Bloqueo nervioso o ningún bloqueo nervioso para el control del dolor después de la cirugía de reemplazo de cadera (artroplastia) electiva en adultos
Resumen
Antecedentes
Se calcula que se realizan más de 300 000 reemplazos de cadera en total cada año en los EE.UU. Para los países europeos, el número de procedimientos de reemplazo de cadera por 100 000 personas realizado en 2007 varió de menos de 50 a más de 250. Para facilitar la rehabilitación posoperatoria, el dolor debe ser tratado de forma adecuada. Se han propuesto los bloqueos nerviosos periféricos y los bloqueos neuraxiales para reemplazar o complementar la analgesia sistémica.
Objetivos
Se procuró comparar los efectos relativos (efectos beneficiosos y perjudiciales) de los diferentes bloqueos nerviosos que pueden usarse para aliviar el dolor después del reemplazo de cadera electivo en adultos.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, número 12, 2016), MEDLINE (Ovid SP) (1946 hasta diciembre, semana 49, 2016), Embase (Ovid SP) (1980 hasta diciembre, semana 49, 2016), CINAHL (EBSCO host) (1982 hasta 6 diciembre 2016), ISI Web of Science (1973 hasta 6 diciembre 2016), Scopus (desde su inicio hasta diciembre 2016), en registros de ensayos y en sitios web relevantes.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorios (ECA) realizados en adultos sometidos al reemplazo de cadera primario electivo y que comparaban los bloqueos nerviosos periféricos con cualquier otra modalidad de tratamiento del dolor. No se aplicaron restricciones de idioma ni de estado de publicación.
Obtención y análisis de los datos
Dos autores de la revisión extrajeron los datos de forma independiente. Se contactó con los autores de los estudios.
Resultados principales
Se incluyeron 51 ECA con 2793 participantes; de estos 45 ECA (2491 participantes: bloqueo nervioso periférico = 1288; comparadores = 1203) se incluyeron en los metanálisis. Hay 11 estudios en curso y tres en espera de clasificación.
En comparación con la analgesia sistémica sola, los bloqueos nerviosos periféricos redujeron: el dolor en reposo al ingresar a la unidad de atención posoperatoria (DME ‐1,12 IC del 95%: ‐1,67 a ‐0,56; nueve ensayos, 429 participantes; equivalente a 3,2 en una escala de 0 a 10; evidencia de calidad moderada); el riesgo de estado de confusión agudo: cociente de riesgos [CR] 0,10; IC del 95%: 0,02 a 0,54; un ensayo, 225 participantes; número necesario a tratar para obtener un beneficio adicional (NNTB) 12; IC del 95%: 11 a 22; evidencia de muy baja calidad); el prurito (CR 0,16; IC del 95%: 0,04 a 0,70; 2 ensayos, 259 participantes para los bloqueos nerviosos periféricos continuos; NNTB 4 (IC del 95%: 4 a 8); evidencia de muy baja calidad); la duración de la estancia hospitalaria (DME ‐0,75; IC del 95%: ‐1,02 a ‐0,48; evidencia de muy baja calidad; dos ensayos, 249 participantes; equivalente a 0,75 días). La satisfacción del participante aumentó (DME 0,67; IC del 95%: 0,45 a 0,89; evidencia de baja calidad; cinco ensayos, 363 participantes; equivalente a 2,4 en una escala de 0 a 10). No se encontró una diferencia para el número de participantes que caminaron durante el día uno posoperatorio (evidencia de muy baja calidad). Se informaron dos complicaciones relacionadas con el bloqueo nervioso: un hematoma local y una paresia persistente tardía.
En comparación con los bloqueos neuraxiales, los bloqueos nerviosos periféricos redujeron el riesgo de prurito (CR 0,33; IC del 95%: 0,19 a 0,58; seis ensayos, 299 participantes; evidencia de calidad moderada; NNTB 6 (IC del 95%: 5 a 9). No se encontró una diferencia para el dolor en reposo al ingresar a la unidad de atención posoperatoria (evidencia de calidad moderada); el número de complicaciones nerviosas relacionadas con el bloqueo (evidencia de baja calidad); el estado de confusión agudo (evidencia de muy baja calidad); la duración de la estancia hospitalaria (evidencia de baja calidad); el tiempo hasta la primera caminata (evidencia de baja calidad); ni en la satisfacción del participante (evidencia de alta calidad).
Se encontró que los bloqueos nerviosos periféricos proporcionan un mejor control del dolor en comparación con la analgesia sistémica sin diferencias principales entre los bloqueos nerviosos periféricos y los bloqueos neuraxiales. También se encontró que los bloqueos nerviosos periféricos pueden asociarse con una reducción del riesgo del estado de confusión agudo posoperatorio y una reducción moderada en la duración de la estancia hospitalaria, que podría ser significativa en cuanto a la reducción de los costos considerando el número cada vez mayor de procedimientos realizados anualmente.
Conclusiones de los autores
En comparación con la analgesia sistémica sola, hay evidencia de calidad moderada de que los bloqueos nerviosos periféricos alivian el dolor posoperatorio, hay evidencia de baja calidad de que la satisfacción del paciente aumenta, y evidencia de muy baja calidad de reducciones en el estado de confusión agudo, el prurito y la duración de la estancia hospitalaria.
Se encontró evidencia de calidad moderada de que los bloqueos nerviosos periféricos reducen el prurito en comparación con los bloqueos neuraxiales.
Los 11 estudios en curso (después de finalizados) y los tres estudios en espera de clasificación pueden alterar las conclusiones de la revisión una vez que se haya hecho la evaluación
PICOs
Resumen en términos sencillos
Bloqueos nerviosos periféricos en comparación con otros tipos de alivio del dolor para pacientes sometidos a la cirugía de reemplazo total de la articulación de la cadera
Antecedentes
El control del dolor después de la cirugía de reemplazo de cadera mejora la comodidad y la participación en la rehabilitación. Estos aspectos ayudan a los pacientes a regresar a su hogar antes y a limitar los costos de tratamiento.
El bloqueo nervioso periférico es un tratamiento para el control del dolor que incluye la inyección de un anestésico local alrededor de los nervios para bloquear o detener la sensación de dolor transmitida al cerebro. Un bloqueo neuraxial es una inyección de un anestésico local en la columna vertebral a través de una aguja o un catéter (tubo pequeño muy delgado) para bloquear la transmisión del dolor de la columna vertebral al cerebro.
Se evaluaron los efectos beneficiosos y perjudiciales de los bloqueos nerviosos en comparación con ningún bloqueo nervioso u otras formas de alivio del dolor luego del reemplazo de cadera en adultos.
Fechas de la búsqueda
Se buscó hasta diciembre 2016.
Características de los estudios
Se incluyeron 51 estudios (2793 participantes) en la revisión y se analizaron los resultados de 45 estudios (2491 participantes). Hay 11 estudios en curso y tres en espera de clasificación.
Fuentes de financiación de los estudios
Las fuentes de financiamiento incluyeron el gobierno, instituciones de beneficencia, instituciones, la industria (en parte, n = 1); más de la mitad no fue especificado (n = 29).
Resultados clave
En comparación con la analgesia sistémica, se encontró que los bloqueos nerviosos periféricos alivian el dolor, reducen el riesgo de confusión (p.ej. no saber la fecha, la hora, ni la ubicación) (por cada 12 pacientes tratados uno menos presentará confusión), reducen la picazón (por cada 4 pacientes tratados uno menos desarrollará comezón) y la duración de la estancia hospitalaria (equivalente a 0,75 días) y aumentan la satisfacción del paciente con el tratamiento del dolor (equivalente a 2,4 puntos más en una escala de 0 a 10). No se encontró una diferencia en el tiempo hasta la primera caminata después de la intervención quirúrgica.
Dos pacientes tuvieron complicaciones: un hematoma local y debilidad muscular persistente tardía.
Calidad de la evidencia
La calidad de la evidencia sobre los bloqueos nerviosos periféricos en comparación con los analgésicos sistémicos se consideró moderada a muy baja.
La calidad de la evidencia sobre los bloqueos nerviosos periféricos en comparación con los bloqueos neuraxiales se consideró alta para la satisfacción del paciente; moderada para la reducción de la picazón; similar para el alivio del dolor; y baja para las complicaciones similares relacionadas con el bloqueo, la duración de la estancia hospitalaria y el tiempo hasta la primera caminata. La evidencia sobre la confusión se evaluó como de muy baja calidad.
La calidad de la evidencia se disminuyó a baja o muy baja debido a las imperfecciones en el diseño del estudio y al número limitado de ensayos y participantes.
Authors' conclusions
Summary of findings
| Peripheral nerve blocks compared to systemic analgesia for elective primary total hip replacement | ||||||
| Patient or population: adults undergoing elective primary total hip replacement | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Peripheral nerve blocks | |||||
| Pain at rest on arrival in postoperative care unit | CD | The mean pain at rest on arrival in postoperative care unit in the intervention groups was | SMD ‐1.12 (‐1.67 to ‐0.56) | 429 | ⊕⊕⊕⊝ | Equivalent to 3.2 on a scale from 0 to 10 |
| Total number of nerve block‐related complications | NA | 1 local haematoma 1 delayed paresis | NA | NA | NA | Examples: erythema, damage to surrounding structures, allergic reactions, infections, transient and lasting neurological damage |
| Acute confusional state | Study population | RR 0.10 | 225 | ⊕⊝⊝⊝ | Number needed to treat for additional benefit: 12 (95% CI 11 to 22) | |
| 133 per 1000 | 13 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 5 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 15 per 1000 | |||||
| Pruritus | Study population | RR 0.16 | 259 | ⊕⊝⊝⊝ | Continuous peripheral nerve blocks only Number needed to treat for additional benefit: 4 (95% CI 4 to 8) | |
| 161 per 1000 | 26 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 8 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 40 per 1000 | |||||
| Hospital length of stay | CD | The mean hospital length of stay in the intervention groups was | SMD 0.75 (‐1.02 to ‐0.48) | 249 | ⊕⊝⊝⊝ | Continuous peripheral nerve blocks only Equivalent to 0.75 day |
| Walking at postoperative day 1 | Number of participants walking at postoperative day 1 | The risk difference was 0.01 (‐0.03 to 0.05) | RD 0.01 (‐0.03 to 0.05) | 278 | ⊕⊝⊝⊝ | One trial evaluated effect with single injection block and the other with continuous peripheral nerve block |
| Patient satisfaction | CD | The mean patient satisfaction in the intervention groups was | SMD 0.67 (0.45 to 0.89) | 363 | ⊕⊕⊝⊝ | Equivalent to 2.4 on a scale from 0 to 10 |
| The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious concerns about study limitations | ||||||
| Peripheral nerve block compared to neuraxial block for elective primary total hip replacement | ||||||
| Patient or population: adults undergoing elective primary total hip replacement | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Neuraxial block | Peripheral nerve block | |||||
| Pain at rest on arrival in postoperative care unit | Visual/verbal analogue scale from 0 to 10 | The mean pain at rest on arrival in postoperative care unit in the intervention groups was | SMD 0.39 (‐0.15 to 0.94) | 118 | ⊕⊕⊕⊝ | |
| Total number of block‐related complications | Study population | RD 0 | 334 | ⊕⊕⊝⊝ | ||
| 48 per 1000 | 48 per 1000 | |||||
| Low | ||||||
| 10 per 1000 | 10 per 1000 | |||||
| High | ||||||
| 100 per 1000 | 100 per 1000 | |||||
| Aute confusional state | Study population | RR 0.29 | 50 | ⊕⊝⊝⊝ | ||
| 43 per 1000 | 13 per 1000 | |||||
| Low | ||||||
| 25 per 1000 | 7 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 43 per 1000 | |||||
| Pruritus | Study population | RR 0.33 | 299 | ⊕⊕⊕⊝ | Number needed to treat for additional benefit: 6 (95% CI 5 to 9) | |
| 258 per 1000 | 88 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 17 per 1000 | |||||
| High | ||||||
| 300 per 1000 | 102 per 1000 | |||||
| Hospital length of stay | The mean hospital length of stay in the control groups was 12.6 days* | The mean hospital length of stay in the intervention groups was | MD 0.19 (‐0.39 to 0.77) | 64 | ⊕⊕⊝⊝ | |
| Time to first walk | The mean time to first walk in the control groups was 3.3 | The mean time to first walk in the intervention groups was | MD ‐0.41 (‐1.09 to 0.27) | 94 | ⊕⊕⊝⊝ | |
| Patient satisfaction | CD | The mean patient satisfaction in the intervention groups was | SMD 0.08 (‐0.32 to 0.48) | 307 | ⊕⊕⊕⊕ | |
| The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious concerns about study limitations | ||||||
Background
Description of the condition
Total hip replacement (arthroplasty) is one of the most successful orthopaedic operations performed for intractable hip pain due to primary and secondary osteoarthritis, osteonecrosis, and rheumatoid arthritis. It is estimated that over 300,000 total hip replacements are performed each year in the USA alone. For European countries, the number of hip replacement procedures performed in 2007 varied from fewer than 50 to over 250 per 100,000 people (WHO 2011). Controlling pain after a hip replacement improves patient comfort and satisfaction and enables patients to participate in rehabilitation more fully, leading to an earlier return home and reduced demand on resources. Current methods of pain control following hip replacement surgery include:
-
systemic opioids or other analgesics;
-
neuraxial blocks (epidural analgesia);
-
peri‐articular/intra‐articular analgesia; and
-
nerve blocks (psoas compartment block, femoral block, fascia iliaca compartment block, combined nerve blocks).
Pain after hip replacement has traditionally been managed using systemic pain medications including acetaminophen (paracetamol), non‐steroidal anti‐inflammatory drugs (NSAIDs) and opioid analgesics. The use of parenteral or oral opioids is associated with significant adverse effects including nausea, vomiting, constipation, drowsiness, and confusion; all may affect recovery and satisfaction and prolong hospital stays.
Central neuraxial blocks provide effective pain control, but there is a higher risk of neurologic complications (transient paraesthesia, motor blockade, seizures) (Auroy 1997; Türker 2003), cardiovascular complications necessitating haemodynamic monitoring (intra‐operative hypotension, postural hypotension, cardiac arrest) (Auroy 1997), pruritus (itch), nausea and vomiting (Horlocker 1998), and urinary retention with central neuraxial blocks compared to peripheral nerve blocks (Horlocker 1998; Fischer 2005). Epidural anaesthesia for non‐obstetric indications has sometimes been associated with a risk of epidural haematoma (Volk 2012). The risk factors predisposing to epidural haematoma, such as higher age, renal insufficiency, and use of anticoagulants, are very common in people undergoing hip replacement. Central neuraxial blocks in people who are anticoagulated increase the risk of epidural/spinal haematoma that may result in permanent neurological damage (Dahlgren 1995;Sternlo 1995). Elderly people undergoing hip replacements may have spinal stenosis, which may increase the risk of neurological complications with central neuraxial blocks (Hebl 2010). A prior Cochrane review studied epidural analgesia for pain relief following hip or knee replacement (Choi 2003).
Peri‐articular analgesia (injecting various combinations of local anaesthetics and analgesics into the hip joint or periarticular tissues) has gained popularity recently. A Cochrane Review protocol has been published on this topic (Hadi 2014).
Because peripheral nerve blocks confine anaesthesia to the surgical region, many disadvantages of neuraxial blocks can be avoided. Peripheral nerve damage can occur after peripheral nerve block, but consequences may be more limited than with central neuraxial blocks. Given the significant differences in safety profiles of peripheral nerve blocks and central neuraxial blocks, we focused specifically on peripheral nerve blocks.
Description of the intervention
Nerve blocks (injection of a local anaesthetic around a nerve) relieve pain by interrupting transmission of pain signals from the peripheral nerves. Nerve blocks for orthopaedic procedures have been shown to facilitate the execution of surgery in ambulatory surgery (day surgery), improve pain control and sleep after surgery, and decrease time to discharge home (Ilfeld 2006a; Ilfeld 2006b). Nerve blocks may also reduce the need for systemic pain medications limiting associated adverse effects.
How the intervention might work
The hip area is innervated by branches of the lumbar plexus. The hip joint is supplied with femoral and obturator nerves, nerve to quadratus femoris, superior gluteal and sciatic nerves. The dermatomal supply of the hip joint is typically from spinal nerve roots lumbar‐4 to as low as sacral‐2. The bony structures of the hip joint are supplied from spinal nerve roots lumbar‐3 to sacral‐1. It is difficult to achieve complete pain relief of the hip with peripheral nerve blocks (de Visme 2000), and some techniques (psoas compartment block) are considered to be expert‐level (practiced only by some anaesthesiologists; Hargett 2005). There are many types and techniques for blocking the lumbar plexus nerves following hip replacement.
-
Lumbar plexus, or psoas compartment block: peripheral regional anaesthetic technique to block the major nerves of the lumbar plexus (femoral, lateral femoral cutaneous and obturator nerves) in the psoas major muscle (Chayen 1976; Capdevila 2002; Karmakar 2015).
-
Femoral nerve block is a safe and widely practiced local anaesthetic technique used to supplement anaesthesia and provide postoperative analgesia after hip surgery (Szucs 2010; Winnie 1973). Local anaesthetic is infiltrated around the femoral nerve, which provides anaesthesia to the anterior thigh (femoral nerve) and the medial lower leg (through the saphenous nerve). However, the cephalad spread of the local anaesthetic may not be sufficient to block the obturator nerve (medial thigh) and the lateral cutaneous nerve of thigh (Marhofer 2000).
-
Fascia iliaca compartment block (FICB) is an anterior‐thigh regional anaesthetic block targeting the lumbar plexus (Dalens 1989; Murgatroyd 2013). This block was initially described by Dalens 1989 for children where sensory blockade of the obturator nerve was believed to be observed. It was believed the local anaesthetic spread underneath the fascia iliaca proximally towards the lumbosacral plexus (Dalens 1989). However, it has since been discovered that nearly half of patients do not have a skin component of the obturator nerve and that assessing adductor strength is the only effective way to measure obturator nerve function (Bouaziz 2002). Kaloul 2004 found that motor obturator nerve blockade is achieved in fewer than 50% of patients undergoing femoral nerve blocks. The effect of the FICB is similar to the femoral nerve block, but may provide a more reliable method of reaching the femoral lateral cutaneous nerve.
-
It is possible to individually block nerves supplying the hip, but this is time consuming.
Why it is important to do this review
Nerve blocks have been used successfully to reduce opioid requirements following other surgical interventions. A Cochrane Review has been published that investigated adding peripheral nerve blocks for hip fracture surgery (Guay 2017). Several RCTs have reported on the use of nerve blocks for pain control after hip replacement. A Cochrane Review focusing on functional improvement with regional analgesia at 3, 6, or 12 months after hip, knee, or shoulder replacement is available (Atchabahian 2015).
This review aimed to provide evidence to assist people undergoing hip replacement to decide which pain management protocol to choose and whether the risks associated with having nerve blocks exceed the benefits.
The rapidly rising volume of hip replacement surgeries being performed annually worldwide will considerably increase the burden on healthcare resources. A systematic review evaluating the current evidence of the short‐ and long‐term safety and efficacy of nerve blocks after hip replacement surgery was necessary.
Objectives
We aimed to compare the relative effects (benefits and harms) of the different nerve blocks that may be used to relieve pain after elective hip replacement in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included all parallel randomized controlled trials (RCTs) comparing different nerve blocks with other pain treatment modalities (control group). We excluded non‐RCTs and observational studies.
Types of participants
We included adults aged over 16 years undergoing hip replacement for the first time. We excluded adults undergoing revision hip replacement or hip replacement for acute fractures.
Types of interventions
We included all peripheral nerve blocks: psoas compartment block, femoral nerve block (or 3‐in‐1) block, fascia iliaca compartment block, obturator nerve block or femoral lateral cutaneous nerve block as the study intervention. For comparators we divided studies according to systemic analgesia, no block or sham block (comparison 1), neuraxial blocks (comparison 2), local anaesthetic infiltration (comparison 3) or intravenous lidocaine infusion (comparison 4).
Types of outcome measures
Primary outcomes
-
Participant‐reported pain at rest and with movement on visual analogue scale (VAS), numeric rating scale (NRS), or other similar scales or on ordinal or qualitative scales (FACES).
-
Total number of nerve block‐related complications (e.g. erythema, damage to surrounding structures, allergic reactions, infections, transient and lasting (more than three months) neurological damage).
Secondary outcomes
-
Analgesic requirements: we assessed amount of oral/parental supplemental analgesic needed.
-
Minimal clinically important improvement in pain: we evaluated if the improvement in pain scores (continuous variable) was clinically important (categorical variable). We considered a pain scale improvement of 2 cm with nerve block on a 0 cm to 10 VAS scale as clinically important. We assessed the proportion of participants with pain VAS scale differences of 2 cm on a 0 cm to 10 cm scale as categorical variable.
-
Complications specific to the method of treatment: We assessed for:
-
allergic reactions;
-
damage to surrounding structures at site of nerve block;
-
other complications as detailed in each study.
-
-
General medical complications within six weeks after surgery: we assessed for the following.
-
Gastrointestinal: nausea, vomiting, constipation, ileus.
-
Pulmonary: pneumonia, bronchitis.
-
Cardiovascular: hypotension, myocardial infarction, blood loss and blood transfusion.
-
Neurological: acute confusional state, drowsiness, cerebrovascular accident, postoperative cognitive dysfunction.
-
Thromboembolic complications: deep vein thrombosis or pulmonary embolism.
-
Other medical complications: pruritus, respiratory depression or other.
-
-
Use of resources. We assessed:
-
length of hospital stay;
-
costs of treatment;
-
re‐hospitalization due to pain;
-
re‐hospitalization due to any other reason, including re‐operation.
-
-
Quality of life, assessed with the 36‐Item Short Form Health Survey (Ware 1992), Sickness Impact Profile, or other quality‐of‐life scales
-
Short‐term rehabilitation milestones (within six weeks after surgery), such as
-
time to start rehabilitation, e.g. time to sit up in bed; and
-
time to achieve rehabilitation milestones, e.g. transfer unassisted in and out of bed and ability to walk unassisted with a walker on a level surface.
-
-
Patient satisfaction on VAS, NRS, or other similar scales, or on ordinal or qualitative scales (FACES)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 12, 2016; Appendix 1), MEDLINE (Ovid SP;1946 to December Week 49, 2016; Appendix 2), Embase (Ovid SP; 1980 to December week 49, 2016; Appendix 3), CINAHL (EBSCO host; 1982 to 6 December 2016; Appendix 4), ISI Web of Science (1973 to 6 December 2016; Appendix 5) and Scopus (from inception to 6 December 2016; Appendix 6).
We applied no language restriction.
Searching other resources
We searched the following resources in April 2016:
-
reference lists of included trials and recent relevant reviews (2012 to 2016);
-
trials registers: www.clinicaltrials.gov and Australian and New Zealand Clinical Trials Register;
-
Google Scholar;
-
Prospect, the Procedure Specific Postoperative Pain Management web site; and
-
conference abstracts of the American Society of Anesthesiologists (2004 to 2015), the American Society of Regional Anesthesia (Spring Meetings 2004 to 2016), the European Society of Anaesthesiology (2004 to 2015) and the European Society of Regional Anaesthesia (2004 to 2015).
Data collection and analysis
Selection of studies
Two review authors (JG and RLJ) independently screened the titles and abstracts of publications identified in the literature search for possible inclusion. We obtained the full published manuscripts of clinical trials that appeared to be eligible to assess their relevance based on the prespecified inclusion criteria. We documented reasons for study exclusion. We planned to, but did not need to involve a third review author (SK) to resolve any disagreements regarding study exclusion. Where there was insufficient published information to make a decision about inclusion, we contacted the study authors.
Data extraction and management
Two review authors (JG and SK) independently extracted study data. We resolved any discrepancies in the extracted data by discussion. We planned to, but did not need to involve a third review author (RLJ) to resolve any disagreements. All study authors were contacted to obtain additional information.
Assessment of risk of bias in included studies
Two review authors (JG and SK) assessed each trial independently, without masking of authors or source, for methodology quality (Higgins 2011). We resolved any discrepancies in extracted data by discussion. We planned to but did not need to involve a third review author (RLJ) to resolve any disagreements.
We assessed each trial for risk of bias based on following parameters: selection bias (random sequence generation and allocation concealment), performance and detection bias (blinding of participants and personnel (healthcare providers) and blinding of outcome assessors), attrition bias (incomplete outcome data) and reporting bias (selective reporting) (Appendix 7). We rated each of those parameters as low risk, high risk, or unclear risk. We considered the risk of bias as 'unclear’ if there was insufficient reporting to permit judgement of low or high risk and made no assumptions.
We also assessed whether studies used intention‐to‐treat analysis methods (Hollis 1999).
Measures of treatment effect
For dichotomous variables, we calculated risk ratios (RR) with 95% confidence intervals (CI) or risk difference (RD) (studies with 0 events on both sides). For continuous data, such as pain intensity, we used mean difference (MD) or standardized mean difference (SMD; different scales from one study to another or data extractable as P value only) with 95% CIs. Data extractable only as P value, number of participants and direction were entered using the calculator in RevMan (Review Manager 2014). Continuous data provided in formats other than mean and SD were extracted as P values, number of participants and direction. For results given as SMD, we calculated a clinical equivalence on a known scale by multiplying the SMD by a typical standard deviation (SD) of one of the studies included in the analysis.
We calculated the number needed to treat for additional benefit (NNTB), or the number needed to treat to harm (NNTH), where appropriate, based on the odds ratio.
Unit of analysis issues
Some trials compared multiple interventions or control groups of interest. Where each arm assessed a different intervention of interest, we analysed the interventions separately in the appropriate meta‐analysis. To address issues of double‐counting and unit‐of‐analysis error of the control group resulting from including several correlated comparisons into a meta‐analysis, we:
-
excluded subgroups not relevant to the review scope (Asano 2010; Nishio 2014; Saksena Shrivastava 2011);
-
split the control group into two groups (shared) with smaller size and included two reasonably independent comparisons (Anis 2011; Biboulet 2004; Celidonio 2008; Lončar 2016; Marino 2009; Nicholson 2002; Nishio 2014; Singelyn 2005; Utebey 2009); or
-
combined subgroups to present one comparison (for some of the analysis, split groups were recombined).
We chose to split a subgroup when we thought it would help to explain sources of heterogeneity without adding a risk of introducing small‐study effect.
Dealing with missing data
All study authors were contacted. Standard deviations were calculated from standard errors of means but not from quartiles or ranges. Medians were not considered equivalent to means. Data given as exact P values were extracted as such. We made no imputations.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of forest plots with consideration of the test for heterogeneity (Chi²) and the I² statistic (Higgins 2003).
We assessed clinical heterogeneity of included trials based on their clinical and methodological diversity ('Risk of bias' assessment). Our a priori hypothesis for sources of clinical heterogeneity were:
-
different types of nerve blocks used;
-
different regimens of analgesic agents across trials, i.e. different types of local analgesic drugs, concentrations, sites, and timing of injections (single injection versus continuous catheter technique) used to administer blocks; and
-
different levels of standard co‐analgesia across trials.
We presented primary analyses using fixed (I² < 25%) or random‐effects (I² ≥ 25%) models. We considered values of I² ≥ 25% to represent significant between‐study heterogeneity (Higgins 2003). If significant heterogeneity existed (I² ≥ 25%), we explored the data according to our predefined criteria for heterogeneity exploration with meta‐regression or subgrouping as appropriate. We also used sensitivity analysis based on risk bias or presence of an outlier. When possible (≥ 3 studies) we tested results for the possibility of a small‐study effect using Egger's regression intercept (Egger 1997).
Assessment of reporting biases
We planned to assess publication bias (≥ 3 studies) by constructing funnel plots and using Duval and Tweedie's trim and fill technique (Duval 2000; Duval 2000a).
