اسیدهای صفراوی توتال سرم یا پروفایل اسید صفراوی سرم، یا هر دو، به منظور تشخیص کلستاز داخل کبدی دوران بارداری
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategies |
| Cochrane Hepato‐Biliary Group Controlled Trials Register | 6 May 2019 | (((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) AND ((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum) AND (pregnan* or obstetric* or gestation*) |
| Cochrane Hepato‐Biliary Group Diagnostic Test Accuracy Studies Register | 6 May 2019 | (((bile or cholic or CA or glycocholic or GCA or choliglycine or chenodeox*cholic or CDCA or deox*cholic or DCA or lithocholic or LCA or ursodeox*cholic or UDCA or glyco‐conjugated or tauro‐conjugated or glycine or taurine) and acid*) AND ((cholestas* and (hepat* or liver*)) or jaundice or icterus graviardum) AND (pregnan* or obstetric* or gestation*) |
| Cochrane Library | 2019, Issue 5 | #1 MeSH descriptor: [Bile Acids and Salts] explode all trees |
| MEDLINE Ovid | 1946 to 6 May 2019 | 1. exp "Bile Acids and Salts"/ |
| Embase Ovid | 1974 to 6 May 2019 | 1. exp bile acid/ |
| Science Citation Index Expanded (Web of Science) | 1900 to 6 May 2019 | #4 #1 AND #2 AND #3 |
| Conference Proceedings Citation Index – Science (Web of Science) | 1900 to 6 May 2019 | #4 #1 AND #2 AND #3 |
| BIOSIS Previews (Web of Science) | 1969 to 6 May 2019 | #4 458 #1 AND #2 AND #3 |
| CINAHL (EBSCOhost) | 1981 to 6 May 2019 | S14 64 S6 AND S9 |
| CNKI (Chinese database) | 1979 to May 2019 | ((bile acid OR bile) AND pregnancy) OR ICP |
| VIP (Chinese database) | 1989 to May 2019 | (bile AND pregnancy) AND diagnosis |
| LILACS (VHL) | 6 May 2019 | 1. (tw:((tw:(cholestasis )) AND (tw:(pregnancy OR obstetric)))) OR (tw:((tw:( colestasis)) AND (tw:(gravídica OR (intrahepática AND embarazo) OR obstétrica)))) OR (tw:((tw:(ictericia)) AND (tw:(embarazo OR gravídica)))) OR (tw:((tw:(colestase)) AND (tw:(gravidez OR gestacional OR obstétrica)))) OR (tw:( (tw:(icterícia)) AND (tw:(gravidez OR colestática)))) AND (instance:"regional") AND ( db:("LILACS")) 2. (tw:(acidos biliares)) AND (tw:(embarazo OR gravidez OR obstétrica OR gestational OR gravidica)) AND (instance:"regional") AND ( db:("LILACS")) |
| SCIELO | 6 May 2019 | 1. ((cholestasis) AND (pregnancy OR obstetric) ) OR ((colestasis) AND (embarazo OR obstétrica) ) OR ((ictericia) AND (embarazo OR gravídica) ) OR ( (icterícia) AND (gravidez OR colestática)) OR ((colestase) AND (gravidez OR gestacional) ) 2. (bile acids) AND (pregnancy OR obstetric) 3. (acidos biliares) AND (embarazo OR gravidez OR obstétrica OR gestational OR gravidica) |
| Evidence search: Health and Social Care, RHL, TRIP, OpenSIGLE, NTIS | 6 May 2019 | cholestasis AND (obstetric OR pregnancy) AND (bile acid) |
Appendix 2. QUADAS‐2
| Domain | Participant selection | Index test | Reference standard | Flow and timing |
| Description | Describe methods of participant selection: describe inclusion criteria for participants (prior testing, presentation, intended use of index test, and setting): The studies that fulfil the inclusion criteria of this review should have included as participants pregnant women recruited in any clinical setting. They should have been evaluated for personal history of skin or liver diseases, presence of pruritus during their pregnancy, and they should have been assessed with any most common liver test (or tests), followed by any of the already mentioned index tests (total bile acids, cholic acid, glycocholic acid, chenodeoxycholic acid, cholic/chenodeoxycholic acid ratio, deoxycholic acid, lithocholic acid, ursodeoxycholic acid, total glyco‐conjugated bile acids, total tauro‐conjugated bile acids, total glyco‐conjugated bile acids/total taurine‐conjugated bile acids ratio) | Describe the index test and how it was be conducted and interpreted: Total bile acids, cholic acid, glycocholic acid, chenodeoxycholic acid, cholic/chenodeoxycholic acid ratio, deoxycholic acid, lithocholic acid, ursodeoxycholic acid, total glyco‐conjugated bile acids, total tauro‐conjugated bile acids, total glyco‐conjugated bile acids/total taurine‐conjugated bile acids ratio, are non‐ invasive laboratory serum tests performed after the first clinical evaluation of the pregnant women for