Lasers for caries removal in deciduous and permanent teeth
Abstract
Background
Despite considerable improvements in oral health, dental caries continue to be a public health issue. The most frequently used, and universally accepted technique, to remove caries is through mechanical ablation of decayed tissues by means of rotating drills (diamond or tungsten carbide, or both). In the past few decades, the introduction of adhesive filling materials (resin composites) has affected cavity filling procedures by reducing its retention needs, with advantages for dental tissue conservation. Consequently, new minimally invasive strategies were introduced into dental practice, such as the use of lasers to perform highly controlled tissue ablation. Laser use has also raised expectations of limiting pain and discomfort compared to using drills, as well as overcoming drill phobia.
Objectives
The main objective of the review was to compare the effects of laser‐based methods to conventional mechanical methods for removing dental caries in deciduous and permanent teeth.
Search methods
We searched the following electronic databases: Cochrane Oral Health's Trials Register (searched 22 June 2016), Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library (searched 22 June 2016), MEDLINE Ovid (1946 to 22 June 2016), Embase Ovid (1980 to 22 June 2016), ProQuest Dissertations and Theses (1980 to 22 June 2016), Zetoc (limited to conference proceedings) (1993 to 22 June 2016), and ISI Web of Knowledge (limited to conference proceedings) (1990 to 22 June 2016). We checked the reference lists of relevant articles to identify additional studies. We searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform for ongoing trials.
Selection criteria
We included randomised controlled trials, split‐mouth trials and cluster‐randomised trials (irrespective of their language) comparing laser therapy to drill ablation of caries. We included participants of any age (children, adolescents and adults).
Data collection and analysis
Two review authors independently screened titles and abstracts of citations identified by the review search strategy. Two review authors independently evaluated the full text of relevant primary studies, assessed risk of bias and extracted data. We used standard methodological procedures expected by Cochrane.
Main results
We included nine randomised trials, published between 1998 and 2014, involving 662 participants. The population consisted of both children and adolescents in four trials, only adults in four trials, and both children/adolescents and adults in one trial. Four studies examined only permanent teeth, and five studies evaluated both deciduous and permanent teeth. Six trials used Er:YAG (erbium‐doped yttrium aluminium garnet) lasers, two trials employed Er,Cr:YSGG (erbium, chromium: yttrium‐scandium‐gallium‐garnet) lasers, and one trial used Nd:YAG (neodymium‐doped yttrium aluminium garnet) laser.
Overall, the trials had small sample sizes, and the majority were at unclear or high risk of bias. The primary outcomes were evaluated in a limited number of trials (removal of caries (four trials (but only two reported quantitative data)); episodes of pain (five studies)). There was insufficient evidence to suggest that either lasers or drill were better at caries removal (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.99 to 1.01; 2 studies; 256 treated caries; P = 0.75; I2 = 0%; low‐quality evidence).
The incidence of moderate or high pain was greater in the drill group compared to the laser group (RR 0.40, 95% CI 0.28 to 0.57; 2 studies; 143 participants; P < 0.001; I2 = 50%). Similarly, the need for anaesthesia was significantly higher in the drill group than in the laser group (RR 0.25, 95% CI 0.10 to 0.65; 3 studies; 217 children/adolescents; P = 0.004; I2 = 0%).
In terms of marginal integrity of restoration, there was no evidence of a difference between laser and drill comparisons evaluated at 6 months (RR 1.00, 95% CI 0.21 to 4.78; 3 studies), 1 year (RR 1.59, 95% CI 0.34 to 7.38; 2 studies), or 2 years (RR 1.00, 95% CI 0.21 to 4.74; 1 study).
There was no evidence of a difference for durability of restoration between laser therapy or drill at 6 months' follow‐up (RR 2.40, 95% CI 0.65 to 8.77; 4 studies), at 1 year (RR 1.40, 95% CI 0.29 to 6.78; 2 studies) or at 2 years' follow‐up (RR 0.50, 95% CI 0.02 to 14.60; 1 study).
Only two trials investigated the recurrence of caries, but no events occurred during 6 months' follow‐up.
There was insufficient evidence of a difference between laser or drill in terms of pulpal inflammation or necrosis at 1 week (RR 1.51, 95% CI 0.26 to 8.75; 3 studies) and at 6 months (RR 0.99, 95% CI 0.10 to 9.41; 2 studies).
Authors' conclusions
Given the low quality of the body of evidence, we concluded that evidence was insufficient to support the use of laser as an alternative to traditional drill therapy for caries removal. We found some evidence in favour of laser therapy for pain control, need of anaesthesia and patient discomfort, but, again, the body of evidence was of low quality. Additional well‐designed, randomised trials investigating the most relevant outcomes are needed.
PICO
Plain language summary
Lasers for the removal of decay in first and permanent teeth
Review question
This review addressed the issue of whether there is an advantage to laser therapy for the removal of decay in teeth and patient pain, compared to a traditional drill, in children, adolescents and adults.
Background
Dental decay is a cavity formation in teeth resulting from the destruction of dental tissue caused by bacteria under certain conditions, including poor oral hygiene and excessive sugar intake. Symptoms may include pain and difficulty with eating, and complications may include tooth loss, infection or inflammation of the gum. Rotating drills are traditionally used to remove decay. However, this mechanical tool may have unexpected side effects, such as the removal of too much or too little decay, in addition to discomfort due to pain, noise and vibration. Laser therapy is a potential alternative to a mechanical drill.
Study characteristics
Cochrane Oral Health provided the search strategies and carried out the search in several electronic databases. We selected nine randomised trials for inclusion in this review that were conducted between 1998 and 2014. The evidence in the review is up to date as of 22 June 2016. The trials involved a total of 662 participants, with 1498 teeth treated. Three studies were conducted in the USA, one in Taiwan, one in China, one in Bulgaria, one in Germany, one in Turkey and one in the UK. The population consisted of both children and adolescents in four trials, only adults in four trials, and both children/adolescents and adults in one trial.
Key results
Despite the number of included studies, only a few trials adequately and completely reported information on the primary outcomes. Two trials reported on removal of decay, and there was not enough evidence to conclude that either lasers or drills were better at decay removal. Only five trials reported on episodes of pain, which was significantly reduced in people treated with lasers. There was no difference in terms of side effects, such as inflammation or death of dental pulp, between the two interventions.
Quality of the evidence
The overall quality of the evidence for the nine studies was low. Only one study adequately randomised participants, and none of the included studies was at low risk of bias. This review highlights the need for high‐quality studies comparing laser therapy and mechanical drills in the treatment of dental decay.
Authors' conclusions
Summary of findings
| Laser compared to standard drill for caries removal in deciduous and permanent teeth | ||||||
| Patient or population: people with caries in deciduous and permanent teeth | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard drill | Laser | |||||
| Caries removal (during treatment) | 995 per 1000 | 995 per 1000 | RR 1.00 | 190 participants; 256 teeth; 256 cavity preparations | ⊕⊕⊝⊝ | |
| Pain ‐ 6‐face rating scale (moderate and high pain) (during treatment) | 760 per 1000 | 304 per 1000 | RR 0.40 | 143 participants | ⊕⊕⊝⊝ | |
| Need for anaesthesia ‐ children (during treatment) | 97 per 1000 | 24 per 1000 | RR 0.25 | 217 participants | ⊕⊕⊝⊝ | |
| Durability of restoration ‐ 6 months follow‐up | 8 per 1000 | 20 per 1000 | RR 2.40 | 236 participants; 682 teeth | ⊕⊕⊝⊝ | |
| Marginal integrity of restorations ‐ 6 months follow‐up | 7 per 1000 | 7 per 1000 | RR 1.00 | 146 participants; 306 teeth | ⊕⊕⊝⊝ | |
| Pulpal inflammation or necrosis ‐ 1 week follow‐up | 5 per 1000 | 7 per 1000 | RR 1.51 | 317 participants; 694 teeth; 752 cavity preparations | ⊕⊕⊝⊝ | |
| Pulpal inflammation or necrosis ‐ 6 months follow‐up | 4 per 1000 | 4 per 1000 | RR 0.99 | 156 participants; 508 teeth; 554 cavity preparations | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The evidence was downgraded by two levels because of very serious concern regarding the risk of bias: (1) the two studies did not report sufficient information regarding the allocation concealment (DenBesten 2001; Hadley 2000); (2) both studies were at high risk of performance bias; (3) neither of the two studies was at low risk of selective reporting bias; (4) serious concern related to funding (other bias). | ||||||
Background
Despite great improvements in oral health, dental caries still persists as a public health issue. The prevalence of dental caries among adults and school‐aged children is high worldwide, affecting nearly 100% of the population in the majority of countries (Petersen 2005; World Health Organization). Caries progressively damages dental tissues, including the dental pulp, leaving teeth weakened and with impaired function. It remains the main cause of tooth loss. The treatment of carious lesions requires the removal of decayed tissues and the provision of dental restorations.
The use of traditional removal systems such as diamond and tungsten carbide rotating instruments (Cianetti 2009; Cianetti 2014; Jackson 2004), has a high potential for triggering dental anxiety and discomfort in many children (and adults) (Bergius 1997; Smith 2003; ten Berge 1998). Although pain may be reduced by local anaesthesia, fear of the needle and of the noise and vibration of mechanical preparation remain causes of anxiety and discomfort. Moreover, the high and low rotating speed drills, used to achieve a complete decayed dentine removal, might lead to caries overexcavation with an increased risk (when caries involves the deeper dentine layers) of pulpal dental exposure or damage, or both (Ricketts 2013). These disadvantages have led to a search for new alternatives such as stepwise, partial, or no dentinal caries removal procedures that show clinical advantage over complete caries removal in the management of dentinal caries (Ricketts 2013). Other innovative dentine excavation tools/techniques include laser, air‐abrasion, sono‐abrasion, and chemo‐mechanical decayed dentine removal (Banerjee 2000), as well as the atraumatic restorative treatment (ART), which uses manual excavators and glass‐ionomer restore materials (at high fluoride concentration) for caries treatment (Frencken 2014). The laser might be classified among these new more conservative and low‐traumatic caries removal intervention procedures.
