Histerectomía con radioterapia o quimioterapia o ambas para mujeres con cáncer de cuello uterino localmente avanzado
Resumen
Antecedentes
Esta es una actualización de la revisión Cochrane publicada en el número 4 de 2015. El cáncer de cuello uterino es una de las causas más frecuentes de muerte por cánceres ginecológicos en todo el mundo. Muchos de los nuevos casos de cáncer de cuello uterino en los países de ingresos bajos se presentan en una fase avanzada. El tratamiento estándar en Europa y EE.UU. para el cáncer de cuello uterino localmente avanzado (CCULA) es la quimiorradioterapia. En los países de ingresos bajos, con acceso limitado a la radioterapia, el CCULA se puede tratar con quimioterapia e histerectomía. No hay certeza de que este procedimiento mejore la supervivencia. Es importante evaluar el valor de la histerectomía con radioterapia o quimioterapia, o ambas, como alternativa.
Objetivos
Determinar si la histerectomía, además del tratamiento estándar con radioterapia o quimioterapia, o ambas, en mujeres con CCULA (estadio IB2 a III) es segura y eficaz en comparación con el tratamiento estándar solo.
Métodos de búsqueda
Se realizaron búsquedas en CENTRAL, MEDLINE a través de Ovid, Embase a través de Ovid, LILACS, en registros de ensayos y la literatura gris hasta el 3 de febrero de 2022.
Criterios de selección
Se buscaron ensayos controlados aleatorizados (ECA) que compararan tratamientos que incluían la histerectomía versus la radioterapia o la quimioterapia, o ambas, en mujeres con CCULA en estadios IB2 a III de la Federación Internacional de Ginecología y Obstetricia (FIGO).
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane. Se evaluó de forma independiente la elegibilidad de los estudios, se extrajeron los datos y se evaluó el riesgo de sesgo. Cuando fue posible, la supervivencia general (SG) y la supervivencia sin progresión (SSP) o sin enfermedad (SSE) se resumió en un metanálisis utilizando un modelo de efectos aleatorios. Los eventos adversos (EA) se informaron de manera incompleta y los resultados de los ensayos se describieron de forma narrativa. Para evaluar la certeza de la evidencia se utilizó el método GRADE.
Resultados principales
A partir de las búsquedas se identificaron 968 estudios. Después de la eliminación de los duplicados, la revisión de los títulos y resúmenes y la evaluación del texto completo, se incluyeron 11 ECA (2683 mujeres) de calidad metodológica variable. Esta actualización identificó cuatro nuevos ECA y tres ECA en curso.
Los estudios incluidos compararon: histerectomía (simple o radical) con radioterapia o quimiorradioterapia o quimioterapia neoadyuvante (QTNA) versus radioterapia sola o quimiorradioterapia (QRT) sola o QRT y braquiterapia. También hay un estudio en curso en el que se comparan tres grupos: histerectomía con QRT versus histerectomía con QTNA versus QRT.
Hubo dos grupos de comparación de los que fue posible hacer un metanálisis.
Histerectomía (radical) con quimioterapia neoadyuvante versus quimiorradioterapia sola
Dos ECA con características de diseño similares (620 y 633 participantes) no encontraron diferencias en la SG a los cinco años entre la QTNA con histerectomía versus la QRT. El metanálisis que evaluó a 1253 participantes no encontró evidencia de una diferencia en el riesgo de muerte (SG) entre las mujeres que recibieron QTNA más histerectomía y las que recibieron QRT sola (CRI 0,94; IC del 95%: 0,76 a 1,16; evidencia de certeza moderada). En ambos estudios, la SSE a los cinco años en el grupo de QTNA más cirugía fue peor (57%) en comparación con el grupo de QRT (65,6%), principalmente para el estadio IIB.
Los resultados de los ensayos no informaron de diferencias manifiestas en las complicaciones graves a largo plazo, la toxicidad aguda de grado 3 ni la toxicidad tardía grave entre los grupos (evidencia de calidad muy baja).
Histerectomía (simple o radical) con quimioterapia neoadyuvante versus radioterapia sola
El metanálisis de tres ensayos de QTNA con histerectomía versus radioterapia sola, que evaluaron a 571 participantes, encontró que las mujeres que recibieron QTNA más histerectomía tuvieron menos riesgo de muerte (SG) que las que recibieron radioterapia sola (CRI 0,71; IC del 95%: 0,55 a 0,93; I2 = 0%; evidencia de calidad moderada). Sin embargo, un número significativo de participantes que recibieron QTNA más histerectomía también recibieron radioterapia. No hubo diferencias en la proporción de mujeres con progresión o recidiva de la enfermedad (SSE y SSP) entre los grupos de QTNA más histerectomía y radioterapia (RR 0,75; IC del 95%: 0,53 a 1,05; I2 = 20%; evidencia de calidad moderada).
La certeza de la evidencia fue baja o muy baja para todas las demás comparaciones en todos los desenlaces.
Ninguno de los ensayos informó sobre los desenlaces de calidad de vida.
Conclusiones de los autores
A partir de los ECA disponibles, no se encontró evidencia suficiente de que la histerectomía con radioterapia, con o sin quimioterapia, mejore la supervivencia de las mujeres con CCULA que son tratadas con radioterapia o QRT sola. La certeza general de la evidencia fue variable en los diferentes desenlaces y se disminuyó unánimemente debido a la preocupación por el riesgo de sesgo. La certeza de la evidencia para la QTNA y la histerectomía radical versus la radioterapia sola para los desenlaces de supervivencia fue moderada. Lo mismo ocurrió con la comparación que incluía la QTNA y la histerectomía en comparación con la QRT sola. La evidencia de otras comparaciones generalmente fue escasa y de certeza baja o muy baja. Esto se debe principalmente a la poca información y a la escasez de datos puesto que los resultados se basan en ensayos por separado. Más ensayos que evalúen el tratamiento médico con y sin histerectomía pueden poner a prueba la solidez de los hallazgos de esta revisión, ya que es probable que estudios de investigación adicionales tengan un impacto importante en la confianza en la estimación del efecto.
PICO
Resumen en términos sencillos
Histerectomía con tratamiento médico para el cáncer de cuello de útero que se ha extendido a los tejidos cercanos solamente
El problema
El cáncer de cuello de útero (cáncer cervical) es el más frecuente entre las mujeres de hasta 65 años. Una alta proporción de mujeres de países pobres son diagnosticadas con cáncer de cuello de útero localmente avanzado (diseminado a los tejidos cercanos, pero sin diseminación distante evidente). Se suelen tratar con radioterapia, con o sin quimioterapia (tratamiento médico). También se utiliza la histerectomía (cirugía para extirpar la matriz y el cuello de útero) con tratamiento médico, especialmente en los países pobres donde el acceso a la radioterapia es limitado.
Objetivo de la revisión
¿La histerectomía con tratamiento médico tiene más efectos beneficiosos en comparación con el tratamiento médico solo en mujeres con cáncer de cuello de útero localmente avanzado?
¿Cómo se realizó la revisión?
Una búsqueda bibliográfica desde 1966 hasta febrero de 2022 identificó 11 ensayos clínicos con un riesgo de sesgo de moderado a alto. Estos ensayos incluyeron 2683 mujeres y compararon: histerectomía con radioterapia frente a radioterapia sola; histerectomía con quimiorradioterapia (quimioterapia más radioterapia) frente a quimiorradioterapia sola; histerectomía con quimiorradioterapia frente a radioterapia interna (braquiterapia) con quimiorradioterapia; e histerectomía precedida de quimioterapia (neoadyuvante, para reducir el tamaño del cáncer) frente a radioterapia sola. También se identificaron tres ensayos en curso.
¿Cuáles son los principales hallazgos?
Histerectomía (simple [útero y cuello de útero] o radical [útero, cuello de útero y tejidos circundantes]) con quimioterapia neoadyuvante frente a radioterapia sola
Al combinar los resultados de tres estudios que evaluaron a 571 mujeres, se determinó que murieron menos mujeres que recibieron quimioterapia neoadyuvante más histerectomía que las que recibieron sólo radioterapia. Sin embargo, muchas mujeres del primer grupo también recibieron radioterapia. No hubo diferencias en el número de mujeres que estuvieron sin enfermedad después del tratamiento.
Histerectomía (radical) con quimioterapia neoadyuvante versus quimiorradioterapia sola
Se combinaron los resultados de dos estudios que evaluaron a 1253 mujeres. No se encontraron diferencias en el riesgo de muerte entre las mujeres que recibieron histerectomía con quimioterapia neoadyuvante y las que recibieron quimiorradioterapia sola.
Los efectos secundarios no se informaron de manera adecuada. Los resultados de los ensayos por separado no mostraron diferencias en los efectos secundarios graves entre los grupos en ninguna comparación. Datos limitados indicaron que las intervenciones parecieron tolerarse razonablemente bien, aunque se necesita más evidencia.
Los estudios no informaron sobre cómo se vio afectada la calidad de vida de las mujeres.
¿Cuáles son las conclusiones?
No se encontró evidencia suficiente de que el agregado de histerectomía a la radioterapia y la quimiorradioterapia mejorara la supervivencia, la calidad de vida ni los efectos secundarios en las mujeres con cáncer de cuello de útero localmente avanzado en comparación con el tratamiento médico solo. En general, la calidad de la evidencia fue variable y preocupa el riesgo de sesgo. Más ensayos que evalúen el tratamiento médico con y sin histerectomía podrían analizar la solidez de los hallazgos de esta revisión. Es probable que los datos adicionales de ensayos planificados cuidadosamente que evalúen el tratamiento médico con y sin histerectomía repercutan en la confianza en estos resultados.
