一线联合治疗对比一线单一治疗原发性高血压
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind trial Follow‐up: 36 months | |
| Participants | Inclusion criteria: aged ≥ 40 years with hypertension (defined as an untreated systolic blood pressure ≥ 130 mmHg or a diastolic blood pressure ≥ 85 mmHg), history of type 2 diabetes mellitus not exceeding 25 years, urinary albumin excretion rate < 20 μg/min, and serum creatinine concentration ≤ 1.5 mg/dL Exclusion criteria: HbA1c > 11%, non‐diabetic renal disease, heart failure, or specific indications or contraindications to ACEI or CCB therapy Country: Italy | |
| Interventions | Monotherapy 1: verapamil SR 240 mg daily Monotherapy 2: trandolapril 2 mg daily Combination therapy: verapamil 180 mg + trandolapril 2 mg daily Target blood pressure 120/80 mmHg. Additional antihypertensive drugs were allowed to achieve the target blood pressure in the following steps: step 1, hydrochlorothiazide or furosemide; step 2, doxazosin, prazosin, clonidine, methyldopa, or beta‐blockers (allowed based on specific indications); and step 3, minoxidil or long‐acting dihydropyridine CCB. Potassium‐sparing diuretics, inhibitors of the renin‐angiotensin system, and non‐dihydropyridine CCBs different from the study drugs were not allowed | |
| Outcomes | Primary endpoint: development of persistent microalbuminuria (urinary albumin excretion ≥ 20 μg/min at 2 consecutive visits) Other outcomes: urinary albumin excretion, blood pressure after 1 month, major cardiovascular events, overall and cardiovascular mortality, HbA1c, retinal changes, adverse effects and safety laboratory parameters | |
| Funding sources | Abbott GmbH & Co | |
| Declarations of interest | Not reported | |
| Notes | Trial started March 1997. We used data of participants without previous antihypertensive treatment (verapamil + trandolapril: 115 participants, verapamil: 106 participants, trandolapril: 109 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to each therapy in a 1:1 ratio according to a computer‐generated randomisation list created by the Biometric Unit of Abbott |
| Allocation concealment (selection bias) | Low risk | The participant randomisation number was requested by telephone or fax and was assigned by the Treatment Assignment Secretariat at the Mario Negri Institute (Ranica, Italy) by an independent investigator unaware of treatments' allocation schemes |
| Blinding of participants and personnel (performance bias) | Low risk | Study treatments were externally non‐distinguishable pink‐ivory, 2‐coloured capsules. Investigators, participants, care providers, endpoint evaluators, monitors, and data analysts were masked throughout the study |
| Blinding of outcome assessment (detection bias) | Low risk | All investigators, participants, care providers, endpoint evaluators, monitors, and data analysts were masked throughout the study |
| Incomplete outcome data (attrition bias) | Low risk | Schematic diagram of the trial |
| Selective reporting (reporting bias) | Low risk | Protocol available. Individual participant data provided |
| Other bias | Unclear risk | Inclusion criteria were changed during the trial (from untreated blood pressure ≥ 140/90 mmHg to ≥ 130/85 mmHg). Blood pressure targets were also changed from 130/85 mmHg to 120/80 mmHg (protocol amendment 3; 27 May 1999). Subgroup of participants naïve to antihypertensives not predefined. Study not designed for our objectives |
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind trial Follow‐up: 52 weeks | |
| Participants | Inclusion criteria: aged 40 to 75 years with type 2 diabetes, hypertension defined as supine systolic blood pressure ≥ 140 mmHg and < 180 mmHg and supine diastolic blood pressure < 110 mmHg, and albumin excretion rate ≥ 20 μg/min and < 500 μg/min in at least 2 of 3 assays Exclusion criteria: HbA1c ≥ 9% during the 3 months before the study, with presumed non‐diabetic kidney disease, serum creatinine ≥ 140 μmol/L, known contraindications to ACEI therapy or indapamide, or other severe disease Countries: Argentina, Austria, Belgium, Brazil, the Czech Republic, France, Germany, Hungary, Ireland, Mexico, Morocco, the Netherlands, Poland, Slovakia, South Africa, Spain, Switzerland, Tunisia, Turkey, the UK | |
| Interventions | Both groups: open 4‐week pre‐randomisation run‐in period of placebo once daily Monotherapy: enalapril 10 mg daily Combination therapy: perindopril 2 mg + indapamide 0.625 mg once daily Target blood pressure was < 140/90 mmHg. Dose adjustment was permitted after week 12 in double‐blind steps: perindopril 4 mg + indapamide 1.25 mg or enalapril 20 mg then perindopril 8 mg + indapamide 2.5 mg or enalapril 40 mg. Non‐study antihypertensive drugs were not permitted. | |