Data synthesis
We conducted meta‐analysis using Review Manager 5.3 (Review Manager 2014) when there were sufficient data from two or more trials. We used fixed‐ (I² < 25%) or random‐effects models (Higgins 2003) and presented data as RR, RD, MD or SMD according to review criteria (Measures of treatment effect). When a result was considered positive using a fixed‐effect model, we also analysed results using random‐effects models to ensure that conclusions were not affected by the type of model used (fixed‐ versus random‐effects).
Where it was not possible to conduct meta‐analyses, we presented data narratively.
Subgroup analysis and investigation of heterogeneity
Our a priori criteria for exploring heterogeneity included:
-
type of local anaesthetic drug;
-
concentration of anaesthetic drug;
-
additional block (e.g. with or without sciatic/obturator block);
-
additional injections or infusions at other sites;
-
repeated injections at the same site;
-
use of adjunct oral analgesics; and
-
use of adjunct parenteral analgesics.
Following examination of forest plots for results presenting more than low level heterogeneity (I² ≥ 25%), we retained the following factors for heterogeneity exploration: participants' age, type of nerve block, type of local anaesthetic used, volume, concentration and dose of local anaesthetic (in lidocaine equivalent) used as loading dose, block administration duration (single injection versus continuous block for the intervention, the comparator or both) and comparator.
Sensitivity analysis
We performed sensitivity analyses based on the risk of bias assessment and presence of outliers.
Summary of findings table and GRADE
We applied the principles of the GRADE system (Guyatt 2008; Guyatt 2011a) to assess the quality of the body of evidence associated with specific outcomes in our review, and constructed 'Summary of findings’ tables (summary of findings Table for the main comparison; summary of findings Table 2) using GRADEpro GDT for:
-
patient‐reported pain at rest on arrival in the postoperative care unit;
-
total number of block‐related complications (comparison 2 only);
-
acute confusional state;
-
pruritus;
-
hospital length of stay;
-
walking on postoperative day one or first time to walk; and
-
patient satisfaction.
We judged the quality of evidence as high when most information was derived from studies at low risk of bias, and downgraded quality by one level when most information was obtained from studies at high or unclear risk of bias (allocation concealment and blinding of outcome assessors). We downgraded quality by two levels when the proportion of information obtained from studies at high risk of bias was sufficient to affect interpretation of results. In relation to inconsistency, we downgraded the quality of evidence by one level when the I² statistic was 50% or higher without satisfactory explanation, and by two levels when the I² statistic was 75% or higher without explanation.
We did not downgrade the quality of evidence for indirectness, because all outcomes were based on direct comparisons, were performed on the population of interest and were not surrogate markers (Guyatt 2011b). For imprecision (Guyatt 2011c), we downgraded the quality of evidence by one level when the CI around the effect size was large or overlapped, an absence of effect and failed to exclude an important benefit or harm, or when the number of participants was fewer than the optimal information size. We downgraded evidence quality by two levels when the CI was very wide and included both appreciable benefit and harm. We downgraded the quality of evidence by one level when correcting for the possibility of publication bias (assessed by Duval and Tweedie’s fill and trim analysis) changed the conclusion (Duval 2000; Duval 2000a).
Where quality of the body of evidence was assessed as high, further research is very unlikely to change our confidence in the estimate of effect. Where quality was assessed as moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Where quality was low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Where the quality was very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
Results of the search
We screened 2856 abstracts from CENTRAL (n = 594), MEDLINE (n = 464), Embase (n = 1030), CINAHL (n = 155), ISI Web of Science (n = 189), Scopus (n = 148), clinicaltrials.gov (n = 65), ANZCTR (n = 53), Google Scholar (n = 49) and from the Procedure Specific Postoperative Pain Management (Prospect) web site (n = 109).
We assessed a total of 2856 records and excluded 2795. We obtained and assessed 89 full‐text articles. We excluded a total of 23 trials that studied populations (n = 9) or interventions that were not relevant to this review (n = 3); were not randomized controlled trial (RCTs) (n = 11); and one was a cross‐over trial (see Characteristics of excluded studies). We identified 11 ongoing trials (Characteristics of ongoing studies).
A flow diagram of the search is presented (Figure 1).

Study flow diagram
Included studies
We included 51 studies with 2793 participants in the review (1448 participants randomized to undergo peripheral nerve blocks and 1345 to control groups). Of the 51 studies, we included 45 trials (2491 participants) in the analysis (1288 ‐ peripheral nerve blocks; 1203 ‐ controls). There was not enough information in the reports to allow us to extract data for six trials, even after trying to contact the study authors (Asano 2010; Fouad 2010; Kai 2010; Kendrisic 2013; Marshall 2008; Saksena Shrivastava 2011).
Setting
Trials were performed in Australia (Stevens 2007), Australia and UK (Marshall 2008), Austria and USA (Goytizolo 2016), Belgium (n = 4; Forget 2009; Singelyn 2005; Thomas 2009; Van Herreweghe 2015), Belgium, France and Switzerland (Bichel 1998), Belgium and USA (Shariat 2013), Bosnia and Hezegovnia (Lončar 2016), Brazil (Duarte 2009), Bulgaria and France (Bakalov 2016), China (n = 2; Chen 2015; Kai 2010), Denmark (n = 3; Jensen 2012; Thybo 2016; Uhrbrand 1992), Egypt (Anis 2011), Egypt and Japan (Asano 2010), France (n = 2; Biboulet 2004; Souron 2003), Germany (n = 4; Ginz 2000; Kratz 2015; Striebel 1993; Wiesmann 2014), India (Saksena Shrivastava 2011), Ireland (Green 2014), Italy (n = 3; Becchi 2008; Celidonio 2008; Frassanito 2008), Japan (n = 2; Fouad 2010; Nishio 2014), Lithuania (Gelmanas 2010), New Zealand (Fredrickson 2015), Romania (Cucereanu Badica 2010), Russia (Borisov 2012), Serbia (Kendrisic 2013), Spain (Nohel 2011), Switzerland (n = 2; Fournier 1998; Stevens 2000), Turkery (n = 4; Aksoy 2014; Köroğlu 2008; Türker 2003; Utebey 2009), UK (n = 4; Kearns 2011; Murray 2005; Nicholson 2002; Twyman 1990), UK and USA (Bhatia 2008) USA (n = 2; Marino 2009; Siddiqui 2007).
Funding
Sources of funding were: governmental (n = 2; Kai 2010; Kearns 2011), charity (n = 3; Fredrickson 2015; Goytizolo 2016; Siddiqui 2007), departmental (n = 16; Aksoy 2014; Bhatia 2008; Chen 2015; Cucereanu Badica 2010; Forget 2009; Ginz 2000; Green 2014; Kratz 2015; Marino 2009; Shariat 2013; Singelyn 2005; Souron 2003; Stevens 2000; Thybo 2016; Twyman 1990; Wiesmann 2014), industry (in part) (Striebel 1993), or unspecified (n = 29; Anis 2011; Asano 2010; Bakalov 2016; Becchi 2008; Biboulet 2004; Bichel 1998; Borisov 2012; Celidonio 2008; Duarte 2009; Fouad 2010; Fournier 1998; Frassanito 2008; Gelmanas 2010; Jensen 2012; Kendrisic 2013; Köroğlu 2008; Lončar 2016; Marshall 2008; Murray 2005; Nicholson 2002; Nishio 2014; Nohel 2011; Saksena Shrivastava 2011; Stevens 2007; Thomas 2009; Türker 2003; Uhrbrand 1992; Utebey 2009; Van Herreweghe 2015).
Trials registered
Eight included trials were registered: Aksoy 2014 (Australian New Zealand Clinical Trials Registry: ACTRN12614000658617); Fredrickson 2015 (Australian and New Zealand Clinical Trials Registry: ACTRN12609000316202); Kearns 2011 (ClinicalTrials.gov: NCT01217294); Kratz 2015 (German Clinical Trial Register (DRKS‐ID): DRKS00000752); Marino 2009 (ClinicalTrials.gov: NCT00790179); Shariat 2013 (ClinicalTrials.gov: NCT01758497); Thybo 2016 (EudraCT: 2013‐004501‐12 and ClinicaTtrials.gov: NCT02289937); and Wiesmann 2014 (German Clinical Trial Register DRKS‐ID: DRKS00000752).
Date of publication
Trials included in analyses were published between 1990 and 2016.
Characteristics of included participants
Partcipants in the included trials had mean (or median age) between 32.1 and 76.5 years. Participants' ASA physical status ranged from 1 to 4 or 1.2 to 3.2.
Types of interventions
Trials used peripheral nerve blocks for surgery without additional anaesthetic techniques other than sedation (Aksoy 2014); with the addition of a neuraxial block (n = 13; Becchi 2008; Borisov 2012; Cucereanu Badica 2010; Gelmanas 2010; Goytizolo 2016; Green 2014; Kai 2010; Kearns 2011; Marino 2009; Marshall 2008; Murray 2005; Stevens 2007; Thybo 2016); or with general anaesthesia (n = 28; Anis 2011; Bhatia 2008; Biboulet 2004; Bichel 1998; Chen 2015; Duarte 2009; Forget 2009; Fouad 2010; Fournier 1998; Frassanito 2008; Fredrickson 2015; Ginz 2000; Kendrisic 2013; Köroğlu 2008; Kratz 2015; Nicholson 2002; Nishio 2014; Shariat 2013; Siddiqui 2007; Singelyn 2005; Souron 2003; Stevens 2000; Striebel 1993; Türker 2003; Twyman 1990; Uhrbrand 1992; Utebey 2009; Wiesmann 2014). The anaesthetic technique used for surgery was unclear for seven trials (Asano 2010; Celidonio 2008; Jensen 2012; Nohel 2011; Saksena Shrivastava 2011; Thomas 2009; Van Herreweghe 2015).
Types of surgery
The types of surgeries performed were reported as: elective hip replacement or arthroplasty (Aksoy 2014; Cucereanu Badica 2010; Cucereanu Badica 2010; Frassanito 2008; Gelmanas 2010; Kendrisic 2013; Kratz 2015; Murray 2005; Souron 2003): partial hip replacement (Türker 2003); total hip arthroplasty or replacement (Asano 2010; Becchi 2008; Bhatia 2008; Biboulet 2004; Bichel 1998; Borisov 2012; Celidonio 2008; Chen 2015; Duarte 2009; Forget 2009; Fouad 2010; Fournier 1998; Fredrickson 2015; Ginz 2000; Goytizolo 2016; Green 2014; Jensen 2012; Kai 2010; Kearns 2011; Köroğlu 2008; Marino 2009; Marshall 2008; Nicholson 2002; Nishio 2014; Nohel 2011; Saksena Shrivastava 2011; Shariat 2013; Siddiqui 2007; Singelyn 2005; Stevens 2000; Stevens 2007; Striebel 1993; Thomas 2009; Thybo 2016; Twyman 1990; Uhrbrand 1992; Utebey 2009; Van Herreweghe 2015); or hip surgery (Anis 2011).
Comparators
Peripheral nerve blocks were compared to no block (Anis 2011; Biboulet 2004; Chen 2015; Cucereanu Badica 2010; Ginz 2000; Goytizolo 2016; Green 2014; Kratz 2015; Marino 2009; Murray 2005; Nicholson 2002; Saksena Shrivastava 2011 (this trial also contained a group who received epidural analgesia, but these data were not retained for analysis), Siddiqui 2007; Stevens 2000; Striebel 1993; Twyman 1990; Uhrbrand 1992; Van Herreweghe 2015; Wiesmann 2014); sham block (Fournier 1998; Jensen 2012; Köroğlu 2008; Shariat 2013; Stevens 2007; Thybo 2016), systemic analgesia (Becchi 2008; Kendrisic 2013; Nishio 2014 (this trial contained a group who received caudal analgesia, but these data were not retained in the analysis), Nohel 2011); systemic analgesia or epidural analgesia (Singelyn 2005; Utebey 2009); epidural analgesia (Asano 2010; Bichel 1998; Borisov 2012; Celidonio 2008; Duarte 2009; Forget 2009; Fouad 2010; Gelmanas 2010; Kai 2010; Marshall 2008; Türker 2003); spinal anaesthesia (Aksoy 2014); spinal analgesia (Bhatia 2008; Frassanito 2008; Fredrickson 2015; Kearns 2011; Souron 2003); or intravenous lidocaine (Thomas 2009).
Because trials where peripheral nerve blocks were compared to no block, sham block or systemic analgesia all reverted to systemic analgesia as the main modality for postoperative pain treatment (except Goytizolo 2016), these were treated as one comparison. Goytizolo 2016 studied the addition of single injection psoas compartment block compared to epidural analgesia.
Types of peripheral nerve blocks
Peripheral nerve blocks were psoas compartment block (single injection: Anis 2011; Frassanito 2008; Goytizolo 2016; Green 2014; Kai 2010; Souron 2003; Stevens 2000; Twyman 1990; repeated doses: Bhatia 2008; Utebey 2009; continuous block: Asano 2010 (this trial also contained a group who received continuous psoas compartment block plus local anaesthetic infiltration, but data were not retained in the analysis); Becchi 2008; Duarte 2009; Fouad 2010; Fredrickson 2015; Gelmanas 2010; Kendrisic 2013; Marshall 2008; Türker 2003), psoas compartment block plus sciatic nerve block plus iliac crest infiltration (single injection: Aksoy 2014), psoas compartment block or femoral nerve block (single injection: Biboulet 2004; continuous nerve block: Marino 2009), femoral nerve block (or 3‐in‐1 block) (single injection: Fournier 1998; Köroğlu 2008; Kratz 2015; Nicholson 2002; Wiesmann 2014; repeated doses: Striebel 1993; continuous block: Bichel 1998; Chen 2015; Nishio 2014; Nohel 2011; Saksena Shrivastava 2011; Singelyn 2005; Thomas 2009), femoral nerve block with sciatic nerve block for one group and without sciatic nerve block for another group (continuous block: Celidonio 2008), fascia iliaca block (single injection: Cucereanu Badica 2010; Forget 2009; Kearns 2011; Murray 2005; Shariat 2013; Stevens 2007; Van Herreweghe 2015; continuous block: Borisov 2012), femoral nerve block plus lateral femoral cutaneous nerve block (single injection: Uhrbrand 1992), femoral nerve block plus obturator nerve block plus lateral femoral cutaneous nerve block (single injection: Jensen 2012), obturator nerve block (single injection: Ginz 2000) or lateral femoral cutaneous nerve block (single injection: Thybo 2016). For fascia iliaca blocks, two studies punctured the skin above the inguinal ligament (Stevens 2007; Van Herreweghe 2015).
Block technique
Psoas compartment, femoral or 3‐in‐1 nerve blocks were performed using a nerve stimulator in 23 studies (Anis 2011; Becchi 2008; Bhatia 2008; Biboulet 2004; Bichel 1998; Duarte 2009; Fournier 1998; Frassanito 2008; Fredrickson 2015; Ginz 2000; Goytizolo 2016; Köroğlu 2008; Kratz 2015; Marino 2009; Nicholson 2002; Siddiqui 2007; Singelyn 2005; Souron 2003; Stevens 2000; Striebel 1993; Türker 2003; Uhrbrand 1992; Wiesmann 2014). Three studies reported using ultrasound guidance alone (Kearns 2011; Shariat 2013; Thybo 2016) and two studies used ultrasound guidance plus nerve stimulator (Aksoy 2014; Nishio 2014). One study used fluoroscopic guidance plus nerve stimulator (Utebey 2009). Landmarks were used in four studies (Borisov 2012; Forget 2009; Stevens 2007; Twyman 1990). One study used injection under direct vision during surgery (Green 2014). Techniques were unspecified in 10 studies (Celidonio 2008; Chen 2015; Cucereanu Badica 2010; Gelmanas 2010; Jensen 2012; Murray 2005; Nohel 2011; Thomas 2009; Van Herreweghe 2015; Türker 2003).
Timing of injection
Local anaesthetics in peripheral nerve blocks were injected before surgical incision in 30 studies (Aksoy 2014; Bhatia 2008; Bichel 1998; Cucereanu Badica 2010; Duarte 2009; Forget 2009; Fouad 2010; Fournier 1998; Frassanito 2008; Fredrickson 2015; Ginz 2000; Goytizolo 2016; Kai 2010; Kearns 2011; Kendrisic 2013; Köroğlu 2008; Kratz 2015; Murray 2005; Nicholson 2002; Siddiqui 2007; Singelyn 2005; Souron 2003; Stevens 2000; Striebel 1993; Thomas 2009; Türker 2003; Twyman 1990; Uhrbrand 1992; Utebey 2009; Wiesmann 2014). Local anaesthetic was injected after surgical incision in nine studies (Anis 2011; Becchi 2008; Biboulet 2004; Borisov 2012; Chen 2015; Green 2014; Marino 2009; Shariat 2013; Thybo 2016). Timing of block performance was unclear in 12 studies (Asano 2010; Bakalov 2016; Celidonio 2008; Gelmanas 2010; Jensen 2012; Lončar 2016; Marshall 2008; Nishio 2014; Nohel 2011; Saksena Shrivastava 2011; Stevens 2007; Van Herreweghe 2015).
Local anaesthetics in peripheral nerve blocks
Local anaesthetics administered as single injection blocks (psoas, femoral or 3‐in‐1) included bupivacaine (12 studies: Aksoy 2014; Anis 2011; Biboulet 2004; Fournier 1998; Ginz 2000; Goytizolo 2016; Green 2014; Köroğlu 2008; Kratz 2015; Stevens 2000; Twyman 1990; Wiesmann 2014), levobupivacaine (3 studies: Forget 2009; Kearns 2011; Murray 2005), ropivacaine (7 studies: Cucereanu Badica 2010; Frassanito 2008; Jensen 2012; Shariat 2013; Souron 2003; Thybo 2016; Van Herreweghe 2015) or a mixture of lidocaine and bupivacaine (2 studies: Nicholson 2002; Uhrbrand 1992). Volumes varied from 8 mL to 40 mL.
If potency equivalences were assumed as: lidocaine = 1, mepivacaine = 0.8, ropivacaine = 3, levobupivacaine = 3.9 and bupivacaine = 4 (Berde 2009), doses in lidocaine equivalents varied from 75 mg to 800 mg and concentrations from 8 mg/mL to 22.5 mg/mL.
Local anaesthetics used as infusions in continuous blocks included bupivacaine 5 studies: (Bichel 1998; Gelmanas 2010; Siddiqui 2007; Singelyn 2005; Türker 2003), lidocaine (Thomas 2009), ropivacaine (6 studies: Becchi 2008; Borisov 2012; Duarte 2009; Fredrickson 2015; Marino 2009; Nishio 2014) or were unspecified (Chen 2015; Nohel 2011). Concentrations in lidocaine equivalents varied from 4.5 to 6 mg/mL. Doses varied form 14 to 66 mg/hour (lidocaine equivalents) and volumes from 3 to 11 mL/hour. Infusions were maintained for 24 hours (Thomas 2009), 36 hours (Siddiqui 2007), 48 hours (7 studies: Becchi 2008; Bichel 1998; Borisov 2012; Celidonio 2008; Duarte 2009; Gelmanas 2010; Singelyn 2005), 72 hours (Chen 2015) or an unspecified duration (Nohel 2011). Local anaesthetics were administered as patient‐controlled analgesia in three studies (Chen 2015; Duarte 2009; Fredrickson 2015). Twelve studies reported that local anaesthesia was administered by continuous infusion (Becchi 2008; Bichel 1998; Borisov 2012; Celidonio 2008; Gelmanas 2010; Marino 2009; Nishio 2014; Nohel 2011; Siddiqui 2007; Singelyn 2005; Thomas 2009; Türker 2003).
Local anaesthetics injected for repeated doses included bupivacaine (Striebel 1993; Utebey 2009) and lidocaine (Bhatia 2008). Trials re‐injected only one dose varying from 4.9 to 20 mg/mL in lidocaine equivalent concentrations with volumes between 13 mL and 30 mL for doses between 130 mg and 600 mg (in lidocaine equivalents).
Adjuvants in peripheral nerve blocks
For single injection blocks, adjuvant drugs added to local anaesthetics included epinephrine (4 studies: Aksoy 2014; Fournier 1998; Stevens 2000; Uhrbrand 1992), and clonidine (6 studies: Anis 2011; Biboulet 2004; Bichel 1998; Celidonio 2008; Kratz 2015; Wiesmann 2014). For one trial (Anis 2011), clonidine was added for one group while no adjuvant was added for another group. Epinephrine plus clonidine was administered in two studies (Forget 2009; Stevens 2007). No adjuvants were added to the local anaesthetic blocks in 16 studies (Anis 2011; Cucereanu Badica 2010; Goytizolo 2016; Green 2014; Jensen 2012; Frassanito 2008; Ginz 2000; Kearns 2011; Köroğlu 2008; Murray 2005; Nicholson 2002; Shariat 2013; Souron 2003; Thybo 2016; Twyman 1990; Van Herreweghe 2015).
For continuous blocks, fentanyl was added in two studies (Gelmanas 2010; Türker 2003), and no adjuvants were added in 11 studies (Becchi 2008; Borisov 2012; Chen 2015; Duarte 2009; Fredrickson 2015; Marino 2009; Nishio 2014; Nohel 2011; Siddiqui 2007; Singelyn 2005; Thomas 2009).
There was no adjuvant added in trials where a repeated dose was administered (Bhatia 2008; Striebel 1993; Utebey 2009).
Narrative summary of included studies
Aksoy 2014 included 80 participants (ASA 3 or 4) undergoing elective hip replacement who were randomized to receive either a combination of psoas compartment block, sciatic nerve block and an iliac crest infiltration, or continuous spinal block. The trial was conducted in Turkey and authors declared no conflicts of interest. We assumed the study was funded by departmental resources.
Anis 2011 included 60 participants (ASA 1 or 2) aged from 18 to 60 years undergoing hip surgery who were randomized to receive either single injection lumbar plexus block with clonidine, single injection lumbar plexus block without clonidine, or no block. The trial was conducted in Cairo, Egypt. The source of funding was unspecified.
Asano 2010 included 45 participants undergoing total hip replacement who were randomized to receive either continuous lumbar plexus block, continuous lumbar plexus block plus local anaesthetic infiltration (this group was not retained for analysis) or intravenous patient‐controlled analgesia. The trial was conducted in Japan and in Egypt. The source of funding was unspecified.
Bakalov 2016 included 40 participants undergoing total hip replacement who were randomized to receive either quadratus lumborum block or no block. The trial was conducted in Bulgaria and France. The source of funding was unspecified.
Becchi 2008 included 73 participants (ASA 1 to 3) undergoing total hip arthroplasty who were randomized to receive either continuous psoas compartment block or continuous morphine infusion. The trial was conducted in Italy. The source of funding was unspecified.
Bhatia 2008 included 87 participants (ASA 1 or 2) aged from 18 to 85 years who were randomized to receive either double dose psoas compartment block or intrathecal diamorphine. The trial was conducted in the UK and in USA and funded by departmental resources.
Biboulet 2004 included 45 participants undergoing elective total hip arthroplasty who were randomized to receive either single injection femoral nerve block, single injection psoas compartment block, or no block. The trial was conducted in France. The source of funding was unspecified.
Bichel 1998 included 24 participants undergoing total hip arthroplasty who were randomized to receive either continuous femoral nerve block or epidural analgesia. The trial was conducted in Belgium, France and Switzerland. The source of funding was unspecified.
Borisov 2012 included 60 participants undergoing total hip arthroplasty who were randomized to receive either continuous fascia iliaca block or continuous epidural block. The trial was conducted in Russia. The source of funding was unspecified.
Celidonio 2008 included 46 participants (ASA 1 to 3) undergoing total hip arthroplasty who were randomized to receive either continuous femoral nerve block, continuous femoral nerve block plus single injection sciatic nerve block, or continuous epidural. The trial was conducted in Italy. The source of funding was unspecified.
Chen 2015 included 102 participants scheduled for total hip replacement who were randomized to receive either continuous femoral nerve block plus low molecular weight heparin injections and intermittent pneumatic pressure or no block plus thromboprophylactic measures according to the attending physician's preference. The trial was conducted in China and was funded by departmental resources.
Cucereanu Badica 2010 included 62 participants undergoing unilateral hip arthroplasty who were alternately allocated (quasi‐randomized trial) to receive either fascia iliaca block or no block. The trial was conducted in Romania and was funded by departmental resources.
Duarte 2009 included 42 adult participants (ASA 1 to 3) scheduled for hip replacement who were randomized to receive either patient‐controlled continuous posterior lumbar plexus block or patient‐controlled continuous epidural block. The trial was conducted in Brazil. The source of funding was unspecified.
Forget 2009 included 20 participants aged from 20 to 80 years undergoing primary elective total hip arthroplasty who were randomized to receive either fascia iliaca block or epidural analgesia. The trial was conducted in Belgium and was funded by departmental resources.
Fouad 2010 included 32 participants undergoing elective total hip arthroplasty who were randomized to receive either continuous psoas compartment block or continuous epidural block. The trial was conducted in Japan. The source of funding was unspecified.
Fournier 1998 included 40 participants (ASA 1 to 3) scheduled for total hip arthroplasty who were randomized to receive either 3‐in‐1 femoral nerve block with local anaesthetics and an adjuvant or sham block. The trial was conducted in Switzerland. The source of funding was unspecified.
Frassanito 2008 included 40 participants (ASA 1 to 3) scheduled for primary unilateral hip arthroplasty who were randomized to receive either psoas compartment block or intrathecal morphine. The trial was conducted in Italy. The source of funding was unspecified.
Fredrickson 2015 included 50 participants (ASA 1 to 3) undergoing total hip joint replacement who were randomized to receive either patient‐controlled continuous psoas compartment block or intrathecal morphine. The trial was conducted in New Zealand and funded by a charity.
Gelmanas 2010 included 34 participants (ASA 1 or 2) undergoing primary hip replacement surgery who were randomized to receive either continuous psoas compartment block or continuous epidural analgesia. The trial was conducted in Lithuania. The source of funding was unspecified.
Ginz 2000 included 40 participants (ASA 1 to 3) undergoing total hip replacement for arthrosis or fracture correction (these participants were excluded from the analysis) who were randomized to receive either obturator nerve block or no block. The trial was conducted in Germany and funded by departmental resources.
Goytizolo 2016 included 92 participants (ASA 1 to 3) aged from 60 to 100 years who were randomized to receive either single injection lumbar plexus block or no block. The trial was conducted in Austria and in the USA and funded by charity.
Green 2014 included 53 participants undergoing primary total hip replacement who were randomized to receive either psoas compartment block or no block. The trial was conducted in Ireland and funded by departmental resources.
Jensen 2012 included 28 participants undergoing total hip arthroplasty who were randomized to receive either a combination of femoral, obturator and lateral cutaneous nerve blocks or placebo blocks. The trial was conducted in Denmark. The source of funding was unspecified.
Kai 2010 included 46 participants (ASA 2 or 3) aged from 67 to 86 years scheduled for total hip replacement who were randomized to receive either psoas compartment block or epidural analgesia. The trial was conducted in China and supported by a grant from the National Neutral Science Foundation of China.
Kearns 2011 included 108 participants (ASA 1 to 3) aged between 18 and 85 years who were randomized to receive either fascia iliaca block or intrathecal morphine and a sham block. The trial was conducted in the UK and funded by governmental and charity sources.
Kendrisic 2013 included 62 participants (ASA 2 or 3) undergoing hip replacement surgery who were randomized to receive either continuous psoas compartment block or intravenous patient‐controlled analgesia. The trial was conducted in Serbia. The source of funding was unspecified.
Köroğlu 2008 included 30 participants (ASA 1 or 2) undergoing elective total hip arthroplasty who were randomized to receive either 3‐in‐1 femoral nerve block with local anaesthetic or a sham block. The trial was conducted in Turkey. The source of funding was unspecified.
Kratz 2015 included 80 participants undergoing hip arthroplasty who were randomized to receive either single injection femoral nerve block or no block. The trial was conducted in Germany and funded using departmental resources.
Lončar 2016 included 30 participants scheduled for elective hip arthroplasty who were randomized to receive single injection femoral nerve block or fascia iliaca block or intravenous morphine. The trial was conducted Bosnia and Herzegovina. The source of funding was unspecified.
