performance and detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective outcome reporting (reporting bias).
-
Other.
For each domain, we judged whether study authors made sufficient attempts to minimize bias in their study design. We made judgements using one of three measures (high, low or unclear risk of bias). We recorded this information in the 'Risk of bias' tables and presented a summary 'Risk of bias'.
Measures of treatment effect
To calculate risk ratios (RR), we collected dichotomous data for outcomes related to mortality, number of participants requiring transfusion of blood products or additional surgery for blood loss and number of participants having an ischaemic event.
Unit of analysis issues
If we had included multi‐arm studies comparing different antiplatelet agents or comparing dual‐ and single‐agent therapies versus a control, we planned to include both comparison groups but split the data for the control group, using a 'halving' method to avoid double‐counting, as recommended by Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We collected risk of ischaemic events as composite data. We reported the number of participants who have had at least one ischaemic event and paid attention to how study authors reported this outcome to avoid a unit of analysis issue in which a participant might have experienced more than one different event.
Dealing with missing data
We assessed whether all measured outcomes had been reported by study authors by comparing, when possible, published reports with protocols or clinical trial registration documents that had been prospectively published.
We assessed whether all randomized participants were included in outcome data. We did not need to contact study authors for missing data.
Assessment of heterogeneity
We assessed evidence of inconsistency within our results through consideration of heterogeneity. We assessed clinical heterogeneity by comparing differences in study design, participants, interventions and outcomes in our included studies using the data we collected during data extraction. We assessed statistical heterogeneity by calculating the Chi² P value or the I² statistic. We judged any heterogeneity above an I² statistic of 60% and a Chi² P value of 0.05 or less to indicate moderate to substantial statistical heterogeneity (Higgins 2011).
As well as looking at the statistical results, we considered point estimates and overlap of confidence intervals (CIs). If the CIs overlap, the results are more consistent. However, combined studies might show a large consistent effect but with significant heterogeneity. Therefore, we planned to interpret heterogeneity with caution (Guyatt 2011).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies using clinical trial registers. We compared published protocols with published study results to assess the risk of selective reporting bias. Had we identified sufficient studies (i.e. more than 10) (Higgins 2011), we planned to generate a funnel plot to assess risk of publication bias in the review; an asymmetrical funnel plot might indicate publication of only positive results (Egger 1997).
Data synthesis
We completed meta‐analysis for outcomes for which we had comparable effect measures from more than one study and when measures of heterogeneity indicated that pooling of results was appropriate. We used Review Manager 5 (Review Manager 2014), to perform meta‐analysis.
For each outcome, we calculated risk ratios (RRs) using the summary data presented in each trial report. We used a Mantel‐Haenszel effects model, unless events were extremely rare (1 per 1000), in which case we planned to use Peto odds ratios (Higgins 2011). We used a random‐effects statistical model to account for the variation in different types of surgical procedures in studies (Borenstein 2010). If evidence suggested moderate statistical or clinical heterogeneity, we planned to investigate this by performing subgroup analyses, as below.
Subgroup analysis and investigation of heterogeneity
We aimed to use subgroup analysis to address potential differences in the population group for which the risk‐benefit ratios might differ according to continuation or discontinuation of the drug; whether people were taking a single or dual antiplatelet therapy; and whether they have coronary stents. We also aimed to address whether there is an optimum point at which antiplatelets can be discontinued; people whose therapy has been discontinued earlier than five days might be at increased risk of ischaemic events (Korte 2011).
We planned to perform subgroup analyses as follows.
-
Single antiplatelet treatment versus dual therapy.
-
Coronary stents versus no coronary stents.
-
Discontinuation of antiplatelet agents within five days before surgery versus discontinuation at more than five days before surgery.
Sensitivity analysis
We planned to explore potential effects of decisions made as part of the review process as follows.
-
Excluding all studies that we judged to be at high or unclear risk of selection bias.
-
Using the alternate meta‐analytical effects model (fixed‐effect).
We compared effect estimates from the above results with effect estimates from the main analysis. We planned to report differences that alter interpretation of the effect. We planned to perform sensitivity analysis on all of our outcomes.
