Rehabilitación multidisciplinaria después del tratamiento del tumor cerebral primario
Resumen
Antecedentes
Los tumores cerebrales pueden causar discapacidad significativa, para la que se puede considerar la rehabilitación multidisciplinaria. Sin embargo, la base de evidencia para la misma es poco clara. Esta revisión es una actualización de una revisión publicada anteriormente en la Base de Datos Cochrane de Revisiones Sistemáticas (Cochrane Database of Systematic Reviews) [2013, número 1, Art. nº CD009509] "Rehabilitación multidisciplinaria después del tratamiento del tumor cerebral primario".
Objetivos
Evaluar la efectividad de la rehabilitación multidisciplinaria en pacientes después del tratamiento del tumor cerebral primario, sobre todo los enfoques que son efectivos (ámbitos, intensidad).
Métodos de búsqueda
Para esta actualización, se hicieron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL, the Cochrane Library hasta el número 12 de 12, 2014), MEDLINE (1950 hasta enero, semana 2, 2015), EMBASE (1980 hasta enero, semana 2, 2015), PEDro (1985 hasta enero, semana 2, 2015) y en LILACS (1982 hasta enero, semana 2, 2015). Se revisaron las bibliografías de los trabajos identificados y se estableció contacto con los autores y con expertos reconocidos en el tema para buscar ensayos publicados y no publicados.
Criterios de selección
Ensayos clínicos controlados (ensayos clínicos aleatorizados y no aleatorizados) que compararan la rehabilitación multidisciplinaria para el tumor cerebral primario con servicios locales normalmente disponibles o con niveles inferiores de intervención; o estudios que compararan la rehabilitación multidisciplinaria en diferentes ámbitos o con diferentes niveles de intensidad.
Obtención y análisis de los datos
Tres autores de la revisión evaluaron de forma independiente la calidad de los estudios, extrajeron los datos y realizaron una síntesis de la «mejor evidencia» sobre la base de la calidad metodológica.
Resultados principales
No se identificó ningún estudio para su inclusión en la versión anterior de esta revisión. Para esta actualización, la búsqueda bibliográfica identificó un ensayo clínico controlado de baja calidad que incluyó a 106 participantes. Los resultados de este estudio indican evidencia de "bajo nivel" que apoya la rehabilitación multidisciplinaria ambulatoria de alta intensidad (paciente ambulatorio) para reducir la discapacidad motora a corto y a largo plazo (continencia, movilidad y movimiento, cognición), en comparación con la atención estándar de los pacientes ambulatorios. Se encontró mejoría en algunos dominios de discapacidad (continencia, comunicación) y se mantuvieron las ganancias psicosociales a los seis meses de seguimiento. No se encontró evidencia de mejoría en la participación general (calidad de vida y relación social). No se informaron eventos adversos como resultado de la rehabilitación multidisciplinaria. No se encontró evidencia de mejoría en la calidad de vida ni en la relación entre costo y efectividad de la rehabilitación. Tampoco fue posible indicar la mejor "dosis" del tratamiento.
Conclusiones de los autores
Desde la última versión de esta revisión, se ha identificado un nuevo estudio para la inclusión. La mejor evidencia hasta la fecha proviene de este ECC, que aporta evidencia de muy baja calidad de que la rehabilitación multidisciplinaria ambulatoria (paciente ambulatorio) de mayor intensidad reduce la discapacidad a corto y a largo plazo en los pacientes con tumor cerebral en comparación con la atención estándar de los pacientes ambulatorios. En el mejor de los casos, estas conclusiones se deben considerar con cautela debido a las brechas actuales en la investigación en esta área. Aunque la solidez de la evidencia ha aumentado con la identificación de un nuevo ensayo clínico controlado en esta revisión actualizada, se necesita más investigación en diseños de estudios apropiados y consistentes, medición de resultados, las necesidades de los cuidadores, la evaluación de ámbitos óptimos, el tipo, la intensidad y la duración de los tratamientos, y la relación entre costo y efectividad de la rehabilitación multidisciplinaria en la población con tumores cerebrales.
PICO
Resumen en términos sencillos
Rehabilitación multidisciplinaria después del tratamiento del tumor cerebral
Los pacientes con tumores cerebrales pueden presentar varios síntomas y discapacidades como problemas psicológicos, dificultades de movilidad o autocuidado, y problemas en sus relaciones sociales y el trabajo, que pueden tener una repercusión significativa sobre la calidad de vida. Estos síntomas y discapacidades pueden tratarse mediante la “rehabilitación multidisciplinaria” aplicada por un equipo de diferentes profesionales sanitarios (p.ej., médicos, enfermeras, terapeutas) que trabajen de manera organizada.
Se encontró un ensayo clínico controlado (calidad deficiente) que comparó la rehabilitación multidisciplinaria con la atención estándar de los pacientes ambulatorios. Los 106 pacientes de este ensayo recibieron tratamiento en el consultorio de atención ambulatoria del hospital. Los participantes estuvieron hasta ocho semanas en el programa de rehabilitación multidisciplinaria y los resultados se midieron a los tres y seis meses después de finalizar el programa.
Hubo alguna evidencia que apoyó el efecto beneficioso de la rehabilitación multidisciplinaria para reducir la discapacidad en los pacientes con tumor cerebral primario. Los pacientes del grupo de rehabilitación multidisciplinaria mostraron mejoría en sus capacidades funcionales (p.ej., continencia, movilidad) y en la función cognitiva en comparación con el grupo de atención estándar sola. La rehabilitación multidisciplinaria no provocó efectos perjudiciales. Las brechas actuales en la investigación destacan la necesidad de estudios de investigación de alta calidad para explorar la efectividad de diversos aspectos de la rehabilitación multidisciplinaria y las necesidades de los cuidadores en esta población de pacientes.
La evidencia de esta revisión está actualizada hasta enero 2015.
Authors' conclusions
Summary of findings
| High‐intensity multidisciplinary rehabilitation compared with wait‐list control group with usual care for primary brain tumour | ||||||
| Patient or population: 106 participants with primary brain tumour Settings: outpatient Intervention: High‐intensity multidisciplinary rehabilitation Comparison: wait‐list control group with usual care | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect* | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care (control group) | High‐intensity multidisciplinary rehabilitation (intervention group) | |||||
| Short‐term disability outcomes at 3‐months postintervention | ||||||
| Change in short‐term disability (function) FIM motor | Median change = 8 points higher | Median change = 18 points higher | Z score: ‐3.13, P < 0.001 R: 0.32 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 13 items with 4 subscales (self care, transfers, locomotion, sphincter control, assessing function (activity) and need for assistance, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Change in short‐term disability (cognition) FIM cognition | Median change = 3 points higher | Median change = 6 points higher | Z score: ‐1.99, P < 0.05 R: 0.20 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 3 items with 3 subscales (communication, psychological, cognition) assessing cognition, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Long‐term disability outcomes at 6‐months postintervention | ||||||
| Change in long‐term disability (function) FIM motor | Median change = 4 points higher | Median change = 12 points higher | Z score: ‐2.33, P < 0.05 R: 0.25 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 13 items with 4 subscales (self care, transfers, locomotion, sphincter control, assessing function (activity) and need for assistance, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Change in long‐term disability (cognition) FIM cognition | Median change = 1.5 points higher | Median change = 6 points higher | Z score: ‐3.09, P < 0.001 R: 0.20 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 3 items with 3 subscales (communication, psychological, cognition) assessing cognition, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Change in short‐term participation outcomes at 3‐months postintervention | ||||||
| Change in short‐term psychological outcomes | Median change = 12 points lower | Median change = 8 points lower | Z score: ‐0.53, P > 0.05 R: 0.05 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 21 items with 3 subscales assessing depression, anxiety, and stress, rated on a 4‐point scale, with higher score indicating higher level of impairment |
| Change in short‐term participation | Median change = 7 points lower | Median change = 6 points lower | Z score: ‐0.40, P > 0.05 R: 0.04 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 23 items with 5 subscales assessing mobility, self care, relationships, participation, and psychological well‐being, rated on a 6‐point scale, with high scores indicating greater impact |
| Change in short‐term QoL CARES‐SF (global) | Median change = 0.2 points lower | Median change = 0.1 points lower | Z score: ‐0.10, P > 0.05 R: 0.01 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 59‐item global scale, with overall score indicating QoL and summary scores for the 5 domains (physical, psychosocial, medical interaction, marital and sexual function), assessing cancer‐specific rehabilitation need and QoL, rated on a 4‐point scale, with higher scores indicating more difficulty or lower QoL |
| Change in long‐term participation outcomes at 6‐months postintervention | ||||||
| Change in long‐term psychological outcomes | Median change = 10 points lower | Median change = 12 points lower | Z score: ‐0.98, P > 0.05 R: 0.11 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 21 items with 3 subscales assessing depression, anxiety, and stress, rated on a 4‐point scale, with higher score indicating higher level of impairment |
| Change in long‐term participation | Median change = 9.5 points higher | Median change = 5 points lower | Z score: ‐0.37, P > 0.05 R: 0.04 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 23 items with 5 subscales assessing mobility, self care, relationships, participation, and psychological well‐being, rated on a 6‐point scale, with high scores indicating greater impact |
| Change in long‐term QoL CARES‐SF (global) | Median change = 0.2 points lower | Median change = 0.2 points lower | Z score: ‐0.42, P < 0.05 R: 0.05 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 59‐item global scale, with overall score indicating QoL and summary scores for the 5 domains (physical, psychosocial, medical interaction, marital and sexual function), assessing cancer‐specific rehabilitation need and QoL, rated on a 4‐point scale, with higher scores indicating more difficulty or lower QoL |
| Change in other outcomes | ||||||
| Cost‐ effectiveness | See comment | See comment | Not estimable | 106 (1 study) | See comment | Not measured |
| Serious adverse events | See comment | See comment | Not estimable | 106 | See comment | No serious adverse events attributed to the intervention |
| *Mann‐Whitney U tests # Effect size statistics (R) were calculated and assessed against Cohen’s criteria (0.1 = small, 0.3 = medium, 0.5 = large effect) CARES‐SF: Cancer Rehabilitation Evaluation System‐Short Form; DASS: Depression Anxiety Stress Scales; FIM: Functional Independence Measure; PIPP: Perceived Impact of Problem Profile; QoL: quality of life | ||||||
| 1GRADE Working Group grades of evidence | ||||||
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews [2013, Issue 1, Art. No. CD009509] on 'Multidisciplinary rehabilitation after primary brain tumour treatment'.
