Comparación de surfactantes de origen animal para la prevención y el tratamiento del síndrome de dificultad respiratoria en lactantes prematuros
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized Single Center | |
| Participants | Total participants = 40 Preterm infants < 37 weeks' gestation Chest radiograph consistent with RDS Required intubation and mechanical ventilation Need for surfactant determined by the care providing team Randomized 40 infants to receive calfactant (n = 19, BW (g) 1621 ± 442 and GA (wk) 30.0 ± 2.1)) and beractant (n = 21, BW (g) 1309 ± 642 and GA (wk) 29.0 ± 3.6)). | |
| Interventions | Calfactant (n = 19) Dose: 100 mg/kg phospholipid Beractant (n = 21) Dose = 100 mg/kg phospholipid Repeat dose given as per manufacturer’s instruction Criteria for re‐dosing: Objective Same technique for surfactant administration | |
| Outcomes | Primary: Dynamic compliance 1 hour following first dose of surfactant administration Secondary outcomes: chronic lung disease (need for oxygen at 36 weeks' PMA); Early onset sepsis PDA; survival (alive at hospital discharge) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to treatment options by sampling replacement. Unclear sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Unmasked |
| Blinding of outcome assessment (detection bias) | High risk | Unmasked |
| Incomplete outcome data (attrition bias) | Low risk | No infants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported on primary outcome (dynamic compliance 1 hour after first dose of surfactant) as well as important secondary outcomes (chronic lung disease (need for oxygen at 36 weeks' PMA); early onset sepsis; PDA; survival (alive at hospital discharge). |
| Other bias | Low risk | |
| Methods | Randomized Single Center | |
| Participants | Total participants = 82 Preterm infants ≤ 32 weeks' gestational age and ≤ 2000 g RDS requiring mechanical ventilation and FiO₂ requirement ≥ 0.30 The groups were comparable for GA and BW (29.0 ± 1.2 wk and 1195 ± 390 g in bovactant (Alveofact) group; 28.7 ± 0.5 wk and 1233 ± 380 g in poractant alfa group; and 29.2 ± 1.0 wk and 1180 ± 410 g in beractant group). | |
| Interventions | Bovactant (Alveofact) (n = 27) Dose 100 mg/kg Poractant alfa (n = 27) Dose: 100 mg/kg Beractant (n = 28) Dose: 100 mg/kg Repeat dose after 12 h | |
| Outcomes | Chronic lung disease (oxygen requirement at 36 weeks' PMA); PDA; pulmonary air leak; ROP; IVH | |
| Notes | Bovactant (Alveofact) and poractant alfa given as rapid bolus Beractant was given slowly by pump via adaptor (not as bolus as recommended by manufacturer) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) | High risk | Different method of administration of surfactant, hence blinding of personnel not possible |
| Blinding of outcome assessment (detection bias) | High risk | Standardized ventilator protocol for initiation and weaning of ventilator, but description of an attempt to blind outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | Randomized Multicenter Double Blinded | |
| Participants | Treatment arm: Total participants: 483 Preterm infants with birth weight < 2000 g with established RDS and < 48 h of age Requiring intubation and mechanical ventilation FiO₂ ≥ 0.40 Prevention arm: Total participants = 499 Preterm infants ≤ 29 weeks' gestation and birth weight ≤ 1250 g Infants in the treatment arm were more mature and heavier compared with the prevention arm. In treatment arm, the mean (SD) gestational age and birth weight were 29.2 ± 2.8 wk & 1162 ± 408 g in calfactant group and 29.2 ± 2.8 wk & 1166 ± 401 g in beractant group. In prevention arm, mean (SD) gestational age and birth weight were 27.1 ± 2.2 wk & 891 ± 221 g in calfactant group and 27.1 ± 2.1 wk and 845 ± 205 g in beractant group. | |
| Interventions | Calfactant Dose: 100 mg/kg Beractant Dose: 100 mg/kg Repeat dosing: 3 repeat treatments given if infant remained intubated for RDS and FiO₂ ≥ 0.30 within first 96 h of life | |
| Outcomes | Primary outcome Decrease in need for third dose of surfactant with established RDS Decrease in need for second repeat dose with prophylactic surfactant administration | |
| Notes | Special 25 mg/ml concentration of calfactant used in the trial to maintain masking. Administration, storage and dispensing of surfactant followed beractant package insert | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment Variable block size randomizations by pseudo‐random number generation |
| Allocation concealment (selection bias) | Low risk | Allocation of patient by selection of the next vial from box of sequentially numbered vials |
| Blinding of participants and personnel (performance bias) | Low risk | Surfactants were provided in a vial covered by two layers of opaque labels. The surfactant products were similar in consistency, concentration and color |
| Blinding of outcome assessment (detection bias) | Low risk | A data co‐ordinator and a neonatologist designated to collect data |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | Randomized Multicenter | |
| Participants | Total participants: Treatment arm (n = 1361) Preterm infants with birth weight 401 to 2000 g and < 36 h of age FiO₂ requirement ≥ 0.40 Prevention arm (n = 749) Preterm infants 23 to 29 + 6/7 weeks' gestation, randomized at birth Infants in the treatment arm were older and larger compared with the prevention arm. In treatment arm, the mean (SD) for GA (wk) and BW (g) were 28.4 ± 2.8 & 1154 ± 402 in beractant group and 28.4 ± 2.7 & 1155 ± 408 in calfactant group. In prevention arm, the mean (SD) GA (wk) and BW (g) were 26.6 ± 1.9 & 907 ± 275 in beractant group and 26.5 ± 2 & 910 ± 287 in calfactant group. | |
| Interventions | Calfactant Dose: 105 mg/kg Beractant Dose: 100 mg/kg Repeat dosing: maximum of 3 repeat doses 6 h apart if infant remained intubated for RDS and had FiO₂ requirement ≥ 0.30 to keep SpO₂ > 90% | |
| Outcomes | Primary outcome: Percent infants alive at 36 weeks' PMA without use of supplemental oxygen requirement Secondary outcome: Death from respiratory failure; air leak; severe brain injury (IVH grade 3 or 4 or PVL) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced randomization schedule Computerized random number generation Twins and multiples randomized as individuals |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) | Low risk | Masked syringes used to administer surfactant by personnel not involved in direct patient care |
| Blinding of outcome assessment (detection bias) | Low risk | Reported to maintain blind during statistical analysis |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Unclear risk | Terminated early due to poor enrollment |
| Methods | Randomized Single Center | |
