مداخلاتی برای هیپوناترمی هیپوتونیک غیر‐هیپوولمیک مزمن
چکیده
پیشینه
هیپوناترمی غیر‐هیپوولمی (non‐hypovolaemic hyponatraemia) مزمن (بیش از 48 ساعت) اغلب اتفاق میافتد، میتواند ناشی از شرایط مختلف باشد و با بقای کوتاهتر و طولانیتر شدن دوره بستری در بیمارستان همراه است. بسیاری از درمانها مانند محدودیت مایع یا آنتاگونیست گیرنده وازوپرسین (vasopressin receptor antagonists) میتوانند برای بهبود هیپوناترمی مورد استفاده قرار گیرند، اما این که این امر منجر به بهبود پیامدهای مهم برای بیمار میشود یا خیر، کمتر مطمئن است.
اهداف
این مرور با هدف 1) بررسی مزایا و آسیبهای مداخلات در مورد هیپوناترمی هیپوتونی غیر‐هیپوولمیک مزمن در مقایسه با دارونما (placebo)، عدم درمان یا سر‐به‐سر؛ و 2) تعیین این که مزایا و آسیبها در شرایط مطلق یا نسبی وابسته به ترکیب خاص از یک کلاس دارویی، مصرف دوز یا اختلال اساسی ناشی از هیپوناترمی با هم متفاوت است یا خیر، انجام شد.
روشهای جستوجو
پایگاه ثبت مطالعات گروه کلیه و پیوند در کاکرین را تا 1 دسامبر 2017 از طریق تماس با متخصص اطلاعات و با استفاده از واژههای جستوجوی مرتبط با این مرور جستوجو کردیم. مطالعات در پایگاه ثبت از طریق جستوجوها در CENTRAL؛ MEDLINE و EMBASE؛ مجموعه مقالات کنفرانس؛ پورتال جستوجوی پایگاه ثبت بینالمللی کارآزماییهای بالینی (ICTRP) و ClinicalTrials.gov شناسایی شدند. همچنین فهرست منابع مطالعات بالقوه مرتبط را غربالگری کرده، با نویسندگان تماس گرفتیم و وبسایتهای آژانسهای تنظیم مقررات را غربالگری کردیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و شبه‐RCTهایی را وارد کردیم که به مقایسه تاثیرات هر نوعی از مداخله با دارونما (placebo)، عدم درمان، مراقبت معمول یا هر نوعی از دیگر مداخلات در بیماران مبتلا به هیپوناترمی هیپوتونیک غیر‐هیپوولمیک مزمن پرداخته بودند. همچنین زیر‐گروههایی را با هیپوناترمی از مطالعات دیگر با معیارهای وسیعتر ورود وارد کردیم (مثلا، افراد مبتلا به نارسایی قلبی مزمن یا افراد مبتلا به سیروز با یا بدون هیپوناترمی) که توانسته بودند پیامدها را از گزارشها یا نویسندگان مطالعه برای شرکتکنندگان مبتلا به هیپوناترمی به دست آورند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم دادهها را استخراج و خطر سوگیری (bias) را ارزیابی کردند. ما اثرات درمان را به صورت تفاوت میانگین (MD) برای پیامدهای پیوسته (کیفیت زندگی مرتبط با سلامت، طول مدت بستری در بیمارستان، تغییر در مقدار اولیه در غلظت سدیم سرمی، عملکرد شناختی) و خطر نسبی (RR) برای پیامدهای دو‐حالتی (مرگومیر، پاسخ و افزایش سریع غلظت سدیم سرمی، هیپرناترمی، پلیاوری، هیپوتانسیون، آسیب حاد کلیه، اختلالات عملکرد کبدی) همراه با 95% فواصل اطمینان (CI) بیان کردیم.
نتایج اصلی
35 مطالعه را با حضور 3429 شرکتکننده شناسایی کردیم. بیستوهشت مطالعه (3189 شرکتکننده) یک آنتاگونیست گیرنده وازوپرسین را در برابر دارنما، مراقبت معمول، عدم درمان یا محدودیت مایع مقایسه کرده بودند. در بزرگسالان مبتلا به هیپوناترمی هیپوتونیک غیر‐هیپوولمیک مزمن، این آنتاگونیست تاثیرات نامطمئنی بر مرگومیر (15 مطالعه؛ 2330 شرکتکننده: RR: 1.11؛ 95% CI؛ 0.92 تا 1.33) به دلیل خطر گزارشدهی انتخابی و عدم دقت جدی؛ و روی کیفیت زندگی مرتبط با سلامت، به دلیل خطر جدی عملکرد، گزارشدهی انتخابی و سوگیری ریزش نمونه (attrition bias)، در شش ماه داشت و از غیر‐مستقیم بودن مرتبط با اعتبارسنجی Short Form Health Survey (یا SF‐12) در زمینه هیپوناترمی رنج میبردند. این مداخله ممکن است مدت اقامت در بیمارستان (شواهد با قطعیت پائین به دلیل خطر سوگیری عملکرد و عدم دقت) را کاهش دهد (3 مطالعه؛ 610 شرکتکننده: MD: ‐1.63 روز؛ 95% CI؛ 2.96‐ تا 0.30‐) و ممکن است تفاوتی اندک یا عدم تفاوت در عملکرد شناختی ایجاد کند (شواهد با قطعیت پائین به دلیل خطر سوگیری عملکرد و عدم دقت). آنها احتمالا پیامدهای میانی غلظت سدیم سرم (21 مطالعه؛ 2641 شرکتکننده: MD: 4.17 میلیمول/لیتر؛ 95% CI؛ 3.18 تا 5.16) را افزایش میدهند، مسئول افزایش دو و نیم، در صورتی که اغلب افراد افزایش 5 تا 6 میلیمول/لیتر در غلظت سدیم داشتند، در مقایسه با دارونما در 4 تا 180 روز (شواهد با قطعیت متوسط به دلیل خطر سوگیری ریزش نمونه) (18 مطالعه؛ 2014 شرکتکننده: RR: 2.49؛ 95% CI؛ 1.95 تا 3.18). اما احتمالا خطر تصحیح سریع سدیم سرم، اغلب به صورت بیش از 12 میلیمول/لیتر/روز تعریف میشود (شواهد با قطعیت متوسط به دلیل غیر‐مستقیم بودن) (14 مطالعه؛ 2058 شرکتکننده: RR: 1.67؛ 95% CI؛ 1.16 تا 2.40) و علت شایع عوارض جانبی مانند تشنگی (13 مطالعه؛ 1666 شرکتکننده: OR: 2.77؛ 95% CI؛ 1.80 تا 4.27) و پلیاوری (6 مطالعه؛ 1272 شرکتکننده: RR: 4.69؛ 95% CI؛ 1.59 تا 13.85) (شواهد با قطعیت بالا) بودند. پتانسیل سمیّت کبدی با توجه به عدم دقت بزرگ، نامطمئن است. تاثیرات بهطور کلی بین عوامل مختلف، همسو و سازگار بودند که نشان از تاثیر کلاس دارویی دارند.
دادههای مربوط به مداخلات دیگر مانند محدودیت مایع، اوره، مانیتول، دیورتیکهای لوپ (loop diuretics)، کورتیکواستروئیدها، دمکلوسیکلین (demeclocycline)، لیتیوم و فنیتوین بهطور عمده وجود نداشت.
نتیجهگیریهای نویسندگان
در افراد مبتلا به هیپوناترمی مزمن، آنتاگونیست گیرنده وازوپرسین افزایش غلظت سدیم سرم را با سرعت متوسط با هزینه افزایش خطر 3% سرعت دادن به این افزایش، بیشتر میکند. تا به امروز شواهد با قطعیت بسیار پائین برای پیامدهای مهم از نظر بیمار وجود دارد؛ تاثیرات بر مورتالیتی و کیفیت زندگی مرتبط با سلامت مشخص نیست و مزایا یا آسیبهای قابل توجه آن را نمیتوان منتفی دانست؛ به نظر میرسد تاثیر مهمی در عملکرد شناختی ندارد اما ممکن است طول مدت اقامت را در بیمارستان کمی کوتاهتر کند، اگر چه دادههای موجود محدود هستند. تصمیمات درمانی باید بر اساس بیشتر بودن مقدار افزایش غلظت سدیم سرم در برابر خطرات کوتاه‐مدت و تاثیرات نامطلوب آن بر پیامدهای مهم بیمار گرفته شوند. شواهد برای درمانهای دیگر تا حد زیادی وجود ندارد.
مطالعات بیشتر برای ارزیابی درمانهای استاندارد مانند محدودیت مایع یا اوره در برابر دارونما و یکی دیگر از این موارد، اطلاعرسانی میشود و ضروری است. با توجه به شواهد محدود موجود برای پیامدهای مهم بیمار، هر مطالعه باید این پیامدها را به شیوهای استاندارد به کار گیرد.
PICOs
خلاصه به زبان ساده
مداخلاتی برای هیپوناترمی هیپوتونیک غیر‐هیپوولمیک مزمن
موضوع چیست؟
غلظت پائین سدیم خون میتواند به علت بسیاری از بیماریها ایجاد شود و به بقای کوتاهتر و طولانیتر شدن اقامت در بیمارستان بستگی دارد. بسیاری از درمانها مانند محدودیت مایع یا قرصهای مخصوص آب به نام آنتاگونیست گیرنده وازوپرسین (vasopressin receptor antagonists) میتوانند برای افزایش غلظت سدیم خون استفاده شوند، تا در درازمدت به آهستگی افزایش یافته و از صدمه مغزی جلوگیری کنند. این که این درمانها نیز پیامدهای بیماران (چگونگی احساس بیمار، عملکرد و بقا) را بهبود میبخشند یا خیر، کمتر روشن است.
ما چه کاری را انجام دادیم؟
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و شبه‐RCTهایی را وارد کردیم که به مقایسه تاثیرات هر نوعی از مداخله با دارونما (placebo)، عدم درمان، مراقبت معمول یا هر نوعی از دیگر مداخلات در بیماران مبتلا به هیپوناترمی هیپوتونیک غیر‐هیپوولمیک مزمن پرداخته بودند.
ما چه چیزی پیدا کردیم؟
جستوجوی سیستماتیک ما (تا دسامبر 2017) 35 مطالعه را شناسایی کرده که 3429 بیمار را به کار گرفته بودند. آنتاگونیست گیرنده وازوپرسین تاثیرات نامشخصی بر خطر مرگومیر و کیفیت زندگی دارند و مطالعات بیشتری برای پاسخ به این سوالات لازم است. آنها احتمالا میزان غلظت سدیم را بهبود میبخشد اما گاهی اوقات خیلی سریع اتفاق میافتد. علاوه بر این، افرادی که از این آنتاگونیست استفاده میکنند ممکن است دچار افزایش تشنگی و تولید ادرار شوند. برای هر یک از دیگر درمانهای موجود، اطلاعات بسیار کمی وجود دارد.
نتیجهگیریها
در افرادی که دارای غلظت کم سدیم هستند، این آنتاگونیست غلظت سدیم را تا حد متوسطی افزایش میدهد. تاثیرات آنها بر مرگومیر و کیفیت زندگی مرتبط با سلامت مشخص نیست و نمیتوان مزایا یا آسیب قابل ملاحظه آنها را منتفی دانست، به نظر میرسد تاثیر مهمی در عملکرد شناختی داشته باشند، اما ممکن است اقامت را در بیمارستان کمی کوتاهتر کنند، اگر چه دادههای موجود محدود است. شواهد برای درمانهای دیگر تا حد زیادی وجود ندارد.
