Topical treatments for blepharokeratoconjunctivitis in children
Abstract
Background
Blepharokeratoconjunctivitis (BKC) is a type of inflammation of the surface of the eye and eyelids that involves changes of the eyelids, dysfunction of the meibomian glands, and inflammation of the conjunctiva and cornea. Chronic inflammation of the cornea can lead to scarring, vascularisation and opacity. BKC in children can cause significant symptoms including irritation, watering, photophobia and loss of vision from corneal opacity, refractive error or amblyopia.
Treatment of BKC is directed towards modification of meibomian gland disease and the bacterial flora of lid margin and conjunctiva, and control of ocular surface inflammation. Although both topical and systemic treatments are used to treat people with BKC, this Cochrane review focuses on topical treatments.
Objectives
To assess and compare data on the efficacy and safety of topical treatments (including antibiotics, steroids, immunosuppressants and lubricants), alone or in combination, for BKC in children from birth to 16 years.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 6), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE ( January 1946 to 11 July 2016), Embase (January 1980 to 11 July 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 11 July 2016. We searched the reference lists of identified reports and the Science Citation Index to identify any additional reports of studies that met the inclusion criteria.
Selection criteria
We searched for randomised controlled trials that involved topical treatments in children up to 16 years of age with a clinical diagnosis of BKC. We planned to include studies that evaluated a single topical medication versus placebo, a combination of treatments versus placebo, and those that compared two or multiple active treatments. We planned to include studies in which participants received additional treatments, such as oral antibiotics, oral anti‐inflammatories, warm lid compresses and lid margin cleaning.
Data collection and analysis
Two review authors independently screened the results of the literature search (titles and abstracts) to identify studies that met the inclusion criteria of the review and applied standards as expected for Cochrane reviews. We graded the certainty of the evidence using GRADE.
Main results
We included one study from the USA that met the inclusion criteria. In the study, 137 children aged zero to six years old with blepharoconjunctivitis were randomised to treatment in one of four trial arms (loteprednol etabonate/tobramycin combination, loteprednol etabonate alone, tobramycin alone or placebo) for 15 days, with assessments on days 1, 3, 7 and 15. We judged the study to be at high risk of attrition bias and bias due to selective outcome reporting. The study did not report the number of children with improvement in symptoms nor with total or partial success as measured by changes in clinical symptoms.
All children showed a reduction in blepharoconjunctivitis grade score, but there was no evidence of important differences between groups. Visual acuity was not fully reported but the authors stated that there was no change in visual acuity in any of the treatment groups. The study reported ocular and non ocular adverse events but was underpowered to detect differences between the groups. Ocular adverse events were as follows: loteprednol/tobramycin 1/34 (eye pain); loteprednol 4/35 (eye pain, conjunctivitis, eye discharge, eye inflammation); tobramycin 0/34; placebo (vehicle) 0/34. The evidence was limited for all these outcomes and we judged it to be very low certainty.
There was no information on clinical signs (aside from grade score), disease progression or quality of life.
Authors' conclusions
There is no high‐quality evidence of the safety and efficacy of topical treatments for BKC, which resulted in uncertainty about the indications and effectiveness of topical treatment. Clinical trials are required to test efficacy and safety of current and any future treatments. Outcome measures need to be developed which can capture both objective clinical and patient‐reported aspects of the condition and treatments.
PICO
Plain language summary
Topical treatment for blepharokeratoconjunctivitis (BKC) in children
What is the aim of this review?
The aim of this Cochrane review was to find out if topical treatment (by eye drops or ointments) for BKC in children improves symptoms and is safe. Cochrane researchers collected and analysed all relevant studies to answer this question and included one study in this review.
Key messages
There is no good evidence as to the benefits and harms of topical treatments for BKC in children.
What was studied in the review?
When the surface of the eye and eyelids becomes inflamed this is known as blepharokeratoconjunctivitis or BKC. This can cause watery, itchy, red eyes that are painful in bright light. Occasionally it can lead to scarring at the front of the eye and result in loss of vision. It is a quite common reason for children attending for eye care. Antibiotics, steroids and lubricants have been considered as possible treatments for this condition and can be given as eye drops or ointments.
What are the main results of the review?
Cochrane researchers found one study that looked at the effects of a combination of steroids and antibiotics in treatment of BKC in children. The study did not report the effects of this treatment clearly and was too small to provide a good assessment of safety.
How up‐to‐date is this review?
The Cochrane researchers searched for studies that had been published up to 11 July 2016.
Authors' conclusions
Summary of findings
| Topical treatments compared with control for blepharokeratoconjunctivitis in children | ||||||
| Patient or population: children with blepharokeratoconjunctivitis Settings: eye clinic Intervention: topical treatments (antibiotics and/or steroids) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (vehicle) | topical treatments (antibiotics/steroids) | |||||
| Improvement in symptoms, reported by the child or by their parents/carers, preferably measured by a validated tool, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (1) | ⊕⊝⊝⊝ | Data on changes in grade of blepharoconjunctivitis measured between baseline and 2 weeks did not suggest any important differences between groups. |
| Elimination of all clinical signs of ocular surface inflammation ('complete success'), preferably measured by a composite grading system, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Improvement of clinical signs of ocular surface inflammation ('partial success'), preferably measured by a composite grading system, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (10 | ⊕⊝⊝⊝ | Data on changes in grade of blepharoconjunctivitis measured between baseline and 2 weeks did not suggest any important differences between groups. |
| Change from baseline in best corrected visual acuity in affected eye(s) in logMAR measured with an ETDRS chart at 4 m, or, in younger children, with a Keeler crowded logMAR chart at 3 m, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (1) | ⊕⊝⊝⊝ | Limited data in a form that could not be extracted; not statistically significant differences between groups. |
| Uncontrolled or poorly controlled disease progression due to treatment failure, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects of medication, at any time during treatment | Ocular adverse events
Non‐ocular adverse events
| 137 (1) | ⊕⊝⊝⊝ | |||
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Some data on blepharoconjunctivitis grade reported on trials registry but we were unable to estimate a measure of effect for this outcome. 2 Data were not fully reported and we were unable to estimate a measure of effect for change in visual acuity. 3 Very low certainty due to very low numbers of events. | ||||||
Background
This Cochrane review is one of two reviews on blepharokeratoconjunctivitis (BKC). We used our previous review on systemic treatments for BKC as the template for this review (O'Gallagher 2016), and some of the text is similar.
Description of the condition
BKC is a type of inflammation of the surface of the eye and eyelids. The diagnosis is clinical and based on changes of the lid margin (telangiectasia, thickening, scarring), meibomian gland dysfunction (MGD), redness of the eye (conjunctival hyperaemia), conjunctival chemosis and inflammation of the cornea (punctate epithelial keratitis, corneal opacities, ulceration, thinning, vascularisation and scarring) (Farpour 2001; Viswalingam 2005). Inflammation of the ocular surface causes symptoms such as watering, itching, foreign body sensation, burning sensation, eye rubbing and sensitivity to light (photophobia) (Viswalingam 2005).
