Intervencije za melanom in situ, uključujući lentigo maligna
Abstract
Background
Malignant melanoma is a form of skin cancer associated with significant mortality once it has spread beyond the skin. Melanoma in situ (MIS) is the earliest histologically recognisable stage of malignant melanoma and represents a precursor of invasive melanoma. Lentigo maligna (LM) represents a subtype of pre‐invasive intraepidermal melanoma associated specifically with chronic exposure to ultraviolet (UV) radiation. Over the past two decades, the incidence of MIS has increased significantly, even more than the invasive counterpart. There are several treatment options for MIS, but no consensus exists on the best therapeutic management of this condition.
Objectives
To assess the effects of all available interventions, surgical and non‐surgical, for the treatment of melanoma in situ, including LM.
Search methods
We searched the following databases up to November 2014: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2014, Issue 10), MEDLINE (from 1946), Embase (from 1974), LILACS (from 1982), African Index Medicus (from inception), IndeMED of India (from inception), and Index Medicus for the South‐East Asia Region (IMSEAR) (from inception). We scanned the references of included and excluded studies for further references to relevant trials and searched five trials registries. We checked the abstracts of major dermatology and oncology conference proceedings, and we shared our lists of included and excluded studies with industry contacts and other experts in the field of melanoma to try to identify further relevant trials.
Selection criteria
We included randomised controlled trials (RCT) on the management of MIS, including LM, that compared any intervention to placebo or active treatment. We included individuals, irrespective of age and sex, diagnosed with MIS, including LM, based on histological examination.
Data collection and analysis
Two authors independently evaluated possible studies for inclusion; extracted data from the included study using a standard data extraction form modified for our review; assessed risk of bias; and analysed data on efficacy, safety, and tolerability. They resolved any disagreements by discussion with a third author. We collected adverse effects information from included studies.
Main results
Our search identified only 1 study eligible for inclusion (and 1 ongoing study in active recruitment stage), which was a single centre, open label, parallel group, 2‐arm RCT with 90 participants, who had 91 histologically proven LM lesions.
Forty‐four participants, with 44 LM lesions, were treated with imiquimod 5% cream 5 days per week plus tazarotene 0.1% gel 2 days/week for 3 months, and 46 participants, with 47 LM lesions, were treated with imiquimod 5% cream 5 days per week for 3 months. Two months after cessation of topical treatment, the initial tumour footprint was excised using 2 mm margins via a staged excision. This study was open label, and analysis was not intention‐to‐treat, leading to a high risk of incomplete outcome data.
Our primary outcome 'Histological or clinical complete response' was measured at 5 months in 29/44 participants (66%) treated with imiquimod plus tazarotene (combination therapy) and 27/46 participants (59%) treated with imiquimod (monotherapy). The difference was not statistically significant (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.81 to 1.55, P value = 0.48).
With regard to our secondary outcomes on recurrence and inflammation, after a mean follow up of 42 months, no local recurrences were observed among complete responders. Difference in overall inflammation score between the 2 groups was significant (mean difference (MD) 0.6, 95% CI 0.2 to 1, P value = 0.004), with the mean overall inflammation score being significantly higher in the combination group.
The study authors did not clearly report on side‐effects. Because of adverse effects, there was a dropout rate of 6/44 participants (13.7%) in the combination group compared with 1/46 (2.2%) in the imiquimod monotherapy group (due to excessive inflammation) before the cessation of topical treatment (first 3 months), but this was not statistically significant (RR 6.27, 95% CI 0.79 to 50.02, P value = 0.08).
Authors' conclusions
There is a lack of high‐quality evidence for the treatment of MIS and LM.
For the treatment of MIS, we found no RCTs of surgical interventions aiming to optimise margin control (square method, perimeter technique, 'slow Mohs', staged radial sections, staged "mapped" excisions, or Mohs micrographic surgery), which are the most widely used interventions recommended as first‐line therapy. The use of non‐surgical interventions in selected cases (patients with contraindications to surgical interventions) may be effective and may be considered preferable for experienced providers and under close and adequate follow up.
For the treatment of LM, we found no RCTs of surgical interventions, which remain the most widely used and recommended available treatment. The use of non‐surgical interventions, such as imiquimod, as monotherapy may be effective and may be considered in selected cases where surgical procedures are contraindicated and used preferentially by experienced providers under close and adequate follow up. The use of topical therapies, such as 5‐fluorouracil and imiquimod, as neoadjuvant therapies warrants further investigation. There is insufficient evidence to support or refute the addition of tazarotene to imiquimod as adjuvant therapy; the current evidence suggests that it can increase topical inflammatory response and withdrawal of participants because of treatment‐related side‐effects.
PICOs
Laički sažetak
Intervencije za liječenje melanoma bez metastaza (melanom in situ), uključujući lentigo maligna
Dosadašnje spoznaje
Melanom in situ (MIS) je najraniji stadij melanoma. Incidencija MIS se povećala tijekom zadnja dva desetljeća. Lentigo maligna (LM) je podtip preinvazivnog melanoma povezan s dugotrajnim izlaganjem ultraljubičastom zračenju, koji prvenstveno zahvaća glavu i vrat. Čini 79% do 83% svih MIS tumora. Često se prepoznaje sa zakašnjenjem.
Istraživačko pitanje
Kakvi su učinci kirurškog i ne‐kirurškog liječenja MIS, uključujući LM?
Značajke istraživanja
Pronađeno je jedno randomizirano kontrolirano kliničko ispitivanje ne‐kirurškog liječenja 90 ljudi sa LM: 44 ih je liječeno imiquimodom u obliku kreme plus tazarotenom u obliku gela, dok ih je 46 liječeno imiquimodom u obliku kreme kroz 3 mjeseca; te intervencije su zatim popraćene kirurškim odstranjenjem u stadijima nakon 2 mjeseca. Nije nađen niti jedan kontrolirani pokus kirurškog liječenja.
Ključni rezultati
Kod onih koji su liječeni imiquimodom i tazarotenom, 66% je imalo potpuni odgovor nakon 5 mjeseci u usporedbi sa 59% onih koji su liječeni samo imiquimodom. Dodatak tazarotena imiquimodu nije doveo do klinički boljeg odgovora, i ljudi u toj grupi su imali jaču upalu. U toj je grupi bilo i više ispitanika koji nisu završili studiju zbog nuspojava.
Kvaliteta dokaza
Kvaliteta dokaza je bila niska. Što se tiče liječenja MIS, kirurške intervencije s ciljem kirurškog odstranjenja tumora bez tumorskih stanica na rubovima postupci su koji se najčešće preporučuju. Dokazi ne podupiru korištenje ne‐kirurških intervencija u odabranim slučajevima (to jest, u starijih osoba kod kojih postoje razlozi zbog kojih se ne mogu primijeniti kirurški zahvati). Ipak, klinički centri bi ih mogli razmotriti kada postoji iskustvo s ovakvim liječenjem, te kada se može poduzeti detaljno i prikladno praćenje.
Što se tiče liječenja LM, kirurške intervencije ostaju dostupno liječenje koje se najčešće preporučuje. Dokazi ne podupiru korištenje ne‐kirurških intervencija, poput imiquimoda, kao jedine terapije u većine slučajeva. Moglo bi ih se razmotriti samo u odabranim slučajevima i u kliničkim centrima s iskustvom. Dosadašnji dokazi ne podupiru korištenje imiquimoda kao neoadjuvantne (odnosno, prije operacije) terapije, ali opravdavaju daljnja istraživanja kako bi se procijenilo bi li korištenje nakon operacije moglo umanjiti vraćanje bolesti, te bi li korištenje prije operacija tumora koji su veliki ili se nalaze na nezgodnom mjestu moglo pomoći da se postigne manji kirurški zahvat. Dokazi ne podupiru dodatak tazarotena imiquimodu kao neoadjuvantnu terapiju.