In sensitivity analysis, we used trial sequential analysis on our primary outcomes using software from The Copenhagen Trial Unit (www.ctu.dk/); this alternative meta‐analytic method accounted for studies with zero events. See Differences between protocol and review. Also, we assessed decisions made on individual studies as part of the review process.
'Summary of findings' table and GRADE
The GRADE approach incorporates assessment of indirectness, study limitations, inconsistency, publication bias and imprecision. We assessed the certainty of the evidence (high, moderate, low, and very low) using these five GRADE considerations and, if required, downgraded the evidence by one or two levels using assessments at each stage of our analysis (Data collection and analysis; Assessment of risk of bias in included studies; Assessment of heterogeneity; Assessment of reporting biases; Data synthesis). This approach gives an overall measure of how certain we can be that our estimate of effect is correct (Guyatt 2008).
We used the principles of the GRADE system to give an overall assessment of evidence certainty related to each of the following outcomes.
-
All‐cause mortality at longest follow‐up (up to six months).
-
All‐cause mortality at longest follow‐up (up to 30 days).
-
Blood loss requiring transfusion of blood products.
-
Blood loss requiring further surgical intervention.
-
Risk of ischaemic events: peripheral thrombosis, cerebral infarction, myocardial infarction within 30 days.
One review author (SL) used GRADEpro software (GRADEpro GDT), to create a 'Summary of findings' table for each comparison (www.guidelinedevelopment.org/). A second author (PA) assessed judgements made and consensus was reached through discussion.
Results
Description of studies
Results of the search
After removing duplicates we screened 10,207 titles and abstracts from database searches, results from clinical trial register searches, grey literature searches, and forward and backward citation searches. We carried out full‐text review of 20 articles. We excluded 10 studies. We identified five eligible studies which were randomized controlled trials (RCTs) (two studies had two publications); we found no quasi‐randomized studies. We found three ongoing studies. See Figure 1.
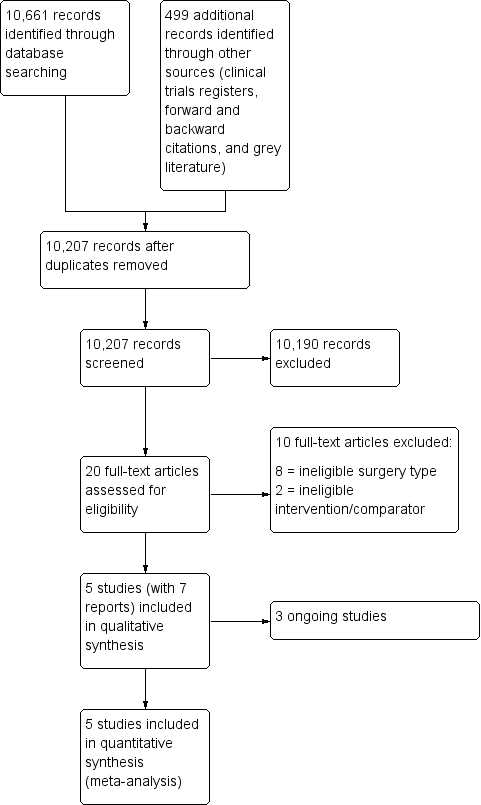
Study flow diagram.
Included studies
We included five RCTs with 666 participants (Antolovic 2012; Chu 2016; Mantz 2011; Nielsen 2000; Oscarsson 2010). All studies were parallel design, three were single‐centre studies (Antolovic 2012; Chu 2016; Nielsen 2000), and two were multi‐centre studies (Mantz 2011; Oscarsson 2010). Two studies had anticipated recruitment of more participants, but stopped early due to recruitment difficulties (Mantz 2011; Oscarsson 2010). In addition, Oscarsson 2010, reported early stopping because of publication during the study period of guidelines about the management of high‐risk patients taking aspirin. See Characteristics of included studies tables.
Study population and setting
Participants were adults scheduled for general elective surgery as follows.