The incidence and prevalence of BKC in children are unknown. In paediatric eye clinics, BKC is a common diagnosis and is estimated to be the reason for referral in 12% to 15% of cases (Gupta 2010; Hammersmith 2005; Hammersmith 2015). The gender distribution differs between published case series; there does not appear to be a definite male or female predilection. Children of South Asian descent may be more frequently affected. In a UK case series of 44 children, 50% were of Indian or Sri Lankan descent, 45.5% white and 4.5% of Middle Eastern origin (Viswalingam 2005). In another UK case series of 27 children, 63% were white, 30% of Indian or Pakistani origin, 4% of Middle Eastern and 4% of Chinese origin (Jones 2007). The age of onset is early childhood, and case series report a mean age of onset of 3.2 to 4.5 years, with a range of five months to 13 years (Farpour 2001; Hammersmith 2005; Jones 2007). The young age of onset means that children are at risk of developing secondary amblyopia, which is a loss of vision due to the brain not learning how to process high‐resolution visual information. Indeed, one paediatric case series recorded reduced visual acuity despite treatment in 70% of affected eyes with a rate of amblyopia of 56% (Jones 2007); all included children in this report from a tertiary referral centre had severe disease with corneal involvement in at least one eye, which may have led to selection bias. Refractive error (both spherical and cylindrical) is common (Gupta 2010; Jones 2007). A particularly severe phenotype with prolonged duration of the condition into adulthood, a systemic association with rosacea and a high risk of corneal complications, such as thinning, vascularisation and perforation, have been observed in a proportion of white patients (Hamada 2012). Whilst this review is concerned with the medical management of BKC, surgical interventions are occasionally indicated, such as the injection of monoclonal antibodies that target molecules of the vascular epithelial growth factor pathway in corneal vascularisation (Chang 2012; Elbaz 2015; Stevenson 2000), gluing or corneal transplantation for corneal perforation, and corneal transplantation for severe scarring; the latter being associated with a high risk of rejection because of corneal vascularisation (Lindsley 2012).
Early features of BKC are lid margin disease and chalazia (cysts within the eyelid) (Jones 2007). Qualitative and quantitative tear film lipid deficiency and the activation of inflammatory pathways may be underlying factors which lead to the conjunctival and corneal signs and symptoms that distinguish BKC from blepharitis (Foulks 2003; Hamada 2012; Suzuki 2015). BKC is a chronic condition and early intervention may help prevent severe corneal disease with loss of vision (Hamada 2012; Jones 2007).
Meibomian gland dysfunction
The meibomian glands, located in the eyelids, secrete a layer of lipids and proteins that protect the tear film against evaporation. Dysfunction of these glands can result in a sensation of dryness or grittiness. Many suggestions about the mechanisms underlying MGD and its management have been made, mainly in adults. We have provided a summary of the recent literature in Appendix 1.
Bacterial flora on lid margin and conjunctiva
Bacteria secrete enzymes, such as lipases, that may further destabilise the tear film (Farpour 2001; Gupta 2010; Nichols 2011; Viswalingam 2005).
Conjunctival cultures from healthy children frequently grow staphylococcal species pluralis (spp.) (42%) and diphtheroids (30%) and, occasionally, streptococcal spp. (13%), Propionibacterium acnes (11%) and Corynebacterium spp. (2%) (Singer 1988). In children with BKC, a case series of four children reported low numbers of coagulase‐negative staphylococci in lid cultures of three children and low numbers of P. acnes in one child (Farpour 2001). Another case series of 44 children with BKC reported culture‐positive lid margin and conjunctival swabs in 15 (34.1%), and of these, 12 showed a moderate or heavy growth of Staphylococcus aureus, one S. epidermidis and two mixed S. aureus/S. epidermidis (Viswalingam 2005). A large case series from a centre in India found positive cultures in 52 of 290 children with BKC (17.9%), of which 34/52 grew S. aureus, 13/52 grew P. acnes and 5/52 grew both (Gupta 2010).
Diagnostic tests
Diagnosis of BKC is based on symptoms and clinical signs, as described above. Lid margin and conjunctival swabs for bacterial culture are not performed routinely in clinical practice. Similarly, lid margin changes are not routinely quantified. However, grading and scoring systems of clinical signs, adapted from systems used in adults, have been proposed (Nichols 2011).
The examination of children is often limited by patient co‐operation, particularly when ocular surface inflammation is severe, and photophobia and discomfort are intense. However, some of the subgroups used in the MGD staging system have been used to develop a staging system for childhood BKC. Viswalingam and colleagues introduced a classification for BKC severity based on bulbar and tarsal conjunctival signs (hyperaemia, infiltration, obscuration of tarsal conjunctival vessels, and the presence of papillae and follicles) and the extent of corneal involvement in degrees at the limbus (less than 120°, 180° to 240°, 240° to 360) (Viswalingam 2005). This system was expanded to include elements of the conjunctival active inflammation score (Elder 1997), the Chronic Stevens Johnson Syndrome/Toxic Epidermal Necrolysis score (Sotozono 2007), and the abbreviated MGD grading system (Bron 2003; Foulks 2003). The resulting grading system has four grades (none, mild, moderate, severe), each with separate grading for disease activity (A) and damage (D) (Hamada 2012). Activity scoring is based on conjunctival hyperaemia/oedema, corneal vascularisation (three clock hours or less, more than three clock hours, peripheral/to pupil margin/into central zone), and conjunctival or corneal ulceration or corneal perforation. Damage scoring is based on lid distortion, subconjunctival fibrosis (fornix shortening), the presence and extent of established vessels/fibrovascular pannus and peripheral/central corneal thinning (Hamada 2012). The latest addition to this system includes the Oxford scoring system of corneal staining (Bron 2003; Hamada 2013). This scoring system can be used to evaluate treatment efficacy, such as: complete success, signified by a reduction in activity scores from any grade to A0 ('no activity'); partial success, signified by a reduction in activity scoring not reaching grade A0; and treatment failure, signified by no change in or a worsening of activity scoring (Hamada 2012, Hamada 2013).
A functional measure of activity and damage is visual acuity; this may also indicate the presence of secondary amblyopia (Jones 2007).
Description of the intervention
Akin to the treatment of adult MGD and blepharitis, the treatment of childhood BKC targets the obstruction of meibomian gland openings (melting, expression and removal of meibomian gland secretions and debris from the lid margin by daily warm lid compresses and lid margin cleaning), the altered bacterial flora of lid margin and conjunctiva (topical and systemic antibiotics), and ocular surface inflammation (topical immunosuppressants and topical/systemic antibiotics inhibiting bacterial lipases, topical lubricants diluting inflammatory mediators in the tear film and compensating for tear film deficiency). Dietary modifications, particularly an increased intake in essential fatty acids (EFAs), may also be of benefit (Hamada 2012; Jones 2007). Rarely, systemic immunosuppression with prednisolone, azathioprine or mycophenolate mofetil may be required to treat sight‐threatening corneal involvement (Hamada 2012).
This review focuses on topical treatments.
How the intervention might work
Topical antibiotics
Topical antibiotics are used in BKC for both their antibiotic and anti‐inflammatory effects. S. aureus and S. epidermidis are common organisms cultured from conjunctival and lid swabs in BKC and topical antibiotics with appropriate spectrum of activity are used (Wong 2010). Ointments are often used to coat the lashes (Raskin 1992). Topical antibiotics may be used alone in mild disease (Wong 2010), or more commonly in conjunction with systemic antibiotics in more severe disease or to aid compliance in children (Gupta 2010; Hamada 2012; Hammersmith 2005; Jones 2007; Viswalingam 2005).
