مقایسه کاپنوگرافی در برابر پایش استاندارد برای بیهوشی و آرامسازی حین انجام پروسیجر در بخش اورژانس
چکیده
پیشینه
بیهوشی و آرامسازی پروسیجرال (procedural sedation and analgesia; PSA) اغلب در بخش اورژانس (emergency department; ED) برای تسهیل پروسیجرها و مداخلات دردناک استفاده شده است. کاپنوگرافی (capnography)، یک روش مدالیته پایش است که بهطور گسترده در اتاق عمل و بخش آندوسکوپی استفاده میشده، بیشتر در محیط ED استفاده میشود و هدف آن کاهش حوادث جانبی قلبیتنفسی است. برخلاف محیطهای خارج از ED، در حال حاضر هیچ توافقی در مورد اینکه اضافه کردن کاپنوگرافی به روشهای پایش استاندارد، حوادث جانبی را در شرایط ED کاهش میدهد، وجود ندارد.
اهداف
ارزیابی اینکه کاپنوگرافی علاوه بر پایش استاندارد (پالس اکسیمتری (pulse oximetry)، فشار خون و پایش قلبی) در مقایسه با پایش استاندارد بهتنهایی برای پیشگیری از حوادث جانبی قلبیتنفسی (مانند عدم اشباع اکسیژن (oxygen desaturation)، هیپوتانسیون (hypotension)، استفراغ (emesis) و آسپیراسیون ریوی (pulmonary aspiration)) در بیماران ED تحت PSA موثرتر است یا خیر.
روشهای جستوجو
پایگاه ثبت مرکزی کارآزماییهای کنترل شده کاکرین (شماره 8، 2016)، و MEDLINE؛ Embase، و CINAHL را تا 9 آگوست 2016 برای کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و کارآزماییهای شبه‐تصادفیسازی شده مربوط به بیماران ED نیازمند PSA را بدون محدودیت زبانی جستوجو کردیم. متارجیستری (www.controlled-trials.com؛ www.clinicalstudyresults.org، و clinicaltrials.gov) از کارآزماییهای در حال انجام را جستوجو کردیم (فوریه 2016). با نویسندگان اصلی مطالعات وارد شده و همچنین مشاوران علمی تولید کنندگان دستگاه کاپنوگرافی برای شناسایی مطالعات منتشر نشده تماس گرفتیم (فوریه 2016). چکیده مقالات کنفرانسهای چهار سازمان را از سال 2010 تا 2015 به صورت دستی جستوجو کردیم.
معیارهای انتخاب
هر گونه RCT یا کارآزمایی شبه‐تصادفی شدهای را وارد کردیم که به مقایسه کاپنوگرافی و پایش استاندارد با پایش استاندارد بهتنهایی برای بیماران ED نیازمند PSA پرداختند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم به انتخاب مطالعه، استخراج دادهها، و ارزیابی کیفیت روششناسی برای ایجاد جداول مربوط به «خطر سوگیری (bias)» پرداختند. یک پژوهشگر مستقل به استخراج دادههای مربوط به هر مطالعه وارد شدهای پرداخت که نویسندگان ما درگیر آن بودند. در صورت امکان، برای دادههای ناقص با نویسندگان مطالعات وارد شده تماس گرفتیم. برای ترکیب دادهها و محاسبه خطر نسبی (RR) و 95% فاصله اطمینان (CI) با استفاده از هر دو مدل اثرات تصادفی و اثر‐ثابت از نرمافزار Review Manager 5 استفاده کردیم.
نتایج اصلی
سه کارآزمایی (κ = 1.00) را با 1272 شرکتکننده شناسایی کردیم. در مقایسه گروه کاپنوگرافی با گروه پایش استاندارد، هیچ تفاوتی در نرخ اشباع اکسیژن (RR: 0.89؛ 95% CI؛ 0.48 تا 1.63؛ 1272 = n؛ 3 کارآزمایی؛ شواهد با کیفیت متوسط) و هیپوتانسیون (RR: 2.36؛ 95% CI؛ 0.98 تا 5.69؛ 986 = n؛ 1 کارآزمایی؛ شواهد با کیفیت متوسط) وجود نداشت. فقط یک اپیزود از استفراغ، بدون تفاوت معنادار بین گروهها، رکورد شد (RR: 3.10؛ 95% CI؛ 0.13 تا 75.88؛ 986 = n؛ 1 کارآزمایی؛ شواهد با کیفیت متوسط). کیفیت شواهد برای پیامدهای اولیه متوسط بود که دلیل اصلی کاهش، ناهمگونی و سوگیری گزارشدهی بود.
تفاوتی در نرخ مداخلات انجام شده روی راههای هوایی وجود نداشت (RR: 1.26؛ 95% CI؛ 0.94 تا 1.69؛ 1272 = n؛ 3 کارآزمایی؛ شواهد با کیفیت متوسط). در تجزیهوتحلیل زیرگروه، نرخ بالاتری از مداخلات راههای هوایی در بزرگسالان در گروه کاپنوگرافی (RR: 1.44؛ 95% CI؛ 1.16 تا 1.79؛ n=1118؛ 2 کارآزمایی؛ شواهد با کیفیت متوسط)، با تعداد افراد مورد نیاز برای درمان تا حصول یک پیامد مضر اضافی از 12 پیامد، یافت شد. اگر چه ناهمگونی آماری کاهش یافت، به دلیل ناهمگونی پیامد تعریف شده و محدودیت سوگیری گزارشدهی، کیفیت شواهد متوسط بود. در هیچ یک از مطالعات، زمان بهبود گزارش نشد.
نتیجهگیریهای نویسندگان
کمبود شواهد قانع کننده نشان میدهد که اضافه کردن کاپنوگرافی به پایش استاندارد در ED PSA نرخ حوادث جانبی قابل توجه از نظر بالینی را کاهش میدهد. بهنظر میرسد کیفیت شواهد با توجه به ناهمگونی جمعیت و پیامد تعریف شده و محدودیت سوگیری گزارشدهی، متوسط باشد. مرور ما به دلیل کم بودن تعداد کارآزماییهای بالینی در این زمینه محدود شد.
