Anticonceptivos orales combinados: riesgo de infarto de miocardio y de accidente cerebrovascular isquémico
Resumen
Antecedentes
Los anticonceptivos orales combinados (AOC) se han asociado con un mayor riesgo de trombosis arterial, es decir, infarto de miocardio o accidente cerebrovascular isquémico. Sin embargo, como estas enfermedades son poco frecuentes en las mujeres jóvenes y existen muchos tipos de anticonceptivos orales combinados, la magnitud del riesgo y el efecto de diferentes contenidos hormonales de las preparaciones de AOC todavía no están claros.
Objetivos
Calcular el riesgo de infarto de miocardio o accidente cerebrovascular isquémico en las usuarias comparadas con las no usuarias de diferentes tipos, dosis y generaciones de anticonceptivos orales combinados.
Métodos de búsqueda
Se hicieron búsquedas de estudios elegibles en las bases de datos electrónicas (MEDLINE (1966 hasta 8 de julio 2015), EMBASE (1980 hasta 8 de julio 2015), Popline (1970 hasta 8 de julio 2015) y LILACS (1985 hasta 8 de julio 2015), sin limitaciones de idioma.
Criterios de selección
Se incluyeron los estudios observacionales que reclutaron a mujeres en el grupo en edad reproductiva (18 a 50 años) y compararon el riesgo de infarto de miocardio o accidente cerebrovascular isquémico entre las usuarias y no usuarias de AOC.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente seleccionaron los estudios relevantes y extrajeron los datos. Se agruparon los riesgos relativos (RR) (odds ratios combinados y un cociente de tasa de incidencia) y los intervalos de confianza (IC) del 95% para el infarto de miocardio o el accidente cerebrovascular isquémico en usuarias versus no usuarias de AOC. Los resultados de infarto de miocardio y el accidente cerebrovascular isquémico se combinaron y también se analizaron por separado. Los análisis se estratificaron según la dosis de estrógeno y el tipo de progestágeno.
Resultados principales
En total se identificaron 1298 publicaciones a través de la estrategia de búsqueda. Se incluyeron 28 publicaciones que informaron sobre 24 estudios. Las usuarias de AOC tuvieron un mayor riesgo de infarto de miocardio o de accidente cerebrovascular isquémico en comparación con las no usuarias (riesgo relativo [RR] 1,6; IC del 95%: 1,3 a 1,9). Estos RR fueron similares para el infarto de miocardio (1,6; IC del 95%: 1,2 a 2,1) y el accidente cerebrovascular isquémico (1,7; IC del 95%: 1,5 a 1,9). Los riesgos no variaron según la generación del progestágeno ni según el tipo de progestágeno. Sin embargo, cuando las preparaciones se estratificaron según la dosis de estrógeno, el riesgo de infarto de miocardio o de accidente cerebrovascular isquémico pareció aumentar con las dosis mayores de estrógeno.
Conclusiones de los autores
Este meta‐análisis mostró que el riesgo de infarto de miocardio o de accidente cerebrovascular isquémico fue 1,6 veces mayor en las mujeres que utilizaron AOC. El riesgo fue mayor para las píldoras con > 50 microgramos de estrógeno. Cuando se combinaron con los resultados de los estudios sobre el riesgo de trombosis venosa en las usuarias de AOC, al parecer la píldora de AOC que contiene levonorgestrel y 30 µg de estrógeno es la forma oral más segura de anticoncepción hormonal.
PICOs
Resumen en términos sencillos
El riesgo de infarto y accidente cerebrovascular en las mujeres que utilizan píldoras anticonceptivas
Antecedentes
Desde su introducción, las píldoras anticonceptivas orales combinadas se han vuelto uno de los métodos de regulación de la natalidad más populares. Estas píldoras contienen dos tipos de hormonas femeninas, estrógeno y progestágeno. Cuando se utilizan correctamente, la tasa de fracaso (es decir, la aparición de un embarazo no deseado) es menor de uno por 100 mujeres por año. A pesar de su fiabilidad, se ha observado que las píldoras anticonceptivas orales aumentan el riesgo de que se forme un coágulo sanguíneo en una arteria, es decir, de una trombosis arterial (infarto o accidente cerebrovascular). Como la trombosis arterial es poco frecuente en las mujeres jóvenes y existen tantos tipos de píldoras anticonceptivas orales, la magnitud del riesgo no está clara. Además, se desconoce el efecto de diferentes tipos de progestágenos o diferentes dosis de estrógeno sobre el riesgo de trombosis arterial.
Pregunta de la revisión
En esta revisión Cochrane se intentó evaluar el riesgo de trombosis arterial con diferentes tipos de píldoras anticonceptivas orales. Para hacer esta evaluación se buscó en la bibliografía el 8 de julio 2015 todos los estudios que evaluaron el riesgo de trombosis arterial asociada con las píldoras anticonceptivas orales en mujeres menores de 50 años.
Características de los estudios
En total, 28 artículos en 24 estudios únicos cumplieron los criterios de inclusión.
Resultados clave
Los resultados mostraron que el riesgo general de trombosis arterial fue 1,6 veces mayor en las mujeres que utilizaron píldoras anticonceptivas orales, en comparación con las mujeres que no las utilizaban. El riesgo no varió claramente según el tipo de progestágeno. Sin embargo, se encontró que el riesgo de trombosis arterial pareció ser dos veces más alto en las mujeres que tomaban píldoras con dosis mayores de estrógeno. No obstante, se debe considerar el riesgo de otros efectos secundarios de las píldoras anticonceptivas orales (como un coágulo de sangre en una vena [trombosis venosa]) antes de prescribir cualquier tipo de píldora anticonceptiva oral. Es probable que la píldora de AOC que contiene levonorgestrel y 30 µg de estrógeno sea la forma oral más segura de anticoncepción hormonal oral combinada.
