Aspirina para el tratamiento agudo de la cefalea tensional episódica en adultos
Resumen
Antecedentes
La cefalea tensional (CT) afecta a cerca de una de cada cinco personas en todo el mundo. Se divide en CT episódica poco frecuente (menos de una cefalea por mes), CT episódica frecuente (dos a 14 cefaleas por mes) y CT crónica (15 días de cefalea al mes o más). La aspirina es uno de varios analgésicos indicados para el tratamiento agudo de la CT episódica.
Objetivos
Evaluar la eficacia y la seguridad de la aspirina para el tratamiento agudo de la cefalea tensional (CT) episódica en adultos en comparación con un placebo o cualquier comparador activo.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase y en la Oxford Pain Relief Database desde el inicio hasta septiembre 2016, y también en listas de referencias de estudios y revisiones relevantes publicados. Se buscaron estudios no publicados consultando a contactos personales y buscando en registros de ensayos clínicos en línea y webs de fabricantes.
Criterios de selección
Se incluyeron estudios aleatorizados, doble ciego, controlados con placebo (grupos paralelos o cruzados) que utilizaron aspirina oral para el alivio sintomático de un episodio agudo de CT. Los estudios debían ser prospectivos, con pacientes de 18 años de edad o más e incluir al menos diez por brazo de tratamiento.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, evaluaron los estudios para inclusión y extrajeron los datos. Para diversos resultados (sobre todo los recomendados por la International Headache Society [IHS]), se calculó el riesgo relativo (RR) y el número necesario a tratar para lograr un resultado beneficioso adicional (NNT), un resultado perjudicial adicional (NNH) o prevenir un evento (NNTp) para la aspirina oral en comparación con un placebo o una intervención activa.
La calidad de la evidencia se evaluó con los criterios GRADE y se creó una tabla de "Resumen de los hallazgos".
Resultados principales
Se incluyeron cinco estudios que incorporaron a adultos con CT episódica frecuente; 1812 participantes tomaron medicación, de los cuales 767 se incluyeron en comparaciones de aspirina 1000 mg con placebo, y 405 en comparaciones de aspirina 500 mg o 650 mg con placebo. No todos estos participantes aportaron datos para los resultados de interés de esta revisión. Cuatro estudios especificaron el uso de los criterios de diagnóstico del IHS; un criterio anterior comúnmente reconocido, pero describió características comparables y excluyó la migraña. Todos los pacientes fueron tratados por cefaleas de intensidad al menos moderada.
Ninguno de los estudios incluidos tuvo bajo riesgo de sesgo en todos los dominios considerados, aunque en la mayoría de los estudios y dominios fue probable que lo anterior se debiera al informe insuficiente en lugar de al uso de métodos deficientes. Se consideró que el riesgo de sesgo de un estudio era alto debido al tamaño pequeño.
No hubo datos para la aspirina en cualquier dosis para el resultado preferido del IHS de estar libre de dolor a las dos horas, o para estar libre de dolor en cualquier otro momento, y sólo un estudio proporcionó datos equivalentes a no tener dolor o a tener dolor leve a las dos horas (evidencia de calidad muy baja). El uso de medicación de rescate fue menor con aspirina 1000 mg que con placebo (dos estudios, 397 participantes); el 14% de los participantes usó medicación de rescate con aspirina 1000 mg en comparación con el 31% con placebo (NNTp 6,0; intervalo de confianza [IC] del 95%: 4,1 a 12) (evidencia de calidad baja). Dos estudios (397 participantes) informaron una evaluación global del paciente al final del estudio; se combinaron las dos categorías principales de ambos estudios para determinar el número de participantes que estaban "satisfechos" con el tratamiento. La aspirina 1000 mg resultó en más pacientes satisfechos (55%) que el placebo (37%) (NNT 5,7; IC del 95%: 3,7 a 12) (evidencia de calidad muy baja).
Los eventos adversos no fueron diferentes entre la aspirina 1000 mg y el placebo (RR 1,1; IC del 95%: 0,8 a 1,5) o la aspirina 500 mg o 650 mg y el placebo (RR 1,3; IC del 95%: 0,8 a 2,0) (evidencia de calidad baja). Los estudios no informaron ningún evento adverso grave.
La calidad de la evidencia, evaluada mediante los criterios GRADE para comparar las dosis de aspirina entre 500 mg y 1000 mg con placebo, fue baja o muy baja. Se disminuyó la calidad de la evidencia debido al número pequeño de estudios y eventos, y porque no se informó la medida de eficacia más importante.
Había datos insuficientes para comparar la aspirina con cualquier comparador activo (paracetamol solo, paracetamol más codeína, aceite de menta o metamizol) en cualquiera de las dosis estudiadas.
Conclusiones de los autores
Con una dosis única de aspirina de entre 500 mg y 1000 mg se consiguieron algunos beneficios en cuanto al uso menos frecuente de la medicación de rescate y más pacientes satisfechos con el tratamiento, en comparación con un placebo, en adultos con CT episódica frecuente con cefalea aguda de intensidad moderada o severa. No hubo diferencias entre una dosis única de aspirina y el placebo para el número de pacientes que presentó eventos adversos. Este dato debe interpretarse con cuidado ya que la cantidad y la calidad de la evidencia fue muy limitada.
PICO
Resumen en términos sencillos
Aspirina oral para el tratamiento de la cefalea tensional episódica aguda en adultos
Conclusión
Esta revisión encontró sólo evidencia de muy baja calidad de que los pacientes con dos a 14 cefaleas tensionales por mes obtienen un alivio aceptable del dolor con aspirina 1000 mg o dosis inferiores. Hay interrogantes acerca de cómo se realizan los estudios de este tipo de cefalea. Estas preguntas incluyen el tipo de pacientes elegidos para los estudios y los tipos de resultados informados. Lo anterior limita la utilidad de los resultados, especialmente en los pacientes que solo presentan una cefalea ocasional.
Antecedentes
Los pacientes con cefalea tensional episódica frecuente padecen de entre dos y 14 episodios por mes. La cefalea tensional impide que los pacientes se concentren y trabajen adecuadamente y provoca mucha discapacidad. Cuando ocurren, las cefaleas mejoran con el transcurso del tiempo, incluso sin tratamiento. La aspirina es un analgésico de uso común y de amplia disponibilidad, que se expende sin prescripción (venta libre). La dosis usual es de 300 mg a 650 mg por vía oral.
Características de los estudios
En septiembre de 2016, se realizaron búsquedas en la bibliografía médica y se encontraron cinco estudios con 1812 pacientes que tomaban aspirina para la cefalea tensional episódica frecuente. Cerca de 1668 pacientes participaron en las comparaciones entre la aspirina en dosis de entre 500 mg y 1000 mg y un placebo (un comprimido inactivo). La International Headache Society recomienda el resultado ningún dolor a las dos horas después de tomar una medicina, pero también se sugieren otros resultados. Ningún estudio informó el estado libre de dolor a dos horas ni otros resultados reconocidos, por lo que la información para analizar los resultados de efectividad de la aspirina fue limitada.
