Fentanilo intranasal para el tratamiento del dolor agudo en niños
Resumen
Antecedentes
El dolor es el síntoma más frecuente en el ámbito de urgencias; sin embargo, el tratamiento oportuno del dolor agudo en los niños sigue siendo subóptimo. La administración de fármacos intranasales ha surgido como un método alternativo para lograr una administración más rápida de los fármacos sin contribuir al malestar para el niño que implica la inserción de una cánula intravenosa.
Objetivos
Se identificaron y examinaron todos los ensayos controlados aleatorizados (ECA) y los ensayos cuasialeatorizados para evaluar los efectos del fentanilo intranasal (FIN) versus intervenciones analgésicas alternativas en niños con dolor agudo, en lo que se refiere a la reducción de la puntuación de dolor, la aparición de eventos adversos, la tolerabilidad de los pacientes, el uso de "analgesia de rescate", la satisfacción del paciente/paterna y la mortalidad del paciente.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane central de ensayos controlados (Cochrane Central Register of Controlled Trials; CENTRAL) (2014, número 1); MEDLINE (Ovid SP, desde 1995 hasta enero de 2014); EMBASE (Ovid SP, desde 1995 hasta enero de 2014); el Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO Host, desde 1995 hasta enero de 2014); la Latin American and Caribbean Health Science Information Database (LILACS) (BIREME, desde 1995 hasta enero de 2014); Commonwealth Agricultural Bureaux (CAB) Abstracts (desde 1995 hasta enero de 2014); Institute for Scientific Information (ISI) Web of Science (desde 1995 hasta enero de 2014); BIOSIS Previews (desde 1995 hasta enero de 2014); China National Knowledge Infrastructure (CNKI) (desde 1995 hasta enero de 2014); International Standard Randomized Controlled Trial Number (ISRCTN) (desde 1995 hasta enero de 2014); ClinicalTrials.gov (desde 1995 hasta enero de 2014); y la Plataforma de registros internacionales de ensayos clínicos (ICTRP) (hasta enero de 2014).
Criterios de selección
Se incluyeron los ECA que compararon FIN versus cualquier otra intervención farmacológica/no farmacológica para el tratamiento del dolor agudo en niños (< 18 años de edad).
Obtención y análisis de los datos
Dos autores de la revisión independientes evaluaron la relevancia de cada título y resumen. Las copias completas de todos los estudios que cumplieron los criterios de inclusión fueron identificadas para una evaluación adicional. Se utilizó la diferencia de medias (DM), el odds ratio (OR) y el intervalo de confianza (IC) del 95% para medir los tamaños del efecto. Dos autores de la revisión evaluaron y calificaron de forma independiente la calidad metodológica de cada ensayo mediante la herramienta Cochrane para evaluar el riesgo de sesgo, según el capítulo 8 del Manual Cochrane para Revisiones Sistemáticas de Intervenciones.
Resultados principales
Tres estudios (313 participantes) cumplieron los criterios de inclusión. Un estudio comparó el FIN con la morfina intramuscular (MIM); otro estudio comparó el FIN con la morfina intravenosa (MIV); y otro estudio comparó el FIN a concentración estándar (FINCE) con el FIN a concentración alta (FINCA). Los tres estudios informaron una reducción en la puntuación de dolor posterior a la administración de FIN. El FIN dio lugar a una reducción mayor en la puntuación de dolor a los diez minutos después de la administración en comparación con la MIM (puntuación de dolor del grupo de FIN: 1/5 versus puntuación de dolor del grupo de MIM: 2/5; valor de p = 0,014). No se informaron otras diferencias estadísticamente significativas en las puntuaciones de dolor en ningún otro punto temporal. Al comparar el FIN con la MIV y el FINCA, no se observaron diferencias estadísticamente significativas en las puntuaciones de dolor entre los brazos de tratamiento, antes de la analgesia o a los cinco, diez, 20 y 30 minutos después de la analgesia. Específicamente, al comparar el FIN con la MIV, se observó que ambos agentes produjeron una reducción estadísticamente significativa en la puntuación de dolor hasta 20 minutos después de la analgesia. No se observó una reducción adicional en la puntuación de dolor después de este periodo de tiempo. Cuando el FINCE se comparó con el FINCA, se observó una reducción estadística y clínicamente significativa en las puntuaciones de dolor durante el periodo de estudio (disminución mediana para ambos grupos de 40 mm, valor de p = 0,000). Ningún estudio informó eventos adversos (p.ej., toxicidad por opiáceos, muerte) después de la administración de FIN. Un estudio describió una mejoría en la tolerancia de los pacientes al FIN en comparación con la MIM, que alcanzó significación estadística. Los otros estudios describieron notificaciones de "sabor desagradable" y vómitos con el FIN. En general, el riesgo de sesgo de todos los estudios se consideró bajo.
