Tratamiento no quirúrgico para la estenosis de la columna lumbar con claudicación neurogénica
Resumen
Antecedentes
La estenosis de la columna lumbar con claudicación neurogénica es una de las afecciones patológicas de la columna lumbar diagnosticada y tratada con más frecuencia. Afecta habitualmente a la población de edad avanzada.
Objetivos
Examinar de forma sistemática las pruebas de la efectividad del tratamiento no quirúrgico de la estenosis de la columna lumbar con claudicación neurogénica.
Métodos de búsqueda
Se realizaron búsquedas en las bases de datos CENTRAL, MEDLINE, CINAHL e Index to Chiropractic Literature (ICL) hasta junio de 2012.
Criterios de selección
Ensayos controlados aleatorios publicados en inglés, en los que al menos un brazo proporcionó datos sobre tratamientos no quirúrgicos
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar recomendados por La Colaboración Cochrane. Dos revisores evaluaron el riesgo de sesgo de cada estudio de forma independiente y utilizaron los 12 criterios recomendados por el Grupo Cochrane de la Espalda (Cochrane Back Review Group) (Furlan 2009). Los resultados dicotómicos se expresaron como riesgo relativo, los resultados continuos como diferencia de medias o diferencia de medias estandarizada; la incertidumbre se expresó con intervalos de confianza (IC) del 95%. Cuando fue posible, se realizó un metanálisis, de locontrario, los resultados se describieron de forma cualitativa. Se utilizó GRADE para evaluar la calidad de las pruebas.
Resultados principales
De las 8635 citas examinadas, se evaluaron 56 artículos de texto completo y se incluyeron 21 ensayos (1851 participantes). Hubo pruebas de calidad muy baja de seis ensayos de que la calcitonina no es mejor que placebo o paracetamol, independientemente de la forma de administración o el resultado evaluado. A partir de ensayos individuales pequeños, hubo pruebas de calidad baja para las prostaglandinas y pruebas de calidad muy baja para la gabapentina o la metilcobalamina, de que mejoraran la distancia de caminata. Hubo pruebas de calidad muy baja de un único ensayo de que las inyecciones epidurales de esteroides mejoraron el dolor, la función y la calidad de vida hasta dos semanas, en comparación con ejercicios en el domicilio o la fisioterapia en pacientes hospitalizados. Hubo pruebas de calidad baja de un único ensayo de que los ejercicios tienen efectos beneficiosos a corto plazo para el dolor de la pierna y la función en comparación con ningún tratamiento. Hubo pruebas de calidad baja y muy baja de seis ensayos de que el tratamiento multimodal no quirúrgico es menos eficaz que la descompresión quirúrgica indirecta o directa con o sin fusión. Un metanálisis de dos ensayos que compararon la descompresión directa con o sin fusión con atención multimodal no quirúrgica no encontró diferencias significativas en la función a los seis meses (diferencia de medias [DM] ‐3,66; IC del 95%: ‐10,12 a 2,80) ni al año (DM ‐6,18; IC del 95%: ‐15,03 a 2,66), pero a los 24 meses se encontró una diferencia significativa que favoreció a la descompresión (DM ‐4,43; IC del 95%: ‐7,91 a ‐0,96).
Conclusiones de los autores
Faltan pruebas de calidad moderada y alta del tratamiento no quirúrgico, por lo que no es posible hacer recomendaciones que orienten la práctica clínica. Debido al ascenso exponencial esperado en la prevalencia de la estenosis de la columna lumbar con claudicación neurogénica, se necesitan urgentemente ensayos grandes de alta calidad.
PICO
Resumen en términos sencillos
Tratamiento no quirúrgico para la estenosis de la columna con dolor en la pierna
Pregunta de la revisión
Se revisaron las pruebas sobre la efectividad de los tratamientos no quirúrgicos para los pacientes con dolor de la pierna causado por la presión sobre los nervios de la columna.
Antecedentes
Si el canal de la columna se estrecha y presiona los nervios (estenosis de la columna), puede causar dolor en las piernas o las nalgas (claudicación neurogénica). La estenosis de la columna se trata con diversos métodos no quirúrgicos, que incluyen analgésicos y otros fármacos, inyecciones en la columna, ejercicios, fisioterapia y tratamientos similares. Se intentó determinar si el uso de métodos no quirúrgicos fue mejor o peor que otras opciones.
Características de los estudios
Se incluyeron 21 ensayos controlados aleatorios que compararon tratamientos no quirúrgicos con placebo, ningún tratamiento o cirugía. Todos los participantes presentaban dolor de la pierna y un diagnóstico confirmado de estenosis de la columna lumbar. Se incluyeron 1851 pacientes, con una edad promedio de 50 años, divididos por igual entre hombres y mujeres. El período de seguimiento varió desde una semana hasta seis años. Las pruebas se actualizaron hasta junio de 2012.
Resultados clave
En general, la revisión indica que la cirugía es más efectiva para aliviar el dolor que los tratamientos no quirúrgicos.
Medicamentos por vía oral Ensayos pequeños, uno de prostaglandinas (en comparación con otra medicina), otro de gabapentina (en comparación con placebo) y otro de vitamina B1 (en comparación con diversos tratamientos) indicaron mejorías en el dolor y la distancia de caminata. Se informaron algunos problemas digestivos con ambos medicamentos en el ensayo de prostaglandinas; y algunos pacientes del ensayo de gabapentina informaron mareo o somnolencia.
Inyecciones epidurales. Dos ensayos pequeños mostraron mejorías a corto plazo en el dolor y la calidad de vida (hasta dos semanas) y dos no mostraron diferencias en comparación con las inyecciones placebo. Ningún ensayo informó reacciones adversas ni problemas.
Inyecciones de calcitonina. Seis ensayos pequeños indicaron que la calcitonina no es mejor que paracetamol o placebo. Varios pacientes informaron sentirse enfermos o presentaron una erupción cutánea.
Enfoques mixtos comparados con cirugía. Cinco ensayos compararon los resultados de la cirugía con los resultados de diversos tratamientos no quirúrgicos. Un ensayo encontró que después de dos años no hubo diferencias entre los tratamientos en lo que respecta al dolor. Los otros cuatro encontraron que la cirugía mejoró el dolor más que los tratamientos no quirúrgicos durante diferentes períodos, pero no necesariamente la capacidad de caminar. Entre el 5% y el 18% presentó efectos secundarios no deseados de la cirugía, algunos importantes.
Terapia física. Cuatro ensayos pequeños que incluyeron alguna forma de ejercicio no lograron demostrar mejorías en la capacidad de caminar después de la fisioterapia. Cada ensayo indicó que el ejercicio es mejor que ningún tratamiento para el dolor de la pierna y que la caminata en cinta rodante y el ciclismo estático producen resultados limitados similares.
