Cirugía laparoscópica para la reparación electiva del aneurisma aórtico abdominal
Resumen
Antecedentes
El aneurisma aórtico abdominal (AAA) es una dilatación anormal de la aorta infradiafragmática igual o mayor que 30 mm, o una dilatación local igual o mayor que un 50% en comparación con el diámetro normal esperado de la arteria. Los AAA ocurren con poca frecuencia en individuos menores de 50 años de edad, aunque posteriormente la prevalencia aumenta de manera extraordinaria con la edad, y se observa un riesgo seis veces mayor de desarrollo de un AAA en los hombres que en las mujeres. Se ha informado que la prevalencia del AAA varía de un 1,3% en las mujeres de 65 a 80 años de edad a entre un 4% y un 7,7% en los hombres de 65 a 80 años de edad.
Hay evidencia de que el riesgo de rotura aumenta a medida que aumenta el diámetro del aneurisma de 50 mm a 60 mm. Por lo tanto, los pacientes con un AAA mayor que 55 mm de diámetro por lo general son derivados para la consideración de la reparación, debido a que el riesgo de rotura excede el riesgo de la reparación. El tratamiento tradicional para el AAA es la reparación quirúrgica abierta (RQA) que incluye una incisión abdominal grande y se asocia con un riesgo significativo de complicaciones. Recientemente, dos procedimientos menos invasivos han comenzado a usarse más ampliamente: la reparación endovascular del aneurisma (REVA) y la reparación laparoscópica. La REVA se realiza a través de vainas insertadas en la arteria femoral en la ingle: posteriormente, se coloca un injerto de stent dentro del saco del aneurisma bajo guía de imágenes radiológicas y se implanta para formar un canal nuevo para el flujo sanguíneo. La reparación laparoscópica incluye el uso de un laparoscopio que se inserta a través de cortes pequeños en el abdomen y el injerto sintético se cose en el lugar para reemplazar el área debilitada de la aorta. La reparación laparoscópica del AAA se clasifica en dos categorías: cirugía laparoscópica asistida manualmente (CLAM), en la que se realiza una incisión para permitir que la mano del cirujano ayude en la reparación; y cirugía laparoscópica total (CLT). Tanto la REVA como la reparación laparoscópica son favorables sobre la RQA debido a que son mínimamente invasivas, son menos dolorosas, se asocian con menos complicaciones y una tasa de mortalidad inferior y tienen una duración más corta de la estancia hospitalaria.
Las pruebas actuales indican que la reparación laparoscópica electiva del AAA tiene un perfil de seguridad favorable equivalente al de la REVA, con tasas bajas de conversión así como tasas similares de mortalidad y morbilidad. Como resultado, se ha sugerido que la reparación laparoscópica electiva del AAA puede tener una función en el tratamiento de los pacientes para los que la REVA no es apropiada.
Objetivos
Evaluar los efectos de la cirugía laparoscópica para la reparación electiva del aneurisma aórtico abdominal.
El objetivo primario de esta revisión era evaluar la mortalidad perioperatoria y la duración de la cirugía de la reparación quirúrgica laparoscópica (total y asistida manualmente) de los aneurismas aórticos abdominales (AAA) en comparación con la reparación quirúrgica abierta tradicional o la REVA. El objetivo secundario era evaluar las tasas de complicación, la mortalidad por todas las causas (> 30 días), la duración de la estancia hospitalaria y en la unidad de cuidados intensivos (UCI), las tasas de conversión y de nueva intervención y la calidad de vida asociada con la reparación quirúrgica laparoscópica (total y asistida manualmente) en comparación con la reparación quirúrgica abierta tradicional o la REVA.
Métodos de búsqueda
El especialista en información del Grupo Cochrane Vascular (Cochrane Vascular Group) buscó en el registro especializado (última búsqueda agosto 2016) y en CENTRAL (2016, número 7). Además, el especialista buscó los detalles de estudios en curso y no publicados en los registros de ensayos. Se hicieron búsquedas en las listas de referencias de los artículos relevantes recuperados mediante las búsquedas electrónicas para obtener citas adicionales.
Criterios de selección
Ensayos controlados aleatorios y ensayos clínicos controlados en los cuales los pacientes con un AAA fueron sometidos a la reparación laparoscópica electiva (reparación laparoscópica total o reparación laparoscópica asistida manualmente) en comparación con la reparación quirúrgica abierta o la REVA.
Obtención y análisis de los datos
Al menos dos autores de la revisión evaluaron de forma independiente los estudios identificados para su posible inclusión.
