Condicionamiento isquémico remoto para la prevención y el tratamiento del accidente cerebrovascular isquémico
Resumen
Antecedentes
El condicionamiento isquémico remoto (CIR) se ha desarrollado como una estrategia neuroprotectora para la prevención y el tratamiento del accidente cerebrovascular isquémico. Habitualmente incluye limitar el flujo sanguíneo a los miembros y luego liberar la sangre isquémica para promover un efecto neuroprotector. Los estudios preclínicos han indicado que el CIR puede tener efectos beneficiosos en los pacientes con accidente cerebrovascular isquémico y en los que tienen riesgo de accidente cerebrovascular isquémico. Sin embargo, la evidencia existente no es suficiente para demostrar la eficacia y la seguridad del CIR para la prevención y el tratamiento del accidente cerebrovascular isquémico.
Objetivos
Evaluar los efectos beneficiosos y perjudiciales del CIR para prevenir el accidente cerebrovascular isquémico, y para tratar a los pacientes con accidente cerebrovascular isquémico y a los que tienen riesgo de accidente cerebrovascular isquémico.
Métodos de búsqueda
Se realizaron búsquedas en el Registro de Ensayos del Grupo Cochrane de Accidentes Cerebrales Vasculares (Cochrane Stroke Group) (16 de enero de 2018), en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2017, número 12) en la Cochrane Library (enero de 2018), MEDLINE Ovid (1946 a enero de 2018), Embase Ovid (1974 a enero de 2018), Web of Science Core Collection (1950 a enero de 2018) y en tres bases de datos chinas (enero de 2018). También se buscó en cuatro registros de ensayos en curso, en listas de referencias y en actas de congresos.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) que compararon el CIR con CIR simulado o tratamiento médico en pacientes con accidente cerebrovascular isquémico o con riesgo de accidente cerebrovascular isquémico.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los estudios, evaluaron la calidad de los ensayos y el riesgo de sesgo, y extrajeron los datos. Se utilizó el enfoque GRADE para evaluar la calidad de la evidencia.
Resultados principales
En esta revisión se incluyeron siete ensayos con 735 participantes. Se analizaron los efectos del CIR sobre la prevención y el tratamiento del accidente cerebrovascular isquémico, respectivamente.
Se evaluó el riesgo de sesgo y se consideró que era bajo para la generación de la secuencia de asignación en seis estudios e incierto en un estudio; incierto para el ocultamiento de la asignación en cuatro estudios y bajo en tres estudios; alto para los datos incompletos de resultado (sesgo de deserción) en cinco estudios y bajo en dos estudios; alto para el cegamiento en tres estudios y bajo en cuatro estudios; bajo para el informe selectivo; y alto para otras fuentes de sesgo en seis estudios y bajo en un estudio.
En el análisis de los efectos del CIR sobre la prevención del accidente cerebrovascular isquémico se incluyeron tres ensayos (con 371 participantes). En los pacientes con estenosis sintomática de la arteria intracerebral, el accidente cerebrovascular recurrente se redujo significativamente mediante el CRI (riesgo relativo [RR] 0,32; intervalo de confianza [IC] del 95%: 0,12 a 0,83; dos ensayos, 182 participantes, evidencia de calidad baja). En los pacientes con estenosis carotídea a los que se les colocaron stents carotídeos, no hubo diferencias significativas en la incidencia de accidentes cerebrovasculares isquémicos entre los participantes tratados con CIR y los no tratados con CIR (RR 0,22; IC del 95%: 0,01 a 4,03; un ensayo, 189 participantes, evidencia de calidad baja); sin embargo, la gravedad del accidente cerebrovascular (evaluada por el volumen del infarto) fue significativamente inferior en los participantes tratados con CIR (diferencia de medias [DM] ‐0,17 ml; IC del 95%: ‐0,23 a ‐0,11; un ensayo, 189 participantes, evidencia de calidad baja). Los eventos adversos asociados con el CIR fueron significativamente mayores en los participantes tratados con CIR (RR 10,91; IC del 95%: 2,01 a 59,28; tres ensayos, 371 participantes, evidencia de calidad baja), pero ningún evento adverso grave fue atribuible al tratamiento con CIR. Ningún participante murió o presentó eventos cardiovasculares durante el período de los estudios y ningún ensayo informó sobre el accidente cerebrovascular hemorrágico o la mejoría en el deterioro neurológico, ficológico o cognitivo.
En el análisis de los efectos del CIR sobre el tratamiento del accidente cerebrovascular isquémico se incluyeron cuatro ensayos (con 364 participantes). En el accidente cerebrovascular isquémico agudo, en los pacientes que reciben trombólisis intravenosa la tasa de muerte o dependencia aumentó significativamente con el tratamiento con CRI en comparación con el tratamiento sin CRI (RR 2,34; IC del 95%: 1,19 a 4,61; un ensayo, 285 participantes, evidencia de calidad baja). En los pacientes con accidente cerebrovascular isquémico agudo, no hubo diferencias significativas entre el CRI y ningún CRI para reducir la gravedad del accidente cerebrovascular según la puntuación de la National Institutes of Health Stroke Scale y el volumen final del infarto (diferencia de medias estandarizada (DME) ‐0,24 ml; IC del 95%: ‐1,02 a 0,54; dos ensayos, 175 participantes, evidencia de calidad muy baja). No hubo diferencias significativas entre el CRI y ningún CRI para mejorar el deterioro psicológico (DME ‐0,37 puntos; IC del 95%: ‐1,15 a 0,41; un ensayo, 26 participantes, evidencia de calidad muy baja) y el deterioro cognitivo (DME ‐0,26 puntos; IC del 95%: ‐0,72 a 0,21; tres ensayos, 79 participantes, evidencia de calidad baja) en los pacientes con accidente cerebrovascular isquémico agudo y enfermedad cerebral de los vasos pequeños. Ningún ensayo informó el accidente cerebrovascular isquémico, el accidente cerebrovascular isquémico recurrente, la mejoría en el deterioro neurológico, el accidente cerebrovascular hemorrágico, los eventos cardiovasculares ni los eventos adversos asociados al CIR.
Conclusiones de los autores
Se encontró evidencia de baja calidad de que el CIR puede reducir el riesgo de accidente cerebrovascular recurrente en los participantes con estenosis de la arteria intracerebral y reducir la gravedad del accidente cerebrovascular en los participantes sometidos a la colocación de un stent carotídeo, pero puede aumentar la muerte o la dependencia en los participantes con accidente cerebrovascular isquémico agudo sometidos a trombólisis intravenosa. Sin embargo, hay considerable incertidumbre acerca de estas conclusiones debido al número pequeño de estudios y a la baja calidad de la evidencia.
PICOs
Resumen en términos sencillos
Condicionamiento isquémico remoto para la prevención y el tratamiento del accidente cerebrovascular causado por reducción del flujo sanguíneo
Pregunta de la revisión
¿Cuáles son los efectos beneficiosos del uso del condicionamiento isquémico remoto (una fisioterapia no invasiva que incluye inflar manguitos de presión arterial para reducir el flujo sanguíneo en los brazos y las piernas y luego liberar la sangre afectada hacia todo el cuerpo) en los pacientes con accidente cerebrovascular o en los que tienen riesgo de accidente cerebrovascular causado por la reducción del flujo sanguíneo?
Antecedentes
El accidente cerebrovascular es la causa principal de discapacidad en los adultos a nivel global y el accidente cerebrovascular isquémico (causado por la reducción del flujo sanguíneo) representa la mayoría de los accidentes cerebrovasculares. Casi un cuarto de los individuos con accidente cerebrovascular isquémico presentará otros eventos (accidente cerebrovascular recurrente). El condicionamiento isquémico remoto (CIR) es una estrategia para proteger y prevenir el daño al tejido cerebral al mejorar su capacidad de tolerar la reducción del flujo sanguíneo. Los estudios han indicado que el CIR puede tener efectos beneficiosos en la prevención y el tratamiento del accidente cerebrovascular isquémico.
