Kurzfristige‐orale Steroide als Zusatztherapie bei chronischer Rhinosinusitis
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 3‐arm, non‐blinded, parallel‐group, with 21 days duration of treatment and follow‐up | |
| Participants | Location: Turkey, 1 site Setting of recruitment and treatment: ear, nose and throat department of Haydarpasa Numune education and research hospital, Istanbul Sample size: 45 Number randomised (and completed): 15 in oral steroids and INCS, 15 in INCS alone, 15 in oral steroids alone (see notes below) Participant (baseline) characteristics:
‐ Grade 1: oral steroids and INCS: 1 (6.7%); INCS alone: 3 (13.3%) ‐ Grade 2: oral steroids and INCS: 9 (60%); INCS alone: 5 (33.3%) ‐ Grade 3: oral steroids and INCS: 5 (33.3%); INCS alone: 8 (53.3%)
Inclusion criteria: none stated. No information on polyp grading criteria (other than Rasp). Exclusion criteria: patients in whom corticosteroid therapy was contraindicated (i.e. those with diabetes mellitus, hypertension, glaucoma, a history of tuberculosis or emotional instability), as well as those who had received corticosteroids within the last month | |
| Interventions | Oral steroids and INCS (n = 15): oral methylprednisolone, 1 mg/kg and reduced progressively over a 21‐day treatment course INCS alone (n = 15): no oral steroid treatment Use of additional interventions (common to both treatment arms): budesonide, unclear method of administration except it states 'intranasal', 400 μg/day, 21 days | |
| Outcomes | Primary outcomes: none reported Secondary outcomes:
Other outcomes reported by the study:
| |
| Funding sources | No information provided | |
| Declarations of interest | "None declared" | |
| Notes | The trial is a 3‐arm trial comparing "oral steroids alone", "INCS alone" and "oral steroids and INCS". The results for the "oral steroids alone" group are not presented here as they are not relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were allocated in turn to either the first, second or third treatment group, depending on their order of presentation." Comment: pg 585, col 1, para 4 |
| Allocation concealment (selection bias) | High risk | Comment: as randomisation was completed based on order of presentation, there is a high risk of bias due to allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Comment: the study was not blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there is no information regarding blinding of outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Comment: no patients dropped out of the study |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes are reported |
| Other bias | Unclear risk | Comment: Rasp classification used for endoscopic polyp staging. Unclear whether this has been validated and no details on stage used. Baseline characteristics are not well described but the polyp grade seems to be unevenly distributed (although not statistically significant) with more severe patients in the placebo group, which may have affected the results. The small size of the trial makes it difficult to draw conclusions. |
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with 15‐day duration of oral steroid treatment and 30‐day duration of follow‐up | |
| Participants | Location: Turkey, 2 sites Setting of recruitment and treatment: paediatric allergy and ear, nose, and throat outpatient clinics of 2 university hospitals Sample size: 48 Number randomised: 24 in oral steroids and antibiotics, 24 in antibiotics alone Number completed: 22 in intervention, 23 in antibiotics alone Participant (baseline) characteristics: note: no. analysed not randomised
