Reducción de la dosis de corticosteroides inhalados para pacientes adultos con asma
Resumen
Antecedentes
El asma es una enfermedad de las vías respiratorias que afecta a más de 300 millones de adultos y niños en todo el mundo. Las guías nacionales e internacionales recomiendan el incremento gradual de la dosis de corticosteroides inhalados (CSI) para lograr el control de los síntomas con la menor dosis posible, debido a que la administración a largo plazo de dosis más altas de CSI se asocia con un riesgo de eventos adversos sistémicos. En los pacientes en los que los síntomas de asma se controlan con dosis de CSI moderadas o más altas, puede ser posible reducir la dosis de CSI sin afectar el control de los síntomas.
Objetivos
Evaluar las pruebas de la reducción de dosis del tratamiento con CSI en adultos con asma bien controlada, que reciben una dosis alta a moderada de CSI.
Métodos de búsqueda
Se identificaron ensayos en el registro especializado de ensayos del Grupo Cochrane de Vías Respiratorias (Specialised Register of the Cochrane Airways Group) y se realizó una búsqueda en ClinicalTrials.gov (www.ClinicalTrials.gov) y en el World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). Se buscó en todas las bases de datos desde su inicio, sin restricción de idioma. También se hicieron búsquedas en las listas de referencias de los estudios incluidos y las revisiones relevantes. La búsqueda más reciente se hizo en julio de 2016.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios (ECA) con al menos 12 semanas de duración y se excluyeron los ensayos cruzados. Se analizaron los estudios en adultos (mayores de 18 años de edad) en los que el asma se mantuvo bien controlada por un mínimo de tres meses con al menos una dosis moderada de CSI. Se excluyeron los estudios que reclutaron participantes con cualquier otra comorbilidad respiratoria.
Se incluyeron los ensayos que compararon una reducción en la dosis de CSI versus ningún cambio en la dosis de CSI en pacientes con asma bien controlada que a) un agonista beta de acción prolongada (ABAP; comparación 1), y b) ABAP de forma concomitante (comparación 2).
Obtención y análisis de los datos
Dos autores de la revisión examinaron de forma independiente los resultados de la búsqueda para la inclusión de los estudios, extrajeron los datos sobre los resultados de interés predeterminados y evaluaron el riesgo de sesgo de los estudios incluidos; los desacuerdos se resolvieron mediante discusión con un tercer autor de la revisión. Los datos dicotómicos se analizaron como odds ratios (OR) y se usaron los participantes del estudio como la unidad de análisis. Los datos continuos se analizaron como diferencias de medias (DM). Se utilizó un modelo de efectos aleatorios. Todos los resultados se calificaron mediante el sistema GRADE (Grades of Recommendation, Assessment, Development and Evaluation) y los resultados se presentaron en las tablas "Resumen de los hallazgos".
Resultados principales
Se incluyeron seis estudios que asignaron al azar a un total de 1654 participantes (reducción de la dosis de CSI, sin ABAP concomitante [comparación 1]: n = 892 participantes, tres ECA; reducción de la dosis de CSI, con ABAP concomitante (comparación 2): n = 762 participantes, tres ECA). Todos los estudios incluidos fueron ECA con un diseño paralelo que compararon una dosis fija de CSI versus una reducción del 50% al 60% de la dosis de CSI en participantes adultos con asma bien controlada. La duración del período de tratamiento varió de 12 a 52 semanas (duración media 21 semanas; duración mediana 14 semanas). Dos estudios se realizaron en un contexto de atención primaria, dos se realizaron en un contexto de atención secundaria y dos no proporcionaron información sobre el contexto.
La realización del metanálisis fue obstaculizada por el escaso número de estudios que contribuyeron a cada comparación, además de la heterogeneidad entre los resultados informados en los estudios incluidos. Se encontró que la calidad de las pruebas resumidas fue baja o muy baja en la mayoría de los resultados considerados debido al riesgo de sesgo (principalmente el informe selectivo), la imprecisión y la indireccionalidad. Aunque se encontró que no hubo diferencias estadísticamente significativas o clínicamente relevantes entre los grupos con respecto a cualquiera de los resultados primarios o secundarios considerados en esta revisión, los datos no fueron suficientes para descartar efectos beneficiosos o perjudiciales.
Conclusiones de los autores
La solidez de las pruebas no es suficiente para determinar si la reducción de la dosis de CSI tiene un efecto beneficioso (en términos de menos efectos adversos) o perjudicial (en términos de una reducción en la efectividad del tratamiento) neto en los pacientes adultos con asma bien controlada. El escaso número de estudios relevantes y la variedad de medidas de resultado limitó el número de metanálisis que fue posible realizar. Se necesitan ECA adicionales, bien diseñados y de mayor duración para informar la práctica clínica con respecto al uso de una estrategia de "reducción de la dosis de CSI" en pacientes con asma bien controlada.
PICOs
Resumen en términos sencillos
Reducción de la dosis de corticosteroides inhalados para pacientes adultos con asma
Antecedentes
El asma es una enfermedad de las vías respiratorias que afecta a más de 300 millones de adultos y niños en todo el mundo. Las guías nacionales e internacionales recomiendan el aumento gradual de la dosis de corticosteroides inhalados (CSI) para lograr el control de los síntomas con la menor dosis posible, debido a que la administración a largo plazo de dosis más altas de CSI se asocia con riesgo de efectos secundarios. En los pacientes en los que los síntomas de asma se controlan con dosis moderadas o más altas de CSI, es posible reducir la dosis de CSI (reducción gradual) sin afectar el control de los síntomas del asma.
Pregunta de la revisión
Se buscaron los estudios (mínimo 12 semanas de duración) en pacientes con asma bien controlada que compararon los efectos de reducir la dosis de CSI versus mantener la dosis de CSI. Los estudios tenían que incluir adultos con 18 años de edad o más con asma bien controlada con una dosis media de CSI durante un mínimo de tres meses. En la presente revisión hubo interés en determinar si la administración de otro tipo de fármaco inhalado para el asma (agonistas beta de acción prolongada [ABAP]) podría influir en los resultados. Dos autores de la revisión examinaron los resultados de la búsqueda de forma independiente y determinaron los estudios relevantes para inclusión en esta revisión. La información relevante de estos estudios también fue agregada a esta revisión por dos autores de la revisión de forma independiente.
Resultados
Se encontraron seis estudios que fueron relevantes para la revisión. En general, no se encontraron diferencias entre los grupos (dosis reducida de CSI versus dosis mantenida de CSI) en cuanto a los ataques de asma, el control del asma, la calidad de vida o los efectos adversos. La administración o no de ABAP al mismo tiempo no pareció afectar los resultados. Sin embargo, la calidad de las pruebas se consideró baja o muy baja debido al escaso número de estudios encontrados y los problemas de informe de los estudios. Este hecho significa que no existe seguridad en los hallazgos; se necesitan estudios adicionales para analizar este tema.
Conclusiones
Se concluye que las pruebas actuales no son suficientes para determinar si los pacientes pueden reducir la dosis de CSI sin que se afecte el control de los síntomas del asma. Tampoco está claro si la reducción de la dosis de CSI podría reducir la ocurrencia de efectos secundarios. Se necesitan estudios adicionales para responder a esta pregunta.
Conclusiones de los autores
Summary of findings
| ICS dose reduction compared with no change in ICS dose (no concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (no concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 8 per 1000 | 14 per 1000 | OR 1.86 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Asthma control | Mean asthma control score in the no change in ICS dose group was 1.79. | MD 0.22 lower | ‐ | 150 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| All‐cause SAEs | 8 per 1000 | 9 per 1000 | OR 1.24 | 742 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Steroid‐related AEs | 31 per 1000 | 23 per 1000 | OR 0.76 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Health‐related quality of life (change from baseline) | Mean change from baseline in health‐related quality of life for the no change in ICS dose group was 0.02. | MD 0.21 lower | ‐ | 554 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence); MCID is 0.5 for AQLQ |