Características de los estudios
Esta revisión incluyó siete estudios (específicamente ensayos controlados aleatorizados), en los que participaron 735 pacientes. Los estudios compararon CIR con CIR simulado o tratamiento médico en pacientes con accidente cerebrovascular isquémico o con riesgo de accidente cerebrovascular isquémico. Tres ensayos (que incluyeron 371 pacientes) fueron elegibles para el análisis del CIR para la prevención del accidente cerebrovascular isquémico, y otros cuatro ensayos (que incluyeron 364 pacientes) fueron elegibles para el análisis del CIR para el tratamiento del accidente cerebrovascular isquémico. Los ensayos incluidos se realizaron en China, Dinamarca y el Reino Unido.
Resultados clave
Los resultados de esta revisión están vigentes hasta enero de 2018. En los pacientes con estrechamiento de las arterias del cerebro, el CIR puede reducir el riesgo de accidente cerebrovascular recurrente. En los pacientes que reciben tratamiento de colocación de un stent (inserción de un tubo metálico o plástico) por el estrechamiento de las arterias del cuello, el CIR puede reducir el tamaño de las nuevas lesiones cerebrales causadas por la reducción del flujo sanguíneo. Sin embargo, su efecto sobre los resultados clínicos (accidente cerebrovascular y muerte) no estuvo claro. Los eventos adversos fueron significativamente más frecuentes en el grupo de CIR, pero no se informó que fueran graves.
Entre los pacientes con accidente cerebrovascular isquémico agudo (en los que solo habían pasado varias horas desde el inicio de los síntomas) que recibieron fármacos para disolver los coágulos, se encontró que el CIR puede aumentar el riesgo de muerte o dependencia (necesitar la ayuda de otros). No se encontraron diferencias significativas en el tamaño del accidente cerebrovascular final. En los pacientes con accidente cerebrovascular isquémico agudo y enfermedad crónica de los vasos sanguíneos del cerebro, el CIR no afectó las medidas de la función nerviosa, el estado de ánimo o la capacidad de pensamiento.
Calidad de la evidencia
Hay evidencia de baja calidad que indica que el CIR puede ayudar a prevenir el accidente cerebrovascular recurrente en los pacientes con estrechamiento de las arterias del cerebro, y puede aumentar la muerte o la dependencia en los pacientes con accidente cerebrovascular isquémico agudo que recibieron fármacos para disolver los coágulos. La evidencia es menos clara para la reducción del volumen del accidente cerebrovascular (tamaño de la lesión del cerebro causada por la reducción del flujo sanguíneo). Los estudios de investigación adicionales probablemente tengan un impacto importante sobre la confianza en estos resultados.
Authors' conclusions
Summary of findings
| RIC versus non‐RIC for preventing ischaemic stroke | ||||||
| Patient or population: people at risk for ischaemic stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐RIC for preventing ischaemic stroke | Risk with RIC | |||||
| Ischaemic stroke or recurrent ischaemic stroke | Symptomatic intracerebral artery stenosis or carotid stenting | RR 0.31 | 371 | ⊕⊕⊝⊝ | ||
| 92 per 1000 | 29 per 1000 | |||||
| Death or dependency | Symptomatic intracerebral artery stenosis or carotid stenting | not estimable | 292 | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Improvement in neurological impairment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Improvement in psychological and cognitive impairment ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Cardiovascular events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events associated with RIC treatment | Symptomatic intracerebral artery stenosis or carotid stenting | RR 10.91 | 371 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Stroke severity | ‐ | MD 0.17 lower | ‐ | 189 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgrade by two levels because of very serious risk of bias and serious imprecision. Very serious risk of bias resulted from: participants were not blinded to the treatment protocol in Meng 2012 and Zhao 2017; Meng 2012 and Meng 2015 used per‐protocol analysis methods; the corresponding author was one of the inventor of the RIC devices used in the studies; and there was large number of participants lost to follow up during the study period. 2 Serious imprecision. Incidence of ischaemic stroke was very lower in Zhao 2017, and the CI for ischaemic stroke was wide. 3 Serious imprecision. Incidence of RIC‐associated adverse events were very lower, and the CI for this outcome was very wide. 4 Downgrade by two levels because of serious risk of bias and serious indirectness. Very serious risk of bias results from: participants were not blinded to the treatment protocol; the corresponding author was one of the inventor of the RIC devices used; and there was large number of participants lost to follow up during the study period in Zhao 2017. 5 Serious indirectness. The infarct volume was used as a surrogate endpoint to assess stroke severity in Zhao 2017. | ||||||
| RIC versus non‐RIC for treating ischaemic stroke | ||||||
| Patient or population: people with ischaemic stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐RIC treating ischaemic stroke | Risk with RIC | |||||
| Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Death or dependency | Acute ischaemic stroke treated with intravenous thrombolysis | RR 2.34 | 285 | ⊕⊕⊝⊝ | ||
| 80 per 1000 | 187 per 1000 | |||||
| Improvement in neurological impairment ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Improvement in psychological and cognitive impairment at completion of follow‐up | The mean psychological function was 55.4 points and the mean cognitive function was 27.08 points. | SMD 0.29 lower | ‐ | 105 | ⊕⊕⊝⊝ | |
| Cardiovascular events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events associated with RIC treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Stroke severity | ‐ | SMD 0.24 lower | ‐ | 175 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgrade two levels by very serious risk of bias and serious imprecision. High risk of bias resulted from the unclear of allocation concealment, the unblinded treatment protocol to the participants, and large number of participants lost to follow up during the study period in Hougaard 2014. 2 Serious imprecision. There was a large number of participants excluded from the study after randomising in Hougaard 2014. 3 Downgrade two levels by very serious risk of bias and serious inconsistency. Very serious risk of bias resulted from the unclear of allocation concealment and high risk of other bias in Mi 2016 and Wang 2017, only a small number of participant were included for this outcome, and the corresponding author was one of the inventors of the RIC devices used in Mi 2016 and Wang 2017. 4 Serious inconsistency. We detected high level of the heterogeneity for the cognitive impairment. 5 Downgrade two levels by very serious risk of bias, serious inconsistency and serious indirectness. High risk of bias resulted from the unclear of allocation concealment, the unblinded treatment protocol to the participants, and large number of participants lost to follow up during the study period in Hougaard 2014; and the small number of participants in England 2016. 6 Serious indirectness. The infarct volume was used as a surrogate endpoint to assess stroke severity in Hougaard 2014. | ||||||
Background
Description of the condition
Stroke is the leading cause of adult disability globally, and its disease burden continues to increase (Feigin 2015; Roth 2015). Ischaemic stroke, which is due to blockage of a brain blood vessel, accounts for nearly 90% of all strokes; and nearly a quarter of individuals who have an ischaemic stroke will have recurrent strokes (Writing Group 2016). In 2013, more than 70% of stroke survivors and more than 50% of deaths from stroke were attributed to ischaemic stroke (Feigin 2015).
Fortunately, most ischaemic strokes are preventable. Prevention and screening of stroke not only reduces the incidence of ischaemic stroke and subsequent disability but may have beneficial effects on cognitive decline and other neuropsychological diseases. Key strategies for preventing ischaemic stroke include control of risk factors, anti‐clotting therapy, intervention for vascular obstruction and lifestyle change (Steiger 2016). Although these strategies play important roles in clinical practice, some are not cost effective and others may result in severe adverse events.
Timely intervention to open the occluded artery and return blood flow to salvageable cerebral tissue via clot‐treating therapies (including systemic and local administration of drugs to break up clots and use of various intra‐arterial minimally‐invasive devices to manually remove clots) are the most effective methods for salvaging brain tissue that had interrupted blood flow but is not already dead. The exact window of time for maximally effective treatment is unknown, but strong evidence shows that systemic administration of a drug to break up clots is only beneficial within 4.5 hours of symptom onset, while minimally‐invasive management with second‐generation mechanical clot‐removal devices is beneficial for patients with specific type of stroke within 24 hours of symptom onset (Powers 2018; Nogueira 2018). Unfortunately, these treatments are expensive and are available only at select comprehensive stroke centres. Furthermore, injury to surviving brain tissue after reestablishment of blood flow (a phenomenon known as ischaemia/reperfusion injury) is a major source of morbidity and mortality (Bai 2015). These disadvantages severely influence the prognosis of ischaemic stroke worldwide, especially in countries with low to medium levels of resources, such as China.
