Uso de guía ecográfica para el bloqueo nervioso neuroaxial y periférico perioperatorio en niños
Resumen
Antecedentes
El uso de la guía ecográfica para la anestesia regional se ha generalizado durante las dos últimas décadas. Sin embargo, no todos los expertos la reconocen como una herramienta esencial, tal vez porque no está claro si la ecografía reduce el riesgo de complicaciones neurológicas graves, y el coste de un aparato de ecografía (22 000 dólares) es significativamente superior al de otras herramientas. Esta revisión fue publicada en 2016 y actualizada en 2019.
Objetivos
Determinar si la guía ecográfica ofrece alguna ventaja clínica al realizar el bloqueo nervioso neuroaxial y periférico en los niños con respecto al aumento de la tasa de éxito o la disminución de la tasa de complicaciones.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL, MEDLINE, Embase y en dos registros de ensayos hasta marzo de 2018, junto con la revisión de las referencias para identificar estudios adicionales y se estableció contacto con los autores de los estudios para obtener información adicional sobre los ensayos.
Criterios de selección
Se incluyeron todos los ensayos controlados aleatorizados paralelos que evaluaron los efectos de la guía ecográfica utilizada para realizar una técnica de bloqueo regional en niños. Se incluyeron los estudios realizados en niños (≤ 18 años de edad) sometidos a cualquier tipo de procedimiento quirúrgico (abierto o laparoscópico), en los cuales se haya realizado un bloqueo nervioso neuraxial (espinal, epidural, caudal o combinado espinal y epidural) o periférico (cualquier bloqueo nervioso periférico incluyendo el fascial (fascia iliaca, plano transversal del abdomen, bloqueos de la vaina del recto o bloqueos perivasculares), para la anestesia quirúrgica (sola o en combinación con la anestesia general) o para la analgesia posoperatoria, con guía ecográfica. Se excluyeron los estudios en los que se utilizó el bloqueo regional para tratar el dolor crónico.
Se incluyeron los estudios en los que se utilizó la guía ecográfica para realizar la técnica en tiempo real (en el plano o fuera del plano), como preexploración antes del procedimiento o para evaluar la propagación del anestésico local para poder ajustar la posición de la aguja o complementar el bloqueo. Para los grupos control, se aceptó cualquier otra técnica utilizada para realizar el bloqueo, que incluye puntos de referencia, pérdida de resistencia (aire o líquido), clic, parestesia, estimulador nervioso, transarterial o infiltración.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane. Los resultados primarios fueron bloqueos fallidos, puntuaciones de dolor a la hora después de la cirugía y la duración del bloqueo. Los resultados secundarios incluyeron el tiempo para realizar el bloqueo, el número de inserciones de agujas y las complicaciones leves y graves. Se utilizaron los criterios GRADE para evaluar la calidad de la evidencia de cada resultado.
Resultados principales
Se incluyeron 33 ensayos con un total de 2293 participantes de 0,9 a 12 (media o mediana) años de edad. La mayoría de los ensayos tuvieron un bajo riesgo de sesgo de selección, detección, desgaste e informe; sin embargo, la falta de cegamiento de los participantes y del personal que los atendía dio lugar a que 25 ensayos se consideraran con alto o incierto riesgo de sesgo. Se han identificado cinco ensayos en curso.
La guía ecográfica probablemente reduce el riesgo de bloqueo fallido (diferencia de riesgo [DR] ‐0,16; intervalo de confianza [IC] del 95%: ‐0,25 a ‐0,07; 22 ensayos; 1789 participantes; evidencia de calidad moderada). Cuando se utilizó la guía ecográfica, hubo una reducción pequeña a moderada del dolor una hora después de la cirugía, equivalente a una reducción de 1,3 puntos en la escala de dolor revisada de Bieri FACES (escala; 0 = ningún dolor, 10 = dolor máximo) (diferencia de medias estandarizada [DME] ‐0,41; IC del 95%: ‐0,74 a ‐0,07 (tamaño medio del efecto); 15 ensayos; 982 participantes; evidencia de calidad moderada). La guía ecográfica aumenta la duración del bloqueo en el equivalente a 42 minutos (DME 1,24; IC del 95%: 0,72 a 1,75; diez ensayos; 460 participantes; evidencia de calidad alta).
Probablemente hay poca o ninguna diferencia en el tiempo necesario para realizar el bloqueo (DME ‐0,46; IC del 95%: ‐1,06 a 0,13; nuevo ensayos; 680 participantes; evidencia de calidad moderada). No se sabe si el número de inserciones de agujas necesarias se reduce con el uso de la guía ecográfica (DME ‐0,63; IC del 95%: ‐1,08 a ‐0,18; tres ensayos; 256 participantes; evidencia de calidad muy baja).
No se produjeron complicaciones importantes ni en los brazos de intervención ni en los brazos control de los ensayos (paro cardíaco por toxicidad anestésica local [22 ensayos; 1576 participantes; evidencia de calidad moderada]; lesión neurológica duradera [19 ensayos; 1250 participantes; evidencia de calidad moderada]).
Puede haber poca o ninguna diferencia en el riesgo de sangre en la punción (DR ‐0,02; IC del 95%: ‐0,05 a 0,00; 13 ensayos; 896 participantes; evidencia de calidad baja) o de lesión neurológica transitoria (DR ‐0,00; IC del 95%: ‐0,01 a 0,01; 18 ensayos; 1230 participantes;evidencia de calidad baja). No hubo casos de convulsiones por toxicidad del anestésico local (22 ensayos; 1576 participantes; evidencia de calidad moderada) ni infecciones del bloqueo sin lesión neurológica (18 ensayos; 1238 participantes; evidencia de calidad baja).
Conclusiones de los autores
La guía ecográfica para el bloqueo regional en niños probablemente disminuye el riesgo de bloqueo fallido. Aumenta la duración del bloqueo y probablemente disminuye las puntuaciones de dolor una hora después de la cirugía. Puede haber poca o ninguna diferencia en los riesgos de algunas complicaciones leves. Los tres estudios en curso pueden alterar las conclusiones de la revisión una vez publicados y evaluados.
PICO
Resumen en términos sencillos
Guía ecográfica para la inyección de anestésicos locales en niños para bloquear la transmisión del dolor
Antecedentes
Un bloqueo regional consiste en inyectar un anestésico local en la columna vertebral o alrededor de los nervios para bloquear la transmisión del dolor. Se puede utilizar para reemplazar la anestesia general o para tratar el dolor después de la cirugía. Encontrar una alternativa eficaz a los analgésicos tradicionales (píldoras o inyecciones que contengan derivados de la morfina) es particularmente importante para los niños, ya que es más probable que presenten los efectos adversos de los analgésicos opiáceos, y también porque el dolor en los primeros años de vida puede causar daños a largo plazo (respuesta exagerada al dolor más adelante en la vida). Tradicionalmente, la búsqueda del lugar exacto donde se debe inyectar el anestésico local se hacía según puntos anatómicos de referencia, es decir, por la palpación de huesos o de un vaso pulsátil (arteria, utilizando los dedos para sentir el pulso). Más tarde, se recomendó el uso de una aguja eléctrica que produce una contracción muscular (estimulador nervioso) por ser más precisa. En las últimas cuatro décadas, los médicos han empezado a utilizar la ecografía para localizar los nervios. Sin embargo, los aparatos de ecografía son caros (22 000 dólares versus 1000 dólares por un estimulador nervioso).
Se deseaba saber si la guía ecográfica puede mejorar la tasa de éxito y reducir la incidencia de complicaciones del bloqueo regional en los niños. Estas complicaciones pueden incluir complicaciones neurológicas duraderas, la introducción accidental de una aguja en un vaso sanguíneo y la convulsión o el paro cardíaco por el exceso de anestesia local o por la inyección accidental en un vaso sanguíneo.
Fecha de la búsqueda
La evidencia está actualizada hasta marzo de 2018.
Características de los estudios
Se incluyeron 33 estudios bien diseñados con un total de 2293 niños en los que se comparó la guía ecográfica con otro método de localización de los nervios (técnicas tradicionales de puntos de referencia o estimulador nervioso) para el bloqueo regional en niños.
Fuentes de financiación de los estudios
Las fuentes de financiamiento incluyeron una organización gubernamental (dos estudios), una organización de beneficencia (un estudio) y un departamento institucional (13 estudios). Dos estudios declararon que recibieron ayuda de la industria (préstamo de equipos). La fuente de financiación no estuvo clara en 14 estudios.
Resultados clave
La guía ecográfica para el bloqueo regional en los niños puede disminuir la ocurrencia de un bloqueo fallido. También puede aumentar la duración del bloqueo y reducir el dolor una hora después de la cirugía. La guía ecográfica puede disminuir el número de inserciones de agujas necesarias para realizar el bloqueo. Sin embargo, debido a que la gran mayoría de los bloqueos en niños se realizan con el niño bajo sedación profunda o anestesia general, el verdadero valor de este hallazgo podría ser discutible. No hubo complicaciones graves en los ensayos incluidos. Puede haber poca o ninguna diferencia entre los grupos de estudio en cuanto a los riesgos de complicaciones leves. En conjunto, para determinar si estos hallazgos justifican o no el coste adicional de la guía ecográfica, probablemente también se deban tener en cuenta los conocimientos especializados del anestesiólogo y los recursos locales. Los tres estudios en curso pueden alterar las conclusiones de la revisión una vez publicados y evaluados.
Calidad de la evidencia
La calidad de la evidencia se consideró moderada para la disminución de la ocurrencia de un bloqueo fallido y la mejoría de las puntuaciones de dolor a la hora; alta para la duración prolongada del bloqueo; y muy baja para la disminución del número de inserciones de agujas.
Authors' conclusions
Summary of findings
| Ultrasound guidance compared with no ultrasound guidance for children | ||||||
| Patient or population: children (≤ 18 years of age) undergoing any type of surgical procedure (open or laparoscopic) for which a neuraxial (spinal, epidural, caudal, or combined spinal and epidural) or peripheral nerve block (any peripheral nerve block including fascial (fascia iliaca, transversus abdominis plane, rectus sheath blocks) or perivascular blocks) for surgical anaesthesia (alone or in combination with general anaesthesia) or for postoperative analgesia was performed with ultrasound guidance | ||||||
| Outcomes | No ultrasound guidance | Ultrasound guidance | Relative effect | Number of participants | Quality of the evidence | Comments |
| Failed blocks | Study population | RD −0.16 | 1789 | ⊕⊕⊕⊝ | NNTB is 6 (95% CI 5 to 8). The effect was more consistent for peripheral nerve block. | |
| 271 per 1000 | 145 per 1000 | |||||
| Pain scores at 1 hour after surgery6 | Mean pain scores at 1 hour after surgery was 0.41 standard deviation lower (‐0.74 to ‐0.07). | 982 | ⊕⊕⊕⊝ | The reduction is equivalent | ||
| Block duration | Mean block duration was 1.24 standard deviation higher (0.72 to 1.75).7 | 460 | ⊕⊕⊕⊕ | Prolongation is equivalent to 42 minutes. | ||
| Time to perform the block | Mean time to perform the procedure was 0.46 standard deviation lower (−1.06 to 0.13). | 680 | ⊕⊕⊕⊝ | |||
| Number of needle passes | Mean number of needle passes was 0.63 standard deviation lower (−1.08 to −0.18). | 256 | ⊕⊝⊝⊝ | Reduction is equivalent to 0.4 needle pass less per participant. | ||
| Minor complications | Bloody puncture | RD −0.02 | 896 | ⊕⊕⊝⊝ | ||
| Study population | ||||||
| 60 per 1000 | 16 per 1000 | |||||
| Transient neurological injury | RD −0.00 (−0.01 to 0.01) | 1230 (18 studies) | ⊕⊕⊝⊝ | |||
| Study population | ||||||
| 7 per 1000 | 3 per 1000 (0.9 to 12 per 1000) | |||||
| Seizure from local anaesthetic toxicity | RD 0.00 (−0.01 to 0.01) | 1576 (22 studies) | ⊕⊕⊕⊝ | |||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 5 per 1000) | |||||
| Block infections without neurological injury | RD 0.00 (−0.01 to 0.01) | 1238 (18 studies) | ⊕⊕⊝⊝ | |||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 6 per 1000) | |||||
| Major complications | Cardiac arrest from local anaesthetic toxicity | RD 0.00 (−0.01 to 0.01) | 1576 (22 studies) | ⊕⊕⊕⊝ | ||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 5 per 1000) | |||||
| Lasting neurological injury | RD 0.00 (−0.01 to 0.01) | 1250 (19 studies) | ⊕⊕⊝⊝ | |||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 6 per 1000) | |||||
| Incidences in study population and their 95% CI were calculated with VassarStat (VassarStats) with no continuity correction. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level for risk of bias. | ||||||
Background
In 2005, in the USA alone, approximately 647,000 children were discharged from a short stay hospital after having undergone a surgical procedure (DeFrances 2007). Anaesthesiologists are involved in these procedures at various steps of the process, amongst which anaesthesia for the procedure itself and treatment of postoperative pain are of the utmost importance. Pain experienced early in life may induce organic brain changes that can make children susceptible to an exaggerated brain response when pain is experienced later in life (Hohmeister 2010). These brain changes are frequently referred to as neuroplasticity. Young children are often unable to understand what is happening to them, and this may increase their distress, leading to an increase in their inability to convey what exactly is making them uncomfortable. In response to this situation, care providers may undertreat or overtreat children experiencing postoperative pain. In one study performed in children, adverse events requiring an intervention (therefore judged as clinically relevant) occurred in 22% and 24% of patients with patient‐controlled analgesia (PCA) administered by trained relatives or nurses and by the patients themselves, respectively (Voepel‐Lewis 2008). Opioid‐based regimens may therefore provide suboptimal treatment for postoperative pain in children.
Description of the condition
Regional blockade interrupts pain transmission to the brain, and may be used during the surgery itself as a replacement for general anaesthesia (regional anaesthesia) or for the treatment of postoperative pain (regional analgesia). In adults, regional analgesic techniques decrease postoperative opioid consumption (Guay 2006), making them a potentially interesting alternative or adjunct to opioid‐based regimens for the treatment of children with postoperative pain. It is also possible that regional anaesthesia can reduce persistent, long‐term pain after surgery, although there is currently little evidence for this in children (Weinstein 2018). Regional blockade techniques are classified as central neuraxial blocks (spinal, epidural, combined spinal and epidural, or caudal) or as peripheral nerve blocks (Guay 2017). These can be performed with local anaesthetics alone, with opioids or with other adjuncts (Pehora 2017). The use of regional blockade in children is today considered reasonably safe (Long 2014; Walker 2018). A large prospective trial on 104,393 blocks placed in 91,701 children and collected between 1 April 2007 and 30 September 2015 reported no permanent neurologic deficits (95% confidence interval 0 to 0.4 per 10,000) (Walker 2018). The most common adverse events were benign catheter‐related failures (4%) (Walker 2018).
