Safety of regular formoterol or salmeterol in adults with asthma: an overview of Cochrane reviews
Abstract
Background
For adults with asthma that is poorly controlled on inhaled corticosteroids (ICS), guidelines suggest adding a long‐acting beta2‐agonist (LABA). The LABA can be taken together with ICS in a single (combination) inhaler. Improved symptom control can be assessed in the individual; however, the long‐term risk of hospital admission or death requires evidence from randomised controlled trials. Clinical trials record these safety outcomes as non‐fatal and fatal serious adverse events (SAEs), respectively.
Objectives
To assess the risk of serious adverse events in adults with asthma treated with regular maintenance formoterol or salmeterol compared with placebo, or when randomly assigned in combination with regular ICS, compared with the same dose of ICS.
Methods
We included Cochrane reviews on the safety of regular formoterol and salmeterol from a June 2013 search of the Cochrane Database of Systematic Reviews. We carried out a search for additional trials in September 2013 and incorporated the new data. All reviews were independently assessed for inclusion and for quality (using the AMSTAR tool). We extracted from each review data from trials recruiting adults (participants older than 12 or 18 years of age).
We combined the results from reviews on formoterol and salmeterol to assess the safety of twice‐daily regular LABA as a class effect, both as monotherapy versus placebo and as combination therapy versus the same dose of ICS.
We did not combine the results of direct and indirect comparisons of formoterol and salmeterol, or carry out a network meta‐analysis, because of concerns over transitivity assumptions that posed a threat to the validity of indirect comparisons.
Main results
We identified six high‐quality, up‐to‐date Cochrane reviews. Of these, four reviews (89 trials with 61,366 adults) related to the safety of regular formoterol or salmeterol as monotherapy or combination therapy. Two reviews assessed safety from trials in which adults were randomly assigned to formoterol versus salmeterol. These included three trials with 1116 participants given monotherapy (all prescribed background ICS) and 10 trials with 8498 adults receiving combination therapy. An additional search for trials in September 2013 identified five new included studies contributing data from 693 adults with asthma treated with combination formoterol/fluticasone in comparison with the same dose of inhaled fluticasone, as well as from 447 adults for whom formoterol monotherapy was compared with placebo.
No trials reported separate results in adolescents. Overall, risks of bias for the primary outcomes were assessed as low.
Death of any cause
None of the reviews found a significant increase in death of any cause from direct comparisons; however, none of the reviews could exclude the possibility of a two‐fold increase in mortality on regular formoterol or salmeterol (as monotherapy vs placebo or as combination therapy versus ICS) in adults with asthma. Pooled mortality results from direct comparisons were as follows: formoterol monotherapy (odds ratio (OR) 4.49, 95% confidence interval (CI) 0.24 to 84.80, 13 trials, N = 4824), salmeterol monotherapy (OR 1.33, 95% CI 0.85 to 2.08, 10 trials, N = 29,128), formoterol combination (OR 3.56, 95% CI 0.79 to 16.03, 25 trials, N = 11,271) and salmeterol combination (OR 0.90, 95% CI 0.31 to 2.6, 35 trials, N = 13,447). In each case, we did not detect heterogeneity, and the quality of evidence was rated as moderate. Absolute differences in mortality were very small, translating into an increase of 7 per 10,000 over 26 weeks on any monotherapy (95% CI 2 less to 23 more) and 3 per 10,000 over 32 weeks on any combination therapy (95% CI 3 less to 17 more).
Very few deaths were reported in the combination therapy trials, and combination therapy trial designs were different from those of monotherapy trials. Therefore we could not use indirect evidence to assess whether regular combination therapy was safer than regular monotherapy.
Only one death occurred in the monotherapy trials comparing formoterol versus salmeterol, so evidence was insufficient to compare mortality.
Non‐fatal serious adverse events of any cause
Direct evidence showed that non‐fatal serious adverse events were increased in adults receiving salmeterol monotherapy (OR 1.14, 95% 1.01 to 1.28, I2 = 0%,13 trials, N = 30,196) but were not significantly increased in any of the other reviews: formoterol monotherapy (OR 1.26, 95% CI 0.78 to 2.04, I2 = 15%, 17 trials, N = 5758), formoterol combination (OR 0.99, 95% CI 0.77 to 1.27, I2 = 0%, 25 trials, N = 11,271) and salmeterol combination (OR 1.15, 95% CI 0.91 to 1.44, I2 = 0%, 35 trials, N = 13,447). This represents an absolute increase on any monotherapy of 43 per 10,000 over 26 weeks (95% CI 6 more to 85 more) and 16 per 10,000 over 32 weeks (95% CI 22 less to 60 more) on any combination therapy.
Direct comparisons of formoterol and salmeterol detected no significant differences between risks of all non‐fatal events in adults (as monotherapy or as combination therapy).
Authors' conclusions
Available evidence from the reviews of randomised trials cannot definitively rule out an increased risk of fatal serious adverse events when regular formoterol or salmeterol was added to an inhaled corticosteroid (as background or as randomly assigned treatment) in adults or adolescents with asthma.
An increase in non‐fatal serious adverse events of any cause was found with salmeterol monotherapy, and the same increase cannot be ruled out when formoterol or salmeterol was used in combination with an inhaled corticosteroid, although possible increases are small in absolute terms.
However, if the addition of formoterol or salmeterol to an inhaled corticosteroid is found to improve symptomatic control, it is safer to give formoterol or salmeterol in the form of a combination inhaler (as recommended by the US Food and Drug Administration (FDA)). This prevents the substitution of LABA for an inhaled corticosteroid if symptom control is improved on LABA.
The results of three large ongoing trials in adults and adolescents are awaited; these will provide more information on the safety of combination therapy under less supervised conditions and will report separate results for the adolescents included.
Plain language summary
Overview of the safety of regular formoterol or salmeterol in adults with asthma
Background
Asthma is a common condition that affects the airways. When a person with asthma comes into contact with an irritant, the muscles around the walls of the airways tighten and the lining of the airways becomes inflamed and starts to swell. This leads to the symptoms of asthma—wheezing, coughing and difficulty in breathing. No cure for asthma is known; however, there are medications that allow most people to control their asthma so they can get on with daily life.
People with asthma can have underlying inflammation in their lungs, and they are generally advised to take inhaled corticosteroids to combat this inflammation. If asthma still is not controlled, additional medications may be used. One type of additional medication is the long‐acting beta2‐agonists, such as formoterol and salmeterol, which work by reversing the narrowing of the airways that occurs during an asthma attack. These drugs improve lung function, symptoms and quality of life, and reduce the number of asthma attacks. However, there are concerns about the safety of long‐acting beta2‐agonists, particularly in people who are not also taking corticosteroids. We prepared this overview to take a closer look at the safety of long‐acting beta2‐agonists, given alone (monotherapy) or in combination with corticosteroids (combination therapy), to adults with asthma.
How the overview was done
We looked at previous Cochrane reviews on long‐acting beta2‐agonists and found a total of six high‐quality reviews on the safety of formoterol or salmeterol. These reviews included a total of 102 studies involving 70,980 adults or teenagers. The most recent search for new studies across all reviews was conducted in September 2013, and we added results from three further studies (1040 participants); these data have been incorporated into the overview.
We compared formoterol or salmeterol monotherapy versus placebo, and formoterol or salmeterol combination therapy versus corticosteroids alone. We then used the results of these comparisons to look for differences between monotherapy and combination therapy. We also looked at formoterol and salmeterol separately to see whether one was safer than the other, either as monotherapy or as combination therapy. For each comparison, we looked first at risks of death and non‐fatal serious adverse events from any cause, and second at risks of death and non‐fatal serious adverse events related to asthma.
What was found
The risk of fatal or non‐fatal serious adverse events was lower overall in trials with adults taking randomly assigned inhaled corticosteroids, but we found no significant difference between monotherapy and combination therapy in the impact of treatment on risk of death or serious adverse events.
We saw no differences between formoterol and salmeterol monotherapy in risk of death or serious adverse events from any cause or in risk of death or serious adverse events related to asthma. We saw no differences between formoterol and salmeterol combination therapy in the number of deaths or serious adverse events from any cause or in the risk of death related to asthma.
We found no clear differences between the safety of monotherapy and that of combination therapy with long‐acting beta2‐agonists, or between the safety of formoterol and that of salmeterol. The lower estimates of risk on combination therapy support current guidelines, which advise that long‐acting beta2‐agonists should be used only in combination with inhaled steroids for adults with asthma. This review suggests that combination therapy is probably safer than use of long‐acting beta2‐agonists alone, but we do not know exactly how much safer. It is important to continue to collect information on the safety of long‐acting beta2‐agonists. Three large ongoing trials may provide more information.
Authors' conclusions
Background
Description of the condition
Despite efforts to define asthma over the past 30 years, there is “still no specific definition or validated diagnostic algorithm for the disease” (Anderson 2008). The definition of asthma in the Global Initiative for Asthma (GINA) guidelines (GINA 2012) is therefore functional:
“Asthma is a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyper‐responsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread, but variable, airflow obstruction within the lung that is often reversible either spontaneously or with treatment.”
Contraction of the smooth muscle around the airways (bronchoconstriction) is the main cause of short‐term wheezing and shortness of breath in asthma. Adults with asthma show airways hyper‐responsiveness to inhaled allergens (Cockcroft 2006) and a variety of chemical stimuli (Boushey 1980). It is by no means clear how airway hyper‐responsiveness relates to the inflammatory changes seen in asthma, or to the inflammatory pathways that mediate these changes (Anderson 2008).
In clinical practice, most adults with asthma are treated in primary care and never suffer from life‐threatening exacerbations. However, there remains a minority who continue to be at risk for hospital admission and even death from asthma, even with advances in available treatment.
In life‐threatening asthma, mucus plugging and oedema of the airways accompany smooth muscle contraction. It is not clear how each of these elements contributes to death from asthma, but it is potentially dangerous to relieve bronchoconstriction without treating the underlying inflammatory changes.
Description of the interventions
Inhaled selective beta2‐agonists were introduced in 1969 to reduce bronchoconstriction (Phillips 1990). This was followed in 1974 by the introduction of inhaled corticosteroids (ICS), and regular ICS treatment has remained the basis of treatment for inflammation in asthma since the early 1990s. The original beta2‐agonists were short‐acting and had a duration of action of four to six hours. Long‐acting beta2‐agonists (salmeterol and formoterol) were introduced in the 1990s; these need to be inhaled only twice daily because they have a duration of action of 12 hours or longer. Of these, salmeterol has a slower onset of action than formoterol (Van Noord 1996). The long‐acting beta2‐agonists (LABA) were introduced first as monotherapy inhalers and then later combined with an ICS in combination inhalers (such as formoterol/budesonide or salmeterol/fluticasone).
Beta2‐agonists relax the airway smooth muscle and relieve bronchoconstriction, and short‐acting beta2‐agonists are recommended as intermittent first‐step treatment for adults and adolescents with asthma (SIGN/BTS 2012). In adults who require treatment (or who have asthma symptoms) more than twice a week, the second step in treatment is to add ICS to reduce inflammation in the airways. The addition of a regular LABA to an ICS is the current recommended next step for adults and children over five years of age whose asthma symptoms are not controlled with regular ICS alone (SIGN/BTS 2012).
How the intervention might work
The mechanism by which beta2‐agonists might cause harm is not currently known. Several theories (Tattersfield 2006) include the possibility of direct toxicity of beta2‐agonists due to adverse cardiac effects. Other possibilities include tolerance induced by regular use of beta2‐agonists so that they become less effective bronchodilators in acute asthma exacerbations (Weinberger 2006) and delay in seeking medical help (if beta2‐agonists mask the severity of an attack). Reduced use of corticosteroids (which are needed to treat bronchial oedema and excess mucus production due to increased inflammation during exacerbations) is a further possible harmful mechanism. For a fuller discussion, please see the appendix in Cates 2008.
Why it is important to do this overview
Two spikes in the rate of global asthma death have been linked to the use of short‐acting beta2‐agonists: isoprenaline forte in the 1960s and fenoterol in the 1980s (Tattersfield 2006). Subsequently two large surveillance studies and a meta‐analysis have reported increased risk of death from asthma with regular use of salmeterol in adults with asthma (Castle 1993; Salpeter 2006; SMART 2006). In 2006 the US Food and Drug Administration (FDA) issued a black triangle warning against the substitution of regular formoterol or salmeterol for an inhaled corticosteroid for control of asthma symptoms. This warning was included in the information leaflets for both inhalers that contained formoterol or salmeterol alone (as monotherapy) and combination inhalers in which they were co‐administered with an inhaled corticosteroid. Given the results of these surveillance studies in adults, the safety of both regular formoterol and salmeterol, with and without ICS, needs to be compared in adults with asthma.
Regular treatment with LABA is not recommended without regular ICS (GINA 2012; Lougheed 2010; SIGN/BTS 2012), but advice from the FDA to use regular LABA for "the shortest duration possible to achieve control of asthma symptoms and then be discontinued" has been challenged as not evidence‐based by the Canadian Thoracic Society Asthma Committee group (Lougheed 2010).
Serious adverse events (SAEs) are uncommon, and although they are routinely recorded in randomised trials, individual clinical trials are not usually powered to detect small but potentially important differences in the risk of SAEs. Moreover, reporting of SAEs in journal articles based on these trials was found to be incomplete (Cates 2012a). Systematic reviews increase statistical power to detect rare events, but the particular challenge is that there are many ways in which SAEs can be described and reported in medical journals (Ioannidis 2001), and only a part of the picture may be seen if analysis of SAEs is restricted to those that investigators considered related to treatment. Evidence suggests that selective reporting does occur, in relation to both efficacy outcomes and adverse events (Whittington 2004; Chan 2004; Chan 2004a), and there has been a call for better reporting of harms in trial reports in journals (Ioannidis 2004). In view of these difficulties, we have sought to summarise evidence from Cochrane systematic reviews that included clinical trial data on SAEs reported on manufacturers' websites and from FDA submissions, in addition to events reported in medical journals.
Objectives
To assess the risk of serious adverse events in adults with asthma treated with regular maintenance formoterol or salmeterol compared with placebo, or when randomly assigned in combination with regular ICS, compared with the same dose of ICS.
Methods
Criteria for considering reviews for inclusion
Types of reviews
Cochrane systematic reviews of randomised trials published in the Cochrane Database of Systematic Reviews (CDSR) that have a primary focus on adverse events.
Participants
Adults and adolescents (over the age of 12 years) with asthma. We included reviews of trials in both adults and children but analysed the results only from trials in adults and adolescents.
Interventions
-
Regular formoterol monotherapy versus placebo.
-
Regular salmeterol monotherapy versus placebo.
-
Regular formoterol in combination with ICS versus the same dose of ICS.
-
Regular salmeterol in combination with ICS versus the same dose of ICS.
-
Regular formoterol versus regular salmeterol.
-
Regular formoterol in combination with ICS versus regular salmeterol in combination with ICS.