| Lung function, FEV1 (L) | Mean FEV1 in the no change in ICS dose group was 3.15 litres. | MD 0.02 litres lower | ‐ | 261 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Exacerbations requiring hospitalisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported by included studies |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). dThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (representative of specialist centres) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded twice for risk of bias (selective reporting and lack of blinding (subjective outcome)) and once for indirectness (single study representative of one setting and drug regimen). fThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ICS dose reduction compared with no change in ICS dose (concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 148 per 1000 | 186 per 1000 | OR 1.31 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring OCS (low‐quality evidence) |
| Asthma control (short asthma morbidity score) | Mean asthma control score was 1.43. | MD 0.16 higher | ‐ | 242 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to asthma control (low‐quality evidence) |
| All‐cause SAEs | 35 per 1000 | 22 per 1000 | OR 0.60 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to all‐cause SAEs (low‐quality evidence) |
| Steroid‐related AEs ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| St. George's Respiratory Scale score (change from baseline) Score 0‐100. 100 = greatest impact of chest disease on life; MCID is 4 units. | Mean change from baseline in HRQoL score was 7.4.c | MD 0.13 higher | ‐ | 229 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to HRQoL (low‐quality evidence) |
| Exacerbation requiring hospitalisation | 4 per 1000 | 14 per 1000 | OR 4.06 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring hospitalisation (low‐quality evidence) |
| Lung function, reduction in FEV1 (% predicted, change from baseline) | Mean change from baseline in % predicted FEV1 was ‐0.75%. | MD 2.45 lower | ‐ | 14 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to lung function (very low‐quality evidence) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cNote that study authors reported the change to the lowest SGRQ score during follow‐up. dThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (single study representative of one setting or drug regimen) and once for imprecision (wide CI). AE, adverse event; CI, confidence interval; FEV1, forced expiratory volume in one second; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; HRQoL, health‐related quality of life; ICS, inhaled corticosteroid; LABA, long‐acting beta agonist; MCID, minimum clinically important difference; MD, mean difference; OCS, oral corticosteroid; OR, odds ratio; RCT, randomised controlled trial; RR, risk ratio; SAE, serious adverse event. | ||||||
| GRADE Working Group grades of evidence | ||||||
Antecedentes
Descripción de la afección
El asma es una afección de las vías respiratorias que afecta a adultos y niños. El número de diagnósticos en todo el mundo se calcula en más de 300 000 000 (Global Asthma Network 2014; Partridge 2006). Durante los ataques de asma (exacerbaciones) se produce un estrechamiento de las vías respiratorias y un aumento en la producción de moco, lo que provoca síntomas de opresión torácica, sibilancias y disnea. Las pruebas de función pulmonar habitualmente muestran una obstrucción en el flujo aéreo con una tasa de flujo espiratorio máximo (TFEM) baja, un volumen espiratorio forzado en un segundo (VEF1) bajo y un cociente VEF1/capacidad vital forzada (CVF) bajo (SIGN/BTS 2016). Con el tratamiento, las anomalías de la función pulmonar mejoran y la funcionalidad puede normalizarse. La variabilidad en las medidas del flujo aéreo es el sello característico del asma.
Las exacerbaciones del asma se pueden desencadenar por los estímulos ambientales. En el asma mediada por la inmunoglobulina E (IgE) (que representa la mitad de los casos de asma) (Pearce 1999), generalmente están implicados los alérgenos inhalados en el domicilio como los ácaros del polvo, los gatos y los perros domésticos (Custovic 2012). Otros estímulos ambientales reconocidos incluyen los contaminantes del aire como el ozono y las partículas finas, la exposición activa y pasiva al tabaquismo (Xepapadaki 2009), los químicos industriales como los ftalatos (Jaakkola 2008), los isocianatos (Fisseler‐Eckhoff 2011), las infecciones virales y el aire frío.
Descripción de la intervención
En los episodios agudos de asma, se administran tratamientos para aliviar los síntomas, habitualmente agonistas beta2 de acción corta (ABAC). Los corticosteroides inhalados (CSI) se utilizan ampliamente como tratamiento de primera línea en los pacientes con asma que no se controla con los tratamientos para aliviar los síntomas solos (SIGN/BTS 2016). Los corticosteroides inhalados que alivian los síntomas de forma efectiva y evitan las exacerbaciones del asma (Adams 2005; Adams 2008), son preferibles para el tratamiento por vía oral, ya que así hay una menor absorción sistémica y muy pocos efectos secundarios. Sin embargo, en algunos países de recursos bajos y medios los factores económicos y sociales pueden contribuir a la falta de cumplimiento con los tratamientos con inhaladores (GINA 2016). Está disponible una variedad de dispositivos para la administración de los CSI a diferentes dosis y tamaños de partículas. Generalmente los CSI se administran dos veces al día, aunque algunas preparaciones más nuevas se administran una vez al día. En los pacientes con asma persistente, con frecuencia los CSI se administran junto con un agonista beta2 de acción prolongada (ABAP), en ocasiones mediante un inhalador combinado. Los CSI se deben comenzar a una dosis apropiada según la gravedad y el control de la enfermedad. Las guías nacionales e internacionales recomiendan ajustar la dosis de CSI mediante un aumento gradual hasta lograr el control de los síntomas a la menor dosis posible. La administración a largo plazo de dosis más altas de CSI tiene el riesgo de causar eventos adversos sistémicos (es decir, efectos secundarios provocados por la acción de los corticosteroides en sitios diferentes a las vías respiratorias) (Lipworth 1999); sin embargo, las dosis más bajas de 800 mcg por día de dipropionato de beclometasona se consideran tolerables (SIGN/BTS 2016). En los pacientes en los que los síntomas de asma se controlan con dosis moderadas o más altas de CSI, puede ser posible reducir la dosis de CSI sin afectar el control de los síntomas (Hawkins 2003).
De qué manera podría funcionar la intervención
Los CSI ofrecen un tratamiento efectivo del asma debido a sus efectos antiinflamatorios y descongestionantes sobre las vías respiratorias (Tse 1984). Los ABAP funcionan al disminuir la hiperreactividad bronquial a los estímulos físicos y químicos y al relajar el músculo liso bronquial (Lipworth 1992). Las guías para el tratamiento del asma se centran en lograr, y luego mantener, el control a la vez que se equilibran los riesgos asociados con la medicación a largo plazo (Bateman 2008). Una vez que se logra el control del asma (es decir, según los criterios GINA 2016) las guías recomiendan la "reducción de la dosis" del tratamiento hasta la menor dosis posible de CSI (SIGN/BTS 2016). Estas recomendaciones se basan en los riesgos conocidos de efectos adversos sistémicos (p.ej. pérdida de la densidad ósea en los adultos, retardo del crecimiento en los niños) asociados con la administración a largo plazo de dosis altas de CSI (Colice 2006; Lipworth 1999; SIGN/BTS 2016).
Por qué es importante realizar esta revisión