Authors' conclusions
Summary of findings
| Imiquimod 5% cream with tazarotene 0.1% gel compared with Imiquimod 5% cream alone for melanoma in situ, including lentigo maligna | ||||||
| Patient or population: participants with melanoma in situ, including lentigo maligna | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Imiquimod 5% cream alone | Imiquimod 5% cream with tazarotene 0.1% gel | |||||
| Primary outcome: Histological complete response (intention‐to‐treat) | 587 per 1000 | 657 per 1000 | RR 1.12 | 90 | ⊕⊕⊝⊝ | ‐ |
| Primary outcome: Histological complete response ( available‐case analysis) | 659 per 1000 | 784 per 1000 | RR 1.19 | 78 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Discontinuation of treatment because of harms | 22 per 1000 | 136 per 1000 | RR 6.27 | 90 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Inflammatory response (number of lesions with grade 2 or 3) | 591 per 1000 | 815 per 1000 | RR 1.38 | 88 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Inflammatory response (overall Inflammation score) for all participants | ‐ | The mean overall inflammation score (inflammatory response) for all participants in the intervention groups was 0.60 higher | ‐ | 88 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| GRADE = Grading of Recommendations Assessment, Development and Evaluation. | ||||||
Background
Please note that we have listed unfamiliar terms in Table 1.
| Medical term | Explanation |
| Intraepidermal | something confined within the epidermis |
| Preinvasive | a malignant tumour that remains constricted in the epithelium, while the dermo‐epidermal junction preserves its integrity |
| Eschar | a dry scab or slough formed on the skin as a result of a burn or by the action of a corrosive or caustic substance |
| Invasive | a malignant tumour that breaks the dermo‐epidermal junction and invades the dermis |
| Lentigo maligna | a subtype of preinvasive intraepidermal melanoma, associated specifically with chronic solar exposure |
| Vehicle | an excipient or a menstruum, a substance, usually without therapeutic action, used as a medium to give bulk for the administration of medicines |
| Apoptosis | a genetically directed process of cell self‐destruction that is marked by the fragmentation of nuclear DNA. It is activated either by the presence of a stimulus or removal of a suppressing agent or stimulus, and is a normal physiological process eliminating DNA‐damaged, superfluous, or unwanted cells. It is a synonym for programmed cell death |
| Actinic keratosis | rough, scaly macule or patch of skin that it is considered precancerous and is associated with chronic sun exposure |
| Lentigo senilis | a flat brownish, pigmented spot on the skin, due to increased deposition of melanin and an increased number of melanocytes |
| Seborrheic keratosis | a benign skin tumour that occurs singly or in clusters on the surface of the skin, is usually light to dark brown or black in colour, and typically has a warty texture often with a waxy appearance |
| Patch | a greater than 1 cm circumscribed area of discolouration on the skin |
| Cytokine | a small protein released by cells that has a specific effect on the interactions between cells, on communications between cells, or on the behavior of cells |
| Innate immunity | immunity that occurs naturally as a result of a person's genetic constitution or physiology and does not arise from a previous infection or vaccination |
| Blinding | the concealment of group assignment ‐ to either the treatment or control group ‐ from the knowledge of participants, investigators in a clinical trial, or both |
| Tumour islands | aggregates of tumour cells |
Description of the condition
Melanoma is one of the deadliest types of skin neoplasm (abnormal growth), resulting from the uncontrollable proliferation of malignant melanocytes (cells within the epidermis) (Bichakjian 2011).
Melanoma in situ (MIS) is the earliest histologically recognisable stage of malignant melanoma, and it is characterised by an increased number of atypical intraepidermal melanocytes (King 2005; Tannous 2000). The disease is clinically relevant because it represents a precursor of invasive melanoma. Individuals diagnosed with melanoma in situ have a higher risk of developing invasive melanoma in their lifetime (Mocellin 2011). Over the past two decades, the incidence of melanoma in situ has increased significantly, even more than the invasive counterpart (Charles 2005), which has led some authors to state that we are witnessing a kind of melanoma epidemic (Beddingfield 2003; Mocellin 2011).
Lentigo maligna (LM) represents a subtype of pre‐invasive intraepidermal melanoma associated specifically with chronic exposure to ultraviolet (UV) radiation, primarily affecting the head and neck region. It is the most prevalent melanoma in situ subtype, accounting for 79% to 83% of all melanoma in situ tumours (Kvaskoff 2011; McKenna 2006).
Lentigo maligna melanoma (LMM) represents 4% to 15% of all melanomas (Clark 1986; McKenna 2006; McLeod 2011). Its incidence is increasing in people older than 45 years, at a greater rate than for any other melanoma subtype (Swetter 2005). Swetter et al report that regional (California) and national data suggest an increasing incidence of LM and LMM, particularly in men above 65 years of age (Swetter 2005). Furthermore, in a recently published population‐based study held in the US, investigators report that the incidence of LM increased significantly among residents of Olmsted County, Minnesota, over a 37‐year period, with incidence being significantly higher among men than women and increasing with age (Mirzoyev 2014). Across 21 years of incidence data for in situ and invasive lesions from the population‐based Queensland Cancer Registry in Australia, MIS increased by 10.4% per year among males and 8.4% per year among females. The incidence of invasive lesions also increased, but not as quickly (Coory 2006). In a study evaluating incidence trends, age incidence relationships, and familial risks in invasive and in situ cutaneous melanoma, investigators in Sweden recorded a marked increase in incidence of MIS, particularly after 1985. Among MIS, lentigo maligna was the most common histogenetic type, almost three times more common than superficially spreading melanoma. The study obtained data from the Swedish Cancer Registry for the years 1961 to 1998 (Hemminki 2003).
The real risk of progression from LM to LMM is not known; Cohen has widely reviewed this and estimated the risk to be "as high as 30% to 50% or as low as 5% based on epidemiologic data" (Cohen 1995). Taking into account that observational, longitudinal, or prospective studies in people with LM are not feasible (since they would require no treatment), the precise lifetime risk of invasive melanoma in this group remains unknown. Investigators have estimated a lifetime risk essentially based on anecdotal experience. There is only one epidemiologic study that has aimed to establish the annual and lifetime risk of malignant transformation of LM (Weinstock 1987). Based on their records, the investigators reported that a person with LM at the age of 45 would have a 3.3% risk of LMM by the age of 75 and a lifetime risk of 4.7% (Weinstock 1987). Diagnosis of LM at the age of 65 was associated with a 1.2% risk of LMM by the age of 75 and a 2.2% lifetime risk (Weinstock 1987). The treatment of MIS, particularly the LM subtype, has been a controversial subject in the literature for over a decade. In the optimal scenario of being able to distinguish LM from LMM clinically, or being able to predict the risk of progression, close monitoring would be a reasonable alternative to treatment. However, since such a scenario is currently impossible, active treatment of LM is recommended (Bichakjian 2011). These considerations have drawn special attention to the treatment of LM (Erickson 2010).
Clinical characteristics and diagnosis
Presentation of melanoma in situ may be quite subtle, and delayed recognition is common. Diagnosis is based on clinical, dermoscopic, and histological features. In terms of clinical presentation, it usually develops as an ill‐defined, darkly pigmented flat lesion, with irregular borders and a diameter larger than 0.7 cm. With regard to LM, the presence of widespread atypical melanocytes in the background of long‐standing sun damage are highly indicative of the disease (Brochez 2002; Farmer 1996; Koehler 2011; Megahed 2002). However, differentiation from lentigo senilis, initial seborrhoeic keratosis, or pigmented actinic keratoses may be difficult (Cognetta 2001). The four most important diagnostic features for LM or LMM on dermoscopy are "asymmetric pigmented follicular openings, dark rhomboidal structures, slate‐gray globules, and slate‐gray dots with a sensitivity of 89% and a specificity of 96%" (Schiffner 2000).
Description of the intervention
Many interventions, including both surgical and non‐surgical therapeutic approaches, are used for the management of melanoma in situ. Surgical excision, including Mohs microsurgery, remains the gold standard therapeutic practice for people with melanoma in situ (Clark 2008; Cohen 1998; Dawn 2007; Kunishige 2011; Mahoney 2005; Osborne 2002).
Surgical excision
1. Regular excision with 5 mm margins
With regard to surgery, in 1992, the National Institutes of Health (NIH) consensus statement established surgical excision with 5 mm clinical margins as the standard treatment for melanoma in situ (National Institutes of Health 1993). However, taking into consideration cumulative evidence from multiple studies over recent years, the conclusion has been made that 5 mm margins are often insufficient to histologically clear the LM variant (National Comprehensive Cancer Network 2009). The National Comprehensive Cancer Network's (NCCN) Guidelines of Care for Melanoma discussed the possible need for > 5 mm margins in larger melanoma lesions of the lentigo maligna subtype (National Comprehensive Cancer Network 2009); these guidelines stated that more extensive histological assessment of the margins may need to be considered in such cases. Thus, the conclusion from these guidelines and Stevenson 2005, who showed that high rates of recurrence following standard excisional surgery, attributed to subclinical extension, is that a 5 mm surgical margin for excision of melanoma in situ is often insufficient for the LM subtype.
