Iridotomy to slow progression of visual field loss in angle‐closure glaucoma
Abstract
Background
Primary angle‐closure glaucoma is a type of glaucoma associated with a physically obstructed anterior chamber angle. Obstruction of the anterior chamber angle blocks drainage of fluids (aqueous humor) within the eye and may raise intraocular pressure (IOP). Elevated IOP is associated with glaucomatous optic nerve damage and visual field loss. Laser peripheral iridotomy (often just called 'iridotomy') is a procedure to eliminate pupillary block by allowing aqueous humor to pass directly from the posterior to anterior chamber through use of a laser to create a hole in the iris. It is commonly used to treat patients with primary angle‐closure glaucoma, patients with primary angle closure (narrow angles and no signs of glaucomatous optic neuropathy), and patients who are primary angle‐closure suspects (patients with reversible obstruction). The effectiveness of iridotomy on slowing progression of visual field loss, however, is uncertain.
Objectives
To assess the effects of iridotomy compared with no iridotomy for primary angle‐closure glaucoma, primary angle closure, and primary angle‐closure suspects.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 9) which contains the Cochrane Eyes and Vision Trials Register; MEDLINE Ovid; Embase Ovid; PubMed; LILACS; ClinicalTrials.gov; and the ICTRP. The date of the search was 18 October 2017.
Selection criteria
Randomized or quasi‐randomized controlled trials that compared iridotomy to no iridotomy in primary angle‐closure suspects, patients with primary angle closure, or patients with primary angle‐closure glaucoma in one or both eyes were eligible.
Data collection and analysis
Two authors worked independently to extract data on study characteristics, outcomes for the review, and risk of bias in the included studies. We resolved differences through discussion.
Main results
We identified two trials (2502 eyes of 1251 participants) that compared iridotomy to no iridotomy. Both trials recruited primary angle suspects from Asia and randomized one eye of each participant to iridotomy and the other to no iridotomy. Because the full trial reports are not yet available for both trials, no data are available to assess the effectiveness of iridotomy on slowing progression of visual field loss, change in IOP, need for additional surgeries, number of medications needed to control IOP, mean change in best‐corrected visual acuity, and quality of life. Based on currently reported data, one trial showed evidence that iridotomy increases angle width at 18 months (by 12.70°, 95% confidence interval (CI) 12.06° to 13.34°, involving 1550 eyes, moderate‐certainty evidence) and may be associated with IOP spikes at one hour after treatment (risk ratio 24.00 (95% CI 7.60 to 75.83), involving 1468 eyes, low‐certainty evidence). The risk of bias of the two studies was overall unclear due to lack of availability of a full trial report.
Authors' conclusions
The available studies that directly compared iridotomy to no iridotomy have not yet published full trial reports. At present, we cannot draw reliable conclusions based on randomized controlled trials as to whether iridotomy slows progression of visual field loss at one year compared to no iridotomy. Full publication of the results from the studies may clarify the benefits of iridotomy.
PICO
Plain language summary
Iridotomy to slow progression of visual field loss in angle‐closure glaucoma
What was the aim of this review?
The aim of this Cochrane Review was to find out whether iridotomy compared to no iridotomy can slow progression of visual field loss in (1) people with primary angle‐closure glaucoma, (2) people with primary angle closure, and (3) people who are suspected of having primary angle closure. We collected and analyzed all relevant clinical trials to answer this question and found two studies awaiting full publication of results.
Key messages
At the time of review, it is uncertain whether iridotomy can slow progression of visual field loss. When they become available, full publication of the results from the two studies may clarify the benefits of iridotomy.
What did we study in this review?
Glaucoma is a group of eye diseases that cause damage to the nerve in the eye. If untreated, glaucoma can lead to blindness. Primary angle‐closure glaucoma is a type of glaucoma which happens when the drainage canals ("angles") in the eyes get blocked, like a sink with something covering the drain. This blockage may lead to increased eye pressure and hence a decrease of the total area in which objects can be seen in side vision ('visual field').
Iridotomy involves using a laser to create a hole in the eye's iris, the colorful disc around the pupil. This opening allows fluid to flow again, which helps control eye pressure and may slow progression of visual field loss.
What were the main results of this review?
At the time of conducting this review, we identified two trials with publication of the full trial results still under preparation. Both trials recruited participants from Asia. One eye of each participant received iridotomy and the other eye did not receive iridotomy. No data are available to assess the effectiveness of iridotomy on slowing progression of visual field loss. Low‐ to moderate‐quality evidence from one trial suggests that iridotomy increases width of the drainage angle ('angle width') at 18 months post‐treatment and may be associated with adverse events, such as 'spikes' of increased eye pressure at one hour post‐treatment.
How up to date is the review?
We searched for studies that have been published up to 18 October 2017.
