Perbekalan sokongan pernafasan berbanding dengan tanpa sokongan pernafasan sebelum pengapitan kord untuk bayi pramatang
Abstract
Background
Placental transfusion (by means of delayed cord clamping (DCC), cord milking, or cord stripping) confers benefits for preterm infants. It is not known if providing respiratory support to preterm infants before cord clamping improves outcomes.
Objectives
To assess the efficacy and safety of respiratory support provided during DCC compared with no respiratory support during placental transfusion (in the form of DCC, milking, or stripping) in preterm infants immediately after delivery.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 5), MEDLINE via PubMed (1966 to 19 June 2017), Embase (1980 to 19 June 2017), and CINAHL (1982 to 19 June 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi‐randomized trials.
Selection criteria
Randomized, cluster randomized, or quasi‐randomized controlled trials enrolling preterm infants undergoing DCC, where one of the groups received respiratory support before cord clamping and the control group received no respiratory support before cord clamping.
Data collection and analysis
All review authors assisted with data collection, assessment, and extraction. Two review authors assessed the quality of evidence using the GRADE approach. We contacted study authors to request missing information.
Main results
One study fulfilled the review criteria. In this study, 150 preterm infants of less than 32 weeks' gestation undergoing 60 second DCC were randomized to a group who received respiratory support in the form of continuous positive airway pressure (CPAP) or positive pressure ventilation during DCC and a group that did not receive respiratory support during the procedure. Mortality during hospital admission was not significantly different between groups with wide confidence intervals (CI) for magnitude of effect (risk ratio (RR) 1.67, 95% CI 0.41 to 6.73). The study did not report neurodevelopmental disability and death or disability at two to three years of age. There were no significant differences between groups in condition at birth (Apgar scores or intubation in the delivery room), use of inotropic agents (RR 1.25, CI 0.63 to 2.49), and receipt of blood transfusion (RR 1.03, 95% CI 0.70 to 1.54). In addition, there were no significant differences in the incidences of any intraventricular haemorrhage (RR 1.50, 95% CI 0.65 to 3.46) and severe intraventricular haemorrhage (RR 1.33, 95% CI 0.31 to 5.75). Several continuous variables were reported in subgroups depending on method of delivery. Unpublished data for each group as a whole was made available and showed peak haematocrit in the first 24 hours and duration of phototherapy did not differ significantly. Overall, the quality of evidence for several key neonatal outcomes (e.g. mortality and intraventricular haemorrhage) was low because of lack of precision with wide CIs.
Authors' conclusions
The results from one study with wide CIs for magnitude of effect do not provide evidence either for or against the use of respiratory support before clamping the umbilical cord. A greater body of evidence is required as many of the outcomes of interest to the review occurred infrequently. Similarly, the one included study cannot answer the question of whether the intervention is or is not harmful.
PICOs
Ringkasan bahasa mudah
Menyediakan sokongan pernafasan sebelum mengapit tali pusat pada bayi pramatang
Soalan ulasan: Adakah bayi pramatang mendapat faedah daripada sokongan pernafasan sebelum kord dikapit?
Latar Belakang: membenarkan bayi pramatang menerima darah dari plasenta selepas kelahiran dan sebelum pengapitan tali pusat mempunyai faedah kesihatan untuk bayi dan tidak berbahaya bagi ibu. Kebanyakan bayi akan mula bernafas atau menangis (atau kedua‐duanya) sebelum kord dikapit. Walau bagaimanapun, sesetengah bayi tidak mempunyai pernafasan teratur pada masa ini. Selepas pengapitan kord, kebanyakkan bayi pramatang diberikan beberapa bentuk sokongan pernafasan seperti tekanan saluran udara positif berterusan (CPAP). CPAP menggunakan tekanan udara rendah yang berterusan untuk memastikan saluran pernafasan terbuka pada bayi yang boleh bernafas sendiri. Persoalan untuk ulasan ini adalah sama ada ia bermanfaat untuk memulakan sokongan pernafasan sebelum kord dikapit.
Ciri‐ciri kajian: Pengulas mencari pangkalan data perubatan dan menemui satu kajian untuk dimasukkan dalam ulasan ini. Bayi pramatang yang dilahirkan sebelum 32 minggu kehamilan (32 minggu dari hari pertama tempoh haid terakhir wanita hingga tarikh semasa) yang mengalami pengapitan tali pusat tertunda selama 60 saat selepas kelahiran dipilih secara rawak untuk memasuki sekumpulan bayi yang mendapat sokongan pernafasan dan sekumpulan bayi yang tidak mendapat sokongan pernafasan. Sokongan pernafasan diberikan selepas kelahiran bayi dan sebelum kord itu dikapit. Sokongan pernafasan merupakan CPAP untuk bayi yang bernafas sendiri atau menerapkan tekanan saluran udara yang terputus‐putus untuk mengembangkan paru‐paru pada bayi yang tidak bernafas dengan baik. Kebanyakan bayi yang dikaji (83%) dilahirkan melalui pembedahan caesarean.
Keputusan utama: kajian tunggal yang dimasukkan dalam ulasan tidak memberikan bukti yang mencukupi sama ada menyokong atau tidak penggunaan sokongan pernafasan sebelum pengapitan kord.
Kualiti bukti: Kualiti bukti adalah rendah, terutamanya kerana lebih banyak bayi perlu dikaji untuk kesimpulan yang pasti.
Authors' conclusions
Summary of findings
| Respiratory support compared with no respiratory support before cord clamping in preterm infants | ||||
| Patient or population: preterm infants Settings: undergoing delayed cord clamping Intervention: respiratory support Comparison: no respiratory support | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Mortality ≤2 years after hospital discharge | RR 1.67 (0.41 to 6.73) | 150 | ⊕⊕⊝⊝ | Secondary study outcome. |
| Inotropic support for hypotension | RR 1.25 (0.63 to 2.49) | 150 | ⊕⊕⊝⊝ | ‐ |
| Peak haematocrit | MD 0.20 (‐1.85 to 2.25) | 150 | ⊕⊕⊕⊝ | Primary outcome. |
| Blood transfusion during neonatal admission | RR 1.03 (0.70 to 1.54) | 150 | ⊕⊕⊕⊝ | 40% with this outcome |
| Phototherapy for hyperbilirubinaemia | MD 0.20 (‐0.31 to 0.71) | 150 | ⊕⊕⊕⊝ | From unpublished data |
| Intraventricular haemorrhage (of any grade) | RR 1.50 (0.65 to 3.46) | 150 | ⊕⊕⊝⊝ | Secondary outcome. |
| Severe intraventricular haemorrhage grade 3 or 4 | RR 1.33 (0.31 to 5.75) | 150 | ⊕⊝⊝⊝ | Uncommon secondary outcome. |
| CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence | ||||
| In the published report, results were presented according to method of delivery. For categorical variables (e.g. mortality), the RR was calculated for the delayed cord clamping with ventilation support (intervention) and delayed cord clamping (control) groups as a whole. For continuous variables (e.g. haematocrit), the authors provided unpublished data that enabled whole group statistics to be determined. 1Downgraded one level due to lack of precision with wide confidence intervals. 2Downgraded two levels due to lack of precision (confidence intervals included both important benefit and harm). The optimal information size for a 30% risk reduction with 80% power and 95% confidence intervals was 3280 infants per group. 3Unpublished data. | ||||
Background
Facilitating placental transfusion before cord clamping has potential benefits for term infants (McDonald 2013), and is increasingly recommended for preterm infants (Perlman 2010; Rabe 2012; McAdams 2014). Reported benefits for preterm infants include reduction in the number of blood transfusions; decreased incidence of intraventricular haemorrhage (IVH) (of all grades); reduced requirement for inotrope support; and improved important neonatal outcomes such as necrotizing enterocolitis and late‐onset sepsis (Rabe 2000; Mercer 2006; Hosono 2008; Kakkilaya 2008; Oh 2011; Rabe 2012).
Different methods are employed to facilitate placental transfusion, including delayed cord clamping (DCC), cord milking, and cord stripping after the cord has been clamped (Hosono 2008; Rabe 2012; March 2013). To date, few direct comparisons between DCC and cord milking have taken place (Rabe 2012; Katheria 2015). Although milking and stripping can be carried out quickly, recommendations for the timing of DCC vary from 30 seconds to one minute or longer for preterm infants (Raju 2013; ACOG 2012). It may be relevant to define early or late clamping according to whether or not respiration is established (Redmond 1965). A limited number of studies has indicated that less blood is retained in the placenta and haematocrit is increased in infants undergoing DCC when breathing is established (Redmond 1965; Kjeldsen 1967; Philip 1973). One study reported placental transfusion in relation to the onset of respiration in small numbers of preterm and low birth weight infants (Kjeldsen 1967). Less blood was retained within the placenta (and by inference, greater placental transfusion occurred) when respiration was established before cord clamping, and placental transfusion appeared to increase over time after birth. This latter finding was thought to reflect that preterm infants took longer to establish regular breathing than term infants.
Description of the condition
Preterm infants show variable respiratory effort while DCC is occurring (Kjeldsen 1967; Nevill 2015). Animal work indicates that positive pressure ventilation is beneficial for anaesthetized preterm lambs before DCC and helps reduce fluctuations in cerebral blood flow (Bhatt 2013). Likewise, observational studies in term and preterm infants confirm better outcomes in infants who establish spontaneous respiration before clamping (Ersdal 2014; Nevill 2015). Preterm infants who were not breathing during DCC had an increased incidence of chronic lung disease and IVH. It is unclear whether preterm infants who were not breathing had more severe respiratory distress or were compromised by lack of placental transfusion. It is also unclear to what extent providing artificial breaths will allow lung expansion to occur before clamping. During DCC, it is thought that placental perfusion remains intact to provide for the metabolic needs of the infant. At the same time, the placenta provides a reservoir of blood to fill the pulmonary vascular bed as the lungs are inflated. As this happens, a rapid drop in pulmonary vascular resistance occurs (Dawes 1953). Venous return from the lungs enters the left atrium and maintains left atrial filling, providing preload (i.e. increased end‐diastolic volume) for left ventricular output and subsequent cerebral perfusion. Ductal shunting rapidly reverses from the in utero pattern of right to left to the dominant postnatal pattern of left to right (Bhatt 2013). This allows some of the left ventricuslar output to enter the low‐resistance pulmonary circulation, thus providing a low‐resistance pathway and reduced left ventricular afterload (i.e. a lower end load against which the left ventricle contracts) during this transitional phase. In addition, the right ventricle pumps into the pulmonary circulation when pulmonary vascular resistance has dropped with lung expansion. Thus, both right and left ventricular afterloads are reduced (Hooper 2015). The net effect of all of this includes increased blood volume and more stable perfusion, especially to the brain (Aladangady 2006; Baenziger 2007; Meyer 2012; Sommers 2012; Bhatt 2013). This is important, as the preterm brain has limited autoregulation. Periods of decreased cerebral perfusion may be followed by reperfusion, and this can result in germinal matrix haemorrhage (Kluckow 2000; Soul 2007; Noori 2014). Facilitating physiological changes during the transition could explain improved outcomes with DCC. In contrast, if respiration is not established and the lungs are not inflated, it follows that DCC may provide less benefit, although evidence shows that placental transfusion still occurs (Redmond 1965; Philip 1977). This raises the question of whether providing respiratory support before clamping could be beneficial and could support physiological changes. Conceivably, breathing assistance could be given to all preterm infants undergoing DCC or only to those who fail to establish respiration. The simplest methods of support would include positive pressure ventilation and continuous positive airway pressure (CPAP) via face mask or nasal prong. More invasive strategies, such as use of a laryngeal mask or endotracheal intubation and even surfactant, are conceivable but would require major deviations from current practice. For this review, our aim was to determine whether providing some form of respiratory support during DCC is beneficial for preterm infants.
Description of the intervention
The intervention was respiratory support provided for preterm infants while DCC was taking place. Types of respiratory assistance were CPAP via face mask or nasal prong and positive pressure ventilation via face mask, as well as more invasive strategies such as endotracheal intubation and use of a laryngeal mask. Tactile stimulation was not considered as respiratory support.
To provide a suitable firm surface for respiratory support, various techniques have been described (Winter 2017; Duley 2018). In some instances, a trolley is placed alongside the mother. This may be a purpose built trolley with resuscitation equipment attached (Katheria 2016), or a trolley with the required equipment (e.g. oxygen blender and T‐piece resuscitator) attached to a pole or pendant (Winter 2017; ABC study (ACTRN12615001026516); te Pas 2016 (NTR6095); VentFirst (NCT02742454)). At caesarean section, a heated gel mattress placed on the maternal thighs has also been found suitable (Katheria 2016). A resuscitaire alongside the mother's bed for vaginal births has also been described (Duley 2018). Temperature maintenance is aided using a plastic wrap, heated gel mattress, or radiant warmer.
Techniques for facilitating placental transfusion have been described in detail elsewhere (Rabe 2012; Al‐Wassia 2015). Cord milking or stripping studies have described variability in terms of speed of milking, the number of times the milking was performed, the length of cord milked, and whether the cord was milked before or after it was clamped (Rabe 2012; Al‐Wassia 2015). Cord stripping was regarded as milking after the cord was clamped.
How the intervention might work
Facilitating respiration during DCC could increase placental transfusion and stabilize the transitional circulation by providing increased preload to both ventricles and reducing afterload. This in turn may result in less fluctuation in cerebral blood flow and decreased likelihood of IVH.
Why it is important to do this review
There are important health benefits for infants undergoing DCC. Although it appears that most infants breathe during the procedure, infants who do not may be subject to worse outcomes. Facilitating a stable transition by providing breathing support or resuscitation could result in reduced IVH, and improved neurodevelopmental and other neonatal outcomes. As noted in the review by Rabe (Rabe 2012), one rationale for early clamping is that it allows respiratory support to commence, but few data are available on respiratory outcomes and the onset of respiration, particularly in preterm infants (Kjeldsen 1967). This highlights the need for additional studies in this area.
Objectives