Authors' conclusions
Summary of findings
| Vasopressin receptor antagonists versus placebo or no treatment for chronic non‐hypovolaemic hypotonic hyponatraemia | ||||||
| Patient or population: chronic non‐hypovolaemic hypotonic hyponatraemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo or no treatment | Vasopressin receptor antagonists | |||||
| Death Follow‐up: range 2 to 180 days | Study population | RR 1.11 | 2330 (15) | ⊕⊝⊝⊝ | Interpretation: effect uncertain; may both result in 11/1000 fewer to 47/1000 more deaths within 6 months | |
| 143 per 1000 1 | 159 per 1000 | |||||
| Health‐related quality of life (assessed with mental component score of SF‐124) Follow‐up: 30 days | The mean change from baseline in health‐related quality of life in the control group ranged between 0.75 and 2.39 on a 0 to 100 point scale (worst to best) 5 | The mean health‐related quality of life in the intervention group was 4.76 higher (0.11 higher to 9.41 higher) | ‐ | 297 (2) | ⊕⊝⊝⊝ | Physical component score also measured in both studies; RR 1.04; CI ‐1.81 to 3.90 Interpretation: anywhere from 0.1 to 9.5/100 points higher increase with treatment, but questionable tool for QoL measurement in hyponatraemia and unclear minimally important clinical difference |
| Length of hospital stay | The mean length of hospital stay in the control group was 6 to 11 days 5 | The mean length of hospital stay in the intervention group was 1.63 days lower (2.96 lower to 0.30 lower) | ‐ | 580 (2) | ⊕⊕⊝⊝ | |
| Cognitive function (assessed with various tools) Follow‐up: 1 to 6 months | Across five studies, confidence intervals spanned the line of no effect and did not include a clinically meaningful effect | ‐ | 1169 (5) | ⊕⊕⊝⊝ LOW 10 | Tools used to assess cognitive function: making test B; reaction time, psychomotor, processing speeds; Mini mental state exam; overall meta‐analysis including all five studies not meaningfully possible | |
| Change from baseline in serum sodium concentration Follow‐up: range 1 to 180 days | The mean change from baseline in serum sodium concentration in the control group was 0.3 to 4.8 mmol/L 5 | The mean change from baseline in serum sodium concentration in the intervention group was 4.17 mmol/L higher (3.18 higher to 5.16 higher) | ‐ | 2641 (21) | ⊕⊕⊕⊝ | |
| Serum sodium concentration (response) Follow‐up: range 4 to 180 days | Study population | RR 2.49 | 2104 (18) | ⊕⊕⊕⊝ | Response most commonly defined by investigators as > 5 to > 6 mmol/L increase or normalisation of serum sodium concentration | |
| 231 per 1000 1 | 576 per 1000 | |||||
| Rapid sodium increase Follow‐up: range 1 to 5 days | Study population | RR 1.67 | 2058 (14) | ⊕⊕⊕⊝ | Rapid increase most commonly defined as > 12 mmol/d | |
| 44 per 1000 1 | 73 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Source of assumed baseline risk was calculated as the unweighted summed event rate in the control groups of the trials included in the meta‐analysis 2 Downgraded one level because studies considered at serious risk of suffering from * selective reporting: 6/16 studies with treatment duration > 1 week did not report death and had no protocol, accounting for 16% of the total number of participants in those studies * commercial sponsorship; possible financial conflict of interest of the authors: all studies sponsored by pharmaceutical companies wanting to commercialize the treatment; all save one had author lists who featured people who had received money for presentations or consultancy, or were employed by the sponsor; only used as supporting reason for downgrading. 3 Downgraded two levels for imprecision. The 95% CI of the pooled estimate includes both important reduction (11/1000 fewer) and increase (47/1000 more) in death with vasopressin receptor antagonists. 4 Choice of the outcome‐measure based on the fact that this was the only one reported in any of the studies. There are concerns around the validity of the SF‐12 as a measure for health‐related quality of life in the field of hyponatraemia as it gauges domains and symptoms not directly attributable to hyponatraemia. 5 Source of the assumed baseline risk was the range of outcomes in the control groups of the trials included in the meta‐analysis 6 Downgraded one level because studies considered seriously at risk of suffering from * Performance bias: self‐reported outcome, participants likely unblinded to treatment due to polyuria as side effect * Selective reporting bias: both mental and physical component score of SF‐12 measured; at week 1 or 2 and day 30 using two different analytic techniques. Only data at day 30 available for analysis. * Attrition bias: overall 37% of data missing, unknown whether missing at random or not. * Commercial sponsorship or possible financial conflict of interest of authors: both studies were sponsored by the company seeking to commercialise the treatment; both had author lists who featured people who had received money for presentations or consultancy, or were employed by the sponsor; only using as supporting argument for downgrading 7 Downgraded one level for indirectness due to concerns around validity of the SF‐12 for measuring quality of life in the context of hyponatraemia and one level for imprecision: only studied in two studies. 8 Downgraded one level because studies considered seriously at risk of suffering from performance bias: participants and personnel likely unblinded to treatment due to polyuria as side effect; this could have influenced self‐reported and professional appreciation of clinical condition and so have influenced decision to discharge from hospital. 9 Downgraded one level for imprecision. The 95% CI of the pooled estimate includes both negligible shortening (0.3 days shorter) and clinically important shortening (3 days shorter) of hospital stay with vasopressin receptor antagonists. 10 Downgraded two levels for indirectness and imprecision. Only studied in 5/28 studies, with most data for lixivaptan, and other studies not reaching the optimal information size. 11 Downgraded one level because we considered studies seriously at risk of suffering from * Attrition bias: 7/21 studies, accounting for 53% of the total number of participants in those studies at high risk of bias either due to true attrition or because a repeated measures analytic technique was used with all measurements of serum sodium concentration included until patient attrition, which we judged would likely have overestimated the treatment effect. *Commercial sponsorship; only used as supporting argument. 12 Downgraded one level for indirectness; risks controlled in tightly organised randomised trial with several measurements of serum sodium concentration daily to avoid rapid correction. In real life, risk of rapid correction likely greater. Commercial sponsorship bias; only used as supporting argument. | ||||||
Background
Description of the condition
Hypotonic hyponatraemia is a common condition, occurring in up to 60% of people admitted to hospitals, depending on the definition of hyponatraemia, the types of patients who are studied and the healthcare facility to which these patients are admitted (Upadhyay 2009). Hypotonic hyponatraemia is usually defined as a serum sodium concentration < 135 mmol/L with an osmolality < 285 mOsm/kg (Reynolds 2006). It develops when the body retains an excess of water relative to the amount of sodium. It can be caused by intrinsic kidney disease but usually results from incomplete suppression of vasopressin activity despite decreased tonicity of the plasma. In situations of decreased circulating blood volume, vasopressin release is increased in a physiologic response to maintain haemodynamic homeostasis. This occurs either with true volume depletion or with reduced effective arterial circulating volume, as seen in heart failure, liver cirrhosis or nephrotic syndrome. In the syndrome of inappropriate antidiuretic hormone secretion, the increased release of vasopressin is non‐haemodynamic and can have multiple causes including ectopic production of vasopressin by a variety of tumours (Verbalis 2013).
When plasma tonicity is low, water tends to enter the cells and causes them to swell. If blood sodium concentrations drop rapidly (within a 48 hour period), the swelling of brain cells may lead to brain oedema, brain stem herniation and eventually even death. Fortunately, when blood sodium concentrations drop more gradually, brain cells adapt to their hypo‐osmolar surroundings and prevent swelling by the transport of solutes from the intracellular to the extracellular compartments. As a consequence, immediate symptoms attributable to chronic hyponatraemia are usually less severe than for acute hyponatraemia (Reynolds 2006). Nevertheless, people with chronic hyponatraemia have reduced attention and less stable gait than those without hyponatraemia (Renneboog 2006). They fall more often and have increased risk of osteoporosis and bone fractures (Arampatzis 2013; Hoorn 2011; Kinsella 2010; Renneboog 2006; Verbalis 2010). Finally, they stay in hospital longer and have an increased risk of death, even when sodium concentrations are only mildly decreased and underlying or comorbid conditions are adjusted for (Wald 2010).
Description of the intervention
It is accepted that acute hypotonic hyponatraemia requires an immediate increase in serum sodium concentration to prevent severe neurologic complications (Ellison 2007). What to do with chronic hypotonic hyponatraemia is less clear. Firstly, chronic non‐hypovolaemic hypotonic hyponatraemia has been treated under the assumption that increasing the sodium concentration improves important health outcomes; that patients live longer, feel better and are hospitalised less frequently. Although several observational studies have indicated an association between hyponatraemia and undesirable outcomes, it is still unclear whether correcting the hyponatraemia improves them (Upadhyay 2009; Wald 2010). Secondly, once brain cells have adapted to their hypo‐osmolar environment, they become vulnerable to osmotic demyelination in case the hypo‐osmolar environment is restored. Although rare, osmotic demyelination is a devastating neurologic complication that may occur when the myelin sheath around pontine and extrapontine neurons breaks down after rapid rises in serum sodium concentration. It very rarely does if the increases stay below 8 to 12 mmol/L/24 h and 18 mmol/L/48 h ‐ accepted limits depending on risk factors such as older age, malnutrition and alcohol abuse (Adrogue 2012; Ellison 2007; Reynolds 2006). Treatment for chronic hypotonic hyponatraemia must balance the uncertain benefit of increasing the sodium concentration against the risk of complications due to overly rapid correction.
How the intervention might work
Whatever the underlying cause, chronic non‐hypovolaemic hypotonic hyponatraemia usually results from urine being insufficiently dilute to maintain serum osmolality within the normal range (Adrogue 2000). Several treatment strategies can be used to try overcoming this (Adrogue 2012; Ellison 2007; Verbalis 2013).
-
Restriction of fluid intake aims to decrease the amount of free water needing excretion.
-
Urea and mannitol improve electrolyte‐free water clearance by increasing urine osmolality and creating osmotic diuresis (Lindner 2012).
-
Loop diuretics, such as furosemide, bumetanide and ethacrynic acid, impair free‐water absorption in the collecting duct by reducing the hypertonicity of the renal medulla.
-
Corticosteroids with a mineralocorticoid effect increase renal sodium retention by active reabsorption of sodium in the principal cells of the cortical collecting tubule.
-
Demeclocycline, lithium, phenytoin and vasopressin receptor antagonists act by pharmacologically inhibiting the effect of antidiuretic hormone on the principal cells of the collecting duct, thereby limiting insertion of water channels in the luminal membrane and thus preventing free water reabsorption.
As hyponatraemia with true volume depletion (chronic hypovolaemic hypotonic hyponatraemia) is treated by restoring volume with water and salt, we do not cover it in this review.
Why it is important to do this review
The benefits and harms of treatments for chronic non‐hypovolaemic hypotonic hyponatraemia have not been formally evaluated in a systematic review. Two systematic reviews have explored the efficacy and safety of vasopressin receptor antagonists (e.g. conivaptan, lixivaptan, satavaptan, tolvaptan) versus placebo, no treatment or fluid restriction (Jaber 2011; Rozen‐Zvi 2010), but to our knowledge there has been no attempt to compare them with any other intervention or to compare any of the other interventions versus placebo or against one another.