-
Inguinal hernia repair, cholecystectomy, colonic/colorectal, laparoscopic (Antolovic 2012).
-
Open inguinal hernia repair, laparoscopic cholecystectomy, laparoscopic or open ventral hernia repair, laparoscopic inguinal repair, Nissen's fundoplication, ostomy closure, open sigmoidectomy and ileostomy (Chu 2016).
-
Orthopaedic, abdominal, urologic, thoracic, oncologic, ears, nose, and throat (ENT) (Mantz 2011).
-
Transurethral prostatectomy (Nielsen 2000) and
-
Abdominal, urologic, orthopaedic, gynaecologic (Oscarsson 2010).
Three studies reported surgery completed under general anaesthesia: in 65.4% participants (Antolovic 2012); in 82% participants (Mantz 2011); and in 22.3% participants (Oscarsson 2010). Surgery was completed using spinal anaesthesia in all participants in Nielsen 2000. One study did not report the type of anaesthesia (Chu 2016).
Three studies reported the number of participants who had coronary stents: 25% participants in Antolovic 2012; 72.1% participants in Chu 2016; and 13.1% participants in Mantz 2011. The studies did not report the length of time since stent insertion. Two studies did not report the number of participants with coronary stents (Nielsen 2000; Oscarsson 2010); one study reported change to study inclusion criteria with a decision to exclude participants with coronary stents in the second year of study (Oscarsson 2010).
Interventions and comparators
In four studies, all participants were on existing antiplatelet therapy (Antolovic 2012; Chu 2016; Mantz 2011; Nielsen 2000), which was: aspirin (100 mg in Antolovic 2012; 150 mg to 300 mg in Nielsen 2000); clopidogrel (75 mg as a single therapy, or in 66.7% participants as dual therapy with aspirin in Chu 2016); and any antiplatelet agent (aspirin, clopidogrel, or ticlopidine and others) in Mantz 2011. In one study, 90% participants were on existing antiplatelet therapy with aspirin (Oscarsson 2010). The studies did not report the length of time that participants had been taking antiplatelet therapy, and we assumed that all participants had been taking antiplatelets for at least two weeks.
Three studies were placebo‐controlled trials (Mantz 2011; Nielsen 2000; Oscarsson 2010). These studies substituted existing therapy with placebo or with aspirin (75 mg aspirin in Mantz 2011 and Oscarsson 2010; 150 mg aspirin in Nielsen 2000).
In two studies, participants continued existing antiplatelet therapy at existing dose or discontinued existing antiplatelet therapy without substitution agent during study period (Antolovic 2012; Chu 2016).
Time points for discontinuation of antiplatelet prior to surgery were at: five days before surgery (Antolovic 2012); seven days before surgery (Chu 2016; Oscarsson 2010); and 10 days before surgery (Mantz 2011; Nielsen 2000).
Sources of funding
Four studies reported the sources of funding (Chu 2016; Mantz 2011; Nielsen 2000; Oscarsson 2010). We noted support from pharmaceutical companies in two of these studies (Nielsen 2000; Oscarsson 2010). One study did not report the sources of funding (Antolovic 2012), and the study authors declared no conflict of interest.
Excluded studies
We excluded 10 studies from review of full‐text articles (Ardekian 2000; Assia 1998; Danino 2007; Devereaux 2014; Duygu 2010; Eapen 2017; Engheta 2016; Gaspar 1999; Medeiros 2011; Varghese 2015). In one study, interruption of long‐term antiplatelet therapy was compared with a substitution agent rather than no agent or a placebo (Danino 2007). One study compared antiplatelets with a placebo, and participants were stratified according to whether they were previously taking antiplatelets; participants who were taking antiplatelets were required to stop treatment for at least three days prior to surgery. We excluded this study because of interruption to treatment (Devereaux 2014). We excluded eight studies because surgery was minor and either under local anaesthetic or sedation (Ardekian 2000; Assia 1998; Duygu 2010; Eapen 2017; Engheta 2016; Gaspar 1999; Medeiros 2011; Varghese 2015). These surgery types were not comparable to other non‐cardiac surgical procedures included in the review. We also noted that the risk of bleeding in the studies was limited to use of local haemostatic measures to control bleeding events. See Characteristics of excluded studies.