To assess the efficacy and safety of respiratory support provided during delayed cord clamping (DCC) compared with no respiratory support during placental transfusion (in the form of DCC, milking, or stripping) in preterm infants immediately after delivery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCT), cluster‐randomized, and quasi‐randomized studies and applied no language or publication status restrictions. The review included only studies in which DCC or placental transfusion was a planned intervention for all participants (i.e. did not include studies in which the control group did not receive intervention to facilitate placental transfusion).
Types of participants
Preterm infants (less than 37 weeks' gestation) undergoing placental transfusion (i.e. DCC, cord milking, or cord stripping) when the treatment group received placental transfusion (i.e. DCC) with ventilation support (V‐DCC) and the control group received placental transfusion (i.e. DCC, cord milking, or cord stripping) without ventilation support (DCC).
Types of interventions
Breathing support included CPAP via face mask, nasal prongs, or nasopharyngeal tube, and positive pressure ventilation provided by face mask, endotracheal tube, or laryngeal mask. Respiratory support was to be provided before cord clamping. Potential comparison groups included infants receiving DCC without support, and infants undergoing cord milking or stripping without support. The intervention could be applied to all preterm infants or only to infants deemed to be not breathing or in need of resuscitation. The intervention above was to be reflected in subgroup analyses, which included types of support provided, whether support was applied to all infants, or only infants not breathing.
Types of outcome measures
Primary outcomes
-
Mortality up to two years after hospital discharge ‐ to be reported as death before 28 days, death before hospital discharge, or death after discharge.
-
Neurodevelopmental disability (mild, moderate, or severe) at age 18 to 24 months (Doyle 2010).
-
Death or neurodevelopmental disability at two to three years of age (Doyle 2010).
Secondary outcomes
-
Condition at birth.
-
-
Apgar score at one, five, and 10 minutes.
-
Cord gas base deficit greater than 10 mmol/L.
-
Need for intubation in delivery room or within first hour of life.
-
Cardiac compression in delivery room.
-
-
Cardiovascular measures and measures of transitional circulation.
-
-
Volume administration in the form of blood products or crystalloid for hypotension (as defined by research authors) during first 24 hours of life, recorded as number of boluses and as total volume administered.
-
Inotropic support for hypotension (mean arterial pressure below gestation in weeks or as defined by research authors) during first 24 hours of life.
-
Superior vena cava (SVC) blood flow (mL/kg/minute) in the first 24 hours of life (Kluckow 2000).
-
Cerebral near‐infrared spectroscopy (NIRS) (total oxygenation index, fractional total oxygen extraction (FTOE) during first 24 hours of life) (van Bel 2008).
-
Urine output (mL/kg/hour) during first 48 hours of life.
-
Metabolic acidosis (base deficit greater than 10 mmol/L) during first 48 hours of life.
-
Poor systemic blood flow during first 24 hours. This included any of the following: volume administration, therapy for low blood pressure, low SVC flow (Groves 2008), abnormal NIRS (van Bel 2008), or decreased urine output (less than 1 mL/kg/hour).
-
-
Haematological outcomes.
-
-
Haemoglobin (g/L) or haematocrit at birth and within 24 hours of birth.
-
Blood transfusion during neonatal admission (number of transfusions and volume transfused).
-
Treatment (phototherapy or other) for hyperbilirubinaemia.
-
-
Respiratory outcomes.
-
-
Use of surfactant in the first 48 hours of life.
-
Days of oxygen therapy.
-
CPAP days.
-
Ventilation days.
-
Oxygen requirement at 28 days.
-
Bronchopulmonary dysplasia as defined by respiratory support or oxygen requirement at 36 weeks' corrected gestational age.
-
Bronchopulmonary dysplasia at 36 weeks' postmenstrual age according to a physiological definition (Walsh 2004).
-
-
Other neonatal outcomes.
-
-
IVH (of any grade) (Papile 1978).
-
Severe IVH grade 3 or 4 (Papile 1978).
-
Periventricular leukomalacia.
-
Necrotizing enterocolitis Bell's stage 2 or greater (Bell 1978).
-
Retinopathy all stages and stage 3 and greater.
-
Sepsis (early‐ and late‐onset more than 48 hours): isolation of an organism from at least one blood or cerebrospinal fluid (CSF) culture with the decision to treat with antibiotics with therapeutic intent against this organism.
-
Pharmacological treatment or surgical ligation for patent ductus arteriosus.
-
Length of hospital stay (days).
-
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal search strategy for specialized register).
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 5) in the Cochrane Library; MEDLINE via PubMed (1966 to 19 June 2017); Embase (1980 to 19 June 2017); and CINAHL (1982 to 19 June 2017) using the following search terms: ((umbilicus OR umbilical OR cord OR navel‐string) AND (clamp OR cut OR ligation OR ligature OR delay* OR defer* OR time OR intact OR late OR early OR immediate) AND (support OR breathing OR respiration OR resuscitation OR continuous positive airway pressure OR CPAP OR positive pressure ventilation OR ventilate*), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We applied no language restrictions.
Searching other resources
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization's International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry).
Data collection and analysis
Selection of studies
All three review authors working independently reviewed search results and each search result was independently reviewed by at least two of the authors. We examined title and abstract in the first instance, then full text if appropriate. We kept a record of the number of studies screened at each step. Disagreements were resolved by consensus and, if necessary, through contact with study authors. Should this fail, the third review author was to make the decision. If a final arbiter was needed, we planned to refer the matter to the Cochrane Neonatal Review Group.
We collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram.
Data extraction and management
Two review authors working independently extracted data from included studies. One review author (MM) extracted the data, and the other (MW) checked the entries. We collected participant demographics (including gestation) and types of interventions (such as mode of respiratory support). We detailed comparison groups (such as DCC without support) and outcomes (in line with the proposed Primary outcomes; and Secondary outcomes) and noted study design. Any disagreements about data extraction were to be resolved by reaching consensus, seeking additional details from study authors, deferring to the opinion of the third review author, and referring the matter to the Neonatal Review Group, in that order. In addition, we contacted the study author to request further data. We used study information to create a Characteristics of included studies table.
Assessment of risk of bias in included studies
Two review authors (MM and MW) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane 'Risk of bias' tool (Higgins 2011) for the following domains.
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
Any disagreements were resolved by discussion or by a third review author. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We used risk ratios (RR) and 95% confidence intervals (CI) for categorical data, and mean differences (MD) between groups for continuous data where provided. We aimed to calculate the number needed to treat for an additional beneficial or harmful outcome.
Unit of analysis issues
The nature of the intervention precluded randomization of infants to more than one group. We planned to include cluster‐randomized studies in this review. We aimed to provide repeat measures (e.g. for haematocrit or repeat SVC blood flow); however, we planned to restrict our review to results of these measures to 48 hours after birth (as noted in the Secondary outcomes).
Dealing with missing data
We planned to ignore data that appeared to be missing. For data deemed not missing at random, we aimed to assign a poor outcome if dichotomous, or the mean value imputed if continuous. We sought to detail any assumptions made and any sensitivity analyses carried out with and without missing data.
Assessment of heterogeneity
We aimed to report heterogeneity in the following groups: less than 25%: none; 25% to 49%: low; 50% to 74%: moderate; and 75% and above: high.
Assessment of reporting biases
We searched for unpublished studies in trial registries. We aimed to prepare funnel plots (if we found at least 10 trials) and to perform Egger's test.
Data synthesis
We aimed to undertake meta‐analysis if studies were sufficiently similar in terms of participants, interventions, and outcome measures. If at least two studies were included in the meta‐analysis, we planned to calculate typical RRs and risk differences, as well as number needed to treat for an additional beneficial effect or an additional harmful effect. For continuous data, we sought to calculate MDs if studies used the same scales or standardized mean differences if studies used difference scales and include 95% CIs. We planned to undertake analysis of studies with high risk of bias omitted to see if review results were affected. We used fixed‐effect models and aimed to note the degree of heterogeneity (see Assessment of heterogeneity). When heterogeneity was high, we planned to undertake sensitivity analysis to identify sources of bias.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes.
-
Mortality (in hospital).
-
IVH (any grade).
-
Severe IVH grade 3 or 4.
-
Use of inotropic agents.
-
Blood transfusion.
-
Haematocrit.
-
Days of phototherapy.
Two review authors independently assessed the quality of the evidence for each outcome. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a 'Summary of findings' table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence into one of four grades:
-
high: we are very confident that the true effect lies close to that of the estimate of the effect;
-
moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different;
-
low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect;
-
very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
-
Type of respiratory support provided to infants undergoing DCC: CPAP (via face mask or nasal prongs or nasopharyngeal tube) and positive pressure ventilation (by face mask or laryngeal mask or endotracheal tube).
-
Gestational age: gestational age less than 28 weeks and gestational age 28 weeks or greater.
-
Infants undergoing DCC that were breathing versus infants undergoing DCC that were not breathing.
Sensitivity analysis
We planned to perform a meta‐analysis of relevant studies to examine the effect of including only certain available studies that met most or all eligibility criteria and omitting those studies that did not. We aimed to undertake a sensitivity analysis to examine risk of bias.
Results
Description of studies
Results of the search
We identified 1809 records through database searching and 39 additional records through other sources. After removing duplicates, 1560 records remained. We excluded 1552 records as obviously irrelevant on reading the titles or abstracts (or both), leaving 8 potentially eligible studies. After reading the full text, we excluded four studies, with reasons (Characteristics of excluded studies table), and identified 3 ongoing studies (Characteristics of ongoing studies). We included one study (Characteristics of included studies) (see Figure 1).
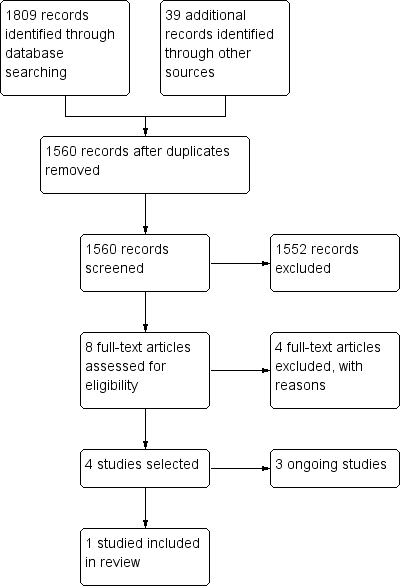
Study flow diagram.
Included studies
One study met the inclusion criteria (see Characteristics of included studies table) (Katheria 2016). This single‐centre RCT performed in San Diego, USA, studied preterm infants of less than 32 weeks' gestation delivered after caesarean or vaginal birth. All infants underwent 60 seconds of DCC while placed on a trolley that was at the level of the incision for caesarean delivery or below the level of the placenta after vaginal birth. Infants were randomized to a standard group (DCC group) or an intervention group (V‐DCC). Infants in the V‐DCC group received ventilation support in the form of face mask CPAP, or if not displaying regular chest movement, positive pressure ventilation via face mask with room air. Infants in the V‐DCC group had a carbon dioxide detection monitor attached to the circuit to assess the effectiveness of respiration better. Infants in the DCC group were dried and given gentle stimulation to help establish respiration. There were 75 infants in each group; 83% of study infants were delivered by caesarean section. The primary outcome was maximum haematocrit in the first 24 hours and neonatal outcomes including IVH, inhospital mortality, blood transfusion, and jaundice were reported. In addition, a variety of physiological measures of the transitional circulation were undertaken.
Excluded studies
We excluded four studies (see Characteristics of excluded studies table). The control group in the study of Aladangady 2006 was randomized to early cord clamping, as were the controls in the Cord pilot trial (Duley 2018). One of the studies was observational (Phillipos 2017), and the other was a pilot feasibility study without a randomized control group (Winter 2017).
Ongoing studies
There were three ongoing studies, the ABC study (ABC study (ACTRN12615001026516)), the VentFirst study (VentFirst (NCT02742454)), and a feasibility study using the Con‐Cord table (te Pas 2016 (NTR6095)) (see Characteristics of ongoing studies table).
Risk of bias in included studies
Overall risk of bias was low.
Allocation
Randomization sequence was computer generated. Allocation of intervention was concealed using numbered sealed envelopes (low risk of bias).
Blinding
Researchers making assessments and measurements were blinded to group allocation at infants' births, but clinicians involved during transition in the delivery room were not blinded to group allocation and may have been the same clinicians caring for the infants (high risk of bias).
Incomplete outcome data
All infants were accounted for (low risk of bias).
Selective reporting
There was no evidence of selective reporting (low risk of bias). Study outcomes were those reported in the protocol (NCT02231411; Katheria 2016).
Other potential sources of bias
There were no other potential sources of bias apparent.
Effects of interventions
One RCT met the inclusion criteria. Reported outcomes were mainly short‐term neonatal outcomes, including inhospital mortality, but not neurodevelopmental outcomes. Study results were reported according to the mode of delivery and most of the infants undergoing DCC were caesarean births. For categorical data, results for each group (i.e. V‐DCC or DCC) could be calculated from the published study. However, this could not be done for continuous data. Subsequently, the authors provided unpublished information, which enabled analysis of continuous data for the V‐DCC and DCC groups as a whole.
See summary of findings Table for the main comparison.
Primary outcomes
Mortality
There was no significant difference in mortality before hospital discharge between groups (5/75 in the V‐DCC group versus 3/75 in the DCC group; RR 1.67, 95% CI 0.41 to 6.73; Analysis 1.1; Figure 2). The study did not report death before 28 days or death after discharge.

