Fulvestrant for hormone‐sensitive metastatic breast cancer
Información
- DOI:
- https://doi.org/10.1002/14651858.CD011093.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 enero 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer de mama
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Clara Lee (CL): screened studies for eligibility, extracted and analysed data, and wrote the review.
Annabel Goodwin (AG): cowrote the second version of the protocol and provided comments, oversight, and feedback on the full review.
Nicholas Wilcken (NW): cowrote the second version of the protocol, screened studies for eligibility, provided oversight for the review, and wrote the review.
Sources of support
Internal sources
-
None, Other.
External sources
-
None, Other.
Declarations of interest
CL: None to disclose associated with this review. Other, unrelated funding has been received from Novartis, Sanofi, Ipsen, Amgen, Roche (conference support) and honoraria from Roche.
AG: Roche virtual ASCO 2015 and 2016 subscriptions. Advisory board for Novartis 2015.
NW: Honoraria from Roche. On advisory boards for Novartis, Pfizer, Roche.
Acknowledgements
We wish to acknowledge the advice received from Dianne O'Connell and Jessica Barrett. We also acknowledge the authors that were involved in the first draft of this protocol and are not involved in further developments of the protocol and review stage: E Amir, J Beyene, P Shah, and H Chen. We acknowledge Orit Freedman and Mark Clemons for their contribution during the protocol phase of our review. We also thank Melina Willson for her invaluable contribution and guidance in the processes and conduct of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Jan 03 | Fulvestrant for hormone‐sensitive metastatic breast cancer | Review | Clara I Lee, Annabel Goodwin, Nicholas Wilcken | |
| 2014 May 01 | Fulvestrant for hormone‐sensitive metastatic breast cancer | Protocol | Clara I Lee, Annabel Goodwin, Orit Freedman, Mark Clemons, Nicholas Wilcken | |
Differences between protocol and review
We revised comparator 2 to "any other standard anticancer agent".
We selected three toxicities for analysis: vasomotor, anthralgia, and gynaecological toxicity based on clinical judgement and prior to review of the results.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anastrozole;
- Antineoplastic Agents, Hormonal [adverse effects, *therapeutic use];
- Breast Neoplasms [*drug therapy, mortality, pathology];
- Disease‐Free Survival;
- Estradiol [adverse effects, *analogs & derivatives, therapeutic use];
- Fulvestrant;
- Neoplasms, Hormone‐Dependent [drug therapy];
- Nitriles [therapeutic use];
- Randomized Controlled Trials as Topic;
- Triazoles [therapeutic use];
Medical Subject Headings Check Words
Female; Humans;
PICO
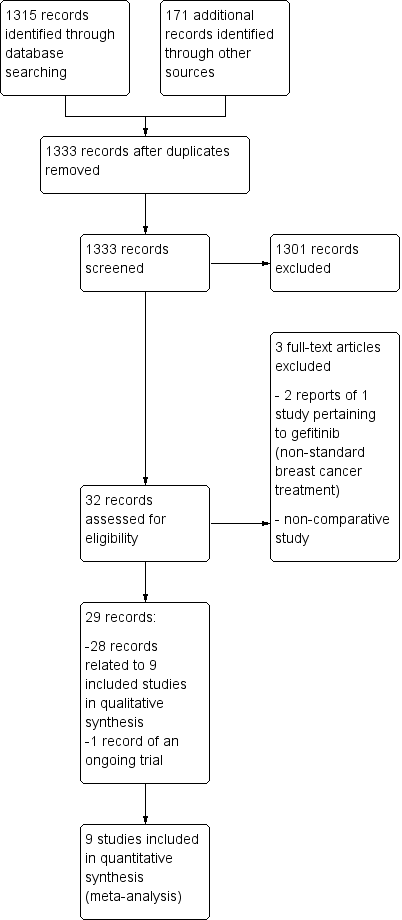
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Progression‐free survival, outcome: 1.1 Fulvestrant vs other endocrine therapy.

Forest plot of comparison: 2 Clinical benefit rate, outcome: 2.1 Fulvestrant vs other endocrine therapy.
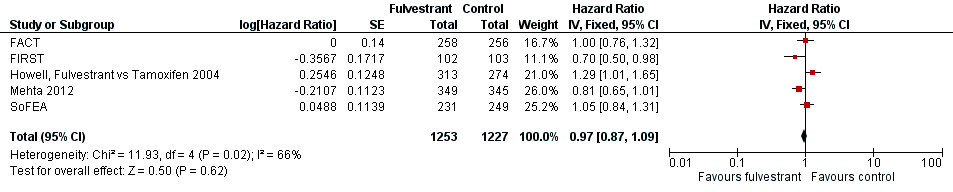
Forest plot of comparison: 3 Overall survival, outcome: 3.1 Fulvestrant vs other endocrine therapy.