Marino 2009 included 225 participants (ASA 1 to 3) aged from 18 to 80 years who were randomized to receive continuous psoas compartment block, continuous femoral nerve block or no block. The trial was conducted in the USA and funded by department resources.
Marshall 2008 included 52 participants scheduled for primary unilateral hip arthroplasty who were randomized to receive either continuous psoas compartment block plus intravenous patient‐controlled analgesia or epidural analgesia. The trial was conducted in Australia and in the UK. The source of funding was unspecified.
Murray 2005 included 50 participants (ASA 1 to 3) undergoing primary hip arthroplasty who were randomized to receive either single injection fascia iliaca block or no block. The trial was conducted in the UK. The source of funding was unspecified.
Nicholson 2002 included 36 female participants aged over 55 years who were randomized to receive either single injection 3‐in‐1 femoral nerve block or no blocks (2 subgroups depending on the agent used for induction of general anaesthesia). The trial was conducted in the UK. The source of funding was unspecified.
Nishio 2014 included 40 participants undergoing primary unilateral hip arthroplasty who were randomized to receive either continuous femoral nerve block or systemic analgesia (2 subgroups: 1 opioid‐based, 1 non opioid‐based). The trial also included another group who received continuous caudal analgesia (not retained in the analysis). This study was conducted in Japan. The source of funding was unspecified.
Nohel 2011 included 60 participants scheduled for total hip arthroplasty who were randomized to receive either patient‐controlled continuous femoral nerve block or intravenous patient‐controlled analgesia. The trial was conducted in Spain. The source of funding was unspecified.
Saksena Shrivastava 2011 included 75 participants scheduled for total hip replacement who were randomized to receive either continuous femoral nerve block or no block. The trial was conducted in India. The source of funding was unspecified.
Shariat 2013 included 32 participants (ASA 1 to 3) aged from 18 to 75 years who had undergone hip arthroplasty presenting with pain scores of 3 or more (scale from 0 to 10) despite intravenous patient‐controlled analgesia for one hour in the post anaesthesia care unit. The trial was conducted in Belgium and in the USA and funded from departmental resources.
Siddiqui 2007 included 34 participants (ASA 1 to 3) aged from 18 to 80 years undergoing elective unilateral hip arthroplasty who were randomized to receive either continuous psoas compartment block or no block. The trial was conducted in the USA and funded by charity.
Singelyn 2005 included 45 participants (ASA 1 to 3) aged from 18 to 80 years undergoing elective unilateral total hip replacement who were randomized to receive continuous femoral nerve block, intravenous patient‐controlled analgesia or epidural analgesia. The trial was conducted in Belgium and funded from departmental resources.
Souron 2003 included 56 participants (ASA 1 or 2) scheduled for primary hip arthroplasty who were randomized to receive either single injection psoas compartment block or intrathecal morphine. The trial was conducted in France and funded from departmental resources.
Stevens 2000 included 60 participants undergoing elective total hip arthroplasty who were randomized to receive either single injection psoas compartment block or no block. The trial was conducted in Switzerland and funded from departmental resources.
Stevens 2007 included 50 participants (ASA 1 to 3) undergoing unilateral total hip replacement who were randomized to receive either single injection fascia iliaca block with local anaesthetics or a sham block. The trial was conducted in Australia. The source of funding was unspecified.
Striebel 1993 included 40 participants undergoing total hip replacement who were randomized to receive either double dose 3‐in‐1 femoral nerve block or no block. The trial was conducted in Germany and received partial industry support.
Thomas 2009 included 20 participants undergoing total hip arthroplasty who were randomized to receive either continuous femoral nerve block and intravenous saline or intravenous lidocaine and saline infusion on the femoral nerve. The trial was conducted in Belgium. The source of funding was unspecified.
Thybo 2016 included 120 participants undergoing primary total hip arthroplasty who were randomized to receive either single injection lateral femoral cutaneous nerve block with local anaesthetic or saline. The trial was conducted in Denmark. The source of funding was unspecified.
Türker 2003 included 30 participants (ASA 1 to 3) undergoing unilateral partial hip replacement who were randomized to receive either continuous psoas compartment block or continuous epidural analgesia. The trial was conducted in Turkey. The source of funding was unspecified.
Twyman 1990 included 20 women undergoing cemented primary total hip replacement who were randomized to receive either single injection psoas compartment block or no block. The trial was conducted in the UK and funded from departmental resources.
Uhrbrand 1992 included 182 participants undergoing total hip arthroplasty who were randomized to receive either single injection 3‐in‐1 femoral nerve block plus single injection lateral femoral cutaneous nerve block or no blocks. The trial was conducted in Denmark. The source of funding was unspecified.
Utebey 2009 included 45 participants (ASA 1 or 2) undergoing total hip arthroplasty who were randomized to receive double dose lumbar plexus block, double dose epidural analgesia or no block. The trial was conducted in Turkey. The source of funding was unspecified.
Van Herreweghe 2015 included 78 participants undergoing total hip arthroplasty who were randomized to receive either single injection fascia iliaca block or no block. The trial was conducted in Belgium; The source of funding was unspecified.
Wiesmann 2014 included 80 participants (ASA 1 or 2) aged from 50 to 70 years undergoing elective total hip arthroplasty who were randomized to receive either single injection femoral nerve block or no block. The trial was conducted in Germany and funded from departmental resources.
Excluded studies
We excluded 23 trials. Eight trials (Adali 2011; Bang 2016; Bogoch 2002; Eyi 2014; Ghabach 2016; Nooh 2016; Segado Jiménez 2010; Sun 2014) investigated populations that were not relevant to this review. Three trials studied interventions that did not match review inclusion criteria (Berge 2004; Dahn 1999; Dahn 2003). Eleven studies were not randomized (Akhtar 2014; Dahl 2012; de Leeuw 2011; Finn 2016; Goitia Arrola 2009; Pandin 1998; Pavy 2007; Perrier 2010; Rowley 2013; Tanzer 2012; Vilchis 2012) and one was a cross‐over trial (Thybo 2016a). (See Characteristics of excluded studies).
Awaiting classification
We found three trials (first identified as ongoing from trial registers), that have been published after the date of the last search (December 2016) (NCT02242201; NCT02344264; NCT02568995) (Characteristics of studies awaiting classification). These trials will be formally evaluated for possible inclusion at the next update.
Ongoing studies
We identified 11 other trials from searching trials registers (NCT01378949; NCT01782612; NCT01875289; NCT01911949; NCT02056145; NCT02108847; NCT02299271; NCT02544269; NCT02658149; NCT02658240; NCT02720471; Characteristics of ongoing studies).
Four trials appear to be unlikely to complete for the following reasons: recruitment status unknown, completion date passed and status not verified in more than two years (NCT01378949; NCT01782612; NCT02056145), or suspended for lack of funding and difficulty to recruit: NCT01378949. These trials have nevertheless been left as ongoing trials to enable verification of their status at the next update.
Two trials are listed as terminated but both mentioned that they recruited some participants (NCT02299271; NCT02544269). Emails have been sent to the study authors to try to obtain preliminary results for possible inclusion at the next update.
Three studies have been completed but we could not find the reports (NCT01875289; NCT02108847; NCT02720471). We wrote emails to the study authors to obtain more information for possible inclusion at the next update.
Two trials are still recruiting participants (NCT02658149; NCT02658240) (Status checked October 2017).
Risk of bias in included studies
All studies had some degree of risk of bias Figure 2; Figure 3. Random sequence generation, allocation concealment and blinding of outcome assessors were the domains where risks of bias were the most frequent.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation concealment was rated as at low risk of bias for more than 25% (14/51) of the included studies (Aksoy 2014; Anis 2011; Borisov 2012; Duarte 2009; Fredrickson 2015; Goytizolo 2016; Kearns 2011; Kratz 2015; Nishio 2014; Shariat 2013; Siddiqui 2007; Souron 2003; Thybo 2016; Wiesmann 2014). For 38 studies (Asano 2010; Bakalov 2016; Becchi 2008; Bhatia 2008; Biboulet 2004; Bichel 1998; Celidonio 2008; Chen 2015; Forget 2009; Fouad 2010; Fournier 1998; Frassanito 2008; Gelmanas 2010; Ginz 2000; Green 2014; Jensen 2012; Kai 2010; Kendrisic 2013; Köroğlu 2008; Lončar 2016; Marino 2009; Marshall 2008; Murray 2005; Nicholson 2002; Nohel 2011; Saksena Shrivastava 2011; Singelyn 2005; Stevens 2000; Stevens 2007; Striebel 1993; Thomas 2009; Türker 2003; Twyman 1990; Uhrbrand 1992; Utebey 2009; Van Herreweghe 2015), there was not enough information in the report and allocation concealment was rated as unclear. Cucereanu Badica 2010 was rated as at high risk because studies authors used alternate allocation, therefore allocation of the next participant could be anticipated by the researcher recruiting participants.
Blinding
Blinding of participants and personnel taking care of participants were rated as at low risk of bias for 25% (13/51) of included studies (Celidonio 2008; Cucereanu Badica 2010; Fournier 1998; Gelmanas 2010; Jensen 2012; Köroğlu 2008; Shariat 2013; Stevens 2000; Stevens 2007; Thomas 2009; Thybo 2016; Van Herreweghe 2015). For 19 studies (Anis 2011; Asano 2010; Bakalov 2016; Bhatia 2008; Biboulet 2004; Duarte 2009; Forget 2009; Fouad 2010; Frassanito 2008; Goytizolo 2016; Green 2014; Kai 2010; Kearns 2011; Lončar 2016; Nicholson 2002; Souron 2003; Türker 2003; Twyman 1990; Uhrbrand 1992), there was not enough information in the report and blinding of participants and personnel taking care of participants was rated as unclear. Blinding of participants or personnel taking care of participants had to be rated as at high risk of bias for 20 studies (Aksoy 2014; Becchi 2008; Bichel 1998; Borisov 2012; Chen 2015; Fredrickson 2015; Ginz 2000; Kendrisic 2013; Kratz 2015; Marino 2009; Marshall 2008; Murray 2005; Nishio 2014; Nohel 2011; Saksena Shrivastava 2011; Siddiqui 2007; Singelyn 2005; Striebel 1993; Utebey 2009; Wiesmann 2014).
Blinding of outcome assessors was rated as at low risk of bias for more than 50% (26/51) of the included studies (Aksoy 2014; Becchi 2008; Bhatia 2008; Biboulet 2004; Celidonio 2008; Cucereanu Badica 2010Duarte 2009; Forget 2009; Fournier 1998; Frassanito 2008; Gelmanas 2010; Goytizolo 2016; Green 2014; Jensen 2012; Kearns 2011; Köroğlu 2008; Kratz 2015; Shariat 2013; Souron 2003; Stevens 2000; Stevens 2007; Thomas 2009; Thybo 2016; Türker 2003; Uhrbrand 1992; Van Herreweghe 2015). For 22 studies (Anis 2011; Asano 2010; Bakalov 2016; Bichel 1998; Borisov 2012; Chen 2015; Fouad 2010; Kai 2010; Kendrisic 2013; Lončar 2016; Marino 2009; Marshall 2008; Murray 2005; Nicholson 2002; Nishio 2014; Nohel 2011; Saksena Shrivastava 2011; Singelyn 2005; Striebel 1993; Twyman 1990; Utebey 2009; Wiesmann 2014), there was not enough information to rate blinding of outcome assessors. Blinding of outcome assessor was rated as at high risk for three studies (Fredrickson 2015; Ginz 2000; Siddiqui 2007).
Incomplete outcome data
This domain was judged as at low risk of bias for more than 75% (44/51) of the included studies (Aksoy 2014; Anis 2011; Bakalov 2016; Becchi 2008; Bhatia 2008; Biboulet 2004; Bichel 1998; Borisov 2012; Celidonio 2008; Chen 2015; Cucereanu Badica 2010; Duarte 2009; Forget 2009; Fouad 2010; Fournier 1998; Frassanito 2008; Fredrickson 2015; Gelmanas 2010; Ginz 2000; Goytizolo 2016; Green 2014; Kai 2010; Kearns 2011; Kendrisic 2013; Köroğlu 2008; Lončar 2016; Marino 2009; Marshall 2008; Murray 2005; Nicholson 2002; Nishio 2014; Shariat 2013; Siddiqui 2007; Singelyn 2005; Souron 2003; Stevens 2000; Striebel 1993; Thomas 2009; Türker 2003; Twyman 1990; Uhrbrand 1992; Utebey 2009; Van Herreweghe 2015; Wiesmann 2014). Incomplete outcome data was rated as unclear for six studies (Asano 2010 (conference abstract and no data suitable for analysis); Jensen 2012 (conference abstract, preliminary report on 28 participants out of 81 scheduled); Nohel 2011 (60 participants enrolled, exact number of participants for whom results are reported not mentioned); Saksena Shrivastava 2011 (conference abstract, no data suitable for analysis, 75 participants enrolled but number for whom results are reported not mentioned); Stevens 2007 (50 participants enrolled, 6 participants excluded from analysis because of study exclusion criteria or breaches in the study protocol or inadequate data collection); Thybo 2016 (20 participants out of the 120 enrolled did not complete the study because inadequate selection or protocol breach or failed spinal anaesthesia)). One trial (Kratz 2015) was rated as at high risk for this domain because 28 of the 80 participants enrolled (> 20%) were excluded from analysis due to violation protocol.
Selective reporting
This domain was judged as at low risk of bias for more than 75% (39/51) of the included studies (Aksoy 2014; Anis 2011; Becchi 2008; Bhatia 2008; Biboulet 2004; Bichel 1998; Borisov 2012; Celidonio 2008; Chen 2015; Cucereanu Badica 2010; Forget 2009; Fournier 1998; Frassanito 2008; Fredrickson 2015; Ginz 2000; Goytizolo 2016; Green 2014; Kai 2010; Kearns 2011; Köroğlu 2008; Kratz 2015; Lončar 2016; Marino 2009; Murray 2005; Nicholson 2002; Nishio 2014; Shariat 2013; Siddiqui 2007; Singelyn 2005; Souron 2003; Stevens 2000; Stevens 2007; Striebel 1993; Thomas 2009; Türker 2003; Twyman 1990; Uhrbrand 1992; Utebey 2009; Wiesmann 2014). For eleven studies (Asano 2010 (conference abstract with no results reported); Bakalov 2016 (conference abstract in which measurements made are not reported in th method section); Fouad 2010 (conference abstract, no number given); Gelmanas 2010 (conference abstract, limited information); Jensen 2012 (preliminary results for 28 participants out of 81 scheduled); Kendrisic 2013 (conference abstract, limited information); Marshall 2008 (conference abstract, limited information); Nohel 2011 (conference abstract, limited information, variance (error bars) not reported); Saksena Shrivastava 2011 (conference abstract, limited information); Thybo 2016 (few data for pain scores at 8 and 12 hours due to "unforeseen difficulties" during data collection) and Van Herreweghe 2015 (conference abstract, limited information)), there was not enough information to rate this domain. Duarte 2009 was rated as at high risk for selective reporting because study authors mention in their method section that they collected and analysed data of adverse effects (nausea, vomiting, pruritus, urinary retention, and motor blockade of the lower limbs) but these results were not provided.
Other potential sources of bias
This domain was judged as at low risk of bias for more than 50% (32/51) of the included studies (Anis 2011; Asano 2010; Bhatia 2008; Bichel 1998; Celidonio 2008; Cucereanu Badica 2010; Forget 2009; Fouad 2010; Fournier 1998; Frassanito 2008; Fredrickson 2015; Gelmanas 2010; Goytizolo 2016; Green 2014; Kearns 2011; Köroğlu 2008; Marino 2009; Nicholson 2002; Nishio 2014; Siddiqui 2007; Singelyn 2005; Souron 2003; Stevens 2000; Stevens 2007; Striebel 1993; Thomas 2009; Türker 2003; Twyman 1990; Uhrbrand 1992; Utebey 2009; Van Herreweghe 2015; Wiesmann 2014). For seventeen studies there was not enough information in the reports to judge this domain or data were not in intention‐to treat analysis and/or groups characteristics before intervention may have differed enough to possibly have an influence on the results (Aksoy 2014 (participants with failed or insufficient blockade were excluded from the analysis); Bakalov 2016 (conference abstract, limited information); Becchi 2008 (not in intention‐to‐treat); Biboulet 2004 (not in intention‐to‐treat); Borisov 2012 (not in intention‐to‐treat); Duarte 2009 (not in intention‐to‐treat); Ginz 2000 (not in intention‐to‐treat); Jensen 2012 (conference abstract, limited information); Kai 2010 (conference abstract, limited information); Kendrisic 2013 (conference abstract, limited information); Kratz 2015 (not in intention‐to‐treat, preoperative pain scores were higher (although not statistically significantly so) in the femoral nerve block group (4.2 ± 2.9 versus 2.9 ± 3.1)); Lončar 2016 (conference abstract, limited information); Murray 2005 (conference abstract, limited information); Nohel 2011 (conference abstract, limited information); Saksena Shrivastava 2011 (conference abstract, limited information); Shariat 2013 (mean pain scores before intervention were 1 point higher in the fascia iliaca group, the difference was not statistically significant); Thybo 2016 (not in intention‐to‐treat)). Chen 2015 (thrombosis prophylaxis differed between groups) and Marshall 2008 (IV patient‐controlled analgesia provided to one group only) were judged as at risk of other bias.
Effects of interventions
See: Summary of findings for the main comparison Peripheral nerve blocks compared to systemic analgesia for elective primary total hip replacement; Summary of findings 2 Peripheral nerve block compared to neuraxial block for elective primary total hip replacement
1.Comparison 1: Peripheral nerve block versus no block, sham block or systemic analgesia for postoperative analgesia
Primary outcomes
1.1 Participant‐reported pain at rest and with movement on visual analogue scale (VAS), numeric rating scale (NRS), or other similar scales or on ordinal or qualitative scales (FACES)
1.1.1 Pain at rest and with movement on arrival in the postoperative care unit
Nine trials (429 participants) evaluated the effect of peripheral nerve blocks on pain at rest on arrival in the postoperative care unit: psoas compartment block (Biboulet 2004; Goytizolo 2016; Utebey 2009) or femoral nerve block (Köroğlu 2008; Kratz 2015; Nishio 2014 (2 subgroups depending on comparison versus opioids or non‐opioids); Stevens 2000; Striebel 1993; Wiesmann 2014). Random sequence generation was rated as at low risk of bias for Goytizolo 2016; Köroğlu 2008; Kratz 2015; Nishio 2014; Striebel 1993 and Wiesmann 2014; and this domain was judged as unclear for Biboulet 2004; Stevens 2000 and Utebey 2009. Allocation concealment was rated as at low risk of bias for Goytizolo 2016; Kratz 2015; Nishio 2014 and Wiesmann 2014; and as unclear for Biboulet 2004; Köroğlu 2008; Stevens 2000; Striebel 1993 and Utebey 2009. Blinding of participants or personnel taking care of the participants was rated as at low risk of bias for Köroğlu 2008 and Stevens 2000; as unclear for Biboulet 2004 and Goytizolo 2016; and as at high risk for Kratz 2015; Nishio 2014; Striebel 1993; Utebey 2009 and Wiesmann 2014. Blinding of outcome assessor was rated as at low risk of bias for Biboulet 2004; Goytizolo 2016; Kratz 2015; Köroğlu 2008 and Stevens 2000; as unclear for Nishio 2014; Striebel 1993; Utebey 2009 and Wiesmann 2014; and at high risk for Kratz 2015. Incomplete outcome data (attrition bias) was rated as at low risk of bias for all trials included in this analysis. Other risks were judged as at low risk of bias for Goytizolo 2016; Köroğlu 2008; Nishio 2014; Stevens 2000; Striebel 1993; Utebey 2009 and Wiesmann 2014. Biboulet 2004 and Kratz 2015 were judged as unclear because data were not analysed according to the intention‐to‐treat principle. Furthermore, for Kratz 2015, although this was not statistically significant, pain scores measured before the intervention were higher for participants included in the femoral nerve block group (4.2 ± 2.9 versus 2.9 ± 3.1).
Peripheral nerve blocks decreased pain at rest on arrival in the postoperative care unit (SMD ‐1.12, 95% CI ‐1.67 to ‐0.56; I² 84%). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill technique indicated imbalance of two studies missing to left of mean for an adjusted point estimate (SMD ‐1.44, 95% CI ‐2.09 to ‐0.80; random‐effects model). The effect was similar for psoas compartment versus femoral nerve block (I² 0%; Analysis 1.1). Volume of solution injected as a loading dose was unknown for Nishio 2014. Excluding this study, the effect was proportional to the volume of local anaesthetic injected (Figure 4; P < 0.00001) and to the dose in lidocaine equivalents (Figure 5; P = 0.0001). Taking a study at low risk of bias for which the standard deviation (SD) is known and with a typical SD in the control group (Wiesmann 2014; SD = 2.2), the difference would be equivalent to 3.2 on a scale from 0 to 10. For a large trial and using the same SD, 68 participants would be required to decrease VAS scores from 5 to 3.5 (Inference for means calculator) (alpha 0.05; beta 0.2; 2‐sided test).
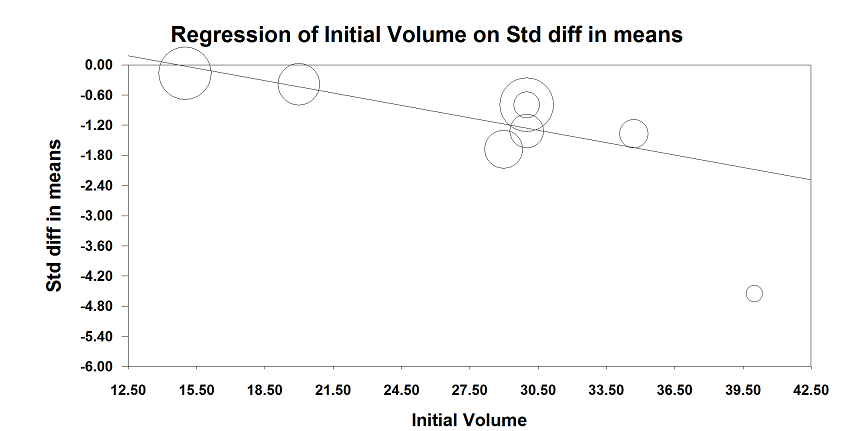
Comparison peripheral nerve block versus no block or sham block or systemic analgesia
Meta‐regression analysis of effect of peripheral nerve block on pain on arrival in postoperative care unit versus volume of the local anaesthetic injected as the loading dose. The effect size was proportional to the concentration used (P < 0.00001).
The largest effect size (standardized mean difference (SMD) ‐4.56, 95% CI ‐5.91 to ‐3.20) was obtained by Köroğlu 2008 where a 3‐in‐1 block with 40 mL 0.25% bupivacaine was compared to a sham block and postoperative analgesia was completed with IV patient‐controlled analgesia with tramadol plus IV meperidine on request.

Comparison peripheral nerve blocks versus no block or sham block or systemic analgesia.
Meta‐regression of pain on arrival in postoperative care unit versus local anaesthetic dose in lidocaine equivalent injected as loading dose. The effect size was proportional to the dose injected (P = 0.0001).
The largest effect size (SMD ‐4.56, 95% CI ‐5.91 to ‐3.20) was obtained by Köroğlu 2008 where a 3‐in‐1 block with 40 mL 0.25% bupivacaine was compared to a sham block and postoperative analgesia was completed with IV patient‐controlled analgesia with tramadol plus IV meperidine on request.
For pain at rest on arrival in the postoperative care unit, we downgraded evidence quality by one level for risk of bias because 50% or more of the included trials were judged at unclear or high risk of bias for allocation concealment, blinding of the outcome assessor or both. We did not downgrade evidence quality for inconsistency because reasonable explanation was found to explain heterogeneity. We did not downgrade for indirectness because all trials were direct comparisons performed with the population of interest and this was not a surrogate marker. We did not downgrade for imprecision because the optimal information size was achieved. We did not downgrade for publication bias because applying a correction would not change the conclusion. We rated the quality of the body of evidence as moderate.
Köroğlu 2008 provided data for pain with movement at arrival in the postoperative care unit (SMD ‐3.62, 95% CI ‐4.78 to ‐2.46).
1.1.2 Pain at rest and with movement from 0.5 to 2 hours after surgery
Nine trials (438 participants) evaluated pain at rest from 0.5 to 2 hours after surgery: femoral nerve block (Kratz 2015; Lončar 2016; Striebel 1993; Wiesmann 2014), fascia iliaca block (Lončar 2016; Shariat 2013) or psoas compartment block (Anis 2011; Green 2014; Siddiqui 2007; Stevens 2000) (SMD ‐0.67, 95% CI ‐1.06 to ‐0.29; I² 71%; Analysis 1.2). In Shariat 2013, participants were recruited only if pain scores remained at ≥ 3 (scale from 0 to 10) despite intravenous patient‐controlled analgesia with morphine for one hour in the postoperative care unit. Egger's regression intercept showed that a small‐study effect could participate to the high I² statistic value (P < 0.05; 2‐tailed). Analysis using Duval and Tweedie's trim and fill method indicated imbalance of two studies missing to right of mean for an adjusted point estimate (SMD ‐0.50, 95% CI ‐0.91 to ‐0.09; random‐effects model). Psoas compartment block decreased pain at rest from 0.5 to 2 hours after surgery (SMD ‐0.91, 95% CI ‐1.20 to ‐0.62; I² 0%) but we did not find a difference for a femoral nerve block: (SMD ‐0.28, 95% CI ‐0.85 to 0.28; I² 68%) or fascia iliaca block (SMD ‐1.21, 95% CI ‐3.34 to 0.92); heterogeneity (I²) between subgroups 49% (P value = 0.14). When Siddiqui 2007 was considered (control group SD = 1.81), the difference in pain score for psoas compartment blocks was equivalent to 1.7 (on a scale from 0 to 10) and 46 participants would be required in a large trial to achieve decrease from 5 to 3.5 in VAS scores (alpha 0.05; beta 0.2; 2‐sided test).
We found no data for pain with movement from 0.5 to 2 hours after surgery or block placement for trials where the block was performed for postoperative analgesia.
1.1.3 Pain at rest and with movement from 4 to 6 hours after surgery
We included 13 trials (599 participants) that evaluated pain at rest between 4 and 6 hours after surgery (SMD ‐0.62, 95% CI ‐0.92 to ‐0.32; I² 66%; Analysis 1.3). Egger's regression intercept showed no evidence of small‐study effect (2‐sided test). Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to right of mean for an adjusted point estimate (SMD ‐0.56, 95% CI ‐0.86 to ‐0.27; random‐effects model).
Femoral nerve block was used in five studies (223 participants) (Kratz 2015; Nishio 2014; Singelyn 2005; Striebel 1993; Wiesmann 2014) (SMD ‐0.30, 95% CI ‐0.98 to 0.38; I² 81%). Two studies (Kratz 2015; Wiesmann 2014) added clonidine to the loading dose of local anaesthetic. Singelyn 2005 added epinephrine, Striebel 1993 added no adjuvant, and the information was not reported by Nishio 2014. Fascia iliaca with a puncture site above (Stevens 2007), or below the inguinal ligament (Cucereanu Badica 2010) was used for two trials (SMD ‐0.36, 95% CI ‐1.06 to 0.33; I² 54%).
Psoas compartment block was used in six trials (303 participants) (SMD ‐0.88, 95% CI ‐1.12 to ‐0.64; I² 0%); I² for heterogeneity between subgroups was 50%. The difference for psoas compartment block was equivalent to 1.4 (on a scale from 0 to 10) (Siddiqui 2007: control group SD = 2.2). Further analysis from data provided by Siddiqui 2007 indicated that 68 participants would be required in a simple trial to decrease VAS scores from 5 to 3.5 (alpha 0.05; beta 0.2; 2‐sided test).
