Intervenciones de autocuidado para la diabetes tipo 2 en pacientes adultos con enfermedades mentales graves
Appendices
Appendix 1. Search strategies
| Cochrane Library |
| 1. [mh "Diabetes Mellitus, Type 2"] 2. ("MODY" or "NIDDM" or T2D*):ti,ab 3. (("non insulin*" next depend*) or (noninsulin* next depend*) or noninsulindepend* or "non" next "insulindepend*"):ti,ab 4. ((typ* next (2 or II)) near/4 diabet*):ti,ab 5. ((("late" or adult* or matur* or "slow" or stabl*) near/4 "onset") and diabet*):ti,ab 6. {or #1‐#5} 7. [mh "Diabetes Insipidus"] 8. (diabet* next "insipidus"):ti,ab 9. #7 or #8 10. #6 not #9 11. [mh ^"Mental Disorders"] 12. [mh "Affective Disorders, Psychotic"] 13. [mh "Personality disorders"] 14. [mh "Schizophrenia and Disorders with Psychotic Features"] 15. ("mental" near/4 (disorder* or "illness")):ti,ab 16. (schizo* or psychos?s or "psychotic"):ti,ab 17. (("bipolar" or "affective" or "personality") next disorder*):ti,ab 18. [mh ^"Depressive Disorder, Major"] 19. (("major" or "unipolar" or "clinical" or "recurrent") next depress*):ti,ab 20. {or #11‐#19} 21. #10 and #20 |
| MEDLINE (Ovid SP) |
| 1. exp Diabetes Mellitus, Type 2/ 2. (MODY or NIDDM or T2D*).tw. 3. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 4. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 5. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 6. or/1‐5 7. exp Diabetes Insipidus/ 8. diabet* insipidus.tw. 9. 7 or 8 10. 6 not 9 11. Mental Disorders/ 12. exp Affective Disorders, Psychotic 13. exp Personality disorders/ 14. exp "Schizophrenia and Disorders with Psychotic Features"/ 15. (mental adj3 (disorder* or illness)).tw. 16. (schizo* or psychos?s or psychotic).tw. 17. ((bipolar or affective or personality) adj disorder*).tw. 18. Depressive Disorder, Major/ 19. ((major or unipolar or clinical or recurrent) adj depress*).tw. 20. or/11‐19 21. 10 and 20 22. Patient Education as Topic/ 23. Patient Compliance/ 24. exp Self Care/ 25. exp Health Promotion/ 26. exp Behavior Therapy/ 27. exp Health Behavior/ 28. Program Evaluation/ 29. Life style/ 30. Weight Loss/ 31. self.tw. 32. (monitor* or manage*).tw. 33. (educat* or knowledge).tw. 34. (behav* or psychoth* or psychosocial).tw. 35. (aware* or adjust*).tw. 36. (adher* or compliance).tw. 37. (intervention? or program? or programme?).tw. 38. (lifestyle or life style).tw. 39. (weight adj3 (management or los* or reduct*)).tw. 40. or/22‐39 41. 21 and 40 [42‐52: Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version] 42. randomised controlled trial.pt. 43. controlled clinical trial.pt. 44. randomi?ed.ab. 45. placebo.ab. 46. drug therapy.fs. 47. randomly.ab. 48. trial.ab. 49. groups.ab. 50. or/42‐49 51. exp animals/ not humans/ 52. 50 not 51 53. 41 and 52 |
| EMBASE (Ovid SP) |
| 1. non insulin dependent diabetes mellitus/ 2. (MODY or NIDDM or T2D*).tw. 3. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 4. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 5. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 6. or/1‐5 7. exp diabetes insipidus/ 8. diabet* insipidus.tw. 9. 7 or 8 10. 6 not 9 11. mental disease/ 12. major affective disorder/ 13. exp personality disorder/ 14. exp psychosis/ 15. (mental adj3 (disorder* or illness)).tw. 16. (schizo* or psychos?s or psychotic).tw. 17. ((bipolar or affective or personality) adj disorder*).tw. 18. major depression/ 19. ((major or unipolar or clinical or recurrent) adj depress*).tw. 20. or/11‐19 21. 