نقش فوتوتراپی در درمان زخمهای پا در افراد مبتلا به دیابت
چکیده
پیشینه
زخمهای پا عارضه ناتوان کننده دیابت هستند که 15% تا 25% از افراد مبتلا به دیابت را در دورههایی از زندگیشان تحت تاثیر قرار میدهند. فوتوتراپی یک روش درمانی نسبتا جدید، غیر‐تهاجمی و بدون درد است، که باعث پیشرفت فرآیند بهبودی زخم از طریق مکانیسمهای متعدد مانند افزایش رشد سلولی و فعالیت عروقی میشود. فوتوتراپی ممکن است به عنوان یک رویکرد جایگزین برای درمان زخمهای پا در افراد مبتلا به دیابت استفاده شود، اما شواهد مربوط به اثر آن در مقایسه با دارونما (placebo) و سایر درمانها هنوز مشخص نشده است.
اهداف
ارزیابی اثرات فوتوتراپی برای درمان زخمهای پا در افراد مبتلا به دیابت.
روشهای جستوجو
پایگاه ثبت تخصصی گروه زخم در کاکرین (11 اکتبر 2016)، پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (CENTRAL) (کتابخانه کاکرین Cochrane Library، شماره 10، 2016)؛ Ovid MEDLINE (11 اکتبر 2016)؛ Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (11 اکتبر 2016)؛ Ovid Embase (11 اکتبر 2016)؛ EBSCO CINAHL Plus (11 اکتبر 2016)؛ و زیرساختهای دانش ملی چین (China National Knowledge Infrastructure) (24 جون 2017) را جستوجو کردیم. همچنین پایگاههای ثبت کارآزماییهای بالینی را برای یافتن مطالعات در حال انجام و منتشر نشده تا 24 جون 2017 جستوجو کرده، و فهرست منابع را برای شناسایی مطالعات بیشتر غربالگری کردیم. هیچ محدودیتی را در رابطه با زبان، تاریخ انتشار، یا شرایط مطالعه اعمال نکردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده یا کارآزماییهای تصادفیسازی و کنترل شده خوشهای که 1) فوتوتراپی را با فوتوتراپی ساختگی، عدم استفاده از فوتوتراپی، یا سایر روشهای ورزش‐درمانی (physical therapy) مقایسه کردند، 2) اشکال مختلف فوتوتراپی را با هم مقایسه کردند، 3) فوتوتراپی را با توان خروجی، طول موج، تراکم قدرت، یا دامنه دوز متفاوت در بزرگسالان مبتلا به دیابت و دارای زخم باز پا با هرگونه شدت، در هر محیطی مقایسه کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم انتخاب مطالعه، استخراج دادهها، و ارزیابی خطر سوگیری (bias) را انجام دادند. در زمان مناسب پیامدهای مطالعه را ترکیب کردیم.
نتایج اصلی
هشت کارآزمایی با 316 شرکتکننده، معیارهای ورود را داشتند. بیشتر مطالعات وارد شده مطالعات تک‐مرکزی بودند که در کلینیکها یا بیمارستانها با حجم نمونه بین 14 تا 84 فرد انجام شدند. از آنجایی که یک دامنه در معرض خطر بالای سوگیری، و سه دامنه یا بیشتر در معرض خطر نامشخص سوگیری قرار داشتند، بهطور کلی، مطالعات وارد شده را در معرض خطر نامشخص یا بالای سوگیری در نظر گرفتیم.
هیچ مطالعهای را شناسایی نکردیم که دادههای معتبری را در مورد زمان سپری شده تا التیام کامل زخم گزارش کرده باشد. متاآنالیز چهار مطالعه شامل 116 شرکتکننده نشان داد بیماران دریافت کننده فوتوتراپی در مقایسه با افرادی که فوتوتراپی دریافت نکردند/ دارونما، ممکن است با نسبت بالاتری از زخمهای کاملا بهبود یافته در طول پیگیری روبهرو شوند (64.5% برای گروه فوتوتراپی و 37.0% برای گروه بدون فوتوتراپی/دارونما؛ خطر نسبی (RR): 1.57؛ 95% فاصله اطمینان (CI): 1.08 تا 2.28؛ شواهد با کیفیت پائین، به دلیل محدودیتهای مطالعه و عدم دقت کاهش یافت). دو مطالعه، در قسمت نتایج اشارهای به عوارض جانبی نکردند؛ یک مطالعه با 16 شرکتکننده نشان داد که عوارض جانبی مرتبط با دستگاه وجود نداشت، و مطالعه دیگر با 14 شرکتکننده به این نتیجه رسید که تفاوت شفافی بین گروه فوتوتراپی و دارونما وجود نداشت.
چهار مطالعه تغییر را در اندازه زخم گزارش کردند، اما در درجه اول به دلیل ناهمگونی زیاد، ترکیب نشدند. نتایج حاصل از کارآزماییهای فردی (شامل 16 تا 84 شرکتکننده) بهطور کلی نشان دادند که پس از دو تا چهار هفته درمان فوتوتراپی ممکن است منجر به کاهش بیشتری در اندازه زخم شود اما کیفیت شواهد به دلیل خطر نامشخص سوگیری در کارآزمایی اولیه و حجم نمونه کوچک پائین بود. تجزیهوتحلیلهای مربوط به کیفیت زندگی و آمپوتاسیون را فقط در یک مطالعه انجام دادیم (به ترتیب 28 و 23 شرکتکننده)؛ هر دو پیامد تفاوت روشنی را بین گروه فوتوتراپی و گروه عدم فوتوتراپی/دارونما نشان ندادند.
نتیجهگیریهای نویسندگان
این مرور سیستماتیک از کارآزماییهای تصادفیسازی شده نشان داد فوتوتراپی، در مقایسه با عدم استفاده از فوتوتراپی/دارونما، ممکن است در طول پیگیری، نسبت زخمهای کاملا بهبود یافته را افزایش و اندازه زخم را در افراد مبتلا به دیابت کاهش دهد، اما شواهدی وجود نداشت که نشان دهد فوتوتراپی کیفیت زندگی را بهبود میبخشد. با توجه به حجم نمونه کوچک و نقایص روششناسی در کارآزماییهای اصلی، سطح کیفیت شواهد پائین بود، که این موضوع اطمینان ما را به این نتایج کاهش میدهد. انجام کارآزماییهای تصادفیسازی و کنترل شده با طراحی خوب مورد نیاز هستند تا تائید شود که فوتوتراپی میتواند گزینه موثری برای درمان زخمهای پا در افراد مبتلا به دیابت باشد یا خیر.
