안정형 만성폐쇄성폐질환 환자를 위한 LAMA와 LABA의 병용요법과 LABA와 ICS의 병용요법 비교
초록
배경
장시간 작용형 β‐자극제(LABA), 장시간 작용형 무스카린성 길항제(LAMA) 및 흡입 코르티코스테로이드(ICS)의 3종류의 흡입 약물은 만성 폐쇄성 폐질환(COPD)의 관리에 사용된다. 두 종류의 약제 병용이 필요한 경우 LAMA+LABA 또는 LABA+ICS가 선택되는 경우가 많다. 왜냐하면 해당 조합은 단일 흡입 장치에 의해 투여하는 것이 가능하기 때문이다. 이전 Global Initiative for Chronic Obstructive Lung Disease(GOLD)의 가이드라인은 범주 C와 D에 속한 고위험 환자들의 안정기 COPD 관리를 위해 주요 치료로 LABA+ICS를 권장했다. 그러나 새로운 GOLD의 2017년도 가이드라인에서는 LABA+ICS보다 LAMA+LABA 투여를 권장하고 있다.
목적
안정기의 COPD 환자의 치료에 있어 LAMA+LABA와 LABA+ICS의 효과와 부작용을 비교하기 위함이다.
검색 전략
Cochrane Airways Group Specialized Register(2016년 2월 2일), ClinicalTrials.gov(2016년 6월 4일), World Health Organization Clinical Trials Search Portal(2016년 6월 4일)를 이용한 전자 검색을 실시한 후 수기 검색(2016년 6월 5일)을 실시했다. 2명의 검토자가 선택한 논문을 조사하여 선별할 논문을 결정했다.
선정 기준
안정기의 COPD에 대한 LAMA+ LABA와 LABA+ICS를 비교한 독립적인 무작위대조시험, 병행군간 대조시험 및 교차대조임상시험을 포함하였다. 시행 기간의 최소값은 1개월로하고, 외래 환자만을 대상으로 했다.
자료 수집 및 분석
2명의 검토자가 독립적으로 데이터를 추출하고 바이어스 위험을 평가했다. 검토자간의 의견불일치는 토론으로 해결했다. 이분된 결과 변수는 교차비(OR), 연속된 결과 변수는 평균차(MD)로 95% 신뢰구간(CI)을 이용하여 RevMan5에서 분석했다. 악화는 0번 또는 1번 이상 악화증상을 겪은 환자의 숫자로 측정하였다.
주요 결과
9,839명의 참가자를 포함한 11건의 연구를 메타분석의 대상으로 하였다. 대부분의 연구는 중등도에서 중증의 COPD 환자를 대상으로 하였으며 최근의 악화는 제외되었다. 최근의 악화를 경험한 사람만을 대상으로 한 제약 회사 주도의 임상시험이 최대의 연구이며, 참가자의 37%를 차지했다. 1건의 연구를 제외한 모든 연구가 제약 회사의 자금 지원을 받고 있었기 때문에 기타 비뚤림 위험이 높다고 평가했다. 제약 회사의 자금 지원을 받지 않은 하나의 연구는 수행, 검출, 출판 비뚤림의 위험이 높았다.
5건의 연구가 GOLD 카테고리 B의 참가자를 모집하였고 1건의 연구는 카테고리 D의 참가자를 모집하였으며 2건의 연구는 카테고리 A/B 참가자를 모집하고 3건의 연구는 카테고리에 관계없이 참가자를 모집했다. 추적 기간은 6˜52주였다.
LABA+ICS 군과 비교하여 LAMA+LABA 군의 통합된 주요 평가 항목의 결과는 다음과 같다: 악화, OR 0.82 (95% CI 0.70˜0.96, p= 0.01, I² =17%, 근거의 질 낮음); 심각한 부작용 0.91(95% CI 0.79˜1.05, p=0.18, I² =0%, 근거의 질 중간); St. George 's Respiratory Questionnaire(SGRQ) 기저선에서의 총 점수 변화, MD ‐1.22(95% CI 2.52˜0.07, p=0.06, I² =71%, 근거의 질 낮음); 1초 강제호기량(FEV1) 기저선에서의 변화, MD 0.08L(95% CI 0.06˜0.09, p<0.0001 , I²=50%, 근거의 질 중간). 마찬가지로 이차 평가 항목의 결과는 다음과 같다: 폐렴, OR 0.57(95% CI 0.42˜0.79, p=0.0006, I²=0%, 근거의 질 낮음); 전체 사망, OR 1.01(95% CI 0.61˜1.67, p=0.88, I²=0%, 근거의 질 낮음), 임상적으로 의미있는 최소 변화량(4점) 이상의 SGRQ의 기저선으로부터의 개선, OR 1.25(95% CI 1.09˜1.44, p=0.002, I²=0%, 근거의 질 중간).
연구진 결론
COPD 치료에서 LAMA+LABA 치료군에서는 악화가 적고, FEV1의 개선이 크게 나타나며, 폐렴의 위험성이 낮고, SGRQ에서 4점 이상의 삶의 질 개선이 높은 빈도로 관찰되었다. 이러한 결과는 중등도에서 중증의 COPD 환자를 대상으로 한 관찰 기간이 1년 미만인 서로 이질적인 연구에 의한, 중간에서 낮은 질의 근거에 근거하고 있다. 이 결과는 GOLD 최신 지침의 변경을 지지한다.
PICOs
쉬운 말 요약
어떤 약물의 조합이 만성 폐쇄성 폐질환(COPD)에 안전하고 효과적인가
배경
만성 폐쇄성 폐질환(COPD)은 오랜 기간 동안 기침, 가래 및 호흡 곤란을 특징으로 하는 폐질환이다. 현재 장시간 작용형 무스카린 길항제+장시간 작용형 β‐작용제(LAMA+LABA), LABA+흡입 코르티코스테로이드(LABA+ICS)의 2종류의 합제가 하나의 흡입기로 사용가능하다. 최근의 지침에서는 LABA+ICS보다 LAMA+LABA 투여를 권장하고있다.
연구 특성
COPD 환자에 대한 LAMA+LABA와 LABA+ICS의 효과와 부작용을 비교한 9,839명의 참가자를 대상으로 한 11건의 임상시험을 통합했다.
주요 결과
심각한 부작용과 사망 위험은 두 치료 모두 유의미한 차이가 없었다. LABA+ICS에 비해 LAMA+LABA는 재발 위험이 낮고, 폐렴의 발병이 적고, 호흡 기능 및 일상생활에서의 증상이 양호하게 개선되었다.
근거의 질
분석된 연구의 대부분은 제약 회사의 지원을 받고 있었기 때문에 결과를 신중하게 해석할 필요가 있다. 그러나 이러한 연구는 대체로 일반적으로 수용가능한 방식으로 실시되고 있었다. 이 데이터는 1년 미만의 기간 동안 연구된 중등도에서 중증의 만성 폐쇄성 폐질환(COPD) 환자의 임상시험에서 얻은 질 낮은 또는 중간 정도의 우수한 근거를 뒷받침한다.
