Tratamiento para pacientes con anemia ferropénica posparto
Resumen
Antecedentes
La anemia ferropénica posparto es causada por hemorragia o ingesta / captación inadecuadas de hierro dietético. Esta afección se define por la carencia de hierro acompañada de una concentración de hemoglobina sanguínea inferior a la normal, aunque puede estar afectada por otros factores aparte de la anemia y se debe interpretar según la presencia de cualquier síntoma concurrente. Los síntomas incluyen fatiga, disnea y mareos. Las opciones de tratamiento incluyen hierro oral o intravenoso, eritropoyetina que estimula la producción de eritrocitos y la sustitución mediante la transfusión de eritrocitos.
Objetivos
Evaluar la eficacia y los efectos perjudiciales de las formas de tratamiento disponibles para las pacientes con anemia ferropénica posparto.
Métodos de búsqueda
registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (9 abril 2015); WHO International Clinical Trials Registry Portal (ICTRP) y la Latin‐American and Caribbean Health Sciences Literature database (LILACS) (8 abril 2015) y listas de referencias de estudios recuperados.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios publicados, no publicados y en curso que compararon un tratamiento para la anemia ferropénica posparto con placebo, ningún tratamiento u otro tratamiento para la anemia ferropénica posparto, incluidos los ensayos descritos solamente como resúmenes. Se consideró la inclusión de ensayos asignados al azar por grupos. Se incluyeron los ensayos abiertos y los ensayos con cegamiento, independientemente de quién se cegó. Las participantes fueron pacientes con una hemoglobina posparto de 120 g por litro (g/l) o menos, en las que el tratamiento se inició en el transcurso de seis semanas después del parto.
Se excluyeron los ensayos no aleatorios, los ensayos cuasialeatorios y los ensayos que utilizaron un diseño cruzado.
Obtención y análisis de los datos
Dos autores de la revisión evaluaron de forma independiente los estudios para la inclusión, examinaron la calidad de los mismos y extrajeron los datos. Se contactó con los autores de los estudios y con la industria farmacéutica para obtener información adicional.
Resultados principales
Se incluyeron 22 ensayos controlados aleatorios (2858 pacientes) y la mayoría tuvo alto riesgo de sesgo en varios dominios. Se realizaron 13 comparaciones. Muchas comparaciones se basan en un escaso número de estudios con tamaños de la muestra pequeños. Ningún análisis de los resultados primarios incluyó más de dos estudios.
El hierro intravenoso se comparó con el hierro oral en diez estudios (1553 pacientes). La fatiga se informó en dos estudios y mejoró significativamente a favor del grupo tratado por vía intravenosa en uno de los estudios. No se informaron otros síntomas de anemia. Una paciente murió debido a miocardiopatía (cociente de riesgos [CR] 2,95; intervalo de confianza [IC] del 95%: 0,12 a 71,96; dos estudios; un evento; 374 pacientes; pruebas de baja calidad). Una paciente desarrolló arritmia. Ambas complicaciones cardíacas ocurrieron en el grupo tratado por vía intravenosa. Ocurrieron reacciones alérgicas en tres pacientes tratadas con hierro intravenoso, que no fue estadísticamente significativo (CR promedio 2,78; IC del 95%: 0,31 a 24,92; ocho estudios; 1454 pacientes; I² = 0%; pruebas de baja calidad). Los eventos gastrointestinales fueron menos frecuentes en el grupo tratado por vía intravenosa (CR promedio 0,31; IC del 95%: 0,20 a 0,47; ocho estudios; 169 eventos; 1307 pacientes; I² = 0%; pruebas de muy baja calidad).
Un estudio evaluó la transfusión de eritrocitos versus ninguna intervención. La fatiga general mejoró significativamente más en el grupo de transfusión a los tres días (DM ‐0,80; IC del 95%: ‐1,53 a ‐0,07; 388 pacientes; pruebas de baja calidad ), pero no se observó diferencias entre los grupos a las seis semanas. No se informó la mortalidad materna.
Las comparaciones restantes evaluaron hierro oral (con o sin otras sustancias alimenticias) versus placebo (tres estudios), hierro intravenoso con hierro oral versus hierro oral (dos estudios) y eritropoyetina (sola o combinada con hierro) versus placebo o hierro (siete estudios). Estos estudios no investigaron la fatiga. La mortalidad materna rara vez se informó.
Conclusiones de los autores
El grupo de pruebas no permitió establecer una conclusión clara con respecto a la eficacia de las intervenciones en la anemia ferropénica posparto. La calidad de las pruebas fue baja.
Rara vez se informaron los resultados clínicos. Es posible que los valores de laboratorio no sean indicadores confiables de la eficacia, ya que no siempre se correlacionan con los efectos clínicos del tratamiento. Todavía no está claro qué forma de tratamiento es la más eficaz para aliviar los síntomas de anemia posparto.
El hierro intravenoso fue superior con respecto a los efectos perjudiciales gastrointestinales, aunque hubo anafilaxia y eventos cardíacos y se necesitan más datos para establecer si fueron causados por el hierro intravenoso.
La significación clínica de alguna mejoría temporal en las puntuaciones de fatiga de las pacientes tratadas con transfusión de sangre es incierta y este efecto moderado se debe equilibrar contra los riesgos conocidos, p.ej. mortalidad materna (no informada) y sensibilización inmunológica materna, que puede dañar potencialmente los embarazos futuros.
Cuando se compara hierro oral con placebo aún se desconoce si la eficacia (alivio de los síntomas de anemia) supera los efectos perjudiciales gastrointestinales documentados.
No fue posible establecer conclusiones con respecto al tratamiento con eritropoyetina debido a la falta de pruebas.
Los estudios de investigación adicionales deben evaluar el efecto del tratamiento mediante resultados clínicos, es decir, presencia y gravedad de los síntomas de anemia equilibrados contra los efectos perjudiciales, es decir supervivencia y morbilidad grave.
PICOs
Resumen en términos sencillos
Tratamiento para las pacientes con anemia ferropénica después del parto
La anemia es una afección en la que la sangre contiene menos hemoglobina que lo normal (recuento sanguíneo bajo), demostrado mediante análisis de sangre. La hemoglobina es la molécula dentro de los eritrocitos que necesita del hierro para transportar el oxígeno. La ingesta / captación insuficiente de hierro y la pérdida de hierro (hemorragia) puede causar anemia ferropénica. Los síntomas de anemia incluyen cansancio, disnea y mareo. Las pacientes pueden tener una hemorragia grave durante el parto y muchas embarazadas ya presentan anemia que puede empeorar como resultado de la hemorragia. La anemia grave puede estar vinculada a las muertes maternas. Es más probable que la anemia ferropénica después del parto ocurra en los países de bajos ingresos.
El tratamiento para la anemia ferropénica incluye comprimidos de hierro o una solución inyectada en una vena (por vía intravenosa). Otra opción es restaurar los eritrocitos mediante la transfusión de sangre de un donante o el estímulo de la formación de eritrocitos con eritropoyetina. Es importante investigar si un tratamiento es mejor que otro en el alivio de los síntomas de anemia y si las opciones de tratamiento son seguras.
Se incluyeron 22 estudios controlados aleatorios con 2858 pacientes y se realizaron 13 comparaciones, muchas de las cuales se basaron en pocos estudios que incluyeron escasos números de pacientes. La calidad general de las pruebas fue baja. La mayoría de los ensayos se realizó en países de ingresos altos.
Diez estudios que incluyeron 1553 pacientes compararon hierro intravenoso con hierro oral. Solamente un estudio mostró un efecto positivo temporal del hierro intravenoso sobre la fatiga. No se informaron otros síntomas de anemia. Una paciente murió debido a complicaciones cardíacas en el grupo intravenoso. Sólo dos estudios informaron sobre las muertes maternas. Ocurrieron reacciones alérgicas en tres pacientes y complicaciones cardíacas en dos pacientes del grupo intravenoso. Los síntomas gastrointestinales fueron frecuentes en el grupo oral y provocaron que algunas participantes abandonaran el tratamiento.
Un estudio comparó la transfusión de eritrocitos con ninguna transfusión. Algunas puntuaciones de fatiga (pero no todas) mejoraron temporalmente en las pacientes que recibieron transfusión. No se informó la mortalidad materna.
Cuando se comparó hierro oral con placebo (tres estudios), no se informaron los síntomas de anemia. Aún se desconoce si los efectos beneficiosos del hierro oral superan los efectos perjudiciales gastrointestinales documentados.
Otras opciones de tratamiento se compararon en otros estudios que no investigaron la fatiga.
Muy pocos estudios informaron el alivio de los síntomas de anemia, que probablemente es el objetivo más importante del tratamiento.
El grupo de pruebas no permitió evaluar completamente la eficacia de los tratamientos para la anemia ferropénica después del parto y se necesitan estudios de investigación adicionales.
Conclusiones de los autores
Summary of findings
| Intravenous iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Intravenous iron | |||||
| Maternal mortality | Study population | RR 2.95 | 374 | ⊕⊕⊝⊝ | 1 maternal death was reported across the included studies. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Fatigue at 14, 28, and 42 days | See comment | See comment | Not estimable | 361 | ⊕⊝⊝⊝ | No statistically significant difference was found at days 14 and 42 days. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Infections | Study population | RR 1.7 | 718 | ⊕⊝⊝⊝ | ||
| 86 per 1000 | 146 per 1000 | |||||
| Moderate | ||||||
| 34 per 1000 | 58 per 1000 | |||||
| Constipation | Study population | RR 0.21 | 1217 | ⊕⊝⊝⊝ | ||
| 114 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 112 per 1000 | 24 per 1000 | |||||
| All gastrointestinal symptoms | Study population | RR 0.31 | 1307 | ⊕⊝⊝⊝ | ||
| 216 per 1000 | 67 per 1000 | |||||
| Moderate | ||||||
| 261 per 1000 | 81 per 1000 | |||||
| Anaphylaxis or evidence of hypersensitivity | Study population | RR 2.78 | 1454 | ⊕⊕⊝⊝ | 3 cases of allergic reactions all occurred in the group treated with intravenous iron. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The outcome is unlikely to be influenced by risk of bias and so we did not downgrade the evidence for this outcome: open‐label design combined with a objective outcome measure. | ||||||
| Red blood cell transfusion compared with non‐transfusion for postpartum iron deficiency anaemia | ||||||
| Patient or population: patients with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐transfusion | RBC transfusion | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue | See comment | See comment | 519 | ⊕⊕⊝⊝ | General fatigue at 3 days was 0.8 lower (1.53 to 0.07) in the transfused group. No statistically significant difference was seen at six weeks. | |
| Persistent anaemia symptoms | Study population | Not estimable | 519 | ⊕⊝⊝⊝ | The outcome was not systematically registered/reported. | |
| See comment | See comment | |||||
| Moderate | ||||||
| Infections | Study population | RR 0.93 | 519 | ⊕⊕⊕⊝ | ||
| 92 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 92 per 1000 | 86 per 1000 | |||||
| Erythrocyte alloantibody formation | Study population | RR 3.03 | 519 | ⊕⊝⊝⊝ | There was no systematical screening for this outcome in the study population. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Thromboembolic events | Study population | RR 1.01 | 519 | ⊕⊕⊝⊝ | ||
| 8 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 8 per 1000 | 8 per 1000 | |||||
| Transfusion reactions | Study population | RR 7.08 | 519 | ⊕⊝⊝⊝ | 3 cases of transfusion reactions occurred in the transfusion group. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: open‐label design combined with a subjective outcome measure. | ||||||
| Oral iron compared with placebo for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oral iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms | Study population | Not estimable | (1) | See comment | Symptoms of anaemia were not reported for the anaemic groups separately. | |
| See comment | See comment | |||||
| Moderate | ||||||
| All gastrointestinal symptoms | Study population | RR 1 | 68 | ⊕⊝⊝⊝ | ||
| 176 per 1000 | 176 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 177 per 1000 | |||||
| Constipation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: open‐label design combined with a subjective outcome measure. | ||||||
| Intravenous iron with oral iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Intravenous iron with oral iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | ‐ | See comment | In 1 study no maternal deaths were reported. The other study did not report on maternal mortality. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ 1 week | Study population | RR 1.75 | 72 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 194 per 1000 | |||||
| Moderate | ||||||
| 111 per 1000 | 194 per 1000 | |||||
| Persistent anaemia symptoms ‐ 2 weeks | Study population | RR 0.6 | 72 | ⊕⊝⊝⊝ | ||
| 139 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 139 per 1000 | 83 per 1000 | |||||
| Persistent anaemia symptoms ‐ 6 weeks | Study population | RR 3 | 72 | ⊕⊝⊝⊝ | ||
| 28 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 28 per 1000 | 84 per 1000 | |||||
| Infections ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Anaphylaxis or evidence of hypersensitivity | Study population | Not estimable | 0 | ⊕⊕⊝⊝ | 1 study reported 0 events, other study pooled adverse events, not reporting allergic reactions separately. Thus the effect was not estimable. | |
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: the included study had high risk of attrition and reporting bias. | ||||||
| Erythropoietin (regardless of rout) with intravenous iron compared with intravenous iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron | EPO (regardless of rout) with IV iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | ‐ | See comment | In 1 study no maternal deaths were reported. The other study did not report on maternal mortality. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO 10,000 U of doses with intravenous iron compared with intravenous iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: patients with women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron | Erythropoietin 10,000 U 2 doses with intravenous iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO with oral iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Subcutaneous EPO with oral iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | 40 | See comment | No maternal deaths were reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO with IV iron and oral iron compared with intravenous iron with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron + oral iron | Subcutaneous EPO + IV iron + oral iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
Antecedentes
Descripción de la afección
Las pacientes que tienen un parto pueden contraer anemia posparto debido a hemorragia excesiva o a afecciones preexistentes en el embarazo. La anemia posparto grave puede ser un problema importante y posiblemente está vinculada al 40% de las muertes maternas en todo el mundo (WHO 2001). La anemia también aumenta el riesgo de muerte materna por otras causas como las infecciones, la desnutrición y la hemorragia (WHO 2012b). En algunas mujeres, en particular en los países de escasos recursos, la anemia posparto es una causa importante de salud deficiente (Bergmann 2010; Gupta 2010; Khan 2006; WHO 2012a).
La anemia, incluida la anemia posparto, se define por un valor de hemoglobina (Hb) inferior al normal, pero los síntomas clínicos son fundamentales para la evaluación de su importancia. La hemoglobina es la molécula contenida dentro de los eritrocitos y es responsable del transporte de oxígeno en el cuerpo. Durante el embarazo, la mayoría de las pacientes presenta una reducción fisiológicamente normal de la concentración de Hb debido a la acumulación de líquido (WHO 2001). La anemia posparto puede ser causada o incrementarse por una baja ingesta o captación de hierro dietético, pérdida de sangre o infecciones (p.ej. paludismo) y los cambios fisiológicos durante el embarazo y la hemorragia asociada con el parto pueden agravar la afección (WHO 1999).
La anemia posparto puede causar síntomas como disnea, palpitaciones (una sensación de aumento de la frecuencia cardíaca) y cansancio, así como un aumento del riesgo de infecciones. Todos estos síntomas pueden repercutir en la capacidad de la mujer de lactar y cuidar a su recién nacido en general (Bergmann 2010; Milman 2011).
Durante el embarazo, el volumen sanguíneo circulante aumenta para preparar a la mujer para la pérdida de sangre en el momento del parto. La cantidad de hemorragia o resorción del líquido excesivo de los tejidos corporales durante y después del parto varía entre las mujeres (Milman 2011), lo que puede tener una repercusión importante sobre las concentraciones de Hb materna. En general se acepta que una concentración baja de Hb, generalmente menos de 120 g por litro (g/l), es indicativa de anemia en las pacientes posparto, aunque hay una considerable variación en la concentración precisa que define la anemia y también en el tiempo después del parto al que se debe medir (Barroso 2011; Bergmann 2010; Bodnar 2005; Breymann 2010; Milman 2011; Richter 1995). Por lo tanto, la anemia posparto no se ha definido bien y el nivel de Hb en el período posparto (seis semanas después del parto) depende en gran medida de a qué tiempo después del parto se mide (WHO 2012a). Se debe recalcar que incluso aunque se ha mostrado una asociación entre una Hb baja y síntomas clínicos en los estudios observacionales basados en la población, el rango normal de Hb es un valor estadístico arbitrariamente definido derivado del promedio poblacional, y el nivel de Hb de una mujer individual no refleja necesariamente los síntomas clínicos que puede experimentar (WHO 2001). Como la correlación entre los diferentes síntomas clínicos y el nivel del Hb en la anemia posparto no se ha descrito bien, la importancia clínica de un cambio en el nivel de Hb como resultado de cualquier tratamiento administrado aún es incierta. Este es el motivo por el cual un cambio en la concentración de Hb es un resultado indirecto en los ensayos de intervenciones para la anemia. Sin embargo, en la práctica clínica un nivel bajo de Hb es la prueba de laboratorio utilizada con mayor frecuencia para apoyar o refutar el diagnóstico clínico de anemia y en general se entiende que es probable que una disminución importante en el nivel de Hb en un plazo corto se correlacione con una pérdida de sangre grande durante el parto, que puede dar lugar a síntomas agudos de anemia y shock.
Durante el embarazo, la anemia también se define por los niveles de Hb bajos y se estratifica en anemia leve, moderada o grave (WHO 2002). Sin embargo, no hay una correlación clara entre el tipo y la gravedad de los síntomas de anemia y estas estratificaciones. La anemia en el embarazo (p.ej. debido a ingesta dietética insuficiente) y la hemorragia durante o después del parto son variables predictivas sólidas para la anemia ferropénica posparto (Bodnar 2005; Milman 2011; Reveiz 2011). La anemia ferropénica es una afección en la que el nivel de Hb bajo es causado por una cantidad insuficiente de hierro en el cuerpo. Hasta donde se conoce, solamente un estudio ha calculado la incidencia de anemia ferropénica posparto, e informó el 4,2% entre las pacientes en los Estados Unidos examinadas en el transcurso de los seis primeros meses después del parto (Bodnar 2002).
La anemia ferropénica posparto no tiene un código específico en la International Classification of Diseases (ICD‐10), pero se incluye en el más código general 099.0 "Anemia que complica el embarazo, el parto y el puerperio" (WHO ICD 2010). Hasta el momento todas las definiciones de anemia posparto dependen solamente de los valores de Hb, no de los síntomas. La clasificación en diferentes estadios de gravedad también se basa solamente en los valores de Hb (Milman 2012). En el período posparto, al igual que durante el embarazo, aún no se ha respondido la pregunta de si cualquier efecto beneficioso del tratamiento de la anemia supera los efectos perjudiciales de dicho tratamiento (Reveiz 2011). Los efectos perjudiciales conocidos dependen de la elección del tratamiento e incluyen p.ej. síntomas gastrointestinales y reacciones alérgicas.
Descripción de la intervención
Hay varias opciones de tratamiento para las pacientes con anemia posparto, y el tratamiento óptimo, la dosis y el equilibrio entre los efectos beneficiosos y perjudiciales pueden variar según el momento y la gravedad de la anemia, los síntomas clínicos, los efectos perjudiciales de la intervención, los recursos disponibles y factores como la ubicación geográfica, el nivel socioeconómico y la educación. Las formas de tratamiento descritas en esta revisión incluyen suplementos de hierro administrados por vía oral o directamente en una vena o el músculo (parenteralmente), eritropoyetina que estimula la producción de eritrocitos y la sustitución de los eritrocitos mediante transfusión de sangre.
De qué manera podría funcionar la intervención
Tratamiento con hierro oral
El tratamiento con hierro oral se ha utilizado durante muchos años como tratamiento para la anemia ferropénica en general (Dudrick 1986), así como durante el embarazo (Pena‐Rosas 2012). El hierro oral es a menudo el tratamiento recomendado para la anemia ferropénica leve a moderada (Bodnar 2005) debido a su bajo costo y facilidad de uso. El cuerpo tiene una capacidad limitada de absorber el hierro de los intestinos y a menudo se requiere un tratamiento prolongado durante varios meses para aumentar la concentración de Hb y aliviar los síntomas de la anemia (Auerbach 2008; Milman 2012; Van Wyck 2007; Westad 2008). Los efectos adversos gastrointestinales (GI) como el estreñimiento y las náuseas son frecuentes con el tratamiento con hierro oral (al‐Momen 1996; Bhandal 2006). Lo anterior puede afectar al cumplimiento de las pacientes con el tratamiento y, por lo tanto, impedir la corrección de la anemia.
Folato
El folato, también llamado ácido fólico y vitamina B9, es una sustancia que se encuentra en muchos alimentos y está naturalmente disponible en las verduras en concentraciones especialmente altas. El folato participa en la síntesis del ADN, la división celular y el crecimiento en las células humanas. La deficiencia de folato puede causar anemia megaloblástica, no anemia ferropénica. Sin embargo, el folato a menudo se agrega como un complemento al hierro oral porque la desnutrición a menudo da lugar a una falta de hierro y de folato en el cuerpo. El efecto a largo plazo de la administración de folato y los niveles continuamente altos de folato en sangre se han asociado con un aumento del riesgo de ciertos cánceres (Almeida 2010). En esta revisión no se considerará la administración de suplementos de folato como un tratamiento independiente de la anemia, pero se aceptarán los estudios en los que forme parte de otros tipos de tratamiento para la anemia ferropénica posparto.
Tratamiento con hierro parenteral
Se ha mostrado que la administración parenteral de hierro produce un aumento más rápido de la concentración de Hb en la anemia ferropénica durante el embarazo (Milman 2012). El hierro administrado parenteralmente se ha asociado con dolor y rubor (eritema) en el sitio de inyección y, muy pocas veces, con reacciones anafilácticas caracterizadas por prurito, rubor y en casos graves angioedema (tumefacción), colapso vascular, broncoespasmo (constricción de las vías respiratorias) y shock (Barish 2012; Breymann 2008; Kochhar 2013; Seid 2008; Wysowski 2010). La administración de compuestos nuevos de hierro de bajo peso molecular (como sacarosa de hierro y carboximaltosa férrica) puede disminuir el riesgo de reacciones anafilácticas, pero estos productos son costosos comparados con el tratamiento con hierro oral, que no produce estos efectos perjudiciales graves (Khalafallah 2012; Kochhar 2013).
Eritropoyetina
La eritropoyetina (EPO) es una hormona producida en los riñones cuando los niveles de oxígeno sanguíneo son bajos. Actúa en la estimulación de la eritropoyesis (formación de sangre) en la médula ósea (Oster 2012). Inicialmente, la EPO se utilizó para la anemia asociada con enfermedades renales (del riñón). Posteriormente, la EPO se ha utilizado para tratar otras formas de anemia y como una opción a la transfusión de sangre para el tratamiento de la anemia ferropénica, incluida la anemia ferropénica posparto (Bergmann 2010; Oster 2012). Los efectos adversos del tratamiento con EPO incluyen síntomas leves similares a los de la gripe como odinofagia, tos, fiebre, mialgias y debilidad, cefalea y fatiga. Efectos adversos poco frecuentes pero más graves incluyen hipertensión, complicaciones tromboembólicas, crisis convulsivas y aplasia pura de eritrocitos (Dodd 2004; Kliger 2012). Estudios de investigación recientes han revelado una asociación con ciertos cánceres hematológicos, lo que dio lugar a advertencias de caja negra de la Food and Drug Agency (FDA) (etiqueta en el producto que advierte contra riesgos graves o potencialmente mortales). La administración de EPO se limita actualmente a grupos específicos de pacientes y se utiliza muy pocas veces en las pacientes con anemia posparto (Bunn 2009; Oster 2012).
Transfusión de sangre
La transfusión de sangre alogénica se puede utilizar en el tratamiento de la anemia posparto y puede ser salvar vidas en caso de hemorragia aguda o grave en el momento del parto (Montufar‐Rueda 2013). Sin embargo, cuando se provocaron disminuciones experimentales en voluntarios sanos de hasta 50 g/l de Hb en un contexto controlado, se produjeron mecanismos compensatorios cardíacos, pero no se comprometió la salud (Weiskopf 1998). Se han encontrado reacciones adversas en lugar de efectos clínicos beneficiosos cuando se transfundieron poblaciones mixtas de pacientes con anemia leve a moderada (Carson 2012; Rohde 2014; Salpeter 2014). Por lo tanto, en general no se recomienda la transfusión después de hemorragias pequeñas a moderadas en pacientes con una respuesta fisiológica normal a la anemia. La transfusión de una unidad de eritrocitos aumenta generalmente la Hb en 10 g/l en pacientes hemodinámicamente estables sin hemorragia (Wiesen 1994). Existen riesgos asociados que incluyen infecciones transmitidas por el donante (en particular la hepatitis y el virus de la inmunodeficiencia humana [VIH]), sobrecarga circulatoria asociada a las transfusión y diversas reacciones inmunológicas como fiebre, urticaria (ronchas), anafilaxia, lesión pulmonar relacionada con la transfusión o formación de anticuerpos que pueden interferir con los embarazos futuros (Fuller 2010; Hendrickson 2009; SHOT Report 2011; Villanueva 2013). La transfusión de sangre algunas veces puede causar hemólisis aguda (deterioro de los eritrocitos) si se administra sangre incompatible por error (Fuller 2010). Las transfusiones de sangre son costosas, ya que los costos incluyen el cribado para infección, el pareamiento cruzado, el almacenamiento y la administración estéril y segura de los productos sanguíneos (Shander 2010). En los países de bajos ingresos, o durante los desastres, la sangre para la transfusión puede no estar fácilmente disponible.
Por qué es importante realizar esta revisión
La anemia posparto causada por la ingesta insuficiente de hierro o hemorragia (anemia ferropénica posparto) es una afección frecuente que afecta a las pacientes después del parto y se puede asociar con síntomas que pueden influir en la supervivencia, en la salud y en la capacidad de cuidar al recién nacido. Las formas de tratamiento disponibles para la anemia ferropénica posparto producen efectos perjudiciales, algunos graves. Como todas las pacientes sangran durante el parto, es una práctica frecuente administrar tratamiento para la anemia ferropénica posparto, para permitirles a las pacientes sintetizar de manera eficaz nuevos eritrocitos. Algunas poblaciones se pueden beneficiar más que otras, y en algunas poblaciones y categorías de gravedad de la enfermedad el tratamiento puede ser innecesario, ineficaz e incluso nocivo. Las pacientes y los cuidadores necesitan estimaciones confiables de los efectos beneficiosos y perjudiciales de los tratamientos disponibles para la anemia posparto para que puedan equilibrarse para cada paciente individual.
Esta revisión es una actualización de una revisión anterior de Dodd 2004.
Objetivos
Evaluar la eficacia y los efectos perjudiciales de las formas de tratamiento disponibles para las pacientes con anemia ferropénica posparto. Se incluyen el hierro oral y parenteral, la eritropoyetina y la transfusión de sangre.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron los ensayos controlados aleatorios publicados, no publicados y en curso que compararon un tratamiento para la anemia ferropénica posparto con placebo, ningún tratamiento u otro tratamiento para la anemia ferropénica posparto, incluidos los ensayos descritos solamente como resúmenes. Se consideró la inclusión de ensayos asignados al azar por grupos. Se incluyeron los ensayos abiertos y los ensayos con cegamiento, independientemente de quién se cegó. Se excluyeron los ensayos no aleatorios, los ensayos cuasialeatorios y los ensayos que utilizaron un diseño cruzado.
Tipos de participantes
Pacientes con un valor de Hb posparto de 120 g/l (7,4 milimoles por litro) o menos, en las que el tratamiento se inició hasta seis semanas después del parto. De ser posible se diferenciaron los grupos socioeconómicos poblacionales, ya que este factor puede afectar la respuesta al tratamiento, pero se incluyeron todos.
Tipos de intervenciones
Tratamiento para la anemia ferropénica posparto comenzado en las seis primeras semanas después del parto comparado con placebo, ningún tratamiento u otro tratamiento.
Actualmente, el tratamiento aceptado para la anemia ferropénica incluye la transfusión de sangre o la administración de suplementos de hierro por vía oral o parenteral, solos o en combinación con folato o eritropoyetina.
La administración de suplementos de folato no se consideró un tratamiento independiente de la anemia ferropénica, pero se aceptó como parte de otros tipos de tratamiento para la anemia ferropénica posparto.
Las nuevas formas de tratamiento apropiadas para la anemia ferropénica se incluirán en las actualizaciones futuras.
Tipos de medida de resultado
Resultados primarios
-
Mortalidad materna: Se consideró que ninguna mujer murió solamente si: a) se señaló explícitamente, o b) no ocurrieron abandonos durante el seguimiento, o c) los autores de contacto proporcionaron esta información cuando se solicitó. Se consideró la presencia de mortalidad solamente si: a) se señaló explícitamente en un informe publicado o b) los autores de contacto proporcionaron esta información cuando se solicitó. La mortalidad se evaluó como no informada si a) no se mencionaron los abandonos o sus causas, b) no se justificaron todos los abandonos, c) no se informó explícitamente si las que abandonaron estaban vivas al final del período de seguimiento.
-
Fatiga: como se informó por las pacientes: expresión verbal de fatiga o falta de energía e incapacidad para mantener las rutinas habituales; medida por una escala o cuestionario; o según la definición de los autores de los ensayos. Resultados a corto plazo y a largo plazo; por lo tanto, tiempo mínimo y máximo a partir del valor inicial.
Resultados secundarios
-
Síntomas de la anemia persistentes durante el tratamiento. Cualquiera de los siguientes síntomas: disnea, taquipnea, taquicardia, palpitaciones, mareo ortostático, síncope, palidez.
-
Bienestar psicológico, que incluye rendimiento cognitivo medido por el Blues Questionnaire (Kennerley 1989), el Self‐report symptom inventory 90 (SCL‐90‐R) (Schmitz 1999), el SF36 (Medical Outcomes Study Short Form) (Ware 2000) o un cuestionario similar; o según la definición de los autores de los ensayos. Solamente resultados a corto plazo; por lo tanto, tiempo mínimo desde el valor inicial.
-
Infección urinaria, endometritis u otras infecciones (según la definición de los autores de los ensayos).
-
Cumplimiento del tratamiento (según la definición de los autores de los ensayos).
-
Lactancia (al alta hospitalaria; seis semanas posparto; seis meses posparto).
-
Duración de la estancia hospitalaria.
-
Cualquier evento adverso durante el tratamiento (cada tipo de efecto perjudicial analizado individualmente, de ser posible).
-
Número de transfusiones de eritrocitos (número de pacientes transfundidas y número de unidades de eritrocitos por paciente).
Para los resultados de otro bienestar psicológico no se aplicaron restricciones con respecto a los períodos de seguimiento para evitar excluir datos sobre cualquier efecto beneficioso o perjudicial a largo plazo. No se aplicó ninguna restricción de idioma.
Se planificó incluir los siguientes resultados en las tablas "Resumen de los hallazgos" de la revisión, mediante el programa Grade Profiler (GRADEpro 2014).
-
Mortalidad materna.
-
Fatiga.
-
Estreñimiento (para la sustitución con hierro oral).
-
Reacciones alérgicas (para el hierro intravenoso).
Las comparaciones incluidas en las tablas "Resumen de los hallazgos" se eligieron sobre la base de su relevancia para las normas de tratamiento actuales según los expertos clínicos. Por lo tanto, se decidió no incluir el tratamiento con eritropoyetina intravenosa (EPO) (IV) o extracto de levadura en las tablas "Resumen de los hallazgos" porque estos métodos ya no se practican. Para los resultados específicos de los tratamientos enumerados anteriormente (estreñimiento y reacciones alérgicas), los resultados se incluyeron en las tablas "Resumen de los hallazgos" si el tratamiento específico estaba presente en solamente uno de los brazos de estudio.
Se decidió incluir en las tablas "Resumen de los hallazgos" los resultados adicionales que se encontró que eran importantes para la toma de decisiones clínica para cada forma de tratamiento individual cuando este tratamiento estaba presente en solamente uno de los brazos de estudio. Para las comparaciones con hierro IV este resultado fue infecciones. Para las comparaciones con hierro oral se incluyeron todos los síntomas GI combinados. Para las comparaciones con transfusiones de eritrocitos se incluyeron las infecciones, los eventos tromboembólicos y los eventos adversos específicos de las transfusiones como la formación de aloanticuerpos y las reacciones a las transfusiones. Para las comparaciones con EPO, los eventos tromboembólicos fueron fundamentales. En todas las comparaciones que cumplieron los criterios anteriormente mencionados, se encontró que era importante incluir los síntomas de anemia.
Results
Description of studies
For an individual description of the studies please see Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies.
Results of the search
We retrieved 57 (9 April 2015) articles from the Pregnancy and Childbirth Group's Trials Register, 16 (8 April 2015) from the WHO International Clinical Trials Registry Portal (ICTRP), and 154 (8 April 2015) from LILACS. After excluding duplicates, 178 records remained. We screened these records for relevance by title and abstracts, and excluded 118. One discontinued study is awaiting classification and four studies based on five reports are ongoing. The remaining 54 full text articles were assessed for eligibility. Of these, 17 studies based on 19 reports were excluded (Figure 1, Study flow diagram). In addition, we assessed seven studies that were excluded by the previous authors of this review, and agreed with their assessment. Of these, one trial also appeared in our electronic search, resulting in 17 excluded studies.