Los pacientes con asma persistente generalmente son tratados con dosis altas de CSI o con una combinación de CSI y ABAP (Ducharme 2010). Dos revisiones Cochrane separadas (Ahmad 2015; Kew 2015) resumieron las pruebas de eliminar el ABAP de la combinación de CSI/ABAP cuando se tratan niños y adultos con asma. La reducción de la dosis de CSI puede disminuir la probabilidad de efectos secundarios no deseados, particularmente los efectos secundarios sistémicos del uso de corticosteroides (Colice 2006; SIGN/BTS 2016). De hecho, las guías de la British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN) recomiendan que los CSI se deben ajustar a la menor dosis posible a la que se mantenga un control efectivo del asma (SIGN/BTS 2016). Sin embargo, se mantiene el debate con respecto al mejor protocolo de reducción de la dosis del tratamiento con CSI, en particular con respecto a la menor dosis posible de CSI y la tasa de reducción gradual de ajuste de la dosis (Rogers 2012). Por lo tanto, es importante resumir las pruebas sobre el "tratamiento de reducción de los CSI". Finalmente, los CSI están entre los fármacos repetidos prescritos con mayor frecuencia, por lo que representan una proporción significativa de los gastos farmacéuticos en el Reino Unido y en otros países (NHS 2013). Por lo tanto, cualquier estrategia para reducir el uso de los CSI puede significar una importante medida de ahorro de costos.
Objetivos
Evaluar las pruebas de la reducción de dosis del tratamiento con CSI en adultos con asma bien controlada, que reciben una dosis alta a moderada de CSI.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron ensayos controlados aleatorios (ECA) de grupos paralelos, de al menos 12 semanas de duración. Se incluyeron los estudios informados como texto completo, los publicados como resumen solamente y los datos no publicados. No se excluyeron los estudios por el idioma o el cegamiento.
Tipos de participantes
Se incluyeron adultos (≥ 18 años de edad) con asma bien controlada durante un mínimo de tres meses con al menos una dosis moderada de CSI (es decir, una dosis de al menos 400 mcg de dipropionato de beclometasona [DPB] o equivalente) (SIGN/BTS 2016). El control del asma se clasificó según criterios predeterminados, por ejemplo, según los criterios descritos en GINA 2016 (es decir, síntomas diarios dos veces o con menos frecuencia por semana, uso de inhalador de rescate dos veces o con menos frecuencia por semana, sin síntomas nocturnos o sin limitación para las actividades cotidianas) o según el cuestionario de control del asma (es decir, una puntuación menor de 1,5). Se excluyeron los pacientes con las siguientes comorbilidades / características: enfermedad pulmonar obstructiva crónica (EPOC), bronquiectasia o cualquier otra comorbilidad respiratoria.
Si los estudios incorporaron adultos y adolescentes (de diez a 17 años de edad) (WHO 2014), y los datos no se informaron por separado, el estudio se incluyó si la media de edad de los participantes de los grupos de intervención y de comparación fue 18 años o más.
Tipos de intervenciones
Se incluyeron los ensayos que compararon las siguientes intervenciones.
-
Reducción en la dosis de CSI versus ningún cambio en la dosis de CSI, en pacientes con asma bien controlada con al menos una dosis moderada de cualquier CSI, pero que un ABAP concomitante.
-
Reducción en la dosis de CSI versus ningún cambio en la dosis de CSI, en pacientes con asma bien controlada con al menos una dosis moderada de cualquier CSI, pero que un ABAP concomitante.
En ambas comparaciones se podía haber utilizado un CSI diferente en los grupos de intervención y de comparación, siempre que ambos grupos utilizaran la misma dosis de CSI equivalente al dipropionato de beclometasona (DPB) (≥ 400 mcg) antes de la asignación al azar. Se excluyeron los estudios en los que se interrumpió el tratamiento con CSI, ya que este caso se relaciona con una pregunta clínica diferente. Se incluyeron los estudios que permitieron la administración de fármacos de acción corta para aliviar los síntomas, siempre que no formaran parte del tratamiento asignado al azar.
Para esta última comparación (pacientes que recibieron un ABAP concomitante), varios estudios incluyeron participantes que utilizaron inhaladores combinados (CSI/ABAP); se excluyeron los estudios en que el tratamiento asignado al azar incluyó una reducción simultánea de la dosis del CSI y el ABAP debido a que esta estrategia está relacionada con una pregunta clínica diferente. También se excluyeron los estudios en los que el tratamiento asignado al azar incluyó una reducción gradual hasta el tratamiento con inhalador solo (es decir, "tratamiento de mantenimiento y alivio de los síntomas con inhalador solo" [en inglés, SMART]) con una dosis más baja de CSI porque también se relaciona con una pregunta clínica diferente que se analizó en otra revisión (Kew 2013).
Tipos de medida de resultado
Resultados primarios
-
Exacerbaciones que requieren corticosteroides orales
-
Control del asma (medido con una escala validada; la medida de preferencia es la puntuación del Asthma Control Questionnaire [ACQ])
-
Eventos adversos graves por todas las causas
-
Eventos adversos relacionados con los corticosteroides
Resultados secundarios
-
Calidad de vida relacionada con la salud (medida con una escala validada; la medida de preferencia es la puntuación del Asthma Quality of Life Questionnaire [AQLQ])
-
Índices de función pulmonar (la medida de preferencia es VEF1)
-
Exacerbaciones que requieren hospitalización
-
Exacerbaciones que requieren consulta en el servicio de urgencias
-
Mortalidad
El informe de uno o más de estos resultados en un ensayo no fue un criterio de inclusión.
Results
Description of studies
The Characteristics of included studies table presents details of the included studies. We reported in the Characteristics of excluded studies table reasons for exclusion of studies considered during review of full‐text articles.
Results of the search
We identified 983 records by performing electronic searches of bibliographic databases and an additional 53 records by searching clinicaltrials.gov. Of a total of 1034 records (two duplicates removed), we excluded most (n = 972) upon screening titles and abstracts. We examined full‐text articles of the remaining 62 records and excluded 53 records (reporting 41 studies), primarily because the intervention did not meet the criteria for inclusion in this review (n = 27 studies). The interventions considered were typically complex and difficult to separate from other components; this resulted in a high rate of exclusions at full‐text review stage. Other reasons for exclusion at this stage included 'wrong study design' (n = 8), 'wrong comparator' (n = 3), 'wrong route of administration' (n = 2) and 'wrong patient population' (n = 1). The remaining 11 records reported the findings of six studies, which we included in this review. Figure 1 depicts the flow of information through the different stages of this systematic review.
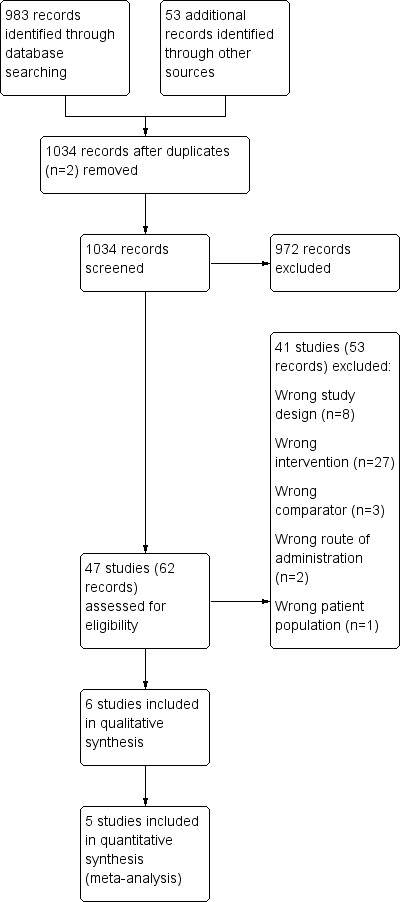
Study flow diagram.
Included studies
Six studies met the inclusion criteria and contributed data to the analyses (Godard 2008; Gunn 1997; Hawkins 2003; Juniper 1991; Knox 2007; Magnussen 2000). The six included studies randomised a total of 1654 participants (ICS dose reduction, no concomitant LABA: n = 892; ICS dose reduction, concomitant LABA: n = 762). The largest (Gunn 1997) and smallest (Juniper 1991) studies included 631 and 28 participants, respectively. All included studies were reported as full peer‐reviewed articles.
Methods
All included studies were RCTs with a parallel design that compared a fixed dose of ICS versus a reduced dose of ICS. Two studies included three arms (Godard 2008; Gunn 1997), and in one of these, only two out of three arms were relevant to this review (Godard 2008). Five studies were performed as double‐blind, and one study was open‐label (Gunn 1997). Five studies reported a run‐in period (duration two to eight weeks), and one study was an extension of a previous 12‐month study (Juniper 1991). Duration of the treatment period ranged from 12 to 52 weeks (mean duration 21 weeks; median duration 14 weeks). Outcome data were reported at the last time point reported for each study. Most studies were performed in Europe (Godard 2008; Gunn 1997; Hawkins 2003; Knox 2007; Magnussen 2000), and one study in Canada (Juniper 1991). Two studies were conducted in the setting of primary care (Gunn 1997; Hawkins 2003), two were conducted in the secondary care setting (Juniper 1991; Magnussen 2000) and two reported no information on setting (Godard 2008; Knox 2007).
