Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy
Abstract
Background
Pregnant women with sickle cell disease (HbSS, HbSC and HbSβThal) may require blood transfusion to prevent severe anaemia or to manage potential medical complications. Preventive blood transfusion in the absence of complications starting from the early weeks of pregnancy or blood transfusion only for medical or obstetric indications have been used as management policies. There is currently no consensus on the blood transfusion policy that guarantees optimal clinical benefits with minimal risks for such women and their babies. The present review replaces and updates a Cochrane review that was withdrawn in 2006.
Objectives
To assess the benefits and harms of a policy of prophylactic versus selective blood transfusion in pregnant women with sickle cell disease.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 October 2013) and reference lists of retrieved studies. We did not apply any language restrictions.
Selection criteria
Randomised and quasi‐randomised trials evaluating the effects of prophylactic versus selective (emergency) blood transfusion in pregnant women with sickle cell disease. Trials were considered for inclusion whether the unit of randomisation was at individual or cluster level, however, no cluster‐randomised trials were identified.
Data collection and analysis
Two review authors independently assessed trials for inclusion and assessed trial quality. Two review authors independently extracted data. Data were checked for accuracy.
Main results
Out of six relevant reports identified by the search strategy, two trials involving 98 women with sickle cell anaemia (HbSS) met our inclusion criteria. The two trials were at moderate risk of bias. Overall, there were few events for most of the reported outcomes and the results were generally imprecise. One trial (involving 72 women) reported no maternal mortality occurring in women who received either prophylactic or selective blood transfusion. The same trial (involving 72 women) indicated no clear differences in maternal mortality, perinatal mortality (risk ratio (RR) 2.85, 95% confidence interval (CI) 0.61 to 13.22) or markers of severe maternal morbidity [pulmonary embolism (no events); congestive cardiac failure (RR 1.00, 95% CI 0.07 to 15.38); acute chest syndrome (RR 0.67, 95% CI 0.12 to 3.75)] between the treatment groups (prophylactic blood transfusion versus selective blood transfusion). Prophylactic blood transfusion reduced the risk of pain crisis compared with selective blood transfusion (RR 0.42, 95% CI 0.17 to 0.99, two trials, 98 women); however, the margin of uncertainty around the effect estimate ranged from very small to substantial reduction. One trial (involving 72 women) indicated no differences in the occurrence of acute splenic sequestration (RR 0.33, 95% CI 0.01 to 7.92) and haemolytic crises (RR 0.33, 95% CI 0.04 to 3.06) and delayed blood transfusion reaction (RR 2.00, 95% CI 0.54 to 7.39) between the comparison groups.
Authors' conclusions
Evidence from two small trials of low quality suggests that prophylactic blood transfusion to pregnant women with sickle cell anaemia (HbSS) confers no clear clinical benefits when compared with selective transfusion. Currently, there is no evidence from randomised or quasi‐randomised trials to provide reliable advice on the optimal blood transfusion policy for women with other variants of sickle cell disease (i.e. HbSC and HbSβThal). The available data and quality of evidence on this subject are insufficient to advocate for a change in existing clinical practice and policy.
PICO
Plain language summary
Blood transfusion policies for sickle cell disease in pregnancy
Sickle cell disease is an inherited disorder of haemoglobin, the protein in red blood cells that carries oxygen. In this condition, an abnormal haemoglobin S from one parent is combined with another abnormal haemoglobin from the other parent. Haemoglobin S inherited from both parents (genotype HbSS), described as sickle cell anaemia is the most common form.
When oxygen tension is low, haemoglobin S crystallizes and makes the red blood cells sickle shaped. Sickling reduces red blood cell capacity to manoeuvre through very small blood vessels causing vascular blockage and early destruction of red cells. The breakdown of red blood cells and massive pooling of damaged red blood cells in the liver and spleen cause anaemia. Acute illnesses include painful crises, pulmonary embolism, acute chest syndrome and congestive cardiac failure. Therefore, pregnant women with sickle cell disease require careful management.
Depending on the institutional policy, blood transfusion can be given at intervals to a pregnant HbSS woman with relatively few or no symptoms to improve the oxygen carrying capacity of blood by increasing haemoglobin blood concentration and lowering haemoglobin S levels; or only when indicated by the development of medical or pregnancy complications. Giving blood at frequent intervals carries the risks of blood‐borne infections and excessive levels of iron.
This review set out to determine whether giving blood at intervals before serious complications occur compared with giving blood only when medically indicated makes a difference to the health of the mother and her baby. The review authors included two controlled trials that randomised 98 women with sickle cell anaemia (haemoglobin SS) before 28 weeks of gestation to one of the two blood transfusion policies. The two trials were of low quality. One trial (72 women) indicated no difference in severe ill health and death of the mother or newborn. There was no difference in the risk of delayed blood transfusion reaction. The two trials suggested giving blood at frequent intervals marginally reduced the risk of pain crisis, with a large degree of uncertainty about the size of the effect, compared with giving blood only when medically indicated. Blood transfusion was delivered at a ratio of four or five to one for prophylactic versus selective blood transfusion, respectively.