Description of the condition
Primary brain tumours are a diverse group of neoplasms that account for 2% of all cancers and affect approximately seven people per 100,000 population annually worldwide (Arber 2010; Parkin 2005). There is evidence to support the increasing overall incidence of primary brain tumours, with the highest increase noted in patients over 60 years of age (Flowers 2000). In 2009, there were an estimated 22,070 new cases of primary brain tumours in the United States (Jemal 2009). Three thousand new cases of primary brain tumours are reported each year in the United Kingdom, with approximately 2500 deaths per annum (Arber 2010). A similarly high incidence rate is also reported in Australia, with approximately 1400 new cases and more than 1200 deaths from malignant and benign brain tumours annually (Brain Foundation 2011).
Significant medical advances in the treatment of primary brain tumours have resulted in a marked increase in the number of survivors (Huang 2011; Poggi 2009). Surgery remains the primary treatment for the majority of people with brain tumours, with radiotherapy and chemotherapy or both as neoadjuvant, concomitant, or adjuvant treatment (Chandana 2008). However, the treatment regimens can produce significant adverse effects (Aziz 2003; Tang 2008). Despite these treatment options, brain tumours remain a significant source of functional and psychosocial impairment for this patient population, limiting them in everyday activity and participation due to many issues (Huang 2011; Tang 2008). Diagnosis of brain tumour can further have a distressing psychological impact, significant costs and socioeconomic implications, increased demand for health care, social, and vocational services, and caregiver burden (Tang 2008).
People with primary brain tumour can present with various combinations of problems, such as physical, cognitive, psychosocial, behavioural, and environmental issues. The World Health Organization (WHO) developed the International Classification of Functioning, Disability and Health (ICF), which defines a common language for describing the impact of disease at different levels: impairment (body structure and function), limitation in activity and participation (WHO 2001). Within this framework, primary brain tumour‐related impairments can limit activity or function and participation in society and life situations, and reduce life span (Khan 2013b). Many people diagnosed with brain tumour may have ongoing concerns (such as relationship, employment, recurrence) (Ownsworth 2009). The limitation in function (disability) can have a cumulative effect over time and cause considerable distress to cancer survivors and their families, and reduce quality of life (QoL) (Ness 2010). Patients discharged back to the community are confronted by various adjustment issues, such as the person's perceptions of self worth, self image, and role reversal within the family. Families or caregivers or both often struggle to cope with the new demands associated with increased care needs, inability to drive and return to work, financial constraints, marital stress, and general limitation in the person's participation. A longitudinal study of people with brain tumours living in the community after treatment (median time since diagnosis 2.1 years) found that over half still had pain (56%); 44% reported ataxia; 43% seizures; 37% paresis, 36% cognitive dysfunction; and 35% visual impairment (Khan 2013c). Ongoing monitoring, education, and counselling of the person (and family) are therefore important (Khan 2013d).
The care needs after treatment for primary brain tumour (surgery, chemotherapy, and radiotherapy) are varied given the complex, multifactorial nature of the condition and multiple disabilities (which may progress) in these people. These are best met with a coordinated, multidisciplinary, multifaceted approach that includes acute medical and surgical care, rehabilitation, palliative and other supportive interventions (Gabanelli 2005).
Description of the intervention
Rehabilitation has been defined as "a problem‐solving educational process aimed at reducing disability and handicap (participation) experienced by someone as a result of disease or injury" (Wade 1992). In this review, multidisciplinary rehabilitation is defined as the coordinated delivery of multidimensional rehabilitation intervention provided by two or more disciplines (such as nursing, physiotherapy, occupational therapy, social work, psychology and other allied health), in conjunction with medical professionals (surgeon; oncologist; rehabilitation, palliative physician), which aims to improve patient symptoms and maximise functional independence and participation (social integration) using a holistic biopsychosocial model, as defined by the ICF (WHO 2001). A multidisciplinary approach provides patients with skills needed to manage their own care to improve their coping ability, knowledge base, and QoL (Corner 2007). It prioritises patient‐centred care and focuses on a person's function and disability, using a goal‐based, functionally oriented approach that is time based. The patients (and family or caregivers) are active participants in the goal‐setting process. The content, intensity, and frequency of therapy in multidisciplinary rehabilitation can vary, as programmes are individualised based on clinical needs. The content can include physical reconditioning, task re‐acquisition strategies, cognitive behavioural therapy, vocational and recreational programmes.
People after primary brain tumour treatment can present to rehabilitation settings with a range of difficulties that may be physical, emotional, psychosocial, and/or environmental (Khan 2013b). Multidisciplinary rehabilitation encompasses the framework and common language for describing the impact of disease at different levels using the ICF (WHO 2001). For example, in people after brain tumour treatment:
-
'impairments' are problems with body (anatomical) structures or function (headaches, seizures, neurocognitive dysfunction, muscle weakness, aphasia, visual impairments);
-
'activity limitations' (disabilities) are difficulties a person has executing everyday tasks (mobility or self care);
-
'restriction in participation' relates to problems a person has that limit involvement in societal participation and life situations (i.e. employment, family life, social re‐integration);
-
'contextual factors' are:
-
‘environmental’ issues, which make up the physical, social, and attitudinal environment in which a person lives their life (i.e. living condition, support and attitudes of friends and family); and
-
‘personal' problems (such as gender, race, coping style, social and educational background) that may affect the person’s experience of living with their condition.
-
Many systematic reviews support various treatment modalities for people with primary brain tumour such as chemotherapy and symptomatic pharmacological therapy (Stewart 2002), radiotherapy (Andrews 2004), or surgery (Pirzkall 1998). A number of reviews also address unidisciplinary rehabilitation for this population, such as psychological interventions (Ownsworth 2009; Sheard 1999). However, none address multidisciplinary rehabilitation in these people.
How the intervention might work
Multidisciplinary rehabilitation in people after primary brain tumour treatment can utilise various categories in domains comprising the structured framework outlined by the ICF, for targeted intervention and therapy. The ICF provides clinicians with specific categories within relevant domains for intervention, for example ‘activity and participation’ domain (relating to mobility, self care, domestic life, major life areas), and environmental factors (transport, access to places, relationships, attitudes).
Many impairments (hemiparesis, dysphasia, cognitive deficits) seen in the brain tumour population are also common in other neurological conditions such as acquired brain injury, stroke, and multiple sclerosis. There is strong evidence to support multidisciplinary rehabilitation in various neurological conditions, such as multiple sclerosis (Khan 2007), acquired brain injury (Turner Stokes 2005), Guillain‐Barre syndrome (Khan 2010), motor neuron disease (Ng 2009), and stroke (SUTC 2007), as well as in oncological conditions, such as breast cancer (Khan 2012a; Khan 2012b). A number of studies have shown that people with brain tumours undergoing rehabilitation appear to make significant functional gains (Geler‐Kulcu 2009; Greenberg 2006; Huang 2001a; Marciniak 2001; O’Dell 1998; Tang 2008), in line with those seen in people affected by other cerebral pathologies such as traumatic brain injury or stroke (Kirshblum 2001). Furthermore, there is strong evidence that unidisciplinary interventions such as exercise and physical therapies enhance physiological and functional outcomes and improve QoL in cancer survivors (MacVicar 1986; MacVicar 1989; Markes 2006; McNeely 2010).
Why it is important to do this review
There are no systematic reviews for multidisciplinary rehabilitation following primary brain tumour treatment to date. Other reasons to do this review include the following:
-
Brain tumour rehabilitation is complex and challenging (Kirshblum 2001), and in light of recent initiatives as outlined in the United States National Coalition for Cancer Survivorship (NCCS 2009), which aims to produce evidence‐based guidelines and implement survivorship care plans, there is a need to address the long‐term requirements of cancer survivors. The previous version of this systematic review aimed to inform the implementation of a National Cancer Survivorship Initiative (Cancer Reform Strategy 2007). This initiative explored individualised approaches to survivorship care (education, nutrition, self management) and the provision of rehabilitation programmes, and was a national service framework in the United Kingdom. This updated review could also be useful for the development of further rehabilitation programmes for those living with and beyond cancer, such as the Living With and Beyond Cancer Programme from United Kingdom (Department of Health 2013).
-
Advances in medical care and increased life expectancy among people with disabilities mean that ongoing health and well‐being become increasingly important and require longer‐term planning (Campbell 1999; Turk 2001). From the rehabilitation perspective, the challenge is not just about helping the brain tumour survivor to overcome the symptoms and improving their performance status; it is also about helping them stay independent in the community in the face of changes associated with tumour progression/recurrence, as well as aging, and helping families to overcome the additional demands and stress.
-
A better understanding of the optimal structure, function, and content of multidisciplinary rehabilitation (with clear delineation of the roles) would further guide improvement of service provision from an organisational and economic perspective.
A systematic review is therefore required to summarise the best available evidence to date. This review aimed to identify the existing evidence for multidisciplinary rehabilitation in people after primary brain tumour treatment, guide treating clinicians, and determine gaps in current knowledge.
Objectives
To assess the effectiveness of multidisciplinary rehabilitation in people after primary brain tumour treatment, and especially the types of approaches that are effective (settings, intensity).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and controlled clinical trials (CCTs), including quasi‐randomised and quasi‐experimental designs with comparative controls (controlled before‐and‐after studies).
Types of participants
Inclusion criteria
-
Adults (aged 18 years and older).
-
Confirmed diagnosis of brain tumour, regardless of time of onset or disease stage according to the WHO classification of tumours of the central nervous system (CNS) (Louis 2007), which include: astrocytic tumours; oligodendroglial tumours; ependymal tumours; choroid plexus tumours; other neuroepithelial tumours; neuronal and mixed neuronal‐glial tumours; tumours of the pineal region; embryonal tumours; tumours of the haemopoietic system; germ cell tumours; meningeal tumours; tumours of the sellar region.
We also included studies involving participants with a range of cancers or other diagnoses that reported data specifically for people with primary brain tumour.
Exclusion criteria
-
Studies recruiting only participants with metastatic (i.e. non‐primary) brain tumour.
-
Studies involving participants with CNS cancers where data were not provided separately for primary brain tumour.
Types of interventions
As described above, multidisciplinary rehabilitation is defined as any intervention delivered by two or more disciplines (such as nursing, physiotherapy, occupational therapy, social work, psychology, and other allied health) in conjunction with medical input (surgeon, oncologist, rehabilitation and/or palliative physician), to maximise activity and participation, as defined by the ICF (WHO 2001).
Multidisciplinary rehabilitation interventions and programmes are broadly described in terms of settings and content (Khan 2007; Turner Stokes 2005). Rehabilitation settings may include "inpatient" settings, where care is delivered 24 hours a day in a hospital ward or specialist rehabilitation or palliative care unit; "ambulatory/outpatient settings", which may be within a hospital or in the community; and "home‐based settings", which are set within the patient’s own home and local community.
The content, intensity, and frequency of therapy provided in multidisciplinary rehabilitation programmes can vary based on individual needs. The content can include physical reconditioning, task re‐acquisition strategies, environmental modification, cognitive behavioural therapy, vocational and recreational programmes.