Description of the intervention
Ultrasound refers to an oscillating sound pressure wave at a frequency above the upper limit audible to the human ear (approximately 20 kHz). In nature, bats use ultrasounds as a guide for night flights. Ultrasounds emitted by the animal are reflected when they hit an obstacle. The same principle has been applied to develop devices in which ultrasound is used to create two‐dimensional (2‐D) or even three‐dimensional (3‐D) pictures (Feinglass 2007). Ultrasound has been used for regional blockade for almost four decades. The pioneers to whom use of ultrasound for regional blockade can be attributed include P La Grange (La Grange 1978), RL Ting (Ting 1989), T‐J Wu (Wu 1993), and S Kapral (Kapral 1994). The probe emitting and receiving ultrasounds is placed over the area of the body in which the local anaesthetic will be injected. After appropriate visualization of the target, the needle may be advanced in‐plane (parallel to the beam), allowing visualization of the entire needle during its trajectory, or out‐of‐plane (perpendicular to the beam). The local anaesthetic is then injected under visualization. For neuraxial blocks, ultrasound guidance can be used in real time to observe advancement of the needle within the epidural space or within the intrathecal canal (Niazi 2014), but can also be used as a pre‐puncture guide to identify the exact vertebral level needed, to find an appropriate intervertebral space sufficient to allow passage of the needle, to determine the depth to which the needle should be advanced for placement of its tip at the chosen location, and to visualize the spread of the local anaesthetic. For peripheral nerve blocks, ultrasound guidance allows visualization of target nerves, advancement of the needle (in‐plane technique), and spread of the local anaesthetic.
How the intervention might work
In children, regional anaesthetic techniques are usually performed with the child under deep sedation or under general anaesthesia. Fortunately, this does not seem to increase the risk of complications associated with regional anaesthesia (Taenzer 2014; Walker 2018). However, as the child cannot express any paraesthesia‐related discomfort (with potential needle placement inside a neural structure), visualization might be even more important in this age group. Ultrasound guidance allows adequate visualization of nerves and other structures relevant to the performance of both neuraxial and peripheral nerve blocks, particularly in children, in whom relevant structures are relatively superficial.
Failed block is the most common problem in paediatric regional anaesthesia (Walker 2018). Severe local anaesthetic systemic toxicity is reported in 0.76 per 10,000 blocks (95% confidence interval 0.3 to 1.6 per 10,000 blocks) (Walker 2018). In adults, ultrasound guidance may produce superior peripheral nerve block success rates and decrease inadvertent vascular puncture (Lewis 2015). Ultrasound guidance for regional anaesthesia in children may thus improve the success rate while decreasing the rate of complications.
Why it is important to do this review
The use of ultrasound guidance for regional anaesthesia has become popular over the past two decades. However, ultrasound is not recognized by all experts as an essential tool. Indeed, many authorities believe that "clinical data demonstrating a reduction in neurologic injury with ultrasound guidance are currently lacking" (Neal 2015; Walker 2018). The cost of an ultrasound machine varies, with an average actually estimated at approximately USD 22,000 (Liu 2010; Ponde 2016). The equipment required to use ultrasound guidance is thus substantially more expensive than other tools, such as those used for nerve stimulation, which can be acquired for approximately USD 1000 or less (Liu 2010).
The main findings of a previously published version of our review were a reduction in the risk of failed peripheral nerve block and a longer block duration when ultrasound guidance was used compared with nerve stimulator (Guay 2016a). We undertook this update to look for new trials and to determine if these claimed advantages still stand.
Objectives
To determine whether ultrasound guidance offers any clinical advantage when neuraxial and peripheral nerve blocks are performed in children in terms of decreasing failure rate or the rate of complications.
Methods
Criteria for considering studies for this review
Types of studies
We included all parallel randomized controlled trials that evaluated the effect of ultrasound guidance when a regional blockade technique was performed in children and that included any of our selected outcomes. We excluded observational studies, quasi‐randomized trials, cross‐over trials, and cluster‐randomized trials. We did not exclude studies based on language of publication or publication status.
Types of participants
We included studies performed in children (≤ 18 years of age) undergoing any type of surgical procedure (open or laparoscopic) for which a neuraxial (spinal, epidural, caudal, or combined spinal and epidural) or peripheral nerve block (any peripheral nerve block including fascial (fascia iliaca, transversus abdominis plane, rectus sheath blocks) or perivascular blocks), for surgical anaesthesia (alone or in combination with general anaesthesia) or for postoperative analgesia, was performed with ultrasound guidance. We excluded studies in which regional blockade was used to treat chronic pain.
Types of interventions
We included studies in which ultrasound guidance was used to perform the technique in real time (in‐plane or out‐of‐plane), as pre‐scanning before the procedure, or to evaluate the spread of the local anaesthetic so the position of the needle could be adjusted or the block complemented. For control groups, we accepted any other technique used to perform the block including landmarks, loss of resistance (air or fluid), click, paraesthesia, nerve stimulator or transarterial. Infiltration of local anaesthetics was accepted. We excluded no studies based on the specific technique used as the comparator.
Types of outcome measures
We evaluated differences between treatment and control groups based on the following outcomes.
Primary outcomes
-
Success rate (study author's definition).
-
Pain scores at one hour after surgery (any ascendent or descendent pain scale used by study authors).
-
Block duration (study author's definition; 0 to 1 day).
Secondary outcomes
-
Time to perform the block (minutes if available).
-
Number of needle passes.
-
Minor complications: bloody puncture, transient neurological injury, seizure from systemic local anaesthetic toxicity, block infections without neurological injury.
-
Major complications: cardiac arrest from local anaesthetic toxicity, lasting neurological injury (lasting more than three months).
Search methods for identification of studies
Electronic searches
We searched for studies with systematic and sensitive search strategies as described in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). There were no language, publication year, or publication status restrictions. We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1; 2018, Issue 3), MEDLINE (OvidSP) (from 1946 to 7 March 2018; Appendix 2), and Embase (OvidSP) (from 1974 to 7 March 2018; Appendix 3). The search strategy was done in consultation with the Information Specialist, and run by the Information Specialist.
Searching other resources
We searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; March 2018) and World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/; March 2018) for unpublished and ongoing studies, and the OpenGrey database (www.opengrey.eu; March 2018) for grey literature.
We scanned the reference lists of all studies retained (during data extraction) and recent meta‐analysis or reviews on the topic (March 2018). We also scanned conference proceedings of American Society of Regional Anesthesia, European Society of Regional Anaesthesia, European Society of Anaesthesiology, and American Society of Anesthesiology for 2012 to 2017. We contacted trial authors for additional information.
Data collection and analysis
Selection of studies
Two review authors (JG and SK) screened the list of all titles and abstracts identified by the search described above. We retrieved and independently read potential articles for inclusion to determine their eligibility. We resolved discrepancies by discussion without the need for help from the third review author (SS). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009). We listed all reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
We selected studies, extracted data (assessment of risk of bias in included studies; types of outcome measures; assessment of heterogeneity), and entered the data onto our data extraction sheet. We first entered the site where the study was performed and the date of data collection (to facilitate exclusion of duplicate publications), then whether the study was included in the review or the reason for rejection. After we reached agreement, one review author (JG) entered the data and the moderators for heterogeneity exploration into Comprehensive Meta‐Analysis (Comprehensive Meta‐Analysis 2007), and the same review author (JG) entered the 'Risk of bias' assessment into Review Manager 5 (Review Manager 2014). Any disagreements were resolved by discussion. We contacted all study authors for additional information. We then transferred data for analysis to Review Manager 5 in the format required to include the maximal number of studies (events and total number of participants for each group; mean, standard deviation, and number of participants included in each group; or generic inverse variance if necessary). When possible, we performed an intention‐to‐treat analysis.
Assessment of risk of bias in included studies
We assessed the risks of bias of the included studies based on the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, attrition bias, reporting bias, and other risk of bias, using Review Manager 5 (Higgins 2011; Review Manager 2014). Any disagreements were resolved by discussion. We assessed the risk of bias based on information presented in the reports or provided by study authors, with no assumptions made.
Measures of treatment effect
We reported results as risk differences (RDs) and 95% confidence intervals (95% CIs) for dichotomous data (success rate, minor complications) and as mean differences (MDs) and 95% CIs for continuous data (pain scores, block duration, time to perform the procedure) to the degree this was feasible. If continuous data were measured on different scales (pain scores), or if results were provided with P values (number of attempts or needle passes), we presented the results as standardized mean differences (SMDs) and 95% CIs. For SMDs, we considered 0.2 a small effect, 0.5 a medium effect, and 0.8 a large effect (Pace 2011). When an effect was noted, we calculated a number needed to treat for an additional beneficial outcome (NNTB) or a number needed to treat for an additional harmful outcome (NNTH) from the odds ratio (OR). We used the odds ratio for calculation of the number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) (Cates 2005), as this value is less likely to be affected by the side (benefit or harm) on which data are entered (Cates 2002; Deeks 2002). When we were unable to demonstrate an effect, we calculated the number of participants required in a large trial to ensure that enough participants were included in the retained studies to justify a conclusion on the absence of effect (Pogue 1998).
Unit of analysis issues
We included only parallel‐group trials. If a study contained more than two groups, we fused two groups (by using the appropriate formula for adding standard deviations when required) if we thought that they were equivalent according to the criteria of our protocol (taking our factors for heterogeneity exploration into account) (Guay 2014), or we separated them and split the control group in half if we thought that they were different.
Dealing with missing data
We contacted all study authors. We did not consider medians as equivalent to means. Instead, we used the P value and the number of participants included in each group to calculate the effect size. We did not impute results. We entered data as intention‐to‐treat to the degree this was feasible. If this was not possible, we assessed the study as having unclear risk of bias for other risk of bias and entered the data on a per‐protocol basis.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results and examined statistical heterogeneity before carrying out any meta‐analysis. We quantified statistical heterogeneity by using the I2 statistic. We quantified the amount of heterogeneity as low (< 25%), moderate (50%), or high (75%), depending on the value obtained for the I2 statistic (Higgins 2003).
Assessment of reporting biases
We examined publication bias by using a funnel plot followed by Duval and Tweedie’s trim and fill technique for each outcome. Duval and Tweedie’s trim and fill analysis yields an estimate of what would be the effect size (odds ratio, risk ratio, etc.) if no publication bias was present.
Data synthesis
We analysed the data by using Review Manager 5 (Review Manager 2014), and Comprehensive Meta‐Analysis (Comprehensive Meta‐Analysis 2007), with random‐effects models. We presented the characteristics of included and excluded studies in Characteristics of included studies and Characteristics of excluded studies tables. We presented the 'Risk of bias' assessment in a 'Risk of bias' graph. We presented results for each comparison as forests plots.
Subgroup analysis and investigation of heterogeneity
We explored any amount of heterogeneity, but focused more specifically on comparisons with more than a small amount of heterogeneity (I2 ≥ 25%) (Higgins 2003). We explored heterogeneity by applying Egger’s regression intercept (to assess the possibility of a small‐study effect; Rucker 2011), and by performing visual inspection of the forest plots with studies placed in order according to a specific moderator, subgroupings (categorical moderators), or meta‐regressions (continuous moderators). We considered the following factors when exploring heterogeneity: type of block (neuraxial versus peripheral nerve block), type of comparator (nerve stimulator versus other), age and type of guidance (pre‐scanning versus real time (in‐plane or out‐of‐plane)), and combined methods (ultrasound plus nerve stimulator compared with other modalities versus ultrasound alone compared with other modalities).
Sensitivity analysis
A sensitivity analysis (based mainly on the 'Risk of bias' assessment (allocation concealment and blinding of outcome assessor) or on an outlier) could also be performed for results with heterogeneity.
'Summary of findings' table and GRADE
We rated the quality of the body of evidence according to the GRADE system (GRADEprofiler; Schünemann 2013). We presented the quality of evidence in a 'Summary of findings' table for all of our outcomes: failed blocks, pain scores at one hour after surgery, block duration, time to perform the block, number of needle passes, and minor and major complications. For risk of bias, we judged the quality of the evidence as high when most information was derived from studies at low risk of bias, and downgraded the quality when most information was derived from studies at high or unclear risk of bias (allocation concealment and blinding of outcome assessors). For inconsistency, we downgraded the quality of evidence when the I2 statistic was 75% or higher without a satisfactory explanation. We did not downgrade the quality of evidence for indirectness, because outcomes were based on direct comparisons performed on the population of interest and were not surrogate markers. For imprecision, we downgraded the quality of evidence when the confidence interval around the effect size was large or overlapped an absence of effect and failed to exclude an important benefit or harm, or when the number of participants was less than the one required in a large trial. For publication bias, we downgraded the quality of evidence when correcting for the possibility of publication bias as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion. It should be noted that while factors influencing the quality of evidence are additive ‐ such that the reduction or increase in each individual factor is added together with the other factors to reduce or increase the quality of evidence for an outcome ‐ grading the quality of evidence involves judgements that are not exclusive. GRADE is therefore not a quantitative system for grading the quality of evidence. Each factor for downgrading or upgrading reflects not discrete categories but a continuum within each category and among the categories (Schünemann 2013). When the body of evidence is intermediate with respect to a particular factor, the decision about whether a study falls above or below the threshold for up‐ or downgrading the quality depends on judgement. Review authors may decide not to downgrade, even if there was some uncertainty around a specific category, when they have already downgraded for another factor, and lowering the quality of evidence further for the outcome would seem inappropriate (Schünemann 2013). When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain. Low‐quality and very low‐quality evidence are considered equivalent to observational studies.
Results
Description of studies
Results of the search
(See Figure 1)
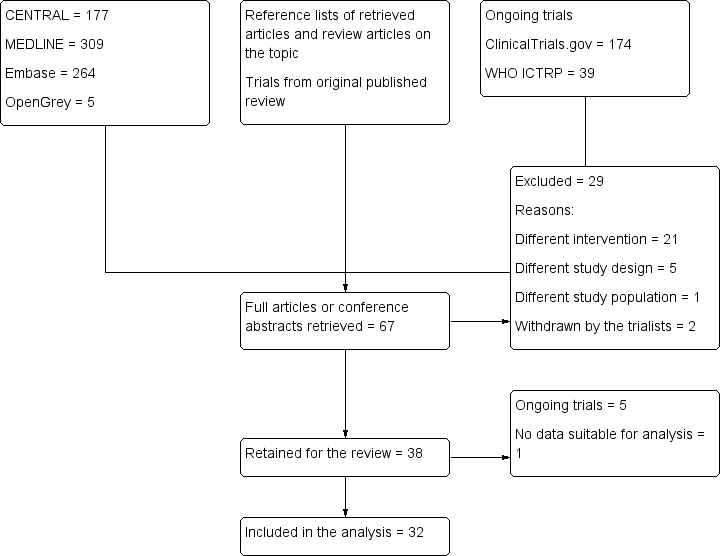
Study flow diagram. Search results for the 2018 update.