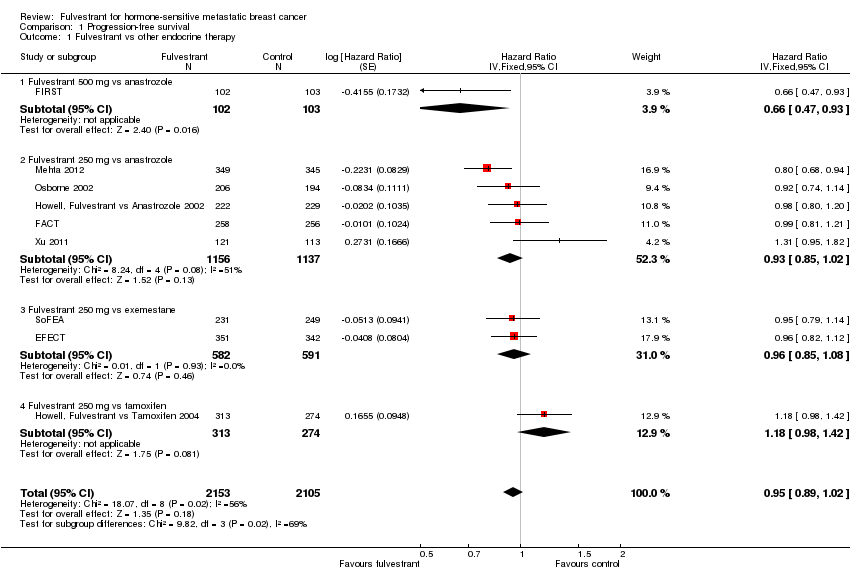
Comparison 1 Progression‐free survival, Outcome 1 Fulvestrant vs other endocrine therapy.

Comparison 1 Progression‐free survival, Outcome 2 First‐line vs second‐line or more.

Comparison 1 Progression‐free survival, Outcome 3 Combination fulvestrant vs single agent with other endocrine therapy.
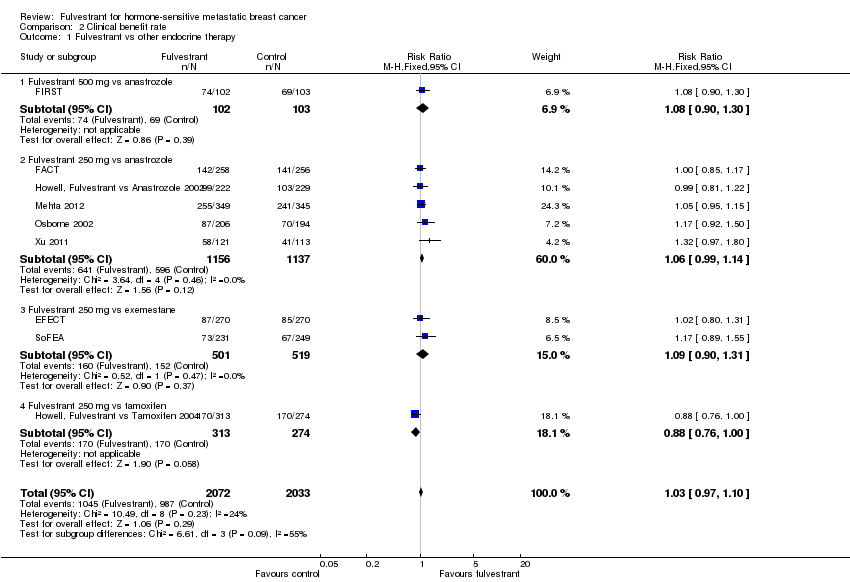
Comparison 2 Clinical benefit rate, Outcome 1 Fulvestrant vs other endocrine therapy.

Comparison 2 Clinical benefit rate, Outcome 2 Clinical benefit rate (first‐line vs second‐line).

Comparison 2 Clinical benefit rate, Outcome 3 Combination vs monotherapy fulvestrant.
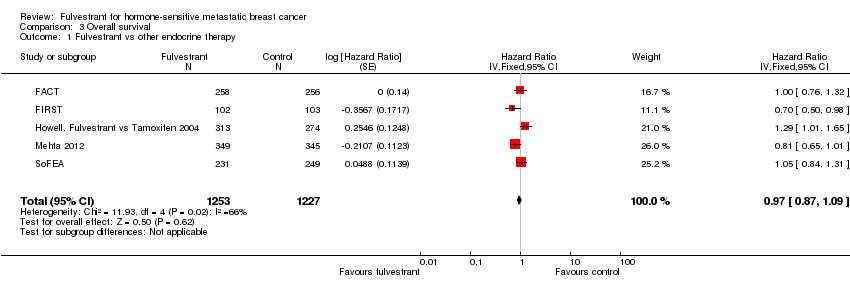
Comparison 3 Overall survival, Outcome 1 Fulvestrant vs other endocrine therapy.

