Agentes inotrópicos y estrategias vasodilatadoras para el tratamiento del shock cardiogénico o síndrome de gasto cardíaco bajo
Resumen
Antecedentes
El shock cardiogénico (SC) y el síndrome de gasto cardíaco bajo (SGCB) como complicaciones del infarto agudo de miocardio (IAM), la insuficiencia cardíaca (IC) o la cirugía cardíaca son afecciones potencialmente mortales. Aunque existe un amplio base de evidencia para el tratamiento de los pacientes con síndrome coronario agudo en condiciones hemodinámicas estables, las estrategias de tratamiento para los pacientes que se vuelven hemodinámicamente inestables o desarrollan SC son menos claras. Por lo tanto, en esta revisión se resume la evidencia sobre el tratamiento de los pacientes con SC o SGCB con diferentes agentes inotrópicos y fármacos vasodilatadores. Ésta es la primera actualización de una revisión Cochrane publicada originalmente en 2014.
Objetivos
Evaluar la eficacia y la seguridad de la atención cardíaca con agentes inotrópicos positivos y estrategias vasodilatadoras en pacientes con SC o SGCB debido a IAM, IC o cirugía cardíaca.
Métodos de búsqueda
Se hicieron búsquedas en CENTRAL, MEDLINE, Embase y CPCI‐S Web of Science en junio 2017. También se buscó en cuatro registros de ensayos en curso, se examinaron las listas de referencias y se contactó con expertos en el tema para obtener más información. No se aplicaron restricciones de idioma.
Criterios de selección
Ensayos controlados aleatorizados en pacientes con infarto de miocardio, insuficiencia cardíaca o cirugía cardíaca complicados por shock cardiogénico o SGCB.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane.
Resultados principales
Se identificaron 13 estudios elegibles con 2001 participantes (rango de edad media o mediana: 58 a 73 años) y dos estudios en curso. Los estudios se categorizaron en ocho comparaciones, todos versus atención cardíaca y otros fármacos activos adicionales o placebo. Estas comparaciones investigaron la eficacia de levosimendán versus dobutamina, enoximona o placebo, epinefrina versus norepinefrina‐dobutamina, amrinona versus dobutamina, dopexamina versus dopamina, enoximona versus dopamina y óxido nítrico versus placebo.
Todos los ensayos se publicaron en revistas revisadas por pares y el análisis se hizo según el principio de intención de tratar (ITT). Doce de 13 ensayos eran pequeños con pocos participantes incluidos. El reconocimiento del financiamiento por la industria farmacéutica o la falta de declaraciones sobre los conflictos de intereses se observaron en cinco de 13 ensayos. En general, la confiabilidad en los resultados de los estudios analizados se redujo debido a graves limitaciones de los estudios, así como a imprecisión o indireccionalidad muy graves. Los dominios de interés, que muestran un alto riesgo de más del 50%, incluyen el sesgo de realización (cegamiento de los participantes y del personal) y el sesgo que afecta la calidad de la evidencia sobre los eventos adversos.
Levosimendán puede reducir la mortalidad a corto plazo en comparación con el tratamiento con dobutamina (CR 0,60; IC del 95%: 0,37 a 0,95; seis estudios; 1776 participantes; evidencia de baja calidad; NNT: 16 [pacientes con riesgo moderado], NNT: 5 [pacientes con SC]). Este efecto beneficioso inicial sobre la supervivencia a corto plazo con levosimendán versus dobutamina no se confirma en el seguimiento a largo plazo. No hay seguridad (debido a la falta de poder estadístico) con respecto al efecto de levosimendán en comparación con tratamiento con placebo (CR 0,48; IC del 95%: 0,12 a 1,94; dos estudios; 55 participantes, evidencia de muy baja calidad) o enoximona (CR 0,50; IC del 95%: 0,22 a 1,14; 1 estudio, 32 participantes, evidencia de muy calidad baja).
Todas las comparaciones de otros fármacos inotrópicos, inodilatadores o vasodilatadores positivos mostraron incertidumbre con respecto a su efecto sobre la mortalidad a corto plazo, con evidencia de muy baja calidad, y se basaron en un solo ECA. Estos estudios únicos compararon epinefrina con norepinefrina‐dobutamina (CR 1,25; IC del 95%: 0,41 a 3,77; 30 participantes), amrinona con dobutamina (CR 0,33; IC del 95%: 0,04 a 2,85; 30 participantes), dopexamina con dopamina (ninguna muerte hospitalaria en 70 participantes), enoximona con dobutamina (dos muertes en 40 participantes) y óxido nítrico con placebo (una muerte en tres participantes).
Conclusiones de los autores
Aparte de la baja calidad de los datos de la evidencia que indican un efecto beneficioso a corto plazo sobre la mortalidad de levosimendán comparado con dobutamina, actualmente no hay datos consistentes y convincentes para apoyar que uno u otro tratamiento farmacológico inotrópico o vasodilatador sea una solución superior para reducir la mortalidad en los pacientes hemodinámicamente inestables con shock cardiogénico o SGCB.
Al considerar la evidencia limitada proveniente de los datos presentes debido al riesgo generalmente alto de sesgo y a la imprecisión, se debe recalcar que aún existe una gran necesidad de ensayos aleatorizados grandes y bien diseñados sobre este tema para cerrar la brecha entre la práctica diaria en la medicina de cuidados intensivos y la evidencia disponible. Parece útil aplicar el concepto de "tratamiento temprano dirigido por objetivos" en el shock cardiogénico y el SGCB, con estabilización hemodinámica temprana dentro de marcos temporales predeterminados. Por lo tanto, los ensayos clínicos futuros deben investigar si este concepto terapéutico influiría en las tasas de supervivencia mucho más que la búsqueda del "mejor" fármaco para el apoyo hemodinámico.
PICOs
Resumen en términos sencillos
Estrategias inotrópicas y vasodilatadoras en los pacientes con shock cardiogénico o gasto cardíaco bajo
Pregunta de la revisión
Se examinó la evidencia del tratamiento con diferentes agentes inotrópicos y fármacos vasodilatadores con respecto a sus efectos sobre la mortalidad en los pacientes con shock cardiogénico (SC) o síndrome de gasto cardíaco bajo (SGCB).
Antecedentes
El SC y el SGCB aún son complicaciones potencialmente mortales. Los fármacos inotrópicos y vasoactivos son agentes potentes, pero posiblemente nocivos. Sus efectos beneficiosos y perjudiciales se asocian con la mortalidad.
Características de los estudios
La evidencia está actualizada hasta junio de 2017. Se incluyeron 13 estudios con 2001 participantes con SC o SGCB como complicaciones del infarto de miocardio, la insuficiencia cardíaca o la cirugía cardíaca, con períodos de seguimiento entre lo que duró el período de recuperación y 12 meses. Cuatro estudios fueron financiados por un fabricante de fármacos.
Resultados clave
Se compararon diferentes enfoques a los tratamientos estándar con el posible agregado de fármacos inotrópicos o vasoconstrictores como levosimendán, dobutamina, enoximona y epinefrina. Esta revisión presenta evidencia de baja calidad de que levosimendán comparado con dobutamina reduce la mortalidad a corto plazo. El efecto beneficioso sobre la supervivencia con levosimendán versus dobutamina no se confirma en el seguimiento a largo plazo. Evidencia de muy baja calidad muestra incertidumbre con respecto al efecto de levosimendán en comparación con placebo o enoximona. Evidencia de muy baja calidad muestra incertidumbre en cuanto a la comparación de epinefrina con norepinefrina‐dobutamina, amrinona o enoximona con dobutamina, dopexamina con dopamina, y óxido nítrico con placebo.
Calidad de la evidencia
La confiabilidad en los resultados de los estudios que se analizaron (evidencia de baja a muy baja calidad) se redujo debidos a graves limitaciones en los estudios, así como imprecisión o indireccionalidad muy grave.
Authors' conclusions
Summary of findings
| Levosimendan compared to dobutamine for cardiogenic shock or low cardiac output syndrome | ||||||
| Patient or population: people with cardiogenic shock or low cardiac output syndrome | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dobutamine | Risk with levosimendan | |||||
| All‐cause, short‐term mortality: range 15 days to 12 months | Moderate1 | RR 0.60 | 1776 | ⊕⊕⊝⊝ | Studies included participants with LCOS or CS due to cardiac surgery, HF or AMI | |
| 154 per 1000 | 92 per 1000 | |||||
| High2 | ||||||
| 500 per 1000 | 300 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from the median risk among the control group risk in included studies with participants with low cardiac output, low cardiac output or cardiogenic shock, or cardiogenic shock. | ||||||
| Levosimendan compared with placebo for cardiogenic shock or low cardiac output syndrome | ||||||
| Patient or population: adults with cardiogenic shock or low cardiac output syndrome Settings: hospital Intervention: levosimendan Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with placebo | Risk with levosimendan | |||||
| All‐cause short‐term mortality: range 4 to 6 months | Moderate1 | RR 0.48 (0.12 to 1.94) | 55 | ⊕⊕⊝⊝ | Studies included participants with LCOS or CS due to HF or AMI | |
| 187 per 1000 | 90 per 1000 | |||||
| High2 | ||||||
| 500 per 1000 | 240 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from median risk among the control group risk in included studies with low cardiac output or cardiogenic shock. | ||||||
| Levosimendan compared with enoximone for cardiogenic shock | ||||||
| Patient or population: adults with cardiogenic shock Settings: hospital Intervention: levosimendan Comparison: enoximone | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with enoximone | Risk with levosimendan | |||||
| All‐cause short‐term mortality: 30 days | 625 per 10001 | 313 per 1000 (138 to 712) | RR 0.50 (0.22 to 1.14) | 32 | ⊕⊝⊝⊝ very low2,3 | Study included participants with CS due to AMI |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from the control group risk in a small included study with low cardiac output or cardiogenic shock. | ||||||
| Epinephrine compared with norepinephrine‐dobutamine for low cardiac output syndrome | ||||||
| Patient or population: adults with low cardiac output syndrome Setting: in‐hospital Intervention: epinephrine Comparison: norepinephrine‐dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with norepinephrine‐dobutamine | Risk with epinephrine | |||||
| All‐cause short‐term mortality: 28 days | 267 per 1000 | 333 per 1000 | RR 1.25 (0.41 to 3.77) | 30 | ⊕⊝⊝⊝ very low1,2 | Study included participants with LCOS due to HF |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two steps for imprecision due to few events, and the confidence interval crosses the line of no difference and includes possible benefit from both approaches. | ||||||
| Amrinone compared with dobutamine for low cardiac output syndrome | ||||||
| Patient or population: adults with low cardiac output syndrome Setting: hospital Intervention: amrinone Comparison: dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dobutamine | Risk with amrinone | |||||
| All‐cause short‐term mortality: 30 days | 200 per 10001 | 66 per 1000 | RR 0.33 (0.04 to 2.85) | 30 | ⊕⊝⊝⊝ very low2,3 | Study included participants with LCOS following cardiac surgery |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from the control group risk in participants with low cardiac output and no cardiogenic shock in the included small study. | ||||||
| Dopexamine compared with dopamine for cardiogenic shock or low cardiac output syndrome | ||||||
| Patient or population: adults with cardiogenic shock or low cardiac output syndrome Setting: hospital Intervention: dopexamine Comparison: dopamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dopexamine | Risk with dopamine | |||||
| All‐cause short‐term mortality: time in hospital | 500 per 10001 | Not estimable2 | RR not estimable2 | 70 | ⊕⊝⊝⊝ very low3,4 | Study included participants with LCOS/CS following elective surgery for CABG |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from a large observational study, due to the small size of included studies in this population (Singh 2007). | ||||||
| Enoximone compared with dobutamine for low cardiac output syndrome | ||||||
| Patient or population: adults with low cardiac output syndrome Setting: hospital Intervention: enoximone Comparison: dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dobutamine | Risk with enoximone | |||||
| All‐cause short‐term mortality: 1 month | 500 per 10001 | Not estimable2 | RR not estimable2 | 40 | ⊕⊝⊝⊝ very low3,4 | Study included participants with LCOS after mitral valve surgery |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from a large observational study, due to the small size of included studies in this population (Singh 2007). | ||||||
| Nitric oxide compared with placebo for cardiogenic shock | ||||||
| Patient or population: adults with cardiogenic shock Setting: in‐hospital Intervention: nitric oxide Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with nitric oxide | Risk with placebo | |||||
| All‐cause short‐term mortality: 1 month | 500 per 10001 | Not estimable2 | RR not estimable2 | 3 | ⊕⊝⊝⊝ | Study included participants with CS due to AMI |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from a large observational study, due to the small size of included studies in this population (Singh 2007). | ||||||
Background
Worldwide, cardiovascular disease is one of the leading causes of morbidity, death, and loss of disability‐adjusted life years (Gaziano 2010; Lozano 2012; Moran 2008; Murray 1996). In 2013 in the USA, the overall rate of death attributable to cardiovascular disease was 222.9 per 100,000 Americans (Mozaffarian 2016). The estimated direct and indirect annual costs for cardiovascular disease and stroke were USD 317 billion for 2011 to 2012 (Mozaffarian 2016). As the population ages, the economic impact of cardiovascular diseases on the nation's healthcare system will become even greater (CDC 2011). Data from the INTERHEART study showed that rates of cardiovascular disease have greatly increased in low‐income and middle‐income countries, with about 80% of the global burden of cardiovascular disease occurring in these countries (Yusuf 2004).
Cardiovascular diseases are the most common cause of cardiogenic shock (CS). AMI (acute myocardial infarction) is complicated by CS in approximately 5% to 10% of cases (Goldberg 1999; Hochman 1999). The incidence of CS remained unchanged between 2001 and 2014 in an analysis of five Italian registries (De Luca 2015). Among people with CS, the proportion of people with hypertension, renal dysfunction and previous primary percutaneous coronary intervention (PCI) has increased over time, whereas the proportion of people with previous heart failure (HF) has declined. PCI was established as a standard therapy for revascularisation in people with AMI complicated by CS. This has led to an increase of PCI from 19% to 60% over the years. In hospital, mortality decreased from 68% in 2001 to 38% in 2014. In 2014 more people presented with CS on admission and fewer developed CS during their stay in hospital (De Luca 2015; WHO 2014).
Therapeutic strategies in people with CS due to AMI rely predominantly on acute and effective revascularisation of the infarct‐related artery and dependent myocardium (Hochman 1999; Hochman 2001; Hochman 2006). Subsequently, drugs like dopamine, dobutamine, norepinephrine or epinephrine are used to increase perfusion pressure and cardiac output (Dickstein 2008; O'Gara 2013; Steg 2012; Werdan 2012). Recently, new therapeutic strategies have been established, such as treatment with phosphodiesterase (PDE) inhibitors or calcium sensitisers (Reyentovich 2016).
Description of the condition
There is no absolute definition of a low cardiac output state. Haemodynamic criteria that are sometimes used include cardiac index less than 1.8 L/min/m2, or less than 2.2 L/min/m2 if inotropic drugs are administered, and a pulmonary capillary wedge pressure (PCWP) of at least 15 mmHg (Reyentovich 2016). However, the definitions in clinical trials vary (Reyentovich 2016). Clinically defined, the condition presents with hypotension (a systolic blood pressure of less than 90 mmHg for at least 30 minutes or the need for supportive measures to maintain a systolic blood pressure of 90 mmHg or more) and end‐organ hypoperfusion (cool extremities, urine output of less than 30 mL per hour, altered mental status, or elevated serum lactate). There is a continuum from low cardiac output syndrome (LCOS) to CS. In CS the low system oxygen delivery going along with low cardiac output is complicated by multi‐organ dysfunction. CS represents an acute, life‐threatening medical condition, which needs immediate attention. Pathogenesis of CS is broad. Apart from CS following AMI as discussed above, it includes unstable angina, valvular heart diseases, etc., but also systemic illnesses that trigger cardiac dysfunction, for example, septic shock with severe cardiac depression. CS with low cardiac output is a complex syndrome that involves a cascade of acute left ventricular dysfunction, decreased cardiac output, hypotension, and tissue hypoperfusion (Hochman 2007).
Description of the intervention
Medical drug therapy can be characterised under different aspects:
-
inotropic myocardial stimulation (positive inotropes that increase contractility) (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Levy 2011; Mebazaa (SURVIVE) 2007);
-
left ventricular unloading (vasodilators) (Baldassarre 2008; Rosseel 1997).
Medical drug therapy in CS is predominantly based on inotropic and vasoactive substances. They are administered for haemodynamic stabilisation through increased cardiac output and perfusion pressures by optimising systemic vascular resistance (SVR). In the early stages, increased SVR often requires vasodilatory drugs. The following stages are characterised by an escalating systemic inflammatory response syndrome so that only vasopressors, often in increasing dosages, can elevate the decreased SVR. Therapeutic approaches of anticoagulation and platelet inhibition may also be applied to modulate the systemic inflammatory response and improve the microcirculatory disturbances.