the diagnosis of intrahepatic cholestasis of pregnancy. The serum concentration of the index test(s) can be assessed through different techniques. Laboratory methods and diagnostic cut‐off values could vary between different studies. | Describe the reference standard and how it was conducted and interpreted: Clinical evaluation including the follow‐up after delivery. The clinical evaluation is the final judgment of the clinician who takes into account the clinical assessment of suggestive signs and symptoms for intrahepatic cholestasis of pregnancy and the presence of any otherwise unexplained, persistent abnormalities of AST, ALT, or bilirubin levels until delivery. The follow‐up after delivery is the assessment of spontaneous relief of symptoms and normalisation of liver tests within eight weeks at most. | Describe any people who did not receive the index test(s) or reference standard (or both) or who will be excluded from the 2 x 2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard: As mentioned in the protocol, we will exclude participants who lack data for the two‐by‐two table. To define a time interval between our index tests and our reference standard is not relevant, as the index tests should be performed when the suspicion of intrahepatic cholestasis of pregnancy arises and the reference standard comprises the follow‐up after delivery. |
| Signalling questions: yes/no/unclear | Was a consecutive or random sample of participants enrolled? Yes: all consecutive participants or random sample of people with suspected intrahepatic cholestasis of pregnancy were enrolled in the study. No: selected participants were not included. Unclear: insufficient data were reported to permit a judgement. | Were the index test results interpreted without knowledge of the results of the reference standard? We think this will not be a relevant question for our review as the index tests are objective laboratory tests and the answer should always be 'Yes'. | Is the reference standard likely to classify the target condition correctly? Yes: if participants underwent a through clinical evaluation excluding all possible differential diagnosis and if they underwent an adequate follow‐up after delivery assessing the spontaneous relief of symptoms and normalisation of the previously found abnormal liver tests. No: clinical evaluation including the follow‐up after delivery was not able to rule out other possible differential diagnosis. Unclear: insufficient data were reported to permit a judgement. | Was there an appropriate interval between index test(s) and reference standard? This is not a relevant question to our review (see above). |
| Was a case‐controldesign avoided? Yes: case‐control design was avoided. No: case‐control design was not avoided. Unclear: insufficient information was reported to permit a judgement. | If a threshold was used, was it pre‐specified? Yes. No. Unclear: it is not reported or not clearly described. | Were the reference standard results interpreted without knowledge of the results of the index test? Yes: clinical evaluation including the follow‐up after delivery was performed without knowledge of the results of total serum bile acids or any component of serum bile acid profile. No: clinical evaluation including the follow‐up after delivery was performed with knowledge of the results of total serum bile acids or any component of serum bile acid profile. Unclear: insufficient data were reported to permit a judgement. _________________ Was the index test evaluation not part of the reference standard? Yes: the index test evaluation was not part of the reference standard. No: index test evaluation was part of the reference standard. Unclear: insufficient data were reported to permit a judgement. | Did all participants receive the reference standard? Yes: all participants underwent the reference standard, i.e., clinical evaluation including the follow‐up after delivery. No: not all participants underwent the reference standard, i.e., clinical evaluation including the follow‐up after delivery. Unclear: insufficient data were reported to permit a judgement. | |
