Whole body vibration exercise training for fibromyalgia
Abstract
Background
Exercise training is commonly recommended for adults with fibromyalgia. We defined whole body vibration (WBV) exercise as use of a vertical or rotary oscillating platform as an exercise stimulus while the individual engages in sustained static positioning or dynamic movements. The individual stands on the platform, and oscillations result in vibrations transmitted to the subject through the legs. This review is one of a series of reviews that replaces the first review published in 2002.
Objectives
To evaluate benefits and harms of WBV exercise training in adults with fibromyalgia.
Search methods
We searched the Cochrane Library, MEDLINE, Embase, CINAHL, PEDro, Thesis and Dissertation Abstracts, AMED, WHO ICTRP, and ClinicalTrials.gov up to December 2016, unrestricted by language, to identify potentially relevant trials.
Selection criteria
We included randomized controlled trials (RCTs) in adults with the diagnosis of fibromyalgia based on published criteria including a WBV intervention versus control or another intervention. Major outcomes were health‐related quality of life (HRQL), pain intensity, stiffness, fatigue, physical function, withdrawals, and adverse events.
Data collection and analysis
Two review authors independently selected trials for inclusion, extracted data, performed risk of bias assessments, and assessed the quality of evidence for major outcomes using the GRADE approach. We used a 15% threshold for calculation of clinically relevant differences.
Main results
We included four studies involving 150 middle‐aged female participants from one country. Two studies had two treatment arms (71 participants) that compared WBV plus mixed exercise plus relaxation versus mixed exercise plus relaxation and placebo WBV versus control, and WBV plus mixed exercise versus mixed exercise and control; two studies had three treatment arms (79 participants) that compared WBV plus mixed exercise versus control and mixed relaxation placebo WBV. We judged the overall risk of bias as low for selection (random sequence generation), detection (objectively measured outcomes), attrition, and other biases; as unclear for selection bias (allocation concealment); and as high for performance, detection (self‐report outcomes), and selective reporting biases.
The WBV versus control comparison reported on three major outcomes assessed at 12 weeks post intervention based on the Fibromyalgia Impact Questionnaire (FIQ) (0 to 100 scale, lower score is better). Results for HRQL in the control group at end of treatment (59.13) showed a mean difference (MD) of ‐3.73 (95% confidence interval [CI] ‐10.81 to 3.35) for absolute HRQL, or improvement of 4% (11% better to 3% worse) and relative improvement of 6.7% (19.6% better to 6.1% worse). Results for withdrawals indicate that 14 per 100 and 10 per 100 in the intervention and control groups, respectively, withdrew from the intervention (RR 1.43, 95% CI 0.27 to 7.67; absolute change 4%, 95% CI 16% fewer to 24% more; relative change 43% more, 95% CI 73% fewer to 667% more). The only adverse event reported was acute pain in the legs, for which one participant dropped out of the program. We judged the quality of evidence for all outcomes as very low. This study did not measure pain intensity, fatigue, stiffness, or physical function. No outcomes in this comparison met the 15% threshold for clinical relevance.
The WBV plus mixed exercise (aerobic, strength, flexibility, and relaxation) versus control study (N = 21) evaluated symptoms at six weeks post intervention using the FIQ. Results for HRQL at end of treatment (59.64) showed an MD of ‐16.02 (95% CI ‐31.57 to ‐0.47) for absolute HRQL, with improvement of 16% (0.5% to 32%) and relative change in HRQL of 24% (0.7% to 47%). Data showed a pain intensity MD of ‐28.22 (95% CI ‐43.26 to ‐13.18) for an absolute difference of 28% (13% to 43%) and a relative change of 39% improvement (18% to 60%); as well as a fatigue MD of ‐33 (95% CI ‐49 to ‐16) for an absolute difference of 33% (16% to 49%) and relative difference of 47% (95% CI 23% to 60%); and a stiffness MD of ‐26.27 (95% CI ‐42.96 to ‐9.58) for an absolute difference of 26% (10% to 43%) and a relative difference of 36.5% (23% to 60%). All‐cause withdrawals occurred in 8 per 100 and 33 per 100 withdrawals in the intervention and control groups, respectively (two studies, N = 46; RR 0.25, 95% CI 0.06 to 1.12) for an absolute risk difference of 24% (3% to 51%). One participant exhibited a mild anxiety attack at the first session of WBV. No studies in this comparison reported on physical function. Several outcomes (based on the findings of one study) in this comparison met the 15% threshold for clinical relevance: HRQL, pain intensity, fatigue, and stiffness, which improved by 16%, 39%, 46%, and 36%, respectively. We found evidence of very low quality for all outcomes.
The WBV plus mixed exercise versus other exercise provided very low quality evidence for all outcomes. Investigators evaluated outcomes on a 0 to 100 scale (lower score is better) for pain intensity (one study, N = 23; MD ‐16.36, 95% CI ‐29.49 to ‐3.23), HRQL (two studies, N = 49; MD ‐6.67, 95% CI ‐14.65 to 1.31), fatigue (one study, N = 23; MD ‐14.41, 95% CI ‐29.47 to 0.65), stiffness (one study, N = 23; MD ‐12.72, 95% CI ‐26.90 to 1.46), and all‐cause withdrawal (three studies, N = 77; RR 0.72, 95% CI ‐0.17 to 3.11). Adverse events reported for the three studies included one anxiety attack at the first session of WBV and one dropout from the comparison group ("other exercise group") due to an injury that was not related to the program. No studies reported on physical function.
Authors' conclusions
Whether WBV or WBV in addition to mixed exercise is superior to control or another intervention for women with fibromyalgia remains uncertain. The quality of evidence is very low owing to imprecision (few study participants and wide confidence intervals) and issues related to risk of bias. These trials did not measure major outcomes such as pain intensity, stiffness, fatigue, and physical function. Overall, studies were few and were very small, which prevented meaningful estimates of harms and definitive conclusions about WBV safety.
PICO
Plain language summary
Whole body vibration training for adults with fibromyalgia
Review question
What are the effects of whole body vibration (WBV) training on health‐related quality of life (HRQL), pain intensity, fatigue (feeling tired), stiffness, physical function, study participant dropout, and adverse events among adults (18 years of age and older) with fibromyalgia?
Background
People with fibromyalgia have chronic widespread body pain, often with increased fatigue, stiffness, depression, and problems sleeping. Vibration training is a new type of exercise that might reduce fibromyalgia symptoms. Vibration training usually consists of a person standing on a vibrating platform with the ability to change body position on the platform to squatting or standing on one leg. The vibration tricks the body into thinking it is falling, forcing the muscles to contract and relax dozens of times each second. These contractions are responsible for most of the benefits attributed to vibration training, including improvements in circulation, muscle strength, balance, and flexibility.
Study characteristics
We searched until December 2016 and found four studies with a total of 150 middle‐aged female participants from the same country (Spain). One study (41 participants) compared WBV against control (standard care); two studies (79 participants) compared WBV plus other exercises (strengthening, flexibility, etc.) against other exercises alone or against control (WBV + MX vs MX); and another study (30 participants) compared WBV plus mixed exercise against mixed exercise alone.
Results
HRQL (0 to 100 Fibromyalgia Impact Questionnaire (FIQ) scale, 0 is best)
WBV vs control
Vibration training was 4% better than control (or 4 points, ranging from 11 improved to 3 worse).
• People who had training rated their quality of life at 55 points.
• People who had no training rated their quality of life at 59 points.
WBV + MX vs MX
Vibration training plus exercise was 16% better than control (or 16 points, ranging from 32 to 0.5).
• People who had training rated their quality of life at 43 points.
• People who had no training rated their quality of life at 59 points.
Pain intensity, fatigue, stiffness, and physical function were not measured for WBV vs control; physical function was not measured for WBV + MX vs MX.
Pain
Vibration training plus exercise was 28% better than control (or 28 points, ranging from 13 to 43).
• People who had training rated their pain at 41 points.
• People who had no training rated their pain at 69 points.
Fatigue
Vibration training plus exercise was 33% better than control (or 33 points, ranging from 16 to 49).
• People who had training rated their fatigue at 42 points.
• People who had no training rated their fatigue at 75 points.
Stiffness
Vibration training plus exercise was 26% better than control (or 26 points, ranging from 10 to 43).
• People who had training rated their fatigue at 42 points.
• People who had no training rated their fatigue at 69 points.
Dropouts from treatment (number)
Four more people dropped out of vibration training (4% more, 16% fewer to 24% more) for any reason than dropped out of the control group.
• 14 out of 100 people dropped out of vibration training.
• 10 out of 100 people dropped out of the control group.
A total of 8 per 100 people dropped out of the vibration plus exercise group for any reason compared with 33 per 100 from the control group (24% risk difference).
Adverse events (narrative)
WBV vs control
One person dropped out because of acute pain in the legs. We are uncertain whether vibration training combined with other exercise provides additional benefits over control or vibration training alone, as evidence was of very low quality because of study design flaws and small numbers of participants.
WBV + MX vs MX
Study authors stated that this program did not make symptoms worse and did not result in injury; one patient exhibited a mild anxiety attack at the first session.
Quality of the evidence
One study that reported on this comparison provided very low‐quality evidence because of study design flaws and small numbers of participants.
Authors' conclusions
Summary of findings
| Whole body vibration versus control | ||||||
| Patient or population: individuals with fibromyalgia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with WBV | |||||
| Health‐related quality of life Follow‐up: 12 weeks | Mean health‐related quality of life was 59 points | Mean health‐related quality of life in intervention group was 3.73 points lower (10.81 lower to 3.35 higher) at post‐test than in control group | ‐ | 41 | ♁◯◯◯ | Absolute improvement: 4% (95% CI 11% better to 3% worse) Relative change: 6.7% improvement (95% CI 19.6% improvement to 6.1% worse) NNTB: n/ac |
| Pain intensity | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Fatigue | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Stiffness | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Physical function | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Adverse events | Gusi 2010: "The program was reasonably safe: only 5% of the participants (n = 1) dropped out of the program because of acute pain in the legs. The program was completed by 85% of the participants, without secondary adverse effects" (page 1076; 1 study) | |||||
| All‐cause withdrawal | Study population | RR 1.43 (0.27 to 7.67) | 41 | ♁◯◯◯ | Absolute risk difference: 4% more events (95% CI 16% fewer to 24% more) Relative change: 43% more (73% fewer to 667% more) NNTH: n/ac | |
| 10 per 100 (2 of 20) | 14 per 100 (3 of 21) | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImpresicion: number of participants lower than 400 rule of thumb; wide confidence interval (downgraded twice) bHigh risk of biases including detection, performance, and reporting biases cNumber needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated with Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated with Wells calculator (CMSG Editorial Office) | ||||||
| Whole body vibration plus mixed exercise versus control | ||||||
| Patient or population: individuals with fibromyalgia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with WBV + MX | |||||
| Health‐related quality of life | Mean health‐related quality of life was 59.64 points at the end of the study | Mean health‐related quality of life in the intervention group was 16.02 points lower (31.57 lower to 0.47 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference: 16% improvement (95% CI 32% to 0.5% improvement). Relative change: 24% (47% to 0.7%)c NNTBd: 3 (2 to 237) |
| Pain Intensity | Mean pain intensity was 69.38 mm in the control group at the end of the study | Mean pain Intensity in the intervention group was 28.22 mm lower (43.26 lower to 13.18 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference: 28% (95% CI 43% to 13%). Relative difference: 39% (95% CI 18% to 60%) NNTBd: 2 (1 to 4) |
| Fatigue | Mean fatigue was 75.17 mm at the end of the study | Mean fatigue in the intervention group was 32.84 mm lower (49.2 lower to 16.48 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference: 33% (95% CI 49% to 16%). Relative difference: 47% (95% CI 23% to 70%) NNTBd: 2 (1 to 4) |
| Stiffness | Mean stiffness was 68.71 mm at the end of the study | Mean stiffness in the intervention group was 26.27 mm lower (42.96 lower to 9.58 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference 26% (95% CI 43% to 10%). Relative difference: 36.5% (95% CI 60% to 23%) NNTBd: 2 (1 to 6) |
| Physical function | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Adverse events (narrative) | Alentorn‐Geli 2008: "This program neither exacerbated FM‐related symptoms nor resulted in musculoskeletal injuries; however, 1 patient exhibited a mild anxiety attack on the first session of WBV" (page 978) Sañudo 2013: "This study, however, demonstrated that WBV training is safe (no adverse events)..." (page 683) | |||||
| All‐cause withdrawal | 33 per 100 (7 of 21) | 8 per 100 | RR 0.25, 95% CI 0.06 to 1.12 | 46 | ♁◯◯◯ | Absolute risk difference: 24% (95% CI 3 to 51) NNTHd: n/a |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecision: Number of participants lower than 400 rule of thumb; wide confidence interval. Need for more studies with more participants to reach optimal information size (downgraded twice) bHigh risk of biases including reporting and selection biases. Need for methodologically better designed and executed studies cBaseline control group mean (SD) = 67 (15.81), n = 10 dNumber needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated with Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated with Wells calculator (CMSG Editorial Office) | ||||||
Background
Description of the condition
Fibromyalgia is a chronic syndrome marked by widespread pain, fatigue, waking unrefreshed, and cognitive symptoms (Wolfe 2010; Wolfe 2011). Most people with fibromyalgia experience concurrent gastrointestinal (eg, abdominal pain, irritable bowel syndrome) and somatosensory symptoms (eg, muscle tenderness, hyperalgesia, allodynia, paraesthesias ‐ see Glossary ‐ Table 1), in addition to disturbances in sleep, mood, and cognition (Mease 2005). The myriad of symptoms significantly affects quality of life and results in both physical and psychosocial disabilities, with far reaching implications for family, employment, and independence (Mease 2005). Moreover, people with fibromyalgia are often intolerant of physical activity and tend to have a sedentary lifestyle, which increases risk of additional morbidity (Park 2007; Raftery 2009). As the result of extensive somatic complaints and disability, people with fibromyalgia typically make a greater number of physician visits each year and enlist more specialists in their care (Park 2007).
| Term | Definition |
| Allodynia | Pain resulting from a stimulus that would not normally provoke pain |
| Amplitude | Absolute value of maximum displacement from a zero value during 1 period of an oscillation |
| Damping | Energy dissipation properties of a material or system under cyclic stress |
| Endurance | Two forms of endurance that refer to health‐related physical fitness include cardiorespiratory endurance (also known as cardiovascular endurance, aerobic fitness, aerobic endurance, exercise tolerance), which "relates to the ability of the circulatory and respiratory systems to supply fuel during sustained physical activity and to eliminate waste products after supplying fuel," and muscle endurance, which "relates to the ability of muscle groups to exert external force for many repetitions" (Caspersen 1985) |
| Frequency | Number of cycles or completed alternations per unit time of a wave or oscillation |
| Hertz | One hertz is 1 cycle per second; therefore, when an individual is exposed to a vibration of 30 Hz, targeted muscles receive 30 cycles of vibration per second, which makes muscles contract and relax 30 times in the same period |
| Hyperalgesia | Increased pain from a stimulus that normally provokes pain |
| Natural frequency | Frequency at which a system oscillates when not subjected to continuous or repeated external forces |
| Paresthesia | Abnormal sensation that is spontaneous or is evoked by a stimulus (eg, numbness) |
| Phase angle | Particular stage or point of advancement in a cycle; fractional part of the period through which time has advanced, measured from some arbitrary origin often expressed as an angle (phase angle); the entire period being taken is 360° |
Prevalence of fibromyalgia in the USA has been estimated at 2% of the population, with greater representation among females than males (3.4% female to 0.5% male; Wolfe 1995). Canadian statistics are similar to those of the USA; self‐reported prevalence of fibromyalgia in Canada has been estimated at 1.1% across all ages, again with female diagnoses outnumbering male diagnoses (1.8% female to 0.3% male; McNalley 2006). Prevalence rates among some European countries (France, Italy, Portugal, Spain) are estimated to range between 1.4% (France) and 3.7% (Italy), with fibromyalgia diagnoses twice as common among females (Branco 2010). The American College of Rheumatology (ACR) recently prepared new diagnostic criteria for fibromyalgia. Some of the published prevalence rates may change as study authors adopt the newer criteria. In Olmsted County, in Minnesota, age‐ and sex‐adjusted criteria led to estimation of 6.4% (Vincent 2013). In Germany, use of the new criteria resulted in a prevalence rate of 2.1%, with a higher rate among females (2.4%) than males (1.8%; Wolfe 2013). In Asia, Nakamura and colleagues found the prevalence rate in Japan to be 2.1% when using the new diagnostic criteria (Nakamura 2014); this is much higher than the prevalence rate of 0.05% in China, which is based on 1990 (older) criteria (Zeng 2008).
To date, no definitive cause or pathophysiology for fibromyalgia has been discerned. However, current evidence supports the model of central amplification of pain perception, which may be developed and maintained by a variety of factors that influence neurotransmitter and neurohormone dysregulation (Bennett 1999; Clauw 2011; Clauw 2014; Desmeules 2003).
Several recent Cochrane systematic reviews and a Cochrane overview on use of medications for treatment of fibromyalgia have produced tier‐2 evidence of moderate pain relief for pregabalin (an antiepileptic; Wiffen 2013; Appendix 1), amitriptyline (a tricyclic antidepressant; Moore 2012), milnacipran (a serotonin–norepinephrine reuptake inhibitor; Derry 2012), and monoamine oxidase inhibitors (one of the first antidepressants developed; Tort 2012). These reviews have helped to inform recent clinical practice guidelines. Systematic reviews of non‐pharmacological methods, including exercise (Bidonde 2014; Busch 2008; Busch 2013), cognitive‐behavioral therapy (Bernardy 2013), and acupuncture (Deare 2013), have presented low‐quality evidence showing positive effects for management of fibromyalgia. Ablin 2013 noted that "recent evidence‐based interdisciplinary guidelines concur on the importance of treatments tailored to the individual patient and further emphasize the necessity of self management strategies which include exercise and psychological techniques."
Description of the intervention