| Outcomes | Primary outcome: change in the albumin excretion rate after 1 year Secondary outcomes: albumin/creatinine ratio, supine blood pressure and blood pressure response defined as a reduction in systolic blood pressure < 140 mmHg and diastolic blood pressure < 90 mmHg or reduction of systolic blood pressure ≥ 20 mmHg or reduction of diastolic blood pressure ≥ 10 mmHg, or a combination of these. Serious adverse events were predefined as those that were fatal or required prolonged hospitalisation. | |
| Funding sources | Institut de Recherches Internationales Servier | |
| Declarations of interest | Not reported | |
| Notes | Trial conducted between March 1997 and January 2001. The trial recruited 481 participants, and we used data of 109 participants without previous antihypertensive treatment (perindopril + indapamide: 55 participants; enalapril: 54 participants). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation method used to assign treatments (personal communication) |
| Allocation concealment (selection bias) | Low risk | At the beginning of the study, investigators received randomised permutation blocks and the corresponding sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | All study products were supplied in the form of capsules of identical appearance. Prior to the study, the investigator received the therapeutic units and the corresponding coded envelopes. Blister packs and boxes were identified with a unique drug code number for each participant. A 2‐part tear‐off label was affixed to each blister pack and box. When the medication was delivered to the participant, the investigator removed the tear‐off portion of the label and attached it to the participant's case report form |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators provided description of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | In our subgroup, there were more withdrawals due to lack of efficacy in the monotherapy group (6 with monotherapy versus 0 with combination therapy) |
| Selective reporting (reporting bias) | Low risk | Investigators provided results data as requested |
| Other bias | Unclear risk | Subgroup of participants naïve to antihypertensives not predefined. Study not designed for our objectives |
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, clinical trial Follow‐up: 18 months | |
| Participants | Aged 30 to 70 years, stage 1 hypertension (140 mmHg to 159 mmHg/90 mmHg to 99 mmHg; > 130 mmHg in people with diabetes), no current use of blood pressure‐lowering medication, no previous cardiovascular disease Country: Brazil | |
| Interventions | Monotherapy: losartan starting dose 50 mg, up to 100 mg daily Combination therapy: chlorthalidone 12.5 mg + amiloride 2.5 mg starting dose up to chlorthalidone 25 mg + amiloride 5 mg in the same pill daily Amlodipine up to 10 mg daily and propranolol up to 160 mg daily, in an open fashion, to be added if blood pressure not controlled (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg) | |
| Outcomes | Primary: blood pressure variation and proportion of use of add‐on drugs, adverse events, development or worsening of microalbuminuria, and left ventricular hypertrophy on electrocardiogram Secondary: fatal or major cardiovascular events: myocardial infarction, stroke, coronary interventions, heart failure, duplication of creatinine | |
| Funding sources | Department of Science and Technology (DECIT), Health Ministry; National Council of Research (CNPq) and Agency for Funding of Studies and Projects (FINEP), Science and Technology Ministry; National Institute of Health Technology Assessment (IATS); and Funding of Incentive to Research (FIPE), Hospital de Clınicas de Porto Alegre, all in Brazil | |
| Declarations of interest | All authors reported no conflicts of interest and financial disclosures with regard to the subject of the manuscript. | |
| Notes | Trial conducted between February 2011 and September 2016. The trial recruited 655 participants, and we used data from 200 participants without previous antihypertensive treatment (chlorthalidone + amiloride: 116 participants; losartan: 84 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a computer‐generated list, using validated software (Random Allocator), with variable block sizes of 4, 6, 8, or 10 and was stratified by centre |
| Allocation concealment (selection bias) | Low risk | To guarantee concealment of the allocation list, randomisation was implemented through a 24‐hour web‐based automated system. |