Both systematic reviews have found an early increase in serum sodium concentration, but no improvement in outcomes important to patients. Indeed, most randomised controlled trials (RCTs) have evaluated short‐term and surrogate outcomes only, making it difficult to adequately asses any expected benefit in the long‐term. Since the most recent systematic review was published, 12 additional RCTs comparing vasopressin receptor antagonists versus control have been completed, increasing the total sample size by at least 50%. Although outcomes are still mostly surrogate and short‐term, the largest study was terminated early due to a numeric imbalance in the number of early deaths in the experimental group (FDA 2012). Two vasopressin receptor antagonists have received wide‐spread regulatory approval, but indications and permitted treatment durations vary among regions. Divergent interpretations of survival and harms data are a likely cause.
Objectives
This review aimed to:
-
Look at the benefits and harms of interventions for chronic non‐hypovolaemic hypotonic hyponatraemia when compared with placebo, no treatment or head‐to‐head;
-
Determine if benefits and harms vary in absolute or relative terms dependent on the specific compound within a drug class, on the dosage used, or the underlying disorder causing the hyponatraemia.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at interventions for chronic, non‐hypovolaemic hypotonic hyponatraemia.
We also included data for hyponatraemia subgroups within studies with broader inclusion criteria (e.g. people with chronic heart failure or people with cirrhosis with or without hyponatraemia) which report outcomes for participants with hyponatraemia, or where we could obtain these subgroup data from the study authors.
Types of participants
Inclusion criteria
-
Adults and children beyond the neonatal period (the interval from birth to 28 days of age)
-
Chronic, hypotonic hyponatraemia: presence of hyponatraemia > 48 hours, serum osmolality < 285 mOsm/kg and serum sodium concentration < 135 mmol/L, or as defined by authors); not requiring immediate treatment and due to:
-
decreased effective circulating volume in the setting of heart failure, liver cirrhosis or nephrotic syndrome;
-
inappropriate antidiuresis, associated with any underlying condition (includes syndrome of inappropriate antidiuretic hormone secretion and nephrogenic syndrome of inappropriate antidiuresis); or
-
impaired renal dilutional capacity due to kidney disease.
-
Sodium concentrations can be measured in any type of blood sample (e.g. serum, plasma, whole blood, venous, arterial, capillary) using any measurement method (e.g. flame emission spectrophotometry, direct or indirect reading potentiometry by an ion‐selective electrode) in any setting (e.g. central laboratory, local laboratory, point of care device).
Exclusion criteria
-
Children in the neonatal period (the interval from birth to 28 days of age)
-
Isotonic or hypertonic hyponatraemia (osmolality ≥ 285 mOsm/kg)
-
Hyponatraemia due to true (extracellular) volume depletion, such as from third spacing (type of fluid leakage into interstitial spaces seen in pancreatitis, bowel obstruction, sepsis), and gastrointestinal, or renal sodium loss
-
Hyponatraemia due to secondary adrenal insufficiency or hypothyroidism
-
Hyponatraemia due to primary psychogenic polydipsia
-
Patients treated with any form of dialysis or extracorporeal ultrafiltration.
Types of interventions
We included studies of any degree of fluid restriction or any drug treatment that has the aim of increasing the sodium concentration. Any dose or route of administration is permitted, and interventions can be compared with placebo, no treatment, a different dose of the same or different interventions, different administration routes of the same or different interventions, or different combinations of interventions.
Treatments included (but were not limited to):
-
vasopressin receptor antagonists (conivaptan, mozavaptan, lixivaptan, satavaptan, tolvaptan)
-
fluid restriction
-
urea
-
mannitol
-
loop diuretics (furosemide, bumetanide, ethacrynic acid)
-
corticosteroids (hydrocortisone or equivalent, fludrocortisone)
-
demeclocycline
-
lithium
-
phenytoin.
We excluded studies in which any form of dialysis treatment was given to correct serum sodium concentration.
Types of outcome measures
We assessed outcomes up to one week, up to one, two and six months, and up to one and five years.
Primary outcomes
-
Death (all‐cause mortality)
-
Health‐related quality of life and specifically symptoms attributed to hyponatraemia by trialists.
Secondary outcomes
-
Length of hospital stay
-
Serum sodium concentration (mmol/L) at end of treatment or change from beginning to end of treatment
-
Response defined as increase of ≥ 5 mmol/L or normalisation of serum sodium concentration (≥ 135 to 145 mmol/L, or as defined by the authors)
-
Outcomes related to over‐correction of serum sodium concentration
-
Incidence of hypernatraemia (serum sodium concentration > 145 mmol/L, or as defined by the authors)
-
Rapid increase in serum sodium concentration (increase in serum sodium concentration > 8 to 12 mmol/L in 24 h or > 18 mmol/L in 48h, or as defined by the authors)
-
Incidence of osmotic demyelination syndrome, previously known as central pontine and extrapontine myelinolysis (diagnosed clinically, by MRI, or post mortem)
-
-
Any treatment‐specific side effects as defined by authors
-
AKI (demeclocycline, mannitol, loop diuretics)
-
Chronic kidney disease (lithium)
-
Hypotension (mannitol, loop diuretics, vasopressin receptor antagonists)
-
Thirst (mannitol, loop diuretics, fluid restriction, vasopressin receptor antagonists)
-
Central nervous system symptoms (phenytoin)
-
Polyuria (mannitol, loop diuretics, vasopressin receptor antagonists)
-
Any other adverse event as reported by trialists
-
-
Treatment discontinuation or switch
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 1 December 2017 through contact with the Information Specialist using search terms relevant to this review. The Specialised Register contains studies identified from the following sources:
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney and transplant journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
-
Reference lists of clinical practice guidelines, review articles and relevant studies.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
-
Point‐of‐care sources such as Dynamed and UpToDate as well as US Food and Drug Administration (FDA) and European Medicines Agency (EMA) applications.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies possibly relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however studies and reviews that possibly included relevant data or information on studies of interest for our analysis were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (e.g. death, number of patients with serum sodium concentration increase of ≥ 5 mmol/L, number of patients that develop hypernatraemia, number of patients with rapid increase in serum sodium concentration, number of patients that develop osmotic demyelination syndrome), individual study results were expressed as risk ratios (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. length of hospital stay, serum sodium concentration at the end of the study or its change from beginning to the end of treatment), results were expressed as the mean difference (MD). For outcomes reported both as dichotomous and continuous data (thirst), we presented individual study results as odds ratios (OR), by converting standardized mean differences to the natural logarithm of the odds ratios (Higgins 2011).
Unit of analysis issues
If studies had multiple treatment groups, we tried to collapse these into one where appropriate to enable single pair wise comparison (e.g. collapsing three groups of different doses of vasopressin receptor antagonists into one group and including them in single pair wise comparison versus placebo) (Higgins 2011).
Dealing with missing data
Any further information required from the original authors was requested by emailing the corresponding author. If no response or insufficient information was retrieved, we subsequently emailed the sponsor. Any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per‐protocol population was performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were investigated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with I2 calculated to measure the proportion of total variation in the estimates of treatment effect that was due to heterogeneity beyond chance (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
Funnel plots were used to assess for the potential existence of small study bias. If we suspected asymmetry on visual inspection and the analysis included > 10 studies, we conducted formal hypothesis testing, using Egger's test for continuous outcomes and Peter's regression test for dichotomous outcomes (Higgins 2011).
Data synthesis
Where feasible and appropriate, data were pooled using the random‐effects model. Dichotomous outcome results were expressed as risk ratio (RR) and continuous outcome results were expressed as mean difference (MD), both with 95% confidence intervals. For outcomes reported both as dichotomous and continuous data (thirst), we converted standardized mean differences to log‐transformed odds ratios, combined them using the generic inverse‐variance method and expressed the overall effect estimate as an odds ratio with its 95% confidence interval (Higgins 2011). Although the underlying conditions causing hyponatraemia are very different, the mechanism by which hyponatraemia develops is similar in that vasopressin activity plays a role in most forms of the disorder. We believe it justified pooled analysis across subgroups of participants with different underlying conditions.
We summarised the quality of the evidence together with absolute treatment effects based on estimated baseline risks by using the Grading of Recommendations Assessment, Development, and Evaluation guidelines (GRADE 2008). To estimate the absolute number of people with hyponatraemia who avoided death or incurred a rapid increase in serum sodium concentration with vasopressin receptor antagonists, the risk estimate and 95% CI were obtained from the control arm of the corresponding meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We analysed data for death, length of hospital stay, change from baseline and response in serum sodium concentration, cognitive function and outcomes related to over correction of serum sodium concentration within subgroups of participants dependent on the type of vasopressin receptor antagonist they were treated with. Additional prespecified subgroup analyses and univariate random effects meta‐regression were conducted to explore potential sources of heterogeneity in effects of vasopressin receptor antagonists on death, change in serum sodium concentration and rapid increase in serum sodium concentration. The potential sources of heterogeneity included type of vasopressin receptor antagonist under evaluation, the underlying condition causing the hyponatraemia (with as non‐prespecified categories studies only including participants with inappropriate antidiuresis; studies only including participants with heart failure or liver cirrhosis or studies including both), mean baseline serum sodium concentration, treatment duration and risk of selection bias. Meta‐regression was undertaken on the log RR scale for categorical outcomes using Comprehensive Meta‐Analysis® software, each study weighting equal to the inverse of the variance of the estimate for that study, with between study variance estimated using the restricted the method of moments. Results were expressed as the ratio of the RR within each subgroup for categorical explanatory variables and per one unit increase for continuous variables.
Sensitivity analysis
In addition to estimating treatment effects using random effects models, we also estimated fixed effects models to ensure robustness of the model chosen and susceptibility to outliers. Finally we also assessed whether including the number of deaths during follow‐up (in contrast to only including those occurring during treatment) affected the estimate of the effect of treatment on all‐cause mortality.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
-
Death
-
Health‐related quality of life, assessed with the mental component score and physical component score of the Short Form Health Survey (SF‐12)
-
Cognitive function
-
Length of hospital stay
-
Change from baseline in serum sodium concentration
-
Response of serum sodium concentration: defined as 4 to 6 mmol/L increase or normalisation
-
Rapid sodium increase
Results
Description of studies
Results of the search
We identified 1954 citations through electronic searches conducted in 1 December 2017. We found 16 additional reports by screening the reference lists, contacting authors, conducting online searches for full reports of included abstracts, searching online trial registries, and screening the website of regulatory agencies. We reviewed in detail 157 reports, which led to the inclusion of 111 reports of 35 unique studies including 3429 participants (Figure 1).

Study flow diagram.
