Paracetamol (acetaminofeno) para el dolor crónico no relacionado con el cáncer en niños y adolescentes
Resumen
Antecedentes
El dolor es una característica común en la niñez y la adolescencia en todo el mundo, y en muchos jóvenes, el dolor es crónico. En las guías de la Organización Mundial de la Salud sobre los tratamientos farmacológicos del dolor persistente en niños se reconoce que el dolor en este grupo etario es un problema de gran interés y significación para la salud pública en la mayoría de los países del mundo. Anteriormente, el dolor se desestimaba en la mayoría de los casos y con frecuencia no se trataba, los criterios sobre el dolor en niños han cambiado con el transcurso del tiempo y en la actualidad su alivio se considera importante.
Se diseñó una serie de siete revisiones sobre dolor crónico no relacionado con el cáncer y dolor por cáncer (en la que se estudiaron antidepresivos, antiepilépticos, antiinflamatorios no esteroideos, opiáceos y paracetamol, como temas prioritarios) para examinar la evidencia sobre el dolor en niños mediante el uso de intervenciones farmacológicas en niños y adolescentes.
Como se trata de la principal causa de morbilidad en niños y adolescentes en el mundo actual, las enfermedades crónicas (y el dolor asociado) es un problema de salud significativo. El dolor crónico (definido como el dolor que dura tres meses o más) se puede presentar en la población pediátrica en diversas clasificaciones fisiopatológicas: nociceptivo, neuropático, idiopático, visceral, dolor por daño en los nervios, dolor musculoesquelético crónico, dolor abdominal crónico y otras causas desconocidas.
El paracetamol (acetaminofeno) es uno de los analgésicos utilizados más ampliamente en adultos y niños. La dosis recomendada en el Reino Unido, Europa, Australia y EE.UU. para los niños y adolescentes es, en general, 10 a 15 mg/kg cada cuatro a seis horas, con intervalos específicos para la edad desde 60 mg (seis a 12 meses) hasta 500 a 1000 mg (más de 12 años de edad). El paracetamol es el único analgésico recomendado para los niños menores de tres meses de vida. El paracetamol ha resultado seguro en dosis apropiadas y controladas; sin embargo, la sobredosis o el abuso del fármaco puede provocar efectos adversos en los niños que incluyen la insuficiencia hepática y renal.
Objetivos
Evaluar la eficacia analgésica y los eventos adversos del paracetamol (acetaminofeno) administrado para tratar el dolor no relacionado con el cáncer en niños y adolescentes, desde el nacimiento hasta los 17 años de edad, en cualquier ámbito.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials; CENTRAL) vía Cochrane Register of Studies Online, MEDLINE vía Ovid y Embase vía Ovid desde su inicio hasta el 6 de septiembre de 2016. También se realizaron búsquedas en las listas de referencias de estudios y revisiones recuperados, y se realizaron búsquedas en registros de ensayos clínicos en línea.
Criterios de selección
Ensayos controlados aleatorizados, con o sin cegamiento, de tratamiento, en cualquier dosis y por cualquier vía de administración, del dolor crónico no relacionado con el cáncer en niños y adolescentes, que comparasen paracetamol con placebo o un comparador activo.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, evaluaron la elegibilidad de los estudios. Se planificó utilizar datos dicotómicos para calcular el cociente de riesgos y el número necesario a tratar, con el uso de métodos estándar, siempre que estuviesen disponibles. Se planificó realizar la evaluación GRADE (Grading of Recommendations Assessment, Development and Evaluation) y crear una tabla de “Resumen de los hallazgos”.
Resultados principales
No hubo estudios que cumplieran los criterios de inclusión para esta revisión.
No existe evidencia para apoyar o refutar el uso del paracetamol en niños con dolor crónico no relacionado con el cáncer.
