Aspirin (single dose) for perineal pain in the early postpartum period
Información
- DOI:
- https://doi.org/10.1002/14651858.CD012129.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 julio 2020see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For this update, Emily Shepherd and Rosalie Grivell re‐assessed the studies awaiting classification. Emily Shepherd drafted the update, with input from Rosalie Grivell.
For the previous version of this review, Sujana Molakatalla and Emily Shepherd assessed studies for inclusion and exclusion; carried out data extraction, and assessed the risk of bias of the included trials. Emily Shepherd entered data into RevMan 5 and performed the analyses. Sujana Molakatalla drafted the review with input from both Emily Shepherd (editorial) and Rosalie Grivell (editorial and clinical).
Sources of support
Internal sources
-
South Australian Health and Medical Research Institute (SAHMRI), Women and Kids, Australia
-
Department of Obstetrics and Gynaecology, Flinders University and Flinders Medical Centre, Australia
External sources
-
No sources of support supplied
Declarations of interest
Emily Shepherd: none known
Rosalie M Grivell: none known
Acknowledgements
As part of the pre‐publication editorial process, three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser (2017) commented on this review.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS), or the Department of Health and Social Care.
We acknowledge the support from the Cochrane Pregnancy and Childbirth editorial team in Liverpool. We thank Sujana Molakatalla for her contribution as an author to previous versions of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Jul 24 | Aspirin (single dose) for perineal pain in the early postpartum period | Review | Emily Shepherd, Rosalie M Grivell | |
| 2017 Feb 09 | Aspirin (single dose) for perineal pain in the early postpartum period | Review | Sujana Molakatalla, Emily Shepherd, Rosalie M Grivell | |
| 2016 Mar 21 | Aspirin (single dose) for perineal pain in early postpartum period | Protocol | Sujana Molakatalla, Emily Shepherd, Rosalie M Grivell | |
Differences between protocol and review
There are some differences between our published protocol (Molakatalla 2016), and this full review.
Methods, data collection, and analysis − assessment of pain − we clarified that our measure was 50% or greater pain relief (our protocol stated 50%). We also clarified equations used for measures of pain in the review.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Pain [*drug therapy];
- Anti-Inflammatory Agents, Non-Steroidal [*administration & dosage];
- Aspirin [*administration & dosage];
- Episiotomy [adverse effects];
- Obstetric Labor Complications [*drug therapy];
- Pain, Postoperative [drug therapy];
- *Perineum;
- Placebo Effect;
- Postpartum Period;
- Randomized Controlled Trials as Topic;
- Time Factors;
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO
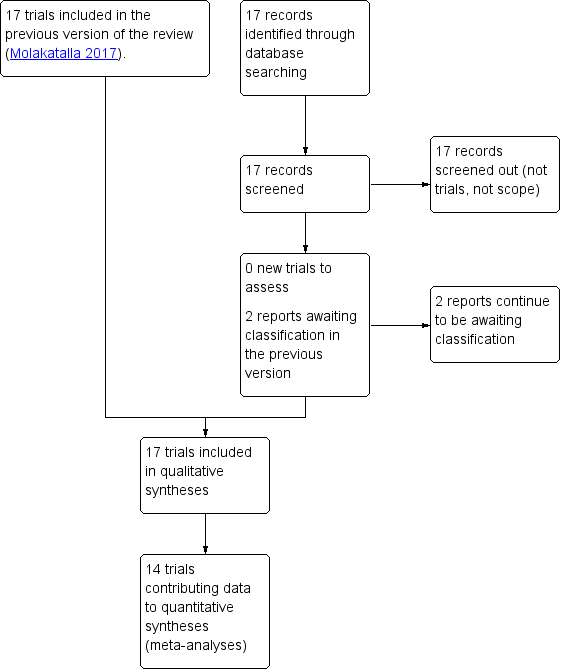
Study flow diagram

Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each 'Risk of bias" item for each included study

Funnel plot of comparison: 1 Aspirin versus placebo for perineal pain, outcome: 1.1 Adequate pain relief as reported by the women

Funnel plot of comparison: 1 Aspirin versus placebo for perineal pain, outcome: 1.2 Need for additional pain relief