We considered all studies that stated or implied multidisciplinary rehabilitation for inclusion in this review, provided they satisfied the definition above and compared multidisciplinary rehabilitation to some form of control condition. The control conditions included:
-
lower‐level or different types of interventions such as 'routinely available local services' (e.g. medical and nursing care);
-
minimal interventions (such as 'information only');
-
'wait list' controls or no treatment, or usual care;
-
interventions given in different settings and of lower intensity.
We excluded studies if they assessed the effect of therapy from a single discipline (for example physiotherapy only) or any unidisciplinary intervention or modality (for example physical exercise).
Types of outcome measures
Primary outcomes
Primary outcomes reflect the burden of disease on patients and on the services provided for them. We categorised these according to the ICF into those that focus on the following (WHO 2001).
-
Impairment, e.g. headache, seizures, muscle weakness, aphasia, visual impairments, pain.
-
Disability (limitation in activity), measured by validated tools such as the Functional Independence Measure (Granger 1998), Barthel Index (Mahoney 1965), Cancer Rehabilitation Evaluation System‐short form (Ganz 1992; Schag 1991), Cancer Survivor Unmet Needs measure (Hodgkinson 2007), and Perceived Impact of Problem Profile (Pallant 2006).
-
Restriction in participation and/or environmental or personal context, e.g. quality of life (SF‐36; Ware 1993), fatigue (Fatigue Impacts Scale; Fisk 1994), psychological (Depression Anxiety Stress Scale; Lovibond 1995) and vocational outcomes (Work Instability Scale; Gilworth 2003), social re‐integration and patient satisfaction measures, and others.
-
Any adverse events that may have resulted from the intervention, defined as those events that are life‐threatening or requiring prolonged hospitalisation.
Secondary outcomes
Secondary outcomes included those that reflected service utilisation, such as the length of hospital stay in both acute and subacute settings, readmission, the cost of care, and the extent of services used at the time of discharge.
Timing of outcome measures
The time points for outcome assessments were: short term (immediately after intervention or up to three months) and long term (greater than three months) from the start of the intervention. We considered participant follow‐up assessments similarly as short term (up to three months) and long term (greater than three months) after cessation of the intervention.
Search methods for identification of studies
We considered articles in all languages, with a view to translation if necessary.
Electronic searches
We searched the following databases:
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library) Issue 12 of 12, 2014 (see Appendix 1)
-
MEDLINE (via OvidSP) (from 1950 to January week 2, 2015) (see Appendix 2)
-
EMBASE (via OvidSP) (from January 1980 to January week 2, 2015) (see Appendix 3)
-
PEDro (from January 1985 to January week 2, 2015) (see Appendix 4)
-
LILACS (from January 1982 to January week 2, 2015) (see Appendix 5)
The initial search strategy for this review also included searches of the Cochrane Cancer Network, CANCERLIT, BIOSIS, and Science Citation Index. We used the same principle to search each database. This included: (i) the terms and phrases identifying RCTs and CCTs combined using the Boolean “OR”; (ii) all the terms and phrases describing brain neoplasm combined with “OR”, and (iii) all terms used to identify the interventions of interest, that is multidisciplinary rehabilitation, combined with “OR”. We then grouped these terms with the Boolean operator “AND” and performed the final search of the articles from the displayed results. We used wild cards and truncation symbols to ensure that we did not miss terms with alternative spellings and endings. We exploded all MeSH terms.
Searching other resources
We searched the WHO International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx), the metaRegister of Controlled Trials (http://www.isrctn.com/), and ClinicalTrials.gov (https://clinicaltrials.gov/) for all prospectively registered and ongoing trials. We checked the bibliographies of identified studies and contacted the study authors and known experts in the field seeking published and unpublished trials. We also handsearched the most relevant journals, which included (but were not limited to): Brain, Cancer, Supportive Care in Cancer, Journal of Cancer Therapy, American Journal of Clinical Oncology: Cancer Clinical Trials, Annals of Cancer Research and Therapy, Journal of Surgical Oncology, Journal of Oncology, European Journal of Cancer and Clinical Oncology, Journal of the Cancer Institute, Neuro‐oncology, Journal of Neuro‐oncology, Journal of Neurology, Neurosurgery and Psychiatry, Physical Therapy, Archives of Physical Medicine and Rehabilitation, and Clinical Rehabilitation.
We also undertook an expanded search using the related articles feature (via PubMed), ProQuest Dissertations & Theses, searching key authors (via Web of Science) and searching OpenGrey (System for Information on Grey Literature in Europe).
Data collection and analysis
Selection of studies
Two review authors (BA, LN) independently screened and short‐listed all abstracts and study titles identified by the search strategy for appropriateness based on the selection criteria. The two review authors (BA, LN) independently evaluated each study from this short‐list for inclusion or exclusion. We obtained the full text of all potential articles for further assessment to determine if the study met the inclusion/exclusion criteria. A consensus was met about the possible inclusion/exclusion of all studies, with the involvement of other review authors. Review authors were not masked to the name(s) of the author(s), institution(s), or publication source at any level of the review. As four authors of this review (FK, BA, KD, MG) are authors of the included study, one review author (LN) further screened the list of potential studies twice.
We had intended to contact trialists of eligible studies to further clarify details of their multidisciplinary rehabilitation if needed, however this was not necessary.
Data extraction and management
Three review authors (BA, LN, MG) independently extracted the data from each study that met the inclusion criteria using a standardised data collection form. We summarised all studies that met the inclusion criteria in the 'Characteristics of included studies' table provided in the Review Manager 5 (RevMan) software developed by The Cochrane Collaboration to include details on design, participants, interventions, and outcomes (RevMan 2014).
Assessment of risk of bias in included studies
Three review authors (BA, LN, MG) independently assessed the methodological quality of the included studies using Cochrane's 'Risk of bias' tool (Higgins 2011). This included the allocation sequence generation; allocation concealment; blinding of participants, therapists, and outcome assessors; incomplete outcome data; and selective outcome reporting. A judgement of ‘low risk’ indicated a low risk of bias, ‘high risk’ indicated a high risk of bias, and ‘unclear’ indicated either unclear or unknown risk of bias.
We considered studies to be of high methodological quality if the risk of bias for all domains was low. We termed these studies 'high‐quality studies'. We rated studies as low methodological quality if there was unclear or high risk of bias for one or more domains, terming these 'low‐quality studies'. Any disagreements or lack of consensus were resolved by consultation with a third review author (FK).
Measures of treatment effect
It was not possible to perform measures of treatment effect or to pool the data for meta‐analysis, due to insufficient data and the type of data available and the diversity of methods in the studies. We entered and analysed all data in RevMan (RevMan 2014). We qualitatively summarised the studies in the 'Characteristics of included studies' table and presented the results of primary and secondary outcomes of included studies, categorised according to ICF framework, in the summary of findings Table for the main comparison. We described the results narratively in the Discussion section. If studies become available, and if meta‐analyses are feasible in future updates, we will analyse treatment effects as described in the protocol version of this review (Khan 2011).
Unit of analysis issues
We anticipated that the appropriate unit of analysis would be by type, intensity, and setting of multidisciplinary rehabilitation.
Dealing with missing data
We would have attempted to contact the primary authors of potentially eligible studies to provide clarification of the data if necessary, however this was not required.
In addition, we excluded studies with 'fatal flaws' (for instance, withdrawals by more than 40% of the participants, nearly total non‐adherence to the protocol, or very poor or non‐adjusted comparability in the baseline criteria).
Assessment of heterogeneity
We followed the statistical analysis method as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, it was not possible to conduct a comprehensive quantitative analysis due to the variability of methods used and the type of available data reported in each study.
Assessment of reporting biases
We minimised publication bias by sourcing unpublished data where possible and would have contacted authors for the full data set or the reason for not publishing the data, however this was not required (Egger 1998).
Data synthesis
As mentioned above, we were unable to conduct a quantitative analysis due to lack of studies identified, clinical heterogeneity, and the variation in methods and available data in the included studies. If studies had been available, we would have attempted a quantitative analysis, provided there was clinical homogeneity and the data in each study allowed for such an analysis. We would also have calculated a weighted treatment effect across trials using the RevMan and expressed the results as risk ratios with 95% confidence intervals (CIs) and risk differences with 95% CIs for dichotomous outcomes and mean differences and 95% CIs for continuous outcomes (RevMan 2014). We would have initially used a fixed‐effect model and approximate Chi2 tests for heterogeneity to assess outcome data for compatibility with the assumption of a uniform risk ratio (P > 0.10). In the presence of significant heterogeneity (P < 0.10), we would have used random‐effects meta‐analysis instead.
We used the GRADE approach to grade evidence quality, as described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We have highlighted the strength of study findings, discussed gaps in current literature, and identified future research directions in the Discussion section. If data becomes available in future updates, we will attempt a quantitative analysis, as described in the protocol version of this review (Khan 2011).
Subgroup analysis and investigation of heterogeneity
Due to lack of available data, it was not possible to perform subgroup analysis for the following:
-
type of multidisciplinary rehabilitation (i.e. inpatient, ambulatory care);
-
intensity of treatment (high‐, low‐intensity (usual outpatient care) multidisciplinary rehabilitation);
-
time from definitive treatment (surgery, radiotherapy, and chemotherapy) to commencement of multidisciplinary rehabilitation (acute: less than six weeks, intermediate: six weeks to six months, and longer term: more than six months).
Factors considered in heterogeneity included: setting, type, and intensity of multidisciplinary rehabilitation.
Sensitivity analysis
We performed no sensitivity analysis. If studies had been available, and heterogeneity existed across trials, we would have conducted sensitivity analyses by omitting trials with a high risk of bias.
Results
Description of studies
See the 'Characteristics of included studies' and 'Characteristics of excluded studies' tables for details.
Results of the search
The initial version of this review identified a total of 5410 references (CENTRAL = 957; MEDLINE = 1853; EMBASE = 2559; PEDro = eight; LILACS = 14; the Cochrane Neuromuscular Disease Group Specialized Register = 19) with our search criteria (up to 13 March 2013) (Khan 2013a). Of these references, 18 passed the first screening review and were selected for closer scrutiny. We also found two potentially relevant articles from bibliographies of papers identified. The updated electronic and manual search (up to 12 January 2015) yielded a further 1609 additional titles (CENTRAL = 103; MEDLINE = 888; EMBASE = 615; PEDro = two; LILACS = one). We finally screened titles and abstracts of 1543 articles after removal of duplicates. Of these, we scrutinised full‐text of 24 potential articles and included one CCT. (See Figure 1 for the study flow chart.)