Overall, available evidence on this subject is insufficient to advocate for a change in clinical practice and policy.
Authors' conclusions
Summary of findings
| Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | ||||||
| Patient or population: patients with sickle cell disease in pregnancy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic versus selective blood transfusion | |||||
| Maternal death | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | 0 participants died in this study. |
| Severe maternal morbidity (pulmonary embolism) | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | 0 participants experienced pulmonary embolism in this study. |
| Severe maternal morbidity (congestive cardiac failure) | 28 per 1000 | 28 per 1000 | RR 1 | 72 | ⊕⊝⊝⊝ | |
| Perinatal death | 54 per 1000 | 154 per 1000 | RR 2.85 | 76 | ⊕⊝⊝⊝ | |
| Sickle cell crisis (pain crisis) | Study population | RR 0.42 | 98 | ⊕⊕⊝⊝ | ||
| 490 per 1000 | 206 per 1000 | |||||
| Moderate | ||||||
| 481 per 1000 | 202 per 1000 | |||||
| Sickle cell crises (acute splenic sequestration) | 28 per 1000 | 9 per 1000 | RR 0.33 | 72 | ⊕⊝⊝⊝ | |
| Blood transfusion reaction | 83 per 1000 | 167 per 1000 | RR 2 | 72 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study at moderate risk of bias due to unclear methods of random sequence generation and allocation concealment and use of unblinded interventions. * Little variation in baseline risks. | ||||||
Background
Sickle cell disease (SCD) is an inherited disorder of haemoglobin S synthesis. The genetic defects of haemoglobin (Hb) are the most common genetic disorders worldwide and homozygous sickle cell anaemia (HbSS) is the most frequent (Hoffbrand 2011).
Description of the condition
The inherited disorders of haemoglobin S are either in the homozygous form (HbSS) or in combination with another Hb variant such as haemoglobin C (HbSC), D (HbSD) or β‐thalassaemia (HbSβo‐thalassaemia and HbSβ+‐thalassaemia). The homozygous form, HbSS (sickle cell anaemia), is the most common followed by HbSC and the HbSβ‐thalassaemias (Hoffbrand 2011).
SCD is a relatively prevalent condition in Africa, the Mediterranean and the Caribbean; about 10% of the Jamaican population is estimated to carry HbS. However, the disease prevalence now has a more global outlook as a result of immigration (RCOG 2011). Population estimates in the United States of America indicated that 72,000 to 98,000 people have SCD (Hassel 2010). Similarly, the United Kingdom currently has the largest population of people with SCD in Europe, a population that increased from approximately 5000 in 1995 (Howard 1995) to about 12,000 to 15,000 in 2011 (RCOG 2011).
Pregnancy in women with SCD carries significant risks of maternal and perinatal morbidity and mortality, particularly in resource‐poor settings where facilities are lacking to adequately manage associated complications (Afolabi 2009; Odum 2002). Complications that may arise include various forms of crises resulting from haemolysis, vascular occlusion and sequestration (massive pooling) of damaged red blood cells in the liver and spleen, all having the potential to cause profound anaemia (Hoffbrand 2011). Another recognised complication, and an important cause of death in women with SCD, particularly in women with sickle cell anaemia is acute chest syndrome (ACS), which is characterised by cough, chest pain, dyspnoea, fever, increasing anaemia and fluid infiltrate on chest X‐ray, all resulting from the sickling of red cells in the lungs. ACS is the most common cause of death in patients with SCD after puberty (Hoffbrand 2011), and it occurs in 7% to 20% of pregnant women with SCD (RCOG 2011). While all these complications are not specific to pregnancy in women with SCD, they are more frequent and exacerbated during pregnancy and are all major causes of severe ill health and death among women with the condition. Although the complications could arise in all forms of SCD, they are more frequent and severe among those with sickle cell anaemia (HbSS) (Nomura 2009; Odum 2002; RCOG 2011).
Pregnancy‐specific complications include increased risk of spontaneous abortion (Serjeant 2004), urinary tract infection (Howard 1995), pre‐eclampsia and thromboembolism (RCOG 2011). Pregnant women with SCD also have an increased risk of preterm birth, repeated antepartum hospitalisations, placenta abruption, induction of labour, caesarean section and postpartum sepsis (ACOG 2007; Asnani 2011; Barfield 2010). As a result of chronic anaemia, women with SCD are also more likely to require blood transfusions (Grossetti 2009) and subsequent alloimmunisation of red cells which also increases the risk of haemolytic disease of the newborn. Fetal complications also include intrauterine growth restriction, prematurity and its sequelae, low birthweight and death (ACOG 2007; Barfield 2010; Howard 1995).
