Estatinas para la estenosis de la válvula aórtica
Resumen
Antecedentes
La estenosis de la válvula aórtica es el tipo más frecuente de cardiopatía valvular en los EE.UU. y Europa. La estenosis de la válvula aórtica se considera similar a la enfermedad ateroesclerótica. Algunos estudios han evaluado las estatinas para la estenosis de la válvula aórtica.
Objetivos
Evaluar la efectividad y la seguridad de las estatinas en la estenosis de la válvula aórtica.
Métodos de búsqueda
Se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase, LILACS ‐ IBECS, Web of Science and CINAHL Plus. Se hicieron búsquedas en estas bases de datos desde su inicio hasta 24 noviembre 2015. También se realizaron búsquedas de ensayos en curso en registros de ensayos. No se aplicaron restricciones de idioma.
Criterios de selección
Ensayos clínicos controlados aleatorizados (ECA) que compararon las estatinas solas o en combinación con otros fármacos sistémicos para reducir los niveles de colesterol versus placebo o atención habitual.
Obtención y análisis de los datos
Los resultados primarios fueron gravedad de la estenosis de la válvula aórtica (evaluada por criterios ecocardiográficos: gradiente de presión media, área de la válvula y velocidad de flujo aórtico), no reemplazo de la válvula y muerte por causa cardiovascular. Los resultados secundarios fueron hospitalización por cualquier motivo, mortalidad general, eventos adversos y calidad de vida del paciente.
Dos autores de la revisión, de forma independiente, seleccionaron los ensayos para su inclusión, extrajeron los datos y evaluaron el riesgo de sesgo. Se utilizó la metodología GRADE para evaluar la calidad de los hallazgos y se utilizó GRADE profiler (GRADEPRO) para importar los datos de Review Manager 5.3 para crear una tabla "Resumen de los hallazgos".
Resultados principales
Se incluyeron cuatro ECA con 2360 participantes que comparaban las estatinas (1185 participantes) con el placebo (1175 participantes). Se encontró evidencia de baja calidad para el resultado primario gravedad de la estenosis de la válvula aórtica, evaluada por el gradiente de presión media diferencia de medias [DM] ‐0,54; intervalo de confianza [IC] del 95%: ‐1,88 a 0,80; participantes = 1935; estudios = 2), el área de la válvula (DM ‐0,07; IC del 95%: ‐0,28 a 0,14; participantes = 127; estudios = 2), y la velocidad del flujo aórtico (DM ‐0,06; IC del 95%: ‐0,26 a 0,14; participantes = 155, estudio = 1). Evidencia de calidad moderada no mostró un efecto sobre el no reemplazo de la válvula con las estatinas (riesgo relativo [RR] 0,93; IC del 95%: 0,81 a 1,06; participantes = 2360; estudios = 4), ni efectos sobre el dolor muscular como un evento adverso (RR 0,91; IC del 95%: 0,75 a 1,09; participantes = 2204, estudios = 3; evidencia de calidad moderada). Evidencia de calidad baja y muy baja mostró falta de confiabilidad alrededor del efecto de las estatinas en la muerte por causa cardiovascular (RR 0,80; IC del 95%: 0,56 a 1,15; participantes = 2297; estudios = 3; evidencia de baja calidad) y la hospitalización por cualquier motivo (RR 0,84; IC del 95%: 0,39 a 1,84; participantes = 155; estudio = 1; evidencia de calidad muy baja). Ninguno de los cuatro estudios incluidos informó la mortalidad general ni la calidad de vida del paciente.
Conclusiones de los autores
Los hallazgos mostraron falta de confiabilidad alrededor del efecto de las estatinas para la estenosis de la válvula aórtica.La calidad de la evidencia de los resultados informados varió de moderada a muy baja. Estos resultados apoyan las guías europeas y de EE.UU. (2012 y 2014, respectivamente) con respecto a que hasta el presente no hay una opción clínica de tratamiento para la estenosis de la válvula aórtica.
PICO
Resumen en términos sencillos
Estatinas para la estenosis de la válvula aórtica
Pregunta de la revisión
¿Cuál es la evidencia con respecto al efecto de las estatinas en los pacientes que presentan estenosis de la válvula aórtica?
Antecedentes
El corazón es el responsable de bombear la sangre a todo el cuerpo. Tiene cuatro válvulas que controlan el flujo sanguíneo en su interior. Una de estas válvulas es la válvula aórtica que controla el flujo de la sangre de la cámara del ventrículo izquierdo al cuerpo. La estenosis de la válvula aórtica es una enfermedad caracterizada por la reducción de esta válvula. Este es el tipo más frecuente de cardiopatía valvular en los EE.UU. y Europa. Su incidencia aumenta con la edad y del 2% al 7% de los adultos con más de 65 años de edad presenta estenosis de la válvula aórtica. La estenosis de la válvula aórtica se considera similar a la enfermedad ateroesclerótica y se sabe que tiene un período asintomático prolongado durante varias décadas. Cuando se manifiesta clínicamente, síntomas como el síncope (lapso breve de pérdida de la conciencia), la angina y la disnea (falta de aire) pueden provocar la muerte. Algunos ensayos prospectivos y retrospectivos han indicado que las estatinas pueden retrasar la progresión de la estenosis de la válvula aórtica. Las estatinas se consideran fármacos muy útiles para reducir el colesterol elevado.
Características de los estudios
La evidencia está actualizadas hasta el 24 noviembre 2015. Se efectuaron búsquedas en las bases de datos electrónicas en busca de los informes de los ensayos controlados aleatorizados que compararan las estatinas solas o en combinación con otros tipos de fármacos que reducen los lípidos en el tratamiento de la estenosis de la válvula aórtica.
Resultados clave
La gravedad de la estenosis de la válvula aórtica se evaluó según los criterios ecocardiográficos siguientes: gradiente de presión media, área de la válvula y velocidad del flujo aórtico. También se evaluaron el no reemplazo de la válvula y la muerte por causa cardiovascular. No hubo diferencias en el efecto para el gradiente de presión media, el área de la válvula, el no reemplazo de la válvula y la muerte por causa cardiovascular en el grupo de estatina en comparación con el grupo placebo. No fue posible realizar un metanálisis para evaluar la velocidad del flujo aórtico ya que solamente un estudio analizó este resultado. También se evaluó la seguridad de las estatinas al analizar otros eventos adversos, entre ellos el dolor muscular. El dolor muscular es el evento adverso más prevalente que puede limitar el uso de las estatinas. El dolor muscular no difirió en el grupo de estatina en comparación con el grupo placebo. Los resultados de cuatro ensayos controlados aleatorizados con 2360 participantes indicaron que las estatinas no retrasaron la progresión de la estenosis de la válvula aórtica.
