Antifibrinolytic therapy for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing minor oral surgery or dental extractions
Abstract
Background
Minor oral surgery or dental extractions (oral or dental procedures) are widely performed and can be complicated by hazardous oral bleeding, especially in people with an inherited bleeding disorder such as haemophilia or Von Willebrand disease. The amount and severity of singular bleedings depend on disease‐related factors, such as the severity of the haemophilia, both local and systemic patient factors (such as periodontal inflammation, vasculopathy or platelet dysfunction) and intervention‐related factors (such as the type and number of teeth extracted or the dimension of the wound surface). Similar to local haemostatic measures and suturing, antifibrinolytic therapy is a cheap, safe and potentially effective treatment to prevent bleeding complications in individuals with bleeding disorders undergoing oral or dental procedures. However, a systematic review of trials reporting outcomes after oral surgery or a dental procedure in people with an inherited bleeding disorder, with or without, the use of antifibrinolytic agents has not been performed to date.
Objectives
The primary objective was to assess the efficacy of local or systemic use of antifibrinolytic agents to prevent bleeding complications in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures. Secondary objectives were to assess if antifibrinolytic agents can replace or reduce the need for clotting factor concentrate therapy in people with haemophilia or Von Willebrand disease and to further establish the effects of these agents on bleeding in oral or dental procedures for each of these populations.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register, compiled from electronic database searches of the Cochrane Central Register of Controlled Trials (CENTRAL), of MEDLINE and from handsearching of journals and conference abstract books. We additionally searched the reference lists of relevant articles and reviews. We searched PubMed, Embase and The Cochrane Library. Additional searches were performed in ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP).
Date of last search of the Cystic Fibrosis and Genetic Disorders Group's Coagulopathies Trials Register: 14 December 2015.
Selection criteria
Randomised and quasi‐randomised controlled trials in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures using antifibrinolytic agents (tranexamic acid or epsilon aminocaproic acid) to prevent perioperative bleeding compared to no intervention or usual care with or without placebo.
Data collection and analysis
Two authors independently screened the titles and abstracts of all identified articles. Full texts were obtained for potentially relevant abstracts and two authors independently assessed these for inclusion based on the selection criteria. A third author verified trial eligibility. Two authors independently performed data extraction and risk of bias assessments using standardized forms.
Main results
While there were no eligible trials in people with Von Willebrand disease identified, two randomised, double‐blind, placebo‐controlled trials (total of 59 participants) in people with haemophilia undergoing dental extraction were included. One trial of tranexamic acid published in 1972 included 28 participants with mild, moderate or severe haemophilia A and B and one of epsilon aminocaproic acid published in 1971 included 31 people with haemophilia with factor VIII or factor IX levels less than 15%. Overall, the two included trials showed a beneficial effect of tranexamic acid and EACA, administered systemically, in reducing the number of bleedings, the amount of blood loss and the need for therapeutic clotting factor concentrates. Regarding postoperative bleeding, the tranexamic acid trial showed a risk difference of ‐0.64 (95% confidence interval ‐0.93 to ‐ 0.36) and the EACA trial a risk difference of ‐0.50 (95% confidence interval 0.77 to ‐0.22). The combined risk difference of both trials was ‐0.57 (95% confidence interval ‐0.76 to ‐0.37), with the quality of the evidence (GRADE) for this outcome is rated as moderate. Side effects occurred once and required stopping epsilon aminocaproic acid (combined risk difference of ‐0.03 (95% CI ‐0.08 to 0.13). There was heterogeneity between the two trials regarding the proportion of people with severe haemophilia included, the concomitant standard therapy and fibrinolytic agent treatment regimens used. We cannot exclude that a selection bias has occurred in the epsilon aminocaproic acid trial, but overall the risk of bias appeared to be low for both trials.
Authors' conclusions
Despite the discovery of a beneficial effect of systemically administered tranexamic acid and epsilon aminocaproic acid in preventing postoperative bleeding in people with haemophilia undergoing dental extraction, the limited number of randomised controlled trials identified, in combination with the small sample sizes and heterogeneity regarding standard therapy and treatment regimens between the two trials, do not allow us to conclude definite efficacy of antifibrinolytic therapy in oral or dental procedures in people with haemophilia. No trials were identified in people with Von Willebrand disease.
PICO
Plain language summary
Drugs that prevent oral bleeding in people with haemophilia or Von Willebrand disease undergoing minor oral surgery or dental extractions
Review question
We reviewed the evidence about whether antifibrinolytic medicine (drugs that promote blood clotting) such as tranexamic acid or epsilon aminocaproic acid, can prevent oral bleeding in people with haemophilia or Von Willebrand disease undergoing minor oral surgery or dental extractions.
Background
Haemophilia and Von Willebrand disease are inherited bleeding disorders. People with these disorders have an increased risk of bleeding complications during and after oral surgery or dental extractions, even if these are relatively minor and commonly performed. The number of bleeds and the severity of each depend on disease‐related factors (such as the severity of the haemophilia), as well as patient‐related factors (such as inflammation of the gums or blood vessel diseases) and intervention‐related factors (such as the type and the number of teeth extracted or how big the surface of the wound is). Measures such as giving clotting factors directly into the blood stream, are commonly used before, during or after surgery to prevent bleeding complications. However, these measures are costly and have risks such as the formation of inhibitors and the transmission of infections. Therefore, it is important to search for alternative methods to prevent bleeding complications. In routine practice antifibrinolytic medicine is often used before, during and after surgery. However, there is currently no clear scientific evidence for this practice .
Search date
The evidence is current to: 14 December 2015.
Trial characteristics
We did not find any trials of antifibrinolytic medicine to prevent bleeding after minor oral surgery or dental extractions in people with Von Willebrand disease. The review does include two trials published in the 1970's in 59 people with haemophilia undergoing dental extraction. In one trial the people were aged between 13 and 65 years and in the second trial the people had an average age of 34 years. One trial lasted five days and used tranexamic acid; the second trial lasted 10 days and used epsilon aminocaproic acid tablets. Both trials compared the active medicine with a substance that contained no medication (a placebo) in addition to clotting factor concentrates.
Key results
Overall, the two included trials showed a reduction in the number of bleeds after dental extraction, in the amount of blood loss, and in the need for clotting factor concentrates in the people treated with tranexamic acid or epsilon aminocaproic acid tablets compared to those who received a placebo. When combining the results of both trials it appeared that antifibrinolytic medication roughly halves the bleeding rate after dental extraction and the trials reported. Side effects of the antifibrinolytic medicine rarely occurred and led to discontinuation of epsilon aminocaproic acid tablets in only one case.
Quality of the evidence
In the epsilon aminocaproic acid trial, the trial physicians assigned each person to receive a placebo or the active treatment based on a pair‐matching technique for age, factor‐assay and the number of extractions. The fact that the trial physicians made this decision may have introduced a selection bias. However, we do not think that this had a major impact on the trial's conclusions. Overall, the two trials were small and differed from each other in terms of how many of the people taking part had severe haemophilia , the simultaneous use of clotting factor concentrates and the different antifibrinolytic treatment schedules. We rated the overall quality of the evidence as low for using antifibrinolytic medicine to prevent bleeding in people with haemophilia after minor oral surgery or dental extractions. No evidence was found for people with Von Willebrand disease. It could however be argued that, if antifibrinolytic medicine works for people with haemophilia, it is likely that the medicine will also work for people with other bleeding disorders undergoing dental extractions or minor oral surgery.