Authors' conclusions
Summary of findings
| LAMA + LABA versus LABA + ICS for stable COPD | ||||||
| Population: stable COPD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effects | Number of participants | Quality of the evidence | Comments | |
| LABA+ICS | LAMA+LABA | |||||
| Exacerbations (number of people experiencing ≥ 1 exacerbations) Follow‐up: 12 to 52 weeks | 377 per 1000 | 332 per 1000 (298 to 368) | OR 0.82 | 8922 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| Serious adverse effects Follow‐up: 12 to 52 weeks | 96 per 1000 | 87 per 1000 | OR 0.91 | 9793 | ⊕⊕⊕⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (MD) Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD ‐1.22 (‐2.52 to 0.07) | 6055 (6 RCTs) | ⊕⊕⊝⊝ | Low MD means favourable outcome |
| Trough FEV1 change from the baseline Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD 0.08 L (0.06 to 0.09) | 6238 (6 RCTs) | ⊕⊕⊕⊝ | High MD means favourable outcome |
| Pneumonia Follow‐up: 12 to 52 weeks | 26 per 1000 | 15 per 1000 | OR 0.57 | 8540 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| All‐cause death Follow‐up: 12 to 52 weeks | 7 per 1000 | 7 per 1000 | OR 1.01 | 8200 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (≥ 4 points, MCID) Follow‐up: 24 to 52 weeks | 445 per 1000 | 500 per 1000 (466 to 535) | OR 1.25 (1.09 to 1.44) | 3192 (2 RCTs) | ⊕⊕⊕⊝ | High OR means favourable outcome |
| *The absolute risk (and its 95% CI) of LAMA+LABA group is based on the assumed risk in the LABA+ICS group and the OR of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect | ||||||
| 1 Every study had at least one domain of high risk of bias mostly due to conflicts of interest. 2 Indirectness due to definition of exacerbation. 3 There was a considerable heterogeneity, I2 = 71%. 4 Downgraded due to imprecision. | ||||||
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is characterised mainly by bronchial obstruction, systemic inflammation, and comorbidities. It is the third leading cause of death in the world, with more than three million people dying as a result of COPD every year (WHO 2015). The condition is a concern not only for pulmonologists, but also for physicians and general practitioners. In addition to active tobacco smoking, air pollution, and occupational exposures play a central role in the development of COPD. The most common symptoms of COPD ‐ shortness of breath on exertion and cough ‐ are present for a prolonged period and typically worsen over time (GOLD 2016).
Since the late 1960s, the definition of COPD has been modified repeatedly. Early definitions of COPD included chronic bronchitis, which is clinically characterised by chronic cough, and emphysema, which is pathologically defined by damaged sacs or alveoli in the lungs (Burrows 1966). Following the 1995 American Thoracic Society Statement, in 2001, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) released its first report, Global Strategy for the Diagnosis, Management, and Prevention of COPD (Pauwels 2001), which supported the definition of COPD that indicates the disorder is recognised primarily by chronic obstruction of lung airflow (Pauwels 2001).
If COPD is properly diagnosed and managed, some symptoms can be ameliorated. Smoking cessation and vaccination are the first steps in COPD management, and daily pharmacological treatment is required for most people with COPD (GOLD 2016).
Description of the intervention
Whilst asymptomatic people with mild airflow limitation can be treated with on‐demand short‐acting bronchodilators, key medications for symptomatic COPD management consist of three classes of inhaler device medications: long‐acting beta‐agonists (LABA), long‐acting muscarinic antagonists (LAMA), and inhaled corticosteroids (ICS) (GOLD 2016). If the disease cannot be controlled adequately with LAMA or LABA monotherapy, administration of two or more medications from different classes may prove beneficial. When two classes of medications are required, LAMA plus LABA (LAMA+LABA) and LABA plus ICS (LABA+ICS) are often selected because these combinations can be administered via one medication device (Frampton 2014; Malerba 2014; Nannini 2013; Schachter 2013), which is most beneficial for improving patient adherence (Horita 2015a).
How the intervention might work
Currently, there is no medication that cures COPD. Thus, the practical goal of COPD treatment is to control symptoms, reduce frequency of exacerbations, and improve exercise tolerance. The treatment of COPD usually consists of smoking cessation, vaccination, inhaled bronchodilators, ICS, oral medication, long‐term oxygen therapy, and pulmonary rehabilitation (GOLD 2017). According to the GOLD approach, people are classified into Categories A to D depending on the degree of symptoms and the risk of exacerbations (GOLD 2017). Medications belonging to specific class are recommended based on the following:
-
category A: bronchodilator (short or long acting); consider switching to another depending on response;
-
category B: long‐acting bronchodilator (LAMA or LABA), or both LAMA and LABA if symptoms not controlled on one drug;
-
category C: LAMA; consider switching to LAMA+LABA or to LABA+ICS if further exacerbations occur (LAMA+LABA now preferred over LABA+ICS);
-
category D: LAMA+LABA initially (unless high blood eosinophil counts or people with asthma‐COPD overlap syndrome (ACOS), in which case LABA+ICS may be preferred); consider triple therapy if symptoms persist. Roflumilast or a macrolide (e.g. azithromycin) (or both) may also be considered.
LAMA: LAMAs dilate the airway by selectively blocking acetylcholine M3 receptors (Alagha 2014), and by inhibiting bronchoconstriction. Since the early 2000s, LAMAs, especially tiotropium, have been regarded as the first‐choice medication for treating people with COPD. LAMAs confer anti‐inflammatory, and even more importantly, anti‐airway remodelling effects (Tashkin 2004).
LABA: beta‐agonists widen the airways by relaxing airway muscles. Studies suggest that LABAs might also provide anti‐inflammatory and protective effects against bronchoconstrictive substances. Regular use of a short‐acting beta‐agonist that works quickly and lasts for four to six hours is not currently recommended for people with asthma or COPD. A LABA that lasts for about 12 to 24 hours is considered to be a maintenance medication (Anderson 2014; Tashkin 2004).
ICS: ICSs reduce inflammation in the airways. Although ICSs are indicated for bronchial asthma in which eosinophils play a key role, they are not so effective when neutrophils are observed in the airways of people with COPD (Barnes 2010; Hanania 2008; Suissa 2009). The previous GOLD guideline recommended that ICS is prescribed combined with LABA for people with COPD with severe airflow limitation or with high risk of exacerbation (GOLD 2016). Studies suggest that LABA+ICSs may be highly effective for people with a high sputum/blood eosinophil count (Pascoe 2015).
Why it is important to do this review
The previous GOLD guidelines recommend first‐line use of ICS only for people with category C and D COPD, that is, people with severe to very severe airflow limitation and two or more exacerbations per year, with one or more hospitalisations for exacerbations (GOLD 2016). The previous guidelines suggested that ICSs reduced the risk of exacerbations (GOLD 2016). Nonetheless, prescription rates for ICSs and combined LABA+ICS agents are high (Drivenes 2014; Price 2014; White 2013). This is probably because many randomised controlled trials (RCTs) have supported the hypothesis that salmeterol (LABA)/fluticasone propionate (ICS) combination, which is the oldest combination treatment, can improve quality of life, especially for people with dyspnoea, and can also decrease acute exacerbations of COPD, and reduce yearly declines in pulmonary function (GOLD 2016).
Although controversial (Wedzicha 2016), blood/sputum eosinophil counts can serve as predictive biomarkers for differentiating people with COPD who will derive the greatest benefit from ICS administration from people who will not benefit from an ICS (Pascoe 2015).
The GOLD 2017 guidance recommends LAMA+LABA over LABA+ICS for people belonging to categories B, C, and D (GOLD 2017).
It should be re‐evaluated which type of combination treatment (LAMA+LABA or LABA+ICS) is most beneficial for people with COPD. Researchers must continue to evaluate the effectiveness of these treatments.
Objectives
To compare the benefits and harms of LAMA+LABA versus LABA+ICS for treatment of people with stable COPD.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include individual and cluster RCTs and cross‐over trials, but not quasi‐RCTs. However, we found no cluster RCTs. We included studies reported as full text, those published as abstract only, and unpublished data. When we could not obtain sufficient data from published articles, we contacted authors and sponsors, and accessed trial registration websites. We included open‐label studies, single‐blinded studies and double‐blinded studies. The minimum accepted trial duration was one month.
Types of participants
We included adults with a diagnosis of COPD according to GOLD guidelines (GOLD 2016). We did not set specific exclusion criteria involving comorbidities. We planned to exclude original studies focusing on ACOS.
Types of interventions
We included trials comparing LAMA+LABA versus LABA+ICS. We permitted treatments administered via a single combined device or via two separate devices. We excluded trials of short‐acting bronchodilators (e.g. ipratropium). We included cointerventions when they were not part of the randomly assigned treatment.