PICOs
خلاصه به زبان ساده
نقش فوتوتراپی در درمان زخمهای پا در افراد مبتلا به دیابت
سوال مطالعه مروری
فوتوتراپی یک روش درمانی بدون درد است که برای درمان بیماریهای مختلف از نور استفاده میکند. ما شواهد مربوط به فوتوتراپی را در درمان زخمهای پا در افراد مبتلا به دیابت بررسی کردیم. ما میخواستیم بدانیم که فوتوتراپی التیام زخم را سرعت میبخشد و کیفیت زندگی بیماران را بهبود میبخشد، و اینکه عوارض جانبی دارد یا خیر.
پیشینه
زخمهای پا عارضه ناتوان کننده دیابت هستند که 15% تا 25% از افراد مبتلا به دیابت را در دورههایی از زندگیشان تحت تاثیر قرار میدهند. زخمهای پای دیابتی دردناک و مستعد ابتلا به عفونت هستند. تمامی زخمهای پای دیابتی لزوما بهبود نمییابند، که در نهایت ممکن است منجر به قطع عضو از طریق جراحی میشود. فوتوتراپی شامل قرار دادن منطقه آسیب دیده در معرض نور ماوراء بنفش، و گاهی اوقات استفاده از لیزر است. به نظر میرسد این روش از طریق مکانیزمهای متعددی مانند افزایش رشد سلولی و فعالیت عروق خونی به التیام زخمها کمک میکند. این روش بهعنوان یک رویکرد جایگزین برای بهبود زخمهای پا در افراد مبتلا به دیابت مورد استفاده قرار گرفته است.
ویژگیهای مطالعه
در اکتبر 2016، برای یافتن کارآزماییهای تصادفیسازی و کنترل شدهای جستوجو کردیم که به مقایسه فوتوتراپیهای مختلف، یا مقایسه فوتوتراپی با درمانهای دیگر یا دارونما (placebo) (درمان ساختگی)، برای زخمهای پا در بزرگسالان مبتلا به دیابت پرداختند. ما هشت کارآزمایی (316 شرکتکننده) را وارد کردیم. اکثر مطالعات در کلینیکها یا بیمارستانها انجام شده و تعداد اندکی شرکتکننده داشتند (14 تا 84). میانگین سنی در مطالعات انتخاب شده بین 53 تا 68 سال، و نسبت زنان به مردان 0.46 تا 1.88 بود. مطالعات وارد شده فوتوتراپی را با دارونما یا عدم استفاده از فوتوتراپی، در سطح بالای مراقبتهای معمول مقایسه کردند (مراقبتهای معمول شامل درمانهایی مانند پانسمانها، آنتیبیوتیکها، یا تمیز کردن زخم بود). دورههای درمان بین 15 روز تا 20 هفته متغیر بودند.
نتایج اصلی
نتایج نشان داد که فوتوتراپی، در مقایسه با عدم استفاده از فوتوتراپی یا دارونما، ممکن است نسبت زخمهای کاملا بهبود یافته را در طول پیگیری افزایش و اندازه زخم را کاهش دهد. با این حال، از آن جایی که مطالعات وارد شده شامل تعداد کمی شرکتکننده و دارای نقاط ضعف در روشهای مطالعه بودند، اطمینان ما به این نتایج محدود است. ما شواهد کافی را مبنی بر اینکه آسیبهای بالقوه یا بروز آمپوتاسیون (amputation) بین گروه فوتوتراپی و گروه بدون فوتوتراپی/دارونما تفاوت داشت یا خیر، نیافتیم.
کیفیت شواهد
سطح کیفیت شواهد را به دلیل فقدان اطلاعات و خطر اینکه نتایج مطالعه دارای سوگیری (bias) باشد، پائین قضاوت کردیم. برای تأیید منافع و آسیبهای فوتوتراپی، انجام مطالعات بیشتر با کیفیت بالا مورد نیاز هستند.
این خلاصه به زبان ساده تا 26 اکتبر 2016 بهروز است.
Authors' conclusions
Summary of findings
| Phototherapy compared with placebo/no phototherapy for foot ulcers in people with diabetes | ||||||
| Patient or population: Diabetes with foot ulcers Settings: Clinics and hospitals Intervention: Phototherapy Comparison: Placebo/no phototherapy | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed absolute effect | Corresponding absolute effect | |||||
| Placebo/no phototherapy | Phototherapy | |||||
| Wound healing ‐ time to complete wound healing (weeks) | No study provided reliable data for this outcome. | |||||
| Wound healing ‐ proportion of wounds completely healed during follow‐up | 330 per 1000 | 568 per 1000 | RR 1.57 (1.08 to 2.28) | 116 (4 studies) | ⊕⊕⊝⊝ | |
| Adverse events | See comment | See comment | See comment | See comment | See comment | In Landau 2011, there were no device‐related adverse events. In Londahl 2013, the authors suggested that there was no difference in adverse events between intervention and control groups, but the number of adverse events was not reported. |
| *The basis for the assumed absolute effect (e.g. the median control group risk across studies) is provided in footnotes. The corresponding absolute effect (and its 95% confidence interval) is based on the assumed absolute effect in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for study limitations (high risk of bias for incomplete outcome data in two studies and potential influence of imbalance in baseline characteristics in one study) and one level for imprecision (small sample size). | ||||||
Background
Description of the condition
Diabetes mellitus is a group of disorders characterised by raised blood glucose levels. It impairs quality of life, and has a strong association with heart disease, stroke, and death (Barr 2007; Ellen 2007). Owing to a rapidly aging population, the number of people with diabetes is increasing throughout the world. The World Health Organization estimated that the global prevalence of diabetes was 9% among adults in 2014, and that diabetes will be the seventh‐leading cause of death in 2030 (Mathers 2006; WHO 2014).
Foot ulcers, defined as wounds extending through the full thickness of the skin below the level of the ankle (Apelqvist 2000a; Lipsky 2012), are a disabling complication of diabetes. People with diabetes are at considerable risk of developing foot ulcers. It has been estimated that 15% to 25% of people with diabetes will be affected by foot ulcers at some time in their lives (Boulton 2008; Singh 2005). The worldwide prevalence of foot ulcers amongst people with diabetes varies by country, with about 2% in high‐income countries, compared with about 15% to 20% in low and middle income countries (Margolis 2011; Shailesh 2012; Tseng 2003). The high risk of foot ulceration in people with diabetes is the result of a complex combination of extrinsic and intrinsic factors. Firstly, peripheral insensitivity due to neuropathy may lead to abnormal loading and increased pressure of the foot, which in turn causes tissue damage (Boulton 2000). The impaired sensation may also reduce a person's awareness of potentially dangerous foreign bodies and injuries. Secondly, the additional presence of peripheral vascular disease may lead to poor healing of the damaged tissue (Apelqvist 2000a; Boulton 2000). Limited joint mobility, foot deformation, and poorly fitting footwear may further increase the risk of foot ulcers in people with diabetes (Apelqvist 2000a).