Macrolides
Erythromycin is the most commonly‐used systemic antibiotic in childhood BKC (Gupta 2010; Hammersmith 2005; Jones 2007; Meisler 2000), and its use has also been reported topically (Meisler 2000; Wong 2010). Newer macrolide antibiotics, such as azithromycin, are also available. Topical azithromycin use has been described in childhood BKC with the reported advantage of a long half‐life due to tissue accumulation of the drug (Doan 2013). Macrolide antibiotics have both antibacterial and anti‐inflammatory properties. They inhibit bacterial protein synthesis by binding to the 50S subunit of bacterial 70S ribosomes (Klein 1997). The effect can be bactericidal or bacteriostatic, depending on the bacterial species, drug concentration, growth phase of the organism and inoculum size (Klein 1997). In vitro, macrolide antibiotics reduce the release of proinflammatory cytokines, particularly IL‐1beta, ‐6, ‐8 and ‐12, tumour necrosis factor‐alpha (TNF‐alpha), and matrix metalloproteinases (MMPs) ‐1, ‐3 and ‐9, and affect neurophil chemotaxis and phagocytosis (Geerling 2011; Li 2010; Murphy 2008). In an animal model of corneal inflammation, azithromycin reduced leucocyte migration into the cornea and decreased mRNA expression levels of IL‐1beta, TNF‐alpha and intercellular adhesion molecule (ICAM)‐1 (Sadrai 2011). The risk of adverse effects is low, and no adverse events have been reported with topical use.
Chloramphenicol
Chloramphenicol is an antibiotic effective mainly against Gram‐positive bacteria; it is bacteriostatic through the inhibition of protein synthesis (Robert 2001). It has been shown to be effective against 94% of bacterial isolates in blepharitis and conjunctivitis (Everett 1995). Its use has been described in BKC in children (Hamada 2012; Jones 2007; Viswalingam 2005; Wong 2010). No adverse effects were reported with its use topically in children for BKC in small case series (Jones 2007).
Other topical antibiotics
Other topical antibiotics used in BKC in children include fusidic acid (Wong 2010), gentamicin (Jones 2007; Wong 2010), and ciprofloxacin (Jones 2007; Wong 2010). No side effects have been reported apart from mild punctate epithelial erosions in one child receiving topical ciprofloxacin (Jones 2007).
Immunosuppressants and ‐modulators
Topical corticosteroids
Glucocorticoids have a potent anti‐inflammatory effect; they bind to cytoplasmic glucocorticoid steroid receptors and translocate to the nucleus of the cell, and induce an increase in the transcription of proteins that inhibit the production of inflammatory mediators, e.g. lipocortin. Corticosteroids also inhibit the transcription of proinflammatory cytokines. Adverse effects of topical corticosteroid therapy include a rise in intraocular pressure, with subsequent damage of the retinal ganglion cell axons (glaucoma), opacification of the crystalline lens (cataract), increased susceptibility of the cornea to infection (bacterial/herpetic keratitis, with risk of corneal scar or perforation), and, as reported in isolated cases only, adrenal suppression, Cushing syndrome and growth retardation (Afandi 2003; Chiang 2006; Krupin 1976; Steelman 2001; Wolthers 2011). Frequently used in BKC are — in decreasing order of potency — topical dexamethasone, prednisolone, fluorometholone (Chen 2012; Hamada 2012; Hosseini 2013; Jones 2007; Mehta 2006; Torkildsen 2011; Viswalingam 2005), and, more recently, a topical corticosteroid with reported reduced risk of adverse events resulting from penetration into the anterior chamber, loteprednol (Chen 2012; Comstock 2010;Comstock 2012b; White 2008). In isolated cases, long‐acting corticosteroids have also been administered as injection into the episcleral space under Tenon's layer (Bondalapati 2014).
Immunomodulators
Calcineurin inhibitors, ciclosporin A (CSA), tacrolimus (FK506) and pimecrolimus, are immunomodulators that specifically inhibit T lymphocyte proliferation by inhibiting interleukin 2 expression by T helper cells (Kacmaz 2010; Tatlipinar 2005).
Ciclosporin A (CSA)
CSA has been used to reduce the need for topical steroids in the treatment of atopic keratoconjunctivitis; no serious adverse events were noted (Tatlipinar 2005). In MGD, CSA used at a low concentration of 0.05% can reduce meibomian gland inclusions, tear film changes and signs and symptoms of corneal dryness by reducing meibomian gland inflammation and by modulating immune cell populations in the conjunctiva and lacrimal gland (Perry 2006; Prabhasawat 2012; Qiao 2013; Rubin 2006; Schechter 2009; Stevenson 2000). CSA may have advantages over topical steroids, as it does not induce profound suppression of the host immune response (Kunert 2000).
In BKC the use of CSA has been described at concentrations of 2% as a rescue medication when topical azithromycin failed (Doan 2007), and at 0.5% as primary treatment (Ismail 2012), with successful control of inflammation and regression of corneal vascularisation within days, as well as at a concentration of 0.05% or 1% as an adjunct to other topical and systemic medication (Auw‐Hädrich 2009; Choi 2013).
Tacrolimus
The successful use of tacrolimus has been described in two cases of BKC (Joseph 2005).
To date, no study has described the use of pimecrolimus in BKC.
Lubricants
Lubricants are used by some in BKC in children in order to address the evaporative dry eye that one study reported to have resulted from meibomian gland disease (Wong 2010). Lubricants are thought to reduce hyperosmolarity of the tears, reduce friction between the tarsal conjunctiva and the ocular surface, improve spreading of the lipid layer of the tear film, and dilute the concentration of proinflammatory substances in the tears (Geerling 2011). Carboxymethylcellulose and hyaluronate have been reported in the management of BKC in children (Hamada 2012). Their benefit in BKC has not been supported by any trial data.
Table 1 summarises the medications and outcomes used for this condition.