10 and 20 22. exp health education/ 23. exp patient attitude/ 24. exp self care/ 25. behavior therapy/ 26. exp health behavior/ 27. exp program evaluation/ 28. lifestyle/ 29. weight reduction/ 30. weight control/ 31. self.tw. 32. (monitor* or manage*).tw. 33. (educat* or knowledge).tw. 34. (behav* or psychoth* or psychosocial).tw. 35. (aware* or adjust*).tw. 36. (adher* or compliance).tw. 37. (intervention? or program? or programme?).tw. 38. (lifestyle or life style).tw. 39. (weight adj3 (management or los* or reduct*)).tw. 40. or/22‐39 41. 21 and 40 [42:Wong 2006a"sound treatment studies" filter – BS version] 42. random*.tw. or clinical trial*.mp. or exp health care quality/ 43. 41 and 42 44. limit 43 to embase |
| PsycINFO (Ovid SP) |
| 1. Diabetes Mellitus/ 2. (MODY or NIDDM or T2D*).tw. 3. (non insulin* depend* or noninsulin* depend* or noninsulin?depend* or non insulin?depend*).tw. 4. ((typ? 2 or typ? II or typ?2 or typ?II) adj3 diabet*).tw. 5. (((late or adult* or matur* or slow or stabl*) adj3 onset) and diabet*).tw. 6. or/1‐5 7. Diabetes Insipidus/ 8. diabet* insipidus.tw. 9. 7 or 8 10. 6 not 9 11. Mental Disorders/ 12. exp Affective Disorders/ 13. exp Personality Disorders/ 14. exp Psychosis/ 15. (mental adj3 (disorder* or illness)).tw. 16. (schizo* or psychos?s or psychotic).tw. 17. ((bipolar or affective or personality) adj disorder*).tw. 18. exp Major Depression/ 19. ((major or unipolar or clinical or recurrent) adj depress*).tw. 20. or/11‐19 21. 10 and 20 22. Health Education/ or Health Literacy/ or Client Education/ 23. Disease Management/ or Coping Behavior/ or Self Care Skills/ 24. Health Behavior/ or Treatment Compliance/ 25. Health Promotion/ or Health Attitudes/ 26. "Physical Illness (Attitudes Toward)"/ or Illness Behavior/ 27. exp Program Evaluation/ 28. exp Behavior Therapy/ 29. exp Lifestyle/ 30. Weight Loss/ or Weight Control/ 31. self.tw. 32. (monitor* or manage*).tw. 33. (educat* or knowledge).tw. 34. (behav* or psychoth* or psychosocial).tw. 35. (aware* or adjust*).tw. 36. (adher* or compliance).tw. 37. (intervention? or program? or programme?).tw. 38. (lifestyle or life style).tw. 39. (weight adj3 (management or los* or reduct*)).tw. 40. or/22‐39 41. 21 and 40 [42:Eady 2008"PsycInfo Search Strategies" filter ‐ BS version] 42. control*.tw. OR random*.tw. OR exp Treatment/ 43. 41 and 42 |
| CINAHL (via EBSCO) |
| S1 MH "Diabetes Mellitus, Type 2+" S2 TX (MODY OR NIDDM OR T2D*) S3 TX ("non insulin* depend*" OR "noninsulin* depend*" OR noninsulin#depend* OR "non insulin#depend*") S4 TX (("typ* 2" OR "typ* II" OR typ#2 OR typ#II) N3 diabet*) S5 TX (((late OR adult* OR matur* OR slow OR stabl*) N3 onset) AND diabet*) S6 S1 OR S2 OR S3 OR S4 OR S5 S7 MH "Mental Disorders" OR MH "Mental Disorders, Chronic" OR MH "Psychotic Disorders+" OR MH "Personality Disorders+" OR (MH "Depression+") S8 TX (mental N3 (disORder* OR disease* OR illness)) S9 TX (schizo* OR psychos#s OR psychotic) S10 TX ((bipolar OR affective OR personality) N1 disorder) S11 TX ((major OR unipolar OR clinical OR recurrent) N1 depress*) S12 S7 OR S8 OR S9 OR S10 OR S11 S13 S6 AND S12 S14 MH "Health Education+" OR MH "Health Behavior+" OR MH "Coping" OR MH "Self Care+" OR MH "Health Promotion" S15 MH "Behavior Therapy+" OR MH "Program Evaluation" S16 MH "Life Style+" OR MH "Weight Loss" OR MH "Weight Control" S17 TX (self OR monitor* OR manage* OR educat* OR knowledge OR behav* OR psychoth* OR psychosocial OR aware* OR adjust* OR adher* OR compliance) S18 TX (intervention# OR program# OR programme# OR lifestyle OR "life style") S19 TX (weight N3 (management OR los* OR reduct*)) S20 S14 OR S15 OR S16 OR S17 OR S18 OR S19 S21 S13 AND S20 [S22:Wong 2006b"therapy studies" filter ‐ BS version] S22 MH "prognosis+" OR MH "study design+" OR random* S23 S21 AND S22 |
