مقایسه استرنوتومی محدود در برابر کامل به منظور تعویض دریچه آئورت
چکیده
پیشینه
اختلال دریچه آئورت، یک عارضه شایع است که با جراحی قلب به آسانی قابل درمان است. این عمل به طور معمول از طریق باز کردن استرنوم به صورت طولی در راستای مرکز («استرنوتومی میانی») و تعویض دریچه تحت بایپس قلبیریوی انجام میشود. استرنوتومی میانی به طور کلی به خوبی تحمل میشود، اما از آنجایی که گزینههای کمتر تهاجمی در دسترس قرار دارند، اثربخشی برشهای محدود زیر سوال رفته است. به طور خاص، تاثیرات کاهش قدرت دید و دسترسی جراحی موجب افزایش نگرانیهای ایمنی در مورد جایگذاری کانولها، خروجی قلب، جایگذاری سیم اپیکاردی و هواگیری از قلب در انتهای پروسیجر شده است. این مشکلات ممکن است باعث افزایش زمان جراحی و تاثیر بر پیامدها شود. تصور میشود مزایای برشهای کوچکتر شامل کاهش درد، بهبود مکانیک تنفس، کاهش عفونت زخمها، خونریزی و نیاز به ترانسفیوژن، کوتاه شدن بستری در بخش مراقبتهای ویژه، زیبایی بیشتر و بازگشت سریعتر به فعالیت طبیعی باشد.
اهداف
ارزیابی تاثیرات تعویض دریچه آئورت با حداقل تهاجم از طریق استرنوتومی محدود در برابر تعویض دریچه آئورت معمول از طریق استرنوتومی میانی در افراد مبتلا به اختلال دریچه آئورت که نیازمند جراحی تعویض هستند.
روشهای جستوجو
ما بدون اعمال محدودیتهای زبانی، در CENTRAL؛ MEDLINE؛ Embase، پایگاههای ثبت کارآزماییهای بالینی و وبسایتهای تولید کنندگان را از آغاز تا جولای 2016 جستوجو کردیم. منابع مقالات انتخاب شده را به دنبال شناسایی مطالعات مرتبط بیشتر مرور کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده مربوط به مقایسه تعویض دریچه آئورت از طریق استرنوتومی میانی در برابر تعویض دریچه آئورت از طریق استرنوتومی محدود را وارد مرور کردیم. کارآزماییهایی را که سایر برشهای کمتر تهاجمی مانند پروسیجرهای مینیتوراکوتومی، دسترسی دریچهای، ترانس‐آپیکال، ترانس‐فمورال یا روباتیک انجام داده بودند، از مرور خارج کردیم. گرچه تعدادی مطالعات مورد‐شاهدی آیندهنگر و گذشتهنگر و کوهورت (همگروهی) با اجرای مناسب وجود داشت، آنها در این مرور وارد نشدند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده مرور بهطور مستقل از هم مقالات کارآزمایی را برای استخراج دادهها، ارزیابی کیفیت و شناسایی خطر سوگیری (bias)، بررسی کردند. در صورت لزوم، نویسنده سوم مرور داوری کرد. کیفیت شواهد را با استفاده از متدولوژی درجهبندی توصیه، ارزیابی، توسعه و ارزشیابی (GRADE) تعیین کرده و نتایج پیامدهای مرتبط با بیمار را در جدول «خلاصهای از یافتهها» خلاصه کردیم.
نتایج اصلی
این مرور شامل هفت کارآزمایی با 511 شرکتکننده بود. این بزرگسالان از مراکزی در اتریش، اسپانیا، ایتالیا، آلمان، فرانسه و مصر وارد شدند. ما 12 مقایسه را برای بررسی تاثیرات تعویض دریچه آئورت با حداقل تهاجم از طریق همی‐استرنوتومی فوقانی محدود در مقابل جراحی انجام گرفته از طریق استرنوتومی میانی کامل، اجرا کردیم.
هیچ شواهدی از تاثیر همی‐استرنوتومی فوقانی بر مورتالیتی در برابر استرنوتومی میانی کامل وجود نداشت (خطر نسبی (RR): 1.01؛ 95% فاصله اطمینان (CI): 0.36 تا 2.82؛ شرکتکنندگان = 511؛ مطالعات = 7؛ کیفیت متوسط). شواهدی از افزایش زمان بایپس قلبیریوی در تعویض دریچه آئورت از طریق همی‐استرنوتومی فوقانی موجود نبود (تفاوت میانگین (MD): 3.02 دقیقه؛ 95% CI؛ 4.10‐ تا 10.14؛ شرکتکنندگان = 311؛ مطالعات = 5؛ کیفیت پائین). شواهدی درباره افزایش زمان کراس‐کلمپ آئورتی (aortic cross‐clamp) وجود نداشت (MD: 0.95 دقیقه؛ 95% CI؛ 3.45‐ تا 5.35؛ شرکتکنندگان = 391؛ مطالعات = 6؛ کیفیت پائین). هیچ یک از مطالعات وارد شده، عوارض قلبی عمده و حوادث مغزیعروقی را به عنوان نقطه پایانی ترکیبی گزارش نکرده بودند.
شواهدی از تاثیر بر طول بستری در بیمارستان در همی‐استرنوتومی محدود موجود نبود (MD:‐1.31 روز؛ 95% CI؛ 2.63 ‐ تا 0.01؛ شرکتکنندگان = 297؛ مطالعات = 5؛ I2 = 89%؛ کیفیت بسیار پائین). از دست دادن خون پس از جراحی، در گروه همی‐استرنوتومی فوقانی کمتر بود (MD: ‐158.00 میلیلیتر؛ 95% CI؛ 303.24‐ تا 12.76‐؛ شرکتکنندگان = 297؛ مطالعات = 5؛ کیفیت متوسط). این شواهد، کاهش عفونتهای زخم استرنوم عمیق (MD: 0.71؛ 95% CI؛ 0.22 تا 2.30؛ شرکتکنندگان = 511؛ مطالعات = 7؛ کیفیت متوسط) یا عمل کنترل خونریزی را تایید نکرد (RR: 1.01؛ 95% CI؛ 0.48 تا 13.2؛ شرکتکنندگان = 511؛ مطالعات = 7؛ کیفیت متوسط). هیچ تغییری در نمرات درد حاصل از همی‐استرنوتومی فوقانی مشاهده نشد (تفاوت میانگین استاندارد شده (SMD): 0.33‐؛ 95% CI؛ 0.85‐ تا 0.20؛ شرکتکنندگان = 197؛ مطالعات = 3؛ I2 = 70%؛ کیفیت بسیار پائین)، اما افزایش اندکی در تستهای عملکرد ریوی پس از جراحی استرنوتومی محدود با حداقل تهاجم وجود داشت (MD: %1.98 از FEV1 پیشبینی شده؛ 95% CI؛ 0.62 تا 3.33؛ شرکتکنندگان = 257؛ مطالعات = 4؛ I2 = 28%؛ کیفیت پائین). کاهش کمی در مدت بستری در بخش مراقبتهای ویژه، به عنوان نتیجه همی‐استرنوتومی محدود با حداقل تهاجم دیده شد (MD: ‐0.57 روز؛ 95% CI؛ 0.93‐ تا 0.20‐؛ شرکتکنندگان = 297؛ مطالعات = 5؛ کیفیت پائین). با تعویض دریچه آئورت کمتهاجمی از طریق استرنوتومی محدود در مقایسه با استرنوتومی کامل، فیبریلاسیون دهلیزی (RR: 0.60؛ 95% CI؛ 0.07 تا 4.89؛ شرکتکنندگان = 240؛ مطالعات = 3؛ کیفیت متوسط) و زمان استفاده از ونتیلاسیون پس از جراحی (MD: ‐1.12؛ 95% CI؛ 3.43‐ تا 1.19؛ شرکتکنندگان = 297؛ مطالعات = 5؛ کیفیت پائین) کاهش نیافته بود. هیچ کدام از مطالعات وارد شده، تجزیهوتحلیل هزینهها را گزارش نکرده بودند.
نتیجهگیریهای نویسندگان
کیفیت شواهد در این مرور، به طور کلی پائین تا متوسط ارزیابی شد. حجم نمونههای مطالعه کوچک بود و قدرت کافی را برای نشان دادن تفاوتهای موجود در پیامدها با نرخ پائین رویدادها نداشت. ناهمگونی بالینی هم بین مطالعات و هم درون مطالعات، یک ویژگی نسبتا ثابت کارآزماییهای جراحی و همچنین مرتبط با نیاز به احتیاط در تفسیر نتایج است.
با در نظر گرفتن این محدودیتها، عدم‐قطعیت نسبت به تاثیر بر مورتالیتی یا زمانهای حمایت برونپیکری (extracorporeal) با همی‐استرنوتومی فوقانی برای تعویض دریچه آئورت در قیاس با استرنوتومی میانی کامل وجود داشت. کیفیت این شواهد برای تایید کاهش در مدت کلی بستری یا ماندن در بخش مراقبتهای ویژه در بیمارستان، پائین بود. درباره هرگونه تفاوت در نرخهای دیگر، معیارهای پیامد ثانویه یا حوادث جانبی رویکردهای استرنوتومی محدود با حداقل تهاجم برای تعویض دریچه آئورت نیز عدم‐قطعیت وجود داشت.
ظاهرا میان تعویض دریچه آئورت با حداقل تهاجم از طریق همی‐استرنوتومی فوقانی و تعویض دریچه آئورت معمول از طریق استرنوتومی میانی کامل عدم‐قطعیت وجود دارد. کارآزمایی تصادفیسازی و کنترل شده آیندهنگر با قدرت کافی و طراحی مناسب پیش از قبول گسترده رویکرد کمتهاجمی، مورد نیاز هستند. انجام یک تجزیهوتحلیل قوی از هزینهها برای چنین مطالعهای مزیت خواهد داشت. ترجیح رو به رشد بیماران برای روشهای کمتهاجمی، سزاوار انتخاب تجزیهوتحلیلهای کیفیت زندگی به عنوان نقاط پایانی و همچنین اندازهگیریهای کمی ذخیره فیزیولوژیک (physiological reserve) است.