How the intervention might work
To stabilise people with CS or LCOS, drugs for positive inotropic support, vasopressors and sometimes vasodilators are commonly used. Drugs like dobutamine, dopexamine, enoximone, milrinone, amrinone, levosimendan and istaroxime are used to increase cardiac contractility and induce additional reduction of SVR for left ventricular unloading (How 2010; Leone 2004; Mattera 2008; McGhie 1992; Pietrangelo 2010; Rognoni 2011; Sehgal 2011).
While there is some evidence that inotropes like levosimendan might be cost effective in treating elective, high‐risk, cardiac‐surgery patients (Severi 2011), there is no comparable evidence in CS. Since there is limited evidence for drug treatment strategies in CS, the beneficial effects on quality of life or cost become much more important (Harjola 2010; HFMA 2010; Komamura 2008; Loisance 1991; Loisance 1993). A follow‐up analysis of the SHOCK trial showed that, although one‐year mortality after emergency revascularisation remained high (54%), most survivors had good functional status. The level of recovery for people with CS undergoing early revascularisation was similar to that of historical controls not in CS and undergoing elective revascularisation (Sleeper 2005). The use of classic inotropic agents activating the beta‐receptor cyclic adenosine monophosphate (cAMP) pathway (that is dobutamine or milrinone) should be restricted to 'rescue' therapy in people with acute HF and signs of peripheral hypoperfusion (hypotension, renal dysfunction) refractory to volume replacement, diuretics and vasodilators. This approach is largely supported by observations from clinical trials suggesting that both short‐term treatment of acute HF without an essential requirement for inotropic support as well as long‐term inotropic therapy in people with severe chronic HF with classical inotropic agents can increase arrhythmias and mortality (Landmesser 2007). Overall, we assume that the potential benefits of inotropic support in CS provide an opportunity for haemodynamic improvement by enhanced myocardial performance. With increased dosages of inotropic support, these potential benefits have to be judged against the background of the increased myocardial oxygen consumption by the ischaemic myocardium. Without myocardial revascularisation, infarct‐related CS inotropic support may show temporary beneficial haemodynamic effects superimposed on the background of expanding AMI. These disadvantages may be seen as general risks or side effects of undergoing inotropic support. At present there is only poor evidence for reduced risks of increased cellular damage or superiority in myocardial protection of the ischaemic myocardium for one of the investigated inotropic drugs (Landmesser 2007; Mentzer 2011; Triposkiadis 2009; Zheng 2009). Pure vasodilators like nitroglycerin or nitroprusside may only be used in certain subgroups of CS (Menon 2000) under conditions of guided haemodynamic monitoring to improve left ventricular performance by left ventricular unloading via vasodilation (Belskii 1987; Den Uil 2009; Hollenberg 2007).
The main strategies in the treatment of people with CS remain re‐establishing adequate macro‐ and microcirculatory conditions for the stabilisation of the oxygen supply at the cellular level, and modulation of the systemic inflammatory response to avoid functional and morphological cellular damage, to prevent multi‐organ dysfunction or failure (De Backer 2010; Hermansen 2011; Shpektor 2010). Once cellular damage has become irreversible every further therapeutic intervention, regardless of whether pharmacological‐ or device‐related, has no significant impact on short‐ or long‐term mortality (De Backer 2010; Hermansen 2011; Shpektor 2010).
Why it is important to do this review
While there is a broad body of evidence for the treatment of people with acute coronary syndromes (ACS) under stable haemodynamic conditions, there is only poor evidence, due to the low number of trials, for treatment strategies for people who become haemodynamically unstable or develop CS. These findings are correlated with limited or controversial treatment recommendations in the case of haemodynamic instability or shock (Buerke 2011).
The German‐Austrian S3 Guideline provides the first dedicated guidance for the treatment of infarct‐related CS (Werdan 2012). These recommendations reveal the lack of evidence for all recommended therapeutic measures (De Waha 2012). In contrast to the established recommendation of intra‐aortic balloon pump (IABP) support in infarct‐related CS (strong recommendation on the basis of small studies), a recent, large randomised controlled trial showed that there is no survival benefit for people treated with IABP (Thiele 2012; Thiele 2013). Randomised clinical trials are difficult to perform and costly in people with CS or LCOS. However, as AMIs are frequent and CS is associated with high mortality, any mortality‐reducing intervention is likely to have major public health implications and should be thoroughly tested.
Vasopressors are relevant to this review but were excluded, as they are the topic of another Cochrane Review on vasopressors in hypotensive shock (Gamper 2016).
Most of the existing randomised trials of people with CS have showed improved haemodynamics without effects on other relevant outcomes (Thiele 2009; Triumph 2007; Unverzagt 2011). Such improved haemodynamic status might not be a suitable surrogate marker for survival. Provided that quality of life is not compromised, all‐cause mortality constitutes the ultimate proof of patient benefit.
Objectives
To assess efficacy and safety of cardiac care with positive inotropic agents and vasodilator strategies in people with CS or LCOS due to AMI, HF or cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) of parallel‐group design that evaluated efficacy and safety within a follow‐up including at least the in‐hospital period (reports of mortality). We excluded cross‐over trials due to the investigation of all‐cause mortality as the primary outcome. Our focus was on the acute setting and, therefore, we excluded prevention trials and long‐term studies (treatment lasting one month or more).
Abstracts or unpublished data were included only if sufficient information on study design, characteristics of participants, interventions and outcomes was available, or if the full information and final results were confirmed by contact with the first author.
Types of participants
Adult patients, aged 18 years and over, with acute LCOS (medium risk study population) or CS (high risk study population) with a follow‐up period that included at least hospitalisation.
Types of interventions
-
Experimental intervention: we summarised treatments with investigational single drugs or combinations (whatever the dosage or intensity and mode, frequency, timing and duration of delivery) in one intervention group per substance. Therapeutic regimens were 'investigational' if they had been recently introduced into clinical practice or were compared to accepted therapeutic strategies, no matter whether these drugs had been investigated in regard to therapeutic efficacy or superiority.
-
Control intervention: treatments without specific experimental single drugs or corresponding combinations, or treatment options including other inotropic or vasodilative drugs. We summarised placebo or no treatment in one control group.
Types of outcome measures
Primary outcomes
-
All‐cause mortality (short term: in hospital or intensive care unit (ICU) up to four months; long term: 6 to 12 months)
Secondary outcomes
-
Major adverse cardiac events (MACE), including in‐hospital death, coronary artery bypass graft (CABG) surgery, stroke or transient ischaemic attack, AMI, and repeat PCI at the same site during the index hospital stay (Moscucci 2005) (in hospital or ICU)
-
Length of hospital stay
-
Quality of life (in hospital or ICU)
-
Haemodynamics (cardiac index, mean arterial pressure (MAP), pulmonary capillary wedge pressure (PCWP) (in hospital or ICU)
-
Adverse events (in hospital or ICU)
-
Costs (in hospital or ICU)
Search methods for identification of studies
We conducted searches in co‐operation with Cochrane Heart to identify published and unpublished RCTs.
Electronic searches
We updated our searches in the following databases on 22 June 2017; Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5) in the Cochrane Library, MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and MEDLINE (Ovid, 1946 to 22 June 2017), Embase Classic and Embase (Ovid, 1947 to 21 June 2017) and CPCI‐S (Conference Proceedings Citation Index‐Science) Web of Science (Thomson Reuters, 1990 to 22 June 2017).
We used a combination of subject headings and text strings relating to CS, LCOS, drug therapy and comparative therapy trials to construct the search strategy for the review (Appendix 1). We applied the Cochrane sensitivity‐maximising RCT search filter to MEDLINE and adaptations of it to Embase and Web of Science (Lefebvre 2011). No language restrictions were imposed.
We also searched the following registers of ongoing and completed trials (Appendix 1).
-
controlled‐trials.com (28 July 2017)
-
centerwatch.com (28 July 2017)
-
clinicalTrials.gov (28 July 2017)
-
The World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) apps.who.int/trialsearch (28 July 2017).
Searching other resources
We contacted members of Cochrane Heart, experts in the field, and manufacturers of the drugs (Carinoharm GmbH Germany, Fresenius Kabi Germany, Orion Corporation Finland, Sanofi Aventis Deutschland GmbH Germany, UCB Pharma GmbH Germany) for further information. In addition, we scanned reference lists from eligible trials and contacted the first authors to obtain further information on study design and to collect individual participant data.
Data collection and analysis
Selection of studies
Two review authors (JS plus HS and EH plus SW) independently screened studies identified using the search strategy described above by title, keywords and abstract. We accessed the full articles for further assessment if the information given suggested that the study:
-
included participants with AMI, HF or cardiac surgery complicated by CS or LCOS;
-
compared
-
cardiac care with versus without inotropic therapies, or
-
cardiac care with versus without therapies having vasodilator properties;
-
-
used designs with randomised allocation of participants; and
-
included primary data.
We settled differences in opinion by consensus with a third review author (SU or SF). After the exclusion of non‐relevant publications and duplicates, we assessed the full‐text versions of the remaining papers against the inclusion and exclusion criteria, extracted data and entered them into standardised data extraction tables. We recorded the selection process in a PRISMA flow chart according to Moher 2009 (Figure 1).
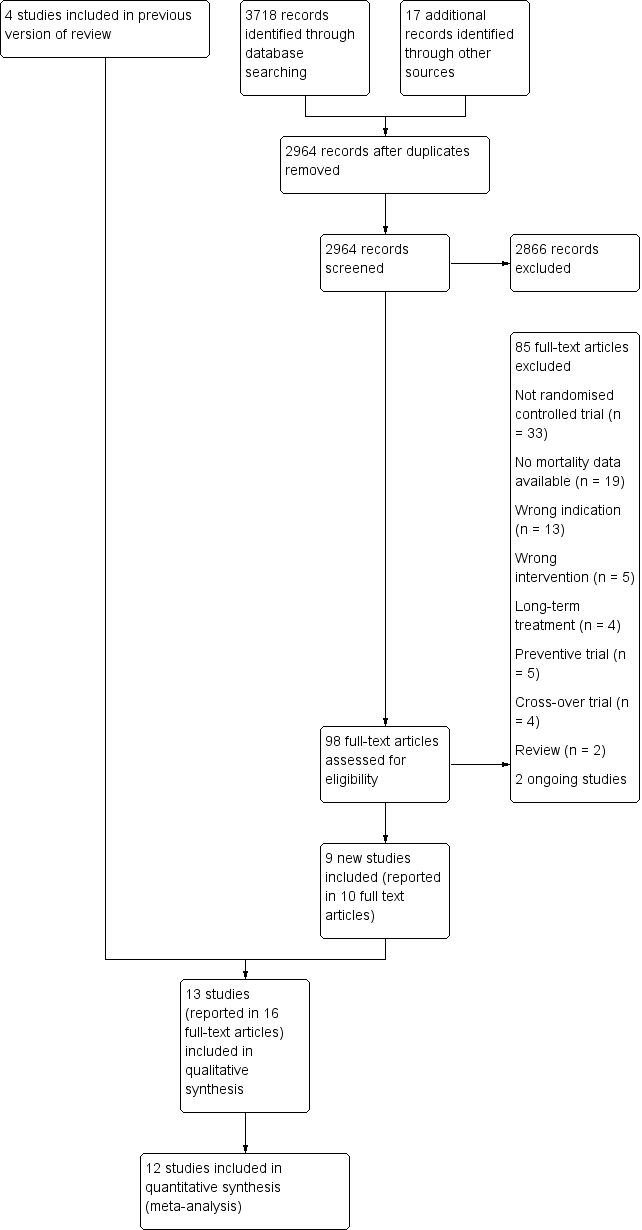
Study flow diagram
Data extraction and management
Two review authors independently extracted the details of study population, interventions and outcomes (EH, HS). The data extraction tables included the following items.
-
General information: title, authors, source, contact address, country, published or unpublished, language and year of publication, sponsoring of trial
-
Trial characteristics including study design, timing and follow‐up, and quality assessment as specified above
-
Participants: inclusion and exclusion criteria, definition of indication, baseline characteristics, similarity of groups at baseline, number of people eligible/randomised/completing/analysed, reasons for withdrawals/loss to follow‐up.
-
Interventions: dosage, route and timing of drug therapy and comparison intervention
-
Outcomes: participants per group, mortality at specific time points (in hospital or ICU, 28 or 30 days, 6 and 12 months), adverse effects (with definitions, methods for monitoring), MACE, haemodynamics (cardiac index, MAP, PCWP), length of hospital and ICU stay, quality of life, costs.
The two review authors who performed data extraction resolved any differences by consensus with a third review author (JS), referring back to the original article. As this review was planned as an individual participant data (IPD) meta‐analysis, we contacted the first authors of all eligible trials (SU) and asked them to provide IPD and other missing information. We compared IPD provided by the trial authors with the extracted, published results and checked them for consistency.
Assessment of risk of bias in included studies
Two review authors (EH, HS) independently assessed the internal validity of eligible studies according to the Cochrane 'Risk of bias' tool (Higgins 2011a), resolving any disagreements by discussion until consensus was obtained. We described risk of bias and judged it as high, low or unclear in six specific domains:
-
random sequence generation;
-
allocation concealment;
-
double blinding of participants, personnel and outcome assessment;
-
incomplete outcome data addressed;
-
selective reporting;
-
other sources of bias (cross‐over, baseline differences regarding the most important prognostic factors, conduct of the study affected by interim results, deviation from the study protocol, not reflecting clinical practice, inappropriate administration of an intervention, contra‐active or similar pre‐randomisation intervention).
We used the following items to assess the quality of evidence on adverse effects (AEs) (Higgins 2011a).
-
Are definitions of reported AEs given?
-
Were the methods that were used for monitoring AEs reported (e.g. use of prospective or routine monitoring; spontaneous reporting; participant checklist, questionnaire or diary; systematic survey of participants)?
-
Were any participants excluded from the AE analysis?
-
Does the report provide numerical data by intervention group?
-
Which categories of AEs were reported by the investigators?
Measures of treatment effect
We presented effect measures for the primary endpoint (all‐cause mortality) of the RCTs as risk ratios (RRs) with their 95% confidence intervals (CIs) and short‐term (less than six months) follow‐up periods.
We used RRs and 95% CIs to compare frequencies of MACE events. We calculated mean differences and 95% CIs as effect measures for haemodynamic measures. The data on haemodynamics (cardiac index, MAP, PCWP), length of hospital and ICU stay were reported differently for the included studies and are summarised in an additional table. No information on quality of life or costs was available from the eligible trials.
Unit of analysis issues
We randomised participants individually into treatment groups. The unit of analysis was the individual participant with one single measurement for each outcome.
Dealing with missing data
If data were not available in the trial report or data collection, we contacted the trial investigators to provide missing data.
Assessment of heterogeneity
This systematic review brings together diverse material, with studies differing in the participants, interventions and exposure times, therefore we did not expect a single‐study effect and planned to apply a random‐effects model. To quantify the extent of variability among the studies we planned to estimate the Q‐test for heterogeneity in order to quantify heterogeneity as a proportion of variability with Thompson’s I2 statistic and to calculate the between‐study variance τ2 (Higgins 2002; Rücker 2008).
The following factors are possible sources of clinically relevant heterogeneity and we have summarised them in the table Characteristics of included studies.
-
Different variations of standard therapies (other vasoactive drugs, revascularisation, IABP, mechanical ventilation, renal replacement therapy)
-
Different variations of the experimental intervention (doses and scheduling)
-
Different variations of control groups (treatment without investigated single drugs or combinations, treatment with placebo, or no treatment)
-
Differences in outcome‐relevant prognostic factors (age, gender, co‐morbidities, cardiac index, ejection fraction, time from symptom onset to intervention)
-
Different definition of the indication (CS versus LCOS)
-
Quality of studies
Assessment of reporting biases
The use of funnel plots for the graphical detection of publication bias was not possible due to the small number of eligible trials.
Data synthesis
The analysis was based on the intention‐to‐treat (ITT) principle. We undertook meta‐analyses on the basis of the random‐effects model of comparable studies with reference to the expected clinical heterogeneity arising from differences in study characteristics and the associated assumption that the effects being estimated in the different studies were not identical, but followed some distribution.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for all‐cause mortality with regard to sex, age, and cause of LCOS/CS. We conducted subgroup analyses for the comparison levosimendan versus control (Analysis 1.2, Analysis 1.4) but not for other treatment strategies due to lack of available data.
Sensitivity analysis
We performed the following sensitivity analyses.
-
Only including studies with a low risk of bias (at least six of seven 'Risk of bias' domains need to be of low risk of bias).
-
Comparing results of the random‐effects model and the fixed‐effect model.
