Omega‐3 masne kiseline za sprječavanje i usporavanje napredovanja senilne makularne degeneracije
Abstract
Background
Evidence from animal models and observational studies in humans has suggested that there is an inverse relationship between dietary intake of omega 3 long‐chain polyunsaturated fatty acids (LCPUFA) and risk of developing age‐related macular degeneration (AMD) or progression to advanced AMD.
Objectives
To review the evidence that increasing the levels of omega 3 LCPUFA in the diet (either by eating more foods rich in omega 3 or by taking nutritional supplements) prevents AMD or slows the progression of AMD.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 1), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2015), EMBASE (January 1980 to February 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to February 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 February 2015.
Selection criteria
We included randomised controlled trials (RCTs) where increased dietary intake of omega 3 LCPUFA was compared to placebo or no intervention with the aim of preventing the development of AMD, or slowing its progression.
Data collection and analysis
Both authors independently selected studies, assessed them for risk of bias and extracted data. One author entered data into RevMan 5 and the other author checked the data entry. We conducted a meta‐analysis for one primary outcome, progression of AMD, using a fixed‐effect inverse variance model.
Main results
We included two RCTs in this review, in which 2343 participants with AMD were randomised to receive either omega 3 fatty acid supplements or a placebo. The trials, which had a low risk of bias, were conducted in the USA and France. Overall, there was no evidence that people who took omega 3 fatty acid supplements were at decreased (or increased risk) of progression to advanced AMD (pooled hazard ratio (HR) 0.96, 95% confidence interval (CI) 0.84 to 1.10, high quality evidence). Similarly, people taking these supplements were no more (or less) likely to lose 15 or more letters of visual acuity (USA study HR 0.96, 95% CI 0.84 to 1.10; French study at 36 months risk ratio (RR) 1.25, 95% CI 0.69 to 2.26, participants = 230). The number of adverse events was similar in the intervention and placebo groups (USA study participants with one or more serious adverse event RR 1.00, 95% CI 0.91 to 1.09, participants = 2080; French study total adverse events RR 1.05, 95% CI 0.97 to 1.13, participants = 263).
Authors' conclusions
This review found that omega 3 LCPUFA supplementation in people with AMD for periods up to five years does not reduce the risk of progression to advanced AMD or the development of moderate to severe visual loss. No published randomised trials were identified on dietary omega 3 fatty acids for primary prevention of AMD. Currently available evidence does not support increasing dietary intake of omega 3 LCPUFA for the explicit purpose of preventing or slowing the progression of AMD.
PICOs
Laički sažetak
Omega‐3 masne kiseline za sprječavanje i usporavanje napredovanja staračke makularne degeneracije
Uvod
Postavljeno je pitanje ima li unos omega‐3 masnih kiselina putem prehrane ulogu u sprječavanju ili usporavanju napredovanja senilne makularne degeneracije.
Dosadašnje spoznaje
Senilna makularna degeneracija (AMD) je bolest oka koja pogađa središnji dio mrežnice (fotoosjetljivo tkivo u stražnjem dijelu oka) AMD je povezan s gubitkom oštrine vida i nepovoljno utječe na obavljanje zadataka kao što su čitanje, vožnja i prepoznavanje lica. Zbog nedostatka lijeka za tu bolest postoji značajno zanimanje za ulogu promjenjivih čimbenika rizika koji bi mogli spriječiti ili usporiti napredovanje AMD‐a. Dokazi dobiveni prethodnim populacijskim studijama upućuju na manju vjerojatnost da će ljudi čija prehrana sadrži razmjerno visoke razine omega‐3 masnih kiselina (kao one iz ribljih ulja) razviti AMD.
Značajke studije
Pretražene su studije objavljene do 2. veljače 2015. godine. Pronađena su dva klinička istraživanja s ukupno 2343 sudionika. Istraživanja su provedena u SAD‐u i Francuskoj, a ispitivala su uporabu ribljeg ulja u ljudi s AMD‐om koji su imali veliki rizik za napredovanje do ozbiljnijih stadija bolesti. Odabrane su studije sa što manjim rizikom od pristranosti. Jednu je studiju financirala država, a drugu proizvođač dodatka prehrani.