Resultados clave
Ninguno de los estudios informó sobre los pacientes que estaban libres de dolor a las dos horas, y sólo un estudio informó un resultado que se consideró equivalente al estado libre de dolor o la presencia de un dolor leve a las dos horas. Para la aspirina 1000 mg, cerca de diez pacientes de cada 100 usaron analgésicos adicionales, en comparación con 30 de cada 100 con placebo (evidencia de calidad muy baja). Al final del estudio, 55 de 100 pacientes estaban "satisfechos" con el tratamiento en comparación con 37 de 100 que recibieron el placebo (evidencia de calidad baja). Cerca de 15 de cada 100 pacientes que tomaban la aspirina 1000 mg informaron un efecto secundario después de una dosis, que fue el mismo que con placebo (evidencia de calidad baja).
Calidad de la evidencia
La calidad de la evidencia fue baja o muy baja para las comparaciones entre la aspirina y el placebo. La evidencia de calidad baja y muy baja significan que no existe mucha seguridad acerca de los resultados.
Authors' conclusions
Summary of findings
| Aspirin 1000 mg compared with placebo for episodic tension‐type headache | ||||||
| Patient or population: adults with episodic tension‐type headache Settings: community Intervention: aspirin 1000 mg Comparison: placebo | ||||||
| Outcomes | Outcome with | Outcome with | RR NNT, NNTp or NNH (95% CI) | No of studies, participants, events | Quality of the evidence | Comments |
| Pain‐free at 2 hours | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free at other time points (1, 4, 24 hours) | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free or mild pain at 2 hours | 61/112 | 78/103 | Not calculated | 1 study, 215 participants, 139 events | Very low | Downgraded three levels due to sparse data: single study with 215 participants in comparison |
| Use of rescue medication | 310 in 1000 | 140 in 1000 | RR 0.47 (0.31 to 0.70) NNTp 6.0 (4.1 to 12) | 2 studies, 397 participants, 91 events | Low | Downgraded two levels due to sparse data: small number of studies, participants and events |
| Patient Global Evaluation at end of study | 370 in 1000 | 550 in 1000 | RR 1.5 (1.2 to 1.8) NNT 5.7 (3.7 to 12) | 2 studies, 397 participants, 181 events | Very low | Downgraded three levels due to sparse data: small number of studies, participants, differences in scales and timing for the outcome, and post‐hoc nature of the analysis |
| Any adverse event | 140 in 1000 | 160 in 1000 | RR 1.1 (0.75 to 1.5) NNH not calculated | 3 studies, 723 participants, 107 events | Low | Downgraded two levels due to sparse data: small number of studies and events |
| Specific adverse events | Inconsistently reported and too few events for analysis Included gastrointestinal upset or dyspepsia, nausea, dizziness, and somnolence | ‐ | 4 studies, 767 participants | Very low | Downgraded three levels due to sparse data: small number of studies, few events, inconsistent reporting | |
| Serious adverse events | No events reported | No events reported | ‐ | 4 studies, 767 participants, no events | Very low | Downgraded three levels due to sparse data: small number of studies and no events |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence (EPOC 2015). High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. † Substantially different: a large enough difference that it might affect a decision. | ||||||
Background
This review is based on a template for reviews of drugs used for acute treatment of episodic tension‐type headache (TTH) in adults. The aim is for all reviews to use the same methods.
Headaches are a commonly reported problem in community‐based surveys worldwide. The lifetime prevalence of headache is estimated to be greater than 90% (Steiner 2004), and the annual prevalence is estimated to be 46% in the general adult population (Stovner 2007). Variations in reported prevalence of TTH may result from differences in study design, population, inclusion or exclusion of cases of infrequent episodic TTH, overlap with probable migraine, cultural and environmental differences, or even genetic factors (Sahler 2012). TTH is more common than migraine, a finding replicated across the world (Oshinaike 2014; Vos 2012).
The management of people with non‐migrainous headaches is, however, largely neglected (Rasmussen 2001; Steiner 2011), and may be fragmented by the involvement of clinicians from different medical specialities (neurology; ear, nose, and throat; ophthalmology; psychiatry). Because headache is rarely life‐threatening and headache pain is generally mild to moderate in intensity, people often self‐medicate and do not seek formal care from health services (Rasmussen 2001). People with TTH have more work absence than people without headaches (Lyngberg 2005), which may lead to loss of productivity (Cristofolini 2008; Pop 2002). Headache‐related characteristics include significant problems with headache management, disability, pain, worry, and dissatisfaction with care, as well as greater use of medical services and worse general health (Harpole 2005).
Headache can be either primary (cause not known) or secondary (due to other systemic or local causes) (Green 2009). TTH belongs to the group of primary headaches and is the most common type; the large number of people affected imposes a significant burden on the healthcare system (Stovner 2007). Generally, episodes of TTH are mild to moderate in intensity and self‐limiting, but in a small group of people they may be more severe and disabling (Green 2009). People with longer lasting or more severe headaches may seek help in a clinical setting, but the majority do not do so, which can result in inadequate and inappropriate management (Kernick 2008). In one Canadian community‐based telephone survey to determine medication patterns of 274 people with frequent headache (of all types) aged 18 to 65 years, only 1% used prescription medication. The majority reported using over‐the‐counter (OTC; non‐prescription) analgesics (56% used paracetamol (acetaminophen) and 15% used aspirin), and the perceived effectiveness of OTC medication was approximately 7 on a scale of 0 to 10 (Forward 1998).
Until recently, professional strategies for the management of TTH were largely extrapolated from those used for migraine. The World Health Organization (WHO) essential drug list, for example, does not include indications for the management of TTH (WHO 2015). In 2010, the British Association for the Study of Headache (BASH; BASH 2010) and the European Federation of Neurological Societies (EFNS; Bendtsen 2010) updated or published guidelines for the management of TTH. The two guidelines reflect ongoing systematic efforts to bridge the gap between clinical trial evidence and clinical practice with the aim of improving practice. While these guidelines represent a step forward, they fail to take into account the quality and methodological limitations of individual studies.
Description of the condition
TTH has been known by several names, including tension headache, muscle contraction headache, psychomyogenic headache, stress headache, ordinary headache, essential headache, idiopathic headache, and psychogenic headache (IHS 2004). TTH is diagnosed mainly by the absence of features found in other headache types, especially migraine. The third edition of the International Classification of Headache Disorders distinguishes between episodic and chronic subtypes of TTH (IHS 2013). Chronic TTH is diagnosed when headache occurs on 15 or more days per month on average for three or more months (180 or more days per year); otherwise TTH is considered to be episodic.