PICOs
خلاصه به زبان ساده
استفاده از کاپنوگرافی (capnography) در بیماران بخش اورژانس دریافت کننده آرامسازی حین انجام پروسیجر
سوال مطالعه مروری
آیا پایش تشخیص دیاکسید کربن به کاهش عوارض قلبی، ریوی، و راههای هوایی برای بیماران بخش اورژانس دریافت کننده آرامسازی حین انجام پروسیجرهای دردناک کمک خواهد کرد؟
پیشینه
داروها اغلب برای کاهش درد یا آگاهی (یا هر دو) در بیماران دریافت کننده پروسیجرهای دردناک استفاده میشوند. گاهی اوقات، عوارض مربوط به قلب بیمار، ریهها، یا راههای هوایی (لولههای تنفسی) میتوانند به علت این داروها (بهعنوان مثال استفراغ به دلیل استنشاق از ریهها) رخ دهند. کارکنان بخش مراقبتهای سلامت، ضربان قلب، فشار خون، نرخ تنفس، و نرخ اکسیژن خون را برای کمک به پیشگیری از وقوع این عوارض کنترل میکنند.
استفاده از کاپنوگرافی (اندازهگیری گاز دیاکسید کربنی که یک بیمار تنفس میکند) برای افزایش بیشتر ایمنی آرامسازی بیماران در بخش اورژانس پیشنهاد شده است. این مطالعه به منظور تعیین اینکه کاپنوگرافی وقتی که به پایش استاندارد اضافه شود تفاوتی ایجاد میکند یا خیر، انجام گرفت.
ویژگیهای مطالعه
مطالعات را با استفاده از بانکهای اطلاعاتی پژوهشی متعدد، مجموعه مقالات پژوهشی، و تماس با متخصصان در این زمینه جستوجو کردیم. شواهد تا آگوست 2016 بهروز است. ما فقط مطالعاتی را در نظر گرفتیم که دارای شرکتکنندگانی بودند که برای پروسیجرهای بخش اورژانس تحت آرامسازی قرار گرفته بودند. فقط مطالعاتی را وارد کردیم که به مقایسه کاپنوگرافی و پایش استاندارد با پایش استاندارد بهتنهایی پرداختند.
پیامدهای اصلی شامل عوارض مربوط به مقدار کم اکسیژن خون، فشار خون پائین، و استفراغ بود. تعداد دفعاتی را که ارائه دهندگان مراقبت سلامت به تنفس آسانتر بیمار کمک کردند نیز رکورد کردیم. این میتواند به معنی اقدامات ساده مانند باز کردن دهان برای مانورهای بسیار جدی مانند تنفس مکانیکی برای بیمار باشد.
سه مطالعه با 1272 فرد، دارای شواهد متوسط، در مطالعه ما وارد شدند.
نتایج کلیدی
هیچ تفاوتی از نظر عوارض قلبی، ریوی، یا راههای هوایی با افزودن کاپنوگرافی وجود نداشت. هنگامی که فقط بزرگسالان مورد مطالعه قرار گرفتند، ارائه دهندگان مراقبت سلامت، مانورهای بیشتری را برای کمک به تنفس بیمار هنگام استفاده از کاپنوگرافی انجام دادند. این میتواند به دلیل آلارمهای دروغین باشد.
کیفیت شواهد
سطح شواهد متوسط تعیین شد.
Authors' conclusions
Summary of findings
| Capnography and standard monitoring compared with standard monitoring for emergency department patients undergoing procedural sedation and analgesia | ||||||
| Patient or population: patients undergoing PSA Settings: emergency departments in North America Intervention: capnography and standard monitoring Comparison: standard monitoring | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard monitoring | Capnography and standard monitoring | |||||
| Oxygen desaturation | Medium risk population | RR 0.89 (0.48 to 1.63) | 1272 participants (3 studies) | ⊕⊕⊕⊝ | ‐ | |
| 8 per 1000a | 7 per 1000 | |||||
| Hypotension | Medium risk population | RR 2.36 (0.98 to 5.69) | 986 participants (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 6 per 1000c | 14 per 1000 | |||||
| Emesis, pulmonary aspiration | Medium risk population | RR 3.10 (0.13 to 75.88) | 986 participants (1 study) | ⊕⊕⊕⊝ | None of the studies recorded pulmonary aspiration events. | |
| 4 per 1000e | 4 per 1000 (1 to 304) | |||||
| Airway interventions | Medium risk population | RR 1.26 (0.94 to 1.69) | 1272 participants (3 studies) | ⊕⊕⊕⊝ | 2 studies included verbal/physical stimulation and supplemental oxygen as airway interventions (not consistent with our definition) but only reported total airway interventions (as dichotomous outcomes). | |
| 150 per 1000f | 189 per 1000 | |||||
| Airway interventions adult subgroup analysis (aged ≥ 18 years)h | Medium risk population | RR 1.44 (1.16 to 1.79) | 1118 participants (2 studies) | ⊕⊕⊕⊝ | ‐ | |
| 190 per 1000i | 274 per 1000 | |||||
| Recovery time | None of the studies reported recovery time. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PSA: procedural sedation and analgesia; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aCampbell 2006; Cudny 2013. No study found to determine assumed risk for all‐age population, combined incidence of these studies used as a surrogate. Hypoxia defined as oxygen saturation < 90% at any time with baseline oxygen saturation ≥ 95% for Campbell 2006. Unknown definition of hypoxia for Cudny 2013. b Although statistics show low to moderate heterogeneity (I2 = 42%, P = 0.18), quality downgraded due to heterogeneity in study designs. cCampbell 2006. Hypotension defined as systolic blood pressure < 85 mmHg at any time with baseline systolic blood pressure ≥ 100 mmHg. d Downgraded for reporting bias in one study. eLanghan 2012. Used this paediatric study as surrogate for all ages population. g Downgraded due to significant heterogeneity (I2 = 53%). h The study by Campbell 2016 reported adults aged 16 years or greater whereas the study by Deitch 2010 reported adults aged greater than 18 years. j Downgraded due to heterogeneity in outcome definitions as well as small number of studies. | ||||||
Background
Description of the condition
Procedural sedation and analgesia (PSA) is used very frequently in the modern emergency department (ED) under the auspices of emergency physicians and allied healthcare professionals. PSA is defined as the use of medications to induce an altered state of consciousness to safely facilitate painful procedures and interventions such as fracture reduction, cardioversion, and incision and drainage of abscesses without loss of spontaneous cardiopulmonary function (Godwin 2005). Despite widespread use, PSA is not without risk. Overshoot in the intended depth of sedation potentially resulting in respiratory compromise, loss of airway protection, and cardiovascular depression occurs in 1.3% to 10% of cases (Campbell 2006; Miner 2003; Swanson 1996). In one study, respiratory depression occurred in up to 39% of patients undergoing PSA with propofol (Deitch 2010). Emesis with or without pulmonary aspiration is of particular concern in patients with full stomachs who may be intoxicated and undergoing PSA in the ED. Safeguards to reduce complications in ED PSA include monitoring heart rate, respiratory rate, blood pressure, and oxygen saturation.