Vibration is a forced mechanical oscillation (ie, periodic alteration of force, acceleration, and displacement over time) whereby energy is transferred from an actuator (ie, the vibration device) to a resonator (ie, the human body, or parts of it; Rittweger 2010). Most vibration exercise devices generate oscillations that have a sinusoidal shape (ie, smooth repetitive oscillation) and are described by amplitude (A), frequency (f), and phase angle (Rittweger 2010). During vibration exercise, the body is accelerated through contact with the device platform, thus causing a reactive force by, and within, the body (Rittweger 2010). The main variables that determine the magnitude of response to whole body vibration (WBV) include:
-
vibration direction (ie, vertical vs oscillatory);
-
vibration amplitude (millimeters, or mm);
-
vibration frequency (Hertz, or Hz);
-
vibration acceleration (gravitational units, 1.0 g = 9.81 m/s²); and
-
body position on the platform (also see List of Abbreviations, Table 2).
| Abbreviation | Description |
| A | amplitude |
| ACR | American College of Rheumatology |
| ACSM | American College of Sports Medicine |
| AE | aerobic exercise |
| EMG | electromyography |
| f | frequency |
| FIQ | Fibromyalgia Impact Questionnaire |
| FX | flexibility |
| g | gravitational load (G‐force) = 1 cm/s2 |
| HR | heart rate |
| HRQL | health‐related quality of life |
| hz | Hertz |
| ITT | intention‐to‐treat |
| kg | kilogram |
| m/s2 | unit of acceleration: 1 Gal = 0.01 m/s2 |
| MCID | minimal clinically important difference |
| MD | mean difference |
| MX | mixed intervention (includes more than 1 mode of physical activity) |
| n | number of studies |
| N | number of individuals |
| RD | risk difference |
| Relax | relaxation |
| RT | resistance training |
| s | seconds |
| SD | standard deviation |
| SE | standard error |
| SMD | standardized mean difference |
| sTNFR1 and sTNFR2 | soluble tumor necrosis factor receptor 1 and 2 |
| VAS | visual analogue scale |
| WBV | whole body vibration |
| wk | week(s) |
| WU | warm‐up |
Vibration exercise is most often practiced as WBV; vibration is applied to a person while he/she stands, generally in a static position, on an oscillating platform or platforms. Among the various platforms, two different types of energy transfer can be distinguished. The first type ‐ synchronous WBV ‐ transfers vibration to both feet synchronously, and the second type operates in a side‐alternating way, so that the right foot is lower when the left foot is higher (and vice versa). It has been argued that side‐alternating vibration evokes rotational movements around the hip and lumbosacral joints (Rittweger 2002). This movement introduces an additional degree of freedom; therefore, whole body mechanical impedance is less in side‐alternating than in synchronous WBV (Abercromby 2007).
WBV platforms have no firm stationary attachment, and when they are used, the only downward force acting on the body is gravity (Yue 2002). Because the structure of the human body is not rigid, muscles and tendons act as spring‐like elements that store and release mechanical energy. In this spring‐mass system, compression occurs during the vibration upstroke, and expansion during the downstroke. As a consequence, less displacement occurs at the body’s center of mass than at the platform level (Cardinale 2005).
When the frequency of the vibration platform matches the frequency of the human body, mechanical energy can accumulate within the body, including its mass and elastic components. This accumulation of energy can result in a situation wherein the vibration amplitude is greater in the resonator (eg, human body) than in the actuator (eg, vibration platform). This amplitude augmentation causes increased internal forces within the resonator (Cheung 2007; Furness 2009).
During WBV, vibrations are transmitted in rapid succession from one segment of the human body to the next (eg, from the foot to the calf, from the calf to the thigh). The quantity of vibration energy transmitted depends on musculoskeletal stiffness and damping of the vibration. Effective axial body stiffness is increased by straightened limbs (Greene 1979; Lafortune 1996) and leads to an increase in resonance frequency. Appropriate posture can help to minimize resonance. Standing on the forefoot will involve ankle joint actuators (ie, the calf muscles) in the damping process, which is not the case when stance is on the mid‐foot. Putting weight on the forefoot can help to avoid resonance and increase damping by the musculature, thus reducing vibration transmission to the trunk.
How the intervention might work
This section briefly reviews the physiological and mechanical effects of vibration exercise on healthy individuals. It is anticipated that individuals with fibromyalgia will experience similar effects, and that these effects may mediate benefits or harms.
Physiological and mechanical effects of vibration exercise on the human body in healthy individuals
Muscle activity and power
During WBV, muscles and tendons will be exposed to a period of elongation (stretch phase), followed by a period of shortening (shortening phase; Abercromby 2007). WBV is characterized by cyclic transitions between eccentric and concentric muscle contractions. A few studies have demonstrated the immediate effects of WBV upon electromyography (EMG) activity. Seated vibration, with frequencies between 0.3 Hz and 5 Hz (peak acceleration levels between 1.2 g and 2.0 g [12 m/s² and 20 m/s²]), has been shown to produce vibration‐synchronous EMG activity in the erector spinae muscle (Abercromby 2007; Cardinale 2003; Cochrane 2009; Seidel 1988). For vibration frequencies usually applied for exercise purposes, effects on vastus lateralis EMG amplitude are larger at 30 Hz than at 40 Hz or 50 Hz (Cardinale 2003). EMG responses generally seem to be more pronounced in side‐alternating than in synchronous WBV.
It can be expected that the vibration‐related increase in muscle activity (and thus muscular energy metabolism) results in the generation of additional heat. Cochrane 2008 reported intramuscular temperature increases during WBV. During WBV, skin blood flow, as measured by laser‐Doppler flowmetry, is increased (Lohman 2007; Maloney‐Hinds 2008; Rittweger 2000). A 100% increase in popliteal blood flow, along with an increase in gastrocnemius and vastus lateralis muscle blood flow, has been found after termination of WBV (f = 26 Hz, A = 3 mm, side‐alternating; Kerschan‐Schindl 2001). Reduced pulse wave velocity and thus reduced arterial stiffness, suggestive of arterial vasodilation in the vibrated musculature, have been reported (Otsuki 2008).
Bosco and colleagues were the first to study acute effects of WBV as an exercise modality on muscular power (Bosco 1999). After ten 60‐second sessions of WBV (frequency = 26 Hz, amplitude = 5 mm, single leg stance), trial authors found an increase in leg press power of 6% to 8% in young elite volleyball players. Similar effects have been reported for jump height in elite hockey players (Cochrane 2005). The literature states that muscular power output can be acutely increased by WBV, but force generation capacity seems to be depressed. The observed power enhancement is probably due to the warming‐up effect (Cochrane 2008), as it is well known that muscle temperature has a strong effect on muscular power but only a very small effect on force generation (de Ruiter 2000).
Posture and stance
The effect of vibration on posture is closely related to the anatomic location of the application (Kavounoudias 1998). Vibratory foot stimulation causes a shift in posture toward the front when applied under the heel, and to the rear when applied under the forefoot (Kavounoudias 1999). The latter effect increases linearly with the frequency of vibration, and vibratory effects from the forefoot and from the heel seem to antagonize each other (Kavounoudias 1999). In a similar way, vibratory stimulation of the tibialis anterior and gastrocnemius muscles seems to have antagonistic effects upon posture (Polonyova 2001). Unilateral application of muscle vibration can also cause sway in the frontal plane (Polonyova 2001). When foot sole vibration and tendon stimulation are compared, an integrated postural response seems to occur (Kavounoudias 2001), the outcome of which is determined by site of application and vibration frequency.
Joint stability
Effects of WBV on joint stability remain unclear. It is presumed, however, that WBV improves the effectiveness of the stretch reflex. Two studies on healthy individuals reported contrasting results. Those from Melnyk 2008 verified that the change in reflex levels caused by vibration helped to improve joint stability and enhanced short latency responses to anterior cruciate ligament stretch. This effect may be due to enhanced efficacy of the monosynaptic pathway, which may result in improved knee stability and reduced anterior tibial displacement upon shock provocation. Hopkins 2008 found no effect of WBV on ankle stability.
Effects of vibration exercise on individuals with chronic pain
Low‐quality evidence emanating from several small randomized controlled trials (RCTs) suggests that WBV exercise reduces pain in various clinical populations. Conditions that have been studied include osteoarthritis (Simao 2012), low back pain in the elderly with osteoporosis (Iwamoto 2005), chronic idiopathic low back pain (del Pozo‐Cruz 2011; Rittweger 2002), and painful diabetic peripheral neuropathy (Kessler 2013).
Several mechanisms of effect have been proposed. The gate control theory suggests that activation of mechanoreceptors and A‐β fibers competes with peripheral and central nociceptive activity at the dorsal horn of the spinal cord, resulting in reduction of second‐order nociceptive activity, with subsequent decreased pain perception (Melzack 1965). WBV exercise could lead to reduced pain through presynaptic inhibition of both nociceptive and motor neurons (Kessler 2013). WBV is also known to diminish excitation of central motor neurons while reducing the Hoffman reflex in healthy adults (Kipp 2011). Other possible explanations include decreased inflammation with repeated WBV, suggested by reduction in biomarkers of inflammation such as cortisol (Cardinale 2010; Elmantaser 2012) and lower levels of soluble tumor necrosis factor receptors sTNFR1 and sTNFR2 (Simao 2012). Gay 2007 proposed that sustained pain relief from vibration therapy may be the result of central mechanisms such as enhanced endogenous inhibition of nociception or cortical reorganization that occurs during rehabilitation.
Does vibration differ from effects of aerobic and strength exercise?
Aerobic exercise (AE) training primarily affects the circulatory system and the respiratory system. After AE training, the heart pumps out more blood per beat, and more capillaries are available to transfer this blood to the working muscles and to the lungs. In addition, the lungs become more efficient in moving air in and out, and in transferring oxygen into the blood and removing carbon dioxide. As a result of these improvements in heart and lung function, people have an increased total work capacity and can perform more work at a given submaximal level (ACSM 2013 9th Guidelines). Currently, little is known about effects of vibration exercise on the cardiovascular system, but it is unlikely that vibration exercise produces the same improvements in heart rate or lung function that are achieved by AE training.
Resistance training (RT) can take several forms, producing more strength, more power, or more endurance in the muscles. Effects of strength training are evident in the muscles and in their neuromuscular effectors (ACSM 2013 9th Guidelines). We know that WBV produces a series of isometric contractions in the thigh muscles. These have been linked to an increase in muscle power produced after WBV, perhaps similar to what we see in strength training. However, studies on the overall effects of WBV are novel in exercise research. Some studies have used WBV in people with fibromyalgia (Chulvi‐Medrano 2013), but results show inconsistencies between study types and outcome measures.
Why it is important to do this review
WBV is a novel exercise intervention that has been reported to improve bone density and balance while exerting a greater strengthening effect among healthy adults and elderly individuals than is seen with traditional modes of exercise (Fort 2012; Iwamoto 2004; Sitja‐Rabert 2012; Torvinen 2002). WBV has been reported to reduce pain and disability among adults with chronic low back pain (del Pozo‐Cruz 2011; Iwamoto 2005). Although benefits have been documented, small sample sizes and other limitations in study design have led to difficulty in interpreting research findings. Researchers have observed a variety of adverse effects including inner ear problems, dizziness, headache, and fracture among those with severe osteoporosis (Kosar 2012). To date, a few RCTs (Alentorn‐Geli 2008; del Pozo‐Cruz 2011; Olivares 2011; Sañudo 2012) and one relevant topical review of vibration exercise (Chulvi‐Medrano 2013) have focused on WBV as an exercise intervention for people with fibromyalgia, but no Cochrane systematic reviews have assessed this research. A systematic review is needed to evaluate research on effects of WBV alone or WBV combined with traditional aerobic and resistance exercises versus traditional exercise or control for symptom management, balance, and health‐related quality of life (HRQL) in people with fibromyalgia.
Objectives
To evaluate benefits and harms of WBV exercise training in adults with fibromyalgia.
Methods
Criteria for considering studies for this review
Types of studies
We included trials described as randomized, even if methods used to generate the random sequence were unclear or unreported, or if the method of allocating participants was likely to be quasi‐random (ie, by alternation, date of birth, or similar pseudo‐randomized method).
Types of participants
We included in the review studies that examined adults with fibromyalgia. We selected studies that used published criteria for diagnosis (or classification) of fibromyalgia. Until recently, the American College of Rheumatology (ACR) 1990 criteria served as the standard for classifying individuals as having fibromyalgia (Wolfe 1990) when they have experienced widespread pain lasting longer than three months, and when pain can be elicited at 11 of 18 specific tender points on the body with 4 kg tactile pressure.
A newer preliminary diagnostic tool ‐ ACR 2010 (Wolfe 2010) ‐ does not rely upon a physical tender point examination and is available as both a clinician‐administered tool and a survey questionnaire (Wolfe 2011). This measure includes a Widespread Pain Index (19 areas representing anterior and posterior axes and limbs) in addition to a Symptom Severity Scale containing items related to secondary symptoms such as fatigue, sleep disturbance, cognition, and somatic complaints. Scores on both measures are used to determine whether a person qualifies as meeting a "case definition" of fibromyalgia. This tool has been used to classify 88.1% of cases that meet ACR 1990 criteria, and it allows ongoing monitoring of symptom change in people with a current or previous diagnosis of fibromyalgia (Wolfe 2010). Although measures focusing on tender point counts have been widely applied in clinic and research settings, methods described by Wolfe 2010 and Wolfe 2011 promise to classify people with fibromyalgia more efficiently while allowing improved monitoring of disease status over time.
Although differences among published fibromyalgia diagnostic/classification criteria are known, for the purposes of this review, we considered all published criteria to be acceptable and comparable.
Types of interventions
We examined trials that studied WBV exercise interventions (ie, moving or holding a standing position while on an oscillating platform) regardless of the frequency, duration, or intensity of exercise sessions. We have provided an example of a WBV exercise intervention in Appendix 2. We categorized interventions by duration of the program (ie, "short" < seven weeks; "intermediate" seven to 12 weeks, "long" > 12 weeks) and by frequency of training per week (ie, once per week, twice per week, and three or more times per week).
Comparator interventions included control (eg, placebo or sham intervention, usual care, wait list control) and other active interventions including physical therapies or non‐exercise interventions (eg, education, self‐management interventions, relaxation, medication).
Types of outcome measures
We designated seven outcomes as major outcomes: HRQL, physical function, pain intensity, fatigue, stiffness, number of participants who withdrew or dropped out, and number of adverse events; and three as minor outcomes: muscle strength, balance, and number of participants with greater than 30% improvement in pain. In selecting these outcome measures, we considered the consensus statement of Choy and associates (Choy 2009) regarding a core set of outcome measures for clinical trials in fibromyalgia and anticipated effects of WBV exercise on physical fitness.
We extracted data for selected outcomes (HRQL, physical function, pain intensity, fatigue, stiffness, muscle strength, and balance) at baseline (pretest), immediately at cessation of treatment (post‐treatment), and at any follow‐up assessment. We required that each included study report measurements for one or more outcomes in such a way that we could evaluate post‐test differences between groups (post‐test or pretest scores with change scores at post‐test). We extracted the number of participants who experienced adverse events, the number with greater than 30% improvement in pain, and the number who withdrew from the study during the intervention phase.
Major outcomes
When an included study used more than one instrument to measure a particular outcome, we applied the following preferred hierarchy in choosing outcomes for analysis.
-
HRQL ‐ This outcome consists of multidimensional indices used to measure general health status or HRQL, or both (Choy 2009). When included studies used more than one instrument to measure HRQL, we preferentially extracted data from the Fibromyalgia Impact Questionnaire (FIQ total), followed by the 36‐Item Short Form Health Survey (SF‐36), the 12‐Item Short Form Health Survey (SF‐12), and the EuroQol Quality of Life Questionnaire (EQ‐5D).
-
Pain ‐ The International Association for the Study of Pain defines pain as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage" (Merskey 1994). For the purposes of this review, we focused on one aspect of the pain experience – pain intensity. When a single study reported more than one measure of pain, we preferentially extracted measures of average pain intensity (as opposed to worst, least, or current pain) assessed by a pain intensity visual analogue scale (VAS) score, the FIQ Pain Scale (English or translated), and the McGill Pain VAS, followed by the Numerical Pain Rating Scale. For studies that did not report unidimensional measures of pain intensity, we extracted composite measures that included pain intensity and interference (SF‐36 Bodily Pain Scale), or pain intensity and suffering from pain (Mulitdimensional Pain Inventory ‐ Pain Severity Scale).
-
Fatigue ‐ Individuals with fibromyalgia and clinicians alike recognize fatigue as an important symptom in fibromyalgia. Fatigue can be measured in a global manner, as when an individual rates fatigue on a single item scale, or by using a multidimensional tool that breaks the experience of fatigue down into two or more dimensions, such as general fatigue, physical fatigue, mental fatigue, reduced motivation, reduced activity, and degree of interference with activities of daily living (Boomershine 2012). We accepted both unidimensional and multidimensional measures for this outcome. When included studies used more than one instrument to measure fatigue, we preferentially extracted the fatigue VAS (FIQ ‐ English or translated, or single‐item fatigue VAS) score, followed by scores on the SF‐36 Vitality Subscale, the Chalder Fatigue Scale (total), the Fatigue Severity Scale, and the Multidimensional Fatigue Inventory.
-
Stiffness ‐ In focus groups conducted by Arnold 2008, individuals with fibromyalgia "... remarked that their muscles were constantly tense. Participants alternately described feeling as if their muscles were 'lead jelly' or 'lead Jell‐O,' and this resulted in a general inability to move with ease and a feeling of stiffness." A commonly used measure of stiffness encountered in this literature is the FIQ Stiffness subscale.
-