Participants
We included studies that recruited adult participants aged ≥ 18 years or in which most participants were adults. When reported, the age range of participants across included studies was 16.2 to 86 years (Godard 2008; Gunn 1997; Hawkins 2003; Knox 2007); in the two studies for which the age range was not reported, the mean age of participants was approximately 40 years (Juniper 1991; Magnussen 2000). Participants in the included studies had asthma that was generally well controlled by regular preventive therapy (i.e. step 2 of the BTS/SIGN guidelines; SIGN/BTS 2016) (Gunn 1997; Juniper 1991; Knox 2007; Magnussen 2000), with the use of high‐dose ICS (≥ 1000 μg BDP) (Hawkins 2003) or with an add‐on therapy (i.e. step 3 of the BTS/SIGN guidelines) (Godard 2008). When reported, most participants were non‐smokers (Godard 2008; Hawkins 2003; Knox 2007).
Interventions
All included studies compared a 50% to 60% reduction in dose of ICS versus no change in ICS dose. In terms of the type and baseline dose of ICS, studies included a variety of comparisons: fluticasone propionate (FP) 250 μg twice daily versus ciclesonide 160 μg once daily (representing a 50% reduction according to Global Initiative for Asthma (GINA) guidelines) (Knox 2007); a 50% reduction in dose of any ICS (as used before the study) (Hawkins 2003); salmeterol/fluticasone combination (SFC) 50/100 μg twice daily versus no change (SFC 50/250 μg twice daily) (Godard 2008); a 50% reduction in dose of budesonide versus no change in budesonide dose (any dose) (Juniper 1991); and chlorofluorocarbon beclomethasone 1000 μg/day versus hydrofluoroalkane beclomethasone 400 μg/day (< 50% reduction) (Magnussen 2000). The study comparison reported by Gunn and colleagues (Gunn 1997) was as follows: Participants on an initial high dose of ICS (budesonide 400 μg twice daily or beclomethasone 400 μg twice daily or beclomethasone 500 μg twice daily delivered via a pressurised metered‐dose inhaler (pMDI) and spacer device) were randomised to receive budesonide 200 μg twice daily via a Turbohaler, or 400 μg once daily (i.e. both groups represent a halving of the initial ICS dose). Participants on an initial low dose of ICS (budesonide or beclomethasone 200 μg twice daily) were randomised to receive budesonide 100 μg twice daily via a Turbuhaler, or 200 μg once daily (i.e. both groups represent a halving of the initial ICS dose). There was no change in initial dose of budesonide or beclomethasone in the control group (Gunn 1997).
Inhaler devices varied across studies but were consistent between intervention and control groups in at least three of the six included studies. One study used a Diskus dry powder inhaler (Godard 2008); another used the Autohaler, a breath‐actuated metered‐dose inhaler (MDI) (Magnussen 2000); one study used a hydrofluoroalkane MDI (Knox 2007); another did not report the device used (Juniper 1991); one study permitted the use of an MDI or a dry powder inhaler as long as the same device was used throughout the study (Hawkins 2003); and another used the Turbohaler for participants in the intervention group and an MDI for those in the comparator group (Gunn 1997) and considered the two inhaler types to be equivalent for a given dose.
Outcomes
Outcomes reported were inconsistent across included studies. All studies reported data on asthma control, although several studies used scales that were not validated and thus did not contribute data to the meta‐analysis. Most studies reported exacerbations requiring oral corticosteroids (OCS) (Godard 2008; Hawkins 2003; Knox 2007; Magnussen 2000), all‐cause serious adverse events (SAEs) (Godard 2008; Gunn 1997; Hawkins 2003; Knox 2007) and lung function (Godard 2008; Gunn 1997; Juniper 1991; Knox 2007; Magnussen 2000), although reported measures of lung function varied across studies. Two studies reported quality of life (QoL): One study used both the Juniper Asthma QoL Questionnaire and the Dupuy Psychological General Well Being Index (Gunn 1997), and the second study used the EuroQoL questionnaire and the St George's Respiratory Questionnaire (Hawkins 2003). Steroid related AEs and exacerbations requiring hospitalisation were each reported by two studies (Knox 2007 and Magnussen 2000; Godard 2008 and Hawkins 2003, respectively). Mortality and exacerbations requiring an emergency department visit were each reported by one study (Godard 2008 and Hawkins 2003, respectively).
Excluded studies
We excluded 53 references (related to 41 studies) following assessment of full‐text articles (Characteristics of excluded studies). We excluded 27 studies as they used an intervention that was not relevant to this review (e.g. a dose reduction of ICS was not used, or a concomitant reduction in ICS and LABA was used). Eight studies were excluded because they used a study design not appropriate for this review (e.g. cross‐over or non‐randomised design). We excluded six studies because they used a comparator not relevant to this review (n = 3; e.g. a dose reduction in the control group) or a route of administration not relevant to this review was used (n = 2; e.g. the intervention was OCS, not ICS) or because the patient population studied was not relevant (n = 1; e.g. participants were children).
Risk of bias in included studies
Please refer to the Characteristics of included studies tables for details on risk of bias and for supporting evidence for each study. Figure 2 provides a summary of risk of bias judgements, presented by study and domain (sequence generation, allocation concealment, blinding, incomplete data, selective reporting and 'other'). Figure 3 depicts the risk of bias for each domain, presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
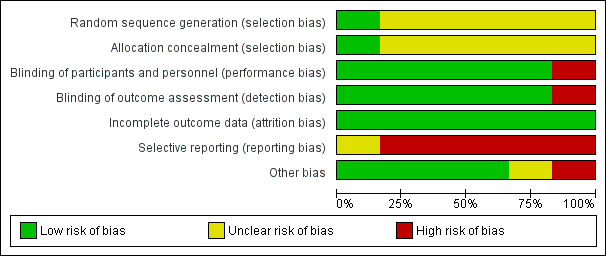
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Most studies (five of six) provided insufficient information regarding methods of random sequence generation and concealment of treatment allocation to allow a judgement on risk of bias (Godard 2008; Gunn 1997; Juniper 1991; Knox 2007; Magnussen 2000); therefore, the risk of bias for these studies was unclear. One study (Hawkins 2003) used a computer‐generated randomisation sequence and concealed allocation method, and was considered to be at low risk for selection bias.
Blinding
We considered five of six studies (Godard 2008; Hawkins 2003; Juniper 1991; Knox 2007; Magnussen 2000) to have low risk of performance and detection bias, as participants, personnel and outcome assessors were blinded to treatment allocation through adequate methods. One study (Gunn 1997) used an open‐label design, in which participants, personnel and outcome assessors were not blinded to treatment allocation; we considered this study to be at high risk of both performance and detection bias.
Incomplete outcome data
We considered all studies to be at low risk of attrition bias on the basis of low and balanced rates of participant withdrawal, which were adequately documented in the trial report.
Selective reporting
We judged five studies (Godard 2008; Gunn 1997; Hawkins 2003; Juniper 1991; Magnussen 2000) to be at high risk of reporting bias because no study protocol was available and there appeared to be either non‐standard presentation of the data or selective reporting of data that were likely recorded. One study (Knox 2007) appeared to report a fairly comprehensive set of outcomes (i.e. exacerbations, steroid‐related AEs, all‐cause SAEs, lung function and asthma control); however, a protocol was not available, so we judged the risk of bias as unclear.
Other potential sources of bias
We judged four studies to be at low risk of other bias, as no other concerns were identified (Hawkins 2003; Juniper 1991; Knox 2007; Magnussen 2000). We considered Godard 2008 to be at high risk of other bias because, contrary to the methods described, investigators randomised a relatively high proportion of participants who had asthma that was not well controlled and included them in the full analysis set. We judged Gunn 1997 to be at unclear risk of bias because there appeared to be some changes in the inhaler used to deliver the ICS at the same time as changes in dose, although we noted that the two inhaler types were considered equivalent for a given dose.
Effects of interventions