Resultados principales
Se incluyó en la revisión un ensayo controlado aleatorio con un total de 100 participantes masculinos. El ensayo comparó la reparación laparoscópica asistida manualmente con la REVA y proporcionó resultados para la mortalidad hospitalaria, la duración de la cirugía, la duración de la estancia hospitalaria y la isquemia de los miembros inferiores. El estudio incluido no informó los otros resultados planificados previamente de esta revisión. No ocurrieron muertes hospitalarias en el estudio. La reparación laparoscópica asistida manualmente se asoció con una duración más larga de la cirugía (DM 53,00 minutos, IC del 95%: 36,49 a 69,51) que la REVA. La incidencia de isquemia del miembro inferior fue similar entre los dos grupos de tratamiento (cociente de riesgos [CR] 0,50; intervalo de confianza [IC] del 95%: 0,05 a 5,34). La duración media de la estancia hospitalaria fue de 4,2 días y 3,4 días en los grupos de reparación laparoscópica asistida manualmente y de REVA respectivamente aunque no se informaron las desviaciones estándar y por lo tanto no fue posible analizar de forma independiente la significación estadística de este resultado. La calidad de las pruebas se disminuyó debido a la imprecisión causada por la inclusión de un estudio pequeño; y a los intervalos de confianza amplios y la imposibilidad para generalizar la evidencia debido a que el estudio incluyó solo participantes masculinos. Ningún estudio comparó la reparación laparoscópica (total o asistida manualmente) con la reparación quirúrgica abierta ni la reparación quirúrgica laparoscópica total con la REVA.
Conclusiones de los autores
Hay evidencia insuficiente para establecer cualquier conclusión acerca de la efectividad y la seguridad de la reparación quirúrgica laparoscópica (total y asistida manualmente) del AAA versus reparación quirúrgica abierta o REVA, debido a que sólo un ensayo aleatorio pequeño reunió los requisitos para la inclusión en esta revisión. Se necesitan ensayos controlados aleatorios de alta calidad.
PICOs
Resumen en términos sencillos
Cirugía laparoscópica para el aneurisma aórtico abdominal
Antecedentes
Un aneurisma aórtico abdominal (AAA) es un ensanchamiento anormal de la aorta abdominal, la arteria principal que provee sangre a los órganos en el abdomen y la parte inferior del cuerpo. Cerca de un 4% a un 7% de los hombres mayores de 65 años de edad tienen un AAA, aunque es menos común en las mujeres. Los aneurismas mayores que 55 mm de diámetro conllevan un alto riesgo de rotura que dan lugar a la muerte; aproximadamente un 60% de los pacientes que presentan la rotura del AAA mueren antes de llegar al hospital. Los pacientes con AAA mayores que 55 mm por lo general son derivados para la reparación, debido a que el riesgo de rotura excede el riesgo de la reparación. Hay tres métodos para reparar un AAA: intervención quirúrgica, reparación endovascular del aneurisma (REVA) y reparación laparoscópica. La intervención quirúrgica incluye la realización de un corte grande en el abdomen, después del cual se expone y se abre la aorta abdominal y se cose un injerto sintético (tubo) en dicho sitio para reemplazar el área debilitada de la aorta. La REVA incluye la realización de un corte en el área de la ingle, después del cual se inserta un injerto de stent en forma colapsada que se abre dentro del aneurisma bajo guía de rayos X y se implanta con un stent. La reparación laparoscópica o la cirugía del AAA mínimamente invasiva (keyhole) se realiza mediante cortes muy pequeños en el abdomen del paciente, después de los cuales se inserta un telescopio fino (laparoscopio) a través de estos cortes y se cose el injerto sintético en dicho sitio. Los beneficios de la REVA y la reparación laparoscópica son que requieren incisiones más pequeñas, son menos dolorosas, presentan menos complicaciones, una tasa de mortalidad inferior y una estancia hospitalaria más corta que la reparación quirúrgica. Las pruebas actuales indican que la REVA es el enfoque preferido para la reparación del AAA. Sin embargo la reparación laparoscópica del AAA se ha sugerido como una opción segura y efectiva para el tratamiento de dichos pacientes para los que la REVA no es apropiada. Esta revisión procuró evaluar los efectos de la cirugía laparoscópica para los aneurismas aórticos abdominales.
Características de los estudios y resultados clave
La revisión incluyó un ensayo controlado aleatorio (actualizado hasta agosto de 2016), que estudió a 100 participantes masculinos y que comparó la reparación laparoscópica asistida manualmente con la REVA. No ocurrieron muertes hospitalarias durante el estudio. El ensayo indicó que la reparación laparoscópica asistida manualmente tuvo una duración mayor que la REVA aunque no hubo diferencias en el número de pacientes con una reducción en el flujo sanguíneo de la pierna luego de cualquiera de los tratamientos.