Authors' conclusions
Summary of findings
| Hysterectomy (radical) with neoadjuvant chemotherapy versus with chemoradiotherapy alone for women with locally advanced cervical cancer | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: NACT + hysterectomy Comparison: CCRT | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence(GRADE) | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 58.5–98.4 months in the 2 trials | HR 0.94 (0.76 to 1.16) | 1253 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | I2 = 0% |
| DFS Median follow‐up 58.5–98.4 months in the 2 trials | HR 1.38 (1.02 to 1.87) | 633 (1 RCT) | ⊕⊕⊝⊝ Lowb | — |
| 5‐year DFS in the NACT + surgery group was 57% vs 65.6% in the chemoradiotherapy group (P = 0.021) | 620 (1 RCT) | |||
| Quality of life | — | — | — | Not reported. |
| SAEs and toxicity | SAEs In first trial, there were no toxic deaths reported. 198 SAEs occurred: 145 in the NACT + surgery arm vs 53 in the CCRT arm. In the second trial there were 114 grade 3 or 4 SAEs: 92 in the NACT + surgery arm vs 22 in the CCRT arm | 198 (1 RCT) 114 (1 RCT) | ⊕⊝⊝⊝ Very lowc | — |
| Toxicity In 1 trial, NACT + surgery group, compared with the chemoradiotherapy group, there was a lower rate of rectal (5.7% with NACT + surgery vs 13.3% with chemoradiotherapy; P = 0.002), bladder (2.8% with NACT + surgery vs 7.3% with chemoradiotherapy; P = 0.017), and vaginal (19.9% with NACT + surgery vs 36.9% with chemoradiotherapy; P = 0.001) toxicity occurring or persisting 90 days after treatment completion. However, 24 months after treatment completion, there was no difference in rectal and bladder toxicities between groups, whereas vaginal toxicity continued to occur at a lower rate in the NACT + surgery group (12.0% with NACT + surgery vs 25.6% with chemoradiotherapy; P = 0.001). | 114 (1 RCT) | |||
| Treatment‐related morbidity No treatment‐related deaths in either chemoradiotherapy or NACT + surgery arm. Overall, 89% of participants in the chemoradiotherapy arm and 73% in the NACT + surgery arm had complications, with 18% in NACT + surgery arm experiencing recurrence and requiring adjuvant radiotherapy. | 111 (1 RCT) | |||
| CI: confidence interval; CCRT: concurrent chemoradiotherapy; DFS: disease‐free survival; HR: hazard ratio; NACT: neoadjuvant chemotherapy; SAE: serious adverse event. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. | ||||
| aDowngraded one level due to concerns regarding the uncertainty of risk of bias in individual trials and only two trials in meta‐analysis (although it is arguable whether the number of included participants represented relatively sparse data). | ||||
| Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone for women with locally advanced cervical cancer | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: neoadjuvant chemotherapy + radical hysterectomy Comparison: radiotherapy alone | ||||
| Outcomes | Relative effect | No of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 39–60 months in the 3 trials | HR 0.71 (0.55 to 0.93) | 571 | ⊕⊕⊕⊝ | — |
| Disease‐ or progression‐free survival Median follow‐up 39–60 months in the 3 trials | HR 0.75 (0.53 to 1.05) | 571 | ⊕⊕⊕⊝ | There were varying definitions of disease‐ and progression‐free survival. However, we did not consider this merited further downgrading to low‐certainty evidence. |
| Quality of life | — | — | — | Not reported. |
| Severe adverse events and toxicity | Acute severe toxicity RR 1.32 (0.47 to 3.71) | 118 (1 RCT) | ⊕⊕⊝⊝ | — |
| Long‐term severe complications RR 0.86 (0.49 to 1.50) | 409 (1 RCT) | |||
| Severe late toxicity RR 0.60 (0.27 to 1.34) | 118 (1 RCT) | |||
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to concerns regarding the uncertainty of risk of bias in individual trials. | ||||
| Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: radiotherapy + hysterectomy (simple or radical) Comparison: radiotherapy alone | ||||
| Outcomes | Relative effect (95% CI) | No of participants | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 9.6 years | HR 0.89 (0.61 to 1.29) | 256 (1 RCT) | ⊕⊕⊖⊖ Lowa,b | 12 participants in each regimen (10% with radiotherapy + hysterectomy vs 9% with radiotherapy) were lost to follow‐up by 5 years. |
| Progression‐free survival Median follow‐up 9.6 years | HR 0.77 (0.54 to 1.10) | 256 (1 RCT) | ⊕⊕⊖⊖ Lowa,b | 12 participants in each regimen (10% with radiotherapy + hysterectomy vs 9% with radiotherapy) were lost to follow‐up by 5 years. |
| Tumour‐free actuarial survival at 5 years | 5‐year, tumour‐free actuarial survival for women with Stage IB was 80% in the preoperative radiotherapy + hysterectomy group and 89% in the radiotherapy group. In Stage IIA, these rates were 79% in the preoperative radiotherapy + hysterectomy group and 56% in the radiotherapy group. | 118 (1 RCT) | ⊕⊖⊖⊖ Very lowa,b,c | — |
| Quality of life | — | — | — | Not reported. |
| Severe/serious adverse events | 1 trial stated that both treatment programmes were well tolerated and there were no differences between groups in adverse effects. There were 18/129 women with a grade 3 or 4 adverse effect in the radiotherapy + hysterectomy group and 19 cases in 18/121 women of severe adverse effects in the radiotherapy group. In another trial, only 1/48 (2%) women with Stage IB disease experienced a severe complication (grade 3) in the radiotherapy + hysterectomy group (ureteral stricture) whereas 5/40 experienced severe complications in the radiotherapy group (including rectovaginal fistula, vesicovaginal fistula, ureteral stricture and pelvic infection) (P > 0.05). Similarly in women with Stage IIA disease, 5/14 (40%) women experienced a severe complication in the radiotherapy + hysterectomy group (including proctitis, rectal stricture, small bowel stricture and ureteral stricture) whereas only 1/16 women experienced a severe complication in the radiotherapy group (rectal stricture) (P > 0.05). | 374 (2 RCTs) | ⊕⊕⊖⊖ Lowa,b | Relative effect measures were not presented due to the crude combining of adverse events or sparse data, or both. |
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to sparse data leading to imprecision. | ||||
| Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: chemoradiotherapy + hysterectomy (simple or radical) Comparison: chemoradiotherapy alone | ||||
| Outcomes | Relative effect | No of participants | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 3.8 years | Overall survival was inadequately reported and it was not possible to calculate a hazard ratio. Overall survival time in the chemoradiotherapy + hysterectomy group was 6–40 months, median survival time was 23 months, and 3‐year survival rate was 82.7%. Total survival time in the chemoradiotherapy group was 5–41 months, median survival time was 22.5 months and 3‐year survival rate was 81.8%. Trial authors reported differences between arms were not statistically significant (P = 0.56). | 102 | ⊕⊝⊝⊝ | — |
| Progression or event‐free survival Median follow‐up 3.8 years | Progression‐free survival was inadequately reported in both trials and it was not possible to calculate a hazard ratio. In 1 trial, progression‐free survival time in the chemoradiotherapy + hysterectomy group was 3–40 months, median survival time was 23 months and 3‐year survival rate was 73.1%. The progression‐free survival time in the chemoradiotherapy alone group was 5–41 months, median survival time was 22 months and 3‐year survival rate was 64.8%. There was no significant difference between arms (P = 0.76). Another trial included 61 women and compared chemoradiotherapy + simple or radical hysterectomy vs chemoradiotherapy alone. There was no difference in 3‐year event‐free (death) survival rate (86% in the chemoradiotherapy + hysterectomy group vs 97% in the chemoradiotherapy alone group; log rank P = 0.15). | 163 | ⊕⊝⊝⊝ | — |
| Quality of life | — | — | — | Not adequately reported. |
| Severe/serious adverse events | — | — | — | Not adequately reported. |
| RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded two levels due to sparse data leading to imprecision. | ||||
| Hysterectomy (simple or radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: chemoradiotherapy + hysterectomy (simple or radical) Comparison: chemoradiotherapy alone | ||||
| Outcomes | Relative effect | No of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 3 years | HR 0.65 (95% CI 0.35 to 1.21) | 211 | ⊕⊕⊝⊝ | — |
| Progression or event‐free survival Median follow‐up 3 years | HR 0.70 (95% CI 0.31 to 1.34) | 211 | ⊕⊕⊝⊝ | — |
| Quality of life | — | — | — | Not reported. |
| Severe late complications | There was no difference in the proportion of women with severe late complications in the brachytherapy and radical hysterectomy groups (P = 0.53). There were 4 cases of grade 3 or 4 proctitis in the brachytherapy group vs 2 cases in the radical hysterectomy group; 3 cases of severe cystitis in the brachytherapy group vs 0 in the radical hysterectomy group; 0 cases of grade 3 or 4 hydronephrosis in either group. Of the 211 participants, chemoradiotherapy with cisplatin and gemcitabine appeared to be reasonably well tolerated, although nearly a third of women experienced severe neutropenia (most grade 3). Of the 86 women who received a radical hysterectomy, the number of intraoperative and early surgical complications appeared to be reasonably low, with bleeding (9/86) being the most common. | 211 | ⊕⊕⊝⊝ | — |
| CI: confidence interval; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to sparse data leading to imprecision. | ||||
Background
Description of the condition
Cervical cancer is the fourth most frequently diagnosed cancer and the fourth leading cause of cancer death in women, with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020 (Global Cancer Statistics 2020). Rates remain disproportionately high in transitioning (countries with a low or medium Human Development Index (a measure that considers income, health and education to assess how well economies are doing)) versus transitioned (countries with a high or very high Human Development Index) countries (incidence: 18.8 per 100,000 in transitioning versus 11.3 per 100,000 in transitioned; mortality: 12.4 per 100,000 in transitioning versus 5.2 per 100,000 in transitioned). Mortality from cervical cancer has declined in many high‐income countries, particularly in countries with organised cervical cancer screening programmes; however, in low‐income countries, mortality has increased and it remains a major international problem (Global Cancer Statistics 2020). Cervical cancer is considered nearly completely preventable with primary and secondary measures such as human papillomavirus vaccination and screening methods; however, these measures have not been equitably implemented across and within countries, resulting in the above inequality on incidence and mortality. The introduction in 1988 of a national cervical screening programme in the UK within a decade led to a halving in the incidence of cervical cancer, from an age‐standardised incidence rate of 16.2 per 100,000 to an age‐standardised incidence rate of 8.3 per 100,000 in 2008 (NCIN 2010). In many transitioning countries, access to health services is limited and screening for cervical cancer is either absent or reaches few of the women who need it. In these areas, cervical cancer is the most common cancer in women and the leading cause of cancer death (Global Cancer Statistics 2020; Mathers 2008).
In 2018, the World Health Organization called for global action to reduce incidence of cervical cancer (to 4 per 100,000 or less worldwide) by adopting a triple intervention strategy of vaccinating young girls by the age of 15 years, screening of women twice in the age range of 35 to 45 years and treating at least 90% of the precancer lesions detected through screening (Global Cancer Statistics 2020). In the meantime, many women are diagnosed at an advanced stage that is more difficult to treat. Naga and colleagues reported that more than two‐thirds of women have advanced disease at diagnosis and approximately 85% occur in transitioning countries (Naga 2018). Another report from a transitioning country has shown that over 80% of new cervical cancer cases are found at advanced stages (Stage IB2 or more), and over half of these are Stage III to IV (Khuhaprema 2010).
Cervical cancer is staged according to the International Federation of Gynecology and Obstetrics (FIGO) system (Appendix 1). Since the publication of the first review, the FIGO staging of cervical cancer has changed (Singh 2019; Table 1). Prior to this, FIGO staging for cervical cancer was based mainly on clinical examination (Percorelli 2009). In 2018, this approach was revised to allow imaging (r) and pathology (p) findings, where available, to assign stage (Bhatla 2018). The most important changes were as follows.
| Stage I (2018): carcinoma strictly confined to the cervix (extension to the uterine corpus should be disregarded) | ||
| 2009 FIGO stage: description | 2018 FIGO stage: description | Comment |
|---|---|---|
| IA: invasive carcinoma diagnosed only by microscopy, with maximum depth of invasion </= 5 mm and largest extension </= 7 mm | IA: invasive carcinoma diagnosed only by microscopy, with maximum depth of invasion < 5 mm | – Lateral extent of the carcinoma is no longer considered in distinguishing between FIGO Stage IA and IB carcinomas – If margins of loop are involved patient is allocated to Stage IB1 |
|
|
| |
|
|
| |
| IB: clinically visible lesions limited to the cervix or preclinical cancers greater than Stage IA | IB: invasive carcinoma with measured deepest invasion >/= 5 mm (greater than Stage IA), lesion limited to the cervix uteri | – See above – LVSI must be commented upon, although does not affect FIGO stage |
|
| IB1: invasive carcinoma >/= 5 mm depth of stromal invasion, and < 2 cm in greatest dimension | – New stage category |
| IB2: invasive carcinoma >/= 2 cm and < 4 cm in greatest dimension | – New stage category | |
| IB2: invasive carcinoma > 4 cm in greatest dimension | IB3: invasive carcinoma >/= 4 cm in greatest dimension | – New stage category |
Adapted from Singh 2019.
LVSI: lymphovascular space invasion.
-
The horizontal dimension is no longer considered in defining the upper boundary of a Stage IA carcinoma.
-
The diagnosis of Stage IA1 and IA2 carcinomas is made on microscopic examination of a surgical specimen, which includes the entire lesion. The margins of an excision specimen should be reported to be negative for disease.
-
If the margins of the cone biopsy are positive for invasive cancer, the patient is assigned to Stage IB1.
-
Stage IB has been subdivided into IB1, IB2 and IB3 based on maximum tumour size.
-
The revised 2018 system includes nodal status; the presence of nodal involvement in a tumour of any size upstages the case to Stage IIIC, with IIIC1 indicating pelvic and IIIC2 indicating para‐aortic nodal involvement. The revised FIGO classification is thereby now more closely aligned with the structure of the TNM classification, which is a classification system of cancer that describes the size of the tumour and any spread of cancer into nearby tissue (T); it describes spread of cancer to nearby lymph nodes (N); and it also describes metastasis (spread of cancer to other parts of the body) (M) (Bhatla 2018).
Remaining or recurrent disease frequently occurs after initial treatment in more than 50% of Stage III to IVA cervical cancer, leading to mortality (Appendix 1).
In this review, women with locally advanced cervical cancer (LACC) were the population of interest. It is likely that the included studies used the 2009 FIGO classification system (Percorelli 2009), as the new system was introduced in 2018 (Bhatla 2018).
Description of the intervention
Treatment decisions for invasive cervical cancer should be individualised and based on factors such as age, medical condition of the women, stage of disease and other tumour‐related factors in order to yield the best cure with minimum complications (Kesic 2006). As a general rule, multiple treatment modalities have more potential complications and adverse effects than one treatment modality.
For Stage IA1, local cervical treatments (large loop or needle excision of the transformation zone (LLETZ/NETZ), knife cone biopsy) or total hysterectomy (surgery to remove the womb and the neck of the womb) can be used, depending on women's preferences and fertility aspirations.
For Stage IA2 to IB1, radical hysterectomy with pelvic lymphadenectomy or chemoradiotherapy have been the accepted treatment modalities with reported similar efficacies (Eifel 1993). This finding was supported mainly by a randomised controlled trial (RCT) before the concurrent chemoradiotherapy (CCRT) era (Landoni 1997). In younger women, surgery is preferred, partly because of the advantage of preservation of ovarian function.
Radical hysterectomy, and bilateral pelvic lymphadenectomy, involves the removal of the uterus, the cervix, the upper part of the vagina and the tissues around the cervix (parametrial tissue), as well as the lymph nodes (glands) in the pelvis to determine if they contain cancer cells (pelvic lymphadenectomy). Although this type of surgery has excellent results, it can result in adverse effects such as organ injury (bladder, bowel, blood vessel, nerve) and long‐term adverse effects such as sexual or bladder dysfunction, pelvic cyst formation and lymphoedema (swelling) of the legs.
Radical trachelectomy may be an alternative to radical hysterectomy in women who want to preserve their fertility, provided they meet certain criteria. These are tumour size 2 cm or less and no metastasis to regional lymph nodes (Shepherd 2012). Radical trachelectomy involves removing the cervix, the upper part of the vagina and the parametrial tissue and the pelvic lymph glands. This treatment is well‐established, appears to be safe and effective in preserving fertility, and has a high chance of conception. Late miscarriage and premature labour are the most serious adverse effects in pregnancies where the women have had a trachelectomy.
For Stage IB2 tumours and above, the incidence of lymph node metastasis increases significantly, as well as the incidence of central, regional and distant recurrences (Alvarez 1989; Burghardt 1978; Chung 1980; Delgado 1990; Piver 1975). If a surgical approach is chosen, there may be difficulties in removing all of the tumour with a margin of normal tissue (that is adequate surgery), therefore there is a high probability of requiring additional treatment (radiotherapy with or without chemotherapy) with the increased morbidity of combined treatment. For women with FIGO Stage IB2 disease and higher, chemoradiotherapy is now standard care; it has been shown to improve disease‐free survival (DFS), progression‐free survival (PFS) and overall survival (OS) (CCCMAC 2010; NCI 1999). It involves administration of cisplatin‐based chemotherapy during the course of radiotherapy, all delivered within seven weeks. The chemotherapy makes the cancer cells more sensitive to the radiotherapy and therefore improves the treatment results.