Calidad de la evidencia
La calidad de la evidencia varió de moderada a muy baja entre los diferentes resultados debido a limitaciones en los estudios originales. Los estudios incluidos tienen al menos una limitación metodológica.
Conclusiones
Según la evidencia de esta revisión no hay seguridad alrededor del efecto de las estatinas para la estenosis de la válvula aórtica. Estos resultados apoyan las guías europeas y de EE.UU. (2012 y 2014, respectivamente) con respecto a que hasta el presente no hay una opción clínica de tratamiento para la estenosis de la válvula aórtica. Una opción podría ser ampliar el conocimiento de la fisiopatología de esta enfermedad e incluir factores de riesgo como el calcio, la herencia, la vitamina D, la inflamación, el estrés oxidativo, la diabetes, la hipertensión entre otros que podrían contribuir a la estenosis de la válvula aórtica. Se necesitan ensayos aleatorizados de alta calidad que incluyan factores de riesgo de estenosis de la válvula aórtica.
Authors' conclusions
Summary of findings
| Statin versus Placebo for aortic valve stenosis | |||||
| Patient or population: patients with aortic valve stenosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Statin | ||||
| Mean pressure gradient (mmHg) Better indicated by lower scores. Follow‐up: median 2.4 to 4.5 years | The mean mean pressure gradient in the control groups was | The mean mean pressure gradient in the intervention groups was | MD ‐0.54 (‐1.88 to 0.80) | 1935 | ⊕⊕⊝⊝ |
| Valve area (cm2) Follow‐up: median 2.4‐ to 3.5 years | The mean valve area in the control groups was | The mean valve area in the intervention groups was | MD ‐0.07 (‐0.28 to 0.14) | 127 | ⊕⊕⊝⊝ |
| Aortic jet velocity (m/s) | The mean aortic jet velocity in the control groups was | The mean aortic jet velocity in the intervention groups was | MD ‐0.06 (‐0.26 to 0.14) | 155 | ⊕⊕⊝⊝ |
| Freedom from valve replacement | Study population | RR 0.93 | 2360 | ⊕⊕⊕⊝ | |
| 281 per 1000 | 261 per 1000 | ||||
| Moderate population | |||||
| 222 per 1000 | 206 per 1000 | ||||
| Death from cardiovascular cause | Study population | RR 0.80 | 2297 | ⊕⊕⊝⊝ | |
| 56 per 1000 | 45 per 1000 | ||||
| Moderate population | |||||
| 39 per 1000 | 31 per 1000 | ||||
| Hospitalisation for any reason | 154 per 1000 | 129 per 1000 | RR 0.84 | 155 | ⊕⊝⊝⊝ |
| Adverse events ‐ Muscle pain | Study population | RR 0.91 | 2204 | ⊕⊕⊕⊝ | |
| 168 per 1000 | 153 per 1000 | ||||
| Moderate population | |||||
| 30 per 1000 | 27 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded by one due to randomisation and allocation being unclear for Rossebø 2008. | |||||
Background
Aortic valve stenosis is the main type of heart valve disease observed in high‐income countries.Calcified aortic stenosis is the main etiology of this disease. There are two more causes of aortic valve stenosis, namely congenital and rheumatic diseases (Vahanian 2012). Currently, aortic valve stenosis is the most common indication for surgical valve replacement in North America and Europe (Vahanian 2010). The number of cases of aortic valve replacements has doubled in the past decade in the USA, due to the ageing population (Dweck 2012). When aortic valve replacement is combined with bypass surgery, operative mortality ranges from 5% to 7% (Vahanian 2010). In the last few years, transcatheter aortic valve implantation (TAVI) has been used as an alternative for patients with contraindications to surgery or for high‐risk patients (Vahanian 2012).
Description of the condition
Aortic valve stenosis is the most prevalent valve illness in adults and the third cause of cardiovascular disorders (after arterial hypertension and coronary artery disease) (Parolari 2011). Aortic valve stenosis is characterised by a reduction of the aortic valve area, and it is mainly caused by the calcification of its three leaflets (Vahanian 2012). These leaflets are the part of the aortic valve designed to open in the direction of the blood flow. The incidence of aortic valve stenosis ranges from 2% to 7% in adults over 65 years old (Vahanian 2012). This calcification process is known to be constant and is evidenced by lipid deposition, inflammation and the accumulation of calcium, and is similar to coronary atherosclerosis in many aspects (Dweck 2012). Other causes of aortic valve stenosis, with varying prevalence rates in different populations, include bicuspid aortic valve and rheumatic disease. Patients with bicuspid aortic valve have shown a higher tendency to have calcification than patients with tricuspid aortic valve. Bicuspid aortic valve is more common in younger adults and its incidence ranges from 0.5% to 2.0% in the population (Siu 2010). Rheumatic disease remains an important public health problem in low‐ and middle‐income countries with a prevalence of around 1% among school children in Africa, Asia and Latin America (Canterin 2010). The prevalence of aortic disease among people with rheumatic disease in these countries ranges from 5% to 24% in the population and its calcification process is less common (Canterin 2009). Aortic valve stenosis is a progressive disease which may last for decades, and which causes obstruction of the left ventricular outflow tract leading to ventricular hypertrophy and subsequently heart failure (Dweck 2012). The onset of typical symptoms such as dyspnoea (shortness of breath), angina and syncope (brief lapse of consciousness) is closely related to the severity of the illness (Vahanian 2010). The degree of valve obstruction can be assessed by Doppler echocardiography, the gold standard for the disease, which is used to measure the valve area, mean pressure gradient and aortic jet velocity (Vahanian 2012; Nishimura 2014). The echocardiographic criteria for aortic valve stenosis (Vahanian 2012, Nishimura 2014) are shown in Table 1.