In this context, given the need for extended margins, alternative techniques aiming to optimise margin control have been employed, including the square method, perimeter technique, 'slow Mohs', staged radial sections, staged "mapped" excisions, and Mohs micrographic surgery (MMS) (Clark 2008).
2. The square method
When using the square method, after drawing the original margins of the lesion, a new margin in the shape of a square or polygon is drawn at a distance of 5 mm from the original 1. Then, an additional square is drawn 2 to 3 mm around the latter polygon. This 2 to 3 mm slide of tissue around the lesion is excised, leaving behind a thin frame‐like defect that is simply sutured closed while waiting for the histopathologic report of the excised tissue. If the report is positive, another square frame is excised from the periphery, and the process is repeated until clear margins are obtained. At the final stage, the central part is excised, and the individual undergoes a reconstruction.
"The key advantages of the 'square' method are that it allows the use of high‐quality permanent sections for total peripheral margin assessment and it avoids creating an open wound for the patient to care for while awaiting closure" (Clark 2008). One big disadvantage is that the individual must wait some time for pathology reports, possibly resulting in delayed complete excision. This important disadvantage of the square method has resulted in adaptations of the surgical technique (Clark 2008; Jejurikar 2007; Johnson 1997). For example, the central part of the tumour is removed at first visit, without performing reconstruction (Agarwal‐Antal 2002; Clark 2008; Mahoney 2005). This modification facilitates "staging prior to reconstruction but creates an open wound. Another common modification is the use of specific polygons [or curved lines] instead of a true square" (Clark 2008). "This allows the surgeon to match the surgical defect with the shape of the melanoma in situ as well as the surrounding cosmetic units and structures" (Clark 2008). Furthermore, straight lines are retained to facilitate histological processing. "Finally, another common modification is the use of 24‐hour rush permanent sections instead of routine processing" (Bub 2004).
3. The "mapped" excision method
Hill and Gramp first described "mapped" excision in 1999 (Hill 1999), as well as the 'radial processing' described by Bub, et al (Bub 2004). "While often included in review articles of melanoma in situ treatment for completeness, these forms of staged excisions do not employ en face [parallel to the surgical margins] histological processing and represent fundamentally different modalities than either the square method and its modifications or Mohs micrographic surgery" (Clark 2008).
4. Mohs micrographic surgery
Mohs micrographic surgery (MMS) is a surgical and pathological procedure used to treat many cutaneous neoplasms (Clark 2008; Mohs 1941). "Its design integrates the role of surgeon and pathologist and is unique in providing assessment of 100% of surgical margins intraoperatively, in contrast to a significantly lower percentage of assessed margins, typically 0.1% (Zitelli 1997) to 5%, by standard specimen analysis" (Shriner 1998). "Mohs micrographic surgery has the advantage of offering intraoperative margin control with assessment of 100% of margins, and it does not require a separate dermatopathologist to be available for rush specimen analysis" (Clark 2008). Even though the overall process may take several hours, achieving definitive excision and closure on the same day is a big advantage of MMS. A drawback of MMS is "the difficulty associated with adequately preparing frozen sections for [visualisation] of melanocytes, including the need for immunohistochemical stains" (Clark 2008). However, besides the progress in the field of excision techniques, the question concerning the initial margin size needed to clear a clinically ill‐defined tumour still remains (Erickson 2010).
Non‐surgical interventions
Localisation of lesions in cosmetically sensitive sites, such as the head and neck area, often raise serious difficulties for surgeons, who aim for complete clearance of the tumour, while seeking to minimise morbidity to the area. Similarly, the difficulty of obtaining wide excision margins in some body areas, together with the higher prevalence of lentigo maligna in the elderly (who may have contraindications to surgical procedures), has led to investigation of other less invasive treatment methods (Erickson 2010). In this context, radiotherapy (Farshad 2002; Tsang 1994); cryosurgery (Collins 1991); laser surgery (Kopera 1995); and topical medication have been used with variable success, especially for individuals with large patches of LM, or those who are not otherwise good candidates for surgery (Rajpar 2006; Ray 2005; Rodriguez Prieto 1993; Ryan 1988). Apart from their use as primary treatments, non‐surgical techniques can contribute as adjuvant therapies after surgical excision, in order to minimise recurrence, or after incomplete excision to further enhance the therapeutic response. A recent trend also includes application of non‐ablative treatments as neoadjuvant therapies, before surgical treatment of large lesions or difficult sites, in order to achieve smaller surgical excisions (Gupta 2004), although there is concern over the potential for creating non‐contiguous tumour islands.
Lasers
Carbon dioxide laser ablation, Q‐switched neodymium‐doped yttrium aluminium garnet (QS Nd:YAG), and alexandrite lasers have all been used in the treatment of LM in those elderly people who may be unsuitable for surgery (Lee 2011; Madan 2009).
Curettage and electrodesiccation
Curettage and electrosurgery have been used for the treatment of lentigo maligna (Coleman 1980). Usually, a looped metal instrument (curettage) is used to 'scrape' the lesion's surface and remove part or the whole of the lesion, and an electric current (electrosurgery) is applied to cauterise and destroy an area of the lesion. Recurrences have been observed. The main drawback of this technique is the lack of histological analysis. It has also been reported that atypical melanocytes in the hair follicle are not likely to be destroyed using this technique (Gaspar 1997).
Radiation therapy
"Radiation therapy is [a] non‐invasive treatment modality that has been used as a primary treatment for lentigo maligna. It is appealing in elderly patients who may be poor surgical candidates and in patients with large lentigo malignas on the face that would require a large surgical excision" (Garbe 2008). Cure rates for lentigo maligna achieved with radiotherapy, with either Grenz rays or soft X‐rays using the Miescher technique, range from 86% to 95% (Farshad 2002; Schmid‐Wendtner 2000; Tsang 1994).
The German Cancer Society suggested "radiotherapy as a potential second line treatment for lentigo maligna in their 2008 guidelines (Garbe 2008). The [therapeutic] protocol reported in these guidelines includes Grenz ray therapy (12 kV) for LM (100 to 120 Gy; 10 Gy twice weekly for 5 to 6 weeks). However, the German consensus statement did conclude that radiation therapy for the primary treatment of melanoma is indicated only in those cases in which surgery is impossible or not reasonable".
Cryosurgery
Cryosurgery has been proposed as an alternative to surgical excision for lentigo maligna (Field 1995). It is recommended in people for whom surgery is not appropriate. The cryosurgery technique varies. Kufiick and Gage suggested "aggressive treatment [using] double freeze‐thaw cycles with a 1 cm margin and temperatures of ‐40 to ‐50°C at the base of the lesion" (Kuflik 1994). A significant drawback in cryosurgery is the lack of a histological evaluation of the destroyed specimen. As a result of the latter, no assessment of microscopic foci of lentigo maligna melanoma or identification of aggressive melanoma subtypes can be made. On the same basis, it does not give any clue to the prognostic outcome based on histological grade in case of an invasive tumour.
Imiquimod
Imiquimod belongs to the synthetic imidazoquinoline amine family. It is used as a topical immune‐response modifier, and is officially approved for the treatment of superficial basal cell carcinoma, actinic keratoses, and external genital warts. Because of the antitumour effect of imiquimod, it has been used for many other neoplasias, including melanoma in situ (Ray 2005). However, success rates are variable, and recurrence with progression to invasive melanoma has been reported (Woodmansee 2009).
Azelaic acid
Azelaic acid has been successfully used in the treatment of lentigo maligna melanoma (Rodriguez Prieto 1993; Vereecken 2002). The precise mechanism of action responsible for this beneficial effect remains obscure. Numerous investigators have used azelaic acid 20% in a cream, or 15% to 55% in ointment, for a number of months to over a year for the treatment of lentigo maligna (Rodriguez Prieto 1993; Vereecken 2002). However, the results have varied between studies.
5‐fluorouracil
Topical chemotherapy of lentigo maligna of the face with 5‐fluorouracil 5% cream has been used (Litwin 1975).