Calidad de la evidencia
Los hallazgos de todos los ensayos en este estudio se basaron en pruebas de calidad baja o muy baja. Los estudios tuvieron un diseño deficiente o no lograron proporcionar información suficiente acerca de lo que se realizó. Debido a lo anterior, no se puede asegurar que los resultados sean confiables y los estudios de investigación adicionales podrían establecer una conclusión diferente.
Authors' conclusions
Background
Description of the condition
Lumbar spinal stenosis (LSS) is characterized by bilateral or unilateral buttock, thigh, or calf discomfort; pain; or weakness precipitated by walking and prolonged standing (Comer 2009). The pathophysiology is thought to be compression or ischemia, or both, of the lumbosacral nerve roots due to narrowing of the lateral and central vertebral canals, usually as a consequence of osteoarthritic thickening of the articulating facet joints, infolding of the ligamentum flavum, and degenerative bulging of the intervertebral discs (Comer 2009; Katz 2008). Neurogenic claudication can have a significant impact on functional ability, quality of life, and independence in the elderly. Those afflicted have greater walking limitations than individuals with knee or hip osteoarthritis (Winter 2010). Lumbar spinal stenosis is the most common reason for spine surgery among individuals older than 65 years (Deyo 2010). The incidence of new cases of neurogenic claudication is expected to rise dramatically over the next 20 years when an estimated 23% to 25% of the population will be older than 65 years (Government of Canada 2006). This will have a significant impact on healthcare resources in the near future.
Description of the intervention
Most patients who seek care for neurogenic claudication are treated nonoperatively. A course of conservative treatment is also recommended prior to surgical intervention (AHRQ 2001). However, what constitutes effective conservative or nonoperative treatment is unknown (AHRQ 2001; Chou 2007). The purpose of this review was to systematically evaluate the clinical effectiveness of nonoperative treatments of lumbar spinal stenosis with neurogenic claudication.
Various nonoperative treatments are available to patients with neurogenic claudication including epidural injections (with steroid or anaesthetic, or both), oral medications (such as non‐steroidal anti‐inflammatory drugs (NSAIDs), analgesics, muscle relaxants, prostaglandins, and neuropathic drugs), vitamin B12, nasal or intramuscular calcitonin, physical therapies (such as exercise therapy, orthoses, electrical modalities, and traction), and manual therapy. Patients receiving nonoperative care often receive a combination of these treatments.
How the intervention might work
The mechanism of action of the various treatments differs. Epidural steroid injections, calcitonin, and NSAIDS aim to reduce nerve root inflammation (Fukusaki 1988; Podichetty 2004). Analgesics, epidural anaesthetic injections, and prostaglandins act to block pain transmission and increase neural blood flow (Fukusaki 1988; Matsudaira 2009). Vitamin B12 is thought to increase nerve root blood flow (Waikakul 2000) and neuropathic medications act to block pain transmission. Physical and manual therapies aim to reduce pain and maximize function by improving lumbar spine and lower extremity flexibility, muscular strength, and endurance (Whitman 2006).
Why it is important to do this review
Lumbar spinal stenosis causing neurogenic claudication is a leading cause of pain, disability, and lost independence in people over the age of 65 years. With the aging population the number of people who suffer from this condition is expected to grow exponentially. The vast majority of people with lumbar spinal stenosis causing neurogenic claudication receive nonoperative treatments. However, what constitutes effective nonoperative treatment is unknown (AHRQ 2001; Chou 2007). This review is an update of a previous review on this topic (Ammendolia 2012).
Objectives
To assess the effects of nonoperative treatments for lumbar spinal stenosis with neurogenic claudication.
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were randomized controlled trials (RCTs) published in English, at least one arm of the trial provided data on the effectiveness of a nonoperative treatment, and at least 80% of participants had neurogenic claudication with lumbar spinal stenosis confirmed by imaging. Studies evaluating participants with radiculopathy due to disc lesions were excluded. Studies with mixed populations were included only if separate data for participants with neurogenic claudication due to lumbar spinal stenosis were provided.
Types of participants
Neurogenic claudication was defined as buttock or leg pain or aching, numbness, tingling, weakness, or fatigue with or without back pain precipitated by standing or walking. Studies evaluating participants with radiculopathy due to disc lesions were excluded.
Types of interventions
Any study that included at least one treatment arm that employed nonoperative treatment, including but not limited to oral and injection‐based medications, manual therapies, and physical therapies.
Nonoperative treatments were compared to placebo, no treatment, other nonoperative treatments, or surgical interventions.
Types of outcome measures
Studies were included if they measured at least one of the following outcomes: walking ability, pain intensity, function, quality of life, and global improvement. Common outcome measures used in this patient population included the following.
The Zurich Claudication Questionnaire (ZCQ), also known as the Swiss Spinal Stenosis questionnaire, is a validated self report, condition specific questionnaire for patients with neurogenic claudication (Comer 2011). It measures symptom intensity, function, and satisfaction with treatment. Walking ability is often measured using subscales of the Oswestry Disability Index (Fairbank 1980) or the Zurich Claudication Questionnaire (Stucki 1996), or objectively using the Self Paced Walking Test, Treadmill Walking Test, and distance walked. Pain intensity is usually measured using a visual analog scale (VAS), verbal response scale (VRS), McGill pain questionnaire (Melzack 2005), or numerical pain rating scale (NPRS). Function in patients with neurogenic claudication can be measured using the Oswestry Disability Index or the Roland Morris Disability Questionnaire (Brouwer 2004). Quality of life can be measured using the Short Form‐36 (SF‐36) questionnaire (Ware 1995). A subjective global rating of change or improvement can also be employed, typically in the form of a Likert scale.
Outcomes were categorized according to these follow‐up periods: immediate (up to one week), short‐term (between one week and three months), intermediate (between three months and one year), and long‐term (one year or longer).
Search methods for identification of studies
Electronic searches
An electronic search was performed by an experienced librarian from the Cochrane Back Review Group in CENTRAL (2012, Issue 6), MEDLINE (1966 to June 2012), EMBASE (1980 to June 2012), CINAHL (1982 to June 2012), and Index to Chiropractic Literature (1985 to June 2012). The terms “spinal stenosis”, “lumbar spinal stenosis”, “neurogenic claudication”, “lumbar radicular pain”, “cauda equina”, and “spondylosis” were combined with a highly sensitive search strategy to identify RCTs. See Appendix 1 for more details.
Data collection and analysis
Selection of studies
Two review authors (CA and KS) independently screened all titles and abstracts identified by the search strategy. The full‐texts of articles deemed to be potentially relevant were independently assessed by two review authors, who made the final decision for inclusion. A third review author was consulted if consensus was not reached.
