Antibiotics for persistent cough or wheeze following acute bronchiolitis in children
Abstract
Background
Bronchiolitis is a common acute respiratory condition with high prevalence worldwide. This clinically diagnosed syndrome is manifested by tachypnoea (rapid breathing), with crackles or wheeze in young children. In the acute phase of bronchiolitis (≤ 14 days), antibiotics are not routinely prescribed unless the illness is severe or a secondary bacterial infection is suspected. Although bronchiolitis is usually self‐limiting, some young children continue to have protracted symptoms (e.g. cough and wheezing) beyond the acute phase and often re‐present to secondary care.
Objectives
To compare the effectiveness of antibiotics versus controls (placebo or no treatment) for reducing or treating persistent respiratory symptoms following acute bronchiolitis within six months of acute illness.
Search methods
We searched the following databases: the Cochrane Airways Group Register of Trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid), Embase (Ovid), the World Health Organization (WHO) trial portal, the Australian and New Zealand Clinical Trials Registry, and ClinicalTrials.gov, up to 26 August 2016.
Selection criteria
We included randomised controlled trials (RCTs) comparing antibiotics versus controls (placebo or no treatment) given in the post‐acute phase of bronchiolitis (> 14 days) for children younger than two years with a diagnosis of bronchiolitis.
Data collection and analysis
Two review authors independently assessed studies against predefined criteria, and selected, extracted, and assessed data for inclusion. We contacted trial authors for further information.
Main results
In this review update, we added one study with 219 children. A total of two RCTs with 249 children (n = 240 completed) were eligible for inclusion in this review. Both studies contributed to our primary and secondary outcomes, but we assessed the quality of evidence for our three primary outcomes as low, owing to the small numbers of studies and participants; and high attrition in one of the studies. Data show no significant differences between treatment groups for our primary outcomes: proportion of children (n = 249) who had persistent symptoms at follow‐up (odds ratio (OR) 0.69, 95% confidence interval (CI) 0.37 to 1.28; fixed‐effect model); and number of children (n = 240) rehospitalised with respiratory illness within six months (OR 0.54, 95% CI 0.05 to 6.21; random‐effects model). We were unable to analyse exacerbation rate because studies used different methods to report this information. Data showed no significant differences between treatment groups for our secondary outcome: proportion of children (n = 240) with wheeze at six months (OR 0.47, 95% CI 0.06 to 3.95; random‐effects model). One study reported bacterial resistance, but only at 48 hours (thus with limited applicability for this review). Another study reported adverse events from which all children recovered and remained in the study.
Authors' conclusions
Current evidence is insufficient to inform whether antibiotics should be used to treat or prevent persistent respiratory symptoms in the post‐acute bronchiolitis phase. Future RCTs are needed to evaluate the efficacy of antibiotics for reducing persistent respiratory symptoms. This is particularly important in populations with high acute and post‐acute bronchiolitis morbidity (e.g. indigenous populations in Australia, New Zealand, and the USA).
PICO
Plain language summary
Antibiotics for persistent cough or wheeze following acute bronchiolitis in children
Review question
How do antibiotics compare with placebo or no treatment for reducing or treating persistent respiratory symptoms in children following acute bronchiolitis?
Background
Bronchiolitis is a lung condition that commonly affects children across the world. Young children with bronchiolitis normally have a cough, fast and difficult breathing, and poor feeding. Antibiotics are not usually prescribed unless the illness is severe, or a secondary bacterial infection is suspected. However, some children continue to have ongoing problems (i.e. wheeze, cough) after the acute infection (> 14 days), increasing the risk of ongoing burden of disease and costs to the health system. These children often re‐present for further medical care in the community (general practitioners and health providers) or in hospital (emergency departments). Antibiotics used to treat these ongoing symptoms may get rid of bacteria in the lungs and may improve long‐term outcomes.
Study characteristics
This review update (up to 26 August 2016) includes two clinical trials that compared antibiotics with placebo for children in the post‐acute bronchiolitis phase (> 14 days). The first was reported from Turkey and enrolled 30 infants aged seven months or younger. The second was reported from Australia and New Zealand and enrolled 249 infants aged 24 months or younger. Both trials initiated treatment during hospitalisation for bronchiolitis and provided follow‐up for six months post hospitalisation.