| Participants | Total participants = 126 Preterm infants < 37 weeks' gestation Clinical and radiological diagnosis of RDS within 6 h of birth FiO₂ ≥ 0.30 to maintain SpO₂ 88% to 96% The median gestational age and birth weight was 28 wk and 1165 g in poractant group; and 28 wk and 1080 g in beractant group. The rate of antenatal steroid coverage was 63% for poractant group and 51% for beractant group | |
| Interventions | Poractant alfa (n = 61) Dose: 200 mg/kg Beractant (n = 65) Dose: 100 mg/kg Repeat dose given if FiO₂ ≥ 0.30 at 100 mg/kg for both poractant alfa and beractant | |
| Outcomes | Primary outcome: FiO₂ requirement after 24 h following surfactant administration Secondary outcomes: Need for repeat dose; duration for respiratory support; duration of hospitalization; and other key morbidities related to prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization process not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | Randomized Single Center "open label" trial | |
| Participants | Total participants = 52 Preterm infants between 24 + 0/7 to 29 + 6/7 weeks’ GA Inborn RDS requiring mechanical ventilation Randomized within 6 h The mean (SD) gestational age and birth weight was 27.1 (1.6) wk and 930 (231) g in poractant group; and 26.7(1.7) wk and 900 (271) g in beractant group. | |
| Interventions | Portactant alfa (n = 25) Dose: 200 mg/kg Beractant (n = 27) Dose: 100 mg/kg Repeat dose at 100 mg/kg for both groups if FiO₂ requirement remains ≥ 0.30 | |
| Outcomes | Primary outcome: Short‐term outcome of prematurity FiO₂ requirement, MAP and MAP X FiO₂ until 72 h Secondary outcomes: Key morbidities related to prematurity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using SAS software with variable block size design |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No blinding for either the participants or personnel was reported. |
| Blinding of outcome assessment (detection bias) | High risk | None |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up. |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | Quasi‐randomized Single Center | |
| Participants | Total participants = 150 Newborns diagnosed with RDS requiring exogenous surfactant The mean (SD) gestational age and birth weight was 29.4 (2.4) wk and 1435 (642) g in poractant group; and 29.5 (2.7) wk and 1450 (519) g in beractant group. The groups were similar in characteristics. The rate of antenatal steroid exposure was only 45.5% for poractant group and 42.3% for beractant group. | |
| Interventions | Poractant alfa (n = 79) Dose: 200 mg/kg Beractant (n = 71) Dose: 100 mg/kg Repeat dosing if FiO₂ requirement ≥ 0.50 and radiographic evidence of RDS in presence of continued respiratory distress | |
| Outcomes | Ventilation support requirement at 7 days of age | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd or even number of admission code |
| Allocation concealment (selection bias) | High risk | Odd or even number of admission code |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome reported by two senior neonatologists who did not know the group assignment |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | As part of her PhD thesis, Halahakoon evaluated the effects of poractant alfa, beractant and colfosceril palmitate (Exosurf Neonatal) | |
| Participants | infants 24 to 32 weeks' gestation with RDS requiring assisted ventilation and FiO₂ > 0.40 at < 12 h of age poractant alfa at 100 mg/kg (n = 17, mean (SD) birth weight of 926 (278) g, mean (SD) gestational age of 26.8 (2.4) wk), colfosceril palmitate at 67.5 mg/kg (n = 12, mean (SD) birth weight of 956 (233) g, mean (SD) gestational age of 26.9 (1.9) wk) and beractant at 100 mg/kg (n = 10, mean (SD) birth weight of 1011 (327) g, mean (SD) gestational age of 27.3 (2.0) wk). | |
| Interventions | poractant alfa (n = 17), beractant (n = 10) and colfosceril palmitate (Exosurf neonatal) (n = 12) | |
| Outcomes | cerebral function, hypoxanthine levels and antioxidant levels | |
| Notes | Clinical data are being sought for possible inclusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened at the time of randomization |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | No |
| Other bias | Low risk | |
| Methods | Randomized Single Center | |
| Participants | Total participants = 109 Preterm infants < 34 weeks' gestation Requiring intubation and mechanical ventilation for RDS within 6 h of birth FiO₂ ≥ 0.40 to maintain SpO₂ > 90% The mean (SD) GA and BW were 28.5 ± 4.4 wk & 1031 ± 298 g in the bovactant (Alveofact) group and 29.2 ± 2.3 wk & 1078 ± 279 g in beractant group | |
| Interventions | Bovactant (n = 54) Dose: 50 mg/kg phospholipid Beractant (n = 55) Dose: 100 mg/kg phospholipid Repeat dosing (up to 3 doses) given if patient still requiring mechanical ventilation and FiO₂ ≥ 0.30 until 48 h of age | |
| Outcomes | Primary outcome: Chronic lung disease (oxygen requirement at 28 days) Secondary outcomes: Severity of RDS as assessed by FiO₂; oxygenation index, a/A PO₂; MAP; Pneumothorax; IVH; PDA; days on mechanical ventilation; days of hospitalization | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | Low risk | |
| Blinding of outcome assessment (detection bias) | Low risk | Assessment team drawn from the NICU who were not involved in surfactant administration |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Methods | The objective of this study was to compare the perfusion index (PI) variability in premature infants with respiratory distress syndrome (RDS) following administration of two different animal‐derived surfactant preparations. | |
| Participants | Prospective study on 92 preterm infants with RDS. The mean (SD) birth weight and gestational age was 1098 (256) g and 29.6 (1.8) wk in poractant alfa group; and 1086 (248) g and 29 (1.9) wk in beractant group. The rate of antenatal steroid coverage was 76% in poractant alfa group and 82% in beractant group. | |
| Interventions | Patients were randomized into two groups. Group 1 (n = 46) received beractant; Group 2 (n = 46) received poractant alfa. | |
| Outcomes | Surfactant dosing, oxygenation index (OI), perfusion index (PI). Clinical outcomes including pulmonary hemorrhage, intraventricular hemorrhage, patent ductus arteriosus, necrotizing enterocolitis, and mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation |
| Allocation concealment (selection bias) | Low risk | Sealed envelops contained cards that were randomly assigned to the groups |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported on key morbidities of prematurity |
| Other bias | Low risk | |
| Methods | Randomized Single Center | |
| Participants | Total participants = 63 Preterm infants with birth weights 500 to 1800 g Clinical or radiological diagnosis of RDS Requiring intubation and mechanical ventilation for RDS The mean (SD) BW and GA were 1038 ± 263 g and 27.5 ± 1.9 wk in the bLES group and 971 ± 299 g and 26.9 ± 2.3 wk in beractant group. | |