However, surgery in LACC (Stage IB2 to III) has been considered in the following cases:
-
after chemoradiotherapy, for those in whom no complete remission is achieved within two to three months following treatment, the tumour is oncologically operable and the woman is clinically fit to undergo additional surgery;
-
after either radiotherapy or chemotherapy are used to shrink the cervical tumour to a size where it can be removed with normal margins;
-
following neoadjuvant chemotherapy (NACT; chemotherapy given before other treatments to reduce the size of the tumour), especially in transitioning countries with limited access to radiotherapy, but it remains unclear whether it offers a benefit over surgery alone or chemoradiotherapy (Rydzewska 2012).
Stage IVA cervical cancer, where the cancer has spread to the adjacent bladder or rectum, is usually treated with chemoradiotherapy (CCCMAC 2010). Some authors have suggested that NACT plus radical surgery (including removal of the affected bladder or rectum) might be a valid alternative to standard treatment (Benedetti Panici 2007).
For Stage IVB, the aim of treatment is generally palliative radiotherapy and chemotherapy (Kesic 2006; NCI 2014).
How the intervention might work
Although surgical resection of advanced non‐metastatic forms of cervical cancer is controversial, it may help improve local control (Houvenaeghel 1998). Many studies report favourable outcomes of hysterectomy for women with advanced cervical cancer after radiotherapy (Classe 2006; Kornovski 2007; Leino 1994; Noterman 2006; Potish 1990; Tsuda 2001; Wang 2002). Whether simple total hysterectomy (Leino 1994; Potish 1990; Wang 2002) or radical hysterectomy (Classe 2006; Kornovski 2007; Noterman 2006; Tsuda 2001; Wang 2002) is needed is unclear (Noterman 2006).
Multimodal treatment of hysterectomy combined with chemotherapy or radiotherapy, or both, may improve survival; but it may (Hequet 2013) or may not (Classe 2006; Perez 1987) cause significantly worse adverse events compared with radiotherapy or chemoradiotherapy alone.
Why it is important to do this review
It is important to assess the value of hysterectomy in addition to chemotherapy, radiotherapy or chemoradiotherapy in the treatment of LACC.
Objectives
To determine whether hysterectomy, in addition to standard treatment with radiotherapy or chemotherapy, or both, in women with LACC (Stage IB2 to III) is safe and effective compared with standard treatment alone.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women (aged 18 years or older) with LACC (Stage IB2 to III).
Types of interventions
We compared hysterectomy in combination with neoadjuvant, concurrent or adjuvant therapy versus non‐surgical interventions.
-
Hysterectomy (radical) with NACT versus chemoradiotherapy alone.
-
Hysterectomy (simple or radical) with NACT versus radiotherapy alone.
-
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone.
-
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone.
-
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy.
-
Hysterectomy (radical) with chemoradiotherapy versus hysterectomy (radical) with NACT versus chemoradiotherapy alone.
Types of outcome measures
Primary outcomes
-
Overall survival (OS): survival until death from all causes assessed from the time when women were enrolled in the study, or as defined by the trial authors.
Secondary outcomes
-
Progression‐free survival (PFS).
-
If authors reported disease‐free survival (DFS) rather than PFS then this was assessed.
-
Quality of life measures using a scale that had been validated through reporting of norms against a validated scale in a peer‐reviewed publication.
-
Severe adverse events
-
Surgery‐related complications: measured as the proportion of women who developed one of the items below (according to the study definition) within 12 weeks. These were classified as either early (before discharge from hospital or within seven days of surgery), late (from seven days to within 12 weeks of surgery), or total complications (early and late):
-
any postoperative infection;
-
surgery‐related injuries (blood vessel, nerve, bladder, bowel);
-
excessive blood loss (according to the study definition);
-
thromboembolic events;
-
any anaesthesiological complications;
-
other severe adverse event;
-
fistula formation;
-
voiding or bladder dysfunction;
-
lymphocysts or lymphoedema;
-
psychosexual dysfunction.
-
-
Chemotherapy‐ and radiotherapy‐related complications: grades of chemotherapeutic and radiotherapeutic toxicity were extracted and grouped as:
-
haematological (leukopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
-
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver toxicity, proctitis);
-
genitourinary;
-
skin (stomatitis, mucositis, alopecia, allergy);
-
neurological (peripheral and central); and
-
pulmonary.
-
-
Search methods for identification of studies
We sought papers in all languages and conducted translations where necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews (gnoc.cochrane.org).
For this update, we searched the following electronic databases on 3 February 2022:
-
the Cochrane Register of Controlled Trials (CENTRAL; 2022, Issue 2), in the Cochrane Library Appendix 2;
-
MEDLINE via Ovid (1946 to 4 February 2022) Appendix 3;
-
Embase via Ovid (1980 to 2022 week 4) Appendix 4;
-
LILACS (February 2022) Appendix 5.
All relevant articles that were found were identified on PubMed and, using the 'related articles' feature, we carried out further searches for newly published articles.
Searching other resources
Unpublished and grey literature
We conducted a Google search for Internet‐based resources and open‐access publications. We searched Metaregister (www.controlled-trials.com/rct), Physicians Data Query (www.nci.nih.gov), ClinicalTrials.gov (www.clinicaltrials.gov), and the National Cancer Institute (www.cancer.gov/clinicaltrials) for ongoing trials. One trial was identified through these searches; this trial closed to recruitment in September 2014. We contacted the principal investigator for more details and preliminary results, but have not yet received a reply. Therefore, we have added this trial to the Ongoing studies section.
We searched conference proceedings and abstracts through ZETOC (zetoc.mimas.ac.uk) and WorldCat Dissertations.
Handsearching
We handsearched the citation lists of included studies, key textbooks and previous systematic reviews.
We handsearched reports/websites/conferences of the following:
-
International Gynecological Cancer Society (IGCS);
-
European Society of Gynaecological Oncology (ESGO);
-
Society of Gynecologic Oncologists (SGO);
-
British Gynaecological Cancer Society (BGCS);
-
Australian Society of Gynaecologic Oncologists (ASGO);
-
American Society of Clinical Oncology (ASCO);
-
European Society of Medical Oncology (ESMO);
-
Clinical Oncological Society of Australia (COSA).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database, Endnote. We removed duplicates and two review authors (AB, FK) independently examined the remaining references. We excluded those studies that clearly did not meet the inclusion criteria and obtained the full texts of potentially relevant references. Two review authors (AB, FK) independently assessed the eligibility of the retrieved papers. We resolved disagreements by discussion between the two review authors with a final review by the other authors (EB, MP, DO). Reasons for exclusion are documented.
Data extraction and management
For included studies, we abstracted data as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The data included the following:
-
author, year of publication and journal citation (including language);
-
country;
-
setting;
-
inclusion and exclusion criteria;
-
study design, methodology;
-
study population:
-
total number enrolled;
-
patient characteristics;
-
age;
-
comorbidities;
-
type of initial or primary treatment (chemotherapy, chemoradiation, or radiotherapy), including details on dose, duration and combination;
-
performance status;
-
-
advanced cervical cancer details at diagnosis:
-
stage;
-
grade;
-
histology;
-
-
intervention (hysterectomy) details:
-
type of hysterectomy (total or subtotal, simple or radical with/without pelvic lymphadenectomy);
-
timing of hysterectomy;
-
prevention of complications (prophylactic antibiotics or any other measures);
-
grade or prior training of surgeon;
-
-
comparison details:
-
details of dose and duration of chemotherapeutic, radiotherapeutic or a combination treatment used;
-
method of primary treatment administration;
-
drug regimen;
-
-
local control, for example, bleeding, pressure symptoms, pain;
-
risk of bias in study (assessment of risk of bias in included studies);
-
duration of follow‐up;
-
outcomes, OS and PFS, quality of life and severe adverse events:
-
for each outcome, outcome definition (with diagnostic criteria if relevant);
-
unit of measurement (if relevant);
-
for scales, upper and lower limits, and whether high or low score is good;
-
results, number of participants allocated to each intervention group and
-
for each outcome of interest, sample size, missing participants.
-
We extracted data on outcomes as follows.
-
For time to event (OS and PFS) data, we extracted the log of the hazard ratio (HR) (log(HR)) and its standard error from trial reports. If these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998.
-
For dichotomous outcomes (e.g. adverse events), we extracted the number of participants in each treatment arm who experience the outcome of interest and the number of participants.
These were assessed at the endpoint in order to estimate a risk ratio (RR) and 95% confidence interval (CI).
Where possible, all the data extracted were those relevant to an intention‐to‐treat analysis in which the participants were analysed in the groups to which they were assigned. We noted the time points at which outcomes were collected and reported.
Two review authors (AB, FK) abstracted data independently onto a data abstraction form specially designed for the review. We resolved differences between review authors by discussion.
For continuous outcomes (e.g. quality of life), we had planned to extract the final value and standard deviation of the outcome of interest and the number of women assessed at the endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardised mean difference (if trials measured outcomes on different scales) between treatment arms and its standard error. However, none of the trials reported continuous outcome data for the quality of life.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included RCTs in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions using the Cochrane’s RoB 1 tool and the criteria specified in Chapter 8 (Higgins 2011). This included assessment of:
-
sequence generation;
-
allocation concealment;
-
blinding (restricted to blinding of outcome assessors as it was not possible to blind participants or investigators to these treatment modalities);
-
incomplete outcome data, we recorded the proportion of participants whose outcomes were not reported at the end of the study. We coded the satisfactory level of losses to follow‐up for each outcome as:
-
yes, if less than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
-
no, if 20% or greater of participants were lost to follow‐up or reasons for loss to follow‐up were different between treatment arms;
-
unclear, if loss to follow‐up was not reported;
-
-
selective reporting of outcomes;
-
other possible sources of bias.
Two review authors (FK, AB) applied the risk of bias tool independently and resolved differences by discussion. Results were summarised in a risk of bias summary and graph (Figure 1; Figure 2). Results of meta‐analyses were interpreted depending on the findings with respect to risk of bias.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We used the following measures of the effect of treatment:
-
for time‐to‐event data, we used HRs with 95% CI, where possible;
-
for dichotomous outcomes, we used RRs with 95% CI.
Unit of analysis issues
There were no unit of analysis issues.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for the heterogeneity were investigated and reported.
Assessment of reporting biases
We did not examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias, as stated in the protocol a priori, due to the fact that meta‐analyses of only three trials were possible.
Data synthesis
If sufficient clinically similar trials were available, we pooled their results in meta‐analyses.
-
For time to event data, we pooled HRs using the generic inverse variance facility of Review Manager 5 (Review Manager 2014).
-
We used random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We had intended to conducted subgroup analysis in the meta‐analysis of progression and DFS, grouping trials by whether the trial measured progression or DFS. However, this was not possible due to the lack of statistical heterogeneity and sparse data, but it may be considered in update of the review (see Differences between protocol and review).
Sensitivity analysis
We performed a meta‐analysis of three trials assessing NACT and hysterectomy versus radiotherapy alone.
We performed a meta‐analysis of two trials assessing NACT and hysterectomy versus chemoradiotherapy alone.
Summary of findings and assessment of the certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (such as directness of results) (Langendam 2013; Schünemann 2020). We created summary of findings tables based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020) and using GRADEpro GDT (GRADEpro GDT). We used the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation and grade as follows.
-
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
-
Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
-
Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
-
Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We presented summary of findings tables reporting the following outcomes listed in order of priority:
-
OS.
-
PFS or DFS.
-
Quality of life.
-
Severe adverse events and toxicity.
Results
Description of studies
We searched for RCTs assessing the role of hysterectomy in combination with chemotherapy or radiotherapy, or both, versus chemoradiotherapy alone.
Results of the search
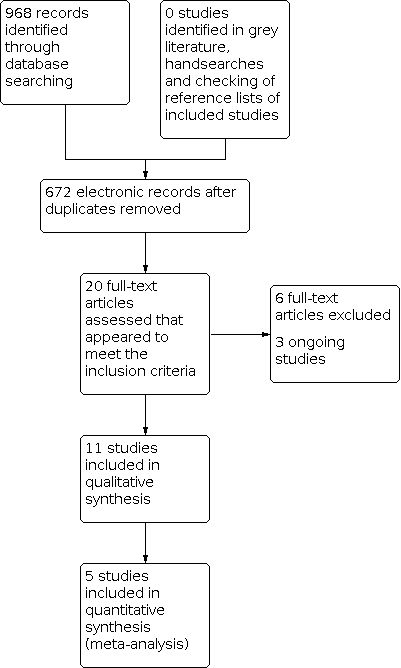
The search strategy identified 968 unique references. We read the abstracts and excluded those that did not meet the inclusion criteria at this stage. We retrieved 20 articles in full and after full‐text screening excluded six references for the reasons described in the Characteristics of excluded studies table. Eleven studies met our inclusion criteria and are described in the Characteristics of included studies table (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017). See PRISMA flow chart for further details of study selection process (Figure 3). Three studies are ongoing (CSEM 006 study; Reis Fihlo 2018; Shanmugam 2019).
Study flow diagram.
Searches of the grey literature did not identify any additional studies.
Included studies
The 11 included studies randomised 2683 women, all of whom were assessed for primary survival outcomes at the end of the studies (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017).
The review identified the following treatment comparisons for the 11 included studies.
-
Hysterectomy (radical) with NACT versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014).
-
Hysterectomy (simple or radical) with NACT versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010).
-
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone (Keys 2003; Perez 1987).