Description of the intervention
Ischaemic conditioning is a strategy by which one or more cycles of a brief period of interruption of blood flow to an organ or tissue followed by resumption of flow confer protection against subsequent, more severe ischaemic events (Hausenloy 2016). This far‐reaching phenomenon was first demonstrated by Murry and colleagues (Murry 1986). Since that time, ischaemic conditioning has been widely studied. However, a major disadvantage of attempting to condition a specific organ is that it requires application of an intervention directly to the target, which is not always feasible in clinical practice. A strategy by which the protective conditioning cycles are applied to an organ or tissue remote from the target organs may offer a far more attractive clinical application. Przyklenk and colleagues introduced a new method of carrying out ischaemic conditioning, in which the conditioning cycles of ischaemia were applied to a separate region (Przyklenk 1993). Later on, this strategy was named 'remote ischaemic conditioning' (RIC). Investigators have evaluated many tissues and organs such as kidney, liver, limbs, and the mesenteric artery as remote conditioning sites in animals. RIC performed on limbs, generally with blood pressure cuffs inflated to a pressure that blocks blood flow in one or both directions, is now considered the most convenient and feasible method that can be used in clinical settings (Hausenloy 2016; Pan 2016). RIC can be divided into three types on the basis of initiation of conditioning: preconditioning (RIC applied before ischaemia), perconditioning (RIC applied after ischaemia onset and before reperfusion), and postconditioning (RIC applied during reperfusion) (Pan 2016).
Many research studies have shown RIC to be a well tolerated and effective strategy for protecting the brain from ischaemia or reperfusion injury (Hahn 2011; Hoda 2012; Hougaard 2014; Pan 2016; Ren 2015). It has been determined that RIC could increase brain tolerance to injury caused by ischaemia, reduce the risk of cerebral infarction, improve blood flow to the brain, and promote the formation of alternately‐routed blood vessels to the brain (Hougaard 2014; Meng 2012; Pan 2016). Several randomised controlled trials (RCTs) have reported that RIC might confer neuroprotection against damage from acute ischaemic stroke and chronic cerebral ischaemia (Hougaard 2014; Meng 2012; Meng 2015). Although evidence suggests potential benefit of RIC for people with ischaemic stroke, many questions remain unanswered. The underlying protective mechanisms of RIC still need to be elucidated, and the optimal duration and number of ischaemia/reperfusion cycles have not been established. Additionally, the potential for complications related to pressure cuffs is regarded as low, and other adverse effects associated with RIC remain unknown. Finally, criteria are needed that can be used to identify ideal candidate people for RIC.
How the intervention might work
Characterisation of the mechanism by which RIC provides protection for the brain is currently incomplete, and most evidence has been provided by studies on cardiac preconditioning and postconditioning. Underlying mechanisms include two windows of protection: the early phase (action immediately after a stimulus that might play a role through affecting critical proteins) and the delayed phase (action days or weeks after a stimulus that might play a role in gene regulation and new protein synthesis) (Hahn 2011). Research findings indicate that protective signals may be transmitted from the conditioned organ to the brain through three routes: humoral, neuronal, and immunological pathways (Weber 2010).
Substances or humoral factors generated by the conditioned site may be circulated to the brain through the blood upon re‐establishment of blood flow of the conditioned site and may exert protective effects through signal pathways. A neuronal pathway at conditioned sites might be stimulated by local production of factors such as adenosine, bradykinin or other neuropeptides, which then stimulate nerve signals to the brain, thus inducing neuroprotection. RIC may derive its protective effects via alteration of gene and small molecule expression within the immune system, which then activates immune cells and alters inflammatory responses (Lim 2012; Pan 2016). Therefore, although the three protective signal pathways are distinct, both neuronal and immunological pathways are linked to the humoral pathway.
Why it is important to do this review
RIC appears to be a well tolerated, cost‐effective, easily implemented strategy that has the potential to provide specialised care for any user (e.g. patients, caregivers, physicians) in any circumstance (e.g. home, ambulance, hospital). Previous Cochrane review authors have evaluated RIC in vascular and endovascular surgical procedures (Desai 2011), and RIC has also be evaluated in coronary artery bypass grafting and kidney ischaemia‐reperfusion injury in theCochrane Database of Systematic Reviews (Menting 2017; Benstoem 2017). RIC is developing as a neuroprotective strategy for ischaemic stroke prevention and treatment, and its implementation in clinical settings is increasing in many parts of the world. Several clinical trials have shown that RIC might reduce stroke recurrence and improve stroke outcomes, and that it is well tolerated without associated severe adverse events (Hougaard 2014; Meng 2012; Meng 2015). However, existing evidence is insufficient to demonstrate the efficacy and safety of RIC.
Objectives
To assess the benefits and harms of RIC for preventing ischaemic stroke and for treating people with ischaemic stroke and those at risk for ischaemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
We included all identified randomised controlled trials (RCTs) comparing remote ischaemic conditioning (RIC) versus non‐RIC (sham RIC or standard medical management alone) in people with ischaemic cerebrovascular diseases. We excluded uncontrolled trials as well as quasi‐RCTs where allocation to treatment or control was not concealed (e.g. allocation by alteration, open random number list, date of birth, day of the week, or hospital number).
Types of participants
We included participants with any of the following: (1) acute ischaemic stroke diagnosed on the basis of the a combination of clinical examination findings and diagnostic test results or according to definitions for acute ischaemic stroke used by researchers to enrol patients in their RCTs; (2) chronic cerebral ischaemia (i.e.14 days after symptom onset) or gradual‐onset cerebral ischaemia (e.g. small vessel disease); (3) a definitive diagnosis of intracranial or extracranial cerebrovascular moderate and severe stenosis (i.e. > 50%) or occlusion confirmed by computed tomography angiography (CTA), magnetic resonance angiography (MRA) or digital subtraction angiography (DSA). We excluded studies of participants undergoing cardiovascular intervention therapies, coronary artery bypass grafting, any other type of cardiac surgery, and people undergoing other surgeries, as the complication risk of ischaemic stroke was low in these patients.
Types of interventions
The RIC protocol consisted of remote ischaemic preconditioning, remote ischaemic perconditioning and remote ischaemic postconditioning, which were the same procedure but divided based on the order of its initiation and the occurrence of ischaemia. A remote stimulus was applied to any organ (e.g. upper and lower limbs). This stimulus consisted of one or more cycles of brief ischaemia and reperfusion of varying duration. The control condition consisted of no intervention or standard medical management and shamRIC,which required application of a blood pressure cuff, tourniquet or other occlusive device without full interruption of blood flow.
Types of outcome measures
Primary outcomes
-
Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up
-
Death or dependency (e.g. Barthel Index (BI) score ≤ 60, modified Rankin Scale (mRS) grade 3 to 6, defined by the trial author) at completion of follow‐up
Secondary outcomes
-
Stroke severity as measured by infarct volume, the National Institutes of Health Stroke Scale (NIHSS), the Canadian Neurological Scale (CNS), the Scandinavian Stroke Scale, the European Stroke Scale, the modified Rankin Scale (mRS) or other scales reported by trial authors
-
Improvement in neurological impairment (e.g. NIHSS, mRS, CNS, other scales defined by trial authors) at completion of follow‐up
-
Improvement in psychological and cognitive impairment (e.g. Mini‐Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Wechsler Memory Scale (WMS), Wechsler Memory Scale‐Revised (WMS‐R), Luria‐Nebraska Neuropsychological Battery (LNNB) Memory Scale, other scales defined by trial authors) at completion of follow‐up
-
Cardiovascular events (e.g. acute myocardial infarction, angina)
-
Haemorrhagic stroke (e.g. intracerebral haemorrhage, subarachnoid haemorrhage)
-
Adverse events associated with RIC treatment (e.g. soft tissue injury, venous thrombosis, artery dissection, events reported by trial authors)
Search methods for identification of studies
See the 'Specialised register' section in the Cochrane Stroke Group module. We attempted to identify all relevant RCTs without restriction on language or publication status, and arranged translation of relevant papers where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (16 January 2018), and the following electronic bibliographic databases.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library (searched 16 January 2018) (Appendix 1)
-
MEDLINE Ovid (from 1946 to 16 January 2018) (Appendix 2)
-
Embase Ovid (from 1974 to 16 January 2018) (Appendix 3)
-
Science Citation Index Expanded (SCI‐EXPANDED) (Web of Science; 1950 to 10 January 2018) (Appendix 4)
-
Conference Proceedings Citation Index‐ Science (CPCI‐S) (Web of Science; 1950 to 10 January 2018) (Appendix 4)
-
China Biological Medicine Database (CBM; www.sinomed.ac.cn/) (from 1978 to 10 January 2018) (Appendix 5)
-
China Doctoral Dissertations Full‐text Database (CDFD) (CNKI; from 1984 to 10 January 2018) (Appendix 6)
-
China Academic Journal Network Publishing Database (CAJD) (CNKI; from 1915 to 10 January 2018) (Appendix 7)
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and adapted it for the other databases (Appendix 2).