Inclusion criteria: children with CRS; CRS diagnosis made on a basis of sinonasal symptoms and signs present for a period of more than 3 months in the presence of abnormalities on coronal sinus computed tomographic (CT) scans. All patients presented with nasal purulence, postnasal purulence or both and 1 or more of the following symptoms: nasal obstruction, cough, halitosis, headache or facial pain/pressure. They had multiple courses (each 10 to 14 days, > 3 courses) of antimicrobial treatment with at least 2 or more of the following broad‐spectrum antibiotics before entry into the study: amoxicillin‐clavulanic acid, second‐generation cephalosporins (mostly cefuroxime) or clarithromycin Patients with allergic rhinitis were also included if they also showed purulent rhinorrhoea, postnasal purulence or both Exclusion criteria: ‐ Systemic corticosteroids in the last 2 months before the study ‐ Systemic antibiotics and inhaler or intranasal corticosteroids in the last 4 weeks before the study ‐ Other respiratory tract disorders (cystic fibrosis, ciliary dyskinesia, nasal polyps, large adenoids and asthma), immune deficiency ‐ Systemic disease, gastroesophageal reflux, aspirin sensitivity ‐ Acquired or congenital sinonasal abnormalities, contraindication to corticosteroid use ‐ Patients with pollen‐induced rhinitis if they were seen during the pollen season | |
| Interventions | Intervention (n = 24): oral methylprednisolone tablets, 15 days according to the following schedule (doses were rounded up to the nearest 4 mg): Comparator group (n = 24): placebo tablets for 15 days Use of additional interventions (common to both treatment arms): antibiotics (oral amoxicillin/clavulanate) was administered at 45/6.4 mg/kg/day (maximum, 2000/285 mg/day) for 30 days | |
| Outcomes | Primary outcomes: 1. Disease severity, assessed by the patients and their parents by using a visual analogue scale (VAS) (range 0 (no symptoms) to 10 (most severe)). The symptoms scored were: purulent nasal discharge, nasal obstruction, postnasal drainage, halitosis, cough, and facial pain or headache. Individual scores were combined to make a rhinosinusitis symptom score (range 0 to 60) at the end of treatment measured at 30 days. Secondary outcomes: 2. CT scan, scored using the Lund‐Mackay staging system (0 to 24) measured at 30 days Other outcomes reported by the study:
| |
| Funding sources | No information provided | |
| Declarations of interest | "The authors have declared that they have no conflicts of interest." | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… a random allocation chart based on a table of random numbers." Comment: pg 349, col 2, para 1 |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization assignments were kept in sealed envelopes" Comment: pg 349, col 2, para 1 |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote: "Placebo tablets contained lactose and were of same size and colour as methylprednisolone (16 mg per tablet). Placebo and methylprednisolone tablets were dispensed in identical packets containing a minimum of 20 tablets each." "The randomization code was kept by the nursing staff in the pediatric allergy department." Comment: pg 349, col 2, para 1 Although the paper states that the tablets were equivalent with respect to size and colour, no mention was made about the taste of the tablets. As the taste of methylprednisolone is different to lactose, it may have been obvious which was the treatment. 1 patient in the treatment group dropped out due to unpalatability. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: the study reports that the participants were blinded and so the patient‐reported outcomes are likely to have been blinded. For the CT scan outcome it is noted that the assessor was blind to treatment and sequence. No mention of whether the analysis was completed blind. |