'Summary of findings' table and GRADE assessment
We created 'Summary of findings' tables using GRADEpro GDT (GRADEpro GDT 2015) to summarise evidence and included our primary outcome (short‐term, all‐cause mortality) (Guyatt 2011a; Guyatt 2013). We estimated the assumed risk of death in the control group with standard cardiac care on the basis of estimated mortality risks from Singh 2007 for people with CS. We used the five GRADE considerations (study limitations, inconsistency, imprecision, indirectness and other considerations) to rate our overall confidence in effect estimates. We used methods and recommendations as described in GRADE to rate the quality of evidence (Balshem 2011; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f) and justified all decisions to downgrade the quality of evidence using footnotes. We added comments to aid the reader's understanding of the review where necessary (Santesso 2016).
We used the median risk among control groups to describe the baseline risk for people with low cardiac output syndrome (moderate risk). In the case of one study with participants with low cardiac output syndrome, we used the control group risk from this study. Due to the small size of included studies of people with CS or mixed populations, we also used the control group risk from a well‐designed observational study to describe the high baseline risk for people with CS (Singh 2007).
Results
Description of studies
Results of the search
The previous version of this review included four studies. We updated the searches to identify any new potentially relevant references and identified a total of 2964 references after duplicates had been removed. In total, we thought 98 full‐text papers were of relevance and assessed them against the inclusion and exclusion criteria. Of these, nine new studies (reported in 10 full‐text papers) met our predefined inclusion criteria (see Characteristics of included studies). The remaining studies are listed in Characteristics of excluded studies. We recorded the process in a PRISMA flow chart (Figure 1).
Included studies
Thirteen randomised controlled trials met the inclusion criteria. Four of these investigated people with AMI complicated by CS or LCOS (Baldassarre 2008; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013), four investigated people with acute HF complicated by CS or LCOS (Adamopoulos 2006; Follath(LIDO) 2002; Levy 2011; Mebazaa (SURVIVE) 2007), and five investigated people with cardiac surgery complicated by CS or LCOS (Alvarez 2006; Atallah 1990; Dupuis 1992; Levin 2008; Rosseel 1997).
The majority of published clinical trials examined levosimendan (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Mebazaa (SURVIVE) 2007). There was only one trial investigating epinephrine (Levy 2011), one trial investigating dopexamine (Rosseel 1997), one trial investigating enoximone (Atallah 1990), one trial investigating amrinone (Dupuis 1992), and one trial investigating nitric oxide (Baldassarre 2008). Control group participants were treated with dobutamine (Adamopoulos 2006; Alvarez 2006; Atallah 1990; Dupuis 1992; Follath(LIDO) 2002; Garcίa‐González 2006; Levin 2008; Mebazaa (SURVIVE) 2007), dopamine (Rosseel 1997), enoximone (Fuhrmann 2008), norepinephrine‐dobutamine (Levy 2011), or placebo (Adamopoulos 2006; Baldassarre 2008; Husebye 2013).
Eight studies were conducted as single‐centre trials in Spain (Alvarez 2006; Garcίa‐González 2006), France (Atallah 1990; Levy 2011), Germany (Fuhrmann 2008), Greece (Adamopoulos 2006), Norway (Husebye 2013), and in Canada (Dupuis 1992). Four studies were conducted as multi‐centre trials in Argentina (Levin 2008), the Netherlands plus Belgium (Rosseel 1997), Europe (Follath(LIDO) 2002), or Europe, Israel and Russia (Mebazaa (SURVIVE) 2007). One trial (Baldassarre 2008) was planned as a multi‐centre trial in Europe and the USA; this trial was stopped early due to low enrolment rates.
Each study characteristic is presented briefly in the table Characteristics of included studies. We included Information from two secondary publications of one included trial (Garcίa‐González 2006). A more comprehensive assessment of the included studies is given below.
Participants
Altogether, 1828 participants were enrolled in the trials on levosimendan; 905 were treated with levosimendan, and 923 served as controls and were treated with dobutamine (23 participants in Adamopoulos 2006, 20 participants in Alvarez 2006, 97 participants in Follath(LIDO) 2002, 11 participants in Garcίa‐González 2006, 68 participants in Levin 2008, 660 participants in Mebazaa (SURVIVE) 2007), enoximone (16 participants in Fuhrmann 2008) or placebo (23 participants in Adamopoulos 2006, five participants in Husebye 2013). Husebye 2013 included 61 participants with AMI complicated by acute HF. The trial authors provided additional information and IPD on all participants with CS (n = 9). The trial on epinephrine (Levy 2011) included 30 participants, with 15 of them receiving norepinephrine‐dobutamine as control. The trial on dopexamine (Rosseel 1997) included 70 participants with 35 of them receiving dopamine as control. The trial on amrinone (Dupuis 1992) included 30 participants with 15 of them receiving dobutamine as control. And the trial on enoximone (Atallah 1990) included 40 participants with 20 of them receiving dobutamine as controls. The trial on nitric oxide (Baldassarre 2008) included only three participants at two centres in the USA. These were two men and one woman, with a mean age of 69 years. Two of them received nitric oxide and one placebo. The trial authors provided no further information on their participants.
The mean or median age varied between 58 and 73 years. Husebye 2013 excluded participants under 20 years of age, Follath(LIDO) 2002 excluded participants under 21 years of age, and Rosseel 1997 excluded participants over 75 years of age. No age restriction was described in Adamopoulos 2006; Alvarez 2006; Atallah 1990; Dupuis 1992; Fuhrmann 2008; Garcίa‐González 2006; Levin 2008; Levy 2011, and Mebazaa (SURVIVE) 2007. Between 44% (Alvarez 2006) and 90% (Dupuis 1992) of participants in the included trials were male. Time of randomisation varied between trials. Participants in Fuhrmann 2008 had to be included within two hours following PCI and 24 hours of CS, participants in Husebye 2013 needed a median time of three hours from start of AMI symptoms to PCI, participants in Alvarez 2006 had to be included within four hours post cardiac surgery, participants in Levin 2008 within six hours post cardiac surgery, and participants in Atallah 1990 within 24 hours post cardiac surgery. Information concerning time of randomisation was unavailable in Adamopoulos 2006; Dupuis 1992; Follath(LIDO) 2002; Garcίa‐González 2006; Levy 2011; Mebazaa (SURVIVE) 2007, and Rosseel 1997.
Baseline MAP varied between 55 ± 9 mmHg and 54 ± 8 mmHg in Levy 2011's two treatment groups, and 85 ± 18 mmHg and 84 ± 14 mmHg in Atallah 1990's two treatment groups. Baseline cardiac index varied between 1.6 ± 0.4 L/min*m2 in both treatment groups of Levy 2011, and 2.3 (interquartile range (IQR) 2.1 to 2.5) L/min*m2 and 2.2 (IQR 1.7 to 2.4) L/min*m2 in the two treatment groups of Fuhrmann 2008. Baseline PCWP varied between 12.6 ± 2.8 mmHg and 13.2 ± 2.4 mmHg in the two treatment groups of Rosseel 1997, and 27 ± 5 mmHg in Garcίa‐González 2006. Information concerning baseline MAP, cardiac index or PCWP was unavailable in Mebazaa (SURVIVE) 2007 and Dupuis 1992 only displayed these data graphically.
Participants in all trials were treated at the time of randomisation with different vasoactive drugs including diuretics (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Levin 2008; Levy 2011; Mebazaa (SURVIVE) 2007), ACE inhibitors (Adamopoulos 2006; Follath(LIDO) 2002; Levy 2011; Mebazaa (SURVIVE) 2007), beta blockers (Adamopoulos 2006; Dupuis 1992; Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007), nitrates (Dupuis 1992; Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007), dopamine (Dupuis 1992; Mebazaa (SURVIVE) 2007), digitalis (Atallah 1990; Garcίa‐González 2006), aldosterone antagonists (Adamopoulos 2006; Levy 2011; Mebazaa (SURVIVE) 2007), digoxin (Alvarez 2006; Follath(LIDO) 2002; Levin 2008), catecholamines (Fuhrmann 2008; Husebye 2013; Levin 2008), and calcium channel blockers (Dupuis 1992; Follath(LIDO) 2002).
According to the inclusion and exclusion criteria described, six studies included solely participants suffering from LCOS (Adamopoulos 2006; Alvarez 2006; Atallah 1990; Dupuis 1992; Levin 2008; Levy 2011), five studies included solely participants suffering from CS (Baldassarre 2008; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Rosseel 1997), and two studies included participants suffering from either LCOS or CS (Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007).
Interventions
Eight included trials investigated the efficacy and safety of the calcium‐sensitiser levosimendan in combination with established therapeutic regimens. The comparisons were the following.
-
Adamopoulos 2006: levosimendan 6 µg/kg over 10 minutes, followed by a constant rate of 0.1 µg/kg/minute for 24 hours compared with either placebo (5% dextrose) or 5 µg/kg/min dobutamine for 24 hours; the infusion rate of dobutamine was gradually doubled if an adequate haemodynamic response was not achieved after two hours.
-
Alvarez 2006: levosimendan 12 μg/kg over 15 to 20 minutes, followed by a constant rate of 0.2 μg/kg/minute for 24 hours compared with 7.5 μg/kg/minute dobutamine for 24 hours.
-
Follath(LIDO) 2002: levosimendan 24 µg/kg over 10 minutes, followed by a constant rate of 0.1 µg/kg/minute compared with 5 µg/kg/min dobutamine; the infusion rate of either levosimendan or dobutamine was doubled if an adequate haemodynamic response was not achieved after two hours.
-
Fuhrmann 2008: levosimendan 12 μg/kg over 10 minutes, followed by a constant rate of 0.1 μg/kg/minute for 50 minutes and 0.2 μg/kg/minute for 23 hours compared with 0.5 μg/kg enoximone for 30 minutes followed by 2 to 10 μg/kg/minute continuously titrated to the best haemodynamic response.
-
Garcίa‐González 2006: levosimendan 24 μg/kg over 10 minutes followed by a constant rate of 0.1 μg/kg/minute for 24 hours compared with 5 μg/kg/min dobutamine for 24 hours; if an adequate haemodynamic response was not achieved after two hours, the infusion rate of dobutamine was doubled until the desired haemodynamic response was achieved.
-
Husebye 2013: levosimendan at a constant rate of 0.2 μg/kg/minute for one hour followed by a constant rate of 0.1 μg/kg/min for 24 hours compared with placebo.
-
Levin 2008: levosimendan 10 µg/kg over one hour followed by a constant rate of 0.1 µg/kg/minute for 24 hours compared with 5 µg/kg/minute dobutamine for 24 hours; the infusion rate of dobutamine was increased at 15‐minute intervals to 7.5/10/12.5 µg/kg/minute if no adequate haemodynamic response was achieved.
-
Mebazaa (SURVIVE) 2007: levosimendan 12 µg/kg over 10 minutes followed by a constant rate of 0.1 µg/kg/minute for 50 minutes followed by a constant rate of 0.2 µg/kg/minute for 23 hours (if tolerated) compared with 5 µg/kg/minute dobutamine for at least 24 hours; the infusion rate of dobutamine could be increased to a maximum rate of 40 µg/kg/minute if no adequate haemodynamic response was achieved.
One included trial investigated the efficacy and safety of epinephrine:
-
Levy 2011: 0.1 μg/kg/minute epinephrine compared with 0.1 μg/kg/min norepinephrine‐dobutamine; both treatment groups were titrated on MAP at 5‐minute intervals to obtain a MAP of between 65 and 70 mmHg with a stable or increased cardiac index.
One included trial investigated the efficacy and safety of dopexamine:
-
Rosseel 1997: 0.5/1.0/2.0 mg/kg/minute dopexamine for six hours compared with 1.5/3.0/6.0 mg/kg/min dopamine for six hours; both treatment groups were titrated in three steps at 15‐minute intervals until a cardiac index greater than 2.5 L/min/m2 was reached.
One included trial investigated the efficacy and safety of enoximone:
-
Atallah 1990: 1 mg/kg enoximone over 10 minutes, followed by a mean dosage of 5 to 10 µg/kg/minute compared with a mean dosage of 5 to 10 µg/kg/min dobutamine.
One included trial investigated the efficacy and safety of amrinone:
-
Dupuis 1992: 0.75 mg/kg amrinone, immediately followed by a constant rate of 10 µg/kg/minute for five minutes (if the treatment objectives were not achieved another 0.75 mg/kg were given) compared with 5 µg/kg/minute dobutamine for 5 to 10 minutes (if the treatment objectives were not achieved, stepwise increase to 15 µg/kg/minute).
One included trial planned to investigate the efficacy and safety of inhaled nitric oxide:
-
Baldassarre 2008: 40 ppm or 80 ppm nitric oxide over eight hours followed by a constant rate of 40 ppm compared with placebo (40 ppm nitrogen gas) over eight hours.
Excluded studies
We excluded 33 trials because they were not RCTs (Affonti 2013; Andriange 1971; Aronski 1978; Belskii 1987; Bussmann 1983; Caimmi 2011; Canella 1981; Clark 1983; De Monte 1986; Delle Karth 2003; Dhainaut 1990; Estanove 1988; Fowler 1980; Friedle 1992; Gray 1981; Hobbs 1998; Lanfear 2009; Lima 2010; Lopez 1997; Lvoff 1972; Nadjamabadi 1980; Orellano 1991; Russ 2009; Santman 1992; Shah 2014; Sterling 1984; Tacon 2012; Tritapepe 1999; Tritapepe 2009; Tzimas 2009; Verma 1992; Wright 1992; Zerkowski 1992). Information on mortality was missing in 19 studies (Carmona 2010; Duygu 2008; Feneck 2001; Galinier 1990; George 1989; Gunnicker 1995; Kikura 1997; Kikura 2002; Lancon 1990; MacGregor 1994; Meissner 1996; Nijhawan 1999; Patel 1993; Seino 1996; Slawsky 2000; Sunny 2016; Timewell 1990; Wimmer 1999; Zwölfer 1995). We excluded 13 trials due to wrong indication (Al‐Shawaf 2006; Barisin 2004; Cotter 2003; Cuffe 2002; Erb 2014; Felker 2003; Landoni 2017; Levin 2012; Lilleberg 1998; Mehta 2017; Meng 2016; O'Connor 1999; Packer 2013), and five trials due to wrong intervention (Avanzini 2002; Beller 1995; Genth‐Zotz 2000; Ochiai 2014; Pouleur 1992). An additional four trials performed long‐term treatment (Berger 2007; Jondeau 1994; Mavrogeni 2007; Stanek 1999). Five studies investigated the preventive use of inotropic agents or vasodilator strategies (Butterworth 1993; De Hert 2007; Hoffman 2003; Lechner 2012; Sharma 2014), and four trials used a cross‐over design (Dominguez‐Rodriguez 2007; Ferrario 1994; Loeb 1971; Richard 1983). Furthermore, we screened two reviews (Kaplan 1980; Perret 1978) for eligible trials. Reasons for exclusion are presented briefly in tabulated form (see Characteristics of excluded studies).
Ongoing studies
We identified two ongoing studies investigating sodium nitroprusside versus dobutamine (NCT02767024) and milrinone versus dobutamine (NCT03207165) for CS treatment. For details of the planned investigations in tabulated form please see Characteristics of ongoing studies.
Risk of bias in included studies
All trials were published in peer‐reviewed journals. Trials acknowledging funding by the pharmaceutical industry were Dupuis 1992 (supported by a grant from Sanofi‐Winthrop); Follath(LIDO) 2002 (supported by Quintiles/Innovex (study management), Ercopharma, and a grant from Orion Pharma, which was involved in the study design, planning/running of the statistical analyses, and preparation of the trial report); Husebye 2013 (received an unrestricted educational grant from Orion Pharma); and Mebazaa (SURVIVE) 2007 (supported by Orion Pharma and Abbott Laboratories). In Levy 2011 conflict of interest was not disclosed. No clinical report or final publication was published on the trial on nitric oxide but the results were confirmed by contact with the responsible investigator.
Included trials were small and the number of included participants ranged from three to 199, with the exception of Mebazaa (SURVIVE) 2007, who enrolled 1320 participants. In all trials analysis was done by ITT. Figure 2 and Figure 3 present a summary of all investigated sources of bias in the thirteen eligible studies.