Painful crisis secondary to vascular occlusion is the most frequent manifestation of SCD, which is often precipitated by conditions such as infection, stress, acidosis, dehydration and hypoxia (low oxygenation state) (Hoffbrand 2011). Furthermore, visceral sequestration crises as a result of pooling of blood within the reticuloendothelial system (liver and spleen), as well as haemolytic and aplastic crises, are all associated with worsening anaemia (Hoffbrand 2011) that often have to be corrected to avert severe morbidity and mortality.
In spite of these complications, successful pregnancy outcomes have been reported in up to 57% of women with HbSS and 85% of women with HbSC (Asnani 2011; Serjeant 2004). These outcomes have been achieved with interventions to improve the complications occurring in these women. Measures to improve pregnancy outcomes have included the use of analgesia, intravenous fluid replacement, antibiotics and packed red cell transfusion for treatment of vaso‐occlusive complications (ACOG 2007; RCOG 2011). However, there is no compelling evidence from randomised trials that regimens comprising the various combination of these interventions actually improve pregnancy outcomes (Marti‐Carvajal 2009).
Description of the intervention
Pregnancy in women with SCD requires management by a multi‐disciplinary team of haematologists, obstetricians and anaesthetists to reduce the risk of likely complications. Although longitudinal follow‐up studies of women with HbSC have reported a relatively benign course of pregnancy (Serjeant 2005), complications occurred in 96.6% of pregnant women with HbSS (Odum 2002). While pregnant women with HbSβ+‐thalassaemia share similarly mild clinical behaviours with those with HbSC, the clinical course of pregnancy in HbSβo‐thalassaemia is quite similar to women with sickle cell anaemia (Hoffbrand 2011).
Blood transfusion for women with SCD during pregnancy could either be selective or prophylactic. Selective blood transfusion is performed when conditions such as anaemia, pain crises, or ACS necessitates blood transfusion. Other indications for selective blood transfusion are malaria infection and sepsis as these may lead to haemolysis of red cells. On the other hand, prophylactic blood transfusion is performed with the aim of optimising the oxygen‐carrying capacity of the blood and reducing complications related to sickled red cells and anaemia in an asymptomatic pregnant woman with SCD. Prophylactic blood transfusion is often started early in pregnancy and performed at intervals to reduce the chances of transfusion on an emergency basis. Whenever blood is transfused, it is aimed at reducing the proportion of HbS in the circulation to less than 40% and also to achieve an Hb concentration of 10 gram/decilitre (ACOG 2007; Grossetti 2009).
Prophylactic blood transfusion can either be simple "top‐up" (transfusion without prior withdrawal of blood from the recipient) or exchange blood transfusion (Howard 1995). Prophylactic exchange blood transfusion was first proposed by Ricks in 1965 (ACOG 2007). He recommended exchange blood transfusion four to six weeks before the delivery date to optimise the woman's Hb level towards the end of pregnancy when complications are most frequent (ACOG 2007). Prophylactic transfusion reduces the risk of sickling by reducing maternal erythropoiesis and thereby, increasing the partial O2 pressure (Grossetti 2009). It has the advantage of avoiding the risk of alloimmunisation from transfusion of inadequately phenotyped red blood cells and the transfusion‐related reaction or overload that could occur with emergency transfusion (Grossetti 2009).
How the intervention might work
Depending on the institutional policy, prophylactic blood transfusion could be started during the first, second or beginning of the third trimester of pregnancy (Grossetti 2009; Howard 1995; Ngo 2010). When it commences during the third trimester of pregnancy, it preferably begins at 28 weeks (Gilli 2007) and repeated every two to four weeks until delivery. This intervention carries the risks of iron overload (from the woman's inability to adequately excrete iron released from sickled and dead red blood cells), blood‐related infections and alloimmunisation (due to exposure to multiple sources of allogeneic blood) with significant cost implications, especially in resource‐poor settings. Compliance to schedules of transfusion and the need for repeated hospitalisation are other issues of concern for affected women as well as health services (Makani 2007). On the other hand, selective blood transfusion, which has a comparatively lower risk of transfusion‐related morbidities, may become indicated and performed at a time when it is already too late to improve maternal, or fetal outcomes.
Why it is important to do this review
Pregnant women with SCD are at increased risk of severe complications as a result of chronic anaemia, sickling of red cells and their consequences. Repeated blood transfusions to optimise the Hb level is an intervention to avert some of the complications encountered despite the potential morbidity that such practice constitutes. In spite of its use, the benefit of prophylactic blood transfusion to improve the outcome of pregnancy is uncertain. For instance, 11.6% of women who had prophylactic transfusion still needed emergency blood transfusions for severe anaemia during the same pregnancy (Ngo 2010). This is partly related to the lack of consensus among clinicians, hospital and even countries regarding the optimal transfusion regimen, which makes evaluation of their impact on pregnancy outcomes difficult, and the fact that the usefulness of the practice had been largely derived from observational studies that have inherent limitations for policy formulation on the subject (Cunningham 1983; Grossetti 2009; Howard 1995; Ngo 2010). The existing variation in the approaches to care requires a systematic evaluation of the evidence in order to balance effectiveness with safety. This systematic review replaces an outdated and withdrawn review (Mahomed 2006). It evaluated the use of blood transfusion in pregnant women with SCD based on rigorous studies derived from up‐to‐date searches.