Description of the intervention
Capnography, the continuous measurement of exhaled carbon dioxide (CO2) partial pressures (PCO2), can be used in conjunction with other monitoring variables to determine the effects of sedation medications on ventilation and potentially prevent hypoxic events. This is accomplished by placement of an infrared sensor at the facemask or nasal cannula. The data output can be interpreted in the form of capnograms (CO2 waveforms versus time or expired volume) or end‐tidal CO2 partial pressure (an estimation of alveolar PCO2 (Kodali 2013). It is a widely accepted modality and used extensively in the operating room and the endoscopy suite in addition to standard PSA monitoring (Swanson 1996). While capnography use in the ED was historically limited to confirmation of endotracheal tube placement and for cardiac arrest, it is being used with increasing frequency for PSA (Krauss 2007).
How the intervention might work
Sedation medications act to depress minute ventilation by decreasing respiration rate or tidal volume, or both. On a physiological level, alterations in PCO2 are used to estimate changing pulmonary CO2 levels, a surrogate of changes in minute ventilation. Capnography can be used to detect decreasing trends of minute ventilation (i.e. ventilatory depression) earlier and steps can be taken (airway intervention or medication or dose change) to correct the undesired physiological disturbance (Burton 2006). Specifically, capnography data can be interpreted by monitoring for changes in end‐tidal PCO2 (PETCO2), the shape of the capnogram, or arterial (a) and end‐tidal PaCO2 ‐ PETCO2 gradients (Kodali 2013). It is theorized that by using capnography in addition to other physiological variables to detect ventilatory depression, cardiorespiratory complications such as hypoxia can be detected and treated earlier.
Why it is important to do this review
There has been a trend towards increased use of capnography in ED PSA despite limited evidence demonstrating its efficacy in this environment. The controlled environments of the operating room and endoscopy suite are in contrast to the ED with respect to both patient population and procedures performed. ED visits are unplanned and patients often present in significant distress with full stomachs and they may have recently consumed alcohol or recreational drugs. Considering this, conclusions derived from studies in operating room and endoscopy settings do not necessarily apply to ED PSA. Only one published randomized controlled trial (RCT) had been performed at the protocol stage studying capnography as a primary intervention in ED patients undergoing PSA (Deitch 2010). This study found that capnography both reduced and provided earlier detection of hypoxic events. The authors used definitions inconsistent with other literature, notably defining hypoxia as a blood oxygen saturation (SpO2) less than 93% for 15 seconds or greater, complicating the interpretation of their conclusions. A complete review of relevant studies is needed to improve generalizability and ultimately to provide a more powerful consensus on the utility of capnography in the ED.
Objectives
To assess whether capnography in addition to standard monitoring (pulse oximetry, blood pressure and cardiac monitoring) is more effective than standard monitoring alone to prevent cardiorespiratory adverse events (e.g. oxygen desaturation, hypotension, emesis, and pulmonary aspiration) in ED patients undergoing procedural sedation and analgesia.
Methods
Criteria for considering studies for this review
Types of studies
We only considered RCTs and quasi‐RCTs for inclusion. We imposed no language restrictions. We included unpublished data if the trial met the inclusion criteria.
Types of participants
We included all ED patients requiring PSA were included, regardless of age. Procedural sedation refers to "the technique of administering sedatives or dissociative agents with or without analgesics to induce an altered state of consciousness that allows the patient to tolerate unpleasant procedures while preserving cardiorespiratory function" (Godwin 2005). We excluded participants receiving PSA in non‐ED environments such as endoscopy suites or operating rooms.
Types of interventions
All participants had to be randomized to either capnography in addition to standard monitoring (pulse oximetry, blood pressure and cardiac monitoring) or standard monitoring alone.
Types of outcome measures
Primary outcomes
-
Oxygen desaturation (percentage of oxygen saturation in arterial blood (SaO2) less than 90% for 30 seconds).
-
Hypotension (systolic blood pressure less than 90 mmHg).
-
Emesis, pulmonary aspiration.
Secondary outcomes
-
Airway interventions performed (airway repositioning manoeuvres, positive pressure ventilation (PPV), oral pharyngeal or nasal pharyngeal airway placement, endotracheal intubation (ETI)).
-
Recovery time (time from end of procedure to cessation of monitoring).
Note: this definition of oxygen desaturation was most consistent with other relevant studies in the literature (Anderson 2007; Campbell 2006; Deitch 2007; Hart 1997; Langhan 2012; Mallory 2011; Miner 2002; Wright 1992). We would argue that this is clinically significant due to the fact that as oxygen saturation falls below 90%, the steep area of the oxyhaemoglobin dissociation curve is entered mandating medical intervention.
Outcomes did not form part of the study eligibility assessment so that studies that met the design, participant, intervention, and comparison criteria could be included in the review even if they reported no relevant outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2016, Issue 8); MEDLINE (1980 to 9 August 2016); Embase (1980 to 9 August 2016); and CINAHL (1982 to 9 August 2016) to identify all clinical trials relating to our objectives. We imposed no language or publication restrictions, or publication status restrictions. We reviewed the reference lists of all available primary studies and review articles to identify potential relevant citations.
See Appendix 1 (CENTRAL), Appendix 2 (MEDLINE), Appendix 3 (Embase), and Appendix 4 (CINAHL) for our search strategies.
Searching other resources
We contacted the authors of the primary studies and scientific advisors of the various capnography device manufacturers to enquire about additional published or unpublished studies (February 2016). We searched for trials in progress using: www.controlled‐trials.com, www.clinicalstudyresults.org, and clinicaltrials.gov (February 2016).
We handsearched Canadian Association of Emergency Physicians (CAEP); American College of Emergency Physicians (ACEP); Society for Academic Emergency Medicine (SAEM); and International Conference on Emergency Medicine (ICEM) conference abstract submissions from 2010 to 2015.
Finally, we personally contacted colleagues, collaborators, and other trialists working in the field of ED PSA to identify potentially relevant studies.
Data collection and analysis
Selection of studies
We combined results from the search strategies into a reference manager program (EndNote X7.4) with duplicates excluded. Two authors (BFW, KDM) selected all trials which appeared relevant on the basis of title, abstract, and MeSH headings for full review.