Rezultati
Ta su istraživanja pokazala da uzimanje dodataka koji sadrže omega‐3 masne kiseline u razdobljima do pet godina nisu usporili napredak senilne makulativne degeneracije ili značajno smanjili gubitak vida u usporedbi s placebom. Pojavnost nuspojava bila je slična u eksperimentalnoj i placebo skupini.
Kvaliteta dokaza
Procijenjeno je da je kvaliteta dokaza o brzini napretka AMD visoka, ali da je kvaliteta dokaza o drugim ishodima umjerena jer su procjene bile neprecizne.
Authors' conclusions
Summary of findings
| Omega 3 fatty acids compared to placebo for slowing the progression of age‐related macular degeneration | ||||||
| Patient or population: people with AMD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No omega 3 fatty acids (placebo) | Omega 3 fatty acids | |||||
| Loss of 3 or more lines of VA at 24 months | 100 per 1000 | 114 per 1000 | RR 1.14, 95% CI 0.53 to 2.45 | 236 | ⊕⊕⊕⊝ | |
| Loss of 3 or more lines of VA at 36 months | 150 per 1000 | 187 per 1000 | RR 1.25, 95% CI 0.69 to 2.26) | 230 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 24 months | 100 per 1000 | 106 per 1000 | RR 1.06, 95% CI 0.47 to 2.40 | 224 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 36 months | 150 per 1000 | 168 per 1000 | RR 1.12, 95% CI 0.53 to 2.38 | 195 | ⊕⊕⊕⊝ | |
| Progression of AMD over 5 years | 300 per 1000 | 290 per 1000 | HR 0.96 | 2343 | ⊕⊕⊕⊕ | |
| Adverse effects | 500 per 1000 | 505 per 1000 | RR 1.01, 95% CI 0.94 to 1.09 | 2343 | ⊕⊕⊕⊕ | AREDS2 reported participants with one or more serious adverse events (AE). NAT‐2 reported total AE including treatment emergent and serious non‐ocular events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision | ||||||
Background
Description of the condition
Age‐related macular degeneration (AMD) is the most common cause of blindness and visual impairment in developed countries, accounting for over 50% of blind and partially sighted certifications in the UK (Bunce 2010). Since the disease predominantly affects individuals aged 55 years and over, its prevalence continues to rise as the population is living longer. AMD is characterised by degenerative changes within the macula, the central area of the retina responsible for detailed vision and colour perception (Lim 2012). The early stage of AMD is associated with an accumulation of small focal yellowish deposits (drusen) under the retinal pigment epithelium, often with an associated pigmentary disturbance. At this stage the patient is generally asymptomatic, however, as the disease progresses large focal areas of retinal pigment epithelial cell loss can occur, referred to as geographic atrophy, which leads to a progressive worsening of central vision. In approximately 10% of cases, an acute neovascular response arises under the retina, with associated blurring or distortion of vision. If untreated, the resulting haemorrhagic and exudative pathology disrupts normal retinal anatomy, eventually leading to the formation of a dense fibrous scar. When both eyes are affected, late AMD causes significant visual impairment, with difficulties in reading, driving and recognising faces. It can, therefore, severely impact on vision‐related quality of life. The estimated prevalence of late AMD in populations of European ancestry is 1.4% at age 70 years, increasing to 5.6% at 80 and 20.1% at 90 years of age (Rudnicka 2012). In the UK, the annual incidence of late AMD in the population aged 50 years and above is 4.1 per 1000 women and 2.6 per 1000 men (Owen 2012).
There is currently no cure for AMD. Vascular endothelial growth factor inhibitors that are injected directly into the vitreous humour of the eye can stabilise vision in neovascular AMD, however no effective treatment is available for geographic atrophy. In the absence of a cure, research has focused on preventing or slowing the progression of AMD through the control of modifiable risk factors, for example smoking or dietary modification (including the use of nutritional supplements).
Description of the intervention
This review considers the evidence for the role of omega 3 fatty acids in the primary prevention and treatment of AMD. Fatty acids are divided into three broad categories, saturated, monounsaturated and polyunsaturated. Although humans can synthesise saturated and monounsaturated fats, they do not have the enzyme systems required to synthesise polyunsaturated fatty acids and therefore dietary sources are essential. Long‐chain polyunsaturated fatty acids (LCPUFA) are classified according to their chemical structure. They have a methyl group at one end of the molecule and a carboxyl group at the other, separated by a chain of 18 or more carbon atoms that contains two or more double bonds. The n‐3 variety of LCPUFA (designated ω‐3 or omega 3) has a double bond positioned three carbon atoms from the methyl end of the molecule. Omega 3 LCPUFA are obtained principally from dietary sources, however they can be synthesised from the short‐chain omega 3 fatty acid alpha linolenic acid, although endogenous synthesis in humans is limited (Burdge 2002).