All reviews specified that the minimum duration of included trials was 12 weeks, but no restriction was placed on the dose of formoterol or salmeterol. We did not include reviews of formoterol combination therapy used for both maintenance and relief of symptoms, as the dose of ICS was higher in the combination therapy arms of the trials.
Outcome measures
-
Primary outcomes: death of any cause and adults with one or more non‐fatal serious adverse events of any cause.
-
Secondary outcomes: asthma‐related deaths and adults with one or more asthma‐related non‐fatal serious adverse events.
The choice of adults with one or more all‐cause serious adverse events as the primary outcome was made because ascertainment bias is a concern for asthma‐related events, as the trialists decided whether an event was listed as asthma‐related. Moreover a participant with a serious adverse event may have this recorded under more than one category, leading to double‐counting of individual participants. The number of participants with at least one serious adverse event of any cause was clear from the manufacturers' trial reports on their websites, with separate reporting of fatal and non‐fatal events. Neither hazard ratios nor count data were available from the trial reports.
Search methods for identification of reviews
We identified relevant systematic reviews by searching the Cochrane Database of Systematic Reviews (CDSR) in The Cochrane Library (2013, Issue 6 of 12) in June 2013. We applied no date restrictions. We did not search for non‐Cochrane reviews. See Appendix 1 for the search strategy.
Although we envisaged this as an overview of Cochrane reviews, we believed that it was important to include the most recent trials in this overview; therefore we updated the searches for each review to September 2013. The search was conducted on the Cochrane Airways Group Register of Trials (CAGR). This register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts. The keywords used to identify relevant trials are provided in the search methods of the individual reviews.
Data collection and analysis
Selection of reviews
Two review authors independently assessed Cochrane reviews (and additional trials from the search in September 2013) for inclusion in this overview. There was no disagreement, so discussion with a third review author was not needed.
Data extraction and management
We extracted data from studies included in the existing Cochrane reviews of serious adverse events in relation to characteristics, risks of bias and data for serious adverse events. These reviews had identified participants with a fatal event and participants with one or more non‐fatal serious adverse events of any cause (as these were well reported in the sponsors' web reports); we analysed the number of participants with one or more events as dichotomous data throughout. We cross‐checked the details of trial identifiers and references in each review to confirm that individual trial arms were counted only once in the analyses conducted for the overview.
We extracted data from the reviews on control group event rates, so we could compare weighted mean event rates between adults in the reviews (both as a proportion of the total number of participants and adjusted for the duration of each trial).
We did not attempt to extract data from other Cochrane reviews in which adverse events were included as secondary outcomes (i.e. an analysis of SAEs was not a primary purpose of those reviews), as they had already been checked in the course of preparation of the six included reviews.
Assessment of methodological quality of included reviews
Quality of included reviews
Two review authors independently assessed the included reviews for methodological quality, with particular emphasis on potential bias in the review process of each review, using the AMSTAR tool (Shea 2007). We assessed incorporation of the risk of bias into each review and planned to carry out a sensitivity analysis based on the results of studies at low or unclear risk of bias for each outcome. We considered risks of bias in relation to selection of studies, ascertainment of serious adverse events and methods of analysis of the results.
Quality of evidence in included reviews
We assessed whether the included reviews relied merely on evidence from reports of trial results published in journals or looked more widely at manufacturers' trial reports and submissions to the FDA (to reduce the risk of publication bias).
Two review authors independently assessed the quality of evidence in the included reviews using the 'Risk of bias' tables in the included reviews (for the trials on adults). We also assessed the limitations of evidence found in the reviews for trials on adults using the 'Summary of findings' tables from the included reviews, and independently reassessed the downgrading decisions made in each review using the GRADE process. The results are summarised in 'Summary of findings' tables for the overview (see Table 1; Table 2; Table 3; Table 4).
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular LABA (salmeterol or formoterol) | |||||
| Adults who died of any cause | ||||||
| Formoterol monotherapy v placebo Follow‐up: mean 14 weeks | 0 per 10000 | not estimable (see comment) | OR 4.49 (0.24 to 84.80) | 4824 (13 studies) | ⊕⊕⊝⊝ | No deaths on placebo, two deaths on formoterol |
| Salmeterol monotherapy v placebo Follow‐up: mean 27 weeks | 23 per 10000 | 31 per 10000 (20 to 48) | OR 1.33 (0.85 to 2.08) | 29,128 (10 studies) | ⊕⊕⊕⊝ | |
| LABA monotherapy v placebo Follow‐up: mean 26 weeks | 20 per 10000 | 27 per 10000 (18 to 43) | OR 1.37 (0.88 to 2.13) | 33,952 | ⊕⊕⊕⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol monotherapy v placebo Follow‐up: mean 14 weeks | 106 per 10000 | 133 per 10000 (83 to 214) | OR 1.26 (0.78 to 2.04) | 5758 (17 studies) | ⊕⊕⊕⊝ | |
| Salmeterol monotherapy v placebo Follow‐up: mean 27 weeks | 345 per 10000 | 391 per 10000 (348 to 437) | OR 1.14 (1.01 to 1.28) | 30,196 (13 studies) | ⊕⊕⊕⊕ | |
| LABA monotherapy v placebo Follow‐up: mean 26 weeks | 316 per 10000 | 359 per 10000 (322 to 401) | OR 1.14 (1.02 to 1.29) | 35,954 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (was the mean control group risk across all studies, including those with no events in either arm of the trial). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
1. Confidence intervals are very wide, as only two deaths occurred (‐2 points)
2. Confidence intervals are wide enough to include important harm and benefit (‐1 for imprecision)
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Salmeterol monotherapy | Formoterol monotherapy | |||||
| Adults who died of any cause | ||||||
| Formoterol monotherapy v salmeterol monotherapy Follow‐up: mean 24 weeks | 18 per 10000 | 2 per 10000 (0 to 115) | OR 0.14 (0.00 to 6.82) | 1116 | ⊕⊝⊝⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol monotherapy v salmeterol monotherapy Follow‐up: mean 24 weeks | 641 per 10000 | 501 per 10000 (305 to 806) | OR 0.77 (0.46 to 1.28) | 1116 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
1. Open studies (‐1 point) and confidence intervals are very wide indeed, as only one death occurred (‐2 points)
2. Open studies and wide confidence intervals (‐1 point each)
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular LABA (salmeterol or formoterol) | |||||
| Adults who died of any cause | ||||||
| Formoterol combination therapy v ICS Follow‐up: mean 29 weeks | 2 per 10000 | 7 per 10000 (2 to 32) | OR 3.56 (0.79 to 16.03) | 11,271 | ⊕⊕⊕⊝ | |
| Salmeterol combination therapy v ICS Follow‐up: mean 34 weeks | 11 per 10000 | 10 per 10000 (3 to 29) | OR 0.90 (0.31 to 2.60) | 13,447 | ⊕⊕⊕⊝ | |
| LABA combination therapy v ICS Follow‐up: mean 32 weeks | 7 per 10000 | 10 per 10000 (4 to 24) | OR 1.42 (0.60 to 3.38) | 24,718 | ⊕⊕⊕⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol combination therapy v ICS Follow‐up: mean 29 weeks | 241 per 10000 | 239 per 10000 (187 to 304) | OR 0.99 (0.77 to 1.27) | 11,271 | ⊕⊕⊕⊝ | |
| Salmeterol combination therapy v ICS Follow‐up: mean 34 weeks | 209 per 10000 | 240 per 10000 (191 to 298) | OR 1.15 (0.91 to 1.44) | 13,447 | ⊕⊕⊕⊝ | |
| LABA combination therapy v ICS Follow‐up: mean 32 weeks | 228 per 10000 | 244 per 10000 (206 to 288) | OR 1.07 (0.90 to 1.27) | 24,718 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies, including those with no events in either arm of the trial). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
1. Confidence intervals are wide and include important harm and benefit (‐1 for imprecision)
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Salmeterol combination therapy | Formoterol combination therapy | |||||
| Adults who died of any cause | ||||||
| Direct comparisons of formoterol combination therapy v salmeterol combination therapy Follow‐up: mean 23 weeks | 3 per 10000 | 8 per 10000 (1 to 48) | OR 2.68 | 6769 | ⊕⊕⊝⊝ | Based on data from all formoterol combination trials |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Direct comparisons of formoterol combination therapy v salmeterol combination therapy Follow‐up: mean 23 weeks | 226 per 10000 | 252 per 10000 (186 to 342) | OR 1.12 | 6769 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
1. Confidence intervals are very wide, as only five deaths occurred (‐2 points)
2. Confidence intervals are wide and include important harm and benefit (‐1 point)
Data synthesis
Direct randomised comparison data
We extracted data on adults in the comparisons included in the systematic reviews (see Figure 1) using Review Manager 5.2 (RevMan 5.2); pooled results from these comparisons are shown in forest plots and in a table of pooled results (see Table 5).
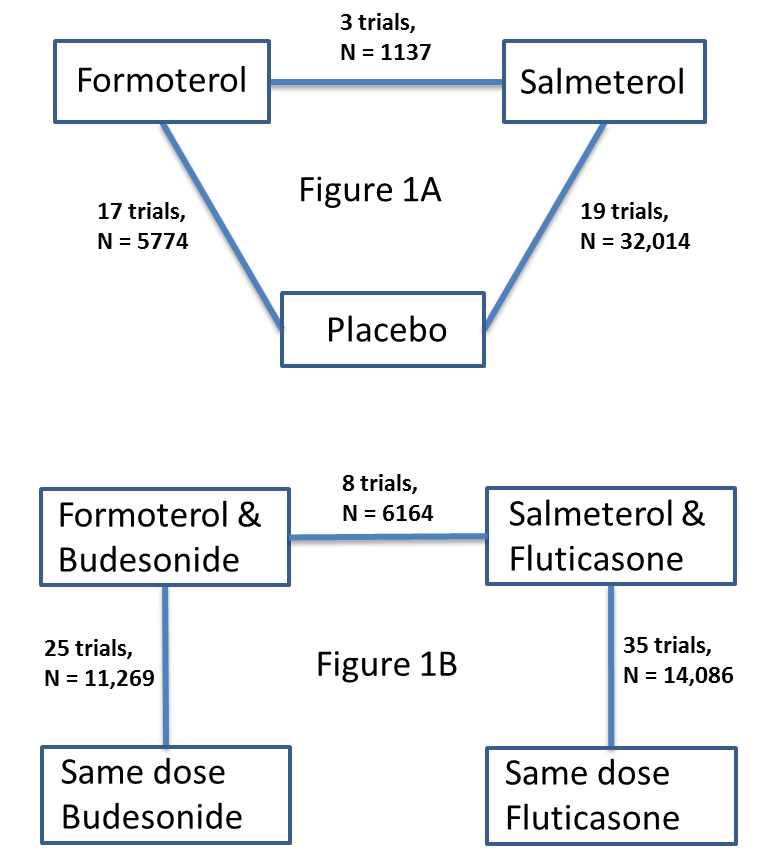
Network of comparisons of serious adverse events from reviews of regular formoterol and salmeterol. Figure 1A shows the numbers of trials and adults on monotherapy versus placebo. Figure 1B shows the numbers of trials and adults on combination therapy versus the same dose of ICS. Adults randomly assigned to other arms in the included trials have not been counted.
| Comparison (with additional data from two new trials) | Formoterol Monotherapy | Placebo | Pooled Effect Size (95% Confidence Interval) | Quality of the evidence | |||
| Outcome (mean duration 14 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 2 | 2924 | 0 | 1900 | Peto OR 4.49 (95% CI 0.24 to 84.80) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 48 | 3401 | 25 | 2357 | Peto OR 1.26 (95% CI 0.78 to 2.04) | 15% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 1 | 2495 | 0 | 1690 | Peto OR 4.54 (95% CI 0.07 to 285.25) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE due to asthma | 17 | 2849 | 10 | 022 | Peto OR 1.09 (95% CI 0.50 to 2.40) | 23% | ⊕⊕⊝⊝ |
| Comparison | Salmeterol Monotherapy | Placebo | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 27 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 44 | 14648 | 33 | 14480 | Peto OR 1.33 (95% CI 0.85 to 2.08) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 587 | 15170 | 518 | 15026 | Peto OR 1.14 (95% CI 1.01 to 1.28) | 0% | ⊕⊕⊕⊕ |
| Mortality due to asthma | 13 | 14648 | 3 | 14480 | Peto OR 3.49 (95% CI 1.31 to 9.31) | Data from single trial | ⊕⊕⊕⊕ |
| Non‐fatal SAE due to asthma | 23 | 1994 | 16 | 1847 | Peto OR 1.43 (95% CI 0.75 to 2.71) | 0% | ⊕⊕⊝⊝ |
| Comparison | Formoterol Monotherapy | Salmeterol Monotherapy | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 26 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 0 | 554 | 1 | 662 | Peto OR 0.14 (95% CI 0.00 to 6.82) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE all‐cause | 28 | 554 | 36 | 662 | Peto OR 0.77 (95% CI 0.46 to 1.28) | 0% | ⊕⊕⊝⊝ |
| Mortality due to asthma | 0 | 554 | 0 | 662 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 6 | 554 | 7 | 662 | Peto OR 0.86 (95% CI 0.29 to 2.57) | 0% | ⊕⊕⊝⊝ |
| Comparison (with additional data from three new trials) | Formoterol Combination Therapy | Inhaled Corticosteroids | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 29 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 6 | 6507 | 1 | 4764 | Peto OR 3.56 (95% CI 0.79 to 16.03) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 145 | 6507 | 115 | 4764 | Peto OR 0.99 (95% CI 0.77 to 1.27) | 0% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 1 | 6507 | 0 | 4764 | Peto OR 7.34 (95% CI 0.15 to 369.72) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE due to asthma | 17 | 6325 | 30 | 4576 | Peto OR 0.49 (95% CI 0.28 to 0.88) | 0% | ⊕⊕⊕⊝ |
| Comparison | Salmeterol Combination Therapy | Inhaled Corticosteroids | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 34 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 7 | 6986 | 7 | 6461 | Peto OR 0.90 (95% CI 0.31 to 2.60) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 167 | 6986 | 135 | 6461 | Peto OR 1.15 (95% CI 0.91 to 1.44) | 0% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 0 | 6986 | 0 | 6461 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 29 | 6986 | 23 | 6461 | Peto OR 1.12 (95% CI 0.65 to 1.94) | 5% | ⊕⊕⊕⊝ |
| Comparison | Formoterol Combination Therapy | Salmeterol Combination Therapy | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 24 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 4 | 3453 | 1 | 3316 | Peto OR 2.68 (95% CI 0.44 to 16.14) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 90 | 3453 | 75 | 3316 | Peto OR 1.12 (95% CI 0.82 to 1.53) | 13% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 0 | 3453 | 0 | 3316 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 17 | 3081 | 25 | 3082 | Peto OR 0.69 (95% CI 0.37 to 1.26) | 33% | ⊕⊕⊝⊝ |
1. Few events were observed leading to wide CIs (including the possibilities of no effect and appreciable harm)
2.There was no independent assessment of the cause of serious adverse events, leading to possible ascertainment bias for disease‐specific outcomes
3. Open studies
We analysed serious adverse event data as odds ratios (ORs) of participants with one or more events using RevMan 5.2; risk differences were compared as sensitivity analyses. Because zero cells were included in many of the studies, the Peto OR was preferred, as it requires no zero cell adjustment (Bradburn 2007). A risk difference analysis was carried out as a sensitivity analysis, as this offers the advantage of including data from trials with no events in either arm, but risk differences tend to have greater heterogeneity than ORs. The risk differences were used to compare all‐cause events and asthma‐related events on the same scale, because ORs would not be expected to be the same if the ratio of all‐cause events was driven by the increase in asthma‐related events. For example, in SMART 2006, 13 deaths due to asthma on salmeterol monotherapy were reported, along with three deaths on placebo, yielding an OR of 3.49. For all‐cause mortality, 10 additional deaths were reported (42 on salmeterol and 32 on placebo), which is exactly the same risk difference as for asthma‐related deaths, but yielding a smaller OR of 1.39 because the 10 additional deaths due to asthma are now diluted by deaths from other causes in this lower odds ratio.