Types of outcome measures
Primary outcomes
-
Exacerbations (participants with one or more).
-
Serious adverse events (SAE) (participants with one or more).
-
St. George's Respiratory Questionnaire (SGRQ) total score change from baseline (mean difference (MD)).
-
Trough forced expiratory volume in one second (FEV1) change from baseline.
Secondary outcomes
-
Pneumonia* (participants with one or more occurrences).
-
All‐cause death.
-
SGRQ total score change from baseline (4 points or greater).
-
Hospitalisations for COPD exacerbations (participants with one or more occurrences).
*Pneumonia was assessed based on X‐ray.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which was maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), and PsycINFO, and by handsearching of respiratory journals and conference abstracts (see Appendix 1 for details). We searched all records in the CAGR on 2 February 2016 using the search strategy provided in Appendix 2.
We conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) Clinical Trials Search Portal (www.who.int/ictrp/en/) on 4 June 2016. We searched all databases from their inception, and we imposed no restrictions on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references, and we searched relevant manufacturers' websites for trial information. We searched for errata or retractions from included studies published as full text on PubMed (www.ncbi.nlm.nih.gov/pubmed), and reported within the review the date when this was done. These handsearches were done up to 5 June 2016.
Data collection and analysis
Selection of studies
Two review authors (NH and YS) independently screened the titles and abstracts of all studies identified by the search for possible inclusion, and coded studies as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text publications. Two review authors (NH and YS) independently screened the full texts to identify studies for inclusion and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, when required, we consulted a third review author (TK). We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form that had been piloted on at least one study in the review to document study characteristics and outcome data. Two review authors (NH and YS) extracted the following study characteristics from the included studies.
-
Methods: study design, duration of study follow‐up and 'run‐in' period, number of study centres and countries, and study start date.
-
Participants: number, mean and standard deviation (SD) age, gender, mean and SD of baseline FEV1 key inclusion criteria, number of participants randomised and completed, and follow‐up duration.
-
Interventions: intervention, comparison, and dosage of the intervention.
-
Outcomes: primary outcomes specified and collected and time points reported.
-
Notes: funding for trial and notable conflicts of interest (COI) of trial authors, trial registration, and other information if necessary.
Two review authors (NH and YS) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by consensus or by consultation with a third review author (TK). One review author (NH) transferred data into the Review Manager 5 (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review versus data provided in study reports. A second review author (YS) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (NH and YS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (EO). We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias.
We graded each potential source of bias as high, low, or unclear and provided an explanation from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality and risk of bias for a participant‐reported outcome might be very different). When we requested information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the previously published protocol (Horita 2016).
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR), and continuous data as MD with 95% confidence intervals (CI). We entered data presented as a scale with a consistent direction of effect (i.e. data in the LAMA+LABA arm minus data in the LABA+ICS arm). Although there is no universal rule to interpret the magnitude of the therapeutic effect from ORs, we believe that an OR greater than 1.5 and an OR of less than 0.7 mean that there is a considerable chance that the outcome is clinically important.
We undertook meta‐analyses only when this was meaningful (i.e. if treatments, participants, and the underlying clinical question were similar enough for pooling to make sense).
We had planned to describe skewed data using medians and interquartile ranges; however, we found no report describing skewed data.
According to the original protocol, when multiple trial arms were reported in a single trial, we planned to include only the relevant arms. However, we did not find such studies.
Unit of analysis issues
We analysed the number of participants, not the number of events, as the unit of analysis for dichotomous data (i.e. participants with one or more events). For continuous data, we used MDs.
Dealing with missing data
We tried to contact investigators, study sponsors, and registration websites to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was only reported in an abstract format). When this was not possible, and when missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by conducting a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis: 0% to 40%: might not be important; 30% to 60%: might represent moderate heterogeneity; 50% to 90%: might represent substantial heterogeneity; 75% to 100%: might show considerable heterogeneity (Higgins 2011). When we identified considerable heterogeneity, we reported this and explored possible causes by performing a prespecified subgroup analysis.
Assessment of reporting biases
We created and examined a funnel plot to explore possible small‐study and publication biases when more than 10 trials could be pooled for an outcome.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis by using a fixed‐effect model (Sensitivity analysis).
'Summary of findings' table
We created a 'Summary of findings' table that includes the following outcomes.
-
Exacerbations (participants with one or more).
-
SAEs (participants with one or more).
-
SGRQ total score change from the baseline (MD).
-
Trough FEV1 change from the baseline.
-
Pneumonia (participants with one or more occurrences).
-
All‐cause death.
-
SGRQ total score change from the baseline (4 points or greater).
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence (very low, low, moderate, and high quality of evidence) as it related to studies that contributed data to meta‐analyses for prespecified outcomes (Guyatt 2008). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) with GRADEpro software (GRADEpro). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we provided comments to aid readers' understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for all primary and secondary outcomes. We believe these subgroup data are especially useful for identifying the cause of heterogeneity, however we were unable to perform the severity subgroup analysis because separate data for participants with different severities were not reported. We used the I2 test to detect heterogeneity as discussed in (Higgins 2003).
-
LAMA+LABA: "combined indacaterol + glycopyrronium bromide (QVA149, IND/GLY)" versus "combined umeclidinium + vilanterol (UMEC/VI)" versus "other LAMA/LABA inhalers".
-
COPD severity: 'including only mild or moderate (or both) (% predicted FEV1 50% or greater)' versus 'including severe and/or very severe (% predicted FEV1 less than 50%)' versus 'including both categories.'
We used the formal test for subgroup interactions provided in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We planned to carry out the following sensitivity analyses. We were only able to carry out the first two analyses.
-
Excluding unblinded studies from the analysis.
-
Analysing the data using a fixed‐effect model.
-
When a study was at high risk of bias for allocation concealment and attrition (greater than 20%), we planned to perform sensitivity analyses for primary outcomes by removing this study.
Results
Description of studies
See Characteristics of included studies, (and Table 1), Characteristics of excluded studies, Characteristics of ongoing studies tables.
| Study | LAMA+LABA | LABA+ICS | Key inclusion criteria | Follow‐up duration (weeks) | Mean/median age (years) | Number randomised |
| Tiotropium/olodaterol (2.5/5 μg) or tiotropium/olodaterol (5/5 μg) | Salmeterol/fluticasone (50/250 μg) twice daily or salmeterol/fluticasone (50/500 μg) twice daily. | %pred FEV1 30% to 80% Ex(‐) | 6 × 4 time periods (cross‐over) | 64 | 229 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 63 | 707 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 64 | 700 | |
| Tiotropium/indacaterol (18/150 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 80%, Ex(‐) | 16 | 71 | 46 | |
| Tiotropium/salmeterol (18/50 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 8 x 2 time periods (cross‐over) | 61 | 344 | |
| Tiotropium/formoterol (18/24 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 6 | 62 | 605 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 62 | 717 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, Ex(‐) | 26 | 63 | 523 | |
| Aclidinium/formoterol (400/12 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 < 80%, CAT ≥ 10, Ex(‐) | 24 | 63 | 933 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 25% to 60%, mMRC ≥ 2, Ex(+) | 52 | 65 | 3362 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, mMRC ≥ 2, Ex(‐) | 26 | 65 | 744 |
%pred FEV1: % predicted forced expiratory volume in one second; CAT: chronic obstructive pulmonary disease assessment test; Ex(‐): without recent exacerbation; Ex(+): with recent exacerbation; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; mMRC: modified Medical Research Council dyspnoea scale.
Results of the search
An electronic search of the CAGR (via the Cochrane Register of Studies) conducted by a trained librarian (ES) on 2 February 2016 identified 393 candidate reports, excluding duplicates. Additional search by ClinicalTrial.gov found 56 reports. Additional search by WHO search portal found 70 reports. Hand search found a report. Update search on 7th September 2016 found 45 reports. Therefore, the total number of candidates were 565. Among the 565, 61 were removed due to duplicate, 452 were removed by screening, 40 were removed by full‐text check. The remaining 12 were used for quantitative synthesis. Among the 12, two ongoing studies without results were not used for quantitative synthesis. One paper reported two independent RCTs (Donohue 2015a; Donohue 2015b).