| Interventions | bLES (n = 29) Dose: 135 mg/kg phospholipid Beractant (n = 31) Dose: 100 mg/kg phospholipid Objective criteria for repeat dosing: FiO₂ > 0.10 compared with FiO₂ requirement after first dose of surfactant | |
| Outcomes | Primary outcome: Oxygenation Index Secondary outcomes: Chronic lung disease (oxygen requirement at 36 weeks' PMA); duration on ventilator; days on oxygen; mortality before hospital discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization based on computer‐generated codes Stratified by birth weight |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | Randomized Single Center | |
| Participants | Total = 58 Preterm infants < 37 weeks' gestation Clinical signs and symptoms of RDS requiring intubation and surfactant as per clinical judgement Surfactant administration routine at ≤ 28 weeks' gestation The mean (SD) gestational age and birth weight was 29.6 (3.6) wk and 1394 (699) g in poractant alfa group; and 29.3 (2.9) wk and 1408 (534) g in beractant group | |
| Interventions | Poractant alfa (n = 29) Dose: 200 mg/kg Beractant (n = 29) Dose: 100 mg/kg Redosing if FiO₂ requirement ≥ 0.30 and infant remains on mechanical ventilation | |
| Outcomes | Primary outcome: FiO₂ requirement at 48 h after first surfactant dose Secondary outcomes: Pneumothorax; PDA; IVH, PVL; BPD (oxygen requirement at 28 days and at 36 weeks' PMA); ROP; death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) | High risk | Not stated, but infants received different re‐treatment schedules that would be obvious to staff. Written standard protocols for ventilator management and oxygen weaning provided and mandated to minimize performance bias |
| Blinding of outcome assessment (detection bias) | High risk | The radiologist and cardiologist reported to be masked Masking for other outcome assessment not reported |
| Incomplete outcome data (attrition bias) | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
| Methods | Randomized Multicenter Masked Trial | |
| Participants | Total participants = 293 Preterm infants with BW 750 to 1750 g and GA < 35 wk Clinical or radiographic evidence of RDS Intubated and mechanically ventilated FiO₂ ≥ 0.30 OR a/A ratio of ≤ 0.33 The groups were comparable with mean BW (SD) being 1511 (259), 1148 (265), 1187 (275) g and mean GA (SD) being 28.7 (2.0), 28.8 (2.0), 28.7 (2.0) wk for high‐dose poractant alfa, low‐dose poractant alfa, and beractant respectively. | |
| Interventions | Poractant alfa (n = 96) Dose 100 mg/kg Poractant alfa (n = 99) Dose 200 mg/kg Beractant (n = 98) Dose 100 mg/kg Repeat doses for both surfactants at 100 mg/kg | |
| Outcomes | Primary outcome: Area of FiO₂ under curve during 6‐hour period after first dose of surfactant Secondary outcome: Change in FiO₂, MAP (measured at baselines, 1, 2, 4 and 6 h after first dose); total number of doses of surfactant needed; median duration of oxygen and mechanical ventilation; complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using random number, stratified by birth weight |
| Allocation concealment (selection bias) | Low risk | Opaque and sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Administration of first dose was masked; repeat doses were "unmasked" and given based on individual product recommendations |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors blinded to type or dose of first dose of surfactant (re: assessment of primary outcome) |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | Randomized Single Center | |
| Participants | Total participants = 44 | |
| Interventions | Surfacen: n = 21 Beractant: n = 23 | |
| Outcomes | Oxygenation and ventilation index; days on ventilator; days on supplemental oxygen; complications of prematurity; mortality | |
| Notes | Article in Spanish ‐ data obtained from abstract and tables. Further translation requested to determine risk of bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Article in Spanish ‐ data obtained from abstract and tables. Further translation requested to determine risk of bias. |
| Other bias | Unclear risk | Spanish article with limited data |
| Methods | Randomized Multicenter Study | |
| Participants | Total participants = 73 Preterm infants with birth weight 700 to 1000 g Clinical and radiological findings consistent with RDS FiO₂ requirement ≥ 0.40 The mean (SD) BW and GA were 1095 ± 225 g and 28.9 ± 2.3 wk in the poractant group and 1082 ± 252 g and 28.8 ± 2.2 wk in the beractant group. | |
| Interventions | Poractant alfa (n = 33) Dose: 200 mg/kg Beractant (n = 40) Dose: 100 mg/kg Repeat dosing with surfactant if FiO₂ ≥ 0.30 and infant remains on mechanical ventilator | |
| Outcomes | Primary outcome: FiO₂ requirement and ventilatory support in first 48 h after surfactant administration Secondary outcomes; Complications diagnosed within first 28 days of life: PIE; PDA; IVH; pulmonary hemorrhage; sepsis; BPD and death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not reported |
| Allocation concealment (selection bias) | Low risk | Opaque and sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | "Because the recommended doses, volumes, dose interval and dose procedures differed between both surfactant preparations, blinding of surfactant administration was not feasible" |
| Blinding of outcome assessment (detection bias) | High risk | Masking of radiologist to the intervention. Masking for other outcome assessment not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
| Methods | Randomized Single Center | |
| Participants | Total participants = 50 Preterm infants < 36 weeks' gestation Radiographic diagnosis of RDS The groups were comparable with respect to BW, GA and antenatal steroid exposure. The mean (SD) BW and GA were 1250 ± 356 g and 30.0 ± 2.6 wk in the calfactant group and 1172 ± 397 g and 29.3 ± 2.9 wk in the beractant group. | |
| Interventions | BovactantAlveofact (n = 25) Dose: 50 mg /kg phospholipid Beractant (n = 25) Dose: 100 mg/kg phospholipid Dose repeated as per manufacturers’ instructions as required based on blood gases and chest X‐ray | |
| Outcomes | Primary outcome: FiO₂, a/A PO₂ and mean airway pressure before and after treatment Duration of mechanical ventilation Secondary outcomes: Bronchopulmonary dysplasia (oxygen requirement at 36 weeks' PMA); pneumothorax; mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. However, it was unlikely as surfactant was administered "according to the manufacturers recommendations, for dosing and method of administration" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Low risk | Report on key morbidities related to prematurity |
| Other bias | Low risk | |
BW: birth weight
g: grams
GA: gestational age
h: hours
IVH: intraventricular hemorrhage
PDA: patent ductus arteriosus
PMA: postmenstrual age
RDS: respiratory distress syndrome