We also conducted a search of the following research registers to identify ongoing matched trials (10 January 2018).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/trialsearch) (Appendix 8)
-
ClinicalTrials.gov (www.clinicaltrials.gov/) (Appendix 9)
-
Stroke Trials Registry (www.strokecenter.org/trials/) (Appendix 10)
-
ISRCTN Registry (www.isrctn.com) (Appendix 11)
Searching other resources
In an effort to identify other published, unpublished, and ongoing studies, we searched reference lists of relevant trials, and relevant journals (Brain Circulation and Conditioning Medicine) and conference proceedings (European Stroke Conference, International Stroke Conference, and Neuroscience Conferences). We also searched for and contacted trialists and researchers in the field where necessary.
Data collection and analysis
Selection of studies
Two review authors (WBZ and JZ) independently screened titles and abstracts of records identified through literature search activities and excluded those that were obviously irrelevant and duplicated. We retrieved the full text of remaining articles, and the same two review authors (WBZ and JZ) independently screened full‐text articles to identify studies for inclusion or exclusion on the basis of our predetermined Criteria for considering studies for this review. The two authors resolved any disagreements through discussion or by consulting a third author (RM).
Data extraction and management
Two review authors (WBZ and JZ) independently extracted and recorded study details on a data extraction form. We extracted the following data: methods, characteristics of participants, interventions, treatment duration, primary and secondary outcomes and time points reported. The same two authors cross‐checked all extracted data and resolved any differences in the extracted data by referring to the original articles and through discussion. We extracted data to allow an intention‐to‐treat (ITT) analysis (including all participants in the groups to which they were originally randomly allocated).
For continuous outcomes, we used means and standard deviations for each group. If median and interquartile ranges were used in the original article, we translated them to means and standard deviations (Wan 2014). For binary outcomes, we extracted the number of participants with the event in each group.
Assessment of risk of bias in included studies
Two review authors (WBZ and JZ) independently assessed risk of bias for each study using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with a third review author (RM) where necessary. We assessed risk of bias according to the following domains: selection bias; performance bias; detection bias; attrition bias; reporting bias and other possible bias.
For each domain, we categorised each study as presenting 'low risk', 'high risk', or 'unclear risk' of bias and provided information from the study report, together with a justification for our judgement, in the 'Risk of bias' tables.
Measures of treatment effect
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data (e.g., ischaemic stroke, recurrent ischaemic stroke, death, dependency, haemorrhagic stroke, cardiovascular events). For continuous data (e.g. scores from scales), we used mean differences (MDs) with 95% CIs for data measured in the same way between trials, and standardised mean differences (SMDs) with 95% CIs to combine data when different scales were used for measurement. We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
In most studies, the unit of analysis was the individual participant and a single measurement for each outcome was collected and analysed. However, for cross‐over trials, the unit of analysis included only the first phase in the analysis. For cluster‐randomised trials, the unit of analysis was the cluster.
Dealing with missing data
We carried out analyses according to the ITT principle: we used the number of initially randomised individuals as the denominator. If data were missing, we contacted the corresponding author or co‐authors through the address or email given in the publication. When this was not possible, we calculated best‐ and worst‐case scenarios for dichotomous data: the best‐case scenario would mean participants with missing data were alive or achieved dependence or did not experience stroke, and the worst‐case scenario would mean that participants with missing data were dead or experienced stroke or did not achieve dependence.
Assessment of heterogeneity
We assessed heterogeneity between study results using the I2 statistic. We defined a value greater than 50% as indicating substantial heterogeneity.
Assessment of reporting biases
We had intended to use funnel plots to assess the potential existence of reporting bias if more than 10 studies were included in this review. However, because we only seven included studies, we assessed reporting bias qualitatively on the basis of the study characteristics.
Data synthesis
We conducted a meta‐analysis using Review Manager 5 (Review Manager 2014). We used RR as a measure of effect for binary outcomes and MD or SMD as a measure of effect for continuous outcomes. We used a random‐effects model to better estimate the effect size of different studies, regardless of whether heterogeneity was significant.
GRADE and 'Summary of findings' table
We summarised the evidence by creating 'Summary of findings' tables using GRADEproGDT (https://gradepro.org/), in which we imported data from Review Manager 5. We used the GRADE approach to assess the quality of evidence as high, moderate, low, or very low, based on the following five considerations: study limitations, imprecision, consistency of effect, indirectness and publication bias (Higgins 2011). The table presents results and our assessments of the quality of evidence for the main outcomes.
Subgroup analysis and investigation of heterogeneity
We had intended to performed subgroup analysis to investigate heterogeneity by analysing differences in the number of RIC cycles and their length, the position of RIC (upper versus lower limb, unilateral versus bilateral), and length of RIC treatment (fractionated versus continuous). However, in this review only seven trials were eligible for analysis: three studies for the analysis of RIC for preventing ischaemic stroke, and another four studies for the analysis of RIC for treating ischaemic stroke. Given the limited number of included studies, there were not enough data for subgroup analysis.
Sensitivity analysis
We had intended to perform sensitivity analyses if the results were substantially heterogeneous, by 1) excluding studies with inadequate allocation concealment; 2) excluding studies that did not use blinding; 3) excluding studies in which loss to follow‐up was not reported or was greater than 10%; 4) excluding unpublished studies; 5) excluding studies that did not use sham as a comparison; and 6) excluding long studies, large studies or those using imputed data to determine their influence on study results. However, we did not perform any sensitivity analyses because of the limited number of included studies, and no substantial heterogeneity was found in this review.
Results
Description of studies
A detailed description of all studies can be found in Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
We identified a total of 8765 records from database searching in January 2018. After removing 1668 duplicates, 7097 records remained, which we screened and excluded 7066 records. We retrieved 31 full‐text articles and abstracts and assessed them for eligibility. Finally, we identified seven trials and assessed them for eligibility as per protocol with refined inclusion criteria. We excluded 16 studies with reasons (see Characteristics of excluded studies), and identified eight ongoing studies. We present details of the seven included trials in Characteristics of included studies. For an illustration of these results, please see the study flow diagram (Figure 1).
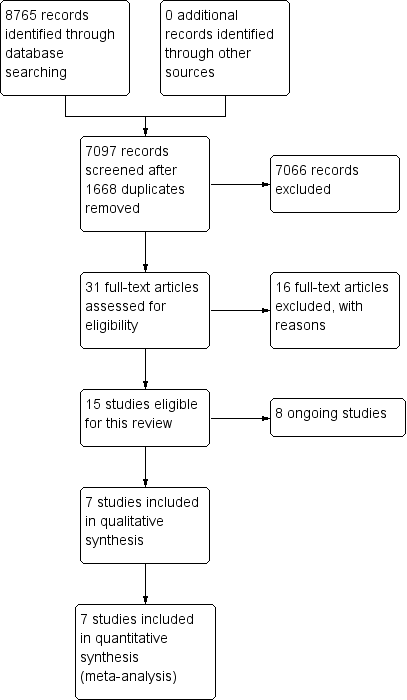
Study flow diagram.
Included studies
Seven trials met our inclusion criteria. The first trial was a single‐centre trial conducted in the UK (England 2016). The trial compared remote ischaemic conditioning (RIC) with sham RIC in 26 participants (13 in each group) with acute ischaemic stroke. RIC and sham RIC were started within 24 hours of stroke onset and performed manually using a standard upper arm blood pressure cuff in the nonparetic arm. RIC consisted of four cycles of five minutes' inflation (20 mmHg above systolic blood pressure) and five minutes' deflation; and sham RIC consisted of four cycles of five minutes' inflation (30 mmHg) and five minutes' deflation. The primary outcome was tolerability and feasibility of RIC after acute ischaemic stroke, and the duration of follow‐up was 90 days.
