Intervenciones para prevenir y mejorar los déficits cognitivos en adultos tratados con irradiación craneal
Resumen
Antecedentes
Los déficits cognitivos son comunes en las personas que han recibido una irradiación craneal y tienen un grave impacto en el funcionamiento diario y la calidad de vida. El efecto beneficioso del tratamiento farmacológico y no farmacológico de los déficits cognitivos en esta población no está claro. Ésta es una versión actualizada de la revisión Cochrane original publicada en el número 12, 2014.
Objetivos
Evaluar la efectividad de las intervenciones para prevenir o mejorar los déficits cognitivos en adultos tratados con irradiación craneal.
Métodos de búsqueda
Para esta actualización de la revisión se realizaron búsquedas en el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials; CENTRAL), MEDLINE vía Ovid, Embase vía Ovid y PsycInfo vía Ovid hasta el 12 de septiembre de 2022.
Criterios de selección
Se incluyeron los ensayos controlados aleatorizados (ECA) que evaluaron intervenciones farmacológicas o no farmacológicas en adultos con irradiación craneal, con el funcionamiento cognitivo objetivo como medida de desenlace principal o secundaria.
Obtención y análisis de los datos
Dos autores de la revisión (MK, JD) extrajeron de forma independiente los datos de los estudios seleccionados y realizaron una evaluación del riesgo de sesgo. Se informaron los desenlaces de la función cognitiva, la fatiga y el estado de ánimo. No se agruparon los datos.
Resultados principales
Ocho estudios cumplieron los criterios de inclusión y se incluyeron en esta revisión actualizada. Seis eran de la versión original de la revisión, y dos más se añadieron cuando se actualizó la búsqueda. Otros 19 estudios se evaluaron como parte de esta actualización, pero no cumplieron los criterios de inclusión.
De los ocho estudios incluidos, cuatro investigaron la "prevención" de los problemas cognitivos (durante la radioterapia y el seguimiento) y cuatro investigaron la "mejoría" (intervenciones para tratar el deterioro cognitivo como complicación tardía de la radioterapia). Hubo cinco estudios farmacológicos (dos estudios de prevención y tres de mejoría) y tres estudios no farmacológicos (dos de prevención y uno de mejoría). Debido a las diferencias entre los estudios en las intervenciones evaluadas, no fue posible realizar un metanálisis.
Estudios en fase temprana del tratamiento de radioterapia (cinco estudios)
Los estudios farmacológicos de la "fase temprana del tratamiento de radioterapia" se diseñaron para prevenir o mejorar los déficits cognitivos e incluyeron fármacos utilizados en la demencia (memantina) y la fatiga (hidrocloruro de d‐treo‐metilfenidato). Los estudios no farmacológicos en la "fase temprana del tratamiento de radioterapia" incluyeron una dieta cetogénica y un programa de rehabilitación cognitiva y resolución de problemas de dos semanas de duración.
En el estudio de memantina, el desenlace cognitivo principal de la memoria a los seis meses no alcanzó la significación, pero hubo una mejoría significativa en la función cognitiva general en comparación con el placebo, con eventos adversos similares en todos los grupos. El estudio del clorhidrato de d‐treo‐metilfenidato no encontró diferencias estadísticamente significativas entre los grupos, con pocos eventos adversos. El estudio de una dieta cetogénica restringida en calorías no encontró efectos, aunque una ingesta de calorías inferior a la esperada en el grupo control complica la interpretación de los resultados. El estudio que investigó la utilidad de un programa de rehabilitación no realizó una comparación estadística del rendimiento cognitivo entre los grupos.
Estudios en fase tardía de radiación o de efecto tardío (cuatro estudios)
Los estudios farmacológicos de "mejoría" para tratar las complicaciones cognitivas de la radioterapia incluyeron fármacos utilizados en la demencia (donepezilo) o psicoestimulantes (metilfenidato y modafinilo). Las medidas no farmacológicas incluían la rehabilitación cognitiva y la resolución de problemas (Goal Management Training). Estos estudios incluyeron a pacientes con problemas cognitivos al inicio que tenían un cáncer cerebral "estable".
El estudio de donepezilo no encontró una mejoría en el desenlace cognitivo principal del rendimiento cognitivo general, pero sí encontró una mejoría en una prueba individual de memoria, en comparación con el placebo. No se informaron eventos adversos. Un estudio que comparó el metilfenidato con el modafinilo encontró mejorías en la función cognitiva tanto en el grupo del metilfenidato como en el del modafinilo. Se notificaron pocos eventos adversos. Otro estudio que comparó dos dosis diferentes de modafinilo combinó los grupos de tratamiento y encontró mejorías en todas las pruebas cognitivas. Sin embargo, se informaron varios eventos adversos. Ambos estudios estaban limitados por el pequeño tamaño muestral. El estudio Goal Management Training indicó un efecto beneficioso de la intervención, una intervención conductual que combinó conciencia plena y entrenamiento de estrategias, sobre la función ejecutiva y la velocidad de procesamiento.
Hubo varias limitaciones entre los estudios y pocos estuvieron exentos de altos riesgos de sesgo.
Conclusiones de los autores
En esta actualización, se encontró evidencia adicional limitada del tratamiento o la mejoría de los déficits cognitivos en adultos tratados con irradiación craneal. Como se concluyó en la revisión original, existe evidencia que respalda que la memantina podría ayudar a prevenir los déficits cognitivos de los adultos con metástasis cerebrales que reciben irradiación craneal. Hay evidencia que apoya que el donepezilo, el metilfenidato y el modafinilo podrían tener una función en el tratamiento de los déficits cognitivos en adultos con tumores cerebrales que han sido tratados con irradiación craneal. El abandono de los pacientes afectó la potencia estadística de estos estudios. Los estudios de investigación que tratan de minimizar el retiro del consentimiento y, por consiguiente, reducir la necesidad de procedimientos de imputación, podrían ofrecer una mayor certeza de la evidencia.
Solo hay evidencia de un pequeño estudio que apoya las intervenciones no farmacológicas en la mejoría de los déficits cognitivos. Se necesitan más estudios de investigación.
PICO
Resumen en términos sencillos
Intervenciones para prevenir y mejorar los déficits cognitivos en adultos tratados con irradiación craneal
Antecedentes
Los problemas con las capacidades/habilidades mentales/cognitivas (efectos secundarios cognitivos) son comunes en las personas que han recibido radiación en el cerebro por un tumor cerebral primario o secundario (metastásico) o para evitar que un tumor se extienda al cerebro desde otra parte del cuerpo. Este efecto secundario tóxico de la radiación cerebral puede ser agudo (durante el tratamiento) o al principio del tratamiento (de uno a seis meses) y puede ser reversible. Sin embargo, los efectos tóxicos posteriores pueden aparecer muchos meses o años después, y cuando se producen suelen ser irreversibles y a menudo lentamente progresivas. Los déficits cognitivos tardíos, como la pérdida de memoria, los problemas de planificación de tareas o los cambios conductuales, pueden tener un grave impacto en la calidad de vida y en la capacidad de realizar actividades con normalidad. Las intervenciones para ayudar a prevenir o tratar estos efectos tóxicos tardíos de la radiación podrían mejorar el bienestar del paciente. En esta revisión se revisan todos los estudios sobre intervenciones farmacológicas (medicamentos) y no farmacológicas (psicológicas) que tienen como objetivo prevenir o tratar los efectos secundarios cognitivos asociados con la radioterapia del cerebro.
Características de los estudios
En la revisión original publicada en agosto de 2014, se realizaron búsquedas en cuatro bases de datos bibliográficas, que se utilizan para identificar artículos de revistas revisadas por pares y otros tipos de publicaciones periódicas. Fueron elegibles para inclusión seis ensayos controlados aleatorizados, en los que las personas fueron asignadas al azar a la intervención o a un grupo de comparación (grupo control). Cada ensayo evaluó diferentes intervenciones, por lo que no se combinaron los resultados. El mayor ensayo investigó el medicamento memantina en 508 personas con un tumor cerebral metastásico. Otro ensayo investigó el donepezilo en 198 pacientes con un tumor cerebral primario o secundario. Los otros ensayos fueron más pequeños e investigaron el modafinilo y el metilfenidato. Se encontró una intervención psicológica para prevenir los déficits cognitivos durante la radiación cerebral.
En esta actualización se realizaron búsquedas en las mismas bases de datos que en la revisión original. Se incluyeron dos nuevos estudios en la revisión. Uno de ellos fue un estudio de prevención no farmacológica que investigó el efecto de una dieta cetogénica restringida en calorías y el ayuno intermitente. El otro estudio identificado fue un estudio de mejoría no farmacológica que evaluó el Goal Management Training, una intervención conductual que combinaba conciencia plena y entrenamiento de estrategias. Un artículo adicional incluido en la revisión fue el texto completo de un procedimiento de conferencia incluido en la revisión original, que investigó el donepezilo en comparación con el placebo.
Hallazgos clave
Los hallazgos sobre la eficacia de la memantina ofrecen evidencia preliminar de respaldo en la prevención de los déficits cognitivos en pacientes con un tumor cerebral secundario que reciben irradiación cerebral. Los hallazgos sobre la eficacia del donepezilo ofrecen un apoyo inicial para su uso en la mejoría de los déficits cognitivos en pacientes con un tumor primario o secundario previamente tratado con radiación. Es importante seguir investigando ambos medicamentos para confirmar su eficacia y sus posibles efectos secundarios. El resto de los estudios no contaban con un número suficiente de participantes para proporcionar resultados fiables. Los efectos secundarios (episodios adversos) no se informaron en todos los estudios, pero en los que sí se notificaron, la mayoría de las veces fueron poco frecuentes y no graves. El reclutamiento y la retención de los participantes en los ensayos de la mayoría de los estudios farmacológicos fue difícil. Por último, aunque se encontró un respaldo limitado a los tratamientos no farmacológicos, esto no indica que estas intervenciones sean inefectivas, sino que es necesario seguir investigando.
Certeza de la evidencia
Se encontraron limitaciones en la certeza de la evidencia entre los estudios. Varios de los ensayos controlados aleatorizados farmacológicos tenían un bajo riesgo de sesgo, aunque algunos tenían un alto riesgo de sesgo debido, por ejemplo, a un diseño abierto o a la falta de un grupo placebo. Las intervenciones no farmacológicas tuvieron un alto riesgo de sesgo, ya que es difícil utilizar una comparación placebo en estos ensayos.
Authors' conclusions
Background
Description of the condition
Cognition refers to the mental abilities that require the high‐level handling of information. Such abilities include learning and memory, executive function, visuo‐spatial processing, language, concentration, attention and intellectual function (Gilroy 2000). Cognitive dysfunction (or deficit) in any of these areas can have a significant impact on a person's ability to function in day‐to‐day life, including work performance, language and communication, social interactions and independent living (Meyers 1998).
Cognitive deficits are common among patients who have received cranial irradiation (Kirkman 2022; Lehrer 2022; Perez 2022) to treat primary or metastatic brain tumours, or as prevention (prophylaxis) of other cancers. Both the brain tumour itself and tumour treatment can cause cognitive deficits (Kirkman 2022; Taphoorn 2004), and patients with brain tumours who receive radiotherapy tend to have worse cognitive deficits than those who are radiotherapy naive, with identified neuroimaging correlates including cortical atrophy and white matter abnormalities (Correa 2007; Postma 2002; Surma‐aho 2001; Sultana 2020). Over 80% of primary and metastatic brain tumour patients have self‐reported cognitive concerns regarding memory or concentration (Lidstone 2003; Mukand 2001). For example, in a prospective study, cognitive functioning was assessed with neuropsychological testing in patients receiving cranial irradiation for the treatment of brain metastases. Results demonstrated cognitive deficits in the domains of learning, delayed recall and recognition six to eight weeks following radiotherapy when compared to baseline scores (Welzel 2008). In another study, patients with lung cancer receiving prophylactic cranial irradiation demonstrated declined cognitive functioning on subjective and objective measures at six‐ and 12‐month follow‐up assessments when compared to baseline scores (Gondi 2013). A randomised trial also documented significant cognitive deficits four months after whole brain radiotherapy (WBRT) compared to patients treated with radiosurgery alone (Chang 2009).
Neurotoxic effects of cranial irradiation
Radiation can be delivered to the brain tumour using large focused doses (stereotactic radiation), as part of standard fractionated treatments, or to the whole brain. Potential risk factors for cognitive decline following brain radiation include receiving fractionated radiation doses greater than 2 Gy (Klein 2002), higher total radiation dose, larger brain volume of irradiation, using a divided‐dose schedule and longer overall treatment time (Lee 2002). Other risk factors may include either combined or subsequent chemotherapy use, age, with those fewer than seven years or greater than 60 years old at higher risk, and comorbid vascular risk factors such as diabetes and hypertension (Crossen 1994; Szerlip 2011). In the identification of acute treatment‐related neurotoxicity it is important to distinguish symptoms from tumour progression, recurrence or metastases, since continuation of treatment may lead to irreversible central nervous system (CNS) injury (Dietrich 2008).
The neurotoxic effects of brain radiation have traditionally been divided into acute, early‐delayed and late‐delayed radiation encephalopathy (Sheline 1980; Wujanto 2021). Acute radiation encephalopathy occurs as a result of disruption to the blood‐brain barrier leading to accumulation of fluid in the tissue (vasogenic oedema). Corticosteroids are used at this stage, and may improve symptoms of drowsiness and headache, and prevent further neurologic decline. Early‐delayed radiation encephalopathy may occur at one to six months following completion of treatment, and symptoms of short‐term memory and attentional deficits are seen alongside drowsiness and worsening of pre‐existing neurological deficits. A return to baseline is often found within 12 months (Vigliani 1996). This phase is associated with reversible damage to the myelin sheath (Sheline 1980). In contrast to early complications, late‐delayed radiation encephalopathy is viewed as irreversible. This complication occurs months to years following radiation therapy and manifests as white matter lesions (i.e. leukoencephalopathy). In more severe forms it can manifest or lead to a formation of dead brain tissue which, as a result, can lead to a pressure effect and associated neurological dysfunction (Fink 2012).
The precise relationship between initial acute changes and late/chronic radiation damage to the brain is unknown. However, more recent evidence indicates that more subtle, early forms of radiation‐induced damage to the CNS could drive chronic pathophysiological processes leading to permanent cognitive decline (Makale 2017). Clinically, late radiation damage is characterised by progressive mental slowing and impairment in attention and memory, with less commonly gait ataxia, urinary incontinence, apathy, and pyramidal and extrapyramidal signs (Taphoorn 2003). The cognitive deficits increase in incidence and severity over time (Klein 2002). However the exact incidence is hard to elucidate due to the large variety of neuropsychological tests used, the heterogeneous study populations and times at which patients are followed up (Taphoorn 2004). Furthermore, deficits are likely to vary depending on the specific anatomic structures irradiated (Haldbo‐Classen 2020). Historical data indicate that up to 90% of adult brain tumour patients who survive for more than six months following WBRT therapy develop (some form of) cognitive impairment (Crossen 1994), and in up to 5% of long‐term survivors the cognitive impairment progresses to dementia necessitating admission to a nursing home (DeAngelis 1989; Vigliani 1996). The incidence of severe cognitive deficits/late delayed radiation encephalopathy has been shown to be even higher in patients with primary CNS lymphoma, reaching nearly 100% in patients older than 60 years old (Abrey 1998). However, improvements in treatments (including hippocampal‐sparing techniques and focal radiotherapy) are likely to have reduced the cognitive effects of radiotherapy treatment (Wujanto 2021). Nevertheless, due to these adverse effects of cranial irradiation, the benefit of radiotherapy treatment for patients with a more favourable prognosis, such as with a low‐grade glioma (LGG), or as prophylactic cranial irradiation for small cell lung carcinoma, has been the subject of much debate in the past decade (Gondi 2013; Xue 2022).
The mechanisms of cranial irradiation‐induced cognitive impairment
The mechanisms by which radiation causes cognitive decline have been proposed to include microvascular damage, oligodendrocyte decline, loss of white matter integrity, neuroinflammation, metabolic changes, and neuronal dendritic spike damage (Makale 2017; Pazzaglia 2020; Sultana 2020). Long‐term radiation‐induced cognitive decline is likely to reflect changes to the white matter, hippocampus and prefrontal cortex, as well as other areas (Makale 2017). Two of the most studied mechanisms of radiation‐induced cognitive impairment include white matter changes and impaired neurogenesis.