Included studies
Twenty‐eight studies (providing data for 3189 participants) compared a vasopressin receptor antagonist versus placebo, usual care or no treatment (ACTIV in CHF 2003; Annane 2009; BALANCE 2010; Decaux 2006; DILIPO 2011; EVEREST 2005; Ghali 2006; Gheorghiade 2003; Gines 2008b; Gerbes 2003; HARMONY 2012; HYPOCAT 2008; INSIGHT 2016; Koren 2011; LIBRA 2012; Naidech 2010; Nevens 2009; Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c; Salahudeen 2014; SALT‐1 2006; SALT‐2 2006; Soupart 2006; Wong 2003b; Yang 2013; Zeltser 2007), or versus fluid restriction (Gheorghiade 2006). Studied vasopressin receptor antagonists included conivaptan (Annane 2009; Ghali 2006; Koren 2011; Naidech 2010; Zeltser 2007), lixivaptan (BALANCE 2010; Gerbes 2003; HARMONY 2012; LIBRA 2012; Wong 2003b), satavaptan (Decaux 2006; DILIPO 2011; Gines 2008b; HYPOCAT 2008; Soupart 2006), tolvaptan (ACTIV in CHF 2003; EVEREST 2005; Gheorghiade 2003; Gheorghiade 2006; INSIGHT 2016; Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c; Salahudeen 2014; SALT‐1 2006; SALT‐2 2006; Yang 2013) and M0002 ‐ also termed SPD556 or RWJ 351647 (Nevens 2009). Two studies assessed different doses conivaptan (Kalra 2011) or tolvaptan (Shoaf 2017)
Singhi 1995 (50 participants) compared fluid restriction with normal maintenance fluid in children with bacterial meningitis and reported data for the subgroup with hyponatraemia (26 participants). Dzau 1984 (14 participants) compared captopril + furosemide versus furosemide alone in adults with decompensated heart failure. Jalan 2007 (24 participants) compared infusion of human salt poor albumin in combination with fluid and sodium restriction versus fluid and sodium restriction alone in patients with liver cirrhosis and ascites. Hayes 1987 (10 participants) compared early versus delayed administration of urea, and NATRIPHAR 2013 (19 participants) compared a change in prescribed medications versus standard care in elderly patients admitted to the internal medicine ward or residents of the nursing home of the same institution.
Twenty‐eight studies were conducted specifically in participants with hyponatraemia (Annane 2009; BALANCE 2010; Decaux 2006; Dzau 1984; Ghali 2006; Gheorghiade 2006; Gines 2008b; Gerbes 2003; HARMONY 2012; Hayes 1987; HYPOCAT 2008; INSIGHT 2016; Jalan 2007; Kalra 2011; Koren 2011; LIBRA 2012; Naidech 2010; Nevens 2009; NATRIPHAR 2013; Otsuka Study 2011b; Otsuka Study 2011c; Salahudeen 2014; SALT‐1 2006; SALT‐2 2006; Soupart 2006; Wong 2003b; Yang 2013; Zeltser 2007). For five studies, participants with hyponatraemia formed a subgroup of a larger study including both participants with and without hyponatraemia (ACTIV in CHF 2003; DILIPO 2011; EVEREST 2005; Gheorghiade 2003Singhi 1995).
Studies included on average mostly older adults (median 65 years, interquartile range 5) with moderate hyponatraemia (median 129 mmol/L; range 124 to 133). Participants had as primary cause of hyponatraemia a syndrome of inappropriate antidiuresis in nine studies (Decaux 2006; HARMONY 2012; LIBRA 2012; Naidech 2010; NATRIPHAR 2013; Otsuka Study 2011a; Salahudeen 2014; Singhi 1995; Soupart 2006), heart failure in seven studies (ACTIV in CHF 2003; BALANCE 2010; Dzau 1984; EVEREST 2005; Gheorghiade 2003; Otsuka Study 2011b; Yang 2013) and liver cirrhosis in six studies (Gines 2008b; Hayes 1987; HYPOCAT 2008; Jalan 2007; Nevens 2009; Otsuka Study 2011c). The others included a mixed group of patients. Sample sizes varied and were generally small (median 69 participants; range 6 to 652). Treatment was mostly short‐term (median 8 days; range 1 to 365). Data for at least one outcome of interest were available from 31 studies and 3365 participants, two studies reported no numeric data (Jalan 2007; Otsuka Study 2011c).
Excluded studies
We excluded 35 studies (46 reports). Thirty‐three did not include the appropriate population, with participants either not having hyponatraemia at randomisation (Albert 2013; De Vita 2012; Galton 2011; Mori 1999; Owen 2014; Ramsay 1988; Zamboli 2011; Zellweger 2001), some participants possibly having hyponatraemia but without available subgroup data (Abraham 2006; Angeli 2010; Ghali 2012; Guyader 2002; K‐STAR 2017; Licata 2003; Matsuzaki 2011a; Matsuzaki 2011b; Okita 2014; Paterna 2000; Sakaida 2014; SECRET of CHF 2017; Shanmugam 2016; Suzuki 2013b; TACT‐ADHF 2016; TACTICS‐HF 2017; Thuluvath 2006; Wong 2009; Wong 2010a; Wong 2012; Yang 2010b); having hyponatraemia but caused by psychogenic polydipsia (Alexander 1991), prolonged exercise (Rogers 2011), or head and neck surgery (Rajan 2015); or having severe symptoms requiring immediate treatment SALSA 2017). One study compared two different salt‐restricted diets in combination with step‐wise increase of diuretic treatment for reducing weight and ascites in patients with decompensated liver cirrhosis (Bernardi 1993); and finally one study (Gines 2007) would have led to double counting of participants as it represented a second randomised trial built on top of a first included study (HYPOCAT 2008) using the same study medication.
Risk of bias in included studies
The risk of bias is described in (Figure 2; Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The allocation sequence was adequately generated in 14 studies (BALANCE 2010; DILIPO 2011; EVEREST 2005; Gerbes 2003; HYPOCAT 2008; Naidech 2010; NATRIPHAR 2013; Otsuka Study 2011a; Otsuka Study 2011b; Salahudeen 2014; SALT‐1 2006; SALT‐2 2006; Singhi 1995; Wong 2003b). and adequately concealed in 12 studies (ACTIV in CHF 2003; BALANCE 2010; EVEREST 2005; Gheorghiade 2003; Gerbes 2003; HYPOCAT 2008; Naidech 2010; NATRIPHAR 2013; Salahudeen 2014; SALT‐1 2006; SALT‐2 2006; Wong 2003b). For the remaining studies the authors provided insufficient information about the procedures to permit a judgement of the risk of bias.
Blinding
In 26 studies, all assessing a vasopressin receptor antagonist, the investigators attempted to blind participants and personnel by providing a matching placebo (ACTIV in CHF 2003; Annane 2009; BALANCE 2010; Decaux 2006; DILIPO 2011; EVEREST 2005; Ghali 2006; Gheorghiade 2003; Gines 2008b; Gerbes 2003; HARMONY 2012; HYPOCAT 2008; INSIGHT 2016; Kalra 2011; Koren 2011; LIBRA 2012; Nevens 2009; Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c; Salahudeen 2014; SALT‐1 2006; SALT‐2 2006; Soupart 2006; Wong 2003b; Zeltser 2007). Although it was probably unlikely for participants and personnel to be fully blinded due to important increases in urine output when treated with a vasopressin receptor antagonist, co‐interventions of fluid restriction or salt‐intake were reported and similar in 12 studies (Annane 2009; DILIPO 2011; Ghali 2006; Gerbes 2003; HARMONY 2012; HYPOCAT 2008; Kalra 2011; Koren 2011; LIBRA 2012; Salahudeen 2014; Soupart 2006; Zeltser 2007). For two studies, fluid restriction could be adapted by both participant and treating physician (e.g. based on urine output). We judged this would not have introduced important risk of bias for death and objective outcomes related to serum sodium concentration, but may have biased health‐related quality of life measures and resulted in biased estimates of risk of rapid increase in serum sodium concentration (SALT‐1 2006; SALT‐2 2006).
Three studies explicitly reported blinding of outcomes assessors (BALANCE 2010; SALT‐1 2006; SALT‐2 2006). In 28 others we judged blinding of outcome assessors would likely have occurred or measured outcomes were objective enough so that the risk of bias was probably low (ACTIV in CHF 2003; Annane 2009; Decaux 2006; DILIPO 2011; Dzau 1984; EVEREST 2005; Ghali 2006; Gheorghiade 2003; Gheorghiade 2006; Gerbes 2003; HARMONY 2012; HYPOCAT 2008; INSIGHT 2016; Jalan 2007; Kalra 2011; Koren 2011; LIBRA 2012; Naidech 2010; NATRIPHAR 2013; Nevens 2009; Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c; Salahudeen 2014; Singhi 1995; Soupart 2006; Wong 2003b; Zeltser 2007).
Incomplete outcome data
In 17 studies, attrition stayed below 20% with either well documented reasons and/or limited opportunity for important bias (ACTIV in CHF 2003; Annane 2009; BALANCE 2010; DILIPO 2011; Dzau 1984; Ghali 2006; Gheorghiade 2006; HYPOCAT 2008; INSIGHT 2016; Kalra 2011; Koren 2011; Naidech 2010; Otsuka Study 2011a; Otsuka Study 2011b; Salahudeen 2014; Singhi 1995; Soupart 2006). Eight studies had attrition rates > 25% and either did not attempt to re‐include participants in the analysis (EVEREST 2005; Gheorghiade 2003NATRIPHAR 2013) or used imputation methods to deal with missing serum sodium concentration values that may have caused overestimation of the effect of the study medication ‐ e.g. last observation carried forward, thus ignoring rebound hyponatraemia on cessation of treatment (HARMONY 2012; LIBRA 2012; SALT‐1 2006; SALT‐2 2006; Wong 2003b; Zeltser 2007). The others provided insufficient information to allow judgement of high or low risk of bias (Decaux 2006; Gheorghiade 2003; Gines 2008b; Gerbes 2003; Hayes 1987; Jalan 2007; Nevens 2009; Otsuka Study 2011c; Yang 2013).
Selective reporting
For 15 studies, we found a registered protocol in a trial registry (ACTIV in CHF 2003; BALANCE 2010; DILIPO 2011; EVEREST 2005; HARMONY 2012; HYPOCAT 2008; INSIGHT 2016; LIBRA 2012; Naidech 2010; NATRIPHAR 2013; Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c; SALT‐1 2006; SALT‐2 2006). The Otsuka Study was registered as a single study but reported was as three separate studies (Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c). Investigators fully reported all expected pre‐registered outcomes at pre‐registered time‐points in two studies (INSIGHT 2016; Naidech 2010). Whether a protocol was provided or not, for 12 studies investigators reported all expected outcomes related to benefit and harms at reasonable time‐points (Annane 2009; Decaux 2006; DILIPO 2011; Ghali 2006; HARMONY 2012; Kalra 2011; Koren 2011; LIBRA 2012; NATRIPHAR 2013; Singhi 1995; Soupart 2006; Zeltser 2007). In seven studies with treatment duration > one week, authors did not report the primary outcomes all‐cause mortality or health‐related quality of life or any outcome related to rapid increases in serum sodium concentration (ACTIV in CHF 2003; Gheorghiade 2003; HYPOCAT 2008; Gines 2008b; Nevens 2009; Salahudeen 2014; Yang 2013). In eight studies with treatment duration ≤ 1 week, authors did not report any secondary outcome related to serum sodium concentration or rapid increases thereof (Dzau 1984; Gheorghiade 2006; Gerbes 2003; Jalan 2007; Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c; Wong 2003b).
Other potential sources of bias
Bias through possible financial conflict of interest of the authors, sponsorship bias, or both
Industry funded 28 studies (ACTIV in CHF 2003; Annane 2009; BALANCE 2010; Decaux 2006; DILIPO 2011; EVEREST 2005; Ghali 2006; Gheorghiade 2003; Gheorghiade 2006; Gerbes 2003; HARMONY 2012; HYPOCAT 2008; INSIGHT 2016; Kalra 2011; LIBRA 2012; Koren 2011; Naidech 2010; Nevens 2009; Otsuka Study 2011a; Otsuka Study 2011b; Otsuka Study 2011c; Salahudeen 2014; Salahudeen 2014; SALT‐1 2006; SALT‐2 2006; Soupart 2006; Wong 2003b; Zeltser 2007). The funding source was unclear for six studies (Dzau 1984; Gines 2008b; Jalan 2007; NATRIPHAR 2013; Singhi 1995; Yang 2013). For 17 studies we retrieved a declaration of interest for the authors featuring on the reports (ACTIV in CHF 2003; Annane 2009; DILIPO 2011; EVEREST 2005; Ghali 2006; Gheorghiade 2003; Gines 2008b; Gerbes 2003; HARMONY 2012; HYPOCAT 2008; Kalra 2011; Koren 2011; LIBRA 2012; Naidech 2010; SALT‐1 2006; SALT‐2 2006; Zeltser 2007). All save one (Naidech 2010) had author lists who featured people who had received money for presentations or consultancy, or were employed by the sponsor.