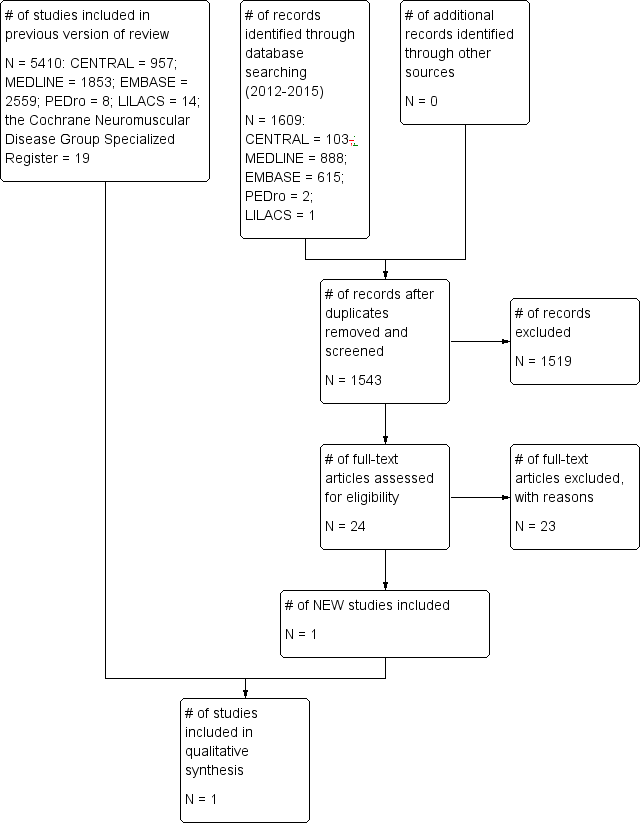
Study flow diagram.
Included studies
From the updated search we found one new CCT (Khan 2014), which compared a high‐intensity multidisciplinary ambulatory (outpatient) rehabilitation programme with a standard outpatient care over six months. (See Characteristics of included studies.) This study involved a total of 106 participants (with gliomas) recruited from a tertiary hospital in Melbourne, Australia, Participants were predominantly women (56%), with mean age of 51 years (standard deviation 13.6 years, range 21 to 77 years), and median time since diagnosis of 2.1 years. Participants were allocated to a treatment group (N = 53) for an individualised high‐intensity multidisciplinary programme or a wait‐list control group (N = 53).
The intensive multidisciplinary rehabilitation programme was an individualised, time‐based, functional, goal‐oriented treatment with active patient participation involving all relevant disciplines based on participant need and team consensus. The programme consisted of one‐hour sessions of uninterrupted therapy two to three times per week for six to eight weeks or more (depending on the individual need). Each session was broken into half‐hour therapy blocks (occupational, social, psychological, and physiotherapy).
The methods used included, for example, physiotherapy for strengthening, endurance, and gait training; occupational therapy to improve everyday function (domestic or community tasks), driving, and return to work; and clinical psychology for counselling and support as required.
The mean duration of the rehabilitation programme was 21 days (range 14 to 32 days). The control group were those people on the wait‐list for the rehabilitation programme, who continued with their usual activity in the community.
The primary outcome measured was change in the Functional Independence Measure (FIM), which assesses independence in daily activities including self care, continence, mobility, locomotion, and cognition. Secondary measures included measures of participation and psychosocial function: change in the Depression Anxiety Stress Scales (DASS), the Perceived Impact of Problem Profile (PIPP), and the Cancer Rehabilitation Evaluation System‐Short Form (CARES‐SF).
All outcome measures were assessed at baseline, three, and six months after programme completion.
Excluded studies
We excluded 23 studies for the reasons described in the 'Characteristics of excluded studies' table. The primary reasons for exclusion were:
-
not RCT or CCT (N = 17)
-
unidisciplinary intervention (N = 5)
-
data not specifically provided for brain tumour subgroup (N = 1)
Of the 17 studies excluded due to study design, 13 reported functional outcomes related to multidisciplinary rehabilitation and are described below in the Discussion section.
Risk of bias in included studies
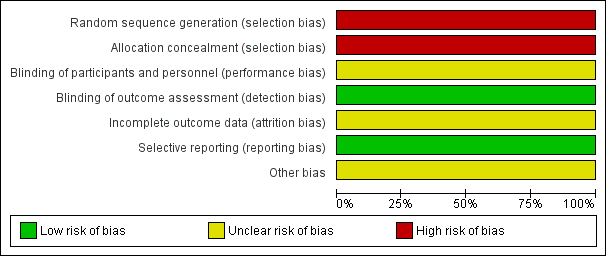
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The newly included CCT, Khan 2014, comprehensively described details of the rehabilitation programme, and the trial investigators performed an a priori power calculation based on the primary outcome. The study had several weaknesses that increased the risk of bias:
-
Participants were not randomised and allocation concealment procedure was not performed.
-
The trial tested a small convenience sample limited to one Australian metropolitan facility, limiting the external validity of the results.
-
The participants were not blinded, owing to the nature of the treatment.
-
Avoidance of co‐interventions or their equal distribution throughout study groups was not reported. Reporting of co‐interventions could have helped judgement of their division among study groups, and whether they would have significantly affected the outcome.
-
Loss of 21 participants (20%) at six‐months follow‐up.
Allocation
Allocation concealment of intervention assignment was not performed in this study.
Blinding
Participants were not blinded, as the rehabilitation intervention itself requires participants to be consented for treatment, hence making it difficult to blind participants. This could have influenced participant‐reported outcomes. However, participants were asked not to discuss their involvement in the study with treating therapists and outcome assessors in order to reduce the risk of breaking blinding of the therapists and assessors. Treating therapists were blinded.
Incomplete outcome data
At three‐months follow‐up, eight participants (7%) dropped out: four in each group. At six‐months follow‐up, the overall dropout rate was 20%: 12 in the intervention group and nine in the control group.
Selective reporting
The authors of the study reported all prespecified (primary and secondary) outcomes in detail.
Other potential sources of bias
The study included a small selective cohort listed on a single database held at a single tertiary institution that agreed to participate in research projects, which may limit the generalisability of findings. Participant follow‐up was short, that is only up to six months.
Avoidance of co‐interventions or their equal distribution throughout study groups was not reported. Reporting of co‐interventions could have helped judgement of their division among study groups, and whether they would have significantly affected the outcome.
Effects of interventions
See: Summary of findings for the main comparison
The CCT, Khan 2014, addressed the effectiveness of outpatient (ambulatory) multidisciplinary rehabilitation for people with primary brain tumours. The findings for all outcomes are presented in Table 1.
| Methods | Case‐control study, Italy |
| Participants | N = 150; Intervention: N = 75 with brain tumours (meningioma and glioblastoma), control: N = 75 with stroke
Inclusion: all admitted patients to an inpatient neurorehabilitation unit after surgery for brain tumours (meningiomas or glioblastomas) over a 2‐year period (2007‐2009). Control participants were stroke patients (ischaemic or haemorrhagic), matched one‐to‐one for age, sex, and side of lesion Exclusion: people with oligoastrocytoma, oligodendroglioma, and ependymomas in order to obtain homogenous group
|
| Interventions | Inpatient multidisciplinary rehabilitation administered by experienced physical therapists, 60‐min session, 6 days/week for 4 consecutive weeks, which included passive/assisted stretching exercises, strengthening exercises, balance exercises, ground‐floor walking (including step control), and 4 weeks of speech therapy (individual 60‐min sessions, once daily, 6 days/week) when aphasia was diagnosed |
| Outcomes | Sitting balance, standing balance, Hauser Index: gait disorders, MGHFAC: severity of gait disorders, FIM |
| Assessment time points | Before and after the intervention |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control study, USA |
| Participants | N = 42; Intervention: N = 21 with low‐grade gliomas, control: N = 21 with high‐grade gliomas
Inclusion: all patients admitted to an inpatient acute rehabilitation programme between 1996 and 2008. 21 of 443 with high‐grade and 21 of 24 with low‐grade astrocytoma were selected |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; LOS; discharge‐to‐home rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: N/A Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Case‐control study, Turkey |
| Participants | N = 42; Intervention: N = 21 with brain tumours (benign and malignant), control: N = 21 with stroke
Inclusion: all admitted patients to an inpatient neurorehabilitation unit, control participants were stroke patients (ischaemic or haemorrhagic), matched by side of lesion Exclusion: people with oligoastrocytoma, oligodendroglioma, and ependymomas in order to obtain homogenous group |
| Interventions | Inpatient "conventional" rehabilitation programme, single 60‐min sessions, 5 days/week for 4 consecutive weeks, which included physiotherapy and occupational therapy (if needed). Physiotherapy focused on positioning, postural control, range of motion, and progressive resistive exercises together with endurance and gait. Patients were discharged when their functional level was considered sufficient to allow them to participate in outpatient rehabilitation |
| Outcomes | PAS for Stroke, BBS, MAS, FIM (mobility) |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control study, Israel |
| Participants | N = 1828; Intervention N = 168 with brain tumours (128 meningiomas, 40 gliomas), control: N = 1660 with stroke (ischaemic or haemorrhagic)
Inclusion: all admitted patients to an inpatient neurorehabilitation unit over an 11‐year period (1993‐2004)
|
| Interventions | Inpatient multidisciplinary rehabilitation provided by PT, medical staff, OT, and speech pathologist. Details of the multidisciplinary rehabilitation not provided |
| Outcomes | FIM, FIM efficiency, LOS days, discharge destination (rate discharge to home) |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: No Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Prospective case series, USA |
| Participants | N = 10 (brain tumour)
Inclusion: all admitted patients to an inpatient neurorehabilitation unit over a 1‐year period (1999‐2000) |
| Interventions | Inpatient multidisciplinary rehabilitation that included: OT, rehabilitation therapy, recreational therapy, speech therapy, PT, rehabilitation nursing and case management |
| Outcomes | FIM, DRS, KPS, FACT‐BR |
| Assessment time points | Admission and discharge, post hoc analysis at 3‐month postdischarge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control, USA |
| Participants | N = 156; Intervention: N = 78 with primary or metastatic brain tumours (benign and malignant), control: N = 78 with traumatic brain injury matched by age and side of lesion
Inclusion: evaluation by a physiatrist for the following criteria: medical stability, need for therapy from more than one discipline, demonstration of gains with acute‐care therapies, potential to tolerate 3 hours of therapy, willingness and motivation to participate in a rehabilitation programme Exclusion: patients who did not complete rehabilitation due to medical complications or death |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to community rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control study, USA |
| Participants | N = 126; Intervention: N = 63 with primary or metastatic brain tumours (benign and malignant), control: N = 63 with stroke, case matched by age, gender, and side of lesion
Inclusion: all patients admitted to an inpatient rehabilitation centre |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to community rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Cohort study without control, Korea |
| Participants | N = 25 with brain tumours Inclusion: all admitted patients to an inpatient neurorehabilitation unit after surgery (resection) for brain tumours (benign or malignant) over a 1‐year period (1 July 2008 to 30 June 2009), able to follow simple commands, as determined by scores ≥ 24 on the Korean version of the Mini‐Mental State Examination Exclusion: those unable to complete a questionnaire because of a severe aphasia or a cognitive deficit, or who were clinically unstable, either medically or surgically |