Physical function ‐ This outcome focuses on the basic actions and complex activities considered "essential for maintaining independence, and those considered discretionary that are not required for independent living, but may have an impact on quality of life" (Painter 1999). Given that cardiorespiratory fitness; neuromuscular attributes such as muscular strength, endurance, and power; and muscle and joint flexibility are important determinants of physical function, this outcome is highly relevant as an outcome of exercise interventions. When more than one measure of physical function was available within a study, we preferentially extracted data from the FIQ (English or translated) Physical Impairment Subscale, followed by the Health Assessment Questionnaire Disability Scale (HAQ), the SF‐36 Physical Function Scale, the Sickness Impact Profile – Physical Disability, and the Multidimensional Pain Inventory Household Chores Scale.
-
Adverse events ‐ This outcome refers to the proportion of participants who experienced an adverse event (injury, exacerbation of pain, and/or other fibromyalgia symptoms). We described the nature of the adverse events in a narrative report.
-
All‐cause withdrawals ‐ This outcome refers to the proportion of participants who withdrew from or dropped out of the study.
Minor outcomes
Among the three outcomes designated as minor outcomes, we included two fitness variables that have the potential to improve with WBV. The following preference listing guided our extraction and reporting when studies provided more than one measure for minor outcomes.
-
Muscle strength ‐ Muscle strength is the measure of a muscle's ability to generate force. It is commonly expressed as maximal voluntary contraction (MVC) during isometric testing; one‐repetition maximum (1RM) during dynamic isotonic testing (Howley 2001); and/or peak torque muscle contraction during isokinetic testing. When more than one measure of strength was reported, we preferentially extracted dynamic test results over isometric test results, lower limb over upper limb tests, and extensor muscle strength over flexor muscle strength.
-
Balance ‐ Balance is "the ability to align body against gravity to maintain or move the body (center of mass) within the available base of support without falling; the ability to move the body in equilibrium with gravity via interaction of the sensory and motor system" (Kisner 2007). Balance is "complex, with contributions from sensory, central nervous, and neuromuscular systems" (Arnold 2011). Balance can be measured by clinical examination through measures such as the Berg Balance Scale (Berg 1992), which consists of a series of daily tasks that progressively challenge balance, or by more objective measures such as stabilimetry (Nordahl 2000; Demir‐Gocmen 2013). Postural sway is measured through a device that records movement of the center of pressure as registered by a force plate while the individual attempts to stand still on two legs (Thomas 2001). Patients' eyes can remain open, for a lower level of challenge, or closed, for a higher level of challenge. Sway is reported in degrees of displacement, with higher degrees indicating greater sway and worse postural control. Preferentially, we extracted data on postural sway.
-
Improvement in pain greater than 30% ‐ A 30% reduction in pain is considered a benchmark for a moderately important change in pain intensity and is promoted as a method for assessing and interpreting change in pain in clinical trials (IMMPACT ‐ Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials) (Dworkin 2008). When available, we extracted data on proportions of participants who met this criterion for intervention efficacy.
Search methods for identification of studies
In this review, a comprehensive search included nine databases to find trials that addressed physical activity interventions in adults and fibromyalgia, and three to find trials that addressed health technology assessments. We screened citations found during electronic and manual searches and classified them by type of exercise training. We included in this review the subset of studies that included a WBV intervention.
Electronic searches
We searched the following databases from database inception using current methods as outlined in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We applied no language restrictions. We have provided in the appendices full search strategies for each database as indicated in the list.
-
MEDLINE (OVID) 1946 to December 2016 (Appendix 3).
-
Embase (OVID), Embase Classic+Embase 1947 to December 2016 (Appendix 4).
-
Cochrane Library (Wiley) to December 2016 (http://www.thecochranelibrary.com/view/0/index.html) (Appendix 5).
-
Cochrane Database of Systematic Reviews (Cochrane Reviews).
-
Database of Abstracts of Reviews of Effects (DARE).
-
Cochrane Central Register of Controlled Trials (CENTRAL).
-
Health Technology Assessment Database (HTA).
-
NHS Economic Evaluation Database (EED).
-
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO) 1982 to December 2016 (Appendix 6).
-
Physiotherapy Evidence Database (PEDro) (www.pedro.org.au/) to December 2016 (Appendix 7).
-
Dissertation Abstracts (ProQuest) to December 2016 (Appendix 8).
-
Current Controlled Trials, accessed to December , 2013 (Appendix 9).
-
Clinicaltrials.gov, accessed from June 2014 to December 2016 (Appendix 9).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/) to December 2016 (Appendix 10).
-
Allied and Complementary Medicine (AMED) (OVID) 1985 to December 2016 (Appendix 11).
Additionally, we searched three sources of health technology assessments from inception to December 2016 to capture technology assessments of vibration trainers for fibromyalgia.
-
Centre for Reviews and Dissemination (http://www.crd.york.ac.uk/CRDWeb/) to December 2016 (Appendix 12).
-
Agency for Healthcare Research and Quality, Technology Assessments (http://www.ahrq.gov/research/findings/evidence‐based‐reports/index.html), to December 2016 (Appendix 13).
-
Canadian Agency for Drugs and Technologies in Health (CADTH) Canadian Search Interface HTA database (https://www.cadth.ca/) to December 2016 (Appendix 14).
Searching other resources
Two review authors independently reviewed reference lists from key journals, identified articles, and reviews of all types of treatment for fibromyalgia; we scrutinized all promising or potential references and added appropriate titles to the search output.
Data collection and analysis
Review authors
Review authors are members of the Cochrane Exercise for Fibromyalgia Team (see Acknowledgments). Authors of this review included a kinesiologist, physical therapists, and an information specialist. Review authors were trained in data extraction via a standardized orientation program, and worked independently and in pairs to extract data. The review team met monthly to discuss progress, to clarify procedures, to make decisions regarding inclusion or exclusion and classification of outcome variables, and to work collaboratively in production of this review.
Selection of studies
Two review authors independently examined the titles and abstracts of studies generated by searches based on a set of criteria (see Appendix 15). We retrieved full‐text publications for all potentially relevant abstracts and translated all non‐English reports. Two review authors independently examined full‐text reports and translations to determine whether studies met the selection criteria (see Appendix 15). Review authors resolved disagreements and questions regarding interpretation of inclusion criteria in discussion with partners, unless the pair agreed to take the issue to the team.
For searches conducted before 2015, we used electronic forms developed and refined for our previous reviews to facilitate independent screening and consensus (Busch 2008). For the final update to the literature search conducted in June 2015, we conducted screening by using Covidence online software, which was recently developed for creating and maintaining systematic reviews (https://www.covidence.org/).
Data extraction and management
Pairs of review authors independently extracted data. We used electronic data extraction forms developed and refined during our previous reviews to facilitate independent data extraction and consensus (Busch 2008). We resolved disagreements by reaching consensus or by involving a third person (AJB). One review author (JB) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review versus those provided in study reports. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way, instances when data were obtained directly from study authors, and times when data were transformed or estimated from a graph.
If both unadjusted and adjusted values were reported for the same outcome, we extracted the adjusted values. If data were analyzed on an intention‐to‐treat (ITT) sample and another sample (eg, per‐protocol, as‐treated), we extracted ITT data.
We extracted the following data from the included studies.
-
Methods: study design, total duration of study, details of any "run‐in" period, number of study centers and locations, study setting, withdrawals, dates of study.
-
Participants: number of participants, mean age, age range, gender, disease duration, diagnostic criteria, inclusion criteria, exclusion criteria.
-
Interventions, comparisons, concomitant treatments:
-
for interventions with a non‐vibration exercise component: mode of exercise or physical activity, frequency, time, duration, intensity, progression model;
-
for interventions with a vibration exercise component: name of the vibration device, characteristics of the vibration, stance or position of the participant, whether any stabilizing support was used during the exercise; and
-
for other interventions: mode, frequency, duration.
-
-
Major outcomes: means and standard deviations for tests at baseline, post‐test intervention, and follow‐up for continuous outcomes (HRQL, physical function, pain, fatigue, and stiffness). If post‐test data were not available, we extracted means and standard deviations of change scores. We extracted the number of participants per group reporting adverse events and the number of participants who withdrew.
-
Minor outcomes: means and standard deviations for tests at baseline, post‐test intervention, and follow‐up for muscle strength and balance, as well as the number of participants who experienced at least 30% improvement in pain intensity.
-
Characteristics of trial design as outlined below in the Assessment of risk of bias in included studies section.
-
Congruence of exercise protocol with American College of Sports Medicine (ACSM) guidelines (Garber 2011 ‐ Appendix 16).
-
Notes: country, language, trial author contact or not, protocol identifier, funding for trial, notable declarations of interest for trial authors.
Assessment of risk of bias in included studies
To assess bias, we followed procedures recommended in the Cochrane Handbook for Systematic Reviews of Interventions. Two review authors independently evaluated risk of bias for each included study by using a customized form based on the Cochrane "Risk of bias" tool (Higgins 2011a). This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. For other sources of bias, we considered items such as baseline inequities despite randomization, or within‐study inequities involving duration of interventions. We rated each criterion as having low, high, or unclear risk of bias. We assigned the criterion "unclear risk" when absence or ambiguity of information blocked assessors' ability to determine the potential for bias. In such cases, we revised assessments if trial authors responded to our requests for more information. We resolved disagreements between review author pairs on classifying risk of bias through discussion at consensus meetings. If we could not reach agreement, we referred the issue to the review team for a decision.
Measures of treatment effect
For continuous data, we used group post‐test means and standard deviations (SDs) and RevMan 2014 software to calculate effect sizes. We expressed effect sizes preferentially in the form of mean differences (MDs) and 95% confidence intervals (CIs), but when different scales were used to measure the same outcome, we calculated standardized mean differences (SMDs) instead, with corresponding 95% CIs. We analyzed dichotomous data as risk differences and 95% CIs. We used RevMan 2014 software to generate forest plots to display measurement results.
In the comments column of the "Summary of findings" table, we provided the absolute percentage difference, the relative percentage change from baseline, and the number needed to treat for an additional beneficial outcome (NNTB). We provided the NNTB only when the outcome showed a statistically significant difference.
For dichotomous outcomes, such as withdrawals, we calculated the NNTB from the control group event rate, and relative risk using the Visual Rx NNT calculator (Cates 2008). We calculated the NNTB for continuous measures using the Wells calculator (available at the CMSG Editorial Office).
For dichotomous outcomes, we calculated the absolute risk difference (RD) using the RD statistic in RevMan 2014 and expressed the result as a percentage. For continuous outcomes, we calculated absolute benefit as improvement in the intervention group minus improvement in the control group, expressed in original units.
We calculated the relative percentage change for dichotomous data as the risk ratio (RR) and expressed this as a percentage. For continuous outcomes, we calculated the relative difference in change from baseline as the MD divided by the pooled baseline mean.
In accordance with Philadelphia Panel guidelines (Philadelphia Panel 2001), we assumed a minimal clinically important difference (MCID) of 15 points on a 100‐point continuous pain scale, and a relative difference of 15% on all functional scales, as clinically relevant.
Unit of analysis issues
Although many randomized trials include only two parallel arms (ie, groups), some have three or four parallel arms; thus, a single randomized trial can yield several relevant comparisons. This review examined any relevant comparison that allowed evaluation of effects of WBV exercise interventions for people with fibromyalgia. For example, a three‐arm trial comparing WBV versus drug treatment versus sham could appear in two separate analyses: WBV versus sham; WBV versus drug treatment. As another example, a trial with one WBV protocol versus a second WBV protocol versus a placebo or sham would yield three analyses: protocol one versus protocol two; protocol one versus sham; protocol two versus sham. When a control group was used as a comparator twice in the same analysis, we halved the sample size of the control group. We identified no cross‐over trials and no cluster‐RCTs (Higgins 2011b).
Dealing with missing data
When numerical data were missing, we contacted the authors of studies using open‐ended questions to request information needed to assess risk of bias or treatment effects, or both. When numerical data were available only in graphic form, we used Engauge version 5.1 to extrapolate means and SDs by digitalizing data points on graphs (Mitchell 2012).
For dichotomous outcomes (eg, number of withdrawals due to adverse events), we calculated the withdrawal rate using the number of participants randomized within the group as the denominator.
For continuous outcomes (eg, mean change in pain score), we calculated the MD or the SMD based on the number of participants analyzed at that time point. If the number of participants analyzed was not presented for each time point, we used the number of randomized participants in each group at baseline.
When post‐test SDs were unavailable, we used SDs of pretest scores as estimates. When variance was expressed via statistics other than SD (eg, standard error, confidence interval, P value), we computed SD according to the methods recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). When missing SDs could not be derived by the above methods, we imputed them (eg, from other studies in the meta‐analysis).
Assessment of heterogeneity
We assessed clinical and methodological diversity of included studies in terms of participants, interventions, outcomes, and study characteristics, to determine whether a meta‐analysis was appropriate. We did this by reviewing data recorded in data extraction tables. We assessed statistical heterogeneity by visually inspecting forest plots to assess for obvious differences in study results, and by using I² and Chi² statistical tests.
As recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), we applied interpretation of an I² value of 0% to 40% as might "not be important"; 30% to 60% as may represent "moderate" heterogeneity; 50% to 90% as may represent "substantial" heterogeneity; and 75% to 100% as represents "considerable" heterogeneity.
We interpreted a P value from the Chi² test that was less than or equal to 0.10 as evidence of statistical heterogeneity.
Assessment of reporting biases
We found an insufficient number of studies (ie, < 10) to produce funnel plots that would have allowed us to investigate publication reporting bias (Sterne 2011).
We screened the Clinical Trial Register at the International Clinical Trials Registry Platform of the World Health Organization (apps.who.int/trialssearch) for a priori trial protocols, and when these were available, we used them to evaluate whether selective reporting of outcomes was present (outcome reporting bias).
Data synthesis
When only one study was available, we used RevMan 2014 to calculate the mean difference in post‐test values (and 95% CIs) of a WBV intervention versus the comparator. When two or more studies reported the same outcome, we used RevMan 2014 to pool data (meta‐analysis). Before pooling data, we ensured that the directionality of data permitted pooling; we reversed selected scales arithmetically as required so that higher values consistently had the same meaning. We ensured that scaling factors were consistent to permit calculation of mean difference, when possible (eg, 10‐cm scales were expressed in mm to match other 100‐mm scales). We presented results grouped by common comparator, for example, WBV versus sham, WBV versus no treatment, or WBV versus active intervention.
We restricted the primary analysis for subjective self‐reported outcomes (HRQL, pain intensity, physical function, fatigue, and stiffness) to studies with low risk of selection and detection biases and low risk of attrition. We included all studies for adverse events and withdrawals.
"Summary of findings" table
We used GradePro (version GDT) to prepare "Summary of findings" table(s) for major outcomes for WBV versus control, and for WBV plus mixed exercise versus control (Schünemann 2011). In the "Summary of findings" table(s), we integrated analysis of quality of evidence and magnitude of effect of the interventions. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence and assign quality to one of four levels.
-
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
-
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
-
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
-
Very low quality: We are very uncertain about the estimate.
We assigned GRADE quality ratings separately for each of the seven major outcomes. We gave HRQL primacy over all other variables in the "Summary of findings" table and in the "Plain language summary" because of the comprehensive nature of the outcome variable.
Subgroup analysis and investigation of heterogeneity
We planned to explore relative effects of age and exercise volume on the impact of WBV exercise for pain intensity and HRQL.
Sensitivity analysis
We planned to perform sensitivity analyses to investigate the impact of statistical heterogeneity and methodological weaknesses (ie, high or unclear risk of selection bias and detection bias, or attrition rates > 20%).
Results
Description of studies
See Characteristics of included studies,Characteristics of excluded studies, and Characteristics of studies awaiting classification.
Results of the search
Our search yielded a total of 3894 records, which include 3802 journal records and 92 trial registry records. After we removed 975 duplicates, 2919 records remained. We excluded 2670 records on citation and abstract screening. We assessed 213 full‐text articles and 20 protocols/trial registry records for eligibility, and we excluded 88 full‐text articles and protocols/trial registry records. A further 11 articles and 1 protocol are awaiting classification. We excluded an additional 127 full‐text articles (primary articles n = 111, companion articles n = 16) and 18 protocols that compared physical activity interventions that did not meet the criteria for WBV training interventions. We included 4 publications (primary and companion full‐text journal articles (n=7) and 2 RCT protocols) describing effects of WBV (see Figure 1).