Calidad de la evidencia
La calidad general de la evidencia de esta revisión fue moderada. La mayoría de los estudios (22 de 28) confirmaron correctamente que a las pacientes se les había diagnosticado una trombosis arterial. Sin embargo, solamente cuatro estudios también comprobaron que se informó correctamente el tipo de píldora que la paciente había utilizado. Además, solamente la mitad de los estudios aseguraron que se hicieron comparaciones correctas entre las pacientes con y sin trombosis arterial. También es importante señalar el hecho de que el análisis sobre el tipo de progestágeno se basó en pocos estudios.
Authors' conclusions
Background
Description of the condition
Worldwide, myocardial infarction and ischemic stroke are two of the most important causes of morbidity and mortality (WHO 2013). It is estimated that over 17 million people die from cardiovascular diseases annually, with over 80% of deaths occurring in low‐ and middle‐income countries (Nabel 2012). Myocardial infarction most often occurs when an atherosclerotic plaque (a collection of fatty acids, fibrous tissue and leukocytes) ruptures in the wall of a coronary artery. The plaque contents obstruct the artery and deprive the downstream part of the heart muscle of oxygen (Nabel 2012). This can cause permanent cell damage or necrosis and leads to heart failure, stroke or death in up to 10% of cases (Brieger 2009; Roe 2010).
Ischemic stroke is characterized by brain ischemia due to the obstruction of a cerebral artery (Caplan 2009). As in myocardial infarction, a cerebral artery can be obstructed due to the rupture of an atherosclerotic plaque. Alternatively, a thrombus can embolize from elsewhere in the body and become lodged in the cerebral vasculature (Caplan 2009). The affected part of the brain rapidly loses function, leading to both transient and permanent disabilities such as loss of speech and movement (Di Carlo 2003).
The most important risk factors for myocardial infarction and ischemic stroke are hyper cholesterolemia, hypertension, diabetes and smoking (Wilson 1998). These risk factors generally accumulate over time, explaining why most patients with a cardiovascular event are aged 50 years or older (Berry 2012). Nevertheless, there are also risk factors that can contribute to myocardial infarction or ischemic stroke in younger (female) individuals. Studies in young women have shown an association between combined oral contraception use and an increased risk of myocardial infarction and ischemic stroke (Zakharova 2011). However, as these diseases are rare in young women and as many types of combined oral contraception exist, the magnitude of the risk and the effect of different hormonal contents of combined oral contraceptive (COC) preparations remain unclear.
Description of the intervention
COCs are the most commonly used reversible form of contraception in developed countries (United Nations 2011). They contain an estrogen and a progestagen that are usually taken together in one pill for the first 21 days of every menstrual cycle, followed by a pill‐free week (Speroff 2011). COCs prevent ovulation, mainly by suppressing the surge in luteinizing hormone (due to the progestagen content) (Speroff 2011). With full compliance, the failure rate (i.e. the occurrence of unwanted pregnancy) is less than one per 100 women per year (Trussell 2011).
How the intervention might work
COCs are associated with an increase in many coagulation factors (e.g. factor VII, VIII, X), increased activity of the fibrinolytic inhibitors Plasminogen Activator Inhibitor (PAI)‐1 and PAI‐2 and a reduced anticoagulant response (activated protein C resistance) (Tchaikovski 2010). Research has recently found hypercoagulability to be an important determinant of atherogenesis and atherosclerosis (Borissoff 2011), which in turn precedes myocardial infarction and ischemic stroke. In addition, COCs have been associated with increases in triglycerides, Low Density Lipoprotein cholesterol and insulin levels, and a reduced glucose tolerance (Godsland 1990), which are all well known risk factors for arterial cardiovascular disease (Wilson 1998).
Why it is important to do this review
In order to reduce the harmful thrombotic side‐effects, the dose of estrogen in COCs has been gradually reduced from 150 µg in the first preparations to ≤ 30 µg today (Speroff 2011). In addition to preparations with so‐called 'first generation' progestagens lynestrenol and norethisterone, preparations containing second (levonorgestrel and norgestrel) or third generation progestagens (desogestrel and gestodene) and preparations containing drospirenone were developed in an attempt to improve the cardio‐metabolic profile of COCs (Godsland 1990; Bringer 1992; Badimon 1999; Krattenmacher 2000). Still, results of studies on the risk of myocardial infarction and ischemic stroke associated with various types of COCs are conflicting. As over 100 million women use COCs worldwide (WHO 2004) and all combined preparations are equally effective for the prevention of unwanted pregnancies (Lawrie 2011), the issue of safety is paramount. Therefore, it is important to obtain a systematic overview of all available evidence on this topic in order to advise women as to the safest choice of combined oral contraception with regards to the risk of myocardial infarction and ischemic stroke.
Objectives
-
To estimate the risk of myocardial infarction and ischemic stroke in combined oral contraception users compared with non‐users.
-
To compare the risk of myocardial infarction and ischemic stroke associated with the three generations of COCs, as well as with preparations containing drospirenone or cyproterone acetate.
