Mefloquina para la prevención del paludismo en embarazadas
Resumen
Antecedentes
La Organización Mundial de la Salud recomienda el tratamiento preventivo intermitente en el embarazo (TPIe) con sulfadoxina‐pirimetamina para el paludismo en todas las pacientes que viven en zonas de transmisión moderada a alta del paludismo en África. Sin embargo, la resistencia de los parásitos a la sulfadoxina‐pirimetamina ha aumentado de forma sostenida en algunas áreas de la región. Además, las pacientes con infección por VIH que reciben profilaxis con cotrimoxazol no pueden recibir sulfadoxina‐pirimetamina debido a las posibles interacciones medicamentosas. Por lo tanto, hay una necesidad urgente de identificar fármacos alternativos para la prevención del paludismo en el embarazo. Uno de dichos candidatos es la mefloquina.
Objetivos
Evaluar los efectos de la mefloquina para la prevención del paludismo en pacientes embarazadas, específicamente, evaluar:
• la eficacia, la seguridad y la tolerabilidad de la mefloquina para la prevención del paludismo en pacientes embarazadas; y
• la repercusión del estado del VIH, el número de gestaciones y el uso de mosquiteros tratados con insecticida sobre los efectos de la mefloquina.
Métodos de búsqueda
Se hicieron búsquedas en el registro especializado del Grupo Cochrane de Enfermedades Infecciosas (Cochrane Infectious Diseases Group Specialized Register), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL) en la Cochrane Library, MEDLINE, Embase, Latin American Caribbean Health Sciences Literature (LILACS), la Malaria in Pregnancy Library y en dos registros de ensayos hasta el 31 enero de 2018. Además, se chequearon las referencias y se estableció contacto con los autores de los estudios para identificar estudios adicionales, datos no publicados, informes confidenciales y datos brutos de los ensayos publicados.
Criterios de selección
Ensayos controlados aleatorios y cuasialeatorios que compararon el TPI con mefloquina o la profilaxis con mefloquina versus placebo, ningún tratamiento o un régimen farmacológico alternativo.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, analizaron todos los registros identificados mediante la estrategia de búsqueda, aplicaron los criterios de inclusión, evaluaron el riesgo de sesgo y extrajeron los datos. Cuando fue necesario, se estableció contacto con los autores de los ensayos para solicitarles información adicional. Se compararon los resultados dicotómicos mediante los cocientes de riesgos (CR), los resultados de los recuentos como cocientes de tasas de incidencia (CTI) y los resultados continuos mediante las diferencias de medias (DM). Se presentaron todas las medidas del efecto con intervalos de confianza (IC) del 95%. La certeza de la evidencia se evaluó mediante el enfoque GRADE para los siguientes resultados principales del análisis: parasitemia periférica materna en el momento del parto, episodios clínicos de paludismo durante el embarazo, paludismo placentario, anemia materna en el momento del parto, bajo peso al nacer, abortos espontáneos y mortinatos, mareos y vómitos.
Resultados principales
Seis ensayos realizados entre 1987 y 2013 de Tailandia (1), Benin (3), Gabón (1), Tanzania (1), Mozambique (2) y Kenia (1) que incluyeron a 8192 embarazadas cumplieron los criterios de inclusión.
Dos ensayos (con 6350 embarazadas sin infección por HIV) compararon dos dosis de TPIe con mefloquina con dos dosis TPIe con sulfadoxina‐pirimetamina. Otros dos ensayos que incluyeron a 1363 mujeres con infección por VIH compararon tres dosis de TPIe con mefloquina más cotrimoxazol versus cotrimoxazol. Un ensayo en 140 mujeres con infección por VIH comparó tres dosis del TPIe con mefloquina versus cotrimoxazol. Finalmente, un ensayo que incluyó a 339 participantes con estado del VIH desconocido comparó la profilaxis con mefloquina versus placebo.
Los estudios incluyeron a pacientes con cualquier número de gestaciones y de todas las edades (cuatro ensayos) o > 18 años de edad (dos ensayos). La edad gestacional en el momento de la incorporación al estudio fue > 20 semanas (un ensayo), entre 16 y 28 semanas (tres ensayos) o ≤ 28 semanas (dos ensayos). Dos de los seis ensayos realizaron el cegamiento de los participantes y el personal, y sólo uno tuvo un bajo riesgo de sesgo de detección para los resultados de seguridad.
En comparación con sulfadoxina‐pirimetamina, el TPIe con mefloquina da lugar a una reducción del 35% de la parasitemia periférica materna en el momento del parto (CR 0,65; IC del 95%: 0,48 a 0,86; 5455 participantes, dos estudios; evidencia de certeza alta), pero puede tener poco o ningún efecto sobre las infecciones placentarias por paludismo (CR 1,04; IC del 95%: 0,58 a 1,86; 4668 participantes, dos estudios; evidencia de certeza baja). La mefloquina da lugar a poca o ninguna diferencia en la incidencia de episodios clínicos de paludismo durante el embarazo (cociente de tasas de incidencia [CTI] 0,83; IC del 95%: 0,65 a 1,05; dos estudios; evidencia de certeza alta). La mefloquina disminuyó la anemia materna en el momento del parto (CR 0,84; IC del 95%: 0,76 a 0,94; 5469 participantes, dos estudios; evidencia de certeza moderada). Los datos muestran poca o ninguna diferencia en las proporciones de lactantes con bajo peso al nacer (CR 0,95; IC del 95%: 0,78 a 1,17; 5641 participantes, dos estudios; evidencia de certeza alta) y en las tasas de mortinatos y de abortos espontáneos (CR 1,20; IC del 95%: 0,91 a 1,58; 6219 participantes, dos estudios; estadística I2 = 0%; evidencia de certeza moderada). El TPIe con mefloquina aumentó los vómitos relacionados con el fármaco (CR 4,76; IC del 95%: 4,13 a 5,49; 6272 participantes, dos estudios; evidencia de certeza alta) y los mareos (CR 4,21; IC del 95%: 3,36 a 5,27; participantes = 6272, dos estudios; evidencia de certeza moderada).
En comparación con cotrimoxazol, el TPIe con mefloquina más cotrimoxazol probablemente da lugar a una reducción del 48% en la parasitemia periférica materna en el momento del parto (CR 0,52; IC del 95%: 0,30 a 0,93; 989 participantes, dos estudios; evidencia de certeza moderada) y una reducción del 72% del paludismo placentario (CR 0,28; IC del 95%: 0,14 a 0,57; 977 participantes, dos estudios; evidencia de certeza moderada), pero tiene poco o ningún efecto sobre la incidencia de episodios clínicos de paludismo durante el embarazo (CTI 0,76; IC del 95%: 0,33 a 1,76; un estudio; evidencia de certeza alta) y probablemente ningún efecto sobre la anemia materna en el momento del parto (CR 0,94; IC del 95%: 0,73 a 1,20; 1197 participantes, dos estudios; evidencia de certeza moderada), las tasas de bajo peso al nacer (CR 1,20; IC del 95%: 0,89 a 1,60; 1220 participantes, dos estudios; evidencia de certeza moderada) y las tasas de aborto espontáneo y mortinatos (CR 1,12; IC del 95%: 0,42 a 2,98; 1347 participantes, dos estudios; evidencia de certeza muy baja). La mefloquina se asoció con riesgos mayores de vómitos relacionados con los fármacos (CR 7,95; IC del 95%: 4,79 a 13,18; 1055 participantes, un estudio; evidencia de certeza alta) y mareos (CR 3,94; IC del 95%: 2,85 a 5,46; 1055 participantes, un estudio; evidencia de certeza alta).
Conclusiones de los autores
La mefloquina fue más eficaz que la sulfadoxina‐pirimetamina en las pacientes sin infección por VIH o que la profilaxis con cotrimoxazol diario en las pacientes embarazadas con infección por VIH para la prevención de la infección por paludismo y se asoció con un riesgo menor de anemia materna, ningún efecto adverso sobre los resultados del embarazo (como mortinatos y abortos) y ningún efecto sobre el bajo peso al nacer y la prematuridad. Sin embargo, la proporción alta de eventos adversos relacionados con la mefloquina constituye una barrera importante a su efectividad para el tratamiento preventivo del paludismo en las pacientes embarazadas.
PICOs
Resumen en términos sencillos
Mefloquina para la prevención del paludismo en embarazadas
¿Cuál es el objetivo de esta revisión?
El objetivo de esta revisión Cochrane fue determinar si el fármaco antipalúdico mefloquina es eficaz y seguro para la prevención del paludismo en las pacientes embarazadas que viven en zonas de transmisión estable. Se encontraron seis estudios relevantes para ayudar a responder esta pregunta.
Mensajes clave
El fármaco antipalúdico mefloquina es eficaz para la prevención del paludismo en las pacientes embarazadas. Se ha encontrado que el fármaco es seguro en cuanto a los resultados adversos del embarazo, como el bajo peso al nacer, la prematuridad, los mortinatos y los abortos, así como las malformaciones congénitas. Sin embargo, presenta una peor tolerabilidad que otros fármacos antipalúdicos.
¿Qué se estudió en la revisión?
Las pacientes embarazadas son vulnerables a la infección por paludismo, especialmente cuando conviven con el VIH. Las consecuencias del paludismo durante el embarazo pueden ser graves e incluir resultados de salud deficientes en las pacientes y sus hijos. Por este motivo, para la prevención de la infección por paludismo en las zonas de transmisión estable en las que el paludismo es endémico, a las pacientes se les recomienda dormir cubiertas con mosquiteros y tomar fármacos efectivos (como la sulfadoxina‐pirimetamina o el cotrimoxazol en el caso de infección por VIH) como quimioprevención contra el paludismo durante todo el embarazo.
Esta revisión Cochrane consideró los efectos de la mefloquina para la prevención del paludismo en pacientes embarazadas con infección por VIH y sin infección por VIH.
¿Cuáles son los principales resultados de la revisión?
Se encontraron cinco estudios relevantes realizados en África subsahariana y uno en Tailandia entre 1987 y 2013. Estos estudios compararon la mefloquina con placebo u otros fármacos antipalúdicos actualmente recomendados para la prevención del paludismo en pacientes embarazadas. La revisión muestra lo siguiente:
• En comparación con sulfadoxina‐pirimetamina, la quimioprevención con mefloquina en las pacientes sin infección por HIV:
◦reduce los riesgos de parasitemia periférica materna (presencia de parásitos de paludismo en la sangre de la mujer) y de anemia en el momento del parto;
◦ no logra cambios en la prevalencia de resultados maternos adversos (como bajo peso al nacer, prematuridad, mortinatos y abortos y malformaciones congénitas) ni en la incidencia de episodios clínicos de paludismo durante el embarazo; y
◦ aumenta los riesgos de eventos adversos relacionados con los fármacos incluidos los vómitos, la fatiga/debilidad y los mareos.
• En comparación con la profilaxis con cotrimoxazol solo, la quimioprevención con mefloquina más cotrimoxazol en las pacientes infectadas por VIH:
◦ reduce el riesgo de parasitemia periférica materna en el momento del parto y el riesgo de paludismo placentario;
◦ no logra cambios en la prevalencia de los resultados adversos del embarazo (como bajo peso al nacer, prematuridad, mortinatos y abortos y malformaciones congénitas) ni en la incidencia de episodios clínicos de paludismo durante el embarazo; y
◦ aumenta el riesgo de eventos adversos relacionados con los fármacos como vómitos y mareos.
En términos generales, la proporción alta de eventos adversos relacionados con la mefloquina constituye una barrera importante a su efectividad para el tratamiento preventivo del paludismo en las pacientes embarazadas.
¿Cuál es el grado de actualización de esta revisión?
Los autores de la revisión buscaron estudios hasta el 31 de enero de 2018.
Conclusiones de los autores
Summary of findings
| Mefloquine compared with sulfadoxine‐pyrimethamine for preventing malaria in pregnant women | ||||||
| Patient or population: HIV‐uninfected pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments (compared with sulfadoxine‐pyrimethamine) | |
| Risk with sulfadoxine‐pyrimethamine | Risk with mefloquine | |||||
| Clinical malaria episodes during pregnancy | ‐ | ‐ | IRR 0.83 | ‐ | ⊕⊕⊕⊕ | Mefloquine results in little or no difference in the incidence of clinical malaria episodes during pregnancy |
| Maternal peripheral parasitaemia at delivery | 43 per 1000 | 28 per 1000 (20 to 37) | RR 0.65 (0.48 to | 5455 (2 RCTs) | ⊕⊕⊕⊕ | Mefloquine results in lower maternal peripheral parasitaemia at delivery |
| Placental malaria | 52 per 1000 | 54 per 1000 | RR 1.04 | 4668 | ⊕⊕⊝⊝ Due to imprecision and heterogeneity | Mefloquine may result in little or no difference in placental parasitaemia |