Authors' conclusions
Summary of findings
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Outcomes | Anticipated absolute effects* | Relative effect | № of participants | Number needed to treat (NNT) | Quality of the evidence | Comments | |
| Risk with placebo or usual care | Risk with antifibrinolytic therapy | ||||||
| Postoperative bleedings requiring intervention | Study population | Risk difference: 57 | 59 | 1.8 | ⊕⊕⊕⊝ | ||
| 67 per 100 (20 events) | 10 per 100 (3 events) | ||||||
| Side effects or other adverse events | Study population | Risk difference: 3.4 | 59 | 0.3 | ⊕⊕⊝⊝ | ||
| 0 events | 1 event | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 The magnitude of effect was considered large in this study, therefore the quality of evidence was rated up one level. 2 Small sample sizes and lack of studies 3 Heterogeneity between the included trials regarding the proportion of severe people with haemophilia included, the concomitant standard therapy and fibrinolytic agent treatment regimens used | |||||||
Background
Description of the condition
Minor oral surgery or dental extractions (oral or dental procedures) are widely performed and can be complicated by hazardous oral bleeding, especially in people with an inherited bleeding disorder such as haemophilia or Von Willebrand disease (VWD). The amount and severity of singular bleedings depend on disease‐related factors, such as the severity of the haemophilia, patient factors both local and systemic such as periodontal inflammation, vasculopathy or platelet dysfunction, and intervention‐related factors, such as the type and the number of teeth extracted or the dimension of the wound surface. It is relevant to search for alternative methods to prevent bleeding complications because of the disadvantages of commonly used perioperative measures. For example clotting factor concentrates include the risk of developing inhibitors, the risk of transmitting infections and are costly. Also, the need for repeated clotting factor replacement by intravenous infusion may well have an impact on the quality of life (Kaufman 2013). Expensive and sometimes dangerous over‐treatment with clotting factor replacement or desmopressin should be avoided. The small risk of thromboembolic events, including coronary occlusion in those with pre‐existent coronary artery disease and the risk of fluid overload and hyponatraemia with desmopressin, precludes its use in individuals with certain co‐morbidities. In practice, antifibrinolytic treatment, administered either topically or systemically, is often used to achieve adequate perioperative haemostasis.
Inherited bleeding disorders impair normal haemostasis resulting in a tendency to bleed, the severity of which depends on the degree of the underlying coagulation factor deficiency. Haemophilia and VWD are the most common inherited bleeding disorders, with a prevalence of 1 in 5000 males and 1%, respectively (Rodeghiero 1987; Stonebraker 2010). Haemophilia is an X‐linked inherited bleeding disorder, caused by a deficiency of factor VIII (FVIII) or factor IX (FIX); VWD is a mostly dominantly inherited congenital bleeding disorder caused by a deficiency or dysfunction of Von Willebrand factor (VWF). Factor VIII, IX and VWF are clotting proteins, necessary for the completion of primary (VWF) and secondary (FIX and FVIII) haemostasis. In addition, VWF is a carrier protein for FVIII in the circulatory system, therefore FVIII deficiency is also seen in moderate and severe VWD, with consequent additional impairment of secondary haemostasis. Severe haemophilia is defined as FVIII or FIX levels less than 1%, moderate haemophilia as 1% to 5% FVIII or FIX and mild haemophilia as 5% to 40% FVIII or FIX. Severe VWD is defined as VWF activity less than 10%, moderate VWD as 10% to 30% and mild VWD as VWF activity more than 30%. Rarer congenital deficiencies or dysfunction of other coagulation factors and platelets can also occur.
Dental extractions and dentoalveolar surgery, such as third molar removal, dental implant placement and periodontal surgery, are among the most common invasive minor oral surgical interventions. In undergoing these interventions, people with congenital bleeding disorders are at an increased risk of bleeding complications during and after the procedure. If no antifibrinolytic agents, local haemostatic measures such as fibrin adhesives, oxidized cellulose or gelatin sponges and suturing are used, postoperative bleeding has been reported to occur in over 73% of people with haemophilia after oral surgery (Ramstrom 1989).
Description of the intervention
The main pharmaceutical interventions used to prevent perioperative bleeding in people with congenital bleeding disorders are the antifibrinolytic agents tranexamic acid (TXA) or epsilon aminocaproic acid (EACA). Antifibrinolytic therapy, as compared to clotting factor concentrates, is a relatively cheap and potentially effective therapy in preventing bleeding complications in oral surgery, in addition to other commonly used perioperative measures such as factor replacement therapy, desmopressin and local haemostatic measures. Considering the disadvantages of factor replacement therapy in this population, it is important to find alternative methods to prevent bleeding complications in those undergoing oral or dental procedures. Despite the potentially beneficial effects for people with congenital bleeding disorders and its routine application in haemophilia treatment, antifibrinolytic therapy has not yet become part of the standard therapeutic guidelines in oral or dental procedures. Antifibrinolytic agents can be administered topically as a mouthwash or systemically as oral or intravenous formulations (see table below). All agents can be used before, during or after dental procedures. In people with renal insufficiency a dose reduction is necessary to ensure renal clearance. Antifibrinolytic agents are contraindicated if there is active thrombotic disease.
Dosing of antifibrinolytic agents
| Antifibrinolytic agent | Available concentration | Dose (adults) | Dose (children) |
| TXA mouthwash | 50 mg/ml | 10 ml, 4‐times‐daily | ≥ 1 year: 20 mg/kg bodyweight/day in 2 to 3 doses a day |
| IV TXA | 100 mg/ml slowly IV (1 ml/min) | 0.5 g to 1 g, 2‐ to 3‐times‐daily | ≥ 1 year: 20 mg/kg bodyweight/day in 2 to 3 doses a day |
| Oral TXA | 500 mg | 1 to 1.5 g, 2‐ to 3‐times‐daily | ≥ 1 year: 20 mg/kg bodyweight/day in 2 to 3 doses a day |
| IV EACA | 250 mg/ml | Starting dose 4 g to 5 g slowly IV (more than 1 hour), followed by continuous infusion of 1 g/hour | 100 mg/kg or 3 g/m2 slowly IV during the first hour, followed by continuous infusion 33.3 mg/kg/hour or 1 g/m2/hour |
| Oral EACA | 500 mg and 1000 mg | Starting dose 4 g to 5 g, followed by 1 to 1.25 g/hour. Maximum dose 24 g per 24 hours | starting 100 mg/kg, followed by 3 g/m2 during the first hour, followed by 33.3 mg/kg or 1 g/m2 every hour, maximum 18 g/m2 (600 mg/kg) in 24 hours |
Table legend: EACA: epsilon aminocaproic acid; IV: intravenous; TXA: tranexamic acid.
