Intervenciones microinvasivas para el tratamiento de las caries dentales proximales en dientes permanentes y primarios
Resumen
Antecedentes
Las lesiones dentales proximales, limitadas a la dentina, se tratan tradicionalmente mediante procedimientos invasivos (obturación y relleno). Las opciones no invasivas (p.ej. barniz de fluoruro, hilo dental) podrían evitar la pérdida de sustancia pero su efectividad depende del cumplimiento de los pacientes. Recientemente se han probado enfoques microinvasivos para tratar las lesiones de caries proximales. Estas intervenciones colocan una barrera encima (sellado) o dentro (infiltración) de la lesión. Actualmente hay diferentes métodos y materiales disponibles para los tratamientos microinvasivos, como el sellado mediante sellantes de resina, parches / cintas (poliuretano), cementos de ionómero de vidrio (CIV) o infiltración de resina.
Objetivos
Evaluar los efectos de los tratamientos microinvasivos para el control de las lesiones de caries proximales en la dentición primaria y permanente en niños y adultos.
Métodos de búsqueda
Se realizaron búsquedas en las siguientes bases de datos hasta el 31 de diciembre de 2014: El Registro de Ensayos del Grupo Cochrane de Salud Oral, el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL), MEDLINE vía OVID, EMBASE vía OVID, LILACs vía BIREME Virtual Health Library, Web of Science Conference Proceedings, ZETOC Conference Proceedings, Proquest Dissertations and Theses, ClinicalTrials.gov, OpenGrey y la Plataforma de Registros Internacionales de Ensayos Clínicos de la Organización Mundial de la Salud (OMS). Se buscó en el metaRegister of Controlled Trials hasta el 1 de octubre de 2014. No hubo restricciones de idioma ni de fecha en las búsquedas en las bases de datos electrónicas.
Criterios de selección
Se incluyeron los ensayos controlados aleatorios con una duración de al menos seis meses que compararon los tratamientos microinvasivos de las caries dentales proximales no cavitadas en los dientes primarios, los dientes permanentes o ambos, versus medidas no invasivas, procedimientos invasivos, ninguna intervención o placebo. También se incluyeron los estudios que compararon diferentes tipos de tratamientos microinvasivos.
Obtención y análisis de los datos
Dos revisores de forma independiente examinaron los resultados de la búsqueda, extrajeron los datos y evaluaron el riesgo de sesgo. Se utilizaron los procedimientos metodológicos estándar esperados por Cochrane para evaluar el riesgo de sesgo y resumir los datos. Los metanálisis se realizaron con el modelo de efectos aleatorios, mediante el método de Balagtas‐Becker para calcular el odds ratio (OR) para la progresión de la lesión. La calidad de las pruebas se calificó mediante los métodos GRADE.
Resultados principales
Se incluyeron ocho ensayos que asignaron al azar a 365 participantes. Todos los ensayos utilizaron un diseño de boca dividida, algunos con más de dos lesiones tratadas en el mismo participante. Los estudios se realizaron en consultorios dentales de salud pública o universitarios en Brasil, Colombia, Dinamarca, Alemania, Tailandia, Groenlandia y Chile. Seis estudios evaluaron los efectos de los tratamientos microinvasivos en la dentición permanente y dos estudios en la dentición primaria, y el riesgo de caries varió de bajo a alto. Los investigadores midieron el riesgo de caries en diferentes estudios mediante la presencia de caries solamente o mediante el programa Cariogram, que combina ocho factores contribuyentes, incluida la presencia de caries, la dieta, la saliva y otros factores relacionados con las caries. El período de seguimiento en los ensayos varió de uno a tres años. Todos los estudios utilizaron la progresión de la lesión como resultado primario, que la evalúa mediante diferentes métodos de lectura de las radiografías. Cuatro estudios recibieron apoyo de la industria para realizar la investigación, y uno fue realizado por los inventores de la intervención.
Se consideró que siete estudios tenían un riesgo general de sesgo alto, principalmente debido a la falta de cegamiento de los participantes y el personal. Se evaluaron los efectos de la intervención para todas los tratamientos microinvasivos y los subgrupos se analizaron según los diferentes métodos de tratamiento informados en los estudios incluidos.
En el metanálisis, que agrupó el grupo de datos más sensible (en cuanto al método de medición) de los estudios que presentaron datos en un formato apropiado para el metanálisis, mostró que el tratamiento microinvasivo redujo significativamente las probabilidades de progresión de la lesión en comparación con el tratamiento no invasivo (p.ej. barniz de fluoruro) o el asesoramiento de higiene bucodental (p.ej. el hilo dental) (OR 0,24; IC del 95%: 0,14 a 0,41; 602 lesiones; siete estudios; I2= 32%). No hubo pruebas de diferencias de subgrupos (p = 0,36).
Los cuatro estudios que midieron los eventos adversos no informaron eventos adversos después del tratamiento microinvasivo. La mayoría de los estudios no informó ningún resultado adicional.
La calidad de las pruebas de los tratamientos microinvasivos se evaluó como moderada. Aún no está claro qué tratamiento microinvasivo es más ventajoso, o si ciertas afecciones o características clínicas de los pacientes son más convenientes para los tratamientos microinvasivos que otras.
Conclusiones de los autores
Las pruebas disponibles indican que el tratamiento microinvasivo de las lesiones de caries proximales detiene las lesiones dentinales no cavitadas e iniciales del esmalte (limitadas al tercio exterior de la dentina, según la radiografía) y es significativamente más eficaz que el tratamiento profesional no invasivo (p.ej. barniz de fluoruro) o el asesoramiento (p.ej. hilo dental). Existe una certeza moderada de que es poco probable que los estudios de investigación adicionales cambien significativamente la estimación del efecto. Debido al escaso número de estudios, aún no está claro qué técnica microinvasiva ofrece el mayor efecto beneficioso, o si los efectos del tratamiento microinvasivo otorgan un efecto beneficioso mayor o menor según diferentes consideraciones clínicas o relacionadas con los pacientes.
PICOs
Resumen en términos sencillos
Tratamientos microinvasivos para el tratamiento de las caries dentales en las superficies de los dientes adyacentes en dientes de niños y adultos
Pregunta de la revisión
El objetivo de esta revisión es evaluar los efectos de los tratamientos microinvasivos sobre el tratamiento de las caries dentales sobre los dientes adyacentes (proximales) en niños y adultos (dientes primarios y permanentes).
Antecedentes
Las caries en las superficies de los dientes que están próximos entre sí (superficies proximales) son frecuentes. Generalmente no han progresado a estadios posteriores de las caries y la superficie del diente todavía no tiene caries.
Se utilizan diferentes métodos para tratar las caries dentales proximales. Un método frecuente es perforar el tejido del diente afectado e insertar un relleno plástico o metálico. Sin embargo, es posible que se elimine mucho tejido sólido en el proceso y este método se considera invasivo. Otros métodos no invasivos en uso incluyen que el dentista aplique barniz de fluoruro o que aconseje a los pacientes el uso regular del hilo dental. Estos métodos no invasivos no requieren eliminar tejido dental.
Los enfoques más recientes (tratamientos microinvasivos) incluyen la preparación (condicionamiento) de la superficie del diente con un ácido y luego colocar un sellado (cubierta) encima de la superficie o "infiltrar" tejido desmineralizado más blando con resinas. Estos métodos más nuevos funcionan al colocar una barrera en la superficie del diente o dentro del tejido desmineralizado para protegerlo contra los ácidos y evitar la pérdida adicional de minerales desde el interior del diente. Lo anterior, en teoría, debe detener las caries. Este enfoque puede ser realizado por un dentista u otro profesional dental e incluye la pérdida de pocos micrómetros de tejido dental debido a la necesidad de condicionar la superficie del diente con ácido.
Aún existe incertidumbre sobre cuán eficaces son los tratamientos microinvasivos para tratar las caries proximales. Tampoco está claro si alguna de estas técnicas es mejor que otras. Por ejemplo, se necesita un ácido más fuerte para el tejido poroso infiltrado con resina que cuando la superficie del diente sencillamente está sellada o cubierta. Aunque la infiltración podría ser un método más eficaz de protección del tejido que el sellado, el uso de un ácido más fuerte también significa la pérdida de más tejido. El objetivo de esta revisión fue investigar el mejor enfoque para tratar dicho deterioro en los adultos y los niños.