Our electronic search identified 755 trials: 177 from CENTRAL, 309 from MEDLINE, 264 from Embase, and 5 from the grey literature. Our search of the trial registries identified a further 213 ongoing trials. After screening the titles/abstracts of trials identified by this search, the reference lists of retained trials and review articles, and conference abstracts, and adding the trials of the previously published version, we retrieved the full texts of 67 trials. We excluded 29 trials, leaving 38 trials in the review, of which 5 are ongoing. We included 33 trials in the review, and 32 in the analysis. One trial did not contain data suitable for analysis (Qiu 2016).
Included studies
We included 33 trials with a total of 2293 participants in the review. Compared with our previously published version (Guay 2016a; 20 trials with 1241 participants), this update contains 13 additional trials with 1052 additional participants. Blocks were performed with ultrasound guidance for 1149 participants, and without ultrasound guidance for 1144 participants. There were no data suitable for analysis for one trial with 100 participants (Qiu 2016), therefore we included 32 trials with 2193 participants in the analysis (Figure 1).
Setting
Trials were performed in Austria (N = 1; Marhofer 2004); Belgium (N = 2; Abasbassi 2017; Faraoni 2010); Canada (N = 1; Lorenzo 2014); China (N = 7; Li 2016; Liu 2012; Liu 2018; Nan 2012; Qiu 2016; Wang 2013; Yang 2015); Egypt (N = 2; Elnour 2009; Shaaban 2014); Greece (N = 1; Gkliatis 2017); India (N = 2; Ponde 2009; Ponde 2013); Ireland (N = 1; O'Sullivan 2011); Japan (N = 2; Tachibana 2012; Uchinami 2017); South Africa (N = 4; Oberndorfer 2007; Weintraud 2009; Willschke 2005; Willschke 2006); Turkey (N = 3; Ahiskalioglu 2018; Kendigelen 2014; Sahin 2013); and the USA (N = 7; Dingeman 2013; Flack 2014; Gurnaney 2011; Hozella 2017; Litz 2017; Relland 2017; Shank 2016). The trials were published between 2004 and 2018.
Funding
The source of funding was a governmental organization (N = 2; Flack 2014; Nan 2012); a charitable organization (N = 2; Dingeman 2013; Shank 2016); departmental/institutional (N = 13; Ahiskalioglu 2018; Faraoni 2010; Kendigelen 2014; Li 2016; Litz 2017; Liu 2018; Lorenzo 2014; O'Sullivan 2011; Ponde 2013; Relland 2017; Uchinami 2017; Wang 2013; Yang 2015); or unspecified (N = 14; Abasbassi 2017; Elnour 2009; Gkliatis 2017; Gurnaney 2011; Hozella 2017; Liu 2012; Marhofer 2004; Oberndorfer 2007; Ponde 2009; Qiu 2016; Sahin 2013; Shaaban 2014; Tachibana 2012; Weintraud 2009). Two studies declared that they received industry help (equipment loan; Willschke 2005; Willschke 2006).
Participants
The mean or median age of participants ranged from 0.9 to 12 years.
The following surgeries were performed: major abdominal or thoracic surgery (Willschke 2006); appendicectomy (Gkliatis 2017; Shaaban 2014); circumcision (Abasbassi 2017; Ahiskalioglu 2018; Faraoni 2010; Li 2016; O'Sullivan 2011); inguinal hernia repair (Kendigelen 2014; Qiu 2016; Sahin 2013; Wang 2013; Weintraud 2009); inguinal hernia repair or hydrocelectomy (Hozella 2017; Yang 2015); inguinal hernia repair or hydrocelectomy or cure of cryptorchidism (Nan 2012; Willschke 2005); lower limb surgery (Oberndorfer 2007; Ponde 2013); laparoscopic surgery (Uchinami 2017); the Nuss procedure for pectus excavatum (Tachibana 2012); open pyeloplasty (Lorenzo 2014); skin grafts (Shank 2016); upper limb surgery (Elnour 2009; Liu 2018; Marhofer 2004; Ponde 2009); umbilical hernia repair (Dingeman 2013; Flack 2014; Gurnaney 2011; Litz 2017; Relland 2017); or urological or perineal surgery (Liu 2012).
The following blocks were performed: brachial plexus block (Elnour 2009; Marhofer 2004; Ponde 2009); a median nerve block (Liu 2018); sciatic and femoral nerve blocks (Oberndorfer 2007; Ponde 2013); a femoral lateral cutaneous nerve block (Shank 2016); ilioinguinal/iliohypogastric nerve blocks (Nan 2012; Weintraud 2009; Willschke 2005; Yang 2015); penile nerve block (Abasbassi 2017; Faraoni 2010; Li 2016; O'Sullivan 2011); rectus sheath block (Dingeman 2013; Flack 2014; Gurnaney 2011; Litz 2017; Qiu 2016; Relland 2017; Uchinami 2017); transversus abdominis plane blocks (Gkliatis 2017; Hozella 2017; Kendigelen 2014; Lorenzo 2014; Sahin 2013; Shaaban 2014); thoracic epidural (Tachibana 2012); thoracic or lumbar epidural (Willschke 2006); and caudal blocks (Ahiskalioglu 2018; Liu 2012; Wang 2013).
Ultrasound guidance
Ultrasound guidance was used in real time with an in‐plane (Elnour 2009; Faraoni 2010; Flack 2014; Gurnaney 2011; Hozella 2017; Kendigelen 2014; Li 2016; Liu 2018; Lorenzo 2014; O'Sullivan 2011; Ponde 2009; Ponde 2013; Qiu 2016; Relland 2017; Sahin 2013; Shaaban 2014; Uchinami 2017; Yang 2015); out‐of‐plane (Abasbassi 2017; Ahiskalioglu 2018; Marhofer 2004; Nan 2012; Oberndorfer 2007; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006); or unspecified technique (Dingeman 2013; Gkliatis 2017; Litz 2017; Shank 2016); or as pre‐scanning (Liu 2012; Tachibana 2012). (See Table 1)
| Study | Intervention | Comparator |
| Ultrasound‐guided out‐of‐plane dorsal penile nerve block with 0.2 mL/kg of levobupivacaine 0.5% | Landmark dorsal penile nerve block with 0.2 mL/kg of levobupivacaine 0.5% (Dalens 1989) | |
| Ultrasound‐guided out‐of‐plane caudal block with 0.125% levobupivacaine plus 10 mcg/kg morphine (total volume: 0.5 mL/kg) | Landmark caudal block with 0.125% levobupivacaine plus 10 mcg/kg morphine (total volume: 0.5 mL/kg) | |
| Bilateral rectus sheath block with real‐time ultrasonographic guidance with 0.5 mL/kg per side of ropivacaine 0.25%, cephalad to the umbilicus | Infiltration by the surgeon (subcutaneous or intradermal) with 0.4 mL/kg of ropivacaine 0.5% | |
| Axillary brachial plexus with ultrasound, 13‐6 MHz linear probe, real‐time in‐plane technique, circumferential spread around each target nerve | Axillary brachial plexus with nerve stimulator 0.5 mA, 0.3 ms distal response for median, radial, and ulnar nerves. A triceps response could also be accepted for the radial nerve. | |
| Real‐time (in‐plane) ultrasound guidance was used to guide bilateral injections into the subpubic space, deep to Scarpa's fascia, with ropivacaine 0.75% 0.1 mL/kg per side plus 0.05 mL/kg at the base of the penis | Landmark dorsal penile nerve block with same volume and locations | |
| Real‐time (in‐plane) ultrasound‐guided rectus sheath block with 0.2 mL/kg 0.25% bupivacaine (1 mg/kg) to each side at least 10 minutes before | Wound infiltration of 0.4 mL/kg 0.25% bupivacaine (1 mg/kg) at the end of surgery | |
| Ultrasound‐guided posterior transversus abdominis plane block Total dose of 0.3 mL/kg ropivacaine 0.2% | Wound infiltration Total dose of 0.3 mL/kg ropivacaine 0.2% | |
| Real‐time (in‐plane) rectus sheath block, 1 cm cephalad to the umbilicus with 0.25% bupivacaine, volume according to a table Total volume: < 12 kg = 0.5 mL/kg; ≥ 12 to 30 = 12 mL; ≥ 30 to 40 = 16 mL; ≥ 40 = 20 mL | Infiltration of 0.25% bupivacaine at the end of surgery. Volume according to a table Total volume: < 12 kg = 0.5 mL/kg; ≥ 12 to 30 = 12 mL; ≥ 30 to 40 = 16 mL; ≥ 40 = 20 mL | |
| Transverse abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine placed with in‐plane ultrasound guidance | Wound infiltration with 0.5 mL/kg of 0.25% ropivacaine | |
| Transverse abdominis plane block with 2 mg/kg of 0.25% bupivacaine (maximal volume 30 mL) placed with in‐plane ultrasound guidance | Wound infiltration with 2 mg/kg of 0.25% bupivacaine | |
| Ultrasound‐guided in‐plane penile block, probe beneath the scrotum with 0.1 mL/kg of lidocaine 1% and ropivacaine 0.375% | Landmark traditional penile block with 0.2 mL/kg of lidocaine 1% and ropivacaine 0.375% | |
| Ultrasound‐guided rectus sheath block with 0.5 mL/kg (maximal volume 10 mL) per side of ropivacaine 0.2% | 0.5 mL/kg (maximal volume 10 mL) per side of ropivacaine 0.2% injected under direct visualization into the rectus sheaths bilaterally by the attending surgeon | |
| Caudal anaesthesia with ultrasound for pre‐scanning; positive reaction in caudal space was monitored simultaneously by ultrasound | Caudal anaesthesia with landmarks; positive reaction in caudal space was monitored simultaneously by classic swoosh test | |
| Ultrasound‐guided in‐plane median nerve block with 0.2% ropivacaine (maximal volume 0.5 mL/kg) | Landmark median nerve block with 0.2% ropivacaine (maximal volume 0.5 mL/kg) plus skin incision infiltration | |
| Real‐time, in‐plane, ultrasound‐guided transversus abdominis plane block with 0.4 mL/kg bupivacaine 0.25% with 1:200,000 epinephrine before incision | Wound infiltration with 0.4 mL/kg bupivacaine 0.25% with 1:200,000 epinephrine before incision | |
| Infraclavicular lateral brachial plexus blocks with 0.5% ropivacaine 0.5 mL/kg guided by ultrasound visualization (out‐of‐plane), injection around the brachial plexus | Infraclavicular brachial plexus blocks with 0.5% ropivacaine 0.5 mL/kg guided by nerve stimulation (coracoid process, 0.3 mA and 0.3 ms) | |
| ilioinguinal or iliohypogastric block under ultrasonic guidance (linear 5 to 10 MHz probe; real time; out‐of‐plane) with a mixture of 0.8% lidocaine and 0.25% levobupivacaine at 0.2 mL/kg | Ilioinguinal or iliohypogastric block performed according to the traditional method (Schulte‐Steinberg's method) of anatomical localization with the same local anaesthetic at 0.3 mL/kg | |
| Penile block under ultrasound guidance. Hockey‐stick probe (6 to 13 MHz, 25 mm), real‐time (in‐plane; information from study authors) guidance. 2 puncture techniques with 0.5% bupivacaine 1 to 2 mL up to 3 years and 1 additional mL per each additional 3 years up to a maximum of 5 to 6 mL | Penile block with landmarks with the same doses | |
| Sciatic and femoral nerve block under ultrasound guidance using a multiple‐injection technique until the nerves were surrounded by levobupivacaine. Portable ultrasound unit with a 5 to 10 MHz linear hockey stick probe, real‐time (out‐of plane) technique | Sciatic and femoral nerve blocks under nerve stimulator guidance using a predefined dose of 0.3 mL/kg of levobupivacaine injected when a current of 0.3 mA over 0.3 ms at 2 Hz elicited plantar flexion (sciatic) or cephalic movement of the patella (femoral) | |
| Real‐time (in‐plane) ultrasound‐guided infraclavicular brachial plexus block with a 38‐millimetre linear 5 to 10 MHz probe and with 0.5 mL/kg of 0.5% of bupivacaine as a 1‐injection technique | Lateral infraclavicular brachial plexus block guided by nerve stimulator with 0.5 mL/kg of 0.5% bupivacaine injected at 0.5 mA over 250 ms. If after 3 redirections a wrist response could not be obtained, an elbow response was accepted. | |
| Femoral and sciatic block under real‐time (in‐plane) ultrasound guidance with a 5 to 10 MHz probe and 0.5 mL/kg of 0.25% bupivacaine for the sciatic nerve and 0.7 mL/kg of lidocaine 1% for the femoral nerve. The procedure was abandoned in the absence of visualization of the sciatic nerve. | Femoral and sciatic block with nerve stimulator. Procedure abandoned if sciatic nerve stimulation was not obtained after 3 attempts (each pass was counted as an attempt). Injection of 0.5 mL/kg of bupivacaine 0.25% with plantar or dorsiflexion (ankle movement for the sciatic block) and 0.7 mL/kg of lidocaine 1% with a quadriceps contraction (femoral block) obtained at 0.5 mA. A fascia iliaca block (loss‐of‐resistance technique) was administered to children in whom a femoral block could not be performed. | |
| Transverse abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine placed with in‐plane ultrasound guidance | Landmark (triangle of Petit) transverse abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine | |
| In‐plane ultrasound‐guided rectus sheath block with 0.1 mL/kg of 0.2% ropivacaine with 5 mcg/mL of epinephrine per side and administered at the T9–T10 level | The surgeon injected either 0.5 mL/kg of 0.5% bupivacaine or 1 mL/kg of 0.25% bupivacaine with 5 mcg/mL of epinephrine based on the surgeon’s discretion in line with his or her standard practice. | |
| Transversus abdominis plane block with ultrasound (real time; in‐plane) and 0.5 mL/kg of 0.25% levobupivacaine. The block was performed after anaesthesia induction with a 10 to 18 MHz probe placed between the iliac crest and the subcostal area at the mid‐axillary line and the needle directed posteriorly. The surgical procedure began 5 to 10 minutes after local anaesthetic administration. | Wound infiltration with 0.2 mL/kg of 0.25% levobupivacaine between external aponeurosis and the skin was performed by surgeons during wound closure. | |
| In‐plane ultrasound‐guided transversus abdominis plane block with 0.4 mL/kg of bupivacaine 0.25% with 1:200,000 epinephrine. Total dose of bupivacaine will not exceed 2 mg/kg, and total volume will not be more than 20 mL. | Wound infiltration with 0.4 mL/kg of bupivacaine 0.25% by the surgeon at the end of surgery | |
| Ropivacaine 0.2% (without epinephrine) around the lateral femoral cutaneous nerve via ultrasound guidance; dose = 20 mL or 1 mL/kg for children < 20 kg | Donor site infiltration with bupivacaine 0.25% with epinephrine 1:200,000; dose = 1 mL/kg (no maximum) | |
| Thoracic epidural. Ultrasound was used to determine the level, the wider space, and the puncture point (shortest depth from the skin). | Landmarks | |
| In‐plane ultrasound‐guided rectus sheath block with 0.2 mL/kg of 0.375% ropivacaine per side in the posterior rectus sheath compartment | Local anaesthetic infiltration with 0.2 mL/kg of 0.75% | |
| Ultrasound guidance. Pre‐scanning with a 5 to 10 MHz linear 38 millimetre probe, real time (out of‐plane), confirmed with upward displacement of the sacrococcygeal ligament upon injection | Landmarks | |
| Ilioinguinal‐iliohypogastric nerve blockade with real‐time (out of‐plane) ultrasound guidance using a 5 to 10 MHz linear hockey stick probe | Ilioinguinal‐iliohypogastric nerve blockade with landmarks (single‐pop) | |
| Real‐time (out of‐plane) ultrasound‐guided ilioinguinal block with levobupivacaine 0.25% in amount sufficient to surround the nerves | Ilioinguinal block using the traditional fascial click method. The needle was inserted vertically through the tented skin, 1 to 2 cm medial and 1 to 2 cm inferior to the anterior superior iliac spine. After the first fascial click was detected, and following a negative aspiration, levobupivacaine 0.25% (0.3 mL/kg) was injected. The spread of local anaesthetic was examined with ultrasound after injection but with no further intervention consequential to this information. | |
| Epidural catheter placement was guided by direct ultrasound visualization (real time, out‐of‐plane for needle placement). Images obtained by an assistant with 5 to 10 MHz hockey stick probe to obtain a paramedian longitudinal view. Midline needle insertion. Confirmation of the position of the tip of the catheter and spread of local anaesthetics through the catheter. Levobupivacaine 0.25% for confirmation of needle placement and through the catheter (0.2 mg/kg for the latter) | Epidural catheter with standard loss‐of‐resistance technique with air or saline. Levobupivacaine 0.2% 0.2 mg/kg through the needle and 0.2 mg/kg through the catheter | |
| In‐plane ultrasound‐guided ilioinguinal nerve block with 0.2 mL/kg mixture of 0.8% lidocaine with 0.25% levobupivacaine | Landmark‐based ilioinguinal nerve block (van Schoor 2005), with 0.3 mL/kg mixture of 0.8% lidocaine with 0.25% levobupivacaine |
T9–T10: vertebral thoracic level 9‐10
Comparator
Ultrasound guidance was compared with infiltration (Dingeman 2013; Flack 2014; Gkliatis 2017; Gurnaney 2011; Hozella 2017; Kendigelen 2014; Lorenzo 2014; Relland 2017; Sahin 2013; Shaaban 2014; Shank 2016; Uchinami 2017); landmarks (Abasbassi 2017; Ahiskalioglu 2018; Faraoni 2010; Li 2016; Liu 2012; Liu 2018; Nan 2012; O'Sullivan 2011; Qiu 2016; Tachibana 2012; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006; Yang 2015); a surgeon‐administered block (Litz 2017); or a nerve stimulator (Elnour 2009; Marhofer 2004; Oberndorfer 2007; Ponde 2009; Ponde 2013).