ROP: retinopathy of prematurity
SD: standard deviation
wk: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Bozdağ and colleagues conducted a prospective randomized controlled trial to compare the efficacy of two animal‐derived surfactants for pulmonary hemorrhage in very low birth weight (VLBW) infants. 42 infants were divided into two groups, poractant alfa (n = 21) and beractant (n = 21). Excluded because patient population was VLBW infants with pulmonary hemorrhage. | |
| Choi and colleagues conducted a multicenter study of a domestically developed bovine surfactant Newfacen compared with Surfacten, another bovine‐derived surfactant for efficacy. A total of 492 preterm infant with established RDS with birth weight < 1500 g were randomly assigned to receive either Newfacen (n = 224) or Surfacten (n = 268). Short‐term responses to surfactant and acute complications such as total doses of surfactant administered and changes in respiratory parameters were studied. This study was excluded because both the surfactants studied belong to the same comparison group (bovine lung lavage surfactant). | |
| Proquitté and colleagues performed a retrospective, observational study comparing the effects of bovactant (Alveofact) and poractant alfa (Curosurf) on gas exchange and outcome in premature infants. During a 5‐year period in one German neonatal intensive care unit (NICU), 187 premature infants were treated with surfactant, with 82 receiving bovactant and 105 receiving poractant alfa. The investigators recorded FiO₂ and gas exchange (PaO₂/FiO₂ ratio, PaCO₂, SaO₂) during the first 72 h after surfactant administration and the incidence of outcome parameters at day 28 (bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH grade III or IV), patent ductus arteriosus (PDA), pneumothorax, necrotizing enterocolitis (NEC) and death). The study was excluded as it is not a randomized controlled trial. | |
| Rebello and colleagues compared the effects of butantan (a porcine surfactant obtained by organic extraction) with other commercially available surfactants (either beractant or poractant) in preterm infants with RDS. A total of 327 preterm infants with BW < 1500 g with RDS were randomly assigned to receive butantan (n = 154) and compared with a control group receiving either beractant or poractant (n = 173). The mean BW (SD) and mean GA (SD) were 990 g (245) and 28 wk (2.1) for butantan group and 996 g (235) and 28.1 wk (2.2) for control group respectively. This study was excluded because control group received both modified bovine lung lavage surfactant and porcine lung lavage surfactant. |
BW: birth weight
RDS: respiratory distress syndrome
SD: standard deviation
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective, longitudinal, single‐center cohort study |
| Participants | infants born at ≤ 1,500 g and/or ≤ 32 wk with RDS Conducted between 2008 and 2009 |
| Interventions | Poractant alfa (n = 113) or beractant (n = 102) |
| Outcomes | Neurological and developmental assessments were performed at a corrected age of 18 to 24 months |
| Notes | Unclear whether the patients reported are related to the study of Didzar 2012 |
| Methods | Randomized clinical trial in Alzahra Hospital, Tabriz, Iran |
| Participants | Preterm newborn infants with gestation age less than 32 wk with RDS |
| Interventions | Poractant alfa (Curosurf) (N = 66) and bovactant (Alveofact) (N = 64) Surfactant was administered using the INSURE method (intubation, surfactant administration, extubation) |
| Outcomes | Ventilator support through 7 days, mean duration of oxygen supplementation and hospital stay and other complications associated with prematurity |
| Notes |
| Methods | Randomized Single Center |
| Participants | Total participants = 40 Preterm infants < 30 wk gestation and birth weight < 1000 g |
| Interventions | Surfactant A (porcine lung extract) (n = 20) Dose: 200 mg/kg, repeat dose at 100 mg/kg Surfactant B (Bovine lung extract)(n = 20) Dose: 100 mg/kg (initial and repeat dose) |
| Outcomes | Primary outcome: Airway inflammatory response (Cytokine IL‐6 and IL‐8) Secondary outcomes: BPD (oxygen requirement at 36 weeks' PMA); FiO₂ and oxygenation index at 7 days of life; days of mechanical ventilation; days of hospitalization |
| Notes |
| Methods | Clinical trial performed during a 2‐year period in Ghaem Center's neonatal care unit. Method of allocation unknown. |
| Participants | 104 preterm infants were treated with surfactant; 74 in beractant (Survanta) group and 30 in the poractant (Curosurf) group. Mean gestational age (beractant (Survanta) 30.58 vs poractant (Curosurf) 29.00 wk) Mean birth weight (beractant (Survanta) 1388 vs poractant (Curosurf) 1330 g) |
| Interventions | Beractant (Survanta) vs poractant (Curosurf) |
| Outcomes | bronchopulmonary dysplasia at 28 days, Intraventricular hemorrhage grades III/IV, pneumothorax, patent ductus arteriosis, and death. |
| Notes |
| Methods | Randomized controlled non‐blinded study |
| Participants | 30 preterm infants with RDS, treated with poractant alfa (n = 15) or beractant (n = 15); 18 preterm infants without RDS served as a control group. |
| Interventions | poractant alfa (n = 15) or beractant (n = 15) |
| Outcomes | Oxygenation and hemodynamic parameters were recorded and compared through the first 6 h of treatment. Perfusion index (PI) and tissue carbon monoxide (TCO) values were measured prior to (Tp), immediately after (T0), and at 5 minutes (T5), 30 minutes (T30), 60 minutes (T60), and 360 minutes (T360) after the bolus surfactant administration. The mean arterial pressure, oxygenation index, pH, and lactate levels were recorded simultaneously. |
| Notes | Both study groups had lower Tp PI and higher Tp TCO levels than controls. Both surfactant preparations improved the PI, TCO, mean arterial pressure, oxygenation index, pH, and lactate levels at the end point of T360. However, the median Tp PI value of 1.3 first decreased to 0.86 at T0 (P < 0.001), and then it increased to 0.99 at T5 (p < 0.001) and to 1.25 at T30 (p = 0.037). The median Tp TCO value of 3 decreased to 2, 1.5, 0, and 0 at T0, T5, T30, and T60, respectively (p < 0.001). PI more quickly recovered to Tp values (30 minutes vs. 60 minutes) and reached the control group values (30 minutes vs. 360 minutes) with beractant compared with that with poractant alfa. TCO recovered to Tp values in both groups at the same time (5 minutes vs. 5 minutes), but reached the control group values more quickly (5 minutes vs. 30 minutes) with poractant alfa than with beractant. |
BPD: bronchopulmonary dysplasia
g: grams
PMA: postmenstrual age
wk: weeks
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 1 Neonatal mortality. | ||||
| 1.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.80] |