Características de los estudios
Esta revisión consideró las pruebas actualizadas hasta el 31 de diciembre de 2014. Se encontraron ocho ensayos relevantes con 365 participantes. En estos ensayos participaron niños y adultos y las lesiones de caries (caries dentales) se asignaron al azar a diferentes tratamientos microinvasivos y no invasivos. Ningún estudio comparó intervenciones microinvasivas con tratamiento invasivo (rellenado). Cuatro estudios recibieron apoyo económico de los inventores o los fabricantes de la intervención para realizar la investigación.
Resultados clave
Las pruebas actuales indican que los tratamientos microinvasivos pueden reducir significativamente la probabilidad de progresión de las caries dentales en comparación con los métodos descritos como no invasivos. Hay muy pocos estudios para decidir qué técnica de tratamiento microinvasiva es mejor o la repercusión de diferentes consideraciones clínicas y relacionadas con los pacientes. No se informaron efectos secundarios negativos; sin embargo, solamente la mitad de los estudios midió este resultado y el período de seguimiento de algunos de los estudios fue relativamente corto.
Calidad de la evidencia
Aunque los estudios de investigación adicionales posiblemente podrían cambiar estos resultados, las pruebas disponibles brindan confianza moderada de que los tratamientos microinvasivos son mucho más eficaces que los tratamientos no invasivos para detener las caries dentales.
Authors' conclusions
Summary of findings
| Micro‐invasive versus non‐invasive treatments for managing dental decay in primary and permanent teeth | ||||||
| Patient or population: people with dental decay on proximal surfaces of primary and permanent teeth Comparison: non‐invasive treatments (e.g. fluoride varnish, advice to floss) Radiographic follow‐up period: 6 months to 3 years | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Odds Ratio | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Sealing | |||||
| Caries progression measured by DSR > pairwise > visual scoring (12 months to 36 months follow‐up) | Study population | OR 0.24 | 602 (7 RCTs) | ⊕⊕⊕⊝ | The quality of evidence for caries progression measured by scoring (12 to 30 months), including 468 participants (5 RCTs), OR 0.27 (95% CI 0.17 to 0.44), was moderatea,b,c. The quality of evidence for caries progression measured by pairwise (18 to 36 months), including 330 participants (4 RCTs), OR 0.31 (95% CI 0.18 to 0.53), was moderatea,b,c. The quality of evidence for caries progression measured by digital substraction radiography (12 months to 18 months), including 270 participants (3 RCTs), OR 0.18 (95% CI 0.06 to 0.50), was moderatea,b,c. | |
| 547 per 1000 | 284 per 1000 | |||||
| Moderate | ||||||
| 649 per 1000 | 337 per 1000 | |||||
| Change in decayed, missing and filled (DMF/dmf) figures at surface, tooth and whole mouth level. | — | — | — | — | — | No studies reported on caries measured as change in decayed, missing and filled (DMF/dmf) figures at surface, tooth or whole mouth level |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOne or more studies lacked sufficient blinding of participants, personnel or both. Downgraded one level. | ||||||
Background
Description of the condition
Dental caries
Dental caries is a sugar‐dependent disease that damages tooth structures and may result in cavity formation in the enamel (hard outer tissues of the teeth), dentine (calcified tissue of the tooth body) and cementum (surface layer of the tooth root) (Kidd 2005). Dental plaque is a biofilm formed on the tooth's surface and frequently contains caries‐producing bacteria. These micro‐organisms metabolise dietary sugars and produce acids on the tooth surfaces. The resulting decreasing pH leads to an altered mineral saturation within the biofilm and the dissolution of tooth minerals (that is, demineralisation). Prolonged loss of mineral components will eventually lead to cavitation of the carious lesions, i.e. decay.
Dental caries is the most prevalent disease worldwide (Marcenes 2013). Caries and its sequelae are considered the most important burden of oral health and are increasingly more prevalent in sociodemographically disadvantaged groups (Antoft 1999; Hannigan 2000; Petersen 2005; Ekstrand 2007; Martignon 2010; Sheiham 2010; Schwendicke 2015). Apart from the high cost of treatment, the related pain and discomfort can affect quality of life (Leal 2012).
Proximal tooth surfaces are frequently affected by caries lesions (Ekstrand 2000; Mejàre 2002; Ekstrand 2006; Rehman 2009), which dental practitioners traditionally treat using conventional invasive restorative (drill and fill) methods (Nielsen 2001). The majority of lesions in adolescents and adults occur proximally (Forsling 1999). This, together with the limited life‐span of dental restorations, led to the development of alternative approaches for sealing instead of restoratively managing proximal caries lesions.
Description of the intervention
The treatment of proximal caries lesions can be invasive or non‐invasive. Recently, micro‐invasive treatments for caries lesions have been proposed for the management of dental decay.
Conventional invasive treatment
Conventional invasive treatment usually involves the use of rotary burs, alone or in conjunction with metal hand instruments (e.g. excavators), to remove carious (i.e. infected and demineralised) dentine and then place a filling (restoration). The conventional cavity preparation for caries lesions on proximal tooth surfaces usually leads to removal of marginal ridges of the tooth, thereby sacrificing a considerable amount of sound hard tissue and weakening the tooth structure. The pain and discomfort associated with conventional cavity preparation methods and fear of local anaesthetic injection, which is used to control pain, may discourage people from seeking dental treatment (Berggren 1984). Furthermore, conventional invasive treatment requires expensive equipment (e.g. handpiece), electricity supply and highly trained dental health personnel. These factors limit the access to dental care where financial, human and structural resources are scarce.
Non‐invasive treatment
Non‐invasive treatments aim at 'managing' rather than removing caries lesions. They include biofilm control via mechanical removal of plaque (e.g. dental floss or interdental brushing by patient), antibacterial treatments (e.g. application of chlorhexidine varnishes by dentist or other members of dental team) or remineralisation treatments (e.g. topical fluoride application by dentist or other members of dental team). The efficacy of measures for biofilm control remains uncertain, especially for proximal lesions (Poklepovic 2013), whilst research has generally found fluoride application to be effective in managing caries lesions (Marinho 2009). In general, the dependence on patient adherence compromises the effectiveness of non‐invasive means, especially with regard to biofilm removal on proximal surfaces via oral home care (e.g. flossing). Moreover, the need for periodic visits, for example for repeated application of topical fluoride, increases treatment costs and decreases the cost‐effectiveness of non‐invasive measures (Ellwood 2003; Martignon 2006; Schwendicke 2014a).
Micro‐invasive treatment
Micro‐invasive treatments involve conditioning the tooth surface prior to treating the caries lesion; this conditioning via organic acids involves the loss of few micrometers of tooth substance (enamel). There are two types of micro‐invasive treatments: sealing and resin infiltration.
Conventionally, sealing is performed to prevent dental caries in the pits and fissures on the occlusal or pitted tooth surfaces. Research has found such sealing to be highly efficacious (Ahovuo‐Saloranta 2013). For non‐sound (carious) surfaces, dental practitioners can also apply sealants therapeutically. Such sealing is thought to prevent the diffusion of bacterial acids into the lesion and minerals out of the lesion. Creating a diffusion barrier may prevent dental caries (Handleman 1973; Ripa 1993; Schwendicke 2015).
Based on the identical pathogenesis of occlusal and proximal caries lesions, sealing might also be efficacious in managing dental caries on proximal tooth surfaces (Splieth 2010). A number of sealant materials and techniques have been used as micro‐invasive treatments on proximal surfaces, such as resin sealants, polyurethane patches (tapes), and glass ionomer cement (GIC). These are applied onto the proximal lesion after separating adjacent teeth from each other, for example via orthodontic bands placed between the teeth for a few days prior to sealing (Gomez 2005; Martignon 2010; Splieth 2010; Alkilzy 2011; Trairatvorakul 2011). After separation, practitioners can protect adjacent tooth surfaces and prepare the lesion surface using phosphoric acid or dentine conditioner (for GIC) to increase the micro‐mechanical retention of the sealant (Cueto 1967). Then, they can apply and light cure flowable or fluidic sealant (with or without the patch) or GIC. Available sealant patches are fabricated based on polyurethane and form an elastic pre‐cured adhesive monomer layer that is bonded to the tooth. Compared to conventional sealants, such patches are supposed to be easier to handle and to provide an even layer of resin (Alkilzy 2011).