Comparator interventions include placebo or no intervention or usual care with or without placebo. Usual care in people with congenital bleeding disorders includes local haemostatic measures (e.g. suturing, local administration of fibrin glue or haemostatic gelatin sponge) except for local antifibrinolytic agents, prophylactic factor replacement therapy and temporary interruption or blockage of anticoagulation drugs. The latter are obviously rarely used in people with congenital bleeding disorders. Factor replacement therapy consists of recombinant or plasma‐derived FVIII or FIX concentrates in case of haemophilia and of plasma‐derived VWF/FVIII concentrates in case of VWD.
How the intervention might work
The antifibrinolytic agents TXA and EACA act by binding reversibly to plasminogen and blocking the interaction of plasminogen with fibrin, thereby preventing degradation of the fibrin clot which supports blood clotting. The agent TXA is more potent than EACA (Pell 1973). Bleeding in haemophilia is due not only to defective prothrombin activation but also to hyperfibrinolysis. An important role of FVIII and FIX is to consolidate and sustain the fibrin clot through the activation of thrombin activatable fibrinolysis inhibitor (TAFI). Decreased FVIII and FIX activity in haemophilia and severe VWD leads to less TAFI activation due to less thrombin generation and a consequent increase in fibrinolysis (Broze 1996). In addition, fibrinolytic activity is particularly high in oral mucosa due to the fibrinolytic activity of saliva and local tissue plasminogen activator (t‐PA) production (Sindet‐Pedersen 1990). Therefore, the inhibition of fibrinolysis with antifibrinolytic agents is a rational approach for limiting oral bleeding after surgery in people with congenital bleeding disorders (Shira 1981).
Large placebo‐controlled randomised trials have proven the effectiveness of TXA in reducing postoperative blood loss and transfusion requirements in cardiac surgery (Katsaros 1996; Later 2009; Maddali 2007). In orthopedic surgery, which is also associated with high perioperative blood loss, a meta‐analysis of large randomised placebo‐controlled trials demonstrated the efficacy of TXA on the same end points without an increased incidence of thrombosis (Gandhi 2013). In emergency surgery, TXA reduced the need for blood transfusion by 30% (Perel 2013). Fewer randomised controlled trials have been conducted in other types of surgery, including oral surgery in people with congenital bleeding disorders or in people using vitamin K antagonists (Forbes 1972; McCormack 2012; Sindet‐Pedersen 1989).
Why it is important to do this review
Within this review, we aimed to analyse the evidence base for the use of antifibrinolytic agents in people with haemophilia or VWD undergoing oral or dental procedures. Given their low cost, high tolerability, and the evidence for effectiveness and safety in other patient groups (cardiac and orthopedic surgery), these agents are an attractive alternative to clotting factor replacement therapy to prevent perioperative bleeding in oral or dental procedures. If this review finds sufficient scientific evidence to support the use of antifibrinolytic agents in people with congenital bleeding disorders undergoing oral or dental procedures, then this treatment is likely to become part of the general standard therapeutic approach, potentially preventing postoperative blood loss without the disadvantages and high costs of clotting factor replacement therapy or desmopressin.
A systematic review on studies reporting outcomes after oral surgery in people receiving oral anticoagulation therapy, excluding antiplatelet agents, found few bleeding complications, which were controlled with local haemostatic measures including TXA mouthwashes (Madrid 2009). However, high‐quality published evidence to support the use of antifibrinolytic agents in people with coagulation disorders undergoing oral surgery is limited. We are unaware of any recent systematic review or meta‐analysis specifically reporting on antifibrinolytic therapy in this population.
Objectives
Primarily, we aim to assess the efficacy of antifibrinolytic agents to prevent bleeding complications in people with haemophilia or VWD undergoing oral or dental procedures.
Secondary objectives are to assess if antifibrinolytic agents can replace or reduce the need for clotting factor concentrate therapy in people with haemophilia or VWD and to establish the effects of these agents on bleeding in oral or dental procedures for each of these patient populations.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials in people with haemophilia or VWD undergoing oral or dental procedures (minor oral surgery).
Types of participants
People of all ages with haemophilia or VWD undergoing any type of oral or dental procedures. In order to avoid too much heterogeneity and very small subgroups of people with rarer coagulation disorders, we have restricted our review to these common congenital bleeding disorders. We defined VWD as VWF activity less than 40% and haemophilia A or B as FVIII or FIX levels less than 40%.
Minor oral surgery was defined as dentoalveolar surgery such as third molar removal, dental implant placement and periodontal surgery.
Types of interventions
Main intervention
Use of the antifibrinolytic agents (TXA or EACA) to prevent perioperative bleeding in people with haemophilia or VWD undergoing oral or dental procedures at any dose, mode of delivery (topical, oral or intravenous), frequency and duration of administration, whether started before, during or immediately after the intervention.
Comparison interventions
Placebo or no intervention or usual care, with or without placebo, in people with haemophilia or VWD.
Usual care included local haemostatic measures except for local antifibrinolytic agents and prophylactic factor replacement therapy. Trials using co‐interventions such as additional preventive coagulation factor replacement and local haemostatic measures were included if these co‐interventions were equally distributed among the intervention and comparison groups and did not seriously hamper the interpretation of the separate effect of the antifibrinolytic agents.
Types of outcome measures
Primary outcomes
-
Number of postoperative bleeding episodes requiring intervention*
-
Side effects or other adverse events
*Intervention is defined as any additional treatment or medical attention needed in addition to usual care to halt postoperative bleeding directly up to 10 days after surgery. Usual care to halt bleeding consists of local pressure and is not considered a medical intervention. Postoperative bleeding episodes include immediate postoperative bleeds, defined as bleeding within 24 hours after surgery, as well as delayed postoperative bleeds, defined as bleeding 24 hours to 10 days after surgery. This includes both clinically relevant (non‐major) bleeding requiring medical attention (e.g. wound dressing or additional sutures) and major bleeding requiring transfusion of packed red blood cells. Side effects are only being considered clinically relevant if they lead to a discontinuation or change of therapy.
Secondary outcomes
-
Number of minor postoperative bleeding episodes (defined as self‐limiting, usually with local pressure, that did not require medical attention)
-
Number of immediate (less than 24 hours) and delayed (24 hours to 10 days) postoperative bleeding episodes requiring intervention
-
Any postoperative complication except bleeding, such as wound infection
-
Change in haemoglobin level from baseline
-
Major bleeding, requiring transfusion of packed red blood cells
-
Bleeding duration (minutes, all types of bleeding minor and major)
-
Amount of postoperative blood loss (ml)
-
Need for and dose of clotting factor concentrates
Search methods for identification of studies
Electronic searches
The Cystic Fibrosis and Genetic Disorders (CFGD) Group identified relevant trials from the Group's Coagulopathies Trials Register using the term: antifibrinolytics.
The CFGD Coagulopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library) and weekly searches of MEDLINE and the prospective handsearching of one journal ‐ Haemophilia. Unpublished work is identified by searching the abstract books of major conferences: the European Haematology Association conference; the American Society of Hematology conference; the International Society of Haemostasis and Thrombosis (ISTH congresses and its Scientific and Standardization Committee (SSC) scientific meetings) and the Congress of the World Federation of Hemophilia. The search term 'antifibrinolytics' will be added to this trials register. For full details of all searching activities for the register, see the relevant section of the Cystic Fibrosis and Genetic Disorders Group Module.