| Did the study avoid inappropriate exclusions? Yes: the study avoided inappropriate exclusions (e.g., women having a previously assessed value of the index test(s) below a defined cut‐off). No: the study excluded participants inappropriately. Unclear: insufficient data were reported to permit a judgement. | Did all participants receive the same reference standard? Yes: all participants received the same reference standard, i.e., clinical evaluation including the follow‐up after delivery. No: not all participants received the same reference standard, i.e., clinical evaluation including the follow‐up after delivery. Unclear: insufficient data were reported to permit a judgement. | |||
| Were all participants included in the analysis? Yes: all participants meeting the selection criteria (selected participants) were included in the analysis, or data on all the selected participants were available so that a 2 x 2 table including all selected participants could be constructed. No: not all participants meeting the selection criteria were included in the analysis or the 2 x 2 table could not be constructed using data on all selected participants. Unclear: insufficient data were reported to permit a judgement. | ||||
| Risk of bias: high/low/unclear | Could the selection of participants have introduced bias? High risk of bias: yes, if the selection of participants have introduced bias. Low risk of bias: no, if the selection of participants have not introduced bias. Unclear risk of bias: insufficient data on participants selection were reported to permit a judgement on the risk of bias. | Could the conduct or interpretation of the index test have introduced bias? High risk of bias: if the answer to the signalling questions on the conduct or interpretation of the index test is 'no'. Low risk of bias: if the answer to the signalling questions on the conduct or interpretation of the index test is 'yes'. Unclear risk of bias: if the answers to the two signalling questions on the conduct or interpretation of the index test is either 'unclear' or any combination of 'unclear' with 'yes' or 'no'. | Could the reference standard, its conduct, or its interpretation have introduced bias? High risk of bias: if the answer to the signalling questions on the reference standard, its conduct, or its interpretation is 'no'. Low risk of bias: if the answer to the signalling questions on the reference standard, its conduct, or its interpretation is 'yes'. Unclear risk of bias: if the answers to the three signalling questions on the reference standard, its conduct, or its interpretation is either 'unclear' or any combination of 'unclear' with 'yes' or 'no'. | Could the participant flow have introduced bias? High risk of bias: if the answer to the signalling questions on flow and timing is 'no'. Low risk of bias: if the answer to the signalling questions on flow and timing is 'yes'. Unclear risk of bias: if the answers to the 4 signalling questions on flow and timing is either 'unclear' or any combination of 'unclear' with 'yes' or 'no'. |
| Concerns regarding applicability: high/low/unclear | Are there concerns that the included participants do not match the review question? High concern: there is high concern that the included participants do not match the review question. Low concern: there is low concern that the included participants do not match the review question. Unclear concern: if it is unclear. | Are there concerns that the index test, its conduct, or interpretation differ from the review question? High concern: there is high concern that the conduct or interpretation of total serum bile acids or any component of serum bile acid profile differs from the way likely to be used in clinical practice. Low concern: there is low concern that the conduct or interpretation of total serum bile acids or any component of serum bile acid profile differs from the way likely to be used in clinical practice. Unclear concern: if it is unclear. | Are there concerns that the target condition as defined by the reference standard does not match the review question? High concern: all participants did not undergo clinical evaluation including the follow‐up after delivery. Low concern: all participants undergo clinical evaluation including the follow‐up after delivery. Unclear concern: If it is unclear. | ‐‐ |