Conclusiones de los autores
El FIN podría ser un analgésico efectivo para el tratamiento de los pacientes con dolor agudo moderado a intenso, y su administración parece causar un malestar mínimo en los niños. Sin embargo, esta revisión de los estudios publicados no permite establecer conclusiones definitivas con respecto a si el FIN es superior, no inferior o equivalente a la morfina intramuscular o intravenosa. Las limitaciones de esta revisión incluyen las siguientes: pocos estudios elegibles para inclusión (tres); ningún estudio examinó la administración del FIN en niños menores de tres años; ningún estudio incluyó a niños con dolor de causa "médica" (p.ej., dolor abdominal observado en la apendicitis); y todos los estudios elegibles se realizaron en Australia. Por lo tanto, los hallazgos podrían no ser generalizables a otros ámbitos de asistencia sanitaria, a los niños menores de tres años de edad ni a los que presentan dolor por una causa "médica".
PICOs
Resumen en términos sencillos
Fentanilo intranasal para el tratamiento del dolor agudo en niños
Antecedentes
El dolor es la razón más común por la que los pacientes acuden a los servicios de urgencias (SU). La naturaleza desafiante del tratamiento de los niños con dolor agudo intenso se refleja en la bibliografía médica a través del tratamiento deficiente del dolor en esta población. Se examinó la evidencia sobre el efecto del fentanilo intranasal (FIN) (un medicamento fuerte para el alivio del dolor, similar a la morfina) en comparación con cualquier otra técnica para el alivio del dolor utilizada en el tratamiento del dolor agudo intenso en los niños.
Características de los estudios
Se incluyeron los estudios con niños (menores de 18 años de edad) que presentaban dolor agudo intenso como resultado de lesiones o enfermedades médicas. La intervención estudiada fue la administración de FIN para el alivio del dolor en comparación con cualquier otra intervención con medicamentos para el alivio del dolor (p.ej., morfina intravenosa) o una intervención sin medicamentos (p.ej., colocación de una férula en la extremidad, un apósito para las heridas) proporcionada en el servicio de urgencias. La evidencia está actualizada hasta enero de 2014.
Resultados clave
Se identificaron tres estudios que incluían a 313 niños con dolor agudo intenso causado por una fractura de los miembros superiores e inferiores. Estos ensayos compararon FIN frente a morfina administrada con una aguja en un músculo (morfina intramuscular) o mediante un goteo en una vena (morfina intravenosa), así como FIN a concentración estándar frente a FIN a concentración alta. La población estudiada en conjunto en estos ensayos incluyó niños de tres a 15 años de edad. Los niños varones representaron aproximadamente dos tercios de la población general estudiada. La revisión concluyó que el FIN podría ser un analgésico eficaz para el tratamiento de los niños con dolor agudo de moderado a intenso, y su administración parece causar una molestia mínima en los niños; sin embargo, la evidencia no es suficiente para poder valorar los efectos del FIN en comparación con la morfina intramuscular o intravenosa. No se informaron eventos adversos graves (p.ej., toxicidad por opiáceos, muerte).
Limitaciones
Las limitaciones de esta revisión incluyen lo siguiente: pocos estudios (tres) fueron elegibles para inclusión; ningún estudio examinó el uso del FIN en niños menores de tres años de edad; ningún estudio incluyó a niños con dolor debido a una causa "médica" (p.ej., el dolor abdominal observado en la apendicitis); y todos los estudios elegibles se realizaron en Australia. Por lo tanto, los hallazgos podrían no ser generalizables a otros ámbitos de asistencia sanitaria, a los niños menores de tres años de edad ni a los que presentan dolor por una causa "médica".