The second trial was a single‐centre trial conducted in Denmark (Hougaard 2014). The trial compared intravenous thrombolysis plus RIC with intravenous thrombolysis alone in 285 participants with acute ischaemic stroke. Because of the imbalanced randomisation and large number of participants excluded from the analysis after randomisation, there were unbalanced numbers of participants between the two groups (160 in the intervention group, and 125 in the control group). RIC was started in the ambulance and induced by four inflations of a standard upper limb blood pressure cuff to either 200 mmHg or 25 mmHg above the systolic blood pressure, each lasting five minutes and separated by five minutes of cuff deflation. The primary outcome was the penumbra salvage, and the duration of follow‐up was 90 days.
The third trial was conducted in two centres in China (Meng 2012). The trial compared standard medical treatment plus RIC and standard medical treatment alone in 103 participants (51 in the intervention group and 52 in the control group) with symptomatic intracranial artery stenosis. RIC, consisting of five cycles of bilateral arms ischaemia (induced by inflating tourniquets to 200 mmHg) for five minutes followed by reperfusion for another five minutes, was performed twice daily for a total of 300 consecutive days. Standard medical treatment including antiplatelet agents, lipid control agents, and antidiabetic agents (if necessary). The primary outcome was time point of the first stroke recurrence event, and the duration of follow up was 300 days.
The fourth trial was a single‐centre trial conducted in China (Meng 2015). The trial compared RIC and sham RIC in 79 octogenarian and nonagenarian participants (40 in the intervention group and 39 in the control group) with symptomatic intracerebral artery stenosis. RIC consisted of five cycles of simultaneous bilateral arms ischaemia (induced by inflating tourniquets to 200 mmHg) for five minutes followed by reperfusion for another five minutes. Sham RIC consisted of five cycles of bilateral arms ischaemia (induced by inflating tourniquets to 30 mmHg) for five minutes, followed by reperfusion for another five minutes. Both RIC and sham RIC were performed for 180 consecutive days. Medical management included either clopidogrel 75 mg/day in combination with atorvastatin 20 mg/day or aspirin 100 mg/day and clopidogrel 75 mg/day in combination with atorvastatin 20 mg/day. The duration of follow‐up was 180 days.
The fifth trial was a single‐centre trial conducted in China (Mi 2016). The trial compared RIC and sham RIC in 17 participants (nine in the intervention group and eight in the control group) with cerebral small vessel disease. Both RIC and sham RIC were achieved using an automated cuff inflator placed on bilateral arms that were inflated to 200 mmHg and 50 mmHg respectively. Both RIC and sham RIC consisted of five cycles of five minutes' ischaemia follow by five minutes' reperfusion. All participants were required to receive RIC or sham RIC treatment twice daily for one year. The primary outcome was changes in brain lesions, evaluated by the number of lacunar infarctions and white matter lesions volume, and the duration of follow‐up was one year.
The sixth trial was a single‐centre trial conducted in China (Wang 2017). The trial compared RIC and sham RIC in 36 participants (18 in the intervention group and 18 in the control group) with cerebral small vessel disease‐related mild cognitive impairment. Both RIC and sham RIC were achieved using an automated cuff inflator placed on bilateral arms that were inflated to 200 mmHg and 50 mmHg respectively. Both RIC and sham RIC consisted of five cycles of five minutes' ischaemia follow by five minutes' reperfusion. All participants were required to receive RIC or sham RIC treatment twice daily for one year. The primary outcome was the change of brain lesions, and the follow‐up duration was one year.
The seventh trial was a single‐centre trial conducted in China (Zhao 2017). The trial compared RIC plus standard medical treatment, sham RIC plus standard medical treatment and standard medical treatment alone in 189 participants (63 in each group) with severe carotid artery stenosis. RIC and sham RIC consisted of five cycles of bilateral arms ischaemia for five minutes, followed by reperfusion for another five minutes, and they were performed twice daily for two weeks before carotid stenting. Both RIC and sham RIC procedures were performed by using an electric autocontrol device with cuffs that inflated to a pressure of 200 mmHg and 60 mmHg respectively during the ischaemic period. The primary outcomes were the presence of one or more new brain lesions on diffusion weighted imaging (DWI) within 48 hours after carotid artery stenting, and the incidence of clinical events (i.e. ischaemic stroke, transient ischaemic attach (TIA), acute myocardial infarction, haemorrhagic stroke, hyperperfusion syndrome, and death) within six months after stenting. The duration of follow‐up was six months.
Excluded studies
We excluded 16 studies from this review. The Characteristics of excluded studies table provides summary details.
We excluded seven studies because the participants underwent percutaneous coronary intervention or cardiac surgery (Cho 2017; Coverdale 2017; Davies 2013; Hoole 2009; Hudetz 2014; Sloth 2014; Zhong 2013). We excluded one study because the included participants underwent transfemoral transcatheter aortic valve implantation (Kahlert 2017). One study randomised participants treated with major vascular surgery, including carotid endarterectomy; we excluded it because we did not obtain the data on participants treated with carotid endarterectomy after emailing the author (Healy 2015). We excluded four studies because they did not assess ischaemic stroke in their outcome assessment (Jing 2011; Meng 2017; Sales 2017; Walsh 2010). Another study was not a randomised controlled trial (Joseph 2015), and another study has passed the completion date but the status has not been verified after more than two years, and no paper or results have been published to date (NCT01672515). We excluded one study because the comparison was between healthy people and patients (Hyngstrom 2016).
Risk of bias in included studies
The 'Risk of bias' assessments for all included studies are summarised in Figure 2 and Figure 3.
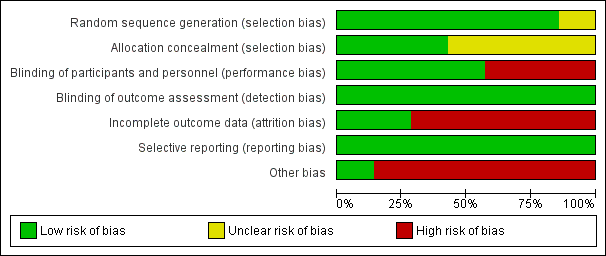
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
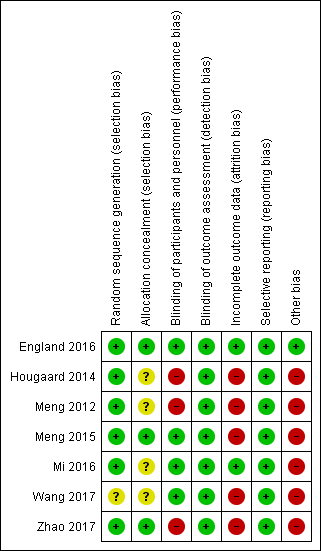
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of randomisation was described and considered adequate with low risk of bias in six included studies (England 2016; Hougaard 2014; Meng 2012; Meng 2015; Mi 2016; Zhao 2017). Four studies reported that the random sequence was generated by using computer‐generated random number sequence (Meng 2012; Meng 2015; Mi 2016; Zhao 2017). One study reported that the randomisation was performed by drawing from a large number of sealed opaque envelopes (Hougaard 2014). One study reported that the randomisation was generated by minimisation (England 2016). Another study only reported that randomisation was performed by an independent statistician; the details were not reported, therefore we judged the risk of bias as unclear (Wang 2017).
Allocation concealment was adequate with low risk of bias in three studies, one of which used a web‐based randomisation method (England 2016), and two of which used sequentially numbered, opaque, sealed envelopes (Meng 2015; Zhao 2017). Allocation concealment was unclear for three trials which only reported that the assignments were sealed, but not whether the assignment was sequentially numbered (Hougaard 2014; Mi 2016; Wang 2017). One study did not report the details of allocation (Meng 2012).
Blinding
Because of the nature of RIC treatment, if no sham RIC treatment was used, it was impossible to blind the participants. Therefore, we judged two studies as being at high risk of bias (Hougaard 2014; Meng 2012), and four as being at low risk of bias (Meng 2015; England 2016; Mi 2016; Wang 2017). One study was comprised of three groups (RIC group, sham RIC group, and control group); although sham RIC was included in the study, it was also impossible to blind the participants (Zhao 2017). Therefore, we also judged this trial as having high risk of performance bias.