See: Summary of findings for the main comparison ICS dose reduction compared with no change in ICS dose (no concomitant LABA) for adults with asthma; Summary of findings 2 ICS dose reduction compared with no change in ICS dose (concomitant LABA) for adults with asthma
Structure of the analysis
As per the protocol, we chose to analyse participants who were receiving a concomitant LABA separately from those who were not receiving a concomitant LABA.
Structure of the meta‐analysis
We created two main comparison headings within the analysis tree. For each comparison, we elected to perform a meta‐analysis only when interventions and outcomes were sufficiently similar for pooling of the data.
Participants not taking concomitant a LABA: ICS reduction versus no change in ICS dose
This comparison comprised all studies that compared a reduction in the dose of ICS versus no change in ICS dose among participantsnot taking a concomitant LABA (Gunn 1997; Knox 2007; Magnussen 2000).
Participants taking a concomitant LABA: ICS reduction versus no change in ICS dose
This comparison comprised all studies that compared a reduction in the dose of ICS versus no change in ICS dose among participants taking a concomitant LABA (Godard 2008; Hawkins 2003; Juniper 1991)
Structure of the narrative synthesis
In the following sections, we present a narrative summary of study results according to the prespecified outcomes. We present primary outcomes (exacerbations requiring OCS, asthma control, all‐cause SAEs, steroid‐related AEs) followed by secondary outcomes (health‐related QoL, lung function, exacerbations requiring hospitalisation, exacerbations requiring an emergency department visit, mortality). For each outcome, we describe the effect of the intervention among participants not taking a concomitant LABA followed by the effect of the intervention among participants taking a LABA.
Primary outcomes
Exacerbations requiring oral corticosteroids
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring treatment with oral steroids (odds ratio (OR) 1.86, 95% confidence interval (CI) 0.16 to 21.09; n = 261 participants, two studies; I2 = 0%; Analysis 1.1; Figure 4). For people who stepped down their dose of ICS, we estimated that six more people per 1000 would have an exacerbation requiring oral steroids, but the confidence intervals ranged from seven fewer to 132 more people per 1000. We rated the quality of the evidence as very low after downgrading twice for imprecision (no events were reported by one of the contributing studies, and confidence intervals include the null effect (risk ratio (RR) 1.0) and appreciable benefit (RR 0.75) or harm (RR 1.25)) and once for indirectness, as the evidence was based on studies operating out of specialist centres.
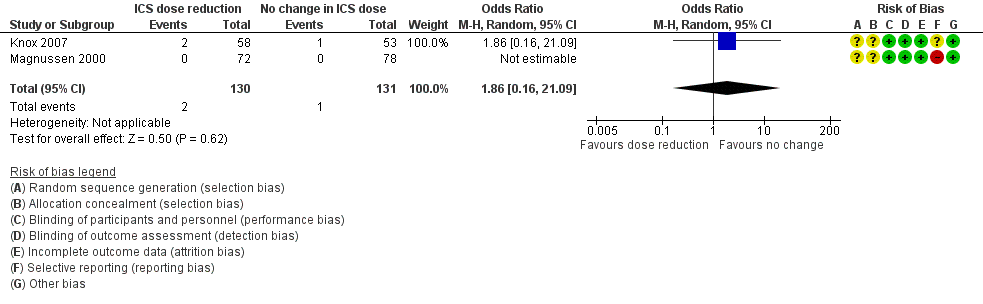
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.1 Exacerbation requiring OCS.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring treatment with oral steroids (OR 1.31, 95% CI 0.82 to 2.08; n = 569 participants; two studies; I2 = 0%; Analysis 2.1; Figure 5). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 38 more people per 1000 would have an exacerbation requiring oral steroids, but the confidence intervals ranged from 23 fewer to 118 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.1 Exacerbation requiring OCS.
Asthma control
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to asthma control (mean difference (MD) ‐0.22, 95% CI ‐1.05 to 0.61; n = 150 participants; one study; Analysis 1.2). We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for indirectness (single study representative of a single setting and drug regimen).
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to asthma control as measured by the short asthma morbidity score (change from baseline: MD 0.16, 95% CI ‐0.34 to 0.66; n = 242 participants; one study; scale 0 (perfect control) to 8 (very poor control); Analysis 2.2). We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for indirectness (single study representative of a single setting and drug regimen). Nor did stepping down the dose of ICS result in clear benefit or harm with respect to asthma control as measured by the Asthma Severity Questionnaire (MD 1.13, 95% CI ‐0.24 to 2.49; scale 0 (best control) to 6 (worst control); Analysis 2.3). We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (single study representative of a single setting and drug regimen) and once for imprecision (confidence intervals include the null effect (MD 0) and appreciable harm (MD 1.5)).
All‐cause SAEs
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to SAEs (OR 1.24, 95% CI 0.25 to 6.25; n = 742 participants; two studies; I2 = 5%; Analysis 1.3; Figure 6). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 1 more person per 1000 would have an SAE, but confidence intervals ranged from six fewer to 37 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include the null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.3 All‐cause SAEs.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to SAEs (OR 0.60, 95% CI 0.11 to 3.33; n = 569 participants; two studies; I2 = 35%; Analysis 2.4; Figure 7). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 13 fewer people per 1000 would have an SAE, but the confidence intervals ranged from 31 fewer to 74 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.4 All‐cause SAEs.
Steroid‐related AEs
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to steroid‐related AEs (OR 0.76, 95% CI 0.16 to 3.54; n = 261 participants; two studies; I2 = 0%; Analysis 1.4; Figure 8). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that eight fewer people per 1000 would have a steroid‐related AE, but confidence intervals ranged from 26 fewer to 69 more people per 1000. We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (representative of specialist centres) and once for imprecision (confidence intervals include the null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.4 Steroid‐related AEs.
ICS stepdown, concomitant LABA
No included studies reported data for steroid‐related AEs.
Secondary outcomes
Health‐related quality of life
ICS stepdown, no concomitant LABA
There was a statistically significant difference in health‐related quality of life (change from baseline) between groups as measured by the Asthma Quality of Life Questionnaire (change from baseline: MD ‐0.21, 95% CI ‐0.33 to ‐0.09; n = 554 participants, one study; scale 0 (worst) to 7 (best); Analysis 1.5). However, the mean difference and 95% confidence limits were below the minimal clinically important difference (MCID) of 0.5, indicating no clinically relevant difference between groups. We rated the quality of the evidence as very low after downgrading twice for risk of bias (selective reporting and lack of blinding for a subjective outcome measure) and once for indirectness (single study representative of a single setting and drug regimen).
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no benefit or harm with respect to health‐related quality of life as measured by St George's Respiratory Scale (change from baseline: MD 0.13, 95% CI ‐2.80 to 3.06; n = 229 participants, one study; scale 0 to 100 (greatest impact of chest disease on life); Analysis 2.6) or the EuroQoL (change from baseline: MD 2.32, 95% CI ‐1.64 to 6.28; n = 219 participants, one study; scale 0 to 100 (best imaginable health state); Analysis 2.5). With regards to the St George's Respiratory Scale, the mean difference and 95% confidence limits were below the MCID of 4 units, indicating no clinically relevant difference between groups. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for indirectness (single study representative of a single setting and drug regimen).
Lung function
ICS stepdown, no concomitant LABA
Stepping down the dose of ICS resulted in no benefit or harm with respect to lung function. There was no statistically significant change in percent predicted FEV1 (MD ‐0.02, 95% CI ‐0.12 to 0.08; n = 261 participants, two studies; I2 = 0%; Analysis 1.7; Figure 9) nor in morning PEFR (MD ‐5.98 L/min, 95% CI ‐19.47 to 7.51; n = 875 participants, three studies; I2 = 0%; Analysis 1.6; Figure 10). We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (confidence intervals include the null effect and appreciable benefit (RR 0.75) or harm (RR 1.25)).