| Maternal anaemia at delivery | 219 per 1000 | 184 per 1000 | RR 0.84 | 5469 | ⊕⊕⊕⊝ Due to imprecision | Mefloquine probably results in fewer women anaemic at delivery |
| Low birth weight | 117 per 1000 | 111 per 1000 | RR 0.95 | 5641 | ⊕⊕⊕⊕ | Mefloquine results in little or no difference in low birth weight |
| Stillbirths and abortions | 31 per 1000 | 37 per 1000 | RR 1.20 | 6219 | ⊕⊕⊕⊝ Due to imprecision | Mefloquine probably results in little or no difference in stillbirths or abortions |
| AEs: vomiting | 82 per 1000 | 390 per 1000 | RR 4.76 | 6272 | ⊕⊕⊕⊕ | Mefloquine results in a four‐fold increase in vomiting |
| AEs: dizziness | 94 per 1000 | 396 per 1000 | RR 4.21 | 6272 | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine probably results in a four‐fold increase in dizziness |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aNot downgraded for risk of bias: although one trial has serious risk of bias, the other is of good quality and exclusion of the smaller trial has little effect on the estimate of effect. | ||||||
| Mefloquine plus cotrimoxazole compared with cotrimoxazole for preventing malaria in pregnant women | ||||||
| Patient or population: HIV‐infected pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments (compared with cotrimoxazole) | |
| Risk with cotrimoxazole | Risk with mefloquine plus cotrimoxazole | |||||
| Clinical malaria episodes during pregnancy | ‐ | ‐ | IRR 0.76 (0.33 to 1.76) | ‐ (1 RCT) | ⊕⊕⊕⊕ | Mefloquine results in little or no difference in the incidence of clinical malaria episodes during pregnancy |
| Maternal peripheral parasitaemia at delivery (PCR) | 66 per 1000 | 34 per 1000 (20 to 62) | RR 0.52 (0.30 to 0.93) | 989 (2 RCTs) | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine probably results in lower maternal peripheral parasitaemia at delivery |
| Placental malaria (PCR) | 68 per 1000 | 19 per 1000 | RR 0.28 | 977 | ⊕⊕⊕⊕ | Mefloquine plus cotrimoxazole results in fewer women with placental malaria at delivery |
| Maternal anaemia at delivery | 178 per 1000 | 168 per 1000 | RR 0.94 | 1197 | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine plus cotrimoxazole probably results in little or no difference in maternal anaemia cases at delivery |
| Low birth weight | 118 per 1000 | 141 per 1000 | RR 1.20 | 1220 | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine plus cotrimoxazole probably results in little or no difference in the proportion of births that are low birth weight |
| Spontaneous abortions and stillbirths | 50 per 1000 | 56 per 1000 | RR 1.12 | 1347 | ⊕⊝⊝⊝ | We do not know if mefloquine plus cotrimoxazole results in a difference in spontaneous abortions and stillbirths |
| AEs: vomiting | 30 per 1000 | 239 per 1000 (144 to 396) | RR 7.95 (4.79 to 13.18) | 1055 (1 RCT)d | ⊕⊕⊕⊕ | Mefloquine plus cotrimoxazole results in an eight‐fold increase in vomiting |
| AEs: dizziness | 75 per 1000 | 296 per 1000 (214 to 411) | RR 3.94 (2.85 to 5.46) | 1055 (1 RCT)e | ⊕⊕⊕⊕ | Mefloquine plus cotrimoxazole results in a four‐fold increase in dizziness |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by 1 for risk of bias: one of the included trials is at serious risk of bias. | ||||||
Antecedentes
Descripción de la afección
El paludismo es la enfermedad parasitaria más importante en todo el mundo y es endémica en zonas de África, Asia y América del Sur. Las embarazadas se encuentran en mayor riesgo de infección por paludismo que las mujeres no embarazadas del mismo grupo etario y están en mayor riesgo de presentar una enfermedad grave (Brabin 1983; Desai 2007). La infección por paludismo durante el embarazo, en particular el primer o segundo embarazo, también se asocia con resultados adversos para la madre (anemia grave) y para el lactante (bajo peso al nacer, mortalidad neonatal; Ataíde 2014; Guyatt 2004; Menendez 2010; Radeva‐Petrova 2014; Schwarz 2008; Steketee 2001). Los síntomas informados más comúnmente por las pacientes embarazadas semiinmunes con paludismo clínico incluyen cefalea, artromialgias y fiebre (Bardaji 2008). En las zonas de transmisión baja, las pacientes embarazadas con parasitemia palúdica con frecuencia inician la enfermedad con síntomas y signos como fiebre, malestar general, cefalea y vómitos. Si no se trata, la infección puede dar lugar a complicaciones graves como paludismo cerebral y edema pulmonar y puede ser una causa de mortalidad materna (Bardaji 2008).
Para reducir la carga y las consecuencias del paludismo en el embarazo, la Organización Mundial de la Salud (OMS) actualmente recomienda que las pacientes embarazadas que viven en zonas de transmisión moderada a alta de paludismo en África duerman cubiertas por un mosquitero tratado con insecticida (MTI), como se describe en Jugar 2006 y reciban tratamiento preventivo intermitente (TPI) con sulfadoxina‐pirimetamina en cada visita programada de atención prenatal (siempre que las dosis se administren con un mes de diferencia) (WHO 2013). El TPI es una forma de quimioprevención del paludismo que se evaluó y adoptó como política en respuesta a los parásitos del paludismo que desarrollaban resistencia a la profilaxis semanal con cloroquina y en respuesta al cumplimiento bajo del régimen semanal (WHO 2004). La vida media de eliminación prolongada de la sulfadoxina‐pirimetamina permite utilizar dosis intermitentes, aunque aun así provee la cobertura profiláctica durante las semanas intermedias (White 2005). El TPI, por lo tanto, se define como la "administración de una dosis curativa de tratamiento de un fármaco antipalúdico efectivo a intervalos predefinidos durante el embarazo" independientemente de la presencia o la ausencia de infección actual (White 2005).
La sulfadoxina‐pirimetamina es el fármaco utilizado para el TPI en el embarazo, aunque la resistencia se ha propagado en muchas partes del sur y el este de África (ter Kuile 2007; OMS 2012a), lo que estimula a los investigadores y a los elaboradores de políticas a buscar alternativas seguras y efectivas a la sulfadoxina‐pirimetamina (Desai 2018).
Descripción de la intervención
La mefloquina es una 4‐metanolquinolina que está relacionada con la quinina. Fue desarrollada originalmente por los militares de los EE.UU. para la prevención del paludismo en los soldados y ha sido utilizada ampliamente para la prevención del paludismo en los viajeros (Schlagenhauf 2010). Al igual que la sulfadoxina‐pirimetamina, la mefloquina tiene una vida media de eliminación prolongada de dos a cuatro semanas; en los viajeros, la dosis semanal consiste en 250 mg (FDA 2004), y en las pacientes embarazadas es factible la dosis mensual en las dosis de tratamiento (Briand 2009).
La mefloquina se investigó por primera vez en los años noventa como tratamiento profiláctico para las pacientes embarazadas. Un estudio observacional generó preocupaciones en cuanto a que la mefloquina se puede asociar con un mayor riesgo de mortinatos (Nosten 1999); sin embargo, otros ensayos no confirmaron este hallazgo (Pekyi 2016; Steketee 1996). Una revisión sistemática consideró la seguridad de la mefloquina en el embarazo y estableció la conclusión de que no hay evidencia que indique que la administración de mefloquina en el embarazo conlleva un mayor riesgo para el feto (Gonzalez 2014). Se sabe que el fármaco se asocia con un rango de efectos secundarios leves transitorios relacionados con la dosis, como vómitos, náuseas y mareos (Bardaji 2012; Lee 2017; Sevene 2010; ter Kuile 1995). Los investigadores han descrito efectos secundarios neurosiquiátricos graves que ocurren en alrededor de uno en 10 000 viajeros que reciben mefloquina como quimioprofilaxis (Phillips‐Howard 1995; Steffen 1993). Los estudios realizados en pacientes embarazadas beninesas encontraron que los mareos y los vómitos son los efectos adversos más frecuentes relacionados con la administración de mefloquina como TPI en el embarazo (Briand 2009; Denoeud‐Ndam 2012).
Los datos muestran resistencia a la mefloquina en las áreas de resistencia a múltiples fármacos de Tailandia (Carrara 2009; Nosten 2000), pero todavía es poco frecuente en África (Aubouy 2007; MacArthur 2001; Oduola 1987).
De qué manera podría funcionar la intervención
Se cree que la quimioprevención del paludismo funciona mediante la eliminación o la supresión de la infección asintomática por paludismo en la sangre periférica de la madre y la placenta (White 2005). Sin embargo, esta reducción de la parasitemia palúdica puede no ser suficiente para justificar las recomendaciones de prescripciones profilácticas generalizadas que no proporcionan un beneficio posterior en resultados clínicamente importantes para la madre y el recién nacido. Estos resultados pueden incluir una reducción de los episodios de paludismo materno, una reducción en el riesgo de anemia y una mejoría en el peso al nacer, así como resultados más significativos como una reducción de la enfermedad materna grave o tasas inferiores de pérdida espontánea del embarazo y de mortalidad materna, neonatal e infantil (ver Figura 1).
Los efectos de la quimioprevención del paludismo pueden depender de la epidemiología local del paludismo y, por lo tanto, del nivel de inmunidad adquirido contra el paludismo en las pacientes embarazadas. En las zonas de transmisión estable, las pacientes en edad fecunda pueden ser parcialmente inmunes al paludismo, y presentar parasitemia sin enfermedades clínicas; sin embargo, las infecciones asintomáticas pueden tener efectos perjudiciales, como anemia y bajo peso al nacer. Por el contrario, en las zonas de transmisión inestable del paludismo, la inmunidad contra el paludismo naturalmente adquirida por lo general es baja entre los adultos y la infección por paludismo se puede asociar con episodios clínicos y enfermedad grave.
Las pacientes primigrávidas se encuentran en mayor riesgo de efectos adversos de la infección por paludismo que las pacientes multigrávidas. Se cree que este hecho se debe a que las pacientes desarrollan anticuerpos específicos para los parásitos de tipo placentario cuando se exponen al Plasmodium falciparum durante el primer embarazo. Estos anticuerpos luego están presentes en los embarazos posteriores (Ataíde 2014). Lo anterior se observa en las pacientes multigrávidas como una respuesta inmunitaria más específica y eficiente y la eliminación de la infección en un estadio más temprano que en las pacientes primigrávidas (Walker 2013).
Otro modificador potencial del efecto de la susceptibilidad a la infección por paludismo es el estado del VIH (Menéndez 2011). En muchas áreas donde el paludismo es endémico, los datos indican que la prevalencia de infección por VIH, que se ha observado aumenta el riesgo de infección por paludismo, es alta entre las pacientes embarazadas (Gonzalez 2012; van Eijk 2003). En comparación con las pacientes sin infección por HIV, las pacientes con infección por VIH tienen una mayor probabilidad de transportar los parásitos del paludismo en la sangre, de tener mayores densidades de parásitos, y de desarrollar parasitemia placentaria, anemia, y síntomas de paludismo (Ayisi 2003; van Eijk 2002; van Eijk 2003). Este mayor riesgo de paludismo es el mismo en las mujeres multigrávidas (mujeres en su tercer embarazo o más) y en las mujeres que cursan su primer o segundo embarazo (ter Kuile 2004; van Eijk 2003). La infección por paludismo placentario también puede aumentar el riesgo de transmisión perinatal maternoinfantil del VIH (Ayisi 2003).
Se ha demostrado que el uso de MTI durante el embarazo tiene un impacto beneficioso sobre los resultados del embarazo (reducción de la prevalencia del bajo peso al nacer, el aborto espontáneo y la parasitemia placentaria) en zonas de África donde el paludismo es endémico (Gamble 2007), y este enfoque podría modificar el efecto del TPI (Menéndez 2008).
Por qué es importante realizar esta revisión
La OMS recomienda el TPI con sulfadoxina‐pirimetamina en todas las pacientes embarazadas que viven en zonas de transmisión moderada a alta de paludismo en África (WHO 2004; WHO 2013). Sin embargo, los estudios han mostrado que la resistencia a la sulfadoxina‐pirimetamina en algunas regiones del este de África ha aumentado de forma sostenida durante las dos últimas décadas (Iriemenam 2012; Mockenhaupt 2008). Por lo tanto, hay una necesidad urgente de antipalúdicos más efectivos para la prevención del paludismo durante el embarazo.