Eventually, the quantitative synthesis included 10 articles representing 11 trials (Figure 1).

Study flow diagram.
Included studies
We included 11 studies comprising 9839 participants. All reports used individual randomisation, 10 used parallel‐group design, and one used a cross‐over design. The number of participants included in each study ranged from 46 to 3362, with a median of 700 participants per study. Most studies included people with moderate to severe COPD without recent exacerbations. One trial that included only people with a recent exacerbation was the largest study and accounted for 37% of total participants. In each study, 65% to 91% (median 72%) were men. Mean age in each study ranged 61 years to 71 years with a median mean of 63 years. Every study included participants with both per cent predicted (%pred) FEV1 less than 50% and %pred FEV1 greater than 50%. One study only included participants with recent exacerbations (Wedzicha 2016), while the other studies only included participants without recent exacerbations. Five studies recruited people with category B COPD, one study recruited people with category D COPD, two studies recruited people with category A/B COPD, and three studies recruited people regardless of category.
Treatment
Treatment duration ranged from 6 to 52 weeks. Of the LABA+ICS treatments used in these studies, one study used uncombined salmeterol/fluticasone propionate (Rabe 2008) and the other studies used combined salmeterol/fluticasone propionate. Of the administered LAMA+LABA treatments, three studies used indacaterol/glycopyrronium (Vogelmeier 2013; Wedzicha 2016; Zhong 2015), three studies used umeclidinium/vilanterol (Donohue 2015a; Donohue 2015b; Singh 2015), one study used tiotropium/olodaterol (Beeh 2016), one study used tiotropium/indacaterol (Hoshino 2015), one study used tiotropium/salmeterol (Magnussen 2012), one study used tiotropium/formoterol (Rabe 2008), and one study used aclidinium/formoterol (Vogelmeier 2016).
Outcomes
With regards to the primary outcomes, eight studies reported FEV1‐related outcomes (Beeh 2016; Donohue 2015a; Donohue 2015b; Rabe 2008; Singh 2015; Vogelmeier 2013; Vogelmeier 2016; Zhong 2015), one study reported airway dimensions (Hoshino 2015), one study reported rate of exacerbations (Wedzicha 2016), and one study reported both forced residual capacity and endurance time as coprimary endpoints (Magnussen 2012) (see Characteristics of included studies table).
Excluded studies
We excluded seven studies with reasons; six due to a no comparison between LAMA+LABA and LABA+ICS (Bruhn 2003; Calverley 2007; Knobil 2004a; Knobil 2004b; NCT00120978; Sciurba 2004), and one because the cost‐effectiveness analysis design used previously published data (Price 2014) (see Characteristics of excluded studies table).
Ongoing studies
We found two ongoing studies awaiting results (NCT02497001; NCT02516592).
One study is a moderate‐sized trial comparing indacaterol/glycopyrronium with salmeterol/fluticasone sponsored by Novartis. The study comparison and inclusion criteria are similar to those of included studies sponsored by Novartis. The second study is a large‐sized four‐arm trial comparing glycopyrronium/formoterol/budesonide (aerosol), glycopyrronium/formoterol (aerosol), formoterol/budesonide (aerosol), and formoterol/budesonide (powder). The primary outcomes of these studies are trough FEV1 change from the baseline at the end of follow‐up. Both studies started in 2015.
Risk of bias in included studies
Included studies had generally low risk of bias for random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. However, all but one studies were sponsored by pharmaceutical companies, thus we marked them as high risk of other bias (Figure 2).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
While seven studies reported centralised randomisation using acceptable methods, the remaining four did not provide information on randomisation (Beeh 2016; Hoshino 2015; Rabe 2008; Vogelmeier 2016). These four studies had unclear risk of bias.
Blinding
While 10 studies were conducted in a double‐blinded manner, one adopted neither double‐ nor single‐blinding methods (Hoshino 2015).
Incomplete outcome data
Our prespecified criteria for high attrition bias was a dropout rate of more than 20% of randomised participants. However, no trial had a dropout rate of more than 20%. One study did not report the completion rate (Rabe 2008).
Selective reporting
Our criteria for rating a study as having a risk of selective reporting bias was if it was a non‐registered trial or considerably deviated from the registered protocol concerning outcome reporting. One trial had a high risk of selective reporting bias due to non‐registration (Hoshino 2015).
Other potential sources of bias
Ten out of 11 trials were sponsored by pharmaceutical companies, thus we marked them as high risk of other bias (Beeh 2016; Donohue 2015a; Donohue 2015b; Magnussen 2012; Rabe 2008; Singh 2015; Vogelmeier 2013; Vogelmeier 2016; Wedzicha 2016; Zhong 2015). We found no other source of bias apart from COIs.
Effects of interventions
See summary of findings Table for the main comparison for the main comparisons.
Primary outcomes
Exacerbations (participants with one or more)
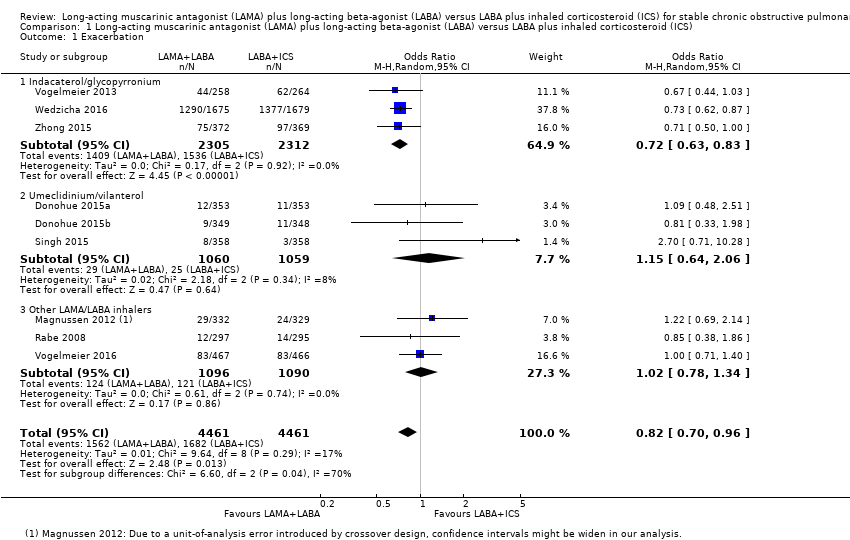
Nine studies with 8932 participants evaluated exacerbations. Based on these nine studies with 12 to 52 weeks of observation, compared to LABA+ICS, there was a significant decrease in the number of people experiencing one or more exacerbations with LAMA+LABA (OR 0.82, 95% CI 0.70 to 0.96; P = 0.01; I2 = 17%; Figure 3; Analysis 1.1; low quality evidence). In the LAMA+LABA subgroup analysis, participants who were treated with indacaterol/glycopyrronium had fewer exacerbations (OR 0.72, 95% CI 0.63 to 0.83; P < 0.001; I2 = 0%) compared to participants treated with LABA+ICS. In contrast, LAMA+LABA was not related to reduced risk of exacerbation in umeclidinium/vilanterol and the other LAMA+LABA subgroups.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.1 Exacerbation.
Although we did not plan to evaluate the time to first exacerbation in the protocol, one trial reported some useful data. The largest study evaluated the time to the first exacerbation (Wedzicha 2016). The hazard ratio for time to the first exacerbation was 0.84 (95% CI 0.78 to 0.91; P < 0.001) in favour of LAMA+LABA arm. The annual exacerbation rate was lower in the LAMA+LABA arm than in the LABA+ICS arm (3.59 per year versus 4.03 per year; rate ratio, 0.89, 95% CI 0.83 to 0.96; P = 0.003).