Data extraction and management
Two review authors independently performed data extraction using a standardized form. The data included characteristics of patients and treatments, and outcomes. Safety data (adverse effects and complications) were also collected when available.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias. Risk of bias was assessed using the 12‐item criteria recommended by the Cochrane Back Review Group (Furlan 2009). See Appendix 2. The criteria were scored as 'high', 'low', or 'unclear' and were reported in the 'Risk of bias' table. A study was considered at low risk of bias if it fulfilled six or more of the 12 criteria, including clearly described and appropriate randomization and allocation concealment, and with no severe flaws. A severe flaw was defined a priori as a serious methodological deficiency not captured by the 12‐item criteria that significantly increases the risk of bias, such as very high dropout or crossover rates. Discrepancies in risk of bias scoring and data extraction were discussed during a consensus meeting.
Data synthesis
Dichotomous outcomes were analysed by calculating the relative risk (RR). Continuous outcomes were analysed by calculating the mean difference (MD) when the same instrument was used to measure outcomes, or the standardized mean difference (SMD) when different instruments were used to measure the outcomes. The uncertainty was expressed with 95% confidence intervals (95% CI). The outcome measures from the individual trials were combined through meta‐analysis where possible (clinical comparability of population, intervention, and outcomes between trials) using a fixed‐effect model unless there was significant statistical heterogeneity, in which case a random‐effects model was used. A P value less than 0.05 of the Chi test indicates significant statistical heterogeneity.
If a meta‐analysis was not possible, the results from clinically comparable trials were described qualitatively in the text.
Regardless of whether there were sufficient data available to use quantitative analyses to summarize the data, we assessed the overall quality of the evidence for each outcome. To accomplish this, we used the GRADE approach as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted in the updated Cochrne Back Review Group (CBRG) method guidelines (Furlan 2009). Factors that may decrease the quality of the evidence are: study design and risk of bias, inconsistency of results, indirectness (not generalisable), imprecision (sparse data), and other factors (for example reporting bias). The quality of the evidence for a specific outcome was reduced by a level according to the performance of the studies against the five factors.
High quality evidence: there are consistent findings among at least 75% of RCTs with low risk of bias; consistent, direct and precise data; and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate quality evidence: one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality evidence: three of the domains are not met. We are very uncertain about the results.
No evidence: no RCTs were identified that addressed this outcome.
Results
Description of studies
A total of 21 RCTs met the inclusion criteria and were included in the review (Figure 1). Table 1 describes the characteristics of the included trials. A total of 1851 participants (926 men and 925 women) were randomized to 1 of 23 comparison groups. Nineteen trials were conducted at tertiary care centers and two at medical or rehabilitation clinics (Goren 2010; Whitman 2006). The mean age of participants was more than 50 years in all but two trials, in which the mean age was just less than 50 years (Cuckler 1985; Zahaar 1991). The duration of symptoms varied considerably among the studies, with a mean range of 12 weeks to 15 years. Follow‐up periods also varied significantly. Three of the four studies evaluating epidural injections provided follow‐up data up to a week after the injections (Cuckler 1985; Fukusaki 1988; Zahaar 1991), whereas all studies comparing multimodal nonoperative care with surgery provided long‐term follow‐up (at least two‐year) outcome data (Amundsen 2000; Malmivaara 2007; Weinstein 2007; Weinstein 2008; Zucherman 2004).

Selection process of included articles.
| Comparisons and trials | Risk of bias | GRADE assessment and outcomes/measures (walking ability, pain, function, and quality of life) at each follow‐up point | ||||||||
| Consistency | Directness | Precision | Selective reporting | Immediate | Short‐term | Intermediate | Long‐term | Quality of the evidence | ||
| Calcitonin | ||||||||||
| Calcitonin injection versus placebo injection | ||||||||||
| High High | No No | Yes Yes | No No | Yes Yes |
| = TWT = VAS | = TWT = VAS | = TWT = VAS | +000 +000 | |
| High | No | Yes | No | Yes |
| ? distance walked | ? distance walked |
| +000 | |
| High High | No No | Yes Yes | No No | Yes Yes |
| = distance walked = VAS |
|
| +000 +000 | |
| Calcitonin nasal spray versus placebo nasal spray | ||||||||||
| High High High High | No No No No | Yes Yes Yes | No No No No | Yes |
| = distance walked = time walked = SF‐36 = VAS |
|
| +000 +000 +000 +000 | |
| High High High High High | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = Shuttle walk = VAS leg = VAS back = ODI = global |
|
| +000 +000 +000 +000 +000 | |
| Calcitonin nasal spray plus physical therapy versus paracetamol plus physical therapy | ||||||||||
| High High High | No No No | Yes Yes Yes | No No No |
|
| = distance walked = VAS = RMDI |
|
| +000 +000 +000 | |
| Oral medication | ||||||||||
| Oral prostaglandin versus etodolac (NSAID) | ||||||||||
|
| Low Low Low Low Low | No No No No No | Yes Yes Yes Yes Yes | No No No No No | Yes |
| > distance walked ? SF‐36 = LBP > Leg pain > global |
|
| ++00 +000 ++00 ++00 ++00 |
| Methylocobalamin (vitamin B12) plus conservative care versus conservative care | ||||||||||
| High | No | Yes | No | Yes | > distance walked | > distance walked | +000 | |||
| Gabapentin versus placebo | ||||||||||
|
| High High High | No No No | Yes Yes Yes | No No No |
|
|
| > distance walked = VAS (1‐2 months) > VAS (3 months) | > distance walked > VAS | +000 +000 +000 |
| Physical therapy | ||||||||||
| Exercise plus ultrasound versus exercise plus sham ultrasound | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back = VAS leg = ODI |
|
| ++00 ++00 ++00 ++00 | |
| Exercise plus ultrasound versus no treatment | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back > VAS leg > ODI |
|
| ++00 ++00 ++00 ++00 | |
| Exercise plus sham ultrasound versus no treatment | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back > VAS leg > ODI |
|
| ++00 ++00 ++00 ++00 | |
| Inpatient physical therapy versus home exercise program plus oral diclofenac | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = NHP |
| +000 +000 +000 +000 | |
| Unweighted treadmill walking plus exercise versus cycling plus exercise | ||||||||||