(See Table 1)
Excluded studies
(See Characteristics of excluded studies)
We excluded 29 trials for the following reasons.
-
Different intervention (Abdellatif 2012; Ahmed 2014; Alsadek 2015; Atta 2008; Aveline 2011; Bryskin 2015; Fiocca 2013; Hamill 2015; Harju 2016; Jagannathan 2009; Matinyan 2015; Micic 2011; Mirjalili 2015; Narasimhan 2017; Oksuz 2017; Sahin 2013; Sethi 2016; Sherif 2011; Shin 2009; Triffterer 2012; Tutuncu 2017).
-
Different study design (Erbuyun 2016; Laserre‐Sartre 2009; Lloyd 2016; Ponde 2017; Pérez‐Pradilla 2015).
-
Different study population (Fusco 2016).
-
Trial withdrawn by study authors (NCT03427437; Sohn 2010).
Ongoing studies
We found five ongoing trials that could fit our inclusion criteria (ACTRN12608000488303; CTRI/2014/09/005023; CTRI/2018/01/011534; NCT02321787; NCT02352519). (See Characteristics of ongoing studies)
Awaiting classification
There are no trials awaiting classification.
Risk of bias in included studies
We have included a summary of our 'Risk of bias' assessment in Figure 2 and Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We judged random sequence generation as at low risk of bias for 27 trials (Abasbassi 2017; Ahiskalioglu 2018; Dingeman 2013; Elnour 2009; Faraoni 2010; Flack 2014; Gurnaney 2011; Kendigelen 2014; Litz 2017; Liu 2012; Liu 2018; Lorenzo 2014; Marhofer 2004; Nan 2012; O'Sullivan 2011; Oberndorfer 2007; Ponde 2009; Ponde 2013; Relland 2017; Sahin 2013; Shaaban 2014; Shank 2016; Uchinami 2017; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006), and as at
unclear risk of bias for the other six trials.
Allocation
We judged allocation concealment as at low risk of bias for 20 trials (Abasbassi 2017; Dingeman 2013; Elnour 2009; Flack 2014; Gurnaney 2011; Kendigelen 2014; Litz 2017; Liu 2018; Lorenzo 2014; Marhofer 2004; O'Sullivan 2011; Oberndorfer 2007; Ponde 2009; Ponde 2013; Sahin 2013; Uchinami 2017; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006). We judged it as at unclear/high risk of bias for the remaining 13 trials.
Blinding
We judged blinding of participants and personnel caring for the participants as at low risk of bias for eight trials (Abasbassi 2017; Faraoni 2010; O'Sullivan 2011; Ponde 2009; Relland 2017; Shaaban 2014; Wang 2013; Weintraud 2009). We judged it as at unclear/high risk of bias for the remaining 25 trials.
We judged blinding of outcome assessment as at low risk of bias for 22 trials (Abasbassi 2017; Ahiskalioglu 2018; Dingeman 2013; Faraoni 2010; Gkliatis 2017; Gurnaney 2011; Hozella 2017; Kendigelen 2014; Litz 2017; Lorenzo 2014; Marhofer 2004; O'Sullivan 2011; Oberndorfer 2007; Ponde 2009; Ponde 2013; Relland 2017; Sahin 2013; Shaaban 2014; Uchinami 2017; Wang 2013; Weintraud 2009; Yang 2015). We judged it as at unclear/high risk of bias for the remaining 11 trials.
Incomplete outcome data
We judged all 33 included trials as at low risk of bias for this domain.
Selective reporting
We judged 28 trials as at low risk of reporting bias (Abasbassi 2017; Ahiskalioglu 2018; Dingeman 2013; Elnour 2009; Faraoni 2010; Gkliatis 2017; Gurnaney 2011; Hozella 2017; Kendigelen 2014; Li 2016; Litz 2017; Liu 2012; Liu 2018; Lorenzo 2014; Nan 2012; O'Sullivan 2011; Oberndorfer 2007; Ponde 2013; Qiu 2016; Sahin 2013; Shaaban 2014; Tachibana 2012; Uchinami 2017; Wang 2013; Weintraud 2009; Willschke 2005; Willschke 2006; Yang 2015). We judged the remaining five trials as at unclear/high risk of bias.
Other potential sources of bias
We judged 18 trials as exempt from other risk of bias (Abasbassi 2017; Ahiskalioglu 2018; Dingeman 2013; Kendigelen 2014; Liu 2012; Liu 2018; Lorenzo 2014; Marhofer 2004; O'Sullivan 2011; Ponde 2009; Ponde 2013; Qiu 2016; Relland 2017; Shank 2016; Tachibana 2012; Wang 2013; Willschke 2005; Willschke 2006). We judged the remaining 15 trials as at unclear risk of bias for this domain.
Effects of interventions
Primary outcomes
1. Success rate (study authors’ definition)
Twenty‐two trials with 1789 participants provided results for failure rate for peripheral nerve blocks (N = 9), fascia blocks (N = 8), or neuraxial blocks (N = 5) (Figure 4; Analysis 1.1). As per our previously published version (Guay 2016a), we did not include data from Lorenzo and colleagues in this analysis because failure reported by these authors was considered a failure of one specific regional anaesthetic for this specific indication and not a failure of ultrasound guidance (Lorenzo 2014). The definitions used by study authors can be found in Table 2. We obtained data from the reports.
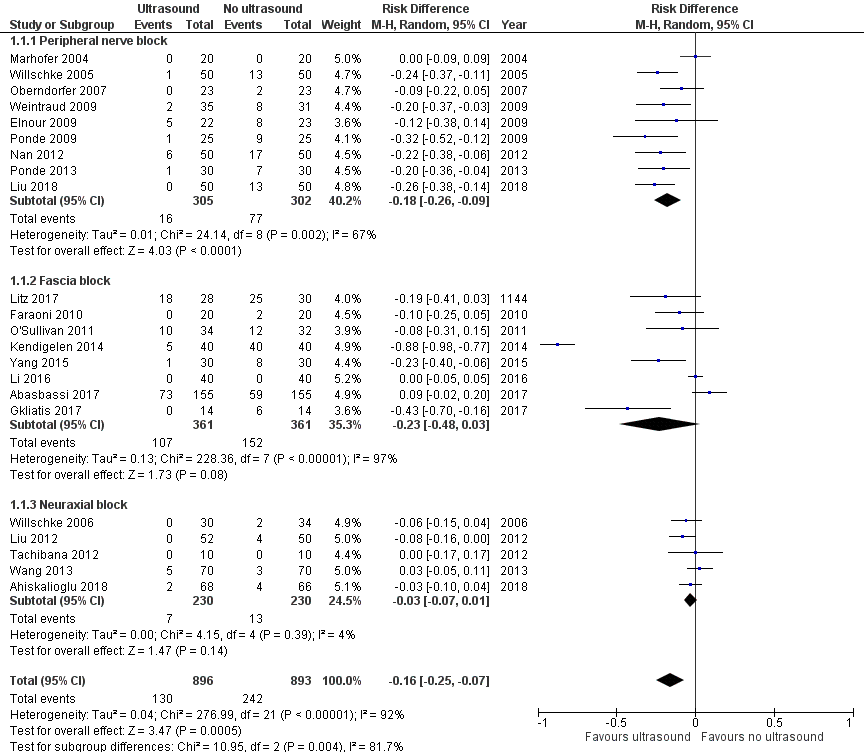
Forest plot of comparison: 1 Ultrasound versus no ultrasound, outcome: 1.1 Success (event = failed block).
| Study | Type of block | Timing of blockade | Definition |
| Dorsal nerve penile block | Not reported | Proportion of participants that needed piritramide (Objective Pain Scale score > 3) were considered as failed blocks. | |
| Caudal block | Under general anaesthesia before surgical incision in both groups | Failed block was defined as significant motor movements following surgical induction or heart and respiratory rates increasing > 20% of the basal levels. | |
| Axillary brachial plexus block | Under general anaesthesia before surgical incision in both groups | The procedure was considered a failure when:
| |
| Penile nerve block | Under general anaesthesia before surgical incision in both groups | Ineffective block was defined as an increase in heart rate and mean arterial pressure > 20% above baseline values. | |
| Transversus abdominis plane block | Not reported | Proportion of participants that needed tramadol were considered as failed blocks. | |
| Transversus abdominis plane block | End of surgery | Proportion of participants that needed tramadol during the first 5 minutes after surgery were considered as failed blocks. | |
| Penile block | Not reported | Not reported | |
| Rectus sheath block | Before surgical incision for ultrasound‐guided technique and before skin closure for the surgeon‐administered technique | Pain scores ≥ 4 in post‐anaesthesia care unit | |
| Caudal block | Under general anaesthesia 10 to 15 minutes before surgical incision in both groups | Unsuccessful caudal puncture after 4 attempts (n = 2 in the control group) or signs of pain, such as body movement, tachycardia, and tachypnoea during surgery | |
| Median nerve block | Before surgical incision | A block was considered unsuccessful if the children met any of the following criteria:
| |
| Infraclavicular brachial plexus block | Propofol sedation, 30 minutes before surgical incision in both groups | Procedure was considered a failure if ≥ 2 of the 4 nerves (ulnar, radial, median, and musculocutaneous) could not be blocked effectively. | |
| Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia before surgical incision in both groups | Inadequate analgesia was defined as an increase of heart rate > 10% of baseline level, which required elevation of the sevoflurane concentration to 3% to 4% during surgery. | |
| Penile nerve block | Under general anaesthesia ≥ 10 minutes before surgical incision in both groups | Procedure was considered a failure if a rise in heart rate or respiratory rate > 25% from baseline occurred in response to surgical stimulus. | |
| Sciatic and femoral nerve blocks | Under general anaesthesia ≥ 20 minutes before surgical incision in both groups | Procedure was considered a failure if a rise in heart rate > 15% of baseline value occurred at skin incision or during surgery. | |
| Infraclavicular brachial plexus block | Under general anaesthesia before surgical incision in both groups | Procedure was considered a failure if a pain response to surgical stimulus occurred, defined as an increase in heart rate and arterial blood pressure > 20% of basal rate or non‐specific body movement in response to surgical stimulus and withdrawal of blocked limb in response to incision. | |
| Sciatic and femoral nerve blocks | Under general anaesthesia ≥ 20 minutes before surgical incision in both groups | Procedure was considered a failure when:
| |
| Thoracic epidural anaesthesia | Under general anaesthesia before surgical incision in both groups | Procedure was considered a failure if a participant complained of severe postoperative pain despite sufficient epidural administration of local anaesthetics. | |
| Caudal block | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if a child had motor or haemodynamic response, as indicated by an increase in mean arterial pressure or heart rate > 15% compared with baseline values obtained just before skin incision and subsequent to surgical procedure. | |
| Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if child had an increase in heart rate or mean arterial blood pressure > 10% compared with baseline during operation. | |
| Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if child had an increase in heart rate or mean arterial pressure > 10% after skin incision or during surgery. | |
| Thoracic or lumbar epidural anaesthesia | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | An increase in heart rate or blood pressure > 20% from baseline was considered to reflect inadequate analgesia and was managed by bolus administration of levobupivacaine 0.25% 0.3 mL/kg of body weight through the epidural catheter. If this was unsuccessful, the epidural block was considered to have failed. | |
| Ilioinguinal nerve block | Under general anaesthesia after induction | Pain score of > 3 was considered a sign of inadequate analgesia. |
CHEOPS: Children's Hospital of Eastern Ontario Pain Scale
As per our previous version, we have presented data as failure rate. Ultrasound guidance reduces the risk of failed blocks (risk difference (RD) −0.16, 95% confidence interval (CI) −0.25 to −0.07; Figure 4; summary of findings Table for the main comparison). The effect was more consistent for peripheral nerve blocks (RD −0.18, 95% CI −0.26 to −0.09; P = 0.004 for subgroups difference). There was no statistically significant evidence of small‐study effect. The asymmetry of the funnel plot leads to a trim and fill estimate (RD −0.24, 95% CI −0.34 to −0.15). Based on a failure rate of 27%, 238 participants (119 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; one‐sided test). The number needed to treat for an additional beneficial outcome (NNTB) is 6 (95% CI 5 to 8). We downgraded the quality of the evidence once for risk of bias, rating the quality as moderate.