The aim of the present systematic review was to assess the scientific evidence in support of laser ablation of carious tissues in deciduous and permanent teeth.
Description of the condition
Dental caries is a pathological condition resulting from an imbalance between pathological factors that lead to demineralisation and protective factors that increase enamel acid resistance or promote its remineralising capacity, or both. Pathological factors include inadequate oral hygiene, diet with excessive (in amount and frequency) sugar intake, and reduced natural cleansing mechanisms (salivary flow, chewing, tongue motility); each of these conditions contributes to promote the microbiological plaque development on tooth surfaces that is essential, with its acid and enzymatic action, in the carious destruction of dental tissues. Protective factors include salivary flow, numerous salivary components, antibacterials (both natural and applied), fluoride from extrinsic sources, and selected dietary components (Featherstone 2004). Dental caries covers the continuum of disease ranging from early enamel demineralisation, through the initial white spot to deeper dentinal cavitations with possible pulpal involvement and tooth loss.
Description of the intervention
Laser is an acronym standing for Light Amplification by Stimulated Emission of Radiation. Laser is a device emitting a high coherence light beam with waves at single frequency (very narrow spectrum). The laser core is constituted of a material (positioned inside a highly reflective optical cavity) termed 'gain medium' with properties that allow it to amplify light deriving from the energy source of the device (Silfvast 2004). Gain medium is a material of controlled purity, size, concentration and shape, and can be of any state: gas (carbon dioxide (CO2), argon), liquid (organic dye), solid (neodymium‐doped yttrium aluminium garnet (Nd:YAG)), or plasma (semiconductors) (Silfvast 2004).
Laser light, like ordinary light, is an electromagnetic wave; there is, however, a very important fundamental difference in the mechanism of light emission by the source of ordinary light and that of the laser light. While the emission from the ordinary light source is spontaneous, and the light spreads uniformly in all directions in space, emission from the laser source is stimulated, and the emitted light beam is highly directional with extremely low divergence in space. When delivered to the target tissue site, the laser light can be (Featherstone 2000; Kutsch 1993):
-
absorbed: the laser energy interacts with the atoms in the target tissue and is generally converted to heat;
-
reflected: the laser light reflects off a surface in a direct or diffuse fashion;
-
scattered: the laser energy spreads out into a larger area; if the light is scattered, it is no longer a coherent beam and it is not delivered where needed; or
-
transmitted: the energy travels directly through tissue, causing no effect. It passes into underlying tissue.
Laser interactions with the dental tissues (enamel and dentine) fall into three major categories: interaction with the mineral (carbonated hydroxyapatite), interaction with the water and interaction with the organic material (proteins and lipids). Dentine has a much higher content of water and protein than enamel, decreasing the contribution of the mineral phase and emphasising the role of water and protein in the light absorption (Featherstone 2000). Like enamel, dentine absorption is low in the visible region, but the tissue scatters more than enamel (Featherstone 2000; Wigdor 1995), which may have negative consequences such as subsurface vaporisation, cracking, and pulpal necrosis. To explore the interactions between laser and dental tissues, investigators have operated different types of lasers whilst varying a number of parameters, including wavelength (λ), pulsed or continuous emission, pulse duration, pulse energy, repetition rate, beam spot size, delivery method, laser beam characteristics, and optical properties of tissue such as the refractive index of the tissue, the scattering coefficient (μs), the absorption coefficient (μa), and the scattering 'anisotropy' (i.e. the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions) (Featherstone 2000).
Laser safety considerations
All laser‐tissue interactions using surgical lasers carry general and specific safety concerns; it is estimated that there continue to be an average of 35 laser injuries per year. Laser safety professionals believe that this number under‐represents the actual number of injuries and that many more accidents occur per year that are not documented (McNeil 2008; Rockwell 1994). Lasers usually used in dental ablation (high‐power lasers) are considered as Class IV lasers according to USA (American National Standards Institute 2007; American National Standards Institute 2011), and international regulations (International Electrotechnical Commission 2001; International Electrotechnical Commission 2007; Parker 2007). These regulations also prescribe required safety measures, such as labelling lasers with specific warnings and wearing laser safety goggles when operating lasers. Class IV lasers (the highest and most dangerous class of laser), due to their power and wavelength, can cause directly or by diffuse reflections of the laser beam severe, permanent damage to eye or skin (laser beam risks). In addition to the risks directly associated with exposure to the beam, other risks (non‐laser beam risks) can be produced by high temperatures (fire risk), chemicals either associated with the laser or the ablation of target tissue (laser plume), and electrical device (electrical shock) (Sweeney 2008; Sweeney 2009).
How the intervention might work
Caries progressively destroys the tissues of the tooth, so the treatment of dental caries includes removal of the decayed material and restoration of the surface of the affected tooth with restorative material (fillings). Traditional treatments comprise the use of drills, alone or in combination with metal hand instruments. However, the use of mechanical means may lead to overexcavation and result in perforation of the pulp chamber or, to the contrary, to incomplete removal of carious dentine (Banerjee 2000; Celiberti 2006). The first lasers used for dental ablation (ruby laser (λ = 0.694 µm), Nd:YAG laser (λ = 1.064 µm), holmium‐doped yttrium aluminum garnet (Ho:YAG) laser (λ = 2.120 µm), and CO2 laser (λ = 9.600 µm)) provoked increased temperature in the dental pulp as well as microcracks and carbonisation (Goldman 1965; Melcer 1984; Melcer 1986). During the mid‐1990s, researchers examined the safety and utility of erbium‐doped yttrium aluminium garnet (Er:YAG) laser for treatment of dental tissues, showing that thermal damage was minimal when appropriate settings were used and an adequate water cooling spray was employed (Li 1992). To achieve caries removal, the laser wavelength must be such that there is a major interaction with either the mineral or the water or both, unless there is plasma‐mediated ablation by ultrashort pulses (Featherstone 2001; Hibst 1989). In particular, the Er:YAG laser and the erbium: yttrium‐scandium‐gallium‐garnet (Er:YSGG) laser irradiations (λ = 2.940 µm and λ = 2.790 µm, respectively) are strongly absorbed by the water (the Er:YSGG is additionally absorbed by the hydroxyl ion (OH‐) group in the carbonated hydroxyapatite mineral of the tooth) so that the primary mechanism of action is to heat the water at the surface and the subsurface, thereby expanding it and causing tissue to be exploded out from the surface (Featherstone 2001; Fried 1996; Hibst 1989). For optimum caries ablation, the pulse duration of the appropriate wavelength laser should be matched approximately with the 'thermal relaxation time', that is the time within which the bulk of the laser energy from a pulse would be absorbed by the tissue. Times dramatically shorter than the thermal relaxation time will provide excessive energy densities, and pulse durations markedly in excess of the thermal relaxation time will distribute unnecessary energy deeper into the tissue (Fried 1998; Fried 2001; Zuerlein 1999).
Why it is important to do this review
In recent years, the introduction of adhesive systems has greatly altered cavity preparation techniques and, as a result, conservative tooth tissue ablation techniques have gained popularity. These systems minimise the requirement for retention and resistance form for cavity preparation. Moreover, unintentional side effects, such as pain, noise, and vibration, are the main factors leading to participant discomfort and fear during conventional dental treatment (Banerjee 2000). Many studies have shown that fear of the rotary burs is often associated with dental anxiety (Alvesalo 1993; Bedi 1992a; Bedi 1992b; Berggren 1984), and although in recent decades (in the UK) an increasing number of dentate adults claim they visit their dentist regularly, one of the main barriers to regular dental care is 'drill fear' (Kelly 2000). Consequently, new and alternative strategies in the treatment of dental caries are warranted to reach a maximal conservation of tissues while minimising the risk of overexcavation and perforation of pulp chamber and patient discomfort and fear related to the use of traditional rotary instruments (Celiberti 2006; Clarkson 2001; Takamori 2003). Among these new emerging techniques, laser, in particular Er:YAG laser, are gaining acceptance by the dental profession and the general public (Chimello 2008; Dommisch 2008; Keller 1998). Randomised controlled trials comparing the efficacy of Er:YAG laser with conventional treatment for caries have shown no significant differences in caries removal (DenBesten 2001; Dommisch 2008), cavity preparation (DenBesten 2001), or pulpal damage (DenBesten 2001; Keller 1998), whilst contradictory results were found on the need for use of local anaesthesia (DenBesten 2001; Keller 1998), treatment experience (DenBesten 2001; Dommisch 2008), and treatment time (DenBesten 2001; Dommisch 2008; Keller 1998).
Moreover, during the removal of carious lesions, it is very difficult to know exactly when to stop excavation because of an apparent lack of objective clinical markers. Whereas a significant correlation has been found between dentine hardness and the level of bacterial infection, the same correlation could not be found for tissue colour (Kidd 1996; Kidd 2004). This issue is relevant when considering lasers, as they have a lack of tactile feedback as a guide to caries removal compared with hand and rotary instruments.
We therefore aimed to produce a high‐quality systematic review that investigated the effects of lasers for the removal of dental caries.