Key results
This review update includes a total of two trials with 249 children (n = 240 completed). Both studies contributed to primary and secondary outcomes, but the quality of evidence was low. Review authors noted no significant differences between treatment groups for our primary outcomes: proportion of children (n = 249) who had persistent symptoms at follow‐up, and number of children (n = 240) rehospitalised with respiratory illness within six months; nor for our secondary outcome: proportion of children (n = 240) with wheeze at six months. One study reported bacterial resistance, but only at 48 hours. One study reported adverse events from which all children recovered and remained in the study.
Quality of evidence
Currently, not enough evidence is available to inform whether antibiotics should be used to treat or prevent persistent respiratory symptoms in the post‐acute phase of bronchiolitis. Clinical trials are needed to evaluate the efficacy of antibiotics for reducing persistent respiratory symptoms, especially in countries where morbidity of bronchiolitis is high (e.g. indigenous populations).
Authors' conclusions
Summary of findings
| Antibiotics compared with placebo or no treatment for persistent respiratory symptoms following acute bronchiolitis | ||||||
| Patient or population: children < 24 months with persistent respiratory symptoms following acute bronchiolitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with antibiotics | |||||
| Number of participants who were not cured at follow‐up | 234 per 1000 | 174 per 1000 | OR 0.69 | 249 | ⊕⊕⊝⊝ | |
| Number of participants rehospitalised for a respiratory illness | 238 per 1000 | 271 per 1000 | OR 1.19 | 240 | ⊕⊕⊝⊝ | |
| Proportion of participants with recurrent wheeze | 123 per 1000 | 99 per 1000 | OR 0.47 (0.06 to 3.95) | 240 | ⊕⊕⊝⊝ | |
| ^Intervention/Comparison group: treatment initiated during child's hospitalisation for acute bronchiolitis *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| We used GRADEPro software to create this table (GRADEpro GDT 2015) GRADE Working Group grades of evidence | ||||||
| aQuality downgraded because of small numbers of studies and participants and high attrition in the Tahan study. Hence, we cannot be confident of the effect estimate | ||||||
Background
Description of the condition
Bronchiolitis is a common acute respiratory condition, with a high prevalence worldwide (Chang 2009). This clinically diagnosed syndrome is manifested by tachypnoea (rapid breathing) with crackles or wheeze in very young children (Ralston 2014). Multiple viruses and some atypical bacteria can cause bronchiolitis, including respiratory syncytial virus (RSV), influenza, human metapneumovirus, rhinovirus, influenza, parainfluenza, adenovirus, and mycoplasma.
In the acute phase (≤ 14 days) of bronchiolitis, antibiotics are usually only recommended if the illness is severe, or if a secondary bacterial infection is suspected (Ralston 2014; NICE 2015). However, a Cochrane review found "minimal evidence to support the use of antibiotics for acute bronchiolitis" (Spurling 2011).
Although bronchiolitis is typically self‐limiting, lasting from three to seven days, a number of children continue to display respiratory symptoms beyond the acute phase. Swingler 2000 reported that 39% of infants were still symptomatic after 14 days, 18% after 21 days, and 9% after 28 days. Other studies have shown that 40% to 50% of those hospitalised have a "grumbling, sometimes protracted, respiratory syndrome of persistent cough and recurrent viral‐induced wheeze" (SIGN 2006). Although symptoms such as cough and wheezing may be mild in post‐acute bronchiolitis, they have the potential to increase the burden of disease (e.g. presenting or re‐presenting to secondary care). Furthermore, among some populations (e.g. indigenous children) (Bailey 2009), recurrent hospitalisations are common, and prolonged symptoms (e.g. wet cough) have been associated with a future diagnosis of bronchiectasis (McCallum 2016). Cohort studies have also suggested that bronchiolitis may trigger, or may be associated with future development of asthma (Sigurs 2000; Carroll 2009).
The possible biological mechanism that may give rise to persistent respiratory symptoms is likely to be multi‐factorial. In bronchiolitis, airway oedema occurs, the airway epithelium is affected, and cilial damage can persist for 13 to 17 weeks (Wong 2005). Cilia are an important component of the airway's clearance mechanism. Damage to airway cilia and possible impairment of innate immunity in severe bronchiolitis (Halfhide 2008) predispose these infants to secondary bacterial infection. Persistent or delayed resolution of airway oedema or the presence of secondary bacterial infection in the airways (endobronchial infection) related to persistent cilial damage can cause wheeze, cough, or both.