Three trials (Becchi 2008; Cucereanu Badica 2010; Singelyn 2005, 120 participants) evaluated pain scores with movement from 4 to 6 hours (SMD ‐0.46, 95% CI ‐1.12 to 0.20; I² 64%; Analysis 1.4). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method showed no evidence of publication bias. Femoral nerve block (Singelyn 2005) (SMD 0.00, 95% CI ‐0.86 to 0.86), fascia iliaca block with the puncture site below the inguinal ligament (Cucereanu Badica 2010) (SMD ‐0.18, 95% CI ‐0.91 to 0.55) or psoas compartment block (Becchi 2008) (SMD ‐1.00, 95% CI ‐1.51 to ‐0.49) were used (I² for heterogeneity between subgroups 64%).
1.1.4 Pain at rest and with movement at 24 hours after surgery
Six trials (Becchi 2008; Kratz 2015; Siddiqui 2007; Singelyn 2005; Stevens 2007; Wiesmann 2014, 303 participants) evaluated pain scores at rest after single injection block with clonidine (Kratz 2015; Stevens 2007; Wiesmann 2014) or continuous peripheral nerve block (Becchi 2008; Siddiqui 2007; Singelyn 2005) (SMD ‐0.66, 95% CI ‐1.05 to ‐0.28; I² 61%; Analysis 1.5). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method showed no evidence of publication bias. Femoral nerve block (Kratz 2015; Singelyn 2005; Wiesmann 2014) (SMD ‐0.68, 95% CI ‐1.27 to ‐0.10; I² 63%), fascia iliaca block (SMD 0.00, 95% CI ‐0.59 to 0.59) or psoas compartment block (SMD ‐0.97, 95% CI ‐1.37 to ‐0.56; I² 0%) were used; I² for heterogeneity between subgroups 72%. Considering a trial with low risk of bias and typical SD (Shariat 2013; control group SD = 2.2), the difference would be equivalent to 1.5 on a scale from 0 to 10.
Three trials with 317 participants (Becchi 2008; Marino 2009 (2 subgroups); Singelyn 2005), evaluated pain scores with movement after continuous peripheral nerve block (there were no data for single injection block for this outcome) at 24 hours after surgery (SMD ‐0.71, 95% CI ‐1.26 to ‐0.17; I² 78%; Analysis 1.6). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method showed no evidence of publication bias (random‐effects model). The effect was seen with psoas compartment block (SMD ‐1.13, 95% CI ‐1.46 to ‐0.80; I² 0%) but we did not find a difference with femoral nerve block (SMD ‐0.24, 95% CI ‐0.59 to 0.12; I² 0%; I² for heterogeneity for subgroups 92%). Taking a trial at low risk of bias and with a typical SD (Marino 2009; control group SD = 2.3), the difference would be equivalent to 1.6 on a scale from 0 to 10.
1.1.5 Pain at rest and with movement at 48 hours after surgery
Data were available for two trials (Becchi 2008; Singelyn 2005; 93 participants) that evaluated pain at rest at 48 hours after surgery for participants who benefited from continuous peripheral nerve block (SMD ‐0.80, 95% CI ‐1.35 to ‐0.25; I² 29%). Singelyn 2005 used continuous femoral nerve block and Becchi 2008 used continuous psoas compartment block (I² for heterogeneity between subgroups 29%; Analysis 1.7). Taking a trial at low risk of bias and with a typical SD (Duarte 2009; control group SD = 1.80), the difference would be equivalent to 1.44 on a scale from 0 to 10.
Three trials (Becchi 2008; Marino 2009; Singelyn 2005; 317 participants) evaluated pain with movement at 48 hours after surgery in participants with continuous peripheral nerve block (SMD ‐0.62, 95% CI ‐1.13 to ‐0.11; I² 75%; Analysis 1.8). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method showed no evidence of publication bias (random‐effects model). Continuous compartment block reduced pain scores with movement at 48 hours after surgery (SMD ‐0.93, 95% CI ‐1.25 to ‐0.61; I² 0%). We did not find a difference with continuous femoral nerve block (SMD ‐0.16, 95% CI ‐0.60 to 0.28; I² 17%; I² for heterogeneity between subgroups 87%). Taking a trial at low risk of bias and with a typical SD (Singelyn 2005; SD of the control group 1.80), the difference would be equivalent to 1.12 on a scale from 0 to 10.
1.2 Total number of nerve block‐related complications (e.g. erythema, damage to surrounding structures, allergic reactions, infections, transient and lasting neurological damage)
One local haematoma in the groin was reported after accidental puncture of the femoral artery during performance of 3‐in‐1 femoral nerve block under nerve stimulator guidance (Uhrbrand 1992). One persistent (at 3 months) delayed paresis was reported by Siddiqui 2007 following psoas compartment catheter insertion under nerve stimulator guidance. An 82 year old female participant developed a delayed quadriceps weakness that was not present when the catheter was removed at 36 hours but was discovered by the surgeon at her postoperative visit four weeks later. Magnetic resonance imaging revealed no abnormality and the participant declined further investigation. Weakness improved but was present at the three month follow‐up visit. Compression from an haematoma was suspected: international normalized ratio of 5.6 was measured on postoperative day 3 while the participant was receiving warfarin. Warfarin was started at 5 mg the evening before surgery and continued at 5 mg per day each night thereafter (Table 1).
| Study | Regional anaesthesia technique | Complications related to regional anaesthesia | Analgesic technique | Complications related to systemic analgesia or general anaesthesia |
| Ultrasound to localize the spinous process of L3. Psoas compartment block with 30 mL 0.25% bupivacaine and epinephrine 5 µg/mL injected when an ipsilateral quadriceps muscle contraction was obtained at 0.5 to 0.8 mA. Subgluteal sciatic nerve block with 20 mL of the same solution (dual guidance and plantar flexion at 0.5 to 0.8 mA Karmakar 2007). Iliac crest block with 5 mL of the same solution. Continuous spinal anaesthesia (22G; catheter over the needle) for the surgery for the comparator | No major complications due to the peripheral nerve block procedure were observed in any participants intra‐ or postoperatively No participant had post dural puncture headache in the continuous spinal anaesthesia group until discharged. Cauda equina syndrome was not observed in any participant | Subcutaneous morphine 0.1 mg/kg for the peripheral nerve block group. Intrathecal morphine 0.2 mg for the comparative group. Rescue analgesia with IV tramadol 50 mg | Complications related to morphine (any route) not reported. No significant difference in terms of peripheral oxygen saturation | |
| Nerve stimulation psoas compartment block (Capdevila 2002) with 15 mL 0.5% bupivacaine plus 15 mL saline ± clonidine 2.5 µg/mL (total 75 µg) injected when a quadriceps contraction was obtained at 0.5 mA and 50 ms | No local anaesthetics side effects occurred during the first 24 hours postoperatively | IM morphine | Respiratory rate was in the normal range after 2 hours postoperatively for systemic analgesia group participants Mean respiratory rate was above 10 per minute for all groups at 2, 6 and 12 hours | |
| Continuous lumbar plexus block ultrasound and nerve stimulation guidance: injection 0.6 mL/kg of 0.375% ropivacaine and 100 µg fentanyl through the stimulating needle, advancement of the catheter to the local anaesthetic pool with ultrasound guidance, followed by continuous infusion of 0.2% ropivacaine at 6 mL to 8 mL/hour plus fentanyl (0.5 µg/kg/hour). Continuous lumbar epidural (L2‐L3) with 4 mL ropivacaine 0.75% plus 100 µg of fentanyl, followed by continuous infusion of 0.2% ropivacaine at 4 mL/hour plus fentanyl (0.5 µg/kg/hour) | Not reported | IV patient controlled analgesia with fentanyl | Not reported | |
| Quadratus lumborum block (Blanco 2015) | Not reported | Paracetamol, ketoprofen and IV morphine patient‐controlled analgesia | Vomiting | |
| Nerve stimulation psoas compartment block (Capdevila 2002). When a quadriceps contraction was obtained between 0.3 and 0.5 mA and 100 ms, the needle bevel was oriented caudally and laterally, the psoas compartment was distended with 5 mL saline, and a 20 G multi‐perforated catheter was introduced through the needle and advanced 5 cm distally to the needle tip. A test dose of 1% lidocaine with epinephrine 5 µg/mL (3 mL) was performed. If the test dose was negative, 10 mL contrast medium (Omnipaques; Amersham Healths, Oslo, Norway) was injected into the catheter, and an antero‐posterior radiograph of the lumbar region was obtained. Catheter location was evaluated by a radiologist. 30 min before the end of surgery, participants received a loading dose 0.75% ropivacaine (0.4 mL/kg) followed by an infusion of 0.2% ropivacaine at 10 mL/hour for 48 hours. Spinal anaesthesia with a 27 G Whitacre needle at L3‐L4 and 15 mg isobaric bupivacaine | No participant developed haematoma There was no epidural spread, dysaesthesia or any other sign of local anaesthetic toxicity. One catheter displacement occurred during the study period and no neurological complications were recorded after catheter withdrawal | IV infusion of morphine 0.1% and ketorolac 0.12% at 2 mL/hour for 48 hours | No participant had itching or respiratory depression | |
| Psoas compartment block with a nerve stimulator and 0.4 mL/kg 0.125% levobupivacaine before surgery, catheter inserted, and re‐injection of the same dose of levobupivacaine at the end of surgery | Not reported | Intrathecal diamorphine 4 µg/kg. IV patient‐controlled analgesia with morphine | Not reported | |
| Femoral nerve block with nerve stimulation (Winnie 1973) and 2 mg/kg 0.375% bupivacaine and 2 µg/kg clonidine or Psoas compartment block at L3 with nerve stimulation (Parkinson 1989) and 2 mg/kg of 0.375% bupivacaine and 2 µg/kg of clonidine | Except for 4 cases of epidural anaesthesia (lasting < 4 hours) in the psoas compartment block group, no complications secondary to the block were noted | IV patient‐controlled analgesia with morphine. All participants systematically received | There was no difference in respiratory rate among the three groups during the study. No participants reported pruritus. Sedation scores in all participants were < 1 for the first 8 postoperative hours and rated 0 after 8 hours | |
| Femoral nerve block with a nerve stimulator and 0.3 mL/kg 0.5% bupivacaine followed by an infusion of bupivacaine 0.125% and sufentanil 0.5 µg/mL at 10 mL/hour. Lumbar epidural (L2‐L3 or L3‐L4) with 0.3 mL/kg 0.5% bupivacaine followed by an infusion of 0.125% bupivacaine and sufentanil 0.5 µg/mL at 6 mL/hour | Not reported | IM piritramide | Not reported | |
| Continuous fascia iliaca block, catheters threaded 3 cm cranially and loaded with 30 mL 0.33% ropivacaine followed by an infusion of 0.2% ropivacaine at 1 mg/10 kg continued for 48 hours. Continuous epidural (combined spinal /epidural) with catheters threaded 3 cm to 5 cm cranially and an infusion of 0.2% ropivacaine started after surgery. Spinal anaesthesia with 15 mg 0.5% hyperbaric 0.5% bupivacaine at L2‐L3 or L3‐L4 with the needle oriented cranially for surgery | There were no complications related to regional anaesthesia, intra‐ or postoperative techniques. One failed catheter in the fascia iliaca block group | Acetaminophen 4 g/day. Ketoprofen 300 mg/day. Tramadol as rescue analgesia | Not reported | |
| Continuous femoral nerve block with 20 mL 0.5% ropivacaine 3 mg/kg (maximum 40 mL), sufentanil and clonidine alone or associated with single injection sciatic nerve block with 20 mL of the same solution. Infusion of 0.2% ropivacaine and sufentanil at 8 mL/hour through the femoral nerve catheter for 48 hours. Continouous lumbar epidural with 0.5% ropivacaine 15 mL to 20 mL and sufentanil, clonidine. Infusion of 0.2% ropivacaine and sufentanil at 8 mL/hour through the epidural catheter for 48 hours | Not reported | Morphine and ketorolac | Not reported | |
| Continuous femoral nerve block (puncture site below the inguinal ligament and outside the femoral artery) with catheter inserted 10 cm past the needle tip connected to a patient‐controlled pump for 72 hours | Not reported | Not reported | Not reported | |
| Fascia iliaca compartment block with 40 mL 0.5% ropivacaine. Spinal anaesthesia with 0.5% bupivacaine for surgery | No significant adverse effects | IV morphine | Not reported | |
| All blocks performed on the operated side in the upper position. Psoas compartment block (Capdevila 2002) with a nerve stimulator and 0.4 mL/kg 0.5% ropivacaine injected when a quadriceps contraction was obtained between 0.35 and 0.5 mA and 50 ms. Catheter inserted after the initial bolus (20 G multi‐orifice) inserted 3 cm to 5 cm cephalad. Patient‐controlled continuous block after the surgery with ropivacaine 0.2%. Epidural catheter with a paramedian approach at L3‐L4 or L4‐L5 with 10 mL to 15 mL 0.5% ropivacaine before catheter insertion (20 G multi‐orifice; inserted 3 cm to 5 cm cephalad). Patient‐controlled continuous epidural analgesia after the surgery with ropivacaine 0.2% and fentanyl 3 µg/mL | Not reported | IV morphine | Not reported | |
| Single injection fascia iliaca (landmarks) with 0.4 mL/kg 0.5% L‐bupivacaine (max 30 mL) with clonidine 2.5 µg/mL and epinephrine 5 µg/mL. Single injection epidural analgesia (iliac crest level) with 10 mL 0.25% L‐bupivacaine and 10 µg sufentanil | Partial transient motor blockade for 4 participants. Side effects and complications similar for both groups | IV paracetamol 1 g every 6 hours. IV patient‐controlled piritramide | Side effects and complications similar for both groups | |
| Continous psoas compartment block with dual guidance (ultrasound (in‐plane) and nerve stimulator), catheter insertion, 25 mL 0.375% ropivacaine. Continous infusion of 0.2% ropivacaine at 4 to 6 mL/h. Continous epidural analgesia with 6 mLropivacaine 0.5% plus fentanyl 100 µg continuous infusion with 0.2% ropivacaine and fentanyl 4 µg/mL at 4 to 6 mL/h | Not reported | IV flurbiprofen 50 mg every 12 hours. Pentazocine | Not reported | |
| Femoral nerve block (Winnie 1973) with nerve stimulation and 40 mL 0.5% bupivacaine with epinephrine 5 µg/mL injected when a quadriceps contraction was obtained at ≤ 0.4 mA (needle angled proximally). Sham block | No side effects such as paraesthesiae or dysaesthesiae in the area covered by the block, haematoma or signs | IM diclofenac and/or subcutaneous morphine 0.1 mg/kg | Not reported | |
| Operated side upper position. Psoas compartment block (Capdevila 2002) with a nerve stimulator and 0.4 mL/kg ropivacaine 0.5% injected when a quadriceps contraction was obtained at 0.5 mA and 0.1 ms. Spinal anaesthesia (L3‐L4) with 15 mg hyperbaric bupivacaine plus 15 µg fentanyl and 0.1 mg morphine | One epidural extension. No failed spinal or psoas compartment block | Intrathecal morphine 0.1 mg. Routine paracetamol 1 g IV 4 x daily and ketorolac 30 mg IV 3 x daily. IV tramadol as rescue | No respiratory depression. Higer risk of pruritus with intrathecal morphine | |
| Continuous psoas compartment block (Heller 2009) with a nerve stimulator, catheter inserted 3 cm past the needle tip and needle bevel oriented laterally when a quadriceps contraction was obtained between 0.5 and 0.8 mA and 0.1 ms and loaded with 40 mL 0.5% ropivacaine followed by patient‐controlled analgesia with 0.2% ropivacaine after surgery. Spinal anaesthesia with 2 mL 0.5% and morphine 0.1 mg | Neurological irritation or injury did not differ between groups. Symptoms suggestive of neurological irritation or injury did not differ (spinal = 8/23 and psoas compartment block = 6/27) between groups. All were mild and short‐lived. No participant demonstrated symptoms or signs of systemic local anaesthetic toxicity and there were no catheter‐related bleeding or infectious complications | Intrathecal morphine 0.1 mg. IV parecoxib 40 mg at the time of surgery. Oral paracetamol 1 g every 6 hours. Sustained release diclofenac 75 mg every 12 hours. Sustained release tramadol 100 mg every 12 hours on request. Oral or IV morphine as rescue analgesia. (Lumbar plexus group participants also received morphine 0.1 mg/kg IV during surgery) | Higher risk of pruritus in the intrathecal group. 2 in‐hospital falls with intrathecal morphine | |
| Continuous psoas compartment block with bupivacaine 0.125% and fentanyl 0.05 mg/mL at 5 to 10 mL/hour. Continuous epidural analgesia with bupivacaine 0.125% and fentanyl 0.05 mg/mL at 3 to 5 mL/hour | Degree of motor block was significantly higher in the lumbar plexus block group | Type of additional analgesic used unspecified | Frequency of side effects did not differ significantly between groups | |
| Obturator nerve block (obturator canal level) with a nerve stimulator and 30 mL 0.25% bupivacaine injected when an adductor muscle contraction was obtained at 0.5 mA | Not reported | IV piritramide on request. Rectal diclofenac 100 mg or metamizole 1 g on request | Not reported | |
| Lumbar plexus block with a nerve stimulator and 30 mL 0.5% bupivacaine injected when a quadriceps contraction was obtained < 1 mA. Combined spinal/epidural with 60 mg 1.5% mepivacaine and 3 mL aliquots of 2% lidocaine through an epidural catheter for surgery and patient‐controlled epidural analgesia with 0.06% bupivacaine and 10 µg/mL hydromorphone for postoperative analgesia for all participants | No adverse events attributable to the lumbar plexus block occurred | Epidural patient‐controlled analgesia and oral opioid | No adverse events attributable to either technique occurred | |
| The psoas compartment block (50 mL (40 mL 0.25% bupivacaine and 10 mL saline) was administered using an 18 G spinal needle. volume of (Green 2011). Spinal anaesthesia for surgery | There were no adverse events perioperatively, directly related to block administration | Routine paracetamol 1 g 4 x daily and diclofenac 75 mg 2 x daily. Oxycontin 10 mg 2 x daily. Oxynorm for | Not reported | |
| Femoral, obturator (anterior branch) and lateral femoral cutaneous nerve block | Not reported | Morphine on request | Not reported | |
| Psoas compartment block with a nerve stimulator with 30 mL 0.375% ropivacaine injected when a quadriceps contraction was obtained at 0.3 mA and isobaric spinal anaesthesia (L2‐L3 or L3‐L4) with bupivacaine 0.12 mg/kg. Epidural (L2‐L3 or L3‐L4) and isobaric spinal anaesthesia (L2‐L3 or L3‐L4) with bupivacaine 0.16 mg/kg for surgery | No participants had post‐dural puncture headache or back pain | Not reported | All participants had oxygen saturation between 98% and 100% | |
| Ultrasound‐guided (out‐of‐plane) fascia iliaca block with levobupivacaine 2 mg/kg diluted to a total volume of 40 mL below the inguinal ligament but proximal to any femoral artery branching | 4 adverse events defined as any untoward medical occurrence and 4 serious adverse events defined as resulting in death or life threatening (at the time of the event) or required hospitalization or prolongation of existing hospitalization or resulting in persistent or significant disability or incapacity or consisting of a congenital anomaly or birth defect or beng otherwise considered medically significant by the investigator (not necessarily related to the trial treatment). Pulmonary embolism (N = 2), femoral nerve palsy (N = 1; resolved at 3 months), late wound infection (N = 1) | Intrathecal morphine 0.1 mg. IV patient‐controlled analgesia with morphine | 3 adverse events defined as any untoward medical occurrence and 2 serious adverse events defined as resulting in death or life threatening (at the time of the event) or required hospitalization or prolongation of existing hospitalization or resulting in persistent or significant disability or incapacity or consisting of a congenital anomaly or birth defect or beng otherwise considered medically significant by the investigator (not necessarily related to the trial treatment). Pulmonary embolism (N = 1), wound infection resulting in multi‐organ failure (N = 1) | |
| Femoral (3‐in‐1) block with nerve stimulation and 40 mL 0.25% bupivacaine injected when a quadriceps contraction was obtained at 0.5 mA and with distal pressure | There was no local anaesthetic toxicity | IV patient‐controlled analgesia with tramadol | Most common side effects were nausea and vomiting | |
| Single injection femoral nerve block with a nerve stimulator and 20 mL 0.25% bupivacaine with 20 µg clonidine injected when a quadriceps contraction was obtained at 0.4 mA and 0.1 ms | Not reported | Metamixol 15 to 25 mg/kg before end of surgery. IV piritramide (in the postoperative care unit). Ibuprofene. Metamizole. Oral oxycodone | Not reported | |
| Single injection femoral nerve or fascia iliaca block | Not reported | IV morphine | Not reported | |
| Continuous psoas compartment block with nerve stimulator, catheter advanced 3 cm past the needle tip when a quadriceps contraction was obtained at 0.5 mA and 100 ms. Catheters were loaded with 0.6 mL/kg ropivacaine 0.5% and the position was verified with 10 mL contrast medium. Participants then received ropivacaine 0.2% infused at 0.15 mL/kg/hour for 48 hours. Continuous femoral nerve block loaded through the needle with 0.6 mL/kg ropivacaine 0.5% injected when a quadriceps contraction was obtained at 0.5 mA and 100 ms, catheter advanced < 10 cm past the needle tip. This was followed by an infusion of ropivacaine 0.2% at 0.15 mL/kg/hour for 48 hours | Lumbar plexus block was associated with an epidural spread in 5/75 (7%) participants. No perineural haematoma recorded | IV patient‐controlled analgesia with hydromorphone | Nausea and pruritus | |
| Continuous lumbar plexus block as patient‐controlled analgesia. Continuous epidural analgesia. Spinal anaesthesia for surgery | Not reported | Parenteral opioids | Not reported | |
| Fascia iliaca block on landmarks with 2 mg/kg 0.5% levobupivacaine. Spinal anaesthesia with isobaric 0.5% bupivacaine 0.1 to 0.2 mg/kg for surgery | Not reported | IV patient‐controlled analgesia with morphine | Not reported | |
| Femoral (3‐in‐1) nerve block with a nerve stimulator and 30 mL of equal parts 0.5% bupivacaine and 2% lidocaine | Not reported | Routine paracetamol 1 g every 6 h. Diclofenac 8 mg on request. IM morphine | Not reported | |
| Dual guidance continuous femoral nerve block (ultrasound and nerve stimulator) with a catheter inserted 10 cm past the needle tip and participants received an infusion of 0.15% ropivacaine at 3 mL/hour | Not reported | IV patient‐controlled analgesia with fentanyl or intrarectal diclofenac or IV flurbiprofen on participant's request and preferences | Nausea and vomiting, drowsiness | |
| Continuous femoral nerve block with 0.1 mL/kg/hour of 0.1% bupivacaine. Continous epidural analgesia with 0.1 mL/kg/hour 0.1% bupivacaine | Not reported | IV patient‐controlled analgesia with fentanyl | Not reported | |
| Ultrasound‐guided (in‐plane), low pressure (20 pounds per square inch) fascia iliaca block with 30 mL 0.5% ropivacaine | Not reported | IV patient‐controlled analgesia with morphine | Not reported | |
| Continuous psoas compartment block (Winnie 1974) with a nerve stimulator and a 4 inch Tuohy needle (quadriceps contraction at 0.5 to 1.0 mA and 50 ms) and 5 mL saline before catheter insertion advanced 4 cm to 6 cm past the needle tip. Injection of 3 mL 2% lidocaine with epinephrine 5 µg/mL followed by 20 mL 0.25% bupivacaine and an infusion of 0.125% bupivacaine at 10 mL/hour for 36 hours | One participant in the continuous lumbar plexus block developed a delayed paresis (first noted 4 weeks after surgery), possibly related to nerve compression by a haematoma, international normalized ratio 5.6 on postoperative day 3. The weakness improved but remained at 3 months | IV patient‐controlled analgesia with morphine | One participant in the systemic analgesia group developed respiratory depression | |
| Continuous femoral nerve block (Winnie 1973) with a nerve stimulator and 40 mL 0.25% bupivacaine with epinephrine 5 µg/mL through a catheter inserted 10 cm past the needle tip followed by an infusion of 0.125% bupivacaine at 10 mL/hour. Epidural analgesia at L2‐L3 or L3‐L4 with catheters inserted 4 cm to 5 cm past the needle tip, a test dose with 3 mL 0.25% bupivacaine with epinephrine 5 µg/mL, followed by 10 mL of the same solution and 10 µg sufentanil and then by an infusion of 0.125% bupivacaine at 10 mL/hour | 3 participants with catheter‐related problems in the epidural group 2 urinary retention for femoral nerve block group and 6 for epidural group | IV patient‐controlled analgesia with morphine | 4 urinary retention in the IV morphine group | |
| Psoas compartment block (Winnie 1975) with a nerve stimulator and with 25 mL ropivacaine 0.475% injected when a quadriceps contraction was obtained at 0.5 mA and 0.1 ms | No major complication occurred. Epidural block did not occur. 2 blood aspirations during psoas compartment block performance | Intrathecal morphine 0.1 mg | No major complications were observed with either technique | |
| Psoas compartment block (Winnie 1974) with a nerve stimulator and 0.4 mL/kg 0.5% bupivacaine and epinephrine 5 µg/mL injected when a quadriceps contraction was obtained between 0.2 and 0.5 mA and 50 ms | Epidural anaesthesia occurred in 3 of 28 plexus group participants No other side effects were noted, total spinal anaesthesia, renal | IV patient‐controlled analgesia with morphine | Nausea and vomiting | |
| Fascia iliaca block with modified landmarks (Dalens 1989 but 1 cm above the inguinal ligament; first pop when the needle traverses the superficial fascia and second pop for the fascia transversalis) and 30 mL 0.5% bupivacaine with epinephrine 5 µg/mL, 150 µg of clonidine and 9 mL saline. Hypothesisis: this puncture point would enable blockade of the ilio‐inguinal, iliohypogastric and genitofemoral nerves as well as the femoral, lateral cutaneous and obturator nerves but sensory block not tested | Not reported | IV patient‐controlled analgesia with morphine | Nausea and vomiting | |
| Continuous femoral (3‐in‐1) nerve block with catheters (inserted 10 cm past the needle tip) with a nerve stimulator (between 0.2 and 2.0 mA on needle) and 30 mL 0.5% bupivacaine administered before surgery (quadriceps motor blockade confirmed before anaesthesia induction) and 10 minutes after surgery | Mean bupivacaine plasma levels ranged between 0.75 and 1.33 µg/mL Placement of the 3‐in‐1 catheters were without complications | IV meperidine on request | Nausea and vomiting | |
| Continuous femoral nerve block with lidocaine (lidocaine 1% bolus followed by intra‐operative infusion of lidocaine 1% 10 mL/hour and lidocaine 0.5% 10 mL/hour during 24 hours postoperatively | Not reported | IV lidocaine infusion: 1.5 mg/kg followed by intra‐operative continuous infusion of 2 mg/kg/hour and postoperatively 1 mg/kg/hour for 24 hours. All participants received paracetamol 4 g for24 hours and IV patient‐controlled analgesia with morphine | Not reported | |
| Ultrasound‐guided (in‐plane) lateral femoral cutaneous nerve block with 8 mL 0.75% ropivacaine. Spinal anaesthesia with 2 mL to 2.5 mL 0.5% isobaric bupivacaine for surgery | No adverse events or harms were observed during the trial | Paracetamol 1 g orally every 6 hours. Ibuprofen 600 mg orally every 8 hours. IV or oral oxycodone on request | No adverse events, or harms, were observed during the trial | |
| Psoas compartment block (side to blocked uppermost, 3 cm caudal and 5 cm lateral to L4 apophysis and redirected upward if the apophysis of L5 was contacted) with a nerve stimulator and when a quadriceps contraction was obtained at 0.5 mA, 10 mL saline was injected before catheter insertion 5 cm past the needle tip. This was followed by a test dose with 3 mL 2% lidocaine with epinephrine 5 µg/mL, then 20 mL contrast solution, followed by 30 mL 0.5% bupivacaine (repeated if surgery lasted > 2 hours). Patient‐controlled analgesia with 0.125% bupivacaine and fentanyl 2 µg/mL for 24 hours. Epidural analgesia at L3‐L4, catheters advanced 3 cm cephalad, test dose with 3 mL 2% lidocaine with epinephrine 5 µg/mL followed by 15 mL 0.5% bupivacaine (repeated if surgery lasted > 2 hours). Patient‐controlled analgesia with 0.125% bupivacaine and fentanyl 2 µg/mL for 24 hours | Psoas: 2 kinked catheters No participants in the psoas group developed epidural Epidural: 1 kinked catheter and 1 lateralization on non‐operated side. No participants in either group developed complications such as cardiac arrest, permanent 7 urinary retention in the epidural group | IM diclofenac 75 mg on request | Nausea and vomiting Pruritus | |
| Psoas compartment block (Chayen 1976), loss of resistance technique, 20 mL air injected to dilate the space followed by 0.42 mL/kg of 0.375% bupivacaine | There were no complications associated with the technique | Unspecified | Not reported | |
| Femoral (3‐in‐1; Brands 1978) nerve block with a nerve stimulator and 20 mL 0.5% bupivacaine plus 20 mL 2% lidocaine with epinephrine Femoral lateral cutaneous nerve block with 5 mL 1% lidocaine on landmarks | One haematoma in the groin after accidental puncture of the femoral artery No toxic reactions due to the local anaesthetics were recorded | Nicomorphine on request | Not reported | |
| Continuous psoas compartment block with a nerve stimulator (Parkinson's approach; Parkinson 1989; L3 confirmation with fluoroscopy in prone position) and with 0.4 mL/kg 0.25% bupivacaine injected before surgery when a quadriceps contraction was obtained at 0.5 mA (position confirmed with 2 mL radiopaque substance and catheter inserted 2 cm past the needle tip) and half dose after 8 hours. Continuous lumbar (L3‐L4; catheters threaded 3 to 3.5 cm cranially) epidural analgesia with a test dose (3 mL 2% lidocaine) and 2 mL per segment until T10‐T12 before general anaesthesia with 0.025% bupivacaine and half dose after 8 hours | Two epidural spreads One urinary retention with epidural analgesia | IV patient‐ controlled analgesia with morphine | Pruritus | |
| Supra‐inguinal fascia iliaca compartment block with 40 mL 0.5% ropivacaine | Not reported | Paracetamol Non steroidal anti‐inflammatory drugs IV patient‐controlled analgesia with morphine | Not reported | |
| Femoral nerve block with a nerve stimulator and 15 mL bupivacaine 0.25% and clonidine 20 µg injected when a quadriceps contraction was obtained at 0.3 mA and 0.1 ms | Not reported | IV piritramide in the postoperative care unit. IV/oral metamizole and oral ibuprofen in both groups on request | No oxygen saturation < 90% No nausea and vomiting |
IM: intramuscular; IV: intravenous; mA: milliampere; ms: millisecond;
Siddiqui 2007 was judged as at low risk of bias for random sequence generation, allocation concealment, incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias; and as at high risk of bias for blinding of participant or personnel taking care of the participant and blinding of outcome assessment. Uhrbrand 1992 was judged as at unclear risk of bias for random sequence generation, allocation concealment and blinding of participant or personnel taking care of the participant and as at low risk of bias for all other domains.