| ICTRP Search Portal (Standard search) |
| diabet* AND mental illness* OR diabet* AND mental disorder* OR diabet* AND mental disease* OR diabet* AND schizo* OR diabet* AND psychosis OR diabet* AND psychoses OR diabet* AND psychotic OR diabet* AND bipolar OR diabet* AND affective disorder* OR diabet* AND personality disorder* OR diabet* AND major depress* OR diabet* AND unipolar depress* OR diabet* AND clinical depress* OR diabet* AND recurrent depress* OR diabet* AND severe depress* |
| ClinicalTrials.gov (Advanced search) |
| Search Terms: (diabetes OR diabetic) AND (mental OR schizophrenia OR psychosis OR psychoses OR psychotic OR bipolar OR affective OR personality OR major depression OR major depressive OR clinical depression OR unipolar depression OR recurrent depression) Study Type: Interventional Studies Age Group: Adult, Senior |
Appendix 2. Description of interventions
| Intervention | Comparator | |
| McKibbin 2010 | The Diabetes Awareness and Rehabilitation Training (DART) intervention was a group, face‐to‐face, 24‐week self management programme. DART comprised 3 modules: (1) basic diabetes education (sessions 1 to 4, repeated at sessions 13 to 16); (2) nutrition (sessions 5 to 8, repeated at sessions 17 to 20); and (3) lifestyle exercise (sessions 9 to 12, repeated at sessions 21 to 24). Each module contained four 90‐minute manualised sessions. Basic education included an explanation of motivation and a review of blood sugar in symptoms of low and high blood sugar, diabetes complications, how to use a glucose meter, doctor visits and how to talk with your doctor and medication. Nutrition education included a review of food groups, portion sizes, healthy meals and food labels, and replacing sugar with fat and fibre. Lifestyle and exercise education reviewed different types of exercise, how exercise impacts blood sugar, tracking exercise using a pedometer and foot care during exercise Personnel adapted educational materials for people of middle age and older with schizophrenia by introducing 1 or 2 topics per session, providing an overview and summary of the materials, implementing a teach and query training method, using mnemonic aids and print materials with larger font and limiting text. Participants were given simple guidelines about how they might lead a healthier lifestyle, such as switching from regular soda or fruit punch to diet soda or water One diabetes‐trained mental health professional delivered the intervention. These facilitators did not make contact with participants' healthcare providers, and they encouraged participants to speak with their physician about their diabetes and provided guidance on how to record laboratory results and examination findings | Usual care plus information (UCI) consisted of usual care delivered by participants' providers and 3 brochures provided by the American Diabetes Association relevant to diabetes management (i.e. basic diabetes education, nutrition, exercise) |
Appendix 3. Baseline characteristics (I)
| Intervention and comparator | Duration of intervention | Description of participants | Trial period | Country | Setting | |
| McKibbin 2010 | I: DART | 24 weeks (6 months post intervention) | Participants with type 2 diabetes and schizophrenia/schizoaffective disorder | ‐ | USA | Community |
| C: UCI | ||||||
| "‐" denotes not reported C: comparator; DART: Diabetes Awareness and Rehabilitation Training; UCI: usual care plus information; I: intervention; SD: standard deviation | ||||||
Appendix 4. Baseline characteristics (II)