Authors' conclusions
Summary of findings
| Iridotomy compared to no iridotomy for patients with primary angle‐closure suspect, primary angle closure, or primary angle‐closure glaucoma | ||||||
| Patient or population: patients with primary angle‐closure suspect, primary angle closure, or primary angle‐closure glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of eyes | Certainty of the evidence | Comments | |
| Risk with no iridotomy | Risk with Iridotomy | |||||
| Proportion of progressive visual field loss at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Intraocular pressure: mean IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Gonioscopic findings: mean angle width at 1 year | The mean angle width was 11.3° in the no iridotomy group | The mean angle width in the iridotomy group was 12.7° higher | MD 12.7 | 1550 | ⊕⊕⊕⊝ | Participants in the study were primary angle‐closure suspects. Data were only available at 18 months. |
| Need for additional surgery: proportion of participants who received additional surgery to control IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Medications: mean number of medications used to control IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Quality of life measures | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Adverse events ‒ IOP spike (rise greater than or equal to 8 mmHg) at 1 hour | 4 per 1000 | 98 per 1000 | RR 24.00 | 1468 | ⊕⊕⊝⊝ | Participants in the study were primary angle‐closure suspects. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias, as the study is at unclear risk of bias for incomplete outcome data, selective outcome reporting, and other sources of bias due to the lack of availability of a full trial report. 2 Downgraded by one level for imprecision, as the confidence interval of the risk ratio between the groups is wide. | ||||||
Background
Description of the condition
Glaucoma refers to a group of similar diseases defined by progressive damage to the optic nerve (optic neuropathy). This damage occurs in a characteristic pattern with associated structural and functional changes, including visual field loss (Foster 2002). Elevated intraocular pressure (IOP) is associated with glaucomatous optic nerve damage. IOP can rise when aqueous humor, a clear fluid that continuously flows through the anterior chamber to nourish and pressurize the eye, does not drain properly (AAO 2015; EGS 2014; Mapstone 1968). There are two broad subtypes of glaucoma, angle‐closure and open‐angle, in which the drainage pathway for aqueous humor is occluded or not, respectively (AAO 2015).
Primary angle‐closure glaucoma, the focus of this review, involves appositional (reversible) or synechial (adhesional) closure of the anterior chamber angle (AAO 2015; Emanuel 2014). Two main mechanisms have been hypothesized as responsible for angle closure: (1) pupillary block; and (2) anterior displacement of the iris. In the former, contact between the iris and lens at the pupillary margin increases resistance to aqueous outflow, as the iris bows forward and comes into contact with the trabecular meshwork (iridotrabecular contact (ITC)) (AAO 2015). In the latter, a large or anteriorly‐positioned ciliary body pushes the peripheral iris forward, often leading to continued ITC (AAO 2015).
For this review, we follow a recently proposed classification of angle‐closure glaucoma (Table 1) (AAO 2015; Aung 2001; Foster 2000; Foster 2002; Ng 2012). This definition rests on the idea of describing an 'occludable' angle, using terms such as 'narrow' to specify the anatomical predisposition to angle closure, further qualified by degrees of ITC, presence of IOP elevated above the population‐based norm, and presence of peripheral anterior synechiae (PAS). The drainage angle is assessable by gonioscopy with a diagnostic contact prism. In brief:
| Primary angle‐closure suspect (PACS) | Primary angle closure (PAC) | Primary angle‐closure glaucoma (PACG) | |
| Iridotrabecular contact greater than or equal to 180° | X | X | X |
| Elevated intraocular pressure OR peripheral anterior synechiae | X | X | |
| Optic nerve damage | X |
-
primary angle‐closure suspects (PACS) are patients who have reversible ITC of 180° or more on gonioscopy; however, there is no evidence of permanent aqueous outflow obstruction, damage to the angle (i.e. no PAS), rise in IOP, or glaucomatous optic neuropathy;
-
primary angle closure (PAC) patients have ITC of 180° or more plus elevated IOP or PAS or both, but no signs of glaucomatous optic neuropathy; and
-
primary angle‐closure glaucoma (PACG) patients have ITC of 180° or more in the presence of glaucomatous optic nerve damage (with or without PAS or elevated IOP at the time of examination).
Epidemiology
Glaucoma is among the leading causes of blindness and, particularly due to the irreversible nature of the disease, a pressing public health challenge (Bourne 2013; Kingman 2004; Resnikoff 2004). The World Health Organization characterizes glaucoma as one of its priority eye diseases, and researchers have approximated that about five million people today are blind as a consequence of glaucoma (Osborne 2003; Quigley 2006). A recent systematic review found a global prevalence of glaucoma in the 40 to 80 years age group of 3.54%, and estimated that prevalence will reach 76 million by 2020 and 111.8 million by 2040 (Tham 2014).