How the intervention might work
Of the main interventions mentioned above, cryosurgery acts through the direct 'killing' of the melanocytes at temperatures of ‐ 4 to ‐7 °C (Gage 1979), and imiquimod activates the innate immune response by binding to receptors on neutrophils, macrophages, and dendritic cells. This leads to synthesis and release of cytokines, including tumour necrosis factor‐α (Kang 2009), which has been shown to have significant antitumour effects. There have been several studies of the mechanism of action of azelaic acid (Addo‐Boadu 1996; Leibl 1985), but "inhibition of DNA synthesis is one of the mechanisms by which azelaic acid prevents growth and proliferation of abnormal melanocytes" (Leibl 1985). Finally, 5‐fluorouracil inhibits a cell's ability to synthesise DNA by inducing cell cycle arrest and apoptosis (programmed cell death) (Desai 2012).
Why it is important to do this review
We think it is very important to systematically evaluate all of the available surgical and non‐surgical therapeutic options for the treatment of melanoma in situ, since it predisposes to the deadliest type of skin cancer (i.e., invasive melanoma), and its incidence is growing rapidly.
We published the plans for this review as a protocol 'Interventions for melanoma in situ, including lentigo maligna' (Apalla 2013).
Objectives
To assess the effects of all available interventions, surgical and non‐surgical, for the treatment of melanoma in situ, including LM.
We also aimed to assess efficacy, harms, and tolerability of postoperative adjuvant therapies after surgical treatment, as well as the efficacy, harms, and tolerability of non‐surgical interventions preoperatively (neoadjuvant therapies), in order to facilitate surgical removal.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials of any design of the management of melanoma in situ, including LM, that compared any intervention to placebo or active treatment.
Types of participants
We included individuals, irrespective of age and sex, diagnosed with melanoma in situ, including LM, based on histological examination by healthcare practitioners.
Types of interventions
We included surgical (regular excision with 5 mm margins, Mohs surgery, staged excision, etc.) and non‐surgical (imiquimod, lasers, cryosurgery, etc.) treatments, alone or in combination with other treatments, for the management of melanoma in situ, including LM.
Types of outcome measures
The outcome measures of interest were those that estimated clinical efficacy, harms, or tolerability in a reliable and valid way.
Primary outcomes
-
Histological or clinical complete response rate.
-
Total number of acute and short‐term (up to one year) treatment‐related adverse events.
Secondary outcomes
-
Clinical or histological local recurrence rate.
-
Time to complete histological or clinical clearance (for surgical and non‐surgical treatments).
-
Time to histological or clinical local recurrence.
-
Incidence of distal metastases.
-
Progression to invasive melanoma.
-
Short‐, medium‐, and long‐term participant satisfaction.
-
Treatment‐related quality of life.
-
Discontinuation of treatment rate because of harms.
-
Inflammatory response.
Main outcomes for the 'Summary of findings' table
We provided data in the summary of findings Table for the main comparison for the primary and secondary outcomes.
Timing of outcomes
We summarised and pooled outcomes according to the following time schedule: up to one year, between one to five years, and longer than five years from treatment initiation, for the short‐, medium‐, and long‐term outcomes, respectively. We classified treatment‐related harms as 'acute' if they arose during treatment or within two weeks of treatment completion. Analysis of participant satisfaction took place at the same time points.
Economic data
We did not consider economic factors in this review.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 26 November 2014:
-
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2014, Issue 10) using the strategy in Appendix 2;
-
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
-
Embase via Ovid (from 1974) using the strategy in Appendix 4; and
-
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
We searched the following databases up to 30 November 2014 using the strategy in Appendix 6:
-
African Index Medicus (indexmedicus.afro.who.int, from inception);
-
IndMED of India (indmed.nic.in, from inception); and
-
Index Medicus for the South‐East Asia Region (IMSEAR) (from inception).
Trials registers
We searched the following trials registers up to 30 November 2014 using the following search string: ["lentigo maligna" OR "lentigo maligna melanoma" OR "Hutchinson Melanotic Freckle" OR "melanoma in‐situ" OR "melanoma in situ" OR "melanoma‐in‐situ" OR (melanoma NEAR in‐situ)].
-
The metaRegister of Controlled Trials (www.controlled‐trials.com).
-
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov).
-
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
-
The World Health Organization International Clinical Trials Registry platform (who.int/trialsearch).
-
The South African National Clinical Trials Register (www.sanctr.gov.za).
Searching other resources
References from published studies
We checked the bibliographies of included and excluded studies for further references to relevant trials.
Unpublished literature
We tried to identify further relevant trials by sharing our lists of included and excluded studies with industry contacts and other experts in the field of melanoma. We asked if they knew of any further relevant trials on melanoma in situ and also whether they were aware of melanoma trials where a subset of participants had melanoma in situ.
Conference proceedings
In order to identify eligible studies from conference proceedings, we checked the Conference Proceedings Citation Index, accessed via Web of Science™ Core Collection, and Cochrane conference proceedings up to 17 February 2014. We scanned the abstracts of the following major dermatology and oncology conference proceedings, where they were not already recorded in the Cochrane Skin Group Specialised Register:
-
American Academy of Dermatology (2008/2009);
-
European Academy of Dermatology and Venereology (EADV) (from 2006);
-
European Academy of Dermatology and Venereology Spring Symposium (from 2006);
-
Society for Investigative Dermatology (SID) (2007/2008/2009); and
-
World Congress of Dermatology (from 2002).
Adverse effects
We did not perform a separate search for adverse effects of the target interventions. We considered adverse effects and side‐effects described in included studies only.
Data collection and analysis
Some parts of the methods section of this review uses text that was originally published in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two authors (TT and AK) independently examined titles and abstracts of each reference identified in the search results. We checked them independently and resolved any differences related to what we should include or exclude by referring to a third author (SM or AWC). We obtained the full text of all articles that either met the inclusion criteria or for which reading the full text was necessary to decide on their eligibility.
Data extraction and management
Two review authors (TT and AK) independently extracted the data on to a standard data extraction form, which we had modified for our review. Although we were blinded to each other's data extraction, we were not blinded to the journal of publication or to the trial author names. We resolved differences by discussion with a third author (SM or AWC). We extracted data according to Table 7.3.a 'Checklist of items to consider in data collection or data extraction' in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (TT and AK) entered data into the 'Characteristics of included studies' table in Review Manager 2014 (RevMan).
Briefly, we tried to extract the following data: general study information, trial methodology, key variables characterising the participants, key variables characterising the lesion, key variables characterising the intervention, outcomes, and results.
-
General study information
-
-
Author
-
Publication status (full report/abstract/unpublished data)
-
Year of trial initiation
-
Publication date
-
Source of study/funding
-
Setting (place trial was conducted)
-
Dual publication
-
Language of original publication
-
-
Trial methodology
-
-
Design
-
Type of trial
-
Study duration
-
Sequence generation
-
Allocation sequence concealment
-
Participant/provider and outcome assessor blinding
-
Number of participants allocated to each treatment group
-
Number of dropouts and reasons for dropouts
-
Intention‐to‐treat analysis or not
-
Method/duration of follow‐up period
-
-
Key variables characterising the participants
-
-
Age
-
Sex
-
Nationality
-
Duration of lesion
-
Comparability of study groups at entry
-
Inclusion and exclusion criteria
-
Recruitment method
-
Number of participants enrolled
-
Previous treatment
-
Immunocompetent or not (e.g., B‐cell lymphoma, transplantation)
-
-
Key variables characterising the lesion
-
-
Type of lesion
-
Site
-
Size
-
How the diagnosis was established (histological criteria used)
-
Melanotic or amelanotic
-
One or multiple lesions
-
Type of biopsy used (punch, excisional, incisional)
-
-
Key variables characterising the intervention
-
-
Type of intervention
-
For surgical intervention: type of surgical procedure (standard excision, Mohs surgery, etc.), size of postoperative surgical defect, number of stages required; type of reconstruction
-
For topical treatment: duration, mode of application, time of application, if occlusion or not, when treatment stopped (e.g., weeping erosion appeared), if concomitant treatment (cryotherapy) applied, if inflammatory response appeared
-
-
Use of concomitant therapy
-
Evidence of compliance monitoring
-
For cryotherapy: mode of application, description of freeze‐thaw cycles
-
For laser: type of laser (Argon, Q‐switched ruby, QS Nd:YAG, alexandrite laser), method of application
-
-
Outcomes
-
-
Outcomes used and time points
-
Diagnostic criteria used and outcome definition
-
-
Results
-
-
Lost to follow up
-
Summary data for each intervention group
-
Mean and standard deviation for continuous data
-
The number of participants with the outcome event and the denominator for categorical outcomes
-
Estimate of effect with confidence intervals
-
Subgroup analyses (specific subsets of participants)
-
Assessment of risk of bias in included studies
Two review authors (TT and AK) independently assessed the quality and the risk of bias of the eligible study. We resolved any disagreement by discussion and arbitration or by consensus with a third author (SM or AWC). Where necessary, we contacted trial authors for clarification. Quality evaluation and assessment of the risk of bias included evaluation of the following components for the included trial, using the 'Risk of bias' tool suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
(a) method of generation of the randomisation sequence;
(b) method of allocation concealment ‐ we considered this as adequate if the assignment could not be foreseen by either participants or investigator;
(c) blinding (who was blinded/not blinded) in order to assess performance bias ‐ we considered this adequate if participants, investigators, and assessors were blinded as to who received the intervention and who received the placebo/other treatment;
(d) loss to follow up (in each arm); and
(e) whether participants were analysed in the groups to which they were originally randomised (intention‐to‐treat).