PICOs
خلاصه به زبان ساده
جراحی قلب به منظور تعویض دریچه آئورت از طریق برش کوچک در برابر برش کامل استاندارد در جلوی قفسه سینه
ما به دنبال پاسخ به چه سوالی بودیم؟
ما شواهد راجع به تاثیر برش از نوع «سوراخ کلید» (keyhole) (به جای برش کامل معمول در زیر استخوان جناغ) را بر جراحی تعویض دریچه آئورت در بزرگسالان مرور کردیم. خواسته ما، مقایسه ایمنی و اثربخشی این دو روش بود.
چرا این موضوع مهم است؟
دریچه آئورت، از بازگشت خون به قلب پیشگیری میکند. بیماری دریچه آئورت، یک عارضه شایع است که به خوبی با جراحی قلب قابل درمان است. شیوه معمول انجام این عمل، از طریق شکافتن تمام استخوان جناغ به صورت طولی برای دسترسی به قلب است، اما این کار دردناک و مخرب به نظر میرسد. حدود 20 سال است که برخی از جراحان، این عمل را از طریق یک سوراخ کوچکتر انجام میدهند و به جای برش طولی کامل استخوان جناغ، قسمتهایی از آن را میبرند. انجام این شیوه باعث کوچکتر شدن اسکار میشود، اما همچنین میتواند جراحی را به چالش بیشتری بکشد، زیرا مشاهده و دستیابی به قلب دشوارتر است. این امر ممکن است باعث افزایش مدت عمل و کاهش ایمنی شود، حتی اگر از بیرون کوچکتر به نظر برسد.
چه مطالعاتی وارد این مرور شدند؟
ما بانکهای اطلاعاتی علمی را برای یافتن مطالعات منتشر شده و منتشر نشدهای بررسی کردیم که باز کردن کامل استخوان جناغ (استرنوتومی میانی) را برای تعویض دریچه آئورت با باز کردن فقط بخشی از استخوان جناغ (استرنوتومی با حداقل تهاجم یا جزئی) برای همین عمل مقایسه کرده باشند. تمام رکوردها را تا جولای 2016 جستوجو کردیم و هفت مطالعه را با 511 شرکتکننده یافتیم که به این سوال پاسخ داده بودند.
طراحی این مطالعات چگونه بود؟
این مطالعات از کشورهایی در اروپا و شمال آفریقا بودند. آنها 511 شرکتکننده نیازمند تعویض دریچه آئورت با آمیزهای از شرایط متفاوت داشتند. سن اغلب این افراد، 60 تا 70 سال و تقریبا نیمی از آنان مرد بودند. شرکتکنندگان در هر گروه، مشابه بودند. بودجه یکی از مطالعات توسط شرکتی تامین شده بود که تجهیزات برای انجام جراحی کمتهاجمی را تولید میکند.
این مطالعات نشان دهنده چه بودند؟
هیچ تفاوتی میان گروهها در تعداد افرادی که در نتیجه جراحی جان خود را از دست دادند، وجود نداشت. اگر 23 نفر از هر 1000 نفر، دچار مرگ ناشی از برش کامل در استخوان جناغ خود شده بودند، حدود 24 نفر (مقداری بین هشت و 66 نفر) در هر 1000 نفر، با استفاده از عمل «سوراخ کلید» فوت کردند. از آنجایی که این طیف از سه برابر کمتر تا سه برابر بیشتر امتداد دارد، سخت است که بگوییم کدام جراحی به طور قطع بهتر یا بدتر است.
همچنین میزان زمانی که جراحان به منظور استفاده از دستگاه قلب‐ریه برای پشتیبانی از قلب در هنگام عمل نیاز دارند، تفاوت نداشت. مدتی که قلب به طور کامل برای انجام عمل میایستد، تفاوتی نداشت.
تفاوت اندکی در مقدار زمان به کارگیری ونتیلاتور یا بستری در بیمارستان برای بیماران وجود داشت، گرچه مدت سپری شده در بخش مراقبتهای ویژه، تا حدود نصف در گروه برش کوچکتر کمتر بود. هیچ یک از مشکلات مهمی که پس از جراحی قلب رخ میدهند، در این گروهها شایعتر از یکدیگر نبود (عفونت در اطراف قلب، ضربان نامنظم قلب یا احتیاج به عمل مجدد به علت خونریزی). خونریزی پس از انجام جراحی با حداقل تهاجم، اندکی کمتر بود. میانگین اتلاف خون در جراحی با برش کوچکتر، 158 میلیلیتر کمتر بود.
کیفیت شواهد
کیفیت شواهد از بسیار پائین تا متوسط متغیر بود. یکی از مشکلات عمده این مطالعات این بود که آنها کوچک بوده و شاید تفاوتهای ظریف بین گروهها را نمایان نسازند. از آنجایی که مشکلات پس از جراحی قلب نادر هستند، نیاز به ارزیابی تعداد زیادی از افراد جراحی شده برای تشخیص تغییرات کوچک داریم. یکی دیگر از مشکلات این است که جراحان تمایل به اجرای روشهای اندکی متفاوت در نحوه انجام جراحیها دارند. تفاوتهایی در عملکرد نیز وجود داشت، به این معنی که اندازهگیریها ممکن است در یک زمان و با استفاده از روشهای مشابه، انجام نشده باشد. برای نتیجهگیری راجع به اینکه کدام تفاوت در گروهها در این مرور ناشی از برش کوچکتر و کدام ناشی از سایر عوامل هستند، باید احتیاط کنیم.
Authors' conclusions
Summary of findings
| Limited upper hemi‐sternotomy versus full median sternotomy for aortic valve replacement | ||||||
| Patient or population: participants requiring aortic valve replacement | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with Full Sternotomy | Risk with Limited Sternotomy | |||||
| Mortality Follow‐up: in‐patient stay | Study population | RR 1.01 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 23 per 1000 | 24 per 1000 | |||||
| Cardiopulmonary bypass time | The mean cardiopulmonary bypass time ranged from 71 to 107 minutes | The mean cardiopulmonary bypass time in the intervention group was 3.02 minutes more (4.1 fewer to 10.14 more) | ‐ | 311 | ⊕⊕⊝⊝ | Cardiopulmonary bypass times tend to have high variability between surgeons according to surgical technique. Differences of up to 15 minutes are unlikely to have clinical significance. |
| Aortic cross‐clamp time | The mean aortic cross‐clamp time ranged from 46 to 72 minutes | The mean aortic cross‐clamp time in the intervention group was 0.95 minutes more (3.45 fewer to 5.35 more) | ‐ | 391 | ⊕⊕⊝⊝ | Ischaemic times tend to have high variability between surgeons according to surgical technique. Differences of up to 10 minutes are unlikely to have clinical significance. |
| Length of hospital stay Follow‐up: in‐patient stay | The mean length of hospital stay ranged from 6.0 to 9.3 days | The mean length of hospital stay in the intervention group was 1.31 days lower (2.63 lower to 0.01 higher) | ‐ | 297 | ⊕⊝⊝⊝ | Expediency of discharge is a quality marker in some healthcare systems, but not universally. |
| Postoperative blood loss Follow‐up: until removal of operative drains | The mean postoperative blood loss ranged from 280 mL to 590 mL | The mean postoperative blood loss in the intervention group was 158 mL lower (303 lower to 12 lower) | ‐ | 297 | ⊕⊕⊕⊝ | ‐ |
| Deep sternal wound infection Follow‐up: not specified | Study population | RR 0.71 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 23 per 1000 | 17 per 1000 | |||||
| Pain scores Follow‐up: 12 hours | The mean pain scores ranged from 1.2 to 16 standard deviations | The mean pain scores in the intervention group was 0.3 standard deviations fewer (0.85 fewer to 0.2 more) | ‐ | 197 | ⊕⊝⊝⊝ | The assessment of pain within and across studies was insufficiently standardised to make strong conclusions about effect on pain |
| Intensive care unit length of stay | The mean intensive care unit stay was 1.4 to 2.1 days | The mean intensive care unit stay in the intervention group was 0.57 days lower (0.93 lower to 0.2 lower) | ‐ | 297 | ⊕⊕⊝⊝ | ‐ |
| Postoperative pulmonary function tests Follow‐up: 5 to 7 days | The mean pulmonary function tests ranged from 53% to 82% predicted FEV1 | The mean pulmonary function tests in the intervention group was 1.98% predicted FEV1 higher (0.62 higher to 3.33 higher) | ‐ | 257 | ⊕⊕⊝⊝ | ‐ |
| Re‐exploration Follow‐up: in‐patient stay | Study population | RR 1.01 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 47 per 1000 | 47 per 1000 | |||||
| Postoperative atrial fibrillation Follow‐up: in‐patient stay | Study population | RR 0.60 | 240 | ⊕⊕⊝⊝ | ‐ | |
| 175 per 1000 | 105 per 1000 | |||||
| Postoperative ventilation time | The mean postoperative ventilation time ranged from 5.3 to 13.2 hours | The mean postoperative ventilation time in the intervention group was 1.12 hours lower (3.43 lower to 1.19 higher) | ‐ | 297 | ⊕⊝⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 4600 (to determine 1% difference using α 0.05, β 0.20). Studies all had fewer than 100 participants. 2 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 120 (to determine 15‐minute difference using α 0.05, β 0.80). Studies all had fewer than 100 participants. 3 Downgraded for inconsistency: use of rapid deployment valves in one study and other variations in surgical technique lead to high heterogeneity. 4 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 100 (to determine 10‐minute difference in mortality using α 0.05, β 0.80). Studies all had fewer than 100 participants. 5 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 140 (to determine 1‐day difference using α 0.05, β 0.80). Studies all had fewer than 100 participants. 6 Downgraded for indirectness: length of stay is a surrogate marker of quality and national variations exist in discharge criteria. 7 Downgraded for high risk of bias: outcome measure sensitive to lack of blinding in study. 8 Downgraded for inconsistency: variations in surgical or anaesthetic technique lead to high heterogeneity. 9 Downgraded for indirectness: different measures of pain used across studies. 10 Downgraded for inconsistency: different timing of postsurgical lung function tests across studies lead to high heterogeneity. 11 Downgraded for imprecision: wide 95% confidence intervals overlapping no effect. | ||||||
Background
Aortic valve disease affects approximately 1% of the adult population in the US and comprises a range of pathologies including senile degeneration and functional regurgitation (Nkomo 2006). Of the 20 million people worldwide estimated to have rheumatic heart disease (Kumar 2013), aortic valve involvement accounts for nearly one‐third of cases (Manjunath 2014). These conditions, spanning stenosis or incompetence of the aortic valve, tend to be progressive, causing angina, breathlessness, and eventually precipitating heart failure and death. Attempts at medical management of the conditions underlying aortic valve disease have not proved fruitful (Coffey 2014; Freeman 2005; Kumar 2013; Scheuble 2005); surgical intervention remains, therefore, the gold standard in treating the condition. Aortic valve surgery has evolved significantly since its inception, such that it can be performed with relatively low mortality; attention is now directed at reducing morbidity.