Conclusiones de los autores
No existe evidencia de ensayos controlados aleatorizados para apoyar o refutar el uso del paracetamol (acetaminofeno) para tratar el dolor crónico no relacionado con el cáncer en niños y adolescentes. No se pueden establecer conclusiones acerca de la eficacia ni los efectos perjudiciales de la administración del paracetamol para tratar el dolor crónico no relacionado con el cáncer en niños y adolescentes.
A partir de ensayos controlados aleatorizados en adultos se sabe que el paracetamol puede ser efectivo, en algunas dosis y en algunas afecciones con dolor (no siempre crónico).
PICO
Resumen en términos sencillos
Paracetamol para el dolor crónico no relacionado con el cáncer en niños y adolescentes
Conclusión
No existe evidencia de ensayos controlados aleatorizados que apoye ni refute la indicación de que el paracetamol (acetaminofeno) en cualquier dosis brinda alivio del dolor para el dolor crónico no relacionado con el cáncer en niños o adolescentes.
Antecedentes
Los niños pueden presentar dolor crónico o recurrente relacionado con trastornos genéticos, daño nervioso, dolor musculoesquelético crónico, dolor abdominal u otras razones desconocidas. El dolor crónico se define como dolor que dura tres meses o más y se acompaña frecuentemente de cambios en la funcionalidad y el estilo de vida, así como por signos y síntomas de depresión y ansiedad.
El paracetamol (acetaminofeno) es uno de los analgésicos más ampliamente utilizados en adultos y niños. La dosis recomendada en el Reino Unido, Europa, Australia y EE.UU. para los niños y adolescentes es, en general, 10 a 15 mg/kg cada cuatro a seis horas, con intervalos específicos para la edad desde 60 mg (seis a 12 meses) hasta 500 a 1000 mg (más de 12 años de edad). El paracetamol es el único analgésico recomendado para los niños menores de tres meses de vida. El paracetamol ha resultado seguro en dosis apropiadas y controladas; sin embargo, la sobredosis o el abuso del fármaco puede provocar efectos adversos en los niños entre los que se encuentran la insuficiencia hepática y renal.
Resultados clave
En septiembre de 2016 se buscaron los ensayos clínicos que administraran paracetamol para tratar el dolor crónico (posiblemente debido a dolor nervioso, trastornos musculoesqueléticos, cólicos menstruales o molestias abdominales).
No se encontraron estudios que cumplieran los criterios para esta revisión. Varios estudios han evaluado el paracetamol en adultos con dolor crónico, pero ninguno incluyó participantes desde el nacimiento hasta los 17 años de edad.
Calidad de la evidencia
Se planificó calificar la calidad de la evidencia de los estudios mediante cuatro niveles: muybajo, bajo, moderado o alto. La evidencia de calidad muybaja significa que hay muy poca seguridad en los resultados. La evidencia de calidad altasignifica que se tiene mucha seguridad en los resultados.
No fue posible calificar la calidad del grupo de evidencia, ya que no hubo evidencia para apoyar o refutar el uso del paracetamol para el dolor crónico no relacionado con el cáncer en niños o adolescentes.
Authors' conclusions
Background
Pain is a common feature of childhood and adolescence around the world, and for many young people, that pain is chronic. The World Health Organization guidelines for pharmacological treatments for persisting pain in children acknowledge that pain in children is a major public health concern of high significance in most parts of the world (WHO 2012). While in the past, pain was largely dismissed and was frequently left untreated, views on children's pain have changed over time, and relief of pain is now seen as important. Since the 1970s, studies comparing child and adult pain management have revealed a variety of responses to pain, fuelling the need for a more in‐depth focus on paediatric pain (Caes 2016).