Diabetic foot problems have a significant impact on a person's quality of life. It often takes several weeks or months for ulcers to heal, even with timely and intensive treatment (Zimny 2002). A substantial proportion of ulcers do not heal, and lower‐extremity amputations are performed (Reiber 2001). Evidence has shown that people with diabetes have a 10‐ to 20‐fold increased risk of losing a part or full lower limb due to non‐traumatic amputation when compared with people without diabetes (Morris 1998; Wrobel 2001). Approximately 30% to 60% of all amputations of the lower extremity are performed in people with diabetes, and over 85% of these amputations are preceded by a foot ulcer deteriorating to deep infection or gangrene (Apelqvist 2000b; CDC 2014; Ziegler‐Graham 2008).
Foot problems in people with diabetes also have a significant financial impact on national health systems around the world. The direct costs of treatment for foot ulcers in people with diabetes include wound dressings, antibiotics, surgery, specialist footwear, staff costs, and hospital admissions (Stockl 2004). The costs other than treatment, including the costs associated with preventive efforts, rehabilitation, home care, and the loss of work time and productivity, are also high. In the United States, ulcer care adds around USD 9 billion to 13 billion to the direct yearly costs associated with diabetes itself (Rice 2014).
Description of the intervention
Though a variety of therapeutic methods such as wound cleansing, debridement, antibiotics, off‐loading and skin grafting are available for the treatment of foot ulcers in people with diabetes, the results of treatment are often unsatisfactory (Khanolkar 2008). Phototherapy is a relatively new, non‐invasive, and pain‐free treatment method that has received clearance from the US Food and Drug Administration for its beneficial effects on tissue healing and pain relief (MedX Health 2012; Vargas 2005). Phototherapy uses light for therapeutic purposes. Accumulating evidence indicates that phototherapy may promote the repair processes of skin, ligament, bone, tendon, and cartilage (Fung 2002; Kana 1981; Posten 2005; Trelles 1987).
Technical parameters determining the therapeutic effects of phototherapy include output power, wavelength, power density, and dose range (Posten 2005; Verma 2012). For wound healing, the commonly used form of phototherapy is low‐level laser therapy (LLLT), which is primarily defined as power with a range of 0.001 to 0.1 W, a wavelength between 300 and 10,600 nm, a pulse rate of 0 to 5000 Hz, intensity of 0.01 to 100 W/cm2, and a dose of 0.01 to 100 J/cm (Posten 2005; Verma 2012). In addition to LLLT, a relatively new type of phototherapy, light‐emitting diode (LED), is also used for wound healing (El‐Deen 2014; MedX Health 2015). This type of phototherapy provides a more superficial, even distribution of energy with lower power density and longer treatment time than LLLT (MedX Health 2015). There is currently no recommendation about the standard procedure of phototherapy for treating foot ulcers in people with diabetes. In some previous clinical studies, phototherapy was performed from approximately 4 to 50 minutes per time, twice per week to twice daily, for a total of 15 to 90 days (Beckmann 2014).
How the intervention might work
It has been shown that phototherapy can directly supply bio‐stimulative light energy to cells, thereby stimulating molecules and atoms of cells without causing significant increase in tissue temperature (Basford 1989; Karu 1989). The effect is closely related to photochemical reactions in the cells. The possible mechanisms for phototherapy to promote the ulcer repair process in people with diabetes are as follows (for a definition of technical terms, please see the glossary in Appendix 1).
-
Increased metabolic activity. Phototherapy may stimulate more efficient electron transfer in the cytochrome oxidase pathway, thereby increasing the capacity of mitochondria to generate adenosine triphosphate (ATP). Increased ATP in turn increases the energy supply for the cell's metabolic processes (Karu 2004; Yu 1997).
-
Rapid cell growth. In vitro and in vivo studies have suggested that laser light accelerates cellular reproduction and growth (Kreisler 2002; Lubart 1992; Schindl 2003). These effects may be attributable to the stimulation of the oxidative metabolic pathway and the increase in overall cell metabolism (Chen 2008).
-
Angiogenesis. A number of animal studies have suggested that LLLT may promote the physiological process through which new blood vessels form from pre‐existing vessels (Cury 2013). This is particularly important for people with diabetes, who are often associated with peripheral vascular disease.
-
Increased vascular activity. In vitro data have suggested that laser light induces temporary vasodilation, increasing blood flow to the injured area (Gorshkova 2013).
-
Faster wound healing. Lasers may promote wound healing by stimulating fibroblast development, accelerating collagen synthesis in damaged tissue, and increasing the release of growth factor (Cury 2013; Dourado 2011; Pereira 2002).
Why it is important to do this review
Given the huge disease burden and the difficulty in treatment, the management of foot ulcers in people with diabetes has become a major challenge worldwide. Phototherapy may be used as an alternative approach for healing ulcers. Though some potential mechanisms of phototherapy have been investigated, the exact process of healing with phototherapy remains unclear. To date a number of randomised controlled trials (RCTs) have been performed to evaluate the efficacy of phototherapy for treating foot ulcers in people with diabetes (Kajagar 2012; Kaviani 2011; Minatel 2009; Schindl 1998; Zhang 2012), but few systematic reviews have been undertaken to summarise the related evidence. In 2014, Beckmann and colleagues performed a systematic literature review of phototherapy for diabetic foot ulcers (Beckmann 2014); however, this was not carried out as a standard systematic review, and some key steps like quality assessment were not undertaken. A Cochrane systematic review would provide decision makers with the best evidence on the effectiveness of phototherapy for treating foot ulcers in people with diabetes.
Objectives
To assess the effects of phototherapy for the treatment of foot ulcers in people with diabetes.
Methods
Criteria for considering studies for this review
Types of studies
RCTs or cluster RCTs, irrespective of publication status or language.
Types of participants
Adults with Type I or Type II diabetes with an open foot ulcer of any severity, in any setting. We placed no restriction on the aetiology of the ulcer; trials recruiting people with diabetes with ulcers of neuropathic, ischaemic, or neuroischaemic causes were all eligible for inclusion.
Types of interventions
LLLT, LED, and other forms of phototherapy were eligible for inclusion. There was no limitation on phototherapy irradiance, wavelength, and frequency of treatment. Participants could receive standard treatment for diabetic foot ulcers such as wound cleansing, debridement, and antibiotics, but the treatment must have been balanced between intervention group and control group (i.e. the phototherapy would be the only systematic difference between groups). We included the following comparisons.
-
Phototherapy compared with sham phototherapy, no phototherapy (usual care alone), or other physical therapy modalities (such as electronic stimulations, electromagnetics, and ultrasound).
-
Comparisons of different forms of phototherapy.
-
Comparisons of phototherapy of different output power, wavelength, power density, or dose range.
Types of outcome measures
Primary outcomes
-
Complete wound healing. A range of different methods of measuring and reporting this outcome are used in studies. We reported the authors' definitions of complete wound healing where possible. We considered RCTs that reported one or more of the following outcomes to provide the most relevant and rigorous measures of wound healing:
-
time to complete wound healing (unit: weeks or days; correctly analysed using survival, time‐to‐event approaches, or mean time to healing if it was clear that all wounds were healed during follow‐up);
-
proportion of wounds completely healed during follow‐up.