| Author, year | Study type | N | Age | Mean/median follow‐up | Mechanical treatment | Systemic (oral) interventions | Topical interventions | Physician‐reported outcomes | Patient‐reported outcomes | Adverse events from condition | Adverse events from treatment |
| Case series | 7 | 6 to 14 years | 6 months | Lid hygiene | Amoxicillin/ clavulanate | Chloramphenicol (PF) drops, chloramphenicol ointment to lids, prednisolone 0.5% (PF) | Eyelid condition, corneal epitheliopathy, stromal defects | Improvement of symptoms | None | None | |
| Case series | 3 | 30 months to 8 years | Variable | Lid hygiene | Azithromycin | Loteprednol 0.2%, CSA 0.05%, | Chalazia, keratitis, corneal ulcer/scar, phlyctenule, MGD | Improvement of itching | None | None | |
| Case series | 16 | 4 to 16 years | Variable | Lid hygiene | Erythromycin (1 participant only) | Azithromycin 1.5%, CSA 2% | Bulbar conjunctival hyperaemia, conjunctival phlycten, corneal inflammation, blepharitis grade | Ocular redness | None | Ocular irritation (redness, burning, stinging) | |
| Case series | 8 | 3.5 to 13 years | 8.3 months | Lid hygiene | Erythromycin suspension 450 mg divided into 3 doses | Prednisolone 0.5% (PF); hydrocortisone acetate 1% ointment nocte | Bulbar conjunctival redness, inferior superficial corneal vascularisation, punctate corneal epithelial staining, inferior subepithelial vascularisation and infiltrate, conjunctival phlyctenules, corneal phlyctenules, circumferential pannus, corneal scar | Red eyes, photophobia, itching, discharge | Corneal scarring and thinning | Stomach disturbance, diarrhoea | |
| Case series | 615 | 7 months to 16 years | Not reported | Lid hygiene | Erythromycin | Topical steroids and antibiotics (not specified) | Outcomes not reported (presenting signs only) | Outcomes not reported (presenting symptoms only) | None | None | |
| Case series | 10 | 6 to 27 years | 4.4 years | Lid hygiene | Azathioprine, mycophenolate mofetil, prednisolone | Steroids (not specified) | Disease remission/ control of inflammation | None | Corneal perforation | none | |
| Case series | 29 | 2 to 12 years | 5.4 months | Warm compresses | Erythromycin, doxycyclin | Prednisolone 1%, dexamethasone 0.1%, antibiotic, fluorometholone, loteprednol etabonate 0.5% | Eyelid inflammation, superficial punctate keratitis, corneal vascularisation, corneal infiltrates, phlyctenules, corneal scarring | None | Amblyopia | Gastrointestinal distress, mouth ulcers (unrelated) | |
| Case series | 27 | 7 months to 15.9 years | 2.3 years | Warm compresses, lid hygiene | Erythromycin, doxycyclin, flaxseed oil | chloramphenicol, ciprofloxacin, gentamicin, prednisolone 1% or 0.5%, fluorometholone 0.1% | Visual acuity, astigmatism | Discomfort, photophobia | Amblyopia | Vaginal candidiasis | |
| Case series | 5 | 4 to 9 years | Not specified | Erythromycin | Lid hyperaemia and swelling, corneal infiltrates | None | None | None | |||
| Case series | 114 | Mean 9.3 years (± 4.2) | 26.4 months | Lid hygiene | Flaxseed oil, erythromycin | Lubricants (hyaluronate, methylcellulose), erythromycin, ciprofloxacin, steroids (dexamethasone 0.1% (PF), loteprednol 0.5%, fluorometholone 0.1%), CSA 0.05% | Visual acuity | None | None reported | None reported | |
| Case series | 51 | Mean 10.2 years (± 3.6) | 58.9 months | Warm compresses, lid hygiene | Antibiotics (erythromycin, amoxicillin/ clavulanate, doxycycline), steroids | Steroids (dexamethasone 1%, prednisolone 0.12 to 1%, fluorometholone 0.1%) , antibiotics (fucidic acid, levofloxacin, tobramycin), CSA 0.5% | Visual acuity | Redness, tearing, blurred vision, pain, irritation, photophobia, white spot, swelling, discharge, itching, rubbing | Corneal perforation | Raised intraocular pressure, cataract, gastrointestinal disturbance | |
| Case series | 44 | 1 to 14 years | 7 years | Lid hygiene | Erythromycin | Chloramphenicol, steroids | Reduction of clinical signs | Redness, watering, itching, grittiness, discharge, photophobia, pain | None reported | None reported |
Abbreviations:
CSA: ciclosporin
MGD: meibomian gland dysfunction
N: number of participants
PF: preservative‐free
Why it is important to do this review
In paediatric eye clinics, BKC is a common and sometimes sight‐threatening condition that can affect a child’s quality of life. A Cochrane review on systemic treatment for BKC has been published (O'Gallagher 2016). New topical treatments with antibiotics and calcineurin inhibitors have become available. Children, their families and clinicians need accurate and unbiased data on the benefits and potential harms of the different management options available so as to inform treatment choice, particularly as medication is often required for prolonged periods of time.
Objectives
To assess and compare data on the efficacy and safety of topical treatments (including antibiotics, steroids, immunosuppressants and lubricants), alone or in combination, for BKC in children from birth to 16 years.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) in the review. We excluded quasi‐RCTs (e.g. those that allocated participants to treatment groups in alternating order of presentation or based on patient identification number or date of presentation).
Types of participants
We included children aged zero to 16 years with a clinical diagnosis of hypersensitivity blepharokeratoconjunctivitis (BKC).
Types of interventions
We evaluated topical treatments, including topical antibiotics, immunosuppressants/immunomodulators and lubricants. We included studies that evaluated a single topical medication versus placebo, a combination of treatments versus placebo, and those that compared two or multiple active treatments. We included studies in which participants received additional treatments, such as oral antibiotics, oral anti‐inflammatories, warm lid compresses and lid margin cleaning, where these were applied to both the control and intervention arms of the trial.
Types of outcome measures
BKC is defined by both subjective symptoms and objective clinical changes. Improvement in symptoms is the major goal of treatment and is usually associated with a reduction in clinical signs.
There is no validated patient‐ or parent‐/carer‐reported evaluation tool by which to quantify the symptoms of BKC. The Quality of Life in Children with Keratoconjunctivitis (QUICK) questionnaire, a tool to assess the impact of a different inflammatory eye surface condition, allergic keratoconjunctivitis, on children’s well‐being (Sacchetti 2007), has not been evaluated in children with BKC. In adults, the Ocular Surface Disease Index (OSDI) is a commonly used symptom measure, although recently Rasch analysis has demonstrated multidimensionality and poor targeting (Dougherty 2011). There is also no validated tool to measure vision‐related quality of life in children with ocular surface disorders; generic health‐related quality of life tools, such as the PedsQL (Varni 2001) or the paediatric health utility measure CHU9D (Ratcliffe 2011), have not been used in this context. However, as quality of life and economic outcomes are increasingly considered to be important, future work may include these or other validated measures.
Regarding clinical signs, the grading system based on BKC activity and damage allows an evaluation of treatment efficacy (Hamada 2012; Hamada 2013; Viswalingam 2005). Treatment success is reflected in a reduction of the activity score, which ranges from zero to three. Outcomes can be defined either as a change in activity score as an ordinal numerical value or, as proposed by Hamada 2013, as complete success (reduction in activity score to zero), partial success (exit value smaller than the baseline value) and no change (lack of treatment effect).
A composite scoring system, such as the BKC activity/damage grading system, may overcome the problem of inconsistent reporting in clinical trials, as observed in adult meibomian gland dysfunction (MGD) trials (Asbell 2011; Nichols 2011).
Primary outcomes
Percentage of children who experienced an improvement in symptoms, reported by the child or by their parents/carers, preferably measured by a validated tool, at three months (± one month) after the start of treatment.
Secondary outcomes
-
Percentage of children with elimination of all clinical signs of ocular surface inflammation ('complete success'), preferably measured by a composite grading system such as those described above (Hamada 2012; Hamada 2013; Viswalingam 2005).
-
Percentage of children with an improvement in clinical signs of ocular surface inflammation ('partial success'), preferably measured by a composite grading system as describe above.
-
Change from baseline in best corrected visual acuity in affected eye(s) in logMAR measured using an Early Treatment of Diabetic Retinopathy Study (ETDRS) chart at 4 m or, in younger children, with a Keeler crowded logMAR chart at 3 m. If a RCT reported visual acuity using a different chart or at a different testing distance, we considered conversion of measurements to logMAR if appropriate.
-
Percentage of children that suffered from uncontrolled or poorly controlled disease progression due to treatment failure.
-
Percentage of children that suffered adverse effects of medication.
-
Adherence to treatment as measured by the percentage of study medication used (study medication issued during the course of the trial minus residual study medication returned at end of trial)/(study medication issued during the course of the trial).
-
Total amount of topical steroids (total number of drops) used during the trial duration.
-
Total amount of systemic immunosuppressants (total dose) used during the trial duration.
-
Cost‐effectiveness or cost‐utility of treatments.
-
Patient‐reported symptom severity or quality of life, measured by any validated tool.