| Blinding of participants and personnel (performance bias) | Low risk | The 2 study drugs were identical in size, shape, colour, taste, and texture |
| Blinding of outcome assessment (detection bias) | Low risk | Participants, members of the steering committee, healthcare staff, data collectors, and outcome assessors but no members from the data safety monitoring committee were blinded as to whether participants had received chlorthalidone/amiloride or losartan |
| Incomplete outcome data (attrition bias) | Low risk | The proportion of incomplete follow‐up is balanced between groups |
| Selective reporting (reporting bias) | Low risk | Investigators provided results data as requested |
| Other bias | Unclear risk | Subgroup of participants naive to antihypertensives not predefined. Study not designed for our objectives |
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind trial Follow‐up: 12 months | |
| Participants | Inclusion criteria: aged 18 to 84 years with essential hypertension defined as a supine systolic blood pressure ≥ 160 mmHg and < 210 mmHg, or a supine diastolic blood pressure ≥ 95 mmHg and < 110 mmHg, or both. In all cases, hypertension was uncomplicated. Exclusion criteria: people receiving medication for diabetes, hypocholesteraemia, or cardiovascular disease Countries: Australia, Austria, Belgium, France, Germany, Ireland, Italy, the Netherlands, Portugal, Spain, Sweden, Switzerland, the UK | |
| Interventions | Both groups: 4‐week placebo period Monotherapy: atenolol 50 mg Combination therapy: perindopril 2 mg + indapamide 0.625 mg In both groups, the medication was taken orally in the morning as a single dose. The dosage was then adapted to the blood pressure, and the dose was doubled (2 capsules once daily) after 3 months if systolic blood pressure remained > 160 mmHg or diastolic blood pressure > 90 mmHg, or both. At the end of the procedure, drug dosage was progressively decreased over 8 to 15 days to avoid any complication caused by atenolol withdrawal | |
| Outcomes | Brachial systolic blood pressure, diastolic blood pressure, pulse pressure, aortic pulse wave velocity, carotid and aortic blood pressures, heart rate, adverse effects Target blood pressure defined as < 140/90 mmHg | |
| Funding sources | INSERM, Association Claude Bernard, GPH‐CV, and Laboratoires Servier | |
| Declarations of interest | Not reported | |
| Notes | Study dates not reported. Trial recruited 471 participants. We used data from 129 participants without previous antihypertensive treatment (perindopril/indapamide: 63 participants; atenolol: 66 participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomisation method used to assign treatments (personal communication) |
| Allocation concealment (selection bias) | Low risk | Prior to the study, the investigator received the therapeutic units and the corresponding coded envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | All study products were supplied in the form of capsules of identical appearance |
| Blinding of outcome assessment (detection bias) | Low risk | All measurements were analysed by 2 physicians blinded to treatment, clinical data, and physical examination |
| Incomplete outcome data (attrition bias) | Unclear risk | In the entire study, 471 participants were randomised and 354 completed active treatment period, but reasons for withdrawals were provided for only 96 participants. Information lacking on 7 participants in the perindopril + indapamide group and 12 participants in the atenolol group |
| Selective reporting (reporting bias) | Low risk | Investigators provided results data as requested |
| Other bias | Unclear risk | Subgroup of participants naïve to antihypertensives not predefined. Study not designed for our objectives |
ACEI: angiotensin‐converting enzyme inhibitor; CCB: calcium channel blocker; HbA1c: glycated haemoglobin; SR: slow release.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Follow‐up only 32 weeks | |
| We requested data for participants naïve to antihypertensive drugs. The authors provided individual participant data, but there were fewer than 50 participants per group (trandolapril: 39 participants, trandolapril + verapamil: 40 participants) | |
| We requested data for participants naïve to antihypertensive drugs. The authors provided individual participant data, but there were fewer than 50 participants per group (delapril: 33 participants, delapril + manidipine: 38 participants) | |
| Single‐blind trial | |
| Participants entered a run‐in period in which they received ramipril 2.5 mg once daily for 3 days, followed by telmisartan 40 mg + ramipril 2.5 mg once daily for 7 days and then ramipril 5 mg + telmisartan 40 mg for 11 to 18 days, so participants were not naïve to antihypertensive treatment at randomisation | |