Secondary outcomes
1.1 Analgesic requirements
We included 12 trials (897 participants) that reported analgesia requirements delivered as single injection blocks (10 studies: Anis 2011; Cucereanu Badica 2010; Fournier 1998; Ginz 2000; Murray 2005; Shariat 2013; Stevens 2007; Thybo 2016; Uhrbrand 1992; Van Herreweghe 2015); repeated doses (Utebey 2009) or continuous nerve block (Marino 2009) and evaluated opioid consumption from 0 to 24 hours (SMD ‐0.62, 95% CI ‐0.89 to ‐0.35; I² 86%; Analysis 1.9). Egger's regression intercept showed no evidence of small‐study effect (P = 0.03; 2‐sided test). Analysis using Duval and Tweedie's trim and fill method indicated imbalance of two studies missing to right of mean for an adjusted point estimate (SMD ‐0.52, 95% CI ‐0.79 to ‐0.24). When trials were subgrouped by type of block, peripheral nerve block was found to reduce opioid consumption when femoral nerve block with or without femoral lateral cutaneous nerve block (SMD ‐0.39, 95% CI ‐0.61 to ‐0.17); fascia iliaca block (SMD ‐0.84, 95% CI ‐1.27 to ‐0.41); or psoas compartment block (SMD ‐0.96, 95% CI ‐1.27 to ‐0.65; I² for heterogeneity between subgroups 86%) was used.
1.2 Minimal clinically important improvement in pain
Data were not available for proportions of participants with pain VAS scale differences of 2 cm on a 0 cm to 10 cm scale as a categorical variable. A mean difference of 2 cm or more between the intervention and control groups was found for pain at rest and with movement on arrival in the postoperative care unit.
1.3 Complications specific to method of treatment
1.3.1 Complications from peripheral nerve blocks (damage to surrounding structures at site of nerve block)
Becchi 2008 reported one instance of catheter dislodgment. Marino 2009 reported that catheters were not functional (N = 2) or were occluded (N = 2). Epidural extension of psoas compartment block was reported by Biboulet 2004 (N = 4), Marino 2009 (N = 5), Stevens 2000 (N = 3) and Utebey 2009 (N = 2). Uhrbrand 1992 reported one local haematoma. One persistent (at 3 months) delayed paresis was reported in Siddiqui 2007 (see details above).
1.3.2 Complications from systemic analgesia or general anaesthesia (other complications as detailed in each study)
Siddiqui 2007 reported that one participant developed respiratory depression from systemic analgesia.
1.4 General medical complications within six weeks after surgery
1.4.a Gastrointestinal: nausea and vomiting, constipation, ileus
We included five trials (282 participants) that reported results for nausea (Goytizolo 2016; Jensen 2012), nausea and vomiting (Stevens 2007; Wiesmann 2014) or vomiting (Bakalov 2016) for the first 24 hours (RR 0.86, 95% CI 0.53 to 1.41; I² 17%; Analysis 1.10). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to right of mean for an adjusted point estimate (RR 0.96, 95% CI 0.59 to 1.58).
We could not obtain data suitable for analysis for constipation or ileus.
1.4.b Pulmonary: pneumonia, bronchitis
No included studies reported this outcome.
1.4.c Cardiovascular: hypotension, myocardial infarction, blood loss and blood transfusion
Four trials (150 participants) reported on the risk of hypotension (RR 0.95, 95% CI 0.50 to 1.81; I² 0%) (Becchi 2008; Siddiqui 2007; Singelyn 2005; Utebey 2009). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method showed no evidence of publication bias.
We could not obtain data suitable for analysis for myocardial infarction.
Based on data from eight trials (323 participants), we did not find differences for intra‐operative blood loss (MD ‐52.47 mL, 95% CI ‐170.88 to 65.95; I² 79%; Analysis 1.12) (Cucereanu Badica 2010; Kratz 2015; Nishio 2014; Siddiqui 2007; Stevens 2000; Twyman 1990; Utebey 2009; Wiesmann 2014). Egger's regression intercept showed no evidence of small‐study effect. Data for amount of blood transfused were available for three trials (Nicholson 2002; Singelyn 2005; Utebey 2009; 82 participants) (MD ‐0.59 units, 95% CI ‐1.25 to 0.08; I² 48%; Analysis 1.13).
1.4.d Neurological: acute confusional state, drowsiness, cerebrovascular accident, postoperative cognitive dysfunction
Only Marino 2009 provided results for acute confusional state (RR 0.10, 95% CI 0.02 to 0.54). Marino 2009 was judged as at low risk of bias for random sequence generation, incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias; at unclear risk of bias for allocation concealment and blinding of outcome assessment; and as at high risk of bias for blinding of participant or blinding of personnel taking care of the participant, The number needed to treat for an additional beneficial outcome (NNTB) = 12 (95% CI 11 to 22). In a large trial, 3158 participants (1579 per group) would be required to eliminate a 25% difference (alpha 0.05; beta 0.2; 1‐sided test).
For grade of the evidence for reduced risk of confusional state, we downgraded by two levels because the included trial was at unclear risk of bias for allocation concealment and blinding of outcome assessor and as at high risk for blinding of participant or blinding of personnel taking care of the participant. Inconsistency and publication bias could not be assessed. The trial was a direct comparison. We downgraded the quality by two levels because the optimal information was not achieved (low number of trials/participants). We rated the level as very low.
Three trials (Jensen 2012; Köroğlu 2008; Nishio 2014; 86 participants) provided results for drowsiness (sedated) up to 48 hours (RR 0.40, 95% CI 0.13 to 1.18; I² 0%; Analysis 1.14). Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to left of mean for an adjusted point estimate (RR 0.39, 95% CI 0.14 to 1.06). Based on an incidence of 26% of excessive sedation (drowsiness) in the control groups, 1304 (652 per group) would be required in a large trial to eliminate a 25% difference (alpha 0.05; beta 0.2; 2‐sided test).
We found no data suitable for analysis for cerebrovascular accident or postoperative cognitive dysfunction.
1.4.e Thromboembolic complications: deep venous thrombosis or pulmonary embolism
We could not obtain data suitable for analysis for deep venous thrombosis.
Chen 2015 reported no pulmonary emboli in 88 participants.
1.4.f Other medical complications
1.4.f.1 Risk of pruritus
Four trials (Goytizolo 2016; Köroğlu 2008; Marino 2009; Siddiqui 2007; 379 participants) reported on the risk of pruritus during the first 48 hours after surgery (RR 0.34, 95% CI 0.09 to 1.27; I² 66%; Analysis 1.15). Random sequence generation was judged as at low risk for Goytizolo 2016; Köroğlu 2008; Marino 2009 and Siddiqui 2007. Allocation concealment was judged as at low risk for Goytizolo 2016 and Siddiqui 2007; and as at unclear risk for Köroğlu 2008 and Marino 2009. Blinding of participant and/or personnel taking care of the participant was judged as at low risk of bias for Köroğlu 2008; as at unclear risk for Goytizolo 2016 and as at high risk of bias for Marino 2009 and Siddiqui 2007. Blinding of outcome assessment was judged as at low risk for Goytizolo 2016 and Köroğlu 2008; as at unclear risk for Marino 2009; and as at high risk for Siddiqui 2007. Incomplete outcome data (attrition bias) was judged as at low risk for Goytizolo 2016; Köroğlu 2008; Marino 2009 and Siddiqui 2007. Selective reporting (reporting bias) was judged as at low risk for Goytizolo 2016; Köroğlu 2008; Marino 2009 and Siddiqui 2007. Other bias were judged as at low risk for Goytizolo 2016; Köroğlu 2008; Marino 2009 and Siddiqui 2007. Egger's regression intercept showed no significant evidence of small‐study effect (2‐sided test). Analysis using Duval and Tweedie's trim and fill method indicated imbalance of two trials missing to right of mean for an adjusted point estimate (RR 0.85, 95% CI 0.25 to 2.81; random‐effects model). The risk was reduced when continuous block was used (RR 0.16, 95% CI 0.04 to 0.70; I² 31%; 2 trials, 259 participants) but not when single injection block was used (RR 0.92, 95% CI 0.50 to 1.68; I² 0%). Considering a basal rate of 20% (control group), the NNTB for continuous nerve blocks would be 4 (95% CI 4 to 8). Considering a basal rate of 20%, 1428 participants (714 per group) would be required in a large trial to eliminate a 25% difference (alpha 0.05; beta 0.2; 1‐sided test).
For grade of evidence for reduced risk of pruritus for continuous nerve blocks, we downgraded by two levels for risk of bias because 75% of the included trials were judged as at unclear/high risk for allocation concealment and/or blinding of outcome assessors. There was no significant inconsistency for this subgroup (I² < 50%). We included direct comparisons only. We downgraded the level by one because the optimal information size was not achieved. Publication bias could not be assessed. We rated the level of evidence as very low.
1.4.f.2 Risk of respiratory depression
Three trials (Becchi 2008; Siddiqui 2007; Wiesmann 2014; 184 participants) reported risk of respiratory depression (RD ‐0.01, 95% CI ‐0.05 to 0.03; I² 0%). Egger's regression intercept showed no significant evidence of small‐study effect (2‐sided test). Analysis using Duval and Tweedie's trim and fill method showed no evidence of publication bias.
1.5 Use of resources
1.5.a Hospital length of stay
Three trials (Marino 2009; Singelyn 2005; Thybo 2016; 348 participants (the forest plot (Analysis 1.17) displays 349 participants due to subgroup division) reported length of hospital stay (SMD ‐0.56, 95% CI ‐0.91 to ‐0.21; I² 54%; Analysis 1.17) (Marino 2009 (2 subgroups); Singelyn 2005; Thybo 2016). Random sequence generation was judged as at low risk for Marino 2009; Singelyn 2005 and Thybo 2016. Allocation concealment was judged as at low risk for Thybo 2016; and as at unclear risk for Marino 2009 and Singelyn 2005. Blinding of participant and/or personnel taking care of the participants was judged as at low risk for Thybo 2016 and as at high risk for Marino 2009 and Singelyn 2005. Blinding of outcome assessment for judged as at low risk for Thybo 2016; and as at unclear risk of bias for Marino 2009 and Singelyn 2005. Incomplete outcome data, selective reporting and other bias were judged as at low risk for Marino 2009; Singelyn 2005; and as at unclear risk for Thybo 2016. Egger's regression intercept showed no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to right of mean for an adjusted point estimate (SMD ‐0.46, 95% CI ‐0.78 to ‐0.13; random‐effects model). Continuous femoral nerve or psoas compartment blocks reduced length of hospital stay (SMD ‐0.75, 95% CI ‐1.02 to ‐0.48: I² 0%). We found no effect from single injection femoral lateral cutaneous nerve block (SMD ‐0.13, 95% CI ‐0.52 to 0.26). Taking one study (Marino 2009; SD of control 1 day), the difference would be equivalent to 0.75 day.
We assessed evidence quality of continuous block only for hospital length of stay. We downgraded the level by two because 75% or more of the included trials were rated as unclear/high risk for allocation concealment and/or blinding of outcome assessor. There was no inconsistency for this subgroup. We included direct comparisons only. We downgraded by one level for low number of trials included. Publication bias could not be assessed. We rated the level of evidence as very low.
1.5.b Costs of treatment
We could not obtain data suitable for analysis for this outcome.
1.5.c Re‐hospitalization due to pain
We could not obtain data suitable for analysis for this outcome.
1.5.d Re‐hospitalization due to any other reason, including re‐operation
We could not obtain data suitable for analysis for this outcome.
1.6 Quality of life, assessed with SF‐36, Sickness Impact Profile, or other quality‐of‐life scales
We could not obtain data suitable for analysis for this outcome.
1.7 Short‐term rehabilitation milestones (within six weeks after surgery)
1.7.1 Time to start rehabilitation, e.g. time to sit up in bed
Thybo 2016 (60 participants) provided results for time to first mobilization (SMD ‐0.30, 95% CI ‐0.81 to 0.22).
1.7.2. Time to achieve rehabilitation milestones, e.g. transfer unassisted in and out of bed and ability to walk unassisted with a walker on a level surface
1.7.2.1 Walking
Based on data from two trials (Green 2014; Marino 2009; 272 participants) we did not find a difference in numbers of participants who walked on postoperative day 1: (RD 0.01, 95% CI ‐0.03 to 0.05; I² 0%; Analysis 1.18). Random sequence generation, incomplete outcome data, selective reporting and other bias were judged as at low risk for Green 2014 and for Marino 2009. Allocation concealment was judged as at unclear risk for Green 2014 and for Marino 2009. Blinding of participant and/or personnel taking care of participant was judged as unclear risk for Green 2014 and as at high risk for Marino 2009. Blinding of outcome assessment was judged as at low risk for Green 2014 and at as unclear risk for Marino 2009. Marino 2009 also reported results for numbers of participants who could walk more than 12.2 m at 48 hours (RR 0.51, 95% CI 0.03 to 7.88) for continuous femoral nerve block and (RR 11.15, 95% CI 0.67 to 184.61) for continuous psoas compartment block (P value for heterogeneity between subgroups 0.12). Given that an overall percentage of 11% of participants were able to walk more than 12.2 m at 48 hours, 618 participants (309 per group) would be required in a large trial to eliminate a difference of 50% (alpha 0.05; beta 0.2; 1‐sided test).
We rated the level of evidence for number of participants who could walk on postoperative day 1. We downgraded the level by two for risk of bias because 75% of included trials were at unclear/high risk for allocation concealment and/or blinding of outcome assessor. There was no inconsistency. We included direct comparisons only. We downgraded by one level for imprecision. Publication bias could not be assessed. We rated the evidence as very low.
1.7.2.2 Hip flexion
Two trials (Biboulet 2004 ‐ two subgroups; Singelyn 2005; 68 participants) reported results for hip flexion at seven days after surgery (MD ‐1.40°, 95% CI ‐9.04° to 6.25°; I² 43 %; Analysis 1.19.
1.7.2.3 Delayed rehabilitation
Nishio 2014 reported delayed rehabilitation due to drowsiness for 1/10 participants receiving continuous femoral nerve blocks versus 5/18 participants for systemic analgesia (RR 0.28, 95% CI 0.04 to 2.06).
1.8 Patient satisfaction on VAS, NRS, or other similar scales, or on ordinal or qualitative scales (FACES)
Köroğlu 2008 (30 participants) reported a higher satisfaction score with single injection femoral nerve block (SMD, 1.65 95% CI 0.83 to 2.48).
Four trials (Marino 2009; Siddiqui 2007; Singelyn 2005; Striebel 1993; 362 participants) (the forest plot (Analysis 1.20) displays 363 due to subgroup division) reported a higher satisfaction score with continuous peripheral nerve blocks (SMD 0.67 (95% CI 0.45 to 0.89; I² 13%; Analysis 1.20). Random sequence generation was judged as at low risk for Marino 2009; Siddiqui 2007; Singelyn 2005 and Striebel 1993. Allocation concealment was judged as at low risk for Siddiqui 2007 and as at unclear risk for Marino 2009; Singelyn 2005 and Striebel 1993. Blinding of participant and/or personnel taking care of the participant was judged as at high risk of bias for Marino 2009; Siddiqui 2007; Singelyn 2005 and Striebel 1993. Blinding of outcome assessment was judged as at unclear risk for Marino 2009; Singelyn 2005 and Striebel 1993; and as at high risk for Siddiqui 2007. Incomplete outcome data, selective reporting and other bias were judged as at low risk for Marino 2009; Siddiqui 2007; Singelyn 2005 and Striebel 1993. Egger's regression intercept showed no evidence of small‐study effect; and Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. Taking Marino 2009 (control group SD = 3.5), the difference would be equivalent to 2.4 on a scale from 0 to 10. Based on the same trial, 50 participants (25 per group) would be required in a large trial to eliminate a difference of 2 (from 9 to 7 on a scale from 0 to 10) (alpha 0.05; beta 0.2; 2‐sided test).
We rated the quality of evidence for continuous peripheral nerve blocks. We downgraded by two levels for risk of bias because 75% or more of the trials were judged as at unclear/high risk for allocation concealment and/or blinding of outcome assessor. There was no significant inconsistency. We included direct comparisons only. The optimal information size was achieved. The was no evidence of publication bias. We rated the evidence as low.
2. Comparison 2: Peripheral nerve block versus neuraxial block for postoperative analgesia
Primary outcomes
2.1 Participant‐reported pain at rest and with movement on VAS, numeric rating scale (NRS), or other similar scales or on ordinal or qualitative scales (FACES)
2.1.1 Pain at rest and with movement on arrival in the postoperative care unit
Fredrickson 2015 (50 participants) compared single injection psoas compartment block with 40 mL 0.5% bupivacaine to single injection spinal injection of 2 mL bupivacaine 0.5% with morphine 0.1 mg. Worst pain scores at rest in the postoperative care unit were higher for participants who received psoas compartment block (SMD 0.99, 95% CI 0.41 to 1.58).
Four trials (Bichel 1998; Duarte 2009; Türker 2003; Utebey 2009; 118 participants) compared continuous femoral nerve block (Bichel 1998), continuous psoas compartment block (Duarte 2009; Türker 2003), or repeated dose psoas compartment block (Utebey 2009) with epidurals. Random sequence generation and allocation concealment were judged as at low risk of bias for Duarte 2009 and as at unclear risk for Bichel 1998; Türker 2003 and Utebey 2009. Blinding of participant and/or personnel taking care of the participants was judged as at unclear risk for Duarte 2009 and Türker 2003; and as at high risk of bias for Bichel 1998 and Utebey 2009. Blinding of outcome assessment was judged as at low risk for Duarte 2009; Türker 2003; and as at unclear risk for Bichel 1998 and Utebey 2009. Incomplete outcome data was judged as at low risk for Bichel 1998; Duarte 2009; Türker 2003 and Utebey 2009. Selective reporting was judged as at low risk for Bichel 1998; Türker 2003 and Utebey 2009; and as at high risk for Duarte 2009. Other bias were judged as at low risk for Bichel 1998; Türker 2003 and Utebey 2009; and as at unclear risk for Duarte 2009. We found no difference for pain at rest on arrival in the postoperative care unit (MD 0.39 95% CI ‐0.15 to 0.94; I² 0%; Analysis 2.1). There was no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to the right of mean for an adjusted point estimate (MD 0.40, 95% CI ‐0.14 to 0.94). In Türker 2003, participants had partial hip replacement. There was no mention concerning hip fracture in the report but this intervention is often performed for patients with hip fracture. We were unable to contact the authors to establish with certainty if participants had hip fracture. When Türker 2003 was excluded from the analysis, MD was 0.41 (95% CI ‐0.47 to 1.29; I² 0%). Based on results reported byDuarte 2009 (control group SD = 3.3), 76 participants (38 per group), would be required in a large trial to decrease pain scores from 5.5 to 4.
For the level of evidence for continuous block or repeated dose, we downgraded by one level for risk of bias because 50% or more of the included trials were judged as being unclear/high risk for allocation concealment and/or blinding of outcome assessor. The optimal information size was achieved. Correcting for the possibility of publication bias would not change the conclusion. We rated the quality of evidence as moderate.
Two trials compared continuous femoral nerve (Bichel 1998) or psoas compartment block (Türker 2003) with continuous epidural for pain with movement. We did not find a difference for pain with movement on arrival in the postoperative care unit (MD 0.08, 95% CI ‐0.46 to 0.62; I² 23%; fixed‐effect model; Analysis 2.2). When Türker 2003 was excluded from the analysis, MD was 0.43 (95% CI ‐0.37 to 1.23).
2.1.2 Pain at rest and with movement from 0.5 to 2 hours after surgery
Two trials (Celidonio 2008; Forget 2009; 66 participants) compared single injection fascia iliaca block with single injection epidural block (Forget 2009), or continuous femoral nerve block with or without the addition of single parasacral sciatic nerve block with continuous epidural (Celidonio 2008). Pain at rest from 0.5 to 2 hours after surgery was higher when femoral nerve or fascia iliaca block without parasacral nerve block was used (MD 3.45, 95% CI 2.01 to 4.90; I² 30%; Analysis 2.3).
Pain with movement at two hours was higher regardless of whether parasacral sciatic nerve block was provided (MD 1.54, 95% CI 0.56 to 2,52) or not (3.50; 95% CI 1.99 to 5.01) (Celidonio 2008).
2.1.3 Pain at rest and with movement from 4 to 6 hours after surgery
Nine trials (398 participants) reported pain at rest from 4 to 6 hours after surgery. Four trials studied a continuous femoral nerve block (alone: Celidonio 2008 1 subgroup; Bichel 1998; Singelyn 2005 or with a parasacral sciatic nerve block: Celidonio 2008 (1 subgroup)). Two trials studied a fascia iliaca block (Borisov 2012; Forget 2009). Three trials studied a psoas compartment block (Duarte 2009; Souron 2003; Türker 2003). Peripheral nerve blocks were compared with an epidural (single injection: Forget 2009; continuous: Bichel 1998; Borisov 2012; Celidonio 2008; Duarte 2009; Singelyn 2005; Türker 2003) in six trials. Two trials compared peripheral nerve blocks with an intrathecal injection. The intrathecal injection contained opioids only in one trial (Souron 2003) or opioids plus a local anaesthetic (Kearns 2011).
Pain was higher with peripheral nerve blocks (SMD 0.41, 95% CI 0.11 to 0.71; I² 49%; Analysis 2.4). There was no statistically significant evidence of small‐study effect, nor significant evidence of publication bias (random‐effects model). When Türker 2003 was excluded from the analysis MD was 0.43 (95% CI 0.10 to 0.75; I² 53%). Excluding Forget 2009 (where piritramide consumption at 1 hour was higher for the peripheral nerve block group: 7.3 ± 3.5 versus 1.8 ± 3.7 mg), SMD was 0.55 (95% CI 0.34 to 0.76; I² 0%). Taking Singelyn 2005 (control group SD = 2.4), this would be equivalent to 1.3.
Seven trials (325 participants) reported results for pain with movement at 4 to 6 hours for continuous femoral nerve block (Bichel 1998; Celidonio 2008; Singelyn 2005), continuous femoral nerve block plus single injection parasacral nerve block (Celidonio 2008), fascia iliaca block (single injection: Kearns 2011; continuous block: Borisov 2012) or continuous psoas compartment block (Duarte 2009; Türker 2003) compared with continuous epidural (Bichel 1998; Borisov 2012; Celidonio 2008; Duarte 2009; Singelyn 2005; Türker 2003) or single injection intrathecal injection of an opioid and a local anaesthetic (Kearns 2011).
Pain with movement at 4 to 6 hours after surgery was higher when a peripheral nerve block was used (SMD 0.53, 95% CI 0.20 to 0.86; I² 48%; Analysis 2.5. There was no evidence of small‐study effect nor publication bias (random‐effects model). When Türker 2003 was excluded from the analysis, MD was 0.63 (95% CI 0.33 to 0.93; I² 29%).The effect size was inversely proportional to the concentration of the local anaesthetic injected as the loading dose, i.e. the difference between the two treatment groups was lower when higher concentrations were used (Figure 6; P = 0.003). Taking Duarte 2009 as a measure of variance (control group SD = 3.0), the difference would be equivalent to 1.6.

Comparison peripheral nerve block versus neuraxial block.
Meta‐regression analysis of the concentration of local anaesthetic in the initial loading dose in lidocaine equivalent versus pain with movement at 4 to 6 hours after surgery. The higher the concentration, the lower was the difference between the treatment groups (P = 0.003)
2.1.4 Pain at rest and with movement at 24 hours after surgery
Eight trials (328 participants) evaluated pain at rest at 24 hours with single injection fascia iliaca (Forget 2009; Kearns 2011), psoas compartment block (Frassanito 2008), continuous femoral nerve block (Bichel 1998; Celidonio 2008; Singelyn 2005), continuous femoral nerve block plus single injection parasacral nerve block (Celidonio 2008) or continuous psoas compartment block (Duarte 2009; Türker 2003) compared with single injection epidural (Forget 2009) or intrathecal injection of an opioid plus a local anaesthetic (Kearns 2011; Frassanito 2008) or continuous epidural (Bichel 1998; Celidonio 2008; Duarte 2009; Singelyn 2005; Türker 2003). At 24 hours, we did not find a difference between the analgesic techniques for pain at rest (SMD 0.10, 95% CI ‐0.22 to 0.42; I² 45%; Analysis 2.6). There was no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to left of mean for an adjusted point estimate 0.04 (95% CI ‐0.27 to 0.35; random‐effects model). When Türker 2003 was excluded from the analysis MD would be 0.08 (95% CI ‐0.28 to 0.44; I² 51%). Results for pain were given on a scale from 0 to 4 where 0 = no pain; 1 = light pain; 2 = moderate pain; 3 = significant pain; 4 = unbearable level of pain in Bichel 1998. Analysis excluding Bichel 1998 indicated SMD 0.20 (95% CI ‐0.03 to 0.43; I² 0%).