We aimed to evaluate all outcomes at three months (± two months) after the start of treatment. As disease activity and severity may differ between the two eyes of the same child (see also the 'Unit of analysis issues' section), we reviewed included papers to ensure that the trial included either only one eye, or, if it included both, it reported improvement for one eye or eyes separately.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 6), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to 11 July 2016), Embase (January 1980 to 11 July 2016), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 11 July 2016.
See appendices for details of search strategies for CENTRAL (Appendix 2), MEDLINE (Appendix 3), Embase (Appendix 4), ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the WHO ICTRP (Appendix 7).
We modified our search strategy from the protocol when we discovered a published report of a potentially relevant trial identified in our search of ClinicalTrials.gov (Comstock 2012). The initial search strategy had not identified this paper due to its use of the term blepharoconjunctivitis (BC) rather than blepharokeratoconjunctivitis or blepharokeratitis. A second search of databases was carried out using the term blepharoconjunctivitis.
Searching other resources
We manually searched the reference lists of the trials included in the review for additional trials. We also used the Science Citation Index to identify reports that cited the studies included in this review. With both of these strategies we aimed to identify any other relevant reports or trials that had not been identified by the electronic searches. We did not handsearch journals or conference proceedings.
Data collection and analysis
Selection of studies
Two review authors independently screened the results of the search (titles and abstracts) to identify studies that loosely met the inclusion criteria of the review. Review authors were not masked with respect to study authors, institution or journal. We divided studies into 'definitely include', 'definitely exclude' and 'possibly include' categories. We made a final judgement as to the inclusion/exclusion of those in the 'possibly include' category after we obtained the full‐text copy of each article. We planned to obtain translations of relevant abstracts and, where necessary, full‐text articles into English before we made a final decision regarding inclusion/exclusion. We took care to identify multiple reports of the same study and, where identified, we linked them together. We independently examined the full‐text reports for compliance with the inclusion criteria. We resolved any disagreement over which studies to include by discussion or by consulting a third review author. We listed the studies that we excluded after we obtained the full‐text articles in a 'Characteristics of excluded studies' table and provided a reason for exclusion. Also, we created a PRISMA diagram to illustrate the study selection process.
Data extraction and management
Two review authors extracted data independently using a data extraction form (Appendix 8), which we developed in conjunction with Cochrane Eyes and Vision (CEV) using Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions for guidance (Higgins 2011a). Where data were missing or unclear, one review author attempted to contact the trial authors for unpublished data or clarification. We made initial contact via email and if there was no response we sent a second email. We entered data into Review Manager (RevMan) (RevMan 2014); one review author entered the data and the second review author checked for any errors.
We aimed to collect the following information on study characteristics.
-
Study design: parallel group RCT/one or both eyes reported.
-
Participants: country, total number of participants, age, sex, inclusion and exclusion criteria.
-
Intervention and comparator details: including number of people (eyes) randomised to each group.
-
Primary and secondary outcomes as measured and reported in the trials, adverse events.
-
Length of follow‐up.
-
Date study conducted.
-
Funding and conflicts of interest.
We also aimed to collect the following data for our predefined outcomes separately for intervention and comparator groups.
-
Dichotomous outcomes: number of participants followed up, number of events.
-
Continuous outcomes: number of participants followed up, mean and standard deviation.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias in the included studies using the Cochrane 'Risk of bias' assessment tool, detailed in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), as guidance. We graded each parameter of trial quality as either low risk of bias, high risk of bias or unclear risk of bias.
The six main domains of the Cochrane 'Risk of bias' tool include the following.
Selection bias
We graded studies as at high risk, low risk or unclear risk based on the method of randomisation (sequence generation) and allocation concealment. Where we made an assessment of 'unclear risk' assessment, we asked the trial authors to provide further information to enable us to make a more detailed risk assessment. Examples indicative of a low risk would be randomisation using computer‐generated sequences or a random number table, the central allocation of treatments, and concealment of allocation. We classified a description of 'randomised controlled trial' without details of the allocation schedule as 'unclear', and we contacted the trial authors for more details. We classified lack of allocation concealment as 'high risk'.
Performance bias
Performance bias could occur if participants/carers or staff know which treatment group the participant has been allocated to. In this review, both objective/physician‐reported outcomes and self‐report of symptoms could be affected by performance bias. We, therefore, made a judgement regarding performance bias for individual studies.
Attrition bias
Attrition (e.g. following withdrawal or loss from follow‐up) can cause bias. We recorded any incomplete outcome data. We documented the rate of withdrawal from each treatment group. Where trial authors had not taken missing data into consideration, we classified the risk of bias as high. We also classified the risk of bias as high if the reason for missing outcome data was likely to be related to the true outcome, if the proportion of missing outcomes compared with observed event may have induced clinically relevant bias in intervention effect estimate (dichotomous data), if plausible effect size (difference in means or standardised difference in means) among missing outcomes may have induced clinically relevant bias in observed effect size (continuous data), if an 'as treated' analysis was performed with substantial departure of the intervention received from that assigned at randomisation, and if there was inappropriate application of simple imputation.
Detection bias
We judged studies on their use of masking strategies. Detection bias can occur if outcome assessors know which treatment participants have received.
Reporting bias
Where a study protocol was available, we compared the published protocol with the final outcomes to assess the risk of selective outcome reporting as high, low or unclear. If no protocol was available, we studied the full‐text article so that we could make this judgement.
Other bias
We judged whether the design of each study was subject to any risk of other bias not detailed above.
We graded the risk of bias as unclear if a publication contained insufficient information to allow a judgement to be made, and our attempts to contact the trial authors to clarify were unsuccessful.
Review authors were not masked to any aspect of the study design and we resolved any disagreement by discussion or by consultation with a third designated review author.
Measures of treatment effect
Our primary outcome was dichotomous, as were the first two secondary outcomes. The risk ratio was to be the measure of treatment effect. LogMAR visual acuity values tend to be normally distributed, and we aimed to use the mean difference as a measure of treatment effect. However, we planned to note whether or not the trial authors assessed the symmetry of their data and also how the investigate the logMAR scores, as different charts may yield different values. We aimed to use odds ratios for adverse events, but where trial reported a variety of adverse events and only one trial reported each type, we planned to simply collate this information. We also planned to collate information on adherence to treatment, simultaneous use of topical steroids and systemic immunosuppressants, and economic data.
Unit of analysis issues
Each child may have one or two affected eyes, and both disease severity and activity may differ between eyes. The topical treatment administered to the two eyes, if severity differs, may also be different. Medication administered topically can in part be absorbed systemically and have an effect on the contralateral eye, though this effect is likely to be minor. Using fellow eyes as controls would not be appropriate.
We foresaw potential for studies to be included in which participants have one or both eyes affected. The primary outcome (i.e. whether or not the child or parent/carer reports an improvement) could be measured at the eye level or at participant level; there is, therefore, potential for a unit of analysis issue. We reviewed papers to ensure that either the trial authors included only one eye in the trial, or, if they included both eyes, they reported improvement for one eye or both eyes separately.
Dealing with missing data
For each outcome we assessed whether the trial authors conducted an intention‐to‐treat analysis. One review author attempted to retrieve any missing data by contacting the authors of the relevant papers, and allowed one month for replies. Where we failed to obtain these data but the trial authors adequately examined the reasons for loss to follow‐up and found that they were similar between treatment groups, we used available‐case analyses. We documented whether the original studies stated that they compared the characteristics of participants with complete data with those of participants with no missing data and whether they provided any information about the possible effects of missing data.