-
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone (Morice 2012; Zheng 2017).
-
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy (Cetina 2013).
The duration of follow‐up of participants varied from 36 to 98 months. In Keys 2003, the women who were last seen alive had a median follow‐up of 9.6 years (range 0.3 to 16.1 years). In EORTC 2019, the median follow‐up was 8.2 years (95% CI 7.8 to 8.6).
The certainty of the evidence in this review was low or very low for all comparisons of outcomes other than for NACT and radical hysterectomy versus radiotherapy alone. The certainty of the evidence for OS and progression or DFS was moderate and was mainly downgraded due to concerns regarding risk of bias in individual trials. The trials in all the comparisons were at high or moderate risk of bias.
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
One multicentre RCT included 620 participants with FIGO Stage IB2, IIA (greater than 4 cm) or IIB cervical cancer (EORTC 2019). Both patient groups received cisplatin‐based chemotherapy. In the NACT plus surgery group, women received neoadjuvant cisplatin‐based chemotherapy on day one. Treatment was repeated every 21 days. Within six weeks after the last chemotherapy course, and with a cumulative minimum of 225 mg/m2, women underwent a type III to V Piver‐Rutledge radical hysterectomy. Women with positive lymph nodes or tumour invasion into the parametria or less than 5 mm from the resection borders after surgery received standard adjuvant external‐beam radiotherapy once daily, five days a week, for 5.0 to 5.6 weeks (25 to 28 treatment days) followed by external boost radiotherapy or brachytherapy for one or two days In the concurrent radiotherapy group, radiation consisted of 45 Gy to 50 Gy plus boost concurrent with weekly cisplatin chemotherapy (40 mg/m2 per week). Adjuvant hysterectomy was allowed, but not recommended, in cases of histologically confirmed residual tumour. Participants in both groups were evaluated for OS at five years (primary endpoint) and OS, PFS, toxicity and quality of life (secondary endpoints).
Gupta 2018 was a single‐centre RCT that included women with cervical cancer Stage 1B2, IIA or IIB disease. The NACT plus surgery group received three cycles of paclitaxel (175 mg/m2) and carboplatin (dosed to an area under curve of 5 to 6) once every three weeks. Participants underwent clinical response assessment after the second and third cycles of chemotherapy. Participants who had no response or disease progression at these time points crossed over to receive definitive CCRT, whereas responders underwent surgery three to four weeks after the third cycle of chemotherapy.
Participants assigned to the NACT plus surgery group underwent Piver‐Rutledge class III radical abdominal hysterectomy, bilateral pelvic lymphadenectomy and lower para‐aortic lymph node sampling by expert gynaecological oncologists. Surgery was abandoned in participants with intraoperative findings of either unresectable primary tumour or lymph node disease, and these participants were treated with definitive concurrent chemoradiation. Participants assigned to the concurrent chemoradiation group and those who were crossed over from the NACT plus surgery group received standard external‐beam radiation to the whole pelvis and brachytherapy. They received an external radiation dose of 40 Gy in 20 fractions with 2 Gy per fraction and a midline shield at 20 Gy, followed by intracavitary radiation to 'point A' as follows: either two applications of a low‐dose rate of 30 Gy each or five applications of a high‐dose rate of 7 Gy each. Radiation doses were modified to respect tumour, rectal and bladder constraints. These women also received five cycles of cisplatin (40 mg/m2), administered once every week starting with external‐beam radiotherapy. Participants in the NACT plus surgery group who underwent radical hysterectomy were given adjuvant therapy (radiotherapy or CCRT) as per protocol‐defined criteria, in accordance with published evidence. On the basis of histopathological evaluation of the surgical specimen, adjuvant chemoradiation was given in the presence of any one of the following features: lymph node metastasis, positive surgical margins or parametrial involvement. Adjuvant radiotherapy alone was given based on the presence of any two of the following features: deep cervical stromal invasion, lymphovascular invasion or tumour size greater than 4 cm. Participants in both groups were evaluated at protocol‐defined time points to evaluate response, monitor for relapse and assess toxicity.
In Khan 2014, both patient groups received platinum‐based chemotherapy. Participants assigned to the chemoradiotherapy group received external beam radiotherapy (EBRT; 45 Gy to 50 Gy) followed by brachytherapy. Participants assigned to the NACT plus surgery group, had radical hysterectomy and pelvic lymphadenectomy. Participants in both groups were evaluated for short‐term complications within 30 days of completion of treatment and long‐term complications that were reported within two years after treatment.
Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone
Three RCTs randomised 571 women compared NACT and hysterectomy (simple or radical) versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). Benedetti‐Panici 2002 included women with Stage IB2 to III cervical cancer, Chang 2000 included women with IB to IIA bulky disease and Noriyuki 2010 had only women with Stage IIIB disease. The median age in each arm was similar in Benedetti‐Panici 2002 and Chang 2000 (range 46 to 52 years), whereas in Noriyuki 2010, women were significantly older in the radiotherapy arm (mean age 53 in the NACT and hysterectomy arm versus 60 years in the radiotherapy arm). All women in Benedetti‐Panici 2002 and Noriyuki 2010 and most in Chang 2000 had squamous cell cancers. In Benedetti‐Panici 2002 and Chang 2000, the Eastern Cooperative Oncology Group performance status was zero for most eligible women. Noriyuki 2010 did not report performance status.
In Benedetti‐Panici 2002, the NACT regimen was not predetermined; minimal requirements were a cisplatin‐containing regimen with a 240 mg/m2 or greater total cisplatin dose with a maximum of two additional drugs, administered over six to eight weeks. After NACT, the women were clinically reassessed and classified as suitable or unsuitable for radical surgery. Participants who were unsuitable for radical surgery were treated with radiotherapy. Surgery consisted of radical hysterectomy (type III to V) plus systematic (at least 20 nodes to be resected) pelvic lymphadenectomy (aortic lymphadenectomy was optional). Postoperative radiotherapy was given in participants with positive surgical resection margins or metastatic nodes, or both. In the case of node metastasis, the choice of adjuvant treatment was based on the institution's policy (e.g. chemotherapy, external‐beam radiotherapy or no further therapy). Adjuvant treatment was given to 48 (29%) participants in the surgical group; 38 (23%) participants in the surgical group underwent adjuvant radiotherapy.
Conventional radiotherapy consisted of external‐beam, megavoltage radiotherapy (45 Gy to 50 Gy) to the whole pelvis over five to six weeks. In the presence of metastatic pelvic nodes an extra dose of 5 Gy to 7 Gy was administered. Low‐dose rate brachytherapy (20 Gy to 30 Gy to the tumour volume) was provided two to four weeks after external radiotherapy. Aortic node metastases, when present, were irradiated (45 Gy per five weeks, followed by a 5 Gy boost if residual disease was eventually detected) with extended fields encompassing pelvic and aortic volume or at the end of pelvic irradiation, in the case of a pelvic complete remission. Salvage treatments were allowed in women who showed progressive disease.
In Chang 2000, the NACT was cisplatin and vincristine, followed by bleomycin. Two to four weeks after the completion of NACT, participants underwent a type III radical abdominal hysterectomy and pelvic lymphadenectomy. The adnexae were usually left in women aged 40 years or less if the gross appearance of the adnexae was normal.
The radiotherapy usually included a combination of external radiotherapy and high‐dose rate brachytherapy; with 40 Gy to 44 Gy whole pelvic irradiation. The para‐aortic lymph nodes were not routinely included in the treatment field. Parametria received up to 50 Gy. If bulky tumour persisted after 44 Gy of irradiation, external‐beam doses to the lower pelvis were increased to 50 Gy to 54 Gy without central block followed by brachytherapy, or to 70 Gy without brachytherapy. The median cumulative dose to 'point A' in this treatment protocol was 70 Gy. Thirty‐seven participants were treated using this method. The postoperative radiotherapy was given by using techniques similar to those described above. The dose to the whole pelvis was 44 Gy to 45 Gy, and that to the true pelvis was 50 Gy to 54 Gy. After external radiotherapy, brachytherapy was given in two to three fractions with a total dose of 4 Gy/0.5 cm to 6 Gy/0.5 cm below the vaginal mucosa.
Participants in the NACT arm had a higher incidence of receiving adjuvant therapy with either radiotherapy or chemotherapy after the scheduled treatment than those in the radiotherapy arm, who received radical hysterectomy as the adjuvant therapy. Of the 68 women in the NACT arm, 62 underwent hysterectomy and 19 of those had adjuvant radiotherapy, six had adjuvant chemotherapy and two had chemoradiotherapy.
In Noriyuki 2010, the NACT regimen consisted of cisplatin, bleomycin and mitomycin for three courses every four weeks. If the tumour was surgically removable, a radical hysterectomy was performed with bilateral salpingo‐oophorectomy and pelvic lymphadenectomy, and then radiotherapy was given at 40 Gy to the whole pelvic region. If the tumour progressed or relapsed, combined chemotherapy of bleomycin, vincristine, mitomycin and cisplatin (BOPM) was given, and then irinotecan with cisplatin as the third line. If the local tumour was inoperable, radiotherapy was given at 40 Gy to the whole pelvic region with 20 Gy brachytherapy, followed by BOPM chemotherapy.
The radiotherapy group received radiotherapy to the whole pelvic region in 20 fractions totalling 40 Gy. The total dose delivered to 'point B' as a boost dose with midline shield coverage was 20 Gy. The total dose delivered by brachytherapy was 24 Gy to 30 Gy. The pelvic field extended from the upper margin of L5 to the mid‐portion of the obturator foramen or the lowest level of disease, with a 3 cm margin, and laterally 1.5 cm to 2 cm beyond the lateral margins of the bony pelvic wall. The duration of the radiotherapy was four weeks. In cases with local recurrence or progression of the primary lesion, chemotherapy was added, which included BOMP, irinotecan with cisplatin, and cisplatin or carboplatin alone. When distant metastasis occurred, the researchers added radiotherapy, or the single lesion was surgically removed.
All three studies assessed OS (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010), one study also assessed PFS (Benedetti‐Panici 2002) and two studies also assessed DFS (Chang 2000; Noriyuki 2010). However, the definition of DFS in Chang 2000 was absence of persistent or recurrent disease, so this appeared to be a combination of progression and DFS. It was possible to include all three studies in a meta‐analyses of OS and PFS or DFS as HR estimates were either explicitly reported, deduced (Parmar 1998), or obtained via personal correspondence (Noriyuki 2010). Benedetti‐Panici 2002 reported severe toxicity and complications and Chang 2000 reported tumour response to treatment and toxicity. Noriyuki 2010 did not report adverse events. None of the three studies reported quality of life outcomes.
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone
Two studies, which included 374 women, compared preoperative radiotherapy and radical hysterectomy versus radiotherapy alone (Keys 2003; Perez 1987). Keys 2003 included 256 eligible women with Stage IB2 disease (tumour size 4 cm to 8 cm); Perez 1987 included 118 women with Stage IIA disease as well as Stage IB (but women with a tumour more than 5 cm were excluded). The age distribution was comparable in the two groups in both trials, but additional information was not reported in Perez 1987. In Keys 2003, just over 75% of the women were 50 years old or under. Most women in both trials had squamous cell carcinoma of the cervix. Additional baseline information was not reported in the Perez 1987 trial; but the performance status of women in Keys 2003 was generally good (more than 76% in both arms with performance status of 0 and more than 20% with performance status of 1). Keys 2003 did not mention whether the participants were evaluated clinically or radiologically after radiotherapy in order to assess the tumour response and residual disease.
In Keys 2003, the daily fraction size was 180 Gy and external treatment carried to a total dose of 40 Gy for the radiation alone and 45 Gy for the adjuvant hysterectomy regimens. Both groups received brachytherapy one to two weeks after completing external treatment. The brachytherapy dose prescription was different between the treatment arms; the radiation alone group received 40 Gy with a total dose of 80 Gy to 'point A', while those who had hysterectomy received only 30 Gy with a total dose of 75 Gy to 'point A'. A minimum dose of 55 Gy was prescribed to 'point B' for both regimens. All irradiation was completed within 10 weeks. The surgical group then underwent simple hysterectomy with removal of tubes and ovaries, if present, two to six weeks after completion of all irradiation.
In Perez 1987, participants who were treated with preoperative radiotherapy and surgery received 20 Gy whole pelvis irradiation and one brachytherapy for 5000 to 6000 milligram‐hours (approximately 5 Gy to 6 Gy low‐dose rate given over six days), followed two to six weeks later by a radical hysterectomy and bilateral pelvic lymphadenectomy (up to the bifurcation of the common iliac vessels). The dose to the cervix was about 70 Gy and to the pelvic lymph nodes 30 Gy.
Treatment with irradiation alone in Perez 1987 consisted of 10 Gy to 20 Gy delivered to the whole pelvis and an additional parametrial dose to total of 50 Gy to the external iliac lymph nodes combined with two brachytherapy insertions for a total of approximately 7500 milligram‐hours (65 Gy to 70 Gy to 'point A'). The dose to the paracervical tissues was about 85 Gy and to the pelvic lymph nodes 60 Gy.
Keys 2003 assessed OS, pelvic‐free survival and the rate of pelvic recurrence. Perez 1987 assessed the five‐year tumour‐free actuarial survival, the sites of failure after therapy and treatment complications.
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone
Morice 2012 included 61 women with FIGO Stage IB2 or II cervical cancer with a complete clinical and radiological response after chemoradiotherapy, randomly allocated to the treatment arms: hysterectomy or no hysterectomy. The median age and stage distribution was similar in both groups (45 years in the chemoradiotherapy and hysterectomy arm and 44 years in the chemoradiotherapy and no hysterectomy arm). Half of the women had FIGO Stage IB2 and half Stage II disease. More than 80% of participants in each group had squamous cell cancer. The performance status of the included women was not described.