| 1.2 Treatment | 3 | 1451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 2 Mortality prior to discharge Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 2 Mortality prior to discharge. | ||||
| 2.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 2.2 Treatment | 6 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 3 Oxygen requirement at 28 to 30 days of age (all infants) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 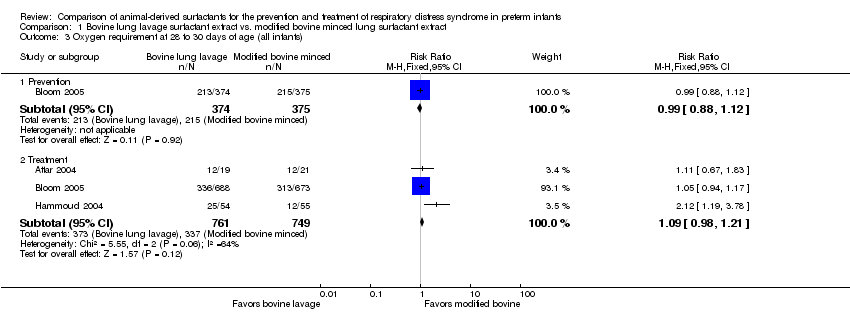 Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 3 Oxygen requirement at 28 to 30 days of age (all infants). | ||||
| 3.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3.2 Treatment | 3 | 1510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.98, 1.21] |
| 4 Oxygen requirement at 36 weeks postmenstrual age (all infants) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age (all infants). | ||||
| 4.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.19] |
| 4.2 Treatment | 5 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.11] |
| 5 Death or oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 5 Death or oxygen requirement at 28 to 30 days of age. | ||||
| 5.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |
| 5.2 Treatment | 2 | 1401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 6 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 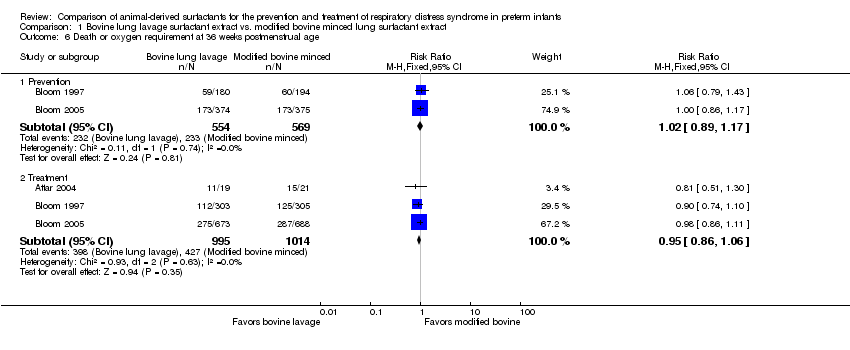 Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 6 Death or oxygen requirement at 36 weeks postmenstrual age. | ||||
| 6.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 6.2 Treatment | 3 | 2009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 7 Received > one dose of surfactant Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 7 Received > one dose of surfactant. | ||||
| 7.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7.2 Treatment | 5 | 2178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 8 Pneumothorax Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 8 Pneumothorax. | ||||
| 8.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.36] |
| 8.2 Treatment | 6 | 2224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.51] |
| 9 Air leak syndromes Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9 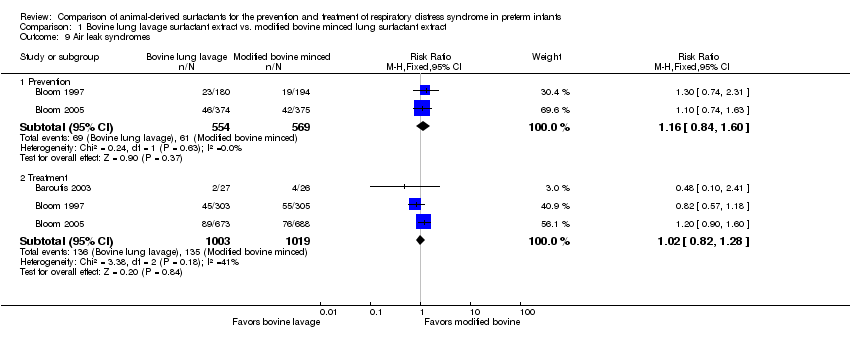 Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 9 Air leak syndromes. | ||||
| 9.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.60] |
| 9.2 Treatment | 3 | 2022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 10 Pulmonary hemorrhage Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10 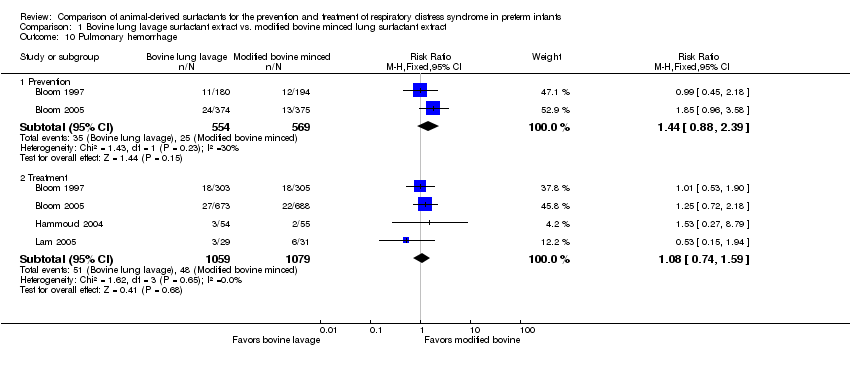 Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 10 Pulmonary hemorrhage. | ||||
| 10.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.88, 2.39] |
| 10.2 Treatment | 4 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.59] |
| 11 Treated patent ductus arteriosus (PDA) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 11 Treated patent ductus arteriosus (PDA). | ||||
| 11.1 Treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.34] |
| 12 Culture‐confirmed bacterial sepsis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 12 Culture‐confirmed bacterial sepsis. | ||||
| 12.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 12.2 Treatment | 6 | 2228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 13 Necrotizing enterocolitis (any stage) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 13 Necrotizing enterocolitis (any stage). | ||||
| 13.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 13.2 Treatment | 5 | 2191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.33] |
| 14 Periventricular leukomalacia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.14 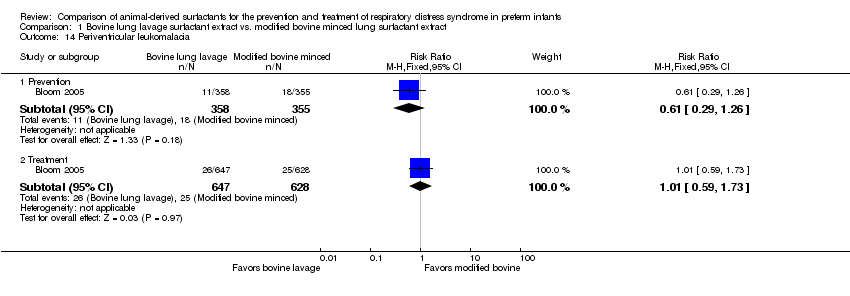 Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 14 Periventricular leukomalacia. | ||||
| 14.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.26] |
| 14.2 Treatment | 1 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.59, 1.73] |
| 15 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 15 Retinopathy of prematurity in infants examined (all stages). | ||||
| 15.1 Prevention | 2 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
| 15.2 Treatment | 3 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
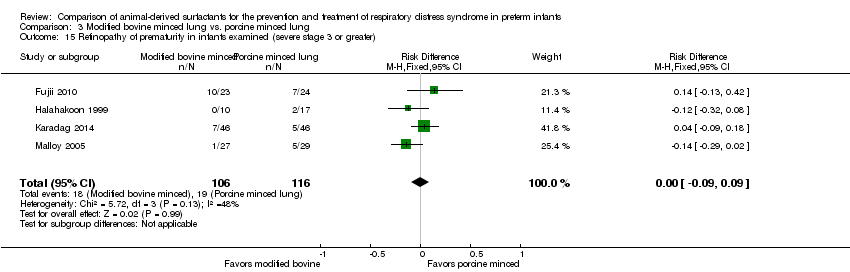
| 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16 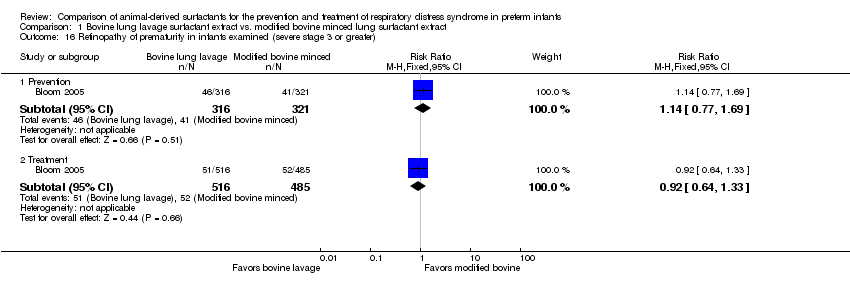 Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater). | ||||
| 16.1 Prevention | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.69] |
| 16.2 Treatment | 1 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades). | ||||
| 17.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 17.2 Treatment | 3 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
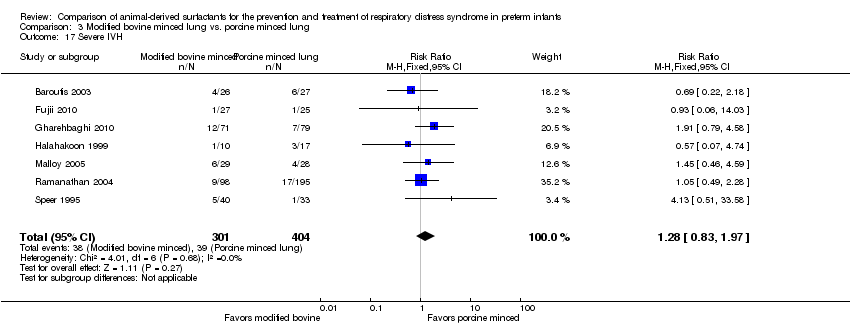
| 18 Severe IVH in infants receiving neuroimaging Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 18 Severe IVH in infants receiving neuroimaging. | ||||
| 18.1 Prevention | 2 | 1087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.89, 1.83] |
| 18.2 Treatment | 5 | 2040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.51, 3.87] |
| Analysis 2.1  Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 1 Mortality prior to discharge. | ||||
| 2 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 3.04] |
| Analysis 2.2  Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 2 Oxygen requirement at 36 weeks postmenstrual age. | ||||
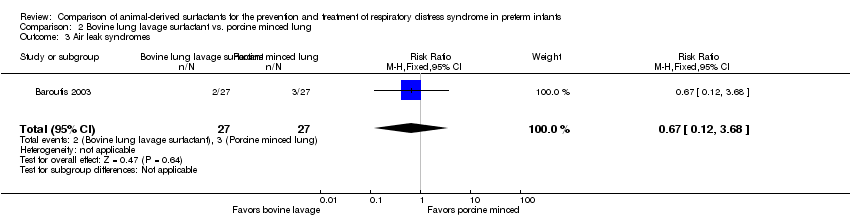
| 3 Air leak syndromes Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| Analysis 2.3 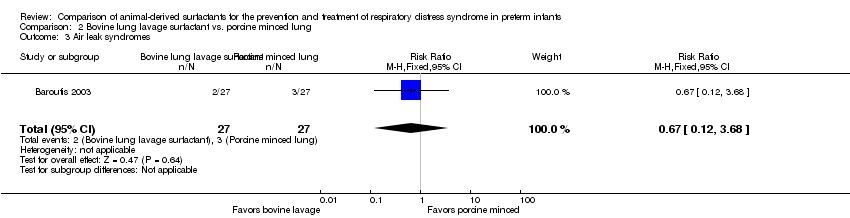 Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 3 Air leak syndromes. | ||||
| 4 Necrotizing enterocolitis (any stage) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| Analysis 2.4  Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 4 Necrotizing enterocolitis (any stage). | ||||
| 5 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.24, 2.66] |
| Analysis 2.5  Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 5 Retinopathy of prematurity in infants examined (all stages). | ||||
| 6 Severe IVH Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.29, 2.41] |
| Analysis 2.6  Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 6 Severe IVH. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.72, 3.07] |
| Analysis 3.1  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 1 Neonatal mortality. | ||||
| 2 Mortality prior to discharge Show forest plot | 9 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.04, 2.00] |
| Analysis 3.2  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 2 Mortality prior to discharge. | ||||
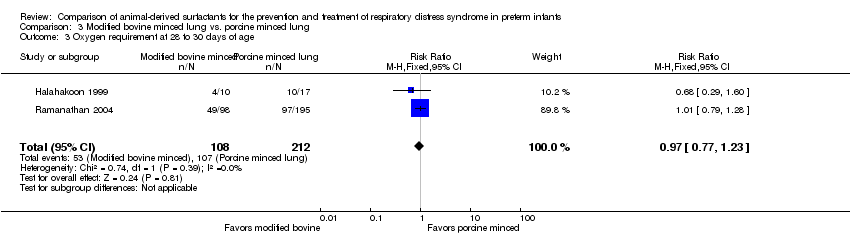
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| Analysis 3.3  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 3 Oxygen requirement at 28 to 30 days of age. | ||||