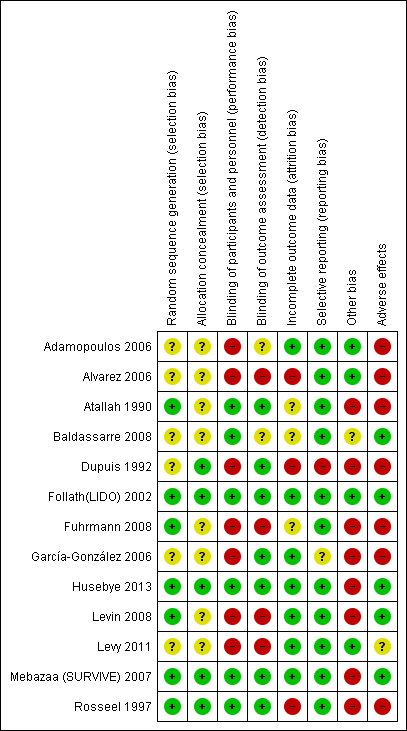
Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Random sequence generation (selection bias)
The method of sequence generation was reported in eight trials (Atallah 1990; Dupuis 1992; Follath(LIDO) 2002; Fuhrmann 2008; Husebye 2013; Levin 2008; Mebazaa (SURVIVE) 2007; Rosseel 1997). Follath(LIDO) 2002; Fuhrmann 2008; Husebye 2013; Levin 2008; and Rosseel 1997 used blocked random tables by means of a computer random number generator with Husebye 2013 using an extra stratum for participants with CS. Atallah 1990 performed sequence generation by drawing of lots. Mebazaa (SURVIVE) 2007 randomised participants centrally, using an interactive, voice‐response system, stratified by a biased coin algorithm with previous acute decompensated heart failure and country as factors. Dupuis 1992 randomised participants according to their ability to separate from cardiopulmonary bypass.
Allocation
Dupuis 1992; Follath(LIDO) 2002; Husebye 2013; Mebazaa (SURVIVE) 2007 and Rosseel 1997 described the method of allocation concealment. Allocation was performed by a blinded investigator according to a pre‐determined list. No information was available from the other eight trials.
Blinding
Risk of bias due to performance or detection was low in Atallah 1990; Follath(LIDO) 2002; Husebye 2013; Mebazaa (SURVIVE) 2007, and Rosseel 1997. In Adamopoulos 2006; Alvarez 2006; Levin 2008, and Fuhrmann 2008 blinding was either not performed or not possible due to different timing of administration of the study drug. In Garcίa‐González 2006 and Dupuis 1992 outcome assessment was blinded but not personnel/participants. Levy 2011 was described both as an open‐label study and as a double‐blind study but no further information was provided.
Incomplete outcome data
The included studies investigated all‐cause mortality, haemodynamics, MACE and AEs. Eight studies (Atallah 1990; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Levy 2011; Mebazaa (SURVIVE) 2007) reported 30‐day follow‐up data on all‐cause mortality distribution. Four trials (Follath(LIDO) 2002; Garcίa‐González 2006; Husebye 2013; Mebazaa (SURVIVE) 2007) reported six‐month follow‐up data on all‐cause mortality distribution. Nine trials with follow‐up times ranging from 6 to 72 hours reported haemodynamic, post‐interventional data (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Levin 2008; Levy 2011; Mebazaa (SURVIVE) 2007; Rosseel 1997), but data concerning CI, MAP, and PCWP were given solely in Adamopoulos 2006; Alvarez 2006; Fuhrmann 2008; Garcίa‐González 2006; Levin 2008; Levy 2011; and Rosseel 1997. MACE events were reported during the study drug infusion, time in hospital, over 30 days or up to six months.
Five studies reported exclusion of participants (Alvarez 2006; Atallah 1990; Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007; Rosseel 1997). Dupuis 1992 presented full data for solely 43% of enrolled participants. Fuhrmann 2008 reported haemodynamic changes in 36 participants but randomised only 32 participants.
Selective reporting
Adamopoulos 2006; Alvarez 2006; Atallah 1990; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Levy 2011; Mebazaa (SURVIVE) 2007, and Rosseel 1997 reported all primary outcomes pre‐specified in the method section. Pre‐specified secondary endpoints were missing in Garcίa‐González 2006. Baldassarre 2008 had restricted reporting on mortality and adverse events. Dupuis 1992 gave only part of the outcomes for subgroups of treatment groups, called "Blocks".
Other potential sources of bias
None of the included trials reported any cross‐over or deviation from the study protocol.
There were some potentially important baseline differences in prognostic factors such as sex, timetable or co‐morbidities in Atallah 1990; Dupuis 1992; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013, and Rosseel 1997. No information on baseline differences was available for the trial with a subgroup of participants with CS (Husebye 2013).
The conduct of two trials was affected by interim results. Fuhrmann 2008 was stopped after recruitment of 36% of the pre‐planned sample size as a result of a planned interim analysis, due to a trend toward reduced mortality for levosimendan. In Mebazaa (SURVIVE) 2007 the originally targeted number of participants (n = 700) was increased to 1320 following a blinded review of mortality after 131 deaths to achieve the target number of 330 deaths.
Three trials reported inappropriate delivery, with interruptions of study drug administration. In Follath(LIDO) 2002 (203 participants enrolled) four participants (2.0%) did not receive the study drug at all (one in levosimendan group, three in dobutamine group), 16 participants (7.8%) were classified as permanent discontinuation before 24 hours owing to adverse events or insufficient clinical response (six in levosimendan group, 10 in dobutamine group), 11 participants (5.4%) were prone to a temporary interruption due to a dose‐limiting event (five in levosimendan group, six in dobutamine group), and 14 participants (6.9%) received the study drug for less than 18 hours (six in levosimendan group, eight in dobutamine group). In Mebazaa (SURVIVE) 2007 (1220 participants receiving study drug) 71 participants (5.8%) discontinued the intervention due to adverse events (30 in levosimendan group, 41 in dobutamine group). In Husebye 2013 discontinuation was necessary in one participant (1.6%) from the levosimendan group due to atrial fibrillation and one participant (1.6%) from the placebo group due to hypotension, although these participants were in CS.
All clinical trials evaluating shock participants addressed the problem of pre‐randomisation drug‐treatment strategies. Most of the included trial participants were not randomised to the study drug at the index event (onset of LCOS/CS) and they were therefore pre‐treated with different inotropic and vasoactive drugs, which could have influenced their microcirculation and thereby affected prognosis.
To the best of our knowledge no trial used a complex standardised study protocol for vasopressor down‐titration for the assessment of the lowest necessary vasopressor dosage in each individual participant.
Although the title and inclusion criteria of the study conducted by Garcίa‐González 2006 implied that the enrolled participants suffered from CS‐complicating AMI, there remained major concerns regarding the eligibility of the included participants. This was because none of them developed multi‐organ failure and the mortality rates appeared very low in comparison to commonly reported data.
Bias affecting the quality of evidence on adverse events
Reports on AEs were missing in two trials (Adamopoulos 2006; Garcίa‐González 2006). Only Husebye 2013 and Levin 2008 gave definitions of the reported AEs. Information on monitoring of AEs was restricted to Follath(LIDO) 2002; Husebye 2013, and Mebazaa (SURVIVE) 2007. Follath(LIDO) 2002 collected AEs as spontaneous reports without breaking blinding. In Husebye 2013, study personnel blinded to treatment allocation throughout the study period of five days and at the six‐week follow‐up monitored AEs. Mebazaa (SURVIVE) 2007 collected AEs for 31 days following initial study drug administration and during all blinded drug re‐administrations. With the exception of Dupuis 1992, who reported AEs solely for particular Blocks of participants (43%), no trial excluded participants from AE analysis.
Although we were aware of the methodological problems and restrictions, especially in regard to the definition of CS in the study of Garcίa‐González 2006, we nevertheless decided to include all studies that randomised participants with AMI complicated by CS or LCOS, mainly because of the limited number of trials that were available. The 'Risk of bias' tables of the individual trials are given in Characteristics of included studies.
Effects of interventions
See: Summary of findings for the main comparison Levosimendan compared to dobutamine for cardiogenic shock or low cardiac output syndrome; Summary of findings 2 Levosimendan compared to placebo for cardiogenic shock or low cardiac output syndrome; Summary of findings 3 Levosimendan compared to enoximone for cardiogenic shock; Summary of findings 4 Epinephrine compared to norepinephrine‐dobutamine for low cardiac output syndrome; Summary of findings 5 Amrinone compared to dobutamine for low cardiac output syndrome; Summary of findings 6 Dopexamine compared to dopamine for cardiogenic shock or low cardiac output syndrome; Summary of findings 7 Enoximone compared to dobutamine for low cardiac output syndrome; Summary of findings 8 Nitric oxide compared to placebo for cardiogenic shock
1. Levosimendan versus dobutamine
Three small, single‐centre trials with 109 participants (Adamopoulos 2006; Alvarez 2006; Garcίa‐González 2006) as well as three multi‐centre trials with 1667 participants (Follath(LIDO) 2002; Levin 2008; Mebazaa (SURVIVE) 2007) investigated levosimendan compared with dobutamine in people with AMI (Garcίa‐González 2006), acute HF (Adamopoulos 2006; Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007), or cardiac surgery (Alvarez 2006; Levin 2008) complicated by CS/LCOS with low‐quality evidence.
All‐cause mortality
Short‐term
A lower all‐cause mortality was reported, with 96 deaths out of 891 participants (10.7%) in the intervention arm with levosimendan compared with 131 deaths out of 885 participants (14.8%) in the control groups treated with dobutamine (RR 0.60, 95% CI 0.37 to 0.95; participants = 1776; studies = 6; low‐quality evidence) with low heterogeneity between single studies (I2 = 35%) (summary of findings Table for the main comparison; Analysis 1.1). Out of 1000 people with CS, approximately 500 would be expected to die with standard cardiac care with dobutamine (Singh 2007) within a short‐term follow‐up period compared to 300 (95% CI 185 to 475) with levosimendan (summary of findings Table for the main comparison; Analysis 1.1). In people at moderate risk, approximately 154 per 1000 would be expected to die with standard cardiac care with dobutamine compared to 92 (95% CI 57 to 146) with levosimendan (summary of findings Table for the main comparison; Analysis 1.1).
Long‐term
The protective effect of levosimendan was reduced in the long‐term follow‐up. Three trials with 1552 participants (Follath(LIDO) 2002; Garcίa‐González 2006; Mebazaa (SURVIVE) 2007) reported 200 deaths out of 778 participants (25.7%) in the levosimendan group compared with 223 deaths out of 774 participants (28.8%) in the dobutamine group (RR 0.85, 95% CI 0.65 to 1.12) (Analysis 1.3).
Subgroup analyses
Treatment effects were higher in studies on participants with LCOS due to cardiac surgery (Alvarez 2006; Levin 2008; RR 0.38, 95% CI 0.17 to 0.87) compared to studies on participants with LCOS due to HF (Adamopoulos 2006; Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007; RR 0.69, 95% CI 0.42 to 1.11) when investigating levosimendan compared to dobutamine. Only one study compared the effect depending on gender, age and history of congestive HF (Mebazaa (SURVIVE) 2007) (Analysis 1.2). They observed a worse efficacy in participants with no history of congestive HF (RR 1.54, 95% CI 0.82 to 2.87) compared to participants with a history of congestive HF (RR 0.76, 95% CI 0.55 to 1.04).
Sensitivity analyses
A sensitivity analysis showed no differences depending on the statistical model, but there is uncertainty on the result from two studies with blinding of personnel and participants (Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007; RR 0.70; 0.39 to 1.27) (Analysis 2.2). Results from three trials regarding long‐term mortality over six months (RR 0.85, 0.65 to 1.12) (Analysis 1.3) were comparable. Sensitivity analysis on the basis of the fixed‐effect model (RR 0.73, 95% CI 0.57 to 0.93) (Analysis 2.1) and on the basis of studies with low risk of bias (RR 0.70, 95% CI 0.39 to 1.27) (Analysis 2.2) stated the results from the main analysis.
Major adverse cardiac events (MACE)
Information on MACE was restricted to Garcίa‐González 2006 and Levin 2008. Garcίa‐González 2006 documented no re‐infarction or cerebrovascular accident in either group during hospitalisation (Table 1). Levin 2008 reported perioperative infarction in one out of 69 participants (1.4%) of the levosimendan intervention arm but eight out of 68 participants (11.8%) of the dobutamine intervention arm, and stroke in two out of 69 participants (2.9%) of the levosimendan intervention arm but six out of 68 participants (8.8%) of the dobutamine intervention arm (Table 1).
| Comparison | Primary studies | MACE | Intervention | Control | RR (95% CI) | ||
| events | total | events | total | ||||
| Levosimendan vs dobutamine | Perioperative infarction | 1 (1.4%) | 69 | 8 (11.8%) | 68 | 0.12 (0.02 to 0.96) | |
| Re‐infarction | 0 (0%) | 11 | 0 (0%) | 11 | Not estimable | ||
| Cerebrovascular accidents | 2 (2.9%) | 69 | 6 (8.8%) | 68 | 0.33 (0.07 to 1.57) | ||
| Cerebrovascular accidents | 0 (0%) | 11 | 0 (0%) | 11 | Not estimable | ||
| Levosimendan vs placebo | MACE (death, non‐fatal myocardial infarction, revascularisation of the infarct‐related artery) | 2 (50.0%) | 4 | 2 (40.0%) | 5 | 1.25 (0.29 to 5.35) | |
| Repeat PCI | 1 (25.0%) | 4 | 0 (0%) | 5 | 3.60 (0.18 to 70.34) | ||
| Amrinone vs dobutamine | Re‐infarction (2 h) | 0 (0%) | 15 | 6 (40.0%) | 15 | 0.08 (0.00 to 1.25) | |
| Dopexamine vs dopamine | Perioperative infarction | 3 (8.6%) | 35 | 2 (5.7%) | 35 | 1.50 (0.27 to 8.43) | |
| Nitric oxide vs placebo | Myocardial infarction | 1 (50.0%) | 2 | 1 (100%) | 1 | 0.67 (0.17 to 2.67) | |
CI: confidence interval; PCI: percutaneous coronary intervention; RR: risk ratio
Length of hospital stay
Information on length of hospital stay was restricted to Levin 2008, which reported a shorter median intensive care unit (ICU) time in the levosimendan intervention arm compared to the dobutamine intervention arm, with high imprecision (66 (IQR 58 to 74) hours compared to 158 (106 to 182) hours) (Table 2).
| Comparison | Primary studies | Reported information | Intervention | Control | ||
| Events/time | Total | Events/time | Total | |||
| Levosimendan vs dobutamine | Stay in ICU (hours, median with IQR) | 66 (58‐74) | 69 | 158 (106‐182) | 68 | |
| Levosimendan vs enoximone | Stay in ICU (days, median with IQR) | 10 (5‐23) | 16 | 13 (7‐19) | 16 | |
| Enoximone vs dobutamine | Stay in ICU (hours, mean) | 92 ± 37 | 18 | 155 ± 129 | 19 | |
ICU: intensive care unit; IQR: intra‐quartile‐range
Quality of life
No results were available from the included studies.