Comparison 4 Toxicity, Outcome 1 Vasomotor.
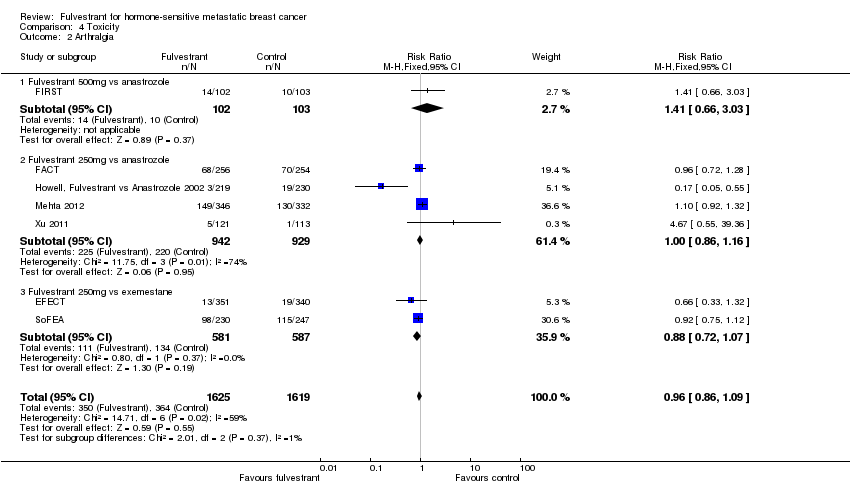
Comparison 4 Toxicity, Outcome 2 Arthralgia.
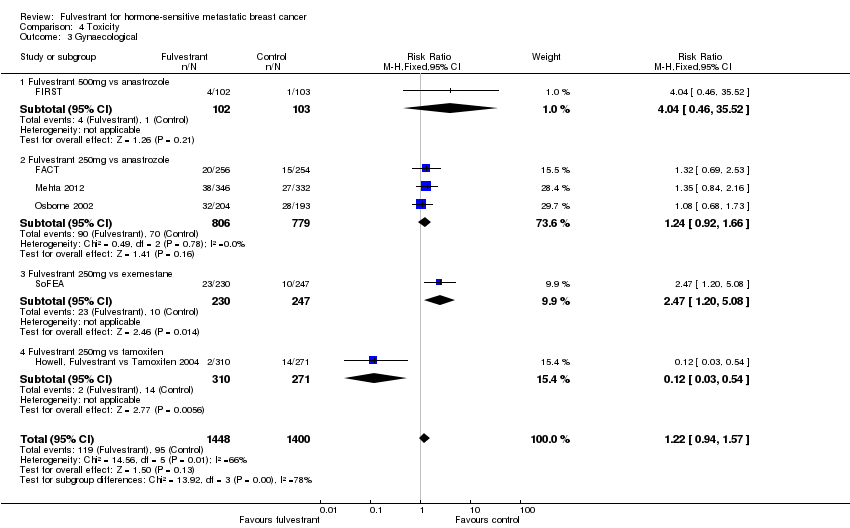
Comparison 4 Toxicity, Outcome 3 Gynaecological.