The omega 3 LCPUFA docosahexaenoic acid (DHA) is present in high concentrations in retinal photoreceptors and is therefore essential for visual function. Although a diet rich in oily fish, eggs, nuts and particular vegetable oils provides a plentiful supply of omega 3 fatty acids, there has been a great deal of interest in the health benefits of omega 3 supplementation, and commercially available supplements in the form of oils and capsules are widely available. Capsules typically contain a mixture of DHA and its precursor eicosapentaenoic acid (EPA), often in combination with antioxidant vitamins and minerals.
How the intervention might work
There is a plausible biological rationale for increasing omega 3 LCPUFA in AMD. DHA accounts for 50% to 60% of the total fatty acid content of the outer segments of photoreceptors. The constant turnover of outer segment membranes requires a continuous dietary supply of DHA, or its precursors, and a deficiency may predispose a person to the development of AMD. Omega 3 LCPUFA may also confer protection against the oxidative, inflammatory and vasogenic processes that play a key role in the pathogenesis of AMD (Kishan 2011; SanGiovanni 2005). In a mouse model that develops a range of AMD‐like retinal lesions, progression of the disease was slowed and in some cases reversed in a group of mice fed on a diet rich in DHA and EPA (Tuo 2009). The protective effect of omega 3 LCPUFA was associated with a reduction in pro‐inflammatory mediators and an increase in the levels of anti‐inflammatory metabolites. An EPA rich diet has also been shown to suppress experimental choroidal neovascularisation (CNV) and CNV‐related inflammatory molecules both in vitro and in vivo (Koto 2007).
Epidemiological studies in humans have provided some evidence that consumption of fish and foods rich in omega 3 LCPUFA could reduce the risk of developing AMD (Chong 2009; Christen 2011; Hodge 2006; Tan 2009). Similarly, a nested cohort study within the Age‐Related Eye Disease Study (AREDS) found that participants at moderate to high risk of progressing to late AMD who reported the highest consumption of omega 3 LCPUFA were 30% less likely to develop geographic atrophy and neovascular AMD when compared to those reporting the lowest consumption (SanGiovanni 2009).
Why it is important to do this review
The high prevalence of AMD and the limited availability of effective treatments for the majority of sufferers highlights the need to search for new treatment strategies to prevent or slow the progression of the disease. There is significant interest in the role of diet and nutritional supplementation in AMD. There is evidence that antioxidant vitamins (beta‐carotene, vitamin C and vitamin E) and zinc supplementation slow down the progression to advanced AMD (Evans 2012b), but there is no evidence to support the use of these supplements in primary prevention (Evans 2012a). Although observational studies have suggested a protective role for omega 3 LCPUFA in the prevention and treatment of AMD, these results may be confounded by other dietary or lifestyle factors. Therefore, a systematic review of randomised controlled trials that examines the effect of increasing dietary intake of omega 3 LCPUFA in AMD was needed. The review considered evidence for both atrophic and neovascular AMD.
Objectives
To review the evidence that increasing the levels of omega 3 LCPUFA in the diet (either by eating more foods rich in omega 3 or by taking nutritional supplements) prevents or slows the progression of AMD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) where increased dietary intake of omega 3 fatty acids was compared to placebo or no intervention.
Types of participants
People from the general population with or without AMD. The definition of AMD was taken as defined by study investigators (for example using a standardised grading scheme, or AMD leading to a reduction in visual acuity).
Types of interventions
Any type and any dose of omega 3 fatty acids, either as fish oil capsules or dietary manipulation (for example increased consumption of oily fish). The intervention could be delivered either as monotherapy or in combination with other measures, where the study design allowed for the effect of the omega 3 treatment to be isolated. We imposed no restriction on the duration of treatment.