Radiotherapy was delivered to the pelvis for a total dose of 45 Gy to 50 Gy, in five fractions of 1.8 Gy to 2 Gy per week, followed one to two weeks later by brachytherapy. Most women in both groups had one application of brachytherapy at 15 Gy. CCRT was cisplatin during external radiotherapy. A complete clinical and radiological response (based on magnetic resonance imaging (MRI)) was evaluated six to eight weeks after brachytherapy.
Hysterectomy could be performed via laparotomy or a laparoscopy and could be extrafascial or radical (type II according to the Piver classification) according to the preoperative examination. A selective or complete pelvic lymphadenectomy was optional and could be performed if lymphadenopathy was detected during surgery.
The trial gave HRs for OS and recurrence‐free survival as well as reporting the site of first recurrence. Morbidity was not reported after confirmation from the study authors. The median duration of follow‐up was 3.8 years (range 0.4 to 5.8 years) when the trial was closed early because of poor accrual.
Zheng 2017 included 102 participants with LACC and compared chemoradiotherapy and radical hysterectomy and pelvic lymph node dissection versus chemoradiotherapy alone. Fifty‐two participants were included in the hysterectomy arm and 50 in the chemoradiotherapy alone arm.
Women who met the inclusion criteria were first treated with CCRT.
The radiotherapy plan was as follows: the linear accelerator was used for external pelvic irradiation, and the intracavitary 192Ir was used for radiotherapy. Stage 1: the whole basin irradiation before and after the field or left and right field irradiation, four or five times a week, each time 2.25 Gy or 1.8 Gy, pelvic centre total dose 30 Gy. Stage 2: the lead block protected the uterus, and the uterus continued to be irradiated from the front and back, five times a week, 1.8 Gy to 2.0 Gy each time, and the total periuterine dose was 15 Gy to 20 Gy. At the beginning of the second stage of external irradiation, intracavitary and the back of the cavity were performed at the same time once a week, with 4.6 Gy to 7.0 Gy at 'point A' and total of 35 Gy to 42 Gy at 'point A'. Chemotherapy regimen: cisplatin alone: 35 mg/m2 to 40 mg/m2, once a week. Surgical treatment: participants in the experimental group underwent radical surgery four to six weeks after the completion of CCRT. For radical total hysterectomy and pelvic lymphadenectomy, 3 cm of paracervical and vaginal tissues were removed.
The outcomes of the study included recurrence and OS rate using. As outcome indicators they used: short‐term efficacy evaluation: evaluate the efficacy according to the tumour regression before and after treatment; complete remission: complete regression of the tumour by gynaecological examination and imaging examination; partial remission: tumour volume reduction of 50% or less; stability of disease: tumour volume reduction of 50% or less; progression of disease: tumour enlargement or presence of new lesions. Complete remission plus partial remission indicated effective treatment.
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy
Cetina 2013 included 211 women aged 18 to 70 years with a histological diagnosis of untreated FIGO Stage IB2 to IIB cervical cancer and no evidence of para‐aortic lymph node involvement. It was reported that these 211 women were randomly allocated to either brachytherapy after external‐beam radiotherapy with chemotherapy or radical hysterectomy after external‐beam radiotherapy with chemotherapy. Women were ineligible for the study if they had previously received chemotherapy or radiotherapy. The median age, and stage distribution, was similar in both groups (44 years in the brachytherapy arm and 45 years in the hysterectomy arm). The median performance status (Karnofsky's) score was 90 and the median tumour size was 32 mm in both arms. Most participants in each treatment arm had FIGO Stage IIB disease (70% of participants in the brachytherapy arm and 74% in the hysterectomy arm). More than 80% of participants in each group had squamous cell cancer.
Participants received 50.4 Gy external‐beam radiotherapy to the entire pelvic region in 28 sessions of 1.8 Gy/day, five days/week, over the six weeks of chemotherapy. Immediately after completion of external‐beam radiotherapy with chemotherapy, participants in the brachytherapy underwent low‐dose rate brachytherapy of 30 Gy to 35 Gy delivered to 'point A', to result in a cumulative dose of 80 Gy to 85 Gy combining external‐beam radiotherapy and brachytherapy. The cumulative external‐beam radiotherapy and brachytherapy dose to 'point B' (the pelvic wall) was 55 Gy to 65 Gy.
Within four to six weeks after the external‐beam radiotherapy with chemotherapy, participants in the hysterectomy group were submitted to type III radical hysterectomy and bilateral pelvic lymph node dissection and para‐aortic lymph node sampling, if the multidisciplinary team judged the disease could be resected obtaining margins free of disease. Postoperative low‐dose rate brachytherapy was mandated in participants in the hysterectomy are only if the surgical specimen revealed positive surgical margins and was administered within four weeks after surgery at a median dose of 30 Gy to the vaginal mucosa delivered to a depth of 0.5 cm.
The trial gave HRs for OS and PFS. The trial also reported pathological response; operative complications; toxicity to chemoradiation with cisplatin and gemcitabine; long‐term complications; and late complications including proctitis, cystitis and hydronephrosis. Only late complications were reported in a breakdown by treatment arm. The median duration of follow‐up was 36 months (range 3 to 80 months).
Excluded studies
We excluded six full‐text studies.
-
Five studies included women who received hysterectomy or surgical staging in both arms (Katsumata 2013; Keys 1999; Sardi 1997; Sun 2013; Yang 2016).
-
One study compared surgery versus radiotherapy in women with early‐stage carcinoma of the cervix (Sundfor 1996).
Studies awaiting classification
We found no studies awaiting classification.
Ongoing studies
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
Reis Fihlo 2018 is an ongoing study and the final results will be available in 2023 (personal communication with the authors). Both participant groups (Stage IB2, IIA, or IIB) will receive platinum‐based chemotherapy. Participants assigned to the NACT and radical hysterectomy group will receive chemotherapy every 21 days for six weeks. Within six weeks after the last chemotherapy course, women will undergo a type III to V Piver‐Rutledge radical hysterectomy. Women with positive lymph nodes or tumour invasion into the parametria or less than 5 mm from the resection borders after surgery will receive standard adjuvant external‐beam radiotherapy once daily, five days a week, for 5.0 to 5.6 weeks (25 to 28 treatment days) followed by external boost radiotherapy or brachytherapy for one or two days. Participants assigned to the CCRT group will receive standard therapy comprising cisplatin‐based chemotherapy once weekly for six weeks. Adjuvant hysterectomy will be allowed, but not recommended, in cases of histologically confirmed residual tumour. Participants in both groups will be evaluated for OS, PFS, toxicity of the regimens and quality of life.
CSEM 006 study is an ongoing study assessing DFS of women with Stage IIB cervical cancer randomised to NACT combined with surgery versus CCRT.
Hysterectomy (radical) with chemoradiotherapy versus hysterectomy with neoadjuvant chemotherapy versus chemoradiotherapy alone
Shanmugam 2019 is an ongoing RCT where all three participant groups with LACC will receive cisplatin and paclitaxel chemotherapy. Women assigned to the CCRT group will receive cisplatin (75 mg/m2) and paclitaxel (175 mg/m2) given within a three‐week interval between the two cycles along with concurrent radiotherapy of EBRT 50 Gy (2 Gy for 25 doses) followed by brachytherapy of 21 Gy (7 Gy for 3 doses) completed within eight weeks. Women assigned to the preoperative chemoradiation plus radical hysterectomy group will receive cisplatin (75 mg/m2) and paclitaxel (175 mg/m2) given within a three‐week interval between the two cycles along with concurrent radiotherapy of 50 Gy EBRT (2 Gy for 25 doses) followed by radical hysterectomy within three weeks after completion of radiotherapy. Women assigned to the preoperative chemotherapy plus radical hysterectomy group will receive cisplatin (75 mg/m2) and paclitaxel (175 mg/m2) given within a three‐week interval for three cycles followed by radical hysterectomy within three weeks after completion of chemotherapy. The outcomes of the study will be OS, PFS, overall response rate, complete clinical response, partial clinical response and quality of life.
For further details of the excluded studies see the Characteristics of excluded studies table.
Risk of bias in included studies
Nine studies were at overall high risk of bias (Cetina 2013; Chang 2000; EORTC 2019; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017), and Benedetti‐Panici 2002 (which satisfied four of the criteria that we used to assess risk of bias) and Chang 2000 (which satisfied three items) were at moderate to high risk of bias (see Figure 1).
Allocation
Only Gupta 2018, Keys 2003, and Perez 1987 reported the method of generation of the sequence of random numbers used to allocate women to the treatment arms, but they did not report concealment of this allocation sequence from participants and the healthcare professionals involved in the trials. The other eight trials did not report on the method of sequence generation, although two trials reported adequate concealment of allocation (Benedetti‐Panici 2002; Chang 2000). Allocation concealment was unclear in nine trials (Cetina 2013; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Morice 2012; Noriyuki 2010; Perez 1987; Zheng 2017).
Blinding
Since it was not possible to blind participants and clinicians to these particular interventions, performance bias may have been an issue in all 11 included trials. Only one trial reported adequate blinding (low‐risk of detection bias; Benedetti‐Panici 2002), so the other 10 trials may have been prone to detection bias.
Incomplete outcome data
At least 80% of eligible women who were randomised were assessed at the endpoint in nine trials (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; Gupta 2018; Keys 2003; Morice 2012; Perez 1987; Noriyuki 2010; Zheng 2017), but this was unclear in two trials (EORTC 2019; Khan 2014). Two trials did not use intention‐to‐treat analyses (Morice 2012; Perez 1987).
Selective reporting
Five studies reported pertinent outcomes, although none reported quality of life outcomes (low risk of reporting bias; Benedetti‐Panici 2002; Cetina 2013; Chang 2000; Gupta 2018; Keys 2003). Two studies did not report adverse events or toxicity (high risk of reporting bias; Morice 2012; Noriyuki 2010). One study did not report OS despite the fact that the number of women who had died would have been known since disease progression was defined as the number of women whose disease had progressed or died (high risk of reporting bias; Perez 1987). This raised concern about a significant reporting bias. EORTC 2019, Khan 2014, and Zheng 2017 were either in abstract form only or inadequately reported primary survival outcomes, so it was difficult to judge whether outcomes were selectively reported (unclear risk of reporting bias).
Other potential sources of bias
It was unclear whether any additional forms of bias may have been present in all studies so this item was scored at unclear risk of bias, although over 25% of participants in each arm deviated from the protocol in Benedetti‐Panici 2002 and so this study was at high risk of bias for this item.
Effects of interventions
See: Summary of findings 1 Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone for women with locally advanced cervical cancer; Summary of findings 2 Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone for women with locally advanced cervical cancer; Summary of findings 3 Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone; Summary of findings 4 Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone; Summary of findings 5 Hysterectomy (simple or radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy
Hysterectomy (radical) with neoadjuvant chemotherapy versus chemoradiotherapy alone
Three trials included 1364 women and compared NACT and hysterectomy versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014). See summary of findings Table 1.
Overall survival
Two studies, assessing 1253 participants, reported OS (EORTC 2019; Gupta 2018). There was no evidence of a difference in risk of death between women who received NACT plus hysterectomy and those who received chemoradiotherapy alone (HR 0.94, 95% CI 0.76 to 1.16; moderate‐certainty evidence; Analysis 1.1). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was not important (I2 = 0%).
EORTC 2019 included 620 participants with Stage IB2 to IIB cervical cancer and compared NACT plus surgery (314 participants) with standard chemoradiotherapy (312 participants). Five‐year OS was 72% in the NACT plus surgery arm and 76% in the standard chemoradiotherapy arm (difference 4.0%, 95% CI –4% to 12%; HR 0.87, 95% CI 0.65 to 1.17; P = 0.332). The trial reported 191 (31%) deaths with a median follow‐up of 8.2 years (interquartile range (IQR) 7.8 to 8.6). Five‐year OS was 72% in the NACT plus surgery arm and 76% in the standard chemoradiotherapy arm (difference 4.0%, 95% CI –4% to 12%). Additional radiotherapy was given to 113 (36.3%) participants in the NACT plus surgery arm. Additional surgery was performed in nine (2.9%) participants in the standard chemoradiotherapy arm.
Gupta 2018 included 633 women aged 18 to 65 years with histologically confirmed squamous cell carcinoma of the cervix with 1994 FIGO Stage IB2, IIA or IIB disease. It compared NACT plus surgery with chemoradiotherapy. Five‐year OS rates in the NACT plus surgery group was 75.4% compared with 74.7% in the concurrent chemoradiation group (HR 1.03, 95% CI 0.75 to 1.40; P = 0.87).
Disease‐free survival
In Gupta 2018, five‐year DFS in the NACT plus surgery group was 69.3% compared with 76.7% in the concurrent chemoradiation group (HR 1.38, 95% CI 1.02 to 1.87; P = 0.038). In subgroup analyses, there was evidence of a difference in DFS detriment in the NACT plus surgery group in participants with FIGO Stage IIB disease, with a significant test of interaction between treatment effect and Stages IIA and IIB disease. In women with Stage IIB disease, the five‐year DFS rates in the NACT plus surgery group was 67.2% and concurrent chemoradiation group was 79.3% (unadjusted HR for DFS in the NACT plus surgery group 1.90, 95% CI 1.25 to 2.89; P = 0.003).