| Incomplete outcome data (attrition bias) | Low risk | Comment: although there was low drop‐out (3/48 = 6%) there are no clear reasons provided for 2 of the patients' "protocol violation" or why their results were not included in the analysis |
| Selective reporting (reporting bias) | High risk | Quote: "No clinically significant adverse events were reported. Twenty seven parents (16 in the methylprednisolone group and 11 in the placebo group) reported that their children's appetite and weight increased after treatment. At the end of the treatment, the mean ± SD changes in patients’ weights from baseline were 0.42 ± 0.26 kg in the methylprednisolone group versus 0.27 ± 0.30 kg in the placebo group. The difference was not significant (P = 0.08)" Comment: although all of the efficacy outcomes are presented in the results section, the methods section classified increase in appetite and weight gain as clinically significant adverse events and the results indicated that there may be a difference between the groups The summed scores for the individual symptom scores as presented in the paper do not add up to the total symptom score as presented |
| Other bias | Unclear risk | Comment: no information regarding the validation of visual analogue scales for reporting CRS symptoms. Nothing was made in the results of the finding that the placebo group benefited substantially from placebo treatment. |
CRS: chronic rhinosinusitis
CT: computerised tomography
INCS: intranasal steroids
RCT: randomised controlled trial
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| INTERVENTION: oral steroids versus surgery | |
| INTERVENTION: oral steroids alone versus no steroid treatment | |
| INTERVENTION: oral steroids alone versus no steroid treatment | |
| INTERVENTION: oral steroids alone versus no steroid treatment | |
| INTERVENTION: oral steroids alone versus no steroid treatment | |
| INTERVENTION: surgery | |
| INTERVENTION: combined medical and surgical treatment | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroid alone versus placebo (Ongoing study) | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroid (12 days) + INCS versus oral steroid (20 days) + INCS | |
| INTERVENTION: oral steroids alone versus placebo | |
| STUDY DESIGN: review of previous oral steroids trials | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroids alone versus placebo | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: 4‐arm trial: (1) oral steroids, (2) intranasal steroids, (3) steroid injection into polyp, (4) no treatment | |
| INTERVENTION: oral steroid alone then INCS versus placebo then INCS (note: oral steroids and INCS not given concurrently) | |
| INTERVENTION: oral steroid alone then INCS versus placebo then INCS (note: oral steroids and INCS not given concurrently) | |
| INTERVENTION: endoscopic polypectomy with ethmoidectomy | |
| INTERVENTION: surgical removal versus systemic corticosteroids | |
| INTERVENTION: surgical polypectomy followed by continuous topical steroid treatment versus a single dose of depot steroid | |
| INTERVENTION: oral steroids alone versus no steroid treatment | |
| INTERVENTION: oral steroids alone versus no steroid treatment (Ongoing study) | |
| INTERVENTION: oral steroids alone versus placebo (Ongoing study) | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: medical versus surgical treatment | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroid versus INCS | |
| POPULATION: allergic fungal sinusitis | |
| STUDY DESIGN: surgical outcomes paper | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroids alone then INCS versus placebo then INCS (note: oral steroids and INCS not given concurrently) | |
| STUDY DESIGN: not randomised | |