| Interventions | Inpatient rehabilitation (4 weeks); details not provided |
| Outcomes | Fatigue severity: PFS, BFI Mood status: BDI Motor impairment: MI Functional status: KPS, MBI QoL: EORTC QLQ‐C30 |
| Assessment time points | Before and after the intervention |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case series, USA |
| Participants | N = 132 participants divided into 4 groups: astrocytomas 26%, meningiomas 33%, metastatic tumours 16%, other tumours 25%. Participants also grouped into those with tumour recurrence and those with initial tumour presentation
Inclusion: all patients > 18 years, inpatient rehabilitation within a 3‐year period (1993‐1996) |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to home rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control, USA |
| Participants | N = 80; Intervention: N = 40 participants with brain tumours (benign and malignant), control: N = 40, case matched by admission FIM score, age, and gender to 40 participants with traumatic brain injury
Inclusion: all patients admitted to an inpatient acute rehabilitation programme over a 2‐year period (1994‐1996) |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; LOS; discharge destination to home rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Prospective case series (before and after) study, Italy |
| Participants | N = 121 with malignant brain tumours
Inclusion: all patients discharged from hospital over 3‐year period (2000‐2003) with neurological deficits |
| Intervention | Home neurorehabilitation programme including physiotherapy 1 hour/3 times a week for 3 months, neurologist evaluation, psychological assistance, nursing and palliative care team if needed (further details not provided) |
| Outcomes | BI, KPS, EORTC QLQ‐C30 |
| Assessment time points | Before and 3 months after rehabilitation |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Patients who completed only basal questionnaire were excluded Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case series, USA |
| Participants | N = 13 (primary malignant brain tumours with a history of surgical resection, radiation, and chemotherapy)
Inclusion: all patients receiving outpatient rehabilitation who had a diagnosis of malignant brain tumour and adequate medical records to characterise their tumour and courses of therapy |
| Intervention | Outpatient rehabilitation with input from psychologists, speech/language pathologists, OT, and vocational specialists. Participants received an average of 2.6 ± 1.9 months of therapy (duration of 5 hours/day) (further details not provided) |
| Outcomes | Level of independence, vocational (productivity) outcomes |
| Assessment time points | Admission, discharge, and 8‐months follow‐up |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Yes Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case series, Canada |
| Participants | N = 63 with primary and metastatic brain tumours, divided into 3 groups: glioblastoma multiforme 29%; metastatic tumours 40%; and various other primary brain tumours 31%
Inclusion: all patients admitted to an inpatient rehabilitation ward over a 3‐year period (2003‐2006) Exclusion: patients with meningiomas |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to home rate; survival |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
BBS: Berg Balance Scale
BDI: Beck Depression Inventory
BFI: Brief Fatigue Inventory
BI: Barthel Index
DRS: Disability Rating Scale
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30
FACT‐BR: Functional Assessment of Cancer Therapy–Brain
FIM: Functional Independence Measure
KPS: Karnofsky Performance Status Scale
LOS: length of stay
MAS: Motor Assessment Scale
MBI: Modified Barthel Index
MGHFAC: Massachusetts General Hospital Functional Ambulation Classification
MI: Motricity Index
N: total number
N/A: not applicable
OT: occupational therapist
PAS: Postural Assessment Scale
PFS: Piper Fatigue Scale
PT: physiotherapist
QoL: quality of life
USA: United States of America
Short‐term subjective outcomes (reduced disability and limitation in participation) at three‐months postintervention
-
The treatment group demonstrated a significantly greater gain in total FIM motor score in comparison with the control group (mean difference 3.5, 95% CI 0.8 to 6.2; P = 0.012). This difference was seen across all domains: self care, sphincter control, mobility, locomotion, and communication (P < 0.01 for all) and cognition subscale (P < 0.05) with small‐to‐moderate effect sizes (Cohen’s R = 0.2 to 0.4).
-
There were no significant short‐term effects on other scores.
Longer‐term subjective outcomes (reduced disability and limitation in participation) at six‐months postintervention
-
Significant improvement in the treatment group was maintained only for FIM 'sphincter', 'communication', and 'cognition' subscales (P < 0.01 for all).
-
We noted no difference in groups on other scales.
Cost‐effectiveness was not evaluated. The study reported no serious adverse events attributed to the rehabilitation intervention. P values are reported as they were stated in the study publications.
Discussion
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews [2013, Issue 1, Art. No. CD009509] on 'Multidisciplinary rehabilitation after primary brain tumour treatment'. The literature search for this update identified one low‐quality controlled clinical trial (CCT) involving 106 participants.
Summary of main results
One small CCT, Khan 2014, provided low‐quality evidence that an individualised, high‐intensity multidisciplinary ambulatory (outpatient) rehabilitation programme reduced disability (mobility, self care, continence, and cognition) when compared with the control group with usual outpatient care at three months. Improvement in some domains of disability (continence, communication) and psychosocial gains were maintained at six‐months follow‐up. The study demonstrated that the multidisciplinary rehabilitation programme positively impacted on participation when compared with usual outpatient care at six months (summary of findings Table for the main comparison). The reported relative effect sizes were modest to large. This updated review increases the body of evidence for high‐intensity multidisciplinary ambulatory (outpatient) rehabilitation, as previously no such studies had been identified.
Brain tumour is a complex and devastating condition that places many demands on patients, caregivers, and health professionals. People with brain tumour can have diverse presentations and a varied level of disability for rehabilitation, requiring an individualised approach. Rehabilitation itself is defined as a 'complex' intervention (when the active ingredient in the intervention is not easily identifiable) (MRC 2000).
For the overall completeness of this review, we have presented data from studies with other designs or observational studies in the section below, with the understanding that the contribution of such studies for best evidence synthesis is limited at best.
Overall completeness and applicability of evidence
The gaps in the evidence base in rehabilitation practice for people with brain tumour include:
-
limited high‐quality evidence for overall effectiveness of multidisciplinary rehabilitation;
-
lack of agreement among treating clinicians with respect to the clinical approach and the most effective forms of intervention;
-
failure to incorporate the perspective of the person with brain tumour and their caregivers;
-
lack of common outcome measures to compare rehabilitation practice across different programmes and populations.
These are similar to evidence gaps that have been outlined previously for people with multiple sclerosis, motor neuron disease, and acquired brain injury (Khan 2007; Ng 2009; Turner Stokes 2005).
We identified one CCT, Khan 2014, evaluating the effectiveness of multidisciplinary rehabilitation in primary brain tumour survivors. Although randomised controlled trials (RCTs) and CCTs are the main scientific and rigorous approach to study comparative effectiveness of interventions and are widely considered as the ‘gold standard’ for high‐level evidence, they are much less suited to studying ‘complex’ interventions such as rehabilitation (Horn 2012; Khan 2007; Turner Stokes 2005). The many challenges for traditional research designs (such as RCTs) in rehabilitation settings include: heterogeneous, interdependent components; different patient populations and contexts; treatments that are multifaceted, multilayered and involve organisational restructure; and individual intervention and ethical considerations (Horn 2012; Khan 2007; Khan 2010). Brain tumour survivors form a diverse group with a wide range of clinical presentations and varied levels of disability, requiring a customised and multifactorial approach to rehabilitation. Hence, such a rehabilitation approach does not lend itself well to RCTs, and as a result many vital questions remain unanswered (Horn 2012; Khan 2007; Whyte 2002).
Additional evaluation of observational studies
Given the paucity of clinical trials, we also evaluated observational studies (with 10 participants or more) in order to provide a more complete picture of the existing literature. The literature search identified 13 observational studies reporting various outcomes following multidisciplinary rehabilitation in people with brain tumours (Bartolo 2012; Fu 2010; Geler‐Kulcu 2009; Greenberg 2006; Huang 1998; Huang 2000; Huang 2001a; Kim 2012; Marciniak 2001; O'Dell 1998; Pace 2007; Sherer 1997; Tang 2008). (See Table 1.) We have summarised the limited evidence from these studies below.
-
Seven studies were conducted in the United States, two in Italy, and one each in Korea, Turkey, Israel, and Canada.
-
Each study was conducted within a single institute/facility, with a total of 830 participants with various types of brain tumours.
-
Eleven studies involved inpatient rehabilitation settings and two ambulatory settings (one outpatient setting, Sherer 1997, and one home based, Pace 2007).
-
Seven studies were retrospective audits of hospital medical records (Fu 2010; Greenberg 2006; Huang 1998; Huang 2000; Marciniak 2001; O'Dell 1998; Tang 2008).
-
Seven studies were case‐control studies, of which six compared the rehabilitation outcomes of brain tumour participants with other non‐oncological neurological condition cohorts (four compared with stroke survivors (Bartolo 2012; Geler‐Kulcu 2009; Greenberg 2006; Huang 1998), two with traumatic brain injury (Huang 2000; O'Dell 1998). One study, Fu 2010, compared the functional outcomes between low‐ and high‐grade astrocytomas.
-
The content, duration, intensity, and nature of the multidisciplinary rehabilitation programmes were not well described.
-
All studies had small sample sizes, making it difficult to detect a possible treatment effect.
-
No adverse effects were reported.
-
We rated all studies as very low quality due to lack of methodological robustness and unsystematic clinical observations (Table 1).