Although angle‐closure glaucoma is less common than open‐angle glaucoma, it is often more severe and more likely to result in irreversible blindness if left untreated (AAO 2015). Among the 64.3 million people with glaucoma aged 40 to 80 years, 20.2 million were estimated to have PACG in 2013; in this sub‐population, 14.5 million were estimated to be living in Asia (Quigley 2006; Tham 2014). For example, the number of people in China with PACS, PAC, and PACG has been estimated as 28.2 million, 9.1 million, and 3.5 million, respectively (Foster 2001). Moreover 91% of the 1.7 million cases of bilateral blindness in this population are attributable to PACG (Foster 2001). The risk of progression from PACS to PAC and from PAC to PACG has also been estimated as 22% and 29%, respectively, over five years (Thomas 2003a; Thomas 2003b). PACG is less common among people of European descent, with pooled prevalence of PACG for people aged 40 years or older estimated to be 0.4% (Day 2012). Other risk factors for angle‐closure diseases include female sex, older age, and family history of angle closure (AAO 2015; Bonomi 2002; Day 2012).
Treatment options
Treatments for angle‐closure glaucoma include medical interventions and surgical interventions (with or without laser) that open the angle to remove blockage of the normal flow of aqueous humor, lower IOP and equalize pressure across the anterior and posterior chambers of the eye. Medical options include miotics such as topical pilocarpine. Other agents, including beta‐blockers, alpha2‐agonists, carbonic anhydrase inhibitors and prostaglandin analogs, can also lower IOP but do not remove the risk of disease progression from PACS to PAC and PACG (AAO 2015; See 2011). Surgical options include lens extraction, iridoplasty, iridectomy, iridotomy, and trabeculectomy (Azuara‐Blanco 2016; See 2011). Today, the standard first‐line treatment for angle closure is iridotomy.
Description of the intervention
Laser peripheral iridotomy ('iridotomy') is an outpatient procedure in which an opening is created in the peripheral iris using a neodymium‐doped yttrium aluminum garnet (Nd:YAG) or argon laser mounted on a slit lamp biomicroscope (AAO 2015; Nolan 2000). Iridotomy is based on the same principle as iridectomy, which involves surgical removal of part of the iris. Iridotomy has largely replaced iridectomy: there are approximately 51 iridotomies for every iridectomy performed (Ramulu 2007).
Iridotomy has some limitations. Changes in aqueous pressure gradients and iris configuration after iridotomy may increase contact between the lens and the iris, theoretically leading to a risk of more rapid development of cataracts (Caronia 1996; Lim 2005). Other potential risks include the rare occurrence of corneal endothelial damage localized to the surgery site, dysphotopsias or stray light symptoms, and the development of posterior synechiae (Pollack 1981; Quigley 1981; Robin 1984). Posterior synechiae can limit vision in dimly‐lit environments and complicate later cataract surgery or other ocular procedures.
How the intervention might work
Iridotomy eliminates the pressure gradient caused by pupillary block by making an opening in the peripheral iris; this hole—created with laser—allows free circulation of aqueous humor from posterior to anterior chambers even if the pupil is blocked (Fleck 1997; Friedman 2001; Ng 2012). By restoring a more posterior iris position, this opening may prevent progression of PAS and further IOP rise, minimize subsequent optic nerve damage, and slow progression of visual field loss. In cases of suspected angle closure, iridotomy is often used as a prophylactic measure to prevent further progression of angle closure (AAO 2015).
Why it is important to do this review
Glaucoma is a leading cause of blindness worldwide. Iridotomy is the most common procedure to treat patients with PACG. Iridotomy has also been used prophylactically in the contralateral eye of people who have previously been diagnosed with PAC or PACG in one eye (Ang 2000; Edwards 1982; Snow 1977). Yet, iridotomy does not directly correct the underlying anatomical defects related to angle closure, and it is unclear if iridotomy is sufficient for long‐term control of IOP in patients with PACG (See 2011). Additionally, a recent survey of glaucoma specialists to set priorities for comparative effectiveness research on the management of angle‐closure disease identified that understanding the role of iridotomy for the prevention of angle‐closure glaucoma is an important unmet evidence gap (Yu 2015). A systematic review of the evidence is needed to evaluate the benefits and risks of iridotomy in patients with PACS, PAC, and PACG.
Objectives
To assess the effects of iridotomy compared with no iridotomy for primary angle‐closure glaucoma, primary angle closure, and primary angle‐closure suspect.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). As we anticipated few RCTs on this intervention, we planned to include quasi‐randomized trials. We defined quasi‐randomized trials as studies that employed a method of allocating patients to a treatment arm that is not strictly random (e.g. by date of birth, hospital record number, in alternation, etc.). We included studies irrespective of their publication status and language. We included reports of secondary analyses of included RCTs and grouped them with the RCT.
Types of participants
We included studies of participants with gonioscopically‐narrow angles—i.e. participants with PACS, PAC, or PACG in one or both eyes. We did not restrict by age, gender or ethnicity.
Types of interventions
We included only trials that compared iridotomy versus no iridotomy or sham treatment. We applied no restrictions with respect to IOP‐lowering medications.