Effects of interventions
Vasopressin receptor antagonists versus placebo or no treatment
Primary outcomes
Vasopressin receptor antagonists had uncertain effects on mortality, results being compatible with both 11 in 1000 fewer to 47 in 1000 more deaths within six months (Analysis 1.1 (15 studies, 2330 participants): RR 1.11, 95% CI 0.92 to 1.33; I2 = 0%). Using GRADE criteria, we downgraded the certainty of the evidence three levels from high to very low, partly because of this imprecision, partly for a risk of selective reporting (Guyatt 2010). Six of 16 long‐term studies reasonably expected to measure death as an outcome did not, and the absence of a protocol did not allow to assess whether its measurement may have been planned, but not reported (Gheorghiade 2003; Gines 2008b; HYPOCAT 2008; Nevens 2009; Wong 2003b; Yang 2013).
Vasopressin receptor antagonists increased scores for the mental component summary of the SF‐12 (Analysis 1.2.1 (2 studies, 297 participants): MD 4.76, 95% CI 0.11 to 9.41, I2 = 63%), but had unclear effects on the physical component summary score (Analysis 1.2.2 (2 studies, 297 participants): MD 1.04, 95% CI ‐1.81 to 3.90, I2 = 0%). We downgraded the certainty of the evidence for these outcomes one level because we judged the results to be at serious risk of performance bias due to likely unblinding of the participants with a self‐reported outcome; of selective reporting bias due to outcomes being measured at two different time‐points with only one time‐point reported; of attrition bias due to more than one third of the overall data being missing. We downgraded two additional levels for imprecision and indirectness, because of concerns around the validity of the SF‐12 for measuring quality of life in the context of hyponatraemia.
Five studies evaluated cognitive function (BALANCE 2010; HARMONY 2012; LIBRA 2012; INSIGHT 2016; Salahudeen 2014). In three studies, two of which provided data that could reliably contribute to meta‐analysis ((BALANCE 2010; LIBRA 2012), investigators used the trail making test part B, a neuropsychological test of visual attention and task switching. They found lixivaptan did not shorten the time to complete the test (Analysis 1.3 (858 participants): MD 6.89 sec, 95% CI ‐6.34 to 20.12). A fourth study tested reaction time, psychomotor and processing speeds and found no difference in change between the groups (INSIGHT 2016, 56 participants, 0.20, 95% CI ‐0.10 to 0.50). The fifth study, assessing the change in mini mental state exam with tolvaptan, found no change from baseline (Salahudeen 2014, 30 participants, MD ‐0.70, 95% CI ‐2.23, 0.83). We considered the evidence of moderate certainty, downgrading one level mainly because of some indirectness and imprecision as it is unclear to what extent the used tools reflect the impairment thought to be caused by hyponatraemia, and the outcome was infrequently studied.
Three studies found vasopressin receptor antagonists shortened hospital stay anywhere between 0.3 and 3 days (Analysis 1.4 (3 studies, 610 participants): MD ‐1.63 days, 95% CI ‐2.96 to ‐0.30, I2 = 0%). We downgraded the certainty of the evidence one level because we judged it at serious risk of suffering from performance bias, as participants and personnel were likely unblinded due to polyuria as a side‐effect and this could have influenced self‐reported and professional appreciation of clinical condition and so have influenced decision to discharge from hospital. We downgraded an additional level for imprecision as the confidence interval of the pooled effect included both a negligible effect and a clinically important one.
Secondary outcomes
Vasopressin receptor antagonists caused a modest increase in serum sodium concentration. At the end of treatment, participants treated with placebo had an average increase in serum sodium concentration ranging from 0.5 to 4.7 mmol/L. In comparison, people treated with a vasopressin receptor antagonist had an average increase that was approximately 4 mmol/L higher (Analysis 1.5 (21 studies, 2641 participants): MD 4.17 mmol/L, 95% CI 3.18 to 5.16; I2 = 91%). These results were generally consistent for studies with shorter and longer follow‐up. Although there was substantial heterogeneity among included studies as described by the I2, individual point estimates all favoured vasopressin receptor antagonists. Investigators often also analysed serum sodium concentration as a dichotomous outcome, defining response as an increase of 5 to 6 mmol/L or normalisation of the absolute value. Defined as such, the previous data translated into two and a half as many people having a response with vasopressin receptor antagonists compared with placebo (Analysis 1.6 (18 studies, 2104 participants): RR 2.49, 95% CI 1.95 to 3.18; I2 = 56%). On average 23% of participants treated with placebo had a response versus 57% treated with a vasopressin receptor antagonist. Overall, in absolute terms this implies treating three adults with hyponatraemia with a vasopressin receptor antagonist could result in one more individual attaining an increase in serum sodium concentration of 5 mmol/L. We downgraded the certainty of the evidence from high to moderate because in 7/21 studies, accounting for 53% of the total number of participants in those studies, we harboured serious concerns around attrition bias; either because outcomes were not measured in the participants that dropped out, or because analytic techniques were used that included all measurements of serum sodium concentration included until patient attrition, both of which we judged would likely have overestimated the treatment effect.
Treatment with a vasopressin receptor antagonist raised the risk of rapid increases in serum sodium concentration by 67%, resulting in three additional people with a rapid increase per 100 treated with a vasopressin receptor antagonist versus placebo (Analysis 1.7 (14 studies, 2058 participants): RR 1.67, 95% CI 1.16 to 2.40; I2 = 0%). The analysis showed no significant heterogeneity. Additional sensitivity analysis including only studies defining an increase in serum sodium concentration of > 12 mmol/L/24 h did not meaningfully change the results (Analysis 1.14.2 (10 studies, 1801 participants): RR 1.63, 95% CI 1.09 to 2.43; I2 = 0%).The effects for hypernatraemia were unclear (Analysis 1.8 (10 studies, 1595 participants): RR 1.37, 95% CI 0.63 to 3.01; I2 = 1%). None of the included studies reported participants developing osmotic demyelination syndrome. For similar concerns related to attrition bias as, we considered the evidence to be of moderate certainty.
Overall, treatment with vasopressin receptor antagonists increased the odds for thirst nearly three times compared versus treatment with placebo (Analysis 1.9 (13 studies, 1666 participants): OR 2.77, 95% CI 1.80 to 4.27; I2 = 66%). Other side‐effects were generally less extensively reported. Nevertheless there was some evidence vasopressin receptor antagonists substantially increased the risk of polyuria (Analysis 1.10.1 (6 studies, 1272 participants): RR 4.69, 95% CI 1.59 to 13.85, I2 = 0%). The risk remained uncertain for hypotension (Analysis 1.10.2 (14 studies, 1748 participants): RR 1.11, 95% CI 0.75 to 1.63; I2 = 0%), AKI (Analysis 1.10.3 (8 studies, 1920 participants): RR 0.89, 95% CI 0.67 to 1.18; I2 = 0%), and liver function abnormalities (Analysis 1.10.4 (3 studies, 811 participants): RR 2.43, 95% CI 0.88 to 6.70; I2 = 0%). Half of the studies in which the vasopressin receptor antagonist was administered intravenously evaluated important adverse events related to the infusion itself. Overall there were almost three times as many patients developing infusion‐site phlebitis (Analysis 1.11.2 (2 studies, 133 participants: RR 3.52, 95% CI 1.00 to 12.41; I2 = 0%); the effects for infusion‐site thrombosis were less clear (Analysis 1.11.3 (2 studies, 133 participants): RR 1.75, 95% CI 0.21 to 14.80; I2 = 0%). There were slightly fewer people who discontinued treatment when given placebo than when given a vasopressin receptor antagonist (Analysis 1.12 (14 studies, 2429 participants): RR 0.93, 95% CI 0.85 to 1.00; I2 = 0%).
Analysis of heterogeneity
Using univariate meta‐regression and subgroup analyses, we explored possible sources of heterogeneity in the effect of vasopressin receptor antagonists on the change in serum sodium concentration. Our prespecified potential sources were: the specific vasopressin receptor antagonist compound, the underlying condition causing the hyponatraemia (with as non‐prespecified categories studies only including participants with inappropriate antidiuresis; studies only including participants with heart failure or liver cirrhosis or studies including both), mean baseline serum sodium concentration, treatment duration and risk of selection bias as sources of heterogeneity. A higher serum sodium concentration at baseline resulted in smaller increases in serum sodium with treatment (Figure 4). Per 1 mmol/L increase in baseline serum sodium concentration between 124 and 133 mmol/L, the mean difference on average decreased from 5.7 by 0.33 mmol/L (95% CI ‐0.89 to ‐0.60) to 2.7 mmol/L (Table 1). There was no evidence that the compound, the cause of hyponatraemia, the treatment duration or risk of selection bias modified the effect of vasopressin receptor antagonists on change from baseline in serum sodium concentration.

Effect of baseline serum sodium concentration on change in natraemia: meta‐regression
| Covariate | Number of studies included in meta‐regression | Scale | Absolute change in mean difference | P value |
| Baseline serum sodium concentration | 21 | Per 1 mmol/L increase | ‐0.33 (‐0.65 to ‐0.02) | 0.04 |
| Compound | 21 | Relative to conivaptan | ‐ | 0.17 |
| Conivaptan | 5 | ‐ | ‐ | ‐ |
| Lixivaptan | 4 | ‐ | ‐2.79 (‐5.47 to ‐0.10) | ‐ |
| Satavaptan | 3 | ‐ | ‐0,23 (‐3.34 to 2.87) | ‐ |
| Tolvaptan | 9 | ‐ | ‐0.82 (‐3.13 to 1.50) | ‐ |
| Cause of hyponatraemia | 21 | Relative to SIADH | ‐ | 0.18 |
| SIADH | 5 | ‐ | ‐ | ‐ |
| Combined SIADH ‐ Heart failure, cirrhosis | 10 | ‐ | 0.67 (‐1.77 to 3.13) | ‐ |
| Heart failure | 6 | ‐ | ‐1.19 (‐3.84 to 1.47) | ‐ |
| Treatment duration | 21 | Per day increase | ‐0.02 (‐0,04 to 0.00) | 0.1 |
| Risk of selection bias | 21 | Relative to low risk | ‐ | 0.86 |
| Low risk | 8 | ‐ | ‐ | ‐ |
| High risk | 13 | ‐ | 0.18 (‐1.90 to 2.27) | ‐ |
We observed asymmetry in the funnel plot for the outcomes of response, suggesting the presence of small‐study effects or publication bias, such that studies with small or null effects are not in the public domain and were not uncovered by our sensitive searching (Figure 5, Peters' regression test, P = 0.002). Sensitivity analysis for this outcome excluding four studies with the largest effect estimates and largest estimate of variance reduced the relative risk with 30% (RR 2.07, 95% CI 1.67 to 2.56). However, the funnel plots for other related outcomes were more symmetrical. No asymmetry was observed in funnel plots for change and rapid increase in serum sodium concentration, thirst, treatment discontinuation, and data for death, length of hospital stay, cognitive function, hypernatraemia or other adverse events were insufficient to allow for detection of small‐study effects.

