نقش آمفتامینها در درمان اختلال نقص توجه و بیشفعالی (ADHD) در کودکان و نوجوانان
چکیده
پیشینه
اختلال نقص توجه و بیشفعالی (attention deficit hyperactivity disorder; ADHD)، یکی از شایعترین اختلالات روانپزشکی است که کودکان و نوجوانان را تحت تاثیر قرار میدهد. آمفتامینها از جمله شایعترین داروهایی هستند که برای کنترل ADHD تجویز میشوند. سه کلاس اصلی از آمفتامینها وجود دارند: دگزامفتامین (dexamphetamine)، لیزدگزامفتامین (lisdexamphetamine) و نمکهای آمفتامین مخلوط، که میتوانند به فرمولاسیونهای کوتاهاثر و طولانیاثر تقسیم شوند. برای ارزیابی اثربخشی و بیخطری (safety) آنها در این جمعیت، یک مرور سیستماتیک هرگز انجام نشده است.
اهداف
ارزیابی اثربخشی و بیخطری مصرف آمفتامینها در درمان ADHD در کودکان و نوجوانان.
روشهای جستوجو
در آگوست 2015، به جستوجو در CENTRAL؛ Ovid MEDLINE؛ Embase؛ PsycINFO، پایاننامه و تزهای ProQuest، و Networked Digital Library of Theses and Dissertations پرداختیم. همچنین ClinicalTrials.gov را جستوجو کرده و فهرست منابع مطالعات و مرورهای مرتبط را که از طریق جستوجو شناسایی شدند، بررسی کردیم. هیچ محدودیتی در مورد زبان نگارش مقاله یا دوره زمانی مطالعه اعمال نشد.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) گروه موازی (parallel‐group) و متقاطع (cross‐over) که مشتقات آمفتامین را با دارونما (placebo) در یک جمعیت از کودکان (کمتر از 18 سال) مبتلا به ADHD مقایسه کردند.
گردآوری و تجزیهوتحلیل دادهها
دو نویسنده بهطور مستقل از هم دادههای مربوط به شرکتکنندگان، محیط، مداخلات، روششناسی (methodology)، و پیامدها را برای هر مطالعه وارد شده استخراج کردند. برای پیامدهای پیوسته (continuous outcome)، تفاوت میانگین استاندارد شده (SMD) و برای پیامدهای دو حالتی (dichotomous outcome)، خطر نسبی (RR) را محاسبه کردیم. در جایی که امکانپذیر بود، متاآنالیزها را با استفاده از مدل اثرات تصادفی (random‐effects model) انجام دادیم. همچنین یک متاآنالیز را از شایعترین عوارض جانبی گزارش شده در مطالعات اولیه انجام دادیم.
نتایج اصلی
تعداد 23 کارآزمایی (8 کارآزمایی گروه موازی (parallel‐group) و 15 کارآزمایی متقاطع (cross‐over)) را، با 2675 کودک سه تا 17 سال، وارد کردیم. همه مطالعات آمفتامین ها را با دارونما (placebo) مقایسه کردند. مدت زمان مطالعه از 14 روز تا 365 روز متغیر بود، که اکثریت آنها کمتر از شش ماه به طول انجامیدند. بیشتر مطالعات در ایالات متحده انجام شدند؛ سه مطالعه در سراسر اروپا صورت گرفتند. تعداد 11 مطالعه وارد شده را به دلیل روشهای کورسازی (blinding) ناکافی، در نظر نگرفتن موارد خروج از مطالعه و حذف از آنالیز، و عدم ارائه گزارش از تمامی پیامدهای تعریفشده قبلی، در معرض خطر بالای سوگیری (bias) قضاوت کردیم. تعداد 12 مطالعه باقیمانده را به دلیل ارائه گزارش ناکافی، با خطر نامشخص سوگیری ارزیابی کردیم.
آمفتامینها موجب بهبود شدت نشانه اصلی ADHD بر اساس رتبهبندی والدین (SMD: ‐0.57؛ 95% فاصله اطمینان (CI): 0.86‐ تا 0.27‐؛ 7 مطالعه؛ 1247 کودک/نوجوان؛ شواهد با کیفیت بسیار پائین)، معلمان (SMD: ‐0.55؛ 95% CI؛ 0.83‐ تا 0.27‐؛ 5 مطالعه؛ 745 کودک/نوجوان؛ شواهد با کیفیت پائین) و پزشکان (SMD: ‐0.84؛ 95% CI؛ 1.32‐ تا 0.36‐؛ 3 مطالعه؛ 813 کودک/نوجوان؛ شواهد با کیفیت بسیار پائین) شدند. از سوی دیگر، زمانی که کودکان آمفتامین مصرف کردند، نسبت پاسخدهندگان به درمان که بر اساس مقیاس برداشت کلی بالینی از بهبودی (Clinical Global Impression ‐ Improvement; CGI‐I) رتبهبندی شد، بیشتر بود (RR: 3.36؛ 95% CI؛ 2.48 تا 4.55؛ 9 مطالعه؛ 2207 کودک/نوجوان؛ شواهد با کیفیت بسیار پائین).
شایعترین عوارض جانبی گزارششده شامل کاهش اشتها، بیخوابی/مشکل در به خواب رفتن، درد شکم، تهوع/استفراغ، سردرد و اضطراب بود. آمفتامینها با نسبت بیشتری از شرکتکنندگانی همراه بودند که دچار کاهش اشتها (RR: 6.31؛ 95% CI؛ 2.58 تا 15.46؛ 11 مطالعه؛ 2467 کودک/نوجوان)، بیخوابی (RR: 3.80؛ 95% CI؛ 2.12 تا 6.83؛ 10 مطالعه؛ 2429 کودک/نوجوان)، و درد شکم (RR: 1.44؛ 95% CI؛ 1.03 تا 2.00؛ 10 مطالعه؛ 2155 کودک/نوجوان) شدند. علاوه بر این، نسبتی از کودکان که دچار حداقل یک عارضه جانبی شدند، در گروه آمفتامین بیشتر بود (RR: 1.30؛ 95% CI؛ 1.18 تا 1.44؛ 6 مطالعه؛ 1742 کودک/نوجوان؛ شواهد با کیفیت پائین).
آنالیزهای زیر گروه را برای محصولات آمفتامین (دگزامفتامین، لیزدگزامفتامین، نمک آمفتامین مخلوط)، فرمولاسیون آزادسازی آمفتامین (طولانیاثر در برابر کوتاهاثر)، و منبع بودجه مالی (صنعت در برابر غیر صنعت) انجام دادیم. تفاوتهای میان گروهی از نظر نسبتی از شرکتکنندگان که دچار کاهش اشتها شدند، در هر دو زیر گروه محصولات آمفتامین (P < 0.00001) و فرمولاسیون آزادسازی آمفتامین (P value = 0.008)، همچنین برای رتنشن (retention) در زیر گروه فرمولاسیون آزادسازی آمفتامین (P value = 0.03) مشاهده شد.
نتیجهگیریهای نویسندگان
اغلب مطالعات وارد شده، خطر بالای سوگیری داشته و کیفیت کلی شواهد در اکثر پیامدها از پائین تا بسیار پائین متغیر بود. اگرچه آمفتامینها در کوتاهمدت در کاهش نشانههای اصلی ADHD موثر به نظر میرسند، برخی از عوارض جانبی را نیز در پی دارند. این مرور هیچ شواهدی را نیافت که از یکی از مشتقات آمفتامین نسبت به دیگری پشتیبانی کند، و هیچ تفاوتی را میان محصولات طولانیاثر و کوتاهاثر آمفتامین نشان نداد. کارآزماییهای آینده باید دوره طولانیتری داشته باشند (یعنی بیش از 12 ماه)، شامل پیامدهای روانیاجتماعی بیشتری شوند (مانند کیفیت زندگی و استرس والدین)، و با جزئیات گزارش شوند.
PICOs
خلاصه به زبان ساده
نقش آمفتامینها در درمان اختلال نقص توجه و بیشفعالی در کودکان و نوجوانان
پیشینه
اختلال نقص توجه و بیشفعالی (attention deficit hyperactivity disorder; ADHD) یک مشکل شایع در کودکان و نوجوانان است. ADHD با بیتوجهی (به راحتی دچار حواس پرتی شدن، ناتوانی در تمرکز روی یک کار و وظیفه)، تحریکپذیری (بیحوصلگی، تحرک مداوم) و بیشفعالی (بیتابی، بدون فکر کردن عمل کردن) مشخص میشود. یکی از شایعترین درمانها برای مدیریت ADHD، کلاس دارویی آمفتامینها است که گروهی هستند از داروهای محرک. تصور میشود که آنها شدت نشانههای مرتبط با ADHD را کاهش میدهند.
سوال مطالعه مروری
آیا درمان با آمفتامینها به منظور کاهش نشانههای اصلی ADHD در مقایسه با دیگر کودکان و نوجوانانی که هیچ دارویی دریافت نمیکنند یا با داروی تقلبی (دارونما (placebo)) درمان میشوند، برای کودکان و نوجوانان (زیر 18 سال) با تشخیص ADHD مزیتی دارد؟
ویژگیهای مطالعه
در جون 2015، تعداد 23 کارآزمایی تصادفیسازی و کنترل شده (RCTها: نوعی بررسی علمی که در آنها افراد به صورت تصادفی به یکی از دو یا چند گروه درمانی اختصاص مییابند) را شناسایی کردیم که شامل 2675 کودک و نوجوان در سنین میان سه و 17 سال بودند. این مطالعات آمفتامینها را با دارونما (placebo) مقایسه کردند. سه نوع مختلف آمفتامین مورد بررسی قرار گرفتند: دگزامفتامین (dexamphetamine)، لیزدگزامفتامین (lisdexamphetamine) و نمک آمفتامین مخلوط. طول دوره مطالعات وارد شده از 14 تا 365 روز متغیر بود. این RCTها در ایالات متحده و اروپا انجام شدند.
نتایج کلیدی
ما دریافتیم که آمفتامینها در بهبود نشانههای اصلی ADHD در کوتاهمدت موثر هستند، اما همچنین با خطر بالاتر ابتلا به عوارض جانبی مانند مشکلات خواب، کاهش اشتها و درد معده همراهی داشتند. هیچ شواهدی را نیافتیم دال بر اینکه یک نوع آمفتامین بهتر از دیگری است، میان آمفتامینهایی که در مدت زمان طولانیتری عمل میکنند در برابر مواردی که در مدت زمان کوتاهتری عمل میکنند، هیچ تفاوتی را مشاهده نکردیم.
کیفیت شواهد
به دلیل مشکلات موجود در طراحی آنها و تفاوتهای زیاد میان مطالعات، کیفیت مطالعات وارد شده در سطح پائین تا بسیار پائین بود. انجام RCTهایی با طراحی خوب و گزارشدهی شفاف با طول دوره بیشتر مورد نیاز است، بنابراین ممکن است تاثیرات طولانیمدت (هم مثبت و هم منفی) آمفتامینها را بهتر درک کنیم.
Authors' conclusions
Summary of findings
| Amphetamines compared with placebo for attention deficit hyperactivity disorder in children and adolescents | ||||||
| Patient or population: children or adolescents with ADHD Settings: Beligum, France, Germany, Hungary, Italy, Netherlands, Norway, Poland, Spain, Sweden, United Kingdom, United States Intervention: amphetamines (i.e. dexamphetamine, lisdexamphetamine, mixed amphetamine salts) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Amphetamine | |||||
| Total ADHD symptom score ‐ parent ratings (ADHD Rating Scale, Fourth Version; Conners' Rating Scale; Conners' Global Index; Conners' Abbreviated Symptom Questionnaire) | ‐ | The mean total score in the intervention groups was 0.57 standard deviations lower (‐0.86 to ‐0.27) | SMD ‐0.57 (‐0.86 to ‐0.27) | 1247 | ⊕⊝⊝⊝ | Moderate effect** |
| Total ADHD symptom score ‐ teacher ratings (ADHD Rating Scale, Fourth Version; Conners' Rating Scale; Conners' Global Index; Conners' Abbreviated Symptom Questionnaire) Follow‐up: 7 to 35 days | ‐ | The mean total score in the intervention groups was 0.55 standard deviations lower (‐0.83 to ‐0.27) | SMD ‐0.55 (‐0.83 to ‐0.27) | 745 | ⊕⊕⊝⊝ | Moderate effect** |
| Total ADHD symptom score ‐ clinician ratings (ADHD Rating Scale, Fourth Version) | ‐ | The mean total score in the intervention groups was 0.84 standard deviations lower (‐1.32 to ‐0.36) | SMD ‐0.84 (‐1.32 to ‐0.36) | 813 | ⊕⊝⊝⊝ | Large effect** |
| Proportion of responders (Clinical Global Impressions ‐ Improvement (CGI‐I) scale) | 187 per 1000 | 605 per 1000 | RR 3.36 (2.48 to 4.55) | 2207 | ⊝⊝⊝⊝ | ‐ |
| Academic performance (Permanent Product Measure of Performance; Wechsler Intelligence Scale for Children ‐ Revised; Barnell Lot, Ltd Math Test; Wide Range Achievement Test) Follow‐up: 7 to 21 days | ‐ | The mean score in the intervention groups was 0.51 standard deviations higher (0.31 to 0.70) | SMD 0.56 (0.39 to 0.73) | 826 | ⊕⊕⊝⊝ | Moderate effect** |
| Retention: proportion of participants who completed the trial | 825 per 1000 | 864 per 1000 | RR 1.03 | 2381 | ⊕⊝⊝⊝ | ‐ |
| Proportion of participants who experienced at least 1 adverse event | 366 per 1000 | 582 per 1000 | RR 1.30 (1.18 to 1.44) | 1742 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADHD: Attention deficit hyperactivity disorder; CI: Confidence interval; GRADE: Grades of recommendation, assessment, development and evaluation; RR: Risk ratio; SMD: Standardized mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to the majority of studies included in this outcome having a high risk of bias. | ||||||
Background
Description of the condition
Attention deficit hyperactivity disorder (ADHD) is one of the most common pediatric psychiatric conditions, affecting around 5% of children worldwide (Polanczyk 2007). ADHD is characterized by three core symptoms: inattention, impulsivity and hyperactivity, which are more frequently displayed than would be typical in children of the same age (APA 2000). The core symptoms are often presented to various degrees in different children, breaking ADHD down into three subtypes: the predominantly inattentive type, the predominantly hyperactive‐impulsive type, and the combined type (i.e. children displaying both inattention and hyperactivity) (APA 2000). The condition is often diagnosed at a young age, usually between the ages of three and six years (NIMH 2009). The potential for comorbidity is extremely high in this population and comorbidities are present in almost two‐thirds of pediatric ADHD cases, with the most common being oppositional defiant disorder (50%), conduct disorder (35%), anxiety disorder (33%), and depression (33%) (AHRQ 1999; Mayes 2009).
The symptoms of ADHD have been shown to permeate a child's performance across multiple settings, having long‐term effects on their academic performance and social development. Studies have also shown that children with ADHD are more likely to be irritable, impatient, and aggressive (NIH 2000). In addition, families who have children with ADHD often experience higher levels of parental stress and frustration, marital disruption, and social isolation (Edwards 1995). It has been estimated that 50% of childhood ADHD cases will persist into adolescence and adulthood (Biederman 1993), making it a chronic lifetime condition for many.
Description of the intervention
A wide variety of treatments have been used for the management of ADHD, including psychosocial interventions, dietary management, herbal and homeopathic remedies, and biofeedback. However, for the past few decades, the psychostimulant, methylphenidate, has been the first line of treatment (APA 2000), and has been found to be effective in 70% to 90% of school‐aged children (NIH 2000; Wigal 1999). Amphetamines are the second most frequently prescribed psychostimulant for pediatric ADHD, and are becoming an increasingly popular alternative for children who fail to respond to methylphenidate (Buck 2002). There are currently three different amphetamine preparations available, including: dexamphetamine (dextroamphetamine or d‐amphetamine sulfate), which comes in both short‐acting and long‐acting formulations; lisdexamphetamine, which is available as a long‐acting formulation (Vyvanase); and mixed amphetamine salts, which also comes in both short‐acting as well as long‐acting preparations (Buck 2002; The Medical Letter 2007).
How the intervention might work
Although the pathophysiology of ADHD is poorly understood, evidence has suggested that ADHD may be the result of insufficient production of norepinephrine and dopamine in the prefrontal cortex (Arnsten 2006). As such, the executive functions carried out by the prefrontal cortex are impaired, resulting in forgetfulness, distractibility, impulsivity, and inappropriate social behaviours (Anderson 1999). Others believe that the limbic system plays a major role in the pathophysiology of ADHD, and it is thought that hyperactivity and impulsivity result from abnormally low tonic dopamine activity within this region of the brain (Moore 2011). In either case, as a psychostimulant, amphetamines are thought to both promote marked neurotransmitter release into the synaptic cleft as well as disrupt normal reuptake of neurotransmitters, thereby increasing levels of norepinephrine and dopamine in these regions of the brain and affecting executive functioning (Arnsten 2006; Swanson 2007). A Cochrane Review of amphetamines for ADHD in adults found they improved short‐term symptom severity (Castells 2011).
Why it is important to do this review
Despite being one of the most thoroughly researched disorders in medicine, one of the major controversies regarding ADHD is the use of psychostimulants as a treatment option. While current evidence suggests that amphetamines may be beneficial for improving the core symptoms of ADHD, their effects on academic and social domains remain inconsistent and unclear (NIH 2000). Wide variations in the use and prescription of amphetamines across communities suggest that there is a lack of consensus among practitioners regarding which people with ADHD should be treated with amphetamines. Charach 2011 and Miller 1999 have conducted reviews assessing amphetamines for pediatric ADHD; however, the former focused only on long‐term effectiveness of amphetamines (i.e. > 12 months), while the latter is not only out of date, but also focused solely on the dexamphetamine preparation. It is imperative for healthcare providers, parents, and those diagnosed with ADHD to be aware of the most suitable treatment options available, and how they differ in terms of their efficacy and safety profiles. Our synthesis of all available, randomized controlled trials assessing the efficacy and safety of amphetamines for pediatric ADHD will provide evidence to better inform clinical practice and further research relating to ADHD management. While assessing amphetamines against other ADHD treatments, such as methylphenidate, psychotherapy and antidepressants is important, establishing whether amphetamines are superior to placebo is a necessary first step. Thus, this review will focus only on the amphetamine versus placebo comparison.
Objectives
To assess the efficacy and safety of amphetamines for ADHD in children and adolescents.
Methods
Criteria for considering studies for this review
Types of studies
Parallel‐group and cross‐over randomized controlled trials (RCTs).
Types of participants