| INTERVENTION: oral steroids alone then INCS versus placebo then INCS (note: oral steroids and INCS not given concurrently) | |
| INTERVENTION: oral steroids alone then INCS versus placebo then INCS (note: oral steroids and INCS not given concurrently) |
INCS: intranasal steroids
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Corticosteroid therapy for chronic rhinosinusitis without nasal polyps (CRSsNP) |
| Methods | 2‐arm, randomised, controlled, parallel‐group, open study |
| Participants | 40 patients (18 to 80 years old) with CRS without nasal polyps |
| Interventions | Group 1: systemic prednisone: starting dose of 40 mg for 5 days followed by a taper decreasing by 10 mg every 5 days. Followed by topical mometasone (INCS) until the end of the study. Group 2: topical mometasone (INCS) at the standard dose of 2 sprays into each nostril once daily until the end of the study. Both groups: 3‐week course of a broad‐spectrum antibiotic, amoxicillin/clavulanate, at a daily dose of 875 mg twice daily. If the participant is allergic to penicillin and its derivatives or has had an adverse reaction to amoxicillin/clavulanate, a 3‐week course of clarithromycin instead. |
| Outcomes | Primary: Lund‐MacKay score from CT scan Secondary: Taskforce symptom inventory, SNOT‐22 questionnaire, medication side effects and compliance inventory All outcomes measured at 4 to 6 weeks and 3 months after initiation of treatment |
| Starting date | August 2012 |
| Contact information | Bruce Tan, MD, Assistant Professor, Dept of Otolaryngology, Northwestern University Feinberg School of Medicine (contact: [email protected]) |
| Notes | Results expected June 2017 |
CRS: chronic rhinosinusitis
CT: computerised tomography
INCS: intranasal corticosteroids
SNOT‐22: Sino‐Nasal Outcome Test‐22
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nasal polyp grading Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.87, ‐0.05] |
| Analysis 1.1 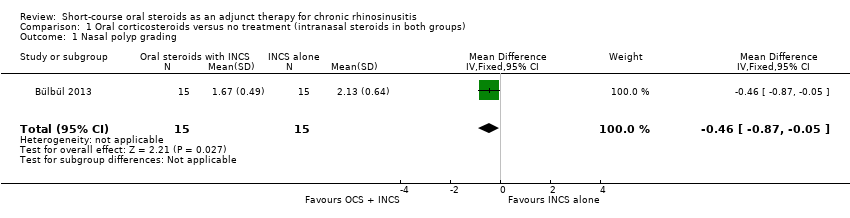 Comparison 1 Oral corticosteroids versus no treatment (intranasal steroids in both groups), Outcome 1 Nasal polyp grading. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total symptom score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐9.59, ‐4.61] |
| Analysis 2.1  Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 1 Total symptom score. | ||||
| 2 Nasal obstruction Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐4.71, ‐2.29] |
| Analysis 2.2  Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 2 Nasal obstruction. | ||||
| 3 Purulent nasal discharge Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.54, 1.14] |
| Analysis 2.3  Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 3 Purulent nasal discharge. | ||||
| 4 Headache/facial pain Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐2.55, ‐0.05] |
| Analysis 2.4 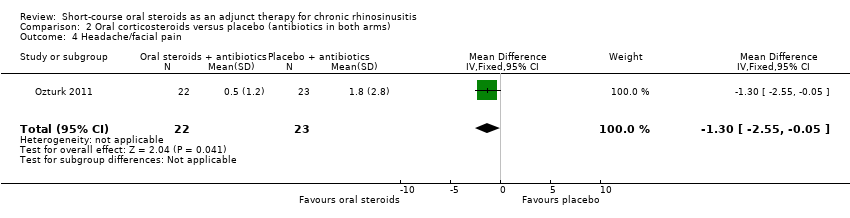 Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 4 Headache/facial pain. | ||||
| 5 Cough Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.35, ‐0.85] |
| Analysis 2.5  Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 5 Cough. | ||||
| 6 CT score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.91, ‐0.89] |
| Analysis 2.6  Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 6 CT score. | ||||
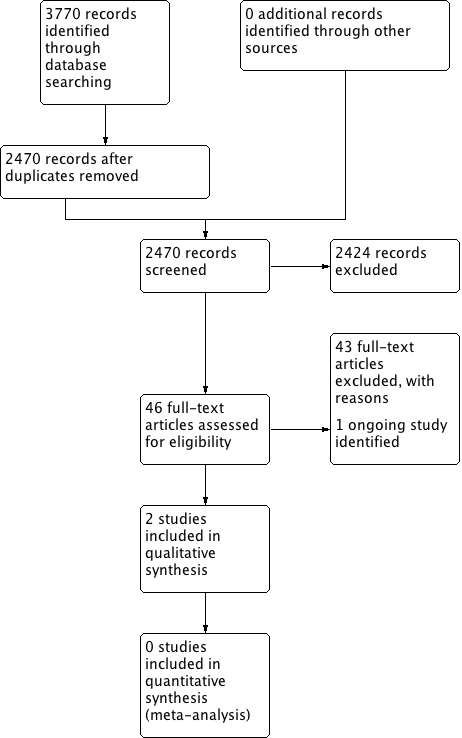
Process for sifting search results and selecting studies for inclusion.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
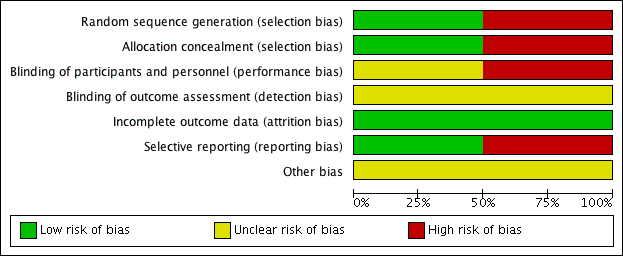
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
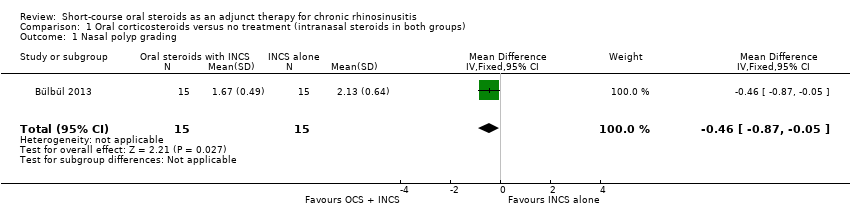
Comparison 1 Oral corticosteroids versus no treatment (intranasal steroids in both groups), Outcome 1 Nasal polyp grading.