One study was an outcome‐blinded, placebo‐controlled trial (England 2016). All other studies reported that the outcome assessors were blinded to the treatment protocol (Hougaard 2014; Meng 2012; Meng 2015; Mi 2016; Wang 2017; Zhao 2017). We therefore judged all the trials as having low risk of detection bias.
Incomplete outcome data
We judged two studies as having low risk of attrition bias as there were no participants lost to follow‐up during the study period (England 2016; Mi 2016). We judged three trials as having high risk of attrition bias because of the following losses to follow‐up: 35 out of 103 (34.0%) participants (Meng 2012); 21 out of 79 (26.6%) participants (Meng 2015); and 6 out of 36 (16.7%) participants (Wang 2017). We judged the two remaining studies as having high risk of attrition bias because 158 out of 443 (35.7%) participants were excluded in the as‐treated analyses (Hougaard 2014); and 27 out of 189 (14.3%) participants were lost to the analyses of imaging and 42 out of 189 (22.2%) participants were lost to the analyses of clinical events (Zhao 2017).
Selective reporting
All included studies registered their study protocols in one of the clinical trial registration websites, and all predefined study outcomes were published. Therefore, we judged all included studies as low risk of reporting bias (England 2016; Hougaard 2014; Meng 2012; Meng 2015; Mi 2016; Wang 2017; Zhao 2017).
Other potential sources of bias
We judged five trials performed in the same centre in China to be high risk of other bias because these trials had the same principle investigator, who was one of the inventors of the electric autocontrol RIC device used in these studies (Meng 2012; Meng 2015; Mi 2016; Wang 2017; Zhao 2017). We judged one other study to be at high risk of other bias because two authors were shareholders in the company where the devices used in the study were made (Hougaard 2014). We judged the remaining study to be at low risk of other potential bias as we identified no other bias (England 2016).
Effects of interventions
See: Summary of findings for the main comparison RIC versus non‐RIC for preventing ischaemic stroke; Summary of findings 2 RIC versus non‐RIC treating ischaemic stroke
See: summary of findings Table for the main comparison and summary of findings Table 2.
Three studies focused on remote ischaemic conditioning (RIC) for preventing ischaemic stroke (Meng 2012; Meng 2015; Zhao 2017), while the other four studies focused on RIC for treating ischaemic stroke (England 2016; Hougaard 2014; Mi 2016; Wang 2017). The outcomes used in these two categories of studies were different. Therefore, we analysed RIC for preventing and treating ischaemic stroke separately. Although multiple relative risks were used for outcomes analyses, caution should be taken when using these results as the presented data were extracted from only one or two studies with small sample sizes and low quality of evidence.
RIC versus non‐RIC for preventing ischaemic stroke
Three studies focused on RIC for preventing ischaemic stroke (Meng 2015; Meng 2012; Zhao 2017). The total number of participants was 371.
Primary outcomes
1.1. Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up
Ischaemic stroke was reported in Zhao 2017 and recurrent ischaemic stroke was reported in Meng 2012 and Meng 2015.
1.1.1 Ischaemic stroke
One study (Zhao 2017), involving 189 participants (50.9% of the total number of participants included in this part of review) presented data on ischaemic stroke. There was no significant difference in the incidence of ischaemic stroke between those allocated to RIC versus those allocated to non‐RIC (risk ratio (RR) 0.22, 95% confidence interval (CI) 0.01 to 4.03; P = 0.31) (Analysis 1.1).
1.1.2 Recurrent ischaemic stroke
Two studies (Meng 2012; Meng 2015), involving 182 participants (49.1% of the total number of participants included in this part of review), presented data on recurrent ischaemic stroke. The incidence of recurrent ischaemic stroke was significantly reduced by RIC treatment (RR 0.32, 95% CI 0.12 to 0.83; P = 0.02) (Analysis 1.1).
1.2. Death or dependency at completion of follow‐up
Death was not reported in Meng 2015. The other two studies (Meng 2012; Zhao 2017) reported that no participant died during the study period, so analysis was not applicable to the outcome of death. None of the trials (Meng 2012; Meng 2015; Zhao 2017) reported any forms of dependency that enabled us to include them for analysis.
Secondary outcomes
1.3. Stroke severity
This outcome was assess by infarct volume in Zhao 2017, which involving 189 participants (50.9% of the total number of participants included in this part of review). Infarct volume was calculated using magnetic resonance imaging (MRI) scans. In participants with multiple lesions, the total infarct volume was added up. The infarct volume was significantly smaller in participant treated with RIC compared with those not treated with RIC (MD ‐0.17 mL, 95% CI ‐0.23 to ‐0.11; P < 0.01; I2 = 3%) (Analysis 1.3).
1.4. Improvement in neurological impairment at completion of follow‐up
None of the three trials reported this outcome in a form that enabled us to include them for this analysis.
1.5. Improvement in phycological and cognitive impairment at completion of follow‐up
None of the three trials reported this outcome in a form that enabled us to include them for this analysis.
1.6. Cardiovascular events
This outcome was not reported in Meng 2015. The other two studies (Meng 2012; Zhao 2017) reported that no participant experienced cardiovascular events during the study period, so analysis was not applicable to this outcome.
1.7. Haemorrhagic stroke
None of the three trials reported this outcome in a form that enabled us to include them for this analysis.
1.8. Adverse events associated with RIC treatment
All studies (371 participants: 100% of the total number of participants included in this part of review) reported data on adverse events associated with RIC treatment. One study reported that no participant experienced any adverse events associated with RIC treatment (Meng 2012). One study reported that 6/63 participants experienced arm skin petechiae from repeated pressure cuff applications (Zhao 2017), and the other study reported that sporadic petechiae in 3/40 participants were observed (Meng 2015). The adverse events associated with RIC treatment were significantly higher in participants treated with RIC (RR 10.91; 95% CI 2.01 to 59.28; P = 0.006; I2 = 0%). No participant experienced severe adverse events associated with RIC treatment.
RIC versus non‐RIC for treating ischaemic stroke
Four studies focused on RIC for treating ischaemic stroke (England 2016; Hougaard 2014; Mi 2016; Wang 2017). The total number of participants was 364.
Primary outcomes
2.1. Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up
None of the four trials reported these outcomes in any forms that enabled us to include them for this analysis.
2.2. Death or dependency at completion of follow‐up
Both death and dependency were was reported by one study including 285 participants (78.3% of the total number of participants included in this part of review) (Hougaard 2014). Dependency, assessed by the mean and median of the modified Ranks Scale score and the Barthel Index, was reported in one study (England 2016); we tried to contact the study authors to get the incidence of dependency, but failed to receive any response. Two other studies did not report any of the outcomes (Mi 2016; Wang 2017). Therefore we only included Hougaard 2014 in this analysis. The rate of death or dependency was significantly increased by RIC treatment compared with non‐RIC treatment (RR 2.34; 95% 1.19 to 4.61; P = 0.01) (Analysis 2.2).
Secondary outcomes
2.3. Stroke severity
This outcome was assessed by the infarct volume in Hougaard 2014 and the NIHSS scores in England 2016.
2.3.1. Final infarct volume
This outcome was assessed by the final infarct volume in one study with 149 participants (40.9% of the total number of participants included in this part of review) (Hougaard 2014). No significant difference was detected between participants allocated to RIC treatment and those allocated to non‐RIC treatment (SMD 0.08 mL; 95% CI ‐0.24 to 0.41; P = 0.61) (Analysis 2.3).
2.3.2. NIHSS scores
Scores on the NIHSS at three‐month follow‐up were reported in England 2016, which included 26 participants (9.1% of the total number of participants included in this part of review). There was no significant difference between participants allocated to RIC treatment and those allocated to non‐RIC treatment (SMD ‐0.73 points; 95% CI ‐1.53 to 0.07; P = 0.07) (Analysis 2.3).
2.4. Improvement in neurological impairment at completion of follow‐up
None of the four trials reported this outcome in a form that enabled us to include them for this analysis.
2.5. Improvement in phycological and cognitive impairment at completion of follow‐up
The phycological function was reported in England 2016 and the cognitive function was reported in three studies (England 2016; Mi 2016; Wang 2017).