We converted pooled ORs (and 95% confidence intervals (CIs)) into absolute differences for the 'Summary of findings' tables with Visual Rx 2012 (using mean control arm event rates from the trials).
Inhaled corticosteroids as an effect modifier of the safety of formoterol and salmeterol
One of the purposes of this overview was to explore the impact of concurrent (randomised) ICS on the safety of LABA, as well as to determine whether this was an import effect modifier. In the monotherapy trials, with only background ICS, there was a danger that adults would discontinue their background ICS because of symptomatic improvement resulting from the LABA. We therefore wanted to explore whether the combination therapy trials had a better safety profile than the monotherapy trials. Randomisation of adults to combination therapy or LABA monotherapy would not have addressed the effect of modification of randomised ICS on LABA safety.
We did not set out to carry out a network meta‐analysis to combine direct and indirect comparisons in relation to fatal and non‐fatal SAEs with formoterol and salmeterol, because the monotherapy and combination therapy networks are not connected (Figure 1). Moreover, participants enrolled into the combination therapy trials were largely suffering from asthma that required regular ICS, whilst the monotherapy trials included variable proportions of participants on ICS. In the most recent trials included in this overview, participants were stratified by previous use of ICS; those already taking ICS were randomly assigned to two arms comparing combination therapy versus ICS, whilst those not receiving ICS were randomly assigned to formoterol monotherapy or placebo. This precludes carrying out a network meta‐analysis (even though all four arms were in the same trial), because no transitivity exists between the combination therapy and monotherapy arms. In other words, participants did not have an equal likelihood of being randomly assigned to combination therapy or monotherapy arms of these trials.
We explored the safety interaction with ICS by indirectly comparing treatment effects of formoterol or salmeterol versus placebo (diagonal lines in Figure 1A) with the treatment effect of formoterol or salmeterol with ICS versus the same dose of ICS (corresponding vertical lines in Figure 1B) using the methods described in Altman 2003 and Bucher 1997 (see Appendix 2 for further details). This comparison was carried out by entering the monotherapy and combination therapy trial results as different subgroups in RevMan 5.2, with the results displayed as a forest plot. Tests for interaction between subgroups were generated for the ORs using RevMan 5.2 and allow us to assess whether we could see any differences in safety outcomes of the monotherapy and combination therapy trials. We preserved the benefits of randomisation by pooling pair‐wise comparisons from each trial (Bucher 1997). However, we recognise that adults in the combination therapy trials suffered from asthma that required ICS, whereas at least some of the adults in the monotherapy trials did not, and this may pose a threat to the transitivity assumption that is inherent in Bucher's method.
Multi‐arm trials
We included multi‐arm trials in the direct and indirect comparisons made. However, no trial arms were included in more than one review. For multi‐arm trials in which participants were randomly assigned to placebo, formoterol, ICS or combination therapy, the comparison between the first two arms would have been included in the formoterol monotherapy review and the comparison between the second two arms in the formoterol combination review. We combined trial arms that used different doses of the same LABA. None of the multi‐arm trials included arms that randomly assigned participants to formoterol or salmeterol in the same trial.
Transitivity assumptions and assessment of inconsistency
Transitivity assumptions were assessed by considering whether important differences in potential effect modifiers of safety could be noted between the trials included in each Cochrane review (Cipriani 2013). The effect modifiers that we considered important were asthma severity (for which previous use of ICS was considered to be a marker), potential safety differences between different doses and types of ICS and study design (because regular LABA might be considered safer in small, closely supervised randomised trials).
Inconsistency between direct and indirect comparisons of formoterol versus salmeterol was assessed by entering pooled results from the trials with direct comparisons and pooled indirect comparisons as separate subgroups in RevMan 5.2. The test for subgroup differences was then reported between pooled direct comparisons and indirect comparisons.
Control group event rates
Major differences between control group event rates present a threat of confounding to indirect comparisons between the results from different reviews and suggest that the transitivity assumptions inherent in Bucher's method may not be met. We therefore extracted control group events from each review and compared mean event rates both as proportions of the total number in the control groups and as weekly rates per 1000 adults (by dividing the proportion by the weighted average duration of the trials). We would not necessarily expect treatment effects to be scalable across widely different control group risks, so we avoided making indirect comparisons when control event rates were not similar.
Results
Results of the search
The search identified 25 reviews, of which 19 were excluded because they were not relevant. The remaining six reviews were included in this overview. Figure 2 shows further details of the inclusion and exclusion processes. We found 92 references from the search update conducted in September 2013. From these, two review authors (CJC and KMK) independently included five new trials on formoterol combination therapy versus inhaled corticosteroids (Corren 2013; Matsunaga 2013; Nathan 2012; Pearlman 2013; Stirbulov 2012).

Review selection flow diagram.
The events found in these new trials are summarised in Appendix 3.
Description of included reviews
Six Cochrane reviews on serious adverse events associated with LABA treatment in asthma were included.
-
Regular treatment with formoterol for chronic asthma: serious adverse events (Cates 2012a).
-
Regular treatment with salmeterol for chronic asthma: serious adverse events (Cates 2008).
-
Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events (Cates 2013b).
-
Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events (Cates 2013a).
-
Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events (Cates 2012b).
-
Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events (Cates 2010).
We present characteristics of the included reviews as summarised in Table 6 and the results of individual reviews (with additional data from the new trials) as summarised in Table 5. The characteristics of included studies in adults and adolescents in each of the reviews are summarised in Table 7 (Cates 2012a), Table 8 (Cates 2008), Table 9 (Cates 2012b), Table 10 (Cates 2013b), Table 11 (Cates 2013a) and Table 12 (Cates 2010).
| Review title | Inclusion criteria | Date of search | No. included studies (all versus placebo or ICS) | No. included studies (adults only versus placebo or ICS) | ||||
| Studies (Randomised trials only) | Participants (Diagnosis of asthma; any age group) | Intervention | Comparison | Primary outcome measures (All‐cause mortality & non‐fatal SAEs) | ||||
| 1. Regular treatment with formoterol for chronic asthma: serious adverse events | Yes | Yes | Inhaled formoterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or SABA | Yes | January 2012 | 20 (versus placebo) | 15 (versus placebo) |
| 2. Regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2008 | Yes | Yes | Inhaled salmeterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or SABA | Yes | August 2011 | 24 (versus placebo) | 19 (versus placebo) |
| 3. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events Cates 2013b | Yes | Yes | ICS and formoterol once or twice/day; at least 12 weeks duration; any dose; any single or separate device | Same dose and type of ICS | Yes | August 2012 | 27 | 20 |
| 4. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events Cates 2013a | Yes | Yes | ICS and salmeterol once or twice/day; at least 12 weeks duration; any dose; any single or separate device | Same dose and type of ICS | Yes | August 2012 | 40 | 35 |
| 5. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2012b | Yes | Yes | Inhaled formoterol; at least 12 weeks duration; not randomised with ICS | Inhaled salmeterol; at least 12 weeks duration; not randomised with ICS | Yes | January 2012 | 4 | 3 |
| 6. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events Cates 2010 | Yes | Yes | Inhaled formoterol with an ICS; at least 12 weeks duration; any dose; any single or separate delivery device | Inhaled salmeterol with an ICS; at least 12 weeks duration; any dose; any single or separate delivery device | Yes | August 2011 | 10 | 10 |
| Study ID from adults in Cates 2012a | % patients on background ICS | (N) | (N) | (N) | Placebo (N) | Mortality data (all‐cause) | Duration (weeks) |
| Formoterol Dose | 48 mcg/day | 24 mcg/day | 12 mcg/day |
|
| ||
| Bensch 2001 | 51 | 135 | 136 | 136 | ✓ | 12 | |
| Busse 2004 | 64 | 80 | 80 | ✓ | 12 | ||
| Corren 2007 | 0 | 123 | 131 | ✓ | 12 | ||
| Corren 2013 | 0 | 111 | 109 | ✓ | 12 | ||
| Ekstrom 1998 | 86 | 135 | 129 |
| 12 | ||
| Ekstrom 1998a | 89 | 114 | 113 |
| 12 | ||
| Fitzgerald 1999 | 100 | 89 | 91 | ✓ | 24 | ||
| LaForce 2005 | 67 | 86 | 91 | ✓ | 12 | ||
| Molimard 2001 | 100 | 130 | 129 | ✓ | 12 | ||
| Nathan 2012 | 0 | 116 | 111 | ✓ | 12 | ||
| Noonan 2006 | 100 | 123 | 125 | ✓ | 12 | ||
| Pleskow 2003 | 44 | 136 | 139 | 141 | ✓ | 12 | |
| SD‐037‐0344 | 100 | 429 | 210 | ✓ | 12 | ||
| Steffensen 1995 | 87 | 103 | 101 |
| 12 | ||
| van der Molen 1997 | 100 | 125 | 114 |
| 24 | ||
| van Schayck 2002 | 95 | 46 | 41 | ✓ | 12 | ||
| Wolfe 2006 | 62 | 525 | 527 | 514 | ✓ | 16 | |
| mean duration 14 weeks |
All trials contributed data for non‐fatal serious adverse events of any cause. Corren 2013 and Nathan 2012 have been added to the trials already included in the Cochrane review.
| Study ID from adults Cates 2008 | % patients on background ICS | Number on salmeterol | **Dose of salmeterol (mcg/bd) | Number on placebo | Data found on mortality (all‐cause) | Data found on non‐fatal SAE (all‐cause) | Duration (weeks) |
| Adinoff 1998 | 64 | 142 | 50 | 244 |
|
| 36 |
| Boyd 1995 | 100 | 55 | 100 | 64 |
| ✓ | 12 |
| Busse 1998 | 67 | 263 | 50 | 275 |
| ✓ | 12 |
| Chervinsky 1999 | 51 | 176 | 50 | 176 | ✓ |
| 52 |
| D'Alonso 1994a | 21 | 106 | 50 | 108 |
|
| 12 |
| D'Urso 2001 | 93 | 455 | 50 | 456 | ✓ | ✓ | 24 |
| Kavuru 2000 | 0* | 92 | 50 | 82 | ✓ | ✓ | 12 |
| Kemp 1998a | 43 | 149 | 50 | 152 |
|
| 12 |
| Kemp 1998b | 100 | 252 | 50 | 254 |
| ✓ | 12 |
| Lazarus 2001 | 0* | 54 | 50 | 56 | ✓ | ✓ | 16 |
| Nathan 1999 | 0* | 128 | 50 | 129 | ✓ | ✓ | 26 |
| Nathan 2006 | 0* | 91 | 50 | 89 |
| ✓ | 12 |
| Pearlman 1992 | 25 | 78 | 50 | 79 | ✓ |
| 12 |
| Pearlman 2004 | 0* | 92 | 50 | 87 |
| ✓ | 12 |
| Rosenthal 1999 | 0 | 202 | 50 | 206 |
|
| 24 |
| Shapiro 2000 | 0* | 88 | 50 | 93 | ✓ | ✓ | 12 |
| SLMF4002 | 100 | 93 | 100 | 95 | ✓ | ✓ | 26 |
| SMART 2006 | 47 | 13,176 | 50 | 13,179 | ✓ | ✓ | 28 |
| Wolfe 2000 | 33 | 331 | 50 | 167 | ✓ | ✓ | 12 |
| mean duration 27 weeks |
* background ICS treatment was withdrawn from all participants
** 50 micrograms is the ex‐actuator dose, but in some studies this is reported as the equivalent delivered dose of 42 micrograms
| Study ID from adults in Cates 2012b | N | Age | Formoterol Device | Salmeterol Device | Location | Sponsor | Duration (weeks) |
| Condemi 2001 | 528 | 18+ | Foradil Aerolizer | Serevent Diskus | USA | Novartis | 26 |
| Gabbay 1998 | 127 | 18+ | Foradil Aerolizer | Serevent Diskus | UK | Novartis | 12 |
| Vervolet 1998 | 482 | 18+ | Foradil Aerolizer | Serevent Diskus | Europe | Novartis | 26 |
| Total | 1137 | mean duration 24 weeks |
All the above trials compared formoterol (Foradil) 12 mcg twice daily with salmeterol 50 mcg twice daily and all participants were taking a background inhaled corticosteroid.
| Study ID from adults in Cates 2013b | Age (Years | N on F&ICS | N on ICS Alone | Daily dose of budesonide or other ICS (mcg metered dose) | Daily Dose of Formoterol (mcg metered dose) | Once daily | Twice daily | Combined inhalers | Separate inhalers | DPI | pMDI | Duration weeks |
| Brown 2012 | 12+ | 377 | 364 | 800 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Buhl 2003 | 18+ | 352 | 171 | 400 | 12 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Chuchalin 2002 | 18+ | 111 | 114 | 400 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Corren 2007 | 12+ | 123 | 121 | 400 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Corren 2013 | 12+ | 110 | 113 | 250 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| D5896C00001 | 12+ | 312 | 153 | 400 | 12/24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Jenkins 2006 | 12+ | 341 | 115 | 1600 | 48 | ✓ | ✓ | ✓ | ✓ | 24 | ||
| Kuna 2006 | 18+ | 409 | 207 | 200 | 12 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Meltzer 2012 | 12+ | 182 | 188 | 200 (mometasone) | 20 | ✓ | ✓ | 26 | ||||
| Morice 2007 | 12+ | 462 | 217 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Nathan 2010 | 12+ | 191 | 192 | 400 (mometasone) | 20 | ✓ | ✓ | ✓ | 26 | |||
| Nathan 2012 | 12+ | 115 | 117 | 100 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| Noonan 2006 | 12+ | 239 | 109 | 400 | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| O'Byrne 2001 | 18+ | 554 | 550 | 400 | 12 | ✓ | ✓ | ✓ | 52 | |||
| O'Byrne 2001a | 18+ | 315 | 312 | 800 | 12 | ✓ | ✓ | ✓ | 52 | |||
| Pauwels 1997 | 18+ | 210 | 213 | 200 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Pauwels 1997a | 18+ | 215 | 214 | 800 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Pearlman 2012 | 12+ | 119 | 119 | 100 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| Peters 2008 | 12+ | 443 | 133 | 1600 | 48 | ✓ | ✓ | ✓ | 52 | |||
| Price 2002 | 12+ | 250 | 255 | 800 | 24 | ✓ | ✓ | ✓ | 24 | |||
| SD‐039‐0726 | 16+ | 301 | 145 | 200 | 12/24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Spector 2012 | 12+ | 156 | 155 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Weinstein 2010 | 12+ | 255 | 240 | 800 (mometasone) | 20 | ✓ | ✓ | ✓ | 12 | |||
| Zangrilli 2011 | 12+ | 127 | 123 | 800 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Zetterstrom 2001 | 18+ | 238 | 124 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| mean duration 29 weeks |
All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause. Corren 2013, Nathan 2012 and Pearlman 2012 have been added to the trials already included in the Cochrane review.