From the potentially relevant articles identified, two authors (BFW, KDM) independently selected trials (based on the full‐text format) for inclusion in this review (see Appendix 5 for the article inclusion form). Agreement was measured using simple agreement and kappa statistics. We resolved disagreements by discussion to reach consensus or third party adjudication (SGC). Authors were not blinded to information about the articles such as publication names, institutions involved, or conclusions made. A list of all included trials was completed (Appendix 6). Adjudication of the final list was performed by a separate author (BFW) and an independent third party (Anna MacDonald) for inclusion or exclusion from the selection of studies conducted by authors of this review.
Data extraction and management
Two authors (BFW, KDM) independently extracted data from the trials using an electronic data collection form (see Appendix 7). Disagreement not resolved by consensus was adjudicated by a third author (SGC). Any additional trial data required was obtained by contacting the first trial author (BFW). Data for trials conducted by authors of this review were extracted by a separate author (BFW) and an independent third party (Anna MacDonald). Data extraction included the following items.
-
General information: title, authors, contact address, publication source, publication year, country.
-
Methodological characteristics and study design.
-
Population characteristics: age, sedation agent used, procedure performed.
-
Intervention: capnography, definition of; capnography alert level.
-
Control: blood pressure, heart rate, oxygen saturation.
-
Outcome measures as noted above.
Assessment of risk of bias in included studies
We performed a methodological quality assessment using the 'Risk of bias' tool detailed in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The quality assessment form is attached as Appendix 8. Two authors (BFW, KDM) independently performed this assessment with disagreement not resolved by consensus arbitrated by a third author (SGC). Assessors were not blinded to the study authors or the results of the studies.
We assessed the following domains: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; selective reporting; and all other biases.
Measures of treatment effect
For dichotomous variables, we calculated individual and pooled statistics as risk ratios (RR) with 95% confidence intervals (CI). For continuous outcomes, we calculated individual and pooled statistics as mean differences (MD) or standardized mean differences (SMD) and 95% CIs.
Unit of analysis issues
We did not include cluster‐randomized trials and cross‐over trials in the review, and therefore, there were no unit of analysis issues.
Dealing with missing data
An intention‐to‐treat (ITT) analysis, which deals with 'missing data' from the individual trials, was completed.
Assessment of heterogeneity
We assessed heterogeneity by analysing the methodological diversity (risk of bias assessment), clinical diversity (participant age, procedure performed, sedation agent used, definition of hypotension, definition of oxygen desaturation), and trial size (publication bias). We visually inspected Forest plots for evidence of heterogeneity and calculated both Chi2 and I2 statistics. Given the small number of included studies, we classified significant heterogeneity as a measured I2 statistic greater than 50% (Higgins 2011). We performed subgroup and sensitivity analyses to address clinical heterogeneity.
Assessment of reporting biases
We addressed publication bias, when found, by adjusting the results using the Egger approach (Egger 1997), and the 'trim and fill' method. In the event that 10 or more studies were included in the review, we planned to create a funnel plot. In addition, we planned to use quality weighting to test the robustness of the results.
Data synthesis
In the absence of significant heterogeneity, we performed a meta‐analysis using Review Manager 5 (RevMan 2014). To ensure the robustness of the results, we used both random‐effects and fixed‐effect modelling. The criteria for statistical significance was a P value less than 0.05 and 95% CIs that did not intersect.
Subgroup analysis and investigation of heterogeneity
As the utility of capnography in ED PSA may be dependent on the age of the participant, the sedation agent used, and the procedure performed, subgroup analysis was planned a priori in the event significant heterogeneity was detected (determined by the forest plot and I2 statistic).
-
Adults (aged over 18 years) versus paediatrics.
-
Sedation agent used (propofol, ketamine, midazolam, lorazepam, fentanyl, etomidate, pentobarbital, methohexital).
-
Procedure performed (fracture reduction, joint reduction, incision and drainage, suturing, cardioversion, lumbar puncture, diagnostic imaging, endoscopy, Foley catheter insertion, central line placement, dressing changes, burn debridement, ophthalmic procedure, dental procedure, temporomandibular joint reduction, chest tube insertion, other).
Sensitivity analysis
In order to test the robustness of our findings, we planned to perform a sensitivity analysis for trials with low risk versus high risk of bias. We planned to use random‐effects and fixed‐effect model estimates for each outcome variable. In the event of any missing data, we planned to use best‐case and worst‐case scenarios for imputation of missing data.
'Summary of findings' table and GRADE
We used the GRADE system to assess the quality of the evidence associated with specific outcomes (oxygen desaturation, hypotension, emesis, pulmonary aspiration, airway interventions performed, recovery time) in our review and to construct a 'Summary of findings' table using the GRADE software (Guyatt 2008). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
Results of the search
The electronic search resulted in 3311 studies after removal of duplicates using bibliographic software (Figure 1). We identified 38 studies for full paper review by screening titles and abstracts. Of these 38 references, we included three in our review (Characteristics of included studies table) and excluded 35 due to the following:

Search flow diagram.
-
10 were review articles;
-
eight were non‐randomized trials;
-
eight involved other interventions;
-
eight were letters or questionnaires;
-
one was a systematic review.
Of these 35 excluded reports, four were identified as potentially relevant but were then excluded (Characteristics of excluded studies table).
Included studies
We included three articles comparing 1272 participants in our review (Characteristics of included studies table).
Setting
All three studies took place in single‐centred academic EDs in the US or Canada. Two studies involved physicians performing the sedation (Deitch 2010; Langhan 2015), and one study involved advanced level paramedics performing the sedation, an evidence‐based standard at that institution (Campbell 2016). All providers involved in procedural sedation were trained in interpreting capnography.
Population
Two of the included studies enrolled adults only with Campbell 2016 enrolling 986 participants (aged greater than 16 years) and Deitch 2010 enrolling 132 participants (aged greater than 18 years). The other study enrolled 154 paediatric participants (aged less than 20 years) (Langhan 2015). All studies included patients undergoing PSA but generally excluded critically ill patients. The paediatric study (Langhan 2015), excluded patients who had supplemental oxygen at baseline whereas the adult studies administered baseline supplemental oxygen as standard of care (Campbell 2016; Deitch 2010).
Interventions
The control groups differed in one study (Campbell 2016), using only standard monitoring without capnography whereas two studies used standard monitoring and blinded capnography (Deitch 2010; Langhan 2015). Standard monitoring was similar in all studies, consisting of cardiac monitoring, blood pressure monitoring, and pulse oximetry. Capnography alert levels were specified and similar in two of the studies (Deitch 2010; Langhan 2015), whereas one study did not specify alert levels (Campbell 2016).