Objectives
To assess the benefits and harms of a policy of prophylactic versus selective blood transfusion in pregnant women with sickle cell disease (SCD).
Methods
Criteria for considering studies for this review
Types of studies
All published randomised controlled trials (including those using a cluster‐randomised design) evaluating the effects of prophylactic versus selective blood transfusion in pregnant women with SCD. We planned to include but did not find any eligible quasi‐randomised trials. Eligible reports presented only as abstract were excluded because there was no evidence that the trial was finally published many years after a conference presentation.
Types of participants
Pregnant women with SCD (genotype HbSS, HbSC and HbSβ‐thalassaemias). Studies of pregnant women with sickle cell trait (HbAS) were not eligible for inclusion.
Types of interventions
Prophylactic blood transfusion to optimise Hb concentration to a specified level versus selective (emergency) blood transfusion when indicated by specific complication or a critically low level of Hb concentration. Studies were included regardless of whether whole blood or packed red cells were transfused.
Types of outcome measures
Primary outcomes
-
Maternal death
-
Severe maternal morbidity (e.g. organ failure, pulmonary embolism, fat embolism, stroke, intensive care unit admission; or as defined by trial authors)
-
Perinatal death
Secondary outcomes
Mother
-
Sickle cell crisis (due to vaso‐occlusion, sequestration or haemolysis)
-
Total units of blood transfused
-
Blood transfusion reaction
-
Iron overload in the woman (as assessed by trial authors)
-
Postpartum haemorrhage (greater than 500 mL blood loss or haemodynamic compromise following any degree of blood loss; or as defined by trial authors)
-
Cumulative duration of hospital stay
Infant ·
-
Admission to neonatal intensive care
-
Haemolytic disease of the newborn
Search methods for identification of studies
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (31 October 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
weekly searches of Embase;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference list of all relevant trials.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors (BO Okusanya and OT Oladapo) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved disagreements through discussion.
Data extraction and management
We designed a form as specified by the Cochrane Pregnancy and Childbirth Group to extract data. For eligible studies, both review authors independently extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2012) and checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details. There was no masking of authors or journals.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes. Where the blinding of study participants and personnel of included studies was poor or not done, this was explained in the 'Risk of bias' table.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes. Where the assessment of outcomes of included studies are poorly blinded or unblinded, this was explained further in the 'Risk of bias' table.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We considered whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at a high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We planned but could not explore the impact of the level of bias through undertaking sensitivity analyses due to small number of included trials.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned but could not use the mean difference where outcomes were measured in the same way between trials due to lack of information on the standard errors or confidence intervals for reported means. For the same reason, we planned but could not use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
The search strategy did not find any eligible cluster‐randomised trials. However, in future updates, if we identify any cluster‐randomised trials we will include them in the analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we assessed levels of attrition. We planned but did not explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis as none of the trials included were at high risk of attrition bias.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. We planned but could not explore substantial heterogeneity where identified by sensitivity analysis because only two small trials were included in the review.
Assessment of reporting biases
There were too few included studies in the meta‐analysis to investigate reporting biases (such as publications bias). In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We planned to use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where we suspected clinical heterogeneity sufficient to suggest that the underlying treatment effects may differ between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, where an average treatment effect across trials was considered clinically meaningful.
Where random‐effects analyses was used, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
There was no subgroup analysis as the included studies did not report any of the criteria pre‐specified in the protocol in a way suitable for such analysis.
In future updates, if more data become available and we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses for the primary outcomes.
-
Number of fetuses (singleton versus multiple).
-
Type/clinical severity of haemoglobinopathy (homozygous (HbSS) versus heterozygous (HbSC and or HbSβ+‐thal).
-
Type of transfused blood (whole blood versus packed red cells).
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not perform sensitivity analysis to explore the effect of trial quality as the two included trials have similar methodological quality. In future updates, as more data become available, we will perform sensitivity analysis based on trial quality, separating high‐quality trials from those of low quality for the primary outcomes.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved six trial reports relating to four studies. We included two studies (Koshy 1987; Koshy 1988) involving 98 women. Two studies have been excluded (Koshy 1991; Cerqueira 1999). See (Figure 1).