Types of outcome measures
Primary outcomes
Proportion of participants with any progression of visual field loss at one year. We planned to assess progression of visual field loss using criteria as defined in included studies as measured using any validated tool, such as automated Humphrey Field Analyzer, Heidelberg Edge Perimeter, or Oculus. We also planned to consider other time points during follow‐up as reported in the included studies and to assess this outcome for studies involving participants with PAC or PACG.
Secondary outcomes
The secondary outcomes for comparison of interventions included the following.
-
Mean change in IOP from baseline to one year, measured by any method of applanation tonometry, e.g. Goldmann or Perkins.
-
Gonioscopic findings in the participant, including angle width and presence of PAS, as reported by the investigators, at one year.
-
Need for additional surgery, as defined by the proportion of participants who received additional surgery to control IOP within one year after iridotomy.
-
Number of medications used to control IOP at one year.
-
Mean change in best corrected visual acuity (BCVA) as measured by logMAR from baseline to one year after iridotomy.
-
Quality‐of‐life data, as recorded by the investigators.
To improve comparability and consistency, we have adapted some of the above outcomes from previous Cochrane Reviews (Friedman 2006; Zhang 2015). If trials did not report outcomes at one year, we considered longer‐term outcomes closest to one year.
Adverse events
We reported adverse effects—including IOP spikes, persistent IOP elevation, hyphema and other adverse effects—as they were recorded by the investigators.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for RCTs and quasi‐randomized trials. There were no language or publication year restrictions. The date of the search was 18 October 2017.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 9) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 18 October 2017) (Appendix 1);
-
MEDLINE Ovid (1946 to 18 October 2017) (Appendix 2);
-
Embase.com (1980 to 18 October 2017) (Appendix 3);
-
PubMed (1948 to 18 October 2017) (Appendix 4);
-
LILACS (1982 to 18 October 2017) (Appendix 5);
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 18 October 2017) (Appendix 6);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)(www.who.int/ictrp; searched 18 October 2017) (Appendix 7).
Searching other resources
We searched the references of included studies for information about further trials. We did not conduct manual searches of journals or conference proceedings for this review.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts identified in the electronic searches using Covidence. We classified each title and abstract as 'Yes' (relevant), 'Maybe' (maybe relevant), or 'No' (not relevant). We retrieved full‐text articles for records classified as 'Yes' or 'Maybe' and reviewed them against the eligibility criteria of the review. We contacted the trial authors to clarify any details necessary to make a complete assessment of the relevance or design of the study. We documented reasons for exclusion for each study assessed as not eligible after reviewing the full‐text reports. We resolved discrepancies between review authors by discussion at each stage of the selection process.
Data extraction and management
Two review authors independently extracted data from included studies into a web‐based, electronic data collection form using Covidence. We extracted information on the study design (e.g. study setting, countries where recruitment took place, sample size, study duration and follow‐up time, study design, analysis choice, sources of funding, and potential conflicts of interests); characteristics of the participants (e.g. inclusion/exclusion criteria, underlying disease conditions, and medical history, including visual acuity and other vision‐related characteristics); interventions and comparators (e.g. type of laser, duration and timing); and outcomes (e.g. domain, specific measurement, specific metric, method of aggregation, and the time frame). Where 2 × 2 tables or means and standard deviations (or standard errors) were not available, we planned to include effect estimates (e.g. odds ratios and regression coefficients), confidence intervals, test statistics, or P values. We contacted study investigators for any missing or unclear information and proceeded with available information when we received no response within two weeks.
The two authors then compared the extracted data and resolved discrepancies by discussion and, when necessary, through consultation with the third author. One review author completed data entry into Review Manager 5 (RevMan 5) (Review Manager 2014); and a second author verified the data entered.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in included studies following the guidance given in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Specific items we considered included random sequence generation and allocation concealment (selection bias); masking of participants and study personnel (performance bias); masking of outcome assessors (detection bias); missing data and intention‐to‐treat analysis (attrition bias); selective outcome reporting (reporting bias); and other potential sources of bias. We assigned each item as having 'low risk,' 'high risk,' or, if the information provided is insufficient to make an assessment, 'unclear risk' using the criteria described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We documented reasons for those assessments.
We resolved discrepancies through discussion. We contacted the study investigators as appropriate.
Measures of treatment effect
We intended to report risk ratios (RR) with 95% confidence intervals (CIs) for any dichotomous outcomes (i.e. proportion of participants with any evidence of progression of visual field loss and proportion of participants who needed additional surgery to control IOP) and mean differences in change from baseline with 95% CIs for continuous outcomes (i.e. mean change in IOP, progressive field loss, number of medications used, and mean change in BCVA). We intended to conduct separate analyses for outcomes in the eyes of participants with PACG, PAC, and PACS. If any trials on eyes with narrow angles compared eyes within individuals (e.g. one eye was randomized to the treatment while the other was randomized to observation), then we planned to note whether or not the study investigators included statistical methods accounting for the correlation between eyes belonging to the same individual.