Seven trials (217 participants) reported results for pain with movement at 24 hours for continuous femoral nerve block (Bichel 1998; Celidonio 2008; Singelyn 2005), continuous femoral nerve block plus single injection parasacral sciatic nerve block (Celidonio 2008), continuous psoas compartment block (Duarte 2009; Fredrickson 2015; Türker 2003) or single injection fascia iliaca block (Kearns 2011) compared with continuous epidural (Bichel 1998; Celidonio 2008; Duarte 2009; Singelyn 2005; Türker 2003) or with single intrathecal injection of a local anaesthetic plus an opioid (Fredrickson 2015; Kearns 2011). We did not find a difference between peripheral nerve blocks and neuraxial blocks for pain with movement at 24 hours (SMD 0.28, 95% CI ‐0.06 to 0.62); I² 51%; Analysis 2.7) and no statistically significant evidence of small‐study effect nor publication bias. When Türker 2003 was excluded from the analysis, MD was 0.37 (95% CI 0.03 to 0.71; I² 43%).
2.1.5 Pain at rest and with movement at 48 hours after surgery
Seven trials (289 participants) evaluated pain at rest at 48 hours and compared continuous femoral nerve block (Bichel 1998; Celidonio 2008; Singelyn 2005),continuous femoral nerve block with the addition of single injection parasacral sciatic nerve block (Celidonio 2008), continuous psoas compartment block (Duarte 2009; Türker 2003) or single injection fascia iliaca block (Duarte 2009; Türker 2003) with continuous epidural (Bichel 1998; Celidonio 2008; Duarte 2009; Singelyn 2005; Türker 2003), or with single injection epidural (Forget 2009) or with single injection intrathecal injection of an opioid plus local anaesthetic (Kearns 2011). We did not find a difference between the two analgesic techniques (SMD 0.07, 95% CI ‐0.16 to 0.31; I² 22%; Analysis 2.8) and there was no statistically significant evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of two trials missing to left of mean for an adjusted point estimate (SMD ‐0.01, 95% CI ‐0.23 to 0.21; fixed‐effect model). When Türker 2003 was excluded from the analysis, MD was 0.04 (95% CI ‐0.21 to 0.29; I² 26%).
Seven trials evaluated pain with movement at 48 hours after surgery. Of these, only five (164 participants) were continuous peripheral nerve blocks: femoral nerve block with (Celidonio 2008) or without the addition of single injection parasacral sciatic nerve block (Bichel 1998; Celidonio 2008; Singelyn 2005) or psoas compartment block (Duarte 2009; Türker 2003) with continuous epidural as the comparator. We did not find a difference between the modalities (SMD 0.33, 95% CI ‐0.13 to 0.79; I² 50%; Analysis 2.9) nor statistically significant evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to left of mean for an adjusted point estimate (SMD 0.21, 95% CI ‐0.24 to 0.67; random‐effects model). When Türker 2003 was excluded from the analysis, MD was 0.41 (95% CI ‐0.12 to 0.94; I² 54%). Neuraxial block was however associated with better pain control when a ropivacaine infusion was used in the peripheral nerve block (SMD 0.80, 95% CI 0.36 to 1.24; I² 0%; P value for heterogeneity between subgroups (ropivacaine versus bupivacaine) = 0.003).
2.2 Total number of nerve block‐related complications (e.g. erythema, damage to surrounding structures, allergic reactions, infections, transient and lasting neurological damage)
Five trials (Aksoy 2014; Borisov 2012; Fredrickson 2015; Kearns 2011; Souron 2003; 334 participants) reported results for block‐related complications (any of the following: erythema, damage to surrounding structures, allergic reactions, infections, transient and lasting neurological damage): RD: 0.00 (95% CI ‐0.05 to 0.05; I² 0%; Analysis 2.10). Random sequence generation and allocation concealment were judged as at low risk for Aksoy 2014; Borisov 2012; Fredrickson 2015; Kearns 2011 and Souron 2003. Blinding of participant and/or personnel taking care of the participant was judged as at unclear risk for ;Kearns 2011 and Souron 2003 and as at high risk for Aksoy 2014; Borisov 2012; Fredrickson 2015. Blinding of outcome assessment for judged as at low risk for Aksoy 2014; Kearns 2011 and Souron 2003; as at unclear risk for Borisov 2012; and as at high risk for Fredrickson 2015. Incomplete outcome data and selective reporting were judged as at low risk for Aksoy 2014; Borisov 2012; Fredrickson 2015; Kearns 2011 and Souron 2003. Other bias were judged as at low risk for Fredrickson 2015; Kearns 2011 and Souron 2003; and as at unclear risk of bias for Aksoy 2014 and Borisov 2012. There was no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to right of mean for an adjusted point estimate (RD 0.01, 95% CI ‐0.02 to 0.04). Based on 5% incidence, a large trial would need to include 6620 participants (3310 per group) to eliminate a 25% difference (alpha 0.05; beta 0.2; 1‐sided test).
For quality of evidence for total number of nerve block‐related complications, we did not downgrade for risk of bias because less than 50% was judged as at high/unclear risk for allocation concealment and/or blinding of outcome assessors. There was no inconsistency. We used direct comparisons only. We downgraded by two levels for imprecision due to a very low number of participants included. We did not downgrade for publication bias because applying a correction for a potential one would not modify the conclusion. We rated the quality as low.
Secondary outcomes
2.1 Analgesic requirements
Four trials (Bhatia 2008; Frassanito 2008; Souron 2003; Utebey 2009; 203 participants) reported an higher dose opioids with psoas compartment blocks (single injection blocks: Frassanito 2008; Souron 2003; repeated doses: Bhatia 2008; Utebey 2009) compared with single injection intrathecal injection of an opioid (Souron 2003), with an opioid plus a local anaesthetic (Bhatia 2008; Frassanito 2008) or with a repeated doses epidural (Utebey 2009) (SMD 0.67, 95% CI 0.04 to 1.31; I² 77%; Analysis 2.11). There was no statistically significant evidence of small‐study effect nor publication bias. The effect was clearer when single injection peripheral nerve blocks were compared to single injection intrathecal injections (P value for heterogeneity between subgroups 0.003; Analysis 2.11).
2.2 Minimal clinically important improvement in pain
A mean difference of 2 or more between peripheral nerve blocks and neuraxial blocks was found for pain at rest and with movement (without the addition of a parasacral sciatic nerve block) from 0.5 to 2 hours after surgery (Analysis 2.3).
2.3 Complications related to specific method of treatment
See Table 1
2.3.1 Complications from peripheral nerve blocks
Five non functional catheters were reported: no analgesia with a fascia iliaca block (Borisov 2012: n = 1), kinked catheters with psoas compartment bocks (Türker 2003: n = 2) and psoas compartment block catheter occlusions (blood clots) (Marino 2009: n = 2). Epidural extension of psoas compartment block was reported in Biboulet 2004 (n = 4), Frassanito 2008 (n = 1), Marino 2009 (n = 5), Stevens 2000 (n = 3) and Utebey 2009 (n = 2). Two vascular punctures during psoas compartment blocks were reported by Souron 2003. Six transient neurological symptom events suggestive of local irritation were reported by Fredrickson 2015 after psoas compartment blocks. Kearns 2011 reported a transient femoral nerve palsy that resolved at three months following single injection ultrasound guided (out‐of‐plane technique) fascia iliaca block.
2.3.2 Complications from neuraxial blocks
Fredrickson 2015 reported transient neurological symptoms suggestive of local irritation (n = 8 for spinal anaesthesia). Six epidural catheters problems were reported: five contralateral analgesia (Singelyn 2005: n = 3 and Türker 2003: n = 2) and one kinked catheter (Türker 2003). Fredrickson 2015 also reported two inpatient falls with intrathecal morphine.
2.4 General medical complications within six weeks after surgery
2.4.a Gastrointestinal: nausea and vomiting, constipation, ileus
Data were provided in two trials (Fredrickson 2015; Souron 2003; 30 participants). We did not find a difference for vomiting (Souron 2003) or postoperative nausea and vomiting (Fredrickson 2015) (RR 0.79, 95% CI 0.44 to 1.39; I² 0%; Analysis 2.12).
2.4.b Pulmonary: pneumonia, bronchitis
None of the included studies reported these outcomes.
2.4.c Cardiovascular: hypotension, myocardial infarction, blood loss and blood transfusion
Eight trials (Aksoy 2014; Bhatia 2008; Frassanito 2008; Kearns 2011; Singelyn 2005; Souron 2003; Türker 2003; Utebey 2009; 429 participants) reported on hypotension. We did not find a difference in risk of hypotension (RD ‐0.09, 95% CI ‐0.22 to 0.03; I² 83%; Analysis 2.13). Egger's regression intercept showed no statistical evidence of small‐study effect (2‐sided test). Analysis using Duval and Tweedie's trim and fill method indicated imbalance of two trials missing to right of mean for an adjusted point estimate (RD ‐0.02, 95% CI ‐0.13 to 0.10; random‐effects model). When Türker 2003 was excluded from the analysis, RD was ‐0.05 (95% CI ‐0.16 to 0.06; I² 78%). Hypotension risk decreased when peripheral nerve blocks were compared to continuous neuraxial blocks (RD ‐0.34, 95% CI ‐0.48 to ‐0.20; 3 trials, 113 participants; I² 0%; NNTB = 4, 95% CI 3 to 6).
Aksoy 2014 (70 participants) reported no myocardial infarctions.
Five trials (Aksoy 2014; Bichel 1998; Duarte 2009; Türker 2003; Utebey 2009; 188 participants) reported intra‐operative blood loss (MD ‐8.84 mL, 95% CI ‐80.82 to 63.14 mL; I² 83%; Analysis 2.14) with no statistically significant evidence of small‐study effect (2‐sided test). Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to left of mean for an adjusted point estimate ‐0.04 (95% CI ‐0.71 to 0.63; random‐effects model). When Türker 2003 was excluded from the analysis, MD was ‐22.75 (95% CI ‐109.88 to 64.38; I² 87%), and when Utebey 2009 was excluded, MD was 39.94 mL (95% CI 18.05 to 61.84 mL; I² 0%).
Three trials with 87 participants provided results for intra‐operative (Utebey 2009) or total number of units transfused (Duarte 2009; Singelyn 2005) (MD ‐0.23 units, 95% CI ‐0.65 to 0.19 units; I² 33%; Analysis 2.15). There was no statistically significant evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of two trials missing to right of mean for an adjusted point estimate (MD 0.10 unit, 95% CI ‐0.36 to 0.55 unit; random‐effects model).
2.4.d Neurological: acute confusional state, drowsiness, cerebrovascular accident, postoperative cognitive dysfunction
Fredrickson 2015 (50 participants) evaluated the number of participants with confusion at 48 hours (RR 0.29, 95% CI 0.01 to 6.69). For Fredrickson 2015, random sequence generation, allocation concealment, incomplete outcome data, selective reporting and other bias were judged as at low risk of bias and blinding of participant, personnel taking care of the participant and outcome assessment were judged as at high risk of bias.
We downgraded the quality of evidence for acute confusional state by one level for risk of bias for absence of blinding of outcome assessor. Inconsistency and publication bias could not be assessed. The trial is a direct comparison. We downgraded the quality by two levels for imprecision. We rated the quality as very low.
Three trials (176 participants) evaluated drowsiness at 1 hour (Forget 2009) or up to 48 hours after surgery (Kearns 2011; Souron 2003) (RD 0.04, 95% CI ‐0.07 to 0.15; I² 75%; Analysis 2.16). There was no statistically significant evidence of small‐study effect nor publication bias (random‐effects model).
Aksoy 2014 (70 participants) measured mini mental tests 24 hours after surgery (SMD 0.05, 95% CI ‐0.42 to 0.52).
2.4.e Thromboembolic complications: deep venous thrombosis or pulmonary embolism
Two trials (Aksoy 2014; Türker 2003) with 100 participants reported no deep venous thrombosis.
Aksoy 2014 (70 participants) assessed pulmonary embolism and reported no events.
2.4.f Other medical complications
2.4.f.1 Risk of pruritus
From six trials (Frassanito 2008; Fredrickson 2015; Kearns 2011; Souron 2003; Türker 2003; Utebey 2009; 299 participants, peripheral nerve blocks reduced the risk of pruritus compared to neuraxial blocks (RR 0.33, 95% CI 0.19 to 0.58; I² 0%; Analysis 2.18). Random sequence generation was judged as at low risk of bias for Fredrickson 2015; Kearns 2011 and Souron 2003; and as at unclear risk for Frassanito 2008; Türker 2003 and Utebey 2009. Allocation concealment was judged as at low risk of bias for Fredrickson 2015; Kearns 2011; Souron 2003; at unclear risk for Frassanito 2008; Türker 2003 and Utebey 2009. Blinding of participant and personnel taking care of the participants was judged as at low risk for Türker 2003; as at unclear risk for Frassanito 2008; Kearns 2011; Souron 2003; and as at high risk for Fredrickson 2015 and Utebey 2009. Blinding of outcome assessment was judged as at low risk for (Frassanito 2008; Kearns 2011; Souron 2003 and Türker 2003; as at unclear risk for Utebey 2009; and as at high risk for Fredrickson 2015. Incomplete outcome data, selective reporting and others bias were judged as at low risk of bias for Frassanito 2008; Fredrickson 2015; Kearns 2011; Souron 2003; Türker 2003 and Utebey 2009.
There was no statistically significant evidence of small‐study effect nor publication bias (fixed‐effect model). When Türker 2003 was excluded from the analysis, RR was 0.33, 95% CI 0.18 to 0.61; I² 0%). Based on an incidence rate of 26% the NNTB would be 6 (95% CI 5 to 9) and 1028 participants (514 per group) would be required in a large trial to eliminate a 25% difference (alpha 0.05; beta 0.2; 1‐sided test).
For level of quality of the body of evidence for reduced pruritus, we did not downgrade for risk of bias because less than 50% of the trials were judged as at unclear/high risk of bias for allocation concealment and/or blinding of outcome assessor. There was no inconsistency, We included trials with direct comparisons only. We downgraded the level by one for imprecision because the optimal information size was not achieved. There was no evidence of publication bias. We rated the level as moderate.
2.4.f.2 Risk of respiratory depression
Based on data from four trials (Frassanito 2008; Fredrickson 2015; Kearns 2011; Souron 2003; 213 participants), we did not find a difference in the risk of respiratory depression between treatments (RD 0.01, 95% CI ‐0.03 to 0.05; I² 0%; Analysis 2.19). There was no statistically significant evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to right of mean for an adjusted point estimate (RD 0.00, 95% CI ‐0.02 to 0.03; fixed‐effect model). Considering an incidence rate of 1%, 34,318 participants (17,159 per group) would be required to eliminate a 25% difference (alpha 0.05; beta 0.2; 1‐sided test).
2.5 Use of resources
2.5.a Hospital length of stay
From two trials (Duarte 2009; Singelyn 2005) with 64 participants we did not find a difference in hospital length of stay (MD 0.19 days, 95% CI ‐0.39 to 0.77 days; I² 0%; Analysis 2.20). Random sequence generation was judged as at low risk of bias for Duarte 2009 and Singelyn 2005. Allocation concealment was judged as at low risk for Duarte 2009 and as at unclear risk for Singelyn 2005. Blinding of participant and/or personnel taking care of the participant was judged as at unclear risk for Duarte 2009 and as at high risk for Singelyn 2005. Blinding of outcome assessment for judged as at low risk of bias for Duarte 2009 and as at unclear risk for Singelyn 2005. Duarte 2009 was judged as at low risk for incomplete outcome data, at high risk for selective reporting and as at unclear risk for other bias. Singelyn 2005 was judged as at low risk for all those three domains.
We downgraded the level of evidence for hospital length of stay by one level for risk of bias because 50% or more of the trials were judged as at unclear/high risk for allocation concealment and/or blinding of outcome assessor. There was no inconsistency and trials were direct comparisons. We downgraded by one level for low number of trials. Publication bias could not be assessed. We rated the quality as low.
2.5.b Cost of treatment
We found no data suitable for analysis for this outcome.
2.6 Quality of life, assessed with the SF‐36, Sickness Impact Profile, or other quality‐of‐life scales
We found no data suitable for analysis for this outcome.
2.7 Short‐term rehabilitation milestones (within 6 weeks after surgery)
2.7.1 Time to start rehabilitation, e.g. time to sit up in bed
Two trials evaluated time from bed to chair while comparing a single‐injection block to morphine intrathecal injection (Kearns 2011; SMD 0.41, 95% CI 0.02 to 0.80) or continuous psoas compartment block to epidural local anaesthetic and opioid infusion (Duarte 2009; SMD ‐0.14, 95% CI ‐0.75 to 0.47; Analysis 2.21).
Fredrickson 2015 provided results for numbers of participants in chairs at 24 hours (20/27 versus 19/23) or at 48 hours (26/27 versus 22/23) for peripheral nerve block versus neuraxial block, respectively.
Kearns 2011 reported results for numbers of participants becoming mobile at first attempt (38/52 versus 44/51) for peripheral nerve block versus neuraxial block, respectively.
2.7.2 Walking
From three trials (Duarte 2009; Singelyn 2005; Türker 2003) with 94 participants we did not find a difference in time to first walk (SMD ‐0.41, 95% CI ‐1.09 to 0.27; I² 69%; Analysis 2.22'). Random sequence generation was judged as at low risk of bias for Duarte 2009 and Singelyn 2005; and as at unclear risk for Türker 2003. Allocation concealment was judged as at low risk for Duarte 2009 and as at unclear risk for Singelyn 2005 and Türker 2003. Blinding of participant and personnel taking care of participant was judged as at low risk for Türker 2003; as at unclear risk for Duarte 2009; and as at high risk for Singelyn 2005. Blinding of outcome assessment was judged as at low risk for Duarte 2009 and Türker 2003; and as at unclear risk for Singelyn 2005. Incomplete outcome data was judged as at low risk for Duarte 2009; Singelyn 2005 and Türker 2003. Selective reporting was judged as at low risk for Singelyn 2005 and Türker 2003; and at high risk for Duarte 2009. Other bias were judged as at low risk for Singelyn 2005 and Türker 2003; and as at unclear risk for Duarte 2009. There was no statistically significant evidence of small‐study effect (2‐sided test) nor evidence of publication bias. When Türker 2003 was excluded from the analysis, MD was ‐0.08 (95% CI ‐0.49 to 0.32; I² 0%). The comparison favoured peripheral nerve blocks when larger doses were used (P value for the meta‐regression 0.03).
We downgraded the level by one because 50% or more of the trials were judged as at unclear/high risk for allocation concealment and/or blinding of outcome assessor. We did not downgrade for inconsistency because reasonable explanation was found for heterogeneity. All trials included were direct comparisons. There was no evidence of publication bias. We rated the quality as low.
Fredrickson 2015 reported numbers of participants walking at 24 hours (15/27 versus 21/23) and 48 hours (24/27 versus 21/23) for peripheral nerve block versus neuraxial block, respectively.
Duarte 2009 reported time required to wean off the walker (MD 0.00, 95% CI ‐1.75 to 1.75).
2.7.3 Hip flexion
Singelyn 2005 reported degree of hip flexion from postoperative days 1 to 10. Mean differences were: MD 2.00° (95% CI ‐12.16° to 16.16°) and 0.00° (95% CI ‐6.59° to 6.59°) at postoperative days three and seven, respectively.
2.7.4 Duration of physiotherapy and daily life activity
Duarte 2009 reported duration of functional physiotherapy required (MD ‐0.30 day. 95% CI ‐1.13 to 0.53 day).
Duarte 2009 also reported time required to perform activities of daily life (MD 0.30 day, 95% CI ‐1.20 to 1.80 day).
2.8 Patient satisfaction on VAS, NRS, or other similar scales, or on ordinal or qualitative scales (FACES)
From six trials (Borisov 2012; Frassanito 2008; Kearns 2011; Singelyn 2005; Souron 2003; Türker 2003) with 307 participants, we did not find a difference in patients satisfaction scores: (SMD 0.08, 95% CI ‐0.32 to 0.48; I² 64%; Analysis 2.23). Random sequence generation was judged as at low risk of bias for Borisov 2012; Kearns 2011; Singelyn 2005 and Souron 2003; and as at unclear risk of bias for Frassanito 2008; Türker 2003. Allocation concealment was judged as at low risk of bias for Borisov 2012; Kearns 2011 and Souron 2003; and as at unclear risk of bias for Frassanito 2008; Singelyn 2005 and Türker 2003. Blinding of participant and/or personnel taking care of the participants was judged as at unclear risk for Frassanito 2008; Kearns 2011; Souron 2003 and Türker 2003; and as at high risk for Borisov 2012 and Singelyn 2005. Blinding of outcome assessment was judged as at low risk for Borisov 2012; Frassanito 2008; Kearns 2011; Souron 2003 and Türker 2003; and as at unclear risk for Singelyn 2005. Incomplete outcome data was judged as at low risk for Borisov 2012; Frassanito 2008; Kearns 2011; Singelyn 2005; Souron 2003 and Türker 2003). Selective reporting was judged as at low risk for Borisov 2012; Frassanito 2008; Kearns 2011; Singelyn 2005; Souron 2003 and Türker 2003. Other bias were judged as at low risk for Frassanito 2008; Kearns 2011; Singelyn 2005; Souron 2003 and Türker 2003; and as at unclear risk for Borisov 2012. There was no evidence of small‐study effect. Analysis using Duval and Tweedie's trim and fill method indicated imbalance of one trial missing to left of mean for an adjusted point estimate SMD ‐0.05, 95% CI ‐0.47 to 0.38; random‐effects model. When Türker 2003 was excluded from the analysis, SMD was 0.06 (95% CI ‐0.41 to 0.53; I² 71%). There may be a difference between treatment modalities in favour of neuraxial blocks when continuous peripheral nerve blocks are used (SMD 0.54, 95% CI 0.16 to 0.31; Analysis 2.23; equivalent to 0.8 on a scale from 0 to 10 (taking Singelyn 2005 with an SD of 1.4 in the control group); P value for heterogeneity between subgroups = 0.008). On the other hand, meta‐regression analysis showed that older participants were more likely to prefer peripheral nerve blocks than neuraxial blocks (P = 0.001; Figure 7). From Singelyn 2005 (control group SD = 1.4), the number of participants included was sufficient to eliminate a change from 9 to 7.

Comparison peripheral nerve block versus neuraxial block
Meta‐regression patient satisfaction versus age. Peripheral nerve block was more appreciated by older participants (P = 0.001).
This figure was generated with Comprehensive Meta‐analysis, the software expressed years with decimals.
We did not downgrade for risk of bias because fewer than 50% of the trials were judged as at unclear/high risk of bias for allocation concealment and/or blinding of outcome assessors. We did not downgrade for inconsistency because we found reasonable explanation for heterogeneity. Trials were direct comparisons. We did not downgrade for imprecision. We did not downgrade for publication bias because applying a correction would not modify the conclusion. We rated the quality as high.
3. Comparison 3: Peripheral nerve block versus local anaesthetic infiltration
We identified three ongoing trials (NCT02658149; NCT02658240; NCT02720471) and one trial published after the search (NCT02568995) comparing peripheral nerve blocks to local anaesthetic infiltration. These trials will be assessed for inclusion and results presented in a future review update if appropriate.
4. Comparison4: Peripheral nerve block versus intravenous lidocaine
One small trial (Thomas 2009), with 20 participants published in abstract form only, compared continuous femoral nerve block with intravenous infusion of lidocaine (1.5 mg/kg followed by 2 mg/kg/hour intra‐operatively and 1.5 mg/kg/hour for 24 hours postoperatively). They did not find a difference for intravenous patient‐controlled morphine consumption at 24 hours (MD ‐2.0 mg, 95% CI ‐18.0 to 14.0 mg). Consumption from 24 to 48 hours was higher for participants who received continuous femoral nerve block (MD 11.0 mg, 95% CI 1.4 to 20.6 mg). The study authors did not report a difference for pain scores at 24 hours (MD ‐0.4, 95% CI ‐2.1 to 1.3), 48 hours (MD 0.2, 95%, CI ‐2.13 to 2.5) or three months (MD ‐0.3, 95% CI ‐1.1 to 0.5) nor for degree of flexion (time point unspecified).
Discussion
We included 51 studies with 2793 participants in the review (1448 participants randomized to undergo peripheral nerve blocks and 1345 to control groups). Of the 51 studies, we included 45 trials (2491 participants) in the analysis (1288 ‐ peripheral nerve blocks; 1203 ‐ controls).
We found that peripheral nerve blocks provide better quality pain relief compared to systemic analgesia (moderate‐quality evidence). The difference was equivalent to 3.2 on a scale from 0 to 10 for pain at rest on arrival in the postoperative care unit (summary of findings Table for the main comparison). Pain control provided by peripheral nerve block was equivalent to neuraxial block (summary of findings Table 2).
In 2000, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) suggested that pain should be considered as the fifth vital sign, and that under‐treatment of pain would constitute abrogation of a fundamental human right (White 2007). After this statement was issued, an increase in the use of opioids for acute postoperative pain treatment was observed, together with increased side effects from opioid use (White 2007). It was also noted that postoperative critical respiratory events were encountered more frequently during the first 24 hours after opioid therapy was introduced (Ramachandran 2011).
We found one reported adverse event where a participant experienced respiratory depression from systemic opioids (intravenous patient‐controlled analgesia with morphine; Siddiqui 2007). Fortunately, the event was without serious consequence. Finding alternatives to systemic opioids for postoperative pain treatment is a clinical goal. Ensuring that alternate treatment provides at least equal analgesia without undue increased risk of harm is imperative.
A previous Cochrane Review found that neuraxial blocks were superior to systemic analgesia for postoperative abdominal surgery pain treatment (Guay 2016a). Results from the current review confirm that peripheral nerve blocks provides superior pain control for elective primary total hip replacement (moderate‐quality evidence). We found no major differences between peripheral nerve block and neuraxial blocks (summary of findings Table 2; moderate‐quality evidence).
We included a range of peripheral nerve blocks in this Review. Although comparing efficacy among peripheral nerve block types was not an objective of this review, some results suggest a higher efficacy of psoas compartment blocks for elective hip replacement. This would seem true for pain at rest at 0.5 to 2 hours (Analysis 1.2), pain at rest and on movement at 4 to 6 hours after surgery (Analysis 1.3; Analysis 1.4) and pain on movement at 24 and 48 hours (Analysis 1.6; Analysis 1.8). However, although psoas compartment blocks seem relatively safe in the hands of well‐trained operators (Njathi 2017), better efficacy needs to be weighed against the fact that psoas compartment blocks require a higher level of anaesthesiologist expertise (Hargett 2005) and are considered to be deep blocks making them relatively contraindicated for people with altered coagulation/haemostasis (Horlocker 2010).
Although the incidence of major complications associated with neuraxial blocks are roughly equivalent to peripheral nerve blocks (2 to 4 per 10,000; Auroy 2002; Barrington 2013; Neal 2015; Orebaugh 2012; Sites 2012), serious adverse events associated with peripheral nerve blocks are often less devastating with better prognosis. We found one reported incidence of neurological complication that lasted for more than three months (Siddiqui 2007). This event related to a participant with prolonged prothrombin time (international normalized ratio 5.6 measured on postoperative day 3) after psoas compartment block. Psoas compartment block should be treated as a neuraxial block (deep block, not a compressible site) (Horlocker 2010). When warfarin is started before surgery, measurement of international normalized ratio before neuraxial/deep block performance is suggested (Horlocker 2010). Additional daily measurements are recommended during the time the catheter is in situ (Horlocker 2010). Catheter withdrawal is advocated when the international normalized ratio is < 1.5 (Horlocker 2010).
A transient (< 3 months) femoral nerve paresis potentially associated with an out‐of‐plane ultrasound‐guided fascia iliaca block was reported (Kearns 2011). Although ultrasound guidance reduces inadvertent intravascular needle penetration in adults (Lewis 2015), there is no clear evidence that ultrasound guidance reduces the risk of neurological complications (Guay 2016b; Lewis 2015; Neal 2015). As opposed to an in‐plane technique where a clear visualization of the needle shaft is required through the entire procedure, an out‐of‐plane needle approach offers only a glimpse of the needle tip position. Although there are scant data to guide the needle approach for ultrasound‐guided regional anaesthesia for hip arthroplasty, evidence extrapolated from ultrasound‐guided vascular access literature suggests that damage to surrounding structures may be reduced by using an in‐plane technique (Berk 2013). Further studies on ultrasound guidance for regional blockade could be useful.
