非抱合型高ビリルビン血症の新生児に対する光線療法における反射材の使用
Abstract
Background
Phototherapy is a well‐established effective therapy for treating babies with significant neonatal jaundice. Studies have shown that increasing light intensity will increase its efficiency. A potentially inexpensive and easy way of increasing the intensity of light on the body of the infant may be to hang reflective materials from the sides of phototherapy units.
Objectives
To assess the effects of reflective materials in combination with phototherapy compared with phototherapy alone for unconjugated hyperbilirubinaemia in neonates.
Search methods
We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 11), in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R); and the Cumulative Index of Nursing and Allied Health Literature (CINAHL), on 1 November 2019. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
We included randomised and quasi‐randomised controlled trials if the participants, who were term or preterm infants, received phototherapy with curtains made of reflective materials of any type in the treatment arm, and if those in the comparison arm received similar phototherapy without curtains or other intensified phototherapy, such as a double bank of lights.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We used the GRADE approach to assess the certainty of evidence.
Main results
Of 15 studies identified, we included 12 (1288 babies) in the review ‐ 11 comparing phototherapy with reflective materials and phototherapy alone, and one comparing a single phototherapy light bank with reflective materials with double phototherapy. All reflective materials consisted of curtains on three or four sides of the cot and were made of white plastic (five studies), white linen (two studies), or aluminium (three studies); materials were not specified in two studies. Only 11 studies (10 comparing reflective materials versus none and one comparing reflective curtains and a single bank of lights with a double (above and below) phototherapy unit) provided sufficient data to be included in the meta‐analysis. Two excluded studies used the reflective materials in a way that did not meet our inclusion criteria, and we excluded one study because it compared four different phototherapy interventions not including reflective materials. The risk of bias of included studies was generally low, but all studies had high risk of performance bias due to lack of blinding of the intervention.
Three studies (281 participants) reported a decline in serum bilirubin (SB) (μmol/L) at four to eight hours (mean difference (MD) ‐14.61, 95% confidence interval (CI) ‐19.80 to ‐9.42; I² = 57%; moderate‐certainty evidence). Nine studies (893 participants) reported a decline in SB over 24 hours and showed a faster decline in SB in the intervention group, but heterogeneity (I² = 97%) was too substantial to permit a meaningful estimate of the actual effect size (very low‐certainty evidence). Subgroup analysis by type of reflective material used did not explain the heterogeneity. Exchange transfusion was reported by two studies; both reported none in either group. Four studies (466 participants) reported the mean duration of phototherapy, and in each of these studies, it was reduced in the intervention group but there was substantial heterogeneity (I² = 88%), precluding meaningful meta‐analysis of data. The only two studies that reported the mean duration of hospital stay in hours showed a meaningful reduction (MD ‐41.08, 95% CI ‐45.92 to ‐36.25; I² = 0; moderate‐certainty evidence).
No studies reported costs of the intervention, parental or medical staff satisfaction, breastfeeding outcomes, or neurodevelopmental follow‐up.
The only study that compared use of curtains with double phototherapy reported similar results for both groups.
Studies that monitored adverse events did not report increased adverse events related to the use of curtains, including acute life‐threatening events, but other rarer side effects could not be excluded.
Authors' conclusions
Moderate‐certainty evidence shows that the use of reflective curtains during phototherapy may result in greater decline in SB. Very low‐certainty evidence suggests that the duration of phototherapy is reduced, and moderate‐certainty evidence shows that the duration of hospital stay is also reduced. Available evidence does not show any increase in adverse events, but further studies are needed.
PICO
一般語訳
黄疸のある乳児への光線療法時の反射材の使用
レビューの論点:光治療器の側面に吊るした反射材のカーテンを使用することで、光治療の効果が向上するかどうかを調べたいと考えた。
背景:黄疸(皮膚の黄色の変色)は、赤ちゃんの最大60%に発生する。これはビリルビンの蓄積によるものである。ビリルビンの軽度の上昇は正常とされているが、非常に高い場合には、ビリルビンが脳に入り込み、脳障害を引き起こす可能性がある(訳注:ビリルビン脳症)。年齢別・リスク別ガイドラインを用いた治療は、ビリルビンがこれらのレベルに到達しないようにすることを目的としている。黄疸には通常、光線療法(特定の波長の光を体に当てる)が行われており、皮膚に当てる光の強さがビリルビンの減少率を決定する要因の一つとなっている。光の強度を高めるための潜在的に安価な方法は、反射材を使用することである。懸念されるのは、このカーテンが赤ちゃんの視界を遮ってしまうのではないかということである。
研究の特性:ランダム化比較試験(RCT)を選択した。検索は2019年11月1日でアップデートされた。
主な結果:合計1288人の赤ちゃんを対象とした12の研究が確認できた。このうち、11個がシングルユニットの光線療法機の周囲に反射材がある場合とない場合を比較した。1つの試験では、光と反射材を1つのユニットにした場合と、カーテンのない2つのユニットにした場合を比較していた。反射材の種類には、白いプラスチック、白いリネン、アルミニウムなどがあり、ベビーベッドの三方または四方に配置されていた。
1132人の赤ちゃんを含む11件の研究で、主要な転帰であるビリルビンの低下について十分なデータが得られた。第1の比較例では10個、第2の比較例では1個である。
3つの研究では、4時間から8時間でビリルビンの減少が報告されていた。小さな、しかし臨床的に重要である違いでカーテンの使用を支持する中程度のエビデンスが示された。24時間にわたるビリルビンの低下は、9つの研究で測定された。どの研究もカーテン群ではビリルビンの低下が早いことを示していたが、低下のばらつきが大きく、効果の大きさを推定する意味がなかった。光線治療の持続時間を報告している4つの研究では、反射型カーテンを使用した場合の方が短いことが示されているが、これは確実性が非常に低い。2つの研究から得られた中程度の確実性のあるエビデンスによると、介入により入院期間がほぼ2日短縮されることが示されている。また、カーテンによる赤ちゃんの遮蔽による体温の不安定化や急性の生命を脅かすような重大な有害事象の報告はなく、その他の軽微な影響の報告もなかった。つまり、全体的な反射カーテンはメリットがあるかもしれないが、弊害があるかどうかは不明である。どの研究も、カーテンに対する親や医療従事者の満足度や、カーテンが授乳率に影響を与えたかどうかを報告していなかった。
一つの試験では、カーテン付きのライトユニット1台の使用と、カーテンなしのライトユニット2台の使用を比較し、介入群と対照群の両方で同様の結果が得られた。
エビデンスの質:エビデンスレベルは中等度である。
Authors' conclusions
Summary of findings
| Phototherapy with reflective curtains compared to phototherapy alone for newborn infants with unconjugated hyperbilirubinaemia | |||||
| Patient or population: newborn infants with unconjugated hyperbilirubinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative | № of | Certainty of the | Comments |
|---|---|---|---|---|---|
| Decline in bilirubin at 4 to 8 hours (μmol/L) | MD 14.61 lower in the phototherapy with curtains group than in the group with phototherapy alone | ‐ | 281 | ⊕⊕⊕⊝ | |
| Decline in serum bilirubin (over 24 hours) | See comment | ‐ | 893 | ⊕⊝⊝⊝ | Although the effect estimate for all studies favoured reflective curtains, no summary effect is available due to substantial heterogeneity |
| Exchange transfusion | Two studies reported no exchange transfusion; remaining studies did not report this outcome | ‐ | (2 RCTs) | ‐ | |
| Acute life‐threatening events | This outcome was not reported in any of the included studies | ‐ | (11 RCTs) | ‐ | |
| Parental satisfaction | This outcome was not reported in any of the included studies | ‐ | (11 RCTs) | ‐ | |
| Breastfeeding on | This outcome was not reported in any of the included studies | ‐ | (11 RCTs) | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aDowngraded one level for moderate inconsistency (heterogeneity). bLack of blinding of caregivers in all studies and possible selection bias, cDowngraded two levels for substantial inconsistency (heterogeneity). | |||||