-
-
Number of adverse events: all adverse events, treatment‐related adverse events, and any specific adverse events reported in the trials.
Secondary outcomes
-
Change in ulcer size expressed in either absolute (unit: mm2) or relative terms (unit: %).
-
Participant health‐related quality of life/health status (measured using a standardised generic questionnaire such as EQ‐5D, SF‐36, SF‐12, SF‐6, Nottingham Health Profile, visual analogue scale, or wound‐specific questionnaires such as the Cardiff Wound Impact Schedule). We did not include ad hoc measures of quality of life that were not likely to be validated and would not be common to multiple trials.
-
Number and level (foot, at the ankle, below the knee, at the knee, above the knee, and at the hip) of amputations at study end.
Search methods for identification of studies
We carried out the literature search according to methods stated in the published protocol (Wang 2015).
Electronic searches
We searched the following electronic databases to identify relevant RCTs:
-
the Cochrane Wounds Specialised Register (searched 11 October 2016);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 10);
-
Ovid MEDLINE (1946 to 11 October 2016);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 11 October 2016);
-
Ovid Embase (1974 to 11 October 2016);
-
EBSCO Cumulative Index to Nursing and Allied Health Literature (CINAHL Plus) (1937 to 11 October 2016);
-
China National Knowledge Infrastructure (CNKI) (1990 to 24 June 2017).
The detailed search strategies are presented in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2017). There were no restrictions with respect to language, date of publication, or study setting.
Searching other resources
We searched the following clinical trial registers for ongoing and unpublished trials:
-
ISRCTN registry (www.isrctn.com/) (searched 24 June 2017);
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/) (searched 24 June 2017);
-
ClinicalTrials.gov (clinicaltrials.gov) (searched 24 June 2017);
-
Chinese Clinical Trial Register (www.chictr.org.cn/enindex.aspx) (searched 24 June 2017).
We also manually searched the reference lists of the included studies and previous reviews, and contacted manufacturers in an effort to identify additional eligible studies.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Wang 2015).
Selection of studies
Two review authors (HW, JY) independently assessed the study eligibility according to the predefined inclusion and exclusion criteria. All citations were initially imported into reference management software, and duplicate citations were removed automatically. We screened the titles and abstracts to determine the eligibility of the remaining studies, retrieving the full texts of potentially eligible studies for assessment. Any disagreements were resolved by discussion with a third review author (DH). We collated multiple reports of a same study so that each study, rather than each report, was the unit of interest in the review. We completed a PRISMA flow chart to summarise the process (Liberati 2009).
Data extraction and management
Two review authors (HW, JY) independently extracted data using a standard form pre‐designed for this review. We consulted the authors of original studies for missing information where necessary. We extracted the following data:
-
study characteristics (e.g. authors, location, title);
-
care setting;
-
participant characteristics (number of participants; participant age, sex, race, diabetes mellitus duration, foot ulcer duration and size, and ulcer aetiology);
-
intervention and comparisons (technical parameters of phototherapy, treatment duration, other treatment received);
-
risk of bias information (randomisation, sequence concealment, blinding, incomplete outcome data, selective reporting);
-
results on our outcomes of interest (e.g. point estimates, standard deviation);
-
length of follow‐up period.
Assessment of risk of bias in included studies
Two review authors (HW, JY) independently evaluated the risk of bias of the included studies using Cochrane's tool for assessing risk of bias (Higgins 2011b). We categorised the risk of bias as low, unclear, or high for each domain. The domains for risk of bias are as follows (Higgins 2011b):
-
selection bias (sequence generation and allocation concealment);
-
performance bias (blinding of participants and personnel);
-
detection bias (blinding of outcome assessment);
-
attrition bias (incomplete outcome data);
-
reporting bias (selective reporting);
-
other bias (design‐specific risks of bias for cluster RCTs, and other problems).
We have presented a detailed description of the tool used for assessing bias in the above domains in Appendix 3. We applied the results of the 'Risk of bias' assessment to the sensitivity analysis, evaluation of the quality of the evidence, and in formulating the conclusions of the review.
Measures of treatment effect
We presented dichotomous outcomes, such as number of ulcers completely healed and adverse events, as risk ratios (RRs) and corresponding 95% CIs. For continuous outcomes, we used mean differences (MD) and 95% CIs as the measure of treatment effects. We planned to present time‐to‐event data as hazard ratios with corresponding 95% CIs, however no such data were reported in the included studies.
Unit of analysis issues
The primary unit of analysis was the participant. We checked the level at which randomisation occurred (individual ulcers, participants, or clusters (wards, clinics, clinicians)), and whether the analysis of original trials properly took into account the level of randomisation. When we included trials that evaluated multiple ulcers on the same participant or other cluster studies, we planned to extract the direct estimate from the analysis that properly accounts for the unit of analysis issues (e.g. multilevel model analysis or variance components analysis). We intended to combine effect estimates and their standard errors using the generic inverse‐variance method (Higgins 2011a). If we found unit of analysis issues for which we were unable to adjust, we planned to report them as part of the 'Risk of bias' assessment. However, we did not identify any included studies with unit of analysis issues.
Dealing with missing data
We contacted the authors of the original studies to clarify methodological ambiguities or to obtain additional results not available from the published data, or both. For dichotomous healing data, where a study presented data on the number of healed ulcers, we assumed that the wounds of randomised participants that were not included in an analysis did not heal. Where a trial did not specify participant group numbers prior to dropout, we presented only complete‐case data (Higgins 2011a). Data on secondary outcomes were analysed using complete cases only.
Assessment of heterogeneity
We considered clinical, methodological, and statistical heterogeneity. We evaluated clinical heterogeneity by considering the variability in important factors (age, duration of diabetes, duration of ulcer, ulcer size, ulcer aetiology, intervention and outcome characteristics) among trials. We assessed statistical heterogeneity among studies with the I2 statistic (Higgins 2003). We determined the level of statistical heterogeneity per the recommendations found in the Cochrane Handbook for Systematic Reviews of Interventions as follows (Higgins 2011a).
-
0% to 40%: might not be important.
-
30% to 60%: may represent moderate heterogeneity.
-
50% to 90%: may represent substantial heterogeneity.
-
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
Had 10 or more studies been included in the meta‐analysis, we would have assessed the risk of publication bias using a funnel plot (Thornton 2000; Trautner 1996). We intended to test funnel plot asymmetry by Egger's test (Egger 1997).
Data synthesis
We combined details of included studies in a narrative review according to comparison between intervention and comparator, population, and time point of the outcome measurement. We used the fixed‐effect model for combining data as it was reasonable to assume that studies were estimating the similar underlying treatment effect, in terms of intervention, study population, and methods. The result of the random‐effects model was similar to the fixed‐effect model as the statistical heterogeneity was minimal.