Study flow diagram.
No additional randomised controlled trials (RCTs) were found through screening of the citation lists of relevant publications.
We attempted to contact the trial authors (contact information from articles or of the Internet) for additional information or clarification of methods used for all included trials and trials with an unclear assessment for eligibility. We received the additional information from 10 individual studies (Backe 2009;Daniilidis 2011; Froessler 2013; Giannoulis 2009; Guerra 2012; Krafft 2011; Prick 2014; Van Wyck 2007; Wagstrom 2007; Westad 2008).
The remaining authors did not respond (Bhandal 2006; Breymann 1996; Breymann 2000; Jain 2013; Makrydimas 1998; Mumtaz 2011; Perello 2014; Seid 2008; Tam 2005; Verma 2011), were not possible to contact due to lack of contact information (Beard 2005; Krauss 1972; Lebrecht 1995; Meyer 1995), or did not have resources to provide the requested information (Breymann 2008).
Included studies
Design and sample sizes
We included 22 RCTs with 2858 women (Beard 2005; Bhandal 2006; Breymann 1996; Breymann 2000; Breymann 2008; Froessler 2013; Guerra 2012; Jain 2013; Krafft 2011; Krauss 1972; Lebrecht 1995; Makrydimas 1998; Meyer 1995; Mumtaz 2011; Perello 2014; Prick 2014; Seid 2008; Tam 2005; Van Wyck 2007; Verma 2011; Wagstrom 2007; Westad 2008).
Participants
All the participants were women with postpartum anaemia who received treatment within six weeks postpartum.
Interventions
Itravenous iron versus oral iron
Intravenous (IV) iron (either iron carboxymaltose or iron sucrose) was compared with oral ferrous sulphate in 10 studies including a total of 1553 women (Bhandal 2006; Breymann 2008; Froessler 2013; Guerra 2012; Jain 2013; Mumtaz 2011; Seid 2008; Van Wyck 2007; Verma 2011; Westad 2008). One study added oral iron to those originally assigned to receive IV iron after four weeks (Westad 2008).
The follow‐up periods varied from 14 to 84 days between the studies. Socioeconomic status was clearly stated as being low in only one study (Froessler 2013). We did not make assumptions regarding socioeconomic status based on the name of the country.
Red blood cell transfusion
Red blood cell (RBC) transfusion was compared with non‐intervention (standard of care) in one study with 519 women (Prick 2014). The treatment of the non‐intervention arm was decided by the clinicians. This trial reported on all pre‐defined outcomes for this review, except maternal mortality. Follow‐up was six weeks.
Oral iron
Oral iron was compared with either placebo or no treatment in three studies with a total of 315 women (Beard 2005; Krauss 1972; Tam 2005). The preparations used in each trial contained various additives, such as vitamin C, vitamin B, and folic acid. Follow‐up varied from 30 days to nine months among studies. One RCT only included women of low socioeconomic status (Beard 2005). The remaining studies did not specify this. The trial by Krauss 1972 included three study arms. The trial by Tam 2005 was based on two anaemic study groups (one treated and one given placebo) and one non‐anaemic group. The study was included based on intervention, which fulfilled our criteria. However, the majority of the results were combined for both anaemic groups, thus not distinguishing between the treated and untreated group.
Inravenous iron and oral iron versus oral iron
Intravenous iron with oral iron was compared with IV placebo and active oral iron treatment in two studies (Breymann 2000; Perello 2014), including a total of 112 women. Follow‐up was two and six weeks, respectively.
Erythropoietin
Erythropoietin and IV iron was compared with IV iron alone in two studies with a total of 100 women (Krafft 2011; Wagstrom 2007). In the trial by Wagstrom 2007, EPO was given subcutaneously (SC) in two different doses in two different EPO groups (total of 40,000 U and 20,000 U). The EPO group with a total dose of 20,000 U was analysed separately. In Krafft 2011, EPO was given IV. Follow‐up was two weeks in both studies.
Erythropoietin combined with IV iron followed by oral iron was compared with IV iron alone followed by oral iron in three studies with a total of 186 women (Breymann 1996; Breymann 2000; Lebrecht 1995). Two of the studies had three study arms (Breymann 1996; Breymann 2000). In one study EPO was given either SC or IV (Breymann 1996), and one study also had a study arm that only received oral iron (Breymann 2000). We compared study arms with similar treatment across studies.
Subcutaneous EPO and oral iron were compared with oral iron in one study with 40 women (Makrydimas 1998). Follow‐up was 40 days.
Intravenous EPO was compared with placebo, without iron supplementation in one study with 71 women (Meyer 1995). Follow‐up was five days.
Outcomes
All of the included publications reported at least one clinical outcome measure that was preplanned for this review. These 22 publications also reported laboratory values such as Hb, ferritin or others.
Of all included studies, six reported on maternal mortality (Breymann 2000; Guerra 2012; Krafft 2011; Lebrecht 1995; Makrydimas 1998; Van Wyck 2007), three on fatigue (Prick 2014; Van Wyck 2007; Westad 2008), three on anaemia symptoms (Perello 2014; Prick 2014; Tam 2005), seven on psychological well being (Beard 2005; Meyer 1995; Perello 2014; Prick 2014; Van Wyck 2007; Wagstrom 2007; Westad 2008), six on infections (Breymann 2008; Guerra 2012; Krafft 2011; Prick 2014; Van Wyck 2007; Wagstrom 2007), nine on compliance (Bhandal 2006; Breymann 2008; Guerra 2012; Jain 2013; Krafft 2011; Prick 2014; Van Wyck 2007; Verma 2011; Westad 2008), four on breastfeeding (Krafft 2011; Makrydimas 1998; Prick 2014; Tam 2005), four on length of hospital stay (Makrydimas 1998; Perello 2014; Prick 2014; Verma 2011), and 20 on adverse events during treatment. The studies that did not report on adverse events were Beard 2005 and Meyer 1995. Eleven studies reported on the use of blood transfusions as a rescue treatment (Bhandal 2006; Breymann 1996; Breymann 2000; Breymann 2008; Froessler 2013; Krafft 2011; Makrydimas 1998; Perello 2014; Prick 2014; Wagstrom 2007; Westad 2008).
We chose not to consider placebo treatment as a type of intervention, based on the lack of evidence for a substantial placebo effect (Hróbjartsson 2010). Groups with inactive placebo were therefore considered comparable with groups not receiving treatment. Also, we chose not to distinguish between SC and IV EPO administration, as we did not expect the effect to be influenced by the route of administration.
This allowed five comparisons based on interventions with more than one study. The rest of the studies and the remaining study arms were analysed separately. Thus, a total of 13 comparisons were conducted in this review.
The included studies are described in detail in the Characteristics of included studies tables. Only our preplanned outcomes chosen for this review were described and analysed.
Excluded studies
We excluded 17 studies. Reasosns for exclusion were inadequate randomisation methods, mixed anaemic and non‐anaemic population without subgroup analysis, summary of two included and one excluded study, analyses based on both antepartum and postpartum anaemia, no definition of the postpartum period (thus including women enrolled more than six weeks postpartum), lack of a control arm, investigation of differences in screening strategies rather than different interventions, and interventions found as not appropriate for treatment of iron deficiency anaemia. For further details, please see Characteristics of excluded studies.
Risk of bias in included studies
The 'Risk of bias' assessment is summarised in Figure 2 and Figure 3.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Low risk of bias was found in 12 studies (Bhandal 2006; Froessler 2013; Guerra 2012; Jain 2013; Krafft 2011; Perello 2014; Prick 2014; Seid 2008; Tam 2005; Van Wyck 2007; Wagstrom 2007; Westad 2008). Ten studies had unclear risk of bias, as the random sequence generation method was not described (Beard 2005; Breymann 1996; Breymann 2000; Breymann 2008; Krauss 1972; Lebrecht 1995; Makrydimas 1998; Meyer 1995; Mumtaz 2011; Verma 2011).
Allocation concealment
Allocation concealment was adequately described in 13 studies (Beard 2005; Bhandal 2006; Breymann 1996; Breymann 2000; Froessler 2013; Guerra 2012; Krafft 2011; Perello 2014; Prick 2014; Tam 2005;Van Wyck 2007;Wagstrom 2007; Westad 2008). The method was not described and thus the risk of bias was unclear in the remaining nine studies (Breymann 2008; Jain 2013; Krauss 1972; Lebrecht 1995; Makrydimas 1998; Meyer 1995; Mumtaz 2011; Seid 2008; Verma 2011).
Blinding
Performance bias
In one study, the method of blinding was adequately described and it was clear who was blinded (Perello 2014).
Six studies had an unclear risk of bias: two placebo‐controlled studies described the blinding method as double‐blind, but it was unclear who was blinded (Lebrecht 1995; Meyer 1995). In the study reported by Beard 2005 it was unclear if all treatment components (iron, folate, vitamin C) were prepared in a single tablet and whether this tablet resembled the placebo tablet. In Tam 2005 it was reported that although the trial was double‐blinded, the participants reported stool discolorations when receiving active treatment.
The majority of studies were open‐label due to different non‐blinded administration routes and thus considered at high risk (Bhandal 2006; Breymann 1996; Breymann 2000; Breymann 2008; Froessler 2013; Guerra 2012; Jain 2013; Krafft 2011; Krauss 1972; Makrydimas 1998; Mumtaz 2011; Prick 2014; Seid 2008; Van Wyck 2007; Verma 2011; Wagstrom 2007; Westad 2008).
Detection bias
Seven studies were rated as having unclear risk of bias: in two studies described as double‐blinded it was not described who was blinded (Lebrecht 1995; Meyer 1995). In Perello 2014, it was not clear whether personnel who handled self‐rated questionnaires were blinded. In Tam 2005 it was unclear whether the women were able to guess their treatment based on the change in stool colour. The outcomes in this trial were subjective and reported by the women. The risk of detection bias therefore depends on the women's knowledge of the correlation between iron treatment and stool discolouration and the clinician's knowledge of the discolouration at the time when the remaining outcomes were registered. This was not described and the risk of bias was therefore rated as unclear.
In the study reported by Beard 2005, it was not clear from study description who exactly was blinded during the trial and whether the placebo tablet and treatment were sufficiently similar to prevent the patients from guessing the group. Thus the subjective (patient registered) outcome of psychological well being may have been affected by insufficient blinding.
High risk of bias was found in seventeen studies due to open‐label trial design (Bhandal 2006; Breymann 1996; Breymann 2000; Breymann 2008; Froessler 2013; Guerra 2012; Jain 2013; Krafft 2011; Krauss 1972; Makrydimas 1998; Mumtaz 2011; Prick 2014; Seid 2008; Van Wyck 2007; Verma 2011; Wagstrom 2007; Westad 2008). Maternal mortality is one of the few outcome measures which is most probably not affected by a lack of blinding. However, this outcome was rarely reported.
Incomplete outcome data
Information on dropouts and withdrawals after randomisation was reported in 19 studies (Beard 2005; Bhandal 2006; Breymann 2000; Breymann 2008; Froessler 2013; Guerra 2012; Jain 2013; Krafft 2011; Krauss 1972; Lebrecht 1995; Makrydimas 1998; Mumtaz 2011; Perello 2014; Prick 2014; Seid 2008; Tam 2005; Van Wyck 2007; Wagstrom 2007; Westad 2008). Three trial authors provided additional information on dropout rates (Froessler 2013; Van Wyck 2007; Wagstrom 2007).
Dropout varied greatly across studies. Dropout rates after randomisation were lower than 5% in six studies (Bhandal 2006; Breymann 2000; Krafft 2011; Lebrecht 1995; Makrydimas 1998; Seid 2008), between 5% and 9.9% in three studies (Krauss 1972;Mumtaz 2011; Van Wyck 2007), between 10% and 19.9% in six studies (Froessler 2013; Guerra 2012; Jain 2013; Perello 2014; Tam 2005; Wagstrom 2007), and 20% or more in four studies (Beard 2005; Breymann 2008; Prick 2014; Westad 2008). However, for Beard 2005, the missing data were given as lost to follow‐up, not as discontinuation of treatment. The numbers are therefore very high and probably overestimate the actual dropout rate. Three studies did not report sufficient information to calculate the dropout rate after randomisation (Breymann 1996; Meyer 1995; Verma 2011).
Low risk of bias was found in nine studies with a low dropout rate or an equal distribution of dropouts across groups (Bhandal 2006; Breymann 2000; Jain 2013; Krafft 2011; Krauss 1972; Lebrecht 1995; Makrydimas 1998; Mumtaz 2011; Seid 2008).
High risk of bias was found in 10 studies, due to a high dropout rate and/or unequal distribution across groups (Beard 2005; Breymann 2008; Froessler 2013; Meyer 1995; Perello 2014; Prick 2014; Tam 2005; Van Wyck 2007; Wagstrom 2007; Westad 2008).
An unclear risk of attrition bias was found in three studies. In one study it was not possible to assess if the dropouts were in fact not related to the trial (Guerra 2012). In three studies it was not mentioned whether or not any patients dropped out after randomisation (Breymann 1996; Verma 2011).
Selective reporting
We applied strict criteria when evaluating reporting bias because we consider mortality and adverse events as extremely important outcomes, and as per our method section we rated the studies as high risk if the study failed to include results of a key outcome that would have been expected to be reported (i.e. mortality). Therefore, only two studies were rated as having low risk of reporting bias (Krafft 2011; Van Wyck 2007).
Often the trial authors stated that the objectives of their trial were efficacy and safety, but did not specify which preplanned outcome measures were going to be used to evaluate efficacy. Two studies were rated as having unclear risk of bias because no preplanned outcomes were specified (Breymann 2000; Guerra 2012).
High risk of bias was found in 18 studies: 16 of these did not report on adverse events and/or maternal mortality (Beard 2005; Bhandal 2006; Breymann 1996; Breymann 2008; Froessler 2013; Jain 2013; Krauss 1972; Meyer 1995; Mumtaz 2011; Perello 2014; Prick 2014; Seid 2008; Tam 2005; Verma 2011; Wagstrom 2007; Westad 2008), and in two studies there was a lack of data to support their conclusions on quality of life (Lebrecht 1995; Makrydimas 1998). The study by Verma 2011 did not report on the following preplanned outcomes which were stated in the 'aims and objectives' section of the published report: patient satisfaction, quality of life, cost of treatment, length of hospital stay, use of blood transfusion, impact on stress, depression, cognitive function, and breastfeeding.
Other potential sources of bias
One study was found to have unclear risk of bias because the Hb level for inclusion was not stated (Krauss 1972). Three studies were found to have a high risk of bias because of significant errors in the published reports (Mumtaz 2011; Van Wyck 2007; Verma 2011). For further description, please see Characteristics of included studies.
Effects of interventions
See: Summary of findings for the main comparison Intravenous iron compared with oral iron for women with postpartum iron deficiency anaemia (Comparison 1); Summary of findings 2 Red blood cell transfusion compared with non‐transfusion (Comparison 2); Summary of findings 3 Oral iron compared with placebo (Comparison 3); Summary of findings 4 Intravenous iron with oral iron compared with oral iron (Comparison 6); Summary of findings 5 Erythropoietin (regardless of rout) with intravenous iron compared with intravenous iron (Comparison 7); Summary of findings 6 Subcutaneous EPO 10,000 U of doses with intravenous iron compared with intravenous iron (Comparison 8); Summary of findings 7 Subcutaneous EPO with oral iron compared with oral iron (Comparison 10); Summary of findings 8 Subcutaneous EPO with intravenous iron and oral iron compared with intravenous iron with oral iron (Comparison 12)
Comparison 1: IV iron versus oral iron
Intravenous (IV) iron treatment was compared with oral iron in 10 studies with a total of 1553 women (Bhandal 2006; Breymann 2008; Froessler 2013; Guerra 2012; Jain 2013; Mumtaz 2011; Seid 2008; Van Wyck 2007; Verma 2011; Westad 2008). IV iron was in the form of either iron sucrose (seven studies) or iron‐carboxymaltose (three studies). Doses differed across the trials with a range of 300 mg to 2500 mg in total dose. In several studies doses were individually calculated using the Ganzoni formula, estimating the iron deficit in each patient. Oral iron was given as ferrous sulphate typically using a fixed dose. The content of elemental iron (the dose of the pure iron ion in the iron sulphate tablet) was rarely reported. Treatment regimens differed between studies with regard to doses, number of tablet per day and number of days of treatment. Non‐elemental iron doses ranged from 100 mg to 325 mg per tablet.
Primary outcomes
Maternal mortality
Maternal mortality was only reported by two studies. There was one maternal death in the group receiving IV iron caused by peripartum cardiomyopathy 13 days postpartum, thus it is not clear whether this death was directly caused by the study medication (Van Wyck 2007). A corresponding author from one other study reported that no women died (Guerra 2012) (risk ratio (RR) 2.95; 95% confidence interval (CI) 0.12 to 71.96; two RCTs; one event; 374 women; low quality evidence; Analysis 1.1). In the remaining studies this information was not clear as per our definition in the Primary outcomes section.
Fatigue
Fatigue was reported by two studies, and a meta‐analysis was not possible due to lack of data. One study reported a statistically significant improvement in fatigue in the group receiving IV treatment (see below) (Westad 2008). The other study showed no difference in fatigue (see below) (Van Wyck 2007).
Van Wyck 2007 used the Fatigue Linear Analog Scale Assessment (Portenoy 2006) for a mean total fatigue score. Westad 2008 used the Fatigue Score (Chalder 1993), where the scores were reported as mean change from baseline for physical, mental and total fatigue. It was not possible to obtain standard deviations from Westad 2008 (standard deviations were only available for baseline data), and thus we could not perform a meta‐analysis. In both studies the higher score indicated higher level of fatigue.
In the trial by Westad 2008, all women received oral iron after four weeks. Results by weeks eight and 12 are described in Comparison 5.
In the published paper by Westad 2008, the authors state that they found a statistically significant improvement in the 'physical fatigue' and 'total fatigue' scores favouring the IV iron group at four weeks with a P value of 0.02 for both scores. They found no between‐group difference in the 'mental fatigue' score.
Van Wyck 2007 provided raw means data on our request. There was no statistically significant differences between groups at 14 days (short term) or 42 days (long term) (very low quality evidence; Analysis 1.2; Analysis 1.3).
Secondary outcomes
Anaemia symptoms
Anaemia symptoms (other than fatigue) were not reported.
Psychological well being
Psychological well being was reported by two studies (Van Wyck 2007; Westad 2008). Both used the SF‐36 questionnaire, where higher scores indicate better health state (Ware 2000). There was no overall difference in psychological well being (see below).
It was not possible to carry out a meta‐analyses as standard deviations were only available for baseline data for the study by Westad 2008. This study reported only on four out of eight SF‐36 items. In the published report the authors found no significant between‐group difference at week four.
Van Wyck 2007 provided raw means on all eight items of the SF‐36, thus 'physical function', 'physical role', 'bodily pain', 'social function', 'mental health', 'general health', 'vitality', and 'emotional role'. From the additional data provided, we found no statistically significant difference between the groups at 14 days (Analysis 1.4 to Analysis 1.11).
There was no difference in the occurrence of depression (Analysis 1.12).
Infections
Infections were analysed as a total for each group based on the assumption that if anaemia can cause immune deficiency, and bioavailable iron can supply microorganisms with nutrition, infections could occur anywhere in body. The results were divergent: In the study by Breymann 2008 infections were more frequent in the IV iron group, whereas there was no difference in the study by Van Wyck 2007. Our analysis found no statistically significant difference in infections (RR 1.70; 95% CI 0.58 to 5.03; three RCTs; 718 women; I² 72 %; T² 0.45; Chi² 3.56; P 0.06; very low quality evidence; Analysis 1.13). We conducted a sensitivity analysis to investigate the high level of heterogeneity in which the difference remained statistically non‐significant after we subtracted the study causing heterogeneity (Analysis 14.1), and when we used fixed‐effect meta‐analysis (Analysis 14.2).
Compliance
Compliance was reported in seven studies (Bhandal 2006; Breymann 2008; Guerra 2012; Jain 2013; Van Wyck 2007; Verma 2011; Westad 2008). Bhandal 2006 and Guerra 2012 reported 100% compliance in both groups. However, Guerra 2012 did not count the remaining pills. In the study by Breymann 2008, compliance in the group receiving IV iron was 99% and over 90% in the group receiving oral iron. Westad 2008 reported a compliance of 95% specifically for IV injections in the group receiving IV iron. The mean daily intake of oral iron was 99 mg by week four, resulting in 50% compliance in the oral group. Van Wyck 2007 reported a compliance of 98% in the group receiving IV iron and 83.9% in the group receiving oral iron. Jain 2013 reported the group receiving oral iron to have a 100% compliance confirmed by pill count, but compliance for the group receiving IV iron was not specified. Verma 2011 mentioned that compliance was better in the group receiving IV iron than in the group receiving oral iron, but data were not available. Thus, compliance was 95% to 100% for IV iron, and 50% to 100% for oral iron (RR 1.17; 95% CI 1.01 to 1.35; five RCTs; 890 women, I² 90 %; T² 0.02; Chi² 38.44; P < 0.00001; Analysis 1.14).
Breastfeeding
Breastfeeding was not reported.
Length of hospital stay
Length of hospital stay was generally not reported. Verma 2011 noted that hospital stays were longer in the IV group, but data were not available.
Adverse events during treatment
Three women experienced anaphylaxis or hypersensitivity, all of whom received IV iron. However, there were few events and a reliable absolute risk estimate could therefore not be calculated (RR 2.78; 95% CI 0.31 to 24.92; eight RCTs; three events; 1454 women (767 in the IV arm versus 687 in the oral arm); I² = 0%; low quality evidence; Analysis 1.32.
One woman who received IV iron developed an arrhythmia during iron infusion (Analysis 1.33).
There was a statistically significant difference in the risk of all combined gastrointestinal (GI) adverse events favouring the group receiving IV iron (RR 0.31; 95% CI 0.20 to 0.47; eight RCTs; 169 events; 1307 women; I² = 0%; very low quality evidence; Analysis 1.15).
The GI symptoms that were significantly less frequent in the IV iron group were: constipation (RR 0.21; 95% CI 0.11 to 0.39; six RCTs; 74 events; 1217 women; I² = 0%; very low quality evidence; Analysis 1.16), nausea (RR 0.30; 95% CI 0.11 to 0.81; four RCTs; 22 events; 745 women; I² = 0%; Analysis 1.17), GI pain (RR 0.18; 95% CI 0.04 to 0.83; four RCTs; 13 events; 543 women; I² = 0%; Analysis 1.18), and diarrhoea (RR 0.11; 95% CI 0.02 to 0.59; three RCTs; 14 events; 569 women; I² = 0%; Analysis 1.19).
There was no difference for the occurrence of vomiting (RR 0.40; 95% CI 0.02 to 9.66; one RCT, 128 women; Analysis 1.20) or dyspepsia (RR 0.36; 95% CI 0.04 to 3.20; two RCTs; 93 women; Analysis 1.21).
In the group receiving IV iron we found an increased risk of dysgeusia (distortion of the sense of taste) (RR 7.20; 95% CI 1.63 to 31.76; four RCTs; 13 events; 543 women; I² = 0%; Analysis 1.22), injection site discomfort (RR 4.72; 95% CI 1.03 to 21.54; four RCTs; 10 events; 702 women; I² = 0%; Analysis 1.25), and flush (RR 9.00; 95% CI 1.18 to 68.81; two RCTs, eight events; 124 women; I² = 0%; Analysis 1.28).
There was no statistically significant difference between IV iron and oral iron regarding other adverse events including headache (RR 1.93; 95% CI 0.87 to 4.29; four RCTs; 1124 women; I² = 0%; Analysis 1.23), skin rash (RR 2.34; 95% CI 0.79 to 6.97; two RCTs; 489 women; I² = 0%; Analysis 1.26), muscle cramps (RR 6.05; 95% CI 0.74 to 49.68; two RCTs, 371 women; I² = 0%; Analysis 1.29), and hepatic involvement (RR 0.45; 95% CI 0.12 to 1.71; three RCTs; 996 women; I² = 51%; T² 0.70; Chi² 4.07; P 0.13; Analysis 1.24). In the analyses for hepatic involvement there was high heterogeneity, therefore we conducted a sensitivity analysis. The difference between groups became statistically significant in favour of the IV group when we removed the study causing heterogeneity (Breymann 2008) (RR 0.22; 95% CI 0.06 to 0.75; two RCTs; 652 women; I² = 0%; Analysis 14.3). The difference was statistically non‐significant when we changed to fixed‐effect meta‐analysis (Analysis 14.4).
Some adverse events were rare and reported only by one study. We found no difference in these outcomes, which were urticaria (reported as an isolated symptom and not as part of an allergic reaction) (Analysis 1.27), unspecified pain (Analysis 1.30), and unspecified serious adverse events (Analysis 1.31).
Red blood cell transfusions
There was no difference between groups the frequency of women receiving blood transfusion as a "rescue treatment" (blood transfusion rates) (RR 0.48; 95% CI 0.19 to 1.23; four RCTs; 18 events; 606 women; I² = 0%; Analysis 1.34). For the trial by Westad 2008, we assumed that the reported number of blood transfusions were received within the first four weeks of treatment, which is clinically most probable. However, this is not specified in the published report. The number of units of RBCs transfused was not reported.
Discontinued study
One trial by Backe 2009 entitled 'A 6‐week randomised, open comparative, multi‐centre study of IV ferric carboxymaltose (Ferinject) and oral iron (Duroferon) for treatment of post partum anaemia' with the trial identification number NCT00929409 was identified. Based on the type of intervention this trial should have been included in Comparison 1.
However, we were informed by the contact person for the trial that "Our controlled trial “A 6‐week randomised, open comparative, multi‐centre study of IV ferric carboxymaltose (Ferinject) and oral iron (Duroferon) for treatment of post partum anemia” was stopped because of slow progress, and the sponsor (Renapharma Vifor) then unfortunately decided to terminate the trial".
We then repeatedly attempted to contract the sponsors for preliminary results and the trial report made after discontinuation. We never received a response from the company. This indicates a high risk of publication bias (Bassler 2010).
Comparison 2: RBC transfusion versus non‐intervention
Prick 2014 was the only trial comparing RBC transfusion to non‐intervention, i.e. other treatment at the clinician's discretion. The trial included 519 women.
Primary outcomes
Maternal mortality
Mortality was not reported.
Fatigue
There was a small and transient, but statistically significant between‐groups difference in fatigue during the first week favouring the group receiving RBC transfusions (see below).
Fatigue was measured by the Multidimentional Fatigue Inventrory (MFI) (Smets 1995). We chose to report only on 'general fatigue', which summarises the remaining domains domains: 'physical fatigue', 'reduced activity', 'reduced motivation', and 'mental fatigue'. High score indicates higher level of fatigue.
The authors provided raw means and standard deviations on our request. However, they pointed out, that it would not be correct to enter the results as raw means while not correcting for baseline differences and mode of delivery. We chose to quote the data from the manuscript, but also import and analyse the data provided by the authors.
In the published report's table S1 (data corrected for baseline differences), the authors found a statistically significant between‐groups difference in mean general fatigue at three days. There was no significant difference between groups at six weeks.
The additionally provided data showed that the group receiving RBC transfusions had significantly better scores than the non‐intervention group in general fatigue at three days (mean difference (MD) ‐0.80; 95% CI ‐1.53 to ‐0.07; women 388; low quality evidence; Analysis 2.1), but not at six weeks (low quality evidence; Analysis 2.2).
Secondary outcomes
Anaemia symptoms
Anaemia symptoms eliciting a RBC transfusion occurred in 28 women in the non‐intervention group. However, the frequency of anaemia symptoms (besides fatigue) was not systematically reported for the remaining, non‐transfused members of the non‐intervention group or for the RBC transfusion group (very low quality evidence).
Psychological well being
Psychological well being improved significantly more in the group receiving RBC transfusions (see below).
Psychological well being was registered using the SF‐36 questionnaires (high score indicates better health state). We chose to quote the SF‐36 data from the manuscript, as well as to report the additionally provided data at one week of follow‐up.The published report (Table S1) showed a statistically significant between‐groups difference in 'physical functioning', where scores were 5.5 points lower at one week of follow‐up in the non‐intervention group, thus favouring the group receiving RBC transfusions. When we entered the additionally provided data we found a statistically significant difference in physical functioning favouring the group receiving RBC transfusions at one week (MD 5.67; 95% CI 0.84 to 10.50; 368 women; Analysis 2.3).
For social function there was a borderline statistically significant difference at one week favouring the group receiving RBC transfusions (MD 5.34; 95% CI 0.11 to 10.57; 369 women; Analysis 2.4). For the remaining items there was no statistically significant difference at one week of follow‐up (Analysis 2.5 to Analysis 2.10). Thus, there was a discrepancy between the reported results and our calculations of additionally provided data, but both sources find effect in favour of RBC transfusions.
Infections
Infection rates were similar (RR 0.93; 95% CI 0.53 to 1.61; 519 women; moderate quality evidence; Analysis 2.11).
Compliance
Compliance to treatment was lower in the non‐intervention group, where 33 women did not comply with allocated treatment versus seven in the RBC group (RR 1.11; 95% CI 1.06 to 1.17; 519 women; Analysis 2.12).
Breastfeeding
Breastfeeding rate at randomisation was 77% in both groups. There was no statistically significant difference in breastfeeding rate between groups at six weeks of follow‐up (RR 0.91; 95% CI 0.78 to 1.07; 297 women; Analysis 2.13).
Length of hospital stay
Length of hospital stay was a median of two days in both groups.
Adverse events during treatment
There was no statistically significant difference in reported adverse events, which were alloantibody formation (very low quality evidence), rash, fever, thromboembolic events (low quality evidence), parenteral iron intolerance, and transfusion reactions (very low quality evidence) (Analysis 2.14 to Analysis 2.19). Transfusion reactions (alloantibodies, fever) only occurred in transfused participants, however, there was no systematical investigation for the presence of new alloantibodies.
Red blood cell transfusions
In the RBC transfusion group, 251 women received transfusion, seven refused. The total number of RBC units given was 517 (median: 2 units per woman; interquartile range 2‐2). In the non‐intervention group 33 women received RBC transfusion and 88 RBC units were given (median: 0 units per woman; interquartile range 0‐0).
Comparison 3: Oral iron versus placebo
Oral iron was compared with placebo by three studies (Beard 2005; Krauss 1972; Tam 2005). The study by Krauss 1972 had three study arms. For this comparison we chose the study arm that received tablet Eryfer containing ferrous sulphate, ascorbic acid and sodium bicarbonate as the intervention arm (group S) and the placebo arm (empty preparation) as the control arm. The remaining arm received oral iron, magnesium oxide, yeast extract (see Comparison 4). The follow‐up periods for the three studies were 30, 42, and 145 days and the trials did not report results at comparable time points. The trials also reported on different outcomes, as a result it was not possible to perform meta‐analyses for this comparison.
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms
Only one study, Tam 2005, reported on persistent anaemia symptoms, but for both study groups combined. These were dyspnoea (n = 6), palpitations (n = 6), chest discomfort (n = 3), dizziness (n = 12), headache (n = 10).
Psychological well being
Psychological well being was significantly better in the placebo group, shown by two different tools (see below).
Psychological well being was reported by Beard 2005. The tools used for the assessment were Digit Symbol Substitution test (high score indicates better cognitive performance) (Hoyer 2004), Edinburgh Postnatal Depression Scale (EPDS), where high scores are associated with depression (Cox 1987), Spielberger State Trait Anxiety Inventory (STAI), where high scores indicate higher anxiety (Marteau 1992), and the Perceived Stress questionnaires, where high scores indicate more stress (Cohen 1983). The Digit Symbol Substitution evaluating cognitive performance showed no difference between groups at 10 weeks (MD 0.0; 95% CI ‐2.76 to 2.76; 51 women, one RCT; Analysis 3.1). The EPDS test did not show any statistical difference between groups at 10 weeks (MD 0.10; 95% CI ‐0.86 to 1.06; 51 women, one RCT; Analysis 3.2). The STAI tool showed no difference at 10 weeks (MD ‐0.40; 95% CI ‐3.18 to 2.38; 51 women, one RCT; Analysis 3.3). The Perceived Stress questionnaire showed a statistically significant difference favouring the placebo group at 10 weeks (MD 4.10; 95% CI 1.70 to 6.50; 51 women, one RCT; Analysis 3.4).
In the published report, the authors do not acknowledge the findings listed above.
Infections
Infections were not reported.
Compliance
Compliance was not reported.
Breastfeeding
Tam 2005 reported on breastfeeding rates at two days postpartum, with no statistically significant difference between groups (RR 0.82; 95% CI 0.58 to 1.17; 122 women; one RCT; Analysis 3.5).
Length of hospital stay
Length of hospital stay was not reported.
Adverse events
Adverse events were reported in two studies (Krauss 1972; Tam 2005). Tam 2005 reported only on one type of adverse events (back pain) for each study group individually, with no difference between groups (RR 0.66; 95% CI 0.42 to 1.03; one RCT, 53 events; 150 women; Analysis 3.6). The remaining adverse events were given for both study groups combined. The authors stated that there was no difference regarding nausea, vomiting, or constipation (Tam 2005). Krauss 1972 reported that six women in each group had GI adverse events such as constipation, low appetite, and morning sickness, but the numbers of each adverse event were not reported (very low quality evidence) (Analysis 3.7). No serious adverse events were reported.
Red blood cell transfusions
Red blood cell transfusions were not reported.
Comparison 4: Oral iron, magnesium oxide and yeast extract versus placebo
One study was included (67 women) in this comparison (Krauss 1972).
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms, psychological well being, infections, compliance, breastfeeding, length of hospital stay, blood transfusions
Not reported.
Adverse events
Different types of GI adverse events were not reported separately. Therefore, we analysed all GI symptoms as a whole for each group and found a statistically significant difference favouring the placebo group. Sixteen women in the intervention group had constipation, anorexia, bloating and vomiting. Six women in placebo group had constipation, low appetite and morning sickness (RR 2.75; 95% CI 1.23 to 6.16; one RCT; 22 events; 67 women; Analysis 4.1). No serious adverse events were reported.
Comparison 5: IV iron and oral iron after four weeks versus oral iron (week five to 12)
One study was included (117 women) (Westad 2008). Group A received IV iron immediately after giving birth and started oral iron after four weeks. Group B started receiving oral iron immediately after delivery.
Primary outcomes
Maternal mortality
Maternal mortality was not reported.
Fatigue
In the published report, the authors state that 'physical fatigue' improved significantly more in group A compared to group B. The improvement was seen at week eight (P = 0.02) and at week 12 (P = 0.03). There was no difference between the groups in their 'mental fatigue' score. The 'total fatigue' score was significantly better in group A at eight and 12 weeks (P = 0.02 at both time points). Standard deviations were not available for fatigue at eight and 12 weeks; therefore we could not carry out a statistical analysis.
Secondary outcomes
Anaemia symptoms
Anaemia symptoms were not reported.
Psychological well being
There was no difference between groups in the SF‐36 scores at week eight according to the published report. Standard deviations were not available for analysis.
Infections
Infections were not reported.
Compliance
Compliance to treatment with oral iron was assessed by counting returned pills. Compliance was reported as less than 50% of the recommended dose in both groups.
Breastfeeding
Breastfeeding was not reported.
Length of hospital stay
Length of hospital stay was not reported.
Adverse events
Adverse events did not differ significantly between groups. These were all GI symptoms, GI pain, constipation, diarrhoea, nausea, dysgeusia (distortion of the sense of taste), flatulence, melena, or headache (Analysis 5.1 to Analysis 5.9).
Red blood cell transfusions
We assumed that the RBC transfusions reported in this study were given within the first four weeks (see Comparison 1).
Comparison 6: IV iron and oral iron versus oral iron
Two studies (112 women) were included (Breymann 2000; Perello 2014). One study administered placebo EPO in the intervention arm (Breymann 2000) and the other study administered placebo IV iron in the comparator arm (Perello 2014). Thus, as per the lack of evidence for a substantial placebo effect (Hróbjartsson 2010) and the similar active treatments in these two studies, we found them comparable. Length of follow‐up was two and six weeks, respectively.
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms
One study evaluated anaemia symptoms by the number of patients who scored the severity as being equal to or more than seven on the Visual Analoge Scale (VAS) (higher score indicates higher severity) (Perello 2014). There was no statistically significant difference between groups at any time point (very low quality evidence; Analysis 6.1 to Analysis 6.3).
Psychological well being