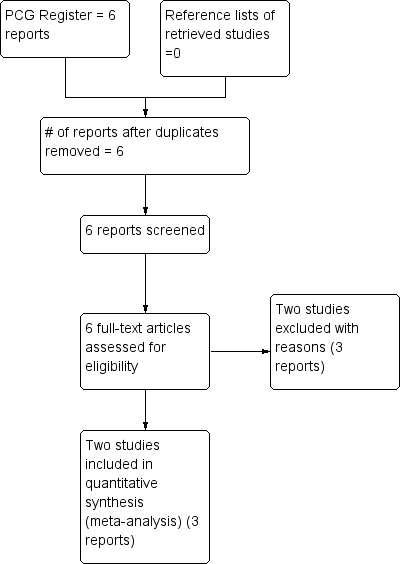
Study flow diagram.
Included studies
We included two trials (Koshy 1987; Koshy 1988), involving 98 women, with outcome data on effectiveness variables available for all participating women. Both trials are very small and were conducted in USA in the late 1980s. One was a single centre (Koshy 1987) and the other (Koshy 1988), a multicentre study conducted in secondary and university hospitals.
Participants
The included trials (Koshy 1987; Koshy 1988) recruited pregnant women with sickle cell anaemia (genotype HbSS) before 28 weeks of gestation. The diagnosis of the sickle cell anaemia in the studies was confirmed with haemoglobin (Hb) electrophoresis on cellulose acetate with citrate agar and solubility testing. In addition, Koshy 1988 also carried out quantitative chromatography while Koshy 1987 performed gene mapping when indicated. Exclusion criteria included pregnant women with other types of sickle cell disease (SCD) (HbSC and HbSβThal); religious belief against blood transfusion (i.e. Jehovah's witness); pregnancy greater than 28 weeks; those presenting with several medical complications; or women who had several red blood cell antibodies.
Interventions
Prophylactic blood transfusion in asymptomatic pregnant women with sickle cell anaemia was compared with selective blood transfusion when indicated by medical or obstetric complications. Prophylactic blood transfusion was commenced prior to 28 weeks of gestation and continued at intervals until delivery. The goal of prophylactic transfusion was to maintain HbS at less than 35% and Hb concentration at 10 to 11 g/dL. Prophylactic blood transfusion took place in an outpatient clinic setting with either partial exchange alone (Koshy 1987), with simple transfusion or partial exchange transfusion (Koshy 1988). In Koshy 1988, women were transfused with two units of packed washed frozen red cells weekly, immediately following trial entry, for three weeks or until goals of prophylactic transfusion were reached; Koshy 1987 did not provide the details of the schedule for prophylactic transfusion.
Selective (emergency) blood transfusion was carried out when indicated by a medical or obstetric complication. For Koshy 1987, such complications included Hb concentration less than 5 g/dL, hypoxaemia, septicaemia, splenic sequestration and preparation for surgery or anaesthesia. In Koshy 1988, the indication for blood transfusion was mainly haematological ‐ Hb concentration less than 6 g/dL (or haematocrit less than 18%) and a reticulocyte count less than 3%.
Outcomes
None of the included studies pre‐specified maternal death as an outcome variable although it was reported by Koshy 1988. Koshy 1988 reported some markers of severe maternal morbidities including pulmonary embolism, congestive heart failure, and acute chest syndrome. One of the two trials (Koshy 1988) reported perinatal death as an outcome.
Regarding the secondary outcomes for this review, pain (vaso‐occlusive) crisis was reported by the two studies but only Koshy 1988 reported haemolytic and acute sequestration crises, total units of blood transfused and blood transfusion reaction. Other relevant maternal outcomes pre‐specified for this review such as cumulative duration of hospital stay, postpartum haemorrhage and iron overload, and infant outcomes were not reported by any of the trials.
For more information about included studies, see Characteristics of included studies.
Excluded studies
Two studies (Cerqueira 1999; Koshy 1991) were excluded. Koshy 1988 was an observational study that compared the findings of two studies on pregnant women with SCD. Cerqueira 1999 was a conference abstract report of the preliminary findings of a trial of more than two decades without any evidence that the trial was completed.
For more information, see Characteristics of excluded studies.
Risk of bias in included studies
Overall, the risk of bias for the included studies was uncertain as the trial reports contained little methodological description. The details of each trial are given in the Characteristics of included studies table.
Allocation
The risk of selection bias was unclear for both trials as their methods of generating and concealing allocation sequence were not described. They only reported that participants were "randomised" or "randomly assigned" into two treatment groups.
Blinding
With regard to blinding of participants and key study personnel, we considered the two trials to be at high risk of performance bias even though the nature of the interventions made it impracticable to blind participants or study personnel. The risk of detection bias was, however, assessed as unclear as both studies gave no description on blinding of outcome assessors.
Incomplete outcome data
The included studies were considered to be at low risk of bias as outcome data were available for all participating women in both treatment groups.
Selective reporting
The risk of reporting bias was considered high for Koshy 1987 and unclear for Koshy 1988. Koshy 1987 reported pain (vaso‐occlusive) crisis as the only outcome of interest without including results for many other recognised morbidities in pregnant women with sickle cell anaemia. For Koshy 1988, comparison of outcome measure in the 'Methods' and 'Results' sections of the report indicated no evidence of selective outcome reporting although there was a reference to occurrence of maternal mortality in the 'Discussion' section.