2.5.1 Psychological impairment
Psychological impairment was only reported in England 2016, which included 26 participants (9.1% of the total number of participants included in this part of review). The Zung Depression Score was used for the assessment. There was no significant difference between participants allocated to RIC treatment and those allocated to non‐RIC treatment (SMD ‐0.37 points; 95% CI ‐1.15 to 0.41; P = 0.35) (Analysis 2.5).
2.5.2 Cognitive impairment
Three studies reported results of the Mini‐Mental State Examination (MMSE) (England 2016; Mi 2016; Wang 2017). Seventy‐nine participants (21.7% of the total number of participants included in this part of review) were included in this analysis. There was no significant difference in cognitive function between participants allocated to RIC treatment and those allocated to non‐RIC treatment (SMD ‐0.26 points; 95% CI ‐0.72 to 0.21; P = 0.28) (Analysis 2.5).
2.6. Cardiovbascular events
None of the trials reported this outcome in a form that enabled us to include them for this analysis.
2.7. Haemorrhagic stroke
None of the trials reported this outcome in a form that enabled us to include them for this analysis.
2.8. Adverse events associated with RIC treatment
None of the trials reported this outcome in a form that enabled us to include them for this analysis.
Subgroup analyses
Given the limited number of included studies, we did not perform subgroup analyses. If we include more studies in future updates of this review, we will explore differences in the results by creating subgroups according to the number of RIC cycles or their length, position of RIC treatment, and length of treatment.
Sensitivity analyses
We did not perform sensitivity analyses because of the limited number of included studies.
Funnel plots
We could not use funnel plots because only seven studies were included in this review, and three focused on RIC for preventing ischaemic stroke and the other four focused on RIC for treating ischaemic stroke.
Discussion
In this Cochrane Review, we have assessed the benefits and harms of remote ischaemic conditioning (RIC) for either preventing or treating ischaemic stroke.
Summary of main results
RIC for preventing ischaemic stroke
Due to the low incidence of death and cardiovascular events in the study population, these analyses were not applicable. Two studies assessed effects of RIC in preventing recurrent ischaemic stroke in participants with symptomatic intracerebral artery stenosis (Meng 2012; Meng 2015), and one study assessed the effects of RIC in preventing ischaemic stroke after carotid stenting in participants with severe carotid stenosis (Zhao 2017). Based on the included studies, we found that RIC might prevent recurrent ischaemic stroke in people with symptomatic intracerebral artery stenosis, and reduce post‐stenting infarct volumes in people undergoing carotid stenting.
Two studies reported adverse events associated with repeated RIC treatment, such as sporadic petechiae in local arm skin (Meng 2015; Zhao 2017). Although the adverse events associated with RIC treatment were significantly higher in participants treated with RIC, no severe adverse events (such as venous thrombosis, arterial dissection, or death) were reported.
RIC for treating ischaemic stroke
Two studies assessed the effects of RIC in treating acute ischaemic stroke (Hougaard 2014; England 2016), and another two studies assessed effects of RIC in treating cerebral small vessel disease (Mi 2016; Wang 2017). Based on the included studies, we found that RIC did not significantly reduce the final infarct volume in people with acute ischaemic stroke who received intravenous thrombolysis, but it might increase the death or dependency of these people. We also found that neurological impairment, assessed by the National Institutes of Health Stroke Scale (NIHSS) score, and psychological impairment were not significantly improved by RIC in this patient population. However, it should be noted that the sample size of one included trial was rather small (n = 26), and the number of participants lost to follow‐up in another included study was rather high.
One study compared cognitive function between acute ischaemic stroke participants treated with RIC and non‐RIC (England 2016), while two studies compared cognitive function between cerebral small vessel disease participants treated with RIC and non‐RIC (Mi 2016; Wang 2017). There was no significant difference in cognitive function between the groups; however, it should be noted that the sample size of the three included trials was rather small (n = 79).
Overall completeness and applicability of evidence
In this review, we widened the protocol inclusion criteria to allow various RIC treatment protocols, and included all trials that recruited people with ischaemic cerebrovascular disease.
The three studies focused on preventing ischaemic stroke were carried out in China, and intracerebral artery atherosclerosis was common in this patient population. This may indicate that the results of RIC for preventing ischaemic stroke are likely to be only applicable to the Chinese population, and possibly to Asians, among whom there is a high prevalence of intracerebral artery atherosclerosis. The three included studies tested the same RIC treatment protocol (bilateral arms, five cycles of five minutes' ischaemia/reperfusion, twice daily) with different treatment duration (two weeks, 180 days, and 300 days). We found low‐quality evidence that RIC could prevent recurrent ischaemic stroke in participants with symptomatic intracerebral artery stenosis, and reduce post‐stenting new infarct volumes in participants undergoing carotid stenting. RIC treatment for preventing recurrent ischaemic stroke and ischaemic stroke should be reviewed in light of this evidence. Due to the low incidence of death and cardiovascular diseases, and the relatively small simple sizes of the included studies, the current review found no evidence that RIC could prevent death and cardiovascular diseases in people with ischaemic cerebrovascular disease. The three included studies reported RIC‐specific adverse events, such as sporadic localised petechiae in arm skin, but none of them reported any severe adverse events related to RIC treatment.
Four studies focused on RIC for treating ischaemic stroke. One recruited participants with acute ischaemic stroke within 24 hours of symptom onset in the UK (England 2016), one recruited ischaemic stroke candidates considered for intravenous thrombolysis within 4.5 hours in Denmark (Hougaard 2014), one recruited participants with cerebral small vessel disease in China (Mi 2016), and one recruited participants with cerebral small vessel disease‐related mild cognitive impairment in China (Wang 2017). This may mean that the results of this review are likely to be applicable to people from these countries. The included studies tested two treatment protocols (four cycles of unilateral arm and five cycles of bilateral arms, five minutes' ischaemia/reperfusion), and treatment duration of RIC varied from once to twice daily for 12 consecutive months. We found that RIC may increase death or dependency in people with acute ischaemic stroke who received intravenous thrombolysis, but we did not find any clear evidence that RIC had any effects on the final infarct volume, or psychological impairment in people with acute ischaemic stroke. Neither did we find any influence of RIC on the cognitive impairment in people with acute ischaemic stroke and cerebral small vessel disease. RIC for treating acute ischaemic stroke and cerebral small vessel disease should be reviewed in light of this evidence. Given the poor prognosis of acute ischaemic stroke, especially in those with large artery occlusion, further evidence relating to the use of RIC in this patient population or in combination with endovascular therapy or intravenous thrombolysis would be useful to better clarify the potential benefits and risks in this scenario.
Quality of the evidence
We assessed the quality of the evidence by using the GRADE approach (Guyatt 2008), and we presented the results in summary of findings Table for the main comparison and summary of findings Table 2.
Four studies did not specify how allocation concealment was achieved, so we assessed them as having unclear risk of selection bias. Three studies were not blinded for participants, so we assessed them as having high risk of performance bias; and six studies had a high percentage of participants lost to follow‐up, so we assessed them as having high risk of attrition bias. Overall, for each of the outcomes reported in our 'Summary of findings' tables, we graded the quality of evidence as low or very low.
From the three studies focused on RIC for preventing ischaemic stroke, there is low‐quality evidence that RIC performed better than non‐RIC in preventing recurrent ischaemic stroke in people with symptomatic intracerebral artery stenosis if treated for several consecutive months, and in reducing incidence of post‐stenting new infarct volume in people undergoing carotid stenting if treated for two consecutive weeks before stenting. However, further research is likely to have a substantial impact on our confidence in the above estimations.
Four studies focused on RIC for treating ischaemic stroke. There is low‐quality evidence that RIC might increase death or dependency in acute ischaemic stroke participants treated with intravenous thrombolysis. There is low‐quality evidence that RIC performed no better or no worse than non‐RIC in improving psychological impairment in people with acute ischaemic stroke if started within 24 hours of ictus, and there is low‐quality evidence that RIC performed no better or no worse than non‐RIC in improving cognitive impairment in people with acute ischaemic stroke and cerebral small vessel disease. There is very low quality evidence that RIC performed no better or no worse than non‐RIC in reducing stroke severity (assessed by NIHSS score and infarct volume) in people with acute ischaemic stroke whether or not treated with intravenous thrombolysis. However, further research is likely to have an important impact on our confidence in the above estimations.