Clinical diagnostic pathway for the diagnosis of intrahepatic cholestasis of pregnancy

Study selection flow diagram.

Risk of bias and applicability concerns summary: review authors' (CM and TS) judgements about each domain for each included study.
N.B. The empty cells stand for the test, not performed in the study.

Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
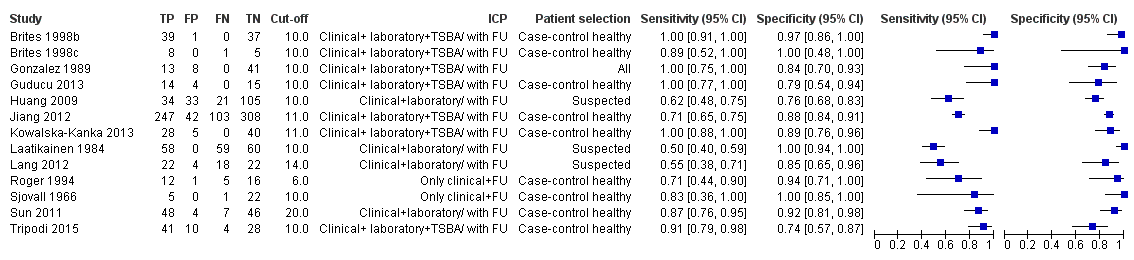
Forest plot of total serum bile acids (TSBA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy

Summary receiver operating characteristic (ROC) plot of total serum bile acids (TSBA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy. Statistical method used: HSROC (hierarchical summary ROC) model.

Forest plot of total serum bile acids (TSBA) with cut‐off = 10 µmol/L for the diagnosis of intrahepatic cholestasis of pregnancy

Summary receiver operating characteristic (ROC) plot of of total serum bile acids (TSBA) with cut‐off = 10 µmol/L for the diagnosis of intrahepatic cholestasis of pregnancy. Statistical method used: bivariate model.
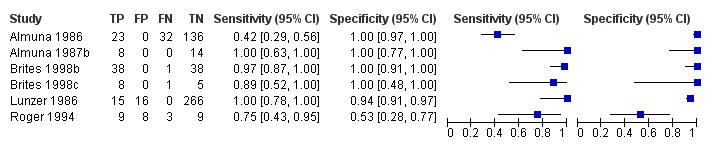
Forest plot of glycocholic acid (GCA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy

Summary receiver operating characteristic (ROC) plot of glycocholic acid (GCA) (all studies) for the diagnosis of intrahepatic cholestasis of pregnancy. Statistical method used: HSROC (hierarchical summary ROC) model.

Forest plots of cholic acid (CA) with different cut‐offs for the diagnosis of intrahepatic cholestasis of pregnancy: a) cut‐off 2 µmol/L; b) cut‐off = 3 µmol/L; c) cut‐off = 4 µmol/L; d) cut‐off = 5 µmol/L
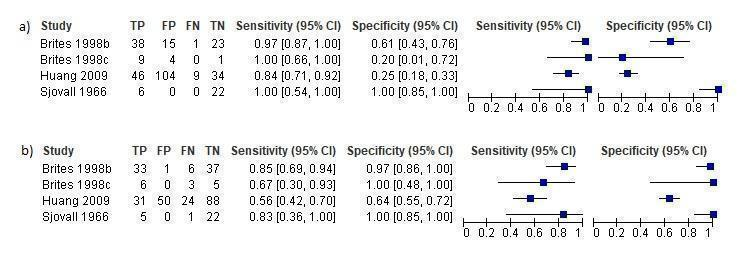
Forest plots of chenodeoxycholic acid (CDCA) at different cut‐offs for the diagnosis of intrahepatic cholestasis of pregnancy: a) cut‐off = 2 µmol/L; b) cut‐off = 3 µmol/L

Forest plot of CA/CDCA with cut‐off = 1.8

Summary ROC Plot of sensitivity analysis of TSBA cut‐off=10 μmol/L excluding studies in which TSBA assessment was part of the reference standard. Statistical method used: HSROC (hierarchical summary ROC) model.