Acute treatment with analgesics is more appropriate for episodic TTH, while both pharmacological and non‐pharmacological treatments are used for managing chronic TTH. Structural changes in the brain have been reported in people with chronic TTH (Fumal 2008). Furthermore, management of TTH in children and adolescents poses a clinically diverse situation (establishing diagnoses, dosages, nature of preparation, pharmacodynamics, etc.; Monteith 2010). For these reasons, this review focused on the acute treatment of episodic TTH in adults.
Diagnosis
Episodic TTH is subdivided into infrequent and frequent subtypes (IHS 2013).
Infrequent episodic TTH is defined by the following criteria.
-
Lifetime history of at least 10 episodes occurring on fewer than one day per month (fewer than 12 days per year) and satisfying criteria 2 through to 4 below.
-
Headache lasting from 30 minutes to seven days.
-
Headache has at least two of the following characteristics:
-
bilateral location;
-
pressing or tightening (non‐pulsating) quality;
-
mild or moderate intensity;
-
not aggravated by routine physical activity such as walking or climbing stairs.
-
-
Both:
-
no nausea or vomiting (anorexia may occur);
-
no more than one of photophobia or phonophobia.
-
-
Not attributed to another disorder.
Frequent episodic TTH is diagnosed when there is a lifetime history of least 10 episodes occurring on at least one day but fewer than 15 days per month for at least three months (at least 12 and fewer than 180 days per year), and when criteria 2 through to 5, above, are also met.
Prevalence
The Global Burden of Diseases Study 2010 reported global prevalence of TTH as 21%, making it the second most prevalent condition after dental caries, and slightly more prevalent than migraine (Vos 2012).
Causation
The exact pathogenesis of TTH is still unknown and is said to be multifactorial, including central dysfunction of pain processing pathways and peripheral myofascial factors. There is a general agreement that peripheral myofascial nociception disturbances play a greater role in the pathogenesis of both frequent and infrequent episodic TTH (Bendtsen 2016; Fernández‐de‐las‐Peñas 2010; Fumal 2008).
Description of the intervention
Medicines derived from willow bark, which is rich in salicylate, have been used for centuries for treating pain, fever, and inflammation. In the mid‐19th century, chemists first synthesised acetylsalicylic acid, and by the end of the century, Bayer had patented and was selling the drug, which the company called aspirin, around the world.
Aspirin is used to treat mild to moderate pain, including migraine headache pain; inflammatory conditions such as rheumatoid arthritis; and, in low doses, it is used as an antiplatelet agent in cardiovascular disease. Its efficacy for treating acute pain has been well demonstrated (Moore 2011). It is a potent gastrointestinal irritant, and may cause discomfort, ulcers, and bleeding. It may aIso cause tinnitus at high doses, and it is no longer used in children and adolescents, in whom it may cause Reye's syndrome (swelling of the brain that may lead to coma and death) (Glasgow 2006). Its use as an analgesic and antipyretic agent has declined, largely due to concerns about these adverse events, as newer products have become available. However, in some countries, it may be the only drug readily available, and for some conditions, such as migraine, some people report it to be an effective and reliable treatment (Kirthi 2013).
Aspirin is available in various strengths, ranging from 75 mg to about 1.5 g (Drugs.com). For the treatment of pain, the British National Formulary recommends 300 mg to 900 mg orally every four to six hours as needed (maximum 4 g daily) or 450 mg to 900 mg rectally (maximum 3.6 g daily), in adults (BNF 2016). Aspirin 500 mg is commonly available in some parts of the world. For oral administration, aspirin is available in three formulations: standard tablet, soluble tablet, and enteric‐coated tablet.
How the intervention might work
Aspirin irreversibly inhibits cyclo‐oxygenase enzymes, which are needed for prostaglandin and thromboxane synthesis. Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in mediating inflammatory and nociceptive processes. Suppression of prostaglandin synthesis is believed to underlie the analgesic effects of aspirin (Vane 1971).
Why it is important to do this review
Episodic TTH is ubiquitous, affecting a large proportion of adults. Despite being generally mild to moderate in intensity, headache results in considerable suffering to the affected person and contributes overall to a significant loss of productivity to society (Mannix 2001; Rasmussen 2001; Steiner 2004; Stovner 2007). The treatment of episodic TTH is essentially pharmacological. Seeking relief, people generally self‐medicate with one or more medicines, and OTC medicines are often used (Forward 1998). Aspirin is a readily accessible OTC analgesic; as a generic drug, it could be a drug of choice or the first‐line drug for management of TTH, particularly in low‐resource settings. It has been shown to work in individual studies and one systematic review (Moore 2014; Steiner 2003).
Authors of two guidelines on the management of TTH have reviewed the effectiveness of treatment modalities. In both they adopted a consensus methodology. The BASH guidelines are based on a limited review of studies (BASH 2010). The EFNS guidelines are based on a more detailed and thorough search of the literature (Bendtsen 2010). Moreover, the EFNS guidelines represent an improvement over the BASH guidelines in that they used a standard, published protocol for developing management guidelines (Brainin 2004). That protocol strongly recommends active and frequent consultation of the Cochrane Library, and this suite of reviews is being carried out to provide relevant evidence (see Derry 2015; Stephens 2016; Veys 2016). One non‐Cochrane systematic review by Verhagen and colleagues followed methods similar to those used in Cochrane Reviews and evaluated the efficacy and tolerability of analgesics for the acute treatment of episodes of TTH in adults, but the authors analysed the non‐standard measure "pain relief or recovery over 2 to 6 hours" as the main efficacy outcome (Verhagen 2006).
Reviews explicitly adopting Cochrane methods and evaluating the more focused outcomes recommended in the International Headache Society (IHS)'s recently updated guidelines for controlled trials of drugs in TTH (IHS 2010) are clearly important. One survey of TTH study methods and reporting demonstrated that these are seldom adhered to in clinical trials, but did report a variety of outcomes, including IHS‐preferred outcomes, for aspirin, ibuprofen, ketoprofen, and paracetamol (Moore 2014).
Objectives
To assess the efficacy and safety of aspirin for acute treatment of episodic tension‐type headache (TTH) in adults compared with placebo or any active comparator.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐controlled studies (parallel‐group or cross‐over) in any setting using aspirin for symptomatic relief of an acute episode of TTH. Studies had to be prospective. We accepted studies reporting treatment of consecutive headache episodes if outcomes for the first, or each, episode were reported separately. Studies were included regardless of publication status or language of publication. We included studies conducted in any setting (home, clinic, physician's office, community centre, etc.) if it was clear that treatment was for an acute episode of TTH.