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.1 Mortality during neonatal admission.
Neurodevelopmental disability
The study did not report neurodevelopmental disability.
Death or neurodevelopmental disability at two to three years of age
The study did not report death or neurodevelopmental disability at two to three years of age.
Secondary outcomes
Condition at birth
Apgar score at one, five, and 10 minutes
There were no significant differences in Apgar score between the V‐DCC and DC groups (unpublished data) and medians were 8 at five minutes in both groups.
The study did not report Apgar score at 10 minutes.
Cord blood base deficit greater than 10 mmol/L
Cord blood base deficit greater than 10 mmol/L was not reported.
Need for intubation in delivery room or within first hour of life
Intubation rates in the delivery room were not significantly different (27/75 in the V‐DCC group versus 33/75 in the DCC group; RR 0.82, 95% CI 0.55 to 1.21; Analysis 1.2; Figure 3).

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.2 Need for intubation in delivery room.
Cardiac compression in delivery room
No cases received cardiac compressions during resuscitation at birth (unpublished information).
Cardiovascular measures and measures of transitional circulation
Volume administration in the form of blood products or crystalloid for hypotension
Receipt of fluid bolus for volume administration was not significantly different between groups (20 cases in the V‐DCC group versus 21 in the DCC group; unpublished data).
Inotropic support for hypotension
Use of inotropic agents in the two groups was not significantly different (15/75 in the V‐DCC group and 12/75 in the DCC group; RR 1.25, 95% CI 0.63 to 2.49; Analysis 1.3; Figure 4).