Serious adverse events (participants with one or more)
Eleven studies with 9793 participants evaluated SAEs with 12 to 52 weeks of observation. However, we discarded data from one study from the analysis because there were no SAEs in either arm (Hoshino 2015). Based on the remaining 10 studies, compared to LABA+ICS, LAMA+LABA was associated with a non‐significant decrease in SAE (OR 0.91, 95% CI 0.79 to 1.05; P = 0.18; I2 = 0; Figure 4; Analysis 1.2; moderate quality of evidence). In the LAMA+LABA subgroup analysis, we observed no heterogeneity (I2 = 0%). Funnel plot did not indicate publication bias (Figure 5).
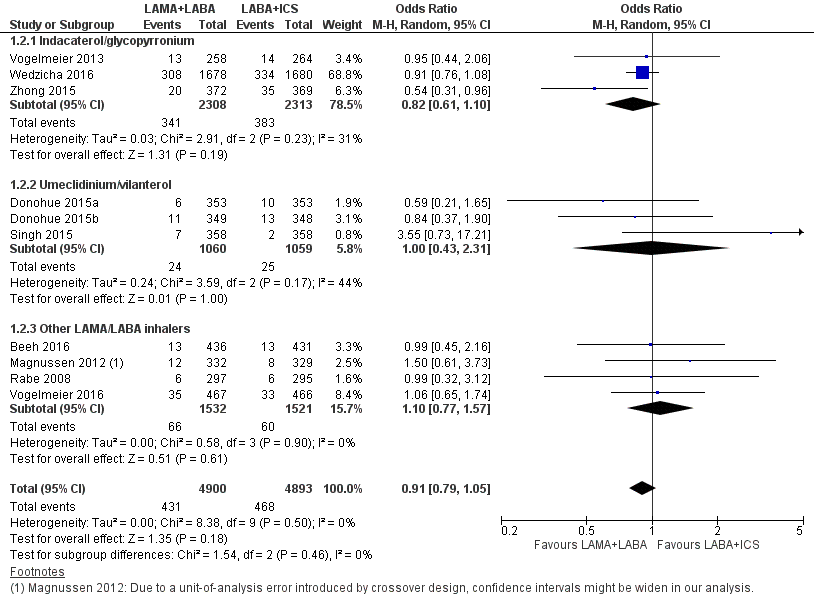
Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.
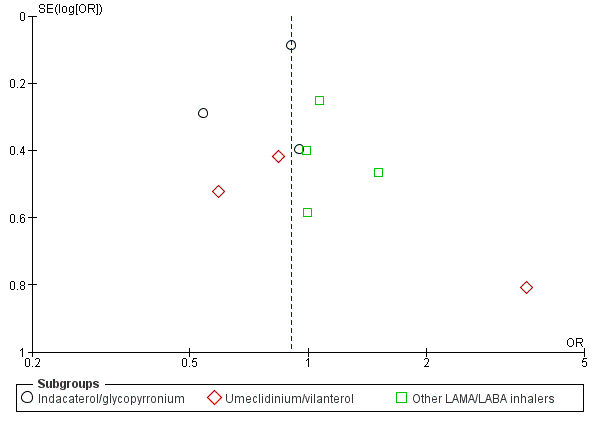
Funnel plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.
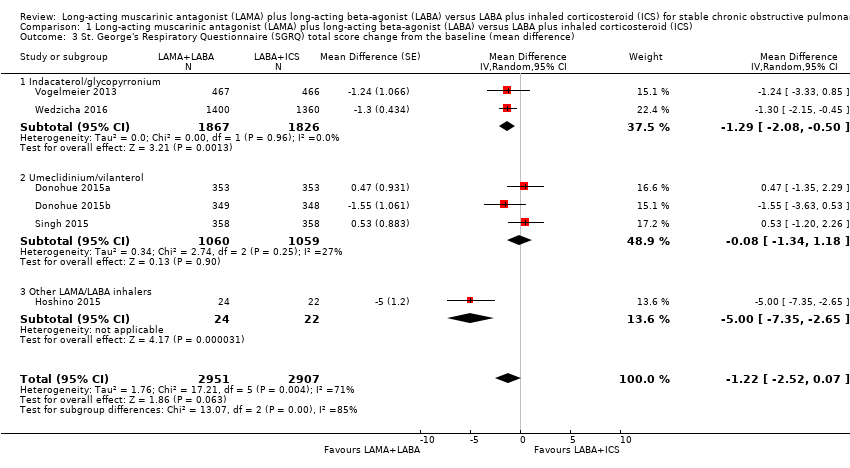
SGRQ total score change from the baseline (mean difference)
Six studies with 5858 participants assessed SGRQ total score change from the baseline with 12 to 52 weeks of observation. In these six studies, compared to LABA+ICS, there was a non‐significant decrease in SGRQ total score change from the baseline with LAMA+LABA, with an MD of ‐1.22 (95% CI ‐2.52 to 0.07; P = 0.06; I2 = 71%; Figure 6; Analysis 1.3, low quality evidence). In the LAMA+LABA subgroup analysis, there was a significant decrease in scores in participants treated with indacaterol/glycopyrronium and 'other LAMA/LABA inhalers' compared to participants treated with LABA+ICS (indacaterol/glycopyrronium: MD ‐1.29, 95% CI ‐2.08 to ‐0.50; P = 0.001; I2 = 0%; other LAMA/LABA inhalers: MD ‐5.00, 95% CI ‐7.35 to ‐2.65, P < 0.0001).
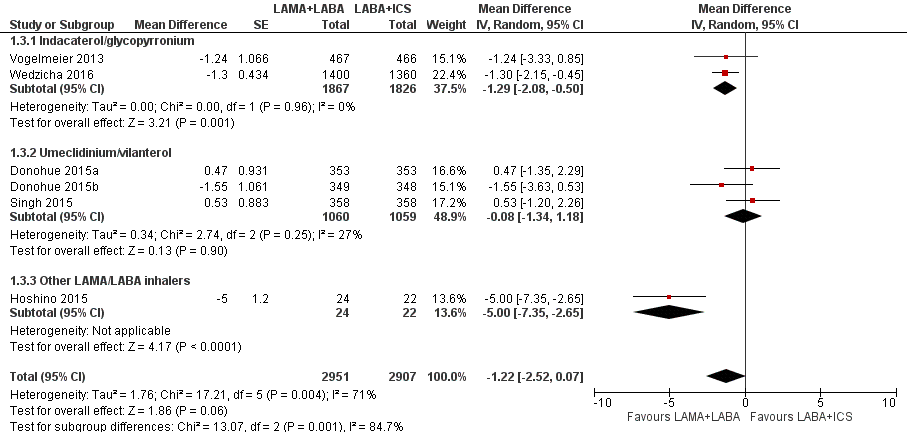
Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), outcome: 1.3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference).
Trough forced expiratory volume in one second change from the baseline
In total, six studies with 7277 participants reported 12 to 52 weeks of observation for trough FEV1 change from the baseline. Compared to LABA+ICS, there was a significant increase in trough FEV1 change from the baseline with LAMA+LABA (MD 0.08 L, 95% CI 0.06 to 0.09; P < 0.0001; I2 = 50%; Figure 7; Analysis 1.4, moderate quality evidence). This was larger than the minimal clinically important difference of 0.05 L. In the LAMA+LABA subgroup analysis, each LAMA+LABA subgroup was consistently associated with an increase in trough FEV1 change from the baseline.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.4 Trough forced expiratory volume in one second (FEV1) change from the baseline.
Secondary outcomes
Pneumonia (participants with one or more occurrences)
Eight studies with 8540 participants evaluated pneumonia with 12 to 52 week of observation. Compared to LABA+ICS, there was a significant reduction in the number of participants experiencing one or more episodes of pneumonia with LAMA+LABA (OR 0.57, 95% CI 0.42 to 0.79; P = 0.0006; I2 = 0%; Analysis 1.5; low quality evidence). In the LAMA+LABA subgroup analysis, the estimated decrease of pneumonia expressed as pooled OR was within the range of 0.37 to 0.50. An OR of 0.57 almost halved the odds and was considered to be a large decrease in risk. Although it would be possible to calculate an absolute risk reduction, we decided not to, as the absolute effect size is highly dependent on study duration.