Calidad de la evidencia
En la actualidad, hay una falta de ensayos controlados aleatorios que examinen la efectividad y la seguridad comparativas de la reparación laparoscópica del AAA. La calidad de la evidencia disponible fue imprecisa debido a la inclusión de un estudio pequeño, los intervalos de confianza amplios, y la imposibilidad de generalizar la evidencia debido a que el estudio incluyó a participantes masculinos solamente.
Conclusiones
Se necesita investigación adicional antes de poder establecer conclusiones.
Authors' conclusions
Summary of findings
| Hand‐assisted laparoscopic repair compared to EVAR for elective abdominal aortic aneurysm repair | ||||||
| Patient or population: People undergoing elective abdominal aortic aneurysm repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with EVAR | Risk with hand‐assisted laparoscopic repair | |||||
| In‐hospital mortality | see comment | Not estimable | 100 | ⊕⊕⊝⊝ | 0 participants died while in hospital in this study | |
| Operative time (minutes) | The mean operative time was 125 minutes | The mean operative time was 53 minutes longer | ‐ | 100 | ⊕⊕⊝⊝ | |
| Major complications ‐ lower limb ischaemia (up to 12 months) | Study population | RR 0.50 | 100 | ⊕⊕⊝⊝ | ||
| 40 per 1000 | 20 per 1000 | |||||
| Long‐term complications (12 months or longer if reported) | see comment | outcome not reported | ||||
| All‐cause mortality/survival (> 30 days) | see comment | outcome not reported | ||||
| Length of ICU stay (days) | see comment | outcome not reported | ||||
| Overall length of hospital stay (days) | The mean length of hospital stay was 3.4 days | The mean length of hospital stay was 4.2 days | Not estimable | 100 | ⊕⊕⊝⊝ | Standard deviations around the mean length of hospital stay were not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias was unclear for detection bias in the included studies but we did not consider it sufficient enough to downgrade the quality of the evidence | ||||||
Background
Description of the condition
Abdominal aortic aneurysm (AAA) is an abnormal dilatation of the infradiaphragmatic aorta that is equal to or greater than 30 mm (Sakalihasan 2005; Wanhainen 2008); or a local dilatation of equal to or greater than 50% compared to the expected normal diameter of the artery (Johnston 1991).
AAAs rarely occur in individuals under 50 years of age (Lederle 2000a), but thereafter the prevalence dramatically increases with age, with men at a six‐fold greater risk of developing an AAA than women (Pleumeekers 1995; Scott 2002).
Prevalence of AAA has been reported to range from 1.3% in women aged 65 to 80 years to between 4% and 7.7% in men aged 65 to 80 years (Ashton 2002; Ashton 2007; Lindholt 2005; Nordon 2011; Norman 2004; Scott 2002). The annual incidence of AAA in Western populations has been estimated at between 0.4% and 0.67% (Forsdahl 2009; Lederle 2000b; Nordon 2011; Vardulaki 1999); however more recent evidence suggests that AAA incidence is decreasing, most likely because of a reduction in tobacco smoking (Anjum 2012).
The development of an AAA is often multifactorial, with a change in the composition of the collagen and elastin matrix in the arterial wall attributed to atherosclerosis and inflammation of the aortic wall (Shah 1997). Aside from age and sex, the main well‐established modifiable risk factor is cigarette smoking, with smokers having a two‐ to three‐fold increased risk of AAA compared to non‐smokers (Lederle 1997; Lederle 2003). There is an apparent genetic correlation, with aneurysms tending to occur more frequently in close relatives of people who have suffered an AAA, but a mode of inheritance has not been demonstrated (Ballard 1999).
The natural progression of AAA can vary considerably between individuals (Ernst 1993), with subsequent variation in presentation ranging from no symptoms to symptoms such as groin, back, or abdominal pain. Findings upon physical examination include pulsating abdominal masses, and co‐existing aneurysmal popliteal or femoral arteries, and bruits. As the dilatation is often asymptomatic, the AAA can expand to such an extent that it ruptures, which is a surgical emergency. Approximately 60% of people with ruptured AAA die before reaching hospital (Ballard 1999), and even when it is possible to perform an emergency open surgical repair, the mortality rate remains high, at between 35% to 70% (Veith 2003). Newer techniques such as endovascular aneurysm repair have been shown to be more cost‐effective in the emergency setting but do not confer a greater chance of survival (Sweeting 2015).
