Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis
Abstract
Background
Xpert MTB/RIF and Xpert MTB/RIF Ultra (Xpert Ultra) are World Health Organization (WHO)‐recommended rapid tests that simultaneously detect tuberculosis and rifampicin resistance in people with signs and symptoms of tuberculosis. This review builds on our recent extensive Cochrane Review of Xpert MTB/RIF accuracy.
Objectives
To compare the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis and detection of rifampicin resistance in adults with presumptive pulmonary tuberculosis. For pulmonary tuberculosis and rifampicin resistance, we also investigated potential sources of heterogeneity.
We also summarized the frequency of Xpert Ultra trace‐positive results, and estimated the accuracy of Xpert Ultra after repeat testing in those with trace‐positive results.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, MEDLINE, Embase, Science Citation Index, Web of Science, LILACS, Scopus, the WHO ICTRP, the ISRCTN registry, and ProQuest to 28 January 2020 with no language restriction.
Selection criteria
We included diagnostic accuracy studies using respiratory specimens in adults with presumptive pulmonary tuberculosis that directly compared the index tests. For pulmonary tuberculosis detection, the reference standards were culture and a composite reference standard. For rifampicin resistance, the reference standards were culture‐based drug susceptibility testing and line probe assays.
Data collection and analysis
Two review authors independently extracted data using a standardized form, including data by smear and HIV status. We assessed risk of bias using QUADAS‐2 and QUADAS‐C. We performed meta‐analyses comparing pooled sensitivities and specificities, separately for pulmonary tuberculosis detection and rifampicin resistance detection, and separately by reference standard. Most analyses used a bivariate random‐effects model. For tuberculosis detection, we estimated accuracy in studies in participants who were not selected based on prior microscopy testing or history of tuberculosis. We performed subgroup analyses by smear status, HIV status, and history of tuberculosis. We summarized Xpert Ultra trace results.
Main results
We identified nine studies (3500 participants): seven had unselected participants (2834 participants). All compared Xpert Ultra and Xpert MTB/RIF for pulmonary tuberculosis detection; seven studies used a paired comparative accuracy design, and two studies used a randomized design. Five studies compared Xpert Ultra and Xpert MTB/RIF for rifampicin resistance detection; four studies used a paired design, and one study used a randomized design. Of the nine included studies, seven (78%) were mainly or exclusively in high tuberculosis burden countries. For pulmonary tuberculosis detection, most studies had low risk of bias in all domains.
Pulmonary tuberculosis detection
Xpert Ultra pooled sensitivity and specificity (95% credible interval) against culture were 90.9% (86.2 to 94.7) and 95.6% (93.0 to 97.4) (7 studies, 2834 participants; high‐certainty evidence) versus Xpert MTB/RIF pooled sensitivity and specificity of 84.7% (78.6 to 89.9) and 98.4% (97.0 to 99.3) (7 studies, 2835 participants; high‐certainty evidence). The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at 6.3% (0.1 to 12.8) for sensitivity and −2.7% (−5.7 to −0.5) for specificity. If the point estimates for Xpert Ultra and Xpert MTB/RIF are applied to a hypothetical cohort of 1000 patients, where 10% of those presenting with symptoms have pulmonary tuberculosis, Xpert Ultra will miss 9 cases, and Xpert MTB/RIF will miss 15 cases. The number of people wrongly diagnosed with pulmonary tuberculosis would be 40 with Xpert Ultra and 14 with Xpert MTB/RIF.
In smear‐negative, culture‐positive participants, pooled sensitivity was 77.5% (67.6 to 85.6) for Xpert Ultra versus 60.6% (48.4 to 71.7) for Xpert MTB/RIF; pooled specificity was 95.8% (92.9 to 97.7) for Xpert Ultra versus 98.8% (97.7 to 99.5) for Xpert MTB/RIF (6 studies).
In people living with HIV, pooled sensitivity was 87.6% (75.4 to 94.1) for Xpert Ultra versus 74.9% (58.7 to 86.2) for Xpert MTB/RIF; pooled specificity was 92.8% (82.3 to 97.0) for Xpert Ultra versus 99.7% (98.6 to 100.0) for Xpert MTB/RIF (3 studies).
In participants with a history of tuberculosis, pooled sensitivity was 84.2% (72.5 to 91.7) for Xpert Ultra versus 81.8% (68.7 to 90.0) for Xpert MTB/RIF; pooled specificity was 88.2% (70.5 to 96.6) for Xpert Ultra versus 97.4% (91.7 to 99.5) for Xpert MTB/RIF (4 studies).
The proportion of Ultra trace‐positive results ranged from 3.0% to 30.4%. Data were insufficient to estimate the accuracy of Xpert Ultra repeat testing in individuals with initial trace‐positive results.
Rifampicin resistance detection
Pooled sensitivity and specificity were 94.9% (88.9 to 97.9) and 99.1% (97.7 to 99.8) (5 studies, 921 participants; high‐certainty evidence) for Xpert Ultra versus 95.3% (90.0 to 98.1) and 98.8% (97.2 to 99.6) (5 studies, 930 participants; high‐certainty evidence) for Xpert MTB/RIF. The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at −0.3% (−6.9 to 5.7) for sensitivity and 0.3% (−1.2 to 2.0) for specificity. If the point estimates for Xpert Ultra and Xpert MTB/RIF are applied to a hypothetical cohort of 1000 patients, where 10% of those presenting with symptoms have rifampicin resistance, Xpert Ultra will miss 5 cases, and Xpert MTB/RIF will miss 5 cases. The number of people wrongly diagnosed with rifampicin resistance would be 8 with Xpert Ultra and 11 with Xpert MTB/RIF.
We identified a higher number of rifampicin resistance indeterminate results with Xpert Ultra, pooled proportion 7.6% (2.4 to 21.0) compared to Xpert MTB/RIF pooled proportion 0.8% (0.2 to 2.4). The estimated difference in the pooled proportion of indeterminate rifampicin resistance results for Xpert Ultra versus Xpert MTB/RIF was 6.7% (1.4 to 20.1).
Authors' conclusions
Xpert Ultra has higher sensitivity and lower specificity than Xpert MTB/RIF for pulmonary tuberculosis, especially in smear‐negative participants and people living with HIV. Xpert Ultra specificity was lower than that of Xpert MTB/RIF in participants with a history of tuberculosis. The sensitivity and specificity trade‐off would be expected to vary by setting. For detection of rifampicin resistance, Xpert Ultra and Xpert MTB/RIF had similar sensitivity and specificity. Ultra trace‐positive results were common.
Xpert Ultra and Xpert MTB/RIF provide accurate results and can allow rapid initiation of treatment for rifampicin‐resistant and multidrug‐resistant tuberculosis.
Plain language summary
Xpert Ultra compared to Xpert MTB/RIF for diagnosing pulmonary tuberculosis and rifampicin resistance in adults
Why is improving the diagnosis of pulmonary tuberculosis important?
Tuberculosis is one of the leading causes of death worldwide. While tuberculosis is largely curable when detected early and effectively treated, around 1.2 million people died of tuberculosis in 2019. Xpert MTB/RIF and Xpert Ultra (the newest version) are World Health Organization‐recommended rapid tests that simultaneously detect tuberculosis and rifampicin resistance in people with tuberculosis symptoms. Rifampicin is an important antituberculosis drug. Not recognizing tuberculosis when it is present (false negative) may result in severe illness and death, and an increased risk of infecting others. An incorrect diagnosis of tuberculosis (false positive) may result in anxiety, additional testing, unnecessary treatment, and medication side effects.
What is the aim of this review?
To determine how accurate Xpert Ultra is compared with Xpert MTB/RIF for diagnosing pulmonary tuberculosis and rifampicin resistance in adults. An extensive review of Xpert MTB/RIF accuracy was recently published as a Cochrane Review.
What was studied in this review?
We compared the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF with results primarily measured against culture (detection of pulmonary tuberculosis) and drug susceptibility testing and line probe assays (detection of rifampicin resistance).
What are the main results in this review?
Nine studies (3500 participants) compared Xpert Ultra to Xpert MTB/RIF for diagnosing pulmonary tuberculosis, and five studies (930 participants) compared Xpert Ultra to Xpert MTB/RIF for rifampicin resistance.
How confident are we in the results of this review?
Confident. The review included sufficient studies and participants and used optimum reference standards. In the comparison between Xpert Ultra and Xpert MTB/RIF, most studies were at low risk of bias.
Who do the results of this review apply to?
People considered to have pulmonary tuberculosis.
What are the implications of this review?
The results of these studies indicate that, in theory, for a population of 1000 people where 100 of those presenting with symptoms have pulmonary tuberculosis, Xpert Ultra will miss 9 cases, and Xpert MTB/RIF will miss 15 cases. The number of people wrongly diagnosed with pulmonary tuberculosis would be 40 with Xpert Ultra, and 14 with Xpert MTB/RIF.
The results of these studies indicate that, in theory, for a population of 1000 people where 100 of those have rifampicin resistance, Xpert Ultra will miss 5 cases, and Xpert MTB/RIF will miss 5 cases. The number of people wrongly diagnosed with rifampicin resistance would be 8 with Xpert Ultra, and 11 with Xpert MTB/RIF.
How up‐to‐date is this review?
28 January 2020.
Authors' conclusions
Summary of findings
| Review question: what is the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis? Patients/population: adults with presumptive pulmonary tuberculosis. Participants were unselected, meaning they were not enrolled in a study based on microscopy smear results or history of tuberculosis Role: an initial test Index tests: Xpert Ultra and Xpert MTB/RIF Threshold for index tests: an automated result is provided Reference standards: solid or liquid culture Studies: cross‐sectional and cohort studies Setting: primary care facilities and local hospitals Xpert Ultra sensitivity 90.9% (86.2 to 94.7) and specificity 95.6% (93.0 to 97.4) Xpert MTB/RIF sensitivity 84.7% (78.6 to 89.9) and specificity 98.4% (97.0 to 99.3) | ||||||||
| Test result | Number of results per 1000 patients tested (95% CrI)** | Number of participants*** | Certainty of the evidence (GRADE) | |||||
|---|---|---|---|---|---|---|---|---|
| Prevalence 2.5% | Prevalence 10% | Prevalence 30% | ||||||
| Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | |||
| True positives (TP) | 23 (22 to 24) | 21 (20 to 22) | 91 (86 to 95) | 85 (79 to 90) | 273 (259 to 284) | 254 (236 to 270) | 983 (7) | ⊕⊕⊕⊕ High |
| 2 more TP in Xpert Ultra | 6 more TP in Xpert Ultra | 19 more TP in Xpert Ultra | ||||||
| False negatives (FN) | 2 (1 to 3) | 4 (3 to 5) | 9 (5 to 14) | 15 (10 to 21) | 27 (16 to 41) | 46 (30 to 64) | ||
| 2 fewer FN in Xpert Ultra | 6 fewer FN in Xpert Ultra | 19 fewer FN in Xpert Ultra | ||||||
| True negatives (TN) | 932 (907 to 950) | 959 (946 to 968) | 860 (837 to 877) | 886 (873 to 894) | 669 (651 to 682) | 689 (679 to 695) | 1852 (7) | ⊕⊕⊕⊕ High |
| 27 fewer TN in Xpert Ultra | 26 fewer TN in Xpert Ultra | 20 fewer TN in Xpert Ultra | ||||||
| False positives (FP) | 43 (25 to 68) | 16 (7 to 29) | 40 (23 to 63) | 14 (6 to 27) | 31 (18 to 49) | 11 (5 to 21) | ||
| 27 more FP in Xpert Ultra | 26 more FP in Xpert Ultra | 20 more FP in Xpert Ultra | ||||||
| Abbreviations: CrI: credible interval | ||||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||||
| *The results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure. **95% credible limits were estimated based on those around the point estimates for pooled sensitivity and specificity. Prevalence estimates were suggested by the World Health Organization Global Tuberculosis Programme. The median tuberculosis prevalence in the included studies was 30.1% (range 12.8% to 72.2%). ***In the Xpert Ultra analysis there were 1851 participants. Piersimoni 2019 reported three non‐determinate results for Xpert Ultra and two for Xpert MTB/RIF, accounting for the small difference in the total number of participants. | ||||||||
| Review question: what is the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for the detection of rifampicin resistance? Patients/population: adults with presumptive pulmonary tuberculosis Role: an initial test Index tests: Xpert Ultra and Xpert MTB/RIF Threshold for index tests: an automated result is provided Reference standards: drug susceptibility testing, line probe assay Studies: cross‐sectional and cohort studies Setting: primary care facilities and local hospitals Xpert Ultra sensitivity 94.9% (88.9 to 97.9) and specificity 99.1% (97.7 to 99.8) Xpert MTB/RIF sensitivity 95.3% (90.0 to 98.1) and specificity 98.8% (97.2 to 99.6) | ||||||||
| Test result | Number of results per 1000 patients tested (95% CrI)** | Number of participants*** | Certainty of the evidence (GRADE) | |||||
|---|---|---|---|---|---|---|---|---|
| Prevalence 2% | Prevalence 10% | Prevalence 15% | ||||||
| Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | |||
| True positives (TP) | 19 (18 to 20) | 19 (18 to 20) | 95 (89 to 98) | 95 (90 to 98) | 142 (133 to 147) | 143 (135 to 147) | 238 (5) | ⊕⊕⊕⊕ High |
| 0 fewer TP in Xpert Ultra | 0 fewer TP in Xpert Ultra | 1 fewer TP in Xpert Ultra | ||||||
| False negatives (FN) | 1 (0 to 2) | 1 (0 to 2) | 5 (2 to 11) | 5 (2 to 10) | 8 (3 to 18) | 7 (3 to 15) | ||
| 0 fewer FN in Xpert Ultra | 0 fewer FN in Xpert Ultra | 1 more FN in Xpert Ultra | ||||||
| True negatives (TN) | 971 (957 to 977) | 968 (953 to 976) | 892 (879 to 897) | 889 (875 to 896) | 842 (830 to 847) | 840 (826 to 847) | 692 (5) | ⊕⊕⊕⊕ High |
| 3 more TN in Xpert Ultra | 3 more TN in Xpert Ultra | 2 more TN in Xpert Ultra | ||||||
| False positive (FP) | 9 (3 to 23) | 12 (4 to 27) | 8 (3 to 21) | 11 (4 to 25) | 8 (3 to 20) | 10 (3 to 24) | ||
| 3 fewer FP in Xpert Ultra | 3 fewer FP in Xpert Ultra | 2 fewer FP in Xpert Ultra | ||||||
| Abbreviations: CrI: credible interval | ||||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||||
| *The results presented in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure. **Prevalence estimates were suggested by the World Health Organization Global Tuberculosis Programme. The median prevalence of rifampicin resistance in the included studies was 23.6% (range 1.9% to 31.8%). Credible limits were estimated based on those around the point estimates for pooled sensitivity and specificity. ***Xpert Ultra included 921 participants, and Xpert MTB/RIF included 930 participants, mainly owing to indeterminate results with Xpert Ultra. | ||||||||
Background
Tuberculosis is a leading cause of infectious disease‐related death and is one of the top 10 causes of death worldwide (WHO Global tuberculosis report 2020). In 2019, 10 million people developed tuberculosis disease, a number that over the past several years has been decreasing slowly (WHO Global tuberculosis report 2020). Of the 10 million tuberculosis cases, approximately 8% occurred among people living with HIV. When tuberculosis is detected early and effectively treated, the disease is largely curable. However, in 2019, around 1.2 million HIV‐negative people and 208,000 HIV‐positive people died from tuberculosis (WHO Global tuberculosis report 2020). The World Health Organization (WHO) estimates that, from 2000 to 2019, more than 60 million lives were saved by diagnosing and treating tuberculosis. The COVID‐19 pandemic threatens to reverse the gains made in recent years. A modelling study by the WHO suggests that there could have been between 200,000 and 400,000 additional tuberculosis deaths in 2020 if, over a period of three months, 25% to 50% fewer people were detected with and treated for tuberculosis (WHO Global tuberculosis report 2020).
Drug‐resistant tuberculosis is a serious threat to global health. For the purpose of surveillance and treatment, drug‐resistant tuberculosis is classified as rifampicin‐resistant tuberculosis, multidrug‐resistant tuberculosis (MDR‐TB), and extensively drug‐resistant tuberculosis (XDR‐TB). MDR‐TB is defined as resistance to at least isoniazid and rifampicin, the two most important first‐line antituberculosis drugs. XDR‐TB is defined as MDR‐TB plus resistance to at least one drug in the fluoroquinolone class and one of the second‐line injectable agents. In 2019, there were approximately half a million new cases of rifampicin‐resistant tuberculosis (of which 78% had MDR‐TB) worldwide, with India (27%), China (14%), and the Russian Federation (9%) accounting for the largest burden, and 12,350 cases of XDR‐TB (WHO Global tuberculosis report 2020). Globally in 2019, 59% of bacteriologically confirmed new cases were tested for rifampicin resistance, an increase from 51% in 2018 (WHO Global tuberculosis report 2020).
In 2014, the World Health Assembly unanimously approved the WHO End TB Strategy, a 20‐year strategy devised to end the global tuberculosis epidemic (WHO 2015a). Early diagnosis of tuberculosis, including universal drug susceptibility testing and systematic screening of contacts and high‐risk groups, is a key part of the strategy.
The same or similar text appears in the Background and Methods sections in related protocols and reviews (Kay 2020; Kohli 2021; Shapiro 2020; Vonasek 2020).
Target condition being diagnosed
Pulmonary tuberculosis
Tuberculosis is caused by the bacterium Mycobacterium tuberculosis (M tuberculosis) and is spread from person to person through the air (CDC 2020). Tuberculosis most commonly affects the lungs (pulmonary tuberculosis), but may affect any organ or tissue outside of the lungs (extrapulmonary tuberculosis). Signs and symptoms of pulmonary tuberculosis include cough, fever, chills, night sweats, weight loss, haemoptysis (coughing up blood), and fatigue. Signs and symptoms of extrapulmonary tuberculosis depend on the site of disease.
Tuberculosis treatment regimens must contain multiple drugs to which the organisms are sensitive to cure tuberculosis and avoid selection for drug resistance.
Rifampicin resistance
Rifampicin inhibits bacterial DNA‐dependent ribonucleic acid (RNA) polymerase, encoded by the RNA polymerase gene (rpoB) (Hartmann 1967). Resistance to this drug has mainly been associated with mutations in a limited region of the rpoB gene (Telenti 1993). Rifampicin resistance may occur alone or in association with resistance to isoniazid and other drugs. In high MDR‐TB settings, the presence of rifampicin resistance alone may serve as a proxy for MDR‐TB (WHO 2011a). People with drug‐resistant tuberculosis can transmit the infection to others. The drugs used to treat MDR‐TB are less potent and more toxic than the drugs used to treat drug‐susceptible tuberculosis, historically requiring two years or more of therapy. The WHO has issued recommendations that all individuals with MDR‐TB or rifampicin‐resistant tuberculosis, including those who are also resistant to fluoroquinolones, may benefit from effective all‐oral treatment regimens (WHO Consolidated Guidelines (Module 4) 2020).
Index test(s)
Xpert MTB/RIF and Xpert MTB/RIF Ultra (Xpert Ultra, the newest version of Xpert MTB/RIF) (Cepheid Inc, Sunnyvale, USA) are the index tests. The index tests are nucleic acid amplification tests (NAAT; i.e. molecular tests) used for diagnosing tuberculosis and rifampicin‐resistant tuberculosis. Xpert MTB/RIF and Xpert Ultra cartridges are used with the GeneXpert system (Cepheid 2018; Cepheid 2019). Xpert MTB/RIF and Xpert Ultra are able to detect both M tuberculosis complex and rifampicin resistance within two hours after starting the test, with minimal hands‐on technical time. With Xpert MTB/RIF and Xpert Ultra, unlike in conventional NAAT, sample processing and polymerase chain reaction (PCR) amplification and detection are integrated into a single, self‐enclosed test unit, the GeneXpert cartridge. Following sample loading, all steps in the assay are completely automated and self‐contained. In addition, the assays' sample reagent, used to liquefy sputum, has potent tuberculocidal (the ability to kill tuberculosis bacteria) properties and so largely eliminates biosafety concerns during the test procedure (Banada 2010). Except as described below for Ultra trace call results, a single Xpert MTB/RIF or Xpert Ultra run will provide both detection of tuberculosis and detection of rifampicin resistance. One cannot deselect testing for rifampicin resistance and only run the assay for tuberculosis detection.
The development of Xpert MTB/RIF was a major step toward improving detection of tuberculosis and rifampicin resistance globally (Boehme 2010; Small 2011). Since Xpert MTB/RIF was released, there have been four generations (G1, G2, G3, and G4) of the test involving different software and cartridge combinations. Although in comparison with smear microscopy, Xpert MTB/RIF has increased sensitivity for pulmonary tuberculosis (Steingart 2014), the test has suboptimal sensitivity in people with smear‐negative and HIV‐associated tuberculosis. A Cochrane Review on the diagnostic accuracy of Xpert MTB/RIF for pulmonary tuberculosis found pooled sensitivity and specificity (95% credible interval (CrI)) of 85% (82 to 88) and 98% (97 to 98) (70 studies, 37,237 unselected participants; high‐certainty evidence) (Horne 2019). However, Xpert MTB/RIF sensitivity was decreased in people with smear‐negative culture‐positive disease, pooled sensitivity of 67% (62 to 72), and people living with HIV, pooled sensitivity of 81% (75 to 86) (Horne 2019). Xpert MTB/RIF versions have also had some limitations in detecting rifampicin resistance.
In order to overcome these limitations, Cepheid developed Xpert Ultra, a re‐engineered assay using a newly developed cartridge that is run on the same device after a software upgrade. To improve sensitivity for tuberculosis detection, Xpert Ultra incorporates two different multi‐copy amplification targets and a larger DNA reaction chamber than Xpert MTB/RIF (WHO 2017). A laboratory study reported that the limit of detection (the lowest number of colony‐forming units (CFUs) per sample that can be reproducibly distinguished from negative samples with 95% confidence) using Xpert Ultra improved to 15.6 CFU/mL of sputum compared to 112.6 CFU/mL for Xpert MTB/RIF (Chakravorty 2017).
Importantly, Xpert Ultra added a new semiquantitative category for tuberculosis detection that was not present in Xpert MTB/RIF: "trace call" corresponds to the lowest bacillary load for M tuberculosis detection (WHO 2017). This new category is reported as MTB trace DETECTED. No rifampicin resistance results are available (reported as INDETERMINATE) for people with trace results. As with Xpert MTB/RIF, Xpert Ultra detects both live and dead bacteria.
To address limitations in rifampicin resistance detection, Xpert Ultra uses melting temperature‐based analysis, in lieu of real‐time PCR analysis with Xpert MTB/RIF. Melting temperature‐based analysis allows Xpert Ultra to better distinguish resistance‐conferring mutations from silent mutations (Global Laboratory Initiative 2017).
The test procedure may be used directly on clinical specimens, either raw sputum specimens or sputum pellets created after decontaminating and concentrating the sputum (Blakemore 2010). In both cases, the test material is combined with the assay sample reagent (sodium hydroxide and isopropanol), mixed by hand or vortex, and incubated at room temperature for 15 minutes. The reagent:sample volume ratio is 2:1 for unprocessed sputum and 3:1 for sputum pellets. After the incubation step, 2 mL of the treated specimen are transferred to the cartridge and the run is initiated. The manufacturer does not specifically mention the use of the index tests with frozen specimens (Cepheid 2018; Cepheid 2019). As with Xpert MTB/RIF, Xpert Ultra using the GeneXpert system requires an uninterrupted and stable electrical power supply, temperature control, and yearly calibration of the cartridge modules (Global Laboratory Initiative 2019). Like previous Xpert cartridge generations, Xpert Ultra can be performed by operators with minimal technical expertise (Theron 2014b). The time to run the assay is shorter for Xpert Ultra (65 to 87 minutes) than for Xpert MTB/RIF (112 minutes) (Global Laboratory Initiative 2017).
Clinical pathway
Xpert Ultra and Xpert MTB/RIF are used for the diagnosis of tuberculosis and rifampicin resistance. Figure 1 shows the clinical pathway and presents the context in which the index tests might be used. The target condition is pulmonary tuberculosis. Individuals to be evaluated for pulmonary tuberculosis are adults with signs or symptoms suggestive of tuberculosis, such as cough, fever, night sweats, weight loss, haemoptysis, and fatigue, or with an abnormal chest x‐ray suggestive of tuberculosis. Additionally, people who are known to have tuberculosis and are at risk for rifampicin‐resistant tuberculosis or MDR‐TB (e.g. those with a previous history of tuberculosis treatment or those who have an inadequate response to antituberculosis treatment) may undergo Xpert Ultra testing to evaluate for rifampicin resistance.
![The clinical pathway describes how people might present and the point in the pathway at which they would be considered for testing with Xpert MTB/RIF or Xpert Ultra.Abbreviations: DST: drug susceptibility testing; INH: isoniazid; MDR‐TB: multidrug‐resistant tuberculosis; MTB: Mycobacterium tuberculosis; mWRD: molecular WHO‐recommended rapid diagnostic; PLHIV: people living with HIV; RIF: rifampicin; TB: tuberculosis; Ultra: Xpert Ultra; WHO: World Health Organization.1Persons to be evaluated for TB include adults and children with signs or symptoms suggestive of TB, or with a chest X‐ray with abnormalities suggestive of TB. This algorithm may also be followed for the diagnosis of extrapulmonary TB using CSF, lymph node and other tissue specimens.
2Programs may consider collecting two specimens upfront. The first specimen should be promptly tested using the molecular WRD test. The second specimen may be used for the additional testing described in this algorithm. For persons being evaluated for pulmonary TB, sputum is the preferred specimen. Tissue biopsy samples are difficult or impossible to obtain repeatedly; therefore, they should be tested with as many methods as possible (e.g. molecular WRD, culture, DST or histology).
3Molecular WRD tests appropriate for this algorithm include Xpert MTB/RIF, Xpert Ultra, Truenat MTB, Truenat MTB Plus and TB‐LAMP.
4“MTB detected (not trace)” includes MTB detected as high, moderate, low or very low. These categories apply to the original Xpert MTB/RIF and Xpert Ultra tests. Results of the Truenat MTB and MTB Plus tests and the TB‐LAMP test also fall into the category of “MTB detected (not trace)”.Additional footnotes are explained in WHO Consolidated Guidelines (Module 4) 2020.This algorithm for the use of a molecular WHO‐recommended rapid diagnostic (WRD), which includes Xpert Ultra and Xpert MTB/RIF, comes from the WHO operational handbook on tuberculosis (WHO Consolidated Guidelines (Module 4) 2020). Copyright © [2020] [World Health Organization]: reproduced with permission.](/cdsr/doi/10.1002/14651858.CD009593.pub5/media/CDSR/CD009593/image_n/nCD009593-FIG-01.jpg)
The clinical pathway describes how people might present and the point in the pathway at which they would be considered for testing with Xpert MTB/RIF or Xpert Ultra.
Abbreviations: DST: drug susceptibility testing; INH: isoniazid; MDR‐TB: multidrug‐resistant tuberculosis; MTB: Mycobacterium tuberculosis; mWRD: molecular WHO‐recommended rapid diagnostic; PLHIV: people living with HIV; RIF: rifampicin; TB: tuberculosis; Ultra: Xpert Ultra; WHO: World Health Organization.
1Persons to be evaluated for TB include adults and children with signs or symptoms suggestive of TB, or with a chest X‐ray with abnormalities suggestive of TB. This algorithm may also be followed for the diagnosis of extrapulmonary TB using CSF, lymph node and other tissue specimens.
2Programs may consider collecting two specimens upfront. The first specimen should be promptly tested using the molecular WRD test. The second specimen may be used for the additional testing described in this algorithm. For persons being evaluated for pulmonary TB, sputum is the preferred specimen. Tissue biopsy samples are difficult or impossible to obtain repeatedly; therefore, they should be tested with as many methods as possible (e.g. molecular WRD, culture, DST or histology).
3Molecular WRD tests appropriate for this algorithm include Xpert MTB/RIF, Xpert Ultra, Truenat MTB, Truenat MTB Plus and TB‐LAMP.
4“MTB detected (not trace)” includes MTB detected as high, moderate, low or very low. These categories apply to the original Xpert MTB/RIF and Xpert Ultra tests. Results of the Truenat MTB and MTB Plus tests and the TB‐LAMP test also fall into the category of “MTB detected (not trace)”.
Additional footnotes are explained in WHO Consolidated Guidelines (Module 4) 2020.
This algorithm for the use of a molecular WHO‐recommended rapid diagnostic (WRD), which includes Xpert Ultra and Xpert MTB/RIF, comes from the WHO operational handbook on tuberculosis (WHO Consolidated Guidelines (Module 4) 2020). Copyright © [2020] [World Health Organization]: reproduced with permission.
The downstream consequences of testing include the following.
-
True‐positive (TP): patients would benefit from rapid diagnosis and appropriate treatment.
-
True‐negative (TN): patients would be spared unnecessary treatment and would benefit from reassurance and pursuit of an alternative diagnosis.
-
False‐positive (FP): patients would probably experience anxiety and morbidity caused by additional testing, unnecessary treatment, and possible adverse events; possible stigma associated with a tuberculosis or MDR‐TB diagnosis; and the chance that a false‐positive result may halt further diagnostic evaluation.
-
False‐negative (FN): increased risk of morbidity and mortality and delayed treatment initiation; risk of ongoing tuberculosis transmission.
Settings of interest
We were interested in how the index tests performed in people with presumptive pulmonary tuberculosis, who were evaluated as they would be in routine practice, most often in local hospitals or primary care centres. The index tests may have the greatest impact on health when used in a setting such as a primary healthcare facility, where treatment can be started the same day as testing or as soon as possible.
Role of index test(s)
We were interested in the following roles for testing.
I. Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis
Index test used as an initial test replacing smear microscopy and culture for the diagnosis of pulmonary tuberculosis in adults with presumptive pulmonary tuberculosis (WHO Consolidated Guidelines (Module 3) 2020). An initial test does not mean that other tests will follow.
II. Xpert Ultra and Xpert MTB/RIF for the detection of rifampicin resistance
Index test used as an initial test replacing culture and phenotypic drug susceptibility testing for the diagnosis of rifampicin‐resistant tuberculosis in adults with presumptive pulmonary tuberculosis (WHO Consolidated Guidelines (Module 3) 2020).
As mentioned, in high MDR‐TB settings the presence of rifampicin resistance alone may serve as a proxy for MDR‐TB. Xpert Ultra and Xpert MTB/RIF do not eliminate the need for subsequent culture and phenotypic drug susceptibility testing (DST), which are required to monitor treatment progress and to detect resistance to drugs other than rifampicin, respectively.
Alternative test(s)
In this section, we describe selected alternative tests for the detection of pulmonary tuberculosis and rifampicin resistance. For a comprehensive review of alternative tests, we refer the reader to several excellent resources (Branigan 2019; Lewinsohn 2017; Unitaid 2017).
Smear microscopy is the examination of smears for acid‐fast bacilli (tuberculosis bacteria) under a microscope. The examination may be performed by light microscopy (Ziehl‐Neelsen), fluorescence microscopy, or light‐emitting diode (LED) fluorescence microscopy. Advantages of smear microscopy include its simplicity, low cost, speed, and high specificity in high tuberculosis burden areas. In addition, smear microscopy identifies the most infectious people with tuberculosis. Smear microscopy can be performed in basic laboratories. Drawbacks of smear microscopy include the need for specialized training and its relatively low sensitivity, 50% to 60% on average for a direct smear (Steingart 2006b). Around 5000 to 10,000 organisms per millilitre must be present in the specimen for tuberculosis bacteria to be visible by microscopy (American Thoracic Society 2000). Although the sensitivity of microscopy can be improved by approximately 10% with fluorescence (Steingart 2006a), a large number of tuberculosis cases will still go undiagnosed. Smear‐negative tuberculosis is disproportionately higher in HIV‐positive than in HIV‐negative individuals, accounting for 24% to 61% of all pulmonary cases in people living with HIV (Getahun 2007; Perkins 2007). Microscopy cannot distinguish between drug‐susceptible tuberculosis and drug‐resistant tuberculosis. The WHO recommends that microscopy as the initial diagnostic test be replaced with WHO‐recommended rapid tests that can simultaneously detect tuberculosis and tuberculosis drug resistance (WHO Consolidated Guidelines (Module 3) 2020).
Mycobacterial culture is a method used to grow bacteria on nutrient‐rich media. In comparison with microscopy, a positive culture requires only around 100 organisms per millilitre, and therefore can detect lower numbers of tuberculosis bacteria (American Thoracic Society 2000). Additionally, culture is essential for species identification and DST. However, culture is a relatively complex and slow procedure. Solid culture typically takes between four to eight weeks for results, and liquid culture, although more sensitive and rapid than solid culture, requires up to six weeks and is more prone to contamination (WHO 2015b). In addition, culture requires specialized laboratories and highly skilled staff. Culture is the reference standard for pulmonary tuberculosis in this review.
NAAT are molecular systems that can detect small quantities of genetic material (DNA or RNA) from microorganisms, such as M tuberculosis. The key advantage of NAAT is that they are rapid diagnostic tests, potentially providing results in a few hours. A variety of molecular amplification methods are available, of which PCR is the most common. NAAT are available as commercial kits and in‐house tests (based on a protocol developed in a laboratory) and are routinely used in high‐income countries for tuberculosis detection. In‐house PCR is widely used in low‐income countries because these tests are less expensive than commercial kits. However, in‐house PCR is known to produce inconsistent results (Flores 2005). In addition to Xpert MTB/RIF and Xpert Ultra, the WHO recommends Truenat tuberculosis technology (Truenat MTB, MTB Plus and MTB‐RIF Dx assays) (Molbio Diagnostics, Goa, India) to detect tuberculosis and rifampicin‐resistant tuberculosis (WHO Consolidated Guidelines (Module 3) 2020).
Alternative molecular methods for DST include the commercial line probe assays GenoType MTBDRplus assay (MTBDRplus, Hain LifeScience, Nehren, Germany) and the Nipro NTM+MDRTB detection kit 2 (Nipro, Tokyo, Japan), which detect the presence of mutations associated with drug resistance to isoniazid and rifampicin (Nathavitharana 2017). MTBDRplus is the most widely studied line probe assay. Advantages of line probe assays are that they can provide a result for the detection of tuberculosis and drug resistance in one to two days. Drawbacks are that line probe assays are expensive and need to be used in intermediate and central laboratories (Unitaid 2017). The WHO recommends that for individuals with a sputum smear‐positive specimen or a cultured tuberculosis isolate, commercial molecular line probe assays may be used as the initial test instead of phenotypic culture‐based DST to detect resistance to rifampicin and isoniazid (WHO Consolidated Guidelines (Module 3) 2020). Other molecular assays for the detection of tuberculosis and resistance to rifampicin and isoniazid are in development (Walzl 2018).
Alere Determine TB LAM Ag (AlereLAM) (Alere Inc, Waltham, USA) is a commercially available point‐of‐care test for tuberculosis disease (pulmonary and extrapulmonary tuberculosis). The test detects lipoarabinomannan (LAM), a component of the bacterial cell wall, which is present in the urine of some people with tuberculosis. AlereLAM is performed by placing urine on one end of a test strip, with results appearing as a band on the strip if tuberculosis is present. The test is simple, requires no special equipment, and shows results in 25 minutes. This urine test has potential advantages over sputum‐based testing due to the ease of sample collection. The accuracy of urinary LAM detection is improved among people living with HIV with advanced immunosuppression (Bjerrum 2019). The use of AlereLAM in HIV‐positive adult inpatients was shown to reduce mortality in two randomized trials (Gupta‐Wright 2018; Peter 2016). Based on evidence from the randomized trials and a Cochrane Review (Bjerrum 2019), the WHO currently recommends that AlereLAM be used to assist in the diagnosis of active tuberculosis in HIV‐positive adults, adolescents, and children (WHO Consolidated Guidelines (Module 3) 2020). The key change from the WHO 2015 guidelines is broadening the indication for the use of lateral flow LAM among HIV‐positive inpatients with signs and symptoms of active tuberculosis (pulmonary and extrapulmonary); the test is now recommended for all such patients, irrespective of their CD4 count (WHO Consolidated Guidelines (Module 3) 2020).
Fujifilm SILVAMP TB LAM (FujiLAM, co‐developed by Foundation for Innovative New Diagnostics (FIND), Geneva, Switzerland and Fujifilm, Tokyo, Japan) is a new, urine‐based, point‐of‐care test for tuberculosis diagnosis in people living with HIV. In an individual participant data meta‐analysis that included five cohorts of people living with HIV, FujiLAM was found to have superior sensitivity, 70.7% (95% confidence interval 59.0 to 80.8), compared to AlereLAM sensitivity of 42.3% (31.7 to 51.8), against a microbiological reference standard; FujiLAM had lower specificity, 90.9% (87.2 to 93.7), compared to AlereLAM specificity of 95.3% (92.2 to 97.7) (Broger 2020).
Rationale
Xpert Ultra and Xpert MTB/RIF are rapid tests that may provide benefits for patients (earlier diagnosis and the opportunity to begin earlier, appropriate treatment) and for public health (opportunities to interrupt tuberculosis transmission), especially in high tuberculosis burden countries.
Since 2010, the WHO has recommended the use of Xpert MTB/RIF as the preferred initial diagnostic test for people thought to have MDR‐TB or HIV‐associated tuberculosis (strong recommendation, moderate‐certainty evidence) (WHO 2011b). In 2013, the WHO expanded the recommendations, stating that Xpert MTB/RIF may be used rather than conventional microscopy and culture as the initial diagnostic test in all adults suspected of having tuberculosis (conditional recommendation acknowledging resource implications, high‐[certainty] evidence) (WHO 2013). In addition, the WHO recommended that following an Xpert MTB/RIF test that demonstrates rifampicin resistance, subsequent drug susceptibility testing (e.g. using a line probe assay to second‐line drugs) remains essential to detect resistance to drugs other than rifampicin (WHO 2013). In 2017, based on a non‐inferiority analysis of Xpert Ultra compared with Xpert MTB/RIF, the WHO stated that recommendations on the use of Xpert MTB/RIF also apply to the use of Xpert Ultra as the initial diagnostic test for all adults and children with signs and symptoms of tuberculosis (WHO 2017).
In December 2019, the WHO convened a Guideline Development Group to update the recommendations on the use of molecular assays intended as initial tests for the diagnosis of pulmonary and extrapulmonary tuberculosis and rifampicin resistance. To extend the work of our previous Cochrane Review (Horne 2019), we performed this review update to inform updates to WHO policy (WHO Consolidated Guidelines (Module 3) 2020).
Objectives
Primary objectives
To compare the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis and detection of rifampicin resistance in adults with presumptive pulmonary tuberculosis.
Secondary objectives
For detection of pulmonary tuberculosis, to investigate the effects of potential sources of heterogeneity such as smear status, HIV status, and history of tuberculosis on test accuracy.
For detection of rifampicin resistance, to investigate the effect of smear status (smear positive and smear negative) on test accuracy.
To summarize the frequency of Xpert Ultra trace‐positive results.
To estimate the accuracy of Xpert Ultra after repeat testing in those with trace‐positive results.
Methods
Criteria for considering studies for this review
Types of studies
We included cross‐sectional and cohort type diagnostic accuracy studies that directly compared the index tests in participants with presumptive pulmonary tuberculosis. These study designs included paired and randomized comparative accuracy studies. Paired comparative accuracy studies are those in which each participant receives both index tests. Randomized comparative accuracy studies are those which randomly allocate participants to index tests, with each participant receiving only one index test. 'Presumptive pulmonary tuberculosis' refers to a patient who presents with symptoms or signs suggestive of tuberculosis. We included studies where the index tests were evaluated for both pulmonary tuberculosis and rifampicin resistance, pulmonary tuberculosis alone, or rifampicin resistance alone. We also included randomized controlled trials that evaluated the use of the index(s) test on patient health outcomes, but that also reported sensitivity and specificity. Although the study design was a randomized trial for the purpose of determining the impact of the test on participant outcomes, the study design was a cross‐sectional study for the purpose of determining the diagnostic accuracy of the index tests in this review. However, we did not identify any randomized controlled trials. We used abstracts to identify published studies and included these publications if they met our inclusion criteria. We only included studies that reported data comparing the index test(s) to an acceptable reference standard from which we could extract true‐positive (TP), true‐negative (TN), false‐positive (FP), and false‐negative (FN) values. The index tests could be assessed alone or together with other tests.
We included studies that evaluated the index tests in HIV‐positive people irrespective of tuberculosis symptoms, for example HIV‐positive people being assessed for antiretroviral therapy. We included these studies for the following reasons: the risk of developing tuberculosis is much higher in people living with HIV, estimated to be 20 to 37 times higher in HIV‐positive individuals than in HIV‐negative individuals (Getahun 2010); signs and symptoms of tuberculosis in people living with HIV vary, which makes it challenging to determine when to consider a diagnosis of tuberculosis; and many HIV‐positive people in low‐income countries develop tuberculosis as the first manifestation of AIDS.
We excluded case reports and studies with a case‐control design, the latter because these types of studies are prone to bias, particularly studies enrolling participants with severe disease and healthy participants without disease. We excluded studies of the index tests in people with diabetes but without tuberculosis symptoms, and studies designed to find people with active tuberculosis in community settings. We excluded drug resistance surveys.
Participants
We included studies that enrolled adults, aged 15 years or older, with presumptive pulmonary tuberculosis, rifampicin‐resistant tuberculosis, or MDR‐TB. For tuberculosis detection, we were interested in people who were not currently on tuberculosis treatment or those on treatment for less than seven days. Tuberculosis treatment might interfere with the confirmation of tuberculosis on culture (the reference standard for this review). If the treatment status of the participants was unclear, we contacted primary study authors for this information.
We included studies that assessed the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF using sputum and other respiratory specimens, such as fluid obtained from bronchial alveolar lavage and tracheal aspiration, consistent with the intended use of the manufacturer (Cepheid 2018), and studies from all types of health facilities and all laboratory levels (peripheral, intermediate, and central) from all countries. We excluded studies where the age of the participants was unknown.
Index tests
The index tests were Xpert Ultra and Xpert MTB/RIF.
Index test results are automatically generated (i.e. there is a single threshold), and the user is provided with a printable test result as follows.
Xpert Ultra
-
MTB (M tuberculosis) DETECTED HIGH; RIF (rifampicin) Resistance DETECTED
-
MTB DETECTED MEDIUM; RIF Resistance DETECTED
-
MTB DETECTED LOW; RIF Resistance DETECTED
-
MTB DETECTED VERY LOW; RIF Resistance DETECTED
-
MTB DETECTED HIGH; RIF Resistance NOT DETECTED
-
MTB DETECTED MEDIUM; RIF Resistance NOT DETECTED
-
MTB DETECTED LOW; RIF Resistance NOT DETECTED
-
MTB DETECTED VERY LOW; RIF Resistance NOT DETECTED
-
MTB DETECTED HIGH; RIF Resistance INDETERMINATE
-
MTB DETECTED MEDIUM; RIF Resistance INDETERMINATE
-
MTB DETECTED LOW; RIF Resistance INDETERMINATE
-
MTB DETECTED VERY LOW; RIF Resistance INDETERMINATE
-
MTB Trace DETECTED; RIF Resistance INDETERMINATE
-
INVALID (the presence or absence of MTB cannot be determined)
-
ERROR (the presence or absence of MTB cannot be determined)
-
NO RESULT (the presence or absence of MTB cannot be determined)
We considered a trace result to mean MTB (M tuberculosis) DETECTED.
Xpert MTB/RIF
-
MTB (M tuberculosis) DETECTED; RIF (rifampicin) Resistance DETECTED
-
MTB DETECTED; RIF Resistance NOT DETECTED
-
MTB detected; RIF Resistance INDETERMINATE
-
MTB NOT DETECTED
-
INVALID (the presence or absence of MTB cannot be determined)
-
ERROR (the presence or absence of MTB cannot be determined)
-
NO RESULT (the presence or absence of MTB cannot be determined
Target conditions
The target conditions were active pulmonary tuberculosis and rifampicin resistance.
Reference standards
For pulmonary tuberculosis, the reference standards were solid culture or automated liquid culture.
-
Pulmonary tuberculosis present was defined as a positive M tuberculosis culture.
-
Pulmonary tuberculosis absent was defined as a negative M tuberculosis culture.
We also included a composite reference standard. The diagnosis of pulmonary tuberculosis was defined as a positive culture or clinical criteria specified by the primary study authors. Clinical criteria might include cough longer than two weeks, fever, night sweats, or weight loss and radiographic findings consistent with pulmonary tuberculosis.
-
Pulmonary tuberculosis present was defined as a positive M tuberculosis culture or meeting composite reference standard criteria.
-
Pulmonary tuberculosis absent was defined as a negative M tuberculosis culture and not meeting composite reference standard criteria.
For rifampicin resistance, the reference standards were culture‐based drug susceptibility testing (DST), and line probe assays (LPA) (WHO Consolidated Guidelines (Module 3) 2020). Acceptable methods for DST are the proportion method, performed on solid media, such as Lowenstein‐Jensen, and use of a commercial liquid culture system, such as Mycobacteria Growth Indicator Tube (MGIT) 960 automated mycobacterial detection system (BD, USA).
-
Rifampicin resistance present was defined as a positive culture‐based DST (or LPA) result for resistance.
-
Rifampicin resistance absent was defined as a negative culture‐based DST (or LPA) result for resistance (i.e. rifampicin susceptible).
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and ongoing).
Electronic searches
We searched the following databases on 11 October 2018, 23 August 2019, and 28 January 2020, using the search terms and strategy described in Appendix 1:
Cochrane Infectious Diseases Group Specialized Register; MEDLINE (Ovid, from 1966); Embase (Ovid, from 1974); Science Citation Index ‐ Expanded (from 1900), Conference Proceedings Citation Index ‐ Science (CPCI‐S, from 1990), and BIOSIS Previews (from 1926); all three from the Web of Science; Scopus (Elsevier, from 1970); Latin American Caribbean Health Sciences Literature database (LILACS) (BIREME, from 1982). We also searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov), the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/trialsearch), and the ISRCTN registry (www.isrctn.com/) for trials in progress, and ProQuest Dissertations & Theses A&I (from 1990) for dissertations.
In order to identify other systematic reviews and meta‐analyses, we performed additional searches on 28 May 2020 in MEDLINE (PubMed), Embase (Ovid), and the Cochrane Library, applying filters for systematic reviews (https://www.sign.ac.uk/what-we-do/methodology/search-filters/) to search terms for Xpert and tuberculosis.
Searching other resources
We reviewed the reference lists of included articles and any relevant review articles identified through the above methods. We also contacted researchers at the Foundation for Innovative New Diagnostics (FIND), the WHO Global Tuberculosis Programme, and other experts in the field of tuberculosis diagnostics for information on ongoing and unpublished studies.
Data collection and analysis
Selection of studies
We used Covidence to manage the selection of studies (Covidence). Two review authors independently scrutinized titles and abstracts identified from literature searching to identify potentially eligible studies. We retrieved the article of any citation identified by any review author for full‐text review. Two review authors independently assessed the full‐text articles for inclusion using predefined inclusion and exclusion criteria, resolving any discrepancies by discussion with a third review author. We recorded all studies excluded after full‐text assessment and their reasons for exclusion in the Characteristics of excluded studies table. We illustrated the study selection process in a PRISMA diagram (Moher 2009). We included search results from the original review and re‐evaluated previously included studies to determine if the studies met the refined inclusion criteria.
Data extraction and management
We extracted data on the following characteristics (Appendix 2).
-
Author, publication year, study design, country where the study was located, level of laboratory services, clinical setting (outpatient, inpatient, or both outpatient and inpatient), and whether the test was run at point of care
-
Population characteristics: age, gender, smear status, HIV status
-
Index test(s), Xpert Ultra and Xpert MTB/RIF
-
Reference standard
-
Condition of the specimen (fresh or frozen)
-
Quality Assessment of Studies of Diagnostic Accuracy ‐ Revised (QUADAS‐2) items, Whiting 2011, and QUADAS‐C, Yang B 2020
-
Number of TP, FP, FN, TN (i.e. true positives, false positives, false negatives, true negatives) and trace results
-
Number of non‐determinate results for the detection of pulmonary tuberculosis
-
Number of indeterminate results for the detection of rifampicin resistance
We classified country income status as either low‐ and middle‐income or high‐income, according to the World Bank List of Economies (World Bank 2020). In addition, we classified ‘country' as being high burden or not high burden for tuberculosis, TB/HIV, or MDR‐TB, according to the WHO post‐2015 era classification (WHO Global tuberculosis report 2020). A country could be classified as high burden for one, two, or all three of the high‐burden categories.
Although the manufacturer recommends use of fresh specimens, several studies used frozen specimens, so we also extracted this information. We investigated the influence of condition of specimen in a sensitivity analysis.
Regarding the definition of smear positivity, as most of the included studies performed the index tests in intermediate‐level or central‐level laboratories, we assumed that these studies adhered to the revised definition of a new sputum smear‐positive pulmonary tuberculosis case based on the presence of at least one acid‐fast bacillus in at least one sputum sample in countries with a well‐functioning external quality assurance system (WHO 2007).
We followed Cochrane policy, which states that "authors of primary studies will not extract data from their own study or studies. Instead, another author will extract these data, and check the interpretation against the study report and any available study registration details or protocol".
Assessment of methodological quality
We used the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool to assess the quality of the included studies (Whiting 2011). QUADAS‐2 consists of four domains: patient selection, index test, reference standard, and flow and timing. We assessed all domains for risk of bias and the first three domains for concerns regarding applicability. Two review authors, working independently, completed QUADAS‐2, resolving any disagreements through discussion. We have presented the results of this quality assessment in text, tables, and graphs. In addition, we used QUADAS‐C (C stands for comparison) to assess risk of bias in the included studies. QUADAS‐C was designed to be an extension to QUADAS‐2, with a set of additional questions. QUADAS‐C results in separate 'Risk of bias' judgements for comparative accuracy studies. QUADAS‐C assesses risk of bias in the same four domains as QUADAS‐2: (1) patient selection, (2) index tests, (3) reference standard, and (4) flow and timing, but does not assess applicability concerns. The version of QUADAS‐C used in this review (v2019.10.10) is a preliminary version which may be revised further (Yang B 2020). QUADAS‐2 and QUADAS‐C tools tailored to this review are described in Appendix 3.
Statistical analysis and data synthesis
We performed descriptive analyses for the results of the included studies using Stata 15 (Stata 2017). We determined sensitivity and specificity estimates and 95% confidence intervals (CIs) for individual studies and generated forest plots using Review Manager 5 (Review Manager 2020). Whenever possible, we included nontuberculous mycobacteria (NTM) as non‐tuberculosis for specificity determinations. We chose to use data that were not subject to discrepant analyses (unresolved data) owing to the potential for bias (Hadgu 2005).
We carried out meta‐analyses to estimate the pooled sensitivity and specificity of the index tests separately for tuberculosis detection and rifampicin resistance detection. We performed analyses separately by reference standard. Whenever possible, we determined pooled estimates using an adaptation of the bivariate random‐effects model of Reitsma 2005, which uses the exact binomial likelihood for the observed proportions (Chu 2006). The bivariate random‐effects approach allowed us to calculate the pooled estimates of sensitivity and specificity while dealing with potential sources of variation caused by (1) imprecision of sensitivity and specificity estimates within individual studies; (2) correlation between sensitivity and specificity across studies; and (3) variation in sensitivity and specificity between studies. For Xpert Ultra and Xpert MTB/RIF for detection of pulmonary tuberculosis among smear‐positive individuals (described below), we performed a univariate analysis. For the primary analysis of Xpert Ultra versus Xpert MTB/RIF for tuberculosis detection, we estimated accuracy using studies that did not preselect participants based on prior microscopy testing or that primarily included participants with a history of tuberculosis. In addition, we determined predictive values at a pretest probability of 10%, a value suggested by the WHO.
Rifampicin resistance detection
For analysis of Xpert Ultra or Xpert MTB/RIF accuracy for detection of rifampicin resistance, we included participants who
-
were culture‐positive;
-
had a valid phenotypic DST or LPA result;
-
were Xpert Ultra or Xpert MTB/RIF tuberculosis‐positive; and
-
had a valid Xpert Ultra or Xpert MTB/RIF result for rifampicin resistance, detected or not detected (susceptible).
Sensitivity = Xpert Ultra (or Xpert MTB/RIF) rifampicin resistance detected/phenotypic DST or LPA rifampicin‐resistant.
Specificity = Xpert Ultra (or Xpert MTB/RIF) rifampicin resistance not detected/phenotypic DST or LPA rifampicin‐susceptible.
Comparison of Xpert Ultra and Xpert MTB/RIF
We performed meta‐analyses of the accuracy of Xpert Ultra and Xpert MTB/RIF in studies that made direct comparisons between Xpert Ultra versus Xpert MTB/RIF (Takwoingi 2013). We extracted the median and the 95% credible interval (CrI) for all parameters of interest from samples of the posterior distributions. The 95% CrI is the Bayesian equivalent of the classical (frequentist) 95% confidence interval (CI). We compared the accuracy of Xpert Ultra versus Xpert MTB/RIF by estimating the difference in their pooled sensitivities and the difference in their pooled specificities and calculated the probability that Xpert Ultra accuracy exceeds (or is less than) that of Xpert MTB/RIF accuracy.
We estimated all models using a Bayesian approach with low‐information prior distributions using OpenBUGS software (Version 3.2.3) (Lunn 2009) and R (R Core Team 2019). Under the Bayesian approach, all unknown parameters must be provided a prior distribution that defines the range of possible values of the parameter and the likelihood of each of those values based on information external to the data. In order to let the observed data determine the final results, we chose to use low‐information prior distributions over the pooled sensitivity and specificity parameters and their between‐study standard deviation parameters. We summarize the model and the OpenBUGS program we used to implement it in the Statistical Appendix (Appendix 4). As meta‐analysis models may be sensitive to the choice of prior distributions over between‐study standard deviation parameters, we performed sensitivity analyses using alternative prior distributions that are less informative, allowing a wider range of possible values. We noted no appreciable change in pooled accuracy parameters but, as expected, found that the posterior credible intervals and prediction intervals were slightly wider. Information from the prior distribution is combined with the likelihood of the observed data in accordance with Bayes theorem to obtain a posterior distribution for each unknown parameter (Appendix 5).
Using a sample from the posterior distribution, we can obtain various descriptive statistics of interest. We estimated the median pooled sensitivity and specificity and their 95% CrIs. The median or the 50% quantile is the value below which lies 50% of the posterior sample. We reported the median because the posterior distributions of some parameters may be skewed, and the median would be considered a better point estimate of the unknown parameter than the mean in such cases. The 95% CrI is the Bayesian equivalent of the classical (frequentist) 95% CI. (We have indicated 95% CI for individual study estimates and 95% CrI for pooled study estimates, as appropriate). The 95% CrI may be interpreted as an interval that has a 95% probability of capturing the true value of the unknown parameter, given the observed data and the prior information.
We generated plots using R (R Core Team 2019).
Approach to inconclusive index test results
The index tests report an inconclusive test result for unexpected results. The proportion of inconclusive (non‐determinate) results for the detection of pulmonary tuberculosis is the number of tests classified as INVALID, ERROR, or NO RESULT divided by the total number of index tests performed. The proportion of inconclusive (indeterminate) results for the detection of rifampicin resistance is the number of tests classified as MTB DETECTED; RIF (rifampicin) resistance INDETERMINATE divided by the total number of index test‐positive results. We used a Bayesian hierarchical model for a single proportion to estimate the pooled proportion of inconclusive tests results. For participants with trace results on Xpert Ultra, rifampicin resistance is always reported as INDETERMINATE. As we found very few inconclusive results reported, we excluded these results from the quantitative analysis and separately reported the pooled proportion of non‐determinate and indeterminate index test results. In addition, we compared the pooled proportion (expressed as a percentage) of indeterminate results for Xpert Ultra versus Xpert MTB/RIF by estimating the difference in their pooled proportions with the probability that these differences exceed zero.
Investigations of heterogeneity
We visually inspected forest plots and summary receiver operating characteristics (SROC) plots to explore heterogeneity in the sensitivity and specificity estimates for Xpert Ultra and Xpert MTB/RIF. We performed the following subgroup analyses.
Detection of pulmonary tuberculosis
For the detection of pulmonary tuberculosis, we performed comparative analyses for Xpert Ultra versus Xpert MTB/RIF with respect to smear status (smear negative and smear positive), HIV status (positive and negative), and history of tuberculosis (yes or no). We performed these analyses by fitting a bivariate model to each subgroup. We extracted the median and the 95% CrI for the difference in the pooled sensitivities and the difference in the pooled specificities, respectively, of Xpert Ultra versus Xpert MTB/RIF. When there were at least four studies in a subgroup, we also calculated the probability that the difference exceeds zero in each case.
Among smear‐positive individuals, we performed a univariate analysis because in several studies the value for true negatives plus false positives was zero, and specificity was inestimable.
Detection of rifampicin resistance
For the detection of rifampicin resistance, we compared Xpert Ultra and Xpert MTB/RIF accuracy with respect to smear status (smear positive and smear negative).
Xpert Ultra trace results
Summary of Xpert Ultra trace‐positive results and repeated testing of Ultra trace specimens
Xpert Ultra added a new result category, trace, that corresponds to the lowest bacillary load for M tuberculosis detection (WHO 2017). This new category is reported as MTB trace DETECTED. We summarized the frequency of Xpert Ultra trace‐positive results, as well as the frequency of trace results in individuals with a history of tuberculosis. We also summarized the accuracy of Xpert Ultra repeated test for diagnosing pulmonary tuberculosis in people who have an initial Ultra trace result.
Nontuberculous mycobacteria
NTM, such as M avium complex and M abscessus, constitute a multi‐species group of environmental mycobacteria that can cause pulmonary disease in humans that clinically resembles tuberculosis. People living with HIV with severe immunosuppression are particularly vulnerable to infections caused by NTM (Gopinath 2010). Previous studies have shown that Xpert MTB/RIF does not cross‐react with other mycobacterial species (Helb 2010). We summarized data for NTM separately by determining the proportion (expressed as a percentage) of false‐positive Xpert Ultra and Xpert MTB/RIF results in specimens that grew NTMs.
Sensitivity analyses
For Xpert Ultra for detection of pulmonary tuberculosis, we performed sensitivity analyses by limiting inclusion in the meta‐analysis based on the following criteria.
-
Studies that included only untreated participants. We excluded studies that did not explicitly state that they included only untreated participants.
-
Studies that used liquid culture as the reference standard.
-
Studies where a consecutive or random sample of participants was enrolled.
-
Studies where the reference standard was blinded.
-
Studies that only used fresh specimens.
-
Studies that accounted for all participants in the analysis. We excluded studies where we answered no or unclear to the QUADAS‐2 flow and timing signalling question: Were all patients included in the analysis?
We did not perform sensitivity analyses for Xpert MTB/RIF, as we performed these analyses in the previous update of this review. Most of these analyses included greater than 50 studies (Horne 2019).
Assessment of reporting bias
We chose not to carry out formal assessments of publication bias using methods such as funnel plots or regression tests, because such techniques have not been helpful for diagnostic test accuracy studies (Macaskill 2010). As Xpert Ultra and Xpert MTB/RIF are produced by only one manufacturer and subjected to considerable scrutiny, we believe that reporting bias was minimal.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of evidence using the GRADE approach for diagnostic studies (Balshem 2011; Schünemann 2008; Schünemann 2016). As recommended, we rated the certainty of the evidence as either high (not downgraded), moderate (downgraded by one level), low (downgraded by two levels), or very low (downgraded by more than two levels) based on five domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. For each outcome, the certainty of evidence started as high when there were high‐quality studies (cross‐sectional or cohort studies) that enrolled participants with diagnostic uncertainty. If there was a reason for downgrading, we used our judgement to classify the reason as either serious (downgraded by one level) or very serious (downgraded by two levels). Two review authors discussed the judgements of the certainty of the evidence and applied GRADE in the following way (GRADEpro GDT; Schünemann 2020a; Schünemann 2020b).
-
Risk of bias: we used QUADAS‐2 to assess risk of bias.
-
Indirectness: we assessed indirectness in relation to the population (including disease spectrum), setting, interventions, and outcomes (accuracy measures). We also used prevalence as a guide to whether there was indirectness in the population.
-
Inconsistency: GRADE recommends downgrading for unexplained inconsistency in sensitivity and specificity estimates. We carried out prespecified analyses to investigate potential sources of heterogeneity and did not downgrade when we believed we could explain inconsistency in the accuracy estimates.
-
Imprecision: we considered a precise estimate to be one that would allow a clinically meaningful decision. We considered the width of the CrI and asked ourselves, would we make a different decision if the lower or upper boundary of the CrI represented the truth? In addition, we worked out projected ranges for TP, FN, TN, and FP for a given prevalence of tuberculosis and made judgements on imprecision from these calculations.
-
Publication bias: we rated publication bias as undetected (not serious). We considered the comprehensiveness of the literature search and outreach to researchers in tuberculosis; the presence of only studies that produce precise estimates of high accuracy despite small sample size; and knowledge about studies that were conducted, but are not published.
Results
Results of the search
We identified and screened a total of 1054 records for inclusion in this review update. Of these, we assessed 74 full‐text papers against our inclusion criteria. We excluded 67 papers for the following reasons: Xpert Ultra not evaluated (n = 52), extrapulmonary tuberculosis (n = 5), paediatric population (n = 3), case‐control study (n = 2), inappropriate reference standard (n = 1), only culture‐positive specimens were tested and Xpert MTB/RIF not evaluated (n = 1), did not include respiratory specimens (n = 1), community‐based screening (n = 1), and could not obtain full text (n = 1).
We identified eight eligible publications including nine unique studies; one publication contributed two distinct cohorts (Mishra 2020a; Mishra 2020b). All included studies compared Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis (Berhanu 2018; Chakravorty 2017; Dorman 2018; Mishra 2020a; Mishra 2020b; Opota 2019; Pereira 2020; Piersimoni 2019; Wang 2019). Of the total nine studies, five studies compared Xpert Ultra and Xpert MTB/RIF for the detection of rifampicin resistance (Chakravorty 2017; Dorman 2018; Mishra 2020b; Piersimoni 2019; Wang 2019). Figure 2 shows the flow of studies in the review. We recorded the excluded studies, including selected studies from the previous Cochrane Review (Horne 2019), and the reasons for their exclusion in the Characteristics of excluded studies table.