All‐cause death
Eight studies with 8200 participants evaluated all‐cause death with 12 to 52 weeks of observation. There was a similar risk of all‐cause death with both treatment regimens (OR 1.01, 95% CI 0.61 to 1.67; P = 0.88; I2 = 0%; Analysis 1.6; low quality evidence). Results were constant across all LAMA+LABA subgroups.
St. George's Respiratory Questionnaire total score change from the baseline (4 points or greater)
Two studies with 3192 participants evaluated SGRQ total score change from baseline (4 points or greater) with 24 to 52 weeks of observation. Compared to LABA+ICS, there was a more frequent change in SGRQ total score (4 points or greater) with LAMA+LABA (OR 1.25, 95% CI 1.09 to 1.44; P = 0.002; I2 = 0%; Analysis 1.7; moderate quality evidence).
Hopsitalisations for COPD exacerbations
Outcome not reported.
Sensitivity analysis using a fixed‐effect model
The sensitivity analysis using a fixed‐effect model suggested a similar pooled OR and a similar pooled MD for all outcomes.
Sensitivity analysis excluding unblinded studies
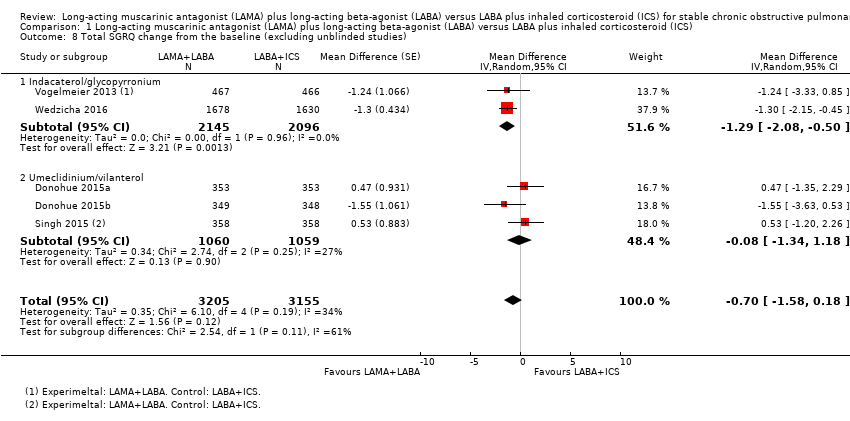
We found one unblinded study that provided data for SGRQ total score change from the baseline (Hoshino 2015). After excluding this study, five studies evaluated SGRQ total score change from the baseline. Compared to LABA+ICS, there as a non‐significant decreasing SGRQ total score change from the baseline with LAMA+LABA (MD ‐0.70, 95% CI ‐1.58 to 0.18; P = 0.12; I2 = 34%) (Analysis 1.8).
Discussion
Summary of main results
We conducted a systematic review and meta‐analysis to compare the efficacy and safety of LAMA+LABA and LABA+ICS for people with stable COPD. We found 11 studies consisting of 9839 participants. Most studies were well designed, but may have been at high risk of bias due to commercial sponsorship. Compared to LABA+ICS, LAMA+LABA led to significantly fewer exacerbations, larger trough FEV1 change from the baseline, a reduced risk of pneumonia, and more frequent SGRQ total score improvement more than minimal clinically important difference. In contrast, we did not detect any significant differences in SAEs, SGRQ total score change from the baseline, and all‐cause death (summary of findings Table for the main comparison).
Overall completeness and applicability of evidence
Most of the studies included in this analysis recruited people with moderate to severe COPD as categorised according to GOLD guidelines. Therefore, we should be careful when applying the results from our analysis to people with mild and very severe COPD.
Furthermore, many new medications have been developed in the LAMA and LABA classes, for example: glycopyrronium, umeclidinium and aclidinium (LAMA); and indacaterol, vilanterol, and olodaterol (LABA). These medications generally lead to larger improvements of FEV1 than previous medications, such as tiotropium and salmeterol. Further investigations into the effects of combining these new medications are needed, namely glycopyrronium/indacaterol (QVA149, Ultibro, Novartis), glycopyrrolate/formoterol (PT003, Pearl Therapeutics), aclidinium/formoterol (AstraZeneca), tiotropium/olodaterol (Spiolt, Boehringer Ingelheim), and umeclidinium/vilanterol (Anoro, GSK). Our subgroup analysis suggested that more recent LAMA+LABA combinations, especially indacaterol/glycopyrronium and umeclidinium/vilanterol, may have better therapeutic potency over previously approved LAMA and LABA (Celli 2014; Horita 2015a). This should be evaluated further. In this review, we evaluated combined medications in the same class collectively; however, a meta‐analysis assessing each combined medication separately would also be interesting.
Once‐daily glycopyrronium 50 μg/indacaterol 110 μg (Ultibro) has been approved for use in many countries including within the EU, Canada, and Japan. Once‐daily glycopyrronium 50 μg/indacaterol 110 μg was evaluated in many RCTs along with studies included in the current systematic review. However, twice‐daily glycopyrronium 15.6 μg/indacaterol 27.5 μg (Utibron) has been approved in the USA. Therefore, the result from glycopyrronium/indacaterol subgroup analysis should be applied with care to people in the USA.
When all LAMA+LABA were evaluated collectively, LAMA+LABA was related with a reduction in the risk of exacerbation. This analysis was based on our original protocol. However, we observed substantial heterogeneity (I2 = 69.7%) and there was a reduced risk only in the glycopyrronium/indacaterol subgroup (Figure 3). Thus, it is still not clear whether only glycopyrronium/indacaterol prevented the exacerbation or LAMA+LABA generally prevented the exacerbation.
Long‐acting beta‐agonist plus inhaled corticosteroid
It is well known that first‐line LABA+ICS is frequently prescribed for people with COPD, particularly in the EU and North America (although the updated GOLD 2017 guidance may impact on this). This applies not just to people with COPD in GOLD categories C and D (Drivenes 2014; Price 2014; Suissa 2009; White 2013). However, the effectiveness of LABA+ICS even for these high‐risk group of people with COPD is questionable for several reasons. First, although LABA+ICS has been repeatedly evaluated in many trials, few trials assessed the efficacy and safety of single‐agent ICS. Thus, the benefits of adding ICS to LABA when treating stable COPD has not yet been sufficiently clarified (Suissa 2009). Second, there is no plausible explanation for the efficacy of adding ICS over LABA to treat COPD. Unlike asthma caused by eosinophilic inflammation, COPD is mainly caused by neutrophilic inflammation (Suissa 2009). Even though some experts have repeatedly warned of the risks of using ICS for stable COPD (Barnes 2010; Suissa 2009), pharmaceutical promotions (Table 2) and GOLD guidelines (GOLD 2016) have continued to recommend the use of LABA+ICS for stable COPD. According to Nannini's review, LABA+ICS is surely effective compared to placebo (Nannini 2013). Nonetheless, superiority of LABA+ICS over LABA monotherapy was still questionable (Nannini 2012). The superiority of LABA+ICS over LABA monotherapy in preventing exacerbations was observed but was supported by low quality evidence (Nannini 2012). They observed an increased risk of pneumonia with LABA+ICS, which is compatible with our analysis (Nannini 2012).
| Sponsor | Record count | % of 1723 |
| GlaxoSmithKline | 134 | 7.78 |
| Novartis | 128 | 7.43 |
| AstraZeneca | 122 | 7.08 |
| Boehringer Ingelheim | 113 | 6.56 |
| Pfizer | 84 | 4.88 |
| Nycomed | 49 | 2.84 |
| GSK | 45 | 2.61 |
| Chiesi | 41 | 2.38 |
| Almirall | 36 | 2.09 |
| Merck | 30 | 1.74 |
Web of Science Core Collection, advanced search for "TI=(COPD) AND TS=(inhal*)" without any restriction hit 1723 reports as of 13 June 2016. "Results analysis" > "Source Titles" output the table above.