Children and adolescents under 18 years of age and diagnosed with ADHD using specified diagnostic criteria such as the Diagnostic and Statistical Manual of Mental Disorders Third Edition (DSM‐III) (APA 1987), Fourth Edition (DSM‐IV) (APA 2000), or equivalent (note: since the fifth edition (DSM‐5) was released during the conduct of this review, studies utilizing this criteria are not included). We included trials that involved children/adolescents with some comorbid conditions (oppositional defiant disorder, conduct disorder, and anxiety). We excluded trials whose inclusion criteria included children/adolescents with psychiatric comorbidity that require highly specialized treatment programs (for example, autism, bipolar disorder, and psychosis).
Types of interventions
Intervention
Any oral form of amphetamine (i.e. amphetamine, dexamphetamine, lisdexamphetamine and mixed amphetamine salts), at any dose.
Control
Placebo.
Types of outcome measures
Primary outcomes
-
Change in core ADHD symptoms* (inattention, hyperactivity, impulsivity), as measured by a validated scale rated by children, parents, teachers, clinicians, or investigators such as Conners’ Parent Rating Scale ‐ Revised (CPRS‐R) (Conners 1998a), Conners’ Teacher Rating Scale ‐ Revised (CTRS‐R) (Conners 1998b), or the ADHD Rating Scale, Fourth Version (ADHD‐RS‐IV) (DuPaul 1998).
Secondary outcomes
-
Clinical improvement*, as measured by, for example, the Clinical Global Impression ‐ Improvement scale (CGI‐I) (Guy 1976).
-
Clinical severity, as measured by, for example, the Clinical Global Impression ‐ Severity scale (CGI‐S) (Guy 1976).
-
Academic performance*, as measured by any validated tool that purports to assess academic performance such as the Wechsler Intelligence Scale for Children (WISC) (Wechsler 1991).
-
Quality of life, as measured by a validated scale such as the Pediatric Quality of Life Inventory ‐ 32 (PedsQL‐32) (Varni 1998).
-
Retention: proportion of randomized participants who completed the trial*.
-
Adverse events (such as nausea, insomnia/sleep problems, and decreased appetite).
-
Proportion of adverse events.
-
Proportion of participants who experienced at least one adverse event*, as reported in the trials.
-
Proportion of participants who withdrew due to any adverse event.
-
Outcomes marked with an asterisk (*) were used to populate summary of findings Table for the main comparison.
Time frames were denoted as short term (up to six months), medium term (between six and 12 months), and long term (over 12 months).
See Table 1 for further information.
| Types of outcome measures | Primary outcomes Multiple perspectives (i.e. teacher, parent, clinician) are considered the gold standard when assessing the core symptoms of ADHD. As such, we will not favor one perspective over another. In the event that reports do not agree with one another, for example, teacher reports disagree with parent reports on the improvement of core symptoms, this may be quite telling about how a child’s environment impacts their ADHD given the varying demands between a school environment and home environment. This will be interpreted accordingly in the discussion. Secondary outcomes We will assess 'parental stress' as a secondary outcome. |
| Measures of treatment effect | Dichotomous outcome data When a single study has utilized more than one measure to assess the same construct (e.g. ADHD core symptoms as assessed by teacher‐rated ADHD‐RS‐IV and teacher ratings of the Conners’ ADHD Rating Scale), treatment effects will be averaged across outcome measures in order to arrive at a single treatment effect for use in the meta‐analysis. Continuous outcome data For continuous outcomes, where the same rating scale has been used for all studies, we will calculate mean differences. |
| Unit of analysis issues | Cross‐over trials For meta‐analyses that use that use a mean difference, we will compute standard deviations for the cross‐over trials taking into account correlation. If correlation coefficients are not available, we will impute them from other studies or use 0.5 as a conservative estimate (Follman 1992). For cross‐over trials where carry‐over is thought to be a problem, where no washout period is present, or when only data from the first period are available, we will analyze data from the first period only. Studies with multiple time points In studies where results are presented for several periods of follow‐up, we will analyze each outcome at each point in a separate meta‐analysis with other comparable studies taking measures at a similar time frame post‐randomization. Time frames will reflect short‐term (up to six months), medium‐term (between 6 months and 12 |
| Assessment of reporting biases | For each primary meta‐analysis in which we have identified a sufficient number of studies (n ≥ 10) for inclusion, we will draw funnel plots in order to assess the possibility of publication bias. |
| Subgroup analysis and investigation of heterogeneity | We will conduct the following subgroup analyses.
|
| Sensitivity analysis | We will conduct the following sensitivity analyses.
|
ADHD: attention deficit hyperactivity disorder.
ADHD‐RS‐IV: Attention Deficit Hyperactivity Rating Scale, Fourth Version.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases in May 2013, July 2014, and again on 12 August 2015.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2015 Issue 7; Ovid), which includes the Specialised Register of the Cochrane Developmental, Psychosocial and Learning Problems Group.
-
Ovid MEDLINE (1948 to Week 1, August 2015).
-
Embase (1974 to Week 1, August 2015; Ovid).
-
PsycINFO (1806 to Week 1, August 2015; Ovid).
-
ProQuest Dissertations and Theses (all available years).
-
Networked Digital Library of Theses and Dissertations (ndltd.org; all available years).
-
ClinicalTrials.gov (clinicaltrials.gov; all available years).
No language or date restrictions were applied.
Please see Appendix 1 to Appendix 7 for our search strategies.
Searching other resources
We inspected the reference lists of identified RCTs and review articles to identify additional publications.
Data collection and analysis
Selection of studies
Two review authors (SP and LS) independently screened all titles and abstracts retrieved from the search to identify those that appeared to meet the inclusion criteria. The same authors then obtained the full‐text articles of those studies and assessed their eligibility. Disagreements were resolved by SV.
Data extraction and management
Two review authors (SP and LS) independently extracted data related to study methods, participant characteristics, and outcomes by using a pre‐designed data collection form. Disagreements were resolved through discussion. SP entered all relevant data into Review Manager (RevMan 2014).
We emailed study authors up to three times (minimum one month wait between contact) to obtain missing or unclear data.
Assessment of risk of bias in included studies
Using the Cochrane 'Risk of bias' tool (Higgins 2011a), two review authors (SP and LS) independently assessed each included study as being at low risk, high risk, or unclear (uncertain) risk of bias for each of the seven domains explained in Appendix 8. Disagreements were resolved through discussion.
Measures of treatment effect
Dichotomous outcome data
We calculated the risk ratio (RR) and 95% confidence intervals (CIs) for dichotomous outcomes.
Continuous outcome data
For continuous outcomes, we used the Hedges’ method to calculate standardized mean differences (SMDs) with individual study weights calculated as the inverse of the variance, presented with 95% CIs (Hedges 1994). To ensure that all scales were pointing in the same direction, we multiplied the mean value of one set by ‐1 (Deeks 2011). We combined change scores and endpoint scores, however when both types of scores were available in the same study, priority was given to change scores since they adjust for any imbalances in baseline characteristics.
See Table 1 for further methods archived for future updates of this review.
Unit of analysis issues
Cross‐over trials
Since we calculated SMDs for all our continuous outcomes, we treated cross‐over studies as if they were parallel and computed a pooled standard deviation. Although this method does not account for the correlation in cross‐over studies, it prevented any overestimation of effect sizes, which is desirable when computing SMDs. Carry‐over was not reported in any of the cross‐over studies.
See Table 1 for further methods archived for future updates of this review.
Studies with multiple comparisons
For studies with more than two independent comparisons, such as amphetamine versus placebo versus psychotherapy, we excluded the psychotherapy arm. We handled studies with multiple and correlated interventions, for example, lisdexamphetamine versus mixed amphetamine salts versus placebo, or 10 mg dexamphetamine versus 20 mg of dexamphetamine versus placebo in the following way. For continuous outcomes of parallel‐group studies, we calculated means using the formulae described in Table 7.7.a of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). For dichotomous outcomes of parallel‐group studies, we summed the number of events across intervention arms. For continuous outcomes of cross‐over studies, we averaged both the means and the standard deviations of the relevant intervention arms across the groups. For dichotomous outcomes of cross‐over studies, we randomly dropped one arm and used the other in the meta‐analysis.
Studies with multiple time points
We analysed studies separately according to their time frame. Time frames were denoted as short term (up to six months), medium term (between six and 12 months), and long term (over 12 months). All but one study (Gillberg 1997) were considered short term. Since Gillberg 1997 was the only medium‐term study, it was excluded from the meta‐analysis.
See Table 1 for further analyses archived for future updates of this review.
Dealing with missing data
We emailed study authors up to three times (with at least one month between contacts) to obtain missing data. For those studies that did not report outcomes using intention‐to‐treat analysis and for which missing data were unobtainable, we used the number of randomized participants as the denominator for dichotomous variables. For continuous outcomes, we used the sample size to calculate the mean and standard deviations in the study. For studies that did not report standard deviations, we calculated it from P values, CIs, or standard errors (as described in section 7.7.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b)). We did not use any imputations to deal with missing data.
Assessment of heterogeneity
We assessed statistical heterogeneity by examining the I² statistic (Higgins 2003), which quantifies the degree of heterogeneity in a meta‐analysis, and Chi² statistic (P value less than 0.10 as evidence of heterogeneity). We also reported Tau² estimates for each random‐effects meta‐analysis (Deeks 2011).
We explored heterogeneity by conducting a series of subgroup analyses (Subgroup analysis and investigation of heterogeneity), which were selected a priori and based on preliminary evidence from other studies (Castells 2011; Lundh 2012).
Assessment of reporting biases
See Table 1 for methods archived for future updates of this review.
Data synthesis
We synthesized the results in a meta‐analysis using the random‐effects model since studies were fairly heterogeneous in terms of their study design (inclusion of parallel‐group and cross‐over trials), intervention protocols, and study duration. We used the inverse variance method for continuous outcomes, and the Mantel‐Haenszel method for dichotomous outcomes.
Summary of findings
In summary of findings Table for the main comparison, we present data on the following outcomes: total ADHD symptom score ‐ parent ratings, total ADHD symptom score ‐ teacher ratings, total ADHD symptom score ‐ clinician ratings, proportion of responders, academic performance, proportion of participants who completed the trial, and proportion of participants who experienced at least one adverse event. We presented continuous outcomes as SMDs and 95% CIs, and dichotomous outcomes as RRs and 95% CIs. Data regarding number of participants and studies were presented for each outcome. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE Working Group 2004) to determine the quality of the evidence, where evidence was downgraded if (1) the majority (> 50%) of included studies had a high risk of bias; (2) the outcome included comparisons of different amphetamine derivatives and release formulations; (3) the outcome had significant statistical heterogeneity (I² > 50%); and (4) the outcome had wide 95% CIs indicating that the intervention effect was highly variable.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses.
-
Type of amphetamine: dextroamphetamine, lisdexamphetamine, or mixed amphetamine salts.
-
Type of amphetamine release formulation: long acting (extended release) or short acting (immediate release).
-
Funding source: with or without pharmaceutical industry funding. Since some studies failed to report their funding source, we grouped studies as 'industry funded', 'publicly funded', or 'not reported'.
We conducted subgroup analyses on the following outcomes, which had a sufficient number of studies (more than five), regardless of the degree of statistical heterogeneity present in the main analysis:
-
total score on core symptom ADHD scale ‐ parent ratings;
-
proportion of responders according to CGI‐I (Guy 1976);
-
academic performance;
-
retention: proportion of participants who completed the trial;
-
proportion of participants who dropped out/withdrew due to an adverse event;
-
proportion of participants experiencing decreased appetite;
-
proportion of participants experiencing insomnia;
-
proportion of participants experiencing abdominal pain; and
-
proportion of participants experiencing headaches.
-
We calculated a pooled effect size for each subgroup.
We were unable to conduct a subgroup analysis when all of the studies in a particular meta‐analysis belonged to only one strata of any subgroup.
See Table 1 for further analyses archived for future updates of this review.
Sensitivity analysis
We repeated our meta‐analyses using a fixed‐effect model.
See Table 1 for further analyses archived for future updates of this review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
Figure 1 summarizes the flow of studies through the screening process. The electronic databases retrieved 7011 records while other sources yielded 198 records. After removing duplicates, we identified 5210 records for further consideration. After screening titles and available abstracts, we examined the full texts of 324 records, 34 met our inclusion criteria. From these, we identified 23 studies, four of which had multiple reports. These were: Borcherding 1990 (four reports), Coghill 2013 (three reports), Donnelly 1989 (two reports; one of which was the pilot, and the other was the full study), and Ramtvedt 2013 (two reports). In addition, we identified two ongoing clinical trials (Fanton 2009; NCT01711021); although recruitment has ended for both of these trials, none of the results have been published. We contacted the authors of both studies three times yielding no response. One non‐English language study is awaiting classification until we can ascertain if it was randomized and whether participants had a formal diagnosis of ADHD (Glos 1973). This information is unobtainable given our inability to contact the author. Another study also awaits classification as only the abstract has been published (Itil 1974). Information on whether treatments were randomized and whether participants had a formal diagnosis is needed. We contacted the authors three times yielding no response.