Funnel plot of comparison: 1 Vasopressin receptor antagonists versus placebo or no treatment, outcome: 1.6 Response in serum sodium concentration.
Sensitivity analysis
Including the number of deaths occurring during follow‐up rather than during treatment had little effect on the estimate of the treatment effect (Analysis 1.13 (16 studies, 2404 participants): RR 1.10, 95% CI 0.91 to 1.32).
For the serum sodium concentration analysis, when we excluded seven studies judged at high or unclear risk of performance bias, the summary treatment estimate remained unchanged (14 studies, 1458 participants: MD 4.89 mmol/L, 95% CI 4.02 to 5.76). When we excluded eight studies judged at high or unclear risk of attrition bias, we found similar treatment effect estimates (13 studies, 1342 participants: MD 4.71 mmol/L, 95% CI 3.34 to 6.08).
Conivaptan versus conivaptan
Kalra 2011 (117 participants) compared four regimens with or without a loading dose with one another and found no significant difference in any of the measured outcomes (death, change from baseline serum sodium concentration, response in serum sodium concentration, thirst, injection‐site phlebitis, injection‐site thrombosis, treatment discontinuation (data not shown).
Tolvaptan versus tolvaptan
Shoaf 2017 (30 participants) compared 3 single doses with one another and found rapid correction in 1, 1 and 2 subjects in the 3.75, 7.5, and 15 mg dose groups respectively, but only abstract data was available and the number of participants in each group was not reported (data not shown).
Fluid restriction versus normal maintenance fluid treatment
Singhi 1995 (26 participants) compared fluid restriction (calculated as 65% of normal) versus normal maintenance intravenous fluid administration in children with bacterial meningitis. At two days, administration of restricted volumes significantly increased the serum sodium concentration (Figure 6 (1 study, 26 participants): MD 4.40 mmol/L, 95% CI 1.79 to 7.01), but had uncertain effects on the risk of death (Figure 6 (1 study, 26 participants): RR 7.80, 95% CI 0.46 to 131.62).

Single study results
Captopril and furosemide versus captopril
Dzau 1984 (14 participants) found after five days of treatment, the combination of captopril and furosemide resulted in a 10 mmol/L higher serum sodium concentration compared with captopril alone (Figure 6 (1 study, 14 participants): MD 10.00 mmol/L, 95% CI 8.60 to 11.40). The report did not include outcomes related to death, quality of life or adverse events due to rapid increases in serum sodium concentration caused by treatment.
Albumin versus no treatment
Jalan 2007 (24 participants) compared infusion of human salt poor albumin in combination with fluid and sodium restriction versus fluid and sodium restriction alone in patients with refractory ascites caused by liver cirrhosis. We only identified an abstract and it did not include any comparative data.
Urea versus urea
Hayes 1987 (10 participants) compared urea given immediately after development of hyponatraemia in people with ascites under diuretics versus urea given after a 'no treatment interval' of three days. We only identified an abstract and it did not include any comparative data.
Medication change versus standard care
NATRIPHAR 2013 (19 participants) compared a change in medication regimen to standard care in older adults suspected of having drug‐induced hyponatraemia. Compared with standard care, changing the medication regimen had uncertain effects on risk of death (Figure 6 (1 study, 19 participants): RR 0.37, 95% CI 0.02 to 8.01), response in serum sodium concentration (Figure 6 (1 study 14 participants): RR 10.11, 95% CI 0.68 to 150.68) and change from baseline in serum sodium concentration at one month (Figure 6 (1 study, 14 participants): MD 1.70, 95% CI ‐1.39 to 4.79). No participant in either group developed hypernatraemia or osmotic demyelination syndrome.
No studies evaluated the effects of urea, mannitol, loop diuretics with or without oral sodium chloride, corticosteroids, demeclocycline, lithium or phenytoin with regard to correction of hyponatraemia.
Discussion
Summary of main results
This review included evidence from 35 randomised controlled trials involving 3429 participants, primarily covering vasopressin receptor antagonists. Generally, the evidence for patient‐important outcomes was limited. Vasopressin receptor antagonists had very uncertain effects on mortality and health‐related quality of life. There was low certainty evidence for a reduction in hospital stay (1.6 days) and for the absence of an important improvement in cognitive function.
Most studies have focused on the intermediate biochemical outcome and there was moderate certainty evidence that vasopressin receptor antagonists increased the serum sodium concentration (4 mmol/L). But they were also associated with an increased risk of rapid serum sodium correction and commonly caused adverse effects such as thirst and polyuria. On average, treating 1000 people would cause 290 additional people to have an increase in serum sodium concentration of at least 5 mmol/L, but it would come at a cost of an additional 29 people having an increase exceeding 8 to 12 mmol/L/d, considered as the threshold from which on there is an increased risk for osmotic demyelination, be it that no such cases were documented in any of included studies. Effects were generally consistent across the different drugs in this class. RCT data for other interventions such as fluid restriction, urea, mannitol, loop diuretics, corticosteroids, demeclocycline, lithium and phenytoin were largely absent.
Overall completeness and applicability of evidence
Although the FDA and the EMA have approved certain vasopressin receptor antagonists for treating people with hyponatraemia, regulatory approval was largely based on intermediate outcomes in short‐term studies. To date, clinically important outcomes (e.g. reduction in all‐cause mortality or improvement in health‐related quality of life, cognitive and general functional status) remain insufficiently investigated. The data we had, indicated with moderate confidence that vasopressin receptor antagonists modestly increased the serum sodium concentration, but there is insufficient evidence to conclude this truly translates in the improvement of patient‐important outcomes.
In the context of intervention studies, a surrogate or intermediate is a measurable outcome such as a laboratory test, which responds to an intervention (e.g. lowering of cholesterol with statins) and is causally associated a clinically important outcome (e.g. reduction in mortality with statins) (Ballinger 2014). Investigators often use surrogates instead of important health outcomes because surrogates can substantially reduce the cost, sample size and duration of a randomised trial. However, not all are valid proxies of clinically important outcomes. It is true that in acute and profound hyponatraemia, the evidence from observational studies is so overwhelming that we readily accept that increasing the serum sodium concentration is life‐saving. But as we move further from the extreme of acute, profound hyponatraemia into chronic, mild hyponatraemia, the evidence for such a consistent causal link weakens. Although it is true that a chronically low serum sodium concentration is strongly and consistently associated with increased mortality and risk of bone fractures (Wald 2010), there is currently insufficient evidence that aside from affecting the surrogate (e.g. increase in serum sodium concentration with a vasopressin receptor antagonist) treatment in case of more chronic hyponatraemia also changes the patient‐important outcomes downstream of the surrogate in the same causal pathway (e.g. reduction in mortality as a consequence of raising serum sodium concentration with a vasopressin receptor antagonist). There are intuitive reasons to assume that such causality can reasonably be extrapolated to the entire spectrum of hypotonic hyponatraemia. But likewise, there are reasons for caution. Lixivaptan primarily failed to obtain regulatory approval by the FDA for hyponatraemia in chronic heart failure due to a numeric ‐ be it statistically non‐significant ‐ imbalance in early deaths. The clinical review team argued that 'while the early death in participants with chronic heart failure and hyponatraemia could reflect the underlying disease, they could not exclude the possibility that some subjects with hyponatraemia associated with acute worsening congestive failure were exquisitely sensitive to intravascular free water shifts and did not tolerate even a small change in intravascular volume status or osmolality, induced by lixivaptan and/or effects resulting from a compensatory neurohumoral activation' (BALANCE 2010).
Studies contributing to this review included mostly participants with mild to moderate hyponatraemia (mean serum sodium concentration at study level ≥ 123 mmol/L). Meta‐regression revealed a modifying effect of the serum sodium concentration at baseline, with lower values associated with larger increases in natraemia. Extrapolation of meta‐regression data would suggest higher increases, but possibly higher risks of rapid correction as the baseline serum sodium decreases. Although no study reported osmotic demyelination, it is unclear what would happen if vasopressin receptor antagonists were used on a larger scale and in people with sodium concentrations below those included in the RCTs that contributed to this review. Likewise, it is possible that while improvements in cognitive function were not readily detected, they would emerge for people with lower serum sodium concentrations at baseline.
Finally, studies evaluating the effectiveness of alternative interventions for increasing serum sodium concentration in people with chronic, non‐hypovolaemic hyponatraemia are largely absent.
Quality of the evidence
Overall, we considered the data evaluating the effects of vasopressin receptor antagonists for people with chronic, non‐hypovolaemic, hypotonic hyponatraemia on patient‐important outcomes such as mortality, health‐related quality of life, and hospital stay limited and of low certainty. Low certainty evidence suggests that additional studies are likely to change our confidence in the effects (GRADE 2008). According to GRADE, RCT data is considered high quality, but may be downgraded for several reasons (Guyatt 2010). These reasons varied for each outcome. For death it was largely driven by the width of the confidence interval, which captured both appreciable benefit and harm, and left us with considerably uncertainty around the treatment effect. For health‐related quality of life, there was some evidence that vasopressin receptor antagonists increased the mental component summary score of the SF‐12, and unclear effects on the physical component summary score. However, we serious questioned the validity of this tool for measuring quality of life in the context of hyponatraemia. For one thing, many items included in the SF‐12 do not reflect readily acknowledged signs and symptoms of hyponatraemia (e.g. bodily pain, anxiety) and similarly, the neurocognitive signs of hyponatraemia are not assessed by the tool. In addition to the general content validity issues, we also judged effects for this self‐reported outcome may have been inflated because participants were likely unblinded due to polyuria as a side‐effect; results were selectively reported for one in two measurement time‐points; and more than one‐third of the data were missing.
We had more extensive data for outcomes related to serum sodium concentration, resulting in moderate confidence in the effect estimates all around. Downgrading occurred mainly for missing outcome data, which we judged could have somewhat overestimated the treatment effect; as well as indirectness, in that tight follow‐up during dose‐titration, is likely to have avoided rapid correction somewhat in the trial setting. It may result in higher risk of rapid correction when used in clinical practice.
Of note, all studies assessing benefits and harms of vasopressin receptor antagonists were likely instigated and sponsored by the pharmaceutical company developing or seeking to commercialise the compound. For the studies that provided a declaration of interest for the authors of study reports, all save one had author lists that featured people who had received money for presentations or consultancy, or were employed by the sponsor. Industry sponsorship does not necessarily introduce bias into the design and conduct of clinical trials, but empiric evidence does show that pharmaceutical industry‐sponsored studies are more likely to have favourable efficacy results (RR 1.32, 95% CI 1.21 to 1.44) and harm results (RR 1.87, 95% CI 1.54 to 2.27) than studies not sponsored by industry (Lundh 2012).
Data for other interventions such as fluid restriction, change in medical regimens, captopril or albumin were sparse and inconclusive.