-
To compare the effect of varying doses of estrogen and types of progestagen in COCs on the risk of myocardial infarction and ischemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
Myocardial infarction and ischemic stroke are potential side effects of COC use. Previous research has suggested that results of observational studies are credible when studying side effects of medication(Vandenbroucke 2004). This was supported by a meta‐analysis that showed no difference between the risk of side‐effects assessed in meta‐analyses of experimental data and the risk of side‐effects assessed in meta‐analyses of observational data(Golder 2011). Therefore in this Cochrane Review we included observational studies with a cohort, case‐control or nested case‐control design and, if available, data from randomised controlled trials (RCTs).
Types of participants
The participants were women in the reproductive age group (18 to 50 years) who either used or did not use COCs. We excluded studies on women using postmenopausal hormone therapy, non‐oral contraceptives or progestagen‐only contraceptives.
Types of interventions
We compared the risk of myocardial infarction and ischemic stroke between combined oral contraception users and non‐users. Both previous combined oral contraception users and never users were considered to be non‐users. The risk of myocardial infarction and ischemic stroke was assessed for different types of COC preparations. We categorized COCs according to their generation (progestagens). We classified preparations containing lynestrenol or norethindrone as first generation preparations, levonorgestrel and norethisterone acetate as second generation progestagens, and desogestrel and gestodene as third generation preparations. In addition, we categorized COCs separately according to the dose of estrogen and the type of progestagen used.
Types of outcome measures
The outcome measures of interest were objectively diagnosed fatal or non‐fatal first myocardial infarction or ischemic stroke. We classified a myocardial infarction as objectively confirmed if diagnosed based on a medical examination and pain assessment combined with an electrocardiogram (ECG), serum cardiac biomarkers or other specified strict diagnostic criteria of myocardial infarction, or by autopsy examination. Ischemic stroke was classified as objectively confirmed if a sudden onset focal neurological deficit was diagnosed on the basis of a medical history and neurological examination combined with brain imaging, or by autopsy examination.
We quantified the risk of developing either myocardial infarction or ischemic stroke in COC users compared with non‐users by obtaining crude numbers of users and non‐users of combined oral contraception, and crude numbers of women with and women without an arterial thrombotic event. We combined these numbers to compute an overall relative risk of myocardial infarction or ischemic stroke in COC users.
Primary outcomes
-
Fatal or non‐fatal arterial thrombosis (i.e. myocardial infarction or ischemic stroke).
Secondary outcomes
-
Fatal or non‐fatal myocardial infarction.
-
Fatal or non‐fatal ischemic stroke.
Search methods for identification of studies
We created the search in association with an expert librarian (C. Manion, Cochrane).
Electronic searches
We searched the following databases: MEDLINE (1966 to July 08, 2015), EMBASE (1980 to July 08, 2015), Popline (1970 to July 08, 2015) and LILACS (1985 to July 08, 2015). The study search was performed without language restrictions.
Searching other resources
The references of the selected studies and of reviews were additionally checked in case we did not capture any relevant studies through our search strategy.
Data collection and analysis
We used the study results to compare the relative risk of myocardial infarction and ischemic stroke between users and non‐users of COCs, and between women using different types and doses of COCs. Most studies included women without oral contraception or women who use preparations containing levonorgestrel with 30 µg of ethinylestradiol as a reference group. Standard meta‐analytic techniques were used, a random effect model being set as default.
Selection of studies
Two review authors (REJR, FMH) independently evaluated the title and abstract of all studies retrieved from the search strategy. This was done using standard piloted forms and specific inclusion and exclusion criteria. We resolved any disagreements by consensus and consulted a third review author (OMD) if necessary.
Data extraction and management
Two review authors (REJR and FMH) independently extracted data using standard, piloted data extraction forms and entered data into Review Manager (RevMan). We resolved all disagreements by consensus.
Assessment of risk of bias in included studies
Our 'Risk of bias' assessment was equipped for observational studies and adapted from the Newcaste Ottowa scale. We examined the risk of bias in the included observational studies based on four aspects that may affect the association between the exposure (COCs) and the outcome (myocardial infarction and ischemic stroke).
Firstly, exposure to combined oral contraception had to be confirmed through a prescription database in order for the risk of bias to be classified as 'low'. We classified other, less objective, methods such as interviews and questionnaires as a 'high' risk of bias, as research has shown that women have difficulty accurately recalling the type of preparations they used (Nischan 1993; Norell 1998).
Secondly, the diagnosis of a myocardial infarction or ischemic stroke had to be ascertained by objective measures. We classified studies in which myocardial infarction had been diagnosed on the basis of an ECG, serum cardiac biomarkers or other specified strict diagnostic criteria of myocardial infarction, or by autopsy examination as having a low risk of bias. For ischemic stroke these criteria were a neurological examination combined with brain imaging or other specified strict diagnostic criteria of ischemic stroke, or autopsy examination.
In cohort studies, loss to follow‐up can lead to biased risk estimates. We classified studies with < 10% loss to follow‐up as having a low risk of bias.
Finally, in case‐control selection, the selection of controls affects the validity of the results. We classified case‐control studies including controls from the source population of the cases (i.e. controls from the same neighbourhood as the cases who would most likely have been admitted to the same hospital as the cases if they had developed myocardial infarction or ischemic stroke) as low risk of bias (Grimes 2005).
As myocardial infarction and ischemic stroke are side effects of using COCs, and it is unethical to perform a RCT for side effects alone, we did not anticipate finding any RCTs on this topic. However, if such studies were found, we would have assessed the risk of bias according to recommended principles (Higgins 2011). We did not use an aggregate 'Risk of bias' score as this is generally discouraged (Jüni 1999).
Two review authors (REJR, FMH) independently assessed the risk of bias using a standard piloted form. Both review authors are trained in Clinical Epidemiology and Study Methodology. We resolved any persistent disagreement by consensus or discussion with a third review author (OMD). We did not use the 'Risk of bias' assessment to accept or reject studies.