The primary mechanism of delayed radiation‐induced white matter changes is associated with secondary endothelial damage and microvascular ischaemic insult (Lyubimova 2004), accompanied by apoptosis (programmed cell death) of oligodendrocyte progenitors (Makale 2017). This leads to a decrease in the volume of cerebral white matter, which is directly associated with cognitive decline (Correa 2004; Mulhern 2004; Reddick 2006). This has been confirmed in a longitudinal study that found medulloblastoma patients receiving a cranial irradiation dose of 36 Gy to show more rapid cerebral white matter volume decrease than those receiving a cranial irradiation dose of 23.4 Gy (Palmer 2002). Rarely, these white matter lesions can increase in size and may progress to frank white matter necrosis characterised by focal cavitations in the white matter within the radiated fields (Anscher 1991). Treatment of radionecrosis involves surgical excision and steroid therapy, and studies using bevacizumab, an angiogenesis inhibitor, have also reported high rates of clinical and radiological responses, albeit with small sample sizes (Gonzales 2007; Levin 2011; Torcuator 2009; Wang 2012).
Neurogenesis refers to the birth of new neurons, and occurs through the division of neural stem cells and subsequent maturation into neural progenitor cells that migrate and mature into neurons (Gage 2019). There exists compelling molecular evidence of the occurrence of neurogenesis throughout the human lifespan (Zhou 2022). A post‐mortem study in patients with medulloblastoma found significantly lower neurogenesis in patients treated with radiotherapy two to 23 years prior to analysis, compared to controls matched for age and sex (Monje 2007). Radiotherapy strategies that attempt to spare the crucial areas of neurogenesis, including the hippocampus, have been shown in several studies to result in improved cognition relative to conventional radiotherapy techniques (Gondi 2014; Kim 2018), including a phase III randomised controlled trial (Brown 2020).
Measuring cognitive deficits
Use of a core battery of validated neuropsychological tests to assess cognitive function have been recommended in order to harmonise studies of cognitive function in patients with cancer (Wefel 2011). These include the Hopkins Verbal Learning Test‐Revised (HVLT‐R) (Benedict 1998) to assess learning and memory, Trail Making Test (TMT) (Reitan 1992) to assess processing speed and executive function, and the Controlled Oral Word Association test of the Multilingual Aphasia Examination (COWA) (Benton 1989) to assess verbal fluency. Other tests have also been used, such as digit span and digit symbol (Wechsler 1981) to assess working memory and information processing speed, respectively. Cognitive function has also been assessed by using brief mental status evaluations, such as the Mini‐Mental State Examination (MMSE) (Folstein 1975). Whilst the MMSE is often shorter than neuropsychological testing, it has been associated with poor sensitivity in detecting cognitive deficits (Meyers 2003). Other studies have used subjective patient reports of cognitive concerns, such as in memory and concentration (Lidstone 2003; Mukand 2001). An additional consistent finding from the research literature is that correlations between subjectively assessed cognitive symptoms and objectively determined cognitive functioning are quite modest, with correlation coefficients generally ranging from 0.20 to 0.30 (Klein 2002). These are suggested to be confounded by some patients' lack of awareness regarding their cognitive impairments, and correlations with fatigue and depression, rather than cognitive test performance (Cull 1996; Gehring 2015).
To evaluate intervention effects, repeated neuropsychological testing is employed. However, practice effects occur resulting in improved test performance (Duff 2012). As a consequence, improvement (or stability) of test scores may be unjustly attributed to the intervention, where perhaps patients would otherwise have demonstrated a decline over time ‐ which is not uncommon in patients with brain tumours). In preventative and ameliorative intervention trials, practice effects should thus be accounted for. The variations in tools available, use of both objective and subjective measures, differences in time points at which cognitive functioning is measured and the differences in intervention duration highlight the caution that must be taken when combining and generalising results and conclusions.
Description of the intervention
This review included all studies on interventions that aim to:
-
prevent, or
-
ameliorate
any cognitive deficits in people who have received therapeutic or prophylactic cranial irradiation prior to, or during, participation in the study. These may include pharmacological and non‐pharmacological (medical, psychological or behavioural) interventions for the management of cognitive deficits. It is worth noting, however, that it is likely that almost all patients in the 'prevention' studies will have deficits in at least one cognitive domain, most commonly in memory and verbal fluency, due to the tumour or possibly prior neurosurgical intervention. A large proportion of patients will have been on steroid therapy and some may be recovering from craniotomy. Thus the distinction between 'prevention' and 'amelioration' is not clear cut.
Pharmacological
We defined pharmacological interventions as a drug, including herbs, given by any route at any therapeutic dose with the intention of preventing or ameliorating cognitive deficits in persons who have received cranial irradiation.
Studies investigating the pharmacological prevention of cognitive impairment have frequently been conducted in patients undergoing cranial irradiation during participation. For example, memantine, used in the treatment of Alzheimer's Disease (Robinson 2006), has been investigated for its neuroprotective role during irradiation.
Studies of pharmacological treatment for cognitive impairment after cranial irradiation have largely focused on psychostimulants, including methylphenidate (Butler 2007, Gehring 2012a) and modafinil (Gehring 2012a, Kaleita 2006). Objective cognitive functioning and patient‐reported outcomes of fatigue, mood and quality of life have been used to assess the efficacy of methylphenidate and modafinil in patients with brain tumour, 83% of whom had received cranial irradiation (Gehring 2012a). Donepezil (Rapp 2015, Shaw 2006) and memantine (Brown 2013), used in the treatment of Alzheimer's Disease, have also been investigated for their use in the treatment/prevention of cognitive symptoms in brain‐irradiated adults. Although the pharmacological mechanism of action is widely assumed to be through 'prevention' of cognitive failure rather than the treatment of existing cognitive impairments, the effect of drugs used in this setting is unclear and may have a 'treatment effect' as well as a 'preventative effect' in slowing the progression of cognitive failure. Chinese medicinal herbs and dietary supplements have also been investigated in irradiated brain tumour patients (Attia 2012).
Non‐pharmacological
We defined non‐pharmacological interventions as any non‐drug intervention given with the intention of ameliorating or preventing cognitive deficits during or following cranial irradiation. These can include, but are not limited to, medical, psychological and behavioural interventions.
Medical interventions include any biomedical intervention given to a person in which the intervention is not primarily investigating cancer treatment or control. For example, one study explored the use of hyperbaric oxygen therapy in cranial irradiated brain tumour patients using 31 neuropsychological tests (Hulshof 2002).
Psychological interventions may include (but are not limited to) education, retraining and compensation strategies. Several studies have evaluated cognitive rehabilitation in patients with brain tumours (Gehring 2009, Locke 2008, Richard 2019).
Behavioural interventions can include exercise (Gehring 2020) as well as behavioural modification interventions such as mindfulness and dietary interventions.
How the intervention might work
Clinical trials have explored the prevention and treatment of cognitive deficits by targeting pharmacological, psychological or behavioural pathways.
Pharmacological
Pharmacological interventions may prevent cognitive deficits via their neuroprotective role during WBRT such as memantine, an N‐Methyl‐D‐aspartate receptor antagonist (Brown 2013).
Pharmacological interventions may ameliorate cognitive deficits via their involvement in critical neurotransmitter pathways. Methylphenidate is a CNS stimulant found to have a positive effect on attention due to its action on the brain centre for attention control, the fronto‐striatal network, by increasing dopamine and noradrenaline concentrations (Volkow 2002). Another centrally acting drug is donepezil, a reversible cholinesterase inhibitor involved in inhibiting the breakdown of the neurotransmitter acetylcholine, often prescribed for patients with Alzheimer's Disease. This may have a cognitive enhancing effect by prolonging and improving cholinergic function, associated with learning and memory (Steinberg 2011).
Non‐pharmacological
Medical interventions have also been considered to help prevent or treat cognitive deficits. Hyperbaric oxygen therapy has been used to improve damage to the nervous system by stimulating angiogenesis, the process through which new blood vessels are formed from pre‐existing blood vessels (Gill 2004).
Psychological interventions may help prevent and improve cognitive deficits by retraining cognitive capacities such as attention and memory, or via compensation strategies such as memory aids. These interventions target the plasticity of the brain, via restoration or reorganisation of function (Miotto 2013; Mora 2013). For example, Cicerone 2011 reviewed 370 cognitive rehabilitation interventions and found supportive evidence for its role in patients with traumatic brain injury and stroke.
Behavioural interventions, such as exercise, may also help ameliorate or prevent cognitive deficits. Exercise has been associated with increases in cerebral blood flow, increased hippocampal neurogenesis, changes in neurotransmitter release and arousal levels and brain structure, and particularly through the involvement of Brain Derived Neurotrophic Factor associated with nerve growth (Gligoroska 2012).
Other non‐pharmacological interventions, such as those involving diet modifications, may also play a role in improving cognitive functioning. The dietary supplement Ginkgo biloba has been associated with regulating signalling pathways, cellular metabolism and gene transcription (Smith 2003).
Why it is important to do this review
As anti‐cancer treatments become more effective and readily available across treatment centres, patients live longer disease‐free, but with long‐term sequelae of the disease and the neurotoxic side effects of treatment (Cochran 2012). Greater emphasis is now being placed on quality of life and with the establishment of neurocognitive function as a predictor of survival (Meyers 2000; van Kessel 2021) and quality of life (Mitchell 2010), cognitive functioning is an essential outcome measure. There is currently no standard policy to direct treatment. With even mild cognitive impairment leading to negative functional and psychiatric consequences, especially if persistent and untreated, it is important to identify ways to reduce the long‐term impact of cranial irradiation on neuropsychological function.
Objectives
To assess the effectiveness of interventions for preventing or ameliorating cognitive deficits in adults treated with cranial irradiation.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomised trials (including those described in conference abstracts) that included: (i) a group receiving an intervention for cognitive function; and (ii) a control group or comparison group receiving no intervention for cognitive function, standard care, or a comparison with a normative data control group, or comparison to another active intervention.
Types of participants
Prevention
For studies investigating the prevention of cognitive deficits, we included studies that involved adult patients (aged 18 years and over) who received an intervention aimed at the prevention of cognitive deficits and underwent cranial irradiation (whole brain or partial brain radiation) during participation in the study, for the treatment of primary or secondary brain cancer, or prophylactic treatment for other cancers.
Since these studies refer to interventions for preventing cognitive deficits, the presence of cognitive deficits at baseline was not an inclusion criterion. However, we only included studies where cognitive functioning was assessed via neuropsychological testing both prior to and following the start of the intervention.
Amelioration
For studies investigating the amelioration of cognitive deficits, we included studies that involved adult patients (aged 18 years and over) with impairment in at least one cognitive domain, who received an intervention aiming to ameliorate cognitive deficits and had previously undergone cranial irradiation (whole brain or partial brain radiation) prior to participation in the study for the treatment of primary or secondary brain cancer, or prophylactic treatment for other cancers. Cognitive impairment was determined prior to participation via neuropsychological testing. Participants could have received cranial irradiation during childhood, but had to be an adult (aged 18 years and over) during participation in the study.
We also included studies that involved only a subset of patients who had undergone cranial irradiation in the review, if this group formed a large majority (> 80%) of the study population or had been explored via subgroup analyses.
Types of interventions
Studies that were included could have utilised pharmacological (e.g. stimulants, or neuroprotective agents) or medical (e.g. hyperbaric oxygen therapy) approaches, or psychological (e.g. cognitive rehabilitation) or behavioural (e.g. exercise) interventions, targeted to prevent or ameliorate radiation‐related cognitive deficits.
Whilst we included studies that investigated the preventative role of an intervention during cranial irradiation, we did not include those where the intervention being investigated was cranial irradiation itself, associated with treating the tumour or improving tumour control. Such excluded studies included those on:
-
hippocampal sparing techniques;
-
techniques limiting radiation dosage to healthy tissue (e.g. intensity‐modulated radiation therapy);
-
the addition of chemotherapy agents (e.g. motexafin gadolinium).
Although these techniques can be associated with reduced or limited cognitive side effects, these techniques would best fit a separate Cochrane systematic review investigating the effect of dose of radiotherapy in causing cognitive problems.
Pharmacological interventions
We investigated the effectiveness of any drug or herb given by any route for any duration, and at any therapeutic dose, with the objective of preventing or treating cognitive deficits in patients who had received, or were receiving, cranial irradiation. Such drugs are likely to include psychostimulants (e.g. methylphenidate, modafinil), and might include drugs to treat cognitive deficits in other neurological conditions (e.g. donepezil, memantine).
For ethical reasons, studies involving drugs may not automatically include a placebo arm. To increase the relevance of the review we included studies without a placebo arm if the study involved a group of participants who have been randomised to a control group of some kind (e.g. treatment as usual, another active drug or allocation to a waiting list), or that have been compared to normative control data with correction of practice effects caused by repeated neuropsychological testing.
Non‐pharmacological interventions
For non‐drug medical interventions, we investigated any medical intervention, such as hyperbaric oxygen therapy, which aimed to prevent or improve cognitive deficits in patients who had received, or were receiving, cranial irradiation.
For psychological and behavioural interventions, we reviewed any cognitive and/or behavioural treatment given with the intention or preventing or treating cognitive deficits in patients who had received, or were receiving, cranial irradiation; these could include, but were not limited to, retraining, education or teaching of compensation strategies, physical exercise interventions or dietary supplements. Due to the nature of these interventions, it can be difficult to blind different arms of trials. To increase the relevance of the review we included studies without a placebo arm if the study involved a group of participants who have been randomised to a control group of some kind (e.g. treatment as usual, another active treatment or allocation to a waiting list), or that have been compared to normative control data with correction of practice effects caused by repeated neuropsychological testing.
Types of outcome measures
Primary outcomes
The primary outcome for this review was cognitive performance as assessed by neuropsychological tests (and not self‐report); this could be a general or composite cognitive score or individual cognitive test scores using validated neuropsychological tests (e.g. Hopkins Verbal Learning Test‐Revised (HVLT‐R (HVLT‐R), Controlled Oral Word Association Test (COWA)). Cognitive functioning had to be measured at baseline and following intervention at any time point.