Potential biases in the review process
Although this review was conducted by two or more independent authors, used a comprehensive search of the published and unpublished research designed by a specialist librarian, and examined all potentially relevant clinical outcomes, potential biases exist in the review process. The major weakness of this review is the paucity of data for treatments other than vasopressin receptor antagonists. First, summary of existing evidence therefore focusses the discourse on new, and expensive, treatments, rather than focusing on existing, and cheaper alternatives. RCTs are extraordinarily expensive and consequently often conducted by the pharmaceutical industry. This results in a catch‐22 situation of evidence being mostly created, and thus only available, for newer interventions in general. Many other interventions are or have been used in clinical practice, but were driven to the background because RCT data were largely absent. Notably, also for fluid restriction, despite it being the currently accepted first‐line treatment for both hypervolaemic and euvolaemic hyponatraemia, there are no randomised trial data available. Currently, there are limited opportunities for use of vasopressin receptor antagonists in practice. Only two vasopressin receptor antagonists have obtained large scale regulatory approval. Conivaptan is FDA approved for euvolaemic and hypervolaemic hyponatraemia in hospitalised patients. It is available only as an intravenous preparation and treatment duration is limited to a maximum duration of four days because of drug‐interaction effects with other agents metabolized by the cytochrome P450 3A4 hepatic isoenzyme (FDA 2012). Tolvaptan, an oral agent, is also FDA‐approved for the treatment of euvolaemic and hypervolaemic hyponatraemia. Although theoretically available for long‐term treatment, recent concerns around the potential for severe liver injury in patients with autosomal dominant polycystic kidney disease ‐ be it at doses far exceeding the ones used in hyponatraemia ‐ has caused the FDA to restrict the use of tolvaptan to 30 days and issue a contra‐indication for patients with underlying liver disease (Mirski 2013). Canadian regulatory authorities mandated monitoring liver injury tests at regular intervals, but did not limit duration of use. In the European Union, tolvaptan is approved only for the treatment of euvolaemic hyponatraemia, due to safety concerns stemming from a numeric ‐ be it statistically non‐significant ‐ difference in treatment‐emergent fatalities in patients with hypervolaemia.
Agreements and disagreements with other studies or reviews
This review largely agreed with the findings of two earlier systematic reviews. However, neither of these previous reviews sought to examine any other treatments beyond vasopressin receptor antagonists for treating chronic non‐hypovolaemic hypotonic hyponatraemia. The first, including 15 RCTs and 1619 participants found vasopressin antagonists on average increased the serum sodium concentration by approximately 5 mmol/L at one week (13 studies, 1119 participants: MD 5.27 mmol/L, 95% CI 4.27 to 6.26), and approximately 3.5 mmol/L beyond the first week (8 studies, 793 participants: MD 3.49 mmol/L, 95% CI 2.56 to 4.41), but it came at a cost of increased risk of overly rapid correction of the serum sodium concentration (8 studies, 860 participants: RR, 2.52, 95% CI 1.26 to 5.08) (Rozen‐Zvi 2010).The second review, including 11 RCTs and 1094 participants, found vasopressin antagonists on average increased the serum sodium concentration by approximately 5 mmol/L at day 4 (11 studies, 1094 participants: MD 4.90 mmol/L, 95% CI 4.10 to 5.80), but was associated with a 6% increased risk of overly rapid correction of the serum sodium concentration (9 studies, 995 participants: RD 0.06, 95% CI 0.03 to 0.10) (Jaber 2011).
We also largely agreed with the interpretation of these data by the respective study authors as presented in their discussion: vasopressin receptor antagonists effectively raise the serum sodium concentration. But whether this translates to meaningful changes in outcomes that matter to patients is not clear. At this stage the available RCTs have insufficiently studied clinical outcomes to conclude that people whose serum sodium concentration increases under treatment will experience changes in end‐points such as general well‐being, cognitive function, gait stability, bone fractures and survival.
In 2013, a guideline group consisting of seven authors (six Americans and one Irishman), published expert recommendations identifying several alternative treatments, including fluid restriction, demeclocycline, urea and vasopressin receptor antagonists. Driven by the emergence of RCT evidence for vasopressin receptor antagonists as effective means for increasing the serum sodium concentration, and the absence of such evidence for other treatments, they projected vasopressin receptor antagonists were likely to become a mainstay of treatment for euvolaemic hyponatraemia and probably represented the best approach to treating hyponatraemia in most oedema‐forming states (Verbalis 2013).
A European guideline published in 2014 ‐ to which four authors of this review contributed ‐ suggested that in moderate or profound hyponatraemia (serum sodium concentration < 130 mmol/L), restricting fluid intake was first‐line treatment (2D); increasing solute intake with urea or a combination of low‐dose loop diuretics and oral sodium chloride were equal second‐line treatments (2D); and vasopressin receptor antagonists were not recommended (1C). (Spasovski 2014). There is almost no RCT evidence for treatments other than vasopressin receptor antagonists. The group's rationale to advocate some of these treatments anyway stemmed from the premise that in the absence of unequivocal evidence that increasing the serum sodium concentration leads to an improvement in patient‐important outcomes, the focus should be on avoiding harm. They gave much weight to avoiding rapid increases in the serum sodium concentration, for it's ‐ albeit very rare ‐ association with osmotic demyelination syndrome. And based on the absence of observational evidence for rapid increases or other important harms for fluid restriction, urea and low‐dose loop diuretics with oral sodium chloride, they formulated a weak recommendation to consider these for treating people with sodium concentrations below 130 mmol/L. Largely because of the documented risk of rapid increase and some concern around its potential for liver disease, a negative recommendation was formulated for vasopressin receptor antagonists.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Effect of baseline serum sodium concentration on change in natraemia: meta‐regression

Funnel plot of comparison: 1 Vasopressin receptor antagonists versus placebo or no treatment, outcome: 1.6 Response in serum sodium concentration.
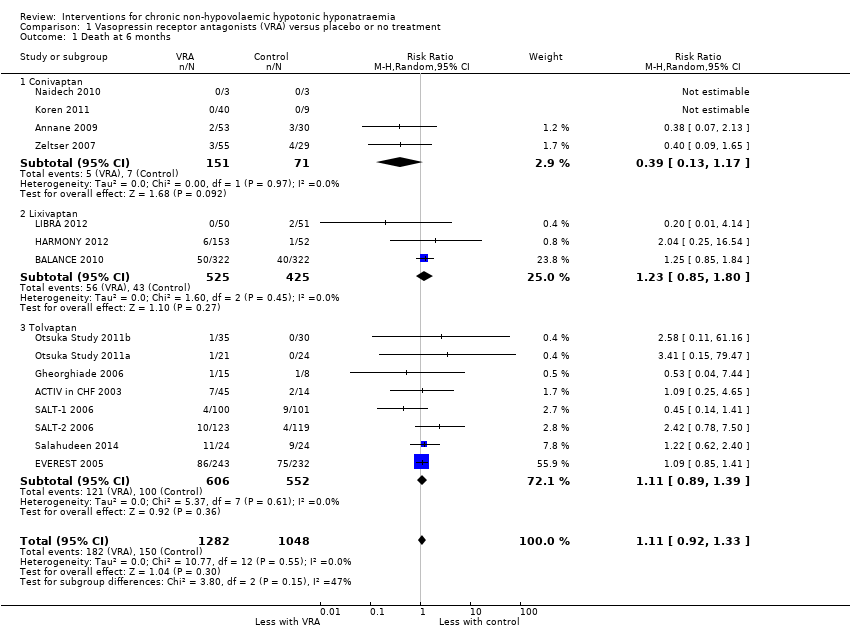
Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 1 Death at 6 months.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 2 Health‐related quality of life.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 3 Cognitive function: trail making test Part B.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 4 Length of hospital stay.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 5 Change from baseline serum sodium concentration.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 6 Response in serum sodium concentration.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 7 Rapid increase in serum sodium concentration.
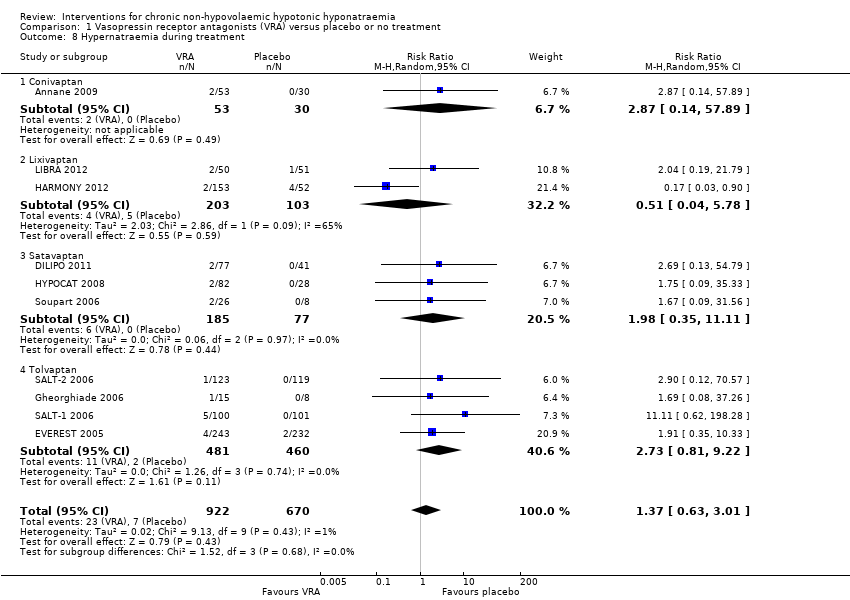
Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 8 Hypernatraemia during treatment.
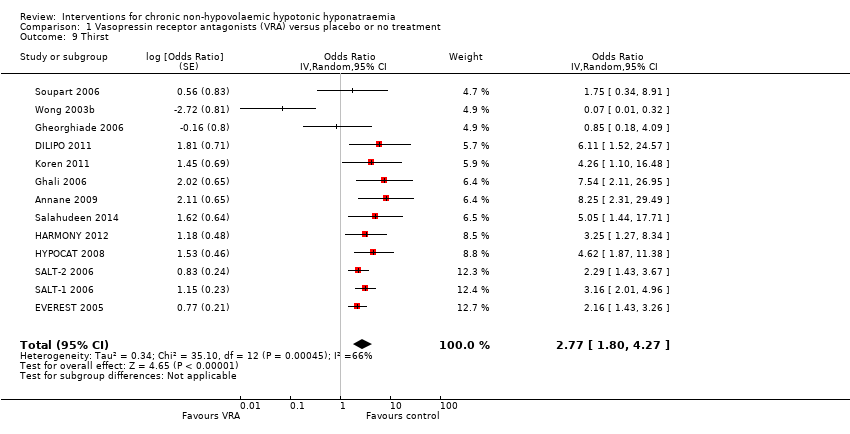
Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 9 Thirst.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 10 Other adverse events.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 11 Injection‐site complications at 2 to 7 days.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 12 Treatment discontinuation.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 13 Death during follow‐up: sensitivity analysis.