Sensitivity analysis
We undertook sensitivity analyses to assess the robustness of our results according to the risk of bias of the included studies. Where possible, we excluded from any meta‐analysis studies that scored high risk of bias for one or more domains according to Cochrane's tool for assessing risk of bias.
'Summary of findings' table and GRADE
We used a 'Summary of findings' table to summarise the treatment effects and quality of the evidence for the main comparisons and primary outcomes.
We evaluated the strength of evidence for all study outcomes using GRADE (Guyatt 2008a; Guyatt 2008b). We initially ranked the evidence as high‐quality because the estimates were based on randomised trials. We downgraded the quality of the evidence for the following factors: study limitations, inconsistency of results, indirectness of evidence, imprecision, and publication bias. We presented the quality of the evidence as follows (Guyatt 2008b).
-
High: further research is very unlikely to change our confidence in the estimated effect.
-
Moderate: further research is likely to have an important impact on our confidence in the estimated effect and may change the estimate.
-
Low: further research is very likely to have an important impact on our confidence in the estimated effect and is likely to change the estimate.
-
Very low: we are very uncertain about the estimate.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
The literature search yielded 446 potentially eligible citations, of which 376 were identified from electronic databases and 70 from other sources. We excluded 425 studies after screening titles and abstracts. We evaluated the full texts of the 21 remaining citations, of which 10 studies were considered as ineligible (see Characteristics of excluded studies table); two studies were ongoing studies (see Characteristics of ongoing studies table); one study pending full‐text retrieval is awaiting further classification (see Characteristics of studies awaiting classification table), and eight studies were included in the review (see Characteristics of included studies table). See flow chart of study selection in Figure 1.

Flow chart of study selection.
Included studies
Eight RCTs with 316 participants met the inclusion criteria for this systematic review (see Characteristics of included studies table). All of the included studies were single‐centre studies except for Londahl 2013, which was a multicentre trial. Most trials had two study arms except for Ortíz 2014, which had three arms. The included studies were generally small, with a sample size from 14 to 84. The included studies were performed in China (Zhang 2012; Zhang 2013; Zhao 2005), Iran (Kaviani 2011), India (Kajagar 2012 ), Colombia (Ortíz 2014 ), Israel (Landau 2011), and Sweden (Londahl 2013). Most studies were performed in clinics or hospitals (Kajagar 2012; Kaviani 2011; Landau 2011; Zhang 2012; Zhang 2013; Zhao 2005); the information about setting was not reported in two studies (Londahl 2013; Ortíz 2014). Most included studies were published as journal articles except for Londahl 2013, which was retrieved from a conference abstract. As for funding sources, two studies were funded by government or university (Kaviani 2011; Ortíz 2014), one by a commercial company (Landau 2011), and the funding sources were not reported in other studies (Kajagar 2012; Londahl 2013; Zhang 2012; Zhang 2013; Zhao 2005).
Participants
In the seven studies that reported age (Kajagar 2012; Kaviani 2011; Landau 2011; Ortíz 2014; Zhang 2012; Zhang 2013; Zhao 2005), the average age of included participants was between 53 and 68 years. Six studies reported the gender of included participants (Kajagar 2012; Kaviani 2011; Landau 2011; Ortíz 2014; Zhang 2012; Zhao 2005), showing a ratio of females to males of 0.46 to 1.88. In the four studies that reported the duration of diabetes (Kaviani 2011; Ortíz 2014; Zhang 2013; Zhao 2005), the mean duration of diabetes of included participants was from 7.1 years to 19.2 years. Four studies reported ulcer duration (Kajagar 2012; Kaviani 2011; Londahl 2013; Ortíz 2014), showing a median ulcer duration of 4.5 weeks to 46 weeks. In the four studies that reported ulcer size at baseline (Kajagar 2012; Kaviani 2011; Landau 2011; Zhao 2005), the average ulcer size was from 0.57 cm2 to 26.8 cm2. Six studies reported the Wagner classification of ulcer (Kajagar 2012; Kaviani 2011; Landau 2011; Londahl 2013; Ortíz 2014; Zhang 2013). All studies included people with stage I to II ulcers, except Zhang 2013, which included people with stage II to VI ulcers. No studies reported the ulcer aetiology of all included participants, except Kajagar 2012, which reported that 9 (26.47%) participants in the phototherapy group and 6 (17.64%) participants in the control group presented with peripheral neuropathy.
Interventions
Three trials compared phototherapy plus usual care with placebo plus usual care (Kaviani 2011; Landau 2011; Londahl 2013), and five studies compared phototherapy plus usual care with usual care (Kajagar 2012; Ortíz 2014; Zhang 2012; Zhang 2013; Zhao 2005). The study by Ortíz and colleagues also compared phototherapy plus usual care with high‐voltage pulsed current plus usual care (Ortíz 2014). Therefore, we included this study under phototherapy versus no phototherapy/placebo and under phototherapy versus high‐voltage pulsed current. The phototherapies used in the included trials included visible red light (Kaviani 2011; Ortíz 2014; Zhang 2012; Zhang 2013), visible red and near‐infrared light (Londahl 2013), visible light (Landau 2011), and a combination of far‐infrared light and ultraviolet light (Zhao 2005). The participants in the intervention and control arms in the included trials generally received usual care, which included debridement, topical treatment, wound dressing, oral antibiotics, or contact cast immobilisation (when necessary). The treatment duration of the included studies was from 15 days to 20 weeks.
Outcomes
Two studies reported the time to complete wound healing (Kaviani 2011; Landau 2011), but the data may not be reliable because not all of the ulcers were healed at study end. Four studies reported the proportion of wounds completely healed during follow‐up (Kaviani 2011; Landau 2011; Ortíz 2014; Zhang 2012). Two studies mentioned adverse events (Landau 2011; Londahl 2013). Four studies reported change in ulcer size (Kajagar 2012; Kaviani 2011; Landau 2011; Zhang 2013), three of which reported this information in relative terms (Kaviani 2011; Landau 2011; Zhang 2013), and one in absolute terms (Kajagar 2012). Ortíz 2014 evaluated quality of life by EQ‐5D self report questionnaire on a 0 to 100 scale (EQ visual analogue scale). Only one study reported the number of amputations at study end (Kaviani 2011).
Excluded studies
We excluded 10 studies from this systematic review: in seven studies the included participants were not people with diabetes and foot ulcers (Hart 2004; ISRCTN21741608; Minatel 2009; Nawfar 2011; Saied 2011; Schindl 1998; Schindl 2002); the design of two studies was not RCT (Chi 2002; Rinaldi 1993); and in the study by Minatel and colleagues the intervention was not phototherapy (Minatel 2010).
Risk of bias in included studies
We generally considered the included studies as at unclear or high risk of bias, as they either had one domain at high risk of bias (Kaviani 2011; Landau 2011; Ortíz 2014), or they had three or more domains at unclear risk of bias (Kajagar 2012; Londahl 2013; Zhang 2012; Zhang 2013; Zhao 2005). (See Characteristics of included studies; Figure 2; Figure 3.)