Description of the condition
Since the mid‐1980s, rheumatic fever, the leading cause of valvular heart disease, has been on the decline in high‐income countries (Rose 1986). In the rest of the world, rheumatic heart disease continues to have a high burden of mortality and morbidity (Carapetis 2005). While it is relatively uncommon in North America (Dare 1993), rheumatic heart disease still represents 22% of valvular heart disease in Europe (Iung 2014). In industrialised nations, senile or degenerative aortic disease typified by aortic stenosis predominates, the incidence of which is increasing in an ageing population. The prevalence of aortic stenosis rises exponentially from the age of 50 years, affecting more than 1 in 50 adults over the age of 75 years (Thaden 2014). Aortic valve disease represents over half of the valvular heart disease in Europe (Iung 2003).
Severe aortic valve disease necessitates surgical intervention for symptomatic relief or prognostic benefit, or both. Previously it was believed that people with severe aortic stenosis maintained a long asymptomatic period with low risk of death (Ross 1968). However, even where symptoms are absent, the outlook is poor for people with severe stenosis; the majority will develop symptoms within five years (Pellikka 2005), and event‐free survival is as low as 21% at two years (Otto 1997). In the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) study from 2001 to 2004, even in people with mild or moderate aortic stenosis, 10% and 38%, respectively, progressed to surgically significant disease within five years (Gohlke‐Bärwolf 2013). It is thought that the burden of valvular heart disease will continue to increase and that indications for surgery will become broader; at present half of diagnoses of aortic stenosis are made postmortem (d'Arcy 2011).
Description of the intervention
The first total aortic valve replacement was performed in 1958 in a person in whom an attempt at repair caused disintegration of the cusps (Lillehei 1962). In the intervening half‐century, aortic valve repair has grown less common, with replacement with tissue or mechanical prosthetic valves now representing 99% of surgical management of aortic valve disease in the Euro Heart Survey (Iung 2003). It is the second‐most common cardiac surgical procedure in North America (Lee 2011). The prognostic benefit of this operation has been known for many years (Schwarz 1982), and since the early 1980s, the mortality from isolated, uncomplicated aortic valve replacement has dropped more than five‐fold to less than 1% (Carabello 2013). The long‐term freedom from serious complications is similar, even with mechanical valves requiring warfarinisation (Braunwald 2000).
Worldwide aortic valve replacement is most commonly performed via median sternotomy, an incision that extends from the sternal notch to the xiphisternum and divides the entire sternum longitudinally.
Rao and Kumar were the first to describe an aortic valve replacement through a right anterior thoracotomy (Rao 1993). The group used central cannulation and an oblique aortotomy. Subsequently, Cosgrove and Sabik used the term "minimally invasive" to describe an aortic valve procedure via a 10‐cm right anterior thoracotomy, excising the second and third costal cartilages, and employing femoral cannulation to establish cardiopulmonary bypass (CPB) (Cosgrove 1996). Various modifications have since been described, including limited upper hemi‐sternotomy in a J‐shape (Liu 1999a), inverted T‐ or Y‐shape (Cohn 1997), or lazy S (Autschbach 1998). These techniques variably allow access to cannulate the ascending aortic and right atrium ‐ as in open surgery ‐ to establish CPB. Due to the limited access, CPB and aortic cross clamp times may be longer, with theoretical effects on neurological and renal morbidity. Other modifications to the open technique may also be necessary, warranting investment in additional equipment and training (Malaisrie 2014; Walther 2006).
How the intervention might work
Median sternotomy is generally well tolerated due to fixation of the sternum on closure (Lee 2004), but the disruption can nonetheless cause pain and affect respiratory dynamics, reduce mobility, and is thought to necessitate restriction of upper body weight‐bearing (Walther 1999a). Minimally invasive surgery, by virtue of preserving the integrity of the thoracic cage, aims to improve pain, mobility and return to normal activities following discharge (Cohn 1997). These benefits are thought to offset any increase in operative time as a result of reduced surgical access, and therefore potentially reduce cost by up to 20% in all but the people at the highest risk (Cohn 1998). However, these benefits are not guaranteed, as disruption of the intercostal nerves with some approaches might paradoxically cause more pain than that associated with sternotomy (Walther 1999a), and additional port sites or groin cannulation may offset the cosmetic advantage, quality of life or satisfaction (Detter 2002a). Where people have any doubt about the efficacy of a minimally invasive approach, many choose full sternotomy (Ehrlich 2000).
Why it is important to do this review
Aortic valve replacement via full sternotomy is well tolerated and demonstrates excellent long‐term event‐free survival and quality of life. At present, few cardiac surgeons offer minimally invasive aortic valve replacement via limited sternotomy as there is uncertainty whether it offers advantages over conventional aortic valve replacement and there is clinical equipoise. If equivalence in key measures of mortality and morbidity, along with evidence of reduced pain, immobility, length of stay, and overall cost could be demonstrated, there would be momentum to make minimally invasive aortic valve replacement the gold standard. This review sought to evaluate the effect of aortic valve replacement through limited upper hemi‐sternotomy on 30‐day mortality, morbidity, health‐related quality of life, and cost compared with conventional aortic valve replacement through a full median sternotomy in people undergoing aortic valve replacement.
At present, there are no guidelines to either recommend or discourage surgeons from using minimally invasive approaches to aortic valve surgery. Neither the US nor the European guidelines on valvular heart disease make any reference to its use (Nishimura 2014; Vahanian 2012). The Society of Thoracic Surgeons Aortic Valve Guidelines for Management and Quality Measures refers to potential and future benefits of minimally invasive surgery, but makes no specific recommendations (Svensson 2013). The International Society for Minimally Invasive Cardiothoracic Surgery have no consensus guidelines on the subject of minimally invasive aortic valve replacement. As these approaches have been used for nearly two decades, however, it is likely that a dearth of strong evidence influences the decision not to offer recommendations.
Previous meta‐analyses have addressed this subject (Brown 2009; Khoshbin 2011; Murtuza 2008; Phan 2014), but results from a recently reported trial, Borger 2015, necessitates a contemporary review. The earliest of these meta‐analyses was a well‐conducted systematic review that included observational studies in order to address the dearth of randomised controlled studies (Murtuza 2008). However, the technical nature of surgery does not lend itself well to comparison in observational studies. Surgeons and centres that elect to undertake minimally invasive surgery tend to be high‐performing with low complication rates from conventional surgery. Well‐designed randomisation methods are therefore required to minimise the effects of selection biases in surgical studies. Furthermore, three randomised controlled trials have been performed since Murtuza's review (Borger 2015; Calderon 2009; Moustafa 2007), and only two of these were incorporated in subsequent reviews. In addition, the application of Cochrane methodology to the systematic review of the literature and meta‐analysis, including quality scoring, would be of benefit to readers of an updated review.
Objectives
To assess the effects of minimally invasive aortic valve replacement via a limited sternotomy versus conventional aortic valve replacement via median sternotomy in people with aortic valve disease requiring surgical replacement.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials. We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included adults (aged 18 years or greater) with a diagnosis of aortic valve disease requiring aortic valve replacement.
Types of interventions
We included trials comparing minimally invasive aortic valve surgery through any form of hemi‐sternotomy with conventional, isolated aortic valve surgery via median sternotomy. We did not consider transapical or transfemoral aortic valve replacement, or any minimally invasive procedures performed through thoracotomies, video‐assisted thoracoscopic surgery, or other access not through a partial sternotomy. We considered any modifications to the surgical technique to facilitate this form of access including femoral cannulation, transvenous pacing, and rapid deployment/sutureless valves.
Types of outcome measures
The following were the outcome measures of interest for this study. We did not exclude studies that did not report any of the outcomes of interest, but we did comment on them, in narrative form, in the 'Discussion' section.
Primary outcomes
-
Mortality (i.e. all‐cause mortality).
-
Major adverse cardiac or cerebrovascular events (MACCE) (cardiac or cerebrovascular death, myocardial infarction, cardiac arrest, stroke, congestive cardiac failure).
-
Extracorporeal support times (CPB and aortic cross‐clamp).
Secondary outcomes
-
Organ failure requiring support (including respiratory, renal, gastrointestinal, or multi‐organ failure).
-
Length of hospital stay.
-
Postoperative blood loss.
-
Deep sternal wound infection.
-
Pain scores (as measured by visual analogue scale).
-
Quality of life (as measured by 36‐item Short Form (SF‐36) or any other validated scale).
-
Cost of surgery.
-
Intensive care unit length of stay.
-
Postoperative pulmonary function tests.
-
Re‐exploration.
-
Postoperative atrial fibrillation.