Limitations
We identified some limitations of the current studies. Although we tried to extract data for exacerbations of any severity, some trials counted only moderate to severe exacerbations. In addition, some outcomes such as exacerbation and adverse effects were dependent on the threshold judged by researchers of the original articles. Concerning a cross‐over study by Magnussen and colleagues, CIs might be wider in our analysis due to a unit‐of‐analysis error introduced by cross‐over design (Magnussen 2012). Nevertheless, an error with this would make our results conservative. Additional limitations are lack of long‐term follow‐up data, the inclusion of category A/B participants for whom LABA+ICS is not currently recommended, and heterogeneous design among studies.
Quality of the evidence
The quality of evidence is summarised in summary of findings Table for the main comparison.
Among the 11 included studies, one study with the smallest number of participants did not have any commercial sponsorship (Hoshino 2015). The other 10 studies had a COI involving a sponsoring manufacturer (Table 2). Stakeholders need not be excluded from medical studies. However, their involvement should be properly justified with independency, transparency, democracy, and compassion toward participants (Cluzeau 2012).
Even though most studies were rated as having a risk of bias due to a COI, the included studies were well designed and had a sufficient number of participants. Except for the SGRQ total score change from the baseline, the LAMA+LABA subgroup analysis, fixed‐effect‐model‐based sensitivity analysis, and sensitivity analysis excluding unblinded studies confirmed the robustness of each pooled outcome.
LAMA+LABA therapies were associated with significantly better results for exacerbation rates, trough FEV1, pneumonia, and SGRQ total score change more than minimal clinically important difference. These findings were also supported by biological plausibility. It is reasonable to assume that the dual bronchodilator therapy had a larger bronchodilating effect than LABA+ICS, which could lead to improved quality of life. In addition, ICS diminishes the local immunity of the airway and increase the risk of pneumonia and viral infection.
We observed a strong heterogeneity in the results for SGRQ total score change from the baseline. This heterogeneity was mainly introduced by an open‐label study by Hoshino 2015 (Figure 7). The pooled MD of this outcome was not sufficiently reliable.
Potential biases in the review process
We included two unpublished studies, for which we extracted data from the ClinicalTrial.gov website (Beeh 2016; Vogelmeier 2016). Publication of the full‐length articles at a later date could affect these results.
We also found two additional ongoing studies (NCT02497001; NCT02516592). The results from these studies may affect our results when this review is updated.
Concerning a cross‐over study by Magnussen and colleagues, CIs might be wider in our analysis (Magnussen 2012).
Agreements and disagreements with other studies or reviews
We conducted a systematic review and meta‐analysis on the same subject in 2015 that included eight studies consisting of 4392 participants (Horita 2015b). The study revealed that LAMA+LABA was associated with a larger improvement in trough FEV1, as well as fewer occurrences of exacerbations and pneumonia. The frequency of SAEs, all‐cause death, and SGRQ change from the baseline were not different between treatment arms. The current review generally confirms the results obtained in the previous review. Oba and colleagues published similar meta‐analysis (Oba 2016). They concluded that, compared to LABA+ICS, LAMA+LABA was associated with greater improvement of FEV1, fewer episodes of pneumonia, and similar risk of exacerbation (Oba 2016). The key discrepancy between our analysis and their analysis is effect on exacerbations. Our study has more power as we included the recently published large trial (Wedzicha 2016).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.1 Exacerbation.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.

Funnel plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.2 Serious adverse events.

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), outcome: 1.3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference).

Forest plot of comparison: 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus ICS (inhaled corticosteroid), outcome: 1.4 Trough forced expiratory volume in one second (FEV1) change from the baseline.
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 1 Exacerbation.

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 2 Serious adverse effect.
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference).

Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline.
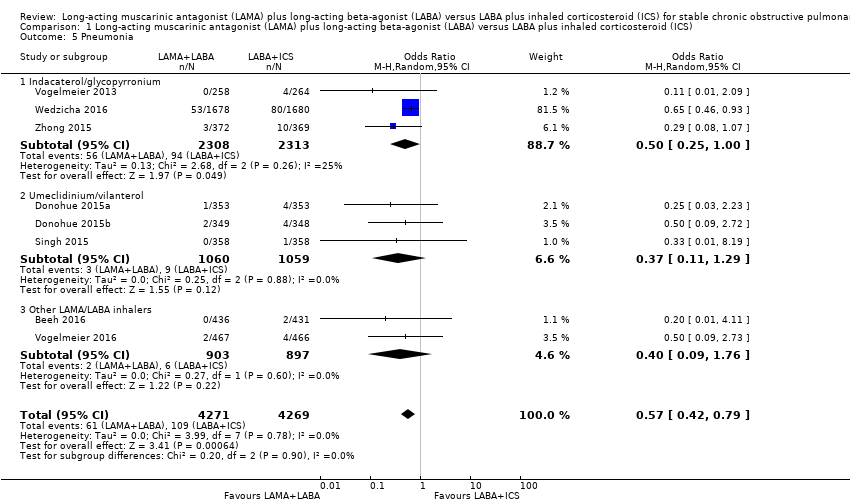
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 5 Pneumonia.
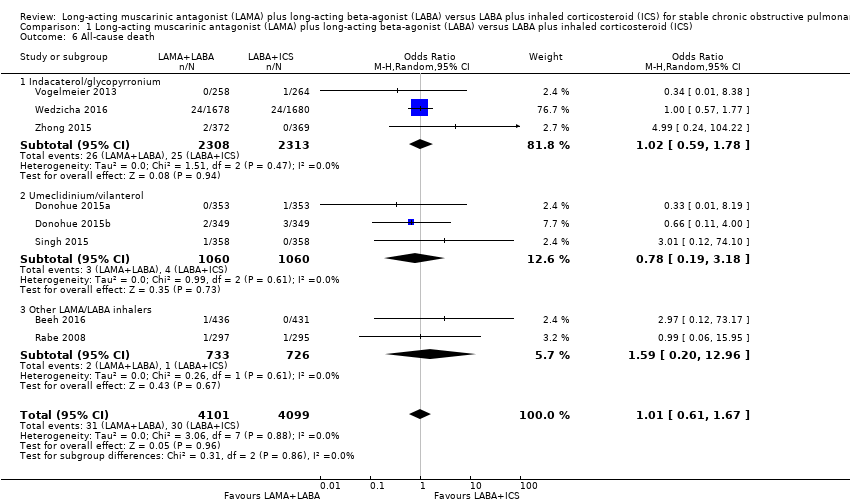
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 6 All‐cause death.
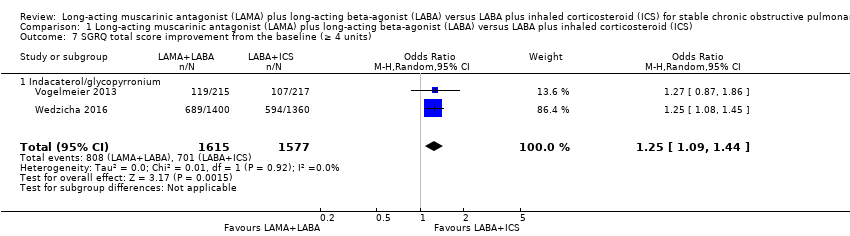
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 7 SGRQ total score improvement from the baseline (≥ 4 units).