Objectives
The main objective of the review was to compare the effects of laser‐based methods to conventional mechanical methods for removing dental caries in deciduous and permanent teeth.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials irrespective of their publication status or language. We also considered split‐mouth trials and cluster randomised trials for inclusion in the review.
Types of participants
We included children and adults with caries, irrespective of race, gender, socioeconomic status, health status, or geographical location. We excluded studies in which participants were selected on the basis of special (general or oral) health conditions. We considered every type of tooth: deciduous and permanent teeth, restored and unrestored teeth, vital or non‐vital/root‐filled teeth.
Types of interventions
We included studies investigating any laser specific to the removal of carious lesions, such as erbium lasers, with different radiation parameters compared with conventional mechanical caries removal techniques (handpiece with a bur) or other systems (e.g. chemo‐mechanical, sono‐abrasion, air‐abrasion). We used no restrictions regarding restorative materials.
Types of outcome measures
Primary outcomes
-
Caries removal (confirmed by clinical, radiological, or other validated assessment tools) (Bader 2001).
-
Episodes of pain (during and after treatment) defined as a clear pain feeling reported by participant during the treatment to distinguish from unpleasant feelings such as vibration, noise, or smell.
Secondary outcomes
-
Marginal integrity of restoration.
-
Durability of restoration: survival time of restoration (in months) from the time of placement.
-
Recurrent caries.
-
Pulpal inflammation or necrosis (at one‐year follow‐up).
-
Participant discomfort.
-
Need for anaesthesia (defined as the requirement by the participant during or before the treatment to receive local anaesthetic injection in order to avoid feelings of pain caused by dentist's used of ablative devices).
-
Operator preference/fatigue in operator.
-
Participant preference.
-
Duration of treatment.
Search methods for identification of studies
We undertook a comprehensive search to identify all relevant studies, regardless of language or publication status (published, unpublished, and ongoing).
Electronic searches
We developed detailed search strategies for each database to identify studies to be included in or considered for this review. These were based on the search strategy developed for MEDLINE but revised appropriately for each database (Appendix 1). The search strategy combined the subject search with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying reports of randomised trials in MEDLINE (2008 revision), as published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We searched the following databases:
-
Cochrane Oral Health's Trials Register (searched 22 June 2016) (Appendix 2);
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 5) in the Cochrane Library (searched 22 June 2016) (Appendix 3);
-
MEDLINE Ovid (1946 to 22 June 2016) (Appendix 1);
-
Embase Ovid (1980 to 22 June 2016) (see Appendix 4);
-
ProQuest Dissertations and Theses (1980 to 22 June 2016) (Appendix 5);
-
Zetoc (limited to conference proceedings) (1993 to 22 June 2016) (Appendix 6);
-
ISI Web of Knowledge (limited to conference proceedings) (1990 to 22 June 2016) (Appendix 7).
Searching other resources
We searched the following databases for ongoing trials:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 22 June 2016) (Appendix 8);
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/; searched 22 June 2016) (Appendix 9).
We manually checked the reference lists of all the included articles to identify any additional studies. We contacted organisations, researchers, and experts known to be involved in the field by email in an effort to trace unpublished or ongoing studies. We also contacted manufacturers to identify any ongoing or unpublished studies. Where we found multiple publications of a single trial, we used only the first publication except in the case that additional data were provided, such as delayed outcome results.
Data collection and analysis
Selection of studies
We imported the records obtained from each database into the bibliographic software package EndNote (EndNote X7) and merged them into one core database, removing duplicate records. We obtained all potentially relevant reports identified when searching other sources (reference lists of relevant trials, reviews, articles, and textbooks) and manually entered the records located from searching these (non‐electronic) sources into EndNote.
Two review authors (Guido Lombardo (GL) and Iosief Abhraha (IA)) independently and in duplicate assessed the titles and abstracts of all trial reports identified by the electronic searching outlined above. We obtained hard copies of the full text of studies that possibly fulfilled the inclusion criteria. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted (Alessandro Montedori (AM)).
We requested further information from the authors of papers containing insufficient information. Where we identified more than one publication of a trial, we reviewed all publications and included the paper with the first publication date as a primary version. We then performed data extraction and 'Risk of bias' assessment for all studies meeting the inclusion criteria. We recorded studies rejected at this or subsequent stages and reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (GL, IA) independently and in duplicate extracted data, and resolved disagreements by a consensus meeting with a third review author (AM). We tested the data extraction sheet in advance. We collected information regarding trial characteristics (year of publication, country of the study, methodological quality items of the study), participants' characteristics (number of participants, age range, sex), intervention characteristics (type of laser, laser beam characteristics), comparator characteristics (type of comparator: none, placebo, or conventional therapy), and outcomes characteristics (primary and secondary outcomes were considered as stated above). We recorded any adverse events reported in the trials. We contacted study authors to provide missing data.
Assessment of risk of bias in included studies
Two review authors independently and in duplicate assessed the risk of bias of all studies considered eligible for the review using Cochrane's 'Risk of bias' tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were resolved by a consensus meeting with a third review author.
We assessed the following seven domains for each included study.
-
Random sequence generation (selection bias) (Savovic 2012; Wood 2008).
-
Allocation concealment (selection bias) (Savovic 2012; Wood 2008).
-
Blinding of participants and personnel (performance bias) (Savovic 2012; Wood 2008).
-
Blinding of outcome assessment (detection bias) (Savovic 2012; Wood 2008).
-
Incomplete outcome data (attrition bias) (Abraha 2015).
-
Selective reporting (reporting bias) (Chan 2004; Macura 2010).
-
Other bias.
For each domain we assigned a judgement of the corresponding risk of bias: low risk of bias, unclear risk of bias, or high risk of bias; and supported this judgement with a description of what was reported to have occurred in the study in a 'Risk of bias' table. We handled additional considerations for split‐mouth trials according to the recommendations of Sections 16.3.2 and 16.4.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
After taking into account the additional information provided by the authors of the trials, we assigned studies into the following categories.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
We constructed a 'Risk of bias' table for each included study and presented results graphically.
Measures of treatment effect
Where possible, for each trial, we calculated risk ratios (RR) with 95% confidence intervals (CI) for all prespecified, dichotomous outcomes. We calculated mean difference (MD) for continuous data. In the case of studies of split‐mouth design, we aimed to calculate log risk ratio and standard error separately for each outcome.
Unit of analysis issues
We checked included studies for unit of analysis errors. We handled any unit of analysis issues in split‐mouth trials according to the recommendations of Sections 16.3 and 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We identified no cluster‐randomised trials.
Dealing with missing data
We did not consider missing data as a reason to exclude a study from the review, and planned to use methods for imputing missing data outlined in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where necessary, we attempted to contact the study authors to request more information. Where we judged data to be 'missing at random', we included only the available data in the analysis and ignored the missing data.
Assessment of heterogeneity
We assessed heterogeneity according to the approach described in Section 9.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the Chi2 test and I2 statistic to assess heterogeneity. We considered heterogeneity to be statistically significant if the P value was less than 0.1. The Cochrane Handbook for Systematic Reviews of Interventions provides a rough guide to the interpretation of the I2 statistic: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
For future updates where more than 10 studies are included in the review, we plan to use a funnel plot to explore publication bias (Egger 1997). We will perform linear regression using the approach described by Egger and colleagues to determine the funnel plot asymmetry (Egger 1997).
Data synthesis
Where there were studies of similar comparisons reporting the same outcome measures, we carried out meta‐analysis using Review Manager software according to Cochrane statistical guidelines (RevMan 2011). We combined RRs for dichotomous data, and MDs for continuous data, using random‐effects models. We combined data from split‐mouth studies with data from parallel‐group trials using the method suggested by Elbourne (Elbourne 2002), employing the generic inverse variance method in Review Manager 5 (RevMan 2011).
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analysis for type of participants (children, adults) but not for type of tooth (deciduous, permanent) or type of intervention (laser and its beam characteristics).
Sensitivity analysis
We did not find a sufficient number of trials that contributed to a meta‐analysis. Our a priori assumption was to undertake sensitivity analysis based on methodological items of study quality and for potential sources of heterogeneity specified a priori as follows: excluding/including unpublished studies; excluding/including studies assessed as being at unclear or high risk of bias for allocation concealment; excluding/including studies assessed as being at unclear or high risk of bias for blinding of outcomes assessment; excluding/including studies assessed as being at unclear or high risk of bias for incomplete outcome data. In future updates, we plan to perform sensitivity analysis to test how different assumptions about missing data and data extracted from trials with particular study design (split‐mouth, cluster‐randomisation) may affect the results.
Presentation of main results
We developed a summary of findings Table for the main comparison for the primary outcomes of the review using GRADEproGDT software (GRADEproGDT 2014). We assessed the quality of the body of evidence with reference to the overall risk of bias of the included studies, directness of the evidence, inconsistency of the results, precision of the estimates, risk of publication bias, and magnitude of the effect. We categorised the quality of the body of evidence for each of the primary outcomes as high, moderate, low, or very low.