| At week 17 all participants received forced open‐label combination, so the double‐blind comparison of monotherapy versus combination lasted only 16 weeks | |
| We requested data for participants naïve to antihypertensive drugs. The authors provided aggregate data, but there were fewer than 50 participants per group (enalapril: 46 participants, perindopril + indapamide: 40 participants) | |
| The participants have pre‐hypertension, a condition not within the scope of this review | |
| Not stated if this was a double‐blind trial. No data for any of our primary outcomes |
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | Multicentre, randomised, double‐blind, clinical trial Follow‐up: 12 months |
| Participants | Inclusion criteria: aged ≥ 18 with stage 1 essential hypertension (defined as sitting systolic blood pressure ≥ 140 mmHg and < 160 mmHg and sitting diastolic blood pressure ≥ 90 mmHg and < 100 mmHg after a 2‐week wash‐out placebo period) Exclusion criteria: type 2 diabetes mellitus, impaired liver or kidney function, anaemia, unstable cardiovascular conditions (e.g. New York Heart Association (NYHA) class I to IV congestive heart failure or a history of myocardial infarction or stroke) or cerebrovascular conditions within 6 months of study enrolment Country: Italy |
| Interventions | Monotherapy 1: olmesartan 20 mg Monotherapy 2: amlodipine 10 mg Combination therapy: olmesartan 20 mg + amlodipine 5 mg in single tablet |
| Outcomes | Body weight, body mass index, systolic and diastolic blood pressures, fasting plasma glucose, fasting plasma insulin, lipid profile, tumour necrosis factor‐α, retinol binding protein‐4, and interleukins 6 and 7 At baseline, and after 6 and 12 months, participants underwent a euglycaemic, hyperinsulinaemic clamp |
| Notes | We requested data for outcomes of interest in the subgroup of participants naïve to antihypertensive treatment but received no response There are 6 publications of the trial with the same data; as of August 2019, 5 of them have been retracted |
| Methods | Multicentre, randomised, double‐blind, clinical trial Follow‐up: 24 months |
| Participants | Inclusion criteria: outpatients aged < 65 years, with a first‐diagnosed essential hypertension (diastolic blood pressure > 90 mmHg and < 110 mmHg or systolic blood pressure > 140 mmHg and < 180 mmHg, or both) and naïve to antihypertensive treatment Exclusion criteria: hypertrophic cardiomyopathies due to aetiologies other than hypertension; history of heart failure, left ventricular ejection fraction ≤ 50%, angina, stroke, transient ischaemic cerebral attack, coronary artery bypass surgery, or myocardial infarction any time prior to first visit; concurrent symptomatic arrhythmia; liver dysfunction; creatinine > 1.5 mg/dL; endocrine, infective, or inflammatory disorders; use of anti‐inflammatory medications Country: Italy |
| Interventions | Monotherapy 1: enalapril 20 mg once a day Monotherapy 2: lercanidipine 10 mg once a day Combination therapy: enalapril 20 mg + lercanidipine 10 mg once a day |
| Outcomes | Body mass index, systolic and diastolic blood pressure, fasting plasma glucose, lipid profile, lipoprotein a, soluble receptor for advanced glycation end products, soluble CD40 ligand, serum myeloperoxidase, high sensitivity C‐reactive protein and tumour necrosis factor‐α |
| Notes | We requested data for outcomes of interest but received no response. There are 2 publications of the trial with the same data; as of August 2019, 1 of them has been retracted |
| Methods | Randomised, double‐blind, clinical trial Follow‐up: 12 months |
| Participants | Inclusion criteria: outpatients aged ≥ 18, overweight, mild to moderate primary hypertension (systolic blood pressure ≥ 140 mmHg and < 180 mmHg and/or diastolic blood pressure ≥ 90 mmHg and < 105 mmHg), well‐controlled type 2 diabetes mellitus (HbA1c ≤ 7.5%), low‐density lipoprotein cholesterol < 160 mg/dL Exclusion criteria: hypertrophic cardiomyopathies due to aetiologies other than hypertension, history of heart failure, history of angina, stroke, transient ischaemic cerebral attack, coronary artery bypass surgery, myocardial infarction, concurrent known symptomatic arrhythmia; liver dysfunction (aspartate aminotransferase or alanine aminotransferase exceeding 2‐fold the upper limit); creatinine > 1.5 mg/dL, hypersensitivity to the study drugs, pregnant women and women of childbearing potential Country: Italy |