| Intervention and comparator | Sex | Age | Ethnicity | Duration of diabetes | Type of severe mental illness | Age of onset of | |
| McKibbin 2010 | I: DART | 38 | 52 (10.1) | White: 45 | 8.9 (5.8) | Schizophrenia: 79 Schizoaffective: 21 | 25.7 (12.3) |
| C: UCI | 38 | 54 (8.4) | White: 72 | 8.6 (6.5) | Schizophrenia: 90 Schizoaffective: 10 | 29.3 (11.8) | |
| "‐" denotes not reported C: comparator; DART: Diabetes Awareness and Rehabilitation Training; I: intervention; SD: standard deviation; UCI: usual care plus information | |||||||
Appendix 5. Baseline characteristics (III)
| Intervention and comparator | HbA1c | BMI | Diastolic blood pressure | Systolic blood pressure | Glucose control agents | Antipsychotic medication | Comorbidities | |
| McKibbin 2010 | I: DART | 7.4 (2.9) | 33.6 (6.8) | 83 (10) | 134 (17) | Diet only: 15 | Apripiprazole or ziprasidone: 25 | ‐ |
| C: UCI | 6.7 (2.1) | 32.9 (6.2) | 85 (13) | 132 (15) | Diet only: 10 | Apripiprazole or ziprasidone: 21 | ‐ | |
| "‐" denotes not reported BMI: body mass index; C: comparator; DART: Diabetes Awareness and Rehabilitation Training; HbA1c: glycosylated haemoglobin A1c; I: intervention; SD: standard deviation; UCI: usual care plus information | ||||||||
Appendix 6. Matrix of trial endpoints (publications and trial documents)
| Endpoints quoted in trial document(s) | Trial results posted [Yes/No] | Publications specified [No/Citation] | Endpoints quoted in publicationb | |
| McKibbin 2010 | N/T | No | No | Diabetes knowledge, self efficacy (Leutwyler 2010 (see McKibbin 2006) Weight, body mass index, waist circumference, blood pressure, fasting blood glucose, HbA1c, cholesterol, high‐density lipoprotein, low‐density lipoprotein, triglycerides, diabetes knowledge, self efficacy, energy expenditure, activity levels, total kilocalories consumed, total minutes of activity (McKibbin 2006) BMI, weight, waist circumference, HbA1c, diabetes knowledge, energy expenditure (McKibbin 2010) |
| aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trial registers) EMA: European Medicines Agency; FDA: Food and Drug Administration (US); N/T: no trial document available | ||||
Appendix 7. Examination of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| McKibbin 2010 | Self care behaviours | N/A | N/A | Total activity, total calories consumed and total minutes of activity were measured immediately following the intervention, but not at 6‐month follow‐up, and were not analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored. Total energy expenditure was measured both immediately following the intervention and at 6‐month follow‐up but was not analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A |
| Diabetes‐related complications | N/I | N/I | N/I | N/I | |
| Adverse events | N/I | N/I | N/I | N/I | |
| All‐cause mortality | N/I | N/I | N/I | N/I | |
| Self efficacy | N/A | N/A | Self efficacy was measured and analysed immediately following the intervention and was analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored. Self efficacy at 6 months post intervention was not reported | ||
| Progression of severe mental illness | N/A | N/A | Symptoms were measured at baseline and following the intervention by the PANSS and the Hamilton Depression Scale, as indicated in Leutwyler 2010 (see McKibbin 2006), but these results are not reported in McKibbin 2006 nor McKibbin 2010 | N/A | |