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.7 Lung function, FEV1 (L).
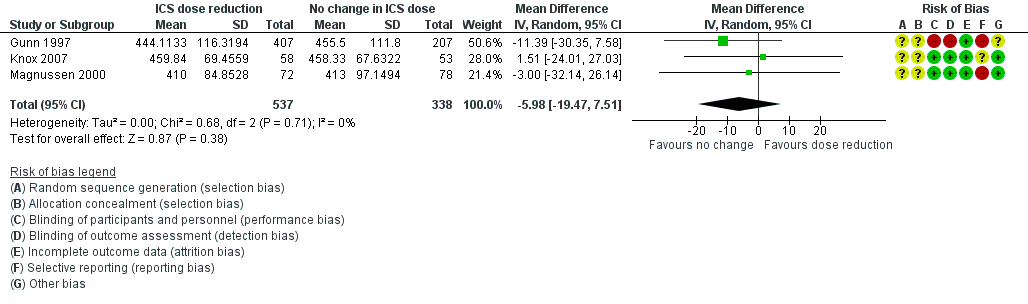
Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.6 Lung function, PEFR morning (L/min).
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no benefit or harm with respect to lung function. There was no statistically significant between‐group differences for change in percent predicted FEV1 from baseline (MD ‐2.45, 95% CI ‐8.88 to 3.98; n = 14 participants, one study; Analysis 2.8) nor for change from baseline in morning PEFR (MD ‐4.54, 95% CI ‐12.08 to 3.00; n = 310 participants, one study; Analysis 2.7). We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (single study representative of a single setting and drug regimen) and once for imprecision (wide CI).
Exacerbations requiring hospitalisation
ICS stepdown, no concomitant LABA
No studies reported the number of participants requiring hospitalisation.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring hospitalisation (OR 4.06, 95% CI 0.45 to 36.86; n = 569 participants, two studies; I2 = 0%; Analysis 2.9; Figure 11). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 10 more people per 1000 would have an exacerbation requiring hospitalisation, but the confidence intervals ranged from 2 fewer to 112 more people per 1000. We rated the quality of the evidence as low after downgrading once for risk of bias (selective reporting) and once for imprecision (wide CI).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.9 Exacerbation requiring hospitalisation.
Exacerbations requiring an emergency department visit
ICS stepdown, no concomitant LABA
No studies reported the number of participants requiring an emergency department visit.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm with respect to exacerbations requiring an emergency department visit (OR 2.00, 95% CI 0.18 to 22.33; n = 259 participants, one study; Analysis 2.10). For people who stepped down their dose of ICS (versus those with no change in ICS dose), we estimated that 7 more people per 1000 would have an exacerbation requiring an emergency department visit, but confidence intervals ranged from 7 fewer to 141 more people per 1000. We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for imprecision (wide CI) and once for indirectness (single study representative of a single setting and drug regimen).
Mortality
ICS stepdown, no concomitant LABA
No studies reported mortality data.
ICS stepdown, concomitant LABA
Stepping down the dose of ICS resulted in no clear benefit or harm in terms of mortality; the single study reporting data (N = 310 participants; Analysis 2.11) reported no deaths in either group. We rated the quality of the evidence as very low after downgrading once for risk of bias (selective reporting), once for indirectness (single study representative of a single setting and drug regimen) and once for imprecision (no events reported).
Subgroup analyses
Magnitude of dose reduction
Review authors did not perform this prespecified subgroup analysis for either comparison because all of the included studies represented a 50% to 60% reduction in ICS dose.
Separate ICS/LABA inhalers versus combination ICS/LABA inhaler
This was relevant only to the second comparison (participants who were permitted to receive a concomitant LABA).
For exacerbations requiring oral corticosteroids, the use of individual ICS and LABA inhalers (Hawkins 2003) versus a combined inhaler (Godard 2008) did not appear to influence the overall OR (1.31, 95% CI 0.82 to 2.08) because results of the two contributing studies were comparable (OR 1.29, 95% CI 0.75 to 2.23 (Hawkins 2003) and OR 1.34, 95% CI 0.55 to 3.28 (Godard 2008), respectively).
We could not perform a subgroup analysis for asthma control as only one study contributed data to each measure of asthma control (short asthma morbidity score (Hawkins 2003) and asthma severity questionnaire (Juniper 1991)).
Sensitivity analyses
It was not possible for review authors to conduct the planned sensitivity analyses because of the paucity of included studies contributing to each outcome examined.
Discusión
Resumen de los resultados principales
Se incluyeron seis estudios que asignaron al azar a un total de 1654 participantes (reducción de la dosis de corticosteroides inhalados [CSI], sin agonista beta de acción prolongada [ABAP] concomitante: n = 892 participantes, tres ensayos controlados aleatorios (ECA); reducción de la dosis de CSI, ABAP concomitante: n = 762, tres ECA). Todos los estudios incluidos fueron ECA con un diseño paralelo que compararon una dosis fija de CSI con una reducción del 50% al 60% en la dosis de CSI entre pacientes adultos con asma bien controlada. La duración del tratamiento varió de 12 a 52 semanas (duración media 21 semanas; duración mediana 14 semanas). Dos estudios se realizaron en un contexto de atención primaria, dos se realizaron en un contexto de atención secundaria y dos no proporcionaron información sobre el contexto.
La realización del metanálisis fue obstaculizada por el escaso número de estudios que contribuyeron a cada comparación, combinado con las diferencias entre los resultados informados en los estudios incluidos. Sin embargo, se observó un bajo nivel de heterogeneidad en los metanálisis que se realizaron. No se encontraron diferencias estadísticamente significativas entre los grupos (reducción gradual del CSI versus ningún cambio en el CSI) con respecto a cualquiera de los resultados primarios o secundarios considerados en esta revisión y, por lo tanto, no fue posible determinar si la reducción de la dosis del CSI en los adultos con asma (en comparación con mantener la dosis previa de CSI) confiere efectos beneficiosos generales. Por un lado, no se identificó una diferencia estadísticamente significativa entre los grupos en medidas de efectividad como el control del asma, la función pulmonar ni el número de participantes que presentaron exacerbaciones, lo que podría apoyar la reducción de la dosis del CSI recomendada por las guías en los pacientes con asma bien controlada. Sin embargo, se detectó una tendencia numérica hacia un aumento en el número de participantes que presentaron exacerbaciones y no se observaron efectos beneficiosos en cuanto a otros resultados de seguridad. Por otra parte, la calidad de la pruebas en general se consideró baja o muy baja, lo que significa que no existe seguridad con respecto a las estimaciones del efecto (ver a continuación). Por último, todavía no está claro si el tratamiento concomitante con ABAP influye en el cociente efectos beneficiosos / perjudiciales de la reducción de la dosis de CSI.
Compleción y aplicabilidad general de las pruebas
Dos de los estudios incluidos se realizaron en un contexto de atención primaria, dos se realizaron en un contexto de atención secundaria y dos no informaron el contexto. Cada comparación (± ABAP) incluyó un estudio en estas tres categorías (atención primaria, atención secundaria, no establecida); sin embargo, debido al escaso número de estudios que contribuyeron a cada resultado, algunos resultados pueden ser más representativos de un contexto particular. Cuando éste fue el caso, se consideró este factor para disminuir la calidad de las pruebas debido a la indireccionalidad. Además, estudios únicos (representativos de un régimen o duración del tratamiento únicos) contribuyeron con algunos de los resultados, y la mayoría de los metanálisis incluyeron sólo dos estudios. Por lo tanto, los resultados de la presente revisión pueden ser relevantes para los regímenes de tratamiento particulares representados en los estudios individuales. Por último, los resultados de esta revisión sólo son relevantes para los pacientes adultos. Es posible que los efectos perjudiciales potenciales debido a los efectos sistémicos asociados con la administración a largo plazo de CSI sean más relevantes en los niños. Para analizar lo anterior sería necesario considerar la inclusión de estudios pediátricos en las actualizaciones futuras de esta revisión o en otra revisión.
Una de las preocupaciones asociadas con la reducción de la dosis de los CSI en los pacientes con asma bien controlada es el posible deterioro lento del control del asma con el transcurso del tiempo con la reaparición lenta de la hiperreactividad bronquial. Además, la exposición a largo plazo a los corticosteroides puede provocar el desarrollo de efectos secundarios sistémicos como la pérdida de la densidad ósea en los adultos y el retardo del crecimiento en los niños (Colice 2006; Lipworth 1999; SIGN/BTS 2016). La duración media de los estudios incluidos fue 21 semanas (duración mediana 14 semanas), que no es potencialmente suficiente para detectar un deterioro a largo plazo en el control del asma / la función pulmonar ni para una evaluación adecuada de los resultados de seguridad a largo plazo.
Calidad de la evidencia
Pocos estudios relevantes cumplieron los criterios de inclusión predeterminados; este hecho, combinado con el uso de una variedad de medidas de resultado entre los estudios incluidos, limitó el número de metanálisis que fue posible realizar. En cuanto al riesgo de sesgo, los estudios incluidos generalmente fueron de calidad moderada, aunque el informe selectivo de resultados introdujo riesgo de sesgo en cinco de seis de los estudios incluidos. Además, no estuvo claro si se utilizaron métodos adecuados de generación de la secuencia de asignación o de cegamiento de la asignación en todos los estudios, excepto uno.
En esta revisión la calidad de las pruebas se evaluó mediante GRADE (Higgins 2011) y el programa informático GRADEpro; los hallazgos se muestran en las "Tablas resumen de los hallazgos". El resumen de los hallazgos para la comparación principal presenta los resultados de la primera comparación (reducción de la dosis de CSI versus ningún cambio en el CSI, en pacientes que no recibieron ABAP concomitante) y el Resumen de los hallazgos 2 presenta los resultados de la segunda comparación (reducción de la dosis de CSI versus ningún cambio en el CSI, en pacientes que recibieron ABAP concomitante). En resumen, en ambas comparaciones la calidad de las pruebas resumidas se consideró baja o muy baja para la mayoría de los resultados debido al riesgo de sesgo (principalmente informe selectivo) la imprecisión (pocos eventos en un escaso número de estudios o intervalos de confianza amplios) y la indireccionalidad (estudios únicos representativos de un contexto o régimen farmacológico único). Sobre la base de la calidad de las pruebas, no hay seguridad con respecto a las estimaciones del efecto presentadas en esta revisión.
Sesgos potenciales en el proceso de revisión
Se siguieron los métodos estándar descritos en el Manual Cochrane para las revisiones sistemáticas de intervenciones (Higgins 2011) para disminuir los sesgos en el proceso de revisión. Con respecto al proceso de revisión, el Especialista de información del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group) diseñó y realizó la búsqueda electrónica principal, dos autores de la revisión de forma independiente examinaron los resultados de la búsqueda y dos autores de la revisión (uno con conocimientos clínicos avanzados) revisaron todos los resultados a texto completo. De acuerdo con los métodos Cochrane, no se excluyó ningún ensayo sobre la base del idioma, el estado de la publicación o los resultados informados, de manera que se puede asegurar que se identificaron todas las pruebas potencialmente relevantes de ECA. En cuanto a los resultados y las conclusiones, dos autores de la revisión realizaron de forma independiente todos los pasos del proceso de revisión en los que se necesitó tomar decisiones subjetivas (p.ej. selección de los estudios, extracción de los datos, evaluación del riesgo de sesgo, evaluación de la calidad general d las pruebas mediante GRADE) y, de ser necesario, un tercer autor de la revisión ayudó a resolver los desacuerdos. Por último, esta revisión se sometió a revisión editorial y por pares de forma tal que se ha considerado la opinión de expertos externos independientes. En conjunto, estos factores deberían garantizar que las conclusiones sean bastante representativas de los resultados resumidos durante el proceso de revisión.
Acuerdos y desacuerdos con otros estudios o revisiones
Los resultados de esta revisión coinciden con los de una revisión sistemática (Gionfriddo 2015) que examinó las pruebas de la reducción de la dosis de CSI desde un régimen programado hasta un régimen basado en las necesidades. Estos autores de la revisión no encontraron pruebas suficientes para asociar la reducción de la dosis de CSI con un efecto sobre el número de exacerbaciones del asma. Por el contrario, los autores encontraron algunas pruebas de menos días libres de síntomas en los pacientes que utilizaron un CSI basado en las necesidades. En otra revisión sistemática, Hagan y colegas encontraron de manera similar que las exacerbaciones del asma no fueron más probables desde el punto de vista estadístico entre los pacientes que redujeron la dosis de CSI que entre los que mantuvieron la dosis de CSI (Hagan 2014). La revisión de Hagan incluyó estudios en adultos y niños y permitió la reducción gradual del CSI basado en las necesidades. Sin embargo, sus hallazgos fueron consistentes con los de la presente revisión.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.1 Exacerbation requiring OCS.