Measures of treatment effect
From each matched case‐control study (matched on age and calendar time) included , we extracted the crude number of women who were exposed to combined oral contraception, the crude number of women not exposed to combined oral contraception, the crude number of women with myocardial infarction or ischemic stroke and the crude number of women without myocardial infarction or ischemic stroke event. In addition we extracted the crude numbers of women in separate subgroups (i.e. different doses of estrogen, different types of progestagen). We used these numbers to calculate odds ratios for COC users versus non‐users at the study level . For one cohort study (Lidegaard 2012a) this matching assumption was not met, and we extracted adjusted estimates (incidence rate ratio).
Unit of analysis issues
Current use of COCs, stratified according to the dose of ethinylestradiol and the type of progestagen, was analysed in women without a history of myocardial infarction or ischemic stroke. Also we studied the effect of previous combined oral contraception use on the risk of cardiovascular disease if data were available.
Dealing with missing data
We only included participants with complete data on exposure to COCs and the outcomes myocardial infarction or ischemic stroke, or both.
Assessment of heterogeneity
For results from standard meta‐analytic techniques, we presented statistical measures of heterogeneity (Chi² test and I² statistics).
Assessment of reporting biases
We made an overview of possible reporting biases for each included study. In addition, for the overall comparison of COC users with non‐users, we performed sensitivity analyses according to the presence or absence of various types of bias.
Data synthesis
We combined data from studies with similar designs, interventions and outcome measures. For the analysis on use versus non‐use and the analysis at the level of estrogen generations, we performed a random effects meta‐analysis based on data that were adjusted for age and calendar time by design or by analysis. For one cohort study (Lidegaard 2012a) we first performed a fixed effect meta‐analysis based on risk estimates for various contraceptives from that study. The pooled analysis was subsequently used in the random effects meta‐analysis. For analyses with 5 or less studies, both fixed and random effect analyses are presented as, in this case, the between study variability cannot be estimated reliably.
The matched case‐control studies provided odds ratios. As these studies were matched on calendar time the odds ratios are a valid estimate of the incidence rate ratio (Knol 2008, Vandenbroucke 2012). Moreover, as the outcome is very rare, relative risks, incidence rate ratios and odds ratios will be similar. We used the term relative risk to denote the pooled effect, even though formally speaking, this consisted of odds ratios and an incidence rate ratio.
We initially aimed to perform a formal network analysis. However, this requires the use of raw data in case of multiple arm studies. Raw data can only be used if unadjusted estimates provide meaningful effect estimates, which was not the case for the cohort study mentioned above Lidegaard 2012a. We therefore applied standard random effects meta‐analysis techniques throughout the review.
Sensitivity analysis
The outcomes myocardial infarction and ischemic stroke were pooled as well as analysed separately. To explore heterogeneity, we performed sensitivity analyses according to funding source and risk of bias. We defined funding as any financial support for the study from pharmaceutical companies.
Results
Description of studies
In total, we identified 1182 unique publications by searching electronic databases and checking the reference lists of the selected articles. Of these, we excluded 1047 publications based on the title or abstract, or both, and excluded a further 107 articles after detailed assessment of the full text (Figure 1). Twenty‐eight articles reporting on 24 separate studies met the inclusion criteria and were eligible for analysis. Heinemann 1998 and Lewis 1997 both presented results from the 'Transnational Study on Oral Contraceptives and the Health of Young Women' study, with Heinemann 1998 reporting on ischemic stroke and Lewis 1997 reporting on myocardial infarction. Similarly, van Kemmeren 2002 reported on ischemic stroke in the RATIO, whereas Tanis 2001 presented the data on myocardial infarction. Shapiro 1979 and Slone 1981 reported different analyses of the same study from the USA. The two articles by Mann 1975a and Mann 1975b reported on different data from a single British study. Finally, the article by Sidney 1998 on myocardial infarction described data from the Kaiser Permanente study and the University of Washington study, from which the risk of ischemic stroke is separately reported in Pettiti 1996 and Schwartz 1997. Thirteen studies were performed in Europe, eight in the USA and three studies in several countries around the world.

Study flow diagram.
The eligible publications included one cohort study (Lidegaard 2012a), 22 case‐control studies and one nested case‐control study. We did not find any RCTs on this topic. Sixteen of the 28 articles reported on the relationship between COCs and myocardial infarction. Eleven articles reported on ischemic stroke and one study assessed both the risk of myocardial infarction and the risk of ischemic stroke. All included articles were published between 1975 and 2010 and reported on data collected between 1968 and 2009. The pharmaceutical industry sponsored six of the 24 included studies.
Risk of bias in included studies
We have presented the risk of bias per publication in Figure 2. In total, only five studies confirmed the use of combined oral contraception through a prescription database and were classified as having a low risk of bias. All other papers assessed the exposure by questionnaire or interview only (high risk of bias).

Risk of bias in the 28 included articles (reporting on 24 included studies). Green: low risk; red: high risk; yellow: unclear risk.
Regarding Lidegaard 2012a "Source population": not applicable as this was a population study.
The outcomes myocardial infarction and ischemic stroke were ascertained by objective measures in 22 studies. Six studies had a high risk of bias as the diagnoses were not objectively confirmed.
The only cohort study that was included in this Cochrane Review had almost complete follow‐up and so was classified as having a low risk of bias. Follow‐up was not applicable to the other included articles as they all reported on case‐control studies.