Infants (zero to 12 months), children (1 to 9 years), and adolescents (10 to 18 years) (WHO 2012), account for 27% (1.9 billion) of the world's population (United Nations 2015); the proportion of those aged 14 years and under ranges from 12% (in Hong Kong) to 50% (in Niger) (World Bank 2014). However, little is known about the pain management needs of this population. For example, in the Cochrane Library, approximately 12 reviews produced by the Cochrane Pain, Palliative and Supportive Care Review Group in the past 18 years have been specifically concerned with children and adolescents, compared to over 100 reviews specific to adults. Additional motivating factors for investigating children's pain include the vast amount of unmanaged pain in the paediatric population and the development of new technologies and treatments. We convened an international group of leaders in paediatric pain to design a suite of seven reviews in chronic pain and cancer pain (looking at antidepressants, antiepileptic drugs, non‐steroidal anti‐inflammatory drugs, opioids, and paracetamol as priority areas) in order to review the evidence under a programme grant for children's pain utilising pharmacological interventions in children and adolescents (Appendix 1).
This review is based on a template for reviews of pharmacotherapies used to relieve pain in infants, children, and adolescents. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence (Appendix 2) (Moore 2010a; Moore 2012). This review focused on paracetamol (acetaminophen) to treat chronic non‐cancer pain.
Description of the condition
This review focused on chronic non‐cancer pain experienced by children and adolescents as a result of any type of chronic disease that occurs throughout the global paediatric population. Children's level of pain can be mild, moderate, or severe, and pain management is an essential element of patient management during all care stages of chronic disease.
As the leading cause of morbidity in children and adolescents in the world today, chronic disease (and its associated pain) is a major health concern. Chronic pain can arise in the paediatric population in a variety of pathophysiological classifications: nociceptive, neuropathic, idiopathic, or visceral. Chronic pain is pain that lasts three months or longer and is commonly accompanied by changes in lifestyle, functional abilities, as well as by signs and symptoms of depression and anxiety (Ripamonti 2008).
Whilst diagnostic and perioperative procedures performed to treat chronic diseases are a known common cause of pain in these patients, this review did not cover perioperative pain or adverse effects of treatments such as mucositis.
Description of the intervention
Paracetamol (also known as acetaminophen) is one of the most widely used analgesics around the world in both the adult and paediatric populations. First marketed in the USA and UK in the 1950s, paracetamol is now recommended in all healthcare setting guidelines as the first‐line analgesic for both adults and children experiencing mild to moderate pain (NICE 2016). Paracetamol is currently available in most countries in healthcare settings and can be accessed without prescription (WHO 2012).
The recommended dosage for paediatric patients under 18 years old is generally 10 to 15 mg/kg (BNF 2016; FDA 2017; TGA 2017). For adolescents (12 years and older) recommended doses are 500 to 1000 mg oral tablet or liquid formula (via rectum if necessary), at a frequency of every four to six hours, with a maximum of 4 g over 24 hours. For children under 12 years, oral and rectal doses are recommended as follows at the same frequency: 500 mg (10 to 12 years), 375 mg (8 to 10 years), 250 mg (6 to 8 years), 240 mg (4 to 6 years), 180 mg (2 to 4 years), 120 mg (6 to 24 months), and 60 mg (3 to 6 months). Paracetamol is the only recommended analgesic for children under 3 months of age (WHO 2012).
Paracetamol has been proven to be safe in appropriate and controlled dosages (Forrest 1982). However, adverse effects of paracetamol in the paediatric population can include hepatic or renal failure with overuse or overdose (Zyoud 2015). Other less common side effects include: malaise; toxic epidermal necrolysis skin reactions (including Stevens‐Johnson syndrome), acute generalised exanthematous pustulosis; blood disorders including neutropenia, leucopenia, thrombocytopenia; and upon infusion, hypotension, flushing, and tachycardia (BNF 2016; Forrest 1982 ).
How the intervention might work
Although paracetamol has been widely used in medical practice, its mechanism of action remains uncertain (Graham 2013). The main proposed mechanism is the inhibition of cyclooxygenase (COX) enzymes through metabolism by the peroxidase function of these isoenzymes. This process results in inhibition of phenoxyl radical formation from a critical tyrosine residue important for the cyclooxygenase activity of COX‐1 and COX‐2 and prostaglandin synthesis (Graham 2013; Jozwiak‐Bebenista 2014). Paracetamol is a preferential inhibitor of COX‐2 due to its gastrointestinal tolerance and poor inhibition of platelet activity (Graham 2013; Hinz 2008; Hinz 2012).