Description of the intervention
In the presence of endobronchial infection, antibiotics are a potentially beneficial intervention for individuals with post‐acute bronchiolitis. When given early (i.e. before the onset of secondary bacterial infection), antibiotics have the potential to prevent persistent symptoms; when given later (i.e. after the acute episode), they are used to treat the bacterial endobronchial infection. Antibiotics may be given orally, intravenously, or via a nebuliser. The efficacy of antibiotics given in the acute or non‐acute phase may be influenced by study setting. For example, in non‐affluent settings, where dense bacterial colonisation of the nasopharynx is higher than in affluent urban settings (Leach 1994), antibiotics may be more effective.
How the intervention might work
Antibiotics may be useful for treating individuals with symptoms of post‐acute bronchiolitis by preventing or eliminating secondary bacterial infection in the lower airways. Lower airway bacterial infection following viral respiratory infection has been well described in airway cells in vitro (Didierlaurent 2008), as well as in epidemiological and clinical studies (McCullers 2006). In addition to antibacterial effects, antibiotics such as macrolides, through their anti‐inflammatory properties, may confer immunomodulatory effects, thus influencing neutrophilic inflammation (Giamarellos‐Bourboulis 2008; Zarogoulisidis 2011) and reducing airway oedema. Elimination of endobronchial infection or inflammation, or both, may reduce airway secretions or oedema, or both, consequently improving persistent respiratory symptoms.
Why it is important to do this review
A small but significant number of children with acute bronchiolitis have persistent problems beyond the acute infection (for a variety of reasons). These children are usually referred to secondary and tertiary practices (i.e. outside of general practice) and are treated with a variety of medications such as antibiotics, bronchodilators, inhaled (Fox 1999) and oral corticosteroids (Blom‐Danielle 2007), and leukotriene receptor antagonists (Kim 2010). This clinical issue has been identified as an area that needs more research (Su 2014). Further, use of any medication may result in adverse events, and persistent symptoms may affect the burden of disease and health economics. Thus, a systematic review of the benefits and other effects achieved by using antibiotics in the post‐acute bronchiolitis phase should prove useful in guiding clinical practice.
Objectives
To compare the effectiveness of antibiotics versus controls (placebo or no treatment) for reducing or treating persistent respiratory symptoms following acute bronchiolitis within six months of acute illness.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) comparing antibiotics versus controls (placebo or no treatment) given during the post‐acute phase of bronchiolitis (> 14 days). Antibiotics could have been started during the acute phase (at the start of illness to prevent post‐bronchiolitis symptoms) or during the post‐acute phase (for treatment of post‐bronchiolitis symptoms).
Types of participants
Included were previously healthy children (aged two years or younger) with bronchiolitis (as defined by study authors) who were treated with antibiotics beyond the acute bronchiolitis period (> 14 days).
Excluded were children with any underlying chronic disease such as lung disease (e.g. cystic fibrosis, bronchopulmonary dysplasia, bronchiectasis, aspiration), cardiac disease, or immunodeficiency (primary or secondary).
Types of interventions
We planned to examine all types of antibiotics given beyond the acute period (> 14 days), including those prescribed for acute bronchiolitis that lasted beyond the acute phase of 14 days.
Types of outcome measures
Primary outcomes
-
Number of participants who were not cured at follow‐up (up to six months) (i.e. wheeze/cough)
-
Exacerbation rate (up to six months)
-
Number of participants rehospitalised for a respiratory illness within six months of onset of illness
Secondary outcomes
-
Mean difference in cough indices (e.g. cough diary, frequency, or scores; quality of life)
-
Mean difference in wheeze indices (e.g. cough diary, frequency, or scores; quality of life)
-
Proportion of participants with wheeze (within six months of intervention)
-
Adverse events (e.g. proportion of participants requiring new medications or developing pneumonia)
-
Bacterial resistance
We selected complete resolution of symptoms as the primary outcome, as previously healthy children should completely recover after an episode of acute bronchiolitis. Cohort studies of bronchiolitis (Bailey 2009) have reported pneumonia and recurrent wheeze (some cases requiring repeat hospitalisation). Thus, we considered re‐admission for a respiratory infection and recurrent wheeze as important outcomes.
Search methods for identification of studies
Electronic searches
The Trial Search Co‐ordinator for the Cochrane Airways Group performed the search, identifying trials through the following databases.
-
Cochrane Airways Group Register of Trials (all years).
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8).
-
MEDLINE (Ovid) September week 4 2012 to August week 3 2016.
-
Embase (Ovid) week 12 2012 to week 34 2016.
-
World Health Organization (WHO) trials portal.
-
Australian and New Zealand Clinical Trials Registry.
We have listed the search strategies in Appendix 1. We searched all databases from their inception to the present and imposed no restriction on language of publication.