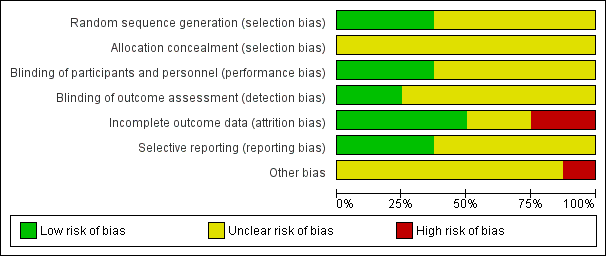
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
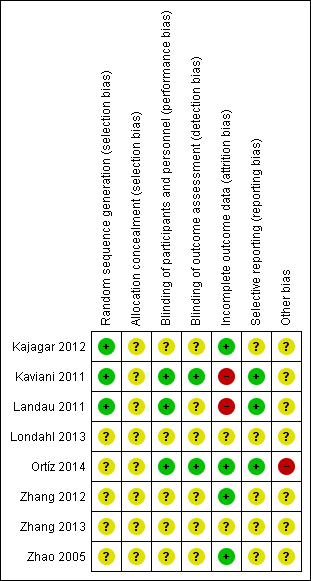
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Adequacy of randomisation process
All of the included studies were described as "randomised". Three studies reported the randomisation methods in detail, therefore we judged them to be at low risk of bias for this domain (Kajagar 2012; Kaviani 2011; Landau 2011). The remaining five studies did not report the detailed randomisation methods, hence we judged them to be at unclear risk of bias for this domain.
Allocation concealment
As none of the included studies reported the allocation procedure, we judged them all to be at unclear risk of bias for this domain.
Blinding
Blinding of participants and personnel
Three studies were double‐blind studies, and the details for blinding were reported, therefore we judged them as being at low risk of bias (Kaviani 2011; Landau 2011; Ortíz 2014). We judged the remaining five studies as at unclear risk of bias, as there was no mention of how blinding of participants and personnel was implemented (Kajagar 2012; Londahl 2013; Zhang 2012; Zhang 2013; Zhao 2005).
Blinding of outcome assessment
The study by Kaviani and colleagues suggested that the outcomes were assessed by two physicians blinded to treatment (Kaviani 2011). The protocol for the study by Ortíz and colleagues suggested that the outcome assessment was blinded (Ortíz 2014). We judged these two studies as at low risk for this domain (Kaviani 2011; Ortíz 2014). We judged the remaining studies as being at unclear risk of bias because there was no mention of how blinding of outcome assessment was implemented.
Incomplete outcome data
We judged the study by Kaviani and colleagues and the study by Landau and colleagues as being at high risk of bias because the reasons for missing outcome data were likely to be related to the outcome (Kaviani 2011; Landau 2011). We judged three studies to be at low risk in this domain because all randomised patients were included in data analysis (Kajagar 2012; Zhang 2012; Zhao 2005). We assessed the study by Ortíz and colleagues as at low risk of bias because this study applied intention‐to‐treat analysis (Ortíz 2014). We considered the remaining two studies to be at unclear risk of bias for this domain (Londahl 2013; Zhang 2013).
Selective reporting
We considered the study by Kaviani and colleagues and the study by Landau and colleagues to be at low risk of bias based on the study report, however we did not obtain the study protocols (Kaviani 2011; Landau 2011). We judged the study by Ortíz and colleagues as being at low risk of bias based on the study protocol (Ortíz 2014).
Other potential sources of bias
We assessed all studies as being at unclear risk of bias, as there was generally insufficient information to judge whether an important risk of bias existed, except for the study by Ortíz and colleagues (Ortíz 2014), which we considered to be at high risk of bias because there appeared to be an imbalance between intervention group and control group in total ulcer size and ulcer duration.
Effects of interventions
Comparison 1. Phototherapy compared with placebo/no phototherapy
Primary outcome
Wound healing ‐ time to complete wound healing
We identified no studies that reported valid data for this outcome. Although Kaviani 2011 and Landau 2011 reported the mean or median time to wound closure, the assessment for the ulcer healing time was not reliable, and we did not include the data in quantitative analysis because not all of the ulcers were healed.
Wound healing ‐ proportion of wounds completely healed during follow‐up
Four studies including 116 participants contributed to the evaluation of proportion of wounds completely healed during follow‐up (Kajagar 2012; Landau 2011; Ortíz 2014; Zhang 2012). The follow‐up time of these four studies was 4 weeks in Zhang 2012, 12 weeks in Landau 2011, 16 weeks in Ortíz 2014, and 20 weeks in Kaviani 2011. All of the ulcers in the four studies were in stage I to II according to the Wagner classification. We pooled the four studies with a fixed‐effect model. Meta‐analysis indicated that the phototherapy group had a higher proportion of wounds completely healed during follow‐up compared with the control group (64.5% for the phototherapy group versus 37.0% for the control group; RR 1.57, 95% CI 1.08 to 2.28; I2 = 0%) (see Analysis 1.1). The quality of the evidence was low due to high risk of bias for incomplete outcome data and a wide confidence interval for the estimate.
Number of adverse events
Two studies mentioned adverse events in the results (Landau 2011; Londahl 2013). Landau 2011 reported no device‐related adverse events. In Londahl 2013, which was a conference abstract, the authors suggested that there was no difference in adverse events between the phototherapy group and the placebo group, however the number of adverse events was not reported.
Secondary outcome
Change in ulcer size
Four studies with 191 participants contributed to the analysis of change in ulcer size (Kajagar 2012; Kaviani 2011; Landau 2011; Zhang 2013). In Kaviani 2011, the mean reduction of ulcer size was greater in the LLLT group than that in the placebo group two weeks after the beginning of treatment (47.5% for LLLT group versus 29.4% for placebo group; MD 18.10%, 95% CI 11.16% to 25.04%) and four weeks after the beginning of treatment (73.7% for LLLT group versus 47.3% for placebo group; MD 26.40%, 95% CI 15.91% to 36.89%) (see Analysis 1.2); the quality of the evidence was low due to high risk of bias for incomplete outcome data and wide confidence interval. In Zhang 2013, the reduction of the ulcer size one week after treatment was 10.8% in the phototherapy group compared with 11.3% in control group (MD 0.50%, 95% CI ‐0.74% to 1.74%) (see Analysis 1.2); the quality of the evidence was low due to unclear risk of bias in the original trial and small sample size. The phototherapy group had greater mean reduction in ulcer size two weeks after treatment (23.8% for LLLT group versus 21.9% for placebo group; MD 1.90%, 95% CI 0.10% to 3.70%) and three weeks after treatment (41.7% for LLLT group versus 38.1% for placebo group; MD 3.60, 95% CI 0.19 to 7.01) (see Analysis 1.2); the quality of the evidence was low due to unclear risk of bias in the original trial and small sample size. In Landau 2011, the Mann‐Whitney U test reported by the authors suggested that the mean reduction in wound size for the treatment group was greater than that for the placebo group (89% versus 54%; reported P = 0.048). In Kajagar 2012, the mean reduction in ulcer area after the completion of 15 days of therapy tended to be larger in the phototherapy group than in the control group (1043.20 mm2 for the phototherapy group versus 322.44 mm2 for the control group; MD 720.76 mm2, 95% CI 626.61 mm2 to 814.91 mm2) (see Analysis 1.3); the quality of the evidence was low due to unclear risk of bias in the original trial and small sample size. We did not combine these studies because i) different measure terms were used (relative term in Kaviani 2011, Zhang 2013, and Landau 2011, and absolute term in Kajagar 2012); ii) the standard deviation was not reported in Landau 2011; and iii) we identified major statistical heterogeneity.