| Interventions | Monotherapy 1: olmesartan 20 mg daily Monotherapy 2: amlodipine 10 mg daily Combination therapy: olmesartan 20 mg + amlodipine 5 mg in single tablet daily |
| Outcomes | Systolic and diastolic blood pressure, lipoprotein (a), myeloperoxidase, isoprostanes, and PON‐1 |
| Notes | We requested data for outcomes of interest in the subgroup of participants naïve to antihypertensive treatment but received no response. |
| Methods | Multicentre, randomised, double‐blind, clinical trial Mean follow‐up: 3.5 years |
| Participants | Inclusion criteria: aged 55 to 80 years with hypertension (blood pressure ≥ 150/95 mmHg or ≥ 160 mmHg systolic) and at least 1 additional cardiovascular risk factor: hypercholesterolaemia; smoker (10 cigarettes per day currently or up to 1 year before entry); family history of myocardial infarction in parent or sibling before age 50 years; current left‐ventricular hypertrophy, coronary heart disease; left‐ventricular strain; peripheral vascular disease Countries: Denmark, France, Israel, Italy, the Netherlands, Norway, Spain, Sweden, the UK |
| Interventions | Monotherapy: initially nifedipine 30 mg daily Combination therapy: hydrochlorothiazide 25 mg + amiloride 2.5 mg daily Dose titration was by dose doubling, and addition of atenolol 25 to 50 mg or enalapril 5 to 10 mg in people whose blood pressure fell by < 20/10 mmHg or was > 140/90 mmHg |
| Outcomes | Primary: cardiovascular death, myocardial infarction, heart failure or stroke Secondary: total mortality; death from a vascular cause; and non‐fatal vascular events including transient ischaemic attacks, angina (new or worsening), and renal failure; serious adverse events |
| Notes | We requested data for outcomes of interest in the subgroup of participants without previous antihypertensive treatment but received no response |
| Methods | Randomised, double‐blind, clinical trial Follow‐up: 24 months |
| Participants | Hypertensive outpatients in sinus rhythm |
| Interventions | Monotherapy 1: telmisartan 20 mg to 80 mg Monotherapy 2: amlodipine 2.5 mg to 10 mg Combination therapy: telmisartan + amlodipine |
| Outcomes | Primary: onset of atrial fibrillation |
| Notes | We asked if the study met our inclusion criteria but received no response |
| Methods | Multicentre, randomised, double‐blind, event‐driven, clinical trial Median follow‐up: 24.7 months |
| Participants | Inclusion criteria: individuals aged ≥ 18 years who had had acute myocardial infarction (0.5 to 10 days previously) that was complicated by clinical or radiological signs of heart failure or evidence of left ventricular systolic dysfunction Countries: Argentina, Australia, Austria, Belgium, Brazil, Canada, the Czech Republic, Denmark, France, Germany, Hungary, Ireland, Italy, the Netherlands, New Zealand, Norway, Poland, Russia, Slovakia, South Africa, Spain, Sweden, the UK, the USA |
| Interventions | Monotherapy 1: valsartan 20 mg twice daily Monotherapy 2: captopril 6.25 mg 3 times daily Combination therapy: valsartan 20 mg twice daily + captopril 6.25 mg 3 times daily Doses were gradually increased with the goal of reaching valsartan 160 mg, captopril 50 mg, or valsartan 80 mg + captopril 50 mg at 3 months. Investigators increased or decreased the doses of the study drugs at their discretion according to the participant's clinical status |
| Outcomes | Primary: all‐cause mortality |
| Notes | Participants were candidates to also receive beta‐blockers Guidelines discourage the studied combination We requested data for outcomes of interest for the subgroup of people with hypertension without previous treatment and without additional antihypertensive drugs but received no response |
HbA1c: glycated haemoglobin; PON‐1: Paraoxonase‐1.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Total mortality Show forest plot | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| Analysis 1.1  Comparison 1: Combination therapy versus monotherapy, Outcome 1: Total mortality | ||||
| 1.1.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| 1.1.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2 Serious adverse events Show forest plot | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.31, 1.92] |
| Analysis 1.2  Comparison 1: Combination therapy versus monotherapy, Outcome 2: Serious adverse events | ||||
| 1.2.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.24, 1.64] |
| 1.2.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.34, 29.42] |
| 1.3 Cardiovascular events Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.22, 4.41] |
| Analysis 1.3  Comparison 1: Combination therapy versus monotherapy, Outcome 3: Cardiovascular events | ||||