Study flow diagram for vibration training interventions.
Included studies
We included in our analysis four studies (Alentorn‐Geli 2008; Gusi 2010; Sañudo 2010; Sañudo 2013) that met our selection criteria. Although we included only four studies, these were reported in seven separate journal articles; we also found two RCT protocols. Olivares 2011 and Adsuar 2012 reported additional variables from the Gusi 2010 primary study; therefore, we included the three reports and counted them as one study for analysis (hereafter identified as Gusi 2010). Likewise, Sañudo 2012 reported on additional variables from Sañudo 2010; we included this pair but also counted them as one study (hereafter both reports are identified as Sañudo 2010).
RCTs included a total of 150 female participants with an average age of 57 years (range of mean ages 52 to 62). One trial author reported disease duration (range 9.8 to 10.5 years; Alentorn‐Geli 2008), and another reported symptom duration (range 12.7 to 13.7 years; Gusi 2010). Inclusion requirements of these studies were that participants were female and postmenopausal and had received a diagnosis of fibromyalgia according to ACR criteria (Wolfe 1990) or rheumatologist assessment, were diagnosed with fibromyalgia for at least three years, or were part of a community association. Exclusion criteria in the four studies included no previous experience with vibratory training; psychological or physical therapy provided or participation in a physical activity program; respiratory or cardiovascular conditions that prevented physical exertion; receipt of medications that could interfere with balance control; medical contraindications (ie, acute hernia, thrombosis, diabetes, epilepsy, metabolic or neuromuscular disease, osteoporosis, osteoarthritis, orthopedic injury, prosthesis); history of severe trauma; frequent migraines; peripheral nerve entrapment; severe psychiatric illness; rheumatic disorders; and pregnancy.
Average sample size was 37 (range 30 to 46; Sañudo 2010 ; Sañudo 2013). A total of 62 individuals were assigned to WBV: 21 in the vibration versus control group, 26 in the vibration and mixed versus control group, and 15 in the vibration and mixed versus other group. Median duration of the intervention was 7 weeks (range 6 to 12 weeks). Two studies provided a short intervention (< 7 weeks; Alentorn‐Geli 2008; Sañudo 2010), and two an intermediate intervention (Gusi 2010; Sañudo 2013) (8 to 12 weeks). All studies were conducted in Spain.
We contacted trial authors using open‐ended questions to request information needed to assess risk of bias and/or treatment effects. We received responses from Sañudo (for his 2010 and 2013 articles).
Three studies (Alentorn‐Geli 2008; Sañudo 2010; Sañudo 2013) that included aerobic exercise in the protocols did not meet ACSM criteria based on frequency. Of the two studies that included resistance in the protocol (Sañudo 2010; Sañudo 2013), only one met ACSM criteria. The other study (Sañudo 2013) did not provide enough information to confirm whether criteria were met. The flexibility provided in two studies (Alentorn‐Geli 2008; Sañudo 2013) adequately met ACSM criteria. Table 3 provides details of the exercise protocols included in these trials.
| Vibration + Mixed vs Mixed + Placebo vs Control | |||||||||
| Author, year | Intervention | Frequency (times per week | length in weeks) | Total duration | Supervised or home program | Aerobic component | Resistance component | Flexibility Component | Other | |
| I (intensity): ACSM classification and physiological measure; M (mode): mode of exercise; T (time): duration of aerobic component in minutes | M (muscle groups, joints or areas of body); I (intensity resistance, repetitions, sets); T (type), T (time) | M (muscle groups, joints or areas of body); T (type of stretch, repetition, set), T (time) | |||||||
| AE + FX + Relax | 2 times/wk | 6 weeks | 90’ | Not specified | I: moderate to vigorous (65%‐85% HR max), T: primarily level ground walking with games and dance, T: 30' | Not applicable | M: 5 whole body stretches involving lower and upper limbs, neck, back; T: dynamic, 5 reps held for 30 s with 30 s rests, T: 25' | Relaxation | |
| Vibration + Mixed vs Mixed | |||||||||
| AE + RT + FX | 2 times/wk | 6 weeks | 60’ | Supervised | I: light to moderate (50%‐69% HR max), M: not specified, T: 4‐6 intervals of 2‐3’, 1‐2’ rest between intervals | M: major muscle groups, I: 8 exercises, 1 × 8‐10 reps with 1‐3 kg, T: not specified | M: not specified, I: 1x 3 reps holding for 30s, T: not specified | ||
| AE + RT + FX | 2 times/wk | 8 weeks | 45‐60’ | Supervised | I: moderate (65%‐70% HR max), M: not specified, T: 10‐15’ | M: deltoids, biceps, neck, hips, back, and chest, I: 1 set of 8‐10 reps for 8 different muscle groups against 1‐3 kg, T: 15‐20’ | M: deltoids, biceps, neck, hips, back, and chest I: 1 set of 3 reps for 8‐9 different ex, maintained for 30 s, T: 10’ | ||
Included studies reported use of two vibration devices: Galileo Fitness Platform (Novotec Medical, Pforzheim, Germany) (see Figure 2) was used by Gusi 2010 and Sañudo 2010, and PowerPlate (PowerPlate International B.V., Badhoevendorp, The Netherlands) (see Figure 3) was used by Alentorn‐Geli 2008 and Sañudo 2013. Three studies used a fixed vertical amplitude (Alentorn‐Geli 2008; Gusi 2010; Sañudo 2013), and one used a variable amplitude (Sañudo 2010) with a range from 2 mm to 4 mm; frequency varied from 12.5 Hz (Gusi 2010) to 30 Hz (Alentorn‐Geli 2008; Sañudo 2013). We have provided in Table 4 additional details about the position and exercise of participants, use of stabilizing support, footwear provided, and types of exercise performed (ie, static/dynamic or unilateral/bilateral).