Haemodynamics
Information on cardiac index was restricted to Adamopoulos 2006; Alvarez 2006; Garcίa‐González 2006, and Levin 2008; information on pulmonary capillary wedge pressure (PCWP) was restricted to Adamopoulos 2006, and information on mean arterial pressure (MAP) was restricted to Alvarez 2006 and Levin 2008. In every case beneficial effects of levosimendan were reported compared to dobutamine (cardiac index: MD between 0.1 L/min/m2; 95%CI 0.06 to 0.14 and 0.7 L/min/m2; 95%CI 0.65 to 0.75; not pooled due to considerable heterogeneity (I2 = 99%); PCWP: MD ‐4.0 mmHg; 95% CI ‐4.6 to ‐3.4; MAP: MD ‐‐2.2 mmHg; 95% CI ‐4.6 to ‐0.3) (Analysis 1.5; Analysis 1.6; Analysis 1.7; Table 3).
| Comparison | Primary studies | Haemodynamics | Intervention | Control | MD (95% CI) | ||
| Intervention vs control | last measurements | mean ± SD or median (IQR) | total | mean ± SD or median (IQR) | total | ||
| Levosimendan vs dobutamine | Cardiac index (after 72 h, L/min/m2) | 1.9 ± 0.1 | 23 | 1.8 ± 0.04 | 23 | 0.10 (0.06 to 0.14) | |
| Cardiac index (after 48 h, L/min/m2) | 2.8 ± 0.3 | 21 | 2.3 ± 0.2 | 20 | 0.50 (0.34 to 0.66) | ||
| Cardiac index (after 30 h, L/min/m2) | 2.9 ± 0.4 | 11 | 2.4 ± 0.2 | 11 | 0.50 (0.24 to 0.76) | ||
| Cardiac index (after 48 hrs, L/min/m2) | 3.4 ± 0.2 | 69 | 2.7 ± 0.1 | 68 | 0.70 (0.65 to 0.75) | ||
| PCWP (after 72 h, mmHg) | 19.0 ± 1 | 23 | 23.0 ± 1.0 | 23 | ‐4.00 (‐4.60 to ‐3.40) | ||
| MAP (after 48 h, mmHg) | 77.0 ± 5 | 21 | 81.0 ± 7.0 | 20 | ‐4.00 (‐7.70 to ‐0.30) | ||
| MAP (after 48 h, mmHg) | 78.8 ± 7 | 69 | 80.1 ± 4 | 68 | ‐1.30 (‐3.20 to 0.60) | ||
| Levosimendan vs placebo | Cardiac index (after 72 h, (L/min/m2) | 1.9 ± 0.1 | 23 | 1.8 ± 0.1 | 23 | 0.10 (0.04 to 0.16) | |
| PCWP (after 72 h, mmHg) | 19.0 ± 1 | 23 | 23.0 ± 1.0 | 23 | ‐4.00 (‐4.60 to ‐3.40) | ||
| Levosimendan vs enoximone | Cardiac index (after 48 h, L/min/m2) | 3.1 (2.5‐3.5) | 16 | 3.1 (2.8‐3.3) | 16 | Not estimable | |
| MAP (after 48 h (mmHg) | 75.0 (58.0‐79.0) | 16 | 70.0 (63.0‐83.0) | 16 | Not estimable | ||
| Epinephrine vs norepinephrine‐dobutamine | Cardiac index (after 24 h, L/min/m2) | 2.9 ± 0.5 | 15 | 2.8 ± 0.4 | 15 | 0.10 (‐0.22 to 0.42) | |
| MAP (after 24 h, mmHg) | 64 ± 9 | 15 | 65.0 ± 11.0 | 15 | ‐1.00 (‐8.20 to 6.20) | ||
| Dopexamine vs dopamine | Cardiac index (after 6 h, L/min/m2) | 3.1 ± 0.7 | 29 | 2.8 ± 0.5 | 30 | 0.30 (‐0.01 to 0.61) | |
| PCWP (after 6 h, mmHg) | 9.3 ± 3.2 | 29 | 10.8 ± 2.9 | 30 | ‐1.50 (‐3.10 to 0.10) | ||
| MAP (after 6 h, mmHg) | 76.3 ± 11.5 | 29 | 78.2 ± 12.8 | 30 | ‐1.90 (‐8.10 to 4.30) | ||
CI: confidence interval; IQR: intra‐quartile‐range; MAP: mean arterial pressure; MD: mean difference; PCWP: pulmonary capillary wedge pressure; SD: standard deviation
Adverse events (AEs)
AEs were reported by Alvarez 2006; Follath(LIDO) 2002; Garcίa‐González 2006; Levin 2008, and (very detailed) Mebazaa (SURVIVE) 2007. In Garcίa‐González 2006, no AEs occurred. Levin 2008 reported a better safety profile of levosimendan compared to dobutamine (Table 4). In contrast, Alvarez 2006; Follath(LIDO) 2002, and Mebazaa (SURVIVE) 2007 did not observed marked differences in the safety profile of the drugs compared (Table 4).
| Comparison | Primary studies | Adverse events (no MACE) | Intervention | Control | ||
| events | total | events | total | |||
| Levosimendan vsdobutamine | Atrial fibrillation | 78 (10.4%) | 750 | 71 (9.5%) | 748 | |
| Ventricular fibrillation | 15 (2.3%) | 660 | 19 (2.9%) | 660 | ||
| Ventricular arrhythmias | 7 (3.6%) | 193 | 25 (13.3%) | 188 | ||
| Ventricular tachycardia | 52 (7.9%) | 660 | 48 (7.3%) | 660 | ||
| Ventricular extrasystoles | 40 (6.1%) | 660 | 24 (3.6%) | 660 | ||
| Tachycardia | 33 (5.0%) | 660 | 33 (5.0%) | 660 | ||
| Bradycardia | 8 (1.2%) | 660 | 17 (2.6%) | 660 | ||
| Headache | 69 (9.0%) | 763 | 36 (4.7%) | 760 | ||
| Cardiac failure | 91 (11.9%) | 763 | 127 (16.7%) | 760 | ||
| Congestive cardiac failure | 26 (3.9%) | 660 | 22 (3.3%) | 660 | ||
| Cardiac arrest | 20 (3.0%) | 660 | 26 (3.9%) | 660 | ||
| Disorder aggravated | 17 (2,2%) | 763 | 27 (3.6%) | 760 | ||
| Gastrointestinal disorders | 54 (7.1%) | 763 | 52 (6.8%) | 760 | ||
| Acute kidney failure | 29 (4.0%) | 729 | 43 (5.9%) | 728 | ||
| Need for dialysis | 2 (2.9%) | 69 | 8 (11.9%) | 68 | ||
| Pneumonia | 34 (4.7%) | 729 | 34 (4.7%) | 728 | ||
| Multiple organ failure | 0 (0%) | 11 | 0 (0%) | 11 | ||
| Stroke | 0 (0%) | 11 | 0 (0%) | 11 | ||
| Vasoplegia | 1 (1.4 %) | 69 | 9 (13.2%) | 68 | ||
| Dyspnoea | 1 (1.4%) | 69 | 4 (5.8%) | 68 | ||
| Inflammatory response syndrome | 4 (5.8%) | 69 | 15 (22.1%) | 68 | ||
| Sepsis | 1 (1.4%) | 69 | 9 (13.2%) | 68 | ||
| Prolonged ventilatory assistance | 6 (8.7%) | 69 | 22 (32.3%) | 68 | ||
| Hypokalaemia | 62 (9.4%) | 660 | 39 (5.9%) | 660 | ||
| Hyperkalaemia | 15 (2.3%) | 660 | 16 (2.4%) | 660 | ||
| Hypotension | 102 (15.5%) | 660 | 92 (13.9%) | 660 | ||
| Nausea | 45 (6.8%) | 660 | 49 (7.4%) | 660 | ||
| Insomnia | 37 (5.6%) | 660 | 29 (4.4%) | 660 | ||
| Chest pain | 32 (4.8%) | 660 | 47 (7.1%) | 660 | ||
| Constipation | 26 (3.9%) | 660 | 28 (4.2%) | 660 | ||
| Pyrexia | 22 (3.3%) | 660 | 19 (2.9%) | 660 | ||
| Urinary tract infection | 21 (3.2%) | 660 | 30 (4.5%) | 660 | ||
| Anexiety | 20 (3.0%) | 660 | 19 (2.9%) | 660 | ||
| Pulmonary oedema | 20 (3.0%) | 660 | 18 (2.7%) | 660 | ||
| Dizziness | 19 (2.9%) | 660 | 16 (2.4%) | 660 | ||
| Cough | 19 (2.9%) | 660 | 21 (3.2%) | 660 | ||
| Pain in extremity | 18 (2.7%) | 660 | 10 (1.5%) | 660 | ||
| Pruritus | 16 (2.4%) | 660 | 7 (1.1%) | 660 | ||
| Anaemia | 15 (2.3%) | 660 | 17 (2.6%) | 660 | ||
| Epistaxis | 14 (2.1%) | 660 | 7 (1.1%) | 660 | ||
| Back pain | 13 (2.0%) | 660 | 18 (2.7%) | 660 | ||
| Angina pectoris | 12 (1.8%) | 660 | 18 (2.7%) | 660 | ||
| Muscle spasms | 12 (1.8%) | 660 | 13 (2.0%) | 660 | ||
| Dyspnoea | 9 (1.4%) | 660 | 17 (2.6%) | 660 | ||
| Hypertension | 9 (1.4%) | 660 | 15 (2.3%) | 660 | ||
| Cataract | 7 (1.1%) | 660 | 14 (2.1%) | 660 | ||
| Agitation | 7 (1.1%) | 660 | 0 (0%) | 660 | ||
| Levosimendan vsplacebo | Non‐sustained ventricular tachycardia | 1 (25.0%) | 4 | 3 (60.0%) | 5 | |
| Atrial fibrillation | 1 (25.0%) | 4 | 0 (0%) | 5 | ||
| Episodes of hypotension during drug infusion (MAP fall > 10 mmHg) | 2 (50.0%) | 4 | 1 (20.0%) | 5 | ||
| Levosimendan vsenoximone | Need of mechanical ventilation | 13 (81.3%) | 16 | 15 (93.8%) | 16 | |
| Acute renal failure | 5 (31.3%) | 16 | 8 (50.0%) | 16 | ||
| Need of continuous renal replacement therapy | 5 (31.5%) | 16 | 8 (50.0%) | 16 | ||
| New onset atrial fibrillation | 7 (43.8%) | 16 | 9 (56.3%) | 16 | ||
| Ventricular tachycardia or fibrillation | 8 (50.0%) | 16 | 11 (68.8%) | 16 | ||
| Development of systemic inflammatory response | 8 (50.0%) | 16 | 13 (81.3%) | 16 | ||
| Pneumonia | 7 (43.8%) | 16 | 7 (43.8%) | 16 | ||
| Urinary infections | 0 (0%) | 16 | 2 (12.5%) | 16 | ||
| Sepsis | 3 (18.8%) | 16 | 2 (12.5%) | 16 | ||
| Epinephrine vs. norepinephrine‐dobutamine | Supraventricular arrhythmia | 2 (13.3%) | 15 | 0 (0%) | 15 | |
| Sustained ventricular tachycardia | 1 (6.7%) | 15 | 0 (0%) | 15 | ||
| Amrinone vs. dobutamine | Cardiac arrhythmias during treatment | 0 (0%) | 15 | 4 (26.7%) | 15 | |
| Myocardial ischemias (within 16 to 20 hrs) | 4 (26.7%) | 15 | 4 (26.7%) | 15 | ||
| Dopexamine vs. dopamine | Cardiac events | 19 (54.3%) | 35 | 22 (62.9%) | 35 | |
| Abnormal blood loss | 2 (5.7%) | 35 | 1 (2.9%) | 35 | ||
| Kidney failure | 1 (2.9%) | 35 | 1 (2.9%) | 35 | ||
| Other adverse events | 5 (14.3%) | 35 | 1 (2.9%) | 35 | ||
| Major adverse events | 0 (0%) | 35 | 0 (0%) | 35 | ||
MACE: major adverse cardiac events; MAP: mean arterial pressure
Costs
No results were available from the included studies.
2. Levosimendan versus placebo
Two small, single‐centre trials with 55 participants investigated levosimendan compared with placebo in context of people suffering from AMI (Husebye 2013) or acute HF (Adamopoulos 2006) complicated by LCOS/CS with very low‐quality evidence.
All‐cause mortality
Short‐term
No benefit of levosimendan treatment was reported compared to placebo in the short‐term follow‐up (RR 0.48, 95% CI 0.12 to 1.94; participants = 55; studies = 2; very low‐quality evidence) with very low heterogeneity between single studies (I2 = 0%) (Adamopoulos 2006; Husebye 2013). Out of 1000 people, approximately 500 people with CS would be expected to die within a short‐term follow‐up period with standard cardiac care (Singh 2007) compared to 240 (95% CI 60 to 970) with levosimendan. In people with moderate risk, approximately 187 per 1000 people would be expected to die with standard cardiac care compared to 90 (95% CI 22 to 363) with levosimendan (summary of findings Table 2; Analysis 1.1).
Long‐term
No benefit of levosimendan treatment was reported compared to placebo in the long‐term follow‐up (RR 0.63, 95% CI 0.08 to 4.66) (Husebye 2013).
Subgroup analyses
Subgroup analysis revealed no difference in treatment effects in studies with participants with LCOS due to AMI (Husebye 2013; RR 0.40, 95% CI 0.02 to 7.82) compared to studies on participants with LCOS due to HF (Adamopoulos 2006; RR 0.50; 95% CI 0.10 to 2.47) when investigating levosimendan compared to placebo (Analysis 1.2).
Sensitivity analyses
Sensitivity analysis on the basis of the fixed‐effect model (RR 0.47, 95% CI 0.12 to 1.93) stated the results from the main analysis (Analysis 2.1).
MACE
Information on MACE was restricted to Husebye 2013, which reported four of nine participants (44%) with CS to suffer from MACE (Table 1).
Length of hospital stay
No results were available from the included studies.
Quality of life
No results were available from the included studies.
Haemodynamics
Information on haemodynamics was restricted to Adamopoulos 2006, which reported beneficial effects of levosimendan compared to placebo for the cardiac index (MD 0.10 L/min/m2, 95% CI 0.04 to 0.16) as well as PCWP (MD ‐4.0 mmHg; 95% CI ‐4.58 to ‐3.42) (Table 3). There were no data available for MAP.
Costs
No results were available from the included studies.
AEs
Information on AEs was restricted to Husebye 2013. From the nine participants with CS, two out of four participants (50%) with levosimendan compared to one out of five (20%) participants with placebo suffered from hypotension during drug infusion, with a decrease in MAP of > 10 mmHg. Furthermore, one out of four participants (25%) from the levosimendan intervention arm each suffered from either non‐sustained ventricular tachycardia or atrial fibrillation compared to three out of five participants (60%) suffering from non‐sustained ventricular tachycardia or no participant suffering from atrial fibrillation in the placebo group (Table 4).
3. Levosimendan versus enoximone
There was only one small, single‐centre study with 32 participants investigating levosimendan compared with enoximone in people with AMI complicated by CS (Fuhrmann 2008) with very low‐quality evidence.
All‐cause mortality
Short‐term
There were five deaths out of 16 participants (31.3%) in the intervention arm with levosimendan compared with ten deaths out of 16 participants (62.5%) in the control groups treated with enoximone, but RR indicated no survival benefit (RR 0.50, 0.22 to 1.14; participants = 32; studies = 1; very low‐quality evidence). Out of 1000 people, approximately 625 would be expected to die with standard cardiac care with enoximone within a short‐term follow‐up period compared to 313 (95% CI 138 to 712) with levosimendan (summary of findings Table 3; Analysis 1.1).
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not possible on the basis of this one trial without reported subgroup analyses.
MACE
No results were available from the included study.
Length of hospital stay
A shorter median ICU time was reported in the levosimendan group compared to the enoximone, with high imprecision (10 (IQR 5 to 23) days compared to 13 (IQR 7 to 19) days) (Table 2).
Quality of life
No results were available from the included studies.
Haemodynamics
We found no differences in cardiac index between participants randomised to levosimendan or enoximone (median cardiac index 3.1 L/min/m2 in both groups; IQR 2.5 to 3.5 on levosimendan versus 2.8 to 3.3 on enoximone). Only small differences were found in MAP between participants randomised to levosimendan and enoximone (median MAP 75 mmHg (IQR 58 to 79) on levosimendan versus 70 mmHg (IQR 63 to 83) on enoximone) (Table 3).
Costs
No results were available from the included study.
Adverse events (AEs)
Reported AEs included requiring mechanical ventilation, acute renal failure, need for continuous renal replacement therapy, new onset atrial fibrillation, ventricular tachycardia or fibrillation, pneumonia, urinary infections, and sepsis (Table 4). Levosimendan showed a slightly better safety profile compared to enoximone.
4. Epinephrine versus norepinephrine‐dobutamine
There was only one small, single‐centre study with 30 participants investigating epinephrine compared with norepinephrine‐dobutamine in the context of acute HF complicated by LCOS (Levy 2011), with very low‐quality evidence.
All‐cause mortality
Short‐term
No reported difference in short‐term mortality with five deaths out of 15 participants (33.3%) in the intervention arm with epinephrine compared with four deaths out of 15 participants (26.7%) in the control groups treated with norepinephrine‐dobutamine (RR 1.25; 95% CI 0.41 to 3.77; participants = 30; studies = 1; very low‐quality evidence) . Out of 1000 people, approximately 267 per 1000 would be expected to die with standard cardiac care compared to 333 (95% CI 109 to 1000) with epinephrine (summary of findings Table 4).
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not possible on the basis of this one trial without reported subgroup analyses.
MACE
No results were available from the included study.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The reported cardiac index and MAP showed no differences between participants randomised to either epinephrine or norepinephrine‐dobutamine. (cardiac index: 2.9 ± 0.5 vs. 2.8 ± 0.4; MAP: 64 ± 9 vs. 65 ± 11) (Table 3). Concerning PCWP there were no data available from the included study.
Costs
No results were available from the included study.
Adverse events (AEs)
In the epinephrine group, two out of 15 (13.3%) participants suffered from supraventricular arrhythmia, and one out of 15 (6.7%) participants suffered from sustained ventricular tachycardia. No such AEs are reported for the participants treated with norepinephrine‐dobutamine (Table 4).
5. Amrinone versus dobutamine
There was only one small, single‐centre study with 30 participants investigating amrinone compared with dobutamine in the context of cardiac surgery complicated by LCOS (Dupuis 1992), with very low‐quality evidence.
All‐cause mortality
Short‐term
Mortality within the recovery period was reported to be one out of 15 participants (6.7%) in the amrinone group, and three out of 15 participants (20%) in the dobutamine group (RR 0.33; 95% CI 0.04 to 2.85; participants = 30; studies = 1; very low‐quality evidence) (summary of findings Table 5). Out of 1000 people, approximately 200 per 1000 would be expected to die with dobutamine compared to 66 (95% CI 8 to 570) with amrinone (summary of findings Table 5).
Subgroup and sensitivity analyses
Subgroup and sensitivity analyses were not possible on the basis of this one trial without reported subgroup analyses.