| Reflective curtains compared to intensified phototherapy for newborn infants with unconjugated hyperbilirubinaemia | |||||
| Patient or population: newborn infants with unconjugated hyperbilirubinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|---|
| Decline in bilirubin (first measurement) | MD 0.17 higher in group with curtains than in group with intensified phototherapy | ‐ | 159 | ⊕⊕⊝⊝ | |
| Duration of phototherapy (in hours) | MD 4.04 higher in group with curtains than in group with intensified phototherapy | ‐ | 159 | ⊕⊕⊝⊝ | |
| Exchange transfusion | Three infants reached exchange transfusion level. No differences between groups | ‐ | (159) (1 RCT) | ‐ | |
| Acute life‐threatening events | No events reported | ‐ | (159) (1 RCT) | ‐ | |
| Patient satisfaction | Not reported | ‐ | (159) (1 RCT) | ‐ | |
| Breastfeeding on discharge | Outcome not reported | ‐ | (159) (1 RCT) | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aAlthough blinding of caregivers was lacking, the outcome assessor was blinded. bDowngraded one level for single study; unable to assess inconsistency with other studies. cSingle study and wide confidence interval, including potential for a decrease and an increase in the outcome. | |||||
Background
Description of the condition
Neonatal jaundice occurs in about 60% of otherwise healthy newborn infants. Although for most infants jaundice does not lead to any major morbidity, jaundice may get so severe that it can lead to kernicterus ‐ deposition of bilirubin in parts of the brain ‐ resulting in permanent brain damage (AAP 2004). Treatment with phototherapy based on age‐ and risk‐specific guidelines or nomograms is aimed at preventing bilirubin from reaching these levels.
When neonates develop jaundice during the first week of life, this most often occurs because the newborn infant has an increased rate of breakdown of haemoglobin in the presence of immature liver function, leading to unconjugated hyperbilirubinaemia. This is considered to be part of a normal physiological process. Pathological jaundice may occur if haemolysis is increased, as occurs when there is incompatibility between mother and infant blood groups, or when extravasation of blood occurs during the delivery, resulting in, for example, cephalohaematoma or subaponeurotic haemorrhage, both of which consist of haemorrhages into the scalp. In certain populations, glucose‐6‐phosphate dehydrogenase (G6PD) deficiency is an important cause of pathological jaundice (Lu 1966; Singh 1986). Other causes include impaired conjugation, as in Gilbert’s syndrome, and increased enterohepatic circulation, as is seen in breastfeeding jaundice. These causes and some others result in an increase in unconjugated bilirubin, and it is this form of bilirubin that can be treated with phototherapy. Conjugated hyperbilirubinaemia, which is regarded as a separate entity, is much less common, generally occurs later in the neonatal period, and is not treated with phototherapy.
Description of the intervention
Before phototherapy was discovered, the mainstay of treatment for hyperbilirubinaemia was exchange transfusion (ET). Although ET is effective in removing bilirubin, it is associated with many complications, including those related to the use of blood products (infection, haemolysis of transfused blood), metabolic derangements (metabolic acidosis, deranged serum calcium), cardiorespiratory complications (arrhythmia, apnoea), and complications related to umbilical venous catheteristion. The morbidity of ET ranges from 5% to 10%, and mortality ranges from 0% to 7% (Ip 2004).
Other modalities of treatment may have no proven effects (e.g. phenobarbitone) (Arya 2004; Murki 2005), or they may be effective only for very specific conditions (e.g. infusion of immunoglobulin) (Alcock 2002).
Phototherapy has been used since 1958 for treatment of neonatal hyperbilirubinaemia (Cremer 1958); it has been the mainstay of treatment since that time.
The energy provided by light converts bilirubin to water‐soluble forms through two main processes. These processes are called photo‐isomerisation and photo‐oxidation. They result in minor changes in the molecular structure of bilirubin that allow it to be excreted via the liver or the kidneys without having to undergo the process of conjugation in the liver. Conjugation, which is the rate‐limiting step of normal bilirubin excretion, is exacerbated by the relative liver immaturity of the newborn (Maisels 2008).
The rate of production of water‐soluble forms of bilirubin is dependent first on the wavelength of light. It is most efficient if the light wavelength is within the range of 450 nm to 490 nm. Second, the intensity of the light is a factor. The rate of bilirubin conversion increases linearly with the intensity of light from about 5 to 30 microWatt*cm‐2*nm‐1. It was initially perceived that light intensities higher than this did not appear to confer clinical benefit (Tan 1982). However, a study using light‐emitting diodes found a linear relation between light irradiance in the range of 20 to 55 microWatt*cm‐2*nm‐1 and a decrease in bilirubin after 24 hours of therapy, with no evidence of a saturation point (Vandborg 2012). To achieve the maximal effective level of light intensity, more or stronger lights can be used and light sources can be brought as close as possible to the skin surface (Kang 1995). Third, the surface area of skin exposed to the light will affect the rate of bilirubin conversion.
Higher light intensities can be obtained by using multiple phototherapy units, but this approach increases the cost of phototherapy and, theoretically, has increased potential to cause hyperthermia. Curtains made of reflective materials placed around the phototherapy unit (while infants are receiving phototherapy) have the potential to increase the irradiance. In some parts of the world, it may be necessary to use curtains at the side of open incubators (or phototherapy units) to protect children from convective heat loss (air flow from fans or air conditioners) or from mosquitoes. Materials used for these purposes often are not reflective in nature.
How the intervention might work
Bright surfaces such as white cloth, mirrors, and aluminium foil can reflect dispersed phototherapy light. Curtains made from reflective materials usually are attached to the phototherapy unit and hung around the infant's cot to capture light that might be dispersed away from the infant and, by their reflective nature, reflect it back onto the infant. This might increase the photo irradiance and hence result in increased bilirubin conversion. The reflective materials used may differ in their ability to reflect dispersed light. The expected increase in light intensity that reflective materials would provide is uncertain, but some comparisons between different materials have been reported in Djokomuljanto 2006. This study reported an increase in irradiance of three materials (a white plastic sheet with a paper backing behind it, a square of white bed sheeting, and a square of white cotton gauze ‐ used to make baby napkins). The greatest increase was noted with the plastic sheet. A 35% increase was reported directly below the light source, and 54% at the side of the baby cot.