Paracetamol is widely considered to be a safe drug when administered in appropriate doses (Jozwiak‐Bebenista 2014); however, there is clear evidence that higher doses or prolonged use of paracetamol can lead to liver failure (where the paracetamol compounds are metabolised), cardiovascular events, and even death (Chan 2006; Daly 2008; Forman 2005; Graham 2013; Roberts 2016; Sheen 2002). Overall, paracetamol is considered to be a safe and effective analgesic option that is tolerable in the majority of paediatric patients. Using the recommended doses, severe side effects can be avoided and adequate relief of chronic pain can be achieved for the infant, child, or adolescent.
Why it is important to do this review
The paediatric population is at risk of inadequate management of pain (AMA 2013). Some conditions that would be aggressively treated in adult patients are being managed with insufficient analgesia in younger populations (AMA 2013). Although there have been repeated calls for best evidence to treat children's pain, such as Eccleston 2003, there are no easily available summaries of the most effective paediatric pain relief.
This review formed part of a Programme Grant addressing the unmet needs of people with chronic pain, commissioned by the National Institute for Health Research (NIHR) in the UK. This topic was identified in June 2015 during consultation with experts in paediatric pain. Please see Appendix 1 for full details of the meeting. The standards used to assess evidence in chronic pain trials have changed substantially in recent years, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change was to encourage a move from using average pain scores, or average change in pain scores, to the number of children who have a large decrease in pain (by at least 50%). Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and quality of life (Moore 2011a). These standards are set out in the reference guide for pain studies (AUREF 2012).
Objectives
To assess the analgesic efficacy and adverse events of paracetamol (acetaminophen) used to treat chronic non‐cancer pain in children and adolescents aged between birth and 17 years, in any setting.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials, with or without blinding, and participant‐ or observer‐reported outcomes.
Full journal publication was required, with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis. We planned to include studies published in any language. We excluded abstracts (usually meeting reports) or unpublished data, non‐randomised studies, studies of experimental pain, case reports, and clinical observations.
Types of participants
We planned to include studies of infants, children, and adolescents, aged from birth to 17 years old, with chronic or recurrent pain (lasting for three months or longer), arising from genetic conditions, neuropathy, or other conditions. These included but were not limited to chronic musculoskeletal pain and chronic abdominal pain.
We excluded studies of perioperative pain, acute pain, cancer pain, headache, migraine, and pain associated with primary disease or its treatment.
We planned to include studies of participants with more than one type of chronic pain, in which case we would analyse results according to the primary condition.
Types of interventions
We planned to include studies reporting interventions prescribing paracetamol for the relief of chronic pain by any route, in any dose, with comparison to a placebo or any active comparator. We did not consider interventions prescribing paracetamol in combination with another drug (such as opioids), as this comparison is covered in the two opioid reviews as part of this suite (Cooper 2017a; Wiffen 2017a).
Types of outcome measures
In order to be eligible for inclusion in this review, studies had to report pain assessment, as well as meeting the other selection criteria.
We planned to include trials measuring pain intensity and pain relief assessed using validated tools such as numerical rating scale (NRS), visual analogue scale (VAS), Faces Pain Scale ‐ Revised (FPS‐R), Colour Analogue Scale (CAS), or any other validated rating scale.
We were particularly interested in Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT) definitions for moderate and substantial benefit in chronic pain studies (PedIMMPACT 2008). These are defined as: at least 30% pain relief over baseline (moderate); at least 50% pain relief over baseline (substantial); much or very much improved on Patient Global Impression of Change scale (PGIC) (moderate); very much improved on PGIC (substantial).