Authors' conclusions
Summary of findings
| Intranasal fentanyl compared with intravenous morphine for the management of acute moderate to severe pain in children | ||||||
| Patient or population: children (aged < 18 years) with acute severe pain Settings: emergency department Intervention: intranasal fentanyl Comparison: intravenous morphine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous morphine | Intranasal fentanyl | |||||
| Pain reduction (mean VAS) Pain assessed before analgesia (0 min) and at 5, 10, 20 and 30 min after analgesia | 0 min = 67 5 min = 42 10 min = 41 20 min = 35 30 min = 33 | 0 min = 68 5 min = 55 10 min = 46 20 min = 37 30 min = 37 | 67 participants (1 study) | ⊕⊕⊕⊕ | Given no statistically significant difference between treatment arms, VAS scores were combined to form an overall VAS score for each time point. Combined VAS scores produced statistically significant reductions in pain at 5, 10 and 20 min after analgesia | |
| Respiratory depression | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) | ⊕⊕⊕ | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than | |
| Hypotension | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) | ⊕⊕⊕ | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than | |
| Decreased level of consciousness | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) | ⊕⊕⊕ | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than | |
| Intolerance to analgesia | 1 participant complained of a momentary flush at the IV site following administration of morphine | 4 participants; 3 participants reported a "bad taste" following INF administration, 1 participant vomited 20 min following INF administration | 67 participants (1 study) | ⊕⊕⊕⊕ | ||
| Use of ED "rescue" analgesia | 1 participant required 5 additional doses of IV morphine (protocol violation) | 1 participant required 6 additional doses of INF (protocol violation) | 67 participants (1 study) | ⊕⊕⊕ | Protocol violation in control and intervention arms of this trial. As per protocol, participants should receive only 4 additional doses of either agent | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| ED: Emergency department. IV: Intravenous. INF: Intranasal fentanyl. VAS: Visual analogue scale. | ||||||
Background
Description of the condition
Pain is the most common presenting symptom in the emergency setting and remains a challenging clinical problem for healthcare providers in both prehospital and emergency department (ED) environments (Alonso‐Serra 2003; Cordell 2002; Groenewald 2012; Paris 1988; Verghese 2010). Timely management of pain in children in the emergency care setting continues to be suboptimal (Rupp 2004; Wilsey 2004; Wilson 1989). Some studies have identified a significant disparity in the assessment and management of acute pain between adults and children, with adults twice as likely as children to receive appropriate analgesia for similar pain scores (Hennes 2005; Schechter 1989). Pain in the very young or in those with neurodevelopmental or cognitive delay has been associated with the worst pain management in this setting (Friedland 1994; Izsak 2008), and evidence shows that more than one‐third of children attending the ED via ambulance report acute pain as a chief complaint (Galinski 2011).
Description of the intervention
Management of acute pain in children in the emergency setting involves both pharmacological and non‐pharmacological interventions (Berde 2002; Kart 1997; Probst 2005; MacLean 2007). Examples of non‐pharmacological interventions to relieve pain in children include verbal reassurance, distraction techniques, wound dressings and splinting of fractures. Pharmacological agents may be administered by the oral, inhalational (e.g. nitrous oxide) or parenteral route. Commonly used oral analgesics include paracetamol (acetaminophen), non‐steroidal anti‐inflammatory drugs (NSAIDs) (e.g. ibuprofen) and opioids (e.g. codeine, morphine). Parenterally administered analgesia (e.g. morphine) is indicated for acute moderate to severe pain.
Although intravenous morphine traditionally has been considered the 'gold standard' analgesic for moderate to severe pain, the skills required to establish vascular access in children, in particular in the prehospital setting, are not universally available. Furthermore, insertion of an intravenous line invariably adds to the distress of most children. Intranasal fentanyl (INF) is increasingly employed as an acceptable alternative to intravenous morphine for the management of moderate or severe acute pain in children in prehospital, primary care and ED settings (Bendall 2011; Borland 2007; Borland 2008; Saunders 2010). The easily accessible rich vascular plexus of the nasal mucosa is an attractive route for drug delivery because it facilitates rapid drug absorption into the systemic circulation (by avoiding gastrointestinal degradation and hepatic first pass metabolism), resulting in an onset of action that compares favourably with intravenously administered analgesics. INF has a bioavailability of 89% with a short onset of action (˜7 minutes) (Panagiotou 2010). Duration of effect is directly related to INF dose, with pain scores returning to predose values at approximately 120 to 200 minutes after a single dose (Foster 2008). Pragmatically the intranasal route of administration is quicker than the intravenous route for all types of drug administration when the time required to insert an intravenous cannula is considered.
How the intervention might work
INF may offer emergency healthcare providers an acceptable alternative to intravenous opiates for achieving earlier effective pain relief for the child in pain, obviating the need for an intravenous cannula.
Why it is important to do this review
The intranasal route of analgesic administration offers several advantages over the intravenous route in the emergency care setting. These include reducing possible distress to the child, minimizing the risk of needle‐stick injuries, reducing staff training needed to undertake the procedure, providing a faster method of drug delivery and providing more rapid drug absorption than is achieved by the intravenous route.
Objectives
We identified and evaluated all randomized controlled trials (RCTs) and quasi‐randomized trials to assess the effects of INF versus alternative analgesic interventions in children with acute pain, with respect to reduction in pain score, occurrence of adverse events, patient tolerability, use of "rescue analgesia," patient/parental satisfaction and patient mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs and quasi‐randomized trials, with an RCT defined as a study in which participants were allocated to treatment groups on the basis of a random method (e.g. using random number tables, hospital number, date of birth).
Types of participants
We included children (< 18 years of age) with acute moderate to severe pain caused by injury (e.g. burns, wounds, suspected fractures) or medical illness.