-
Postoperative ventilation times.
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases on 6 July 2016:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 6);
-
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily, and MEDLINE (Ovid, 1946 to 6 July 2016);
-
Embase (Ovid, 1980 to week 27, 2016).
We adapted the preliminary search strategy for MEDLINE (Ovid) for use in the other databases (Appendix 1). We applied the Cochrane sensitivity‐maximising randomised controlled trial filter to MEDLINE (Ovid) and for Embase we applied terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/), which was performed on 28 December 2016.
We searched all databases from their inception and we imposed no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information (performed in July 2015 and updated in January 2016):
-
St Jude Medical (professional‐intl.sjm.com/);
-
Edwards Lifesciences (www.edwards.com/products/mivs/pages/avr.aspx);
-
Medtronic (www.medtronic.com/for‐healthcare‐professionals/products‐therapies/cardiovascular/index.htm);
-
On‐X (www.onxlti.com/);
-
Sorin/LivaNova (www.livanova.sorin.com/).
Where the information from initial screening of papers identified studies with uncertain value for this review, we contacted authors to gain access to missing data.
Data collection and analysis
Selection of studies
Two review authors (BHK, SGJ) independently screened titles and abstracts for inclusion of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. In case of disagreement, we asked a third review author (SCM) to arbitrate. We retrieved the full‐text study reports/publication, and two review authors (BHK, SGJ) independently screened the full text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, by consulting a third review author (SCM). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table.
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. Two review authors (BHK, SGJ) extracted study characteristics from included studies. We extracted the following study characteristics.
-
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: n, mean age, age range, gender, pathophysiology of aortic disease (stenotic or regurgitant), severity of condition, EuroSCORE or Society of Thoracic Surgeons score, left ventricular ejection fraction, prevalence of diabetes, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention including mode of access and modifications to cannulation strategy, comparison group, CPB time, and aortic cross‐clamp time.
-
Outcomes: primary and secondary outcomes as specified and collected, and time points reported.
-
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (BHK, SGJ) independently extracted outcome data from included studies. We resolved any disagreements by consensus or by involving a third review author (DC, RJNNW, SCM). One review author (BHK) transferred data into Review Manager 5 (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (SGJ) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (BHK, SGJ) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (SCM). We assessed the risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias (e.g. industry funding or small‐study bias).
We graded each potential source of bias as high, low, or unclear risk of bias and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to a published protocol and reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). The reason we chose RRs in preference to odds ratios was because they are considered easier to interpret (Higgins 2011), and uniformly presenting data with a consistent presentation would allow simpler comparison of effects on complications or risks of surgery. We analysed continuous data as mean difference (MD) (or standardised mean difference (SMD) if different scales were used for measurement of the same outcome measure) with 95% CIs. We entered data presented as a scale with a consistent direction of effect.
We narratively described skewed data reported as medians and interquartile ranges (IQR).
Unit of analysis issues
We did not anticipate unit of analysis issues as we expected all trials to be parallel design.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. Where we identified substantial heterogeneity (I2 greater than 50%), we reported it and explored possible causes.
Assessment of reporting biases
Where we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study biases for the primary outcomes.
Data synthesis
We undertook meta‐analyses only where this was meaningful: that is if the treatments, participants, and the underlying clinical question were considered similar enough for pooling to make sense.
We used a fixed‐effect model on the assumption that surgical techniques for aortic valve replacement were sufficiently standardised in the key components of the procedure to be comparable. If there was substantial heterogeneity (I2 greater than 50%), we used a random‐effects model for pooling, to account for the small but cumulative differences in surgical technique and aftercare that exist between surgeons and units.
'Summary of findings' table
We created a 'Summary of findings' table for our outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it related to the studies which contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software. We justified all decisions to downgrade or upgrade the quality of studies using footnotes and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We did not anticipate performing any subgroup analyses.
Sensitivity analysis
We planned to carry out the following sensitivity analysis: only including studies with a low risk of bias. As none of the included studies were of overall low risk of bias, our final sensitivity analyses were performed by excluding studies evaluated to be at high risk of bias. We also performed a separate sensitivity analysis excluding studies where rapid‐deployment valves were utilised.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice, and our implications for research suggested priorities for future research and outlined what the remaining uncertainties are in the area.
Results
Description of studies
Results of the search
We retrieved 203 references through electronic searching of CENTRAL, MEDLINE, and Embase, following de‐duplication. After review of titles and abstracts, 151 references were screened out as not relevant. From the remaining 52 references, 45 studies were excluded following full‐text review (Figure 1).
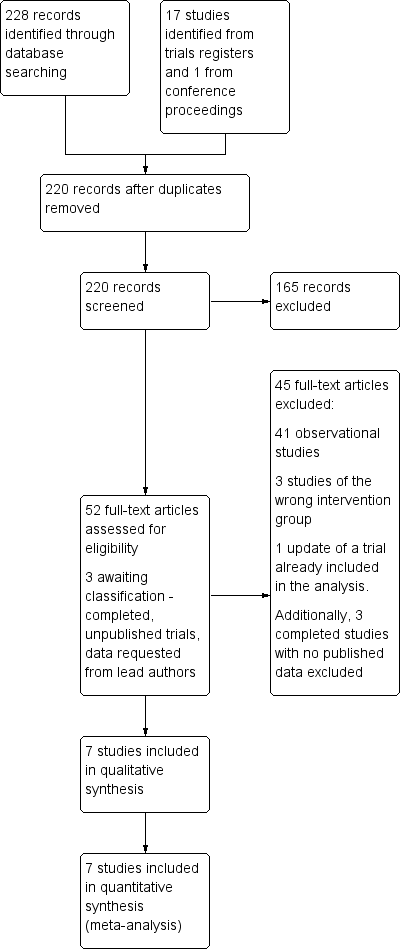
Study flow diagram.
The search for ongoing trials revealed 17 unique studies. Ten were not relevant to the current review, one had been terminated before completion (NCT00221663), one was a proposed long‐term registry (NCT02278666 (SATURNO)) and two were still recruiting (NCT02272621; NCT02726087 (QUALITY‐AVR)). Three were now completed without any published results as yet, although the primary investigators had been contacted (ISRCTN29567910 (MAVRIC); ISRCTN58128724 (MiniStern); NCT01972555 (CMILE)). These were therefore still awaiting classification. One study had been identified in conference proceedings, but excluded as it had not been prospectively randomised.
Included studies
Following the search, screening, and exclusion of irrelevant studies, we identified seven studies that met the inclusion criteria. These seven randomised controlled trials represented 511 participants in studies of between 40 and 120 participants, performed between 1999 and 2015. The studies were performed in Austria (Mächler 1999), Spain (Aris 1999a), Italy (Bonacchi 2002), Germany (Borger 2015; Dogan 2003), France (Calderon 2009), and Egypt (Moustafa 2007). All were undertaken in cardiothoracic surgical settings and only one was a multi‐centre trial, conducted by 12 surgeons across five German centres (Borger 2015).
All seven trials included participants undergoing elective, isolated aortic valve replacement. The majority of studies included both aortic stenosis and aortic regurgitation pathologies except one, which excluded participants with pure aortic regurgitation (Borger 2015). Acute pathology of the aortic valve (i.e. endocarditis), calcified ascending aorta, and other recent potential confounding comorbidities (e.g. myocardial infarction, cerebrovascular accident, significant neurological impairment) were variably described as exclusion criteria, but by definition of the inclusion criteria, all studies were likely to have excluded such participants empirically.
Variations in the participant population may have existed as three studies excluded people with very poor left ventricular ejection fraction under 25% (Bonacchi 2002; Borger 2015; Moustafa 2007). One study excluded participants with moderate left ventricular function under 40% and participants with chronic airway disease or renal impairment (Calderon 2009).
Only one of the studies reported power calculations (Calderon 2009), and four cited the outcome measures a priori in the methods (Aris 1999a; Borger 2015; Calderon 2009; Dogan 2003). All sought institutional ethical approval prior to conduct of the study.
All but one study used reversed L‐shaped upper hemi‐sternotomy as the limited sternotomy; one study used a reversed C‐shaped incision according to anatomy (Bonacchi 2002). For clarity, we will refer to the minimally invasive incision as a limited upper hemi‐sternotomy for the remainder of this review. The surgical technique remained similar between studies, with all employing aortic and right atrial cannulation, rather than femoral cannulation, to institute normothermic or mild hypothermic CPB. Cross‐clamping was exclusively across the ascending aorta and cardioplegia techniques varied in terms of delivery and type. All studies used antegrade, both as root and ostial cardioplegia, but some also gave retrograde cardioplegia for open cases. The choice of cardioplegia solution included blood and crystalloid (both St Thomas' and Bretschneider's solutions).
Choice of prostheses varied across studies. Some studies used exclusively mechanical valves (Aris 1999a; Moustafa 2007, although the former had a single participant exception), whilst others varied the valve choice dependent on participant age. The valve insertion technique was not stipulated in the majority of cases (e.g. interrupted, pledgeted, semi‐continuous, etc.) except for one study which compared rapid deployment balloon expandable stented valves for the mini‐sternotomy arm (Borger 2015). Venting strategies also varied between studies with pulmonary vein, pulmonary artery, aortic root, or no venting used.
Of the primary outcome measures, all studies reported perioperative mortality (as either in‐hospital or 30‐day mortality). Bonacchi 2002 did not provide data for CPB time and Mächler 1999 reported this as median with IQR, precluding it from inclusion in the quantitative analysis. All studies reported aortic cross‐clamp times, but again reported as median and ranges in one study (Mächler 1999), which was therefore excluded from meta‐analysis. None of the studies reported major adverse outcomes as a composite, but all reported major complications. Long‐term follow‐up beyond six months was not described.