Psychological well being was measured using the EPDS (Cox 1987) and the STAI (Marteau 1992) tools by Perello 2014. A clinically significant EPDS score was defined as being equal to or more than 11. There was no statistical difference between groups at one week of follow‐up (Analysis 6.4).
Infections
Infections were not reported.
Lenght of hospitalisation
Lenght of hospitalisation was reported by Perello 2014 and did not differ between groups (Analysis 6.5).
Adverse events
Adverse events were given as the number of patients who scored severity as being equal to or more than seven on the VAS in one study (Perello 2014). There was no statistically significant difference between groups at any time point (Analysis 6.6 to Analysis 6.8). The other study reported that there were no serious adverse events, including hypersensitivity or thromboembolic events (very low quality evidence). Five cases of dysgeusia, three cases of warm flushes and five cases of GI complaints were reported, but not by group (Breymann 2000).
Red blood cell transfusions
Blood transfusion rates did not differ between groups (Analysis 6.9).
Comparison 7: Erythropoietin (regardless of route) and IV iron versus IV iron
Two studies were included (80 women) (Krafft 2011; Wagstrom 2007). For the study by Wagstrom 2007, which had three study arms, we chose to compare group one (SC EPO 40,000 U + IV iron) and three (IV iron), for optimal resemblance to the study by Krafft 2011. Arm two received SC EPO 20,000 U + IV iron (Comparison 8).
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms
Anaemia symptoms were not reported
Psychological well being
One woman developed postpartum depression (RR 0.33; 95% CI 0.01 to 7.72; one RCT; 40 women; Analysis 7.1)
Infections
Infection rates were similar (RR 2.00; 95% CI 0.72 to 5.59; two RCTs; 80 women; Analysis 7.2).
Compliance
Compliance was reported as 100% in both groups since no women refused injections (Krafft 2011; Analysis 7.3).
Breastfeeding
All women in both groups of one study breast fed (Analysis 7.4) (Krafft 2011).
Length of hospital stay
Length of hospital stay was not reported.
Adverse events
Adverse events did not differ significantly between groups. These were dysgeusia, flush, diarrhoea, headache, itching including increased liver enzymes, dizziness, or thrombophlebitis (Analysis 7.5 to Analysis 7.11). There were no thromboembolic events.
Red blood cell transfusions
There was no difference between groups regarding blood transfusion rate (RR 3.00; 95% CI 0.13 to 69.52; two RCTs; 80 women; Analysis 7.12).
Comparison 8: Subcutaneous EPO 10,000 U two doses and IV iron versus IV iron
We included one study (40 women) Wagstrom 2007, with three study arms. For this comparison we used the arm that received SC EPO 20,000 U + IV iron (group two) and IV iron (group three). The last arm received SC EPO 40,000 U + IV iron (Comparison 7).
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms
Anaemia symptoms were not reported
Psychological well being
One woman developed postpartum depression (RR 0.33; 95% CI 0.01 to 7.72; one RCT; 40 women; Analysis 8.1)
Infection
Infection rates were similar (RR 0.75; 95% CI 0.19 to 2.93; Analysis 8.2).
Compliance, breastfeeding, length of hospital stay
Not reported.
Adverse events
There was no between‐group difference for other adverse events including headache, low blood pressure, diarrhoea, dizziness, or itching with increased liver enzymes (Analysis 8.3 to Analysis 8.7). There was no thromboembolic events.
Red blood cell transfusions
No women received RBC transfusions (Analysis 8.8).
Comparison 9: IV EPO, IV iron and oral iron versus IV iron and oral iron
Three studies were included (Breymann 1996; Breymann 2000; Lebrecht 1995). Two of them had three study arms. For the study by Breymann 1996, we chose the groups receiving IV EPO + IV iron + oral iron (group three) and IV iron + oral iron (group one) for this comparison. The remaining group received SC EPO + IV iron + oral iron (Comparison 12). For the study by Breymann 2000, we chose the group that received IV EPO + IV iron + oral iron (group one) and the group that received IV placebo‐EPO + IV iron + oral iron (group two) for this comparison. The last group received oral iron alone (Comparisons 6 and 13). The follow‐up periods for the three studies were between 14 and 42 days, with no common time point.
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms, psychological well being, infections, compliance, breastfeeding, length of hospital stay
Not reported
Adverse events
All three studies reported that there were no serious adverse events such as anaphylactic reactions or thromboembolic events. Leg paraesthesia was the only adverse event reported by group, with no statistically significant difference (RR 0.72; 95% CI 0.08 to 6.65; two RCTs; two events; 76 women; I² = 0%; Analysis 9.1). Two women experienced a warm sensation during iron infusion, dysgeusia was observed in 27 women and 10 women complained of a burning sensation during EPO injection (Breymann 1996). There were five cases of GI adverse events, five cases of dysgeusia and three cases of warm flushes (Breymann 2000). However, these numbers were not given for each group, but were combined, thus it is unknown to which study arm these women were randomised.
Red blood cell transfusions
No women RBC received blood transfusions (Breymann 1996; Breymann 2000; Analysis 9.2).
Comparison 10: Subcutaneous EPO and oral iron versus oral iron
One study was included (40 women) (Makrydimas 1998).
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms, psychological well being, infections, compliance
Not reported.
Breastfeeding
More women were breastfeeding in the EPO group (RR 1.90; 95% CI 1.21 to 2.98; Analysis 10.1). The time point for this observation was not stated.
Length of hospital stay
Length of hospital stay was reported as a median with 11 days (range six to 11) for 'EPO + oral iron' group and 14 days (range 11 to 19) for the oral group. The reason for the prolonged hospitalisation was not given. The available data were not sufficient to perform a statistical analysis.
Adverse events
Adverse events including GI symptoms were not reported.
Red blood cell transfusions
Two women in the oral group showed haemodynamic instability and received blood transfusions (RR 0.20; 95% 0.01 to 3.92; Analysis 10.2).
Comparison 11: IV EPO versus IV placebo
One study was included (71 women) (Meyer 1995).
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms
Anaemia symptoms were not reported
Psychological well being
According to the study authors, there was no statistically significant difference between the groups regarding psychological well being, which was measured using selected items from the 'Blues Questionnaire' (low score indicates absence of blues) (Kennerley 1989) and the 'Self‐report symptom inventory 90 [SCL‐90‐R]' (high scores indicate high levels of unfavourable symptoms) (Schmitz 1999). Data were not eligible for analysis.
Infections, compliance, breastfeeding, length of hospital stay, adverse events, red blood cell transfusions
Not reported
Comparison 12: Subcutaneous EPO, IV iron and oral iron versus IV iron and oral iron
One study was included (60 women) (Breymann 1996). The study had three study arms. For this comparison, we chose the arm receiving SC EPO + IV iron + oral iron (group two) and IV iron + oral iron (group 1). The remaining study arm received IV EPO + IV iron + oral iron (Comparison 9).
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms, psychological well being, infections, compliance, breastfeeding, length of hospital stay
Not reported
Adverse events
The study authors reported that there were no GI events, anaphylactic reactions or serious adverse events. Two women felt a warm sensation during iron infusion, 27 women experienced dysgeusia and 10 women experienced a burning sensation during EPO injection. However, these numbers were not provided per group.
Red blood cell transfusions
No women received blood transfusions.
Comparison 13: IV EPO, IV iron and oral iron versus oral iron
One study was included (40 women) (Breymann 2000). The study had three study arms. For this comparison, we used the arm that received IV EPO + IV iron + oral iron (group one) and oral iron (group three). The remaining study arm received IV placebo‐EPO + IV iron + oral iron (Comparisons 6 and 9).
Primary outcomes
None of our primary outcomes were reported.
Secondary outcomes
Anaemia symptoms, psychological well being, infections, compliance, breastfeeding, length of hospital stay
Not reported
Adverse events
The study reported that there were no serious adverse events, including hypersensitivity or thromboembolic events. Five cases of dysgeusia, three cases of warm flushes and five cases of GI complaints were reported, but not by group.
Red blood cell transfusions
No women received blood transfusions.
Planned analyses
We used random‐effects meta‐analyses for all dichotomous outcomes due to variation in length of treatment and dosages.
We found important statistical heterogeneity in two meta‐analyses in Comparison 1, and therefore we performed a sensitivity analyses for these, although the meta‐analyses were based on secondary outcomes.
Sensitivity analyses regarding trial design could not be performed due to lack of blinding throughout the main comparison and the other comparisons did not include enough studies for a meaningful sensitivity analysis.
It was not meaningful to perform subgroup analyses as planned, due to few included studies.
We did not produce a funnel plot as we had a maximum of eight studies in any single meta‐analysis.
'Summary of findings' tables
Please see summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4; summary of findings Table 5; summary of findings Table 6; summary of findings Table 7; summary of findings Table 8.
Discusión
Resumen de los resultados principales
Esta revisión incluyó 22 estudios con un total de 2858 mujeres. La mayoría de los análisis se basan en un pequeño número de estudios. Muy pocos estudios informan los resultados primarios mortalidad materna y fatiga. Los principales resultados se resumen en el "Resumen de los hallazgos para la comparación principal; Resumen de los hallazgos 2; Resumen de los hallazgos 3; Resumen de los hallazgos 4; Resumen de los hallazgos 5; Resumen de los hallazgos 6; Resumen de los hallazgos 7; Resumen de los hallazgos 8".
Resumen
Para el hierro intravenoso (IV) comparado con el hierro oral, la fatiga mejoró significativamente en el grupo de hierro IV en uno de dos estudios. No hubo datos sobre otros síntomas de la anemia. El bienestar psicológico no fue significativamente diferente entre los grupos. Hubo muy pocos datos o ninguno sobre la lactancia materna y la duración de la estancia hospitalaria. Las tasas de infección no fueron significativamente diferentes entre los grupos. La mortalidad materna se informó de manera insuficiente; Sin embargo, se informó una muerte debido a miocardiopatía en el grupo de hierro IV. Además, una paciente del grupo de hierro IV desarrolló arritmia. Solamente ocurrieron reacciones alérgicas en el grupo de hierro IV, pero no hubo diferencias estadísticamente significativas. La disgeusia, el malestar en el sitio de inyección y los sofocos solamente se observaron en las pacientes tratadas con hierro IV. Los síntomas gastrointestinales como el estreñimiento, las náuseas, el dolor gastrointestinal y la diarrea fueron mucho más frecuentes con el hierro oral. El compromiso hepático fue más frecuente en el grupo tratado por vía oral, aunque solo después del análisis de sensibilidad. El cumplimiento del tratamiento fue inferior en las pacientes tratadas por vía oral, posiblemente asociado con la alta frecuencia de síntomas GI. Las tasas de transfusión de sangre no difirieron significativamente.
Para la transfusión de eritrocitos en comparación con ninguna intervención no hubo datos sobre la mortalidad. Para la fatiga y el bienestar psicológico hubo una mejoría transitoria pequeña pero estadísticamente significativa a favor del grupo transfundido con eritrocitos. No hubo diferencia en las tasas de infección. Más pacientes del grupo de transfusión de eritrocitos cumplieron con el tratamiento asignado. No se observaron diferencias en la lactancia materna, la duración de la estancia hospitalaria o los eventos adversos informados, incluidas las reacciones a la transfusión. Una paciente desarrolló aloanticuerpos a los eritrocitos como resultado de la transfusión.
Para el hierro oral comparado con placebo no se informaron los resultados primarios. El bienestar psicológico no mejoró en el grupo de tratamiento. No hubo diferencias en la lactancia o los eventos adversos. No se informaron eventos adversos graves. El tratamiento con hierro oral con óxido de magnesio y el extracto de levadura mostraron significativamente más eventos adversos GI en comparación con placebo.
Para el hierro IV con hierro oral versus el hierro oral (semanas cinco a 12) no se informó la mortalidad. La fatiga mejoró más en el grupo tratado inicialmente con hierro IV. El cumplimiento fue igualmente deficiente en ambos grupos. No hubo diferencias en los eventos adversos informados. No se informaron eventos adversos graves. Se supuso que las transfusiones de eritrocitos tuvieron lugar en las cuatro primeras semanas de este estudio.
Para el hierro IV y hierro oral versus el hierro oral no se informaron los resultados primarios. No hubo eventos adversos graves. En general, no hubo diferencias entre los grupos.
Para la eritropoyetina (EPO) con hierro IV versus el hierro IV no se informaron los resultados primarios. No hubo diferencias con respecto a las infecciones. Todas las pacientes lactaron. No hubo diferencias con respecto a los eventos adversos. No se informaron eventos adversos graves. No se observaron diferencias en cuanto a las transfusiones sanguíneas.
Para la EPO IV con hierro IV y hierro oral versus el hierro IV con hierro oral no se informaron los resultados primarios. No se produjeron eventos adversos graves. No hubo diferencias en otros eventos adversos. Ninguna mujer recibió transfusiones.
Para la EPO subcutánea (SC) con hierro oral versus el hierro oral solo no se informaron los resultados primarios. Significativamente más pacientes lactaron en el grupo tratado con EPO. La duración de la estancia hospitalaria fue bastante larga en ambos grupos. No hubo diferencias en las tasas de transfusión.
Para la EPO IV versus el placebo IV no se informaron los resultados primarios. No hubo diferencias estadísticamente significativas en cuanto al bienestar psicológico.
Para la EPO SC, hierro IV y hierro oral versus el hierro IV con hierro oral no se informaron los resultados primarios. No hubo eventos adversos graves. Ninguna mujer recibió transfusiones. Algunas pacientes sintieron una sensación de ardor durante la inyección de EPO.
Para la EPO IV, hierro IV y hierro oral versus el hierro oral no se informaron los resultados primarios. No hubo eventos adversos graves. Ninguna mujer recibió transfusiones.
En todos los estudios incluidos, los datos sobre las reacciones alérgicas se obtuvieron de 1003 pacientes que recibieron tratamiento con hierro IV (solo o combinado con otro tratamiento). Tres de estas mujeres desarrollaron una reacción anafiláctica.
Interpretación de los métodos y los resultados de los estudios
Comparación principal: Hierro IV versus hierro oral
El hierro intravenoso es quizás más eficaz para reducir la fatiga que el hierro oral, pero este efecto beneficioso solamente se mostró en un estudio pequeño, por lo que la cantidad de pruebas es muy limitada y la importancia clínica todavía no está clara. No se evaluaron otros síntomas de la anemia. Por lo tanto, no es posible establecer conclusiones al respecto.
Ocurrió una muerte materna en el grupo IV debido a miocardiopatía periparto. Además, una paciente desarrolló arritmia durante la infusión de hierro IV. Por lo tanto, se observaron dos casos de eventos adversos cardíacos graves. Esto no se había descrito anteriormente como un efecto adverso del tratamiento con hierro IV y no es posible establecer conclusiones con respecto a una relación causal. Sin embargo, se sugiere que los eventos cardíacos se monitoricen cuidadosamente y se describan en los estudios futuros.
La anafilaxia se informó en tres pacientes y todas habían recibido hierro IV. Debido al escaso número de eventos y a la falta de poder estadístico, estos datos no demuestran que la anafilaxia u otros eventos adversos graves ocurran con mayor frecuencia debido al hierro IV que al hierro oral. Sin embargo, se necesitaría un número muy alto de participantes en los estudios para descartar una asociación entre el hierro IV y la anafilaxia. Como la anafilaxia es un efecto secundario comprobado del hierro IV y es una reacción peligrosa y potencialmente mortal, el French Medicines Agency ha planteado inquietudes debido a algunos casos observados. El French Medicines Agency emitió por lo tanto un informe de seguridad (French Medicines Agency 2013) y una recomendación de control de riesgos (French Medicines Agency 2013a) con respecto a las reacciones alérgicas al hierro IV. Se concluyó que el tratamiento con hierro IV conlleva un riesgo pequeño de reacciones alérgicas que pueden ser potencialmente mortales.
La urticaria puede ser una manifestación de una reacción alérgica y, por lo tanto, las reacciones de urticaria informadas pueden estar incluidas en la anafilaxia o en las pruebas del resultado de hipersensibilidad, con la condición de que la urticaria también puede ser causada por otros factores.
La distorsión gustativa (disgeusia), el malestar en el sitio de inyección y los sofocos fueron síntomas asociados con el tratamiento con hierro IV. Estas reacciones son desagradables pero transitorias y no se consideran perjudiciales. Sin embargo, no es posible excluir que los sofocos y el malestar en el sitio de inyección podrían ser pródromos que anuncian reacciones de hipersensibilidad más graves y se deben observar estrechamente.
Los eventos adversos gastrointestinales estreñimiento, náuseas, dolor GI y diarrea combinados y específicos fueron mucho más frecuentes con el hierro oral. Este hecho se corresponde bien con el conocimiento y las inquietudes generales entre los médicos de que los síntomas GI son frecuentes, molestos y pueden afectar negativamente el cumplimiento. Lo anterior significa que los pacientes que no pueden controlar los efectos adversos del tratamiento a menudo no reciben un tratamiento suficiente. Sin embargo, estos son pacientes que probablemente tienen la mayor necesidad de tratamiento y depende de los médicos detectar este subgrupo menos tolerante de pacientes y considerar métodos de tratamiento alternativos. El cumplimiento alto del tratamiento con hierro IV se puede explicar por el hecho de que los médicos administraron el tratamiento IV en pocas dosis, a diferencia de las numerosas dosis diarias autoadministradas de hierro oral durante varias semanas.
Para el compromiso hepático, los resultados fueron estadísticamente significativos a favor del tratamiento IV, cuando se realizó un análisis de sensibilidad. Es sorprendente que el compromiso hepático se mostró en el grupo oral. No fue posible encontrar bibliografía que apoye la asociación entre el hierro oral y la toxicidad hepática en los seres humanos. Sin embargo, este efecto del sulfato ferroso oral se describe en las ratas (Toblli 2008; Toblli 2013). Se debe señalar que los dos estudios incluidos en el análisis de sensibilidad para el compromiso hepático tuvieron una dosis semanal casi cinco veces mayor de hierro oral que el estudio que causó la heterogeneidad. Estos resultados indican una relación dosis‐respuesta entre el sulfato ferroso oral y el efecto hepatotóxico. El anterior fue un resultado poco habitual, aunque puede ser más frecuente porque la función hepática no se midió en todos los estudios. Además, la mayoría de los estudios con un brazo de hierro IV excluyó a las participantes con enfermedad hepática conocida. Por lo tanto, hay una selección de poblaciones que favorece a las pacientes con un hígado sano también en el brazo oral de estos estudios. Se necesitan estudios de investigación adicionales para examinar el efecto del sulfato ferroso sobre la función hepática.
Se encontró una alta heterogeneidad para la infección, lo que indicó la incertidumbre del resultado. Para las infecciones no fue posible predecir con certeza qué estudio tuvo mayores probabilidades de causar la heterogeneidad. Sin embargo, se sabe que el estudio Breymann 2008 causó la heterogeneidad en el metanálisis para el compromiso hepático, que tuvo un nivel alto de sesgo de deserción y que el método de asignación al azar no se describió. Los resultados para las infecciones se mantuvieron estadísticamente no significativos en el análisis de sensibilidad.
Eficacia
Efectos perjudiciales del tratamiento
Otras modalidades de tratamiento
Para las transfusiones de eritrocitos comparadas con los datos de ninguna intervención (Comparación 2), se observó una mejoría pequeña y transitoria pero estadísticamente significativa y los datos del SF 36 favorecieron al tratamiento con eritrocitos. Los datos del SF 36 proporcionados tuvieron desviaciones estándar muy amplias y el análisis de las medias brutas difirió de lo que se señaló en el informe publicado. Sin embargo, ambas fuentes encontraron un efecto estadísticamente significativo a favor de las transfusiones de eritrocitos. Es debatible si el efecto de la transfusión de eritrocitos sobre la fatiga y el bienestar psicológico es clínicamente significativo en las pacientes hemodinámicamente estables sin síntomas graves de anemia, ya que la ganancia pequeña y transitoria en las puntuaciones de fatiga y bienestar psicológico se debe equilibrar contra los efectos secundarios graves potenciales de las transfusiones de eritrocitos. Además, el alivio de los síntomas subjetivos relativamente leves con un tratamiento costoso no es eficaz en función de los costos (Prick 2014).
Este estudio no informó sistemáticamente todos los síntomas de anemia en los dos grupos. Sin embargo, los síntomas graves de anemia dieron lugar a la transfusión de eritrocitos en 28 pacientes asignadas al azar al grupo ninguna intervención. Lo anterior ilustra la importancia del registro y el informe de los síntomas de anemia como un resultado y de la necesidad de evaluarlos como una indicación para la transfusión, ya que la consecuencia de estos síntomas puede desencadenar un tratamiento potencialmente perjudicial.
El ensayo se realizó en los Países Bajos en los que el procedimiento para la transfusión de sangre, incluido el cribado y el pareamiento cruzado y la administración, está muy desarrollado y por lo tanto es seguro. La misma falta de diferencia con respecto a las infecciones, la lactancia materna, la duración de la estancia hospitalaria o los eventos adversos puede no ser la misma en un contexto diferente, es decir, en países de bajos ingresos. Una paciente desarrolló aloanticuerpos después de la transfusión. Como no hay un examen sistemático postransfusión para los aloanticuerpos recién formados en todos los participantes transfundidos, y como estos anticuerpos a menudo tienen consecuencias clínicas sutiles o retardadas en las semanas posteriores a la transfusión, es muy probable que la frecuencia esté subestimada. En general, la incidencia de aloanticuerpos recién generados depende en gran medida de la población de pacientes y no se conoce la incidencia precisa de anticuerpos producidos mediante la transfusión posparto. Un estudio reciente reveló que los aloanticuerpos inesperados aparecen en el 3% de las pacientes obstétricas y aproximadamente un tercio de estos casos se asoció con enfermedades hemolíticas (destrucción de los eritrocitos) en el recién nacido (Smith 2013). Aunque los anticuerpos como consecuencia de la transfusión pueden solamente representar algunos de estos casos, potencialmente pueden dañar al feto en el futuro y, por lo tanto, tener consecuencias significativas (Klein 2014). Hubo un cumplimiento bajo en el grupo ninguna intervención que se puede explicar por el diseño de los ensayos: Si las pacientes asignadas al grupo ninguna intervención recibieron una transfusión, p.ej. debido a hemorragia posparto secundaria, automáticamente no lograron cumplir con el tratamiento asignado, mientras que las pacientes asignadas al grupo de transfusión de eritrocitos no tuvieron un motivo obvio para rechazar la transfusión después de dar el consentimiento para participar en el ensayo.
Cuando se comparó el hierro oral con placebo no fue posible realizar un metanálisis debido a que no hubo suficientes datos. Además, los períodos de seguimiento fueron muy diferentes y no hubo puntos temporales comunes. En la evaluación del bienestar psicológico una prueba indicó que el tratamiento en realidad redujo el bienestar psicológico en comparación con placebo. Sin embargo, los autores del ensayo no llegaron a la misma conclusión. Cuando se evalúan medidas como el bienestar psicológico es importante utilizar herramientas validadas para la población específica (es decir, mujeres posparto).
Las pacientes que comenzaron su período posparto con una infusión de hierro IV y luego se complementaron con hierro oral a las cuatro semanas tuvieron significativamente menos fatiga que las pacientes que recibieron hierro oral solo desde el comienzo del período posparto. Sin embargo, al final de este estudio la tasa de abandonos fue muy alta y la población estuvo muy expuesta al sesgo de deserción, por lo que es posible que se seleccionaran pacientes más fuertes y más sanas.
Los datos de un estudio pequeño (Makrydimas 1998) indicaron que las pacientes pudieron lactar más en el grupo tratado con EPO y hierro oral en comparación con el grupo de hierro oral. Este efecto de la EPO no se ha informado en otro sitio. Por el contrario, el estudio con igual tamaño de la muestra realizado por Krafft 2011 mostró que las pacientes tratadas con EPO y hierro IV tuvieron la misma tasa alta de lactancia materna que las tratadas con hierro IV solo. Por lo tanto, la diferencia en la tasa de lactancia observada en Makrydimas 1998 probablemente coincidió debido al material pequeño de estudio.
Control de la fatiga y datos de la calidad de vida
La fatiga y el bienestar psicológico son conceptos muy difíciles de definir y de delimitar uno del otro. La fatiga se puede percibir como un fenómeno unidimensional (Escala Analógica Visual [EAV]), se puede subdividir en aspectos mentales y físicos (Fatigue Score) e incluso puede consistir en dimensiones adicionales (reducción de la motivación y la actividad), como se observa en el Multidimentional Fatigue Inventrory (MFI). Estos aspectos adicionales de la fatiga tienen ciertas semejanzas con los ítems de las herramientas utilizadas para medir el bienestar psicológico. El bienestar psicológico medido con el SF‐36 puede estar influenciado por una depresión puerperal. Como se define en la International Classification of Diseases (ICD‐10), uno de los síntomas fundamentales de la depresión es "reducción de la energía o aumento del cansancio", o lo que se comprende habitualmente como "fatiga". En realidad, el SF‐36 contiene preguntas dirigidas específicamente a revelar los síntomas de fatiga resumidos en el ítem "vitalidad". Por lo tanto, aunque la fatiga y el bienestar psicológico se definieron como resultados separados, se reconoce la complejidad de estos síntomas y se desarrollaron herramientas para medirlos. Por lo tanto, no es aconsejable disecar los datos de los cuestionarios en un intento de extraer solamente los resultados relacionados con la fatiga.
La evaluación de la calidad de vida relacionada con la salud es muy subjetiva y las herramientas creadas para medir la calidad de vida simplemente traducen sentimientos en números que solamente se aproximan a la realidad, y la significación estadística en el cálculo de las puntuaciones no equivale necesariamente a la significación clínica. Por lo tanto, podría ser más informativo y útil establecer una diferencia o umbral mínimo clínicamente relevante en las puntuaciones por encima o por debajo de las cuales se confirma que un paciente presenta una afección o una emoción determinadas (p.ej. Perello 2014).
Para los resultados de la calidad de vida solamente se informaron los puntos temporales más relevantes. La intención fue limitar el número de análisis a una cantidad de información manejable y evitar informar puntos temporales demasiado cercanos entre sí.
Para el bienestar psicológico, se encontró que los puntos temporales a corto plazo fueron más importantes cuando se compararon dos regímenes de tratamiento, ya que es clínicamente relevante la rapidez con la que un tratamiento administrado puede aliviar los síntomas, y es probable que las diferencias a largo plazo estén afectadas por un número desconocido de factores de confusión, y la anemia a menudo se resolvería con el transcurso del tiempo debido a factores fisiológicos.
La discrepancia en la significación estadística entre las medias brutas introducidas en RevMan 2014 y los resultados en los informes publicados se puede deber a la falta de cálculos de ajuste para los datos adicionales proporcionados (Prick 2014). Sin embargo, esta explicación no se aplica a los datos publicados (p.ej. Beard 2005).
Compleción y aplicabilidad general de las pruebas
Después de incluir 22 estudios, era de esperar que hubiera datos suficientes para realizar un metanálisis exhaustivo de los resultados clínicamente relevantes para la eficacia de diferentes métodos de tratamiento de la anemia ferropénica posparto. En cuanto a la eficacia del tratamiento, la bibliografía disponible proporcionó información sorprendentemente escasa sobre los resultados clínicos. En cambio, se hizo énfasis en resultados substitutos como los valores de laboratorio. Fue sorprendente que solamente tres estudios investigaran la fatiga, seleccionada como un resultado primario debido a su gran frecuencia en las pacientes con anemia.
Los estudios identificados proporcionan una apreciación general aceptable de diversos síntomas perjudiciales, aunque el informe fue heterogéneo, los síntomas fueron en ocasiones difíciles de agrupar y la mortalidad materna se informó muy pocas veces. Se conoce que la anafilaxia, la complicación más temida del hierro IV, es poco frecuente y se ha confirmado que los eventos adversos GI asociados con el hierro oral fueron comunes.
Podría ser difícil distinguir entre los síntomas de una afección y los efectos adversos de un tratamiento. En teoría, los síntomas de anemia deben disminuir con el transcurso del tiempo, a la vez que aumentan los eventos adversos debido a la exposición a los fármacos. Los investigadores generalmente no informaron el momento en el cual ocurrió un síntoma. Sin embargo, es importante informar el punto temporal al cual el paciente presenta un malestar de cualquier clase (p.ej. cefalea) para distinguir entre los síntomas de la anemia y los efectos perjudiciales del tratamiento. Los estudios no coincidieron en los puntos temporales comunes clínicamente relevantes para recopilar los datos, que es importante en el metanálisis.
Sorprendentemente, solo tres estudios pequeños investigaron el efecto del hierro oral versus placebo y uno de ellos se realizó hace más de 40 años. El costo de los diferentes fármacos y tratamientos varía según el contexto, por lo que no fue posible generalizarlo. Sin embargo, es seguro que el hierro IV cuesta significativamente más que el hierro oral (Khalafallah 2012) y la infusión a menudo requiere hospitalización. Cuando se investiga el tratamiento de una afección que tiene grandes probabilidades de ocurrir en ámbitos de bajos ingresos, en el que los factores nutricionales, los períodos más largos de lactancia materna y el número de embarazos en la paciente pueden ser diferentes de los de los países de ingresos altos, se le debe brindar atención especial a los tipos de opciones de tratamiento disponibles en estos contextos particulares, es decir, el hierro oral.
La mayoría de los ensayos se realizó en países de ingresos altos y solamente en dos informes se señaló específicamente que las participantes procedían de un contexto de bajos ingresos. Lo anterior cuestiona la validez externa de los resultados y los médicos deben tener presente que las pacientes de diferentes contextos pueden responder de manera diferente al mismo tratamiento. Las pacientes que viven en ámbitos de bajos ingresos pueden ser más propensas a la desnutrición y las infecciones. Pueden tener un grado inferior de educación y según las normas sociales, es de esperar que tengan más hijos. Estos factores pueden afectar el cumplimiento, los efectos adversos del tratamiento, el tratamiento de los eventos adversos (búsqueda de ayuda) y la respuesta al tratamiento.
Entre los estudios aleatorios elegibles para inclusión en esta revisión hubo una variación significativa en el diseño de los ensayos y muchos fueron pequeños. Este hecho, junto con la falta de resultados clínicos, hizo muy difícil agrupar los datos en los metanálisis.
Calidad de la evidencia
El grupo de pruebas en esta revisión no permite establecer una conclusión consistente sobre la eficacia clínica. La conclusión sobre los efectos perjudiciales es más exhaustiva, aunque presenta graves limitaciones, especialmente con respecto a la mortalidad materna.
Cuando se utilizó la herramienta "Riesgo de sesgo", se encontró que en su mayoría los estudios incluidos tuvieron alto riesgo de sesgo en al menos dos dominios. Además, 11 estudios no describieron el método de asignación al azar. Lo anterior puede disfrazar un grave sesgo de selección (ver Características de los estudios excluidos para ejemplos). Muchos autores de contacto no respondieron a las cartas y aún no se han revelado posibles violaciones de la ética de la investigación.
Al utilizar el enfoque GRADE se disminuyó el nivel de calidad del grupo de pruebas debido a los siguientes motivos. En su mayoría los estudios fueron abiertos y en algunos de los estudios cegados no estuvo claro si el cegamiento tuvo éxito. Las medidas de resultado clínicas como la salud autoinformada y la mayoría de los eventos adversos, están muy afectados por el sesgo de realización y de detección. Sin embargo, algunos resultados están menos afectados o no están afectados por la subjetividad de la paciente, por ejemplo las infecciones con ascenso en la temperatura corporal, las enzimas hepáticas elevadas y la mortalidad. Una gran parte de los estudios incluidos tuvo una tasa alta de abandonos (especialmente con el hierro oral), lo que indica un mayor riesgo de sesgo de deserción. Por lo tanto, las poblaciones de estudio pueden haber sido seleccionadas si las que abandonaron representan la parte más vulnerable de la población. En dichos casos, es importante informar por qué las participantes abandonaron y realizar análisis por intención de tratar.
La inconsistencia solamente fue significativa en dos resultados (compromiso hepático e infecciones, Comparación 1) debido al alto nivel de heterogeneidad, que se abordó mediante análisis de sensibilidad.
No hubo dificultades con respecto a la falta de direccionalidad.
No obstante, la imprecisión fue un problema frecuente debido a los tamaños de la muestra pequeños y a los intervalos de confianza amplios, lo que naturalmente disminuye la confianza en los efectos.
El riesgo de sesgo de informe fue alto. Muchos estudios no informaron los efectos perjudiciales, es decir la mortalidad materna o la supervivencia, aunque lo anterior se debe considerar un conocimiento fundamental cuando se prueba la seguridad de los fármacos. La falta de informe de los efectos perjudiciales plantea la grave inquietud de que pueden haber ocurrido eventos no favorables pero no se informaron. En varios ensayos hubo discrepancia entre los resultados preplanificados e informados, lo que también indica un alto riesgo de informe selectivo. Además, se sabe de un ensayo apropiado para inclusión que se terminó prematuramente por los patrocinadores del ensayo. Este hecho se agrega al alto riesgo de sesgo de informe.
Sesgos potenciales en el proceso de revisión
Los criterios de inclusión se basaron en los valores de hemoglobina (Hb), aunque se señaló que los criterios de inclusión se deben basar en la presencia y la gravedad de los síntomas de anemia. Sin embargo, como los estudios incluidos decidieron incluir a las pacientes según los valores de Hb como criterios de inclusión, no fue posible utilizar los síntomas de anemia como criterios de inclusión, ya que casi nunca las pacientes se cribaron de forma sistemática para detectarlos.
Debido a la falta de detalles en las secciones de método de los estudios incluidos, se pueden haber agrupado datos en parte incompatibles, es decir, datos de diferentes puntos temporales durante el seguimiento y diferentes dosis. Además, no fue posible distinguir claramente los eventos adversos del tratamiento de los síntomas de anemia.
Solamente se informaron los resultados preespecificados para esta revisión. Por lo tanto, no se informa el efecto de las intervenciones sobre los valores de laboratorio.
Algunos datos fueron proporcionados gráficamente en los informes publicados. Se solicitaron constantemente estos datos en forma numérica junto con las desviaciones estándar correspondientes. Cuando los autores no respondieron, se leyeron los valores de los gráficos. Sin embargo, la falta de datos como la desviación estándar impidió realizar ciertos análisis.
Acuerdos y desacuerdos con otros estudios o revisiones
Sólo se informaron los resultados que se consideraron clínicamente relevantes. La versión original de esta revisión (Dodd 2004) también informó los resultados de laboratorio (Hb y hematócrito [Htc]). La revisión anterior tenía la limitación de que había muy pocos estudios incluidos. En la presente revisión se intentó determinar el efecto clínico del tratamiento, pero este objetivo no se pudo lograr en la mayoría de los estudios ya que se centraron en los valores de laboratorio.
Se identificaron varios otros artículos de revisión sobre el tratamiento de la anemia, incluida la anemia ferropénica posparto (Breymann 2010; Khalafallah 2012; Milman 2011; Milman 2011a; Milman 2012). En general, la gravedad de la anemia, la evaluación del efecto del tratamiento y los tratamientos propuestos se basaron solamente en los valores de laboratorio. Los métodos de búsqueda no se describieron claramente y por tanto, se asume que la búsqueda no fue sistemática.
Se identificó una revisión sistemática sobre la seguridad y eficacia del tratamiento con hierro IV que incluyó algunas medidas de resultado clínicas además de los valores de Hb (Litton 2013). Sin embargo, dicha revisión se basó en todos los tipos de pacientes y no se realizó un análisis de subgrupos en las pacientes posparto. Por lo tanto, sus resultados pueden no ser aplicables a esta población específica. Los resultados mostraron un mayor riesgo de infecciones en el grupo tratado de forma intravenosa pero sin diferencias en la mortalidad, los eventos adversos o las transfusiones de sangre. Los resultados sobre las infecciones no se pudieron confirmar con el presente metanálisis, pero se observó una tendencia similar y la falta de significación se puede deber al menor número de participantes en el presente análisis.
Una revisión sistemática abordó la prevención y el tratamiento de la anemia materna y mostró una falta de pruebas con respecto al tratamiento de la anemia en el período posnatal y el informe deficiente de medidas de resultado clínicas (Parker 2012).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Intravenous iron versus oral iron, Outcome 1 Maternal mortality.