We excluded from the review patients who received INF for the preemptive treatment of pain (i.e. patients who received INF as part of procedural sedation in the emergency setting). We also excluded children younger than three months of age because of opiate sensitivity.
Types of interventions
The target intervention was INF administered (via droplet, atomizer or spray) for pain relief in children with painful clinical conditions. INF concentration was noted. This treatment was compared with the following interventions.
-
Administration of other pharmacological interventions for pain relief (e.g. intravenous morphine) as an active control (including 'double‐dummy' study designs).
-
Non‐pharmacological intervention for pain relief (e.g. limb splinting).
-
Placebo administration.
Types of outcome measures
Primary outcomes
-
Reduction in pain score or intensity assessed by validated age‐appropriate pain scores (e.g. 'Faces, Legs, Activity, Cry, Consolability' (FLACC); Wong Baker; numerical rating scale (NRS); visual analogue scale (VAS)).
We sought outcome assessments at multiple time points post INF administration, if reported. We also sought to identify reductions in reported pain in terms of reductions across the spectrum of pain, that is, mild, moderate or severe pain, and no pain, such as reductions in pain from severe to moderate, or reductions in pain from pain to no pain (VAS < 2), as reported by study authors.
Secondary outcomes
-
Occurrence of all adverse events associated with INF (e.g. opiate toxicity).
-
Participant tolerance of INF including the incidence of nausea, vomiting or reported discomfort.
-
Use of 'rescue' analgesia before and after hospital arrival.
-
Participant satisfaction as defined by study authors.
-
Parental satisfaction as defined by study authors.
-
Cost as defined by study authors.
-
Mortality.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1; see Appendix 1); MEDLINE (Ovid SP, from 1995 to January 2014; see Appendix 2); EMBASE (Ovid SP, from 1995 to January 2014; see Appendix 3); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO Host, from 1995 to January 2014; see Appendix 4); Commonwealth Agricultural Bureaux (CAB) Abstracts (from 1995 to January 2014); the Institute for Scientific Information (ISI) Web of Science (from 1995 to January 2014; see Appendix 5); the Latin American and Caribbean Health Science Information Database (LILACS) (BIREME, from 1995 to January 2014; see Appendix 6); BIOSIS Previews (from 1995 to January 2014); the China National Knowledge Infrastructure (CNKI) (from 1995 to January 2014); International Standard Randomized Controlled Trial Number (ISRCTN) (from 1995 to January 2014); ClinicalTrials.gov (from 1995 to January 2014); and the International Clinical Trials Registry Platform (ICTRP) (to January 2014).
We identified published, unpublished and ongoing studies by searching these databases from 1995, as we understand no studies were conducted on the use of INF in children before this date. We modelled subject strategies for databases on the search strategy designed for MEDLINE (see Appendix 2). When appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomized controlled trials and controlled clinical trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2, Box 6.4.b (Higgins 2011).
We imposed no language restrictions.
Searching other resources
We searched the reference lists of review articles and relevant trials, textbooks and abstracts of scientific meetings to identify further RCTs. We reviewed the titles and abstracts to identify all potentially eligible RCTs. We obtained the full‐text versions of these articles. We made additional efforts to identify RCTs potentially relevant to the topic by using the following data sources.
-
Foreign language literature.
-
Grey literature (theses, internal reports, non–peer‐reviewed journals).
-
References (and references of references) cited in primary sources.
-
Other unpublished sources known to experts in the speciality (to be sought by personal communication).
-
Raw data from published trials (sought by personal communication).
Data collection and analysis
Selection of studies
Two independent review authors (JC and MB) assessed each title and abstract for relevance. Disagreements on eligibility were resolved by discussion or by referral to a third party (ROS). If no abstract was available, we obtained and assessed the full paper. We obtained the full copies of all studies that met the inclusion criteria for further assessment.
Data extraction and management
Two review authors (JH and SMC) independently extracted the data; two separate review authors (JC and NK) entered the data into Review Manager software (RevMan 5.2) for statistical analysis (see Appendix 7).
Assessment of risk of bias in included studies
Two review authors (JC and MB) independently undertook assessment of risk of bias of the included trials. They took the following into consideration, guided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of personnel, participants and outcome assessment.
-
Incomplete outcome data.
-
Selective reporting.
-
Other bias.
We used the Cochrane risk of bias tool in RevMan 5.2, which involves describing each of the domains as reported in the trial and then assigning a judgement about the adequacy of each entry (low risk of bias, high risk of bias or unclear (or unknown) risk of bias).
We considered a trial as having low risk of bias when all domains were assessed as adequate. We considered a trial as having high risk of bias when one or more domains were assessed as inadequate or unclear.
We included the 'Risk of bias' table as part of the Characteristics of included studies section and present a 'Risk of bias summary' figure, which details all judgements made for all studies included in the review.