| Low Low Low Low Low | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = distance walked = ODI = RMDI = VAS = global |
|
| ++00 ++00 ++00 ++00 ++00 | |
| Manual therapy, exercise and unweighted treadmill versus flexion exercise, walking and sham ultrasound | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT > global = ODI = NPRS |
| = global | +000 +000 +000 +000 | |
| Epidural injection | ||||||||||
| Translaminar epidural steroid injections versus placebo injections | ||||||||||
| High | No | Yes | No | = global | =global | +000 | ||||
| Translaminar epidural steroids plus epidural block versus placebo injections | ||||||||||
| High | No | Yes | No |
| > distance walked | = distance walked |
|
| +000 | |
| Translaminar epidural steroids plus epidural block versus epidural block injections | ||||||||||
| High | No | Yes | No |
| = distance walked | = distance walked |
|
| +000 | |
| Translaminar epidural block versus placebo | ||||||||||
| High | No | Yes | No |
| > distance walked | = distance walked |
|
| +000 | |
| Intralaminar epidural steroid plus epidural block versus home exercise program plus oral diclofenac | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes Yes Yes Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = HNP |
| +000 +000 +000 +000 | |
| Intralaminar epidural steroid plus epidural block versus inpatient physical therapy | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes Yes Yes Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = HNP |
| +000 +000 +000 +000 | |
| Caudal epidural steroids versus placebo injections | ||||||||||
| High | No | Yes | No |
| = global |
|
| = global | +000 | |
| Multimodal nonoperative treatment | ||||||||||
| Indirect decompression using interspinous spacer (X‐Stop) versus multimodal nonoperative care for degenerative spondylolisthesis | ||||||||||
| Zucherman 2004, Anderson 2006 | High | No | Yes | No | SR (short & intermediate) |
| > ZCQ | > ZCQ | > ZCQ | +000 |
| Indirect decompression using interspinous spacer (X_Stop) versus multimodal nonoperative care | ||||||||||
| Zucherman 2004, 2005, Hsu 2006 | High | No No | Yes Yes | No No | SR SR |
| > ZCQ > SF‐36 | > ZCQ > SF‐36 | > ZCQ > SF‐36 | +000 +000 |
| Direct decompression ± fusion versus multimodal nonoperative care for degenerative spondylolisthesis | ||||||||||
| High High High High High | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = SF‐36 = ODI = LBPBS = LPBI = SBS | = SF‐36 = ODI = LBPBS = LPBI = SBS | = SF‐36 = ODI = LBPBS = LPBI = SBS | +000 +000 +000 +000 +000 | |
| Direct decompression ± fusion versus multimodal nonoperative care | ||||||||||
| High High | No No | Yes Yes | No No |
|
| ?* pain severity | ?* global | ?* pain severity ? global | +000 +000 | |
| Low Low Low Low Low Low | No No No No No No | Yes Yes Yes Yes Yes Yes | No No No No No No |
|
| = TWT = SW > VAS leg walk > VAS leg > VAS LB ODI (see Figure 4) | = TWT = SW > VAS leg walk > VAS leg > VAS LB ODI (see Figure 4) | ++00 ++00 ++00 ++00 ++00 +000 | ||
| High High High High High High | No No No No No No | Yes Yes Yes Yes Yes Yes | No No No No No No |
|
| = SF‐36 BP = SF‐36 PF = LBPBS = LPBI = SBS = ODI | = SF‐36 BP = SF‐36 PF = LBPBS = LPBI = SBS ODI (see Figure 4) | > SF‐36 BP** = SF‐36 PF = LBPBS = LPBI = SBS ODI (Figure 4) | +000 +000 +000 +000 +000 +000 | |
Follow‐up points: Immediate = up to 1 week, short‐term = >1 week ‐ 3 months, intermediate = 3 months ‐ 1 year, long‐term = > 1 year
> statistically significant favouring intervention (first comparison), < statistically significant favouring control (second comparison), = no statistically significant difference between intervention and control groups
TWT = Treadmill Walking Test, VAS = Visual Analog Scale for Pain Intensity, RMDI = Roland‐Morris Back Disability Index, NHP = Nottingham Health Profile, Global = Patient Perceived Improvement, SR = Selective Reporting, ODI = Oswestry Back Disability Index, ? = insufficient data, LBP = Low back Pain Severity Scale, Leg pain = Leg Pain Severity Scale, ? SF‐36 = No data on overall score, improvement in some subscales, NPRS = Numeric Pain Rating Scale, SF‐36 BP = SF‐36 Bodily Pain Subscale, SF‐36‐ PF = SF‐36 Physical Function Subscale, LBPBS ‐ Low Back Pain Bothersome Scale, LPBI = Leg Pain Bothersome Index, SBS ‐ Stenosis Bothersome Scale, SW = Subjective Walking, VAS leg = Visual Analog Scale for Leg Pain, VAS LB = Visual Analog Scale for Low Back Pain, VAS leg walking = Visual Analog Scale for Leg pain while walking, ?* = no between group statistical comparisons, ** = SF‐36 BP significantly better at 2 years but not 4 years.
GRADE evidence: +000 = Very low quality evidence, ++00 = Low quality evidence, +++0 = Moderate quality evidence, ++++ = High quality evidence
Results of the search
We obtained 8635 records that were screened, and 56 articles were eligible for full‐text review.
Included studies
Types of studies: all studies were randomized controlled trials (RCTs)
Study population: all participants had clinical findings and imaging confirmed lumbar spinal stenosis with neurogenic claudication
Technique: type, practitioner, number and duration of treatments varied
Outcome measures, follow‐up and safety: there was a wide variation in outcomes measures used, follow‐up, and safety profiles
Excluded studies
Reasons for exclusion included publication duplication (n = 1), studies that were not RCTs (n = 16), studies that lacked imaging confirmation (n = 2), studies that did not have neurogenic claudication as an inclusion criterion (n = 9), articles not published in English (n = 3), and studies that did not report results for spinal stenosis patients separately (n = 4).
Risk of bias in included studies
Although 13 studies met six or more criteria for risk of bias, only four studies were considered to have low risk of bias (Goren 2010; Malmivaara 2007; Matsudaira 2009; Pua 2007). Specifically, among the remaining nine studies that met six or more criteria, seven failed to explicitly describe or use an appropriate randomization procedure, allocation concealment, or both (Cuckler 1985; Fukusaki 1988; Podichetty 2004; Tafazal 2007; Whitman 2006; Zahaar 1991; Zucherman 2004); and two had severe flaws due to high crossover rates, which made the intention‐to‐treat analyses uninterpretable (Weinstein 2007; Weinstein 2008). Other common sources of risk of bias included failure to blind the participants receiving the intervention or control (Amundsen 2000; Fukusaki 1988; Goren 2010; Koc 2009; Malmivaara 2007; Matsudaira 2009; Porter 1983; Sahin 2009; Waikakul 2000; Weinstein 2007; Weinstein 2008; Whitman 2006; Yaski 2007; Zucherman 2004), failure to blind the treating healthcare provider (Amundsen 2000; Fukusaki 1988; Goren 2010; Koc 2009; Malmivaara 2007; Matsudaira 2009; Porter 1983; Sahin 2009; Waikakul 2000; Weinstein 2007; Weinstein 2008; Whitman 2006; Yaski 2007; Zucherman 2004), and selective reporting (Eskola 1992; Koc 2009; Matsudaira 2009; Porter 1983; Porter 1988; Waikakul 2000). No studies reported co‐interventions. See Figure 2 and Figure 3.