| Mild Aortic stenosis | Moderate Aortic stenosis | Severe Aortic stenosis | |
| Valve area | 1.5 cm² | 1.0 to 1.5 cm² | < 1.0 cm² |
| Mean pressure gradient | < 20 mmHg | 20 to 39 mmHg | > 40 mmHg |
| Aortic jet velocity | < 2.0 to 2.9 m per second | 3.0 to 3.9 m per second | > 4.0 m per second |
Description of the intervention
Statins are lipid‐lowering drugs recommended for the treatment of some disease such as diabetes, acute infarction myocardial and dyslipidaemia (abnormal blood levels of cholesterol) (Stone 2014). Randomised controlled trials (RCTs) have demonstrated the importance of statins in decreasing cardiovascular events (Ridker 2008). Statins act inside the liver by blocking hydroxymethylglutaryl coenzyme A (HMG CoA) reductase enzyme which plays a key role in cholesterol synthesis (Vaughan 2004; Kapur 2008). Besides inhibiting the enzyme responsible for the formation of cholesterol, statins have several pleiotropic effects such as nitric oxide increase, endothelial function improvement, antioxidant, anti‐thrombotic and anti‐inflammatory actions, atherosclerotic plaque stabilisation and anticoagulant action (Kapur 2008). Most people have shown good tolerance to statins. However, adverse effects such as liver dysfunction and myopathy may occur. Muscle pain is the main symptom of myopathy that can limit the use of statins. It occurs in 1.5 % to 5% of patients treated with statins (Mancini 2011). A recent systematic review (Macedo 2014) regarding the safety of statins in 86 included studies demonstrated the presence of the following adverse effects: myopathy, liver enzymes and diabetes (respectively: odds ratio (OR) 2.63 [95% confidence interval (CI) 1.50 to 4.61]; OR 1.54 [95% CI 1.47 to 1.62]; OR 1.31 [95% CI 0.99 to 1.73]). However, the review considered them minor adverse effects when compared with the beneficial effects of statins in major cardiovascular events (Macedo 2014).
How the intervention might work
Calcified aortic valve stenosis has recently come to be considered an active and progressive inflammatory process, and similar to coronary atherosclerosis in many aspects (Dweck 2012). What occurs is a build‐up of fat on the valve leaflets promoting oxidation of low‐density lipoprotein (LDL) by macrophages, activation of myofibroblasts, infiltration of T lymphocytes and cell proliferation from pro‐inflammatory cytokines IL 1(beta), as well as tumour necrosis factor (TNF alpha) (Liebe 2006). Considering the possible relationship between aortic valve stenosis and atherosclerotic disease, statins may halt the progression, or even induce regression of aortic valve stenosis by inhibiting HMG‐CoA reductase, which decreases fat deposition on the valve leaflets (Pedersen 2008). Statins also have anti‐inflammatory actions such as reducing cytokines, blocking smooth muscle cell and T cell depletion, as well as blocking macrophage‐oxidised LDL which hinders foam cells formation (Liebe 2006; Dweck 2012). Studies to verify the possible benefit of statins for the treatment of aortic valve stenosis have already been conducted. A retrospective non‐randomised observational study with the lowest level of evidence showed that statin therapy was successful in delaying the progression of aortic valve stenosis based on a slow reduction in the valve area (P = 0.02) and a small increase in peak pressure gradient (P = 0.01) (Novaro 2001; Pedersen 2008). Other randomised control trials (SALTIRE, SEAS, ASTRONOMER) have also evaluated the role of various statins in halting the progression of aortic valve stenosis over the past 10 years (Cowell 2005; Rossebø 2008; Chan 2010). The SALTIRE study (A randomised trial of intensive lipid‐lowering therapy in calcific aortic stenosis, Cowell 2005) evaluated the effect of 80 mg of atorvastatin in the progression of aortic valve stenosis. A total of 155 patients (mostly men) with a mean age of 68 years were included. The trial did not show any benefit of atorvastatin to slow the progression and calcification of aortic valve stenosis (P = 0.93) (Cowell 2005). The SEAS trial (Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis, Rossebø 2008) evaluated the effect of the association of 40 mg of simvastatin plus 10 mg of ezetimibe in 1873 patients (mostly men) with mild to moderate aortic valve stenosis and a mean age of 67 years (Rossebø 2008). The conclusion of this trial was that simvastatin plus ezetimibe did not reduce the progression of aortic valve stenosis (P = 0.77). The ASTRONOMER trial (Effect of lipid lowering with rosuvastatin on progression of aortic stenosis, Chan 2010) evaluated the effect of 40 mg of rosuvastatin in 269 patients (mostly men) with mild to moderate aortic stenosis and a mean age 58 years. There were no significant differences between the two groups (rosuvastatin versus placebo) in the progression of the disease (mean pressure gradient and aortic valve area) (P = 0.32), cardiovascular death and the need for aortic valve replacement (P = 0.446), as well as adverse events (P = 0.64) (Chan 2010). Currently, there is no recommended dosage of statins to treat aortic valve stenosis (Vahanian 2012; Nishimura 2014).
Why it is important to do this review
The current evidence shows there is still no effective clinical treatment to halt the progression of aortic valve stenosis (Cowell 2005, Rossebø 2008, Chan 2010, Van der Linde 2011). Many patients with aortic valve stenosis who are eventually submitted to invasive procedures such as valve replacement are often diagnosed in the advanced stages of the disease (Katz 2010). Many of these patients also suffer from common chronic diseases such as diabetes, hypertension, dyslipidaemia, as well as the risks associated with smoking (Rajamannan 2008), which hinder post‐operative recovery. They are also subjected to long periods of hospitalisation which represents high costs for health services. Therefore, in order to promote a new rational decision‐making process regarding clinical interventions such as statins for the treatment of aortic valve stenosis, we proposed a systematic review of randomised controlled trials (Mulrow 1994) using Cochrane criteria (Higgins 2011). This review is important as it uses the best available evidence to assess the effects of statins on the progression of aortic valve stenosis, thus limiting the risk of systematic and random errors in the analyses and providing credible results (Antman 1992; Oxman1993; Higgins 2011).
Objectives
General objective
To evaluate the effectiveness and safety of statins in aortic valve stenosis.
Specific objectives
-
To evaluate the effectiveness of statins in the following subgroups: mild aortic valve stenosis, moderate aortic valve stenosis and severe aortic valve stenosis.
-
To investigate the safety of statins in aortic valve stenosis by analysing the adverse effects observed in the included studies.
-
To assess the quality of life of patients suffering from aortic valve stenosis and undergoing treatment with statins.
Methods
Criteria for considering studies for this review
Types of studies
Published or ongoing randomised controlled clinical trials (RCTs) in which statins alone or in association with other systemic drugs to reduce cholesterol levels compared with placebo or control groups were eligible for inclusion. We considered studies with at least one‐year follow‐up.
Types of participants
Adults (age ranged 18 to 85 years) of both sexes suffering from aortic valve stenosis and diagnosed by means of clinical and echocardiographic criteria. For more details, see Table 1. We considered only patients with tricuspid and bicuspid aortic valve disease. We excluded rare causes of valve disease and rheumatic disease.
Types of interventions
Statins alone or in association with other systemic drugs to reduce cholesterol levels versus placebo or control groups.
Types of outcome measures
Primary outcomes
-
Severity of aortic valve stenosis (evaluated by echocardiographic criteria such as mean pressure gradient, valve area and aortic jet velocity)
-
Freedom from valve replacement
-
Death from cardiovascular cause
Secondary outcomes
-
Hospitalisation for any reason
-
Overall mortality
-
Adverse events
-
Patient quality of life
Search methods for identification of studies
We searched for every published or ongoing randomised controlled clinical trial in which statins, alone or in association with other systemic drugs, to reduce cholesterol levels compared with placebo or control groups (date of the search: 24 November, 2015). Searches were conducted by Information Scientists of the Cochrane Heart Group and the Brazilian Cochrane Center .