Types of outcome measures
Primary outcomes
We defined the primary outcomes as follows:
-
risk of developing incident AMD or new visual loss attributed to AMD; and
-
risk of progression of AMD in people previously diagnosed with the disease.
Secondary outcomes
We defined the secondary outcomes as follows:
-
quality of life; and
-
any adverse outcomes reported.
We assessed primary and secondary outcomes as reported by study authors either in the short term (less than two years) or following a longer‐term intervention (more than two years).
We used the following definitions in the review.
-
AMD: there are a number of internationally recognised classification systems that rely on photographic grading of fundus images, e.g. Wisconsin Age‐related Maculopathy Grading System (Klein 1991) or the Age‐Related Eye Disease Study (AREDS) Classification System (AREDS 1999) which encompasses five categories of increasing severity from 'early' to 'advanced' AMD. However, study‐specific definitions may also be used, e.g. following ophthalmoscopic examination of the retina or confirmation from medical records of participant self reporting of AMD.
-
AMD progression: may be defined by a change in severity based on fundus appearance or progressive visual loss due to AMD.
-
Visual loss: any well‐defined outcome based on visual acuity measured using a standardised measurement technique.
-
Quality of life: any validated measurement scale which aims to measure vision functioning or vision‐specific quality of life.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2015, Issue 1), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2015), EMBASE (January 1980 to February 2015), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to February 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 2 February 2015.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We reviewed the reference list of included studies to identify additional trials for this review.
Data collection and analysis
Selection of studies
We screened the titles and abstracts of articles retrieved by the searches independently. We obtained full‐text copies of articles that definitely or potentially met the inclusion criteria. We independently reviewed these and selected studies according to the definitions in the Criteria for considering studies for this review. We documented reasons for excluding studies at this stage and resolved any disagreements by discussion.
We wrote to authors of included studies to ask if they were aware of any published or unpublished studies on omega 3 acids in relation to AMD.
Data extraction and management
We independently extracted data from eligible studies using a standardised form developed by the Cochrane Eyes and Vision Group. We compared the results and resolved any discrepancies by discussion. One author cut and pasted the data into Review Manager 5 (RevMan 2014) and the other checked that this was done correctly.
Assessment of risk of bias in included studies
We used the 'risk of bias' assessment tool developed by Cochrane, described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess the quality of included studies. The tool uses the following criteria.
-
Sequence generation.
-
Allocation concealment.
-
Blinding (masking).
-
Incomplete outcome data.
-
Selective reporting.
-
Other sources of bias.
We performed the 'risk of bias' assessment independently and resolved any discrepancies by discussion.
Measures of treatment effect
In general for binary outcomes we used the risk ratio (RR). For the analysis of progression to advanced AMD, the available data were reported from Cox proportional hazard models and therefore for this outcome the results were expressed as a hazard ratio.
Unit of analysis issues
The majority of studies in this area are parallel‐group RCTs. Cluster randomisation (where individuals are randomised in groups) and cross‐over trials are unlikely to be used to investigate the role of omega 3 LCPUFA in AMD but will be considered in the future if these studies become available.
We analysed the results by person since the individual is the unit of randomisation (as the intervention is applied to the individual, not the eye).
Dealing with missing data
The data included in this review represented an 'available case analysis'. This makes the assumption that data are missing at random. We recorded the amount of missing data and reasons for exclusions and attrition, where available, and contacted investigators for clarification. We did not impute missing data.
Assessment of heterogeneity
We assessed heterogeneity by examining the forest plot, along with the Chi2 test and the I2 statistic.
Assessment of reporting biases
There were insufficient numbers of studies to carry out a funnel plot analysis to investigate the relationship between treatment effect and study size.
Data synthesis
For data on progression of AMD, log hazard ratios and standard errors were obtained from Cox proportional hazards regression models. These results were combined using the generic inverse‐variance method and since only two trials were available for analysis, a fixed‐effect model was used.
Subgroup analysis and investigation of heterogeneity
Insufficient data were available to perform any subgroup analyses.
Sensitivity analysis
No sensitivity analysis was planned.
Results
Description of studies
See: the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables for more information.