Furthermore, we assessed if baseline comparisons of groups regarding important confounding variables were made, in order to assess selection bias.
We also carefully assessed the following:
-
if method and duration of follow‐up period were adequate;
-
definition of partial clearance;
-
definition of complete clinical clearance; and
-
definition of clinical local recurrence.
Measures of treatment effect
We expressed dichotomous outcomes as risk ratios (RR), with 95% confidence intervals (CIs). We expressed continuous outcomes as mean differences (MD), with 95% CIs. We expressed continuous outcomes that were measured with different methodologies across studies as standardised mean differences (SMD), with 95% CIs.
Unit of analysis issues
If we had found cross‐over trials, we would have only considered data from the first period. We would have pooled data from the first period of cross‐over trials with data from parallel‐group studies. If multiple‐treatment group trials were eligible, we would have combined groups to create a single pair‐wise comparison following recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 16) (Higgins 2011). If there were no relevant intervention groups that we could combine, we would have considered multiple‐treatment meta‐analyses. For intraindividual studies, we would have considered each body part as the unit of analysis.
Dealing with missing data
We analysed the data in our included trial as if the analysis was undertaken using intention‐to‐treat. If the analysis of the trial data had been performed using per‐protocol analysis or it was clear that the number of participants analysed for the outcome were not the same as those randomised, thereby excluding intention‐to‐treat analysis, we would have contacted the original investigators to obtain missing data. If contact with the author was not fruitful, we would have used imputation techniques, if possible, based on assumptions ‐ the validity of which we would have assessed through sensitivity analyses. If the results of the sensitivity analysis were suggestive of bias, we would have performed available‐case analysis and reported (with the intention of comparing the two) the results of both methods.
Assessment of heterogeneity
We would have assessed statistical heterogeneity using I² statistic. If I² statistic for the primary outcomes was greater than 50%, we would have ascertained that significant heterogeneity was present. Thus, we would have explored this using prespecified subgroup analyses taking into consideration reasons, such as differences in participants and treatment factors.
Assessment of reporting biases
If we had included more than 10 studies in the meta‐analysis, we had planned to use funnel plots to visually analyse the existence of publication bias. Additionally, we aimed to perform the Egger's test (Egger 1997) to formally assess the symmetry of funnel plots.
Data synthesis
If there had been sufficient studies and if no evidence of heterogeneity between eligible studies existed (I² statistic < 25%), we aimed to synthesise data using fixed‐effect models. Otherwise, we would have used random‐effects models. For dichotomous outcomes, we would have pooled risk ratios (RR) and corresponding 95% confidence intervals (CI) from individual studies. For continuous outcomes, we would have pooled the mean difference (MD) and corresponding 95% CIs from individual studies.
Subgroup analysis and investigation of heterogeneity
Following assessment of heterogeneity, we aimed to perform exploratory subgroup analysis to identify factors that could explain efficacy, safety, or tolerability, or that could explain heterogeneity.
The following were possible subgroups.
-
Study factors
-
-
Design
-
Risk of bias of included studies
-
Length of follow up
-
-
Treatment factors
-
-
Length of treatment
-
Treatment schedule
-
The number of studies included was insufficient, and we were not able to consider these factors.
Sensitivity analysis
We had planned to perform sensitivity analyses to examine the effects of excluding study subgroups or certain studies of low methodological quality.
Results
Description of studies
We report the characteristics of studies in the 'Characteristics of included studies' table, 'Characteristics of excluded studies' tables, and 'Characteristics of ongoing studies' table. A summary of findings is presented in summary of findings Table for the main comparison.
Results of the search
Figure 1 depicts a study flow diagram. In summary, we identified 809 records in total. We removed 27 duplicates, leaving 782 records for screening. Following exclusion of 774 records on the basis of title and abstract, we screened 8 for eligibility and inclusion with full text assessment. From these 8 studies, we excluded 4 and provided reasons in the 'Characteristics of excluded studies' tables; we cannot currently make a decision about 2 studies, so they are detailed in the 'Characteristics of studies awaiting classification' tables; 1 other study is ongoing in the active recruitment phase and fulfilled the criteria for inclusion (NCT01720407). One study, Hyde 2012, was completed with results and fulfilled criteria for inclusion.
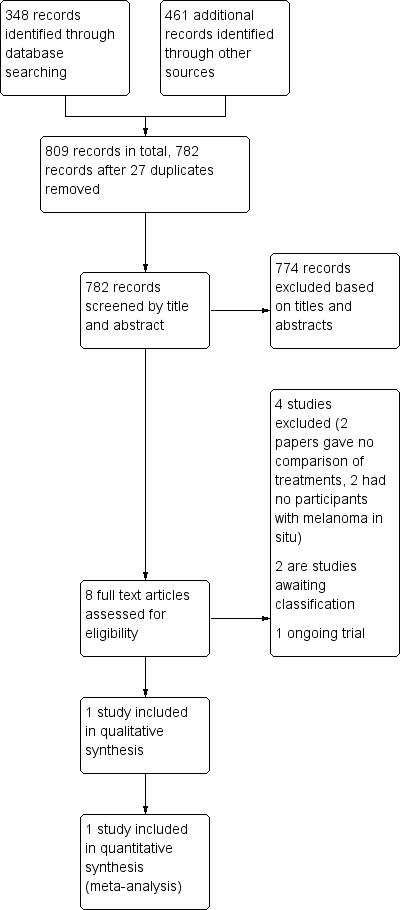
Study flow diagram
Included studies
Our search identified only one study eligible for inclusion, Hyde 2012, with 90 participants.
Design
This was a randomised, single centre, open label, parallel group, two‐arm, controlled trial.
Participants
Ninety participants (24 women, 66 men), who had 91 lentigo maligna (LM) lesions with histologically proven LM, participated and underwent initial shave excision of any visible tumour. The mean age of the participants was 68.2 years (age range: 35 to 92).
Interventions
Forty‐four participants (13 women, 31 men), with 44 LM lesions, were treated with imiquimod 5% cream 5 days/week (from Monday to Friday) plus tazarotene 0.1% gel 2 days/week (Saturday and Sunday) for 3 months, and 46 participants (11 women, 35 men), with 47 LM lesions, were treated with imiquimod 5% cream 5 days/week (from Monday to Friday) for 3 months.
Participants in all groups treated an area extending 1 inch (2.54 cm) beyond the outlined tumour margins. Two months after cessation of topical treatment, the initial tumour footprint was excised using 2 mm margins via a staged excision with radial frozen sections and melan‐A immunostaining to confirm negative margins.
Outcomes
The primary outcome of the study was histological complete response (histological complete clearance) at month five after the initiation of treatment. The secondary outcomes included local recurrence rate, progression to invasive melanoma, discontinuation of treatment because of harms, and overall inflammation score (inflammatory response).
Setting
Only one centre in a university‐setting participated in this study. The study had no funding support. Contact with the corresponding author of the included study (Dr Mark Hyde) was successful, and he provided access to primary study data and replied, when possible, to all of our inquiries.