| Study ID from adults in Cates 2013a | Age of Participants (Years) | N on FSC | N on ICS | Daily dose of fluticasone (mcg) | Daily dose of salmeterol (mcg) | Combined Inhaler | Separate Inhalers | Duration (weeks) |
| Aubier 1999 | 12+ | 338 | 165 | 1000 | 100 | ✓ | ✓ | 28 |
| Bailey 2008 | 12+ | 239 | 236 | 200 | 100 | ✓ | 52 | |
| Bateman 2001 | 12+ | 1709 | 1707 | 200 | 100 | ✓ | 52 | |
| GOAL 2004 | 12+ | 333 | 165 | 200/500/1000 | 100 | ✓ | 12 | |
| Godard 2008 | 18+ | 159 | 159 | 500 | 100 | ✓ | 24 | |
| Ind 2003 | 16+ | 336 | 160 | 500 | 100 | ✓ | 28 | |
| Katial 2011 | 12+ | 306 | 315 | 500 | 100 | ✓ | 52 | |
| Kavuru 2000 | 12+ | 310 | 318 | 200 | 100 | ✓ | 52 | |
| Kerwin 2011 | 12+ | 92 | 90 | 500 | 100 | ✓ | 12 | |
| Koenig 2008 | 12+ | 156 | 156 | 200/500/1000 | 100 | ✓ | 40 | |
| Koopmans 2006 | 18+ | 173 | 177 | 500 | 100 | ✓ | 12 | |
| Lundback 2006 | 18+ | 101 | 102 | 500 | 100 | ✓ | 12 | |
| Murray 2004 | 12+ | 94 | 91 | 200 | 100 | ✓ | 12 | |
| Nathan 2006 | 12+ | 171 | 168 | 220 | 100 | ✓ | 16 | |
| Nelson 2003 | 12+ | 95 | 97 | 200 | 100 | ✓ | 12 | |
| Pearlman 2004 | 12+ | 92 | 89 | 200 | 100 | ✓ | 12 | |
| Renzi 2010 | 12+ | 262 | 270 | 200 | 100 | ✓ | 24 | |
| Rojas 2007 | 12+ | 180 | 182 | 500 | 100 | ✓ | 12 | |
| SAM30007 | 18+ | 29 | 32 | 200/500/1000 | 100 | ✓ | 30 | |
| SAM40004 | 18+ | 42 | 21 | 200 | 100 | ✓ | 52 | |
| SAM40008 | 18+ | 93 | 93 | 1000 | 100 | ✓ | 26 | |
| SAM40031 | 18+ | 41 | 41 | 200/500/1000 | 100 | ✓ | 52 | |
| SAM40065 | 12+ | 150 | 150 | 200/500/1000 | 100 | ✓ | 40 | |
| SAS30022 | 12+ | 210 | 212 | 500 | 50 | ✓ | 12 | |
| SAS30023 | 12+ | 151 | 155 | 100 | 50 | ✓ | 12 | |
| SAS40036 | 15+ | 172 | 159 | 200 | 100 | ✓ | 16 | |
| SAS40037 | 15+ | 161 | 161 | 200 | 100 | ✓ | 16 | |
| SAS40068 | 12+ | 262 | 270 | 200 | 100 | ✓ | 24 | |
| SFA103153 | 12+ | 239 | 236 | 200 | 100 | ✓ | 52 | |
| SFCF4026 | 18+ | 159 | 159 | 500 | 100 | ✓ | 24 | |
| Shapiro 2000 | 12+ | 84 | 84 | 500 | 100 | ✓ | 12 | |
| SLGF75 | 16+ | 14 | 17 | 200 | 100 | ✓ | 12 | |
| Strand 2004 | 18+ | 78 | 72 | 200 | 100 | ✓ | 12 | |
| van Noord 2001 | 12+ | 337 | 172 | 1000 | 100 | ✓ | 12 | |
| Wallin 2003 | 12+ | 18 | 19 | 400 | 100 | ✓ | 12 | |
| mean duration 34 weeks |
All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause
| Study ID from adults in Cates 2010 | N | Duration (weeks) | Formoterol device | Formoterol dose | ICS type and dose | Salmeterol device | Salmeterol dose | ICS type and dose | Duration (weeks) |
| Aalbers 2004 | 439 | 26 | DPI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 26 |
| Bodzenta‐Lukaszyk 2011 | 202 | 12 | HFA pMDI with AeroChamber | 10 µg bd | Fluticasone 100 µg or 250 µg bd | HFA pMDI with AeroChamber | 50 µg bd | Fluticasone 100 µg or 250 µg bd | 12 |
| Busse 2008 | 833 | 30 | pMDI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 30 |
| Dahl 2006 | 1397 | 24 | DPI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 24 |
| Kuna 2007 | 2218 | 24 | DPI | 12 µg bd | Budesonide 400 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg bd | 24 |
| Maspero 2010 | 404 | 52 | pMDI | 10 µg bd | Mometasone 200 µg or 400 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg or 500 µg bd | 52 |
| Papi 2007 | 228 | 12 | pMDI | 12 µg bd | Beclomethasone extra fine 200 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| Ringdal 2002 | 428 | 12 | DPI two separate inhalers | 12 µg bd | Budesonide 800 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| SAM 40010 | 373 | 12 | DPI | 6 µg bd | Budesonide 200 µg bd | DPI | 50 µg bd | Fluticasone 100 µg bd | 12 |
| SAM 40048 | 247 | 12 | DPI | 6 µg bd | Budesonide 200 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| mean duration 23 weeks | |||||||||
All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause
All reviews used the same inclusion criteria apart from the treatments themselves (randomised controlled trials in participants of any age with a diagnosis of asthma) and outcome measures (all‐cause mortality, all‐cause non‐fatal serious adverse events, asthma‐related mortality and serious adverse events). The included studies were not restricted to products approved for adults by the FDA, and most of the trials on LABA and inhaled steroids delivered both treatments in a single (combination) inhaler, as shown in Table 10 and Table 11. The definition of serious adverse events was uniform across the reviews (see Appendix 4), and data were well reported for fatal and non‐fatal serious adverse events of any cause (our primary outcomes).
A total of 89 studies on 61,366 adults and adolescents were included in the reviews of monotherapy versus placebo and combination therapy versus ICS (Cates 2008; Cates 2012a; Cates 2013a; Cates 2013b). The three new studies contributed an additional 447 participants to the formoterol monotherapy comparison and 693 participants to the formoterol combination therapy comparison. Three trials including 1116 adults and adolescents directly compared formoterol monotherapy versus salmeterol monotherapy (Cates 2012b), and ten trials including 8498 adults and adolescents directly compared combination therapies (Cates 2010). All studies were conducted in adults and adolescents over 12 years of age in a range of settings, between 1992 and 2010. Separate data on adolescents over the age of 12 years were not available from these trials, so we carried out our analyses for all participants over the age of 12 years. The early studies primarily randomly assigned adults to formoterol or salmeterol monotherapy versus placebo, with variable proportions of participants using ICS as background therapy. In later years, studies tended to randomly assign participants to ICS treatment in control and intervention groups, and almost all studies gave the ICS in a combination inhaler with formoterol or salmeterol.
Methodological quality of included reviews
Quality of the included reviews
We assessed the methods used in the reviews by using the AMSTAR tool (Shea 2007). As all included reviews were Cochrane reviews, they were conducted according to the rigorous methods presented in the Cochrane Handbook for Systematic Reviews of Interventions; therefore the AMSTAR ratings were high (all achieved a score of at least nine of a possible 11). We had sought additional data from the manufacturers' websites and from FDA reports for each individual review to minimise publication bias. This had the effect of reducing the risk of bias in the overview as well as in the reviews. The numbers of participants with fatal and non‐fatal serious adverse events were clearly reported on the manufacturers' websites.
Because one of the authors of this overview (CJC) is also the lead author of all of the included reviews, quality assessments were conducted by Susan Wieland and Elizabeth Stovold. Complete agreement between the assessors was reached, and the full quality assessment is summarised in Table 13.
| AMSTAR Criteria | ||||||
| 1. Was an 'a priori' design provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2a. Was there duplicate study selection? (0.5 point) | Yes | Yes | Yes | Yes | Yes | No |
| 2b. Was there duplicate data extraction? (0.5 point) | No | No | Yes | Yes | Yes | No |
| 3. Was a comprehensive literature search performed? | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | No | No | No | No | No | No |
| 5. Was a list of studies (included and excluded) provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Were the characteristics of the included studies provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was the scientific quality of the included studies assessed and documented? | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the methods used to combine the findings of studies appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Was the likelihood of publication bias assessed? | Yes | Yes | Yes | Yes | Not applicable | Not applicable |
| 11. Was the conflict of interest stated? | Yes | Yes | Yes | Yes | Yes | Yes |
| Total criteria met: | 10.5 | 10.5 | 11 | 11 | 10 | 9 |
| Note: item 4 is met with the assessment 'NO', all others 'YES'. We felt that item 2 was 2 separate questions, so we split it into two parts and awarded half a point for each. This differs from the published version of the tool. | ||||||
Risk of bias of the included studies in each review
Each review assessed the risk of bias of included studies related to adults suffering an all‐cause serious adverse event (SAE) and an asthma‐related SAE; the results are summarised as figures in each review. As a very large number of studies were included, we have summarised study findings in narrative form. Although reporting of sequence generation and allocation concealment was patchy in the trial reports, discussion with the trial sponsors revealed that standard procedures adopted in the trials would lead to a uniformly low risk of selection bias. The included studies were double‐blind in design, with the exception of two studies comparing formoterol monotherapy versus placebo (Molimard 2001 and van Schayck 2002), two studies comparing formoterol combination therapy versus salmeterol combination therapy (Aalbers 2004 and Busse 2008) and all three studies comparing formoterol monotherapy versus salmeterol monotherapy. Complete all‐cause SAE outcome data were obtained with the exceptions shown in Table 7 and Table 8 for some of the monotherapy trials. The primary outcome results were not downgraded because of risks of bias, except for open studies in the review comparing formoterol versus salmeterol monotherapy (see Table 5).
However, no independent assessment of the causation of SAEs was performed in any of the studies (with the single exception of asthma‐related mortality in SMART). This means that the trials were not clearly protected from ascertainment bias for asthma‐related events. Even with double‐blinding, if the threshold was high for assessing any SAE as asthma‐related across all participants in a trial, this could reduce the numbers of events deemed to be asthma‐related and could introduce bias by reducing apparent differences between the groups for asthma‐related events. The possibility of different thresholds between trials is a particular threat to the validity of indirect comparisons of asthma‐related events between trials.
We therefore have lower confidence in the findings for asthma‐related serious adverse events (see Table 5).
Transitivity assumptions
We did not attempt to carry out a network meta‐analysis and did not make indirect comparisons between monotherapy and combination therapy trials, because we did not consider that the transitivity assumptions needed were justifiable. In other words, any individual participant would not have been equally likely to have been randomly assigned to any of the trials included in the indirect comparison. Although some recent trials included randomised arms for combination therapy, ICS, monotherapy and placebo, the randomisation process was stratified such that adults receiving previous ICS were randomly assigned to the first two arms of the trial, and those not taking ICS to the second two arms. Adults with asthma therefore were not equally likely to be randomly assigned to combination therapy or monotherapy. This is a confounding factor when monotherapy is compared with combination therapy, which makes the proposed indirect comparisons unreliable (because they violate the transitivity assumptions needed for an indirect comparison). Moreover the study design used in SMART 2006 resulted in much lower levels of supervision of adults than that used in the smaller trials, and this was thought to be an important potential effect modifier. Similarly, although we carried out indirect comparisons of the safety of formoterol and salmeterol and detected no inconsistency between direct and indirect comparisons, we did not combine direct and indirect comparisons of formoterol versus salmeterol because of potential effect modification caused by different inhaled corticosteroids and differences in trial design between SMART 2006 and the smaller trials.
Effect of interventions
None of the studies included in the reviews reported separate data for adolescents, so we analysed all participants over 12 years of age who were randomly assigned to studies in adults. We refer to this group as "adults" when describing the results.
We have created four new 'Summary of findings' tables for this overview (see Table 1; Table 2; Table 3; Table 4). Table 1 summarises the relative and absolute impact of regular formoterol or salmeterol (as monotherapy) on all‐cause mortality and non‐fatal serious adverse events of any cause in adults with asthma. Table 2 summarises data comparing formoterol monotherapy versus salmeterol monotherapy, and Table 3 summarises regular formoterol or salmeterol randomly assigned in conjunction with inhaled corticosteroids versus the same dose of inhaled corticosteroids. Finally Table 4 summarises formoterol combination therapy versus salmeterol combination therapy.
The forest plots show the pooled results of trials from the comparison in each review using the convention of a box to indicate the weight and point estimate, and horizontal lines to display the 95% confidence interval of the pooled results from each review. When appropriate, the pooled results from the formoterol and salmeterol reviews have been combined to show a class effect of LABA; these combined results are shown as a diamond, in which the centre of the diamond represents the point estimate of the combined results, and the width of the diamond shows its 95% confidence interval for the class effect of LABA. Heterogeneity between pooled formoterol and salmeterol results is reported as Chi2 and I2 statistics on the forest plots.
Formoterol or salmeterol monotherapy versus placebo (with variable background use of inhaled corticosteroids)
An analysis of outcomes from the formoterol and salmeterol monotherapy reviews is shown in Figure 3 and is summarised in Table 1 and Table 5. A total of 17 studies with 5774 adults compared formoterol versus placebo (see Table 7), and 19 studies with 32,014 adults compared salmeterol versus placebo (see Table 8). The proportion of adults using background inhaled corticosteroids was variable, and the proportion in each study is shown in Table 7 and Table 8. However, we have no information that shows whether any of the adults who died in these studies were actually taking inhaled corticosteroids at the time. Most of the deaths on monotherapy of any kind (42 on salmeterol and 32 on placebo) occurred among the 26,355 participants in SMART 2006.