Such curtains may also reflect heat, thereby increasing the risk of hyperthermia. It is possible that such curtains could obstruct the visibility of the infant and affect nursing observation; this obstruction could also affect mother‐infant bonding. When properly used, these curtains should not confer increased infection risk.
Why it is important to do this review
A recent Cochrane Review suggests that light sources from light‐emitting diodes are as effective as conventional light sources (Kumar 2011). These lights are power efficient with low heat production and have an extremely long life span (Kumar 2011). However, light‐emitting diodes are currently expensive, and further data on their safety are needed. Therefore, conventional phototherapy is likely to continue to be used for some time to come. The reflective materials used with conventional phototherapy are inexpensive; if they are found to increase the intensity and, more important, the efficiency of phototherapy while shortening the duration of treatment with little or no extra cost, this would be important in resource‐constrained settings. However, a growing body of evidence suggests that phototherapy has sufficient adverse effects that in any setting, unnecessary prolongation of phototherapy should be avoided. Increased oxidative stress markers have been found in infants who underwent phototherapy (El‐Farrash 2019). Phototherapy has been associated with childhood cancer (Auger 2019; Newman 2016), and its use is a risk factor for breastfeeding failure (Waite 2016) ‐ both reasons for shortening the duration of phototherapy. The aim of this review is to systematically compile and assess available evidence from randomised and quasi‐randomised trials comparing effects of phototherapy provided with and without reflective curtains.
Objectives
To assess the effects of reflective materials in combination with phototherapy compared with phototherapy alone for unconjugated hyperbilirubinaemia in neonates.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) and quasi‐RCTs. We included only studies with individual participant allocation and did not include cross‐over studies.
Types of participants
We included studies of term and preterm neonates up to the age of 14 days (for term infants) and 21 days (for preterm infants) with unconjugated hyperbilirubinaemia receiving phototherapy.
Types of interventions
We included studies in which participants received phototherapy in combination with curtains made of reflective materials of any type in the treatment arm(s), and phototherapy alone in the comparison arm(s). The setup of the phototherapy units and its relation to the infants should be similar in both arms, except for the use of reflective materials. The reflective materials used are hung from overhead phototherapy units around the cot on at least the two long sides of the cot. If incubators are used, the surface of the reflective materials and the position should be similar as for their use in cots.
Because this review is exploring the effects of reflective materials, we made a post hoc decision to include studies comparing single phototherapy with reflective materials versus other interventions such as different reflecting materials and comparing phototherapy with reflecting curtains versus other forms of phototherapy (e.g. fibreoptic phototherapy, double phototherapy).
Types of outcome measures
Primary outcomes
-
Decline in serum bilirubin levels per unit of time over the first four to eight hours, at 24 hours, or until the first measurement of bilirubin (μmol/L per unit of time)
Secondary outcomes
-
Duration of treatment with phototherapy (hours)
-
Number of exchange transfusions within the neonatal period and number of babies requiring exchange transfusion
-
All‐cause mortality at discharge
-
Acute life‐threatening event (ALTE) during phototherapy
-
Cost of the intervention (because there may be large variation depending on materials used and the type of phototherapy units used, we will adopt a purely descriptive approach for individual studies)
-
Parental satisfaction (questionnaire‐based assessment, during or within a reasonable time after admission)
-
Medical staff satisfaction (questionnaire‐based assessment, during or within a reasonable time after admission)
-
Exclusive breastfeeding on discharge
-
Partial breastfeeding on discharge
-
Neurodevelopmental follow‐up
-
Actual measures of light intensity on infant skin during phototherapy
Adverse effects
-
Dehydration (more than expected weight loss for age during phototherapy or by clinical assessment)
-
Hyperthermia (axillary temperature > 37.5 °C)
-
Hypothermia (axillary temperature < 36.5 °C)
-
Body rash
-
Bronze discolouration of the skin
-
Interference with mother‐infant interaction (through observational or questionnaire‐based assessment)
-
Adverse effects related to problems with observation of infant (e.g. intravenous line problems)
Search methods for identification of studies
We used the standard search strategy of Cochrane Neonatal, as documented in the Cochrane Library. See the Cochrane Neonatal search strategy at http://neonatal.cochrane.org/resources-review-authors.
Electronic searches
We conducted a comprehensive search including the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 11), in the Cochrane Library; Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 1 November 2019); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981 to 1 November 2019). We have included the search strategies for each database in Appendix 1. We did not apply language restrictions.
We searched clinical trial registries for ongoing and recently completed trials. We searched the World Health Organization’s International Clinical Trials Registry Platform (ICTRP) at www.who.int/ictrp/search/en/ and the US National Library of Medicine’s ClinicalTrials.gov at clinicaltrials.gov via Cochrane CENTRAL. Additionally, we searched the ISRCTN Registry for any unique trials not found through the Cochrane CENTRAL search.
Searching other resources
We communicated with experts and searched the reference lists of any identified reviews and included trials for references to other trials. We searched abstracts and conference and symposium proceedings of the Perinatal Society of Australia and New Zealand. For identified unpublished trials, we contacted the corresponding investigator for information. We considered unpublished studies and studies reported only as abstracts as eligible for review. We also contacted the corresponding or first author of identified RCTs for additional information about studies when further data were required.
Data collection and analysis
Selection of studies
The lead review author performed the search for trials with the assistance of Cochrane Neonatal. Two review authors (LCH and IJ) independently screened titles and abstracts obtained through the electronic searches to create a pool of eligible studies. The lead review author obtained the full articles of the latter, which two review authors (HVR and LCH) then independently scrutinised for relevance using a standardised eligibility form with pre‐defined inclusion criteria. Any disagreement was handled by a third review author (JJH). Possible duplicate publications were assessed by comparing author names, locations and settings, specific details of the intervention, numbers of participants and their baseline data, and date and duration of studies. We obtained data sets that are as complete as possible. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), along with the Characteristics of excluded studies table.
Data extraction and management
For included studies, we extracted data concerning study identity (title, authors, reference), design, methods, eligibility, risk of bias, clinical features of participants, interventions and outcomes, and treatment effects, using a specially designed data extraction form. For studies that we initially considered eligible for inclusion but that we excluded after reading the full report, we documented the reason for exclusion.
Two review authors (LCH and IJ) independently extracted and compared all data; they resolved any discrepancies by discussion or by consultation with a third review author (JJH). Unresolved disagreements were referred for arbitration by a third review author or mentor.
Assessment of risk of bias in included studies
Two review authors (HVR and IJ) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane "Risk of bias" tool for the following domains (Higgins 2019).
-
Sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Any other bias.