Results of the search
The electronic searches run in January 2012 yielded a total of 337 references. The Trials Search Co‐ordinator scanned the search results, removed duplicates and removed 135 references which were not relevant to the scope of the review. We screened the remaining 116 reports to identify potentially relevant studies. We obtained full‐text copies of four studies and excluded them after reading the full reports (Feher 2005; Huang 2008; Johnson 2008; Scorolli 2002). We also excluded two studies after reading their unpublished reports (NCT01258335; OPAL). We identified two ongoing studies (AREDS2; VITAL‐AMD) and another completed trial (ISRCTN98246501/NAT2) was awaiting publication. These studies were to be assessed for inclusion in the review when data became available.
An update search run in February 2015 identified a further 180 references (Figure 1). After de‐duplication we screened 153 references and discarded 146 as not being relevant to the scope of the review. We obtained seven full‐text reports for potential inclusion in the review. After assessment we included three reports of two trials (AREDS2; NAT2) and excluded three trials (Arnold 2013; García Layana 2013; Ziegler 2013). We have identified an ongoing trial (NCT02264938) and will assess this trial when results become available. In the previous version of this review there were three trials awaiting assessment, which would be included when data became available. The VITAL‐AMD trial has not yet been completed and is still classed as ongoing. The study protocols for AREDS2 and NAT2 have been removed from the review as the results of these trials have now been published and incorporated into the review as the two new included studies.
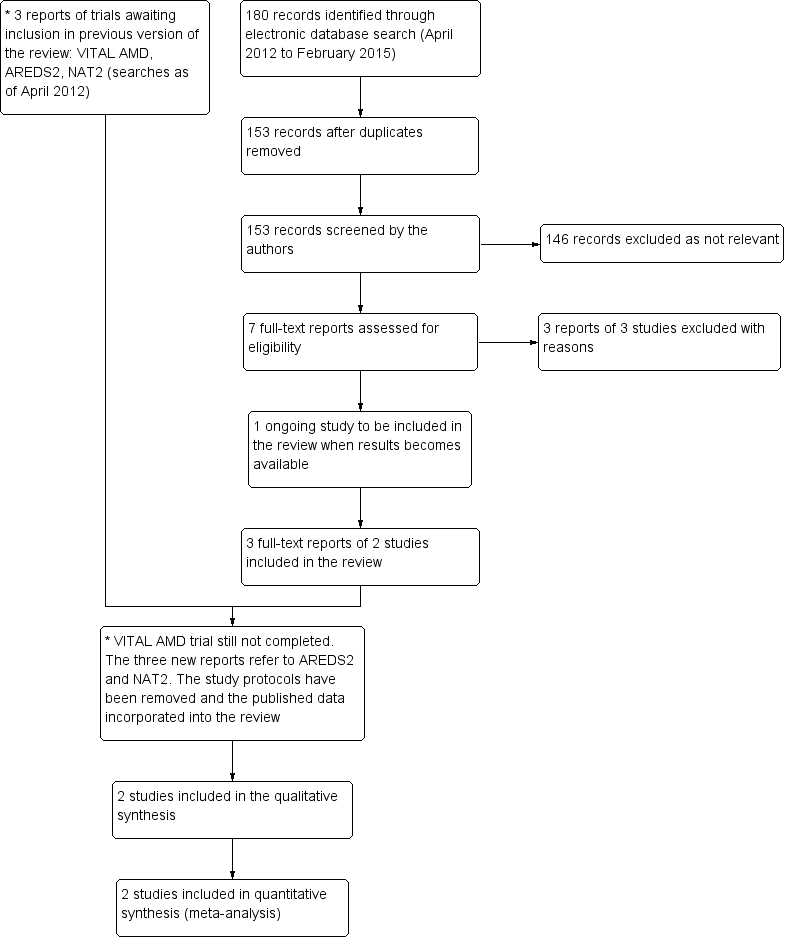
Results from searching for studies for inclusion in the review.
Included studies
Below is a summary of the two trials included in this review. See Characteristics of included studies for detailed information on individual trials.
Types of participants
Both trials included participants at high risk of developing advanced AMD; with either bilateral large drusen or large drusen in one eye and advanced AMD in the fellow eye (AREDS2), or early AMD in the study eye and CNV in the fellow eye (NAT2). The mean age for people participating in the trials was 74 years. On average, slightly more women than men were recruited (mean percentage that were female 56%). People taking part in the trials were either recruited from a single hospital clinic (NAT2), recruited from specialist retinal clinics or were volunteers from the general population (AREDS2).