Unit of analysis issues
We planned for our unit of analysis to be one study eye per individual participant, therefore accounting for non‐independence of eyes would not be necessary. When both eyes from the same individual were randomized, we planned to use the estimates that had accounted for the correlation.
Dealing with missing data
We addressed any missing study data for the outcomes of interest or any unclear information by writing to the authors. We planned to consider multiple imputation or other imputation approaches for missing data. In the event that the quality of the available data prevented any meaningful analysis, we planned to omit the study from the analyses and would have noted this decision in the discussion.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by examining participant characteristics, iridotomy procedures, and outcomes by carefully reviewing the available data and taking into consideration potential risk of bias. We planned to assess statistical heterogeneity by assessing forest plots and examining the I² value (Deeks 2011; Higgins 2003). The I² value describes the proportion of total variation across studies that is due to heterogeneity rather than chance (Higgins 2011). We considered I² greater than 70% as the cut‐off point to identify the presence of considerable heterogeneity (Higgins 2011). We planned to give consideration to the consistency of the effect estimates. For example, had we found that all effect estimates were in the same direction, we might have reported a meta‐analysis even though there might have been substantial statistical heterogeneity.
Assessment of reporting biases
We intended to examine selective outcome reporting as part of the 'Risk of bias' assessment, by comparing the outcomes reported in included studies and outcomes listed in study registration or study protocols (where available). We planned to examine funnel plots of intervention effect estimates for evidence of asymmetry, if there were a sufficient number of included studies (i.e. 10 or more). An asymmetrical funnel plot may imply possible publication bias or exaggeration of treatment effects in small, low‐quality studies (Sterne 2001).
Data synthesis
We planned to follow Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions for data analysis (Deeks 2011). In the absence of substantial clinical and methodological heterogeneity, we would have used a random‐effects model to compute a quantitative synthesis. Had the number of studies included in the quantitative synthesis been less than three with no evidence of substantial statistical heterogeneity, we would have considered a fixed‐effect meta‐analysis. We provided a descriptive, qualitative synthesis of studies and their results, based on the information available.
Subgroup analysis and investigation of heterogeneity
We planned to consider the following pre‐specified subgroups: (1) with or without use of IOP‐lowering medications; and (2) by ethnic/racial groups. The effect of iridotomy may vary based on the use of IOP‐lowering medication; and ethnicity/race is a known risk factor for angle‐closure glaucoma (AAO 2015).
Sensitivity analysis
We planned to conduct two sensitivity analyses to determine the effect of excluding studies at high risk of bias for incomplete outcome data (i.e. the amount or distribution of missing outcomes differ between treatment groups) (Higgins 2011); and the effect of excluding studies that were quasi‐randomized trials. If appropriate, we would have also conducted additional sensitivity analyses to determine the impact of any post‐hoc decisions made during the review process.
'Summary of findings' table
We prepared a 'Summary of findings' table for each available outcome. We assessed the certainty of the evidence using the GRADE approach with GRADEpro software (GRADEpro 2015). BR did the initial assessment, which was checked by JL. We considered risk of bias, inconsistency, indirectness, and imprecision when judging the certainty of the evidence. We included the following outcomes in the summary.
-
Proportion of participants with progressive visual field loss at one year.
-
Mean change in IOP from baseline to one year.
-
Gonioscopic findings in the participants at one year.
-
Need for additional surgery within one year.
-
Number of medications used to control IOP at one year.
-
Quality of life measures.
-
Adverse effects as documented.
Results
Description of studies
Results of the search
Our search, which we conducted on 18 October 2017, returned 4040 titles and abstracts. We identified five additional potentially relevant records through reviewing reference lists. After removing duplicates, we screened 2572 unique records (Figure 1). Of the records screened, we classified 29 records as 'relevant' or 'maybe relevant' and reviewed the full‐text reports of these records. We excluded 16 records (14 records described studies using a non‐randomized design; 2 records described studies using iridoplasty as the intervention). The remaining 13 records represented two studies with outcomes partially reported: the Asymptomatic Narrow Angles ‒ Laser Iridotomy Study (ANA‐LIS); and the Zhongshan Angle Closure Prevention (ZAP) Trial. At the time of conducting this review, the preparations of the full reports of both trials are underway (per correspondence with trial authors) (ANA‐LIS; ZAP).

Study flow diagram.
Included studies
We included two RCTs (2502 eyes, 1251 participants) in this review (ANA‐LIS and ZAP) (Characteristics of included studies). Both trials compared iridotomy versus no iridotomy. Both trials used a paired‐eye design, where one eye from each participant was randomized to the iridotomy group and the fellow eye to the no iridotomy group. A full report with results is not available for either trial. For the ANA‐LIS trial, preliminary results were reported in a conference abstract (ANA‐LIS; Mani 2016 report). For the ZAP trial, safety results for up to two weeks post‐treatment and anterior chamber angle configuration results for up to 18 months of follow‐up are reported in published journal articles (ZAP; Jiang 2012 report; Jiang 2014 report). The other reports we identified for the two included studies include trial registry records, design papers, and results for nested observational studies.