| 1.3.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.10, 3.95] |
| 1.3.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.13, 75.69] |
| 1.4 Cardiovascular mortality Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Analysis 1.4  Comparison 1: Combination therapy versus monotherapy, Outcome 4: Cardiovascular mortality | ||||
| 1.4.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Withdrawals due to adverse effects Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| Analysis 1.5  Comparison 1: Combination therapy versus monotherapy, Outcome 5: Withdrawals due to adverse effects | ||||
| 1.5.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.49, 1.35] |
| 1.5.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.45] |
| 1.6 Reaching blood pressure control Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1: Combination therapy versus monotherapy, Outcome 6: Reaching blood pressure control | ||||
| 1.6.1 People with diabetes, target ≤ 120/80 mmHg | 1 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.18] |
| 1.6.2 People with diabetes, target ≤ 140/90 mmHg | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.24, 3.22] |
| 1.6.3 People without diabetes, target ≤ 140/90 mmHg | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.62, 1.28] |
| 1.7 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 3 | 548 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐5.39, 1.27] |
| Analysis 1.7  Comparison 1: Combination therapy versus monotherapy, Outcome 7: Systolic blood pressure change from baseline at end of 1 year | ||||
| 1.7.1 People with diabetes | 2 | 419 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐8.27, 3.19] |
| 1.7.2 People without diabetes | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐7.28, 2.62] |
| 1.8 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 2 | 443 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.21, 0.96] |
| Analysis 1.8  Comparison 1: Combination therapy versus monotherapy, Outcome 8: Diastolic blood pressure change from baseline at end of 1 year | ||||
| 1.8.1 People with diabetes | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.56, 0.78] |
| 1.8.2 People without diabetes | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.45 [‐1.40, 4.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 1: Serious adverse events | ||||
| 2.1.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.52, 3.00] |
| 2.1.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 2.2 Withdrawals due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 2: Withdrawals due to adverse effects | ||||
| 2.2.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.43, 3.73] |
| 2.2.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.66] |
| 2.3 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 3: Systolic blood pressure change from baseline at end of 1 year | ||||
| 2.3.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [‐2.10, 5.58] |
| 2.3.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐3.25, 1.19] |
| 2.4 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 4: Diastolic blood pressure change from baseline at end of 1 year | ||||
| 2.4.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐1.96, 2.90] |
| 2.4.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐2.08, 0.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Total mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.87] |
| Analysis 3.1  Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 1: Total mortality | ||||
| 3.2 Serious adverse events Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.04] |
| Analysis 3.2  Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 2: Serious adverse events | ||||
| 3.3 Cardiovascular events Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.13, 15.71] |
| Analysis 3.3  Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 3: Cardiovascular events | ||||
| 3.4 Cardiovascular mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Analysis 3.4  Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 4: Cardiovascular mortality | ||||
| 3.5 Withdrawals due to adverse effects Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| Analysis 3.5  Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 5: Withdrawals due to adverse effects | ||||
| 3.6 Reaching blood pressure control Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.42] |
| Analysis 3.6  Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 6: Reaching blood pressure control | ||||
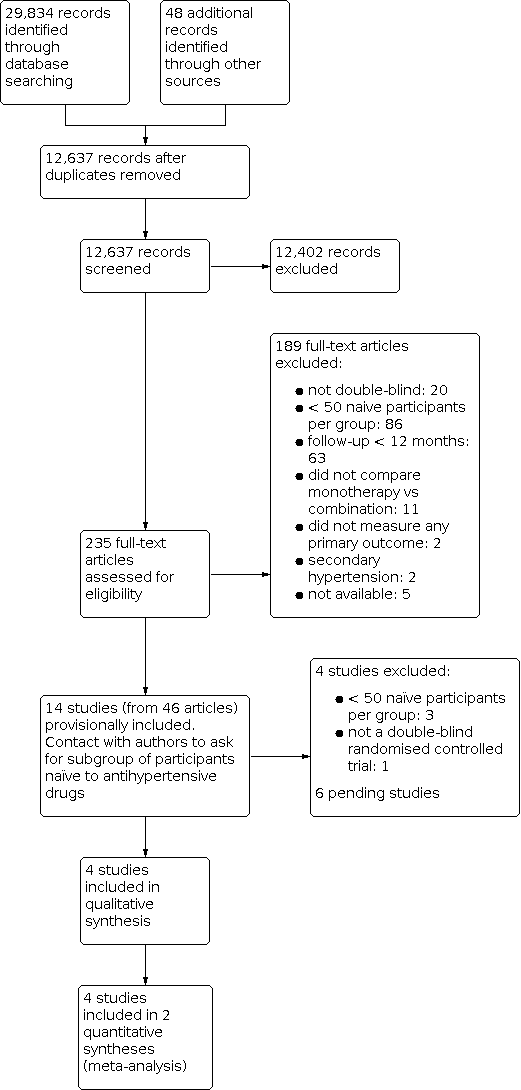
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Combination therapy versus monotherapy, Outcome 1: Total mortality