We have summarised the effects of interventions and results of these studies in Table 2.
| Statistical analysis | Student’s t test, Chi2 test, Wilcoxon matched‐pairs signed‐rank test, Mann‐Whitney U test, Kruskal–Wallis ANOVA |
| Results |
|
| Author’s conclusions | Rehabilitation after surgery can improve functional outcome, justifying the delivery of rehabilitation services, even during the acute phase, to brain tumour inpatients, irrespective of tumour type |
| Statistical analysis | Descriptive analysis, Chi2 test, Kruskal‐Wallis test, Mann‐Whitney U test |
| Results |
|
| Author’s conclusion | All participants made significant functional gains from admission to discharge. Compared with people with low‐grade astrocytoma, people with high‐grade astrocytoma had higher total FIM gain but also longer LOS. FIM efficiencies were comparable between the groups |
| Statistical analysis | Freidman test, Chi2 test, Mann‐Whitney U test, ANOVA |
| Results |
|
| Author’s conclusions | People with brain tumour progressed as well as people with stroke in a post‐acute inpatient rehabilitation programme |
| Statistical analysis | Descriptive statistics, analysis of variance |
| Results |
|
| Author’s conclusions | Both people with gliomas and people with meningiomas hospitalised for inpatient rehabilitation improved their FIM ratings after a short inpatient multidisciplinary rehabilitation. Both groups had high rates of discharge to the community |
| Statistical analysis | ANOVA, Spearman’s correlation analysis, Bonferroni statistical test |
| Results |
|
| Author’s conclusion | Although participants made functional gains during and after inpatient multidisciplinary rehabilitation, gains in QoL were not significant until 1‐month postdischarge. QoL does not appear to correlate well with functional outcomes. Furthermore, the KPS is less sensitive than the FIM and DRS in detecting change in functional status |
| Statistical analysis | ANOVA, Chi2 test |
| Results |
|
| Author’s conclusion | People with brain tumour can achieve comparable functional outcome and have a shorter rehabilitation length of stay and greater discharge to community rate than people with traumatic brain injury |
| Statistical analysis | ANOVA, Chi2 test |
| Results |
|
| Author’s conclusion | People with brain tumour can achieve comparable functional outcome and discharge to community rate, and have a shorter rehabilitation length of stay than people with stroke |
| Statistical analysis | Mann–Whitney test, Spearman’s correlation analysis, Wilcoxon signed‐rank tests |
| Results |
|
| Author’s conclusion | The findings suggest that people with brain tumours commonly complain of a moderate level of fatigue, which may reduce daily functioning and quality of life, with sleep disturbance being a significant predictor of fatigue. During rehabilitation, functional outcomes and motor power showed improvements in those people, not aggravating fatigue |
| Statistical analysis | Descriptive analysis, analysis of variance |
| Results |
|
| Author’s conclusion | Metastatic or primary brain tumour type does not affect the efficiency of functional improvement during inpatient multidisciplinary rehabilitation. People receiving concurrent radiation therapy make greater functional improvement per day than those not receiving radiation. People with recurrent tumours make significantly smaller functional motor gains than those completing inpatient multidisciplinary rehabilitation after the initial diagnosis of the tumour |
| Statistical analysis | Descriptive analysis, Chi2 test, Kruskal‐Wallis test, Mann‐Whitney U test |
| Results |
|
| Author’s conclusion | Daily functional gains made by people with brain tumour undergoing multidisciplinary rehabilitation were similar to those made by people with traumatic brain injury matched by age, gender, and admission functional status |
| Statistical analysis | Chi2 test, Student t test (paired or not, as appropriate) |
| Results | At 3‐months follow‐up:
|
| Author’s conclusion | Multidisciplinary rehabilitation at home in people with brain tumour was associated with significant functional gain measured both with BI and KPS. The benefit of multidisciplinary rehabilitation may influence patient's perception of quality of life |
| Statistical analysis | Descriptive analyses only |
| Results |
|
| Author’s conclusion | People with primary malignant brain tumours achieved increased community independence and vocational outcomes (such as employment, education) after individualised outpatient multidisciplinary rehabilitation. Such treatment programme appears to be an attractive, relatively low‐cost option for these patients, however additional investigation is needed. |
| Statistical analysis | ANOVA, Chi2 test, Kruskal‐Wallis and post‐hoc tests using Mann‐Whitney U test with Bonferroni adjustment, Wilcoxon signed‐ranks test, logistic regression, Kaplan‐Meier analyses |
| Results |
|
| Author’s conclusion | People with primary and metastatic brain tumours achieved functional gains after multidisciplinary rehabilitation. High functional improvement is a significant predictor of longer survival in brain metastases and GBM. |
ADL: activities of daily living
ANOVA: analysis of variance
BBS: Berg Balance Scale
BFI: Brief Fatigue Inventory
BI: Barthel Index
DRS: Disability Rating Scale
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30
FACT‐BR: Functional Assessment of Cancer Therapy–Brain
FIM: Functional Independence Measure
GBM: glioblastoma multiforme
KPS: Karnofsky Performance Status Scale
LOS: length of stay
MAS: Motor Assessment Scale
MBI: Modified Barthel Index
MGHFAC: Massachusetts General Hospital Functional Ambulation Classification
MI: Motricity Index
OT: occupational therapist
PASS: Postural Assessment Scale for Stroke
PFS: Piper Fatigue Scale
PT: physiotherapist
QoL: quality of life
USA: United States of America
Findings based on observational studies
Eleven studies addressed the efficacy of inpatient multidisciplinary rehabilitation (N = 696 participants) and reported significant reduction in disability (improvement in function) after a period of multidisciplinary rehabilitation as measured by various functional measurement tools (Functional Independence Measure (FIM), Barthel Index (BI), Modified Barthel Index (MBI), Karnofsky Performance Status Scale (KPS), Motricity Index (MI), Piper Fatigue Scale (PFS), Brief Fatigue Inventory (BFI); Table 2) (Bartolo 2012; Fu 2010; Geler‐Kulcu 2009; Greenberg 2006; Huang 1998; Huang 2000; Huang 2001a; Kim 2012; Marciniak 2001; O'Dell 1998; Tang 2008). One study, Huang 2001a, reported continued functional improvements three months after discharge in a post‐hoc analysis. Six studies compared multidisciplinary rehabilitation outcomes of people with brain tumours with people with other neurological conditions (stroke or traumatic brain injury). All six reported significantly greater gain in total FIM score when compared to stroke or traumatic brain injury (Bartolo 2012; Geler‐Kulcu 2009; Greenberg 2006; Huang 1998; Huang 2000; O'Dell 1998). One study, Fu 2010, compared functional outcomes between different types of brain tumour and found no significant differences. Four studies noted shorter length of hospital stay (LOS) in people with brain tumour compared with people with either stroke or traumatic brain injury (Greenberg 2006; Huang 1998; Huang 2000; Huang 2001a), in contrast with findings of O'Dell 1998, which reported no difference in LOS between brain tumour and traumatic brain injury groups. The percentage of participants discharged to home/community environments was comparable or greater in the brain tumour group, compared with people with stroke or traumatic brain injury (Greenberg 2006; Huang 1998; Huang 2000; O'Dell 1998). Another study, Kim 2012, investigating fatigue severity and the relationship between fatigue and other associated factors in patients with brain tumours found that after four weeks of inpatient rehabilitation, both motor function (MI scores) and functional status (KPS, MBI scores) improved significantly, whereas there was no change in fatigue severity (PFS and BFI scores).
Two studies (N = 134 participants) evaluated the effectiveness of ambulatory multidisciplinary rehabilitation (Pace 2007; Sherer 1997). One study, Sherer 1997, reported favourable participation outcomes (community independence and employment) after outpatient multidisciplinary rehabilitation in people with brain tumours. These gains were generally maintained at eight months after discharge. Another study, Pace 2007, showed significant functional gain (BI, KPS scores) and improved quality of life after home‐based multidisciplinary rehabilitation (Table 2).
These observational studies addressed a broad spectrum of outcomes with limited follow‐up. The study participants were heterogeneous (disease severity, diagnostic criteria used) with varying rehabilitation practices across countries (United States, Turkey, Italy, Israel, Korea), limiting generalisability of findings.
Limitations of findings
This review highlighted a number of limitations in the existing literature for multidisciplinary rehabilitation in the brain tumour population. These include the following.
-
Limited number of methodologically rigorous studies (RCTs or CCTs).
-
Due to paucity of data, comparison of multidisciplinary rehabilitation in different settings or at different intensities was not possible.
-
Only one low‐quality study provided direct evidence for organised multidisciplinary rehabilitation in achieving better outcomes when compared with control conditions.
-
No studies addressed cost benefits of multidisciplinary rehabilitation, nor information about caregiver burden or needs.
Issues for consideration in brain tumour multidisciplinary rehabilitation
Despite the lack of robust evidence for multidisciplinary brain tumour rehabilitation, significant progress in the management of cancer survivors has led to increased prominence for integrated multidisciplinary rehabilitation (Franklin 2007; Gabanelli 2005; Kirshblum 2001). Many issues need to be considered for improved care for brain tumour survivors, including those discussed below.
Brain tumours are complex and can be rapidly progressive, characterised by heterogeneous symptoms associated with increased intracranial pressure and focal symptoms related to tumour location (Vargo 2011). Brain tumours can present for rehabilitation with a diverse clinical picture, varying levels of disability ranging from cognitive impairment, alterations in functional status to the presence of neuropsychiatric symptoms, requiring an individualised approach (Sherwood 2006). It is often associated with a poor prognosis, particularly malignant tumours, which have a median overall survival of about 12 months (Arber 2010). In addition to optimising standard medical treatments (surgery, radiotherapy, chemotherapy) and minimising complications (pain, hemiparesis, dysphasia), these patients require comprehensive multidisciplinary rehabilitation, which goes beyond simple physical recovery (Gabanelli 2005; Kirshblum 2001). The goals of multidisciplinary rehabilitation include post‐acute psychosocial adjustment and participation (independence, economic stability, employment, leisure activities, education) and palliative care (if required), ultimately optimising quality of life of the person (Huang 2011; Kirshblum 2001). Compared to other similar neurological impairments (such as traumatic brain injury, stroke), the time frames for intervention in people with brain tumour are shorter (due to high mortality rate), thus requiring well‐defined functional goals. Furthermore, detrimental effects of treatment in this population are significantly higher (Kirshblum 2001).
Despite advances in brain tumour treatment, rehabilitation has not gained similar momentum among treating healthcare professionals (Kirshblum 2001; Tang 2008). A survey in the United States found that half of rehabilitation hospitals do not treat more than 10 people with brain tumours annually (Boake 1993). This may be due to either poor awareness of the extent of rehabilitation services available on the part of healthcare professionals (neurosurgeons, neurologists, neuro‐oncologists), or lack of understanding of the principles/benefits of rehabilitation (Kirshblum 2001; Tang 2008). There are well‐defined conceptual frameworks and models for designing successful rehabilitation programmes for people with cancer diagnoses (Dietz 1969; Franklin 2007). These serve as a tool for identification of issues, symptoms, and functional deficits that occur most frequently at each stage of the cancer journey (that is staging/pretreatment, primary treatment, post‐treatment, recurrence, end‐of‐life) and help formulate needs of patients to establish a framework for providing multidisciplinary rehabilitation over time (Franklin 2007). However, none of the observational studies identified in this review used this approach.
There is no optimal and universally accepted outcome measure that incorporates the full spectrum of problems for people with cancer (Franklin 2007). Generic measures used in brain tumour (and other cancer) populations in general rehabilitation settings (for example the FIM or BI) are not sensitive enough to capture the relevant gains following intervention, and have ceiling effects (Franklin 2007; Khan 2009). The FIM constitutes an ordinal rating scale and therefore should not be summed to a single total score nor subjected to mathematical manipulation (such as division by LOS) to derive a measure of efficiency (Khan 2009). The KPS is frequently used in brain tumour research, but it does not provide sufficient/specific information to guide the selection of appropriate and timely rehabilitation interventions (Franklin 2007). More information is thus required to determine whether the functional efficiency reported in the identified observational studies has real implications for clinical practice.