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.3 Inotropic support for hypotension.
Superior vena cava blood flow
There was no significant difference between groups in SVC blood flow (reported as subgroups according to delivery method).
Cerebral near‐infrared spectroscopy
There was no significant difference between groups in cerebral NIRS readings (reported graphically).
Urine output
There was no significant difference between groups in urine output (reported as subgroups according to delivery method).
Metabolic acidosis
There was no significant difference in umbilical venous pH when the groups as a whole were analyzed (MD 0.02, 95% CI ‐0.05 to 0.01; unpublished data).
Poor systemic blood flow during first 24 hours
The study did not report poor systemic flow.
Haematological outcomes
Haemoglobin or haematocrit at birth and within 24 hours of birth
The study found no significant difference in peak haematocrit within 24 hours of birth (according to method of delivery) between groups. When analyzed for the V‐DCC group versus DCC group as a whole, the MD was 0.20 (95% CI ‐1.85 to 2.25; unpublished data; Analysis 1.4; Figure 5).
![Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.4 Peak haematocrit in first 24 hours [%].](/cdsr/doi/10.1002/14651858.CD012491.pub2/media/CDSR/CD012491/image_n/nCD012491-AFig-FIG05.png)
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.4 Peak haematocrit in first 24 hours [%].
Blood transfusion during neonatal admission
In the V‐DCC group, 30 infants were transfused compared to 29 in the DCC group (RR 1.03, 95% CI 0.70 to 1.54; Analysis 1.5; Figure 6). The volume transfused was not specified. There was no significant difference in number of transfusions (MD 1.31, 95% CI ‐2.50 to 5.12; unpublished).