In studies involving preventative interventions, we determined efficacy as a statistically significant improvement in cognitive functioning, or no change/decline from baseline. Studies could have objective cognitive functioning as a primary outcome or include cognitive performance as the secondary outcome to an alternative primary quality of life measure (e.g. fatigue, mood).
In studies involving ameliorating interventions, we determined efficacy as a statistically significant improvement, or no change, in cognitive functioning from baseline. To increase the relevance of the review, we did not restrict eligible studies with respect to the time point at which cognitive functioning was measured at baseline or at follow‐up. We noted and discussed the time points at which cognitive functioning was measured.
Secondary outcomes
-
Self‐reported cognitive functioning via interviews or questionnaires.
-
General functioning including mood/psychiatric symptoms (e.g. Hospital Anxiety and Depression Scale (HADS)), self‐reported fatigue (e.g. Brief Fatigue Inventory (BFI)) and quality of life measurements (e.g. FACT‐Br).
-
Adverse events (e.g. nausea, skin reactions, headache).
We noted and reviewed the secondary outcomes if recorded, but these were not eligibility criteria for this review.
Search methods for identification of studies
Electronic searches
For this review update, we searched the following databases up to 12 September 2022:
-
Cochrane Register of Controlled Trials (CENTRAL, 2022, Issue 9), in the Cochrane Library;
-
MEDLINE via Ovid (August 2014 to September 11 2022);
-
Emase via Ovid (August 2014 to 2022 week 36);
-
PsycInfo via Ovid (August 2014 to 12 September 2022).
The search strategies are listed in Appendix 1 (MEDLINE), Appendix 2 (Embase), Appendix 3 (CENTRAL), and Appendix 4 (PsycINFO). The search strategies were not restricted by year of publication, language or publication type. Following de‐duplication the references were run through the Cochrane 'RCT Classifier' to identify records more than 10% likely to be a randomised controlled trial ( RCT).
Original Searches
The original searches for the review of 'Interventions for preventing and ameliorating cognitive deficits in adults treatment with cranial irradiation' (Day 2014b) were as follows:
-
Cochrane Register of Controlled Trials (CENTRAL, 2014, Issue 8);
-
MEDLINE (1950 to August 2014);
-
Embase (1980 to August 2014);
-
PsycINFO (1974 to August 2014).
Searching other resources
-
We searched the reference lists of included studies.
-
We searched for ongoing trials using ClinicalTrials.gov (www.clinicaltrials.gov), the Physicians Data Query (www.cancer.gov/clinicaltrials) and the metaRegister of Controlled Trials (www.controlled-trials.com/mrct).
Data collection and analysis
Selection of studies
We used the reference management database EndNote to download all titles and abstracts retrieved by electronic searching. We removed duplicates and two review authors (MK, JD) independently examined the remaining references. The review authors were not blinded to the authors or affiliations of the studies. We obtained full‐text copies of potentially relevant references and excluded studies clearly not meeting the inclusion criteria. Two review authors (MK, JD) independently assessed the eligibility of retrieved papers, with disagreements planned to be resolved by discussion with a third review author (KG), which was not necessary. We documented reasons for the exclusion of studies.
Data extraction and management
Data extraction
We used the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions to extract data from included trials using a data extraction form specifically designed for this review (Higgins 2011). Two review authors (MK, JD) completed data extraction independently. Differences between review authors were resolved by discussion.
Data extracted included the following:
-
article details (author, year of publication, journal, country and language);
-
aim of the study (preventative or ameliorative);
-
methodology (study design, participant recruitment method, inclusion and exclusion criteria, informed consent, ethical approval, statistical analyses including corrections for practice effects);
-
population demographics (geographical location, setting, age, gender, ethnicity, total number included in trial and analyses);
-
details of participants health status (including disease status, tumour pathology, tumour treatment details including proportion receiving radiotherapy, antiepileptic medication, corticosteroid use);
-
intervention (characteristics such as drug dose, preparation and route of administration, frequency and duration, detail of providers, timing of intervention in relation to radiotherapy);
-
outcomes (primary and secondary outcomes assessed, method and timing of assessments);
-
results of cognitive functioning measure (neuropsychological test performance);
-
results of other outcome measures (including self‐reported cognitive questionnaires on cognitive symptoms, quality of life, depression, fatigue and adverse events);
-
risk(s) of bias.
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants are analysed in the groups to which they are assigned.
Data management
We used Review Manager 5.4 to collate data (RevMan 2020). For continuous outcomes (e.g. cognitive performance and quality of life measures), we extracted the final values and standard deviations (SDs) and the number of patients assessed at endpoint for each treatment arm to estimate the mean difference (MD) between treatment arms and its standard error (SE). We noted and reviewed the time points for outcome assessment. Where participant and study details were missing, we noted these as a potential limitation of the study.
Assessment of risk of bias in included studies
We used the Cochrane Handbook for Systematic Reviews of Interventions risk of bias tool to assess the risk of bias in included studies (Higgins 2011), including the assessment of:
-
selection bias: random sequence generation and allocation concealment;
-
performance bias: blinding of participants, personnel (patients and treatment providers) and outcome assessors;
-
attrition bias: incomplete outcome data;
-
reporting bias: selective reporting of outcomes;
-
other possible sources of bias.
A full risk of bias item list with specific criteria for each item can be found in Appendix 5.
We interpreted and reported all bias criteria as having a low, high or unclear risk of bias. We reported an unclear risk of bias when insufficient information was provided, or when uncertainty over the potential for bias was present. Two review authors (MK, JD) applied the risk of bias tool independently and resolved differences by discussion. We summarised results in a risk of bias graph and risk of bias summary and interpreted the results with respect to risk of bias.
Measures of treatment effect
For continuous outcomes, we used the between‐group mean difference (MD) with 95% confidence interval (CI). We planned to use the standardised mean difference (SMD) with 95% CIs to combine trials that measured the same outcome, but used different methods. For dichotomous outcomes we used the risk ratio (RR) with 95% CI.
Unit of analysis issues
We did not identify any study designs with unit‐of‐analysis issues.
Dealing with missing data
We did not impute missing outcome data for any outcomes.
Assessment of heterogeneity
If a meta‐analysis had been possible, we aimed to assess heterogeneity between studies by a formal statistical test to indicate the significance of the heterogeneity (Deeks 2001). We planned to Investigate and report heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and via visual inspection of forest plots.
Assessment of reporting biases
Two review authors (MK, JD) reviewed and recorded any details of reporting bias. We aimed to examine funnel plots, if a meta‐analysis that included more than 10 trials was possible, to assess potential small‐study effects, such as publication bias.
Data synthesis
If sufficient clinically similar trials had been available, we intended to combine data for meta‐analysis using the Cochrane Review Manager software 5.4 (RevMan 2020), as follows:
-
for continuous outcomes, we planned to pool MDs between treatment arms at the end of follow‐up if trials measured the outcome on the same scale and at the same primary study endpoint, otherwise we planned to pool SMDs;
-
we intended to use random‐effects models for all meta‐analyses, with 95% CIs (DerSimonian 1986);
-
for dichotomous data, we planned to pool RRs (RevMan 2020).
Subgroup analysis and investigation of heterogeneity
If sufficient data had been available, we would have reviewed studies separately using the following categories:
-
drug dose;
-
World Health Organization (WHO) tumour grade (low‐grade/high‐grade).
Sensitivity analysis
If sufficient data had been available, we would have considered the following factors:
-
differing risk of bias profiles;
-
different classes of agents, doses or scheduling differences.
We anticipated that additional types of sensitivity analyses would have been identified during the conduct of the review.
Summary of findings and assessment of the certainty of the evidence
Summary of findings tables were created where appropriate and adequate data were provided in relation to the topic of this review. If sufficient data had been available, we would have used the GRADE approach to assess certainty of the body of evidence. Table 1; Table 2.
| Cognitive functioning measure (standardised scores) | Memantine | Placebo |
P | ||
| N | Median change after 24 weeks (IQR) | N | Median change after 24 weeks (IQR) | ||
| Short‐term verbal memory | 77 | ‐0.23 (‐1.16 to 0.70) | 90 | ‐0.415 (‐1.86 to 0.46) | 0.21 |
| Long‐term verbal memory (recall) | 76 | 0 (‐1.67 to 0.59) | 90 | ‐0.90 (‐2.22 to 0.55) | 0.06 |
| Long‐term verbal memory (recognition) | 76 | 0 (‐1.12 to 1.43) | 90 | ‐0.72 (‐2.73 to 0.71) | 0.01* |
| Verbal Fluency | 78 | ‐0.10 (‐0.62 to 0.53) | 90 | ‐0.16 (‐0.83 to 0.61) | 0.31 |
| Trail Making Test A | 76 | 0.08 (‐1.01 to 1.82) | 92 | ‐0.37 (‐2.08 to 0.50) | 0.02* |
| Trail Making Test B | 74 | ‐0.45 (‐2.37 to 1.04) | 90 | ‐0.49 (‐2.60 to 0.62) | 0.30 |
| Cognitive composite score | 73 | ‐0.03 (‐0.90 to 0.72) | 90 | ‐0.41 (‐1.30 to 0.12) | 0.02* |
* P < 0.05
IQR: interquartile range
| Cognitive performance measure | Goal Management Training | Brain Health Program | Wait‐listed control | P | Goal Management Training | Brain Health Program | Wait‐listed control | P | ||||||
| N | Domain composite score change post‐training compared to baseline (SD) | N | Domain composite score change post‐training compared to baseline (SD) | N | Domain composite score change post‐training compared to baseline (SD) |
| N | Domain composite score change at 4‐month follow‐up compared to baseline (SD) | N | Domain composite score change at 4‐month follow‐up compared to baseline (SD) | N | Domain composite score change at 4‐month follow‐up compared to baseline (SD) |
| |
| Executive composite | 10 | 0.33 (0.54) | 6 | 0.08 (0.43) | 4 | ‐0.44 (0.45) | 0.077 | 10 | 0.69 (0.51) | 6 | 0.13 (0.50) | 3 | ‐0.07 (0.44) | 0.046 |
| Memory composite | 10 | 0.40 (0.84) | 6 | 0.11 (0.91) | 4 | 0.43 (0.76) | 0.778 | 10 | 1.08 (0.82) | 6 | 0.86 (1.01) | 3 | 0.84 (0.52) | 0.842 |
| Processing speed | 10 | 0.44 (0.55) | 6 | 0.47 (0.43) | 4 | ‐0.65 (1.53) | 0.071 | 10 | 0.54 (0.57) | 6 | ‐0.14 (0.94) | 3 | ‐0.18 (1.03) | 0.179 |
SD: standard deviation
P values refer to time‐by‐group interaction (intervention) effects on change compared to baseline, using analysis of variance
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
Details can be found in Figure 1.
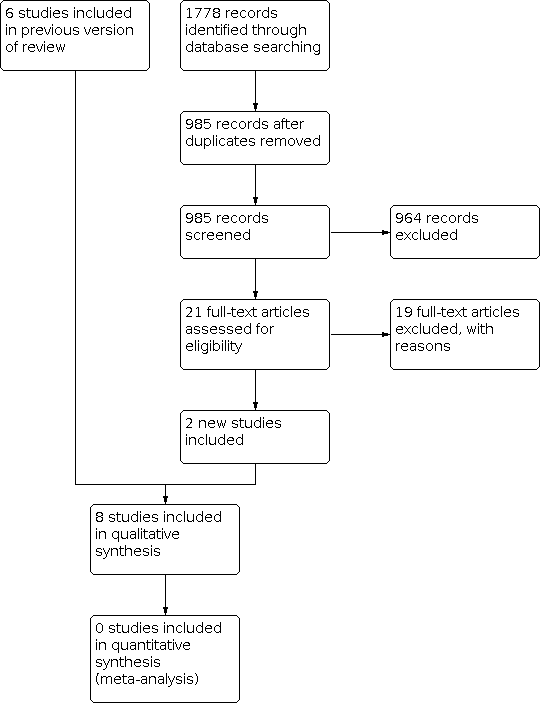
In the original review, six studies were identified for inclusion. Four published trials met our inclusion criteria for analysis; two trials investigating the prevention of cognitive deficits and two trials investigating the amelioration of cognitive deficits. Three conference abstracts were also identified; data were available for one study (Kaleita 2006) and, following correspondence, data were obtained for another study (Rapp 2013). In addition, we identified one study when searching clinical trial databases for ongoing trials (Umphrey 2013). A further study was identified as awaiting classification (Shaw 2013).
In the updated search, an additional 1778 citations were identified through database searching, which left 985 records for screening following de‐duplication of the results. Upon screening of titles, this narrowed the results to 21 articles. Of these 21 articles, the updated search identified two new additional studies for inclusion (Richard 2019; Voss 2022). In addition, the full‐text article (Rapp 2015) for a conference abstract included in the original review (Rapp 2013) was identified. The full‐text article of the study identified in the original review was awaiting classification (Shaw 2013) was also identified (Page 2015), which did not meet the criteria for inclusion in the review. In addition, the full‐text article of a study identified in the original review as an ongoing trial (Umphrey 2013) was identified (Porter 2022), which also did not meet the criteria for inclusion in the review. An ongoing study was identified when searching clinical trial databases for ongoing trials (Chan 2018).
Included studies
For detailed information on included studies see the 'Characteristics of included studies' table.
Prevention
Two included studies investigated a pharmacological intervention (d‐threo‐methylphenidate hydrochloride and memantine) for the prevention of cognitive deficits during cranial irradiation (Brown 2013; Butler 2007). One included study investigated a cognitive rehabilitation and problem‐solving program for the prevention of cognitive deficits in patients with primary brain tumours receiving radiotherapy (Locke 2008). Another study investigated the effect of a calorie‐restricted ketogenic diet and intermittent fasting on progression‐free survival in patients with glioblastoma, with cognitive outcomes as one of a number of secondary outcomes (Voss 2022).
Pharmacological Studies
Memantine versus placebo
One study recruited 554 eligible patients with brain metastases primarily from lung cancer, with breast, colon and other cancers also included; 46 patients did not meet the inclusion criteria, therefore 508 participants were allocated to intervention (N = 256; median age [range], 60 [31‐84] years; M:F, 115:141; 84% white); placebo arm (N = 252; median age [range], 59 [29‐86] years; M:F, 107:145; 83.3% white) (Brown 2013). This was greater than the calculated 221 participants required in each arm to reach 80% statistical power, although at eight weeks there were 129 and 139 patients in the intervention and placebo arms, respectively; of the 508 eligible patients, 55 withdrew consent and 271 died prior to completion of the study. Overall, 31% and 33% of participants completed 24 weeks of drug use as per the study protocol in the memantine and control groups, respectively. Imputation was carried out for participants still alive at the time of missed assessment. Wilcoxon rank‐sum test, Gray’s Test, Cox proportional hazards regression model, stratified log‐rank test statistical analyses were performed.
d‐threo‐methylphenidate hydrochloride versus placebo
One study recruited 68 patients (intervention arm N = 34; median age [range], 52 [31‐79] years; M:F, 20:14; 76% white; placebo arm N = 34; median age [range], 60 [28‐83] years; M:F, 17:17; 91% white) of the 81 projected patients, calculated for a 90% statistical power, but with much fewer patients for analysis over time. Patients had a primary (N = 33) or metastatic brain tumour (Butler 2007); further details concerning the brain tumours were not reported. Two participants withdrew consent prior to receiving the intervention, 11 participants withdrew consent following the baseline assessment, 12 following radiation, and 11 after four weeks. Participant withdrawal of consent after eight weeks was not reported. This study was terminated early due to low accrual. Two sample t‐tests, analysis of covariance, mixed‐model analysis of covariance and autoregressive covariance structure statistical analyses were performed.
Comparisons between the pharmacological prevention studies
Both pharmacological prevention studies recruited participants in the USA, and both of these USA studies were multi‐centre studies involving four centres (Butler 2007), and 143 centres, including Canada (Brown 2013). Both studies reported obtaining ethical approval and informed consent from participants, and described the recording of adverse events. Cranial irradiation schedule requirements varied between the two studies, with patients receiving 37.5 Gy of WBRT via 15 fractions of 2.5 Gy (Brown 2013) or partial or WBRT of at least 25 Gy in at least 10 fractions of 1.8 to 3.0 Gy/fraction (Butler 2007). Both studies reported using a randomisation method, which was confirmed via correspondence as random number generation using a computer program, and compared the interventions with a matched placebo group (Brown 2013; Butler 2007). In addition, both studies reported the use of a double‐blinding and allocation concealment technique, which was confirmed via correspondence to have been through the use of a pharmaceutical company providing matched drug containers (Brown 2013; Butler 2007).
Interventions included d‐threo‐methylphenidate hydrochloride (d‐MPH; Butler 2007) and memantine (Brown 2013). One study commenced administration of the study drug no later than three days after commencing radiotherapy (Brown 2013), and the other within five days of commencing radiotherapy (Butler 2007). Both pharmacological prevention studies included dose‐escalation techniques, continued for eight (Butler 2007) or 24 (Brown 2013) weeks. Dose reduction and withdrawal techniques were included if patients experienced severe adverse events (Butler 2007), or when the patient's creatine clearance levels declined (Brown 2013).