Funnel plot of comparison: 1 Aspirin versus placebo for perineal pain, outcome: 1.3 Maternal adverse effects

Comparison 1: Aspirin versus placebo for perineal pain, Outcome 1: Adequate pain relief as reported by the woman

Comparison 1: Aspirin versus placebo for perineal pain, Outcome 2: Need for additional pain relief

Comparison 1: Aspirin versus placebo for perineal pain, Outcome 3: Maternal adverse effects

Comparison 2: 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 1: Adequate pain relief as reported by the woman

Comparison 2: 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 2: Need for additional pain relief

Comparison 2: 300 mg aspirin versus 600 mg aspirin for perineal pain, Outcome 3: Maternal adverse effects

Comparison 3: 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 1: Adequate pain relief as reported by the woman

Comparison 3: 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 2: Need for additional pain relief

Comparison 3: 600 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 3: Maternal adverse effects

Comparison 4: 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 1: Adequate pain relief as reported by the woman

Comparison 4: 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 2: Need for additional pain relief

Comparison 4: 300 mg aspirin versus 1200 mg aspirin for perineal pain, Outcome 3: Maternal adverse effects
| Aspirin compared with placebo for perineal pain in the early postpartum period | ||||||
|---|---|---|---|---|---|---|
| Patient or population: women with perineal pain in the early postpartum period Settings: hospitals in USA, Venezuela, Belgium, Canada, India Intervention: aspirin (single dose) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Certainty of the evidence | Comments | |
| Assumed risk for placebo | Corresponding risk for aspirin | |||||
| Adequate pain relief as reported by the woman (4 to 8 hours) | Study population | RR 2.03 (1.69 to 2.42) | 1001 (13 RCTs) | ⊕⊕⊝⊝ | ||
| 253 per 1000 | 513 per 1000 | |||||
| Need for additional pain relief (4 to 8 hours) | Study population | RR 0.25 (0.17 to 0.37) | 744 (10 RCTs) | ⊕⊝⊝⊝ | ||
| 267 per 1000 | 67 per 1000 | |||||
| Maternal adverse effects (4 to 8 hours) | Study population | RR 1.08 (0.57 to 2.06) | 1067 (14 RCTs) | ⊕⊝⊝⊝ | ||
| 27 per 1000 | 29 per 1000 (15 to 55) | |||||
| Neonatal adverse effects | (0 RCTs) | Not reported by any of the included RCTs | ||||
| Perineal pain at six weeks postpartum | (0 RCTs) | Not reported by any of the included RCTs | ||||
| *The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWe downgraded 2 levels for very serious limitations in study design: most of the trials contributing data were at unclear risk of selection bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Adequate pain relief as reported by the woman Show forest plot | 13 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.69, 2.42] |
| 1.1.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.36, 18.88] |
| 1.1.2 500 mg to 650 mg aspirin | 11 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.64, 2.39] |
| 1.1.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.84, 3.99] |
| 1.1.4 1200 mg aspirin | 3 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.25, 6.06] |
| 1.2 Need for additional pain relief Show forest plot | 10 | 744 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.17, 0.37] |
| 1.2.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.79] |
| 1.2.2 500 mg to 650 mg aspirin | 9 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.17, 0.41] |
| 1.2.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.60] |
| 1.2.4 1200 mg aspirin | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.06, 0.70] |
| 1.3 Maternal adverse effects Show forest plot | 14 | 1067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.57, 2.06] |
| 1.3.1 300 mg aspirin | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3.2 500 mg to 650 mg aspirin | 13 | 892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.51, 2.53] |
| 1.3.3 900 mg aspirin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [0.55, 11.41] |
| 1.3.4 1200 mg aspirin | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Adequate pain relief as reported by the woman Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2 Need for additional pain relief Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.3 Maternal adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Adequate pain relief as reported by the woman Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.39] |
| 3.2 Need for additional pain relief Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.30, 5.68] |
| 3.3 Maternal adverse effects Show forest plot | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Adequate pain relief as reported by the woman Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.2 Need for additional pain relief Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.3 Maternal adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