The secondary outcome measures for the meta‐analysis were also variably reported. Studies frequently documented organ failure requiring support, but not universally in the outcome tables. All but two study reported total hospital stay (Borger 2015; Mächler 1999), both from Germany where length of stay is not considered a quality marker. Blood loss was described by several different methods which were not universally comparable. Only one study measured quality of life (Borger 2015), and no trials reported a cost analysis. Pulmonary function tests included forced expiratory volume in one second (FEV1) in all studies that reported this outcome.
None of the studies described their protocols for transfusion, return to theatre, discharge from intensive care unit, or discharge from hospital.
This information is summarised in the Characteristics of included studies tables.
Excluded studies
The Characteristics of excluded studies table effectively summarises the reasons for excluding the 45 studies not included in the meta‐analysis. Five studies were not randomised, 35 were observational, and four were not via partial sternotomies (two via thoracotomy, one port access, and one robotic).
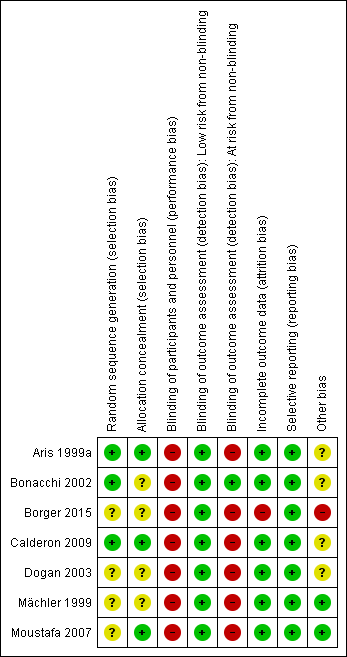
Risk of bias in included studies
The risk of bias is summarised in the 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary table (Figure 3). We made overall judgements on the risk of bias per study based on the number of domains assessed as high risk. Due to the nature of studies on surgical incisions, nearly all included studies were at high risk of bias for lack of blinding, but this will have affected the various outcome measures inconsistently (e.g. pain was likely to have been highly influenced by lack of blinding whereas deep sternal wound infection was not). Studies with risk of bias thought to be high that was not related to blinding were therefore considered high risk of bias and excluded in the sensitivity analyses.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three studies used computer‐generated random allocation sequence generation (Aris 1999a; Bonacchi 2002; Calderon 2009); this was unclear in four studies (Borger 2015; Dogan 2003; Mächler 1999; Moustafa 2007). Three studies achieved allocation concealment by sealed enveloped opened at the time of surgery (Aris 1999a; Calderon 2009; Moustafa 2007); this was unspecified for the other four studies.
Blinding
Although blinding of the participants following minimally invasive surgery has previously been described by use of standardised dressings, none of the trials included here employed participant blinding. The surgeons were not blinded in any trial and it was not clear who the outcome assessors were in the majority of trials. Bonacchi 2002 employed blinded outcome assessors for pain score measurements, but not for any of the remaining outcomes. Nonetheless, for several quantifiable outcome measures (e.g. chest tube drainage), lack of blinding should not have significantly affected detection bias.
Incomplete outcome data
The majority of studies reported outcomes on all randomised participants (Aris 1999a; Bonacchi 2002; Calderon 2009; Dogan 2003; Mächler 1999; Moustafa 2007). In one study, there were six withdrawals from the study after randomisation (five in the limited sternotomy and one in the full sternotomy group), and the data were reported for participants who underwent treatment as intended (Borger 2015). Three of the participants in this study were withdrawn because participants randomised to minimally invasive surgery "eventually received a conventional valve because of problems with their anatomy". As such, these participants would have constituted a failure to proceed with intended surgery because of the intervention and would contribute to attrition bias.
Selective reporting
The majority of studies had not widely published a trial protocol citing their intended outcome measures. Aris and colleagues had a protocol approved with their Departmental Research Committee but did not register this with an international registry (Aris 1999a). Bonacchi and coworkers did not describe having a protocol prior to starting the trial (Bonacchi 2002). Borger and colleagues published a protocol (CADENCE‐MIS), but did not report on four prespecified secondary outcome measures (velocity‐time index, left ventricular outflow tract diameter, annular size, or septal thickness) (Borger 2015). Calderon's group had published a protocol with similar characteristics to the published study (NCT00221663), but this was updated as "Terminated ‐ due to slow recruitment" (Calderon 2009). Only one proposed outcome measure from the retracted protocol was not included (cytokine levels from tracheal aspirates). The published study described approval from the local ethics committee. Dogan and colleagues' study was approved by the institutional ethics committee but the protocol not published a priori (Dogan 2003). Mächler and coworkers did not describe a prestudy protocol and Moustafa and colleagues stated that their study had received approval from the protocol research committee at their institution, but did not have a published protocol in a registry (Mächler 1999; Moustafa 2007).
Whilst specific outcomes may have had variable reporting within studies, we adopted an approach of assessing selective reporting bias at a study‐level, in accordance with recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered which outcome measures for aortic valve replacement were important and could be reasonably expected to be reported and found that all studies had included information about the most important measures. We did not downgrade the judgement for Borger and colleagues' study on the basis of the missing variables as we did not consider these to be important clinical measures.
Other potential sources of bias
The minor differences in the surgical techniques between studies was not thought to have contributed a significant risk of bias, although it may have introduced so explicable heterogeneity.
Within studies, one trial had a significant confounding factor in the methodology in that the limited upper hemi‐sternotomy group also received rapid deployment balloon‐expandable valves, whereas the full‐sternotomy group received conventional surgically implanted stented prostheses (Borger 2015). This study was also funded by the manufacturer of the expanding valve.
Four studies did not report detailed demographic differences between the two groups at baseline (Aris 1999a; Bonacchi 2002; Calderon 2009; Dogan 2003), and it is unclear if this may have introduced bias.
Effects of interventions
The summary of findings Table for the main comparison provides an overview of the aggregated results of the studies.
Primary outcomes
Mortality
All seven trials reported mortality either as in‐hospital or 30‐day mortality, but zero events in both groups in two trials meant that the effect was not estimable for these studies (Dogan 2003; Moustafa 2007). The overall effect estimate for 511 participants suggested no difference between limited and full sternotomy on perioperative mortality (RR 1.01, 95% CI 0.36 to 2.82; participants = 511; studies = 7; I2 = 0%; quality of evidence = moderate) (Analysis 1.1). A sensitivity analysis, removing two studies at high risk of bias (Borger 2015; Mächler 1999), did not change this outcome (RR 0.64, 95% CI 0.17 to 2.35; participants = 297; studies = 5; I2 = 0%).
Major adverse cardiac or cerebrovascular events
None of the included studies reported a composite of MACCE.
Cardiopulmonary bypass time
Five studies reported CPB times in formats that could be pooled (Aris 1999a; Borger 2015; Calderon 2009; Dogan 2003; Moustafa 2007). One study did not cite CPB times but noted that there was no statistically significant difference between the two groups (Bonacchi 2002). Another study provided median CPB times, with IQR, that was not amenable to meta‐analysis (Mächler 1999).
The remainder of the studies showed significant heterogeneity, likely to represent the cumulative effects of intraoperative differences between surgeons, hospitals, and countries. It is unlikely that this clinical heterogeneity can be corrected for by trial methodology, and we therefore elected to use a random‐effects model to mitigate these differences to some degree. As CPB time is such an important surrogate marker of clinical outcome following cardiac surgery, we chose not to exclude this outcome measure completely from quantitative meta‐analysis, but downgraded the quality level of evidence. The overall effect was of no difference (MD 3.02 minutes, 95% CI ‐4.10 to 10.14; participants = 311; studies = 5; I2 = 84%; quality of evidence = low) (Analysis 1.2). Sensitivity analysis performed by exclusion of the study using rapid‐deployment valves in the limited sternotomy arm of the study did not change the results (Borger 2015).
Aortic cross‐clamp time
We excluded only one study in the analysis of aortic cross‐clamp times, as data were presented as median and IQR (Mächler 1999). The estimate of effect for the remaining studies suggested no difference in the outcome between limited and full sternotomy (MD 0.95 minutes, 95% CI ‐3.45 to 5.35; participants = 391; studies = 6; I2 = 82%, quality of evidence = low) (Analysis 1.3). Several explanations might exist for the heterogeneity in these studies. Because of variations in the type of aortic pathology across studies, some aortic annuli may have required more extensive decalcification than others. The use of rapid deployment valves in one study may also have affected the clinical heterogeneity (Borger 2015). Borger and colleague used Edwards Intuity rapid deployment valves which do not require aortic decalcification (unlike some other rapid deployment valves) and this will have also contributed to the reduction in aortic cross‐clamp time. As with our meta‐analysis of CPB times, we felt that the clinical importance of this measure warranted quantification with appropriate consideration of reasons for differences across studies. Sensitivity analysis, removing the study by Borger 2015, that may have been biased by the use of rapid‐deployment valves in the minimally invasive group, did not substantially change the overall effect.
Secondary outcomes
Organ failure requiring support
No studies reported a composite of multi‐organ failure.
Length of hospital stay
Five studies assessed length of hospital stay following aortic valve replacement via either full or upper hemi‐sternotomy. Aris 1999a, Calderon 2009, and Dogan 2003 had results that clustered around the point of equipoise, with Bonacchi 2002 demonstrating a 95% CI that just fell in favour of surgery via limited hemi‐sternotomy. The study from Egypt showed a much greater advantage of upper hemi‐sternotomy, though the length of stay in the full sternotomy group was substantially higher than other studies (mean stay more than two weeks) (Moustafa 2007). As the discharge criteria for institutions can vary and the mean stay in this study was likely to have been affected by a long‐staying outlier, this may explain the high heterogeneity. The overall estimate of effect almost favoured limited sternotomy (MD ‐1.31 days, 95% CI ‐2.63 to 0.01; participants = 297; studies = 5; I2 = 89%; quality of evidence = very low) (Analysis 1.4). When the study by Moustafa 2007 was excluded, the heterogeneity fell considerably, but the overall effect was still of no difference (MD ‐0.30 days, 95% CI ‐0.67 to 0.08; participants = 237; studies = 4; I2 = 5%).