These outcomes differ from those used in most earlier reviews, concentrating as they do on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. People with chronic pain desire high levels of pain relief, ideally more than 50% pain intensity reduction, and ideally having no worse than mild pain (Moore 2013a; O'Brien 2010).
We planned to record any reported adverse events. We planned to report the timing of outcome assessments.
Primary outcomes
-
Participant‐reported pain relief of 30% or greater
-
Participant‐reported pain relief of 50% or greater
-
PGIC much or very much improved
In the absence of self reported pain, we planned to consider the use of 'other‐reported' pain, typically by an observer such as a parent, carer, or healthcare professional (Stinson 2006; von Baeyer 2007).
Secondary outcomes
We identified the following with reference to the PedIMMPACT recommendations, which suggest core outcome domains and measures for consideration in paediatric acute and chronic/recurrent pain clinical trials (PedIMMPACT 2008).
-
Carer Global Impression of Change
-
Requirement for rescue analgesia
-
Sleep duration and quality
-
Acceptability of treatment
-
Physical functioning as defined by validated scales
-
Quality of life as defined by validated scales
-
Any adverse events
-
Withdrawals due to adverse events
-
Any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
Search methods for identification of studies
We developed the search strategy based on previous strategies used by the Cochrane Pain, Palliative and Supportive Care Review Group and carried out the searches.
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Cochrane Register of Studies Online), searched 6 September 2016;
-
MEDLINE (via Ovid) (1946 to September week 2 2016);
-
Embase (via Ovid) (1974 to 2016 week 38).
We used medical subject headings (MeSH) or equivalent and text word terms. We restricted our search to randomised controlled trials and clinical trials. There were no language or date restrictions. The focus of the key words in our search terms was on chronic pain and paracetamol. We tailored searches to individual databases. The search strategy for MEDLINE is in Appendix 3, Embase in Appendix 4, and CENTRAL in Appendix 5.
Searching other resources
We searched ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) for ongoing trials up to June 2017. In addition, we checked reference lists of reviews and retrieved articles for additional studies, and performed citation searches on key articles. We planned to contact experts in the field for unpublished and ongoing trials, however this was not necessary. We planned to contact study authors where necessary for additional information.
Data collection and analysis
We planned to perform separate analyses according to particular chronic pain conditions. We planned to combine different chronic pain conditions in analyses for exploratory purposes only.
Selection of studies
Two review authors independently determined study eligibility by reading the abstract of each study identified by the search. Review authors independently eliminated studies that clearly did not satisfy inclusion criteria, and obtained full copies of the remaining studies. Two review authors independently read these studies to select those that met the inclusion criteria, a third review author adjudicating in the event of disagreement. We did not anonymise the studies in any way before assessment. We included a PRISMA flow chart to illustrate the results of the search and the process of screening and selecting studies for inclusion in the review (Moher 2009), as recommended in section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to include studies in the review irrespective of whether measured outcome data were reported in a ‘usable’ way.
Data extraction and management
We planned to obtain full copies of the studies with two review authors independently carrying out data extraction. Where available, we would have extracted information about the pain condition, number of participants treated, drug and dosing regimen, study design (placebo or active control), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event or serious adverse event). We planned to collate multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We planned to collect characteristics of the included studies in sufficient detail to populate a Characteristics of included studies table.
We planned to use a template data extraction form and checked for agreement before entry into Cochrane's statistical software Review Manager 5 (RevMan 2014).
If a study had more than two intervention arms, we planned to only include the intervention groups and control groups that met the eligibility criteria. If multi‐arm studies were included, we planned to analyse multiple intervention groups in an appropriate way that avoided arbitrary omission of relevant groups and double‐counting of participants.
Assessment of risk of bias in included studies
We planned for two review authors to independently assess risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We planned to complete a 'Risk of bias' table for each included study using the Cochrane 'Risk of bias' tool in Review Manager 5 (RevMan 2014).