Study flow diagram
Included studies
Twenty‐three studies met the inclusion criteria: 15 studies were cross‐over trials (Barkley 2000; Biederman 2007a; Borcherding 1990; Childress 2015; Donnelly 1989; James 2001; Manos 1999; McCracken 2003; Nemzer 1986; Ramtvedt 2013; Sharp 1999; Shekim 1986; Short 2004; Swanson 1998a; Wigal 2009a), while eight studies were parallel‐group trials (Biederman 2002; Biederman 2007b; Coghill 2013; Findling 2011; Giblin 2011; Gillberg 1997; Pliszka 2000; Spencer 2006a).
Ten studies included a single comparison of an amphetamine derivative versus placebo (Borcherding 1990; Childress 2015; Coghill 2013; Donnelly 1989; Gillberg 1997; Nemzer 1986; Pliszka 2000; Ramtvedt 2013; Sharp 1999; Shekim 1986), and 11 studies compared more than one dose of an amphetamine derivative with placebo (Barkley 2000; Biederman 2002; Biederman 2007b; Findling 2011; Giblin 2011; Manos 1999; McCracken 2003; Short 2004; Spencer 2006a; Swanson 1998a; Wigal 2009a); two studies compared more than one amphetamine derivative, each at various doses, versus placebo (Biederman 2007a; James 2001).
Participants
In total, the 23 included studies recruited 2675 children and adolescents aged between three years and 17 years, 72% (n = 1925) of whom were boys; one study did not report the number of included boys and girls (Pliszka 2000).
Twenty‐two studies used various versions of the DSM criteria to confirm ADHD diagnosis in their participants, including criteria from the Third Edition (DSM‐III; four trials; n = 102; Borcherding 1990; Donnelly 1989; Nemzer 1986; Shekim 1986); Third Edition Revised (DSM‐III‐R; one trial; n = 62; Gillberg 1997); Fourth Edition (DSM‐IV; eight trials; n = 899; Barkley 2000; Biederman 2002; James 2001; Manos 1999; McCracken 2003; Sharp 1999; Short 2004; Swanson 1998a), and Fourth Edition, Text Revision (DSM‐IV‐TR; nine trials; n = 1553; Biederman 2007a; Biederman 2007b; Childress 2015; Coghill 2013; Findling 2011; Giblin 2011; Ramtvedt 2013; Spencer 2006a; Wigal 2009a). Pliszka 2000 (n = 59) diagnosed ADHD using the Diagnostic Interview Schedule for Children (Costello 1985).
Interventions
Twelve studies assessed mixed amphetamine salts (Barkley 2000; Biederman 2002; Biederman 2007a; Childress 2015; Gillberg 1997; James 2001; Manos 1999; McCracken 2003; Pliszka 2000; Short 2004; Spencer 2006a; Swanson 1998a); seven studies used dextroamphetamine (Borcherding 1990; Donnelly 1989; James 2001; Nemzer 1986; Ramtvedt 2013; Sharp 1999; Shekim 1986); and six studies looked at lisdexamphetamine (Biederman 2007a; Biederman 2007b; Coghill 2013; Findling 2011; Giblin 2011; Wigal 2009a). Two studies assessed two amphetamine derivatives (Biederman 2007a; James 2001).
Twelve studies randomized children and adolescents to set doses or dosing schedules (Barkley 2000; Biederman 2002; Biederman 2007b; Coghill 2013; Findling 2011; Giblin 2011; Manos 1999; McCracken 2003; Ramtvedt 2013; Short 2004; Spencer 2006a; Swanson 1998a). Seven studies used weight‐based dosing (Borcherding 1990; Donnelly 1989; James 2001; Nemzer 1986; Pliszka 2000; Sharp 1999; Shekim 1986), while six studies titrated children and adolescents to their optimal dose (Biederman 2007a; Childress 2015; Gillberg 1997; Pliszka 2000; Shekim 1986; Wigal 2009a). Two studies used both weight‐based dosing and titration (Pliszka 2000; Shekim 1986). The mean (range) doses investigated in the included studies were 34.22 mg/day (7.8 mg/day to 90 mg/day) for dextroamphetamine, 50.24 mg/day (30 mg/day to 70 mg/day) for lisdexamphetamine, and 19.86 mg/day (5 mg/day to 120 mg/day) for mixed amphetamine salts.
Duration
Study intervention length ranged from 14 days to 365 days, with a median of 28 days. Only one study was longer than 63 days (Gillberg 1997).
Location
Twenty studies were conducted in the United States (Barkley 2000; Biederman 2002; Biederman 2007a; Biederman 2007b; Borcherding 1990; Childress 2015; Donnelly 1989; Findling 2011; Giblin 2011; James 2001; Manos 1999; McCracken 2003; Nemzer 1986; Pliszka 2000; Sharp 1999; Shekim 1986; Short 2004; Spencer 2006a; Swanson 1998a; Wigal 2009a). One multicenter trial was conducted in 48 centers across 10 countries: Belgium, France, Germany, Hungary, Italy, Netherlands, Poland, Spain, Sweden, and the United Kingdom (Coghill 2013). The two remaining studies were conducted in Sweden (Gillberg 1997) and Norway (Ramtvedt 2013).
Outcomes
Details of all ADHD core symptom outcome measures used by study can be found in the Characteristics of included studies and Table 2. The most commonly used outcome tool for the primary outcome included the Conners' Rating Scales (Conners 1998a; Conners 1998b) and the ADHD‐RS‐IV (DuPaul 1998).
For secondary outcomes, the most commonly utilized outcome tool for academic performance was the Permanent Product Measure of Performance (PERMP; Swanson 1998b). Only one study assessed quality of life (Findling 2011), and used the Youth Quality of Life ‐ Research Version questionnaire (YQOL‐R; Salum 2012)
| Outcome | Outcome measure (respondent) | Studies | Measure used in meta‐analysis |
| Inattention | ADHD Rating Scale, Fourth Version (parent ratings) | No (data presented in an unusable format) | |
| ADHD Rating Scale, Fourth Version (clinician ratings) | Yes | ||
| Yes | |||
| Yes | |||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Yes | ||
| Conners’ Rating Scale (parent ratings) | Yes | ||
| No (only study that included long‐term data) | |||
| Conners’ Rating Scale (teacher ratings) | No (only study that included long‐term data) | ||
| IOWA Conners’ Rating Scale | Yes | ||
| SKAMP scale (teacher ratings) | No (data not available) | ||
| SKAMP scale (investigator/research personnel ratings) | Yes | ||
| Yes | |||
| Hyperactivity/impulsivity | ADHD Rating Scale, Fourth Version (parent ratings) | No (data presented in an unusable format) | |
| ADHD Rating Scale, Fourth Version (clinician ratings) | Yes | ||
| Yes | |||
| Yes | |||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Yes | ||
| Conners’ Rating Scale (parent ratings) | No (only study that included long‐term data) | ||
| Yes | |||
| Conners’ Rating Scale (teacher ratings) | No (only study that included long‐term data) | ||
| Yes | |||
| Total core symptom score | ADHD Rating Scale, Fourth Version (parent ratings) | Yes | |
| Yes | |||
| ADHD Rating Scale, Fourth Version (teacher ratings) | Yes | ||
| ADHD Rating Scale, Fourth Version (clinician ratings) | Yes | ||
| Yes | |||
| Yes | |||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Yes | ||
| No (no data available) | |||
| Conners’ Rating Scale (parent ratings) | No (data presented in an unusable format) | ||
| Yes | |||
| No (data not available) | |||
| No (only study that included long‐term data) | |||
| Yes | |||
| No (data not available) | |||
| No (data presented in an unusable format) | |||
| Conners’ Rating Scale (teacher ratings) | No (no data available) | ||
| Yes | |||
| No (only study that included long‐term data) | |||
| Yes | |||
| No (data not available) | |||
| No (data presented in an unusable format) | |||
| Conners’ Global Index (parent ratings) | Yes | ||
| Yes | |||
| Conners’ Global Index (teacher ratings) | Yes | ||
| Conners’ Abbreviated Symptom Questionnaire (parent ratings) | Yes | ||
| Conners’ Abbreviated Symptom Questionnaire (teacher ratings) | Yes | ||
| ADHD Questionnaire (developed within study) (parent ratings) | No (data presented in an unusable format) | ||
| ADHD Questionnaire (developed within study) (teacher ratings) | No (data presented in an unusable format) | ||
| SKAMP scale (investigator/research personnel ratings) | Yes |
ADHD: attention deficit hyperactivity disorder.
IOWA: inattention/overactivity with aggression.
SKAMP: Swanson, Kotkin, Agler, M‐Flynn and Pelham scale.
Excluded studies
We excluded a total of 290 studies. We excluded 264 clearly irrelevant reports and formally excluded 26 studies for the following reasons: 17 studies because they were not RCTs or used multiple cross‐over designs (this review only included single cross‐over RCTs); three studies because there was no placebo comparison, three studies because they did not use formal ADHD diagnostic criteria; one study because it had no direct amphetamine ‐ placebo comparison; one study because participants had ineligible comorbid conditions; and one study because it included adults.
See also Characteristics of excluded studies tables.
Risk of bias in included studies
A more in depth risk of bias assessment for each study can be found in Characteristics of included studies. In addition, Figure 2 provides a summary of this assessment.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
Only three studies reported on how the random sequence was generated and were assessed as being at 'low' risk of bias on this domain (Biederman 2007b; Childress 2015; Findling 2011). We rated the other 20 studies as 'unclear' risk of bias as they did not adequately describe their methods of randomization.
Allocation concealment
Four studies described the methods used to conceal the allocation sequence and were rated as being at 'low' risk of bias on this domain (Biederman 2007a; Coghill 2013; Findling 2011; Manos 1999). The rest of the studies we assessed as 'unclear' risk of bias, as they did not sufficiently describe their methods of allocation concealment.
Blinding
Performance bias
Although blinding was intended in all of the studies, we assessed risk of bias by how authors described their amphetamine and placebo capsules and rated 10 studies as being at 'low' risk of bias on this domain (Biederman 2007b; Coghill 2013; James 2001; Manos 1999; Nemzer 1986; Pliszka 2000; Sharp 1999; Short 2004; Swanson 1998a; Wigal 2009a). We rated one study as being at 'high' risk of bias on this domain since they described their intervention and placebo as not being identical (Ramtvedt 2013). The other 12 studies we marked as being at 'unclear' risk of bias since they were not explicit about the similarities between the two interventions.
Detection bias
Only two studies explicitly stated that outcome assessors were blinded to interventions and therefore we judged them to be at 'low' risk of bias (Manos 1999; Short 2004). The other 21 studies we rated as 'unclear' risk of bias since they were not explicit about which parties were blinded to the intervention assignment.
Incomplete outcome data
We rated thirteen studies that adequately addressed dropouts and used appropriate statistical methods to compensate for dropouts as having a 'low' risk of bias on this domain (Biederman 2002; Biederman 2007a; Biederman 2007b; Childress 2015; Donnelly 1989; Findling 2011; Gillberg 1997; James 2001; McCracken 2003; Pliszka 2000; Sharp 1999; Spencer 2006a; Wigal 2009a). Seven studies failed to provide reasons for dropouts and failed to address any exclusions from their analyses, and therefore we rated them as having a 'high' risk of bias (Barkley 2000; Borcherding 1990; Coghill 2013; Ramtvedt 2013; Shekim 1986; Short 2004; Swanson 1998a). The three remaining studies did not discuss dropouts in their reports and we rated them as being at 'unclear' risk of bias (Giblin 2011; Manos 1999; Nemzer 1986).
Selective reporting
We assessed 17 studies as having 'unclear' risk of bias on this domain as the study protocols for most of them were not available, so we could not assess reporting bias (Barkley 2000; Biederman 2002; Biederman 2007b; Borcherding 1990; Donnelly 1989; Giblin 2011; Gillberg 1997; James 2001; Manos 1999; McCracken 2003; Nemzer 1986; Pliszka 2000; Sharp 1999; Shekim 1986; Short 2004; Swanson 1998a; Wigal 2009a). We rated four studies as having a 'low' risk of bias, as they appropriately reported on all outcomes defined in their protocols (Biederman 2007a; Childress 2015; Findling 2011; Spencer 2006a). Two studies we assessed as having a 'high' risk of bias since they failed to report on all outcomes mentioned in their registered protocols (Coghill 2013; Ramtvedt 2013).
Other potential sources of bias
We rated three studies as being at 'unclear' risk of bias on this domain since the validity of their primary outcome tools were not described (Borcherding 1990; Donnelly 1989; Ramtvedt 2013). The other 20 studies appeared to be free of other potential sources of bias and therefore we rated them as being at 'low' risk of bias on this domain.
Effects of interventions
See: Summary of findings for the main comparison
We included 19 studies in meta‐analyses, however, two of those studies had measured other outcomes that were relevant to this review, but were not reported in their results (Borcherding 1990; Swanson 1998a). Biederman 2007b had reported some of their results as bar graphs, which, when extracted using graphic digitizer software, gave implausible results and therefore was excluded from the meta‐analysis on those outcomes. Four studies were excluded from all meta‐analyses: Giblin 2011 had not reported data on any of the relevant outcomes in this review; Gillberg 1997 was the only medium‐term study and therefore could not be combined with the other short‐term studies; Ramtvedt 2013 had aggregated their parent‐ and teacher‐rated ADHD scores; and Short 2004 assessed amphetamines versus methylphenidate versus placebo and pooled the amphetamine and methylphenidate data in their results; we were unable to isolate the amphetamine versus placebo comparison.
Primary outcome
Change in core ADHD symptoms
We conducted a series of meta‐analyses for the primary outcome, change in core ADHD symptoms (inattention, hyperactivity, impulsivity), as measured by a validated scale rated by children, parents, teachers, clinicians, or investigators (Analysis 1.1 to Analysis 1.11).
For all 11 outcomes, amphetamines were superior to placebo for reducing the core symptoms of ADHD.
-
Total ADHD symptom score ‐ parent ratings (SMD ‐0.57; 95% CI ‐0.86 to ‐0.27; Tau² = 0.10; I² = 77%; 7 studies; 1247 children/adolescents; Analysis 1.1).
-
Hyperactivity/impulsivity ‐ parent ratings (SMD ‐0.54; 95% CI ‐0.89 to ‐0.19; Tau² = 0.00; I² = 0%; 2 studies; 132 children/adolescents; Analysis 1.2).
-
Total ADHD symptom score ‐ teacher ratings (SMD ‐0.55; 95% CI ‐0.83 to ‐0.27; Tau² = 0.04; I² = 41%; 5 studies; 745 children/adolescents; Analysis 1.3).
-
Hyperactivity/impulsivity ‐ teacher ratings (SMD ‐1.13; 95% CI ‐1.63 to ‐0.62; 1 study; 70 children/adolescents; Analysis 1.4).
-
Inattention ‐ teacher ratings (SMD ‐1.43; 95% CI ‐2.35 to ‐0.52; 1 study; 24 children/adolescents; Analysis 1.5).
-
Total ADHD symptom score ‐ clinician ratings (SMD ‐0.84; 95% CI ‐1.32 to ‐0.36; Tau² = 0.16; I² = 88%; 3 studies; 813 children/adolescents; Analysis 1.6).
-
Hyperactivity/impulsivity ‐ clinician ratings (SMD ‐0.75; 95% CI ‐1.28 to ‐0.23; Tau² = 0.20; I² = 90%; 3 studies; 813 children/adolescents; Analysis 1.7).
-
Inattention ‐ clinician ratings (SMD ‐0.78; 95% CI ‐1.26 to ‐0.30; Tau² = 0.16; I² = 88%; 3 studies; 813 children/adolescents; Analysis 1.8).
-
Total ADHD symptom score ‐ investigator/research personnel ratings (SMD ‐1.15; 95% CI ‐1.87 to ‐0.44; Tau² = 0.37; I² = 94%; 3 studies; 630 children/adolescents; Analysis 1.9).
-
Hyperactivity/impulsivity ‐ investigator/research personnel ratings (SMD ‐1.46; 95% CI ‐ 1.83 to ‐1.08; Tau² = 0.03; I² = 41%; 2 studies; 280 children/adolescents; Analysis 1.10).
-
Inattention ‐ investigator/research personnel ratings (SMD ‐0.73; 95% CI ‐1.42 to ‐0.04; Tau² = 0.46; I² = 94%; 4 studies; 634 children/adolescents; Analysis 1.11).
It is important to note, however, that the majority of these meta‐analyses included between one and three studies, and that Analysis 1.1, Analysis 1.6, Analysis 1.7, Analysis 1.8, Analysis 1.9, and Analysis 1.11 had considerable heterogeneity present with I² ranging from 77% to 94%. Only three outcomes included more than three studies: total ADHD symptom score ‐ parent ratings (seven studies; Analysis 1.1; Figure 3), total ADHD symptom score ‐ teacher ratings (five studies; Analysis 1.3; Figure 4), and total ADHD symptom score ‐ investigator/research personnel ratings (four studies; Analysis 1.11).
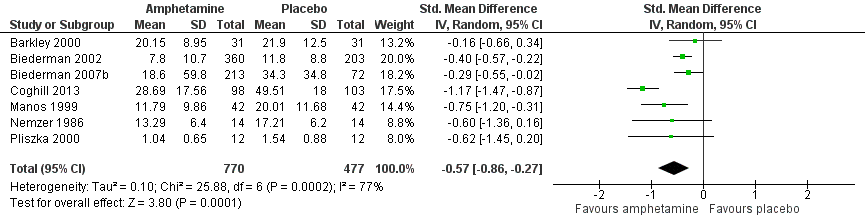
Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.1 Total ADHD symptom score ‐ parent ratings.

Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.3 Total ADHD symptom score ‐ teacher ratings.
Secondary outcomes
We conducted meta‐analyses that compared amphetamines versus placebo on five of our six secondary outcomes (see Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16).
Clinical improvement
The proportion of responders was higher in the amphetamine group (RR 3.36; 95% CI 2.48 to 4.55; Tau² = 0.14; I² = 72%; 9 studies; 2207 children/adolescents; Analysis 1.12)
Clinical severity
We found evidence of a significant difference between the two groups on the CGI‐S (Guy 1976), in favour of amphetamine (SMD ‐0.86; 95% CI ‐1.72 to ‐0.01; Tau² = 0.25; I² = 64%; 2 studies; 86 children/adolescents; Analysis 1.13).
Academic performance
We found evidence that amphetamines may improve academic performance as compared to placebo (SMD 0.56; 95% CI 0.39 to 0.73; Tau² = 0.02; I² = 28%; 8 studies; 826 children/adolescents; Analysis 1.14).
Quality of life
As shown in the illustrative forest plot, the one study that provided data on quality of life (Findling 2011), found no difference between the two groups (SMD ‐0.01; 95% CI ‐0.27 to 0.25; 309 children/adolescents; see Analysis 1.15).
Retention
There was no difference between those given amphetamine and those given placebo for retention (RR 1.03; 95% CI 0.97 to 1.10; Tau² = 0.01; I² = 83%; 11 studies; 2381 children/adolescents; Analysis 1.16).
Adverse events
Proportion of adverse events
We performed a series of meta‐analyses of the most commonly reported adverse events. A higher proportion of children and adolescents in the amphetamine group as compared to placebo group experienced decreased appetite (RR 6.31; 95% CI 2.58 to 15.46; Tau² = 1.59; I² = 85%; 11 studies; 2467 children/adolescents; Analysis 1.17); insomnia/trouble sleeping (RR 3.80; 95% CI 2.12 to 6.83; Tau² = 0.42; I² = 59%; 10 studies; 2429 children/adolescents; Analysis 1.18); abdominal pain (RR 1.44; 95% CI 1.03 to 2.00; Tau² = 0.04; I² = 13%; 10 studies; 2155 children/adolescents; Analysis 1.19); and nausea/vomiting (RR 1.63; 95% CI 1.04 to 2.56; Tau² = 0.08; I² = 26%; 6 studies; 1579 children/adolescents; Analysis 1.20). There were no differences between the amphetamine and placebo groups in the proportion of children and adolescents who experienced headaches (RR 0.93; 95% CI 0.75 to 1.16; Tau² = 0.00; I² = 0%; 9 studies; 2091 children/adolescents; Analysis 1.21) and anxiety/nervousness (RR 1.22; 95% CI 0.78 to 1.93; Tau² = 0.09; I² = 32%; 5 studies; 1088 children/adolescents; Analysis 1.22).
Proportion of participants who experienced at least one adverse event
The proportion of children and adolescents who experienced at least one adverse event was higher in the amphetamine group as compared to the placebo group (RR 1.30; 95% CI 1.18 to 1.44; Tau² = 0.00; I² = 1%; 6 studies; 1742 children/adolescents; see Analysis 1.23; Figure 5).

Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.23 Proportion of participants who experienced at least one adverse event.
Proportion of participants who withdrew due to any adverse event
There was no difference between the amphetamine and placebo groups in the proportion of children and adolescents who withdrew due to an adverse event (RR 1.60; 95% CI 0.86 to 2.98; Tau² = 0.00; I² = 0%; 9 studies; 2160 children/adolescents; Analysis 1.24).
Subgroup analyses
Type of amphetamine
We conducted a series of subgroup analyses according to type of amphetamine (dexamphetamine, lisdexamphetamine, and mixed amphetamine salts). Mixed amphetamine salts were associated with improved parent ratings of total ADHD symptoms as compared to dexamphetamine and lisdexamphetamine, however, there appeared to be no between‐group differences (Chi² = 0.55, P value = 0.76; Analysis 2.1). Only one subgroup yielded between‐group differences (Chi² = 26.06, P < 0.00001; Analysis 2.6; Figure 6). There were no between‐group differences for any of the other outcomes.

Forest plot of comparison: 2 Subgroup analysis 1: Type of amphetamine, outcome: 2.6 Proportion of participants experiencing decreased appetite.
Type of amphetamine release formulation
We conducted a subgroup analysis to explore the influence of long‐acting versus short‐acting amphetamine release formulations (Analysis 3.1 to Analysis 3.6). Both rentention: proportion of participants who completed the trial (Chi² = 4.50, P value = 0.03; Analysis 3.4) and proportion of participants experiencing decreased appetite (Chi² = 6.93, P value = 0.008; Analysis 3.5) yielded between‐group differences.
Funding source
We wanted to explore the influence of industry‐funded versus publicly‐funded sources (Analysis 4.1 to Analysis 4.3). Since five studies did not report their source of funding, we introduced another subgroup, 'not reported'. No between‐group differences were found.
Sensitivity analysis
We repeated the analyses by changing the statistical model from a random‐effects model to a fixed‐effect model. The results were similar (see Analysis 5.1 to Analysis 5.24).
Discussion
Summary of main results
We included 23 studies in this review, 19 of which we included in meta‐analyses. Overall, this review found that amphetamines were more efficacious than placebo for reducing ADHD core symptom severity in the short‐term, however, they did not influence retention in the trial and were associated with a number of adverse events. According to conventional cut‐offs (Cohen 1988), the largest effect sizes observed (i.e. an SMD > 0.8) were teacher ratings of hyperactivity/impulsivity, teacher ratings of inattention, and clinician ratings of total ADHD symptoms. The meta‐analyses with the most available data included parent and teacher ratings of total ADHD symptoms, both of which yielded low to moderate effect sizes.
The median study duration was only 28 days, therefore it was not possible to assess the long‐term efficacy and safety of amphetamines for pediatric ADHD. This is particularly problematic in a condition such as ADHD, which may require treatment for years.
There was a lot of variation in the amphetamine derivatives and release formulations utilized in the included studies. As such, we conducted subgroup analyses to explore their differences. Minimal between‐group differences were found, however, conclusions should not be drawn from these analyses as they are based on observational and not randomized comparisons. Furthermore, given that the majority of studies assessed mixed amphetamine salts and long‐acting release formulations, the subgroups assessing other amphetamine derivatives (i.e. lisdexamphetamine, dexamphetamine), and short‐acting release formulations, may not have been adequately powered to detect a true difference.
We performed a meta‐analysis of the most commonly reported adverse events in the primary studies. These included decreased appetite, insomnia/trouble sleeping, abdominal pain, nausea/vomiting, headaches and anxiety. Meta‐analysis revealed that most adverse events occurred more often in the amphetamine groups than in the placebo groups.
Overall completeness and applicability of evidence
This review focused only on the amphetamine versus placebo comparison. While it is important to assess amphetamines versus other active therapies, such as other stimulants, psychotherapy or antidepressants, we believe it is important to first establish whether or not amphetamines are superior to placebo. We were unable to assess the long‐term efficacy of amphetamines (i.e. beyond 12 months of use). The average duration of included studies was five weeks long, excluding one long‐term study that was 12 months long (Gillberg 1997). Short‐term trials are particularly problematic for chronic conditions, such as ADHD, as children will likely be on stimulant medications for much longer periods than have been studied. As mentioned earlier, adverse events occurred more frequently when children and adolescents were treated with amphetamines than when they were treated with placebo, however, it is important to point out the poor reporting around adverse events in the included studies. Some studies only reported on adverse events that were experienced by a certain percentage of children and adolescents, thereby potentially ignoring additional adverse events experienced at less than that fraction. Futhermore, many studies were unclear regarding their methods of collecting adverse events and whether they assessed the causality of these adverse events as it related to the interventions. Heterogeneity of terms used to describe adverse events was also a major hurdle when conducting this review, and limited our ability to appropriately synthesize the data. Finally, as with efficacy data, we were unable to assess the long‐term safety profile of amphetamines given the lack of long‐term trials.
Only one study explored the impact of amphetamines on children and adolescents' quality of life (Findling 2011). It found no effect, although this may be because the study was underpowered. This is a significant gap in the available evidence on the effects of amphetamines, and studies should include a focus on psychosocial factors such as parental stress or quality of life.
The external validity of our results was also limited by the inclusion/exclusion criteria of the included studies. Since we excluded studies that included children and adolescents with comorbidity other than conduct disorder, oppositional defiant disorder and anxiety, we cannot extrapolate the results of our review to patients with other commonly occurring comorbidity such as depression, and tic disorder.
Few trials reported on the ADHD subtype of their included children and adolescents. Therefore, we were unable to make any conclusions as regards the potential heterogeneity of effect of different formulations of amphetamines across different ADHD subtypes. Furthermore, since primary studies did not subgroup their results according to important prognostic factors, such as age and gender, we were unable to subgroup our meta‐analyses, which would have been particularly relevant for clinicians.
Although our review did assess the proportion of children and adolescents who dropped out due to an adverse event and found no differences between amphetamine and placebo groups (Analysis 1.24), it is worth noting that many studies in the meta‐analysis had zero events in the placebo group, resulting in potentially invalid results that overestimate variance. The high number of zero events may be attributable to a lack of power to detect these events given the much smaller sample sizes of the placebo groups (n = 900) as compared to the amphetamine groups (n = 1532).
Finally, it was difficult for us to accurately assess our results in a clinically meaningful way, since interpretation of scores is both sex‐ and age‐dependent.
Quality of the evidence
The overall quality of the evidence ranged from low to very low as assessed by the GRADE approach, indicating that there is room for improvement amongst the current evidence about the efficacy of amphetamines in managing ADHD in children and adolescents. The studies appear to have a multitude of methodological issues making it difficult to draw strong clinical conclusions. Moreover, most studies failed to report on how the random sequence was generated (90%), how allocation was concealed (85%), the methods used to blind participants and personnel (55%), and the methods used to blind outcome assessors (90%). As such, we were unable to determine whether it was a reporting problem or a methodological problem.
Potential biases in the review process
Limitations of our review include not being able to account for correlation in cross‐over studies given the formula for calculating SMDs, thereby yielding less precise effect sizes, which may result in overlooking any potential statistical heterogeneity between the studies. Furthermore, caution is warranted in the interpretation of our meta‐analyses of adverse events that contain rare events. Most methods of meta‐analysis, including the chosen Mantel‐Haenszel approach, perform poorly with rare events; they can yield misleading results, have very wide CIs or the statistical power can be too low to detect a difference.
Given the small number of studies included in the primary meta‐analyses (maximum of seven studies), we felt that a funnel plot would not provide meaningful information about potential publication bias, which may have led to an overestimation of treatment effects. Furthermore, the exclusion of one potentially eligible non‐English study may have also biased our findings. Egger 1997 found that non‐English studies tend to be negative, and so excluding them may have yielded an overestimation of treatment effects. On the other hand, other researchers have found that excluding trials reported in languages other than English do not significantly affect the results of a meta‐analysis (Moher 2003).
Another limitation arises from the subgroup comparisons. It must be noted that these analyses are based on observational and not randomized comparisons, and therefore should not be interpreted as conclusive evidence.
Another potential bias of our review is that one of the authors, Dr. Catherine J Nikles, has published a study on amphetamines for ADHD; however, two independent authors assessed the eligibility of this study, which was subsequently excluded due to the nature of the design (Nikles 2006).
Agreements and disagreements with other studies or reviews
We identified two previously conducted systematic reviews prior to conducting ours (Charach 2011; Miller 1999). Charach 2011 only assessed the long‐term (i.e. more than 12 months) efficacy and safety of amphetamines for pediatric ADHD, and included only one RCT that assessed amphetamines versus placebo, which was also included in our systematic review (Gillberg 1997). Miller 1999 also systematically assessed amphetamines for pediatric ADHD, however, reviewers only included studies that assessed the dexamphetamine derivative of amphetamines. Furthermore, this review was published in 1999, making it over 14 years old. As such, Miller 1999 included only three relevant RCTs in their review, which were also included in this review (Borcherding 1990; Donnelly 1989; Gillberg 1997). The majority of meta‐analyses conducted in the Miller 1999 review included RCTs that assessed any stimulant medication (methylphenidate or amphetamine) versus placebo. The one meta‐analysis that was restricted to the amphetamine versus placebo comparison showed that amphetamines improve total ADHD symptoms as rated by the Abbreviated Conners Teacher Rating Scale (ATRS; Conners 1990): MD ‐4.77; 95% CI ‐6.43 to ‐2.99. This is consistent with the results of our review for this outcome, which also showed improvement in this outcome in favour of amphetamine (SMD ‐0.55; 95% CI ‐0.83 to ‐0.27).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.1 Total ADHD symptom score ‐ parent ratings.

Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.3 Total ADHD symptom score ‐ teacher ratings.

Forest plot of comparison: 1 Amphetamines versus placebo, outcome: 1.23 Proportion of participants who experienced at least one adverse event.

Forest plot of comparison: 2 Subgroup analysis 1: Type of amphetamine, outcome: 2.6 Proportion of participants experiencing decreased appetite.
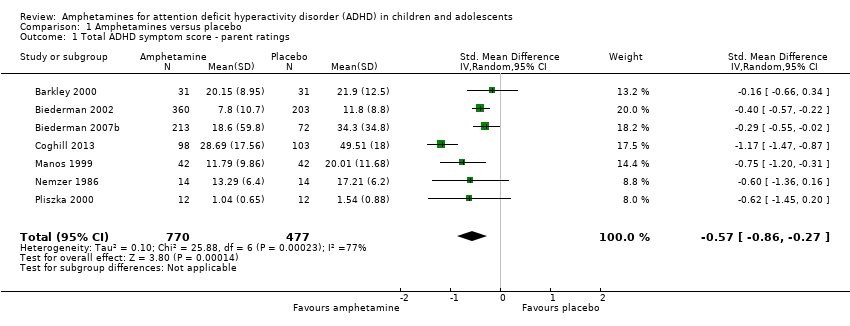
Comparison 1 Amphetamines versus placebo, Outcome 1 Total ADHD symptom score ‐ parent ratings.

Comparison 1 Amphetamines versus placebo, Outcome 2 Hyperactivity/impulsivity ‐ parent ratings.

Comparison 1 Amphetamines versus placebo, Outcome 3 Total ADHD symptom score ‐ teacher ratings.

Comparison 1 Amphetamines versus placebo, Outcome 4 Hyperactivity/impulsivity ‐ teacher ratings.
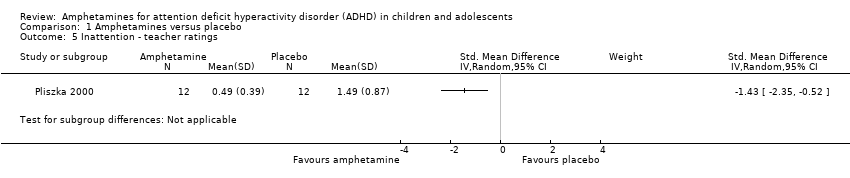
Comparison 1 Amphetamines versus placebo, Outcome 5 Inattention ‐ teacher ratings.

Comparison 1 Amphetamines versus placebo, Outcome 6 Total ADHD symptom score ‐ clinician ratings.
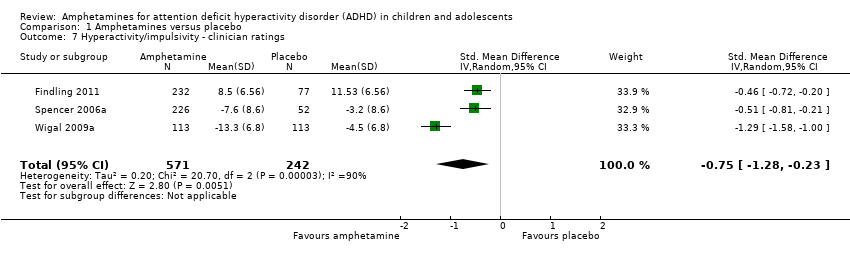
Comparison 1 Amphetamines versus placebo, Outcome 7 Hyperactivity/impulsivity ‐ clinician ratings.

Comparison 1 Amphetamines versus placebo, Outcome 8 Inattention ‐ clinician ratings.

Comparison 1 Amphetamines versus placebo, Outcome 9 Total ADHD symptom score ‐ investigator/research personnel ratings.
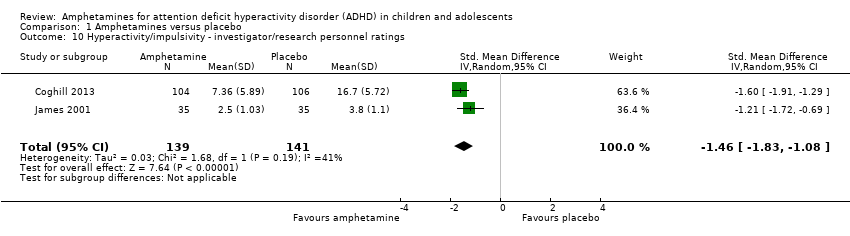
Comparison 1 Amphetamines versus placebo, Outcome 10 Hyperactivity/impulsivity ‐ investigator/research personnel ratings.

Comparison 1 Amphetamines versus placebo, Outcome 11 Inattention ‐ investigator/research personnel ratings.

Comparison 1 Amphetamines versus placebo, Outcome 12 Proportion of responders (Clinical Global Impression ‐ Improvement; CGI ‐ I).
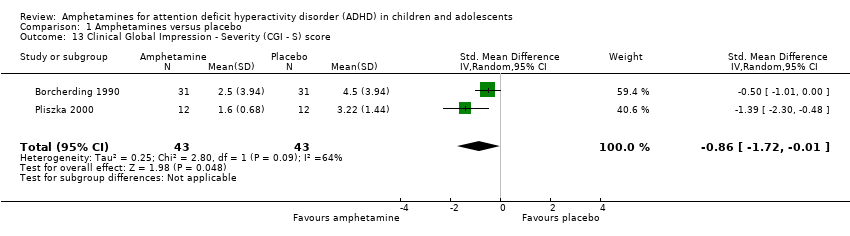
Comparison 1 Amphetamines versus placebo, Outcome 13 Clinical Global Impression ‐ Severity (CGI ‐ S) score.
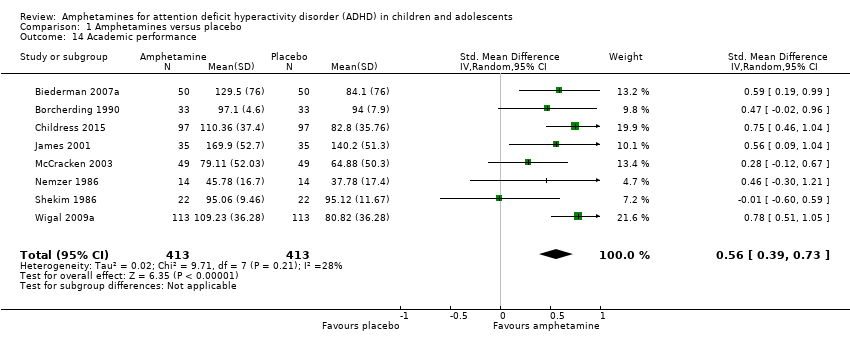
Comparison 1 Amphetamines versus placebo, Outcome 14 Academic performance.

Comparison 1 Amphetamines versus placebo, Outcome 15 Quality of life.

Comparison 1 Amphetamines versus placebo, Outcome 16 Retention: proportion of participants who completed the trial.

Comparison 1 Amphetamines versus placebo, Outcome 17 Proportion of participants experiencing decreased appetite.

Comparison 1 Amphetamines versus placebo, Outcome 18 Proportion of participants experiencing insomnia/trouble sleeping.

Comparison 1 Amphetamines versus placebo, Outcome 19 Proportion of participants experiencing abdominal pain.