An included study reported two instances of falls by inpatients (Fredrickson 2015). Both falls occurred in participants who had received 0.1 mg morphine intrathecally. Some concerns about the risk of inpatient falls have been raised in relation to use of continuous lower limb peripheral nerve blocks for postoperative analgesia. Detailed analysis revealed that attributable risk for patients who had undergone continuous peripheral nerve block was not outside the expected probability of postoperative falls after orthopaedic surgery (Johnson 2013). A large retrospective trial found that risk for inpatient falls was higher among older patients, those with higher comorbidity burden and more major complications, but that the use of peripheral nerve blocks was not significantly associated with inpatient falls (Memtsoudis 2014). Inpatient falls occur mainly while patients are in their own rooms (while in the bathroom, going to and from the bathroom, or using a bedside commode) (Johnson 2014). Regardless of use of peripheral nerve block, fall prevention strategies should continue to provide education for all patients and reinforce practices to monitor patients in their hospital rooms (Johnson 2014).
We also found that peripheral nerve blocks may be associated with reduced risk of postoperative acute confusional state (very low quality‐evidence; summary of findings Table for the main comparison). The pathophysiology of acute confusional state may be multifactorial and include side effects of medications used, hypoxaemia, immobilization, infection and systemic inflammation (Mouzopoulos 2009). Peripheral nerve blocks (or local anaesthetics) may influence these factors. Further studies are needed to confirm these findings and shed some light on the exact mechanisms involved.
Pruritus is an annoying side effect associated with opioid administration and may be challenging to treat without partial reversal of analgesic effects, especially when administered intrathecally (Alhashemi 1997). Interestingly, continuous peripheral nerve blocks reduced the risk of pruritus compared to systemic analgesia (NNTB 4, 95% CI 4 to 8; very low‐quality evidence; summary of findings Table for the main comparison) or neuraxial blocks (NNTB 6, 95% CI 5 to 9; moderate‐quality evidence; summary of findings Table 2). Compared with systemic analgesia, we speculated that continuous peripheral nerve blocks conferred a reduced risk of pruritus attributable to reduced systemic opioids (SMD ‐0.62, 95% CI ‐0.89 to ‐0.35; Analysis 1.9).
We also found a modest reduction of hospital length of stay associated with peripheral nerve blocks compared to systemic analgesia (equivalent to 0.75 day; very low‐quality evidence). Although the reduction was relatively small, considering the large numbers of procedures performed each year (over 300,000 total hip replacements annually in the USA alone) and that projections estimate further future increases (Kurtz 2007), even a small reduction may have important economic impact. Future studies on costs would be useful.
We did not find differences in short‐term rehabilitation milestones.
Compared to systemic analgesia, patient satisfaction was higher when peripheral nerve blocks were used for postoperative analgesia following primary elective hip arthoplasty (equivalent to 2.4 on a scale from 0 to 10; low‐quality evidence; summary of findings Table for the main comparison) and comparable to satisfaction reported for neuraxial blocks (summary of findings Table 2).
Summary of main results
Compared to systemic analgesia alone, there is evidence that peripheral nerve blocks reduce postoperative pain, acute confusional status, pruritus and hospital length of stay.
Compared to neuraxial blocks, there is evidence that peripheral nerve blocks reduce pruritus.
Adverse events were rare. With peripheral nerve blocks, there were two neurological injuries (Kearns 2011; Siddiqui 2007). One injury lasted less than three months and the other one was still partially unresolved at three months. The latter might may have been caused by abnormal blood clotting. It is therefore possible that the injury that was not fully resolved at three months may have been avoidable had guidelines and recommendations regarding block placement in patients receiving medications altering blood coagulation been followed (Horlocker 2010). Two inpatient falls were reported in patients receiving morphine‐like substances in the spine (Fredrickson 2015). One patient with intravenous morphine experienced respiratory depression (Siddiqui 2007).
Overall completeness and applicability of evidence
We are confident that our results reflect the actual available evidence on the benefits of peripheral nerve blocks in patients undergoing elective primary hip arthroplasty. Although techniques used in the included trials required a certain amount of expertise, they reflect contemporary clinical practice. Until recently, peripheral nerve blocks were generally used only by a limited group of experienced practitioners. However, widespread use of ultrasound guidance technique has increased and the number of clinicians using peripheral nerve blocks for postoperative analgesia is rapidly growing.
Pain has been the most frequent outcome studied to illustrate the potential benefits of peripheral nerve blocks. The body of evidence for other important outcomes, such as reduced risk of acute confusional state, faster mobilization and reduced length of hospital stay, is still relatively sparse and could be enhanced.
Although ultrasound guidance has increased the number of anaesthesiologists providing peripheral nerve blocks to their patients, a recent study performed on 12,911,056 patients undergoing ambulatory surgery indicate that only 3.3% of the patients who could have benefited from a peripheral nerve block did indeed received one (Gabriel 2017). Authors concluded that future studies are necessary to identify barriers and disparities in care.
Quality of the evidence
We assessed the evidence according to GRADE recommendations (GRADEpro GDT) for all outcomes and presented results in 'Summary of findings' tables (summary of findings Table for the main comparison; summary of findings Table 2)..
For peripheral nerve blocks compared to systemic analgesia, the evidence quality was rated as moderate for pain, low for patient satisfaction and very low for pruritus, acute confusional state, hospital length of stay and number of participants walking at postoperative day one.
For peripheral nerve blocks compared to neuraxial blocks, the quality of evidence was rated as high for patient satisfaction, moderate for pain and pruritus, low for total number of block‐related complications, hospital length of stay and time to first walk, and very low for acute confusional state.
Evidence quality was downgraded to low or very low due to flawed study designs and limited numbers of trials and participants.
Potential biases in the review process
Following an extensive search, we are confident that our results reflect the published evidence relating to nerve blocks following primary hip arthroplasty. We also think that our extensive heterogeneity exploration, based on a priori chosen factors, allowed us to draw valid conclusions on the benefits of the various peripheral nerve blocks used for elective primary hip arthroplasty. We examined the effects of interventions based on technique (psoas compartment blocks, femoral nerve blocks, fascia iliaca blocks etc.) localization methods (ultrasound or nerve stimulator guidance), drug type (lidocaine, bupivacaine, ropivacaine etc.) and doses, concentrations or administration methods (single injection versus continuous infusion). Assessments of these trial aspects are clinically relevant and strengthen review findings.
Agreements and disagreements with other studies or reviews
This is the first version of this review. Our finding that peripheral nerve blocks reduce pain compared to systemic analgesia is consistent with other non‐Cochrane reviews (Barreveld 2013; Højer Karlsen 2015; Stein 2012). This also stands for reductions in opioid use at 24 hours and risk of pruritus with psoas compartment blocks (Højer Karlsen 2015). A reduced length of hospital stay was also advocated by McGraw 2012. In their review on the effects of peripheral nerve blocks on long‐term functional outcome after elective large joint replacement, Atchabahian 2015 did not detect a difference in range of motion with the use of peripheral nerve blocks for postoperative analgesia. This finding is consistent with our results; we did not detect a difference for walking on postoperative day one or for hip flexion at 7 days with peripheral nerve blockade versus systemic analgesia alone (summary of findings Table for the main comparison).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison peripheral nerve block versus no block or sham block or systemic analgesia
Meta‐regression analysis of effect of peripheral nerve block on pain on arrival in postoperative care unit versus volume of the local anaesthetic injected as the loading dose. The effect size was proportional to the concentration used (P < 0.00001).
The largest effect size (standardized mean difference (SMD) ‐4.56, 95% CI ‐5.91 to ‐3.20) was obtained by Köroğlu 2008 where a 3‐in‐1 block with 40 mL 0.25% bupivacaine was compared to a sham block and postoperative analgesia was completed with IV patient‐controlled analgesia with tramadol plus IV meperidine on request.

Comparison peripheral nerve blocks versus no block or sham block or systemic analgesia.
Meta‐regression of pain on arrival in postoperative care unit versus local anaesthetic dose in lidocaine equivalent injected as loading dose. The effect size was proportional to the dose injected (P = 0.0001).
The largest effect size (SMD ‐4.56, 95% CI ‐5.91 to ‐3.20) was obtained by Köroğlu 2008 where a 3‐in‐1 block with 40 mL 0.25% bupivacaine was compared to a sham block and postoperative analgesia was completed with IV patient‐controlled analgesia with tramadol plus IV meperidine on request.

Comparison peripheral nerve block versus neuraxial block.
Meta‐regression analysis of the concentration of local anaesthetic in the initial loading dose in lidocaine equivalent versus pain with movement at 4 to 6 hours after surgery. The higher the concentration, the lower was the difference between the treatment groups (P = 0.003)

Comparison peripheral nerve block versus neuraxial block
Meta‐regression patient satisfaction versus age. Peripheral nerve block was more appreciated by older participants (P = 0.001).
This figure was generated with Comprehensive Meta‐analysis, the software expressed years with decimals.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 1 Pain at rest on arrival at postoperative care unit.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 2 Pain at rest from 0.5 to 2 hours after surgery.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 3 Pain at rest at 4 to 6 hours after surgery.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 4 Pain on movement at 4 to 6 hours after surgery.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 5 Pain at rest at 24 hours after surgery for single injection block with clonidine or continuous nerve block.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 6 Pain on movement at 24 hours after surgery for continuous peripheral nerve block.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 7 Pain at rest at 48 hours after surgery.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 8 Pain on movement at 48 hours after surgery.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 9 Analgesic requirements: opioids consumption from 0 to 24 hours.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 10 Gastrointestinal complications: postoperative nausea and vomiting.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 11 Cardiovascular complications: hypotension.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 12 Cardiovascular complications: intra‐operative blood losses (mL).

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 13 Cardiovascular complications: number of blood units transfused.
Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 14 Neurological complications: drowsiness up to 48 hours after surgery.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 15 Pruritus first 48 hours.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 16 Respiratory depression.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 17 Hospital length of stay.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 18 Walking on postoperative day 1.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 19 Hip flexion at 7 days.

Comparison 1 Peripheral nerve blocks versus no block, sham block or systemic analgesia, Outcome 20 Patient satisfaction for continuous peripheral nerve block.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 1 Pain at rest on arrival in postanaesthesia care unit.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 2 Pain with movement at arrival in postanaesthesia care unit.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 3 Pain at rest from 0.5 to 2 hours after surgery.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 4 Pain at rest at 4 to 6 hours after surgery.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 5 Pain with movement at 4 to 6 hours after surgery.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 6 Pain at rest at 24 hours after surgery.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 7 Pain with movement at 24 hours.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 8 Pain at rest at 48 hours after surgery.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 9 Pain with movement at 48 hours (continuous peripheral nerve blocks only).

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 10 Total number of nerve block‐related complications.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 11 Analgesic requirements: opioids consumption from 0 to 24 hours.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 12 Gastrointestinal complications: postoperative nausea and vomiting.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 13 Cardiovascular complications: hypotension.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 14 Cardiovascular complications: intra‐operative blood losses (mL).

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 15 Cardiovascular complications: number of units transfused.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 16 Neurological complications: drowsiness up to 48 hours after surgery.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 17 Thromboembolic complications: deep venous thrombosis.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 18 Pruritus.
Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 19 Respiratory depression.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 20 Hospital length of stay.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 21 Time to start rehabilitation: bed to chair.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 22 Walking.

Comparison 2 Peripheral nerve blocks versus neuraxial blocks, Outcome 23 Patient satisfaction.
| Peripheral nerve blocks compared to systemic analgesia for elective primary total hip replacement | ||||||
| Patient or population: adults undergoing elective primary total hip replacement | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Peripheral nerve blocks | |||||
| Pain at rest on arrival in postoperative care unit | CD | The mean pain at rest on arrival in postoperative care unit in the intervention groups was | SMD ‐1.12 (‐1.67 to ‐0.56) | 429 | ⊕⊕⊕⊝ | Equivalent to 3.2 on a scale from 0 to 10 |
| Total number of nerve block‐related complications | NA | 1 local haematoma 1 delayed paresis | NA | NA | NA | Examples: erythema, damage to surrounding structures, allergic reactions, infections, transient and lasting neurological damage |
| Acute confusional state | Study population | RR 0.10 | 225 | ⊕⊝⊝⊝ | Number needed to treat for additional benefit: 12 (95% CI 11 to 22) | |
| 133 per 1000 | 13 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 5 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 15 per 1000 | |||||
| Pruritus | Study population | RR 0.16 | 259 | ⊕⊝⊝⊝ | Continuous peripheral nerve blocks only Number needed to treat for additional benefit: 4 (95% CI 4 to 8) | |
| 161 per 1000 | 26 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 8 per 1000 | |||||
| High | ||||||
| 250 per 1000 | 40 per 1000 | |||||
| Hospital length of stay | CD | The mean hospital length of stay in the intervention groups was | SMD 0.75 (‐1.02 to ‐0.48) | 249 | ⊕⊝⊝⊝ | Continuous peripheral nerve blocks only Equivalent to 0.75 day |
| Walking at postoperative day 1 | Number of participants walking at postoperative day 1 | The risk difference was 0.01 (‐0.03 to 0.05) | RD 0.01 (‐0.03 to 0.05) | 278 | ⊕⊝⊝⊝ | One trial evaluated effect with single injection block and the other with continuous peripheral nerve block |
| Patient satisfaction | CD | The mean patient satisfaction in the intervention groups was | SMD 0.67 (0.45 to 0.89) | 363 | ⊕⊕⊝⊝ | Equivalent to 2.4 on a scale from 0 to 10 |
| The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious concerns about study limitations | ||||||
| Peripheral nerve block compared to neuraxial block for elective primary total hip replacement | ||||||
| Patient or population: adults undergoing elective primary total hip replacement | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Neuraxial block | Peripheral nerve block | |||||
| Pain at rest on arrival in postoperative care unit | Visual/verbal analogue scale from 0 to 10 | The mean pain at rest on arrival in postoperative care unit in the intervention groups was | SMD 0.39 (‐0.15 to 0.94) | 118 | ⊕⊕⊕⊝ | |
| Total number of block‐related complications | Study population | RD 0 | 334 | ⊕⊕⊝⊝ | ||
| 48 per 1000 | 48 per 1000 | |||||
| Low | ||||||
| 10 per 1000 | 10 per 1000 | |||||
| High | ||||||
| 100 per 1000 | 100 per 1000 | |||||
| Aute confusional state | Study population | RR 0.29 | 50 | ⊕⊝⊝⊝ | ||
| 43 per 1000 | 13 per 1000 | |||||
| Low | ||||||
| 25 per 1000 | 7 per 1000 | |||||
| High | ||||||
| 150 per 1000 | 43 per 1000 | |||||
| Pruritus | Study population | RR 0.33 | 299 | ⊕⊕⊕⊝ | Number needed to treat for additional benefit: 6 (95% CI 5 to 9) | |
| 258 per 1000 | 88 per 1000 | |||||
| Low | ||||||
| 50 per 1000 | 17 per 1000 | |||||
| High | ||||||
| 300 per 1000 | 102 per 1000 | |||||
| Hospital length of stay | The mean hospital length of stay in the control groups was 12.6 days* | The mean hospital length of stay in the intervention groups was | MD 0.19 (‐0.39 to 0.77) | 64 | ⊕⊕⊝⊝ | |
| Time to first walk | The mean time to first walk in the control groups was 3.3 | The mean time to first walk in the intervention groups was | MD ‐0.41 (‐1.09 to 0.27) | 94 | ⊕⊕⊝⊝ | |
| Patient satisfaction | CD | The mean patient satisfaction in the intervention groups was | SMD 0.08 (‐0.32 to 0.48) | 307 | ⊕⊕⊕⊕ | |
| The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious concerns about study limitations | ||||||
| Study | Regional anaesthesia technique | Complications related to regional anaesthesia | Analgesic technique | Complications related to systemic analgesia or general anaesthesia |
| Ultrasound to localize the spinous process of L3. Psoas compartment block with 30 mL 0.25% bupivacaine and epinephrine 5 µg/mL injected when an ipsilateral quadriceps muscle contraction was obtained at 0.5 to 0.8 mA. Subgluteal sciatic nerve block with 20 mL of the same solution (dual guidance and plantar flexion at 0.5 to 0.8 mA Karmakar 2007). Iliac crest block with 5 mL of the same solution. Continuous spinal anaesthesia (22G; catheter over the needle) for the surgery for the comparator | No major complications due to the peripheral nerve block procedure were observed in any participants intra‐ or postoperatively No participant had post dural puncture headache in the continuous spinal anaesthesia group until discharged. Cauda equina syndrome was not observed in any participant | Subcutaneous morphine 0.1 mg/kg for the peripheral nerve block group. Intrathecal morphine 0.2 mg for the comparative group. Rescue analgesia with IV tramadol 50 mg | Complications related to morphine (any route) not reported. No significant difference in terms of peripheral oxygen saturation | |
| Nerve stimulation psoas compartment block (Capdevila 2002) with 15 mL 0.5% bupivacaine plus 15 mL saline ± clonidine 2.5 µg/mL (total 75 µg) injected when a quadriceps contraction was obtained at 0.5 mA and 50 ms | No local anaesthetics side effects occurred during the first 24 hours postoperatively | IM morphine | Respiratory rate was in the normal range after 2 hours postoperatively for systemic analgesia group participants Mean respiratory rate was above 10 per minute for all groups at 2, 6 and 12 hours | |
| Continuous lumbar plexus block ultrasound and nerve stimulation guidance: injection 0.6 mL/kg of 0.375% ropivacaine and 100 µg fentanyl through the stimulating needle, advancement of the catheter to the local anaesthetic pool with ultrasound guidance, followed by continuous infusion of 0.2% ropivacaine at 6 mL to 8 mL/hour plus fentanyl (0.5 µg/kg/hour). Continuous lumbar epidural (L2‐L3) with 4 mL ropivacaine 0.75% plus 100 µg of fentanyl, followed by continuous infusion of 0.2% ropivacaine at 4 mL/hour plus fentanyl (0.5 µg/kg/hour) | Not reported | IV patient controlled analgesia with fentanyl | Not reported | |
| Quadratus lumborum block (Blanco 2015) | Not reported | Paracetamol, ketoprofen and IV morphine patient‐controlled analgesia | Vomiting | |
| Nerve stimulation psoas compartment block (Capdevila 2002). When a quadriceps contraction was obtained between 0.3 and 0.5 mA and 100 ms, the needle bevel was oriented caudally and laterally, the psoas compartment was distended with 5 mL saline, and a 20 G multi‐perforated catheter was introduced through the needle and advanced 5 cm distally to the needle tip. A test dose of 1% lidocaine with epinephrine 5 µg/mL (3 mL) was performed. If the test dose was negative, 10 mL contrast medium (Omnipaques; Amersham Healths, Oslo, Norway) was injected into the catheter, and an antero‐posterior radiograph of the lumbar region was obtained. Catheter location was evaluated by a radiologist. 30 min before the end of surgery, participants received a loading dose 0.75% ropivacaine (0.4 mL/kg) followed by an infusion of 0.2% ropivacaine at 10 mL/hour for 48 hours. Spinal anaesthesia with a 27 G Whitacre needle at L3‐L4 and 15 mg isobaric bupivacaine | No participant developed haematoma There was no epidural spread, dysaesthesia or any other sign of local anaesthetic toxicity. One catheter displacement occurred during the study period and no neurological complications were recorded after catheter withdrawal | IV infusion of morphine 0.1% and ketorolac 0.12% at 2 mL/hour for 48 hours | No participant had itching or respiratory depression | |
| Psoas compartment block with a nerve stimulator and 0.4 mL/kg 0.125% levobupivacaine before surgery, catheter inserted, and re‐injection of the same dose of levobupivacaine at the end of surgery | Not reported | Intrathecal diamorphine 4 µg/kg. IV patient‐controlled analgesia with morphine | Not reported | |
| Femoral nerve block with nerve stimulation (Winnie 1973) and 2 mg/kg 0.375% bupivacaine and 2 µg/kg clonidine or Psoas compartment block at L3 with nerve stimulation (Parkinson 1989) and 2 mg/kg of 0.375% bupivacaine and 2 µg/kg of clonidine | Except for 4 cases of epidural anaesthesia (lasting < 4 hours) in the psoas compartment block group, no complications secondary to the block were noted | IV patient‐controlled analgesia with morphine. All participants systematically received | There was no difference in respiratory rate among the three groups during the study. No participants reported pruritus. Sedation scores in all participants were < 1 for the first 8 postoperative hours and rated 0 after 8 hours | |
| Femoral nerve block with a nerve stimulator and 0.3 mL/kg 0.5% bupivacaine followed by an infusion of bupivacaine 0.125% and sufentanil 0.5 µg/mL at 10 mL/hour. Lumbar epidural (L2‐L3 or L3‐L4) with 0.3 mL/kg 0.5% bupivacaine followed by an infusion of 0.125% bupivacaine and sufentanil 0.5 µg/mL at 6 mL/hour | Not reported | IM piritramide | Not reported | |
| Continuous fascia iliaca block, catheters threaded 3 cm cranially and loaded with 30 mL 0.33% ropivacaine followed by an infusion of 0.2% ropivacaine at 1 mg/10 kg continued for 48 hours. Continuous epidural (combined spinal /epidural) with catheters threaded 3 cm to 5 cm cranially and an infusion of 0.2% ropivacaine started after surgery. Spinal anaesthesia with 15 mg 0.5% hyperbaric 0.5% bupivacaine at L2‐L3 or L3‐L4 with the needle oriented cranially for surgery | There were no complications related to regional anaesthesia, intra‐ or postoperative techniques. One failed catheter in the fascia iliaca block group | Acetaminophen 4 g/day. Ketoprofen 300 mg/day. Tramadol as rescue analgesia | Not reported | |
| Continuous femoral nerve block with 20 mL 0.5% ropivacaine 3 mg/kg (maximum 40 mL), sufentanil and clonidine alone or associated with single injection sciatic nerve block with 20 mL of the same solution. Infusion of 0.2% ropivacaine and sufentanil at 8 mL/hour through the femoral nerve catheter for 48 hours. Continouous lumbar epidural with 0.5% ropivacaine 15 mL to 20 mL and sufentanil, clonidine. Infusion of 0.2% ropivacaine and sufentanil at 8 mL/hour through the epidural catheter for 48 hours | Not reported | Morphine and ketorolac | Not reported | |
| Continuous femoral nerve block (puncture site below the inguinal ligament and outside the femoral artery) with catheter inserted 10 cm past the needle tip connected to a patient‐controlled pump for 72 hours | Not reported | Not reported | Not reported | |
| Fascia iliaca compartment block with 40 mL 0.5% ropivacaine. Spinal anaesthesia with 0.5% bupivacaine for surgery | No significant adverse effects | IV morphine | Not reported | |
| All blocks performed on the operated side in the upper position. Psoas compartment block (Capdevila 2002) with a nerve stimulator and 0.4 mL/kg 0.5% ropivacaine injected when a quadriceps contraction was obtained between 0.35 and 0.5 mA and 50 ms. Catheter inserted after the initial bolus (20 G multi‐orifice) inserted 3 cm to 5 cm cephalad. Patient‐controlled continuous block after the surgery with ropivacaine 0.2%. Epidural catheter with a paramedian approach at L3‐L4 or L4‐L5 with 10 mL to 15 mL 0.5% ropivacaine before catheter insertion (20 G multi‐orifice; inserted 3 cm to 5 cm cephalad). Patient‐controlled continuous epidural analgesia after the surgery with ropivacaine 0.2% and fentanyl 3 µg/mL | Not reported | IV morphine | Not reported | |
| Single injection fascia iliaca (landmarks) with 0.4 mL/kg 0.5% L‐bupivacaine (max 30 mL) with clonidine 2.5 µg/mL and epinephrine 5 µg/mL. Single injection epidural analgesia (iliac crest level) with 10 mL 0.25% L‐bupivacaine and 10 µg sufentanil | Partial transient motor blockade for 4 participants. Side effects and complications similar for both groups | IV paracetamol 1 g every 6 hours. IV patient‐controlled piritramide | Side effects and complications similar for both groups | |
| Continous psoas compartment block with dual guidance (ultrasound (in‐plane) and nerve stimulator), catheter insertion, 25 mL 0.375% ropivacaine. Continous infusion of 0.2% ropivacaine at 4 to 6 mL/h. Continous epidural analgesia with 6 mLropivacaine 0.5% plus fentanyl 100 µg continuous infusion with 0.2% ropivacaine and fentanyl 4 µg/mL at 4 to 6 mL/h | Not reported | IV flurbiprofen 50 mg every 12 hours. Pentazocine | Not reported | |
| Femoral nerve block (Winnie 1973) with nerve stimulation and 40 mL 0.5% bupivacaine with epinephrine 5 µg/mL injected when a quadriceps contraction was obtained at ≤ 0.4 mA (needle angled proximally). Sham block | No side effects such as paraesthesiae or dysaesthesiae in the area covered by the block, haematoma or signs | IM diclofenac and/or subcutaneous morphine 0.1 mg/kg | Not reported | |
| Operated side upper position. Psoas compartment block (Capdevila 2002) with a nerve stimulator and 0.4 mL/kg ropivacaine 0.5% injected when a quadriceps contraction was obtained at 0.5 mA and 0.1 ms. Spinal anaesthesia (L3‐L4) with 15 mg hyperbaric bupivacaine plus 15 µg fentanyl and 0.1 mg morphine | One epidural extension. No failed spinal or psoas compartment block | Intrathecal morphine 0.1 mg. Routine paracetamol 1 g IV 4 x daily and ketorolac 30 mg IV 3 x daily. IV tramadol as rescue | No respiratory depression. Higer risk of pruritus with intrathecal morphine | |