Types of participants
Both trials included only participants with bilateral asymptomatic primary angle‐closure suspects. ZAP further specified that participants with an IOP rise greater than 15 mmHg from baseline in the dark room prone provocative test were excluded (ZAP; Jiang 2010 report).
The ANA‐LIS trial reported that participants came from three hospital sites in Singapore (ANA‐LIS; Mani 2016 report). Participants in the ZAP trial were enrolled from a tertiary specialized hospital in Guangzhou, China. In both trials, there were more female participants than male participants (76% in ANA‐LIS and 83% in ZAP) and the populations comprised older adults (mean age of 62.8 years in ANA‐LIS and 59.4 years in ZAP).
Types of interventions
Both trials used neodymium:yttrium–aluminum–garnet (Nd:YAG) laser iridotomy in the laser group while the control group received no iridotomy. In ANA‐LIS, investigators noted that laser peripheral iridotomy was performed in one randomly selected eye per patient by sequential argon and Nd:YAG laser after pretreatment with 2% pilocarpine instilled into the eye (ANA‐LIS; How 2012 report). In this trial, argon settings of 500 mW to 1000 mW power with a spot size of 50 μm for a duration of 0.05 seconds and a yttrium–aluminum–garnet setting of 2 mJ to 5 mJ were used (ANA‐LIS; How 2012 report). In the ZAP trial, the trial authors noted specifically that participants also received one drop of brimonidine 0.15% and pilocarpine 2% in the intervention eye 15 minutes before treatment (ZAP; Jiang 2012 report). Iridotomy was performed using the YAG laser, starting at an initial setting of 1.5 mJ (ZAP; Jiang 2012 report).
Types of outcomes
Proportion of participants with progressive visual field loss
The ANA‐LIS trial specified measuring visual field loss by automated perimetry as an outcome in the trial registration (ANA‐LIS; NCT00347178 report). It is unclear how the ZAP trial assesses visual field loss. At the time of this review, no data are available on the proportion of participants with progressive visual field loss at one year.
Mean change in IOP
Both the ANA‐LIS and ZAP trial specified measuring changes in IOP (ANA‐LIS; NCT00347178 report; ZAP; ISRCTN45213099 report). At the time of this review, no data are available on the mean change in IOP at one year. The ZAP trial reported findings for this outcome at the 1‐hour and 2‐weeks follow‐up periods (ZAP; Jiang 2012 report). We did not assess these results because they fall outside of our pre‐specified time point of one year and are intended to assess acute risks of treatment rather than benefit.
Gonioscopic findings
Both the ANA‐LIS and ZAP trial specified reporting on gonioscopic findings (ANA‐LIS; NCT00347178 report; ZAP; ISRCTN45213099 report). The ZAP trial reported angle width as measured by a Goldmann‐type, 1‐mirror gonioscopic lens as well as anterior segment optical coerence tomography at 18 months (ZAP; Jiang 2014 report). At the time of this review, no other data are available on gonioscopic findings at one year.
Need for additional surgery
Neither the ANA‐LIS nor the ZAP trial specified measuring need for additional surgery. At the time of this review, no data are available on the need for additional surgery at one year.
Number of medications to control IOP
Neither the ANA‐LIS nor the ZAP trial specified measuring number of medications to control IOP. At the time of this review, no data are available on the number of medications to control IOP at one year.
Mean change in BCVA
Neither the ANA‐LIS nor the ZAP trial specified measuring change in BCVA. At the time of this review, no data are available on the mean change in BCVA at one year.
Quality of life
Neither the ANA‐LIS nor the ZAP trial specified measuring quality of life. At the time of this review, no data are available on quality of life.
Adverse events
Both the ANA‐LIS and ZAP trial specified reporting adverse events. The ZAP trial has reported adverse events in terms of IOP spikes (defined as IOP increase ≥ 8 mmHg at 1 hour post‐treatment) (ZAP; Jiang 2012 report). At the time of this review, no other data are available on adverse events.
Excluded studies
We excluded 16 articles after reviewing full‐text reports (Figure 1): 14 reports were not of RCTs and two reports examined iridoplasty. We provide our reasons for exclusion in the Characteristics of excluded studies.