Cross‐over studies are well‐suited to study acute episodic TTH and eliminate within‐person variation; however, they pose challenges during analysis related to dropouts, inadequate reporting (reporting only the first period), and inappropriate reporting (reporting as parallel‐group trials instead of paired observations). We included cross‐over trials only if there was adequate washout (48 hours or more) between treatments and after ascertaining that the participants were adequately randomised to the first treatment period.
We excluded trials using alternation, date of birth, hospital record number, or other 'quasi‐random' methods of allocation of treatment.
Types of participants
Study participants were adults (18 years of age or older) with episodic TTH. We excluded studies involving participants with chronic TTH.
The diagnosis of episodic TTH ideally conformed to IHS criteria (IHS 2013). We considered other definitions if they conformed in general to IHS diagnostic criteria and reasonably distinguished TTH from other headache types by specifying distinctive features of TTH, for example, absence of nausea or vomiting, mild to moderate head pain, character and location of pain, absence of obvious phonophobia or photophobia and aura, and differentiated from chronic daily headache.
We analysed data only for people with acute TTH episodes. Studies including participants with 'mixed' migraine and TTH or 'combination' headaches would have posed problems, as these terms may refer to people with discrete episodes of migraine and discrete episodes of TTH, or to people with headaches which (in the view of the investigators) combined features of migraine and TTH. The IHS criteria assign a dual diagnosis of migraine and TTH or 'probable migraine', respectively, to such people. In these situations, we described the headache pattern denoted by these terms, and considered the inclusion of such trials or treatment groups in, or their exclusion from, the review on a case‐by‐case basis. We excluded secondary headache disorders using criteria based on the International Classification of Headache Disorders (ICHD) (IHS 2013).
Types of interventions
Included studies had at least one arm in which aspirin was given orally for an acute episode of TTH. There was no restriction on dose. Included studies could use either a single dose to treat a discrete headache episode or investigate different dosing strategies.
A placebo comparator is essential to demonstrate that aspirin is effective in this condition. The placebo used had to be identical to aspirin in appearance (size, colour, etc.) and number of tablets or capsules, or a double‐dummy technique had to be used. We looked primarily for studies using aspirin alone, but also sought studies that compared a combination of aspirin and another active oral treatment with the non‐aspirin component alone. We would have considered active‐controlled trials without a placebo as secondary evidence, but our searches did not identify any.
Types of outcome measures
Primary and secondary outcomes selected for analysis reflect the updated guidelines for controlled trials of drugs in TTH (IHS 2010).
Primary outcomes
-
Pain‐free rate at the end of two hours using any standard method of pain assessment and without the use of rescue medication.
Secondary outcomes
-
Pain‐free rate at different time points, without the use of rescue medication; we adopted one hour, four hours, and 24 hours as clinically important endpoints and analysed them separately.
-
Pain‐free or mild pain at two hours (equivalent to headache response in migraine trials); this is an outcome regarded as useful by most people with acute or chronic pain (Moore 2013).
-
Pain Intensity Difference (PID) and Sum of Pain Intensity Difference (SPID), without the use of rescue medication, analysed separately.
-
Use of rescue medication. When participants used rescue medication they were considered to have withdrawn from the study because of a lack of efficacy.
-
Adverse events: number of participants with any adverse event, identity and rates (where data permitted) of specific adverse events, serious adverse events, and number of withdrawals due to adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (via Cochrane Register of Studies Online) on 7 September 2016;
-
MEDLINE (via Ovid, 1946 to 7 September 2016);
-
Embase (via Ovid, 1974 to 7 September 2016);
-
Oxford Pain Relief Database (Jadad 1996a).
The search strategies for CENTRAL, MEDLINE, and Embase are reported in Appendix 1, Appendix 2, and Appendix 3, respectively.
Searching other resources
We searched the International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov (ClinicalTrials.gov) for completed or ongoing trials using the key words 'headache' or 'cephalalgia' or their variations (using wildcards). We also examined web‐based clinical trials registries of relevant manufacturers and drug companies including GlaxoSmithKline, Novartis, Bayer, and RB.
We searched the reference lists of all eligible trials and previous systematic reviews for additional studies.
Data collection and analysis
Selection of studies
Two authors independently reviewed the titles and abstracts of all studies identified through searching and excluded any that clearly did not satisfy inclusion criteria, and read full copies of the remaining studies to identify those suitable for inclusion. We resolved disagreements by mutual discussion; it was not necessary to involve a third author.
Data extraction and management
We adapted the Cochrane Pain, Palliative and Supportive Care Review Group's (PaPaS) data extraction form to suit the requirements of this review. Two authors independently extracted data from each study using the form. We resolved disagreements by mutual discussion; it was not necessary to involve a third author. One author entered data into Review Manager 5 (RevMan 2014), and another checked the entries.
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion (Jadad 1996b), limiting inclusion to studies that were randomised and double‐blind as a minimum. The scores for each study are reported in a Characteristics of included studies table.
Two authors independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
-
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number).
-
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (e.g. open list).
-
Blinding of participants and personnel (checking for possible performance bias), and blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
-
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (fewer than 10% of participants did not complete the study or the study used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' (LOCF) analysis); high risk of bias (used 'completer' analysis).
-
Size of study (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised (Dechartres 2013; Nüesch 2010). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We used risk ratios (RR) to establish statistical difference, and numbers needed to treat for an additional beneficial outcome (NNT) and pooled percentages as absolute measures of benefit or harm with 95% confidence intervals (CI).
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
-
When significantly fewer adverse outcomes occurred with aspirin than with control (placebo or active), we used the number needed to treat to prevent one event (NNTp).
-
When significantly more adverse outcomes occurred with aspirin compared with control (placebo or active), we used the number needed to treat for an additional harmful outcome or to cause one event (NNH).
We have reported continuous data as the mean difference, with 95% CIs where appropriate. We did not carry out any analysis of continuous data.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
The most likely source of missing data was expected to be cross‐over studies; we planned to use only first‐period data where possible, but the only cross‐over study that we included did not provide any usable data for analysis.
For all outcomes, we carried out analyses, as far as possible, on a modified intention‐to‐treat basis, in which we included all participants who were randomised and received an intervention. Where sufficient information was reported, we re‐included missing data in the analyses undertaken. We planned to exclude data from outcomes where results from 10% or more of participants were missing with no acceptable reason provided or apparent, but this was not necessary. We have noted where there were substantial amounts of missing data in any study, and planned to perform sensitivity analyses to investigate their effect in any analyses; in the event, this was not necessary.
Assessment of heterogeneity
We planned to assess heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987), but there were insufficient data. Where we pooled data, we reported the I2 statistic.
Assessment of reporting biases
We planned to assess publication bias by examining the number of participants in trials with zero effect (RR of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as an NNT of 10 or greater for pain‐free at two hours, and NNT of 8 or greater for no or mild pain at two hours. In the event, there were insufficient data to make this assessment.