MACE
From the participants randomised to dobutamine, six out of 15 (40%) suffered from MACE (re‐infarction within two hours) whereas no MACE were reported for participants randomised to amrinone (Table 1).
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
No results were available from the included study.
Costs
No results were available from the included study.
Adverse events (AEs)
From the participants randomised to dobutamine four out of 15 (26.7%) suffered from cardiac ischaemia whereas no such events were reported for participants randomised to amrinone. There were no differences between treatment groups with regard to myocardial ischaemia (four out of 15 participants (26.7%) randomised to either amrinone or dobutamine) (Table 4).
6. Dopexamine versus dopamine
There was only one small, multi‐centre study with 70 participants investigating dopexamine compared with dopamine in the context of cardiac surgery complicated by LCOS/CS (Rosseel 1997) (summary of findings Table 6) with very low‐quality evidence. No RR and resulting estimations on absolute risk reduction were possible.
All‐cause mortality
Concerning in‐hospital mortality no deaths were reported in either intervention arm. Subgroup and sensitivity analyses were not possible.
MACE
Perioperative infarctions were reported for three out of 35 participants (8.6%) in the dopexamine intervention arm, and two out of 35 (5.7%) participants in the dopamine intervention arm (Table 1).
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
The reported cardiac index, PCWP, and MAP showed no significant differences between participants randomised to either dopexamine or dopamine after six hours of treatment (cardiac index: MD 0.30 L/min/m2, 95% CI ‐0.01 to 0.61; PCWP: MD ‐1.5 mmHg, 95% CI ‐3.1 to 0.1; MAP: MD ‐1.9 mmHg, 95% CI ‐8.1 to 4.3) (Table 3).
Costs
No results were available from the included study.
Adverse events (AEs)
In the dopexamine group 19 out of 35 participants (54.3%) suffered from cardiac events, two out of 35 participants (5.7%) suffered from abnormal blood loss, and one out of 35 participants (2.9%) suffered from kidney failure. In the dopamine group, cardiac events occurred in 22 out of 35 participants (62.9%), and both abnormal blood loss and kidney failure occurred in one out of 35 participants (2.9%), but no major AEs occurred in either group (Table 4).
7. Enoximone versus dobutamine
There was only one small, single‐centre trial with 40 participants investigating enoximone compared with dobutamine in the context of cardiac surgery complicated by LCOS/CS (Atallah 1990) (summary of findings Table 7) with very low‐quality evidence. No RR and resulting estimations on absolute risk reduction were possible.
All‐cause mortality
Within one month, two deaths were reported, which were not specified between treatment groups. Subgroup and sensitivity analyses were not possible.
MACE
No results were available from the included study.
Length of hospital stay
A shorter stay in the ICU was reported in the enoximone group compared to the dobutamine group, with high imprecision in particular in the dobutamine intervention arm (92 ± 37 hours compared to 155 ± 129 hours) (Table 2).
Quality of life
No results were available from the included study.
Haemodynamics
No results were available from the included study.
Costs
No results were available from the included study.
Adverse events (AEs)
No results were available from the included study.
8. Nitric oxide versus placebo
There was only one small, single‐centre trial investigating nitric oxide compared with placebo in people with AMI complicated by CS (Baldassarre 2008) (summary of findings Table 8) with very low‐quality evidence. The study authors reported three included participants.
All‐cause mortality
The study authors claim that no participant with nitric oxide and one participant with placebo died (Table 2). Subgroup and sensitivity analyses were not possible.
MACE
The study authors claim AMIs for one out of two participants (50%) in the nitric‐oxide intervention arm, and one out of one participants (100%) in the placebo intervention arm (Table 1). No RR and resulting estimations on absolute risk reduction were possible.
Length of hospital stay
No results were available from the included study.
Quality of life
No results were available from the included study.
Haemodynamics
No results were available from the included study.
Costs
No results were available from the included study.
Adverse events (AEs)
No results were available from the included study.
Discussion
Summary of main results
This systematic review includes thirteen RCTs that analysed 2001 participants in trials with greatly differing mortality rates of between 0% and 47%.
Drugs examined
Eight studies investigated levosimendan and compared its efficacy and safety with standard cardiac care and dobutamine, enoximone or placebo. One trial investigated epinephrine compared with norepinephrine and continued dobutamine, one trial investigated amrinone compared with dobutamine, one trial investigated dopexamine compared with dopamine, and one trial investigated enoximone compared with dobutamine. One small RCT on vasodilator strategies compared the effects of nitric oxide, a gas for inhalation, with placebo.
Endpoints
All studies reported mortality outcomes, while length of hospital and ICU stay were reported in three trials only (Atallah 1990; Levin 2008; Fuhrmann 2008). Haemodynamic parameters (as a surrogate marker for morbidity) were available in seven trials (Adamopoulos 2006; Alvarez 2006; Garcίa‐González 2006; Levin 2008; Fuhrmann 2008; Levy 2011; Rosseel 1997), and MACE/adverse events were reported in 11 studies (Alvarez 2006; Baldassarre 2008; Dupuis 1992; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Levy 2011; Mebazaa (SURVIVE) 2007; Rosseel 1997). No data were available for quality of life or costs in any of the trials.
As regards the development of multi‐organ failure, it became obvious that the participants included in some of the trials (Atallah 1990; Garcίa‐González 2006; Rosseel 1997) must have been less severely compromised compared to the participants in the other eligible trials because none or only a few of these participants developed multi‐organ failure. Organ failure determines the clinical course and outcome of CS patients much more than haemodynamics alone (Prondzinsky 2010).
Mortality
There was low‐quality evidence from six trials that participants on levosimendan had lower short‐term mortality rates compared to those on dobutamine. Very low‐quality evidence shows uncertainty around the effect of levosimendan compared to placebo or enoximone. All studies investigating the comparison of epinephrine with norepinephrine‐dobutamine, amrinone or enoximone with dobutamine, dopexamine with dopamine, and nitric oxide with placebo presented uncertainty on their effect on short‐term mortality, with very low‐quality evidence and based on only one single RCT.
Haemodynamics
Levosimendan showed beneficial effects in cardiac index, MAP, and PCWP in comparison to dobutamine (Adamopoulos 2006; Alvarez 2006; Garcίa‐González 2006; Levin 2008) and placebo (Adamopoulos 2006). No clinically relevant differences in cardiac index, MAP, and PCWP were reported for levosimendan compared with enoximone (Fuhrmann 2008), epinephrine compared with norepinephrine‐dobutamine (Levy 2011) as well as dopexamine compared with dopamine (Rosseel 1997). No data were available regarding the comparisons of either amrinone or enoximone with dobutamine (Atallah 1990; Dupuis 1992), and of nitric oxide with placebo (Baldassarre 2008).
Length of hospital and ICU stay
Only three of the thirteen trials reported length of stay in ICU (Atallah 1990; Fuhrmann 2008; Levin 2008). Levin 2008 showed a shorter time in the ICU on levosimendan compared to dobutamine, Fuhrmann 2008 on levosimendan compared to enoximone, and Atallah 1990 on enoximone compared to dobutamine, but in all of these studies the results of comparison groups showed a high level of uncertainty.
Quality of life and costs
No data were available to address quality of life and costs in any of these trials.
Adverse events (AEs)
Levin 2008 reported a better safety profile of levosimendan compared to dobutamine, but this was not found in the studies of Alvarez 2006; Follath(LIDO) 2002, and Mebazaa (SURVIVE) 2007. Reporting on AEs in the comparison of levosimendan with enoximone or placebo, epinephrine with norepinephrine‐dobutamine, amrinone or enoximone with dobutamine, dopexamine with dopamine, and nitric oxide with placebo presented uncertainty, and were based on only one single RCT.
Overall completeness and applicability of evidence
Data were too limited to justify clinical strategies on the basis of the derived evidence on the efficacy and safety of levosimendan or nitric oxide. This statement is strictly related to the limited evidence from RCTs. It is not a judgement concerning the potential benefits of the investigated drugs and does not rule out the possibility that larger RCTs might in future verify the expected beneficial effects.
Quality of the evidence
We identified a total of thirteen eligible studies with 2001 participants and included these studies in eight comparisons to current standard therapies. All these studies were published as full texts, four of them were funded by manufacturers of the drugs (Dupuis 1992; Follath(LIDO) 2002; Husebye 2013; Mebazaa (SURVIVE) 2007).
Effect estimates for our primary outcome, all‐cause mortality are based on the results from one to six RCTs of small to moderate size (between three and 660 participants). This may raise the possibility of publication bias, but the number of studies was insufficient to meet rigorous criteria to create funnel plots. The mortality rates reported by Atallah 1990; Garcίa‐González 2006 and Rosseel 1997 were surprisingly low and in marked contrast to the expected mortality rates of between 40% and 80%. The limited data available for haemodynamic parameters showed clinically relevant differences in cardiac index at baseline in the different studies. The heterogeneity in the baseline haemodynamic characteristics introduces relevant concerns regarding the definitions of CS and LCOS used in these trials. This could also be an explanation for the surprising differences in mortality rates.
We downgraded high‐quality evidence of our eligible RCTs due to relevant study limitations, imprecision or indirectness. We downgraded the quality of the evidence for the following outcomes for study limitations (risk of bias) as recommended in Guyatt 2011b. We downgraded the quality of the evidence for study limitations with high risk of performance bias due to lack of blinding of participants and physicians, high risk of attrition bias due to loss to follow‐up or selection bias due to baseline imbalances. We downgraded the quality of the evidence for imprecision if clinical action would differ if the lower or the upper boundary of the CI represented the truth (Guyatt 2011d). We strongly suspected indirection due to the assumption that participants were included in the studies on the basis of different definition of LCOS or CS due to very low mortality and downgraded the quality of the evidence (Guyatt 2011c).
Levosimendan reduces short‐term mortality compared to a standard therapy with standard cardiac care with dobutamine (RR 0.60, 95% CI 0.37 to 0.95; low‐quality evidence). Six studies with 1776 participants generated the evidence (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Garcίa‐González 2006; Levin 2008; Mebazaa (SURVIVE) 2007). Two studies (Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007) were funded by the manufacturers of levosimendan. We judged the evidence to be low quality and downgraded the evidence by one step for serious study limitations with lack of blinding of participants and physicians in four studies (Adamopoulos 2006; Alvarez 2006; Garcίa‐González 2006; Levin 2008), high risk of bias due to loss to follow‐up and per‐protocol analysis in one study (Alvarez 2006) and baseline differences in prognostic‐relevant factors in one study (Garcίa‐González 2006). We additionally downgraded the evidence by a second step due to imprecision due to few events (summary of findings Table for the main comparison).
There is uncertainty surrounding the effect of levosimendan compared to therapy with standard cardiac care with placebo on short‐term mortality (RR 0.48, 95% CI 0.12 to 1.94; very low‐quality evidence). The very low‐quality evidence was based on two studies (Adamopoulos 2006; Husebye 2013) with 55 participants and we downgraded it by two steps for very serious imprecision due to few events and because the cardiac index crosses the line of no difference, which includes possible benefit from both approaches, and by one additional step for study limitations with high risk of performance bias due to lack of blinding of participants and physicians and missing information on randomisation in Adamopoulos 2006 (summary of findings Table 2). One of these studies was funded by the manufacturer of levosimendan.
There is uncertainty surrounding the effect of levosimendan compared to standard cardiac care with enoximone on short‐term mortality (RR 0.50; 95% CI 0.22 to 1.14; very low‐quality evidence). This evidence was based on one study (Fuhrmann 2008) with 32 participants and we downgraded it by one step for serious imprecision because the cardiac index crosses the line of no difference, which results in possible benefit from both approaches, and by two steps due to very serious study limitations with lack of blinding of participants and physicians, baseline differences in prognostic‐relevant factors and being stopped early for benefit (summary of findings Table 3).
There is uncertainty surrounding the effect of epinephrine compared with standard cardiac care with norepinephrine and continued dobutamine on short‐term mortality (RR 1.25, 95% CI 0.41 to 3.77; very low‐quality evidence). This evidence was based on one study (Levy 2011) with 30 participants and we downgraded it by two steps for very serious imprecision due to few events and because the CI crosses the line of no difference, which results in possible benefit from both approaches. We downgraded the evidence by one more step due to serious study limitation and resulting high risk of performance bias due to lack of blinding of participants and physicians (summary of findings Table 4).
There is uncertainty surrounding the effect of amrinone compared to standard cardiac care with dobutamine on short‐term mortality (RR 0.33, 95% CI 0.04 to 2.85; 1 study; 30 participants; very low‐quality evidence). The evidence was based on one study, which was funded by the manufacturer of amrinone (Dupuis 1992). We downgraded the evidence by two steps for very serious imprecision due to few events and because the cardiac index crosses the line of no difference, which results in possible benefit from both approaches, and by one additional step due to serious study limitation and resulting high risk of performance bias due to lack of blinding of participants and physicians (summary of findings Table 5).
There is uncertainty surrounding the effect of dopexamine compared to standard cardiac care with dopamine on short‐term mortality. The eligible study (Rosseel 1997) reported no in‐hospital deaths out of 70 participants with LCOS after elective surgery for coronary artery bypass graft surgery. We downgraded the evidence to very low‐quality; downgrading by two steps based on serious imprecision because the RR and cardiac index were not estimable, which results in possible benefit from both approaches, and by one step based on suspected indirectness due to the very low mortality in the study population. We assume that the decision to include participants was based on a different definition of LCOS (summary of findings Table 6).
There is uncertainty surrounding the effect of enoximone compared to standard cardiac care with dobutamine on short‐term mortality. The eligible study (Atallah 1990) reported two deaths out of 40 participants with LCOS after mitral valve surgery. We downgraded the evidence by two steps to very low‐quality for serious imprecision because the RR and CI were not estimable which results in possible benefit from both approaches and by one additional step for indirectness due to the very low mortality in the study population. We assume that the decision to include participants was based on a different definition of LCOS (summary of findings Table 7).
There is uncertainty surrounding the effect of nitric oxide compared to standard cardiac care with placebo. The eligible study (Baldassarre 2008) was stopped due to low recruitment after the inclusion of three participants. One participant in the placebo arm died, two participants in the nitric‐oxide arm survived. We downgraded the evidence by two steps to very low‐quality for serious imprecision due to few events and participants, and because the RR and CI were not estimable, which results in possible benefit from both approaches, and by one additional step due to study limitation, with the study being stopped early due to lack of enrolment (summary of findings Table 8).
Potential biases in the review process
We contacted all authors of eligible trials with a request for IPD. Considering that the total number of eligible studies and included participants was relatively small, bias could have been introduced by the mere fact that IPD were not provided, especially in trials reporting favourable effects for the study drug.
As CS is a haemodynamically defined diagnostic term, it is of concern that haemodynamic parameters were not available for all participants. The result was that inclusion criteria and CS definitions relied on the diagnostic definitions being established and reported in the included studies. For this reason we cannot be sure that all reported data refer to people with CS, as commonly defined in the SHOCK trial. The clinical criteria were hypotension (a systolic blood pressure of less than 90 mmHg for at least 30 minutes or the need for supportive measures to maintain a systolic blood pressure of greater than 90 mmHg), end‐organ hypoperfusion (cool extremities or a urine output of less than 30 mL per hour), and a heart rate of greater than 60 beats per minute. The haemodynamic criteria were a cardiac index of no more than 2.2 L/m2/minute of body surface area and a PCWP of at least 15 mmHg (Hochman 1999).
All except one trial investigating levosimendan administered the drug by an initial bolus application. Bolus application of levosimendan might be associated with hypotensive side effects, so we cannot rule out the possibility that the beneficial effects of the drug might have been limited by the bolus application.
One limitation of this review might be the exclusion of all studies not reporting on all‐cause mortality, which possibly lessens the informative value with regard to haemodynamics.
Agreements and disagreements with other studies or reviews
During the last decades several RCTs, cohort studies and systematic reviews have investigated levosimendan and included participants with CS or acute LCOS. These trials have recently been investigated and analysed in ten systematic reviews and meta‐analyses (Delaney 2010; Harrison 2013; Huang 2013; Koster 2015; Landoni 2010a; Landoni 2010b; Landoni 2012; Maharaj 2011; Ribeiro 2010; Thackray 2002).
Delaney 2010 described the efficacy and safety of levosimendan for the treatment of acute severe HF. The systematic search was finalised in June 2007. The meta‐analysis included 19 randomised trials with 3650 participants with acute severe HF. Six studies with a total of 1578 participants, including one trial included in this review (Adamopoulos 2006), compared levosimendan with placebo and reported a non‐significant reduction in mortality for levosimendan (OR 0.83, 95% CI 0.62 to 1.10) with low‐level heterogeneity between the results of the individual trials (I2 = 25.7%). Eight studies with a total of 1979 participants, including four trials included in this review (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Mebazaa (SURVIVE) 2007), compared levosimendan to dobutamine and reported a significant reduction in mortality on levosimendan (OR 0.75, 95% CI 0.61 to 0.92) with low heterogeneity (I2 = 44.6%).