Measures of treatment effect
When the measure of the outcome was sufficiently consistent across trials, we used odds ratios (ORs) for dichotomous data and mean differences (MDs) for continuous data with corresponding 95% confidence intervals (CIs).
Unit of analysis issues
The unit of analysis is based on the individual participant (the unit to be randomly assigned for interventions to be compared). For included trials using a cross‐over design, we used only pre‐cross‐over data.
Dealing with missing data
When data seemed to be missing from a study, they were obtained, if possible, through correspondence with study authors. By using sensitivity analysis, we explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include in the analyses all participants randomly assigned to each group and to analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomly assigned minus the number of participants whose outcomes were known to be missing.
Assessment of heterogeneity
We evaluated clinical heterogeneity (differences between studies in key characteristics of participants, interventions or outcome measures) (Deeks 2001). In the absence of clinical heterogeneity, we used the I2 statistic to describe the percentage of total variation across studies that is due to heterogeneity rather than to chance. We considered an I2 value > 50% to represent significant statistical heterogeneity. We also used visual inspection of the graphical representation of study results with their 95% CIs to assess heterogeneity. We analysed results using both fixed‐effect and random‐effects model analysis because for each model, the result is counterintuitive in some situations. In the presence of significant statistical heterogeneity (I2 > 50%) and when differences in results were of practical importance, we gave greater emphasis to the random‐effects model, which takes into account between‐study variability as well as within‐study variability. We also used the fixed‐effect model to test the robustness of the analysis and to look for outliers.
Assessment of reporting biases
Detecting publication bias is difficult, and avoidance is a better strategy (Glasziou 2001). We avoided publication bias by comprehensively searching the literature and by using study registries (Glasziou 2001). Publication bias is associated with asymmetry (Light 1984). We looked for asymmetry and explored potential reasons other than publication bias, for example, high risk of bias of smaller studies, true heterogeneity, artefact or chance (Egger 1997).
Data synthesis
We performed meta‐analysis using RevMan software (RevMan 5.2). Our primary outcome measure was a reduction in pain score or intensity using a validated age‐appropriate pain score. For dichotomous (or binary) data, we described results both as a relative measure (risk ratio (RR)) and as an absolute measure (number needed to treat for an additional beneficial outcome (NNTB) and risk difference (RD)). Relative measures can be used to combine studies, but absolute measures can be more informative than relative measures because they reflect baseline risk as well as changes in risk associated with the intervention. For continuous data, we used the mean difference (MD) when outcomes were measured in a standard way across studies. This provided the advantage of summarizing results in natural units that are easily understood. When it was desirable to summarize results across studies with outcomes that are conceptually the same but are measured in different ways, we used standardized mean differences (SMDs).
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis on the use of intranasal fentanyl in the prehospital environment.
Sensitivity analysis
We carried out a sensitivity analysis to test how sensitive study results were to reasonable changes in the assumptions made and in the protocol for combining data (Lau 1998). We performed sensitivity analysis for 'randomized versus quasi‐randomized' and 'low risk of bias' versus 'high risk of bias' studies.
Summary of findings
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes. We included the following outcomes in our review and constructed a 'Summary of findings' (SoF) table using RevMan (summary of findings Table for the main comparison).
-
Pain score reduction (using age‐appropriate validated pain scales) following administration of INF at multiple time points.
-
Occurrence of adverse events post INF administration.
-
Acceptability of INF administration among participants.
-
Use of 'rescue analgesia' post INF administration.
-
Participant and parental satisfaction as defined by study authors.
-
Cost as defined by study authors.
-
Mortality.
The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search of electronic databases yielded a total of 4875 publications. After titles and abstracts of all studies had been reviewed, six full papers were retrieved for possible inclusion. After the full texts had been examined, three papers were excluded and three studies were included. The study selection process is summarized in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram shown in Figure 1.

Study flow diagram.
Included studies
We included three trials (313 participants) comparing INF with alternative interventions for the treatment of children in acute pain (Borland 2007; Borland 2011; Younge 1999). All three studies included two comparison arms and were conducted at single sites in Australia. One study compared INF versus intramuscular morphine (IMM) (Younge 1999); another study compared INF versus intravenous morphine (IVM) (Borland 2007); and the final included study compared two different concentrations of INF for the treatment of children in acute pain (Borland 2011).
Each of the three selected studies included children who had experienced pain as a result of suspected limb fracture. One study recruited participants three to 10 years of age (Younge 1999), another study recruited participants three to 15 years of age (Borland 2011) and the final study recruited participants seven to 15 years of age (Borland 2007). Participant sex was not considered among the inclusion criteria. However, the intervention arm of one study consisted of 62.5% male participants (Younge 1999) and 62.9% male participants in another study (Borland 2011). Borland 2007 indicated that baseline characteristics were similar in control and intervention arms. Exclusion criteria were similar for all three studies (head injury impairing judgement, known allergy to opiates, blocked/traumatized nose, participants requiring immediate IV access, inability to perform pain scoring).