The decision to repair an AAA is made on an individual basis, balancing the risk of treatment against the risk of aneurysm rupture. There is evidence that the risk of rupture increases as the aneurysm diameter increases from 50 mm to 60 mm (Brewster 2003). People with an AAA over 55 mm in diameter are therefore generally referred for consideration of repair, as the risk of rupture exceeds the risk of repair. Elective open surgical repair has a mortality of just over 5% (Bush 2006). Aneurysms below or equal to 55 mm are termed small AAAs and are at a low risk of rupture. Despite the improved mortality rates for elective versus emergency aneurysm repair, the management of patients with small AAAs is one of surveillance, whereby the AAA is routinely monitored for growth through ultrasound imaging (Filardo 2015). However, women with a small AAA have a higher rupture rate than men, so that a lower threshold (52 mm) is suggested (Brewster 2003).
Description of the intervention
The traditional treatment for AAA is open surgical repair (OSR): the abdominal aorta which lies in the retroperitoneum is exposed and the aneurysm is clamped and opened; then a prosthetic graft is anastomosed proximally to the normal aorta and distally to the dilated aorta and the clamps are released with the return of normal blood flow. OSR involves a large abdominal incision and is associated with a significant risk of complications including myocardial infarction, arrhythmias, bleeding, injury to the bowel, limb ischaemia, embolus, infection, and kidney damage (Badger 2014). The 30‐day mortality associated with open repair of AAA in the UK Small Aneurysm Trial was 5.8% (TUKSAT 1998), and reported rates range from 2% to 7% in otherwise fit individuals (Paravastu 2014).
A less invasive procedure known as endovascular aneurysm repair (EVAR) has recently become widely used. EVAR is carried out through sheaths inserted in the femoral artery in the groin. A modular covered stent graft is placed within the aneurysm sac, under radiological image guidance. The graft is manipulated into place using guidewires and anchored in place to form a new channel for blood flow (Parodi 1991). The aneurysm is excluded from inside to prevent further expansion and possible rupture. EVAR is favourable over OSR, as it is minimally invasive compared to major abdominal surgery of OSR. EVAR procedures are less painful and have a shorter recovery time and therefore a shorter duration of hospital stay (Paravastu 2014). Furthermore, in EVAR the blood flow to organs and the lower limbs is not disrupted to the same extent as in OSR, there are fewer respiratory side effects, and less blood is lost during the procedure (Paravastu 2014).
A third treatment for AAA is laparoscopic repair of AAA. There are two well‐described methods of laparoscopic repair of AAA. The first is hand‐assisted laparoscopic surgery (HALS), where an incision is made to allow the surgeon's hand to assist in the repair. The second, and more challenging technically, is total laparoscopic surgery (TLS) (NICE 2007). Both procedures require a laparoscope inserted through the abdominal wall via access ports and an induction of a pneumoperitoneum with further access ports. Laparoscopic instruments are used to dissect and clip the lumbar arteries and the inferior mesenteric artery (NICE 2007). The areas above and below the aneurysm are clamped, the sac of the aneurysm is cut open, any thrombus is removed, and a prosthetic vascular graft is anastomosed to both sides of the aorta. The posterior parietal peritoneum or aneurysm sac is then closed over the graft (NICE 2007).
Hand‐assisted laparoscopic surgery
This is generally performed with the patient supine and the use of a mini‐laparotomy incision at the midline for the placement of the laparoscopic port. A further incision is made (7 cm to 9 cm) in an appropriate position as determined by preoperative imaging, to allow the non‐dominant hand of the surgeon to be introduced into the abdominal cavity. A seal and pneumoperitoneum is created. The table is then tilted to the right and the abdominal bowel loops are pushed away from the midline. Further laparoscopic ports are inserted to allow dissection of the aortic neck. The exposure of the aorta and the procedure follows much the same process as the open AAA repair. Once the proximal and distal anastomosis sites are exposed and dissected, the abdomen is deflated and the proximal clamps applied. Thereafter the aneurysm is opened and the distal back bleeding is stopped using occlusive balloons (such as a Fogarty catheter). The lumbar vessels are ligated using sutures in the standard fashion. The proximal anastomosis is performed using long but standard instruments with the mini‐laparotomy incision being auto‐retracted and moved cranially to allow visualisation. Following successful proximal anastomosis, the distal anastomoses (either single in a tube graft or bifurcated in a 'Y' graft) are performed. If the anastomosis is at the level of the external iliac then an oblique suprainguinal incision is required. The peritoneum is then irrigated, closed, and the mini‐laparotomy incision closed (Ferrari 2006)
Total laparoscopic surgery
This is performed through a transperitoneal left retro‐renal approach. The patient is placed in a dorsal decubitus position with appropriate support and straps. A pneumoperitoneum is induced using a Veress needle. Multiple laparoscopic ports are inserted to allow the mobilisation of the left lateral colonic border, the kidney and spleen, so that they drop medially towards the patient's right side. The aorta is exposed and the left renal artery by fixation of the left kidney under traction sutures. The aorta is dissected and exposed from the iliac to the renal arteries taking care to ligate lumbar arteries and isolate the left ureter. The clamp is placed via the laparoscopic trocars on the proximal aorta, and the aortic wall is held under tension by a suture to allow opening of the aorta. The iliac arteries are then clamped using laparoscopic clamps. A longitudinal arteriotomy is then made in the aorta on the left side and thrombus evacuated. A tied tube graft is then sutured into the proximal neck. Next the distal anastomosis is performed after evacuating any new clot in the graft. Sutures and ties are performed intracorporally and the ports removed under vision with release of the pneumoperitoneum (Javerliat 2006)
How the intervention might work
Current evidence suggests that EVAR is the preferred approach for AAA repair in suitable candidates, due to shorter hospital stay and lower perioperative morbidity and mortality rates, as opposed to an open surgical approach. Evidence also suggests that elective laparoscopic AAA repair has a favourable safety profile comparable with that of EVAR, with low conversion rates as well as similar mortality and morbidity rates (Ahmed 2014). As a result, it has been suggested that elective laparoscopic AAA repair may have a role in treating those patients for whom EVAR is unsuitable.