The Cochrane Airways Group (Elizabeth Stovold) performed the search on 26 August 2016.
Searching other resources
We checked the reference lists of included studies and of relevant review articles for additional references. We contacted authors of identified trials, when appropriate, to ask about other published and unpublished studies.
Data collection and analysis
Selection of studies
Using article titles, abstracts, or descriptors, two review authors (GBM and ABC in the original review; GBM and EJP for the search from 2012 to 2016) independently reviewed literature searches to identify potentially relevant trials for full review. They conducted searches of bibliographies and texts and other portals to look for additional eligible studies (e.g. Clinicaltrials.gov; WHO trial registry). From the full‐text articles, the two review authors independently assessed trials for inclusion on the basis of specific predefined criteria.
Data extraction and management
We had no disagreements but had planned to resolve disagreements through discussion or through consultation with another review author (PSM). We extracted data using a standardised data collection form, and entered the data into Review Manager 5.3 (RevMan 2014), in accordance with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When required, we sought further information from study authors. We recorded the selection process in a PRISMA flow diagram (Figure 1).

Study flow diagram.
Assessment of risk of bias in included studies
Two review authors (GBM and ABC for the original search; GBM and EJP for the 2016 search) independently assessed risk of bias for each study using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned that we would resolve disagreements by discussion or by consultation with a third party. We assessed risk of bias according to the following domains (Figure 2).
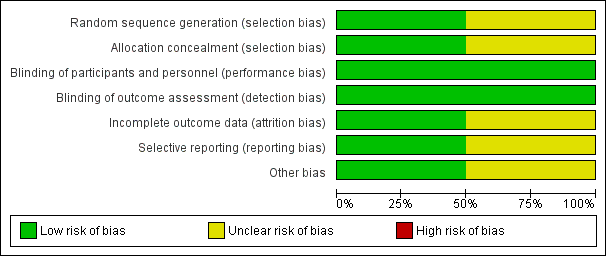
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
-
Allocation sequence generation (selection bias).
-
Concealment of allocation (selection bias).
-
Blinding of participants (performance bias).
-
Outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective outcome reporting (reporting bias).
-
Other bias.
We graded risk for each potential source of bias as high, low, or unclear, and provided justification for our judgements in the 'Risk of bias' table (Figure 2; Figure 3).

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs), and continuous data as mean differences (MDs) or standardised mean differences (SMDs) (as required). We then entered data presented as a scale with a consistent direction of effect. We undertook meta‐analyses only when this was meaningful and considered differences in study populations, inclusion/exclusion criteria, interventions, outcome assessments, and estimated effect sizes.
Unit of analysis issues
For dichotomous data, we reported the proportion of participants contributing to each outcome in comparisons versus the total number randomised. Cross‐over and cluster‐randomised trials are not appropriate for this target population; therefore we did not include them.
Dealing with missing data
Review authors planned to contact investigators or study sponsors to verify key study characteristics and to request missing numerical outcome data when necessary. For the previous review, we attempted to contact investigators from Tahan 2007 to verify study characteristics and to obtain missing numerical outcome data; however, we were unsuccessful in making contact. One of the review authors (EJP) who was not involved in McCallum 2015 obtained additional data from study authors upon request.
Assessment of heterogeneity
We proposed that we would describe any heterogeneity between study results and would test the data using a Chi2 test to see whether heterogeneity reached statistical significance. We would include the 95% confidence interval (CI), estimated via a random‐effects model, when we had concerns about statistical heterogeneity. We considered heterogeneity to be significant when P was less than 0.10 (Higgins 2011).
Assessment of reporting biases
If reporting bias was suspected (see Selective reporting (reporting bias) in the Risk of bias in included studies table below), we planned to contact study authors to request missing outcome data. We planned that if missing data were not provided, and if this was thought to introduce serious bias, we would explore the impact of including such studies in the overall assessment by performing a sensitivity analysis.
Data synthesis
We created summary of findings Table for the main comparison (SoF) in accordance with methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis based on:
-
type of control arm (placebo/no treatment);
-
severity of disease (hospitalised vs non‐hospitalised);
-
treatment with macrolides versus other types of antibiotics;
-
short (≤ 7 days) versus longer (> 7 days) courses of antibiotics;
-
time of commencement of antibiotics (within ≤ 14 days or > 14 days of onset of bronchiolitis); and
-
setting of the study (affluent vs non‐affluent setting).
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the impact of the following potentially important factors on overall outcomes.
-
Study quality (adequate allocation concealment and blinding).
-
Variation in inclusion criteria.