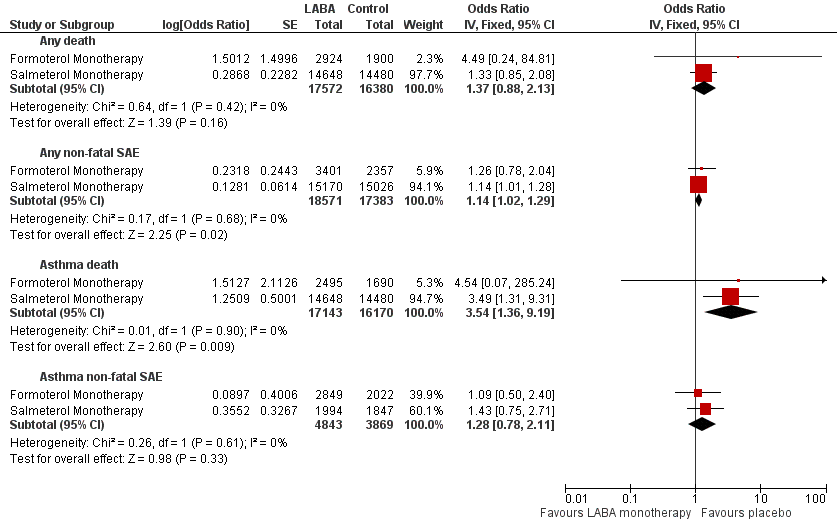
Formoterol or salmeterol monotherapy versus placebo (with variable background use of ICS).
Death of any cause
Formoterol monotherapy: 13 trials contributed 4824 adults; two deaths occurred on formoterol and none on placebo. The pooled OR was 4.49 (95% CI:0.24 to 84.80), I2 = 0%, with a GRADE rating of low confidence. The absolute increase (and 95% CI) could not be calculated from the pooled OR, as no deaths on placebo were reported.
Salmeterol monotherapy: 10 trials contributed 29,128 adults; 44/14,648 deaths occurred on salmeterol and 33/14,480 on placebo. The pooled OR was 1.33 (95% CI 0.85 to 2.08), I2 = 0%. This represents an absolute increase of 8 per 10,000 treated for 27 weeks (95% CI 3 less to 25 more), GRADE rating moderate.
All LABA monotherapy: When all 23 of the above trials were combined, they contributed 33,952 adults with 46 deaths on LABA and 33 on placebo. The pooled OR was 1.37 (95% CI 0.88 to 2.13), I2 = 0%. This represents an absolute increase of 7 per 10,000 over 26 weeks (95% CI 2 less to 23 more), GRADE rating moderate.
No significant heterogeneity was found between the formoterol and salmeterol subgroups (Figure 3).
Non‐fatal SAEs of any cause
Formoterol monotherapy: 17 trials contributed 5758 adults; 48/3401 adults with non‐fatal events were reported on formoterol and 25/2357 on placebo. The pooled OR was 1.26 (95% CI 0.78 to 2.04) , I2 = 15%. This represents an absolute increase of 27 per 10,000 over 14 weeks (95% CI 23 fewer to 108 more), GRADE rating moderate.
Salmeterol monotherapy: 13 trials contributed 30,196 adults; 587/15,170 adults with a non‐fatal SAE were reported on salmeterol and 518/15,026 on placebo. The pooled OR was 1.14 (1.01 to 1.28), I2 = 0%. This represents an absolute increase of 46 per 10,000 over 27 weeks (95% CI 3 more to 92 more), GRADE rating high.
All LABA monotherapy: When all 30 trials were combined, they contributed 35,954 adults; 635/18,571 adults with events were reported on LABA and 543/17,383 on placebo. The pooled OR was 1.14 (1.02 to 1.29), I2 = 2%, high confidence. This represents an absolute increase of 43 per 10,000 over 26 weeks (95% CI 6 more to 85 more), GRADE rating high. No significant heterogeneity was found between the formoterol and salmeterol subgroups (Figure 3).
Asthma‐related deaths
Formoterol monotherapy: 12 trials contributed 4185 adults; one asthma‐related death was reported on formoterol and none on placebo. The pooled OR was 4.54 (95% CI 0.07 to 285.25), with a GRADE rating of low confidence. The absolute increase (and 95% CI) could not be calculated from the pooled OR.
Salmeterol monotherapy: 10 trials contributed 29,128 adults; 13/14,648 deaths were reported on salmeterol and three/14,480 on placebo (all in SMART 2006, which was the only trial that reported using independent assessment of the cause of death). The pooled OR was 3.49 (95% CI 1.31 to 9.31), I2 = 0%. This represents an absolute increase of 5 per 10,000 treated for 27 weeks (95% CI 1 more to 17 more), GRADE rating high.
All LABA monotherapy: When all 22 of the above trials were combined, they contributed 33,313 adults with 14 deaths on LABA and three on placebo. The pooled OR was 3.54 (95% CI 1.36 to 9.19), I2 = 0%, moderate confidence. This represents an absolute increase of five per 10,000 over 26 weeks (95% CI 1 more to 16 more), GRADE rating high.
No significant heterogeneity was found between the formoterol and salmeterol subgroups (Figure 3).
Asthma non‐fatal SAEs
Formoterol monotherapy: 15 trials contributed 4871 adults; 17/2849 adults with non‐fatal events were reported on formoterol and 10/2022 on placebo. The pooled OR was 1.09 (95% CI 0.50 to 2.40) , I2 = 20%. An absolute increase of 4 per 10,000 was seen over 14 weeks (95% CI 24 fewer to 68 more), GRADE rating low.
Salmeterol monotherapy: 12 trials contributed 3841 adults; 23/1994 adults with a non‐fatal SAE were reported on salmeterol and 16/1847 on placebo. The pooled OR was 1.43 (0.75 to 2.71), I2 = 0%. This represents an absolute increase of 37 per 10,000 over 18 weeks (95% CI 22 fewer to 145 more), GRADE rating low. SMART 2006 did not contribute to this analysis, as data on this outcome were not available.
All LABA monotherapy: When all 27 trials were combined, they contributed 8712 adults; 40/4843 adults with events were reported on LABA and 26/3869 on placebo. The pooled OR was 1.28 (0.78 to 2.11), I2 = 0%, high confidence. This represents an absolute increase of 19 per 10,000 over 16 weeks (95% CI 15 fewer to 73 more), GRADE rating low. No significant heterogeneity was found between the formoterol and salmeterol subgroups (Figure 3).
Formoterol monotherapy versus salmeterol monotherapy
One of the systematic reviews (Cates 2012b) looked for evidence from trials that randomly assigned adults to receive either regular formoterol or salmeterol. These trials were considered to provide monotherapy, as ICS was not part of the randomly assigned treatment, but investigators reported that all adults were taking background ICS (see Table 9 for details of the studies included in this review). Direct comparisons from three open trials on 1116 adults comparing formoterol (Foradil) versus salmeterol in this review are summarised for each outcome in Figure 4, and the primary outcomes appear in Table 2. The confidence intervals were very wide because of the small number of participants studied, and no significant differences were found in mortality or non‐fatal serious adverse events of all‐causes or were attributed to asthma. The GRADE rating for these comparisons was low or very low and is shown with a summary of results for the outcomes in Table 2 and Table 5.
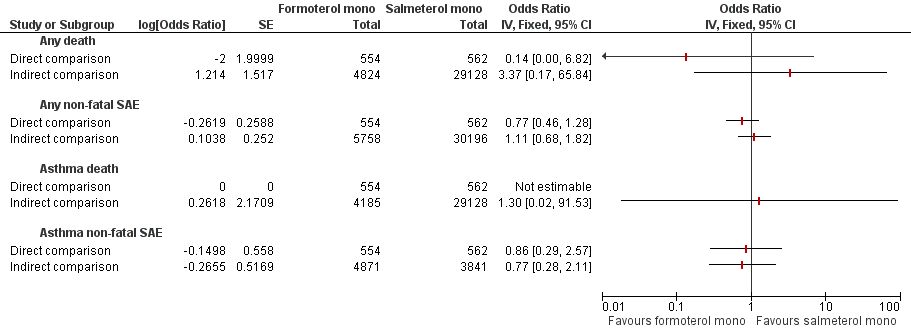
Formoterol monotherapy versus salmeterol monotherapy.
We planned to combine the above direct comparisons with indirect comparisons between pooled results of the trials that compared formoterol versus placebo (Cates 2012a) and pooled results of the trials that compared salmeterol versus placebo (Cates 2008), as shown in Figure 1A. The indirect comparisons are shown alongside the direct comparisons for each outcome in Figure 4. However, the design of SMART 2006 was quite different from that of all other included studies, and this was reflected in much higher weekly rates of serious adverse events in the control arms of the salmeterol monotherapy trials (see Table 14). We did not proceed to attempt to combine indirect comparisons between formoterol and salmeterol monotherapy with direct comparisons because of the risk that serious confounding from the different study designs would violate the transitivity assumption.
| Comparison | Adults with an event on control (n) | Total number of adults on control (N) | SAE per 10,000 adults (95% CI) | Mean duration of trials (weeks) | SAE per 10,000 adults per week |
| Formoterol v Placebo | 25 | 2357 | 106 (65 to 147) | 14 | 7.6 |
| Salmeterol v Placebo | 518 | 15026 | 345 (317 to 375) | 27 | 12.8 |
| Formoterol & ICS v ICS | 115 | 4764 | 241 (197 to 285) | 29 | 8.3 |
| Salmeterol & ICS v ICS | 135 | 6461 | 209 (177 to 247) | 34 | 6.1 |
Formoterol or salmeterol combination therapy versus the same dose of inhaled corticosteroids
An analysis of the outcomes from formoterol and salmeterol combination therapy versus ICS reviews is shown in Figure 5 and is summarised in Table 3 and Table 5. A total of 25 studies with 11,269 adults compared formoterol combination versus the same dose of ICS (budesonide, mometasone or fluticasone), as detailed in Table 10. A total of 35 studies with 14,086 adults compared salmeterol combination versus the same dose of ICS (fluticasone), as detailed in Table 11.

Formoterol or salmeterol combination therapy versus the same dose of ICS.
Death of any cause
Formoterol combination therapy: 25 trials contributed 11,271 adults; 6/6507 adults died on combination formoterol and 1/4764 on ICS alone. The pooled OR was 3.56 (95% CI 0.79 to 16.03), I2 = 0%. The absolute increase was 5 per 10,000 over 29 weeks (95% CI 0 to 30 more), with a GRADE rating of moderate confidence.
Salmeterol combination therapy: 35 trials contributed 13,447 adults; seven/6986 deaths were reported on salmeterol and 7/6461 on placebo. The pooled OR was 0.90 (95% CI 0.31 to 2.6), I2 = 0%. This represents an absolute decrease of 1 per 10,000 treated for 34 weeks (95% CI 8 fewer to 18 more), GRADE rating moderate.
All LABA combination therapy: When all 60 of the above trials were combined, they contributed 24,718 adults; 13/13,493 deaths were reported on LABA combination therapy and 8/11,225 on the same ICS alone. The pooled OR was 1.42 (95% CI 0.60 to 3.38), I2 = 0%. This represents an absolute increase of 3 per 10,000 over 32 weeks (95% CI 3 fewer to 17 more), GRADE rating moderate.
Some heterogeneity was found between the formoterol and salmeterol subgroups for this outcome, as shown in Figure 5 (Chi2 = 2.13, df = 1, P = 0.14, I2 = 53%), but this did not reach statistical significance and may have resulted from the play of chance, as the number of deaths in each subgroup was small (seven and 14, respectively).
Non‐fatal SAEs of any cause
Formoterol combination therapy: 25 trials contributed 11,271 adults; 145/6507 adults suffered a non‐fatal serious adverse event on combination formoterol and 115/4764 on ICS alone. The pooled OR was 0.99 (95% CI 0.77 to 1.27), I2 = 0%. The absolute decrease was 2 per 10,000 over 29 weeks (95% CI 54 fewer to 63 more), with a GRADE rating of moderate confidence.
Salmeterol combination therapy: 35 trials contributed 13,447 adults; 167/6986 non‐fatal events were reported on salmeterol and 135/6461 on placebo. The pooled OR was 1.15 (95% CI 0.91 to 1.44), I2 = 0%. This represents an absolute increase of 31 per 10,000 treated for 34 weeks (95% CI 18 fewer to 89 more), GRADE rating moderate.
All LABA combination therapy: When all 60 of the above trials were combined, they contributed 24,718 adults; 312/13,493 events were reported on LABA combination therapy and 250/11,225 on the same ICS alone. The pooled OR was 1.07 (95% CI 0.90 to 1.27), I2 = 0%. This represents an absolute increase of 16 per 10,000 over 32 weeks (95% CI 22 less to 60 more), GRADE rating moderate.
No significant heterogeneity was found between the formoterol and salmeterol subgroups, as shown in Figure 5.
Asthma‐related deaths
Formoterol combination therapy: 25 trials contributed 11,271 adults; one adult died on combination formoterol and none on ICS alone. The pooled OR was 7.34 (95% CI 0.15 to 369.71). The absolute increase could not be calculated, and the GRADE rating indicated low confidence.
Salmeterol combination therapy: No deaths were reported in either arm of the 35 trials on 13,447 adults.
All LABA combination therapy: When all 60 of the above trials were combined, they contributed 24,718 adults, but only a single death was reported, so the pooled OR remained very uncertain at 7.34 (95% CI 0.15 to 369.71), as shown in Figure 5.
Asthma‐related non‐fatal SAEs
Formoterol combination therapy: 24 trials contributed 10,901 adults; 17/6325 adults suffered a non‐fatal serious adverse event related to asthma on combination formoterol and 30/4576 on ICS alone. The pooled OR was 0.49 (95% CI 0.28 to 0.88), I2 = 0%. The absolute decrease was 34 per 10,000 over 29 weeks (95% CI 47 fewer to 8 fewer), with a GRADE rating of moderate confidence, as no independent assessment of the cause of the serious adverse events was performed.
Salmeterol combination therapy: 35 trials contributed 13,447 adults; 29/6986 non‐fatal events were reported on salmeterol and 23/6461 on ICS alone. The pooled OR was 1.12 (95% CI 0.65 to 1.94), I2 = 5%. This represents an absolute increase of 5 per 10,000 treated for 34 weeks (95% CI 15 less to 40 more), GRADE rating low.
All LABA combination therapy: When all 59 of the above trials were combined, they contributed 24,348 adults with 36/13,311 events on LABA combination therapy and 53/11,037 on the same ICS alone. The pooled OR was 0.76 (95% CI 0.51 to 1.13).
Significant statistical heterogeneity was found between the formoterol and salmeterol subgroups (Chi2 = 4.06, df = 1, P = 0.04, I2 = 75%), as shown in Figure 5. Possible reasons for this heterogeneity include potential differences between the formoterol and salmeterol trials in the way in which causation was attributed, and, as previously noted, no independent adjudication of causation was see in any of these trials.