2. Pain scores at one hour after surgery
Fifteen trials with 982 participants provided results for pain at 1 hour after surgery (Analysis 1.2). The trials compared ultrasound guidance with landmarks (N = 4), a nerve stimulator (N = 2), wound infiltration (N = 8) or a surgeon‐administered block (Litz 2017). We obtained data from the reports or from study authors (N = 8).
The study authors measured pain scores on various scales (Appendix 4). We extracted data as means and standard deviations (SDs) or as P values due to non‐normal distribution (Kolmogorov‐Smirov test; N = 4) or because they were not available as means and SDs (N = 4).
Ultrasound guidance reduces pain at one hour after surgery (standardized mean difference (SMD) −0.41, 95% CI −0.74 to −0.07; Figure 5; summary of findings Table for the main comparison). There was no statistically significant evidence of a small‐study effect or evidence of publication bias. Taking Gurnaney 2011 (SD in the control group 3.1), the difference would be equivalent to −1.3 on the revised Bieri FACES pain scale (scale: 0 = no pain, 10 = maximal pain; Chambers 1999; Hicks 2001). On the basis of Gurnaney 2011 (mean value 4.35; SD 3.1 for the control group), 238 (119 per group) would be required for a large trial to eliminate a difference of 1 on a score from 0 to 10 (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of the evidence once for risk of bias, rating the quality as moderate.
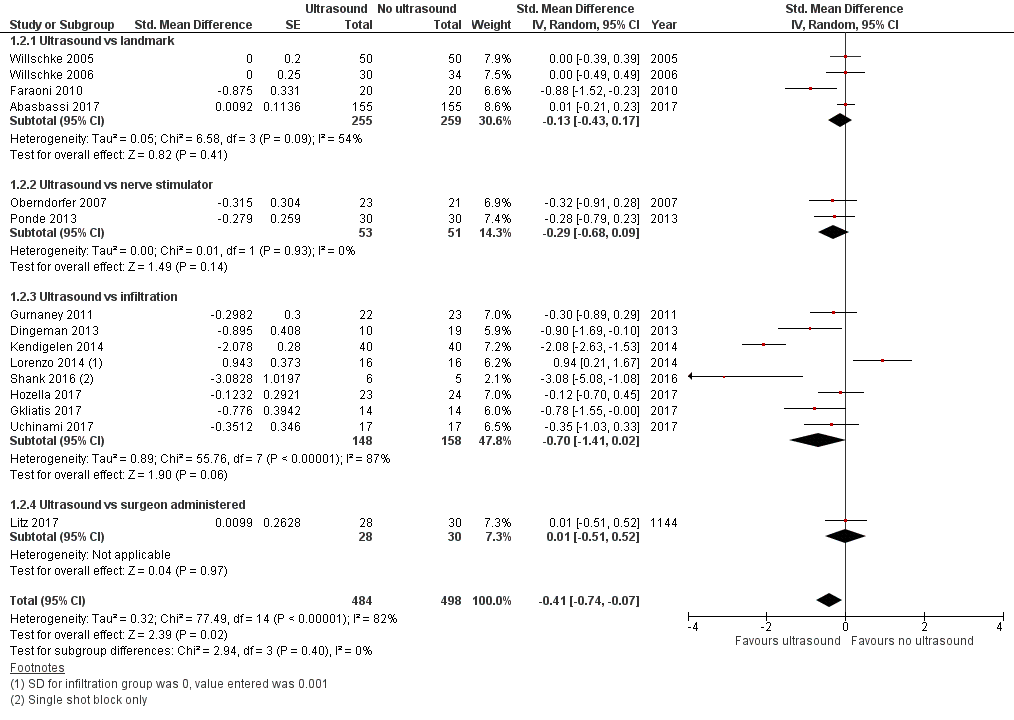
Forest plot of comparison: 1 Ultrasound versus no ultrasound, outcome: 1.2 Pain at one hour after surgery.
3. Block duration (study authors’ definition; 0 to 1 day)
Ten trials with 460 participants provided results for duration of analgesia when ultrasound guidance was compared with landmarks (N = 1), a nerve stimulator (N = 3), wound infiltration (N = 5), or surgeon‐administered block (Analysis 1.3) (Litz 2017). We obtained data from reports or from study authors (N = 2). We extracted data as means and SDs or as P value (non‐normality distribution, Kolmogorov‐Smirov test; N = 1) (not available as means and SDs: N = 3).
Ultrasound guidance prolongs block duration (SMD 1.24, 95% CI 0.72 to 1.75; Figure 6; summary of findings Table for the main comparison). Egger's regression intercept showed that a small‐study effect might be present (P = 0.005; two‐sided test). Correcting the asymmetry of the funnel plot leads to an estimate (SMD 1.38, 95% CI 0.88 to 1.87). Based on Ponde 2013 (SD of the control group 0.57 hour), this would be equivalent to 0.71 hour or 42 minutes. For the six trials where we extracted the data as means and SDs, the mean time was 7.6 hours (SD 6.3 hours) for ultrasound guidance and 4.0 hours (SD 3.0 hours) for the comparators (Appendix 5). We did not downgrade the quality of the evidence and rated it as high.
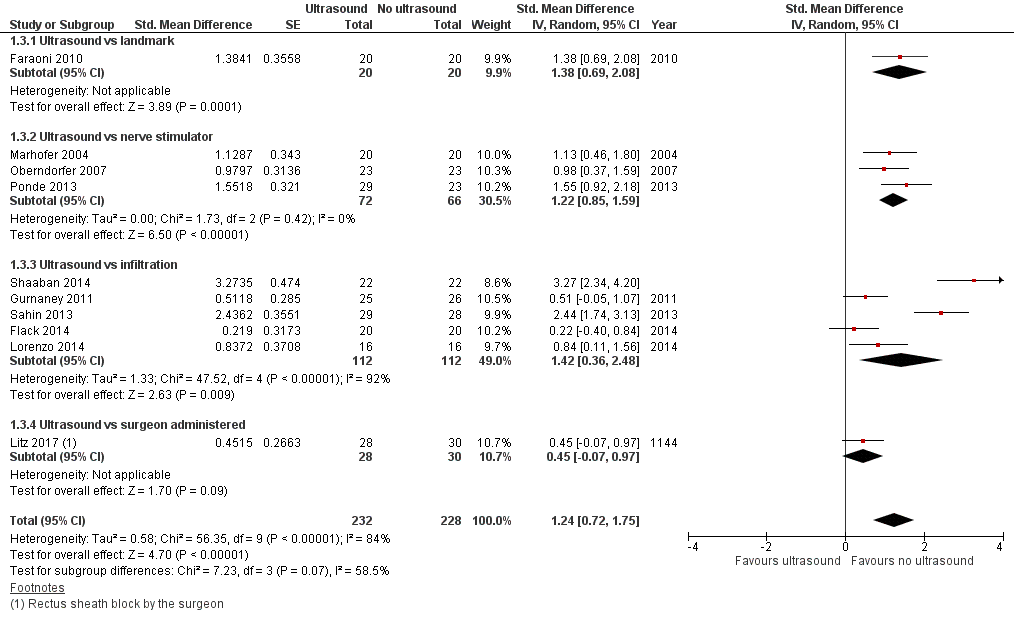
Forest plot of comparison: 1 Ultrasound versus no ultrasound, outcome: 1.3 Block duration.
Secondary outcomes
1. Time to perform the block
Nine trials with 680 participants provided results for time to perform the block (Analysis 1.4). Ultrasound guidance was used in real‐time in‐plane (N = 4), real‐time out‐of‐plane (N = 3), or as pre‐scanning (N = 2). We obtained data from reports and extracted data as mean and SDs or as P value (not available as means and SDs: N = 2).
We did not find a difference a difference in time to perform the block (SMD −0.46, 95% CI −1.06 to 0.13). There was no evidence of small‐study effect. Correcting the asymmetry of the funnel plot leads to an estimate (SMD −0.09, 95% CI −0.77 to 0.58). For the seven trials for which data were extracted as means and SDs, means and SDs were 4.6 minutes ± 4.6 minutes for ultrasound guidance and 5.2 minutes ± 5.0 minutes for the comparators (Appendix 6). We downgraded the quality of the evidence by one level for risk of bias, rating the quality as moderate.
2. Number of needle passes
Three trials with 256 participants provided results for number of needle passes for a neuraxial block (Analysis 1.5) (Ahiskalioglu 2018; Liu 2012; Tachibana 2012). We obtained data from the reports and extracted data as means and SDs or as P value (Tachibana 2012). Ultrasound guidance reduces the number of needle passes required to perform the block (SMD −0.63, 95% CI −1.08 to −0.18). There was no evidence of small‐study effect. Correcting for the asymmetry of the funnel plot would lead to an estimate (SMD −0.32, 95% CI −0.82 to 0.17). From Liu 2012 (SD of the control group 0.6), the difference would be equivalent to 0.4 needle pass less per participant. For trials extracted as means and SDs, means and SDs were 1.2 ± 0.2 for ultrasound guidance and 1.5 ± 0.1 for the comparator (Appendix 7). We downgraded the quality of the evidence for risk of bias, imprecision and, and the possibility of publication bias, rating the quality as very low.
3. Minor complications
3a. Bloody puncture
Thirteen trials with 896 participants provided results for bloody punctures for peripheral nerve blocks (N = 5), fascia blocks (N = 5), or neuraxial blocks (N = 3) (Analysis 1.6). We obtained data from reports or from study authors (N = 4).
We did not find a difference in the risk of bloody puncture (RD −0.02, 95% CI −0.05 to 0.00). There was no evidence of small‐study effect. Correcting for the asymmetry of the funnel plot would lead to an estimate (RD −0.02, 95% CI −0.04 to −0.01). Based on a rate of 5.3%, 1404 participants (702 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of the evidence for imprecision and risk of publication bias, rating the quality as low.
3b. Transient neurological injury
Eighteen trials with 1230 participants provided results for transient neurological injury (paraesthesia) (RD −0.00, 95% CI −0.01 to 0.01; Analysis 1.7). We obtained data from reports or from study authors (N = 2). We downgraded the quality of the evidence by one level for risk of bias due to lack of clarity on specific methods and time points of assessment and by one level for imprecision, rating the quality as low.
3c. Seizures from local anaesthetic toxicity
Twenty‐two trials with 1576 participants provided results for seizures from systemic local anaesthetic toxicity (RD 0.00, 95% CI −0.01 to 0.01; Analysis 1.8). We obtained data from reports or from study authors (N = 2). Based on an incidence of 0.05% (Walker 2018), 148,324 participants (74,162 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of the evidence by one level for imprecision, rating the quality as moderate.
3d. Block infections without neurological injury
Eighteen trials with 1238 participants provided results for infection without neurological injury (RD 0.00, 95% CI −0.01 to 0.01; Analysis 1.9). We obtained data from reports or from study authors (N = 2). Based on an incidence of 0.5% of cutaneous infections with catheter insertions (Walker 2018), 18,766 participants (9383 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; two‐sided test). We downgraded the quality of the evidence by one level for risk of bias (uncertainty about follow‐up) and by one level for imprecision, rating the quality as low.
4. Major complications
No major complications were reported in any of the included studies (see Table 3).
| Peripheral nerve block | |||
| Study | Type of block | Minor complications | Major complications |
| Axillary brachial plexus block | No intravascular injection. Local bruising 2/17 in the ultrasound group vs 3/15 in the nerve stimulator group. Local axillary pain 3/17 in the ultrasound group and 8/15 in the nerve stimulator group. Transient postblock paraesthesia 2/17 in the ultrasound group and 4/15 in the nerve stimulator group (which resolved within 5 days as reported by parents and participants in the follow‐up surgical clinic 1 week later). | Major complications (e.g. unintentional intravascular injection, persistent neurological deficit) did not occur in either group. | |
| Median nerve block | No adverse events were observed in the 2 groups. | No adverse events were observed in the 2 groups. | |
| Infraclavicular brachial plexus block | No clinical signs of inadvertent puncture of major vessels | No clinical signs of pneumothorax, infection, or haematoma | |
| Ilioinguinal or iliohypogastric nerve block | 1 case in no‐ultrasound group had needle puncturing into blood vessels. | No other adverse event was observed in the 2 groups. | |
| Sciatic and femoral nerve blocks | No clinical signs of inadvertent puncture of major vessels | No clinical signs of nerve damage, infection, or haematoma | |
| Infraclavicular brachial plexus block | No complications were related to the regional anaesthetic technique. | No complications were related to the regional anaesthetic technique. | |
| Sciatic and femoral nerve blocks | Not reported | Not reported | |
| Lateral femoral cutaneous block or fascia‐iliaca block | No adverse events occurred performing these blocks. | No adverse events occurred performing these blocks. | |
| Ilioinguinal‐iliohypogastric nerve block | Not reported | The ultrasound‐guided technique resulted in higher Cmax (SD) and AUC values (Cmax: 1.78 (0.62) vs 1.23 (0.70) mcg/mL, P < 0.01; AUC: 42.4 (15.9) vs 27.2 (18.1) mcg 30 min/mL, P < 0.001). No signs of clinical toxicity | |
| Ilioinguinal block | All anaesthetic procedures were uneventful; no clinical evidence of complications such as small bowel or major vessel puncture. | All anaesthetic procedures were uneventful. | |
| Ilioinguinal nerve block | No adverse events other than 1 bloody puncture (landmark) were reported during or after the operation. | No adverse events other than 1 bloody puncture (landmark) were reported during or after the operation. | |
| Fascia block | |||
| Study | Type of block | Minor complications | Major complications |
| Penile nerve block | No adverse events were reported. | No adverse events were reported. | |
| Rectus sheath block | No adverse events requiring immediate medical attention associated with the surgical procedure or the postoperative course were reported in either group. | No adverse events requiring immediate medical attention associated with the surgical procedure or the postoperative course were reported in either group. | |
| Penile nerve block | No complications | No complications | |
| Rectus sheath block | Not reported | Peak plasma bupivacaine concentration was higher following ultrasound rectus sheath block (median 631.9 ng/mL, IQR: 553.9 to 784.1 vs 389.7 ng/mL, IQR: 250.5 to 502.7; P = 0.002). Time to peak concentration was longer in the USGRSB group (median 45 minutes, IQR: 30 to 60 vs 20 minutes, IQR: 20 to 45; P = 0.006). | |
| Posterior transversus abdominis plane block | No complication was recorded in transversus abdominis plane block group. | No complication was recorded in transversus abdominis plane block group. | |
| Rectus sheath block | Not reported | Not reported | |
| Transversus abdominis plane block | No adverse events due to research interventions | No adverse events due to research interventions | |
| Transversus abdominis plane block | No adverse effects related to the transversus abdominis plane block were identified. No complications were observed during or after the block intervention. | No major complications | |
| Penile block | No significant difference in the incidence of respiratory depression between the 2 groups | No significant difference in the incidence of respiratory depression between the 2 groups | |
| Rectus sheath block | 1 child in the surgeon‐administered rectus sheath block group developed a superficial surgical site infection; there were no complications in the ultrasound‐guided group. | No anaesthetic complications reported. | |
| Transversus abdominis plane block | None reported. | No local anaesthetic‐specific adverse events were noted. | |
| Penile nerve block | No complications were reported of either technique. | No complications were reported of either technique. | |
| Transversus abdominis plane block | Not reported | Not reported | |
| Rectus sheath block | No complications | No complications | |
| Transversus abdominis plane block | Not reported | Not reported | |
| Transversus abdominis plane block | There were no complications attributable to the ultrasound‐guided block. | There were no complications attributable to the ultrasound‐guided block. | |
| Rectus sheath block | No procedure‐related complications were observed in either group. | No procedure‐related complications were observed in either group. No adverse events associated with the nerve block procedure such as neuropathy or local anaesthetic intoxication were reported for any child. | |
| Neuraxial block | |||
| Study | Type of block | Minor complications | Major complications |
| Caudal block | Dural puncture and systemic local anaesthetic toxicity were not observed in any of the groups. No intraoperative desaturation was observed. | Dural puncture and systemic local anaesthetic toxicity were not observed in any of the groups. No intraoperative desaturation was observed. | |
| Caudal | Not reported | Not reported | |
| Thoracic epidural | Not reported | No children experienced severe side effects. | |
| Caudal | There was an incidence of bloody puncture of 18.6% in group landmarks and 5.7% in group ultrasound (P < 0.05). | No dural puncture or systemic reaction to local anaesthetic was reported in either group. | |
| Thoracic (n = 59) or lumbar (n = 5) epidural | Blood was aspirated in 1 child in the control group. | No dural puncture occurred in either group. | |
AUC: area under the curve for blood concentrations of local anaesthetics; Cmax : maximal blood concentration of local anaesthetic; IQR: interquartile range; SD: standard deviation; USGRSB: ultrasound‐guided rectus sheath block.