Electronic searches
We searched the following electronic databases.
-
Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 10 2015) in the Cochrane Library.
-
MEDLINE (Ovid, 1946 to 24 November, 2015).
-
Embase (Embase CLASSIC and Embase Ovid, 1947 to 23 November, 2015).
-
Web of Science (Thomson Reuter, 1970 to 23 November, 2015).
-
CINAHL Plus (EBSCO ‐1937 to 24 November, 2015).
-
LILACS‐IBECS (1982 to 24 November, 2015).
Details of the search strategies used can be found in Appendix 1. We used medical subject headings (MeSH) for MEDLINE. The terms used to search MEDLINE were modified when necessary to search the other databases listed. No language or date restrictions were applied to the searches. The following RCT filter was applied to MEDLINE (OVID) and adaptations of it were used for Embase (OVID) and Web of Science: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) (Higgins 2011).
Searching other resources
We checked the reference lists of the included studies to obtain other articles that were not found by the search strategy. We contacted pharmaceutical companies and authors of published articles to get relevant information. We also searched the WHO's International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/), ClinicalTrials.gov (www.clinicaltrials.gov) and Current Controlled Trials Register (www.controlled‐trials.com/) to identify ongoing trials using the terms "aortic valve stenosis" as condition and "Hydroxymethylglutaryl CoA Reductases" as intervention. We searched Grey literature in OpenGrey (System for Information on Grey Literature in Europe) (http://www.opengrey.eu/) and materials from international congresses of the European Society of Cardiology (www.escardio.org), American Heart Association (AHA) (www.heart.org) and American College of Cardiology (ACC) (www.acc.org) with analogous terms. We imposed no language restrictions.
Data collection and analysis
Two review authors (LT, AFTG) independently collected data using a standard form and two review authors (LT, CRM) independently input the data into the Review Manager (RevMan 5.3) software (RevMan 2014).
Selection of studies
Two review authors (LT, AFTG) independently selected the studies based on a search strategy conducted in the database. Two authors (LT, AFTG) independently identified potentially eligible studies by reading all the titles and abstracts of the articles found. We obtained the full text of relevant studies and selected studies that satisfied the inclusion criteria. The CONSORT (Consolidated Standards of Reporting Trials) statement was used to identify the RCTs when selecting the studies (Schulz 2010) and the PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) statement was applied to check and report each step of the review process (Moher 2009). We resolved disagreements by consensus and asked a third review author (ANA) to make a decision when necessary. There was a high degree of agreement between the authors. The excluded studies were presented and justified in a standard form. For more details, see below Characteristics of excluded studies section.
Data extraction and management
Two review authors (LT, AFTG) independently extracted the data from the included studies. We used a standard form to extract the following data: study objective, study design, study place, date, country, follow‐up, participants (total number, age, gender, comorbidities, inclusion and exclusion criteria, losses and withdrawals), severity of the disease, aortic valve morphology, statistical power of the study, sample size calculation, intervention (statins, types of statins, dosage, association with other drugs to reduce cholesterol levels, side effects, route of administration and placebo), results (outcome measures, adverse events and risk of bias), and funding sources. We solved any disagreement by consensus involving a third author when necessary (ANA). We contacted the authors of the studies when the extracted data were insufficient or unclear (Higgins 2011). We summarised the extracted data in a meta‐analysis using the Review Manager (RevMan 5.3) software (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (LT, AFTG) independently assessed the risk of bias by applying the Cochrane Collaboration 'Risk of bias' tool (Higgins 2011). The differences were settled through discussion or by resorting to a third review author (CRM or ANA). We analysed the following items associated with the risk of bias in the included studies: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data and selective outcome reporting among other sources of bias. We classified each of these criteria as high, low or unclear bias categories according to the Cochrane Collaboration tool and compiled them in a 'Risk of bias' graph and 'Risk of bias' summary.
Measures of treatment effect
We calculated the mean difference (MD) between the treatment groups for continuous outcomes (e.g. valve area). We calculated risk ratio (RR) in both groups for dichotomous outcomes (e.g. death from cardiovascular cause). (Higgins 2011). All outcomes were reported with 95% confidence intervals (CIs).
Unit of analysis issues
Cross‐over trials and cluster‐randomised trials were not eligible for inclusion because the range of this review was a progressive disease (aortic valve stenosis), and the unit of randomisation was the individual. Four RCTs were included (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011) and the participants were individually randomised into treatment groups. The result of the intervention was evaluated and analysed for each outcome in this review.
Dealing with missing data
We contacted the study authors to clarify and recover missing data. Only assessable data were considered in this review and the impact of missing data are addressed in the Discussion section of this review.
Assessment of heterogeneity
The presence of statistical heterogeneity was analysed using the I2 statistical test and the Chi2 test for each outcome. When no heterogeneity was found, we performed a fixed‐effect model. When substantial heterogeneity (I² statistic above 50%) was found, we used the random‐effects model in order to quantify the effect of the findings and verify if they were statistically significant (Higgins 2011). The reasons for the presence of these large differences are explored in the Discussion.
Assessment of reporting biases
We strived to include both published and unpublished studies during the selection process; however the funnel plots and the test of asymmetry to assess possible publication bias were not done due to the insufficient number of trials found (less than10) (Higgins 2011).
Data synthesis
We carried out a meta‐analysis of the data using the Review Manager software, version 5.3 (RevMan 2014). Had there been studies with substantial heterogeneity and for which a meta‐analysis could not be made, we would have prepared a narrative synthesis of the data. We used fixed‐effect and random‐effects models to measure the effect size of the results. We used the fixed‐effect model when the studies did not show heterogeneity. We planned to use the random‐effects model if the studies showed substantial heterogeneity (an I² statistic above 50%) (Higgins 2011). We presented the effect size of the included studies in the forest plots with their respective confidence intervals (CIs).
Subgroup analysis and investigation of heterogeneity
The number of included studies was insufficient to carry out subgroup analysis (less than 10). Therefore, we were unable to evaluate the effectiveness of statins in the following subgroups: mild aortic valve stenosis, moderate aortic valve stenosis and severe aortic valve stenosis as we mentioned in the objective section of this review. In future updates, we plan to conduct subgroup analyses according to age, gender, type of statin and severity of illness, if we have a sufficient number of eligible studies (more than 10).