Any disagreements were resolved by discussion or by consultation with a third assessor (JJH). See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We carried out data analysis using Review Manager 5 (RevMan 2014). If it was possible to conduct a meta‐analysis of identified trials, the effect measures for binary outcomes were risk ratio (RR) and risk difference (RD). For binary outcome(s), we planned to calculate the number needed to benefit (NNTB) or the number needed to harm (NNTH) when the RD is statistically significant. For continuous outcomes, the effect measure is the mean difference (MD). If scales of different lengths are used, and if we judged that the outcome measured is similar enough, we used the standardised mean difference (SMD). For all estimates, we provide 95% confidence intervals (CIs).
Unit of analysis issues
We did not anticipate any problems with unit of analysis issues.
Dealing with missing data
We contacted the respective investigators in cases where adequate information was not available within the papers.
Assessment of heterogeneity
We estimated the amount of heterogeneity of treatment effect across trials using the I² statistic. We used the following cutoffs and labels: < 25%: no heterogeneity, 25% to 49%: low heterogeneity, 50% to 74%: moderate heterogeneity, and > 75%: substantial heterogeneity. If substantial heterogeneity was present, we explored its potential sources, taking into account differences in study design, participants, and interventions used in the trials. We explored possible differences using a limited number of pre‐specified subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We planned to create a funnel plot if 10 or more studies were included in the meta‐analysis of the same outcome. If there would be skewing with positive results being published and negative results not being published, we planned to report this and attempt to explain, recognising that not all funnel plot asymmetry is due to publication bias.
Data synthesis
We used a fixed‐effect model for analysis as recommended by the Cochrane Neonatal Group (http://neonatal.cochrane.org/resources-review-authors). When meta‐analysis was deemed appropriate, we performed the analysis using RevMan 5 software supplied by Cochrane (RevMan 2014).
Certainty of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the certainty of evidence for the following (clinically relevant) outcomes: decline in bilirubin, exchange transfusion, acute life‐threatening events, parental satisfaction, and breastfeeding at discharge.
Two review authors independently assessed the certainty of evidence for each of the outcomes above. We considered evidence from RCTs as high certainty but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a "Summary of findings" table to report the certainty of evidence.
The GRADE approach results in an assessment of the certainty of a body of evidence according to one of four grades.
-
High: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We visually inspected the forest plot, if indicated. If we had sufficient data, we intended to perform the following subgroup analyses.
-
Types, sizes, and configurations of different reflective materials (as stated by study authors).
-
Phototherapy methods and irradiance of units used in conjunction.
-
Preterm versus term gestational age (< 28 weeks, 28 to 32 weeks, > 32 weeks to 38 weeks, and term).
-
Severity of baseline jaundice (≤ 340 micromol/L and > 340 micromol/L).
The exact cutoffs that we use for these subgroups will depend on the availability of subgroups in the included studies.
For the purpose of creating subgroups according to the severity of jaundice, we used the cutoff value of 300 μmol/L.
Sensitivity analysis
We conducted a sensitivity analysis based on trial quality to test judgements made in our risk of bias assessment.
Results
Description of studies
Results of the search
The search resulted in 1402 studies (1015 after removal of duplicates). After screening, we retrieved full‐text articles for 15 articles; 12 of these were included in the qualitative analysis and 11 in the quantitative analysis of this review, as shown in Figure 1.
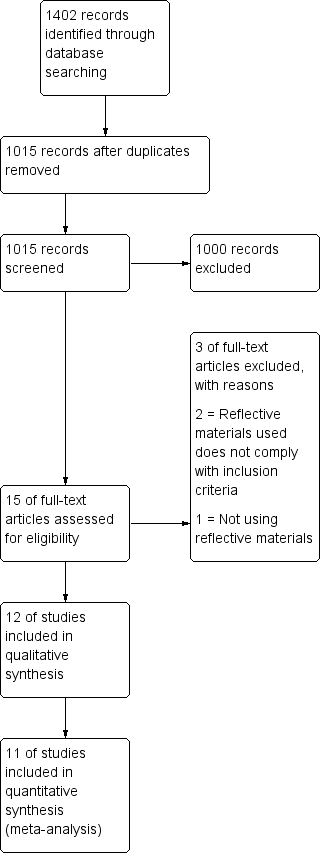
Flow diagram.
Included studies
We included 12 studies (Abd Hamid 2013; Babaei 2013; Dachlan 2015; Devpura 2017; Djokomuljanto 2006; Eggert 1988; Kurniasih 2011; Lahiri 2016; Lee 2014; Magaspi 2014; Rashmi 2015; Sivanandan 2009): 11 in the first comparison and 1 in the second comparison (Abd Hamid 2013).
Babaei 2013 included 182 term infants, more than 48 hours but less than 14 days of age, with SB of 300 to 360 μmol/L without haemolysis. The intervention consisted of standard phototherapy with a white shiny plastic cover around three sides of the phototherapy unit. The intervention was compared with standard phototherapy without curtains. Outcomes included SB on admission and after 12 and after 24 hours of phototherapy, duration of phototherapy, and duration of hospital stay. Adverse effects were also reported.
Dachlan 2015 included 70 term neonates, more than 24 hours of age, with neonatal jaundice requiring phototherapy. Asphyxia, G6PD deficiency, severe infection, and unknown birth weight were exclusion criteria for this study. The intervention consisted of a phototherapy unit with aluminium reflecting materials on four sides of the unit. Controls received similar phototherapy but without reflecting materials. Outcome measures included SB after 12, 24, and 48 hours after the start of phototherapy and the duration of phototherapy. Adverse effects were not reported.
Devpura 2017 included 100 healthy term neonates with non‐haemolytic jaundice between 24 hours and 14 days of age. The intervention consisted of a phototherapy unit with white reflecting materials on three sides of the unit. The type of material used was not reported. Controls received similar phototherapy but without reflecting materials. Outcome measures included the fall of SB at the end of 12 hours and 24 hours, the rate of fall of SB in the first 12 hours and between 12 hours and 24 hours, and the duration of phototherapy. There were no pre‐specified adverse events, and there was no report of whether any adverse events were detected.
Djokomuljanto 2006 included 100 term newborns with uncomplicated neonatal jaundice presenting in the first week of life. Researchers compared phototherapy with curtains (white plastic) with identical phototherapy without curtains. Outcomes were mean decrease in SB after four hours of phototherapy, median duration of phototherapy, and adverse effects of phototherapy.
Eggert 1988 included 70 newborns above the age of 40 hours with uncomplicated hyperbilirubinaemia, who were cared for in incubators. Children treated with antibiotics and with blood group incompatibility were excluded. The intervention was phototherapy (special blue light) with white cloth draped at the four outer walls of the phototherapy unit; the intervention was compared with the same type of phototherapy without white cloth around it at four sides (a third group was also studied with a different type of phototherapy, not relevant to our research question). Main outcomes were SB at 24 hours and duration of phototherapy. Adverse effects were not reported.
Kurniasih 2011 included 63 infants with uncomplicated hyperbilirubinaemia without haemolytic disease. The intervention was phototherapy (compact blue lights) with white plastic curtains around the phototherapy.units, and the control was similar phototherapy units without any curtains. Main outcomes were mean decrease in bilirubin after 12 and 24 hours of phototherapy and total duration of phototherapy. Adverse effects were reported.