Thirteen studies had a low risk of bias as they included control subjects from the same source population as the cases. The 15 other studies included hospital controls as control subjects which is associated with a high risk of bias.
Effects of interventions
Current versus none use
All 24 included studies assessed the risk of myocardial infarction or ischemic stroke in current versus non‐users of combined oral contraception (Table 1). We performed a standard random‐effects meta‐analysis to assess the risk of myocardial infarction or ischemic stroke in these groups. (Figure 3) COC users were at increased risk of myocardial infarction or ischemic stroke compared with non‐users: relative risk 1.6 (95% CI 1.3‐1.9).These RRs were similar for myocardial infarction ( 1.6, 95% CI 1.2 to 2.1) and ischemic stroke (1.7, 95% CI 1.5 to 1.9).
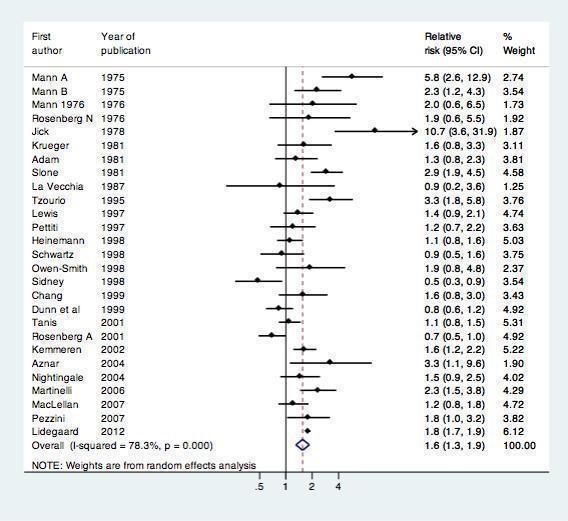
Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users.
| Study | P ublication year | Study design | Outcomea |
| 1981 | Case control | Myocardial infarction | |
| 2004 | Case control | Ischemic stroke | |
| 1999 | Case control | Ischemic stroke | |
| 1999 | Case control | Myocardial infarction | |
| 1998/1997 | Case control | Both | |
| 1978 | Case control | Myocardial infarction | |
| 2002/2001 | Case control | Both | |
| 1981 | Case control | Myocardial infarction | |
| 1987 | Case control | Myocardial infarction | |
| 2012 | Cohort | Both | |
| 2007 | Case control | Ischemic stroke | |
| 1975/1976 | Case control | Myocardial infarction | |
| 1975 | Case control | Myocardial infarction | |
| 2006 | Case control | Ischemic stroke | |
| 2004 | Nested case control | Ischemic stroke | |
| 1998 | Case control | Ischemic stroke | |
| 1997 | Case control | Ischemic stroke | |
| 2007 | Case control | Ischemic stroke | |
| 1976 | Case control | Myocardial infarction | |
| 2001 | Case control | Myocardial infarction | |
| 1998 | Case control | Ischemic stroke | |
| 1998 | Case control | Myocardial infarction | |
| 1981/1981 | Case control | Myocardial infarction | |
| 1995 | Case control | Ischemic stroke |
aDenotes both myocardial infarction and ischemic stroke.
Progestagen generation
Overall, eight studes assessed the risk of myocardial infarction or ischemic stroke for different generations of COC preparations (Table 2, Figure 4). The results of the meta‐analysis showed that first generation preparations were not clearly associated with an increased risk of myocardial infarction or ischemic stroke: pooled RR 1.2 (95% CI 0.8 to 1.9) compared with non‐use. The pooled relative risks for second generation versus non‐use and third generation versus non‐use were 1.1 (95% CI 0.8‐1.7) and 1.1 (95% CI 0.7‐1.7) respectively.
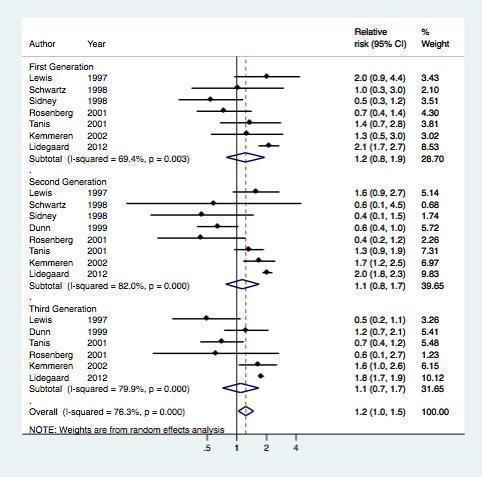
Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified per generation
| Study | Design | Outcome | Non‐use Event (n)/Total (n) | 1st generation Event (n)/Total (n) | 2nd generation Event (n)/Total (n) | 3rd generation Event (n)/Total (n) |
| Case‐control | Myocardial infarction | 386/1853 | — | 20/139 | 20/81 | |
| Case‐control | Ischemic stroke | 101/669 | 7/38 | 52/225 | 32/142 | |
| Case‐control | Ischemic stroke | 125/397 | 14/28 | 27/62 | 8/41 | |
| Cohort | Both | * | * | * | * | |
| Case‐control | Myocardial infarction | 591/3301 | 11/79 | 4/46 | 2/17 | |
| Case‐control | Ischemic stroke | 52/476 | 4/36 | 1/15 | — | |
| Case‐control | Myocardial infarction | 255/1159 | 8/60 | 3/27 | — | |
| Case‐control | Myocardial infarction | 146/714 | 11/42 | 59/232 | 20/130 |
Abbreviations: COC: combined oral contraceptives; n: number.
1st generation: preparations containing lynestrenol or norethisterone acetate.