Results
Description of studies
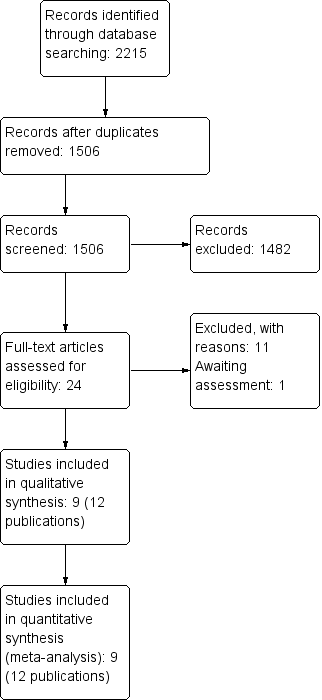
Results of the search
The search strategy identified 2215 records. After removing duplicates and assessing titles and abstracts, we evaluated 24 full‐texts for inclusion in the review. Nine trials (with 12 publications) fulfilled the inclusion criteria and were contained in the final analysis (Belcheva 2014; DenBesten 2001; Evans 2000; Hadley 2000; Harris 2000; Keller 1998; Liu 2006; Yazici 2010; Zhang 2013) (Figure 1). Study characteristics are described in the Characteristics of included studies table. All of the trials were reported in English except Zhang 2013, for which the authors provided an adequate summary in English.

Study flow diagram.
Included studies
Study design
The nine included trials were published between 1998 and 2014. Six studies were split‐mouth randomised controlled trials (Evans 2000; Hadley 2000; Keller 1998; Liu 2006; Yazici 2010; Zhang 2013), and three studies were parallel‐group randomised trials (Belcheva 2014; DenBesten 2001; Harris 2000).
Overall 1498 teeth were treated, 777 only with lasers, 732 only with drills, and 12 with both intervention techniques used in the same tooth on separate caries.
Participants
The nine trials recruited 662 participants, ranging from 27 to 123 participants per study. The age range of the participants was 3.5 to 84 years. Four studies involved only children and adolescents (Belcheva 2014; DenBesten 2001; Liu 2006; Zhang 2013); four studies included only adults (Hadley 2000; Harris 2000; Keller 1998; Yazici 2010); and one study included participants between 3.5 to 68 years of age (Evans 2000).
In seven studies (DenBesten 2001; Hadley 2000; Harris 2000; Keller 1998; Liu 2006; Yazici 2010; Zhang 2013), the male percentage ranged from 22% to 63%, whereas no information regarding gender was reported in the remaining two studies (Belcheva 2014; Evans 2000).
The trials differed in terms of dentition, types of teeth, and caries treated. Four trials examined only permanent teeth (Hadley 2000; Harris 2000; Keller 1998; Yazici 2010), while five studies evaluated both primary and permanent teeth (Belcheva 2014; DenBesten 2001; Evans 2000; Liu 2006; Zhang 2013). None of the studies dealt with primary teeth alone.
In terms of types of teeth, one study examined only maxillary anterior dental elements (Liu 2006); one trial investigated only third permanent molars (Harris 2000); one study considered anterior teeth and molars (Zhang 2013); one trial examined first and second permanent molars (Yazici 2010); one study evaluated unspecified molars (Belcheva 2014); and one trial assessed molar, premolar, and incisors (Evans 2000). Three studies did not provide information about the type of teeth treated (DenBesten 2001; Hadley 2000; Keller 1998).
In terms of caries, occlusal and proximal caries were described in Keller 1998, while occlusal and buccal caries were reported in Harris 2000. Caries dental location was not described in Zhang 2013. In the remaining six studies, instead of caries location, the type of prepared cavity for caries removal, using Black's classification, was described (Belcheva 2014; DenBesten 2001; Evans 2000; Hadley 2000; Liu 2006; Yazici 2010). Class I cavities were described in Yazici 2010; Classes I and II in both publications of Belcheva 2014; Classes III and IV in Liu 2006; Classes I and V in DenBesten 2001; Classes I, II, III and V in Evans 2000; and Classes I, II and V in Hadley 2000.
The depth of caries varied from enamel alone to deeper layers of dentine in four trials (DenBesten 2001; Evans 2000; Keller 1998; Zhang 2013). In one study, the depth of caries reached the upper half layers of dentine (Belcheva 2014), and in another two trials, the caries did not involve the dentine (Harris 2000; Yazici 2010).
None of included studies distinguished between primary and secondary caries; only one reported as an exclusion criterion the presence of restorations (need to justify secondary caries) in the analysed teeth (Harris 2000).
Treatment
In six of the nine trials, the same type of laser was employed: erbium‐doped yttrium aluminium garnet (Er:YAG) laser (Belcheva 2014; DenBesten 2001; Evans 2000; Keller 1998; Liu 2006; Zhang 2013). However, device characteristics varied across the studies in terms of wavelength (from 2.78 µ to 2.94 µ). These parameters did not overlap in any of the six included trials. Among the remaining three studies, two trials employed an erbium, chromium: yttrium‐scandium‐gallium‐garnet (Er,Cr:YSGG) laser (2.78 µ; 0 mJ to 300 mJ; 20 Hz; 140 µsec) (Hadley 2000), and one trial used a neodymium‐doped yttrium aluminium garnet (Nd:YAG) laser (1.06 µ; 100 mJ; 2 W; 10 Hz to 20 Hz; 150 µsec) (Harris 2000). In four studies (five papers) laser light ray was cooled with a water spray alone (Belcheva 2014; Evans 2000; Keller 1998; Liu 2006), while in four studies the cooling system was composed by both air and water spray together (DenBesten 2001; Hadley 2000; Yazici 2010; Zhang 2013). In one study laser was used without any cooling system (Harris 2000).
The number of operators varied across the studies. The greatest number of operators was described in Evans 2000, where 15 dentists (nine general dental practitioners, five hospital‐based dentists, and one community dental practitioner) trained to use lasers to treat caries were recruited. Hadley 2000 described two operators qualified to use lasers. Liu 2006, Yazici 2010 and Zhang 2013 reported that all dental treatments were performed by only one dentist. DenBesten 2001 reported the presence of operators, but did not provide the number, and three studies did not provide sufficient information (Belcheva 2014; Harris 2000; Keller 1998).
Setting
Three studies were performed in the USA (DenBesten 2001; Hadley 2000; Harris 2000), one in the UK (Evans 2000), one in Germany (Keller 1998), one in Taiwan (Liu 2006), one in Bulgaria (Belcheva 2014) and one in Turkey (Yazici 2010). Seven studies were conducted at university clinics (DenBesten 2001; Hadley 2000; Harris 2000; Keller 1998; Liu 2006; Yazici 2010; Zhang 2013), one trial related to two publications of Belcheva 2014 was carried out in a paediatric hospital department, and one was performed in mixed centres located in hospitals and in private dental offices (Evans 2000). Moreover, while four studies were multicentric (DenBesten 2001; Evans 2000; Harris 2000; Keller 1998), five studies (six papers) were monocentric (Belcheva 2014; Hadley 2000; Liu 2006; Yazici 2010; Zhang 2013).
Outcomes evaluated
Caries removal
Four studies evaluated caries removal (DenBesten 2001; Hadley 2000; Harris 2000; Zhang 2013). In all four studies, the evaluation of caries removal was conducted before the prepared cavities were filled with restoration materials. The removal of caries was classified dichotomously as 'complete' or 'incomplete'. In two studies, the evaluation of caries removal was performed only on caries that were not previously treated (DenBesten 2001; Harris 2000). In addition, in DenBesten 2001, the removal of caries was established by operators through tactile and visual examination by a trained, independent evaluator. When any procedure was considered incomplete, it was repeated until an agreement between evaluator and operator was reached. In Hadley 2000, 66 pairs of teeth were treated. During the treatment the completeness of caries removal was assessed by means of a blinded evaluation. Until caries removal was not assessed as complete, the decayed tissues removing procedure was reapplied. In Harris 2000, no information was provided about diagnostic instruments or the methods used, although it was reported that two blinded evaluators assessed the degree of caries elimination. However, no data was reported. Similarly, in Zhang 2013, although the caries removal assessment was described no results were reported in terms of caries removal.
Episodes of pain and the need for anaesthesia
Four trials assessed both outcomes (DenBesten 2001; Keller 1998; Liu 2006; Zhang 2013). In Keller 1998 pain was reported as a component of several participant's discomfort items including unpleasant sensations of noise, smell and vibration. Another trial (Belcheva 2014) reported only the outcome pain felt by participants.
Belcheva 2014 measured pain through a six‐face rating scale. DenBesten 2001 classified the level of pain by means of a six‐face pain scale with values from 0 to 10. Liu 2006 measured pain with a modified simple four‐face scale, whereas Zhang 2013 ranked the pain felt by participants during dental care with a six‐face scale from 0 to 5 (from "no or little pain" to "more than moderate pain"). The evaluation of pain was performed only among participants who had not received local anaesthesia before treatment.
In three split‐mouth studies (Keller 1998; Liu 2006; Zhang 2013), the participants expressed their level of pain or discomfort, during each intervention, in a direct drill‐versus‐laser comparison, whereas in the remaining two trials, each participant, randomly allocated to either the laser or drill group, expressed their level of pain for either ablation approach, without a direct comparison between the two types of intervention (Belcheva 2014; DenBesten 2001).
The four trials that evaluated need for anaesthesia evaluated children or adolescents. In DenBesten 2001 and Liu 2006 need for anaesthesia was assessed during treatment and participants received anaesthesia on request; in Keller 1998 participants might ask for local anaesthesia during preparation; whereas in Zhang 2013 all treatments were started with anaesthesia and during treatment children had the possibility of asking for local anaesthesia.
Marginal integrity and durability of restorations (retention)
The marginal defect represents the first phase of restoration failure by constituting a fissure where plaque deposition is favoured. The enhanced plaque occurring between filling and preparation margins could lead to an increased risk of secondary caries (Dennison 2012). Three studies reported data about marginal integrity of restorations (Hadley 2000; Yazici 2010; Zhang 2013). These studies, together with Harris 2000, also evaluated the durability of restorations. Hadley 2000 and Yazici 2010 performed objective and tactile (with a dental explorer) evaluation to assess both marginal integrity and restoration durability, while Zhang 2013 provided insufficient information about the method and tools used for this evaluation.