Data synthesis
We planned to analyse studies using a single dose of aspirin in established pain of at least moderate intensity separately from studies in which medication was taken before pain was well established, or in which a second dose of medication was permitted. In the event, all the studies treated established headaches and reported moderate or severe baseline pain intensity, or a mean baseline pain of moderate intensity. None specifically treated early, or when pain was mild. None of the studies allowed a second dose of study medication.
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). We calculated RR for benefit or harm with 95% CIs using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp and NNH with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR for benefit or harm included one.
We planned to use the z test to determine significant differences between NNT, NNTp, and NNH for different groups in subgroup and sensitivity analyses (Tramèr 1997).
We have described data from comparisons and outcomes with only one study or fewer than 200 participants in the text and summary tables where appropriate for information and comparison, but did not carry out any quantitative analysis.
Quality of evidence
We used the GRADE approach to assess the quality of evidence related to each of the key outcomes, and reported our judgement on the quality of the evidence in the 'Summary of findings' table (Chapter 12, Higgins 2011; Appendix 4).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there are so few data that the results are highly susceptible to the random play of chance, or if studies use LOCF imputation in circumstances where there are substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where there were no data reported for an outcome, we reported the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We included a 'Summary of findings' table as set out in the PaPaS author guide (PaPaS 2012; Section 4.6.6, Higgins 2011). The table included outcomes of pain‐free at two hours, pain‐free at other time points (one, four, and 24 hours), pain‐free or mild pain at two hours, use of rescue medication, Participant Global Evaluation at end of study, any adverse event, specific adverse events (where these were reported), and serious adverse events.
For the 'Summary of findings' table, we used the following descriptors for levels of evidence (EPOC 2015).
-
High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low.
-
Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate.
-
Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high.
-
Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high.
† Substantially different: a large enough difference that it might affect a decision.
Subgroup analysis and investigation of heterogeneity
Possible issues for subgroup analysis were dose, formulation, and route of administration. A minimum of two studies and 200 participants have to be available for any sensitivity analysis. In the event, there were too few data for subgroup analysis.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more). A minimum of two studies and 200 participants have to be available for any sensitivity analysis. In the event, there were too few data for sensitivity analysis.
Results
Description of studies
Results of the search
Our searches identified 345 potentially relevant reports in CENTRAL, 653 in MEDLINE, 1595 in Embase, and four in clinical trial registries. After removing duplicates and screening titles and abstracts, we obtained and read 12 full reports of published studies and four clinical trial registry reports. Two of the clinical trial reports related to studies that had been published and identified in our database searches. We included five studies in this review, and excluded eight. See Figure 1. One study appeared to satisfy our inclusion criteria but we were unable to identify any results and the sponsors have not responded to our request for information (NCT01464983).

Study flow diagram.
Included studies
We included five studies (1812 participants took medication, 1668 included in efficacy analyses), all of which enrolled adults with episodic TTH (see Characteristics of included studies table). Four studies specified using IHS diagnostic criteria (Gatoulis 2012; Göbel 2001; Martínez‐Martín 2001; Steiner 2003), and one predated commonly recognised criteria, but described comparable characteristics and specifically excluded migraine (Peters 1983). All participants treated headaches of at least moderate pain intensity.
Participants were aged 18 to 66 years (mean about 40 years, where reported), and there were more women than men (60% to 80% in individual studies). Studies did not report baseline frequency of headache, but it is likely (from inclusion criteria) that the majority of participants were experiencing frequent episodic TTH (2 to 14 headache days/month). Studies typically excluded participants who had a contraindication to one of the study medications or who were regularly taking medication that could influence the study results (e.g. analgesics, tranquillisers, or muscle relaxants). People who experienced migraines were either excluded or required to be able to distinguish between TTH and migraine. One study specified that participants should have been previously responsive to aspirin or paracetamol (Peters 1983).
Four studies used a parallel design (Gatoulis 2012; Martínez‐Martín 2001; Peters 1983; Steiner 2003), and one used a cross‐over design (Göbel 2001).
While the included studies reported outcomes described in their methods, they did not consistently report outcomes of interest for this review. None of the studies reported pain‐free at any time point. None of the studies reported pain‐free or mild pain, but one study reported an equivalent measure at two hours (Steiner 2003). Two studies reported a Patient Global Evaluation (PGE) of 'good' or better (Martínez‐Martín 2001; Steiner 2003). Although this was not a prespecified outcome, it is widely recognised, and we chose to review it in view of the paucity of other data. Three studies reported on some measure of pain intensity or PID at two hours, and the use of rescue medication (Gatoulis 2012; Martínez‐Martín 2001; Steiner 2003). In addition, all studies provided some information on adverse events, serious adverse events, and adverse event withdrawals.
Four studies used aspirin 1000 mg (Gatoulis 2012; Göbel 2001; Martínez‐Martín 2001; Steiner 2003), one study used aspirin 650 mg (Peters 1983), and one study used aspirin 500 mg (Steiner 2003). All studies used the oral route of administration, included a placebo comparator, and made no mention of the particular formulation used, except for Gatoulis 2012 which specified Extra Strength Bayer (aspirin) and Tylenol (paracetamol). It seems likely that the studies all used a standard tablet formulation.
Active comparators were:
-
paracetamol 500 mg (Steiner 2003) and paracetamol 1000 mg (Peters 1983; Steiner 2003);
-
paracetamol 300 mg plus codeine 30 mg (Gatoulis 2012);
-
metamizole 500 mg and 1000 mg (Martínez‐Martín 2001);
-
peppermint oil (oleum menthae piperitae) solution Ll 170, 10 g (Göbel 2001).
There were no studies comparing a combination of aspirin and another active oral treatment with the non‐aspirin component alone.
Since outcomes of interest were so poorly reported and because many participants received active comparators, the number of participants with data for analyses for aspirin was very much smaller than the total number of participants who took study medication. For subgroup analysis of dose, we considered two groups: aspirin 1000 mg, and aspirin 500 mg or 650 mg. There were no data for subgroup analysis by formulation or route of administration.
Excluded studies
We excluded seven published studies, five because they included participants who experienced headaches on 15 or more days per month or participants with mixed headache types (or both); one because it tested the effect of adding a tranquilliser to aspirin; and one because it did not clearly state that it was randomised. We also excluded one clinical trial report because the trial was terminated early and had enrolled only nine participants (see Characteristics of excluded studies table).
Risk of bias in included studies
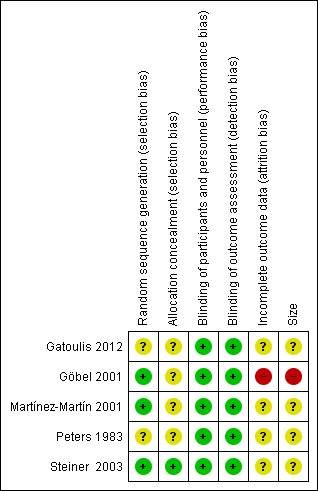
Figure 2 provides a summary of the risk of bias assessment for each study.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were randomised, but only three adequately described the method used to generate the random sequence (Göbel 2001; Martínez‐Martín 2001; Steiner 2003). Only one study described the method used to conceal the random allocation (Steiner 2003).