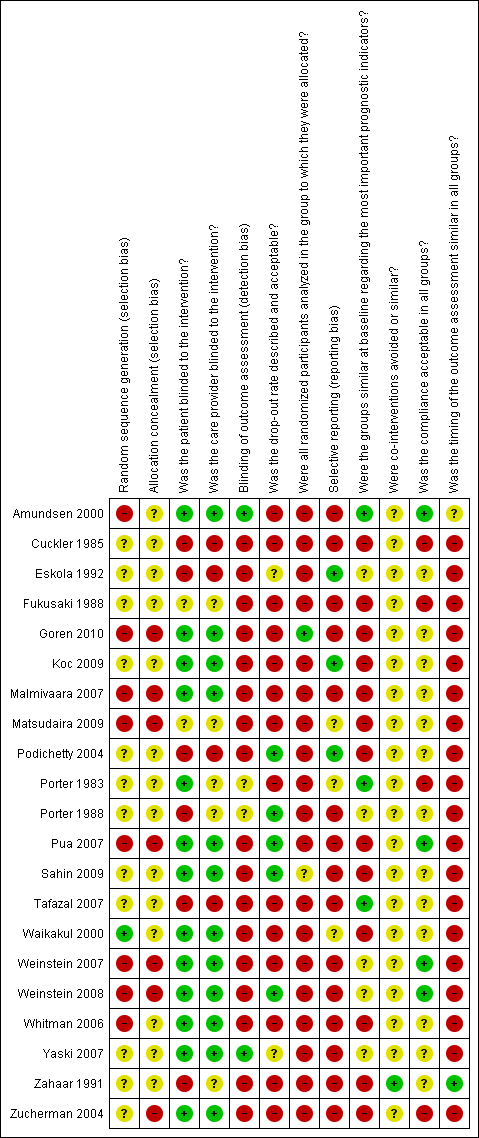
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Nineteen of the 23 comparisons were examined in a single trial, most with small sample sizes. It was possible to combine data from only two trials for one outcome in a meta‐analysis (Malmivaara 2007; Weinstein 2008) (Analysis 1.1). Heterogeneity in source population, intervention, and outcome instruments precluded pooling of data for the other trials. Table 1 summarizes the quality of the evidence for the outcomes for each comparison. The results below are reported on the basis of statistically significant differences between comparators for each outcome.
Oral medication
There was low‐quality evidence based on one trial (N = 79) that prostaglandins improved walking distance and leg pain in the short‐term compared with etodolac (an NSAID), with 5% of participants in both groups reporting gastrointestinal upset (Matsudaira 2009). A small trial evaluating gabapentin (N = 55) provided very low‐quality evidence for improved walking distance and pain intensity compared with placebo in the intermediate and long‐term follow‐up periods (Yaski 2007). This trial reported that some participants randomized to the gabapentin group (no data specified) experienced mild to moderate drowsiness or dizziness, or both. There was very low‐quality evidence from one trial (N = 152) that methylcobalamin (vitamin B12) plus conservative care improved walking distance in the intermediate and long‐term follow‐up compared with conservative treatment alone (Waikakul 2000). There were no reported adverse effects for methylocabalin.
Epidural injections
All four trials evaluating epidural injections provided very low‐quality evidence for all outcomes (Cuckler 1985; Fukusaki 1988; Koc 2009; Zahaar 1991). One trial (N = 53) comparing translaminar epidural block injections, with or without steroids, with placebo showed improved walking distance only immediately after the injection (Fukusaki 1988). Another small trial (N = 29)evaluating intralaminar epidural steroid injection plus epidural block compared with home exercise or inpatient physical therapy demonstrated improvements in pain intensity, function, and quality of life at two weeks follow‐up (Koc 2009). One trial evaluating caudal (Zahaar 1991) (N = 30) and another translaminar (Cuckler 1985) (N = 37) epidural steroid injections showed no difference in global improvement compared with placebo injections. Two trials did not mention adverse events (Cuckler 1985; Zahaar 1991), whereas the other two trials reported no complications after the injections (Fukusaki 1988; Koc 2009).
Calcitonin injections
There was very low‐quality evidence from six trials (N = 231) that calcitonin was no better than placebo or paracetamol, regardless of mode of administration or outcome assessed (Eskola 1992; Porter 1983; Porter 1988; Podichetty 2004; Sahin 2009; Tafazal 2007). Adverse effects of the calcitonin injections were reported as minor (nausea and rash) and were experienced among 40% to 89% of the participants.
Multimodal nonoperative treatment
Five trials compared multimodal nonoperative care with indirect or direct surgical decompression (Amundsen 2000; Malmivaara 2007; Weinstein 2007; Weinstein 2008; Zucherman 2004). In general, multimodal nonoperative treatment was used to simulate usual nonoperative care in the community and varied considerably within and across the trials. Nonoperative treatments included orthosis, rehabilitation, physical therapy, exercise, NSAIDs, analgesics, education, heat and cold applications, transelectrical nerve stimulation, ultrasound, and epidural injections. Details on the frequency or duration of nonoperative care were lacking.
There was very low‐quality evidence from one trial (N = 191) that indirect decompression using interspinous spacers (X‐Stop, St Francis Medical Technologies, Concord, CA) with or without grade 1 spondylolisthesis provided long‐term global improvement and improved quality of life compared with multimodal nonoperative care (Zucherman 2004). No complications were reported for participants receiving nonoperative care. Complications were reported in 11% of participants undergoing interspinous spacer implants; these included spinous process fracture, coronary ischemia, respiratory distress, hematoma, and death due to pulmonary edema.
There was very low‐quality evidence based on intention‐to‐treat analysis among randomized participants from one trial (N = 304) that direct surgical decompression with or without fusion for spondylolisthesis was no better than multimodal nonoperative care for all outcomes assessed (Weinstein 2007). At two‐year follow‐up, about 40% of participants crossed over in either direction. One trial (N = 94) provided low‐quality evidence that direct surgical decompression improved back and leg pain but not walking ability compared with nonoperative care at six months and two years (Malmivaara 2007). The intention‐to‐treat analysis in another trial (N = 289) with a high crossover rate (51% of participants assigned to nonoperative care received surgery) provided very low‐quality evidence that surgical decompression improved bodily pain at two years but not four years compared with nonoperative treatment (Weinstein 2008). A meta‐analysis was performed for one outcome, the Oswestry Disability Index, among randomized participants in two trials comparing direct decompression with or without fusion to multimodal nonoperative care (Malmivaara 2007; Weinstein 2008). There was no significant difference at six months (MD ‐3.66, 95% CI ‐10.12 to 2.80) and one year (MD ‐6.18, 95% CI ‐15.03 to 2.66). At 24 months, a significant difference was found favouring decompression (MD ‐4.43, 95% CI ‐7.91 to ‐0.96). See Figure 4. The quality of the evidence for each follow‐up period was very low. Longer‐term data were available but not combined due to larger differences in the follow‐up periods.