Assessment of heterogeneity
We planned to examine studies for sources of methodological and clinical heterogeneity. We then planned to assess clinical, methodological and statistical heterogeneity by performing the following.
-
We planned to look for different directions of effects and poor overlap of the confidence intervals (CIs) on the forest plot.
-
We planned to examine the result of the Chi² test and calculation of the I² statistic with CIs. We planned to interpret the I² statistic values as advised in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We did not plan to adopt strict thresholds for the I² statistic but typically would have been concerned if values were 50% or higher.
Assessment of reporting biases
If we had a sufficient number of trials (more than 10) we planned to conduct a funnel plot to assess the evidence of publication bias, although we acknowledge that asymmetry in such a plot does not always indicate publication bias.
Data synthesis
We planned to perform data analyses by following the guidelines in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
We attempted to collate all data that were relevant to our primary and secondary outcomes. If appropriate, we planned to meta‐analyse our primary outcome and the first two secondary outcomes. We foresaw potential for some studies to report composite scores for signs and symptoms, and others to report individual scores for signs and symptoms. We intended to perform meta‐analyses on the proportion of participants categorised into each of the three outcome groups based on composite scores.
We planned to collate the results of the studies that reported only individual scores for signs and symptoms; we did not plan to include them in a meta‐analysis.
We aimed to collate the data for other secondary outcomes, but to avoid any multiplicity issues and for review clarity, we did not plan to meta‐analyse these data but would have provided a summary in tabulated form.
We planned to use a random‐effects model unless there was a very small number of studies (fewer than three), in which case we would have used a fixed‐effect model. If we detected substantial heterogeneity, either methodological (by review of studies) or by large values of the I² statistic (as outlined in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b)), we did not plan to conduct a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We intended to conduct subgroup analyses based on participant age (less than eight years versus greater than or equal to eight years), as adherence to treatment may increase with age. From clinical experience, children become more understanding and allow treatment more easily from around the age of eight; also, secondary amblyopia is a risk in children under this age threshold only.
Sensitivity analysis
We planned to conduct a sensitivity analysis to assess how robust our review results were to the inclusion of studies at high risk of bias (by which we mean those that we judged to be at high risk of bias in any of the 'Risk of bias' domains we assessed).
'Summary of findings' table
We planned to prepare a 'Summary of findings' table (Higgins 2011b), describing populations, interventions and outcomes as outlined above. We did not plan to distinguish between low‐/medium‐/high‐risk populations. We have presented the proposed outline for a 'Summary of findings' table in Appendix 9. We planned to use the GRADE approach (see below) to assess the certainty of the evidence and to use the GRADEpro Guideline Development Tool (GDT) to create the 'Summary of findings' table (GRADEpro 2014).
We have listed the GRADE Working Group grades of evidence below.
-
High certainty: further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low certainty: we are very uncertain about the estimate.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 440 references (Figure 1). The Cochrane Information Specialist scanned the search results, removed 196 duplicates and then removed 199 references which were irrelevant to the scope of the review. We screened the remaining 45 reports and obtained seven full‐text reports for further assessment. We included two reports of one study (Comstock 2012), and excluded five reports of five studies (see the 'Characteristics of excluded studies' table for details).
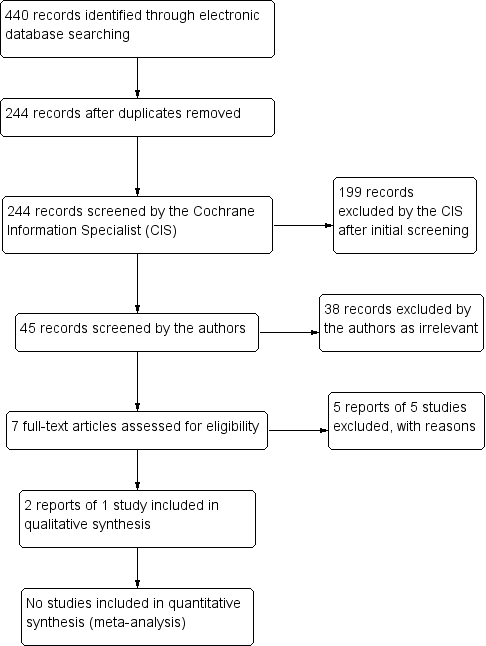
Study flow diagram.
Included studies
Comstock 2012 reported a study of the use of loteprednol etabonate/tobramycin combination drop in children with BC. This was a randomised controlled trial (RCT) with four treatment arms (loteprednol etabonate/tobramycin combination, loteprednol etabonate alone, tobramycin alone or placebo). The trial randomised 137 children with BC aged zero to six years to treatment in one of the four arms for 15 days, with assessments on days 1, 3, 7 and 15.
Excluded studies
Bloom 1994, Chen 2012 and White 2008 were RCTs but we excluded them because they only included adult participants.
Hosseini 2013 presented a RCT of azithromycin 1.0%/dexamethasone 0.1% combination drop versus azithromycin 1.0% alone or dexamethasone 0.1% alone in the treatment of BC. Of 417 participants, 19 were under 16 years of age. We contacted the study authors and their industry partners, but were unable to obtain detailed data about the paediatric subgroup, so we excluded the study.
Shulman 1982 reported findings from an RCT of gentamicin‐betamethasone ointment versus gentamicin ointment alone, betamethasone ointment alone, or placebo in the treatment of acute BC. The study included people aged between 10 and 86 (N = 87). Again, we contacted the study authors, but we were unable to obtain the results for the paediatric subgroup and consequently we excluded the study.
Table 2 summarises the characteristics of the two excluded studies that included children.
| Author, year | Study type | N | Age | Mean/median follow‐up | Mechanical treatment | Systemic (oral) interventions | Topical interventions | Physician‐reported outcomes | Patient‐reported outcomes | Adverse events from condition | Adverse events from treatment |
| RCT | 137 | 0 to 6 years | 15 days | None | None | Loteprednol 0.5% , tobramycin 0.3% | Visual acuity | None | None | Eye pain, conjunctivitis, eye discharge, and eye inflammation | |
| RCT | Total 417; 19 children | Not specified | 15 days | None | None | Azithromycin 1%, dexamethasone 0.1% | Complete bacterial eradication from conjunctiva and eyelids, complete resolution of clinical signs | Complete resolution of symptoms | None | Eye disorder, reduced visual acuity, punctate keratitis, blurred vision, conjunctival oedema, discharge, lid oedema, irritation, pain, itching | |
| RCT | Total 71; number of children not specified | 10 to 86 years | 14 to 15 days | None | None | Gentamycin 0.3%, betamethasone 0.1% | Ocular sign score, specific ocular inflammatory signs, bacterial eradication | None | None | Conjunctival hyperaemia |
Abbreviations:
N: number of participants
RCT: randomised controlled trial
Risk of bias in included studies
See Figure 2

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Comstock 2012 used a computerised randomisation code developed by an independent statistician and we felt this represented a low risk of selection bias.
Blinding
Participants in Comstock 2012 were masked to allocation and drugs were dispensed in identical containers. Due to slight differences in the consistency and appearance of the eye drops, observers were not permitted to be present during instillation of drops. We thought this represented a low risk of performance bias. The risk of detection bias was unclear.
Incomplete outcome data
In Comstock 2012 there were some participants whose parents withdrew consent, or who were lost to follow‐up, but these were accounted for and were balanced across treatment groups. Comstock 2012 provided the safety outcome data for all participants who had received at least one dose of the intervention. We felt this represented a low risk of attrition bias.