Outcomes
All three studies recorded the primary outcome of oxygen desaturation but there was some variance in the definition.
-
One study defined hypoxia as less than 93% for greater than 15 seconds (Deitch 2010). The author was unable to be reached for unpublished data.
-
One study defined hypoxia as less than 90% for greater than 30 seconds (Campbell 2016).
-
One study defined hypoxia as less than 95% (Langhan 2015). We were able to contact the authors and collect unpublished data for episodes of hypoxia less than 90%.
Campbell 2016 recorded vomiting and hypotensive events, with hypotension defined as systolic blood pressure (SBP) less than 100 mmHg or less than 85 mmHg if baseline less than 100 mmHg. Langhan 2015 did not measure vomiting and hypotension. Deitch 2010 did not report vomiting and hypotension despite the methods section stating the recording of such events. None of the studies recorded pulmonary aspiration events.
All three studies recorded the secondary outcome of airway interventions but there was significant variance in classification of such interventions. Only Campbell 2016 and Langhan 2015 provided data detailing the number of specific interventions performed. Deitch 2010 and Langhan 2015 published dichotomous data, whereas we contacted the authors of Campbell 2016 to obtain dichotomous data (only continuous outcome data was published).
None of the studies reported recovery time (excluding procedure time).
Funding
The Campbell 2016 and Langhan 2015 studies were supported by independent government grants. Deitch 2010 had capnography equipment donated by a private medical company.
Excluded studies
We excluded four studies due to retrospective analysis of capnography data in RCTs comparing other interventions (Deitch 2007;Deitch 2008; Hart 1997; Sivilotti 2010) (see Characteristics of excluded studies table).
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We have included the risk of bias in Figure 2 and Figure 3. Overall quality of evidence was moderate with downgrades resulting from outcome definition heterogeneity and limited reporting bias in one study.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All three studies used random sequence generation using a computer (Campbell 2016; Deitch 2010; Langhan 2015). Allocation concealment was appropriate in all studies. Although none of the studies mentioned use of an ITT protocol, there were no participants given an intervention other than their assigned group intervention. We found that there was low risk of allocation bias.
Blinding
None of the studies blinded either treating personnel or participants to the intervention. We deemed this an inherent bias due to the monitoring nature of the intervention; treating personnel are required to see the monitoring technique to perform the procedure. Likewise, the logistics of blinding a patient to bedside monitoring while maintaining safety is understandably difficult. We deemed this to be low risk of bias because future studies would likely not be able to improve upon this.
Incomplete outcome data
Two studies accounts for all data, with low risk for attrition bias (Campbell 2016; Langhan 2015). One study excluded 12% of participants enrolled in the study from analysis based on a priori exclusion criteria of greater than 35% data loss (Deitch 2010). This was deemed a high source of attrition bias.
Selective reporting
Given the small number of studies included in the meta‐analysis, it was difficult to assess for publication bias. The review did not include the minimum number of studies stipulated to produce a funnel plot. However, we are confident that our robust search strategy identified all relevant published studies. With respect to intra‐study reporting bias, one study collected data on adverse events (hypotension, emesis) but did not report them in the results section (Deitch 2010). This study reported only absolute numbers of airway interventions and not specific types although these data were also reported to be collected. We found this study to be at a high risk of reporting bias due to these discrepancies between the methods and results reported. One study did not enrol in a trial registry and thus reporting bias was unknown in this study (Campbell 2016).
Other potential sources of bias
All three included studies defined oxygen desaturation differently. A priori, we defined oxygen desaturation as an oxygen saturation of less than 90% for 30 seconds, the same definition used by Campbell 2016. This definition was most consistent with other relevant studies in the literature (Anderson 2007; Campbell 2006; Deitch 2007; Hart 1997; Langhan 2012; Mallory 2011; Miner 2002; Wright 1992), and we would argue, makes physiological sense based on the oxyhaemoglobin dissociation curve and is clinically significant mandating medical intervention. Although Langhan 2015 defined hypoxia as an oxygen saturation of less than 95%, the authors provided unpublished individual participant data such that rates of oxygen desaturation, as defined above, could be calculated. Deitch 2010 defined hypoxia as an oxygen saturation of less than 93% for greater than 15 seconds. Attempts to contact the authors were unsuccessful and we were required to include these participants in the analysis despite an obvious difference in outcome definition that would introduce more oxygen desaturation events. We performed a sensitivity analysis to explore the effect of this heterogeneity on the overall results.
Two studies were supported by independent government grants (Campbell 2016; Langhan 2015). One study had a capnography device donated by a private medical manufacturing company (Deitch 2010).
Effects of interventions
Primary outcomes
1. Oxygen desaturation
A total of 74 hypoxic events occurred in 1272 participants with an incidence of 33.3% in Deitch 2010, 9.1% in Langhan 2015, and 1.6% in Campbell 2016. The significantly higher incidence in Deitch 2010 likely represents the much more conservative definition of hypoxia used in this study. There was no significant difference in the rate of hypoxia between the intervention and control groups (RR 0.89, 95% CI 0.48 to 1.63; I2 = 42%) (Analysis 1.1; Figure 4). A sensitivity analysis to address heterogeneity was performed excluding one study with a different hypoxia definition (Deitch 2010). This proved to reduce heterogeneity but did not show a significant difference in the rate of hypoxia with the use of capnography (RR 1.33, 95% CI 0.66 to 2.69; I2 = 0%) (Figure 5). The quality of the evidence was downgraded to moderate quality due to heterogeneity between outcome definitions.

Forest plot of comparison: capnography plus standard monitoring versus standard monitoring, outcome: 1.1 oxygen desaturation.
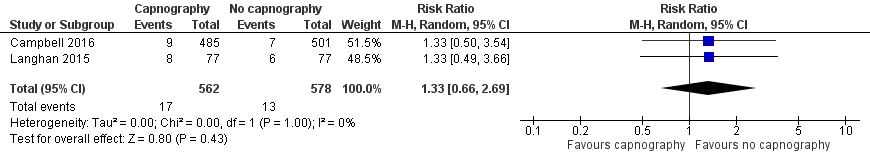
Forest plot of comparison: capnography plus standard monitoring) versus standard monitoring, outcome: 1.6 oxygen desaturation (sensitivity analysis based on definition of oxygen desaturation. Deitch 2010 excluded).