The wide confidence intervals of the risk differences for both asthma‐related non‐fatal events and all‐cause non‐fatal events (see Appendix 5) meant that we could not be sure whether the variation in point estimates for disease‐specific and all‐cause outcomes was due to the play of chance.
Formoterol combination therapy versus salmeterol combination therapy
Direct comparisons
Trials comparing formoterol combination therapy directly versus salmeterol combination therapy were assessed in Cates 2010. The pooled results of these trials are shown in the forest plot in Figure 6 for each outcome (under the label of direct comparisons), and the primary outcomes are shown in Table 4.

Formoterol combination therapy versus salmeterol combination therapy.
Indirect comparisons
Combination therapy trials in Cates 2013b comparing formoterol combination therapy versus the same dose of ICS were of similar design and duration to the trials in Cates 2013a comparing salmeterol and fluticasone (FPS) versus the same dose of fluticasone (see Table 14). The ICS arm event rates were also reasonably similar (see Table 14), so we did not demonstrate a difference between the safety of budesonide and that of fluticasone in the control arms of these trials. We therefore decided to proceed with an indirect comparison between these sets of trials. The indirect comparison subtracted the log OR of the pooled FPS versus fluticasone results from the log OR of the pooled formoterol combination versus ICS; the odds ratios for all indirect comparisons are shown on the second line for the outcomes in Figure 6.
Although comparison of direct and indirect results indicated no significant inconsistency, and the control event rates were similar, nevertheless, we decided not to proceed with combining the direct and indirect comparisons of the safety of formoterol and salmeterol because of differences in trial design and unknown potential differences between fluticasone and budesonide given at the different doses used in the trials. Any indirect differences found between the formoterol and salmeterol trials might have been caused by differences between the inhaled corticosteroids (or other differences between the trials, including ascertainment of events), rather than by differences between the safety of formoterol and that of salmeterol.
Death of any cause
No significant difference in all‐cause mortality was found between formoterol and salmeterol combination therapy from the small number of trials that directly compared the two treatments (OR 2.68, 95% CI 0.44 to 16.14, I2 = 0%,10 studies, N = 6769). A total of 59 trials on 24,348 adults compared each combination product versus the same dose of inhaled corticosteroids. Even so, considerable uncertainty was still noted around the odds ratio from the indirect comparison of all‐cause mortality on formoterol combination therapy versus salmeterol combination therapy (OR 3.93, 95% CI 0.62 to 24.74). The causes of all deaths in the combination therapy trials are shown in Table 15 and Table 16.
| Study ID | Treatment arm | Cause of death |
| Buhl 2003 | Formoterol and budesonide | Cardiac arrest |
| O'Byrne 2001 | Formoterol and budesonide (separate inhalers) | Status asthmaticus, followed by septic shock |
| Pauwels 1997a | Formoterol and budesonide (separate inhalers) | Suicide |
| Zetterstrom 2001 | Formoterol and budesonide | Suicide |
| Brown 2012 | Formoterol and budesonide | Cerebro‐vascular accident |
| Brown 2012 | Budesonide | Homicide |
| Nathan 2010 | Formoterol and mometasone | Uterine Leiomyosarcoma |
| Jenkins 2006 | Formoterol and budesonide | Pulmonary embolus (but the death occurred after the control budesonide arm was discontinued so was not included in the meta‐analysis) |
| Study ID | Treatment arm | Cause of death |
| Aubier 1999 | salmeterol and fluticasone (separate inhalers) | Bronchial carcinoma (one death) |
| GOAL 2004 | salmeterol/fluticasone | Myocardial infarction (two deaths) and pneumonia (one death) |
| GOAL 2004 | fluticasone | Myocardial infarction (two deaths) |
| Ind 2003 | salmeterol and fluticasone (separate inhalers) | Pneumothorax (one death) |
| Kerwin 2011 | salmeterol/fluticasone | Cardiac disease (one death) |
| Kerwin 2011 | fluticasone | Breast cancer (one death) |
| Koenig 2008 | fluticasone | Cardiac arrest and deep vein thrombosis (one death) |
| Renzi 2010 | fluticasone | Cardiac arrest (one death) |
| SAS40068 | fluticasone | Ventricular hypertrophy and aortic hypoplasia (one death) |
| Strand 2004 | fluticasone | Unknown cause (one death) |
| van Noord 2001 | salmeterol/fluticasone | Leukaemia (one death) |
Non‐fatal SAEs of any cause
Direct evidence from trials that randomly assigned the combination products head‐to‐head showed no significant differences between formoterol and salmeterol (OR 0.69, 95% CI 0.37 to 1.26, I2 = 33%, 8 studies, N = 6163). Indirect evidence from trials comparing formoterol combination therapy versus salmeterol combination therapy also showed no significant differences (OR 0.86, 95% CI 0.61 to 1.22).
Asthma‐related deaths
Only a single death from asthma was reported (in a trial comparing formoterol combination therapy versus budesonide), so no comparison of pooled estimates was possible for this outcome.
Asthma‐related non‐fatal SAEs
Direct evidence from trials that randomly assigned the combination products head‐to‐head showed no significant differences between formoterol and salmeterol (OR 0.69, 95% CI 0.37 to 1.26, I2 = 33%, 8 studies, N = 6163). However, pooled results from adult trials of formoterol combination therapy revealed a significant reduction in the risk of serious adverse events attributed to asthma, whilst trials of salmeterol combination therapy found a non‐significant increase in risk (see Figure 5 and Table 5).
When findings are compared, the indirect evidence shows a significant advantage for formoterol combination therapy (OR 0.44, 95% CI 0.20 to 0.98). This indirect evidence remains subject to differences between the inhaled corticosteroids used and between formoterol and salmeterol. An additional complication is seen for asthma‐related events, in that these may have been attributed in a different way between the formoterol and salmeterol trials. We are therefore very unsure of the causation of the indirect difference found between the formoterol and salmeterol trials.
Discussion
Summary of main results
How we assessed the safety of regular formoterol and salmeterol
We have summarised the safety evidence from Cochrane reviews that included randomised controlled trials in which regular formoterol or salmeterol was compared with placebo (with varying proportions of adults who had been prescribed background treatment with ICS) and trials in which the same products were randomly assigned with ICS (usually in a single combination inhaler) and compared with the same dose of ICS alone. We have supplemented the results of six Cochrane reviews with additional data from three recently published trials of formoterol (alone and in combination with fluticasone). We have not carried out a network meta‐analysis, as the networks shown in Figure 1 are not connected. Moreover we have not combined the direct and indirect comparisons of formoterol and salmeterol because of unknown safety differences between fluticasone and budesonide in the combination therapy trials, and because of differences in trial design in the monotherapy trials. However we have contrasted the safety of formoterol and salmeterol when used with and without randomly assigned ICS, to try to find out whether we can see an improved safety profile when combination therapy is used (as the use of combination therapy prevents the substitution of LABA for ICS).
Is the risk of dying increased on regular formoterol or salmeterol?
None of the reviews found a significant increase in death of any cause, nor could any of the reviews exclude the possibility of a two‐fold increase in mortality on regular formoterol or salmeterol (as monotherapy or combination therapy) in adults with asthma. The pooled mortality results were as follows: formoterol monotherapy OR 4.49 (95% CI 0.24 to 84.80, 13 trials, N = 4824), salmeterol monotherapy OR 1.33 (95% CI 0.85 to 2.08, 10 trials, N = 29,128), formoterol combination OR 3.56 (95% CI 0.79 to 16.03, 25 trials, N = 11,271) and salmeterol combination OR 0.90 (95% CI 0.31 to 2.6, 35 trials, N = 13,447). In each case, I2 = 0%, and the quality of evidence was rated as moderate. Absolute differences in mortality were very small, translating into an increase of 7 per 10,000 over 26 weeks on any monotherapy (95% CI 2 less to 23 more), as shown in Table 1 and 3 per 10,000 over 32 weeks on any combination therapy (95% CI 3 less to 17 more), as shown in Table 3.
Very few deaths were reported in the combination therapy trials, and trial designs were not the same for combination therapy and monotherapy trials. Therefore we could not assess whether the risks of mortality on regular combination therapy were different from the risks on regular monotherapy.
Only one death occurred in the monotherapy trials comparing formoterol versus salmeterol, so evidence was insufficient to compare mortality from the direct comparisons in these trials.
Is risk of non‐fatal serious adverse events increased on regular formoterol or salmeterol?
Adults with a non‐fatal serious adverse event were more commonly reported on salmeterol monotherapy (OR 1.14, 95% CI 1.01 to 1.28, I2 = 0%,13 trials, N = 30,196), but this finding was not significantly different in any of the other reviews: formoterol monotherapy OR 1.26 (95% CI 0.78 to 2.04, I2 = 15%, 17 trials, N = 5758), formoterol combination OR 0.99 (95% CI 0.77 to 1.27, I2 = 0%, 25 trials, N = 11,271) and salmeterol combination OR 1.15 (95% CI 0.91 to 1.44, I2 = 0%, 35 trials, N = 13,447). This represents an absolute increase on any monotherapy of 43 per 10,000 over 26 weeks (95% CI 6 more to 85 more), as shown in Table 1, and 16 per 10,000 over 32 weeks (95% CI 22 less to 60 more) on any combination therapy (see Table 3).
We detected no significant differences between the risks of non‐fatal events in adults on regular formoterol and salmeterol, given as monotherapy or as combination therapy.
Overall completeness and applicability of evidence
The key question for people making decisions about treating asthma is how each individual will respond to different treatment regimens. In some instances, immediate symptom relief can act as a guide to management, but for each adult or adolescent, the balance between longer‐term risks and benefits of regular LABA is unknown. The risk of asthma exacerbation, hospitalisation or death cannot be judged from the symptomatic impact of treatment for an individual in the short term, and so evidence is needed from trials to give clinicians and patients an idea of the long‐term risk of harms. Evidence from systematic reviews of randomised trials on large populations of adults and adolescents over a prolonged period is needed to assess such risks and to potentially allow both patient and care‐giver to balance potential risks and benefits of treatment. At present, no separate data on adolescents have been published from any of the completed trials, although McMahon 2011 includes an analysis of risks stratified by age group from data submitted by the sponsors to the FDA.
Although individual participant results in relation to death from asthma have been reported for SMART 2006, it was not possible to ascertain from the records whether the people who died from asthma were taking inhaled corticosteroids at the time of their final illness. Records indicate whether an inhaled corticosteroid was prescribed at enrolment into the studies, but this information is of limited value because we do not know whether the prescribed treatment was actually taken by any given individual.
Almost all trials that randomly assigned adults to LABA with ICS used a combination inhaler containing both products, so we were not able to compare the safety of adults randomly assigned to combination therapy in a single inhaler versus adults randomly assigned to two separate inhalers to deliver LABA and ICS.
To take into account the duration that adults remained in the trials, it is preferable to use an analysis of hazard ratios. The only trial to report hazard ratios was SMART 2006, and in the case of this trial, the hazard ratios were very similar to the odds ratios (which we obtained from all other trials).
Chowdhury 2011 highlighted the fact that the FDA has required safety trials of combination therapy with regular LABA and ICS. Each of four of these trials is aiming to recruit 11,700 adults and adolescents over 12 years of age. These trials will compare treatments given for six months and will study budesonide and formoterol (NCT01444430), mometasone and formoterol (NCT01471340), fluticasone and salmeterol (NCT01475721) and Foradil. It has been stipulated that 10% of participants recruited to these trials must be younger than 18 years of age, and we believe it is important that data from the adolescent population are reported separately. A further trial will report findings in 6200 children aged four to 11 years receiving fluticasone and salmeterol (NCT01462344). These ongoing trials are expected to be completed in 2016 to 2017.
It remains to be seen whether these additional trials have sufficient power to resolve the issue of the comparative safety of combination therapy in adults, or whether one type of combination therapy is safer than another. However the design of these large studies is more similar to that of SMART 2006; therefore valuable information will be obtained from trials that may have lower levels of supervision of enrolled adults and adolescents. Arguably, lower levels of supervision may be a closer reflection of daily clinical practice.
Quality of the evidence
All of the included reviews were Cochrane reviews and were judged to be of good quality with high AMSTAR scores. The quality of individual studies was assessed in reviews using the Cochrane risk of bias tool. Although sequence generation and method of allocation concealment were not clearly reported in most of the trials in the reviews, we judged that risk of selection bias was low, as almost all of the trials were sponsored by the manufacturers and used standard methods designed for regulatory purposes. Almost all of the trials were double‐blind in design, and all trials in reviews of combination therapy contributed data on mortality and non‐fatal serious adverse events (although this was not the case for the monotherapy trials, as shown in Table 7 and Table 8). We sought data from manufacturers' websites and FDA reports for the included Cochrane reviews. Therefore combination therapy review results were not downgraded because of risks of bias in the included trials, but monotherapy review results may be subject to reporting bias in view of missing data from some trials.
We chose all‐cause SAEs as the primary outcome for this overview because ascertainment bias is a concern for asthma‐related events. Even in double‐blind trials, a high threshold for labelling events as asthma‐related could lead to an underestimation of the true effect of treatment on such events. Moreover a participant with an SAE may have this recorded under more than one category (leading to double‐counting of individual participants), whereas data on the number of participants with at least one SAE of any cause are more reliably available from the manufacturers' trial reports on their websites. Indirect comparisons of asthma‐related events will be subject to additional bias if there is a difference in threshold between the studies.
Potential biases in the overview process
Sensitivity analyses were carried out using risk differences; these gave very similar results to the point estimates derived from the odds ratios (see Appendix 5 and Cates 2011 for full details of risk difference meta‐analysis results).
The indirect comparisons made between formoterol combination therapy and salmeterol combination therapy are subject to potential confounding due to differences between the individual trials, which would not have been protected by their randomised design. In particular, assessment of causation may not have been the same across trials, so indirect comparisons of asthma‐related serious adverse events are at particular risk of bias.
Chris Cates was an author of all included Cochrane systematic reviews of adverse events. He therefore played no part in the independent quality assessment of these reviews.
Agreements and disagreements with other studies or reviews
We were unable to reach a conclusion regarding the risks of mortality or the relative safety of formoterol versus salmeterol in children in our recent overview of the safety of formoterol and salmeterol monotherapy and combination therapy in children (Cates 2012c). However, although we were unable to conclude that LABA combination therapy was risk free in children, the absolute increase in risk of non‐fatal serious adverse events (three per thousand over three months) for children on combination therapy was smaller than the absolute increase in risk for children on LABA monotherapy.
In adults the increase in risk of an all‐cause non‐fatal serious adverse event on combination therapy is about half that found in children, but the wide confidence intervals mean that we cannot be sure if there is a difference between the safety of combination therapy in the different age groups. McMahon 2011 in their overview of the safety data submitted to the FDA expressed the same uncertainty about the relation between age and the safety of combination therapy.