Comparison 1: Combination therapy versus monotherapy, Outcome 2: Serious adverse events

Comparison 1: Combination therapy versus monotherapy, Outcome 3: Cardiovascular events

Comparison 1: Combination therapy versus monotherapy, Outcome 4: Cardiovascular mortality

Comparison 1: Combination therapy versus monotherapy, Outcome 5: Withdrawals due to adverse effects

Comparison 1: Combination therapy versus monotherapy, Outcome 6: Reaching blood pressure control

Comparison 1: Combination therapy versus monotherapy, Outcome 7: Systolic blood pressure change from baseline at end of 1 year

Comparison 1: Combination therapy versus monotherapy, Outcome 8: Diastolic blood pressure change from baseline at end of 1 year

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 1: Serious adverse events

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 2: Withdrawals due to adverse effects

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 3: Systolic blood pressure change from baseline at end of 1 year

Comparison 2: Combination therapy versus monotherapy (men versus women), Outcome 4: Diastolic blood pressure change from baseline at end of 1 year

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 1: Total mortality

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 2: Serious adverse events

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 3: Cardiovascular events

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 4: Cardiovascular mortality

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 5: Withdrawals due to adverse effects

Comparison 3: Combination with potassium‐sparing diuretics versus monotherapy, Outcome 6: Reaching blood pressure control
| Combination therapy compared to monotherapy for primary hypertension | |||||
| Patient or population: people with primary hypertension | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with monotherapy | Risk with combination therapy | ||||
| Total mortality Follow‐up: 12 to 36 months | 3 per 1000 | 4 per 1000 | RR 1.35 | 568 | ⊕⊝⊝⊝ |
| Cardiovascular mortality Follow‐up: 12 to 36 months | 0 per 1000 | 0 per 1000 | Not estimable | 568 | ⊕⊝⊝⊝ |
| Cardiovascular events Follow‐up: 12 to 36 months | 9 per 1000 | 9 per 1000 | RR 0.98 | 568 | ⊕⊝⊝⊝ |
| Serious adverse events Follow‐up: 12 to 36 months | 176 per 1000 | 136 per 1000 | RR 0.77 | 568 | ⊕⊝⊝⊝ |
| Withdrawals due to adverse effects Follow‐up: 12 to 36 months | 128 per 1000 | 109 per 1000 | RR 0.85 | 568 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for serious risk of bias because all data came from subgroups of participants not predefined in the original studies, and the outcomes of our review were not the primary outcome in any included trial. | |||||
| Combination with potassium‐sparing diuretics versus monotherapy for primary hypertension | |||||
| Patient or population: people with primary hypertension | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| Risk with monotherapy | Risk with combination with potassium‐sparing diuretics | ||||
| Total mortality Follow‐up: 18 months | 12 per 1000 | 3 per 1000 | RR 0.24 | 200 | ⊕⊝⊝⊝ |
| Cardiovascular mortality Follow‐up: 18 months | 0 per 1000 | 0 per 1000 | Not estimable | 200 | ⊕⊝⊝⊝ |
| Cardiovascular events Follow‐up: 18 months | 12 per 1000 | 17 per 1000 | RR 1.45 | 200 | ⊕⊝⊝⊝ |
| Serious adverse events Follow‐up: 18 months | 24 per 1000 | 17 per 1000 | RR 0.72 | 200 | ⊕⊝⊝⊝ |
| Withdrawals due to adverse effects Follow‐up: 18 months | 0 per 1000 | 0 per 1000 | Not estimable | 200 | ⊕⊝⊝⊝ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aWe downgraded by one level for serious risk of bias because all data came from a subgroup of participants not predefined in the original study, and outcomes of our review were not the primary outcome in the trial. | |||||
| Characteristic | Treatment | Mean (standard deviation) | |||
|---|---|---|---|---|---|
| Number of participants | Combination | 115 | 55 | 63 | 116 |
| Monotherapy | 215 | 54 | 66 | 84 | |
| Total participants included in the trial (%) | Combination | 38.08% | 22.78% | 28.09% | 34.83% |
| Monotherapy | 35.54% | 22.54% | 25.82% | 26.09% | |
| Age (years) | Combination | 60.98 (7.62) | 57.27 (8.53) | 52.49 (12.68) | 51.8 (8.2) |
| Monotherapy | 60.62 (8.36) | 59.93 (8.75) | 50.38 (10.57) | 54.0 (9.0) | |
| Sex (% men) | Combination | 67.83% | 74.55% | 71.43% | 56.90% |
| Monotherapy | 69.30% | 77.78% | 62.12% | 61.90% | |
| Ethnicity (% white people) | Combination | 100.00% | 96.36% | 98.41% | 62.1% |