Lahiri 2016 included 100 term infants between 24 hours and 10 days of age, who were exclusively breastfed, with birth weight > 2500 grams and SB < 340 μmol/L. Infants with haemolysis were excluded. The intervention was compact fluorescent phototherapy light with white cotton cloth with an inner reflecting surface hung from three sides of the phototherapy unit. The control group received similar phototherapy without curtains. Main outcomes included SB at 4, 12, and 24 hours of phototherapy; mean SB decline; duration of phototherapy; and mean spectral irradiance. Adverse effects were not reported.
Rashmi 2015 included 30 term infants weighing more than 2500 grams with SB between 300 and 340 μmol/L, who were aged 48 hours to 14 days. Researchers excluded infants with haemolysis and infants on phenobarbitone or herbal preparations. They compared phototherapy with and without curtains, but the nature of the curtains was not reported. Outcomes were SB after 24 and 48 hours of phototherapy. Adverse effects were monitored but were not reported.
Magaspi 2014 included 201 term babies below seven days of age without evidence of haemolysis. The intervention consisted of phototherapy with aluminium foil on three sides of the cot. The control arm was partially enclosed with a white material at the level of the cot (there was a third arm, which was not relevant to this research question). Outcomes were SB at 24 hours of phototherapy and duration of phototherapy. Adverse effects were not reported.
Lee 2014 (available only in abstract format) included 108 preterm and term healthy infants with jaundice requiring phototherapy. The intervention group had white curtains hanging around the phototherapy unit. The control group received phototherapy without the addition of a white curtain. Outcomes were SB at 24 hours of phototherapy and mean duration of hospitalisation. Adverse effects, including skin rash, were reported. However, no quantitative data were included in the abstract. Therefore this study could not be included in the meta‐analysis.
Sivanandan 2009 included healthy term neonates with non‐haemolytic jaundice between 24 hours and 10 days of age with SB < 357 μmol/L. Babies requiring exchange transfusion, or having evidence of haemolysis, were excluded. The intervention consisted of a phototherapy unit with white plastic sheets with an inner reflective surface on three sides of the unit. Controls received similar phototherapy but without curtains. Outcomes reported were rate of fall of SB per hour during the first eight hours, reduction in SB after 24 hours, number of exchange transfusions, all‐cause mortality, and the following side effects: loose stools, feeding intolerance, skin rashes, temperature, vomiting, decreased urine output, and others.
Abd Hamid 2013 included 156 infants with birth weight more than 2.3 kg and with SB more than 300 μmol/L for babies more than 48 hours of age, and more than 250 μmol/L for babies less than 48 hours of age. The comparison was between double phototherapy and single therapy with reflective curtains (silver‐coloured reflecting cloth) on three sides of the phototherapy unit. Main outcomes reported included mean decrease in SB four and 10 hours after the start of phototherapy, duration of phototherapy, number of exchange transfusions, all‐cause mortality, ALTE, and adverse effects.
Participants
All studies included term babies with jaundice requiring phototherapy according to international or national guidelines (AAP 2004). Only Lee 2014 included term and preterm babies. Ten studies reported exclusion of at least one cause of haemolysis. For six studies, the lower limit of age at study entry was 24 hours after birth, for another study 48 hours (Babaei 2013), and for the remaining four studies, this was not specified. The upper limit of age varied from 10 to 14 days. Only one study was performed in a high‐income country (Eggert 1988). All others were conducted in middle‐income countries. Baseline bilirubin levels at study entry were similar for intervention and control groups.
Interventions
Eleven of the 12 included studies compared phototherapy with reflective materials versus an identical phototherapy unit without reflective materials. One study compared a single phototherapy unit with reflective materials versus a double phototherapy unit without curtains (Abd Hamid 2013).
All included studies used reflective materials hanging from three or four sides of the phototherapy unit. Nine studies reported the use of white reflective materials. In five studies, the reflective materials were reported to be made of plastic (Babaei 2013; Devpura 2017; Djokomuljanto 2006; Kurniasih 2011; Sivanandan 2009), two studies used linen (Eggert 1988; Lahiri 2016), and two studies did not report the exact nature of the materials used (besides that they were white) (Lee 2014; Rashmi 2015). Three studies, including the one study in comparison two (Abd Hamid 2013), used aluminium or aluminium‐like reflective materials (Abd Hamid 2013; Dachlan 2015; Magaspi 2014). The control group in all studies in comparison one had similar phototherapy units to those in the intervention group but without any reflective materials, and the control for the study in comparison two used double phototherapy consisting of one light above and one below the baby. Six studies reported use of an irradiance meter to measure irradiance in the two groups (Abd Hamid 2013; Devpura 2017; Djokomuljanto 2006; Eggert 1988; Kurniasih 2011; Sivanandan 2009). The distance between the baby and the phototherapy light was reported in nine studies (Abd Hamid 2013; Babaei 2013; Devpura 2017; Djokomuljanto 2006; Eggert 1988; Kurniasih 2011; Lahiri 2016; Magaspi 2014; Sivanandan 2009). No studies reported using light‐emitting diode lights.
Outcomes
The primary outcome was reported in all 12 studies, but data were sufficient for analysis in only 11 of these. There were differences in the timing of measurement. Four studies reported the first bilirubin assessment at four to eight hours (Abd Hamid 2013; Djokomuljanto 2006; Lahiri 2016; Sivanandan 2009), and nine studies reported assessment of bilirubin at 24 hours, but in only four studies was this the first measurement. For Abd Hamid 2013, we obtained from study authors bilirubin levels measured at 24 hours. Decline in bilirubin was reported as decline from baseline or as absolute bilirubin values post intervention (after specified time periods of phototherapy). The outcome measure reported by the authors of each study was used for analysis of primary outcomes.
Ten studies pre‐specified the adverse effects they would report; Dachlan 2015 and Eggert 1988 did not.
Excluded studies
A total of three studies were excluded from this review. We excluded two studies because they used reflective mirrors behind the lights of phototherapy units and did not have reflective materials hanging around the unit (Hashim 2015; Salehzadeh 2010). Standard specifications of many commonly used and commercially available phototherapy units already include a reflective aluminium coloured surface behind the lamps to increase irradiance. Based on this and on our inclusion criteria, we judged that these studies should not be included in the review. Another RCT, initially retrieved as full text, examined the effects of several interventions on neonatal jaundice, but we excluded this study because the interventions did not include use of reflective materials (Martinez 1992). We identified no ongoing studies.
Risk of bias in included studies
Please see Figure 2 and Figure 3 for a summary of risk of bias.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies reported that allocation to the intervention or control group was random. For three studies, available information was sufficient to conclude that risk of bias for sequence generation was low; for the remaining nine studies, risk of bias was unclear. Concealment of allocation was adequately documented for five studies. In the other seven studies, this was judged as unclear.