Sensitivity analysis
The number of included studies was not enough to carry out sensitivity analysis (less than 10). We would have used sensitivity analysis to test the robustness of any results that appeared to be based on heterogeneous combinations of the studies. Had we included more than 10 studies, we would have applied the sensitivity analysis including and excluding lower quality studies to assess the robustness of the observed effects. In this analysis, we would have considered death from cardiovascular cause as the most important outcome. We hope to be able to plan sensitivity analysis in future updates if we have a sufficient number of eligible studies (more than 10).
Overall quality of the body of evidence: 'Summary of findings' table
We generated a 'Summary of findings' table using GRADEPRO software (GRADE PRO 2011). This table evaluated the overall quality of the body of evidence for the review outcomes using GRADE criteria (study limitations [i.e. risk of bias] consistency of effect, imprecision, indirectness and publication bias). The outcomes analysed in the 'Summary of findings' table were mean pressure gradient, freedom from valve replacement, death from cardiovascular cause, hospitalisation for any reason, aortic jet velocity, valve area and severe adverse events (muscle pain). The GRADE assessments were incorporated into the reported result findings for each outcome.
Results
Description of studies
Results of the search
The database searches generated 946 citations and 645 after de‐duplication. The de‐duplication is a tool to identify indexed articles in more than one of the databases. We found 450 citations in the LILACS‐IBECS database . We found two citations by searching other resources. We identified nine eligible papers for inclusion or exclusion screening the titles and abstracts of the 1097 records. Of these nine papers, four were included (Cowell 2005; Rossebø 2008; Chan 2010; Chan 2011). We excluded three papers (Ditchl 2008; Chan 2011; Panahi 2013). We identified only two ongoing trials (Schuler 2005; Kindo 2008).The flow of studies throughout the review is presented in the PRISMA diagram in Figure 1.

Study flow diagram.
Included studies
Four randomised controlled trials (RCTs) were included in this review (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). A total of 2360 adults with aortic valve stenosis were randomised. All participants, mostly men (66%) and white, were followed up for at least two years. Three of the studies analysed tricuspid aortic valve (Cowell 2005; Rossebø 2008; Chan 2010) and the other study analysed mostly younger patients with bicuspid aortic valve (Van der Linde 2011). Rosuvastatin was the intervention drug of two studies (Chan 2010; Van der Linde 2011). Another study used atorvastatin as intervention drug (Cowell 2005). Only one study used a statin in association with other systemic drugs to reduce cholesterol levels (simvastatin plus ezetimibe) as the intervention drugs (Rossebø 2008). Two studies had participants with other comorbidities such as hypertension, coronary heart disease, β‐blocker and aspirin use, high levels of low‐density lipoprotein (LDL) and who were current smokers (Cowell 2005; Rossebø 2008). For further information about study design, sites, follow‐up, participants, comorbidities, inclusion and exclusion criteria, aortic valve morphology, severity of aortic valve stenosis, intervention and outcomes for each of the included studies in this review see Characteristics of included studies.
We also identified two ongoing trials (Schuler 2005; Kindo 2008). One trial is analysing the effect of 40 mg of fluvastatin per day in asymptomatic aortic valve stenosis. The primary outcomes of this study are the progression of calcified aortic valve stenosis measured by transthoracic echocardiography (mean pressure gradient, valve area and aortic jet velocity) and catheterisation (peak‐to‐peak gradient, left ventricular function and compliance) (Schuler 2005).The recruitment status of this study is unknown as the authors have not updated it. Another trial is analysing the effect 80 mg of atorvastatin per day in surgical aortic valve stenosis and is currently recruiting participants. The primary outcomes of this study are changes on inflammatory markers after aortic valve replacement and changes in left ventricular mass (Kindo 2008). For more details, see Characteristics of ongoing studies.
No studies are awaiting classification.
Excluded studies
Three of the nine eligible studies were excluded after full‐text reading (Ditchl 2008; Chan 2011; Panahi 2013). For more details, see Characteristics of excluded studies. The main reason to exclude these studies was the type of design (a sub‐study of ASTRONOMER trial) (Chan 2011), methodological flaws and high risk of bias (Ditchl 2008; Panahi 2013).
Risk of bias in included studies
We assessed the risk of bias in the included studies as 'low', 'high' or 'unclear'. For more details, see below Figure 2 , Figure 3 and Characteristics of included studies section.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
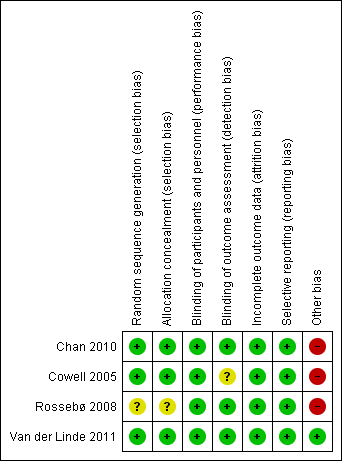
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Three studies mentioned the method of sequence generation and they were considered as low risk of bias (Cowell 2005; Chan 2010; Van der Linde 2011). Two of these studies used a computer program which did not have access to the rest of the data (Chan 2010; Van der Linde 2011). One of these studies used a minimisation technique with a computer program (Cowell 2005). One study did not mention how the random sequence generation was made and it was considered as having an unclear risk of bias (Rossebø 2008).
Allocation concealment
Three studies were considered as low risk of bias for allocation concealment (Cowell 2005; Chan 2010; Van der Linde 2011). One of these studies used a randomisation number from the study database via a secure Internet line (Chan 2010). Another study used numbered containers (Cowell 2005) and Van der Linde 2011 used a randomisation number that was sent to the site co‐ordinator by the pharmacology department and the study medication was sent to the participants. Rossebø 2008 did not mention how the allocation concealment was made and it was considered as unclear risk of bias (Rossebø 2008).
Blinding
Blinding of participants and personnel
We considered all four studies as low risk of bias for blinding of participants and personnel (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). All four studies were reported to be double‐blind, placebo‐controlled trials.
Blinding of outcome assessment
Three studies were classified as low risk of bias for blinding of outcome assessment (Rossebø 2008; Chan 2010; Van der Linde 2011). All members of the committees of these studies who evaluated the outcomes were blinded. One of the studies was classified as unclear risk of bias because it did not mention if the investigators were blinded to evaluate the data. This paper just mentioned what each author did in the study (Cowell 2005).
Incomplete outcome data
We considered all four studies as low risk of bias for incomplete outcome data (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). Withdrawn participants were well‐described in each study.
Selective reporting
We classified all four studies as low risk of bias for selective reporting (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). These four studies evaluated their primary and secondary outcomes and reported the results. Three of these four studies reported the study protocol (Cowell 2005; Rossebø 2008; Chan 2010).