A second technique for proximal micro‐invasive treatment is resin infiltration (caries infiltration). This technique uses light‐curable low‐viscosity resins (infiltrants), which are soaked up into the porous enamel lesion body. Thus, resin infiltration creates the diffusion barrier inside rather than on top of the caries lesion like other sealants (Meyer‐Lueckel 2007). Another difference in resin infiltration is the fact that the pseudo‐intact surface layer present in non‐cavitated proximal lesions needs to be removed prior to infiltration via acid‐etching, using hydrochloric instead of phosphoric acid (Meyer‐Lueckel 2007). Thus, the amount of lost enamel is greater when infiltrating than when sealing a lesion. After etching, the lesion requires desiccation. Resin infiltration does not necessarily require separation of teeth before treatment, since available kits use wedges and applicator foils to separate and isolate adjacent teeth from each other. Hence, practitioners are supposed to administer resin infiltration in a single visit (Ekstrand 2010; Paris 2010a). Moreover, as resin infiltration is not placed on top, but inside the enamel, retention loss is unlikely to occur (as this would mean losing the 'hybridised' tooth tissue). Some of the described materials are resin based, and may therefore cause skin or mucosal reactions, including anaphylactoid, lichenoid or other allergic reactions (FDI 2009).
How the intervention might work
As described, proximal sealing or infiltrating caries lesions prevents diffusion of acids from biofilms into the lesion or mineral out of the lesion, thereby arresting the lesions or reducing their progression. Current applications of these micro‐invasive techniques are restricted to non‐cavitated lesions only.
Why it is important to do this review
Dental caries in proximal surfaces is an important health problem, especially in children, adolescents and young adults. Micro‐invasive treatment via proximal sealing or infiltrating might reduce the loss of dental hard tissue and the risk for follow‐up treatments emanating from invasive conventional treatment methods (Qvist 2008; Schwendicke 2014a). Consequently, they might help to reduce pain or discomfort during treatment, which could in turn lower dental anxiety and avert costs related to late treatment. Moreover, micro‐invasive treatments could be less dependent on patients' adherence than non‐invasive means, with subsequently increased efficacy. Finally, they should minimise the number of repeated treatment visits, thus increasing the cost‐effectiveness of the therapy.
There is still uncertainty within the dental profession with regard to the efficacy of micro‐invasive treatments compared to invasive or non‐invasive treatments for controlling proximal caries lesions. Therefore, it is important to assess the evidence for such strategies. The findings of this review can inform dental practitioners' clinical decision‐making and enhance evidence‐based practice.
Objectives
To evaluate the effects of micro‐invasive treatments for managing proximal caries lesions in primary and permanent dentition in children and adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials with at least six months follow‐up, as this is the shortest recommended length of intervals between radiographic exposures (ADA 2012). Both parallel group and split‐mouth study designs were eligible for inclusion. The unit of randomisation could be a group (e.g. school, class), an individual, a tooth or lesion, or tooth and lesion pairs.
Types of participants
Dentate children and adults, with proximal carious lesions, extended into enamel or dentine (but not the pulp), as detected by radiographs. We only considered teeth with no existing filling at the site of lesion (primary caries lesions).
Types of interventions
Interventions included proximal micro‐invasive treatments using resin or glass ionomer cement (GIC) sealants, polyurethane tapes or resin infiltration, placed or performed by dentists or dental care professionals (DCPs) (e.g. hygienist, therapist, etc.), versus: another micro‐invasive treatment, non‐invasive measures (e.g. dental floss, fluoride varnish, oral hygiene advice), invasive means (conventional restorations), placebo or no intervention.
Types of outcome measures
Primary outcomes
-
Progression of existing carious lesion into enamel or dentine, as detected by radiographs, over a minimum period of six months.
-
Change in decayed, missing and filled (DMF for permanent dentition/dmf for primary dentition) figures at surface, tooth and whole mouth level. Studies were to assess this over a minimum period of six months.
Caries progression could be assessed by any of the following methods.
-
Lesion progression, as scored via digital subtraction radiography, assessing radiographs obtained at baseline and follow‐up.
-
Lesion progression, as scored via pairwise comparison of radiographs obtained at baseline and follow‐up.
-
Lesion progression, as scored by independent assessment of radiographs obtained at baseline and follow‐up.
-
Clinical lesion progression using visual scoring, for example, the International Caries Detection and Assessment System (ICDAS).
We considered digital subtraction radiography (DSR) to be the most sensitive of the caries progression methods.
Secondary outcomes
-
Material deficiency (e.g. retention loss, or number of re‐treatments)
-
Participant and operator perception, as measured by standardised/validated questionnaires
-
Adverse events
-
Direct costs of treatment, indirect costs incurred from time off school or work to attend the dental visits by the patient/parent/caregiver (opportunity costs), or both
Search methods for identification of studies
We identified all relevant studies regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
For the identification of studies included or considered for this review, detailed search strategies were developed for each database to be searched. These were based on the search strategy developed for MEDLINE, but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We provide details of the MEDLINE search in Appendix 1. The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs.
The following bibliographic databases and trials registers were searched:
-
The Cochrane Oral Health Group Trials Register (to 31 December 2014) (see Appendix 2);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2014, Issue 11) (see Appendix 3);
-
MEDLINE via OVID (1946 to 31 December 2014) (see Appendix 1);
-
EMBASE via OVID (1980 to 31 December 2014) (see Appendix 4);
-
LILACS via Bireme Virtual Health Library (1982 to 31 December 2014) (see Appendix 5);
-
Web of Science Conference Proceedings (1990 to 31 December 2014) (see Appendix 6);
-
Zetoc Conference Proceedings (1993 to 31 December 2014) (see Appendix 7);
-
Proquest Dissertations and Theses (1861 to 31 December 2014) (see Appendix 8);
-
OpenGrey (1985 to 31 December 2014) (see Appendix 9).
There were no language or date limitations in the searches of the electronic databases.
Searching other resources
We searched the following trials registries for ongoing trials (see Appendix 10):
-
metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/) (to 1 October 2014, the database was no longer available after this point);
-
The US National Institutes of Health Trials Registry (http://www.clinicaltrials.gov/) (to 31 December 2014);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/default.aspx) (to 31 December 2014).
Handsearching
We only included handsearching done as part of the Cochrane Worldwide Handsearching Programme and uploaded to CENTRAL.
Reference lists
We examined the reference lists of relevant trials in an attempt to identify studies not identified in the previous searches.
Correspondence
We contacted organisations, researchers and experts known to be involved in the field by post, email or in person during scientific meetings and conferences, in an effort to trace unpublished or ongoing studies. We also contacted dental equipment manufacturers to identify any ongoing or unpublished studies. We contacted one company known to manufacture a kit for caries infiltration, and we requested and obtained data and references for all published and unpublished studies on sealants.
Data collection and analysis
We imported the downloaded set of records from each database to the bibliographic software package EndNote and merged them into one core database to remove duplicate records and to facilitate retrieval of relevant articles. For all potentially relevant reports identified when searching other non‐electronic sources (e.g. reference lists of relevant trials, reviews, articles and textbooks), we obtained the records and manually entered them into Endnote.
Selection of studies
Two review authors (MD and FS) independently screened the retrieved studies. Review authors were not blinded to the names of the authors, institutions, journal of publication or results of the studies. We first checked all records identified by the searches on the basis of title, then by abstract, keywords or both. We excluded records that were obviously irrelevant (according to study design/duration, participants, or interventions/comparisons) and obtained the full text of all remaining records. If the information relevant to the inclusion criteria was not available in the abstract or if the title was relevant but the abstract was not available, we obtained the full text of the report. The review authors could read reports in English, Persian, Arabic and German. For publications in other languages, we would have had the title and abstract of the retrieved studies translated by a native speaker who was fluent in English. If we thought the study was potentially eligible, we would have had the full text translated by two translators who were native speakers and fluent in English. One of the authors (MD) was to compare two versions and resolve disagreements, if any, by discussion with the translators. We did not identify any non‐English studies eligible to be included in this review.
MD and FS independently assessed the full reports obtained from all the electronic and other methods of searching to establish whether the studies met the inclusion criteria or not, using an inclusion criteria form that we had previously prepared and piloted. We resolved disagreements by discussion. Where resolution was not possible, we consulted a third review author (SD).