Both studies assessed cognitive functioning using the Mini‐Mental State Examination (MMSE). One study included a neuropsychological test battery that assessed memory, processing speed, executive function and verbal fluency (Brown 2013). The other study also included self‐report measures of fatigue, depression and quality of life (Butler 2007). Timing of cognitive outcome assessment varied between studies, with patients assessed at baseline and then at eight, 16 and 24 weeks of drug use (Brown 2013), or at the end of radiation therapy and at eight weeks of drug use (Butler 2007). Both studies carried out a final follow‐up assessment after the drug was stopped, at 12 (Brown 2013) and 52 (Butler 2007) weeks.
Non‐Pharmacological Studies
Cognitive rehabilitation versus standard care
Locke 2008 recruited 19 participants (intervention arm N = 12; median age [range], 47 [30 to 78] years; M:F, 7:5; ethnicity not reported; control arm N = 7; median age [range], 60 [31 to 71] years; M:F, 4:3) receiving cranial irradiation for the treatment of a primary brain tumour (17 glioma, two meningioma), with complete data in 14 of these participants. Six participants withdrew consent prior to completion of the study due to time commitments, tumour progression, fatigue and the unwillingness of a caregiver to attend appointments. Results were reported for 13 participants and their caregivers at post‐intervention and three months follow‐up. Recruitment was carried out at a single radiation oncology clinic. Ethical approval was obtained and informed consent sought. Patients were required to have a caregiver available to accompany them to each follow‐up to complete a quality‐of‐life questionnaire. Radiation schedule requirements were not reported. The use of a randomisation method was reported, however this was abandoned due to low accrual and the final three participants were enrolled into the intervention arm. Due to the nature of the study, participants were not blinded. Blinding of personnel was not reported.
The intervention included six 50‐minute sessions of cognitive rehabilitation and six 50‐minute sessions of problem‐solving therapy over two weeks, compared with standard medical care. The cognitive rehabilitation intervention was particularly aimed at memory. This involved the education and use of a calendar to compensate for cognitive problems. The problem‐solving intervention involved the education and training of a positive problem‐solving model via constructive thinking, using feelings as cues and reversed advocacy role play.
The primary aim of the study was to assess the tolerability and feasibility of the program. This was assessed through the use of the Mayo‐Portland Adaptability Inventory (Malec 2003), primarily used in the evaluation of rehabilitation programmes designed for patients with acquired brain injury, and via patient feedback questionnaires. Cognitive functioning was assessed using the cognitive test battery Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph 1998). Self‐reported quality of life, mood and fatigue were also assessed. Assessments were taken at baseline, following the two‐week intervention, and at three months. RBANS was only reported at baseline and post‐intervention as the majority of participants choosing a telephone follow‐up for their final clinic appointment, and consequently cognitive assessment could not be carried out. Wilcoxon signed rank statistical tests were used to assess functional status only. Means and standard deviations for the RBANS subtest were reported for baseline and post‐intervention; mean change and standard deviation of mean change were not reported.
Calorie‐restricted ketogenic diet with intermittent fasting versus standard diet
Voss 2022 recruited 50 patients (intervention arm N = 25; median age [range], 56 [39 to 71] years; gender and ethnicity not reported; control arm N = 25; median age [range], 58 [26 to 75] years) with recurrent glioblastoma receiving re‐irradiation from three German university hospitals. This was greater than the 42 patients necessary to have 80% statistical power. Ethical approval was obtained and informed consent provided by all participants. The radiotherapy regimen proposed by the study authors was 5 x 4 Gy from days four to eight in both groups, but other regimens were allowed. Randomisation, completed using computer software that generated random numbers, and allocation concealment were confirmed by correspondence with the study authors. Blinding of the participants and personnel were not possible due to the nature of the intervention.
The intervention comprised of three days of ketogenic diet (21–23 kcal per kg of body weight per day, with carbohydrate intake limited to 50 g per day), followed by three days of fasting and then another three days of ketogenic diet. During the fasting period, patients had an unlimited intake of fluid. From day 10 onwards, no dietary restrictions were implemented. The control group comprised a 'standard diet', with nutritional counselling in line with German Society of Nutrition guidelines, recommending a calorie intake of approximately 30 kcal per kg of body weight per day. Four participants (two from each group) withdrew consent to take part in the trial before the diet, and a further four participants stopped the diet during treatment (three from the intervention group and one from the standard diet group). There were 20 participants in the intervention group and 22 participants in the standard diet group who completed the evaluation as set by the protocol. Cognitive functioning was assessed as a secondary outcome using the MMSE and the d2 Test of Attention at baseline, day 6, day 12 and one month after radiotherapy. Of these, data on results of the d2 Test of Attention were available for 29 participants at baseline, 27 participants at day 6, 28 participants at day 12, and 27 participants at one month. The number of participants with data available for the MMSE were not specified.
The primary endpoint of the study was progression‐free survival at six months, and secondary endpoints included: progression‐free survival; local progression‐free survival at six weeks, three months and 12 months; overall survival; frequency of epileptic seizures; extent of ketosis; quality of life; and cognitive function. Dependent and independent samples t tests, Wilcoxon tests, chi‐squared tests, Kaplan‐Meier survival analysis and log‐rank tests were performed.
Amelioration
Three pharmacological studies (Gehring 2012a; Kaleita 2006; Rapp 2015) and one non‐pharmacological study (Richard 2019) were included that investigated the treatment of cognitive deficits.
Pharmacological Studies
Modafinil at two different doses
One study recruited 30 of 30 expected patients (mean age [standard deviation], 45.3 [11.7] years; M:F, 19:11, ethnicity not reported) with a primary brain tumour, 87% of whom had received radiotherapy (Kaleita 2006); the distribution of tumour grade was almost equal between grade II, III and IV tumours, with two patients with a grade I tumour. Results were reported for all participants at baseline, and at eight and 12 weeks of drug use. Groups were combined for statistical analysis, and paired t‐tests and Wilcoxon Signed Rank tests were used to assess change from baseline.
Methylphenidate versus modafinil
Another study recruited 34 of the 75 planned patients with a primary brain tumour, calculated to have 90% statistical power. Four participants were excluded from the study; three due to tumour progression (two methylphenidate arm; one modafinil arm), and one due to infection‐related delirium requiring hospitalisation (modafinil arm). A further six participants dropped out; three due to the prescription not being filled (two methylphenidate arm; one modafinil arm), one due to nausea (modafinil arm), one due to steroid‐induced hyperactivity (modafinil arm), and one missed the follow‐up appointment (methylphenidate arm). Results were reported for 24 participants at four weeks of treatment (21 glioma, one medulloblastoma, one primary central nervous system (CNS) lymphoma, one hemangiopericytoma; methylphenidate arm N = 19; mean age [standard deviation], 42.5 [10.2] years; M:F, 8:11; ethnicity not reported; modafinil arm N = 5; mean age [standard deviation], 54.4 [7.7] years; M:F, 5:0); 83% of whom had received cranial irradiation prior to participation and 62.5% were receiving chemotherapy during participation (Gehring 2012a). Despite randomisation, there were significantly more males in the modafinil group, compared to the methylphenidate group (P = 0.03). An exploratory statistical analysis approach was used via t‐tests and repeated measures analyses of covariance. Due to low accrual, the two methylphenidate arms were combined during analysis; further analysis also combined all interventions and compared findings with normative data. Practice effect adjusted reliable change index was used to assess individual change in cognitive test scores relative to baseline.
Donepezil versus placebo
A further study recruited 198 of the required 200 patients (intervention arm N = 99; median age [range], 56 [19‐84] years; M:F, 43:56; 91% white; control arm N = 99; median age [range], 54 [19‐81] years; M:F, 49:50; 91% white), required to reach 90% statistical power, from 26 sites; 130 had a primary brain tumour, 53 a metastatic brain tumour, and 15 had received prophylactic cranial irradiation (Rapp 2015). Fifty‐two participants dropped out of the study; reasons were not reported. Results were presented for 146 participants at 24 weeks follow‐up. Chi2, Fisher exact and Wilcoxon rank‐sum statistical tests were used. Imputation was carried out in all participants who provided at least baseline data.
Comparisons between the pharmacological amelioration studies
Two studies initially reported their results as a conference abstract (Kaleita 2006; Rapp 2015), with data from only one of these appearing in a full manuscript at the time this review was conducted (Rapp 2015). All three studies were conducted in the USA. Ethical approval and informed consent was reported for two studies (Gehring 2012a; Rapp 2015) and all reported adverse events. Two studies did not restrict patients to those receiving cranial irradiation (Gehring 2012a; Kaleita 2006). In one study, patients were eligible to participate following partial or whole brain irradiation of 30 Gy or greater (Rapp 2015). The use of a randomisation method was reported by all studies, and correspondence confirmed this and was through the use of a computer program in two studies (Gehring 2012a; Rapp 2015). Two studies used double‐blinding (Kaleita 2006; Rapp 2015), and one study also reported an allocation concealment method via a pharmaceutical company (Rapp 2015). One study used an open‐label design, although all treatment arms were experimental (Gehring 2012a).
Intervention arms varied between studies. Kaleita 2006 compared two dosages of modafinil followed by an extended treatment phase using a titrated dose between 50 mg and 600 mg/day for eight weeks. Gehring 2012a included three intervention arms using two forms of methylphenidate (immediate release and sustained release, combined for analysis), compared to a modafinil arm. Rapp 2015 included one intervention arm of donepezil, with an increasing dosage from 5 mg/day for six weeks and 10 mg/day for 18weeks if tolerated, compared with placebo.
All studies assessed cognitive functioning using neuropsychological testing, and one also calculated a cognitive composite score (Rapp 2015). All studies included self‐reported measures of mood and fatigue, and one also included a measure of quality of life (Gehring 2012a). Assessments were taken at baseline and at four weeks of drug use (Gehring 2012a), at baseline, 12 and 24 weeks of drug use (Rapp 2015), or at baseline and at one, three, four, eight and 12 weeks of drug use (Kaleita 2006). Gehring 2012a used alternate test forms when possible and statistical corrections to minimise re‐test effects. Assessments were not carried out following withdrawal of the drug, but were recorded in one study during a washout period prior to the extension phase (Kaleita 2006).
Non‐Pharmacological Studies
Goal Management Training versus active control and wait‐list control
Richard 2019 recruited 26 patients with meningioma, low‐grade glioma or high‐grade glioma, one of whom experienced disease progression before baseline testing and was thus excluded, leaving 25 patients (demographic data not reported by group allocation; overall median age [range], 48 [21‐68] years; M:F, 15:10; ethnicity not reported) for analysis (20 for post‐intervention analysis). No formal power calculation was described for this study. The study was conducted in Canada. Ethical approval and informed consent was reported. The study did not restrict inclusion to those receiving cranial irradiation (85% received cranial irradiation). Patients were randomised using a random number generator and allocation concealment was achieved through the allocation spreadsheet being viewable only to the intervention provider, confirmed by correspondence with the study authors. Blinding of participants was not possible due to the nature of the interventions.
There were three groups in this study. The intervention comprised of Goal Management Training, a behavioural intervention that combined mindfulness and strategy training, delivered in eight two‐hour individual sessions by a clinical neuropsychologist. An active control group underwent a novel psycho‐educational Brain Health Program that included content on brain and cognition but no cognitive strategy training, also delivered in eight two‐hour individual sessions by a clinical neuropsychologist. The third group was a wait‐list passive control group that received usual care but no neuropsychological intervention. In the Goal Management Training group, one participant only completed six out of eight sessions (due to new employment). In the active control group, one participant withdrew after baseline assessments without providing a reason, and another after completing seven out of eight sessions (due to increased work responsibilities). In the wait‐list control group, one participant withdrew after baseline assessment with no reason provided, one declined assessment at post‐training, and an additional two declined at follow‐up (all citing a lack of time). An analysis comparing study completers (n = 21) and non‐completers (n = 4) showed that non‐completers were younger at enrolment and had worse language abilities, slower processing speed, and higher apathy scores.
Following randomisation, 11, eight and six participants were allocated to the Goal Management Training, active control and wait‐list control groups, respectively, with data on 10, six, and four available for post‐intervention analyses. Cognitive function was assessed using neuropsychological testing covering the domains of executive functioning, memory, and processing speed. The study also assessed self‐reported measures of cognitive symptoms, emotional functioning, coping and adjustment and everyday life function. Assessments were completed at baseline, immediately following the training and at four months. Analysis of variance, Pearson's Chi2, Kruskal‐Wallis and Mann‐Whitney U statistical tests were performed. The primary outcome measure was change in the executive functioning composite score. Secondary endpoints included changes in processing speed, memory, self‐reported cognitive symptoms, emotional functioning and coping/adjustment, programme adherence and patient‐reported use and effects of programme contents, including functional goal attainment.
Excluded studies
For detailed information on excluded studies see the 'Characteristics of excluded studies' table.
In the original review, after screening 16 full‐text articles, 10 studies were excluded in the original review (Attia 2012; Boele 2013; Chan 2003; Gehring 2009; Hulshof 2002; Jatoi 2005; Levin 2011; Meyers 1998; Schellart 2011; Shaw 2006).
For this update, 21 full‐text articles were screened, and a further 19 studies were excluded:
-
Seven amelioration studies investigating lidocaine (Peng 2016), Internet‐based guided self‐help (Boele 2018), cognitive rehabilitation (Van der Linden 2018; van der Linden 2021), home‐based exercise (Gehring 2018; Gehring 2020), and home‐based psychotherapy and rehabilitation (Ownsworth 2012) did not include a majority (80%) of participants who had received cranial irradiation, or did not analyse these patients separately. Two of these studies (Gehring 2018; Van der Linden 2018) also did not include cognitive impairment as an inclusion criterion, and one study (Boele 2018) did not include cognitive assessment via neuropsychological testing.
-
One amelioration study investigating cognitive rehabilitation (Maschio 2015) did not include a control group.
-
Three amelioration studies investigating armodafinil (Page 2015; Porter 2022) and dexamphetamine sulfate (Laigle‐donadey 2018) did not include cognitive impairment in at least one domain as an inclusion criterion.
-
One prevention study investigating the radioprotective effect of memantine (Wong 2016) was a sub‐study of a study already included in the review (Brown 2013)
-
One study investigating donepezil (Naughton 2018) did not include cognitive assessment via neuropsychology testing.
-
Two amelioration studies investigating the role of metformin (Ayoub 2020) and aerobic exercise training (Cox 2020) on cognition focused on childhood cancer survivors that were largely still children at the point of recruitment into the study.
-
One study evaluating the role of exercise therapy focused on postmenopausal breast cancer survivors (Campbell 2017).
-
One study evaluating the role of online couple‐based meditation for patients in primary or metastatic brain tumours did not formally assess cognitive outcomes (Milbury 2020).
-
One amelioration study evaluating the role of virtual reality training on cognitive outcomes in brain tumour patients with cognitive dysfunction did not specify how many patients received radiotherapy (Yang 2014).
-