Galileo Fitness Platform (Copyright © 2008‐2015 Novotec Medical GmbH; reproduced with permission)

Copyright © 2012 Wellsports GmbH Krefeld ‐ PowerPlate International B.V., The Netherlands ‐ awaiting response.Sept15
| Study name/year | Name of device | Vibration frequency and amplitude | Position of participant | Stabilizing support | Footwear | Static/Dynamic; unilateral/bilateral |
| PowerPlate (PowerPlate International B.V., Badhoevendorp, The Netherlands) | 30 Hz; 2 mm vertical amplitude | The following 6 exercises were performed for 30 s each during whole body vibration (WBV) and were repeated 6 times with recovery of 3 minutes between repetitions (a) static squat at 100° of knee flexion (b) dynamic squat between 90° and 130° of knee flexion (c) maintained ankle plantar‐flexion with legs in extension (d) flexion‐extension of right leg between 100° and 130° of knee flexion (e) flexion‐extension of left leg between 100° and 130° of knee flexion (f) squat at 100° of knee flexion shifting body weight from 1 leg to the other For adaptation purposes, only tasks (a), (b), and (c) (repeated 3 times) were performed during first 2 sessions | Yes ‐ for all tasks, individuals held onto the supporting bar | Does not state | Static bilateral | |
| Galileo Fitness Platform (Novotec Medical, Pforzheim, Germany) | 12.5 Hz; 3 mm vertical amplitude | Participants alternated between 2 stances for each repetition Stance A: feet perpendicular to midline axis of the platform with right foot placed slightly ahead of left foot. Toes of right foot and heel of left foot lifted 4 mm above surface of the platform. Knees bent to 45° angle. Back and head kept straight Stance B: as per Stance A, except with left foot placed slightly ahead of right foot | Not reported and not pictured in Figure 2 | Balance testing was performed barefoot. Does not specify that exercise was done barefoot, but Figure 2 indicates this | Static and dynamic both unilateral and bilateral | |
| Galileo Fitness Platform (Novotec Medical, Pforzheim, Germany) | 20 Hz; variable amplitude of 2‐3 mm | Three sets of 45 s of bilateral static squat with 120 s recovery between sets (amplitude = 3 mm) followed by 4 sets of 15 s of unilateral static squat on each leg (amplitude = 2 mm). During WBV, participants stood with both knees in 120° isometric knee flexion (half‐squatting position) as measured by a goniometer | Does not state | Does not state | Static unilateral and bilateral | |
| PowerPlate, North America Inc., Northbrook, IL, United States | 30 Hz; vertical displacement of 4 mm (71.1 m/s‐2 ≈ 7.2 g) | Standing on the platform, with knees in 120º isometric knee flexion (measured by a goniometer) and trunk upright Bilateral static squat: 6 sets of 30 s, with 45‐s recovery between sets Unilateral static squat: 4 sets of 30 s each leg | Does not state | All participants wore sport shoes for vibration exercises | Static unilateral and bilateral |
Excluded studies
See Characteristics of excluded studies.
After screening citations and abstracts, we excluded 77 full‐text articles when studies did not meet the inclusion criteria on the basis of diagnosis of fibromyalgia (n = 8); physical activity intervention (n = 144); study design (n = 50); or specified outcomes not measured (n = 2) (see Figure 1). We excluded 126 full‐text articles among 134 describing physical activity interventions (primary articles n = 110, companion articles n = 16) and 18 RCT protocols because they did not provide a WBV exercise intervention. We excluded one study that provided a WBV intervention (Alentorn‐Geli 2009) because researchers measured none of the specified outcomes.
Risk of bias in included studies
We have provided in Figure 4 and Figure 5 results of our risk of bias assessment for the four included studies. We based risk of bias assessments on primary article data supplemented by responses from trial authors. We have provided additional information in the Characteristics of included studies table.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three studies (75% of the sample; Gusi 2010; Sañudo 2010; Sañudo 2013) used an acceptable method of random sequence generation (eg, random table, computer‐generated random number table or sequence), so we rated risk of bias as low. One study (Alentorn‐Geli 2008) did not specify details of how randomization was conducted, and we deemed risk for selection bias as unclear.
Two studies (50% of the sample) utilized acceptable allocation concealment methods and presented low risk of bias (Gusi 2010;Sañudo 2013); the remaining two studies did not provide enough information to allow a definitive judgement, and we deemed that risk was unclear (Alentorn‐Geli 2008; Sañudo 2010).
Blinding
Performance bias
In exercise studies, investigators found blinding of participants and care providers to be very rare and difficult to achieve. Among the included studies, we rated blinding of participants and personnel (performance bias) as low risk in one study (Alentorn‐Geli 2008), unclear risk in one study (Sañudo 2010), and high risk in two studies (Gusi 2010;Sañudo 2013).
Detection biases (objective and subjective outcome assessments)
Three studies (Gusi 2010; Sañudo 2013; Sañudo 2010) provided information on blinding of outcome assessors; one had no objective outcomes (Alentorn‐Geli 2008), and we rated it as having low risk. In this regard, it is important to note that the current Cochrane "Risk of bias" tool does not allow review authors to choose "not applicable" or to leave the section blank; therefore, we chose to rate Alentorn‐Geli 2008 as low risk despite the lack of outcome measures.
We rated one study as having high risk (Gusi 2010) for self‐reported outcomes, and two studies as having low risk (one included no self‐reported outcomes, and another included a placebo control by which participants were unaware of the intervention) (Alentorn‐Geli 2008; Sañudo 2013). We deemed one study with a head‐to‐head intervention as having unclear risk owing to lack of information on participant awareness of the intervention (Sañudo 2010).
Incomplete outcome data
Four studies (Alentorn‐Geli 2008; Gusi 2010; Sañudo 2010; Sañudo 2013) reported complete outcome data, and we rated risk for attrition bias as low.
Selective reporting
A priori research protocols were available for two studies (Alentorn‐Geli 2008;Gusi 2010). We rated risk of reporting bias for these two studies as high. We rated selective reporting risk of bias as low for the other two studies (Sañudo 2010;Sañudo 2013).
Other potential sources of bias
Two studies (Alentorn‐Geli 2008; Gusi 2010) appear to be free of any other bias, and in two studies (Sañudo 2010; Sañudo 2013), risk for other potential sources of bias is unclear.
Effects of interventions
See: Summary of findings for the main comparison Whole body vibration versus control; Summary of findings 2 Whole body vibration plus mixed exercise versus control
See summary of findings Table for the main comparison and summary of findings Table 2 for the main comparisons "WBV vs control" and "WBV plus mixed exercise versus control." We have grouped results related to effects of the interventions below to correspond to comparison groups of the review.
WBV versus control
Although the study included in this category is detailed under Characteristics of included studies, briefly the interventions compared were:
-
WBV versus control (Gusi 2010).
HRQL (self‐reported, FIQ total, 0 to 100 scale, lower is better health)
One study (Gusi 2010) (41 participants) provided data for the major outcome HRQL: HRQOL was 3.73 points at 12‐week follow‐up in the control group (MD ‐3.73, 95% CI ‐10.8 to 3.3; Analysis 1.1). Absolute difference was 4% (95% CI ‐11% to 3%), and relative change was ‐6.7% (95% CI ‐19.6% to 6.1%).
Balance (degree of displacement, lower scores indicate better balance)
One study (Gusi 2010) (41 participants) provided data for this minor outcome; at post‐test, participants in the intervention group were measured as having 0.35° less sway than those in the control group, as measured by the Biodex Balance System in degrees of displacement (MD 0.35°, 95% CI ‐0.74 to 0.04; Analysis 1.2). Relative change was ‐25% (95% CI ‐52.9% to 2.9%).
All‐cause withdrawal
Rates for WBV (n1/N1) versus control (n2/N2) were 3/21 versus 2/20 (Gusi 2010). Data show no significant differences in all‐cause withdrawal between groups (RR 1.43, 95% CI 0.27 to 7.67). Footnotes in the meta‐analysis show reasons participants withdrew from these studies (Analysis 1.3). Reasons for withdrawal per group: WBV n = 1 due to pain, n = 2 due to schedule interference; control n = 2 for lack of interest.
Adverse events
Gusi 2010 reported adverse events in narrative form (this was not one of the outcomes of the trial): "The program was reasonably safe: only 5% of the participants (n = 1) dropped out of the program because of acute pain in the legs. The program was completed by 85% of the participants, without secondary adverse effects" (page 1076).
Pain intensity, fatigue, stiffness, physical function, strength, and improvement in pain greater than 30%
The trial protocol did not include these outcomes, and they were not measured.
None of the outcomes in the WBV versus control comparison reached a clinically relevant difference of 15%.
WBV plus mixed exercise versus control
Although the two studies included in this category are detailed in the Characteristics of included studies, briefly the interventions compared were:
-
WBV + Mixed exercise (AE + FX + Relax) versus control (Alentorn‐Geli 2008); and
-
WBV + Mixed exercise (AE + RT + FX) versus control (Sañudo 2013).
HRQL (self‐reported, FIQ total, 0 to 100 scale, lower is better health)
One study (Alentorn‐Geli 2008) (21 participants) provided data for the major outcome HRQL: The mean difference between groups was statistically significant. WBV plus mixed exercise intervention showed improvement of 16 FIQ units compared with control (MD ‐16.02, 95% CI ‐31.57 to ‐0.47; Analysis 2.1). Absolute difference was ‐16% (95% CI ‐32% to ‐0.5%), and relative change was ‐23.9% (95% CI ‐47% to ‐1%; NNTB 3 [2 to 237]).
Pain intensity (self‐reported, FIQ pain scale, 0 to 100, lower is less pain)
One study (Alentorn‐Geli 2008) (21 participants) provided data for the major outcome pain intensity: The mean effect was statistically significant. WBV plus mixed exercise intervention showed improvement of 28 mm compared with control (MD ‐28.22, 95% CI ‐43.26 to ‐13.18; Analysis 2.2). Absolute difference was 28% (95% CI 43% to 13%), and relative change was ‐39.2% (95% CI ‐60.1% to ‐18.3%; NNTB 2 [1 to 4]).
Fatigue (self‐reported, FIQ fatigue scale, 0 to 100, lower is less fatigue)
One study (Alentorn‐Geli 2008) (21 participants) provided data for the major outcome fatigue: The mean effect was statistically significant. WBV plus mixed exercise intervention showed improvement of 33 mm compared with control (MD ‐32.84, 95% CI ‐49.20 to ‐16.48; Analysis 2.3). Absolute difference was 33% (95% CI 49% to 16%), and relative change was ‐46.9% (95% CI ‐70.3 to ‐23.5%; NNTB 2 [1 to 4]).
Stiffness (self‐reported, FIQ stiffness scale, 0 to 100, lower is better)
One study (Alentorn‐Geli 2008) (21 participants) provided data for the major outcome stiffness: The mean effect was statistically significant. WBV plus mixed exercise intervention showed improvement of 25 mm compared with control (MD ‐26.27, 95% CI ‐42.96 to ‐9.58; Analysis 2.4). Absolute difference was 26% (95% CI 43% to 10%), and relative change was ‐36.5% (95% CI ‐59.7 to ‐22.9%; NNTB 2 [1 to 6]).
Balance (degree of tilt, lower scores indicate better balance)
One study (Sañudo 2013) (24 participants) provided data for the minor outcome balance: This outcome showed evidence of no effect. Absolute difference at post‐test between intervention and control groups was 0.48° less sway when standing on the balance platform (MD ‐0.48°, 95% CI ‐3.1 to 2.2; Analysis 2.5). Relative change was ‐5.67% (95% CI ‐37.5% to 26.2%).
All‐cause withdrawal
Withdrawal rates for WBV plus mixed exercise (n1/N1) versus control (n2/N2) were 1/11 versus 2/10 (Alentorn‐Geli 2008) and 1/14 versus 5/11 (Sañudo 2013). Data show no differences in all‐cause withdrawal between groups (RR ‐0.24, 95% CI ‐0.5 to 0.03). We footnoted in the meta‐analysis reasons participants withdrew from studies (Analysis 2.6): WBV + MX n = 1 no show for testing; control n = 2 no show for testing (Alentorn‐Geli 2008); WBV + MX n = 1 for illness; control n = 5 for personal reasons (Sañudo 2013).
Adverse events
Trial authors reported adverse events in narrative form and did not consider them an outcome. Overall reported events are minor and are described below as direct quotations from trial authors.
Alentorn‐Geli 2008: "This program neither exacerbated FM‐related symptoms nor resulted in musculoskeletal injuries; however, one patient exhibited a mild anxiety attack on the first session of WBV" (page 978).
Sañudo 2013: "This study, however, demonstrated that WBV training is a safe (no adverse effects), suitable (no dropouts due to the intervention) and effective (increased lower limb muscle strength) way to exercise the musculoskeletal system, and potentially a feasible intervention for those patients who cannot participate in conventional strength training" (page 683).
Physical function, strength, and improvement in pain greater than 30%
No studies performing this comparison reported on these outcomes.
Several outcomes in this comparison met the 15% threshold for clinical relevance: HRQL (improved by 16%), pain intensity (improved by 28%), fatigue (improved by 34%), and stiffness (improved by 26%).
WBV plus mixed exercise versus other exercise
We have detailed the studies under Characteristics of included studies and briefly described bellow:
-
WBV + Mixed exercise (AE + FX + Relax) versus mixed exercise (AE + FX + Relax) + placebo WBV (Alentorn‐Geli 2008);
-
WBV + Mixed exercise (AE + RT + FX) versus mixed exercise (AE + RT + FX) (Sañudo 2010); and
-
WBV + Mixed exercise (AE + RT + FX) versus mixed exercise (AE + RT + FX) (Sañudo 2013).
HRQL (self‐reported, FIQ total, 0 to 100, lower is better health)
Two studies (Alentorn‐Geli 2008; Sañudo 2010) (49 participants) provided data for this major outcome: The mean HRQL showed evidence of no effect (MD ‐6.67, 95% CI ‐14.65 to 1.31; Analysis 3.1). Absolute difference was ‐7% (95% CI ‐15 to 1), and relative change was ‐11.75% (95% CI ‐29.32% to 2.31%).
Pain intensity (self‐reported, FIQ pain scale, 0 to 100, lower is less pain)
One study (Alentorn‐Geli 2008) (23 participants) provided data for this outcome. Pain intensity showed a statistically significant effect favoring WBV by 16 mm (MD ‐16.36, 95% CI ‐29.49 to ‐3.23; Analysis 3.2). Absolute difference was 16% (95% CI ‐29% to ‐3%), and relative change was ‐24% (95% CI ‐45% to ‐5%).
Fatigue (self‐reported, FIQ fatigue scale, 0 to 100, lower is less fatigue)
One study (Alentorn‐Geli 2008) (23 participants) provided data for this outcome. Fatigue showed a statistically significant effect favoring WBV by 14 mm (MD ‐14.41, 95% CI ‐29.47 to 0.65; Analysis 3.3). Absolute difference was 14% (95% CI ‐29% to 1%), and relative change was ‐22.52% (95% CI ‐46% to ‐1%).
Stiffness (self‐reported, FIQ stiffness scale, 0 to 100, lower is less stiffness)
One study (Alentorn‐Geli 2008) (23 participants) provided data for this outcome. Evidence showed no effect for stiffness (MD ‐12, 95% CI ‐26.90% to 1.46%; Analysis 3.4 ). Absolute difference was 11% (95% CI ‐27% to 1%), and relative change was ‐17.67% (95% CI ‐37.36% to ‐2.03%).