| Fulvestrant versus any other endocrine therapy for hormone‐sensitive advanced breast cancer | ||||||
| Patient or population: women with hormone‐sensitive advanced breast cancer | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with any other standard endocrine therapy | Risk with fulvestrant | |||||
| Time to progression | Low‐risk population | HR 0.95 | 4258 | ⊕⊕⊕⊝ | ||
| 500 events per 10001 | 482 events per 1000 | |||||
| High‐risk population | ||||||
| 600 events per 10001 | 581 events per 1000 | |||||
| Clinical benefit rate | Study population | RR 1.03 | 4105 | ⊕⊕⊕⊕ | ||
| 486 per 1000 | 501 per 1000 | |||||
| Mortality | Low‐risk population | HR 0.97 | 2480 | ⊕⊕⊕⊕ | ||
| 350 deaths per 10005 | 342 deaths per 1000 | |||||
| High‐risk population | ||||||
| 400 deaths per 10005 | 391 deaths per 1000 | |||||
| Vasomotor toxicity | Study population | RR 1.02 | 3544 | ⊕⊕⊕⊕ | ||
| 170 per 1000 | 174 per 1000 | |||||
| Arthralgia | Study population | RR 0.96 | 3244 | ⊕⊕⊕⊕ | ||
| 225 per 1000 | 216 per 1000 | |||||
| Gynaecological toxicity | Study population | RR 1.22 | 2848 | ⊕⊕⊕⊕ | ||
| 68 per 1000 | 83 per 1000 | |||||
| Quality of life | None of the studies reported a difference in quality of life between women receiving fulvestrant and other endocrine treatments | ‐ | (4 RCTs) | ⊕⊕⊕⊕ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The baseline risk for the control groups was calculated at 12 months for those women at relatively low risk (first‐line treatment) and at 6 months for those women at high risk (after first‐line treatment). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fulvestrant vs other endocrine therapy Show forest plot | 9 | 4258 | Hazard Ratio (Fixed, 95% CI) | 0.95 [0.89, 1.02] |
| 1.1 Fulvestrant 500 mg vs anastrozole | 1 | 205 | Hazard Ratio (Fixed, 95% CI) | 0.66 [0.47, 0.93] |
| 1.2 Fulvestrant 250 mg vs anastrozole | 5 | 2293 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.85, 1.02] |
| 1.3 Fulvestrant 250 mg vs exemestane | 2 | 1173 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 1.4 Fulvestrant 250 mg vs tamoxifen | 1 | 587 | Hazard Ratio (Fixed, 95% CI) | 1.18 [0.98, 1.42] |
| 2 First‐line vs second‐line or more Show forest plot | 9 | 4251 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.89, 1.01] |
| 2.1 Firstline | 4 | 1996 | Hazard Ratio (Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 2.2 Secondline or more | 5 | 2255 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.88, 1.04] |
| 3 Combination fulvestrant vs single agent with other endocrine therapy Show forest plot | 9 | 4251 | Hazard Ratio (Fixed, 95% CI) | 0.94 [0.89, 1.01] |
| 3.1 Single agent fulvestrant with other endocrine therapy | 7 | 3046 | Hazard Ratio (Fixed, 95% CI) | 0.97 [0.90, 1.04] |
| 3.2 Combination vs other endocrine therapy | 2 | 1205 | Hazard Ratio (Fixed, 95% CI) | 0.87 [0.77, 0.99] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fulvestrant vs other endocrine therapy Show forest plot | 9 | 4105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.10] |
| 1.1 Fulvestrant 500 mg vs anastrozole | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.30] |
| 1.2 Fulvestrant 250 mg vs anastrozole | 5 | 2293 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.99, 1.14] |
| 1.3 Fulvestrant 250 mg vs exemestane | 2 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.31] |
| 1.4 Fulvestrant 250 mg vs tamoxifen | 1 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.00] |
| 2 Clinical benefit rate (first‐line vs second‐line) Show forest plot | 9 | 4104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| 2.1 Firstline | 4 | 1999 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.07] |
| 2.2 Secondline | 5 | 2105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.15] |
| 3 Combination vs monotherapy fulvestrant Show forest plot | 9 | 4104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| 3.1 Monotherapy fulvestrant vs other endocrine therapy | 7 | 2896 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.09] |
| 3.2 Combination with fulvestrant vs other endocrine therapy | 2 | 1208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Fulvestrant vs other endocrine therapy Show forest plot | 5 | 2480 | Hazard Ratio (Fixed, 95% CI) | 0.97 [0.87, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Vasomotor Show forest plot | 8 | 3544 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.18] |
| 1.1 Fulvestrant 500 mg vs anastrozole | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.89] |
| 1.2 Fulvestrant 250 mg vs anastrozole | 4 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.01, 1.58] |
| 1.3 Fulvestrant 250 mg vs exemestane | 2 | 1168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.77, 1.19] |
| 1.4 Fulvestrant 250 mg vs tamoxifen | 1 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.46, 1.00] |
| 2 Arthralgia Show forest plot | 7 | 3244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.09] |
| 2.1 Fulvestrant 500mg vs anastrozole | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.66, 3.03] |
| 2.2 Fulvestrant 250mg vs anastrozole | 4 | 1871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.16] |
| 2.3 Fulvestrant 250mg vs exemestane | 2 | 1168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.07] |
| 3 Gynaecological Show forest plot | 6 | 2848 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.94, 1.57] |
| 3.1 Fulvestrant 500mg vs anastrozole | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.04 [0.46, 35.52] |
| 3.2 Fulvestrant 250mg vs anastrozole | 3 | 1585 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.92, 1.66] |
| 3.3 Fulvestrant 250mg vs exemestane | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.20, 5.08] |
| 3.4 Fulvestrant 250mg vs tamoxifen | 1 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.03, 0.54] |