We attempted to contact authors of papers that we could not classify in order to ascertain whether they met inclusion criteria. We identified and checked all reports related to the same included study; in case of any discrepancy, we contacted the authors. If we identified more than one publication of a trial, we reviewed all of them and considered that the paper with the earliest publication date was the primary reference.
We recorded details about the studies rejected at the full‐text or subsequent stages in the 'Characteristics of excluded studies' tables, along with the reasons for exclusion.
Data extraction and management
The review authors (MD, FS and TW) independently extracted data from all included studies using a pilot‐tested data extraction form. Two review authors then separately entered the data into the 'Characteristics of included studies' table in Review Manager 5 (RevMan 2011) and checked for differences. We resolved any disagreements through discussion with another review author (SD) until reaching a consensus. We contacted the trial authors for clarification or missing information, where necessary. We treated studies with duplicate publications as a single source of data. If publications reported data from different time points, we extracted all data sets but only used the last for synthesis (see below).
We recorded the following details for each trial in the data extraction form: study design of randomised controlled trial (e.g. parallel, split‐mouth, cluster); country where trial took place; setting (e.g. primary or secondary care); number of centres; recruitment period; funding source; inclusion criteria; exclusion criteria; number of patients randomised/number of patients evaluated; test and control interventions; mode of administration; duration of interventions; primary and secondary outcomes; time point(s) when outcomes were measured; method of sample size calculation; duration of follow‐up; comparability of groups at baseline; any co‐interventions; risk of bias; and any other relevant data.
Assessment of risk of bias in included studies
Two review authors (MD and FS) independently undertook the 'Risk of bias' assessment for included trials. We resolved disagreements by discussion with a third review author (SD) until reaching a consensus. Where needed, we contacted study authors to obtain further data. We carried out the assessment according to the criteria described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Sequence generation: Was the method used to generate the allocation sequence appropriate to produce comparable groups? We graded this domain as having a low risk of bias if the authors described a random component in the sequence generation process (e.g. random number table, coin tossing, drawing of lots).
-
Allocation sequence concealment: Was the method used to conceal the allocation sequence appropriate to prevent the allocation being known in advance of, or during, enrolment? We graded this domain as having a low risk of bias if the authors described adequate concealment (e.g. by means of central randomisation, sequentially numbered, opaque envelopes), and graded high risk of bias if inadequate concealment was documented (e.g. alternation, use of case record numbers, dates of birth or day of the week) or if allocation was not concealed.
-
Blinding of participants and personnel: Was knowledge of the allocated intervention adequately prevented during the study? If the study ensured adequate blinding of participants, operators and assessors, for example by using placebo or sham treatments, we graded this domain as having a low risk of bias. For radiographic outcomes, examples of adequate assessment blinding could be anonymising the radiographs, masking the stage of treatment (i.e. baseline or follow‐up) or both. For clinical assessment of sealants, we assumed blinding was not possible. We graded this domain as having a high risk of bias if the study did not use any blinding of participants, operators or assessors.
-
Incomplete outcome data: How complete were the outcome data for the primary outcomes? Did authors report drop‐out rates and reasons for withdrawals? Did they impute missing data appropriately? We graded this domain as having a low risk of bias if the proportion of the missing outcome data was less than 25% and the groups were balanced in numbers and reasons for drop‐outs, or if investigators imputed missing data using appropriate methods. If drop‐out was above 25% and there was no information on reasons for drop‐outs across groups, but attrition was balanced, we graded the risk of bias as unclear. We graded it as high if the proportion of missing outcome data was over 25% and not balanced between groups.
-
Selective outcome reporting: Did investigators report appropriate outcomes or were key outcomes missing? We graded this domain as having a low risk of bias if authors reported all pre‐specified outcomes. If they did not report prespecified or expected data, we assumed the risk of bias to be high.
-
Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias? These include information on the baseline characteristics of the intervention and control groups and the similarity in using co‐interventions between groups. We graded the trials as having a high risk of bias if there were important differences in demographic characteristics or caries risk level at baseline between the study groups, or if the groups received different co‐interventions during the trial.
We developed a standardised 'Risk of bias' assessment form, which included the criteria for assessing the above domains, and we entered data in the 'Risk of bias' tables in RevMan (RevMan 2011). We summarised the potential risk of bias for each study and grouped them into the following three categories, as described in Section 8.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Low risk of bias: plausible bias not likely to seriously alter the results (if low risk of bias for all items).
-
Unclear risk of bias: plausible bias that raises some doubt about the results (if unclear risk of bias for one or more key items).
-
High risk of bias: plausible bias that seriously weakens confidence in the results (if high risk of bias for one or more key items).
We completed a 'Risk of bias' table for each included study (Characteristics of included studies) and presented the results graphically by domain over all studies and by study.
Measures of treatment effect
According to different outcomes, we planned to convert data obtained from visual analogue scales and any categorical outcomes into dichotomous data prior to analysis. For continuous data, we calculated the effect estimate as the mean difference (MD) and reported it along with 95% confidence intervals (CIs). For dichotomous data, we calculated the effect estimate as the odds ratio (OR) and also reported it along with the 95% CI.
Unit of analysis issues
The unit of analysis for trials with cluster or body part (e.g. split‐mouth design) randomisation was each tooth surface. We anticipated that the majority of studies would be split‐mouth studies that included one or more pairs of tooth surfaces per individual, with the interventions randomly allocated to tooth surfaces within each pair. Strict multiple pairing of tooth surfaces within an individual means that the data are not independent and should be analysed as 'paired data' on an individual basis. However, we decided to analyse the pairs independently, as otherwise we would be excluding most of the trials and losing useful information from these studies (we are unaware of any widely used methods to correct and account for dependence of the tooth pairs when, for example, only marginals are reported). This meant that the confidence intervals would be slightly narrower than they should be, and we took this into consideration when we interpreted the results. We undertook a sensitivity analysis excluding studies where the number of pairs far exceeded the number of individuals.
Dealing with missing data
We contacted the study authors to clarify incompletely reported data related to trial characteristics, methodology and outcomes and to ascertain if data was missing at random or not. We planned, but did not perform, imputation of data not missing at random, since we assumed all missing data to be missing at random.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the characteristics of the studies: the similarity between the types of participants, the interventions and the outcomes as specified in the criteria for included studies. We assessed statistical heterogeneity using a Chi2 test and the I2 statistic, where I2 values over 50% indicate moderate to high heterogeneity (Higgins 2003).
Assessment of reporting biases
We assessed publication bias or small‐study effects graphically using a funnel plot (Egger 1997). A symmetrical plot indicates the absence of bias. An asymmetrical plot may indicate the presence of reporting bias. If there was asymmetry, we planned to further investigate the possible causes. Apart from publication bias, other sources of asymmetry in funnel plots include poor methodological quality leading to spuriously inflated effects in smaller studies, true heterogeneity and chance (Higgins 2011).
Data synthesis
We evaluated the efficacy of micro‐invasive treatments in different subgroups of interventions. Moreover, we synthesised studies according to the measure of radiographic progression used (i.e. digital subtraction radiography, pairwise reading, visual scoring). To calculate one effect estimate for all micro‐invasive interventions versus control interventions regardless of the measure used, we combined different measures, preferring more sensitive rather than less sensitive methods for studies where more than one measure was available (digital subtraction radiography over pairwise reading over scoring). We calculated the number needed to treat for an additional beneficial outcome (NNTB) for the overall pooled estimates.
For the split‐mouth studies, we calculated ORs for differences of paired tooth surfaces being carious or not, along with the appropriate standard errors and 95% CIs. To calculate the ORs, we used the Becker‐Balagtas method outlined in Curtin 2002 by means of R software version 2.13.1. We chose the Becker‐Balagtas method because in this review we also included studies that reported data only in marginal form (as parallel group studies, not as 2 x 2 cross‐classification for paired data) and this method facilitated data synthesis. We chose the intracluster correlation coefficient (ICC) in the studies with data only as marginals to be the conservative 0.05. In the studies with data presented as tooth pairs, we calculated the ICC from the data.
We conducted the meta‐analyses with RevMan 2011, using the generic inverse variance method with either the fixed‐effect or the random‐effects model. In meta‐analyses including two or three studies, we used the fixed‐effect model, and in meta‐analyses including four or more studies, we used the random‐effects model. For the sensitivity analyses, we evaluated the effect on the results of split‐mouth studies with high numbers of pairs compared to the number of subjects as well as the 'Risk of bias' grading. We were unable to meta‐analyse the data from split‐mouth studies with different numbers of lesions in each group.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses for the different types of sealing methods/materials (resin sealant, resin infiltration, sealant patch and GIC).