Excluded studies
We excluded four studies and provide reasons for their exclusion in the 'Characteristics of excluded studies' tables. After evaluation of the full text, we found that two did not include people with melanoma in situ (Khayat 2003; Kim 2008), and two did not compare treatments (Chagpar 2007; Morganroth 2009).
Studies awaiting classification
Two studies that were randomised controlled trials were unclear if they included participants with melanoma in situ (Amatetti 1994; Foster 2007). One of these studies compared efficacy of horizontal Mohs tissue processing to vertical breadloaf tissue processing in the surgical management of superficial melanoma (Foster 2007), and the other studied adjuvant therapy with recombinant alpha‐interferon in non‐metastatic melanoma (Amatetti 1994). We contacted the contact authors of these studies in order to obtain more information, but they did not respond to our request. We have included them in the studies awaiting classification section and provide their known characteristics in the 'Characteristics of studies awaiting classification' tables. Therefore, we were unable to include them in the current systematic review.
Ongoing studies
Our search identified one ongoing study (NCT01720407), of which we provide details in the 'Characteristics of ongoing studies' table. This is a randomised, parallel group, two‐arm, open label trial in active recruitment status. The aim of this study is to assess the relevance of imiquimod as neoadjuvant treatment to reduce excision size and the risk of intralesional excision in LM of the face. This study includes participants with LM of the face or neck, histologically confirmed by biopsy. Group A is treated with imiquimod (5 times per week) applied to and extending 1 cm beyond the lesion for a period of 4 weeks, and group B, with vehicle (5 times per week) applied to and extending 1 cm beyond the lesion for a period of 4 weeks. In both groups, surgery performed four weeks after the last application of the topical treatment follows local treatment. The primary outcome of this study is margin of resection. An at least 5 mm healthy tissue margin beyond lesion periphery defines successful resection with proper margins. This study started on October 2012, and MEDA Pharma GmbH & Co. KG sponsors it.
Risk of bias in included studies
We categorised judgements in order to indicate a low, high, or unclear risk of bias. The 'Risk of bias' graph (Figure 2) summarises the results, in addition to the 'Risk of bias' table within the 'Characteristics of included studies' table.
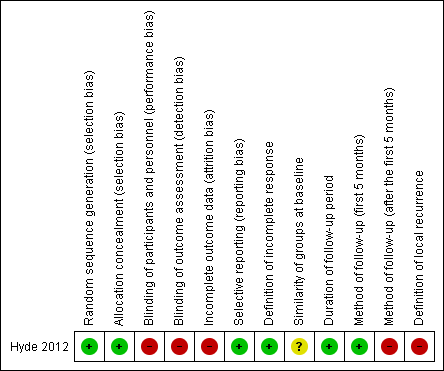
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Randomisation
We deemed the risk of selection bias from the random sequence generation as low; this is because the study used a 1:1 random number table for 2 interventions generated by the clinical trials office at the institution.
Allocation
We initially judged the risk of selection bias from allocation concealment as unclear since authors did not report on this. However, contact with the study authors clarified that the study used a centralised service, so we deemed the risk of selection bias from allocation concealment as low.
Blinding
We judged the risk of bias as high since this was an open label trial and study participants, medical staff, and study staff were not blinded after the assignment was made. The study authors reported that blinding was not possible because of the nature of the study. However, a vehicle (placebo) treatment could have been used instead of tazarotene in the group that received imiquimod monotherapy. Further, blinded outcome assessment is usually possible for pathologists and clinical assessors.
Incomplete outcome data
We judged the risk of bias as high because 5/46 participants from the imiquimod‐alone group and 7/44 participants from the imiquimod‐tazarotene group withdrew consent or dropped out because of adverse effects. The study partially provided reasons. The study did not report intention‐to‐treat analysis.
Selective reporting
We judged the risk of bias as low since the study reported all prespecified outcomes. The corresponding author of the study, Dr Hyde, provided us with all the data needed to evaluate it.
Other potential sources of bias
Definition of incomplete response
We judged the risk of bias as low since the study clearly reported definition of incomplete response, which we deemed sufficient.
Similarity of groups at baseline
We judged the risk of bias as unclear. Although we deemed the randomisation process sufficient and correct, the study authors did not present important baseline characteristics of the two groups, such as size of lesions, which could be potential confounding variables.
Method and duration of follow up
We judged the risk of bias regarding duration of follow‐up period as low as we deemed the follow‐up period adequate (mean was 42 months).
We judged the risk of bias regarding method of follow up for the initial five months as low as we deemed the method of follow up for this time period sufficient.
The original article did not report the method of follow up after the initial five months (after surgery). The corresponding author of the study, Dr Mark Hyde, kindly provided additional data about this. He reported that follow up was done in clinic where possible. For those participants who did not come back to clinic, researchers made phone calls and asked them if they had seen another dermatologist or asked them to look at the scar and tell them if they saw any pigment in or around the scar. Unfortunately, Dr Hyde did not have records to tell us how many came back to clinic and how many follow‐ups were completed by phone. We therefore judged the risk of bias regarding method of follow up after the initial five months as high and deemed the method insufficient.
Definition of local recurrence
We judged the risk of bias regarding definition of local recurrence as high. The study authors did not report the definition of local recurrence. The corresponding author of the study, Dr Hyde, was unable to provide any further information.
Effects of interventions
We provided data in the summary of findings Table for the main comparison for the primary and secondary outcomes.
Imiquimod 5% cream with tazarotene 0.1% gel is the 'combination' therapy and is compared with imiquimod 5% cream alone, which is referred to as the 'monotherapy'.
Primary outcomes
1. Histological or clinical complete response rate
This outcome was measured at five months, which fits our classification of short‐term (up to one year). The study authors reported that in the imiquimod monotherapy group, 27 out of 41 (65%) participants who reached the end point of the study had complete histological response. In the combination group (imiquimod with tazarotene), 29 out of 37 participants (78%) who reached the intended treatment duration had complete histological response. We contacted the corresponding author to obtain information regarding the participants who dropped out, and he kindly gave us access to primary data.
We employed intention‐to‐treat analysis, characterising all participants who dropped out as treatment failures or not complete responders. We decided to do so taking into account the nature of the disease under investigation. Twenty‐nine out of 44 participants (66%) treated with imiquimod and tazarotene and 27 out of 46 participants (59%) treated with imiquimod monotherapy had complete clearance at 5 months. The difference was not statistically significant (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.81 to 1.55, P value = 0.48; Analysis 1.1).
We also employed analysis only for the participants who finished the study, as it had been reported by the study authors. Results did not show a statistically significant difference between groups (combination therapy versus monotherapy). Τhe complete response RR (risk ratio) was 1.19 (95% CI 0.90 to 1.57, P value = 0.22; Analysis 1.2).
We carefully analysed the primary data that Dr Hyde provided. Of the five participants who dropped out from the imiquimod monotherapy group, three never started the treatment and withdrew consent. The other two started the topical treatment but did not finish it. These last two participants had complete response after subsequent surgery. From the seven participants who dropped out of the combination group, four had complete response; for one participant, data were missing; and the other two did not have a complete response. After evaluation of the data and taking into account the nature of the disease, we decided not to impute these data in any analysis.
Because we identified only one study with a modest number of participants, we graded the quality of the evidence as low. See summary of findings Table for the main comparison.
2. Total number of acute and short‐term (up to one year) treatment‐related adverse events
The protocol of Hyde 2012 did not include overall treatment‐related adverse events from non‐surgical or surgical interventions, but it did include total inflammation score (inflammatory response) (see below: Secondary outcome 9: Inflammatory response). Although this outcome can be considered as a treatment‐related adverse event, it is also an outcome that has been suggested to be associated with the efficacy of local treatment with imiquimod.
Secondary outcomes
1. Clinical or histological local recurrence rate
The study did not report any recurrences in the mean follow‐up period of 42 months. This fits with our classification of medium‐term (from year one up to year five). The study authors reported that prior to initiation of topical treatment (in order to avoid the risk of treating invasive lentigo maligna melanoma (LMM)) with topical imiquimod, all visible signs of LM were removed using a shave excision at the first visit. The corresponding author of the study informed us that there were eight people who had died of unrelated causes during this follow‐up period. Of those who dropped out of the study, follow‐up data were available for only one person.