We searched the following resources: PubMed (Appendix 1), Embase (Appendix 2) and Cochrane. Additional searches were performed at ClinicalTrials.gov (Appendix 3), in the CINAHL database (Appendix 4), WHO International Clinical Trials Registry Platform (ICTRP) (Appendix 5), abstract books of the annual scientific meeting of the Internation Society for Thrombosis and Haemostasis (Appendix 6) and the Proquest dissertation database (Appendix 7). We did not use inbuilt filter options from the databases, however we only included studies written in English, Dutch, French or German.
Date of the most recent search of the CFGD Group's Coagulopathies Trials Register: 14 December 2015.
Date of most recent MEDLINE search: 14 September 2015.
Searching other resources
In order to find additional relevant trials, we screened the reference lists of all included trials by hand.
Furthermore, we searched the ongoing trials registers: ClinicalTrials.gov (http://www.clinicaltrials.gov/); and the WHO International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/), abstract books of the annual scientific meeting of the International Society for Thrombosis and Haemostasis, the CINAHL database of nursing and allied health services, trial registries and the Proquest dissertation database using database‐specific search terms equivalent to those used for the search in PubMed. The final search strategies are published in the appendices. There were no restrictions on publication years; all articles published up until the date of the search were screened.
The final search strategies are published as appendices.
Data collection and analysis
Selection of studies
Two authors (EE content area expert, KG content area expert and methodologist) independently screened titles and abstracts of all articles obtained through the search strategy and identified abstracts of trials that appeared to be potentially relevant. Of the identified abstracts, full texts were obtained and two authors (EE, KG) independently assessed these trials for inclusion based on the previously described selection criteria. For this purpose we used a separate data collection form for assessing trial eligibility. Multiple reports of the same trial were identified by comparing authors of the reports, trial dates, trial durations, number of participants, details on the interventions and location and setting of the reported trials. When multiple reports on one or more trials were identified these reports were linked together. A third author (RS content area expert, supervising author) verified the assessment of trials identified for inclusion. There was no disagreement between the authors (EE, KG, RS) in selection of trials. Duplicate records of the same reports were removed using reference manager software (RefMan® 2005). We recorded the articles retrieved from the search databases in the RevMan software (RevMan 2011). Excluded trials were listed, except if obviously not fulfilling the selection criteria of this review, and the primary reason for exclusion was given.
Data extraction and management
Two authors (EE, KG) independently extracted data from published reports using a data extraction form containing the characteristics of the included trials and trial participants, all outcome measures and a risk of bias table. We prepared the data extraction form using the general template for ‘Summary of findings’ tables and a Cochrane checklist of items to consider in data collection or data extraction (Higgins 2011a), which was authorized by all authors. A third author verified the data extraction of trials identified for inclusion (RS). There were no differences in data extraction between the authors (EE, KG, RS). It was not necessary to request further information from the original authors to clarify data.
Assessment of risk of bias in included studies
We assessed the risk of bias of the included trials by using a risk of bias table that included judgments of the adequacy of the sequence generation (selection bias), allocation concealment (selection bias), blinding of the outcome assessments (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other potential sources of bias (Higgins 2011b). This table was completed for each included trial by two authors (EE, KG) independently. Any differences were resolved by discussion between three authors (EE, KG, RS). We rated the risk of bias for each domain as low, unclear or high and summarized this information in a 'risk of bias' plot.
Measures of treatment effect
The treatment effect is the proportion of participants with postoperative bleedings needing intervention, immediate as well as delayed postoperative bleedings lumped together, in the intervention group compared to the control group. For meta‐analysis, this treatment effect was expressed as a risk difference (RD) with corresponding 95% confidence interval (CI). We converted this measure to a number needed to treat (NNT), after calculation of the absolute risk reduction (ARR) using the RR and the control group risk (CGT), which is the risk of postoperative bleeding in the control group. The NNT was useful for better interpretation of the results of our meta‐analysis.
Formulas: ARR = 100 x CGR x (1‐RR), NNT = 100/ARR.
The combined risk difference of the combined results of both trials regarding the number of people with postoperative bleeding and the number of side effects were calculated using the same formulas and are presented in a 'Summary of findings table' (summary of findings Table for the main comparison).
Unit of analysis issues
Trials with non‐standard randomised controlled designs (other than parallel patient‐control designs) were also considered for eligibility, given the rare occurrence of haemophilia and VWD.
Cross‐over trials
In the population of interest, individuals with a rare bleeding disorder, cross‐over trials are especially advantageous because fewer participants are required to obtain the same power and because the intervention evaluated has a temporary effect in the treatment of the stable, chronic condition of a congenital bleeding disorder. A prerequisite, however, is that single participants need comparable dental procedures more than once at different times during the study period. When the interventions are sufficiently spaced in time there is no risk of a carry‐over effect. Furthermore, every participant receives the intervention and control treatment, which allows the determination of the best treatment or preference for an individual participant (Higgins 2011c). Treatment and control intervention could also be given at the same time, when comparable oral or dental procedures are performed on both sides of the mouth, such as in one of the excluded studies (Lee 2005) and one side of the mouth receives the intervention and the other side the control treatment. If, for future updates of this review, cross‐over trials are included, we plan to include data from such trials in the meta‐analysis as if the trial was a parallel group trial.
Trials with multiple treatment groups
If included clinical trials randomise participants to one of several intervention groups, only the groups where the intervention consists of the administration of antifibrinolytic agents to prevent bleeding in oral or dental procedures and the comparison groups with placebo, no intervention or usual care with or without placebo will be included in the meta‐analysis using the treatment effect measure as stated above. If, for future updates of this review, trials of this design meet the inclusion criteria, we plan to present the effect measure separately in the meta‐analysis if more than two groups are relevant with regard to differences in the administration of antifibrinolytic agents, using a proportional part of the comparison group, to allow for any subgroup analyses (Higgins 2011c).
Cluster‐randomised trials
If for future updates, we include cluster‐randomised trials we plan to pay special attention in the risk of bias assessment to the possibility of recruitment bias, baseline imbalances, loss of clusters, incorrect analysis, herd effect and contamination (Higgins 2011c). In the meta‐analysis we plan to use the same treatment effect measure as in the included parallel group trials if the original analysis properly accounts for the cluster design, based for example on a multilevel model or generalized estimating equations (GEEs) and we will seek statistical advice if needed.
Dealing with missing data
Possible sources of missing data are: missing outcomes; selective or incomplete reporting; and lack of intention‐to‐treat analysis. Since there were no missing data in the included trials, it was not necessary to request original authors for additional data (Higgins 2011c).
Assessment of heterogeneity
Differences were expected in the specific interventions and participant characteristics. These differences give rise to clinical heterogeneity between the included trials. To assess the heterogeneity we considered a visual inspection of the forest plot to see whether CIs overlap. In addition, significant statistical heterogeneity arises from methodological differences between trials (Deeks 2011). To quantify inconsistency between trials we planned to calculate the I2 statistic to describe the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (change) (Higgins 2003).