Comparison 1 Intravenous iron versus oral iron, Outcome 2 Fatigue ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 3 Fatigue ‐ 42 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 4 SF‐36: Physical F(x) ‐ 14 days.
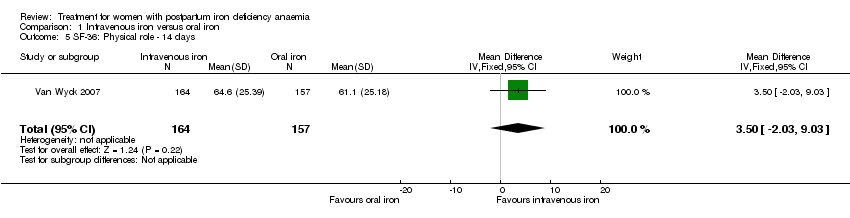
Comparison 1 Intravenous iron versus oral iron, Outcome 5 SF‐36: Physical role ‐ 14 days.
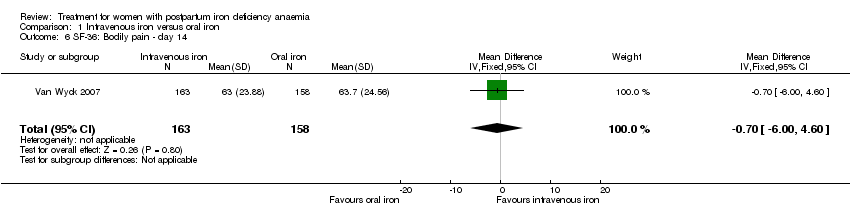
Comparison 1 Intravenous iron versus oral iron, Outcome 6 SF‐36: Bodily pain ‐ day 14.