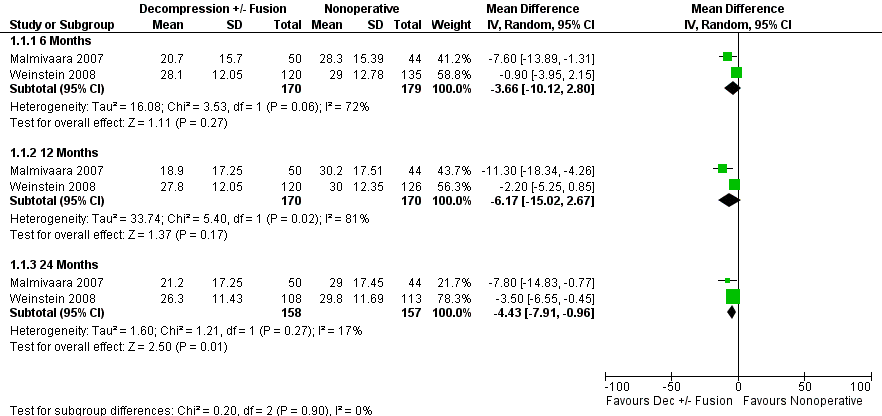
Forest plot of comparison: 1 Direct decompression ± fusion versus multimodal nonoperative care for Oswestry Disability Index, outcome: 1.1 Oswestry Disability Index.
Perioperative complication rates reported for direct decompression with or without fusion ranged from 5.4% to 14%, with dural tears being the most commonly reported. Postoperative complications ranged from 8.2% to 18% of participants and included pulmonary edema, peridural hematoma, and sepsis.
Physical therapy
Four trials evaluated various physical therapy interventions, each of which included some form of exercise (Goren 2010; Koc 2009; Pua 2007; Whitman 2006). None of the trials demonstrated improved walking ability. There was low‐quality evidence from one small trial (N = 45) that exercise was better than no treatment for leg pain and function in the short term (Goren 2010). Another small trial (N = 68) provided low‐quality evidence that unweighted treadmill walking was no better than stationary cycling in the short term regardless of outcome (Pua 2007). The other two trials provided very low‐quality evidence for all outcomes. One trial (N = 29) demonstrated that inpatient physical therapy improved pain intensity, function, and quality of life in the short term compared with a home exercise program plus oral diclofenac (Koc 2009). The other trial (N = 68) showed short‐term global improvement using a combination of manual therapy, exercise, and unweighted treadmill walking compared with flexion exercises, walking, and sham ultrasound (Whitman 2006). Among the physical therapy trials, one patient reported a mild increase in symptoms with exercises (Pua 2007) and in another one patient developed angina pectoris (Koc 2009).
Discussion
Neurogenic claudication is an important and growing cause of pain, disability, and loss of independence in the elderly. The purpose of this review was to evaluate the effectiveness of nonoperative treatments for neurogenic claudication. Our findings suggest that the current evidence is of low and very low quality. This prohibits the ability to make any conclusions about the effectiveness of nonoperative treatment and suggests that future research is very likely to have an important impact on the estimates of the effect, and is likely to change the estimates found in our review. We found low or very low‐quality evidence from single, generally small trials that gabapentin, methylcobalamin, and prostaglandins may improve walking distance. Walking distance was also improved after the use of translaminar epidural block injections, with or without steroids, but only immediately after the injection. Benefits beyond two weeks were not seen with epidural injections regardless of dose, mode of administration, or outcome. Despite the lack of evidence, 25% of all epidural injections are administered for symptoms of lumbar spinal stenosis and their use is growing (Chou 2007). Calcitonin failed to show any benefit, whether administered by injection or nasal spray. Physical therapy is a recommended treatment for neurogenic claudication; however, current evidence has not established its role. What constitutes physical therapy varied considerably among the trials. A common denominator was exercise. Exercise was of short‐term benefit for leg pain and function compared with no treatment, but it is uncertain what the important components of an exercise program are and whether supervised exercise is more effective than a home‐based program. Among the nonoperative trials that reported statistically significant differences in outcomes, the effect sizes were small and unlikely to be of clinical significance. Larger effect sizes were seen favoring indirect decompression using interspinous spacers (X‐Stop) (Zucherman 2004) and direct decompression with or without fusion (Malmivaara 2007) compared with multimodal nonoperative treatment. However, the nonoperative care used in the surgical trials varied significantly, was typically unstructured, and often consisted of failed therapy (the surgical protocol required patients to have failed conservative care prior to surgery). The relationship between symptoms of neurogenic claudication (lower extremity pain numbness, tingling, burning, weakness, and heaviness) and standing or walking ability is unknown. This review found that participants who reported significantly improved back and leg pain (Koc 2009; Malmivaara 2007), back pain–related disability (Goren 2010; Malmivaara 2007), and global improvement (Koc 2009; Whitman 2006) did not have corresponding improvement in their ability to walk. This could be explained in part by the way walking ability was evaluated. The Treadmill Walking Test was used in a third of trials measuring walking distance. However, the Treadmill Walking Test has been found to underestimate walking ability (Tomkins 2009) and patients often refuse to walk on a treadmill because of their fear of falling (Whitman 2006). More valid methods of assessing walking ability include the Self Pace Walking Test (Tomkins 2009).
Summary of main results
We identified 21 RCTs including 1851 participants randomized to 23 different comparisons, in which at least one comparison was nonoperative treatment. Nonoperative treatments included calcitonin, epidural injections, oral medications, physical therapy, and multimodal nonoperative care.
Only four trials had a low risk of bias. There is low quality evidence that prostaglandins improve walking ability compared with etodolac (NSAID); exercise improves leg pain and function compared with no treatment; that unweighted treadmill walking provides similar improvements in pain, function, and walking ability compared with stationary cycling; and direct surgical decompression improves leg pain compared with multimodal nonoperative treatment. There is very low‐quality evidence that gabapentin and methylcobalamin improve walking ability compared with placebo and conservative treatment, respectively; calcitonin is no better than placebo or paracetamol; epidural steroid injections improve pain, function, and quality of life, up to two weeks, compared with home exercise or inpatient physical therapy; and indirect surgical decompression (interspinous spacers) improves quality of life and global recovery compared with multimodal nonoperative care.