4a. Cardiac arrest from local anaesthetic toxicity
Twenty‐two trials with 1576 participants provided results for cardiac arrest from systemic local anaesthetic toxicity (RD 0.00, 95% CI −0.01 to 0.01; Analysis 1.10). We obtained data from reports or from study authors (N = 2). Based on an incidence of 0.05% (Walker 2018), 148,324 participants (74,162 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of the evidence by one level for imprecision, rating the quality as moderate.
4b. Lasting neurological injury (lasting more than three months)
Nineteen trials with 1250 participants provided results for lasting neurological injury (RD 0.00, 95% CI −0.01 to 0.01; Analysis 1.11). We obtained data from reports or from study authors (N = 2). Based on a maximal incidence of 0.004% (Walker 2018), 1,854,710 participants (927,355 per group) would be required in a large trial to eliminate a 50% difference (alpha 0.05; beta 0.2; one‐sided test). We downgraded the quality of the evidence by one level for risk of bias (uncertainty about follow‐up) and by one level for imprecision, rating the quality as low.
Discussion
Summary of main results
Failed block is the most common problem encountered in paediatric regional anaesthesia (Walker 2018). Our meta‐analysis showed that ultrasound guidance may decrease the risk of failed blocks (summary of findings Table for the main comparison). However, results for this outcome revealed some heterogeneity when all studies were included. The increased success rate was most evident for peripheral nerve block (Analysis 1.1).
Pain scores at one hour after surgery were reduced when ultrasound guidance was used; the difference was equivalent to −1.3 on a scale from 0 to 10 (Analysis 1.2). Ultrasound guidance may also significantly prolong block duration (Analysis 1.3).
We could not demonstrate a difference in minor complications (bloody punctures), and the difference between subgroups (neuraxial blocks versus peripheral nerve blocks versus fascia blocks) was not statistically different (Analysis 1.6). Reducing bloody punctures may be interesting for some patients (Suresh 2015), particularly those undergoing surgery requiring full heparinization (e.g. cardiac surgery) (Monahan 2019). For cardiac surgery, official societies suggest delaying surgery for 24 hours in case of traumatic attempt of neuraxial or deep block (Horlocker 2018). Postponing surgery may have an emotional and economical impact. Additional data are required before firm conclusions can be drawn regarding this.
None of the included studies reported major complications (Analysis 1.10; Analysis 1.11; Table 3). Walker and colleagues reported no lasting neurological complications in 104,393 blocks placed in 91,701 children (95% CI 0 to 0.4 per 10,000 blocks) (Walker 2018). It is therefore not surprising than none of these complications were reported in any of the randomized controlled trials included in our review. As a result of the extremely low incidence of major complications associated with paediatric regional anaesthesia, the incidence of these very rare events is probably best evaluated by large prospective studies. For adults, the risk of long‐term neurological injury is estimated at 2 to 4 per 10,000 procedures, and there is no data demonstrating that any given technique would reduce the risk of major postoperative neurological complications (Neal 2015).
Although some bloody punctures were reported in our review (Analysis 1.6), there was no case of systemic local anaesthetic toxicity reported in any the trials included in the present review (seizure or cardiac arrest; Analysis 1.8; Analysis 1.10). In their large prospective trial, Walker and colleagues reported seven cases of severe local anaesthetic toxicity (seizures or cardiac arrest), for an incidence of 0.76 per 10,000 (95% CI 0.3 to 1.6 per 10,000) (Walker 2018). All cases of severe local anaesthetic systemic toxicity involved bolus dosing. This suggests that local anaesthetic toxicity (seizures or cardiac arrests) may have occurred due to either increased individual susceptibility, relative overdose, or inadvertent intravascular injection (Walker 2018). A possible reduction of bloody punctures in neuraxial blocks (RD −0.08, 95% CI −0.15 to −0.02; Analysis 1.6), eventually possibly leading to a decreased risk of inadvertent intravascular injection, might thus be interesting. In adults, a retrospective analysis of regional anaesthesia registries indicated that ultrasound guidance may have reduced the risk of local anaesthetic toxicity, actual risk 8.7 per 10,000 peripheral nerve blocks (95% CI 5.4 to 1.3 per 10,000 procedures) (Barrington 2013).
Infections after a single shot block are rarely observed if at all in immunocompetent individuals (Walker 2018). However, infections are reported with catheters (neuraxial or peripheral nerve blocks). Walker and colleagues reported 92 local cutaneous infections out of 18,065 continuous catheters, for an incidence of 53 per 10,000 catheters (95% CI 43 to 64 per 10,000) (Walker 2018). Antibiotics were prescribed in 29 of the 92 cases (Walker 2018). One child had a deep infection, epidural abscess requiring laminectomy (Walker 2018). The child made a full recovery (Walker 2018). None of these complications were reported in any of the trials included in our review (Analysis 1.9).
The overall incidence of transient postblock paraesthesia (less than 1%) found in the present review is in agreement with numbers reported in adults and children (Analysis 1.7). While reviewing 1614 axillary blocks performed on 607 adults with a nerve stimulator, paraesthesia technique, or transarterial technique, Horlocker and colleagues found seven neurological complications related to the anaesthetic technique, for an incidence of 43 per 10,000 procedures (95% CI 21 to 89 per 10,000 procedures) (Horlocker 1999). Symptoms had a median duration of four weeks (Horlocker 1999). In children, Walker and colleagues reported 25 neurological complications, for an incidence of 2.4 per 10,000 blocks (95% CI 1.6 to 3.6 per 10,000) (Walker 2018). Neurologic complications were more common in children more than 10 years of age, primarily sensory in nature and all resolved over a period of weeks to months, with only 2 cases demonstrating a sensory deficit beyond 3 months (Walker 2018). All events reported in the present review were from one small trial (Elnour 2009), on axillary brachial plexus block. Elnour and colleagues reported 6 postblock paraesthesia out of 50 participants: 4 when axillary brachial plexus blocks were performed with a nerve stimulator (distal motor response at 0.5 mA or less and 0.3 milliseconds, Locoplex needle, Vygon, Ecouen, France), and 2 with ultrasound guidance (in‐plane technique, selective injections to target each nerve, unspecified type of needle). All paraesthesia were classified as transient and resolved within five days after surgery (Elnour 2009). In adults, ultrasound guidance may reduce the risk of procedural paraesthesia (Lewis 2015). In children, performing regional blockade under general anaesthesia or deep sedation is considered standard of care (Ivani 2015), therefore reducing procedural paraesthesia in children with ultrasound guidance may not be as relevant as it is for adults. It is unclear if reducing procedural paraesthesia would have any beneficial effect on the risk of postoperative transient paraesthesia in children.
Overall completeness and applicability of evidence
Our review included both peripheral and neuraxial blocks in children from birth to 18 years of age undergoing a wide variety of surgeries. For this reason, we had to subgroup the studies according to predefined criteria in our exploration of heterogeneity. We are confident that the evidence obtained is sufficient to allow us to draw valid conclusions for failed blocks. Due to their extremely low incidence, major complications might be best evaluated with large prospective trials. More data may be useful regarding the possibility of decreased bloody puncture when ultrasound guidance is used for neuraxial blocks.
Quality of the evidence
Details of our reasons for upgrading or downgrading the quality of the evidence are provided in the Effects of interventions section. We rated the quality of evidence as high for block duration, moderate for success rate and pain at one hour after surgery, and very low for number of needle passes required to perform the block (summary of findings Table for the main comparison). Risk of bias and imprecision were the major reasons for downgrading the quality of the evidence. For neurological injury (transient or lasting), lack of clarity on follow‐up was also considered to be an issue.
Potential biases in the review process
We are confident that given our extensive search we have included the available literature on this topic. The included studies were all relatively recent (from 2004 to 2018) and therefore likely well reflect actual medical practice and technology. Although doses and volumes of injected solutions varied, they seemed appropriate for the surgical indications for which they were used in most studies.
Agreements and disagreements with other studies or reviews
Unlike the Cochrane Review on ultrasound guidance for peripheral nerve block in adults (Lewis 2015), we could not demonstrate a reduction in the risk of bloody punctures. However, we believe that additional paediatric data are required before firm conclusions can be drawn on a possible decreased incidence of bloody punctures when ultrasound guidance is used for caudal blocks. Indeed, bloody punctures may be decreased with ultrasound guidance for caudal blocks (Analysis 1.6). Our finding on decreased failed blocks is in agreement with Lewis and colleagues, Lewis 2015, and with the previous version of the present review (Guay 2016a).
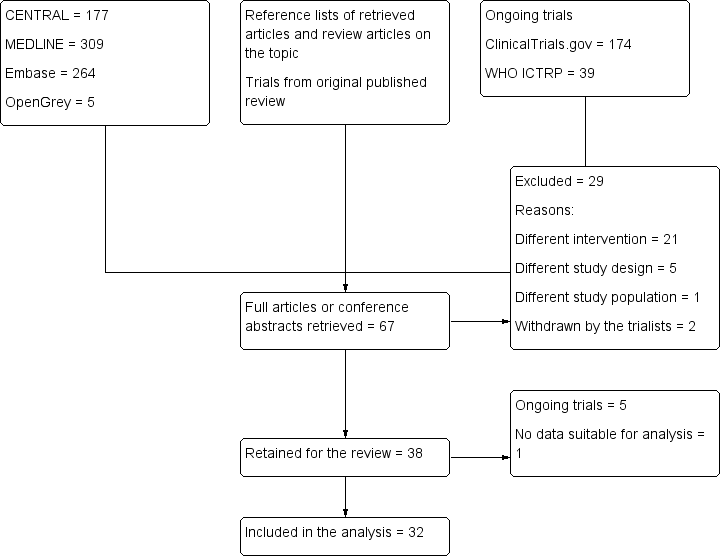
Study flow diagram. Search results for the 2018 update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Ultrasound versus no ultrasound, outcome: 1.1 Success (event = failed block).

Forest plot of comparison: 1 Ultrasound versus no ultrasound, outcome: 1.2 Pain at one hour after surgery.

Forest plot of comparison: 1 Ultrasound versus no ultrasound, outcome: 1.3 Block duration.

Comparison 1 Ultrasound versus no ultrasound, Outcome 1 Success (event = failed block).

Comparison 1 Ultrasound versus no ultrasound, Outcome 2 Pain at 1 hour after surgery.

Comparison 1 Ultrasound versus no ultrasound, Outcome 3 Block duration.
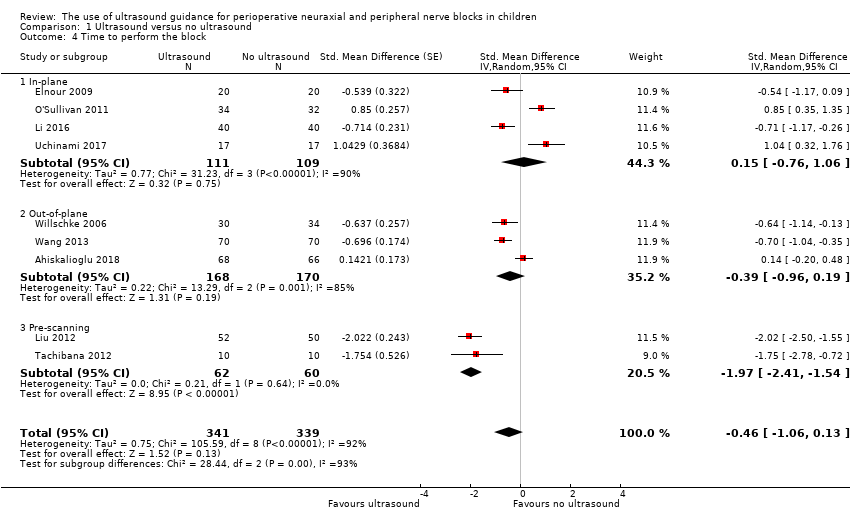
Comparison 1 Ultrasound versus no ultrasound, Outcome 4 Time to perform the block.

Comparison 1 Ultrasound versus no ultrasound, Outcome 5 Number of needle passes.

Comparison 1 Ultrasound versus no ultrasound, Outcome 6 Minor complications: bloody puncture.

Comparison 1 Ultrasound versus no ultrasound, Outcome 7 Minor complications: transient neurological injury.

Comparison 1 Ultrasound versus no ultrasound, Outcome 8 Minor complications: seizure from local anaesthetic toxicity.