Summary ROC Plot of sensitivity analysis of TSBA cut‐off=10 μmol/L excluding studies with case‐control design (95% confidence region not estimable because of too few studies included in the analysis). Statistical method used: HSROC (hierarchical summary ROC) model.

TSBA (all studies).
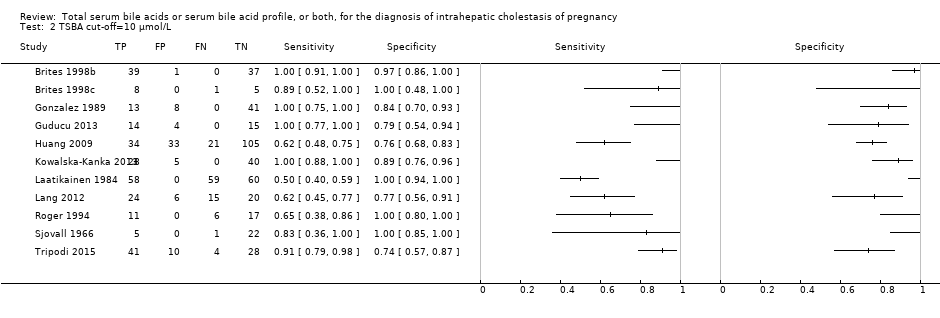
TSBA cut‐off=10 μmol/L.

CA cut‐off=2 μmol/L.

CA cut‐off=3 μmol/L.

CA cut‐off=4 μmol/L.
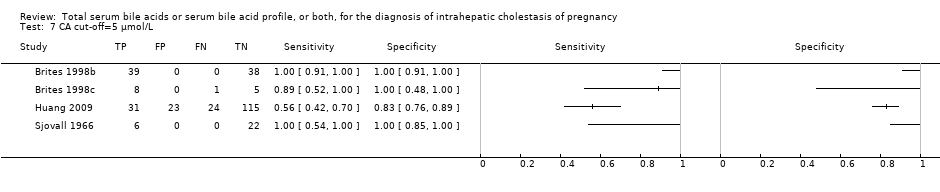
CA cut‐off=5 μmol/L.

CDCA cut‐off=2 μmol/L.

CDCA cut‐off=3 μmol/L.

GCA (all studies).

GCA cut‐off=0.7 μmol/L.

GCA cut‐off=1.5 μmol/L.

GCA cut‐off=2 μmol/L.

CA/CDCA cut‐off=1.8.