Study follow‐up visits varied from 1 week to 2 years. Zhang 2013 performed three follow‐ups, at 3, 6 and 12 months. Yazici 2010 carried out four follow‐up visits, at 6, 12, 18 and 24 months. Hadley 2000 visited the participants at baseline and 1 and 6 months, while Harris 2000 performed four recall visits at 1 week and 1, 3 and 6 months after treatment.
Recurrent caries
Two studies assessed recurrent caries that affected dental tissue restored with filling materials (Hadley 2000; Yazici 2010). In both studies, the evaluation was made at a single follow‐up time. Hadley 2000 re‐evaluated participants at 6 months, whereas Yazici 2010 reassessed participants at 2 years. Both authors assessed this outcome through a two‐point scale (caries or no caries). No radiographs, particularly useful for diagnosis of secondary caries affecting the inner dentine layers under the dental restoration or next to its proximal margins, were performed at any follow‐up visit. The presence of recurrent caries was evaluated by blinded assessors in Hadley 2000, and by two independent investigators not involved with the treatment procedures in Yazici 2010.
Pulpal inflammation or necrosis
Four studies reported information about dental pulpal health during cavity preparation and caries removal (DenBesten 2001; Hadley 2000; Harris 2000; Keller 1998). Two trials described cases of pulpal inflammation (DenBesten 2001; Harris 2000), and another two trials reported cases of pulpal necrosis (Hadley 2000; Keller 1998). DenBesten 2001 evaluated dental pulpal inflammation at three follow‐ups: 1 week and 1 month after treatment, ice/ethylene‐oxide was applied to the tooth (thermal test), while at the 3‐month follow‐up, in addition to the thermal test, an X‐ray evaluation was performed to verify possible apical lesions. Harris 2000 employed a thermal test using an ice stick at 0° C and an electrical pulp tester, as well as performing X‐rays over four follow‐up visits (1 week and 1, 3 and 6 months). Hadley 2000 examined tooth pulpal vitality with an electrical pulpal tester during two recall visits at 1 and 6 months. Finally, Keller 1998 employed a thermal test using ice, only at the end of treatment. All of the studies evaluated pulpal vitality at baseline before treatment initiation.
Participant discomfort
Four studies evaluated participant discomfort using different methods (Evans 2000; Hadley 2000; Keller 1998; Liu 2006). In these studies, discomfort was defined as sensations of vibration, smell and noise experienced by participants during treatment. Two studies classified participants' treatment perception with a rating scale ranging from "comfortable" to "very uncomfortable"; Keller 1998 used a three‐level scale, while Hadley 2000 employed a five‐level discomfort scale based on a standard Likert interval from 1 to 5 (1 = no discomfort, 2 = mild discomfort, 3 = moderate discomfort, 4 = high level of discomfort, 5 = extreme discomfort). In another trial, Liu 2006 measured participants' level of discomfort through a dentist's assessment of the participant's head and body movements. In the last study (Evans 2000), each participant at the end of treatment directly expressed which of the two interventions, laser or drill, had been more uncomfortable. In the second publication of Belcheva 2014, the participants were asked to fill a questionnaire describing discomfort experienced due to vibration, noise and smell during both laser and drill treatments. Moreover, in addition to the aforementioned discomfort factors, the sight of the drill and laser and the taste experienced during treatment were also assessed by Belcheva 2014.
Ablation preference of operator/operator fatigue
Only one trial evaluated dentist preference (Evans 2000). In this study, 11 dentists reported their preference for lasers or drills to remove caries. Each dentist expressed their preference through a rating scale ranging from 0 to 100 and reported the difficulties they experienced with both interventions.
Participant preference
Three studies reported sufficient information about participant preferences regarding the intervention received (Evans 2000; Liu 2006; Zhang 2013). Only participants who did not require local anaesthesia before or during the treatment were asked to express their preference, following completion of treatment. Liu 2006 did not report information about the method used to record participant preference (e.g. filling a form), or whether an independent evaluator or the same treatment operator performed the evaluation. In Evans 2000, each participant indicated his or her choice between laser and drill for future treatment on a specific questionnaire. Finally, Zhang 2013 reported that the examined children indicated their preference through a specific questionnaire where they also answered other questions about their feelings (pain, discomfort, or unpleasant sensations).
Duration of treatment
Four studies assessed the duration treatment of both laser and drill interventions (DenBesten 2001; Keller 1998; Liu 2006; Zhang 2013). Only Keller 1998 indicated the tool (stopwatch) used to measure the time employed to realise a complete cavity preparation. The authors of this study also specified the range of time used to calculate the duration of treatment: from first laser pulse or first contact between bur until the last laser pulse or last contact of the bur with the preparation. The remaining three studies generically referred to the duration of treatment or time average employed to perform a cavity preparation without specifying the starting and finishing treatment times (DenBesten 2001; Liu 2006; Zhang 2013).
Funding
Five studies declared that they have received financial support for their trials from device manufacturers of laser (DenBesten 2001; Evans 2000;Hadley 2000; Harris 2000; Liu 2006). No mention of funding was reported for the remaining trials (Belcheva 2014; Keller 1998; Yazici 2010; Zhang 2013).
Excluded studies
We excluded 11 studies for the following reasons: two studies were not clinical but 'in vitro studies' (Neves 2011; Sirin Karaarslan 2012); one study was a controlled clinical trial (Bohari 2012); one study was a narrative review (Najeeb 2016); four studies used a questionable method of randomisation, given that participant allocation depended on the availability of laser instrumentation (Cozean 1997; Cozean 1998a; Cozean 1998b; Pelagalli 1997); two studies were not available in full text and attempts to contact the authors failed (White 1995; White 1996); and one randomised trial was excluded due to its inadequate unit of analysis: each caries was ideally divided in two halves and each of them was randomly treated with laser or with a drill (Dommisch 2008).
Risk of bias in included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
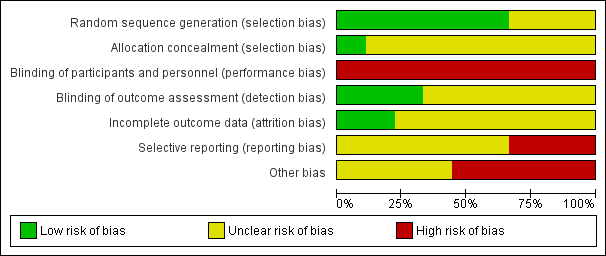
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Six studies reported adequate means to randomise participants (DenBesten 2001; Evans 2000; Keller 1998; Liu 2006; Yazici 2010; Zhang 2013). Evans 2000 used a computer‐generated random number list placed in opaque sealed envelopes, therefore we considered the allocation concealment for this study to be adequate. In DenBesten 2001 the generated number and assignment was placed in a sealed envelope, hence concealed (information obtained by contacting authors). However, it was unclear whether the envelopes were opaque or serially numbered, therefore we considered the allocation concealment for this study to be unclear. Keller 1998 employed a computer program to generate the random number sequence, but provided no detail regarding allocation concealment. Yazici 2010 similarly used a random number table but no details were included on allocation concealment. Zhang 2013 and Liu 2006 used a coin flip to generate the randomised list, however although we considered this to be an adequate method to generate the random sequence, it was unclear whether the allocation was concealed. The remaining three trials reported insufficient data to make judgements about sequence generation and allocation concealment and were therefore considered to have an unclear risk of selection bias (Belcheva 2014;Hadley 2000;Harris 2000). In conclusion, we considered only one study to be at low risk of selection bias.
Blinding
Given the nature of the intervention, it was not possible to blind participants or personnel. Hence, we considered all of the included studies to be at high risk of performance bias. In terms of blinding of the outcome assessor, three studies clearly reported the blinding of assessors of the quality of cavity preparation as well as the integrity of restorations (Hadley 2000; Harris 2000; Yazici 2010); hence, we considered these studies as at low risk of detection bias. Two studies reported that the outcome evaluators were independent, but provided no clear statement on the blinding procedure and were considered to be at unclear risk of detection bias (DenBesten 2001; Zhang 2013). The remaining four studies reported no information about blinding of outcome evaluators and were therefore also considered to be at unclear risk of detection bias (Belcheva 2014; Evans 2000; Keller 1998; Liu 2006).
Incomplete outcome data
Two studies were considered at low risk of bias. Liu 2006 evaluated episodes of pain and included in the analysis all the respective participants and was therefore considered to be at low risk of attrition bias. Yazici 2010 did not report any of the primary outcomes but there was no apparent attrition.
All the remaining studies reported either dropouts or incomplete data but it was not possible to determine the level of attrition bias and were deemed unclear. DenBesten 2001 was the only study to evaluate both primary outcomes. The investigators were able to perform caries removal in both groups of participants with no missing data. However, evaluation of episodes of pain was performed only on participants that did not receive any anaesthesia: 78 out of 82 in the laser group and 31 out of 42 in the drill group. Hence, we judged this study as at unclear risk of bias for incomplete outcome data. Belcheva 2014 examined episodes of pain (but not caries removal). As the study provides only descriptive results regarding pain, we considered it to be at unclear risk of attrition bias. Harris 2000 reported a dropout of three cases but it was unclear from which of the two samples they combined for analysis. Hadley 2000 reported an overall dropout of nine participants during the three follow‐ups after treatment (30 days, 3 and 6 months). In Keller 1998, for the outcome participant discomfort, the scoring of 13 participants was excluded from the study due to incomplete data. In Evans 2000, 5 of 82 cases were not completed. In Zhang 2013 at 6 months' visit, 32 recalled children, 4 children dropped out, and at 12 months' visit a further 8 children were lost to follow‐up.