Risk of bias in included studies
A summary of risk of bias assessments for each trial is shown in Figure 2 and Figure 3. No information on method of randomization or allocation is available for the ANA‐LIS trial, and we assessed this trial as having unclear risk of selection bias. We assessed the ZAP trial at low risk of bias for both reporting the random sequence generation procedure and allocation concealment before randomization (ZAP; Jiang 2010 report). The trial registry record for ANA‐LIS describes this trial as an "open‐label" trial, therefore we assessed this trial as having high risk of bias for performance and detection bias (ANA‐LIS; NCT00347178 report). The ZAP trial registry record describes the trial as "not masked" and we assessed this trial as having high risk of performance bias (ZAP; ISRCTN45213099 report). The research nurse who assessed IOP using Goldmann applanation tonometry in the ZAP trial “was unaware of the treatment status of each eye” (ZAP; Jiang 2012 report). Gonioscopy was performed by "an examiner who was masked to the findings collected at other visits" (ZAP; Jiang 2014 report). No information has been reported on the masking of outcome assessors for other outcomes. Accordingly, we assessed this trial as having unclear risk of detection bias overall. Because the full trial reports are not yet available, we assessed both trials as having unclear risk of bias for incomplete outcome data, selective reporting, and other potential sources of bias. We have noted that neither trial received industry funding.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
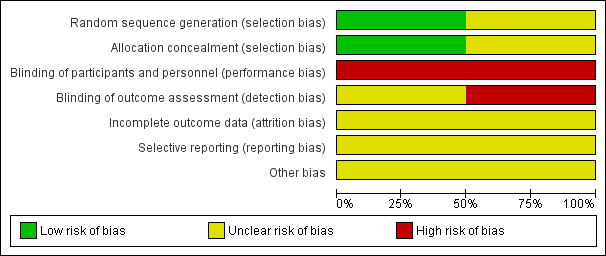
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Proportion of participants with progressive visual field loss
No trial has reported on proportion of participants with progressive visual field loss.
Mean change in IOP
No trial has reported on change in IOP at one year.
Gonioscopic findings
Data for angle width were reported in an analysis of longitudinal changes in 1550 eyes in the ZAP trial (ZAP; Jiang 2014 report). Eyes randomized to iridotomy had a larger angle width (24.0°, 95% CI 23.5° to 24.5°) than eyes randomized to no iridotomy (11.3°, 95% CI 10.9° to 11.7°) (MD 12.70°, 95 CI 12.06° to 13.34°). Using the GRADE approach, we assessed the certainty of the evidence for this outcome as moderate, downgrading for risk of bias.
Need for additional surgery
No trial has reported need for additional surgery.
Number of medication to control IOP
No trial has reported number of medication needed to control IOP.
Mean Change in BCVA
No trial has reported mean change in BCVA.
Quality of life
No trial has reported on quality of life measures.
Adverse events
Data for IOP spikes were reported for 1468 eyes in the ZAP trial. The trial found that eyes undergoing iridotomy were significantly more likely to experience IOP spikes than untreated eyes (9.8% of treated eyes vs 0.4% of untreated eyes, RR 24.00, 95% CI 7.60 to 75.83) (ZAP; Jiang 2012 report). We assessed the certainty of the evidence for this outcome as low, downgrading for risk of bias and imprecision.
Discussion
Summary of main results
We found two RCTs (2502 eyes of 1251 participants) evaluating iridotomy with Nd:YAG laser compared with no iridotomy (the ZAP and ANA‐LIS trials). We were unable to synthesize data quantitatively in a meta‐analysis because full reports of these trials are not yet available, and data were sparse for outcomes specified in this Cochrane Review. We were not able to assess the benefit of iridotomy compared to no iridotomy in eyes of patients with PACS, PAC, or PACG based on available data from RCTs. Evidence from one trial suggests that iridotomy increases angle width up to 18 months after treatment (ZAP; Jiang 2014 report) and may be associated with IOP spikes (ZAP; Jiang 2012 report).
Overall completeness and applicability of evidence
For all but two outcomes specified for this review, no data were available for analysis. Information for one of the two trials was available in a conference abstract (ANA‐LIS), which provided limited details on study design and methods, participant characteristics, and outcome measures. For the ZAP trial, there are some 2‐week and 18‐month follow‐up data available, but these data were published before the trial finished enrollment. We were not able to conduct any meta‐analyses.
Both studies took place in Asia (Singapore and China), the region with the highest prevalence of angle‐closure glaucoma (Quigley 2006; Tham 2014). All participants were adults over 50 years of age who had been diagnosed with bilateral PACS on gonioscopy. The full trial reports, when available, may help in assessing the overall completeness and applicability of the evidence.
Quality of the evidence
Based on currently available information, we assessed the overall certainty of the evidence as low to moderate due to concerns with risk of bias and imprecision. We identified no RCTs reporting on progression of visual field loss at one year.
Potential biases in the review process
We followed the standard Cochrane methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions to minimize potential for introducing biases in the review process (Higgins 2011). We worked with a medical informationist to conduct a highly sensitive search to identify trials meeting our pre‐specified eligibility criteria. We also searched trial registries, anticipating finding few or no RCTs on this topic. The review team involved two methodologists and a clinical expert. The team members worked in pairs to independently screen, review, and extract data to minimize errors and reduce bias.