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 9 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| Analysis 3.4  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age. | ||||
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.64] |
| Analysis 3.5  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age. | ||||
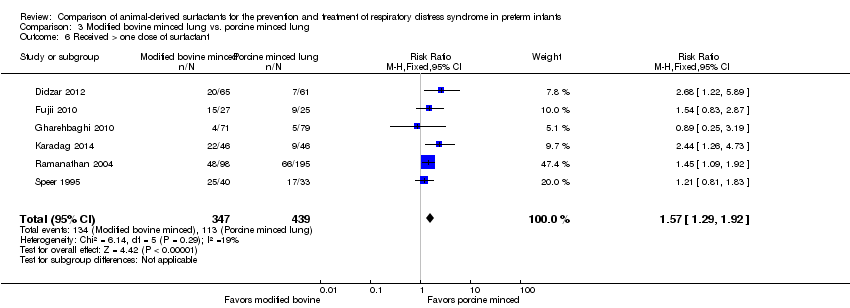
| 6 Received > one dose of surfactant Show forest plot | 6 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.29, 1.92] |
| Analysis 3.6  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 6 Received > one dose of surfactant. | ||||
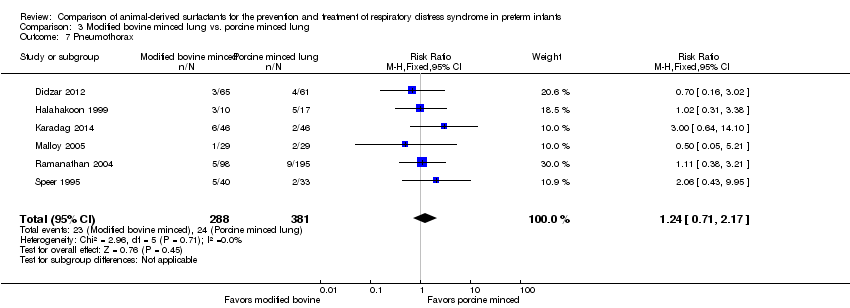
| 7 Pneumothorax Show forest plot | 6 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.17] |
| Analysis 3.7  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 7 Pneumothorax. | ||||
| 8 Air leak syndromes Show forest plot | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.98, 6.68] |
| Analysis 3.8  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 8 Air leak syndromes. | ||||
| 9 Pulmonary hemorrhage Show forest plot | 8 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.02] |
| Analysis 3.9 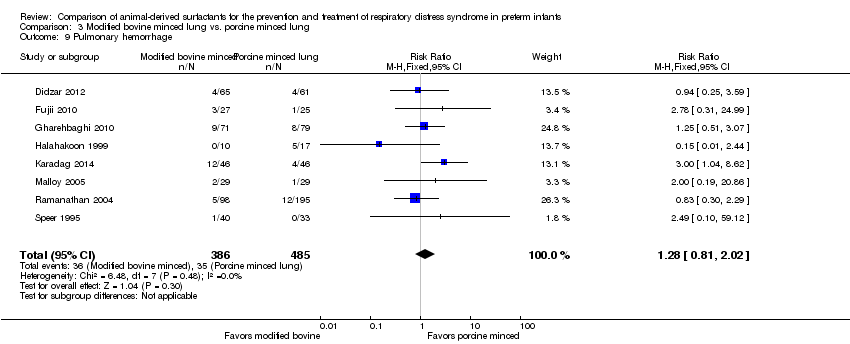 Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 9 Pulmonary hemorrhage. | ||||
| 10 Treated patent ductus arteriosus (PDA) Show forest plot | 3 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.28, 2.70] |
| Analysis 3.10  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 10 Treated patent ductus arteriosus (PDA). | ||||
| 11 Culture‐confirmed bacterial sepsis Show forest plot | 6 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.46] |
| Analysis 3.11  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 11 Culture‐confirmed bacterial sepsis. | ||||
| 12 Necrotizing enterocolitis (any stage) Show forest plot | 7 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.33] |
| Analysis 3.12 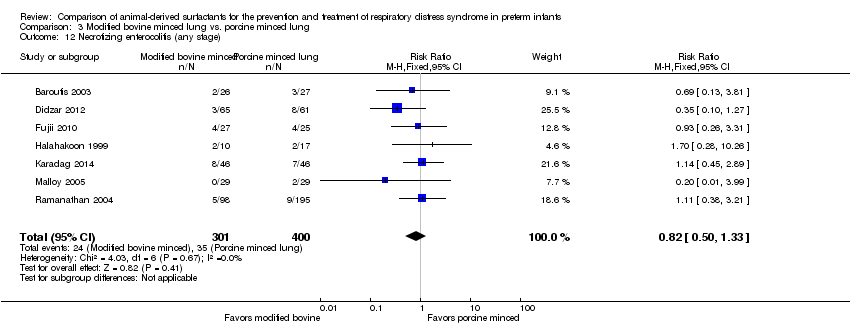 Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 12 Necrotizing enterocolitis (any stage). | ||||
| 13 Periventricular leukomalacia Show forest plot | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.72] |
| Analysis 3.13 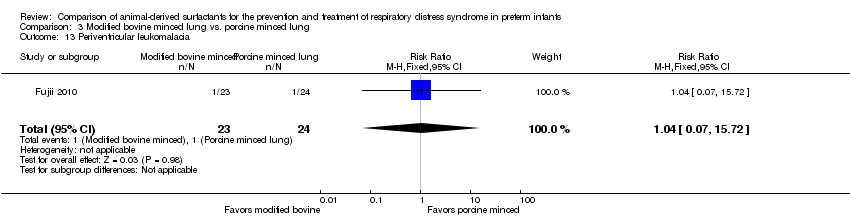 Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 13 Periventricular leukomalacia. | ||||
| 14 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| Analysis 3.14 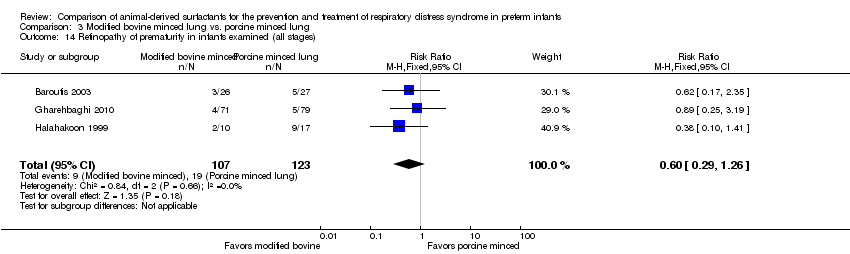 Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 14 Retinopathy of prematurity in infants examined (all stages). | ||||
| 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 4 | 222 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.09, 0.09] |
| Analysis 3.15 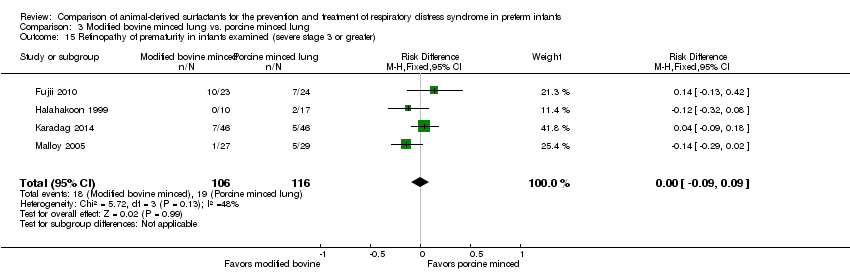 Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater). | ||||
| 16 Intraventricular hemorrhage (all grades) Show forest plot | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.64, 1.50] |
| Analysis 3.16 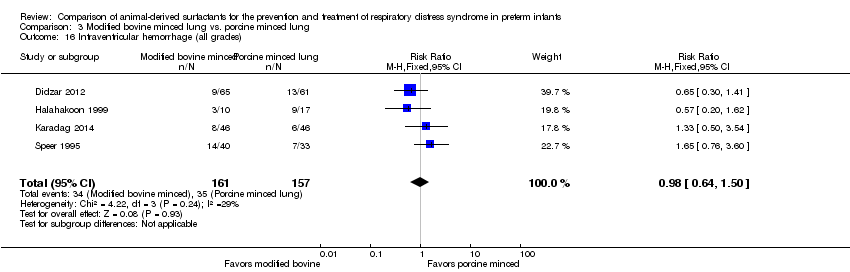 Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 16 Intraventricular hemorrhage (all grades). | ||||
| 17 Severe IVH Show forest plot | 7 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.83, 1.97] |
| Analysis 3.17  Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 17 Severe IVH. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.60, 1.99] |
| Analysis 4.1 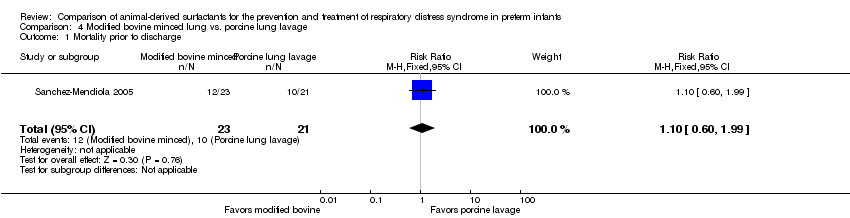 Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 1 Mortality prior to discharge. | ||||
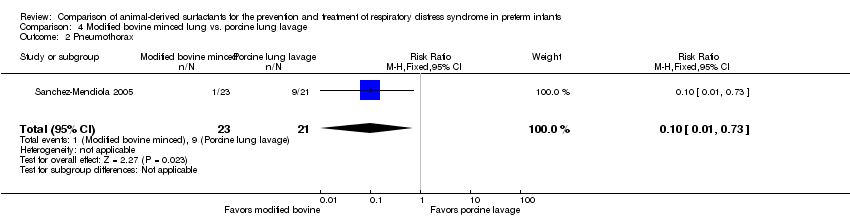
| 2 Pneumothorax Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.73] |
| Analysis 4.2  Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 2 Pneumothorax. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 1 Neonatal mortality. | ||||
| 1.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.55, 2.62] |
| 1.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.74, 9.86] |
| 2 Mortality prior to discharge Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2 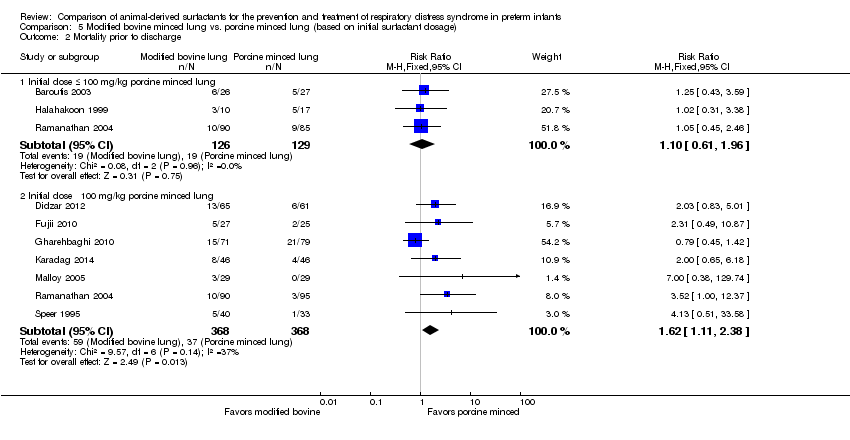 Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 2 Mortality prior to discharge. | ||||
| 2.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 1.96] |
| 2.2 Initial dose ˃ 100 mg/kg porcine minced lung | 7 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.11, 2.38] |
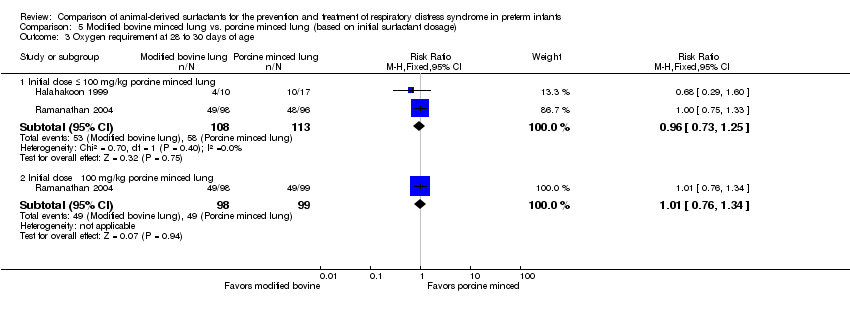
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 3 Oxygen requirement at 28 to 30 days of age. | ||||
| 3.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.25] |
| 3.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 4 Oxygen requirement at 36 weeks postmenstrual age. | ||||
| 4.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.37] |
| 4.2 Initial dose ˃ 100 mg/kg porcine minced lung | 6 | 608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.38] |
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age. | ||||
| 5.1 Initial dose ≤ 100 mg/kg porcine minced lung | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.76, 1.43] |
| 5.2 Initial dose ˃ 100 mg/kg porcine minced lung | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.08, 1.79] |

Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.2 Mortality prior to discharge.
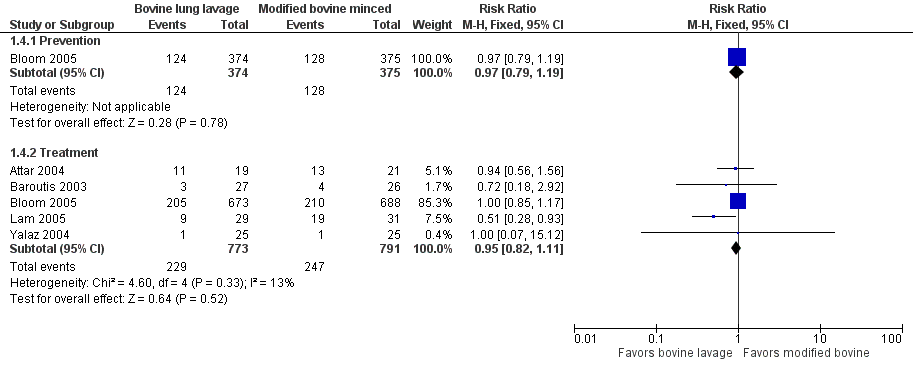
Forest plot of comparison: 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, outcome: 1.4 Oxygen requirement at 36 weeks postmenstrual age (all infants).
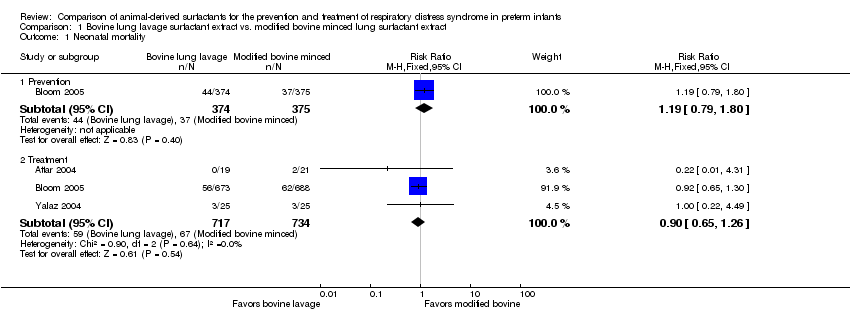
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 1 Neonatal mortality.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 2 Mortality prior to discharge.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 3 Oxygen requirement at 28 to 30 days of age (all infants).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age (all infants).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 5 Death or oxygen requirement at 28 to 30 days of age.
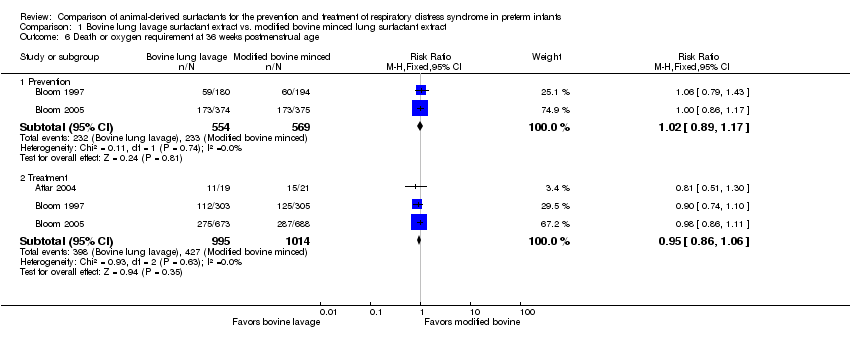
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 6 Death or oxygen requirement at 36 weeks postmenstrual age.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 7 Received > one dose of surfactant.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 8 Pneumothorax.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 9 Air leak syndromes.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 10 Pulmonary hemorrhage.

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 11 Treated patent ductus arteriosus (PDA).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 12 Culture‐confirmed bacterial sepsis.
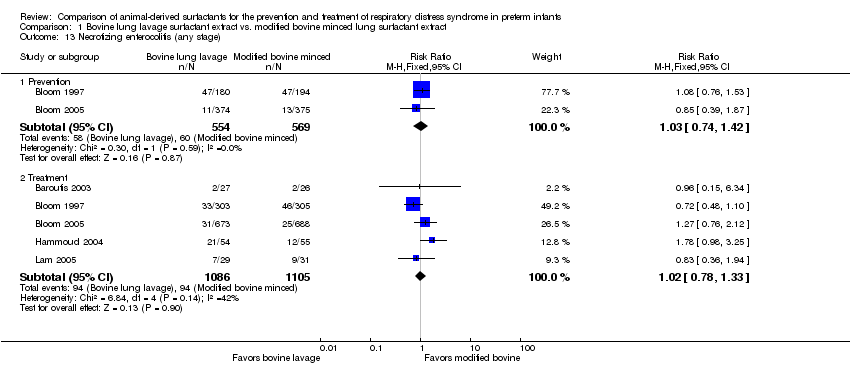
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 13 Necrotizing enterocolitis (any stage).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 14 Periventricular leukomalacia.