Selective reporting
One study evaluated both primary outcomes but did not provide sufficient data for episodes of pain and was thus judged at unclear risk (DenBesten 2001). One study provided results for caries removal but not for pain and was thus judged at unclear risk (Hadley 2000). Three studies that evaluated only episodes of pain but not caries removal were also considered to be at unclear risk of reporting bias (Belcheva 2014; Keller 1998; Liu 2006). Harris 2000 did not report on episodes of pain and although it reported on caries removal in the methods, no data was provided, and therefore was judged at high risk of reporting bias. Similarly, in Zhang 2013, although the caries removal assessment was described no results were reported and was thus judged at unclear risk. The remaining two trials did not report any of the primary outcomes of interest and were therefore considered to be at high risk of selective reporting bias (Evans 2000; Yazici 2010).
Other potential sources of bias
Five studies declared that they have received financial support for their trials from device manufacturers of laser. None of these studies explicitly reported that the sponsors were not involved in the analyses which arises serious concern of other bias (DenBesten 2001; Evans 2000;Hadley 2000; Harris 2000; Liu 2006). The remaining studies did not declare whether they received funding or not and were deemed at unclear risk for other bias (Belcheva 2014; Keller 1998; Yazici 2010; Zhang 2013).
Effects of interventions
Seesummary of findings Table for the main comparison.
Primary outcomes
Caries removal
Of the four studies that evaluated caries removal (DenBesten 2001; Hadley 2000; Harris 2000; Zhang 2013), only two reported quantitative data that allowed meta‐analysis (DenBesten 2001; Hadley 2000). In this studies no cases of residual caries were described in either intervention group. Results from pooling the data showed no evidence of a difference between treatments (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.99 to 1.01; 2 studies; 256 treated caries; P = 0.75; I2 = 0%) (Analysis 1.1; Figure 4)

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.1 Caries removal (clinical).
Episodes of pain
Five trials evaluated episodes of pain during treatment (Belcheva 2014; DenBesten 2001; Keller 1998; Liu 2006; Zhang 2013).
Belcheva 2014 and Zhang 2013 assessed pain through a six‐face rating scale. Results from pooling the outcome data regarding moderate or high pain were significantly in favour of the laser treatment with moderate, but not statistically significant, heterogeneity (RR 0.40, 95% CI 0.28 to 0.57; P < 0.001; heterogeneity: Chi2 = 1.99, degrees of freedom (df) = 1 (P = 0.16); I2 = 50%) (Analysis 1.2; Figure 5).
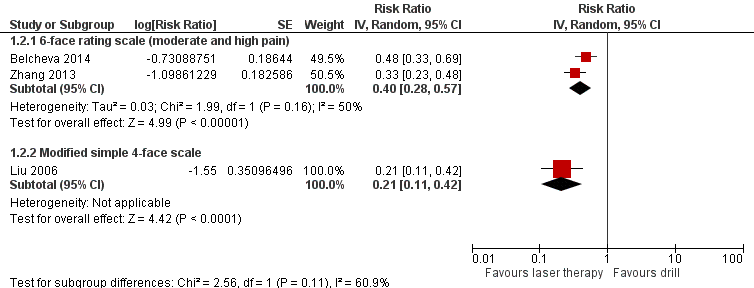
Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.2 Pain.
DenBesten 2001 also used a six‐face rating scale to evaluate pain with values ranging from 0 to 10. The authors reported that there was no difference between the two comparison groups. However, they did not provide any data, which prevented us from carrying out meta‐analysis.
Liu 2006 evaluated pain with a simple modified face scale for self evaluation (four levels: no pain, mild pain, moderate pain and severe pain). The results showed that laser limited the incidence of pain compared to drill (RR 0.21, 95% CI 0.11 to 0.42; P < 0.001) (Analysis 1.2; Figure 5).
In Keller 1998 pain was part of the composite outcome "discomfort" that in addition to pain included unpleasant sensations of noise, smell and vibration. During cavity preparation 74% of the participants considered laser preparation to be comfortable compared to 31% during mechanical preparation (P = 0.002). The study authors did not provide complete data regarding pain except for reporting that the reason for discomfort during laser treatment was mostly reported as "pain" (20) and "bad smell" (13).
Secondary outcomes
Marginal integrity of restorations
Three studies dealt with marginal integrity of restorations (Hadley 2000; Yazici 2010; Zhang 2013), with follow‐up visits at 1 month (1 trial), 6 months (3 trials), 12 months (2 trials), 18 months (1 trial) and 24 months (1 trial). Results from the meta‐analysis (for the 6‐, 12‐ and 24‐month follow‐ups) showed no difference in marginal integrity of restorations between laser and drill (Analysis 1.3).
Durability of restorations
Four studies assessed this outcome, with follow‐ups that ranged from 1 week to 2 years (Hadley 2000; Harris 2000; Yazici 2010; Zhang 2013). Results from pooling the data at 6 months (4 trials), 1 year (2 trials), and 2 years (1 trial) showed no evidence in favour of either of the treatments being compared (Analysis 1.4; Figure 6).

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.4 Durability of restoration.
Recurrent caries
Two trials reported data on the prevalence of secondary caries (Hadley 2000; Yazici 2010). The assessment was made at a 6 months' and a 2 years' follow‐up respectively. No occurrence of caries was reported in either study.
Pulpal inflammation or necrosis
Four studies reported data on pulpal health after dental treatment (DenBesten 2001; Hadley 2000; Harris 2000; Keller 1998). The overall meta‐analysis did not show any evidence of a difference in pulpal inflammation or necrosis between laser therapy and drill (RR 1.29, 95% CI 0.32 to 5.14; 1202 teeth treated; P = 0.72; I2 = 0%) (Analysis 1.5; Figure 7).

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.5 Pulpal inflammation or necrosis.
Participant discomfort
Five studies reported data on participant discomfort in terms of vibration, smell and noise experienced during treatment (Belcheva 2014; Evans 2000; Hadley 2000; Keller 1998; Liu 2006). Two trials evaluated participant discomfort using a rating scale completed by each participant at the end of treatment (Hadley 2000; Keller 1998).
Keller 1998 used a three‐degree rating scale to assess discomfort in 90 adult participants: 1 participant (1%) in the laser group versus 16 participants (14.4%) in the drill group defined the treatment "very uncomfortable"; 23 participants (20.7%) in the laser group versus 46 participants (41.4%) in the drill group defined the treatment "uncomfortable". Thirteen participants were excluded due to incomplete data. An analysis showed that the rate of participant discomfort was significantly lower with the laser therapy than with drill treatment (Analysis 1.6).
Hadley 2000 used a five‐degree rating scale to assess discomfort in 66 adult participants. In the laser group 98.5% of participants did not feel discomfort while 1.5% of them felt a moderate discomfort; in the drill group 87.9% of participants did not complain of any unpleasant feeling while 12.1 % of them felt some mild or moderate discomfort. A statistically significant difference was reported between the two interventions, with laser as being more comfortable (P = 0.007).
Liu 2006 performed an evaluation of children's behaviours in the dental chair based on a dentist's assessment. Thirty‐eight out of 40 children (95%) showed no head movements when treated with a laser versus only 3 children (7.5%) treated with a drill. The average head movement was 0.05 times for the laser group and 0.925 times for the drill group. Regarding body movements, the average was 0.025 times when children were treated with a laser versus 0.3 times with a drill. The authors reported that these differences were statistically different.
Belcheva 2014 evaluated 90 children, half of them treated with laser and other half with drill, in terms of noise, vibration and smell experienced during treatment. In addition to these mentioned discomfort factors, the sight of the drill and laser and taste experienced were also evaluated. The vibration was found to be an unpleasant factor by 86.7% of children in the drill group versus 2.2% of children in the laser group (P < 0.001). Likewise, the laser and drill sight as well as its sound during treatment were found to be unpleasant more often in the drill group than in the laser group. The noise (sound) was assessed as unpleasant by 62.2% of children in the drill group versus 15.6% of children in the laser group (P < 0.05), while the sight was assessed as unpleasant by 20% of children in the laser group versus 40% of children in the drill group (P < 0.01). Conversely, laser was assessed as less unpleasant than drill in terms of taste (42.2% versus 22.2% of children, P < 0.005) and smell (66.7% versus 17% of children, P < 0.001).
In Evans 2000, the evaluation of discomfort was performed by recording each participant's (n = 62 children/adolescents and adults) crude preference between laser and drill expressed at the end of treatment. No significant differences were reported between the two types of treatment (P < 0.05; Wilcoxon matched‐pairs signed‐ranks test).
Need for anaesthesia
Three studies reported the need for anaesthesia in children (DenBesten 2001; Liu 2006; Zhang 2013), and one trial reported data in adults (Keller 1998). Our subgroup meta‐analysis showed that the need for anaesthesia was significantly lower in the laser therapy than in the drill group (RR 0.25, 95% CI 0.10 to 0.65; 3 studies; 217 children/adolescents; P = 0.004; I2 = 0%) (Analysis 1.8; Figure 8). The trial that evaluated only adults showed no evidence in favour of one of the assigned interventions (RR 0.55, 95% CI 0.21 to 1.42). When an overall meta‐analysis was performed, the results were significantly in favour of the laser treatment with low heterogeneity (RR 0.37, 95% CI 0.19 to 0.72; 4 studies; 320 participants; P = 0.004; I2 = 0%).

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.8 Need for anaesthesia.