| Continuous psoas compartment block (Heller 2009) with a nerve stimulator, catheter inserted 3 cm past the needle tip and needle bevel oriented laterally when a quadriceps contraction was obtained between 0.5 and 0.8 mA and 0.1 ms and loaded with 40 mL 0.5% ropivacaine followed by patient‐controlled analgesia with 0.2% ropivacaine after surgery. Spinal anaesthesia with 2 mL 0.5% and morphine 0.1 mg | Neurological irritation or injury did not differ between groups. Symptoms suggestive of neurological irritation or injury did not differ (spinal = 8/23 and psoas compartment block = 6/27) between groups. All were mild and short‐lived. No participant demonstrated symptoms or signs of systemic local anaesthetic toxicity and there were no catheter‐related bleeding or infectious complications | Intrathecal morphine 0.1 mg. IV parecoxib 40 mg at the time of surgery. Oral paracetamol 1 g every 6 hours. Sustained release diclofenac 75 mg every 12 hours. Sustained release tramadol 100 mg every 12 hours on request. Oral or IV morphine as rescue analgesia. (Lumbar plexus group participants also received morphine 0.1 mg/kg IV during surgery) | Higher risk of pruritus in the intrathecal group. 2 in‐hospital falls with intrathecal morphine | |
| Continuous psoas compartment block with bupivacaine 0.125% and fentanyl 0.05 mg/mL at 5 to 10 mL/hour. Continuous epidural analgesia with bupivacaine 0.125% and fentanyl 0.05 mg/mL at 3 to 5 mL/hour | Degree of motor block was significantly higher in the lumbar plexus block group | Type of additional analgesic used unspecified | Frequency of side effects did not differ significantly between groups | |
| Obturator nerve block (obturator canal level) with a nerve stimulator and 30 mL 0.25% bupivacaine injected when an adductor muscle contraction was obtained at 0.5 mA | Not reported | IV piritramide on request. Rectal diclofenac 100 mg or metamizole 1 g on request | Not reported | |
| Lumbar plexus block with a nerve stimulator and 30 mL 0.5% bupivacaine injected when a quadriceps contraction was obtained < 1 mA. Combined spinal/epidural with 60 mg 1.5% mepivacaine and 3 mL aliquots of 2% lidocaine through an epidural catheter for surgery and patient‐controlled epidural analgesia with 0.06% bupivacaine and 10 µg/mL hydromorphone for postoperative analgesia for all participants | No adverse events attributable to the lumbar plexus block occurred | Epidural patient‐controlled analgesia and oral opioid | No adverse events attributable to either technique occurred | |
| The psoas compartment block (50 mL (40 mL 0.25% bupivacaine and 10 mL saline) was administered using an 18 G spinal needle. volume of (Green 2011). Spinal anaesthesia for surgery | There were no adverse events perioperatively, directly related to block administration | Routine paracetamol 1 g 4 x daily and diclofenac 75 mg 2 x daily. Oxycontin 10 mg 2 x daily. Oxynorm for | Not reported | |
| Femoral, obturator (anterior branch) and lateral femoral cutaneous nerve block | Not reported | Morphine on request | Not reported | |
| Psoas compartment block with a nerve stimulator with 30 mL 0.375% ropivacaine injected when a quadriceps contraction was obtained at 0.3 mA and isobaric spinal anaesthesia (L2‐L3 or L3‐L4) with bupivacaine 0.12 mg/kg. Epidural (L2‐L3 or L3‐L4) and isobaric spinal anaesthesia (L2‐L3 or L3‐L4) with bupivacaine 0.16 mg/kg for surgery | No participants had post‐dural puncture headache or back pain | Not reported | All participants had oxygen saturation between 98% and 100% | |
| Ultrasound‐guided (out‐of‐plane) fascia iliaca block with levobupivacaine 2 mg/kg diluted to a total volume of 40 mL below the inguinal ligament but proximal to any femoral artery branching | 4 adverse events defined as any untoward medical occurrence and 4 serious adverse events defined as resulting in death or life threatening (at the time of the event) or required hospitalization or prolongation of existing hospitalization or resulting in persistent or significant disability or incapacity or consisting of a congenital anomaly or birth defect or beng otherwise considered medically significant by the investigator (not necessarily related to the trial treatment). Pulmonary embolism (N = 2), femoral nerve palsy (N = 1; resolved at 3 months), late wound infection (N = 1) | Intrathecal morphine 0.1 mg. IV patient‐controlled analgesia with morphine | 3 adverse events defined as any untoward medical occurrence and 2 serious adverse events defined as resulting in death or life threatening (at the time of the event) or required hospitalization or prolongation of existing hospitalization or resulting in persistent or significant disability or incapacity or consisting of a congenital anomaly or birth defect or beng otherwise considered medically significant by the investigator (not necessarily related to the trial treatment). Pulmonary embolism (N = 1), wound infection resulting in multi‐organ failure (N = 1) | |
| Femoral (3‐in‐1) block with nerve stimulation and 40 mL 0.25% bupivacaine injected when a quadriceps contraction was obtained at 0.5 mA and with distal pressure | There was no local anaesthetic toxicity | IV patient‐controlled analgesia with tramadol | Most common side effects were nausea and vomiting | |
| Single injection femoral nerve block with a nerve stimulator and 20 mL 0.25% bupivacaine with 20 µg clonidine injected when a quadriceps contraction was obtained at 0.4 mA and 0.1 ms | Not reported | Metamixol 15 to 25 mg/kg before end of surgery. IV piritramide (in the postoperative care unit). Ibuprofene. Metamizole. Oral oxycodone | Not reported | |
| Single injection femoral nerve or fascia iliaca block | Not reported | IV morphine | Not reported | |
| Continuous psoas compartment block with nerve stimulator, catheter advanced 3 cm past the needle tip when a quadriceps contraction was obtained at 0.5 mA and 100 ms. Catheters were loaded with 0.6 mL/kg ropivacaine 0.5% and the position was verified with 10 mL contrast medium. Participants then received ropivacaine 0.2% infused at 0.15 mL/kg/hour for 48 hours. Continuous femoral nerve block loaded through the needle with 0.6 mL/kg ropivacaine 0.5% injected when a quadriceps contraction was obtained at 0.5 mA and 100 ms, catheter advanced < 10 cm past the needle tip. This was followed by an infusion of ropivacaine 0.2% at 0.15 mL/kg/hour for 48 hours | Lumbar plexus block was associated with an epidural spread in 5/75 (7%) participants. No perineural haematoma recorded | IV patient‐controlled analgesia with hydromorphone | Nausea and pruritus | |
| Continuous lumbar plexus block as patient‐controlled analgesia. Continuous epidural analgesia. Spinal anaesthesia for surgery | Not reported | Parenteral opioids | Not reported | |
| Fascia iliaca block on landmarks with 2 mg/kg 0.5% levobupivacaine. Spinal anaesthesia with isobaric 0.5% bupivacaine 0.1 to 0.2 mg/kg for surgery | Not reported | IV patient‐controlled analgesia with morphine | Not reported | |
| Femoral (3‐in‐1) nerve block with a nerve stimulator and 30 mL of equal parts 0.5% bupivacaine and 2% lidocaine | Not reported | Routine paracetamol 1 g every 6 h. Diclofenac 8 mg on request. IM morphine | Not reported | |
| Dual guidance continuous femoral nerve block (ultrasound and nerve stimulator) with a catheter inserted 10 cm past the needle tip and participants received an infusion of 0.15% ropivacaine at 3 mL/hour | Not reported | IV patient‐controlled analgesia with fentanyl or intrarectal diclofenac or IV flurbiprofen on participant's request and preferences | Nausea and vomiting, drowsiness | |
| Continuous femoral nerve block with 0.1 mL/kg/hour of 0.1% bupivacaine. Continous epidural analgesia with 0.1 mL/kg/hour 0.1% bupivacaine | Not reported | IV patient‐controlled analgesia with fentanyl | Not reported | |
| Ultrasound‐guided (in‐plane), low pressure (20 pounds per square inch) fascia iliaca block with 30 mL 0.5% ropivacaine | Not reported | IV patient‐controlled analgesia with morphine | Not reported | |
| Continuous psoas compartment block (Winnie 1974) with a nerve stimulator and a 4 inch Tuohy needle (quadriceps contraction at 0.5 to 1.0 mA and 50 ms) and 5 mL saline before catheter insertion advanced 4 cm to 6 cm past the needle tip. Injection of 3 mL 2% lidocaine with epinephrine 5 µg/mL followed by 20 mL 0.25% bupivacaine and an infusion of 0.125% bupivacaine at 10 mL/hour for 36 hours | One participant in the continuous lumbar plexus block developed a delayed paresis (first noted 4 weeks after surgery), possibly related to nerve compression by a haematoma, international normalized ratio 5.6 on postoperative day 3. The weakness improved but remained at 3 months | IV patient‐controlled analgesia with morphine | One participant in the systemic analgesia group developed respiratory depression | |
| Continuous femoral nerve block (Winnie 1973) with a nerve stimulator and 40 mL 0.25% bupivacaine with epinephrine 5 µg/mL through a catheter inserted 10 cm past the needle tip followed by an infusion of 0.125% bupivacaine at 10 mL/hour. Epidural analgesia at L2‐L3 or L3‐L4 with catheters inserted 4 cm to 5 cm past the needle tip, a test dose with 3 mL 0.25% bupivacaine with epinephrine 5 µg/mL, followed by 10 mL of the same solution and 10 µg sufentanil and then by an infusion of 0.125% bupivacaine at 10 mL/hour | 3 participants with catheter‐related problems in the epidural group 2 urinary retention for femoral nerve block group and 6 for epidural group | IV patient‐controlled analgesia with morphine | 4 urinary retention in the IV morphine group | |
| Psoas compartment block (Winnie 1975) with a nerve stimulator and with 25 mL ropivacaine 0.475% injected when a quadriceps contraction was obtained at 0.5 mA and 0.1 ms | No major complication occurred. Epidural block did not occur. 2 blood aspirations during psoas compartment block performance | Intrathecal morphine 0.1 mg | No major complications were observed with either technique | |
| Psoas compartment block (Winnie 1974) with a nerve stimulator and 0.4 mL/kg 0.5% bupivacaine and epinephrine 5 µg/mL injected when a quadriceps contraction was obtained between 0.2 and 0.5 mA and 50 ms | Epidural anaesthesia occurred in 3 of 28 plexus group participants No other side effects were noted, total spinal anaesthesia, renal | IV patient‐controlled analgesia with morphine | Nausea and vomiting | |
| Fascia iliaca block with modified landmarks (Dalens 1989 but 1 cm above the inguinal ligament; first pop when the needle traverses the superficial fascia and second pop for the fascia transversalis) and 30 mL 0.5% bupivacaine with epinephrine 5 µg/mL, 150 µg of clonidine and 9 mL saline. Hypothesisis: this puncture point would enable blockade of the ilio‐inguinal, iliohypogastric and genitofemoral nerves as well as the femoral, lateral cutaneous and obturator nerves but sensory block not tested | Not reported | IV patient‐controlled analgesia with morphine | Nausea and vomiting | |
| Continuous femoral (3‐in‐1) nerve block with catheters (inserted 10 cm past the needle tip) with a nerve stimulator (between 0.2 and 2.0 mA on needle) and 30 mL 0.5% bupivacaine administered before surgery (quadriceps motor blockade confirmed before anaesthesia induction) and 10 minutes after surgery | Mean bupivacaine plasma levels ranged between 0.75 and 1.33 µg/mL Placement of the 3‐in‐1 catheters were without complications | IV meperidine on request | Nausea and vomiting | |
| Continuous femoral nerve block with lidocaine (lidocaine 1% bolus followed by intra‐operative infusion of lidocaine 1% 10 mL/hour and lidocaine 0.5% 10 mL/hour during 24 hours postoperatively | Not reported | IV lidocaine infusion: 1.5 mg/kg followed by intra‐operative continuous infusion of 2 mg/kg/hour and postoperatively 1 mg/kg/hour for 24 hours. All participants received paracetamol 4 g for24 hours and IV patient‐controlled analgesia with morphine | Not reported | |
| Ultrasound‐guided (in‐plane) lateral femoral cutaneous nerve block with 8 mL 0.75% ropivacaine. Spinal anaesthesia with 2 mL to 2.5 mL 0.5% isobaric bupivacaine for surgery | No adverse events or harms were observed during the trial | Paracetamol 1 g orally every 6 hours. Ibuprofen 600 mg orally every 8 hours. IV or oral oxycodone on request | No adverse events, or harms, were observed during the trial | |
| Psoas compartment block (side to blocked uppermost, 3 cm caudal and 5 cm lateral to L4 apophysis and redirected upward if the apophysis of L5 was contacted) with a nerve stimulator and when a quadriceps contraction was obtained at 0.5 mA, 10 mL saline was injected before catheter insertion 5 cm past the needle tip. This was followed by a test dose with 3 mL 2% lidocaine with epinephrine 5 µg/mL, then 20 mL contrast solution, followed by 30 mL 0.5% bupivacaine (repeated if surgery lasted > 2 hours). Patient‐controlled analgesia with 0.125% bupivacaine and fentanyl 2 µg/mL for 24 hours. Epidural analgesia at L3‐L4, catheters advanced 3 cm cephalad, test dose with 3 mL 2% lidocaine with epinephrine 5 µg/mL followed by 15 mL 0.5% bupivacaine (repeated if surgery lasted > 2 hours). Patient‐controlled analgesia with 0.125% bupivacaine and fentanyl 2 µg/mL for 24 hours | Psoas: 2 kinked catheters No participants in the psoas group developed epidural Epidural: 1 kinked catheter and 1 lateralization on non‐operated side. No participants in either group developed complications such as cardiac arrest, permanent 7 urinary retention in the epidural group | IM diclofenac 75 mg on request | Nausea and vomiting Pruritus | |
| Psoas compartment block (Chayen 1976), loss of resistance technique, 20 mL air injected to dilate the space followed by 0.42 mL/kg of 0.375% bupivacaine | There were no complications associated with the technique | Unspecified | Not reported | |
| Femoral (3‐in‐1; Brands 1978) nerve block with a nerve stimulator and 20 mL 0.5% bupivacaine plus 20 mL 2% lidocaine with epinephrine Femoral lateral cutaneous nerve block with 5 mL 1% lidocaine on landmarks | One haematoma in the groin after accidental puncture of the femoral artery No toxic reactions due to the local anaesthetics were recorded | Nicomorphine on request | Not reported | |
| Continuous psoas compartment block with a nerve stimulator (Parkinson's approach; Parkinson 1989; L3 confirmation with fluoroscopy in prone position) and with 0.4 mL/kg 0.25% bupivacaine injected before surgery when a quadriceps contraction was obtained at 0.5 mA (position confirmed with 2 mL radiopaque substance and catheter inserted 2 cm past the needle tip) and half dose after 8 hours. Continuous lumbar (L3‐L4; catheters threaded 3 to 3.5 cm cranially) epidural analgesia with a test dose (3 mL 2% lidocaine) and 2 mL per segment until T10‐T12 before general anaesthesia with 0.025% bupivacaine and half dose after 8 hours | Two epidural spreads One urinary retention with epidural analgesia | IV patient‐ controlled analgesia with morphine | Pruritus | |
| Supra‐inguinal fascia iliaca compartment block with 40 mL 0.5% ropivacaine | Not reported | Paracetamol Non steroidal anti‐inflammatory drugs IV patient‐controlled analgesia with morphine | Not reported | |
| Femoral nerve block with a nerve stimulator and 15 mL bupivacaine 0.25% and clonidine 20 µg injected when a quadriceps contraction was obtained at 0.3 mA and 0.1 ms | Not reported | IV piritramide in the postoperative care unit. IV/oral metamizole and oral ibuprofen in both groups on request | No oxygen saturation < 90% No nausea and vomiting | |
| IM: intramuscular; IV: intravenous; mA: milliampere; ms: millisecond; | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at rest on arrival at postoperative care unit Show forest plot | 9 | 429 | Std. Mean Difference (Random, 95% CI) | ‐1.12 [‐1.67, ‐0.56] |
| 1.1 Femoral nerve block | 6 | 287 | Std. Mean Difference (Random, 95% CI) | ‐1.22 [‐2.06, ‐0.38] |
| 1.2 Psoas compartment block | 3 | 142 | Std. Mean Difference (Random, 95% CI) | ‐0.91 [‐1.25, ‐0.56] |
| 2 Pain at rest from 0.5 to 2 hours after surgery Show forest plot | 9 | 438 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐1.06, ‐0.29] |
| 2.1 Femoral nerve block | 4 | 187 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.85, 0.28] |
| 2.2 Fascia iliaca block | 2 | 47 | Std. Mean Difference (Random, 95% CI) | ‐1.21 [‐3.34, 0.92] |
| 2.3 Psoas compartment block | 4 | 204 | Std. Mean Difference (Random, 95% CI) | ‐0.91 [‐1.20, ‐0.62] |
| 3 Pain at rest at 4 to 6 hours after surgery Show forest plot | 13 | 599 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐0.92, ‐0.32] |
| 3.1 Femoral nerve block | 5 | 223 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.98, 0.38] |
| 3.2 Fascia iliaca block | 2 | 73 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐1.06, 0.33] |
| 3.3 Psoas compartment block | 6 | 303 | Std. Mean Difference (Random, 95% CI) | ‐0.88 [‐1.12, ‐0.64] |
| 4 Pain on movement at 4 to 6 hours after surgery Show forest plot | 3 | 120 | Std. Mean Difference (Random, 95% CI) | ‐0.46 [‐1.12, 0.20] |
| 4.1 Femoral nerve block | 1 | 23 | Std. Mean Difference (Random, 95% CI) | 0.0 [‐0.86, 0.86] |
| 4.2 Fascia iliaca block | 1 | 29 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.91, 0.55] |
| 4.3 Psoas compartment block | 1 | 68 | Std. Mean Difference (Random, 95% CI) | ‐1.0 [‐1.51, ‐0.49] |
| 5 Pain at rest at 24 hours after surgery for single injection block with clonidine or continuous nerve block Show forest plot | 6 | 303 | Std. Mean Difference (Random, 95% CI) | ‐0.66 [‐1.05, ‐0.28] |
| 5.1 Femoral nerve block | 3 | 155 | Std. Mean Difference (Random, 95% CI) | ‐0.68 [‐1.27, ‐0.10] |
| 5.2 Fascia iliaca block | 1 | 44 | Std. Mean Difference (Random, 95% CI) | 0.0 [‐0.59, 0.59] |
| 5.3 Psoas compartment block | 2 | 104 | Std. Mean Difference (Random, 95% CI) | ‐0.97 [‐1.37, ‐0.56] |
| 6 Pain on movement at 24 hours after surgery for continuous peripheral nerve block Show forest plot | 3 | 317 | Std. Mean Difference (Random, 95% CI) | ‐0.71 [‐1.26, ‐0.17] |
| 6.1 Femoral nerve block | 2 | 136 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.59, 0.12] |
| 6.2 Psoas compartment block | 2 | 181 | Std. Mean Difference (Random, 95% CI) | ‐1.13 [‐1.46, ‐0.80] |
| 7 Pain at rest at 48 hours after surgery Show forest plot | 2 | 93 | Std. Mean Difference (Random, 95% CI) | ‐0.80 [‐1.35, ‐0.25] |
| 7.1 Continuous femoral nerve block | 1 | 23 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐1.25, 0.47] |
| 7.2 Continuous psoas compartment block | 1 | 70 | Std. Mean Difference (Random, 95% CI) | ‐0.99 [‐1.48, ‐0.50] |
| 8 Pain on movement at 48 hours after surgery Show forest plot | 3 | 317 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐1.13, ‐0.11] |
| 8.1 Continuous femoral nerve block | 2 | 136 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.60, 0.28] |
| 8.2 Continuous psoas compartment block | 2 | 181 | Std. Mean Difference (Random, 95% CI) | ‐0.93 [‐1.25, ‐0.61] |
| 9 Analgesic requirements: opioids consumption from 0 to 24 hours Show forest plot | 12 | 897 | Std. Mean Difference (Random, 95% CI) | ‐0.62 [‐0.89, ‐0.35] |
| 9.1 Femoral nerve block with or without lateral cutaneous nerve block | 3 | 332 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.61, ‐0.17] |
| 9.2 Fascia iliaca block | 5 | 233 | Std. Mean Difference (Random, 95% CI) | ‐0.84 [‐1.27, ‐0.41] |
| 9.3 Psoas compartment | 3 | 196 | Std. Mean Difference (Random, 95% CI) | ‐0.96 [‐1.27, ‐0.65] |
| 9.4 Obturator nerve block | 1 | 36 | Std. Mean Difference (Random, 95% CI) | ‐0.4 [‐1.07, 0.27] |
| 9.5 Lateral femoral cutaneous alone | 1 | 100 | Std. Mean Difference (Random, 95% CI) | 0.31 [‐0.08, 0.70] |
| 10 Gastrointestinal complications: postoperative nausea and vomiting Show forest plot | 5 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.53, 1.41] |
| 11 Cardiovascular complications: hypotension Show forest plot | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.81] |
| 12 Cardiovascular complications: intra‐operative blood losses (mL) Show forest plot | 8 | 323 | Mean Difference (IV, Random, 95% CI) | ‐52.47 [‐170.88, 65.95] |
| 12.1 Femoral nerve block | 3 | 160 | Mean Difference (IV, Random, 95% CI) | 33.81 [‐27.12, 94.75] |
| 12.2 Fascia iliaca block | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐3.0 [‐216.07, 210.07] |
| 12.3 Psoas compartment block | 4 | 134 | Mean Difference (IV, Random, 95% CI) | ‐181.06 [‐451.65, 89.53] |
| 13 Cardiovascular complications: number of blood units transfused Show forest plot | 3 | 82 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.25, 0.08] |
| 13.1 Femoral nerve block | 2 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.97, 0.07] |
| 13.2 Psoas compartment block | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.88, ‐0.12] |
| 14 Neurological complications: drowsiness up to 48 hours after surgery Show forest plot | 3 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.13, 1.18] |
| 15 Pruritus first 48 hours Show forest plot | 4 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.09, 1.27] |
| 15.1 Single injection blocks | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.50, 1.68] |
| 15.2 Continuous peripheral nerve blocks | 2 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.70] |
| 16 Respiratory depression Show forest plot | 3 | 184 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 17 Hospital length of stay Show forest plot | 3 | 349 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐0.91, ‐0.21] |
| 17.1 Continuous femoral nerve or psoas compartment block | 2 | 249 | Std. Mean Difference (Random, 95% CI) | ‐0.75 [‐1.02, ‐0.48] |
| 17.2 Single injection femoral lateral cutaneous nerve block | 1 | 100 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.52, 0.26] |
| 18 Walking on postoperative day 1 Show forest plot | 2 | 278 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 18.1 Single injection block | 1 | 53 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [‐0.14, 0.23] |
| 18.2 Continuous nerve block | 1 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 19 Hip flexion at 7 days Show forest plot | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐9.04, 6.25] |
| 19.1 Single injection femoral nerve or psoas compartment block | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐6.12 [‐14.53, 2.29] |
| 19.2 Continuous femoral nerve block | 1 | 23 | Mean Difference (IV, Random, 95% CI) | 4.0 [‐3.01, 11.01] |
| 20 Patient satisfaction for continuous peripheral nerve block Show forest plot | 4 | 363 | Std. Mean Difference (Fixed, 95% CI) | 0.67 [0.45, 0.89] |
| 20.1 Femoral nerve block | 3 | 176 | Std. Mean Difference (Fixed, 95% CI) | 0.47 [0.15, 0.78] |
| 20.2 Psoas compartment block | 3 | 187 | Std. Mean Difference (Fixed, 95% CI) | 0.88 [0.57, 1.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain at rest on arrival in postanaesthesia care unit Show forest plot | 4 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.15, 0.94] |
| 2 Pain with movement at arrival in postanaesthesia care unit Show forest plot | 2 | 54 | Std. Mean Difference (Fixed, 95% CI) | 0.08 [‐0.46, 0.62] |
| 3 Pain at rest from 0.5 to 2 hours after surgery Show forest plot | 2 | 66 | Mean Difference (IV, Random, 95% CI) | 2.63 [0.77, 4.49] |
| 3.1 Femoral nerve or fascia iliaca block | 2 | 42 | Mean Difference (IV, Random, 95% CI) | 3.45 [2.01, 4.90] |
| 3.2 Femoral plus sciatic nerve block | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐0.02, 2.14] |
| 4 Pain at rest at 4 to 6 hours after surgery Show forest plot | 9 | 398 | Std. Mean Difference (Random, 95% CI) | 0.41 [0.11, 0.71] |
| 4.1 Single injection peripheral nerve block | 3 | 176 | Std. Mean Difference (Random, 95% CI) | 0.29 [‐0.61, 1.19] |
| 4.2 Continuous peripheral nerve blocks | 6 | 222 | Std. Mean Difference (Random, 95% CI) | 0.40 [0.13, 0.67] |
| 5 Pain with movement at 4 to 6 hours after surgery Show forest plot | 7 | 325 | Std. Mean Difference (Random, 95% CI) | 0.53 [0.20, 0.86] |
| 6 Pain at rest at 24 hours after surgery Show forest plot | 8 | 328 | Std. Mean Difference (Random, 95% CI) | 0.10 [‐0.22, 0.42] |
| 6.1 Single injection block peripheral nerve block | 3 | 163 | Std. Mean Difference (Random, 95% CI) | 0.05 [‐0.26, 0.36] |
| 6.2 Continuous peripheral nerve block | 5 | 165 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.42, 0.65] |
| 7 Pain with movement at 24 hours Show forest plot | 7 | 317 | Std. Mean Difference (Random, 95% CI) | 0.28 [‐0.06, 0.62] |
| 7.1 Single injection peripheral nerve block | 1 | 103 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.52, 0.26] |
| 7.2 Continuous peripheral nerve block | 6 | 214 | Std. Mean Difference (Random, 95% CI) | 0.38 [0.01, 0.75] |
| 8 Pain at rest at 48 hours after surgery Show forest plot | 7 | 289 | Std. Mean Difference (Fixed, 95% CI) | 0.07 [‐0.16, 0.31] |
| 8.1 Single injection shot peripheral nerve block | 2 | 123 | Std. Mean Difference (Fixed, 95% CI) | ‐0.29 [‐0.65, 0.07] |
| 8.2 Continuous peripheral nerve block | 5 | 166 | Std. Mean Difference (Fixed, 95% CI) | 0.35 [0.04, 0.67] |
| 9 Pain with movement at 48 hours (continuous peripheral nerve blocks only) Show forest plot | 5 | 164 | Std. Mean Difference (Random, 95% CI) | 0.33 [‐0.13, 0.79] |
| 9.1 Bupivacaine infusion | 3 | 77 | Std. Mean Difference (Random, 95% CI) | ‐0.16 [‐0.62, 0.30] |
| 9.2 Ropivacaine infusion | 2 | 87 | Std. Mean Difference (Random, 95% CI) | 0.80 [0.36, 1.24] |
| 10 Total number of nerve block‐related complications Show forest plot | 5 | 334 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 11 Analgesic requirements: opioids consumption from 0 to 24 hours Show forest plot | 4 | 203 | Std. Mean Difference (Random, 95% CI) | 0.67 [0.04, 1.31] |
| 11.1 Single injection peripheral nerve block | 2 | 93 | Std. Mean Difference (Random, 95% CI) | 1.24 [0.79, 1.69] |
| 11.2 Repeated doses peripheral nerve blocks | 2 | 110 | Std. Mean Difference (Random, 95% CI) | 0.17 [‐0.37, 0.71] |
| 12 Gastrointestinal complications: postoperative nausea and vomiting Show forest plot | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.39] |
| 13 Cardiovascular complications: hypotension Show forest plot | 8 | 429 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.22, 0.03] |
| 13.1 Compared to a single injection block or two doses | 5 | 306 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.04, 0.08] |
| 13.2 Compared to a continuous neuraxial block | 3 | 123 | Risk Difference (M‐H, Random, 95% CI) | ‐0.34 [‐0.48, ‐0.20] |
| 14 Cardiovascular complications: intra‐operative blood losses (mL) Show forest plot | 5 | 188 | Mean Difference (IV, Random, 95% CI) | ‐8.84 [‐80.82, 63.14] |
| 15 Cardiovascular complications: number of units transfused Show forest plot | 3 | 87 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.65, 0.19] |
| 16 Neurological complications: drowsiness up to 48 hours after surgery Show forest plot | 3 | 176 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.07, 0.15] |
| 16.1 Compared to intrathecal opioids | 2 | 156 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 16.2 Compared to epidural analgesia | 1 | 20 | Risk Difference (M‐H, Random, 95% CI) | 0.4 [0.04, 0.76] |
| 17 Thromboembolic complications: deep venous thrombosis Show forest plot | 2 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 18 Pruritus Show forest plot | 6 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.19, 0.58] |
| 19 Respiratory depression Show forest plot | 4 | 213 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 20 Hospital length of stay Show forest plot | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.39, 0.77] |
| 21 Time to start rehabilitation: bed to chair Show forest plot | 2 | 144 | Std. Mean Difference (Random, 95% CI) | 0.19 [‐0.34, 0.72] |
| 21.1 Single injection block peripheral nerve block | 1 | 103 | Std. Mean Difference (Random, 95% CI) | 0.41 [0.02, 0.80] |
| 21.2 Continuous peripheral nerve block | 1 | 41 | Std. Mean Difference (Random, 95% CI) | ‐0.14 [‐0.75, 0.47] |
| 22 Walking Show forest plot | 3 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.09, 0.27] |
| 23 Patient satisfaction Show forest plot | 6 | 307 | Std. Mean Difference (Random, 95% CI) | 0.08 [‐0.32, 0.48] |
| 23.1 Single injection peripheral nerve block | 3 | 196 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.68, 0.19] |
| 23.2 Continuous peripheral nerve block | 3 | 111 | Std. Mean Difference (Random, 95% CI) | 0.54 [0.16, 0.93] |