TSBA cut‐off=10 μmol/L sensitivity excl TSBA in reference standard.
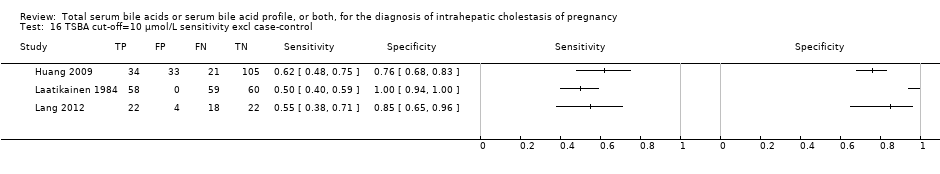
TSBA cut‐off=10 μmol/L sensitivity excl case‐control.
| What is the diagnostic accuracy of total serum bile acids (TSBA), cholic acid (CA), glycocholic acid (GCA), chenodeoxycholic acid (CDCA), or CA/CDCA for intrahepatic cholestasis of pregnancy (ICP), at different cut‐off values? | |||||
| Patients/population | Pregnant women with onset of pruritus from the second trimester or later | ||||
| Prior testing | History, serum tests, liver ultrasound | ||||
| Settings | Obstetrics and Gynaecology departments | ||||
| Index test | TSBA, CA, GCA, CDCA, CA/CDCA | ||||
| Importance | Early diagnosis, treatment and follow‐up to reduce fetal adverse events | ||||
| Reference standard | Clinical evaluation comprising common liver function tests, with exclusion of other possible underlying liver or dermatological diseases, and follow‐up after delivery assessing spontaneous normalization of signs and symptoms. | ||||
| Studies | Cross‐sectional and case‐control studies. Each study can be present in more than one subgroup and for more than one index test | ||||
| Test/Subgroup | Summary accuracy (95% CI) | N° part. (studies) | Median prevalence of ICP in pregnant women with pruritus | Implications for an hypothetical population of 100 pregnant women with pruritus | Quality and Comments |
| TSBA, any cut‐off | Sensitivity 0.88 (0.73 to 0.95) Specificity 0.90 (0.84 to 0.95) | 1645 (13) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 36 (15 to 81) women with ICP would be missed, and 70 (35 to 112) without ICP would be falsely diagnosed. | The overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| TSBA cut‐off = 10 μmol/L | Sensitivity 0.91 (0.72 to 0.98) Specificity 0.93 (0.81 to 0.97) | 839 (11) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 27 (6 to 84) women with ICP would be missed, and 49 (21 to 133) without ICP would be falsely diagnosed. | The overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| CA cut‐off = 2 μmol/L | Sensitivity 0.99 (0.33 to 1.00) Specificity 0.61 (0.23to 0.89) | 312 (4) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, both for sensitivity and specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| CA cut‐off =3 μmol/L | Sensitivity 0.94 (0.66 to 0.99) Specificity 0.82 (0.68 to 0.91) | 312 (4) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 18 (3 to 102) women with ICP would be missed, and 126 (63 to 224) without ICP would be falsely diagnosed. | The overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| GCA, all cut‐offs | Sensitivity 0.92 (0.65 to 0.99) Specificity 0.99 (0.06 to 1.00) | 630 (6) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| GCA cut‐off = 0.7 μmol/L | Sensitivity 0.97 (0.38 to 1.00) Specificity 0.86 (0.02 to 1.00) | 333 (5) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, both for sensitivity and specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| GCA cut‐off =1.5 μmol/L | Sensitivity 0.99 (0.08 to 1.00) Specificity 0.90 (0.75 to 0.97) | 417 (4) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for sensitivity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| GCA cut‐off = 2 μmol/L | Sensitivity 0.99 (0.07 to 1.00) Specificity 0.97 (0.82 to 1.00) | 125 (3) | ‐‐‐ | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for sensitivity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. |
| CDCA cut‐off = 2 | Sensitivity 0.98 (0.62 to 1.00) Specificity 0.66 (0.19 to 0.94) | 312 (4) | ‐‐‐ | The estimate of accuracy is too imprecise (i.e. very wide CI, especially for specificity), owing to the extreme heterogeneity between study results. Moreover, too few studies and of low quality were included for this index test. This makes impossible a judgment on applicability of the index test in a real clinical setting. | |