Sears 2013 found a significant reduction in asthma‐related serious adverse events (but not all‐cause events) in an updated overview of the safety of formoterol in combination with inhaled corticosteroids. This is in keeping with the findings of the Cochrane review on formoterol combination therapy (Cates 2013b).

Network of comparisons of serious adverse events from reviews of regular formoterol and salmeterol. Figure 1A shows the numbers of trials and adults on monotherapy versus placebo. Figure 1B shows the numbers of trials and adults on combination therapy versus the same dose of ICS. Adults randomly assigned to other arms in the included trials have not been counted.

Review selection flow diagram.

Formoterol or salmeterol monotherapy versus placebo (with variable background use of ICS).
Formoterol monotherapy versus salmeterol monotherapy.

Formoterol or salmeterol combination therapy versus the same dose of ICS.

Formoterol combination therapy versus salmeterol combination therapy.
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular LABA (salmeterol or formoterol) | |||||
| Adults who died of any cause | ||||||
| Formoterol monotherapy v placebo Follow‐up: mean 14 weeks | 0 per 10000 | not estimable (see comment) | OR 4.49 (0.24 to 84.80) | 4824 (13 studies) | ⊕⊕⊝⊝ | No deaths on placebo, two deaths on formoterol |
| Salmeterol monotherapy v placebo Follow‐up: mean 27 weeks | 23 per 10000 | 31 per 10000 (20 to 48) | OR 1.33 (0.85 to 2.08) | 29,128 (10 studies) | ⊕⊕⊕⊝ | |
| LABA monotherapy v placebo Follow‐up: mean 26 weeks | 20 per 10000 | 27 per 10000 (18 to 43) | OR 1.37 (0.88 to 2.13) | 33,952 | ⊕⊕⊕⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol monotherapy v placebo Follow‐up: mean 14 weeks | 106 per 10000 | 133 per 10000 (83 to 214) | OR 1.26 (0.78 to 2.04) | 5758 (17 studies) | ⊕⊕⊕⊝ | |
| Salmeterol monotherapy v placebo Follow‐up: mean 27 weeks | 345 per 10000 | 391 per 10000 (348 to 437) | OR 1.14 (1.01 to 1.28) | 30,196 (13 studies) | ⊕⊕⊕⊕ | |
| LABA monotherapy v placebo Follow‐up: mean 26 weeks | 316 per 10000 | 359 per 10000 (322 to 401) | OR 1.14 (1.02 to 1.29) | 35,954 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (was the mean control group risk across all studies, including those with no events in either arm of the trial). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Confidence intervals are very wide, as only two deaths occurred (‐2 points) 2. Confidence intervals are wide enough to include important harm and benefit (‐1 for imprecision) | ||||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Salmeterol monotherapy | Formoterol monotherapy | |||||
| Adults who died of any cause | ||||||
| Formoterol monotherapy v salmeterol monotherapy Follow‐up: mean 24 weeks | 18 per 10000 | 2 per 10000 (0 to 115) | OR 0.14 (0.00 to 6.82) | 1116 | ⊕⊝⊝⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol monotherapy v salmeterol monotherapy Follow‐up: mean 24 weeks | 641 per 10000 | 501 per 10000 (305 to 806) | OR 0.77 (0.46 to 1.28) | 1116 | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Open studies (‐1 point) and confidence intervals are very wide indeed, as only one death occurred (‐2 points) 2. Open studies and wide confidence intervals (‐1 point each) | ||||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Regular LABA (salmeterol or formoterol) | |||||
| Adults who died of any cause | ||||||
| Formoterol combination therapy v ICS Follow‐up: mean 29 weeks | 2 per 10000 | 7 per 10000 (2 to 32) | OR 3.56 (0.79 to 16.03) | 11,271 | ⊕⊕⊕⊝ | |
| Salmeterol combination therapy v ICS Follow‐up: mean 34 weeks | 11 per 10000 | 10 per 10000 (3 to 29) | OR 0.90 (0.31 to 2.60) | 13,447 | ⊕⊕⊕⊝ | |
| LABA combination therapy v ICS Follow‐up: mean 32 weeks | 7 per 10000 | 10 per 10000 (4 to 24) | OR 1.42 (0.60 to 3.38) | 24,718 | ⊕⊕⊕⊝ | |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Formoterol combination therapy v ICS Follow‐up: mean 29 weeks | 241 per 10000 | 239 per 10000 (187 to 304) | OR 0.99 (0.77 to 1.27) | 11,271 | ⊕⊕⊕⊝ | |
| Salmeterol combination therapy v ICS Follow‐up: mean 34 weeks | 209 per 10000 | 240 per 10000 (191 to 298) | OR 1.15 (0.91 to 1.44) | 13,447 | ⊕⊕⊕⊝ | |
| LABA combination therapy v ICS Follow‐up: mean 32 weeks | 228 per 10000 | 244 per 10000 (206 to 288) | OR 1.07 (0.90 to 1.27) | 24,718 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies, including those with no events in either arm of the trial). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Confidence intervals are wide and include important harm and benefit (‐1 for imprecision) | ||||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Salmeterol combination therapy | Formoterol combination therapy | |||||
| Adults who died of any cause | ||||||
| Direct comparisons of formoterol combination therapy v salmeterol combination therapy Follow‐up: mean 23 weeks | 3 per 10000 | 8 per 10000 (1 to 48) | OR 2.68 | 6769 | ⊕⊕⊝⊝ | Based on data from all formoterol combination trials |
| Adults with a non‐fatal serious adverse event of any cause | ||||||
| Direct comparisons of formoterol combination therapy v salmeterol combination therapy Follow‐up: mean 23 weeks | 226 per 10000 | 252 per 10000 (186 to 342) | OR 1.12 | 6769 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (was the mean control group risk across all studies). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Confidence intervals are very wide, as only five deaths occurred (‐2 points) 2. Confidence intervals are wide and include important harm and benefit (‐1 point) | ||||||
| Comparison (with additional data from two new trials) | Formoterol Monotherapy | Placebo | Pooled Effect Size (95% Confidence Interval) | Quality of the evidence | |||
| Outcome (mean duration 14 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 2 | 2924 | 0 | 1900 | Peto OR 4.49 (95% CI 0.24 to 84.80) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 48 | 3401 | 25 | 2357 | Peto OR 1.26 (95% CI 0.78 to 2.04) | 15% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 1 | 2495 | 0 | 1690 | Peto OR 4.54 (95% CI 0.07 to 285.25) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE due to asthma | 17 | 2849 | 10 | 022 | Peto OR 1.09 (95% CI 0.50 to 2.40) | 23% | ⊕⊕⊝⊝ |
| Comparison | Salmeterol Monotherapy | Placebo | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 27 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 44 | 14648 | 33 | 14480 | Peto OR 1.33 (95% CI 0.85 to 2.08) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 587 | 15170 | 518 | 15026 | Peto OR 1.14 (95% CI 1.01 to 1.28) | 0% | ⊕⊕⊕⊕ |
| Mortality due to asthma | 13 | 14648 | 3 | 14480 | Peto OR 3.49 (95% CI 1.31 to 9.31) | Data from single trial | ⊕⊕⊕⊕ |
| Non‐fatal SAE due to asthma | 23 | 1994 | 16 | 1847 | Peto OR 1.43 (95% CI 0.75 to 2.71) | 0% | ⊕⊕⊝⊝ |
| Comparison | Formoterol Monotherapy | Salmeterol Monotherapy | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 26 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 0 | 554 | 1 | 662 | Peto OR 0.14 (95% CI 0.00 to 6.82) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE all‐cause | 28 | 554 | 36 | 662 | Peto OR 0.77 (95% CI 0.46 to 1.28) | 0% | ⊕⊕⊝⊝ |
| Mortality due to asthma | 0 | 554 | 0 | 662 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 6 | 554 | 7 | 662 | Peto OR 0.86 (95% CI 0.29 to 2.57) | 0% | ⊕⊕⊝⊝ |
| Comparison (with additional data from three new trials) | Formoterol Combination Therapy | Inhaled Corticosteroids | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 29 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 6 | 6507 | 1 | 4764 | Peto OR 3.56 (95% CI 0.79 to 16.03) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 145 | 6507 | 115 | 4764 | Peto OR 0.99 (95% CI 0.77 to 1.27) | 0% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 1 | 6507 | 0 | 4764 | Peto OR 7.34 (95% CI 0.15 to 369.72) | Data from single trial | ⊕⊕⊝⊝ |
| Non‐fatal SAE due to asthma | 17 | 6325 | 30 | 4576 | Peto OR 0.49 (95% CI 0.28 to 0.88) | 0% | ⊕⊕⊕⊝ |
| Comparison | Salmeterol Combination Therapy | Inhaled Corticosteroids | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 34 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 7 | 6986 | 7 | 6461 | Peto OR 0.90 (95% CI 0.31 to 2.60) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 167 | 6986 | 135 | 6461 | Peto OR 1.15 (95% CI 0.91 to 1.44) | 0% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 0 | 6986 | 0 | 6461 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 29 | 6986 | 23 | 6461 | Peto OR 1.12 (95% CI 0.65 to 1.94) | 5% | ⊕⊕⊕⊝ |
| Comparison | Formoterol Combination Therapy | Salmeterol Combination Therapy | Pooled Effect Size (95% Confidence Interval) | ||||
| Outcome (mean duration 24 weeks) | Events | Total | Events | Total | Peto Odds Ratio | I2 | |
| Mortality all‐cause | 4 | 3453 | 1 | 3316 | Peto OR 2.68 (95% CI 0.44 to 16.14) | 0% | ⊕⊕⊕⊝ |
| Non‐fatal SAE all‐cause | 90 | 3453 | 75 | 3316 | Peto OR 1.12 (95% CI 0.82 to 1.53) | 13% | ⊕⊕⊕⊝ |
| Mortality due to asthma | 0 | 3453 | 0 | 3316 | Not estimable | Not applicable | |
| Non‐fatal SAE due to asthma | 17 | 3081 | 25 | 3082 | Peto OR 0.69 (95% CI 0.37 to 1.26) | 33% | ⊕⊕⊝⊝ |
| 1. Few events were observed leading to wide CIs (including the possibilities of no effect and appreciable harm) 2.There was no independent assessment of the cause of serious adverse events, leading to possible ascertainment bias for disease‐specific outcomes 3. Open studies | |||||||
| Review title | Inclusion criteria | Date of search | No. included studies (all versus placebo or ICS) | No. included studies (adults only versus placebo or ICS) | ||||
| Studies (Randomised trials only) | Participants (Diagnosis of asthma; any age group) | Intervention | Comparison | Primary outcome measures (All‐cause mortality & non‐fatal SAEs) | ||||
| 1. Regular treatment with formoterol for chronic asthma: serious adverse events | Yes | Yes | Inhaled formoterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or SABA | Yes | January 2012 | 20 (versus placebo) | 15 (versus placebo) |
| 2. Regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2008 | Yes | Yes | Inhaled salmeterol twice/day; at least 12 weeks duration; any dose; any delivery device | Placebo or SABA | Yes | August 2011 | 24 (versus placebo) | 19 (versus placebo) |
| 3. Regular treatment with formoterol and inhaled steroids for chronic asthma: serious adverse events Cates 2013b | Yes | Yes | ICS and formoterol once or twice/day; at least 12 weeks duration; any dose; any single or separate device | Same dose and type of ICS | Yes | August 2012 | 27 | 20 |
| 4. Regular treatment with salmeterol and inhaled steroids for chronic asthma: serious adverse events Cates 2013a | Yes | Yes | ICS and salmeterol once or twice/day; at least 12 weeks duration; any dose; any single or separate device | Same dose and type of ICS | Yes | August 2012 | 40 | 35 |
| 5. Regular treatment with formoterol versus regular treatment with salmeterol for chronic asthma: serious adverse events Cates 2012b | Yes | Yes | Inhaled formoterol; at least 12 weeks duration; not randomised with ICS | Inhaled salmeterol; at least 12 weeks duration; not randomised with ICS | Yes | January 2012 | 4 | 3 |
| 6. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events Cates 2010 | Yes | Yes | Inhaled formoterol with an ICS; at least 12 weeks duration; any dose; any single or separate delivery device | Inhaled salmeterol with an ICS; at least 12 weeks duration; any dose; any single or separate delivery device | Yes | August 2011 | 10 | 10 |
| Study ID from adults in Cates 2012a | % patients on background ICS | (N) | (N) | (N) | Placebo (N) | Mortality data (all‐cause) | Duration (weeks) |
| Formoterol Dose | 48 mcg/day | 24 mcg/day | 12 mcg/day |
|
| ||
| Bensch 2001 | 51 | 135 | 136 | 136 | ✓ | 12 | |
| Busse 2004 | 64 | 80 | 80 | ✓ | 12 | ||
| Corren 2007 | 0 | 123 | 131 | ✓ | 12 | ||
| Corren 2013 | 0 | 111 | 109 | ✓ | 12 | ||
| Ekstrom 1998 | 86 | 135 | 129 |
| 12 | ||
| Ekstrom 1998a | 89 | 114 | 113 |
| 12 | ||
| Fitzgerald 1999 | 100 | 89 | 91 | ✓ | 24 | ||
| LaForce 2005 | 67 | 86 | 91 | ✓ | 12 | ||
| Molimard 2001 | 100 | 130 | 129 | ✓ | 12 | ||
| Nathan 2012 | 0 | 116 | 111 | ✓ | 12 | ||
| Noonan 2006 | 100 | 123 | 125 | ✓ | 12 | ||
| Pleskow 2003 | 44 | 136 | 139 | 141 | ✓ | 12 | |
| SD‐037‐0344 | 100 | 429 | 210 | ✓ | 12 | ||