Strength (newtons and repetitions, higher is better strength)
Two studies (Sañudo 2010; Sañudo 2013) (54 participants) provided data for this outcome showing a statistically significant effect for strength (SMD 0.77, 95% CI 0.20% to 1.35%), representing a moderate effect (Cohen 1988). Absolute difference was 0.77 SDs higher in the WBV plus mixed exercise group, and relative change was 0.71% (95% CI 0.18% to 1.24%).
Balance (degree of displacement/tilt, lower scores indicate better balance)
Two studies (Sañudo 2010; Sañudo 2013) (54 participants) provided data for this outcome showing no effect for balance. Absolute difference at post‐test between intervention and control groups was 0.22° less sway when standing on the balance platform with eyes closed (MD ‐0.22°, 95% CI ‐1.56° to 1.11), and relative change was ‐2.12% (95% CI ‐15% to 11%).
All‐cause withdrawal
Rates for WBV plus mixed exercise (n1/N1) versus other exercise (n2/N2) were 1/11 versus 0/12 (Alentorn‐Geli 2008), 1/14 versus 3/12 (Sañudo 2010), and 1/14 versus 1/14 (Sañudo 2013). Data show no difference in all‐cause withdrawal between groups (RD ‐0.01, 95% CI ‐0.15 to 0.13). We have footnoted in the meta‐analysis reasons participants withdrew from studies (Analysis 3.7).
Adverse events
The authors of these trials did not specify adverse events as an outcome but reported them in narrative form. Overall trial authors described minor events as below.
Alentorn‐Geli 2008: "This program neither exacerbated FM‐related symptoms nor resulted in musculoskeletal injuries; however, one patient exhibited a mild anxiety attack on the first session of WBV" (page 978).
Sañudo 2010: Study authors clarified that one person in the comparison group ("other exercise group") dropped out owing to an injury that was not related to the program (participant fell down on the street).
Sañudo 2013: "This study, however, demonstrated that WBV training is a safe (no adverse effects), suitable (no dropouts due to the intervention), and effective (increased lower limb muscle strength) way to exercise the musculoskeletal system, and potentially a feasible intervention for those patients who cannot participate in conventional strength training" (page 683).
Physical function and improvement in pain greater than 30%
No studies in this comparison reported on these outcomes.
One outcome in this comparison met the 15% threshold for clinical relevance; pain intensity improved by 16% (Alentorn‐Geli 2008).
Subgroup and sensitivity analyses
Given the small number of studies performing the three comparisons and evaluating outcomes, data were insufficient for review authors to perform planned subgroup (ACSM guidelines and outcomes stratified by age) and sensitivity analyses (selection, detection, and attrition).
Discussion
Summary of main results
This review evaluated benefits and harms of whole body vibration (WBV) in adults with fibromyalgia. Results of this review show that WBV exercise training used as an intervention for individuals with fibromyalgia is in its infancy. Our extensive literature search yielded four randomized controlled trials (RCTs) conducted in the same country, which included only middle‐age female participants. The only study comparing WBV only versus control concentrated on two of our outcomes and found no significant effects for either (health‐related quality of life [HRQL] and balance). Two studies compared WBV combined with mixed exercise versus control and found evidence of positive effects on HRQL, pain intensity, fatigue, stiffness, and strength, but not on balance, and showed no overlap in outcomes measured; therefore, results were not replicated across studies. The final comparison examined WBV plus exercise versus exercise alone. When adding WBV to an exercise protocol, this group of studies found no evidence of enhanced effect in terms of HRQL, fatigue, stiffness, or balance, but noted positive effects on pain intensity. No comparisons revealed significant differences in terms of withdrawal, and investigators documented only a few minor adverse events.
Although some results reported on major outcomes were statistically significant, the overall quality of the evidence was very low. In light of uncertainties surrounding currently available evidence, we wonder whether costs of accessing or purchasing WBV machines to carry out this type of exercise warrant their general use by people with fibromyalgia.
Given the small number of trials, small sample sizes, and narrow inclusion criteria reported in the published research, along with the very low quality of evidence, it is difficult to comment on benefits and harms of WBV exercise training. Future research will likely change our understanding of this intervention. We have provided more detailed information on main results in summary of findings Table for the main comparison and summary of findings Table 2.
Overall completeness and applicability of evidence
This review includes four studies. Samples recruited by the included studies consisted of women from Spain ranging from 52 to 62 years old. Sample sizes were small, and pooled samples included fewer than 100 individuals for the few outcomes that could be meta‐analyzed. Therefore, we recommend caution in generalizing results of this review to the wider population of individuals with fibromyalgia.
Studies included in this review address only a few of the WBV regimens (see Table 4 and Table 3) that are possible to provide and make a small start in amassing the evidence required to adequately assess benefits and harms of WBV for individuals with fibromyalgia. Those who wish to use this form of exercise must consider a myriad of options. In addition to variable frequency and duration of exercise sessions, one needs to consider vibration platform characteristics (manufacturer, model), waveform (vertical or rotary oscillations, frequency and amplitude of vibration, g‐force, pulse features), and exercise features (double or single limb support, static joint position or dynamic exercise). Therefore, review authors have concluded that readers should carefully inspect the details regarding WBV interventions provided in included studies with awareness that other WBV interventions may produce different effects.
We are confident that the outcomes selected for this review are valued by people with fibromyalgia and clinicians (Choy 2009). However, the small sample of studies precludes adequate investigation of these outcomes. At present, estimates of treatment effects are imprecise, and it is likely that these estimates will change as additional studies are carried out. Adverse events appear to be minimal, but at this point we cannot be completely sure of the safety of WBV, especially as the interventions examined were restricted and included trials were too small and reported events too few to provide a robust estimate of the risk of adverse events.
Quality of the evidence
Review authors obtained the evidence presented in this review from trials published in academic journals, as well as from registered RCT protocols and trial author responses to requests for information. The overall quality of the evidence was very low after downgrading due to imprecision and study design flaws. Available evidence is limited by the number and quality of included trials, and this does not allow us to reach robust conclusions regarding benefits and harms of WBV. Studies showed methodological flaws resulting in a mix of low (eg, attrition), unclear (eg, selection), and high risks of bias (eg, performance). The sample size of included trials was often small; even after pooling of data in the meta‐analysis, participant numbers were smaller than desired, leading to issues of imprecision. Studies did not consider adverse events as a major outcome but did report them in narrative form.
It is uncertain whether WBV, when compared with control, improves quality of life (mean difference [MD] 10.81 lower to 3.35 higher) or decreases participant withdrawal (risk ratio [RR] 0.27 higher to 7.67 higher). The quality of evidence is very low owing to the paucity of research (single study evidence), imprecision (small sample size and wide confidence intervals), and issues related to risk of bias.
When compared with control, WBV + MX seemed to improve HRQL (MD 31.57 lower to 0.47 lower), pain (MD 43.26 lower to 13.18 lower), fatigue (49.20 lower to 16.48 lower), and stiffness (MD 42.96 lower to 9.58 lower), or made a difference in relation to participant withdrawal (RR 0.06 higher to 1.12 higher). However, the quality of evidence is very low owing to imprecision (small sample size and wide confidence intervals) and issues related to risk of bias. Thus we are uncertain about the effect of WBV + MX on adults with fibromyalgia.
It remains uncertain whether WBV + MX, when compared with other interventions, improves HRQL (MD 14.65 lower to 1.31 higher), fatigue (MD 29.47 lower to 0.65 higher), or stiffness (MD 26.90 lower to 1.46 higher), or decreases participant withdrawal (RR 0.17 higher to 3.11 higher). Data show that the intervention improved pain (24.49 lower to 3.23 lower). However, the quality of evidence is very low owing to the paucity of research (single study evidence), imprecision (small sample size and wide confidence intervals), and issues related to risk of bias (see Table 5).
| Quality assessment | No. of participants | Quality | Importance | |||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Aerobic exercise (AE) intervention | AE control | ||
| Health‐related quality of life (HRQL), 0‐100, lower is best | ||||||||||
| 2 | Randomized trial | Very serious1 | Not serious | Not serious | Serious2 | 23 | 26 | ⊕⊝⊝⊝ | CRITICAL | |
| Pain intensity, 0‐100, lower is best | ||||||||||
| 1 | Randomized trial | Serious3 | Not serious | Not serious | Serious2 | One very small study | 11 | 12 | ⊕⊝⊝⊝ | CRITICAL |
| Fatigue, 0‐100, lower is best | ||||||||||
| 1 | Randomized trial | Serious1 | Not serious | Not serious | Serious2 | One very small study | 11 | 12 | ⊕⊝⊝⊝ | IMPORTANT |
| Stiffness, 0‐100, lower is best | ||||||||||
| 1 | Randomized trial | Serious1 | Not serious | Not serious | Serious2 | One very small study | 11 | 12 | ⊕⊝⊝⊝ | IMPORTANT |
| Physical function, not reported | ||||||||||
| Withdrawals | ||||||||||
| 3 | Randomized trial | Very serious4 | Not serious | Not serious | Serious2 | 3/39 (7.69%) | 4/38 (10.52%) | ⊕⊝⊝⊝ | IMPORTANT | |
| Adverse events:Alentorn‐Geli 2008: "This program neither exacerbated FM‐related symptoms nor resulted in musculoskeletal injuries; however, one patient exhibited a mild anxiety attack on the first session of WBV" (page 978); Sañudo 2010: Trial authors clarified that one person in the comparison group ("other exercise group") dropped out owing to an injury that was not an injury related to the program (participant fell down on the street); Sañudo 2013: "This study, however, demonstrated that WBV training is a safe (no adverse effects), suitable (no dropouts due to the intervention), and effective (increased lower limb muscle strength) way to exercise the musculoskeletal system, and potentially a feasible intervention for those patients who cannot participate in conventional strength training" (page 683) | ||||||||||
Potential biases in the review process
We aimed to minimize systematic error and bias in locating, selecting, including and excluding, appraising, and aggregating/synthesizing studies for this review. Strengths related to preventing bias include the following.
-
Source bias/databases bias: Our search included a comprehensive list of medical, health‐related, and health technology databases supplemented by handsearching or use of other sources; we updated searches periodically.
-
Selective reporting bias: By searching clinical trial registries (eg, clincialtrials.gov), we were able to identify original RCT protocols and to appraise whether reported elements were present in the protocols and publications for two RCTs (Alentorn‐Geli 2008; Gusi 2010). We judged this item as high risk after finding discrepancies between registered protocols and publications. We provided details under Characteristics of included studies.
-
Scope bias: Our inclusion and/or exclusion criteria were not constrained by geography (ie, particular region), time (ie, time limits), or language (ie, English).
-
Publication bias: We applied no language restrictions.
-
We contacted primary authors for clarification and additional information when indicated, although we did not always obtain responses. We asked open‐ended questions to avoid leading answers.
-
We collected information on funding for individual studies, as funding may be seen as a potential source of bias (this information can be seen under Characteristics of included studies). We did not find information on funding for three studies; one study author stated, "no grant supported the investigation" (Alentorn‐Geli 2008).
Limitations of this review include the following.
-
Despite efforts to reduce the impact of publication bias in this review, the possibility remains that some studies may have not been identified by the search.
-
Generalization of review findings is restricted to middle‐aged women of Spanish origin and the characteristics (frequency, amplitude, etc.) of machines utilized.
Agreements and disagreements with other studies or reviews
We found one previous review on whole body vibration and fibromyalgia (Chulvi‐Medrano 2013). This review lacked some important features of a systematic review, that is, it relied on one electronic database (PubMed) instead of performing a broad search, it did not evaluate risk of bias of included studies, and it did not provide numerical data regarding outcomes of the literature search and treatment effects. In addition to the five included studies, review authors drew upon results from a variety of studies excluded from our review to support their conclusions. On the basis of poorly documented evidence, Chulvi‐Medrano 2013 was optimistic about effects of WBV for individuals with fibromyalgia, stating, “research suggests that patients with FMS can achieve significant improvements in functional capacity, lower limb strength, balance, and quality of life (reductions in pain and fatigue) if they take part in a personalized program.” The reader who is not careful could be convinced that evidence showing positive effects of WBV for fibromyalgia is more meaningful than it is.
Other systematic reviews on this topic in the Cochrane Library have examined different populations (ie, neurodegenerative disease, multiple sclerosis) (Amatya 2013; Sitja Rabert 2012). Despite differences among study populations, reviews are similar in providing findings of insufficient evidence of low quality regarding beneficial effects on HRQL, symptoms, and muscle strength. In keeping with the findings of the present review, review authors concluded that further research is needed before robust recommendations regarding the safety of WBV can be provided.
To date, three sets of evidence‐based interdisciplinary guidelines for treatment of fibromyalgia have been published (Ablin 2013; Fitzcharles 2012; Hauser 2010). None of these guidelines have referenced WBV exercise training or recommended such training. As this novel technique is further studied via larger trials in this population, future treatment guidelines may begin to discuss possible benefits.
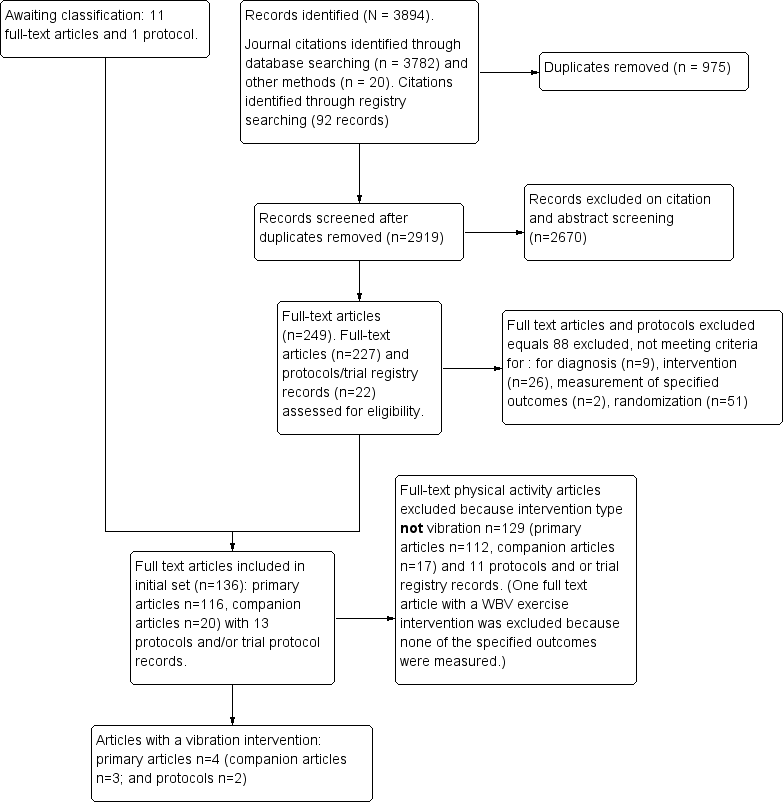
Study flow diagram for vibration training interventions.