| CDCA cut‐off = 3 | Sensitivity 0.75 (CI not calc) Specificity 0.94 (0.88 to 0.97) | 312 (4) | ‐‐‐ | ‐‐‐ | The CI of sensitivity was not calculable and the CI of specificity was too wide. Hence, we cannot know the precision of the estimates obtained and their applicability in a real clinical scenario. |
| CA/CDCA cut‐off = 1.8 | Sensitivity 0.89 (0.54 to 0.98) Specificity 0.92 (0.85 to 0.96) | 312 (4) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 33 (6 to 138) women with ICP would be missed, and 56 (28 to 105) without ICP would be falsely diagnosed | The cut‐off used has been chosen among the best ones, comparing Youden indexes at multiple cut‐offs applied to all studies. This may have led to biased results. Moreover, sensitivity estimate has a wide CI. This makes hard to judge the applicability the index test in a real clinical setting. |
| Subgroup analysis for TSBA cut‐off = 10 μmol/L: timing (P = 0.027) | |||||
| Onset of symptoms | Sensitivity 0.87 (0.68 to 0.96) Specificity 0.87 (0.76 to 0.94) | 839 (11) | 30% (30 out of 100 pregnant women with pruritus having ICP) | 39 (12 to 96) women with ICP would be missed, and 91 (42 to 168) without ICP would be falsely diagnosed | Sensitivity and specificity seem to be quite good if TSBA are tested when symptoms of ICP arise. However, the overall accuracy found may be not applicable to a real clinical context, as most studies were at high risk of bias for patient selection and reference standard. |
| Peak value among multiple assessments | Sensitivity 0.7 (0.24 to 0.94) Specificity 1.00 (CI not calc) | 839 (11) | ‐‐‐ | ‐‐‐ | The CI of sensitivity was too wide and the CI of specificity was not calculable. Hence, we cannot know the precision of the estimates obtained and their applicability in a real clinical scenario. |
| Delivery | Sensitivity 1.00 (1.00 to 1.00) Specificity 0.87 (0.68 to 0.95) | 839 (11) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 0 women with ICP would be missed, and 91 (35 to 224) without ICP would be falsely diagnosed | Sensitivity seems to be higher when TSBA are tested at the time of delivery, while specificity seems to be the same as when symptoms of ICP arise. However, clinicians need to diagnose ICP as soon as possible during pregnancy to monitor and strictly follow up diseased woman, in order to find possible signs of fetal distress and plan the timing of delivery. Delivery time is too late to make a diagnosis. |
| Sensitivity analysis for TSBA cut‐off=10 μmol/L: exclusion of studies with TSBA as part of reference standard | |||||
| Sensitivity 0.57 (0.49 to 0.65) Specificity 0.98 (0.53 to 1.00) | 497 (5) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 129 (105 to 153) women with ICP would be missed, and 14 (0 to 329) without ICP would be falsely diagnosed | The overall accuracy of TSBA, especially sensitivity, seems to be lower when considering only studies without TSBA inclusion in the reference standard. The accuracy of the index test in a real clinical context may be similar to this. However CIs are wide, and estimates too imprecise to judge with certainty their applicability in a real clinical setting. | |
| Sensitivity analysis for TSBA cut‐off=10 μmol/L: exclusion of case‐control studies | |||||
| Sensitivity 0.57 (0.48 to 0.66) Specificity 0.92 (0.52 to 0.99) | 436 (3) | 30% (300 out of 1000 pregnant women with pruritus having ICP) | 129 (102 to 156) women with ICP would be missed, and 56 (7 to 336) without ICP would be falsely diagnosed | The overall accuracy of TSBA, especially sensitivity, seems to be lower when excluding case‐control studies. The accuracy of the index test in a real clinical context may be similar to this. However CIs are wide, and estimates too imprecise to judge with certainty their applicability in a real clinical setting. | |
| CAUTION: The results on this table should not be interpreted in isolation from the results of the individual included studies contributing to each summary test accuracy measure. These are reported in the main body of the text of the review. | |||||