Comparison 1 Ultrasound versus no ultrasound, Outcome 9 Minor complications: block infection without neurological injury.

Comparison 1 Ultrasound versus no ultrasound, Outcome 10 Major complications: cardiac arrest from local anaesthetic toxicity.
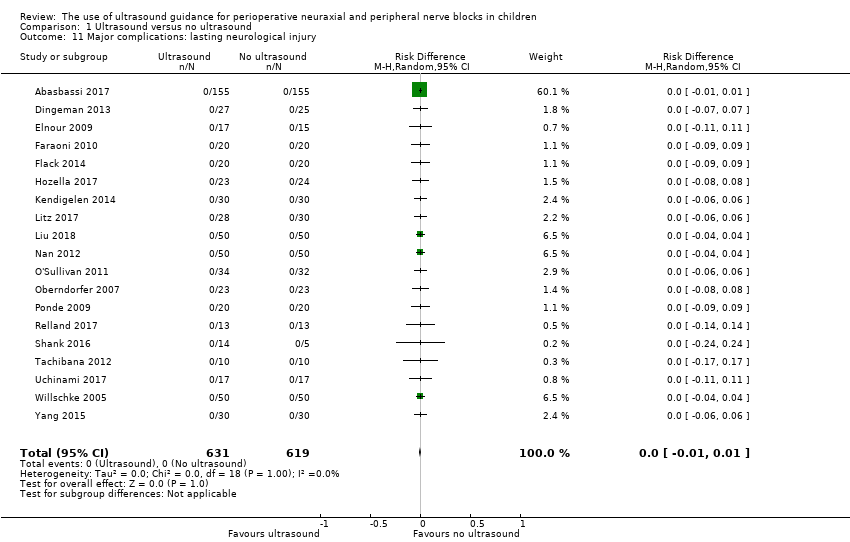
Comparison 1 Ultrasound versus no ultrasound, Outcome 11 Major complications: lasting neurological injury.
| Ultrasound guidance compared with no ultrasound guidance for children | ||||||
| Patient or population: children (≤ 18 years of age) undergoing any type of surgical procedure (open or laparoscopic) for which a neuraxial (spinal, epidural, caudal, or combined spinal and epidural) or peripheral nerve block (any peripheral nerve block including fascial (fascia iliaca, transversus abdominis plane, rectus sheath blocks) or perivascular blocks) for surgical anaesthesia (alone or in combination with general anaesthesia) or for postoperative analgesia was performed with ultrasound guidance | ||||||
| Outcomes | No ultrasound guidance | Ultrasound guidance | Relative effect | Number of participants | Quality of the evidence | Comments |
| Failed blocks | Study population | RD −0.16 | 1789 | ⊕⊕⊕⊝ | NNTB is 6 (95% CI 5 to 8). The effect was more consistent for peripheral nerve block. | |
| 271 per 1000 | 145 per 1000 | |||||
| Pain scores at 1 hour after surgery6 | Mean pain scores at 1 hour after surgery was 0.41 standard deviation lower (‐0.74 to ‐0.07). | 982 | ⊕⊕⊕⊝ | The reduction is equivalent | ||
| Block duration | Mean block duration was 1.24 standard deviation higher (0.72 to 1.75).7 | 460 | ⊕⊕⊕⊕ | Prolongation is equivalent to 42 minutes. | ||
| Time to perform the block | Mean time to perform the procedure was 0.46 standard deviation lower (−1.06 to 0.13). | 680 | ⊕⊕⊕⊝ | |||
| Number of needle passes | Mean number of needle passes was 0.63 standard deviation lower (−1.08 to −0.18). | 256 | ⊕⊝⊝⊝ | Reduction is equivalent to 0.4 needle pass less per participant. | ||
| Minor complications | Bloody puncture | RD −0.02 | 896 | ⊕⊕⊝⊝ | ||
| Study population | ||||||
| 60 per 1000 | 16 per 1000 | |||||
| Transient neurological injury | RD −0.00 (−0.01 to 0.01) | 1230 (18 studies) | ⊕⊕⊝⊝ | |||
| Study population | ||||||
| 7 per 1000 | 3 per 1000 (0.9 to 12 per 1000) | |||||
| Seizure from local anaesthetic toxicity | RD 0.00 (−0.01 to 0.01) | 1576 (22 studies) | ⊕⊕⊕⊝ | |||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 5 per 1000) | |||||
| Block infections without neurological injury | RD 0.00 (−0.01 to 0.01) | 1238 (18 studies) | ⊕⊕⊝⊝ | |||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 6 per 1000) | |||||
| Major complications | Cardiac arrest from local anaesthetic toxicity | RD 0.00 (−0.01 to 0.01) | 1576 (22 studies) | ⊕⊕⊕⊝ | ||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 5 per 1000) | |||||
| Lasting neurological injury | RD 0.00 (−0.01 to 0.01) | 1250 (19 studies) | ⊕⊕⊝⊝ | |||
| Study population | ||||||
| 0 per 1000 | 0 per 1000 (0 to 6 per 1000) | |||||
| Incidences in study population and their 95% CI were calculated with VassarStat (VassarStats) with no continuity correction. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level for risk of bias. | ||||||
| Study | Intervention | Comparator |
| Ultrasound‐guided out‐of‐plane dorsal penile nerve block with 0.2 mL/kg of levobupivacaine 0.5% | Landmark dorsal penile nerve block with 0.2 mL/kg of levobupivacaine 0.5% (Dalens 1989) | |
| Ultrasound‐guided out‐of‐plane caudal block with 0.125% levobupivacaine plus 10 mcg/kg morphine (total volume: 0.5 mL/kg) | Landmark caudal block with 0.125% levobupivacaine plus 10 mcg/kg morphine (total volume: 0.5 mL/kg) | |
| Bilateral rectus sheath block with real‐time ultrasonographic guidance with 0.5 mL/kg per side of ropivacaine 0.25%, cephalad to the umbilicus | Infiltration by the surgeon (subcutaneous or intradermal) with 0.4 mL/kg of ropivacaine 0.5% | |
| Axillary brachial plexus with ultrasound, 13‐6 MHz linear probe, real‐time in‐plane technique, circumferential spread around each target nerve | Axillary brachial plexus with nerve stimulator 0.5 mA, 0.3 ms distal response for median, radial, and ulnar nerves. A triceps response could also be accepted for the radial nerve. | |
| Real‐time (in‐plane) ultrasound guidance was used to guide bilateral injections into the subpubic space, deep to Scarpa's fascia, with ropivacaine 0.75% 0.1 mL/kg per side plus 0.05 mL/kg at the base of the penis | Landmark dorsal penile nerve block with same volume and locations | |
| Real‐time (in‐plane) ultrasound‐guided rectus sheath block with 0.2 mL/kg 0.25% bupivacaine (1 mg/kg) to each side at least 10 minutes before | Wound infiltration of 0.4 mL/kg 0.25% bupivacaine (1 mg/kg) at the end of surgery | |
| Ultrasound‐guided posterior transversus abdominis plane block Total dose of 0.3 mL/kg ropivacaine 0.2% | Wound infiltration Total dose of 0.3 mL/kg ropivacaine 0.2% | |
| Real‐time (in‐plane) rectus sheath block, 1 cm cephalad to the umbilicus with 0.25% bupivacaine, volume according to a table Total volume: < 12 kg = 0.5 mL/kg; ≥ 12 to 30 = 12 mL; ≥ 30 to 40 = 16 mL; ≥ 40 = 20 mL | Infiltration of 0.25% bupivacaine at the end of surgery. Volume according to a table Total volume: < 12 kg = 0.5 mL/kg; ≥ 12 to 30 = 12 mL; ≥ 30 to 40 = 16 mL; ≥ 40 = 20 mL | |
| Transverse abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine placed with in‐plane ultrasound guidance | Wound infiltration with 0.5 mL/kg of 0.25% ropivacaine | |
| Transverse abdominis plane block with 2 mg/kg of 0.25% bupivacaine (maximal volume 30 mL) placed with in‐plane ultrasound guidance | Wound infiltration with 2 mg/kg of 0.25% bupivacaine | |
| Ultrasound‐guided in‐plane penile block, probe beneath the scrotum with 0.1 mL/kg of lidocaine 1% and ropivacaine 0.375% | Landmark traditional penile block with 0.2 mL/kg of lidocaine 1% and ropivacaine 0.375% | |
| Ultrasound‐guided rectus sheath block with 0.5 mL/kg (maximal volume 10 mL) per side of ropivacaine 0.2% | 0.5 mL/kg (maximal volume 10 mL) per side of ropivacaine 0.2% injected under direct visualization into the rectus sheaths bilaterally by the attending surgeon | |
| Caudal anaesthesia with ultrasound for pre‐scanning; positive reaction in caudal space was monitored simultaneously by ultrasound | Caudal anaesthesia with landmarks; positive reaction in caudal space was monitored simultaneously by classic swoosh test | |
| Ultrasound‐guided in‐plane median nerve block with 0.2% ropivacaine (maximal volume 0.5 mL/kg) | Landmark median nerve block with 0.2% ropivacaine (maximal volume 0.5 mL/kg) plus skin incision infiltration | |
| Real‐time, in‐plane, ultrasound‐guided transversus abdominis plane block with 0.4 mL/kg bupivacaine 0.25% with 1:200,000 epinephrine before incision | Wound infiltration with 0.4 mL/kg bupivacaine 0.25% with 1:200,000 epinephrine before incision | |
| Infraclavicular lateral brachial plexus blocks with 0.5% ropivacaine 0.5 mL/kg guided by ultrasound visualization (out‐of‐plane), injection around the brachial plexus | Infraclavicular brachial plexus blocks with 0.5% ropivacaine 0.5 mL/kg guided by nerve stimulation (coracoid process, 0.3 mA and 0.3 ms) | |
| ilioinguinal or iliohypogastric block under ultrasonic guidance (linear 5 to 10 MHz probe; real time; out‐of‐plane) with a mixture of 0.8% lidocaine and 0.25% levobupivacaine at 0.2 mL/kg | Ilioinguinal or iliohypogastric block performed according to the traditional method (Schulte‐Steinberg's method) of anatomical localization with the same local anaesthetic at 0.3 mL/kg | |
| Penile block under ultrasound guidance. Hockey‐stick probe (6 to 13 MHz, 25 mm), real‐time (in‐plane; information from study authors) guidance. 2 puncture techniques with 0.5% bupivacaine 1 to 2 mL up to 3 years and 1 additional mL per each additional 3 years up to a maximum of 5 to 6 mL | Penile block with landmarks with the same doses | |
| Sciatic and femoral nerve block under ultrasound guidance using a multiple‐injection technique until the nerves were surrounded by levobupivacaine. Portable ultrasound unit with a 5 to 10 MHz linear hockey stick probe, real‐time (out‐of plane) technique | Sciatic and femoral nerve blocks under nerve stimulator guidance using a predefined dose of 0.3 mL/kg of levobupivacaine injected when a current of 0.3 mA over 0.3 ms at 2 Hz elicited plantar flexion (sciatic) or cephalic movement of the patella (femoral) | |
| Real‐time (in‐plane) ultrasound‐guided infraclavicular brachial plexus block with a 38‐millimetre linear 5 to 10 MHz probe and with 0.5 mL/kg of 0.5% of bupivacaine as a 1‐injection technique | Lateral infraclavicular brachial plexus block guided by nerve stimulator with 0.5 mL/kg of 0.5% bupivacaine injected at 0.5 mA over 250 ms. If after 3 redirections a wrist response could not be obtained, an elbow response was accepted. | |
| Femoral and sciatic block under real‐time (in‐plane) ultrasound guidance with a 5 to 10 MHz probe and 0.5 mL/kg of 0.25% bupivacaine for the sciatic nerve and 0.7 mL/kg of lidocaine 1% for the femoral nerve. The procedure was abandoned in the absence of visualization of the sciatic nerve. | Femoral and sciatic block with nerve stimulator. Procedure abandoned if sciatic nerve stimulation was not obtained after 3 attempts (each pass was counted as an attempt). Injection of 0.5 mL/kg of bupivacaine 0.25% with plantar or dorsiflexion (ankle movement for the sciatic block) and 0.7 mL/kg of lidocaine 1% with a quadriceps contraction (femoral block) obtained at 0.5 mA. A fascia iliaca block (loss‐of‐resistance technique) was administered to children in whom a femoral block could not be performed. | |
| Transverse abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine placed with in‐plane ultrasound guidance | Landmark (triangle of Petit) transverse abdominis plane block with 0.5 mL/kg of 0.25% ropivacaine | |
| In‐plane ultrasound‐guided rectus sheath block with 0.1 mL/kg of 0.2% ropivacaine with 5 mcg/mL of epinephrine per side and administered at the T9–T10 level | The surgeon injected either 0.5 mL/kg of 0.5% bupivacaine or 1 mL/kg of 0.25% bupivacaine with 5 mcg/mL of epinephrine based on the surgeon’s discretion in line with his or her standard practice. | |
| Transversus abdominis plane block with ultrasound (real time; in‐plane) and 0.5 mL/kg of 0.25% levobupivacaine. The block was performed after anaesthesia induction with a 10 to 18 MHz probe placed between the iliac crest and the subcostal area at the mid‐axillary line and the needle directed posteriorly. The surgical procedure began 5 to 10 minutes after local anaesthetic administration. | Wound infiltration with 0.2 mL/kg of 0.25% levobupivacaine between external aponeurosis and the skin was performed by surgeons during wound closure. | |
| In‐plane ultrasound‐guided transversus abdominis plane block with 0.4 mL/kg of bupivacaine 0.25% with 1:200,000 epinephrine. Total dose of bupivacaine will not exceed 2 mg/kg, and total volume will not be more than 20 mL. | Wound infiltration with 0.4 mL/kg of bupivacaine 0.25% by the surgeon at the end of surgery | |
| Ropivacaine 0.2% (without epinephrine) around the lateral femoral cutaneous nerve via ultrasound guidance; dose = 20 mL or 1 mL/kg for children < 20 kg | Donor site infiltration with bupivacaine 0.25% with epinephrine 1:200,000; dose = 1 mL/kg (no maximum) | |
| Thoracic epidural. Ultrasound was used to determine the level, the wider space, and the puncture point (shortest depth from the skin). | Landmarks | |
| In‐plane ultrasound‐guided rectus sheath block with 0.2 mL/kg of 0.375% ropivacaine per side in the posterior rectus sheath compartment | Local anaesthetic infiltration with 0.2 mL/kg of 0.75% | |
| Ultrasound guidance. Pre‐scanning with a 5 to 10 MHz linear 38 millimetre probe, real time (out of‐plane), confirmed with upward displacement of the sacrococcygeal ligament upon injection | Landmarks | |
| Ilioinguinal‐iliohypogastric nerve blockade with real‐time (out of‐plane) ultrasound guidance using a 5 to 10 MHz linear hockey stick probe | Ilioinguinal‐iliohypogastric nerve blockade with landmarks (single‐pop) | |
| Real‐time (out of‐plane) ultrasound‐guided ilioinguinal block with levobupivacaine 0.25% in amount sufficient to surround the nerves | Ilioinguinal block using the traditional fascial click method. The needle was inserted vertically through the tented skin, 1 to 2 cm medial and 1 to 2 cm inferior to the anterior superior iliac spine. After the first fascial click was detected, and following a negative aspiration, levobupivacaine 0.25% (0.3 mL/kg) was injected. The spread of local anaesthetic was examined with ultrasound after injection but with no further intervention consequential to this information. | |
| Epidural catheter placement was guided by direct ultrasound visualization (real time, out‐of‐plane for needle placement). Images obtained by an assistant with 5 to 10 MHz hockey stick probe to obtain a paramedian longitudinal view. Midline needle insertion. Confirmation of the position of the tip of the catheter and spread of local anaesthetics through the catheter. Levobupivacaine 0.25% for confirmation of needle placement and through the catheter (0.2 mg/kg for the latter) | Epidural catheter with standard loss‐of‐resistance technique with air or saline. Levobupivacaine 0.2% 0.2 mg/kg through the needle and 0.2 mg/kg through the catheter | |
| In‐plane ultrasound‐guided ilioinguinal nerve block with 0.2 mL/kg mixture of 0.8% lidocaine with 0.25% levobupivacaine | Landmark‐based ilioinguinal nerve block (van Schoor 2005), with 0.3 mL/kg mixture of 0.8% lidocaine with 0.25% levobupivacaine | |
| T9–T10: vertebral thoracic level 9‐10 | ||
| Study | Type of block | Timing of blockade | Definition |
| Dorsal nerve penile block | Not reported | Proportion of participants that needed piritramide (Objective Pain Scale score > 3) were considered as failed blocks. | |
| Caudal block | Under general anaesthesia before surgical incision in both groups | Failed block was defined as significant motor movements following surgical induction or heart and respiratory rates increasing > 20% of the basal levels. | |