Other potential sources of bias
We considered Koshy 1988 to be at unclear risk of other potential sources of bias as it was led by the investigator who conducted the Koshy 1987 trial. It is unclear what level of bias the knowledge of results from a similar trial by the same group will have on the implementation of the trial procedures. In addition, the significant difference in previous perinatal mortality in Koshy 1988 (as one of the baseline characteristics) between intervention and control groups questions the effectiveness of the randomisation procedures.
Effects of interventions
Prophlactic versus selective blood transfusion
Overall, there were few events in the small trials included and the results were generally imprecise.
Primary outcomes
Maternal death
One trial (Koshy 1988; 72 women) reported no maternal mortality occurring in women who received either prophylactic or selective blood transfusion.
Severe maternal morbidity (Outcomes 1.2 to 1.4)
One trial (Koshy 1988; 72 women) reported the occurrence of markers of severe acute maternal morbidity including pulmonary embolism, acute chest syndrome and congestive cardiac failure. The trial suggested no difference in the risk of pulmonary embolism between the prophylactic blood transfusion and the selective blood transfusion group (Koshy 1988; 72 women). The trial also indicated no significant difference in the risk of congestive cardiac failure (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.07 to 15.38, Analysis 1.3) and acute chest syndrome (RR 0.67, 95% CI 0.12 to 3.75, Analysis 1.4) between the two comparison groups.
Perinatal death
Perinatal death was reported by one trial (Koshy 1988; 72 women). The trial indicated no significant difference in the risk of perinatal death between women with sickle cell anaemia (HbSS) who received prophylactic blood transfusion compared with those who received transfusion when indicated by medical or obstetric complications (RR 2.85, 95% CI 0.61 to 13.22, Analysis 1.5).
Secondary outcomes
Sickle cell crises
Two trials (Koshy 1987, 26 women; Koshy 1988, 72 women) reported pain crisis as an outcome, while only Koshy 1988 reported sequestration and haemolytic crises.
The two trials (98 women) indicated that prophylactic blood transfusion reduced the risk of pain crises compared with selective blood transfusion in pregnant women with HbSS (RR 0.42, 95% CI 0.17 to 0.99, T²= 0.16, I² 42%, Analysis 1.6). However, the effect was inconsistent across the two trials and the few events and small sample size widen the uncertainty around the average treatment effect estimate. One trial (Koshy 1988, 72 women) suggested no difference in the occurrence of sequestration (RR 0.33, 95% CI 0.01 to 7.92, Analysis 1.7) and haemolytic crises (RR 0.33, 95% CI 0.04 to 3.06).
Total units of blood transfused
Two trials (Koshy 1987, 26 women; Koshy 1988, 72 women) reported the amount of blood transfused. The trials reported the total units of blood transfused per treatment group as well as the average units of blood received per participant in each group. Koshy 1987 reported transfusion of a total of 140 units (with mean of 11 units) of packed red cells to women with HbSS in the prophylactic blood transfusion arm compared with 26 units (with mean of 2 units) to those in the selective blood transfusion arm, while Koshy 1988 reported transfusion of 432 units (with mean of 12 units) and 108 units (with mean of 3 units) to women with HbSS in the prophylactic and selective transfusion groups, respectively. The lack of information on either standard deviation, standard error or confidence intervals of the means precluded the inclusion of the data in the meta‐analysis table.
Blood transfusion reaction
One trial (Koshy 1988, 72 women) indicated no significant difference in the risk of delayed blood transfusion reaction between women with HbSS who received prophylactic compared with those who received blood transfusion only when indicated (RR 2.00, 95% CI 0.54 to 7.39; Analysis 1.9).
Discussion
Sickle cell disease (SCD) poses a threat to the well being of a pregnant woman and her unborn child. Preventing the primary underlying pathophysiological process ‐ sickling of red blood cells and reduction in oxygen‐carrying capacity of the blood ‐ is expected to improve the outcome for both mother and baby. In view of the pregnancy demand on the persistent state of anaemia, there is no doubt that a significant proportion of pregnant women with SCD may require blood transfusion during the course of their pregnancies. However, whether this intervention should be preventive or therapeutic was the question for this review. In spite of its importance, it is surprising to note that very few rigorous studies have been conducted on the subject.