In EORTC 2019, the five‐year DFS in the NACT plus surgery group was 56.9% compared with 65.6% in the CCRT group (P = 0.021), mostly for Stage IIB disease. However, the authors noted that the first imaging measurement occurred systematically earlier in the neoadjuvant plus surgery arm than in the chemoradiotherapy arm.
Quality of life
None of the studies reported quality of life.
Severe adverse events
EORTC 2019 reported no toxic deaths. Short‐term severe adverse events (G3 or higher) occurred more frequently with NACT plus surgery (35%) than with chemoradiotherapy (21%; P < 0.001). In total, there were 198 serious adverse events (SAEs): 145 in the NACT plus surgery arm versus 53 in the standard chemoradiotherapy arm. Within the group of SAEs, there were 109 serious adverse reactions (SARs) with NACT plus surgery, and 35 SARs with chemoradiotherapy. Nearly all were chemotherapy related. In the NACT plus surgery arm, 238 (76%) women underwent surgery.
Main reasons for not having surgery as per protocol were toxicity (25/74, 34%), progressive disease (18/74, 24%) and insufficient response to NACT (12/74, 16%).
Toxicity
In Gupta 2018, in the NACT plus surgery group compared with the concurrent chemoradiation group, there was a lower rate of rectal (5.7% with NACT plus surgery versus 13.3% with chemoradiotherapy; P = 0.002), bladder (2.8% with NACT plus surgery versus 7.3% with chemoradiotherapy; P = 0.017), and vaginal (19.9% with NACT plus surgery versus 36.9% with chemoradiotherapy; P = 0.001) toxicity occurring or persisting 90 days after treatment completion. However, 24 months after treatment completion, there was no difference in rectal and bladder toxicities between groups, whereas vaginal toxicity continued to occur at a lower rate in the NACT plus surgery group (12.0% with NACT plus surgery versus 25.6% with chemoradiotherapy; P = 0.001).
Treatment‐related morbidity
Khan 2014 included 111 women with Stage IB2 to III cervical cancer comparing the toxicity‐related morbidity of CCRT with NACT plus surgery. Participants were evaluated for short‐term complications within 30 days of completion of treatment and long‐term complications that were reported within two years after treatment. There were no treatment‐related deaths. Overall 89% of participants in the chemoradiotherapy arm and 73% in the NACT plus surgery arm had complications, with 18% in the NACT plus surgery arm experiencing recurrence and requiring adjuvant radiotherapy.
In EORTC 2019 there were grade 3/4 complications related to surgery: 8 (3.3%) participants had bleeding, 10 (4.2%) operative lesions to ureter or bladder, 3 (1.2%) fistula, 7 (2.9%) others (sepsis, urinary tract infection and wound dehiscence).
Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone
Three studies compared hysterectomy (simple or radical) with NACT versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). See summary of findings Table 2.
Overall survival
Meta‐analysis of three studies, assessing 571 participants, found that women who received NACT plus hysterectomy had a lower risk of death compared with women who received radiotherapy alone (HR 0.71, 95% CI 0.55 to 0.93; moderate‐certainty evidence; Analysis 2.1) (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%).
Progression‐free survival and disease‐free survival
Meta‐analysis of three studies, assessing 571 participants, found no difference in the risk of disease progression between women who received NACT plus hysterectomy and those who received radiotherapy alone (HR 0.75, 95% CI 0.53 to 1.05; moderate‐certainty evidence; Analysis 2.2) (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance might not have been important (I2 = 20%).
Severe adverse events
Acute toxicity
Benedetti‐Panici 2002 reported short‐term complications but it was not possible to make comparisons because participants were compared in terms of those who received NACT, hysterectomy and radiotherapy separately, and participants may have experienced more than one toxicity in each category (low‐certainty evidence).
Chang 2000 reported 9/68 (13%) cases of grade 3 acute toxicity in the NACT plus hysterectomy group and 7/50 (22%) cases of severe acute toxicity (5/7 were grade 3) in the radiotherapy alone group. There was no evidence of a difference between groups (RR 1.32, 95% CI 0.47 to 3.71; low‐certainty evidence). Acute toxicities included nausea, vomiting, diarrhoea, liver and dermatological adverse effects.
Long‐term complications and toxicity
In Benedetti‐Panici 2002, long‐term severe complications occurred in 32 (19.5%) women in the NACT arm and late severe morbidity with radiotherapy was observed in 39 (22%) women. There was no evidence of a difference in long‐term severe complications between NACT plus hysterectomy and radiotherapy alone (RR 0.86, 95% CI 0.49 to 1.50; low‐certainty evidence).
Chang 2000 reported 9/68 (13%) cases of severe late toxicity (8/9 were grade 3) in the NACT plus hysterectomy group and 11/50 (22%) grade 3 cases in the radiotherapy alone group. There was no evidence of a difference between groups (RR 0.60, 95% CI 0.27 to 1.34; low‐certainty evidence). Late toxicities included intestinal obstruction, radiation cystitis, radiation proctitis and lower leg oedema.
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone
Keys 2003 and Perez 1987 included 374 women and compared preoperative radiotherapy and radical hysterectomy versus radiotherapy alone. The trialists gave a breakdown by FIGO Stage and intervention group in Perez 1987 but did not report OS or use appropriate survival techniques to allow the trial to be pooled. See summary of findings Table 3.
Overall survival
In Keys 2003, there was no evidence of a difference in the risk of death between women who received radiotherapy plus extrafascial hysterectomy and those who received radiotherapy alone (HR 0.89, 95% CI 0.61 to 1.29; low‐certainty evidence).
Progression‐free survival
In Keys 2003, there was evidence of a difference in the risk of disease progression or death between women who received radiotherapy plus hysterectomy and those who received radiotherapy alone (HR 0.77, 95% CI 0.54 to 1.10; low‐certainty evidence).
Tumour‐free actuarial survival at five years
In Perez 1987, five‐year, tumour‐free actuarial survival for women with Stage IB was 80% with preoperative radiotherapy and surgery and 89% with radiotherapy alone. It was not reported how many women were Stage IB1 and how many Stage IB2 (the Stage IB2 group was of interest for this Cochrane Review). Women with barrel‐shaped cervix (endocervical lesion with cervix diameter larger than 5 cm) were excluded. In Stage IIA, the five‐year tumour‐free survival was 79% in the preoperative radiotherapy and surgery group and 56% in the radiotherapy alone group. These differences were not significant (very low‐certainty evidence).
Quality of life
None of the studies reported quality of life.
Severe/serious adverse events
In the women with Stage IB disease in Perez 1987, only 1/48 (2%) women experienced a severe complication (grade 3) in the radiotherapy and surgery group (ureteral stricture) whereas 5/40 experienced severe complications in the radiotherapy alone group (including rectovaginal fistula, vesicovaginal fistula, ureteral stricture and pelvic infection). This difference was not significant. Similarly in women with Stage IIA disease, 5/14 (40%) women experienced a severe complication in the radiotherapy and surgery group (including proctitis, rectal stricture, small bowel stricture and ureteral stricture) whereas only 1/16 experienced a severe complication in the radiotherapy alone group (rectal stricture). This difference was not significant (low‐certainty evidence).
Keys 2003 stated that both treatment programmes were well tolerated and there did not appear to be a difference between groups in terms of adverse effects. There were 18/129 women with a grade 3 or 4 adverse effect in the hysterectomy and radiotherapy group and 19 cases in 18/121 women of severe adverse effects in the radiotherapy alone group. Two women in each group received no radiotherapy and were not included (low‐certainty evidence).
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone
Two studies compared hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone (Morice 2012; Zheng 2017)
Morice 2012 included 61 women and compared chemoradiotherapy and simple or radical hysterectomy versus chemoradiotherapy alone. Zheng 2017 included 102 women with LACC and compared chemoradiotherapy and radical hysterectomy with pelvic lymph node dissection versus chemoradiotherapy alone. Fifty‐two participants were included in the hysterectomy arm and 50 in the chemoradiotherapy alone arm. See summary of findings Table 4.
Overall survival
OS was inadequately reported in Zheng 2017 and it was not possible to calculate an HR. OS time of the chemoradiotherapy plus surgery group was 6 to 40 months, median survival time was 23 months and three‐year survival rate was 82.7%. Total survival time of the chemoradiotherapy group was 5 to 41 months, the median survival time was 22.5 months and the three‐year survival rate was 81.8%. The trial authors reported no evidence of a difference between arms (Chi2 = 0.338, P = 0.56; very low‐certainty evidence).
The postoperative pathological data in the hysterectomy arm showed that the residual rate of non‐cancer was 82.7%, and the residual rate of cancer was 5.8%.
Progression‐free survival
PFS was inadequately reported in Zheng 2017 and it was not possible to calculate an HR. PFS time of the chemoradiotherapy plus surgery group was 3 to 40 months, median survival time was 23 months and three‐year survival rate was 73.1%. PFS time of the chemoradiotherapy alone group was 5 to 41 months, median survival time was 22 months and three‐year survival rate was 64.8%. There was no evidence of a difference between groups (Chi2 = 0.092, P = 0.76; very low‐certainty evidence).
Morice 2012 reported no evidence of a difference in three‐year event‐free (death) survival rate (86% in the chemoradiotherapy plus hysterectomy group and 97% in the chemoradiotherapy alone group; log rank P = 0.15; very low‐certainty evidence).
Recurrence‐free survival at three years
Morice 2012 reported no evidence of a difference in three‐year event‐free (recurrence) survival rate (72% in the chemoradiotherapy plus hysterectomy group and 89% in the chemoradiotherapy alone group; log rank P = 0.17; low‐certainty evidence). We did not attempt to calculate an HR using the methods of Parmar 1998 due to this being a single trial analysis (very low‐certainty evidence).
The authors reported that morbidity was studied in a further publication, but when we contacted them, they could not provide data on morbidity.
Quality of life
The trial did not report quality of life.
Severe adverse events
The trial did not adequately report SAEs.
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy
One trial included 211 women and compared brachytherapy versus radical hysterectomy in women who had already received external‐beam chemoradiotherapy with gemcitabine plus cisplatin (Cetina 2013). See summary of findings Table 5.
Overall survival
There was no evidence of a difference in the risk of death between women in the brachytherapy group and those in the radical hysterectomy group (HR 0.65, 95% CI 0.35 to 1.21; P = 0.19; low‐certainty evidence).
Progression‐free survival
There was no evidence of a difference in the risk of disease progression or death between women in the brachytherapy group and those in the radical hysterectomy group (HR 0.70, 95% CI 0.31 to 1.34; P = 0.24; low‐certainty evidence).
Quality of life
The study did not report quality of life.
Severe adverse events
Severe late complications
There was no evidence of a difference in the proportion of women with severe late complications between groups (P = 0.53; low‐certainty evidence). There were four cases of grade 3 or 4 proctitis in the brachytherapy group and two cases in the radical hysterectomy group. There were three cases of severe cystitis in the brachytherapy group and none in the radical hysterectomy group, and there were no reported cases of grade 3 or 4 hydronephrosis in either group (low‐certainty evidence).
Of the 211 participants in the trial, chemoradiotherapy with cisplatin and gemcitabine appeared to be reasonably well tolerated, although nearly a third of women experienced severe neutropenia (most grade 3). Of the 86 women who received a radical hysterectomy, the number of intraoperative and early surgical complications appeared to be reasonably low, with bleeding (9/86) being the most common (low‐certainty evidence).
Discussion
Summary of main results
We found 11 studies including 2683 women that met our inclusion criteria. These studies assessed the role of hysterectomy (radical or simple) in combination with chemotherapy or radiotherapy, or both, in the treatment of LACC.
The RCTs were of varying methodological quality; most were at high risk of bias. These trials compared the following treatments for women with LACC (Stage IB2 to III).
-
Hysterectomy (radical) with NACT versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014).
-
Hysterectomy (simple or radical) with NACT versus radiotherapy alone (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010).
-
Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone (Keys 2003; Perez 1987).
-
Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone (Morice 2012; Zheng 2017).
-
Hysterectomy (radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy (Cetina 2013).
Three trials included 1364 women and compared NACT and hysterectomy versus chemoradiotherapy alone (EORTC 2019; Gupta 2018; Khan 2014).
EORTC 2019 compared NACT plus surgery with standard CCRT. The preliminary results of this study revealed no difference in five‐year OS between NACT plus hysterectomy and CCRT, indicating that quality of life and long‐term toxicity are important to decide optimal treatment. Overall toxicity was acceptable, occurred more frequently in the NACT plus surgery arm and was mainly related to NACT. The five‐year DFS in the NACT plus surgery group was worse compared with the concurrent chemoradiation group (P = 0.021), mostly for Stage IIB disease. However. the authors noted that the first imaging measurement occurred systematically earlier in the neoadjuvant plus surgery arm than in the chemoradiation arm and this may have affected this result. These first data also indicate that in the surgery arm short‐term toxicity due to NACT has influenced further treatment. Of note, additional treatment was given to a significantly greater number of participants in the NACT and surgery group versus the number of participants in the concurrent chemoradiation group.
Gupta 2018, similarly to the EORTC trial, compared NACT plus surgery with standard CCRT. The five‐year DFS in the NACT plus surgery group was worse compared with the concurrent chemoradiation group, whereas there was no evidence of a difference in the corresponding five‐year OS rates. In subgroup analyses, the DFS detriment in the NACT plus surgery group was significant in women with FIGO Stage IIB disease, with a significant test of interaction between treatment effect and Stages IIA and IIB disease.
Meta‐analysis of three studies, assessing 571 women, found that women who received NACT plus hysterectomy had less risk of death than those who received radiotherapy alone, but there was no difference in the proportion of women with disease progression or recurrence between groups (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010).