All three studies described reduction in pain intensity as the primary outcome measure. Pain scores were documented at five‐minute intervals for 30 minutes (Borland 2007; Younge 1999) and at 10‐minute intervals for 30 minutes (Borland 2011). Secondary outcome measures included participant tolerance to the medication administered (all studies); occurrence of opiate toxicity (respiratory depression, hypotension or decreased level of consciousness) was documented in two studies (Borland 2011; Younge 1999), and the use of "rescue analgesia" was identified in two studies (Borland 2007; Borland 2011).
Pooling of data was not possible, given that no studies employed the same comparator arms. Details of included studies are listed in Characteristics of included studies and Figure 1.
Excluded studies
Three trials were excluded because each involved preemptive treatment of children in pain in advance of lumbar puncture/bone marrow aspiration (Sandler 1992), children requiring a dressing change for burn injury (Borland 2005) and children undergoing catheterization for voiding cystourethrography (Chung 2010).
Risk of bias in included studies
Allocation
Borland 2011 and Younge 1999 appeared to provide sufficient detail in terms of random sequence generation and allocation of concealment. However, Borland 2007 was considered to have unclear risk of selection bias (Figure 2; Figure 3).
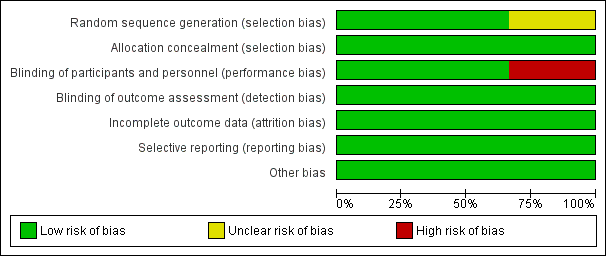
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
Blinding of participants and personnel was reported in two studies (Borland 2007; Borland 2011).
Incomplete outcome data
Two studies were limited by incomplete outcome data (Borland 2007; Borland 2011).
Selective reporting
Reporting bias was considered of low risk in all studies, given that each study's prespecified outcomes of interest in the review have been reported in the prespecified way.
Other potential sources of bias
No other potential sources of bias were identified in either study.
Effects of interventions
See: Summary of findings for the main comparison
Primary outcome
Reduction in pain score
All three studies reported a reduction in pain score/intensity—the primary outcome measure of this review.
Borland 2007 utilized a 100‐mm unmarked VAS for pain assessment. This study reported no statistically significant differences in visual analogue scale scores between treatment arms (INF or intravenous morphine) before analgesia or at 5, 10, 20 and 30 minutes post analgesia (P value 0.333). Statistically significant reductions in the combined VAS score were noted in both treatment arms at 5 minutes post analgesia of 20 mm (P value 0.000), at 10 minutes of 4 mm (P value 0.012) and at 20 minutes of 8 mm (P value 0.000). No further significant reductions in VAS score were reported beyond 20 minutes (P value 0.753) (Table 1). See also summary of findings Table for the main comparison.
| 0 min | 5 min | 10 min | 20 min | 30 min | |
| Intravenous morphine (mm) | 67 | 42 | 41 | 35 | 33 |
| Intranasal fentanyl (mm) | 68 | 55 | 46 | 37 | 37 |
| Difference (mm) (95% CI) | ‐1 (‐12 to 9) | ‐13 (‐23 to ‐3) | ‐5 (‐16 to 7) | ‐2 (‐13 to 10) | ‐4 (‐16 to 8) |
Borland 2007: Mean visual analogue score (mm) over time.
Borland 2011 reported no statistically significant differences in median pain scores between treatment arms (high concentration INF vs standard concentration INF) at any of the study time points. Each agent demonstrated a statistically and clinically significant reduction in pain scores over the duration of the study (median decrease for both groups 40 mm, P value 0.000) (Table 2).
| SINF | HINF | P value | |
| Before analgesia | 80.0 (60.0‐95.5) | 77.5 (60.0‐100) | 0.881 |
| 10 min | 49.5 (26.5‐68.5) | 43.0 (15.2‐66.0) | 0.176 |
| 20 min | 27.5 (18.5‐56.5) | 35.0 (9.0‐57.0) | 0.758 |
| 30 min | 20.0 (10.0‐46.0) | 21.5 (4.75‐51.0) | 0.662 |
Borland 2011: Median visual analogue pain score (mm) over time.