| HbA1c | N/A | N/A | HbA1c was measured immediately following the intervention and at 6‐month follow‐up but was not looked at as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Body mass index | N/A | N/A | BMI was measured and analysed immediately following the intervention and at 6‐month follow‐up but was not looked at as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Weight | N/A | N/A | Weight was measured and analysed immediately following the intervention and at 6‐month follow‐up but was not looked at as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Blood pressure | N/A | N/A | Blood pressure was measured and analysed immediately following the intervention, but results are not reported at 6‐month follow‐up and were not analysed as an outcome when moderating effects of symptoms on effectiveness of the intervention were explored | N/A | |
| Change in antipsychotic treatment type | The article reports no significant changes in antipsychotic treatment type from baseline to 6‐month follow‐up; however, no data were provided | N/A | N/A | N/A | |
| Change in diabetes treatment type | The article reports no significant changes in antipsychotic treatment type between trial arms over time; however, no data were provided | N/A | N/A | N/A | |
| Socioeconomic effects | N/I | N/I | N/I | N/I | |
| aClear that outcome was measured and analysed; trial report states that outcome was analysed but reports only that result was not significant N/A: not applicable N/I: not investigated | |||||
Appendix 8. Definition of endpoint measurement (I)
| Self care behaviours | Diabetes‐related complications | Adverse events | All‐cause mortality | Health‐related quality of life | Diabetes knowledge | Self efficacy | Progression of severe mental illness | |
| McKibbin 2010 | For measure of dietary intake, participants were asked to rank how often they consumed 70 different foods in the past month on the Block Brief 2000 Revision of the Health and Habits and History Questionnaire. Outcome is total calories consumed, lower is positive (SO) For measure of physical activity, participants completed the Yale Physical Activity Scale (YPAS). The YPAS provides 2 indices: total energy expenditure (TEE) and total activity summary index (TASI). Higher scores are positive (SO) Physical activity was also measured by an accelerometer (AM7164) (Computer Science and Applications (CSA), a small, lightweight device that is worn on a belt around the waist. The number of minutes of moderate and vigorous activity (MVA) was derived for each valid day of monitoring (i.e. ≥ 3 days of data, 10 hours per day) and averaged across those days. Higher scores positive (IO) | N/I | N/I | N/I | N/I | 23‐Item diabetes knowledge test. Higher scores reflect greater knowledge (SO) | 28‐Item Diabetes Empowerment Scale. Higher scores reflect higher confidence (SO) | Depressive symptom severity was measured using the 28‐item Hamilton Depression Rating Scale (HAM‐D). Unable to tell whether higher is positive (SO) Positive and negative mood was measured using the Positive and Negative Syndrome Scale (PANSS). Unable to tell whether higher is positive (SO) |
| aMethod of endpoint evaluation. N/I: not investigated | ||||||||
Appendix 9. Definition of endpoint measurement (II)
| HbA1c | Body mass index | Weight | Blood pressure | Change in medication or intensity of drug treatment | Socioeconomic effects | |
| McKibbin 2010 | A 10‐mL blood sample was collected after a 12‐hour fast and was assayed by the UCSD Clinical Research Center using established protocols. Lower scores are positive (IO) | Calculated from height and weight as kg/m2 measured at awakening in light clothing. Lower scores are positive (IO) | Weight in kg measured at awakening in light clothing. Lower scores are positive (IO) | A single‐seated blood pressure reading was obtained after a 5‐minute rest with a validated automated oscillometric sphygmomanometric device (Omron model HEM‐705‐CP, Omron Healthcare Inc., Vernon Hills, IL, USA). Biceps circumference was measured to select the appropriate size cuff, and participants were seated with the forearm resting on the table. Lower scores are positive (IO) | N/I | N/I |