The outcome measures used in the brain tumour population vary and need to reflect its complex constructs, with a focus on activity (disability) and especially restriction in participation, as advocated by the International Classification of Functioning, Disability and Health (ICF) (WHO 2001). The ICF provides a comprehensive framework and classification system for a universal language for health professionals, researchers, patients (and caregivers), and consumer organisations to facilitate communication and agreement among treating clinicians with respect to clinical approach (Khan 2007; Khan 2010; Turner Stokes 2005). An ICF ‘core set’ for head and neck cancers (lists of ICF categories selected by experts for targeted management) has been developed (Tschiesner 2010a) and validated (Leib 2011; Tschiesner 2010b), and in the future can assist with scale development using ICF item‐banking techniques (Cieza 2008). A prospective community cross‐sectional survey used the ICF to evaluate patient‐reported disability in primary brain tumours (Khan 2013b). The authors compared participants’ responses with categories within core sets for stroke and traumatic brain injury and found the existing comprehensive stroke and traumatic brain injury core sets incorporated the majority of issues relevant to brain tumour survivors in post‐acute settings. The authors advocated that the findings not only assist in defining a future core set for brain tumour, but also have a potential of developing a single core set relevant to most long‐term neurological conditions (Khan 2013b).
Cancer registries exist in many countries and contain data mainly for survival, medical, and treatment outcomes. However, data in post‐acute settings that provide information about residual disability and restriction in participation after brain cancer treatment (including rehabilitation and palliative care input) is not routinely available, especially over a longer time period. In Australia, the Australasian Rehabilitation Outcomes Centre (AROC), the national rehabilitation data set, collates inpatient and ambulatory data from 182 accredited public and private rehabilitation facilities across the country (AROC 2011). The AROC data set provides a national benchmarking system to improve clinical rehabilitation outcomes and produce information on the efficacy of interventions through the systematic collection of outcomes information in both inpatient and ambulatory settings. It currently provides generic measures of global disability and essential rehabilitation outcome data only (such as the degree of reduction in disability, length of hospital stay, and discharge destinations) (AROC 2011). A review is underway to refine and collect information in specific domains over time, relevant to brain cancer survivors in the AROC, so that information obtained on outcomes will make this data set more clinically relevant in the future.
Quality of the evidence
One low‐quality CCT provides support for outpatient (ambulatory) multidisciplinary rehabilitation in the brain tumour population, including:
-
low‐quality evidence for the effectiveness of outpatient multidisciplinary rehabilitation in reducing longer‐term disability (at six‐months postintervention);
-
low‐quality evidence for short‐term reduction of disability (at three‐months postintervention);
-
no evidence for improvement in quality of life or participation or both following multidisciplinary rehabilitation or cost‐effectiveness of such programmes;
-
no adverse effects attributable to multidisciplinary rehabilitation;
-
no evidence for caregiver burden, cost, or vocational outcomes (such as return to work, education, etc.).
In addition, we evaluated 13 observational studies (with methodological limitations) in order to provide a more complete picture of the existing literature. The best evidence synthesis from these observational studies provides ‘very‐low‐level’ evidence, suggesting that multidisciplinary rehabilitation (both inpatient and home based) may improve function and that ambulatory programmes may improve vocational outcomes and quality of life in people with brain tumour in the short term.
Potential biases in the review process
The conclusions from this review are limited by the lack of robust clinical trials and 'observational' studies of poor methodological quality with diverse approaches to multidisciplinary rehabilitation as described above. In addition, the review authors recognise a number of limitations in the methodology of the review itself and the completeness of the retrieved literature.
-
The possibility of selection bias from the literature search (van Tulder 2003). Our search strategy principally encompassed cited literature. However, we used an extended range of terms to capture the widest possible selection of the relevant literature.
-
Publication bias cannot be ruled out as we cannot exclude the possibility of negative trials that have not reached the published literature (Egger 1998).
-
Similarly, reference bias is a further possible confounder (Goetzsche 1987), although our search strategy included searching of reference lists within the relevant articles for other possible articles missed in our electronic searches.
-
Searches on the foreign language databases (LILACS) were limited mainly to English language terms, so it is possible that we missed relevant studies in other languages.
We therefore welcome contact from any readers who are aware of important high‐quality studies that would meet the criteria for this review, but are so far not included. We did not find or were not aware of any ongoing studies.
Agreements and disagreements with other studies or reviews
The findings of this review highlight the existing gaps in the literature and emphasise the lack of robust evidence to support multidisciplinary rehabilitation for people with brain tumours. These findings are consistent with other reviews in this field (Huang 2001b; Huang 2011; Vargo 2011).

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| High‐intensity multidisciplinary rehabilitation compared with wait‐list control group with usual care for primary brain tumour | ||||||
| Patient or population: 106 participants with primary brain tumour Settings: outpatient Intervention: High‐intensity multidisciplinary rehabilitation Comparison: wait‐list control group with usual care | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect* | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care (control group) | High‐intensity multidisciplinary rehabilitation (intervention group) | |||||
| Short‐term disability outcomes at 3‐months postintervention | ||||||
| Change in short‐term disability (function) FIM motor | Median change = 8 points higher | Median change = 18 points higher | Z score: ‐3.13, P < 0.001 R: 0.32 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 13 items with 4 subscales (self care, transfers, locomotion, sphincter control, assessing function (activity) and need for assistance, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Change in short‐term disability (cognition) FIM cognition | Median change = 3 points higher | Median change = 6 points higher | Z score: ‐1.99, P < 0.05 R: 0.20 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 3 items with 3 subscales (communication, psychological, cognition) assessing cognition, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Long‐term disability outcomes at 6‐months postintervention | ||||||
| Change in long‐term disability (function) FIM motor | Median change = 4 points higher | Median change = 12 points higher | Z score: ‐2.33, P < 0.05 R: 0.25 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 13 items with 4 subscales (self care, transfers, locomotion, sphincter control, assessing function (activity) and need for assistance, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Change in long‐term disability (cognition) FIM cognition | Median change = 1.5 points higher | Median change = 6 points higher | Z score: ‐3.09, P < 0.001 R: 0.20 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 3 items with 3 subscales (communication, psychological, cognition) assessing cognition, rated on a 7‐point scale (1‐7), with higher score indicating higher independence and lower need for assistance |
| Change in short‐term participation outcomes at 3‐months postintervention | ||||||
| Change in short‐term psychological outcomes | Median change = 12 points lower | Median change = 8 points lower | Z score: ‐0.53, P > 0.05 R: 0.05 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 21 items with 3 subscales assessing depression, anxiety, and stress, rated on a 4‐point scale, with higher score indicating higher level of impairment |
| Change in short‐term participation | Median change = 7 points lower | Median change = 6 points lower | Z score: ‐0.40, P > 0.05 R: 0.04 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 23 items with 5 subscales assessing mobility, self care, relationships, participation, and psychological well‐being, rated on a 6‐point scale, with high scores indicating greater impact |
| Change in short‐term QoL CARES‐SF (global) | Median change = 0.2 points lower | Median change = 0.1 points lower | Z score: ‐0.10, P > 0.05 R: 0.01 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 59‐item global scale, with overall score indicating QoL and summary scores for the 5 domains (physical, psychosocial, medical interaction, marital and sexual function), assessing cancer‐specific rehabilitation need and QoL, rated on a 4‐point scale, with higher scores indicating more difficulty or lower QoL |
| Change in long‐term participation outcomes at 6‐months postintervention | ||||||
| Change in long‐term psychological outcomes | Median change = 10 points lower | Median change = 12 points lower | Z score: ‐0.98, P > 0.05 R: 0.11 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 21 items with 3 subscales assessing depression, anxiety, and stress, rated on a 4‐point scale, with higher score indicating higher level of impairment |
| Change in long‐term participation | Median change = 9.5 points higher | Median change = 5 points lower | Z score: ‐0.37, P > 0.05 R: 0.04 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 23 items with 5 subscales assessing mobility, self care, relationships, participation, and psychological well‐being, rated on a 6‐point scale, with high scores indicating greater impact |
| Change in long‐term QoL CARES‐SF (global) | Median change = 0.2 points lower | Median change = 0.2 points lower | Z score: ‐0.42, P < 0.05 R: 0.05 | 106 (1 study) | ⊕⊕⊕⊕ Low1 | 59‐item global scale, with overall score indicating QoL and summary scores for the 5 domains (physical, psychosocial, medical interaction, marital and sexual function), assessing cancer‐specific rehabilitation need and QoL, rated on a 4‐point scale, with higher scores indicating more difficulty or lower QoL |
| Change in other outcomes | ||||||
| Cost‐ effectiveness | See comment | See comment | Not estimable | 106 (1 study) | See comment | Not measured |
| Serious adverse events | See comment | See comment | Not estimable | 106 | See comment | No serious adverse events attributed to the intervention |
| *Mann‐Whitney U tests # Effect size statistics (R) were calculated and assessed against Cohen’s criteria (0.1 = small, 0.3 = medium, 0.5 = large effect) CARES‐SF: Cancer Rehabilitation Evaluation System‐Short Form; DASS: Depression Anxiety Stress Scales; FIM: Functional Independence Measure; PIPP: Perceived Impact of Problem Profile; QoL: quality of life | ||||||
| 1GRADE Working Group grades of evidence | ||||||
| Methods | Case‐control study, Italy |
| Participants | N = 150; Intervention: N = 75 with brain tumours (meningioma and glioblastoma), control: N = 75 with stroke
Inclusion: all admitted patients to an inpatient neurorehabilitation unit after surgery for brain tumours (meningiomas or glioblastomas) over a 2‐year period (2007‐2009). Control participants were stroke patients (ischaemic or haemorrhagic), matched one‐to‐one for age, sex, and side of lesion Exclusion: people with oligoastrocytoma, oligodendroglioma, and ependymomas in order to obtain homogenous group
|
| Interventions | Inpatient multidisciplinary rehabilitation administered by experienced physical therapists, 60‐min session, 6 days/week for 4 consecutive weeks, which included passive/assisted stretching exercises, strengthening exercises, balance exercises, ground‐floor walking (including step control), and 4 weeks of speech therapy (individual 60‐min sessions, once daily, 6 days/week) when aphasia was diagnosed |
| Outcomes | Sitting balance, standing balance, Hauser Index: gait disorders, MGHFAC: severity of gait disorders, FIM |
| Assessment time points | Before and after the intervention |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control study, USA |
| Participants | N = 42; Intervention: N = 21 with low‐grade gliomas, control: N = 21 with high‐grade gliomas