We planned to assess the following for each study. We would have resolved any disagreements by discussion between review authors or when necessary by consulting a third review author.
-
Random sequence generation (checking for possible selection bias). We planned to assess the method used to generate the allocation sequence as: low risk of bias (i.e. any truly random process, e.g. random number table; computer random number generator); or unclear risk of bias (when the method used to generate the sequence was not clearly stated). We excluded studies that used a non‐random process and were therefore at high risk of bias (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We planned to assess the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); or unclear risk of bias (when the method was not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias). We planned to assess any methods used to blind the participants and personnel from knowledge of which intervention a participant received. We planned to assess the methods as: low risk of bias (study states that the participants and personnel involved were blinded to treatment groups); unclear risk of bias (study does not state whether or not participants and personnel were blinded to treatment groups); or high risk of bias (participants or personnel were not blinded) (as stated in Types of studies, we included trials with or without blinding, and participant‐ or observer‐reported outcomes).
-
Blinding of outcome assessment (checking for possible detection bias). We planned to assess any methods used to blind the outcome assessors from knowledge of which intervention a participant received. We planned to assess the methods as: low risk of bias (e.g. study states that it was single‐blinded and describes the method used to achieve blinding of the outcome assessor); unclear risk of bias (study states that outcome assessors were blinded but does not provide an adequate description of how this was achieved); or high risk of bias (outcome assessors were not blinded) (as stated in Types of studies, we included trials with or without blinding, and participant‐ or observer‐reported outcomes).
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We planned to assess the methods used to deal with incomplete data as: low risk of bias (i.e. less than 10% of participants did not complete the study or 'baseline observation carried forward' (BOCF) analysis was used, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); or high risk of bias (used 'completer' analysis).
-
Selective reporting (checking for possible reporting bias). We planned to assess the methods used to report the outcomes of the study as: low risk of bias (if all planned outcomes in the protocol or methods were reported in the results); unclear risk of bias (if there was not a clear distinction between planned outcomes and reported outcomes); high risk of bias (if some planned outcomes from the protocol or methods were clearly not reported in the results).
-
Size of study (checking for possible biases confounded by small size). We planned to assess studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
-
Other bias. We planned to assess studies for any additional sources of bias as low, unclear, or high risk of bias, and provide rationale.
Measures of treatment effect
Where dichotomous data were available, we planned to calculate a risk ratio (RR) with 95% confidence interval (CI) and meta‐analyse the data as appropriate. We planned to calculate number needed to treat for an additional beneficial outcome (NNTB) where appropriate (McQuay 1998); for unwanted effects the NNTB becomes the number needed to treat for an additional harmful outcome (NNTH) and is calculated in the same manner. Where continuous data were reported, we planned to use appropriate methods to combine these data in the meta‐analysis.
Unit of analysis issues
We planned to accept randomisation to the individual participant only. We planned to split the control treatment arm between active treatment arms in a single study if the active treatment arms were not combined for analysis. We only accepted studies with minimum 10 participants per treatment arm.
Dealing with missing data
We planned to use intention‐to‐treat analysis where the intention‐to‐treat population consisted of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one postbaseline assessment. We would have assigned missing participants zero improvement wherever possible.
Assessment of heterogeneity
We planned to identify and measure heterogeneity as recommended in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to deal with clinical heterogeneity by combining studies that examined similar conditions. We planned to undertake and present a meta‐analysis only if we judged participants, interventions, comparisons, and outcomes to be sufficiently similar to ensure a clinically meaningful answer. We planned to assess statistical heterogeneity visually and by using the I² statistic (L'Abbé 1987). When I² was greater than 50%, we planned to consider the possible reasons.