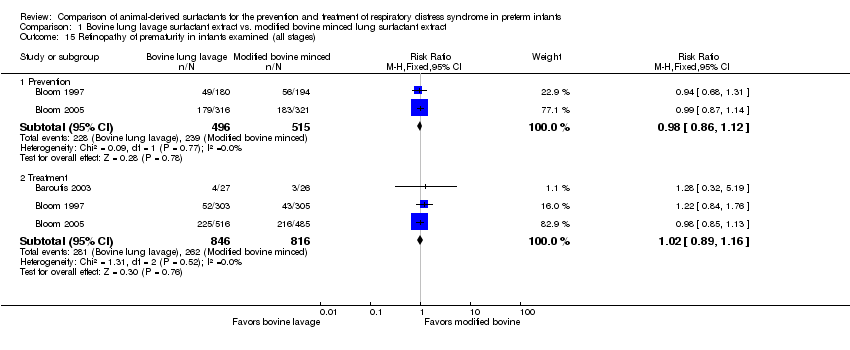
Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 15 Retinopathy of prematurity in infants examined (all stages).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades).

Comparison 1 Bovine lung lavage surfactant extract vs. modified bovine minced lung surfactant extract, Outcome 18 Severe IVH in infants receiving neuroimaging.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 1 Mortality prior to discharge.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 2 Oxygen requirement at 36 weeks postmenstrual age.
Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 3 Air leak syndromes.

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 4 Necrotizing enterocolitis (any stage).

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 5 Retinopathy of prematurity in infants examined (all stages).

Comparison 2 Bovine lung lavage surfactant vs. porcine minced lung, Outcome 6 Severe IVH.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 1 Neonatal mortality.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 2 Mortality prior to discharge.
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 3 Oxygen requirement at 28 to 30 days of age.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 4 Oxygen requirement at 36 weeks postmenstrual age.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age.
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 6 Received > one dose of surfactant.
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 7 Pneumothorax.
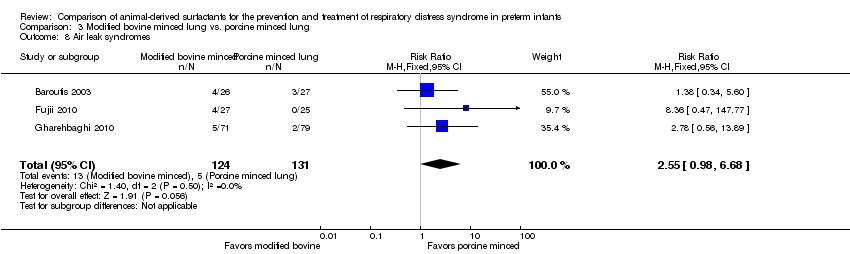
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 8 Air leak syndromes.
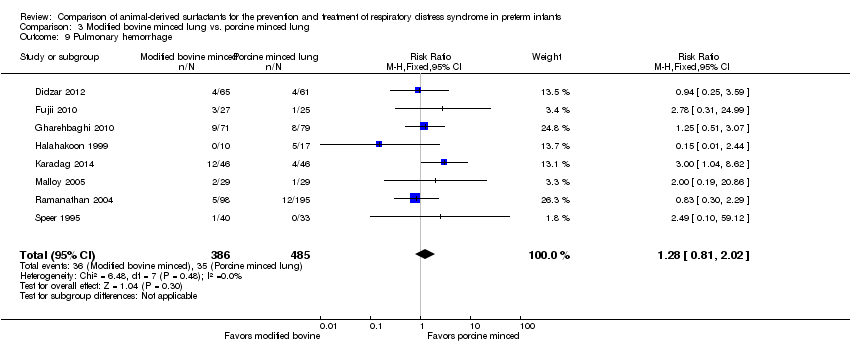
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 9 Pulmonary hemorrhage.

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 10 Treated patent ductus arteriosus (PDA).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 11 Culture‐confirmed bacterial sepsis.
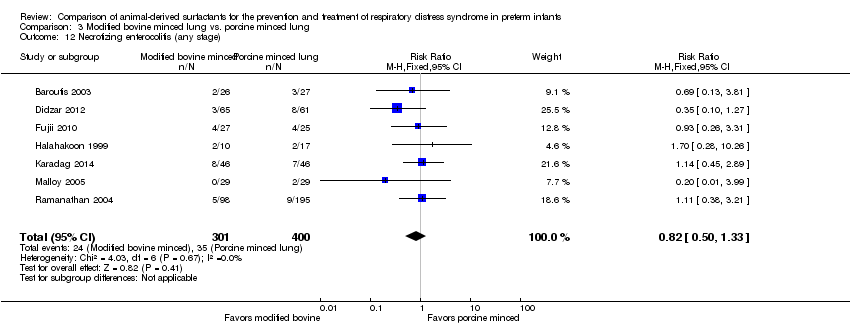
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 12 Necrotizing enterocolitis (any stage).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 13 Periventricular leukomalacia.
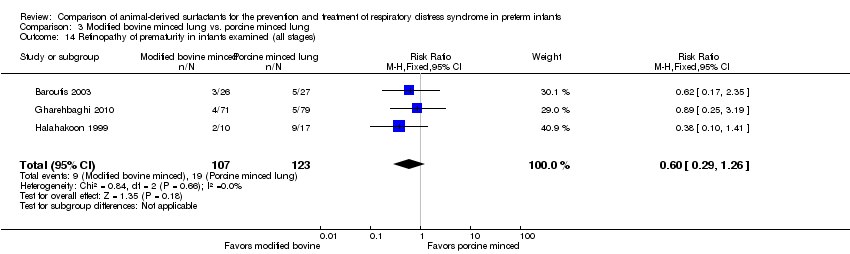
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 14 Retinopathy of prematurity in infants examined (all stages).
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater).

Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 16 Intraventricular hemorrhage (all grades).
Comparison 3 Modified bovine minced lung vs. porcine minced lung, Outcome 17 Severe IVH.

Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 1 Mortality prior to discharge.
Comparison 4 Modified bovine minced lung vs. porcine lung lavage, Outcome 2 Pneumothorax.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 1 Neonatal mortality.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 2 Mortality prior to discharge.
Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 3 Oxygen requirement at 28 to 30 days of age.

Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 4 Oxygen requirement at 36 weeks postmenstrual age.
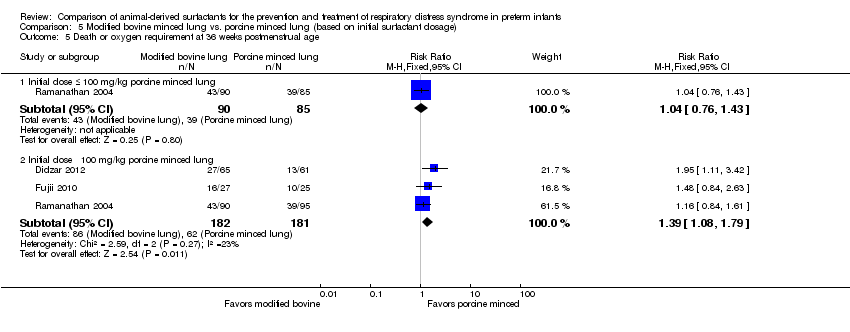
Comparison 5 Modified bovine minced lung vs. porcine minced lung (based on initial surfactant dosage), Outcome 5 Death or oxygen requirement at 36 weeks postmenstrual age.