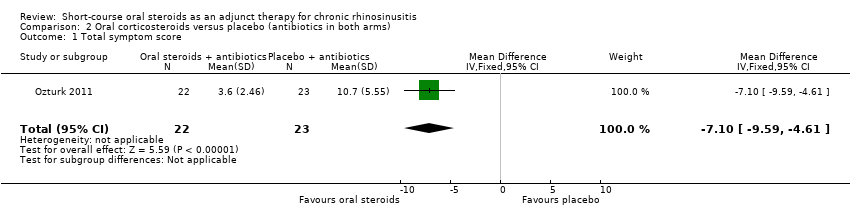
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 1 Total symptom score.
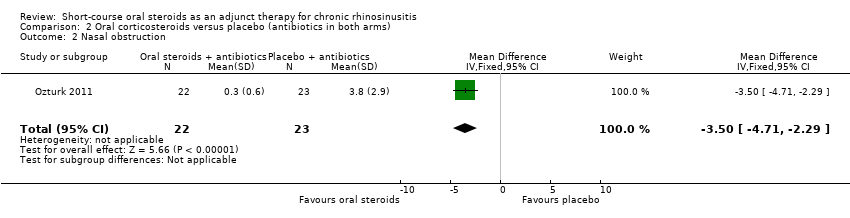
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 2 Nasal obstruction.
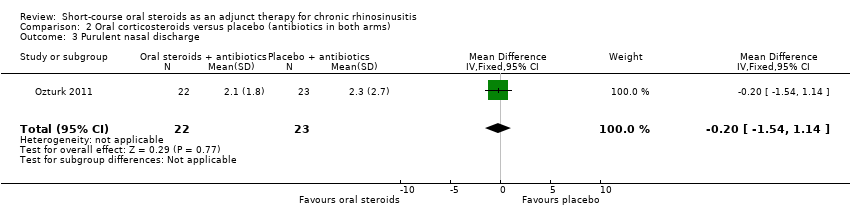
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 3 Purulent nasal discharge.
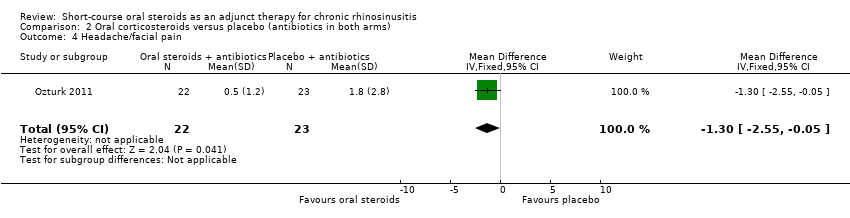
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 4 Headache/facial pain.
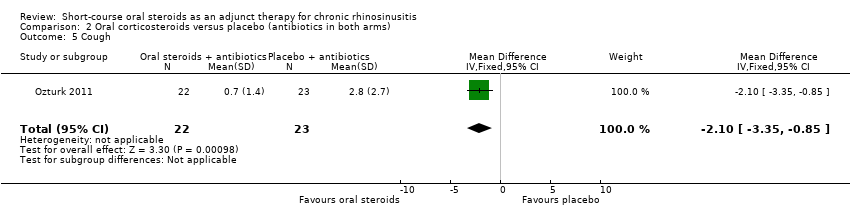
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 5 Cough.