| Monotherapy | 100.00% | 88.89% | 93.94% | 64.3% | |
| Body mass index (kg/m2) | Combination | 28.68 (5.19) | 28.23 (3.18) | 26.85 (3.11) | 29.1 (5.0) |
| Monotherapy | 28.34 (4.42) | 29.22 (3.51) | 26.99 (2.38) | 28.8 (4.7) | |
| Systolic blood pressure (mmHg) | Combination | 151.61 (9.70) | 154.56 (9.86) | 162.56 (11.24) | 140.4 (8.8) |
| Monotherapy | 152.11 (11.57) | 154.04 (11.67) | 158.74 (12.84) | 142.0 (8.4) | |
| Diastolic blood pressure (mmHg) | Combination | 88.72 (7.17) | 90.98 (8.43) | 97.65 (6.89) | 91.7 (7.4) |
| Monotherapy | 89.54 (6.32) | 91.00 (8.26) | 98.94 (5.07) | 90.3 (7.0) | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Total mortality Show forest plot | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| 1.1.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.08, 21.72] |
| 1.1.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2 Serious adverse events Show forest plot | 3 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.31, 1.92] |
| 1.2.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.24, 1.64] |
| 1.2.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.34, 29.42] |
| 1.3 Cardiovascular events Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.22, 4.41] |
| 1.3.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.10, 3.95] |
| 1.3.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.13, 75.69] |
| 1.4 Cardiovascular mortality Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.4.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.5 Withdrawals due to adverse effects Show forest plot | 3 | 568 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| 1.5.1 People with diabetes | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.49, 1.35] |
| 1.5.2 People without diabetes | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.45] |
| 1.6 Reaching blood pressure control Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 People with diabetes, target ≤ 120/80 mmHg | 1 | 314 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 3.18] |
| 1.6.2 People with diabetes, target ≤ 140/90 mmHg | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.24, 3.22] |
| 1.6.3 People without diabetes, target ≤ 140/90 mmHg | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.62, 1.28] |
| 1.7 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 3 | 548 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐5.39, 1.27] |
| 1.7.1 People with diabetes | 2 | 419 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐8.27, 3.19] |
| 1.7.2 People without diabetes | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐7.28, 2.62] |
| 1.8 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 2 | 443 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐1.21, 0.96] |
| 1.8.1 People with diabetes | 1 | 314 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.56, 0.78] |
| 1.8.2 People without diabetes | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 1.45 [‐1.40, 4.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Serious adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.52, 3.00] |
| 2.1.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.45, 1.24] |
| 2.2 Withdrawals due to adverse effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.2.1 Women | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.43, 3.73] |
| 2.2.2 Men | 1 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.42, 1.66] |
| 2.3 Systolic blood pressure change from baseline at end of 1 year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 1.74 [‐2.10, 5.58] |
| 2.3.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐3.25, 1.19] |
| 2.4 Diastolic blood pressure change from baseline at end of 1 year Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.4.1 Women | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐1.96, 2.90] |
| 2.4.2 Men | 1 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐2.08, 0.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Total mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.01, 5.87] |
| 3.2 Serious adverse events Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.10, 5.04] |
| 3.3 Cardiovascular events Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.13, 15.71] |
| 3.4 Cardiovascular mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.5 Withdrawals due to adverse effects Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.6 Reaching blood pressure control Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.42] |