Esta revisión procura evaluar la eficacia y la seguridad de la mefloquina para la prevención del paludismo en las pacientes embarazadas. Estos resultados podrían servir de base para las guías futuras sobre los agentes preventivos para el paludismo en pacientes embarazadas.
Objetivos
Evaluar los efectos de la mefloquina para la prevención del paludismo en las pacientes embarazadas ‐ específicamente, evaluar:
-
la eficacia, la seguridad y la tolerabilidad de la mefloquina para la prevención del paludismo en las pacientes embarazadas; y
-
el impacto del estado del VIH, el número de gestaciones y el uso de mosquiteros tratados con insecticidas (MTI) en los efectos de la mefloquina.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos controlados aleatorios (ECA) y cuasialeatorios.
Tipos de participantes
Embarazadas con un número cualquiera de gestaciones, independientemente del estado del VIH, que vivieran en áreas donde el paludismo es endémico (CDC 2017).
Tipos de intervenciones
Intervenciones
Mefloquina administrada a las pacientes embarazadas como tratamiento preventivo intermitente o como quimioprofilaxis.
Controles
Placebo, ninguna intervención o un régimen medicamentoso alternativo.
Tipos de medida de resultado
Maternos
-
Parasitemia periférica materna durante el embarazo
-
Parasitemia periférica materna en el momento del parto
-
Paludismo placentario¹
-
Hemoglobina media y anemia materna (moderada y grave) en el momento del parto
-
Episodios clínicos de paludismo durante el embarazo
Fetales/infantiles
-
Parasitemia en la sangre del cordón
-
Hemoglobina y anemia en la sangre del cordón (según lo definido en los estudios originales)
-
Media del peso al nacer
-
Prevalencia del bajo peso al nacer (< 2500 g)
-
Prevalencia de prematuridad (< 37 semanas de gestación)
-
Morbilidad en el primer año de vida
Eventos adversos.
-
Eventos adversos graves (EAG)²
-
Enfermedades potencialmente mortales o que requirieron la hospitalización durante el embarazo (EAG en el embarazo)
-
Resultados adversos del embarazo: aborto espontáneo, mortinatos, malformación congénita
-
Mortalidad materna
-
Mortalidad perinatal, neonatal, infantil
-
Frecuencia de la transmisión maternoinfantil del VIH (a las seis semanas de edad)
-
-
Eventos adversos no graves
-
Frecuencia y gravedad de los eventos adversos informados por todas las causas y relacionados con los fármacos
-
¹Paludismo placentario diagnosticado mediante histología, microscopía o cualquier método usado en el estudio incluido. La Figura 2 muestra las relaciones entre diferentes resultados.
²Los autores de la revisión reconocen la limitación del análisis de los eventos adversos graves poco frecuentes debido a que los ensayos controlados aleatorios generalmente no tienen el poder estadístico suficiente para detectarlos.
Métodos de búsqueda para la identificación de los estudios
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Búsquedas electrónicas
We searched the following databases using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register (up to 31 January 2018); the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (January 2018); MEDLINE (PubMed; from 1966 to 31 January 2018); Embase (OVID; 1974 to 31 January 2018); and Latin American Caribbean Health Sciences Literature (LILACS) (BIREME; 1982 to 31 January 2018). We also searched the Malaria in Pregnancy (MiP) Library (www.mip‐consortium.org/resources/index.htm), the WHO International Clinical Trial Registry Platform (ICTRP; www.who.int/ictrp/search/en), ClinicalTrials.gov, and the International Standard Randomized Controlled Trial Number (ISRCTN) registry (www.isrctn.com/), using ‘mefloquine', ‘malaria', and ‘pregnan*' as search terms.
Búsqueda de otros recursos
We contacted researchers working in the field to ask for unpublished data, confidential reports, and raw data from published trials. We also checked the citations of all trials identified by the methods described.
Obtención y análisis de los datos
Selección de los estudios
Two review authors independently screened all trials identified by the search strategy by title or abstract, or both (Appendix 1). We coded studies as ‘retrieve' or ‘do not retrieve'. We retrieved the full‐text copies of trials deemed potentially relevant. Two review authors then independently assessed study eligibility using a form based on the review inclusion criteria. We resolved disagreements through discussion or by consultation with a third review author. Any review author who participated in trials that potentially met the review inclusion criteria did not participate in the procedure to select studies for inclusion. We listed all studies excluded after full‐text assessment and reasons for their exclusion in a ‘Characteristics of excluded studies' table. We illustrated the study selection process in a PRISMA diagram.
Extracción y manejo de los datos
Three review authors (RG, CPD, and MP) used a data extraction form to independently extract data on trial characteristics, including trial site, year, local malaria transmission estimates, antimalarial resistance pattern of mefloquine and the comparator drug (when possible), trial methods, participants, interventions, doses, and outcomes.
We extracted the number of participants randomized and the number of participants analyzed in experimental and control groups for each outcome. For dichotomous outcomes, we extracted the number of participants experiencing the event and the number assessed in each treatment group. For continuous outcomes, we extracted the arithmetic means, standard deviations for each treatment group (when provided), and the number of participants assessed in each group. We also extracted medians and ranges when provided. For outcomes reported as incidences, we extracted the number of participants experiencing the event (cases) and the person‐years at risk.
Any review author who participated in any of the trials included in the review did not participate in data extraction nor ‘Risk of bias' assessment of their own articles.
Evaluación del riesgo de sesgo de los estudios incluidos
Two review authors independently assessed the risk of bias for each included trial using the Cochrane ‘Risk of bias' assessment tool (Higgins 2011). This approach assesses the risk of bias across seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other potential sources of bias (Higgins 2011). For each domain, we assigned a judgment of low, high, or unclear risk of bias. We judged the risk of bias for blinding on the presence of blinding and whether lack of blinding could potentially influence the results.
Medidas del efecto del tratamiento
We presented dichotomous outcomes using risk ratios (RRs), count outcomes as incidence rate ratios (IRRs), and continuous outcomes as mean differences (MDs). We presented all measures of effect with 95% confidence intervals (CIs).
Cuestiones relativas a la unidad de análisis
When conducting a meta‐analysis, we ensured that participants and cases in the placebo group were not counted more than once.
Manejo de los datos faltantes
We aimed to conduct the analysis according to the intention‐to‐treat principle. However, when there was loss to follow‐up, we used a complete‐case analysis such that participants for whom no outcome was reported were excluded from the analysis. This analysis assumes that participants for whom an outcome is available are representative of the original randomized patients. We aimed to conduct a sensitivity analysis to evaluate the robustness of this method, but this was not possible, as described below. If data from trial reports were insufficient, unclear, or missing, we contacted the study authors for additional information.
Evaluación de la heterogeneidad
We calculated the I2 statistic using values of 30% to 59%, 60% to 89%, and 90% to 100% to denote moderate, substantial, and considerable levels of heterogeneity, respectively.
Evaluación de los sesgos de notificación
We aimed to assess the risk of publication bias by constructing funnel plots and looking for asymmetry, but the small number of trials included in each comparison of the meta‐analysis made this assessment impossible.
Síntesis de los datos
We performed data analysis using Review Manager 5 (RevMan 5) (RevMan 2014). We intended to perform subgroup analysis by gravidity and HIV status when possible. HIV status subgroup analysis was not possible in any case owing to different study designs for different HIV status populations. In the absence of heterogeneity, we used a fixed‐effect model for the meta‐analysis; when we detected moderate or considerable heterogeneity, we used a random‐effects model. Additionally, we assessed the certainty of evidence using the GRADE approach (GRADEpro GDT 2015) for the following main outcomes of analysis: maternal peripheral parasitaemia at delivery, clinical malaria episodes during pregnancy, placental malaria, maternal anaemia at delivery, low birth weight, spontaneous abortion and stillbirth, dizziness, and vomiting.
Análisis de subgrupos e investigación de la heterogeneidad
We aimed to investigate heterogeneity by conducting prespecified subgroup analysis to evaluate the contributions of differences in trial characteristics such as risk of bias, geographical region, malaria transmission pattern, antimalarial resistance, drug regimen, use of ITNs, gravidity (primigravidae versus multigravidae), HIV status (uninfected, infected, unknown), and trial methods. Only the gravidity subgroup analysis was possible for one outcome of the main comparison. The other subgroup analyses were not possible because of the small number of trials included in each comparison.
Análisis de sensibilidad
We planned to conduct a sensitivity analysis to restore the integrity of the randomization process and to test the robustness of our results; however, the small number of trials included in each comparison – two at most – made this impossible. Additionally, missing outcome data were balanced in numbers across intervention groups, and reasons for missing data were similar across groups.
Results
Description of studies
Results of the search
The literature search, conducted up to 31 January 2018, identified 254 references, of which two were duplicate trial reports. Of the 252 remaining articles, we excluded 231 articles and one ongoing trial after title/abstract screening. We assessed 20 full‐text articles for eligibility, of which we excluded 14 articles. Six trials (in six publications) met the inclusion criteria of the review (Figure 3).
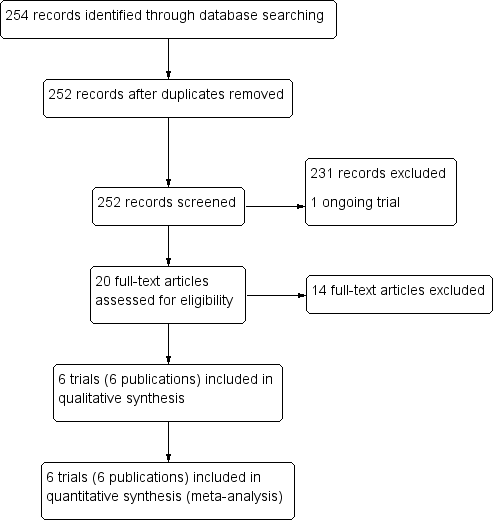
Study flow diagram.
Included studies
Six chemoprevention trials that included 8192 pregnant women met our inclusion criteria (see the Characteristics of included studies section). These trials were conducted between 1987 and 2013 in Thailand (one trial), Benin (three trials), Gabon (one trial), Kenya (one trial), Mozambique (two trials), and Tanzania (two trials).
The included trials recruited women of all gravidities of all ages (four trials) or over 18 years of age (two trials). Gestational age at recruitment was greater than 20 weeks (one trial), between 16 and 28 weeks (three trials), or ≤ 28 weeks (two trials).
Two trials evaluated mefloquine against sulfadoxine‐pyrimethamine as IPTp in HIV‐uninfected pregnant women. Three trials evaluated mefloquine IPTp alone (or in combination with daily cotrimoxazole) against cotrimoxazole in HIV‐infected pregnant women. Finally, one trial in Thailand compared weekly mefloquine prophylaxis against placebo in women of unknown HIV status. All included trials reported that drug administration was supervised.
All included trials recruited women in all gravidity groups; five reported aggregate results and one disaggregated by gravidity for the primary outcome. In five trials, all women in both intervention and control groups received a long‐lasting ITN at recruitment and iron, and investigators routinely administered folic acid.
Excluded studies
We excluded one trial for the reasons given in the ‘Characteristics of excluded studies' table.
Risk of bias in included studies
See Figure 4 and Figure 5 for a summary of the ‘Risk of bias' assessments. We have presented further details in the ‘Characteristics of included studies' table.

‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
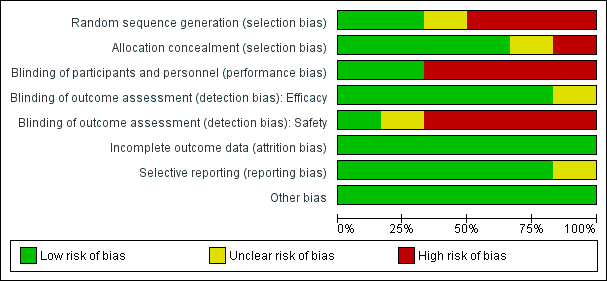
‘Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation (selection bias)
Two trials adequately described methods of sequence generation (Gonzalez 2014a BEN GAB MOZ TAN; Gonzalez 2014b KEN MOZ TAN), three described a non‐random component in the sequence generation process (Briand 2009 BEN; Denoeud‐Ndam 2014a BEN; Denoeud‐Ndam 2014b BEN), and in the remaining trial, the risk was unclear (Nosten 1994 THA).
Allocation concealment (selection bias)
Four trials described adequate methods of allocation concealment (Denoeud‐Ndam 2014a BEN; Denoeud‐Ndam 2014b BEN; Gonzalez 2014a BEN GAB MOZ TAN; Gonzalez 2014b KEN MOZ TAN), one trial reported no concealment of allocation (Briand 2009 BEN), and in the remaining trial, the risk was unclear (Nosten 1994 THA).
Blinding
Blinding of participants and personnel (performance bias)
Four trials were open (Briand 2009 BEN; Denoeud‐Ndam 2014a BEN; Denoeud‐Ndam 2014b BEN; Gonzalez 2014a BEN GAB MOZ TAN), and we assessed these as having high risk of performance risk. Two trials were double‐blind and placebo‐controlled (Gonzalez 2014b KEN MOZ TAN; Nosten 1994 THA), and we assessed these as having low risk of performance bias.
Blinding of efficacy outcome assessment (detection bias)
For five trials, we judged the efficacy outcome as not influenced by blinding or lack of blinding. In the remaining trial, the risk of detection bias for efficacy outcomes was unclear (Nosten 1994 THA).
Blinding of safety outcome assessment (detection bias)
For the four open trials, we judged the risk of detection bias as high for assessment of safety outcomes (Briand 2009 BEN; Denoeud‐Ndam 2014a BEN; Denoeud‐Ndam 2014b BEN; Gonzalez 2014a BEN GAB MOZ TAN). In one trial, the risk of detection bias was unclear (Nosten 1994 THA). For the remaining trial, which was double‐blinded, we judged the risk of detection bias as low (Gonzalez 2014b KEN MOZ TAN).
Incomplete outcome data
In all included trials, missing outcome data were balanced in numbers across groups, and we judged the risk of attrition bias to be low.
Selective reporting
We considered the risk of reporting bias as low in five trials and unclear in one (Nosten 1994 THA).
Other potential sources of bias
All included trials appeared to be free of other sources of bias, and we judged this risk as low.
Effects of interventions
See: Summary of findings for the main comparison Mefloquine compared with sulfadoxine‐pyrimethamine for preventing malaria in pregnant women; Summary of findings 2 Mefloquine plus cotrimoxazole compared with cotrimoxazole for preventing malaria in pregnant women
Comparison 1: Mefloquine versus sulfadoxine‐pyrimethamine (HIV‐uninfected pregnant women)
See summary of findings Table for the main comparison.
Maternal outcomes
We included in this comparison two trials that evaluated two doses of IPTp (Briand 2009 BEN; Gonzalez 2014a BEN GAB MOZ TAN). Data show a decrease in the number of clinical malaria episodes during pregnancy among mefloquine recipients, but this does not clearly constitute an effect of mefloquine because the 95% CIs do not exclude the possibility of no different effects (IRR 0.83, 95% CI 0.65 to 1.05; 2 studies; high‐certainty evidence; Analysis 1.1). Overall, IPTp‐mefloquine was associated with a 35% reduction in the risk of maternal peripheral parasitaemia at delivery (RR 0.65, 95% CI 0.48 to 0.86; 5455 participants, 2 studies; I2 statistic = 16%; high‐certainty evidence; Analysis 1.2), but the absolute difference between treatments was small. We found no significant evidence of an effect of mefloquine or sulfadoxine‐pyrimethamine on placental malaria infections (RR 1.04, 95% CI 0.58 to 1.86; 4668 participants, 2 studies; I2 statistic = 63%; low‐certainty evidence; Analysis 1.3). The mefloquine group showed a slight increase in the mean haemoglobin level at delivery (MD 0.10, 95% CI 0.01 to 0.19; 5588 participants, 2 studies; I2 statistic = 0%; Analysis 1.4) and a decrease in maternal anaemia cases at delivery (RR 0.84, 95% CI 0.76 to 0.94; 5469 participants, 2 studies; I2 statistic = 0%; moderate‐certainty evidence; Analysis 1.5), but the data show no significant differences in severe maternal anaemia at delivery between groups (RR 0.93, 95% CI 0.58 to 1.48; 5469 participants, 2 studies; I2 statistic = 41%; Analysis 1.6). The original definitions of maternal moderate anaemia and severe maternal anaemia were different in the two trials included in the analysis (Gonzalez 2014a BEN GAB MOZ TAN defined anaemia as haemoglobin < 11 g/dL and severe anaemia as haemoglobin < 7 g/dL), but we homogenized data for the analysis as < 9.5 g/dL and < 8 g/dL (as defined in Briand 2009 BEN), respectively.
Foetal/infant outcomes
No effect was evident for the outcomes of cord blood parasitaemia (RR 0.44, 95% CI 0.13 to 1.46; 5309 participants, 2 studies; I2 statistic = 33%; Analysis 1.7) and cord blood anaemia (RR 1.04, 95% CI 0.87 to 1.23; 4006 participants, 1 study; Analysis 1.8).
Regarding newborn outcomes, mean birth weight did not show significant differences between groups (MD 2.52, 95% CI ‐25.66 to 30.69; 5241 participants, 2 studies; I2 statistic = 0%; Analysis 1.9). Low birth weight (RR 0.95, 95% CI 0.78 to 1.17; 5641 participants, 2 studies; I2 statistic = 33%; high‐certainty evidence; Analysis 1.10) and prematurity prevalence (RR 1.03, 95% CI 0.76 to 1.40; 4640 participants, 2 studies; I2 statistic = 0%; Analysis 1.12) also showed no differences between groups. Subgroup analysis of low birth weight by gravidity yielded results that did not vary (primigravidae: RR 1.02, 95% CI 0.80 to 1.30; 1576 participants, 2 studies; I2 statistic = 3%; Analysis 1.11; multigravidae: RR 0.94, 95% CI 0.78 to 1.14; 4065 participants, 2 studies; I2 statistic = 0%; Analysis 1.11).
Only one trial reported data on infant morbidity, and results followed the same trend; the IRR was near 1, and the CIs did not discard the possibility of no difference between mefloquine and sulfadoxine‐pyrimethamine. Chosen proxies for infant morbidity were malaria in the first year of life (IRR 0.97, 95% CI 0.82 to 1.15; 1 study; Analysis 1.13) and hospital admissions in the first year of life (IRR 0.93, 95% CI 0.75 to 1.17; 1 study; Analysis 1.14).
Safety outcomes
No difference was evident between mefloquine and sulfadoxine‐pyrimethamine in overall serious adverse events reporting (RR 0.98, 95% CI 0.81 to 1.20; 4674 participants, 1 study; Analysis 1.15). Definitions of stillbirth and abortion were different for the two trials included in this comparison; therefore we aggregated both outcomes into a single outcome (RR 1.20, 95% CI 0.91 to 1.58; 6219 participants, 2 studies; I2 statistic = 0%; moderate‐certainty evidence; Analysis 1.16). Congenital malformation cases were also similar in both intervention groups (RR 1.10, 95% CI 0.51 to 2.37; 5931 participants, 2 studies; I2 statistic = 33%; Analysis 1.17).
Regarding maternal mortality, one of the trials reported maternal deaths only in the mefloquine group, and the other trial showed a similar proportion of maternal deaths in both IPTp groups; the CI of the meta‐analysis was wide, and heterogeneity was moderate (RR 2.41, 95% CI 0.27 to 21.23; 6219 participants, 2 studies; I2 statistic = 54%; Analysis 1.18). Only one of the trials reported neonatal and infant mortality (Gonzalez 2014a BEN GAB MOZ TAN), but we obtained neonatal mortality rates for the other trial by contacting the study authors (Briand 2009 BEN). Neither of the two outcomes showed a significant effect of mefloquine or sulfadoxine‐pyrimethamine (neonatal deaths: RR 0.98, 95% CI 0.67 to 1.43; 6134 participants, 2 studies; I2 statistic = 0%; Analysis 1.19; incidence of infant deaths: IRR 1.00, 95% CI 0.66 to 1.52; 1 study; Analysis 1.20).
Overall, IPTp‐mefloquine increased the risk of adverse events; results of individual trials and of meta‐analyses were significant for vomiting (RR 4.76, 95% CI 4.13 to 5.49; 6272 participants, 2 studies; I2 statistic = 0%; high‐certainty evidence; Analysis 1.21), fatigue/weakness (RR 4.62, 95% CI 1.80 to 11.85; 6272 participants, 2 studies; I2 statistic = 91%; high‐certainty evidence; Analysis 1.22), and dizziness (RR 4.21, 95% CI 3.36 to 5.27; 6272 participants, 2 studies; I2 statistic = 66%; moderate‐certainty evidence; Analysis 1.23), with the exception of headache (RR 0.70, 95% CI 0.25 to 1.94; 6272 participants, 2 studies; I2 statistic = 85%; Analysis 1.24).
Comparison 2: Mefloquine plus cotrimoxazole versus cotrimoxazole (HIV‐infected pregnant women)
See summary of findings Table 2.
Maternal outcomes