Other potential sources of bias
We considered only one study as low risk of bias for other potential sources of bias (Van der Linde 2011). Three industry funded studies were considered high risk of bias (Cowell 2005; Rossebø 2008; Chan 2010). One of the studies received funding from the Canadian Institute of Health Research and Astra Zeneca Canada, but Astra Zeneca had no input into the study design and analysis (Chan 2010). In another study, the drug was provided by Pfizer which had no access to the rest of the data (Cowell 2005). The PROCAS trial did not received funding from any organisation (Van der Linde 2011). The SEAS trial received funding from Merck, but the scientific responsibility of the study remained with the independent steering committee (Rossebø 2008).
Effects of interventions
See: Summary of findings for the main comparison Statin versus Placebo for aortic valve stenosis
Primary outcomes
1. Severity of aortic valve stenosis
1.1 Mean pressure gradient
Two studies included mean pressure gradient (Rossebø 2008; Van der Linde 2011). The mean pressure gradient did not differ in the participants using statin compared with the participants using placebo (mean difference (MD) ‐0.54, 95% confidence interval (CI) ‐1.88 to 0.80; studies = 2; participants = 1935; low‐quality evidence) Analysis 1.1. There was low heterogeneity in this comparison (I² = 37%).
1.2 Valve area
Two studies included valve area (Chan 2010; Van der Linde 2011). The valve area did not differ in the participants using statin compared with the participants using placebo (MD ‐0.07, 95% CI ‐0.28 to 0.14; studies = 2; participants = 127; low‐quality evidence ) Analysis 1.2 . There was low heterogeneity in this comparison (I² = 10%).
1.3 Aortic jet velocity
Only one study included aortic jet velocity as outcome (MD ‐0.06, 95% CI ‐0.26 to 0.14; participants = 155; study = 1; low‐quality evidence) (Cowell 2005). There were no more studies for comparison Analysis 1.3. Heterogeneity was not applicable.
2. Freedom from valve replacement
Four studies included freedom from valve replacement (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). Freedom from valve replacement did not differ in the participants using statin (309/1185, 26%) compared with the participants using placebo (330/1175, 28%) (risk ratio (RR) 0.93, 95% CI 0.81 to 1.06; studies = 4; participants = 2360; moderate‐quality evidence) Analysis 1.4. There was no substantial heterogeneity in this comparison (I² = 0%).
3. Death from cardiovascular cause
Three studies included death from cardiovascular cause (Cowell 2005; Rossebø 2008; Chan 2010). Death from cardiovascular cause did not differ in the participants using statin (52/1155, 4.5 %) compared with the participants using placebo (64/ 1142, 5.6%) (RR 0.80, 95% CI 0.56 to 1.15; studies = 3; participants = 2297; low‐quality evidence) Analysis 1.5.There was no substantial heterogeneity in this comparison (I² = 0%).
Secondary Outcomes
1. Hospitalisation for any reason
Only one study included hospitalisation for any reason as outcome (RR 0.84, 95% CI 0.39 to 1.84; participants = 155; study = 1; very low‐quality evidence) (Cowell 2005). This outcome occurred in the participants using statin (10/77, 12.9%) compared with the participants using placebo (12/ 78, 15.3%) Analysis 1.6. There were no more studies for comparison. Heterogeneity was not applicable.
2. Overall mortality
Studies did not report data on overall mortality as an outcome.
3. Adverse events
We evaluated a total of five adverse events that were present in at least two studies (muscle pain, hepatic enzymes, hepatitis, gastrointestinal condition and creatine kinase). We considered muscle pain as the main adverse event because it is the most prevalent adverse event that can limit the use of statin.
3.1 Muscle pain
Three studies included muscle pain (Rossebø 2008; Chan 2010; Van der Linde 2011). Muscle pain did not differ in the participants using statin (169/1107, 15.26%) compared with the participants using placebo (184/1097, 16.77%) (RR 0.91, 95% CI 0.75 to 1.09; studies = 3; participants = 2204; moderate‐quality evidence) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
3.2 Hepatic enzymes elevation
Two studies included hepatic enzymes elevation (Rossebø 2008; Chan 2010). Hepatic enzymes elevation differed in the participants using statin (24/1059, 2.26%) compared with the participants using placebo (9/1050, 0.85%) (RR 2.66, 95% CI 1.24 to 5.67; studies = 2; participants = 2109) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
3.3 Hepatitis
Two studies included hepatitis (Rossebø 2008; Chan 2010). Hepatitis did not differ in the participants using statin (7/1077, 0.64 %) compared with the participants using placebo (6/1064, 0.56%) (RR 1.14, 95% CI 0.40 to 3.25; studies = 2; participants = 2141) Analysis 1.7. There was low heterogeneity in this comparison (I² = 18%).
3.4 Gastrointestinal condition, gastrointestinal serious event or gastrointestinal symptoms
Three studies included gastrointestinal condition, gastrointestinal serious event or gastrointestinal symptoms respectively (Cowell 2005; Rossebø 2008; Chan 2010). Gastrointestinal condition, gastrointestinal serious event or gastrointestinal symptoms did not differ in the participants using statin (318/1154, 27%) compared with the participants using placebo (286/1142, 25%) (RR 1.10, 95% CI 0.96 to 1.25; studies = 3; participants = 2296) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
3.5 Creatine kinase elevation
Two studies included creatine kinase elevation (Rossebø 2008; Chan 2010). Creatine kinase elevation did not differ in the participants using statin (3/1059, 0.28%) compared with the participants using placebo (4/1050, 0.38%) (RR 0.75, 95% CI 0.17 to 3.33; studies = 2; participants = 2109) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
4. Patient quality of life
Studies did not report data on patient quality of life as an outcome.
Overall quality of the body of evidence: 'Summary of findings' table
The quality of evidence of our the obtained results was very low to moderate. For more details, see summary of findings Table for the main comparison.
Discussion
The general objective of this review was to evaluate the effectiveness and safety of statins in aortic valve stenosis. We evaluated the effectiveness of statins in aortic valve stenosis through the proposed outcomes found in the included studies. We checked the safety of statins as an important factor for the success of the treatment by analysing the adverse events found in the included studies. Though we had intended to conduct a subgroup analysis (mild, moderate and severe aortic valve stenosis groups) to check the effect of statins on the different subgroups, we were unable to do so due to insufficient number of trials (less than 10). None of the included studies reported on quality of life and overall mortality in their outcomes.