Comparison 1 Amphetamines versus placebo, Outcome 20 Proportion of participants experiencing nausea/vomiting.
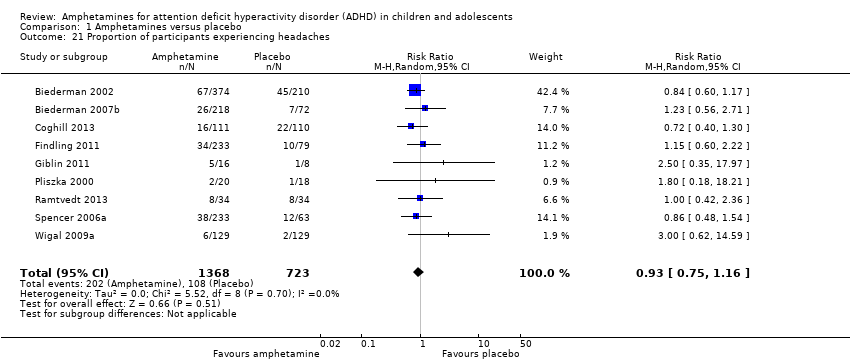
Comparison 1 Amphetamines versus placebo, Outcome 21 Proportion of participants experiencing headaches.

Comparison 1 Amphetamines versus placebo, Outcome 22 Proportion of participants experiencing anxiety/nervousness.
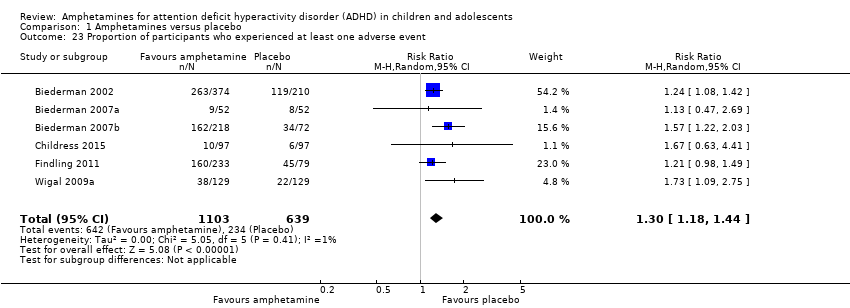
Comparison 1 Amphetamines versus placebo, Outcome 23 Proportion of participants who experienced at least one adverse event.

Comparison 1 Amphetamines versus placebo, Outcome 24 Proportion of participants who dropped out/withdrew due to an adverse event.

Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 1 Total ADHD symptom score ‐ parent ratings.

Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 2 Proportion of responders (CGI ‐ I).

Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 3 Academic performance.
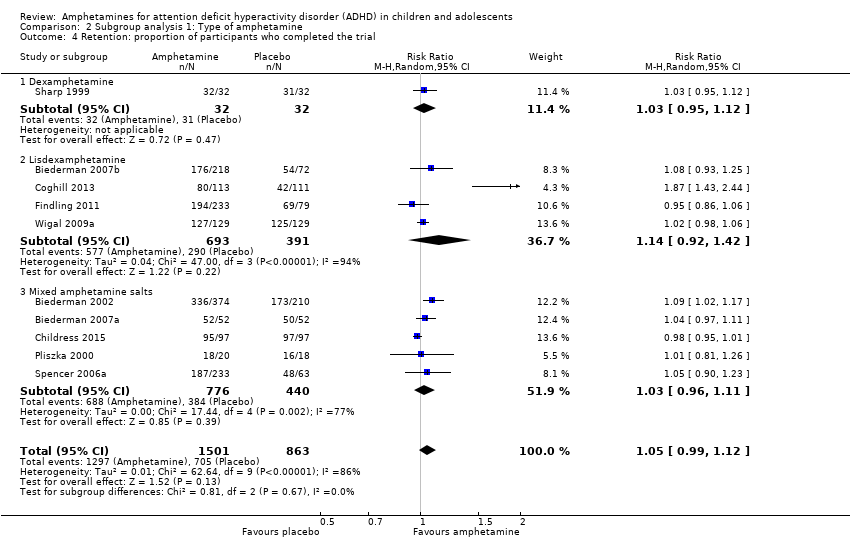
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 4 Retention: proportion of participants who completed the trial.

Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 5 Proportion of participants who dropped out/withdrew due to an adverse event.
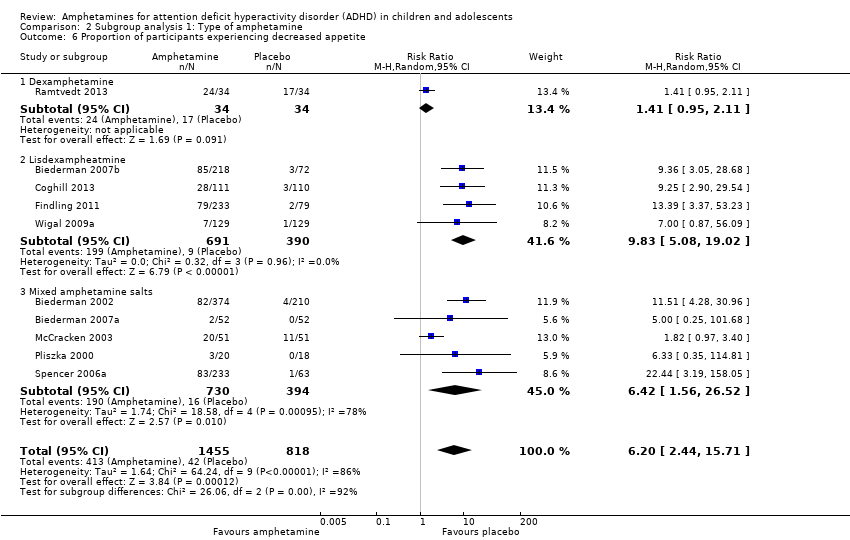
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 6 Proportion of participants experiencing decreased appetite.
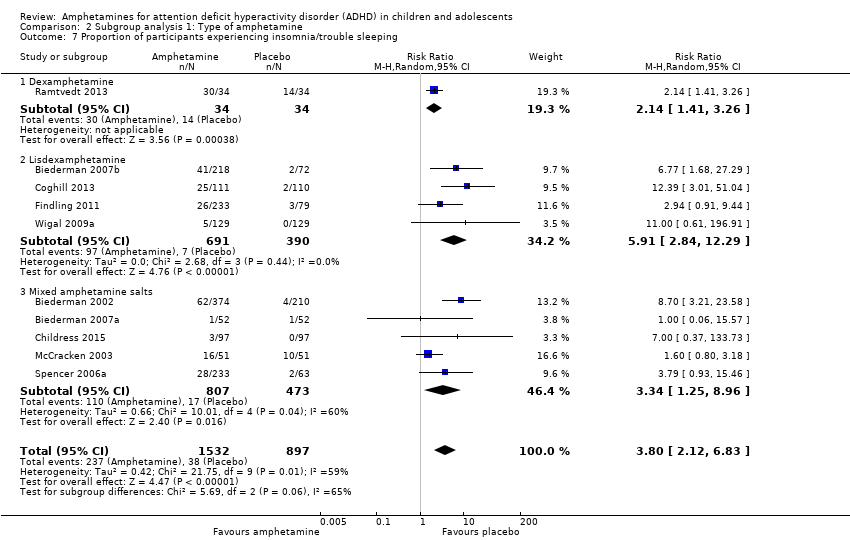
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 7 Proportion of participants experiencing insomnia/trouble sleeping.
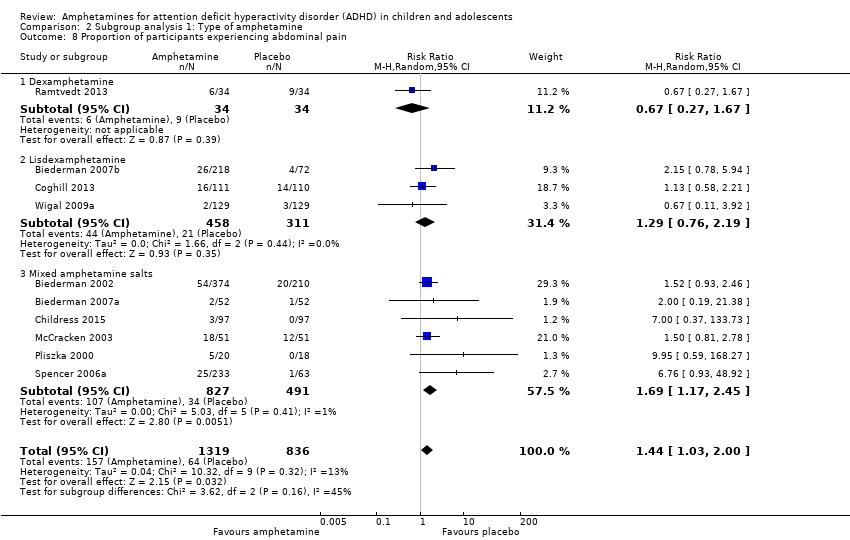
Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 8 Proportion of participants experiencing abdominal pain.

Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 9 Proportion of participants experiencing headaches.

Comparison 2 Subgroup analysis 1: Type of amphetamine, Outcome 10 Proportion of participants experiencing nausea/vomiting.

Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 1 Total ADHD symptom score ‐ parent ratings.

Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 2 Proportion of responders (CGI ‐ I).
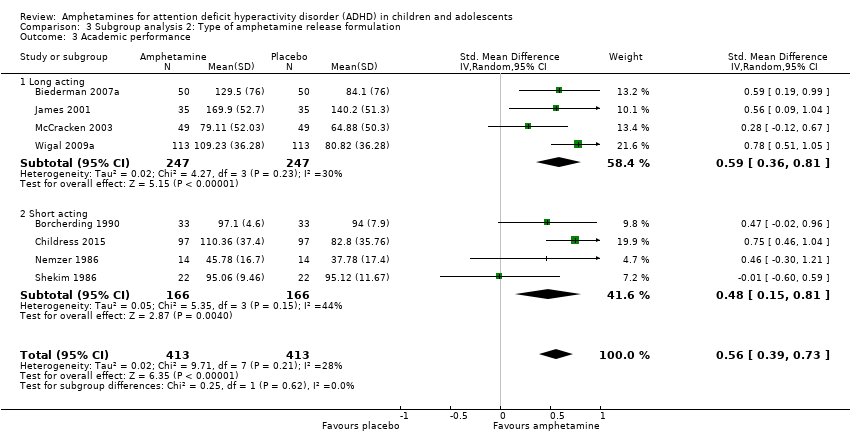
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 3 Academic performance.

Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 4 Retention: proportion of participants who completed the trial.

Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 5 Proportion of participants experiencing decreased appetite.
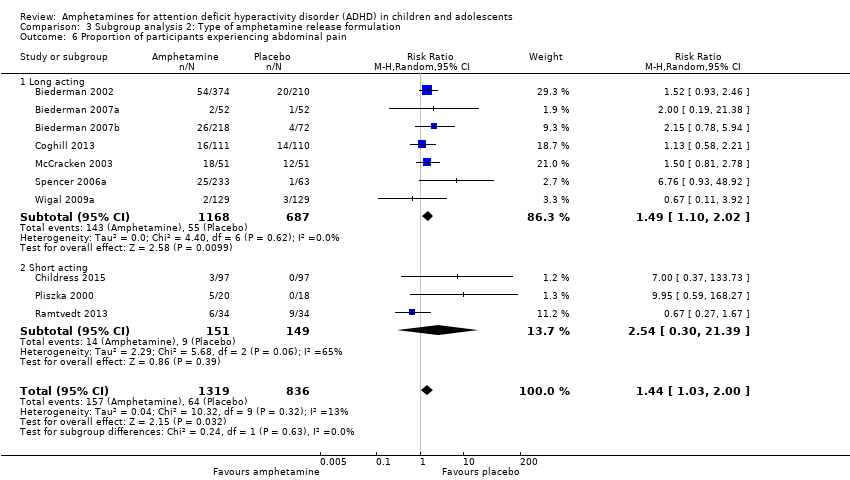
Comparison 3 Subgroup analysis 2: Type of amphetamine release formulation, Outcome 6 Proportion of participants experiencing abdominal pain.

Comparison 4 Subgroup analysis 3: Funding source, Outcome 1 Total ADHD symptom score ‐ parent ratings.
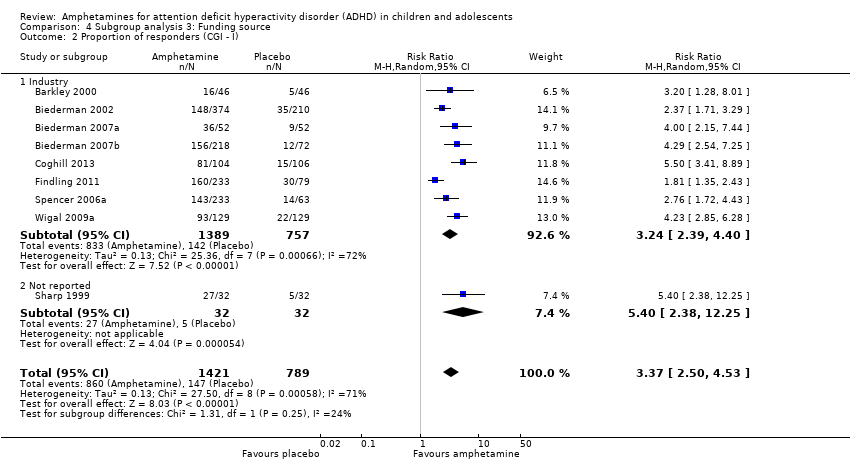
Comparison 4 Subgroup analysis 3: Funding source, Outcome 2 Proportion of responders (CGI ‐ I).