Assessment of reporting biases
To address reporting bias, we set up the literature search to be as comprehensive as possible so as to prevent missing potentially eligible trials. We also searched the trial registration database (www.clinicaltrials.gov) for this purpose. We did not construct a funnel plot as we did not include enough trials (less than 10).
Data synthesis
We assumed that included trials would use different outcome definitions of postoperative bleeding. Therefore, if possible, we recorded the number of postoperative bleedings needing intervention. This outcome measure was used in the meta‐analysis. We combined all interventions using antifibrinolytic agents. We used a random‐effects model for meta‐analysis. We made this decision based on expected small trial populations, due to the rare occurrence of congenital bleeding disorders and on the expected heterogeneity between the trials due to different outcome measures and differences in the administration of antifibrinolytic agents and use of co‐interventions. We summarized the extracted data in a 'Summary of findings' table (summary of findings Table for the main comparison).
Subgroup analysis and investigation of heterogeneity
Given that we only included two trials, we were not able to undertake the planned subgroup analyses (Deeks 2011).
-
Hemophilia versus VWD
-
Severe versus moderate versus mild forms of haemophilia, defined as FVIII or FIX levels of less than 1%, 1% to 5%, and 6% to 40% respectively
-
Severe versus moderate versus mild forms of VWD, defined as VWF activity of < 10%, 10% to 30%, and > 30% respectively
-
Administration form of antifibrinolytic agents: topical versus systemic
-
Antifibrinolytic agent used: TXA versus EACA
-
Different outcome definitions of perioperative bleeding: clinically significant versus minor versus major postoperative bleedings; and immediate versus delayed postoperative bleedings
Sensitivity analysis
Given that we only included two trials, we were not able to undertake the planned sensitivity analyses.
-
high risk of bias trials (e.g. non‐blinded);
-
trials published as abstracts whose results cannot be confirmed in subsequent publications;
-
trials using local haemostatic measures without systemic haemostatic support;
-
non‐standard randomised trial designs;
-
reporting bias: including versus excluding small trials.
Summary of findings table
The findings of the included trials are presented in a 'Summary of findings' table that contains the primary outcome measures. The absolute risks and risk differences (RD (95% CI)) are presented. This table also includes quality assessments for each primary outcome measure according to the GRADE approach (high, moderate, low or very low). The numbers of participants and trials addressing these outcomes are summarized in the Characteristics of included studies tables. Risk of bias assessment is presented in the risk of bias tables in the section Characteristics of included studies and summarized in Figure 1. (Higgins 2011b; Higgins 2011c).
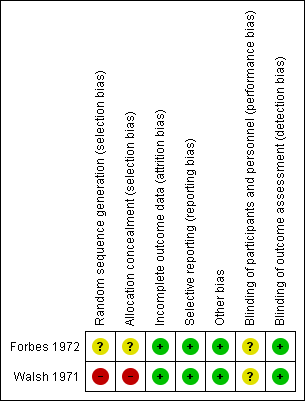
Risk of bias summary of the included studies
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
The initial search yielded 313 articles as presented in the trial flow diagram (Figure 2) (Liberati 2009). After removal of duplicates, 232 articles were screened on title and abstract. Full texts of the 30 articles that appeared to be potentially relevant were obtained. Two articles were eligible for inclusion; the included trials are summarized in the 'Summary of findings table' (summary of findings Table for the main comparison). No trials on people with VWD were considered eligible for this review.

Trial flow diagram
Included studies
The characteristics of the included trials are summarized in the Characteristics of included studies table.
Trial design
Of the two included trials, one was a randomised, double‐blind, placebo‐controlled trial (Forbes 1972), and a second was a quasi‐randomised trial in people with haemophilia (Walsh 1971). The Walsh trial was undertaken in two centres (Walsh 1971).
Country
Both trials were performed in the United Kingdom.
Participants
The Forbes trial evaluated 28 mild, moderate and severe males with haemophilia A or B (range FVIII/IX 0% to 23% of normal) aged 13 to 65 years undergoing dental extractions in 32 separate episodes under local anaesthesia (Forbes 1972). The Walsh trial evaluated 31 males with haemophilia A or B, 23 (nine with severe haemophilia) from the centre in Oxford and eight (three with severe haemophilia) from the centre in Cardiff, aged 12 years or older, with factor VIII or IX levels of 15% or less who were admitted to hospital for extraction of permanent teeth under general anaesthesia (Walsh 1971).
Interventions
In the Forbes trial both the intervention and the control group received the FVIII or FIX equivalent of 1000 ml of human plasma intravenously one hour preoperatively (Forbes 1972). Participants in the intervention group (N = 14) then received TXA tablets starting two hours preoperatively and continued TXA 1 g three times a day for five days postoperatively. Participants in the control group (N = 14) received placebo tablets following the same regimen as the intervention group. In the Walsh trial both the intervention and the control group received one initial dose of plasma‐derived cryoprecipitate of FVIII or IX concentrate within one hour preoperatively aimed to raise the factor VIII or FIX level to 50% of normal (Walsh 1971). In the intervention group (N = 15), the plasma replacement therapy was immediately followed by intravenous infusion of 6 g EACA in 250 ml isotonic saline and 6 g orally four times daily for 7 to 10 days. In the control group (N = 16), placebo (isotonic saline only) was infused followed by the administration of placebo tablets using the same treatment scheme as the intervention group. Tooth sockets were protected with acrylic plates and all patients received oral antibiotics postoperatively.
Outcome measures
Both trials reported the number of participants with bleeding episodes and side effects, the change in haemoglobin level from baseline and the requirement of additional FVIII or FIX replacement therapy. The Forbes trial also discussed the amount of blood loss (Forbes 1972). The Walsh trial also reported transfusion requirement (Walsh 1971). In both trials bleeding was defined as postoperative intra‐oral bleeding. Participants were followed up for five days in the Forbes trial and for seven to 10 days in the Walsh trial (Forbes 1972; Walsh 1971).
Excluded studies
The characteristics of the excluded trials are summarized in the Characteristics of excluded studies table.
The main reasons for exclusion were trial design (case series, case reports, letters and editorials), domain (e.g. articles not on dental or oral surgery) and intervention (e.g. articles not on TXA or EACA) (Characteristics of excluded studies).
Risk of bias in included studies
Detailed quality assessments are presented in the 'Risk of bias' tables presented with each trial in the Characteristics of included studies table. In addition, the risk of bias of each trial is summarized in Figure 1.
Allocation
Sequence generation
In the Forbes trial, the risk of a selection bias due to the sequence generation was unclear, since the process of the random sequence generation was not reported (Forbes 1972).
In the Walsh trial, eligibility of participants was determined by physicians responsible for the care of the participant (Walsh 1971). The risk of bias was therefore considered to be high.
Allocation concealment
The risk of selection bias due to allocation concealment was considered unclear in the Forbes trial and high in the Walsh trial (Forbes 1972; Walsh 1971). The Forbes trial did not report any details (Forbes 1972). In the Walsh trial, it was reported that "...the physician responsible for administration of the test agents assigned the patients to the treatment groups (EACA or placebo) on the basis of a pair‐matching technique." (Walsh 1971).