Types of intervention
AREDS2 compared a daily dose of 650 mg EPA and 350 mg DHA to placebo. All participants were additionally instructed to take the original AREDS formula of antioxidant vitamins and zinc (AREDS 1999) or were entered into a secondary randomisation to investigate the effects of variations of this formula. NAT2 compared 840 mg DHA and 270 mg EPA daily to placebo (olive oil capsules). In AREDS2 the duration of supplementation was five years and for NAT2 participants were supplemented for three years.
Types of outcome measures
The main outcome measures for both trials were the development of advanced AMD and loss of visual acuity corresponding to 15 letters or more from baseline. For AREDS2 advanced AMD was defined as central geographical atrophy (GA) or choroidal neovascularisation (CNV) and was assessed by grading stereoscopic fundus images using masked graders. For NAT2 the AMD outcome was the occurrence of CNV in the study eye (confirmed by angiography). In both trials visual acuity was measured using a standard ETDRS chart (15 letters corresponds to a loss of 3 lines on the chart and is equivalent to a doubling of the visual angle).
Excluded studies
We excluded nine studies in total. Four randomised trials were identified with AMD endpoints (Arnold 2013; Feher 2005; García Layana 2013; Scorolli 2002), however these were excluded since the supplement used contained additional antioxidants and it was not possible to isolate the effects of the omega 3 LCPUFA. One study (Ziegler 2013) was a commentary. Two further trials (Huang 2008; Johnson 2008) investigated the bioavailability of omega 3 LCPUFA following supplementation with DHA and EPA with or without the xanthophylls lutein and zeaxanthin. Another study (NCT01258335) used multifocal electroretinograms to assess the safety of high‐dose omega 3 fatty acids, but did not report AMD outcomes. Similarly for the OPAL study, which investigated omega 3 LCPUFA supplements for cognitive impairment, eye data were collected in a subset of participants but no AMD outcomes were available.
Risk of bias in included studies
Figure 2 and Figure 3 summarise the 'risk of bias' assessment. Overall, we considered the trials to be at low risk of bias for the main types of bias.
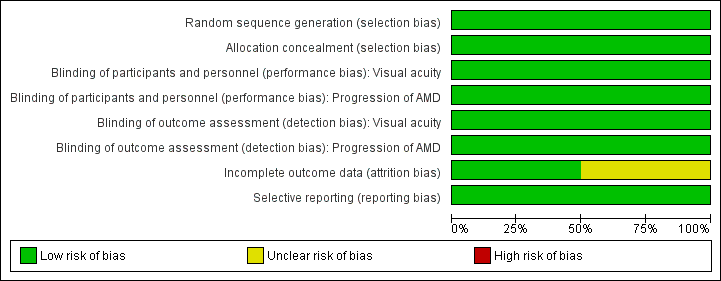
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
AREDS2 randomised participants using a random block design, which was conducted by the AREDS2 Co‐ordinating Centre. NAT2 used a computer generated random sequence that was carried out by an independent auditor.
Masking
In AREDS2 and NAT2 participants and investigators were masked to the treatment assignment during the study. In AREDS2 the study supplements were identical in appearance, size, smell and taste to their placebo counterparts. In NAT2 the placebo contained olive oil and had the same appearance and weight as the active treatment.
Incomplete outcome data
In AREDS2 missing outcome data were balanced in numbers across the intervention and placebo groups with similar reasons for missing data. NAT2 used a per protocol analysis. The main reason for protocol deviation was premature withdrawal, which occurred at a similar rate in the DHA and placebo groups. Other protocol deviations included ‘non‐compliance with study medication or use of non‐permitted medication’. Two hundred and sixty three of the original 300 participants randomised were included in the analysis.
Selective reporting
For both trials all outcomes that are of interest in this review were reported in the pre‐specified way.
Effects of interventions
Prevention of AMD
We did not identify any trials of omega 3 fatty acids in people without AMD.
Slowing the progression of AMD
New visual loss attributed to AMD
Visual acuity data was reported by both trials as a dichotomous outcome (loss of 3 or more lines of visual acuity).