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.5 Blood transfusion during neonatal admission.
Treatment (phototherapy or other) for hyperbilirubinaemia
There was no significant difference in duration of phototherapy for hyperbilirubinaemia. Among caesarean deliveries, the mean duration was 4.1 days in the V‐DCC group and 3.8 days in the DCC group (P = 0.32). When the unpublished data were reviewed, the MD was 0.20 (95% CI ‐0.31 to 0.71; Analysis 1.6; Figure 7). Likewise, there was no significant difference in peak bilirubin levels (reported as subgroups according to method of delivery with 125 patients in the cesarean section group).
![Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.6 Phototherapy for hyperbilirubinaemia [days].](/cdsr/doi/10.1002/14651858.CD012491.pub2/media/CDSR/CD012491/image_n/nCD012491-AFig-FIG07.png)
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.6 Phototherapy for hyperbilirubinaemia [days].
Respiratory outcomes
Use of surfactant in the first 48 hours of life
There were 24/75 infants in the V‐DCC group and 29/75 in the DCC group who received surfactant in the first 48 hours of life after leaving the delivery room (RR 0.83, 95% CI 0.54 to 1.28; Analysis 1.7; Figure 8).
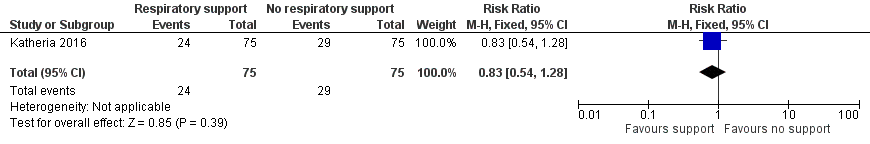
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.7 Use of surfactant in the first 48 hours of life.
Days of oxygen therapy
There was no difference in the number of days of oxygen therapy received between groups (MD 2.80 days, 95% CI ‐8.20 to 13.81; unpublished data).
Continuous positive airway pressure days
The study did not report the number of CPAP days.
Ventilation days
There was no significant difference in the median days of mechanical ventilation between groups (P = 0.99 as reported for caesarean deliveries) or for the groups as a whole (unpublished data).
Oxygen requirement at 28 days
The study did not report oxygen requirement at 28 days.
Bronchopulmonary dysplasia as defined by respiratory support or oxygen requirement at 36 weeks' corrected gestational age (Outcome 1.8)
The number of cases with an oxygen requirement at 28 days was not reported, while there were 11 in the V‐DCC group and 10 in the DCC group with an oxygen requirement at 36 weeks' corrected gestational age (RR 1.10, 95% CI 0.50 to 2.43; Analysis 1.8; Figure 9).
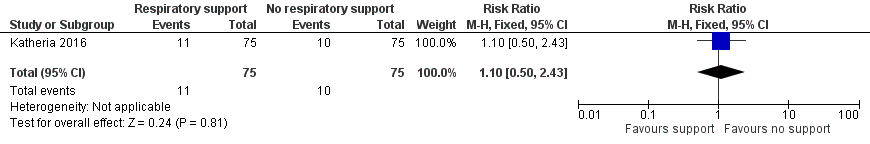
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.8 Bronchopulmonary dysplasia.
Bronchopulmonary dysplasia at 36 weeks' postmenstrual age according to a physiological definition
The study did not report bronchopulmonary dysplasia at 36 weeks' postmenstrual age according to a physiological definition (Walsh 2004).
Other neonatal outcomes
Intraventricular haemorrhage (of any grade)
There was IVH (of any grade) in 12 of the infants in the V‐DCC group and eight in the DCC group (RR 1.50, 95% CI 0.65 to 3.46; Analysis 1.9; Figure 10).
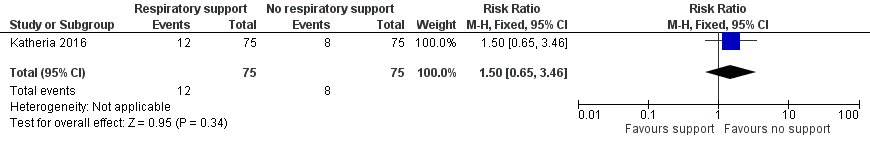
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.9 Intraventricular haemorrhage (of any grade).
Severe intraventricular haemorrhage grade 3 or 4
There was severe IVH grade 3 or 4 in four infants in the V‐DCC group and three in the DCC group (RR 1.33, 95% CI 0.31 to 5.75; Analysis 1.10; Figure 11).