This comparison included two trials evaluating three IPTp doses of mefloquine (Denoeud‐Ndam 2014a BEN; Gonzalez 2014b KEN MOZ TAN). Only one of the trials reported clinical malaria episodes during pregnancy, noting no significant differences in malaria episodes between groups (IRR 0.76, 95% CI 0.33 to 1.76; 1 study; high‐certainty evidence; Analysis 2.1). IPTp‐mefloquine plus cotrimoxazole prophylaxis was associated with a 48% reduction in the risk of maternal peripheral parasitaemia at delivery measured by polymerase chain reaction (PCR) (RR 0.52, 95% CI 0.30 to 0.93; 989 participants, 2 studies; I2 statistic = 0%; moderate‐certainty evidence; Analysis 2.2), a 49% reduction in the risk of placental malaria measured by blood smear (RR 0.51, 95% CI 0.29 to 0.89; 1144 participants, 2 studies; I2 statistic = 0%; Analysis 2.3), and a 72% reduction in the risk of placental malaria measured by PCR (RR 0.28, 95% CI 0.14 to 0.57; 977 participants, 2 studies; I2 statistic = 0%; moderate‐certainty evidence; Analysis 2.4). The other maternal‐related outcomes at delivery included in this comparison did not show evidence that they were effects of mefloquine owing to the wideness of the CIs (mean haemoglobin: MD 0.07, 95% CI ‐0.32 to 0.46; 1167 participants, 2 studies; I2 statistic = 62%; Analysis 2.5; maternal anaemia: RR 0.94, 95% CI 0.73 to 1.20; 1197 participants, 2 studies; I2 statistic = 12%; moderate‐certainty evidence; Analysis 2.6; severe maternal anaemia: RR 0.93, 95% CI 0.41 to 2.08; 1167 participants, 2 studies; I2 statistic = 0%; Analysis 2.7). The original definitions of maternal anaemia were different in the two trials included in the analysis (Gonzalez 2014b KEN MOZ TAN defined anaemia as haemoglobin < 11 g/dL), but we homogenized definitions for the analysis as < 9.5 g/dL (as defined in Denoeud‐Ndam 2014a BEN). The two trials defined severe maternal anaemia as haemoglobin < 7 g/dL.
Foetal/infant outcomes
Meta‐analyses of foetal and neonatal outcomes were underpowered to detect significant effects of mefloquine on cord blood parasitaemia (RR 0.33, 95% CI 0.03 to 3.13; 1166 participants, 2 studies; I2 statistic = 0%; Analysis 2.8), mean birth weight (MD ‐25.75, 95% CI ‐86.99 to 35.49; 1220 participants, 2 studies; I2 statistic = 0%; Analysis 2.9), low birth weight rates (RR 1.20, 95% CI 0.89 to 1.60; 1220 participants, 2 studies; I2 statistic = 0%; moderate‐certainty evidence; Analysis 2.10), and prematurity rates (RR 1.07, 95% CI 0.58 to 1.72; 824 participants, 2 studies; I2 statistic = 32%; Analysis 2.11). These CIs did not exclude the possibility of no different effects between groups.
Safety outcomes
Overall, serious adverse events during pregnancy were significantly less frequent in the group of IPTp‐mefloquine plus cotrimoxazole prophylaxis than in the cotrimoxazole alone group (RR 0.69, 95% CI 0.50 to 0.95; 1347 participants, 2 studies; I2 statistic = 0%; Analysis 2.12). However, analysis of individual adverse events did not show differences between groups, for example, spontaneous abortions and stillbirths (RR 1.12, 95% CI 0.42 to 2.98; 1347 participants, 2 studies; I2 statistic = 69%; very low‐certainty evidence; Analysis 2.13) and congenital malformations (RR 0.61, 95% CI 0.22 to 1.67; 1312 participants, 2 studies; I2 statistic = 0%; Analysis 2.14). Definitions of spontaneous abortion and stillbirth were different in the two included trials (that is, difference in the gestational age cutoff for classifying miscarriage or stillbirth); therefore, we combined both indicators and analyzed them as one. Only one trial included information on maternal deaths (Gonzalez 2014b KEN MOZ TAN), and we obtained this information by contacting the authors in the other trial (Denoeud‐Ndam 2014a BEN). Analyses of maternal deaths revealed no significant differences between groups (RR 0.51, 95% CI 0.13 to 2.01; 1347 participants, 2 studies; I2 statistic = 0%; Analysis 2.15). Also, we found that neonatal mortality rates were not significantly different among groups, as revealed by the CI (RR 1.32, 95% CI 0.65 to 2.69; 1239 participants, 2 studies; I2 statistic = 0%; Analysis 2.16). It is important to note that mefloquine plus cotrimoxazole recipients were at 1.92 times greater risk of mother‐to‐child transmission of HIV than the group that took only cotrimoxazole (RR 1.92, 95% CI 1.13 to 3.25; 1019 participants, 2 studies; I2 statistic = 0%; Analysis 2.17).
Vomiting, fatigue/weakness, and dizziness displayed substantial and considerable levels of heterogeneity in the meta‐analysis. Individual trials showed significant increases in three drug‐related adverse events in the groups given IPTp‐mefloquine plus cotrimoxazole prophylaxis, but random‐effects analyses show a significant effect of IPTp‐mefloquine only in the case of vomiting (RR 20.88, 95% CI 1.40 to 311.66; 1347 participants, 2 studies; I2 statistic = 74%; Analysis 2.18), while fatigue (RR 2.95, 95% CI 0.26 to 32.93; 1347 participants, 2 studies; I2 statistic = 91%; Analysis 2.19) and dizziness (RR 16.34, 95% CI 0.39 to 684.99; 1347 participants, 2 studies; I2 statistic = 86%; Analysis 2.20) show no significant evidence. In the three cases, CIs are considerably wide. Headache cases were not significantly different across groups (RR 0.76, 95% CI 0.28 to 2.10; 1347 participants, 2 studies; I2 statistic = 30%; Analysis 2.21).
Comparison 3: Mefloquine versus cotrimoxazole (HIV‐infected pregnant women)
Maternal outcomes
Only one trial conducted in Benin provided data on this comparison of three IPTp‐mefloquine doses versus cotrimoxazole prophylaxis (Denoeud‐Ndam 2014b BEN). The few observations reported in the trial made the analyses, in general, underpowered to detect differences between groups. Efficacy outcomes directly related to malaria yielded RR indicating beneficial effects of IPTp‐mefloquine in reducing infection, but CIs did not exclude the possibility of no difference between groups (maternal peripheral parasitaemia during pregnancy measured by PCR: RR 0.21, 95% CI 0.03 to 1.72; 98 participants, 1 study; Analysis 3.1; placental malaria measured by PCR: RR 0.73, 95% CI 0.13 to 4.15; 94 participants, 1 study; Analysis 3.2; placental malaria measured by blood smear: RR 0.35, 95% CI 0.01 to 8.30; 108 participants, 1 study; Analysis 3.3). Data show no differences across groups for mean haemoglobin (MD ‐0.10, 95% CI ‐0.67 to 0.47; 100 participants, 1 study; Analysis 3.4) or maternal anaemia at delivery (RR 0.90, 95% CI 0.26 to 3.16; 100 participants, 1 study; Analysis 3.5).
Foetal/infant outcomes
All newborn outcomes included in the trial displayed wide CIs, providing no evidence of differences between groups (mean birth weight: MD ‐102.00, 95% CI ‐255.52 to 51.52; 120 participants, 1 study; Analysis 3.6; low birth weight rate: RR 1.52, 95% CI 0.56 to 4.13; 120 participants, 1 study; Analysis 3.7; prematurity rate: RR 1.08, 95% CI 0.33 to 3.56; 125 participants, 1 study; Analysis 3.8).
Safety outcomes
Serious adverse events reported in the trial were balanced across groups and were infrequent. The CIs reveal the possibility of no different effects between interventions in overall serious adverse events (RR 1.06, 95% CI 0.28 to 4.07; 140 participants, 1 study; Analysis 3.9), stillbirths (RR 4.30, 95% CI 0.49 to 37.49; 139 participants, 1 study; Analysis 3.10), spontaneous abortions (RR 1.07, 95% CI 0.07 to 16.84; 139 participants, 1 study; Analysis 3.11), and congenital malformations (RR 1.07, 95% CI 0.16 to 7.41; 139 participants, 1 study; Analysis 3.12). No maternal deaths occurred during the trial (139 participants, 1 study; Analysis 3.13), and only one neonate in each intervention group died (RR 1.05, 95% CI 0.07 to 16.39; 129 participants, 1 study; Analysis 3.14). The trial did not record infant mortality and regarded infant deaths after seven days of birth until six weeks of age as a proxy; small numbers of observations and infant deaths made demonstration of differences between groups impossible (RR 2.10, 95% CI 0.19 to 22.54; 129 participants, 1 study; Analysis 3.15).
Drug‐related adverse events were significantly more frequent in the mefloquine group. Despite wide CIs, results show an effect of mefloquine in increasing the frequency of vomiting (RR 13.43, 95% CI 3.31 to 54.54; 139 participants, 1 study; Analysis 3.16), fatigue/weakness (RR 6.99, 95% CI 1.64 to 29.81; 139 participants, 1 study; Analysis 3.17), and dizziness (RR 52.60, 95% CI 3.26 to 848.24; 139 participants, 1 study; Analysis 3.18). Data show no differences between groups in drug‐related headache (RR 0.21, 95% CI 0.01 to 4.39; 139 participants, 1 study; Analysis 3.19).
Comparison 4: Mefloquine versus placebo (pregnant women of unknown HIV status)
Maternal and foetal/infant outcomes
Only one trial provided data on this comparison, which comprised two phases of mefloquine prophylaxis with different doses of the drug (Nosten 1994 THA); the results belong to the pooled samples of both trial phases. This trial did not report clinical malaria episodes during pregnancy, maternal anaemia at delivery, cord blood parasitaemia and anaemia, serious adverse events, neonatal mortality, and adverse events, or data reporting was incomplete.
The only observed significant effect that could be attributed to mefloquine was the decrease in maternal peripheral parasitaemia at delivery (RR 0.13, 95% CI 0.05 to 0.33; 339 participants, 1 study; Analysis 4.1). The other efficacy outcomes evaluated in this trial ‐ both maternal and newborn‐related outcomes ‐ showed wide CIs and did not demonstrate different effects between placebo and mefloquine prophylaxis (placental malaria: RR 0.14, 95% CI 0.01 to 2.68; 220 participants, 1 study; Analysis 4.2; mean birth weight: MD ‐80.00, 95% CI ‐184.65 to 24.65; 290 participants, 1 study; Analysis 4.3; low birth weight: RR 1.39, 95% CI 0.78 to 2.48; 290 participants, 1 study; Analysis 4.4; prematurity: RR 0.48, 95% CI 0.15 to 1.53; 199 participants, 1 study; Analysis 4.5).
Safety outcomes