Blinding
The risk of a detection bias was considered low in both included trials. The Forbes trial stated that the clinician did not know the results of performed laboratory assays or which treatment the participants were receiving (Forbes 1972). The Walsh trial stated that physicians unaware of the assigned treatment were responsible for the treatment of potential bleedings (Walsh 1971).
A performance bias cannot be excluded, as the blinding process is not discussed in detail in both trials.
Incomplete outcome data
The risk of an attrition bias was considered low in both trials. There was no loss to follow up in either of the included trials. One participant from the Walsh trial withdrew from the trial, but was still included in the analysis (Walsh 1971).
Selective reporting
The risk of a reporting bias was considered low in both trials. All pre‐specified outcome measures were reported in both trials.
Other potential sources of bias
No other potential sources of bias were identified (Higgins 2011c).
Effects of interventions
See: Summary of findings for the main comparison Summary of findings table
Detailed assessments of our primary outcomes that are reported in both trials are described in the 'Summary of findings table' (summary of findings Table for the main comparison).
The primary aim of the review was to assess the efficacy of antifibrinolytic agents (TXA or EACA) to prevent bleeding complications in people with haemophilia or VWD undergoing oral or dental procedures. Two trials on people with haemophilia were included (Forbes 1972; Walsh 1971). No trials on VWD were included.
Primary outcomes
1. Number of postoperative bleeding episodes requiring intervention
Both included trials evaluated the efficacy of antifibrinolytic agents for preventing bleeding complications in people with haemophilia undergoing dental extraction. Each trial showed a beneficial effect of TXA or EACA compared with placebo, the Forbes trial showing a risk difference of ‐0.64 (95% CI ‐0.93 to ‐0.36) (Forbes 1972), and the Walsh trial showing a risk difference of ‐0.50 (95% CI ‐0.77 to ‐0.22) (Walsh 1971) (Table 1). Analysis of the combined results of both trials showed a combined risk difference of ‐0.57 (95% CI ‐0.76 to ‐0.37) (Forbes 1972; Walsh 1971) (Analysis 1.1; summary of findings Table for the main comparison; Table 2).
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Primary outcomes | 1. Postoperative bleedings requiring intervention | 2. Side effects or other adverse events | |||||
| Absolute risks % (n/ntotal) | Risk difference (RD) | Number needed to treat (NNT) | Absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | |||
| Control | Intervention | Control | Intervention | ||||
| 79% (11/14) | 14% (2/14) | 65% (37 ‐ 93) | 1.5 | 0% (0/14) | 0% (0/14) | 0% | |
| 56% (9/16) | 7% (1/15) | 49% (21 ‐ 77) | 2.0 | 0% (0/16) | 7% (1/15) | 7% (‐0.06 ‐ 20) | |
| n: number; CI: confidence interval | |||||||
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | ||||||||
| 1. Number of people with postoperative bleeding | 2. Side effects or other adverse events | |||||||
| Combined absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | Number needed to treat (NNT) | Combined absolute risks % (n/ntotal) | Risk difference (RD)(% (95% CI) | Number needed to treat (NNT) | |||
| Control | Intervention | Control | Intervention | |||||
| 67% (20/30) | 10% (3/29) | 56% (36% ‐ 76%) | 1.8 | 0% (0/30) | 3.4 (1/29) | 3.4 (‐32 ‐ 38) | 0.3 | |
2. Side effects or other adverse events
None of the participants in the Forbes trial experienced side effects (Forbes 1972). In the Walsh trial, one of the 15 participants from the intervention group experienced side effects requiring treatment withdrawal on the third postoperative day (Walsh 1971) (Table 1). These side effects included postural dizziness, headache, tingling in fingers and nausea. The risk difference in the Walsh trial was 0.07 (95% CI ‐0.10 to 0.23). Combined analysis of the results of both trials resulted in a combined risk difference of ‐0.03 (95% CI ‐0.08 to 0.13) (Forbes 1972; Walsh 1971) (Analysis 1.2; summary of findings Table for the main comparison; Table 1 ).
Secondary outcomes
1. Number of minor postoperative bleeding episodes
This outcome was not reported in either of the trials.
2. Number of immediate (less than 24 hours) and delayed (24 hours to 10 days) postoperative bleeding episodes requiring intervention
This outcome was not reported in either of the trials.
3. Any postoperative complication except bleeding, such as wound infection
This outcome was not reported in either of the trials.
4. Change in haemoglobin level from baseline
The mean fall in haemoglobin was reported in g/100 mL by Forbes and in percentages by Walsh (Forbes 1972; Walsh 1971).
The Forbes trial described a mean fall in haemoglobin of 1.4 g/100 mL in the placebo group and of 0.3 g/100 mL in the TXA group (Forbes 1972).
The Walsh trial showed a mean fall in haemoglobin of 6.3% in both placebo groups at the two trial centres, and a mean fall of haemoglobin in the EACA groups of 3.9% and 3.7% in the first and second trial centre, respectively (Walsh 1971). The differences in the mean fall in haemoglobin were not significant between the placebo and EACA groups (Table 3).
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||
| Patient or population: Patients with haemophilia or Von Willebrand disease Settings: Hospitals, Hemophilia centres Intervention: Tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: Placebo or no intervention or usual care with or without placebo | ||||||
| Secondary outcomes | 1. (Mean) fall in haemoglobin level from baseline | 2. Amount of postoperative blood loss: mean per patient mL (range) | 3. Need for and dose of clotting factor concentrates: Mean number of units IU (range) of replacement therapy per root extracted (A) / per patient (B) | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| 1.4 g/100mL | 0.3 g/100mL | 84.1 mL (4‐323) | 61.2 mL (1‐749) | 617 IU (0‐15800) in eleven participants | 30 IU and 65 IU in two participants | |
| Trial centre 1: 6.3% Trial centre 2: 6.3% | Trial centre 1: 3.9% Trial centre 2: 3.7% | NR | NR | Trial centre 1: 2331 IU Trial centre 2: 1881 IU | Trial centre 1: 145 IU Trial centre 2: 0 IU | |
| Abbreviations:NR: not reported | ||||||
5. Major bleeding, requiring transfusion of packed red blood cells
This outcome was only reported in the Walsh trial (Walsh 1971). In none of the 31 included participants was blood transfusion required.
6. Bleeding duration
This outcome was not reported in either of the trials.
7. Amount of postoperative blood loss (ml)
This outcome was only reported in the Forbes trial (Forbes 1972). During the five‐day follow up, the mean blood loss per participant was 84.1 mL (range four to 323) in the placebo group (n = 14) and 61.2 mL (range one to 749) in the TXA group (n = 14, P = 0.02) (Table 3).
8. Need for and dose of clotting factor concentrates
Both trials presented this outcome, however in different ways; in the Forbes trial as the mean number of units of replacement therapy needed per root extracted (Forbes 1972) and in the second trial as the mean number of units needed per participant (Walsh 1971). The Forbes trial reported a mean number of units FVIII/100 mL replacement therapy per root extracted of 617 (range 0 to 15.800) in 11 participants in the placebo group, and of 30 and 65 units per root extracted in two participants in the TXA group (no statistical testing performed). In the second trial, the mean number of units of replacement therapy needed per participant was 2331 and 1881 in the placebo group of the first and second trial centre, respectively, and 145 and 0 in the EACA group of the first and second trial centre, respectively (Table 3). The number of participants needing postoperative therapeutic factor replacement therapy was significantly higher in the placebo group (statistical testing was only performed on results from the Oxford centre; seven versus one participant needed therapeutic replacement therapy, P = 0.02).