Blinding
Because of the nature of the intervention, it was not possible to blind parents and healthcare professionals to the intervention. We judged that lack of blinding may have influenced the way babies were taken care of; thus we concluded that all studies included in this review were at high risk of performance bias. The main outcome in this review is based on laboratory measurements of SB. It was specifically stated in only one study that the outcome assessor was blinded to the intervention. However we judged measurement of SB to be an objective measure that would be unlikely to be affected by lack of blinding. Thus even if the outcome assessor were not blinded, we judged this to show low risk for detection bias. We judged that other outcomes such as duration of phototherapy, length of stay, or detection of adverse effects would be at high risk for detection bias.
Incomplete outcome data
We judged nine studies to have low risk for attrition bias because the dropout rate or non‐reported data were less than 10% and were balanced across groups. However, we judged three studies to have unclear risk for attrition bias (Eggert 1988; Lee 2014; Magaspi 2014).
Selective reporting
The initial protocols were not available for all included studies. However for all studies, all expected outcomes were reported. We judged seven studies to be at low risk for reporting bias. Two studies reported outcomes in their methods section that were not reported in the results section; therefore we judged them to have high risk for reporting bias. For the other three studies, available information was insufficient to permit a conclusion, and we judged them to have unclear reporting bias.
Other potential sources of bias
For all included studies, we identified no other additional sources of bias.
Effects of interventions
See: Summary of findings 1 Phototherapy with reflective curtains compared to phototherapy alone for newborn infants with unconjugated hyperbilirubinaemia; Summary of findings 2 Reflective curtains compared to intensified phototherapy for newborn infants with unconjugated hyperbilirubinaemia
Comparison 1. Reflective materials versus single phototherapy
Primary outcome
Decline in bilirubin
Decline in SB at four to eight hours (Analysis 1.1)
Three studies reported this outcome (Djokomuljanto 2006; Lahiri 2016; Sivanandan 2009). The mean difference (MD) at four to eight hours was ‐14.61 (95% confidence interval (CI) ‐19.80 to ‐9.42; I² = 57%; 3 studies; 281 participants; moderate‐certainty evidence (Analysis 1.1).
Decline in SB at 24 hours (Analysis 1.2)
Nine studies reported this outcome (Babaei 2013; Dachlan 2015; Devpura 2017; Eggert 1988; Kurniasih 2011; Lahiri 2016; Magaspi 2014; Rashmi 2015; Sivanandan 2009). Therefore in addition to our pre‐specified primary outcome of bilirubin at four to eight hours, we made a post hoc decision to report bilirubin at 24 hours as a primary outcome. Of the nine studies that reported bilirubin at 24 hours, this the first measurement for only four studies. For all nine studies, effect estimates for the mean difference favoured the use of curtains; however heterogeneity was substantial (I² = 97%), so we were not able to provide a pooled estimate (Analysis 1.2; very low‐certainty evidence). Heterogeneity was not explained by the type of curtain used (Analysis 1.2). Within subgroups, heterogeneity was also substantial (I² varied between 85% and 98%). Subgroup analyses for irradiance were not possible due to insufficient data. Studies that clearly described the light source reported the use of compact fluorescent lights (six studies: Abd Hamid 2013; Devpura 2017; Djokomuljanto 2006; Kurniasih 2011; Lahiri 2016; Sivanandan 2009), or they reported the use of conventional fluorescent lights (four studies: Babaei 2013; Dachlan 2015; Eggert 1988; Magaspi 2014); one of these studies used daylight white light (Magaspi 2014), and one did not specify the light used (Rashmi 2015). Because evidence suggests no differences in terms of irradiance or effectiveness between compact and standard fluorescent lights (Sarin 2006), and because the number studies using other light source types was small, subgroup analysis based on the type of light source would not be meaningful. We were also unable to perform subgroup analysis according to gestational age because no studies included solely preterm babies.
Decline in bilirubin at first measurement (Analysis 1.3)
We included nine studies in this analysis (Babaei 2013; Dachlan 2015; Devpura 2017; Djokomuljanto 2006; Eggert 1988; Kurniasih 2011; Lahiri 2016; Rashmi 2015; Sivanandan 2009). Results of the meta‐analysis were similar to results of the analysis at 24 hours. All effect estimates favoured the use of curtains, but heterogeneity was substantial (I² = 97%; Analysis 1.3;very low‐certainty evidence).
Mean decline in SB per hour
Sivanandan 2009 reported the rate of decline of SB per hour: Mean difference per hour was ‐3.39 (95% CI ‐6.88 to 0.10; 1 study; 84 participants; very low‐certainty evidence).
Secondary outcomes
Duration of phototherapy
Duration of phototherapy was reported in five studies; however, Djokomuljanto 2006 reported this as a median with interquartile range and hence was not included in the analysis. For the four studies with analysable data (Babaei 2013; Devpura 2017; Lahiri 2016; Sivanandan 2009), all effect estimates favoured the use of reflective curtains. However heterogeneity between studies was substantial (I² = 88%), precluding a pooled estimate (Analysis 1.5;very low‐certainty evidence). Djokomuljanto 2006 also reported that the median duration of phototherapy was reduced in the reflective curtains group.
Duration of hospital stay
Babaei 2013 and Djokomuljanto 2006 reported this outcome for 179 infants. Mean difference in hospital stay (hours) was reduced in the intervention group at ‐41.08 (95% CI ‐45.92 to ‐36.25; moderate‐certainty evidence).
Other secondary outcomes
Two studies reported no exchange transfusion; the remaining nine studies did not report this outcome.
Mortality was not specifically reported in any study; however, Kurniasih 2011 reported one death in the treatment group during the study period but provided no further details. We contacted study authors twice for further information, but they have not responded.
The difference in irradiance between the two groups was reported in six studies (Abd Hamid 2013; Devpura 2017; Djokomuljanto 2006; Eggert 1988; Kurniasih 2011; Sivanandan 2009). Information was insufficient to permit conclusions or pooling of results.
Other pre‐defined outcomes, including ALTE during phototherapy, cost of the intervention, parental and medical staff satisfaction, exclusive or partial breastfeeding upon discharge, and long‐term neurological outcomes, were not reported in any of the studies.
Comparison 2. Reflective materials versus double phototherapy
Only one trial included a total of 156 infants and compared phototherapy with reflective curtains versus double phototherapy (two compact fluorescent phototherapy units from above) (Abd Hamid 2013). Of our pre‐specified outcomes, study authors reported the decline in bilirubin at four hours and duration of phototherapy. There was little or no difference in the mean decline in bilirubin at four hours (MD 0.17, 95% CI ‐8.58 to 8.92; low‐certainty evidence) nor in the duration of phototherapy (hours) (MD 4.04, 95% CI ‐1.56 to 9.64; low‐certainty evidence).
Adverse events
Data on adverse events are presented in the table below. Listed pre‐specified events are those reported in the methods section as being monitored. Ten studies pre‐specified specific adverse events, and two did not. These two studies also did not report whether any adverse events were detected. Adverse events are shown in Table 1.