Operator preference/fatigue in operator
Dentist preference for laser or traditional drill use for caries removal and cavity preparation was reported in only one study (Evans 2000). In this trial, authors reported that 11 dental practitioners were involved that expressed a preference in 73 out of 77 'dentist/patient encounters'. Authors reported that the preference was in favour of conventional cavity preparation methods rather than the laser (P < 0.001). The principal difficulty reported when using the laser was that of accessing the dental caries with 9 dentists reporting difficulty in 25 'dentist/patient encounters' in which difficulty was reported.
Participant preference
Three studies reported participants' preference for lasers or drills for future treatment of decayed teeth (Evans 2000; Liu 2006; Zhang 2013). In Zhang 2013, 37 of 53 children (72%) preferred a laser, 10 children (22%) preferred a drill and the remaining 6 children (6%) did not express any preference. In Liu 2006, 36 of 40 children (90%) preferred a laser for future treatment, whereas 4 children (5%) preferred a drill. In Evans 2000, 57 of 74 participants (77%) expressed their preference, of which 46 were older than 10 years and 11 were younger than 10 years; 17 other children under 10 years of age (23%) did not express any preference. Thirty‐nine participants over 10 years of age (52.6%) preferred laser treatment, whereas 7 participants (9.5%) chose drill treatment. Among 11 children under 10 years, 6 children (8.1%) preferred laser and 5 children (6.8%) chose drill. Considering children both under and over 10 years of age, 45 (60.8%) preferred a laser for future treatment and 12 (16.2%) preferred a drill.
Duration of treatment
Four studies evaluated the time employed to realise an adequate cavity preparation without residual caries (DenBesten 2001; Keller 1998; Liu 2006; Zhang 2013).
In Liu 2006, which performed removal of caries only on incisors, the mean duration was 1.6 (standard deviation (SD) ± 0.8) minutes in the laser group versus 0.7 (SD ± 0.6) minutes in the drill group. The difference between the two groups was statistically significant (mean difference (MD) 0.90, 95% CI 0.59 to 1.21; P < 0.001).
The remaining three trials evaluated posterior teeth but did not provide sufficient data to allow the production of meta‐analysis. DenBesten 2001 reported an average treatment time of 7.7 minutes for the laser and 6.6 minutes for the drill with no statistical difference between the groups. Keller 1998 reported that laser preparation required more than double the time that for mechanical preparation (mean time for laser preparation 7.5 ± 4.6 minutes compared to 4.3 ± 3.9 minutes for the mechanical means; median for laser preparation time was 7.3 minutes compared to 3 minutes for the mechanical means) without reporting data about significance. Zhang 2013 reported that the treatment time of the laser group (mean 6.4 ± 3.0 minutes) was longer than of the mechanical bur group (mean 3.9 ± 2.0 minutes).
Discussion
Summary of main results
Using data from published and unpublished literature, this review summarised the evidence supporting the use of laser‐based methods compared to conventional mechanical methods to treat caries. The review's significant outcomes were: (I) effectiveness outcomes, such as the removal of caries, recurrent caries, treatment duration, and duration and marginal integrity of restoration; (II) participants' perception outcomes, such as pain, discomfort, need for anaesthesia during or before treatment, and preference for future treatments; and (III) safety outcomes, such as vitality and inflammation of tooth pulpal tissues. Overall we included nine randomised trials with 662 participants. Unfortunately, the included trials did not assess all of the outcomes, in particular the primary outcomes. Only two studies provided data useful for our analysis concerning the outcome removal of dental caries, and the results showed no statistical difference between use of a laser and a drill (low‐quality evidence). In addition, the tooth restorations performed with either intervention showed comparable results in terms of retention rate (four studies) and marginal integrity (three studies), over two years of follow‐up visits. Five trials assessed pain, and the results were generally in favour of lasers (low‐quality evidence). In four of five studies reporting pain data, the participants reported laser to be significantly better compared to drill in terms of pain felt during treatment, while the remaining study reported no difference between the two devices for this outcome. This result was supported by the results of participants' perception outcomes, including need for anaesthesia, participant discomfort and participants' preference.
In addition, when practitioners were asked for their preference, they largely preferred drills to lasers.
Overall completeness and applicability of evidence
The number of studies and the number of participants included in the trials, would have been adequate to sufficiently address the efficacy of lasers to remove caries. The characteristics of the included participants, the clinical setting in which participants received the treatment and the technical features of both lasers and drills were appropriate in most of the studies. However, most of the studies did not address the most relevant outcome (removal of caries), and only five studies addressed the outcome episodes of pain. Other concerns in terms of completeness of the evidence were that only four studies addressed safety issues, such as pulpal inflammation and necrosis, and the lack of consideration regarding any potential side effects due to dental fear associated with the use of traditional dental ablation tools (i.e. drills) (Bergius 1997; Smith 2003; ten Berge 1998). Hence, despite some encouraging results, the applicability of lasers in current clinical practice is uncertain.
Lasers represent an advanced technological device that is expected to be more expensive than traditional drills for dental treatments, which could possibly influence its use in daily clinical practice. However, laser use could be theoretically justifiable in certain circumstances, such as in children showing high dental anxiety. Dental anxiety is a worldwide condition particularly present in children; it is reported that almost 1 in 10 children show a high level of dental fear, preventing a complete and adequate dental treatment (Klingberg 2007). Often this anxiety is generated by both the sight and noise of the drill (Chhabra 2012; Muppa 2013; Nakai 2005; Olak 2013). In these patients, laser could be an alternative and effective caries removal method to avoid the use of conscious sedation or general anaesthesia practices to complete dental treatment (Merigo 2015).
However, we found no clinical studies in the literature describing laser filling removal ability in the treatment of secondary caries. Only a few authors (performing in vitro studies) dealt with the theoretical ability of laser to selectively remove resin composite restorations by neighbouring enamel or dentine without damage to these tissues (Chan 2011; Chan 2014; Louie 2005). Regarding other dental filling materials such as amalgam, glass ionomer cement and glass ionomer cement composite, laser removal ability was not described in any in vitro or in vivo study. This lack of information about laser ability in removal dental restorations limits use of the device in clinical practice for a relevant part of caries treatment, such as secondary caries. Further studies of high methodological quality in this field are required in order to understand whether laser use could also be extended in future to secondary caries care.
Quality of the evidence
All included studies were randomised controlled trials. However, several methodological issues limited the overall quality of the evidence. Despite most of the studies using adequate methods to generate the random sequence, they were at high or unclear risk of selection bias due to lack of or unclear allocation concealment. Given that participants and personnel could not be blinded, it was not possible to avoid performance bias, however, although detection bias could be avoided, blinding of the outcome assessor was carried out and clearly reported in only three studies. With the exception of two trials, all studies were at unclear risk of attrition bias. In addition, none of the studies resulted to be at low risk of selective reporting bias either because one or both of the primary outcomes were not assessed or reported. Regarding other types of bias, none of the trials were at low risk with 2/3 being funded by for‐profit companies raising serious concern of bias.
Overall, we judged none of the nine trials as at low risk of bias, highlighting methodological limits concerning the validity of the evidence related to any single outcome evaluated.
Potential biases in the review process
We carried out a comprehensive search for interventions, across several major electronic databases, in this review. In addition, the review authors screened the reference lists of systematic reviews and contacted trial authors for clarification. Two review authors independently carried out screening of titles and abstracts, full‐text assessment of potentially relevant reviews and data extraction. One review author performed analyses, which a second review author checked.
Agreements and disagreements with other studies or reviews
We identified only one systematic review that investigated laser technology for the removal of caries in the medical literature (Jacobsen 2011). The authors launched a search in January 2009 in PubMed, the Cochrane Library, Embase and Inspec (grey literature was not searched). They identified seven trials (DenBesten 2001; Dommisch 2008; Evans 2000; Hadley 2000; Keller 1998; Liu 2006; Pelagalli 1997); the authors rated the quality and relevance of each study as high, medium or low using a study checklist and concluded that no meta‐analyses could be performed due to high heterogeneity.
Despite the authors' conclusion being similar to ours, Jacobsen's review differs from ours on the following points: (a) two studies were excluded from our review: Dommisch 2008 because the unit of analysis (the study authors randomised part of the cavity of the same tooth caries) was not adequate for our analysis, and Pelagalli 1997 because the allocation of participants depended on the availability of a laser; (b) contrary to our approach, Jacobsen 2011 did not perform any meta‐analysis; and (c) the method used for quality assessment was not clear in Jacobsen 2011.
Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
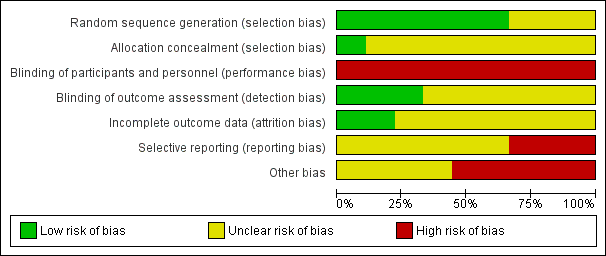
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.1 Caries removal (clinical).

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.2 Pain.

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.4 Durability of restoration.

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.5 Pulpal inflammation or necrosis.

Forest plot of comparison: 1 Laser versus standard drill, outcome: 1.8 Need for anaesthesia.

Comparison 1 Laser versus standard drill, Outcome 1 Caries removal (clinical).

Comparison 1 Laser versus standard drill, Outcome 2 Pain.