Comparison 1 Intravenous iron versus oral iron, Outcome 7 SF‐36: General health ‐ 14 days.
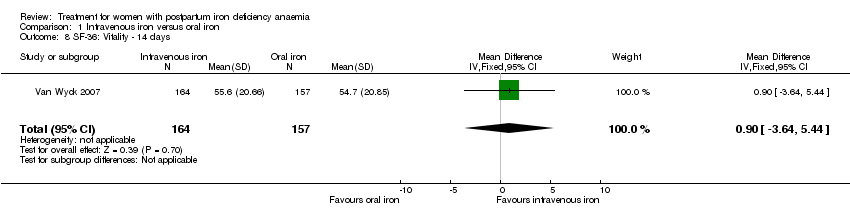
Comparison 1 Intravenous iron versus oral iron, Outcome 8 SF‐36: Vitality ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 9 SF‐36: Emotional role ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 10 SF‐36: Social function ‐ 14 days.

Comparison 1 Intravenous iron versus oral iron, Outcome 11 SF‐36: Mental health ‐ 14 days.
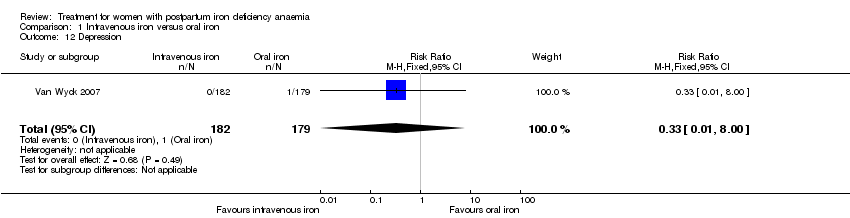
Comparison 1 Intravenous iron versus oral iron, Outcome 12 Depression.
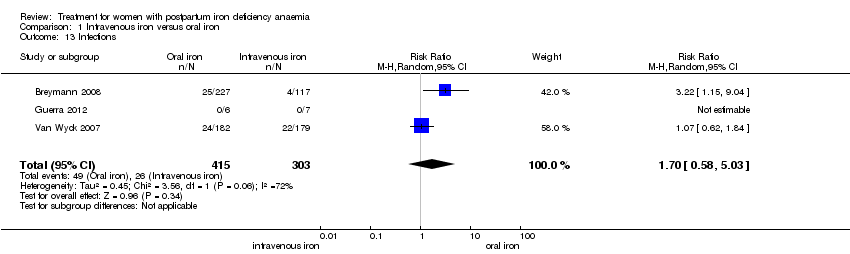
Comparison 1 Intravenous iron versus oral iron, Outcome 13 Infections.