2. Hypotension
Only one study reported hypotension with a total of 23 events in 986 participants, an incidence of 2.3% (Campbell 2016). There was a trend of more hypotensive events in the capnography group compared to the control group that was not statistically significant (RR 2.36, 95% CI 0.98 to 5.69) (Analysis 1.2). One study stated in the methods section it would record hypotensive events but did not report on these findings in the results (Deitch 2010). Due to this reporting bias, the quality of evidence was downgraded to moderate.
3. Emesis, pulmonary aspiration
Only one study reported emesis outcomes with one event occurring in 986 participants, an incidence of 0.1% (Campbell 2016). There was no significant difference between the intervention and control groups (RR 3.10, 95% CI 0.13 to 75.88) (Analysis 1.3). Although one study stated in their methods that episodes of vomiting would be recorded, no such events were reported in the results section (Deitch 2010). Attempts to contact the authors to clarify this were unsuccessful. Due to this reporting bias, the quality of evidence was downgraded to moderate. None of the studies recorded pulmonary aspiration events.
Secondary outcomes
1. Airway interventions
A total of 335 airway interventions were performed in 1272 participants, an incidence of 26.3%. In two studies, there were no interventions recorded involving ETI and only two interventions of PPV (Campbell 2016; Langhan 2015). One study did not report the specific interventions (Deitch 2010). There was no significant difference between the capnography and standard monitoring groups (RR 1.26, 95% CI 0.94 to 1.69) (Analysis 1.4). There was significant heterogeneity (I2 = 53%) and thus the quality of evidence was downgraded to moderate. A sensitivity analysis was performed using a fixed‐effect model resulting in a significant difference favouring the standard monitoring group (RR 1.31, 95% CI 1.09 to 1.58; I2 = 53%) with a number needed to treat for an additional harmful outcome (NNTH) of 14. We believe that due to the heterogeneity in outcome definitions between the studies, along with a high I2 value, that the random‐effects model was more appropriate for this outcome.
2. Recovery time
None of the included studies reported recovery time.
Subgroup analysis for oxygen desaturation
Age subgroup analysis did not reveal any significant differences between the control and intervention groups (Analysis 1.5).
Subgroup analysis for airway interventions
Analysis of the adults‐only group reduced heterogeneity and revealed a significant difference with respect to the use of airway interventions favouring the control group over the capnography group (RR 1.44, 95% CI 1.16 to 1.79; I2 = 0) with an NNTH of 12 (Figure 6). The paediatrics‐only group showed no significant difference between the intervention and control group (RR 0.97, 95% CI 0.71 to 1.34). The quality of the evidence was downgraded to moderate quality due to variance in the definition of airway interventions.

Forest plot of comparison: capnography plus standard monitoring versus standard monitoring, outcome: 1.7 airway interventions (subgroup analysis based on participant age).
Subgroup analysis of sedation agent used
None of the included studies recorded data for analysis.
Subgroup analysis of procedure performed
None of the included studies recorded data for analysis.
Inter‐observer agreement
There was complete agreement between both evaluators regarding article selection (Kappa statistic 1.0).
Discussion
Summary of main results
Primary and secondary outcomes
This review summarized the results of three studies comprising 1272 participants, demonstrating a lack of convincing evidence that the addition of capnography to standard monitoring for PSA in the ED affects the rate of oxygen desaturation or hypotension. Similarly, there was no significant difference in the rate of airway interventions across the three studies based on moderate quality evidence. There was only one recorded event of emesis, and no significant difference, in the one study that reported this outcome.
Capnography is an instrument, and like any tool, its effectiveness is heavily dependent upon the skill of the user. The reality of emergency medicine is that the variety of practitioners using capnography, from physicians to allied health providers, introduces inherent heterogeneity for any review in this subject area. Even if one were to only include studies where ED PSA was performed by physicians, the skill set and training of emergency physicians is highly variable not only from country to country but also within countries. Likewise, the variety of patient presentations and procedures performed in the ED also has associated heterogeneity. While some might argue that this heterogeneity precludes the conduction of a meta‐analysis, this is the very nature of emergency medicine and such reviews are important for both clinicians and policy decision‐makers. Although this review found no evidence supporting the use of capnography for ED PSA, these results should be viewed with caution; they may not be applicable to certain practitioners, for patients in an unstable condition, or for procedures known to have higher risk of adverse events. Considering this, we do not condemn the wholesale use of capnography, but rather find no evidence supporting the mandatory use of capnography for routine ED PSA at this point in time.
Varying definitions of oxygen desaturation accounted for one source of heterogeneity in this meta‐analysis. A sensitivity analysis excluding the one study that defined oxygen desaturation as less than 93% for 15 seconds eliminated heterogeneity but failed to produce a different conclusion than what is reported above (Deitch 2010). When the definition of an oxygen saturation of less than 90% was used, the event incidence was dramatically reduced from 33.3% (Deitch 2010), to 2.6% (Campbell 2016; Langhan 2015). The trend favouring the use of capnography was reversed to favour standard monitoring with this sensitivity analysis but did not reach significance (RR 1.33, 95% CI 0.66 to 2.69). The quality of the evidence was downgraded due to the varying outcome definitions.
The question of what constitutes a clinically significant level of oxygen saturation is difficult to answer. The partial pressure of oxygen in the blood decreases exponentially below 90% in accordance with the standard oxygen dissociation curve, the primary physiological reason we used this definition of hypoxia. This definition has been cited in other relevant studies in the literature (Anderson 2007; Campbell 2006; Deitch 2007; Hart 1997; Langhan 2012; Mallory 2011; Miner 2002; Wright 1992).
In the paediatric setting, there is no consensus on providing baseline supplemental oxygen for PSA. In fact, studies generally refer to supplemental oxygen as an adverse event. Despite this, the paediatric study (Langhan 2015) found no significant heterogeneity in the oxygen desaturation outcome compared to the two adult studies (Campbell 2016; Deitch 2010). There was heterogeneity shown in the secondary outcome of airway interventions. This result may be partially explained by pulse oximetry detecting respiratory depression earlier than capnography in patients breathing room air, a conclusion reached in multiple studies (Deitch 2008; Sivilotti 2010). A lower rate of respiratory depression events detected by capnography than the standard monitoring may have necessitated fewer airway interventions in these paediatric participants breathing room air.