Discussion
Summary of main results
In this systematic review on antifibrinolytic therapy for preventing oral bleeding in people with a haemophilia or Von Willebrand disease (VWD) undergoing oral or dental procedures we could only include two double‐blind, placebo‐controlled trials (one randomised, one quasi‐randomised) in people with haemophilia undergoing dental extraction, one on tranexamic acid (TXA) (Forbes 1972) and one on epsilon aminocaproic acid (EACA) (Walsh 1971). No eligible trials on people with VWD regarding this subject were identified. Overall, the two included trials showed a beneficial effect of TXA and EACA in reducing the number of bleedings, amount of blood loss and the need for additional therapeutic clotting factor concentrates or cryoprecipitate, without the occurrence of relevant side effects. However, the limited number of trials (N = 2), the small sample sizes (N = 28 and N = 31) and the heterogeneity regarding standard therapy and treatment regimens between the two trials do not allow to conclude definite efficacy of antifibrinolytic therapy in oral or dental procedures in people with haemophilia (summary of findings Table for the main comparison).
Overall completeness and applicability of evidence
Our primary objective was to assess the efficacy of antifibrinolytic agents to prevent bleeding complications in people with haemophilia or VWD undergoing oral or dental procedures. The two included trials discussed only people with haemophilia. Generalising the currently available evidence is mainly limited by the few available trials and the previously discussed limitations of these trials.
The participant population in both trials slightly differed in severity of disease; in the Forbes trial 53.6% of the included people with haemophilia were severely affected (Forbes 1972) and in the Walsh trial 38.7% of the participants were severely affected (Walsh 1971), where severe haemophilia is defined as an endogenous factor VIII or IX level less than 1%. The standard treatment also differed to some extent, where in the Forbes trial the factor levels were brought preoperatively to a predefined level of 50% in all participants, in the Walsh trial a standard dose of replacement therapy was administered to each participant. However, neither of the trials reported the increase in preoperative FVIII of FIX levels. Furthermore, in the Forbes trial TXA was administered orally starting two hours before the procedure and in the Walsh trial, EACA was administered intravenously preoperatively and orally postoperatively (Forbes 1972; Walsh 1971). Also, there was no uniform definition of postoperative bleeding in the included trials which was described as the amount of blood loss or transfusion requirement within five days of follow up in one trial (Forbes 1972) and as postoperative intra‐oral bleeding not further specified in the second trial (Walsh 1971).
Quality of the evidence
Only two trials with 59 people with haemophilia undergoing oral or dental extractions met the inclusion criteria of the review. Overall, we rated the quality of the evidence as low. This is mainly due to the small sample sizes, the lack of trials and the heterogeneity between the trials. The evidence found in this review does not allow a robust conclusion regarding the objectives of the review, despite the well‐chosen trial design and consistent results of both trials.
Potential biases in the review process
Given the comprehensive literature searches undertaken, it is likely that all relevant trials were identified. Article screening and data collection were performed independently by two authors. There were no differences between the authors regarding trial selection and data extraction. It is therefore unlikely that these items could have introduced bias.
Agreements and disagreements with other studies or reviews
The two trials from this review were also included in another Cochrane review that examined the effectiveness and safety of different haemostatic regimens (type, dose and duration, modality of administration and target haemostatic levels) administered in people with a congenital bleeding disorder for preventing bleeding complications during and after surgery (Coppola 2015). The trials were also part of a second previously published review examining the optimal level and duration of replacement therapy required to prevent bleeding complications (Hermans 2009). No other relevant trials were included in these reviews. The trial findings from this review are in correlation with the previously published articles.
Evidence from non‐randomised studies was not systematically reviewed. Non‐randomised studies included mostly retrospective analyses, case series and case reports. In general, all studies were supportive of the use of TXA or EACA with few side effects. We found no studies contradicting the efficacy of TXA or EACA in oral or dental procedures. The positive effect depends on factors such as the severity of the bleeding disorder and the intensity of the minor oral surgery or dental extractions. One representative study considered 50 consecutive patients with inherited bleeding disorders (haemophilia A: n = 26; haemophilia B: n = 11; VWD: n = 13) undergoing 31 surgical dental extractions (e.g. wisdom teeth or other surgical extractions) and 82 simple extractions (Hewson 2011). In all included patients, the tooth socket(s) were filled with 5% TXA and Surgicel® before suturing. All patients received 5% TXA mouthwash three times a day for seven days or gauze was applied to the socket in the case of moderate bleeding. There was no factor infusion requirement additional to the usual requirements of patients who were already on prophylaxis, allowing the patients to be treated as outpatients in the dental clinic for simple extractions. For surgical extractions under general anaesthesia, patients were admitted overnight in the hospital as a precaution. No patient had a bleed during this overnight admission. The authors therefore concluded that adequate postoperative haemostasis in these patients was achieved. As a result, previous protocols requiring the clotting factor level to be 70% to 100% before oral surgery, were abandoned and the use of clotting factor concentrates for oral surgery was significantly reduced.
Although VWD is the most common inherited bleeding disorder, no randomised controlled trials discussing antifibrinolytic therapy in people with VWD met the inclusion criteria. The study with the largest sample size considering people with VWD that we found was a retrospective analysis of 63 consecutive patients with VWD (type 1: n = 31; type 2: n = 22; type 3: n = 10) undergoing dental extractions or periodontal surgery (Federici 2000). All included participants received local TXA treatment before surgery and for seven days after surgery. No placebo arm was included. Local TXA during surgery included irrigation with two ampoules of TXA 500 mg. Postoperatively, participants were prescribed with TXA mouthwash and were instructed to rinse for two minutes four‐times daily, for seven days postoperatively. Local therapy only (TXA or TXA and fibrin glue) was compared to local therapy and additional systemic therapy (desmopressin (DDAVP) or Von Willebrand factor (VWF) concentrates). This study showed no significant difference between the two groups regarding postoperative bleeding.