Benedetti‐Panici 2002 reported no difference in long‐term severe complications between NACT plus hysterectomy and radiotherapy alone. Moreover, it has to be considered that 38 (23%) women who were operated on also underwent adjuvant radiotherapy and that 30% of these women were likely to present with severe late complications.
Chang 2000 found no difference in grade 3 acute toxicity and severe late toxicity between the NACT plus hysterectomy group and the radiotherapy alone group.
In summary these data demonstrate a possible beneficial effect of NACT plus hysterectomy versus radiotherapy in terms of survival, but no difference in DFS. This difference may be due to the NACT, the adjuvant treatment,]or both, rather than hysterectomy since these also differed between groups.
Keys 2003 and Perez 1987 included 374 women and compared preoperative radiotherapy and hysterectomy versus radiotherapy alone. These two trials reported no differences in the risk of death or disease progression, five‐year tumour‐free actuarial survival and severe complications between women who received radiotherapy plus hysterectomy and those who received radiotherapy alone.
Only Keys 2003 described the OS and PFS in the subgroup of women with residual disease in the hysterectomy specimen. Women with grossly positive hysterectomy specimens progressed and died at almost seven times the rate compared to those with negative specimens.
Morice 2012 included 61 women and reported no difference in overall and recurrence‐free survival at three years between chemoradiotherapy and hysterectomy (simple or radical) versus chemoradiotherapy alone. The study did not report adverse events and morbidity data.
Similarly, Cetina 2013 compared brachytherapy versus radical hysterectomy in 211 women who had already received chemoradiotherapy with gemcitabine plus cisplatin. They found no difference in the risk of death, disease progression or severe late complications between women in the brachytherapy group and those in the hysterectomy group.
Harms of treatment, especially in terms of quality‐of‐life data, were poorly reported. Studies comparing the more modern standard treatment of chemoradiotherapy did not demonstrate a benefit with the addition of hysterectomy.
Overall completeness and applicability of evidence
All 11 included studies are relevant in terms of the patient population, types of interventions, effectiveness and outcomes. However, in six studies, the role of hysterectomy as adjuvant treatment is more difficult to assess because the women received different types of primary or neoadjuvant treatment compared with the group who had a hysterectomy (Benedetti‐Panici 2002; Chang 2000; EORTC 2019; Gupta 2018; Khan 2014; Noriyuki 2010). Three trials that compared NACT and hysterectomy versus radiotherapy alone appeared to have external validity and represented a wide geographic area including Italy, China and Japan (Benedetti‐Panici 2002; Chang 2000; Noriyuki 2010). Benedetti‐Panici 2002 was a multicentre trial. The generalisability of other studies was less strong as the comparisons differed across studies and only results of single studies could be reported, although some were from multiple centres, which strengthens their representativeness.
Morice 2012 included women who had a complete response after chemoradiotherapy. The remaining studies did not provide an assessment of the response before surgery. It is important to note that women with a complete response to treatment before surgery potentially have a better prognosis compared to women with residual disease, therefore, the role of adjuvant hysterectomy should be assessed in subgroups with similar prognostic factors (Gadducci 2013; Hequet 2013; Landoni 2014). EORTC 2019 is a multicentre, RCT comparing neoadjuvant hysterectomy plus surgery compared to chemoradiotherapy alone; however, at the moment we only have preliminary results.
Overall, studies reported survival data well, although data on harms were poorly reported. These data are insufficient to recommend, outside of clinical trials, adding hysterectomy to chemoradiotherapy/radiotherapy in women with LACC.
Currently, the standard treatment for LACC is platinum‐based CCRT (CCCMAC 2010; NCI 1999). Although in this review we included older RCTs that did not use chemotherapy, the more relevant studies in modern clinical practice are those that use platinum‐based treatment.
The evidence appears to be of low or very low‐certainty for all comparison outcomes other than for NACT and radical hysterectomy versus radiotherapy alone (GRADE Working Group 2004). The certainty of the evidence for overall and progression or DFS was moderate and was mainly downgraded due to concerns regarding risk of bias in individual trials. The trials in all of the comparisons were at high or moderate risk of bias. More trials that assess identical medical management with and without hysterectomy may test the robustness of the findings of this review as further research is likely to have an important impact on our confidence in the estimates of effect. The meta‐analyses in the review found that women who received NACT plus hysterectomy had less risk of death (so prolonged survival) than those who received radiotherapy alone (HR 0.71, 95% CI 0.55 to 0.93; see Analysis 2.1), but there was no difference in disease progression. However, it is difficult to assess the impact of the hysterectomy given that it was in combination with NACT, since much of this difference may have been due to the chemotherapy component controlling microscopic distant disease rather than improving local control. Using the GRADE approach (GRADE Working Group 2004), the evidence summarised by this review is not sufficient to drive changes in clinical practice. Uncertainty about the additive effects of hysterectomy on a number of different outcomes justifies its evaluation in addition to chemoradiotherapy in future clinical trials.
Quality of the evidence
We reviewed 11 heterogeneous studies, assessing 2683 women, that evaluated the role of hysterectomy with radiotherapy or chemotherapy, or both, in women with LACC. Losses to follow‐up were small but the trials generally scored poorly for other risk of bias items and were potentially at high risk of bias. The number of women in the trials varied considerably with the largest including 633 women (Gupta 2018), and the smallest including only 42 women (Noriyuki 2010).
We included trials with LACC but these trials had a different number of cases for each stage of disease (Stage IB2 to IIIB), therefore, the results may have differed across trials.
The type and dose of medical treatment (chemotherapy, radiotherapy or both) were heterogeneous across the trials.
The baseline indicators to measure the general health of participants in the studies were incompletely reported. Six studies mentioned the performance status of women (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003), and not all studies reported important clinical details such as the size of the tumour and information on residual disease.
Primary survival outcomes were largely well reported in the trials included in the first publication of this review (Kokka 2015). HRs were reported explicitly, deduced or obtained via correspondence so time‐to‐event data were analysed using appropriate survival methods in meta‐analyses or, where possible, in single study reports. However, updated trials included in this update of the review were inadequately reported to include survival outcomes in meta‐analyses.
There was incomplete reporting of harms and not all studies reported quality of life. Eight studies reported morbidity by treatment arm (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; EORTC 2019; Gupta 2018; Keys 2003; Khan 2014; Perez 1987), but three trials did not (Morice 2012; Noriyuki 2010; Zheng 2017). It is important to describe the adverse effects of adjuvant hysterectomy in women with LACC receiving multiple treatments as the available literature suggests severe morbidity (Hequet 2013; Mabuchi 2017).
In Benedetti‐Panici 2002, 28% of women deviated from the protocol in each arm (58/210 in the NACT and hysterectomy arm, 55/199 in the radiotherapy arm), which is high. An additional concern was that of the 210 women in the NACT and hysterectomy arm, 75 received radiotherapy, 37 due to not being suitable for hysterectomy and 38 after hysterectomy. Only 164/210 (78%) in the NACT and hysterectomy arm had surgery. This cross‐over and the protocol deviation are potentially highly significant sources of bias.
Four studies did not mention the route of hysterectomy, that is open or laparoscopic (Benedetti‐Panici 2002; Cetina 2013; Chang 2000; Keys 2003). Morice 2012 indicated how many cases had laparoscopic or open procedures; however the study outcomes, including morbidity, were not subgrouped regarding this factor. Evidence suggests that laparoscopic procedures may have less morbidity than open procedures when performed for the appropriate group of women (Bijen 2009; Colombo 2009; Park 2013). Future studies should ideally consider this factor.
In Keys 2003, surgical staging of lymph nodes was optional and was performed on 57 (22%) women equally divided between the two study arms. Any women with metastasis to the para‐aortic nodes was ineligible for the RCT. Of the 103 women who did not have prerandomisation surgical staging, 54 (52%) had a hysterectomy and lymph node sampling procedure. Of these, seven (13%) had positive para‐aortic nodes. Since the surgical staging of lymph nodes was optional and it was performed in a subgroup of women, it is likely that this study was biased as far as the homogeneity of the staging and the prognosis of included women.
In Chang 2000, there were three different dose ranges for brachytherapy during the study period.
For the update of this review, we identified two RCTs with similar design characteristics (EORTC 2019; Gupta 2018). The EORTC trial results have been given so far in abstracts and oral presentations; the full paper is yet to be published. We understand there is a plan for a meta‐analysis of these two studies.
We identified three ongoing trials that may provide more evidence (CSEM 006 study; Reis Fihlo 2018; Shanmugam 2019).
The overall certainty of the evidence for NACT and radical hysterectomy versus radiotherapy alone was moderate for survival outcomes and low for adverse events. All other comparisons provided low‐certainty evidence, mainly because of poor reporting of outcomes and sparse data where results were based on single trials. The imprecision in single trials may be due to there being no significant difference between two treatments or an absence of evidence, which may come to light with greater statistical power. None of the studies reported quality of life and adverse events were incompletely reported, so the certainty of the evidence was low or very low for these outcomes in all comparisons. The trials in all comparisons were at high or moderate risk of bias. Further research is likely to have an important impact on our confidence in the estimates of effects and may change the estimates in the treatment comparisons based on single trial results and for outcomes that were incompletely reported; but we are quite confident of the reliability of the meta‐analysis of NACT and hysterectomy versus radiotherapy alone (571 participants) for the assessment of survival outcomes.
The certainty of the evidence in all other comparisons was low or very low for all outcomes.
This review identified that more evidence is needed and there is justification for evaluating the role of hysterectomy in combination with other adjuvant and neoadjuvant treatment options in clinical trials.
Potential biases in the review process
We performed a comprehensive search including electronic databases and the grey literature. Two review authors independently assessed references and extracted data. We restricted the included studies to RCTs, which provide the strongest level of evidence available. Hence, we have attempted to reduce bias in the review process.
One significant threat to the validity of the review is the possibility of publication bias, that is, studies that had negative results did not find the treatments to have been effective may not have been published. We were unable to assess this possibility as the meta‐analyses included just three studies and the review 11 studies in total.
Agreements and disagreements with other studies or reviews
Treatment of LACC should be individualised and limited to the minimum number of treatment modalities to yield the best cure with minimal complications. The US National Cancer Institute alert in February 1999 stated that chemoradiotherapy should be considered for all women with cervical cancer. This was based on significant improvements in PFS and OS when cisplatin‐based chemotherapy was administered during radiotherapy for various stages of cervical cancer (Morris 1999; NCI 1999; Rose 1999; Whitney 1999).
Chemoradiotherapy is considered by many groups (in North America, Europe) as the standard treatment for LACC (CCCMAC 2010; Green 2001). This includes pelvic external‐beam radiotherapy with concurrent platinum‐based chemotherapy followed by brachytherapy to boost the central disease response. Alternatively, LACC has been treated with primary radiotherapy alone.
In other countries, the lack of access to radiotherapy and the presumed poor control of metastatic disease has necessitated the use of NACT and hysterectomy. Chemotherapy is administered before other treatments to reduce the tumour volume and, therefore, to make women with clinically inoperable disease amenable to surgery (Sardi 1990; Sardi 1997).
With adjuvant hysterectomy the primary site of cervical cancer is removed. This approach may be preferred by women and the physicians as the 'initial site' of the tumour is removed. However, it is not certain if this approach results in improved survival. This systematic review of the currently available published trials found no evidence that adjuvant hysterectomy improves OS in women with LACC treated with radiotherapy or chemotherapy, or both. In women with a complete response to chemoradiotherapy or radiotherapy there is no obvious benefit. Women with a partial response to chemoradiotherapy or radiotherapy represent a poorer prognostic group; the role of adjuvant hysterectomy in this group of women is still debated (Azria 2005; Hequet 2013; Houvenaeghel 2007; Ota 2008; Sun 2014).
In Keys 2003, women with grossly positive hysterectomy specimens progressed and died at almost seven‐times the rate of those with negative specimens. None of the rest of the included RCTs provided information or appeared to have analysed the subgroup of women with residual disease following concurrent chemotherapy; this subgroup of women is quite challenging to treat and involves debate at the multidisciplinary team meetings. Hysterectomy after radiotherapy/concurrent chemotherapy is not the standard of care in most high‐income countries. In cases of suboptimal chemoradiotherapy, due to poor radiotherapy resources, adjuvant hysterectomy may have a role (Kundargi 2013), as it is impossible to deliver a curative dose of radiation via external beam alone. In other settings, hysterectomy for residual central (i.e. cervix or uterus) disease after completion of radiotherapy/concurrent chemotherapy (external beam and brachytherapy) the usual practice is to wait as a minimum of 12 weeks as it is possible that the disease will respond slowly and surgery after radiotherapy can be challenging. In situations where it has not been possible to place a brachytherapy applicator (the brachytherapy may have failed because there is still very bulky disease), then careful consideration of surgical excision is required (whether one will achieve complete clearance surgically before proceeding). In such cases, surgery may involve an exenteration; management in all such cases needs to be individualised as exenterative surgery may not be appropriate even if technically feasible. One of the concerns regarding surgery following chemoradiotherapy is surgery‐related morbidity. The CCCMAC 2010 meta‐analysis showed that chemoradiotherapy alone can cause severe adverse effects; chemotherapy can cause significant acute toxicity and radiotherapy can cause late complications that are difficult to reverse. Surgery following these modalities can be challenging as the quality of the tissues and the potential for healing are adversely affected by the preceding treatments. Hequet 2013 and Ferrandina 2014 found that the morbidity following surgery was high, suggesting an under‐reporting of morbidity data in the included studies. Well‐designed studies are required to assess the adverse effects of adjuvant hysterectomy following concurrent chemotherapy/radiotherapy of women with LACC, including studies where advanced radiotherapy techniques such as intensity‐modulated radiotherapy are used; intensity‐modulated radiotherapy has been associated with reduced toxicity (Baojuan 2012; Lin 2019; Marjanovic 2019), hence adjuvant hysterectomy may cause less morbidity.