We had planned to perform subgroup analyses if the included trials had different designs (e.g. split‐mouth or parallel). However, all included studies used a split‐mouth design. Moreover, we had planned to analyse the effects of age (children under 16 or adults), setting (e.g. primary or secondary care), site (e.g. mesial or distal), tooth type (e.g. premolar or molar), operator (e.g. dentist or DCP) and moisture control (e.g. cotton rolls or rubber dam).
In a change to the protocol, we had also planned to undertake a subgroup analysis according to dentition (primary or permanent) and caries risk (low to moderate, moderate, mostly high or high).
Sensitivity analysis
As described, we had planned to compare the effect estimates in studies with overall low risk of bias compared to the effect estimate from studies at any level of overall risk of bias to assess the robustness of our review results.
Summary of findings table and quality of the evidence
In 'Summary of findings' tables, we present a summary of key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on all important outcomes for a given comparison. We used GRADEprofiler software version 3.2 (GRADEpro) to provide the overall grading of the quality of evidence for the caries outcomes according to recommendations outlined by the GRADE network (GRADE 2004). We performed the following comparison: proximal sealing versus placebo or non‐invasive measures (summary of findings Table for the main comparison).
Results
Description of studies
See: 'Characteristics of included studies'; 'Characteristics of excluded studies'; 'Characteristics of ongoing studies'.
Results of the search
We retrieved 3198 records from searches of the Cochrane Oral Health Group Trials Register (413), CENTRAL (625), MEDLINE (975), EMBASE (587), LILAC (28), Web of Sciences proceedings (543), ZETOC conference proceedings (3), Proquest dissertation and theses services (8), metaRegister of Controlled Trials (2), ClinicalTrials.gov (11), OpenGrey (0) and the WHO International Clinical Trials Registry Platform (3). We identified two further studies through communication with the experts in the field and by checking reference lists from identified trials and review articles (Figure 1).

Study flow diagram
After de‐duplication, we had 2051 records. We screened the title or abstract, or both, and on this basis, rejected 2012 records because they did not meet inclusion criteria. We identified two ongoing studies (see 'Characteristics of ongoing studies'). We obtained and evaluated 37 full‐text manuscripts. We did not identify any non‐English language reports as potentially eligible, and therefore, there were no full‐text reports that needed translation. Of the 37 full‐text articles, we excluded 28 and included 9 (8 studies). The main reasons for exclusion were that the study was a non‐randomised trial, a non‐clinical trial, or it did not include micro‐invasive interventions. We present the reason for the exclusion of each study in 'Characteristics of excluded studies'.
Included studies
We included eight trials in the review (see 'Characteristics of included studies'), all of them using split‐mouth design, some with more than one pair of lesions treated within the same participant. Studies took place in university dental clinics in Brazil, Denmark, Germany, and Thailand, as well as in public health dental clinics in Greenland and Chile. The follow‐up times ranged from one to three years.
The sample sizes in the included studies ranged from 7 to 91 participants, with both males and females evaluated. Participant age ranged from 4 to 39 years, and they had either only enamel or enamel‐dentine lesions extending into the outer third of the dentine. Treated patients had high caries risk in two studies; moderate or high risk in two studies; low, moderate or high risk in two studies; and unknown risk in two studies. Five of the studies assessed the caries risk using the Cariogram programme, which combines several caries‐related factors, including caries experience (number of decayed, missing and filled teeth/surfaces, or DMFT/DMFS); related caries diseases; diet (amount and frequency); plaque amount; fluoride intake; salivary flow rate and buffering capacity (Martignon 2006; Ekstrand 2010; Martignon 2010; Paris 2010a; Martignon 2012). Six studies evaluated the effects of micro‐invasive treatments in the permanent dentition (Gomez 2005; Martignon 2006; Paris 2010a; Alkilzy 2011; Trairatvorakul 2011; Martignon 2012), and two studies focused on the primary dentition (Ekstrand 2010; Martignon 2010). Different control conditions used in the studies included: flossing advice only (three studies); fluoride varnish only (two studies); oral hygiene instruction, flossing and fluoride toothpaste advice (one study); fluoride varnish and fluoride toothpaste advice (one study); fluoride varnish, oral hygiene instruction and diet advice (one study).
Four studies received industry support to carry out the research or had other conflicts of interests. Companies with a financial interest in the study results funded three trials (Ekstrand 2010; Alkilzy 2011; Martignon 2012). The authors of Paris 2010a are inventors of various patents for a resin infiltration technique for dental caries lesions, which is held by Charité‐Universitätsmedizin Berlin. The authors further stated that they receive royalties from the manufacturer of the infiltrant.
All trials used lesion progression as primary outcome and applied radiographic techniques to assess lesion progression. Investigators evaluated caries progression using conventional independent reading of radiographs in five studies (Martignon 2006; Ekstrand 2010; Martignon 2010; Paris 2010a; Trairatvorakul 2011); using pairwise reading of radiographs in three studies (Martignon 2006; Paris 2010a; Martignon 2012) and using digital subtraction radiography in three studies (Martignon 2006; Paris 2010a; Martignon 2012). Ekstrand 2010 additionally assessed lesion progression using ICDAS.
All studies compared micro‐invasive with non‐invasive treatments. Excepting Gomez, the trials also used the non‐invasive intervention in the intervention arm.
-
Three studies, randomising 212 tooth pairs in 212 participants, compared resin sealant plus flossing advice versus flossing advice alone in secondary care settings (Martignon 2006; Martignon 2010; Martignon 2012). The radiographic follow‐up periods were between one and three years.
-
One study, randomising 71 lesions in seven participants, compared resin sealant versus fluoride varnish (Gomez 2005). Trials followed up participants for two years with radiograph.
-
Three studies, randomising 116 tooth pairs in 109 participants evaluated resin infiltration. Ekstrand 2010 compared resin infiltration plus 2.26% sodium fluoride varnish versus 2.26% sodium fluoride varnish alone (48 lesion pairs in 48 children), and Paris 2010a compared resin infiltration plus flossing advice versus sham infiltration plus flossing advice (22 participants and 29 lesions). The study by Martignon 2012 had a third arm for resin infiltration plus flossing advice (39 lesions randomised in 39 participants). The trials followed up participants with radiograph for one to three years. Ekstrand 2010 also followed up participants clinically for one year.
-
One study, randomising 41 tooth pairs in 26 participants, compared glass ionomer sealant plus 12300 ppm fluoride gel plus 1000 ppm dentifrice versus 12300 ppm fluoride gel plus 1000 ppm dentifrice (Trairatvorakul 2011). Radiographic follow‐up was 6 to 12 months.
-
One study, randomising 50 tooth pairs in 50 participants, compared adhesive patch versus no treatment (Alkilzy 2011). All participants recieved advice to use dental floss and a fluoridated toothpaste. Clinical follow‐up was 6 to 12 months, and radiographic follow‐up was two to three years.
We included Gomez 2005 in the review as it meets the eligibility criteria, but we could not include it in the meta‐analysis as the data were not presented in the correct format, and there was a very high ratio of tooth pairs to children.
Excluded studies
We excluded 28 studies after full‐text screening, for the following reasons: 18 studies did not use micro‐invasive treatments as intervention, 7 studies were non‐clinical, 2 studies did not use a randomised controlled design, and 1 study did not include proximal lesions. The 'Characteristics of excluded studies' table presents the reasons for the exclusion of each study.
Risk of bias in included studies
Allocation
All the included studies reported adequate methods for random sequence generation and we therefore assessed them as being at low risk of bias.
We graded allocation concealment as having a low risk' of bias in three studies (Ekstrand 2010; Paris 2010a; Trairatvorakul 2011). The remaining five studies did not provide sufficient information to judge allocation concealment, so we graded them as having an unclear risk of bias.
Blinding
One study reported blinding participants (sham infiltration), but we assessed the risk of bias for this domain as unclear because operators were not blinded, and it was unclear how this might affect outcomes (Paris 2010a). Ekstrand 2010 assumed that the participating children and their parents were blind to which treatment they were receiving, as the children were young and the parents were at some distance from the dental chair during the treatment. We did not consider this adequate and judged the study to have a high risk of bias in this domain. Martignon 2012 did not blind operators, and it was unclear if participants would have been blinded. We decided to assess this study as at high risk of bias. The remaining five studies did not state they were blinded, and as we considered this possibility unlikely, we also assessed them as being at high risk of bias.