Awaiting classification
There are no studies awaiting classification.
Ongoing studies
We found three ongoing studies in clinical trials register searches (NCT01806090; NCT02797548; NCT03184805). The studies have an anticipated recruitment of 4616 participants, and compare continuation versus discontinuation of antiplatelet therapy before non‐cardiac surgery in adults. See Characteristics of ongoing studies.
Risk of bias in included studies
See Characteristics of included studies tables, and 'Risk of bias' summary and 'Risk of bias' graph (Figure 2; Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies were described as randomized, and we judged three of the five studies to have a low risk of bias because they provided sufficient details on the method of randomization (Chu 2016; Mantz 2011; Oscarsson 2010). Two studies reported no details of the method of randomization, and we judged these studies to have an unclear risk of bias (Antolovic 2012; Nielsen 2000).
Two studies reported sufficient detail about methods used to conceal allocation, and we judged these studies to have low risk of bias for allocation concealment (Antolovic 2012; Mantz 2011). The three remaining studies reported no details of allocation concealment, and we judged these studies to have an unclear risk of bias.
Blinding
Three studies were placebo‐controlled trials and study authors reported, or we assumed, that participants and personnel were blinded to group allocation. We judged these three studies to have low risk of performance bias (Mantz 2011; Nielsen 2000; Oscarsson 2010). One study reported that participants were not blinded to group allocation (Chu 2016), and one study reported no information about blinding of participants and personnel (Antolovic 2012).We judged these studies to have and unclear risk of performance bias. However, we acknowledge that it is possible that awareness of group allocation might influence outcome data because some participants might not have adhered to study protocol if requested to discontinue regular antiplatelet therapy.
Three studies reported that outcome assessors were blinded, and we judged these studies to be at low risk of detection bias (Antolovic 2012; Mantz 2011; Oscarsson 2010). The two remaining studies reported insufficient information about blinding of outcome assessors, and we judged these studies to have an unclear risk of bias (Chu 2016; Nielsen 2000).
Incomplete outcome data
Four studies reported no participant losses during the trial or few losses, and we judged these to have low risk of attrition bias (Antolovic 2012; Chu 2016; Nielsen 2000; Oscarsson 2010). One study reported a large number of losses (Mantz 2011), but study authors reported reasons for these losses. We judged this study to have unclear risk of bias because we noted more losses in the continuation group, and we could not be certain of the effect of these losses on the results.
Selective reporting
We found registration with clinical trial registers for two studies, and outcomes were reported according to these documents.We judged these studies to have low risk of selective reporting bias (Mantz 2011; Oscarsson 2010). Two studies reported retrospective clinical trial registration, and it was not feasible to use these documents to assess risk of selective reporting bias (Antolovic 2012; Chu 2016). One study did not report details of a published protocol (Nielsen 2000). We judged these studies to have unclear risk of selective reporting bias.
Other potential sources of bias
One study reported limited baseline characteristics, and details of existing antiplatelet therapies (Nielsen 2000). We noted a difference in ranges of ages between groups in one study (Antolovic 2012). We were unclear whether information that was not reported might have influenced the study results, and we judged these studies to have an unclear of risk of other bias. We noted no other sources of bias in the three remaining studies which we judged to have low risk of bias (Chu 2016; Mantz 2011; Oscarsson 2010).
Effects of interventions
Primary outcomes
1. All‐cause mortality at longest follow‐up (up to six months)
All five studies reported mortality (Antolovic 2012; Chu 2016; Mantz 2011; Nielsen 2000; Oscarsson 2010). Longest follow‐up time points for data were 30 days after surgery in four studies (Antolovic 2012; Mantz 2011; Nielsen 2000; Oscarsson 2010), and at 90 days in one study (Chu 2016).