Table 1. Adverse events
| Studies | Adverse events pre‐specified in study report | Adverse events reported* |
| Skin changes, hypothermia, hyperthermia, dehydration, changes in stool frequency | No adverse events detected | |
| Skin rash, dehydration, hyperthermia, hypothermia, urine output, sunken fontanel | Skin rash (intervention 18/91, control 16/91) and hyperthermia (intervention 3/91, control 4/91) reported | |
| None | Adverse events not reported | |
| Loose stool, hyperthermia, feed intolerance, vomiting, dehydration | Adverse events (not specified) mild in nature and not significant | |
| Hypo/hyperthermia, dehydration, weight loss, skin rash, bronze baby syndrome, loose stool, feed intolerance | No adverse events detected | |
| None | Adverse events not reported | |
| Diarrhoea, dehydration, skin problems, hyperthermia, loose stool, feed intolerance | Hyperthermia (intervention 3/31, control 2/32) | |
| Hyperthermia, hypothermia, skin rash, diarrhoea, dehydration | No adverse events detected | |
| Poor skin turgor, skin rash, loose stool, hyperthermia, abnormal pulse, respiration, blood pressure | Adverse events not reported | |
| Hyperthermia, dehydration, burns, diarrhoea, skin rash | No adverse events detected | |
| None in abstract | Skin rash ("no difference") | |
| Hyperthermia, hypothermia, feed intolerance, skin rash | No adverse events detected |
* Not reported signifies that adverse events were not mentioned in the results section. None detected means that adverse events were specifically reported, but none were identified.
Sensitivity analysis
Sensitivity analysis performed by removing trials at high risk of bias did not substantively change the level of heterogeneity (Magaspi 2014; Rashmi 2015).
Discussion
Summary of main results
Eleven trials including a total of 1180 participants met the inclusion criteria for this review. Only three studies reported the pre‐specified outcome ‐ bilirubin after four to eight hours of phototherapy. We found moderate‐certainty evidence showing that use of reflective curtains resulted in a decline in bilirubin at this time point. The difference resulted in a small decline of 14 μmol/dL (mean difference (MD) ‐14.61, 95% confidence interval (CI) ‐19.80 to ‐9.42). Although no data are available to inform what a clinically important difference might be, we believe that most clinicians would consider this decline, although small, to be clinically meaningful and sufficient to prevent brain damage, particularly for infants who have jaundice severe enough to threaten the brain.
Nine studies reported a drop in bilirubin after 24 hours of phototherapy. For this outcome, the effect estimate for each trial favoured the use of curtains, but heterogeneity was too substantial to permit a sensible estimate of the effect size through meta‐analysis. Similar results were obtained when we analysed serum bilirubin (SB) at first measurement. Overall, these results support the use of reflective curtains, but there is uncertainty about the extent of the decline. Subgroup analyses (based on type of reflective material used or baseline levels of SB) could not explain the heterogeneity. Adverse effects, when reported, were mild and showed no differences between groups. The secondary outcome ‐ duration of phototherapy ‐ was reported in six studies. Each trial favoured the use of reflective materials, but again heterogeneity was too substantial for meta‐analysis of the data. Duration of hospital admission was reported in two trials and was reduced with the use of reflective materials. One trial compared the use of one phototherapy unit with reflective curtains versus the use of two phototherapy units and provided low‐certainty evidence suggesting that there was little to no difference between these two interventions.
Overall completeness and applicability of evidence
Because most of the included studies excluded infants with haemolysis, our results apply mainly to infants with non‐haemolytic causes of jaundice.
The purpose of phototherapy is to prevent exchange transfusion, and the purpose of exchange transfusion is to prevent kernicterus. Unfortunately, we found insufficient information about either of these outcomes. Exchange transfusion is infrequent, and because of interventions now in place to prevent rhesus disease and the advent of phototherapy, kernicterus is now quite rare. Therefore, randomised controlled trials (RCTs) may not serve as the best way to measure the impact of our intervention on these important outcomes. This also applies to some of the adverse effects that might occur with reflective curtains, including acute life‐threatening events (ALTEs) ‐ an important perceived risk that might arise due to curtains obscuring the infant. However, breastfeeding and parental satisfaction are important outcomes that could be measured in the context of an RCT. We did not find information on either of these outcomes in any of the included studies.
We preferred to measure decline in SB at four to eight hours for our primary outcome for several reasons. First, the rate of decline in SB is exponential. The higher the SB, the greater the decline that would be expected. Second, the first four to eight hours is the most critical time in the management of hyperbilirubinaemia. Third, by 24 hours, there may be some attrition because some babies are well enough for discharge home. Three studies reported attrition 24 hours after the start of the intervention, and for four it is unclear whether there might have been attrition because it is unclear how many infants were analysed.
We observed substantial heterogeneity between studies for the outcome decline in bilirubin. This is to be expected for several reasons. First, age at recruitment would be important because babies would be at different stages in the natural history of neonatal jaundice. Bilirubin production increases over the first few days of the condition and then levels off and declines. The SB increase and decline mirrors this, peaking often at about 96 hours of life. Therefore babies might respond to the intervention differently depending on their age at recruitment. Across studies, the mean age at recruitment ranged from 30 hours to 6 days of age. Second, preterm infants might respond differently. When the irradiance of phototherapy is increased, efficacy is increased, and reflective curtains increase irradiance by reflecting scattered light from the light source back to the baby. This varies depending on the type of reflective material used and the distance between the light source and the curtains, as well as the distance between the curtains and the baby. The exponential relationship between irradiance and distance from the light source would mean that small differences in distance could have a considerable effect on irradiance, thus exaggerating differences between studies. For this same reason, the distance between the patient and the light source could also contribute to the heterogeneity we encountered. Reported increases in irradiance resulting from the use of reflecting curtains of a different nature varied among studies, and some studies did not report irradiance, nor the distance between the phototherapy unit and the patient. Subgroup analysis suggests that heterogeneity is not explained by the type of reflective material used nor by the baseline SB. It is not possible to look at the contribution of distance because this varies widely. We were unable to look at the effects of gestation.
Quality of the evidence
We used the GRADE approach to assess the certainty of evidence.
We considered evidence to be of moderate certainty for decline in bilirubin after four to eight hours of phototherapy and for duration of stay in the hospital. We downgraded for study limitations (one level because of the nature of the intervention, or because hospital staff and caregivers of patients could not be blinded to the intervention) and for imprecision (because the number of trials reporting these two outcomes was low).
For the other outcomes reported in this review, we graded evidence as very low certainty. We downgraded evidence based on study limitations (one level for lack of blinding) and on imprecision (one level), as well as for inconsistency (heterogeneity) (one level), making pooling of data not sensible.
For the second comparison, we downgraded the evidence two levels because this involved only one small study with wide confidence intervals, which included the possibility of a decline or an increase in the outcome. In addition, we could not assess whether there was inconsistency because there was only one study.
Potential biases in the review process
One study that met our inclusion criteria and included our primary outcome was available only in abstract form and did not include any quantitative data, although study authors concluded that reflective materials resulted in a more rapid decline in SB and a shorter hospital stay. Another study was available only in the form of a "slide" presentation, which included sufficient quantitative data to be included in the analysis. Both of these studies were identified through additional searches, suggesting that there could yet be other unidentified studies.