We considered the blinding of clinical assessors to be inadequate for the two studies reporting clinical lesion progression. Investigators reported radiographic assessment of lesion progression to be performed blind, and we therefore rated them as having a low risk of bias. Given that we considered radiographic outcome assessment to be more relevant than clinical assessment, we eventually graded all studies as having a low risk in this domain.
Incomplete outcome data
We judged six studies with low (less than 25%) attrition to have a low risk of attrition bias (Gomez 2005; Martignon 2006; Ekstrand 2010; Paris 2010a; Martignon 2012; Trairatvorakul 2011). Two studies had an unclear risk of bias, since attrition was more than 25%, but it was unclear if this affected overall risk of bias given that the studies used a split‐mouth design (Martignon 2010; Alkilzy 2011).
Selective reporting
Six studies reported all pre‐specified outcomes adequately, so we assessed them as having a low risk of selective reporting bias (Gomez 2005; Martignon 2006; Martignon 2010; Paris 2010a; Alkilzy 2011; Trairatvorakul 2011). Two studies were at high risk of bias for this domain: Ekstrand 2010 did not report the outcomes of the six‐month clinical evaluation, and the radiographic evaluation report was incomplete; Martignon 2012 reported the findings of the digital subtraction radiographic evaluation only after one year, not after the complete follow‐up.
Other potential sources of bias
Gomez 2005 did not provide information on the distribution of different lesion depths among study groups, and there was unbalanced allocation of lesions according to ICDAS scores in Ekstrand 2010. We therefore judged both studies to be at high risk for other sources of bias.
We only judged one study to be at overall unclear risk of bias (Paris 2010a); we considered all other studies to be at overall high risk of bias (Figure 2; Figure 3).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
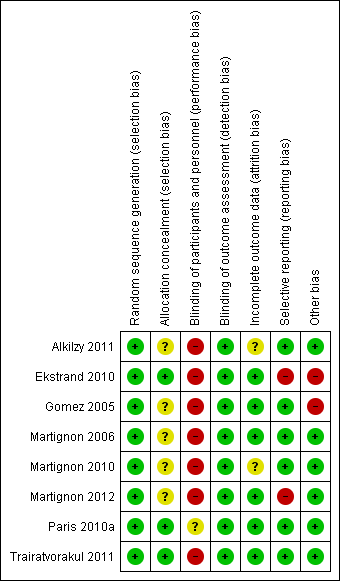
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Effects of interventions
We assessed intervention effects as overall therapies (proximal micro‐invasive treatment) and subgrouped according to different methods (resin sealant, resin infiltration, glass ionomer sealant and sealant patch). We performed separate meta‐analyses for caries progression as assessed by each of three different methods of radiographic readings used in the included studies as well as a meta‐analysis using the most sensitive outcome available (DSR > pairwise > scoring) from each study.
Micro‐invasive treatment versus non‐invasive control/placebo for managing dental decay in primary and permanent teeth
Primary outcomes
Caries progression
The clinical and radiographic follow‐up times in the included studies ranged from 6 to 12 months, and 6 months to 3 years, respectively. We evaluated lesion progression at the most sensitive level of measurement presented in each of the seven studies reporting data in a format suitable for meta‐analysis (summary of findings Table for the main comparison). Our meta‐analysis showed that micro‐invasive treatment significantly reduced the odds of lesion progression compared with non‐invasive or no treatment (OR 0.24, 95% CI 0.14 to 0.41; 602 lesions; seven studies; I2 = 32%; Analysis 1.1; Figure 4). We also analysed different radiographic measures separately, finding that micro‐invasive treatment was effective where caries progression was measured using radiographic scoring (OR 0.27, 95% CI 0.17 to 0.44; 468 lesions; five studies; I2 = 0%; Analysis 1.2; Figure 5), pairwise reading (OR 0.31, 95% CI 0.18 to 0.52; 330 lesions; four studies; I2 = 3%; Analysis 1.3; Figure 6) and DSR (OR 0.18, 95% CI 0.06 to 0.50; 270 lesions; three studies; I2 = 60%; Analysis 1.4; Figure 7). Ekstrand 2010 reported clinical results of caries progression, finding that resin infiltration plus varnish significantly reduced the odds of lesion progression compared with varnish alone (OR 0.22, 95% CI 0.09 to 0.55; 84 lesions).

Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.1 Caries progression follow‐up 12 to 36 months ‐ DSR>Pairwise>Scoring

Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.2 Caries progression follow‐up 12 to 30 months ‐ Scoring

Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.2 Pairwise
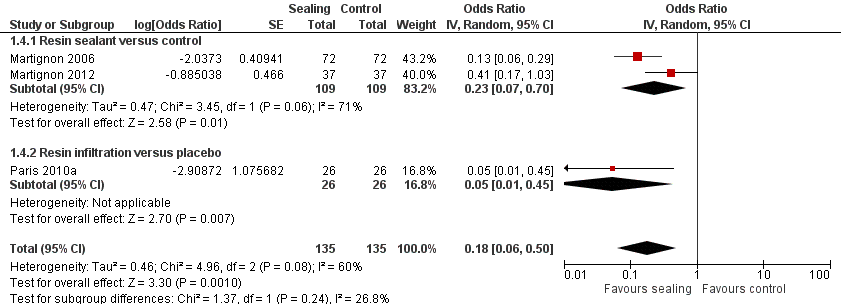
Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.4 Caries progression follow‐up 12 to 18 months ‐ Digital Substraction Radiography.
We were unable to fully include two studies in the meta‐analysis (Gomez 2005; Martignon 2012). Gomez 2005 compared resin infiltration with fluoride varnish but inappropriately reported the data, and due to the substantial clustering of lesions within participants, we were unable to re‐analyse the data. Martignon 2012 included a third trial arm for resin infiltration. We were unable to include the results of this trial arm in the meta‐analysis due to problems arising from double counting of the control group. However, the results indicated that micro‐invasive treatment significantly reduced the odds of lesion progression compared with non‐invasive measures as assessed by both pairwise reading of radiographs (OR 0.20, 95% CI 0.08 to 0.53; 64 lesions) and digital subtraction radiography (OR 0.20, 95% CI 0.08 to 0.53; 64 lesions).
We calculated the NNTB as 7 (scoring), 4 (pairwise reading) and 3 (DSR), depending on the detection measure. NNTB was 5 when pooling all detection measures.
Change in decayed, missing and filled (DMF/dmf) figures at surface, tooth and whole mouth level
No studies measured this outcome.
Secondary outcomes
No studies reported re‐application of sealant patch or re‐infiltration on follow‐up appointments.
One study comparing proximal sealant patch versus non‐invasive control reported on material deficiency (Alkilzy 2011). Dentists different from operators assessed retention and adaptation of patches according to modified Ryge criteria. Clinically, 10% and 23% of proximal sealants had total and partial loss, respectively, after three years. Also, 53% of the proximal sealants placed had maintained perfect margins, and none was reported to have open margins, after three years.
None of the studies reported on participant or operator perception or costs for micro‐invasive treatment or non‐invasive control were reported.
No adverse events for micro‐invasive treatment or non‐invasive control were reported.
Subgroup analysis
We ran subgroup analyses based on the different interventions for the outcome 'caries progression'. We present results separately according to the method of radiographic measurement.
-
Micro‐invasive treatments, as assessed by independent scoring of radiographs, were effective in reducing the odds of lesion progression with resin sealant versus control (OR 0.33, 95% CI 0.18 to 0.59; 256 lesions; two studies) and resin infiltration versus control or placebo (OR 0.19, 95% CI 0.08 to 0.46; 130 lesions; two studies). However, there was no significant difference of the odds of lesion progression between glass ionomer sealant and control (OR 0.13, 95% CI 0.01 to 2.52; 82 lesions; one study). There was no statistically significant difference according to subgroup (test for subgroup differences: Chi2 = 1.20, degrees of freedom (df) = 2; P = 0.55; Figure 5).