2. Time to complete histological or clinical clearance (for surgical and non‐surgical treatments)
The study did not measure or report this outcome.
3. Time to histological or clinical local recurrence
The study did not measure or report this outcome.
4. Incidence of distal metastases
The study did not assess or report Incidence of distal metastases.
5. Progression to invasive melanoma
One participant out of the 44 participants from the combination‐therapy group (imiquimod plus tazarotene) developed invasive melanoma (0.32 Breslow depth and Clark level III) at the treatment site in the 2‐month interval between topical therapy completion and surgery. This fits our classification of short‐term (up to one year). The imiquimod monotherapy group reported no cases of invasive melanoma.
6. Short‐, medium‐, and long‐term participant satisfaction
The study did not measure or report this outcome.
7. Treatment‐related quality of life
The study did not measure or report this outcome.
8. Discontinuation of treatment because of harms
One participant out of 46 (2.2%) in the imiquimod monotherapy group dropped out due to excessive inflammation (inflammation grade of 3) before the cessation of topical treatment (first 3 months). Six out of 44 participants (13.7%) dropped out due to adverse events before the cessation of topical treatment (first 3 months). The result of the comparison was not statistically significant (RR 6.27, 95% CI 0.79 to 50.02, P value = 0.08; Analysis 1.3).
As this single study was insufficiently powered to document a real difference between the two groups, we graded the quality of the evidence as low. See summary of findings Table for the main comparison.
9. Inflammatory response
The study graded inflammatory response by the maximum intensity observed using a scale of zero to three (none: no inflammation; one: pink; two: red; three: presence of erosions, oozing, or eschar). The study authors reported that participants in the combination therapy group had a significantly higher overall inflammation score (mean = 2.3) than those in the monotherapy group (mean = 1.8). These values correspond to the participants who reached the end point of the trial. The study authors did not report on the participants who dropped out; however, we contacted them and obtained primary data for all participants.
We first analysed the primary data of the participants who completed the study. Mean overall inflammation score for the monotherapy group was 1.738 and standard deviation was 0.989 (41 participants). Mean overall inflammation score for the combination group was 2.297 and standard deviation was 0.9 (37 participants). Difference in overall inflammation score between the 2 groups was significant (mean difference (MD) 0.56, 95% CI 0.14 to 0.98, P value = 0.009; Analysis 1.4). Mean overall inflammation score was significantly higher in the combination group compared to the monotherapy group.
We also analysed all data for all participants regarding the inflammatory response. For three participants from the imiquimod monotherapy group, no topical treatment was initiated and no data recorded. We decided to exclude these three participants from the analysis. For the other 43 participants, we imputed the highest grade of inflammation recorded per case. Mean inflammation score was 1.7 and standard deviation was 1 in the monotherapy group (43 participants). Regarding the 44 participants from the combination group, we imputed the highest grade of inflammation recorded per case. Mean inflammation score was 2.3, and standard deviation was 0.93 (44 participants). Difference in overall inflammation score between the 2 groups was statistically significant (MD 0.6, 95% CI 0.2 to 1, P value = 0.004; Analysis 1.5). Mean overall inflammation score was significantly higher in the combination group compared with the monotherapy group.
Given that for one of the groups the mean score (2.29) is close to the maximum (3), these methods that are suitable for normally distributed data might under‐ or over‐estimate the mean difference (Higgins 2011). Because we had contacted the study authors and the original data values were available, we re‐analysed the inflammation scores using non‐parametric methods suitable for ordinal data (Mann‐Whitney U test). Mann‐Whitney U test ‐ using ranks ‐ yielded an identical P value (P value = 0.004), with the mean difference method supported by Review Manager (Review Manager 2014). Even though this difference is most probably real, because we identified only one study with a modest number of participants, we graded the quality of the evidence as low. See summary of findings Table for the main comparison.
We conducted a posthoc analysis regarding the number of lesions that achieved a grade two or three inflammatory response. Analysis of all primary data indicated that 26 of the 44 lesions in the monotherapy group achieved a grade 2 or 3 inflammatory response. In the combined therapy group, 36 of 44 lesions achieved a grade 2 or 3 inflammatory response. The difference between the 2 groups was statistically significant in favour of monotherapy (RR 1.38, 95% CI 1.04 to 1.84, P value = 0.02; Analysis 1.6).
As we only identified one study with a modest number of participants, we graded the quality of the evidence as low. See summary of findings Table for the main comparison.
Discussion
Summary of main results
This systematic review tried to summarise all available interventions for the treatment of melanoma in situ, including lentigo maligna. We identified only one randomised, single centre, open label, parallel group, two‐arm, controlled trial. The trial treated 44 participants with imiquimod 5% cream plus tazarotene 0.1% gel (combination therapy group) and 46 participants with Imiquimod 5% cream (monotherapy group).
Regarding our primary outcome 'Histological or clinical complete response rate', there was no statistically significant difference (RR 1.12, 95% CI 0.81 to 1.55, P value = 0.48, intention‐to‐treat (ITT) analysis) between the combination (66%) and monotherapy groups (59%). The best complete response rate was 78% for the combination group and 66% for the monotherapy group.
With regard to our secondary outcomes, discontinuation of treatment because of harms was not statistically significant. Inflammatory response was reported as mean inflammatory score; this is an interesting outcome that is a treatment‐related adverse event. However, it has also been suggested that it could be an outcome related to efficacy of imiquimod. Mean overall inflammation score was statistically significantly higher in the combination group. Interestingly, although combination therapy exhibited a significantly higher degree of inflammation, this did not translate to a significant increase in the completed histological response rate. This suggests that the quantity of inflammation may not be a factor associated with increased efficacy. Enhancing drug penetration with tazarotene did not increase the complete response rate but did lead to substantially more inflammation. One person out of the 44 participants from the combination therapy group developed invasive melanoma. This emphasises the need for close, adequate, and structured follow up at predefined periods for people treated with imiquimod as monotherapy. In the mean follow‐up period of 42 months, the study reported no local recurrences. Previously published recurrence rates for stage excision alone are approximately 6% (Bub 2004; Lee 2008; Walling 2007).
Whether or not addition of imiquimod as neoadjuvant treatment can decrease local recurrence rate remains a question that future studies should properly address.
Overall completeness and applicability of evidence
This systematic review identified only one study eligible for inclusion and one ongoing study. It is unlikely that the results of the only included study can facilitate clinicians in making evidence‐based treatment decisions. Important outcomes for the evaluation of imiquimod as neoadjuvant treatment, such as reduction of excision size, morbidity, and quality of life, are not properly addressed, although these may be important clinically relevant outcomes. There is insufficient evidence to support or refute the addition of tazarotene to imiquimod as adjuvant therapy. Since tazarotene is one of the strongest topical retinoids (with only isotretinoin 0.05% gel being stronger), addition of any other retinoid is not recommended and is likely to highly increase inflammation and dropout of patients from treatment with no significant efficacy gain.
Quality of the evidence
There is a paucity of randomised controlled trials (RCTs) and high‐quality evidence for the treatment of melanoma in situ and lentigo maligna. Appropriate studies of the most important clinically relevant outcomes, such as recurrence rate; reduction of excision size with the use of neoadjuvant treatments; morbidity; and participant‐rated outcomes, such as satisfaction rate and quality of life, are needed. Our included study was a single centre, open label, parallel group trial with only 90 participants allocated in 2 arms, thus, not adequately powered; no sample size calculation according to any specific clinically relevant outcome was made. The comparisons of interventions in the study were relevant to this disease, but do not cover the range of outcomes this review is aimed at.
We downgraded all the outcomes we examined by two levels to reflect the fact that the single eligible study by Hyde 2012 was subject to a high risk of bias due to lack of blinding, partially incomplete outcome data, and method of follow up. Overall, we judged the evidence to be of low quality, meaning that future studies will modify confidence in the estimate of effect, which in turn could be reversed.
Potential biases in the review process
We assessed risk of bias as low for random sequence generation and selective reporting, definition of incomplete response, and duration of follow up. There was no blinding of participants, personnel, or outcome assessors; therefore, we judged blinding as at high risk of bias. Analysis was not intention‐to‐treat, leading to a high risk of incomplete outcome data. We deemed method of follow up after the initial five months and definition of local recurrence as at high risk of bias. These parameters are of high significance. The study did not report information on similarity of groups at baseline for important confounding variables such as the size of the lesion.