One prevention study investigating Shenqi fuzheng (Chen 2019) was published as a randomised controlled trial although some aspects of the exclusion criteria needed clarification. At the time of publication of this systematic review update no further clarification was available from the authors. As a result, we have placed the study in the 'Studies awaiting Clarification' section.
Studies Awaiting Classification
In the original review there was one study published in conference proceedings (Shaw 2013). The updated search identified the full published article (Page 2015) which did not meet the inclusion criteria to be included in the review as it did not include cognitive impairment in at least one cognitive domain as an inclusion criterion (see 'Characteristics of excluded studies' table). In addition, as mentioned above one study (Chen 2019) was published as a randomised controlled trial although some aspects of the exclusion criteria needed clarification that was not available from the authors at the time of publication of this systematic review update.
Ongoing Studies
In the original review there was one ongoing study identified (Umphrey 2013). The full‐text version of this study has now been published (Porter 2022), which was excluded because the study did not meet the criteria to be included in the review as it did not include cognitive impairment in at least one cognitive domain as an inclusion criterion (see Characteristics of excluded studies table). The updated search found one additional ongoing study, evaluating the effect of ramipril on memory loss in patients with glioblastoma receiving chemoradiation (Chan 2018).
Risk of bias in included studies
We assessed studies using the Cochrane risk of bias tool (Higgins 2011). Between the two review authors of the original (JD, KZ) and updated (MK, JD) reviews, there was agreement in the risk of bias scores following discussion. Attempts to contact authors were carried out where there was an unclear risk of bias. A summary of the risk of bias is presented in Figure 2 and Figure 3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three of the five pharmacological studies were at a low risk of bias in relation to random sequence generation and allocation concealment as they used a stratification randomisation method and a pharmaceutical company to create identical drug containers (Brown 2013; Butler 2007; Rapp 2015). One pharmacological study was at an unclear risk of bias in relation to random sequence generation as it reported the use of a randomisation method, however the method used could not be identified (Kaleita 2006). The same study had a low risk of bias in relation to allocation concealment as blinding was used (Kaleita 2006). Two studies were at a high risk of bias regarding allocation concealment: one of these was a pharmacological study that used an open‐label design (Gehring 2012a); the other was a non‐pharmacological study that reported the use of a randomisation method but this was abandoned due to low accrual, which also placed it at a high risk of bias in relation to random sequence generation (Locke 2008). The pharmacological study at a high risk of bias regarding allocation concealment was at a low risk of bias regarding random sequence generation as a computer was used for randomisation (Gehring 2012a). Two of the three non‐pharmacological studies had a low risk of bias in relation to random sequence generation and allocation concealment as patients were randomised using a random number generator and allocation concealment was confirmed by correspondence with the authors (Richard 2019; Voss 2022).
Blinding
Four of the five pharmacological studies were at a low risk of bias and blinded participants and personnel to the intervention group (Brown 2013; Butler 2007; Kaleita 2006; Rapp 2015). The other pharmacological study used an open‐label design (Gehring 2012a) and was at a high risk of bias. The three non‐pharmacological studies (Locke 2008; Richard 2019; Voss 2022) were at a high risk of bias; blinding of participants to the intervention was not possible due to the nature of the intervention, and in two of these studies blinding of assessors was also not carried out (Locke 2008; Voss 2022).
Incomplete outcome data
The majority of studies included reasons for participant dropout, which were unlikely to be related to the outcomes and all studies were judged at a low risk of bias. Three studies performed intention‐to‐treat analyses (Brown 2013; Butler 2007; Rapp 2015). One study was terminated early (Butler 2007). Three studies recruited the projected number of participants (Brown 2013; Kaleita 2006; Voss 2022). However, no study was able to include the number of participants required to reach the desired statistical power due to withdrawal from participation as a result of patient death, tumour progression or withdrawal of consent. One study reported the use of a multiple imputation procedure via the Markov chain Monte Carlo method for patients still alive at the time of assessments; this included the imputation of scores for 47% of recruited participants at 24 weeks (Brown 2013). One study reported the use of an imputation method in all participants (Rapp 2015). Two studies carried out statistical comparisons between patients who withdrew consent, and those who remained in the study (Brown 2013; Butler 2007). Butler 2007 reported that patients who dropped out were significantly older and with worse performance status, but did not differ in any of the outcome measures. Brown 2013 reported that patients who could not complete cognitive assessments were more likely to have worse neurological function and a shorter survival time and had not undergone surgery or radiosurgery. In one study (Locke 2008), most patients did not return in‐person for follow‐up, instead electing for telephone follow‐up; as the cognitive test used (RBANS) cannot be administered via telephone, most patients did not complete the RBANS at follow‐up.
Selective reporting
All outcomes were reported by all included studies and therefore were all judged at a low risk of bias. It was noted that some studies did not report assessments carried out following withdrawal of the drug (Brown 2013; Butler 2007), and did not report interim assessments (Kaleita 2006; Rapp 2015).
Other potential sources of bias
An unclear risk of bias was reported for three studies. Due to a small sample size, two studies combined intervention groups of participants receiving different forms of the drug methylphenidate (Gehring 2012a) and different doses of modafinil (Kaleita 2006) when analysing results. One study did not perform statistical analyses on the cognitive data pre‐/post‐intervention, instead providing only descriptive data (Locke 2008).
Effects of interventions
In the original review, study interventions and comparisons were heterogenous, and were too different clinically to be able to pool the data. Following the addition of two additional studies in this updated review, the same issue still prevents pooling of data. Eight studies were identified for inclusion in the review. Due to differences in interventions and aim of interventions (prevention versus amelioration) investigated, including the use of drugs with different modes of actions and dosage schedules, and use of different non‐pharmacological interventions, the results are reviewed separately in this review.
Prevention
Pharmacological Studies
The large differences in the mode of action of the drugs investigated, and the unavailability of mean changes in scores, standard deviations or P values, meant pooling of the data was inappropriate. Therefore, results of the studies are reviewed separately and the data are reviewed as reported in the study.
Memantine versus placebo
Brown 2013 compared memantine, which acts on the glutamatergic system as an N‐Methyl‐D‐aspartate receptor antagonist, with placebo intervention in patients with brain metastases. Mean cognitive decline was reported for 280 participants at eight, 16 and 24 weeks assessments. The primary endpoint was Hopkins Verbal Learning Test‐Revised (HVLT‐R) at 24 weeks, but between‐group (n = 149) differences in decline did not reach significance (P =0.059); this was attributed to attrition. The memantine arm had significantly longer time to cognitive decline (hazard ratio (HR) 0.78, 95% confidence interval (CI) 0.62‐0.99, P = 0.01), which was a secondary endpoint; the probability of cognitive function failure at 24 weeks was 53.8% in the memantine arm and 64.9% in the placebo arm. Median change and interquartile ranges (IQRs) were also reported for individual neuropsychological tests at 24 weeks, which are summarised in Table 1. A cognitive functioning composite score was also calculated and the median change was ‐0.41 (IQR) ‐1.30 to 0.12) in the control group and ‐0.03 (IQR ‐0.90 to 0.72) in the intervention group at 24 weeks, which was reported to be significantly different (P = 0.02). This indicated a stability of cognitive function in the intervention group and a decline in the control group. The most common adverse events were fatigue, alopecia, nausea and headache; there was no difference in the adverse events reported between groups (risk ratio (RR) 1.00; 95% CI 0.76 to 1.32). It was noted that more participants in the memantine group were receiving steroids at entry into the study than the control group (P = 0.05), which may have indicated more symptoms and mass effect from brain metastases in the memantine group and would be expected to portend worse cognitive outcome at this time point, although this difference in steroid use did not continue over time.
d‐threo‐methylphenidate hydrochloride (d‐MPH) versus placebo
Butler 2007 evaluated d‐MPH, a central nervous system (CNS) stimulant that acts as a norepinephrine‐dopamine reuptake inhibitor, compared with placebo in patients with primary or metastatic brain tumours. Mean cognitive functioning, as assessed with the Mini‐Mental State Examination (MMSE), in the control group ranged from 26.5 (3.39 SD) to 27.8 (6.12 SD) at the end of radiation to 25.6 (11.54 SD) at eight weeks follow‐up. Mean overall cognitive functioning in the intervention group ranged from 27.2 (2.92 SD) at baseline to 26.4 (5.92 SD) at the end of radiation to 23.3 (10.46 SD) at eight weeks follow‐up. This difference was not significant and the standard deviation of mean change could not be calculated as P values were not reported. No other measures of cognitive performance were used, and it is noted that the MMSE is not considered a sensitive measure of cognitive function (Meyers 2003). Fatigue was the primary outcome in this study, but was not found to be significantly different between groups at eight weeks follow‐up of drug use (mean difference (MD) 3.30, 95% CI ‐10.37 to 16.97). Depression and quality of life were also assessed, reporting no significant difference between groups, although again P values were not provided. Four adverse events were reported in total, although they were not all reported specifically to the arm and therefore the risk ratio could not be calculated; two participants experienced nausea and vomiting, one participant experienced tachycardia (control arm) and one participant was withdrawn from the study due to an increase in liver enzymes.
Non‐Pharmacological Studies
Cognitive rehabilitation versus standard care
Locke 2008 evaluated a two‐week cognitive rehabilitation and problem‐solving program, compared with standard care in patients with primary brain tumours. Control participants were reported as more significantly impaired on a measure of immediate memory than intervention participants at baseline (P = 0.03). Using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), the mean total cognitive functioning score remained stable at 73 from baseline (SD 13.4) to post‐intervention (SD 9.3) in the control groups and improved from 79 (SD 20.0) at baseline to 80 (SD 18.6) at post‐intervention in the intervention group; a statistical comparison was not carried out. Mood, fatigue, quality of life and caregiver burden measures were also recorded, but again no statistical comparisons were made. The primary outcomes of the study were feasibility, strategy implementation and patient satisfaction; 7/8 of the intervention group participants were using strategies at least once per week at the eight‐week follow‐up and 7/8 of both the participants and the caregivers found the intervention 'very helpful'.
Calorie‐restricted ketogenic diet with intermittent fasting versus standard diet
Voss 2022 evaluated the effect of a calorie‐restricted ketogenic diet with intermittent fasting compared to a standard diet in patients with recurrent glioblastoma. Cognition was evaluated using the MMSE and d2 Test of Attention. Median MMSE scores were reported to not be significantly different between baseline and follow‐up for both groups (median = 29 points in both groups). However, baseline evaluation with the d2 Test of Attention revealed severely impaired median cognitive function percentile corrected for age, 5% (range: <1 to 42%) in the intervention group and 16% (range: <1 to 76%) in the standard diet group. At day 6, a non‐significant increase in d2 Test of Attention scores was found in both groups, with a median age‐corrected attention score of 12% in the intervention group (P = 0.187) and 23.5% in the standard diet group (P = 0.103). These scores increased significantly until one‐month follow‐up, with median age‐correct attention scores of 17% in the intervention group (P = 0.035) and 27% in the standard diet group (P = 0.049). Cognitive function was not affected by the diet. Of note, the standard diet group had a lower calorie intake than expected, 21 kcal per kg per day instead of 30 kcal per kg per day. Significant metabolic changes were found. Adverse events were reported in an original paper focusing on the primary outcome of the trial (Voss 2020); until day 12, nine adverse events were reported (four in the intervention group and five in the standard diet group), three of which were seizures and the remainder were headaches, nausea, and possible epileptic seizures. From day 12 until one month, 11 adverse events were reported (five in the intervention group, six in the standard diet group), the majority of which were epileptic seizures (five focal epileptic seizures).
Amelioration
Pharmacological Studies
Due to the differences in control groups or differences in the drug investigated, pooling of the data was inappropriate. Therefore, results of the studies are reviewed separately.
Modafinil at two different doses
Kaleita 2006, published as a conference abstract only, compared two doses of the CNS stimulant modafinil, 200 mg/day or 400 mg/day in divided doses, in patients with primary brain tumours. A significant improvement from baseline was seen at 12 weeks across all cognitive tests; Trail Making Test A (P = 0.002) and B (P < 0.0001), Verbal Fluency (P = 0.002) and Symbol Digit Modalities‐Oral (P = 0.006) and ‐Manual (P = 0.004). Significant differences were also found at eight weeks for all tests. Improvements in fatigue and mood were found at eight and 12 weeks. Adverse events were reported, however the distribution of adverse events between treatment arms was not reported. Thirteen participants experienced symptoms of headache, eight of insomnia, seven of dizziness, seven of dry mouth, five of depressed consciousness and four of nausea.
Methylphenidate versus modafinil
Gehring 2012a compared two CNS stimulants, in three treatment arms; sustained‐release methylphenidate, immediate‐release methylphenidate and modafinil in patients with primary brain tumours. The mean cognitive functioning scores comparison for each test is summarised in Analysis 4.2; Digit Span (MD 0.38; 95% CI 0.03 to 0.73), favouring methylphenidate, and Trail Making Test A (MD ‐2.48, 95% CI ‐4.82 to ‐0.14), favouring modafinil were the only tests to show a significant difference between groups. The two stimulant groups together demonstrated significant improvement of individual Trail Making Test B scores P = < 0.01), corrected for practice effects. Mood, fatigue, quality of life and sleep also significantly improved in both groups, but with no significant difference between groups (see Analysis 4.5; Analysis 4.6; Analysis 4.8; Analysis 4.9; Analysis 4.11). None of the participants who completed the study experienced adverse events in either treatment arm.
Donepezil versus placebo
Rapp 2015 compared donepezil, an acetylcholinesterase inhibitor, with placebo in patients with primary and metastatic brain tumours, and patients undergoing prophylactic cranial irradiation. The mean cognitive functioning score comparison for each test is reported in Analysis 5.2, based on raw data provided by the authors. The primary outcome was a calculated cognitive composite score, in which both groups were found to significantly improve after 24 weeks; there was no significant difference between groups (MD 0.03, 95% CI ‐0.06 to 0.13). A significant difference was found in scores of tests of long‐term memory recognition (MD 0.57, 95% CI 0.07 to 1.07), long‐term memory discrimination (MD 0.94, 95% CI 0.26 to 1.62), and dominant hand psychomotor functioning (MD ‐11.92, 95% CI ‐21.58 to ‐2.27) favouring donepezil. Furthermore, the benefits of donepezil were greater for those who were more cognitively impaired prior to study treatment. The commonest toxicity reported was fatigue (58% of those receiving donepezil and 67% receiving placebo), and the only toxicity that was significantly different between the two arms was diarrhoea (25% of those receiving donepezil and 9% receiving placebo; P = 0.005).
Non‐Pharmacological Studies
Goal Management Training versus active control and wait‐list control