Why it is important to do this review
There is currently no meta‐analysis of the literature to help guide evidence‐based recommendation for laparoscopic surgery for AAA. The aim of this review is to study the benefits and harms of laparoscopic surgery in people with AAA, by critical appraisal and meta‐analysis of the existing literature.
Objectives
To assess the effects of laparoscopic surgery for elective abdominal aortic aneurysm repair.
The primary objective of this review was to assess the perioperative mortality and operative time of laparoscopic (total and hand‐assisted) surgical repair of abdominal aortic aneurysms (AAA) compared to traditional open surgical repair or EVAR. The secondary objective was to assess complication rates, all‐cause mortality (> 30 days), hospital and intensive care unit (ICU) length of stay, conversion and re‐intervention rates, and quality of life associated with laparoscopic (total and hand‐assisted) surgical repair compared to traditional open surgical repair or EVAR.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). If we had found no trials of this sort, we would also have considered controlled clinical trials (CCTs). We excluded case reports, case series, and retrospective studies. We applied no limitation on the language of publication or country.
Types of participants
People of any age undergoing elective repair of an AAA.
Types of interventions
Laparoscopic repair (total laparoscopic repair or hand‐assisted laparoscopic repair) compared with either open surgical repair or EVAR.
We considered the following comparisons.
-
Laparoscopic repair (total or hand‐assisted laparoscopic repair) versus open surgical repair.
-
Laparoscopic repair (total or hand‐assisted laparoscopic repair) versus EVAR.
Types of outcome measures
Primary outcomes
-
30‐day or in‐hospital mortality.
-
Operative time (minutes).
Secondary outcomes
-
Major complications (e.g. myocardial infarction, stroke, renal failure, respiratory failure, bowel ischaemia, lower limb ischaemia, pneumonia, infection) up to 12 months.
-
Long‐term (12 months or longer if reported) major complications.
-
All‐cause mortality/survival (> 30 days).
-
Length of ICU stay (days).
-
Overall length of hospital stay (days).
-
Open conversion rates (conversion of the repair from a primarily laparoscopic approach to a total open surgical approach, inferring difficulty or complications).
-
Re‐intervention rates and device‐related complications.
-
Minor complications (e.g. haematoma, wound infection).
-
Completion of repair (successful completion of the anastomosis and repair by the intended method i.e. starting as a laparoscopic repair and completing this laparoscopically).
-
Quality of life.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
-
The Cochrane Vascular Specialised Register (searched 10 August 2016).
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 7) in the Cochrane Library (searched 10 August 2016).
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries (10 August 2016) for details of ongoing and unpublished studies using the terms 'laparoscopic AND aneurysm';
-
ClinicalTrials.gov (www.clinicaltrials.gov).
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch).
-
ISRCTN Register (www.isrctn.com/).
See Appendix 2 for details of the search strategies used.
Searching other resources
We searched citations within identified studies. We planned, if needed, to contact authors of relevant papers by email to identify any unpublished randomised controlled trials.
Data collection and analysis
Selection of studies
Two review authors (LR, SN) independently reviewed the results of all searches to identify potentially eligible articles. The two review authors (LR, SN) discussed each study to confirm eligibility for inclusion in the systematic review, excluding those not fulfilling the criteria as described in Criteria for considering studies for this review and stating the reasons for exclusion in the review.