As determined in one large case study of 979 adults, PSA in the ED setting without the use of capnography is a safe procedure with clinically significant complications of hypoxia (1%) and hypotension (0.8%) occurring very rarely (Campbell 2006). Using data from this meta‐analysis and assuming an adverse event rate ranging from 3% to 23%, anywhere from 1600 to nearly 10,000 participants would need to be enrolled in each arm of the study to show a 25% decrease in the rate of complications (Sealed 2012). The magnitude and costs of such a trial would likely be unfeasible and, as such, future studies showing a difference in complication rate is improbable. Likewise, in the paediatric ED population, one case study of 204 sedations showed incidences of hypoxia of 0.5% and airway interventions of 2.0% (Cudny 2013). There were no reports of PPV, ETI, hypotension, or aspiration.
This type of monitoring intervention study has inherent bias because of the inability to blind providers to the group assignment (after randomization). This exposes results to the John Henry Effect (Saretsky 1972); theoretically, providers may alter and improve their standard monitoring practice because they know they do not have the additional capnography tool. This may have biased results in favour of the control group. Likewise, providers may have acted according to the Hawthorne Effect (McCarney 2007), increasing quality of the standard monitoring secondary to observation only. Theoretically, this would affect both intervention groups and be unlikely to bias the results in a particular direction. It is impossible to design a study without these sources of bias and therefore the evidence is still deemed high quality.
We encountered some reporting bias from one study that resulted in downgrading the quality of the evidence in multiple outcomes (Deitch 2010). Data were stated to have been collected in the methods section (hypotension, emesis) but then not reported in the results section. With such a small meta‐analysis, this impacted our outcomes significantly. In future updates, we hope to continue to make attempts to contact the authors for unpublished data.
Subgroup analysis
The subgroup analysis based on participant age revealed a significant increase in the rate of airway interventions for adults in the capnography group, with an NNTH of 12. This significant difference was not shown in the paediatrics subgroup. One explanation accounting for this would be that the highly sensitive capnography detects respiratory depression early in patients on supplemental oxygen without an increase in detection of clinically significant oxygen desaturation, causing an increase in unnecessary interventions. A second theory would be that the use of capnography increased clinician confidence resulting in higher rate or dose (or both) of sedative administration.
It should be noted that the airway interventions recorded were largely not serious in nature, mostly comprising airway repositioning with no ETIs reported in any study. So while there was a statistically significant increase in the rate of airway interventions in the capnography group, the clinical significance of this is uncertain.
Overall completeness and applicability of evidence
This study identified and analysed three trials to address our review question. Despite the small number of studies, the number of participants was significant and reflected the population seen in modern western academic EDs. Significant sources of heterogeneity were largely explained by differences in outcome definitions and study populations (adults versus paediatric). Sensitivity analyses did not change the conclusion that there is a lack of evidence that capnography decreases the rate of major adverse events in all age groups, and in fact, it may increase unnecessary airway interventions in adult populations. Airway interventions were noted to be minor in nature but may lead to waking the patient and theoretically result in additional sedative administration in order to successfully finish the procedure.
The three studies included in this meta‐analysis were performed in busy North American academic centres by skilled, experienced clinicians. The applicability of the evidence to settings that PSA is infrequently performed or where training differs is uncertain and could be the subject of future trials.
Quality of the evidence
Overall quality of the three studies was moderate after accounting for inherent bias associated with interventional studies comparing monitoring modalities (performance bias). Variance in outcome definition contributed to qualitative heterogeneity and resulted in moderate quality evidence. There were too few studies to properly assess for publication bias. There may have been some degree of selective reporting bias in one of the studies when comparing methods to reported results for the secondary outcomes (Deitch 2010). One of the studies was not enrolled in a clinical trials registry and thus may have also been affected by selective reporting bias (Campbell 2016).
We assessed heterogeneity through sensitivity and subgroup analyses, with the major source deemed to be due to slight variations in outcome definitions. In future updates, we will make further attempts to contact authors for unpublished data.
Potential biases in the review process
Three co‐authors of this study (KDM, SGC, PJZ) were also authors of one of the studies included in this meta‐analysis (Campbell 2016). Another author (BFW) and an independent researcher (Anna MacDonald) extracted data from this study since neither were involved in the included study. This method was stated a priori (Wall 2013).
A decision was made to perform a sensitivity analysis to address heterogeneity in the oxygen desaturation outcome after it was discovered there was variance in the outcome definition. This possibility was outlined a priori in the previously published protocol to address sources of heterogeneity (Wall 2013).
The safety of using paramedics as sedation providers under the supervision of emergency physicians may be contested by some. The Campbell 2006 study, the largest observational trial studying complications in ED PSA, showed that outcomes were similar to when the providers were emergency physicians.
As mentioned in the 'Discussion', an inability to reach one of the author of an included study to collect unpublished data resulted in significant downgrades to the evidence quality.
Agreements and disagreements with other studies or reviews
We found no other systematic reviews studying capnography. Burton 2012, a short cut review, stated that "capnography may provide early warning of ventilatory changes that could result in hypoxia." This did not necessarily disagree with our findings but the conclusion was primarily based on one well‐designed RCT (Deitch 2010).
One clinical policy guideline stated Level B recommendation ("based on evidence from one or more class of evidence II studies or strong consensus of class of evidence III studies") that capnography may be used to detect hypoventilation and apnoea earlier than standard monitoring in ED patients undergoing PSA (Godwin 2014). The authors noted that serious adverse events were quite rare in this setting and that evidence was lacking showing that capnography reduced these complications. Conclusions were also based heavily on the findings of Deitch 2010 in addition to multiple non‐RCT reviews and RCTs performed in settings other than the ED.
These reviews were written before two of the studies included in this review were published. Their conclusions disagreed with our results and promoted the routine use of capnography for ED PSA but were based only on one RCT rather than the three included in this review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: capnography plus standard monitoring versus standard monitoring, outcome: 1.1 oxygen desaturation.

Forest plot of comparison: capnography plus standard monitoring) versus standard monitoring, outcome: 1.6 oxygen desaturation (sensitivity analysis based on definition of oxygen desaturation. Deitch 2010 excluded).

Forest plot of comparison: capnography plus standard monitoring versus standard monitoring, outcome: 1.7 airway interventions (subgroup analysis based on participant age).

Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 1 Oxygen desaturation.

Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 2 Hypotension.
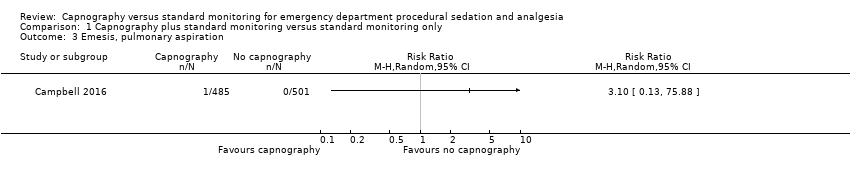
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 3 Emesis, pulmonary aspiration.