2nd generation: preparations containing levonorgestrel.
3rd generation: preparations containing desogestrel or gestodene.
* Adjusted effect estimates extracted
Estrogen dose
Seven studies provided data on the risk of myocardial infarction or ischemic stroke according to the dose of estrogen (Table 3 , Figure 5). The risk of myocardial infarction or ischemic stroke increased with increasing doses of estrogen (RR 1.6, 95% CI 1.4 to 1.8) for preparations containing 20 µg of estrogen (RR 2.0, 95% CI 1.4 to 3.0) for 30 to 49 µg of estrogen and RR 2.4 (95% CI 1.8 to 3.3) for ≥ 50 µg of estrogen.
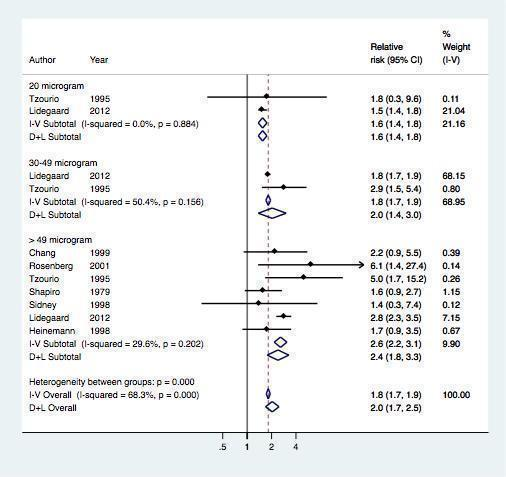
Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified by estrogen dose
| Study | Design | Outcome | Non‐use Event (n)/Total (n) | 20 µg E2 Event (n)/Total (n) | 30 to 49 µg E2 Event (n)/Total (n) | ≥ 50 µg E2 Event (n)/Total (n) |
| Case‐control | Ischemic stroke | 42/188 | — | — | 9/23 | |
| Case‐control | Ischemic stroke | 96/353 | — | — | 15/38 | |
| Cohort | Both | * | * | * | * | |
| Case‐control | Myocardial infarction | 591/3301 | — | — | 4/7 | |
| Case‐control | Myocardial infarction | 205/1812 | — | — | 18/107 | |
| Case‐control | Myocardial infarction | 255/1159 | — | — | 2/7 | |
| Case‐control | Ischemic stroke | 25/135 | 2/7 | 30/76 | 8/15 |
Abbreviations: µg: micrograms; E2: ethinylestradiol; n: number.
* Adjusted effect estimates extracted
Progestagen type
Finally, seven studies assessed the risk of myocardial infarction or ischemic stroke for various types of progestagen (Table 4, Figure 6). Of note, only few studies contributed data per progestagen type (maximum 5 studies, minimum 1 study).

Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified per prosgestagen type
| Study | Design | Outcome | Event (n)/Total (n) | ||||||||
| Non‐use | Norethindron | Levonorgestrel | Norethisterone acetate | Desogestrel | Gestodene | Norgestimate | Drospirenone | Cyproterone acetate | |||
| Case‐control | Myocardial infarction | 386/1853 | — | 18/123 | 2/16 | 9/46 | 11/35 | — | — | — | |
| Case‐control | Ischemic stroke | 101/669 | — | 52/225 | — | — | — | — | — | ||
| Case‐control | Myocardial infarction | 125/397 | — | 8/41 | — | — | — | — | — | — | |
| Cohort | Both | * | * | * | — | * | * | * | * | * | |
| Case‐control | Myocardial infarction | 591/ 3301 | 11/79 | 4/46 | — | — | — | — | — | — | |
| Case‐control | Ischemic stroke | 156/ 921 | 10/64 | — | — | — | — | 4/24 | — | — | |
| Case‐control | Myocardial infarction | 255/ 1159 | 8/60 | — | — | — | — | 3/27 | — | — | |
Abbreviations: n: number.
* Adjusted effect estimates extracted
Check for consistency
Most analyses showed evidence of considerable heterogeneity. The overall analyses for use versus non‐use showed clear evidence of between study heterogeneity beyond chance (i2 78%).
Sensitivity analysis
We performed sensitivity analyses for the risk of myocardial infarction or ischemic stroke in users compared with non‐users of combined oral contraception according to funding source, and according to the risk of bias. Risk estimates were similar in studies sponsored by a pharmaceutical company: RR 1.6 (95% CI 0.9 to 2.4) versus non‐industry studies RR 1.5 (95% CI 1.2 to 1.9). Also no clear difference was found between studies with and without a objective diagnostic measures (p=0.3 from meta‐regression), or between studies with and without a biased selection of control subjects (p=0.2).
Discussion
Summary of main results
In this Cochrane Review, we performed a meta‐analysis of 28 publications to assess the risk of myocardial infarction or ischemic stroke in women using COCs. Overall, the risk of myocardial infarction or ischemic stroke was 1.6 fold increased in women that used COC compared with non‐users. This risk did also not vary according to the generation of progestagen or according to progestagen type. When we stratified preparations according to estrogen dose, the risk of myocardial infarction or ischemic stroke seemed to increase with higher doses of estrogen.
Overall completeness and applicability of the evidence
We have discussed this topic under the 'Potential biases in the review process' section.
Quality of the evidence
We have discussed this topic under the 'Potential biases in the review process' section and presented it in Figure 2.