Blinding
All studies were double‐blind and adequately described the methods used to conceal the intervention from participants and study personnel.
Incomplete outcome data
We judged four studies at unknown risk of bias due to lack of information. Gatoulis 2012 had a large number of withdrawals (24% to 32%), which were mostly due to lack of qualifying headache and non‐compliance with study protocol. Martínez‐Martín 2001 did not explain how data from two consecutive attacks were analysed for some outcomes. Peters 1983 may have randomised participants who did not complete, and of those who did, there was attrition greater than 10% due to protocol violations, with no mention of discontinuations by headache type, or of imputation. Steiner 2003 reported an intention‐to‐treat analysis with LOCF imputation. We considered one study at high risk of bias; Göbel 2001 reported only on participants who completed all four treatment phases, and did not provide any information about participants who did not complete.
Other potential sources of bias
We judged one study at high risk of bias due to small size (Göbel 2001; 44 participants per treatment group). The remaining studies had group sizes between 50 and 199 and we judged them at unknown risk of bias.
Effects of interventions
Results for individual studies are presented in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals).
Aspirin 1000 mg and 500 mg or 650 mg versus placebo
Pain‐free at two hours
No studies reported the outcome of pain‐free at two hours.
Pain‐free at different time points
No studies reported the outcome of pain‐free at any other time point.
Pain‐free or mild pain at two hours
Steiner 2003 reported the proportion of participants who recorded 'total relief' or 'worthwhile effect' at two hours, which we considered equivalent to pain‐free or mild pain at two hours; 78/111 with aspirin 500 mg, 78/103 with aspirin 1000 mg, and 61/112 with placebo (from graph).
No other study reported this, or an equivalent, outcome.
Pain Intensity Difference (PID) and Sum of Pain Intensity Difference (SPID) at two hours
Four studies reported an outcome related to group mean pain intensity and differences between groups. Only Steiner 2003 provided information about imputation for missing data (in this case, LOCF). Studies used scales to measure pain intensity and reported different outcomes, so that no pooled analysis was possible.
Gatoulis 2012 measured pain intensity on a categorical scale (0 = no pain, 3 = severe pain) and presented the data for PID at two hours in graphical form. We estimated scores of 1.3 for aspirin 1000 mg, 1.35 for paracetamol 300 mg plus codeine 30 mg, and 0.8 for placebo.
Martínez‐Martín 2001 measured pain intensity on a 100 mm visual analogue scale (0 = no pain, 100 = unbearable pain) and reported group mean intensities at two hours, using a scale of 0 to 10, of 3.1 for aspirin 1000 mg, 2.6 for metamizole 500 mg, 2.7 for metamizole 1000 mg, and 3.7 for placebo.
Peters 1983 measured pain intensity on a categorical scale (0 = no pain, 2 = moderate pain) and presented the data for SPID at two hours in graphical form. We estimated scores of 3.2 for aspirin 650 mg, 3.2 for paracetamol 1000 mg, and 2.0 for placebo.
Steiner 2003 measured pain intensity on a 100 mm visual analogue scale (0 = no pain, 100 = severe pain) and presented the data for PID at two hours in graphical form, using a scale of 0 to 10. We estimated scores of 3.6 for aspirin 500 mg, 3.9 for aspirin 1000 mg, 3.0 for paracetamol 500 mg, 3.5 for paracetamol 1000 mg, and 2.7 for placebo.
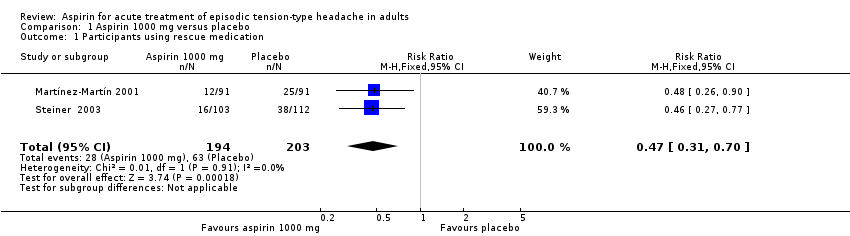
Use of rescue medication
Two studies (397 participants) provided information on the number of participants who used rescue medication (Martínez‐Martín 2001; Steiner 2003). Both studies had a duration of four hours and allowed use of rescue medication if adequate relief was not obtained two hours after treatment. We used data from the first episode treated in Martínez‐Martín 2001.
-
The proportion of participants who used rescue medication with aspirin 1000 mg was 14% (28/194, range 13% to 16%).
-
The proportion of participants who used rescue medication with placebo was 31% (63/203, range 27% to 34%).
-
The RR for aspirin 1000 mg compared with placebo was 0.5 (95% CI 0.3 to 0.7); the NNTp was 6.0 (95% CI 4.1 to 12) (Analysis 1.1).
We judged the quality of this evidence as low, downgraded due to the small number of studies (moderate number of participants).
Only Steiner 2003 reported this outcome for aspirin 500 mg: 18/111 participants used rescue medication following treatment with aspirin 500 mg and 38/112 with placebo.
Patient Global Evaluation (PGE) at the end of the study
Two studies (397 participants) reported a PGE at the end of the study. This was not a prespecified outcome in this review, but it is frequently used as a measure of overall efficacy and tolerability in pain studies, where the top two categories (usually 'good' and 'excellent' on a 5‐point scale) are considered equivalent to 'moderate response' (Dworkin 2008). We chose to analyse this outcome because of the lack of other information in these studies and have included it here as a post‐hoc analysis. Martínez‐Martín 2001 reported at four hours and Steiner 2003 at 24 hours. We combined the top two categories (4‐point‐scale: satisfactory, good (Martínez‐Martín 2001), and 5‐point scale: good, very good (Steiner 2003)) from both studies to determine the number of participants who were 'satisfied' with treatment.
-
The proportion of participants who were 'satisfied' at the end of the study with aspirin 1000 mg was 55% (106/194, range 52% to 57%).
-
The proportion of participants who were 'satisfied' at the end of the study with placebo was 37% (75/203, range 29% to 47%).
-
The RR for aspirin 1000 mg compared with placebo was 1.5 (95% CI 1.2 to 1.8); the NNT was 5.7 (95% CI 3.7 to 12) (Analysis 1.2).
We judged the quality of this evidence as low due to the small number of studies (moderate number of participants), differences in scales and timing for the outcome, and post‐hoc nature of the analysis.
Only Steiner 2003 reported this outcome for aspirin 500 mg: 47/111 participants were 'satisfied' with treatment with aspirin 500 mg, and 32/112 with placebo.