Future studies should pay special attention to evidence‐based clinical criteria for neurogenic claudication and provide appropriate and clear descriptions of randomization and concealment of treatment allocation. Trials on epidural injections and oral medication should ensure participant, provider, and assessor blinding. Trials on physical therapies and surgery, in which participant blinding and provider blinding are not possible, should ensure that there is independent assessment of outcomes. All trials should include valid measures of walking ability, have high follow‐up rates, provide sufficient data on all primary outcomes, base conclusions on intention‐to‐treat analysis, and track and report co‐interventions. Adequate description of nonoperative treatments is also needed.
Overall completeness and applicability of evidence
Considerable variation in eligibility criteria exists among trials evaluating interventions for lumbar spinal stenosis with neurogenic claudication (Genevay 2010). This presents challenges with the selection, synthesis, and interpretation of the evidence on effective interventions for this population. A set of internationally agreed‐upon diagnostic criteria for neurogenic claudication due to lumbar spinal stenosis is needed. The findings in our review are concurrent with other recent systematic reviews evaluating exercise (Iwamoto 2010), calcitonin (Podichetty 2011), epidural injections (Chou 2009), oral medications (Chou 2007), interspinous spacers (Chou 2009b; Kabir 2010), and surgical decompression (Chou 2009b; Kovacs 2011). However, across these reviews there was variation on how the study population was defined.
Potential biases in the review process
The strengths of this review are the inclusion of all nonoperative interventions and the consistent inclusion and exclusion criteria for neurogenic claudication, which included the corroboration of a diagnosis of lumbar spinal stenosis with imaging. The use of these criteria to define the study population increases the likelihood that the presenting symptoms are caused by narrowing of the central or lateral foramina (Chou 2009b; Kovacs 2011; Suri 2010). However, a high degree of reliance on imaging alone can lead to an incorrect diagnosis because 20% of asymptomatic individuals older than 60 years have lumbar spinal stenosis on imaging (Boden 1990). Another strength of this review is the use of the rigorous methods recommended by The Cochrane Collaboration, the World Health Organization, and the Cochrane Back Review Group (Furlan 2009; Higgins 2011). This included the use of the GRADE method to analyse the quality of the evidence. To our knowledge, this is the first systematic review on this topic to use the GRADE method.
Limitations of this review include the potential for publication bias because only English articles were accepted. The definition of a severe flaw and the criteria used to assess risk of bias (low versus high) were arbitrary and therefore alternative definitions and criteria could have impacted the findings and conclusions of this review. The lack of high‐ or moderate‐quality evidence found in this review prohibits any recommendations for clinical practice. To resolve this uncertainty, more research is needed with special attention to evidence‐based clinical criteria for neurogenic claudication,and appropriate and clearly described methods of randomization and allocation concealment. Trials on epidural injections and oral medication should ensure blinding of all trial participants. Trials on physical interventions (physical therapy, manual therapy, surgery), in which participant blinding and provider blinding are not possible, should ensure that there is independent assessment of outcomes. All trials should use valid measures of walking ability, ensure that follow‐up rates are above 80%, provide sufficient data on all primary outcomes, base conclusions on intention‐to‐treat analysis, and track and report co‐interventions. Adequate description of nonoperative treatments is also needed.

Selection process of included articles.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Direct decompression ± fusion versus multimodal nonoperative care for Oswestry Disability Index, outcome: 1.1 Oswestry Disability Index.
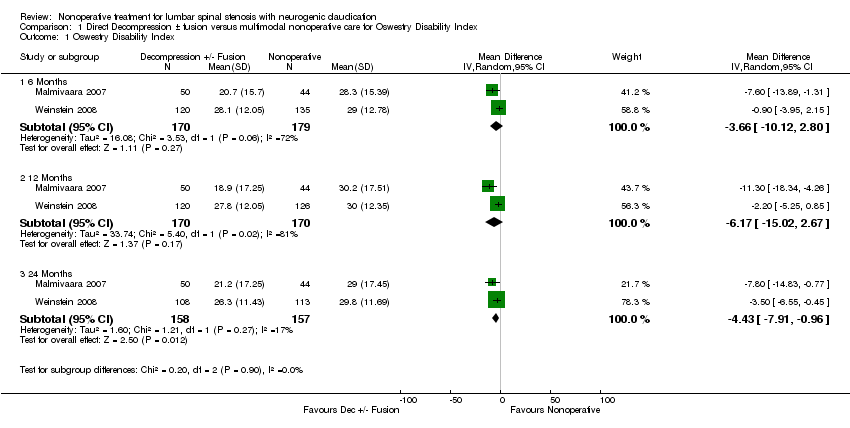
Comparison 1 Direct Decompression ± fusion versus multimodal nonoperative care for Oswestry Disability Index, Outcome 1 Oswestry Disability Index.
| Comparisons and trials | Risk of bias | GRADE assessment and outcomes/measures (walking ability, pain, function, and quality of life) at each follow‐up point | ||||||||
| Consistency | Directness | Precision | Selective reporting | Immediate | Short‐term | Intermediate | Long‐term | Quality of the evidence | ||
| Calcitonin | ||||||||||
| Calcitonin injection versus placebo injection | ||||||||||
| High High | No No | Yes Yes | No No | Yes Yes |
| = TWT = VAS | = TWT = VAS | = TWT = VAS | +000 +000 | |
| High | No | Yes | No | Yes |
| ? distance walked | ? distance walked |
| +000 | |
| High High | No No | Yes Yes | No No | Yes Yes |
| = distance walked = VAS |
|
| +000 +000 | |
| Calcitonin nasal spray versus placebo nasal spray | ||||||||||
| High High High High | No No No No | Yes Yes Yes | No No No No | Yes |
| = distance walked = time walked = SF‐36 = VAS |
|
| +000 +000 +000 +000 | |
| High High High High High | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = Shuttle walk = VAS leg = VAS back = ODI = global |
|
| +000 +000 +000 +000 +000 | |
| Calcitonin nasal spray plus physical therapy versus paracetamol plus physical therapy | ||||||||||
| High High High | No No No | Yes Yes Yes | No No No |
|
| = distance walked = VAS = RMDI |
|
| +000 +000 +000 | |
| Oral medication | ||||||||||
| Oral prostaglandin versus etodolac (NSAID) | ||||||||||
|
| Low Low Low Low Low | No No No No No | Yes Yes Yes Yes Yes | No No No No No | Yes |
| > distance walked ? SF‐36 = LBP > Leg pain > global |
|
| ++00 +000 ++00 ++00 ++00 |
| Methylocobalamin (vitamin B12) plus conservative care versus conservative care | ||||||||||
| High | No | Yes | No | Yes | > distance walked | > distance walked | +000 | |||