Harrison 2013 performed a meta‐analysis investigating the effects of levosimendan in cardiac surgery patients with and without preoperative systolic dysfunction. Timing of levosimendan treatment in included studies (14 RCTs with 1155 participants) varied from preoperative to intraoperative and postoperative. The search was finalised in May 2012 and included one study included in this review (Alvarez 2006). Pooled results demonstrated a significant reduction in the risk of death with levosimendan (‐4.2%, 95% CI ‐7.2% to ‐1.1%) with low‐level heterogeneity (I2 = 28%), which was not significantly affected by the timing of levosimendan administration or the type of control (either placebo or dobutamine or milrinone or IABP). Subgroup analysis showed that the levosimendan‐associated benefit was restricted to studies investigating participants with a lower ejection fraction (mean ejection fraction < 40%), than those in our included trial, Alvarez 2006.
Huang 2013 analysed the clinical efficacy of levosimendan versus dobutamine in any setting in critically ill patients. The search was finalised in February 2012 and included 22 RCTs with a total of 3052 participants, including five trials included in this review (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Levin 2008; Mebazaa (SURVIVE) 2007). Compared with dobutamine, levosimendan was found to be associated with a significant reduction in mortality (RR 0.81, 95% CI 0.70 to 0.92), with small heterogeneity between the results of individual studies (I2 = 6%). Subgroup analysis indicated that the benefit from levosimendan could be found in the subpopulations of cardiac surgery, ischemics HF, and concomitant beta blocker therapy, but not in the subpopulations of hypotension or (supra‐)ventricular arrhythmias. The studies by Alvarez 2006 and Levin 2008 were included in the cardiac surgery setting, the studies by Adamopoulos 2006; Follath(LIDO) 2002, and Mebazaa (SURVIVE) 2007 were included in the cardiology setting.
Koster 2015 assessed the benefits and harms of levosimendan for LCOS in any setting in critically ill patients. The electronic literature search strategy was last updated in February 2014 and included 49 trials with a total of 6688 participants including eight studies included in this review (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Husebye 2013; Levin 2008; Mebazaa (SURVIVE) 2007). Pooled analysis of all studies including critically ill patients not having cardiac surgery comprising any type of control showed an association between levosimendan and mortality (RR 0.83, 95% CI 0.59 to 0.97). Likewise, pooled analysis of all trials including cardiac surgery patients comprising any type of control showed an association between levosimendan and mortality (RR 0.52, 95% CI 0.37 to 0.73). However, in a subgroup analysis with previously defined trials with lower risk of bias, no association of levosimendan and mortality could be shown for either critically ill patients not having cardiac surgery (RR 0.83, 95% CI 0.48 to 1.55) or cardiac surgery patients (RR 1.02, 95% CI 0.48 to 2.16).
Landoni 2010a studied whether levosimendan was associated with improved survival in people undergoing cardiac surgery. The search was updated in January 2009 and identified 10 RCTs with 440 participants, including two studies included in this review (Alvarez 2006; Levin 2008). Levosimendan was associated with a significant reduction in postoperative mortality in the levosimendan intervention arm compared to the control arm (either placebo or dobutamine or milrinone) with OR 0.35 (95% CI 0.18 to 0.71) with low heterogeneity (I2 = 27.4%).
Landoni 2010b investigated the impact of levosimendan on mortality in any setting dealing with critically ill patients. The systematic search was updated in November 2008 and identified 27 RCTs that compared levosimendan versus control, with a total of 3350 participants, including five studies included in this review (Adamopoulos 2006; Alvarez 2006; Follath(LIDO) 2002; Levin 2008; Mebazaa (SURVIVE) 2007). Levosimendan was associated with a significant reduction in mortality (OR 0.74, 95% CI 0.62 to 0.89) with low heterogeneity between the results of individual studies (I2 = 11.3%), and an increase in the number of hypotensive participants (OR 1.38; 95% CI 1.06 to 1.80) with low heterogeneity (I2 = 37.7%).
Landoni 2012 devised an updated meta‐analysis of all RCTs of levosimendan to reach a definite conclusion for this substance in the management of patients requiring inotropic drugs. The search was updated in November 2010 and identified 45 RCTs with 5480 participants. Levosimendan was associated with a significant reduction in mortality (RR 0.80, 95% CI 0.72 to 0.89) and low heterogeneity between study results (I2 = 15.4%). This result was confirmed in studies with different control groups and in different settings. Five of our included studies (Adamopoulos 2006; Follath(LIDO) 2002; Fuhrmann 2008; Garcίa‐González 2006; Mebazaa (SURVIVE) 2007) were in the subgroup of trials performed in cardiology, where a similar reduction of mortality was confirmed (RR 0.75, 95% CI 0.63 to 0.91) with low heterogeneity (I2 = 25.5%). Two of our studies (Alvarez 2006; Levin 2008) were in the subgroup of trials performed in cardiac surgery, where the reduction in mortality was confirmed as well (RR 0.52, 95% CI 0.35 to 0.76) with no heterogeneity between the results of individual studies (I2 = 0%).
Maharaj 2011 evaluated the effect of levosimendan versus control on mortality after coronary revascularisation. This systematic review was based on a search period up to August 2010 and included 17 RCTs involving 729 participants. Levosimendan was associated with a mortality reduction after coronary revascularisation (OR 0.40, 95% CI 0.21 to 0.76) with small heterogeneity of study results (I2 = 12%). Elective revascularisation showed a significant benefit (OR 0.36, 95% CI 0.18 to 0.72) compared with emergency revascularisation (OR 0.61, 95% CI 0.19 to 1.89). The elective revascularisation group included two of our included studies (Alvarez 2006; Levin 2008); the emergency revascularisation group included one of our included studies (Fuhrmann 2008).
Ribeiro 2010 analysed morbidity and mortality reduction associated with levosimendan in the treatment of acute decompensated HF. The search was set to an end date of July 2009 and included 19 RTCs with 3719 participants. A non‐significant reduction in relative risk for overall death was found for both the comparison of levosimendan with placebo (seven trials including 1652 participants, including one trial included in this review (Adamopoulos 2006); RR 0.87, 95% CI 0.65 to 1.18) with small heterogeneity between the results of individual studies (I2 = 12%), and the comparison of levosimendan with dobutamine (10 trials including 2067 participants, including three trials included in this review (Adamopoulos 2006; Alvarez 2006; Mebazaa (SURVIVE) 2007); RR 0.87, 95% CI 0.75 to 1.02) with no heterogeneity between the results of individual studies (I2 = 0%).
Thackray 2002 systematically reviewed the use of intravenous inotropic drugs acting through the adrenergic pathway in people with heart failure. In total 21 RCTs involving 632 participants were included. Three studies comprising 75 participants, including one trial included in this review (Atallah 1990), compared dobutamine with enoximone. No differences on mortality were identified between dobutamine and alternative inotropic agents (OR 1.37, 95 % CI 0.23 to 8.46).
In conclusion, while some of our included studies have been used in recently published reviews, our systematic review differs from previously published reviews for several major reasons.
-
This review comprises participants with AMI, HF or cardiac surgery complicated by CS or LCOS.
-
With the exception of Koster 2015 none of the other meta‐analyses were based on a previously published protocol, as recommended in Shea 2009.
-
Our literature search was upgraded in June 2017 and is more up‐to‐date.
-
Finally, this review is not restricted to levosimendan but investigates other inotropic or vasodilative drugs including epinephrine, amrinone, dopexamine, enoximone, and nitric oxide.
This systematic review focusses on CS and LCOS in the acute setting. Outpatient trials, as discussed in Nieminen 2014 and Silvetti 2014, are not within the scope of this meta‐analysis.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Levosimendan versus control, Outcome 1 All‐cause short‐term mortality.
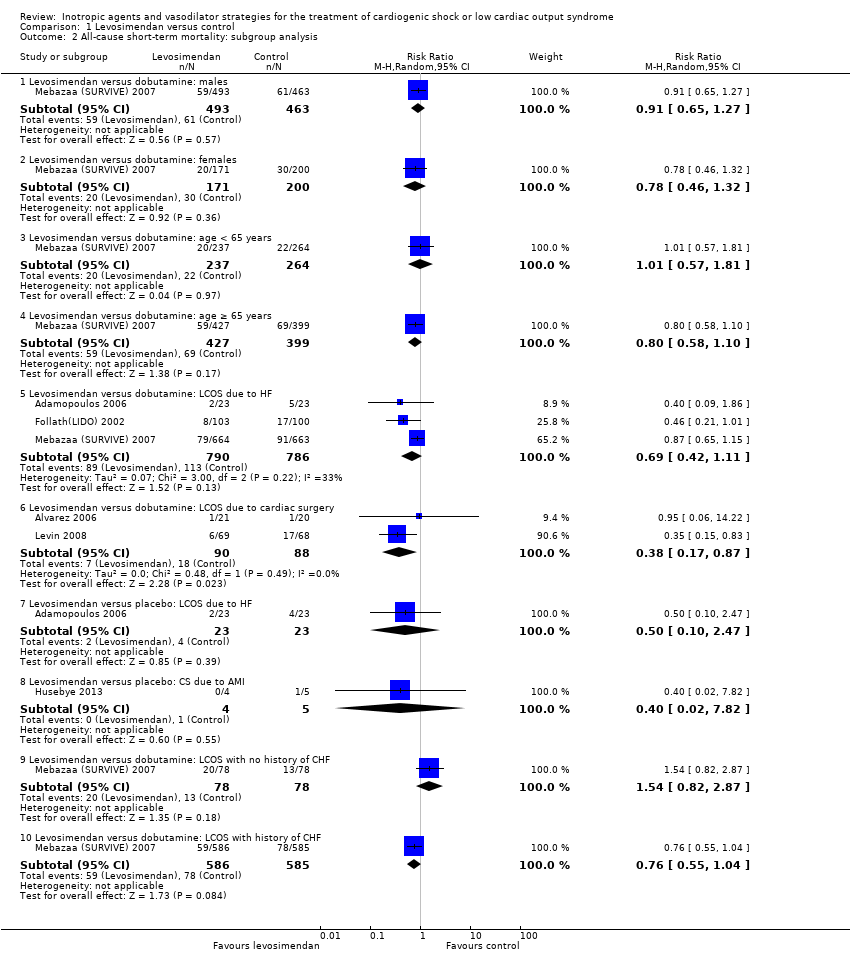
Comparison 1 Levosimendan versus control, Outcome 2 All‐cause short‐term mortality: subgroup analysis.

Comparison 1 Levosimendan versus control, Outcome 3 All‐cause long‐term mortality.

Comparison 1 Levosimendan versus control, Outcome 4 All‐cause long‐term mortality:subgroup analysis.

Comparison 1 Levosimendan versus control, Outcome 5 Cardiac index.

Comparison 1 Levosimendan versus control, Outcome 6 Pulmonary capillary wedge pressure.

Comparison 1 Levosimendan versus control, Outcome 7 Mean arterial pressure.
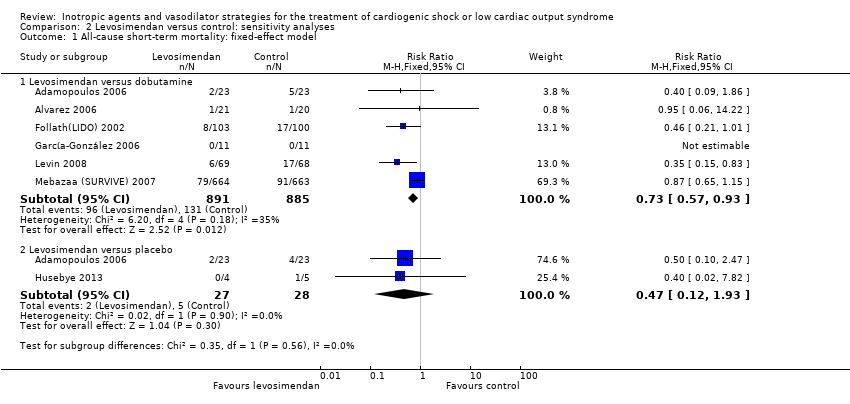
Comparison 2 Levosimendan versus control: sensitivity analyses, Outcome 1 All‐cause short‐term mortality: fixed‐effect model.
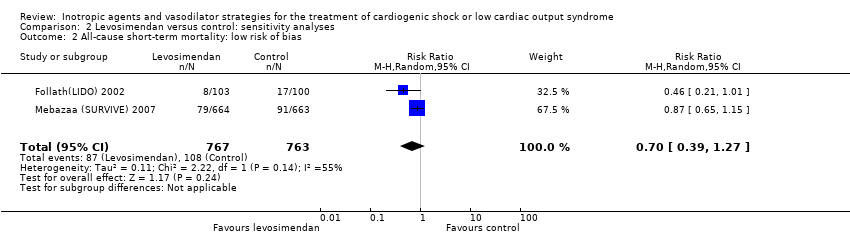
Comparison 2 Levosimendan versus control: sensitivity analyses, Outcome 2 All‐cause short‐term mortality: low risk of bias.