Postoperative blood loss
There was substantial heterogeneity in the results of the five studies that assessed blood loss in the postoperative period and a random‐effects model was therefore employed. The reasons for this heterogeneity were considered and the benefits of performing a quantitative meta‐analysis weighed. As the total measured blood loss may vary across studies as a result of the type of drainage tubes, haemostatic protocols, and postoperative thromboprophylaxis measures employed, this outcome measure was considered at high risk of clinical heterogeneity across cardiac surgical units. However, minimally invasive procedures are more susceptible to field flooding with small amounts of bleeding, and more meticulous haemostasis is required during dissection, which may have reduced the overall bleeding in this group. In addition, the use of transpleural drains in people undergoing upper hemi‐sternotomies (due to the sub‐xiphoid site being difficult to reach) may have allowed some pericardial bleeding to evacuate into the pleura, thereby reducing the estimated blood loss in this group. All but the oldest study by Aris 1999a demonstrated an advantage in this domain for minimally invasive surgery via limited sternotomy and the cumulative effect was that upper hemi‐sternotomy reduced postoperative bleeding (MD ‐158.00 mL, 95% CI ‐303.24 to ‐12.76; participants = 297; studies = 5; I2 = 93%, quality of evidence = moderate) (Analysis 1.5). No sensitivity analyses were performed for this outcome measure.
Deep sternal wound infection
All seven studies reported the incidence of postoperative sternal wound infections but only three had any events in either group (Bonacchi 2002; Borger 2015; Mächler 1999), increasing the possibility of a Type II error. The estimate of effect suggested no differences between full or limited sternotomy (RR 0.71, 95% CI 0.22 to 2.30; participants = 511; studies = 7; I2 = 0%, quality of evidence = moderate) (Analysis 1.6), but the wide variation in the effects both within and between studies implied that these were not powered to identify a difference. Sensitivity analyses had no effect on the estimate as removal of the studies by Borger 2015 and Mächler 1999 left only one study with events (Bonacchi 2002) that showed no difference in outcome.
Pain scores
Three studies described pain scores between the two groups. Bonacchi 2002 used self‐reported pain scores at one and 12 hours measured by nurses blinded to the treatment groups. The figures for 12 hours were compared here. Participants experiencing moderate pain were treated with morphine and non‐steroidal anti‐inflammatory medications. Calderon 2009 employed a 40‐mm visual analogue scale for pain measurements at two days postoperatively. All participants were given paracetamol 1 g every six hours and a morphine patient‐controlled analgesia device to deliver 1‐mg boluses up to every seven minutes. Non‐steroidal analgesia was added to this regimen if participants were still in pain. Unlike the other two studies that reported pain levels, participants in this study reported more pain in the limited sternotomy group than in the full sternotomy group (not reaching statistical significance), but the analgesia usage was also lower in the upper hemi‐sternotomy group. The effects of non‐blinding may have been responsible for this disparity as participants with limited upper hemi‐sternotomy surgery may have felt that they should not require as much analgesia and therefore ended up with higher pain scores. The study by Dogan 2003 also utilised a visual analogue scale to measure pain on the second postoperative day. These were repeated at day five but not included for comparison. The overall estimate of effect using a random‐effects model for the heterogenous data did not show any advantage to surgery via limited upper hemi‐sternotomy (SMD ‐0.33, 95% CI ‐0.85 to 0.20; participants = 197; studies = 3; I2 = 70%; quality of evidence = very low) (Analysis 1.7). There were no sensitivity analyses performed.
Quality of life
Only one study examined quality of life using a validated tool (Borger 2015). There was no difference between full and upper hemi‐sternotomy groups (MD 0.00, 95% CI ‐0.04 to 0.04; participants = 100; studies = 1; I2 = 0%) (Analysis 1.8).
Cost of surgery
None of the included studies reported cost analyses.
Intensive care unit length of stay
Five papers described intensive care stay, but due to a small standard deviation in the reporting of one paper (Calderon 2009), the analysis was weighted on the basis of the remaining four studies only (Aris 1999a; Bonacchi 2002; Dogan 2003; Moustafa 2007). All but the oldest (Aris 1999a) demonstrated an advantage of upper hemi‐sternotomy on length of stay in intensive care, though heterogeneity was high. The overall effect was of a reduction in critical care stay for participants undergoing minimally invasive surgery through limited upper hemi‐sternotomy (MD ‐0.57 days, 95% CI ‐0.93 to ‐0.20; participants = 297; studies = 5; I2 = 79%; quality of evidence = low) (Analysis 1.9). Lack of blinding was thought to have a greater influence on intensive care length of stay than some other outcome measures: trial participants undergoing limited sternotomy were likely to have been promoted for discharge from the critical care area in order to facilitate their mobilisation and recovery. In addition, clinical heterogeneity will have been influenced by the differing practices of monitoring and discharge from intensive care across surgical departments. No sensitivity analyses were performed.
Postoperative pulmonary function tests
Four papers assessed the effects of aortic valve replacement through limited upper hemi‐sternotomy on lung function, although FEV1 (either as an absolute measurement or as a percentage of predicted) was the only common parameter assessed in all these studies. Aris 1999a performed lung function tests preoperatively and again at discharge, finding a statistically significant drop in lung function following surgery, but no difference in the drop between full and upper hemi‐sternotomy groups. Bonacchi 2002 performed lung function tests at five days postoperatively and again at one to two months. The figures for the fifth postoperative day were included in this comparison. Baseline reference pulmonary function tests were not described. The study by Calderon 2009 included preoperative baseline lung function tests and again at 24 hours', 48 hours', and seven days' postoperatively. The data for day seven were used for the analysis. Moustafa 2007 also performed lung function tests at baseline (preoperative), one week, and one month. The data were not clearly annotated and the variability was assumed to be standard error (rather than standard deviation) due to the small differences and we converted it accordingly. The figures for FEV1 at one week were used in the analysis. Despite the differences in time of measurement, there was relatively little heterogeneity in the studies included and the overall effect was of an increase in FEV1 postoperatively in participants undergoing upper hemi‐sternotomy compared to full sternotomy (MD 1.98 % predicted FEV1, 95% CI 0.62 to 3.33; participants = 257; studies = 4; I2 = 28%; quality of evidence = low) (Analysis 1.10).
Re‐exploration
All seven included studies described re‐exploration for bleeding, although one had no events in either group and the effect was therefore not estimable in the analysis (Aris 1999a). The CIs for each of the studies crossed over the line of no effect and therefore the net effect was of no difference between full and upper hemi‐sternotomy (RR 1.01, 95% CI 0.48 to 2.13; participants = 511; studies = 7; I2 = 0%; quality of evidence = moderate) (Analysis 1.11). The effects of a sensitivity analysis (removing Borger 2015 and Mächler 1999, the studies at highest risk of bias) did not change this outcome (RR 0.78, 95% CI 0.30 to 2.04; participants = 297; studies = 5; I2 = 29%).
Postoperative atrial fibrillation
Three studies included data on rates of postoperative atrial fibrillation. Management of atrial fibrillation varies considerably from surgeon to surgeon, with some adopting a prophylactic approach, most treating at onset and pharmacological options being quite wide. This, along with the cannulation strategy, choice of cardioplegia, and degree of atrial manipulation, may explain the heterogeneity in this group. There was no evidence in a combined comparison of an effect on atrial fibrillation by minimally invasive aortic valve replacement through limited hemi‐sternotomy (RR 0.60, 95% CI 0.07 to 4.89; participants = 240; studies = 3; I2 = 79%; quality of evidence = low) (Analysis 1.12). A sensitivity analysis made no change to this overall effect.
Postoperative ventilation time
All but one study (Borger 2015) reported length of invasive ventilation postoperatively. The study by Mächler 1999 presented this as median and IQRs and was not included in the quantitative comparison. They found a statistically significant reduction in the postoperative ventilation time in limited compared to full sternotomy (median 7 hours (IQR 5.3 to 11) with limited versus 10 hours (IQR 8.5 to 12) with full, P < 0.0001). The data from the remaining studies were highly heterogeneous and this is likely to have been due to clinical differences in extubation protocols between units. Only one study described their criteria for extubation (Bonacchi 2002). The overall estimate of effect was of no difference (MD ‐1.12 hours, 95% CI ‐3.43 to 1.19; participants = 297; studies = 5; I2 = 97%; quality of evidence = very low) (Analysis 1.13). Making the assumption that the study by Mächler 1999 had presented normally distributed data as median and IQRs and making an approximated conversion did not change the overall effect (Section 7.7.3.5, Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011).
Discussion
Summary of main results
This systematic review assessed the effects of full versus limited sternotomy on mortality, CPB time, aortic cross‐clamp time, length of hospital and intensive care unit stay, postoperative blood loss, deep sternal wound infection, pain scores, quality of life measures, pulmonary function tests, re‐exploration for bleeding, postoperative atrial fibrillation, and ventilation times. We found seven randomised controlled trials with 511 participants that answered the study question, which were generally of low‐to‐moderate quality of evidence. Some of the outcome measures were of very low quality evidence. The inherent difficulties of blinding surgical access, relatively small study sizes (and corresponding failure to meet Optimal Information Size criteria), and clinically heterogeneous populations were the main reasons for downgrading quality of evidence.
All the identified studies used upper hemi‐sternotomy as the mode of limited sternotomy. Meta‐analysis found no evidence of survival benefit, or increase in risk, with minimally invasive surgery via limited upper hemi‐sternotomy. This correlates with other, recent literature (Phan 2014). The wide CIs of the studies, including the null‐events in two of the studies demonstrate that few of these studies were powered to demonstrate differences in perioperative mortality and the aggregate effect did not cross the Optimal Information Size criteria either.