| Gabapentin versus placebo | ||||||||||
|
| High High High | No No No | Yes Yes Yes | No No No |
|
|
| > distance walked = VAS (1‐2 months) > VAS (3 months) | > distance walked > VAS | +000 +000 +000 |
| Physical therapy | ||||||||||
| Exercise plus ultrasound versus exercise plus sham ultrasound | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back = VAS leg = ODI |
|
| ++00 ++00 ++00 ++00 | |
| Exercise plus ultrasound versus no treatment | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back > VAS leg > ODI |
|
| ++00 ++00 ++00 ++00 | |
| Exercise plus sham ultrasound versus no treatment | ||||||||||
| Low Low Low Low | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT = VAS back > VAS leg > ODI |
|
| ++00 ++00 ++00 ++00 | |
| Inpatient physical therapy versus home exercise program plus oral diclofenac | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = NHP |
| +000 +000 +000 +000 | |
| Unweighted treadmill walking plus exercise versus cycling plus exercise | ||||||||||
| Low Low Low Low Low | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = distance walked = ODI = RMDI = VAS = global |
|
| ++00 ++00 ++00 ++00 ++00 | |
| Manual therapy, exercise and unweighted treadmill versus flexion exercise, walking and sham ultrasound | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No |
|
| = TWT > global = ODI = NPRS |
| = global | +000 +000 +000 +000 | |
| Epidural injection | ||||||||||
| Translaminar epidural steroid injections versus placebo injections | ||||||||||
| High | No | Yes | No | = global | =global | +000 | ||||
| Translaminar epidural steroids plus epidural block versus placebo injections | ||||||||||
| High | No | Yes | No |
| > distance walked | = distance walked |
|
| +000 | |
| Translaminar epidural steroids plus epidural block versus epidural block injections | ||||||||||
| High | No | Yes | No |
| = distance walked | = distance walked |
|
| +000 | |
| Translaminar epidural block versus placebo | ||||||||||
| High | No | Yes | No |
| > distance walked | = distance walked |
|
| +000 | |
| Intralaminar epidural steroid plus epidural block versus home exercise program plus oral diclofenac | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes Yes Yes Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = HNP |
| +000 +000 +000 +000 | |
| Intralaminar epidural steroid plus epidural block versus inpatient physical therapy | ||||||||||
| High High High High | No No No No | Yes Yes Yes Yes | No No No No | Yes Yes Yes Yes |
| = TWT > VAS > RMDI > NHP | = TWT = VAS = RMDI = HNP |
| +000 +000 +000 +000 | |
| Caudal epidural steroids versus placebo injections | ||||||||||
| High | No | Yes | No |
| = global |
|
| = global | +000 | |
| Multimodal nonoperative treatment | ||||||||||
| Indirect decompression using interspinous spacer (X‐Stop) versus multimodal nonoperative care for degenerative spondylolisthesis | ||||||||||
| Zucherman 2004, Anderson 2006 | High | No | Yes | No | SR (short & intermediate) |
| > ZCQ | > ZCQ | > ZCQ | +000 |
| Indirect decompression using interspinous spacer (X_Stop) versus multimodal nonoperative care | ||||||||||
| Zucherman 2004, 2005, Hsu 2006 | High | No No | Yes Yes | No No | SR SR |
| > ZCQ > SF‐36 | > ZCQ > SF‐36 | > ZCQ > SF‐36 | +000 +000 |
| Direct decompression ± fusion versus multimodal nonoperative care for degenerative spondylolisthesis | ||||||||||
| High High High High High | No No No No No | Yes Yes Yes Yes Yes | No No No No No |
|
| = SF‐36 = ODI = LBPBS = LPBI = SBS | = SF‐36 = ODI = LBPBS = LPBI = SBS | = SF‐36 = ODI = LBPBS = LPBI = SBS | +000 +000 +000 +000 +000 | |
| Direct decompression ± fusion versus multimodal nonoperative care | ||||||||||
| High High | No No | Yes Yes | No No |
|
| ?* pain severity | ?* global | ?* pain severity ? global | +000 +000 | |
| Low Low Low Low Low Low | No No No No No No | Yes Yes Yes Yes Yes Yes | No No No No No No |
|
| = TWT = SW > VAS leg walk > VAS leg > VAS LB ODI (see Figure 4) | = TWT = SW > VAS leg walk > VAS leg > VAS LB ODI (see Figure 4) | ++00 ++00 ++00 ++00 ++00 +000 | ||
| High High High High High High | No No No No No No | Yes Yes Yes Yes Yes Yes | No No No No No No |
|
| = SF‐36 BP = SF‐36 PF = LBPBS = LPBI = SBS = ODI | = SF‐36 BP = SF‐36 PF = LBPBS = LPBI = SBS ODI (see Figure 4) | > SF‐36 BP** = SF‐36 PF = LBPBS = LPBI = SBS ODI (Figure 4) | +000 +000 +000 +000 +000 +000 | |
| Follow‐up points: Immediate = up to 1 week, short‐term = >1 week ‐ 3 months, intermediate = 3 months ‐ 1 year, long‐term = > 1 year > statistically significant favouring intervention (first comparison), < statistically significant favouring control (second comparison), = no statistically significant difference between intervention and control groups TWT = Treadmill Walking Test, VAS = Visual Analog Scale for Pain Intensity, RMDI = Roland‐Morris Back Disability Index, NHP = Nottingham Health Profile, Global = Patient Perceived Improvement, SR = Selective Reporting, ODI = Oswestry Back Disability Index, ? = insufficient data, LBP = Low back Pain Severity Scale, Leg pain = Leg Pain Severity Scale, ? SF‐36 = No data on overall score, improvement in some subscales, NPRS = Numeric Pain Rating Scale, SF‐36 BP = SF‐36 Bodily Pain Subscale, SF‐36‐ PF = SF‐36 Physical Function Subscale, LBPBS ‐ Low Back Pain Bothersome Scale, LPBI = Leg Pain Bothersome Index, SBS ‐ Stenosis Bothersome Scale, SW = Subjective Walking, VAS leg = Visual Analog Scale for Leg Pain, VAS LB = Visual Analog Scale for Low Back Pain, VAS leg walking = Visual Analog Scale for Leg pain while walking, ?* = no between group statistical comparisons, ** = SF‐36 BP significantly better at 2 years but not 4 years. GRADE evidence: +000 = Very low quality evidence, ++00 = Low quality evidence, +++0 = Moderate quality evidence, ++++ = High quality evidence | ||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oswestry Disability Index Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 Months | 2 | 349 | Mean Difference (IV, Random, 95% CI) | ‐3.66 [‐10.12, 2.80] |
| 1.2 12 Months | 2 | 340 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐15.02, 2.67] |
| 1.3 24 Months | 2 | 315 | Mean Difference (IV, Random, 95% CI) | ‐4.43 [‐7.91, ‐0.96] |