Comparison 1 Intravenous iron versus oral iron, Outcome 14 Compliance to treatment.
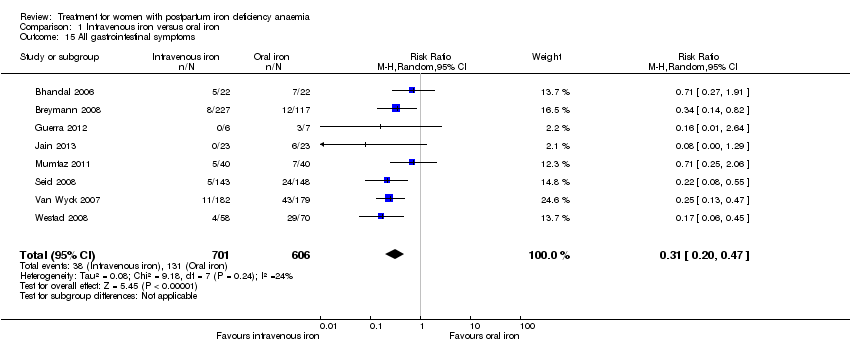
Comparison 1 Intravenous iron versus oral iron, Outcome 15 All gastrointestinal symptoms.
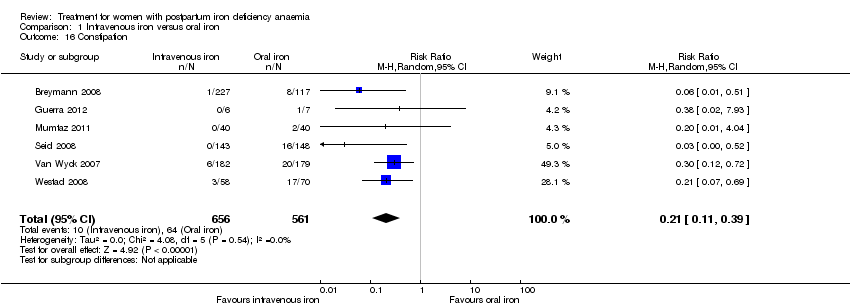
Comparison 1 Intravenous iron versus oral iron, Outcome 16 Constipation.