There were no treatment effects for extracorporeal support and ischaemic times between the two groups. The oldest study showed the largest difference between full and minimally invasive surgery with a significant disadvantage to performing limited sternotomy (Aris 1999a), but this was less apparent in subsequent trials, presumably as a result of the technique being refined. In one trial, the use of rapid deployment valves meant that the disadvantage of limited sternotomy on operative times was negated (Borger 2015). In fact, the advantage conferred by these valves meant that operative times were uniquely shorter for the limited sternotomy cohort in this trial. This may have confounded the comparison between the two groups and the authors acknowledged this in their discussion. Removing the study for sensitivity analysis, however, did not change the estimate of effect, which remained equivalent between the two groups.
Length of hospital stay was no shorter with minimally invasive surgery via limited sternotomy, although the effect bordered on significance. As enhanced recovery programmes and expedited discharge plans become more commonplace, even for major operations, this is expected. The analysis showed an advantage, however, in the reduction of intensive care unit stay with limited sternotomy. It was unclear why this occurred, however, as there were no differences in surgical times, pain scores, respiratory function, rates of atrial fibrillation, or return to theatre for bleeding between the two groups. A number of the studies included also described ventilator time in their outcomes, and these too showed no overall difference between limited or full sternotomy. This raises the possibility of unblinded minimally invasive patients with limited sternotomies being expedited out of the critical care unit.
Overall completeness and applicability of evidence
All the studies included in this systematic review directly addressed the review question and allowed meta‐analyses on a variety of outcome measures. The trial populations seemed to be representative of the people who might undergo aortic valve replacements, including people with aortic regurgitation and aortic stenosis. People at high risk from cardiac surgery were typically excluded (e.g. people with need for multiple or complex procedures or people with left ventricular impairment) and in the present climate, such people would not be eligible for minimally invasive surgery in any case. The surgical techniques appeared to be consistent with current practice in aortic valve implantation, with a combination of mechanical and tissue prosthetic valves implanted. One study used aprotinin routinely (Aris 1999a), which was in the interim withdrawn by the manufacturers because of an increased risk of mortality. This suspension was lifted in 2012. All studies employed aorto‐atrial CPB unless exposure dictated femoral cannulation necessary. As such, the techniques employed appeared to be relevant to modern practice, although clinically heterogeneous.
One study had an overall mortality of 10% for uncomplicated isolated aortic valve replacements via either approach (Aris 1999a). The remainder of the studies appeared to have mortality rates consistent with the expected rates for selected participants. CPB times and aortic cross‐clamp times appeared to be consistent with expected operating times. The remainder of the outcome measures in the conventional approach (full sternotomy) group appeared to correlate with equivalent data in the literature, suggesting that in all studies, the operating surgeons had already passed their learning curves for the procedures.
Quality of the evidence
This meta‐analysis represented 511 participants in seven randomised controlled studies. Most of the studies were underpowered to identify differences in the outcome measures cited. The overall quality of the evidence was low. The summary of findings Table for the main comparison demonstrated the main factors affecting quality within and between studies.
Limitations in study design and implementation
Four studies described adequate control of sequence generation and allocation concealment using computerised systems and envelopes. Blinding was not performed in any study with the exception of pain scores in the paper by Bonacchi 2002. There was no evidence of selective outcome reporting of important outcome measures, but only one study had a pretrial protocol published in an international registry (Borger 2015). This study presented data in a per‐protocol analysis rather than intention‐to‐treat. Participants who dropped out of particular arms of the study could represent failure of the procedure in that case (especially for minimally invasive approaches where the intended valve could not be deployed). The follow‐up was complete in all cases.
Indirectness of evidence
In general, there was no serious concerns of systematic bias as a result of indirectness. Two outcome measures were downgraded on quality of evidence for indirect measures. Pain was measured on a number of different scales across studies. Length of stay was also considered a poor surrogate marker of surgical outcome as different healthcare systems have different philosophies on discharging from hospital: some consider an expedited discharge an indication of participant well‐being, whereas others do not construe early discharge in this way.
Unexplained heterogeneity or inconsistency of results
Eight of the outcome measures that were amenable to quantitative analysis showed substantial heterogeneity. The individual reasons for these have been explored in the discussions for each outcome, but broadly can be attributed to the array of surgical and postoperative differences in practice across departments. Many of these cumulative, minor differences in practice were not protocolised or described in the studies and will have contributed to the clinical heterogeneity in this study. In addition, the use of a novel rapid deployment valve in the minimally invasive arm of one study introduced further heterogeneity in the results (Borger 2015), and this has been elaborated on elsewhere. Inclusion and exclusion criteria were broadly similar, but the variation in aortic valve pathology across studies may have introduced differences in operating times (due to the need for annular decalcification in stenotic valves) and different risk profiles (absence of calcium deposition in the aortic root may reduce the risk of neurological complications). The absence of clear protocols for transfusion, discharge from intensive care unit, discharge from hospital, and return to theatre may also have caused differences in outcome measures, but this should have been standardised between groups within studies. One study had within‐study differences of pain control according to which measure was used (visual analogue scale of self‐reported pain versus total dose of morphine delivered via patient‐controlled analgesia), confirming that surrogate markers may not always be reliable indicators (Calderon 2009).
Imprecision of results
The studies were all underpowered according to Optimal Information Size to measure mortality, deep sternal wound infection, and re‐exploration for bleeding. The details of this calculation are outlined in the footnotes of the summary of findings Table for the main comparison.
Publication bias
A number of randomised controlled trials that had been registered but not completed may reflect attempts to perform aortic valve replacement via minimally invasive approaches that were deemed unsuccessful. There may, therefore, be some potential for publication bias if centres that have demonstrated poor results have terminated their programmes or failed to publish their results.
Potential biases in the review process
Two of the review authors have practices that include minimally invasive aortic valve replacement and one of the review authors consults for a manufacturer of minimally invasive surgical equipment. However, the literature search, review and analysis has been performed in a transparent and reproducible manner. This should have reduced any risk of bias in this review.
We had a number of postprotocol changes to the review methodology which might indicate a bias in the process resulting from prior knowledge of the findings. Several outcome measures were added following aggregation of data: these were not known to us at the time of writing the protocol and we do not believe this could have been foreseen.
Agreements and disagreements with other studies or reviews
One large meta‐analysis incorporating randomised controlled trials, propensity matched studies, and observational studies, found similar outcomes to this review (Phan 2014). They found a significant reduction in perioperative mortality for minimally invasive aortic value replacement (including but not exclusive to upper hemi‐sternotomy), but in subgroup analysis, this difference was only evident in the non‐randomised studies. Similarly, the differences in cross‐clamp and CPB times were significant only when randomised trials were excluded. With more recent studies, both mortality and operative times came closer to each other in both groups, suggesting an early learning curve to minimally invasive surgery. For other outcome measures, Phan and colleagues aggregated mini‐sternotomy and mini‐thoracotomy approaches to aortic value replacement and the comparisons are therefore not applicable for a comparison of limited versus full sternotomy.
Our findings correlated with the trend in the literature: that minimally invasive aortic valve surgery via limited upper hemi‐sternotomy can be performed at least as safely as conventional surgery via a full sternotomy. We did not identify reduction in pain as a result of less‐invasive approaches, but did find a reduction in intensive care stay and a trend towards reduction in hospital stay.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
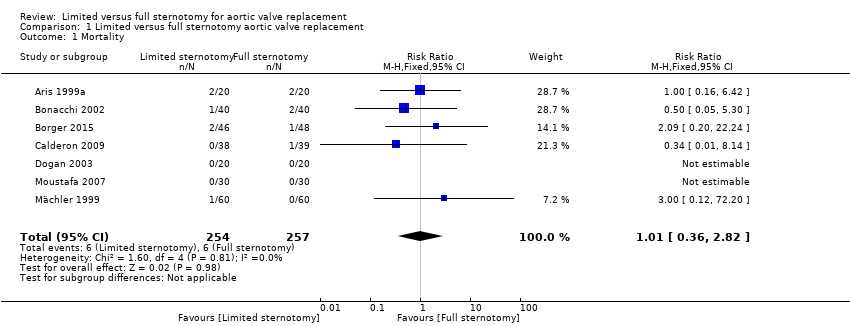
Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 1 Mortality.
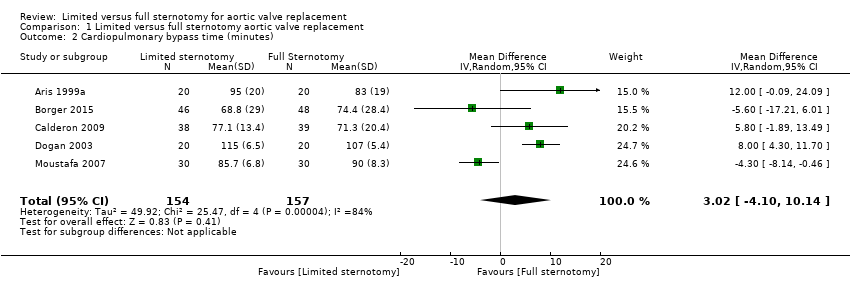
Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 2 Cardiopulmonary bypass time (minutes).
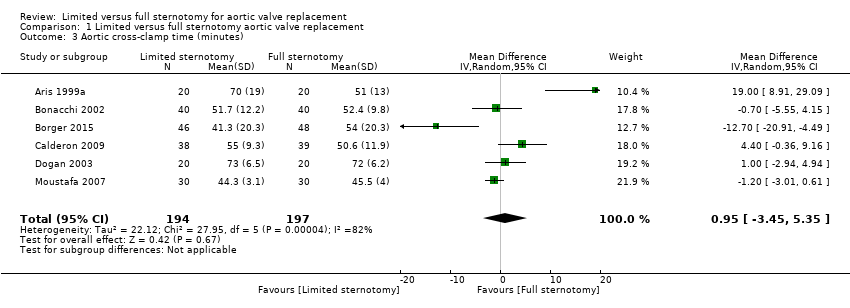
Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 3 Aortic cross‐clamp time (minutes).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 4 Length of hospital stay (days).