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for prevention of RDS (Comparision 1: Prevention studies) | ||||||
| Patient or population: Preterm infants for prevention of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge (from any cause) | 107 per 1000 | 133 per 1000 | RR 1.24 | 1123 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and and the total number of events does not meet the optimal information size (OIS). |
| Oxygen requirement at 36 weeks' postmenstrual age | 341 per 1000 | 331 per 1000 | RR 0.97 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 409 per 1000 | 418 per 1000 | RR 1.02 | 1133 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the no effect. The total number of events meets the OIS |
| Pneumothorax. | 67 per 1000 | 51 per 1000 | RR 0.76 | 749 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Pulmonary hemorrhage | 44 per 1000 | 63 per 1000 | RR 1.44 | 1123 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Severe IVH in infants receiving neuroimaging | 87 per 1000 | 112 per 1000 | RR 1.28 | 1087 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) 95% CI includes both no effect and appreciable harm.2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 2000). |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefit, no effect and appreciable harm and the total number of events does not meet the optimal information size 2 95% CI includes benefit, no effect and appreciable harm 3 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 3000 4 95% CI includes benefit, no effect and appreciable harm and the OIS to detect a clinically beneficial effect if there is one is > 2000 | ||||||
| Bovine lung lavage surfactant extract compared with modified bovine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with modified bovine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 131 per 1000 | 128 per 1000 | RR 0.98 | 2231 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the OIS. |
| Oxygen requirement at 36 weeks' postmenstrual age (all infants) | 312 per 1000 | 297 per 1000 | RR 0.95 | 1564 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 421 per 1000 | 400 per 1000 | RR 0.95 | 2009 | ⨁⨁⨁⨁ | We did not downgrade evidence for imprecision as it was considered that 95% CI is narrow and precise around the probability of no effect. Estimations are based in more than 300 events in each arm. |
| Pneumothorax | 73 per 1000 | 83 per 1000 | RR 1.14 | 2224 | ⨁⨁◯◯ | Downgraded two levels due to: 1) Serious imprecision (95% CI includes both no effect and appreciable harm). 2) Inconsistency: Unexplained heterogeneity, with point estimates widely different; 95% CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) |
| Pulmonary hemorrhage | 44 per 1000 | 48 per 1000 | RR 1.08 | 2138 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm) |
| Severe IVH in infants receiving neuroimaging | 125 per 1000 | 108 per 1000 | RR 0.86 | 2040 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes benefits, no effect and appreciable harm). The optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI includes benefits, no effect and appreciable harm, and the total number of events does not meet the OIS 2 Unexplained heterogeneity, with point estimates widely different and CI not overlapping and leading to different conclusions (P value 0.03, Chi² 10.66, I² = 62%) 3 95% CI of the pooled effect crosses 1 and the optimal information size to reliably detect a clinically beneficial effect if there is one is > 7000 | ||||||
| Bovine lung lavage surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Bovine lung lavage surfactant extract | |||||
| Mortality prior to discharge | 185 per 1000 | 259 per 1000 | RR 1.40 | 54 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Oxygen requirement at 36 weeks' postmenstrual age | 148 per 1000 | 111 per 1000 | RR 0.75 | 54 | ⨁⨁◯◯ | Downgraded two levels due to: 1. Serious imprecision (95% CI includes both no effect and appreciable harm). 2. Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | see comments | see comments | Not reported in any of the studies | |||
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | 222 per 1000 | 184 per 1000 | RR 0.83 | 54 | ⨁◯◯◯ | Downgraded three levels due to: 1. potential risk of bias (lack of blinding of outcome assessment) 2. very serious imprecision: (95% CI includes both no effect and appreciable harm) and the total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 1000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 2 We downgraded because lack of blinding of patients, providers and blinding of outcome assessment 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 1000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine minced lung surfactant extract | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 113 per 1000 | 162 per 1000 | RR 1.44 | 901 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Oxygen requirement at 36 weeks' postmenstrual age | 282 per 1000 | 293 per 1000 | RR 1.04 (0.83 to 1.31) | 773 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (95% CI includes both no effect and appreciable harm). Despite the high risk of bias4 we did not downgrade the quality because of its lower impact on this outcome. |
| Death or oxygen requirement at 36 weeks' postmenstrual age | 380 per 1000 | 494 per 1000 | RR 1.30 | 448 | ⨁⨁⨁◯ | Downgraded one level due to imprecision (total number of events does not meet the OIS) |
| Pneumothorax | 63 per 1000 | 78 per 1000 | RR 1.24 | 669 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Pulmonary hemorrhage | 72 per 1000 | 92 per 1000 | RR 1.28 | 871 | ⨁⨁◯◯ | Downgraded two levels due to very serious imprecision: 1) the 95% CI includes both no effect and appreciable harm. 2) The total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 5000) |
| Severe intraventricular hemorrhage in infants who received neuroimaging | 97 per 1000 | 124 per 1000 | RR 1.28 | 705 | ⨁◯◯◯ | Downgraded three levels due to: 1. Potential risk of bias and 2. serious imprecision: 1) the 95% CI includes both no effect and appreciable harm; and 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 3000) |
| Neurodevelopmental outcome at approximately two years’ corrected age | see comments | see comments | Not reported in any studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The total number of events does not meet the OIS 2 95% CI includes benefit, no effect and appreciable harm. Total number of events does not meet the optimal information size. 3 95% CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 5000 4 Studies that carried large weight for the overall effect estimate are classified as high or unclear risk of bias due to lack of blinding in patients, and outcome assessment 5 95% CI of the pooled effect widely crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 3000 | ||||||
| Modified bovine minced lung surfactant extract compared with porcine lung lavage surfactant in preterm infants for treatment of RDS | ||||||
| Patient or population: Preterm infants for treatment of RDS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with porcine lung lavage surfactant | Risk with Modified bovine minced lung surfactant extract | |||||
| Mortality prior to hospital discharge (from any cause) | 476 per 1000 | 524 per 1000 | RR 1.10 | 44 | ⨁⨁◯◯ | Downgraded two levels due to serious imprecision: 1) The 95% CI includes both no effect and appreciable harm. 2) Total number of events does not meet the optimal information size (OIS to detect a clinically beneficial effect if there is one is > 700). |
| Oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Death or oxygen requirement at 36 weeks' postmenstrual age | see comments | see comments | Not reported in any of the studies | |||
| Pneumothorax | 429 per 1000 | 43 per 1000 | RR 0.10 | 44 | ⨁⨁⨁⨁ | |
| Pulmonary hemorrhage | see comments | see comments | Not reported in any of the studies | |||
| Severe intraventricular hemorrhage in infants who received neuroimaging | see comments | see comments | Not reported in any of the studies | |||
| Neurodevelopmental outcome at approximately two years corrected age | see comments | see comments | Not reported in any of the studies | |||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 95 % CI of the pooled effect crosses 1 and the optimal information size to detect a clinically beneficial effect if there is one is > 700 | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.79, 1.80] |
| 1.2 Treatment | 3 | 1451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.26] |
| 2 Mortality prior to discharge Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.90, 1.71] |
| 2.2 Treatment | 6 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.21] |
| 3 Oxygen requirement at 28 to 30 days of age (all infants) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 3.2 Treatment | 3 | 1510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.98, 1.21] |
| 4 Oxygen requirement at 36 weeks postmenstrual age (all infants) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.79, 1.19] |
| 4.2 Treatment | 5 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.11] |
| 5 Death or oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.13] |
| 5.2 Treatment | 2 | 1401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.96, 1.15] |
| 6 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.17] |
| 6.2 Treatment | 3 | 2009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 7 Received > one dose of surfactant Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 7.2 Treatment | 5 | 2178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 8 Pneumothorax Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Prevention | 1 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.36] |
| 8.2 Treatment | 6 | 2224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.85, 1.51] |
| 9 Air leak syndromes Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.84, 1.60] |
| 9.2 Treatment | 3 | 2022 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 10 Pulmonary hemorrhage Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.88, 2.39] |
| 10.2 Treatment | 4 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.74, 1.59] |
| 11 Treated patent ductus arteriosus (PDA) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Treatment | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.34] |
| 12 Culture‐confirmed bacterial sepsis Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.28] |
| 12.2 Treatment | 6 | 2228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.87, 1.15] |
| 13 Necrotizing enterocolitis (any stage) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Prevention | 2 | 1123 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 13.2 Treatment | 5 | 2191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.33] |
| 14 Periventricular leukomalacia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.26] |
| 14.2 Treatment | 1 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.59, 1.73] |
| 15 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Prevention | 2 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
| 15.2 Treatment | 3 | 1662 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.16] |
| 16 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Prevention | 1 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.69] |
| 16.2 Treatment | 1 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.64, 1.33] |
| 17 Intraventricular hemorrhage in infants receiving neuroimaging (all grades) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Prevention | 1 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 17.2 Treatment | 3 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 18 Severe IVH in infants receiving neuroimaging Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Prevention | 2 | 1087 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.89, 1.83] |
| 18.2 Treatment | 5 | 2040 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.68, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.51, 3.87] |
| 2 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.19, 3.04] |
| 3 Air leak syndromes Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| 4 Necrotizing enterocolitis (any stage) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.68] |
| 5 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.24, 2.66] |
| 6 Severe IVH Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.29, 2.41] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.72, 3.07] |
| 2 Mortality prior to discharge Show forest plot | 9 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.04, 2.00] |
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 9 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.79, 1.12] |
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.04, 1.64] |
| 6 Received > one dose of surfactant Show forest plot | 6 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.29, 1.92] |
| 7 Pneumothorax Show forest plot | 6 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.71, 2.17] |
| 8 Air leak syndromes Show forest plot | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.98, 6.68] |
| 9 Pulmonary hemorrhage Show forest plot | 8 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.81, 2.02] |
| 10 Treated patent ductus arteriosus (PDA) Show forest plot | 3 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.28, 2.70] |
| 11 Culture‐confirmed bacterial sepsis Show forest plot | 6 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.87, 1.46] |
| 12 Necrotizing enterocolitis (any stage) Show forest plot | 7 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.50, 1.33] |
| 13 Periventricular leukomalacia Show forest plot | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 15.72] |
| 14 Retinopathy of prematurity in infants examined (all stages) Show forest plot | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| 15 Retinopathy of prematurity in infants examined (severe stage 3 or greater) Show forest plot | 4 | 222 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.09, 0.09] |
| 16 Intraventricular hemorrhage (all grades) Show forest plot | 4 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.64, 1.50] |
| 17 Severe IVH Show forest plot | 7 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.83, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality prior to discharge Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.60, 1.99] |
| 2 Pneumothorax Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neonatal mortality Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.55, 2.62] |
| 1.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.74, 9.86] |
| 2 Mortality prior to discharge Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 1.96] |
| 2.2 Initial dose ˃ 100 mg/kg porcine minced lung | 7 | 736 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.11, 2.38] |
| 3 Oxygen requirement at 28 to 30 days of age Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Initial dose ≤ 100 mg/kg porcine minced lung | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.25] |
| 3.2 Initial dose ˃ 100 mg/kg porcine minced lung | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 4 Oxygen requirement at 36 weeks postmenstrual age Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Initial dose ≤ 100 mg/kg porcine minced lung | 3 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.37] |
| 4.2 Initial dose ˃ 100 mg/kg porcine minced lung | 6 | 608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.38] |
| 5 Death or oxygen requirement at 36 weeks postmenstrual age Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Initial dose ≤ 100 mg/kg porcine minced lung | 1 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.76, 1.43] |
| 5.2 Initial dose ˃ 100 mg/kg porcine minced lung | 3 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.08, 1.79] |