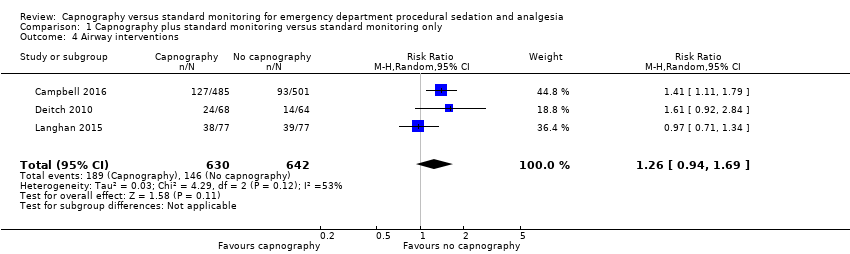
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 4 Airway interventions.

Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 5 Oxygen desaturation (subgroup analysis).
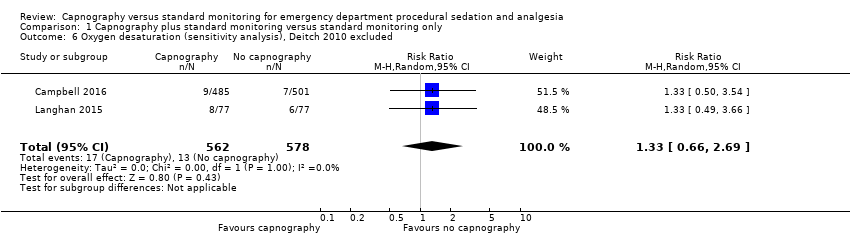
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 6 Oxygen desaturation (sensitivity analysis), Deitch 2010 excluded.
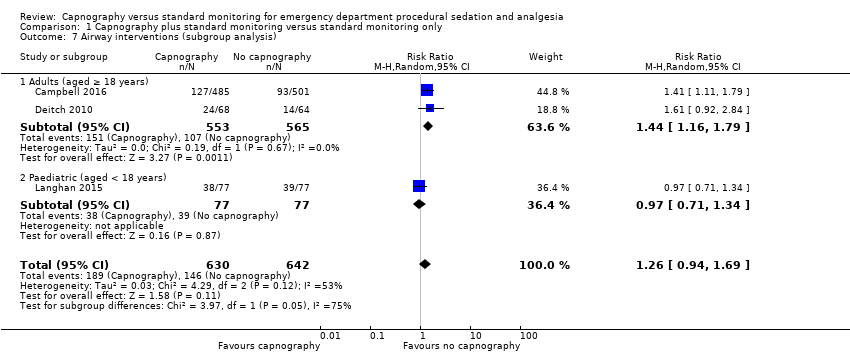
Comparison 1 Capnography plus standard monitoring versus standard monitoring only, Outcome 7 Airway interventions (subgroup analysis).
| Capnography and standard monitoring compared with standard monitoring for emergency department patients undergoing procedural sedation and analgesia | ||||||
| Patient or population: patients undergoing PSA Settings: emergency departments in North America Intervention: capnography and standard monitoring Comparison: standard monitoring | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard monitoring | Capnography and standard monitoring | |||||
| Oxygen desaturation | Medium risk population | RR 0.89 (0.48 to 1.63) | 1272 participants (3 studies) | ⊕⊕⊕⊝ | ‐ | |
| 8 per 1000a | 7 per 1000 | |||||
| Hypotension | Medium risk population | RR 2.36 (0.98 to 5.69) | 986 participants (1 study) | ⊕⊕⊕⊝ | ‐ | |
| 6 per 1000c | 14 per 1000 | |||||
| Emesis, pulmonary aspiration | Medium risk population | RR 3.10 (0.13 to 75.88) | 986 participants (1 study) | ⊕⊕⊕⊝ | None of the studies recorded pulmonary aspiration events. | |
| 4 per 1000e | 4 per 1000 (1 to 304) | |||||
| Airway interventions | Medium risk population | RR 1.26 (0.94 to 1.69) | 1272 participants (3 studies) | ⊕⊕⊕⊝ | 2 studies included verbal/physical stimulation and supplemental oxygen as airway interventions (not consistent with our definition) but only reported total airway interventions (as dichotomous outcomes). | |
| 150 per 1000f | 189 per 1000 | |||||
| Airway interventions adult subgroup analysis (aged ≥ 18 years)h | Medium risk population | RR 1.44 (1.16 to 1.79) | 1118 participants (2 studies) | ⊕⊕⊕⊝ | ‐ | |
| 190 per 1000i | 274 per 1000 | |||||
| Recovery time | None of the studies reported recovery time. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PSA: procedural sedation and analgesia; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aCampbell 2006; Cudny 2013. No study found to determine assumed risk for all‐age population, combined incidence of these studies used as a surrogate. Hypoxia defined as oxygen saturation < 90% at any time with baseline oxygen saturation ≥ 95% for Campbell 2006. Unknown definition of hypoxia for Cudny 2013. b Although statistics show low to moderate heterogeneity (I2 = 42%, P = 0.18), quality downgraded due to heterogeneity in study designs. cCampbell 2006. Hypotension defined as systolic blood pressure < 85 mmHg at any time with baseline systolic blood pressure ≥ 100 mmHg. d Downgraded for reporting bias in one study. eLanghan 2012. Used this paediatric study as surrogate for all ages population. g Downgraded due to significant heterogeneity (I2 = 53%). h The study by Campbell 2016 reported adults aged 16 years or greater whereas the study by Deitch 2010 reported adults aged greater than 18 years. j Downgraded due to heterogeneity in outcome definitions as well as small number of studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oxygen desaturation Show forest plot | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.48, 1.63] |
| 2 Hypotension Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Emesis, pulmonary aspiration Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Airway interventions Show forest plot | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.94, 1.69] |
| 5 Oxygen desaturation (subgroup analysis) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Adults (aged ≥ 18 years) | 2 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.37, 1.71] |
| 5.2 Paediatric (aged <18 years) | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.49, 3.66] |
| 6 Oxygen desaturation (sensitivity analysis), Deitch 2010 excluded Show forest plot | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.66, 2.69] |
| 7 Airway interventions (subgroup analysis) Show forest plot | 3 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.94, 1.69] |
| 7.1 Adults (aged ≥ 18 years) | 2 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.16, 1.79] |
| 7.2 Paediatric (aged < 18 years) | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.34] |