Richard 2019 compared Goal Management Training, an active control (Brain Health Program) and a wait‐list control group in patients with primary brain tumours. The comparison in group changes from baseline to immediately post‐training and four‐month follow‐up for the cognitive performance measures is summarised in Table 2, and analyses for the cognitive performance measures (executive functioning composite, memory composite and processing speed) as well as the self‐reported outcome measures are shown in Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4; Analysis 6.5; Analysis 6.6. A significant intervention effect on the executive composite was observed at four months follow‐up (with a time‐by‐group interaction F(2,16) = 3.760, P = 0.046, partial eta‐squared value = 0.320), which reflected a large improvement in the Goal Management Training group (effect size = 1.08, P = 0.002, corrected effect size = 1.09) and minimal change in the Brain Health Program (effect size = 0.09, P > 0.1) and wait‐control (effect size = ‐0.06, P > 0.1) groups. There was a statistically significant difference in the proportion of participants that scored in the impaired range on one or more tests of executive functioning between groups by follow‐up (Goal Management Training: 91% at baseline to 20% at four months; Brain Health Program, 88% at baseline to 67% at four months; wait‐list control, 100% throughout; chi‐square value = 7.234, P = 0.027). For the memory composite, all groups improved at follow‐up (main effect of time: F(1,16) = 6.435, P < 0.001, partial eta‐squared = 0.524). Effect sizes were moderate to large, although within‐group simple effects tests for the Brain Health Program and wait‐list control groups were not significant (Goal Management Training effect size = 1.37, P > 0.002; Brain Health Program effect size = 0.74, P = 0.093; wait‐list control effect size = 1.06, P > 0.1). Regarding processing speed, at follow‐up the Goal Management Training group had moderate gains relative to baseline (effect size = 0.61, P = 0.015, corrected effect size = 0.68), whereas no improvement was observed in the Brain Health Program (effect size = ‐0.09, P > 0.1) and wait‐list control (effect size = ‐0.30, P > 0.1) groups. Both the Goal Management Training and Brain Health Program groups reported fewer cognitive concerns immediately post‐training (P = 0.049) and at 4‐months follow‐up (P < 0.001).
Discussion
Summary of main results
The aim of this review was to evaluate the effect of any pharmacological or non‐pharmacological intervention on cognitive functioning during or following cranial irradiation. In the original review, we included three randomised trials for the prevention of cognitive deficits, recruiting a total of 641 heterogeneous patients and three randomised trials for the amelioration of cognitive deficits, recruiting a total of 199 heterogeneous patients. In this update, we included an additional randomised trial for preventing cognitive deficits that recruited 50 patients and an additional trial for ameliorating cognitive defects that randomised 25 patients.
Prevention
The two trials that compared drug versus placebo used very different drug agents, dosage schedules and time points for follow‐up; a meta‐analysis was therefore inappropriate.
Brown 2013 compared memantine in a large sample of 554 patients with brain metastases from 143 centres across the USA and Canada, and carried out imputation in participants who had not withdrawn from the study as a result of death. The primary outcome measure, memory as measured using the Hopkins Verbal Learning Test‐Revised (HVLT‐R for Delayed Recall) at 23 weeks, showed a non‐significantly reduced rate of decline in the memantine group. Significant differences between groups were found in other cognitive domains.
Butler 2007 compared d‐threo‐methylphenidate hydrochloride (d‐MPH) in a small sample of 68 patients with primary and metastatic brain tumours with a high drop‐out rate; no difference was found between the intervention group and control group in the primary outcome (fatigue), or in cognitive functioning, depression and quality of life.
The one prevention trial that investigated a cognitive rehabilitation and problem‐solving program, compared with standard care, had the primary aim of assessing the tolerability and feasibility of the intervention (Locke 2008). Therefore, no statistical comparisons were made of the difference between groups in cognitive functioning. Consequently, this study provides very little evidence for the prevention of cognitive deficits, but did offer a tolerable and feasible intervention for further investigation.
Voss 2022 compared calorie‐restricted ketogenic diet and intermittent fasting to standard diet in 50 patients undergoing re‐irradiation for glioblastoma. The study's primary endpoint measure was progression‐free survival at six months (reported in a different manuscript), and cognitive functioning was evaluated as one of several secondary endpoints. The study found no significant change in the primary endpoint measure, or in median Mini‐Mental State Examination (MMSE) scores in either group, at baseline and at follow‐up. Attention was severely impaired at baseline but increased significantly until follow‐up at one month in both groups. Cognitive function was not affected by the diet, and the standard diet group had a lower calorie intake than expected, which complicated interpretation of the results.
Amelioration
The three pharmacological trials that investigated the amelioration of cognitive deficits used different drug agents and/or control groups and different time points for follow‐up; a meta‐analysis was therefore inappropriate.
Gehring 2012a compared two forms of methylphenidate with modafinil, in a small sample of 24 mostly glioma patients. Inconsistent, differential effects were found in cognitive performance between groups in attention, favouring methylphenidate, and processing speed, favouring modafinil. However, when treatment groups were combined, and after corrections for practice effects, there was evidence of a beneficial effect on test performance in speed of processing and executive function requiring divided attention.
Kaleita 2006, published as a conference abstract, also combined intervention arms in a study of 30 patients with mainly gliomas and found an overall significant improvement (but without correction of practice effects) in all cognitive assessments at eight and 12 weeks, compared to baseline.
Rapp 2015 with a larger sample size (146 patients, from 24 sites, with primary or metastatic brain tumours or receiving prophylactic cranial irradiation) than either Gehring 2012a or Kaleita 2006 compared donepezil with placebo. Treatment with donepezil did not significantly improve the primary outcome measure, a cognitive composite score, but did result in modest improvements in several cognitive functions, especially among patients with greater pre‐treatment impairments. However, the clinical relevance of some of these improvements, particularly in the HVLT‐R, are unclear.
Only one study investigated the amelioration of cognitive deficits via a non‐pharmacological intervention. Richard 2019 compared two active interventions, Goal Management Training and Brain Health Program, to a wait‐list control group in a group of 25 patients with primary brain tumours from a single Canadian centre. The study found executive functions improved only in the Goal Management Training group and were maintained at four months follow‐up. Processing speed also improved in the Goal Management Training and Brain Health Program groups, although this was only maintained at four months in the Goal Management Training group. All three groups improved in the memory tests, suggesting a practice effect.
Overall completeness and applicability of evidence
We included eight randomised controlled trials (RCTs) examining the efficacy of interventions for the prevention or amelioration of cognitive deficits in adults treated with cranial irradiation. Due to differences in drug agents, it was inappropriate to combine data into a meta‐analysis.
The evidence identified in this review is insufficient to address the objective of this review and to make generalisations about the applicability (external validity) of the described interventions to the wider population of patients with brain tumours that have received cranial irradiation. This is due in part to the low sample sizes of several of the included studies, as well as the wide heterogeneity in study populations, interventions, study designs, outcome measures and timing of assessments. Most of the included studies were restricted by these and the following limitations, which further reduced both the internal and external validity of the evidence; it is also of note that the completeness of the evidence is hampered by the lack of randomised studies evaluating interventions such as exercise therapy, hyperbaric oxygen therapy and dietary supplements.
Several of the studies included in this review were at high risk of bias in at least one domain. Generally, the non‐pharmacological studies were more likely to be at high risk of bias due to the nature of the intervention and the inability to provide a placebo condition. This negatively affected the internal validity of the included studies.
Many studies were limited by low accrual. Studies that recruited from few centres were unable to reach the necessary number of participants required for sufficient statistical power (Butler 2007; Gehring 2012a; Kaleita 2006; Locke 2008). It may also be that patients may be reluctant to take any additional medication, especially if they are not subjectively experiencing cognitive impairment at the time of enrolment.
Most studies were limited by high attrition rates. One study was able to recruit the projected participants required for 80% statistical power with the involvement of 143 centres (Brown 2013). However, 34% of participants died at 24 weeks of drug use and 47% of the remaining 280 participants missed their assessment appointment. It is important to understand the reasons for missed appointments. For example, in studies that focus on prevention rather than treatment, a lack of interest may be found in patients not experiencing cognitive impairment at that stage. Therefore, it is important to make sufficient attempts to keep patients engaged in study participation, such as by emphasising the importance of ongoing cognitive function assessment. It is also notable that, in some studies, patients withdrawing from the trial tended to be older, and/or had worse performance status/neurological function and/or had shorter survival.
Differences in the time points at which cognitive functioning is measured are also present, both in pharmacological and non‐pharmacological intervention studies. One study carried out assessments at baseline, and at four weeks of modafinil or methylphenidate use (Gehring 2012a), whereas another continued to follow up patients at eight, 16, 24 and 52 weeks following initiation of the drug memantine (Brown 2013). In cognitive rehabilitation studies, patients were assessed at baseline and at the end of a two‐week intervention and at three months (Locke 2008). These studies also demonstrate the variations in duration of the intervention.
Only one study mentioned specifically attempting to minimise practice effects (Gehring 2012a).
A number of assessment tools were used to evaluate cognitive function in the included studies, ranging from a battery of numerous tests (Brown 2013; Gehring 2012a; Kaleita 2006; Rapp 2015; Richard 2019), to the MMSE and d2 Test of Attention (Voss 2022), or the MMSE (Butler 2007) or RBANS (Locke 2008) alone. The use of a single cognitive outcome measure is unlikely to fully and comprehensively represent a participant's cognitive status. In addition, although neuropsychological tests provide objective evidence of cognitive performance, it is largely unclear whether any improvements in test scores translate to genuine long‐term benefits in everyday life/quality of life. More extensive and longer‐term study of neuropsychological performance alongside self‐ and caregiver‐reported measures are required to elucidate further on this issue.
Failure to report all study and patient characteristics meant that limitations remained a concern. The lack of adequately presented data, or lack of reporting of mean differences and standard deviation or P values, meant that the reporting of results as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) could not be carried out in five studies (Brown 2013; Butler 2007; Kaleita 2006; Locke 2008; Voss 2022). Further correspondence to obtain these data were unsuccessful. The use and changes in use of medications associated with cognitive functioning were rarely reported. Only one study reported the use, and change in use of steroids during participation (Brown 2013), and reported that more intervention participants were receiving steroids at study entry than control participants. No studies reported the use, or change in use of anti‐epileptic drugs during participation. As these drugs may also play a role in cognitive functioning (Kirkman 2022), findings may be attributable to changes in these medications during the study period. Assessment of cognitive functioning following withdrawal of the drug under study may have provided additional information relating to the efficacy of the drug. Two studies carried out post‐drug withdrawal assessments (Brown 2013; Butler 2007), but these data were not reported.
Quality of the evidence
Overall, this review summarises limited evidence for the effect of pharmacological and non‐pharmacological interventions for the prevention or amelioration of cognitive deficits in adults treated with cranial irradiation. Two of the studies were at high risk of bias in three or more domains, with additional domains at an unclear risk of bias (Gehring 2012a; Locke 2008). Blinding was not carried out in four studies (Gehring 2012a; Locke 2008; Richard 2019; Voss 2022) and, in two studies, randomisation could not be concluded (Gehring 2012a) or was abandoned (Locke 2008). Four RCTs were at a low risk of bias across all (Brown 2013; Butler 2007; Rapp 2015) or almost all (Richard 2019) domains.
Although a formal GRADE assessment was not conducted as part of this review, it is clear from the above evaluation of the studies that the evidence identified is largely of 'low' and 'very low' certainty, indicating that any new evidence is likely to have an impact on the existing results; this reinforces the need for further high‐quality studies evaluating interventions to prevent and improve cognitive dysfunction in adult patients that have received cranial irradiation.
Potential biases in the review process
We carried out extensive searches in four databases, which included published studies and conference proceedings as well as searching the reference lists of included studies. However, we may have failed to identify all eligible studies. We were somewhat successful in our attempts to obtain further information about the methodology of the studies by emailing the authors. Overall, our attempts to obtain further data on the included studies did not allow us to report all studies as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Agreements and disagreements with other studies or reviews
In the search results we found one other review reporting interventions for preventing or ameliorating cognitive deficits in adults treated with cranial irradiation (Tallet 2013), but did not find any additional studies from the review that were eligible to include in this review.
Other reviews identified were associated more generally with all brain tumour patients, all cancer patients, or more widely in all clinical conditions, who had not necessarily received cranial irradiation. For example, Gehring 2008 conducted a systematic review of interventions for cognitive deficits in patients with all types of brain tumour. Davis 2013 and Gehring 2012b conducted reviews of ongoing studies investigating pharmacological interventions to treat cancer patients with cognitive dysfunction. Rooney 2014 reviewed pharmacological interventions to treat or prevent neurocognitive decline after brain radiation. Challman 2000 reviewed the use of methylphenidate in many clinical conditions, including patients with a brain tumour. Wefel 2015 reviewed evidence relating to cognitive impairment in patients with non‐CNS tumours and focused on patients with breast cancer. Coomans 2019 reviewed studies of antitumour treatment strategies that aimed to prevent or minimise cognitive deficits in brain tumour patients and incorporated all treatment modalities, including surgery and chemotherapy. Karschnia 2019 focused on the pharmacologic management of cognitive impairment induced by cancer therapy.
The studies included in these reviews did not provide additional evidence specifically for cranial irradiated patients. Similar conclusions were made, emphasising that the evidence is limited by significant methodological limitations; such as the absence of a control group, lack of statistical comparisons between groups when control groups were present (due to small sample sizes), the use of unreliable cognitive tests, and the overall need for larger trials with more statistical power. Gehring 2012b suggested the use of home assessments and/or Internet‐based programmes as potential solutions to help with accrual and attrition.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Cognitive functioning composite score | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Brown 2013 | |||||
Comparison 1: Prevention: Memantine versus placebo, Outcome 1: Cognitive functioning composite score
| Cognitive functioning sub‐tests | |||||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 | Heading 6 | Heading 7 |
|---|---|---|---|---|---|---|---|
| Short‐term verbal memory | |||||||
| Brown 2013 | |||||||
| Long‐term verbal memory (recall) | |||||||
| Brown 2013 | |||||||
| Long‐term verbal memory (recognition) | |||||||
| Brown 2013 | |||||||
| Trail making test A | |||||||
| Brown 2013 | |||||||
| Trail making test B | |||||||
| Brown 2013 | |||||||
| Verbal fluency | |||||||
| Brown 2013 | |||||||
Comparison 1: Prevention: Memantine versus placebo, Outcome 2: Cognitive functioning sub‐tests