| aMethod of endpoint evaluation. AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement; SO: self reported outcome measurement HbA1c: glycosylated haemoglobin A1c; N/I: not investigated | ||||||
Appendix 10. Survey of trial investigators providing information on included trials
| Date trial author contacted | Date trial author replied | Date trial author was asked for additional information | Date trial author provided data | |
| McKibbin 2010 | 08/07/15 12/10/15 | 08/07/15 No reply |
| For Table 1 in 2010 paper, the column on the right should reflect the DART programme participant data. Twenty‐six participants were included in each arm for 6‐month follow‐up |
| DART: Diabetes Awareness and Rehabilitation Training | ||||
Appendix 11. Checklist to aid consistency and reproducibility of GRADE assessments
| Diabetes‐related complications | All‐cause mortality | Adverse events | Health‐related quality of life | Self care behaviours | HbA1c | Socioeconomic effects | ||
| Trial limitations | Was random sequence generation used (i.e. no potential for selection bias)? | N/A | N/A | N/A | N/A | Unclear | Unclear | N/A |
| Was allocation concealment used (i.e. no potential for selection bias)? | Unclear | Unclear | ||||||
| Were participants and personnel blinded (i.e. no potential for performance bias)? | Unclear | Unclear | ||||||
| Was outcome assessment blinded (i.e. no potential for detection bias)? | Unclear | Unclear | ||||||
| Was an objective outcome used? | No (↓) | Yes | ||||||
| Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | Yes | Yes | ||||||
| Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | No (↓) | No (↓) | ||||||
| No other biases reported (i.e. no potential for other bias)? | Yes | Yes | ||||||
| Did trials end up as scheduled (i.e. not stopped early)? | Yes | Yes | ||||||
| Inconsistencyb | Point estimates did not vary widely? | Yes | Yes | |||||
| To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least 1 included studies point estimate; | N/A | N/A | ||||||
| Was the direction of effect consistent? | N/A | N/A | ||||||
| What was the magnitude of statistical heterogeneity (as measured by I²) ‐ low (I² < 40%), moderate (I² = 40% to 60%), high I² > 60%)? | N/A | N/A | ||||||
| Was the test for heterogeneity statistically significant (P value < 0.1)? | N/A | N/A | ||||||
| Indirectnessa | Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | |||||
| Were the interventions in included studies applicable to the decision context? | Highly applicable | Highly applicable | ||||||
| Was the included outcome not a surrogate outcome? | No (↓) | No (↓) | ||||||
| Was the outcome time frame sufficient? | Sufficient | Sufficient | ||||||
| Were the conclusions based on direct comparisons? | Yes | Yes | ||||||
| Imprecisionc | Was the confidence interval for the pooled estimate not consistent with benefit and harm? | N/A | N/A | |||||
| What is the magnitude of the median sample size (high: 300 participants, intermediate: 100 to 300 participants, low: < 100 participants)?e | Low (↓) | Low (↓) | ||||||
| What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5 to 10 studies, small: < 5 studies)?e | Small (↓) | Small (↓) | ||||||
| Was the outcome a common event (e.g. occurs more than 1/100)? | N/A | N/A | ||||||