NAT2 reported visual acuity outcomes at 12, 24 and 36 months. At each of these time points there was no evidence of a protective effect of omega 3 fatty acids, but the estimates were uncertain with wide confidence intervals (CIs): at 12 months, RR 6.57, 95% CI 0.82 to 52.65, participants = 254; at 24 months, RR 1.14, 95% CI 0.53 to 2.45, participants = 236; and at 36 months, RR 1.25, 95% CI 0.69 to 2.26, participants = 230.
AREDS2 reported visual acuity outcomes at 60 months (adjusted for baseline AMD status) as a hazard ratio (HR). Again there was no little or no evidence of an effect: HR 0.96 (95% CI 0.84 to 1.10, participants = 2080).
Progression of AMD
Both of the included trials reported data on progression of AMD as time to occurrence of advanced AMD. This was defined as either GA or CNV in either eye in AREDS2 and CNV in the study eye in NAT2. The pooled analysis included 2343 people who experienced 1071 advanced AMD events. The results were reasonably consistent and provided no evidence of any beneficial effect of omega 3 supplementation, pooled HR 0.96 (95% CI 0.84 to 1.10) (Figure 4).

Forest plot of comparison (Analysis 1.1): 1 Omega 3 fatty acids versus control, outcome: 1.10 Progression of AMD.
NAT2 reported the incidence of CNV at 12, 24 and 36 months. At each of these time points there was no evidence of a protective effect of omega 3 fatty acids, but the estimates were uncertain with wide CIs (at 12 months, RR 1.12, 95% CI 0.54 to 2.34, participants = 263; at 24 months, RR 1.06, 95% CI 0.47 to 2.40, participants = 224; and at 36 months, RR 1.12, 95% CI 0.53 to 2.38, participants = 195).
Quality of life
Neither of the trials reported data on quality of life.
Adverse effects
AREDS2 reported 'serious' adverse events only (Table 1). The number of events was similar in the intervention and placebo groups (RR 1.00, 95% CI 0.91 to 1.09, participants = 2080). In NAT2, the frequency of adverse events similarly did not differ between the two arms of the study (RR 1.05, 95% CI 0.97 to 1.13, participants = 263). Five participants (3.7%) in the omega 3 group and two participants (1.6%) in the placebo group experienced adverse reactions that were considered by the authors to be potentially treatment‐related (including gastrointestinal disorders, allergic dermatitis and breath odour).
Discussion
Summary of main results
This review provides evidence that dietary omega 3 LCPUFA supplementation does not slow the progression of AMD or reduce the risk of developing moderate to severe visual loss. The review included data from two RCTs that randomised 2343 individuals with AMD to receive either omega 3 fatty acid supplements or placebo. Duration of supplementation ranged from three to five years. No statistically significant effect on incidence of advanced AMD, progression to advanced AMD or on moderate to severe visual loss were observed. The number of adverse events was similar between the intervention and placebo groups.
Overall completeness and applicability of evidence
The results from both trials were reasonably consistent although the main evidence for a null effect comes from the AREDS2 trial. This was a large well‐conducted randomised study and therefore any potential biases would have been minimised. There are still some unanswered questions, for example we do not know whether the effects of the intervention differ in different populations (for example different ethnicities, nutritional states) or stage of the disease, and whether the composition (EPA:DHA ratio) or source of omega 3 PUFA (oily fish versus fish oil supplements) is important.
We did not identify any trials of supplementation in the general population, that is we do not know whether omega 3 supplementation prevents AMD.
Quality of the evidence
Overall we judged the quality of the evidence to be moderate or high. We downgraded some outcomes for imprecision.
Potential biases in the review process
We followed standard procedures expected by The Cochrane Collaboration.
Agreements and disagreements with other studies or reviews
A systematic review of the effect of dietary omega 3 fatty acids on progression of AMD, including only one RCT and one prospective cohort study, found inconclusive evidence for a beneficial effect (Hodge 2007). Although two systematic reviews (Chong 2009; Hodge 2006) of omega 3 fatty acids for primary prevention of AMD found some evidence based on observational studies that consumption of fish and foods rich in omega 3 LCPUFA was associated with a lower risk of AMD, the authors of both reviews concluded that the lack of evidence from RCTs precluded recommending increasing dietary intake omega 3 fatty acids specifically for AMD prevention.