Data extraction and management
Two review authors (LR, SN) independently extracted from each study information about the study characteristics, participants, interventions, duration of follow‐up, and outcome parameters using standardised forms. We extracted data on the following items, where available.
-
Study design.
-
Number of study participants.
-
Details of participants, including age, sex, diameter of AAA, diagnosis (clinical or ultrasound), and presence of co‐morbidities.
-
Stratification of low‐ and high‐risk patients defined by co‐morbidities, Vascular Physiological and Operative Severity Score for the enUmeration of Mortality and Morbidity (V(p)‐Possum) or Glasgow Aneurysm Score (GAS) where appropriate.
-
Interventions, including type of repair, adverse events (major and minor), and length of hospital stay.
-
Outcome measures as stated above.
-
Length of follow‐up.
Assessment of risk of bias in included studies
Two review authors (LR, SN) independently assessed the design and execution of each study according to the following criteria: random sequence generation; allocation concealment of treatment; blinding of participants, personnel, and outcomes; incomplete outcome data; selective outcome reporting; and other sources of bias in accordance with Cochrane's tool for assessing risk of bias (Higgins 2011). We judged the studies as either low risk of bias, high risk of bias, or unclear (due to either lack of information or uncertainty over the potential for bias). We resolved any disagreements by consensus between the two review authors.
Measures of treatment effect
We assessed dichotomous data using risk ratios (RRs) with 95% confidence intervals (CIs). We analysed continuous outcomes using mean differences (MDs) with 95% CIs where the scales were the same; and where scales were different but outcome measured was the same, we planned to use the standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
We did not include cross‐over trials. The individual participant was the unit of analysis.
Dealing with missing data
Where information was missing, we contacted the authors of the relevant study. If unsuccessful, we planned to exclude the data from the meta‐analysis but report it in the review. We intended to include outcome measures in this systematic review only if it was the intention of the study authors to perform the necessary assessments in all randomised participants. When fewer than 50% of the participants in a study had an acceptable follow‐up for a particular outcome measure, we planned on not reporting the results of this outcome measure due to the associated high risk of attrition bias.
Assessment of heterogeneity
It was intended that if the included studies were comparable with regard to age, sex, treatment, and used outcome definitions, we would perform a pooled analysis. We planned on assessing heterogeneity with the use of forest plots; and by a formal statistical test for heterogeneity, the I² statistic. Substantial heterogeneity was defined as I² greater than 50% (Higgins 2011). We planned on exploring possible causes of heterogeneity and taking appropriate measures.
Assessment of reporting biases
If more than 10 studies had been included in a meta‐analysis, we would have constructed a funnel plot to graphically ascertain the existence of publication bias (Higgins 2011).
Data synthesis
We entered the data into the Cochrane Review Manager 5 software (Review Manager 2014), and analysed them according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to use a fixed‐effect model where no substantial heterogeneity was found and a random‐effects model if heterogeneity (I² greater than 50%) was found.
Subgroup analysis and investigation of heterogeneity
We planned on performing subgroup analysis according to the following.
-
Age.
-
Gender.
-
Body mass index > 30 kg/m².
-
Diabetes.
-
Type of laparoscopic repair (total or hand‐assisted).
-
Previous cardiopulmonary co‐morbidities such as ischaemic heart disease, myocardial infarction, or chronic obstructive pulmonary disease (COPD) as determined by a preoperative cardiopulmonary exercise test (CPEX).
-
Participants at high risk of complications, identified where possible using well‐validated and reliable scoring systems such as the GAS, V(p)‐Possum, or other tests.
We intended to examine heterogeneity for these by visual inspection of forest plots and the I² test.
Sensitivity analysis
We planned on performing a sensitivity analysis by excluding studies at high risk of selection, performance, detection, attrition, and reporting bias.
Summary of findings table
We presented the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data for outcomes — 30‐day or in‐hospital mortality, operative time, major complications up to 12 months, long‐term (12 months or longer if reported) major complications, all‐cause mortality/survival (> 30 days), length of ICU stay, and overall length of hospital stay — in a 'Summary of findings' table, according to Higgins 2011 and Atkins 2004. Since we planned to assess different comparisons of interventions, we intended developing a 'Summary of findings' table for each comparison. We used the GRADEpro (GRADEproGDT) software (www.guidelinedevelopment.org/) to assist in the preparation of the 'Summary of findings' table.