Comparison 1 Vasopressin receptor antagonists (VRA) versus placebo or no treatment, Outcome 14 Rapid increase in serum sodium concentration: sensitivity analysis.
| Vasopressin receptor antagonists versus placebo or no treatment for chronic non‐hypovolaemic hypotonic hyponatraemia | ||||||
| Patient or population: chronic non‐hypovolaemic hypotonic hyponatraemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo or no treatment | Vasopressin receptor antagonists | |||||
| Death Follow‐up: range 2 to 180 days | Study population | RR 1.11 | 2330 (15) | ⊕⊝⊝⊝ | Interpretation: effect uncertain; may both result in 11/1000 fewer to 47/1000 more deaths within 6 months | |
| 143 per 1000 1 | 159 per 1000 | |||||
| Health‐related quality of life (assessed with mental component score of SF‐124) Follow‐up: 30 days | The mean change from baseline in health‐related quality of life in the control group ranged between 0.75 and 2.39 on a 0 to 100 point scale (worst to best) 5 | The mean health‐related quality of life in the intervention group was 4.76 higher (0.11 higher to 9.41 higher) | ‐ | 297 (2) | ⊕⊝⊝⊝ | Physical component score also measured in both studies; RR 1.04; CI ‐1.81 to 3.90 Interpretation: anywhere from 0.1 to 9.5/100 points higher increase with treatment, but questionable tool for QoL measurement in hyponatraemia and unclear minimally important clinical difference |
| Length of hospital stay | The mean length of hospital stay in the control group was 6 to 11 days 5 | The mean length of hospital stay in the intervention group was 1.63 days lower (2.96 lower to 0.30 lower) | ‐ | 580 (2) | ⊕⊕⊝⊝ | |
| Cognitive function (assessed with various tools) Follow‐up: 1 to 6 months | Across five studies, confidence intervals spanned the line of no effect and did not include a clinically meaningful effect | ‐ | 1169 (5) | ⊕⊕⊝⊝ LOW 10 | Tools used to assess cognitive function: making test B; reaction time, psychomotor, processing speeds; Mini mental state exam; overall meta‐analysis including all five studies not meaningfully possible | |
| Change from baseline in serum sodium concentration Follow‐up: range 1 to 180 days | The mean change from baseline in serum sodium concentration in the control group was 0.3 to 4.8 mmol/L 5 | The mean change from baseline in serum sodium concentration in the intervention group was 4.17 mmol/L higher (3.18 higher to 5.16 higher) | ‐ | 2641 (21) | ⊕⊕⊕⊝ | |
| Serum sodium concentration (response) Follow‐up: range 4 to 180 days | Study population | RR 2.49 | 2104 (18) | ⊕⊕⊕⊝ | Response most commonly defined by investigators as > 5 to > 6 mmol/L increase or normalisation of serum sodium concentration | |
| 231 per 1000 1 | 576 per 1000 | |||||
| Rapid sodium increase Follow‐up: range 1 to 5 days | Study population | RR 1.67 | 2058 (14) | ⊕⊕⊕⊝ | Rapid increase most commonly defined as > 12 mmol/d | |
| 44 per 1000 1 | 73 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Source of assumed baseline risk was calculated as the unweighted summed event rate in the control groups of the trials included in the meta‐analysis 2 Downgraded one level because studies considered at serious risk of suffering from * selective reporting: 6/16 studies with treatment duration > 1 week did not report death and had no protocol, accounting for 16% of the total number of participants in those studies * commercial sponsorship; possible financial conflict of interest of the authors: all studies sponsored by pharmaceutical companies wanting to commercialize the treatment; all save one had author lists who featured people who had received money for presentations or consultancy, or were employed by the sponsor; only used as supporting reason for downgrading. 3 Downgraded two levels for imprecision. The 95% CI of the pooled estimate includes both important reduction (11/1000 fewer) and increase (47/1000 more) in death with vasopressin receptor antagonists. 4 Choice of the outcome‐measure based on the fact that this was the only one reported in any of the studies. There are concerns around the validity of the SF‐12 as a measure for health‐related quality of life in the field of hyponatraemia as it gauges domains and symptoms not directly attributable to hyponatraemia. 5 Source of the assumed baseline risk was the range of outcomes in the control groups of the trials included in the meta‐analysis 6 Downgraded one level because studies considered seriously at risk of suffering from * Performance bias: self‐reported outcome, participants likely unblinded to treatment due to polyuria as side effect * Selective reporting bias: both mental and physical component score of SF‐12 measured; at week 1 or 2 and day 30 using two different analytic techniques. Only data at day 30 available for analysis. * Attrition bias: overall 37% of data missing, unknown whether missing at random or not. * Commercial sponsorship or possible financial conflict of interest of authors: both studies were sponsored by the company seeking to commercialise the treatment; both had author lists who featured people who had received money for presentations or consultancy, or were employed by the sponsor; only using as supporting argument for downgrading 7 Downgraded one level for indirectness due to concerns around validity of the SF‐12 for measuring quality of life in the context of hyponatraemia and one level for imprecision: only studied in two studies. 8 Downgraded one level because studies considered seriously at risk of suffering from performance bias: participants and personnel likely unblinded to treatment due to polyuria as side effect; this could have influenced self‐reported and professional appreciation of clinical condition and so have influenced decision to discharge from hospital. 9 Downgraded one level for imprecision. The 95% CI of the pooled estimate includes both negligible shortening (0.3 days shorter) and clinically important shortening (3 days shorter) of hospital stay with vasopressin receptor antagonists. 10 Downgraded two levels for indirectness and imprecision. Only studied in 5/28 studies, with most data for lixivaptan, and other studies not reaching the optimal information size. 11 Downgraded one level because we considered studies seriously at risk of suffering from * Attrition bias: 7/21 studies, accounting for 53% of the total number of participants in those studies at high risk of bias either due to true attrition or because a repeated measures analytic technique was used with all measurements of serum sodium concentration included until patient attrition, which we judged would likely have overestimated the treatment effect. *Commercial sponsorship; only used as supporting argument. 12 Downgraded one level for indirectness; risks controlled in tightly organised randomised trial with several measurements of serum sodium concentration daily to avoid rapid correction. In real life, risk of rapid correction likely greater. Commercial sponsorship bias; only used as supporting argument. | ||||||
| Covariate | Number of studies included in meta‐regression | Scale | Absolute change in mean difference | P value |
| Baseline serum sodium concentration | 21 | Per 1 mmol/L increase | ‐0.33 (‐0.65 to ‐0.02) | 0.04 |
| Compound | 21 | Relative to conivaptan | ‐ | 0.17 |
| Conivaptan | 5 | ‐ | ‐ | ‐ |
| Lixivaptan | 4 | ‐ | ‐2.79 (‐5.47 to ‐0.10) | ‐ |
| Satavaptan | 3 | ‐ | ‐0,23 (‐3.34 to 2.87) | ‐ |
| Tolvaptan | 9 | ‐ | ‐0.82 (‐3.13 to 1.50) | ‐ |
| Cause of hyponatraemia | 21 | Relative to SIADH | ‐ | 0.18 |
| SIADH | 5 | ‐ | ‐ | ‐ |
| Combined SIADH ‐ Heart failure, cirrhosis | 10 | ‐ | 0.67 (‐1.77 to 3.13) | ‐ |
| Heart failure | 6 | ‐ | ‐1.19 (‐3.84 to 1.47) | ‐ |
| Treatment duration | 21 | Per day increase | ‐0.02 (‐0,04 to 0.00) | 0.1 |
| Risk of selection bias | 21 | Relative to low risk | ‐ | 0.86 |
| Low risk | 8 | ‐ | ‐ | ‐ |
| High risk | 13 | ‐ | 0.18 (‐1.90 to 2.27) | ‐ |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death at 6 months Show forest plot | 15 | 2330 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.92, 1.33] |
| 1.1 Conivaptan | 4 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.13, 1.17] |
| 1.2 Lixivaptan | 3 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.85, 1.80] |
| 1.3 Tolvaptan | 8 | 1158 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.89, 1.39] |
| 2 Health‐related quality of life Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Mental component SF‐12 | 2 | 297 | Mean Difference (IV, Random, 95% CI) | 4.76 [0.11, 9.41] |
| 2.2 Physical component SF‐12 | 2 | 300 | Mean Difference (IV, Random, 95% CI) | 1.04 [‐1.81, 3.90] |
| 3 Cognitive function: trail making test Part B Show forest plot | 2 | 858 | Mean Difference (IV, Random, 95% CI) | 6.89 [‐6.34, 20.12] |
| 4 Length of hospital stay Show forest plot | 3 | 610 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.96, ‐0.30] |
| 4.1 Satavaptan | 1 | 139 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐3.80, 0.20] |
| 4.2 Tolvaptan | 2 | 471 | Mean Difference (IV, Random, 95% CI) | ‐1.50 [‐3.28, 0.29] |
| 5 Change from baseline serum sodium concentration Show forest plot | 21 | 2641 | Mean Difference (IV, Random, 95% CI) | 4.17 [3.18, 5.16] |
| 5.1 Conivaptan | 5 | 292 | Mean Difference (IV, Random, 95% CI) | 5.17 [2.65, 7.69] |
| 5.2 Lixivaptan | 4 | 1070 | Mean Difference (IV, Random, 95% CI) | 2.24 [0.78, 3.70] |
| 5.3 Satavaptan | 3 | 257 | Mean Difference (IV, Random, 95% CI) | 4.91 [2.88, 6.94] |
| 5.4 Tolvaptan | 9 | 1022 | Mean Difference (IV, Random, 95% CI) | 4.22 [3.55, 4.89] |
| 6 Response in serum sodium concentration Show forest plot | 18 | 2104 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [1.95, 3.18] |
| 6.1 Conivaptan | 4 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.54, 4.01] |
| 6.2 Lixivaptan | 4 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [1.19, 4.10] |
| 6.3 Satavaptan | 4 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 3.33 [1.88, 5.89] |
| 6.4 Tolvaptan | 6 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [1.75, 3.18] |
| 7 Rapid increase in serum sodium concentration Show forest plot | 14 | 2058 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.16, 2.40] |
| 7.1 Conivaptan | 4 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 3.77 [0.89, 15.98] |
| 7.2 Lixivaptan | 4 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.90, 2.17] |
| 7.3 Satavaptan | 4 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.73, 9.30] |
| 7.4 Tolvaptan | 2 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 6.70 [0.82, 54.56] |
| 8 Hypernatraemia during treatment Show forest plot | 10 | 1592 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.63, 3.01] |
| 8.1 Conivaptan | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.14, 57.89] |
| 8.2 Lixivaptan | 2 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.04, 5.78] |
| 8.3 Satavaptan | 3 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.35, 11.11] |
| 8.4 Tolvaptan | 4 | 941 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [0.81, 9.22] |
| 9 Thirst Show forest plot | 13 | Odds Ratio (Random, 95% CI) | 2.77 [1.80, 4.27] | |
| 10 Other adverse events Show forest plot | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Polyuria | 6 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [1.59, 13.85] |
| 10.2 Hypotension | 14 | 1748 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.75, 1.63] |
| 10.3 Acute kidney injury | 8 | 1920 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.67, 1.18] |
| 10.4 Liver function abnormalities | 3 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [0.88, 6.70] |
| 11 Injection‐site complications at 2 to 7 days Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Reactions | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 7.56 [0.49, 115.93] |
| 11.2 Phlebitis | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 3.52 [1.00, 12.41] |
| 11.3 Thrombosis | 2 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.21, 14.80] |
| 12 Treatment discontinuation Show forest plot | 14 | 2429 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.85, 1.00] |
| 12.1 Conivaptan | 4 | 288 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.39, 1.30] |
| 12.2 Lixivaptan | 4 | 1008 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.84, 1.03] |
| 12.3 Satavaptan | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.46] |
| 12.4 Tolvaptan | 4 | 988 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.66, 1.13] |
| 13 Death during follow‐up: sensitivity analysis Show forest plot | 16 | 2404 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.32] |
| 13.1 Conivaptan | 5 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.19, 1.15] |
| 13.2 Lixivaptan | 3 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.37, 3.15] |
| 13.3 Tolvaptan | 8 | 1158 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.89, 1.39] |
| 14 Rapid increase in serum sodium concentration: sensitivity analysis Show forest plot | 14 | 2058 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.16, 2.40] |
| 14.1 > 8 mmol/L/d | 4 | 257 | Risk Ratio (M‐H, Random, 95% CI) | 3.22 [0.65, 15.94] |
| 14.2 > 12 mmol/L/d | 10 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.09, 2.43] |