PRISMA flow diagram of studies in the review.
*One publication contributed two distinct studies, which were classified as Mishra 2020a and Mishra 2020b.
Methodological quality of included studies
Studies evaluating Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis
QUADAS‐2
Figure 3 shows the risk of bias and applicability concerns for nine studies evaluating Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis.
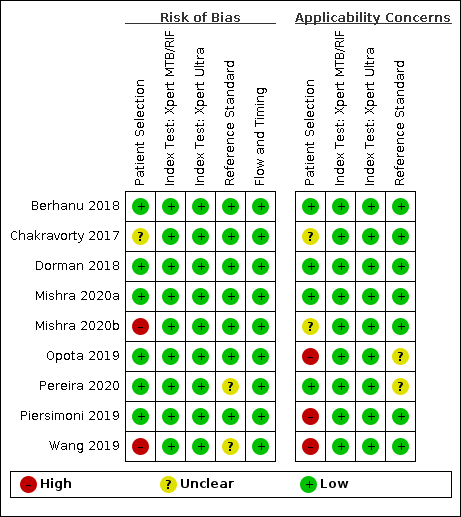
Risk of bias and applicability concerns summary for detection of pulmonary tuberculosis: review authors' judgements about each domain for each included study.
In the patient selection domain, we considered six studies (67%) to have low risk of bias because the study enrolled a consecutive or random sample of eligible participants and avoided inappropriate exclusions (Berhanu 2018; Dorman 2018; Mishra 2020a; Opota 2019; Pereira 2020; Piersimoni 2019). We considered two studies (22%) to have high risk of bias: one study exclusively enrolled participants who had recently received tuberculosis treatment (Mishra 2020b), and one study exclusively enrolled smear‐negative participants (Wang 2019). We considered one study to have unclear risk of bias because the manner of patient selection was not reported (Chakravorty 2017). With respect to applicability, we considered four studies (44%) to have low concern because participants in these studies were evaluated in primary care facilities, local hospitals, or both settings (Berhanu 2018; Dorman 2018; Mishra 2020a; Pereira 2020). We considered three studies (33%) to have high concern: two studies because participants were evaluated exclusively as inpatients in tertiary care centres (Piersimoni 2019; Wang 2019), and one study because the setting was a hospital performing a laboratory‐based evaluation for the purpose of airborne isolation (Opota 2019). We considered two studies (22%) to have unclear concern because we could not tell (Chakravorty 2017; Mishra 2020b).
In the index test domain, we considered all studies to have low risk of bias because the results of the index tests (Xpert Ultra and Xpert MTB/RIF) are automatically generated. Regarding applicability, we considered all studies to have low concern.
In the reference standard domain, we considered seven studies (78%) to have low risk of bias because the results of the reference standard were interpreted without knowledge of the results of the index test (Berhanu 2018; Chakravorty 2017; Dorman 2018; Mishra 2020a; Mishra 2020b; Opota 2019; Piersimoni 2019). We considered two studies (22%) to have unclear risk of bias because information about blinding was not reported (Pereira 2020; Wang 2019). Regarding applicability, we considered seven studies (78%) to have low concern because these studies performed a test to identify M tuberculosis species (speciation) (Berhanu 2018; Chakravorty 2017; Dorman 2018; Mishra 2020a; Mishra 2020b; Piersimoni 2019; Wang 2019), and two studies (22%) to have unclear concern because we could not tell (Opota 2019; Pereira 2020).
In the flow and timing domain, we considered all studies (100%) to have low risk of bias because all participants were included in the analysis.
QUADAS‐C
Appendix 6 shows risk of bias for nine studies comparing Xpert Ultra and Xpert MTB/RIF. Seven studies used a paired diagnostic accuracy design (Chakravorty 2017; Dorman 2018; Mishra 2020a; Opota 2019; Pereira 2020; Piersimoni 2019; Wang 2019), and two studies used a randomized design (Berhanu 2018; Mishra 2020b).
In the patient selection domain, we considered six studies (78%) to have low risk of bias: in five studies participants were consecutively enrolled (Dorman 2018; Mishra 2020a; Opota 2019; Pereira 2020; Piersimoni 2019), and in one study participants were randomly enrolled (Berhanu 2018). In Berhanu 2018, all participants received Xpert MTB/RIF, and the order by which participants were selected to receive Xpert Ultra or a third index test (not included in this review) was randomized. We considered three studies (33%) to have high risk of bias: one study did not report the manner of participant selection (Chakravorty 2017); one study exclusively enrolled participants who had recently received tuberculosis treatment (Mishra 2020b); and one study exclusively enrolled smear‐negative participants (Wang 2019).
In the index test domain, we judged low risk of bias for all studies.
In the reference standard domain, we considered seven studies (78%) to have low risk of bias because the results of the reference standard were interpreted without knowledge of the results of the index test (Berhanu 2018; Chakravorty 2017; Dorman 2018; Mishra 2020a; Mishra 2020b; Opota 2019; Piersimoni 2019). We considered two studies (22%) to have unclear risk of bias because information about blinding was not reported (Pereira 2020; Wang 2019).
In the flow and timing domain, we judged low risk of bias for all studies.
Studies evaluating Xpert Ultra and Xpert MTB/RIF for the detection of rifampicin resistance
QUADAS‐2
Figure 4 shows risk of bias and applicability concerns for the five studies evaluating Xpert Ultra and Xpert MTB/RIF for rifampicin resistance detection.

Risk of bias and applicability concerns summary for detection of rifampicin resistance: review authors' judgements about each domain for each included study.
In the patient selection domain, we considered two studies (40%) to have low risk of bias because the studies enrolled a consecutive or random sample of eligible participants and avoided inappropriate exclusions (Dorman 2018; Piersimoni 2019). We considered two studies (40%) to have high risk of bias: one study exclusively enrolled participants who had recently received tuberculosis treatment (Mishra 2020b), and one study preselected participants on the basis of their sputum specimens being paucibacillary (smear‐negative) (Wang 2019). We considered one study (20%) to have unclear risk of bias because the manner of participant selection was not reported (Chakravorty 2017). Regarding applicability, we considered one study (20%) to have low concern because participants in this study were evaluated in primary care facilities and local hospitals (Dorman 2018). We considered two studies (40%) to have high concern because participants were evaluated exclusively as inpatients in tertiary care centres (Piersimoni 2019; Wang 2019). We considered the remaining two studies (40%) to have unclear concern because we could not tell (Chakravorty 2017; Mishra 2020b).
In the index test domain, we considered all studies to have low risk of bias because the results of the index tests (Xpert Ultra and Xpert MTB/RIF) are automatically generated; the user is provided with printable test results; and the test threshold is prespecified. Regarding applicability, with respect to both Xpert Ultra and Xpert MTB/RIF, we considered all studies to have low concern.
In the reference standard domain, we considered four studies (80%) to have low risk of bias because the results of the reference standard were interpreted without knowledge of the results of the index test (Chakravorty 2017; Dorman 2018; Mishra 2020b; Piersimoni 2019). We considered one study (20%) to have unclear risk of bias because information on blinding was not reported (Wang 2019). With respect to applicability in the reference standard domain, we considered all studies to have low concern because all specimens had already been speciated and identified as M tuberculosis in these studies.
In the flow and timing domain, we considered four studies (80%) to have low risk of bias because all participants were included in the analysis. We considered one study to have unclear risk of bias because, in comparison to Xpert MTB/RIF, Xpert Ultra had a higher number of rifampicin resistance indeterminate results which were not included in the accuracy estimates (Mishra 2020b).
QUADAS‐C
Appendix 7 shows risk of bias for five studies comparing Xpert Ultra and Xpert MTB/RIF. Four studies used a paired diagnostic accuracy design (Chakravorty 2017; Dorman 2018; Piersimoni 2019; Wang 2019), and one study used a randomized design (Mishra 2020b).
In the patient selection domain, we considered two studies (40%) to have low risk of bias (Dorman 2018; Piersimoni 2019); and three studies to have high risk of bias: one study did not report the manner of participant selection (Chakravorty 2017); one study exclusively enrolled participants who had recently received tuberculosis treatment (Mishra 2020b); and one study preselected paucibacillary specimens (Wang 2019).
In the index test domain, we considered all studies (100%) to have low risk of bias.
In the reference standard domain, we considered four studies (80%) to have low risk of bias (Chakravorty 2017; Dorman 2018; Mishra 2020b; Piersimoni 2019), and one study to have unclear risk of bias because information on blinding was not reported (Wang 2019).
In the flow and timing domain, we considered four studies (80%) to have low risk of bias because all participants were included in the analysis (Chakravorty 2017; Dorman 2018; Piersimoni 2019; Wang 2019). We considered one study to have unclear risk of bias because, in comparison with Xpert MTB/RIF, Xpert Ultra had a higher number of rifampicin resistance indeterminate results which were not included in the accuracy estimates (Mishra 2020b).
Findings
Of the total nine studies, seven (78%) were conducted in low‐ or middle‐income countries, and seven (78%) were mainly or exclusively conducted in high tuberculosis burden countries. Seven studies (78%) reported the HIV status of participants (Berhanu 2018; Dorman 2018; Mishra 2020a; Mishra 2020b; Pereira 2020; Piersimoni 2019; Wang 2019), which ranged from 0%, Wang 2019, to 62%, Berhanu 2018. Four studies (44%) evaluated only fresh specimens (Berhanu 2018; Dorman 2018; Mishra 2020a; Pereira 2020); two studies (22%) evaluated only archived frozen samples (Mishra 2020b; Piersimoni 2019); and three studies (33%) evaluated both fresh and frozen specimens (Chakravorty 2017; Opota 2019; Wang 2019). For the culture reference standard, one study used only solid culture (Pereira 2020); five studies (56%) used only liquid culture (Dorman 2018; Mishra 2020a; Mishra 2020b;Opota 2019; Piersimoni 2019); and three studies (33%) used both solid and liquid cultures (Berhanu 2018; Chakravorty 2017; Wang 2019). Key characteristics of the included studies are described in the Characteristics of included studies table and Table 1.
| Study, year ID | Country | Study design | Number of participants | Age (mean or median; years) | Female sex | HIV‐positive | History of tuberculosis | Pulmonary tuberculosis reference standard | Rifampicin resistance reference standard |
|---|---|---|---|---|---|---|---|---|---|
| South Africa | Prospective cohort | 237 | 36 | 33% | 62% | 18% | LJ and MGIT; composite | LJ, MGIT, and MTBDRplus | |
| FIND biobank frozen specimens (Peru, Vietnam, South Africa) and clinical specimens (Georgia, India) | Cross‐sectional | 277 | Not reported | Not reported | Not reported | Not reported | LJ and MGIT | LJ and MGIT | |
| Belarus, Brazil, China, Georgia, India, Kenya, South Africa, Uganda | Prospective cohort | 1439 for detection of MTB, 551 for detection of rifampicin resistance | 28 | 40% | 44% | 21% | LJ and MGIT | LJ and MGIT | |
| South Africa | Prospective cohort | 239 | 37 | 49% | 20% | 39% | MGIT | MTBDRplus | |
| South Africa | Cross‐sectional | 346 | 38 | 40% | 44% | 100% | MGIT | MTBDRplus | |
| Switzerland | Cross‐sectional | 196 | Not reported | Not reported | Not reported | Not reported | MGIT; composite | MGIT | |
| Brazil | Cross‐sectional | 180 | 50 | 44% | 2% | 0% | Ogawa‐Kudoh | N/A | |
| Italy | Cross‐sectional | 266 | 42 | 37% | Not reported | Excluded | MGIT | MGIT | |
| China | Prospective cohort | 498 | 47 | 34% | 0% | 50% | LJ and MGIT | LJ |
Abbreviations: FIND: Foundation for Innovative New Diagnostics; LJ: Löwenstein–Jensen; MGIT: Mycobacteria Growth Indicator Tube; MTB; Mycobacterium tuberculosis; N/A: not applicable.
I. Xpert Ultra versus Xpert MTB/RIF for the detection of pulmonary tuberculosis
We identified seven studies that compared Xpert Ultra and Xpert MTB/RIF in unselected participants against culture (Berhanu 2018; Chakravorty 2017; Dorman 2018; Mishra 2020a; Opota 2019; Pereira 2020; Piersimoni 2019). The median sample size was 239 (interquartile range (IQR) 217 to 272). The prevalence of pulmonary tuberculosis in the studies ranged from 12.8% to 72.2%. The sensitivity of Xpert Ultra ranged from 86% to 100%, and the sensitivity of Xpert MTB/RIF from 81% to 100%. The specificity of Xpert Ultra ranged from 89% to 99%, and the specificity of Xpert MTB/RIF from 94% to 100% (Figure 5).

Forest plots of Xpert Ultra versus Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in adults, unselected participants by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative
Xpert Ultra pooled sensitivity and specificity (95% CrI) were 90.9% (86.2 to 94.7) and 95.6% (93.0 to 97.4) (2834 participants, 983 (34.7%) with tuberculosis); Xpert MTB/RIF pooled sensitivity and specificity were 84.7% (78.6 to 89.9) and 98.4% (97.0 to 99.3) (2835 participants, 983 (34.7%) with tuberculosis) (Table 2). Piersimoni 2019 reported three non‐determinate results for Xpert Ultra and two for Xpert MTB/RIF, accounting for the small difference in the total number of participants in this analysis. The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at 6.3% (0.1 to 12.8) for sensitivity and −2.7% (−5.7 to −0.5) for specificity. We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF as 0.98. We estimated the probability that the pooled specificity of Xpert Ultra was less than that of Xpert MTB/RIF as 0.99 (Table 3).
| Test (analysis) | Reference standard | No. studies (participants) | No. (%) with pulmonary TB or rifampicin resistance | Median pooled sensitivity | Median pooled specificity | Positive predictive value (95% CrI) * | Negative predictive value |
|---|---|---|---|---|---|---|---|
| Xpert Ultra, unselected participants* (pulmonary tuberculosis detection) | Culture | 7 (2834)** | 983 (34.7%) | 90.9% (86.2 to 94.7) | 95.6% (93.0 to 97.4) | 69.6% (58.7 to 79.8) | 99.0% (98.4 to 99.4) |
| Xpert MTB/RIF (pulmonary tuberculosis detection) | Culture | 7 (2835) | 983 (34.7%) | 84.7% (78.6 to 89.9) | 98.4% (97.0 to 99.3) | 85.4% (75.8 to 93.1) | 98.3% (97.6 to 98.9) |
| Xpert Ultra (rifampicin resistance detection) | DST, line probe assays | 5 (921) | 240 (26.1%) | 94.9% (88.9 to 97.9) | 99.1% (97.7 to 99.8) | 91.7% (82.1 to 97.4) | 99.4% (98.7 to 99.8) |
| Xpert MTB/RIF (rifampicin resistance detection) | DST, line probe assays | 5 (930) | 238 (25.6%) | 95.3% (90.0 to 98.1) | 98.8% (97.2 to 99.6) | 99.5% (98.9 to 99.8) | 99.4% (98.7 to 99.8) |
Abbreviations: CrI: credible interval; DST: drug susceptibility testing with solid or liquid culture methods
* Positive and negative predictive values were determined at a pretest probability of 10%
**This analysis included studies that did not preselect participants based on microcopy results or those who had received previous antituberculosis treatment.
***Piersimoni 2019 reported three non‐determinate results for Xpert Ultra and two for Xpert MTB/RIF, accounting for the small difference in the total number of participants in this analysis.
| Detection of pulmonary tuberculosis | ||||
|---|---|---|---|---|
| Test (studies, participants) | Xpert Ultra (7, 2834) | Xpert MTB/RIF (7, 2835) | Difference (Xpert Ultra minus Xpert MTB/RIF)* | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 90.9% (86.2 to 94.7) | 84.7% (78.6 to 89.9) | 6.3% (0.1 to 12.8) | 0.98 |
| Specificity (95% CrI) | 95.6% (93.0 to 97.4) | 98.4% (97.0 to 99.3) | −2.7% (−5.7 to −0.5) | 0.01 |
| Smear‐positive (tuberculosis detection) | ||||
| Test (studies, participants) | Xpert Ultra (6, 593) | Xpert MTB/RIF (6, 598) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 99.3% (98.1 to 99.8) | 98.9% (97.5 to 99.6) | 0.3% (−1.0 to 1.8) | 0.72 |
| Specificity (95% CrI) | Not estimated | Not estimated | N/A | N/A |
| Smear‐negative (tuberculosis detection) | ||||
| Test (studies, participants) | Xpert Ultra (6, 2049) | Xpert MTB/RIF (6, 2051) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 77.5% (67.6 to 85.6) | 60.6% (48.4 to 71.7) | 16.7% (2.1 to 31.8) | 1.00 |
| Specificity (95% CrI) | 95.8% (92.9 to 97.7) | 98.8% (97.7 to 99.5) | −3.0% (‐5.9 to −0.9) | 0.00 |
| History of tuberculosis | ||||
| Test (studies, participants) | Xpert Ultra (4, 602) | Xpert MTB/RIF (4, 610) | Difference (Xpert Ultra minus Xpert MTB/RIF)* | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 84.2% (72.5 to 91.7) | 81.8% (68.7 to 90.0) | 2.4% (−11.9 to 17.2) | 0.64 |
| Specificity (95% CrI) | 88.2% (70.5 to 96.6) | 97.4% (91.7 to 99.5) | −8.9% (−27.0 to 0.6) | 0.03 |
| Detection of rifampicin resistance | ||||
| Test (studies, participants) | Xpert Ultra (5, 921) | Xpert MTB/RIF (5, 930) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 94.9% (88.9 to 97.9) | 95.3% (90.0 to 98.1) | −0.3% (−6.9 to 5.7) | 0.45 |
| Specificity (95% CrI) | 99.1% (97.7 to 99.8) | 98.8% (97.2 to 99.6) | 0.3% (−1.2 to 2.0) | 0.67 |
| Smear‐positive (rifampicin resistance detection) | ||||
| Test (studies, participants) | Xpert Ultra (4, 686) | Xpert MTB/RIF (4, 699) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 93.9% (84.4 to 97.7) | 95.5% (88.4 to 98.6) | −1.5% (−10.9 to 6.0) | 0.32 |
| Specificity (95% CrI) | 99.3% (97.8 to 99.9) | 99.1% (97.3 to 99.9) | 0.1% (−1.5 to 2.0) | 0.59 |
| Smear‐negative (rifampicin resistance detection) | ||||
| Test (studies, participants) | Xpert Ultra (4, 412) | Xpert MTB/RIF (4, 416) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 92.0% (75.0 to 95.8) | 95.4% (82.3 to 99.3) | −3.1% (−20.7 to 11.7) | 0.30 |
| Specificity (95% CrI) | 99.4% (96.2 to 100) | 99.2% (94.8 to 100) | 0.1% (−3.0 to 4.5) | 0.58 |
Abbreviations: CrI: credible interval
* We determined absolute differences for sensitivity and specificity when there were at least four studies in a subgroup.
** Slight differences in numerical values are likely due to rounding errors.
Figure 6 presents the SROC plot for Xpert Ultra and Xpert MTB/RIF pooled sensitivity and specificity estimates together with the credible and prediction regions for pulmonary tuberculosis. The summary point (pooled value) appears close to the upper left‐hand corner of the plots, suggesting high accuracy of both Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis. The 95% credible regions around the summary points of sensitivity and specificity, the regions that contain likely combinations of the pooled sensitivity and specificity, are relatively narrow. The 95% prediction region is slightly wider for Xpert Ultra, displaying more uncertainty as to where the likely values of sensitivity and specificity might occur in a future study.

Summary plot of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of pulmonary tuberculosis. Each individual study is represented by a shaded circle. The size of the circle is proportional to the sample size of the study such that larger studies are represented by larger circles. The filled circle is the median pooled estimate for sensitivity and specificity, Xpert Ultra (red) and Xpert MTB/RIF (black). The dotted lines represent the 95% credible region around the summary estimate; the dashed lines represent the 95% prediction region. The range is truncated to consider only those regions of the receiver operator characteristic (ROC) space where data have been observed.
We identified two studies that compared the accuracy of Xpert Ultra and Xpert MTB/RIF against a composite reference standard based on clinical and radiographic findings. In Berhanu 2018, Xpert Ultra sensitivity and specificity (95% CI) were 80% (68 to 89) and 96% (92 to 98) versus Xpert MTB/RIF sensitivity and specificity of 72% (59 to 82) and 100% (98 to 100). In Opota 2019, Xpert Ultra sensitivity and specificity (95% CI) were 96% (87 to 100) and 100% (97 to 100) versus Xpert MTB/RIF sensitivity and specificity of 84% (71 to 93) and 100% (97 to 100) (Figure 5).
Subgroup analyses
The results of the subgroup analyses by smear status, HIV status, and history of tuberculosis are shown in Table 3.
Xpert Ultra versus Xpert MTB/RIF in participants with smear‐negative sputum specimens
Seven studies reported data for participants with smear‐negative specimens (Figure 7) (Berhanu 2018; Chakravorty 2017; Dorman 2018; Mishra 2020a; Opota 2019; Piersimoni 2019; Wang 2019). The sensitivity of Xpert Ultra ranged from 63% to 92%, and the sensitivity of Xpert MTB/RIF from 41% to 77%. The lowest sensitivity for Xpert Ultra (63%) was reported by Dorman 2018. This was a multicentre study which took place in Belarus, Brazil, China, Georgia, India, Kenya, South Africa, and Uganda, and assessed Xpert Ultra accuracy based on a reference standard of multiple cultures. The lowest sensitivity for Xpert MTB/RIF (41%) was reported by Berhanu 2018. In this study, which took place in South Africa, 62% of participants were HIV‐positive. The specificity of Xpert Ultra ranged from 71% to 99%. The lowest specificity for Ultra (71%) was reported by Wang 2019, which preselected smear‐negative participants based on prior microscopy testing. The specificity of Xpert MTB/RIF ranged from 78% to 100%, and the lowest specificity (78%) was again reported by Wang 2019.
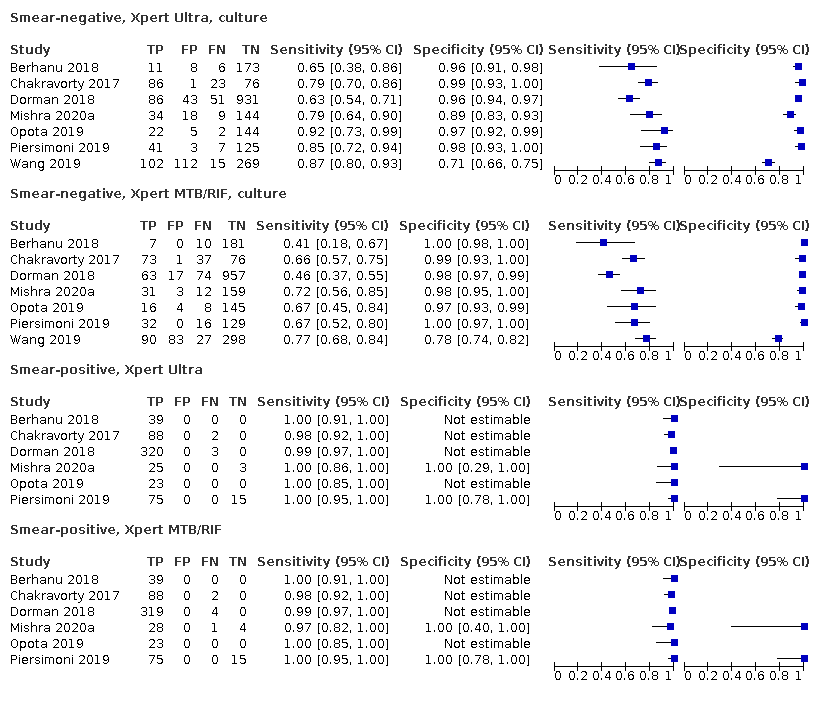
Forest plots of Xpert Ultra versus Xpert MTB/RIF sensitivity and specificity for the detection of pulmonary tuberculosis by smear status. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI). TP = true positive; FP = false positive; FN = false negative; TN = true negative
In a meta‐analysis of studies with unselected participants (excluding Wang 2019), Xpert Ultra pooled sensitivity was 77.5% (67.6 to 85.6) and pooled specificity was 95.8% (92.9 to 97.7) (6 studies, 2049 participants). Xpert MTB/RIF pooled sensitivity and specificity were 60.6% (48.4 to 71.7) and 98.8% (97.7 to 99.5) (6 studies, 2051 participants). The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at 16.7% (2.1 to 31.8) for sensitivity and −3.0% (−5.9 to −0.9) for specificity. We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF as 0.99. We estimated the probability that the pooled specificity of Xpert Ultra was less than that of Xpert MTB/RIF as 1.00.
Repeating the meta‐analysis including Wang 2019, Xpert Ultra pooled sensitivity was slightly higher at 79.4% (70.2 to 87.0) and specificity (95% CrI) slightly lower at 94.2% (88.3 to 97.5) (7 studies, 2547 participants); Xpert MTB/RIF pooled sensitivity and specificity (95% CrI) were 63.0% (51.7 to 73.0) and 98.2% (94.9 to 99.6) (7 studies, 2549 participants).
Xpert Ultra versus Xpert MTB/RIF in participants with smear‐positive sputum specimens
Six studies reported data for participants with smear‐positive specimens (Figure 7) (Berhanu 2018; Chakravorty 2017; Dorman 2018; Mishra 2020a; Opota 2019; Piersimoni 2019). For both index tests, sensitivity estimates were 97% or greater in all studies. For smear‐positive pulmonary tuberculosis, Xpert Ultra pooled sensitivity (95% CrI) was 99.3% (98.1 to 99.8) (6 studies, 593 participants); Xpert MTB/RIF pooled sensitivity (95% CrI) was 98.9% (97.5 to 99.6) (6 studies, 598 participants) (Table 3). We did not determine pooled specificity because in four studies the value for true negatives plus false positives was zero, and specificity was not estimable (Berhanu 2018; Chakravorty 2017; Dorman 2018; Opota 2019). The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at 0.3% (−1.0 to 1.8) for sensitivity. We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF as 0.72.
Xpert Ultra versus Xpert MTB/RIF in people living with HIV
Three studies reported data in people living with HIV (Berhanu 2018; Dorman 2018; Mishra 2020a). The sensitivity of Xpert Ultra ranged from 81% to 90%, and the sensitivity of Xpert MTB/RIF from 68% to 77%. The specificity of Xpert Ultra ranged from 78% to 96%; the specificity of Xpert MTB/RIF was higher, ranging from 99% to 100% (Figure 8). Xpert Ultra pooled sensitivity and specificity (95% CrI) were 87.6% (75.4 to 94.1) and 92.8% (82.3 to 97.0) (627 participants); Xpert MTB/RIF pooled sensitivity and specificity were 74.9% (58.7 to 86.2) and 99.7% (98.6 to 100.0) (635 participants) (Table 4).

Forest plots of Xpert Ultra versus Xpert MTB/RIF sensitivity and specificity for the detection of pulmonary tuberculosis by HIV status and history of tuberculosis. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative
| Analysis | Test | No. of studies (participants) | Median pooled sensitivity | Median pooled specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|---|
| HIV‐negative | Xpert Ultra | 3 (755) | 90.3% (80.3 to 95.6) | 94.3% (79.8 to 98.7) | 63.5% (45.6 to 79.7) | 98.9% (97.7 to 99.5) |
| HIV‐negative | Xpert MTB/RIF | 3 (755) | 89.0% (78.3 to 94.8) | 98.1% (95.3 to 99.4) | 83.8% (67.6 to 94.0) | 98.8% (97.6 to 99.4) |
| HIV‐positive | Xpert Ultra | 3 (627) | 87.6% (75.4 to 94.1) | 92.8% (82.3 to 97.0) | 57.4% (34.5 to 76.8) | 98.5% (97.0 to 99.3) |
| HIV‐positive | Xpert MTB/RIF | 3 (635) | 74.9% (58.7 to 86.2) | 99.7% (98.6 to 100.0) | 96.3% (85.4 to 99.6) | 97.3% (95.6 to 98.5) |
Abbreviations: CI: confidence interval; CrI: credible interval
* Positive and negative predictive values were determined at a pretest probability of 10%
Xpert Ultra versus Xpert MTB/RIF in HIV‐negative people
Three studies reported data for HIV‐negative people (Berhanu 2018; Dorman 2018; Mishra 2020a). The sensitivity of Xpert Ultra ranged from 88% to 91%, and the sensitivity of Xpert MTB/RIF from 86% to 90%. The specificity of Xpert Ultra ranged from 91% to 97%; the specificity of Xpert MTB/RIF was higher, ranging from 97% to 100% (Figure 8). Xpert Ultra pooled sensitivity and specificity (95% CrI) were 90.3% (80.3 to 95.6) and 94.3% (79.8 to 98.7) (755 participants); Xpert MTB/RIF pooled sensitivity and specificity (95% CrI) were 89.0% (78.3 to 94.8) and 98.1% (95.3 to 99.4) (755 participants) (Table 4).
Xpert Ultra versus Xpert MTB/RIF in people with a history of tuberculosis
Four studies reported data for people with a history of tuberculosis (Berhanu 2018; Dorman 2018; Mishra 2020a; Mishra 2020b). The sensitivity of Xpert Ultra ranged from 80% to 86%, and the sensitivity of Xpert MTB/RIF from 70% to 92%. The specificity of Xpert Ultra ranged from 69% to 97%, and the specificity of Xpert MTB/RIF from 84% to 100% (Figure 8). The lowest specificity (69% for Xpert Ultra) was reported by Mishra 2020b, which was notable for preselecting patients who had previously received antituberculosis treatment within the last two years. Xpert Ultra pooled sensitivity and specificity (95% CrI) were 84.2% (72.5 to 91.7) and 88.2% (70.5 to 96.6) (602 participants). Xpert MTB/RIF pooled sensitivity and specificity (95% CrI) were 81.8% (68.7 to 90.0) and 97.4% (91.7 to 99.5) (610 participants). The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at 2.4% (−11.9 to 17.2) for sensitivity and −8.9% (−27.0 to 0.6) for specificity. We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF as 0.64. We estimated the probability that the pooled specificity of Xpert Ultra was less than that of Xpert MTB/RIF as 0.97.
Repeating the meta‐analysis excluding Mishra 2020b, Xpert Ultra pooled sensitivity decreased to 83.3% (66.9 to 92.7), and specificity increased to 91.5% (81.3 to 96.7) (3 studies, 434 participants). Xpert MTB/RIF pooled sensitivity decreased to 76.6% (58.6 to 88.8), and specificity increased to 99.0% (97.7 to 99.8) (3 studies, 432 participants).
Non‐determinate results, detection of pulmonary tuberculosis
Regarding Xpert Ultra, six studies reported non‐determinate results for tuberculosis detection: 14/253 (5.5%) Mishra 2020a; 64/2001 (3.2%) Dorman 2018; 5/173 (2.9%) Mishra 2020b; 3/269 (1.1%) Piersimoni 2019; 5/503 (1.0%) Wang 2019; 0/237 (0%) Berhanu 2018. Among six studies involving 3436 tests, the pooled proportion of non‐determinate test results for Xpert Ultra was low, at 2.0% (0.9 to 3.6).
Regarding Xpert MTB/RIF, five studies reported non‐determinate results for tuberculosis detection: 14/301 (4.6%) Mishra 2020a; 14/811 (1.7%) Wang 2019; 28/2001 (1.4%) Dorman 2018; 2/269 (0.7%) Piersimoni 2019; 1/179 (0.6%) Mishra 2020b. Among five studies involving 3561 tests, the pooled proportion of non‐determinate test results for Xpert MTB/RIF was low, at 1.6% (0.8 to 3.0).
II. Detection of rifampicin resistance
Xpert Ultra versus Xpert MTB/RIF for the detection of rifampicin resistance
We identified five studies that compared Xpert Ultra and Xpert MTB/RIF accuracy for the detection of rifampicin resistance (Chakravorty 2017; Dorman 2018; Mishra 2020b; Piersimoni 2019; Wang 2019). The median sample size was 107 (IQR 90 to 139). The prevalence of rifampicin resistance in the studies ranged from 1.9% to 31.8%. The sensitivity of Xpert Ultra ranged from 83% to 100%, and the sensitivity of Xpert MTB/RIF from 93% to 100%. The specificity of Xpert Ultra ranged from 98% to 100%, and the specificity of Xpert MTB/RIF from 95% to 100% (Figure 9).
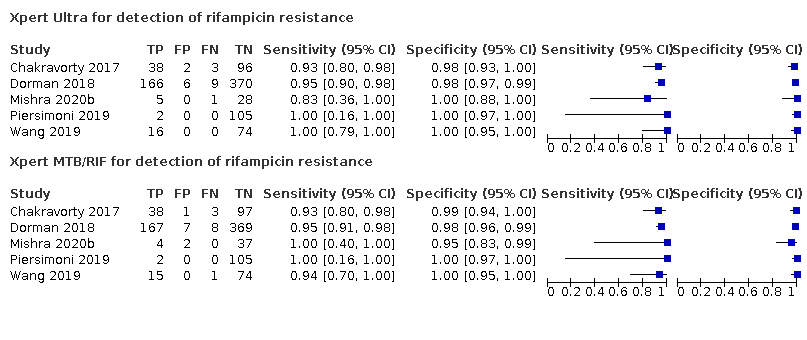
Forest plot of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of rifampicin resistance. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative
Xpert Ultra pooled sensitivity and specificity were 94.9% (88.9 to 97.9) and 99.1% (97.7 to 99.8) (5 studies, 921 participants; high‐certainty evidence) versus Xpert MTB/RIF pooled sensitivity and specificity of 95.3% (90.0 to 98.1) and 98.8% (97.2 to 99.6) (5 studies, 930 participants; high‐certainty evidence) (Table 2). The pooled sensitivity and specificity estimates for Xpert Ultra and Xpert MTB/RIF were similar. The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at −0.3% (−6.9 to 5.7) for sensitivity and 0.3% (−1.2 to 2.0) for specificity. We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF as 0.45. We estimated the probability that the pooled specificity of Xpert Ultra was less than that of Xpert MTB/RIF as 0.33.
Figure 10 presents Xpert Ultra and Xpert MTB/RIF pooled sensitivity and specificity estimates together with the credible and prediction regions for rifampicin resistance. The summary point (pooled value) appears close to the upper left‐hand corner of the plots, suggesting high accuracy of both Xpert Ultra and Xpert MTB/RIF for the detection of rifampicin resistance. The 95% confidence regions around the summary points of sensitivity and specificity, the regions that contain likely combinations of the pooled sensitivity and specificity, are relatively narrow. The 95% prediction regions, the regions that contain the likely values of sensitivity and specificity in a future study, are also relatively narrow.

Summary plot of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of rifampicin resistance. Each individual study is represented by a shaded circle. The size of the circle is proportional to the sample size of the study such that larger studies are represented by larger circles. The filled circle is the median pooled estimate for sensitivity and specificity, Xpert Ultra (red) and Xpert MTB/RIF (black). The dotted lines represent the 95% credible region around the summary estimate; the dashed lines represent the 95% prediction region. The range is truncated to consider only those regions of the receiver operator characteristic (ROC) space where data have been observed.
Xpert Ultra versus Xpert MTB/RIF accuracy for the detection of rifampicin resistance with respect to smear status
We identified four studies that compared Xpert Ultra and Xpert MTB/RIF accuracy for rifampicin resistance detection by smear status (Figure 11) (Chakravorty 2017; Dorman 2018; Mishra 2020b; Piersimoni 2019).
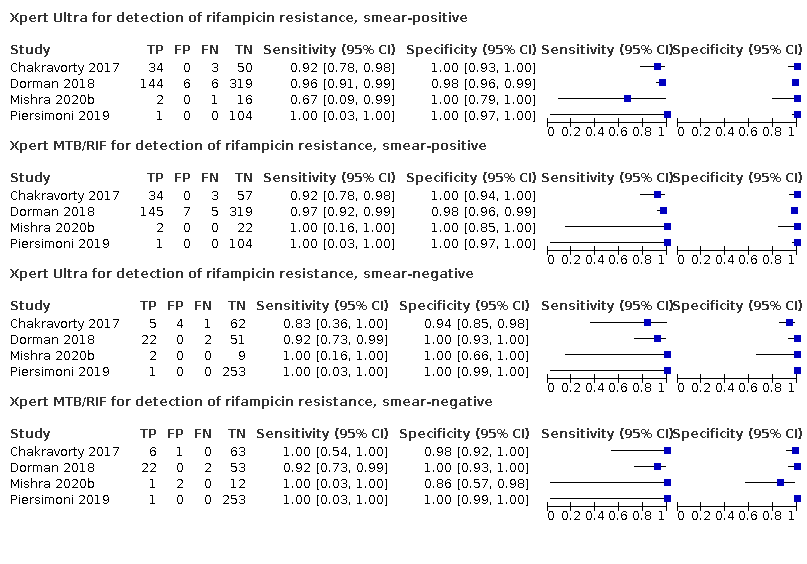
Forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of rifampicin resistance by smear status. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI). TP = true positive; FP = false positive; FN = false negative; TN = true negative
For smear‐positive specimens, Xpert Ultra pooled sensitivity and specificity were 93.9% (84.4 to 97.7) and 99.3% (97.8 to 99.9) (4 studies, 686 participants) versus Xpert MTB/RIF pooled sensitivity and specificity of 95.5% (88.4 to 98.6) and 99.1% (97.3 to 99.9) (4 studies, 699 participants). The pooled specificity estimates for Xpert Ultra and Xpert MTB/RIF were similar. The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at −1.5% (−10.9 to 6.0) for sensitivity and 0.1% (−1.5 to 2.0) for specificity. We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF as 0.32. We estimated the probability that the pooled specificity of Xpert Ultra was less than that of Xpert MTB/RIF as 0.41 (Table 3).
For smear‐negative specimens, Xpert Ultra pooled sensitivity and specificity were 92.0% (75.0 to 95.8) and 99.4% (96.2 to 100) (4 studies, 412 participants) versus Xpert MTB/RIF pooled sensitivity and specificity of 95.4% (82.3 to 99.3) and 99.2% (94.8 to 100) (4 studies, 416 participants). The pooled specificity estimates for Xpert Ultra and Xpert MTB/RIF were similar. The difference in the accuracy of Xpert Ultra minus Xpert MTB/RIF was estimated at −3.1% (−20.7 to 11.7) for sensitivity and 0.1% (−3.0 to 4.5) for specificity. We estimated the probability that the pooled sensitivity of Xpert Ultra exceeds that of Xpert MTB/RIF as 0.30. We estimated the probability that the pooled specificity of Xpert Ultra was less than that of Xpert MTB/RIF as 0.42 (Table 3).
Indeterminate results, detection of rifampicin resistance
Regarding Xpert Ultra, four studies reported indeterminate results for rifampicin resistance: 21/76 (27.6%) Mishra 2020b; 14/80 (17.5%) Mishra 2020a; 16/684 (2.3%) Dorman 2018; 5/214 (2.3%) Wang 2019. Among four studies involving 1054 tests, the pooled proportion of indeterminate rifampicin resistance results for Xpert Ultra was 7.6% (2.4 to 21.0). Importantly, two studies reported the number of trace results that contributed to indeterminate rifampicin resistance results. In both studies, all or almost all indeterminate results were due to trace results, 13/14 (92.9%) in Mishra 2020a and 21/21 (100%) in Mishra 2020b.
Regarding Xpert MTB/RIF, three studies reported indeterminate results for rifampicin resistance: 1/61 (1.6%) Mishra 2020a; 1/67 (1.5%) Mishra 2020b; 4/684 (0.6%) Dorman 2018. Among three studies involving 812 tests, the pooled proportion of indeterminate test results for Xpert MTB/RIF was low, at 0.8% (0.2 to 2.4).
The estimated difference in the pooled proportion of indeterminate rifampicin resistance results for Xpert Ultra versus Xpert MTB/RIF was 6.7% (1.4 to 20.1). We estimated the probability that the pooled proportion of indeterminate results for Xpert Ultra exceeds that for Xpert MTB/RIF as 1.00.
Xpert Ultra trace results
Summary of Xpert Ultra trace positive results
Eight studies reported the number of Xpert Ultra positive results that were trace‐positives (Berhanu 2018; Dorman 2018; Mishra 2020a; Mishra 2020b; Opota 2019; Pereira 2020; Piersimoni 2019; Wang 2019). The percentage of trace‐positive results ranged from 3.0% to 30.4% (Table 5). Among participants with trace‐positive results, four studies reported the percentage of participants with a history of tuberculosis: 20% (10.3% trace) in Berhanu 2018, 57.9% (7.1% trace) in Dorman 2018, 46.2% (18.6% trace) in Mishra 2020a, and 100% (27.6% trace) in Mishra 2020b. Mishra 2020b recruited participants with a recent history of tuberculosis (within the last two years) (Table 5).
| Study | Country | Culture‐positive MTB/total | Trace results (% of Ultra positive) | Number (%) of trace results with history of tuberculosis | Culture‐positive trace/total trace | Additional testing on trace results (not including retesting) | Trace results repeated? |
|---|---|---|---|---|---|---|---|
| South Africa | 56/237 | 6 (10.3%) | 1/5 (20.0%); this 1 patient was culture positive | 2/6 | Sputum re‐collected at day 60 in 1 participant was MGIT negative. | No | |
| FIND biobank samples (Peru, Vietnam, South Africa) and clinical samples (Georgia, India) | 200/277 | Not reported | ‐ | ‐ | ‐ | No | |
| Belarus, Brazil, China, Georgia, India, Kenya, South Africa, Uganda | 462/1439 | 32 (7.1%) | Of 19 culture‐negative trace results, 11 (57.9%) had history of TB. | 13/32 | Among culture‐negative, a follow‐up culture at 2 months was positive in 2/10. | Yes: 13 of the retested samples were culture+; of these 9 were Ultra + on repeat. 19 of the samples were culture‐, of which 10 were Ultra false + | |
| South Africa | 72/239 | 13 (18.6%) | 6/13 (46.2%) | 4/13 | ‐ | Yes: new sample collected median 444 days, after the initial testing. 4 samples retested (1 culture+, 3 culture‐); all culture‐ were not detected, and culture+ was Ultra + | |
| South Africa | 44/168 | 21 (27.6%) | 21/21 (100%) | 2/21 | ‐ | No | |
| Switzerland | 47/196 | 5 (10.0%) | Not reported | 4/5 | The 1 culture‐negative patient was culture‐positive on a lymph node specimen. | No | |
| Brazil | 157/180 | 1 (3.0%) | Not reported | Not reported | ‐ | No | |
| Italy | 123/266 | 8 (6.7%) | Excluded from study | 5/8 | ‐ | Yes: 4 respiratory samples retested (1 culture+, 3 culture‐); all culture‐ were not detected, and culture+ was Ultra + | |
| China | 117/498 | 65 (30.4%) | Not reported | Not reported | ‐ | No |
Abbreviations: FIND: Foundation for Innovative New Diagnostics; MGIT: Mycobacteria Growth Indicator Tube; MTB: Mycobacterium tuberculosis; TB: tuberculosis
‐ Could not determine
Xpert Ultra repeated test for diagnosing pulmonary tuberculosis in people who have an initial Ultra trace result
We identified three studies where an Xpert Ultra repeated test was used to diagnose pulmonary tuberculosis in people who had an initial Ultra trace result, against culture: Mishra 2020a (4 participants), Piersimoni 2019 (4 participants), and Dorman 2018 (32 participants). Piersimoni 2019 retested the same initial samples. Dorman 2018 retested on a separately collected sputum sample. Mishra 2020a retested only those participants with discrepant (Ultra trace‐positive/culture‐negative) results, and retesting was performed on new specimens obtained a median of 444 days (range 245 to 526 days) after initial testing. Xpert Ultra accuracy of a second (repeat) test in Mishra 2020a and Piersimoni 2019 was 100% for both sensitivity and specificity. Dorman 2018 found sensitivity of 69% (95% CI 39 to 91) and specificity of 47% (24 to 71) (Table 5, Figure 12).

Forest plots of repeated Xpert Ultra sensitivity and specificity for detection of pulmonary tuberculosis in adults with initial trace result, culture reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative
Nontuberculous mycobacteria
Three studies reported the number of NTMs that grew from the specimens tested (total of 26 NTMs): Berhanu 2018 (4/244); Piersimoni 2019 (15/269); and Wang 2019 (7/498). Only one of these studies reported on Xpert Ultra and Xpert MTB/RIF results in those with NTM (Piersimoni 2019), and found neither test was positive in those who grew NTMs.
Sensitivity analyses
For Xpert Ultra for the detection of pulmonary tuberculosis, we undertook sensitivity analyses by limiting inclusion in the meta‐analysis to the following.
-
Studies where a single specimen yielded a single Xpert Ultra result for a given participant. We excluded studies that included more specimens than participants.
-
Studies that only included untreated participants.
-
Studies that used liquid culture as the reference standard.
-
Studies where a consecutive or random sample of participants was enrolled.
-
Studies where the reference standard was blinded.
-
Studies that only used fresh specimens.
For Xpert Ultra for the detection of pulmonary tuberculosis, these sensitivity analyses made little difference to any of the findings (Table 6). We planned to perform a sensitivity analysis for studies that accounted for all participants in the analysis; however, for the detection of pulmonary tuberculosis, this criterion was satisfied by all studies.
| Type of analysis (no. of studies, participants) | Median pooled sensitivity (95% Crl) | Median pooled specificity (95% Crl) | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| Xpert Ultra sensitivity and specificity for pulmonary tuberculosis detection in studies with unselected patients (7, 2834) | 90.9% (86.2 to 94.7) | 95.6% (93.0 to 97.4) | 69.6% (58.7 to 79.8) | 99.0% (98.4 to 99.4) |
| Studies that only included untreated participants (exclude studies with some percentage of participants who were receiving tuberculosis treatment) (5, 2361) | 90.9% (84.7 to 95.3) | 94.9% (91.3 to 97.2) | 66.4% (53.2 to 78.4) | 98.9% (98.2 to 99.5) |
| Studies that used liquid culture only as the reference standard for tuberculosis detection (4, 978) | 91.1% (84.0 to 95.5) | 96.1% (91.7 to 98.5) | 72.1% (54.5 to 87.2) | 99.0% (98.2 to 99.5) |
| Studies where consecutive or random participants were selected (6, 2557) | 91.6% (86.6 to 95.4) | 95.3% (92.4 to 97.2) | 68.2% (56.9 to 78.5) | 99.0% (98.5 to 99.5) |
| Studies where the reference standard was blinded (6, 2654) | 90.2% (85.2 to 93.8) | 95.9% (93.0 to 97.7) | 70.8% (58.5 to 81.8) | 98.9% (98.3 to 99.3) |
| Studies using fresh specimens (4, 2095) | 89.8% (82.1 to 95.1) | 94.1% (89.3 to 96.8) | 62.7% (47.9 to 75.8) | 98.8% (97.9 to 99.4) |
Abbreviations: Crl: credible interval.
Discussion
This Cochrane Review on the diagnostic accuracy of Xpert Ultra compared to Xpert MTB/RIF for the detection of pulmonary tuberculosis and rifampicin resistance in adults summarizes the current literature. For the detection of pulmonary tuberculosis, we identified nine studies, of which seven were conducted in unselected participants. Estimation of accuracy in unselected patients is consistent with the intended use of these tests. For the detection of rifampicin resistance, we identified five studies.
Summary of main results
-
For the detection of pulmonary tuberculosis, Xpert Ultra sensitivity and specificity were 90.9% (86.2 to 94.7) and 95.6% (93.0 to 97.4).
-
For the detection of pulmonary tuberculosis, Xpert MTB/RIF sensitivity and specificity were 84.7% (78.6 to 89.9) and 98.4% (97.0 to 99.3).
-
Xpert Ultra sensitivity and specificity were 77.5% (67.6 to 85.6) and 95.8% (92.9 to 97.7) for smear‐negative, culture‐positive tuberculosis.
-
Xpert MTB/RIF sensitivity and specificity were 60.6% (48.4 to 71.7) and 98.8% (97.7 to 99.5) for smear‐negative, culture‐positive tuberculosis.
-
Xpert Ultra sensitivity and specificity for pulmonary tuberculosis were 87.6% (75.4 to 94.1) and 92.8% (82.3 to 97.0) in people living with HIV.
-
Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis were 74.9% (58.7 to 86.2) and 99.7% (98.6 to 100) in people living with HIV.
-
Xpert Ultra sensitivity and specificity for pulmonary tuberculosis in people with a history of tuberculosis were 84.2% (72.5 to 91.7) and 88.2 (70.5 to 96.6).
-
Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in people with a history of tuberculosis were 81.8% (68.7 to 90.0) and 97.4% (91.7 to 99.5).
-
For the detection of pulmonary tuberculosis, the pooled proportion of Xpert Ultra non‐determinate test results was low, 2.0% (0.9 to 3.6).
-
For the detection of pulmonary tuberculosis, the pooled proportion of Xpert MTB/RIF non‐determinate test results was low, 1.6% (0.8 to 3.0).
-
For the detection of rifampicin resistance, Xpert Ultra sensitivity and specificity were 94.9% (88.9 to 97.9) and 99.1% (97.7 to 99.8).
-
For the detection of rifampicin resistance, Xpert MTB/RIF sensitivity and specificity were 95.3% (90.0 to 98.1) and 98.8% (97.2 to 99.6).
-
For the detection of rifampicin resistance, the pooled proportion of Xpert Ultra indeterminate test results was 7.6% (2.4 to 21.0).
-
For the detection of rifampicin resistance, the pooled proportion of Xpert MTB/RIF indeterminate test results was 0.8% (0.2 to 2.4).
Detection of pulmonary tuberculosis
If the point estimates for Xpert Ultra and Xpert MTB/RIF are applied to a hypothetical cohort of 1000 people, where 10% of those presenting with symptoms have pulmonary tuberculosis, Xpert Ultra will miss 9 cases, and Xpert MTB/RIF will miss 15 cases. The number of people wrongly diagnosed with pulmonary tuberculosis would be 40 with Xpert Ultra and 14 with Xpert MTB/RIF (summary of findings Table 1).
Detection of rifampicin resistance
If the point estimates for Xpert Ultra and Xpert MTB/RIF are applied to a hypothetical cohort of 1000 people, where 10% of those presenting with symptoms have rifampicin resistance, Xpert Ultra will miss 5 cases, and Xpert MTB/RIF will miss 5 cases. The number of people wrongly diagnosed with rifampicin resistance would be 8 with Xpert Ultra and 11 with Xpert MTB/RIF (summary of findings Table 2).
Xpert Ultra performance in different subgroups
Xpert MTB/RIF detects DNA sequences of M tuberculosis after amplification, and has a lower limit of detection of 131 CFUs/mL (Helb 2010). The cycle threshold value (CT) is the number of PCR cycles after which Xpert MTB/RIF probes successfully detect M tuberculosis DNA in a given sample. Xpert MTB/RIF CT values are strongly correlated with acid‐fast bacillus (AFB) smear status (Lange 2017). The lower sensitivity of Xpert MTB/RIF in individuals with AFB smear‐negative pulmonary tuberculosis is related to the lower bacillary burden and higher associated CT value compared to individuals with AFB smear‐positive pulmonary tuberculosis. Individuals with pulmonary tuberculosis and HIV co‐infection are more likely to have smear‐negative tuberculosis, which implies a lower bacillary burden and higher mean CT values on Xpert testing (Beynon 2018; Lange 2017); this is the likely mechanism for the lower sensitivity of Xpert Ultra for the diagnosis of tuberculosis in people living with HIV.
Xpert Ultra was developed to improve sensitivity in the detection of pulmonary tuberculosis, in particular in people with smear‐negative disease and people living with HIV. In smear‐negative, culture‐positive pulmonary tuberculosis, we found Xpert Ultra sensitivity of 77.5% as compared to Xpert MTB/RIF sensitivity of 60.6%; in people living with HIV, Xpert Ultra sensitivity was 87.6% as compared to Xpert MTB/RIF sensitivity of 74.9%. The improvement in Xpert Ultra sensitivity came at the expense of a slight reduction in specificity as compared to Xpert MTB/RIF.
In individuals with a history of tuberculosis, we found that Xpert Ultra pooled specificity (88.2%) was considerably lower than the pooled specificity in the primary analysis (95.6%). Hence, the increase in sensitivity of Xpert Ultra as compared to Xpert MTB/RIF comes at the expense of specificity. Dorman and colleagues found that specificity improved as time since the previous diagnosis of tuberculosis increased, and approximated to that of participants without a history of tuberculosis when elapsed time was seven years (Dorman 2018). In comparison, for Xpert MTB/RIF in individuals with a history of tuberculosis, we found that Xpert MTB/RIF pooled specificity (97.4%) was only slightly lower than the pooled specificity in the primary analysis (98.4%). Other studies have reported that Xpert MTB/RIF may be positive at the end of tuberculosis treatment despite cure (Friedrich 2013; Theron 2016; Theron 2018), and may rarely remain positive for up to five years after tuberculosis treatment (Boyles 2014).
Regarding the prevalence of tuberculosis, in comparing settings with a higher or lower prevalence of tuberculosis, we previously reported that for both Xpert MTB/RIF sensitivity and specificity, the 95% CrIs in the two groups did not overlap, suggesting an association of prevalence of tuberculosis with the accuracy estimates (Horne 2019). In comparing settings with a higher or lower prevalence of rifampicin resistance, we also previously found that the Crls for specificity did not overlap, suggesting an association of prevalence of rifampicin resistance with the specificity estimates (Horne 2019). Changes in disease prevalence have often been found to be associated with other important changes, such as in the disease spectrum, which may affect diagnostic accuracy estimates (Leeflang 2013). In this review, we did not analyse the effect of tuberculosis prevalence on Xpert Ultra accuracy. However, we acknowledge that as Xpert Ultra is rolled out globally, differences in accuracy may have important ramifications depending on the prevalence of tuberculosis and rifampicin resistance (Kendall 2017).
For the detection of rifampicin resistance, Xpert Ultra and Xpert MTB/RIF had similar sensitivity and specificity. Of interest, a recent prospective population‐based study in Rwanda, a country with a low prevalence of rifampicin resistance, found that among patients with rifampicin resistance on initial Xpert MTB/RIF testing, 47% (57/121) had a false‐positive rifampicin resistance result, in particular in specimens with a low tuberculosis bacillary burden (Ngabonziza 2020). As mentioned above, in order to address limitations in rifampicin resistance detection, Xpert Ultra uses melting temperature‐based analysis, in lieu of real‐time PCR analysis with Xpert MTB/RIF. Melting temperature‐based analysis allows Xpert Ultra to better distinguish resistance‐conferring mutations from silent mutations (Global Laboratory Initiative 2017).
To improve the sensitivity of Xpert Ultra, a new result category, trace call, was added, which corresponds to the lowest bacillary burden for M tuberculosis detection. The results of our systematic review suggest that Xpert Ultra trace calls are not a rare finding. In the included studies, Xpert Ultra positive results that were trace‐positives ranged from 7% to 30%. Of interest, Dorman 2018 performed several post hoc analyses that evaluated the impact of changing the classification of Xpert Ultra trace calls, which in the primary analysis were considered positive for the identification of M tuberculosis. Reclassifying all trace calls as a negative result increased Xpert Ultra specificity and decreased its sensitivity. When reclassifying trace calls as negative in participants with a history of tuberculosis, or repeating trace calls with the second result determining the ultimate classification, both resulted in sensitivity estimates close to those observed in the primary analysis, with only slightly compromised specificity.
We identified a higher number of rifampicin resistance indeterminate results with Xpert Ultra (7.6%) compared to Xpert MTB/RIF (0.8%). Notably, in studies that reported the number of trace results that contributed to indeterminate rifampicin resistance results, all, or almost all, Xpert Ultra indeterminate rifampicin resistance results were due to trace‐positive results: 92.9% in Mishra 2020a and 100% in Mishra 2020b. The interpretation of and need for additional testing in patients with trace results will depend on clinical and epidemiological considerations.
We identified very limited data from patients who underwent repeated Ultra testing after an initial trace‐positive result. With repeated testing against culture as the reference test, Dorman 2018 found Xpert Ultra sensitivity of 69% and specificity of 47% (32 participants), whereas Mishra 2020a (4 participants) and Piersimoni 2019 (4 participants) found Xpert Ultra sensitivity of 100% and specificity of 100%. Based on the findings in Dorman 2018, WHO recommended that "among persons without HIV infection with an initial trace call positive result, a fresh specimen from the patient should undergo repeat testing and the result of the second Ultra test be used for clinical decisions" (WHO 2017). The issue of how trace call results should be interpreted was recently reconsidered by the WHO with the following guidance: “For patients with Xpert Ultra trace results, decisions regarding treatment initiation should include considerations of the clinical presentation and the patient context (including prior treatment history, probability of relapse and other test results)" (WHO Consolidated Guidelines (Module 3) 2020).
We summarized data for NTM separately by determining the per cent of false‐positive Xpert Ultra results in specimens that grew NTMs. We found that among specimens that were culture‐positive for NTM, false‐positive Xpert Ultra results did not occur. In an analytical study, Chakravorty assessed in triplicate the specificity of Xpert Ultra on 30 different NTMs for cross‐reactivity, and found “MTB not detected” for all replicates tested (Chakravorty 2017).
We previously assessed whether Xpert MTB/RIF accuracy differs according to setting in which the test is performed, that is point of care or peripheral settings compared with central and intermediate laboratories (Horne 2019). Although we did not repeat this analysis for Xpert Ultra (both index tests are run identically), we consider it important to mention the findings from the previous review. When comparing results from studies by test setting, we found the pooled point estimates of Xpert MTB/RIF sensitivity and specificity to be lower in peripheral settings than in central and intermediate laboratories. However, there was considerable overlap in the credible intervals of these estimates, and evidence is insufficient to suggest a difference in Xpert MTB/RIF accuracy by setting. One of the confounding factors may be participant spectrum, the direction of which we cannot predict with certainty (Horne 2019). Of note, Theron and colleagues found no difference in Xpert MTB/RIF accuracy when performed by trained nurses in a primary care setting compared to performance by laboratory technicians at a centralized facility (Theron 2014b).
Patient‐important outcomes are especially relevant to patients, decision‐makers, and the wider tuberculosis community. We are not aware of direct evidence of the effect of Xpert Ultra on patient outcomes; however, two meta‐analyses of the impact of Xpert MTB/RIF compared the effect of Xpert MTB/RIF and smear microscopy on all‐cause mortality. Di Tanna and colleagues summarized the accuracy of Xpert MTB/RIF in an individual patient‐level data meta‐analysis (3 trials, 8143 participants) (Di Tanna 2019), and Haraka and colleagues performed a systematic review and meta‐analysis (5 trials, 10,409 participants) (Haraka 2018; WHO Consolidated Guidelines (Module 3) 2020). In both analyses, Xpert MTB/RIF did not show a statistically significant effect on all‐cause mortality, though the direction of effect was towards mortality reduction. These findings require careful interpretation, as the lack of statistical significance of impact of Xpert MTB/RIF on mortality may not indicate a lack of impact, but rather a lack of evidence of a difference (Altman 1995; Greenland 2016). Insufficient power to detect mortality in randomized trials measuring the impact of diagnostic tests on patient‐important outcomes has been previously discussed as a limitation of such trials (Di Tanna 2019; Schumacher 2019). Early detection of tuberculosis and rifampicin resistance may not lead to improved patient outcomes if the test result is not linked to appropriate treatment and other healthcare services (Pai 2018).
In a systematic review of economic evaluations (28 studies), Zwerling and colleagues summarized costs, cost‐effectiveness, and affordability of molecular tests for tuberculosis, including Xpert MTB/RIF, Xpert Ultra, and Truenat (Molbio Diagnostics, Goa, India). Most studies evaluated Xpert MTB/RIF; no studies evaluated Xpert Ultra; and one study evaluated Truenat (WHO Consolidated Guidelines (Module 3) 2020). Variations in costing, effectiveness, and epidemiological parameters were present in the included studies, making direct comparisons across studies challenging. The review found that the cost‐effectiveness of Xpert MTB/RIF improved among populations with higher tuberculosis and HIV prevalence and in settings where rates of empirical tuberculosis treatment were low. Cost‐effectiveness of Xpert MTB/RIF is dependent on a number of factors, including placement of GeneXpert machines (in centralized or decentralized facilities), testing volume, tuberculosis prevalence, level of empirical tuberculosis treatment, and pretreatment loss to follow‐up (WHO Consolidated Guidelines (Module 3) 2020).
After the WHO recommended the use of Xpert MTB/RIF, country‐level uptake was rapid. A 2018 survey of market penetration of Xpert MTB/RIF in high tuberculosis burden countries found greater use of Xpert MTB/RIF compared to smear microscopy for tuberculosis diagnosis (Cazabon 2018). There are currently no publications regarding market penetration of Xpert Ultra, which only requires new cartridges and a software update to existing GeneXpert machines. However, by the end of 2019, over 80 countries had procured Xpert Ultra tests. In more than 20 of these countries, Xpert Ultra conversion from MTB/RIF was greater than 90%. Examples of countries fully converted to Xpert Ultra are Eswatini (high TB/HIV burden country); Lesotho (high tuberculosis burden and high TB/HIV burden country); Morocco; South Africa (high tuberculosis burden, high TB/HIV burden, high MDR‐TB burden country); Uganda (high TB/HIV burden country); Ukraine (high MDR‐TB burden country); and Zimbabwe (high tuberculosis burden, high TB/HIV burden, high MDR‐TB burden country) (Denamps 2020 [pers comm]).
This review represents the most comprehensive review of the diagnostic accuracy of Xpert Ultra, including comparative accuracy studies of Xpert Ultra and Xpert MTB/RIF. Regarding Xpert MTB/RIF, previous reviews have provided additional findings (Horne 2019; Steingart 2014). These reviews provide evidence that may help countries make decisions about scaling up the tests for programmatic management of tuberculosis and drug‐resistant tuberculosis. Although the information in this review will help inform such decisions, other factors such as resource requirements and feasibility (including stable electrical power supply, temperature control, and maintenance of the cartridge modules) will also be important considerations.
Application of the meta‐analysis to a hypothetical cohort
summary of findings Table 1 and summary of findings Table 2 summarize the findings of our review by applying the results to a hypothetical cohort of 1000 individuals with presumptive pulmonary tuberculosis or rifampicin resistance. We have presented several different scenarios. For Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis, we used prevalences of tuberculosis of 2.5%, 10%, and 30%. For the detection of rifampicin resistance, we used prevalences of rifampicin resistance of 2%, 10%, and 15% (5% is estimated to be equivalent to the upper limit for rifampicin resistance prevalence in new cases; 15% is estimated to be the lower limit for rifampicin resistance prevalence among previously treated cases). The consequences of false‐positive results are patient anxiety, morbidity from additional testing and unnecessary treatment, and possible delay in further diagnostic evaluation. The consequences of false‐negative results are increased risk of patient morbidity and mortality, and continued risk of community transmission of tuberculosis.
Strengths and weaknesses of the review
Completeness of evidence
The findings in this review are based on comprehensive searching, strict inclusion criteria, and standardized data extraction. We had repeated correspondence with study authors to obtain additional data and missing information. The search strategy included studies published in all languages. Although we may have missed some studies despite the comprehensive search, we think it is unlikely that the findings would have changed. We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses of Diagnostic Test Accuracy (PRISMA‐DTA) (McInnes 2018).
Accuracy of the reference standards used
Culture is regarded as the best available reference standard for active tuberculosis disease and was the reference standard for tuberculosis in this review (Lewinsohn 2017). We considered the type of culture used in the included studies because liquid culture is more sensitive than solid culture (American Thoracic Society 2000). Most studies did use liquid culture or a combination of solid and liquid culture; only one of the total nine studies exclusively used solid culture. For the culture reference standard, one study used only solid culture (Pereira 2020); five studies (56%) used only liquid culture (Dorman 2018; Mishra 2020a; Mishra 2020b;Opota 2019; Piersimoni 2019); and three studies (33%) used both solid and liquid cultures (Berhanu 2018; Chakravorty 2017; Wang 2019).
Phenotypic culture‐based DST methods using WHO‐recommended critical concentrations and line probe assays, WHO‐recommended tests (WHO Consolidated Guidelines (Module 3) 2020), were the reference standards for rifampicin resistance. Regarding phenotypic culture‐based DST, following completion of this review, the WHO published recommendations lowering critical concentrations for rifampicin resistance testing (MGIT and 7H10) to reduce misclassification of false resistance (WHO 2021). We will incorporate the new recommendations in future updates of this review. In this review, two of the total five studies used line probe assays (i.e. MTBDRplus) alone as the reference standard.
We assessed the number of specimens with NTMs that were Xpert Ultra‐ and Xpert MTB/RIF‐positive. Three studies reported a total of 26 NTMs that grew from the specimens tested. Only one of these studies reported on Xpert Ultra and Xpert MTB/RIF results in those with NTM (Piersimoni 2019), and found neither test was positive in those that grew NTMs. In the previous review, among 10 studies that reported information comprising 141 NTM, Xpert MTB/RIF was negative in all specimens (Horne 2019). Similarly, a study that assessed Xpert Ultra specificity using 20 culture‐positive NTM specimens (covering a total of 18 species) found that Xpert Ultra was negative for all specimens (Perez‐Risco 2018).
Quality of the included studies
Most studies used consecutive selection of participants and interpreted the reference standard results without knowledge of index test results. Xpert Ultra and MTB/RIF results are generated automatically, without requiring subjective interpretation. For pulmonary tuberculosis detection, using QUADAS‐C, for patient selection, six studies had low risk of bias. We considered three studies to have high risk of bias: one study did not report the manner of participant selection (Chakravorty 2017); one study exclusively enrolled participants who had recently received tuberculosis treatment (Mishra 2020b); and one study exclusively enrolled smear‐negative participants (Wang 2019). In general, studies were fairly well reported, although we corresponded with authors for additional data and missing information. We encourage the authors of future studies to follow the recommendations in the STARD (Standards for Reporting of Diagnostic Accuracy) statement to improve the quality of reporting (Bossuyt 2015).
Interpretability of subgroup analyses
We investigated potential sources of heterogeneity in different subgroups. For tuberculosis detection, Xpert Ultra had higher sensitivity in smear‐positive and HIV‐negative participants. Importantly, we found Xpert Ultra to have higher sensitivity and lower specificity than Xpert MTB/RIF in smear‐negative participants and people living with HIV, two subgroups in which Xpert MTB/RIF has suboptimal sensitivity. In individuals with a history of tuberculosis, we found that Xpert Ultra pooled specificity was considerably lower than the pooled specificity in the primary analysis. Hence, the increase in sensitivity of Xpert Ultra as compared to Xpert MTB/RIF comes at the expense of specificity. As there were small numbers of studies in these analyses, results should be interpreted with caution.
Comparison with other systematic reviews
We are aware of one previously published systematic review that estimated the diagnostic accuracy of Xpert Ultra for pulmonary tuberculosis and rifampicin resistance in adults (Zhang 2019). For the detection of pulmonary tuberculosis, this review found pooled sensitivity of 88.5% (95% CI 82.1 to 92.9) and specificity of 96.7% (95% CI 95.1 to 97.8), similar to the findings of our review (pooled sensitivity 90.9%, 95% CrI 86.2 to 94.7 and pooled specificity 95.6%, 95% CrI 93.0 to 97.4). For the detection of rifampicin resistance, Xpert Ultra accuracy estimates were also similar to those in our review. Another study included adults and children and assessed Xpert Ultra and Xpert MTB/RIF performance in both pulmonary and extrapulmonary tuberculosis (Jiang 2020). We identified several systematic reviews on the diagnostic accuracy of Xpert MTB/RIF, which are summarized in Table 7.
| Author, year (see descriptions of systematic reviews in footnotes) | Date searched up to | No. of studies (participants) | Test | Pulmonary tuberculosis, summary estimates | No. of studies | Rifampicin resistance, summary estimates (95% CrI)* | ||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |||||
| October 2011 | 15 (8117) | Xpert MTB/RIF | 90% (89 to 91) | 98% (98 to 99) | 7 | See footnote for this study | See footnote for this study | |
| (smear‐negative) | May | 15 (2046) | Xpert MTB/RIF | 67% (62 to 71) | 98% (97 to 99) | N/A | N/A | N/A |
| December | 27 (6026) | Xpert MTB/RIF | 89% (85 to 92) | 99% (98 to 99) | Sensitivity: 17 | 95% (90 to 97) | 98% (97 to 99) | |
| Not reported | 12 (8122) | Xpert MTB/RIF | 89% (87 to 90) | 98% (98 to 99) | N/A | N/A | N/A | |
| June | 24 (2486) | Xpert MTB/RIF | 87% (83 to 90) | 97% (96 to 98) | N/A | N/A | N/A | |
| December | N/A | Xpert MTB/RIF | N/A | N/A | 8 | See footnote for this study | See footnote for this study | |
| January | 85 (41,965) | Xpert MTB/RIF | 85% (82 to 87) | 98% (97 to 98) | 48 (8020) | 96% (94 to 97) | 98% (98 to 99) | |
| May 2019 | 10 (not reported) | Xpert Ultra | 89% (82 to 94) | 97% (95 to 98) | 4 (856) | 95% (92 to 97) | 99% (98 to 100) | |
| April 2020 | 19 (5855) | Xpert Ultra and Xpert MTB/RIF | Xpert MTB/RIF: 69% (57 to 78) Xpert Ultra: 84% (76 to 90) | Xpert MTB/RIF: 99% (98 to 99) Xpert Ultra: 97% (96 to 98) | N/A | N/A | N/A | |
Abbreviations: CI: confidence interval; Crl: credible interval; N/A: not applicable.
*Summary sensitivity and specificity estimates are provided for Xpert MTB/RIF, except for Zhang 2019 and Jiang 2020, which evaluated Xpert Ultra.
Chang 2012 included adults and children; Xpert MTB/RIF for detection of rifampicin resistance, sensitivity range 17% to 100%, specificity range 72% to 100%.
Walusimbi 2013 only included smear‐negative participants.
Steingart 2014 is a previous Cochrane Review.
Yan 2016 only included studies that provided data by smear and HIV status.
Li 2017 106 studies (52,410 specimens) for both pulmonary and extrapulmonary tuberculosis.
Alvis‐Zakzuk 2017 summarized accuracy of Xpert MTB/RIF for the detection of rifampicin resistance, sensitivity range 33% to 100%, specificity range 91% to 100%.
Horne 2019 is a previous Cochrane Review update.
Zhang 2019 included adults and children.
Jiang 2020 included adults and children, and assessed Xpert Ultra and Xpert MTB/RIF accuracy for the detection of both pulmonary and extrapulmonary tuberculosis.
Systematic reviews not included in this table:
Kaur 2016 did not provide summary sensitivity and specificity estimates.
Lange 2017 provided sensitivity and specificity with respect to Xpert cycle threshold (CT) values.
Maynard‐Smith 2014 provided accuracy estimates for pulmonary tuberculosis on gastric aspirates and stool.
Wang 2015 only included children.
Compared with previous systematic reviews, our review had a more recent search date thus increasing the number of potential studies for inclusion. Our strict inclusion criteria, for example including only studies that used culture as the reference standard and excluding case‐control studies, meant that some studies included in other reviews were excluded from our review.
Completeness and relevance of the review
Our review included studies using all four previous generations of Xpert MTB/RIF (G1, G2, G3, G4 cartridges) and the newest version, Xpert Ultra. We have included studies that compared the accuracy of Xpert Ultra and Xpert MTB/RIF for diagnosing pulmonary tuberculosis and rifampicin resistance. Our review, plus information previously reported in Horne 2019, present a reasonably complete assessment of the accuracy of these tests. A Cochrane Review on Xpert MTB/RIF for extrapulmonary tuberculosis (including 11 studies evaluating Xpert Ultra) was published (Kohli 2021). This review found that in people with presumptive extrapulmonary tuberculosis, Xpert Ultra and Xpert MTB/RIF may be helpful in confirming the diagnosis. Test sensitivity varied across different extrapulmonary specimens, while for most specimens specificity was high. In addition, Xpert Ultra and Xpert MTB/RIF had similar accuracy for the detection of rifampicin resistance (Kohli 2021). A Cochrane Review update on Xpert MTB/RIF and Xpert Ultra for extrapulmonary tuberculosis is under way. A Cochrane Review on Xpert MTB/RIF and Xpert Ultra for active tuberculosis (pulmonary and extrapulmonary) in children was recently published (Kay 2020).
Applicability of findings to the review question
For the detection of pulmonary tuberculosis, we had low concern for most studies in the index test and reference standard domains. In the patient selection domain, we considered only four studies (44%) to have low concern because participants in these studies were evaluated in primary care facilities, local hospitals, or both settings consistent with the intended use of the test. For the detection of rifampicin resistance, we also had low concern for all QUADAS‐2 domains except for patient selection, where we considered only one of five studies to have low concern for applicability.
![The clinical pathway describes how people might present and the point in the pathway at which they would be considered for testing with Xpert MTB/RIF or Xpert Ultra.Abbreviations: DST: drug susceptibility testing; INH: isoniazid; MDR‐TB: multidrug‐resistant tuberculosis; MTB: Mycobacterium tuberculosis; mWRD: molecular WHO‐recommended rapid diagnostic; PLHIV: people living with HIV; RIF: rifampicin; TB: tuberculosis; Ultra: Xpert Ultra; WHO: World Health Organization.1Persons to be evaluated for TB include adults and children with signs or symptoms suggestive of TB, or with a chest X‐ray with abnormalities suggestive of TB. This algorithm may also be followed for the diagnosis of extrapulmonary TB using CSF, lymph node and other tissue specimens.
2Programs may consider collecting two specimens upfront. The first specimen should be promptly tested using the molecular WRD test. The second specimen may be used for the additional testing described in this algorithm. For persons being evaluated for pulmonary TB, sputum is the preferred specimen. Tissue biopsy samples are difficult or impossible to obtain repeatedly; therefore, they should be tested with as many methods as possible (e.g. molecular WRD, culture, DST or histology).
3Molecular WRD tests appropriate for this algorithm include Xpert MTB/RIF, Xpert Ultra, Truenat MTB, Truenat MTB Plus and TB‐LAMP.
4“MTB detected (not trace)” includes MTB detected as high, moderate, low or very low. These categories apply to the original Xpert MTB/RIF and Xpert Ultra tests. Results of the Truenat MTB and MTB Plus tests and the TB‐LAMP test also fall into the category of “MTB detected (not trace)”.Additional footnotes are explained in WHO Consolidated Guidelines (Module 4) 2020.This algorithm for the use of a molecular WHO‐recommended rapid diagnostic (WRD), which includes Xpert Ultra and Xpert MTB/RIF, comes from the WHO operational handbook on tuberculosis (WHO Consolidated Guidelines (Module 4) 2020). Copyright © [2020] [World Health Organization]: reproduced with permission.](/es/cdsr/doi/10.1002/14651858.CD009593.pub5/media/CDSR/CD009593/image_n/nCD009593-FIG-01.jpg)
The clinical pathway describes how people might present and the point in the pathway at which they would be considered for testing with Xpert MTB/RIF or Xpert Ultra.
Abbreviations: DST: drug susceptibility testing; INH: isoniazid; MDR‐TB: multidrug‐resistant tuberculosis; MTB: Mycobacterium tuberculosis; mWRD: molecular WHO‐recommended rapid diagnostic; PLHIV: people living with HIV; RIF: rifampicin; TB: tuberculosis; Ultra: Xpert Ultra; WHO: World Health Organization.
1Persons to be evaluated for TB include adults and children with signs or symptoms suggestive of TB, or with a chest X‐ray with abnormalities suggestive of TB. This algorithm may also be followed for the diagnosis of extrapulmonary TB using CSF, lymph node and other tissue specimens.
2Programs may consider collecting two specimens upfront. The first specimen should be promptly tested using the molecular WRD test. The second specimen may be used for the additional testing described in this algorithm. For persons being evaluated for pulmonary TB, sputum is the preferred specimen. Tissue biopsy samples are difficult or impossible to obtain repeatedly; therefore, they should be tested with as many methods as possible (e.g. molecular WRD, culture, DST or histology).
3Molecular WRD tests appropriate for this algorithm include Xpert MTB/RIF, Xpert Ultra, Truenat MTB, Truenat MTB Plus and TB‐LAMP.
4“MTB detected (not trace)” includes MTB detected as high, moderate, low or very low. These categories apply to the original Xpert MTB/RIF and Xpert Ultra tests. Results of the Truenat MTB and MTB Plus tests and the TB‐LAMP test also fall into the category of “MTB detected (not trace)”.
Additional footnotes are explained in WHO Consolidated Guidelines (Module 4) 2020.
This algorithm for the use of a molecular WHO‐recommended rapid diagnostic (WRD), which includes Xpert Ultra and Xpert MTB/RIF, comes from the WHO operational handbook on tuberculosis (WHO Consolidated Guidelines (Module 4) 2020). Copyright © [2020] [World Health Organization]: reproduced with permission.
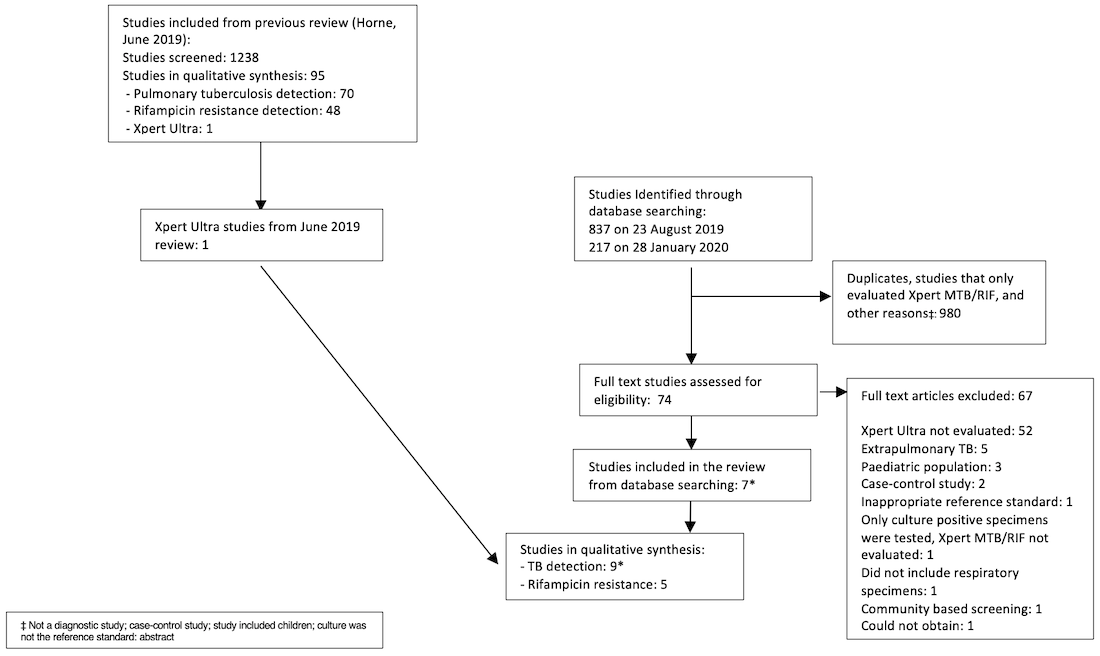
PRISMA flow diagram of studies in the review.
*One publication contributed two distinct studies, which were classified as Mishra 2020a and Mishra 2020b.
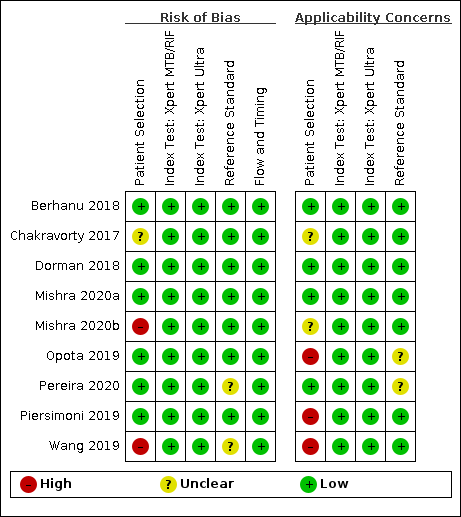
Risk of bias and applicability concerns summary for detection of pulmonary tuberculosis: review authors' judgements about each domain for each included study.

Risk of bias and applicability concerns summary for detection of rifampicin resistance: review authors' judgements about each domain for each included study.

Forest plots of Xpert Ultra versus Xpert MTB/RIF sensitivity and specificity for pulmonary tuberculosis in adults, unselected participants by reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative

Summary plot of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of pulmonary tuberculosis. Each individual study is represented by a shaded circle. The size of the circle is proportional to the sample size of the study such that larger studies are represented by larger circles. The filled circle is the median pooled estimate for sensitivity and specificity, Xpert Ultra (red) and Xpert MTB/RIF (black). The dotted lines represent the 95% credible region around the summary estimate; the dashed lines represent the 95% prediction region. The range is truncated to consider only those regions of the receiver operator characteristic (ROC) space where data have been observed.
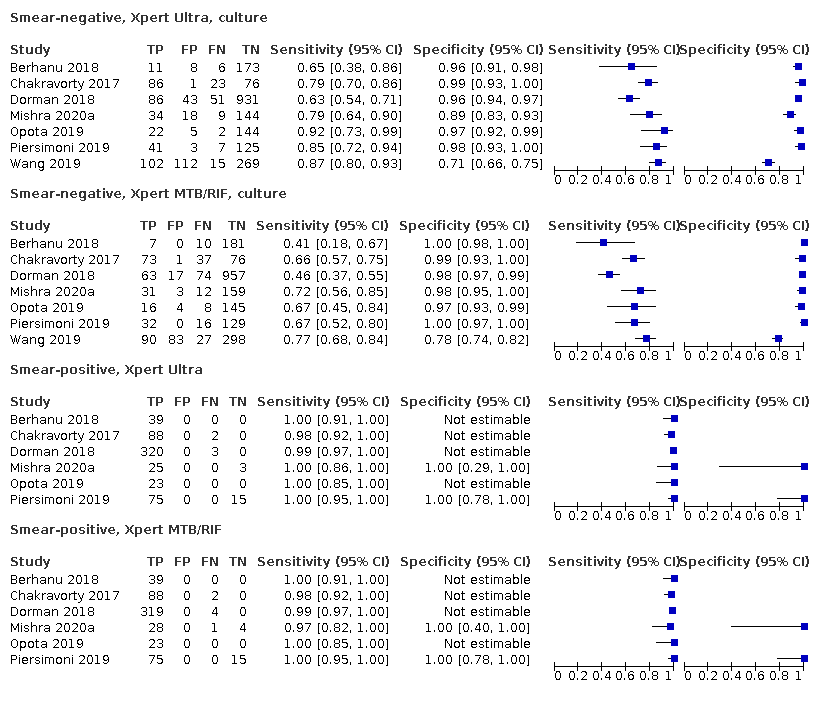
Forest plots of Xpert Ultra versus Xpert MTB/RIF sensitivity and specificity for the detection of pulmonary tuberculosis by smear status. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI). TP = true positive; FP = false positive; FN = false negative; TN = true negative

Forest plots of Xpert Ultra versus Xpert MTB/RIF sensitivity and specificity for the detection of pulmonary tuberculosis by HIV status and history of tuberculosis. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative

Forest plot of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of rifampicin resistance. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative

Summary plot of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of rifampicin resistance. Each individual study is represented by a shaded circle. The size of the circle is proportional to the sample size of the study such that larger studies are represented by larger circles. The filled circle is the median pooled estimate for sensitivity and specificity, Xpert Ultra (red) and Xpert MTB/RIF (black). The dotted lines represent the 95% credible region around the summary estimate; the dashed lines represent the 95% prediction region. The range is truncated to consider only those regions of the receiver operator characteristic (ROC) space where data have been observed.

Forest plots of Xpert Ultra and Xpert MTB/RIF sensitivity and specificity for the detection of rifampicin resistance by smear status. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI). TP = true positive; FP = false positive; FN = false negative; TN = true negative

Forest plots of repeated Xpert Ultra sensitivity and specificity for detection of pulmonary tuberculosis in adults with initial trace result, culture reference standard. The squares represent the sensitivity and specificity of one study, the black line its confidence interval (CI).
TP = true positive; FP = false positive; FN = false negative; TN = true negative

Bayesian bivariate hierarchical model, likelihood.

Bayesian bivariate hierarchical model, prior distributions.

Table. Risk of bias concerns summary for detection of pulmonary tuberculosis: review authors' judgements about each domain for each included study, QUADAS‐C judgements.
P: Patient selection, I: Index test, R: Reference standard, FT: Flow and Timing
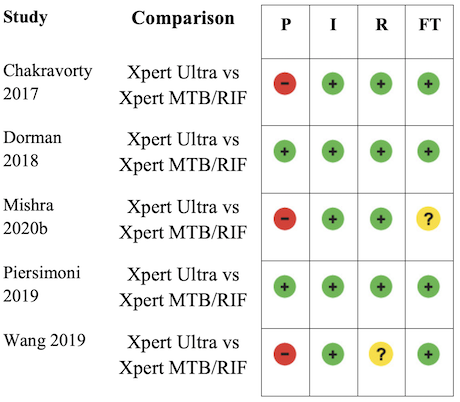
Table. Risk of bias concerns summary for detection of rifampicin resistance: review authors' judgements about each domain for each included study, QUADAS‐C judgements.
P: Patient selection, I: Index test, R: Reference standard, FT: Flow and Timing

Xpert Ultra for detection of pulmonary TB

Xpert MTB/RIF for detection of pulmonary TB

Xpert Ultra for detection of pulmonary TB, composite reference standard

Xpert MTB/RIF for detection of pulmonary TB, composite reference standard
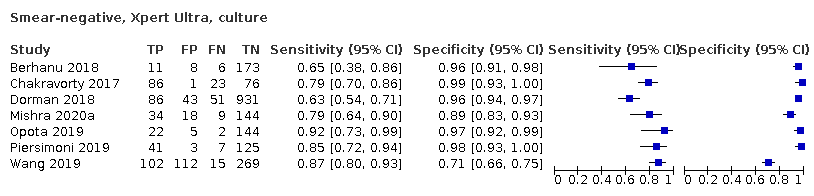
Smear‐negative, Xpert Ultra, culture

Smear‐negative, Xpert MTB/RIF, culture

Smear‐positive, Xpert Ultra

Smear‐positive, Xpert MTB/RIF
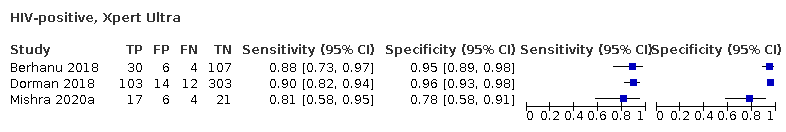
HIV‐positive, Xpert Ultra

HIV‐positive, Xpert MTB/RIF
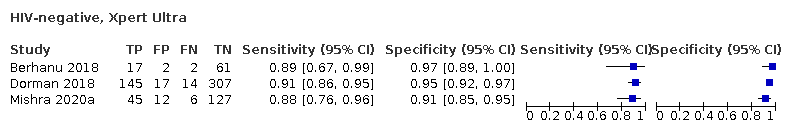
HIV‐negative, Xpert Ultra

HIV‐negative, Xpert MTB/RIF
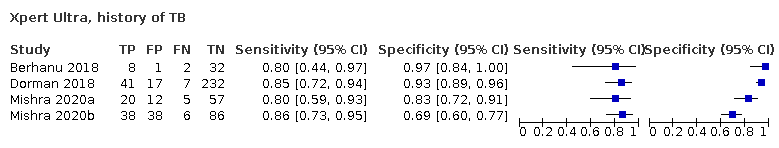
Xpert Ultra, history of TB

Xpert Ultra, no history of TB
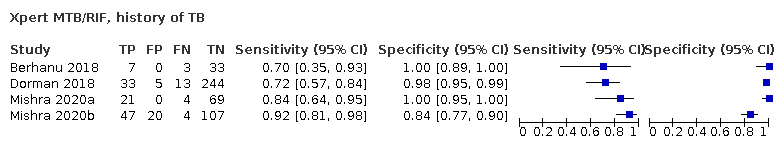
Xpert MTB/RIF, history of TB

Xpert MTB/RIF, no history of TB
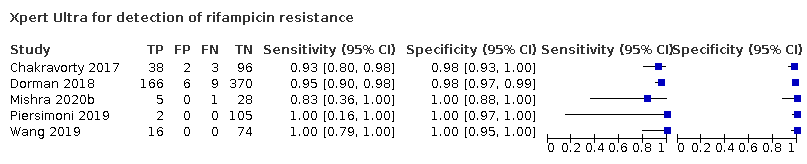
Xpert Ultra for detection of rifampicin resistance
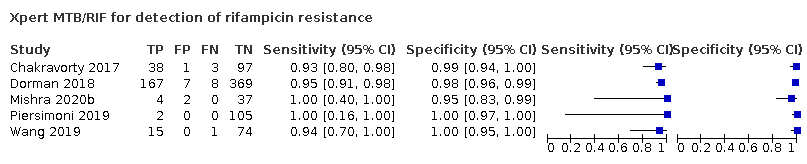
Xpert MTB/RIF for detection of rifampicin resistance

Xpert Ultra repeated test in adults with initial trace result, microbiological reference standard

Xpert Ultra for detection of rifampicin resistance, smear‐positive

Xpert MTB/RIF for detection of rifampicin resistance, smear‐positive

Xpert Ultra for detection of rifampicin resistance, smear‐negative

Xpert MTB/RIF for detection of rifampicin resistance, smear‐negative
| Review question: what is the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for the detection of pulmonary tuberculosis? Patients/population: adults with presumptive pulmonary tuberculosis. Participants were unselected, meaning they were not enrolled in a study based on microscopy smear results or history of tuberculosis Role: an initial test Index tests: Xpert Ultra and Xpert MTB/RIF Threshold for index tests: an automated result is provided Reference standards: solid or liquid culture Studies: cross‐sectional and cohort studies Setting: primary care facilities and local hospitals Xpert Ultra sensitivity 90.9% (86.2 to 94.7) and specificity 95.6% (93.0 to 97.4) Xpert MTB/RIF sensitivity 84.7% (78.6 to 89.9) and specificity 98.4% (97.0 to 99.3) | ||||||||
| Test result | Number of results per 1000 patients tested (95% CrI)** | Number of participants*** | Certainty of the evidence (GRADE) | |||||
|---|---|---|---|---|---|---|---|---|
| Prevalence 2.5% | Prevalence 10% | Prevalence 30% | ||||||
| Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | |||
| True positives (TP) | 23 (22 to 24) | 21 (20 to 22) | 91 (86 to 95) | 85 (79 to 90) | 273 (259 to 284) | 254 (236 to 270) | 983 (7) | ⊕⊕⊕⊕ High |
| 2 more TP in Xpert Ultra | 6 more TP in Xpert Ultra | 19 more TP in Xpert Ultra | ||||||
| False negatives (FN) | 2 (1 to 3) | 4 (3 to 5) | 9 (5 to 14) | 15 (10 to 21) | 27 (16 to 41) | 46 (30 to 64) | ||
| 2 fewer FN in Xpert Ultra | 6 fewer FN in Xpert Ultra | 19 fewer FN in Xpert Ultra | ||||||
| True negatives (TN) | 932 (907 to 950) | 959 (946 to 968) | 860 (837 to 877) | 886 (873 to 894) | 669 (651 to 682) | 689 (679 to 695) | 1852 (7) | ⊕⊕⊕⊕ High |
| 27 fewer TN in Xpert Ultra | 26 fewer TN in Xpert Ultra | 20 fewer TN in Xpert Ultra | ||||||
| False positives (FP) | 43 (25 to 68) | 16 (7 to 29) | 40 (23 to 63) | 14 (6 to 27) | 31 (18 to 49) | 11 (5 to 21) | ||
| 27 more FP in Xpert Ultra | 26 more FP in Xpert Ultra | 20 more FP in Xpert Ultra | ||||||
| Abbreviations: CrI: credible interval | ||||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||||
| *The results presented in this table should not be interpreted in isolation from the results of individual included studies contributing to each summary test accuracy measure. **95% credible limits were estimated based on those around the point estimates for pooled sensitivity and specificity. Prevalence estimates were suggested by the World Health Organization Global Tuberculosis Programme. The median tuberculosis prevalence in the included studies was 30.1% (range 12.8% to 72.2%). ***In the Xpert Ultra analysis there were 1851 participants. Piersimoni 2019 reported three non‐determinate results for Xpert Ultra and two for Xpert MTB/RIF, accounting for the small difference in the total number of participants. | ||||||||
| Review question: what is the diagnostic accuracy of Xpert Ultra and Xpert MTB/RIF for the detection of rifampicin resistance? Patients/population: adults with presumptive pulmonary tuberculosis Role: an initial test Index tests: Xpert Ultra and Xpert MTB/RIF Threshold for index tests: an automated result is provided Reference standards: drug susceptibility testing, line probe assay Studies: cross‐sectional and cohort studies Setting: primary care facilities and local hospitals Xpert Ultra sensitivity 94.9% (88.9 to 97.9) and specificity 99.1% (97.7 to 99.8) Xpert MTB/RIF sensitivity 95.3% (90.0 to 98.1) and specificity 98.8% (97.2 to 99.6) | ||||||||
| Test result | Number of results per 1000 patients tested (95% CrI)** | Number of participants*** | Certainty of the evidence (GRADE) | |||||
|---|---|---|---|---|---|---|---|---|
| Prevalence 2% | Prevalence 10% | Prevalence 15% | ||||||
| Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | Xpert Ultra | Xpert MTB/RIF | |||
| True positives (TP) | 19 (18 to 20) | 19 (18 to 20) | 95 (89 to 98) | 95 (90 to 98) | 142 (133 to 147) | 143 (135 to 147) | 238 (5) | ⊕⊕⊕⊕ High |
| 0 fewer TP in Xpert Ultra | 0 fewer TP in Xpert Ultra | 1 fewer TP in Xpert Ultra | ||||||
| False negatives (FN) | 1 (0 to 2) | 1 (0 to 2) | 5 (2 to 11) | 5 (2 to 10) | 8 (3 to 18) | 7 (3 to 15) | ||
| 0 fewer FN in Xpert Ultra | 0 fewer FN in Xpert Ultra | 1 more FN in Xpert Ultra | ||||||
| True negatives (TN) | 971 (957 to 977) | 968 (953 to 976) | 892 (879 to 897) | 889 (875 to 896) | 842 (830 to 847) | 840 (826 to 847) | 692 (5) | ⊕⊕⊕⊕ High |
| 3 more TN in Xpert Ultra | 3 more TN in Xpert Ultra | 2 more TN in Xpert Ultra | ||||||
| False positive (FP) | 9 (3 to 23) | 12 (4 to 27) | 8 (3 to 21) | 11 (4 to 25) | 8 (3 to 20) | 10 (3 to 24) | ||
| 3 fewer FP in Xpert Ultra | 3 fewer FP in Xpert Ultra | 2 fewer FP in Xpert Ultra | ||||||
| Abbreviations: CrI: credible interval | ||||||||
| GRADE certainty of the evidence High: we are very confident that the true effect lies close to that of the estimate of the effect. | ||||||||
| *The results presented in this table should not be interpreted in isolation from results of the individual included studies contributing to each summary test accuracy measure. **Prevalence estimates were suggested by the World Health Organization Global Tuberculosis Programme. The median prevalence of rifampicin resistance in the included studies was 23.6% (range 1.9% to 31.8%). Credible limits were estimated based on those around the point estimates for pooled sensitivity and specificity. ***Xpert Ultra included 921 participants, and Xpert MTB/RIF included 930 participants, mainly owing to indeterminate results with Xpert Ultra. | ||||||||
| Study, year ID | Country | Study design | Number of participants | Age (mean or median; years) | Female sex | HIV‐positive | History of tuberculosis | Pulmonary tuberculosis reference standard | Rifampicin resistance reference standard |
|---|---|---|---|---|---|---|---|---|---|
| South Africa | Prospective cohort | 237 | 36 | 33% | 62% | 18% | LJ and MGIT; composite | LJ, MGIT, and MTBDRplus | |
| FIND biobank frozen specimens (Peru, Vietnam, South Africa) and clinical specimens (Georgia, India) | Cross‐sectional | 277 | Not reported | Not reported | Not reported | Not reported | LJ and MGIT | LJ and MGIT | |
| Belarus, Brazil, China, Georgia, India, Kenya, South Africa, Uganda | Prospective cohort | 1439 for detection of MTB, 551 for detection of rifampicin resistance | 28 | 40% | 44% | 21% | LJ and MGIT | LJ and MGIT | |
| South Africa | Prospective cohort | 239 | 37 | 49% | 20% | 39% | MGIT | MTBDRplus | |
| South Africa | Cross‐sectional | 346 | 38 | 40% | 44% | 100% | MGIT | MTBDRplus | |
| Switzerland | Cross‐sectional | 196 | Not reported | Not reported | Not reported | Not reported | MGIT; composite | MGIT | |
| Brazil | Cross‐sectional | 180 | 50 | 44% | 2% | 0% | Ogawa‐Kudoh | N/A | |
| Italy | Cross‐sectional | 266 | 42 | 37% | Not reported | Excluded | MGIT | MGIT | |
| China | Prospective cohort | 498 | 47 | 34% | 0% | 50% | LJ and MGIT | LJ | |
| Abbreviations: FIND: Foundation for Innovative New Diagnostics; LJ: Löwenstein–Jensen; MGIT: Mycobacteria Growth Indicator Tube; MTB; Mycobacterium tuberculosis; N/A: not applicable. | |||||||||
| Test (analysis) | Reference standard | No. studies (participants) | No. (%) with pulmonary TB or rifampicin resistance | Median pooled sensitivity | Median pooled specificity | Positive predictive value (95% CrI) * | Negative predictive value |
|---|---|---|---|---|---|---|---|
| Xpert Ultra, unselected participants* (pulmonary tuberculosis detection) | Culture | 7 (2834)** | 983 (34.7%) | 90.9% (86.2 to 94.7) | 95.6% (93.0 to 97.4) | 69.6% (58.7 to 79.8) | 99.0% (98.4 to 99.4) |
| Xpert MTB/RIF (pulmonary tuberculosis detection) | Culture | 7 (2835) | 983 (34.7%) | 84.7% (78.6 to 89.9) | 98.4% (97.0 to 99.3) | 85.4% (75.8 to 93.1) | 98.3% (97.6 to 98.9) |
| Xpert Ultra (rifampicin resistance detection) | DST, line probe assays | 5 (921) | 240 (26.1%) | 94.9% (88.9 to 97.9) | 99.1% (97.7 to 99.8) | 91.7% (82.1 to 97.4) | 99.4% (98.7 to 99.8) |
| Xpert MTB/RIF (rifampicin resistance detection) | DST, line probe assays | 5 (930) | 238 (25.6%) | 95.3% (90.0 to 98.1) | 98.8% (97.2 to 99.6) | 99.5% (98.9 to 99.8) | 99.4% (98.7 to 99.8) |
| Abbreviations: CrI: credible interval; DST: drug susceptibility testing with solid or liquid culture methods * Positive and negative predictive values were determined at a pretest probability of 10% **This analysis included studies that did not preselect participants based on microcopy results or those who had received previous antituberculosis treatment. | |||||||
| Detection of pulmonary tuberculosis | ||||
|---|---|---|---|---|
| Test (studies, participants) | Xpert Ultra (7, 2834) | Xpert MTB/RIF (7, 2835) | Difference (Xpert Ultra minus Xpert MTB/RIF)* | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 90.9% (86.2 to 94.7) | 84.7% (78.6 to 89.9) | 6.3% (0.1 to 12.8) | 0.98 |
| Specificity (95% CrI) | 95.6% (93.0 to 97.4) | 98.4% (97.0 to 99.3) | −2.7% (−5.7 to −0.5) | 0.01 |
| Smear‐positive (tuberculosis detection) | ||||
| Test (studies, participants) | Xpert Ultra (6, 593) | Xpert MTB/RIF (6, 598) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 99.3% (98.1 to 99.8) | 98.9% (97.5 to 99.6) | 0.3% (−1.0 to 1.8) | 0.72 |
| Specificity (95% CrI) | Not estimated | Not estimated | N/A | N/A |
| Smear‐negative (tuberculosis detection) | ||||
| Test (studies, participants) | Xpert Ultra (6, 2049) | Xpert MTB/RIF (6, 2051) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 77.5% (67.6 to 85.6) | 60.6% (48.4 to 71.7) | 16.7% (2.1 to 31.8) | 1.00 |
| Specificity (95% CrI) | 95.8% (92.9 to 97.7) | 98.8% (97.7 to 99.5) | −3.0% (‐5.9 to −0.9) | 0.00 |
| History of tuberculosis | ||||
| Test (studies, participants) | Xpert Ultra (4, 602) | Xpert MTB/RIF (4, 610) | Difference (Xpert Ultra minus Xpert MTB/RIF)* | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 84.2% (72.5 to 91.7) | 81.8% (68.7 to 90.0) | 2.4% (−11.9 to 17.2) | 0.64 |
| Specificity (95% CrI) | 88.2% (70.5 to 96.6) | 97.4% (91.7 to 99.5) | −8.9% (−27.0 to 0.6) | 0.03 |
| Detection of rifampicin resistance | ||||
| Test (studies, participants) | Xpert Ultra (5, 921) | Xpert MTB/RIF (5, 930) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 94.9% (88.9 to 97.9) | 95.3% (90.0 to 98.1) | −0.3% (−6.9 to 5.7) | 0.45 |
| Specificity (95% CrI) | 99.1% (97.7 to 99.8) | 98.8% (97.2 to 99.6) | 0.3% (−1.2 to 2.0) | 0.67 |
| Smear‐positive (rifampicin resistance detection) | ||||
| Test (studies, participants) | Xpert Ultra (4, 686) | Xpert MTB/RIF (4, 699) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 93.9% (84.4 to 97.7) | 95.5% (88.4 to 98.6) | −1.5% (−10.9 to 6.0) | 0.32 |
| Specificity (95% CrI) | 99.3% (97.8 to 99.9) | 99.1% (97.3 to 99.9) | 0.1% (−1.5 to 2.0) | 0.59 |
| Smear‐negative (rifampicin resistance detection) | ||||
| Test (studies, participants) | Xpert Ultra (4, 412) | Xpert MTB/RIF (4, 416) | Difference (Xpert Ultra minus Xpert MTB/RIF)** | Probability (Xpert Ultra minus Xpert MTB/RIF) |
| Sensitivity (95% CrI) | 92.0% (75.0 to 95.8) | 95.4% (82.3 to 99.3) | −3.1% (−20.7 to 11.7) | 0.30 |
| Specificity (95% CrI) | 99.4% (96.2 to 100) | 99.2% (94.8 to 100) | 0.1% (−3.0 to 4.5) | 0.58 |
| Abbreviations: CrI: credible interval * We determined absolute differences for sensitivity and specificity when there were at least four studies in a subgroup. | ||||
| Analysis | Test | No. of studies (participants) | Median pooled sensitivity | Median pooled specificity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|---|
| HIV‐negative | Xpert Ultra | 3 (755) | 90.3% (80.3 to 95.6) | 94.3% (79.8 to 98.7) | 63.5% (45.6 to 79.7) | 98.9% (97.7 to 99.5) |
| HIV‐negative | Xpert MTB/RIF | 3 (755) | 89.0% (78.3 to 94.8) | 98.1% (95.3 to 99.4) | 83.8% (67.6 to 94.0) | 98.8% (97.6 to 99.4) |
| HIV‐positive | Xpert Ultra | 3 (627) | 87.6% (75.4 to 94.1) | 92.8% (82.3 to 97.0) | 57.4% (34.5 to 76.8) | 98.5% (97.0 to 99.3) |
| HIV‐positive | Xpert MTB/RIF | 3 (635) | 74.9% (58.7 to 86.2) | 99.7% (98.6 to 100.0) | 96.3% (85.4 to 99.6) | 97.3% (95.6 to 98.5) |
| Abbreviations: CI: confidence interval; CrI: credible interval * Positive and negative predictive values were determined at a pretest probability of 10% | ||||||
| Study | Country | Culture‐positive MTB/total | Trace results (% of Ultra positive) | Number (%) of trace results with history of tuberculosis | Culture‐positive trace/total trace | Additional testing on trace results (not including retesting) | Trace results repeated? |
|---|---|---|---|---|---|---|---|
| South Africa | 56/237 | 6 (10.3%) | 1/5 (20.0%); this 1 patient was culture positive | 2/6 | Sputum re‐collected at day 60 in 1 participant was MGIT negative. | No | |
| FIND biobank samples (Peru, Vietnam, South Africa) and clinical samples (Georgia, India) | 200/277 | Not reported | ‐ | ‐ | ‐ | No | |
| Belarus, Brazil, China, Georgia, India, Kenya, South Africa, Uganda | 462/1439 | 32 (7.1%) | Of 19 culture‐negative trace results, 11 (57.9%) had history of TB. | 13/32 | Among culture‐negative, a follow‐up culture at 2 months was positive in 2/10. | Yes: 13 of the retested samples were culture+; of these 9 were Ultra + on repeat. 19 of the samples were culture‐, of which 10 were Ultra false + | |
| South Africa | 72/239 | 13 (18.6%) | 6/13 (46.2%) | 4/13 | ‐ | Yes: new sample collected median 444 days, after the initial testing. 4 samples retested (1 culture+, 3 culture‐); all culture‐ were not detected, and culture+ was Ultra + | |
| South Africa | 44/168 | 21 (27.6%) | 21/21 (100%) | 2/21 | ‐ | No | |
| Switzerland | 47/196 | 5 (10.0%) | Not reported | 4/5 | The 1 culture‐negative patient was culture‐positive on a lymph node specimen. | No | |
| Brazil | 157/180 | 1 (3.0%) | Not reported | Not reported | ‐ | No | |
| Italy | 123/266 | 8 (6.7%) | Excluded from study | 5/8 | ‐ | Yes: 4 respiratory samples retested (1 culture+, 3 culture‐); all culture‐ were not detected, and culture+ was Ultra + | |
| China | 117/498 | 65 (30.4%) | Not reported | Not reported | ‐ | No | |
| Abbreviations: FIND: Foundation for Innovative New Diagnostics; MGIT: Mycobacteria Growth Indicator Tube; MTB: Mycobacterium tuberculosis; TB: tuberculosis ‐ Could not determine | |||||||
| Type of analysis (no. of studies, participants) | Median pooled sensitivity (95% Crl) | Median pooled specificity (95% Crl) | Positive predictive value | Negative predictive value |
|---|---|---|---|---|
| Xpert Ultra sensitivity and specificity for pulmonary tuberculosis detection in studies with unselected patients (7, 2834) | 90.9% (86.2 to 94.7) | 95.6% (93.0 to 97.4) | 69.6% (58.7 to 79.8) | 99.0% (98.4 to 99.4) |
| Studies that only included untreated participants (exclude studies with some percentage of participants who were receiving tuberculosis treatment) (5, 2361) | 90.9% (84.7 to 95.3) | 94.9% (91.3 to 97.2) | 66.4% (53.2 to 78.4) | 98.9% (98.2 to 99.5) |
| Studies that used liquid culture only as the reference standard for tuberculosis detection (4, 978) | 91.1% (84.0 to 95.5) | 96.1% (91.7 to 98.5) | 72.1% (54.5 to 87.2) | 99.0% (98.2 to 99.5) |
| Studies where consecutive or random participants were selected (6, 2557) | 91.6% (86.6 to 95.4) | 95.3% (92.4 to 97.2) | 68.2% (56.9 to 78.5) | 99.0% (98.5 to 99.5) |
| Studies where the reference standard was blinded (6, 2654) | 90.2% (85.2 to 93.8) | 95.9% (93.0 to 97.7) | 70.8% (58.5 to 81.8) | 98.9% (98.3 to 99.3) |
| Studies using fresh specimens (4, 2095) | 89.8% (82.1 to 95.1) | 94.1% (89.3 to 96.8) | 62.7% (47.9 to 75.8) | 98.8% (97.9 to 99.4) |
| Abbreviations: Crl: credible interval. | ||||
| Author, year (see descriptions of systematic reviews in footnotes) | Date searched up to | No. of studies (participants) | Test | Pulmonary tuberculosis, summary estimates | No. of studies | Rifampicin resistance, summary estimates (95% CrI)* | ||
|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |||||
| October 2011 | 15 (8117) | Xpert MTB/RIF | 90% (89 to 91) | 98% (98 to 99) | 7 | See footnote for this study | See footnote for this study | |
| (smear‐negative) | May | 15 (2046) | Xpert MTB/RIF | 67% (62 to 71) | 98% (97 to 99) | N/A | N/A | N/A |
| December | 27 (6026) | Xpert MTB/RIF | 89% (85 to 92) | 99% (98 to 99) | Sensitivity: 17 | 95% (90 to 97) | 98% (97 to 99) | |
| Not reported | 12 (8122) | Xpert MTB/RIF | 89% (87 to 90) | 98% (98 to 99) | N/A | N/A | N/A | |
| June | 24 (2486) | Xpert MTB/RIF | 87% (83 to 90) | 97% (96 to 98) | N/A | N/A | N/A | |
| December | N/A | Xpert MTB/RIF | N/A | N/A | 8 | See footnote for this study | See footnote for this study | |
| January | 85 (41,965) | Xpert MTB/RIF | 85% (82 to 87) | 98% (97 to 98) | 48 (8020) | 96% (94 to 97) | 98% (98 to 99) | |
| May 2019 | 10 (not reported) | Xpert Ultra | 89% (82 to 94) | 97% (95 to 98) | 4 (856) | 95% (92 to 97) | 99% (98 to 100) | |
| April 2020 | 19 (5855) | Xpert Ultra and Xpert MTB/RIF | Xpert MTB/RIF: 69% (57 to 78) Xpert Ultra: 84% (76 to 90) | Xpert MTB/RIF: 99% (98 to 99) Xpert Ultra: 97% (96 to 98) | N/A | N/A | N/A | |
| Abbreviations: CI: confidence interval; Crl: credible interval; N/A: not applicable. *Summary sensitivity and specificity estimates are provided for Xpert MTB/RIF, except for Zhang 2019 and Jiang 2020, which evaluated Xpert Ultra. Chang 2012 included adults and children; Xpert MTB/RIF for detection of rifampicin resistance, sensitivity range 17% to 100%, specificity range 72% to 100%. Systematic reviews not included in this table: Kaur 2016 did not provide summary sensitivity and specificity estimates. | ||||||||
| Protocol section | Refreshed protocol |
|---|---|
| Background and research question | This review update will describe the burden of pulmonary tuberculosis worldwide based on the latest World Health Organization (WHO) Global Tuberculosis Report. The Background will describe the updated WHO guidelines on molecular methods for diagnosing tuberculosis, including Xpert MTB/RIF and Xpert Ultra. The WHO Meeting to update the guidelines will take place 3 to 6 December 2019. This Cochrane Review update will have informed these guidelines. |
| Inclusion criteria | This is a diagnostic test accuracy review. Participants, index tests, and target condition will be the same as in Horne 2019. We will add a composite reference standard for Xpert Ultra defined as culture or clinical criteria as defined by the primary study authors, or both. The primary objectives are to assess the diagnostic accuracy of Xpert Ultra for the diagnosis of pulmonary tuberculosis and to assess the diagnostic accuracy of Xpert Ultra for the diagnosis rifampicin resistance in adults. Secondary objectives are as follows:
Concerning patient outcomes, the Discussion will summarize and refer to key findings in the test‐treatment Cochrane Review by Haraka 2018. |
| Methods | We will use QUADAS‐2 to appraise methodological quality of the included studies, consistent with Horne 2019. If there are sufficient data, we will perform meta‐analyses using a bivariate random‐effects model. The analyses will include:
We will create 'Summary of findings' tables for the two primary objectives of the review. |
| *This table was approved by the Cochrane Infectious Diseases Group editorial team on 23 October 2019. | |
| Test | No. of studies | No. of participants |
| 1 Xpert Ultra for detection of pulmonary TB Show forest plot | 9 | 3500 |
| 2 Xpert MTB/RIF for detection of pulmonary TB Show forest plot | 7 | 2835 |
| 3 Xpert Ultra for detection of pulmonary TB, composite reference standard Show forest plot | 2 | 433 |
| 4 Xpert MTB/RIF for detection of pulmonary TB, composite reference standard Show forest plot | 2 | 433 |
| 5 Smear‐negative, Xpert Ultra, culture Show forest plot | 7 | 2547 |
| 6 Smear‐negative, Xpert MTB/RIF, culture Show forest plot | 7 | 2549 |
| 7 Smear‐positive, Xpert Ultra Show forest plot | 6 | 593 |
| 8 Smear‐positive, Xpert MTB/RIF Show forest plot | 6 | 598 |
| 9 HIV‐positive, Xpert Ultra Show forest plot | 3 | 627 |
| 10 HIV‐positive, Xpert MTB/RIF Show forest plot | 3 | 635 |
| 11 HIV‐negative, Xpert Ultra Show forest plot | 3 | 755 |
| 12 HIV‐negative, Xpert MTB/RIF Show forest plot | 3 | 755 |
| 13 Xpert Ultra, history of TB Show forest plot | 4 | 602 |
| 14 Xpert Ultra, no history of TB Show forest plot | 3 | 1476 |
| 15 Xpert MTB/RIF, history of TB Show forest plot | 4 | 610 |
| 16 Xpert MTB/RIF, no history of TB Show forest plot | 3 | 1476 |
| 17 Xpert Ultra for detection of rifampicin resistance Show forest plot | 5 | 921 |
| 18 Xpert MTB/RIF for detection of rifampicin resistance Show forest plot | 5 | 930 |
| 19 Xpert Ultra repeated test in adults with initial trace result, microbiological reference standard Show forest plot | 3 | 40 |
| 20 Xpert Ultra for detection of rifampicin resistance, smear‐positive Show forest plot | 4 | 686 |
| 21 Xpert MTB/RIF for detection of rifampicin resistance, smear‐positive Show forest plot | 4 | 699 |
| 22 Xpert Ultra for detection of rifampicin resistance, smear‐negative Show forest plot | 4 | 412 |
| 23 Xpert MTB/RIF for detection of rifampicin resistance, smear‐negative Show forest plot | 4 | 416 |