| Author | Year | Country | # | Study design | ICP definition | Index test(s) | Cut‐off (μmol/l)a | Laboratory technique | Timing |
| Almuna R | 1986 | Chile | 241 | case‐control | Only clinical/with FU | GCA | 0,7 | Immunoenzymatic assay | Onset |
| Almuna R | 1987 | Chile | 22 | case‐control | Clinical + lab/with FU | GCA | 0,7 | Immunoenzymatic assay | Delivery |
| Brites D * | 1998 | Portugal | 77 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic fluorimetric | Onset |
| CA§ | not given | HPLC | Onset | ||||||
| CDCA§ | not given | HPLC | Onset | ||||||
| GCA | not given | HPLC | Onset | ||||||
| CA/CDCA§ | not given | ||||||||
| Brites D * | 1998 | Portugal | 14 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic fluorimetric | Delivery |
| CA§ | not given | HPLC | Onset | ||||||
| CDCA§ | not given | HPLC | Onset | ||||||
| GCA | not given | HPLC | Onset | ||||||
| CA/CDCA§ | not given | ||||||||
| Gonzalez MC | 1989 | Chile | 62 | Cross‐sectional | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic assay | Onset |
| Guducu N* | 2013 | Turkey | 33 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic assay | Peak |
| Huang W* | 2009 | USA | 193 | Cross‐sectional | Clinical + lab/with FU | TSBA | 10 | LC‐MS | Onset |
| CA | not given | LC‐MS | Onset | ||||||
| CDCA | not given | LC‐MS | Onset | ||||||
| CA/CDCA | 3,4 | ||||||||
| Jiang Y | 2012 | China | 700 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 11 | Enzymatic colorimetric | Onset |
| Kowalska‐Kanka A* | 2013 | Poland | 73 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 11 | Enzymatic colorimetric | Peak |
| Laatikanen T | 1984 | Finland | 177 | case‐control | Clinical + lab/with FU | TSBA | 10 | Enzymatic assay | Delivery |
| Lang T* | 2012 | UK | 66 | Cross‐sectional | Clinical + lab/with FU | TSBA | 14 | Enzymatic colorimetric | Onset |
| Lunzer M | 1986 | Australia | 297 | case‐control | Clinical + lab/with FU | GCA | 1,5 | Radioimmuno assay | Onset |
| Roger D* | 1994 | France | 34 | case‐control | Only clinical/with FU | TSBA | 6 | Enzymatic assay | Onset |
| GCA | 0,7 | Immunoenzymatic assay | Onset | ||||||
| Sjovall K* | 1966 | Sweden | 28 | case‐control | Only clinical/with FU | TSBA | not given | Gas‐liquid chromatography | Onset |
| CA | not given | Gas‐liquid chromatography | Onset | ||||||
| CDCA | not given | Gas‐liquid chromatography | Onset | ||||||
| CA/CDCA§ | not given | ||||||||
| Sun Y | 2011 | China | 105 | case‐control | Clinical + lab/with FU | TSBA | 20 | Enzymatic colorimetric | Onset |
| Tripodi V*b | 2006‐2015 | Argentina | 83 | case‐control | Clinical + lab + TSBA/with FU | TSBA | 10 | Enzymatic assay | Onset |
| ICP ‐ Intrahepatic cholestasis of pregnancy *Studies with individual participant data (provided in the publications or received from authors by email) | |||||||||
| Test | No. of studies | No. of participants |
| 1 TSBA (all studies) Show forest plot | 13 | 1645 |
| 2 TSBA cut‐off=10 μmol/L Show forest plot | 11 | 839 |
| 4 CA cut‐off=2 μmol/L Show forest plot | 4 | 312 |
| 5 CA cut‐off=3 μmol/L Show forest plot | 4 | 312 |
| 6 CA cut‐off=4 μmol/L Show forest plot | 4 | 312 |
| 7 CA cut‐off=5 μmol/L Show forest plot | 4 | 312 |
| 8 CDCA cut‐off=2 μmol/L Show forest plot | 4 | 312 |
| 9 CDCA cut‐off=3 μmol/L Show forest plot | 4 | 312 |
| 10 GCA (all studies) Show forest plot | 6 | 630 |
| 11 GCA cut‐off=0.7 μmol/L Show forest plot | 5 | 333 |
| 12 GCA cut‐off=1.5 μmol/L Show forest plot | 4 | 417 |
| 13 GCA cut‐off=2 μmol/L Show forest plot | 3 | 120 |
| 14 CA/CDCA cut‐off=1.8 Show forest plot | 4 | 312 |
| 15 TSBA cut‐off=10 μmol/L sensitivity excl TSBA in reference standard Show forest plot | 5 | 497 |
| 16 TSBA cut‐off=10 μmol/L sensitivity excl case‐control Show forest plot | 3 | 436 |