We noted no evidence of a difference in deaths according to whether participants continued or discontinued antiplatelet therapy (risk ratio (RR) 1.21, 95% confidence interval (CI) 0.34 to 4.27; 659 participants; Analysis 1.1).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by two levels for imprecision because we noted a wide CI for this effect and because event data were limited to only three of the five studies. See summary of findings Table for the main comparison.
2. All‐cause mortality (up to 30 days)
Four studies reported mortality at 30 days (Antolovic 2012; Mantz 2011; Nielsen 2000; Oscarsson 2010).
We noted no evidence of a difference in deaths according to whether participants continued or discontinued antiplatelet therapy (RR 1.21, 95% CI 0.34 to 4.27; 616 participants; Analysis 1.2).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by two levels for imprecision because we noted a wide CI for this effect and because event data were limited to only three of the four studies. See summary of findings Table for the main comparison.
Secondary outcomes
1. Blood loss requiring transfusion of blood products (intraoperatively and postoperatively)
Four studies reported blood loss requiring transfusion (Antolovic 2012; Chu 2016; Nielsen 2000; Oscarsson 2010). We interpreted data in Nielsen 2000, from a graph in the published report. One study reported major bleeding events but did not define major bleeding by need for transfusion or surgery and we could not include these data in the analysis (Mantz 2011). We noted no difference between groups in these data and have reported the results separately in Table 1. Bleeding was not monitored in any of these trials (Wikkelsø 2016).
| Outcome: major bleeding event | |||
| Study | Continuation group; n = 145 | Discontinuation group; n = 146 | Effect estimate* |
| 10 participants had at least 1 major bleeding event | 10 participants had at least 1 major bleeding event | OR 0.99, 95% CI 0.36 to 2.8; P = 1.0 | |
* as reported by study authors
CI: confidence interval
n: number of randomized participants
OR: odds ratio
We noted no evidence of a difference in the number of participants requiring blood transfusions according to whether participants continued or discontinued antiplatelets (RR 1.37, 95% CI 0.83 to 2.26; 368 participants; Analysis 1.3).
We used the GRADE approach to downgrade the evidence to moderate quality. We downgraded by one level for imprecision because event data were limited to only three of four studies. See summary of findings Table for the main comparison.
2. Blood loss requiring further surgical intervention (intraoperatively and postoperatively)
Four studies reported blood loss requiring surgical intervention (Antolovic 2012; Chu 2016; Nielsen 2000; Oscarsson 2010). One study reported major bleeding events but did not define major bleeding by need for transfusion or surgery, and we could not include these data in analysis (Mantz 2011). We noted no difference between groups in these data and have reported it separately in Table 1.
We noted no difference in number of participants with blood loss requiring surgical interventions (RR 1.54, 95% CI 0.31 to 7.58; 368 participants; Analysis 1.4).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by two levels for imprecision because event data were limited to only three of four studies and because we noted a wide CI for this effect. See summary of findings Table for the main comparison.
3. Risk of ischaemic events: peripheral thrombosis, cerebral infarction, myocardial infarction within 30 days
Four studies reported ischaemic events (Antolovic 2012; Mantz 2011; Nielsen 2000; Oscarsson 2010). We reported data collected for major thrombotic events (defined as acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, severe pulmonary embolism, transient ischaemic attack, or stroke in Antolovic 2012; and defined as stroke, transient ischaemic attack, acute coronary syndrome, peripheral arterial ischaemia, mesenteric arterial ischaemia, deep proximal and distal venous thrombosis, and pulmonary embolism in Mantz 2011); for myocardial infarction in Nielsen 2000; and for cardio‐cerebrovascular events (which included acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, transient ischaemic attack or stroke) in Oscarsson 2010.
We noted no evidence of a difference in number of participants with an ischaemic event (RR 0.67, 95% CI 0.25 to 1.77; 616 participants; Analysis 1.5).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by one level for imprecision because event data were limited to only three of four studies. We downgraded by one level for inconsistency because we noted visual differences in results. See summary of findings Table for the main comparison.
Subgroup analysis
We did not conduct subgroup analysis because we included insufficient studies for each outcome to justify meaningful subgroup analysis (Higgins 2011).