This trial reported only serious adverse events, and adverse events data were not complete in the published article. Stillbirths were more prevalent in the group given mefloquine prophylaxis, but the small number of observed events made the analysis unpowered to detect differences between groups (RR 2.63, 95% CI 0.86 to 8.08; 311 participants, 1 study; Analysis 4.6). Investigators reported only three spontaneous abortions and five congenital malformations, thus the CIs of analyses were very wide to detect differences in effects (spontaneous abortion: RR 0.48, 95% CI 0.04 to 5.22; 311 participants, 1 study; Analysis 4.7; congenital malformation: RR 3.82, 95% CI 0.43 to 33.83; 311 participants, 1 study; Analysis 4.8). During the trial, only one maternal death occurred in the mefloquine group, but the power of the analysis was too low to attribute the effects to an intervention (RR 2.95, 95% CI 0.12 to 71.85; 339 participants, 1 study; Analysis 4.9). Infant deaths were equally frequent in both trial groups (RR 1.04, 95% CI 0.63 to 1.74; 288 participants, 1 study; Analysis 4.10).
Discusión
Resumen de los resultados principales
En esta revisión Cochrane se incluyeron seis ensayos, que incluyeron 8192 mujeres embarazadas.
En las pacientes sin infección por HIV, dos dosis de tratamiento preventivo intermitente con mefloquina en el embarazo (TPIe con mefloquina) redujeron el riesgo de parasitemia periférica materna en el momento del parto en el 35% (evidencia de certeza alta) y el riesgo de anemia en el 16% (evidencia de certeza moderada) en comparación con dos dosis del tratamiento preventivo intermitente con sulfadoxina‐pirimetamina en el embarazo (TPIe con sulfadoxina‐pirimetamina). Los investigadores no presentaron evidencia significativa de un efecto de la mefloquina sobre el paludismo placentario, la parasitemia y la anemia en la sangre del cordón, la media del peso al nacer, la prevalencia del bajo peso al nacer, la prematuridad, los mortinatos y los abortos y las malformaciones congénitas. En general, el TPIe con mefloquina aumenta en cerca de cuatro veces el riesgo de eventos adversos relacionados con los fármacos incluidos los vómitos, la fatiga/debilidad y los mareos (evidencia de certeza alta o moderada), en comparación con sulfadoxina‐pirimetamina.
En las pacientes con infección por VIH, tres dosis del TPIe con mefloquina más profilaxis con cotrimoxazol en comparación con cotrimoxazol solo redujeron el riesgo de parasitemia periférica materna en el momento del parto (medido por la reacción en cadena de la polimerasa [RCP]) en el 48% (evidencia de certeza moderada) y el riesgo de paludismo placentario (medido de acuerdo a la RCP) en el 72% (evidencia de certeza alta). Los metanálisis tuvieron poco poder estadístico para detectar diferencias entre los efectos de la mefloquina más cotrimoxazol y el cotrimoxazol sobre otros resultados maternos, fetales y neonatales. Con respecto a los eventos adversos relacionados con los fármacos, los análisis de efectos aleatorios mostraron un efecto significativo del TPIe con mefloquina más profilaxis con cotrimoxazol en comparación con cotrimoxazol solo únicamente en el caso de los vómitos (CR 7,95; IC del 95%: 4,79 a 13,18; 1055 participantes; evidencia de certeza alta). Es importante observar que los receptores de mefloquina más cotrimoxazol presentaron un riesgo 1,92 veces mayor de transmisión maternoinfantil del VIH que el grupo que recibió cotrimoxazol solo (CR 1,92; IC del 95%: 1,13 a 3,25; 1019 participantes). Un análisis secundario de uno de los ensayos incluidos reveló este resultado (Gonzalez 2014b KEN MOZ TAN).
Un ensayo entre pacientes con infección por VIH que comparó tres dosis de TPIe con mefloquina y cotrimoxazol tuvo poco poder estadístico para detectar un efecto de la mefloquina en los resultados maternos, fetales, neonatales y de seguridad, excepto por los eventos adversos relacionados con los fármacos, que fueron más frecuentes en el grupo de mefloquina.
Finalmente, el único ensayo realizado en Tailandia (donde coexiste el Plasmodium vivax) encontró un efecto significativo atribuible a la profilaxis semanal con mefloquina (en comparación con placebo) solamente en cuanto a la reducción del riesgo de parasitemia periférica materna en el momento del parto (CR 0,13; IC del 95%: 0,05 a 0,33; 339 participantes).
Compleción y aplicabilidad general de las pruebas
Los ensayos se realizaron en el África subsahariana, a excepción de uno realizado en Tailandia, y se publicaron entre 1994 y 2014. Los hallazgos mostraron que la quimioprevención con mefloquina reduce el riesgo de parasitemia materna en el momento del parto en las pacientes con infección por VIH y sin infección por HIV en comparación con otros antipalúdicos o placebo. Además, en las pacientes con infección por VIH, se encontró que la mefloquina redujo el riesgo de paludismo placentario. Los resultados de estos ensayos muestran beneficios clínicamente importantes bastante consistentes para las pacientes y los lactantes. Sin embargo, el riesgo de eventos adversos relacionados con los fármacos aumentó entre las que recibieron mefloquina, y se debe destacar que la mefloquina aumentó el riesgo de transmisión maternoinfantil en un ensayo.
Los ensayos incluidos evaluaron dos o tres dosis de TPIe con sulfadoxina‐pirimetamina de acuerdo a las recomendaciones de la Organización Mundial de la Salud (OMS), mientras que la evidencia actual indica que las dosis mensuales pueden proporcionar un mejor efecto profiláctico (Kayentao 2013). Además, la OMS actualmente recomienda la administración de TPIe en cada visita prenatal programada (WHO 2012b).
Los resultados de esta revisión, derivados de diversos contextos de África subsahariana, que comparó la quimioprevención con mefloquina en el embarazo con diversos fármacos antipalúdicos y placebo, se pueden aplicar en todo el mundo. La mefloquina actualmente se recomienda como quimioprevención del paludismo para las pacientes embarazadas de todas las edades gestacionales que viajan a zonas donde el paludismo es endémico (CDC 2016). Este fármaco también se recomienda para el tratamiento de los episodios de paludismo no complicado en combinación con artesunato (WHO 2015) y se dispone de una forma farmacéutica de dosis fija en algunos países en los que el paludismo es endémico. En 2013 el Evidence Review Group (ERG) de la OMS sobre el TPIe se reunió para evaluar la evidencia obtenida de los ensayos del TPIe con mefloquina, y el Malaria Policy Advisory Committee (MPAC) de la OMS examinó las recomendaciones del ERG y acordó que el régimen de dosis de 15 mg/kg de mefloquina no se debe recomendar para el TPIe, debido a los eventos adversos y la tolerabilidad deficiente (WHO MPAC 2013).
Certeza de la evidencia
La certeza de la evidencia en esta revisión se evaluó mediante el uso del enfoque GRADE y la evidencia se presentó en dos tablas de "Resumen de los hallazgos" para los resultados de eficacia y seguridad (Resumen de los hallazgos tabla 1; Resumen de los hallazgos tabla 2).
En las pacientes embarazadas sin infección por HIV, la evidencia de que el TPIe con mefloquina fue superior al TPIe con sulfadoxina‐pirimetamina para reducir el riesgo de parasitemia periférica y anemia materna en el momento del parto fue de certeza moderada, y la evidencia de que el TPIe con mefloquina aumentó los efectos adversos relacionados con los fármacos (es decir, vómitos y mareos) en comparación con el TPIe con sulfadoxina‐pirimetamina fue de certeza alta y moderada (respectivamente). Los efectos del TPIe con mefloquina para reducir el riesgo de paludismo placentario en comparación con el TPIe con sulfadoxina‐pirimetamina se consideraron de certeza baja debido a la heterogeneidad significativa entre los ensayos. Finalmente, la evidencia de ningún efecto de la mefloquina comparada con sulfadoxina‐pirimetamina en el bajo peso al nacer y los mortinatos y los abortos se consideró de certeza moderada.
En las pacientes con infección por VIH, la evidencia de que el cotrimoxazol más TPIe con mefloquina fue superior al cotrimoxazol para reducir el riesgo de parasitemia periférica y anemia materna en el momento del parto fue de certeza moderada, mientras que la evidencia con respecto a la falta de efecto sobre el riesgo de paludismo placentario fue de certeza alta. La evidencia de ningún efecto del cotrimoxazol más TPIe con mefloquina en comparación con cotrimoxazol en el bajo peso al nacer y los mortinatos y los abortos fue de certeza moderada y muy baja, respectivamente, debido al riesgo de sesgo grave de uno de los ensayos incluidos y la heterogeneidad significativa. Finalmente, la evidencia en cuanto al aumento de los riesgos de vómitos y mareos causados por la mefloquina se consideró de certeza baja debido a que la heterogeneidad entre los ensayos fue significativa y el IC del 95% fue amplio.
Sesgos potenciales en el proceso de revisión
Parece poco probable la omisión de algún ensayo que examinara la mefloquina para la prevención del paludismo en las pacientes embarazadas.
Acuerdos y desacuerdos con otros estudios o revisiones
Una revisión Cochrane anterior sobre los fármacos para la prevención del paludismo en las pacientes embarazadas en zonas endémicas analizó los efectos de la mefloquina para la prevención del paludismo (Radeva‐Petrova 2014). Los resultados son compatibles con los informados previamente aunque incluyen más ensayos y por lo tanto pueden ser más consistentes.
Los resultados de esta revisión Cochrane son también compatibles con los de una revisión sistemática anterior que evaluó la seguridad y la tolerabilidad de la mefloquina en el embarazo (González 2013).