In five included studies there was no difference in the rate of local and distant recurrences between the two arms (Cetina 2013; Keys 2003; Morice 2012; Noriyuki 2010; Perez 1987). In Chang 2000, there was a reduction in the local recurrence rate (9% versus 21%) and a slight decrease in the distant recurrence rate (10% versus 13%) in women who received concurrent radiation and cisplatin and radical hysterectomy compared with those who received radiotherapy alone. The beneficial role of hysterectomy on this difference is unclear because, as mentioned earlier, evidence suggests that it is the addition of cisplatin that reduces the risk of local and distant recurrence (Morris 1999; NCI 1999; Peters 2000; Rose 1999; Whitney 1999).
The most recent randomised studies have highlighted a possible worse result of NACT and surgery versus CCRT in the women with Stage IIB disease (EORTC 2019; Gupta 2018). Meta‐analyses of these two studies and the results of the ongoing trial, CSEM 006 study, should provide more evidence about the best practice in this subgroup of women.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Study flow diagram.

Comparison 1: Hysterectomy (radical) with neoadjuvant chemotherapy (NACT) versus chemoradiotherapy alone, Outcome 1: Overall survival

Comparison 2: Hysterectomy (simple or radical) with neoadjuvant chemotherapy (NACT) versus radiotherapy alone, Outcome 1: Overall survival

Comparison 2: Hysterectomy (simple or radical) with neoadjuvant chemotherapy (NACT) versus radiotherapy alone, Outcome 2: Disease‐ or progression‐free survival
| Hysterectomy (radical) with neoadjuvant chemotherapy versus with chemoradiotherapy alone for women with locally advanced cervical cancer | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: NACT + hysterectomy Comparison: CCRT | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence(GRADE) | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 58.5–98.4 months in the 2 trials | HR 0.94 (0.76 to 1.16) | 1253 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | I2 = 0% |
| DFS Median follow‐up 58.5–98.4 months in the 2 trials | HR 1.38 (1.02 to 1.87) | 633 (1 RCT) | ⊕⊕⊝⊝ Lowb | — |
| 5‐year DFS in the NACT + surgery group was 57% vs 65.6% in the chemoradiotherapy group (P = 0.021) | 620 (1 RCT) | |||
| Quality of life | — | — | — | Not reported. |
| SAEs and toxicity | SAEs In first trial, there were no toxic deaths reported. 198 SAEs occurred: 145 in the NACT + surgery arm vs 53 in the CCRT arm. In the second trial there were 114 grade 3 or 4 SAEs: 92 in the NACT + surgery arm vs 22 in the CCRT arm | 198 (1 RCT) 114 (1 RCT) | ⊕⊝⊝⊝ Very lowc | — |
| Toxicity In 1 trial, NACT + surgery group, compared with the chemoradiotherapy group, there was a lower rate of rectal (5.7% with NACT + surgery vs 13.3% with chemoradiotherapy; P = 0.002), bladder (2.8% with NACT + surgery vs 7.3% with chemoradiotherapy; P = 0.017), and vaginal (19.9% with NACT + surgery vs 36.9% with chemoradiotherapy; P = 0.001) toxicity occurring or persisting 90 days after treatment completion. However, 24 months after treatment completion, there was no difference in rectal and bladder toxicities between groups, whereas vaginal toxicity continued to occur at a lower rate in the NACT + surgery group (12.0% with NACT + surgery vs 25.6% with chemoradiotherapy; P = 0.001). | 114 (1 RCT) | |||
| Treatment‐related morbidity No treatment‐related deaths in either chemoradiotherapy or NACT + surgery arm. Overall, 89% of participants in the chemoradiotherapy arm and 73% in the NACT + surgery arm had complications, with 18% in NACT + surgery arm experiencing recurrence and requiring adjuvant radiotherapy. | 111 (1 RCT) | |||
| CI: confidence interval; CCRT: concurrent chemoradiotherapy; DFS: disease‐free survival; HR: hazard ratio; NACT: neoadjuvant chemotherapy; SAE: serious adverse event. | ||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. | ||||
| aDowngraded one level due to concerns regarding the uncertainty of risk of bias in individual trials and only two trials in meta‐analysis (although it is arguable whether the number of included participants represented relatively sparse data). | ||||
| Hysterectomy (simple or radical) with neoadjuvant chemotherapy versus radiotherapy alone for women with locally advanced cervical cancer | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: neoadjuvant chemotherapy + radical hysterectomy Comparison: radiotherapy alone | ||||
| Outcomes | Relative effect | No of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 39–60 months in the 3 trials | HR 0.71 (0.55 to 0.93) | 571 | ⊕⊕⊕⊝ | — |
| Disease‐ or progression‐free survival Median follow‐up 39–60 months in the 3 trials | HR 0.75 (0.53 to 1.05) | 571 | ⊕⊕⊕⊝ | There were varying definitions of disease‐ and progression‐free survival. However, we did not consider this merited further downgrading to low‐certainty evidence. |
| Quality of life | — | — | — | Not reported. |
| Severe adverse events and toxicity | Acute severe toxicity RR 1.32 (0.47 to 3.71) | 118 (1 RCT) | ⊕⊕⊝⊝ | — |
| Long‐term severe complications RR 0.86 (0.49 to 1.50) | 409 (1 RCT) | |||
| Severe late toxicity RR 0.60 (0.27 to 1.34) | 118 (1 RCT) | |||
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to concerns regarding the uncertainty of risk of bias in individual trials. | ||||
| Hysterectomy (simple or radical) with radiotherapy versus radiotherapy alone | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: radiotherapy + hysterectomy (simple or radical) Comparison: radiotherapy alone | ||||
| Outcomes | Relative effect (95% CI) | No of participants | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 9.6 years | HR 0.89 (0.61 to 1.29) | 256 (1 RCT) | ⊕⊕⊖⊖ Lowa,b | 12 participants in each regimen (10% with radiotherapy + hysterectomy vs 9% with radiotherapy) were lost to follow‐up by 5 years. |
| Progression‐free survival Median follow‐up 9.6 years | HR 0.77 (0.54 to 1.10) | 256 (1 RCT) | ⊕⊕⊖⊖ Lowa,b | 12 participants in each regimen (10% with radiotherapy + hysterectomy vs 9% with radiotherapy) were lost to follow‐up by 5 years. |
| Tumour‐free actuarial survival at 5 years | 5‐year, tumour‐free actuarial survival for women with Stage IB was 80% in the preoperative radiotherapy + hysterectomy group and 89% in the radiotherapy group. In Stage IIA, these rates were 79% in the preoperative radiotherapy + hysterectomy group and 56% in the radiotherapy group. | 118 (1 RCT) | ⊕⊖⊖⊖ Very lowa,b,c | — |
| Quality of life | — | — | — | Not reported. |
| Severe/serious adverse events | 1 trial stated that both treatment programmes were well tolerated and there were no differences between groups in adverse effects. There were 18/129 women with a grade 3 or 4 adverse effect in the radiotherapy + hysterectomy group and 19 cases in 18/121 women of severe adverse effects in the radiotherapy group. In another trial, only 1/48 (2%) women with Stage IB disease experienced a severe complication (grade 3) in the radiotherapy + hysterectomy group (ureteral stricture) whereas 5/40 experienced severe complications in the radiotherapy group (including rectovaginal fistula, vesicovaginal fistula, ureteral stricture and pelvic infection) (P > 0.05). Similarly in women with Stage IIA disease, 5/14 (40%) women experienced a severe complication in the radiotherapy + hysterectomy group (including proctitis, rectal stricture, small bowel stricture and ureteral stricture) whereas only 1/16 women experienced a severe complication in the radiotherapy group (rectal stricture) (P > 0.05). | 374 (2 RCTs) | ⊕⊕⊖⊖ Lowa,b | Relative effect measures were not presented due to the crude combining of adverse events or sparse data, or both. |
| CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to sparse data leading to imprecision. | ||||
| Hysterectomy (simple or radical) with chemoradiotherapy versus chemoradiotherapy alone | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: chemoradiotherapy + hysterectomy (simple or radical) Comparison: chemoradiotherapy alone | ||||
| Outcomes | Relative effect | No of participants | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 3.8 years | Overall survival was inadequately reported and it was not possible to calculate a hazard ratio. Overall survival time in the chemoradiotherapy + hysterectomy group was 6–40 months, median survival time was 23 months, and 3‐year survival rate was 82.7%. Total survival time in the chemoradiotherapy group was 5–41 months, median survival time was 22.5 months and 3‐year survival rate was 81.8%. Trial authors reported differences between arms were not statistically significant (P = 0.56). | 102 | ⊕⊝⊝⊝ | — |
| Progression or event‐free survival Median follow‐up 3.8 years | Progression‐free survival was inadequately reported in both trials and it was not possible to calculate a hazard ratio. In 1 trial, progression‐free survival time in the chemoradiotherapy + hysterectomy group was 3–40 months, median survival time was 23 months and 3‐year survival rate was 73.1%. The progression‐free survival time in the chemoradiotherapy alone group was 5–41 months, median survival time was 22 months and 3‐year survival rate was 64.8%. There was no significant difference between arms (P = 0.76). Another trial included 61 women and compared chemoradiotherapy + simple or radical hysterectomy vs chemoradiotherapy alone. There was no difference in 3‐year event‐free (death) survival rate (86% in the chemoradiotherapy + hysterectomy group vs 97% in the chemoradiotherapy alone group; log rank P = 0.15). | 163 | ⊕⊝⊝⊝ | — |
| Quality of life | — | — | — | Not adequately reported. |
| Severe/serious adverse events | — | — | — | Not adequately reported. |
| RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded two levels due to sparse data leading to imprecision. | ||||
| Hysterectomy (simple or radical) with chemoradiotherapy versus internal radiotherapy (brachytherapy) with chemoradiotherapy | ||||
| Patient or population: women with locally advanced cervical cancer Settings: outpatient Intervention: chemoradiotherapy + hysterectomy (simple or radical) Comparison: chemoradiotherapy alone | ||||
| Outcomes | Relative effect | No of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Overall survival Median follow‐up 3 years | HR 0.65 (95% CI 0.35 to 1.21) | 211 | ⊕⊕⊝⊝ | — |
| Progression or event‐free survival Median follow‐up 3 years | HR 0.70 (95% CI 0.31 to 1.34) | 211 | ⊕⊕⊝⊝ | — |
| Quality of life | — | — | — | Not reported. |
| Severe late complications | There was no difference in the proportion of women with severe late complications in the brachytherapy and radical hysterectomy groups (P = 0.53). There were 4 cases of grade 3 or 4 proctitis in the brachytherapy group vs 2 cases in the radical hysterectomy group; 3 cases of severe cystitis in the brachytherapy group vs 0 in the radical hysterectomy group; 0 cases of grade 3 or 4 hydronephrosis in either group. Of the 211 participants, chemoradiotherapy with cisplatin and gemcitabine appeared to be reasonably well tolerated, although nearly a third of women experienced severe neutropenia (most grade 3). Of the 86 women who received a radical hysterectomy, the number of intraoperative and early surgical complications appeared to be reasonably low, with bleeding (9/86) being the most common. | 211 | ⊕⊕⊝⊝ | — |
| CI: confidence interval; RCT: randomised controlled trial. | ||||
| GRADE Working Group grades of evidence | ||||
| aDowngraded one level due to sparse data leading to imprecision. | ||||
| Stage I (2018): carcinoma strictly confined to the cervix (extension to the uterine corpus should be disregarded) | ||
| 2009 FIGO stage: description | 2018 FIGO stage: description | Comment |
|---|---|---|
| IA: invasive carcinoma diagnosed only by microscopy, with maximum depth of invasion </= 5 mm and largest extension </= 7 mm | IA: invasive carcinoma diagnosed only by microscopy, with maximum depth of invasion < 5 mm | – Lateral extent of the carcinoma is no longer considered in distinguishing between FIGO Stage IA and IB carcinomas – If margins of loop are involved patient is allocated to Stage IB1 |
|
|
| |
|
|
| |
| IB: clinically visible lesions limited to the cervix or preclinical cancers greater than Stage IA | IB: invasive carcinoma with measured deepest invasion >/= 5 mm (greater than Stage IA), lesion limited to the cervix uteri | – See above – LVSI must be commented upon, although does not affect FIGO stage |
|
| IB1: invasive carcinoma >/= 5 mm depth of stromal invasion, and < 2 cm in greatest dimension | – New stage category |
| IB2: invasive carcinoma >/= 2 cm and < 4 cm in greatest dimension | – New stage category | |
| IB2: invasive carcinoma > 4 cm in greatest dimension | IB3: invasive carcinoma >/= 4 cm in greatest dimension | – New stage category |
| Adapted from Singh 2019. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Overall survival Show forest plot | 2 | Hazard Ratio (IV, Random, 95% CI) | 0.94 [0.76, 1.16] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Overall survival Show forest plot | 3 | Hazard Ratio (IV, Random, 95% CI) | 0.71 [0.55, 0.93] | |
| 2.2 Disease‐ or progression‐free survival Show forest plot | 3 | Hazard Ratio (IV, Random, 95% CI) | 0.75 [0.53, 1.05] | |