Summary of main results
This review shows that there is weak evidence indicating that prophylactic blood transfusion to achieve set levels of haemoglobin (Hb) concentration and sickle haemoglobin (HbS) confers no clear advantage in clinical outcomes beyond marginal reduction in the risk of pain crisis compared to emergency blood transfusion indicated by either medical or obstetric complications in women with sickle cell anaemia. This evidence was derived from two small studies (a total of 98 women), at unclear risk of bias suggesting that prophylactic transfusion may reduce the risk of pain crisis by 58% but with uncertainty lying somewhere between 83% and 1% reduction. Other clinical end‐points such as maternal mortality and severe morbidity and perinatal death may not be significantly different between the two transfusion regimens for women with HbSS as indicated by one of the included studies. The marginal benefits in term of pain crisis reduction in women with HbSS was achieved at the expense of blood transfusion at a ratio of four or five to one for prophylactic versus selective blood transfusion groups, respectively. This expected difference in the frequency of blood transfusion between the comparison groups also translates to significant difference in healthcare resource use given the required clinic visits and hospital admissions for both policies.There is no information from randomised or quasi‐randomised trials to assess the benefits or risks of a policy of prophylactic versus selective blood transfusion in women with other variants of SCD, i.e. HbSC and HbSβThal.
Overall completeness and applicability of evidence
In general, there is paucity of studies required to generate evidence regarding the primary question for this review. The available evidence pertains to the most severe form of SCD ‐ sickle cell anaemia ‐ and precludes other variants that are also important. There is a general agreement that these other variants do not carry the same risk of pregnancy‐associated adverse outcomes as sickle cell anaemia as their baseline level of anaemia is somewhat better. In fact, in the two trials included for this review, the trialists primarily excluded women with HbSC and HbSβThal with the justification that complications are less frequent and repeated blood transfusion was not necessary. While this assumption might be true in settings where women with such variants already know their Hb genotype status, it could prove dangerous in settings where many women are first diagnosed of SCD when presenting with related complications in pregnancy. As demonstrated among such women excluded in the included studies for this review, serious complications similar to those found in women with HbSS sometimes occur with considerable frequency and frequent blood transfusion might also be necessary to save lives (Koshy 1988). As the underlying pathophysiological process of SCD variants is the same, it is reasonable to assume that the findings of this review may be applicable across board to other sickle cell haemoglobinopathies. However, in view of the relatively fewer frequency of crises in HbSC and HbSβThal, it is possible that the benefit of prophylactic blood transfusion in terms of reduction of pain crisis may become less apparent due to reduced frequency of events.
Repeated allogeneic blood transfusion has many challenges including allo‐immunisation of red blood cells, blood transfusion reactions and increased risks of blood‐borne infections and iron overload in women with SCD. It is uncertain whether the same level of refinement in blood typing, grouping, cross‐matching and red cell processing techniques, albeit in late 1980s, as performed in the included trials can be achieved in resource‐constrained settings. Where such standards cannot be met, prophylactic transfusion of an average of 11 to 12 units of packed red cells might increase transfusion‐related complications for prophylactic transfusion in excess of the findings of this review.
As a result of the growing concern about blood‐borne infections, particularly HIV and hepatitis virus, selective blood transfusion is now generally favoured in most clinical practice and it is unlikely that the marginal benefit in favour of prophylactic transfusion would be enough to influence such practice.
Quality of the evidence
The review found only two trials (involving 98 women) led by the same investigator suitable for inclusion with most of the outcome data coming from one study. Overall, the studies were at unclear risk for many of their 'Risk of bias' domains. Particularly, there was no description of random sequence generation and allocation concealment. The nature of the intervention also would not allow blinding of the intervention for the participants and personnel and as such puts it at high risk of detection bias particularly for a somewhat subjective outcome such as pain crises, the only outcome with borderline significant difference between the comparison groups. The small number of the participants and the methodological limitations of the included studies does not permit confident conclusion on the safety and effectiveness of prophylactic blood transfusion for pregnant women with sickle cell anaemia.
Potential biases in the review process
We minimised potential biases by the use of a comprehensive search strategy and restriction of the study design to randomised and quasi‐randomised trials. As the search strategy found trials as far back as the late 1980s, it is unlikely that we missed out any important study. It could be that the authors' conclusions in the small trials found (included and excluded), the changing trend towards fewer blood transfusion and the challenges of setting up this type of trial have discouraged further work in more recent time. As a crucial step to limit bias as much as possible, we limited the studies considered for inclusion to those with some form of random component in participants' recruitment given the impracticability of blinding of the intervention, which already exposed them to significant risk of detection bias.
Agreements and disagreements with other studies or reviews
In a Cochrane review (Marti‐Carvajal 2009) evaluating intervention regimens (including prophylactic blood transfusion) to treat sickle cell crises, the review authors found no randomised trials to examine the safety and effectiveness of different regimens that have been used. The lack of prophylactic blood transfusion as an important component of any regimen to treat sickle cell crises supports the marginal benefit regarding pain crisis reduction as demonstrated by the current review.

Study flow diagram.

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 1 Maternal death.