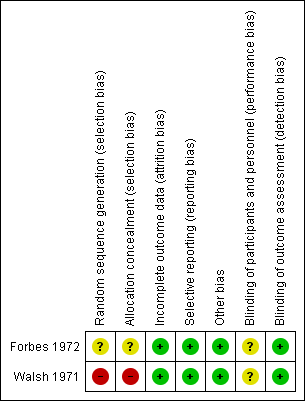
Risk of bias summary of the included studies
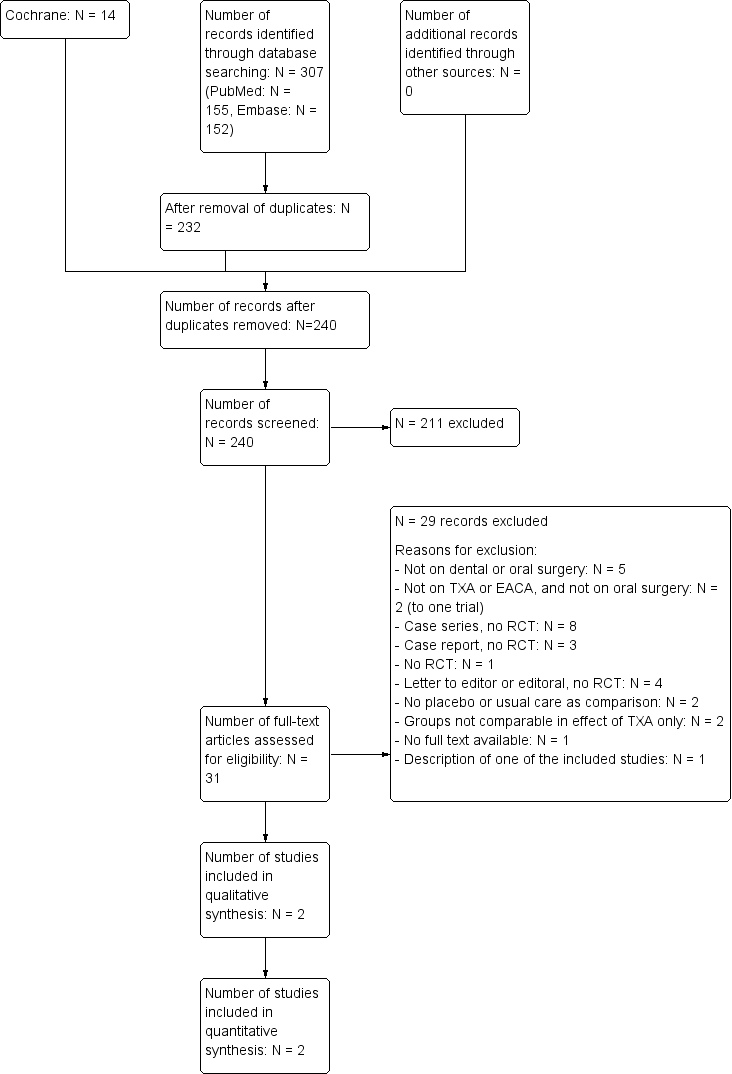
Trial flow diagram
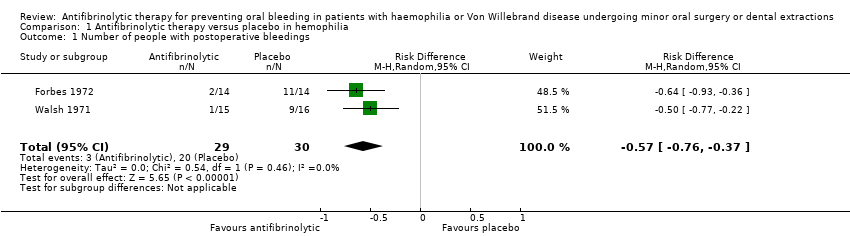
Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 1 Number of people with postoperative bleedings.

Comparison 1 Antifibrinolytic therapy versus placebo in hemophilia, Outcome 2 Number of side effects requiring withdrawal.
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Outcomes | Anticipated absolute effects* | Relative effect | № of participants | Number needed to treat (NNT) | Quality of the evidence | Comments | |
| Risk with placebo or usual care | Risk with antifibrinolytic therapy | ||||||
| Postoperative bleedings requiring intervention | Study population | Risk difference: 57 | 59 | 1.8 | ⊕⊕⊕⊝ | ||
| 67 per 100 (20 events) | 10 per 100 (3 events) | ||||||
| Side effects or other adverse events | Study population | Risk difference: 3.4 | 59 | 0.3 | ⊕⊕⊝⊝ | ||
| 0 events | 1 event | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1 The magnitude of effect was considered large in this study, therefore the quality of evidence was rated up one level. 2 Small sample sizes and lack of studies 3 Heterogeneity between the included trials regarding the proportion of severe people with haemophilia included, the concomitant standard therapy and fibrinolytic agent treatment regimens used | |||||||
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | |||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | |||||||
| Primary outcomes | 1. Postoperative bleedings requiring intervention | 2. Side effects or other adverse events | |||||
| Absolute risks % (n/ntotal) | Risk difference (RD) | Number needed to treat (NNT) | Absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | |||
| Control | Intervention | Control | Intervention | ||||
| 79% (11/14) | 14% (2/14) | 65% (37 ‐ 93) | 1.5 | 0% (0/14) | 0% (0/14) | 0% | |
| 56% (9/16) | 7% (1/15) | 49% (21 ‐ 77) | 2.0 | 0% (0/16) | 7% (1/15) | 7% (‐0.06 ‐ 20) | |
| n: number; CI: confidence interval | |||||||
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in people with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||||
| Patient or population: people with haemophilia or Von Willebrand disease Settings: hospitals, hemophilia centres Intervention: tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: placebo or no intervention or usual care with or without placebo | ||||||||
| 1. Number of people with postoperative bleeding | 2. Side effects or other adverse events | |||||||
| Combined absolute risks % (n/ntotal) | Risk difference (RD) (% (95% CI) | Number needed to treat (NNT) | Combined absolute risks % (n/ntotal) | Risk difference (RD)(% (95% CI) | Number needed to treat (NNT) | |||
| Control | Intervention | Control | Intervention | |||||
| 67% (20/30) | 10% (3/29) | 56% (36% ‐ 76%) | 1.8 | 0% (0/30) | 3.4 (1/29) | 3.4 (‐32 ‐ 38) | 0.3 | |
| Antifibrinolytic therapy compared with placebo or usual care for preventing oral bleeding in patients with haemophilia or Von Willebrand disease undergoing oral or dental procedures | ||||||
| Patient or population: Patients with haemophilia or Von Willebrand disease Settings: Hospitals, Hemophilia centres Intervention: Tranexamic acid (TXA) or epsilon aminocaproic acid (EACA) Comparison: Placebo or no intervention or usual care with or without placebo | ||||||
| Secondary outcomes | 1. (Mean) fall in haemoglobin level from baseline | 2. Amount of postoperative blood loss: mean per patient mL (range) | 3. Need for and dose of clotting factor concentrates: Mean number of units IU (range) of replacement therapy per root extracted (A) / per patient (B) | |||
| Control | Intervention | Control | Intervention | Control | Intervention | |
| 1.4 g/100mL | 0.3 g/100mL | 84.1 mL (4‐323) | 61.2 mL (1‐749) | 617 IU (0‐15800) in eleven participants | 30 IU and 65 IU in two participants | |
| Trial centre 1: 6.3% Trial centre 2: 6.3% | Trial centre 1: 3.9% Trial centre 2: 3.7% | NR | NR | Trial centre 1: 2331 IU Trial centre 2: 1881 IU | Trial centre 1: 145 IU Trial centre 2: 0 IU | |
| Abbreviations:NR: not reported | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of people with postoperative bleedings Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | ‐0.57 [‐0.76, ‐0.37] |
| 2 Number of side effects requiring withdrawal Show forest plot | 2 | 59 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.13] |