Assessment of reporting biases
We planned to assess the risk of reporting bias, as recommended in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The aim of this review was to use dichotomous outcomes of known utility and of value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what the authors of the original studies chose to report or not, though clearly difficulties arose in studies failing to report any dichotomous results. We planned to extract and report continuous data in a narrative way, which probably reflect efficacy and utility poorly, and is useful for illustrative purposes only.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a number needed to treat (NNT) of 10 or higher) (Moore 2008).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We planned to use a random‐effects model for meta‐analysis if there was significant clinical heterogeneity and combining studies was considered to be appropriate. We planned to conduct our analysis using the primary outcomes of pain and adverse events, and to calculate the NNTHs for adverse events. We planned to use the Cochrane software program Review Manager 5 (RevMan 2014).
Quality of the evidence
To analyse data, two review authors would have independently rated the quality of each outcome. We would have used the GRADE approach to assess the quality of the body of evidence related to each of the key outcomes, and planned to report our judgement in a 'Summary of findings' table per Chapter 12 of the Cochrane Handbook (Appendix 6) (Higgins 2011).
In addition, there may be circumstances where the overall rating for a particular outcome would need to be adjusted per GRADE guidelines (Guyatt 2013a). For example, if there were so few data that the results were highly susceptible to the random play of chance, or if studies used LOCF imputation in circumstances where there were substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where no data were reported for an outcome, we planned to report the level of evidence as 'no evidence to support or refute' (Guyatt 2013b).
'Summary of findings' table
We planned to include a 'Summary of findings' table as set out in the Cochrane Pain, Palliative and Supportive Care Review Group’s author guide (AUREF 2012), and recommended in section 4.6.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to justify and document all assessments of the quality of the body of evidence.
In an attempt to reliably interpret the findings of this systematic review, we planned to assess the summarised data using the GRADE guidelines (Appendix 6) to rate the quality of the body of evidence of each of the key outcomes listed in Types of outcome measures per Chapter 12 of the Cochrane Handbook, as appropriate (Guyatt 2011; Higgins 2011). Utilising the explicit criteria against study design, risk of bias, imprecision, inconsistency, indirectness, and magnitude of effect, we planned to summarise the evidence in an informative, transparent, and succinct 'Summary of findings' table or 'Evidence profile' table (Guyatt 2011).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses where a minimum number of data were available (at least 200 participants per treatment arm). We planned to analyse according to age group; type of drug; geographical location or country; type of control group; baseline measures; frequency, dose, and duration of drugs; and nature of drug.
We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of confidence intervals and by performing the test for subgroup differences available in Review Manager 5.
Sensitivity analysis
We did not plan to carry out any sensitivity analysis because the evidence base is known to be too small to allow reliable analysis; we did not plan to pool results from chronic pain of different origins in the primary analyses. We planned to examine details of dose escalation schedules in the unlikely circumstance that this could provide some basis for a sensitivity analysis.
Results
Description of studies
Results of the search
A PRISMA flow diagram of the search results is shown in Figure 1.
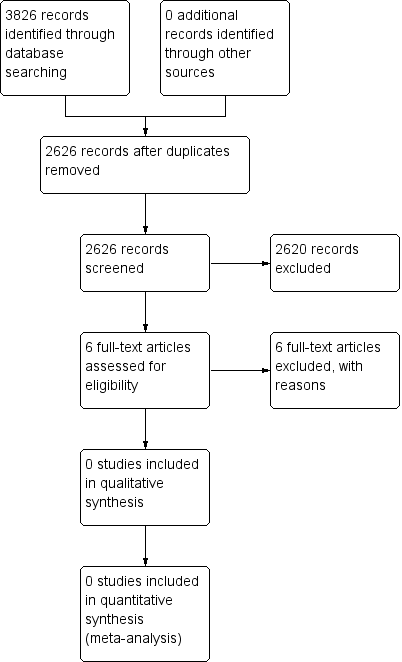
Study flow diagram.
Searches of the three main databases revealed 3826 records of titles and abstracts, of which 1200 duplicates were removed. Our searches of ClinicalTrials.gov and the WHO ICTRP yielded no additional eligible studies.
We screened the remaining 2626 titles and abstracts for eligibility, finding 2620 to be ineligible.
We read the full texts of the remaining six studies, of which all six were found to be ineligible and excluded. We identified no ongoing studies. No studies fulfilled the inclusion criteria or were eligible to be entered into a quantitative analysis.
Included studies
No studies met our inclusion criteria for this review.
Excluded studies
See Characteristics of excluded studies.
We excluded six studies in this review. Five investigated adult populations, and one study was not randomised. (McGuinness 1969).
Risk of bias in included studies
No studies were eligible for inclusion in this review, therefore we did not perform a 'Risk of bias' assessment.
Effects of interventions
No studies were eligible for inclusion in this review, therefore we could not assess the efficacy of paracetamol to treat chronic non‐cancer pain in children and adolescents. We were also unable to examine any adverse effects. Due to the lack of evidence in this field, we were unable to judge the quality of evidence and therefore there is no evidence to support or refute the use of paracetamol for children with chronic non‐cancer pain.
Discussion
Summary of main results
We were unable to find any randomised controlled trials for inclusion in this review, therefore we were unable to comment about efficacy or harm from the use of paracetamol to treat chronic non‐cancer pain in children and adolescents. Similarly, we could not comment on our remaining secondary outcomes: Carer Global Impression of Change; requirement for rescue analgesia; sleep duration and quality; acceptability of treatment; physical functioning; and quality of life. Due to the lack of evidence in this field, we are unable to judge the quality of evidence and therefore there is no evidence to support or refute the use of paracetamol for children with chronic non‐cancer pain.
Overall completeness and applicability of evidence
In adults, the efficacy of paracetamol in chronic pain conditions is being challenged. Paracetamol alone is no better than placebo for low back pain (Saragiotto 2016), spinal pain, or osteoarthritis (Machado 2015), and there is very little evidence of efficacy in neuropathic pain, despite its widespread use (Wiffen 2016). The efficacy of paracetamol in acute pain is established in acute postoperative pain, Moore 2015, and migraine (Derry 2013). Paracetamol is generally less effective than non‐steroidal anti‐inflammatory drugs (Marjoribanks 2015; Moore 2015). Widespread use of paracetamol combined with new evidence about harm has challenged the common assumption of safety (Moore 2016; Roberts 2016).
In children, there is little evidence concerning the pain‐relieving effects of paracetamol in neonates (Ohlsson 2016), for acute otitis media (Sjoukes 2016), or for relief of fever (Wong 2013).
The suite of reviews
This review is part of a suite of reviews on pharmacological interventions for chronic pain and cancer‐related pain in children and adolescents (Appendix 1). Taking a broader view on this suite of reviews, some pharmacotherapies (investigated in our other reviews) are likely to provide more data than others. The results of this review were thus as expected considering that randomised controlled trials in children are known to be limited. The results have the potential to inform policymaking decisions for funding future clinical trials into paracetamol treatment of child and adolescent pain, therefore any results (large or small) are important in order to capture a snapshot of the current evidence for paracetamol.
Quality of the evidence
No studies were eligible for inclusion in this review. We were unable to find any published randomised controlled trials to support or refute the use of paracetamol to treat chronic non‐cancer pain in children and adolescents. We were unable to examine any adverse effects.
This review shows that there is an absence of evidence from trials that paracetamol is effective in chronic non‐cancer pain in children. While it may be the case that this absence of evidence reflects the inadequacy of paracetamol for this purpose and that its use as a monotherapy analgesic is more likely to cause harm than benefit, the opposite may also pertain, as the data are lacking. It is difficult to conduct long‐term randomised controlled trials in children with chronic non‐cancer conditions, and few observational/clinical data have been published.
Potential biases in the review process
We carried out extensive searches of major databases using broad search criteria, and also searched two large clinical trial registries. We consider it to be unlikely that we have missed relevant studies.
Agreements and disagreements with other studies or reviews
We were not able to identify any published systematic reviews on this topic.

Study flow diagram.