Adverse events
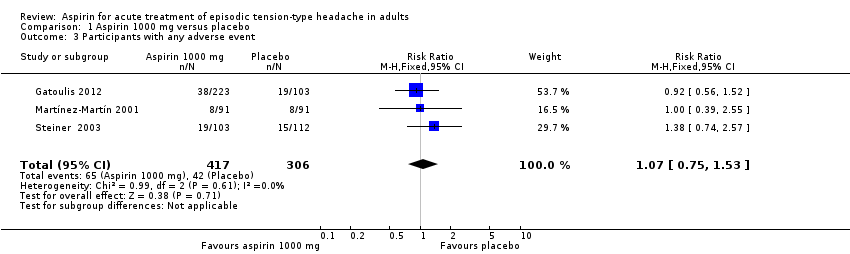
Any adverse event
Three studies (723 participants) contributed data for this analysis for aspirin 1000 mg (Gatoulis 2012; Martínez‐Martín 2001; Steiner 2003). Gatoulis 2012 and Martínez‐Martín 2001 collected data for four hours, and Steiner 2003 for 24 hours after taking study medication. Since there was no obvious difference between rates over the different time periods, we combined the data for analysis.
-
The proportion of participants who experienced any adverse event with aspirin 1000 mg was 16% (65/417; range 9% to 18%).
-
The proportion of participants who experienced any adverse event with placebo was 14% (42/306; range 9% to 18%).
-
The RR for aspirin 1000 mg compared with placebo was 1.1 (95% CI 0.8 to 1.5); the NNH was not calculated (Analysis 1.3).
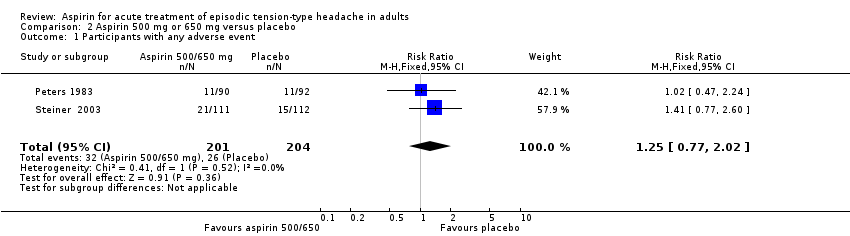
Two studies (405 participants) contributed data for this analysis for aspirin 500 mg or 650 mg (Peters 1983; Steiner 2003). Peters 1983 collected data for six hours, and Steiner 2003 for 24 hours after taking study medication. Since there was no obvious difference between rates over the different time periods, we combined the data for analysis.
-
The proportion of participants who experienced any adverse event with aspirin 500 mg or 650 mg was 16% (32/201; range 12% to 19%).
-
The proportion of participants who experienced any adverse event with placebo was 13% (26/204; range 12% to 13%).
-
The RR for aspirin 500 mg or 650 mg compared with placebo was 1.3 (95% CI 0.8 to 2.0); the NNH was not calculated (Analysis 2.1).
We judged the quality of this evidence as low, downgraded due to the small number of studies and events.
Göbel 2001 reported that adverse events were not different between aspirin and placebo.
Specific adverse events
Individual adverse events were not consistently reported, and no analysis was possible. Specific events that were reported included gastrointestinal upset or dyspepsia, nausea, dizziness, and somnolence.
Serious adverse events
There were no serious adverse events in any of the studies.
Withdrawals due to adverse events
There were no withdrawals due to adverse events in any of the studies.
Aspirin versus active comparators
There were insufficient data for any analysis of aspirin versus an active comparator. Individual studies reported no significant differences between aspirin and paracetamol alone, paracetamol plus codeine, peppermint oil, or metamizole, at any of the doses tested.
Discussion
Summary of main results
Our searches identified only five studies that satisfied our inclusion criteria, with 767 participants included in comparisons of aspirin 1000 mg with placebo, and 405 participants in comparisons of aspirin 500 mg or 650 mg with placebo. It is likely that the studies all used a standard tablet formulation. Participants had moderate or severe pain at the start of treatment, and took just one treatment dose per headache episode.
The primary outcome of this review was pain‐free at two hours using any standard method of pain assessment and without the use of rescue medication, reflecting the updated guidelines for controlled trials of drugs in TTH issued by the IHS (IHS 2010). None of the studies reported our primary outcome, or pain‐free at any other time point. Steiner 2003 reported the number of participants who experienced 'total relief' and 'worthwhile effect' at two hours, an outcome that we considered equivalent to the secondary outcome of no or mild pain at two hours. This was reported by 78/111 participants with aspirin 500 mg, 78/103 with aspirin 1000 mg, and 61/112 with placebo (very low quality evidence).
Four studies reported an outcome related to group mean pain intensity and differences between groups (Gatoulis 2012; Martínez‐Martín 2001; Peters 1983; Steiner 2003), but they used scales to measure pain intensity and reported different outcomes, so that no pooled analysis was possible. Only one of the studies reported on how missing data were handled (Steiner 2003, used LOCF). Numerically better results were reported for aspirin 500 mg, 650 mg, and 1000 mg than for placebo (very low quality evidence).
Fewer participants used rescue medication with aspirin 1000 mg than with placebo, giving an NNTp of 6.0 (95% CI 4.1 to 12) (2 studies, 397 participants, low quality evidence).
Two studies (397 participants) provided dichotomous data for PGE of treatment at the end of the study. We considered the top two categories ('satisfied' with treatment) to be equivalent to 'moderate response' (Dworkin 2008). Comparing aspirin 1000 mg with placebo, the RR for being satisfied with treatment was 1.5 (95% CI 1.2 to 1.8), and the NNT was 5.7 (95% CI 3.7 to 12). Given the sparse data, slightly different scales used to measure the outcome, different time points at which it was measured, and post‐hoc nature of the analysis, these results should be regarded as exploratory and interpreted with caution. For these reasons, we judged the quality of the evidence as very low.
There was no significant difference between aspirin 1000 mg and placebo for the number of participants experiencing any adverse event (3 studies, 723 participants, low quality evidence), and there were no serious adverse events in any of the studies (5 studies, 1060 participants in comparisons of aspirin 500 mg, 650 mg, and 1000 mg with placebo, very low quality evidence). Specific adverse events were inconsistently reported and there were too few events for analysis. They included gastrointestinal upset or dyspepsia, nausea, dizziness, and somnolence (very low quality evidence). These adverse event results for single doses cannot be extrapolated to more frequent use of aspirin.
There were no studies that compared a combination of aspirin and another active oral treatment with the non‐aspirin component alone, and there were insufficient efficacy data to compare aspirin with any active comparator.