We need to mention several limitations of this systematic review. "As with any [review] there is the potential for publication bias to over‐estimate differences in outcomes if studies identifying such differences are more likely to be published in peer‐reviewed journals" (Sladden 2009). This review has the limitation of solely depending on a single study to detect real differences in outcomes. The total number of participants recruited in the included study was not based on a sample size calculation, and the study was insufficiently powered to rule out clinically relevant differences.
We made a substantial effort to obtain information on studies that might include a subset of people with melanoma in situ or lentigo maligna melanoma (LMM). We identified two studies that might be eligible for inclusion in this review, but it was unclear if they included participants with melanoma in situ or LMM (Amatetti 1994; Foster 2007), so this makes our review somewhat incomplete. We contacted the study authors to obtain further information, but to date, they have not responded, so we have detailed these in the 'Characteristics of studies awaiting classification' tables.
Agreements and disagreements with other studies or reviews
One systematic review, Fogarty 2014, tried to summarise the published evidence for the use of radiotherapy in the treatment of LM, focusing on technical aspects in order to develop the best evidence‐based radiotherapy protocol. The authors included any type of studies and could identify only retrospective case series. They concluded that there is a lack of standardisation of the studies, and overall, they could not identify the optimum parameters for radiotherapy.
A review of treatments for LM tried to comprehensively review available evidence (McLeod 2011). The authors employed a specific search strategy with prespecified key search terms, which did not have any restrictions regarding the type of studies, and they included all investigational studies of any design, both prospective and retrospective. They found a number of studies: 12 examining staged surgical excision, 9 using Mohs micrographic surgery, 6 investigating cryosurgery, 22 investigating imiquimod, 7 using lasers, 9 investigating radiation therapy, and 2 investigating electrosurgery and curettage. The authors presented tables with important outcomes and variables. They concluded that staged surgical excision and Mohs micrographic surgery are linked with the lowest recurrence rates for LM and that cryotherapy and radiation therapy may be contemplated as the options for treatment of LM in people who cannot endure surgery (McLeod 2011). They also suggested that imiquimod may have a future role to play in the treatment of LM.
Another review summarised the treatment options specifically for melanoma in situ (Erickson 2010). The authors employed a specific search strategy that did not restrict the types of studies included and presented tables for many important variables. They concluded that "topical imiquimod therapy appears to provide relatively low cure rates" and should be used with extreme caution. "Radiation therapy may be a useful second‐line therapy if surgery is contraindicated". They clearly suggest that "excisional surgery is an appropriate therapy for clinically well‐defined melanomas in situ. However, margins larger than 5 mm may be required when treating larger or indistinct lesions."
We excluded from this systematic review all of the studies (except one) included in the aforementioned reviews because they were case series, retrospective, and uncontrolled studies. These types of studies were not eligible for inclusion in this systematic review, since we restricted the predefined inclusion criteria to randomised controlled trials. Our review and all of the aforementioned three reviews reported limited evidence.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 1 Primary outcome: Histological complete response (ITT).

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 2 Primary outcome: Histological complete response (available‐case analysis).

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 3 Secondary outcome: Discontinuation of treatment because of harms.
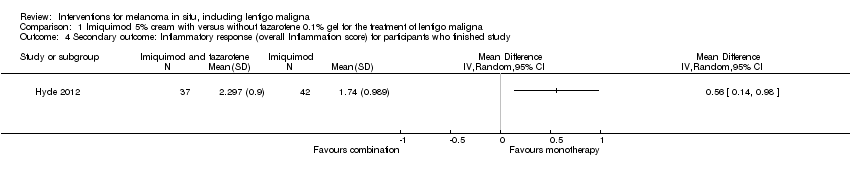
Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 4 Secondary outcome: Inflammatory response (overall Inflammation score) for participants who finished study.
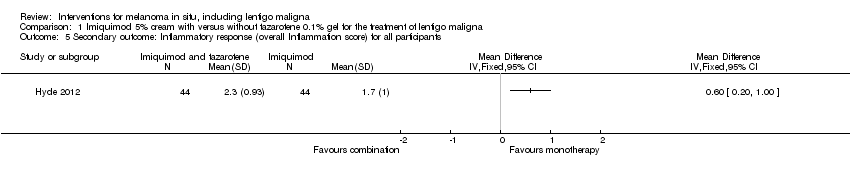
Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 5 Secondary outcome: Inflammatory response (overall Inflammation score) for all participants.

Comparison 1 Imiquimod 5% cream with versus without tazarotene 0.1% gel for the treatment of lentigo maligna, Outcome 6 Secondary outcome: Inflammatory response (number of lesions with grade 2 or 3).
| Imiquimod 5% cream with tazarotene 0.1% gel compared with Imiquimod 5% cream alone for melanoma in situ, including lentigo maligna | ||||||
| Patient or population: participants with melanoma in situ, including lentigo maligna | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Imiquimod 5% cream alone | Imiquimod 5% cream with tazarotene 0.1% gel | |||||
| Primary outcome: Histological complete response (intention‐to‐treat) | 587 per 1000 | 657 per 1000 | RR 1.12 | 90 | ⊕⊕⊝⊝ | ‐ |
| Primary outcome: Histological complete response ( available‐case analysis) | 659 per 1000 | 784 per 1000 | RR 1.19 | 78 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Discontinuation of treatment because of harms | 22 per 1000 | 136 per 1000 | RR 6.27 | 90 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Inflammatory response (number of lesions with grade 2 or 3) | 591 per 1000 | 815 per 1000 | RR 1.38 | 88 | ⊕⊕⊝⊝ | ‐ |
| Secondary outcome: Inflammatory response (overall Inflammation score) for all participants | ‐ | The mean overall inflammation score (inflammatory response) for all participants in the intervention groups was 0.60 higher | ‐ | 88 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| GRADE = Grading of Recommendations Assessment, Development and Evaluation. | ||||||
| Medical term | Explanation |
| Intraepidermal | something confined within the epidermis |
| Preinvasive | a malignant tumour that remains constricted in the epithelium, while the dermo‐epidermal junction preserves its integrity |
| Eschar | a dry scab or slough formed on the skin as a result of a burn or by the action of a corrosive or caustic substance |
| Invasive | a malignant tumour that breaks the dermo‐epidermal junction and invades the dermis |
| Lentigo maligna | a subtype of preinvasive intraepidermal melanoma, associated specifically with chronic solar exposure |
| Vehicle | an excipient or a menstruum, a substance, usually without therapeutic action, used as a medium to give bulk for the administration of medicines |
| Apoptosis | a genetically directed process of cell self‐destruction that is marked by the fragmentation of nuclear DNA. It is activated either by the presence of a stimulus or removal of a suppressing agent or stimulus, and is a normal physiological process eliminating DNA‐damaged, superfluous, or unwanted cells. It is a synonym for programmed cell death |
| Actinic keratosis | rough, scaly macule or patch of skin that it is considered precancerous and is associated with chronic sun exposure |
| Lentigo senilis | a flat brownish, pigmented spot on the skin, due to increased deposition of melanin and an increased number of melanocytes |
| Seborrheic keratosis | a benign skin tumour that occurs singly or in clusters on the surface of the skin, is usually light to dark brown or black in colour, and typically has a warty texture often with a waxy appearance |
| Patch | a greater than 1 cm circumscribed area of discolouration on the skin |
| Cytokine | a small protein released by cells that has a specific effect on the interactions between cells, on communications between cells, or on the behavior of cells |
| Innate immunity | immunity that occurs naturally as a result of a person's genetic constitution or physiology and does not arise from a previous infection or vaccination |
| Blinding | the concealment of group assignment ‐ to either the treatment or control group ‐ from the knowledge of participants, investigators in a clinical trial, or both |
| Tumour islands | aggregates of tumour cells |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: Histological complete response (ITT) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Primary outcome: Histological complete response (available‐case analysis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Secondary outcome: Discontinuation of treatment because of harms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Secondary outcome: Inflammatory response (overall Inflammation score) for participants who finished study Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Secondary outcome: Inflammatory response (overall Inflammation score) for all participants Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Secondary outcome: Inflammatory response (number of lesions with grade 2 or 3) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