-
Micro‐invasive treatments, as assessed by pairwise visual scoring of radiographs, were effective in reducing the odds of lesion progression with resin sealant versus control (OR 0.31, 95% CI 0.18 to 0.54; 218 lesions; two studies) and resin infiltration versus placebo (OR 0.08, 95% CI 0.01 to 0.63; 52 lesions; one study). However, there was no significant difference of the odds of lesion progression in sealant patch versus control (OR 1.00, 95% CI 0.14 to 7.23; 60 lesions; one study). There was also no statistically significant difference according to subgroup (test for subgroup differences: Chi2 = 3.05, df = 2; P = 0.22; Figure 6).
-
Micro‐invasive treatments, as assessed by digital subtraction radiography, were effective in reducing the odds of lesion progression with resin sealant versus control (OR 0.23, 95% CI 0.07 to 0.70; 218 lesions; two studies) and resin infiltration versus placebo (OR 0.05, 95% CI 0.01 to 0.45; 52 lesions; one study). There was no statistically significant difference according to subgroup (test for subgroup differences: Chi2 = 1.37, df = 1; P = 0.24; Figure 7).
Treated participants varied according to their age, caries risk and the dentition evaluated (primary or permanent teeth treated).
We had planned to perform subgroup analyses as outlined in our Methods section (Subgroup analysis and investigation of heterogeneity); however, as there were a limited number of included studies and a lack of the information in the included trials, we did not perform these subgroup analyses.
Sensitivity analysis
We had planned to compare the effect estimate from studies judged as having an overall low risk of bias to the effect estimate from studies at any level of overall risk of bias to assess the robustness of our review results. However, seven of the eight included studies were at overall high risk of bias, so a sensitivity analysis was not possible.
Discussion
Summary of main results
Overall, this review found that micro‐invasive treatments of proximal caries lesions significantly reduced the risks of lesion progression compared with non‐invasive professional or home care. No studies compared micro‐invasive with invasive treatment. Only four studies indicated measuring adverse events after micro‐invasive treatments. No adverse events were reported after micro‐invasive treatments, which suggests they are not detrimental for gingival or subjective health; however, the limited duration of follow‐up (one to three years) in most studies limits the chance of detecting possible adverse events, especially given the relatively small number of included participants and lesions. Overall, we graded the evidence for micro‐invasive versus non‐invasive interventions as being of moderate quality, which reflects the risks of bias, the potential impact of sponsorship bias, and the limited follow‐up and number of events, while also taking into account the large size of the effect and precision of the estimate.
Our synthesis tended to find resin infiltration slightly more efficacious than the application of resin sealant. Resin sealant patches did not appear to be more efficacious than control interventions, but only one study reported on this therapy (Alkilzy 2011). Glass ionomer sealant seemed to have an efficacy similar to that of infiltration, but only one study reported on that method (Trairatvorakul 2011). Given that only one study directly compared resin sealant versus resin infiltration, it remains unclear as to which technique is more advantageous (Martignon 2012). Network meta‐analysis might be an option to further explore this issue. However, given that control groups differed regarding the non‐invasive treatment used (e.g. professional application of fluoride varnish, flossing advice, standard oral home care), one cannot necessarily assume transitivity, which might limit such analyses.
The study participants who received micro‐invasive treatments differed in terms of age groups, settings and caries risk. Moreover, the treated lesions differed in their depth, which might affect the risk of lesion progression after both test and control treatment. Included studies were not amenable to subgroup or meta‐regression analyses that could explore if such factors confound the efficacy of micro‐invasive treatment, as they were limited in number and reported insufficient data on confounders. Further studies should explore these issues to identify the clinical conditions under which proximal micro‐invasive treatments should be performed or avoided.
Overall completeness and applicability of evidence
As discussed, reporting on confounders was limited in the included studies, so our review could not identify a differential efficacy of micro‐invasive treatments in groups with different caries risk or for lesions of different depths. Four studies reported including only active lesions, and other studies did not indicate the state of the included lesions. Active caries lesions were detected by either gingival inflammations of adjacent papilla or plaque stagnation. Experienced dental examiners performed activity assessment. It therefore remains unclear if less effective activity assessment and the resulting treatment of non‐active lesions would affect the efficacy of micro‐invasive treatments. A lack of power in the performed subgroup comparison, stemming from the low number of studies, might cause the detected indifference of treatment effects in different dentitions, and future studies might be useful to evaluate the influence of participants' age or dentition.
Similarly, the included studies did not allow conclusions regarding which sealant material (resin or GIC sealants) or micro‐invasive technique (sealant or infiltration) is most suitable for treating proximal caries. Given the different retention of sealant materials, combined with potentially different antibacterial or remineralising effects of GIC and resin sealants (Mickenautsch 2013), further well‐designed trials might be needed to elucidate this issue. Similarly, the effects of varying losses of enamel due to the use of different acids when conditioning tooth surfaces for sealant versus infiltration, as well as the potential different retention losses when using these two techniques, merit further investigation.
The discussed duration and sample size of most studies limit their external validity, as both patients and dentists would be interested in long‐term efficacy of lesion arrest. Similarly, the efficacy of micro‐invasive treatments was compared only with non‐invasive treatment, whilst the standard for treating proximal lesions (even those confined to enamel) in many dental practices might be invasive therapy. Therefore, pragmatic practice‐based trials should compare the long‐term effects of performing different interventions for proximal carious lesions. Moreover, such trials should consider different outcomes. For example, instead of using lesion progression as an outcome parameter with limited direct impact on the patient, studies should additionally analyse parameters that might be relevant to patients (e.g. pain, preference, costs), dentists (e.g. applicability, technical feasibility, time required for treatment) and other decision‐makers (e.g. costs, equity effects, adherence‐dependency). This might eventually also shed light on which technique (sealant, patch, infiltration) is most suitable in a real‐life setting. Furthermore, such trials should take place not only in realistic primary care settings, but on patients with different risk profiles, as discussed. To decrease the risk of industry bias, public non‐industrial funding and independent data analysis might be advisable.
If assessing radiographic lesion progression, trials should aim at standardising the outcome measure used (i.e. scoring, pairwise, DSR) to facilitate combining trials for quantitative synthesis. To decide between outcome measures, investigators should explore and compare the relevance of each method's sensitivity, specificity and reliability. A further aspect to consider when choosing an outcome measure in such clinical trials might be its applicability in a non‐trial primary care setting. In this sense, future trials should also aim at reflecting the diagnostic challenges associated with leaving and sealing radiographically detectable lesions (Schwendicke 2014b).
Quality of the evidence
We graded evidence to account for study design, risk of bias, consistency, directness of comparisons, precision, presence of publication bias and magnitude of effect estimate. All studies were randomised controlled trials, but we downgraded their quality due to the high risk of bias associated with insufficient blinding of participants, personnel or both. Moreover, the low number of events, (i.e. the limited quantity of data) led to additional downgrading for imprecision of the effect estimate. It is important to highlight that several trials were prone to bias by industry sponsoring, but we did not downgrade them further for this given that we already rated the overall risk of bias to be high. Rather, we upgraded the evidence, as effect estimates all showed a large effect (OR < 0.5). We graded the overall quality of the evidence as moderate for the comparison of interest: proximal sealing compared with no treatment or placebo. This was consistent over all three different methods of caries progression measurement (visual scoring, pairwise and digital subtraction radiography), as well as for the most sensitive caries progression outcome measurement.
Although we judged the overall risk of bias for the included studies as high, the risk of bias was due to performance bias (seven studies), reporting bias (two studies) and other bias (two studies). Several trials did not report allocation concealment, and presumably none of the trials blinded operators. Indeed, this is very difficult as interventions are clearly distinguishable, with operators possibly being able to identify test and control intervention even if they perform mock‐up sealing or infiltration. Only two trials aimed to blind participants. We found examiner blinding during radiographic assessment to be sufficient in most trials. Blinding of examiners when assessing clinical outcomes might be feasible for infiltration, but it remains unclear if sealants and patches are reliably detectable via clinical (visual‐tactile) means, which would generally impede blinding of clinical outcomes.
Another point of note stemmed from bias caused by industry sponsorship and professional interest. All trials studying resin infiltration received some or all of their funding from the patent‐holding company for this technique, and the inventors of the technique themselves performed one trial. For trials using resin sealant or patches, we assumed (or at least did not exclude) industry bias due to insufficient information in all but two cases. As discussed, there is a need for pragmatic, practice‐based, blinded, independently assessed and reported trials with external, non‐industry funding to overcome this limitation.