Overall completeness and applicability of evidence
IHS recommendations regarding outcomes of headache trials are well regarded (IHS 2010), and often, if not always, followed (Bendtsen 2010; Moore 2014). Studies included in this review largely predated those recommendations and were inconsistent in reporting them, which limited the ability to draw useful conclusions about the efficacy of aspirin compared with placebo or active comparators.
Our prespecified outcomes were poorly reported and there were sufficient data for efficacy analysis of only the 1000 mg dose. Consistent reporting of clinically useful outcomes, more information on different dosages, particularly the lower dose in combination with another agent, and on fast‐acting formulations, would improve our understanding of the role of aspirin in TTH.
All studies required participants to have frequent episodic TTH; this is defined as anywhere between 2 and 14 headache days per month. None of the included studies provided information on the mean number of headaches experienced by participants before study entry. We do not know whether the participants in the other studies were typically experiencing 2 to 5, or 10 to 14 headaches per month. This might influence the efficacy of treatments tested in these TTH studies, but we do not know because the information was missing. Neither do we know if these results are applicable to people with infrequent episodic TTH (one headache day per month or less), which may represent a large proportion of people experiencing this type of headache who do not consult their doctors or need medical management, but who use simple analgesics for pain relief.
The overwhelming majority of participants in the included studies had moderate or severe baseline pain. There is good reason for this in clinical trials, because it gives sensitivity to demonstrate a reduction in pain. However, the pain of TTH is usually described as being of mild to moderate intensity (IHS 2010), so the participants in these trials may represent a population with headaches that are more severe and possibly more difficult to treat than is the norm.
To understand these important methodological points, analysis of clinical trials at the level of the individual participant is required, using substantial amounts of data. Such an analysis seems unlikely at present, but would probably be highly informative for the development of existing IHS guidance (Bendtsen 2010).
Quality of the evidence
All included studies were both randomised and double‐blind; none contributing data for analysis were considered at high risk of bias for study conduct. Inconsistent reporting of outcomes; the small number of studies and lack of data for different doses and for active comparators; and the small size of some studies were the major problems that severely limited analyses and downgraded our assessments of the quality of the results. The direction of results was consistent within efficacy analyses.
We judged the overall quality of the evidence as low to very low.
We did not assess selective reporting bias because clinical trials in TTH have until recently had no framework and so are inconsistent in outcomes used (Moore 2014). There is no indication of how selective reporting might create bias in this situation.
Potential biases in the review process
Martínez‐Martín 2001 treated two headache episodes with single doses of the same medication, but it was not clear how the data were combined for some outcomes; for use of rescue medication, we were able to use first‐episode data only. It is unclear how this might have affected the PGE of treatment and adverse event outcomes. Göbel 2001 used a cross‐over study design and reported only on participants who completed all four periods of treatment, but did not contribute to any pooled analyses.
We carried out extensive searches to identify relevant studies, and consulted widely and internationally for an earlier review (Moore 2014). We think it unlikely that there is a substantial number of studies that we have missed or are unpublished. However, with so few data, the existence of only one or two additional trials could change the results, so the estimates from this review must be interpreted with caution.
Agreements and disagreements with other studies or reviews
These results are broadly in agreement with previous reviews that concluded that ibuprofen, paracetamol, and ketoprofen were better than placebo (Moore 2014; Verhagen 2006), as well as the guideline from the EFNS, which recommends ibuprofen as drug of choice among non‐steroidal anti‐inflammatory drugs or paracetamol or aspirin for acute treatment of TTH (Bendtsen 2010). That guideline was not based on a systematic review. The German evidence‐based recommendations for self‐medication of migraine and TTH were based on systematic reviews (Haag 2011), and included only seven studies that recruited at least some people with TTH. For self‐medication of TTH, it recommended paracetamol in combination with other analgesics or caffeine, but not paracetamol alone.

Study flow diagram.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Aspirin 1000 mg versus placebo, Outcome 1 Participants using rescue medication.

Comparison 1 Aspirin 1000 mg versus placebo, Outcome 2 Participant global evaluation: 'satisfied'.
Comparison 1 Aspirin 1000 mg versus placebo, Outcome 3 Participants with any adverse event.
Comparison 2 Aspirin 500 mg or 650 mg versus placebo, Outcome 1 Participants with any adverse event.
| Aspirin 1000 mg compared with placebo for episodic tension‐type headache | ||||||
| Patient or population: adults with episodic tension‐type headache Settings: community Intervention: aspirin 1000 mg Comparison: placebo | ||||||
| Outcomes | Outcome with | Outcome with | RR NNT, NNTp or NNH (95% CI) | No of studies, participants, events | Quality of the evidence | Comments |
| Pain‐free at 2 hours | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free at other time points (1, 4, 24 hours) | No data | No data | ‐ | ‐ | Very low | No data |
| Pain‐free or mild pain at 2 hours | 61/112 | 78/103 | Not calculated | 1 study, 215 participants, 139 events | Very low | Downgraded three levels due to sparse data: single study with 215 participants in comparison |
| Use of rescue medication | 310 in 1000 | 140 in 1000 | RR 0.47 (0.31 to 0.70) NNTp 6.0 (4.1 to 12) | 2 studies, 397 participants, 91 events | Low | Downgraded two levels due to sparse data: small number of studies, participants and events |
| Patient Global Evaluation at end of study | 370 in 1000 | 550 in 1000 | RR 1.5 (1.2 to 1.8) NNT 5.7 (3.7 to 12) | 2 studies, 397 participants, 181 events | Very low | Downgraded three levels due to sparse data: small number of studies, participants, differences in scales and timing for the outcome, and post‐hoc nature of the analysis |
| Any adverse event | 140 in 1000 | 160 in 1000 | RR 1.1 (0.75 to 1.5) NNH not calculated | 3 studies, 723 participants, 107 events | Low | Downgraded two levels due to sparse data: small number of studies and events |
| Specific adverse events | Inconsistently reported and too few events for analysis Included gastrointestinal upset or dyspepsia, nausea, dizziness, and somnolence | ‐ | 4 studies, 767 participants | Very low | Downgraded three levels due to sparse data: small number of studies, few events, inconsistent reporting | |
| Serious adverse events | No events reported | No events reported | ‐ | 4 studies, 767 participants, no events | Very low | Downgraded three levels due to sparse data: small number of studies and no events |
| CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence (EPOC 2015). High: this research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different† is low. Moderate: this research provides a good indication of the likely effect. The likelihood that the effect will be substantially different† is moderate. Low: this research provides some indication of the likely effect. However, the likelihood that it will be substantially different† is high. Very low: this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different† is very high. † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants using rescue medication Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.31, 0.70] |
| 2 Participant global evaluation: 'satisfied' Show forest plot | 2 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.18, 1.83] |
| 3 Participants with any adverse event Show forest plot | 3 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with any adverse event Show forest plot | 2 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.77, 2.02] |