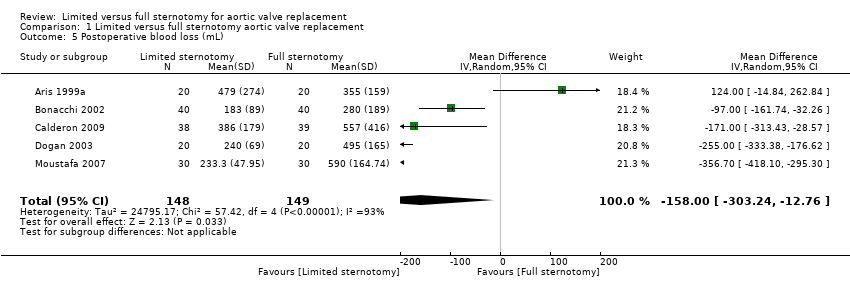
Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 5 Postoperative blood loss (mL).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 6 Deep sternal wound infection.
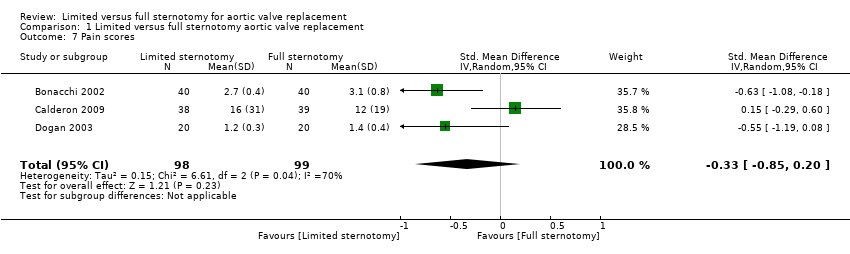
Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 7 Pain scores.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 8 Quality of life.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 9 Intensive care unit length of stay (days).

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 10 Postoperative pulmonary function tests (% FEV1).
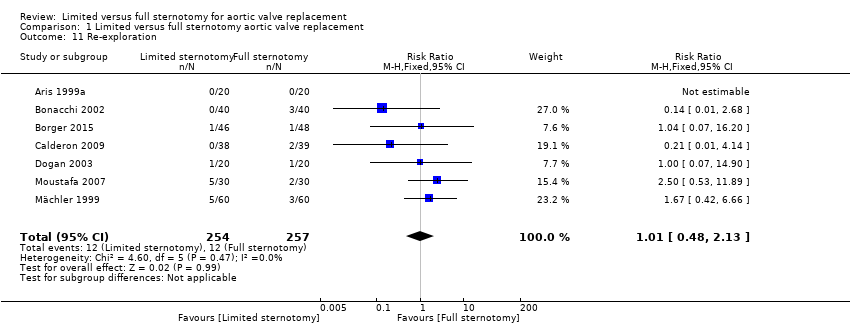
Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 11 Re‐exploration.
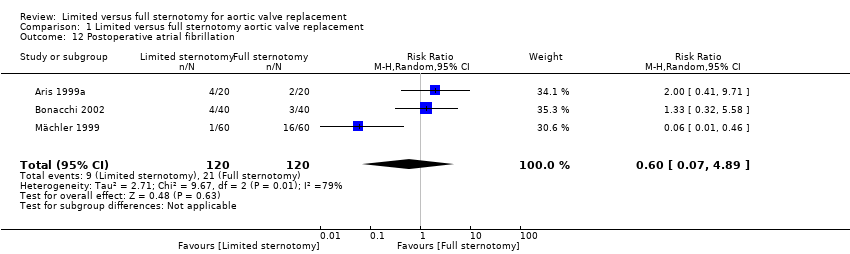
Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 12 Postoperative atrial fibrillation.

Comparison 1 Limited versus full sternotomy aortic valve replacement, Outcome 13 Postoperative ventilation time (hours).
| Limited upper hemi‐sternotomy versus full median sternotomy for aortic valve replacement | ||||||
| Patient or population: participants requiring aortic valve replacement | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with Full Sternotomy | Risk with Limited Sternotomy | |||||
| Mortality Follow‐up: in‐patient stay | Study population | RR 1.01 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 23 per 1000 | 24 per 1000 | |||||
| Cardiopulmonary bypass time | The mean cardiopulmonary bypass time ranged from 71 to 107 minutes | The mean cardiopulmonary bypass time in the intervention group was 3.02 minutes more (4.1 fewer to 10.14 more) | ‐ | 311 | ⊕⊕⊝⊝ | Cardiopulmonary bypass times tend to have high variability between surgeons according to surgical technique. Differences of up to 15 minutes are unlikely to have clinical significance. |
| Aortic cross‐clamp time | The mean aortic cross‐clamp time ranged from 46 to 72 minutes | The mean aortic cross‐clamp time in the intervention group was 0.95 minutes more (3.45 fewer to 5.35 more) | ‐ | 391 | ⊕⊕⊝⊝ | Ischaemic times tend to have high variability between surgeons according to surgical technique. Differences of up to 10 minutes are unlikely to have clinical significance. |
| Length of hospital stay Follow‐up: in‐patient stay | The mean length of hospital stay ranged from 6.0 to 9.3 days | The mean length of hospital stay in the intervention group was 1.31 days lower (2.63 lower to 0.01 higher) | ‐ | 297 | ⊕⊝⊝⊝ | Expediency of discharge is a quality marker in some healthcare systems, but not universally. |
| Postoperative blood loss Follow‐up: until removal of operative drains | The mean postoperative blood loss ranged from 280 mL to 590 mL | The mean postoperative blood loss in the intervention group was 158 mL lower (303 lower to 12 lower) | ‐ | 297 | ⊕⊕⊕⊝ | ‐ |
| Deep sternal wound infection Follow‐up: not specified | Study population | RR 0.71 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 23 per 1000 | 17 per 1000 | |||||
| Pain scores Follow‐up: 12 hours | The mean pain scores ranged from 1.2 to 16 standard deviations | The mean pain scores in the intervention group was 0.3 standard deviations fewer (0.85 fewer to 0.2 more) | ‐ | 197 | ⊕⊝⊝⊝ | The assessment of pain within and across studies was insufficiently standardised to make strong conclusions about effect on pain |
| Intensive care unit length of stay | The mean intensive care unit stay was 1.4 to 2.1 days | The mean intensive care unit stay in the intervention group was 0.57 days lower (0.93 lower to 0.2 lower) | ‐ | 297 | ⊕⊕⊝⊝ | ‐ |
| Postoperative pulmonary function tests Follow‐up: 5 to 7 days | The mean pulmonary function tests ranged from 53% to 82% predicted FEV1 | The mean pulmonary function tests in the intervention group was 1.98% predicted FEV1 higher (0.62 higher to 3.33 higher) | ‐ | 257 | ⊕⊕⊝⊝ | ‐ |
| Re‐exploration Follow‐up: in‐patient stay | Study population | RR 1.01 | 511 | ⊕⊕⊕⊝ | ‐ | |
| 47 per 1000 | 47 per 1000 | |||||
| Postoperative atrial fibrillation Follow‐up: in‐patient stay | Study population | RR 0.60 | 240 | ⊕⊕⊝⊝ | ‐ | |
| 175 per 1000 | 105 per 1000 | |||||
| Postoperative ventilation time | The mean postoperative ventilation time ranged from 5.3 to 13.2 hours | The mean postoperative ventilation time in the intervention group was 1.12 hours lower (3.43 lower to 1.19 higher) | ‐ | 297 | ⊕⊝⊝⊝ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 4600 (to determine 1% difference using α 0.05, β 0.20). Studies all had fewer than 100 participants. 2 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 120 (to determine 15‐minute difference using α 0.05, β 0.80). Studies all had fewer than 100 participants. 3 Downgraded for inconsistency: use of rapid deployment valves in one study and other variations in surgical technique lead to high heterogeneity. 4 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 100 (to determine 10‐minute difference in mortality using α 0.05, β 0.80). Studies all had fewer than 100 participants. 5 Downgraded for imprecision: sample size did not meet Optimal Information Size criteria and 95% confidence intervals overlapped no effect. Optimal Information Size estimated at 140 (to determine 1‐day difference using α 0.05, β 0.80). Studies all had fewer than 100 participants. 6 Downgraded for indirectness: length of stay is a surrogate marker of quality and national variations exist in discharge criteria. 7 Downgraded for high risk of bias: outcome measure sensitive to lack of blinding in study. 8 Downgraded for inconsistency: variations in surgical or anaesthetic technique lead to high heterogeneity. 9 Downgraded for indirectness: different measures of pain used across studies. 10 Downgraded for inconsistency: different timing of postsurgical lung function tests across studies lead to high heterogeneity. 11 Downgraded for imprecision: wide 95% confidence intervals overlapping no effect. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.36, 2.82] |
| 2 Cardiopulmonary bypass time (minutes) Show forest plot | 5 | 311 | Mean Difference (IV, Random, 95% CI) | 3.02 [‐4.10, 10.14] |
| 3 Aortic cross‐clamp time (minutes) Show forest plot | 6 | 391 | Mean Difference (IV, Random, 95% CI) | 0.95 [‐3.45, 5.35] |
| 4 Length of hospital stay (days) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐2.63, 0.01] |
| 5 Postoperative blood loss (mL) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐158.00 [‐303.24, ‐12.76] |
| 6 Deep sternal wound infection Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.22, 2.30] |
| 7 Pain scores Show forest plot | 3 | 197 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.85, 0.20] |
| 8 Quality of life Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 9 Intensive care unit length of stay (days) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.93, ‐0.20] |
| 10 Postoperative pulmonary function tests (% FEV1) Show forest plot | 4 | 257 | Mean Difference (IV, Fixed, 95% CI) | 1.98 [0.62, 3.33] |
| 11 Re‐exploration Show forest plot | 7 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.48, 2.13] |
| 12 Postoperative atrial fibrillation Show forest plot | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.07, 4.89] |
| 13 Postoperative ventilation time (hours) Show forest plot | 5 | 297 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐3.43, 1.19] |