Sensitivity analysis
We completed the following sensitivity analyses which were specified in the protocol.
-
Studies at high or unclear risk of selection bias. We judged only one study to have low risk of selection bias in both domains (sequence generation and allocation concealment), and sensitivity analysis was not feasible.
-
Meta‐analytical effects model. We assessed whether using a fixed‐effect model would alter effect estimates for our outcomes. We found no difference in interpretation of effect estimates depending on meta‐analytical effects model for each of our outcomes.
We completed the following sensitivity analyses which were performed as posthoc decisions (see Differences between protocol and review).
-
Assessment of the primary outcomes using trial sequential analysis. We used a conventional test boundary and a random‐effects model (DerSimonian‐Laird method) to perform trial sequential analysis using software from The Copenhagen Trial Unit (www.ctu.dk/) with continuity corrected for studies with zero events. We found no difference in interpretation of the result for all‐cause mortality at the end of follow‐up (up to six months), and all‐cause mortality (up to 30 days) when analysis accounted for studies with zero events (RR 1.17, 95% CI 0.37 to 3.67).
-
Decisions made during the review process. We included one study in which 10% of participants were not on antiplatelet therapy before the start of the study (Oscarsson 2010). In sensitivity analysis, we excluded this study. We noted no difference to interpretation of effect estimates for each outcome. In one study, we interpreted data for the number of participants with a bleeding event requiring transfusion from a graph (Nielsen 2000). In sensitivity analysis, we excluded this study for this outcome and noted no difference to interpretation of the effect estimate.
Discussion
Summary of main results
We included five studies comparing continuation of antiplatelet therapy with discontinuation of antiplatelet before non‐cardiac surgery. The five studies included 666 adults. We found three ongoing studies.
We found no evidence of a difference in mortality up to six months, and up to 30 days, after surgery, and no evidence of a difference in bleeding requiring further surgery or in ischaemic events. We used the GRADE approach to downgrade evidence for these outcomes to low certainty. We found no evidence of a difference in bleeding requiring transfusion, and we used the GRADE approach to downgrade evidence to moderate certainty.
Overall completeness and applicability of evidence
We included studies of adults who were taking antiplatelet drugs and were scheduled for a variety of different surgical procedures. The study authors did not report the length of time that the participants had been on antiplatelet therapy. Three studies reported the number of people who had a coronary stent, and this was balanced between the groups. However, the length of time since insertion of stent was not reported by study authors. We noted that the number of people with coronary stents differed between these three studies.
Evidence was limited to five studies. Because data were limited, we did not conduct subgroup analysis to explore whether potential differences between studies (e.g. whether participants were on single or dual therapy, had coronary stents, or discontinuation of therapy was before or after five days) could influence the results.
Quality of the evidence
Three studies reported adequate methods of randomization, and two reported methods to conceal allocation. Three studies were placebo‐controlled trials and were at low risk of performance bias, and three studies reported adequate methods to blind outcome assessors to group allocation. Attrition was limited in four studies and two studies had reported prospective registration with clinical trial registers and were at low risk of selective outcome reporting bias.
We used the GRADE approach to downgrade evidence for this review. We noted limited available data for each outcome, a wide confidence interval in evidence for mortality and for blood loss requiring further surgery, and visual differences noted between studies for ischaemic events; we used these reasons to downgrade evidence to low certainty, or moderate certainty.
Potential biases in the review process
We conducted a thorough search and used two review authors to assess study eligibility, to extract data, and to assess risk of bias in included studies, and believe that we reduced potential bias in the review process by using two review authors. However, we did not contact study authors to clarify missing information (such as length of follow‐up, funding sources, or missing information for 'Risk of bias' assessments), and judgements made in the review were based only on information in published reports.
We included one study in which some participants were not on antiplatelets before the start of the trial (Oscarsson 2010). We carried out sensitivity analysis and results were unaffected if this study was excluded from analysis; we believed that including this study did not introduce bias to the results.