Quality of life
Ortíz 2014 evaluated quality of life with the EQ visual analogue scale (EQ VAS) of the EQ‐5D. The median (interquartile range) EQ VAS at study end was 80 (44) for the phototherapy group, 80 (29.5) for the high‐voltage pulsed current group, and 90 (39.5) for the control group. We did not perform a test for the difference in quality of life between the phototherapy group and the control group due to insufficient data. The analysis of covariance (ANCOVA) test reported in the original study did not demonstrate clear differences between these three groups (reported P = 0.18).
Number and level of amputations at study end
In Kaviani 2011, two participants from the placebo group needed to be hospitalised and amputated due to extended gangrene, while no participants from the phototherapy group received amputation (RR 0.16, 95% CI 0.01 to 2.95) (see Analysis 1.4); the quality of the evidence was low due to high risk of bias for incomplete outcome data and wide confidence interval. The level of amputation in these two participants was not reported.
Comparison 2. Phototherapy compared with high‐voltage pulsed current
In Ortíz 2014, nine participants treated with phototherapy were compared with 10 participants treated with high‐voltage pulsed current. During the 16 weeks of intervention, wounds were completely healed in 7 of the 9 participants in the phototherapy group and in 8 of the 10 participants in the high‐voltage pulsed current group (RR 0.97, 95% CI 0.61 to 1.55) (see Analysis 2.1); the quality of the evidence was low due to study limitation and imprecision. Ortíz 2014 evaluated quality of life with the EQ VAS. The median (interquartile range) EQ VAS at study end was 80 (44) for the phototherapy group and 80 (29.5) for the high‐voltage pulsed current group. A test for the difference in quality of life between these groups was not performed.
Sensitivity analysis
We performed a sensitivity analysis according to study quality on phototherapy versus no phototherapy/placebo for the proportion of wounds completely healed during follow‐up. We removed three out of the four studies included in the overall analysis (Kaviani 2011; Landau 2011; Ortíz 2014); the RR of the remaining study was 1.78 (95% CI 0.94 to 3.37) (Zhang 2012), suggesting that there was no major influence on the results of the primary analysis, though the precision of the estimated effect was reduced due to small sample size.
Discussion
Summary of main results
This systematic review included a total of eight RCTs evaluating the effectiveness of phototherapy for the treatment of foot ulcers in people with diabetes (summary of findings Table for the main comparison) (Kajagar 2012; Kaviani 2011; Landau 2011; Londahl 2013; Ortíz 2014; Zhang 2012; Zhang 2013; Zhao 2005). The quality of the evidence for study outcomes was generally low. We did not find any studies reporting valid data on the time to complete wound healing. Pooled data from four RCTs (Kaviani 2011; Landau 2011; Ortíz 2014; Zhang 2012) found that more people may have a healed wound during follow‐up in the phototherapy group compared with the no phototherapy/placebo group (low quality evidence); the result was stable in sensitivity analysis by removing studies with high risk of bias. Our review found no current evidence that the number of adverse events differed between the phototherapy group and the no phototherapy/placebo group, however adverse events were mentioned in only two studies (Landau 2011; Londahl 2013), and the exact number of adverse events was not reported in Londahl 2013 so this current information is very limited.
We found four studies reporting change in ulcer size (Kajagar 2012; Kaviani 2011; Landau 2011; Zhang 2013). Though the original data were not suitable for pooling, results from individual trials generally suggested that, after two to four weeks of treatment, phototherapy may be associated with a greater reduction in ulcer size in both relative and absolute terms (low quality evidence). We found one study that reported the number of amputations at study end (Kaviani 2011), which indicated that there was no clear evidence of a difference in risk of amputations than with placebo. The difference in quality of life between the phototherapy group and the control group was unclear (Ortíz 2014).
We only found one study that compared phototherapy with another ulcer treatment device (high‐voltage pulsed current) (Ortíz 2014). The results suggested that there was no clear difference in ulcer healing rate between these two treatment methods. The difference in quality of life was unclear between the two groups.
Overall completeness and applicability of evidence
The participants in the original trials included inpatients, in Zhang 2012, Zhang 2013, and Zhao 2005, and outpatients, in Kaviani 2011, Landau 2011, and Ortíz 2014. The included studies were performed in Asia (Kajagar 2012; Kaviani 2011; Landau 2011; Zhang 2012; Zhang 2013; Zhao 2005), Sweden (Londahl 2013), and Colombia (Ortíz 2014), and the main ethnicity of participants was Asians, in Kajagar 2012, Zhang 2012, Zhang 2013, and Zhao 2005, and Caucasians, in Kaviani 2011, Landau 2011, and Londahl 2013. We identified no study primarily undertaken in other ethnic groups. Most of the included participants were diagnosed with Meggitt‐Wagner grade I to II foot ulcers (Kajagar 2012; Kaviani 2011; Landau 2011; Londahl 2013; Ortíz 2014).
All of the included studies compared phototherapy with no phototherapy/placebo. Only one study compared phototherapy with other physical therapy modalities (high‐voltage pulsed current) (Ortíz 2014), so the effectiveness of phototherapy compared with other types of physical therapies for treating foot ulcers in people with diabetes, such as shockwave therapy, remains unclear. The phototherapies used in the included trials included visible red light (Kaviani 2011; Ortíz 2014; Zhang 2012; Zhang 2013), visible red and near‐infrared light (Londahl 2013), visible light (Landau 2011), and combination of far‐infrared light and ultraviolet light (Zhao 2005). None of the included studies compared different forms of phototherapy or phototherapy of different output power, wavelength, power density, or dosage range.
In terms of study outcomes, all outcomes of interests in our protocol were reported. However, data for time to complete wound healing may not be valid, and data for adverse events, quality of life, and number and level of amputations at study end were sparsely reported, thus we were unable to obtain a reliable evaluation of these outcomes.