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.10 Severe intraventricular haemorrhage grade 3 or 4.
Periventricular leukomalacia
There was periventricular leukomalacia present in one infant in the V‐DCC group and no infants in the DCC group (RR 3.00, 95% CI 0.12 to 72.49; Analysis 1.11).
Necrotizing enterocolitis Bell's stage 2 or greater
There was necrotizing enterocolitis (Bell's stage 2 or greater) in two infants in the V‐DCC group and one infant in the DCC group (RR 2.00, 95% CI 0.19 to 21.59; Analysis 1.12).
Retinopathy all stages and stage 3 and greater
There was retinopathy of prematurity requiring treatment reported in four infants in the V‐DCC group and two infants in the DCC group (RR 2.00, 95% CI 0.38 to 10.59; Analysis 1.13).
Sepsis
There was culture‐positive sepsis reported in five infants in the V‐DCC group and three infants in the DCC group (RR 1.67, 95% CI 0.41 to 6.73; Analysis 1.14).
Pharmacological treatment or surgical ligation for patent ductus arteriosus
Fourteen infants in the V‐DCC group and 19 in the DCC group were treated for patent ductus arteriosus (RR 0.74, 95% CI 0.40 to 1.36; Analysis 1.15).
Four infants in each group had duct ligation performed (RR 1.0, 95% CI 0.26 to 3.85; Analysis 1.16).
Length of hospital stay
The study did not report length of hospital stay but length of neonatal stay from the unpublished data was similar in the V‐DCC versus DCC groups (MD 0.05, 95% CI ‐12.02 to 12.13).
Over 90% of infants established respiration by the time of cord clamping. In a subgroup analysis of infants who did not establish respiration during DCC, whether vaginal or caesarean births, mortality, and severe IVH rates were not significantly different from infants who did establish breathing.
Discussion
Summary of main results
Our search identified one trial where preterm infants undergoing DCC were randomized to a group receiving ventilation support before clamping (V‐DCC group) compared to a group not receiving such support (DCC group) (see summary of findings Table for the main comparison) (Katheria 2016).
There was no significant difference in mortality during neonatal admission between the groups. Likewise, there were no statistically significant differences in condition at birth, cardiovascular outcomes, haematological parameters (including bilirubin levels), respiratory outcomes, and other neonatal outcomes including IVH between the groups.
Overall completeness and applicability of evidence
The included study was the first RCT to provide respiratory support during delayed cord clamping (Katheria 2016). As such it provided preliminary evidence in relation to the study question as to whether providing respiratory support to preterm infants before cord clamping is beneficial. The study reported inhospital mortality but not neurodevelopmental outcomes. The study was not powered to detect differences in mortality and the wide CIs indicated lack of precision and included both potential benefit and potential harm. Therefore, we could draw no conclusions. Similar limitations applied to the interpretation of IVH rates and other neonatal outcomes. However, the study did indicate that the intervention was feasible and was carried out in a high percentage (95%) of eligible infants.
It is of interest that over 90% of study infants established respiration during the period of DCC and before cord clamping. This means that there was little separation between groups with regard to the effectiveness or otherwise of the intervention in promoting breathing movements during DCC. Many of the theoretical benefits of 'resuscitation with an intact cord' would be expected to occur in those infants who were not breathing on their own but commenced breathing after being given respiratory support. As there were few infants of this type, it remains to be seen if true benefits or harms exist with this approach and definite conclusions cannot be made.
We found no evidence of publication bias, as we found no unpublished studies.
Quality of the evidence
Although the study had an overall low risk of bias, the quality of the evidence for several of the key outcomes for the review was low to very low (GRADE) (Katheria 2016). This is because the study was underpowered to detect these outcomes. This means that, for important but relatively uncommon outcomes such as mortality and severe IVH, the estimates of effect lack precision with wide CIs encompassing the potential both for significant harm and significant benefit or indeed for no effect at all. For other outcomes such as blood transfusion and haematocrit, the quality of evidence was moderate (GRADE) and CIs were narrower. Of note, of the 150 infants in the study, approximately 10% were not displaying evidence of breathing and these were the infants most likely to be in need of respiratory support.
Potential biases in the review process
We found no apparent biases.
Agreements and disagreements with other studies or reviews
We found no other systematic reviews on this topic.