Comparison 1 Long‐acting muscarinic antagonist (LAMA) plus long‐acting beta‐agonist (LABA) versus LABA plus inhaled corticosteroid (ICS), Outcome 8 Total SGRQ change from the baseline (excluding unblinded studies).
| LAMA + LABA versus LABA + ICS for stable COPD | ||||||
| Population: stable COPD | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effects | Number of participants | Quality of the evidence | Comments | |
| LABA+ICS | LAMA+LABA | |||||
| Exacerbations (number of people experiencing ≥ 1 exacerbations) Follow‐up: 12 to 52 weeks | 377 per 1000 | 332 per 1000 (298 to 368) | OR 0.82 | 8922 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| Serious adverse effects Follow‐up: 12 to 52 weeks | 96 per 1000 | 87 per 1000 | OR 0.91 | 9793 | ⊕⊕⊕⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (MD) Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD ‐1.22 (‐2.52 to 0.07) | 6055 (6 RCTs) | ⊕⊕⊝⊝ | Low MD means favourable outcome |
| Trough FEV1 change from the baseline Follow‐up: 12 to 52 weeks | ‐ | ‐ | MD 0.08 L (0.06 to 0.09) | 6238 (6 RCTs) | ⊕⊕⊕⊝ | High MD means favourable outcome |
| Pneumonia Follow‐up: 12 to 52 weeks | 26 per 1000 | 15 per 1000 | OR 0.57 | 8540 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| All‐cause death Follow‐up: 12 to 52 weeks | 7 per 1000 | 7 per 1000 | OR 1.01 | 8200 | ⊕⊕⊝⊝ | Low OR means favourable outcome |
| SGRQ total score change from the baseline (≥ 4 points, MCID) Follow‐up: 24 to 52 weeks | 445 per 1000 | 500 per 1000 (466 to 535) | OR 1.25 (1.09 to 1.44) | 3192 (2 RCTs) | ⊕⊕⊕⊝ | High OR means favourable outcome |
| *The absolute risk (and its 95% CI) of LAMA+LABA group is based on the assumed risk in the LABA+ICS group and the OR of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect | ||||||
| 1 Every study had at least one domain of high risk of bias mostly due to conflicts of interest. 2 Indirectness due to definition of exacerbation. 3 There was a considerable heterogeneity, I2 = 71%. 4 Downgraded due to imprecision. | ||||||
| Study | LAMA+LABA | LABA+ICS | Key inclusion criteria | Follow‐up duration (weeks) | Mean/median age (years) | Number randomised |
| Tiotropium/olodaterol (2.5/5 μg) or tiotropium/olodaterol (5/5 μg) | Salmeterol/fluticasone (50/250 μg) twice daily or salmeterol/fluticasone (50/500 μg) twice daily. | %pred FEV1 30% to 80% Ex(‐) | 6 × 4 time periods (cross‐over) | 64 | 229 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 63 | 707 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 64 | 700 | |
| Tiotropium/indacaterol (18/150 μg) | Salmeterol/fluticasone (50/250 μg) twice daily | %pred FEV1 30% to 80%, Ex(‐) | 16 | 71 | 46 | |
| Tiotropium/salmeterol (18/50 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 8 x 2 time periods (cross‐over) | 61 | 344 | |
| Tiotropium/formoterol (18/24 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 ≤ 65%, Ex(‐) | 6 | 62 | 605 | |
| Umeclidinium/vilanterol (62.5/25 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 30% to 70%, mMRC ≥ 2, Ex(‐) | 12 | 62 | 717 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, Ex(‐) | 26 | 63 | 523 | |
| Aclidinium/formoterol (400/12 μg) twice daily | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 < 80%, CAT ≥ 10, Ex(‐) | 24 | 63 | 933 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | %pred FEV1 25% to 60%, mMRC ≥ 2, Ex(+) | 52 | 65 | 3362 | |
| Indacaterol/glycopyrronium bromide (110/50 μg) | Salmeterol/fluticasone (50/500 μg) twice daily | Stage II/III, mMRC ≥ 2, Ex(‐) | 26 | 65 | 744 | |
| %pred FEV1: % predicted forced expiratory volume in one second; CAT: chronic obstructive pulmonary disease assessment test; Ex(‐): without recent exacerbation; Ex(+): with recent exacerbation; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; mMRC: modified Medical Research Council dyspnoea scale. | ||||||
| Sponsor | Record count | % of 1723 |
| GlaxoSmithKline | 134 | 7.78 |
| Novartis | 128 | 7.43 |
| AstraZeneca | 122 | 7.08 |
| Boehringer Ingelheim | 113 | 6.56 |
| Pfizer | 84 | 4.88 |
| Nycomed | 49 | 2.84 |
| GSK | 45 | 2.61 |
| Chiesi | 41 | 2.38 |
| Almirall | 36 | 2.09 |
| Merck | 30 | 1.74 |
| Web of Science Core Collection, advanced search for "TI=(COPD) AND TS=(inhal*)" without any restriction hit 1723 reports as of 13 June 2016. "Results analysis" > "Source Titles" output the table above. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation Show forest plot | 9 | 8922 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.70, 0.96] |
| 1.1 Indacaterol/glycopyrronium | 3 | 4617 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.63, 0.83] |
| 1.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.15 [0.64, 2.06] |
| 1.3 Other LAMA/LABA inhalers | 3 | 2186 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.78, 1.34] |
| 2 Serious adverse effect Show forest plot | 10 | 9793 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.05] |
| 2.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.61, 1.10] |
| 2.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.43, 2.31] |
| 2.3 Other LAMA/LABA inhalers | 4 | 3053 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.77, 1.57] |
| 3 St. George's Respiratory Questionnaire (SGRQ) total score change from the baseline (mean difference) Show forest plot | 6 | 5858 | Mean Difference (Random, 95% CI) | ‐1.22 [‐2.52, 0.07] |
| 3.1 Indacaterol/glycopyrronium | 2 | 3693 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 3.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |
| 3.3 Other LAMA/LABA inhalers | 1 | 46 | Mean Difference (Random, 95% CI) | ‐5.0 [‐7.35, ‐2.65] |
| 4 Trough forced expiratory volume in 1 second (FEV1) change from the baseline Show forest plot | 6 | 7277 | Mean Difference (Random, 95% CI) | 0.08 [0.06, 0.09] |
| 4.1 Indacaterol/glycopyrronium | 2 | 4291 | Mean Difference (Random, 95% CI) | 0.08 [0.04, 0.12] |
| 4.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | 0.09 [0.07, 0.11] |
| 4.3 Other LAMA/LABA inhalers | 1 | 867 | Mean Difference (Random, 95% CI) | 0.05 [0.02, 0.08] |
| 5 Pneumonia Show forest plot | 8 | 8540 | Odds Ratio (M‐H, Random, 95% CI) | 0.57 [0.42, 0.79] |
| 5.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.25, 1.00] |
| 5.2 Umeclidinium/vilanterol | 3 | 2119 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.11, 1.29] |
| 5.3 Other LAMA/LABA inhalers | 2 | 1800 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.09, 1.76] |
| 6 All‐cause death Show forest plot | 8 | 8200 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.61, 1.67] |
| 6.1 Indacaterol/glycopyrronium | 3 | 4621 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.59, 1.78] |
| 6.2 Umeclidinium/vilanterol | 3 | 2120 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.19, 3.18] |
| 6.3 Other LAMA/LABA inhalers | 2 | 1459 | Odds Ratio (M‐H, Random, 95% CI) | 1.59 [0.20, 12.96] |
| 7 SGRQ total score improvement from the baseline (≥ 4 units) Show forest plot | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| 7.1 Indacaterol/glycopyrronium | 2 | 3192 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [1.09, 1.44] |
| 8 Total SGRQ change from the baseline (excluding unblinded studies) Show forest plot | 5 | 6360 | Mean Difference (Random, 95% CI) | ‐0.70 [‐1.58, 0.18] |
| 8.1 Indacaterol/glycopyrronium | 2 | 4241 | Mean Difference (Random, 95% CI) | ‐1.29 [‐2.08, ‐0.50] |
| 8.2 Umeclidinium/vilanterol | 3 | 2119 | Mean Difference (Random, 95% CI) | ‐0.08 [‐1.34, 1.18] |