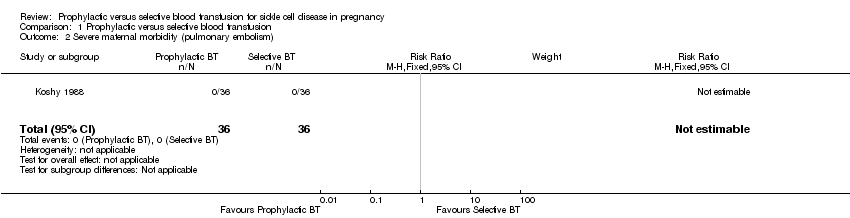
Comparison 1 Prophylactic versus selective blood transfusion, Outcome 2 Severe maternal morbidity (pulmonary embolism).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 3 Severe maternal morbidity (congestive cardiac failure).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 4 Severe maternal morbidity (acute chest syndrome).
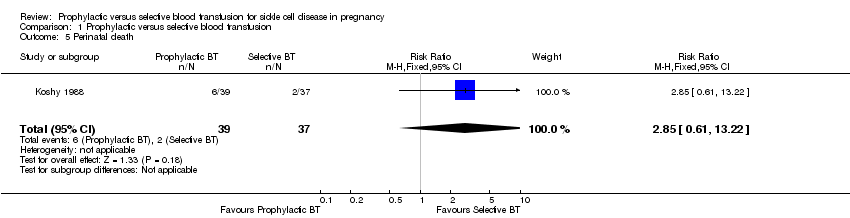
Comparison 1 Prophylactic versus selective blood transfusion, Outcome 5 Perinatal death.
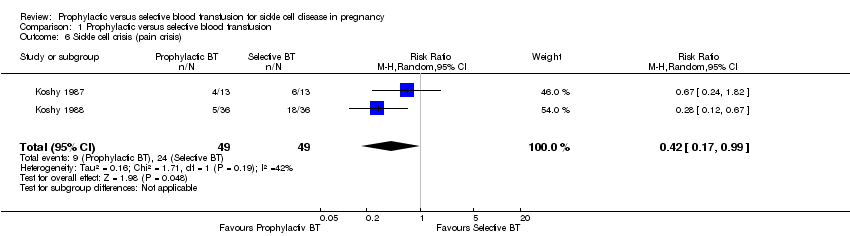
Comparison 1 Prophylactic versus selective blood transfusion, Outcome 6 Sickle cell crisis (pain crisis).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 7 Sickle cell crises (acute splenic sequestration).
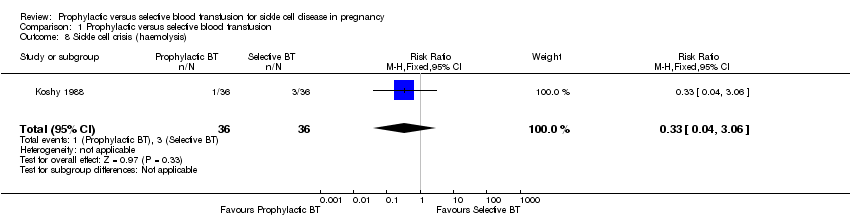
Comparison 1 Prophylactic versus selective blood transfusion, Outcome 8 Sickle cell crisis (haemolysis).

Comparison 1 Prophylactic versus selective blood transfusion, Outcome 9 Blood transfusion reaction.
| Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy | ||||||
| Patient or population: patients with sickle cell disease in pregnancy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Prophylactic versus selective blood transfusion | |||||
| Maternal death | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | 0 participants died in this study. |
| Severe maternal morbidity (pulmonary embolism) | See comment | See comment | Not estimable | 72 | ⊕⊝⊝⊝ | 0 participants experienced pulmonary embolism in this study. |
| Severe maternal morbidity (congestive cardiac failure) | 28 per 1000 | 28 per 1000 | RR 1 | 72 | ⊕⊝⊝⊝ | |
| Perinatal death | 54 per 1000 | 154 per 1000 | RR 2.85 | 76 | ⊕⊝⊝⊝ | |
| Sickle cell crisis (pain crisis) | Study population | RR 0.42 | 98 | ⊕⊕⊝⊝ | ||
| 490 per 1000 | 206 per 1000 | |||||
| Moderate | ||||||
| 481 per 1000 | 202 per 1000 | |||||
| Sickle cell crises (acute splenic sequestration) | 28 per 1000 | 9 per 1000 | RR 0.33 | 72 | ⊕⊝⊝⊝ | |
| Blood transfusion reaction | 83 per 1000 | 167 per 1000 | RR 2 | 72 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study at moderate risk of bias due to unclear methods of random sequence generation and allocation concealment and use of unblinded interventions. * Little variation in baseline risks. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal death Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Severe maternal morbidity (pulmonary embolism) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Severe maternal morbidity (congestive cardiac failure) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.38] |
| 4 Severe maternal morbidity (acute chest syndrome) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.75] |
| 5 Perinatal death Show forest plot | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.61, 13.22] |
| 6 Sickle cell crisis (pain crisis) Show forest plot | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 0.99] |
| 7 Sickle cell crises (acute splenic sequestration) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.92] |
| 8 Sickle cell crisis (haemolysis) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.06] |
| 9 Blood transfusion reaction Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.54, 7.39] |