Comparison 1 Intravenous iron versus oral iron, Outcome 17 Nausea.

Comparison 1 Intravenous iron versus oral iron, Outcome 18 Gastrointestinal pain.

Comparison 1 Intravenous iron versus oral iron, Outcome 19 Diarrhoea.

Comparison 1 Intravenous iron versus oral iron, Outcome 20 Vomiting.

Comparison 1 Intravenous iron versus oral iron, Outcome 21 Dyspepsia.

Comparison 1 Intravenous iron versus oral iron, Outcome 22 Dysgeusia.

Comparison 1 Intravenous iron versus oral iron, Outcome 23 Headache.

Comparison 1 Intravenous iron versus oral iron, Outcome 24 Hepatic involvement.

Comparison 1 Intravenous iron versus oral iron, Outcome 25 Injection site discomfort.

Comparison 1 Intravenous iron versus oral iron, Outcome 26 Skin rash.
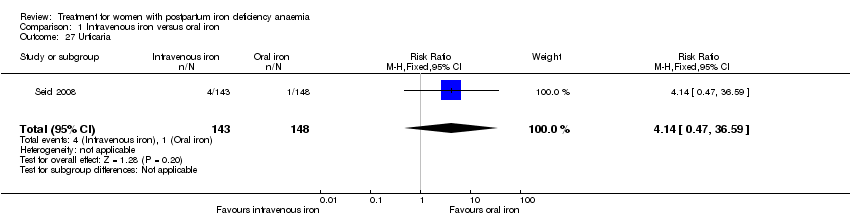
Comparison 1 Intravenous iron versus oral iron, Outcome 27 Urticaria.

Comparison 1 Intravenous iron versus oral iron, Outcome 28 Flush.

Comparison 1 Intravenous iron versus oral iron, Outcome 29 Muscle cramp.
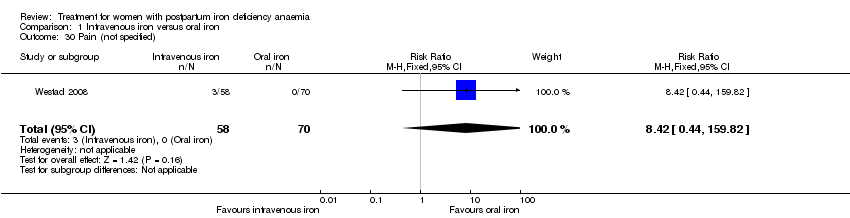
Comparison 1 Intravenous iron versus oral iron, Outcome 30 Pain (not specified).

Comparison 1 Intravenous iron versus oral iron, Outcome 31 Seriouse adverse events (not specified).
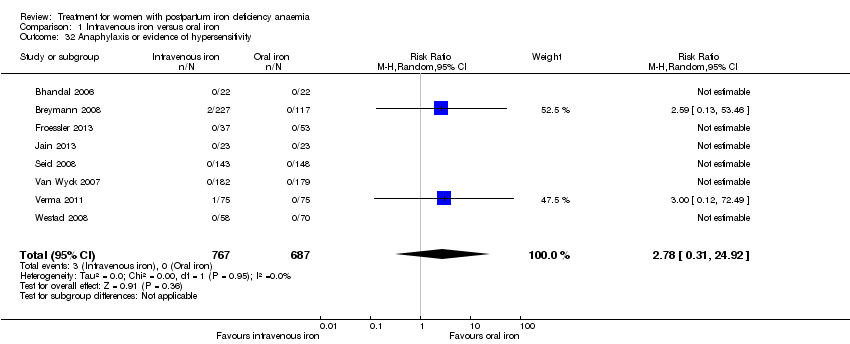
Comparison 1 Intravenous iron versus oral iron, Outcome 32 Anaphylaxis or evidence of hypersensitivity.

Comparison 1 Intravenous iron versus oral iron, Outcome 33 Arythmia.

Comparison 1 Intravenous iron versus oral iron, Outcome 34 Red blood cell transfusion.
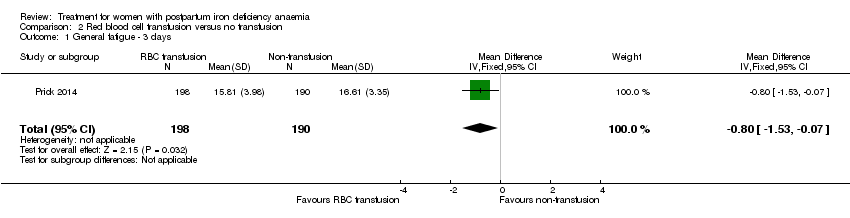
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 1 General fatigue ‐ 3 days.
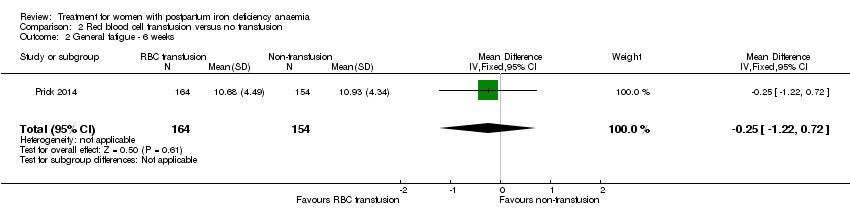
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 2 General fatigue ‐ 6 weeks.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 3 SF‐36: Physical functioning ‐ 1 week.
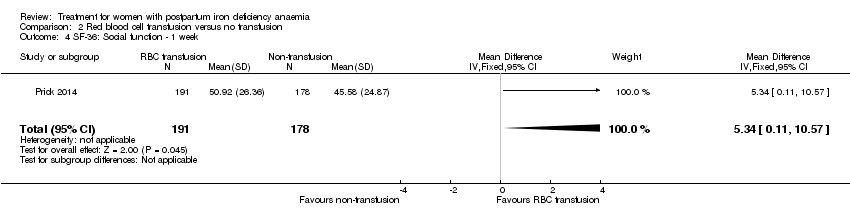
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 4 SF‐36: Social function ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 5 SF‐36: Physical role ‐ 1 week.
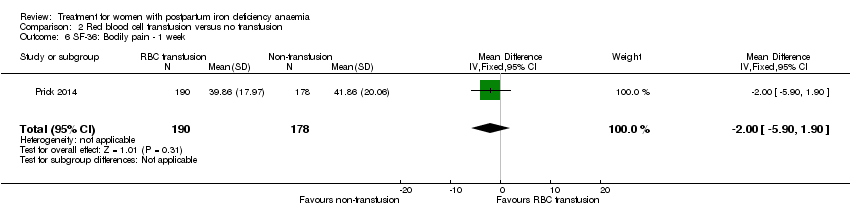
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 6 SF‐36: Bodily pain ‐ 1 week.
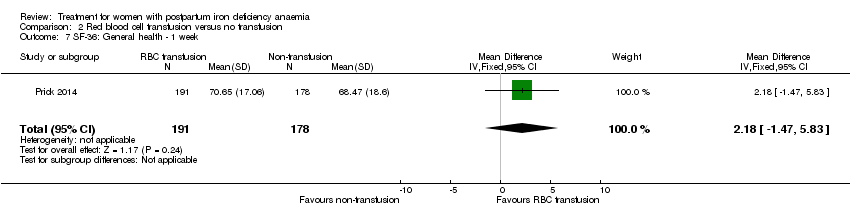
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 7 SF‐36: General health ‐ 1 week.
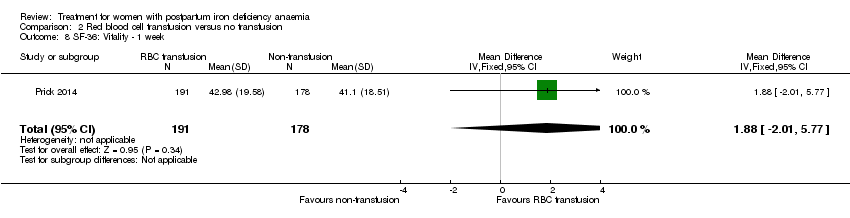
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 8 SF‐36: Vitality ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 9 SF‐36: Emotional role ‐ 1 week.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 10 SF‐36: Mental health ‐ 1 week.
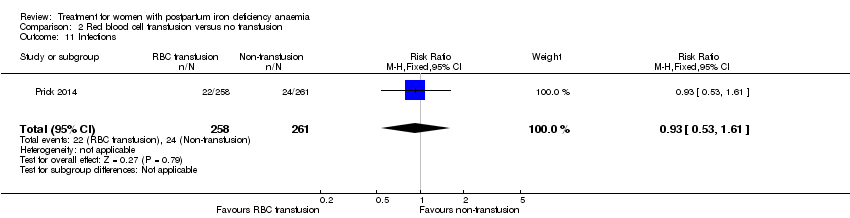
Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 11 Infections.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 12 Compliance to treatment.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 13 Breastfeeding at six weeks.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 14 Erythrocyte alloantibody formation.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 15 Rash.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 16 Fever.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 17 Thromboembolic events.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 18 Parenteral iron intolerance.

Comparison 2 Red blood cell transfusion versus no transfusion, Outcome 19 Transfusion reactions.
Comparison 3 Oral iron versus placebo, Outcome 1 Digit Symbol Substitution test ‐ 10 weeks.

Comparison 3 Oral iron versus placebo, Outcome 2 EPDS ‐ 10 weeks.

Comparison 3 Oral iron versus placebo, Outcome 3 STAI ‐ 10 weeks.

Comparison 3 Oral iron versus placebo, Outcome 4 Percieved Stress ‐ 10 weeks.

Comparison 3 Oral iron versus placebo, Outcome 5 Breastfeeding at two days postpartum.
Comparison 3 Oral iron versus placebo, Outcome 6 Back pain.
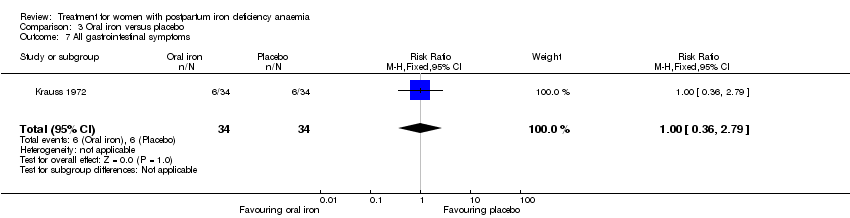
Comparison 3 Oral iron versus placebo, Outcome 7 All gastrointestinal symptoms.

Comparison 4 Oral iron, magnesium oxide and yeast extract versus placebo, Outcome 1 All gastrointestinal symptoms.
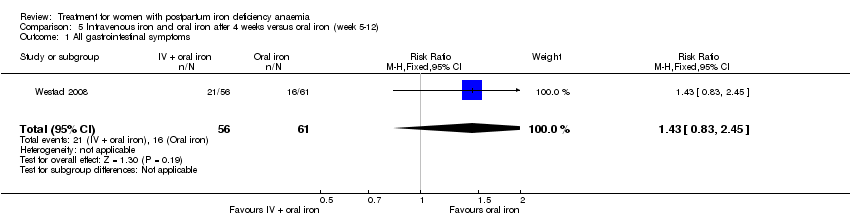
Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 1 All gastrointestinal symptoms.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 2 Abdominal pain.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 3 Constipation.
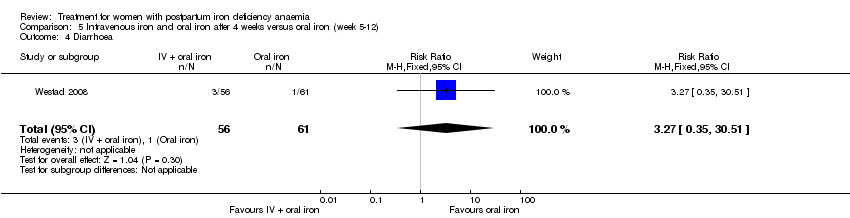
Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 4 Diarrhoea.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 5 Nausea.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 6 Dysgeusia.

Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 7 Flatulence.
Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 8 Melaena.
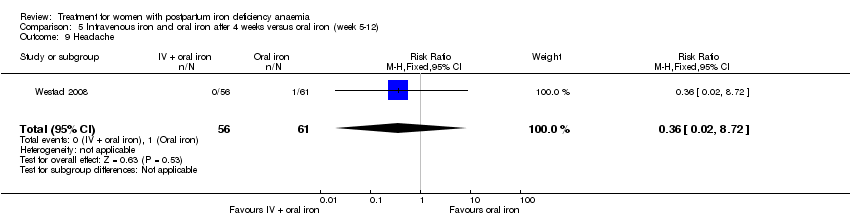
Comparison 5 Intravenous iron and oral iron after 4 weeks versus oral iron (week 5‐12), Outcome 9 Headache.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 1 Persistent anaemia symptoms on a VAS scale: 1 week.
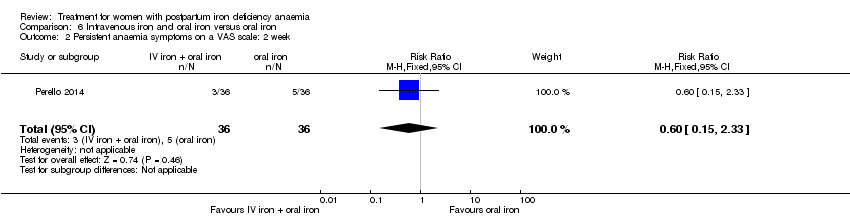
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 2 Persistent anaemia symptoms on a VAS scale: 2 week.
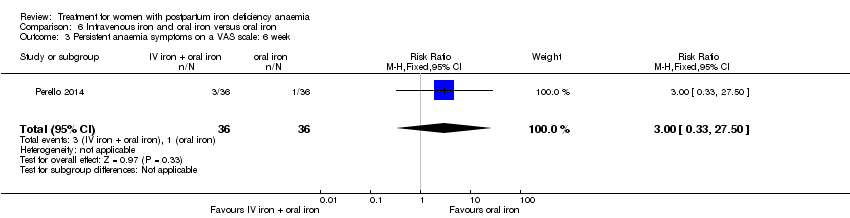
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 3 Persistent anaemia symptoms on a VAS scale: 6 week.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 4 EPDS ‐ 1 week.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 5 Length of hospital stay.
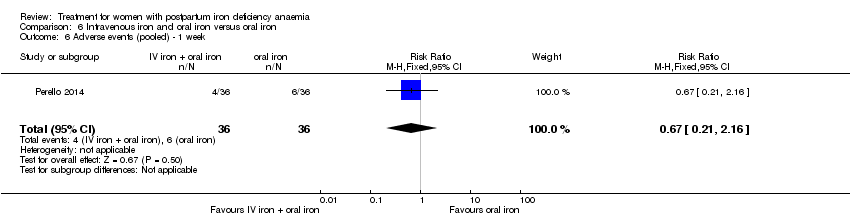
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 6 Adverse events (pooled) ‐ 1 week.
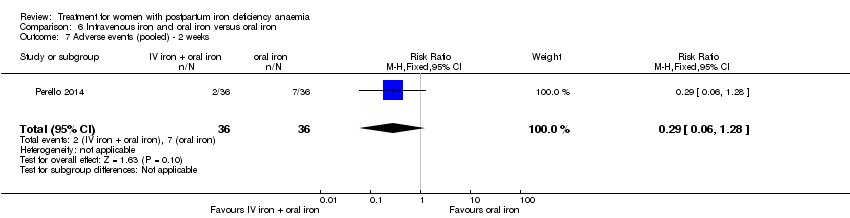
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 7 Adverse events (pooled) ‐ 2 weeks.
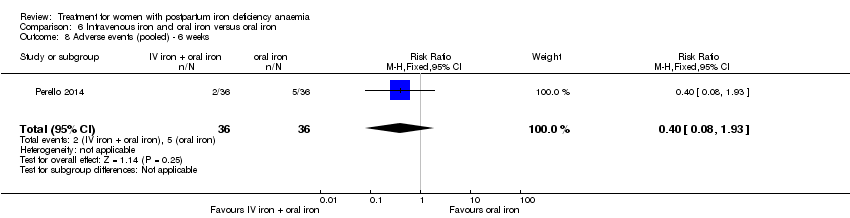
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 8 Adverse events (pooled) ‐ 6 weeks.

Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 9 Red blood cell transfusion.
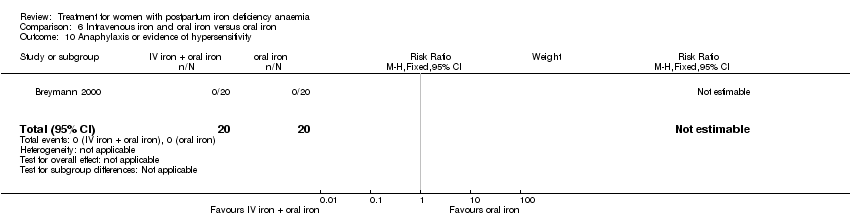
Comparison 6 Intravenous iron and oral iron versus oral iron, Outcome 10 Anaphylaxis or evidence of hypersensitivity.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 1 Postpartum depression.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 2 Infections.
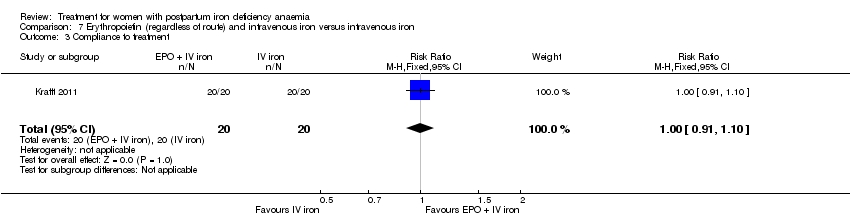
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 3 Compliance to treatment.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 4 Breasfeeding.
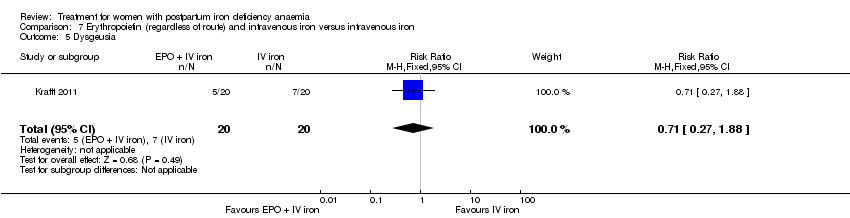
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 5 Dysgeusia.
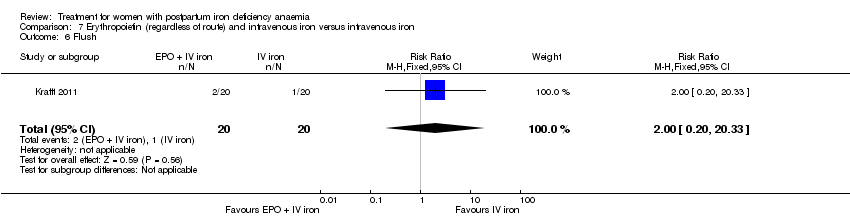
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 6 Flush.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 7 Diarrhoea.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 8 Headache.
Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 9 Itching (including elevated liver enzymes).

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 10 Dizziness.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 11 Thrombophlebitis.

Comparison 7 Erythropoietin (regardless of route) and intravenous iron versus intravenous iron, Outcome 12 Red blood cell transfusion.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 1 Postpartum depression.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 2 Infections.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 3 Headache.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 4 Low blood pressure.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 5 Diarrhoea.
Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 6 Dizziness.

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 7 Itching (including elevated liver enzymes).

Comparison 8 Subcutaneous EPO 10,000 U two doses and intravenous iron versus intravenous iron, Outcome 8 Red blood cell transfusion.
Comparison 9 Intravenous EPO, intravenous iron and oral iron versus intravenous iron and oral iron, Outcome 1 Leg paraesthesia.

Comparison 9 Intravenous EPO, intravenous iron and oral iron versus intravenous iron and oral iron, Outcome 2 Red blood cell transfusion.
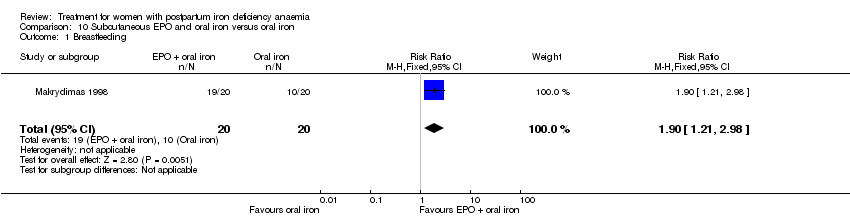
Comparison 10 Subcutaneous EPO and oral iron versus oral iron, Outcome 1 Breastfeeding.
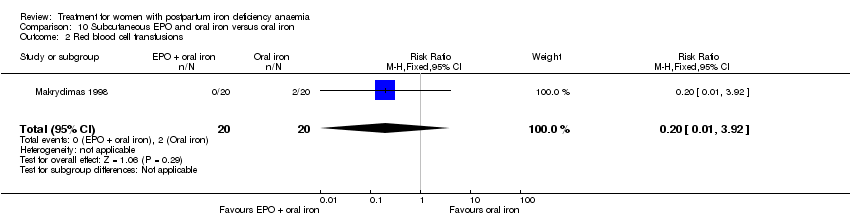
Comparison 10 Subcutaneous EPO and oral iron versus oral iron, Outcome 2 Red blood cell transfusions.

Comparison 14 Sensitivity analysis, Outcome 1 Heterogeneity ‐ Infections ‐ comparison 1.

Comparison 14 Sensitivity analysis, Outcome 2 Heterogeneity, fixed effect ‐ Infections ‐ comparison 1.
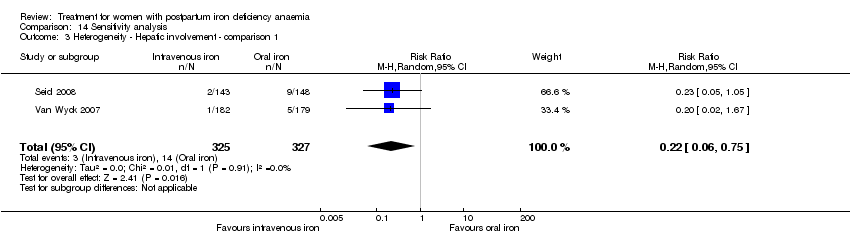
Comparison 14 Sensitivity analysis, Outcome 3 Heterogeneity ‐ Hepatic involvement ‐ comparison 1.

Comparison 14 Sensitivity analysis, Outcome 4 Heterogeneity, fixed effect ‐ Hepatic involvement ‐ comparison 1.
| Intravenous iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Intravenous iron | |||||
| Maternal mortality | Study population | RR 2.95 | 374 | ⊕⊕⊝⊝ | 1 maternal death was reported across the included studies. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Fatigue at 14, 28, and 42 days | See comment | See comment | Not estimable | 361 | ⊕⊝⊝⊝ | No statistically significant difference was found at days 14 and 42 days. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Infections | Study population | RR 1.7 | 718 | ⊕⊝⊝⊝ | ||
| 86 per 1000 | 146 per 1000 | |||||
| Moderate | ||||||
| 34 per 1000 | 58 per 1000 | |||||
| Constipation | Study population | RR 0.21 | 1217 | ⊕⊝⊝⊝ | ||
| 114 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 112 per 1000 | 24 per 1000 | |||||
| All gastrointestinal symptoms | Study population | RR 0.31 | 1307 | ⊕⊝⊝⊝ | ||
| 216 per 1000 | 67 per 1000 | |||||
| Moderate | ||||||
| 261 per 1000 | 81 per 1000 | |||||
| Anaphylaxis or evidence of hypersensitivity | Study population | RR 2.78 | 1454 | ⊕⊕⊝⊝ | 3 cases of allergic reactions all occurred in the group treated with intravenous iron. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The outcome is unlikely to be influenced by risk of bias and so we did not downgrade the evidence for this outcome: open‐label design combined with a objective outcome measure. | ||||||
| Red blood cell transfusion compared with non‐transfusion for postpartum iron deficiency anaemia | ||||||
| Patient or population: patients with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐transfusion | RBC transfusion | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue | See comment | See comment | 519 | ⊕⊕⊝⊝ | General fatigue at 3 days was 0.8 lower (1.53 to 0.07) in the transfused group. No statistically significant difference was seen at six weeks. | |
| Persistent anaemia symptoms | Study population | Not estimable | 519 | ⊕⊝⊝⊝ | The outcome was not systematically registered/reported. | |
| See comment | See comment | |||||
| Moderate | ||||||
| Infections | Study population | RR 0.93 | 519 | ⊕⊕⊕⊝ | ||
| 92 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 92 per 1000 | 86 per 1000 | |||||
| Erythrocyte alloantibody formation | Study population | RR 3.03 | 519 | ⊕⊝⊝⊝ | There was no systematical screening for this outcome in the study population. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Thromboembolic events | Study population | RR 1.01 | 519 | ⊕⊕⊝⊝ | ||
| 8 per 1000 | 8 per 1000 | |||||
| Moderate | ||||||
| 8 per 1000 | 8 per 1000 | |||||
| Transfusion reactions | Study population | RR 7.08 | 519 | ⊕⊝⊝⊝ | 3 cases of transfusion reactions occurred in the transfusion group. | |
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: open‐label design combined with a subjective outcome measure. | ||||||
| Oral iron compared with placebo for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oral iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms | Study population | Not estimable | (1) | See comment | Symptoms of anaemia were not reported for the anaemic groups separately. | |
| See comment | See comment | |||||
| Moderate | ||||||
| All gastrointestinal symptoms | Study population | RR 1 | 68 | ⊕⊝⊝⊝ | ||
| 176 per 1000 | 176 per 1000 | |||||
| Moderate | ||||||
| 177 per 1000 | 177 per 1000 | |||||
| Constipation ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: open‐label design combined with a subjective outcome measure. | ||||||
| Intravenous iron with oral iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Intravenous iron with oral iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | ‐ | See comment | In 1 study no maternal deaths were reported. The other study did not report on maternal mortality. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ 1 week | Study population | RR 1.75 | 72 | ⊕⊝⊝⊝ | ||
| 111 per 1000 | 194 per 1000 | |||||
| Moderate | ||||||
| 111 per 1000 | 194 per 1000 | |||||
| Persistent anaemia symptoms ‐ 2 weeks | Study population | RR 0.6 | 72 | ⊕⊝⊝⊝ | ||
| 139 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 139 per 1000 | 83 per 1000 | |||||
| Persistent anaemia symptoms ‐ 6 weeks | Study population | RR 3 | 72 | ⊕⊝⊝⊝ | ||
| 28 per 1000 | 83 per 1000 | |||||
| Moderate | ||||||
| 28 per 1000 | 84 per 1000 | |||||
| Infections ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Anaphylaxis or evidence of hypersensitivity | Study population | Not estimable | 0 | ⊕⊕⊝⊝ | 1 study reported 0 events, other study pooled adverse events, not reporting allergic reactions separately. Thus the effect was not estimable. | |
| See comment | See comment | |||||
| Moderate | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to risk of bias: the included study had high risk of attrition and reporting bias. | ||||||
| Erythropoietin (regardless of rout) with intravenous iron compared with intravenous iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron | EPO (regardless of rout) with IV iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | ‐ | See comment | In 1 study no maternal deaths were reported. The other study did not report on maternal mortality. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO 10,000 U of doses with intravenous iron compared with intravenous iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: patients with women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron | Erythropoietin 10,000 U 2 doses with intravenous iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO with oral iron compared with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral iron | Subcutaneous EPO with oral iron | |||||
| Maternal mortality | See comment | See comment | Not estimable | 40 | See comment | No maternal deaths were reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Subcutaneous EPO with IV iron and oral iron compared with intravenous iron with oral iron for women with postpartum iron deficiency anaemia | ||||||
| Patient or population: women with postpartum iron deficiency anaemia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous iron + oral iron | Subcutaneous EPO + IV iron + oral iron | |||||
| Maternal mortality ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Fatigue ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Persistent anaemia symptoms ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| Thromboembolic events ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal mortality Show forest plot | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.12, 71.96] |
| 2 Fatigue ‐ 14 days Show forest plot | 1 | 322 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐8.04, 1.44] |
| 3 Fatigue ‐ 42 days Show forest plot | 1 | 329 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐6.77, 2.57] |
| 4 SF‐36: Physical F(x) ‐ 14 days Show forest plot | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.84, 5.64] |
| 5 SF‐36: Physical role ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 3.50 [‐2.03, 9.03] |
| 6 SF‐36: Bodily pain ‐ day 14 Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐6.00, 4.60] |
| 7 SF‐36: General health ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐3.09, 4.49] |
| 8 SF‐36: Vitality ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.64, 5.44] |
| 9 SF‐36: Emotional role ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐4.06, 6.26] |
| 10 SF‐36: Social function ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.08, 6.08] |
| 11 SF‐36: Mental health ‐ 14 days Show forest plot | 1 | 321 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐4.84, 2.44] |
| 12 Depression Show forest plot | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.00] |
| 13 Infections Show forest plot | 3 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.58, 5.03] |
| 14 Compliance to treatment Show forest plot | 5 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.35] |
| 15 All gastrointestinal symptoms Show forest plot | 8 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.47] |
| 16 Constipation Show forest plot | 6 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.11, 0.39] |
| 17 Nausea Show forest plot | 4 | 745 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.11, 0.81] |
| 18 Gastrointestinal pain Show forest plot | 4 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.83] |
| 19 Diarrhoea Show forest plot | 3 | 569 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.02, 0.59] |
| 20 Vomiting Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.66] |
| 21 Dyspepsia Show forest plot | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.04, 3.20] |
| 22 Dysgeusia Show forest plot | 4 | 543 | Risk Ratio (M‐H, Random, 95% CI) | 7.20 [1.63, 31.76] |
| 23 Headache Show forest plot | 4 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.87, 4.29] |
| 24 Hepatic involvement Show forest plot | 3 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.12, 1.71] |
| 25 Injection site discomfort Show forest plot | 4 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 4.72 [1.03, 21.54] |
| 26 Skin rash Show forest plot | 2 | 489 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [0.79, 6.97] |
| 27 Urticaria Show forest plot | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [0.47, 36.59] |
| 28 Flush Show forest plot | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [1.18, 68.81] |
| 29 Muscle cramp Show forest plot | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 6.05 [0.74, 49.68] |
| 30 Pain (not specified) Show forest plot | 1 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [0.44, 159.82] |
| 31 Seriouse adverse events (not specified) Show forest plot | 1 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.26, 4.06] |
| 32 Anaphylaxis or evidence of hypersensitivity Show forest plot | 8 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 2.78 [0.31, 24.92] |
| 33 Arythmia Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.26 [0.18, 101.86] |
| 34 Red blood cell transfusion Show forest plot | 4 | 606 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.19, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 General fatigue ‐ 3 days Show forest plot | 1 | 388 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.53, ‐0.07] |
| 2 General fatigue ‐ 6 weeks Show forest plot | 1 | 318 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.22, 0.72] |
| 3 SF‐36: Physical functioning ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | 5.67 [0.84, 10.50] |
| 4 SF‐36: Social function ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 5.34 [0.11, 10.57] |
| 5 SF‐36: Physical role ‐ 1 week Show forest plot | 1 | 366 | Mean Difference (IV, Fixed, 95% CI) | 4.56 [‐1.41, 10.53] |
| 6 SF‐36: Bodily pain ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.90, 1.90] |
| 7 SF‐36: General health ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [‐1.47, 5.83] |
| 8 SF‐36: Vitality ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 1.88 [‐2.01, 5.77] |
| 9 SF‐36: Emotional role ‐ 1 week Show forest plot | 1 | 368 | Mean Difference (IV, Fixed, 95% CI) | 4.37 [‐4.51, 13.25] |
| 10 SF‐36: Mental health ‐ 1 week Show forest plot | 1 | 369 | Mean Difference (IV, Fixed, 95% CI) | 1.21 [‐2.29, 4.71] |
| 11 Infections Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.61] |
| 12 Compliance to treatment Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.06, 1.17] |
| 13 Breastfeeding at six weeks Show forest plot | 1 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.07] |
| 14 Erythrocyte alloantibody formation Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.15] |
| 15 Rash Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.15] |
| 16 Fever Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.06 [0.24, 104.84] |
| 17 Thromboembolic events Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.13] |
| 18 Parenteral iron intolerance Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.24] |
| 19 Transfusion reactions Show forest plot | 1 | 519 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.08 [0.37, 136.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Digit Symbol Substitution test ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.76, 2.76] |
| 2 EPDS ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.86, 1.06] |
| 3 STAI ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.18, 2.38] |
| 4 Percieved Stress ‐ 10 weeks Show forest plot | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [1.70, 6.50] |
| 5 Breastfeeding at two days postpartum Show forest plot | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.17] |
| 6 Back pain Show forest plot | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.03] |
| 7 All gastrointestinal symptoms Show forest plot | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.79] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All gastrointestinal symptoms Show forest plot | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.23, 6.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All gastrointestinal symptoms Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.83, 2.45] |
| 2 Abdominal pain Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [0.55, 13.48] |
| 3 Constipation Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.55, 2.60] |
| 4 Diarrhoea Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.35, 30.51] |
| 5 Nausea Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| 6 Dysgeusia Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [0.14, 78.49] |
| 7 Flatulence Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| 8 Melaena Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| 9 Headache Show forest plot | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent anaemia symptoms on a VAS scale: 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.46] |
| 2 Persistent anaemia symptoms on a VAS scale: 2 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.33] |
| 3 Persistent anaemia symptoms on a VAS scale: 6 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.50] |
| 4 EPDS ‐ 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.65, 13.88] |
| 5 Length of hospital stay Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.02, 0.42] |
| 6 Adverse events (pooled) ‐ 1 week Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.21, 2.16] |
| 7 Adverse events (pooled) ‐ 2 weeks Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.28] |
| 8 Adverse events (pooled) ‐ 6 weeks Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.93] |
| 9 Red blood cell transfusion Show forest plot | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.72] |
| 10 Anaphylaxis or evidence of hypersensitivity Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postpartum depression Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 2 Infections Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.72, 5.59] |
| 3 Compliance to treatment Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| 4 Breasfeeding Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.91, 1.10] |
| 5 Dysgeusia Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.27, 1.88] |
| 6 Flush Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 20.33] |
| 7 Diarrhoea Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 8 Headache Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.60] |
| 9 Itching (including elevated liver enzymes) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 10 Dizziness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 11 Thrombophlebitis Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
| 12 Red blood cell transfusion Show forest plot | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Postpartum depression Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 2 Infections Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 2.93] |
| 3 Headache Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.57] |
| 4 Low blood pressure Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 5 Diarrhoea Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 6 Dizziness Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 7 Itching (including elevated liver enzymes) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 8 Red blood cell transfusion Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leg paraesthesia Show forest plot | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.08, 6.65] |
| 2 Red blood cell transfusion Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breastfeeding Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.9 [1.21, 2.98] |
| 2 Red blood cell transfusions Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heterogeneity ‐ Infections ‐ comparison 1 Show forest plot | 2 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.62, 1.84] |
| 2 Heterogeneity, fixed effect ‐ Infections ‐ comparison 1 Show forest plot | 3 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.93, 2.38] |
| 3 Heterogeneity ‐ Hepatic involvement ‐ comparison 1 Show forest plot | 2 | 652 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.75] |
| 4 Heterogeneity, fixed effect ‐ Hepatic involvement ‐ comparison 1 Show forest plot | 3 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.21, 1.07] |