Comparison 4 Subgroup analysis 3: Funding source, Outcome 3 Academic performance.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 1 Total ADHD symptom score ‐ parent ratings.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 2 Hyperactivity/impulsivity ‐ parent ratings.
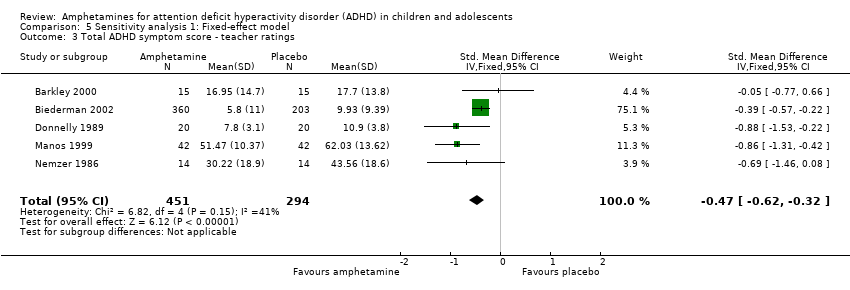
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 3 Total ADHD symptom score ‐ teacher ratings.
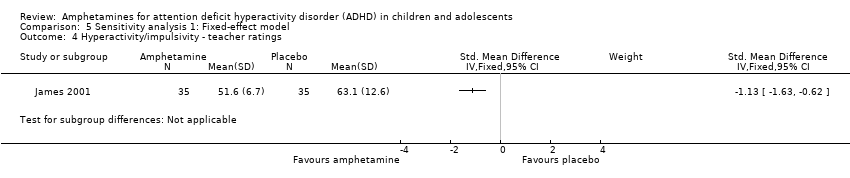
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 4 Hyperactivity/impulsivity ‐ teacher ratings.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 5 Inattention ‐ teacher ratings.
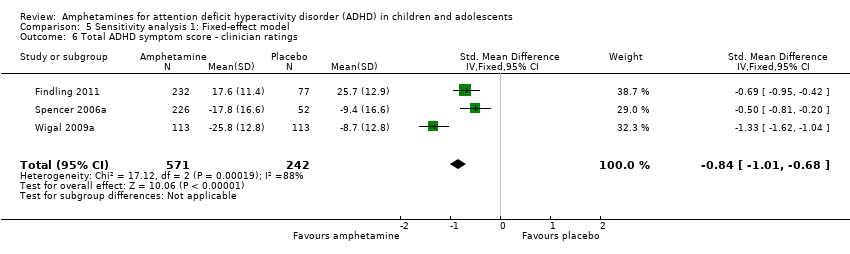
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 6 Total ADHD symptom score ‐ clinician ratings.
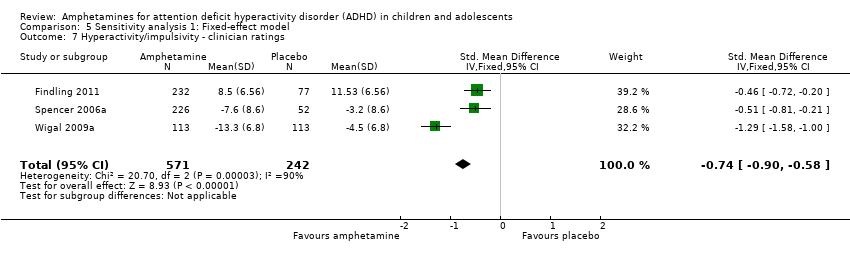
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 7 Hyperactivity/impulsivity ‐ clinician ratings.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 8 Inattention ‐ clinician ratings.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 9 Total ADHD symptom score ‐ investigator/research personnel ratings.
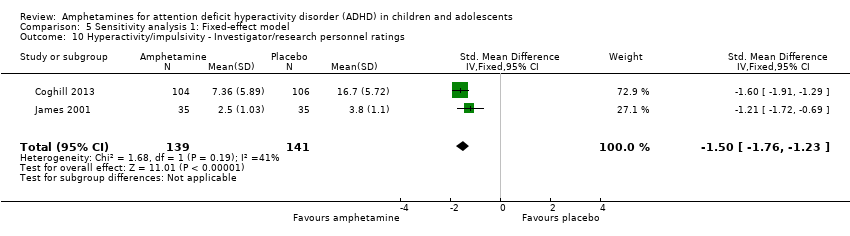
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 10 Hyperactivity/impulsivity ‐ Investigator/research personnel ratings.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 11 Inattention ‐ investigator/research personnel ratings.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 12 Proportion of responders (CGI ‐ I).
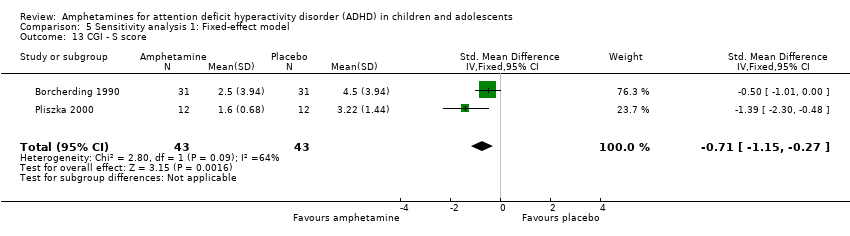
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 13 CGI ‐ S score.
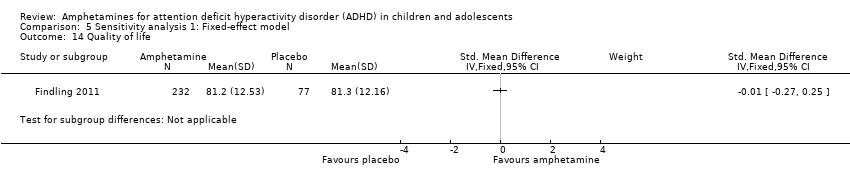
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 14 Quality of life.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 15 Academic performance.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 16 Retention: proportion of participants who completed the trial.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 17 Proportion of participants who experienced at least one adverse event.
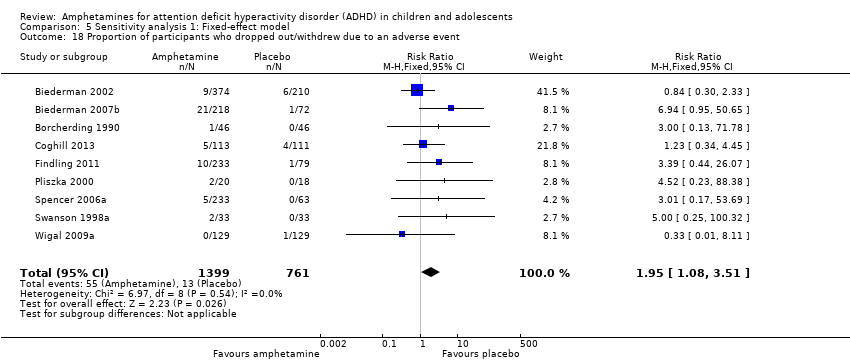
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 18 Proportion of participants who dropped out/withdrew due to an adverse event.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 19 Proportion of participants experiencing decreased appetite.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 20 Proportion of participants experiencing insomnia/trouble sleeping.
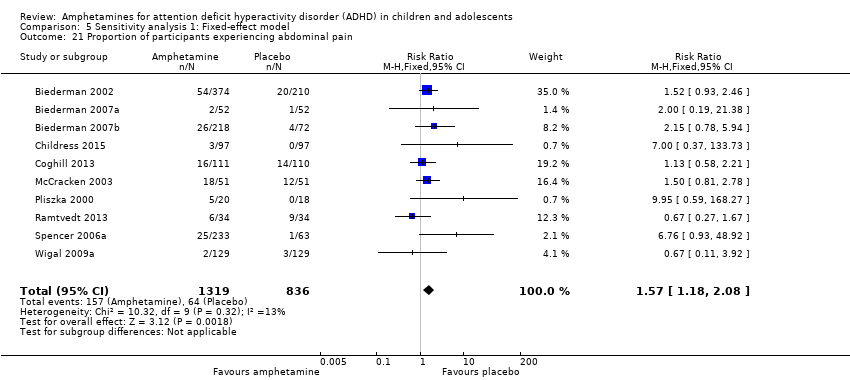
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 21 Proportion of participants experiencing abdominal pain.
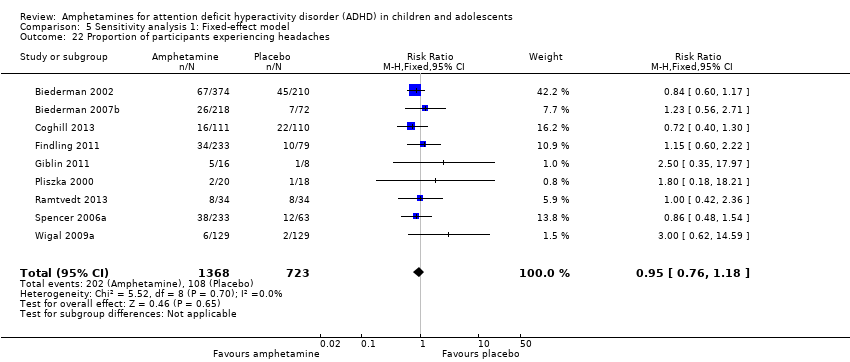
Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 22 Proportion of participants experiencing headaches.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 23 Proportion of participants experiencing anxiety/nervousness.