Lastly, the small numbers of trials and the short follow‐up period limit the quality of evidence and its external validity. For rare adverse events or relevant late endpoints in cariology trials (e.g. the need to restore or to endodontically intervene, or tooth loss), it is necessary to run longer trials with a larger sample size. Moreover, to evaluate if the existing evidence allows firm conclusions, additional analyses might be applicable (Wetterslev 2008).
Potential biases in the review process
We conducted this review according to guidelines established by Cochrane. However, we cannot exclude certain biases within the review process. Inclusion of only randomised trials certainly narrows the available evidence, especially with regard to outcomes other than efficacy (e.g. applicability, technical feasibility, patients' preferences, costs). However, it increases the overall quality of studies and their internal validity, as lesions have been randomly allocated to different treatment arms. The assessment of risk of industry bias did not follow any guidelines, since none have been published. However, we felt that including this information in the 'Characteristics of included studies' tables was required to reflect the potential influences of industry sponsorship, especially when considering that other means (e.g. standard 'Risk of bias' assessment, funnel plot analyses) do not seem to be able to detect these risks (Lundh 2012).
Agreements and disagreements with other studies or reviews
This review included all available randomised trials regarding micro‐invasive treatment of proximal caries lesions. Our results agree with non‐ or pre‐clinical studies, which found both sealing and infiltration efficacious for arrest of carious lesions in vitro, ex vivo or in situ (Paris 2010b; Meyer‐Lueckel 2012). Moreover, our results confirm that sealing can arrest caries lesions, as reported for occlusal or pitted surfaces (Griffin 2008; Oong 2008).
The lack of data regarding the retention of sealants is worrisome, since other reviews found occlusal sealants—with presumably higher mechanical retention, but also higher masticatory forces and wear—to be prone to loss. However, the same research did not find loss of sealants to necessarily lead to re‐activation of the formerly sealed lesion (Mickenautsch 2013). Given that loss of sealant is only possible for resin sealants or patches, not infiltration, further research should investigate this issue.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.1 Caries progression follow‐up 12 to 36 months ‐ DSR>Pairwise>Scoring

Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.2 Caries progression follow‐up 12 to 30 months ‐ Scoring

Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.2 Pairwise

Forest plot of comparison: 1 Proximal sealing versus control/placebo, outcome: 1.4 Caries progression follow‐up 12 to 18 months ‐ Digital Substraction Radiography.

Comparison 1 Proximal sealing versus control/placebo, Outcome 1 Caries progression follow‐up 12 to 36 months ‐ DSR>Pairwise>Scoring.

Comparison 1 Proximal sealing versus control/placebo, Outcome 2 Caries progression follow‐up 12 to 30 months ‐ Scoring.
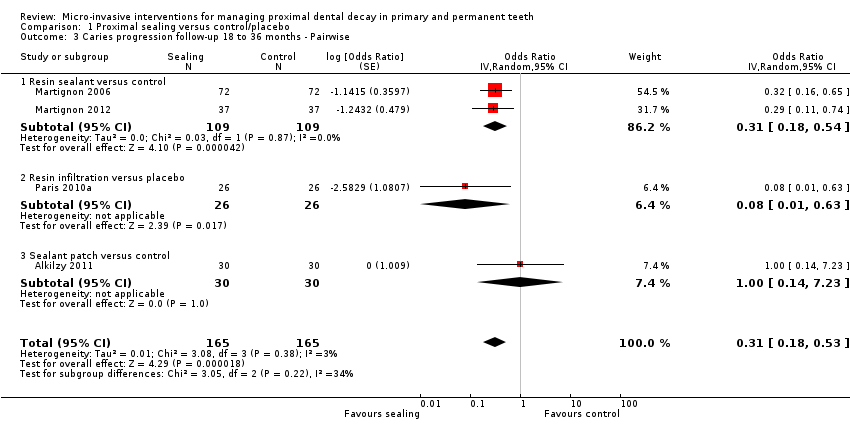
Comparison 1 Proximal sealing versus control/placebo, Outcome 3 Caries progression follow‐up 18 to 36 months ‐ Pairwise.

Comparison 1 Proximal sealing versus control/placebo, Outcome 4 Caries progression follow‐up 12 to 18 months ‐ Digital Substraction Radiography.
| Micro‐invasive versus non‐invasive treatments for managing dental decay in primary and permanent teeth | ||||||
| Patient or population: people with dental decay on proximal surfaces of primary and permanent teeth Comparison: non‐invasive treatments (e.g. fluoride varnish, advice to floss) Radiographic follow‐up period: 6 months to 3 years | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Odds Ratio | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Sealing | |||||
| Caries progression measured by DSR > pairwise > visual scoring (12 months to 36 months follow‐up) | Study population | OR 0.24 | 602 (7 RCTs) | ⊕⊕⊕⊝ | The quality of evidence for caries progression measured by scoring (12 to 30 months), including 468 participants (5 RCTs), OR 0.27 (95% CI 0.17 to 0.44), was moderatea,b,c. The quality of evidence for caries progression measured by pairwise (18 to 36 months), including 330 participants (4 RCTs), OR 0.31 (95% CI 0.18 to 0.53), was moderatea,b,c. The quality of evidence for caries progression measured by digital substraction radiography (12 months to 18 months), including 270 participants (3 RCTs), OR 0.18 (95% CI 0.06 to 0.50), was moderatea,b,c. | |
| 547 per 1000 | 284 per 1000 | |||||
| Moderate | ||||||
| 649 per 1000 | 337 per 1000 | |||||
| Change in decayed, missing and filled (DMF/dmf) figures at surface, tooth and whole mouth level. | — | — | — | — | — | No studies reported on caries measured as change in decayed, missing and filled (DMF/dmf) figures at surface, tooth or whole mouth level |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOne or more studies lacked sufficient blinding of participants, personnel or both. Downgraded one level. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries progression follow‐up 12 to 36 months ‐ DSR>Pairwise>Scoring Show forest plot | 7 | 602 | Odds Ratio (Random, 95% CI) | 0.24 [0.14, 0.41] |
| 1.1 Resin sealant versus control | 3 | 330 | Odds Ratio (Random, 95% CI) | 0.26 [0.13, 0.53] |
| 1.2 Resin infiltration versus control/placebo | 2 | 130 | Odds Ratio (Random, 95% CI) | 0.15 [0.06, 0.39] |
| 1.3 Glass ionomer sealant versus control | 1 | 82 | Odds Ratio (Random, 95% CI) | 0.13 [0.01, 2.51] |
| 1.4 Sealant patch versus control | 1 | 60 | Odds Ratio (Random, 95% CI) | 1.0 [0.14, 7.22] |
| 2 Caries progression follow‐up 12 to 30 months ‐ Scoring Show forest plot | 5 | 468 | Odds Ratio (Random, 95% CI) | 0.27 [0.17, 0.44] |
| 2.1 Resin sealant versus control | 2 | 256 | Odds Ratio (Random, 95% CI) | 0.33 [0.18, 0.59] |
| 2.2 Resin infiltration versus control/placebo | 2 | 130 | Odds Ratio (Random, 95% CI) | 0.19 [0.08, 0.46] |
| 2.3 Glass ionomer sealant versus control | 1 | 82 | Odds Ratio (Random, 95% CI) | 0.13 [0.01, 2.52] |
| 3 Caries progression follow‐up 18 to 36 months ‐ Pairwise Show forest plot | 4 | 330 | Odds Ratio (Random, 95% CI) | 0.31 [0.18, 0.53] |
| 3.1 Resin sealant versus control | 2 | 218 | Odds Ratio (Random, 95% CI) | 0.31 [0.18, 0.54] |
| 3.2 Resin infiltration versus placebo | 1 | 52 | Odds Ratio (Random, 95% CI) | 0.08 [0.01, 0.63] |
| 3.3 Sealant patch versus control | 1 | 60 | Odds Ratio (Random, 95% CI) | 1.0 [0.14, 7.23] |
| 4 Caries progression follow‐up 12 to 18 months ‐ Digital Substraction Radiography Show forest plot | 3 | 270 | Odds Ratio (Random, 95% CI) | 0.18 [0.06, 0.50] |
| 4.1 Resin sealant versus control | 2 | 218 | Odds Ratio (Random, 95% CI) | 0.23 [0.07, 0.70] |
| 4.2 Resin infiltration versus placebo | 1 | 52 | Odds Ratio (Random, 95% CI) | 0.05 [0.01, 0.45] |