-
Analysis via a random‐effects model.
-
Analysis by "treatment received."
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies for full details.
Results of the search
For the previous review (McCallum 2012), the Cochrane Airways Group Specialised Register (CAGR) revealed 270 potentially relevant titles (the 2012 review identified seven abstracts but included only one study (Tahan 2007)). For this update, we identified 128 additional articles through the updated search (up to August 2016), and three others from reference lists. After removing duplicates and assessing titles, we retrieved eight articles for full review, but only a single new study (McCallum 2015) fulfilled the inclusion criteria for this update (Figure 1). The seven remaining studies did not meet review inclusion criteria (Friis 1984; Kneyber 2008; Kabir 2009; Mazumder 2009; Pinto 2012; McCallum 2013; Beigelman 2015).
Included studies
We added one new study (McCallum 2015), thus including two studies in this updated review (see Characteristics of included studies). This review includes a total of 249 children who had been enrolled (n = 240 completed). One trial was a single‐centre study (Tahan 2007), and the other a multi‐centre study (McCallum 2015). Both studies provided follow‐up for six months post hospitalisation.
Participants
Included studies used different inclusion and exclusion criteria for participants. Tahan 2007 enrolled infants aged seven months or younger who had been admitted to a paediatric hospital in Turkey with RSV‐confirmed bronchiolitis. Investigators randomised infants to receive oral clarithromycin 15 mg/kg/d or placebo for three weeks, commencing in hospital. McCallum 2015 enrolled Indigenous infants aged 24 months or younger who had been admitted to Royal Darwin Hospital (Australia), Townsville Hospital (Australia), or Auckland Starship Children's Hospital (New Zealand) with a clinical diagnosis of bronchiolitis. Researchers randomised infants to receive azithromycin 30 mg/kg/dose or an equal volume of placebo, given weekly for three weeks commencing in hospital.
Outcomes
Both studies (Tahan 2007; McCallum 2015) reported length of stay (hospitalisation) and oxygen supplementation as their primary outcomes. Secondary outcomes varied between studies. Tahan 2007 reported wheezing episodes with parents surveyed over the phone at six months. McCallum 2015 reported wheezing of infants at the day 21 clinical review, adverse events, bacterial resistance (at 48 hours), and hospital re‐admissions for respiratory illness at six months.
We have described all study characteristics in the Characteristics of included studies table.
Excluded studies
We excluded studies from the review (Friis 1984; Kneyber 2008; Kabir 2009; Mazumder 2009; Pinto 2012; McCallum 2013; Beigelman 2015) usually because interventions were provided during the acute phase (e.g. ≤ 14 days) (see Characteristics of excluded studies).
Risk of bias in included studies
We have summarised risk of bias for included studies in Figure 2 and Figure 3.
Allocation
We assessed only McCallum 2015 as having low risk for both domains. Investigators adequately described the method of randomisation, reporting that an independent statistician who 'used a computer‐generated, permuted block design' generated randomisation sequences. Tahan 2007 reported that participants were "randomised by a single study nurse" but did not clearly describe how allocation or concealment was done or maintained.
Blinding
We assessed McCallum 2015 as having low risk for both domains. Researchers in the McCallum 2015 study adequately described that the study team, which included investigators/study nurses, families, and participants, remained blinded to randomisation until the analysis had been completed. Treatment allocation was concealed in a sealed opaque envelope. We assessed Tahan 2007 as having low risk of bias. Trial authors described that participants, families, and investigators remained blinded to randomisation until the study had been completed.
Incomplete outcome data
We assessed McCallum 2015 as having low risk of bias, as more than 90% of participants completed study follow‐up. We assessed Tahan 2007 as having unclear risk. Of 30 infants enrolled, nine were excluded (three from the clarithromycin group and six from the placebo group), as they had received corticosteroid treatment in hospital (Tahan 2007). The attrition rate was high (31%), and investigators did not report data for these children, but we remain uncertain about the impact of this bias on results of the review.
Selective reporting
We assessed Tahan 2007 as having unclear risk. It is not clear whether an intention‐to‐treat (ITT) analysis was performed. It also is not clear whether the trial had been registered. We assessed McCallum 2015 as having low risk. Researchers adequately described the method of analysis used (e.g. ITT analysis) and had registered the trial with the Australian and New Zealand Clinical Trials Registry (ACTRN1261000036099).
Other potential sources of bias
We identified no other potential sources of bias.