Results
Description of studies
Results of the search
See Figure 1
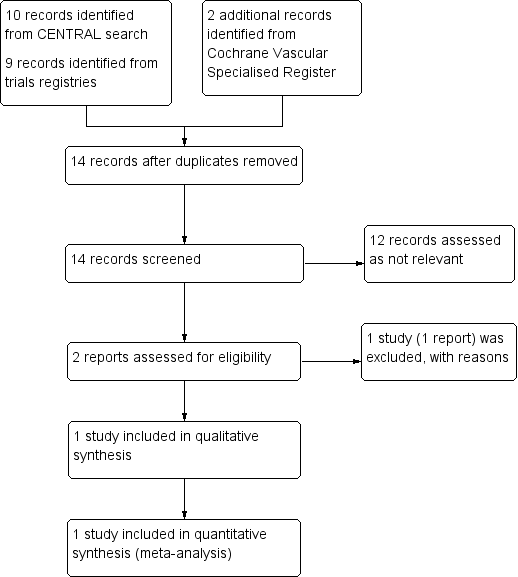
Study flow diagram.
Included studies
One randomised controlled trial with 100 male participants was eligible for inclusion in the review (Veroux 2010); it compared hand‐assisted laparoscopic repair with EVAR. Although the primary outcome of the study was perioperative sexual dysfunction, the study also measured in‐hospital mortality, operative time, length of hospital stay and the major complication incidence of lower limb ischaemia (Veroux 2010).
Excluded studies
One study comparing laparoscopic versus open AAA repair was withdrawn before enrolment and therefore excluded from this review (NCT00821145).
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
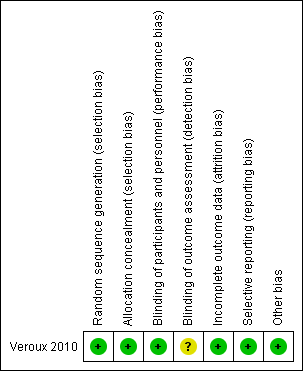
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The included study was judged to be at low risk of selection bias as the study authors reported that randomisation codes were computer‐generated and sealed envelopes labelled with participant numbers were used and only opened when a participant was admitted to AAA repair.
Blinding
The included study did not report whether participants were blinded to treatment. However, we judged that the study outcomes and outcome measurements reported by the included study were not likely to have been influenced by lack of blinding and therefore classified this study as low risk of performance bias. The study authors did not report whether outcome assessors were blinded to treatment and we therefore judged this study to be at unclear risk of detection bias.
Incomplete outcome data
The included study had no missing outcome data and was therefore judged to be at low risk of attrition bias.
Selective reporting
The included study reported all pre‐specified outcomes and was therefore judged to be at low risk of reporting bias.
Other potential sources of bias
The included study appeared to be free from other sources of bias.
Effects of interventions
Laparoscopic repair (total or hand‐assisted) versus open surgical repair
We identified no studies which compared either total or hand‐assisted laparoscopic repair with open surgical repair of AAA.
Laparoscopic repair (total or hand‐assisted) versus endovascular aneurysm repair
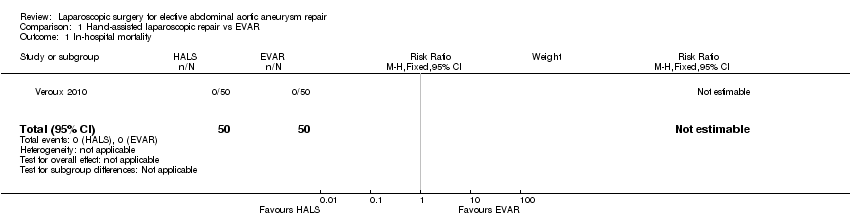
We identified one study which compared hand‐assisted laparoscopic repair with EVAR. There were no cases of in‐hospital mortality and the incidence of the major complication 'lower limb ischaemia' was similar between the two treatment groups (relative risk (RR) 0.50, 95% CI 0.05 to 5.34). Hand‐assisted laparoscopic repair was associated with longer operative time than EVAR (mean difference (MD) 53.00 minutes, 95% CI 36.49 to 69.51). The study reported mean length of hospital stay as 4.2 days and 3.4 days in the hand‐assisted and EVAR groups respectively, but standard deviations for the mean length of hospital stay were not reported and therefore it was not possible to independently test the statistical significance of this result. The study did not report on the other outcomes of this review (major complications other than lower limb ischaemia, long‐term major complications, all‐cause mortality/survival (> 30 days), length of ICU stay, open conversion rates, re‐intervention rates, device‐related complications, minor complications, completion of repair and quality of life).
We identified no studies which compared total laparoscopic repair with EVAR.
Discussion
Summary of main results
We identified one randomised controlled trial with a total of 100 male participants which fulfilled the eligibility criteria for inclusion in this review (Veroux 2010).
Laparoscopic repair (total or hand‐assisted) versus open surgical repair
We identified no studies which compared either total or hand‐assisted laparoscopic repair with open surgical repair of AAA.