Potential biases in the review process
We mostly included raw data for the analysis in our review. Therefore, we cannot rule out that our results may have been confounded by factors that influence both the type of COC preparation that is prescribed and the risk of myocardial infarction or ischemic stroke (e.g. age, body mass index, smoking and calendar time). However, all except one (Lidegaard 2012a) of the included studies were case‐control studies, in which adjustment for age and calendar time is usually dealt with by design (matching). In addition, body mass index and smoking are only weakly associated with COC use and so we do not expect that the lack of adjustment for these variables will have introduced important bias. Indeed, in the six studies that presented both crude and (extensively) adjusted risk estimates for myocardial infarction or ischemic stroke, adjustment only affected the results in two instances (Table 5). Furthermore, if certain types of COCs had been preferentially prescribed to 'less healthy' women (e.g. obese women or smokers) who have a higher risk of myocardial infarction of ischemic stroke, the number of arterial thrombotic events associated with these preparations would have been overestimated. As our results showed no increased risk of myocardial infarction or ischemic stroke in women using COC preparations, any further reduction in this risk estimate due to elimination of confounding would not change the conclusion of our meta‐analysis.
| Study | Crude OR (95% CI) | Adjusted OR (95% CI) | Adjustment variables per study |
| 1.8 (1.3 to 2.5) | 2.8 (1.8 to 4.5) | Age, hypertension, body mass index, lipid levels, diabetes, smoking, alcohol, family history of stroke, duration of COC use, study centre | |
| 2.1 (0.7 to 7.1) | 1.8 (0.3 to 11.5) | Age, geographic area, marital status, education, social class, smoking, alcohol and coffee consumption, parity, age at menopause, diabetes, hypertension, obesity, hyperlipidemia, family history of ischemic heart disease | |
| 2.3 (1.5 to 3.8) | 2.3 (1.4 to 3.8) | Age, educational level, hypertension, hypercholesterolemia, obesity, smoking | |
| 1.6 (0.9 to 2.9) | 2.3 (1.2 to 4.6) | Heart disease, diabetes, hypertension, previous venous thrombosis, migraine, alcohol, smoking | |
| 2.9 (1.0 to 8.7) | 2.9 (0.9 to 9.6) | Social class, smoking status, history of hypertension | |
| 1.0 (0.5 to 1.9) | 1.2 (0.5 to 2.6) | Hypertension, diabetes, smoking, race, body mass index |
Abbreviations: OR: odds ratio; COC: combined oral contraception.
For all other studies, the crude OR or the adjusted OR, or both, were not presented in the manuscript.
A second point that warrants comment, is the lack of a generally accepted way of classifying oral contraceptives. For instance, in some studies, norgestimate is classified as a third generation preparation, whereas most studies only consider desogestrel and gestodene to be third generation preparations. In this meta‐analysis, we chose not to classify norgestimate as a third generation preparation, but only to include desogestrel and gestodene in this definition. However, as we did not find any large differences between the risk of myocardial infarction or ischemic stroke associated with desogestrel, gestodene and norgestimate in the analysis of each progestagen type separately , we do not expect that this choice has greatly influenced our results.
A third point is that some analyses (especially the analyses based on progestagen type) are based on very few studies only. It goes without saying that these results are therefore accompanied by much uncertainty.
Finally, in the analysis on estrogen dose, we did not take the type of progestagen into account. The reason for this was that most studies that provided data on the dose of estrogen did not specify the type of progestagen. However, we consider it unlikely that this classification greatly influenced our results, as preparations containing ≥ 50 µg of estrogen almost always contain levonorgestrel and we did not find this preparation to be associated with an increased risk of myocardial infarction or ischemic stroke in the analysis according to generation (second generation) or the analysis according to progestagen type. For the same reason, we could not take the estrogen dose into account in the analysis on progestagen type. However, as most preparations contained 30 to 49 µg of estrogen (Table 3) a large confounding effect of estrogen dose seems unlikely.
Agreements and disagreements with other studies or reviews
A previous systematic review on the relationship between COCs and myocardial infarction or ischemic stroke found the risk of myocardial infarction or ischemic stroke to be increased with COC use (Plu‐Bureau 2013). However, the review only included studies that were set up after 1990 and only assessed preparations containing low doses of estrogen, third generation progestagens (in which norgestimate was included) or progestin‐only contraceptives, making the two reviews incomparable. Four previous meta‐analyses, two on the risk of myocardial infarction or ischemic stroke (Baillargeon 2005; Peragallo‐Urrutia 2013), one on the risk of myocardial infarction alone (Khader 2003) and one on the risk of ischemic stroke alone (Gillum 2000), similarly found an increased risk in combined oral contraception users compared with non‐users. However, the inclusion criteria for these studies were less strict than for our current meta‐analysis, e.g. defining COC use within the past year as current use, including studies with non‐incident arterial events, analyzing studies with women up to 60 years of age, and including studies that did not present crude numbers of exposed or diseased cases and controls. To our knowledge, our Cochrane Review is the first meta‐analysis to assess the risk of incident myocardial infarction or ischemic stroke in women < 50 years of age who used COCs at or within one month before the date of inclusion.

Risk of bias in the 28 included articles (reporting on 24 included studies). Green: low risk; red: high risk; yellow: unclear risk.
Regarding Lidegaard 2012a "Source population": not applicable as this was a population study.

Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users.

Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified per generation

Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified by estrogen dose

Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified per prosgestagen type
| Study | P ublication year | Study design | Outcomea |
| 1981 | Case control | Myocardial infarction | |
| 2004 | Case control | Ischemic stroke | |
| 1999 | Case control | Ischemic stroke | |
| 1999 | Case control | Myocardial infarction | |
| 1998/1997 | Case control | Both | |
| 1978 | Case control | Myocardial infarction | |
| 2002/2001 | Case control | Both | |
| 1981 | Case control | Myocardial infarction | |
| 1987 | Case control | Myocardial infarction | |
| 2012 | Cohort | Both | |
| 2007 | Case control | Ischemic stroke | |
| 1975/1976 | Case control | Myocardial infarction | |
| 1975 | Case control | Myocardial infarction | |
| 2006 | Case control | Ischemic stroke | |
| 2004 | Nested case control | Ischemic stroke | |
| 1998 | Case control | Ischemic stroke | |
| 1997 | Case control | Ischemic stroke | |
| 2007 | Case control | Ischemic stroke | |
| 1976 | Case control | Myocardial infarction | |
| 2001 | Case control | Myocardial infarction | |
| 1998 | Case control | Ischemic stroke | |
| 1998 | Case control | Myocardial infarction | |
| 1981/1981 | Case control | Myocardial infarction | |
| 1995 | Case control | Ischemic stroke | |
| aDenotes both myocardial infarction and ischemic stroke. | |||
| Study | Design | Outcome | Non‐use Event (n)/Total (n) | 1st generation Event (n)/Total (n) | 2nd generation Event (n)/Total (n) | 3rd generation Event (n)/Total (n) |
| Case‐control | Myocardial infarction | 386/1853 | — | 20/139 | 20/81 | |
| Case‐control | Ischemic stroke | 101/669 | 7/38 | 52/225 | 32/142 | |
| Case‐control | Ischemic stroke | 125/397 | 14/28 | 27/62 | 8/41 | |
| Cohort | Both | * | * | * | * | |
| Case‐control | Myocardial infarction | 591/3301 | 11/79 | 4/46 | 2/17 | |
| Case‐control | Ischemic stroke | 52/476 | 4/36 | 1/15 | — | |
| Case‐control | Myocardial infarction | 255/1159 | 8/60 | 3/27 | — | |
| Case‐control | Myocardial infarction | 146/714 | 11/42 | 59/232 | 20/130 | |
| Abbreviations: COC: combined oral contraceptives; n: number. * Adjusted effect estimates extracted | ||||||
| Study | Design | Outcome | Non‐use Event (n)/Total (n) | 20 µg E2 Event (n)/Total (n) | 30 to 49 µg E2 Event (n)/Total (n) | ≥ 50 µg E2 Event (n)/Total (n) |
| Case‐control | Ischemic stroke | 42/188 | — | — | 9/23 | |
| Case‐control | Ischemic stroke | 96/353 | — | — | 15/38 | |
| Cohort | Both | * | * | * | * | |
| Case‐control | Myocardial infarction | 591/3301 | — | — | 4/7 | |
| Case‐control | Myocardial infarction | 205/1812 | — | — | 18/107 | |
| Case‐control | Myocardial infarction | 255/1159 | — | — | 2/7 | |
| Case‐control | Ischemic stroke | 25/135 | 2/7 | 30/76 | 8/15 | |
| Abbreviations: µg: micrograms; E2: ethinylestradiol; n: number. * Adjusted effect estimates extracted | ||||||
| Study | Design | Outcome | Event (n)/Total (n) | ||||||||
| Non‐use | Norethindron | Levonorgestrel | Norethisterone acetate | Desogestrel | Gestodene | Norgestimate | Drospirenone | Cyproterone acetate | |||
| Case‐control | Myocardial infarction | 386/1853 | — | 18/123 | 2/16 | 9/46 | 11/35 | — | — | — | |
| Case‐control | Ischemic stroke | 101/669 | — | 52/225 | — | — | — | — | — | ||
| Case‐control | Myocardial infarction | 125/397 | — | 8/41 | — | — | — | — | — | — | |
| Cohort | Both | * | * | * | — | * | * | * | * | * | |
| Case‐control | Myocardial infarction | 591/ 3301 | 11/79 | 4/46 | — | — | — | — | — | — | |
| Case‐control | Ischemic stroke | 156/ 921 | 10/64 | — | — | — | — | 4/24 | — | — | |
| Case‐control | Myocardial infarction | 255/ 1159 | 8/60 | — | — | — | — | 3/27 | — | — | |
| Abbreviations: n: number. * Adjusted effect estimates extracted | |||||||||||
| Study | Crude OR (95% CI) | Adjusted OR (95% CI) | Adjustment variables per study |
| 1.8 (1.3 to 2.5) | 2.8 (1.8 to 4.5) | Age, hypertension, body mass index, lipid levels, diabetes, smoking, alcohol, family history of stroke, duration of COC use, study centre | |
| 2.1 (0.7 to 7.1) | 1.8 (0.3 to 11.5) | Age, geographic area, marital status, education, social class, smoking, alcohol and coffee consumption, parity, age at menopause, diabetes, hypertension, obesity, hyperlipidemia, family history of ischemic heart disease | |
| 2.3 (1.5 to 3.8) | 2.3 (1.4 to 3.8) | Age, educational level, hypertension, hypercholesterolemia, obesity, smoking | |
| 1.6 (0.9 to 2.9) | 2.3 (1.2 to 4.6) | Heart disease, diabetes, hypertension, previous venous thrombosis, migraine, alcohol, smoking | |
| 2.9 (1.0 to 8.7) | 2.9 (0.9 to 9.6) | Social class, smoking status, history of hypertension | |
| 1.0 (0.5 to 1.9) | 1.2 (0.5 to 2.6) | Hypertension, diabetes, smoking, race, body mass index | |
| Abbreviations: OR: odds ratio; COC: combined oral contraception. | |||