Potential biases in the review process
We carried out a comprehensive search across major databases, and we believe that we identified all relevant studies. In addition, we contacted the study authors for additional data when needed. However, as less than 10 trials were included this review and all included studies had a small sample size, potential known and unknown biases cannot be excluded. In addition, this meta‐analysis was slightly hampered by missing data because of studies reporting outcome data as medians and interquartile ranges. We were unable to obtain raw data for these studies, and this may cause biases to our analyses.
Agreements and disagreements with other studies or reviews
The findings of our review regarding RIC for preventing ischaemic stroke are different from other meta‐analyses in the literature. Two Cochrane Reviews (one of which included 29 studies involving 5392 participants who underwent cardiovascular surgery, and the other four studies involving 232 participants who underwent vascular and endovascular surgery) and a non‐Cochrane review (which included 23 studies involving a total of 2200 participants who underwent cardiovascular surgery) analysed the effectiveness of RIC on preventing stroke (Benstoem 2017; Desai 2011; Healy 2014). These three previous reviews concluded that RIC had no effect on stroke. This discrepancy may be attributable to the different study populations included in different reviews. In this review, we analysed the effects of RIC in preventing ischaemic stroke in people with symptomatic intracerebral artery stenosis or undergoing carotid stenting, and all these participants had a high possibility of recurrent stroke or new brain infarct. However, in people undergoing cardiovascular surgery, vascular surgery and endovascular surgery, the incidence of stroke is relatively very low, and many studies found no participants developing this complication.
There has been no previous systematic review of RIC versus non‐RIC in treating ischaemic stroke. The evidence in this review that RIC should not be used routinely in treating ischaemic stroke disagrees with the conclusions of the authors of the four included studies. However, due to the flaws identified in the included studies, further studies on the efficacy of RIC for treating ischaemic stroke are welcomed.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 RIC versus non‐RIC for preventing ischaemic stroke, Outcome 1 Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up.

Comparison 1 RIC versus non‐RIC for preventing ischaemic stroke, Outcome 2 Death or dependency at completion of follow‐up.

Comparison 1 RIC versus non‐RIC for preventing ischaemic stroke, Outcome 3 Stroke severity.

Comparison 1 RIC versus non‐RIC for preventing ischaemic stroke, Outcome 6 Cardiovascular events.

Comparison 1 RIC versus non‐RIC for preventing ischaemic stroke, Outcome 8 Adverse events associated with RIC treatment.

Comparison 2 RIC versus non‐RIC treating ischaemic stroke, Outcome 2 Death or dependency at the completion of follow up.

Comparison 2 RIC versus non‐RIC treating ischaemic stroke, Outcome 3 Stroke severity.

Comparison 2 RIC versus non‐RIC treating ischaemic stroke, Outcome 5 Improvement in psychological and cognitive impairment at completion of follow‐up.
| RIC versus non‐RIC for preventing ischaemic stroke | ||||||
| Patient or population: people at risk for ischaemic stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐RIC for preventing ischaemic stroke | Risk with RIC | |||||
| Ischaemic stroke or recurrent ischaemic stroke | Symptomatic intracerebral artery stenosis or carotid stenting | RR 0.31 | 371 | ⊕⊕⊝⊝ | ||
| 92 per 1000 | 29 per 1000 | |||||
| Death or dependency | Symptomatic intracerebral artery stenosis or carotid stenting | not estimable | 292 | ‐ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Improvement in neurological impairment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Improvement in psychological and cognitive impairment ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Cardiovascular events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events associated with RIC treatment | Symptomatic intracerebral artery stenosis or carotid stenting | RR 10.91 | 371 | ⊕⊕⊝⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Stroke severity | ‐ | MD 0.17 lower | ‐ | 189 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgrade by two levels because of very serious risk of bias and serious imprecision. Very serious risk of bias resulted from: participants were not blinded to the treatment protocol in Meng 2012 and Zhao 2017; Meng 2012 and Meng 2015 used per‐protocol analysis methods; the corresponding author was one of the inventor of the RIC devices used in the studies; and there was large number of participants lost to follow up during the study period. 2 Serious imprecision. Incidence of ischaemic stroke was very lower in Zhao 2017, and the CI for ischaemic stroke was wide. 3 Serious imprecision. Incidence of RIC‐associated adverse events were very lower, and the CI for this outcome was very wide. 4 Downgrade by two levels because of serious risk of bias and serious indirectness. Very serious risk of bias results from: participants were not blinded to the treatment protocol; the corresponding author was one of the inventor of the RIC devices used; and there was large number of participants lost to follow up during the study period in Zhao 2017. 5 Serious indirectness. The infarct volume was used as a surrogate endpoint to assess stroke severity in Zhao 2017. | ||||||
| RIC versus non‐RIC for treating ischaemic stroke | ||||||
| Patient or population: people with ischaemic stroke | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with non‐RIC treating ischaemic stroke | Risk with RIC | |||||
| Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Death or dependency | Acute ischaemic stroke treated with intravenous thrombolysis | RR 2.34 | 285 | ⊕⊕⊝⊝ | ||
| 80 per 1000 | 187 per 1000 | |||||
| Improvement in neurological impairment ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Improvement in psychological and cognitive impairment at completion of follow‐up | The mean psychological function was 55.4 points and the mean cognitive function was 27.08 points. | SMD 0.29 lower | ‐ | 105 | ⊕⊕⊝⊝ | |
| Cardiovascular events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events associated with RIC treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Stroke severity | ‐ | SMD 0.24 lower | ‐ | 175 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgrade two levels by very serious risk of bias and serious imprecision. High risk of bias resulted from the unclear of allocation concealment, the unblinded treatment protocol to the participants, and large number of participants lost to follow up during the study period in Hougaard 2014. 2 Serious imprecision. There was a large number of participants excluded from the study after randomising in Hougaard 2014. 3 Downgrade two levels by very serious risk of bias and serious inconsistency. Very serious risk of bias resulted from the unclear of allocation concealment and high risk of other bias in Mi 2016 and Wang 2017, only a small number of participant were included for this outcome, and the corresponding author was one of the inventors of the RIC devices used in Mi 2016 and Wang 2017. 4 Serious inconsistency. We detected high level of the heterogeneity for the cognitive impairment. 5 Downgrade two levels by very serious risk of bias, serious inconsistency and serious indirectness. High risk of bias resulted from the unclear of allocation concealment, the unblinded treatment protocol to the participants, and large number of participants lost to follow up during the study period in Hougaard 2014; and the small number of participants in England 2016. 6 Serious indirectness. The infarct volume was used as a surrogate endpoint to assess stroke severity in Hougaard 2014. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up Show forest plot | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.12, 0.76] |
| 1.1 Ischaemic stroke | 1 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.01, 4.03] |
| 1.2 Recurrent ischaemic stroke | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.12, 0.83] |
| 2 Death or dependency at completion of follow‐up Show forest plot | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 Death | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Dependency | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Stroke severity Show forest plot | 1 | 189 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.23, ‐0.11] |
| 3.1 RIC vs sham control | 1 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.22, ‐0.08] |
| 3.2 RIC vs blank control | 1 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.30, ‐0.12] |
| 4 Improvement in neurological impairment | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Improvement in psychological and cognitive impairment at completion of follow‐up | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Cardiovascular events Show forest plot | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 RIC vs sham control | 1 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 RIC vs blank control | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Haemorrhagic stroke | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Adverse events associated with RIC treatment Show forest plot | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 10.91 [2.01, 59.28] |
| 8.1 RIC vs sham control | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 9.78 [1.23, 77.71] |
| 8.2 RIC vs blank control | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 13.58 [0.72, 255.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ischaemic stroke or recurrent ischaemic stroke at completion of follow‐up | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Death or dependency at the completion of follow up Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Stroke severity Show forest plot | 2 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.02, 0.54] |
| 3.1 NIHSS scores | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.53, 0.07] |
| 3.2 Infarct volume | 1 | 149 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.24, 0.41] |
| 4 Improvement in neurological impairment | 0 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Improvement in psychological and cognitive impairment at completion of follow‐up Show forest plot | 3 | 105 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.68, 0.11] |
| 5.1 Psychological impairment | 1 | 26 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.15, 0.41] |
| 5.2 Cognitive impairment | 3 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.72, 0.21] |
| 6 Cardiovascular events | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Hemorrhagic stroke | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Adverse events associated with RIC treatment | 0 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