Comparison 5 Sensitivity analysis 1: Fixed‐effect model, Outcome 24 Proportion of participants experiencing nausea/vomiting.
| Amphetamines compared with placebo for attention deficit hyperactivity disorder in children and adolescents | ||||||
| Patient or population: children or adolescents with ADHD Settings: Beligum, France, Germany, Hungary, Italy, Netherlands, Norway, Poland, Spain, Sweden, United Kingdom, United States Intervention: amphetamines (i.e. dexamphetamine, lisdexamphetamine, mixed amphetamine salts) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Amphetamine | |||||
| Total ADHD symptom score ‐ parent ratings (ADHD Rating Scale, Fourth Version; Conners' Rating Scale; Conners' Global Index; Conners' Abbreviated Symptom Questionnaire) | ‐ | The mean total score in the intervention groups was 0.57 standard deviations lower (‐0.86 to ‐0.27) | SMD ‐0.57 (‐0.86 to ‐0.27) | 1247 | ⊕⊝⊝⊝ | Moderate effect** |
| Total ADHD symptom score ‐ teacher ratings (ADHD Rating Scale, Fourth Version; Conners' Rating Scale; Conners' Global Index; Conners' Abbreviated Symptom Questionnaire) Follow‐up: 7 to 35 days | ‐ | The mean total score in the intervention groups was 0.55 standard deviations lower (‐0.83 to ‐0.27) | SMD ‐0.55 (‐0.83 to ‐0.27) | 745 | ⊕⊕⊝⊝ | Moderate effect** |
| Total ADHD symptom score ‐ clinician ratings (ADHD Rating Scale, Fourth Version) | ‐ | The mean total score in the intervention groups was 0.84 standard deviations lower (‐1.32 to ‐0.36) | SMD ‐0.84 (‐1.32 to ‐0.36) | 813 | ⊕⊝⊝⊝ | Large effect** |
| Proportion of responders (Clinical Global Impressions ‐ Improvement (CGI‐I) scale) | 187 per 1000 | 605 per 1000 | RR 3.36 (2.48 to 4.55) | 2207 | ⊝⊝⊝⊝ | ‐ |
| Academic performance (Permanent Product Measure of Performance; Wechsler Intelligence Scale for Children ‐ Revised; Barnell Lot, Ltd Math Test; Wide Range Achievement Test) Follow‐up: 7 to 21 days | ‐ | The mean score in the intervention groups was 0.51 standard deviations higher (0.31 to 0.70) | SMD 0.56 (0.39 to 0.73) | 826 | ⊕⊕⊝⊝ | Moderate effect** |
| Retention: proportion of participants who completed the trial | 825 per 1000 | 864 per 1000 | RR 1.03 | 2381 | ⊕⊝⊝⊝ | ‐ |
| Proportion of participants who experienced at least 1 adverse event | 366 per 1000 | 582 per 1000 | RR 1.30 (1.18 to 1.44) | 1742 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADHD: Attention deficit hyperactivity disorder; CI: Confidence interval; GRADE: Grades of recommendation, assessment, development and evaluation; RR: Risk ratio; SMD: Standardized mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to the majority of studies included in this outcome having a high risk of bias. | ||||||
| Types of outcome measures | Primary outcomes Multiple perspectives (i.e. teacher, parent, clinician) are considered the gold standard when assessing the core symptoms of ADHD. As such, we will not favor one perspective over another. In the event that reports do not agree with one another, for example, teacher reports disagree with parent reports on the improvement of core symptoms, this may be quite telling about how a child’s environment impacts their ADHD given the varying demands between a school environment and home environment. This will be interpreted accordingly in the discussion. Secondary outcomes We will assess 'parental stress' as a secondary outcome. |
| Measures of treatment effect | Dichotomous outcome data When a single study has utilized more than one measure to assess the same construct (e.g. ADHD core symptoms as assessed by teacher‐rated ADHD‐RS‐IV and teacher ratings of the Conners’ ADHD Rating Scale), treatment effects will be averaged across outcome measures in order to arrive at a single treatment effect for use in the meta‐analysis. Continuous outcome data For continuous outcomes, where the same rating scale has been used for all studies, we will calculate mean differences. |
| Unit of analysis issues | Cross‐over trials For meta‐analyses that use that use a mean difference, we will compute standard deviations for the cross‐over trials taking into account correlation. If correlation coefficients are not available, we will impute them from other studies or use 0.5 as a conservative estimate (Follman 1992). For cross‐over trials where carry‐over is thought to be a problem, where no washout period is present, or when only data from the first period are available, we will analyze data from the first period only. Studies with multiple time points In studies where results are presented for several periods of follow‐up, we will analyze each outcome at each point in a separate meta‐analysis with other comparable studies taking measures at a similar time frame post‐randomization. Time frames will reflect short‐term (up to six months), medium‐term (between 6 months and 12 |
| Assessment of reporting biases | For each primary meta‐analysis in which we have identified a sufficient number of studies (n ≥ 10) for inclusion, we will draw funnel plots in order to assess the possibility of publication bias. |
| Subgroup analysis and investigation of heterogeneity | We will conduct the following subgroup analyses.
|
| Sensitivity analysis | We will conduct the following sensitivity analyses.
|
| ADHD: attention deficit hyperactivity disorder. | |
| Outcome | Outcome measure (respondent) | Studies | Measure used in meta‐analysis |
| Inattention | ADHD Rating Scale, Fourth Version (parent ratings) | No (data presented in an unusable format) | |
| ADHD Rating Scale, Fourth Version (clinician ratings) | Yes | ||
| Yes | |||
| Yes | |||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Yes | ||
| Conners’ Rating Scale (parent ratings) | Yes | ||
| No (only study that included long‐term data) | |||
| Conners’ Rating Scale (teacher ratings) | No (only study that included long‐term data) | ||
| IOWA Conners’ Rating Scale | Yes | ||
| SKAMP scale (teacher ratings) | No (data not available) | ||
| SKAMP scale (investigator/research personnel ratings) | Yes | ||
| Yes | |||
| Hyperactivity/impulsivity | ADHD Rating Scale, Fourth Version (parent ratings) | No (data presented in an unusable format) | |
| ADHD Rating Scale, Fourth Version (clinician ratings) | Yes | ||
| Yes | |||
| Yes | |||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Yes | ||
| Conners’ Rating Scale (parent ratings) | No (only study that included long‐term data) | ||
| Yes | |||
| Conners’ Rating Scale (teacher ratings) | No (only study that included long‐term data) | ||
| Yes | |||
| Total core symptom score | ADHD Rating Scale, Fourth Version (parent ratings) | Yes | |
| Yes | |||
| ADHD Rating Scale, Fourth Version (teacher ratings) | Yes | ||
| ADHD Rating Scale, Fourth Version (clinician ratings) | Yes | ||
| Yes | |||
| Yes | |||
| ADHD Rating Scale, Fourth Version (investigator/research personnel ratings) | Yes | ||
| No (no data available) | |||
| Conners’ Rating Scale (parent ratings) | No (data presented in an unusable format) | ||
| Yes | |||
| No (data not available) | |||
| No (only study that included long‐term data) | |||
| Yes | |||
| No (data not available) | |||
| No (data presented in an unusable format) | |||
| Conners’ Rating Scale (teacher ratings) | No (no data available) | ||
| Yes | |||
| No (only study that included long‐term data) | |||
| Yes | |||
| No (data not available) | |||
| No (data presented in an unusable format) | |||
| Conners’ Global Index (parent ratings) | Yes | ||
| Yes | |||
| Conners’ Global Index (teacher ratings) | Yes | ||
| Conners’ Abbreviated Symptom Questionnaire (parent ratings) | Yes | ||
| Conners’ Abbreviated Symptom Questionnaire (teacher ratings) | Yes | ||
| ADHD Questionnaire (developed within study) (parent ratings) | No (data presented in an unusable format) | ||
| ADHD Questionnaire (developed within study) (teacher ratings) | No (data presented in an unusable format) | ||
| SKAMP scale (investigator/research personnel ratings) | Yes | ||
| ADHD: attention deficit hyperactivity disorder. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total ADHD symptom score ‐ parent ratings Show forest plot | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 2 Hyperactivity/impulsivity ‐ parent ratings Show forest plot | 2 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.89, ‐0.19] |
| 3 Total ADHD symptom score ‐ teacher ratings Show forest plot | 5 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.83, ‐0.27] |
| 4 Hyperactivity/impulsivity ‐ teacher ratings Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Inattention ‐ teacher ratings Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Total ADHD symptom score ‐ clinician ratings Show forest plot | 3 | 813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.32, ‐0.36] |
| 7 Hyperactivity/impulsivity ‐ clinician ratings Show forest plot | 3 | 813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.28, ‐0.23] |
| 8 Inattention ‐ clinician ratings Show forest plot | 3 | 813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.26, ‐0.30] |
| 9 Total ADHD symptom score ‐ investigator/research personnel ratings Show forest plot | 3 | 630 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐1.87, ‐0.44] |
| 10 Hyperactivity/impulsivity ‐ investigator/research personnel ratings Show forest plot | 2 | 280 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐1.83, ‐1.08] |
| 11 Inattention ‐ investigator/research personnel ratings Show forest plot | 4 | 634 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.42, ‐0.04] |
| 12 Proportion of responders (Clinical Global Impression ‐ Improvement; CGI ‐ I) Show forest plot | 9 | 2207 | Risk Ratio (M‐H, Random, 95% CI) | 3.36 [2.48, 4.55] |
| 13 Clinical Global Impression ‐ Severity (CGI ‐ S) score Show forest plot | 2 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.72, ‐0.01] |
| 14 Academic performance Show forest plot | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 15 Quality of life Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16 Retention: proportion of participants who completed the trial Show forest plot | 11 | 2381 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.97, 1.10] |
| 17 Proportion of participants experiencing decreased appetite Show forest plot | 11 | 2467 | Risk Ratio (M‐H, Random, 95% CI) | 6.31 [2.58, 15.46] |
| 18 Proportion of participants experiencing insomnia/trouble sleeping Show forest plot | 10 | 2429 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [2.12, 6.83] |
| 19 Proportion of participants experiencing abdominal pain Show forest plot | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.00] |
| 20 Proportion of participants experiencing nausea/vomiting Show forest plot | 6 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.04, 2.56] |
| 21 Proportion of participants experiencing headaches Show forest plot | 9 | 2091 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.16] |
| 22 Proportion of participants experiencing anxiety/nervousness Show forest plot | 5 | 1088 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.78, 1.93] |
| 23 Proportion of participants who experienced at least one adverse event Show forest plot | 6 | 1742 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.18, 1.44] |
| 24 Proportion of participants who dropped out/withdrew due to an adverse event Show forest plot | 9 | 2160 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.86, 2.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total ADHD symptom score ‐ parent ratings Show forest plot | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 1.1 Dexamphetamine | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.36, 0.16] |
| 1.2 Lisdexamphetamine | 2 | 486 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.59, 0.14] |
| 1.3 Mixed amphetamine salts | 4 | 733 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.63, ‐0.24] |
| 2 Proportion of responders (CGI ‐ I) Show forest plot | 9 | 2205 | Risk Ratio (M‐H, Random, 95% CI) | 3.38 [2.51, 4.55] |
| 2.1 Dexamphetamine | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.4 [2.38, 12.25] |
| 2.2 Lisdexamphetamine | 4 | 1065 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [2.04, 6.41] |
| 2.3 Mixed amphetamine salts | 4 | 1076 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [2.14, 3.45] |
| 3 Academic performance Show forest plot | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 3.1 Dexamphetamine | 4 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.12, 0.67] |
| 3.2 Lisdexamphetamine | 1 | 226 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [0.51, 1.05] |
| 3.3 Mixed amphetamine salts | 3 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.29, 0.84] |
| 4 Retention: proportion of participants who completed the trial Show forest plot | 10 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.12] |
| 4.1 Dexamphetamine | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.12] |
| 4.2 Lisdexamphetamine | 4 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.92, 1.42] |
| 4.3 Mixed amphetamine salts | 5 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 5 Proportion of participants who dropped out/withdrew due to an adverse event Show forest plot | 9 | 2161 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.86, 2.98] |
| 5.1 Dexamphetamine | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.78] |
| 5.2 Lisdexamphetamine | 4 | 1085 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.70, 5.91] |
| 5.3 Mixed amphetamine salts | 4 | 984 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.53, 3.06] |
| 6 Proportion of participants experiencing decreased appetite Show forest plot | 10 | 2273 | Risk Ratio (M‐H, Random, 95% CI) | 6.20 [2.44, 15.71] |
| 6.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.95, 2.11] |
| 6.2 Lisdexampheatmine | 4 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 9.83 [5.08, 19.02] |
| 6.3 Mixed amphetamine salts | 5 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 6.42 [1.56, 26.52] |
| 7 Proportion of participants experiencing insomnia/trouble sleeping Show forest plot | 10 | 2429 | Risk Ratio (M‐H, Random, 95% CI) | 3.80 [2.12, 6.83] |
| 7.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.41, 3.26] |
| 7.2 Lisdexamphetamine | 4 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 5.91 [2.84, 12.29] |
| 7.3 Mixed amphetamine salts | 5 | 1280 | Risk Ratio (M‐H, Random, 95% CI) | 3.34 [1.25, 8.96] |
| 8 Proportion of participants experiencing abdominal pain Show forest plot | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.00] |
| 8.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.27, 1.67] |
| 8.2 Lisdexamphetamine | 3 | 769 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.76, 2.19] |
| 8.3 Mixed amphetamine salts | 6 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.17, 2.45] |
| 9 Proportion of participants experiencing headaches Show forest plot | 9 | 2063 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.75, 1.16] |
| 9.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.42, 2.36] |
| 9.2 Lisdexamphetamine | 5 | 1077 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.73, 1.57] |
| 9.3 Mixed amphetamine salts | 3 | 918 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.14] |
| 10 Proportion of participants experiencing nausea/vomiting Show forest plot | 6 | 1579 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.04, 2.56] |
| 10.1 Dexamphetamine | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.68, 4.07] |
| 10.2 Lisdexamphetamine | 4 | 927 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.61, 3.61] |
| 10.3 Mixed amphetamine salts | 1 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [1.04, 3.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total ADHD symptom score ‐ parent ratings Show forest plot | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 1.1 Long acting | 3 | 1049 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.08, ‐0.13] |
| 1.2 Short acting | 4 | 198 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.82, ‐0.23] |
| 2 Proportion of responders (CGI ‐ I) Show forest plot | 9 | 2105 | Risk Ratio (M‐H, Random, 95% CI) | 3.31 [2.44, 4.49] |
| 2.1 Long acting | 6 | 1662 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [2.63, 4.79] |
| 2.2 Short acting | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.39, 6.02] |
| 3 Academic performance Show forest plot | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 3.1 Long acting | 4 | 494 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.36, 0.81] |
| 3.2 Short acting | 4 | 332 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [0.15, 0.81] |
| 4 Retention: proportion of participants who completed the trial Show forest plot | 10 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.99, 1.12] |
| 4.1 Long acting | 6 | 1756 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.24] |
| 4.2 Short acting | 4 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 5 Proportion of participants experiencing decreased appetite Show forest plot | 10 | 2271 | Risk Ratio (M‐H, Random, 95% CI) | 6.18 [2.44, 15.63] |
| 5.1 Long acting | 8 | 2165 | Risk Ratio (M‐H, Random, 95% CI) | 7.67 [3.33, 17.65] |
| 5.2 Short acting | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.69, 3.62] |
| 6 Proportion of participants experiencing abdominal pain Show forest plot | 10 | 2155 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.03, 2.00] |
| 6.1 Long acting | 7 | 1855 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.10, 2.02] |
| 6.2 Short acting | 3 | 300 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.30, 21.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total ADHD symptom score ‐ parent ratings Show forest plot | 7 | 1247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐0.86, ‐0.27] |
| 1.1 Industry | 5 | 1135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.89, ‐0.16] |
| 1.2 Public | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.20, ‐0.31] |
| 1.3 Not reported | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.36, 0.16] |
| 2 Proportion of responders (CGI ‐ I) Show forest plot | 9 | 2210 | Risk Ratio (M‐H, Random, 95% CI) | 3.37 [2.50, 4.53] |
| 2.1 Industry | 8 | 2146 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [2.39, 4.40] |
| 2.2 Not reported | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.4 [2.38, 12.25] |
| 3 Academic performance Show forest plot | 8 | 826 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.39, 0.73] |
| 3.1 Industry | 5 | 688 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [0.46, 0.81] |
| 3.2 Public | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.60, 0.59] |
| 3.3 Not reported | 2 | 94 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.06, 0.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total ADHD symptom score ‐ parent ratings Show forest plot | 7 | 1247 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.64, ‐0.40] |
| 2 Hyperactivity/impulsivity ‐ parent ratings Show forest plot | 2 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐0.89, ‐0.19] |
| 3 Total ADHD symptom score ‐ teacher ratings Show forest plot | 5 | 745 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.62, ‐0.32] |
| 4 Hyperactivity/impulsivity ‐ teacher ratings Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5 Inattention ‐ teacher ratings Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Total ADHD symptom score ‐ clinician ratings Show forest plot | 3 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.01, ‐0.68] |
| 7 Hyperactivity/impulsivity ‐ clinician ratings Show forest plot | 3 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐0.90, ‐0.58] |
| 8 Inattention ‐ clinician ratings Show forest plot | 3 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐0.94, ‐0.61] |
| 9 Total ADHD symptom score ‐ investigator/research personnel ratings Show forest plot | 3 | 630 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐1.25, ‐0.91] |
| 10 Hyperactivity/impulsivity ‐ Investigator/research personnel ratings Show forest plot | 2 | 280 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐1.76, ‐1.23] |
| 11 Inattention ‐ investigator/research personnel ratings Show forest plot | 4 | 634 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐0.93, ‐0.60] |
| 12 Proportion of responders (CGI ‐ I) Show forest plot | 9 | 2207 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [2.68, 3.61] |
| 13 CGI ‐ S score Show forest plot | 2 | 86 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.15, ‐0.27] |
| 14 Quality of life Show forest plot | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15 Academic performance Show forest plot | 8 | 826 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.59 [0.45, 0.73] |
| 16 Retention: proportion of participants who completed the trial Show forest plot | 10 | 2364 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.04, 1.12] |
| 17 Proportion of participants who experienced at least one adverse event Show forest plot | 6 | 1742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.20, 1.47] |
| 18 Proportion of participants who dropped out/withdrew due to an adverse event Show forest plot | 9 | 2160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.08, 3.51] |
| 19 Proportion of participants experiencing decreased appetite Show forest plot | 11 | 2467 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [4.03, 7.68] |
| 20 Proportion of participants experiencing insomnia/trouble sleeping Show forest plot | 10 | 2429 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [2.82, 5.41] |
| 21 Proportion of participants experiencing abdominal pain Show forest plot | 10 | 2155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.18, 2.08] |
| 22 Proportion of participants experiencing headaches Show forest plot | 9 | 2091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 23 Proportion of participants experiencing anxiety/nervousness Show forest plot | 5 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.56] |
| 24 Proportion of participants experiencing nausea/vomiting Show forest plot | 6 | 1579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.20, 2.46] |