Younge 1999 assessed pain on a six‐point pain scale (0 = no pain, 5 = worst pain). No significant differences in presenting pain scores were noted between treatment arms (P value 0.46). This study reported a significant difference in pain scores at 10 minutes, with a lower pain score seen in the INF group (pain score of 1 vs pain score of 2 for INF vs intramuscular morphine, respectively; P value 0.014). The median pain score difference at 20 minutes did not reach statistical significance (P value 0.64), and no further difference in pain scores was noted at any other time (Table 3).
| 0 min | 5 min | 10 min | 20 min | 30 min | |
| Intranasal fentanyl | 4 | 3 | 1 | 1 | 1 |
| Intramuscular morphine | 4 | 3 | 2 | 2 | 1 |
Younge 1999: Median pain score (Wong Baker Faces, ordinal scoring 0‐5) over time.
Secondary outcomes
Occurrence of all adverse events associated with INF
No adverse events (e.g. opiate toxicity, death) were reported in any study following INF administration.
Participant tolerance of INF (including incidence of nausea, vomiting or reported discomfort)
Younge 1999 described better tolerance to INF (P value < 0.001), with one child crying during administration and a second child vomiting following administration.
Borland 2007 described three children who reported a bad taste in their mouth and one child who experienced an episode of vomiting 20 minutes post INF administration. One child complained of a momentary flush at the intravenous line site following administration of morphine.
Similarly, Borland 2011 identified one child who reported dislike of the taste of INF solution when swallowed.
Use of 'rescue' analgesia before and after hospital arrival
Younge 1999 identified one child and Borland 2007 identified one child who required rescue analgesia following INF administration.
Borland 2011 did not impose a restriction on participants receiving more than one additional analgesic agent (e.g. oral paracetamol, ibuprofen), and the decision to administer additional analgesia was made at the discretion of the treating nurse. Additional analgesia was offered as per standard fracture management in that ED, with a desire to have these agents active before the effects of fentanyl had worn off. More than one‐third (35.4%) of the overall study population (67/189) was administered additional analgesia. In all, 42 participants received standard concentration INF and 25 received high concentration INF. The standard concentration INF group was given significantly more additional analgesia compared with the high concentration INF group (P value 0.028). Given no demonstrable difference in pain scores between treatment arms, the clinical significance of this finding is difficult to determine.
Participant satisfaction as defined by study authors
No study addressed this outcome.
Parental satisfaction as defined by study authors
No study addressed this outcome.
Cost as defined by study authors
No study addressed this outcome.
Mortality
No study participant died as a result of INF administration.
Discussion
Summary of main results
This review assessed RCT evidence comparing outcomes of INF and alternative analgesic therapy in the treatment of children in acute pain. Our review found no evidence to support a difference in the primary outcome measure—relief of pain—between INF and either intravenous morphine or high concentration INF (Borland 2007; Borland 2011). One study, however, did show a statistically significant reduction in pain intensity at 10 minutes post drug administration when INF was compared with intramuscular morphine (Younge 1999). It was not possible to pool the results of these three trials because the comparator interventions were different, and timing of pain score assessment was inconsistent between two of the three studies. All three trials enrolled children with clinically deformed closed long bone fractures.
This review found no evidence of adverse events (e.g. opiate toxicity, anaphylaxis) associated with administration of INF in any of the included studies. One study described improved acceptability of INF administration compared with administration of intramuscular morphine (Younge 1999), in contrast to four participants in the other trials who reported a "bad taste" following administration of INF. "Rescue analgesia" was administered to three children in two studies (Borland 2007; Younge 1999), whereas the third study (Borland 2011) posed no limitation on participants receiving more than one additional analgesic agent. In this study, more than one‐third (35.4%) of study participants were administered additional analgesia. No study documented participant or parental satisfaction with INF. No participant died as a result of INF administration.
Overall completeness and applicability of evidence
We were unable to perform a valid sensitivity analysis as planned a priori because all RCTs included in this review were randomized (no quasi‐randomized trials were eligible for inclusion).
Quality of the evidence
Some limitations were noted in the design and implementation of all three studies. Borland 2007 used a convenience sample for enrolments that was dependent on identification of suitable participants at triage. No record was kept of potential participants who were not enrolled, so no conclusion can be drawn about potential selection bias. Enrolment in Borland 2011 was not compulsory but was actively encouraged by study investigators. The fact that not all potential participants were screened for inclusion in the study may influence external validity. However, patients meeting inclusion criteria through the "Drugs of Dependence" register in the study ED were recorded and reasons for failure to enrol in the study documented. Based on similarities between cohorts of included and non‐included patients, this potential source of selection bias was minimized. Younge 1999 was limited in design by the open nature of the trial and did not meet the criteria for a good quality study (one that includes all of the following domains: adequate allocation concealment, blinding of outcome assessment and analysis performed according to intention‐to‐treat principles), suggesting a likely potential source of bias.