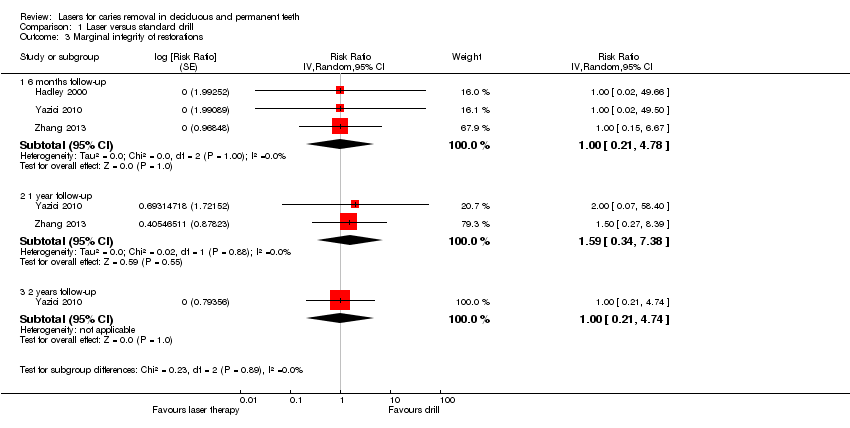
Comparison 1 Laser versus standard drill, Outcome 3 Marginal integrity of restorations.
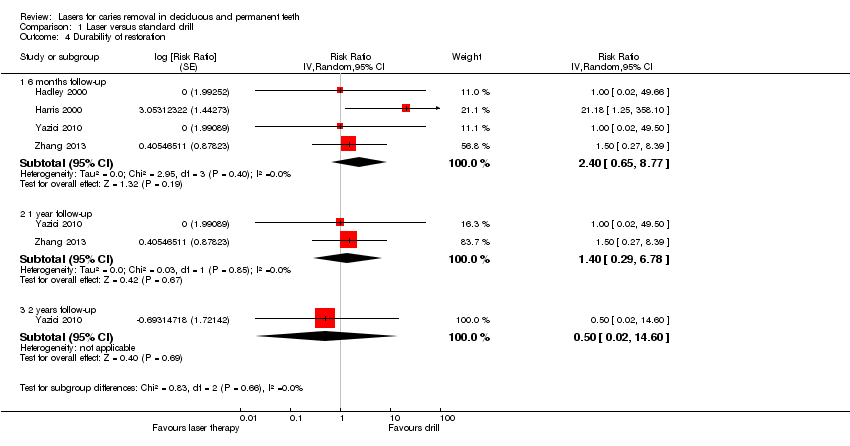
Comparison 1 Laser versus standard drill, Outcome 4 Durability of restoration.

Comparison 1 Laser versus standard drill, Outcome 5 Pulpal inflammation or necrosis.
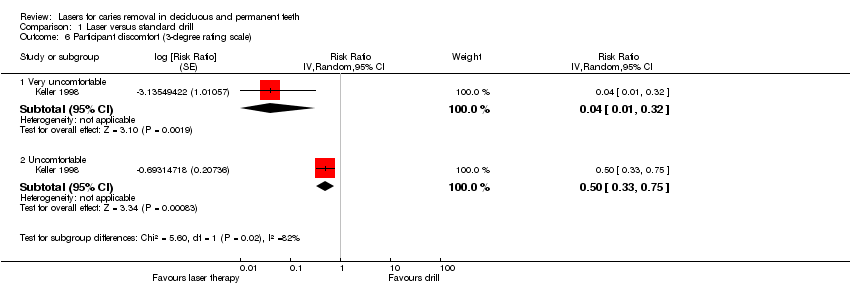
Comparison 1 Laser versus standard drill, Outcome 6 Participant discomfort (3‐degree rating scale).

Comparison 1 Laser versus standard drill, Outcome 7 Participant discomfort (5‐degree rating scale).
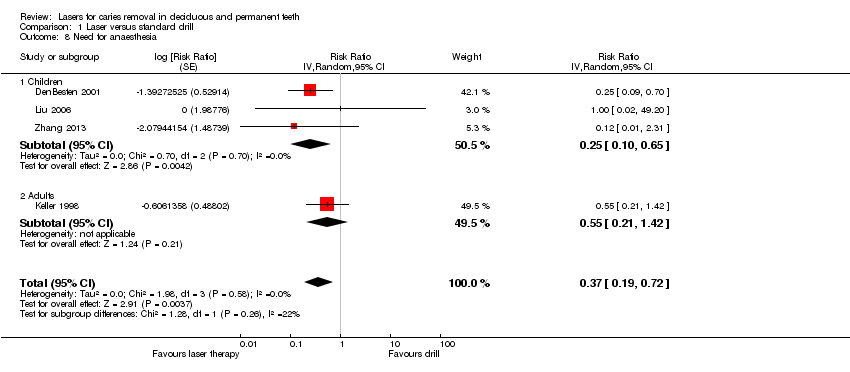
Comparison 1 Laser versus standard drill, Outcome 8 Need for anaesthesia.
| Laser compared to standard drill for caries removal in deciduous and permanent teeth | ||||||
| Patient or population: people with caries in deciduous and permanent teeth | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard drill | Laser | |||||
| Caries removal (during treatment) | 995 per 1000 | 995 per 1000 | RR 1.00 | 190 participants; 256 teeth; 256 cavity preparations | ⊕⊕⊝⊝ | |
| Pain ‐ 6‐face rating scale (moderate and high pain) (during treatment) | 760 per 1000 | 304 per 1000 | RR 0.40 | 143 participants | ⊕⊕⊝⊝ | |
| Need for anaesthesia ‐ children (during treatment) | 97 per 1000 | 24 per 1000 | RR 0.25 | 217 participants | ⊕⊕⊝⊝ | |
| Durability of restoration ‐ 6 months follow‐up | 8 per 1000 | 20 per 1000 | RR 2.40 | 236 participants; 682 teeth | ⊕⊕⊝⊝ | |
| Marginal integrity of restorations ‐ 6 months follow‐up | 7 per 1000 | 7 per 1000 | RR 1.00 | 146 participants; 306 teeth | ⊕⊕⊝⊝ | |
| Pulpal inflammation or necrosis ‐ 1 week follow‐up | 5 per 1000 | 7 per 1000 | RR 1.51 | 317 participants; 694 teeth; 752 cavity preparations | ⊕⊕⊝⊝ | |
| Pulpal inflammation or necrosis ‐ 6 months follow‐up | 4 per 1000 | 4 per 1000 | RR 0.99 | 156 participants; 508 teeth; 554 cavity preparations | ⊕⊕⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The evidence was downgraded by two levels because of very serious concern regarding the risk of bias: (1) the two studies did not report sufficient information regarding the allocation concealment (DenBesten 2001; Hadley 2000); (2) both studies were at high risk of performance bias; (3) neither of the two studies was at low risk of selective reporting bias; (4) serious concern related to funding (other bias). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caries removal (clinical) Show forest plot | 2 | Risk Ratio (Random, 95% CI) | 1.00 [0.99, 1.01] | |
| 2 Pain Show forest plot | 3 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 6‐face rating scale (moderate and high pain) | 2 | Risk Ratio (Random, 95% CI) | 0.40 [0.28, 0.57] | |
| 2.2 Modified simple 4‐face scale | 1 | Risk Ratio (Random, 95% CI) | 0.21 [0.11, 0.42] | |
| 3 Marginal integrity of restorations Show forest plot | 3 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 6 months follow‐up | 3 | Risk Ratio (Random, 95% CI) | 1.0 [0.21, 4.78] | |
| 3.2 1 year follow‐up | 2 | Risk Ratio (Random, 95% CI) | 1.59 [0.34, 7.38] | |
| 3.3 2 years follow‐up | 1 | Risk Ratio (Random, 95% CI) | 1.0 [0.21, 4.74] | |
| 4 Durability of restoration Show forest plot | 4 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 6 months follow‐up | 4 | Risk Ratio (Random, 95% CI) | 2.40 [0.65, 8.77] | |
| 4.2 1 year follow‐up | 2 | Risk Ratio (Random, 95% CI) | 1.40 [0.29, 6.78] | |
| 4.3 2 years follow‐up | 1 | Risk Ratio (Random, 95% CI) | 0.50 [0.02, 14.60] | |
| 5 Pulpal inflammation or necrosis Show forest plot | 4 | Risk Ratio (Random, 95% CI) | 1.29 [0.32, 5.14] | |
| 5.1 1 week | 3 | Risk Ratio (Random, 95% CI) | 1.51 [0.26, 8.75] | |
| 5.2 6 months | 2 | Risk Ratio (Random, 95% CI) | 0.99 [0.10, 9.41] | |
| 6 Participant discomfort (3‐degree rating scale) Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Very uncomfortable | 1 | Risk Ratio (Random, 95% CI) | 0.04 [0.01, 0.32] | |
| 6.2 Uncomfortable | 1 | Risk Ratio (Random, 95% CI) | 0.50 [0.33, 0.75] | |
| 7 Participant discomfort (5‐degree rating scale) Show forest plot | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 7.1 Mild discomfort | 1 | Risk Ratio (Random, 95% CI) | 0.12 [0.01, 2.32] | |
| 7.2 Moderate discomfort | 1 | Risk Ratio (Random, 95% CI) | 0.33 [0.04, 3.12] | |
| 8 Need for anaesthesia Show forest plot | 4 | Risk Ratio (Random, 95% CI) | 0.37 [0.19, 0.72] | |
| 8.1 Children | 3 | Risk Ratio (Random, 95% CI) | 0.25 [0.10, 0.65] | |
| 8.2 Adults | 1 | Risk Ratio (Random, 95% CI) | 0.55 [0.21, 1.42] | |