Selective reporting
The original protocol for Comstock 2012 from ClinicalTrials.gov listed the primary and secondary outcomes as change in total blepharoconjunctivitis grade at various time points. These data were not reported in the published study. The investigators have reported a mean composite blepharoconjunctivitis on clinicaltrials.gov (www.clinicaltrials.gov) for each trial arm with no statistical analysis comparing groups and no details as to how they derived this grade. This represents a high risk of bias.
Other potential sources of bias
Comstock 2012 reported on visual acuity outcomes but the numbers of participants able to complete Snellen acuity varied from 33 to 59% across treatment groups (probably due to the age of the participants). The publication mentioned other methods of visual acuity testing but gave no account of the results of these other than 94.5% of those giving visual behaviour responses could fix and follow. We felt this represented a high risk of bias.
Effects of interventions
See: Summary of findings for the main comparison
Comstock 2012 did not present any efficacy data in the published report. Clinicaltrials.gov (www.clinicaltrials.gov) reports blepharoconjunctivitis grade (on a scale of 0‐32) at day 3, 7 and 15 (Table 3). The registry entry contains no details on how the blepharoconjunctivitis grade was assessed. We were unable to obtain further information despite several attempts to contact the trial authors. There was little evidence of any important differences between groups. The trial authors reported visual acuity data in the publication subject to the limitations mentioned above. There was no change in visual acuity in any of the treatment groups but the presentation of the data precluded detailed analysis.
| Follow‐up | Loteprednol Etabonate and Tobramycin | Loteprednol Etabonate | Tobramycin | Vehicle |
| Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | |
| Day 3 | ‐7.32 (3.27) 34 | ‐7.74 (3.90) 34 | ‐5.94 (4.00) 32 | ‐6.58 (3.46) 31 |
| Day 7 | ‐11.03 (3.20) 34 | ‐10.94 (4.69) 34 | ‐9.90 (3.80) 30 | ‐10.03 (4.63) 30 |
| Day 15 | ‐11.41 (3.29) 34 | ‐11.23 (3.98) 35 | ‐10.68 (4.71) 34 | ‐10.30 (5.19) 33 |
Safety data indicated that there was no statistical difference in the incidence of adverse events between treatment arms. Ocular adverse events included conjunctivitis, eye pain, discharge and inflammation. Non‐ocular adverse events reported were ear infection, ear pain, otitis media, diarrhoea, gastroenteritis, lip swelling, vomiting, pyrexia, urticaria, bronchiolitis, upper respiratory infection, nasopharyngitis, tonsillitis, varicella, dehydration, cough, pharyngolaryngeal pain, respiratory distress, dermatitis and rash. There were no ocular serious adverse events and the authors considered it unlikely for most of the adverse events to be due to treatment. Data for ocular adverse events included any treated fellow eyes, which made further analysis difficult. Table 4 summarises the adverse events seen in each study group.
| Loteprednol/tobramycin | Loteprednol | Tobramycin | Vehicle | |
| Ocular AEs | 1/34 (eye pain) | 4/35 (eye pain, conjunctivitis, eye discharge, eye inflammation) | 0/34 | 0/33 |
| Non‐Ocular AEs | 2/34 3 AEs (gastroenteritis, pyrexia, bronchiolitis) | 6/35 9 AEs (ear infection, lip swelling, vomiting, URI, varicella, cough, phyarngolaryngeal pain, rash) | 6/34 9 AEs (ear infection, otitis media acute, diarrhea, pyrexia, bronchioltis, URI, nasophayngitis, respirator distress, dermatitis (diaper) ) | 5/33 7 AEs (ear pain, pyrexia, urticaria, bronchioloitis, URI, tonsilitis, dehydration) |
Abbreviations:
AEs: adverse events
Discussion
Summary of main results
Only one of the included publications presented sufficient data on children that we could include in this review. It presented data on adverse events only, and did not present sufficient data on improvement in symptoms or signs. The authors presented visual acuity data in a form that precluded extraction for use in this review but they reported no statistically significant changes in visual acuity with either treatment or placebo. There was no difference in adverse events with treatments compared to placebo but it is unclear if this trial was designed with appropriate statistical power to detect a difference.
Overall completeness and applicability of evidence
While we identified three randomised controlled trials (RCTs) with paediatric participants, two trials pertained to mixed participant groups of adults and children, and did not clearly match the definition of blepharokeratoconjunctivitis (BKC). The third trial, Comstock 2012, reported primarily on adverse effects and did not report completely on changes in symptoms and signs. Treatment was limited to two weeks in duration. No RCTs on long‐term treatments met the inclusion criteria of this review.
The treatment in the included study consisted of topical corticosteroid and antibiotic drops, both of which are used in clinical practice as noted in the Description of the intervention section. The duration of treatment is short in comparison to the long term nature of this condition (Description of the condition).
Quality of the evidence
One RCT including 137 participants met the inclusion criteria of this review. Although the randomisation of participants was rigorous, we have identified some risk of bias in this trial. We do not have a large body of high quality evidence to support our conclusions.
Potential biases in the review process
Our approach to this review was based on a broad search, and we included any reports on this condition. We amended and broadened our search strategy further when it became apparent that we might have missed a potentially relevant study. With the enhanced strategy it is less likely that the search would have overlooked any relevant studies. The review authors reviewed the results independently, thus eliminating selection bias. We sought to obtain all relevant data from authors where it was not available in published reports but we were not successful leading to potential omission of relevant data.
Agreements and disagreements with other studies or reviews
We are unaware of any other systematic review of high‐quality evidence that has been undertaken on this topic. There are some narrative reviews about BKC and its treatment, but they have neither followed systematic review methodology nor assessed the quality of evidence (Hammersmith 2015, Wong 2010).