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.1 Mortality during neonatal admission.

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.2 Need for intubation in delivery room.

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.3 Inotropic support for hypotension.
![Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.4 Peak haematocrit in first 24 hours [%].](/cdsr/doi/10.1002/14651858.CD012491.pub2/media/CDSR/CD012491/image_n/nCD012491-AFig-FIG05.png)
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.4 Peak haematocrit in first 24 hours [%].

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.5 Blood transfusion during neonatal admission.
![Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.6 Phototherapy for hyperbilirubinaemia [days].](/cdsr/doi/10.1002/14651858.CD012491.pub2/media/CDSR/CD012491/image_n/nCD012491-AFig-FIG07.png)
Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.6 Phototherapy for hyperbilirubinaemia [days].

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.7 Use of surfactant in the first 48 hours of life.

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.8 Bronchopulmonary dysplasia.

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.9 Intraventricular haemorrhage (of any grade).

Forest plot of comparison: 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, outcome: 1.10 Severe intraventricular haemorrhage grade 3 or 4.
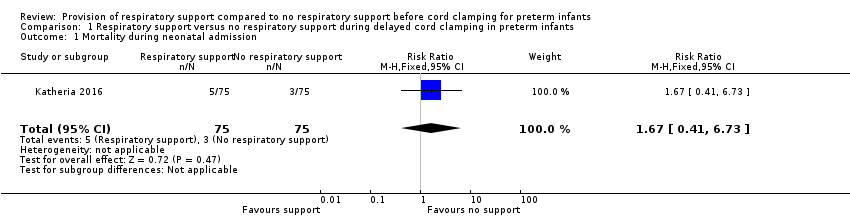
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 1 Mortality during neonatal admission.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 2 Need for intubation in delivery room.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 3 Inotropic support for hypotension.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 4 Peak haematocrit in first 24 hours.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 5 Blood transfusion during neonatal admission.
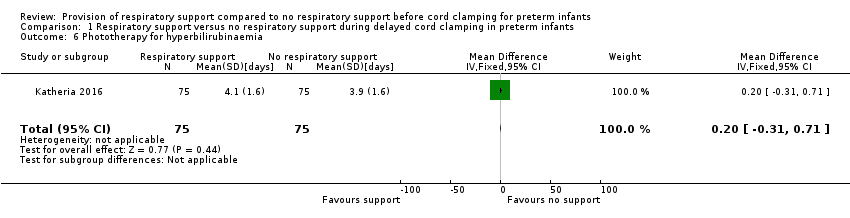
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 6 Phototherapy for hyperbilirubinaemia.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 7 Use of surfactant in the first 48 hours of life.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 8 Bronchopulmonary dysplasia.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 9 Intraventricular haemorrhage (of any grade).

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 10 Severe intraventricular haemorrhage grade 3 or 4.
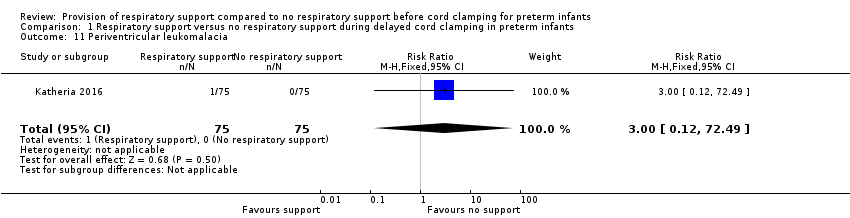
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 11 Periventricular leukomalacia.

Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 12 Necrotizing enterocolitis ≥ Bell's stage 2.
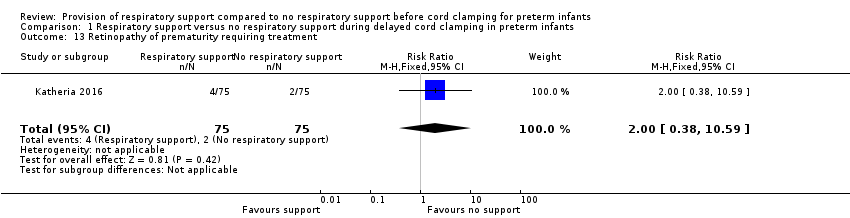
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 13 Retinopathy of prematurity requiring treatment.
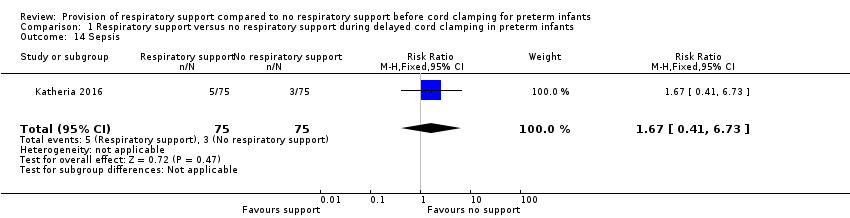
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 14 Sepsis.
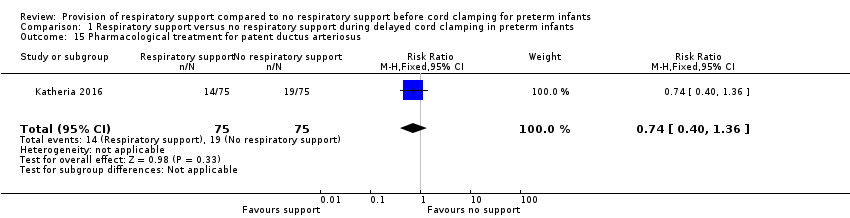
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 15 Pharmacological treatment for patent ductus arteriosus.
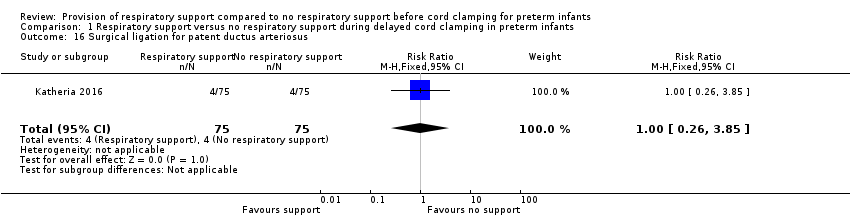
Comparison 1 Respiratory support versus no respiratory support during delayed cord clamping in preterm infants, Outcome 16 Surgical ligation for patent ductus arteriosus.
| Respiratory support compared with no respiratory support before cord clamping in preterm infants | ||||
| Patient or population: preterm infants Settings: undergoing delayed cord clamping Intervention: respiratory support Comparison: no respiratory support | ||||
| Outcomes | Relative effect | No of participants | Quality of the evidence | Comments |
| Mortality ≤2 years after hospital discharge | RR 1.67 (0.41 to 6.73) | 150 | ⊕⊕⊝⊝ | Secondary study outcome. |
| Inotropic support for hypotension | RR 1.25 (0.63 to 2.49) | 150 | ⊕⊕⊝⊝ | ‐ |
| Peak haematocrit | MD 0.20 (‐1.85 to 2.25) | 150 | ⊕⊕⊕⊝ | Primary outcome. |
| Blood transfusion during neonatal admission | RR 1.03 (0.70 to 1.54) | 150 | ⊕⊕⊕⊝ | 40% with this outcome |
| Phototherapy for hyperbilirubinaemia | MD 0.20 (‐0.31 to 0.71) | 150 | ⊕⊕⊕⊝ | From unpublished data |
| Intraventricular haemorrhage (of any grade) | RR 1.50 (0.65 to 3.46) | 150 | ⊕⊕⊝⊝ | Secondary outcome. |
| Severe intraventricular haemorrhage grade 3 or 4 | RR 1.33 (0.31 to 5.75) | 150 | ⊕⊝⊝⊝ | Uncommon secondary outcome. |
| CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence | ||||
| In the published report, results were presented according to method of delivery. For categorical variables (e.g. mortality), the RR was calculated for the delayed cord clamping with ventilation support (intervention) and delayed cord clamping (control) groups as a whole. For continuous variables (e.g. haematocrit), the authors provided unpublished data that enabled whole group statistics to be determined. 1Downgraded one level due to lack of precision with wide confidence intervals. 2Downgraded two levels due to lack of precision (confidence intervals included both important benefit and harm). The optimal information size for a 30% risk reduction with 80% power and 95% confidence intervals was 3280 infants per group. 3Unpublished data. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality during neonatal admission Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.73] |
| 2 Need for intubation in delivery room Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.21] |
| 3 Inotropic support for hypotension Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.63, 2.49] |
| 4 Peak haematocrit in first 24 hours Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.85, 2.25] |
| 5 Blood transfusion during neonatal admission Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.54] |
| 6 Phototherapy for hyperbilirubinaemia Show forest plot | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.31, 0.71] |
| 7 Use of surfactant in the first 48 hours of life Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.54, 1.28] |
| 8 Bronchopulmonary dysplasia Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.50, 2.43] |
| 9 Intraventricular haemorrhage (of any grade) Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.65, 3.46] |
| 10 Severe intraventricular haemorrhage grade 3 or 4 Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.75] |
| 11 Periventricular leukomalacia Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.49] |
| 12 Necrotizing enterocolitis ≥ Bell's stage 2 Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.59] |
| 13 Retinopathy of prematurity requiring treatment Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.59] |
| 14 Sepsis Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.41, 6.73] |
| 15 Pharmacological treatment for patent ductus arteriosus Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.40, 1.36] |
| 16 Surgical ligation for patent ductus arteriosus Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.85] |