We identified neither indirectness of evidence (indirect population, intervention, control, outcomes) nor unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses) in any included study. Similarly no evidence suggested imprecision of results (wide confidence intervals) in any included study. The overall risk of publication bias was thought to be low in all included studies.
Potential biases in the review process
We encountered no potential bias in the review process.
Agreements and disagreements with other studies or reviews
Our search yielded two other systematic reviews on the use of intranasal fentanyl in the context of acute pain management ( Hansen 2013, Mudd 2010). Hansen et al (2013) conducted a systematic review of INF trials (randomized and non‐randomized) completed in emergency department and prehospital settings and imposed no age restriction. These review authors concluded that only two of the 12 studies identified were adequately randomized and double‐blinded (Borland 2007; Borland 2011), emphasizing the fact that "further well‐performed double‐blinded randomized controlled trials are required to thoroughly validate the use of INF in this context." Nevertheless, the review authors acknowledged the fact that these studies demonstrated the non‐inferiority of INF compared with intravenous and intramuscular morphine.
Mudd (2010) conducted a systematic review of INF trials (randomized and non‐randomized) in children. Similarly, this review concluded that INF was "equivalent or superior to morphine that is administered orally, intravenously, and intramuscularly." In addition, this review illustrates that INF may be more favourable over intravenous or intramuscular morphine, given the painless nature of its administration.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Intranasal fentanyl compared with intravenous morphine for the management of acute moderate to severe pain in children | ||||||
| Patient or population: children (aged < 18 years) with acute severe pain Settings: emergency department Intervention: intranasal fentanyl Comparison: intravenous morphine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intravenous morphine | Intranasal fentanyl | |||||
| Pain reduction (mean VAS) Pain assessed before analgesia (0 min) and at 5, 10, 20 and 30 min after analgesia | 0 min = 67 5 min = 42 10 min = 41 20 min = 35 30 min = 33 | 0 min = 68 5 min = 55 10 min = 46 20 min = 37 30 min = 37 | 67 participants (1 study) | ⊕⊕⊕⊕ | Given no statistically significant difference between treatment arms, VAS scores were combined to form an overall VAS score for each time point. Combined VAS scores produced statistically significant reductions in pain at 5, 10 and 20 min after analgesia | |
| Respiratory depression | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) | ⊕⊕⊕ | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than | |
| Hypotension | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) | ⊕⊕⊕ | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than | |
| Decreased level of consciousness | No cases were reported in this study | No cases were reported in this study | 67 participants (1 study) | ⊕⊕⊕ | Dosage regimen for this study was calculated for 3 weight intervals. Inclusion of 21 children outside the weight intervals (1 less than 20 kg and 20 greater than | |
| Intolerance to analgesia | 1 participant complained of a momentary flush at the IV site following administration of morphine | 4 participants; 3 participants reported a "bad taste" following INF administration, 1 participant vomited 20 min following INF administration | 67 participants (1 study) | ⊕⊕⊕⊕ | ||
| Use of ED "rescue" analgesia | 1 participant required 5 additional doses of IV morphine (protocol violation) | 1 participant required 6 additional doses of INF (protocol violation) | 67 participants (1 study) | ⊕⊕⊕ | Protocol violation in control and intervention arms of this trial. As per protocol, participants should receive only 4 additional doses of either agent | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| ED: Emergency department. IV: Intravenous. INF: Intranasal fentanyl. VAS: Visual analogue scale. | ||||||
| 0 min | 5 min | 10 min | 20 min | 30 min | |
| Intravenous morphine (mm) | 67 | 42 | 41 | 35 | 33 |
| Intranasal fentanyl (mm) | 68 | 55 | 46 | 37 | 37 |
| Difference (mm) (95% CI) | ‐1 (‐12 to 9) | ‐13 (‐23 to ‐3) | ‐5 (‐16 to 7) | ‐2 (‐13 to 10) | ‐4 (‐16 to 8) |
| Borland 2007: Mean visual analogue score (mm) over time. | |||||
| SINF | HINF | P value | |
| Before analgesia | 80.0 (60.0‐95.5) | 77.5 (60.0‐100) | 0.881 |
| 10 min | 49.5 (26.5‐68.5) | 43.0 (15.2‐66.0) | 0.176 |
| 20 min | 27.5 (18.5‐56.5) | 35.0 (9.0‐57.0) | 0.758 |
| 30 min | 20.0 (10.0‐46.0) | 21.5 (4.75‐51.0) | 0.662 |
| Borland 2011: Median visual analogue pain score (mm) over time. | |||
| 0 min | 5 min | 10 min | 20 min | 30 min | |
| Intranasal fentanyl | 4 | 3 | 1 | 1 | 1 |
| Intramuscular morphine | 4 | 3 | 2 | 2 | 1 |
| Younge 1999: Median pain score (Wong Baker Faces, ordinal scoring 0‐5) over time. | |||||