We made a post‐hoc decision to exclude studies in which people were scheduled for minor surgeries under local anaesthetic or sedation. These type of surgeries would introduce considerable clinically heterogeneity in the review, and are unlikely to lead to major bleeding requiring transfusion or additional surgery. For this review, eight of the 10 excluded studies were studies of minor surgeries under local anaesthetic or sedation (dental extraction, cataract surgery, removal of skin tumour). These studies did not measure outcome data or provide useful information for this review and, therefore, exclusion of these studies did not influence effect estimates for this review. See Differences between protocol and review.
Agreements and disagreements with other studies or reviews
We found no systematic reviews that considered only randomized controlled trials (RCTs) for non‐cardiac surgery. One review recommended discontinuation of clopidogrel for at least five days before surgery, but this judgement was based on limited evidence from observational studies of non‐cardiac surgery, and evidence from studies of cardiac surgery (Au 2012). Another review concluded no evidence of excessive bleeding from continuation of aspirin using limited evidence from both RCTs and observational studies (Sahebally 2014). Burger 2005 noted an increase in the risk of bleeding complications with aspirin use but without an increase in the severity of the bleeding event; this review included both RCTs and observation studies. We excluded one large RCT from this review because participants taking aspirin were required to stop at least three days before surgery, and was not comparable with other studies in this review (Devereaux 2014). We noted that this large study of non‐cardiac surgery similarly found little or no difference in all‐cause mortality at 30 days or in ischaemic events. Devereaux 2014 found an increased risk of major bleeding in participants who were taking aspirin in the perioperative period.
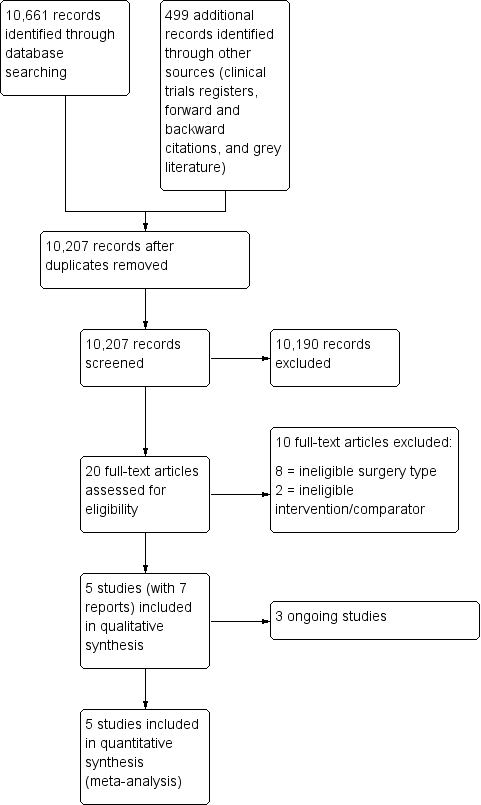
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
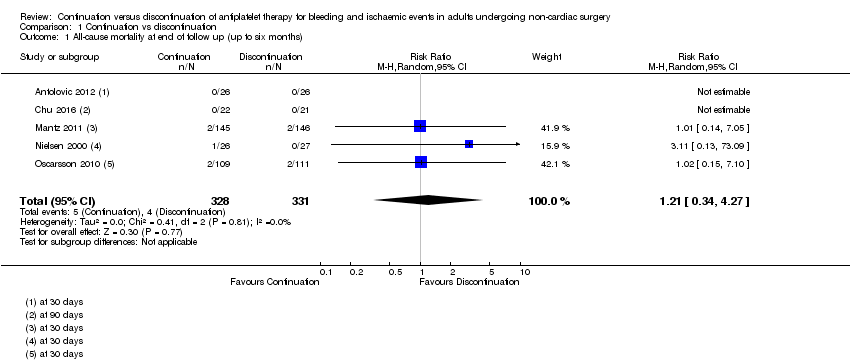
Comparison 1 Continuation vs discontinuation, Outcome 1 All‐cause mortality at end of follow up (up to six months).

Comparison 1 Continuation vs discontinuation, Outcome 2 All‐cause mortality (up to 30 days).

Comparison 1 Continuation vs discontinuation, Outcome 3 Blood loss requiring transfusion of blood products (intraoperatively and postoperatively).