Agreements and disagreements with other studies or reviews
We found no other published systematic reviews evaluating the effectiveness of iridotomy versus no iridotomy for angle closure. Determining the effectiveness and safety of iridotomy is important but full trial data are not available. We found one trial randomizing 4725 participants to screening or no screening, which we excluded from this review because the intervention was not iridotomy (Yip 2010). Participants who screened positive with PAC, however, were offered iridotomy. This trial found that screening with iridotomy offered as a prophylactic treatment did not reduce incidence of PACG at six years (Yip 2010). Additionally, there is growing interest in examining other modalities for treating PACG, such as removing pupillary block through extraction of the lens. The EAGLE trial, which randomized 419 participants to surgical lens extraction or iridotomy favored lens extraction for reducing IOP and improving quality of life (Azuara‐Blanco 2016). Among patients randomized to iridotomy, the investigators observed that IOP decreased from 30.3 mmHg (standard deviation = 8.1 mmHg) to 18.4 mmHg (standard deviation = 4.3 mmHg) at one year, but required more medical treatment to achieve this. An update of the Cochrane Review of lens extraction for management of angle‐closure glaucoma is currently underway. Despite the paucity of evidence from RCTs comparing iridotomy to no iridotomy, this intervention continues to be widely used and recommended for treatment of patients with angle‐closure glaucoma (AAO 2015).
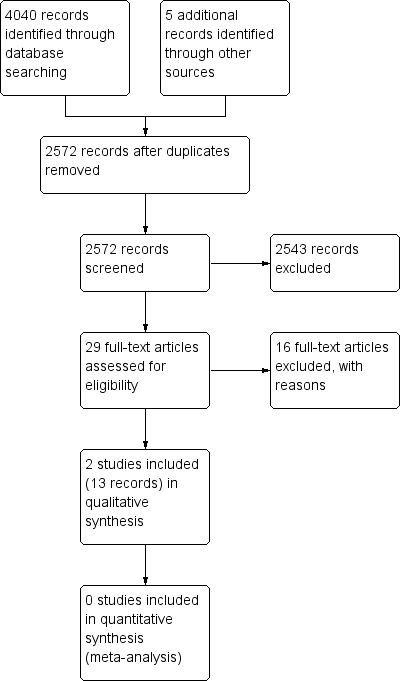
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
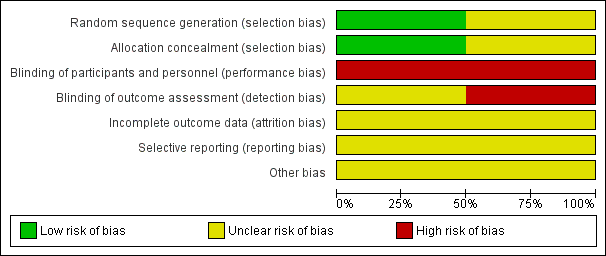
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Iridotomy vs No treatment, Outcome 1 Angle Width.

Comparison 1 Iridotomy vs No treatment, Outcome 2 Adverse events.
| Iridotomy compared to no iridotomy for patients with primary angle‐closure suspect, primary angle closure, or primary angle‐closure glaucoma | ||||||
| Patient or population: patients with primary angle‐closure suspect, primary angle closure, or primary angle‐closure glaucoma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of eyes | Certainty of the evidence | Comments | |
| Risk with no iridotomy | Risk with Iridotomy | |||||
| Proportion of progressive visual field loss at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Intraocular pressure: mean IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Gonioscopic findings: mean angle width at 1 year | The mean angle width was 11.3° in the no iridotomy group | The mean angle width in the iridotomy group was 12.7° higher | MD 12.7 | 1550 | ⊕⊕⊕⊝ | Participants in the study were primary angle‐closure suspects. Data were only available at 18 months. |
| Need for additional surgery: proportion of participants who received additional surgery to control IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Medications: mean number of medications used to control IOP at 1 year | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Quality of life measures | Not reported | Not reported | ‐ | ‐ | ‐ | |
| Adverse events ‒ IOP spike (rise greater than or equal to 8 mmHg) at 1 hour | 4 per 1000 | 98 per 1000 | RR 24.00 | 1468 | ⊕⊕⊝⊝ | Participants in the study were primary angle‐closure suspects. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded by one level for risk of bias, as the study is at unclear risk of bias for incomplete outcome data, selective outcome reporting, and other sources of bias due to the lack of availability of a full trial report. 2 Downgraded by one level for imprecision, as the confidence interval of the risk ratio between the groups is wide. | ||||||
| Primary angle‐closure suspect (PACS) | Primary angle closure (PAC) | Primary angle‐closure glaucoma (PACG) | |
| Iridotrabecular contact greater than or equal to 180° | X | X | X |
| Elevated intraocular pressure OR peripheral anterior synechiae | X | X | |
| Optic nerve damage | X |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Angle Width Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 IOP spike (rise greater than or equal to 8 mmHg) at 1 hour | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