The initially planned primary outcome (decline in SB after four to eight hours of phototherapy) was reported in only three trials for the first comparison. We added post hoc a primary outcome (decline in SB after 24 hours) because this was reported in nine trials. Without this added outcome, assessment of available evidence would have been extremely incomplete.
Our search revealed an article that described the use of reflective materials with a single phototherapy unit versus the use of two phototherapy units without curtains. All study authors deemed this sufficiently important to be added as a second comparison post hoc.
Two review authors were involved in studies included in this review: HVR in Abd Hamid 2013 and Djokomuljanto 2006; and IJAH in Abd Hamid 2013. Extreme care was taken to minimise involvement of these two review authors in decisions concerning their own studies.
A potential bias of this review could be our definition of what constitutes our intervention. We wanted to study the use of additional reflective materials hung around the perimeter of the light bank. We do not have a definition of a reflective material, and only one of the included studies made an attempt to show that the material used by researchers was reflective (Djokomuljanto 2006). We excluded two studies because we judged that the intervention used did not meet our inclusion criteria. The intervention in these two studies consisted of a reflective surface behind the bank of lights. Almost all commercially available phototherapy lights have such a reflective surface behind the light bank, hence our reason for these exclusions. Nevertheless, making these judgements could be conceived as a bias in the review process.
Agreements and disagreements with other studies or reviews
A previously published systematic review included only five trials, all of which we identified (Lee 2018). The method of assessment of quality used in this review differed from our method. Review authors assessed the quality of trials by using the Physiotherapy Evidence Database Scale and the Consolidated Standards of Reporting Trials Guidelines. They did not identify the between‐study heterogeneity that we encountered but similarly concluded that use of reflective materials could improve the "efficacy" of phototherapy. They concluded that this did not cause adverse effects.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Phototherapy with reflective curtains versus phototherapy alone, Outcome 1: Decline in bilirubin at 4 to 8 hours

Comparison 1: Phototherapy with reflective curtains versus phototherapy alone, Outcome 2: Decline in serum bilirubin (over 24 hours)

Comparison 1: Phototherapy with reflective curtains versus phototherapy alone, Outcome 3: Decline in bilirubin (first measurement)

Comparison 1: Phototherapy with reflective curtains versus phototherapy alone, Outcome 4: Rate of decline in bilirubin (micromol/L/h)

Comparison 1: Phototherapy with reflective curtains versus phototherapy alone, Outcome 5: Duration of phototherapy (in hours)

Comparison 1: Phototherapy with reflective curtains versus phototherapy alone, Outcome 6: Duration of hospital stay (in hours)

Comparison 1: Phototherapy with reflective curtains versus phototherapy alone, Outcome 7: Decline in bilirubin at 24 hours (low and high baseline SB)

Comparison 2: Reflective curtains versus intensified phototherapy, Outcome 1: Decline in bilirubin (first measurement)

Comparison 2: Reflective curtains versus intensified phototherapy, Outcome 2: Duration of phototherapy (in hours)
| Phototherapy with reflective curtains compared to phototherapy alone for newborn infants with unconjugated hyperbilirubinaemia | |||||
| Patient or population: newborn infants with unconjugated hyperbilirubinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative | № of | Certainty of the | Comments |
|---|---|---|---|---|---|
| Decline in bilirubin at 4 to 8 hours (μmol/L) | MD 14.61 lower in the phototherapy with curtains group than in the group with phototherapy alone | ‐ | 281 | ⊕⊕⊕⊝ | |
| Decline in serum bilirubin (over 24 hours) | See comment | ‐ | 893 | ⊕⊝⊝⊝ | Although the effect estimate for all studies favoured reflective curtains, no summary effect is available due to substantial heterogeneity |
| Exchange transfusion | Two studies reported no exchange transfusion; remaining studies did not report this outcome | ‐ | (2 RCTs) | ‐ | |
| Acute life‐threatening events | This outcome was not reported in any of the included studies | ‐ | (11 RCTs) | ‐ | |
| Parental satisfaction | This outcome was not reported in any of the included studies | ‐ | (11 RCTs) | ‐ | |
| Breastfeeding on | This outcome was not reported in any of the included studies | ‐ | (11 RCTs) | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aDowngraded one level for moderate inconsistency (heterogeneity). bLack of blinding of caregivers in all studies and possible selection bias, cDowngraded two levels for substantial inconsistency (heterogeneity). | |||||
| Reflective curtains compared to intensified phototherapy for newborn infants with unconjugated hyperbilirubinaemia | |||||
| Patient or population: newborn infants with unconjugated hyperbilirubinaemia | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|---|
| Decline in bilirubin (first measurement) | MD 0.17 higher in group with curtains than in group with intensified phototherapy | ‐ | 159 | ⊕⊕⊝⊝ | |
| Duration of phototherapy (in hours) | MD 4.04 higher in group with curtains than in group with intensified phototherapy | ‐ | 159 | ⊕⊕⊝⊝ | |
| Exchange transfusion | Three infants reached exchange transfusion level. No differences between groups | ‐ | (159) (1 RCT) | ‐ | |
| Acute life‐threatening events | No events reported | ‐ | (159) (1 RCT) | ‐ | |
| Patient satisfaction | Not reported | ‐ | (159) (1 RCT) | ‐ | |
| Breastfeeding on discharge | Outcome not reported | ‐ | (159) (1 RCT) | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence. | |||||
| aAlthough blinding of caregivers was lacking, the outcome assessor was blinded. bDowngraded one level for single study; unable to assess inconsistency with other studies. cSingle study and wide confidence interval, including potential for a decrease and an increase in the outcome. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Decline in bilirubin at 4 to 8 hours Show forest plot | 3 | 281 | Mean Difference (IV, Fixed, 95% CI) | ‐14.61 [‐19.80, ‐9.42] |
| 1.2 Decline in serum bilirubin (over 24 hours) Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 White plastic | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 White linen | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.3 Aluminium | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.4 White not defined | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Decline in bilirubin (first measurement) Show forest plot | 9 | 796 | Mean Difference (IV, Fixed, 95% CI) | ‐29.08 [‐31.93, ‐26.22] |
| 1.4 Rate of decline in bilirubin (micromol/L/h) Show forest plot | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐3.39 [‐6.88, 0.10] |
| 1.5 Duration of phototherapy (in hours) Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Duration of hospital stay (in hours) Show forest plot | 2 | 279 | Mean Difference (IV, Fixed, 95% CI) | ‐41.08 [‐45.92, ‐36.25] |
| 1.7 Decline in bilirubin at 24 hours (low and high baseline SB) Show forest plot | 9 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 Mean baseline SB < 300 micromol/L | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7.2 Mean baseline SB ≥ 300 micromol/L | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Decline in bilirubin (first measurement) Show forest plot | 1 | 159 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐8.58, 8.92] |
| 2.2 Duration of phototherapy (in hours) Show forest plot | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [‐1.56, 9.64] |