| Steffensen 1995 | 87 | 103 | 101 |
| 12 | ||
| van der Molen 1997 | 100 | 125 | 114 |
| 24 | ||
| van Schayck 2002 | 95 | 46 | 41 | ✓ | 12 | ||
| Wolfe 2006 | 62 | 525 | 527 | 514 | ✓ | 16 | |
| mean duration 14 weeks | |||||||
| All trials contributed data for non‐fatal serious adverse events of any cause. Corren 2013 and Nathan 2012 have been added to the trials already included in the Cochrane review. | |||||||
| Study ID from adults Cates 2008 | % patients on background ICS | Number on salmeterol | **Dose of salmeterol (mcg/bd) | Number on placebo | Data found on mortality (all‐cause) | Data found on non‐fatal SAE (all‐cause) | Duration (weeks) |
| Adinoff 1998 | 64 | 142 | 50 | 244 |
|
| 36 |
| Boyd 1995 | 100 | 55 | 100 | 64 |
| ✓ | 12 |
| Busse 1998 | 67 | 263 | 50 | 275 |
| ✓ | 12 |
| Chervinsky 1999 | 51 | 176 | 50 | 176 | ✓ |
| 52 |
| D'Alonso 1994a | 21 | 106 | 50 | 108 |
|
| 12 |
| D'Urso 2001 | 93 | 455 | 50 | 456 | ✓ | ✓ | 24 |
| Kavuru 2000 | 0* | 92 | 50 | 82 | ✓ | ✓ | 12 |
| Kemp 1998a | 43 | 149 | 50 | 152 |
|
| 12 |
| Kemp 1998b | 100 | 252 | 50 | 254 |
| ✓ | 12 |
| Lazarus 2001 | 0* | 54 | 50 | 56 | ✓ | ✓ | 16 |
| Nathan 1999 | 0* | 128 | 50 | 129 | ✓ | ✓ | 26 |
| Nathan 2006 | 0* | 91 | 50 | 89 |
| ✓ | 12 |
| Pearlman 1992 | 25 | 78 | 50 | 79 | ✓ |
| 12 |
| Pearlman 2004 | 0* | 92 | 50 | 87 |
| ✓ | 12 |
| Rosenthal 1999 | 0 | 202 | 50 | 206 |
|
| 24 |
| Shapiro 2000 | 0* | 88 | 50 | 93 | ✓ | ✓ | 12 |
| SLMF4002 | 100 | 93 | 100 | 95 | ✓ | ✓ | 26 |
| SMART 2006 | 47 | 13,176 | 50 | 13,179 | ✓ | ✓ | 28 |
| Wolfe 2000 | 33 | 331 | 50 | 167 | ✓ | ✓ | 12 |
| mean duration 27 weeks | |||||||
| * background ICS treatment was withdrawn from all participants ** 50 micrograms is the ex‐actuator dose, but in some studies this is reported as the equivalent delivered dose of 42 micrograms | |||||||
| Study ID from adults in Cates 2012b | N | Age | Formoterol Device | Salmeterol Device | Location | Sponsor | Duration (weeks) |
| Condemi 2001 | 528 | 18+ | Foradil Aerolizer | Serevent Diskus | USA | Novartis | 26 |
| Gabbay 1998 | 127 | 18+ | Foradil Aerolizer | Serevent Diskus | UK | Novartis | 12 |
| Vervolet 1998 | 482 | 18+ | Foradil Aerolizer | Serevent Diskus | Europe | Novartis | 26 |
| Total | 1137 | mean duration 24 weeks | |||||
| All the above trials compared formoterol (Foradil) 12 mcg twice daily with salmeterol 50 mcg twice daily and all participants were taking a background inhaled corticosteroid. | |||||||
| Study ID from adults in Cates 2013b | Age (Years | N on F&ICS | N on ICS Alone | Daily dose of budesonide or other ICS (mcg metered dose) | Daily Dose of Formoterol (mcg metered dose) | Once daily | Twice daily | Combined inhalers | Separate inhalers | DPI | pMDI | Duration weeks |
| Brown 2012 | 12+ | 377 | 364 | 800 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Buhl 2003 | 18+ | 352 | 171 | 400 | 12 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Chuchalin 2002 | 18+ | 111 | 114 | 400 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Corren 2007 | 12+ | 123 | 121 | 400 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Corren 2013 | 12+ | 110 | 113 | 250 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| D5896C00001 | 12+ | 312 | 153 | 400 | 12/24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Jenkins 2006 | 12+ | 341 | 115 | 1600 | 48 | ✓ | ✓ | ✓ | ✓ | 24 | ||
| Kuna 2006 | 18+ | 409 | 207 | 200 | 12 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Meltzer 2012 | 12+ | 182 | 188 | 200 (mometasone) | 20 | ✓ | ✓ | 26 | ||||
| Morice 2007 | 12+ | 462 | 217 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Nathan 2010 | 12+ | 191 | 192 | 400 (mometasone) | 20 | ✓ | ✓ | ✓ | 26 | |||
| Nathan 2012 | 12+ | 115 | 117 | 100 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| Noonan 2006 | 12+ | 239 | 109 | 400 | 24 | ✓ | ✓ | ✓ | ✓ | ✓ | 12 | |
| O'Byrne 2001 | 18+ | 554 | 550 | 400 | 12 | ✓ | ✓ | ✓ | 52 | |||
| O'Byrne 2001a | 18+ | 315 | 312 | 800 | 12 | ✓ | ✓ | ✓ | 52 | |||
| Pauwels 1997 | 18+ | 210 | 213 | 200 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Pauwels 1997a | 18+ | 215 | 214 | 800 | 24 | ✓ | ✓ | ✓ | 52 | |||
| Pearlman 2012 | 12+ | 119 | 119 | 100 (fluticasone) | 12 | ✓ | ✓ | ✓ | 12 | |||
| Peters 2008 | 12+ | 443 | 133 | 1600 | 48 | ✓ | ✓ | ✓ | 52 | |||
| Price 2002 | 12+ | 250 | 255 | 800 | 24 | ✓ | ✓ | ✓ | 24 | |||
| SD‐039‐0726 | 16+ | 301 | 145 | 200 | 12/24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Spector 2012 | 12+ | 156 | 155 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| Weinstein 2010 | 12+ | 255 | 240 | 800 (mometasone) | 20 | ✓ | ✓ | ✓ | 12 | |||
| Zangrilli 2011 | 12+ | 127 | 123 | 800 | 24 | ✓ | ✓ | ✓ | 12 | |||
| Zetterstrom 2001 | 18+ | 238 | 124 | 800 | 24 | ✓ | ✓ | ✓ | ✓ | 12 | ||
| mean duration 29 weeks | ||||||||||||
| All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause. Corren 2013, Nathan 2012 and Pearlman 2012 have been added to the trials already included in the Cochrane review. | ||||||||||||
| Study ID from adults in Cates 2013a | Age of Participants (Years) | N on FSC | N on ICS | Daily dose of fluticasone (mcg) | Daily dose of salmeterol (mcg) | Combined Inhaler | Separate Inhalers | Duration (weeks) |
| Aubier 1999 | 12+ | 338 | 165 | 1000 | 100 | ✓ | ✓ | 28 |
| Bailey 2008 | 12+ | 239 | 236 | 200 | 100 | ✓ | 52 | |
| Bateman 2001 | 12+ | 1709 | 1707 | 200 | 100 | ✓ | 52 | |
| GOAL 2004 | 12+ | 333 | 165 | 200/500/1000 | 100 | ✓ | 12 | |
| Godard 2008 | 18+ | 159 | 159 | 500 | 100 | ✓ | 24 | |
| Ind 2003 | 16+ | 336 | 160 | 500 | 100 | ✓ | 28 | |
| Katial 2011 | 12+ | 306 | 315 | 500 | 100 | ✓ | 52 | |
| Kavuru 2000 | 12+ | 310 | 318 | 200 | 100 | ✓ | 52 | |
| Kerwin 2011 | 12+ | 92 | 90 | 500 | 100 | ✓ | 12 | |
| Koenig 2008 | 12+ | 156 | 156 | 200/500/1000 | 100 | ✓ | 40 | |
| Koopmans 2006 | 18+ | 173 | 177 | 500 | 100 | ✓ | 12 | |
| Lundback 2006 | 18+ | 101 | 102 | 500 | 100 | ✓ | 12 | |
| Murray 2004 | 12+ | 94 | 91 | 200 | 100 | ✓ | 12 | |
| Nathan 2006 | 12+ | 171 | 168 | 220 | 100 | ✓ | 16 | |
| Nelson 2003 | 12+ | 95 | 97 | 200 | 100 | ✓ | 12 | |
| Pearlman 2004 | 12+ | 92 | 89 | 200 | 100 | ✓ | 12 | |
| Renzi 2010 | 12+ | 262 | 270 | 200 | 100 | ✓ | 24 | |
| Rojas 2007 | 12+ | 180 | 182 | 500 | 100 | ✓ | 12 | |
| SAM30007 | 18+ | 29 | 32 | 200/500/1000 | 100 | ✓ | 30 | |
| SAM40004 | 18+ | 42 | 21 | 200 | 100 | ✓ | 52 | |
| SAM40008 | 18+ | 93 | 93 | 1000 | 100 | ✓ | 26 | |
| SAM40031 | 18+ | 41 | 41 | 200/500/1000 | 100 | ✓ | 52 | |
| SAM40065 | 12+ | 150 | 150 | 200/500/1000 | 100 | ✓ | 40 | |
| SAS30022 | 12+ | 210 | 212 | 500 | 50 | ✓ | 12 | |
| SAS30023 | 12+ | 151 | 155 | 100 | 50 | ✓ | 12 | |
| SAS40036 | 15+ | 172 | 159 | 200 | 100 | ✓ | 16 | |
| SAS40037 | 15+ | 161 | 161 | 200 | 100 | ✓ | 16 | |
| SAS40068 | 12+ | 262 | 270 | 200 | 100 | ✓ | 24 | |
| SFA103153 | 12+ | 239 | 236 | 200 | 100 | ✓ | 52 | |
| SFCF4026 | 18+ | 159 | 159 | 500 | 100 | ✓ | 24 | |
| Shapiro 2000 | 12+ | 84 | 84 | 500 | 100 | ✓ | 12 | |
| SLGF75 | 16+ | 14 | 17 | 200 | 100 | ✓ | 12 | |
| Strand 2004 | 18+ | 78 | 72 | 200 | 100 | ✓ | 12 | |
| van Noord 2001 | 12+ | 337 | 172 | 1000 | 100 | ✓ | 12 | |
| Wallin 2003 | 12+ | 18 | 19 | 400 | 100 | ✓ | 12 | |
| mean duration 34 weeks | ||||||||
| All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause | ||||||||
| Study ID from adults in Cates 2010 | N | Duration (weeks) | Formoterol device | Formoterol dose | ICS type and dose | Salmeterol device | Salmeterol dose | ICS type and dose | Duration (weeks) |
| Aalbers 2004 | 439 | 26 | DPI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 26 |
| Bodzenta‐Lukaszyk 2011 | 202 | 12 | HFA pMDI with AeroChamber | 10 µg bd | Fluticasone 100 µg or 250 µg bd | HFA pMDI with AeroChamber | 50 µg bd | Fluticasone 100 µg or 250 µg bd | 12 |
| Busse 2008 | 833 | 30 | pMDI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 30 |
| Dahl 2006 | 1397 | 24 | DPI | 12 µg bd | Budesonide 400 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 24 |
| Kuna 2007 | 2218 | 24 | DPI | 12 µg bd | Budesonide 400 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg bd | 24 |
| Maspero 2010 | 404 | 52 | pMDI | 10 µg bd | Mometasone 200 µg or 400 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg or 500 µg bd | 52 |
| Papi 2007 | 228 | 12 | pMDI | 12 µg bd | Beclomethasone extra fine 200 µg bd | pMDI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| Ringdal 2002 | 428 | 12 | DPI two separate inhalers | 12 µg bd | Budesonide 800 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| SAM 40010 | 373 | 12 | DPI | 6 µg bd | Budesonide 200 µg bd | DPI | 50 µg bd | Fluticasone 100 µg bd | 12 |
| SAM 40048 | 247 | 12 | DPI | 6 µg bd | Budesonide 200 µg bd | DPI | 50 µg bd | Fluticasone 250 µg bd | 12 |
| mean duration 23 weeks | |||||||||
| All trials of combination salmeterol and ICS contributed data on fatal and non‐fatal serious adverse events of any cause | |||||||||
| AMSTAR Criteria | ||||||
| 1. Was an 'a priori' design provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 2a. Was there duplicate study selection? (0.5 point) | Yes | Yes | Yes | Yes | Yes | No |
| 2b. Was there duplicate data extraction? (0.5 point) | No | No | Yes | Yes | Yes | No |
| 3. Was a comprehensive literature search performed? | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | No | No | No | No | No | No |
| 5. Was a list of studies (included and excluded) provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 6. Were the characteristics of the included studies provided? | Yes | Yes | Yes | Yes | Yes | Yes |
| 7. Was the scientific quality of the included studies assessed and documented? | Yes | Yes | Yes | Yes | Yes | Yes |
| 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the methods used to combine the findings of studies appropriate? | Yes | Yes | Yes | Yes | Yes | Yes |
| 10. Was the likelihood of publication bias assessed? | Yes | Yes | Yes | Yes | Not applicable | Not applicable |
| 11. Was the conflict of interest stated? | Yes | Yes | Yes | Yes | Yes | Yes |
| Total criteria met: | 10.5 | 10.5 | 11 | 11 | 10 | 9 |
| Note: item 4 is met with the assessment 'NO', all others 'YES'. We felt that item 2 was 2 separate questions, so we split it into two parts and awarded half a point for each. This differs from the published version of the tool. | ||||||
| Comparison | Adults with an event on control (n) | Total number of adults on control (N) | SAE per 10,000 adults (95% CI) | Mean duration of trials (weeks) | SAE per 10,000 adults per week |
| Formoterol v Placebo | 25 | 2357 | 106 (65 to 147) | 14 | 7.6 |
| Salmeterol v Placebo | 518 | 15026 | 345 (317 to 375) | 27 | 12.8 |
| Formoterol & ICS v ICS | 115 | 4764 | 241 (197 to 285) | 29 | 8.3 |
| Salmeterol & ICS v ICS | 135 | 6461 | 209 (177 to 247) | 34 | 6.1 |
| Study ID | Treatment arm | Cause of death |
| Buhl 2003 | Formoterol and budesonide | Cardiac arrest |
| O'Byrne 2001 | Formoterol and budesonide (separate inhalers) | Status asthmaticus, followed by septic shock |
| Pauwels 1997a | Formoterol and budesonide (separate inhalers) | Suicide |
| Zetterstrom 2001 | Formoterol and budesonide | Suicide |
| Brown 2012 | Formoterol and budesonide | Cerebro‐vascular accident |
| Brown 2012 | Budesonide | Homicide |
| Nathan 2010 | Formoterol and mometasone | Uterine Leiomyosarcoma |
| Jenkins 2006 | Formoterol and budesonide | Pulmonary embolus (but the death occurred after the control budesonide arm was discontinued so was not included in the meta‐analysis) |
| Study ID | Treatment arm | Cause of death |
| Aubier 1999 | salmeterol and fluticasone (separate inhalers) | Bronchial carcinoma (one death) |
| GOAL 2004 | salmeterol/fluticasone | Myocardial infarction (two deaths) and pneumonia (one death) |
| GOAL 2004 | fluticasone | Myocardial infarction (two deaths) |
| Ind 2003 | salmeterol and fluticasone (separate inhalers) | Pneumothorax (one death) |
| Kerwin 2011 | salmeterol/fluticasone | Cardiac disease (one death) |
| Kerwin 2011 | fluticasone | Breast cancer (one death) |
| Koenig 2008 | fluticasone | Cardiac arrest and deep vein thrombosis (one death) |
| Renzi 2010 | fluticasone | Cardiac arrest (one death) |
| SAS40068 | fluticasone | Ventricular hypertrophy and aortic hypoplasia (one death) |
| Strand 2004 | fluticasone | Unknown cause (one death) |
| van Noord 2001 | salmeterol/fluticasone | Leukaemia (one death) |