Laparoscopic repair (total or hand‐assisted) versus endovascular aneurysm repair
We identified one study which compared hand‐assisted laparoscopic repair with EVAR. There were no cases of in‐hospital mortality and the incidence of the major complication 'lower limb ischaemia' was similar between the two treatment groups. Hand‐assisted laparoscopic repair was associated with a longer operative time than EVAR. The study reported mean length of hospital stay but standard deviations for the mean duration were not reported and therefore it was not possible to independently test the statistical significance of this result. The study did not report on the other outcomes of this review (major complications other than lower limb ischaemia, long‐term major complications, all‐cause mortality/survival (> 30 days), length of ICU stay, open conversion rates, re‐intervention rates and device‐related complications, minor complications, completion of repair and quality of life).
We identified no studies which compared total laparoscopic repair with EVAR.
Overall completeness and applicability of evidence
At present, there is no randomised controlled trial evidence regarding the efficacy and safety of laparoscopic repair compared with open surgical repair of AAA. Furthermore there is exceptionally limited randomised controlled trial evidence on laparoscopic repair compared with EVAR. Only one study on a total of 100 male participants met the inclusion criteria for this review (Veroux 2010). Furthermore, the study looked at mortality, operative time, length of hospital stay and lower leg ischaemia as its secondary outcomes. Other outcomes of interest for this review, such as long‐term major complications, all‐cause mortality (> 30 days), conversion and re‐intervention rates, device‐related complications and quality of life, were not studied and, therefore, remain unknown.
Quality of the evidence
The only study included in this review was judged to be at low risk of bias for all domains except for detection bias where the risk was unclear due to insufficient information to permit a judgement of low or high risk.
For all outcomes, the quality of the evidence was downgraded to low due to the inclusion of only one study with a small sample size and wide confidence intervals. Furthermore, as the study only included males, the quality of the evidence was downgraded further due to indirectness.
Potential biases in the review process
None of the authors have any commercial or other conflict of interest. The Cochrane Vascular Information Specialist performed a comprehensive search of the literature; and review authors selected studies in accordance with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion.
Agreements and disagreements with other studies or reviews
To date, no other systematic review has assessed the efficacy and safety of laparoscopic repair of AAA.
Ahmed 2014 conducted a review to determine how elective laparoscopic abdominal aortic aneurysm (AAA) repair compares to endovascular aneurysm repair (EVAR) in terms of survival. Eight papers (five prospective studies, one retrospective study, one RCT and one systematic review) were deemed to be the best available evidence. The RCT included by Ahmed 2014 was also the only RCT included in our Cochrane Review (Veroux 2010). Ahmed 2014 concluded that laparoscopic AAA repair is just as safe as EVAR, with similar mortality and morbidity rates and length of hospital stay.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Hand‐assisted laparoscopic repair vs EVAR, Outcome 1 In‐hospital mortality.

Comparison 1 Hand‐assisted laparoscopic repair vs EVAR, Outcome 2 Operative time (minutes).

Comparison 1 Hand‐assisted laparoscopic repair vs EVAR, Outcome 3 Major complications.
| Hand‐assisted laparoscopic repair compared to EVAR for elective abdominal aortic aneurysm repair | ||||||
| Patient or population: People undergoing elective abdominal aortic aneurysm repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with EVAR | Risk with hand‐assisted laparoscopic repair | |||||
| In‐hospital mortality | see comment | Not estimable | 100 | ⊕⊕⊝⊝ | 0 participants died while in hospital in this study | |
| Operative time (minutes) | The mean operative time was 125 minutes | The mean operative time was 53 minutes longer | ‐ | 100 | ⊕⊕⊝⊝ | |
| Major complications ‐ lower limb ischaemia (up to 12 months) | Study population | RR 0.50 | 100 | ⊕⊕⊝⊝ | ||
| 40 per 1000 | 20 per 1000 | |||||
| Long‐term complications (12 months or longer if reported) | see comment | outcome not reported | ||||
| All‐cause mortality/survival (> 30 days) | see comment | outcome not reported | ||||
| Length of ICU stay (days) | see comment | outcome not reported | ||||
| Overall length of hospital stay (days) | The mean length of hospital stay was 3.4 days | The mean length of hospital stay was 4.2 days | Not estimable | 100 | ⊕⊕⊝⊝ | Standard deviations around the mean length of hospital stay were not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias was unclear for detection bias in the included studies but we did not consider it sufficient enough to downgrade the quality of the evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 In‐hospital mortality Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Operative time (minutes) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 53.0 [36.49, 69.51] |
| 3 Major complications Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.34] |
| 3.1 Lower limb ischaemia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.34] |