Summary of main results
Only four randomised controlled trials from the electronic searches were included in this review (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). A total of 2360 adults were randomised (age ranged 18 to 85 years). We analysed primary and secondary outcomes considered for this review in each included study. We did not identify differences between the group using statins alone or statins in association with other drugs to reduce cholesterol levels when compared with the placebo group for the following primary outcomes: mean pressure gradient (low‐quality evidence) Analysis 1.1, valve area (low‐quality evidence) Analysis 1.2, freedom from valve replacement (moderate‐quality evidence) Analysis 1.4 and death from cardiovascular cause (low‐quality evidence) Analysis 1.5. We did not carry out meta‐analysis to assess aortic jet velocity as only one study included this outcome (low‐quality evidence) Analysis 1.3. For secondary outcomes, we identified differences between the group using statins alone or statins in association with other drugs to reduce cholesterol levels when compared with the placebo group only for the adverse events. We analysed five adverse events, among which was muscle pain. We considered muscle pain as the main adverse event because it is the most prevalent adverse event that can limit the use of statin (moderate‐quality evidence) Analysis 1.7. The quality of evidence of the obtained results for the analysed outcomes was very low to moderate. For more details, see summary of findings Table for the main comparison. We did not carry out meta‐analysis to assess other secondary outcomes as hospitalisation for any reason (very low‐quality evidence), as only one study reported this outcome Analysis 1.6. None of the four included studies evaluated the secondary outcomes of overall mortality nor patient quality of life. We could not find sufficient number of trials to conduct subgroup analysis and sensitivity analysis (more than 10). The findings of this systematic review did not show that statins may be able to delay the progression of aortic valve stenosis.
Overall completeness and applicability of evidence
The general objective of this review was to evaluate the effectiveness and safety of statins in aortic valve stenosis. We summarised the studies with the best evidence available about this subject. Considering the analysed outcomes, our results are applicable for people suffering from aortic valve stenosis. However, we were unable to find enough studies in the electronic database search that allowed us to assess all objectives of the review. All types of participants and interventions cited in the method section were investigated in the included studies, but we could not carry out a meta‐analysis of the outcomes aortic jet velocity and hospitalisation for any reason as both outcomes were only reported in one study (Cowell 2005). We did not find the outcomes overall mortality and patient quality of life reported in the included studies. Two studies included participants who were current smokers and had other comorbidities such as hypertension, coronary heart disease, β‐blocker and aspirin use, high levels of LDL (Cowell 2005; Rossebø 2008). These comorbidities could have adversely affected the analysis of the results and they require analyses of their effect on each of these subgroups. These difficulties encountered during the review process did not allow us to find a high‐quality evidence in our findings, but our obtained results confirm the current international guidelines which do not recommend statins to slow down the progression of aortic valve stenosis.
Quality of the evidence
The GRADE approach was employed to assess the quality of result findings and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager 5.3 to create 'Summary of findings' tables. The quality of evidence ranged from moderate to very low across the different outcomes, mainly due to risk of bias and imprecise results. One of the four included studies was low risk of bias for the following criteria: random sequence generation; allocation concealment; blinding of participants; personnel and outcome assessors; incomplete outcome data and selective reporting among other sources of bias (Van der Linde 2011). One study had unclear risk of bias for random sequence generation and allocation concealment (selection bias) (Rossebø 2008). Three studies were high risk of bias for other bias because they were industry funded trials (Cowell 2005; Rossebø 2008; Chan 2010). We could not elucidate the risk of bias for these studies even having contacted their authors. Overall, the studies were small and had short follow‐ups (Cowell 2005; Chan 2010; Van der Linde 2011) which may have meant a lack of rigor in their methods and may have produced inconsistent results. Regarding the findings of the studies, particularly the continuous outcomes of the severity of aortic valve stenosis were not affected by dropouts. In the largest trial (Rossebo et al, 1873 of the 2360 patients considered in the meta‐analysis), 1857 patients (99%) were followed until the end of the study (median 52.2 months) and 1693 (90%) were included in an echocardiographic substudy (Rossebø 2008). In the Van der Linde study, 55/63 (87%) of patients completed follow‐up (Van der Linde 2011). In Cowell 2005, 11/155 patients (7%) discontinued treatment because of adverse effects and 134/155 (86.5%) completed the echocardiographic follow‐up. In Chan 2010, the discontinuation rate was high (123/269, 45%). There were 51 cases due to adverse events, but only three patients were lost during the follow‐up. Per‐protocol analysis yielded similar results to intention‐to‐treat analysis. We were unable to assess the publication bias in funnel plots due to limited number of the included studies (less than 10). All of these considerations were taken into account when interpreting the results of this systematic review.
Potential biases in the review process
We attempted to include all relevant studies in this review from an electronic sensitised search. Two review authors independently selected, collected and analysed the data in order to minimise bias. One limitation was that we could not find sufficient studies to perform sensitivity analysis, subgroup analysis, or to assess publication bias. Another limitation was that we could not carry out meta‐analysis to assess the secondary outcome of hospitalisation for any reason as only one trial reported this outcome, and the secondary outcomes of overall mortality and patient quality of life were not reported in any of the trials.
Agreements and disagreements with other studies or reviews
A meta‐analysis published by Teo KK et al in 2011 (2344 participants) (Teo 2011) did not find differences of effect between statin or placebo groups for similar outcomes such as aortic valve stenosis severity, freedom from valve replacement and cardiovascular death either. However, we analysed the safety of statins through their adverse events in this paper. We found differences in one adverse event between statin and placebo group: hepatic enzymes (RR 2.66, 95% CI 1.24 to 5.67; studies = 2; participants = 2109). Nevertheless, a recent review demonstrated that drug‐induced liver injury statin is rare (22 cases from 1188 drug‐induced liver injury cases, eight years of follow‐up) (Russo 2014).
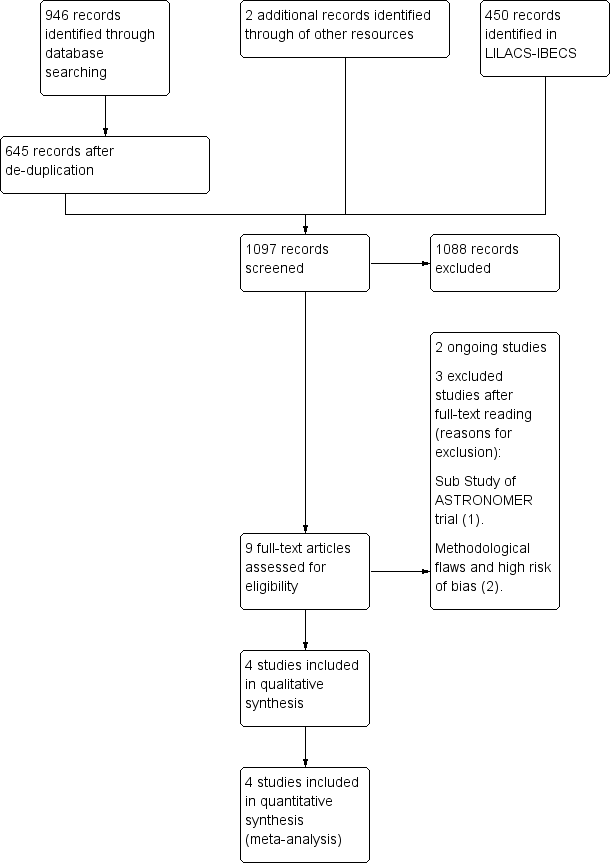
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Statin versus Placebo, Outcome 1 Mean pressure gradient.