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Topical treatments compared with control for blepharokeratoconjunctivitis in children | ||||||
| Patient or population: children with blepharokeratoconjunctivitis Settings: eye clinic Intervention: topical treatments (antibiotics and/or steroids) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty (quality) of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (vehicle) | topical treatments (antibiotics/steroids) | |||||
| Improvement in symptoms, reported by the child or by their parents/carers, preferably measured by a validated tool, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (1) | ⊕⊝⊝⊝ | Data on changes in grade of blepharoconjunctivitis measured between baseline and 2 weeks did not suggest any important differences between groups. |
| Elimination of all clinical signs of ocular surface inflammation ('complete success'), preferably measured by a composite grading system, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Improvement of clinical signs of ocular surface inflammation ('partial success'), preferably measured by a composite grading system, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (10 | ⊕⊝⊝⊝ | Data on changes in grade of blepharoconjunctivitis measured between baseline and 2 weeks did not suggest any important differences between groups. |
| Change from baseline in best corrected visual acuity in affected eye(s) in logMAR measured with an ETDRS chart at 4 m, or, in younger children, with a Keeler crowded logMAR chart at 3 m, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | 137 (1) | ⊕⊝⊝⊝ | Limited data in a form that could not be extracted; not statistically significant differences between groups. |
| Uncontrolled or poorly controlled disease progression due to treatment failure, at three months (± one month) after start of treatment | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse effects of medication, at any time during treatment | Ocular adverse events
Non‐ocular adverse events
| 137 (1) | ⊕⊝⊝⊝ | |||
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Some data on blepharoconjunctivitis grade reported on trials registry but we were unable to estimate a measure of effect for this outcome. 2 Data were not fully reported and we were unable to estimate a measure of effect for change in visual acuity. 3 Very low certainty due to very low numbers of events. | ||||||
| Author, year | Study type | N | Age | Mean/median follow‐up | Mechanical treatment | Systemic (oral) interventions | Topical interventions | Physician‐reported outcomes | Patient‐reported outcomes | Adverse events from condition | Adverse events from treatment |
| Case series | 7 | 6 to 14 years | 6 months | Lid hygiene | Amoxicillin/ clavulanate | Chloramphenicol (PF) drops, chloramphenicol ointment to lids, prednisolone 0.5% (PF) | Eyelid condition, corneal epitheliopathy, stromal defects | Improvement of symptoms | None | None | |
| Case series | 3 | 30 months to 8 years | Variable | Lid hygiene | Azithromycin | Loteprednol 0.2%, CSA 0.05%, | Chalazia, keratitis, corneal ulcer/scar, phlyctenule, MGD | Improvement of itching | None | None | |
| Case series | 16 | 4 to 16 years | Variable | Lid hygiene | Erythromycin (1 participant only) | Azithromycin 1.5%, CSA 2% | Bulbar conjunctival hyperaemia, conjunctival phlycten, corneal inflammation, blepharitis grade | Ocular redness | None | Ocular irritation (redness, burning, stinging) | |
| Case series | 8 | 3.5 to 13 years | 8.3 months | Lid hygiene | Erythromycin suspension 450 mg divided into 3 doses | Prednisolone 0.5% (PF); hydrocortisone acetate 1% ointment nocte | Bulbar conjunctival redness, inferior superficial corneal vascularisation, punctate corneal epithelial staining, inferior subepithelial vascularisation and infiltrate, conjunctival phlyctenules, corneal phlyctenules, circumferential pannus, corneal scar | Red eyes, photophobia, itching, discharge | Corneal scarring and thinning | Stomach disturbance, diarrhoea | |
| Case series | 615 | 7 months to 16 years | Not reported | Lid hygiene | Erythromycin | Topical steroids and antibiotics (not specified) | Outcomes not reported (presenting signs only) | Outcomes not reported (presenting symptoms only) | None | None | |
| Case series | 10 | 6 to 27 years | 4.4 years | Lid hygiene | Azathioprine, mycophenolate mofetil, prednisolone | Steroids (not specified) | Disease remission/ control of inflammation | None | Corneal perforation | none | |
| Case series | 29 | 2 to 12 years | 5.4 months | Warm compresses | Erythromycin, doxycyclin | Prednisolone 1%, dexamethasone 0.1%, antibiotic, fluorometholone, loteprednol etabonate 0.5% | Eyelid inflammation, superficial punctate keratitis, corneal vascularisation, corneal infiltrates, phlyctenules, corneal scarring | None | Amblyopia | Gastrointestinal distress, mouth ulcers (unrelated) | |
| Case series | 27 | 7 months to 15.9 years | 2.3 years | Warm compresses, lid hygiene | Erythromycin, doxycyclin, flaxseed oil | chloramphenicol, ciprofloxacin, gentamicin, prednisolone 1% or 0.5%, fluorometholone 0.1% | Visual acuity, astigmatism | Discomfort, photophobia | Amblyopia | Vaginal candidiasis | |
| Case series | 5 | 4 to 9 years | Not specified | Erythromycin | Lid hyperaemia and swelling, corneal infiltrates | None | None | None | |||
| Case series | 114 | Mean 9.3 years (± 4.2) | 26.4 months | Lid hygiene | Flaxseed oil, erythromycin | Lubricants (hyaluronate, methylcellulose), erythromycin, ciprofloxacin, steroids (dexamethasone 0.1% (PF), loteprednol 0.5%, fluorometholone 0.1%), CSA 0.05% | Visual acuity | None | None reported | None reported | |
| Case series | 51 | Mean 10.2 years (± 3.6) | 58.9 months | Warm compresses, lid hygiene | Antibiotics (erythromycin, amoxicillin/ clavulanate, doxycycline), steroids | Steroids (dexamethasone 1%, prednisolone 0.12 to 1%, fluorometholone 0.1%) , antibiotics (fucidic acid, levofloxacin, tobramycin), CSA 0.5% | Visual acuity | Redness, tearing, blurred vision, pain, irritation, photophobia, white spot, swelling, discharge, itching, rubbing | Corneal perforation | Raised intraocular pressure, cataract, gastrointestinal disturbance | |
| Case series | 44 | 1 to 14 years | 7 years | Lid hygiene | Erythromycin | Chloramphenicol, steroids | Reduction of clinical signs | Redness, watering, itching, grittiness, discharge, photophobia, pain | None reported | None reported | |
| Abbreviations: CSA: ciclosporin | |||||||||||
| Author, year | Study type | N | Age | Mean/median follow‐up | Mechanical treatment | Systemic (oral) interventions | Topical interventions | Physician‐reported outcomes | Patient‐reported outcomes | Adverse events from condition | Adverse events from treatment |
| RCT | 137 | 0 to 6 years | 15 days | None | None | Loteprednol 0.5% , tobramycin 0.3% | Visual acuity | None | None | Eye pain, conjunctivitis, eye discharge, and eye inflammation | |
| RCT | Total 417; 19 children | Not specified | 15 days | None | None | Azithromycin 1%, dexamethasone 0.1% | Complete bacterial eradication from conjunctiva and eyelids, complete resolution of clinical signs | Complete resolution of symptoms | None | Eye disorder, reduced visual acuity, punctate keratitis, blurred vision, conjunctival oedema, discharge, lid oedema, irritation, pain, itching | |
| RCT | Total 71; number of children not specified | 10 to 86 years | 14 to 15 days | None | None | Gentamycin 0.3%, betamethasone 0.1% | Ocular sign score, specific ocular inflammatory signs, bacterial eradication | None | None | Conjunctival hyperaemia | |
| Abbreviations: N: number of participants | |||||||||||
| Follow‐up | Loteprednol Etabonate and Tobramycin | Loteprednol Etabonate | Tobramycin | Vehicle |
| Mean (SD) N | Mean (SD) N | Mean (SD) N | Mean (SD) N | |
| Day 3 | ‐7.32 (3.27) 34 | ‐7.74 (3.90) 34 | ‐5.94 (4.00) 32 | ‐6.58 (3.46) 31 |
| Day 7 | ‐11.03 (3.20) 34 | ‐10.94 (4.69) 34 | ‐9.90 (3.80) 30 | ‐10.03 (4.63) 30 |
| Day 15 | ‐11.41 (3.29) 34 | ‐11.23 (3.98) 35 | ‐10.68 (4.71) 34 | ‐10.30 (5.19) 33 |
| Loteprednol/tobramycin | Loteprednol | Tobramycin | Vehicle | |
| Ocular AEs | 1/34 (eye pain) | 4/35 (eye pain, conjunctivitis, eye discharge, eye inflammation) | 0/34 | 0/33 |
| Non‐Ocular AEs | 2/34 3 AEs (gastroenteritis, pyrexia, bronchiolitis) | 6/35 9 AEs (ear infection, lip swelling, vomiting, URI, varicella, cough, phyarngolaryngeal pain, rash) | 6/34 9 AEs (ear infection, otitis media acute, diarrhea, pyrexia, bronchioltis, URI, nasophayngitis, respirator distress, dermatitis (diaper) ) | 5/33 7 AEs (ear pain, pyrexia, urticaria, bronchioloitis, URI, tonsilitis, dehydration) |
| Abbreviations: AEs: adverse events | ||||