Effects of interventions
Primary outcomes
Number of participants who were not cured at follow‐up (up to six months) (i.e. wheeze/cough)
Both studies (Tahan 2007; McCallum 2015) reported numerical data for this outcome that were based on re‐admissions to hospital with wheezing six months after hospital discharge. Data showed no significant differences between treatment groups (odds ratio (OR) 0.69; 95% confidence interval (CI) 0.37 to 1.28; Analysis 1.1; Figure 4).
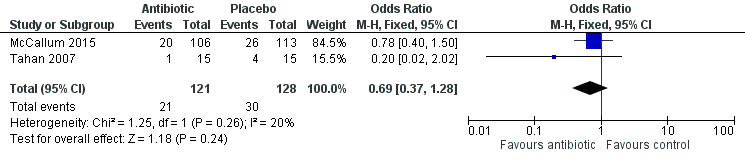
Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.1 Number of participants who were not cured at follow‐up (up to 6 months).
Exacerbation rate (up to six months)
Both studies (Tahan 2007; McCallum 2015) reported respiratory exacerbations up to six months after hospital discharge. Tahan 2007 conducted a phone interview with parents. Five children were rehospitalised with a wheezing illness within six months of discharge (clarithromycin group n = 1; placebo group n = 4) (Tahan 2007). McCallum 2015 conducted a medical chart review at six months and records showed 81 respiratory hospitalisations (azithromycin group n = 47; placebo group n = 34) among 56 participants (azithromycin group n = 31; placebo group n = 25). Of these, 60 admissions were for a wheezing‐associated illness.
Number of participants rehospitalised for a respiratory illness within six months of illness onset
Both McCallum 2015 and Tahan 2007 found no significant differences between groups in numbers of participants re‐admitted within six months (OR 1.19, 95% CI 0.67 to 2.12). Trial results showed statistical heterogeneity for this outcome (I2 = 75%; P = 0.05), which was likely due to the small number of participants included in Tahan 2007. Random‐effects analysis revealed that differences between groups remained non‐significant (OR 0.54, 95% CI 0.05 to 6.21; Analysis 1.2; Figure 5).

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.2 Number of participants who were rehospitalised within 6 months.
Secondary outcomes
Mean differences in cough indices (e.g. cough diary, frequency, or scores; quality of life)
Nil studies reported this outcome.
Mean differences in wheeze indices (e.g. cough diary, frequency, or scores; quality of life)
Nil studies reported this outcome.
Proportion of participants with wheeze (within six months of intervention)
Both studies (Tahan 2007; McCallum 2015) reported wheeze within six months of the intervention. In Tahan 2007, five children presented to hospital with a wheezing illness (clarithromycin group n = 1; placebo group n = 4). McCallum 2015 reported wheeze at two time points: the first at the day 21 clinical review, where wheeze was documented in 22 children (azithromycin group n = 11; placebo group n = 11), the second at a medical chart review performed at six months, where 60 children were re‐admitted to hospital with a wheezing‐associated illness. Data showed no statistically significant differences between treatment groups for the outcome of reported wheeze at six months (OR 0.78, 95% CI 0.35 to 1.73). Results revealed statistical heterogeneity for this outcome (I2 = 75%; P = 0.05), likely due to the small number of participants included in Tahan 2007. Random‐effects analysis revealed that differences between groups remained non‐significant (OR 0.47, 95% CI 0.06 to 3.95; Analysis 1.3; Figure 6).
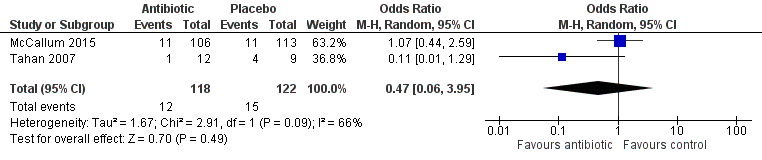
Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.3 Proportion of participants with wheeze (within 6 months of intervention).
Adverse events (e.g. proportion of participants requiring new medications or developing pneumonia)
Tahan 2007, did not report adverse events. McCallum 2015 reported three adverse events. From the azithromycin group, one infant presented to hospital with vomiting and diarrhoea, and a second vomited the trial medication. One infant in the placebo group presented to hospital with wheezing and rash. All three recovered and remained in the study.
Bacterial resistance
Only one study reported on bacterial antimicrobial resistance (McCallum 2015). In this study, bacterial antimicrobial resistance was measured at 48 hours after enrolment and included (e.g. Streptococcus pneumoniae, non‐typableHaemophilus influenza, Moraxella catarrhalis, and Staphylococcus aureus) (McCallum 2015). We have not analysed bacterial resistance for this review, as results of this analysis are not clinically meaningful for post‐acute bronchiolitis.