Indicators and impact of malaria infection in mothers and infants.

Conceptual framework of malaria chemoprevention. Reproduced under the terms of a Creative Commons Licence from Radeva‐Petrova 2014.

‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

‘Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 1 Clinical malaria episodes during pregnancy.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 2 Maternal peripheral parasitaemia at delivery.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 3 Placental malaria.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 4 Mean haemoglobin at delivery.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 5 Maternal anaemia at delivery.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 6 Severe maternal anaemia at delivery.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 7 Cord blood parasitaemia.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 8 Cord blood anaemia.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 9 Mean birth weight.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 10 Low birth weight.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 11 Low birth weight by gravidity.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 12 Prematurity.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 13 Malaria in first year of life.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 14 Hospital admissions in first year of life.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 15 SAEs during pregnancy.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 16 Stillbirths and abortions.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 17 Congenital malformations.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 18 Maternal mortality.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 19 Neonatal mortality.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 20 Infant mortality.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 21 AEs: vomiting.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 22 AEs: fatigue/weakness.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 23 AEs: dizziness.

Comparison 1 Mefloquine versus sulfadoxine‐pyrimethamine, Outcome 24 AEs: headache.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 1 Clinical malaria episodes during pregnancy.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 2 Maternal peripheral parasitaemia at delivery (PCR).

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 3 Placental malaria (blood smear).

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 4 Placental malaria (PCR).

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 5 Mean haemoglobin at delivery.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 6 Maternal anaemia at delivery (< 9.5 g/dL).

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 7 Maternal severe anaemia at delivery.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 8 Cord blood parasitaemia.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 9 Mean birth weight.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 10 Low birth weight.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 11 Prematurity.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 12 SAEs during pregnancy.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 13 Spontaneous abortions and stillbirths.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 14 Congenital malformations.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 15 Maternal mortality.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 16 Neonatal mortality.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 17 Mother‐to‐child transmission HIV.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 18 AEs: vomiting.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 19 AEs: fatigue/weakness.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 20 AEs: dizziness.

Comparison 2 Mefloquine plus cotrimoxazole versus cotrimoxazole, Outcome 21 AEs: headache.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 1 Maternal peripheral parasitaemia at delivery (PCR).

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 2 Placental malaria (PCR).

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 3 Placental malaria (blood smear).

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 4 Mean haemoglobin at delivery.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 5 Maternal anaemia at delivery (< 9.5 g/dL).

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 6 Mean birth weight.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 7 Low birth weight.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 8 Prematurity.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 9 SAEs during pregnancy.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 10 Stillbirths.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 11 Spontaneous abortions.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 12 Congenital malformations.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 13 Maternal mortality.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 14 Neonatal mortality.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 15 Infant deaths after 7 days.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 16 AEs: vomiting.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 17 AEs: fatigue/weakness.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 18 AEs: dizziness.

Comparison 3 Mefloquine versus cotrimoxazole, Outcome 19 AEs: headache.

Comparison 4 Mefloquine versus placebo, Outcome 1 Maternal peripheral parasitaemia during pregnancy.

Comparison 4 Mefloquine versus placebo, Outcome 2 Placental malaria.

Comparison 4 Mefloquine versus placebo, Outcome 3 Mean birth weight.

Comparison 4 Mefloquine versus placebo, Outcome 4 Low birth weight.

Comparison 4 Mefloquine versus placebo, Outcome 5 Prematurity.

Comparison 4 Mefloquine versus placebo, Outcome 6 Stillbirths.

Comparison 4 Mefloquine versus placebo, Outcome 7 Spontaneous abortions.

Comparison 4 Mefloquine versus placebo, Outcome 8 Congenital malformations.

Comparison 4 Mefloquine versus placebo, Outcome 9 Maternal mortality.