| Levosimendan compared to dobutamine for cardiogenic shock or low cardiac output syndrome | ||||||
| Patient or population: people with cardiogenic shock or low cardiac output syndrome | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dobutamine | Risk with levosimendan | |||||
| All‐cause, short‐term mortality: range 15 days to 12 months | Moderate1 | RR 0.60 | 1776 | ⊕⊕⊝⊝ | Studies included participants with LCOS or CS due to cardiac surgery, HF or AMI | |
| 154 per 1000 | 92 per 1000 | |||||
| High2 | ||||||
| 500 per 1000 | 300 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from the median risk among the control group risk in included studies with participants with low cardiac output, low cardiac output or cardiogenic shock, or cardiogenic shock. | ||||||
| Levosimendan compared with placebo for cardiogenic shock or low cardiac output syndrome | ||||||
| Patient or population: adults with cardiogenic shock or low cardiac output syndrome Settings: hospital Intervention: levosimendan Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with placebo | Risk with levosimendan | |||||
| All‐cause short‐term mortality: range 4 to 6 months | Moderate1 | RR 0.48 (0.12 to 1.94) | 55 | ⊕⊕⊝⊝ | Studies included participants with LCOS or CS due to HF or AMI | |
| 187 per 1000 | 90 per 1000 | |||||
| High2 | ||||||
| 500 per 1000 | 240 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from median risk among the control group risk in included studies with low cardiac output or cardiogenic shock. | ||||||
| Levosimendan compared with enoximone for cardiogenic shock | ||||||
| Patient or population: adults with cardiogenic shock Settings: hospital Intervention: levosimendan Comparison: enoximone | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with enoximone | Risk with levosimendan | |||||
| All‐cause short‐term mortality: 30 days | 625 per 10001 | 313 per 1000 (138 to 712) | RR 0.50 (0.22 to 1.14) | 32 | ⊕⊝⊝⊝ very low2,3 | Study included participants with CS due to AMI |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from the control group risk in a small included study with low cardiac output or cardiogenic shock. | ||||||
| Epinephrine compared with norepinephrine‐dobutamine for low cardiac output syndrome | ||||||
| Patient or population: adults with low cardiac output syndrome Setting: in‐hospital Intervention: epinephrine Comparison: norepinephrine‐dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with norepinephrine‐dobutamine | Risk with epinephrine | |||||
| All‐cause short‐term mortality: 28 days | 267 per 1000 | 333 per 1000 | RR 1.25 (0.41 to 3.77) | 30 | ⊕⊝⊝⊝ very low1,2 | Study included participants with LCOS due to HF |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two steps for imprecision due to few events, and the confidence interval crosses the line of no difference and includes possible benefit from both approaches. | ||||||
| Amrinone compared with dobutamine for low cardiac output syndrome | ||||||
| Patient or population: adults with low cardiac output syndrome Setting: hospital Intervention: amrinone Comparison: dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dobutamine | Risk with amrinone | |||||
| All‐cause short‐term mortality: 30 days | 200 per 10001 | 66 per 1000 | RR 0.33 (0.04 to 2.85) | 30 | ⊕⊝⊝⊝ very low2,3 | Study included participants with LCOS following cardiac surgery |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from the control group risk in participants with low cardiac output and no cardiogenic shock in the included small study. | ||||||
| Dopexamine compared with dopamine for cardiogenic shock or low cardiac output syndrome | ||||||
| Patient or population: adults with cardiogenic shock or low cardiac output syndrome Setting: hospital Intervention: dopexamine Comparison: dopamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dopexamine | Risk with dopamine | |||||
| All‐cause short‐term mortality: time in hospital | 500 per 10001 | Not estimable2 | RR not estimable2 | 70 | ⊕⊝⊝⊝ very low3,4 | Study included participants with LCOS/CS following elective surgery for CABG |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from a large observational study, due to the small size of included studies in this population (Singh 2007). | ||||||
| Enoximone compared with dobutamine for low cardiac output syndrome | ||||||
| Patient or population: adults with low cardiac output syndrome Setting: hospital Intervention: enoximone Comparison: dobutamine | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with dobutamine | Risk with enoximone | |||||
| All‐cause short‐term mortality: 1 month | 500 per 10001 | Not estimable2 | RR not estimable2 | 40 | ⊕⊝⊝⊝ very low3,4 | Study included participants with LCOS after mitral valve surgery |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from a large observational study, due to the small size of included studies in this population (Singh 2007). | ||||||
| Nitric oxide compared with placebo for cardiogenic shock | ||||||
| Patient or population: adults with cardiogenic shock Setting: in‐hospital Intervention: nitric oxide Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | No of participants | Quality | Comments | |
| Risk with nitric oxide | Risk with placebo | |||||
| All‐cause short‐term mortality: 1 month | 500 per 10001 | Not estimable2 | RR not estimable2 | 3 | ⊕⊝⊝⊝ | Study included participants with CS due to AMI |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Control group risk estimate comes from a large observational study, due to the small size of included studies in this population (Singh 2007). | ||||||
| Comparison | Primary studies | MACE | Intervention | Control | RR (95% CI) | ||
| events | total | events | total | ||||
| Levosimendan vs dobutamine | Perioperative infarction | 1 (1.4%) | 69 | 8 (11.8%) | 68 | 0.12 (0.02 to 0.96) | |
| Re‐infarction | 0 (0%) | 11 | 0 (0%) | 11 | Not estimable | ||
| Cerebrovascular accidents | 2 (2.9%) | 69 | 6 (8.8%) | 68 | 0.33 (0.07 to 1.57) | ||
| Cerebrovascular accidents | 0 (0%) | 11 | 0 (0%) | 11 | Not estimable | ||
| Levosimendan vs placebo | MACE (death, non‐fatal myocardial infarction, revascularisation of the infarct‐related artery) | 2 (50.0%) | 4 | 2 (40.0%) | 5 | 1.25 (0.29 to 5.35) | |
| Repeat PCI | 1 (25.0%) | 4 | 0 (0%) | 5 | 3.60 (0.18 to 70.34) | ||
| Amrinone vs dobutamine | Re‐infarction (2 h) | 0 (0%) | 15 | 6 (40.0%) | 15 | 0.08 (0.00 to 1.25) | |
| Dopexamine vs dopamine | Perioperative infarction | 3 (8.6%) | 35 | 2 (5.7%) | 35 | 1.50 (0.27 to 8.43) | |
| Nitric oxide vs placebo | Myocardial infarction | 1 (50.0%) | 2 | 1 (100%) | 1 | 0.67 (0.17 to 2.67) | |
| CI: confidence interval; PCI: percutaneous coronary intervention; RR: risk ratio | |||||||
| Comparison | Primary studies | Reported information | Intervention | Control | ||
| Events/time | Total | Events/time | Total | |||
| Levosimendan vs dobutamine | Stay in ICU (hours, median with IQR) | 66 (58‐74) | 69 | 158 (106‐182) | 68 | |
| Levosimendan vs enoximone | Stay in ICU (days, median with IQR) | 10 (5‐23) | 16 | 13 (7‐19) | 16 | |
| Enoximone vs dobutamine | Stay in ICU (hours, mean) | 92 ± 37 | 18 | 155 ± 129 | 19 | |
| ICU: intensive care unit; IQR: intra‐quartile‐range | ||||||
| Comparison | Primary studies | Haemodynamics | Intervention | Control | MD (95% CI) | ||
| Intervention vs control | last measurements | mean ± SD or median (IQR) | total | mean ± SD or median (IQR) | total | ||
| Levosimendan vs dobutamine | Cardiac index (after 72 h, L/min/m2) | 1.9 ± 0.1 | 23 | 1.8 ± 0.04 | 23 | 0.10 (0.06 to 0.14) | |
| Cardiac index (after 48 h, L/min/m2) | 2.8 ± 0.3 | 21 | 2.3 ± 0.2 | 20 | 0.50 (0.34 to 0.66) | ||
| Cardiac index (after 30 h, L/min/m2) | 2.9 ± 0.4 | 11 | 2.4 ± 0.2 | 11 | 0.50 (0.24 to 0.76) | ||
| Cardiac index (after 48 hrs, L/min/m2) | 3.4 ± 0.2 | 69 | 2.7 ± 0.1 | 68 | 0.70 (0.65 to 0.75) | ||
| PCWP (after 72 h, mmHg) | 19.0 ± 1 | 23 | 23.0 ± 1.0 | 23 | ‐4.00 (‐4.60 to ‐3.40) | ||
| MAP (after 48 h, mmHg) | 77.0 ± 5 | 21 | 81.0 ± 7.0 | 20 | ‐4.00 (‐7.70 to ‐0.30) | ||
| MAP (after 48 h, mmHg) | 78.8 ± 7 | 69 | 80.1 ± 4 | 68 | ‐1.30 (‐3.20 to 0.60) | ||
| Levosimendan vs placebo | Cardiac index (after 72 h, (L/min/m2) | 1.9 ± 0.1 | 23 | 1.8 ± 0.1 | 23 | 0.10 (0.04 to 0.16) | |
| PCWP (after 72 h, mmHg) | 19.0 ± 1 | 23 | 23.0 ± 1.0 | 23 | ‐4.00 (‐4.60 to ‐3.40) | ||
| Levosimendan vs enoximone | Cardiac index (after 48 h, L/min/m2) | 3.1 (2.5‐3.5) | 16 | 3.1 (2.8‐3.3) | 16 | Not estimable | |
| MAP (after 48 h (mmHg) | 75.0 (58.0‐79.0) | 16 | 70.0 (63.0‐83.0) | 16 | Not estimable | ||
| Epinephrine vs norepinephrine‐dobutamine | Cardiac index (after 24 h, L/min/m2) | 2.9 ± 0.5 | 15 | 2.8 ± 0.4 | 15 | 0.10 (‐0.22 to 0.42) | |
| MAP (after 24 h, mmHg) | 64 ± 9 | 15 | 65.0 ± 11.0 | 15 | ‐1.00 (‐8.20 to 6.20) | ||
| Dopexamine vs dopamine | Cardiac index (after 6 h, L/min/m2) | 3.1 ± 0.7 | 29 | 2.8 ± 0.5 | 30 | 0.30 (‐0.01 to 0.61) | |
| PCWP (after 6 h, mmHg) | 9.3 ± 3.2 | 29 | 10.8 ± 2.9 | 30 | ‐1.50 (‐3.10 to 0.10) | ||
| MAP (after 6 h, mmHg) | 76.3 ± 11.5 | 29 | 78.2 ± 12.8 | 30 | ‐1.90 (‐8.10 to 4.30) | ||
| CI: confidence interval; IQR: intra‐quartile‐range; MAP: mean arterial pressure; MD: mean difference; PCWP: pulmonary capillary wedge pressure; SD: standard deviation | |||||||
| Comparison | Primary studies | Adverse events (no MACE) | Intervention | Control | ||
| events | total | events | total | |||
| Levosimendan vsdobutamine | Atrial fibrillation | 78 (10.4%) | 750 | 71 (9.5%) | 748 | |
| Ventricular fibrillation | 15 (2.3%) | 660 | 19 (2.9%) | 660 | ||
| Ventricular arrhythmias | 7 (3.6%) | 193 | 25 (13.3%) | 188 | ||
| Ventricular tachycardia | 52 (7.9%) | 660 | 48 (7.3%) | 660 | ||
| Ventricular extrasystoles | 40 (6.1%) | 660 | 24 (3.6%) | 660 | ||
| Tachycardia | 33 (5.0%) | 660 | 33 (5.0%) | 660 | ||
| Bradycardia | 8 (1.2%) | 660 | 17 (2.6%) | 660 | ||
| Headache | 69 (9.0%) | 763 | 36 (4.7%) | 760 | ||
| Cardiac failure | 91 (11.9%) | 763 | 127 (16.7%) | 760 | ||
| Congestive cardiac failure | 26 (3.9%) | 660 | 22 (3.3%) | 660 | ||
| Cardiac arrest | 20 (3.0%) | 660 | 26 (3.9%) | 660 | ||
| Disorder aggravated | 17 (2,2%) | 763 | 27 (3.6%) | 760 | ||
| Gastrointestinal disorders | 54 (7.1%) | 763 | 52 (6.8%) | 760 | ||
| Acute kidney failure | 29 (4.0%) | 729 | 43 (5.9%) | 728 | ||
| Need for dialysis | 2 (2.9%) | 69 | 8 (11.9%) | 68 | ||
| Pneumonia | 34 (4.7%) | 729 | 34 (4.7%) | 728 | ||
| Multiple organ failure | 0 (0%) | 11 | 0 (0%) | 11 | ||
| Stroke | 0 (0%) | 11 | 0 (0%) | 11 | ||
| Vasoplegia | 1 (1.4 %) | 69 | 9 (13.2%) | 68 | ||
| Dyspnoea | 1 (1.4%) | 69 | 4 (5.8%) | 68 | ||
| Inflammatory response syndrome | 4 (5.8%) | 69 | 15 (22.1%) | 68 | ||
| Sepsis | 1 (1.4%) | 69 | 9 (13.2%) | 68 | ||
| Prolonged ventilatory assistance | 6 (8.7%) | 69 | 22 (32.3%) | 68 | ||
| Hypokalaemia | 62 (9.4%) | 660 | 39 (5.9%) | 660 | ||
| Hyperkalaemia | 15 (2.3%) | 660 | 16 (2.4%) | 660 | ||
| Hypotension | 102 (15.5%) | 660 | 92 (13.9%) | 660 | ||
| Nausea | 45 (6.8%) | 660 | 49 (7.4%) | 660 | ||
| Insomnia | 37 (5.6%) | 660 | 29 (4.4%) | 660 | ||
| Chest pain | 32 (4.8%) | 660 | 47 (7.1%) | 660 | ||
| Constipation | 26 (3.9%) | 660 | 28 (4.2%) | 660 | ||
| Pyrexia | 22 (3.3%) | 660 | 19 (2.9%) | 660 | ||
| Urinary tract infection | 21 (3.2%) | 660 | 30 (4.5%) | 660 | ||
| Anexiety | 20 (3.0%) | 660 | 19 (2.9%) | 660 | ||
| Pulmonary oedema | 20 (3.0%) | 660 | 18 (2.7%) | 660 | ||
| Dizziness | 19 (2.9%) | 660 | 16 (2.4%) | 660 | ||
| Cough | 19 (2.9%) | 660 | 21 (3.2%) | 660 | ||
| Pain in extremity | 18 (2.7%) | 660 | 10 (1.5%) | 660 | ||
| Pruritus | 16 (2.4%) | 660 | 7 (1.1%) | 660 | ||
| Anaemia | 15 (2.3%) | 660 | 17 (2.6%) | 660 | ||
| Epistaxis | 14 (2.1%) | 660 | 7 (1.1%) | 660 | ||
| Back pain | 13 (2.0%) | 660 | 18 (2.7%) | 660 | ||
| Angina pectoris | 12 (1.8%) | 660 | 18 (2.7%) | 660 | ||
| Muscle spasms | 12 (1.8%) | 660 | 13 (2.0%) | 660 | ||
| Dyspnoea | 9 (1.4%) | 660 | 17 (2.6%) | 660 | ||
| Hypertension | 9 (1.4%) | 660 | 15 (2.3%) | 660 | ||
| Cataract | 7 (1.1%) | 660 | 14 (2.1%) | 660 | ||
| Agitation | 7 (1.1%) | 660 | 0 (0%) | 660 | ||
| Levosimendan vsplacebo | Non‐sustained ventricular tachycardia | 1 (25.0%) | 4 | 3 (60.0%) | 5 | |
| Atrial fibrillation | 1 (25.0%) | 4 | 0 (0%) | 5 | ||
| Episodes of hypotension during drug infusion (MAP fall > 10 mmHg) | 2 (50.0%) | 4 | 1 (20.0%) | 5 | ||
| Levosimendan vsenoximone | Need of mechanical ventilation | 13 (81.3%) | 16 | 15 (93.8%) | 16 | |
| Acute renal failure | 5 (31.3%) | 16 | 8 (50.0%) | 16 | ||
| Need of continuous renal replacement therapy | 5 (31.5%) | 16 | 8 (50.0%) | 16 | ||
| New onset atrial fibrillation | 7 (43.8%) | 16 | 9 (56.3%) | 16 | ||
| Ventricular tachycardia or fibrillation | 8 (50.0%) | 16 | 11 (68.8%) | 16 | ||
| Development of systemic inflammatory response | 8 (50.0%) | 16 | 13 (81.3%) | 16 | ||
| Pneumonia | 7 (43.8%) | 16 | 7 (43.8%) | 16 | ||
| Urinary infections | 0 (0%) | 16 | 2 (12.5%) | 16 | ||
| Sepsis | 3 (18.8%) | 16 | 2 (12.5%) | 16 | ||
| Epinephrine vs. norepinephrine‐dobutamine | Supraventricular arrhythmia | 2 (13.3%) | 15 | 0 (0%) | 15 | |
| Sustained ventricular tachycardia | 1 (6.7%) | 15 | 0 (0%) | 15 | ||
| Amrinone vs. dobutamine | Cardiac arrhythmias during treatment | 0 (0%) | 15 | 4 (26.7%) | 15 | |
| Myocardial ischemias (within 16 to 20 hrs) | 4 (26.7%) | 15 | 4 (26.7%) | 15 | ||
| Dopexamine vs. dopamine | Cardiac events | 19 (54.3%) | 35 | 22 (62.9%) | 35 | |
| Abnormal blood loss | 2 (5.7%) | 35 | 1 (2.9%) | 35 | ||
| Kidney failure | 1 (2.9%) | 35 | 1 (2.9%) | 35 | ||
| Other adverse events | 5 (14.3%) | 35 | 1 (2.9%) | 35 | ||
| Major adverse events | 0 (0%) | 35 | 0 (0%) | 35 | ||
| MACE: major adverse cardiac events; MAP: mean arterial pressure | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause short‐term mortality Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Levosimendan versus dobutamine | 6 | 1776 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.37, 0.95] |
| 1.2 Levosimendan versus placebo | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.12, 1.94] |
| 1.3 Levosimendan versus enoximone | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.22, 1.14] |
| 2 All‐cause short‐term mortality: subgroup analysis Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Levosimendan versus dobutamine: males | 1 | 956 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.27] |
| 2.2 Levosimendan versus dobutamine: females | 1 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.46, 1.32] |
| 2.3 Levosimendan versus dobutamine: age < 65 years | 1 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.81] |
| 2.4 Levosimendan versus dobutamine: age ≥ 65 years | 1 | 826 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.58, 1.10] |
| 2.5 Levosimendan versus dobutamine: LCOS due to HF | 3 | 1576 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.42, 1.11] |
| 2.6 Levosimendan versus dobutamine: LCOS due to cardiac surgery | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.17, 0.87] |
| 2.7 Levosimendan versus placebo: LCOS due to HF | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.47] |
| 2.8 Levosimendan versus placebo: CS due to AMI | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 0.4 [0.02, 7.82] |
| 2.9 Levosimendan versus dobutamine: LCOS with no history of CHF | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.82, 2.87] |
| 2.10 Levosimendan versus dobutamine: LCOS with history of CHF | 1 | 1171 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.55, 1.04] |
| 3 All‐cause long‐term mortality Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Levosimendan versus dobutamine | 3 | 1552 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.12] |
| 3.2 Levosimendane versus dobutamine | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.37, 24.58] |
| 3.3 Levosimendan versus placebo | 1 | 9 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.08, 4.66] |
| 4 All‐cause long‐term mortality:subgroup analysis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Levosimendan versus dobutamine: males | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Levosimendan versus dobutamine: females | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Levosimendan versus dobutamine: age < 65 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Levosimendan versus dobutamine: age ≥ 65 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Cardiac index Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Levosimendan versus dobutamine | 4 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Levosimendan versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Epinephrine versus norepinephrine‐dobutamine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Dopexamine versus dopamine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pulmonary capillary wedge pressure Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Levosimendan versus dobutamine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Levosimendan versus placebo | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Dopexamine versus dopamine | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Mean arterial pressure Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Levosimendan versus dobutamine | 2 | 178 | Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐4.61, 0.31] |
| 7.2 Epinephrine versus norepinephrine‐dobutamine | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐8.19, 6.19] |
| 7.3 Dopexamine versus dopamine | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐8.10, 4.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause short‐term mortality: fixed‐effect model Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Levosimendan versus dobutamine | 6 | 1776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.93] |
| 1.2 Levosimendan versus placebo | 2 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.93] |
| 2 All‐cause short‐term mortality: low risk of bias Show forest plot | 2 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.39, 1.27] |