Comparison 1: Prevention: Memantine versus placebo, Outcome 3: Adverse events
| General cognitive functioning | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Butler 2007 | |||||
Comparison 2: Prevention: d‐threo‐methylphenidate versus placebo, Outcome 1: General cognitive functioning
| Depression | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Butler 2007 | |||||
Comparison 2: Prevention: d‐threo‐methylphenidate versus placebo, Outcome 2: Depression

Comparison 2: Prevention: d‐threo‐methylphenidate versus placebo, Outcome 3: Fatigue
| Quality of life | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| FACT | |||||
| Butler 2007 | |||||
| Brain subscale | |||||
| Butler 2007 | |||||
Comparison 2: Prevention: d‐threo‐methylphenidate versus placebo, Outcome 4: Quality of life
| Cognitive functioning | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Locke 2008 | |||||
Comparison 3: Prevention: Cognitive rehabilitation versus standard care, Outcome 1: Cognitive functioning
| Cognitive functioning sub‐test | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Attention | |||||
| Locke 2008 | |||||
| Short‐term verbal memory | |||||
| Locke 2008 | |||||
| Short‐term visual memory | |||||
| Locke 2008 | |||||
| Long‐term verbal/visual memory | |||||
| Locke 2008 | |||||
| Language | |||||
| Locke 2008 | |||||
Comparison 3: Prevention: Cognitive rehabilitation versus standard care, Outcome 2: Cognitive functioning sub‐test
| Mood | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Locke 2008 | |||||
Comparison 3: Prevention: Cognitive rehabilitation versus standard care, Outcome 3: Mood
| Fatigue | |||||
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Locke 2008 | |||||
Comparison 3: Prevention: Cognitive rehabilitation versus standard care, Outcome 4: Fatigue