Subgroup analysis
We were unable to perform any subgroup analysis for either study (Tahan 2007; McCallum 2015).
Sensitivity analysis
Data were insufficient to allow performance of any sensitivity analysis.
Discussion
Summary of main results
This review updated a study from the previous review (McCallum 2012) and added a new study (McCallum 2015). Thus, our updated review consists of two randomised controlled trials (RCTs) with 249 children hospitalised with bronchiolitis. One was a single‐centre study, and the other a multi‐centre study with duration of six months. We were unable to analyse many primary and secondary outcomes owing to lack of available data. Investigators reported improvement in cure rate and in the proportion of infants with wheeze by six months but found no statistically significant between‐group differences. A single study examined bacterial antimicrobial resistance only at 48 hours, thus data were not relevant for the post‐acute phase (i.e. we did not perform analysis). This review is limited by the small numbers of available trials and participants. Thus, we cannot provide new evidence to support (or refute) the efficacy of antibiotics for reducing or treating persistent symptoms within six months of acute bronchiolitis.
Overall completeness and applicability of evidence
Use of antibiotics for treatment of bronchiolitis in the acute phase, is recommended only when a secondary bacterial infection is suspected (Ralston 2014; NICE 2015). For some populations, however, the burden of prolonged respiratory symptoms such as wheezing and cough and respiratory re‐admissions after an acute episode of bronchiolitis is increasing and is associated with ongoing poorer respiratory outcomes (e.g. future diagnosis of bronchiectasis) (McCallum 2016).
The findings of our review are limited by few eligible studies, high rates of attrition (Tahan 2007), and lack of available data, precluding the possibility of combining outcomes for meta‐analysis. The data presented in Tahan 2007 suggest that antibiotics may be effective in preventing ongoing respiratory symptoms after acute bronchiolitis. However, when these results are combined with those reported by the larger McCallum 2015 study, intention‐to‐treat (ITT) analysis reveals that none of the clinical outcomes showed statistically significant differences between treatment groups. Given the small numbers of included studies and participants, a type 1 error (inadequate sample size) may be present. Also, review authors noted differences between studies in methods of reporting. Thus, we downgraded the level of evidence in summary of findings Table for the main comparison.
Both studies included hospitalised children, hence data may not be relevant for community‐treated children. Although children with severe bronchiolitis (i.e. hospitalised children) are more likely to have persistent symptoms than those with milder bronchiolitis (i.e. community‐treated children), no studies have examined this.
This review was also limited by the fact that only one study reported on adverse events, including antibiotic resistance (McCallum 2015). By 48 hours, few children harboured nasal bacteria that were antibiotic resistant, and data showed no differences between treatment groups. However, data were limited to 48 hours after admission to hospital; this study ideally would have assessed antibiotic resistance at the day 21 review, but the study design precluded longer‐term assessment.
Both studies used macrolide antibiotics, but it remains unknown whether a non‐macrolide antibiotic would have produced similar findings. Some strains of common lower airway respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis) are macrolide resistant, and it possible that another antibiotic type (e.g. amoxicillin‐clavulanate) may yield different findings. In addition to being an anti‐microbial, macrolides have additional benefits (such as immunomodulatory) and thus data presented here cannot be extrapolated to non‐macrolide antibiotics. Also, in light of the fact that investigators commenced antibiotics at the onset of acute bronchiolitis, Tahan 2007 and McCallum 2015 could be classified as examining 'prevention' of post‐bronchiolitis syndrome. We found no eligible studies that examined 'treatment' of post‐bronchiolitis syndrome (i.e. no studies examined the efficacy of antibiotics for improving clinical outcomes at the point of development of chronic cough, defined by international chronic cough guidelines as > 4 weeks (Leconte 2008; Chang 2017)).
Quality of the evidence
In summary of findings Table for the main comparison, we have summarised available evidence for our main primary and some secondary outcomes related to the effectiveness of antibiotics following acute bronchiolitis. The quality of the evidence was low owing to small numbers of studies and participants and high rates of attrition in Tahan 2007, hence we cannot be confident of the effect estimate in spite of the high quality of one of the studies.
Potential biases in the review process
Three of the authors of this review were senior authors on the McCallum 2015 paper. EJP sought additional data through direct contact with Dr. McCallum, and the Cochrane Editor (Dr. Cates) checked the data. Review author Erin Plumb, who was not involved in the McCallum 2015 trial, performed risk of bias and GRADE assessments.