Omega 3 fatty acids have been extensively studied for their potential health benefits. However, there is continuing controversy regarding their effectiveness. For example, a Cochrane review (Hooper 2004) which included data from 48 RCTs and 41 cohort studies concluded that dietary or supplemental omega 3 fats did not alter total mortality, combined cardiovascular events or cancers in people with, or at high risk of, cardiovascular disease or in the general population. Randomisation to omega 3 fats increased the risk of dropping out due to side effects (RR 1.62, 95% CI 1.10 to 2.40). The most common side effects included a bad or fishy taste or belching (RR 3.63, 95% CI 1.97 to 6.67) and nausea (RR 3.88, 95% CI 1.42 to 10.58).

Results from searching for studies for inclusion in the review.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison (Analysis 1.1): 1 Omega 3 fatty acids versus control, outcome: 1.10 Progression of AMD.
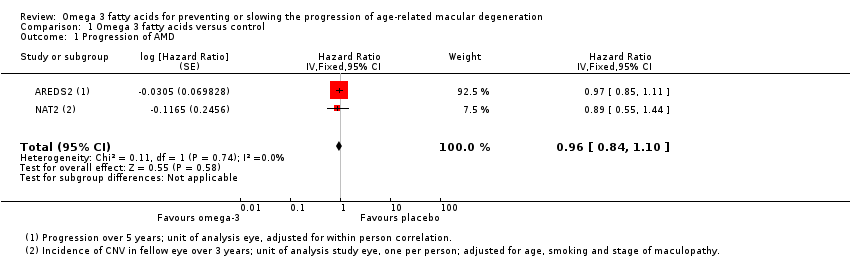
Comparison 1 Omega 3 fatty acids versus control, Outcome 1 Progression of AMD.
| Omega 3 fatty acids compared to placebo for slowing the progression of age‐related macular degeneration | ||||||
| Patient or population: people with AMD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No omega 3 fatty acids (placebo) | Omega 3 fatty acids | |||||
| Loss of 3 or more lines of VA at 24 months | 100 per 1000 | 114 per 1000 | RR 1.14, 95% CI 0.53 to 2.45 | 236 | ⊕⊕⊕⊝ | |
| Loss of 3 or more lines of VA at 36 months | 150 per 1000 | 187 per 1000 | RR 1.25, 95% CI 0.69 to 2.26) | 230 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 24 months | 100 per 1000 | 106 per 1000 | RR 1.06, 95% CI 0.47 to 2.40 | 224 | ⊕⊕⊕⊝ | |
| Incidence of CNV at 36 months | 150 per 1000 | 168 per 1000 | RR 1.12, 95% CI 0.53 to 2.38 | 195 | ⊕⊕⊕⊝ | |
| Progression of AMD over 5 years | 300 per 1000 | 290 per 1000 | HR 0.96 | 2343 | ⊕⊕⊕⊕ | |
| Adverse effects | 500 per 1000 | 505 per 1000 | RR 1.01, 95% CI 0.94 to 1.09 | 2343 | ⊕⊕⊕⊕ | AREDS2 reported participants with one or more serious adverse events (AE). NAT‐2 reported total AE including treatment emergent and serious non‐ocular events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded for imprecision | ||||||
| Adverse effects | Omega 3 N (%) | Placebo N (%) |
| AREDS 2 | ||
| Total number of participants | N = 1068 | N = 1012 |
| Participants with ≥ 1 adverse event · Cardiac disorders · Gastrointestinal disorders · Infections · Neoplasms · Nervous system disorders · Respiratory and chest disorders | 505 (47.3) 119 (11.1) 58 (5.4) 103 (9.6) 83 (7.8) 72 (6.7) 37 (3.5) | 479 (47.3) 96 (9.5) 76 (7.5) 90 (8.9) 80 (7.9) 66 (6.5) 44 (4.3) |
| NAT‐2 | ||
| Total number of participants | N = 134 | N = 129 |
| Total adverse events · Treatment emergent adverse events* · Ocular · Serious non‐ocular | 125 (83.3) 5 (4.7) 88 (58.4) 31 (23.1) | 115 (77.7) 2 (1.6) 74 (50.0) 30 (23.6) |
| * As defined by the study authors (including gastrointestinal disorders, allergic dermatitis and breath odour) | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Progression of AMD Show forest plot | 2 | Hazard Ratio (Fixed, 95% CI) | 0.96 [0.84, 1.10] | |