Quality of the evidence
The quality of the evidence was generally low due to bias in the included studies and imprecision of the estimated effects. Some studies did not clearly report detailed methods for randomisation, allocation concealment, and blinding. We also assessed some included studies as at high risk of bias for incomplete outcome data (Kaviani 2011; and Landau 2011), and imbalanced baseline characteristics (Ortíz 2014). In addition, the sample sizes of the included studies were small; the participant numbers in five out of the eight included studies were less than 30.
Potential biases in the review process
Though we did not limit the language in searching and searched both of the main English and Chinese databases, we might have missed potentially eligible studies that were published in other languages. However, as English is the dominant language in research, the potential influence would be minor. In addition, the full texts of two potentially eligible studies were not available, and some information, such as the detailed study methodology, were not reported by the authors. Attempts to obtain these missing data, such as by contacting study authors by email, were generally unsuccessful. Lastly, the results were likely to be influenced by factors such as ulcer aetiology, however the potential influence was unclear as most included studies did not report this information.
Agreements and disagreements with other studies or reviews
Our results were consistent with the study undertaken by Minatel and colleagues (Minatel 2009), which compared phototherapy with placebo for diabetic patients with leg ulcers. We excluded this study because not all the leg ulcers in this study were foot ulcers. The mean ulcer granulation and healing rates were higher for the phototherapy group than the placebo group throughout the study period. By day 90, 58.3% of ulcers in the phototherapy group had fully healed, compared with 10% in the placebo group. In Schindl 1998, Saied 2011, and Schindl 2002, phototherapy was shown to be effective in improving skin circulation in people with diabetic microangiopathy, supporting a beneficial effect of phototherapy.
In addition to clinical trials, systematic reviews have been performed to evaluate multiple interventions for chronic ulcers of the foot in diabetes, and phototherapy is one of the interventions to have been evaluated (Beckmann 2014; Game 2012; Kwan 2013). These studies summarised the results from original trials only in a narrative manner, generally suggesting that only a few studies were conducted on phototherapy; however, positive findings were reported and further large RCTs to confirm their results were encouraged.

Flow chart of study selection.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
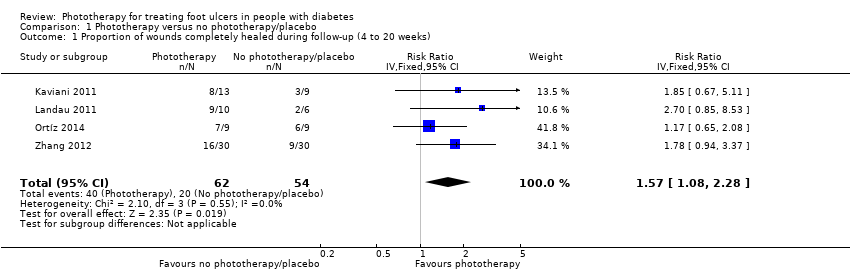
Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 1 Proportion of wounds completely healed during follow‐up (4 to 20 weeks).
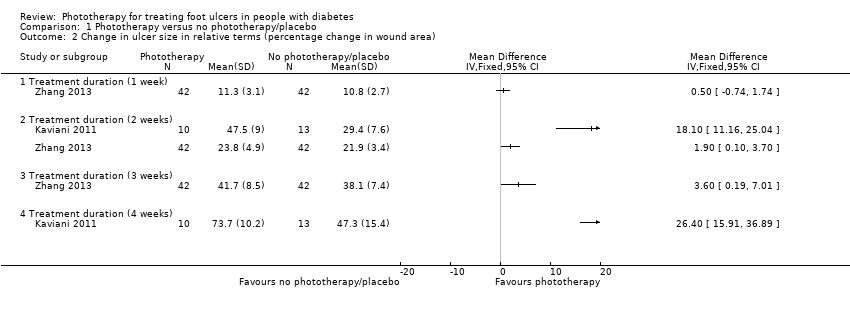
Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 2 Change in ulcer size in relative terms (percentage change in wound area).

Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 3 Change in ulcer size in absolute terms (mean change in wound area).
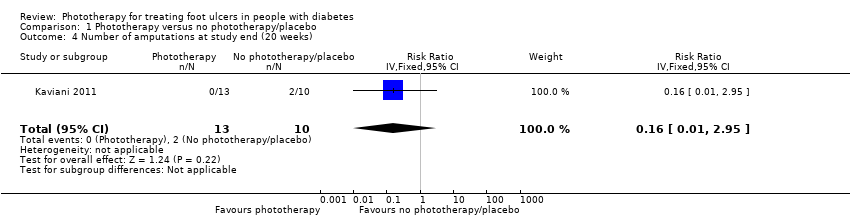
Comparison 1 Phototherapy versus no phototherapy/placebo, Outcome 4 Number of amputations at study end (20 weeks).

Comparison 2 Phototherapy versus high‐voltage pulsed current, Outcome 1 Proportion of wounds completely healed during follow‐up.
| Phototherapy compared with placebo/no phototherapy for foot ulcers in people with diabetes | ||||||
| Patient or population: Diabetes with foot ulcers Settings: Clinics and hospitals Intervention: Phototherapy Comparison: Placebo/no phototherapy | ||||||
| Outcomes | Anticipated absolute effects*(95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed absolute effect | Corresponding absolute effect | |||||
| Placebo/no phototherapy | Phototherapy | |||||
| Wound healing ‐ time to complete wound healing (weeks) | No study provided reliable data for this outcome. | |||||
| Wound healing ‐ proportion of wounds completely healed during follow‐up | 330 per 1000 | 568 per 1000 | RR 1.57 (1.08 to 2.28) | 116 (4 studies) | ⊕⊕⊝⊝ | |
| Adverse events | See comment | See comment | See comment | See comment | See comment | In Landau 2011, there were no device‐related adverse events. In Londahl 2013, the authors suggested that there was no difference in adverse events between intervention and control groups, but the number of adverse events was not reported. |
| *The basis for the assumed absolute effect (e.g. the median control group risk across studies) is provided in footnotes. The corresponding absolute effect (and its 95% confidence interval) is based on the assumed absolute effect in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for study limitations (high risk of bias for incomplete outcome data in two studies and potential influence of imbalance in baseline characteristics in one study) and one level for imprecision (small sample size). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds completely healed during follow‐up (4 to 20 weeks) Show forest plot | 4 | 116 | Risk Ratio (IV, Fixed, 95% CI) | 1.57 [1.08, 2.28] |
| 2 Change in ulcer size in relative terms (percentage change in wound area) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Treatment duration (1 week) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Treatment duration (2 weeks) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Treatment duration (3 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Treatment duration (4 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in ulcer size in absolute terms (mean change in wound area) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Treatment duration (2 weeks) | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 720.76 [626.61, 814.91] |
| 4 Number of amputations at study end (20 weeks) Show forest plot | 1 | 23 | Risk Ratio (IV, Fixed, 95% CI) | 0.16 [0.01, 2.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of wounds completely healed during follow‐up Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |