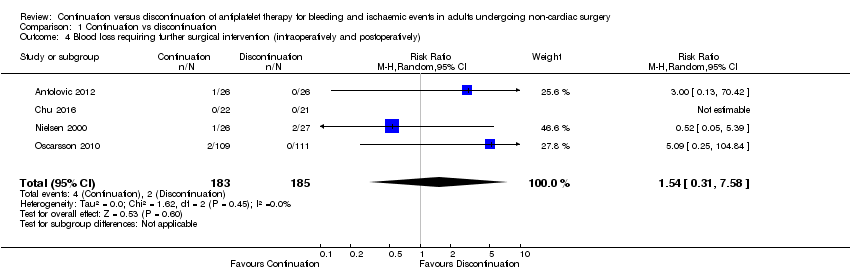
Comparison 1 Continuation vs discontinuation, Outcome 4 Blood loss requiring further surgical intervention (intraoperatively and postoperatively).

Comparison 1 Continuation vs discontinuation, Outcome 5 Risk of ischaemic events (within 30 days).
| Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery | ||||||
| Population: adults undergoing non‐cardiac surgery (including abdominal, urological, orthopaedic, and gynaecological surgery) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of randomized participants | Certainty of the evidence | Comments | |
| Risk with discontinuation | Risk with continuation | |||||
| All‐cause mortality (at longest follow‐up reported by study authors up to 6 months) | Study population | RR 1.21 | 659 | ⊕⊕⊝⊝ | 4 studies reported longest follow‐up at 30 days; 1 study reported longest follow‐up at 90 days | |
| 12 per 1,000 | 15 per 1,000 | |||||
| All‐cause mortality (up to 30 days) | Study population | RR 1.21 | 616 | ⊕⊕⊝⊝ | 4 studies reported mortality at 30 days | |
| 13 per 1,000 | 16 per 1,000 | |||||
| Blood loss requiring transfusion of blood products (intraoperatively and postoperatively) | Study population | RR 1.37 | 368 | ⊕⊕⊕⊝ Moderate2 | ||
| 114 per 1,000 | 156 per 1,000 | |||||
| Blood loss requiring further surgery (intraoperatively and postoperatively) | Study population | RR 1.54 | 368 | ⊕⊕⊝⊝ | ||
| 11 per 1,000 | 17 per 1,000 | |||||
| Ischaemic events: peripheral thrombosis, cerebral myocardial infarction (within 30 days of surgery) | Study population | RR 0.67 | 616 | ⊕⊕⊝⊝ | 2 studies reported data for major thrombotic events (defined as acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, stroke, transient ischaemic attack, acute coronary syndrome, peripheral arterial ischaemia, mesenteric arterial ischaemia, deep proximal or distal venous thrombosis, and pulmonary embolism); 1 study reported data for myocardial infarction; and 1 study reported data for cardio‐cerebrovascular events (which included acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, transient ischaemic attack or stroke) | |
| 52 per 1,000 | 35 per 1,000 | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded by 2 levels for imprecision because we noted a wide CI for this effect, and because event data were limited to few studies | ||||||
| Outcome: major bleeding event | |||
| Study | Continuation group; n = 145 | Discontinuation group; n = 146 | Effect estimate* |
| 10 participants had at least 1 major bleeding event | 10 participants had at least 1 major bleeding event | OR 0.99, 95% CI 0.36 to 2.8; P = 1.0 | |
| * as reported by study authors | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality at end of follow up (up to six months) Show forest plot | 5 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.34, 4.27] |
| 2 All‐cause mortality (up to 30 days) Show forest plot | 4 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.34, 4.27] |
| 3 Blood loss requiring transfusion of blood products (intraoperatively and postoperatively) Show forest plot | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.83, 2.26] |
| 4 Blood loss requiring further surgical intervention (intraoperatively and postoperatively) Show forest plot | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.31, 7.58] |
| 5 Risk of ischaemic events (within 30 days) Show forest plot | 4 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.25, 1.77] |