Inclusion: all patients admitted to an inpatient acute rehabilitation programme between 1996 and 2008. 21 of 443 with high‐grade and 21 of 24 with low‐grade astrocytoma were selected |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; LOS; discharge‐to‐home rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: N/A Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Case‐control study, Turkey |
| Participants | N = 42; Intervention: N = 21 with brain tumours (benign and malignant), control: N = 21 with stroke
Inclusion: all admitted patients to an inpatient neurorehabilitation unit, control participants were stroke patients (ischaemic or haemorrhagic), matched by side of lesion Exclusion: people with oligoastrocytoma, oligodendroglioma, and ependymomas in order to obtain homogenous group |
| Interventions | Inpatient "conventional" rehabilitation programme, single 60‐min sessions, 5 days/week for 4 consecutive weeks, which included physiotherapy and occupational therapy (if needed). Physiotherapy focused on positioning, postural control, range of motion, and progressive resistive exercises together with endurance and gait. Patients were discharged when their functional level was considered sufficient to allow them to participate in outpatient rehabilitation |
| Outcomes | PAS for Stroke, BBS, MAS, FIM (mobility) |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control study, Israel |
| Participants | N = 1828; Intervention N = 168 with brain tumours (128 meningiomas, 40 gliomas), control: N = 1660 with stroke (ischaemic or haemorrhagic)
Inclusion: all admitted patients to an inpatient neurorehabilitation unit over an 11‐year period (1993‐2004)
|
| Interventions | Inpatient multidisciplinary rehabilitation provided by PT, medical staff, OT, and speech pathologist. Details of the multidisciplinary rehabilitation not provided |
| Outcomes | FIM, FIM efficiency, LOS days, discharge destination (rate discharge to home) |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: No Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Prospective case series, USA |
| Participants | N = 10 (brain tumour)
Inclusion: all admitted patients to an inpatient neurorehabilitation unit over a 1‐year period (1999‐2000) |
| Interventions | Inpatient multidisciplinary rehabilitation that included: OT, rehabilitation therapy, recreational therapy, speech therapy, PT, rehabilitation nursing and case management |
| Outcomes | FIM, DRS, KPS, FACT‐BR |
| Assessment time points | Admission and discharge, post hoc analysis at 3‐month postdischarge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control, USA |
| Participants | N = 156; Intervention: N = 78 with primary or metastatic brain tumours (benign and malignant), control: N = 78 with traumatic brain injury matched by age and side of lesion
Inclusion: evaluation by a physiatrist for the following criteria: medical stability, need for therapy from more than one discipline, demonstration of gains with acute‐care therapies, potential to tolerate 3 hours of therapy, willingness and motivation to participate in a rehabilitation programme Exclusion: patients who did not complete rehabilitation due to medical complications or death |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to community rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control study, USA |
| Participants | N = 126; Intervention: N = 63 with primary or metastatic brain tumours (benign and malignant), control: N = 63 with stroke, case matched by age, gender, and side of lesion
Inclusion: all patients admitted to an inpatient rehabilitation centre |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to community rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Cohort study without control, Korea |
| Participants | N = 25 with brain tumours Inclusion: all admitted patients to an inpatient neurorehabilitation unit after surgery (resection) for brain tumours (benign or malignant) over a 1‐year period (1 July 2008 to 30 June 2009), able to follow simple commands, as determined by scores ≥ 24 on the Korean version of the Mini‐Mental State Examination Exclusion: those unable to complete a questionnaire because of a severe aphasia or a cognitive deficit, or who were clinically unstable, either medically or surgically |
| Interventions | Inpatient rehabilitation (4 weeks); details not provided |
| Outcomes | Fatigue severity: PFS, BFI Mood status: BDI Motor impairment: MI Functional status: KPS, MBI QoL: EORTC QLQ‐C30 |
| Assessment time points | Before and after the intervention |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case series, USA |
| Participants | N = 132 participants divided into 4 groups: astrocytomas 26%, meningiomas 33%, metastatic tumours 16%, other tumours 25%. Participants also grouped into those with tumour recurrence and those with initial tumour presentation
Inclusion: all patients > 18 years, inpatient rehabilitation within a 3‐year period (1993‐1996) |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to home rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case‐control, USA |
| Participants | N = 80; Intervention: N = 40 participants with brain tumours (benign and malignant), control: N = 40, case matched by admission FIM score, age, and gender to 40 participants with traumatic brain injury
Inclusion: all patients admitted to an inpatient acute rehabilitation programme over a 2‐year period (1994‐1996) |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; LOS; discharge destination to home rate |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Prospective case series (before and after) study, Italy |
| Participants | N = 121 with malignant brain tumours
Inclusion: all patients discharged from hospital over 3‐year period (2000‐2003) with neurological deficits |
| Intervention | Home neurorehabilitation programme including physiotherapy 1 hour/3 times a week for 3 months, neurologist evaluation, psychological assistance, nursing and palliative care team if needed (further details not provided) |
| Outcomes | BI, KPS, EORTC QLQ‐C30 |
| Assessment time points | Before and 3 months after rehabilitation |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Patients who completed only basal questionnaire were excluded Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case series, USA |
| Participants | N = 13 (primary malignant brain tumours with a history of surgical resection, radiation, and chemotherapy)
Inclusion: all patients receiving outpatient rehabilitation who had a diagnosis of malignant brain tumour and adequate medical records to characterise their tumour and courses of therapy |
| Intervention | Outpatient rehabilitation with input from psychologists, speech/language pathologists, OT, and vocational specialists. Participants received an average of 2.6 ± 1.9 months of therapy (duration of 5 hours/day) (further details not provided) |
| Outcomes | Level of independence, vocational (productivity) outcomes |
| Assessment time points | Admission, discharge, and 8‐months follow‐up |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Yes Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| Methods | Retrospective case series, Canada |
| Participants | N = 63 with primary and metastatic brain tumours, divided into 3 groups: glioblastoma multiforme 29%; metastatic tumours 40%; and various other primary brain tumours 31%
Inclusion: all patients admitted to an inpatient rehabilitation ward over a 3‐year period (2003‐2006) Exclusion: patients with meningiomas |
| Intervention | Inpatient multidisciplinary rehabilitation (details not provided) |
| Outcomes | FIM; FIM efficiency; LOS; discharge destination to home rate; survival |
| Assessment time points | Admission and discharge |
| Risk of bias | Adequate sequence generation: No Adequate allocation concealment: No Blinding: No Incomplete outcome data addressed: Unclear Free of selective reporting: Yes Other bias:
|
| Quality rating of the study | Very low |
| BBS: Berg Balance Scale | |
| Statistical analysis | Student’s t test, Chi2 test, Wilcoxon matched‐pairs signed‐rank test, Mann‐Whitney U test, Kruskal–Wallis ANOVA |
| Results |
|
| Author’s conclusions | Rehabilitation after surgery can improve functional outcome, justifying the delivery of rehabilitation services, even during the acute phase, to brain tumour inpatients, irrespective of tumour type |
| Statistical analysis | Descriptive analysis, Chi2 test, Kruskal‐Wallis test, Mann‐Whitney U test |
| Results |
|
| Author’s conclusion | All participants made significant functional gains from admission to discharge. Compared with people with low‐grade astrocytoma, people with high‐grade astrocytoma had higher total FIM gain but also longer LOS. FIM efficiencies were comparable between the groups |
| Statistical analysis | Freidman test, Chi2 test, Mann‐Whitney U test, ANOVA |
| Results |
|
| Author’s conclusions | People with brain tumour progressed as well as people with stroke in a post‐acute inpatient rehabilitation programme |
| Statistical analysis | Descriptive statistics, analysis of variance |
| Results |
|
| Author’s conclusions | Both people with gliomas and people with meningiomas hospitalised for inpatient rehabilitation improved their FIM ratings after a short inpatient multidisciplinary rehabilitation. Both groups had high rates of discharge to the community |
| Statistical analysis | ANOVA, Spearman’s correlation analysis, Bonferroni statistical test |
| Results |
|
| Author’s conclusion | Although participants made functional gains during and after inpatient multidisciplinary rehabilitation, gains in QoL were not significant until 1‐month postdischarge. QoL does not appear to correlate well with functional outcomes. Furthermore, the KPS is less sensitive than the FIM and DRS in detecting change in functional status |
| Statistical analysis | ANOVA, Chi2 test |
| Results |
|
| Author’s conclusion | People with brain tumour can achieve comparable functional outcome and have a shorter rehabilitation length of stay and greater discharge to community rate than people with traumatic brain injury |
| Statistical analysis | ANOVA, Chi2 test |
| Results |
|
| Author’s conclusion | People with brain tumour can achieve comparable functional outcome and discharge to community rate, and have a shorter rehabilitation length of stay than people with stroke |
| Statistical analysis | Mann–Whitney test, Spearman’s correlation analysis, Wilcoxon signed‐rank tests |
| Results |
|
| Author’s conclusion | The findings suggest that people with brain tumours commonly complain of a moderate level of fatigue, which may reduce daily functioning and quality of life, with sleep disturbance being a significant predictor of fatigue. During rehabilitation, functional outcomes and motor power showed improvements in those people, not aggravating fatigue |
| Statistical analysis | Descriptive analysis, analysis of variance |
| Results |
|
| Author’s conclusion | Metastatic or primary brain tumour type does not affect the efficiency of functional improvement during inpatient multidisciplinary rehabilitation. People receiving concurrent radiation therapy make greater functional improvement per day than those not receiving radiation. People with recurrent tumours make significantly smaller functional motor gains than those completing inpatient multidisciplinary rehabilitation after the initial diagnosis of the tumour |
| Statistical analysis | Descriptive analysis, Chi2 test, Kruskal‐Wallis test, Mann‐Whitney U test |
| Results |
|
| Author’s conclusion | Daily functional gains made by people with brain tumour undergoing multidisciplinary rehabilitation were similar to those made by people with traumatic brain injury matched by age, gender, and admission functional status |
| Statistical analysis | Chi2 test, Student t test (paired or not, as appropriate) |
| Results | At 3‐months follow‐up:
|
| Author’s conclusion | Multidisciplinary rehabilitation at home in people with brain tumour was associated with significant functional gain measured both with BI and KPS. The benefit of multidisciplinary rehabilitation may influence patient's perception of quality of life |
| Statistical analysis | Descriptive analyses only |
| Results |
|
| Author’s conclusion | People with primary malignant brain tumours achieved increased community independence and vocational outcomes (such as employment, education) after individualised outpatient multidisciplinary rehabilitation. Such treatment programme appears to be an attractive, relatively low‐cost option for these patients, however additional investigation is needed. |
| Statistical analysis | ANOVA, Chi2 test, Kruskal‐Wallis and post‐hoc tests using Mann‐Whitney U test with Bonferroni adjustment, Wilcoxon signed‐ranks test, logistic regression, Kaplan‐Meier analyses |
| Results |
|
| Author’s conclusion | People with primary and metastatic brain tumours achieved functional gains after multidisciplinary rehabilitation. High functional improvement is a significant predictor of longer survival in brain metastases and GBM. |
| ADL: activities of daily living | |