| Publication biasd | Was a comprehensive search conducted? | Yes | Yes | |||||
| Was grey literature searched? | Yes | Yes | ||||||
| Were no restrictions applied to study selection on the basis of language? | Yes | Yes | ||||||
| Was no industry influence noted in studies included in the review? | Yes | Yes | ||||||
| Was no evidence of funnel plot asymmetry found? | N/A | N/A | ||||||
| Was no discrepancy in findings noted between published and unpublished trials? | Unclear | Unclear | ||||||
| aQuestions on risk of bias are answered in relation to most of the aggregated evidence in the meta‐analysis rather than to individual trials cWhen judging the width of the confidence interval, it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful (↓): key item for possible downgrading of the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s); GRADE: Grading of Recommendations Assessment, Development and Evaluation; N/A: not applicable | ||||||||
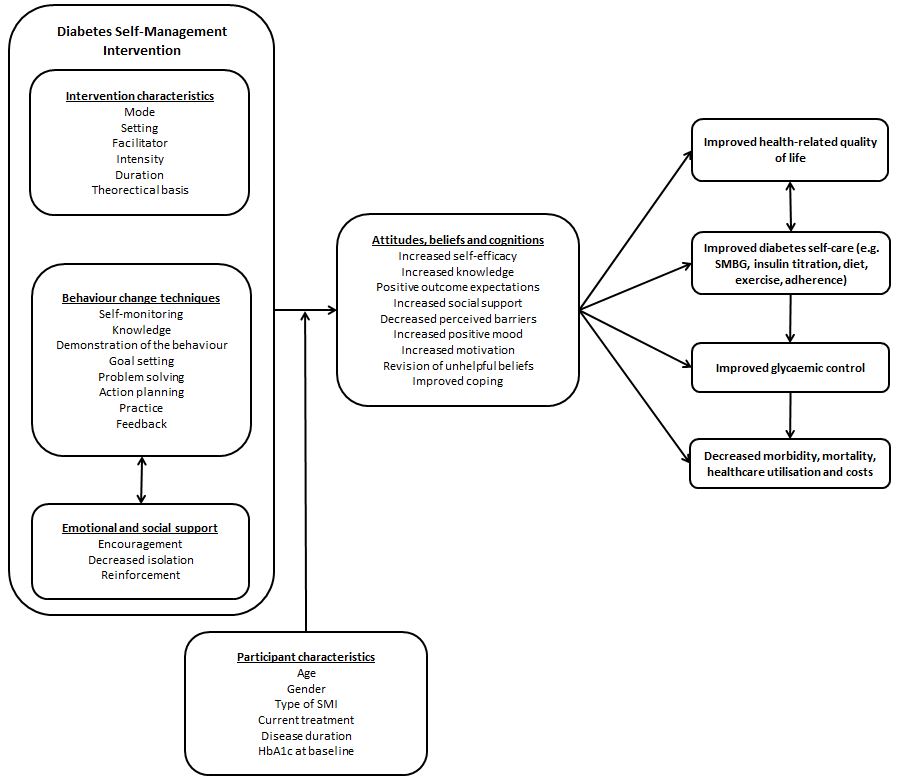
Schematic representation of diabetes self management.

Study flow diagram.
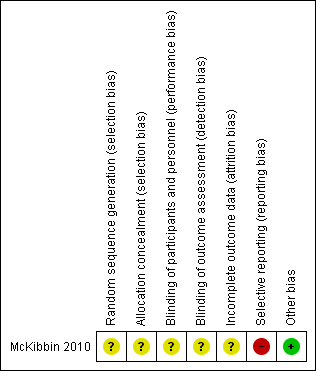
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
| Self management interventions for type 2 diabetes in adult people with severe mental illness | ||||||
| Population: adults with type 2 diabetes and severe mental illness Setting: community Intervention: diabetes self management Comparison: usual care + information | ||||||
| Outcomes | Usual care + information | Diabetes self management | Relative effect | Number of participants | Quality of the evidence | Comments |
| Diabetes‐related complications | See comment | See comment | See comment | See comment | See comment | Not reported |
| All‐cause mortality | See comment | See comment | See comment | See comment | See comment | Not reported |
| Adverse events | See comment | See comment | See comment | See comment | See comment | Not reported |
| Health‐related quality of life | See comment | See comment | See comment | See comment | See comment | Not reported |
| Self care behaviours: physical activity (measured by total energy expenditure in kcal) Follow‐up: 6 months (6 months after the end of the intervention) | Mean energy expenditure was 2148 kcal | Mean energy expenditure was 652 kcal higher | ‐ | 52 (1) | ⊕⊝⊝⊝ | Trial authors stated that this difference reflected no improvement |
| HbA1c [%] Follow‐up: 6 months (6 months after the end of the intervention) | Mean HbA1c was 7.9% | Mean HbA1c was 1% lower | ‐ | 52 (1) | ⊕⊝⊝⊝ | Trial authors stated that this difference reflected no improvement |
| Socioeconomic effects | See comment | See comment | See comment | See comment | See comment | Not reported |
| CI: confidence interval; HbA1c: glycosylated haemoglobin A1c; kcal: kilocalories | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded by three levels because of selective reporting bias, indirectness and imprecision | ||||||
| Intervention and comparator | Sample sizea | Screened/eligible | Randomised | Analysed | Finishing trial | Randomised finishing trial | Follow‐upb | |
| McKibbin 2010 | I: Diabetes Awareness and Rehabilitation Training (DART) | ‐ | 77 | 32 | 26 | 26 | 81.3 | 24 weeks (6 months post intervention) |
| C: usual care plus information (UCI) | 32 | 26 | 26 | 81.3 | ||||
| Total: | 64 | 52 | 52 | 81.3 | ||||
| aAccording to power calculation in trial publication or report bDuration of intervention and/or follow‐up under randomised conditions until end of trial "‐" denotes not reported C: comparator; I: intervention | ||||||||