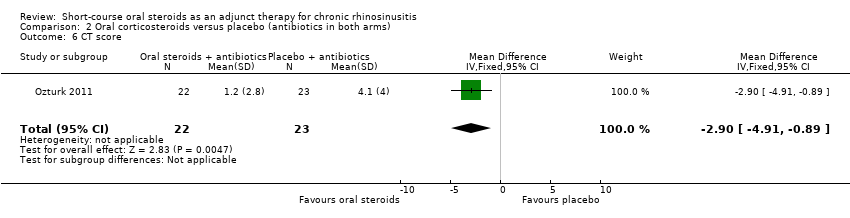
Comparison 2 Oral corticosteroids versus placebo (antibiotics in both arms), Outcome 6 CT score.
| Short‐course oral corticosteroids compared to no oral corticosteroid treatment (intranasal steroids in both arms) for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis | ||||||
| Outcomes No. of participants (studies) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | ||||
| Disease‐specific health‐related quality of life | — | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score | — | No RCT reported this outcome | ||||
| Adverse effect: mood or behavioural disturbances | — | No RCT reported this outcome | ||||
| Health‐related quality of life | — | No RCT reported this outcome | ||||
| Adverse effect: insomnia | — | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances ‐ not measured | — | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Short‐course oral corticosteroids compared to placebo (antibiotics in both groups) for chronic rhinosinusitis | |||||
| Patient or population: chronic rhinosinusitis | |||||
| Outcomes No of participants (studies) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| Without oral steroids | With oral steroids | Difference | |||
| Disease‐specific health‐related quality of life | No RCT reported this outcome | ||||
| Disease severity ‐ patient‐reported symptom score, assessed with: 4 individual symptoms measured on 0 to 10 visual analogue scale summed to provide a range of 0 to 40 No. of participants: 45 | The mean disease severity score without oral steroids was 15.2 | The mean disease severity score with oral steroids was 3.6 | The mean disease severity score in the intervention group was 7.10 lower (9.59 lower to 4.61 lower) | ⊕⊕⊝⊝ | A lower score indicates less severe symptoms. The results relate to a standardised mean difference of 1.61 standard deviations lower (‐2.29 to 0.93 lower), corresponding to a large difference. |
| Adverse effect: mood or behavioural disturbances | No RCT reported this outcome | ||||
| Health‐related quality of life | No RCT reported this outcome | ||||
| Adverse effect: insomnia | No RCT reported this outcome | ||||
| Adverse effect: gastrointestinal disturbances | No RCT reported this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Study completed only in children (mean age 8 years old). Study follow‐up time was less than 3 months (1 month). Scales were not validated and were completed by "parents and children". 2Symptoms included in this score were: purulent nasal discharge, nasal obstruction, cough and facial pain/headache. | |||||
| System | Adverse events | Notes |
| Musculoskeletal | Osteoporosis | Largely limited to long‐term use Significantly increased risk of fractures with prolonged use |
| Osteonecrosis | Rare; appears to be dose‐dependent | |
| Endocrine | Hyperglycaemia | Common; dose‐dependent, usually reversible |
| Cardiovascular | Hypertension | Common; dose‐dependent, usually reversible |
| Dermatological | Striae, bruising | Dose‐dependent, occurs after > 1 month usage |
| Ophthalmological | Cataracts | Irreversible; largely related to long‐term usage |
| Glaucoma | High risk with pre‐existing disease | |
| Gastrointestinal tract | Peptic ulceration | Increased risk largely due to concomitant NSAIDs |
| Psychological | Psychosis | Common; increased risk with dosages > 40 mg/day |
| References: Da Silva 2006; Naber 1996; Stanbury 1998 NSAIDs: non‐steroidal anti‐inflammatory drugs | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nasal polyp grading Show forest plot | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.87, ‐0.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total symptom score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐7.10 [‐9.59, ‐4.61] |
| 2 Nasal obstruction Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐3.50 [‐4.71, ‐2.29] |
| 3 Purulent nasal discharge Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.54, 1.14] |
| 4 Headache/facial pain Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.3 [‐2.55, ‐0.05] |
| 5 Cough Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐3.35, ‐0.85] |
| 6 CT score Show forest plot | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐4.91, ‐0.89] |