Comparison 4 Mefloquine versus placebo, Outcome 10 Infant mortality.
| Mefloquine compared with sulfadoxine‐pyrimethamine for preventing malaria in pregnant women | ||||||
| Patient or population: HIV‐uninfected pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments (compared with sulfadoxine‐pyrimethamine) | |
| Risk with sulfadoxine‐pyrimethamine | Risk with mefloquine | |||||
| Clinical malaria episodes during pregnancy | ‐ | ‐ | IRR 0.83 | ‐ | ⊕⊕⊕⊕ | Mefloquine results in little or no difference in the incidence of clinical malaria episodes during pregnancy |
| Maternal peripheral parasitaemia at delivery | 43 per 1000 | 28 per 1000 (20 to 37) | RR 0.65 (0.48 to | 5455 (2 RCTs) | ⊕⊕⊕⊕ | Mefloquine results in lower maternal peripheral parasitaemia at delivery |
| Placental malaria | 52 per 1000 | 54 per 1000 | RR 1.04 | 4668 | ⊕⊕⊝⊝ Due to imprecision and heterogeneity | Mefloquine may result in little or no difference in placental parasitaemia |
| Maternal anaemia at delivery | 219 per 1000 | 184 per 1000 | RR 0.84 | 5469 | ⊕⊕⊕⊝ Due to imprecision | Mefloquine probably results in fewer women anaemic at delivery |
| Low birth weight | 117 per 1000 | 111 per 1000 | RR 0.95 | 5641 | ⊕⊕⊕⊕ | Mefloquine results in little or no difference in low birth weight |
| Stillbirths and abortions | 31 per 1000 | 37 per 1000 | RR 1.20 | 6219 | ⊕⊕⊕⊝ Due to imprecision | Mefloquine probably results in little or no difference in stillbirths or abortions |
| AEs: vomiting | 82 per 1000 | 390 per 1000 | RR 4.76 | 6272 | ⊕⊕⊕⊕ | Mefloquine results in a four‐fold increase in vomiting |
| AEs: dizziness | 94 per 1000 | 396 per 1000 | RR 4.21 | 6272 | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine probably results in a four‐fold increase in dizziness |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aNot downgraded for risk of bias: although one trial has serious risk of bias, the other is of good quality and exclusion of the smaller trial has little effect on the estimate of effect. | ||||||
| Mefloquine plus cotrimoxazole compared with cotrimoxazole for preventing malaria in pregnant women | ||||||
| Patient or population: HIV‐infected pregnant women | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments (compared with cotrimoxazole) | |
| Risk with cotrimoxazole | Risk with mefloquine plus cotrimoxazole | |||||
| Clinical malaria episodes during pregnancy | ‐ | ‐ | IRR 0.76 (0.33 to 1.76) | ‐ (1 RCT) | ⊕⊕⊕⊕ | Mefloquine results in little or no difference in the incidence of clinical malaria episodes during pregnancy |
| Maternal peripheral parasitaemia at delivery (PCR) | 66 per 1000 | 34 per 1000 (20 to 62) | RR 0.52 (0.30 to 0.93) | 989 (2 RCTs) | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine probably results in lower maternal peripheral parasitaemia at delivery |
| Placental malaria (PCR) | 68 per 1000 | 19 per 1000 | RR 0.28 | 977 | ⊕⊕⊕⊕ | Mefloquine plus cotrimoxazole results in fewer women with placental malaria at delivery |
| Maternal anaemia at delivery | 178 per 1000 | 168 per 1000 | RR 0.94 | 1197 | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine plus cotrimoxazole probably results in little or no difference in maternal anaemia cases at delivery |
| Low birth weight | 118 per 1000 | 141 per 1000 | RR 1.20 | 1220 | ⊕⊕⊕⊝ Due to risk of bias | Mefloquine plus cotrimoxazole probably results in little or no difference in the proportion of births that are low birth weight |
| Spontaneous abortions and stillbirths | 50 per 1000 | 56 per 1000 | RR 1.12 | 1347 | ⊕⊝⊝⊝ | We do not know if mefloquine plus cotrimoxazole results in a difference in spontaneous abortions and stillbirths |
| AEs: vomiting | 30 per 1000 | 239 per 1000 (144 to 396) | RR 7.95 (4.79 to 13.18) | 1055 (1 RCT)d | ⊕⊕⊕⊕ | Mefloquine plus cotrimoxazole results in an eight‐fold increase in vomiting |
| AEs: dizziness | 75 per 1000 | 296 per 1000 (214 to 411) | RR 3.94 (2.85 to 5.46) | 1055 (1 RCT)e | ⊕⊕⊕⊕ | Mefloquine plus cotrimoxazole results in a four‐fold increase in dizziness |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by 1 for risk of bias: one of the included trials is at serious risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria episodes during pregnancy Show forest plot | 2 | Rate Ratio (Fixed, 95% CI) | 0.83 [0.65, 1.05] | |
| 2 Maternal peripheral parasitaemia at delivery Show forest plot | 2 | 5455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.48, 0.86] |
| 3 Placental malaria Show forest plot | 2 | 4668 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.58, 1.86] |
| 4 Mean haemoglobin at delivery Show forest plot | 2 | 5588 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.01, 0.19] |
| 5 Maternal anaemia at delivery Show forest plot | 2 | 5469 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.94] |
| 6 Severe maternal anaemia at delivery Show forest plot | 2 | 5469 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.58, 1.48] |
| 7 Cord blood parasitaemia Show forest plot | 2 | 5309 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.13, 1.46] |
| 8 Cord blood anaemia Show forest plot | 1 | 4006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.23] |
| 9 Mean birth weight Show forest plot | 2 | 5241 | Mean Difference (IV, Fixed, 95% CI) | 2.52 [‐25.66, 30.69] |
| 10 Low birth weight Show forest plot | 2 | 5641 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.78, 1.17] |
| 11 Low birth weight by gravidity Show forest plot | 2 | 5641 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.84, 1.13] |
| 11.1 Primigravidae | 2 | 1576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.80, 1.30] |
| 11.2 Multigravidae | 2 | 4065 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 12 Prematurity Show forest plot | 2 | 4640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.40] |
| 13 Malaria in first year of life Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | 0.97 [0.82, 1.15] | |
| 14 Hospital admissions in first year of life Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | 0.93 [0.75, 1.17] | |
| 15 SAEs during pregnancy Show forest plot | 1 | 4674 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.20] |
| 16 Stillbirths and abortions Show forest plot | 2 | 6219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.91, 1.58] |
| 17 Congenital malformations Show forest plot | 2 | 5931 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.37] |
| 18 Maternal mortality Show forest plot | 2 | 6219 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [0.27, 21.23] |
| 19 Neonatal mortality Show forest plot | 2 | 6134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.67, 1.43] |
| 20 Infant mortality Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | 1.00 [0.66, 1.52] | |
| 21 AEs: vomiting Show forest plot | 2 | 6272 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [4.13, 5.49] |
| 22 AEs: fatigue/weakness Show forest plot | 2 | 6272 | Risk Ratio (M‐H, Random, 95% CI) | 4.62 [1.80, 11.85] |
| 23 AEs: dizziness Show forest plot | 2 | 6272 | Risk Ratio (M‐H, Random, 95% CI) | 4.21 [3.36, 5.27] |
| 24 AEs: headache Show forest plot | 2 | 6272 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.25, 1.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical malaria episodes during pregnancy Show forest plot | 1 | Rate Ratio (Fixed, 95% CI) | 0.76 [0.33, 1.76] | |
| 2 Maternal peripheral parasitaemia at delivery (PCR) Show forest plot | 2 | 989 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.93] |
| 3 Placental malaria (blood smear) Show forest plot | 2 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.29, 0.89] |
| 4 Placental malaria (PCR) Show forest plot | 2 | 977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.14, 0.57] |
| 5 Mean haemoglobin at delivery Show forest plot | 2 | 1167 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.32, 0.46] |
| 6 Maternal anaemia at delivery (< 9.5 g/dL) Show forest plot | 2 | 1197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| 7 Maternal severe anaemia at delivery Show forest plot | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.41, 2.08] |
| 8 Cord blood parasitaemia Show forest plot | 2 | 1166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.13] |
| 9 Mean birth weight Show forest plot | 2 | 1220 | Mean Difference (IV, Random, 95% CI) | ‐25.75 [‐86.99, 35.49] |
| 10 Low birth weight Show forest plot | 2 | 1220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.89, 1.60] |
| 11 Prematurity Show forest plot | 2 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.58, 1.96] |
| 12 SAEs during pregnancy Show forest plot | 2 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.50, 0.95] |
| 13 Spontaneous abortions and stillbirths Show forest plot | 2 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.42, 2.98] |
| 14 Congenital malformations Show forest plot | 2 | 1312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.22, 1.67] |
| 15 Maternal mortality Show forest plot | 2 | 1347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 2.01] |
| 16 Neonatal mortality Show forest plot | 2 | 1239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.65, 2.69] |
| 17 Mother‐to‐child transmission HIV Show forest plot | 2 | 1019 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.13, 3.25] |
| 18 AEs: vomiting Show forest plot | 2 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 20.88 [1.40, 311.66] |
| 19 AEs: fatigue/weakness Show forest plot | 2 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.26, 32.93] |
| 20 AEs: dizziness Show forest plot | 2 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 16.34 [0.39, 684.99] |
| 21 AEs: headache Show forest plot | 2 | 1347 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.28, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal peripheral parasitaemia at delivery (PCR) Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.03, 1.72] |
| 2 Placental malaria (PCR) Show forest plot | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.13, 4.15] |
| 3 Placental malaria (blood smear) Show forest plot | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 4 Mean haemoglobin at delivery Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.67, 0.47] |
| 5 Maternal anaemia at delivery (< 9.5 g/dL) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.26, 3.16] |
| 6 Mean birth weight Show forest plot | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐102.0 [‐255.52, 51.52] |
| 7 Low birth weight Show forest plot | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.56, 4.13] |
| 8 Prematurity Show forest plot | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.56] |
| 9 SAEs during pregnancy Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.28, 4.07] |
| 10 Stillbirths Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.49, 37.49] |
| 11 Spontaneous abortions Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.07, 16.84] |
| 12 Congenital malformations Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.16, 7.41] |
| 13 Maternal mortality Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal mortality Show forest plot | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.39] |
| 15 Infant deaths after 7 days Show forest plot | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.54] |
| 16 AEs: vomiting Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.43 [3.31, 54.54] |
| 17 AEs: fatigue/weakness Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.99 [1.64, 29.81] |
| 18 AEs: dizziness Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 52.60 [3.26, 848.24] |
| 19 AEs: headache Show forest plot | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal peripheral parasitaemia during pregnancy Show forest plot | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.05, 0.33] |
| 2 Placental malaria Show forest plot | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
| 3 Mean birth weight Show forest plot | 1 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐80.0 [‐184.65, 24.65] |
| 4 Low birth weight Show forest plot | 1 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.78, 2.48] |
| 5 Prematurity Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.15, 1.53] |
| 6 Stillbirths Show forest plot | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.86, 8.08] |
| 7 Spontaneous abortions Show forest plot | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.22] |
| 8 Congenital malformations Show forest plot | 1 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.82 [0.43, 33.83] |
| 9 Maternal mortality Show forest plot | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 71.85] |
| 10 Infant mortality Show forest plot | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.74] |