| Axillary brachial plexus block | Under general anaesthesia before surgical incision in both groups | The procedure was considered a failure when:
| |
| Penile nerve block | Under general anaesthesia before surgical incision in both groups | Ineffective block was defined as an increase in heart rate and mean arterial pressure > 20% above baseline values. | |
| Transversus abdominis plane block | Not reported | Proportion of participants that needed tramadol were considered as failed blocks. | |
| Transversus abdominis plane block | End of surgery | Proportion of participants that needed tramadol during the first 5 minutes after surgery were considered as failed blocks. | |
| Penile block | Not reported | Not reported | |
| Rectus sheath block | Before surgical incision for ultrasound‐guided technique and before skin closure for the surgeon‐administered technique | Pain scores ≥ 4 in post‐anaesthesia care unit | |
| Caudal block | Under general anaesthesia 10 to 15 minutes before surgical incision in both groups | Unsuccessful caudal puncture after 4 attempts (n = 2 in the control group) or signs of pain, such as body movement, tachycardia, and tachypnoea during surgery | |
| Median nerve block | Before surgical incision | A block was considered unsuccessful if the children met any of the following criteria:
| |
| Infraclavicular brachial plexus block | Propofol sedation, 30 minutes before surgical incision in both groups | Procedure was considered a failure if ≥ 2 of the 4 nerves (ulnar, radial, median, and musculocutaneous) could not be blocked effectively. | |
| Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia before surgical incision in both groups | Inadequate analgesia was defined as an increase of heart rate > 10% of baseline level, which required elevation of the sevoflurane concentration to 3% to 4% during surgery. | |
| Penile nerve block | Under general anaesthesia ≥ 10 minutes before surgical incision in both groups | Procedure was considered a failure if a rise in heart rate or respiratory rate > 25% from baseline occurred in response to surgical stimulus. | |
| Sciatic and femoral nerve blocks | Under general anaesthesia ≥ 20 minutes before surgical incision in both groups | Procedure was considered a failure if a rise in heart rate > 15% of baseline value occurred at skin incision or during surgery. | |
| Infraclavicular brachial plexus block | Under general anaesthesia before surgical incision in both groups | Procedure was considered a failure if a pain response to surgical stimulus occurred, defined as an increase in heart rate and arterial blood pressure > 20% of basal rate or non‐specific body movement in response to surgical stimulus and withdrawal of blocked limb in response to incision. | |
| Sciatic and femoral nerve blocks | Under general anaesthesia ≥ 20 minutes before surgical incision in both groups | Procedure was considered a failure when:
| |
| Thoracic epidural anaesthesia | Under general anaesthesia before surgical incision in both groups | Procedure was considered a failure if a participant complained of severe postoperative pain despite sufficient epidural administration of local anaesthetics. | |
| Caudal block | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if a child had motor or haemodynamic response, as indicated by an increase in mean arterial pressure or heart rate > 15% compared with baseline values obtained just before skin incision and subsequent to surgical procedure. | |
| Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if child had an increase in heart rate or mean arterial blood pressure > 10% compared with baseline during operation. | |
| Ilioinguinal and iliohypogastric nerve blocks | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | Procedure was considered a failure if child had an increase in heart rate or mean arterial pressure > 10% after skin incision or during surgery. | |
| Thoracic or lumbar epidural anaesthesia | Under general anaesthesia ≥ 15 minutes before surgical incision in both groups | An increase in heart rate or blood pressure > 20% from baseline was considered to reflect inadequate analgesia and was managed by bolus administration of levobupivacaine 0.25% 0.3 mL/kg of body weight through the epidural catheter. If this was unsuccessful, the epidural block was considered to have failed. | |
| Ilioinguinal nerve block | Under general anaesthesia after induction | Pain score of > 3 was considered a sign of inadequate analgesia. | |
| CHEOPS: Children's Hospital of Eastern Ontario Pain Scale | |||
| Peripheral nerve block | |||
| Study | Type of block | Minor complications | Major complications |
| Axillary brachial plexus block | No intravascular injection. Local bruising 2/17 in the ultrasound group vs 3/15 in the nerve stimulator group. Local axillary pain 3/17 in the ultrasound group and 8/15 in the nerve stimulator group. Transient postblock paraesthesia 2/17 in the ultrasound group and 4/15 in the nerve stimulator group (which resolved within 5 days as reported by parents and participants in the follow‐up surgical clinic 1 week later). | Major complications (e.g. unintentional intravascular injection, persistent neurological deficit) did not occur in either group. | |
| Median nerve block | No adverse events were observed in the 2 groups. | No adverse events were observed in the 2 groups. | |
| Infraclavicular brachial plexus block | No clinical signs of inadvertent puncture of major vessels | No clinical signs of pneumothorax, infection, or haematoma | |
| Ilioinguinal or iliohypogastric nerve block | 1 case in no‐ultrasound group had needle puncturing into blood vessels. | No other adverse event was observed in the 2 groups. | |
| Sciatic and femoral nerve blocks | No clinical signs of inadvertent puncture of major vessels | No clinical signs of nerve damage, infection, or haematoma | |
| Infraclavicular brachial plexus block | No complications were related to the regional anaesthetic technique. | No complications were related to the regional anaesthetic technique. | |
| Sciatic and femoral nerve blocks | Not reported | Not reported | |
| Lateral femoral cutaneous block or fascia‐iliaca block | No adverse events occurred performing these blocks. | No adverse events occurred performing these blocks. | |
| Ilioinguinal‐iliohypogastric nerve block | Not reported | The ultrasound‐guided technique resulted in higher Cmax (SD) and AUC values (Cmax: 1.78 (0.62) vs 1.23 (0.70) mcg/mL, P < 0.01; AUC: 42.4 (15.9) vs 27.2 (18.1) mcg 30 min/mL, P < 0.001). No signs of clinical toxicity | |
| Ilioinguinal block | All anaesthetic procedures were uneventful; no clinical evidence of complications such as small bowel or major vessel puncture. | All anaesthetic procedures were uneventful. | |
| Ilioinguinal nerve block | No adverse events other than 1 bloody puncture (landmark) were reported during or after the operation. | No adverse events other than 1 bloody puncture (landmark) were reported during or after the operation. | |
| Fascia block | |||
| Study | Type of block | Minor complications | Major complications |
| Penile nerve block | No adverse events were reported. | No adverse events were reported. | |
| Rectus sheath block | No adverse events requiring immediate medical attention associated with the surgical procedure or the postoperative course were reported in either group. | No adverse events requiring immediate medical attention associated with the surgical procedure or the postoperative course were reported in either group. | |
| Penile nerve block | No complications | No complications | |
| Rectus sheath block | Not reported | Peak plasma bupivacaine concentration was higher following ultrasound rectus sheath block (median 631.9 ng/mL, IQR: 553.9 to 784.1 vs 389.7 ng/mL, IQR: 250.5 to 502.7; P = 0.002). Time to peak concentration was longer in the USGRSB group (median 45 minutes, IQR: 30 to 60 vs 20 minutes, IQR: 20 to 45; P = 0.006). | |
| Posterior transversus abdominis plane block | No complication was recorded in transversus abdominis plane block group. | No complication was recorded in transversus abdominis plane block group. | |
| Rectus sheath block | Not reported | Not reported | |
| Transversus abdominis plane block | No adverse events due to research interventions | No adverse events due to research interventions | |
| Transversus abdominis plane block | No adverse effects related to the transversus abdominis plane block were identified. No complications were observed during or after the block intervention. | No major complications | |
| Penile block | No significant difference in the incidence of respiratory depression between the 2 groups | No significant difference in the incidence of respiratory depression between the 2 groups | |
| Rectus sheath block | 1 child in the surgeon‐administered rectus sheath block group developed a superficial surgical site infection; there were no complications in the ultrasound‐guided group. | No anaesthetic complications reported. | |
| Transversus abdominis plane block | None reported. | No local anaesthetic‐specific adverse events were noted. | |
| Penile nerve block | No complications were reported of either technique. | No complications were reported of either technique. | |
| Transversus abdominis plane block | Not reported | Not reported | |
| Rectus sheath block | No complications | No complications | |
| Transversus abdominis plane block | Not reported | Not reported | |
| Transversus abdominis plane block | There were no complications attributable to the ultrasound‐guided block. | There were no complications attributable to the ultrasound‐guided block. | |
| Rectus sheath block | No procedure‐related complications were observed in either group. | No procedure‐related complications were observed in either group. No adverse events associated with the nerve block procedure such as neuropathy or local anaesthetic intoxication were reported for any child. | |
| Neuraxial block | |||
| Study | Type of block | Minor complications | Major complications |
| Caudal block | Dural puncture and systemic local anaesthetic toxicity were not observed in any of the groups. No intraoperative desaturation was observed. | Dural puncture and systemic local anaesthetic toxicity were not observed in any of the groups. No intraoperative desaturation was observed. | |
| Caudal | Not reported | Not reported | |
| Thoracic epidural | Not reported | No children experienced severe side effects. | |
| Caudal | There was an incidence of bloody puncture of 18.6% in group landmarks and 5.7% in group ultrasound (P < 0.05). | No dural puncture or systemic reaction to local anaesthetic was reported in either group. | |
| Thoracic (n = 59) or lumbar (n = 5) epidural | Blood was aspirated in 1 child in the control group. | No dural puncture occurred in either group. | |
| AUC: area under the curve for blood concentrations of local anaesthetics; Cmax : maximal blood concentration of local anaesthetic; IQR: interquartile range; SD: standard deviation; USGRSB: ultrasound‐guided rectus sheath block. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Success (event = failed block) Show forest plot | 22 | 1789 | Risk Difference (M‐H, Random, 95% CI) | ‐0.16 [‐0.25, ‐0.07] |
| 1.1 Peripheral nerve block | 9 | 607 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.26, ‐0.09] |
| 1.2 Fascia block | 8 | 722 | Risk Difference (M‐H, Random, 95% CI) | ‐0.23 [‐0.48, 0.03] |
| 1.3 Neuraxial block | 5 | 460 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.07, 0.01] |
| 2 Pain at 1 hour after surgery Show forest plot | 15 | 982 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.74, ‐0.07] |
| 2.1 Ultrasound vs landmark | 4 | 514 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.43, 0.17] |
| 2.2 Ultrasound vs nerve stimulator | 2 | 104 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.68, 0.09] |
| 2.3 Ultrasound vs infiltration | 8 | 306 | Std. Mean Difference (Random, 95% CI) | ‐0.70 [‐1.41, 0.02] |
| 2.4 Ultrasound vs surgeon administered | 1 | 58 | Std. Mean Difference (Random, 95% CI) | 0.01 [‐0.51, 0.52] |
| 3 Block duration Show forest plot | 10 | 460 | Std. Mean Difference (Random, 95% CI) | 1.24 [0.72, 1.75] |
| 3.1 Ultrasound vs landmark | 1 | 40 | Std. Mean Difference (Random, 95% CI) | 1.38 [0.69, 2.08] |
| 3.2 Ultrasound vs nerve stimulator | 3 | 138 | Std. Mean Difference (Random, 95% CI) | 1.22 [0.85, 1.59] |
| 3.3 Ultrasound vs infiltration | 5 | 224 | Std. Mean Difference (Random, 95% CI) | 1.42 [0.36, 2.48] |
| 3.4 Ultrasound vs surgeon administered | 1 | 58 | Std. Mean Difference (Random, 95% CI) | 0.45 [‐0.07, 0.97] |
| 4 Time to perform the block Show forest plot | 9 | 680 | Std. Mean Difference (Random, 95% CI) | ‐0.46 [‐1.06, 0.13] |
| 4.1 In‐plane | 4 | 220 | Std. Mean Difference (Random, 95% CI) | 0.15 [‐0.76, 1.06] |
| 4.2 Out‐of‐plane | 3 | 338 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.96, 0.19] |
| 4.3 Pre‐scanning | 2 | 122 | Std. Mean Difference (Random, 95% CI) | ‐1.97 [‐2.41, ‐1.54] |
| 5 Number of needle passes Show forest plot | 3 | 256 | Std. Mean Difference (Random, 95% CI) | ‐0.63 [‐1.08, ‐0.18] |
| 6 Minor complications: bloody puncture Show forest plot | 13 | 896 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.00] |
| 6.1 Peripheral nerve bock | 5 | 326 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 6.2 Fascia block | 5 | 232 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.03] |
| 6.3 Neuraxial block | 3 | 338 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.15, ‐0.02] |
| 7 Minor complications: transient neurological injury Show forest plot | 18 | 1230 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 8 Minor complications: seizure from local anaesthetic toxicity Show forest plot | 22 | 1576 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 9 Minor complications: block infection without neurological injury Show forest plot | 18 | 1238 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 10 Major complications: cardiac arrest from local anaesthetic toxicity Show forest plot | 22 | 1576 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 11 Major complications: lasting neurological injury Show forest plot | 19 | 1250 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |