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.1 Exacerbation requiring OCS.

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.3 All‐cause SAEs.

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.4 All‐cause SAEs.

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.4 Steroid‐related AEs.

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.7 Lung function, FEV1 (L).

Forest plot of comparison: 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), outcome: 1.6 Lung function, PEFR morning (L/min).

Forest plot of comparison: 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), outcome: 2.9 Exacerbation requiring hospitalisation.

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 1 Exacerbation requiring OCS.

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 2 Asthma control.

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 3 All‐cause SAEs.

Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 4 Steroid‐related AEs.
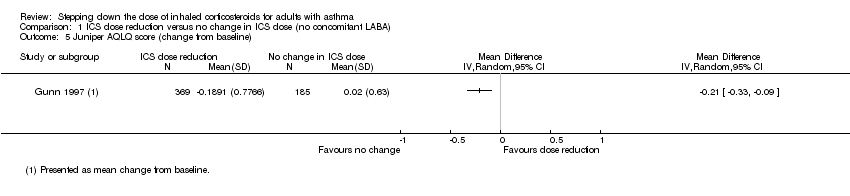
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 5 Juniper AQLQ score (change from baseline).
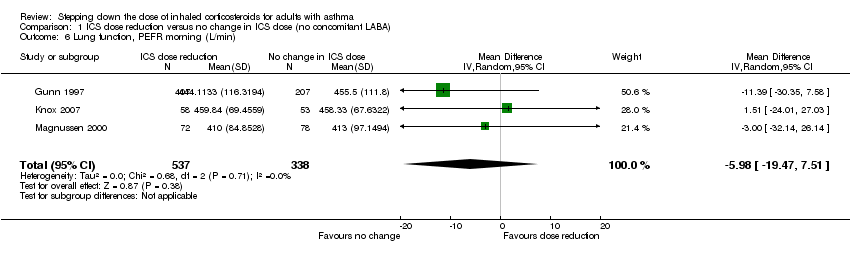
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 6 Lung function, PEFR morning (L/min).
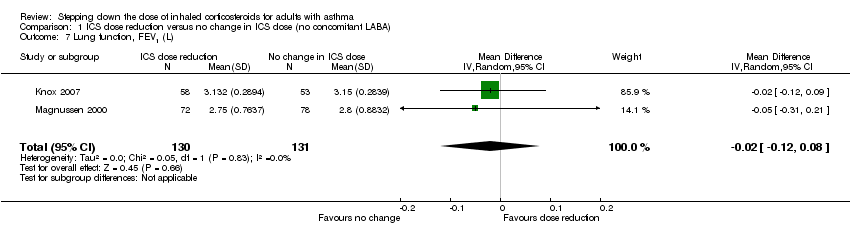
Comparison 1 ICS dose reduction versus no change in ICS dose (no concomitant LABA), Outcome 7 Lung function, FEV1 (L).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 1 Exacerbation requiring OCS.
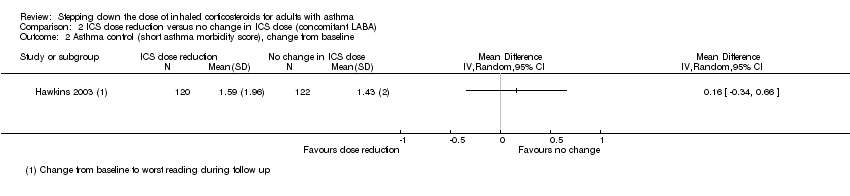
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 2 Asthma control (short asthma morbidity score), change from baseline.