Galileo Fitness Platform (Copyright © 2008‐2015 Novotec Medical GmbH; reproduced with permission)

Copyright © 2012 Wellsports GmbH Krefeld ‐ PowerPlate International B.V., The Netherlands ‐ awaiting response.Sept15

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
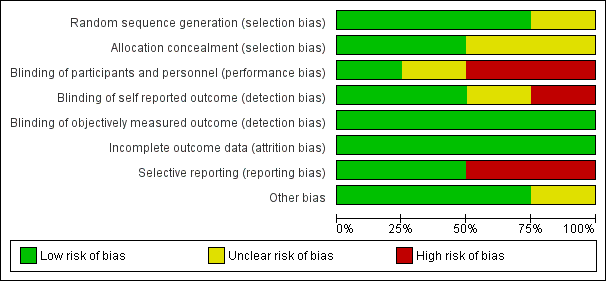
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 WBV vs C, Outcome 1 HRQL, 1‐100 scale, lower means better HRQL.

Comparison 1 WBV vs C, Outcome 2 Balance, degrees of displacement, lower is best.

Comparison 1 WBV vs C, Outcome 3 Withdrawal.

Comparison 2 WBV + MX vs C, Outcome 1 WBV + MX vs C: HRQL, 0‐100 scale, lower is best.
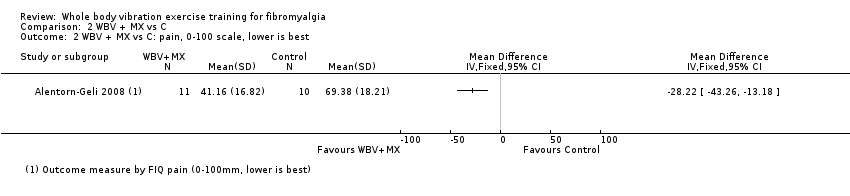
Comparison 2 WBV + MX vs C, Outcome 2 WBV + MX vs C: pain, 0‐100 scale, lower is best.
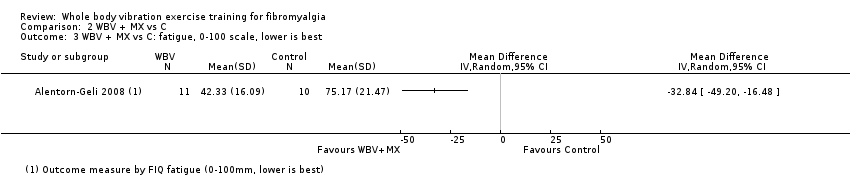
Comparison 2 WBV + MX vs C, Outcome 3 WBV + MX vs C: fatigue, 0‐100 scale, lower is best.

Comparison 2 WBV + MX vs C, Outcome 4 WBV + MX vs C: stiffness, 0‐100 scale, lower is best.

Comparison 2 WBV + MX vs C, Outcome 5 WBV + MX vs C: balance, overall stability index ‐ eyes closed (degrees of displacement 0 to 20 scale, lower is best).

Comparison 2 WBV + MX vs C, Outcome 6 Withdrawal.

Comparison 3 WBV + MX vs Other, Outcome 1 WBV + MX vs Other: HRQL, 0‐100 scale, lower is best.

Comparison 3 WBV + MX vs Other, Outcome 2 WBV + MX vs Other: pain, 0‐100 scale, lower is best.

Comparison 3 WBV + MX vs Other, Outcome 3 WBV + MX vs Other: fatigue, 0‐100 scale, lower is best.

Comparison 3 WBV + MX vs Other, Outcome 4 WBV + MX vs Other: stiffness, 0‐100 scale, lower is best.

Comparison 3 WBV + MX vs Other, Outcome 5 WBV + MX vs Other: strength, measured in newtons and number of reps, higher values are best.
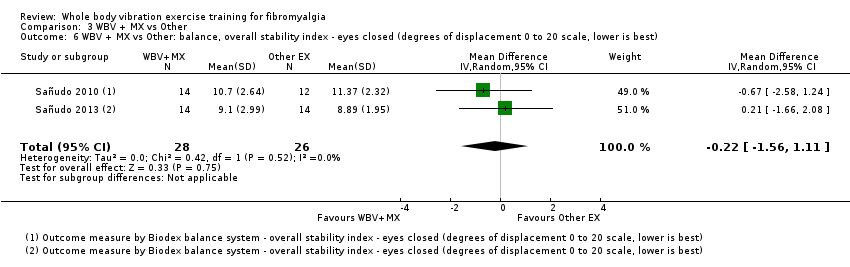
Comparison 3 WBV + MX vs Other, Outcome 6 WBV + MX vs Other: balance, overall stability index ‐ eyes closed (degrees of displacement 0 to 20 scale, lower is best).
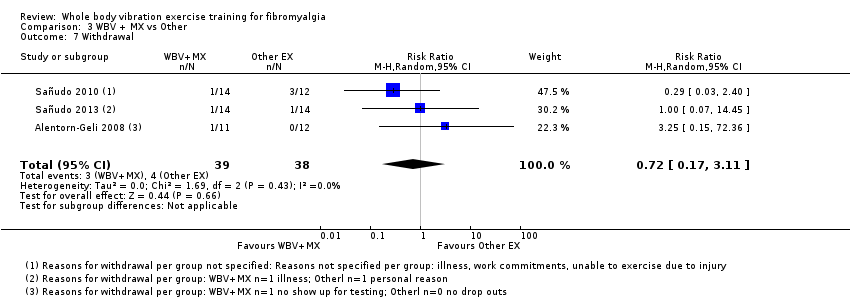
Comparison 3 WBV + MX vs Other, Outcome 7 Withdrawal.
| Whole body vibration versus control | ||||||
| Patient or population: individuals with fibromyalgia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with WBV | |||||
| Health‐related quality of life Follow‐up: 12 weeks | Mean health‐related quality of life was 59 points | Mean health‐related quality of life in intervention group was 3.73 points lower (10.81 lower to 3.35 higher) at post‐test than in control group | ‐ | 41 | ♁◯◯◯ | Absolute improvement: 4% (95% CI 11% better to 3% worse) Relative change: 6.7% improvement (95% CI 19.6% improvement to 6.1% worse) NNTB: n/ac |
| Pain intensity | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Fatigue | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Stiffness | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Physical function | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Adverse events | Gusi 2010: "The program was reasonably safe: only 5% of the participants (n = 1) dropped out of the program because of acute pain in the legs. The program was completed by 85% of the participants, without secondary adverse effects" (page 1076; 1 study) | |||||
| All‐cause withdrawal | Study population | RR 1.43 (0.27 to 7.67) | 41 | ♁◯◯◯ | Absolute risk difference: 4% more events (95% CI 16% fewer to 24% more) Relative change: 43% more (73% fewer to 667% more) NNTH: n/ac | |
| 10 per 100 (2 of 20) | 14 per 100 (3 of 21) | |||||
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImpresicion: number of participants lower than 400 rule of thumb; wide confidence interval (downgraded twice) bHigh risk of biases including detection, performance, and reporting biases cNumber needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated with Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated with Wells calculator (CMSG Editorial Office) | ||||||
| Whole body vibration plus mixed exercise versus control | ||||||
| Patient or population: individuals with fibromyalgia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with WBV + MX | |||||
| Health‐related quality of life | Mean health‐related quality of life was 59.64 points at the end of the study | Mean health‐related quality of life in the intervention group was 16.02 points lower (31.57 lower to 0.47 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference: 16% improvement (95% CI 32% to 0.5% improvement). Relative change: 24% (47% to 0.7%)c NNTBd: 3 (2 to 237) |
| Pain Intensity | Mean pain intensity was 69.38 mm in the control group at the end of the study | Mean pain Intensity in the intervention group was 28.22 mm lower (43.26 lower to 13.18 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference: 28% (95% CI 43% to 13%). Relative difference: 39% (95% CI 18% to 60%) NNTBd: 2 (1 to 4) |
| Fatigue | Mean fatigue was 75.17 mm at the end of the study | Mean fatigue in the intervention group was 32.84 mm lower (49.2 lower to 16.48 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference: 33% (95% CI 49% to 16%). Relative difference: 47% (95% CI 23% to 70%) NNTBd: 2 (1 to 4) |
| Stiffness | Mean stiffness was 68.71 mm at the end of the study | Mean stiffness in the intervention group was 26.27 mm lower (42.96 lower to 9.58 lower) at post‐test than in the control group | ‐ | 21 | ♁◯◯◯ | Absolute difference 26% (95% CI 43% to 10%). Relative difference: 36.5% (95% CI 60% to 23%) NNTBd: 2 (1 to 6) |
| Physical function | Not measured | Not measured | ‐ | Not measured | Not measured | Not measured |
| Adverse events (narrative) | Alentorn‐Geli 2008: "This program neither exacerbated FM‐related symptoms nor resulted in musculoskeletal injuries; however, 1 patient exhibited a mild anxiety attack on the first session of WBV" (page 978) Sañudo 2013: "This study, however, demonstrated that WBV training is safe (no adverse events)..." (page 683) | |||||
| All‐cause withdrawal | 33 per 100 (7 of 21) | 8 per 100 | RR 0.25, 95% CI 0.06 to 1.12 | 46 | ♁◯◯◯ | Absolute risk difference: 24% (95% CI 3 to 51) NNTHd: n/a |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| aImprecision: Number of participants lower than 400 rule of thumb; wide confidence interval. Need for more studies with more participants to reach optimal information size (downgraded twice) bHigh risk of biases including reporting and selection biases. Need for methodologically better designed and executed studies cBaseline control group mean (SD) = 67 (15.81), n = 10 dNumber needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH) not applicable (n/a) when result is not statistically significant. NNT for dichotomous outcomes calculated with Cates NNT calculator (http://www.nntonline.net/visualrx/). NNT for continuous outcomes calculated with Wells calculator (CMSG Editorial Office) | ||||||
| Term | Definition |
| Allodynia | Pain resulting from a stimulus that would not normally provoke pain |
| Amplitude | Absolute value of maximum displacement from a zero value during 1 period of an oscillation |
| Damping | Energy dissipation properties of a material or system under cyclic stress |
| Endurance | Two forms of endurance that refer to health‐related physical fitness include cardiorespiratory endurance (also known as cardiovascular endurance, aerobic fitness, aerobic endurance, exercise tolerance), which "relates to the ability of the circulatory and respiratory systems to supply fuel during sustained physical activity and to eliminate waste products after supplying fuel," and muscle endurance, which "relates to the ability of muscle groups to exert external force for many repetitions" (Caspersen 1985) |
| Frequency | Number of cycles or completed alternations per unit time of a wave or oscillation |
| Hertz | One hertz is 1 cycle per second; therefore, when an individual is exposed to a vibration of 30 Hz, targeted muscles receive 30 cycles of vibration per second, which makes muscles contract and relax 30 times in the same period |
| Hyperalgesia | Increased pain from a stimulus that normally provokes pain |
| Natural frequency | Frequency at which a system oscillates when not subjected to continuous or repeated external forces |
| Paresthesia | Abnormal sensation that is spontaneous or is evoked by a stimulus (eg, numbness) |
| Phase angle | Particular stage or point of advancement in a cycle; fractional part of the period through which time has advanced, measured from some arbitrary origin often expressed as an angle (phase angle); the entire period being taken is 360° |
| Abbreviation | Description |
| A | amplitude |
| ACR | American College of Rheumatology |
| ACSM | American College of Sports Medicine |
| AE | aerobic exercise |
| EMG | electromyography |
| f | frequency |
| FIQ | Fibromyalgia Impact Questionnaire |
| FX | flexibility |
| g | gravitational load (G‐force) = 1 cm/s2 |
| HR | heart rate |
| HRQL | health‐related quality of life |
| hz | Hertz |
| ITT | intention‐to‐treat |
| kg | kilogram |
| m/s2 | unit of acceleration: 1 Gal = 0.01 m/s2 |
| MCID | minimal clinically important difference |
| MD | mean difference |
| MX | mixed intervention (includes more than 1 mode of physical activity) |
| n | number of studies |
| N | number of individuals |
| RD | risk difference |
| Relax | relaxation |
| RT | resistance training |
| s | seconds |
| SD | standard deviation |
| SE | standard error |
| SMD | standardized mean difference |
| sTNFR1 and sTNFR2 | soluble tumor necrosis factor receptor 1 and 2 |
| VAS | visual analogue scale |
| WBV | whole body vibration |
| wk | week(s) |
| WU | warm‐up |
| Vibration + Mixed vs Mixed + Placebo vs Control | |||||||||
| Author, year | Intervention | Frequency (times per week | length in weeks) | Total duration | Supervised or home program | Aerobic component | Resistance component | Flexibility Component | Other | |
| I (intensity): ACSM classification and physiological measure; M (mode): mode of exercise; T (time): duration of aerobic component in minutes | M (muscle groups, joints or areas of body); I (intensity resistance, repetitions, sets); T (type), T (time) | M (muscle groups, joints or areas of body); T (type of stretch, repetition, set), T (time) | |||||||
| AE + FX + Relax | 2 times/wk | 6 weeks | 90’ | Not specified | I: moderate to vigorous (65%‐85% HR max), T: primarily level ground walking with games and dance, T: 30' | Not applicable | M: 5 whole body stretches involving lower and upper limbs, neck, back; T: dynamic, 5 reps held for 30 s with 30 s rests, T: 25' | Relaxation | |
| Vibration + Mixed vs Mixed | |||||||||
| AE + RT + FX | 2 times/wk | 6 weeks | 60’ | Supervised | I: light to moderate (50%‐69% HR max), M: not specified, T: 4‐6 intervals of 2‐3’, 1‐2’ rest between intervals | M: major muscle groups, I: 8 exercises, 1 × 8‐10 reps with 1‐3 kg, T: not specified | M: not specified, I: 1x 3 reps holding for 30s, T: not specified | ||
| AE + RT + FX | 2 times/wk | 8 weeks | 45‐60’ | Supervised | I: moderate (65%‐70% HR max), M: not specified, T: 10‐15’ | M: deltoids, biceps, neck, hips, back, and chest, I: 1 set of 8‐10 reps for 8 different muscle groups against 1‐3 kg, T: 15‐20’ | M: deltoids, biceps, neck, hips, back, and chest I: 1 set of 3 reps for 8‐9 different ex, maintained for 30 s, T: 10’ | ||
| Study name/year | Name of device | Vibration frequency and amplitude | Position of participant | Stabilizing support | Footwear | Static/Dynamic; unilateral/bilateral |
| PowerPlate (PowerPlate International B.V., Badhoevendorp, The Netherlands) | 30 Hz; 2 mm vertical amplitude | The following 6 exercises were performed for 30 s each during whole body vibration (WBV) and were repeated 6 times with recovery of 3 minutes between repetitions (a) static squat at 100° of knee flexion (b) dynamic squat between 90° and 130° of knee flexion (c) maintained ankle plantar‐flexion with legs in extension (d) flexion‐extension of right leg between 100° and 130° of knee flexion (e) flexion‐extension of left leg between 100° and 130° of knee flexion (f) squat at 100° of knee flexion shifting body weight from 1 leg to the other For adaptation purposes, only tasks (a), (b), and (c) (repeated 3 times) were performed during first 2 sessions | Yes ‐ for all tasks, individuals held onto the supporting bar | Does not state | Static bilateral | |
| Galileo Fitness Platform (Novotec Medical, Pforzheim, Germany) | 12.5 Hz; 3 mm vertical amplitude | Participants alternated between 2 stances for each repetition Stance A: feet perpendicular to midline axis of the platform with right foot placed slightly ahead of left foot. Toes of right foot and heel of left foot lifted 4 mm above surface of the platform. Knees bent to 45° angle. Back and head kept straight Stance B: as per Stance A, except with left foot placed slightly ahead of right foot | Not reported and not pictured in Figure 2 | Balance testing was performed barefoot. Does not specify that exercise was done barefoot, but Figure 2 indicates this | Static and dynamic both unilateral and bilateral | |
| Galileo Fitness Platform (Novotec Medical, Pforzheim, Germany) | 20 Hz; variable amplitude of 2‐3 mm | Three sets of 45 s of bilateral static squat with 120 s recovery between sets (amplitude = 3 mm) followed by 4 sets of 15 s of unilateral static squat on each leg (amplitude = 2 mm). During WBV, participants stood with both knees in 120° isometric knee flexion (half‐squatting position) as measured by a goniometer | Does not state | Does not state | Static unilateral and bilateral | |
| PowerPlate, North America Inc., Northbrook, IL, United States | 30 Hz; vertical displacement of 4 mm (71.1 m/s‐2 ≈ 7.2 g) | Standing on the platform, with knees in 120º isometric knee flexion (measured by a goniometer) and trunk upright Bilateral static squat: 6 sets of 30 s, with 45‐s recovery between sets Unilateral static squat: 4 sets of 30 s each leg | Does not state | All participants wore sport shoes for vibration exercises | Static unilateral and bilateral |
| Quality assessment | No. of participants | Quality | Importance | |||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Aerobic exercise (AE) intervention | AE control | ||
| Health‐related quality of life (HRQL), 0‐100, lower is best | ||||||||||
| 2 | Randomized trial | Very serious1 | Not serious | Not serious | Serious2 | 23 | 26 | ⊕⊝⊝⊝ | CRITICAL | |
| Pain intensity, 0‐100, lower is best | ||||||||||
| 1 | Randomized trial | Serious3 | Not serious | Not serious | Serious2 | One very small study | 11 | 12 | ⊕⊝⊝⊝ | CRITICAL |
| Fatigue, 0‐100, lower is best | ||||||||||
| 1 | Randomized trial | Serious1 | Not serious | Not serious | Serious2 | One very small study | 11 | 12 | ⊕⊝⊝⊝ | IMPORTANT |
| Stiffness, 0‐100, lower is best | ||||||||||
| 1 | Randomized trial | Serious1 | Not serious | Not serious | Serious2 | One very small study | 11 | 12 | ⊕⊝⊝⊝ | IMPORTANT |
| Physical function, not reported | ||||||||||
| Withdrawals | ||||||||||
| 3 | Randomized trial | Very serious4 | Not serious | Not serious | Serious2 | 3/39 (7.69%) | 4/38 (10.52%) | ⊕⊝⊝⊝ | IMPORTANT | |
| Adverse events:Alentorn‐Geli 2008: "This program neither exacerbated FM‐related symptoms nor resulted in musculoskeletal injuries; however, one patient exhibited a mild anxiety attack on the first session of WBV" (page 978); Sañudo 2010: Trial authors clarified that one person in the comparison group ("other exercise group") dropped out owing to an injury that was not an injury related to the program (participant fell down on the street); Sañudo 2013: "This study, however, demonstrated that WBV training is a safe (no adverse effects), suitable (no dropouts due to the intervention), and effective (increased lower limb muscle strength) way to exercise the musculoskeletal system, and potentially a feasible intervention for those patients who cannot participate in conventional strength training" (page 683) | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HRQL, 1‐100 scale, lower means better HRQL Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Balance, degrees of displacement, lower is best Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Withdrawal Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WBV + MX vs C: HRQL, 0‐100 scale, lower is best Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 WBV + MX vs C: pain, 0‐100 scale, lower is best Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 WBV + MX vs C: fatigue, 0‐100 scale, lower is best Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 WBV + MX vs C: stiffness, 0‐100 scale, lower is best Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 WBV + MX vs C: balance, overall stability index ‐ eyes closed (degrees of displacement 0 to 20 scale, lower is best) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Withdrawal Show forest plot | 2 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 WBV + MX vs Other: HRQL, 0‐100 scale, lower is best Show forest plot | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐6.67 [‐14.65, 1.31] |
| 2 WBV + MX vs Other: pain, 0‐100 scale, lower is best Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 WBV + MX vs Other: fatigue, 0‐100 scale, lower is best Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 WBV + MX vs Other: stiffness, 0‐100 scale, lower is best Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 WBV + MX vs Other: strength, measured in newtons and number of reps, higher values are best Show forest plot | 2 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [0.20, 1.35] |
| 6 WBV + MX vs Other: balance, overall stability index ‐ eyes closed (degrees of displacement 0 to 20 scale, lower is best) Show forest plot | 2 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐1.56, 1.11] |
| 7 Withdrawal Show forest plot | 3 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.17, 3.11] |