Comparison 3: Prevention: Cognitive rehabilitation versus standard care, Outcome 5: Functional capacity

Comparison 3: Prevention: Cognitive rehabilitation versus standard care, Outcome 6: Quality of life

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 1: Cognitive functioning (calculated score)

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 2: Cognitive functioning (timed tasks)

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 3: Cognitive functioning (total score)

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 4: Self‐reported confusion

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 5: Anxiety

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 6: Depression

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 7: Anger

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 8: Fatigue

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 9: Sleep

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 10: Activity

Comparison 4: Amelioration: Methylphenidate versus modafinil, Outcome 11: Quality of life

Comparison 5: Amelioration: Donepezil versus placebo, Outcome 1: Cognitive composite score

Comparison 5: Amelioration: Donepezil versus placebo, Outcome 2: Cognitive functioning (timed tasks)

Comparison 5: Amelioration: Donepezil versus placebo, Outcome 3: Cognitive functioning (total score)

Comparison 6: Amelioration: Goal management training versus brain health program versus wait‐listed control, Outcome 1: Cognitive performance measures: Goal management training versus wait‐list control

Comparison 6: Amelioration: Goal management training versus brain health program versus wait‐listed control, Outcome 2: Cognitive performance measures: Goal management training versus brain health program

Comparison 6: Amelioration: Goal management training versus brain health program versus wait‐listed control, Outcome 3: Cognitive performance measures: Brain health program versus wait‐list control

Comparison 6: Amelioration: Goal management training versus brain health program versus wait‐listed control, Outcome 4: Patient‐reported outcome measures: Goal management training versus wait‐list control

Comparison 6: Amelioration: Goal management training versus brain health program versus wait‐listed control, Outcome 5: Patient‐reported outcome measures: Goal management training versus brain health program

Comparison 6: Amelioration: Goal management training versus brain health program versus wait‐listed control, Outcome 6: Patient‐reported outcome measures: Brain health program versus wait‐list control
| Cognitive functioning measure (standardised scores) | Memantine | Placebo |
P | ||
| N | Median change after 24 weeks (IQR) | N | Median change after 24 weeks (IQR) | ||
| Short‐term verbal memory | 77 | ‐0.23 (‐1.16 to 0.70) | 90 | ‐0.415 (‐1.86 to 0.46) | 0.21 |
| Long‐term verbal memory (recall) | 76 | 0 (‐1.67 to 0.59) | 90 | ‐0.90 (‐2.22 to 0.55) | 0.06 |
| Long‐term verbal memory (recognition) | 76 | 0 (‐1.12 to 1.43) | 90 | ‐0.72 (‐2.73 to 0.71) | 0.01* |
| Verbal Fluency | 78 | ‐0.10 (‐0.62 to 0.53) | 90 | ‐0.16 (‐0.83 to 0.61) | 0.31 |
| Trail Making Test A | 76 | 0.08 (‐1.01 to 1.82) | 92 | ‐0.37 (‐2.08 to 0.50) | 0.02* |
| Trail Making Test B | 74 | ‐0.45 (‐2.37 to 1.04) | 90 | ‐0.49 (‐2.60 to 0.62) | 0.30 |
| Cognitive composite score | 73 | ‐0.03 (‐0.90 to 0.72) | 90 | ‐0.41 (‐1.30 to 0.12) | 0.02* |
| * P < 0.05 IQR: interquartile range | |||||
| Cognitive performance measure | Goal Management Training | Brain Health Program | Wait‐listed control | P | Goal Management Training | Brain Health Program | Wait‐listed control | P | ||||||
| N | Domain composite score change post‐training compared to baseline (SD) | N | Domain composite score change post‐training compared to baseline (SD) | N | Domain composite score change post‐training compared to baseline (SD) |
| N | Domain composite score change at 4‐month follow‐up compared to baseline (SD) | N | Domain composite score change at 4‐month follow‐up compared to baseline (SD) | N | Domain composite score change at 4‐month follow‐up compared to baseline (SD) |
| |
| Executive composite | 10 | 0.33 (0.54) | 6 | 0.08 (0.43) | 4 | ‐0.44 (0.45) | 0.077 | 10 | 0.69 (0.51) | 6 | 0.13 (0.50) | 3 | ‐0.07 (0.44) | 0.046 |
| Memory composite | 10 | 0.40 (0.84) | 6 | 0.11 (0.91) | 4 | 0.43 (0.76) | 0.778 | 10 | 1.08 (0.82) | 6 | 0.86 (1.01) | 3 | 0.84 (0.52) | 0.842 |
| Processing speed | 10 | 0.44 (0.55) | 6 | 0.47 (0.43) | 4 | ‐0.65 (1.53) | 0.071 | 10 | 0.54 (0.57) | 6 | ‐0.14 (0.94) | 3 | ‐0.18 (1.03) | 0.179 |
| SD: standard deviation P values refer to time‐by‐group interaction (intervention) effects on change compared to baseline, using analysis of variance | ||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Cognitive functioning composite score Show forest plot | 1 | Other data | No numeric data | |
| 1.2 Cognitive functioning sub‐tests Show forest plot | 1 | Other data | No numeric data | |
| 1.2.1 Short‐term verbal memory | 1 | Other data | No numeric data | |
| 1.2.2 Long‐term verbal memory (recall) | 1 | Other data | No numeric data | |
| 1.2.3 Long‐term verbal memory (recognition) | 1 | Other data | No numeric data | |
| 1.2.4 Trail making test A | 1 | Other data | No numeric data | |
| 1.2.5 Trail making test B | 1 | Other data | No numeric data | |
| 1.2.6 Verbal fluency | 1 | Other data | No numeric data | |
| 1.3 Adverse events Show forest plot | 1 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.76, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 General cognitive functioning Show forest plot | 1 | Other data | No numeric data | |
| 2.2 Depression Show forest plot | 1 | Other data | No numeric data | |
| 2.3 Fatigue Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐10.37, 16.97] |
| 2.4 Quality of life Show forest plot | 1 | Other data | No numeric data | |
| 2.4.1 FACT | 1 | Other data | No numeric data | |
| 2.4.2 Brain subscale | 1 | Other data | No numeric data | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Cognitive functioning Show forest plot | 1 | Other data | No numeric data | |
| 3.2 Cognitive functioning sub‐test Show forest plot | 1 | Other data | No numeric data | |
| 3.2.1 Attention | 1 | Other data | No numeric data | |
| 3.2.2 Short‐term verbal memory | 1 | Other data | No numeric data | |
| 3.2.3 Short‐term visual memory | 1 | Other data | No numeric data | |
| 3.2.4 Long‐term verbal/visual memory | 1 | Other data | No numeric data | |
| 3.2.5 Language | 1 | Other data | No numeric data | |
| 3.3 Mood Show forest plot | 1 | Other data | No numeric data | |
| 3.4 Fatigue Show forest plot | 1 | Other data | No numeric data | |
| 3.5 Functional capacity Show forest plot | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐8.55 [‐22.57, 5.47] |
| 3.6 Quality of life Show forest plot | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 6.00 [‐21.55, 33.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Cognitive functioning (calculated score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1.1 Attention (Digit Span) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.03, 0.73] |
| 4.1.2 Speed of processing (Digit symbol) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.30, 1.04] |
| 4.2 Cognitive functioning (timed tasks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.2.1 Speed of processing (TMTA) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐4.82, ‐0.14] |
| 4.2.2 Executive function | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐0.50, 1.88] |
| 4.2.3 Psychomotor functioning (dominant hand) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐1.36, 2.58] |
| 4.2.4 Psychomotor functioning (non‐dominant hand) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐5.38 [‐51.16, 40.40] |
| 4.3 Cognitive functioning (total score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.3.1 Verbal fluency | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.16, 0.82] |
| 4.3.2 Short‐term verbal memory | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.35, 1.19] |
| 4.3.3 Long‐term verbal memory (recall) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐2.00, 1.24] |
| 4.3.4 Long‐term verbal memory (recognition) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 1.62 [‐0.56, 3.80] |
| 4.4 Self‐reported confusion Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.76, 2.68] |
| 4.5 Anxiety Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.5.1 STAI State anxiety | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐11.66, 8.96] |
| 4.5.2 STAI Trait anxiety | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐22.21, 20.55] |
| 4.5.3 POMS anxiety | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 3.56 [‐0.37, 7.49] |
| 4.6 Depression Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.6.1 BDI‐II | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐1.93, 2.81] |
| 4.6.2 POMS depression‐dejection | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.48, 1.22] |
| 4.7 Anger Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐5.99, 4.99] |
| 4.8 Fatigue Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.8.1 Brief Fatigue Inventory | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐12.56, 2.16] |
| 4.8.2 POMS‐Fat | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.30, 1.76] |
| 4.9 Sleep Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.39 [‐6.27, 1.49] |
| 4.10 Activity Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.94 [‐1.79, 5.67] |
| 4.11 Quality of life Show forest plot | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 2.71 [‐14.46, 19.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Cognitive composite score Show forest plot | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.05, 0.11] |
| 5.2 Cognitive functioning (timed tasks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Speed of processing | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐1.82 [‐11.49, 7.85] |
| 5.2.2 Executive function | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | 1.61 [‐15.98, 19.20] |
| 5.2.3 Psychomotor functioning (dominant hand) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐11.93 [‐21.51, ‐2.35] |
| 5.2.4 Psychomotor functioning (non‐dominant hand) | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐5.21, 5.19] |
| 5.3 Cognitive functioning (total score) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 Attention (Digit span (total)) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.12, 0.80] |
| 5.3.2 Attention (Digit span (backward)) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.01, 0.21] |
| 5.3.3 Attention (Digit Span (forward)) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.57, 0.69] |
| 5.3.4 Verbal fluency | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐3.19, 1.35] |
| 5.3.5 Short‐term verbal memory | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.93, 1.57] |
| 5.3.6 Long‐term verbal memory (recall) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.36, 1.06] |
| 5.3.7 Long‐term verbal memory (retention) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 3.96 [‐4.40, 12.32] |
| 5.3.8 Long‐term verbal memory (recognition) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [0.07, 1.07] |
| 5.3.9 Long‐term verbal memory (discrimination) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [0.27, 1.61] |
| 5.3.10 Short‐term visual memory (copy) | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.63, 0.25] |
| 5.3.11 Short‐term visual memory (recall) | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.51, 0.51] |
| 5.3.12 Long‐term visual memory (retention) | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.48, 0.70] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Cognitive performance measures: Goal management training versus wait‐list control Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1.1 Executive composite ‐ immediately post‐training compared to baseline | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [0.22, 1.32] |
| 6.1.2 Executive composite ‐ 4 months compared to baseline | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.17, 1.35] |
| 6.1.3 Memory composite ‐ immediately post‐training compared to baseline | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.94, 0.88] |
| 6.1.4 Memory composite ‐ 4 months compared to baseline | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.54, 1.02] |
| 6.1.5 Processing speed ‐ immediately post‐training compared to baseline | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 1.09 [‐0.45, 2.63] |
| 6.1.6 Processing speed ‐ 4 months compared to baseline | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [‐0.50, 1.94] |
| 6.2 Cognitive performance measures: Goal management training versus brain health program Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.2.1 Executive composite ‐ immediately post‐training compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.23, 0.73] |
| 6.2.2 Executive composite ‐ 4 months compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.05, 1.07] |
| 6.2.3 Memory composite ‐ immediately post‐training compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.61, 1.19] |
| 6.2.4 Memory composite ‐ 4 months compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.73, 1.17] |
| 6.2.5 Processing speed ‐ immediately post‐training compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.51, 0.45] |
| 6.2.6 Processing speed ‐ 4 months compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.68 [‐0.15, 1.51] |
| 6.3 Cognitive performance measures: Brain health program versus wait‐list control Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 Executive composite ‐ immediately post‐training compared to baseline | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.04, 1.08] |
| 6.3.2 Executive composite ‐ 4 months compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.44, 0.84] |
| 6.3.3 Memory composite ‐ immediately post‐training compared to baseline | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐1.36, 0.72] |
| 6.3.4 Memory composite ‐ 4 months compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.98, 1.02] |
| 6.3.5 Processing speed ‐ immediately post‐training compared to baseline | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 1.12 [‐0.42, 2.66] |
| 6.3.6 Processing speed ‐ 4 months compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐1.35, 1.43] |
| 6.4 Patient‐reported outcome measures: Goal management training versus wait‐list control Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.4.1 Cognitive symptoms composite ‐ immediately post‐training compared to baseline | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.23, 1.19] |
| 6.4.2 Cognitive symptoms composite ‐ 4 months compared to baseline | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.24, 0.74] |
| 6.4.3 Emotional functioning composite ‐ immediately post‐training compared to baseline | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.24, 0.78] |
| 6.4.4 Emotional functioning composite ‐ 4 months compared to baseline | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.73, 0.65] |
| 6.4.5 Coping/adjusting composite ‐ immediately post‐training compared to baseline | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.69, 0.83] |
| 6.4.6 Coping/adjusting composite ‐ 4 months compared to baseline | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.82, 0.44] |
| 6.5 Patient‐reported outcome measures: Goal management training versus brain health program Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.5.1 Cognitive symptoms composite ‐ immediately post‐training compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.68, 0.12] |
| 6.5.2 Cognitive symptoms composite ‐ 4 months compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.05, ‐0.23] |
| 6.5.3 Emotional functioning composite ‐ immediately post‐training compared to baseline | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.51, 0.11] |
| 6.5.4 Emotional functioning composite ‐ 4 months compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.69, 0.11] |
| 6.5.5 Coping/adjustment composite ‐ immediately post‐training compared to baseline | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 6.5.6 Coping/adjustment composite ‐ 4 months compared to baseline | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.60, 0.12] |
| 6.6 Patient‐reported outcome measures: Brain health program versus wait‐list control Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.6.1 Cognitive symptoms composite ‐ immediately post‐training compared to baseline | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.04, 1.48] |
| 6.6.2 Cognitive symptoms composite ‐ 4 months compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.89 [0.34, 1.44] |
| 6.6.3 Emotional functioning composite ‐ immediately post‐training compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐0.04, 0.98] |
| 6.6.4 Emotional functioning composite ‐ 4 months compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.39, 0.89] |
| 6.6.5 Coping/adjustment composite ‐ immediately post‐training compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.61, 0.89] |
| 6.6.6 Coping/adjustment composite ‐ 4 months compared to baseline | 1 | 9 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.57, 0.67] |