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 3 Asthma control (Asthma Severity Questionnaire).
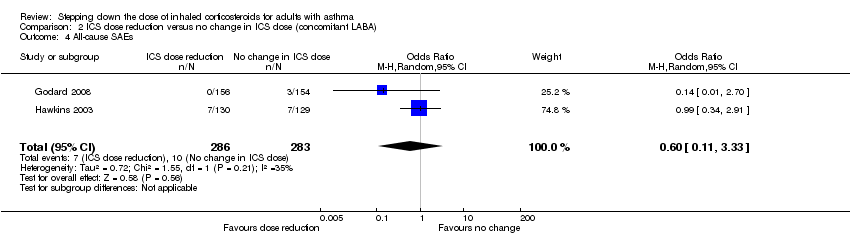
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 4 All‐cause SAEs.

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 5 EuroQoL score (change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 6 St. George's Respiratory Scale score (change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 7 Lung function, PEFR morning (L/min) (change from baseline).
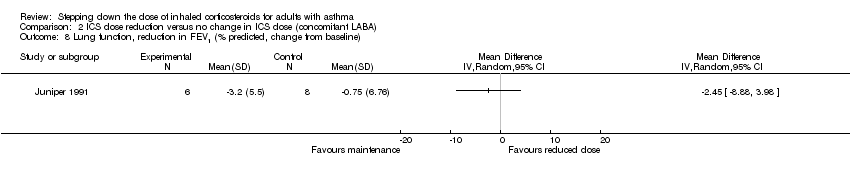
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 8 Lung function, reduction in FEV1 (% predicted, change from baseline).

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 9 Exacerbation requiring hospitalisation.
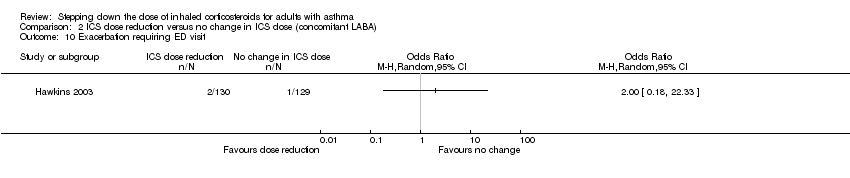
Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 10 Exacerbation requiring ED visit.

Comparison 2 ICS dose reduction versus no change in ICS dose (concomitant LABA), Outcome 11 Mortality.
| ICS dose reduction compared with no change in ICS dose (no concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (no concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 8 per 1000 | 14 per 1000 | OR 1.86 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Asthma control | Mean asthma control score in the no change in ICS dose group was 1.79. | MD 0.22 lower | ‐ | 150 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| All‐cause SAEs | 8 per 1000 | 9 per 1000 | OR 1.24 | 742 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Steroid‐related AEs | 31 per 1000 | 23 per 1000 | OR 0.76 | 261 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence) |
| Health‐related quality of life (change from baseline) | Mean change from baseline in health‐related quality of life for the no change in ICS dose group was 0.02. | MD 0.21 lower | ‐ | 554 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (very low‐quality evidence); MCID is 0.5 for AQLQ |
| Lung function, FEV1 (L) | Mean FEV1 in the no change in ICS dose group was 3.15 litres. | MD 0.02 litres lower | ‐ | 261 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS (low‐quality evidence) |
| Exacerbations requiring hospitalisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported by included studies |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). dThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (representative of specialist centres) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded twice for risk of bias (selective reporting and lack of blinding (subjective outcome)) and once for indirectness (single study representative of one setting and drug regimen). fThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ICS dose reduction compared with no change in ICS dose (concomitant LABA) for adults with asthma | ||||||
| Patient or population: adults with asthma | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with no change in ICS dose (concomitant LABA) | Risk with ICS dose reduction | |||||
| Exacerbation requiring OCS | 148 per 1000 | 186 per 1000 | OR 1.31 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring OCS (low‐quality evidence) |
| Asthma control (short asthma morbidity score) | Mean asthma control score was 1.43. | MD 0.16 higher | ‐ | 242 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to asthma control (low‐quality evidence) |
| All‐cause SAEs | 35 per 1000 | 22 per 1000 | OR 0.60 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to all‐cause SAEs (low‐quality evidence) |
| Steroid‐related AEs ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| St. George's Respiratory Scale score (change from baseline) Score 0‐100. 100 = greatest impact of chest disease on life; MCID is 4 units. | Mean change from baseline in HRQoL score was 7.4.c | MD 0.13 higher | ‐ | 229 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to HRQoL (low‐quality evidence) |
| Exacerbation requiring hospitalisation | 4 per 1000 | 14 per 1000 | OR 4.06 | 569 | ⊕⊕⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to exacerbations requiring hospitalisation (low‐quality evidence) |
| Lung function, reduction in FEV1 (% predicted, change from baseline) | Mean change from baseline in % predicted FEV1 was ‐0.75%. | MD 2.45 lower | ‐ | 14 | ⊕⊝⊝⊝ | No clear benefit or harm of stepping down the dose of ICS with respect to lung function (very low‐quality evidence) |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). bThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for indirectness (single study representative of one setting and drug regimen). cNote that study authors reported the change to the lowest SGRQ score during follow‐up. dThe quality of the evidence was downgraded once for risk of bias (selective reporting) and once for imprecision (confidence intervals include null effect and appreciable benefit or harm). eThe quality of the evidence was downgraded once for risk of bias (selective reporting), once for indirectness (single study representative of one setting or drug regimen) and once for imprecision (wide CI). AE, adverse event; CI, confidence interval; FEV1, forced expiratory volume in one second; GRADE, Grades of Recommendation, Assessment, Development and Evaluation; HRQoL, health‐related quality of life; ICS, inhaled corticosteroid; LABA, long‐acting beta agonist; MCID, minimum clinically important difference; MD, mean difference; OCS, oral corticosteroid; OR, odds ratio; RCT, randomised controlled trial; RR, risk ratio; SAE, serious adverse event. | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.86 [0.16, 21.09] |
| 2 Asthma control Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 All‐cause SAEs Show forest plot | 2 | 742 | Odds Ratio (M‐H, Random, 95% CI) | 1.24 [0.25, 6.25] |
| 4 Steroid‐related AEs Show forest plot | 2 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.54] |
| 5 Juniper AQLQ score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Lung function, PEFR morning (L/min) Show forest plot | 3 | 875 | Mean Difference (IV, Random, 95% CI) | ‐5.98 [‐19.47, 7.51] |
| 7 Lung function, FEV1 (L) Show forest plot | 2 | 261 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation requiring OCS Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.08] |
| 2 Asthma control (short asthma morbidity score), change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Asthma control (Asthma Severity Questionnaire) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 All‐cause SAEs Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.11, 3.33] |
| 5 EuroQoL score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 St. George's Respiratory Scale score (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Lung function, PEFR morning (L/min) (change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Lung function, reduction in FEV1 (% predicted, change from baseline) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Exacerbation requiring hospitalisation Show forest plot | 2 | 569 | Odds Ratio (M‐H, Random, 95% CI) | 4.06 [0.45, 36.86] |
| 10 Exacerbation requiring ED visit Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11 Mortality Show forest plot | 1 | 310 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |