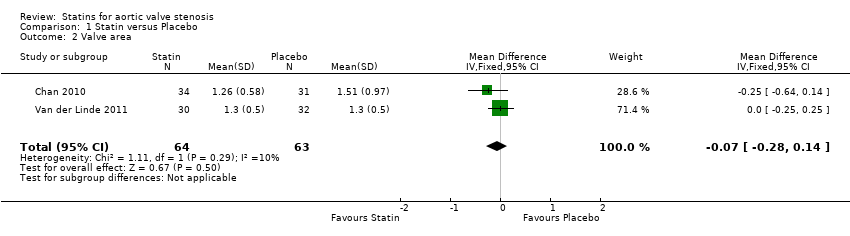
Comparison 1 Statin versus Placebo, Outcome 2 Valve area.
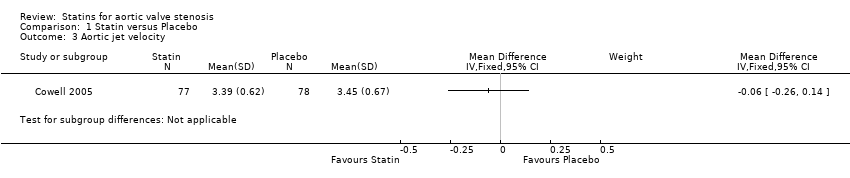
Comparison 1 Statin versus Placebo, Outcome 3 Aortic jet velocity.
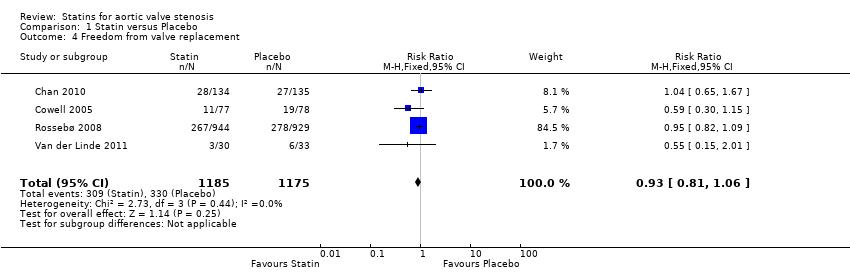
Comparison 1 Statin versus Placebo, Outcome 4 Freedom from valve replacement.

Comparison 1 Statin versus Placebo, Outcome 5 Death from cardiovascular cause.
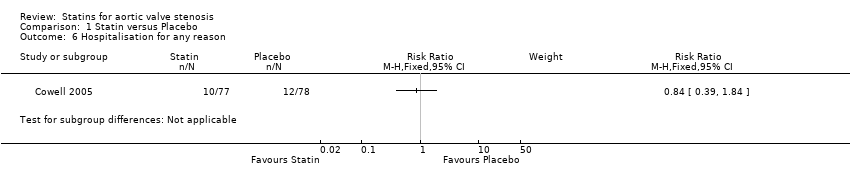
Comparison 1 Statin versus Placebo, Outcome 6 Hospitalisation for any reason.

Comparison 1 Statin versus Placebo, Outcome 7 Severe adverse events.
| Statin versus Placebo for aortic valve stenosis | |||||
| Patient or population: patients with aortic valve stenosis | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Statin | ||||
| Mean pressure gradient (mmHg) Better indicated by lower scores. Follow‐up: median 2.4 to 4.5 years | The mean mean pressure gradient in the control groups was | The mean mean pressure gradient in the intervention groups was | MD ‐0.54 (‐1.88 to 0.80) | 1935 | ⊕⊕⊝⊝ |
| Valve area (cm2) Follow‐up: median 2.4‐ to 3.5 years | The mean valve area in the control groups was | The mean valve area in the intervention groups was | MD ‐0.07 (‐0.28 to 0.14) | 127 | ⊕⊕⊝⊝ |
| Aortic jet velocity (m/s) | The mean aortic jet velocity in the control groups was | The mean aortic jet velocity in the intervention groups was | MD ‐0.06 (‐0.26 to 0.14) | 155 | ⊕⊕⊝⊝ |
| Freedom from valve replacement | Study population | RR 0.93 | 2360 | ⊕⊕⊕⊝ | |
| 281 per 1000 | 261 per 1000 | ||||
| Moderate population | |||||
| 222 per 1000 | 206 per 1000 | ||||
| Death from cardiovascular cause | Study population | RR 0.80 | 2297 | ⊕⊕⊝⊝ | |
| 56 per 1000 | 45 per 1000 | ||||
| Moderate population | |||||
| 39 per 1000 | 31 per 1000 | ||||
| Hospitalisation for any reason | 154 per 1000 | 129 per 1000 | RR 0.84 | 155 | ⊕⊝⊝⊝ |
| Adverse events ‐ Muscle pain | Study population | RR 0.91 | 2204 | ⊕⊕⊕⊝ | |
| 168 per 1000 | 153 per 1000 | ||||
| Moderate population | |||||
| 30 per 1000 | 27 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Downgraded by one due to randomisation and allocation being unclear for Rossebø 2008. | |||||
| Mild Aortic stenosis | Moderate Aortic stenosis | Severe Aortic stenosis | |
| Valve area | 1.5 cm² | 1.0 to 1.5 cm² | < 1.0 cm² |
| Mean pressure gradient | < 20 mmHg | 20 to 39 mmHg | > 40 mmHg |
| Aortic jet velocity | < 2.0 to 2.9 m per second | 3.0 to 3.9 m per second | > 4.0 m per second |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean pressure gradient Show forest plot | 2 | 1935 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.88, 0.80] |
| 2 Valve area Show forest plot | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.28, 0.14] |
| 3 Aortic jet velocity Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Freedom from valve replacement Show forest plot | 4 | 2360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| 5 Death from cardiovascular cause Show forest plot | 3 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.15] |
| 6 Hospitalisation for any reason Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Severe adverse events Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Muscle pain | 3 | 2204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.09] |
| 7.2 Hepatic enzymes elevation | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.24, 5.67] |
| 7.3 Hepatitis | 2 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.25] |
| 7.4 Gastrointestinal condition | 3 | 2296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.25] |
| 7.5 Creatine kinase | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.33] |