Agreements and disagreements with other studies or reviews
We are not aware of any other reviews or studies against which these results can be compared. Updated high‐quality bronchiolitis guidelines (Ralston 2014; NICE 2015) did not include interventions for post‐bronchiolitis syndrome. The findings of our review are similar to findings for acute bronchiolitis (Spurling 2011); Spurling found "minimal evidence to support the use of antibiotics for acute bronchiolitis," although the Spurling review did not include the new study.

Study flow diagram.
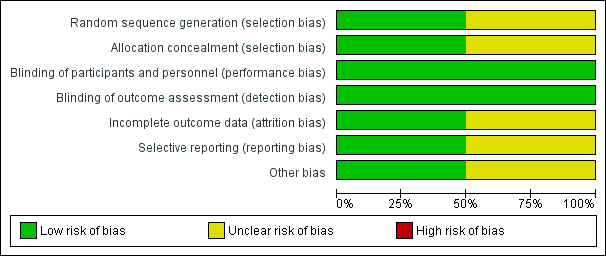
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.1 Number of participants who were not cured at follow‐up (up to 6 months).

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.2 Number of participants who were rehospitalised within 6 months.

Forest plot of comparison: 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), outcome: 1.3 Proportion of participants with wheeze (within 6 months of intervention).
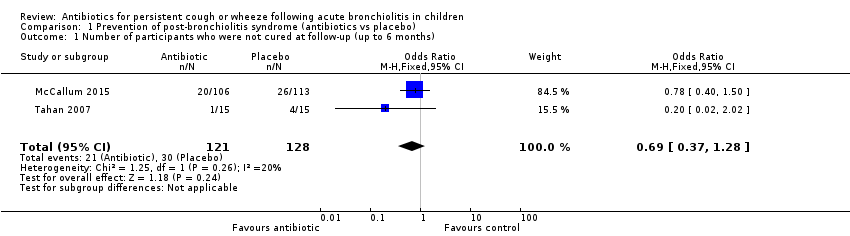
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 1 Number of participants who were not cured at follow‐up (up to 6 months).
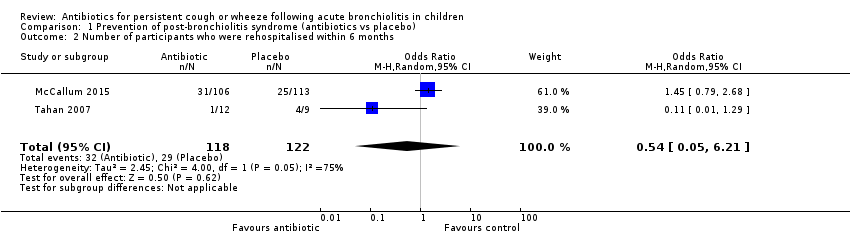
Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 2 Number of participants who were rehospitalised within 6 months.

Comparison 1 Prevention of post‐bronchiolitis syndrome (antibiotics vs placebo), Outcome 3 Proportion of participants with wheeze (within 6 months of intervention).
| Antibiotics compared with placebo or no treatment for persistent respiratory symptoms following acute bronchiolitis | ||||||
| Patient or population: children < 24 months with persistent respiratory symptoms following acute bronchiolitis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with antibiotics | |||||
| Number of participants who were not cured at follow‐up | 234 per 1000 | 174 per 1000 | OR 0.69 | 249 | ⊕⊕⊝⊝ | |
| Number of participants rehospitalised for a respiratory illness | 238 per 1000 | 271 per 1000 | OR 1.19 | 240 | ⊕⊕⊝⊝ | |
| Proportion of participants with recurrent wheeze | 123 per 1000 | 99 per 1000 | OR 0.47 (0.06 to 3.95) | 240 | ⊕⊕⊝⊝ | |
| ^Intervention/Comparison group: treatment initiated during child's hospitalisation for acute bronchiolitis *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| We used GRADEPro software to create this table (GRADEpro GDT 2015) GRADE Working Group grades of evidence | ||||||
| aQuality downgraded because of small numbers of studies and participants and high attrition in the Tahan study. Hence, we cannot be confident of the effect estimate | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants who were not cured at follow‐up (up to 6 months) Show forest plot | 2 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.37, 1.28] |
| 2 Number of participants who were rehospitalised within 6 months Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.05, 6.21] |
| 3 Proportion of participants with wheeze (within 6 months of intervention) Show forest plot | 2 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.06, 3.95] |

